Completely humanized anti-dengue virus monoclonal antibody and preparation and application methods thereof
A monoclonal antibody and dengue virus technology, applied in antiviral agents, antiviral immunoglobulins, botanical equipment and methods, etc., can solve problems such as fully human monoclonal antibodies that do not have dengue virus infection diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1. Construction and expression of fully human monoclonal antibody AD847
[0043] Using a large-capacity fully human phage library (Qiao Yuanyuan, Wang Yan, Chen Xiaosui, etc. Construction and identification of a large-capacity phage antibody library [J]. Chinese Journal of Microbiology and Immunology, 2004, 24(3):194-197.) , and according to the literature "Hu Shi, Shen Yafeng, Li Tian, etc. Screening, preparation and biological activity research of fully human monoclonal antibodies against short-chain neurotoxins from flat-chin sea snakes [J]. PLA Medical Journal, 2017, 42(7): 612-616.)", multiple rounds of screening of monoclonal antibodies against dengue virus ED3 protein were carried out, and finally a fully human anti-dengue virus ED3 protein antibody was obtained, named AD847.
[0044] After sequencing, analyze the sequence and synthesize the full-length heavy and light chains of the full-length human monoclonal antibody. The heavy and light chains are r...
Embodiment 2
[0047] Embodiment 2, Biacore analysis
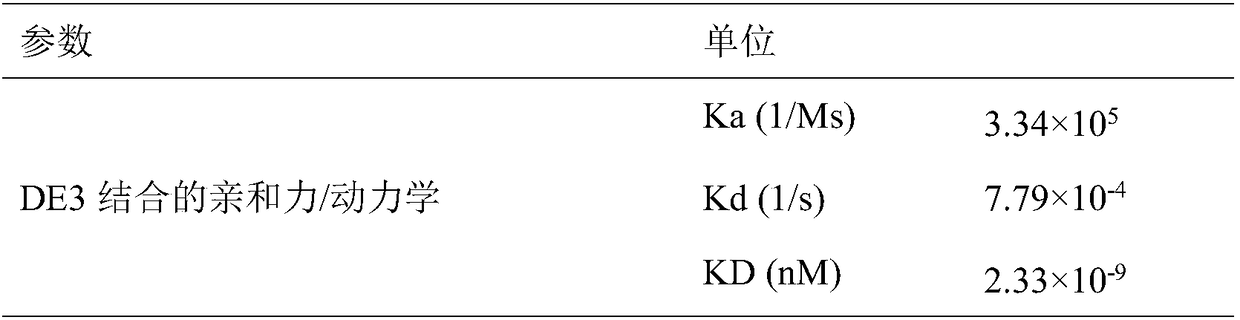
[0048] The polyclonal anti-human Fc antibody (Jackson ImmunoResearch Company) was coated on the CM5M5 chip (GE Company), and after the detected antibody was captured, the affinity of each fusion protein was detected with Biacore T100 (GE Healthcare). The specific detection affinity values are shown in Table 1 .
[0049] Table 1 Biacore analysis results
[0050]
Embodiment 3
[0051] Embodiment 3, in vitro neutralization test
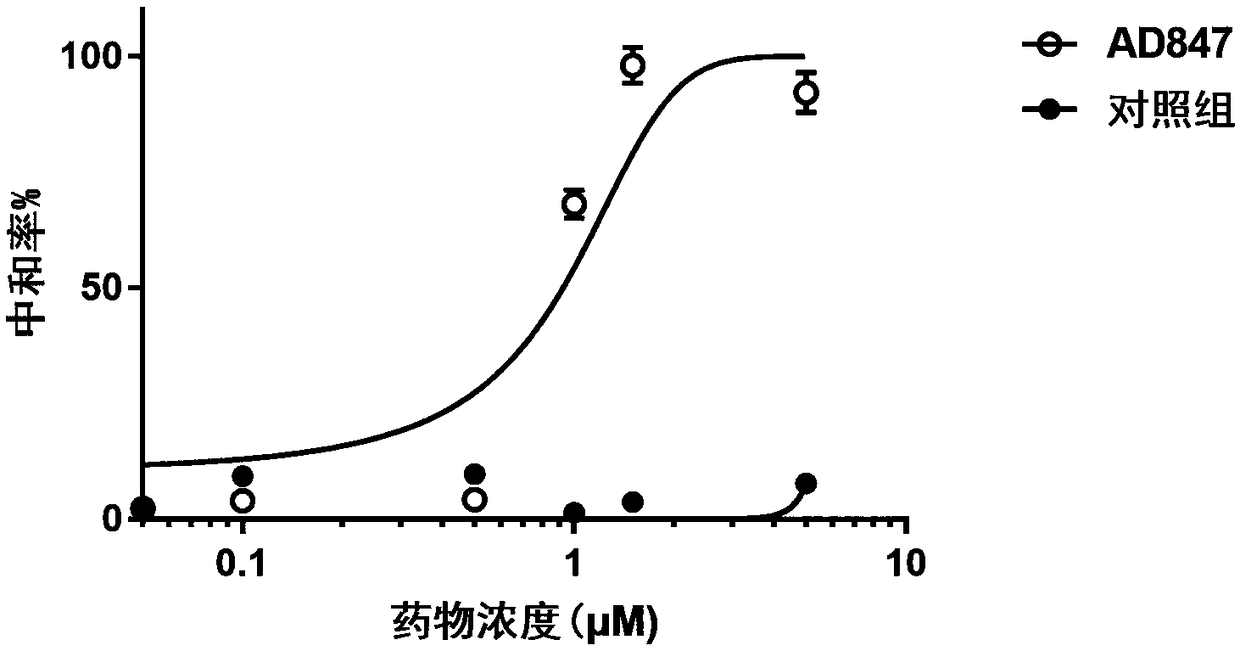
[0052] The plaque reduction neutralization test was carried out by using the method of fixing the virus and diluting the antibody: the different concentrations of the antibody were mixed with the dengue type 4 virus suspension containing 50-100 PFU in equal amounts, and the BHK21 cells were cultured in a 6-well plate in a 37°C water bath for 1 hour. Incubate at 37°C for 1 h, discard the mixture, and wash the cells with PBS buffer. Add the nutrient agar cover, continue to culture for 5 fills, fix the staining, count the number of plaques, and calculate the neutralizing activity of the antibody.
[0053] Such as figure 1 As shown, the monoclonal antibody has dengue type 4 virus-specific neutralizing activity, and the virus neutralizing activity has a concentration-dependent relationship. As the antibody concentration increases, the virus infection rate decreases significantly.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com