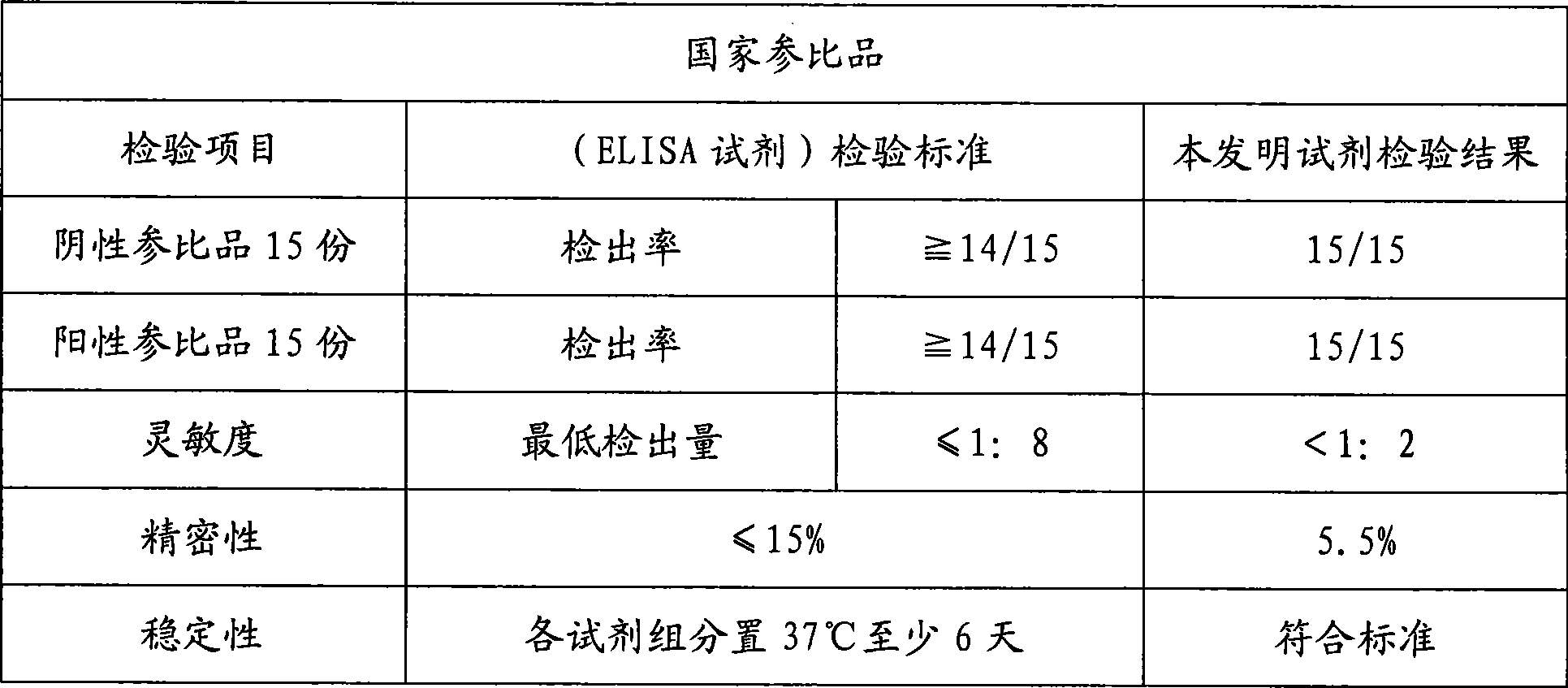
Patents
Literature
71 results about "Hepatitis A viruses" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
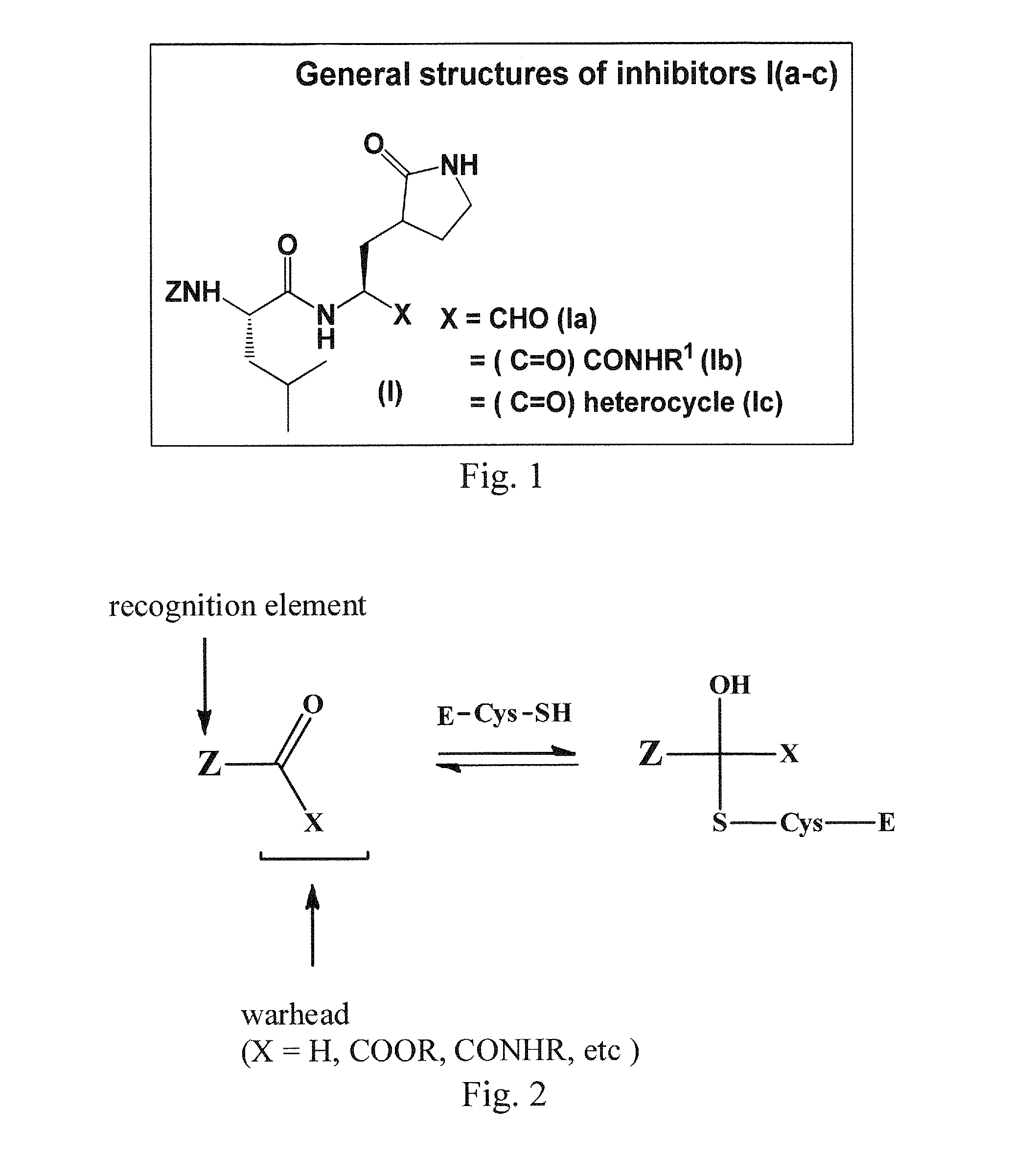
Macrocyclic and peptidomimetic compounds as broad-spectrum antivirals against 3c or 3c-like proteases of picornaviruses, caliciviruses and coronaviruses
Antiviral protease inhibitors, including macrocylic transition state inhibitors and peptidomimetics are disclosed, along with related antiviral compounds, and methods of using the same to treat or prevent viral infection and disease. The compounds possess broad-spectrum activity against viruses that belong to the picornavirus-like supercluster, which include important human and animal pathogens including noroviruses, sapoviruses, enteroviruses, poliovirus, foot-and-mouth disease virus, hepatitis A virus, human rhinovirus (cause of common cold), human coronavirus (another cause of common cold), transmissible gastroenteritis virus, murine hepatitis virus, feline infectious peritonitis virus, and severe acute respiratory syndrome coronavirus.
Owner:WICHITA STATE UNIVERSITY +1
Rapid diagnostic kit based on loop-mediated isothermal amplification technique for hepatitis A virus genes and detection method thereof
ActiveCN101660005AHigh sensitivityEasy to identifyMicrobiological testing/measurementMicroorganism based processesHepatitis A virusesReverse transcriptase
The invention discloses a rapid diagnostic kit based on a loop-mediated isothermal amplification technique for hepatitis A virus genes and a detection method thereof. The kit comprises two pairs of primers, Bst DNA polymerase, revertase, an RNase inhibitor, a stabilizing solution, a reaction solution, a chromogenic solution and a positive contrast solution, wherein the nucleotide sequences of thetwo pairs of primers are shown in SEQ ID NO: 1-4; and the eight solutions are respectively contained in containers. The kit and the detection method can detect the hepatitis A virus with high efficiency and high specificity, are based on the loop-mediated isothermal amplification technique, apply six segments, four primers and one constant temperature to complete an amplification reaction within less than one hour, and have the advantages of low detection cost, short time consumption, high yield, high specificity, significant chromogenic difference between a positive result and a negative result, high authentication rate, distinctness and reliability.
Owner:GUANGZHOU HUAFENG BIOTECH
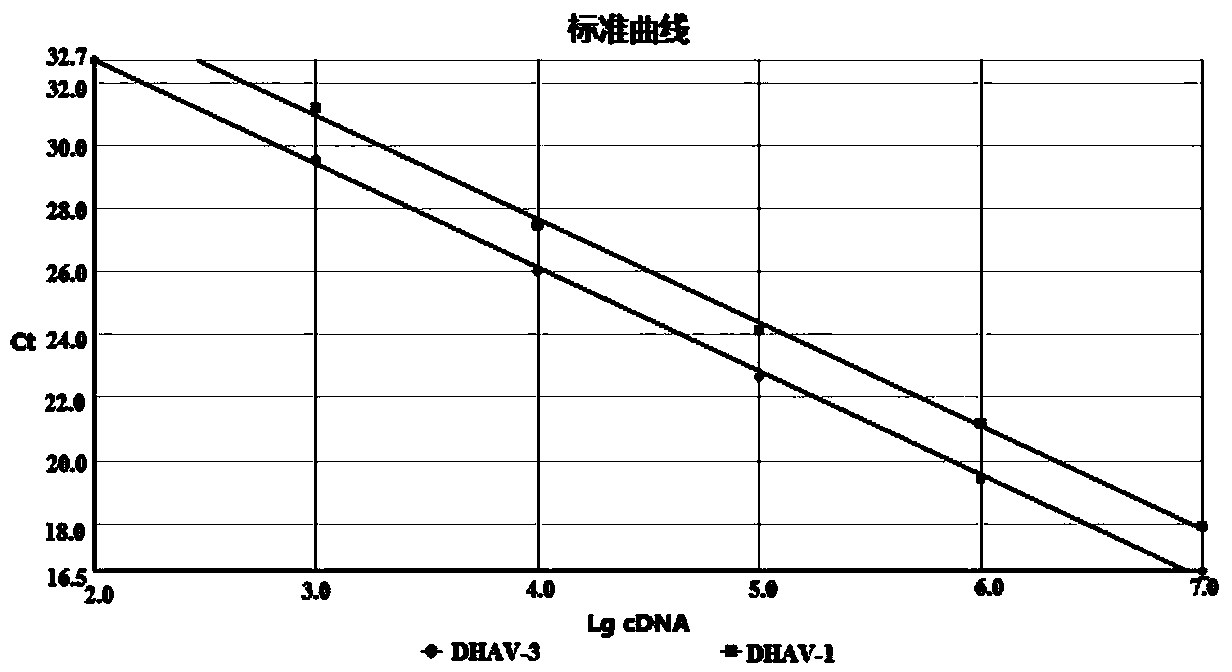
Dual fluorescence quantitative method for quickly identifying type 1 and type 3 duck hepatitis A viruses
InactiveCN104046704ARapid identificationOptimization parametersMicrobiological testing/measurementAgainst vector-borne diseasesDuck hepatitis A virusSpecific detection
The invention provides a dual fluorescence quantitative RT-PCR (reverse transcription-polymerase chain reaction) specific primer for detecting type 1 and type 3 duck hepatitis A viruses. A dual fluorescence quantitative method comprises the following steps: comparing sequences of DHAV-1 and DHAV-3 published on GenBank, and designing a pair of specific detection primers SEQ1 and SEQ2 aiming at DHAV-1 and a Taqman probe (probe1) as well as a pair of specific detection primers SEQ3 and SEQ4 aiming at DHAV-3 and a Taqman probe (probe2) respectively in a region with conservation and relatively large difference between gene sequences of the two viruses; confirming the concentration of the dual fluorescence quantitative RT-PCR specific primer and the Taqman probe aiming at DHAV-1 and DHAV-3. The dual fluorescence quantitative method built by virtue of the group of primers is good in specificity and high in sensitivity, and can be used for quick serum type identification and real-time quantitative analysis of the type 1 and type 3 duck hepatitis A viruses.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
NDV (Newcastle disease virus) recombinant virus expressing DHAV-1 and DHAV-3 VP1 genes and application thereof
ActiveCN105754959AReduce manufacturing costAvoid interferenceSsRNA viruses negative-senseSsRNA viruses positive-senseDuck hepatitis A virusHepatitis A viruses
The invention belongs to the technical field of molecular biology and particularly relates to NDV (Newcastle disease virus) recombinant virus expressing DHAV-1 and DHAV-3 VP1 genes.The recombinant virus is recorded as rLS-1VP1-2A-3VP1 and is obtained inserting serially connected DHAV-1 and DHAV-3 VP1 genes into an NDV vector and carrying out saving.The NDV (Lasota strain) is used as the vector for the recombinant virus, the serially connected DHAV-1 and DHAV-3 VP1 genes are inserted into the NDV (Lasota strain) to obtain a vector, and the DNV recombinant virus co-expressing DHAV-1 and DHAV-3 VP1 genes is obtained by determining an optimal insertion site to insert the DHAV VIP1 gene; the recombinant virus is useful in preventing duck hepatitis A viruses (type 1 and type 3) and duck Newcastle disease and filling the current blank of DHAV-3 vaccines.
Owner:POULTRY INST SHANDONG ACADEMY OF AGRI SCI SHANDONG SPECIFIC PATHOGEN FREEN CHICKS RES CENT
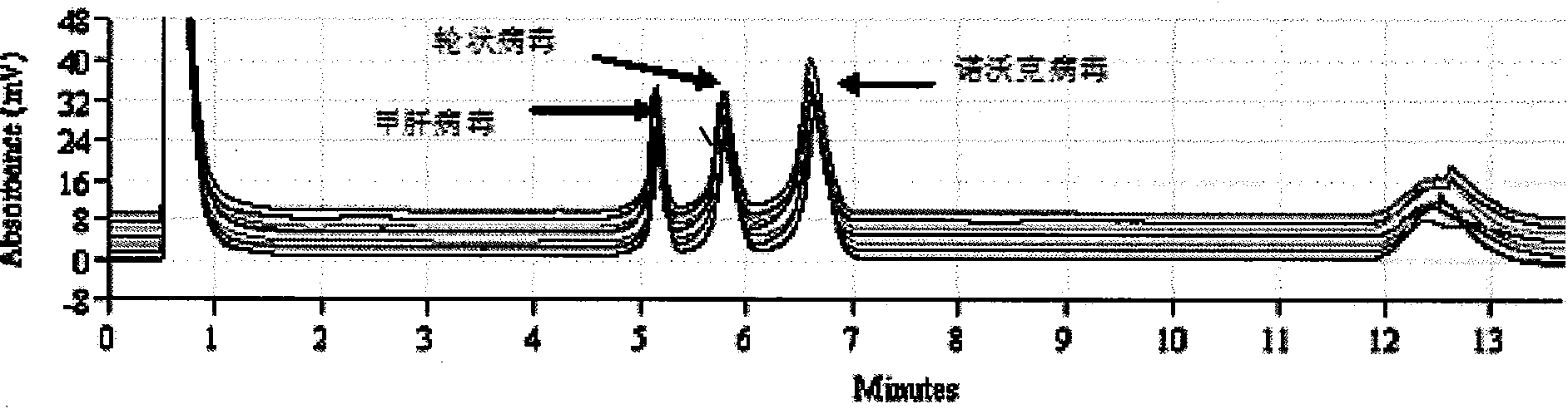
Detection kit and detection method for 3 species of food-borne viruses in marine products
InactiveCN101570798AHigh detection sensitivityHigh-throughput detectionComponent separationMicrobiological testing/measurementRotavirusGram
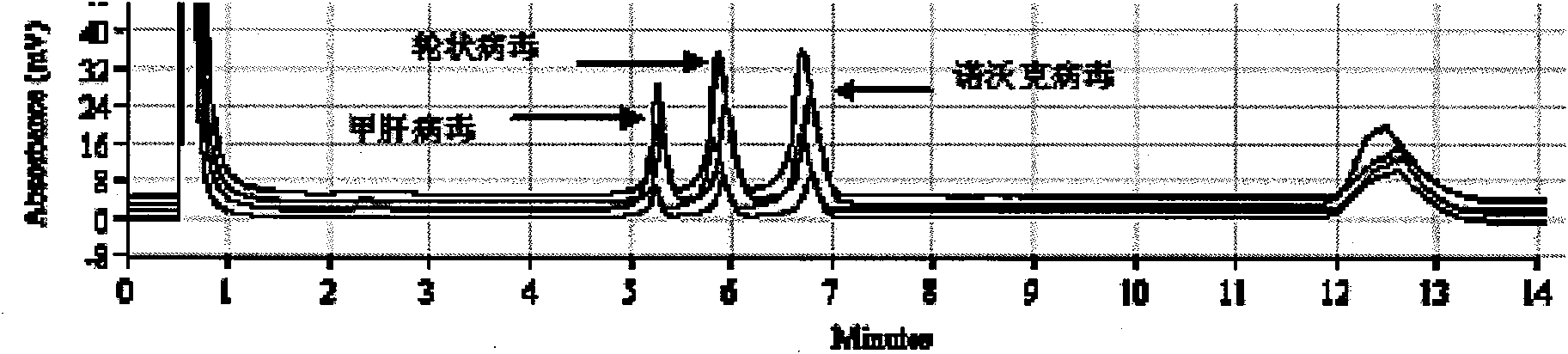
The invention discloses a detection kit and a detection method for 3 species of food-borne viruses in marine products. The kit comprises reverse transcription Tag DNA polymerase with a concentration of 5U / mu L, inverse transcriptase with a concentration of 5U / muL and an RT-PCR reaction solution, wherein the RT-PCR reaction solution contains 10 millimols of Tris.HCl, 50 millimols of KCl, 25 millimols of MgCl2, 10 millimols of dNTP, a ribonuclease inhibitor with a concentration of 40 U / mu L, and 20 mu mols of downstream primer pair and 20 mu mols of upstream primer pairs of the food-borne viruses. The kit can synchronously detect hepatitis A viruses, rotaviruses and norwalk viruses. The kit has extremely high sensitivity, can detect 1*10 nano gram of viral nuclei and is superior to the prior reported detection method for viruses in the marine products. In addition, the method is short in detection time, simple and quick, and suitable for quick detection.
Owner:曹际娟 +2
Hepatitis A virus monoclonal antibody and its application
ActiveCN103923881AEfficient neutralizationMicroorganism based processesImmunoglobulins against virusesAntigenHybridoma cell
The invention provides a hepatitis A virus monoclonal antibody, which is generated by secreting of a hybridoma cell strain with preservation number of CGMCC No.7859. The monoclonal antibody can specifically neutralize the antigen of hepatitis A virus with high efficiency, and has important meaning for diagnosis, prevention and treatment of the infection of hepatitis A virus.
Owner:SINOVAC BIOTECH
Test and quality control kit and test method of Hepatitis A virus and Norovirus in vegetable
InactiveCN105177185AMonitor Lysis EfficiencyAccurate Lysis EfficiencyMicrobiological testing/measurementAgainst vector-borne diseasesEscherichia coliPositive control
The invention discloses a test and quality control kit and test method of Hepatitis A virus and Norovirus in a vegetable. The kit comprises one tube of HAV 2*RT-PCR mix, NV GI+GII parting 2*RT-PCR mix, MS2 2*RT-PCR mix, RNase free water, HAV positive control, GI+GII positive control and coliphage MS2 (2.5*1010 pfu / mL) respectively. The test method comprises the following steps: (1) enrichment of virus in a vegetable sample; (2) extraction of virus RNA; (3) establishment of a coliphage MS2 standard curve; (4) test of Hepatitis A virus, Norovirus GI type and GII type and coliphage MS2; (5) determination of the recovery rate of virus RNA; (6) determination of results. According to the test and quality control kit and test method of the Hepatitis A virus and Norovirus in the vegetable, the quality is effectively guaranteed in the whole virus detection process of critical steps of sample pretreatment, virus concentration, quality control, test, result determination and the like, stability, accuracy and controllability of the test process are enhanced, and the detection technology blank in the field is filled.
Owner:INSPECTION & QUARANTINE TECH CENT SHANDONG ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Preparation method of disinfectant health care paper napkin
InactiveCN103882762AImprove textureTight textureNon-macromolecular organic additionVegetable material additionHepatitis A virusesDisinfectant
The invention discloses a preparation method of disinfectant health care paper napkin; the paper napkin is prepared form a paper napkin raw material by beating, grooving, pressing, printing, embossing and drying, and a disinfectant liquid is added in the beating process. The disinfectant liquid is a mixture of a natural drug distillation extract and ethanol, the natural drugs include ageratum, isatis root, mint and radix scutellariae; the raw materials is one of cotton pulp, wood pulp and straw pulp, and is preferably the wood pulp; and the volume fraction of the ethanol is 70%-75%. The distillation extract of the natural drugs of the ageratum, isatis root, mint and radix scutellariae is added in the beating process for effective killing of hepatitis A virus, hepatitis B virus, human immunodeficiency virus and various bacteria, the disinfectant health care paper napkin is low in cost, simple in process and wide in application range; the wood pulp is preferably used as the raw material, and is non-pollution and non-stimulation, and the prepared napkin paper is exquisite, compact, good in toughness and non-wrinkling.
Owner:吕艳
Hepatitis A virus strain, method for preparing hepatitis A inactivated vaccine and obtained vaccine
InactiveCN1443844AHigh yieldHigh productivityInactivation/attenuationAntibody medical ingredientsVero cellVirus strain
Owner:YUXI WALVAX BIOTECH CO LTD
Chemoluminescent immunoassay kit of hepatitis A virus IgM antibody and preparation method thereof
InactiveCN101533025AEase of mass productionGuaranteed SensitivityChemiluminescene/bioluminescenceAgainst vector-borne diseasesPositive controlIgm antibody
The invention relates to the field of immunoassay medical science, and in particular provides a chemoluminescent immunoassay kit of a hepatitis A virus IgM antibody and a preparation method thereof. The kit comprises: 1) negative and positive control varieties of the hepatitis A virus IgM antibody; 2) a dermatate solid phase carrier; 3) a hepatitis A virus antigen liquid; 4) an enzyme label; 5) a chemoluminescent substrate; and 6) a condensed washing solution. Furthermore, the method for preparing the kit comprises the following steps: 1) preparing control varieties from negative and positive serum of the hepatitis A virus IgM antibody; 2) dermatating the solid phase carrier by an anti-human-mu chain antibody (monoclonal antibody or polyclonal antibody); 3) preparing an antigen liquid from the hepatitis A virus; 4) labeling the hepatitis A virus antibody (the monoclonal antibody or the polyclonal antibody) by enzyme; 5) preparing the chemoluminescent substrate solution; 6) preparing the condensed washing solution; 7) sub-packaging the negative and positive control varieties of the hepatitis A virus IgM antibody, the hepatitis A virus antigen liquid, the enzyme label, the chemoluminescent substrate solution and the condensed washing solution; and 8) assembling the substances into the finished products. The kit has the advantages of simpleness, convenience, quickness, sensitiveness, stability, and the like.
Owner:CHEMCLIN DIAGNOSTICS CO LTD
Hepatitis a virus detecting method in food
InactiveCN101550458ATime-consuming to solveResolve SensitivityMicrobiological testing/measurementMicroorganism based processesRNA extractionHepatitis A viruses
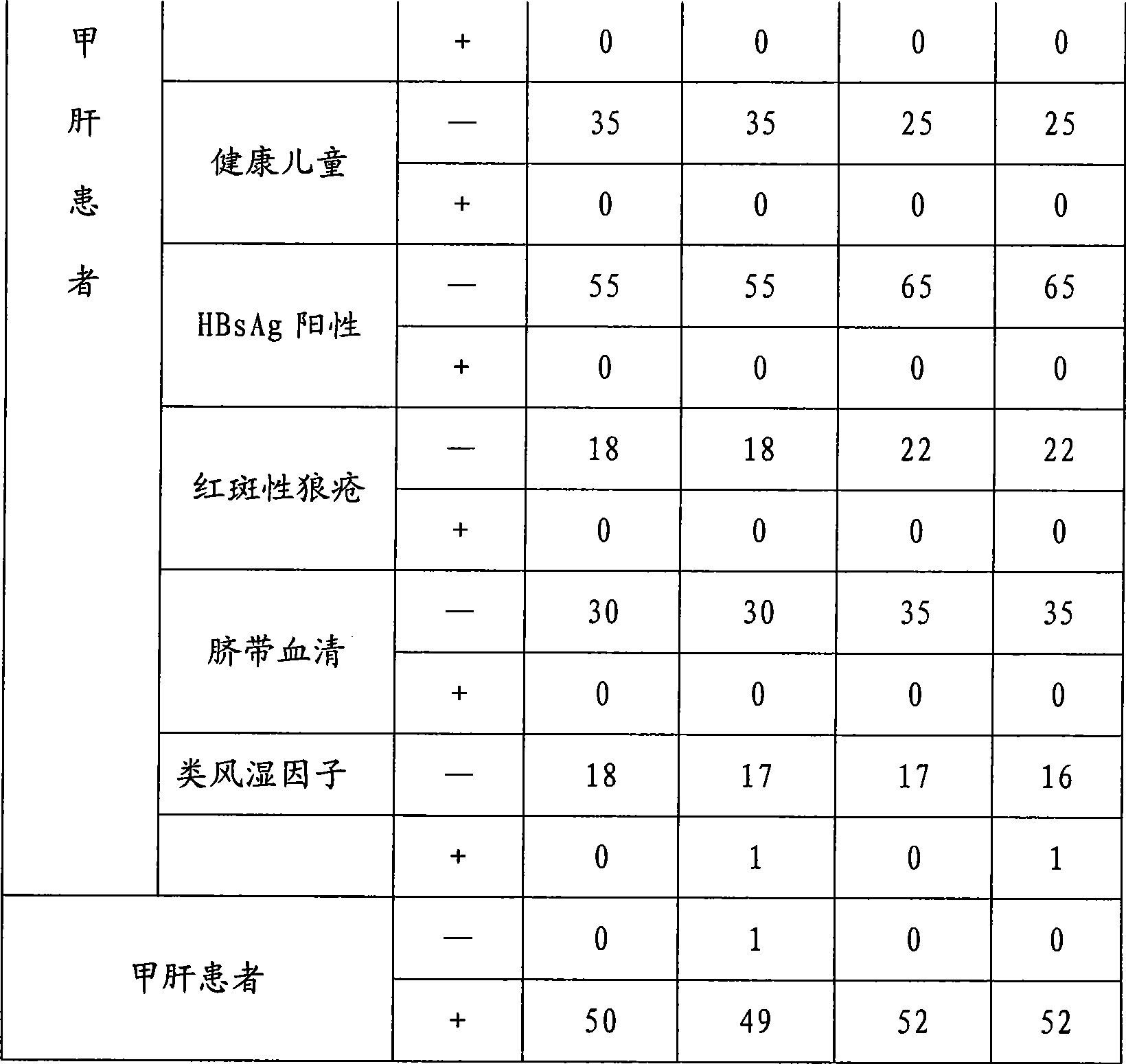
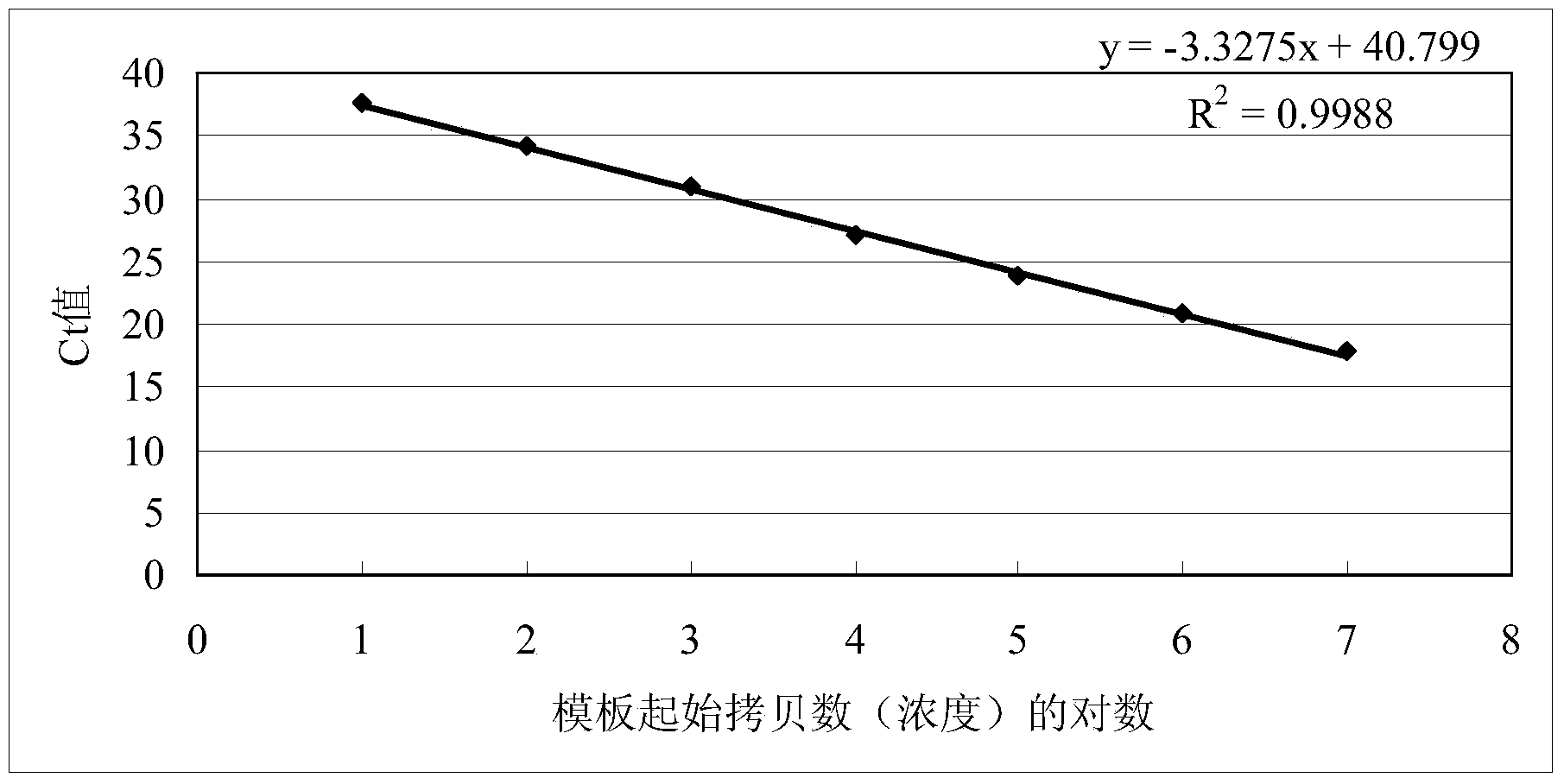
The present invention discloses an hepatitis a virus detecting method in food, the detecting method including following steps : 1) processing of sample under test; 2) RNA extraction of sample under test; 3) RNA reverse transcription; 4) SYBR Green I Real-time PCR reaction; 5) judgement of detected results: when cyclic threshold value of the sample under test is less than 33, judging as hepatitis a virus positive; when cyclic threshold value of the sample under test is more than 35, judging as hepatitis a virus negative; when cyclic threshold value of the sample under test is less than 35 and more than 33, treating as suspicious sample, if the renewed detected results is still in the range, then judging as hepatitis a virus negative. The detecting method of the invention is provided with advantages of rapid, high sensitivity and specificity and quantitative. judging as hepatitis a virus positive.
Owner:INSPECTION & QUARANTINE TECH CENT OF GUANGDONG ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Colloidal gold test strip capable of simultaneously detecting type-1 and type-3 duck hepatitis A viruses and preparation method of colloidal gold test strip
InactiveCN106990247AHigh sensitivityStrong specificityBiological material analysisDuck hepatitis A virusHepatitis A viruses
The invention discloses a colloidal gold test strip capable of simultaneously detecting type-1 and type-3 duck hepatitis A viruses and a preparation method of the colloidal gold test strip. The test strip consists of a bottom plate, a nitrocellulose membrane, a sample pad, a colloidal gold pad and an absorbing pad; anti-mouse IgG coats a quality control line on the nitrocellulose membrane; an anti-type-1 duck hepatitis A virus polyclonal antibody coats a detection line; and while colloidal gold coats the colloidal gold pad, the colloidal gold pad recognizes monoclonal antibodies of the type-1 and type-3 duck hepatitis A viruses. The test strip has high sensitivity, specificity, repeatability and stability, and therefore, a broad-spectrum, sensitive, specific, simple and rapid detecting technology which does not cause missing detection of the type-1 and type-3 duck hepatitis A viruses is provided for clinical and establishment units.
Owner:SICHUAN AGRI UNIV
Detection and quality control kit for hepatitis A viruses and noroviruses in water sample, as well as detecting method
InactiveCN105154590AMonitor Lysis EfficiencyAccurate Lysis EfficiencyMicrobiological testing/measurementMicroorganism based processesEscherichia coliPositive control
The invention discloses a detection and quality control kit for hepatitis A viruses and noroviruses in a water sample, as well as a detecting method. The kit comprises a tube of HAV 2*RT-PCR mix, a tube of NV GI+GII type 2*RT-PCR mix, a tube of MS2 2*RT-PCR mix, a tube of RNase-free water, a tube of HAV positive control, a tube of NV GI+GII positive control and a tube of coliphage MS2(2.5*1010 pfu / mL). The detecting method comprises the following steps: 1, virus concentration; 2, virus RNA extraction; 3, coliphage MS2 standard curve establishment; 4, hepatitis A viruses, norovirus GI type, norovirus GII type and coliphage MS2 detection; 5, virus RNA recovery ratio determination; 6, result judgment. According to the detection and quality control kit for the hepatitis A viruses and noroviruses in the water sample, as well as the detecting method, the quality of the whole virus detection process is effectively controlled through the key steps of sample pretreatment, detection, result judgment and the like, the stability, accuracy and controllability of the detection process are ensured, and the detection technology blank in the field is filled.
Owner:INSPECTION & QUARANTINE TECH CENT SHANDONG ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Hepatitis A virus antibody assay kit and preparation method thereof
InactiveCN101762702AAvoid influenceReduce labor intensityMaterial analysisAgainst vector-borne diseasesCelluloseSerum ige
The invention relates to a hepatitis A virus antibody assay kit which comprises an assay box, a gold labeled working solution bottle containing gold labeled working solution and a washing solution bottle containing washing solution, wherein the assay box comprises a box body and a box cover of the assay box, a cellulose nitrate membrane is arranged on the inner side of the box cover, a reaction hole is arranged on the box cover which corresponds to the position of the cellulose nitrate membrane, a water-absorbing pad is arranged in the box body of the assay box, an assay point and a quality control point are arranged on the cellulose nitrate membrane, the assay point is coated by solid-phase anti-human IgM, and the quality control point is coated by goat anti-mouse antibody. The hepatitis A virus antibody assay kit can fast diagnosis hepatitis A virus specific antibody by only dripping serum to be assayed on the cellulose nitrate membrane, and the hepatitis A virus antibody assay kit has the advantages of simple and convenient use, fast assay speed, no need of any instrument during clinical assay and simple structure and can avoid various factors during the experimental process from affecting the result and reduce the labor intensity of operation staff.
Owner:杨致亭
Detecting kit for hepatitis a virus IgM and IgG antibodies through colloidal gold method
InactiveCN102854316ARelieve painShorten the timeMaterial analysisAgainst vector-borne diseasesAntigenAssay
The invention discloses a detecting kit for hepatitis a virus IgM and IgG antibodies through a colloidal gold method. The detecting kit comprises a kit body, sample diluting liquid and detecting test paper. The detecting test paper is composed of a plastic supporting plate, a sample feeding pad, a colloidal gold pad of antigen VP1 marked with hepatitis a virus specifity, a nitrocellulose NC film, a detecting line T1, a detecting line T2, a quality control C line and a sample absorbing pad and arranged in the kit body. The kit detects that IgM and IgG in serum of the sample are positive simultaneously, the sample result is completely identical with an enzyme-linked immunosorbent assay (ELISA) reagent, no remarkable difference exists, and the kit is applicable to clinical detecting. The kit is low in cost and simple in preparation process and has practical value.
Owner:上海博沃生物科技有限公司
Hepatitis A virus antigen saliva fast detection test paper strip
ActiveCN101614740ASolve the problem that it is not suitable for on-site inspection and the inspection cost is highEasy to operateMaterial analysisAgainst vector-borne diseasesAnti-Hepatitis A virus IgGAdditive ingredient
The invention discloses a hepatitis A virus antigen saliva fast detection test paper strip which comprises a bottom plate that is sequentially stuck with a sample pad, an aurosol pad, a nitrocellulose membrane and a sample absorbing pad; wherein the aurosol pad is attached with polyclonal antibody or first monoclonal antibody of anti-hepatitis A virus antigen marked by aurosol; the nitrocellulosemembrane is coated with a detection line formed by second monoclonal antibody of the anti-hepatitis A virus antigen, and a quality control line formed by double anti-antibody resisting the polyclonalantibody or the first monoclonal antibody; the polyclonal antibody or the first monoclonal antibody is matched with the second monoclonal antibody in pairs. The test paper strip adopts aurosol labeling technique to detect the hepatitis A virus antigen ingredient in saliva, and can be used for judging whether the patient is infected by hepatitis B virus; furthermore, the test paper strip has simple operation, rapid reaction speed, high sensibility, strong specificity, being economical and practical and the like, so as to be suitable for field test and self-test.
Owner:杭州艾力康医药科技有限公司
Universal indirect ELISA kit for detecting type 1 and type 3 duck hepatitis A virus serum antibodies, and application thereof
ActiveCN110095607AReduce testing costsEasy to operateMaterial analysisDuck hepatitis A virusSerum ige
The invention discloses a universal indirect ELISA kit for detecting type 1 and type 3 duck hepatitis A virus serum antibodies, and an application thereof. The invention establishes a universal indirect ELISA detecting method for detecting type 1 and type 3 DHAV serum antibodies based on VP0 recombinant proteins. The method takes prokaryotically expressed type 1 DHAV VP0 recombinant proteins as detection antigens, and verifies that the VP0 recombinant proteins can specifically react with type 1 DHAV serum antibodies and can specifically react with type 3 DHAV serum antibodies by adopting Western blotting and indirect ELSIA methods, thus whether detected duck serum contains antibodies of type 1 and type 3 duck hepatitis A viruses and its antibody level can be determined by conducting indirect ELISA detection through taking the type 1 DHAV VP0 recombinant proteins as the detection antigens. The universal indirect ELISA kit and its application provided by the invention provide new and rapid detection means for effectively preventing and treating duck hepatitis A.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
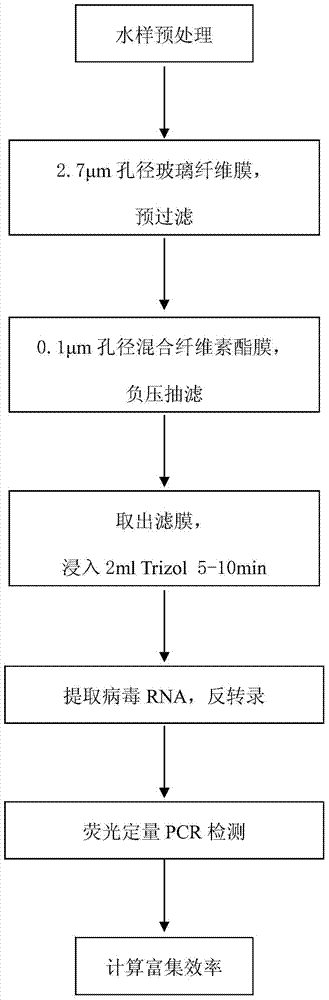
Method for enriching water body hepatitis A viruses based on mixed cellulose ester membrane
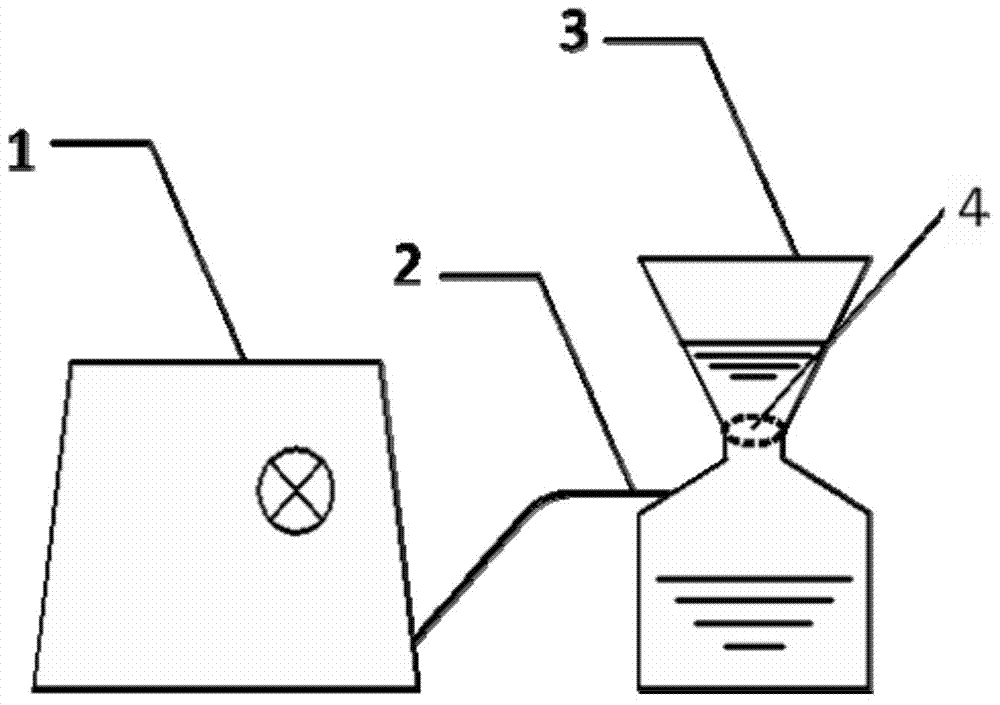
InactiveCN103789275AEasy to installLow costMicrobiological testing/measurementMicroorganism based processesCellulose ester membraneHepatitis A viruses
The invention provides a method for enriching water body hepatitis A viruses based on a mixed cellulose ester membrane. The method specifically comprises the following steps: pre-treating a water sample; filtering the water sample by using a glass fiber membrane; enriching the hepatitis A viruses in the water sample by using the mixed cellulose ester membrane; extracting RNA (Ribonucleic Acid) of the viruses and carrying out reverse transcription; manufacturing a standard curve; detecting the hepatitis A viruses by using fluorescence quantitative PCR (Polymerase Chain Reaction); calculating the enriching efficiency of the hepatitis A viruses. The method provided by the invention is reasonable in concept; the device is simple to operate, is light and is suitable for laboratory operation and wild work; conventional secondary concentration of adding an eluting agent to elute the viruses and adding an organic or inorganic flocculent precipitation agent to precipitate the viruses is saved and the volume can be concentrated for about 250 times; particularly, the enriching efficiency is high and the enriching efficiency in a PBS (Phosphate Buffered Saline) water sample averagely reaches (92.62+ / -5.17)%; the enriching efficiency in an East Lake water sample averagely reaches (79.45+ / -12.10)%. The method has very important meanings on efficient and accurate detection and monitoring on the hepatitis A viruses in the water bodies of various environments.
Owner:WUHAN INST OF VIROLOGY CHINESE ACADEMY OF SCI
Vero cell HCP test kit and application thereof
The invention belongs to the biotechnology field, in particular relates to a Vero cell HCP test kit and an application thereof. The Vero cell HCP test kit has strong specificity, high sensitivity and good repetitiveness, not only can be applied to nonsecreting-type viral vaccines, such as hepatitis A virus, and the like, but also can be applied to quality control and analysis in the preparation process of other Vero cell produced vaccines.
Owner:YUXI WALVAX BIOTECH CO LTD
Quantitative detection kit of hepatitis A virus
InactiveCN103805716AHigh detection specificityEliminate difference noiseMicrobiological testing/measurementAgainst vector-borne diseasesHepatitis A virusesHepatovirus
The invention relates to a quantitative detection kit of a hepatitis A virus. Specifically, a primer pair which detects the specificity of the hepatitis A virus is designed and verified; the optimal fluorescent quantitative PCR (Polymerase Chain Reaction) condition suitable for qualitatively and quantitatively detecting the hepatitis A virus is found by optimizing a fluorescent quantitative PCR system in real time; a standard substance and a quality control article for preparing a standard curve are additionally arranged in the detection kit to eliminate the differential interference among samples to ensure the precision and accuracy of quantitative detection to the maximum extent, thereby breaking through the defects that the previous detection methods of the hepatitis A virus are low in precision and great in uncertainty and the like. The detection method disclosed by the invention is high in sensitivity, and the lowest limit of detection can reach up to 10 copy / microlitre. Meanwhile, the kit is good in repeatability, and no significant differences (p is less than 0.05) exist in the group and among the groups after experiments are repeated for 6 times. The method disclosed by the invention can quickly and accurately detect the precise content of the hepatitis A virus.
Owner:WUHAN INST OF VIROLOGY CHINESE ACADEMY OF SCI
Compositions and method for detecting human parvovirus nucleic acid and for detecting hepatitis A virus nucleic acids in single-plex or multiplex assays
Nucleic acid oligomers specific for human parvovirus genomic DNA are disclosed. An assay for amplifying and detecting human parvovirus genotypes 1, 2 and 3 nucleic acid in biological specimens is disclosed. Compositions for amplifying and detecting the presence of human parvovirus genotypes 1, 2 and 3 genomic DNA in human biological specimens are disclosed.
Owner:GEN PROBE INC
Preparing method for HAV virus-like particle and application thereof
InactiveCN105505926AEfficient and convenient to getFix stability issuesSsRNA viruses positive-senseMicrobiological testing/measurementEscherichia coliBeta hairpin
The invention discloses a preparing method for a virus-like particle containing a hepatitis A virus (HAV)5'-UTR gene. The virus-like particle is an RNA-protein complex formed by wrapping HAV5'-UTR RNA with MS2 bacteriophage encoding coat protein; the encoding histidine label sequence is inserted into a beta hairpin loop structure sequence of the MS2 bacteriophage encoding coat protein, and the MS2 bacteriophage encoding coat protein containing a recombined histidine label and pNH-MS2his recombinant plasmid expressing a maturase protein gene are constructed. The RT-RCR is used for amplifying the HAV5'-UTR gene, the HAV5'-UTR gene is cloned into pNH-MS2his, and pronucleus recombinant expression plasmid is constructed and named pNH-MS2his-HAV5'-UTR. The obtained pNH-MS2his-HAV5'-UTR plasmid is converted into expression escherichia coli BL21 for inducible expression. A purification column of Ni-NTA is adopted for purifying the virus-like particle. The obtained virus-like particle is the virus-like particle containing the HAV5'-UTR gene and is named HAV5'-UTR-VLPs. The HAV5'-UTR-VLPs can serve as standards and quality control products for detecting RT-PCR, is free of infectivity, safe, reliable and good in stability, and has the advantage of resisting ribonuclease.
Owner:INSPECTION & QUARANTINE TECH CENT SHANDONG ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Duck hepatitis A virus universal detection kit
InactiveCN111500786ANo cross reactionRapid diagnosis of infectionMicrobiological testing/measurementAgainst vector-borne diseasesDuck hepatitis A virusHepatitis A viruses
The invention provides a duck hepatitis A virus universal detection kit and a detection method thereof. The kit comprises a universal primer pair for three types of duck hepatitis A viruses (DHAV-1, DHAV-2 and DHAV-3), and the sequences of the primer pair are shown as SEQ ID NO.1-2. The specific primers adopted by the invention can be used for effectively amplifying the three types of duck hepatitis A viruses (DHAV-1, DHAV-2 and DHAV-3), and can be used for quickly judging whether the duck hepatitis A viruses (DHAV-1, DHAV-2 and DHAV-3) exist in a sample to be detected or not. At present, no related research report of the duck hepatitis A virus universal detection kit exists at home and abroad, and the establishment of the kit provided by the invention can fill the blank in related fieldsat home and abroad.
Owner:INST OF ANIMAL HUSBANDRY & VETERINARY FUJIAN ACADEMY OF AGRI SCI
Instant cleaning powder
InactiveCN102041198AExtended shelf lifeImprove performanceInorganic non-surface-active detergent compositionsDetergent powders/flakes/sheetsHepatitis A virusesHepatitis B virus
The invention discloses instant cleaning powder which comprises 10-30 wt% of sodium dichloro isocyanurate and 60-90 wt% of heavy sodium carbonate. The high-power cleaning and sterilizing power has the advantages of wide bactericidal range, wide application range, high dissolubility in water, and small smell, can effectively kill various bacteria, and has strong functions of algae removal, deodorization, water purification, cleaning and blanching. The instant cleaning powder can effectively kill various bacteria, and has specially good effects on inactivating spores, fungi, viruses, hepatitis A virus and hepatitis B virus. In addition, the instant cleaning powder also has the effects of algae removal, deodorization, water purification and blanching, and can effectively remove stubborn stains. The instant cleaning powder can be used for killing bacteria on most surfaces, including surfaces of white cloth, desktops, ground, lavatories, elevators, escalators and the like, has strong permeability and cleaning property, and can not cause stain redeposition.
Owner:CHENGDU AIRCRAFT INDUSTRY GROUP
Rapid diagnostic kit based on loop-mediated isothermal amplification technique for hepatitis A virus genes and detection method thereof
ActiveCN101660005BHigh sensitivityEasy to identifyMicrobiological testing/measurementMicroorganism based processesHepatitis A virusesReverse transcriptase
The invention discloses a rapid diagnostic kit based on a loop-mediated isothermal amplification technique for hepatitis A virus genes and a detection method thereof. The kit comprises two pairs of primers, Bst DNA polymerase, revertase, an RNase inhibitor, a stabilizing solution, a reaction solution, a chromogenic solution and a positive contrast solution, wherein the nucleotide sequences of thetwo pairs of primers are shown in SEQ ID NO: 1-4; and the eight solutions are respectively contained in containers. The kit and the detection method can detect the hepatitis A virus with high efficiency and high specificity, are based on the loop-mediated isothermal amplification technique, apply six segments, four primers and one constant temperature to complete an amplification reaction within less than one hour, and have the advantages of low detection cost, short time consumption, high yield, high specificity, significant chromogenic difference between a positive result and a negative result, high authentication rate, distinctness and reliability.
Owner:GUANGZHOU HUAFENG BIOTECH
Duck hepatitis A virus type 2 detection kit and detection method thereof
InactiveCN109576398AIncreased sensitivityAccurate identificationMicrobiological testing/measurementAgainst vector-borne diseasesDiseaseDuck hepatitis A virus
The invention provides a duck hepatitis A virus type 2 detection kit and a detection method thereof. The kit comprises a primer pair of duck hepatitis A viruses type 2, and a sequence is as shown in SEQ ID NO. 1 to 2. A specific primer adopted by the invention selects the 2A2 protein area design with lowest amino acid homology of DHAV-2, DHAV-1 and DHAV-3, the adopted primer can accurately and rapidly determine whether a disease material contains the duck hepatitis A virus type 2 or not. At present, no related research report about a detection kit for detecting duck hepatitis A virus type 2 byusing specific detection and the detection method thereof exists both at home and abroad, and the establishment of the duck hepatitis A virus type 2 detection kit and the detection method thereof canfill the blank in related fields at home and abroad.
Owner:INST OF ANIMAL HUSBANDRY & VETERINARY FUJIAN ACADEMY OF AGRI SCI
Health product containing selenium and vitamin and preparation method thereof
InactiveCN105361152AAntioxidantReduce the side effects of radiotherapy and chemotherapyFood scienceBeta-CaroteneSide effect
The invention relates to a health product containing selenium and vitamin. A proper weight ratio of seaweed selenium polysaccharide, vitamin E and beta-carotene are added to a mixed fine powder containing Chinese wolfberry, lotus seeds, buckwheat, Chinese yam and konjac; and the synergistic effect of the components endows the prepared health product containing selenium and vitamin with the efficacies of resisting oxidation, removing harmful free radicals, reducing the side effects of radiotherapy and chemotherapy, as well as the unique effects of detoxifying, resisting virus and pollution, repairing islet cells, clearing hepatitis A virus, invigorating spleen, nourishing stomach, promoting blood circulation to remove blood stasis, detoxifying, relieving swelling, softening blood vessels, improving microcirculation, tonifying kidney, astringing essence, nourishing heart, tranquilizing the nerves, and delaying aging. For long-term consumption, the health product can invigorate spleen, nourish stomach, enhance immunity, resist and prevent cancer, and prolong life span.
Owner:吕庆彬
Growth of wild-type hepatitis a virus in cell culture
The invention provides recombinant Hepatitis A Virus (HAV) nucleic acids and host cells that are permissive for their growth and replication. The recombinant Hepatitis A Virus nucleic acids not particularly limited, except that they incorporate at least one heterologous nucleic acid fragment. The heterologous nucleic acid can encode a selectable marker gene and such recombinant HAV nucleic acids are useful for selecting cells that are permissive for growth and replication of wild type HAV. Alternatively, the heterologous nucleic acid may encode a vaccine antigen or other expression product that is desirable to express in a cell harboring the recombinant HAV nucleic acid. The invention further provides cell lines permissive for growth and replication of wild type HAV or HAV having minimal mutations for growth in cell culture. The invention further provides methods for producing HAV vaccines and for monitoring environmental and patient samples for the presence of HAV.
Owner:美利坚合众国政府,由卫生与人类服务部部长代表
Single-domain antibodies against hepatitis A viruses and derived proteins of single-domain antibodies
ActiveCN111138533AIncrease credibilityHigh affinityAntibody mimetics/scaffoldsBiological material analysisHepatitis A virusesHepatitis A Virus Antibody
The present invention relates to the technical field of biotechnology or immunology and relates to single-domain antibodies against hepatitis A virus and derived proteins of the single-domain antibodies. An amino acid sequence of CDR1 of the single-domain antibodies is shown in any one of SEQ ID NO:83-SEQ ID NO:117; an amino acid sequence of CDR2 is shown in any one of SEQ ID NO:118-SEQ ID NO:143;and an amino acid sequence of CDR3 is shown in any one of SEQ ID NO:144-SEQ ID NO:174. Beneficial effects are that: the traditional method for indirect detection of hepatitis A virus antigen (HAV-Ag)by detecting hepatitis A virus antibodies (HAV-IgM and HAV-IgG) has many detection steps and many influence factors. The present invention provides a detection method based on ELISA that directly detects the hepatitis A virus antigen (HAV-Ag) without RT-PCR.
Owner:REGENECORE BIOTECH CO LTD
Application of single domain antibody for hepatitis a viruses
ActiveCN111138532AIncrease credibilityHigh affinityBiological material analysisImmunoglobulins against virusesHepatitis A virusesHepatitis A Virus Antibody
The invention relates to the field of a biological technique or an immunological technique, in particular to an application of a single domain antibody for hepatitis a viruses. The amino acid sequenceof CDR1 of the single domain antibody is as shown in any of SEQID NO: 83-SEQID NO: 117, the amino acid sequence of CDR2 is as shown in any of SEQID NO: 118-SEQ NO: 143, and the amino acid sequence ofCDR3 is as shown in SEQID NO: 144-SEQ NO: 174. The application disclosed by the invention has the beneficial effects that by a traditional method for indirectly detecting hepatitis A virus antigens (HAV-Ag) through detecting hepatitis A virus antibodies (HAV-IgM, HAV-IgG), many detection steps need to be operated, and many influence factors also exist. The invention provides a detection method for directly detecting hepatitis A virus antigens (HAV-Ag) without RT-PCR based on ELISA.
Owner:REGENECORE BIOTECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com