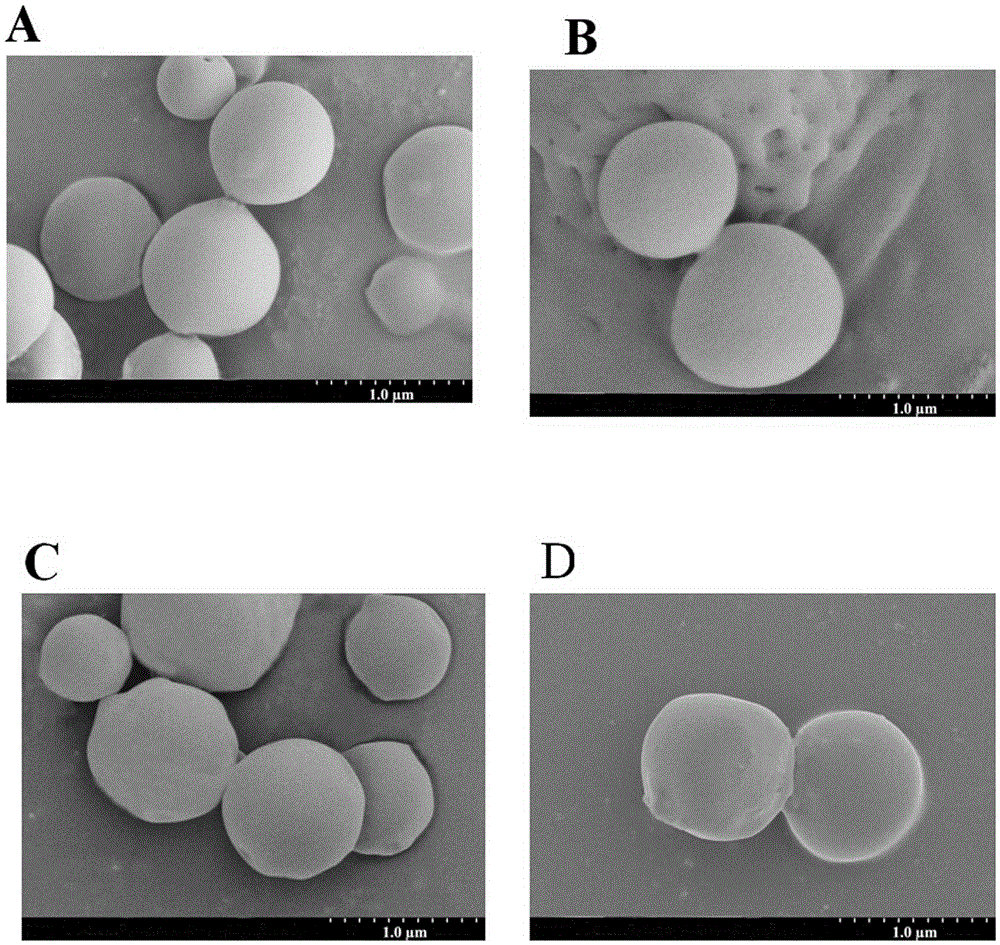
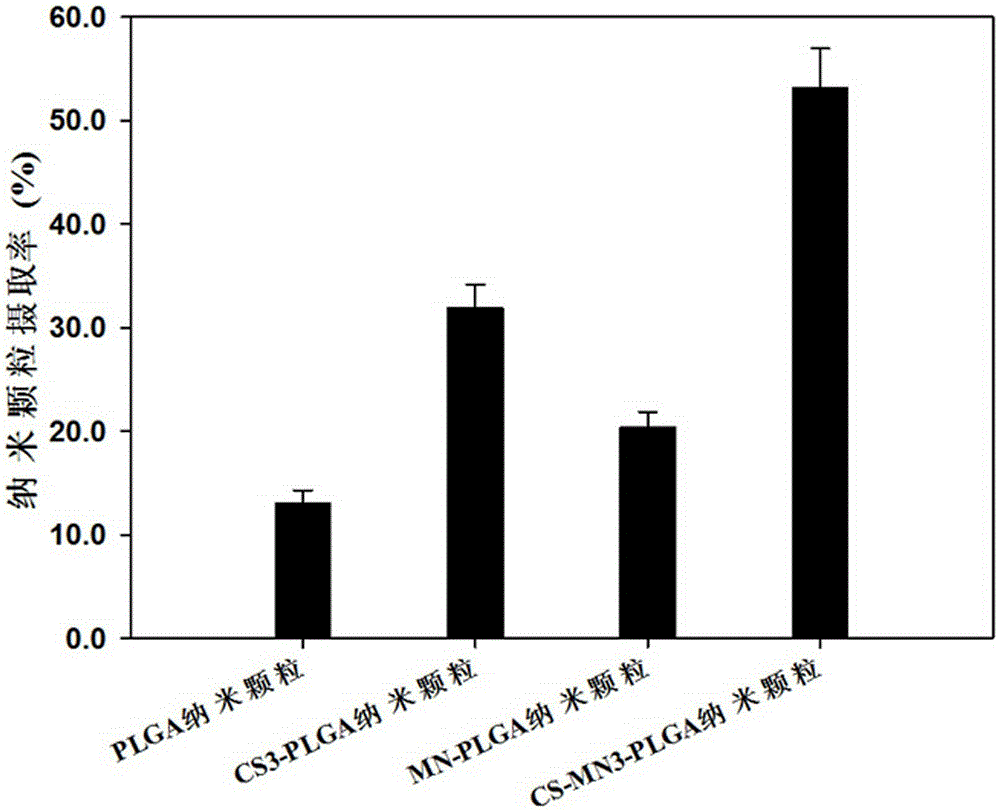
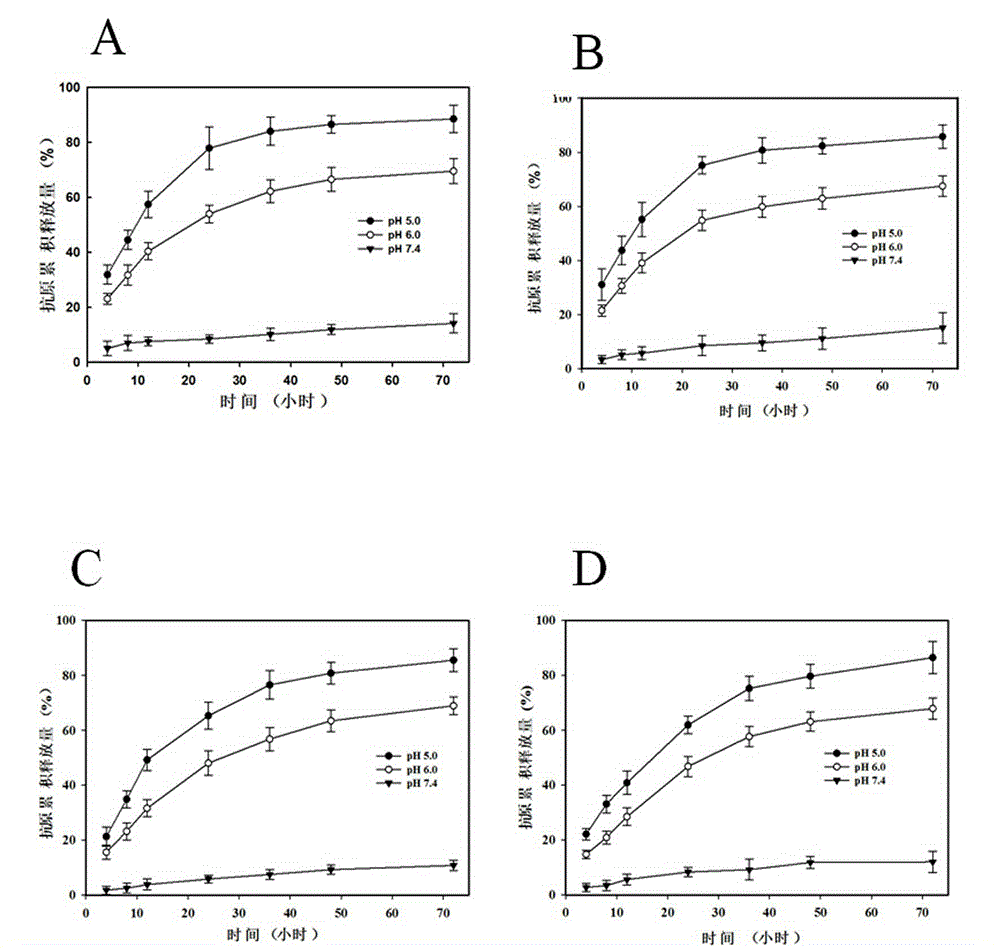
Nanoparticle vaccine preparation containing recombinant hepatitis B surface antigen and preparation method thereof
A hepatitis B surface antigen and nanoparticle technology, applied in the field of life science, can solve the problems of inability to induce mucosal immunity and cellular immunity, and achieve the effect of enhancing ability, enhancing immune response, and enhancing uptake
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0038] Preparation of encapsulated FITC-HSA nanoparticles: In the process of preparing nanoparticles, FITC-HSA is directly dissolved in the inner water phase instead of HSA, and the process remains unchanged to obtain encapsulated FITC-HSA nanoparticles
[0039] Nanoparticle uptake by macrophages: Seed macrophages in 96-well cell culture plates (approximately 5×10 5 cells / mL), 200 μL per well, cultured until the cells adhered to the wall, then added 200 μl of encapsulated FITC-HSA nanoparticle suspension, and the concentration of nanoparticles was set at 100 μg / ml. After incubating together for 12 hours, discard all the supernatant, wash twice with PBS, add cell lysate (PBS buffer containing 1% TritonX-100 and 2% SDS) to each well, and immerse in ice for 30 minutes to fully break up the macrophages , using a fluorescence spectrophotometer to measure the fluorescence intensity at 525nm to study the effect of mannan and chitosan modification on the uptake of nanoparticles by mac...
Embodiment 1
[0043] Sodium phosphate buffer solution (pH7.4) containing human serum albumin, HBsAg, sodium bicarbonate and PVA is used as the inner water phase. In this mixed solution, the concentration of HBsAg is 1mg / ml, and the concentration of human serum albumin is 5mg / ml , the concentration of sodium bicarbonate is 2.5mg / ml, and the concentration of PVA is 5.0mg / ml. Take 5.0ml of this solution and add it to 10ml containing 600mg polylactic acid-polyglycolic acid block copolymers with molecular weights of 9.0kDa, 13kDa, 23kDa, 43kDa and 87kDa (the mass ratio of polylactic acid to polyglycolic acid (PLA:PLG) is 75:25) in dichloromethane solution, the mixture was emulsified at a colostrum speed of 17,500 rpm for 2 min in a high-speed homogenizer to obtain a stable W / O colostrum. Then slowly inject the primary emulsion into 20ml of sodium phosphate buffer (pH7.0) containing 20mg / mlNaCl and 25mg / mlPVA, and emulsify at 17500 rpm for 10 minutes to form a W / O / W emulsion , add 20ml of phosph...
Embodiment 2
[0047] Sodium phosphate buffer solution (pH7.0) containing human serum albumin, HBsAg, sodium bicarbonate and PVA is used as the inner water phase. In this mixed solution, the concentration of HBsAg is 1mg / ml, and the concentration of human serum albumin is 5mg / ml , the concentration of sodium bicarbonate is 1.25mg / ml, and the concentration of PVA is 20.0mg / ml. Take 5.0ml of this solution and add it to 20ml of dichloromethane solution containing 1000mg2.3kDa polylactic acid-polyglycolic acid block copolymer (where the mass ratio of polylactic acid to polyglycolic acid (PLA:PLG) is 85:15). The mixture was emulsified with a high-speed homogenizer at a speed of 17,500 rpm for 10 minutes to obtain a stable W / O colostrum. Then slowly inject the primary emulsion into 20ml of sodium phosphate buffer (pH7.0) containing 100mg / mlNaCl and 10mg / mlPVA, and emulsify at 9500 rpm for 20 minutes to form a W / O / W emulsion , add 20ml of phosphate (pH7.4) buffer solution containing 100mg / mlNaCl t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com