Patents
Literature
102 results about "Sodium phosphate buffer" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
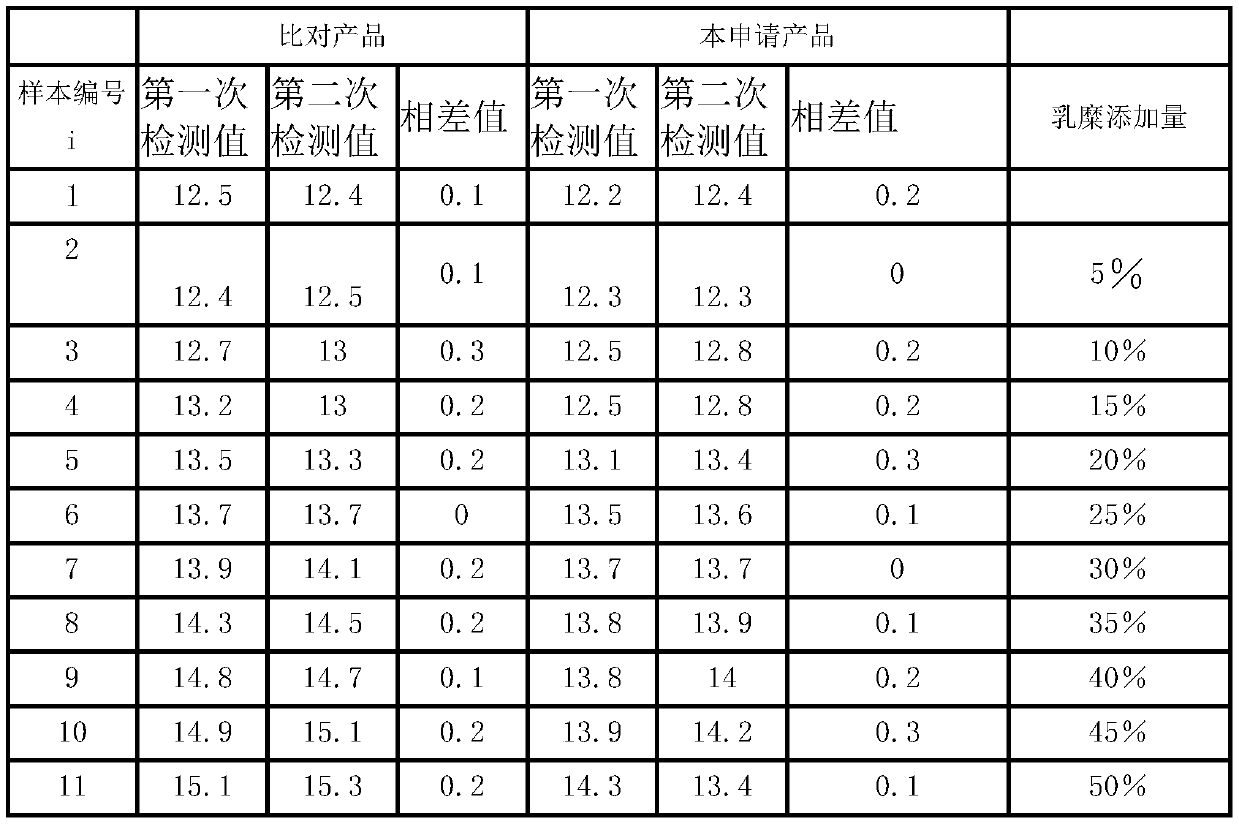
Patent Type
Patent Status
Application Year
Inventor
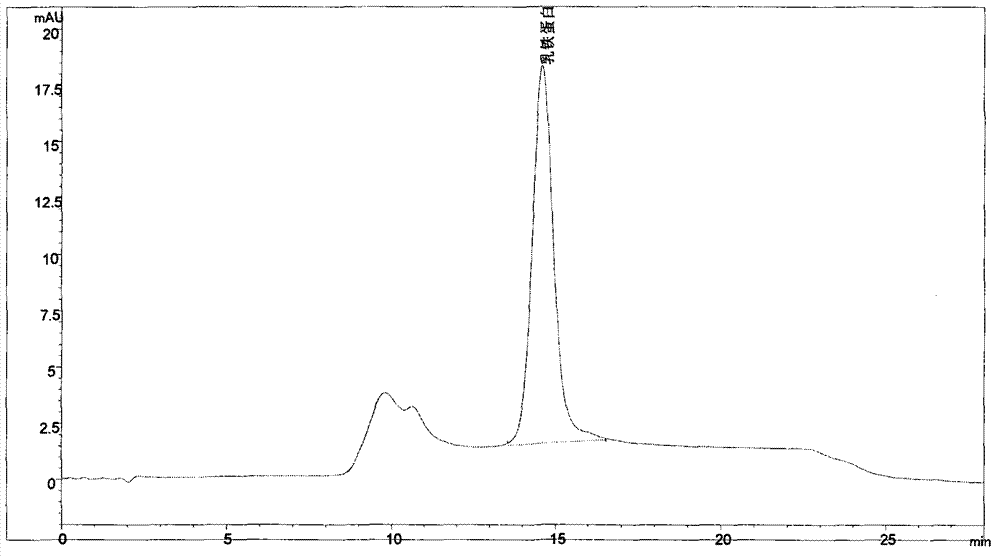
Phosphate Buffered Saline, pH 7.4 P-900 | Phosphate Buffered Saline, pH 7.4, abbreviated as PBS, is used in biological research as a buffer solution containing sodium chloride and sodium phosphate, as well as potassium phosphate and potassium chloride in some formulations. It is.
Malleable paste for filling bone defects
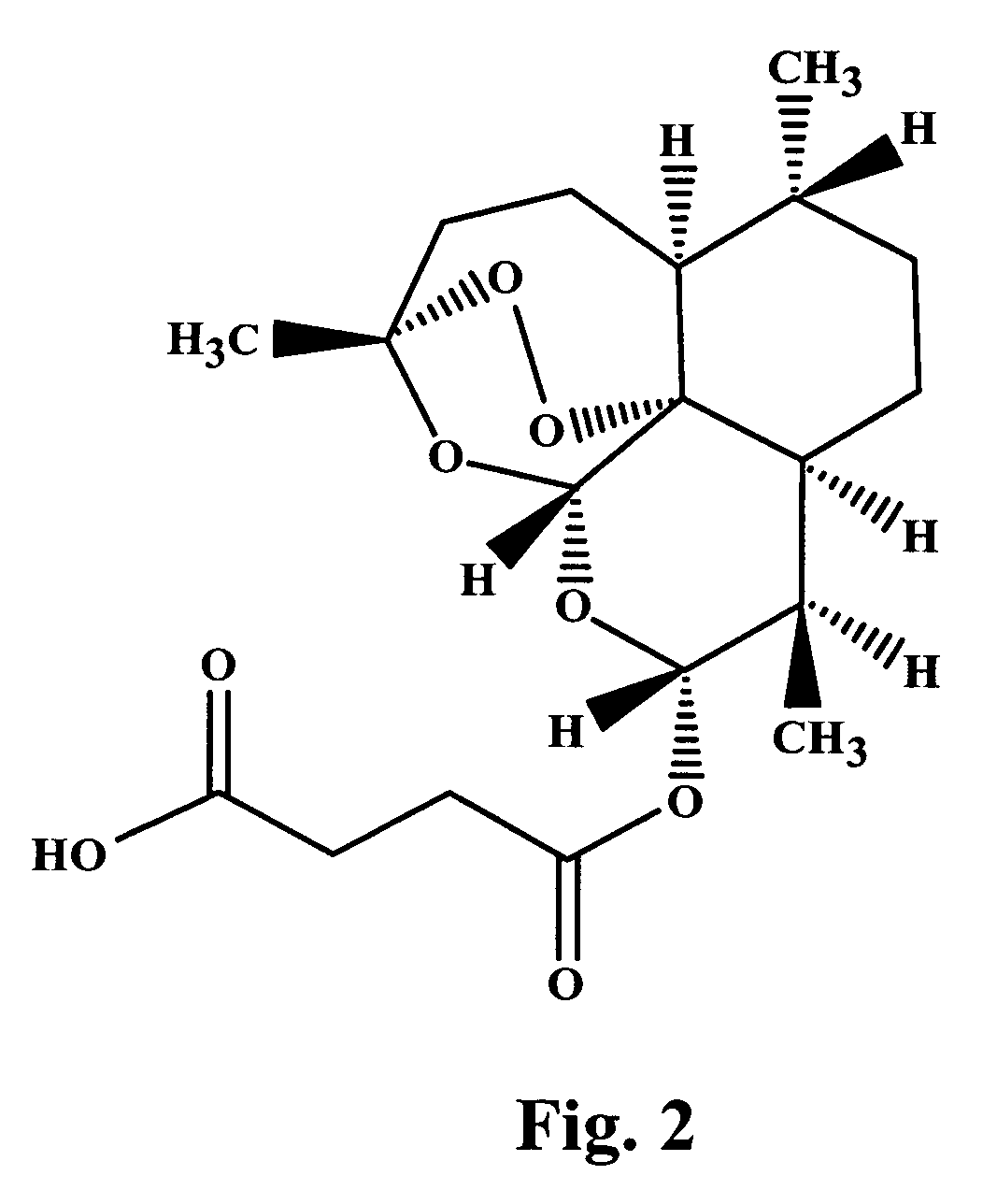
InactiveUSRE38522E1Easy to packFast absorptionSurgical adhesivesPeptide/protein ingredientsBone defectBiomedical engineering
The invention is directed toward a malleable bone putty and a flowable gel composition for application to a bone defect site to promote new bone growth at the site which comprises a new bone growth inducing compound of demineralized lyophilized allograft bone powder. The bone powder has a particle size ranging from about 100 to about 850 microns and is mixed in a high molecular weight hydrogel carrier, the hydrogel component of the carrier ranging from about 0.3 to 3.0% of the composition and having a molecular weight of about at least 10,000 Daltons. The composition contains about 25% to about 40% bone powder and can be additionally provided with BMP's and a sodium phosphate buffer.
Owner:MUSCULOSKELETAL TRANSPLANT FOUND INC
Malleable paste for filling bone defects
InactiveUSRE39587E1Useful bulk viscosityAbsorb more quicklySurgical adhesivesPeptide/protein ingredientsBone defectBiomedical engineering
The invention is directed toward a malleable bone putty and a flowable gel composition for application to a bone defect site to promote new bone growth at the site which comprises a new bone growth inducing compound of demineralized lyophilized allograft bone powder. The bone powder has a particle size ranging from about 100 to about 850 microns and is mixed in a high molecular weight hydrogel carrier, the hydrogel component of the carrier ranging from about 0.3 to 3.0% of the composition and having a molecular weight of about at least 10,000 Daltons. The composition contains about 25% to about 40% bone powder and can be additionally provided with BMP's and a sodium phosphate buffer.
Owner:MUSCULOSKELETAL TRANSPLANT FOUND INC
A liquid-based cell preservation solution
The invention relates to a pathological examination, in particular to a liquid-based cell preservation solution used in cytopathological examination of human cervical mucus, sputum, urine, pleural fluid, tracheal mucus and the like. It is characterized in that it is prepared from alcohols, sodium phosphate buffer, edetate disodium, sodium chloride 0.08%-0.12%, potassium chloride, formaldehyde, dithiothreitol, calcium acetate, magnesium acetate, etc. As a result, it can not only maintain the stability of the cell structure. It can reduce the agglomeration and precipitation of cell mucus and the loss of cell rupture, and can also make cells easy to stain, improve the clarity of cell preparation, facilitate the smooth progress of pathological examination, and the cost is low, which is conducive to popularization and use.
Owner:XIAOGAN CENT HOSPITAL +1
Preparation method for linaclotide
InactiveCN104628826AGuaranteed stabilitySuitable for oxidation capacityPeptide preparation methodsBulk chemical productionSide chainWang resin
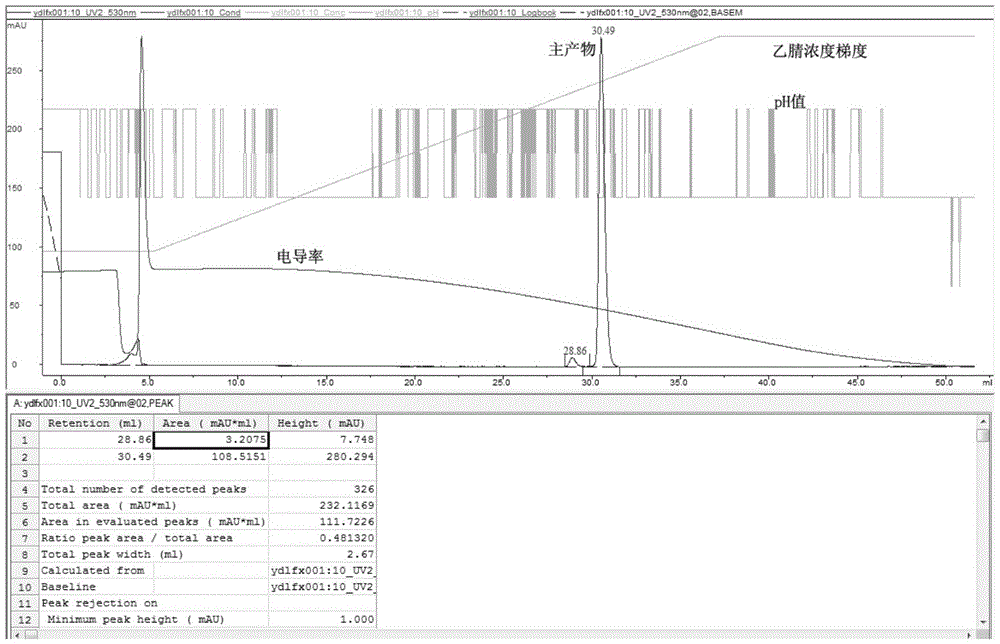
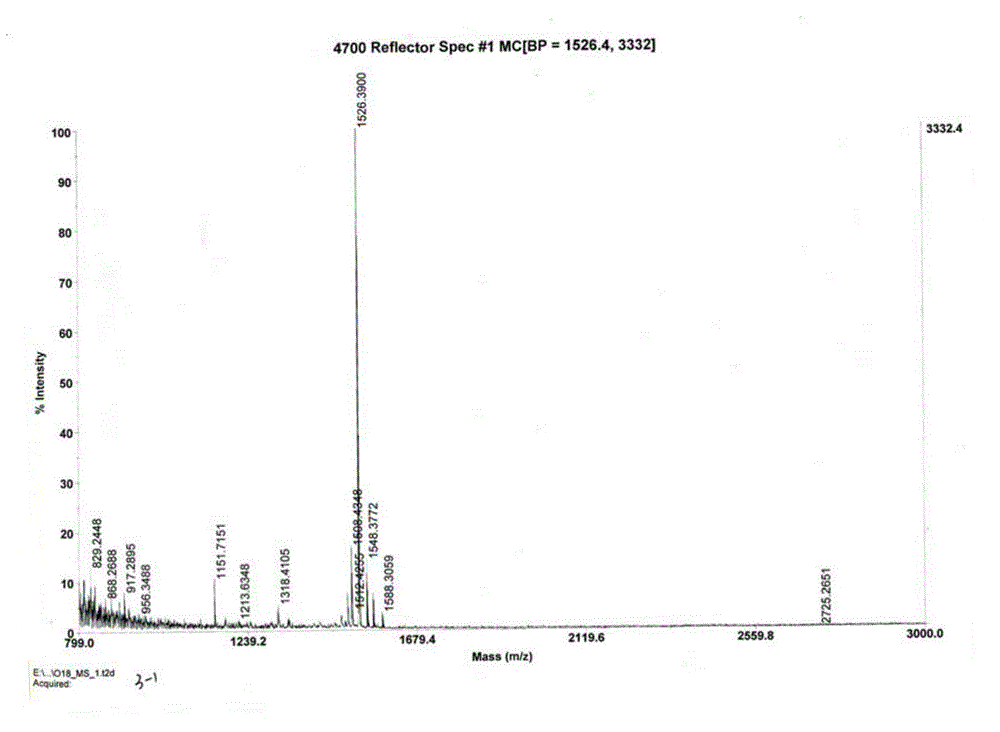
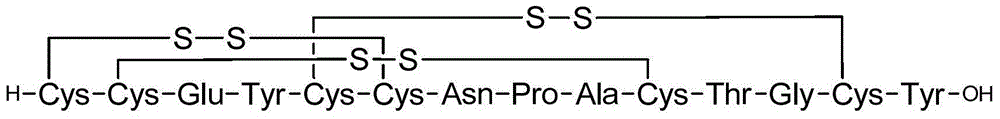
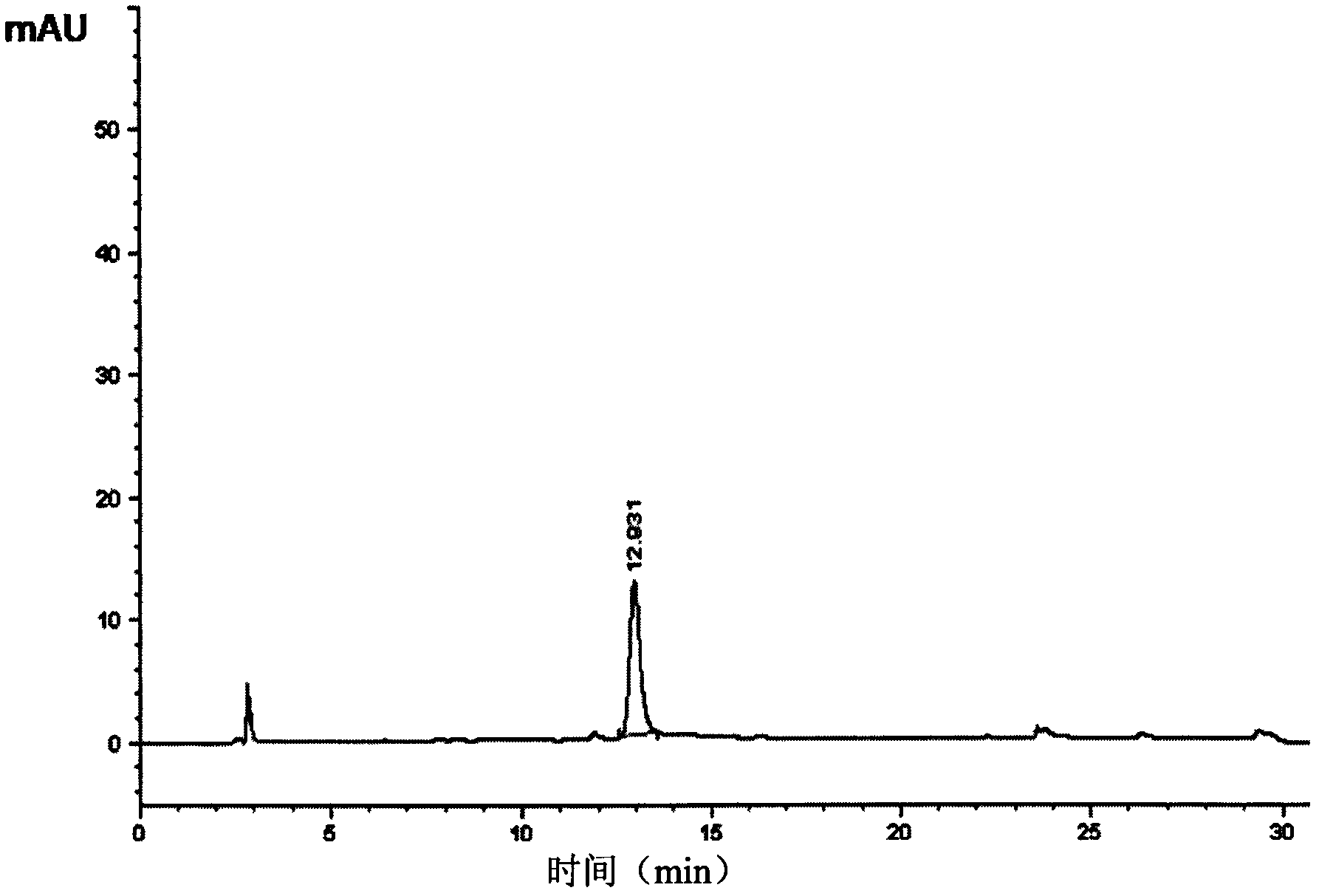
The invention provides a preparation method for linaclotide. The method includes: utilizing a standard Fmoc technology to connect a side chain protected amino acid with Wang resin, adding a condensing agent HBTU and alkali DIPEA, carrying out condensation reaction in a DMF solvent, employing a DMF solution containing 20% hexahydropiperidine to perform de-Fmoc protection, then conducting cutting from the solid phase resin, adding elemental iodine into a sodium phosphate buffer solution to carry out Cysteine oxidation, thus obtaining linaclotide. The preparation method for linaclotide provided by the invention is the technology for highyield synthesis of linaclotide, simplifies the cyclization process and enhances the cyclization yield, and the final product yield reaches 30%-60%. The preparation method has the advantages of simplicity, mild reaction conditions, high yield, and high product purity, is a feasible preparation method for industrialization of linaclotide, and provides good prospects for industrial production.
Owner:EAST CHINA UNIV OF SCI & TECH
Liquid-based cell preservation solution
InactiveCN105409925ABrightly dyedLess inflammatory cellsDead animal preservationPotassiumCervical mucus
The present invention relates to pathological examination, especially to a liquid-based cell preservation solution used in pathologically examining cells such as human cervical mucus, sputum, urine, thoracic liquid, and tracheal mucus. The liquid-based cell preservation solution is characterized by being prepared from alcohols, a sodium phosphate buffer solution, disodium ethylenediamine tetraacetate, 0.08-0.12% sodium chloride, potassium chloride, formaldehyde, dithiothreitol, calcium acetate, magnesium acetate and the like. The cell preservation solution cannot only maintain the cell structure stability and reduce the agglomerated precipitation of the cell mucus and the cell breakage loss, but also enables the cells easily to be stained, improves the clarity of the cell sheets, and facilitates smooth performance of the examination work. Moreover, the cell preservation solution is low in cost and easy to promote and use.
Owner:孝感宏翔生物医械技术有限公司
Azithromycin eye drops and preparing process thereof
InactiveCN101103992AReduce dosageEnsure clarityOrganic active ingredientsSenses disorderSide effectWhole body
The invention discloses azithromycin eye drops and the preparation. The eye drops is a stable ophthalmic preparation made by azithromycin or axithromycin salt as the active ingredient which is assisted by a stabilizer, a pH regulator, an antiseptic, an isotonic agent and a pasting agent; wherein the optimized stabilizer is propanetriol, the pH regulator is sodium phosphate buffer, the antiseptic is Ethyl p-hydroxybenzoate, the isotonic agent is sodium chloride and the pasting agent is sodium hyaluronate. Compared with oral delivery, the invention has the advantages of less dose, small side effect on a whole body, direct absorption at the ocular region, quick achieving effective antibacterial concentration and quick developing treating function; and compared with ophthalmic gel or ophthalmic ointment, the invention has the advantages of convenient application and good tolerance for a patient; thus the reasonable preparation process and stable performance of the invention guarantees the clarity and stability of eye drops.
Owner:陕西省眼科研究所
Determination method for lactoferrin content in dairy products
ActiveCN102590418ASolve for quick extractionResolve accuracyComponent separationTrifluoroacetic acidGradient elution
The invention discloses a determination method for lactoferrin content in dairy products, which includes the steps: (1) degreasing mixed liquid of dairy products and water in centrifugal mode, removing fat to obtain separated milk; (2) passing an affinity column: the separated milk passes through the pre-activated affinity column, washing the column through sodium phosphate buffer solution with pH ranging from 6.8 to 7.4, eluting with eluent (1), collecting the eluent to obtain sample introduction solution; the eluent (1) is sodium phosphate buffer solution containing 1.0 mol / L sodium chloride, and the pH of the eluent (1) is 6.8-7.4; (3) detecting the sample introduction solution through high performance liquid chromatography: eluent (2) of the high performance liquid chromatography is formed by a moving phase A and a moving phase B, the moving phase A is acetonitrile, the moving phase B is 0.1wt% trifluoroacetic acid solution, gradient elution is conducted, and a chromatographic column is a C18 chromatographic column. The determination method has the advantages of specificity and repeatability and capability of removing other disturbance objects and environment change effects and the like, reduces detecting cost, is high in accuracy, and simplifies a lactoferrin detecting method.
Owner:上海德诺产品检测有限公司
Method for preparing carboxylated modified cellulose aerogel under aqueous conditions
InactiveCN104311840AWide variety of sourcesSimple processOther chemical processesFreeze-dryingOxygen
The invention discloses a method for preparing carboxylated modified cellulose aerogel under aqueous conditions. The method comprises the following steps: 1 preparing a carboxylated modified cellulose aqueous dispersion, namely adding a microcrystalline cellulose, 2, 2, 6, 6-tetramethyl-piperidine-1-oxygen free radical (TEMPO), NaClO2 and NaClO solution to a sodium phosphate buffer solution, stirring to react for a period of time under certain conditions, adding absolute ethyl alcohol to terminate the reaction, pouring the solution into a conical flask to keep standing and layered, pouring out a supernatant, and then adding 50% ethanol and water to give the clean and thickly gelatinous carboxylated modified cellulose dispersion to replace the supernatant; and 2 carrying out freeze drying to give the carboxylated modified cellulose aerogel. The method disclosed by the invention has the advantages that the obtained carboxylated modified cellulose aerogel has the maximum Ag<+> adsorption capacity of 1.734mmol / g and the maximum Pb2<+> adsorption capacity of 1.696mmol / g.
Owner:GUANGXI UNIV
Vinorelbine long circulation liposome preparation and preparation method thereof
InactiveCN101933904AImprove stabilityEasy to useOrganic active ingredientsPharmaceutical non-active ingredientsVinorelbineLiposome
The invention relates to a Vinorelbine long circulation liposome preparation and a preparation method thereof, the preparation comprises three sub-packaging units, i.e. an empty liposome, sodium phosphate buffer solution and heavy tartaric acid Vinorelbine; and before being used, the three sub-packaging units are mixed together and heated appropriately. The preparation method has the advantages of simple process and easy operation and is suitable for industrial production, and the Vinorelbine long circulation liposome which is prepared by adopting the method has high encapsulation rate and good stability.
Owner:QILU PHARMA CO LTD
Extraction method of spirulina phycocyanin
InactiveCN107298710ASimple extraction processHigh activityPeptide preparation methodsDepsipeptidesDiseaseUltrafiltration
The invention discloses an extraction method of spirulina phycocyanin. The extraction method comprises the following steps: crude cell disruption, salting out, double-aqueous phase extraction and ultrafiltration, and is characterized in that the cell disruption step comprises the following steps: adding functional protein peptide in algae liquid, selecting a sodium phosphate buffer solution as an extraction solvent, and carrying out repeated freezing and thawing and centrifugation to obtain supernate as a crude protein extract. By adopting the extraction method, the problem of low protein extraction rate caused by low sporoderm-broken rate in the prior art is solved; the phycocyanin extracted by the extraction method provided by the invention can improve the activity of lymphocytes, comprehensively strengthens the disease prevention ability and the disease resistance ability of an organism by improving the organism immune function by a lymphatic system, also has excellent functions of oxidation resistance, radiation resistance, anti-inflammation and bacterium inhibition, and can be used in the field of feed or beverages. The extraction process of the spirulina phycocyanin is simple, is strong in operability and low in production cost, and can be used for producing the spirulina phycocyanin in batch.
Owner:浦江县欧立生物技术有限公司
Andrias davidianus collagen glycopeptide and application thereof
ActiveCN105399815ASmall granularityImprove immunityCosmetic preparationsConnective tissue peptidesFruit juiceOyster
The invention discloses Andrias davidianus collagen glycopeptide and an application thereof. The Andrias davidianus collagen glycopeptides is prepared according to the following method: Andrias davidianus skin is used as a raw material, and Andrias davidianus skin acid-soluble collagen is prepared; the Andrias davidianus skin acid-soluble collagen is used as a raw material, and Andrias davidianus skin collagen peptide is prepared; 10g of oyster polysaccharide is added into 100mL of 0.01mol / L sodium phosphate buffer whose pH value is 6.5, stirring is carried out for 2 hours, 5g of Andrias davidianus collagen peptide and 10g of oyster polysaccharide are added, stirring is continuously carried out for 2 hours, the solution is permitted to stand overnight at 4 DEG C, stirring is carried out for 4 hours at 60 DEG C, the solution is cooled to room temperature, centrifugation at a speed of 9000r / min is carried out for 30 minutes, a supernatant is obtained, the supernatant is frozen and dried to obtain the Andrias davidianus collagen glycopeptide, and the collagen glycopeptide can be applied to cosmetics and fruit juice beverages.
Owner:张家界金驰天问农业科技有限公司
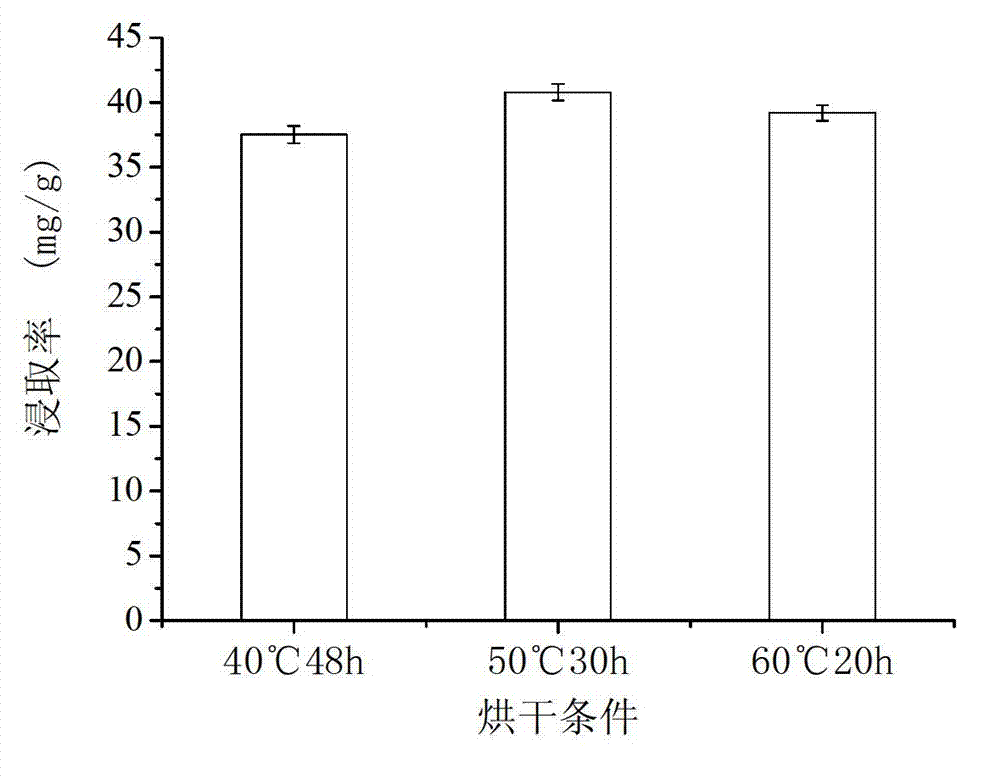
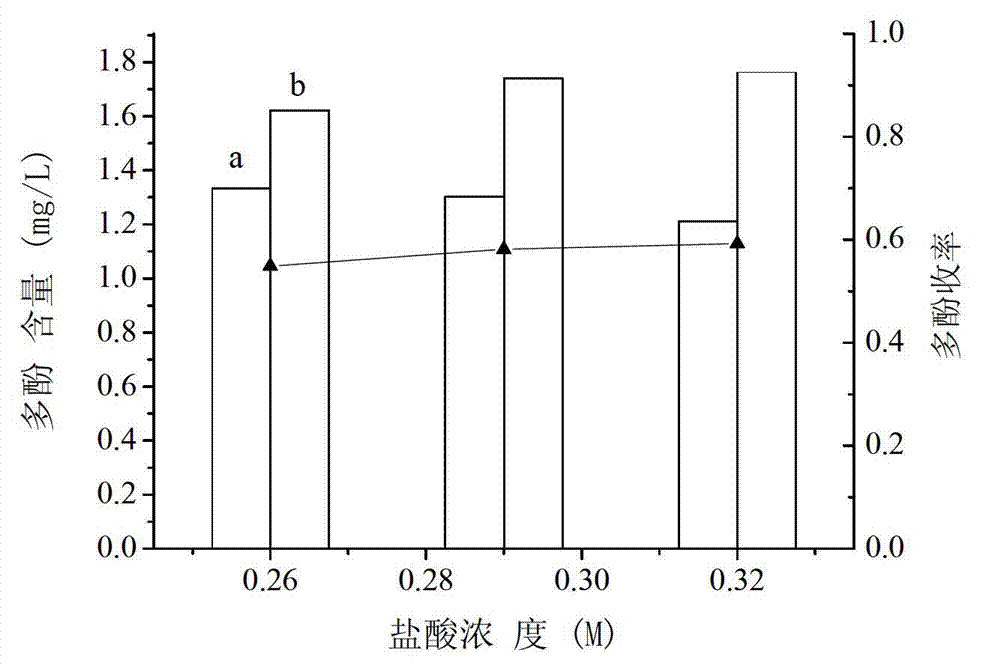
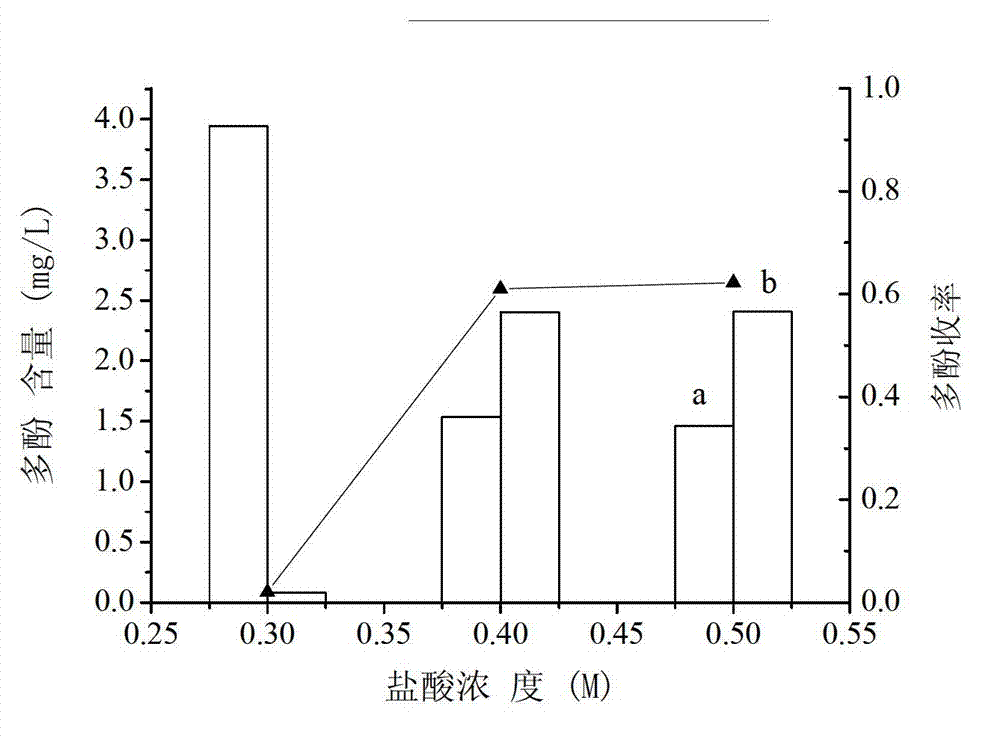
Extraction method of longan seed polyphenol
InactiveCN102961486AReduce oxidase activityReduce pollutionCosmetic preparationsToilet preparationsEconomic benefitsPolyphenol
The invention relates to an extraction method of longan seed polyphenol, relating to a processing method of a longan. The extraction method of the longan seed polyphenol can enhance the economic benefit of a longan industry and increase the income of fruit growers. The extraction method of the longan seed polyphenol comprises the following steps of: drying cleaned longan seeds; smashing raw material longan seeds to obtain longan seed powder; adding a sodium phosphate buffer solution to the longan seed powder to obtain a leach solution; pouring out the leach solution, filtering, removing leaching residues, and recovering immersion liquid; concentrating obtained filter liquor to obtain a concentrated solution; adding hydrochloric acid to the concentrated solution, stirring, and then standing for precipitation to obtain a precipitation solution; centrifugalizing the precipitation solution, removing a supernatant, washing precipitates by using the hydrochloric acid, and then drying to obtain fine longan seed polyphenol powder.
Owner:XIAMEN UNIV
Pepsinogen II detection kit and preparation method thereof
PendingCN111122866AElimination of chylolysisGuaranteed stabilityDisease diagnosisPepsinogen IPepsinogen II
The invention discloses a pepsinogen II detection kit, which comprises a reagent R1 and a reagent R2, wherein the reagent R1 is prepared from 50-150 mmol / L of sodium phosphate buffer solution, 2-3 g / Lof bovine serum albumin, methylisothiazolinone with the concentration of 2-3%, 1.0-5.0 g / L of fatty alcohol and ethylene oxide condensate, 3-5 mmol / L of magnesium chloride and 20-30 mmol / L of ammonium formate, and the balance of deionized water.; and the reagent R2 is prepared from 1.3-2 mg / ml of pepsinogen II antibody emulsion solution, 2-3 g / L of bovine serum albumin, methylisothiazolinone withthe concentration of 2-3%, and the balance of deionized water. The prepared pepsinogen II detection kit and the preparation method of the pepsinogen II detection kit have the advantages that the chyle problem can be effectively solved, the anti-interference capability is high, and meanwhile, the stability and the sensitivity are high.
Owner:浙江强盛生物科技有限公司
Preparation method of pH responsive material
The invention discloses a preparation method of a pH responsive material. The method comprises the following steps: adding a certain mass of starch and TEMPO (Tetramethylpiperidinooxy), NaClO2 and NaClO solutions into a sodium phosphate buffer solution, stirring and reacting for 24 hours under certain conditions, adding anhydrous ethanol to terminate the reaction, pouring the solution into a conical flask to stand and layer, pouring away supernatant liquid, replacing with ethanol with a volume concentration of 50 percent and water, obtaining a clean and thick gelatinous carboxyl modified starch solution, drying and finally obtaining a product. The preparation method has the benefits that 1, the starch is taken as a raw material in the preparation method, is wide in source and low in cost, still returns to the nature in a form of carbon dioxide and water after degradation and is a natural renewable material which is zero-pollution; 2, the preparation process is simple, the product biocompatibility is optimal, the degradation is complete or a degradation product has no harm to bodies, and the preparation method can be widely applied to the fields of material science, biology and medicine.
Owner:GUANGXI UNIV
L-aspartic acid making process
InactiveCN104531797AImprove stabilityStrong oxidation abilityMicroorganism based processesOn/in organic carrierL-AspartateDistilled water
The invention discloses an L-aspartic acid making process which comprises the following steps: (1) strain selection; (2) cell immobilization; (3) conversion; (4) modification, to be more specific, dissolving 1g of dextran in 15ml distilled water, dissolving a certain amount of NaIO4 in 10ml hot water, mixing two solutions, reacting at the temperature of 4 DEG C in a refrigerator for 18h, after dialyzing for 4h with distilled water, dialyzing with 5L of 0.1mol / L sodium phosphate buffer fluid with the pH of 7.4, adding the dialyzed liquid into a conversion solution in the step (3), and fully mixing to obtain a mixed liquid; (5) preparation of a crude product; and (6) crystallization; the mass L-aspartic acid can be produced in a short period, after modification, antigenicity is weak, the human body immune response may not be caused, drug efficacy is good, production is fixed, stability is good, and the L-aspartic acid making process is suitable for popularization and application.
Owner:GUANGXI UNIV
A method of efficiently catalyzing dehydroepiandrosterone by utilization of a resting cell of colletotrichum linli
InactiveCN103911418AIncrease profitPromote growthMicroorganism based processesFermentationResting CellColletotrichum
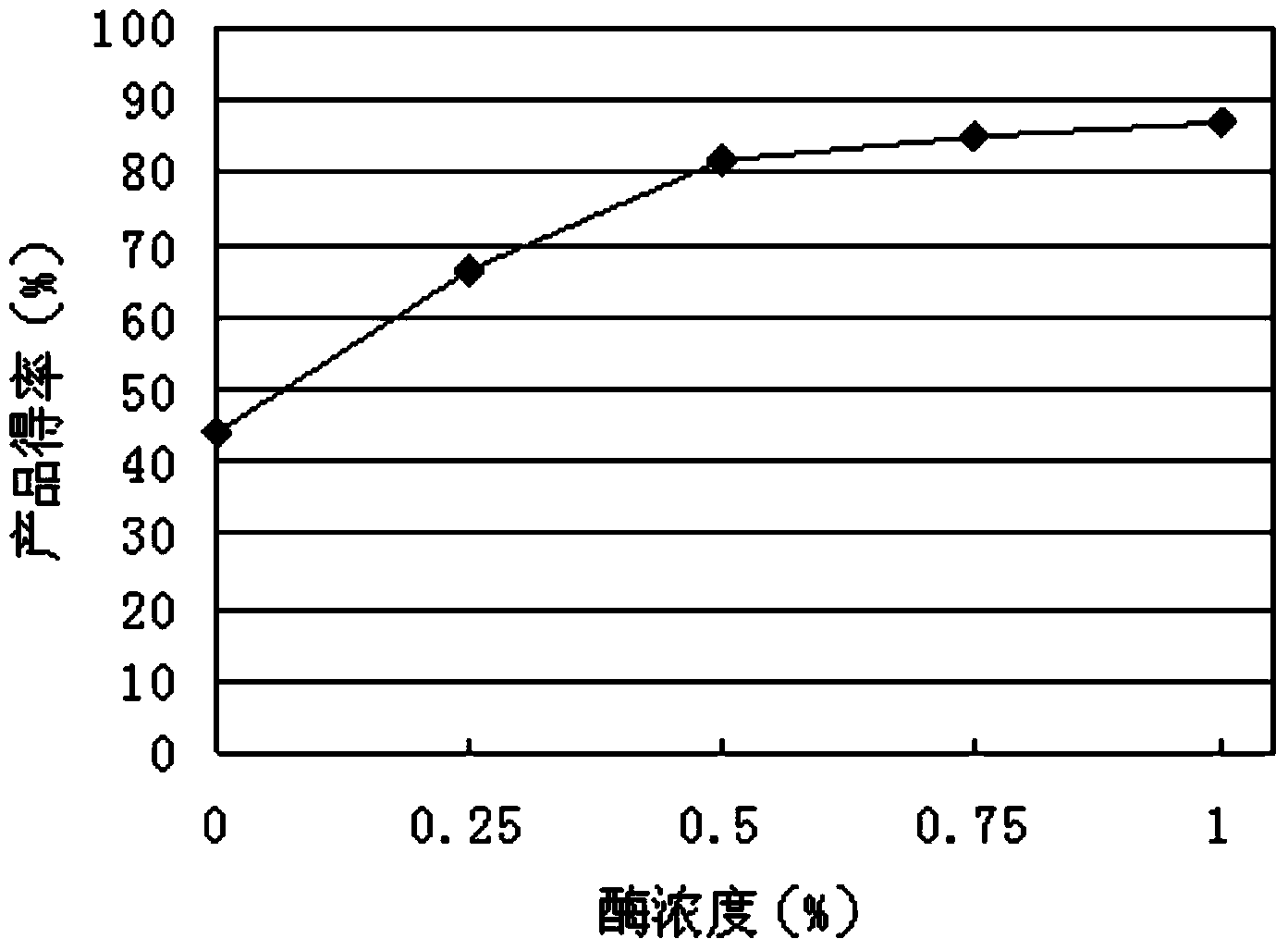
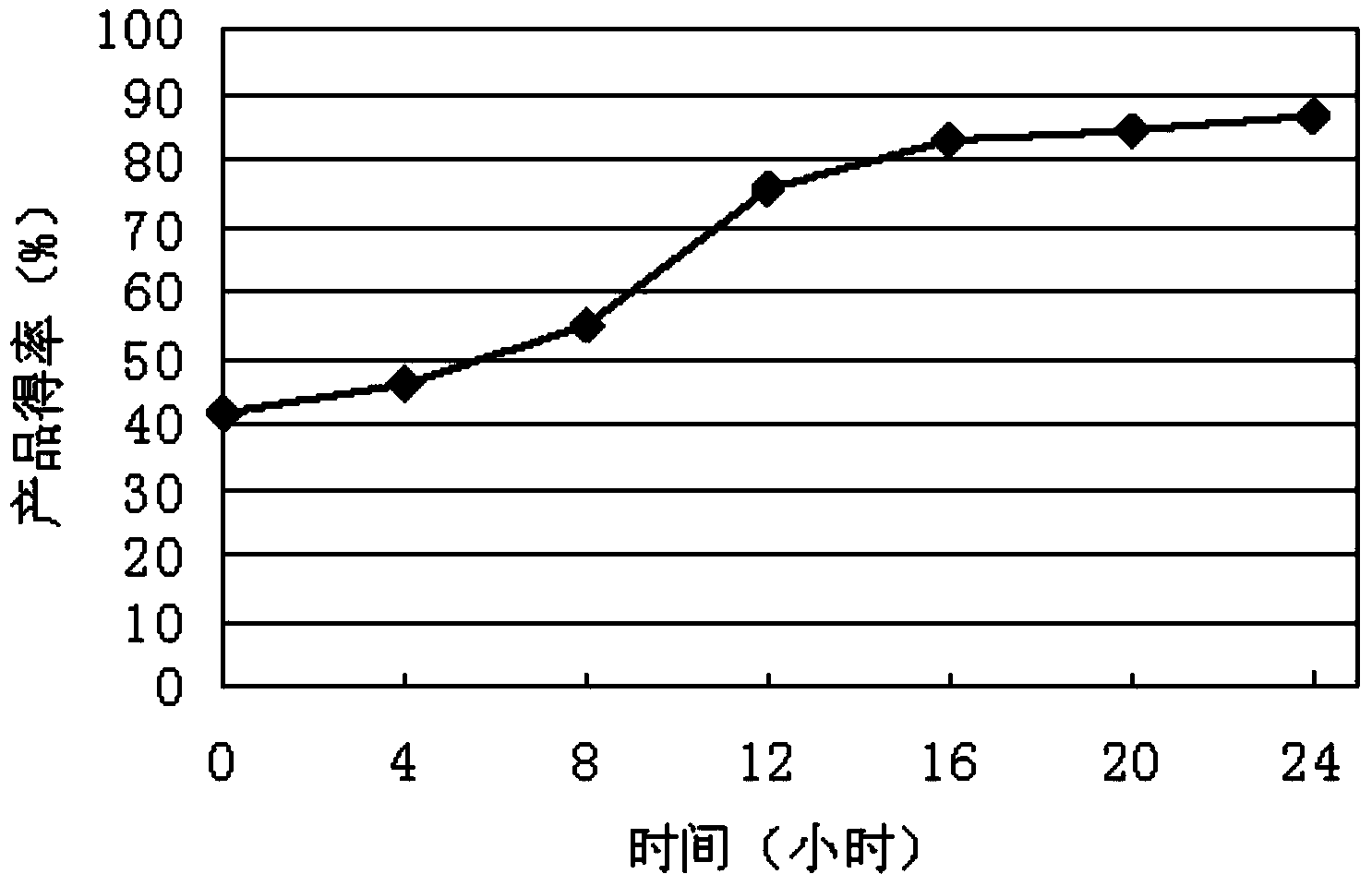
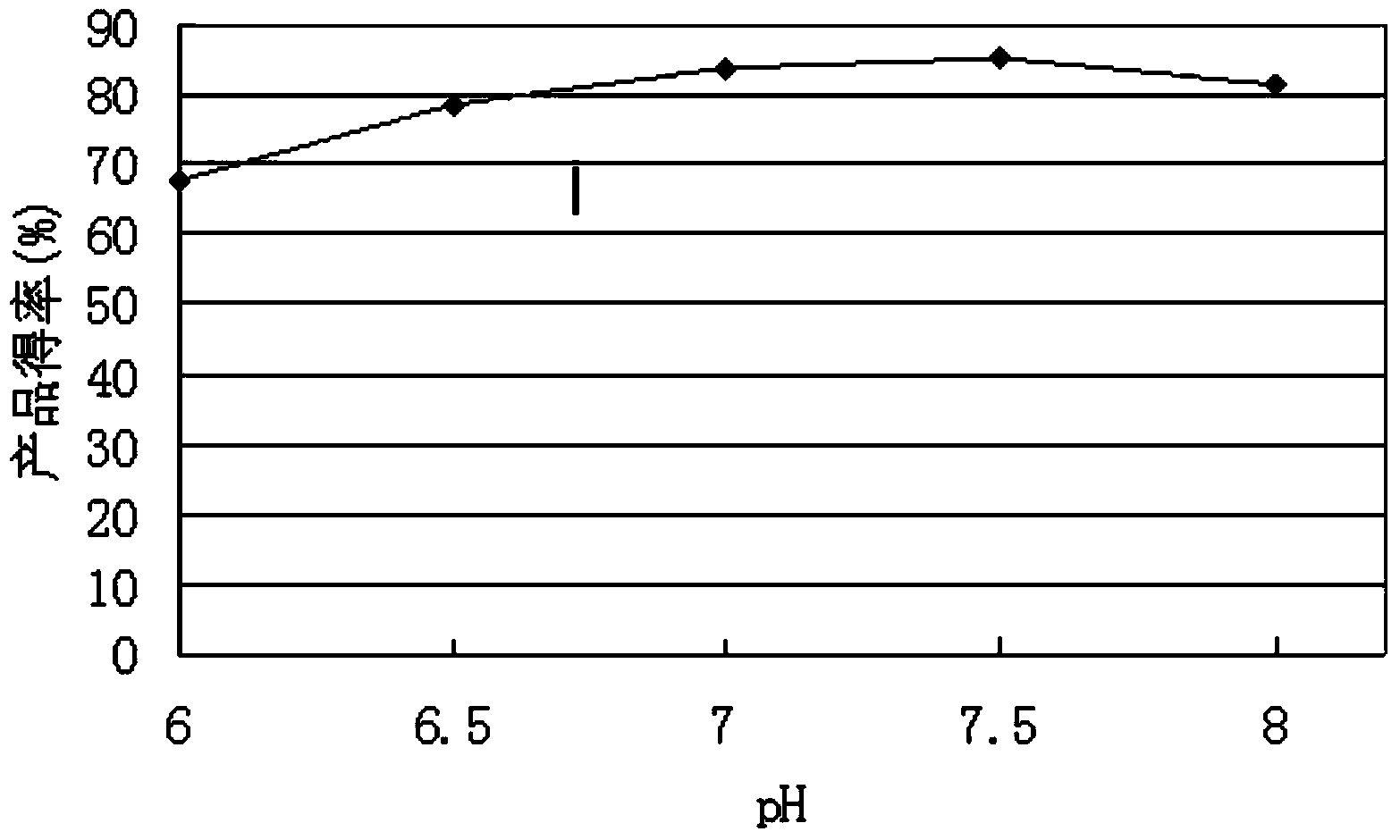
The invention discloses a method of efficiently catalyzing dehydroepiandrosterone by utilization of a resting cell of colletotrichum linli ST-1. The method combines resting cell conversion and substrate assisted solubilization, and increases the utilization rate of a substrate and the product yield. A sodium phosphate buffer provides a stable pH environment for the conversion reaction. Tween 80 can assist solubilization and promote thallus growth. By utilization of a novel conversion process, the molar product yield is increased by 78.3% than that of traditional conversion methods. The conversion rate of the product in the method is 90.4% while the conversion rate of the product of the traditional methods is 67.3%.
Owner:JIANGNAN UNIV
Pepsinogen I detection kit and preparation method thereof
PendingCN111122867AElimination of chylolysisGuaranteed stabilityDisease diagnosisPepsinogen IAntiendomysial antibodies
The invention discloses a pepsinogen I detection kit, which comprises a reagent R1 and a reagent R2, wherein the reagent R1 is prepared from 50-150 mmol / L of sodium phosphate buffer solution, 2-3 g / Lof bovine serum albumin, methylisothiazolinone with the concentration of 2-3%, 1.0-5.0 g / L of fatty alcohol and ethylene oxide condensate, 3-5 mmol / L of magnesium chloride and 20-30 mmol / L of ammoniumformate, and the balance of deionized water.; and the reagent R2 is prepared from 1.3-2 mg / ml of pepsinogen I antibody emulsion solution, 2-3 g / L of bovine serum albumin, methylisothiazolinone with the concentration of 2-3%, and the balance of deionized water. The prepared pepsinogen I detection kit and the preparation method of the pepsinogen I detection kit have the advantages that the chyle problem can be effectively solved, the anti-interference capability is high, and meanwhile, the stability and the sensitivity are high.
Owner:浙江强盛生物科技有限公司
Chlorella polysaccharide
InactiveCN107573430AHigh yieldSimple extraction processImmunological disordersAntineoplastic agentsAlcoholFreeze-drying
The invention provides a chlorella polysaccharide and belongs to the technical field of polysaccharide extraction. The extraction of the chlorella polysaccharide comprises the following steps: (1) preparing an alga solution by chlorella powder, adding an active polypeptide, selecting a sodium phosphate buffer solution as an extracting agent, repeatedly performing freezing and thawing, ultrasonic treatment and centrifuging, and taking supernate to obtain a crude protein extracting solution; (2) performing vacuum concentration on the supernate until the volume of the supernate is 1 / 10-3 / 10 of the volume of the supernate before the vacuum concentration, and performing alcohol precipitation and centrifuging to obtain a crude polysaccharide; (3) performing redissolving and removing proteins byusing a trichloroacetic acid method; and (4) performing vacuum concentration and freeze drying to obtain the chlorella polysaccharide. The extracted chlorella polysaccharide provided by the inventionhas excellent effects of promoting organism immunity and the resisting tumors and can be used in health care products. The extraction process of the chlorella polysaccharide is simple, the operabilityis high, and the production cost is low.
Owner:兰溪市哥特生物技术有限公司
Methods for the formulation and manufacture of artesunic acid for injection
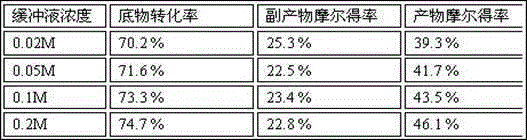
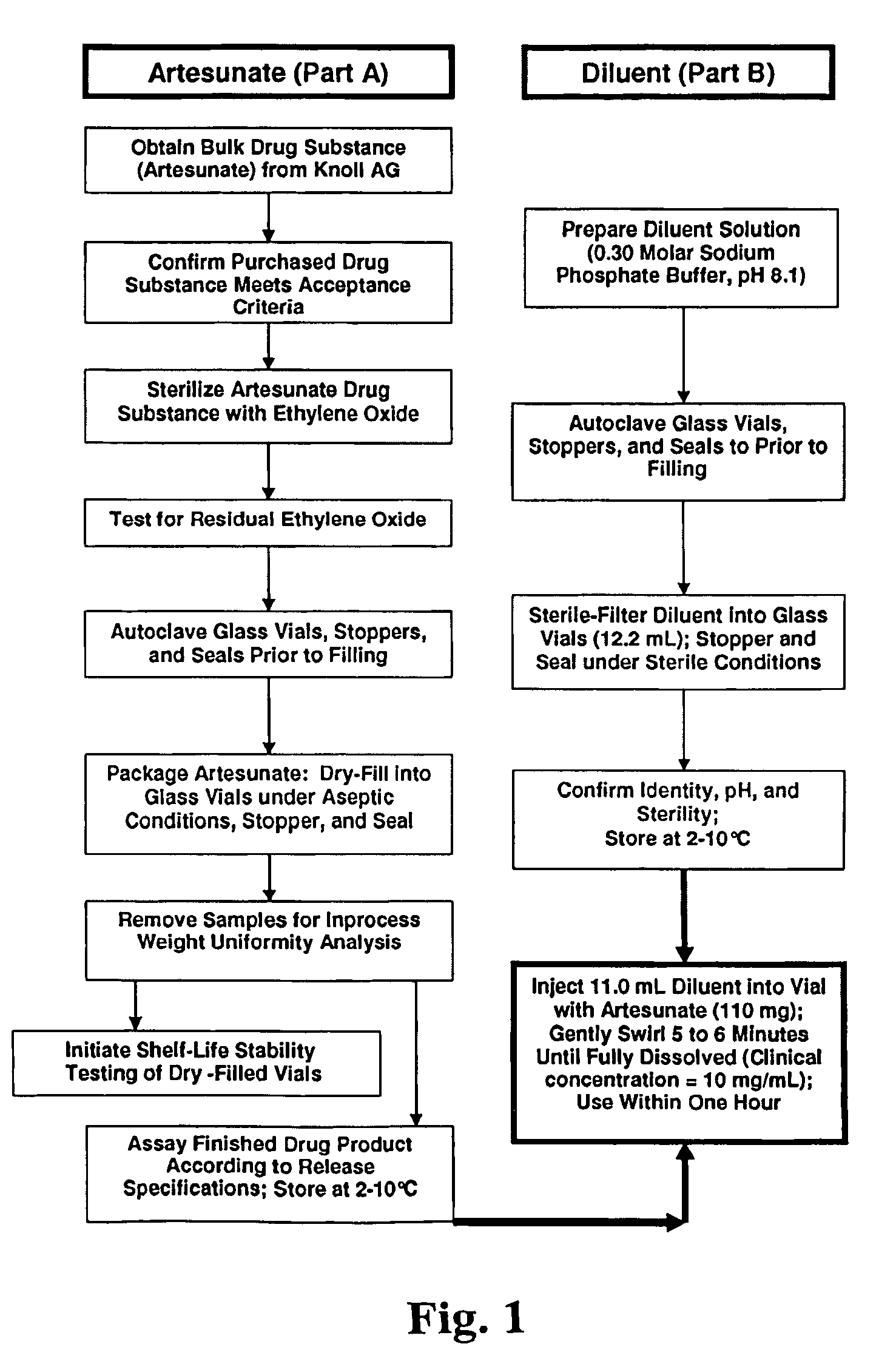
A method for the manufacture of a sterile intravenous or intramuscular formulation of artesunic acid and the formulation are the subject of this invention. First the artesunic acid powder is sterilized with ethylene oxide and placed into a sterile container. The contained sterilized powder is then dissolved in sterile sodium phosphate buffered solution to produce an injectable intravenous or intramuscular formulation. The sodium phosphate dissolves and dilutes the artesunic acid powder without caking or frothing resulting in an improved drug product. The invention also relates to the formulation and a method of treating a patient with either uncomplicated or severe and complicated malaria.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Preparation method of yeast extract
The invention discloses a preparation method of a yeast extract. N-PNGase F is selected and used in the preparation method. The preparation method comprises the following steps: (1) adding 50mM of a sodium phosphate buffer solution with the pH value of 7-8 into 19g-21g of yeast paste to make up the volume to be 100ml; suspending the yeast paste in the sodium phosphate buffer solution to obtain a yeast solution; and (2) adding 0.5g-1.0g of N-PNGase F into the yeast solution, enzymatically hydrolyzing at the speed of 120-180rpm and the temperature of 36.5-37.5 DEG C for 16-24 hours, adding 0.8g-1.2g of sodium chloride, adjusting the pH value to be 6.0, autolyzing at the constant temperature of 45-55 DEG C for 18-28 hours, performing enzyme deactivation, centrifuging at the speed of 3,500-4,000rpm for 13-17 minutes, collecting supernatant liquid, and drying to the constant weight at 105 DEG C to obtain the yeast extract.
Owner:HANGZHOU JINGYINKANG BIOTECH CO LTD
Preparation method of (S)-N-Boc-3-hydroxypiperidine
InactiveCN107841516AConducive to loadIncrease contactOxidoreductasesOn/in organic carrierDistillationPhosphoric acid
The invention discloses a preparation method of (S)-N-Boc-3-hydroxypiperidine, wherein the preparation method includes the following steps: adding N-Boc-3-piperidone and water into a reaction kettle,adding a sodium phosphate buffer solution, mixing and stirring evenly, adding glucose, then adjusting the pH to 7 with phosphoric acid, controlling the temperature to 25-35 DEG C, then adding immobilized mixed enzyme particles prepared in the step (2), stirring, carrying out a reaction for 24 h, followed by, filtering, recycling the immobilized mixed enzyme particles, extracting the filtrate withethyl acetate, carrying out reduced pressure distillation, and recrystallizing with petroleum ether to obtain (S)-N-Boc-3-hydroxypiperidine. The preparation method has the advantages of simple operation, mild conditions, fewer by-products, high product purity and relatively high product yield.
Owner:ITIC MEDCHEM CO LTD
High-performance affinity chromatography for determining lactoferrin in dairy product
InactiveCN102854262AStrong specificityQuantitatively accurateComponent separationGradient elutionChromatography column
The invention discloses a high-performance affinity chromatography method for determining lactoferrin in a dairy product. The method comprises the steps of: (1) extracting the dairy product with water, adjusting pH value by organic acid to precipitate casein, and centrifuging to obtain a test solution; and (2) separating and detecting the test solution by a high-performance liquid chromatography system, by using an affinity chromatography column as a chromatographic column, and a diode array detector as a detector. A mobile phase of the high-performance liquid chromatography system comprises A:0.01mol / L of a sodium phosphate buffer solution with pH of 7.0, and B:0.01mol L of a sodium phosphate buffer solution with pH of 7.0 and containing 1mol / L sodium chloride; and gradient elution is employed. A correlation coefficient of a regression equation of the method is higher than 0.999, and a minimum detectable amount is 2mg / 100g. The invention adopts the affinity chromatography method to solve the problems of complex sample matrix and difficulty in accurate quantifying, and has technical characteristics of simple pretreatment, good separation effect, strong specificity, high accuracy, and little change caused by environmental influence.
Owner:青岛谱尼测试有限公司
Method for measuring content of cozymase I and derivative thereof
ActiveCN1865928AMicrobiological testing/measurementColor/spectral properties measurementsHydrazine compoundPyrophosphate
The disclosed content detection method for coenzyme I and its derivative comprises: preparing sodium pyrophosphate buffer with 8.0-11.0 pH value contained 0.01-0.3v% hydrazine hydrate; adding substrate solution and the target solution; with a spectrophotometer, after the maximal absorption peak, adding 3alpha-steroid dehydrogenase till absorbance stopping; calculating the content according to absorbance calculation.
Owner:阿里生物技术泰州有限公司
Pharmaceutical composition of humanized antibody for vascular endothelial growth factor
ActiveCN105435221AImprove stabilityInorganic non-active ingredientsAntibody ingredientsChemical industryHumanized antibody
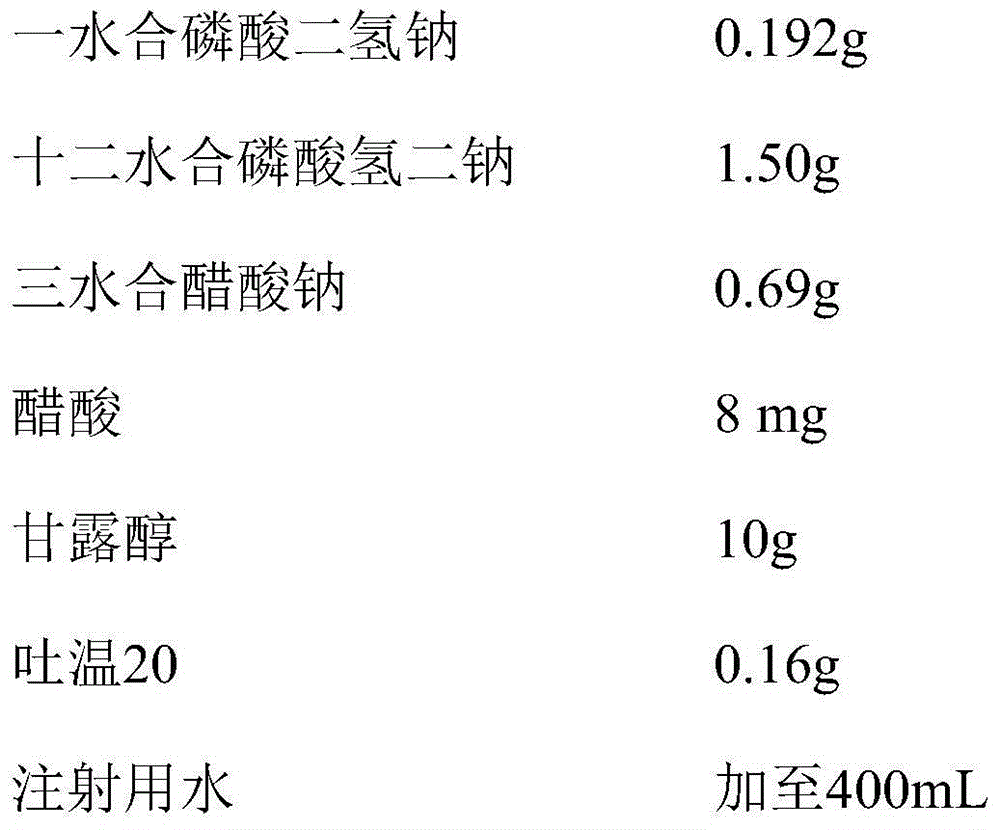
The invention belongs to the fields of medicine chemical industry, relates to a pharmaceutical composition of a humanized antibody for a vascular endothelial growth factor, and specifically relates to a stability-improving bevacizumab pharmaceutical composition. The pharmaceutical composition adopts a combination buffer system of a sodium phosphate buffer agent and a second buffer agent, and adopts one or two of mannitol or sodium chloride as an osmotic pressure regulator; compared with Avastin only adopting a sodium phosphate buffer agent and adopting alpha,alpha-trehalose as an osmotic pressure regulator, the pharmaceutical composition has polymers and degraded materials significantly reduced and has the stability significantly improved, so that the pharmaceutical composition is particularly suitable for requirements of mass production and long-term storage.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
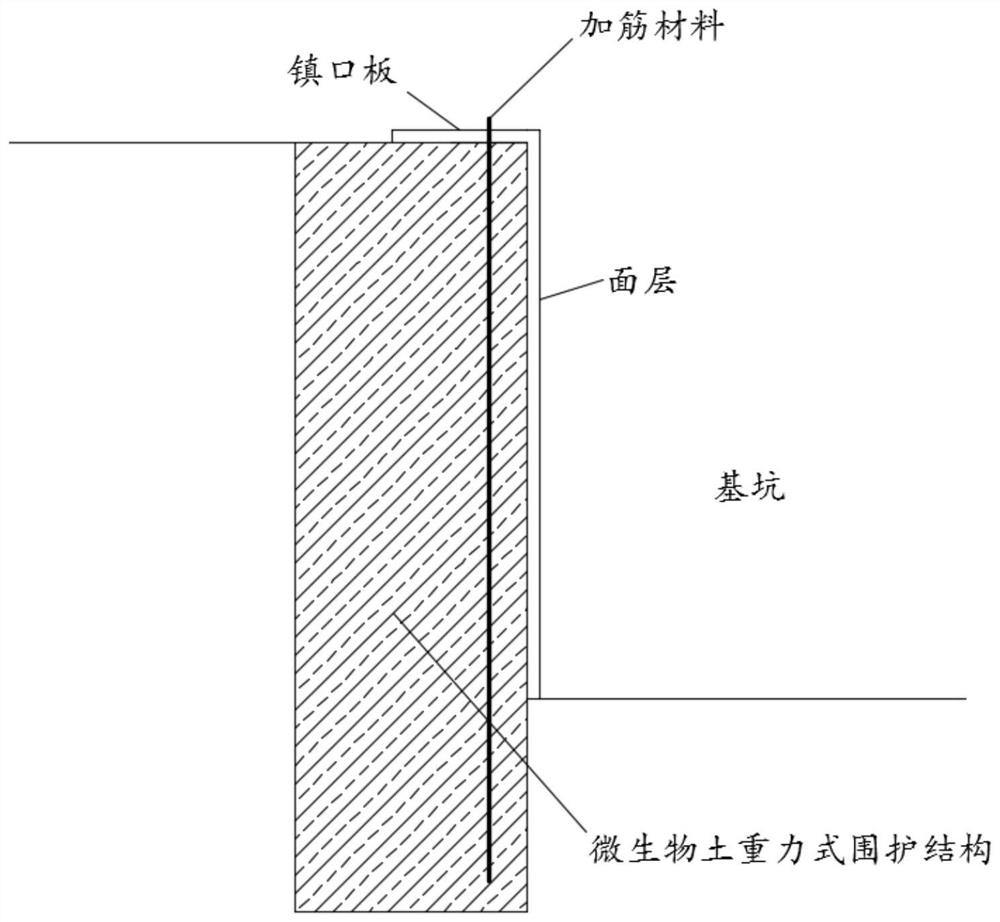
Foundation pit microbial soil gravity type enclosure structure and construction method thereof
ActiveCN113417295AIncreased durabilityUniform curingBacteriaLandfill technologiesMicroorganismSoil science
The invention provides a foundation pit microbial soil gravity type enclosure structure and a construction method thereof. According to the microbial soil gravity type enclosure structure, a stirring pile machine or a high-pressure jet grouting machine stirs and solidifies a composite bacteria solution, a cementation solution and an immobilization material with a soil body to form a continuously-lapped microbial soil columnar reinforced body retaining wall; the immobilization material is formed by mixing straw fiber powder and expanded perlite powder according to the mass ratio of (5-8) : (1-3); and the cementation solution is a cementation solution containing 0.05-0.1 mol / L of a sodium phosphate buffer solution, and the pH value of the sodium phosphate buffer solution is 5.5-6.8. According to the enclosure structure and the construction method thereof, the composite bacteria solution, the cementation solution and the immobilization material are stirred and solidified with the soil body, so that the cementation reaction rate induced by microorganisms can be effectively improved, and the uniformity and durability of the solidified soil body are improved.
Owner:HAINAN UNIVERSITY
Phycocyanin hypoglycemic polypeptide
InactiveCN107312085ALower blood pressureLower blood fatMetabolism disorderMicroorganism lysisProtein solutionFreeze-drying
The invention discloses a phycocyanin hypoglycemic polypeptide. The phycocyanin hypoglycemic polypeptide is obtained after the following steps: 1) cell disruption: adding a functional protein peptide into an algae solution, selecting a sodium phosphate buffer solution as an extraction solvent, and performing repeated freezing and thawing and centrifugation to obtain a supernatant namely a protein crude extract; 2) salting-out: adding a (NH4)2SO4 solution into the protein crude extract, performing standing at 3-5 DEG C for 11-13h, and performing centrifugation to obtain a salting-out sediment; 3) aqueous two-phase extraction: mixing glycol and an inorganic salt until the pH value is 5.8-6.5, adding the salting-out sediment, performing magnetic stirring, then transferring the mixture into a separating funnel for layering to obtain an upper phase namely a protein solution; 4) ultrafiltration: putting the protein solution into an ultrafiltration centrifuge tube, and performing centrifugation and freeze-drying to obtain the phycocyanin hypoglycemic polypeptide. The phycocyanin hypoglycemic polypeptide disclosed by the invention has functions of lowering blood pressure and blood fat and activating blood vessels, and the polypeptide yield is significantly improved via cell disruption during preparation, so that the polypeptide yield is high, and the phycocyanin hypoglycemic polypeptide can be applied to foods or beverages.
Owner:浦江县欧立生物技术有限公司
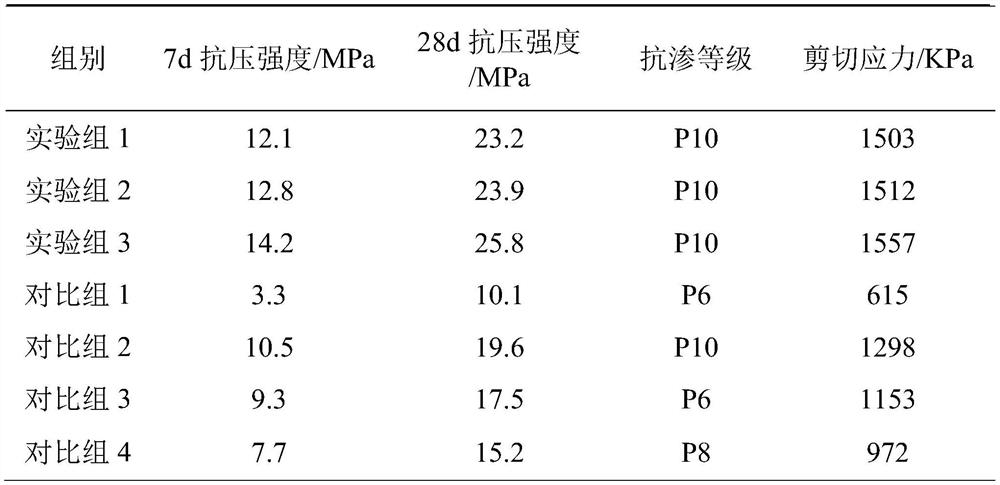
C2-fluoro substituted piperazine linked pyrrolo[2,1-C][1,4] benzodiazepine dimers and a process for the preparation thereof
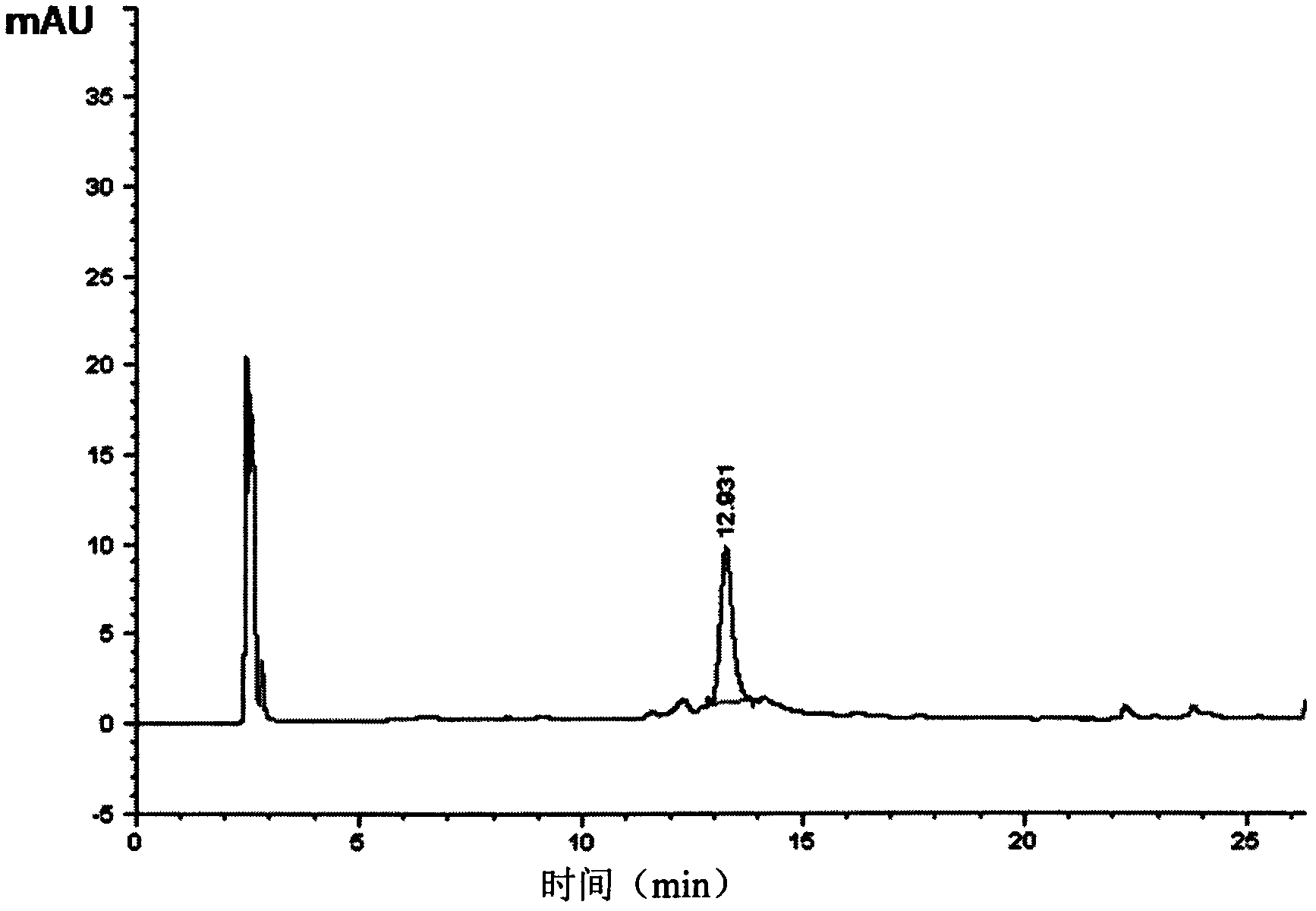
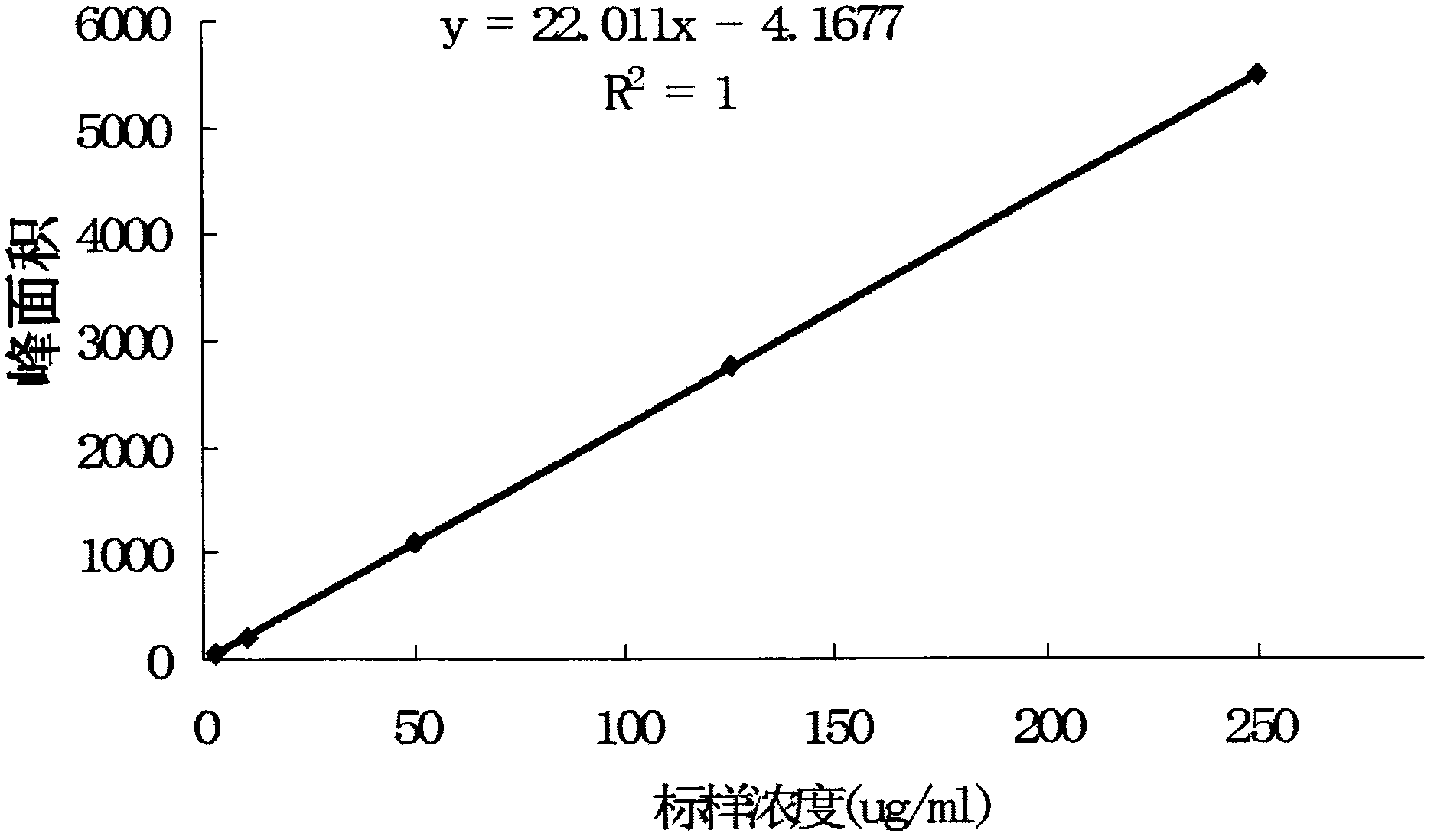
The present invention provides a compounds of general formula IXa-d, useful as potential antitumour agents and pharmaceutical composition comprising these compounds exhibits binding affinity with calf thymus (CT) DNA at a molar ratio of 1:5 in aqueous sodium phosphate buffer at pH of 7.00. The present invention further provides a process for the preparation of C2-Fluoro substituted piperazine linked pyrrolo[2,1c][1-4], benzodiazepine of formula (IX).
Owner:COUNCIL OF SCI & IND RES
Method for rapidly determining xylanase in fermentation liquor
InactiveCN103900981ARapid and accurate determinationReduce human errorColor/spectral properties measurementsWater bathsExoxylanase activity
The invention relates to a method for rapidly determining xylanase in fermentation liquor. The method comprises the following steps: centrifuging 1000*g of fermentation liquor for 10 minutes, taking the supernatant, determining the activity of xylanase, and preheating properly diluted supernatant, 10g / L of xylanase solution and 50mM of sodium phosphate buffer solution with the pH value of 7.0 at the temperature of 39 DEG C for 15-30 minutes, adding 75micronL of sodium phosphate buffer solution and 50 micronL of xylanase solution into 75micronL of diluted supernatant, reacting at the temperature of 39 DEG C for 15-30 minutes, adding 300micronL of DNS solution and ending the reaction; heating in a boiling water bath for 5 minutes, and cooling to room temperature; putting 200-300 micronL of solution on a dried and clean 96-pore ELISA plate, detecting a light absorption value at 530-550nm by using a full-automatic microplate reader, and calculating the activity of xylanase according to a standard curve of xylose. According to the technology, a method for measuring the xylanase in the fermentation liquor is revised, the activity of the xylanase can be rapidly, greatly and accurately measured, and personal errors are reduced. Meanwhile, fewer samples can be detected.
Owner:NORTHWEST A & F UNIV
Bis-2-difluoro-pyrrolo[2,1-C][1,4]benzodiazepine dimers
Owner:COUNCIL OF SCI & IND RES
Photovoltaic cell panel cleaning agent with good anti-freezing performance, and preparation method thereof
InactiveCN110734813AGood antifreezeEasy to cleanInorganic/elemental detergent compounding agentsNon-ionic surface-active compoundsSodium phosphatesCleansing Agents
The invention relates to the field of solar cleaning supplies, particularly to a photovoltaic cell panel cleaning agent with good anti-freezing performance, and a preparation method thereof, wherein the cleaning agent comprises: polyoxyethylene fatty alcohol ether, dihexyl sodium sulfosuccinate, coconut acid diethanolamide, sodium pyrophosphate, a buffer solution, isopropanol and distilled water.According to the invention, the prepared cleaning agent has excellent anti-freezing performance; the pH value of the component B is kept at 7-8 by utilizing the buffer solution in the preparation process of the cleaning agent, so that the component B is weakly alkaline so as to effectively enhance the cleaning effect; sodium hydroxide and epoxypropane are introduced into the fatty alcohol polyoxyethylene ether, and the polyether molecular structure can reduce the viscosity to improve the fluidity, so that the modified fatty alcohol polyoxyethylene ether has low foaming property so as to improve the washing property; and the compatibility of the modified fatty alcohol polyoxyethylene ether, the dihexyl sodium sulfosuccinate, the coconut acid diethanolamide and the sodium pyrophosphate is good, so that the photovoltaic cell panel can be efficiently cleaned through the mutual synergistic effect of the four components.
Owner:丁娜娜
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
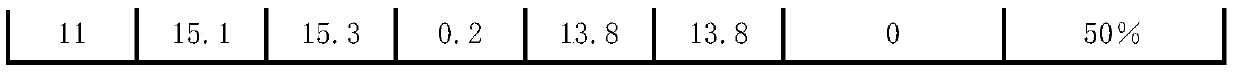



![C2-fluoro substituted piperazine linked pyrrolo[2,1-C][1,4] benzodiazepine dimers and a process for the preparation thereof C2-fluoro substituted piperazine linked pyrrolo[2,1-C][1,4] benzodiazepine dimers and a process for the preparation thereof](https://images-eureka.patsnap.com/patent_img/c58fca9f-2d96-48e3-9a47-863fe56e90be/US08383618-20130226-C00001.png)
![C2-fluoro substituted piperazine linked pyrrolo[2,1-C][1,4] benzodiazepine dimers and a process for the preparation thereof C2-fluoro substituted piperazine linked pyrrolo[2,1-C][1,4] benzodiazepine dimers and a process for the preparation thereof](https://images-eureka.patsnap.com/patent_img/c58fca9f-2d96-48e3-9a47-863fe56e90be/US08383618-20130226-C00002.png)
![C2-fluoro substituted piperazine linked pyrrolo[2,1-C][1,4] benzodiazepine dimers and a process for the preparation thereof C2-fluoro substituted piperazine linked pyrrolo[2,1-C][1,4] benzodiazepine dimers and a process for the preparation thereof](https://images-eureka.patsnap.com/patent_img/c58fca9f-2d96-48e3-9a47-863fe56e90be/US08383618-20130226-C00003.png)
![Bis-2-difluoro-pyrrolo[2,1-C][1,4]benzodiazepine dimers Bis-2-difluoro-pyrrolo[2,1-C][1,4]benzodiazepine dimers](https://images-eureka.patsnap.com/patent_img/fa3cd0dc-c8b1-47e8-9e43-2ce1ca4b0cb5/US07476664-20090113-C00001.png)
![Bis-2-difluoro-pyrrolo[2,1-C][1,4]benzodiazepine dimers Bis-2-difluoro-pyrrolo[2,1-C][1,4]benzodiazepine dimers](https://images-eureka.patsnap.com/patent_img/fa3cd0dc-c8b1-47e8-9e43-2ce1ca4b0cb5/US07476664-20090113-C00002.png)
![Bis-2-difluoro-pyrrolo[2,1-C][1,4]benzodiazepine dimers Bis-2-difluoro-pyrrolo[2,1-C][1,4]benzodiazepine dimers](https://images-eureka.patsnap.com/patent_img/fa3cd0dc-c8b1-47e8-9e43-2ce1ca4b0cb5/US07476664-20090113-C00003.png)