Egg yolk antibody for preventing and treating porcine epidemic diarrhea and preparation method of egg yolk antibody
A technology for porcine epidemic diarrhea and egg yolk antibody, which is applied in the field of egg yolk antibody to achieve the effects of good immunogenicity and reduced incidence of diarrhea
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1 Evolutionary analysis of porcine epidemic diarrhea virus PEDV / CH / 2014 S gene
[0024] The porcine epidemic diarrhea virus involved in the present invention, named PEDV / CH / BJ / 2014, was deposited in the General Microbiology Center of the China Committee for the Collection of Microorganisms on December 4, 2014, with a preservation number of CGMCC No.10111. Preservation address: Institute of Microbiology, Chinese Academy of Sciences, No. 3, Yard 1, Beichen West Road, Chaoyang District, Beijing.
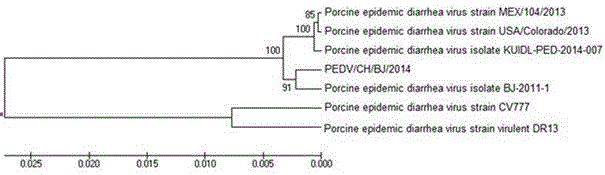
[0025] Using DNAStar software to analyze the sequencing results, the results showed that the PEDV / CH / BJ / 2014 strain and the Chinese isolate 2011-BJ-1 constituted a new branch, and the American reported strains MEX / 104 / 2013, USA / Colorado / 2013 and KUIDL-PED-2014-007 has high homology; compared with classic reference strains such as Belgian CV777, Korean strain DR13, etc., it is not on the same evolutionary branch, and has undergone obvious mutations, indicating that the newl...
Embodiment 2
[0026] Example 2 Preparation of Porcine Epidemic Diarrhea Virus Liquid
[0027] The PEDV / CH / BJ / 2014 virus solution was tested for sterility, mycoplasma and exogenous virus with reference to the appendix of "Chinese Veterinary Pharmacopoeia", and the results showed no contamination by bacteria, mold, mycoplasma and exogenous virus.
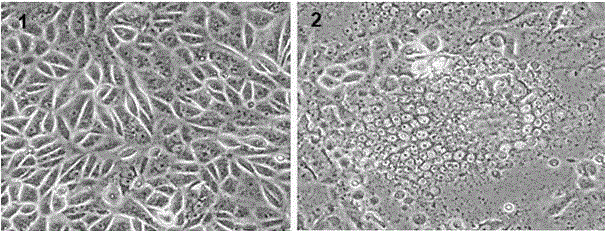
[0028] The 39th passage culture of PEDV / CH / BJ / 2014 was taken, inserted into a confluent monolayer of Vero cells at 0.5%, absorbed for 1.5 h, discarded the virus liquid, supplemented with maintenance solution containing 5 μg / mL trypsin, and incubated at 37 °C, 5% CO 2 Harvest the virus liquid when 90% of the cells develop lesions under the same conditions, measure the virus price, and the virus content is 3*10^7 TCID 50 / mL, the virus was stored at -80°C for later use.
Embodiment 3
[0029] The preparation of embodiment 3 PEDV inactivated vaccine
[0030] Inactivation of PEDV virus: Take the above virus liquid, add 0.2% β-propiolactone, shake well, put it in a refrigerator at 4°C for 24 hours, and shake it every 2 hours during this period. After the inactivation is completed, put it at 37°C for inactivation Degrade β-propiolactone and store at 4°C for later use. Take an appropriate amount of inactivated virus liquid to inoculate healthy Vero cells for inactivation detection, and observe continuously for 7 days. If the cells have no lesions, it means that the virus inactivation is complete.
[0031] PEDV Freund's complete / incomplete adjuvant vaccine: Take the inactivated virus liquid, add the same amount of Freund's complete adjuvant / Freund's incomplete adjuvant, use a glass syringe to connect the three-way valve for emulsification, and prepare the inactivated Vaccines are stored at 4°C for later use.
[0032] Example 3 Preparation and Purification of PED...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com