Synthetic method of deoxyribonucleic acid (DNA) methyl transferase inhibitor
A technology of methyltransferase and synthesis method, applied in the field of medicinal chemistry synthesis, can solve the problems of complex synthesis method, leukopenia, lack of DNMT, etc., and achieve the effects of simple synthesis route, improved reaction rate, and accelerated reaction rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
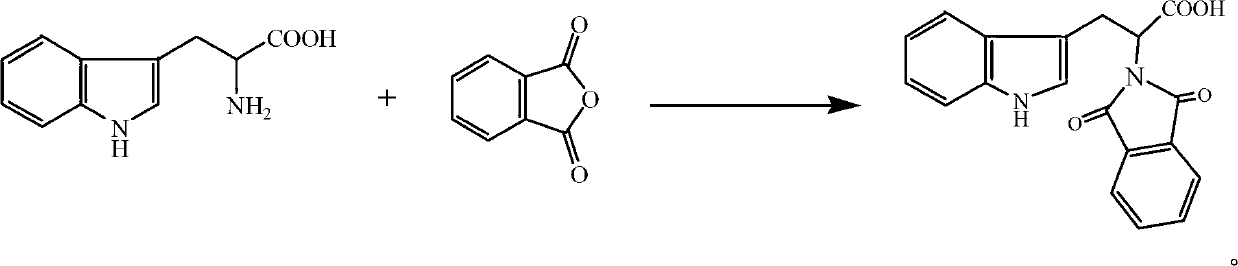
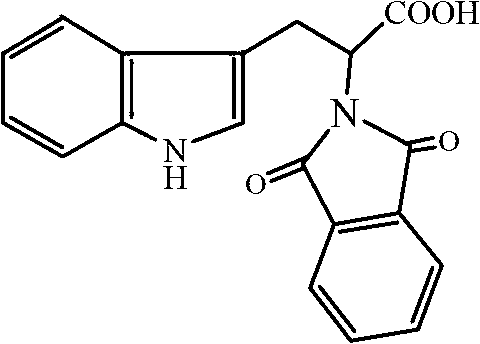
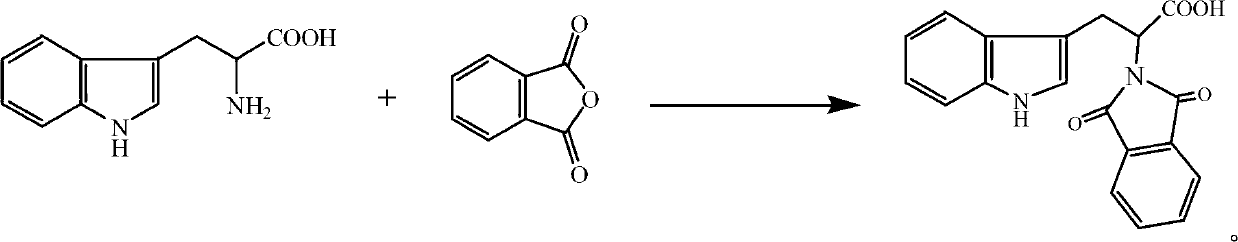
[0023] Dissolve 0.011mol phthalic anhydride (1.63g) in acetone (20mL), then add 0.010mol tryptophan (2.03g) in acetone (20mL) solution, stir at room temperature for 1h, then add 0.015mol acetic anhydride (dehydrating agent) and 0.005mol triethylamine (catalyst), reflux at a temperature of 56°C for 3 hours for acylation reaction; after the reaction is completed, the reaction solution is cooled and poured into ice water (100mL) and allowed to stand , precipitated a white solid, filtered it with suction, washed the filter cake with water three times, recrystallized from absolute ethanol, and dried in vacuo to obtain 2-(1,3-dioxo-1,3-dihydro-2H- Isoindole)-3-(1H-indole)propionic acid, white solid, 2.72g, yield 81.5%, melting point 163°C-164°C. 1 H-NMR (CD 3 OD)δ: 9.76 (s, 1H); 7.72 (s, 4H); 7.46 (d, 1H); 7.20 (d, 1H); 6.98 (t, 1H); 6.93 (s, 1H); 1H); 5.19 (m, 1H); 3.65 (m, 2H); IR(KBr)ν / cm -1 : 3340 (NH); 2916 (CH 2 ); 1751 (C=O); 1710 (C=O); 1637 (=CH); 1549, 1450 (skeletal v...
Embodiment 2
[0025] Dissolve 0.010mol phthalic anhydride (1.48g) in acetone (20mL), then add 0.010mol tryptophan (2.03g) in acetone (20mL) solution, stir at room temperature for 1h, then add 0.020mol acetic anhydride (dehydrating agent) and 0.003mol pyridine (catalyst), reflux at a temperature of 50°C for 5 hours for acylation reaction; after the reaction is completed and the reaction solution is cooled, pour the reaction solution into ice water (100mL), let stand, and precipitate White solid, filtered with suction, washed the filter cake with water three times, recrystallized from absolute ethanol, and dried in vacuo to obtain 2-(1,3-dioxo-1,3-dihydro-2H-isoindol Indole)-3-(1H-indole)propionic acid, white solid, 2.67g, yield 80%, melting point 163°C-164°C.
Embodiment 3
[0027] Dissolve 0.014mol phthalic anhydride (2.07g) in acetone (30mL), then add 0.010mol tryptophan (2.03g) in acetone (20mL) solution, stir at room temperature for 1h, then add 0.010mol acetic anhydride (dehydrating agent) and 0.010mol triethylamine (catalyst), reflux for 4h at a temperature of 60°C for acylation reaction; after the reaction is completed and the reaction solution is cooled, pour the reaction solution into ice water (100mL) and let stand , precipitated a white solid, filtered it with suction, washed the filter cake with water three times, recrystallized from absolute ethanol, and dried in vacuo to obtain 2-(1,3-dioxo-1,3-dihydro-2H- Isoindole)-3-(1H-indole)propionic acid, white solid, 2.74g, yield 82.1%, melting point 163°C-164°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com