Coxsackie virus A16 virus strain, uses of strain, vaccine and preparation method of vaccine
A technology of coxsackie virus and virus strain, applied in antiviral immunoglobulins, biochemical equipment and methods, antiviral agents, etc., can solve the difference in antigenicity and immunogenicity, the gap is large, and the pathogenic mechanism is unclear and other problems, to achieve the effect of effective immune activity and strong virulence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
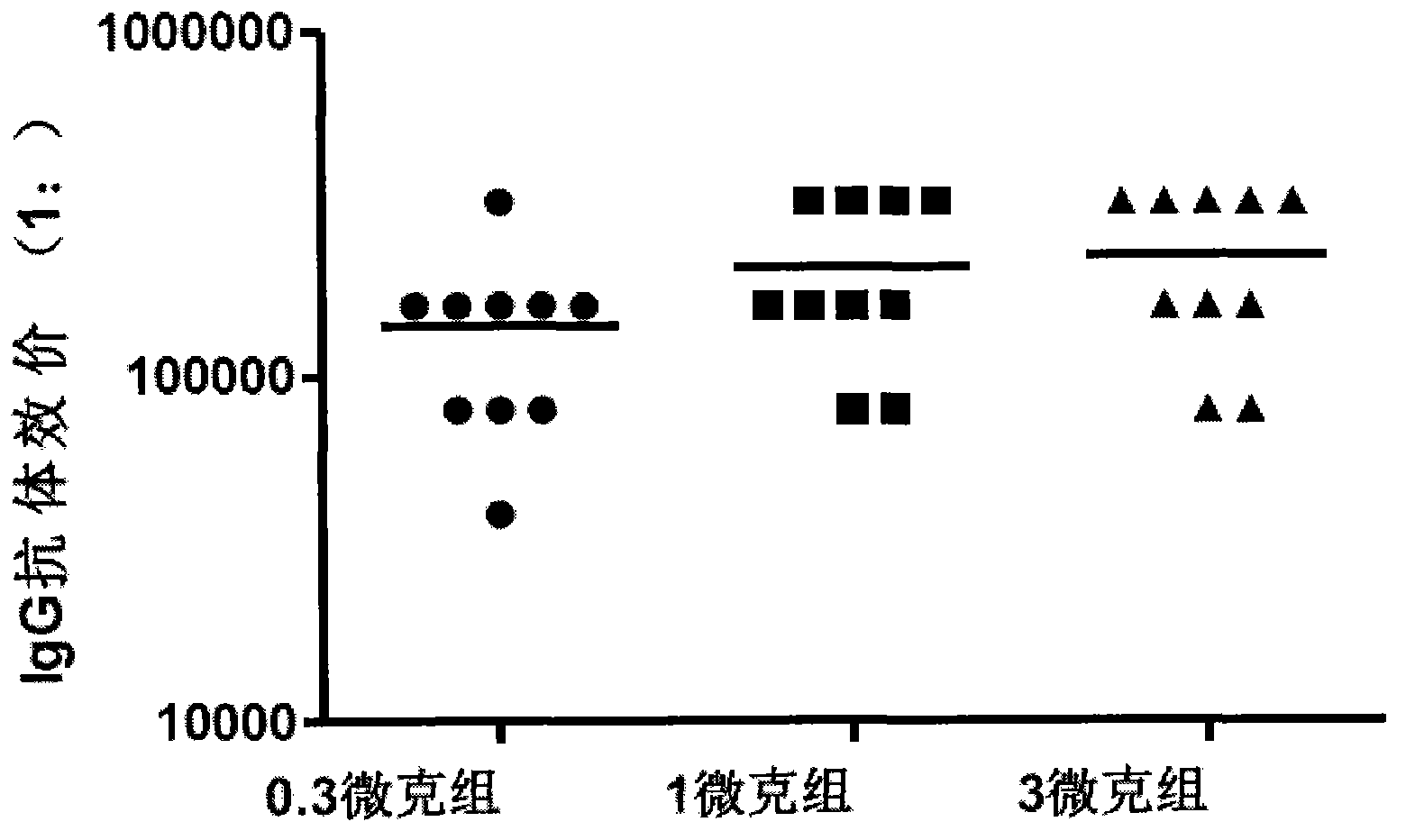
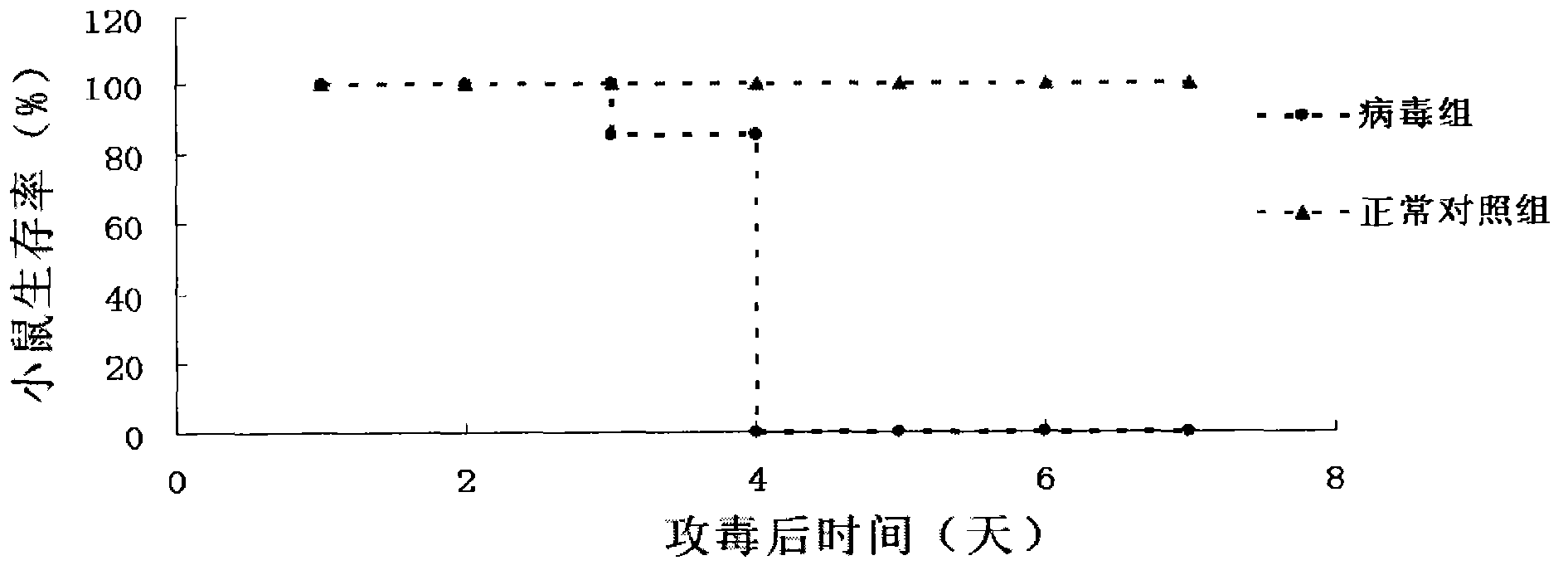
Image
Examples
preparation example Construction
[0051] The fourth aspect of the present invention provides the preparation method of the vaccine, which includes: cultivating the A16 strain of Coxsackievirus, inactivating and purifying it, so as to prepare the vaccine. Preferably, said purification is performed before said inactivation and / or after said inactivation.
[0052] Preferably, the preparation method of the present invention comprises:
[0053] 1) Provide Vero cells or human diploid cells;
[0054] 2) In the Vero cells or human diploid cells obtained in step 1), the Coxsackievirus A16 strain is cultured to obtain a virus suspension;
[0055] 3) purifying and inactivating the virus suspension obtained in step 2), so as to obtain the vaccine stock solution; and
[0056] 4) Diluting the vaccine stock solution obtained in step 3) to obtain the vaccine.
[0057] Preferably, those skilled in the art can prepare the Coxsackie virus A16 type inactivated vaccine by conventional methods as required, wherein the routine pr...
Embodiment 1
[0079] Embodiment 1: Isolation of CA16 virus strain
[0080]Collect throat swab specimens from patients with HFMD (isolated from Feng XX, a 3-year-old child with severe HFMD in Shijiazhuang City, Hebei Province), centrifuge at 2000-4000rpm / min for 30min at 4°C, and take the supernatant with 0.2 Sterilize with a μm filter, and inoculate Vero cells or human diploid cells with the filtered supernatant. Before inoculating the specimen, pour off the growth solution, and each bottle of cells (8×10 6 Each / bottle) inoculated with 0.2-1ml of sample suspension, cultured at 36±1°C; after 1-2 hours of adsorption, replaced with 10ml of DMEM medium containing 2% (v / v) bovine serum to continue the culture. Use an inverted microscope to observe the cytopathic changes day by day. If there is no cytopathic effect (CPE) in 7 days, continue to observe for 7 days until 75% to 90% of the cells change, and then store them at -20°C for secondary use. Subpassaging. When suspicious cell lesions are ...
Embodiment 2
[0081] Embodiment 2: the identification method of CA16 virus strain
[0082] (1) Morphological observation: The lesions of the CA16 virus strain of Example 1 on the Vreo cells were observed under an optical microscope, and the cells were rounded and dispersed, and the particles in the cytoplasm increased. After concentration by ultrafiltration and negative staining with 2% phosphotungstic acid solution, spherical virus particles with a diameter of about 30nm can be observed under an electron microscope.
[0083] (2) Immunological test identification: After neutralizing the virus culture fluid of CA16 virus strain and the same amount of anti-CA16 immune serum at 36±1°C for 1 to 2 hours, inoculate it on a cell culture plate, continue culturing for 7 days, and observe the lesions The number of cell wells, set serum and cell controls at the same time, the titer of the virus control should not be lower than 500CCID 50 / ml. The results showed that all the wells of the virus contro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com