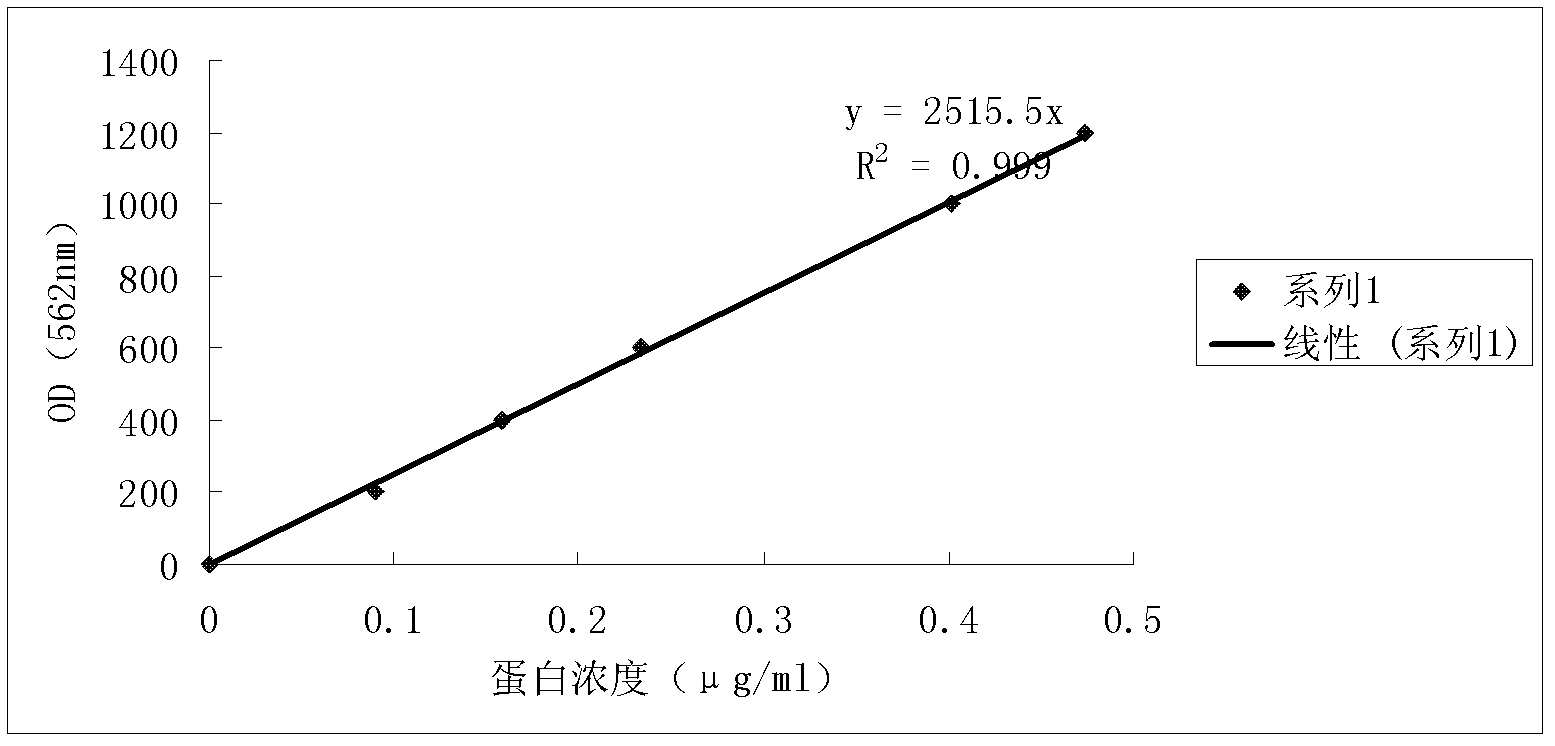
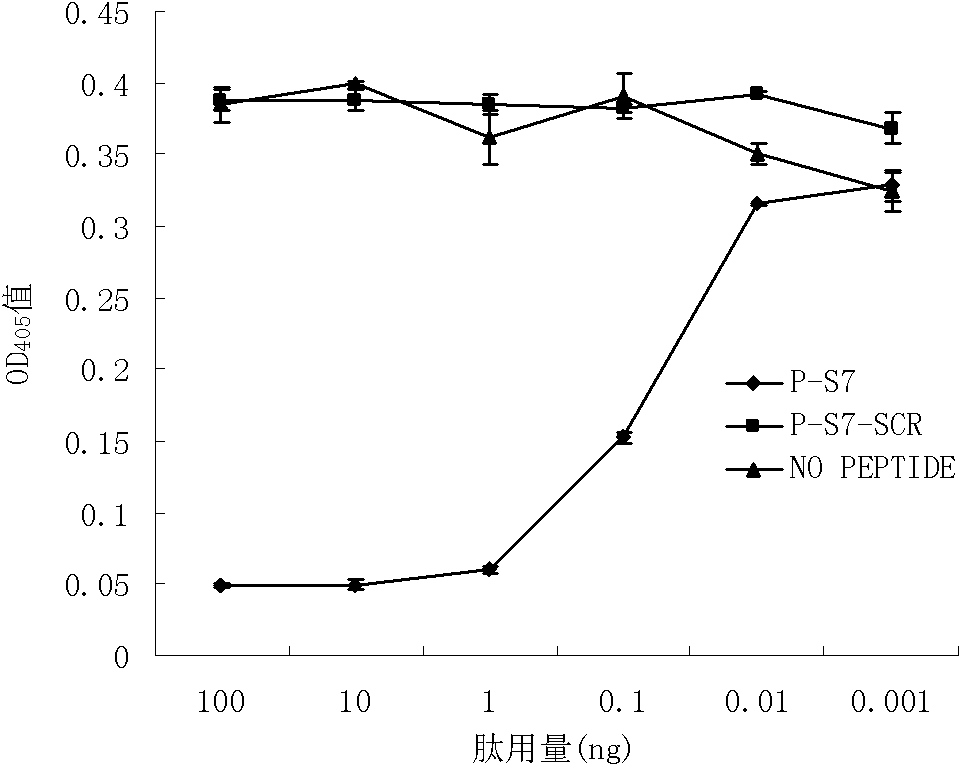
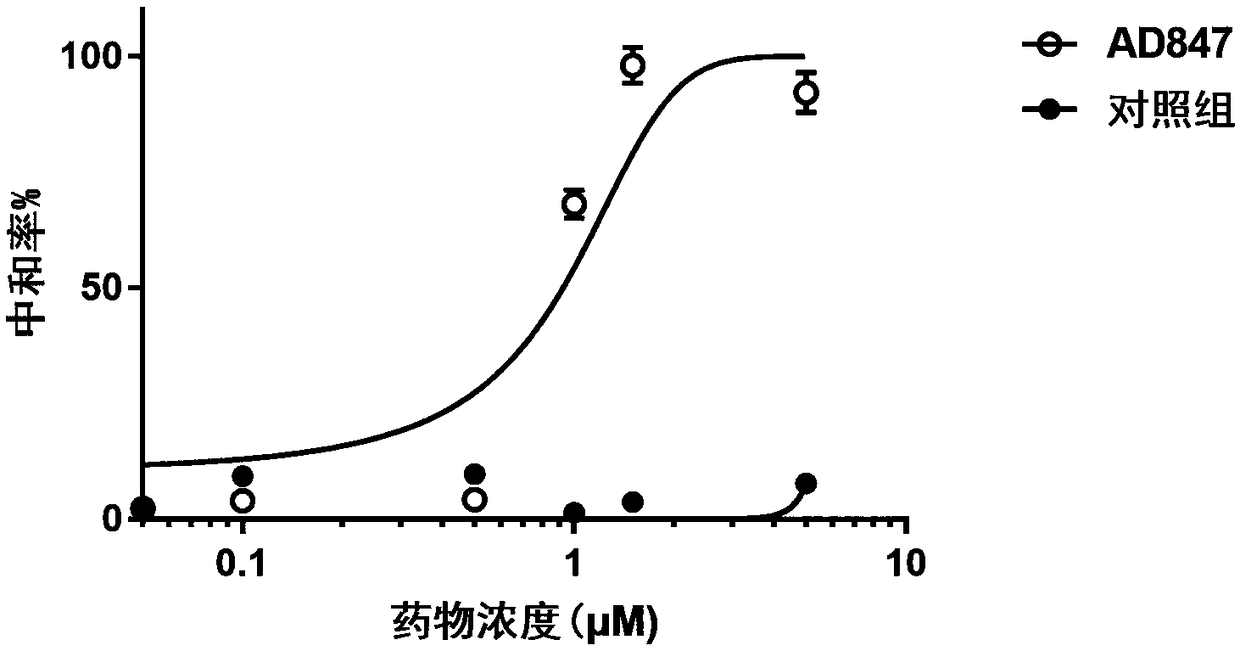
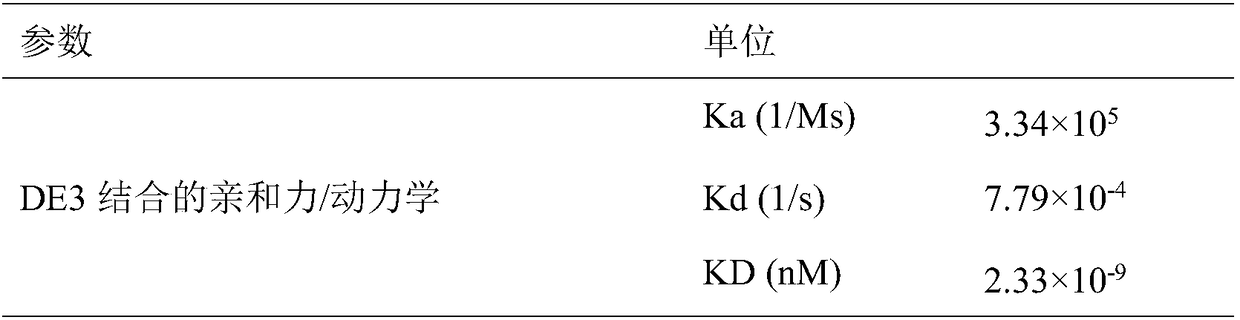
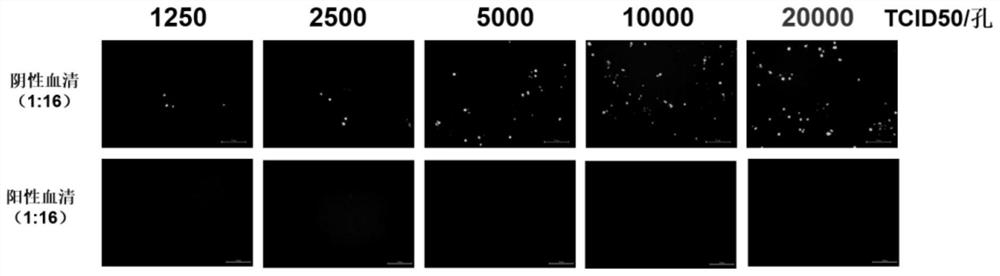
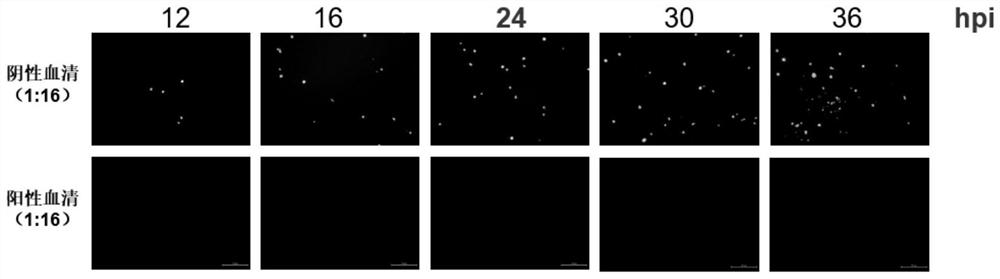
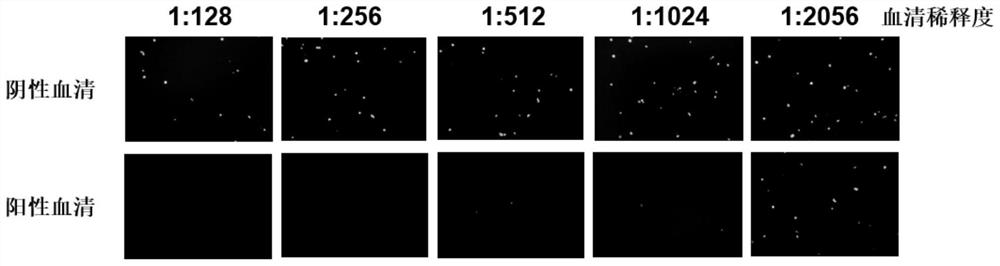
Patents
Literature
52 results about "Neutralization test" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method for preparing porcine circovirus type 2 colloidal gold antibody fast test strip
InactiveCN101881770AMicroorganism based processesFermentationGenetic engineeringEnzyme linked immunoassay
The invention relates to a method for preparing a porcine circovirus type 2 colloidal gold antibody fast test strip. In the method, a porcine circovirus type 2 ORF2 protein is expressed by utilizing genetic engineering, and the test strip is prepared by the principle of enzyme-linked immunoassay and membrane chromatography to fast test antibodies in the blood or serum of pigs. The test strip can be widely used for clinically testing porcine circovirus diseases, has no cross reaction with other viruses, and obtains test results 98.63 percent and 95.83 percent consistent with those obtained by the two methods of the ELISA and neutralization test. Compared with the ELISA, a recombinant antigen immune colloidal gold has the remarkable advantages of high security, no need of culturing the viruses per se, the avoidance of virus spread caused by the operation of the viruses, large-batch preparation, simple process, low production cost, stable and homogeneous antigen components, simple, convenient and labor-saving operation, no apparatus, high detection result specificity, high repeatability, the short time of 15 minutes for the whole test, simple, convenient, fast and accurate operation, high sensitivity, intuition and easy result judgment.
Owner:QINGDAO AGRI UNIV +2
Foot-and-mouth disease virus (FMDV) resistant monoclonal antibody and identified epitope and application thereof
ActiveCN101724605AGood passive immunityImprove immunityVirus peptidesImmunoglobulins against virusesIn vivoAmino acid
The invention discloses a foot-and-mouth disease virus (FMDV) resistant monoclonal antibody and an identified epitope and application thereof, and belongs to the field of prevention and control of the FMDV. The microbial collection number of a hybridoma cell line, which can secrete the neutralizing monoclonal antibody resisting to Asia-1 FMDV, is CGMCC No.2692; and the microbial collection number of the hybridoma cell line, which can secrete the neutralizing monoclonal antibody resisting to O-type FMDV, is CGMCC No.2691. The invention also discloses amino acid sequences of a conformational neutralizing epitope of the Asia-1 FMDV VP1 protein and a linear neutralizing epitope of the O-type FMDV VP1 protein which are identified by the two monoclonal antibodies respectively. In-vitro neutralization tests and in-vivo animal protection tests show that both the two monoclonal antibodies have excellent passive immunity effect, can be applied to emergency prevention of the FMDV and have excellent immunity effect on the passive immunity of the FMDV.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Hybridoma cell strain capable of secreting CPIV3 antibody and ELISA kit
ActiveCN105838678AHas a blocking effectMicroorganism based processesImmunoglobulins against virusesElisa kitSerum samples
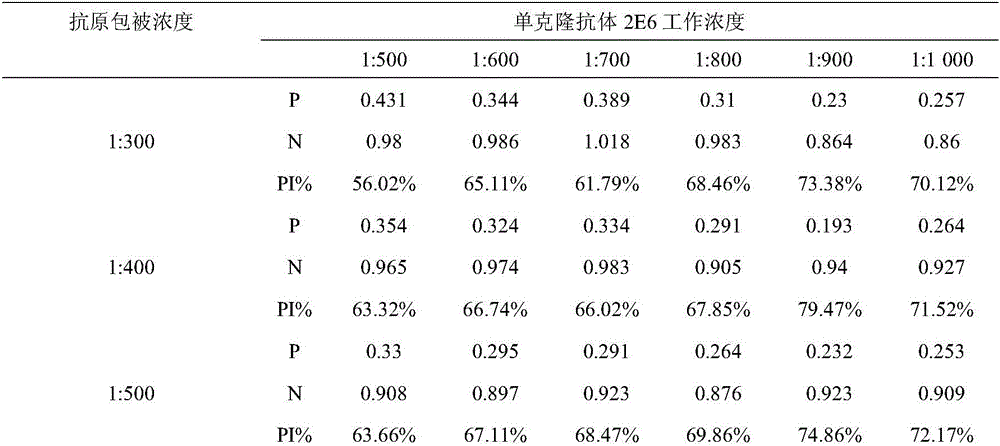
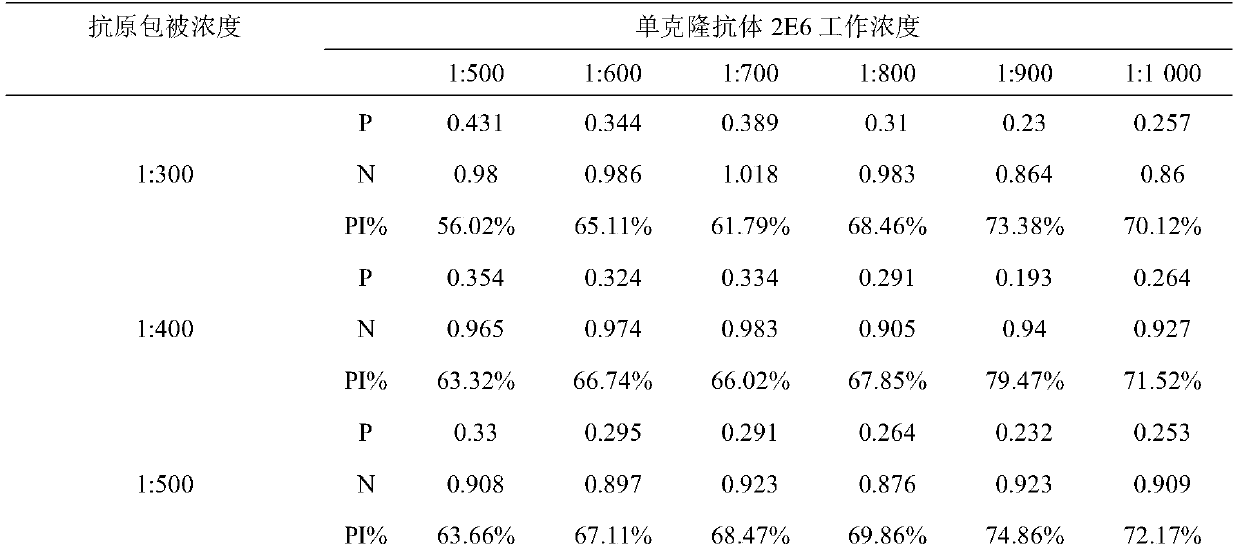
The invention belongs to the field of molecular biology, and relates to a hybridoma cell strain capable of secreting a CPIV3 antibody and an ELISA kit. A caprine parainfluenza virus 3 CPIV3 immunogen used in the invention is a CPIV3 JS2013 viral strain isolated clinically, and a hybridoma cell strain 2E6 is screened from an established hybridoma cell bank capable of secreting the CPIV3 antibody. The monoclonal antibody secreted by the hybridoma cell strain has the blocking effect, and is applied to the CPIV3 blocking ELISA kit. The CPIV3 blocking ELISA kit can specifically detect the antibody generated after the CPIV3 infection or caprine immunization. 212 caprine clinical serum samples are colleted, and the CPIV3 antibody in the serum is tested by the established blocking ELISA method and a neutralization test. Through the comparison between the result of the blocking ELISA method and the result of the neutralization test, the coincidence rate is 98.8%.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Pseudo-virus and packaging method thereof and drug evaluation system
ActiveCN111662884AAvoid the risk of secondary infectionImprove infection abilitySsRNA viruses positive-senseMicrobiological testing/measurementDiseaseReceptor
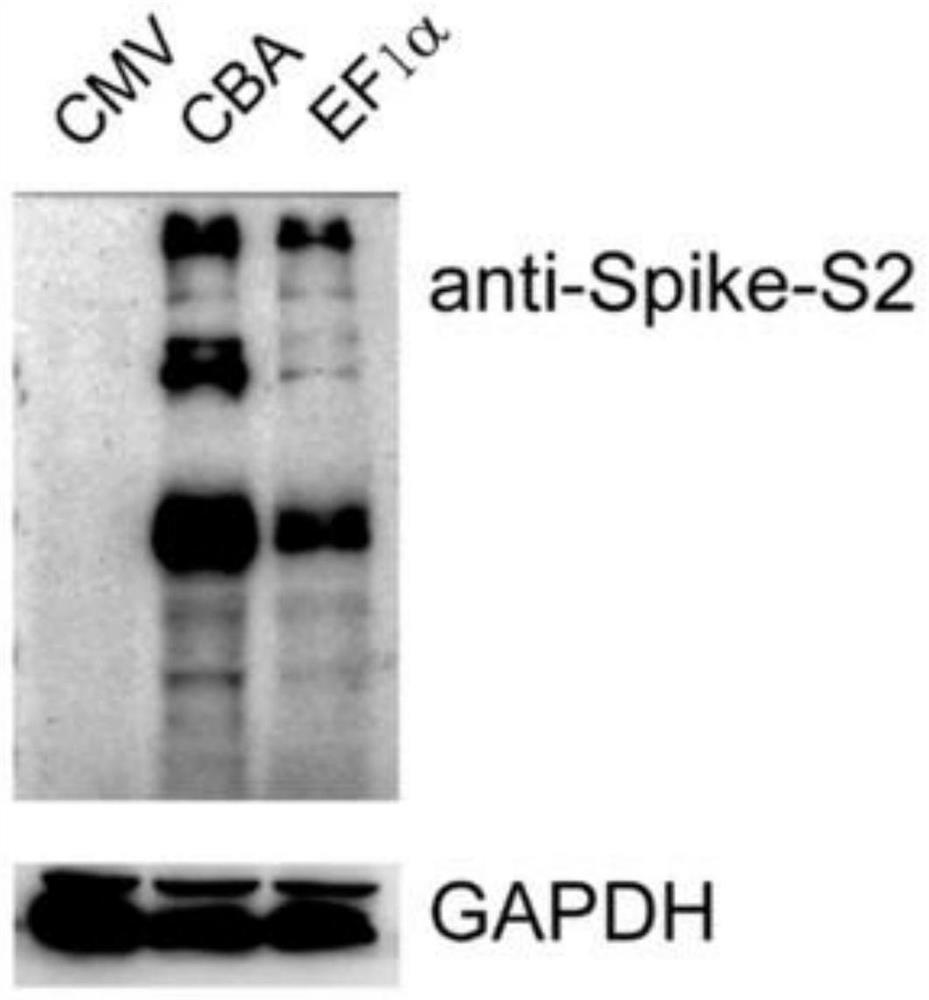
The invention relates to pseudo-virus. The pseudo-virus comprises a plasmid for expressing envelope protein Spike, a reporter gene plasmid and a packaging helper plasmid, wherein the plasmid for expressing the envelope protein Spike contains a CBA promoter and a human optimized Spike expression sequence, and is used for driving the expression of the envelope protein Spike. The pseudo-virus disclosed by the invention can simulate the process of infecting cells by wild novel coronavirus, can be used for researching the relationship between virus and host cells, cloning a receptor of virus and replacing a traditional neutralization test, and is used for researching differential diagnosis of diseases, evaluation and screening of antiviral drugs, evaluation of vaccine immune effect and the like. The invention also relates to a packaging method of the pseudo-virus and a novel coronavirus virus drug evaluation system containing the pseudo-virus, wherein the pseudo-virus is used for infectingthe 293T monoclonal cell which overexpresses the human ACE2 gene under the condition of drug interference, and by detecting the expression quantity of a reporter gene in the pseudo-virus (such as fluorescence intensity detection), the effectiveness of the drug is preliminarily evaluated.
Owner:中吉当康(北京)基因技术有限公司
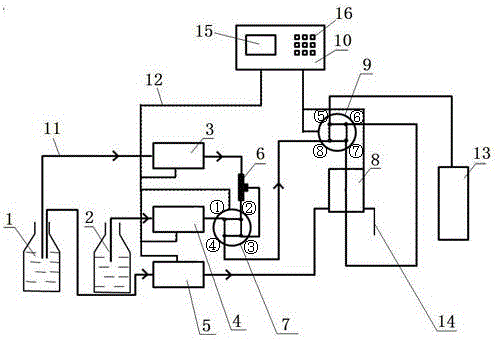
On-line sample dilution and neutralization test device
ActiveCN104897820AAccurate valueReliable test conditionsComponent separationPeristaltic pumpFour-way valve
The invention discloses an on-line sample dilution and neutralization test device which consists of a dilution device, a neutralizer, an instruction control device, a silica gel pipe and a control line, wherein an ultrapure water storage bottle and a sample bottle are connected to a peristaltic pump, an injection pump, a three-way mixer, an electric two-position four-way valve, a three-way mixer and the neutralizer through the dilution silica gel pipe; a control box is connected with the peristaltic pump, the injection pump, the three-way mixer, the electric two-position four-way valve and the neutralizer through the control main line in a control manner; automatic sample dilution and sample neutralization are completed on line through a pipeline and a circuit; samples are provided for a chromatographic analysis system to complete analysis. According to the on-line sample dilution and neutralization test device, the problem caused by ion pollution of an existing neutralization method is effectively solved; no suction pipe or no volumetric flask is used in the whole neutralization process any more, so that the pollution source is reduced, a precise value of experimental data measurement is guaranteed, and a reliable test condition is provided for research on ion chromatographic analysis of snow and ice samples.
Owner:NORTHWEST INST OF ECO-ENVIRONMENT & RESOURCES CAS
DHAV (duck hepatitis A virus) JS strain and application of DHAV JS strain in duck virus hepatitis prevention and cure
ActiveCN103013931AReduce the injection doseReduce stress responseMicroorganism based processesAntiviralsDuck hepatitis A virusDisease
The invention discloses a novel DHAV (duck hepatitis A virus) JS strain and application of the DHAV JS strain in duck virus hepatitis prevention and cure. The DHAV JS strain is obtained through the steps of separation culture, duck embryo neutralization test, PCR (polymerase chain reaction) detection of viruses and sequencing detection experiment of the viruses, and the strain is determined to be the DHAV serotype 3; and the microbial preservation number is CGMCCNo.6852. According to the invention, through taking the novel DHAV JS strain as the virus strain for producing vaccine, the novel DHAV inactivated vaccine is prepared through the steps of inoculating 10-day-old SPF (specific pathogen free) duck embryos, selecting the embryos died after incubating for 48 hours, collecting the embryo liquid, inactivating and emulsifying. The clinical application proves that the prepared inactivated vaccine is favorable in safety and conducive to prevention and control of the novel DHAV disease, can prevent the infection and outbreak of the novel DHAV in a targeted way, and can be in mass production and clinical application.
Owner:哈药集团生物疫苗有限公司
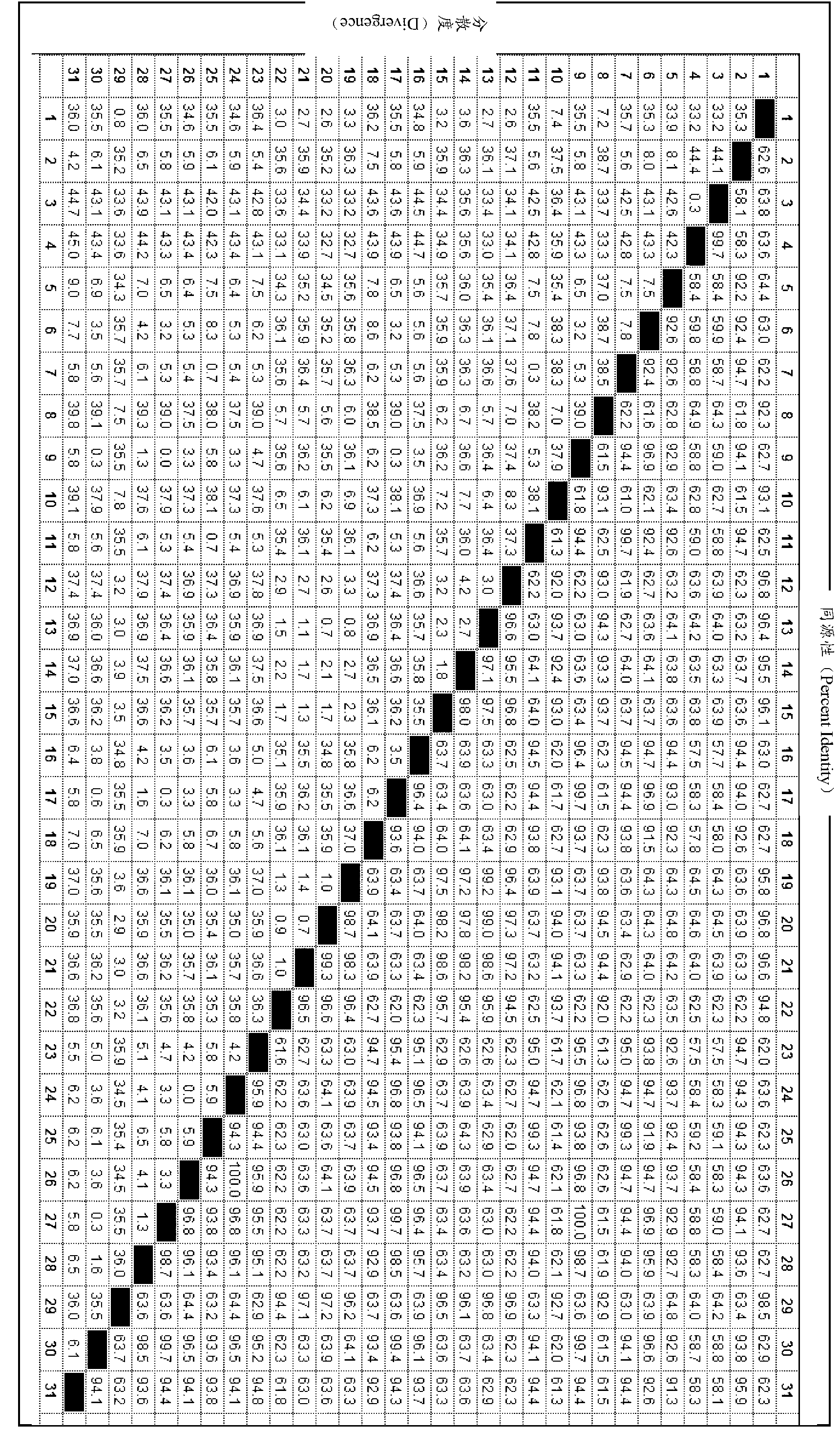
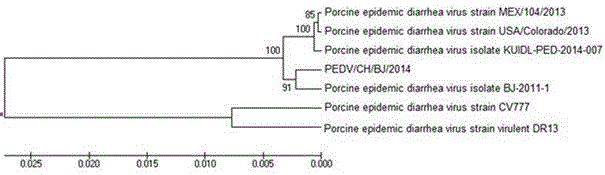
Egg yolk antibody for preventing and treating porcine epidemic diarrhea and preparation method of egg yolk antibody
ActiveCN107177001AResist attackImproving immunogenicityEgg immunoglobulinsImmunoglobulins against virusesEpidemic diarrheaAnimal science
The invention discloses an egg yolk antibody for preventing and treating porcine epidemic diarrhea and a preparation method of the egg yolk antibody. The preparation method comprises the following steps: using an inactivated vaccine of a porcine epidemic diarrhea virus as immunogen for immunizing a laying hen; harvesting egg yolk liquid and purifying from the egg yolk liquid to obtain the egg yolk antibody, wherein a porcine epidemic diarrhea virus strain is PEDV / CH / 2014, with a preservation number of CGMCC (China General Microbiological Culture Collection Center) No. 10111. The egg yolk antibody prepared by the preparation method disclosed by the invention has the advantages of good characters, easiness in storage, high safety and good preventing and treating effects; by an in vitro neutralization test, the neutralizing titer of the egg yolk antibody is measured to reach 1:128; by means of a clinical prevention test, the diarrhea incidence of tested pigs can be reduced by 20 percent, and the cure rate of artificial infection reaches 100 percent.
Owner:BEIJING DAWEIJIA BIOTECH SHARE CO LTD
Porcine circovirus-resistant type 2 egg yolk antibody, and preparation method and application thereof
ActiveCN102617731AImprove the level ofExpression continues to be stableEgg immunoglobulinsImmunoglobulins against virusesAnimal scienceCircovirus
The invention discloses a porcine circovirus-resistant egg yolk antibody and a preparation method and application thereof. The porcine circovirus-resistant egg yolk antibody is prepared by the following steps of: (1) immunizing a nonimmune egg-laying hen by using porcine circoviruses; (2) collecting eggs which laid by the nonimmune egg-laying hen; and (3) extracting the egg yolk antibody from the collected eggs and purifying. The level of the porcine circovirus-resistant egg yolk antibody prepared by the method is high; the expression of the egg yolk antibody is continuous, stable and small in fluctuation; and the titer of the egg yolk antibody is up to 1:640, and can be maintained for over 8 weeks. In-vitro and in-vivo neutralization tests prove that the porcine circovirus-resistant egg yolk antibody prepared by the method can effectively inhibit the porcine circoviruses, has a good immune protective effect, and can be prepared into a medicine or a preparation for preventing or treating diseases caused by the porcine circoviruses.
Owner:WUHAN HUAYANG ANIMAL PHARMA
Envelope protein VP28 idiotype monoclonal antibody against shrimp white spot syndrome virus (WSSV) and preparation method thereof
InactiveCN101691403APrevention and Control of Shrimp WSSV DiseaseImmunoglobulins against virusesAntiviralsAntigenBinding site
The invention discloses an envelope protein VP28 idiotype monoclonal antibody against shrimp white spot syndrome virus (WSSV) and a preparation method thereof. The antibody is secreted by a hybridoma cells with the collection number of CCTCC-CT200938, is prepared by taking anti-WSSV-VP28 monoclonal antibody (Ab1) as antigen, can bind with anti-WSSV-VP28 antibody of hare, and has the capability of competing with WSSV to bind with the anti-WSSV-VP28 antibody of the hare. The anti-WSSV-VP28 idiotype monoclonal antibody (Ab3) prepared by taking the antibody as antigen can bind with the WSSV, the binding site of the anti-WSSV-VP28 idiotype monoclonal antibody (Ab3) and the WSSV is located on an envelope, and the Ab3 can neutralize WSSV infection and has Ab1 properties. In the invention, the idiotype antibody is applied in the research of WSSV for the first time; a screening system is established, which uses an indirect enzyme-linked immunosorbent assay (ELISA) method and a competitive enzyme-linked immunosorbent assay (ELISA) method for detection; the fact that Ab3 has properties of Ab1 is proved by adopting an indirect immnnofluotesent method (IIF), a gold labeling immunoelectron microscopic method and crayfish in vivo neutralization tests, thus proving that the monoclonal antibody in the invention has the property to simulate original antigen WSSV-VP28.
Owner:OCEAN UNIV OF CHINA
Preparation method of avian infectious brunchitis virus HA antigen
InactiveCN101455838AExtended shelf lifeEasy to storeAntiviralsAntibody medical ingredientsAntigenSerum ige
The invention discloses a method for preparing a chicken infectious bronchitis virus HA antigen. The chicken infectious bronchitis virus HA antigen is prepared by the steps of virus multiplication, virus solution concentration and phospholipase C treatment, and antigen inactivation and stabilization. The stability of the prepared HA antigen at a temperature of 4 DEG C is prolonged to more than 6 months from the original 24 hours; besides, the valence of antigen is stable so as to solve the difficult problems which are not solved for a long time that the antigen is temporarily prepared for the test of each time and needs to be detected before use, and reduce the workload of the HI test. The prepared IBV HA antigen can be successfully applied to detecting the valence of antibody of serum HI after the chicken IBV vaccine immunization, and solves the problem that the detection of the IBV vaccine immunization effect is realized through neutralization tests in China for a long time, thereby wasting time and energy and having the danger of poison diffusion.
Owner:HENAN AGRICULTURAL UNIVERSITY
Anti-Foot-and-Mouth Disease Virus Monoclonal Antibody and Its Recognized Epitope and Application
ActiveCN102277333AGood passive immunityImprove immunityImmunoglobulins against virusesMicroorganism based processesIn vivoNeutralization epitope
The invention discloses a monoclonal antibody resisting the foot and mouth disease virus, epitope identified by the monoclonal antibody, as well as application of the monoclonal antibody, and belongs to the field of prevention and control of the foot and mouth disease. The hybridoma cell line capable of excreting the neutralizing monoclonal antibody resisting O-type FMDV (foot and mouth disease virus) has a microbial preservation number of CGMCC (China General Microbiological Culture Collection Center) 2691. The invention also discloses amino acid sequences of linear neutralization epitope of O-type FMDV VP1 protein identified by the monoclonal antibody respectively. In-vitro neutralization tests and in-vivo animal protection tests prove that: the monoclonal antibody disclosed by the invention has excellent passive immune effect, can be applied to emergency prevention of the foot and mouth disease, and plays an excellent immune effect to the passive immunity of the foot and mouth disease.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Chicken Newcastle disease virus and its separation method
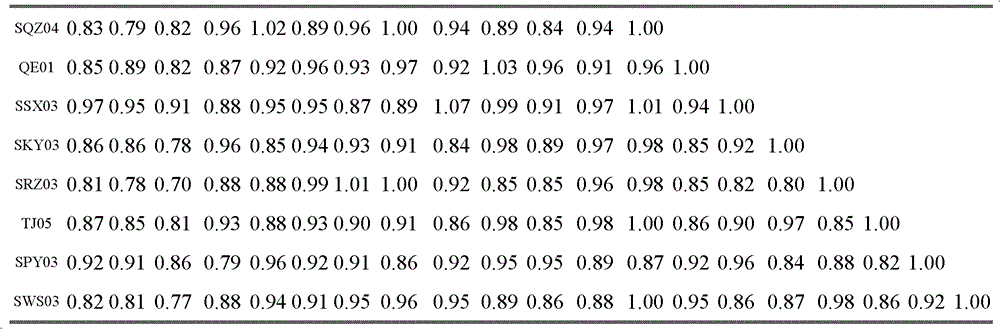
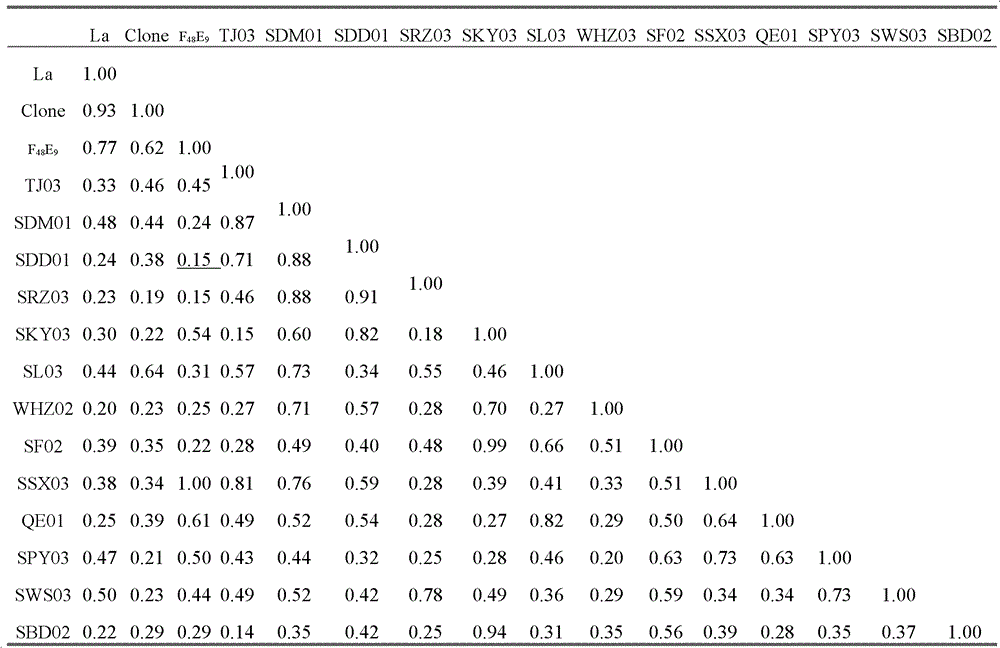
The invention relates to the field of animal virology and provides a chicken Newcastle disease virus and its separation method. The virus is code-named SDM01 and the invention provides its separation and preparation method. The inventor carries out biological collection for the strain and its collection number is CCTCC NO:V201109. Through chicken embryo passage, biological characteristics tests such as a hemagglutinin and serum neutralization test, an animal regress test and exogenous virus testing, are carried out. The test results confirm that the virus is the Newcastle disease virus. It isproved through chicken embryo mean death time (MDT), one-day-old chick intracebral pathogenicity index (ICPI) measurement and 6-week-old chick intravenous inoculation pathogenicity index (IVPI) measurement that the Newcastle disease virus is a virulent strain. Positive serum is prepared through separated strain and standard strain to carry out an HI cross neutralization test, a chicken embryo neutralization test, a cell neutralization test, an F and HN gene sequence sequencing and comparison and an immunity protective test. Results confirm that there exist large differences between the Newcastle disease virus SDM01 strain and traditional strains (La Sota strain and Clone30 strain) in both genetic typing and antigenicity.
Owner:秦卓明 +1
Immunity-chropadography test paper for detecting botulinum toxin and preparation method thereof
The invention provides an immunity-chropadography test paper for detecting botulinum toxin, comprising a layer of cellulose nitrate film, wherein the anti-botulinum toxin antibody solution is sprayedon the cellulose nitrate film and the cellulose nitrate film is arranged on a water absorption pad and a gold-label pad is arranged on the cellulose nitrate film and the gold-label is glass fiber filmor resin and immune colloidal gold probe solution for resisting botulinum toxin antibody is arranged in the gold-label pad and and a sample pad is arranged on the gold-label pad. The invention also provides a preparation method of the immunity-chropadography test paper for detecting botulinum toxin. Monoclonal antibody is used and the botulinum toxin is detected using immune response principle. The sensitivity is high and the specificity is strong and the conventional animal death-causing and neutralization test detection method is substituted and the immune colloidal gold immuno-chropadographic technology is used. The invention has features of short detection period, wide application range, simple sample processing, simple use operation and low cost.
Owner:SHANGHAI HAITAI JINXIN BIOMOLECULAR DETECTION TECH
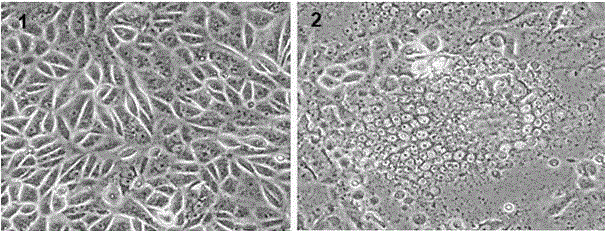
Green fluorescent protein marked recombinant swine fever virus,?its rescue method and application
ActiveCN103255111AEasy to detectStrong specificityMicroorganism based processesViruses/bacteriophagesSwine Fever VirusFluorescence microscope
The invention discloses a green fluorescent protein marked recombinant swine fever virus, its rescue method and application.?According to the invention, green fluorescent protein encoding gene is inserted between the 13th and the 14th amino acid of a classical swine fever virus infectious clone Npro protein coding region. By transfection into PK-15 cells and passage, the visual green fluorescent protein marked recombinant swine fever virus is rescued. The growth characteristics of the recombinant swine fever virus are same as that of its parental virus. After infection of PK-15 cells, the virus is directly visible by the use of fluorescence microscope. According to the modified neutralization test method (EGFP-NT) established by the recombinant swine fever virus, the existence of classical swine fever virus can be observed and the titer of neutralizing antibody in serum to be tested can be determined directly by the use of fluorescence microscope with no additional immunofluorescence test step. The method has the same specificity and sensitivity as the traditional neutralization immunofluorescence assay, but is more convenient and efficient, and can replace the neutralization immunofluorescence assay method for the use of serological test and epidemiological investigation of swine fever.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
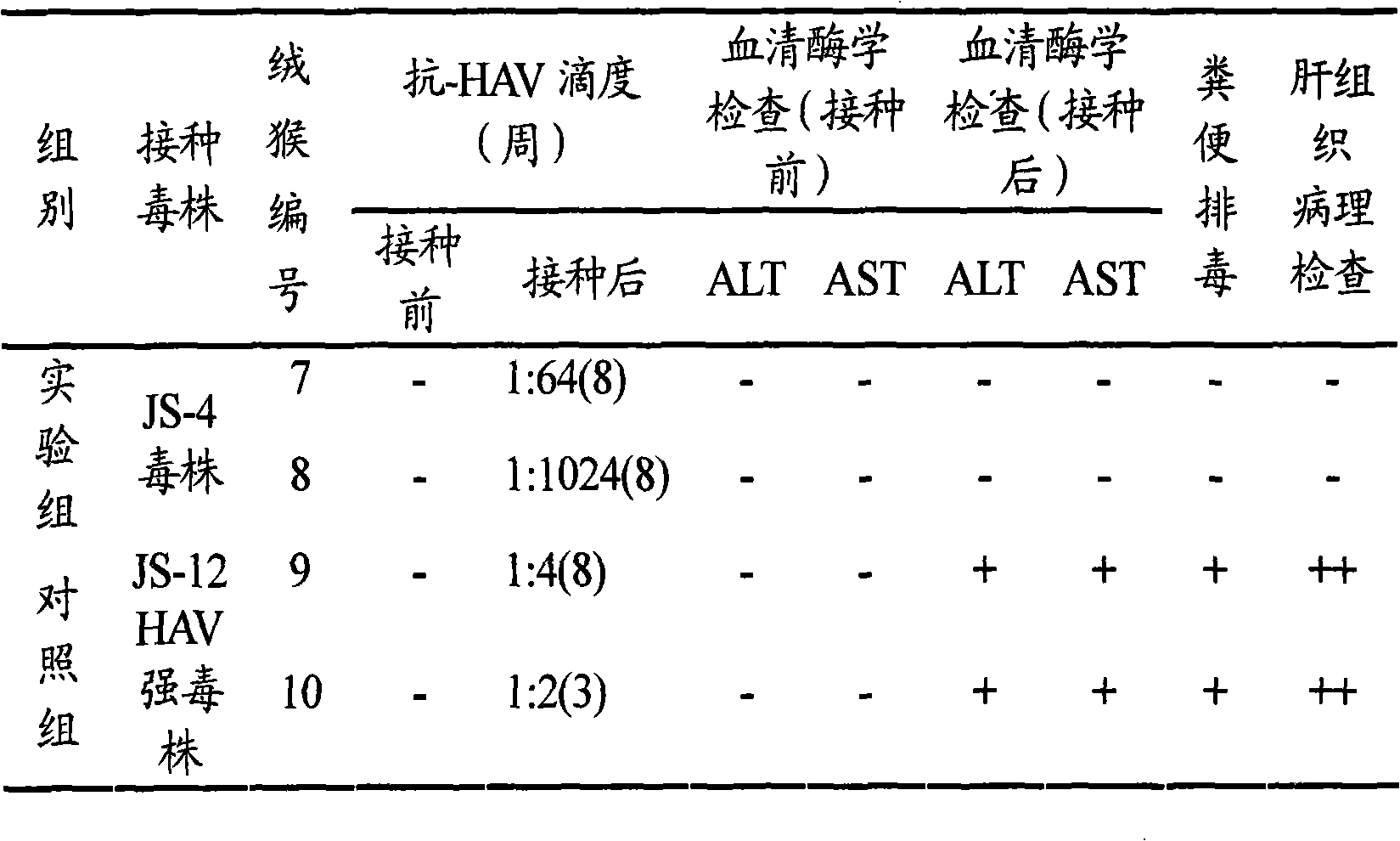
New hepatitis A inactivated vaccine virus strain and method for culturing same
ActiveCN101525597AIncrease productionQuality improvementInactivation/attenuationAntiviralsEarly generationAntigen
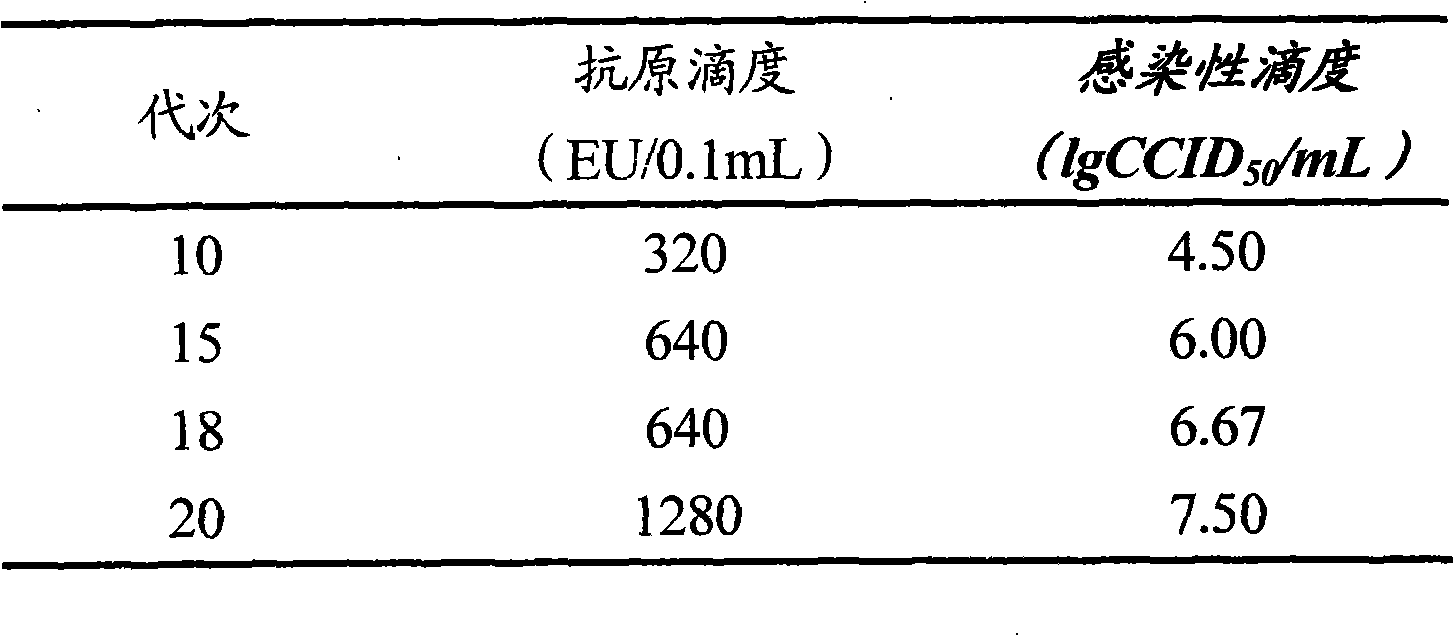
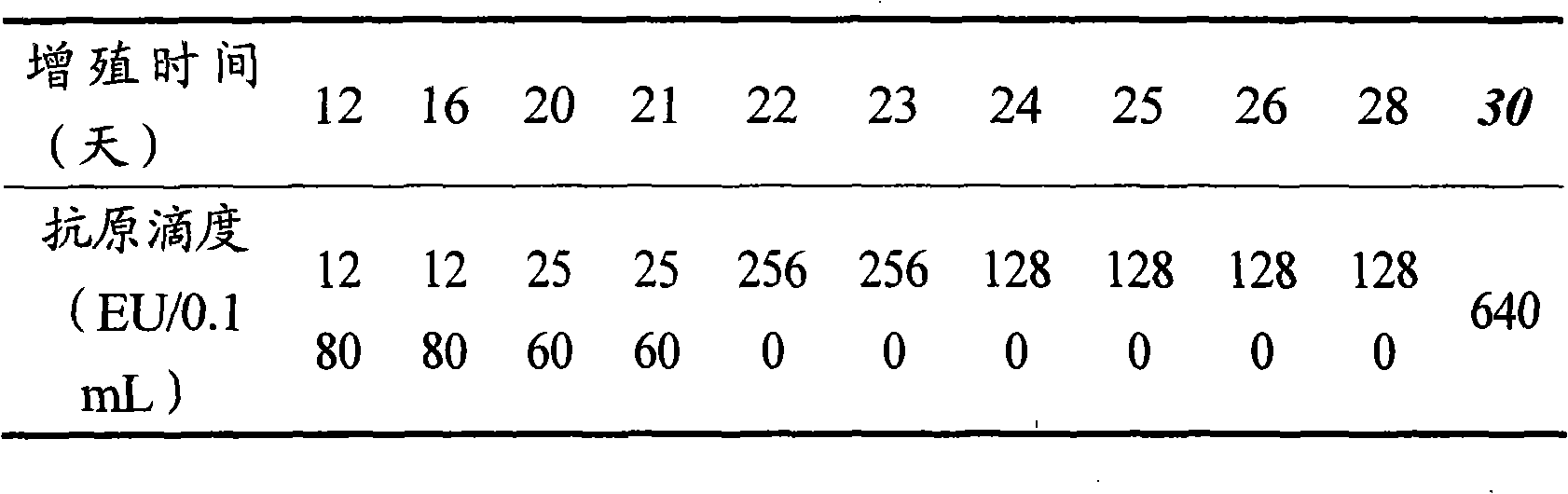
The invention provides a new hepatitis A virus JS-4 strain for preparing hepatitis A inactivated vaccine and a method for culturing the same. The strain is separated from dejecta of a patient infected with acute hepatitis A and is transmitted to a Vero cell for adapted culture; through neutralization tests and other methods, the strain is proved to be hepatitis A virus; in the adaptation process, the subculture cycle of early generation is 28 days; when the strain is transmitted to the tenth generation, the cycle is shortened to 21 days; the strain is continuously cultured for ten generations; the culture temperature is between 35 and 36 DEG C; the cycle for virus propagation is shortened from 28 to 21 days; the antigen titer can reach 1:640-1:1,280; and the infectious titer is between 106.67 and 107.50 CCID50 / ml. The marmoset virulence test proves that the strain has weak virulence, is reliable in safety and has good immunogenicity and protective effect when the strain is used for producing the hepatitis A inactivated vaccine and is an ideal strain for producing the hepatitis A inactivated vaccine.
Owner:JIANGSU SIMCERE VAXTEC BIO PHARMA
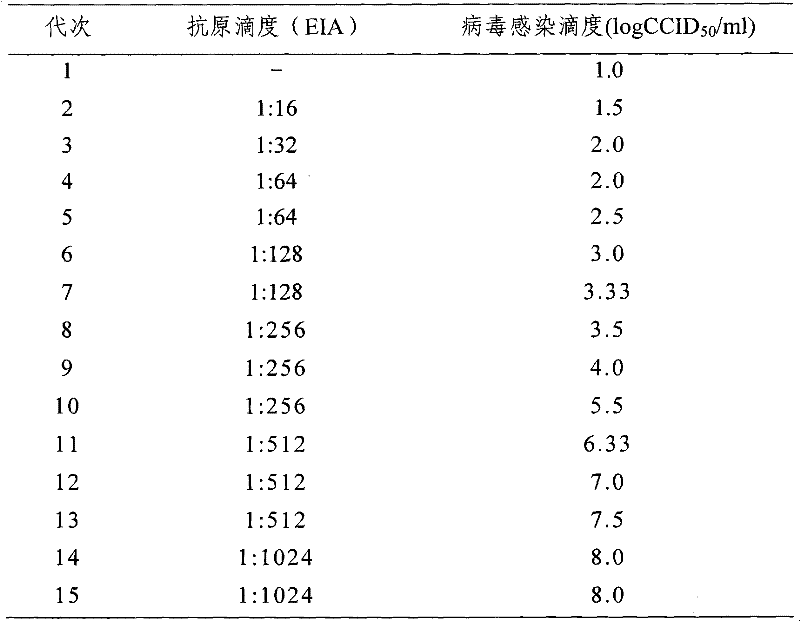
Hepatitis A virus strain SH and diploid cell adaptation method thereof
ActiveCN102174477AShorten the growth cycleImprove reproductive efficiencySsRNA viruses positive-senseViral antigen ingredientsAntigenEarly generation
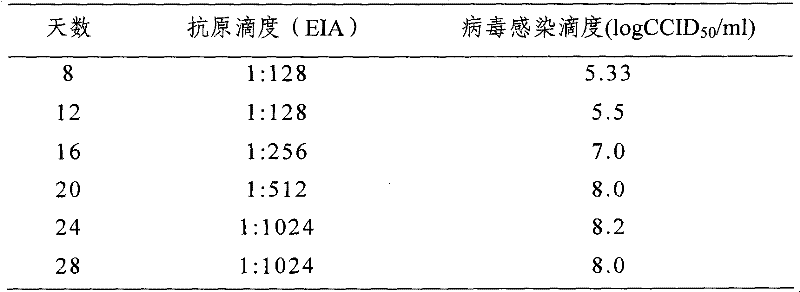
The invention provides a new hepatitis A vaccine virus strain SH and a separation method as well as an MRC-5 cell adaptation method thereof. The virus is separated from excrement of a hepatitis A acute infection patient and is transferred to a diploid cell MRC-5 for adaptation culture. The virus strain SH is proved to be a hepatitis A virus through methods such as gene sequencing, neutralization test and the like; and during adaptation, early-generation sub-culturing period is 35 days, and culturing period is shortened to be 24 days after the strain is sub-cultured for 8 generations. After the strain is continuously cultured for 8 generations, antigen titer can reach (1:512)-(1:1,024), and the virus infection titer can reach 7.0 to 8.01gCCID50 / ml. Immunogenicity tests and cross protection tests show that the strain has a good immunogenicity protection effect during production of a hepatitis A inactivated vaccine, is suitable for industrially producing the hepatitis A inactivated vaccine strain on a large scale and is an ideal strain for producing the hepatitis A inactivated vaccine.
Owner:SHENZHEN KANGTAI BIOLOGICAL PROD
Hybridoma cell line against akabane virus monoclonal antibody, monoclonal antibody as well as reagent kit and uses thereof
ActiveCN101200709AGood repeatabilityConducive to high-throughput screeningImmunoglobulins against animals/humansTissue cultureNeutralization testHybridoma technology
The present invention discloses an anti-akabane virus monoclonal antibody hybridoma cell system, a monoclonal antibody, a kit and a purpose thereof. A hybridoma technology is used to obtain rat-anti-akabane virus monoclonal antibody, the akabane virus is considered as a detection system target antigen, and the akabane virus antibody of a sample for detecting is detected by a competitive enzyme-linked immunosorbent mensuration method. The whole detection process only needs 2.5 hours and all test steps are processed inside a 96-hole enzyme label plate; compared with the traditional neutralization test, the present invention saves time and labor and has high throughput and good repeatability.
Owner:UNION STEMCELL & GENE ENG +1
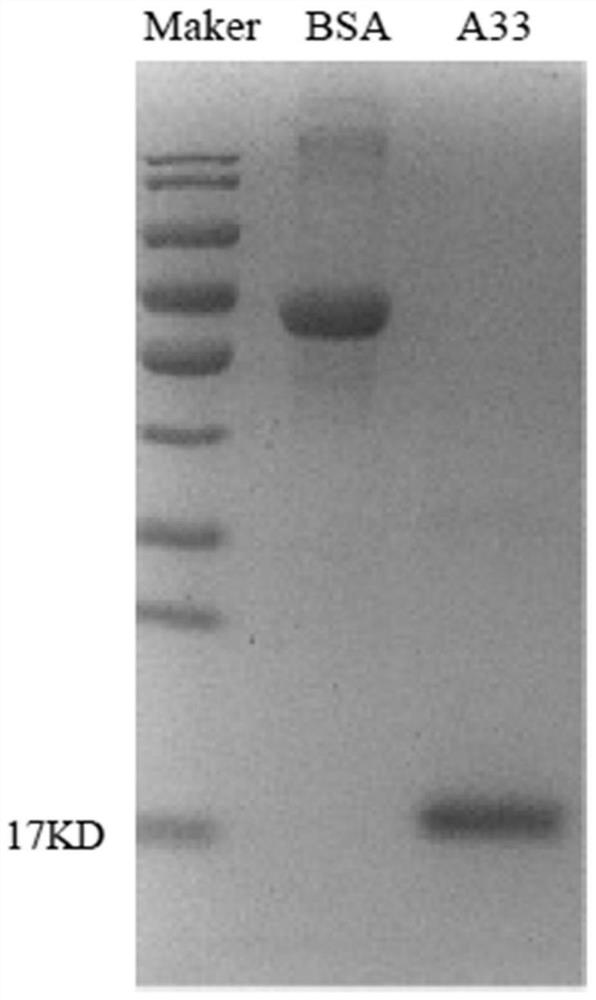
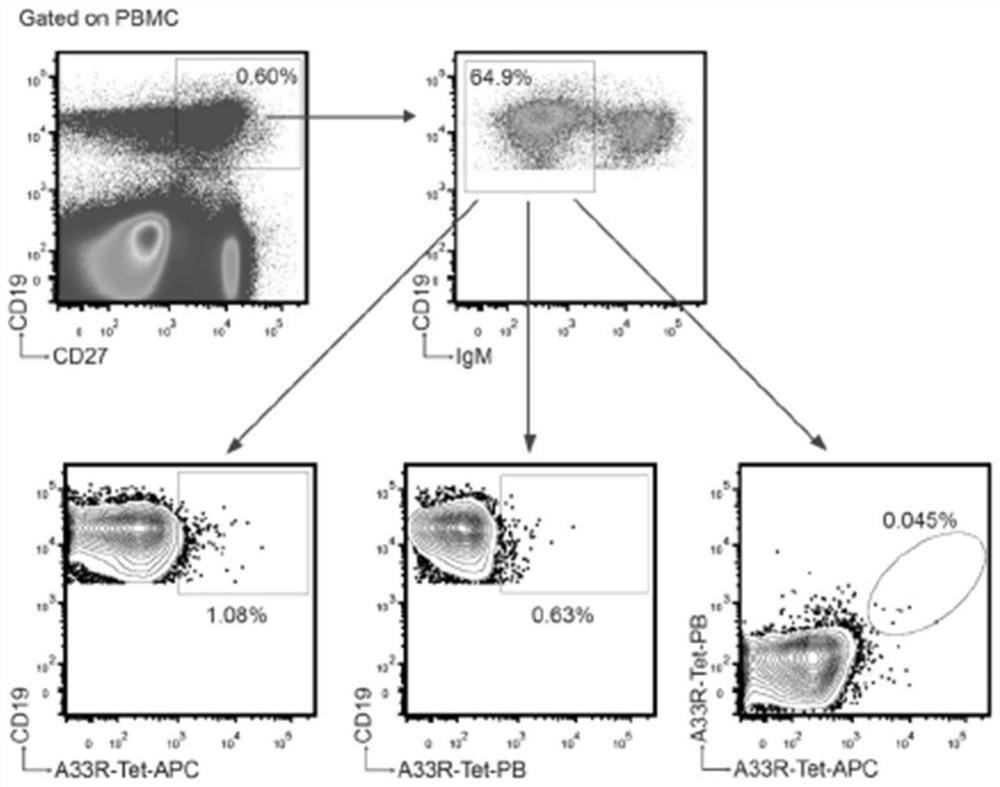
Poxvirus human monoclonal antibody and application thereof
ActiveCN113861285ALow heterogeneityHigh precisionImmunoglobulins against virusesAntiviralsEscherichia coliAntigen
The invention discloses a poxvirus human monoclonal antibody and application thereof, and belongs to the technical field of medicine. According to the invention, vaccinia virus surface film antigen A33 protein expressed by Escherichia coli is taken as an antigen; specific memory B cells of the A33 protein are screened from PBMCs of a volunteer inoculated with a ceiling vaccine; then, the specific B cells are reversed and amplified to obtain a variable region fragment of the antibody; and the variable region fragment and a constant region are further connected into an expression vector to obtain the human monoclonal antibody capable of protecting the pox virus through mammalian cell expression and purification and a series of function detections. Through ELISA detection, the antibody has good fixation capability with the antigen, and in addition, the high protection effect is achieved in the in vitro neutralization test and the mouse infection. The human antibody has an application value in clinical treatment and prevention of the pox virus.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Antibody and encoding gene of mycobacterium tuberculosis Acr protein and application thereof
The invention discloses an antibody and an encoding gene of mycobacterium tuberculosis Acr protein and application of the antibody and the encoding gene of the mycobacterium tuberculosis Acr protein. The invention provides polypeptide which is the antibody of the mycobacterium tuberculosis Acr protein. The mycobacterium tuberculosis Acr protein can be any one of (1)-(15), including (1) polypeptide composed of amino acid sequences shown in a sequence 2 of a sequence table, (2) polypeptide composed of amino acid sequences shown in a sequence 4 of the sequence table, (3) polypeptide composed of amino acid sequences shown in a sequence 6 of the sequence table, and the like. According to experimental evidence, a humanized antibody bank is adopted to screen humanized antibodies of the mycobacterium tuberculosis Acr protein, a humanized antibody with high-neutralization activity is screened out according to an external neutralization test, and fourteen strains of humanized antibody sequences are obtained. Good foundation is provided for construction of humanized single-chain antibody preparations which are used for preventing mycobacterium tuberculosis and have good medical value, and the like.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
A hybridoma cell line secreting cpiv3 antibody and elisa kit
ActiveCN105838678BHas a blocking effectMicroorganism based processesImmunoglobulins against virusesElisa kitSerum samples
Owner:JIANGSU ACAD OF AGRI SCI
Recombinant Newcastle disease LaSota vaccine strain for expressing West Nile virus (WNV) PrM/E protein
InactiveCN104630160AViral antigen ingredientsMicroorganism based processesSpecific iggNeutralization test
The invention provides a recombinant Newcastle disease virus LaSota vaccine strain rLa-WNV-PrM / E for expressing a West Nile virus (WNV) PrM / E protein. The collection number of the vaccine strain is CGMCC No. 10199; the body fluid generated by the recombinant virus and the cellular immune response are systematically evaluated in a mouse mold. Results indicate that the vaccine strain is capable of correctly expressing the PrM / E protein. A C57BL / 6 mouse is immunized twice by use of the recombined virus, and then an ELISA result indicates that the immunized mouse is capable of generating high-level PrM / E protein-specific IgG; a neutralization test result indicates that the immunized mouse is capable of generating high-level WNV neutralization antibodies; the flow cytometry indicates that the immunized mouse is capable of generating WNVE protein epitope-specific CD4+ and CD8+T cell immune response. The vaccine strain is a safe, efficient West Nile virus prevention and control candidate vaccine having perspectiveness and reservation property, and has great strategic importance for China to cope with the potential threats of the virus.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Application of lettuces as host to expression of Hepatitis B vaccines
The invention relates to the technical field of biology, in particular to expression of a human hepatitis B vaccine (namely hepatitis B virus (Hepatitis B virus, HBV) surface antigen protein) throughplants. The plants such as lettuces are used as an effective expression platform for producing recombinant protein, and the human Hepatitis B vaccine is expressed by a simple and effective agrobacterium tumefaciens mediated vacuum osmotic method. The expression system determines that plant foreign protein can be collected after agrobacterium tumefaciens is subjected to infection for 4d. By an SDS-PAGE method, successful expression of the recombinant human Hepatitis B vaccine protein is determined. After expressed recombinant protein is prepared into virus like particles through self-assembly,New Zealand white rabbits can be immunized, and obtained serum can prove that the human Hepatitis B vaccine expressed by the plants has biology activity by an enzyme-linked immunoadsorbent assay method (ELISA) and a pseudovirion granule neutralization test.
Owner:王跃驹
Method for expressing human papilloma virus protein or preparing human cervical cancer vaccine by utilizing lettuce as host
The invention relates to the technical field of biology, and particularly relates to a method for expressing human cervical cancer vaccine (Human Papillomaviruses, HPV) protein) by utilizing plants. The method utilizes the plants such as lettuce as an effective expression platform for producing recombinant protein, and utilizes a simple and effective agrobacterium-mediated vacuum infiltration method to express the human cervical cancer vaccine. The expression system determines that plant foreign protein can be collected after being infected by agrobacterium for 4 days. The SDS-PAGE method is utilized to determine that the recombinant human cervical cancer vaccine protein is successfully expressed. The expressed recombinant protein is self-assembled into virus-like particles and then used for immunizing New Zealand white rabbits, and the obtained serum proves that the human cervical cancer vaccine expressed by the plants has biological activity through an enzyme-linked immunosorbent assay (ELISA) and a pseudovirus particle neutralization test.
Owner:王跃驹
An online sample dilution and neutralization test device
ActiveCN104897820BAccurate valueReliable test conditionsComponent separationPeristaltic pumpFour-way valve
The invention discloses an on-line sample dilution and neutralization test device which consists of a dilution device, a neutralizer, an instruction control device, a silica gel pipe and a control line, wherein an ultrapure water storage bottle and a sample bottle are connected to a peristaltic pump, an injection pump, a three-way mixer, an electric two-position four-way valve, a three-way mixer and the neutralizer through the dilution silica gel pipe; a control box is connected with the peristaltic pump, the injection pump, the three-way mixer, the electric two-position four-way valve and the neutralizer through the control main line in a control manner; automatic sample dilution and sample neutralization are completed on line through a pipeline and a circuit; samples are provided for a chromatographic analysis system to complete analysis. According to the on-line sample dilution and neutralization test device, the problem caused by ion pollution of an existing neutralization method is effectively solved; no suction pipe or no volumetric flask is used in the whole neutralization process any more, so that the pollution source is reduced, a precise value of experimental data measurement is guaranteed, and a reliable test condition is provided for research on ion chromatographic analysis of snow and ice samples.
Owner:NORTHWEST INST OF ECO ENVIRONMENT & RESOURCES CAS
Porcine circovirus-resistant type 2 egg yolk antibody, and preparation method and application thereof
ActiveCN102617731BImprove the level ofExpression continues to be stableEgg immunoglobulinsImmunoglobulins against virusesAnimal scienceCircovirus
The invention discloses a porcine circovirus-resistant egg yolk antibody and a preparation method and application thereof. The porcine circovirus-resistant egg yolk antibody is prepared by the following steps of: (1) immunizing a nonimmune egg-laying hen by using porcine circoviruses; (2) collecting eggs which laid by the nonimmune egg-laying hen; and (3) extracting the egg yolk antibody from the collected eggs and purifying. The level of the porcine circovirus-resistant egg yolk antibody prepared by the method is high; the expression of the egg yolk antibody is continuous, stable and small in fluctuation; and the titer of the egg yolk antibody is up to 1:640, and can be maintained for over 8 weeks. In-vitro and in-vivo neutralization tests prove that the porcine circovirus-resistant egg yolk antibody prepared by the method can effectively inhibit the porcine circoviruses, has a good immune protective effect, and can be prepared into a medicine or a preparation for preventing or treating diseases caused by the porcine circoviruses.
Owner:WUHAN HUAYANG ANIMAL PHARMA
Completely humanized anti-dengue virus monoclonal antibody and preparation and application methods thereof
ActiveCN109456408AEasy to solveGood treatment effectImmunoglobulins against virusesAntiviralsHeavy chainNeutralization test
The invention provides a completely humanized anti-dengue virus monoclonal antibody and preparation and application methods thereof. The completely humanized anti-dengue virus monoclonal antibody is composed of two identical heavy chains and two identical light chains, wherein the amino acid sequence of the heavy chains is shown as SEQ ID NO.1, the amino acid sequence of the light chains is shownas SEQ ID NO.2, the nucleotide sequence for coding the heavy chains is shown as SEQ ID NO.3, and the nucleotide sequence for coding the light chains is shown as SEQ ID NO.4. In vitro neutralization tests verify that the completely humanized anti-dengue virus monoclonal antibody can achieve effective protection to achieve preventing effects before virus infection as well as still achieve virus infection treating effects within a period after a patient is infected, and when combined with other anti-dengue virus drugs, can achieve a broad clinical application prospect.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Method for detecting serum type 4 fowl adenovirus neutralizing antibody based on recombinant fluorescent virus
PendingCN113917139AHigh biosecurityThere is no hidden danger of spreading strong poisonBiological testingImmunoassaysFluorescence microscopeTGE VACCINE
The invention discloses a method for detecting a serum type 4 fowl adenovirus neutralizing antibody based on a recombinant fluorescent virus. The method comprises the following steps: S1, diluting serum to be detected, mixing the diluted serum with 10000-20000 TCID50 / 50 [mu] L of fluorescent virus FAdV-4-EGFP, and carrying out constant temperature incubation for 1-2 h; s2, inoculating a mixture of the incubated virus and serum into LMH cells, culturing the mixture for 24-36 hours, and observing the neutralized condition of the fluorescent virus under a fluorescence microscope. On the basis of a recombinant virus FAdV-4-EGFP which is rescued in the early stage and expresses green fluorescent protein, the strain is a highly attenuated vaccine candidate strain, the chicken is not infected even if the chicken is infected by high-dose intramuscular injection, the growth characteristics of the strain are similar to those of a parent strain, the biological safety is high, and the risk of spreading of a strong virus does not exist. Compared with a traditional neutralization test, the scheme has the advantages that the result can be observed only by infecting the recombinant virus for 24 hours at least, the neutralization test result can be realized 2-3 days ahead of time, and the detection time is greatly shortened.
Owner:YANGZHOU UNIV +1
Method for preparing porcine circovirus type 2 colloidal gold antibody fast test strip
The invention relates to a method for preparing a porcine circovirus type 2 colloidal gold antibody fast test strip. In the method, a porcine circovirus type 2 ORF2 protein is expressed by utilizing genetic engineering, and the test strip is prepared by the principle of enzyme-linked immunoassay and membrane chromatography to fast test antibodies in the blood or serum of pigs. The test strip can be widely used for clinically testing porcine circovirus diseases, has no cross reaction with other viruses, and obtains test results 98.63 percent and 95.83 percent consistent with those obtained by the two methods of the ELISA and neutralization test. Compared with the ELISA, a recombinant antigen immune colloidal gold has the remarkable advantages of high security, no need of culturing the viruses per se, the avoidance of virus spread caused by the operation of the viruses, large-batch preparation, simple process, low production cost, stable and homogeneous antigen components, simple, convenient and labor-saving operation, no apparatus, high detection result specificity, high repeatability, the short time of 15 minutes for the whole test, simple, convenient, fast and accurate operation, high sensitivity, intuition and easy result judgment.
Owner:QINGDAO AGRI UNIV +2
New hepatitis A inactivated vaccine virus strain and method for culturing same
The invention provides a new hepatitis A virus JS-4 strain for preparing hepatitis A inactivated vaccine and a method for culturing the same. The strain is separated from deject a of a patient infected with acute hepatitis A and is transmitted to a Vero cell for adapted culture; through neutralization tests and other methods, the strain is proved to be hepatitis A virus; in the adaptation process, the subculture cycle of early generation is 28 days; when the strain is transmitted to the tenth generation, the cycle is shortened to 21 days; the strain is continuously cultured for ten generations; the culture temperature is between 35 and 36 DEG C; the cycle for virus propagation is shortened from 28 to 21 days; the antigen titer can reach 1:640-1:1,280; and the infectious titer is between 106.67 and 107.50 CCID50 / mL. The marmoset virulence test proves that the strain has weak virulence, is reliable in safety and has good immunogenicity and protective effect when the strain is used for producing the hepatitis A inactivated vaccine and is an ideal strain for producing the hepatitis A inactivated vaccine.
Owner:JIANGSU SIMCERE VAXTEC BIO PHARMA
Chicken Newcastle disease virus and its separation method
InactiveCN102766603BViral antigen ingredientsMicroorganism based processesHemagglutininCross neutralization
Owner:秦卓明 +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com