Patents
Literature
141 results about "Hepatitis a virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Hepatitis A is a liver disease caused by the hepatitis A virus (HAV). The virus is primarily spread when an uninfected (and unvaccinated) person ingests food or water that is contaminated with the faeces of an infected person.
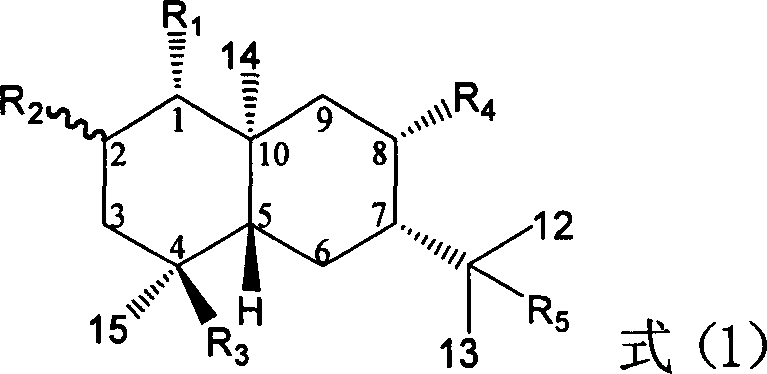
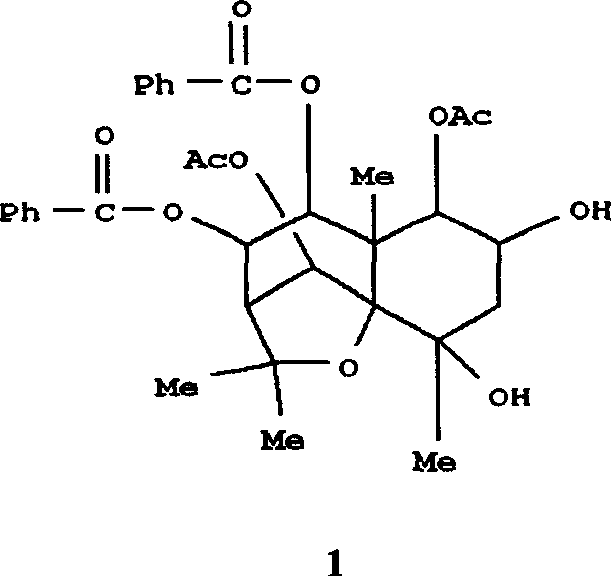
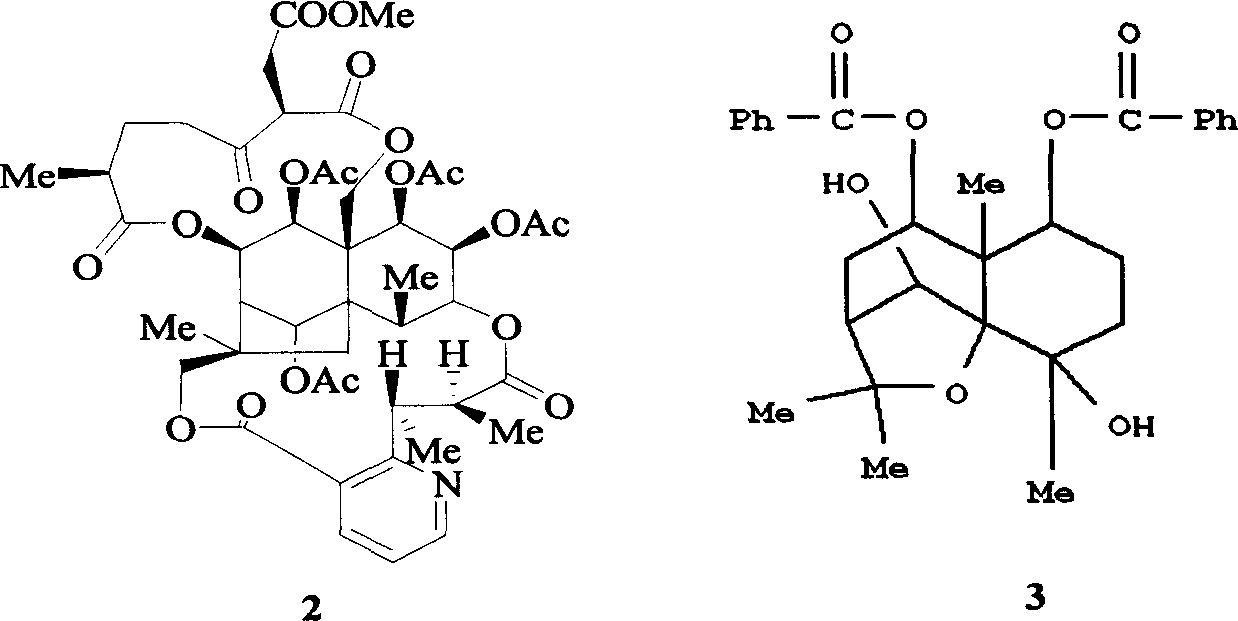
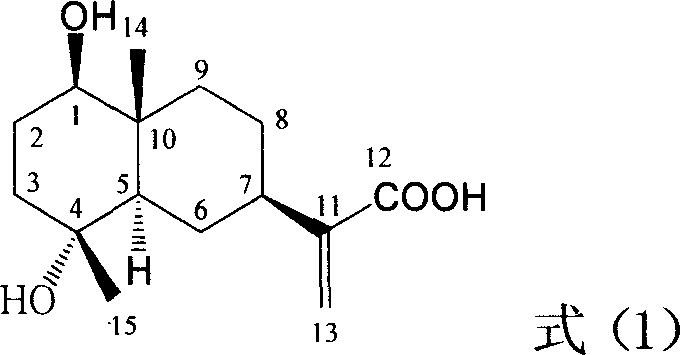
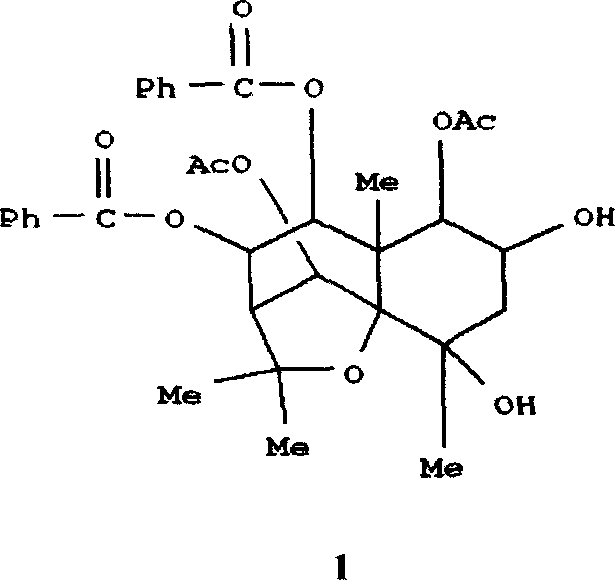
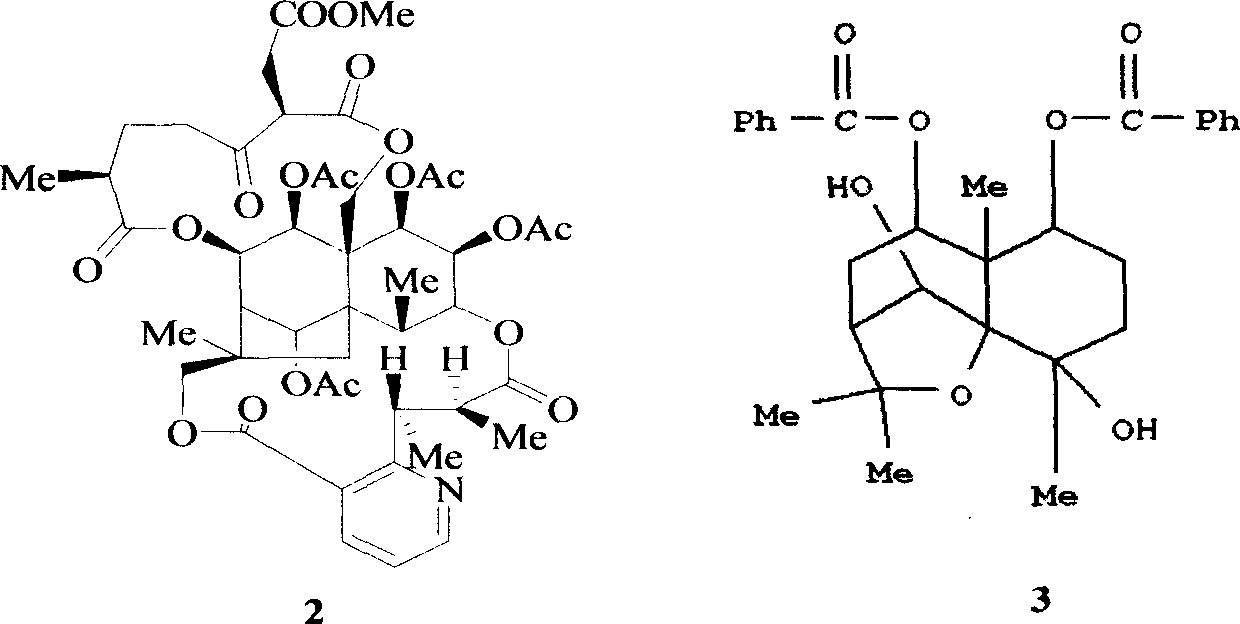
Pharmaceutical use of ent-eudesmane alcohol type sesquiterpene for inhibiting hepatitis virus
InactiveCN1935762APrevention and treatment of viral hepatitis BHBsAg reductionSugar derivativesHydroxy compound active ingredientsDiseaseSolvent
The invention relates to an enantiomorphic amine alkyl sesquiterpene alcohol and glucoside and the medicated salt or solvent thereof, as well as the effect and activity of the composed medicine combination, mainly relating to the medical use in reducing HBV-DNA replication activity. And it has considerably strong inhibiting effect on HBsAG screted by HepG2.2.15 and HBV-DNA replication as compared with positive contrast Lamivudine; and it has obvious inhibition activity to HBV-DNA replication at large dosage (100 mug / mL) and medium dosage(20 mug / mL) as contrasted with Lamivudine, and can be expected to apply to preparing medicines for curing HB virus infection disease.
Owner:赵昱
Pharmaceutical use of 1 beta-hydroxy ilexolic acid for inhibiting hepatitis virus
InactiveCN1935131APrevention and treatment of viral hepatitis BHBsAg reductionOrganic active ingredientsOrganic chemistryChemical structureDisease
The present invention relates to an eudesmane type sesquiterpene derivative 1 beta-hydroxyilicic acid, namely 1 beta-hydroxy-5 alpha H-eudesmane-11 (13)-ethylene-12-acid, its medicineal salt or solvent compound and its medicine composition and medicinal application for preparing medicine capable of curing hepatitis B virus infective disease and resisting hepatitis B virus. Said invention also provides its chemical structure formula.
Owner:WENZHOU MEDICAL UNIV
Methods and reagents to detect and characterize norwalk and related viruses
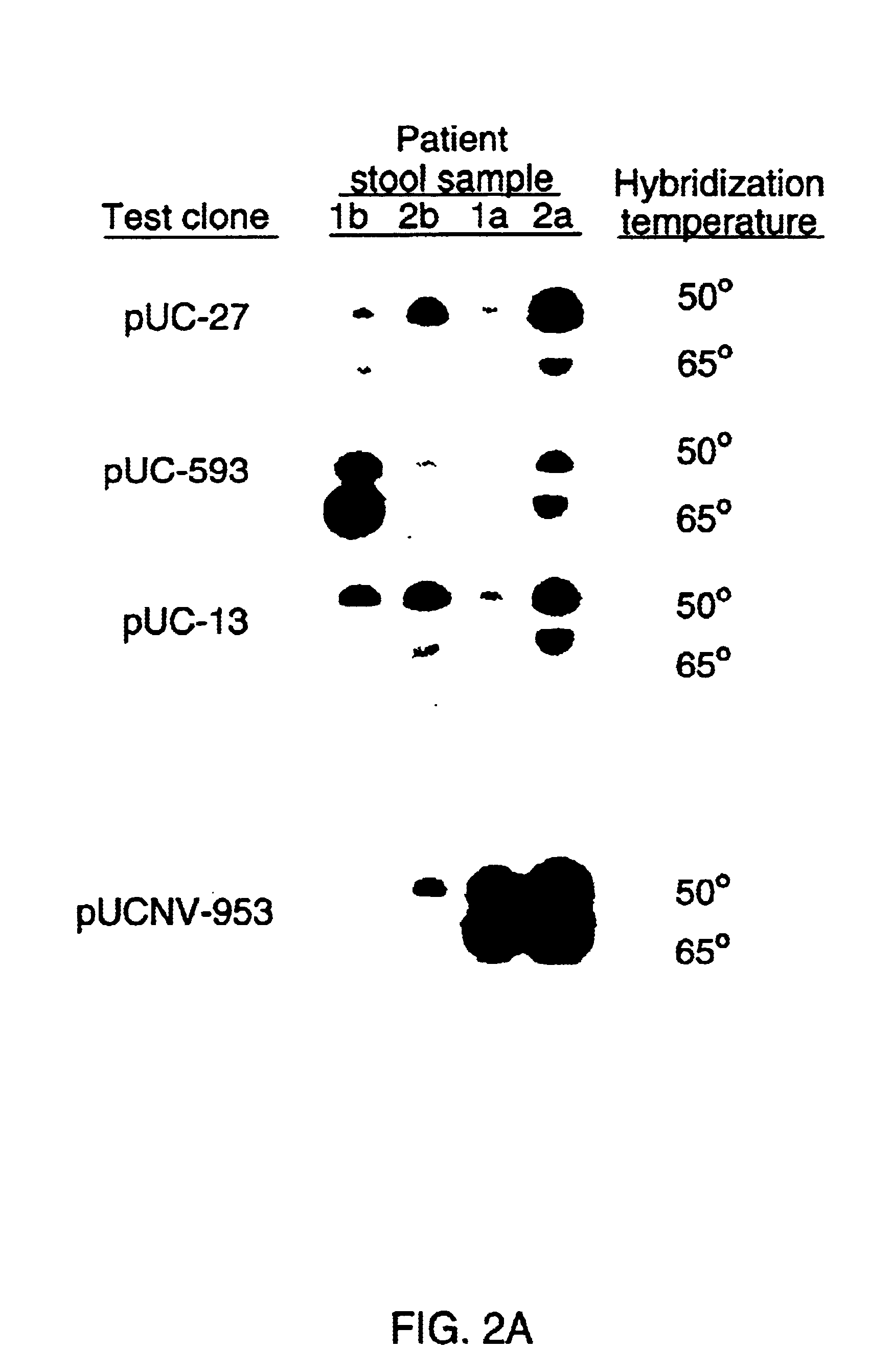
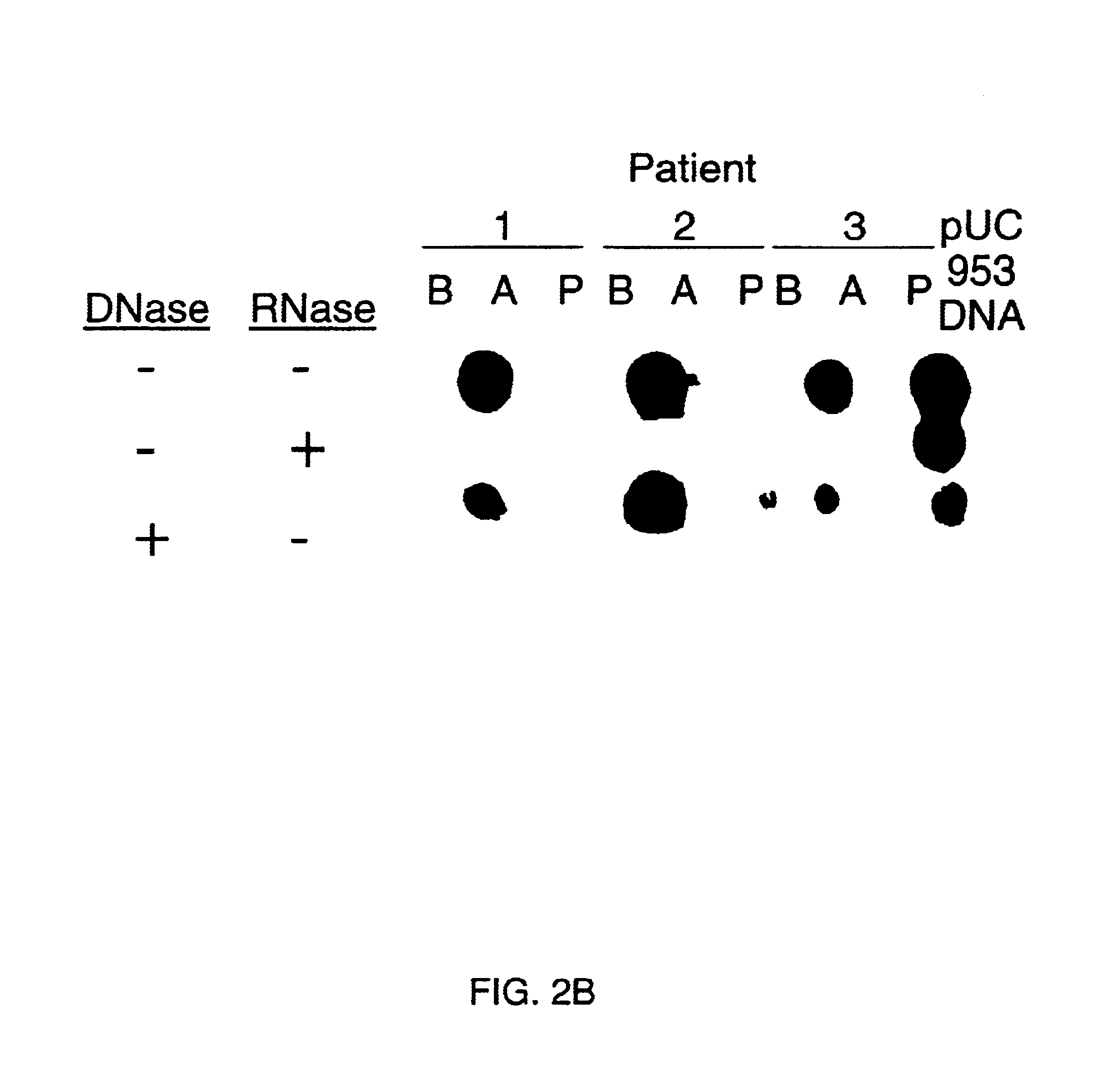
Double-stranded cDNA was synthesized from nucleic acid extracted from Norwalk virus purified from stool specimens of volunteers. One clone was isolated from a cDNA library constructed in a pUC-13 vector after amplification of the cDNA. The specificity of this cDNA (pUCNV-953) was shown by hybridization assays. The cDNA reacted with post (but not pre-) infection stool samples from Norwalk volunteers and with highly purified Norwalk virus, but not with other common enteric viruses such as hepatitis A virus and rotavirus. Finally, the probe detected virus in the same fractions of CsCl gradients in which viral antigen was detected using a specific Norwalk virus radioimmunoassay, and particles were detected by immune electron microscopy. Single-stranded RNA probes derived from the DNA clone after subcloning into an in vitro transcription vector were also used to show that the Norwalk virus contains a ssRNA genome of about 8 kb in size. The original clone was also used to detect additional cDNAs which represent at least 7 kb of nucleic acid of the Norwalk genome. The availability of a Norwalk-specific cDNA and the first partial genome sequence information allow rapid cloning of the entire genome and of establishment of sensitive diagnostic assays. Such assays can be based on detection of Norwalk virus nucleic acid or Norwalk viral antigen using polyclonal or monoclonal antibodies to proteins expressed from the cDNA or to synthetic peptides made based on the knowledge of the genome sequence. Assays using proteins deduced from the Norwlk virus genome and produced in expression systmes can measure antibody responses. Vaccines made by recombinant DNA technology are now feasible.
Owner:BAYLOR COLLEGE OF MEDICINE
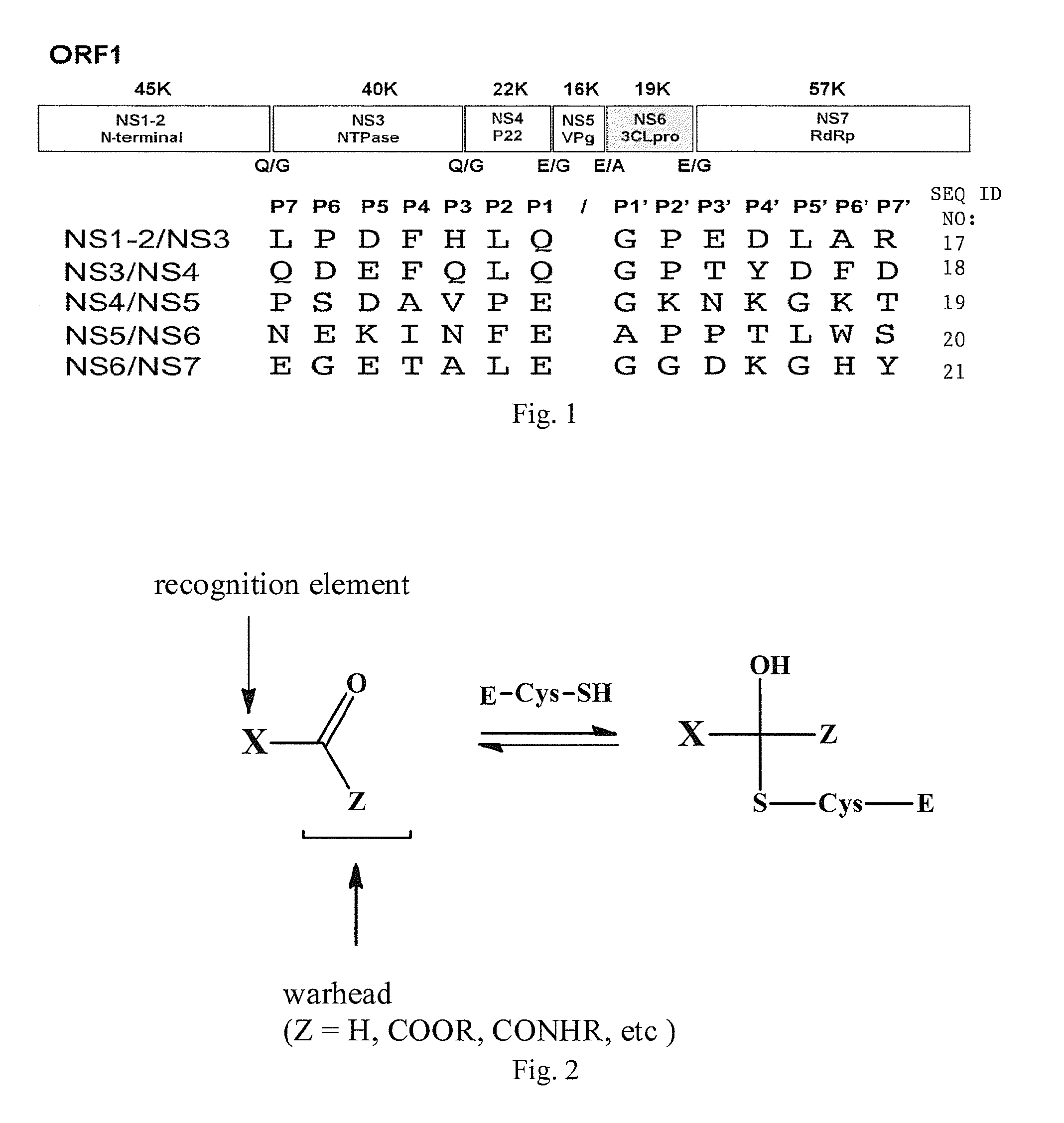
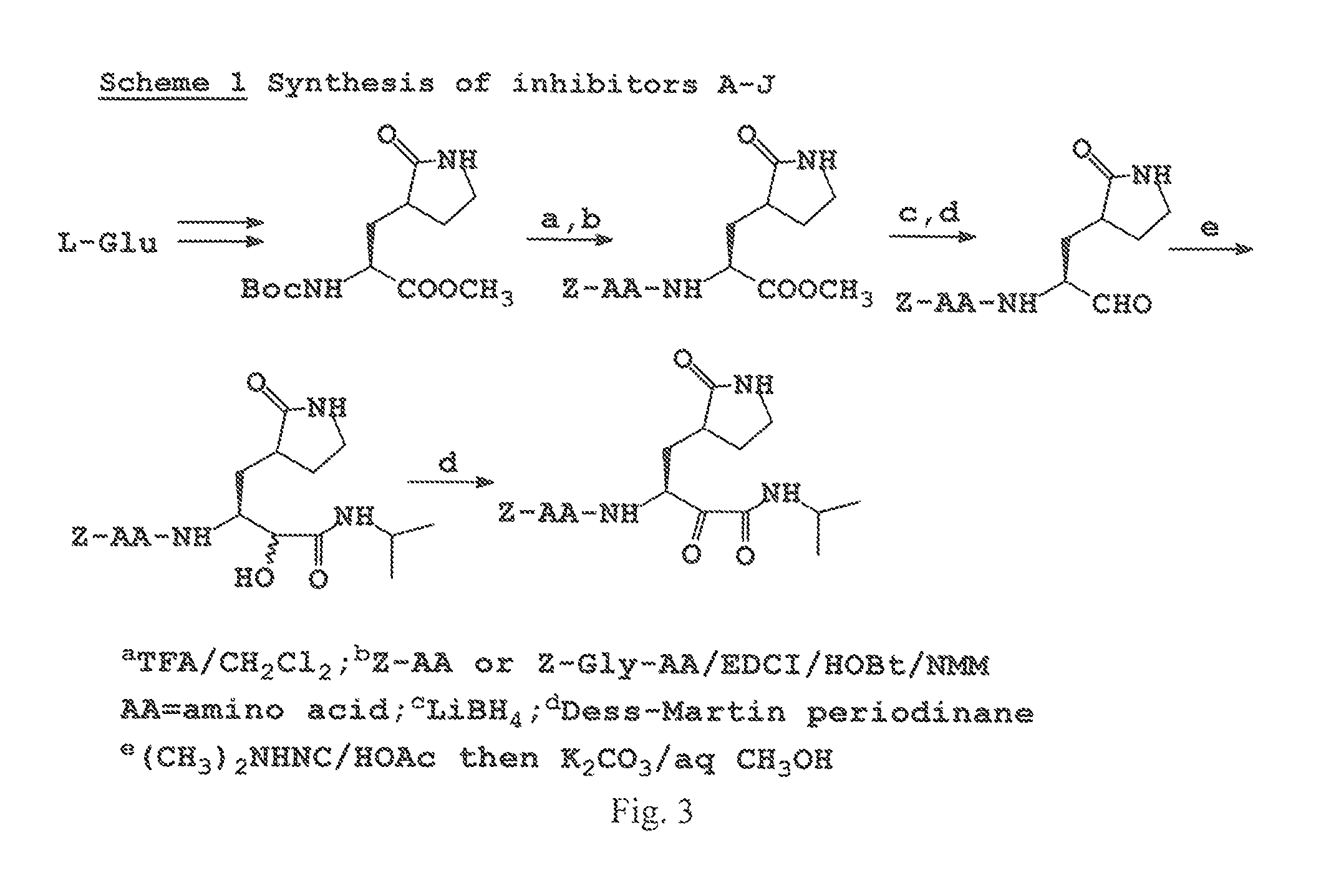
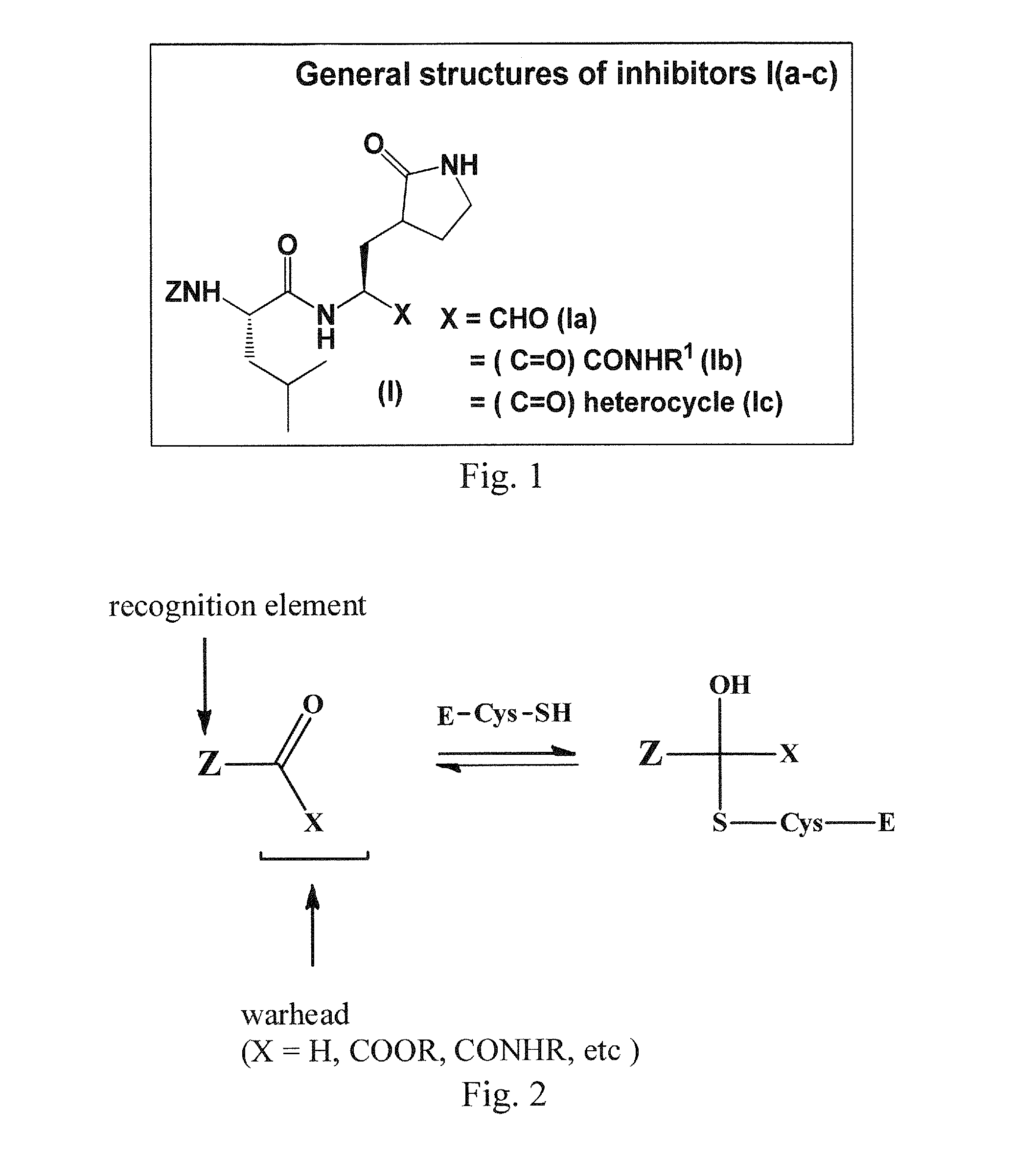
Broad-spectrum antivirals against 3c or 3c-like proteases of picornavirus-like supercluster: picornaviruses, caliciviruses and coronaviruses
ActiveUS20140243341A1Preventing and inhibiting replicationBiocideSsRNA viruses positive-senseEnterovirusDisease
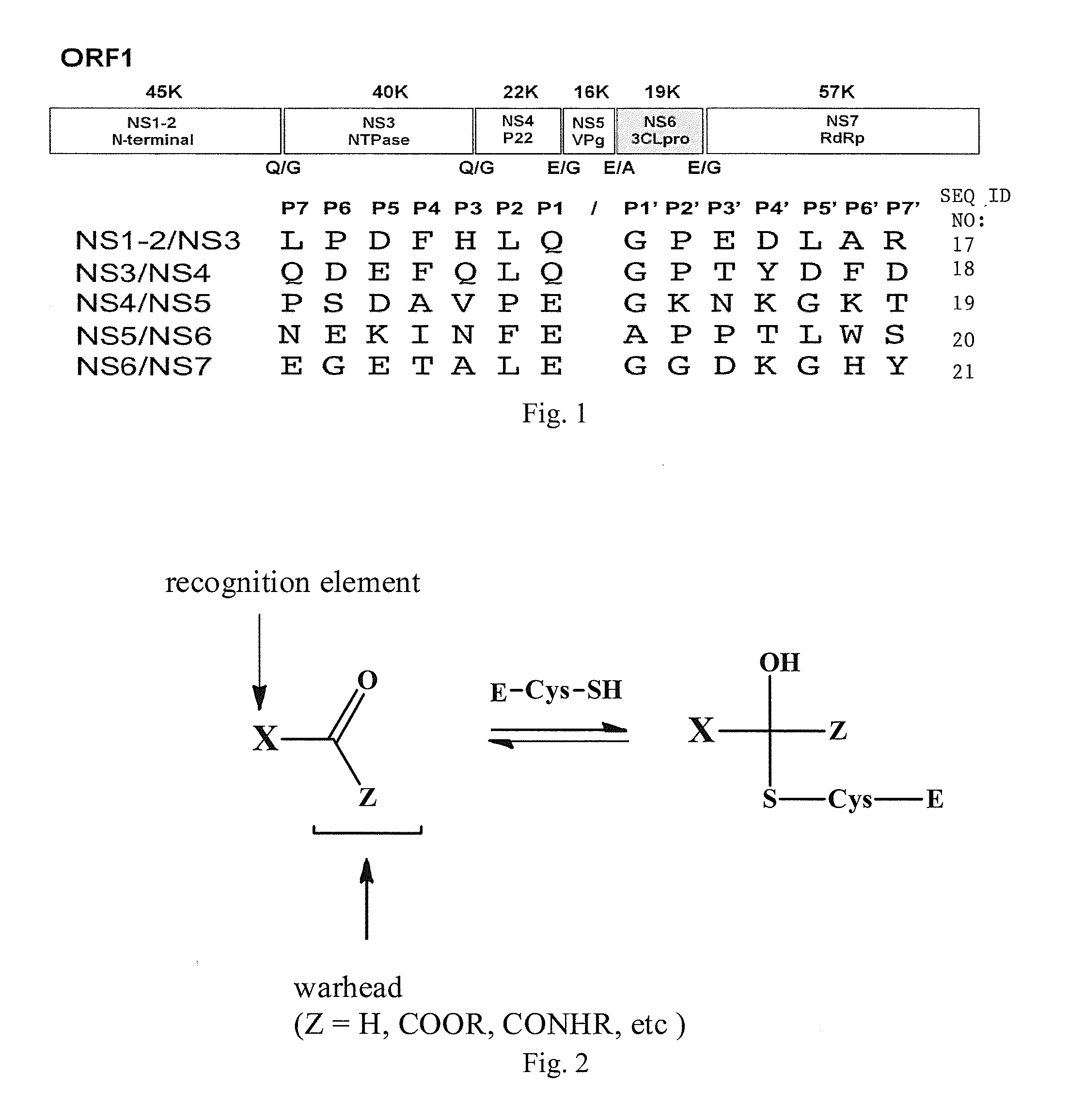
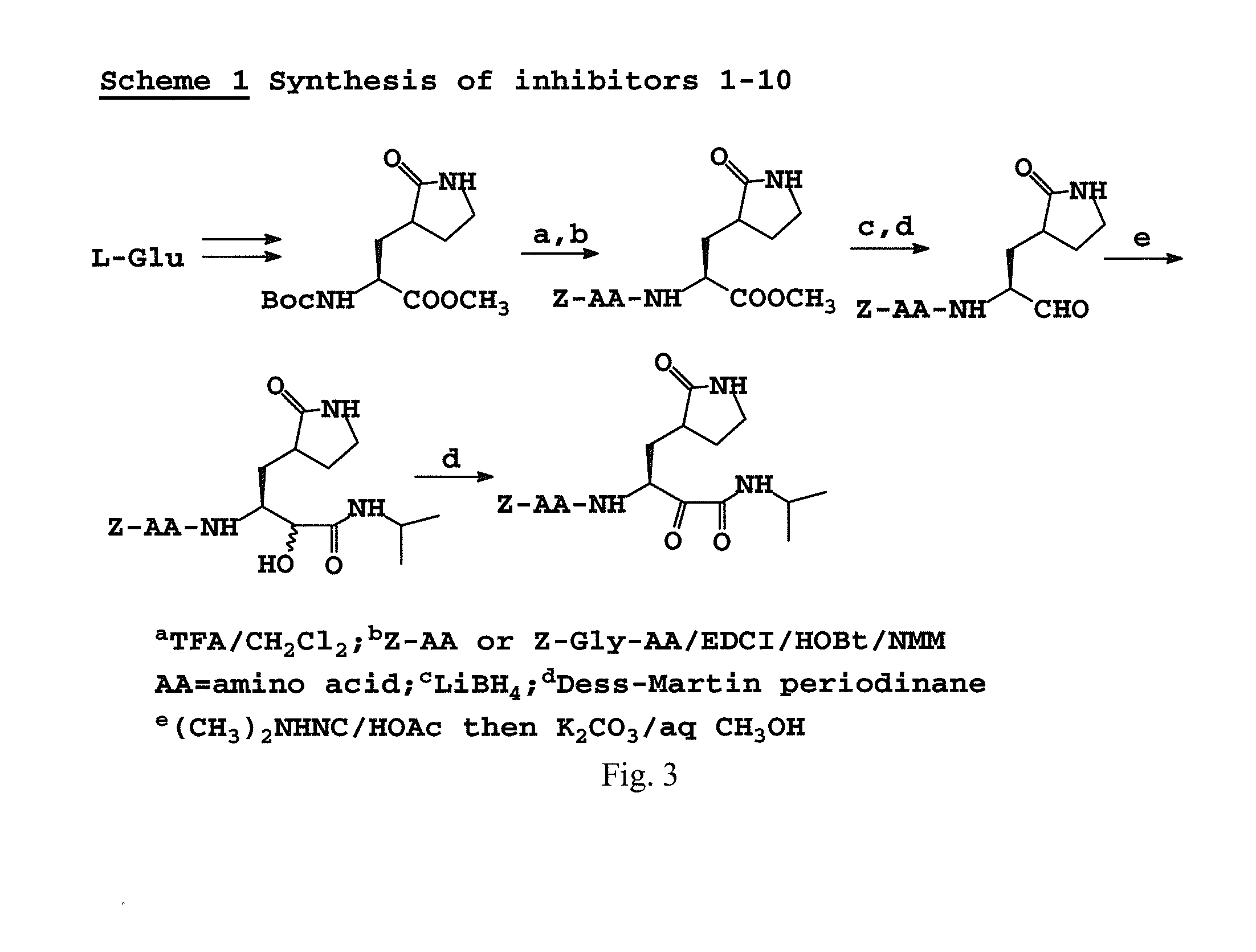
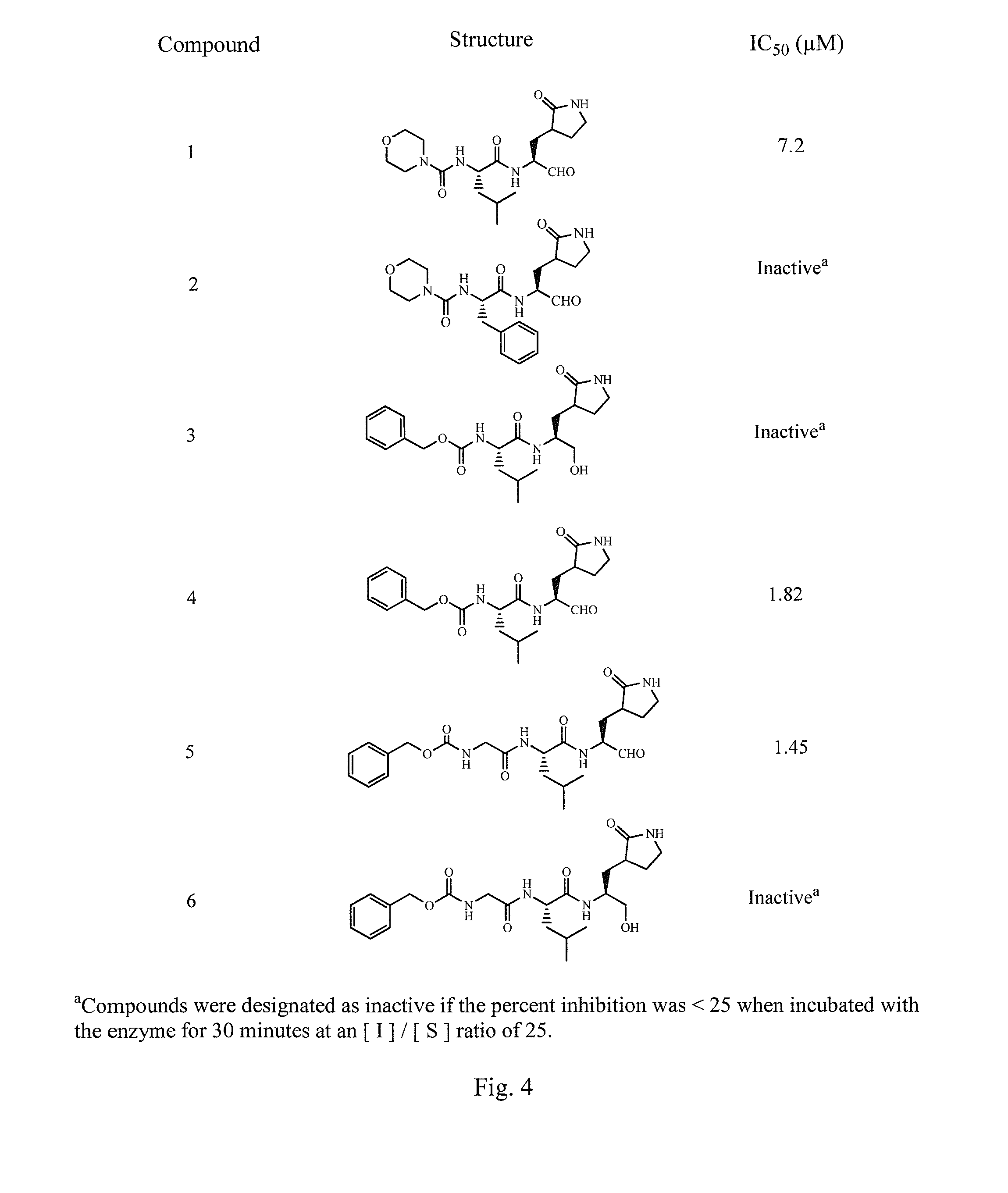
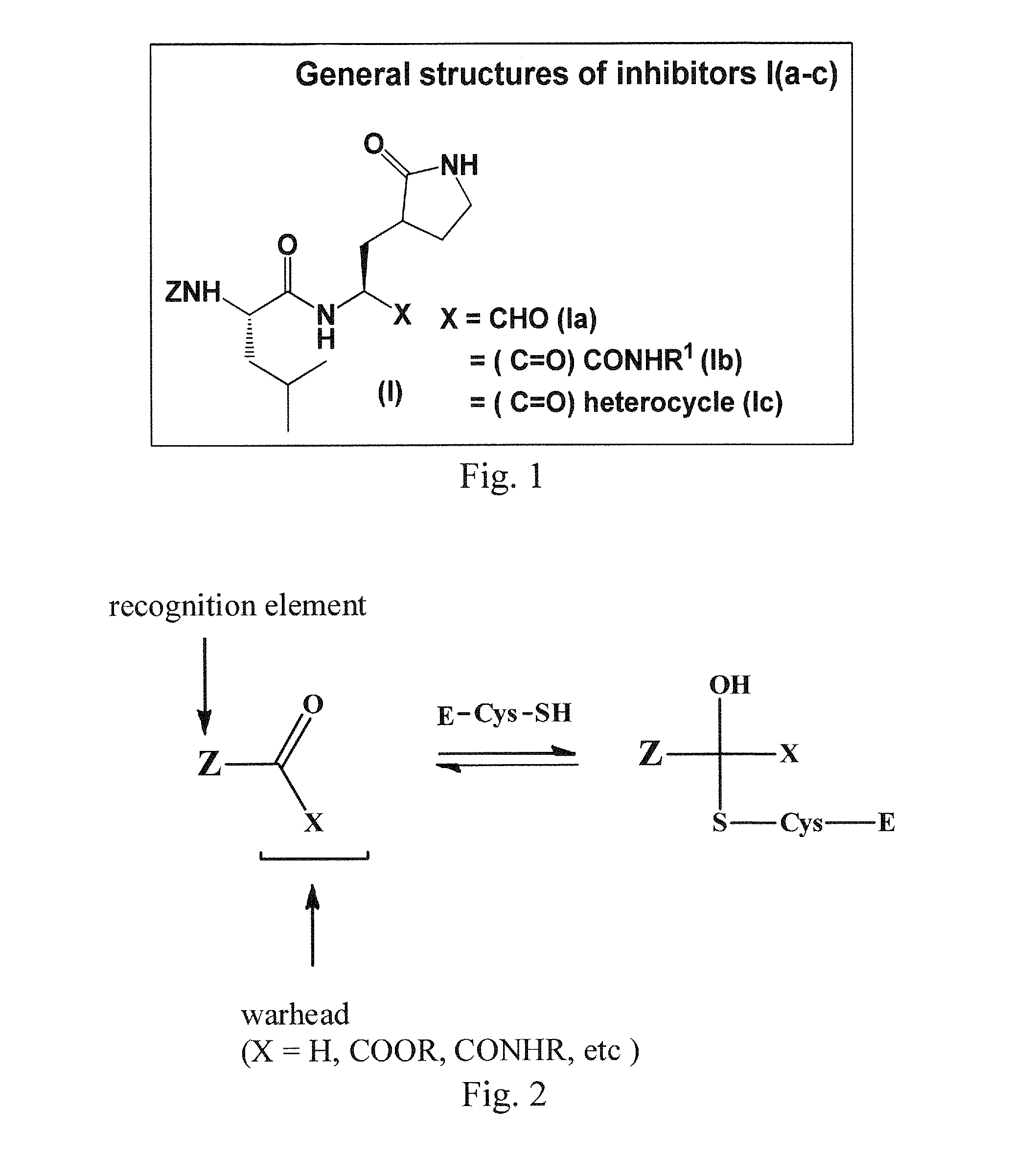
Antiviral protease inhibitors, including peptidyl aldehydes, peptidyl α-ketoamides, peptidyl bisulfite salts, and peptidyl heterocycles, are disclosed, along with related antiviral compounds, and methods of using the same to treat or prevent viral infection and disease. The compounds possess broad-spectrum activity against viruses that belong to the picornavirus-like supercluster, which include important human and animal pathogens including noroviruses, enteroviruses, poliovirus, foot-and-mouth disease virus, hepatitis A virus, human rhinovirus (cause of common cold), human coronavirus (another cause of common cold), transmissible gastroenteritis virus, murine hepatitis virus, feline infectious peritonitis virus, and severe acute respiratory syndrome coronavirus.
Owner:WICHITA STATE UNIVERSITY +2
Compositions and methods for detection of hepatitis A virus nucleic acid
InactiveUS20060014142A1Sugar derivativesMicrobiological testing/measurementOligomerNucleic acid sequencing
Nucleic acid oligomeric sequences and in vitro nucleic acid amplification and detection methods for detecting the presence of HAV RNA sequences in samples are disclosed. Kits comprising nucleic acid oligomers for amplifying and detecting HAV nucleic acid sequences are disclosed.
Owner:GEN PROBE INC
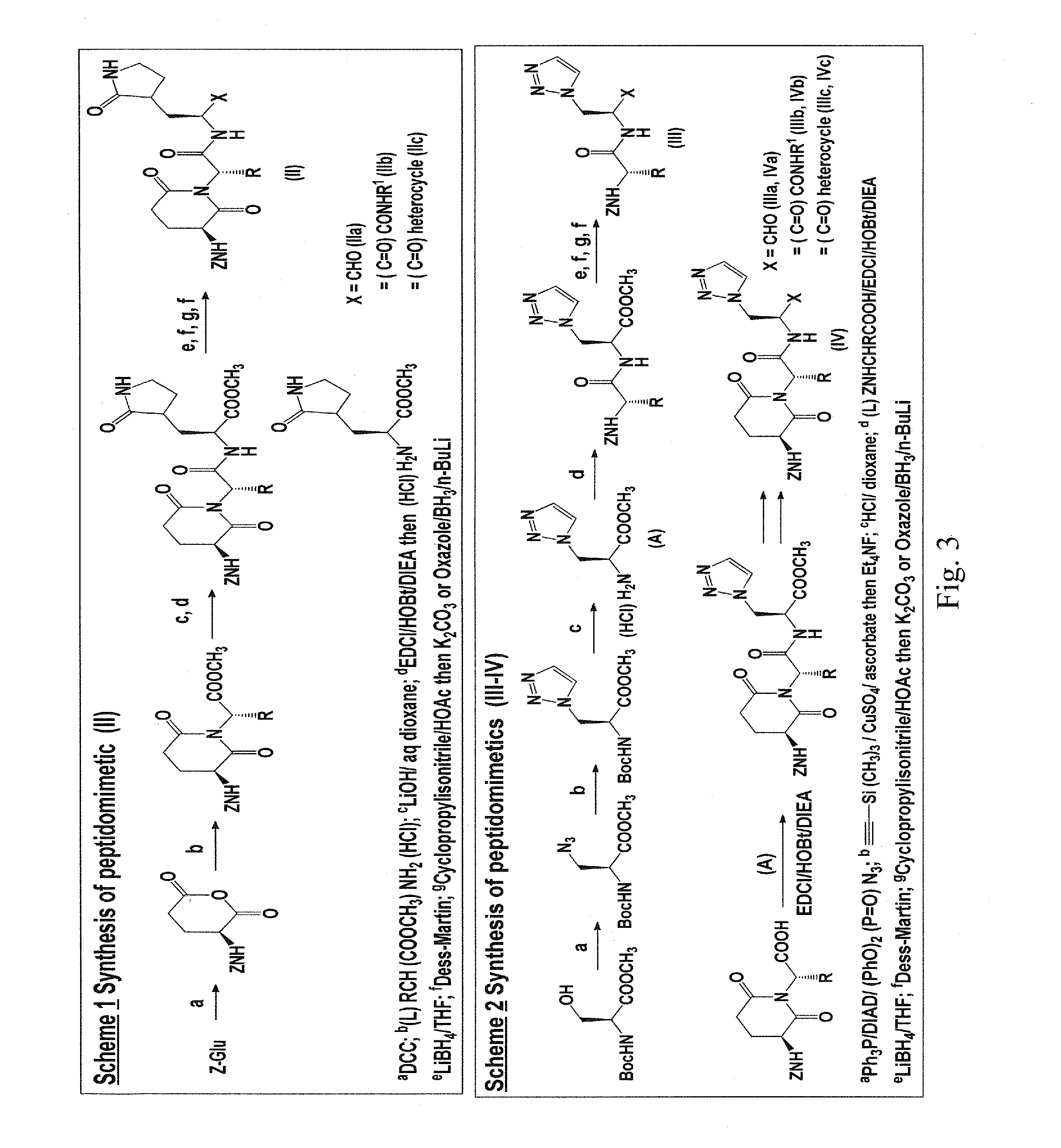
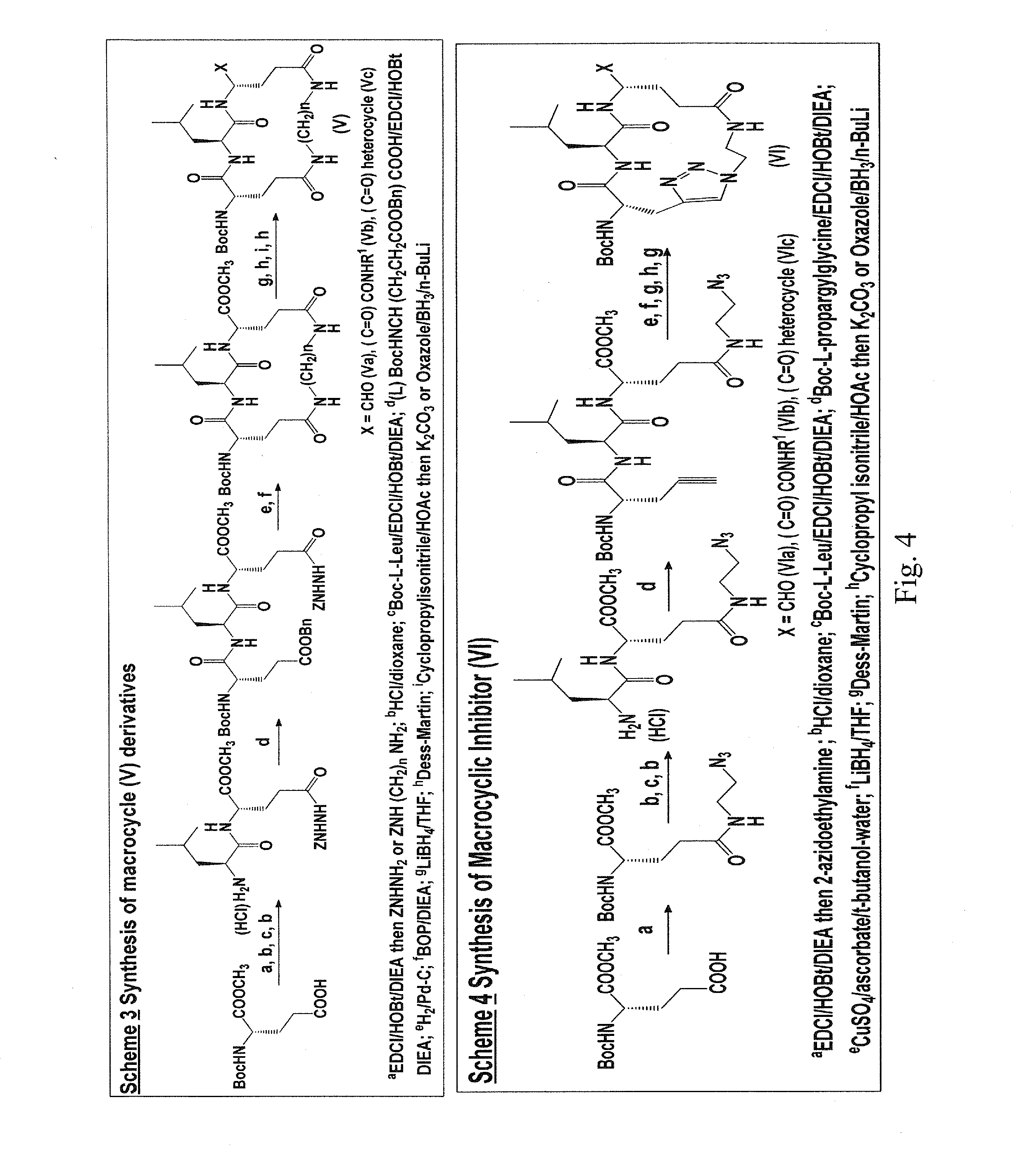
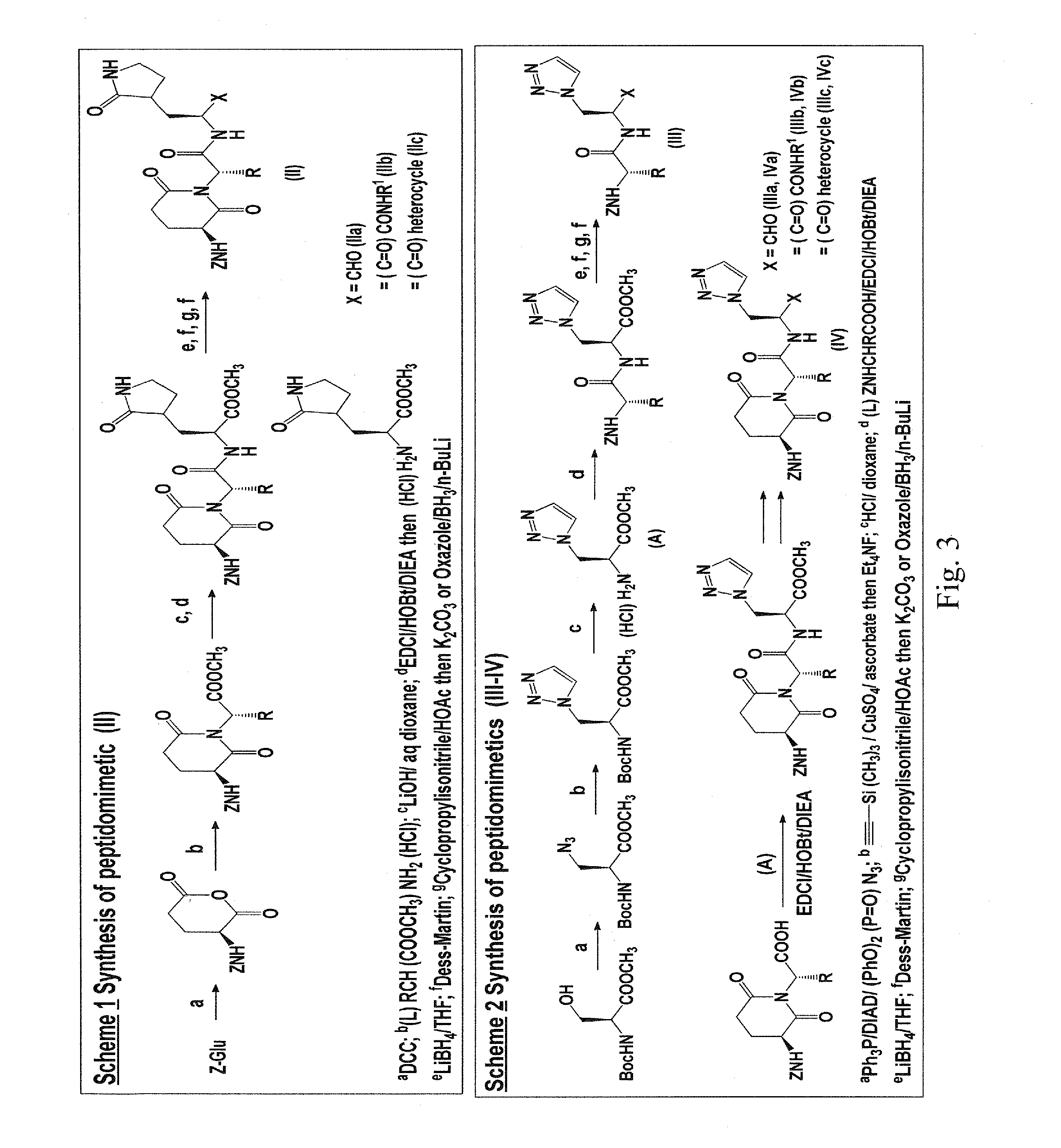
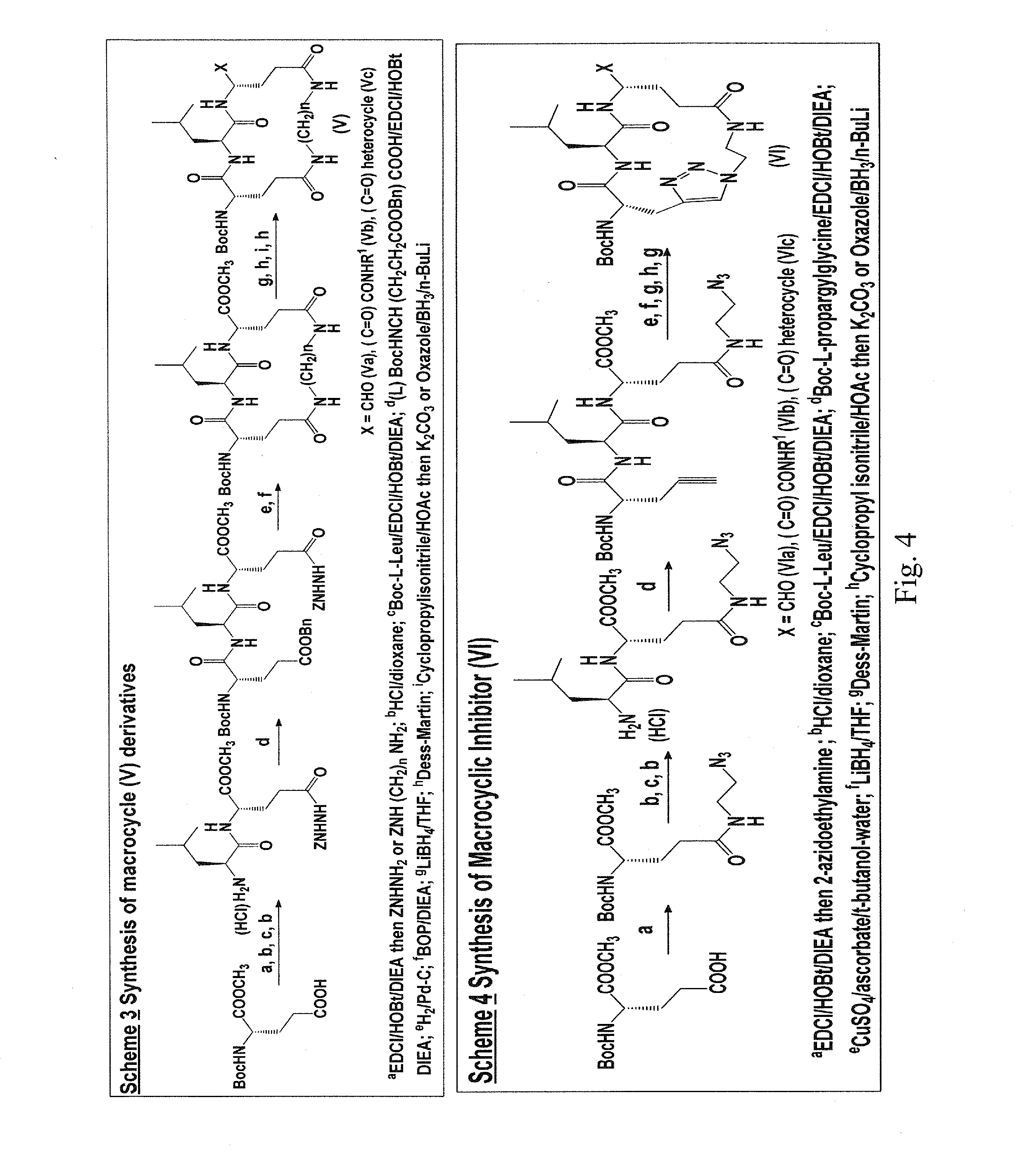
Macrocyclic and peptidomimetic compounds as broad-spectrum antivirals against 3c or 3c-like proteases of picornaviruses, caliciviruses and coronaviruses
Antiviral protease inhibitors, including macrocylic transition state inhibitors and peptidomimetics are disclosed, along with related antiviral compounds, and methods of using the same to treat or prevent viral infection and disease. The compounds possess broad-spectrum activity against viruses that belong to the picornavirus-like supercluster, which include important human and animal pathogens including noroviruses, sapoviruses, enteroviruses, poliovirus, foot-and-mouth disease virus, hepatitis A virus, human rhinovirus (cause of common cold), human coronavirus (another cause of common cold), transmissible gastroenteritis virus, murine hepatitis virus, feline infectious peritonitis virus, and severe acute respiratory syndrome coronavirus.
Owner:WICHITA STATE UNIVERSITY +1
Broad-spectrum antivirals against 3C or 3C-like proteases of picornavirus-like supercluster: picornaviruses, caliciviruses and coronaviruses
Antiviral protease inhibitors, including peptidyl aldehydes, peptidyl α-ketoamides, peptidyl bisulfate salts, and peptidyl heterocycles, are disclosed, along with related antiviral compounds, and methods of using the same to treat or prevent viral infection and disease. The compounds possess broad-spectrum activity against viruses that belong to the picornavirus-like supercluster, which include important human and animal pathogens including noroviruses, enteroviruses, poliovirus, foot-and-mouth disease virus, hepatitis A virus, human rhinovirus (cause of common cold), human coronavirus (another cause of common cold), transmissible gastroenteritis virus, murine hepatitis virus, feline infectious peritonitis virus, and severe acute respiratory syndrome coronavirus.
Owner:WICHITA STATE UNIVERSITY +2
T cell regulatory genes associated with immune disease
InactiveUS20050095593A1Diminish and terminate immunological disorderAvoid developmentImmunoglobulin superfamilySugar derivativesInflammatory Bowel DiseasesAutoimmune responses
A genetic locus and corresponding family of proteins associated with regulation of immune development, function, and cell survival are provided. The locus comprising the TIM family is genetically associated with immune dysfunction, including atopy, autoimmunity, inflammatory bowel disease, dysplasia, and susceptibility to blood-bourne infectious diseases. Polymorphisms in the human TIM-1 gene and exposure to Hepatitis A Virus (HAV) are shown to be associated with protection from the development of atopy.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV +1
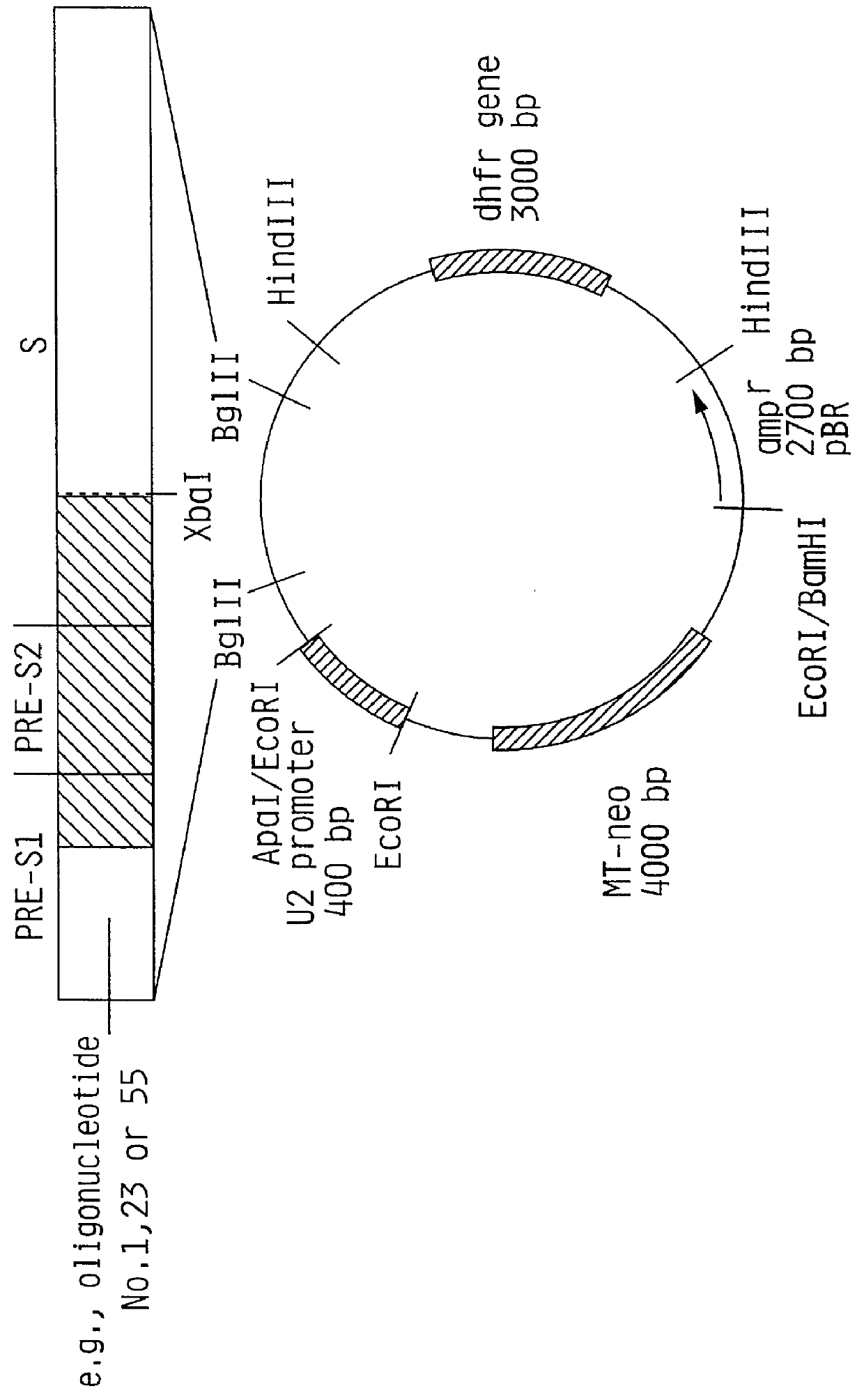
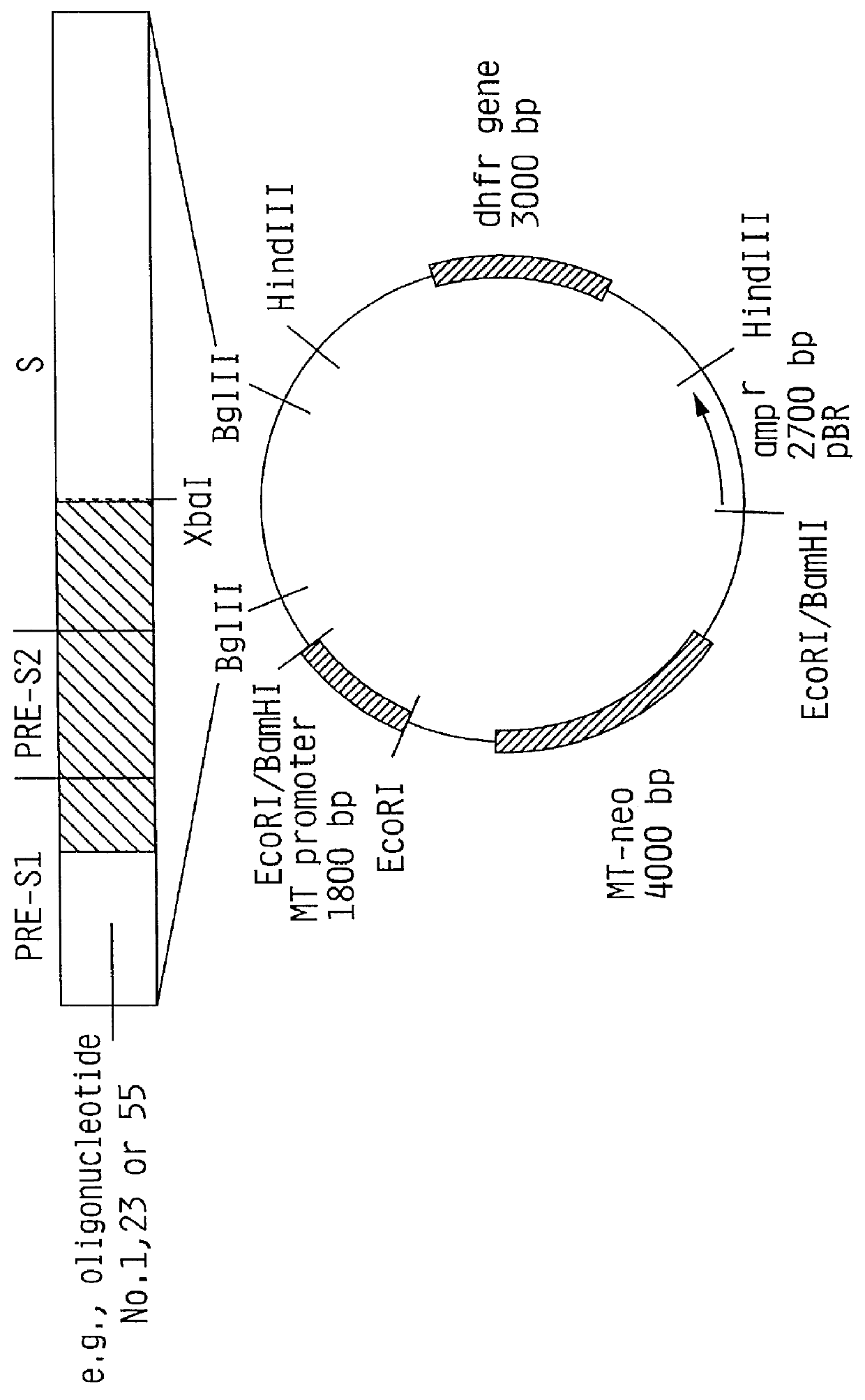
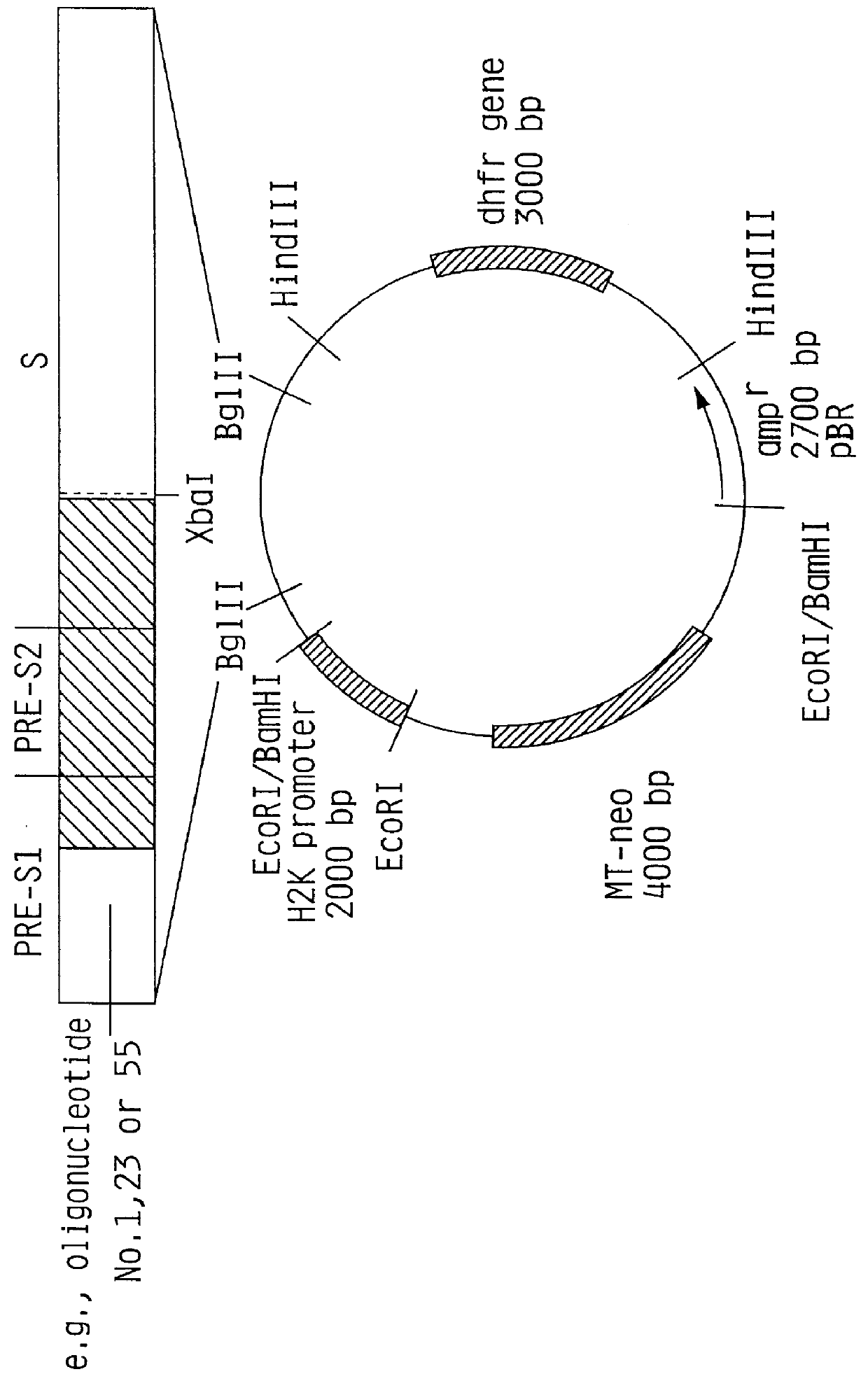
Hepatitis B surface antigen vaccine
InactiveUS6022543AGood antigenicityStimulate immune responseSsRNA viruses positive-senseBacteriaEukaryotic plasmidsHepatitis B virus
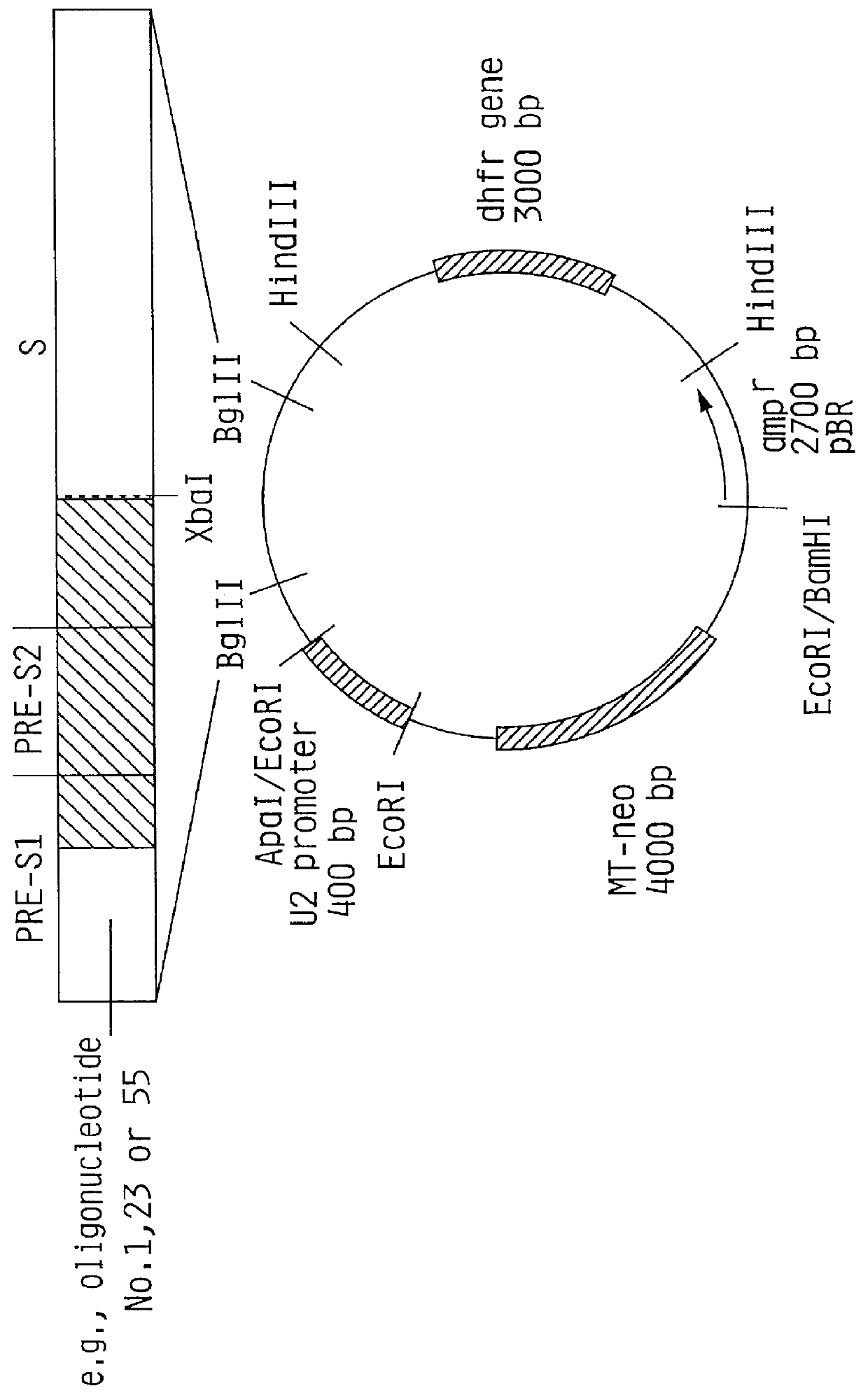
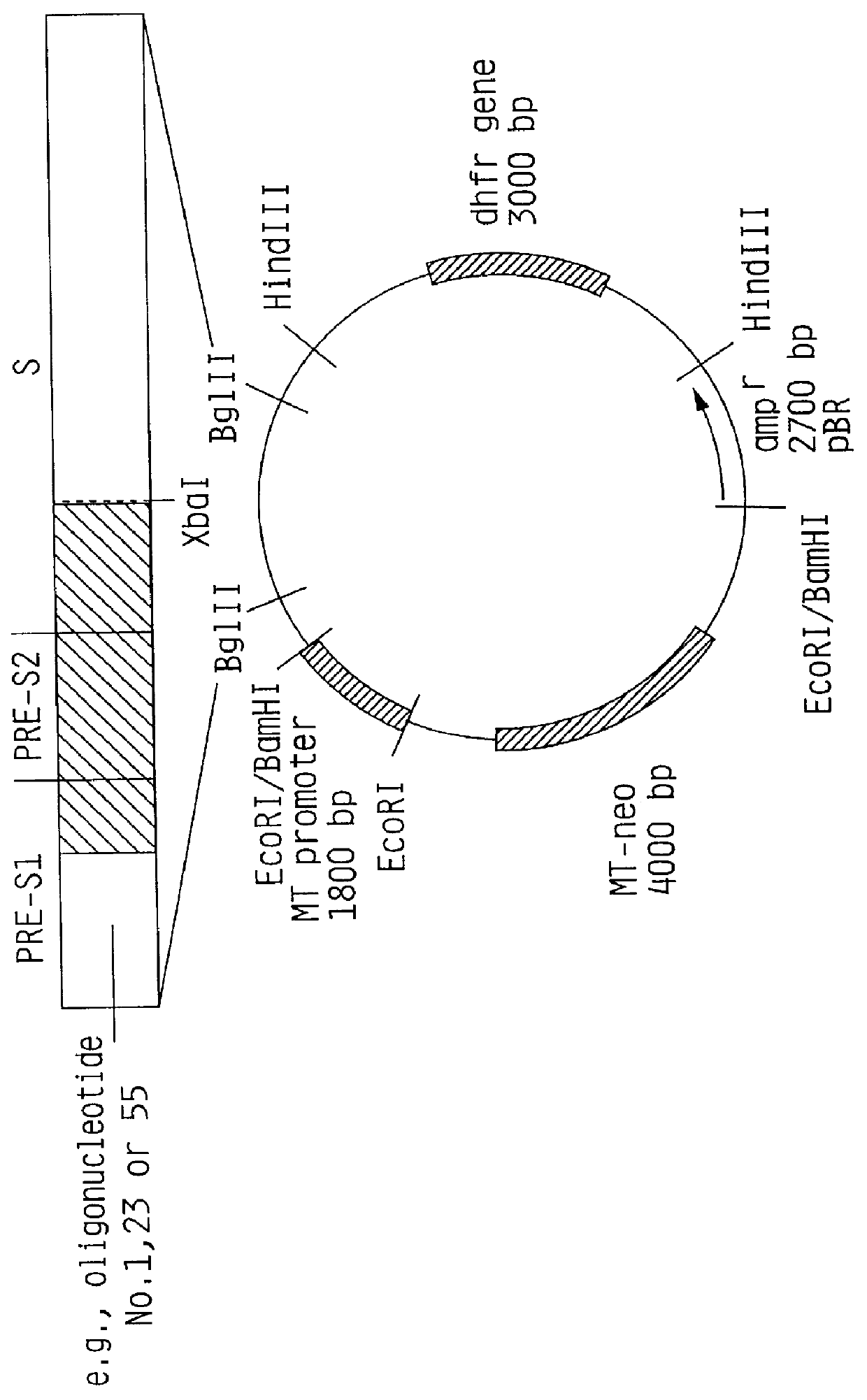
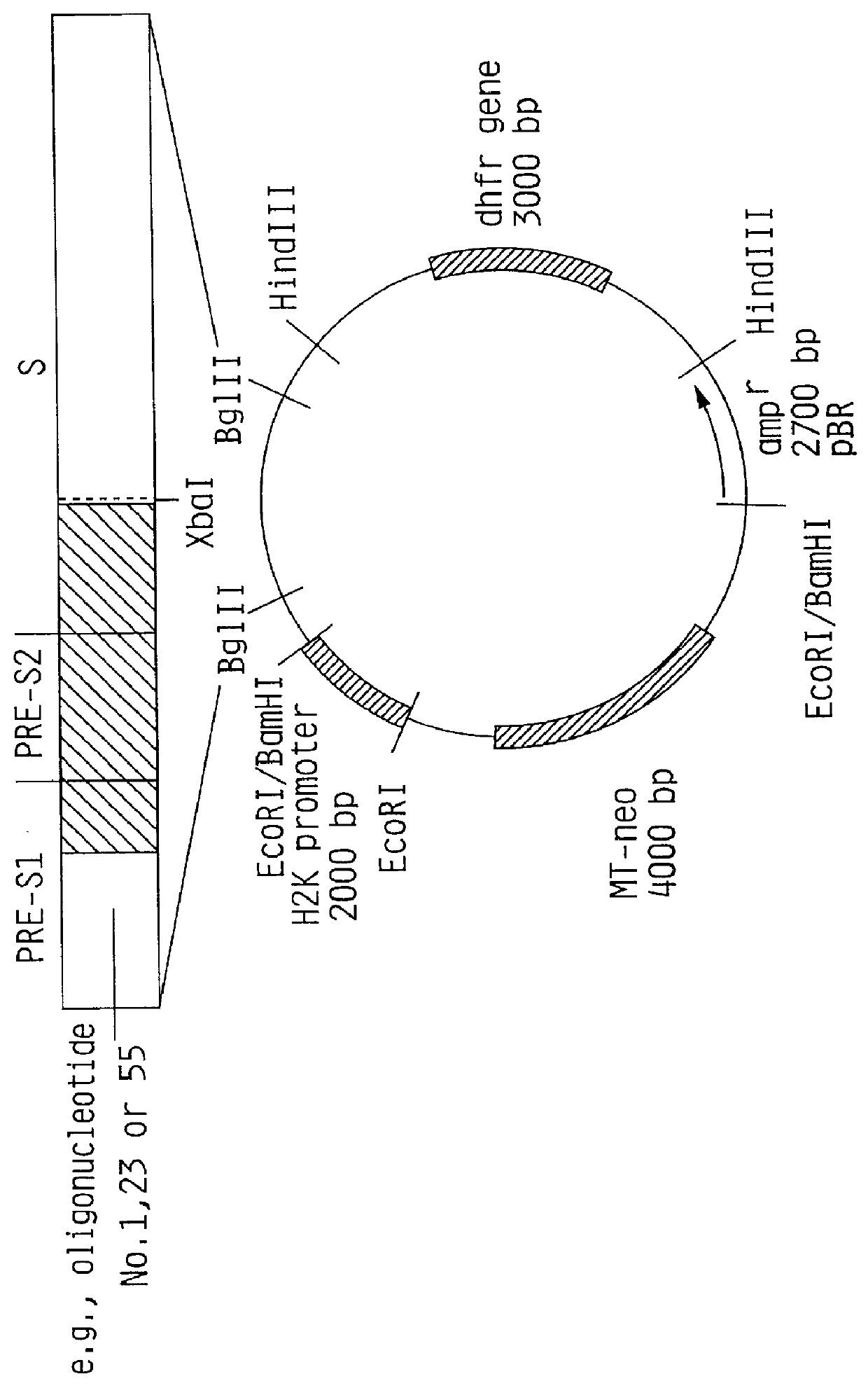
HBV surface antigen particles, prepared by recombinant DNA technology are described, said particles being composed of epitopes from the group of surface peptides and / or core peptide of non-A, non-B hepatitis virus, hepatitis virus A and / or hepatitis virus B. Respective particles are especially characterized by a composition of different epitopes selected from pre-S and S peptides. There are also described DNA-sequences, plasmids and cell lines coding for respective HBV surface antigen particles as well as a new vaccine containing the same.
Owner:MEDEVA HLDG
Macrocyclic and peptidomimetic compounds as broad-spectrum antivirals against 3C or 3C-like proteases of picornaviruses, caliciviruses and coronaviruses
Antiviral protease inhibitors, including macrocylic transition state inhibitors and peptidomimetics are disclosed, along with related antiviral compounds, and methods of using the same to treat or prevent viral infection and disease. The compounds possess broad-spectrum activity against viruses that belong to the picornavirus-like supercluster, which include important human and animal pathogens including noroviruses, sapoviruses, enteroviruses, poliovirus, foot-and-mouth disease virus, hepatitis A virus, human rhinovirus (cause of common cold), human coronavirus (another cause of common cold), transmissible gastroenteritis virus, murine hepatitis virus, feline infectious peritonitis virus, and severe acute respiratory syndrome coronavirus.
Owner:THE WICHITA STATE UNIV +1
NANBV diagnostics and vaccines
A family of cDNA sequences derived from hepatitis C virus (HCV) are provided. These sequences encode antigens which react immunologically with antibodies present in individuals with non-A non-B hepatitis (NANBH), but which are absent from individuals infected with hepatitis A virus, or hepatitis B virus, and also are absent in control individuals. The HCV cDNA sequences lack substantial homology to the sequences of hepatitis delta virus (HDV) and HBV. A comparison of the sequences of amino acids encoded in the HCV cDNA with the sequences of Flaviviruses indicates that HCV may be related to the Flaviviruses.The HCV cDNA sequences and the polypeptides encoded therein are useful as reagents for the detection and therapy of HCV. The reagents provided in the invention are also useful for the isolation of NANBH agent(s), for the propagation of these agents in tissue culture, and for the screening of antiviral agents for HCV.
Owner:NANBV DIAGNOSTICS & VACCINES +3
Hepatitis B surface antigen vaccine
InactiveUS6072049AGood antigenicityStimulate immune responseVirusesBacteriaEukaryotic plasmidsHepatitis B virus
HBV surface antigen particles, prepared by recombinant DNA technology are described, said particles being composed of epitopes from the group of surface peptides and / or core peptide of non-A, non-B hepatitis virus, hepatitis virus A and / or hepatitis virus B. Respective particles are especially characterized by a composition of different epitopes selected from pre-S and S peptides. There are also described DNA-sequences, plasmids and cell lines coding for respective HBV surface antigen particles as well as a new vaccine containing the same.
Owner:MEDEVA HLDG
Molecular biology identification method of i-form duck hepatitis virus
InactiveCN101358247ARapid diagnosisIncreased sensitivityMicrobiological testing/measurementFluorescence/phosphorescenceDuck hepatitis A virusTissue sample
The invention discloses a molecular biological identification method for I type duck hepatitis virus, which comprises the steps of treating samples to be tested, preparing RNA of the samples, obtaining cDNA of the samples by reverse transcription, identifying I type duck hepatitis virus by the fluorescent quantitive RT-PCR method or the nested PCR method, etc. The molecular biological identification method has the advantages of fast diagnosis, high sensitivity and wide applied range, can be used for identifying DHV-1 from the tissue samples, cotton swabs, ducks or chicken embryo allantoic liquid or cell cultures.
Owner:SOUTH CHINA AGRI UNIV
T cell regulatory genes associated with immune disease
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV +1
Combination vaccine with acellular pertussis
The present invention relates to a combination vaccine comprising a mixture of antigens for protection against diseases such as diphtheria, tetanus, acellular pertussis, and infections caused by Haemophilus influenzae and polio viruses. The present invention also relates to inclusion of antigens for protection against infections caused Hepatitis virus and other pathogens, such that administration of the vaccine can simultaneously immunize a subject against more than one pathogen. The invention in particular relates to a fully liquid stable combination vaccine comprising the antigens as indicated above and the methods for manufacturing the same.
Owner:PANACEA BIOTEC
Method and kit for detection of hepatitis a virus neutralizing antibodies
A rapid immunoassay method for the detection of anti-Hepatitis A Virus (HAV) neutralizing antibodies is described herein. This microplate-based enzymatic assay may be applicable in virological diagnostics, in evaluating the immunogenicity of candidate immunogenic compositions, such as HAV vaccines, or in quantifying functional neutralizing antibodies during the course of HAV infection.
Owner:VARIATION BIOTECHNOLOGIES INC
Preparation method of room air virus pathogenic-bacteria jinx
ActiveCN101491243AResolve inhibitionSolving the Killing ProblemBiocideGaseous substancesBacterial virusStaphylococcus aureus
The invention discloses a method for preparing an indoor air virus pathogen invincible opponent. The indoor air virus pathogen invincible opponent prepared by menthol, thymol, weeping forsythia oil and radix bupleuri oil has the killing rate of between 76 and 99.9 percent to idemic encephalitis, influenza viruses, hand-foot-and-mouth disease, avian influenza, legionnella, pneumoniae, hepatitis C viruses, hepatitis A viruses, Staphylococcus albus, Staphylococcus aureus, Klebsiella pneumoniae, Candida albicans, typhoid viruses, Escherichia coli, cholera viruses, infectious germs and bacteria, and harmful germs and bacteria. The indoor air virus pathogen invincible opponent is mainly applied to rooms, schools, hospitals, hotels, air conditioners, automobiles, public places, and the like, and has obvious effect on the control of diseases such as influenza, upper respiratory tract infection. The indoor air virus pathogen invincible opponent has outstanding substantive characteristics and broad application prospect.
Owner:上海钮爱环保科技有限公司
Indirect ELISA method and kit for detecting serum 3-type duck hepatitis virus a antibody
The invention discloses an indirect ELISA method and kit for detecting serum 3-type duck hepatitis virus a antibody and belongs to the technical field of serum antibody detection. The indirect ELISA method includes that serum 3-type duck hepatitis virus VP1 protein is utilized as an envelope antigen with the peridium quantity as 0.1-1mug per hole, HRP-mice anti-duck IgY is utilized as the HRP with the use concentration as 0.2-2mug / mL, and the coloration time is 10 minutes. The kit comprises a VP1 protein antigen envelope board, the HRP-mice anti-duck IgY, sample diluent, a scrubbing solution, TMB solutions A and B and a stop solution. The method and the kit can be used for detecting the serum 3-type duck hepatitis virus a antibody in duck serum and duck egg yolk, and the serum does not have cross reaction with positive serum of other viruses. Compared with a traditional neutral reaction detection method, the method has the advantage of being convenient and accurate.
Owner:WUHAN CHOPPER BIOLOGY
Gene chip and reagent box for detecting food-borne virus
InactiveCN101328505AGuaranteed reproducibilityGuaranteed accuracyMicrobiological testing/measurementAgainst vector-borne diseasesFood borneRotavirus RNA
The invention relates to a gene chip for detecting food-home virus and a kit, belonging to the inspection field. The surface of a solid phase carrier is fixed with a plurality of detection probes and quality control contrast probes, wherein, the detection probes consist of the probes for detecting hepatitis A virus, human astrovirus, norwalk virus G1, norwalk virus G2, rotavirus, polio virus 1, polio virus 2 and polio virus 3; and the quality control contrast probes consist of a spotting positive quality control probe, a chip hybridization positive quality control probe and a chip negative quality control probe. The gene chip and the kit have the advantages of: (1) high throughout: five common viruses are integrated and detected simultaneously, and the practicability is strong; (2) rapidness: the detection time is only 4 hours; (3) specificity: the false positive caused by the cross reaction is avoided; (4) flexibility: the detection flexibility of the chip is 10<8> virus particles per gram of the tissue sample, which is higher than that of RT-PCR; and (5) good repetitiveness and stable results. The gene chip and the kit can be widely applied to the food safety inspection of the inspections system.
Owner:PEOPLES REPUBLIC OF CHINA BEIJING ENTRY EXIT INSPECTION & QUARANTINE BUREAU +1
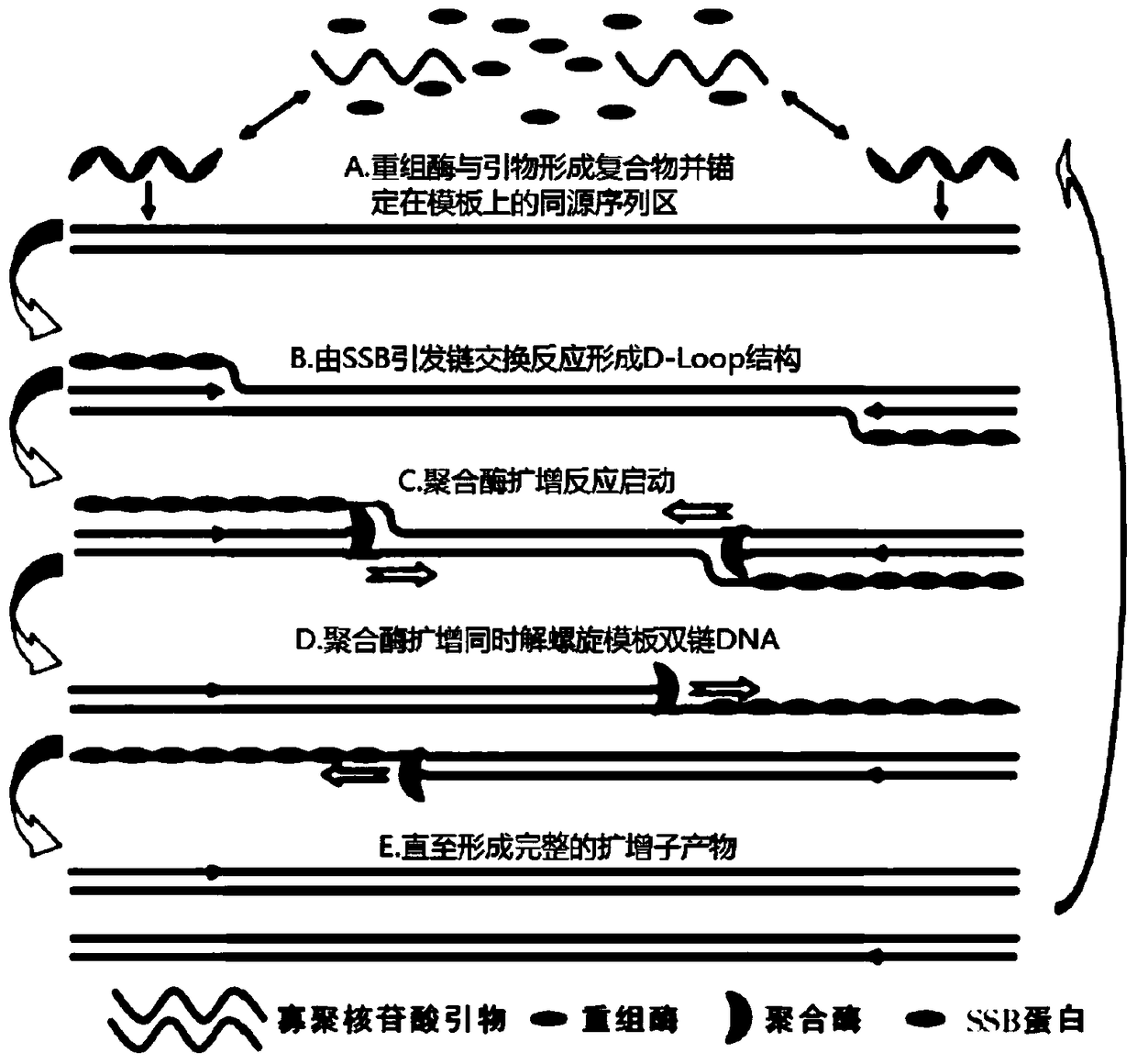
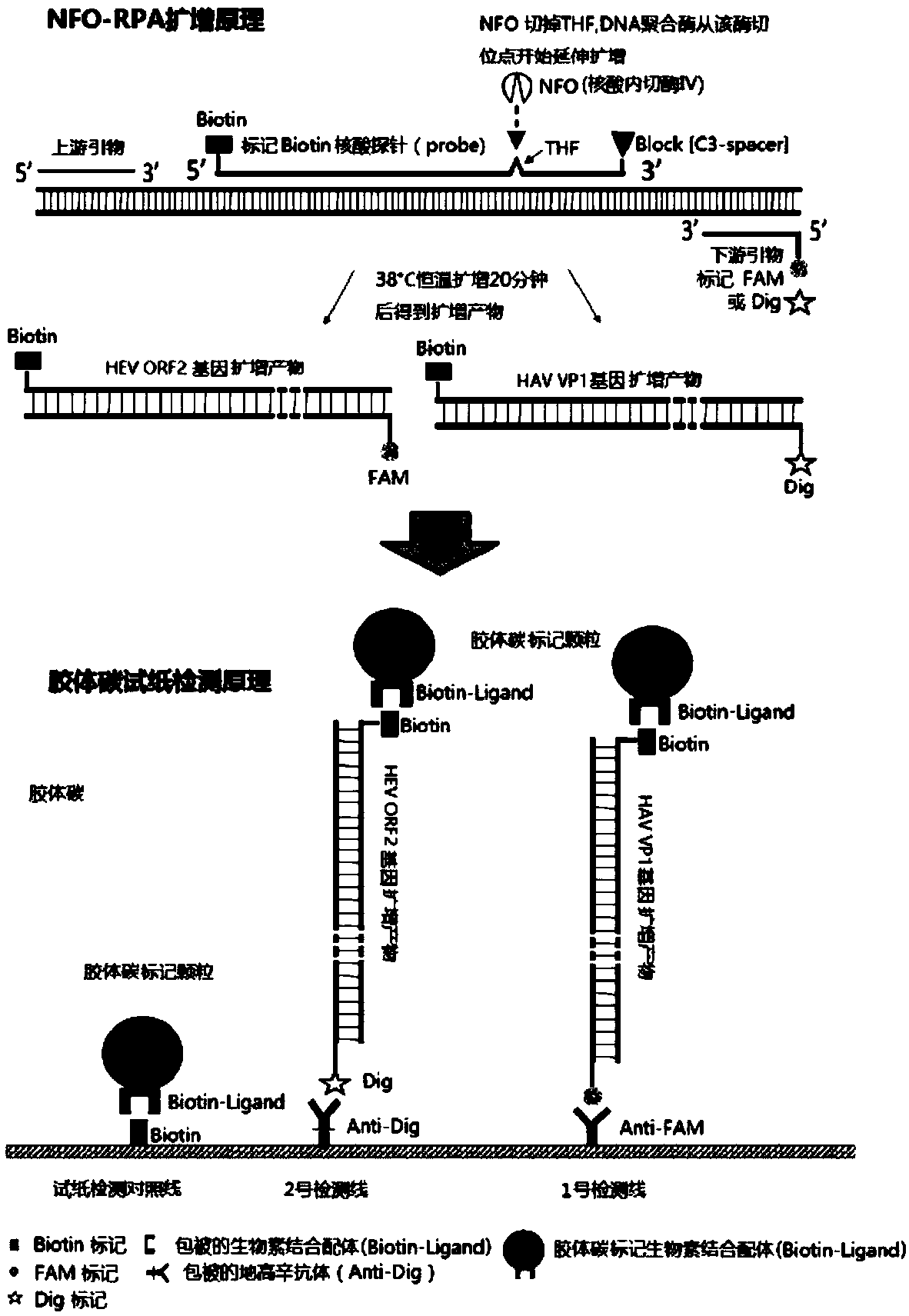
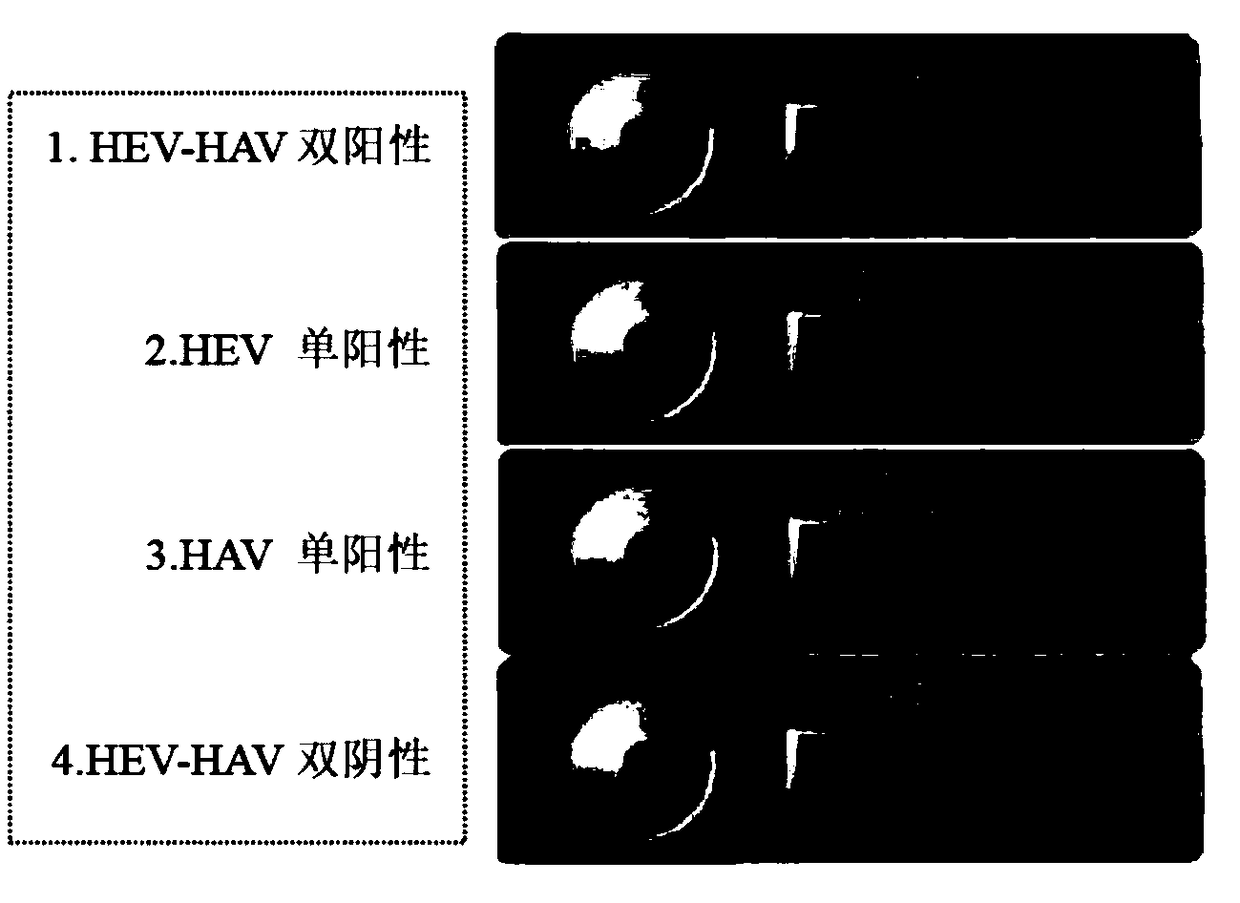
Primer for doubly detecting hepatitis e virus and hepatitis a virus through RT-RPA-lateral flow tomography, probe and kit
ActiveCN108841926AShorten detection timeThe reaction procedure is simpleMicrobiological testing/measurementAgainst vector-borne diseasesControl lineHepacivirus
The invention discloses a RT-RPA-lateral flow tomography kit for doubly detecting hepatitis e virus and hepatitis a virus. The kit comprises an upstream primer, an intermediate probe and a downstreamprimer which are applicable to hepatitis e virus ORF2 gene sequence in the RT-RAP amplification technology, and / or an upstream primer, an intermediate probe and a downstream primer which are applicable to hepatitis a virus VP1 gene sequence, general agents for recombinase polymerase amplification technology, and a lateral flow tomography test strip, wherein the lateral flow tomography test stripcomprises a sample adding pad, a control line, a #1 detecting line and / or a #2 detecting line; the control line, the detecting lines and the primers are combined with a probe label. With the adoptionof the kit, the hepatitis e virus ORF2 gene and / or hepatitis a virus VP1 gene in a sample can be rapidly, sensitively and specifically detected on site in a non-lab environment.
Owner:JINZHOU MEDICAL UNIV
Method of preparing hepatitis A inactivated vaccine
ActiveCN102058882AIncrease production capacityHigh purityAntiviralsViruses/bacteriophagesAdjuvantCell factory
The invention provides a method of preparing a hepatitis A inactivated vaccine, which comprises the following steps of inoculating mixed and absorbed hepatitis A virus strain SH and a human embryo diploid cell MRC-5 to hepatitis A virus propagated in a cell factory, digesting the cell in the virus propagation peak to obtain cell virus liquid, removing impurity proteins of the cell by ultrasonication, chloroform extraction and ultrafiltration through ultrafiltration membranes, degerming and filtering by gel filtration chromatography and purification, and absorbing by an aluminium hydroxide adjuvant so that the hepatitis A inactivated vaccine is prepared. The result of in vivo effectiveness experiments performed on a mouse shows that the hepatitis A inactivated vaccine prepared by the method of the invention has higher ED50 and better immunogenicity than the contract strain. The method of the invention can improve the safety of the vaccine, simplify the technique, shorten the productionperiod, reduce the production cost, and realize the scale production of the hepatitis A inactivated vaccine.
Owner:SHENZHEN KANGTAI BIOLOGICAL PROD
Method of large scale production of Hepatitis A virus
InactiveUS6855535B2Readily apparentSsRNA viruses positive-senseViral antigen ingredientsCell culture supernatantVero cell
The present invention provides methods of large scale production of Hepatitis A Virus (HAV) on VERO cells bound to microcarrier. The invention also provides for methods of isolation of HAV from the cell culture supernatant of HAV infected VERO cells.
Owner:BAXTER HEALTHCARE SA
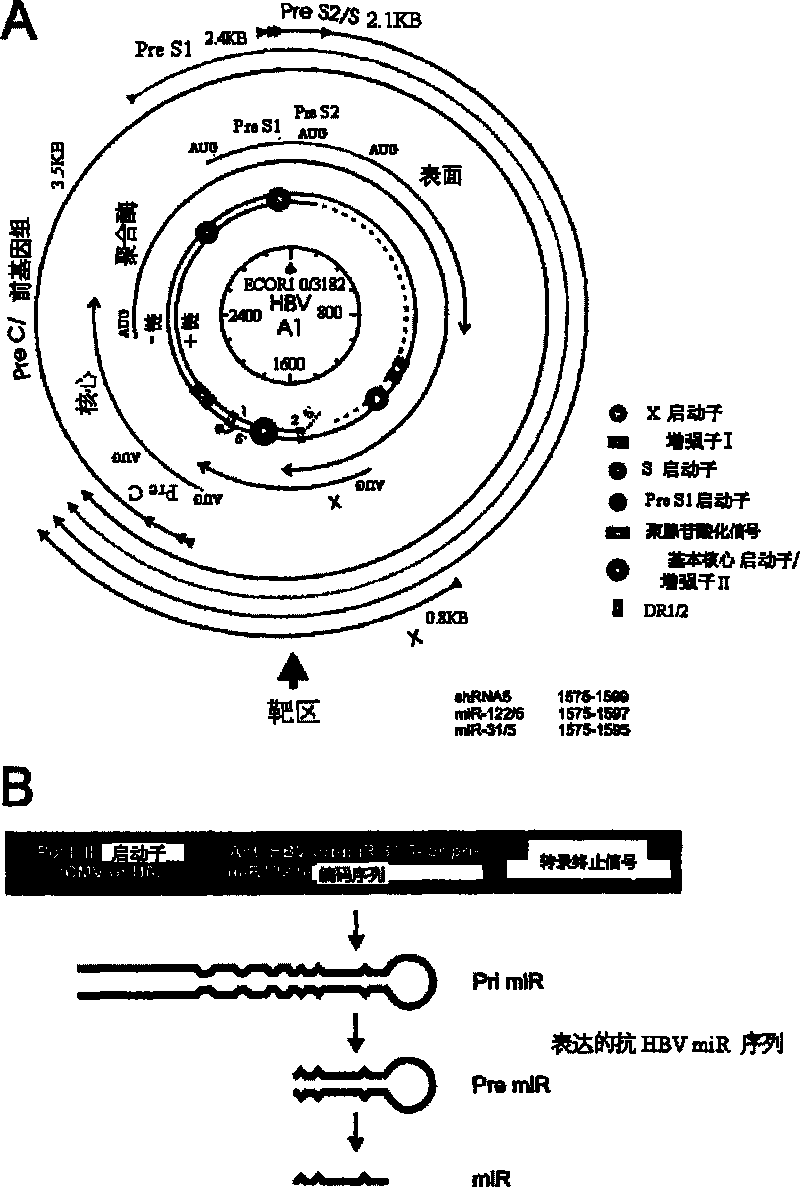
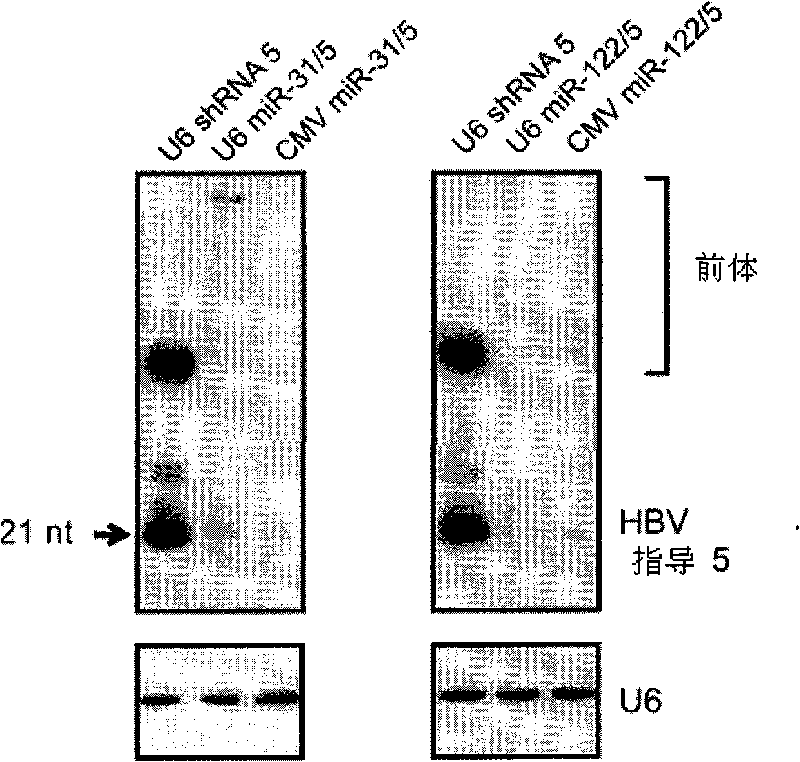
A primary micro RNA expression cassette
This invention relates to inhibition of hepatitis gene expression. More specifically, the invention relates to a method of using RNA sequences to inhibit Hepatitis B and C Virus replication. Expression cassettes that include DNA sequences derived from endogenous micro RNAs (miRs) are used in the method and are transcribed by Pol Il promoters, and then processed to generate sequences that are specific to target hepatitis virus sequences (RNAi effecter sequences). The RNAi effecter sequences can target the selected hepatitis virus sequences resulting in gene silencing or transcriptional inhibition of the hepatitis virus gene. The expression cassettes may be delivered in vitro or in vivo to host cells. A pharmaceutical composition containing the expression cassettes is also claimed.
Owner:UNIVERSITY OF THE WITWATERSRAND
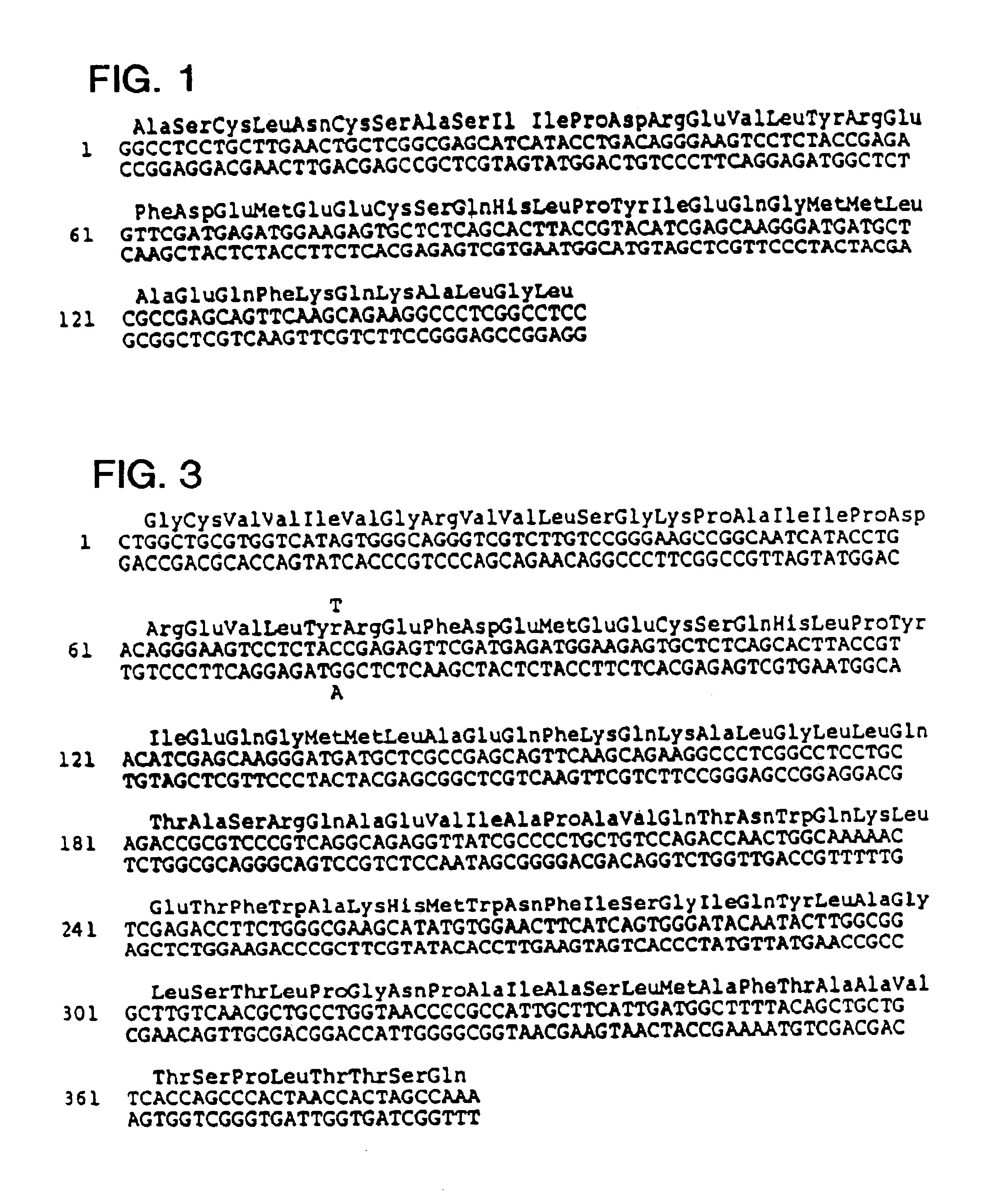
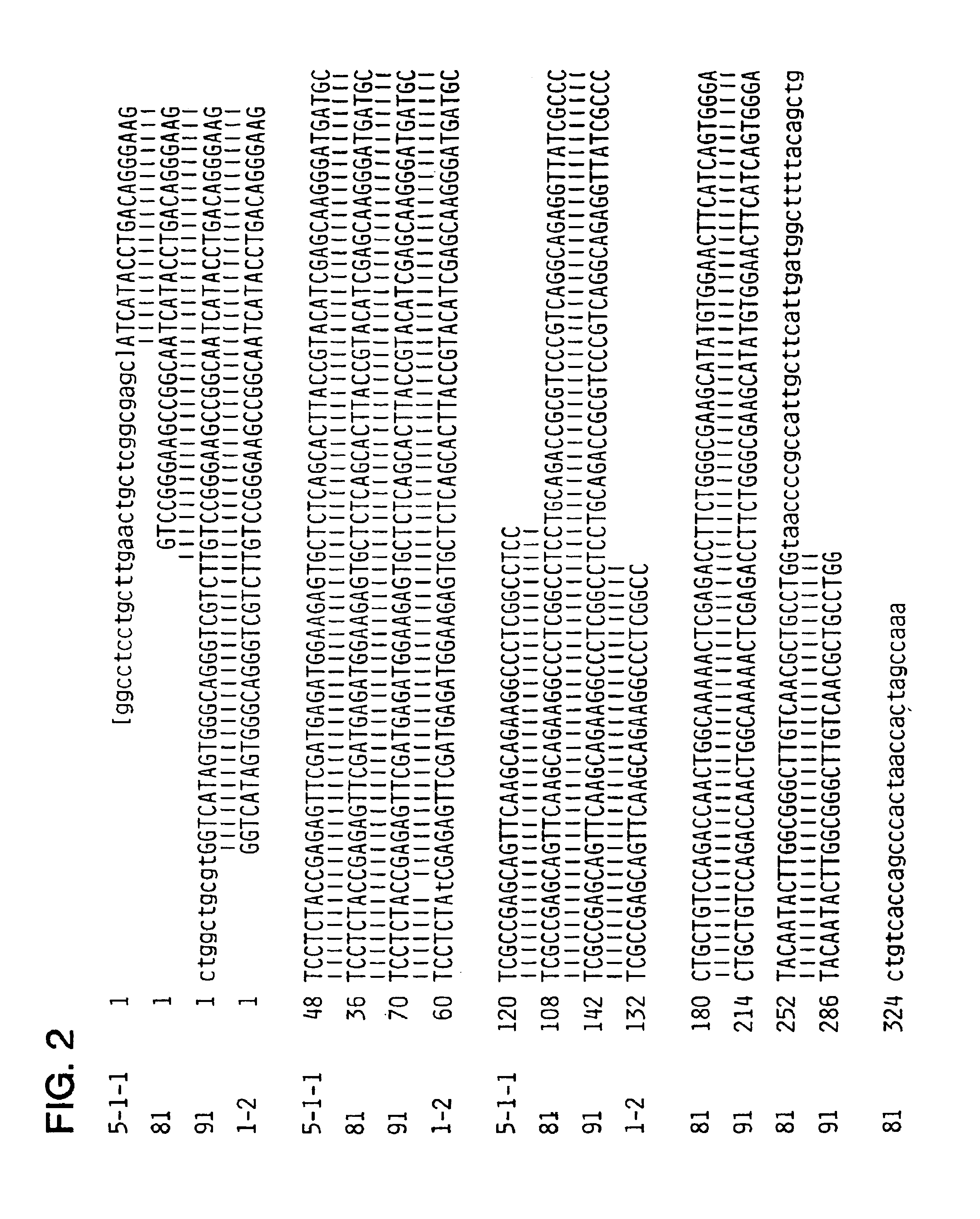
Identification of oligonucleotides for the capture, detection and quantitation of hepatitis A viral nucleic acid
InactiveCN1675378ASugar derivativesMaterial analysis by observing effect on chemical indicatorViral nucleic acidGenome
Hepatitis A virus-specific primers and probes derived from conserved regions of the hepatitis A virus genome are disclosed. Also disclosed are nucleic acid-based assays using the capture oligonucleotides, primers and probes.
Owner:CHIRON CORP
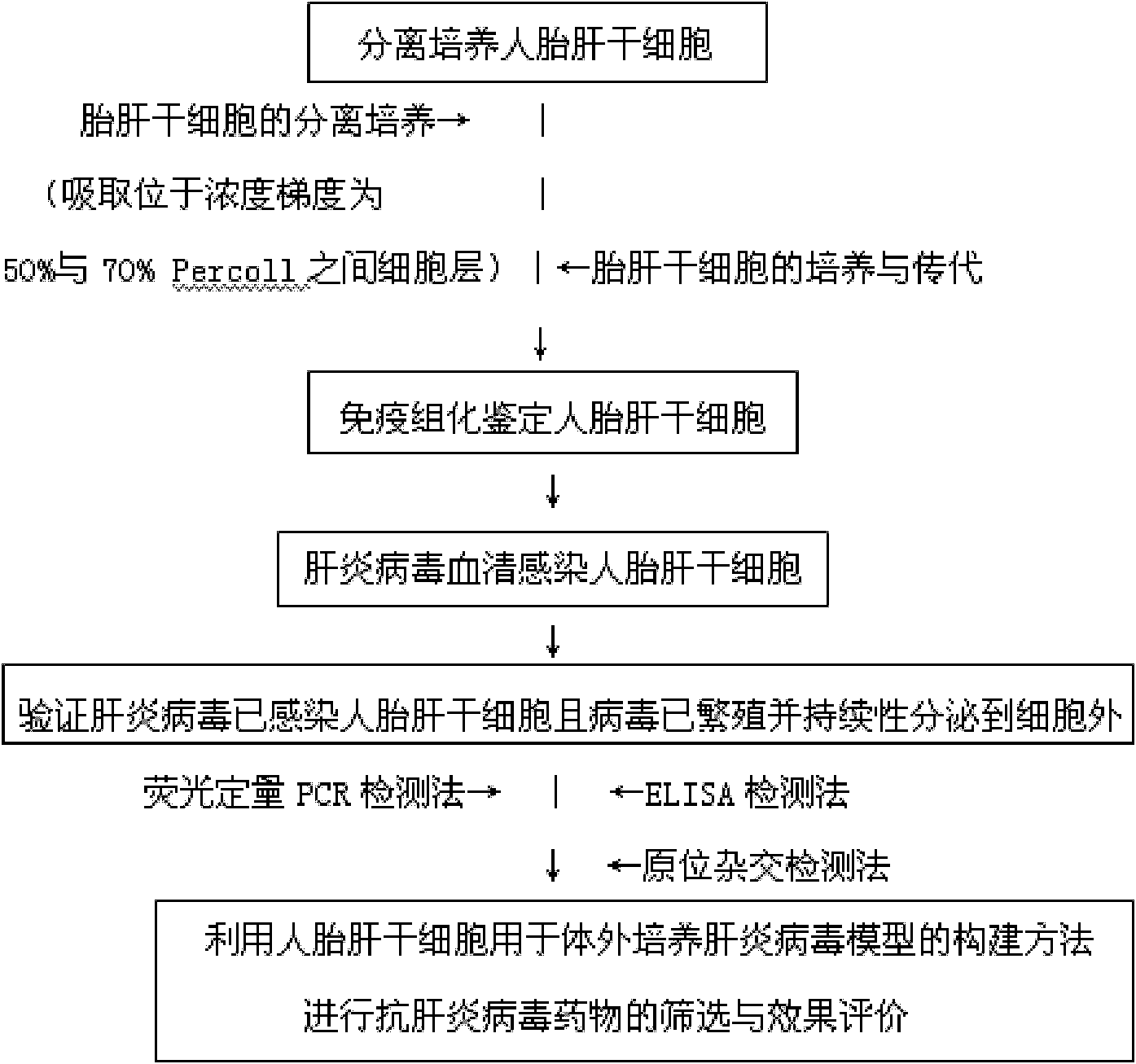
Hepatitis virus in-vitro culture model as well as construction method and application thereof
InactiveCN101914489ASimple methodGood repeatabilitySsRNA viruses positive-senseMicrobiological testing/measurementLiver Stem CellHuman fetal
The invention relates to a hepatitis virus in-vitro culture model as well as a construction method and application thereof. The hepatitis virus in-vitro culture model is constructed by adopting human fetal liver stem cells and comprises the steps of carrying out separate culture on human fetal liver stem cell, immunohistochemically identifying human fetal liver stem cell and infecting human fetal liver stem cell with hepatitis virus by blood serum and verifying whether hepatitis virus infects the human fetal liver stem cells or not and the virus propagate and continuously secrete outside the cell or not; the model can be used for the screening and effect evaluation of an in-vitro hepatitis virus resistant medicament; the human fetal liver stem cells used in the hepatitis virus in-vitro culture model can be infected with hepatitis virus and can continuously secrete hepatitis virus; the human fetal liver stem cells can be subcultured; and the invention has simple and convenient method and high repeatability.
Owner:INST OF HYGIENE & ENVIRONMENTAL MEDICINE PLA ACAD OF MILITARY MEDICAL
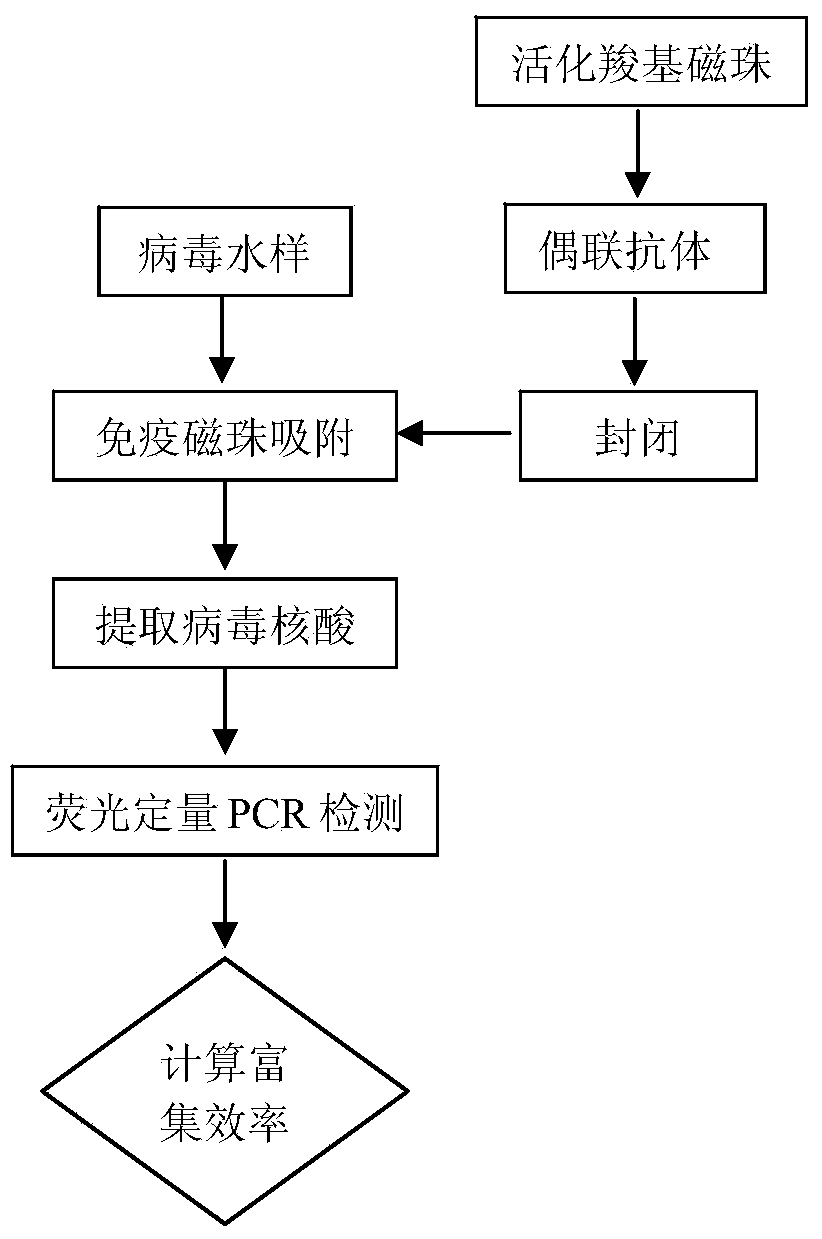
Method for enriching water body hepatitis A viruses based on immunomagnetic beads
InactiveCN103805574ASimple and fast operationStrong specificityMicrobiological testing/measurementMicroorganism based processesMagnetic beadSpecific immunity
The invention relates to a method for enriching water body hepatitis A viruses based on immunomagnetic beads. The method comprises the following steps: preparing specific immunomagnetic beads for enriching hepatitis A viruses; enriching the hepatitis A viruses by using the immunomagnetic beads; quantitatively detecting the enriching efficiency of the hepatitis A viruses. By enriching the hepatitis A viruses in water bodies through the immunomagnetic beads, the method has the advantages of reasonable concept, easiness and convenience in operation, high specificity, high separating speed, high repeatability and no need of expensive instrument equipment. Moreover, when 1mg of activated magnetic beads are coupled with 75 micrograms of hepatitis A virus monoclonal antibodies, the enriching efficiency of 300 microliters of hepatitis A virus water sample of which the virus concentration is 1.52*10<3> copies / microliter can be up to 64.54 percent in maximum, and the enriching efficiency of 300 microliters of hepatitis A virus water sample of which the virus concentration is 1.52*10<6> copies / microliter can be up to 91.45 percent in maximum. The method has important significance to enriching and detection of the hepatitis A viruses in water bodies.
Owner:JIANGXI AGRICULTURAL UNIVERSITY
Hepatitis A virus strain, method for preparing hepatitis A inactivated vaccine and obtained vaccine
InactiveCN1443844AHigh yieldHigh productivityInactivation/attenuationAntibody medical ingredientsVero cellVirus strain
Owner:YUXI WALVAX BIOTECH CO LTD
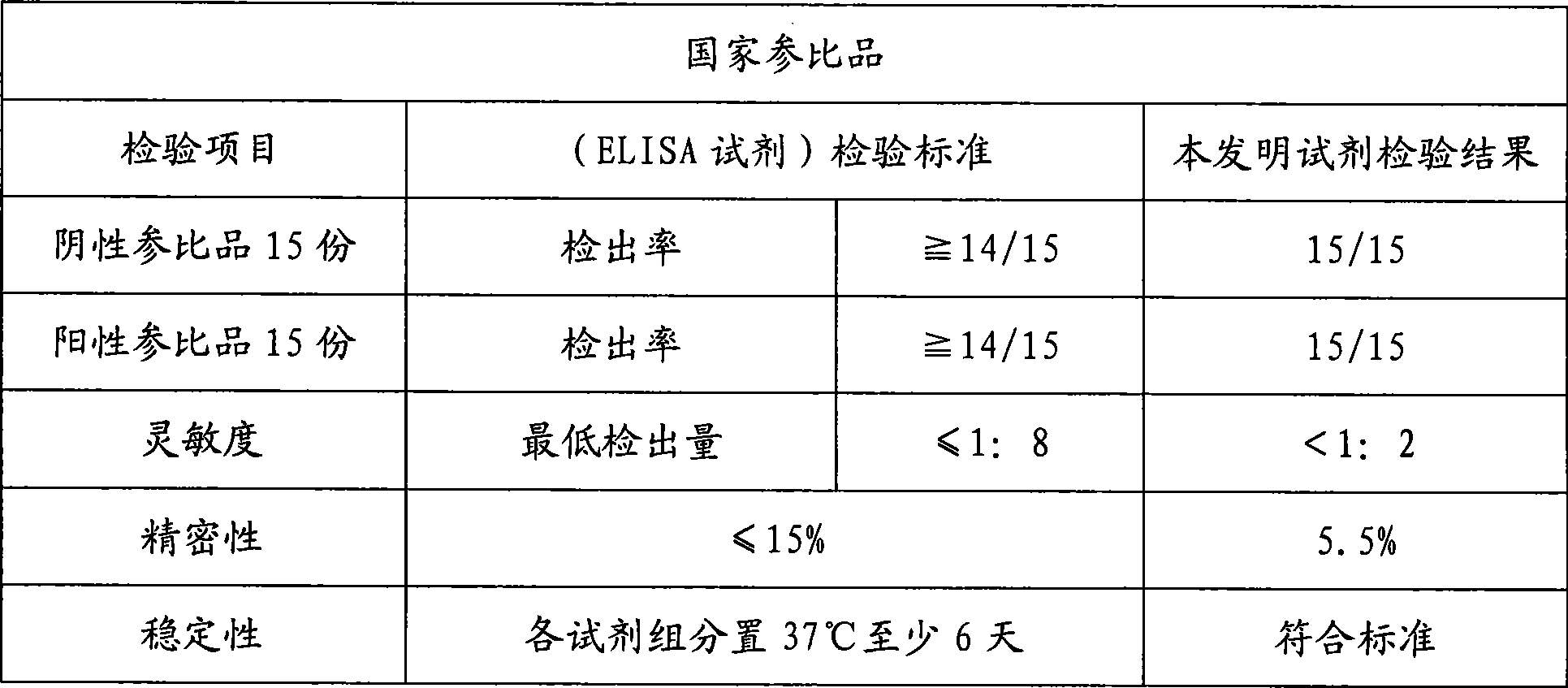
Chemoluminescent immunoassay kit of hepatitis A virus IgM antibody and preparation method thereof
InactiveCN101533025AEase of mass productionGuaranteed SensitivityChemiluminescene/bioluminescenceAgainst vector-borne diseasesPositive controlIgm antibody
The invention relates to the field of immunoassay medical science, and in particular provides a chemoluminescent immunoassay kit of a hepatitis A virus IgM antibody and a preparation method thereof. The kit comprises: 1) negative and positive control varieties of the hepatitis A virus IgM antibody; 2) a dermatate solid phase carrier; 3) a hepatitis A virus antigen liquid; 4) an enzyme label; 5) a chemoluminescent substrate; and 6) a condensed washing solution. Furthermore, the method for preparing the kit comprises the following steps: 1) preparing control varieties from negative and positive serum of the hepatitis A virus IgM antibody; 2) dermatating the solid phase carrier by an anti-human-mu chain antibody (monoclonal antibody or polyclonal antibody); 3) preparing an antigen liquid from the hepatitis A virus; 4) labeling the hepatitis A virus antibody (the monoclonal antibody or the polyclonal antibody) by enzyme; 5) preparing the chemoluminescent substrate solution; 6) preparing the condensed washing solution; 7) sub-packaging the negative and positive control varieties of the hepatitis A virus IgM antibody, the hepatitis A virus antigen liquid, the enzyme label, the chemoluminescent substrate solution and the condensed washing solution; and 8) assembling the substances into the finished products. The kit has the advantages of simpleness, convenience, quickness, sensitiveness, stability, and the like.
Owner:CHEMCLIN DIAGNOSTICS CO LTD
NANBV diagnostics and vaccines
InactiveUS6861212B1Polypeptide with localisation/targeting motifSsRNA viruses positive-senseAntigenTGE VACCINE
A family of cDNA sequences derived from hepatitis C virus (HCV) are provided. These sequences encode antigens which react immunologically with antibodies present in individuals with non-A non-B hepatitis (NANBH), but which are absent from individuals infected with hepatitis A virus, or hepatitis B virus, and also are absent in control individuals. The HCV cDNA sequences lack substantial homology to the sequences of hepatitis delta virus (HDV) and HBV. A comparison of the sequences of amino acids encoded in the HCV cDNA with the sequences of Flaviviruses indicates that HCV may be related to the Flaviviruses.The HCV cDNA sequences and the polypeptides encoded therein are useful as reagents for the detection and therapy of HCV. The reagents provided in the invention are also useful for the isolation of NANBH agent(s), for the propagation of these agents in tissue culture, and for the screening of antiviral agents for HCV.
Owner:CHIRON CORP
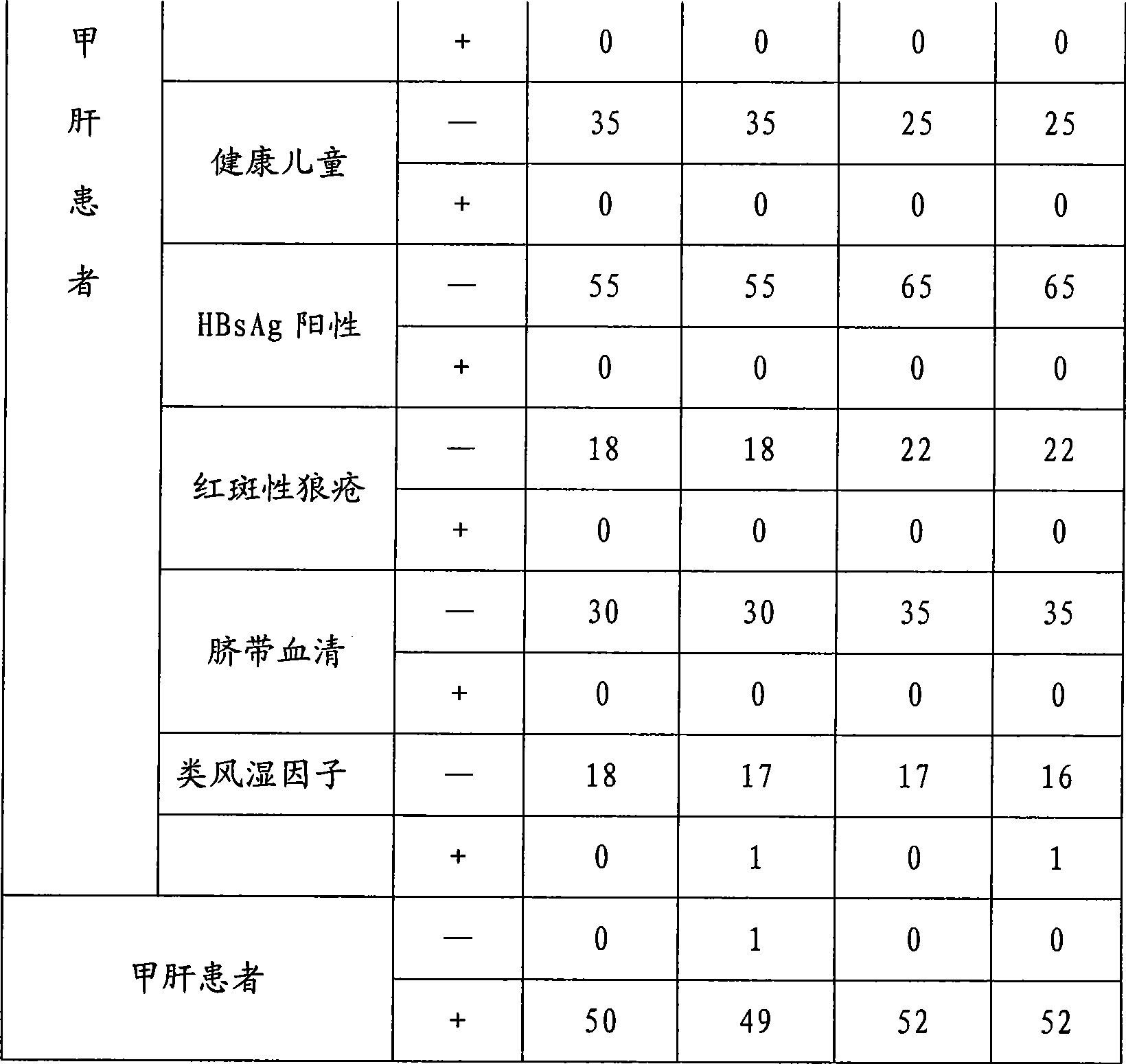
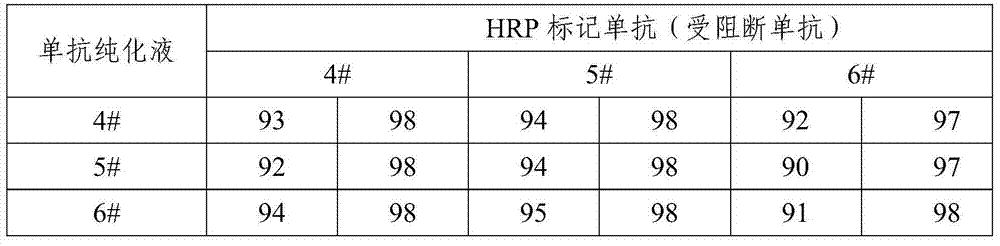
Hepatitis A virus monoclonal antibody and its application
ActiveCN103923881AEfficient neutralizationMicroorganism based processesImmunoglobulins against virusesAntigenHybridoma cell
The invention provides a hepatitis A virus monoclonal antibody, which is generated by secreting of a hybridoma cell strain with preservation number of CGMCC No.7859. The monoclonal antibody can specifically neutralize the antigen of hepatitis A virus with high efficiency, and has important meaning for diagnosis, prevention and treatment of the infection of hepatitis A virus.
Owner:SINOVAC BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com