Reagent kit for screening infectious diseases and quantitating hepatitis B surface antigen and applications
A hepatitis B surface antigen and kit technology, applied in the field of medical detection, can solve the problems of inability to quantitatively detect hepatitis B surface antigen, inconvenient, fast, and incapable of quantitative detection, and reduce the detection cost, without washing, and with high accuracy. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] This example is used to illustrate the preparation methods of the kit standard, quality control, sample buffer and positive control of the present invention.
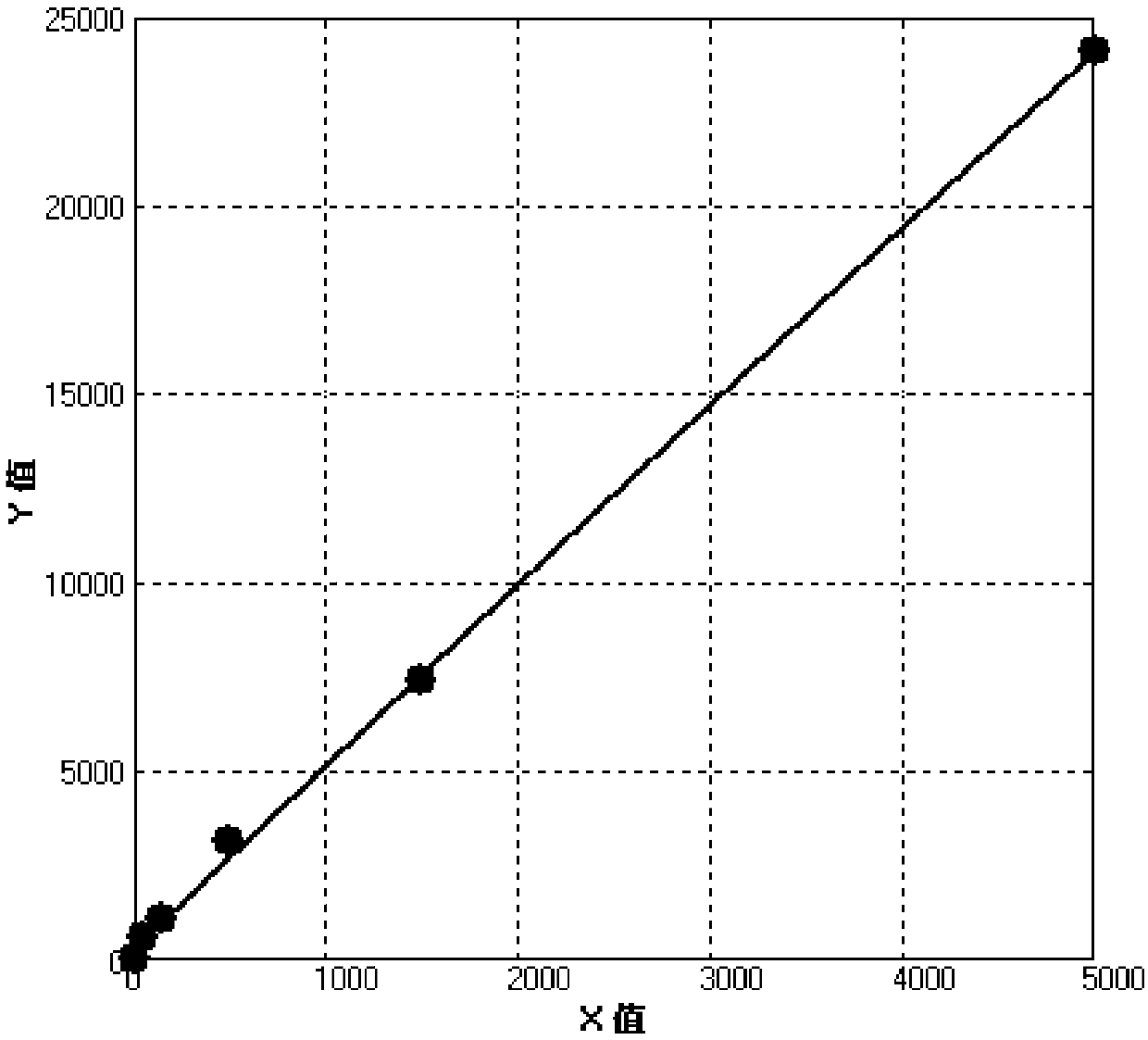
[0088] The preparation of the concentration gradient standard substance and quality control substance of sAg: with the 10mM sodium phosphate buffer solution (PH7.4) that contains 2% neonatal calf serum, 0.05% Proclin300, 5% bovine serum albumin, 0.10% Triton X-100 The purchased sAg antigen 4.4 mg / ml was formulated into concentration gradient standards with marked concentrations of 0, 50, 150, 500, 1500, 5000 ng / ml and quality controls of 80 and 3000 ng / ml; then made into lyophilized powder, 4 Store at ℃;
[0089] Preparation of sample buffer: add 1% bovine serum albumin and 0.05% Proclin 300 respectively to 100 mM phosphate buffer (pH 7.4), and mix well.
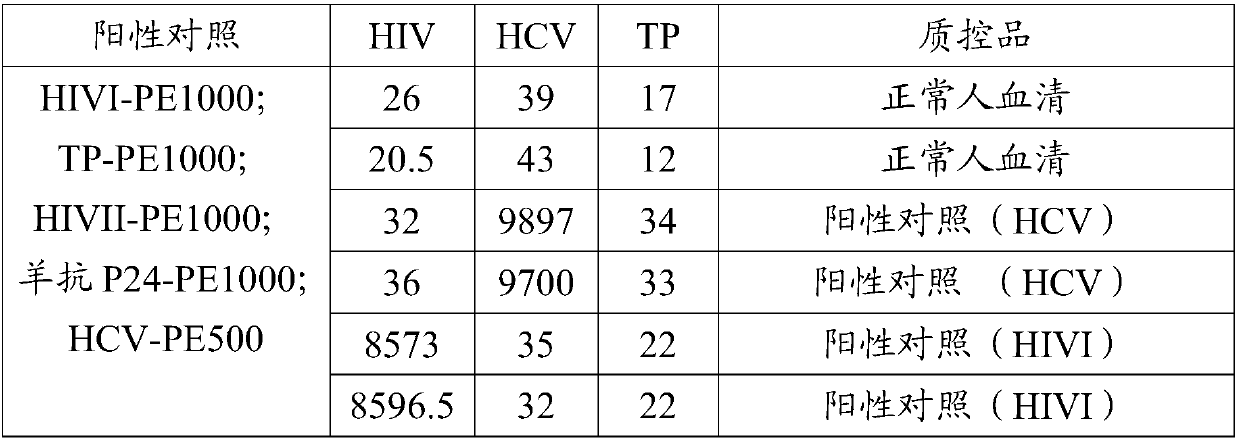
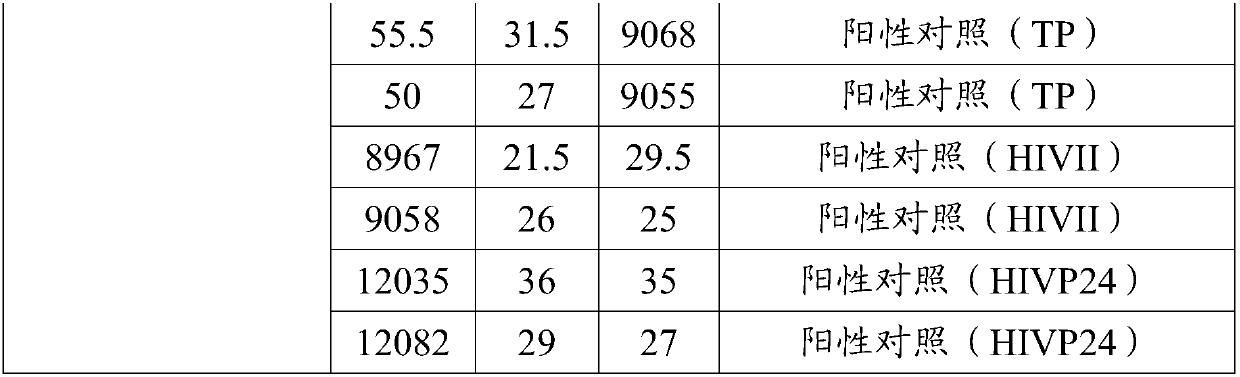
[0090] The positive controls of hepatitis C virus antibody, HIV antibody and Treponema pallidum antibody were prepared by extracting goat serum and purifying ...
Embodiment 2
[0093] This example is used to illustrate the preparation of the hepatitis B virus surface antigen qualitative single item kit.
[0094] 1. Preparation of Hepatitis B surface antibody S11 coated Luminex No. 48 magnetic microspheres.
[0095] Principle: Use EDC to activate the carboxyl groups on the surface of the magnetic microspheres, and couple with the amino groups of the S11 antibody, so that the S11# antibody is coated on the magnetic microspheres.
[0096] method:
[0097] 1. Thoroughly mix Luminex No. 48 magnetic microspheres, and take out 0.2mL 1.25×10 7 pieces / ml.
[0098] 2. Use a magnetic stand for 60 seconds to separate the magnetic microspheres, and carefully aspirate the supernatant. 3. Add 0.1mL deionized water, shake for 60 seconds, sonicate for 20 seconds, separate on a magnetic stand for 60 seconds, suck out the supernatant, and repeat 2 times. 4. Add 0.08mL 50mm sodium dihydrogen phosphate pH4.0, shake for 20 seconds.
[0099] 5. Add 0.01 mL each of 50 ...
Embodiment 3
[0114] This example is used to illustrate the preparation of HIVP24 antigen single reagent.
[0115] 1. Preparation of P24 antibody-coated Luminex No. 72 magnetic microspheres.
[0116] Principle: Use EDC to activate the carboxyl groups on the surface of the magnetic microspheres, and couple with the amino groups of the HIVP24 antibody, so that the P24 antibody is coated on the magnetic microspheres.
[0117] method:
[0118] 1. Thoroughly mix Luminex No. 72 magnetic microspheres, and take out 0.2mL 1.25×10 7 pieces / ml.
[0119] 2. Use a magnetic stand for 60 seconds to separate the magnetic microspheres, and carefully aspirate the supernatant.
[0120] 3. Add 0.1mL deionized water, shake for 60 seconds, sonicate for 20 seconds, separate on a magnetic stand for 60 seconds, suck out the supernatant, and repeat 2 times. 4. Add 0.08mL 50mm sodium dihydrogen phosphate pH4.0, shake for 20 seconds.
[0121] 5. Add 0.01 mL each of 50 mg / mL EDC and NHS and mix well.
[0122] 6. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com