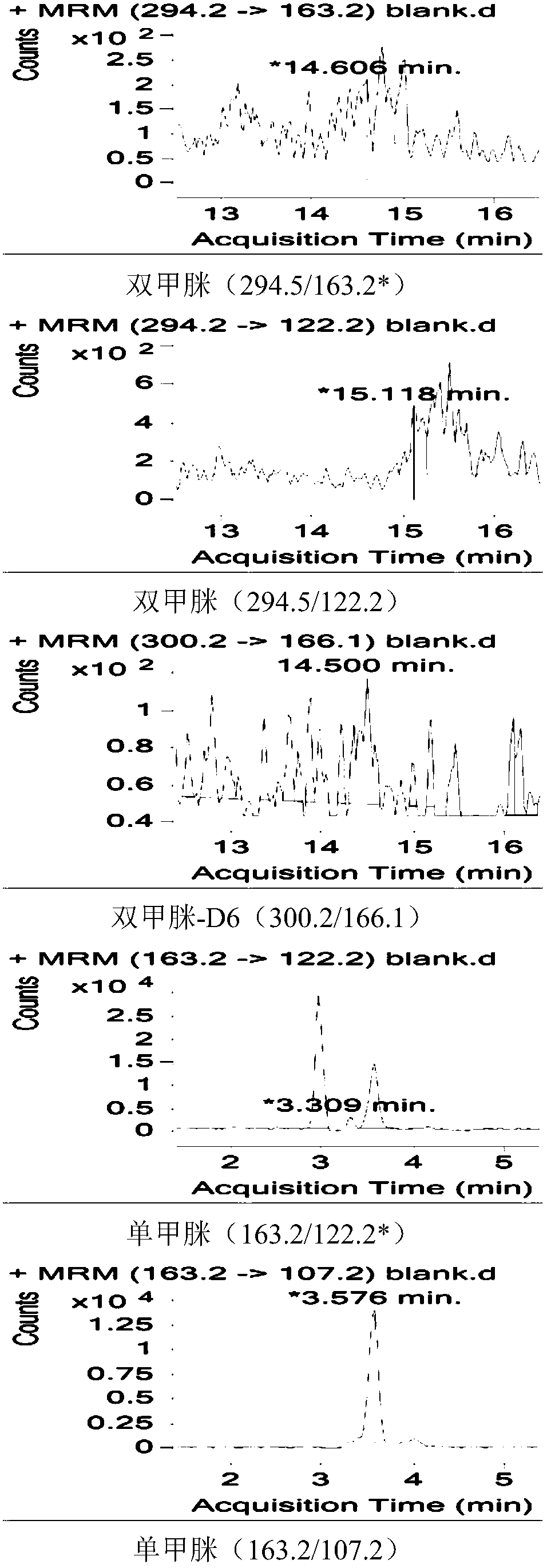
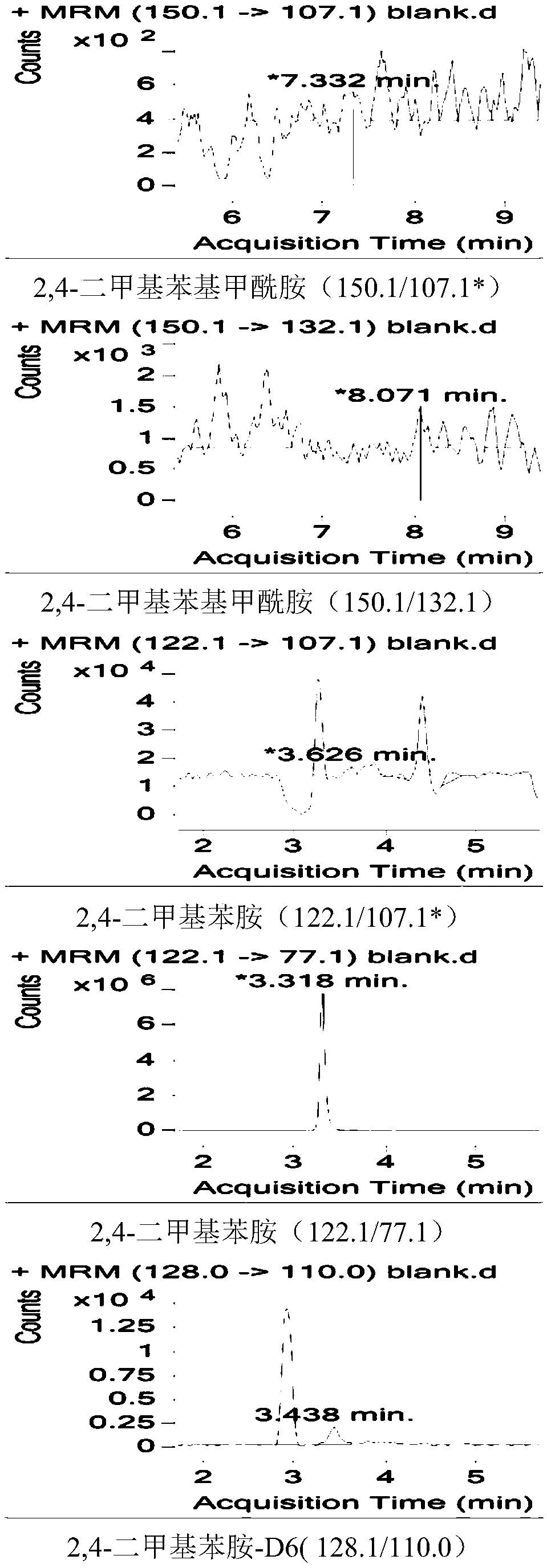
Method for simultaneously determining residual quantities of amitraz and metabolites in royal jelly through dispersive solid phase extraction-liquid chromatography-mass spectrometry/mass spectrometry method
A liquid chromatography and disperse solid-phase technology, which is applied in the field of determination of amitraz and its metabolite residues in royal jelly, can solve the problems of large liver damage, toxic side effects, mutagenicity, etc., and achieve low resource consumption and food maintenance. Safety and low detection cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0037] Dispersive solid phase extraction-liquid chromatography-mass spectrometry / mass spectrometry is a method for simultaneous determination of bisformamidine and its metabolites in royal jelly. The method includes the following steps:
[0038] One, extraction
[0039] Due to the instability of amitraz, it is necessary to first investigate the stability of the compound under the extraction conditions. Due to the particularity of royal jelly samples, protein precipitation is a key step of pretreatment. Therefore, it is necessary to determine the drug residues in royal jelly samples. Remove protein treatment from the sample to reduce the influence of protein on the determination. The experiment comprehensively investigated the effect of organic solvent precipitation and extraction of protein in acidic solution, neutral solution and alkaline solution environment. The study found that acid solution (pH=5) bisformamidine is prone to hydrolysis, and the recovery rate is less than 30%, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com