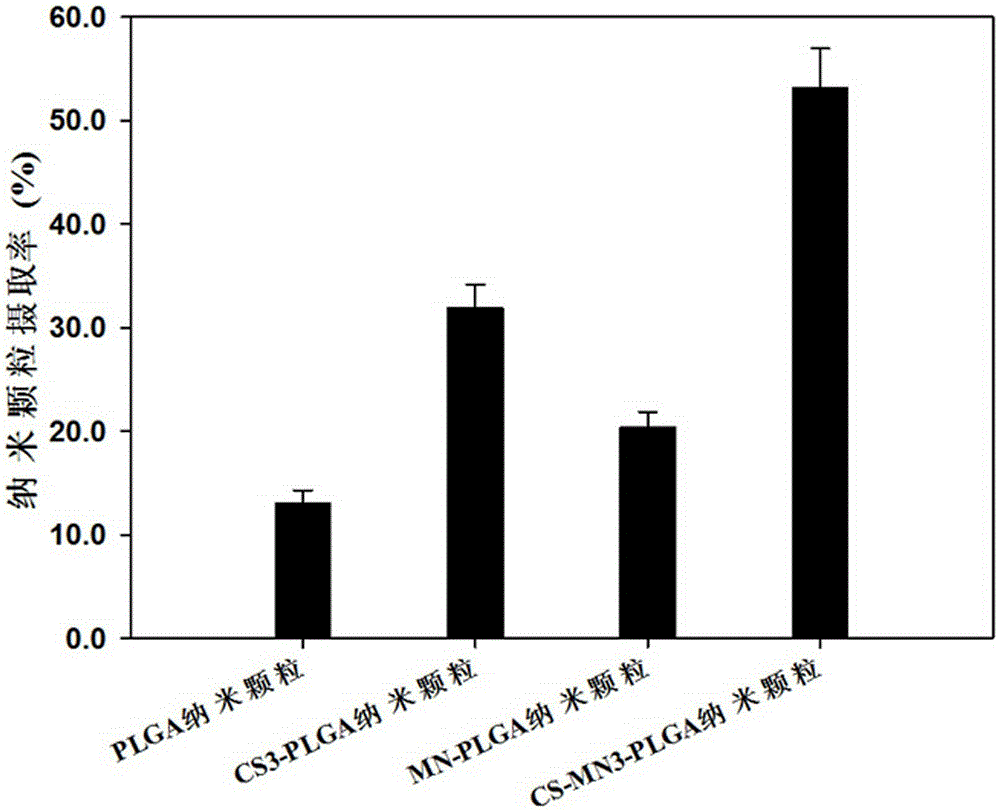
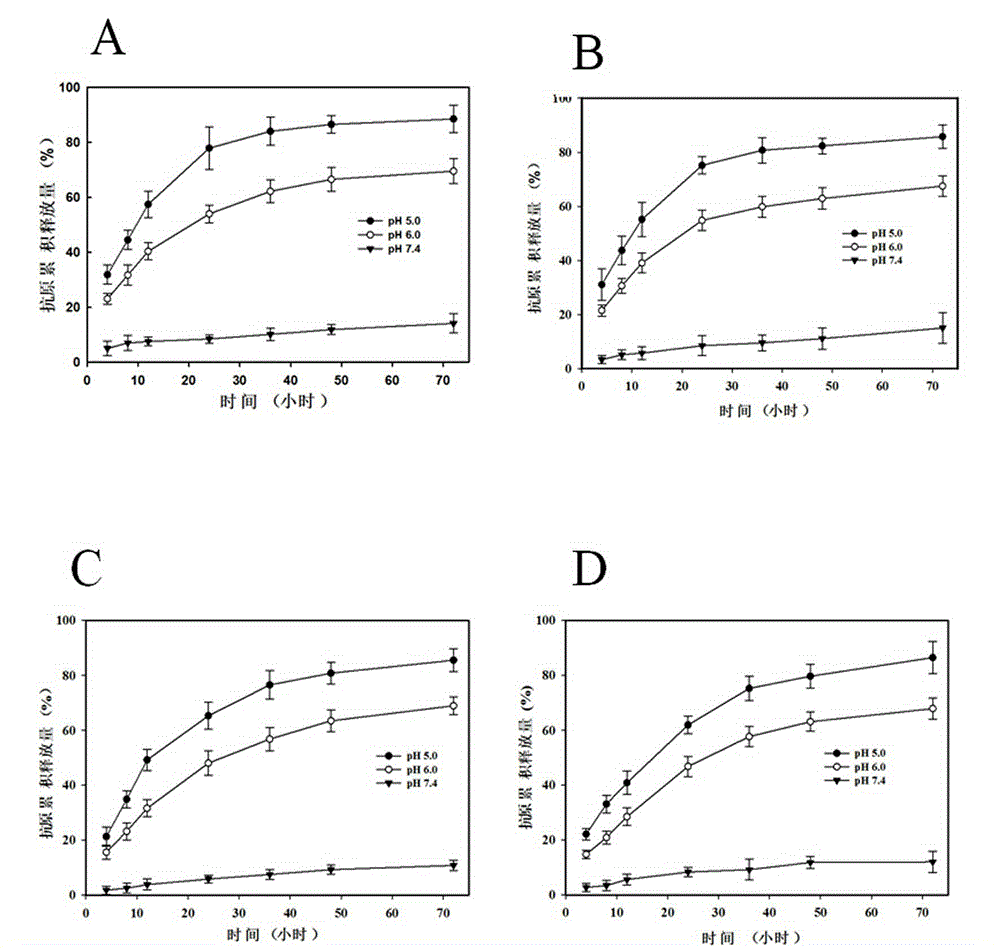
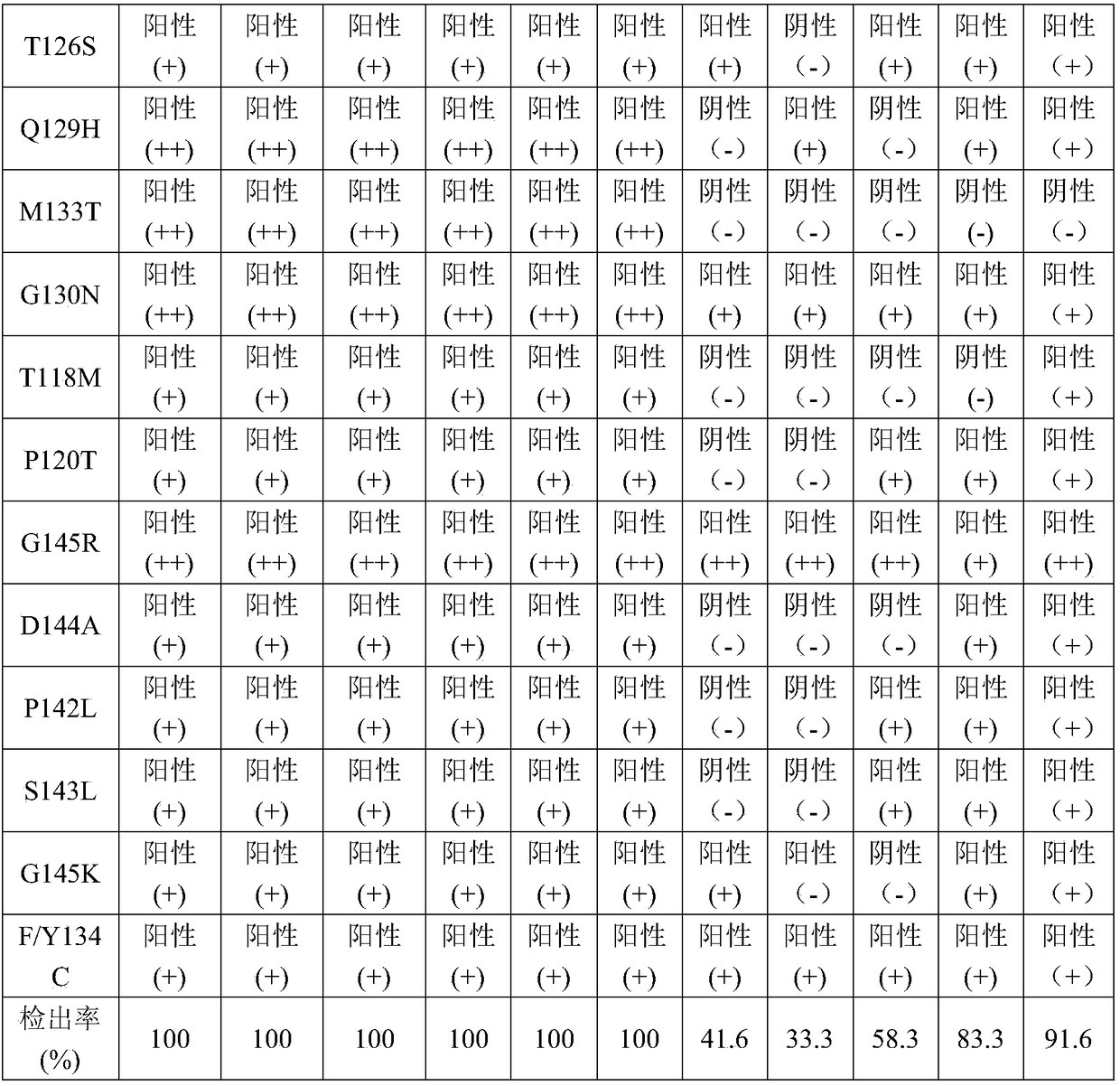
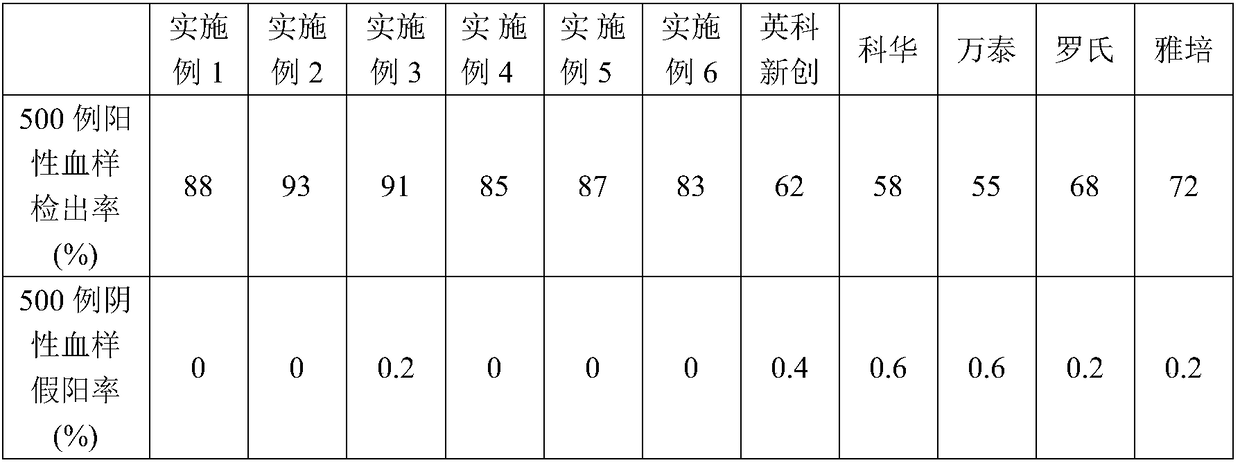
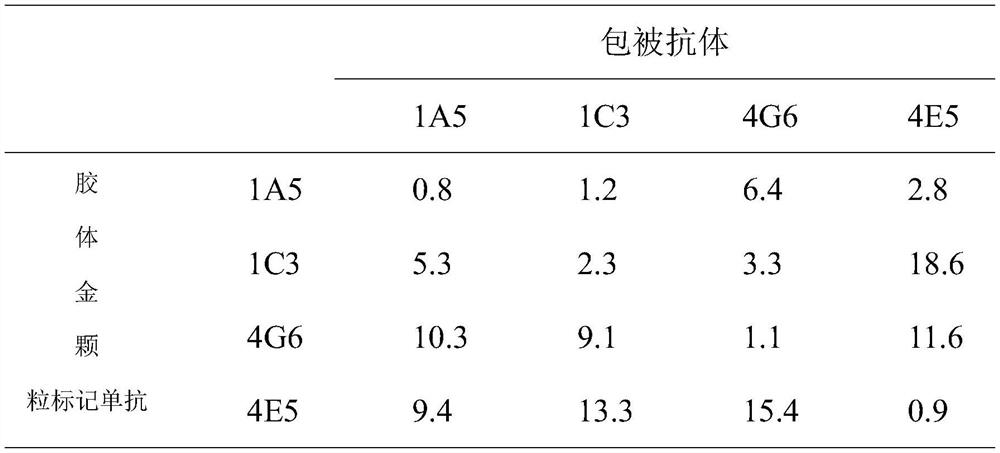
Patents
Literature
122 results about "Hepatitis b surface antigen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
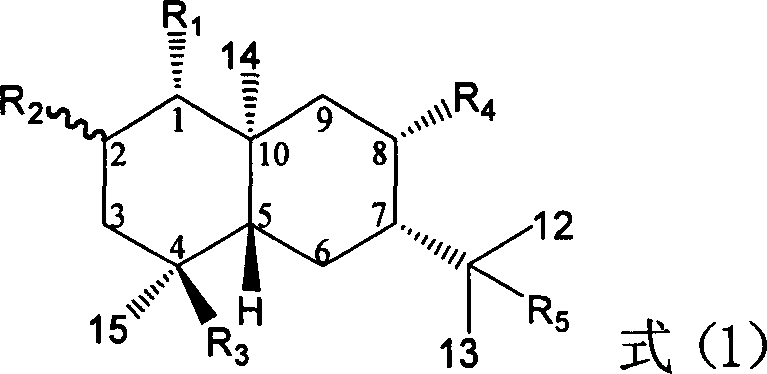
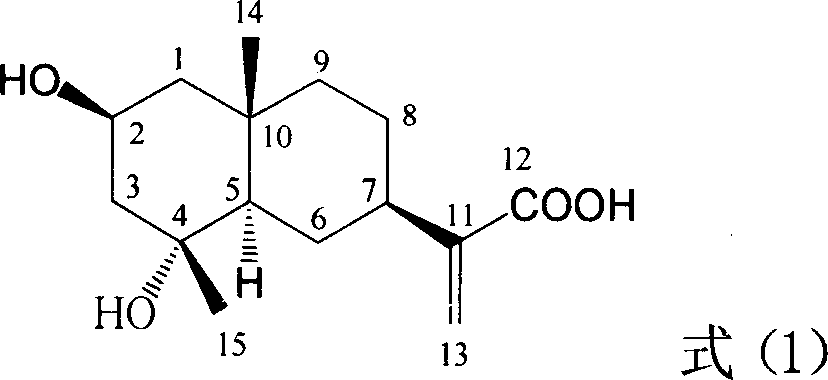
Pharmaceutical use of ent-eudesmane alcohol type sesquiterpene for inhibiting hepatitis virus
InactiveCN1935762APrevention and treatment of viral hepatitis BHBsAg reductionSugar derivativesHydroxy compound active ingredientsDiseaseSolvent
The invention relates to an enantiomorphic amine alkyl sesquiterpene alcohol and glucoside and the medicated salt or solvent thereof, as well as the effect and activity of the composed medicine combination, mainly relating to the medical use in reducing HBV-DNA replication activity. And it has considerably strong inhibiting effect on HBsAG screted by HepG2.2.15 and HBV-DNA replication as compared with positive contrast Lamivudine; and it has obvious inhibition activity to HBV-DNA replication at large dosage (100 mug / mL) and medium dosage(20 mug / mL) as contrasted with Lamivudine, and can be expected to apply to preparing medicines for curing HB virus infection disease.
Owner:赵昱
Medical usage of 2beta-hydroxyilicicacid in inhibiting hepatitis B
InactiveCN1951378APrevention and treatment of viral hepatitis BHBsAg reductionOrganic active ingredientsAerosol deliveryDiseaseHepatitis b surface antigen
The invention relates to a hemiterpene derivant 2-Hydroxyilicic acid, as formula (1) 2beta-hydroxy-5alphaH-eudesmane-11(13)-allyl-12-acid and relative compounds which can be used to prepare the drug treating hepatitis B disease. The inventive compound can restrain the copy of hepatitis B surface antigen (HBsAg) and the hepatitis B deoxyribonucleic acid (HBV-DNA), while its HBsAg restrain ability is higher than positive contrast difuradin; in the density as 100mug / ml, 20mug / ml, and 4mug / ml, it can restrain the copy of hepatitis B virus HBV-DNA.
Owner:WENZHOU MEDICAL UNIV +1
Enantiomorphous eremophilanic acid and its medical use for inhibiting hepatitis B surface antigen
The invention relates to the medicine technical field and concretely relates to a mixture formed by two enantiomorphous eremophilane acids separated from murrey ligularia and the officinal salt as well as the medicine combined material. The enantiomorphous eremophilane acid can be used for preparing the medicine curing hepatitis B virus infectious diseases due to the ability of suppressing the antigenic activity on the surface of hepatitis B virus.
Owner:WENZHOU MEDICAL UNIV
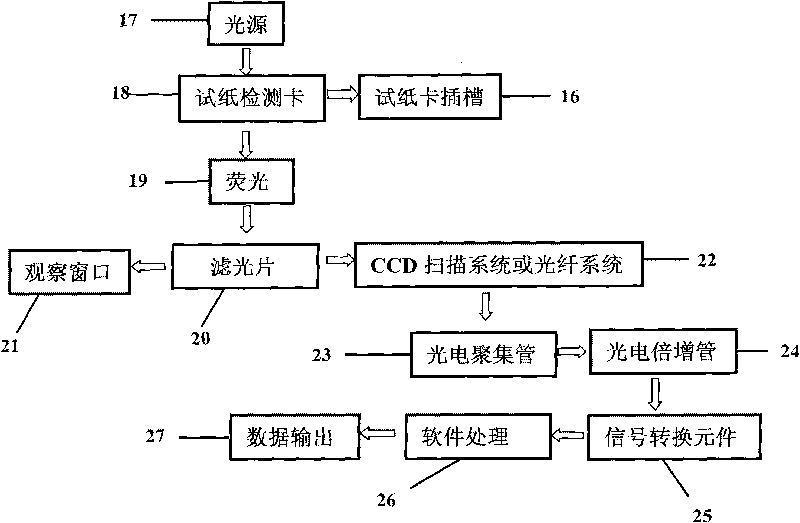
Fluorescent microsphere immunochromatographic testing card for testing five indexes of hepatitis b and method for preparing same
InactiveCN101726596ASimple and fast operationHigh sensitivityBiological testingLuminescent compositionsCelluloseHepatitis B virus
The invention discloses a fluorescent microsphere immunochromatographic testing card for testing five indexes of hepatitis b and a method for preparing the same. The testing card comprises a hepatitis b surface antigen test paper strip, a hepatitis b e surface antigen test paper strip, a hepatitis b surface antibody test paper strip, a hepatitis b e surface antibody test paper strip, and a hepatitis b core antibody test paper strip. Each test paper strip is formed by overlapping and bonding filter paper, a sample pad, a glass fiber film spray-coated with fluorescent microspheres, a cellulose nitrate film and water absorption paper on a bottom plate by glue in sequence, wherein the cellulose nitrate film is coated with antigens serving as a testing area and anti-rabbit antibodies serving as a quality control area; and during a test, after emitted fluorescent light passes a filter, the emitted spectrum is collected, accumulated and multiplied by the CCD scanning technology and then converted into a numerical signal, the numerical signal is multiplied by a correction factor, and the strength of the corrected fluorescent light is substituted in a standard curve of a fluorescence analyzer, so that the concentrations of the five indexes of hepatitis b of the sample can be automatically worked out. The test of hepatitis b viruses by the testing card has the characteristics of specificity, sensitivity, simpleness and accuracy.
Owner:WUXI ZODOLABS BIOTECH
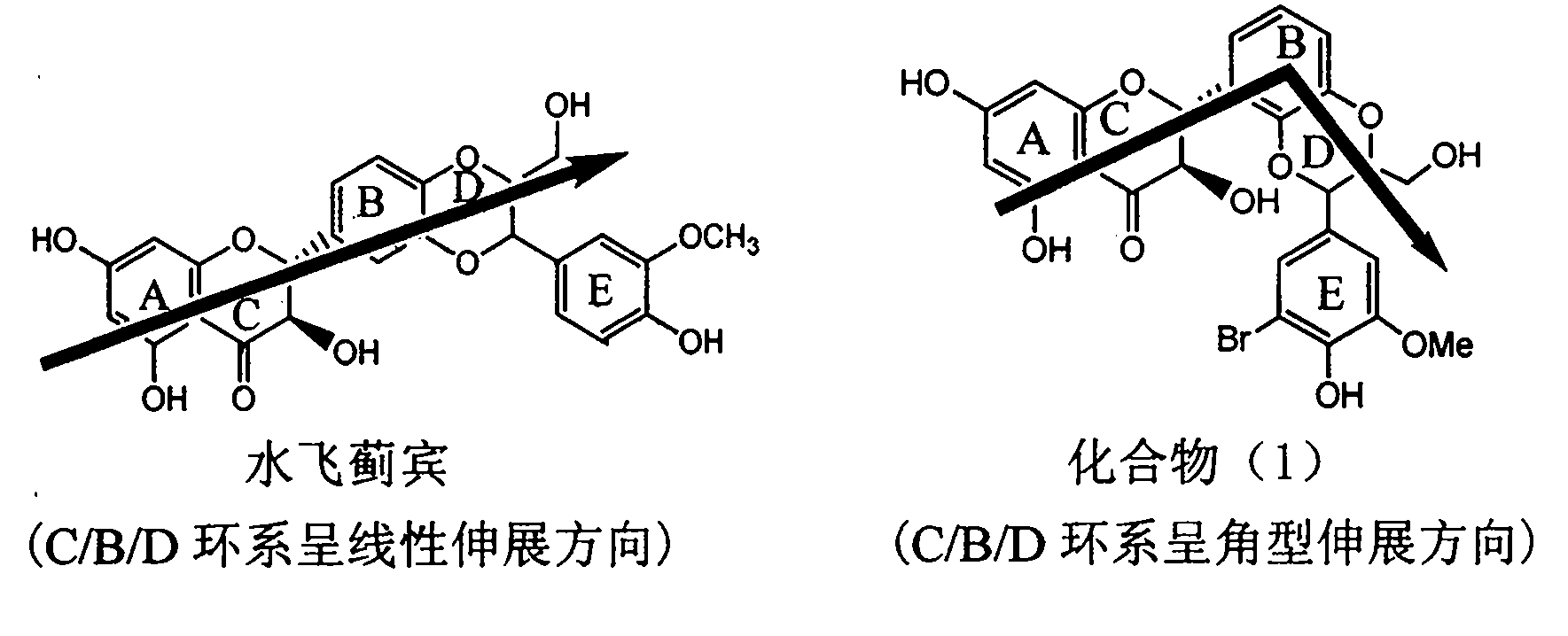
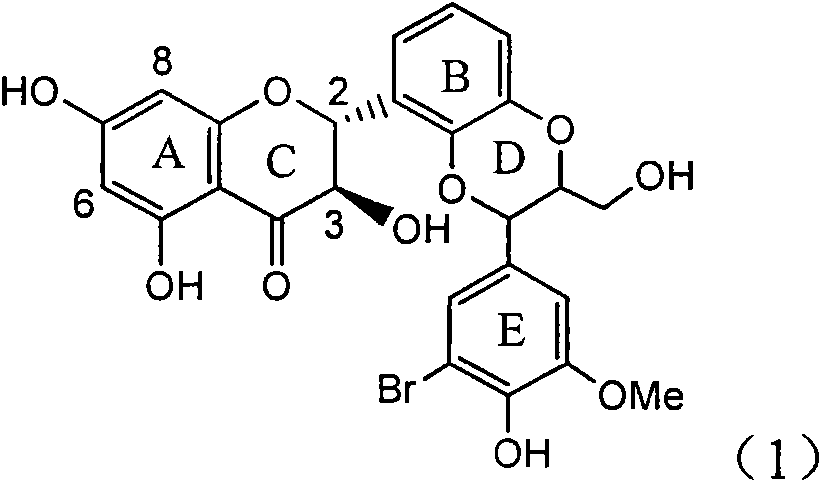
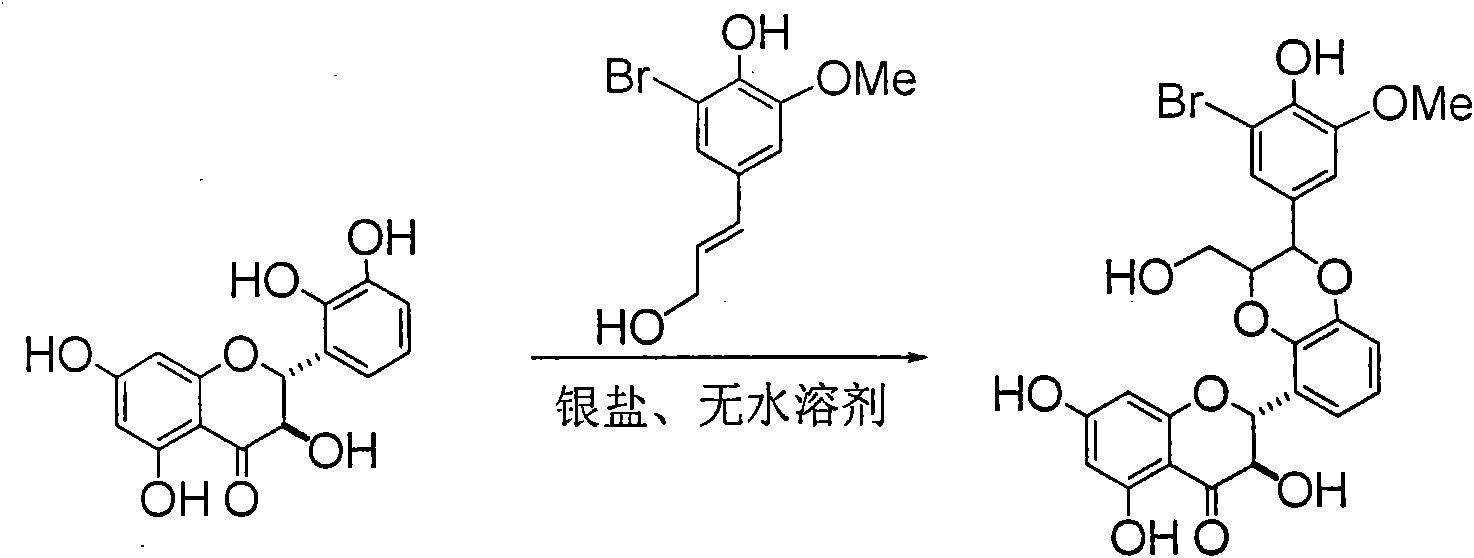
Preparation of brominated flavanonollignan and application in medicine for treating viral hepatitis B
InactiveCN101955478AConvenient sourceThe source is easy to getOrganic active ingredientsOrganic chemistryPositive controlInterferon alpha
The invention relates to the preparation of brominated flavanonollignan and an application in medicines for treating viral hepatitis B, in particular to a B cyclo-dioxane flavanonollignan compound and a preparation method thereof as well as the application of the compound or pharmaceutically acceptable salts thereof in the preparation of medicines for eliminating hepatitis B surface antigens (HBsAg) and hepatitis B e antigens (HBeAg) and medicines for inhibiting HBV DNA replication. The compound has obvious activity of inhibiting HBsAg and HBeAg, and the intensities of the compound for eliminating HBsAg and HBeAg under the concentration of 20 microgram / millimeter are respectively 2.1 times and 1.2 times larger than the corresponding activity of a positive control medicine alpha-interferon; meanwhile, the compound displays high inhibition ratio more than 57% on HBV DNA at the concentration. The results show that the favonolignan or pharmaceutically acceptable salts thereof can be expected to be used for preparing non-nucleoside type medicines for eliminating HBsAg and HBeAg, inhibiting HBV DNA replication and treating HBV infected diseases.
Owner:DALI UNIV
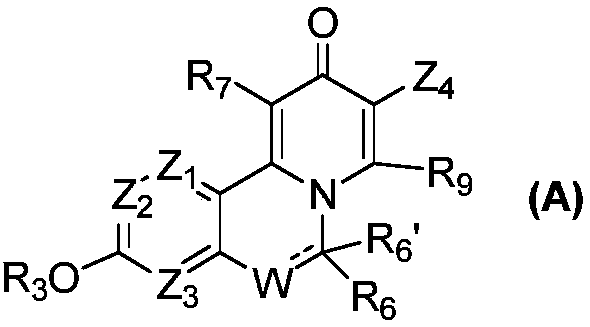
Novel isoquinoline compound and medical application thereof
InactiveCN108727378AImprove bioavailabilityLow toxicityGroup 5/15 element organic compoundsBoron compound active ingredientsAntigenViral infectious disease
The invention relates to an isoquinoline compound shown as a formula (A) or a stereoisomer, a pharmaceutically acceptable salt, a hydrate, a solvate or a crystal thereof, a medicinal composition thereof and application thereof as antiviral medicines. The isoquinoline compound inhibits hepatitis B DNA activity and hepatitis B surface antigen activity at the same time. The invention particularly relates to application thereof to preparation of medicines for treating and / or preventing hepatitis B, hepatitis B viruses (HBV) thereof and other viral infectious diseases, in particular to treatment and / or prevention of the hepatitis B and the hepatitis B viruses as HBV Surface antigen inhibitor medicines and HBV DNA production inhibitor medicines.
Owner:GINKGO PHARMA
Vaccine
InactiveCN101601860ASimple preparation processGentle preparationDigestive systemAntiviralsBody fluidCytokine
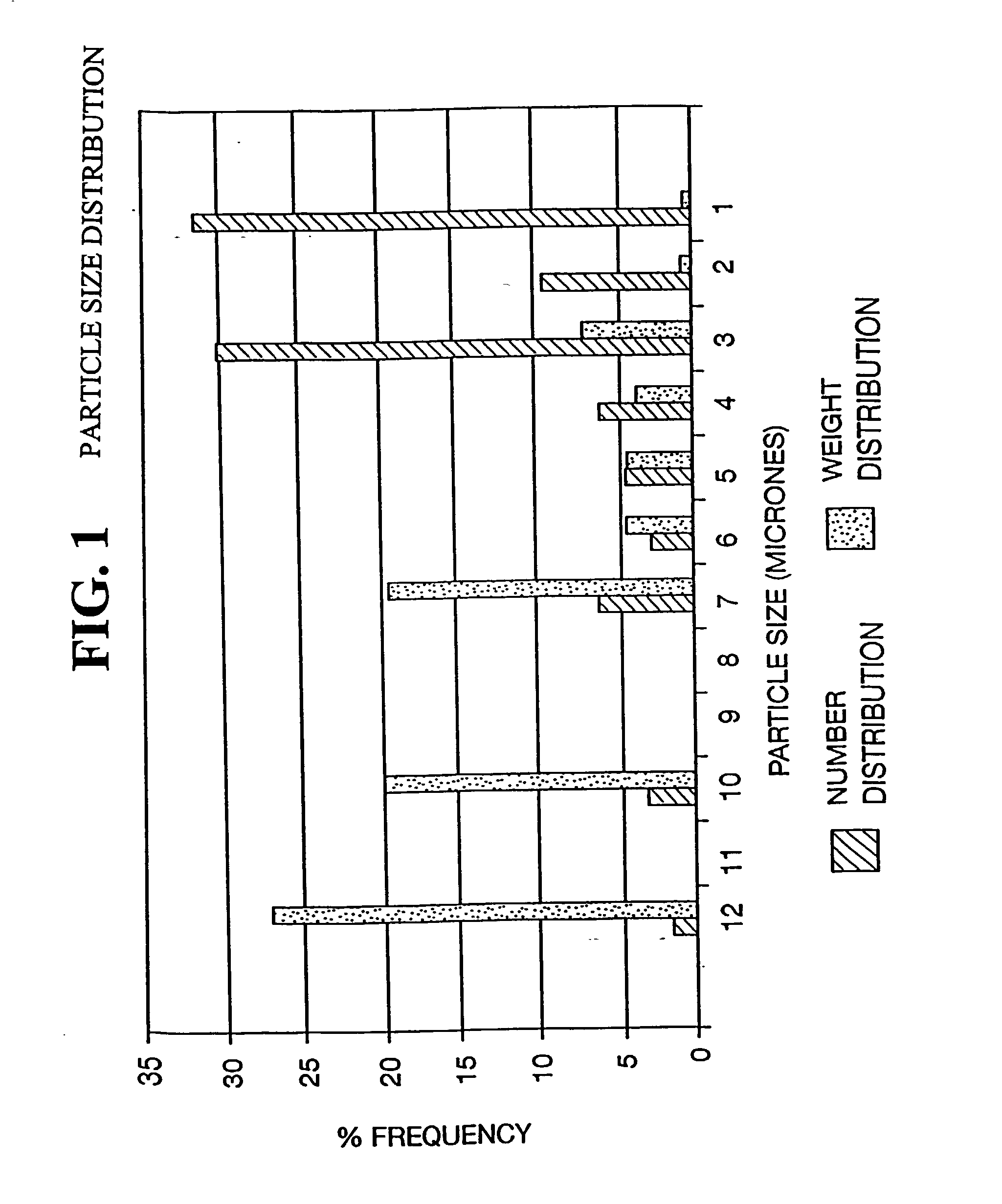
The invention discloses a vaccine for prevention and treatment, wherein the antigen is in two forms with one absorbed on a biodegradable polymer particle and the other unabsorbed (free antigen). The average grain diameter of the particles is 0.1-20um; the antigen has specific structure and antiviral effect, preferably hepatitis B surface antigen and vaccines used for human being. The vaccine is prepared by the following methods: mixing polymer particles with antigen solution to obtain suspension under the absorption of the polymer particles and antigen solution, that is vaccine product; and adding cryoprotector in the obtained suspension-like vaccine to obtain lyophilized powder of the vaccine. The vaccine can induce immune response rapidly in the engine body, induces the engine body to generate high-level humor immune and cell immune, and eliminates or controls virus by secretoryantibody or cytokine. Besides, the vaccine has simple preparation process and mild preparation process.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI +1
Preparation method of liquid phase protein chip
InactiveCN101144815AEarly detectionEarly treatmentMaterial analysisHigh risk populationsFluorescence
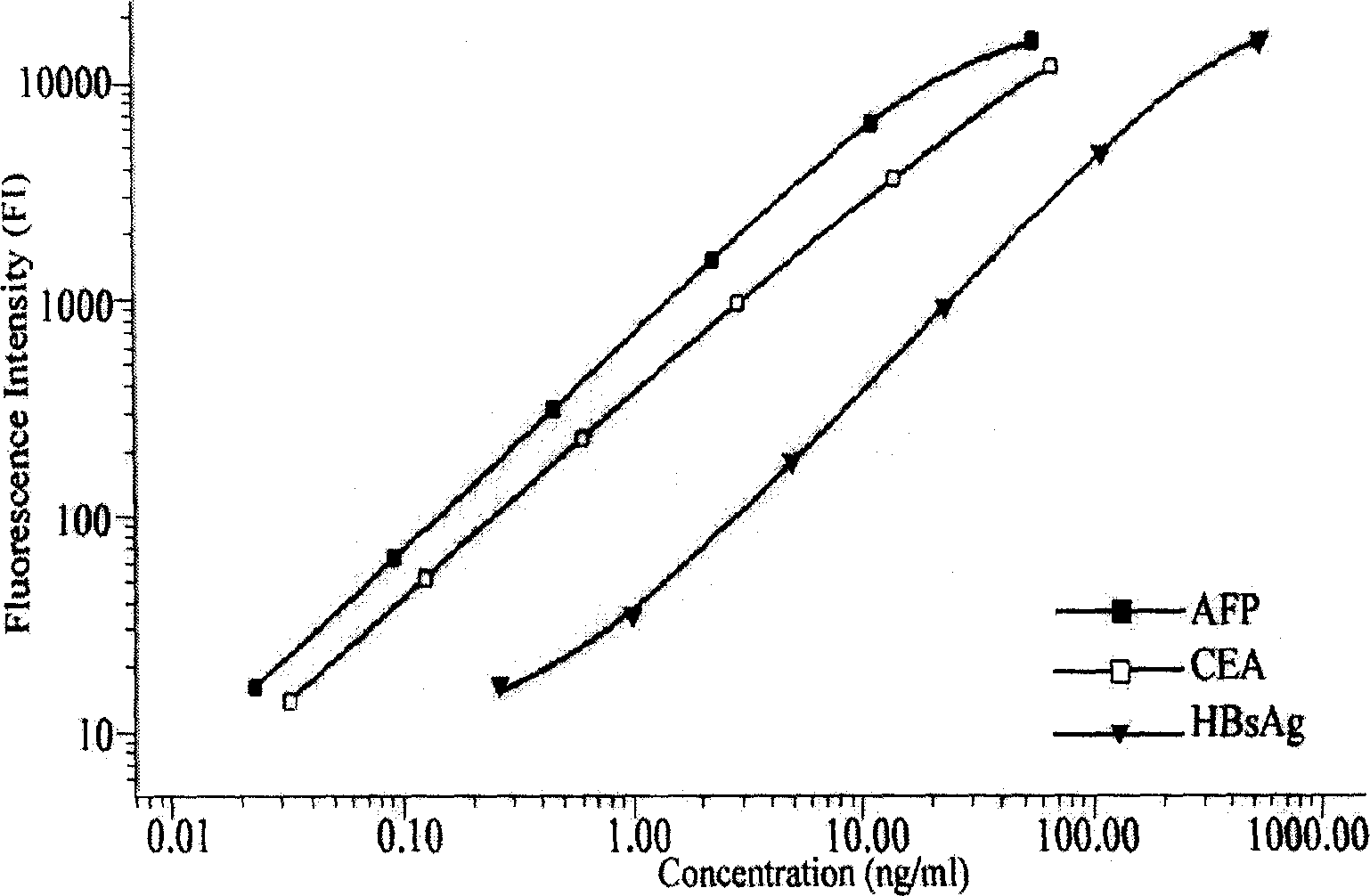
The present invention relates to the biologic technology field, and discloses a liquid phase albumen chip and the preparation and the usage method thereof which can simultaneously test human serum carcinoma embryonic antigen (CEA), Alpha fetoprotein (AFP), and hepatitis B surface antigen (HBsAg). The present invention couples the specificity antibody of CEA, AFP, and HBsAg on different fluorescence micro-spheres, and uses the test antibody marked by biotin or phycoerythrin to determine the nature quickly and quantitatively analyze the above three indexes with the double antibody sandwich method. The present invention uses the filtering membrane board when testing, and washes the board 3 times after each reaction is finished to increase the signal and improve the sensitivity. The present invention has high sensitivity, strong specificity, stable result, excellent repeatability, and simple operation; 1 micro liter serum sample can test three indexed simultaneously. The present invention is applicable to the health test and the general examination as well as the clinic test of the high risk population, and can facilitate the early diagnosis and the early treatment of the knub.
Owner:GUANGZHOU DARUI BIOTECH
Vaccines against diseases caused by enteropathogenic organisms using antigens encapsulated within biodegradable-biocompatible microspheres
InactiveUS20030161889A1Bacterial antigen ingredientsPeptide/protein ingredientsAdjuvantPoly dl lactide
This invention relates to an immunostimulating composition comprising encapsulating microspheres, which may contain a pharmaceutically-acceptable adjuvant, wherein said microspheres having a diameter between 1 nanometer (nm) to 10 microns (um) are comprised of (a) a biodegradable-biocompatible poly(DL-lactide-co-glycolide) as the bulk matrix, wherein the relative ratio between the amount of lactide and glycolide components are within the range of 40:60 to 0:100 and wherein said poly (DL-lactide-co-glycolide) is present in an uncapped form and an end-capped form wherin a ratio of uncapped to end-capped forms is 99 / 1 to 1 / 99, and (b) an immunogenic substance comprising Colony Factor Antigen (CFA / II), hepatitis B surface antigen (HbsAg), or a physiologically similar antigen that serves to elicit the producton of antibodies in animal subjects. The preparation of its composition and its use as a vaccine is also disclosed.
Owner:REID ROBERT H +5
HBV vaccine and a process of preparing the same
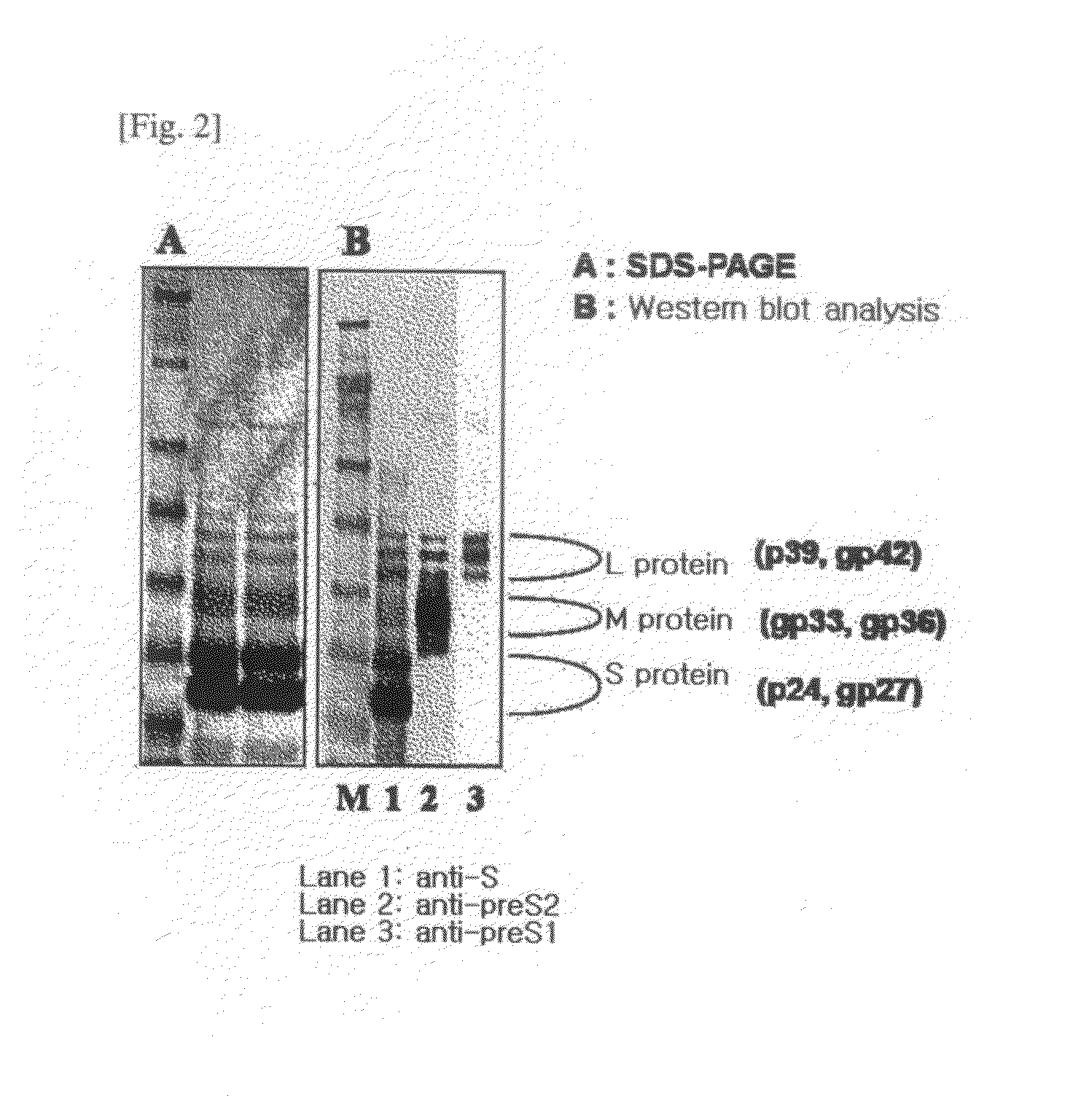
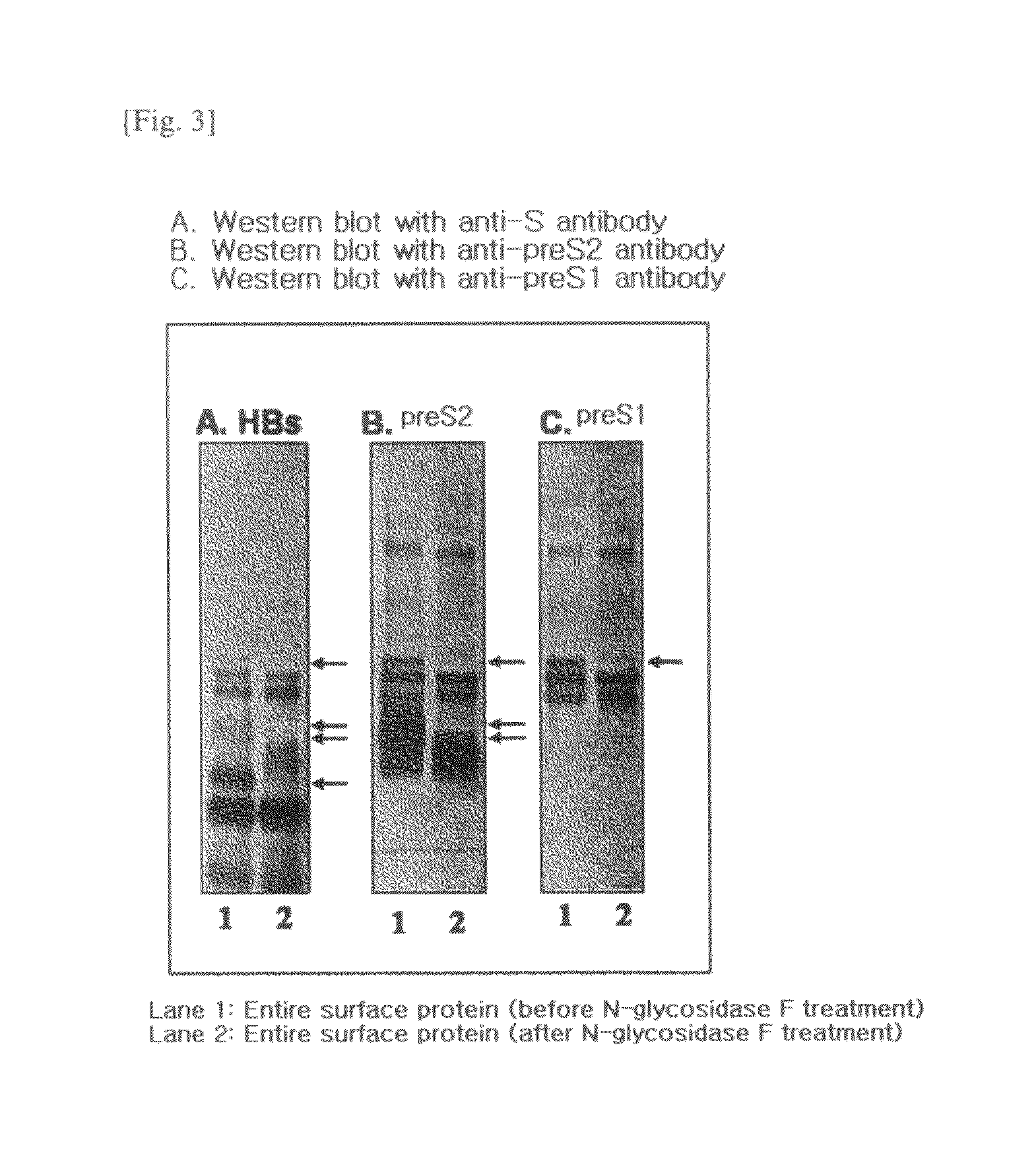
The present invention relates to an HBV vaccine comprising an entire hepatitis B surface antigen of L protein, M protein and S protein, in which the produced antigens form virus-like particles, and a multi-antigen vaccine further comprising an HBV core antigen in addition to the entire surface antigen, and a method for preparing the same. The vaccines provide various epitopes and have excellent immunogenicity to induce a strong humoral immune response as well as a cell-mediated immune response.
Owner:CHA VACCINE RES INST CO LTD
Application of andrographolide C15 substituted series derivatives to preparation of medicine for resisting hepatitis B
ActiveCN102302487AClear anti-HBV activityExpand the range of optionsOrganic active ingredientsOrganic chemistryBULK ACTIVE INGREDIENTViral hepatitis b
The invention belongs to the technical field of medicinal chemistry, and discloses application of andrographolide C15 substituted series derivatives to the preparation of a medicine for resisting hepatitis B. HepG2.2.15 cells are used for detecting the secretion amount of hepatitis B surface antigen (HBsAg) in culture solution supernatant, a large number of andrographolide derivative compounds are screened, and compounds which have high hepatitis B virus (HBV) resistance are optimally selected and have a structure shown in a general formula 1; and the compounds have high HBV-resistant activity, high efficiency and low toxicity and are taken as active ingredients to be used for preparing a medicine for resisting viral hepatitis B, and a new medicine is provided for treating hepatitis, so that the selectable range of clinical medicines is expanded.
Owner:ZHENGZHOU UNIV
Hepatitis b virus surface antigen chemiluminescence immune assay determination kit and method for preparing same
InactiveCN101363861AGuaranteed SensitivityEasy to produce and operateChemiluminescene/bioluminescencePolyclonal antibodiesBiotin-binding proteins
The invention particularly relates to a kit for determining the surface antigen of the hepatitis B virus, and a preparation method thereof, which belongs to the medical field of immunological analysis. The kit comprises (1) a hepatits B virus surface antigen calibration material; (2) an avidin-coated carrier; (3) the polyclonal antibody or monoclonal antibody of a biotinylated hepatitis B virus surface antibody; (4) the polyclonal antibody or monoclonal antibody enzyme-labeled of the surface antibody; and (5) a chemiluminescent primer. Furthermore, the preparation method comprises the following steps of (1) preparing a calibration material from the pure surface antigen; (2) coating the carrier with avidin; (3) performing biotinylation of the polyclonal antibody or monoclonal antibody of the surface antibody; labeling the polyclonal antibody or monoclonal antibody of the surface body with an enzyme; (4) sub-packaging the calibration material and the chemiluminescent primer; and (5) assembling. The kit has the advantages of simpliness, rapidness, sensitiveness, stability and the like.
Owner:北京科美东雅生物技术有限公司
Application of polyguluronate sulfate to anti-hepatitis B virus medicine preparation
ActiveCN105343121AInhibit expressionEnhance production and secretionOrganic active ingredientsAntiviralsAnti virusInterferon
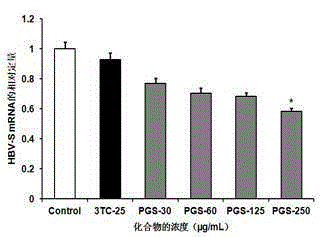
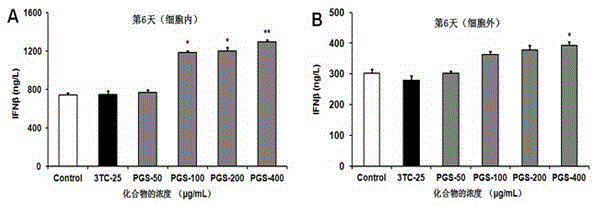
The invention provides application of polyguluronate sulfate to anti-hepatitis B virus medicine preparation. Experiments prove that the polyguluronate sulfate is capable of well inhibiting expression of an HBV (hepatitis B virus) HBsAg (hepatitis B surface antigen) and an HBV HBeAg (hepatitis Be antigen) in a hepatocellular carcinoma cell line HepG2.2.15, remarkably inhibiting expression of HBV-S mRNA (messenger ribonucleic acid) in the hepatocellular carcinoma cell line HepG2.2.15, enhancing generation and secretion of anti-virus immunity-associated IFN (interferon)-beta in cells and activating NF (nuclear factor)-kappa B and MAPK (mitogen-activated protein kinase) signal pathways. The polyguluronate sulfate which is a marine sulfated polysaccharide compound has the advantages of rich resources, low cost, high safety and the like, and is obviously superior to positive control drug lamivudine in anti-HBV effect, thereby being promising in application prospect of anti-hepatitis B virus medicine preparation.
Owner:MARINE BIOMEDICAL RES INST OF QINGDAO CO LTD +1
Hepatitis B virus surface antigen detection particles, preparation thereof and use thereof
InactiveCN101666801AReduce sensitivityHigh sensitivityChemiluminescene/bioluminescenceBiological testingSerum igeAntigen testing
The invention relates to a reagent for the diagnosis of hepatitis B and discloses hepatitis B surface antigen detection particles, which are luminous particles coated by a hepatitis B surface antigen.The invention also discloses the preparation and use of the hepatitis B surface antigen detection particles. In addition, the invention also discloses an in-vitro diagnostic kit for determining the hepatitis B surface antigen in a sample and also discloses the using method of the kit. The kit of the invention can be used in combination with other serums and clinic information to diagnose the acute or chronic hepatitis B infection condition of an individual and can also be used for screening hepatitis B in perinatal females to judge the hepatitis B infection risk of the newborns.
Owner:BEYOND DIAGNOSTICS (SHANGHAI) CO LTD
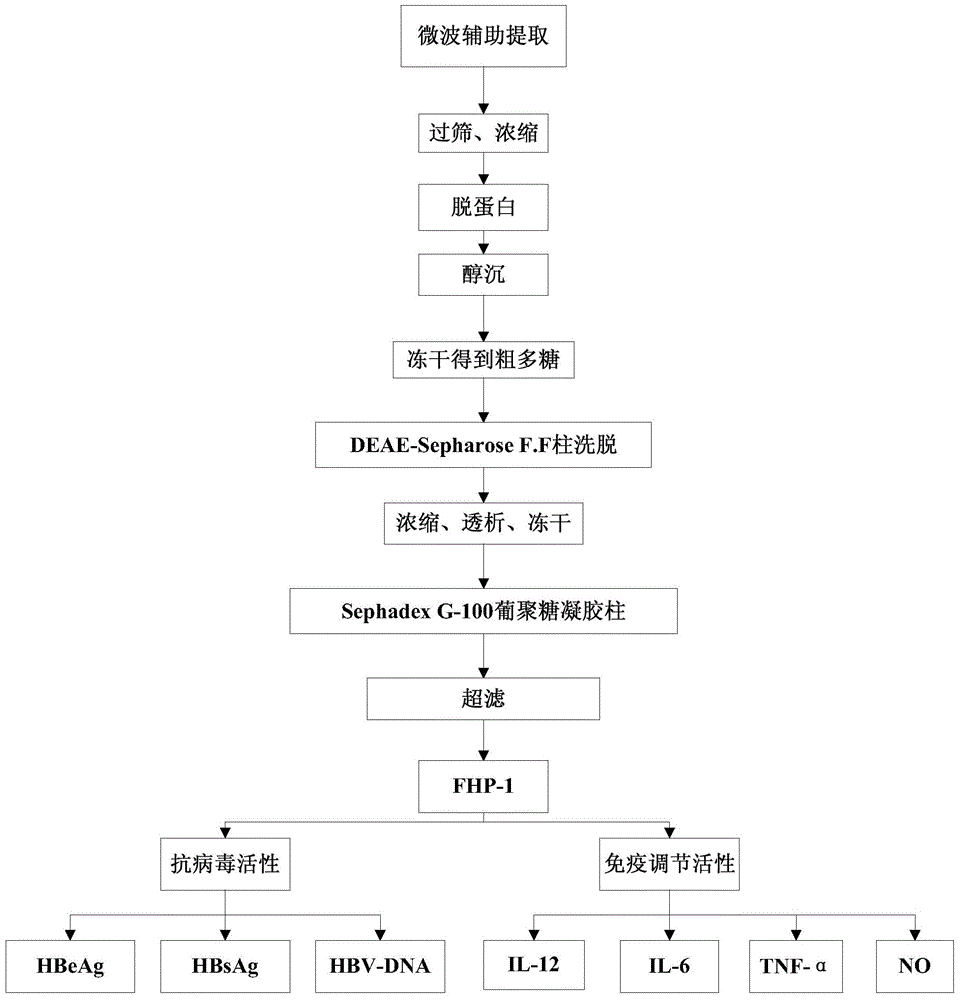
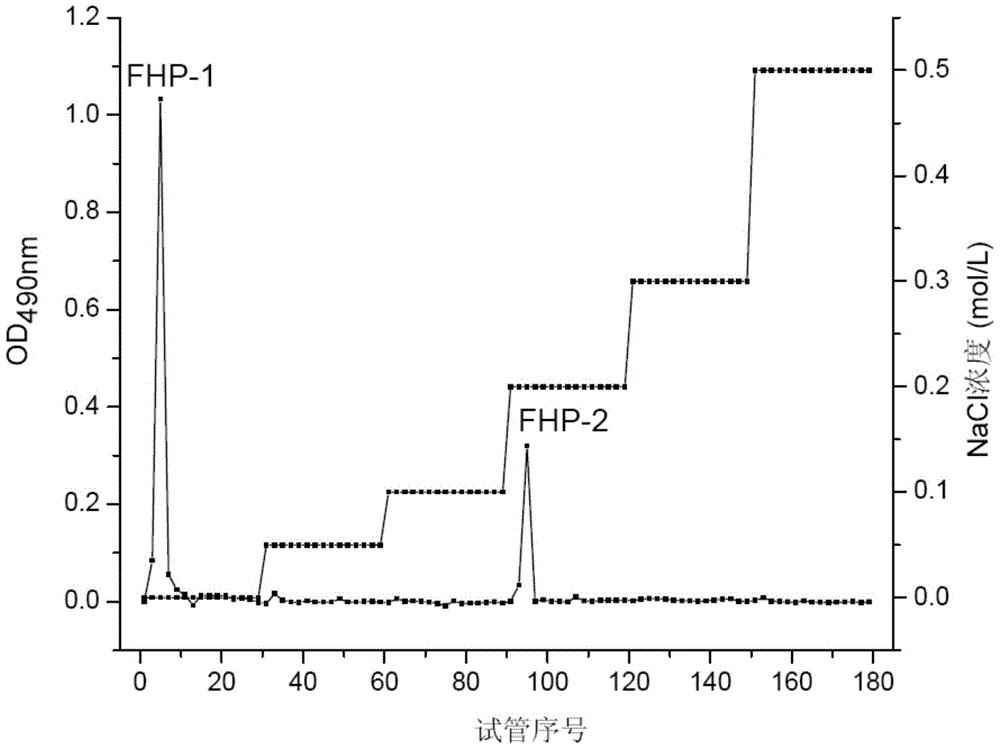
Preparation method and application of flaxseed polysaccharides with antiviral and immune activity
ActiveCN105037573AEfficient destructionEasy extractionOrganic active ingredientsAntiviralsAntigenSulfated polysaccharides
The invention discloses a preparation method and an application of flaxseed polysaccharides with antiviral and immune activity. The preparation method adopting flaxseeds as a raw material comprises the following steps: crushing flaxseeds, carrying out shell-kernel separation, carrying out microwave assisted hot water extraction, carrying out Sevage technology protein removal, carrying out ethanol precipitation, freeze-drying to obtain crude flaxseed polysaccharides, and carrying out ion exchange column chromatography, carrying out Sephadex column chromatography, and carrying out ultrafiltration to prepare flaxseed sulfated polysaccharides. FHP-1 obtained in the invention has homogeneous composition, and the molecular weight is 2626kDa. Polysaccharide with sulfate and triple-helical structure are separated from the flaxseeds in the invention for the first time. Cell biology experiments prove that the polysaccharides can reduce expression of hepatitis B surface antigen and hepatitis B e antigen and inhibit replication of hepatitis B viruses, can activate immune response, and improves secretion of the tumor necrosis factor TNF-a, interleukins IL-6 and IL-12, and an inflammation factor NO by immune cells.
Owner:JINAN UNIVERSITY
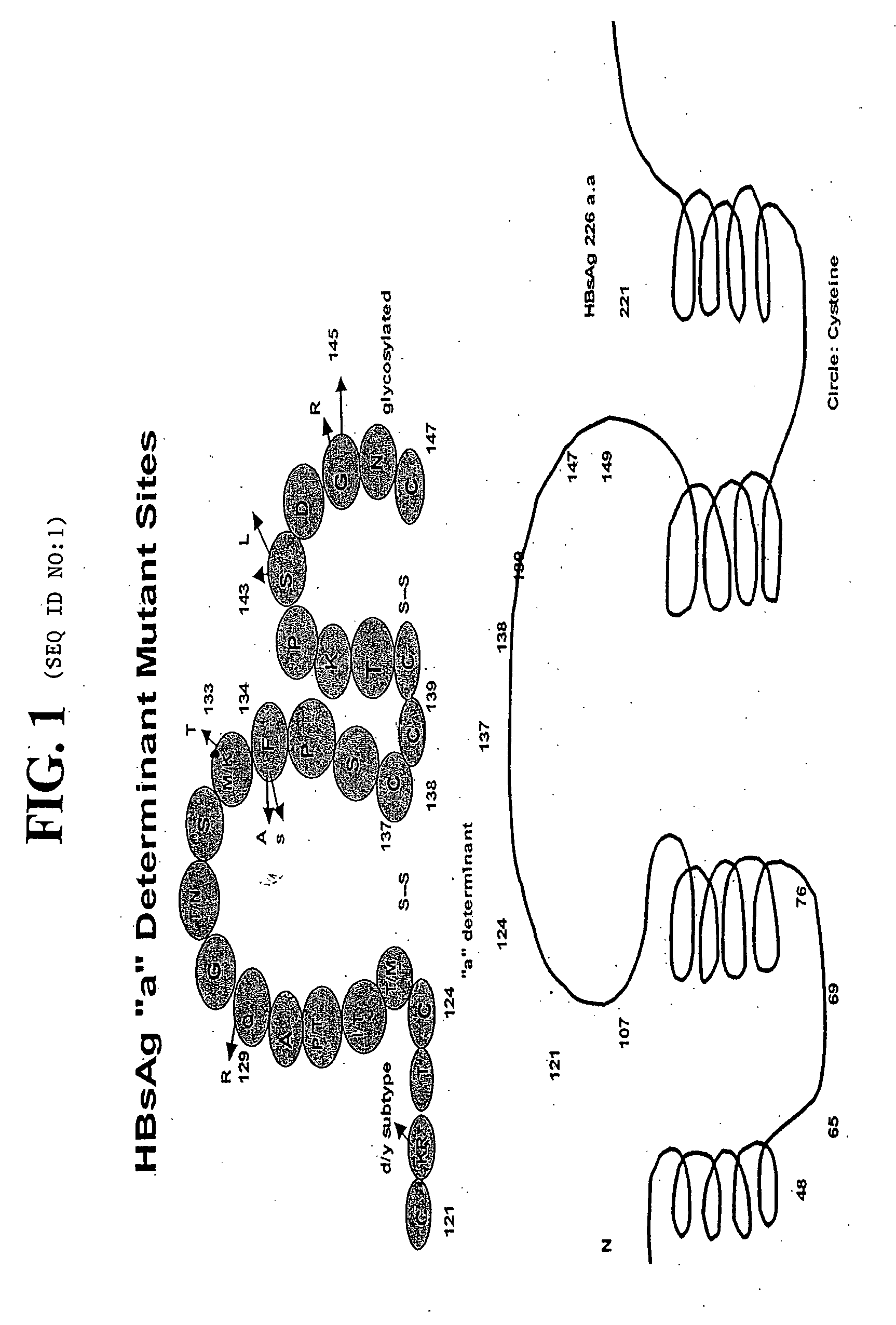
Hepatitis B virus surface antigen mutant and methods of detection thereof
The subject invention relates to a novel hepatitis B surface antigen mutant and methods of detecting this mutant, and / or antibodies thereto, in patient samples. In particular, the mutant contains a substitution of amino acid threonine for the amino acid alanine at position 123 in the amino acid sequence of the hepatitis B surface antigen (HBsAg) protein.
Owner:ABBOTT LAB INC
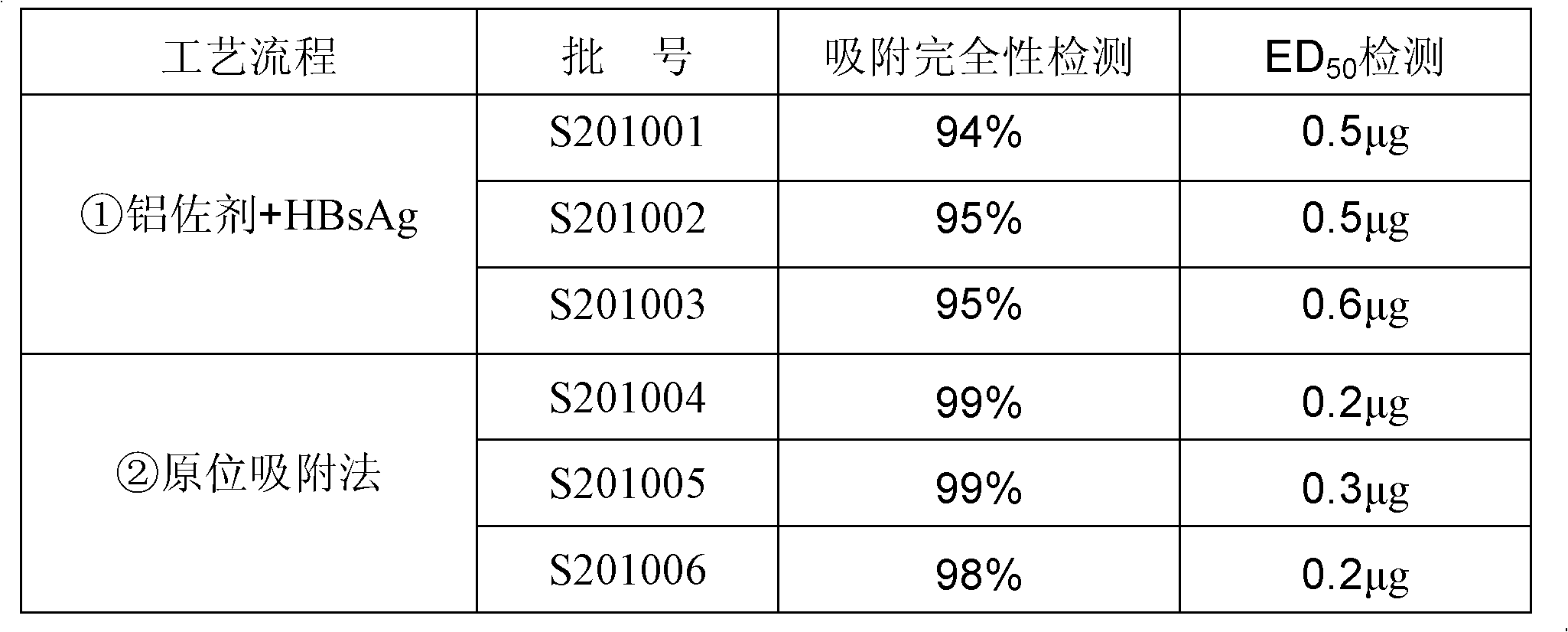
Preparation method of aluminum-containing adjuvant hepatitis B vaccine
ActiveCN102198270AIncrease inoculum volumeHigh positive conversion rateViral antigen ingredientsDigestive systemAdjuvantIn situ adsorption
The invention discloses a preparation method of an aluminum-containing adjuvant hepatitis B vaccine, belonging to the biotechnology field. The preparation method is characterized in that an aluminum adjuvant Al(OH)3 is produced by an on-line reaction, i.e. after a phosphate buffer solution (PBS), a KAl(SO4)2 solution and a hepatitis B surface antigen stock solution are mixed, an NaOH solution is added to the mixed solution, an Al(OH)3 adjuvant is continuously produced, and simultaneously, hepatitis B surface antigens are continuously coated and adsorbed; and the process is called 'in-situ adsorption'. In the invention, the Al(OH)3 adjuvant is produced by an in-situ reaction to greatly improve the adsorption rate of the hepatitis B surface antigens, thereby improving the immunogenicity of the antigens, being capable of more effectively causing organisms to generate an immune response, and producing more protective antibodies. The practice proves that the aluminum adjuvant hepatitis B vaccine produced by the method disclosed by the invention has the advantages of small inoculation amount, few adverse responses, high antibody positive conversion rate and the like, and can induce high-level antibody response after being immunized. Simultaneously, the processing steps are also simplified, and the production cost is greatly lowered.
Owner:DALIAN HISSEN BIO-PHARM CO LTD
Nanoparticle vaccine preparation containing recombinant hepatitis B surface antigen and preparation method thereof
ActiveCN105288613AExtended stayEnhance immune responseDigestive systemAntiviralsSodium bicarbonatePolyvinyl alcohol
The invention provides a nanoparticle vaccine preparation containing a recombinant hepatitis B surface antigen. The preparation comprises the following components: a core which is composed of human serum albumin, the recombinant hepatitis B surface antigen and sodium bicarbonate; an encapsulated layer which is a polylactic acid-polyglycolic acid segmented copolymer for encapsulating the core; an external layer which is composed of chitosan or mannan or a mixture thereof for coating. The preparation method comprises the following steps: (1) adding the recombinant hepatitis B surface antigen, the human serum albumin and sodium bicarbonate are added into a phosphate buffered solution containing polyvinyl alcohol to obtain an internal water phase; (2) dissolving the polylactic acid-polyglycolic acid segmented copolymer into an organic solvent for preparing an oil phase, adding the internal water phase obtained in the step (1) into the oil phase, and stirring at a high speed for forming a W / O primary emulsion; (3) adding the W / O primary emulsion into an external water phase solution containing polyvinyl alcohol, stirring at a high speed for forming a W / O / W complex emulsion, stirring at a low speed, carrying out a centrifugal washing, collecting nanoparticles, carrying out a vacuum freeze drying to obtain the finished product nanoparticle vaccine preparation containing the recombinant hepatitis B surface antigen.
Owner:HEBEI NORMAL UNIV
Hepatitis B surface antigen chemiluminiscence immune detection reagent kit and application thereof
ActiveCN108344869AHigh sensitivityStrong specificityChemiluminescene/bioluminescenceBiological testingEpitopeProtein.monoclonal
The invention relates to a hepatitis B surface antigen chemiluminiscence immune detection reagent kit and application thereof. The reagent kit comprises a magnetic particle reagent indirectly connected with an anti-HBsAg antibody 1, an HBsAg antibody 2 solution marked by a chemiluminiscence marker, a chemiluminiscence substrate solution and a cleaning solution; the anti-HBsAg antibody 1 at least comprises two antibodies or immune reaction fragments of antibodies, and at least comprises an anti-S-protein monoclonal antibody 1, the anti-HBsAg antibody 2 at least comprises two antibodies or immune reaction fragments of the antibodies and at least comprises an anti-S-protein monoclonal antibody 2, the epitopes of HBsAg corresponding to the anti-S-protein monoclonal antibody 1 and the anti-S-protein monoclonal antibody 2 are different, the reagent kit is the first case internationally and has the high sensitivity and accuracy during HBsAg detection, the HBsAg mutant strain detection capability is greatly improved, and the safety for the clinic blood transfusion, operation and the like is remarkably improved.
Owner:SUZHOU HYBIOME BIOMEDICAL ENG CO LTD
Rapid diagnosis kit for pre-S1 antigens of hepatitis B viruses and method for preparing same
The invention relates to a rapid diagnosis kit for pre-S1 antigens of hepatitis B viruses and a method for preparing the same. The rapid diagnosis kit mainly comprises hepatitis B virus pre-S1 antigen detection test paper formed by adhering a sample pad, a conjugate pad, a reaction film and a water absorbing layer to a lining card in sequence, and the test paper is put in a card box, wherein the conjugate pad is prepared by loading anti-HBs polyclonal antibodies on a glass fiber pad, and a detection carrier nitrocellulose film connected with the glass fiber pad is coated with anti-HBs monoclonal antibodies to form a detection line and simultaneously coated with recombined hepatitis B surface antigens to form a quality control line. By using the rapid diagnosis kit, when the sample to be detected contains the pre-S1 antigens and two magenta lines of the detection line and the quality control line are formed, the sample is judged to be positive; and otherwise, only one magenta line is formed, the sample is judged to be negative. The rapid diagnosis kit is used for detecting the pre-S1 antigens of the hepatitis B viruses in human blood serum or blood plasma and has the advantages of easy operation, rapid diagnosis, good specificity and high sensitivity.
Owner:威海威高生物科技有限公司
Rabbit monoclonal antibodies to hepatitis B surface antigens and methods of using the same
ActiveUS20060008798A1Simple and accurate and efficient diagnosisAvoid deathMicrobiological testing/measurementImmunoglobulins against virusesB hepatitis surfaceHepatitis b surface antigen
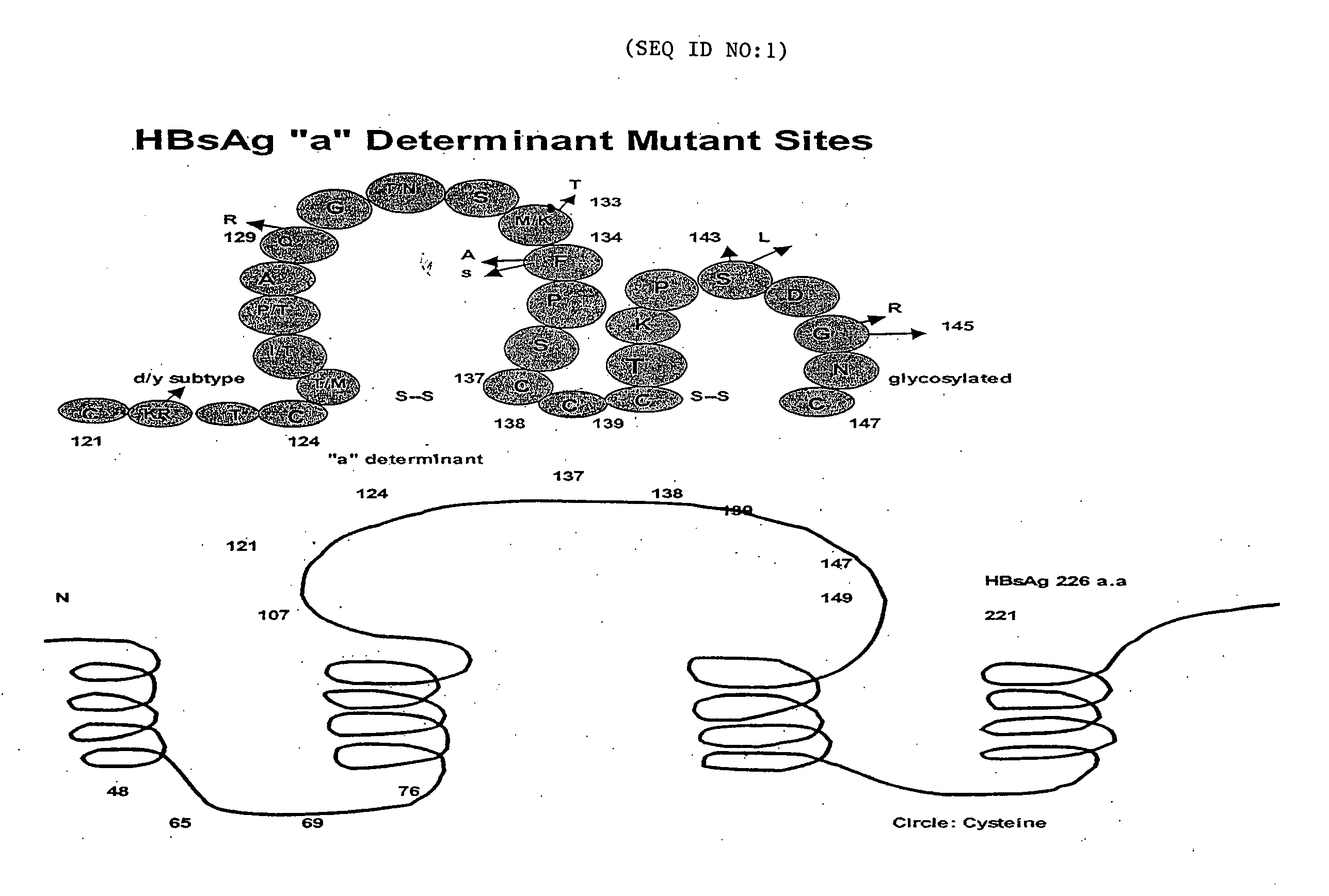
Reagents, methods and immunodiagnostic test kits for the accurate detection of hepatitis B virus (HBV) infection are disclosed. The methods and kits employ novel rabbit monoclonal antibodies directed against HBV surface antigens (HBsAg) with mutations in the “a” determinant region of HBsAg.
Owner:GRIFOLS WORLDWIDE OPERATIONS
Immunochromatographic test paper for detecting hepatitis B surface antigens and preparation method thereof
InactiveCN103558399AHigh sensitivityStrong specificityBiological testingCoatingsMedicineImmunochromatographic test
The invention relates to an immunochromatographic test paper for detecting hepatitis B surface antigens and a preparation method thereof, belonging to the field of a hepatitis B surface antigen detection technique. The immunochromatographic test paper comprises a sample pad, a nitrocellulose membrane and a water absorption pad which are sequentially connected, wherein the sample pad contains a superparamagnetic composite particle labeled anti-HBsAg antibody; the nitrocellulose membrane is coated with a detection line and a quality control line which are mutually separate; the detection line contains an anti-HBsAg coating antibody; and the quality control line contains goat anti-mouse IgG which can be specifically combined with the anti-HBsAg coating antibody. The immunochromatographic test paper has the advantages of high sensitivity and high specificity, is quick and convenient, and can implement objective determination.
Owner:昆明云大生物技术有限公司
Pichia pastoris of high copy expression recombination hepatitis B surface antigen, preparation and application thereof
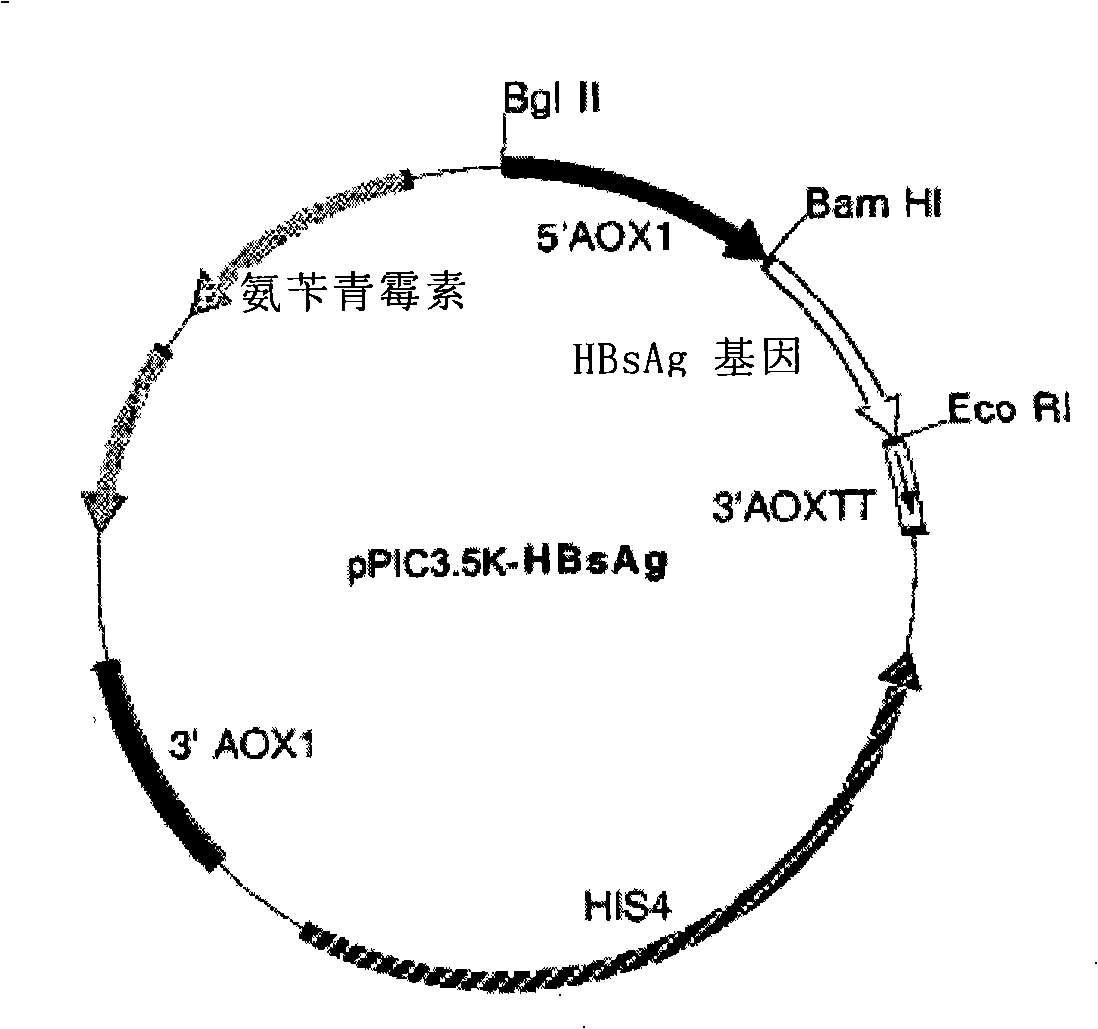
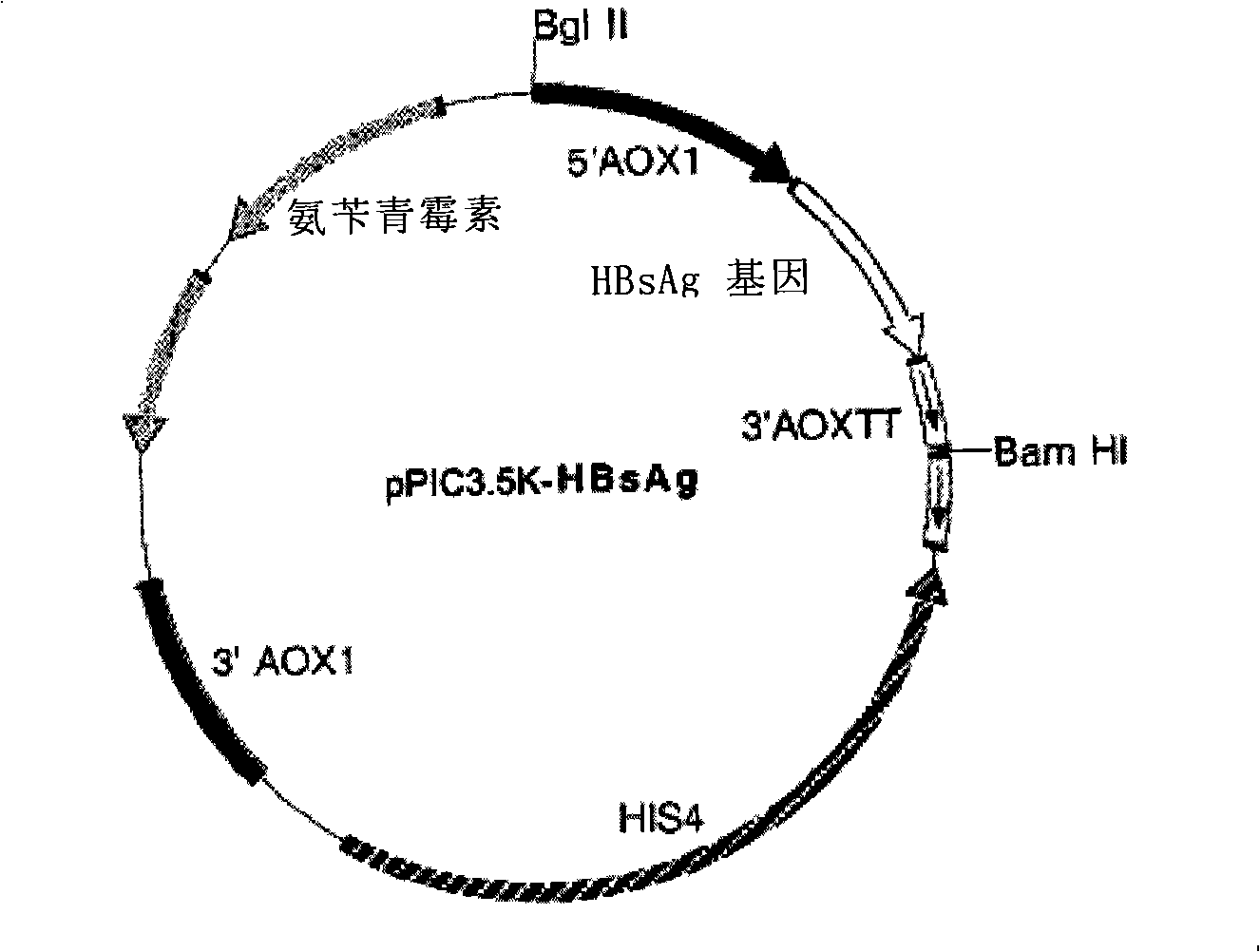
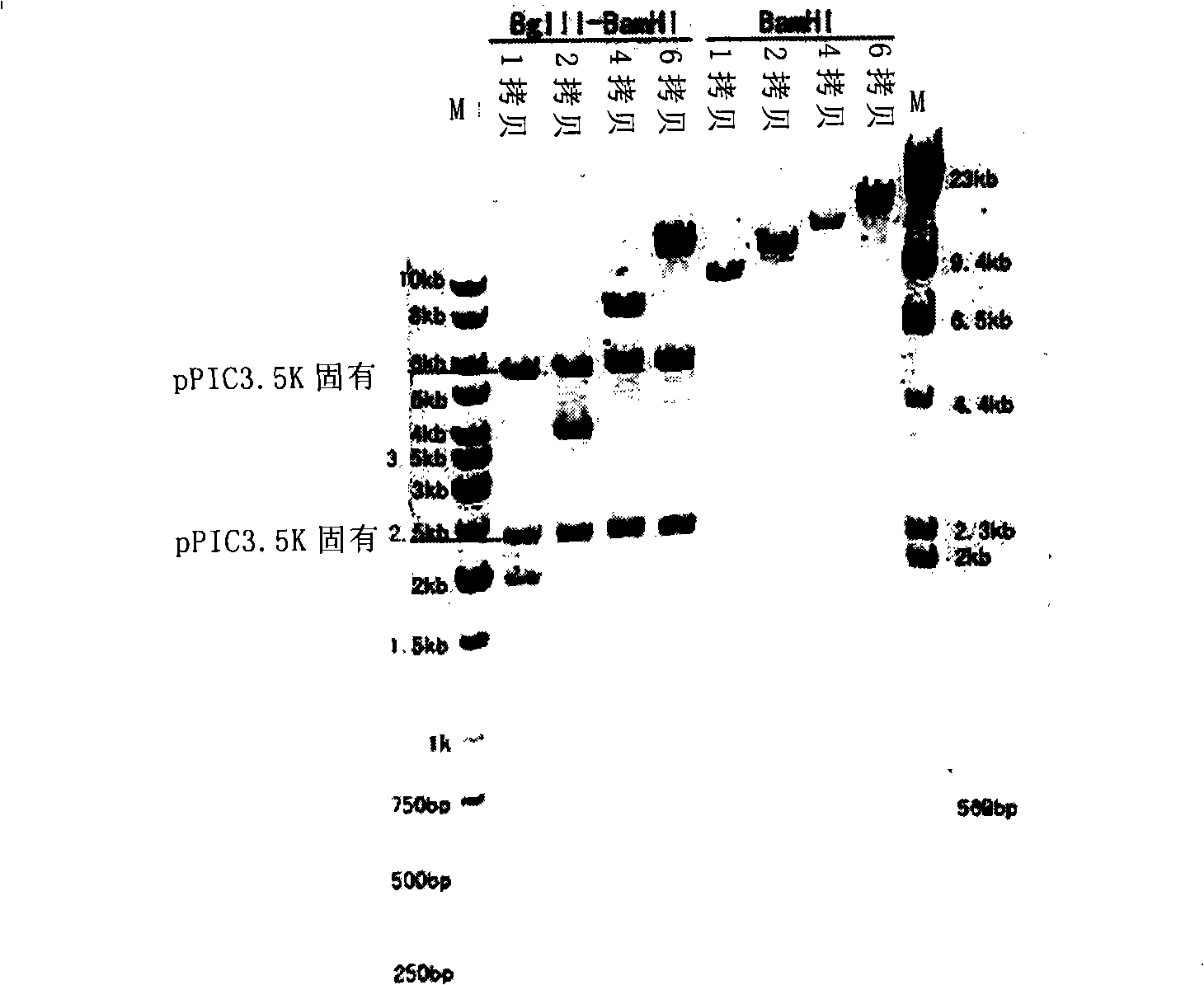
The invention provides a yeast cell for expressing hepatitis B virus surface antigen. The yeast cells contain the constitution matter for expressing the hepatitis B virus surface antigen, and the constitution matter contain 5-15 tandem-arranged hepatitis B virus surface antigen expression cassettes. Each expression cassette includes the elements as follows: (a) an initial signal element AOX; (b) hepatitis B virus surface antigen genes and (c) a terminal signal element AOX (TT). The yeast cells are induced by methanol to express the hepatitis B virus surface antigen and form hepatitis B virus surface antigen virus particles with the expression amount being at least 30mg / L fermented solution. The invention also provides the constitution matter, the preparation method thereof, and a preparation method for the yeast cells and hepatitis B virus surface antigen virus particles expressed by the same. The constituted yeast cell with high copy number and high expression can be used for increasing the yield, reducing the cost, and is suitable for the large-scale production of the hepatitis B virus surface antigen vaccine.
Owner:SHANGHAI INST OF BIOLOGICAL PROD CO LTD
Hepatitis B virus multi-epitope fusion protein and preparation method and application thereof
ActiveCN102199217AHighly inhibitoryEfficient removalDigestive systemAntiviralsEscherichia coliFusion Protein Expression
The invention relates to a hepatitis B virus multi-epitope fusion protein and a preparation method and application thereof. The fusion protein is obtained by inserting hepatitis B virus multi-epitope fusion peptide (with the sequence shown as SEQIDNo.1) formed by serially connecting HBsAg313-321, HBsAg335-343, Pol150-159, Pol455-463 and Padre epitopes through connecting peptide between amino acidat the 78th position and amino acid at the 79th position of a hepatitis B virus core protein; and the preparation method comprises the following steps of: constructing a hepatitis B virus multi-epitope fusion protein expression plasmid pET28-HBc-HP; performing isopropyl thiogalactoside (IPTG) inducing expression by using an Escherichia coli expression system; and purifying by using affinity chromatography. The fusion protein carries a plurality of supertype epitopes of hepatitis B surface antigen (HBsAg), hepatitis B core antigen (HBcAg) and polymerase, is viral particles, has the advantages of strong immunogenicity, wide applicable range and the like, and can be used for preparing therapeutic hepatitis B vaccines.
Owner:ARMY MEDICAL UNIV
Reagent kit for screening infectious diseases and quantitating hepatitis B surface antigen and applications
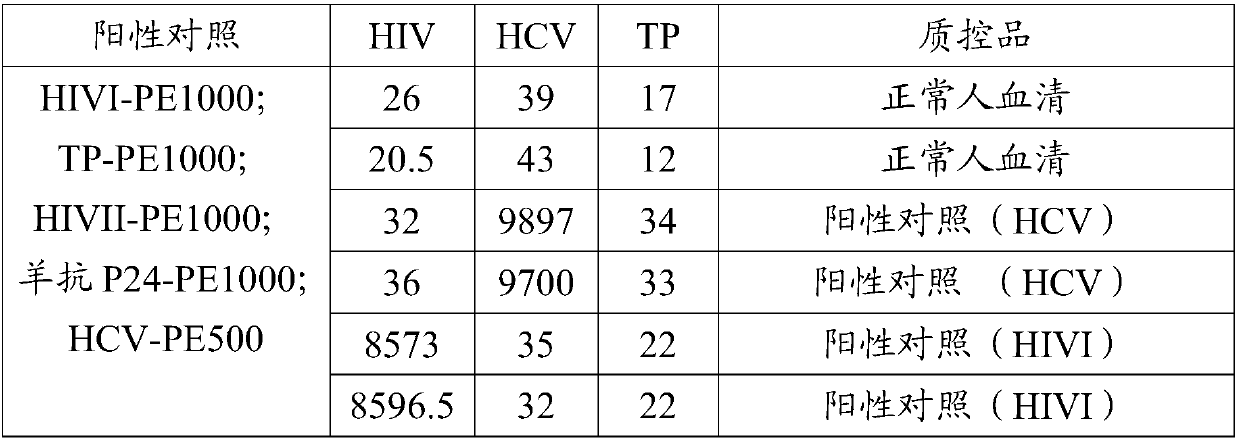
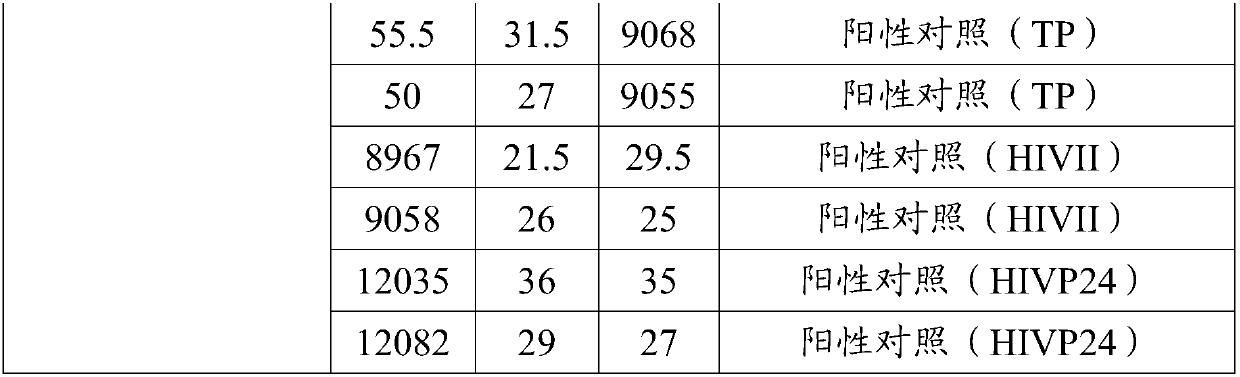
PendingCN110275022AImprove throughputHigh speedBiological material analysisBiological testingAntigenTransmissible disease
The invention provides a reagent kit for screening infectious diseases and quantitating a hepatitis B surface antigen and applications. The technology of the invention has the advantages of free combination, high throughput, high speed, low cost, high accuracy, god repeatability, wide linear range, no need for washing and simple operation. The introduction of liquid chip technology and products in clinical diagnosis can greatly improve the detection efficiency and reduce the detection cost. On a platform of the invention, the detection of human sAg concentration with high throughput, high speed, low cost, high accuracy and god repeatability can be realized, and quantitative experiments of HIV, HCV and TP can be carried out. Compared with conventional immunoassay, the invention aims to provide the high-throughput, rapid and accurate technical platform and reagent kit which can detect a plurality of infectious disease antigens and antibodies at the same time.
Owner:北京协和洛克生物技术有限责任公司
Preparation of hepatitis B surface antigen monoclonal antibody
The invention belongs to the technical field of bioengineering. The invention relates to a hepatitis B surface antigen (HBsAg) recombinant protein. An amino acid sequence of the recombinant protein isformed by connecting two dominant antigen epitopes of HBsAg in series, an escherichia coli preferred codon is adopted to convert the amino acid sequence of the recombinant protein into a corresponding nucleotide sequence, the nucleotide sequence is chemically synthesized, and a recombinant expression vector is constructed. Therefore, the expression quantity of the recombinant protein in escherichia coli is increased. The invention also relates to a phage library established by immunizing mice with the recombinant protein, a corresponding recombinant protein single-chain antibody scfv sequenceis obtained through selecting and screening, the obtained scfv sequence is constructed into a complete mouse IgG1 antibody sequence expression vector, a monoclonal antibody is expressed through transient HEK293F cells, the monoclonal antibody is purified, colloidal gold particles are marked respectively, the optimal monoclonal antibody pairing combination is determined through an orthogonal experiment, and therefore, the hepatitis B surface antigen monoclonal antibody has important significance for early diagnosis and prevention and treatment of hepatitis B.
Owner:杭州贤至生物科技有限公司
Recombinant humanized anti-hepatitis B surface antigen (HBs Ag) Fab antibody and its preparaticon method
The present invention provides a recombinant humanized anti-hepatitis B surface antigen (HBsAg) Fab antibody as human hepatitis B surface antibody and its preparation method. The preparation method includes the following steps: utilizing phage display technique to obtain the gene of a humanized anti-HBsAg Fab fragment, making expression in prokaryotic expression system, on this basis utilizing PCK technique to amplifying the light-heavy chain gene of Fab, constructing it into the methyl alcohol yeast expression vector, using two-step process to convert yeast, constructing Fab engineering bacterium, secreting, expressing and producing Fab antbody fragment. The Fab antibody has strong HBsAG combination activity and antigen specificity, and the expression product of said gene has high application value in clinical therapy of hepatic disease.
Owner:余宙耀 +1
Method for removing residual hose cell DNA in recombinant Hansenula polymorpha hepatitis B surface antigen
The invention provides a method for removing residual hose cell DNA (Deoxyribonucleic Acid) in a recombinant Hansenula polymorpha hepatitis B surface antigen. The method comprises the following steps: step a, dissolving a recombinant Hansenula polymorpha hepatitis B surface antigen sample for removing the residual hose cell DNA into a 10-30 mN of PB solution containing 400-500 mM of NaC1; step b, taking the PB solution containing the NaC1 in which the recombinant Hansenula polymorpha hepatitis B surface antigen sample is dissolved as a loading solution and performing chromatographic purification by utilizing an Monoliths anion exchange column to obtain the recombinant Hansenula polymorpha hepatitis B surface antigen sample for removing the residual hose cell DNA. The method provided by the invention can effectively remove the residual DNA in the recombinant Hansenula polymorpha hepatitis B surface antigen sample, so that prepared vaccine reaches the regulations on the content of the residual DNA in the Part III of the National Formulary (Edition 2010).
Owner:北京生物制品研究所有限责任公司
Lucid ganoderma-Cordyceps sinensis granule and preparation method thereof
InactiveCN103505478AHas immunomodulatory functionSolve difficult problemsOrganic active ingredientsGranular deliveryB hepatitis surfaceMedicine
The invention discloses a lucid ganoderma-Cordyceps sinensis granule. A preparation method for the granule comprises the following steps: subjecting purely natural lucid ganoderma to reflux extraction with 70 to 90% ethanol, carrying out pressure reduced condensation, settlement with a ZTC clarifying agent and combination of extracts obtained after extraction three times, filtering the combined extract, then carrying out standing for 10 to 25 h and centrifugation, collecting a precipitate, adding distilled water to dissolve the precipitate, boiling the dissolved precipitate, filtering out insoluble substances while the dissolved precipitate is hot, adding ethanol into an obtained filtrate with stirring until alcohol content reaches 80%, carrying out standing to allow a grayish purple precipitate to be precipitated, drying the grayish purple precipitate at a low temperature to obtain a crude lucid ganoderma polysaccharide product, subjecting the crude lucid ganoderma polysaccharide product to repeated washing and precipitation with 50 to 80% ethanol, carrying out continuous elution and dissolution by using distilled water, concentrating an eluate, adding ethanol until alcohol content reaches 70%, carrying out standing to allow a precipitate to be filtered and drying the precipitate at a low temperature so as to obtain lucid ganoderma polysaccharide; and processing a lucid ganoderma extract particle from 50 to 80% of the lucid ganoderma polysaccharide and mixing the particle and Cordyceps sinensis mycelia according to a ratio of 1: 9 so as to prepare the lucid ganoderma-Cordyceps sinensis granule. The lucid ganoderma-Cordyceps sinensis granule provided by the invention has an immunoloregulation function, enables negative conversion of a hepatitis B surface antigen to be realized and has energy invigorating, foundation strengthening, liver and kidney nourishing, blood circulation promoting and blood stasis removing effects.
Owner:朱文峰
Magnetic immunochromatographic strip for detection of hepatitis B surface antigen and preparation method thereof
InactiveCN101762701AEasy to manufactureSuitable for mass productionMaterial analysisFluoresceinHepatitis B surface antibody
The invention relates to a magnetic immunochromatographic strip for detection of hepatitis B surface antigens and a preparation method thereof. The strip is prepared through the following steps: pasting a coating film, a magnetic particle pad combined with hepatitis B surface antibodies, a sample pad and an absorbent pad on a bottom board in sequence at intervals of 2mm, and using a transparent plastic seal film to cover the upper layer. The coating film is pre-coated with hepatitis B surface antigen detection lines and quality control lines. The invention introduces the magnetic immunochromatographic technique and the fluorescein isothiocyanate system into the detection of the hepatitis B surface antigens, and has the advantages of simple operation, high sensitivity and good specificity.
Owner:CHEMCLIN DIAGNOSTICS CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com