Chemoluminescent immunoassay kit of hepatitis A virus IgM antibody and preparation method thereof
A hepatitis A virus and chemiluminescence immunological technology, applied in the field of immunoassay medicine, can solve problems such as unseen use, and achieve the effects of ensuring sensitivity, facilitating large-scale production, and reducing gray area values.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
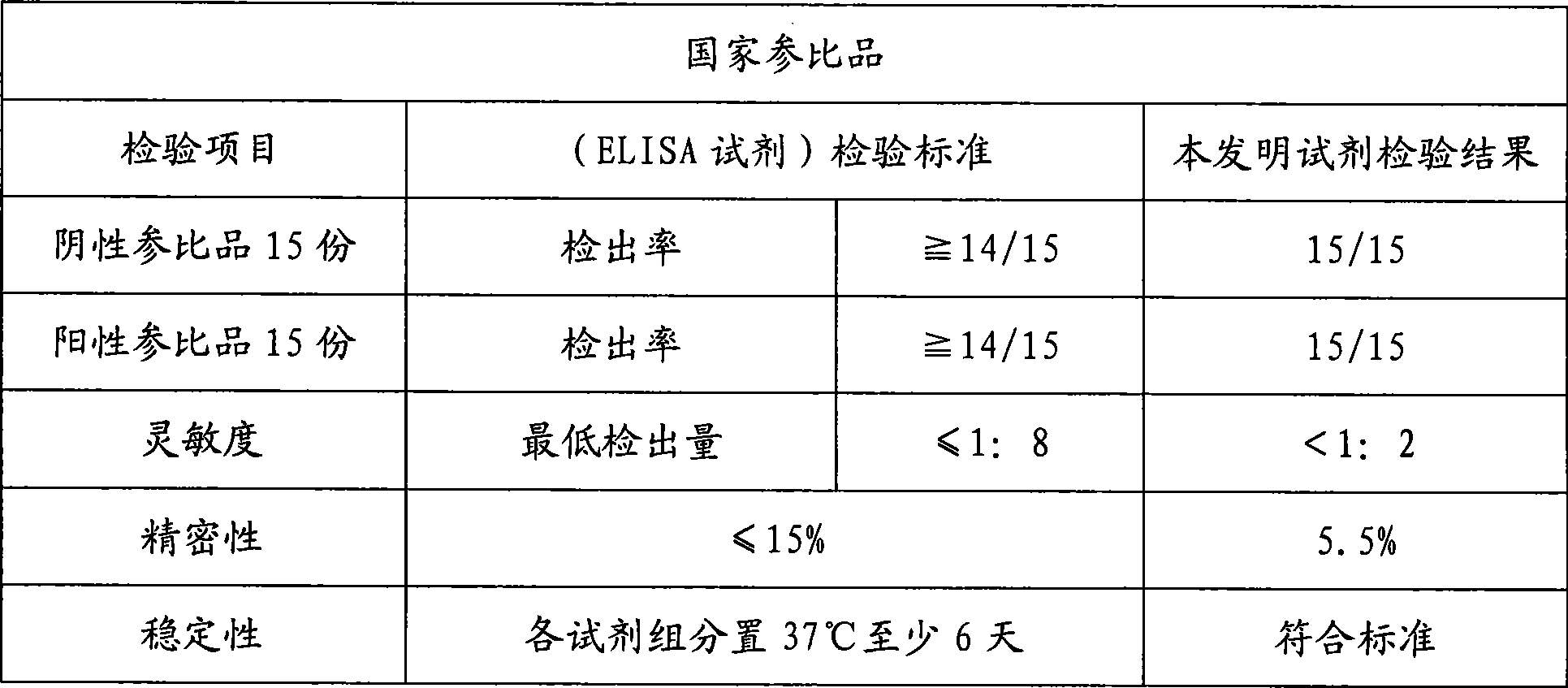
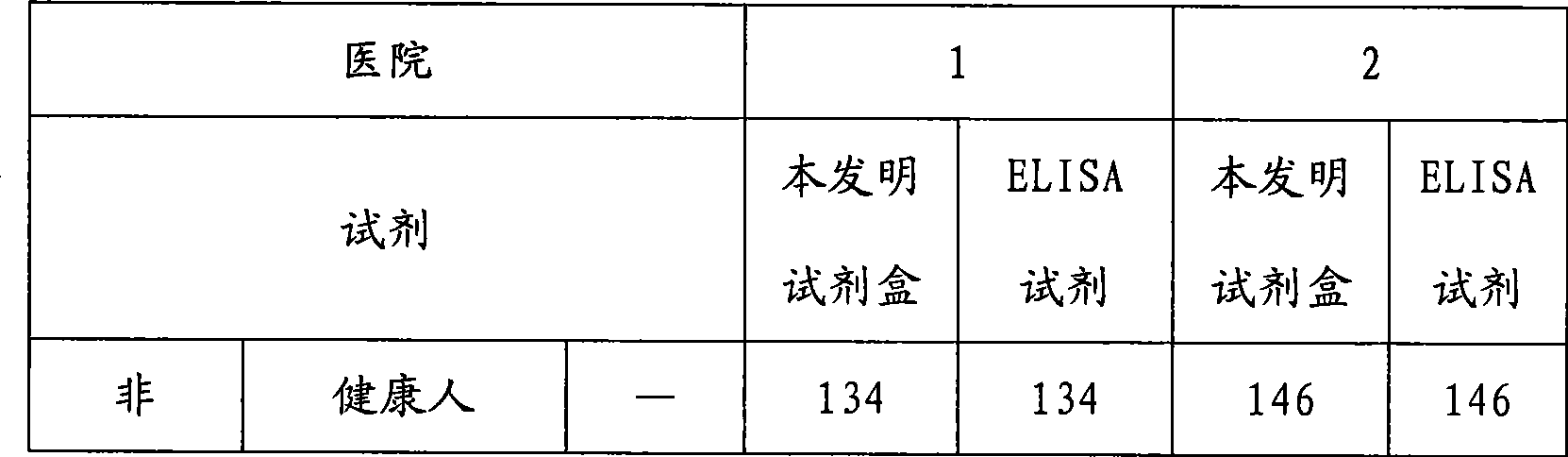
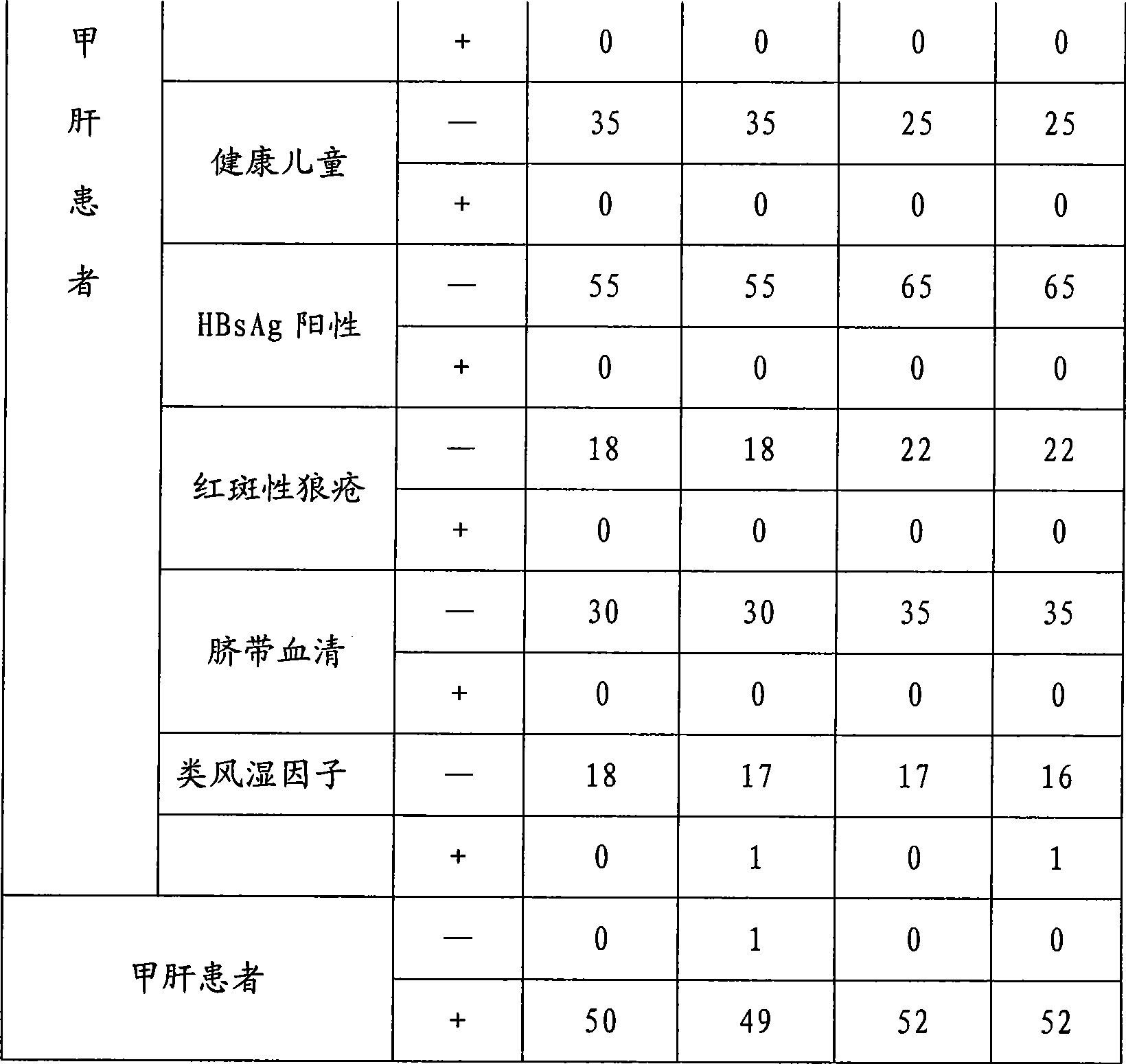
[0028] Example 1 Preparation of the hepatitis A virus IgM antibody chemiluminescent immunoassay assay kit of the present invention
[0029] In the research process of the present invention, the inventor of the present invention firstly carried out screening experiment and quality appraisal to the raw material used, comprise the activity of antibody marker and coated antibody, carrier (such as opaque white microwell plate) Adsorption performance and variability, HRP activity, luminescence intensity and luminescence duration of chemiluminescent substrates, etc. Then the coating method was studied, experiments were carried out with different coating buffers and protection solutions, the most suitable coating buffer and protection solutions were selected, and the best concentration conditions were found through experiments with different coating concentrations of antibodies. There are different methods for HRP labeling. Through repeated exploration and comparative experiments, a s...
Embodiment 2~3
[0085] Examples 2-3 Preparation of Hepatitis A virus IgM antibody chemiluminescent immunoassay assay kit of the present invention
[0086] Except that the hepatitis A virus antibody was labeled with alkaline phosphatase, AMPPD was used as the chemiluminescent substrate, plastic beads and plastic tubes were used as the carrier, the pH value of the coating solution was 4.5, and the pH value of the blocking solution was 7.5. The hepatitis A virus IgM antibody chemiluminescent immunoassay assay kit was prepared in the same manner as in Example 1.
Embodiment 4
[0087] Example 4 Preparation of the hepatitis A virus IgM antibody chemiluminescence immunoassay assay kit of the present invention
[0088] Except that magnetic particles were used as the solid phase carrier, and the magnetic particle anti-human-μ chain antibody solid phase carrier was prepared by the glutaraldehyde coupling method, the rest were prepared by the same method as in Example 1. The hepatitis A virus IgM antibody chemiluminescence immunoassay Analytical assay kits.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com