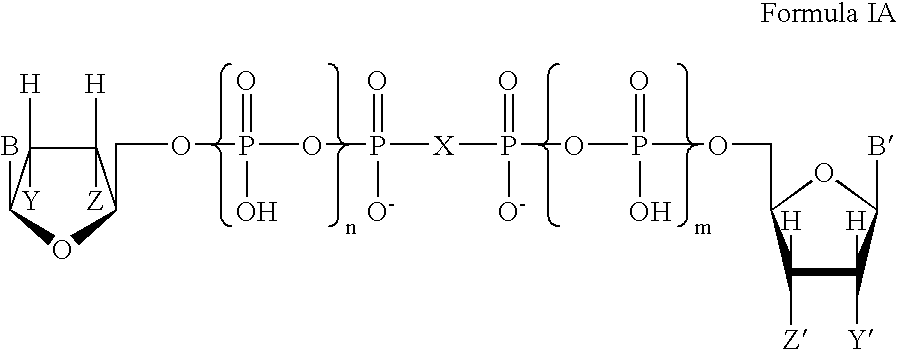Patents
Literature
348 results about "Respiratory mucus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
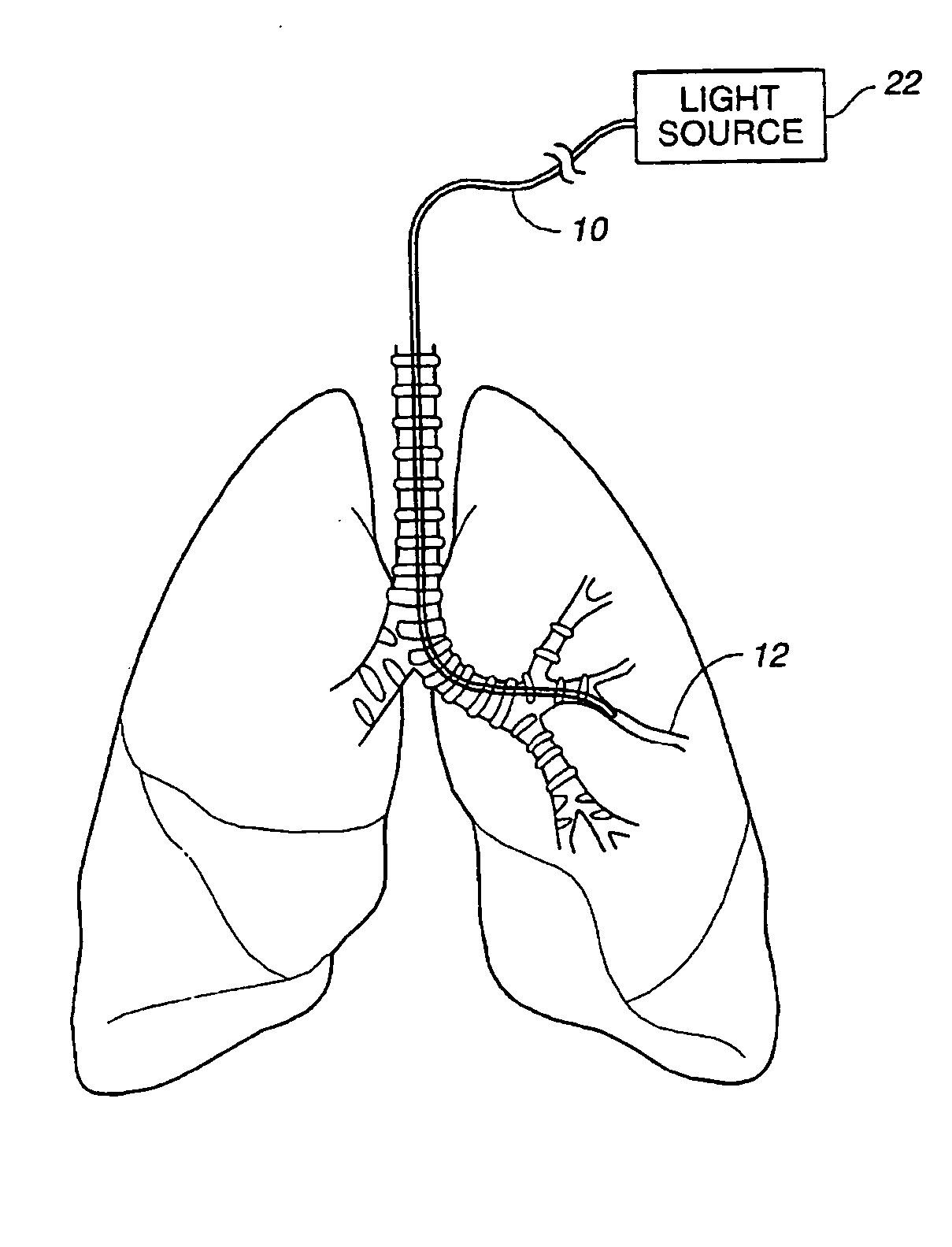
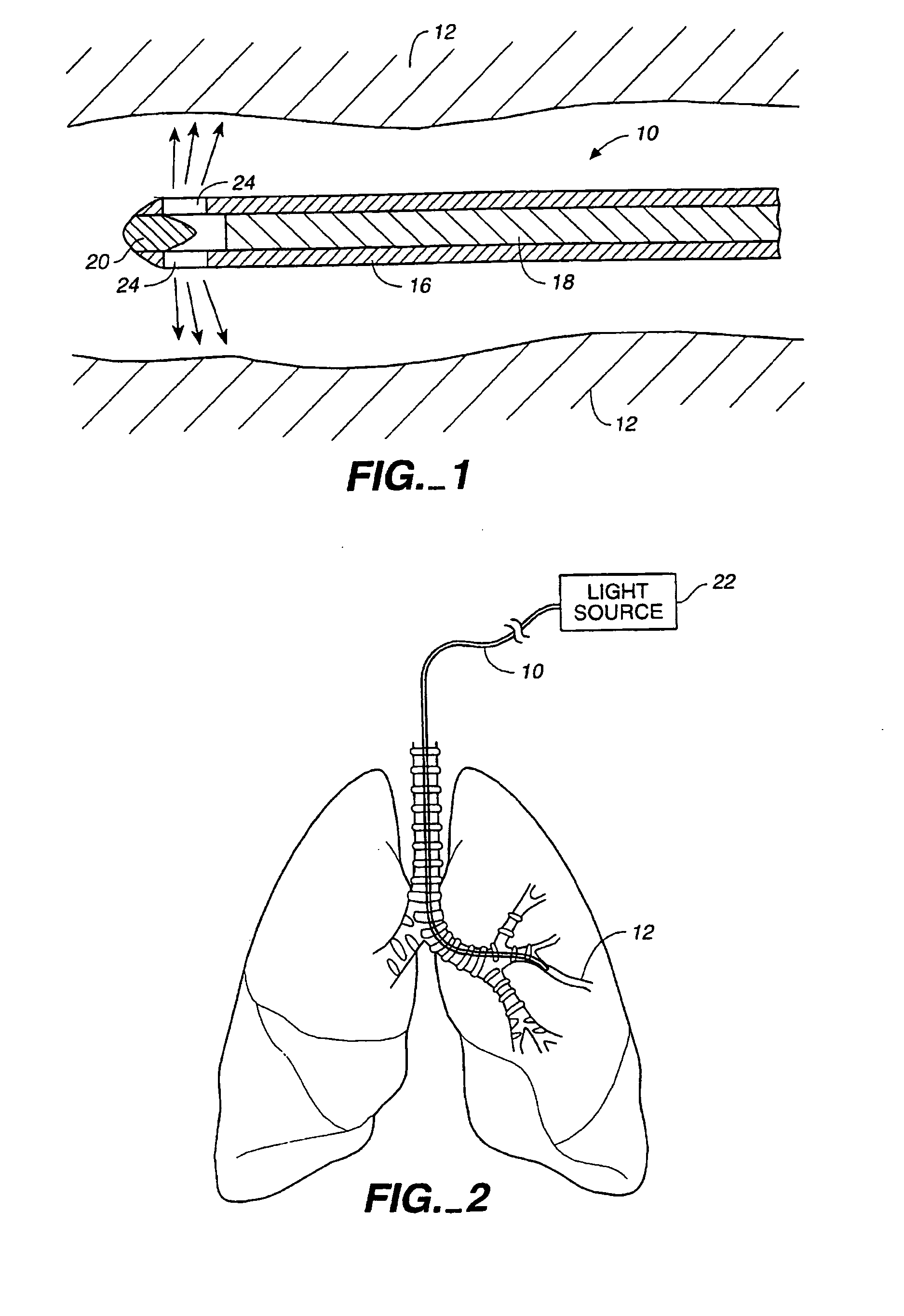
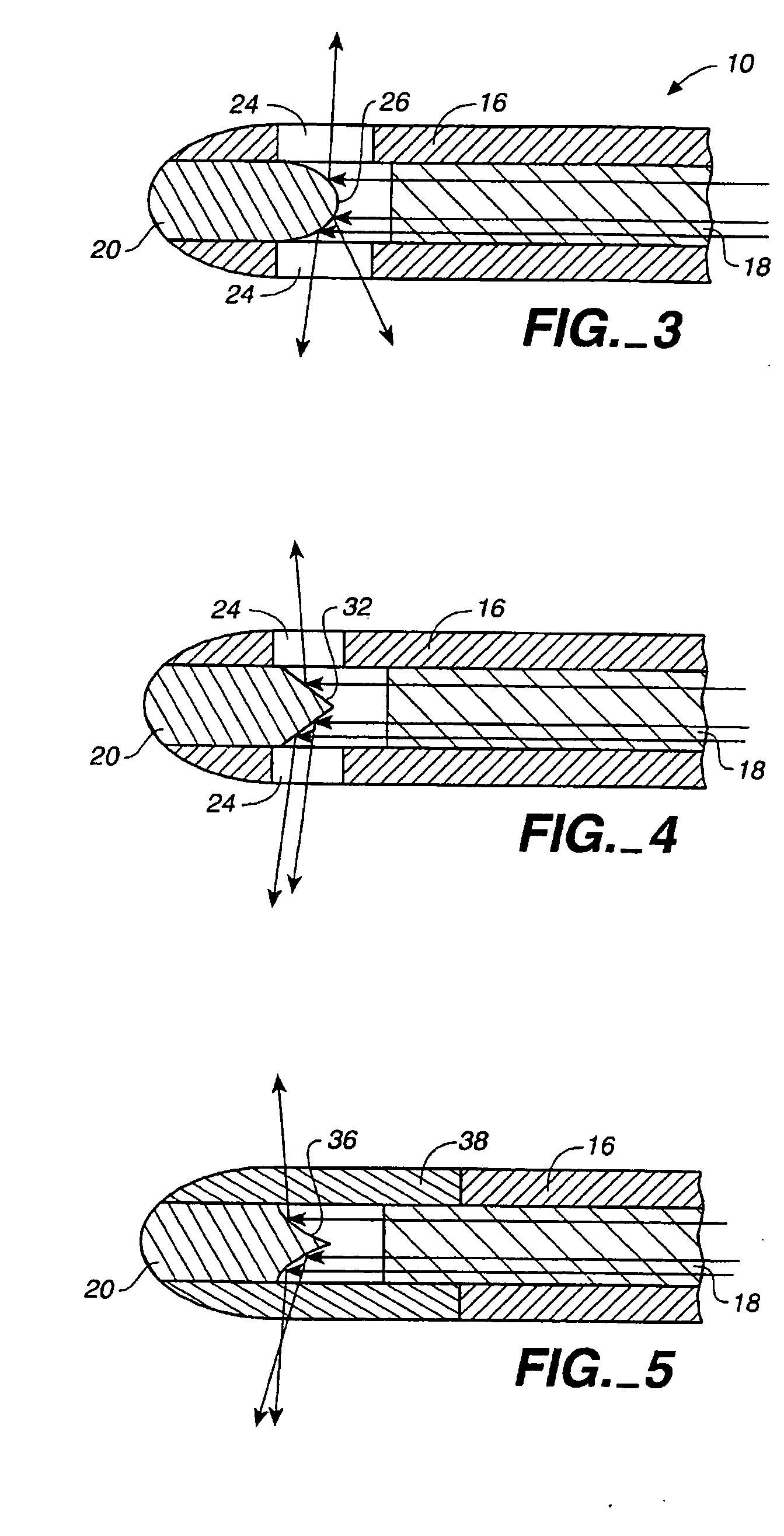
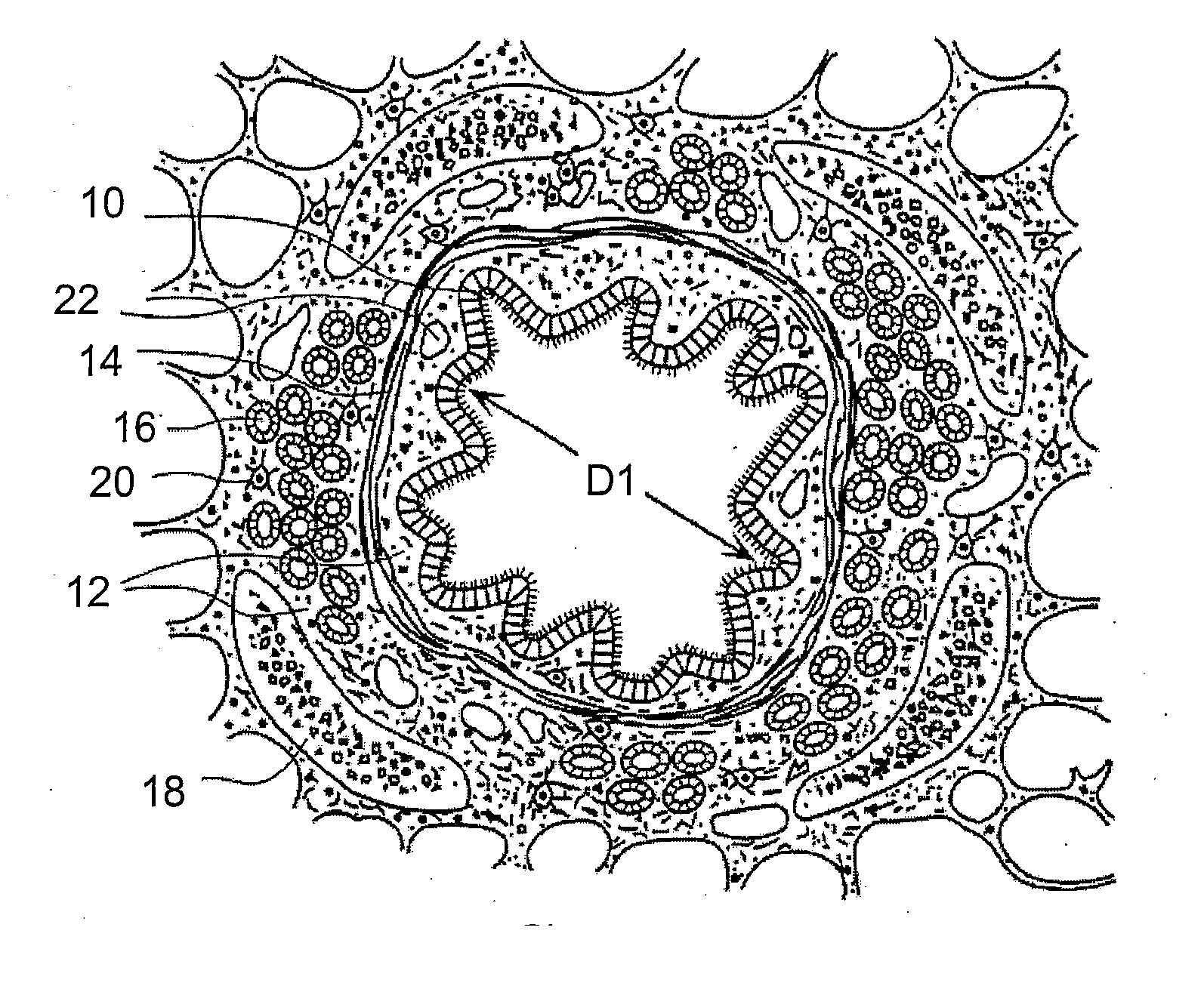
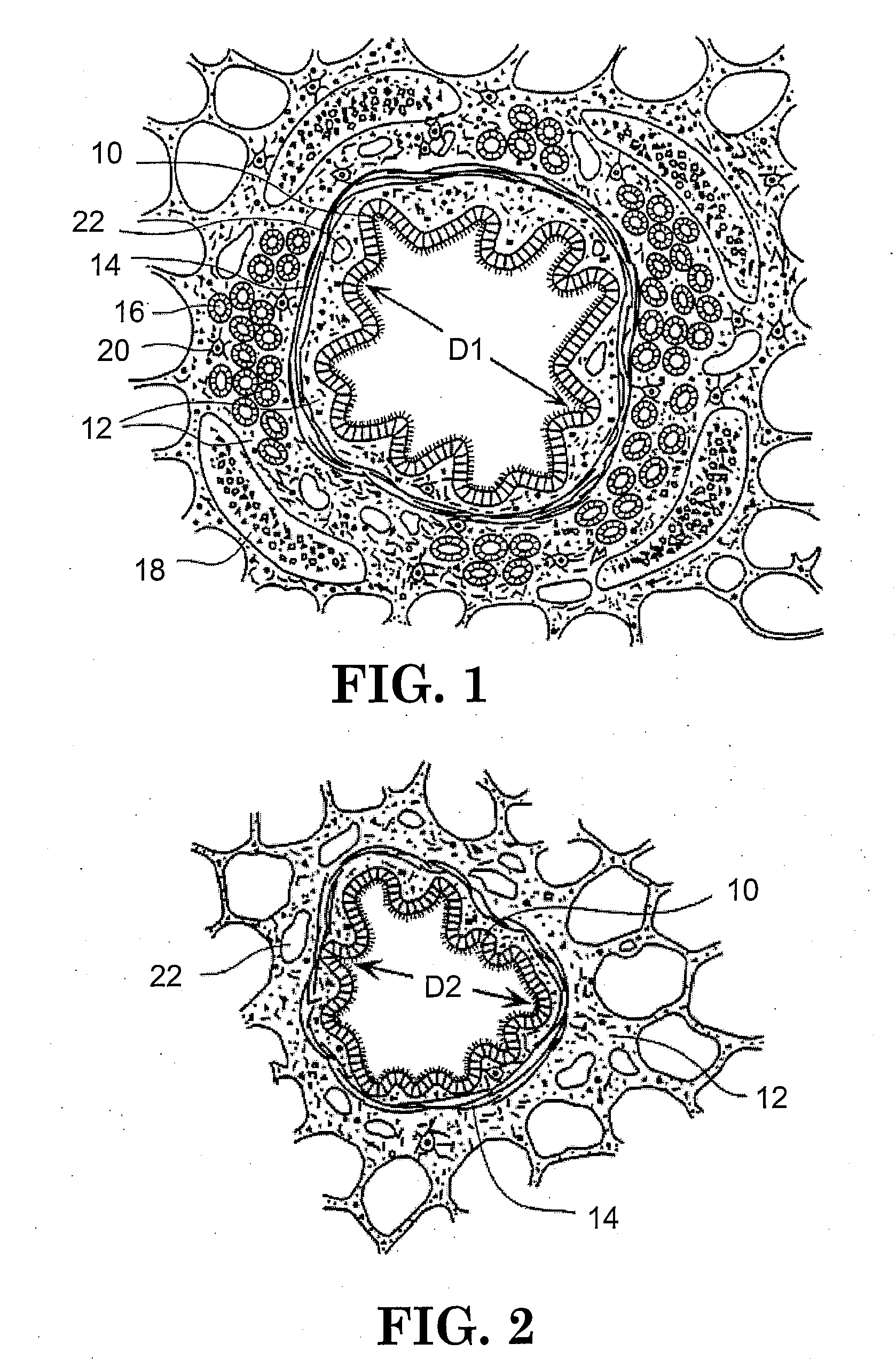
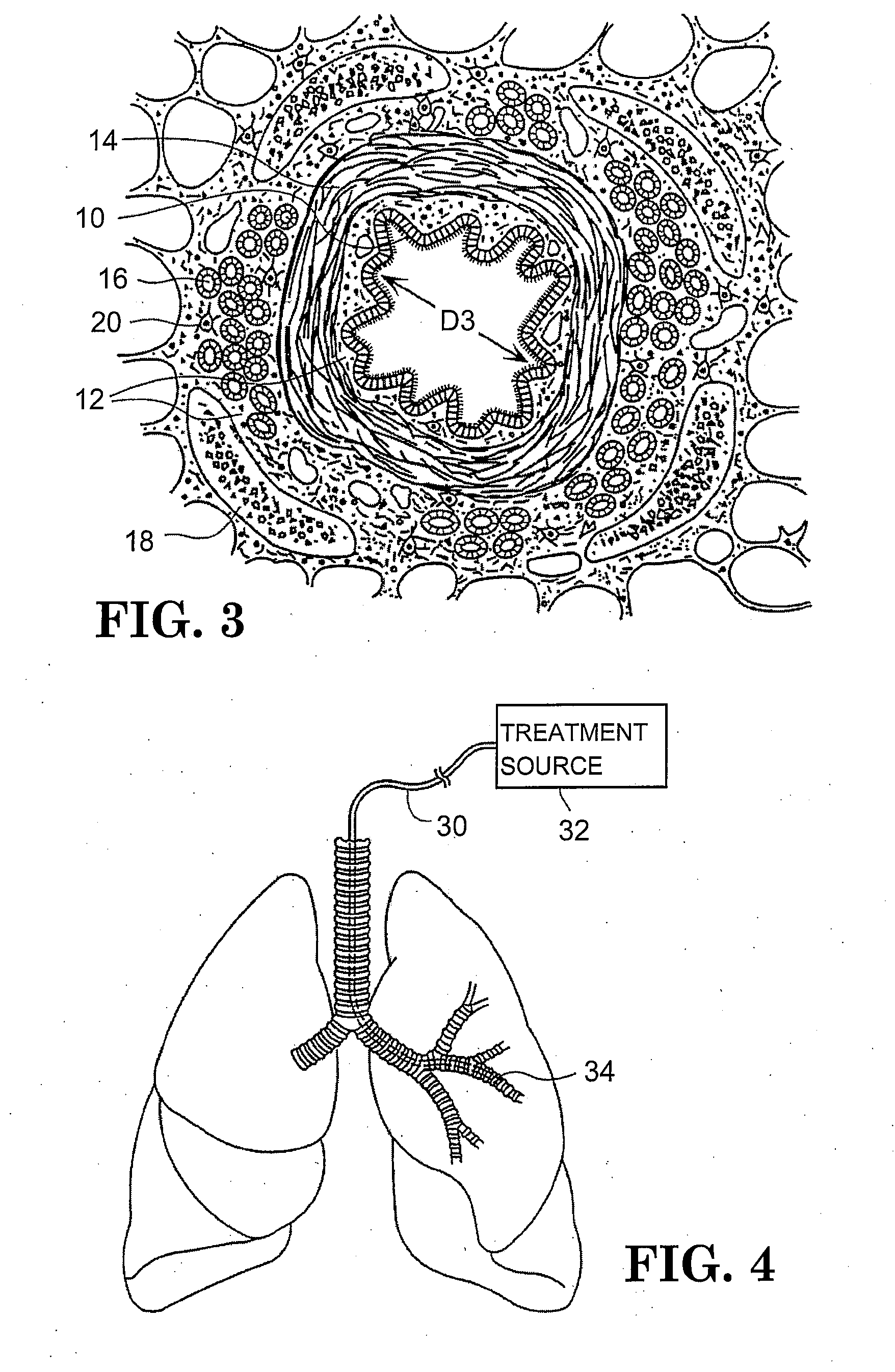
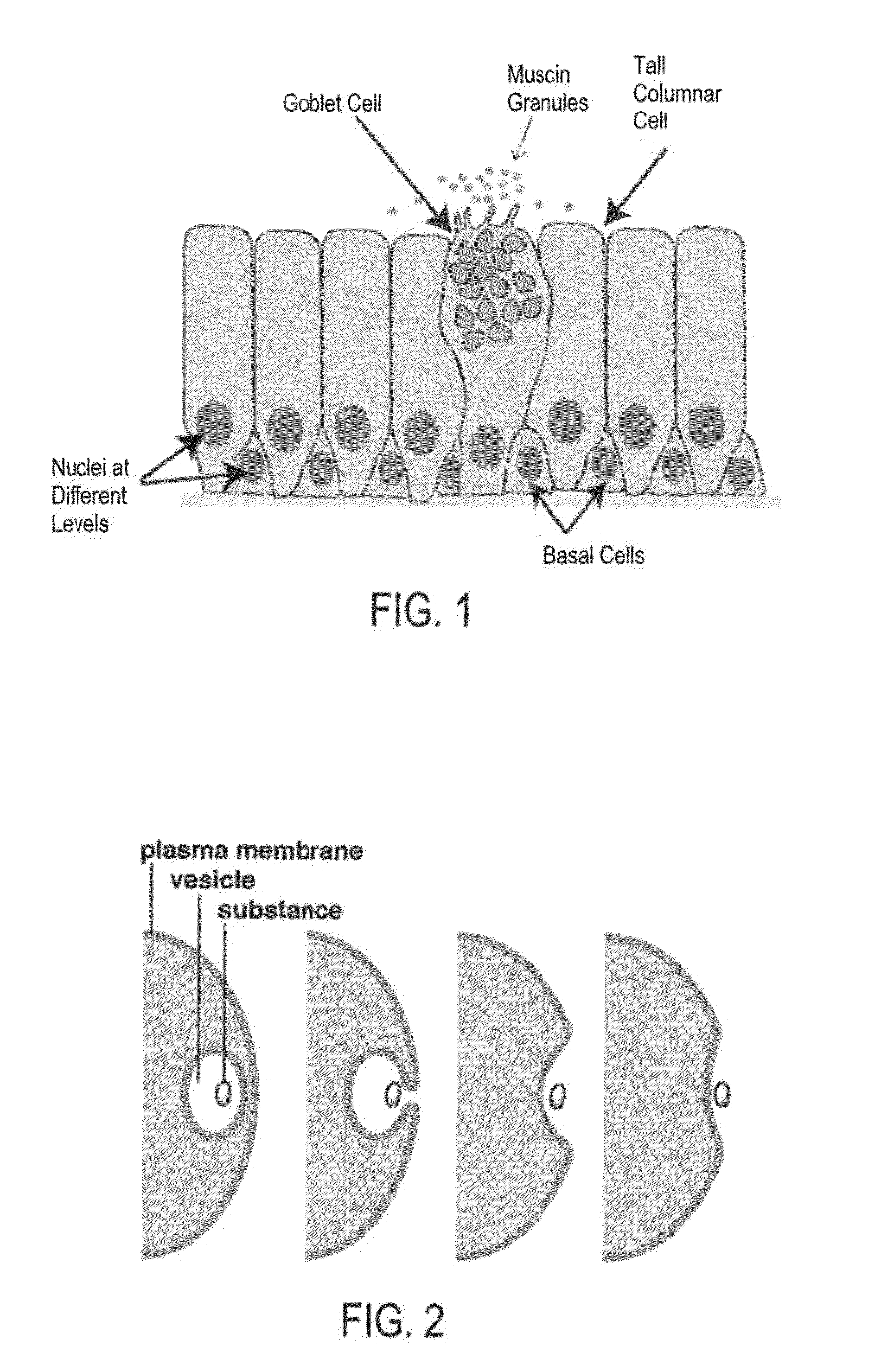
In the human respiratory system, mucus, also known as airway surface liquid (ASL), aids in the protection of the lungs by trapping foreign particles that enter them, in particular, through the nose, during normal breathing.
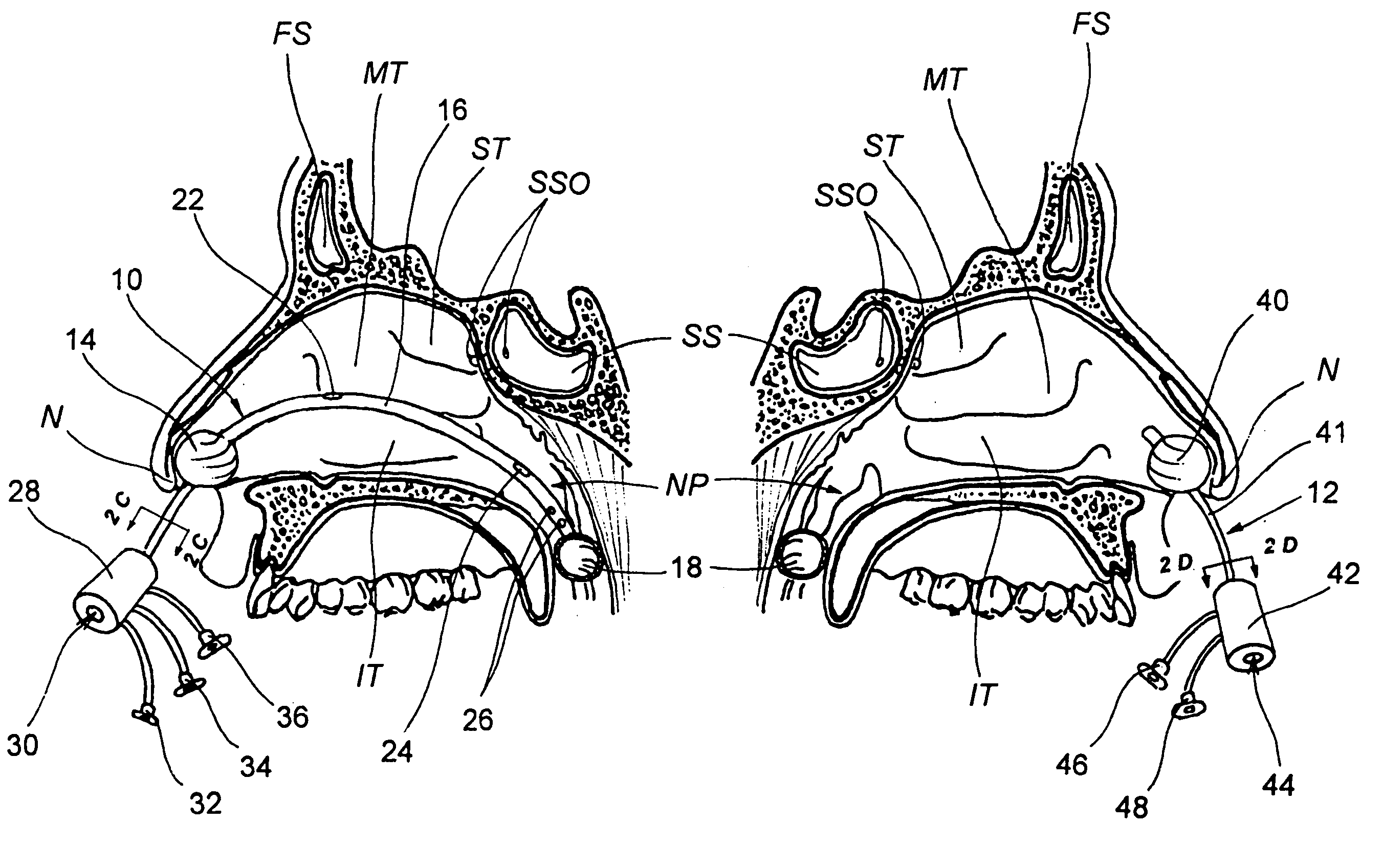
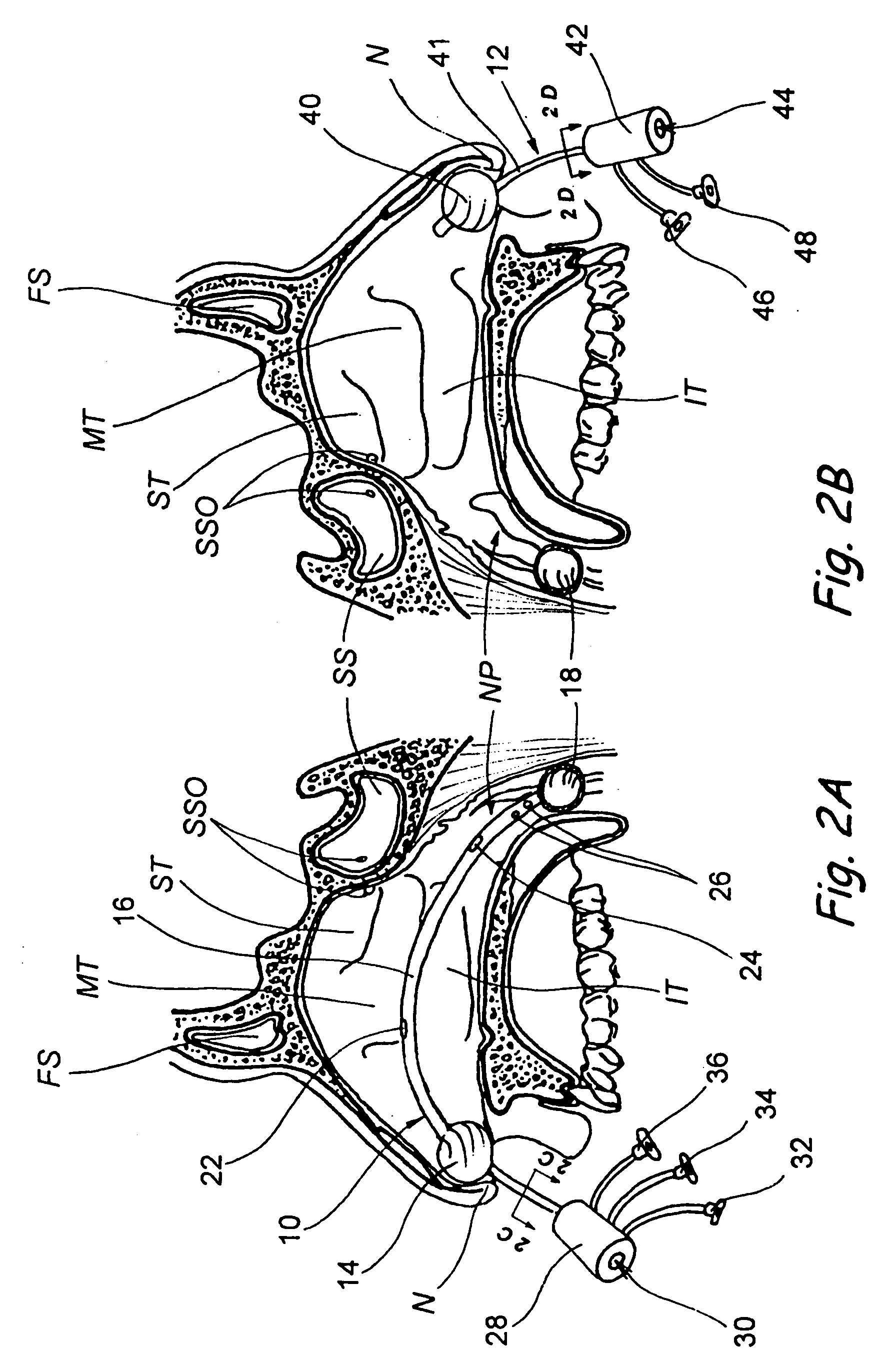
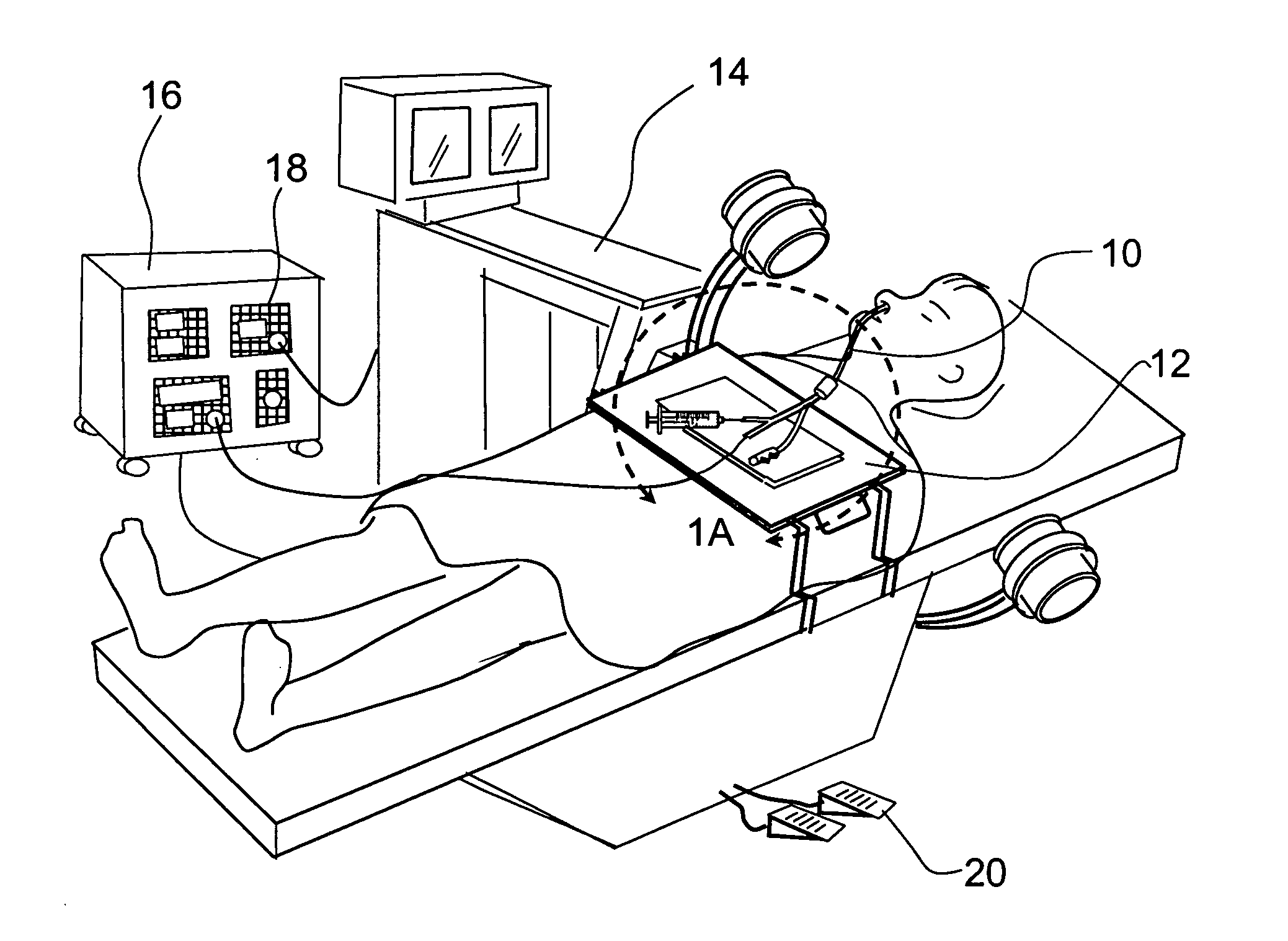
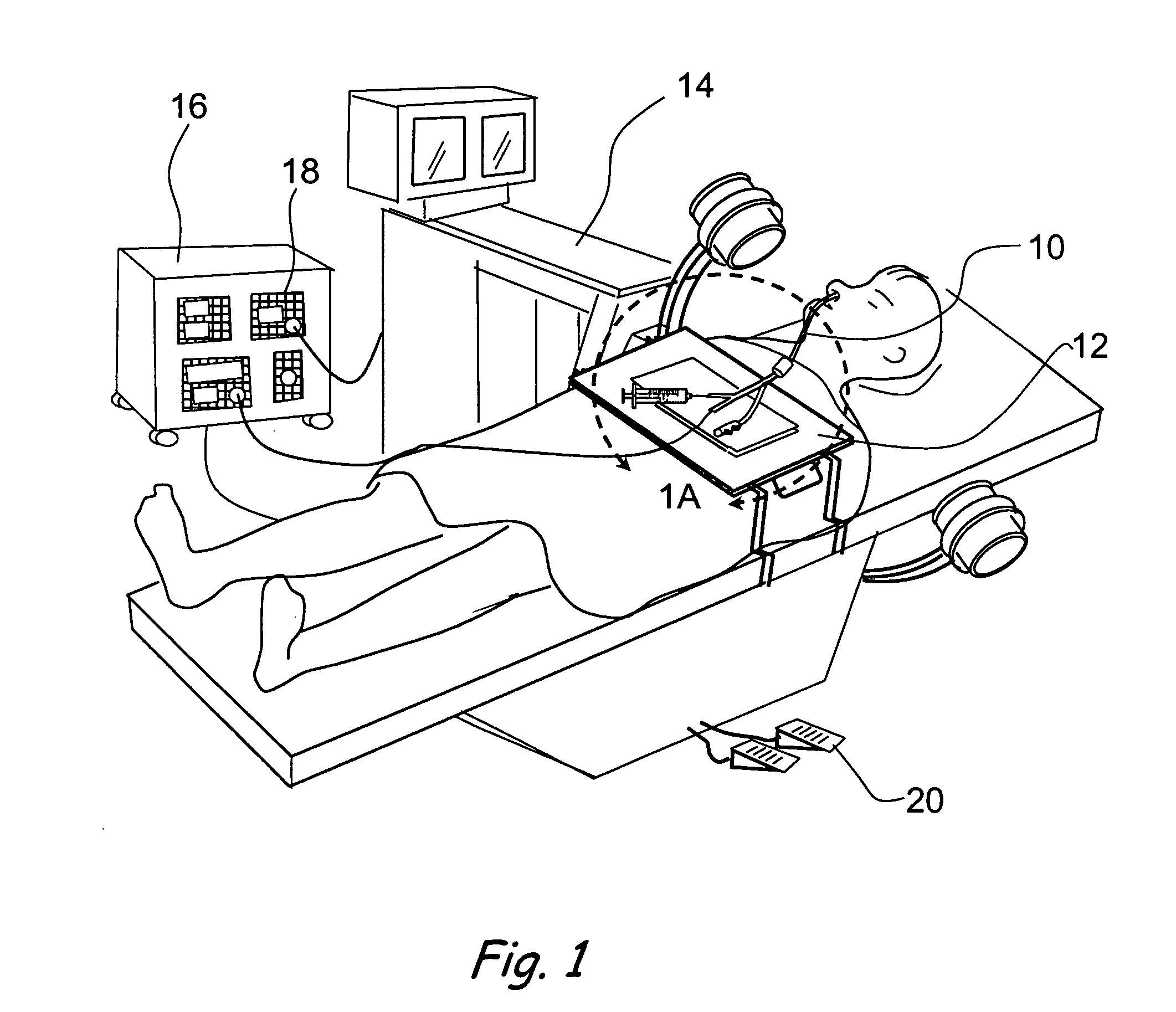
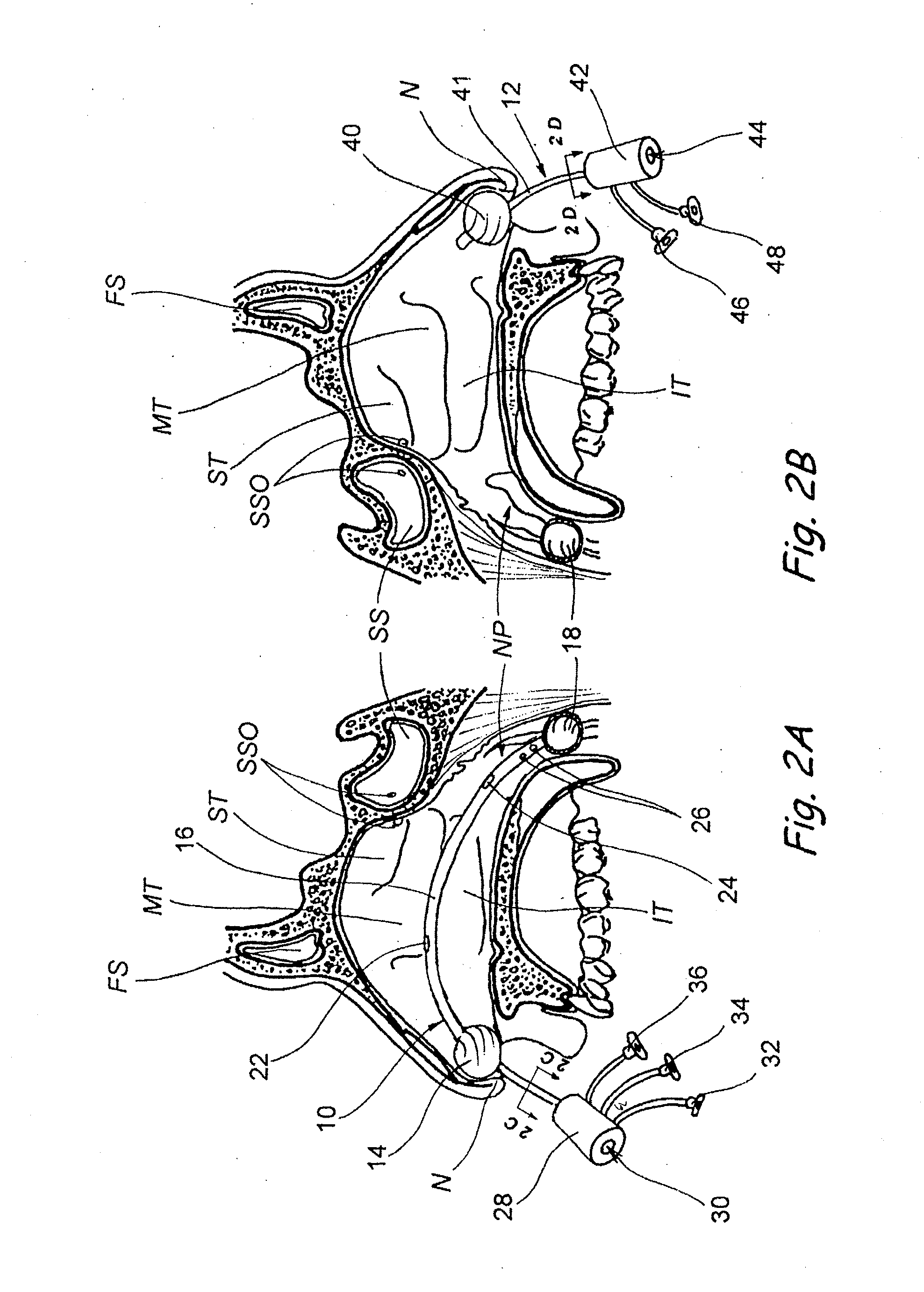
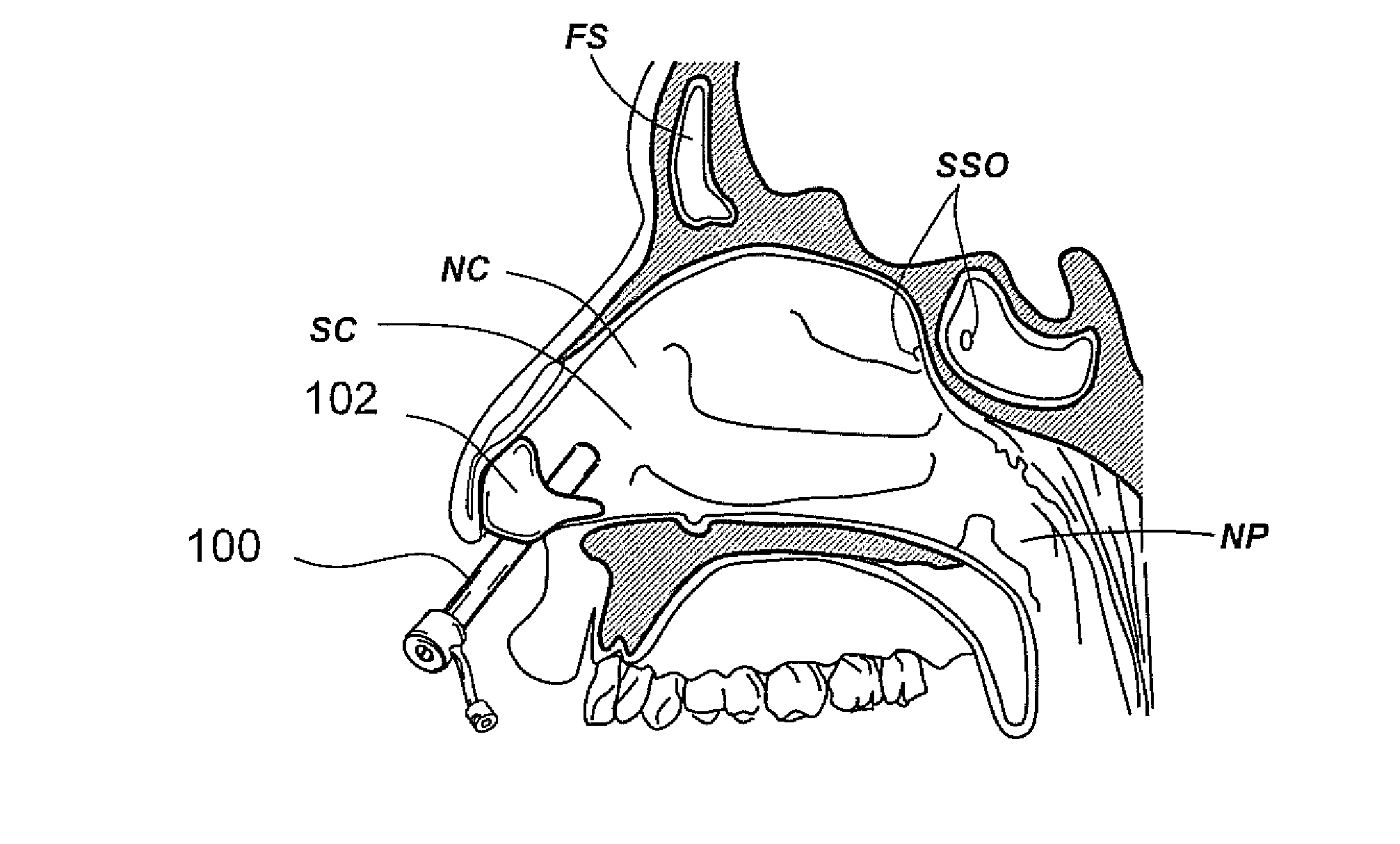
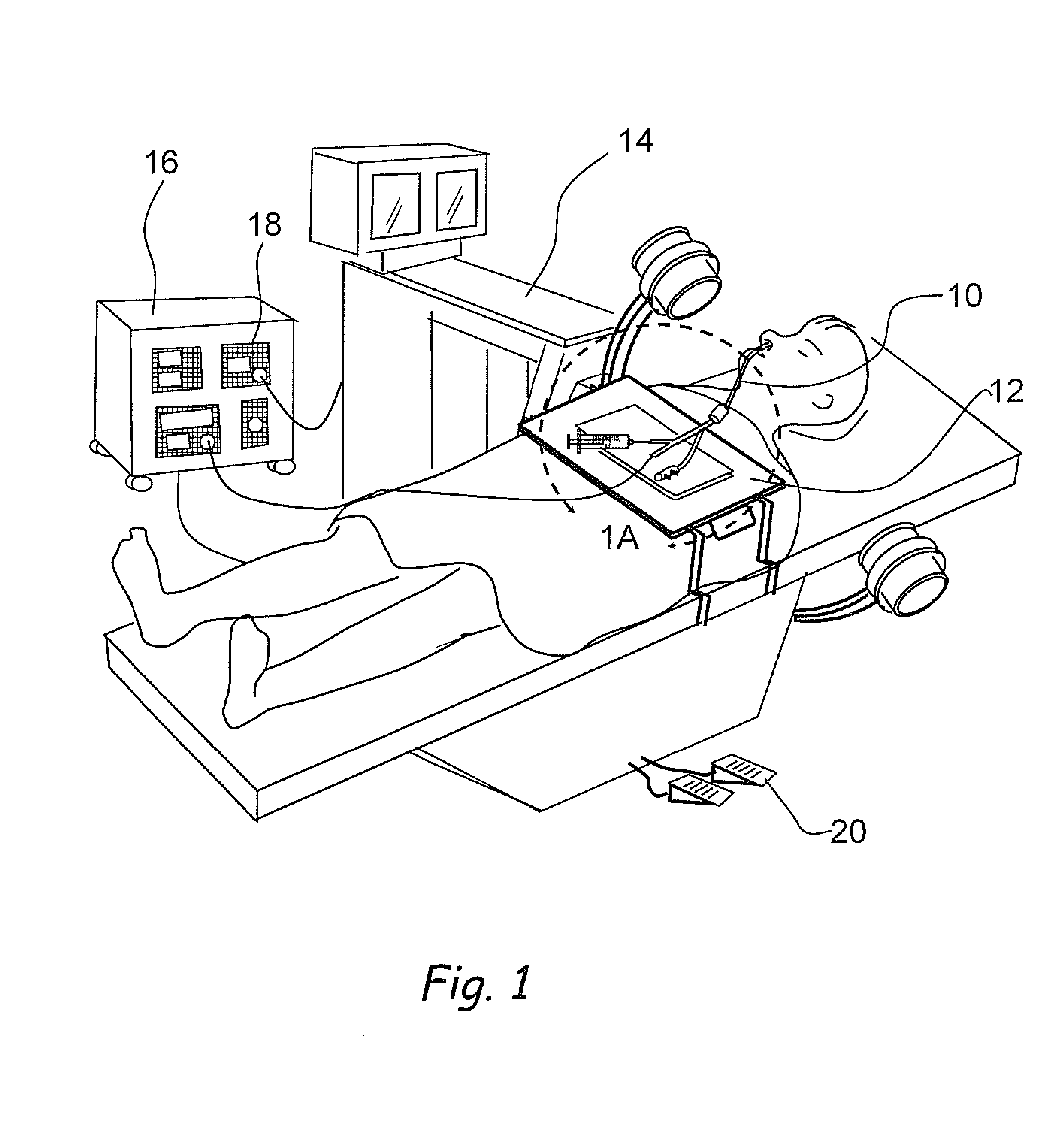
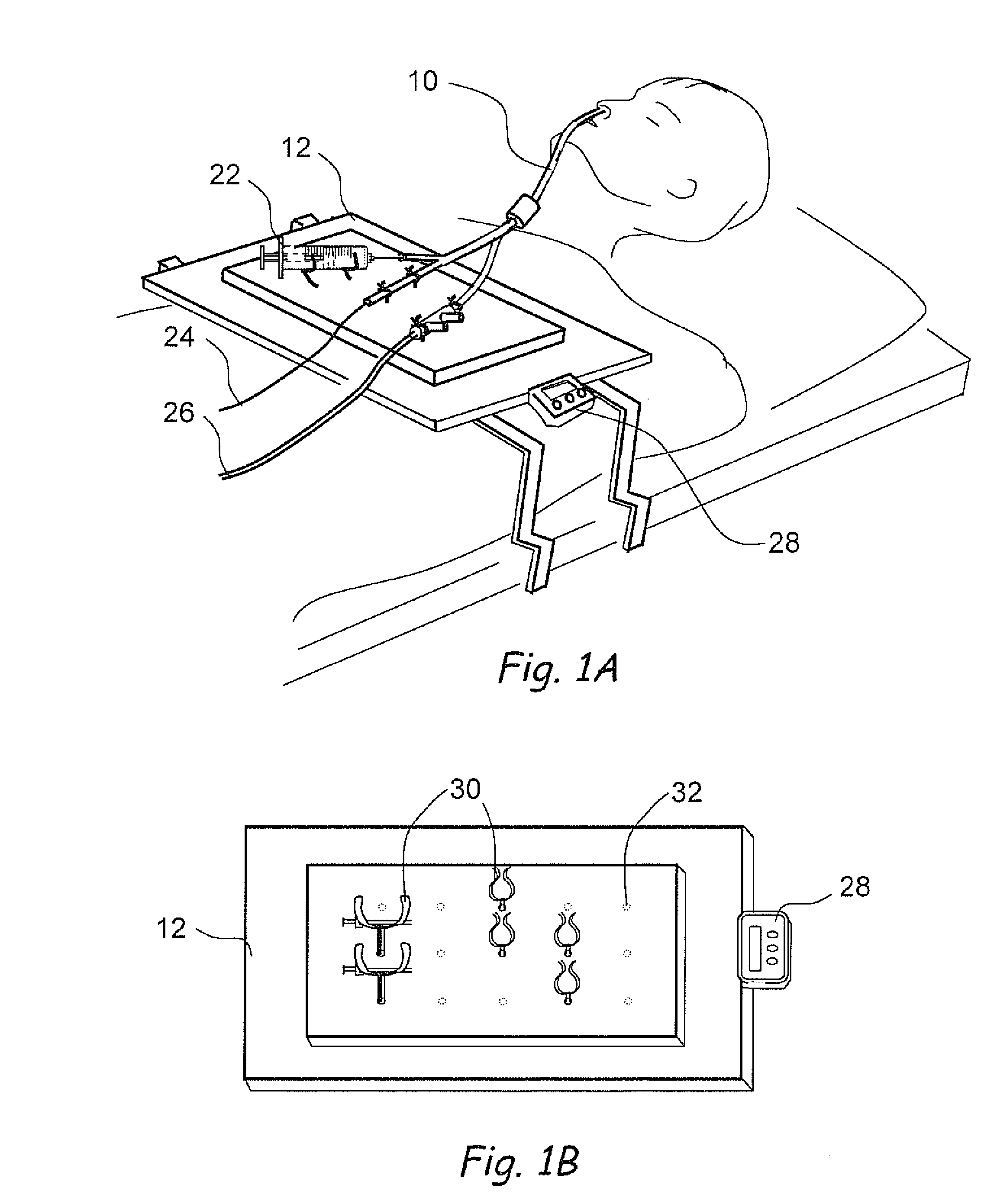
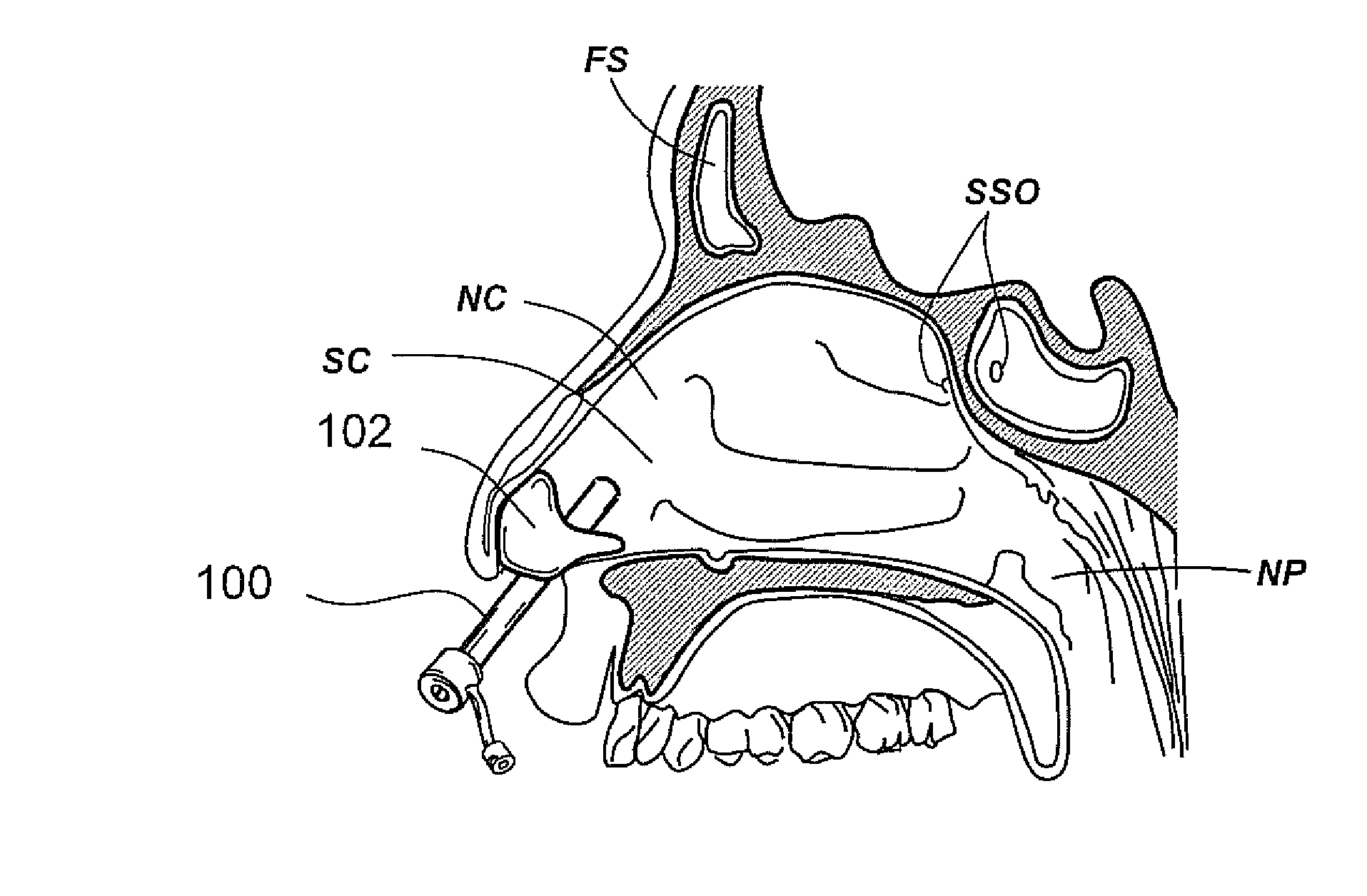
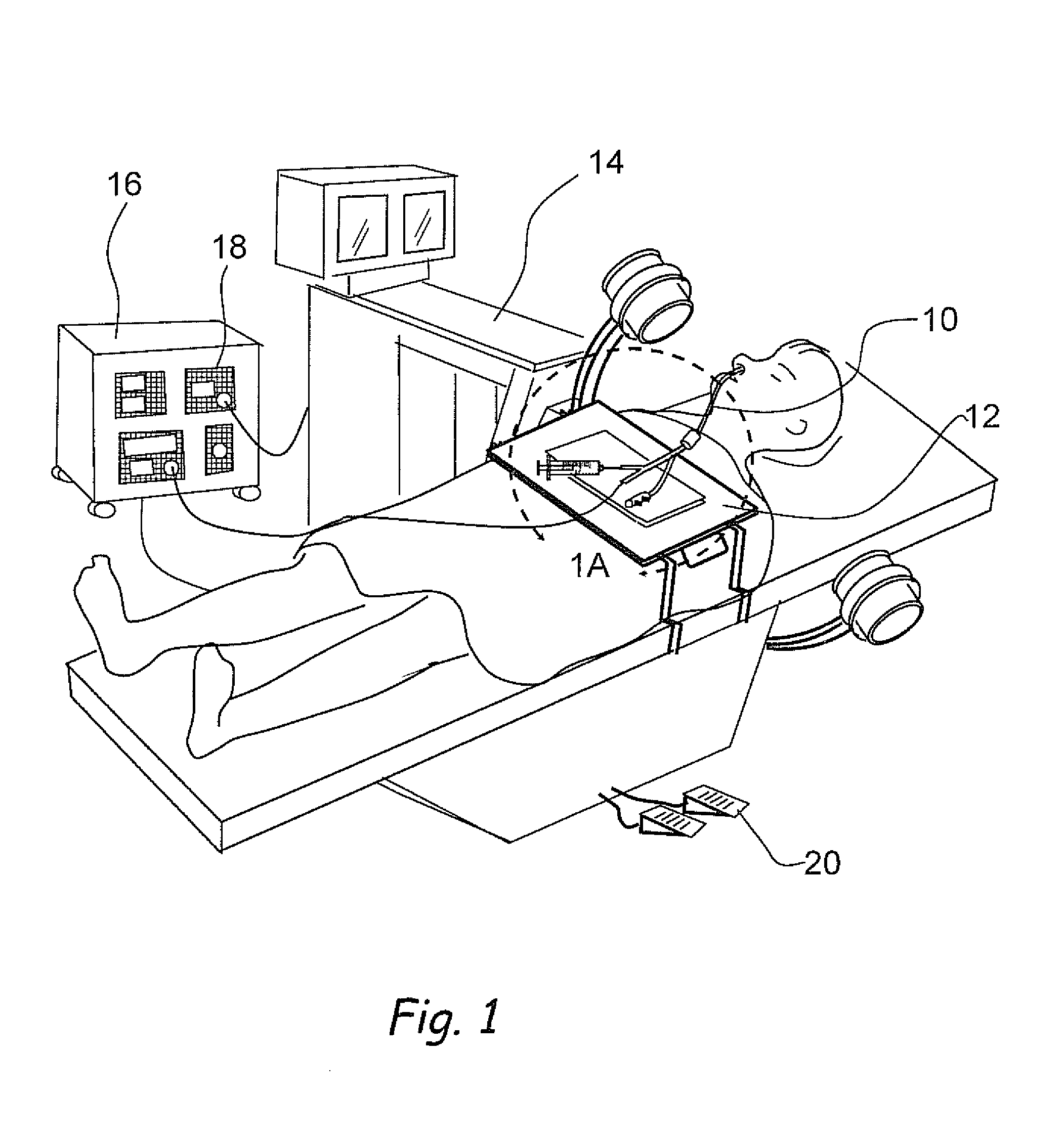
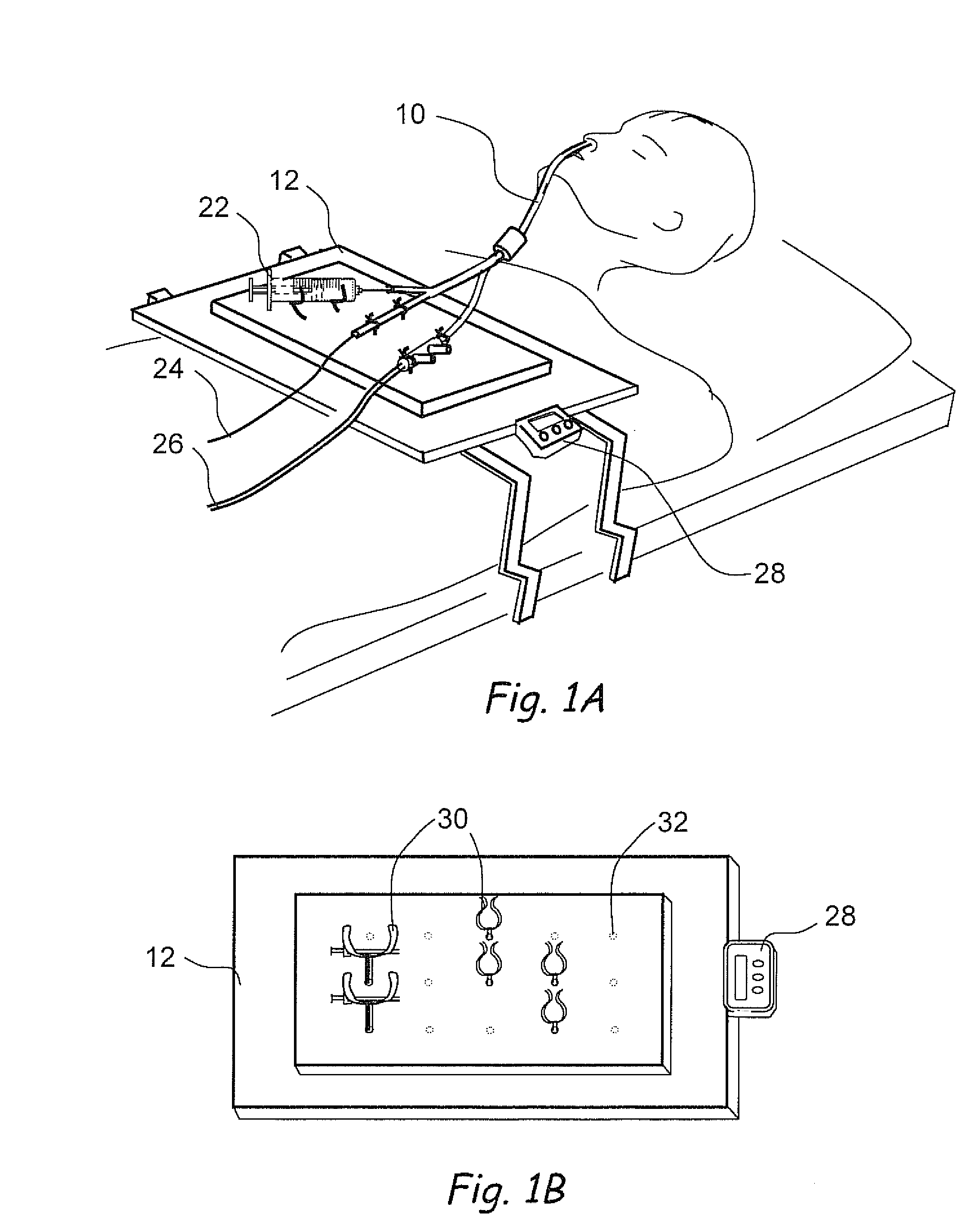
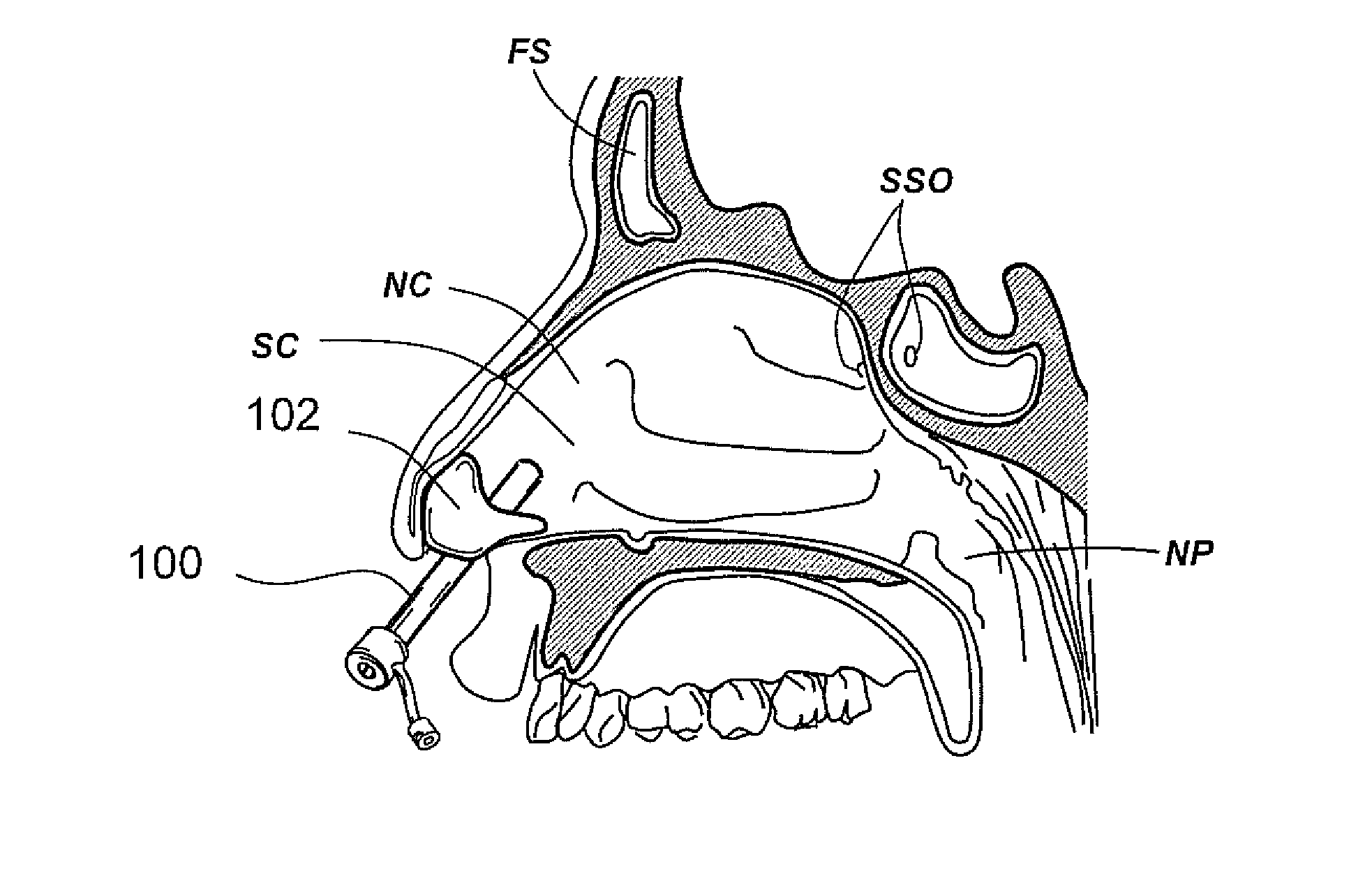
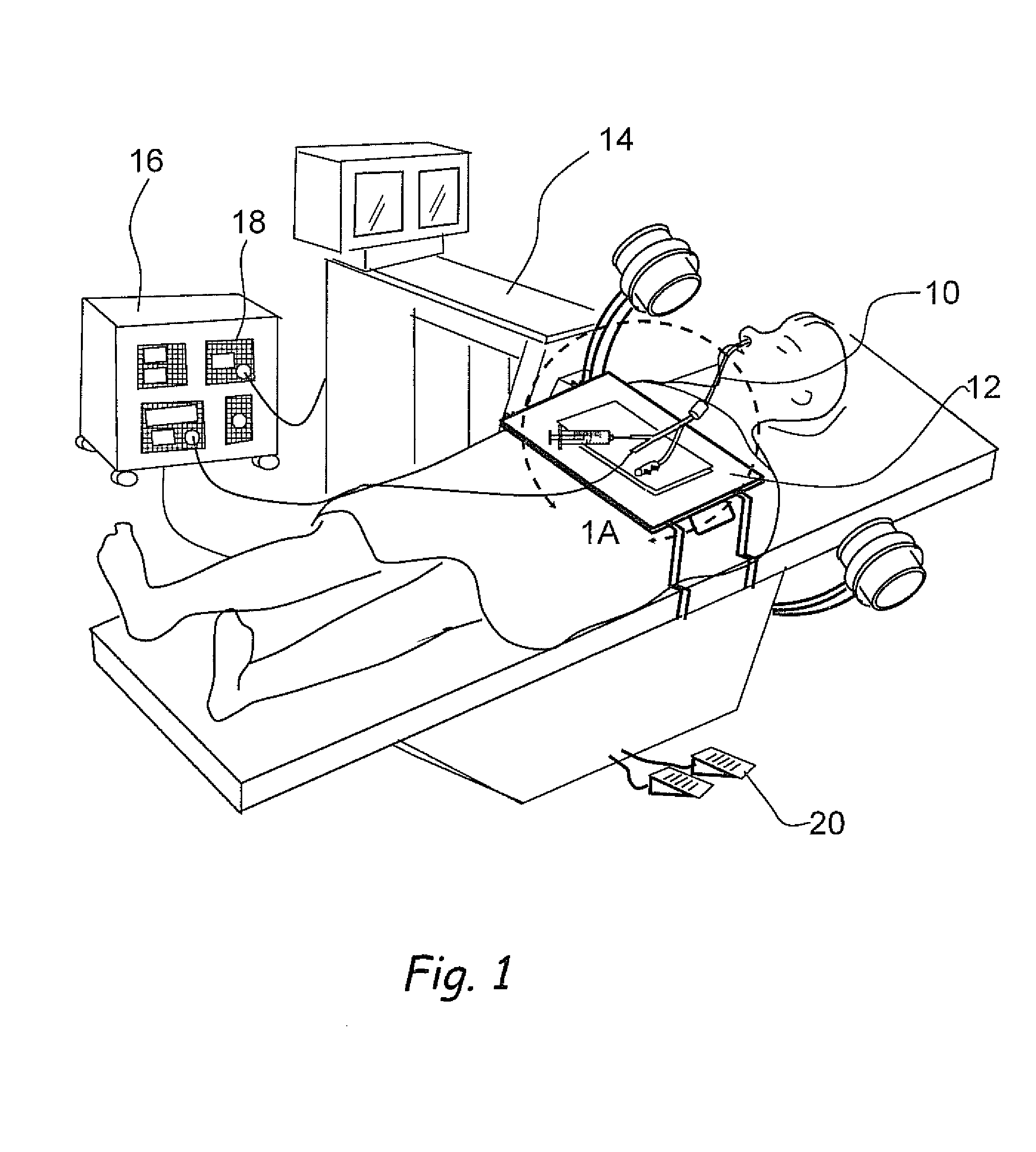
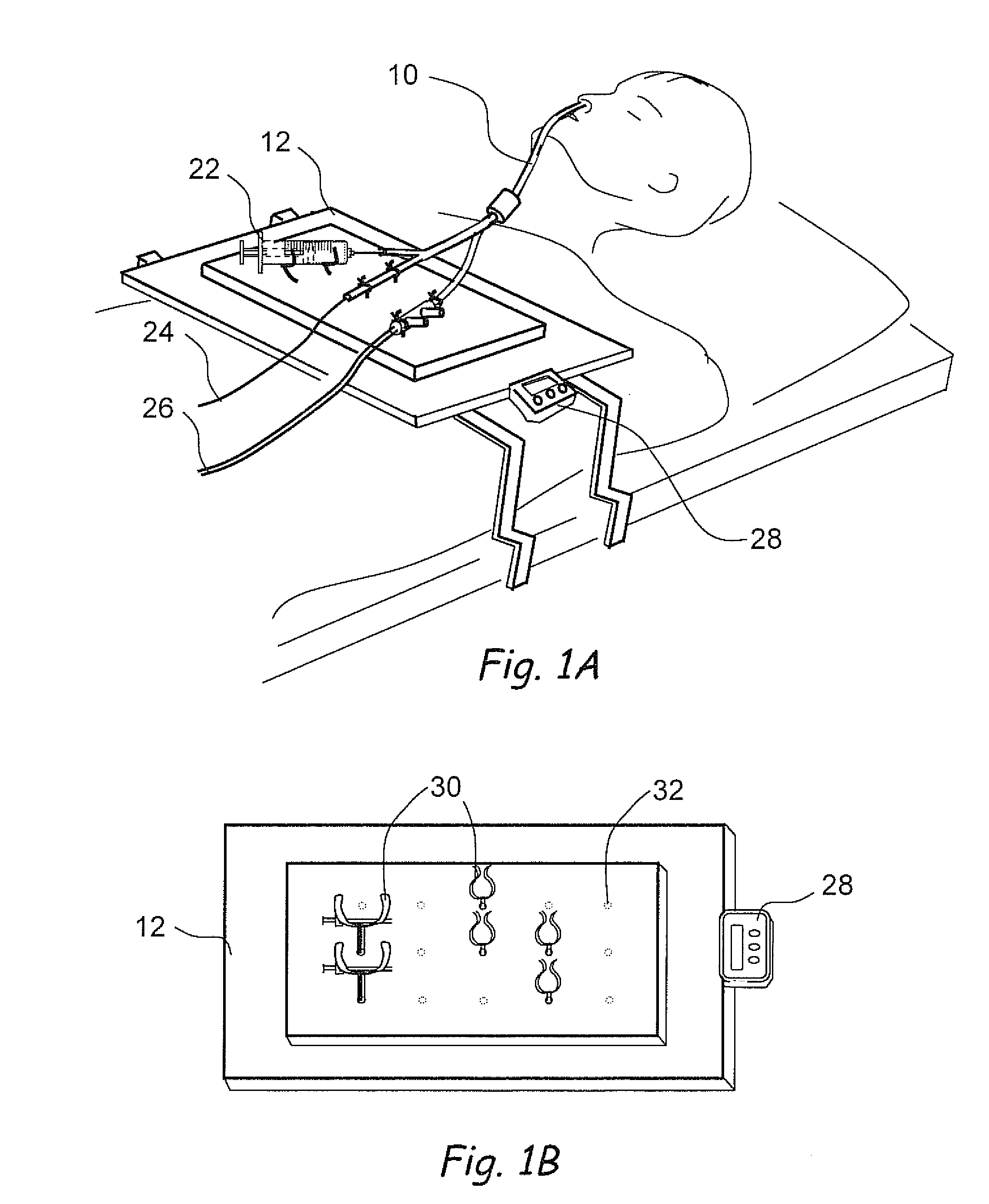
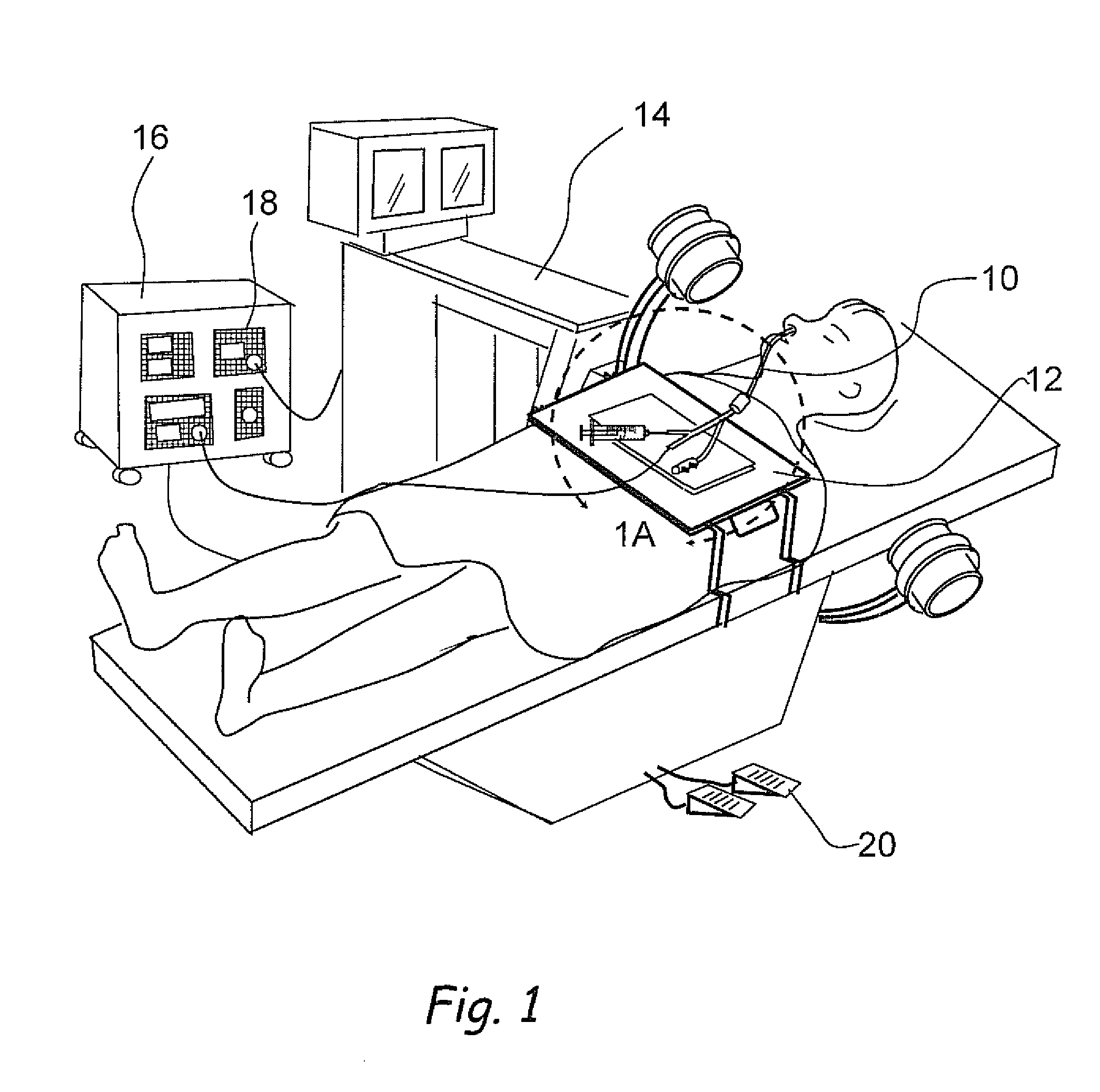
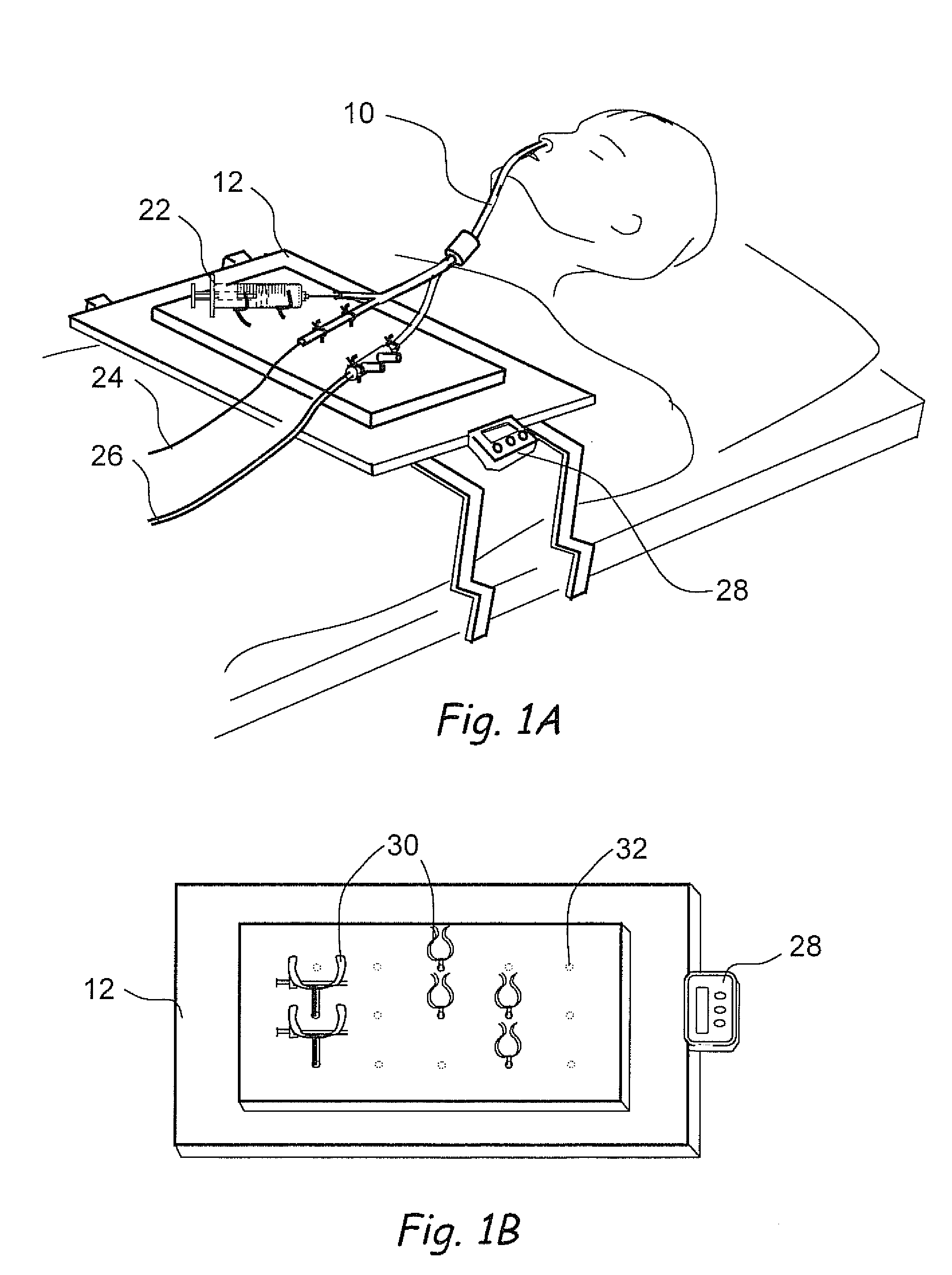
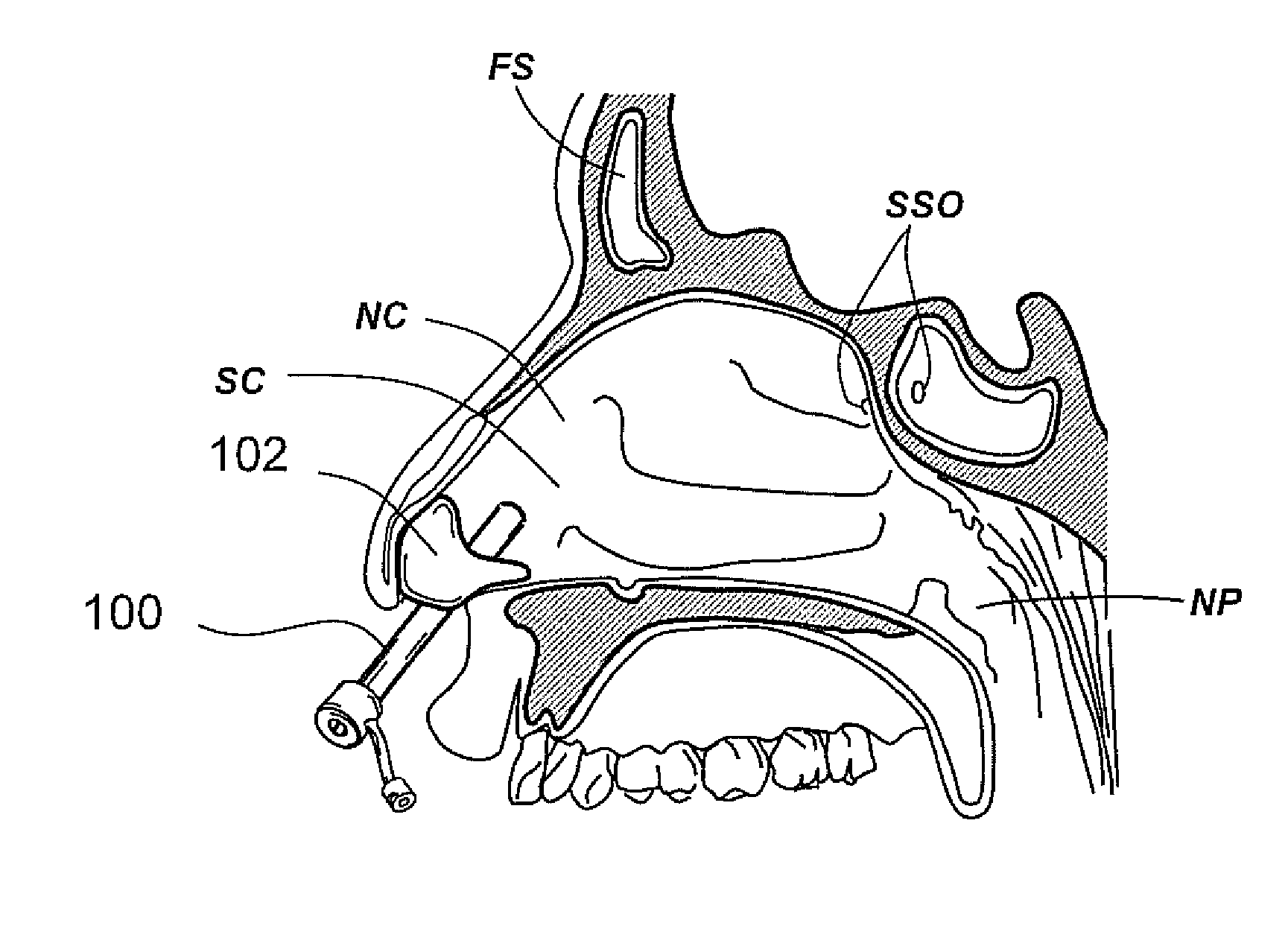
Devices, systems and methods for diagnosing and treating sinusitus and other disorders of the ears, nose and/or throat
Sinusitis, enlarged nasal turbinates, tumors, infections, hearing disorders, allergic conditions, facial fractures and other disorders of the ear, nose and throat are diagnosed and / or treated using minimally invasive approaches and, in many cases, flexible catheters as opposed to instruments having rigid shafts. Various diagnostic procedures and devices are used to perform imaging studies, mucus flow studies, air / gas flow studies, anatomic dimension studies, endoscopic studies and transillumination studies. Access and occluder devices may be used to establish fluid tight seals in the anterior or posterior nasal cavities / nasopharynx and to facilitate insertion of working devices (e.g., scopes, guidewires, catheters, tissue cutting or remodeling devices, electrosurgical devices, energy emitting devices, devices for injecting diagnostic or therapeutic agents, devices for implanting devices such as stents, substance eluting devices, substance delivery implants, etc.
Owner:ACCLARENT INC
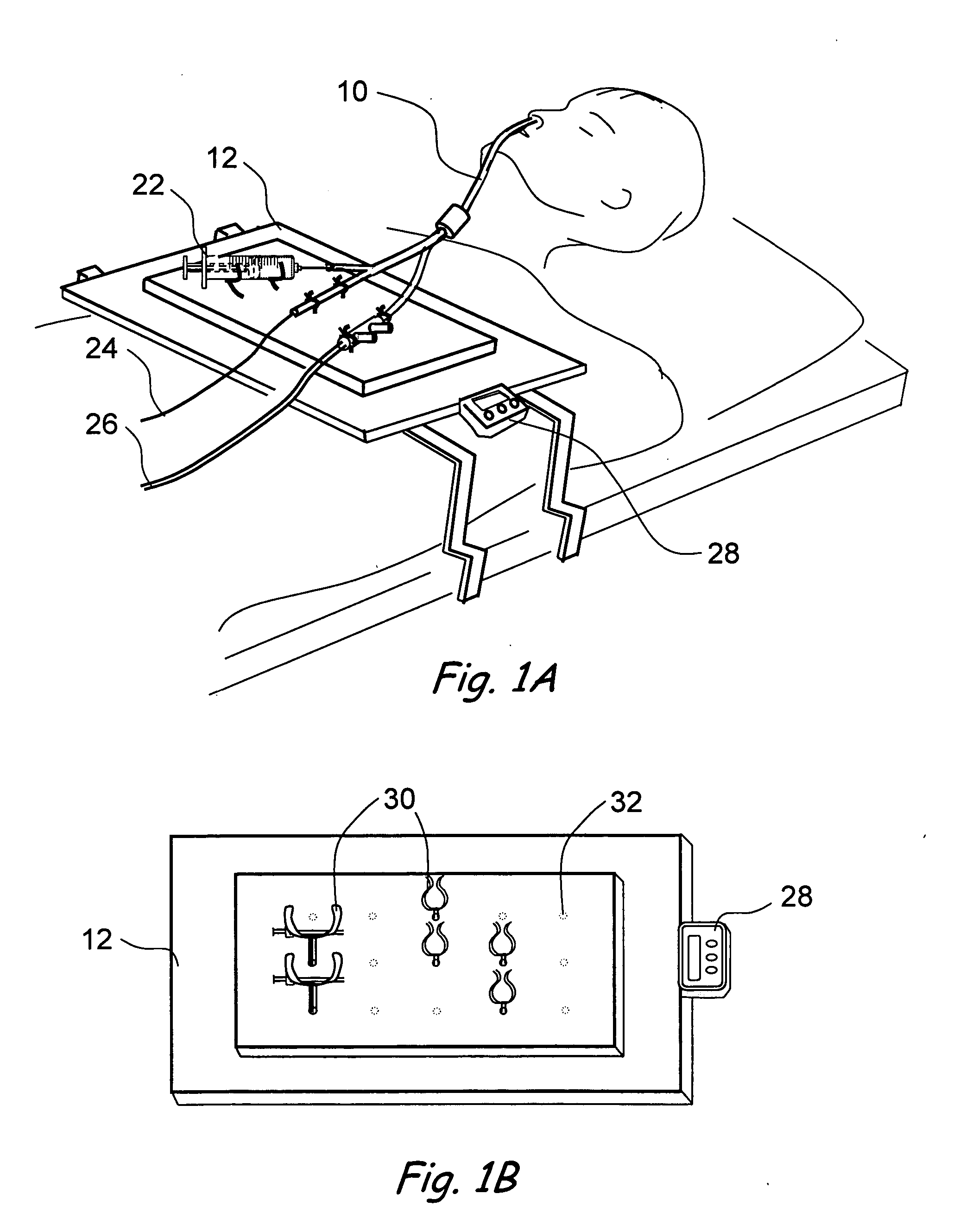
Devices, systems and methods for treating disorders of the ear, nose and throat
Sinusitis, mucocysts, tumors, infections, hearing disorders, choanal atresia, fractures and other disorders of the paranasal sinuses, Eustachian tubes, Lachrymal ducts and other ear, nose, throat and mouth structures are diagnosed and / or treated using minimally invasive approaches and, in many cases, flexible catheters as opposed to instruments having rigid shafts. Various diagnostic procedures and devices are used to perform imaging studies, mucus flow studies, air / gas flow studies, anatomic dimension studies and endoscopic studies. Access and occluding devices may be used to facilitate insertion of working devices such asendoscopes, wires, probes, needles, catheters, balloon catheters, dilation catheters, dilators, balloons, tissue cutting or remodeling devices, suction or irrigation devices, imaging devices, sizing devices, biopsy devices, image-guided devices containing sensors or transmitters, electrosurgical devices, energy emitting devices, devices for injecting diagnostic or therapeutic agents, devices for implanting devices such as stents, substance eluting or delivering devices and implants, etc.
Owner:ACCLARENT INC
Devices, Systems and Methods For Diagnosing and Treating Sinusitis and Other Disorders of the Ears, Nose and/or Throat
Sinusitis, enlarged nasal turbinates, tumors, infections, hearing disorders, allergic conditions, facial fractures and other disorders of the ear, nose and throat are diagnosed and / or treated using minimally invasive approaches and, in many cases, flexible catheters as opposed to instruments having rigid shafts. Various diagnostic procedures and devices are used to perform imaging studies, mucus flow studies, air / gas flow studies, anatomic dimension studies, endoscopic studies and transillumination studies. Access and occluder devices may be used to establish fluid tight seals in the anterior or posterior nasal cavities / nasopharynx and to facilitate insertion of working devices (e.g., scopes, guidewires, catheters, tissue cutting or remodeling devices, electrosurgical devices, energy emitting devices, devices for injecting diagnostic or therapeutic agents, devices for implanting devices such as stents, substance eluting devices, substance delivery implants, etc.
Owner:ACCLARENT INC
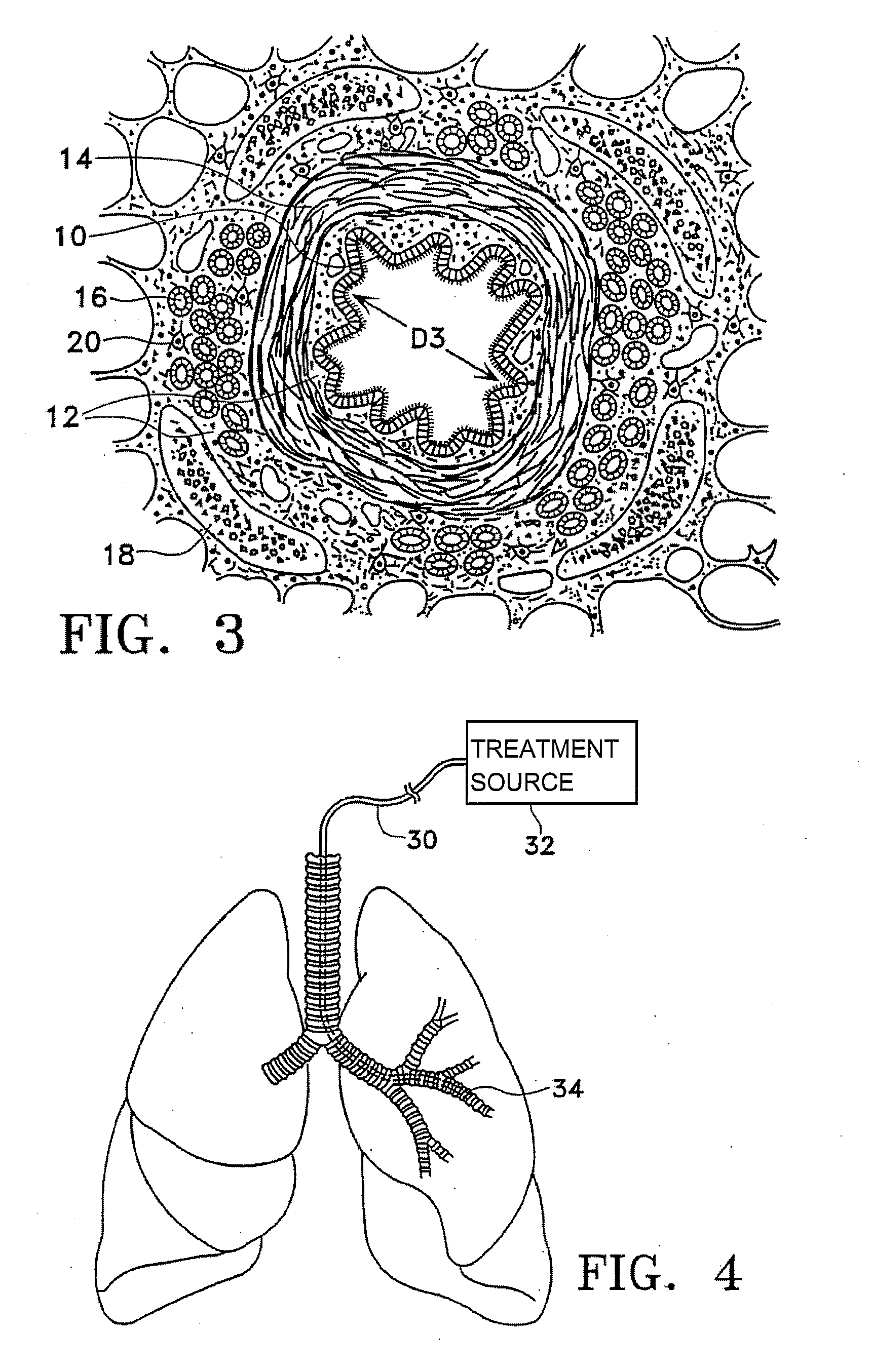
Method of treating airways in the lung
InactiveUS20050010270A1Reduce the amount requiredIncrease inner diameterSurgical instrument detailsLight therapyBronchospasmSmooth muscle spasm
A device and method for treating bodily conduits involves the application of energy to the smooth muscle tissue of the conduit walls to reduce the bulk of smooth muscle tissue and mucus glands. The irradiation treatment of the smooth muscle tissue causes a reduction in the amount of smooth muscle tissue over time which increases the inner diameter of the body conduit for improved fluid flow and prevents smooth muscle spasms. The treatment is particularly useful in the lungs for treatment of asthma to prevent bronchospasms, increase the airway diameter for improved air exchange, and reduce mucus secretions in the lungs.
Owner:BOSTON SCI SCIMED INC
Devices, Systems and Methods for Treating Disorders of the Ear, Nose and Throat
Sinusitis, mucocysts, tumors, infections, hearing disorders, choanal atresia, fractures and other disorders of the paranasal sinuses, Eustachian tubes, Lachrymal ducts and other ear, nose, throat and mouth structures are diagnosed and / or treated using minimally invasive approaches and, in many cases, flexible catheters as opposed to instruments having rigid shafts. Various diagnostic procedures and devices are used to perform imaging studies, mucus flow studies, air / gas flow studies, anatomic dimension studies and endoscopic studies. Access and occluding devices may be used to facilitate insertion of working devices such asendoscopes, wires, probes, needles, catheters, balloon catheters, dilation catheters, dilators, balloons, tissue cutting or remodeling devices, suction or irrigation devices, imaging devices, sizing devices, biopsy devices, image-guided devices containing sensors or transmitters, electrosurgical devices, energy emitting devices, devices for injecting diagnostic or therapeutic agents, devices for implanting devices such as stents, substance eluting or delivering devices and implants, etc.
Owner:ACCLARENT INC
Devices, Systems and Methods for Treating Disorders of the Ear, Nose and Throat
Sinusitis, mucocysts, tumors, infections, hearing disorders, choanal atresia, fractures and other disorders of the paranasal sinuses, Eustachian tubes, Lachrymal ducts and other ear, nose, throat and mouth structures are diagnosed and / or treated using minimally invasive approaches and, in many cases, flexible catheters as opposed to instruments having rigid shafts. Various diagnostic procedures and devices are used to perform imaging studies, mucus flow studies, air / gas flow studies, anatomic dimension studies and endoscopic studies. Access and occluding devices may be used to facilitate insertion of working devices such asendoscopes, wires, probes, needles, catheters, balloon catheters, dilation catheters, dilators, balloons, tissue cutting or remodeling devices, suction or irrigation devices, imaging devices, sizing devices, biopsy devices, image-guided devices containing sensors or transmitters, electrosurgical devices, energy emitting devices, devices for injecting diagnostic or therapeutic agents, devices for implanting devices such as stents, substance eluting or delivering devices and implants, etc.
Owner:ACCLARENT INC
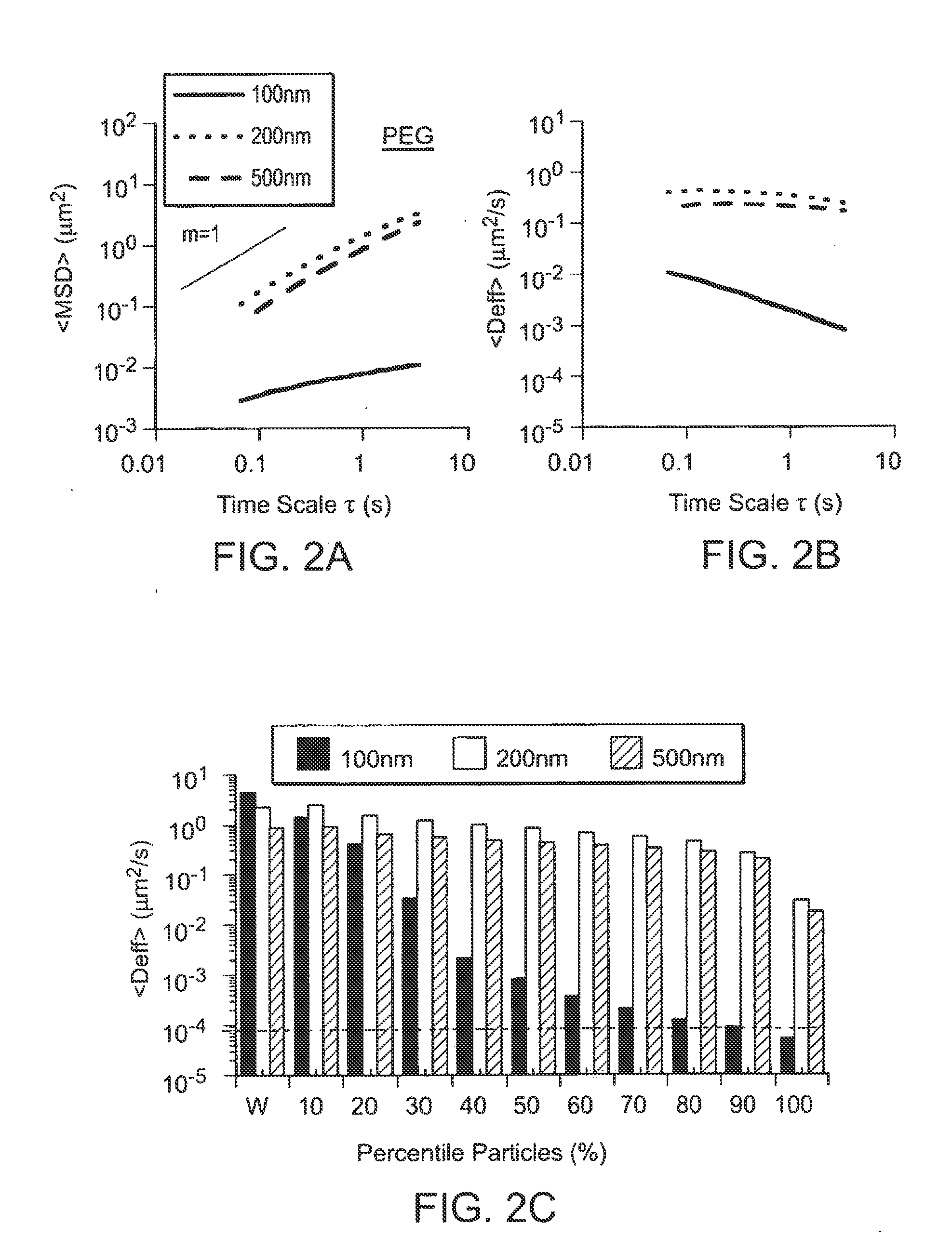
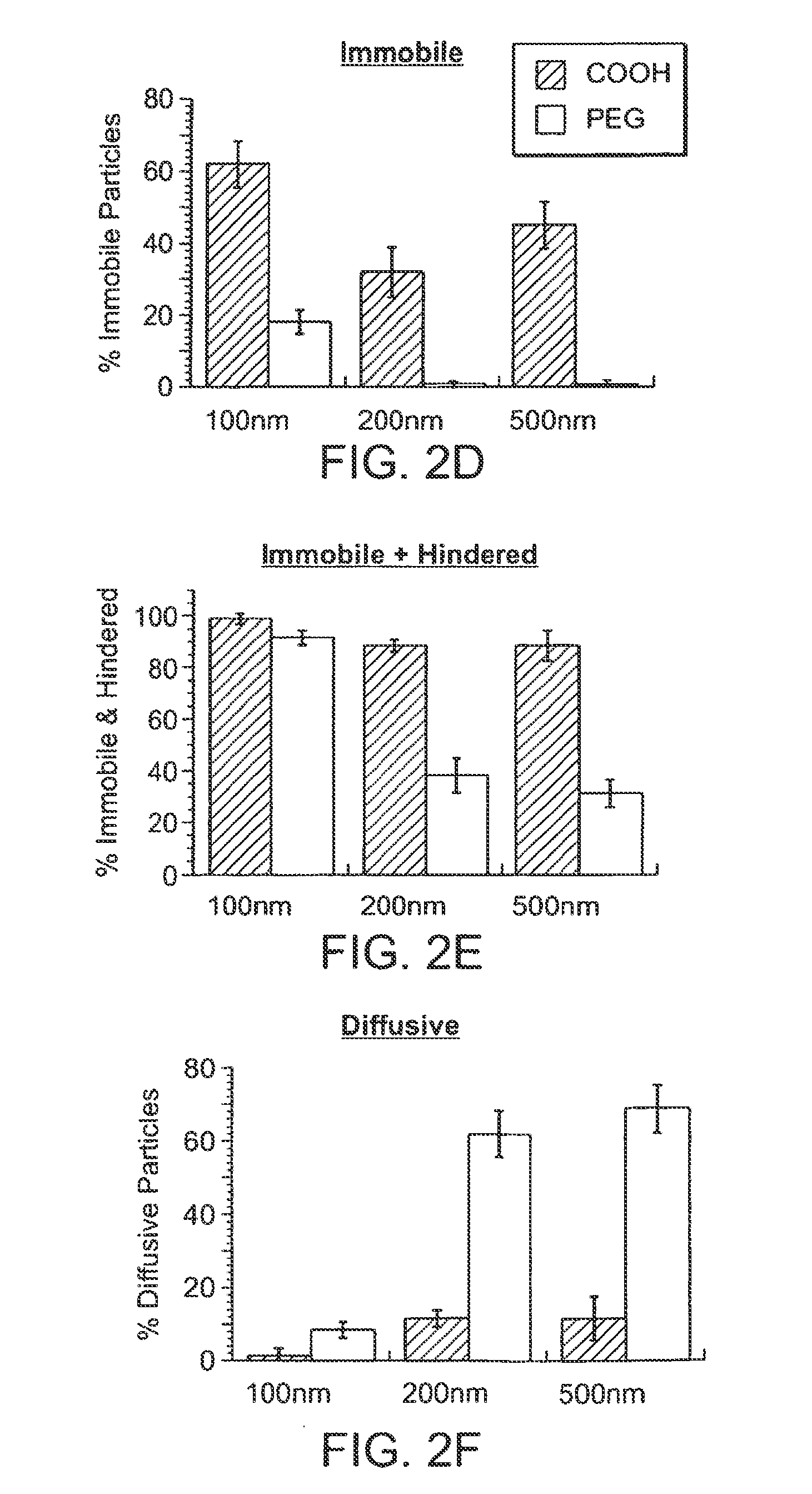
Compositions and methods for enhancing transport through mucus
InactiveUS20130164343A1Diffusion fastPromote adhesion and complexationPowder deliveryNanomedicineRespiratory mucusChemistry
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Combined nasal spray and aspirator device
ActiveUS7862536B2Present invention is physically more favorable for usersGood for healthEar treatmentCannulasNasal cavityNebulizer
Owner:AVITA CORP
Bronchoscopic lung volume reduction valve
ActiveUS20070096048A1Permit flowAvoid flowStentsBronchiPorous coatingBronchoscopic lung volume reduction
A valve to perform lung volume reduction procedures is described. The valve is formed of a braided structure that is adapted for endoscopic insertion in a bronchial passage of a patient's lung. The braided structure has a proximal end and a distal end and is covered with a non porous coating adapted to prevent flow of air into the. A constricted portion of the braided structure is used to prevent flow of air through a central lumen of the structure, and to define at least one funnel shaped portion. The funnel shaped portion blocks the flow of air towards the constriction, i.e. towards the core of the lung. At least one hole is formed in the braided structure to permit flow of mucus from the distal end to the proximal end, to be expelled out of the lungs.
Owner:BOSTON SCI SCIMED INC
Method for treating airways in the lung
InactiveUS20070106348A1Reduce the amount requiredIncrease inner diameterDiagnosticsMedical devicesBronchospasmSmooth muscle spasm
A device and method for treating bodily conduits involves the application of energy to the smooth muscle tissue of the conduit walls to reduce the bulk of smooth muscle tissue and mucus glands. The irradiation treatment of the smooth muscle tissue causes a reduction in the amount of smooth muscle tissue over time which increases the inner diameter of the body conduit for improved fluid flow and prevents smooth muscle spasms. The treatment is particularly useful in the lungs for treatment of asthma to prevent bronchospasms, increase the airway diameter for improved air exchange, and reduce mucus secretions in the lungs.
Owner:BOSTON SCI SCIMED INC
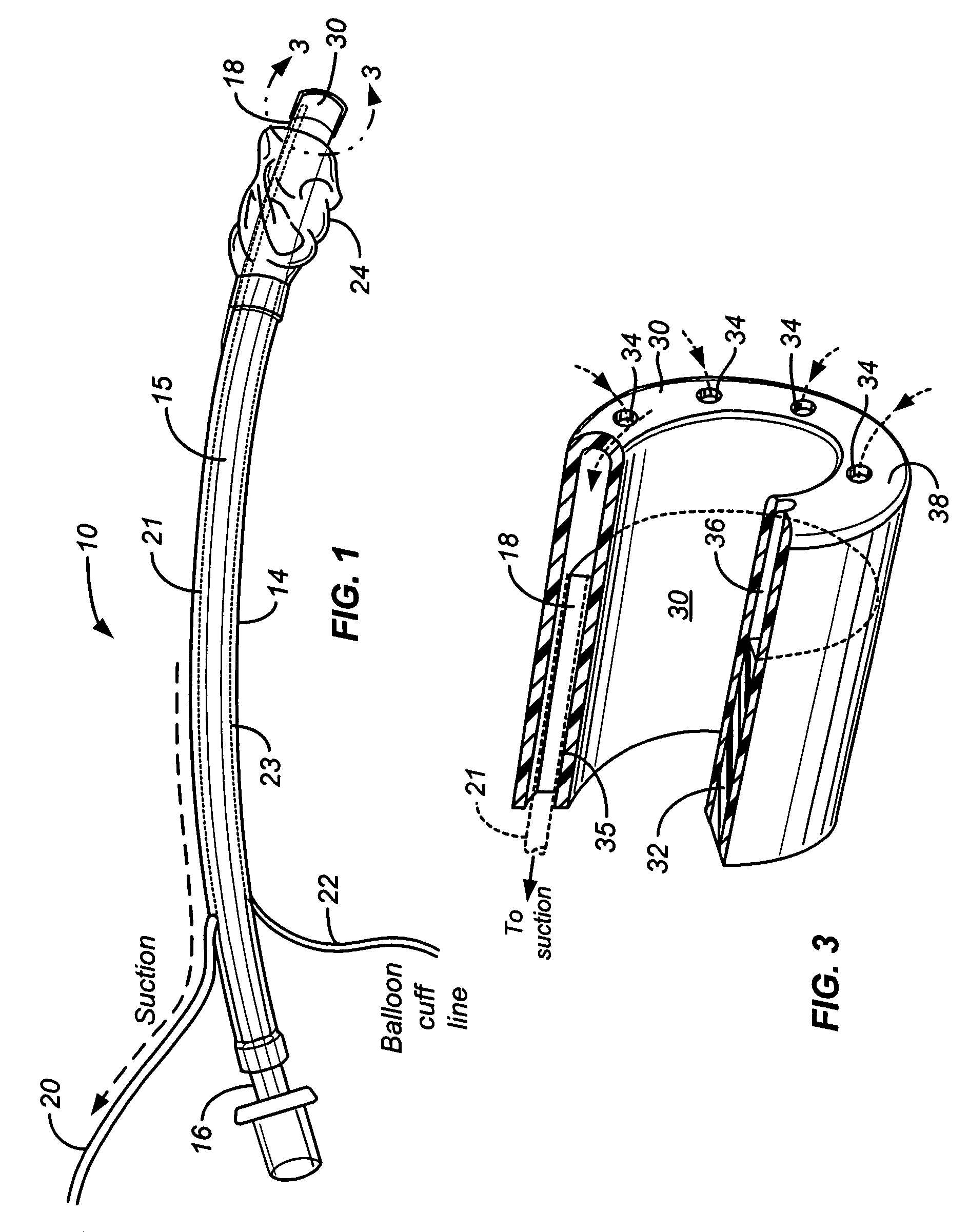
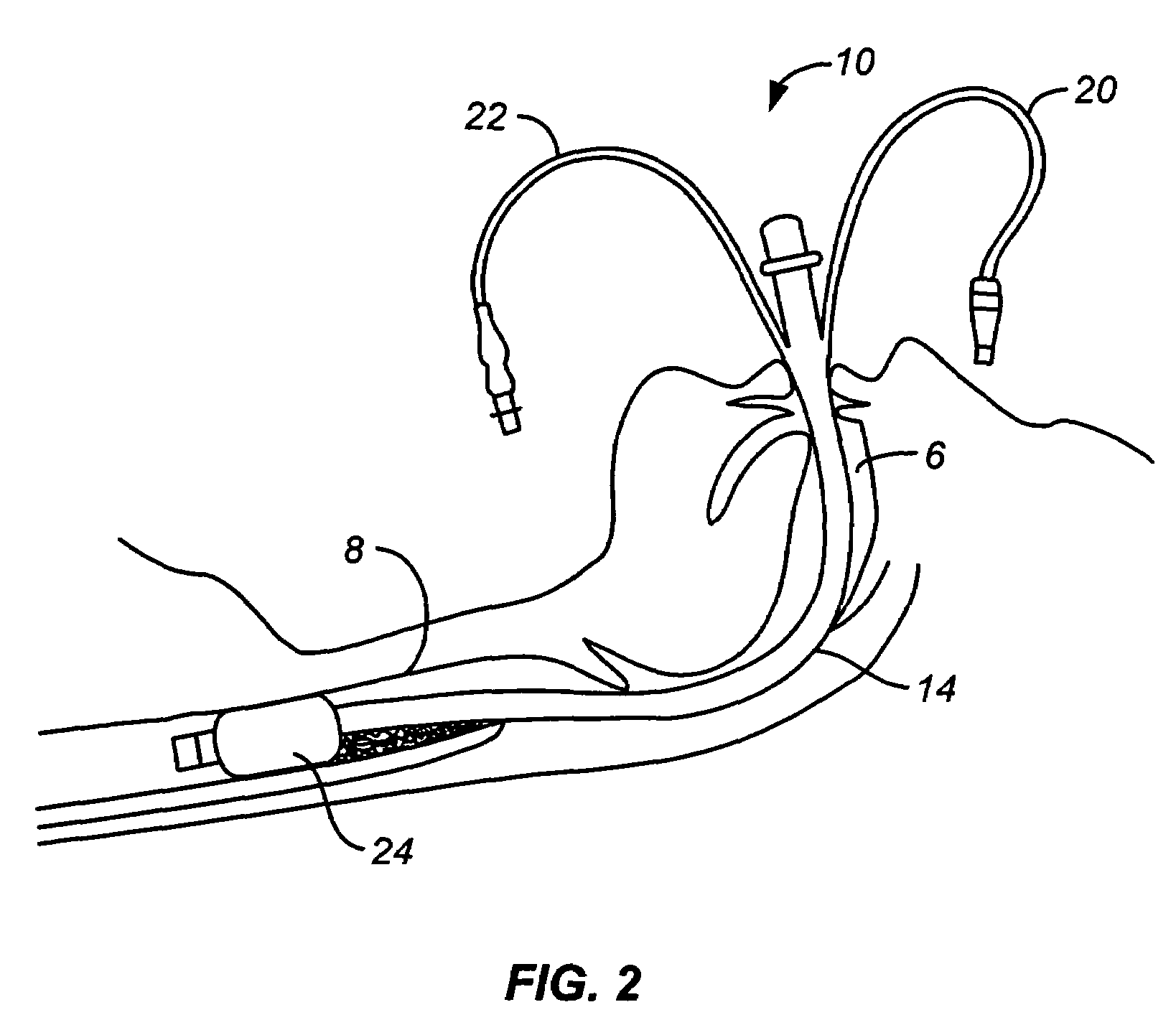
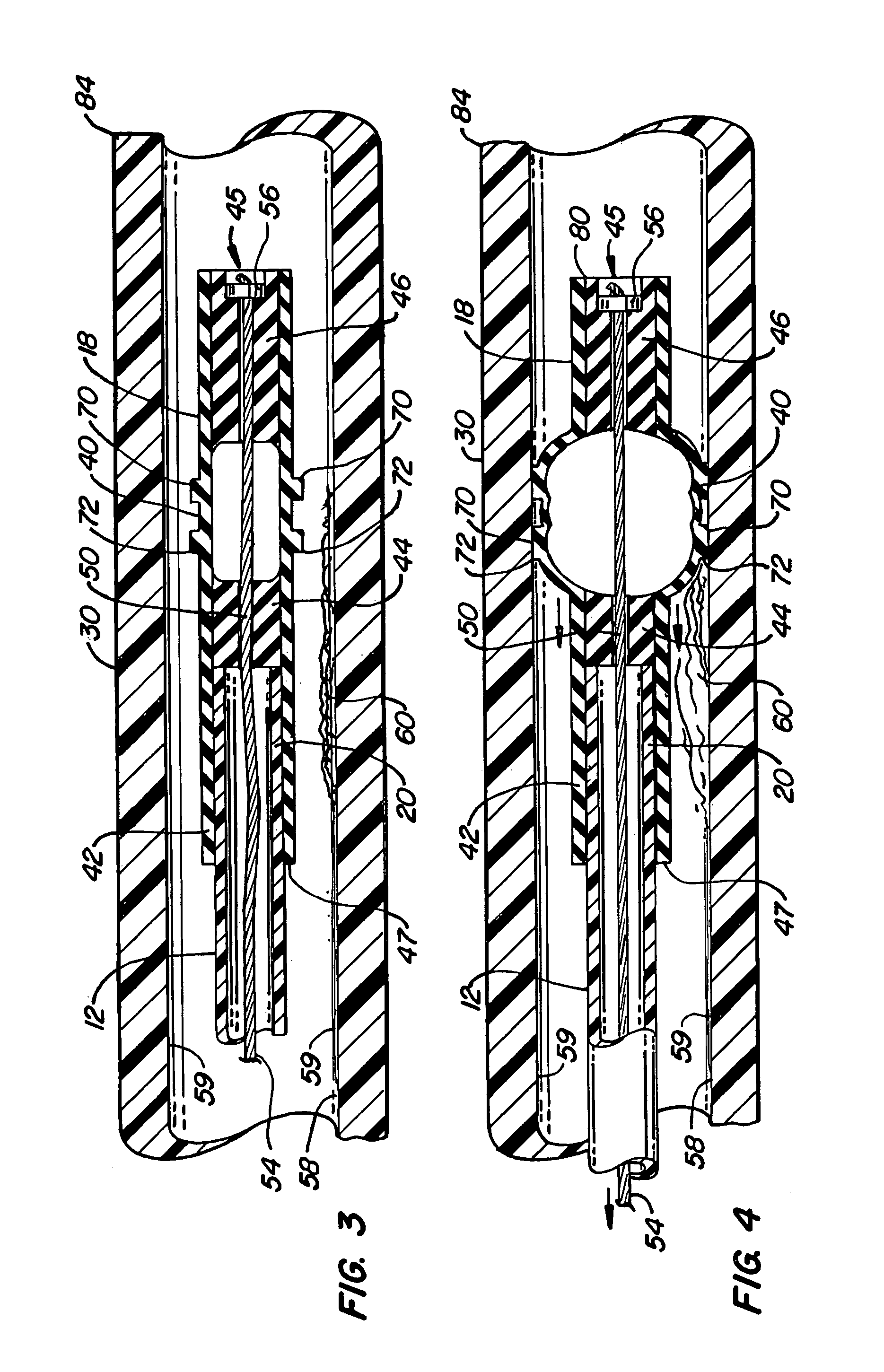
Mucus slurping endotracheal tube
ActiveUS7503328B2Greater assurance of cleanlinessRespiratorsMedical devicesTracheal tubeDisinfectant
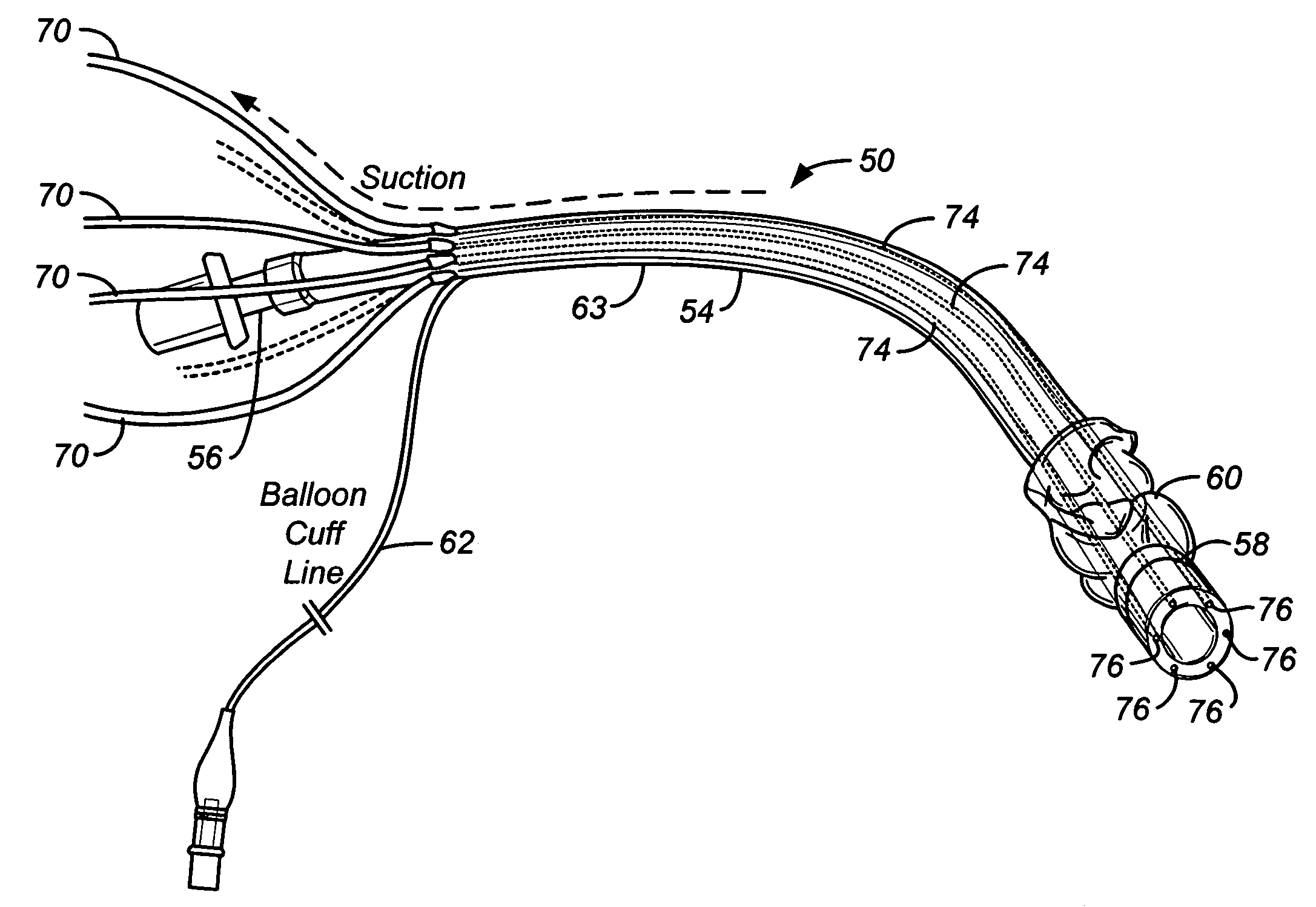
An endotracheal tube assembly (10) is disclosed which suctions away bacteria multiplying mucus before such mucus can accumulate on the inside walls of the endotracheal tube (14). In the preferred embodiment, one or more suctioning tubes (20, 21) are formed into the walls of an endotracheal tube so that they extend along length of the endotracheal tube. A plurality of mucus slurping holes (34) are then formed at or near the distal end of the endotracheal tube and connected to the suctioning tubes. In operation, suctioning through the mucus slurping holes is preferably performed intermittently during patient expiration. By timing this intermittent suctioning with patient expiration, the suctioning flow will be in the same direction as patient breathing. While the mucus slurper of the present invention has been found to be effective at keeping the inner walls of the endotracheal tube free of mucus deposits, it can nonetheless be combined with other cleaning and disinfectant techniques for greater assurance of cleanliness.
Owner:UNITED STATES OF AMERICA
Method and apparatus for alleviating nasal congestion
InactiveUS20090056709A1Reduce riskLimit insertionRespiratorsBreathing masksNasal passageNasal cavity
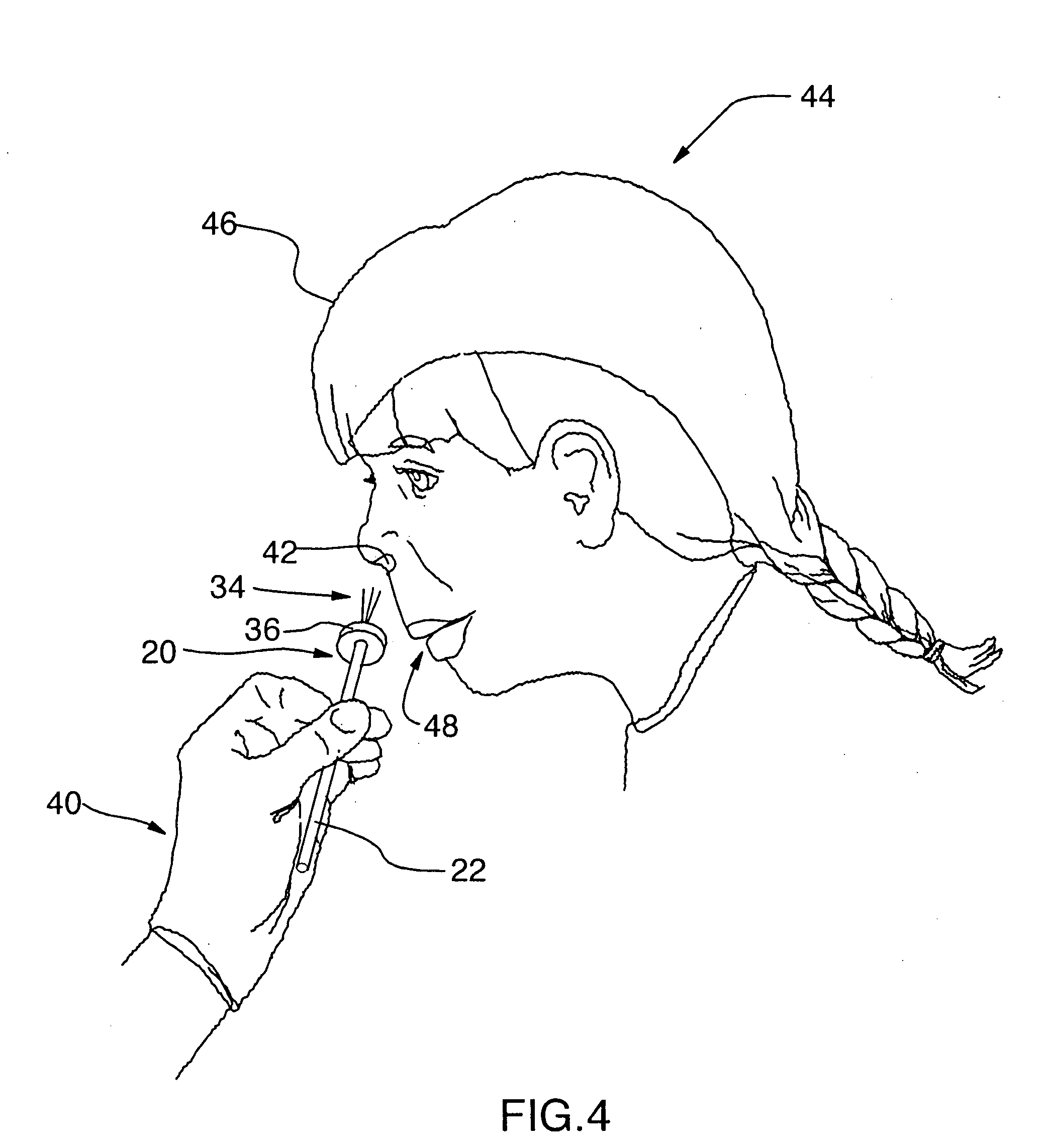
The present invention relates generally to the field of treatments for nasal congestion, and in particular, to a method and apparatus for alleviating nasal congestion in a patient by mechanically stimulating the sneezing reflex in the patient to urge drainage of the nasal passageways. The method includes inserting an instrument having a work tip portion provided with at least one filament into the nostril of the patient. The nasal mucosa of the patient is then probed with the at least one filament to stimulate in the patient the sneeze reflex. This probing action causes the patient to sneeze. With the patient's mouth closed, the sneeze urges at least one of mucus, fluid and debris in the nasal passages of the patient to be forcibly expelled through the nostrils of the patient thereby draining the nasal cavity of the patient. The instrument used in the performance of this method includes a handle portion upon which the work tip portion is carried. The handle portion may be integrally formed with the work tip portion or releasably attachable thereto. The instrument is further provided with a physical stop associated with one of the work tip portion and the handle portion to prevent injury resulting from the working tip portion being inserted too deeply into the nasal passages of the patient.
Owner:WORSOFF MAYA
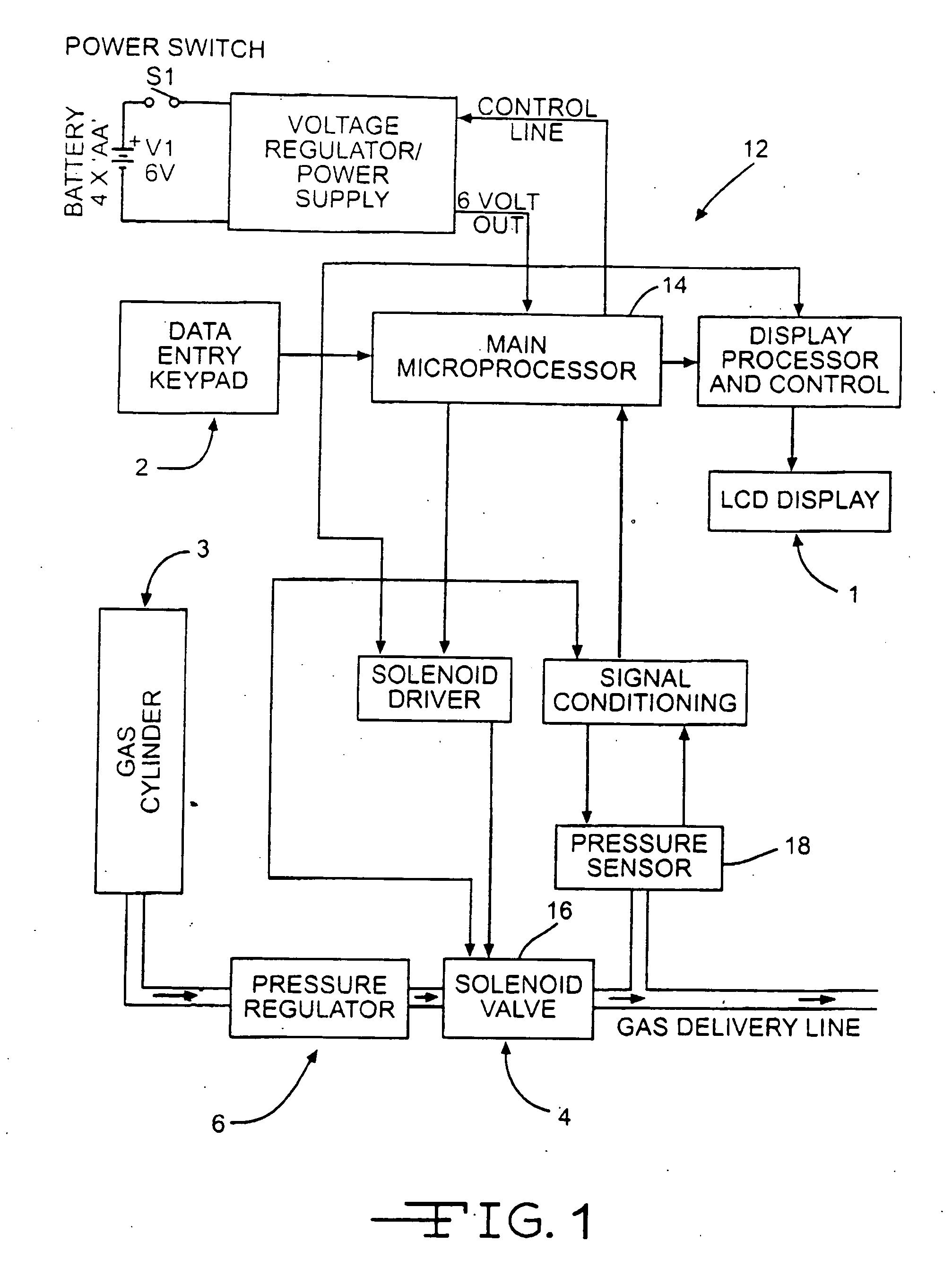
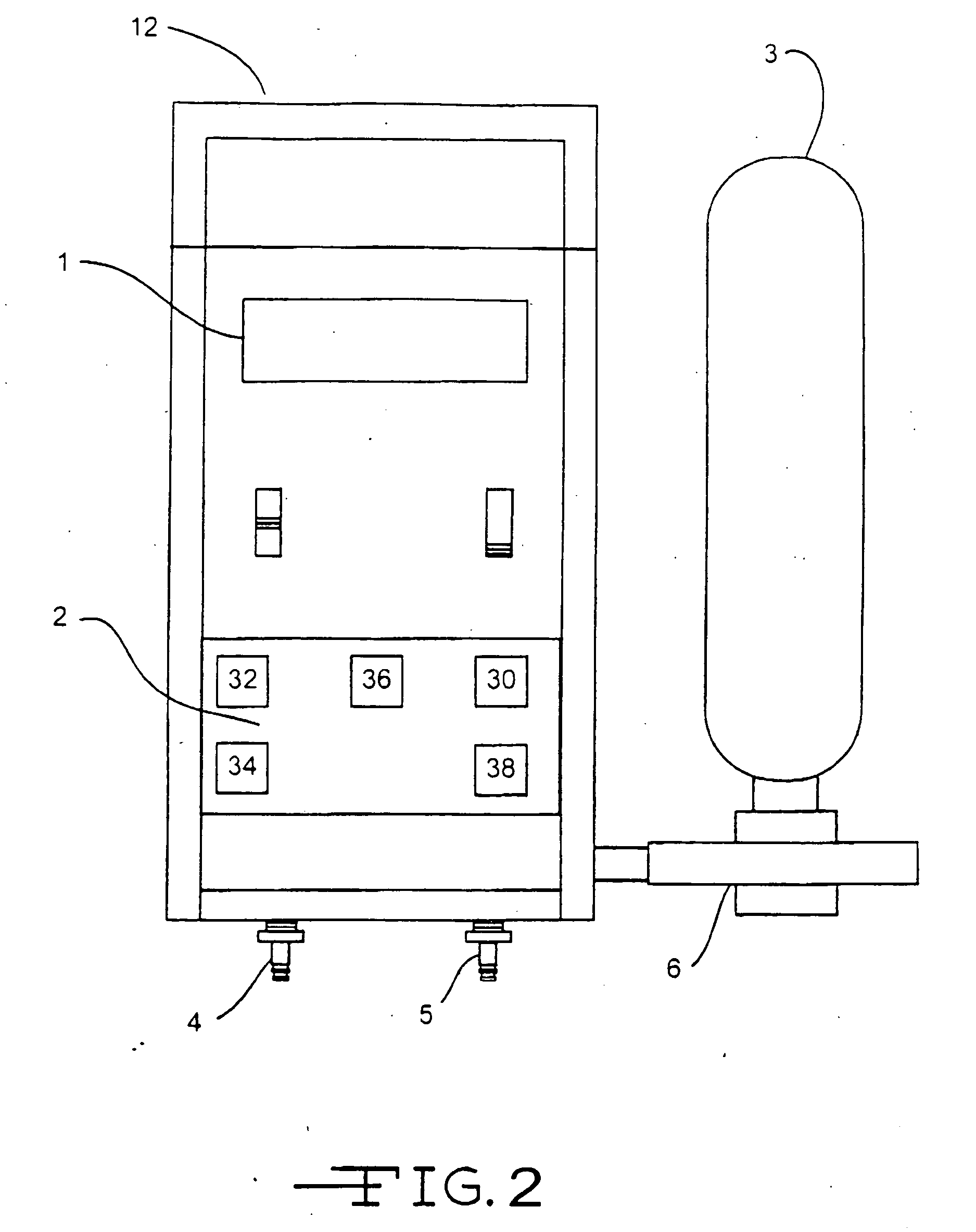
Acoustic respiratory therapy apparatus
ActiveUS20070113843A1Easy to operateGuaranteed uptimeRespiratorsElectrotherapyBreathing passagesTherapeutic Devices
An active respiratory therapeutic device for clearing breathing passages, loosening and breaking up mucus plugs and phlegm in a patient's sinuses, trachea, bronchial passages and lungs while a patient is breathing normally through the device is disclosed. The apparatus preferably includes a C shaped curved hollow housing having a closed end portion and an open threaded end portion. The open end portion forms at least part of an acoustic coupling chamber. A generally funnel shaped tapered mouthpiece tapers to a small end portion sized to be inserted into a patient's mouth. The mouthpiece forms another part of the acoustic coupling chamber. An acoustic signal generator housed within the hollow housing generates and directs acoustic vibrations into and through the coupling chamber. The mouthpiece preferably includes a valve permitting a patient breathing through the mouthpiece to inhale through a valve opening and exhale through a bypass passage around the valve while at the same time coupling the acoustic coupling chamber into the patient's airways.
Owner:VIBRALUNG
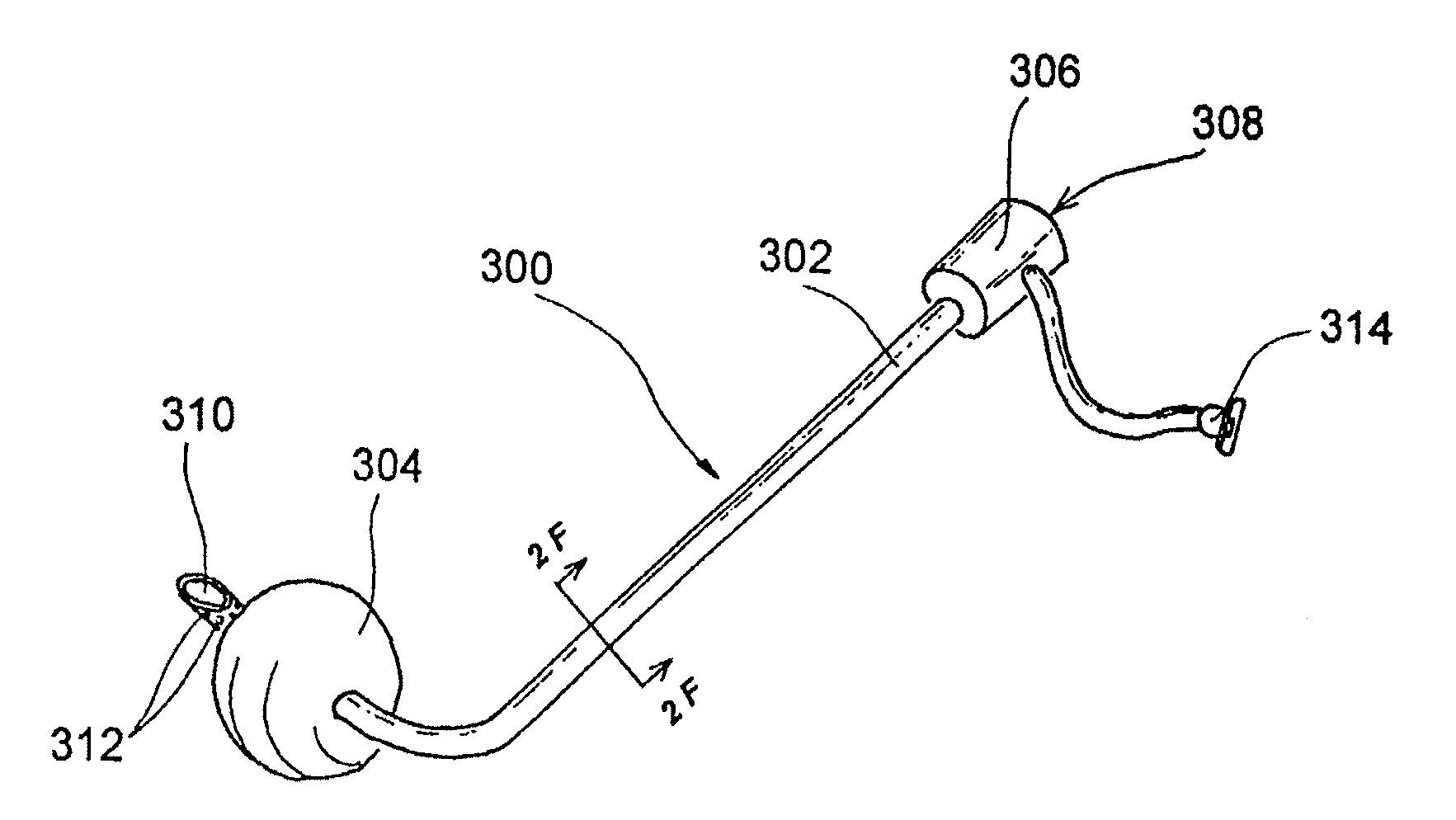
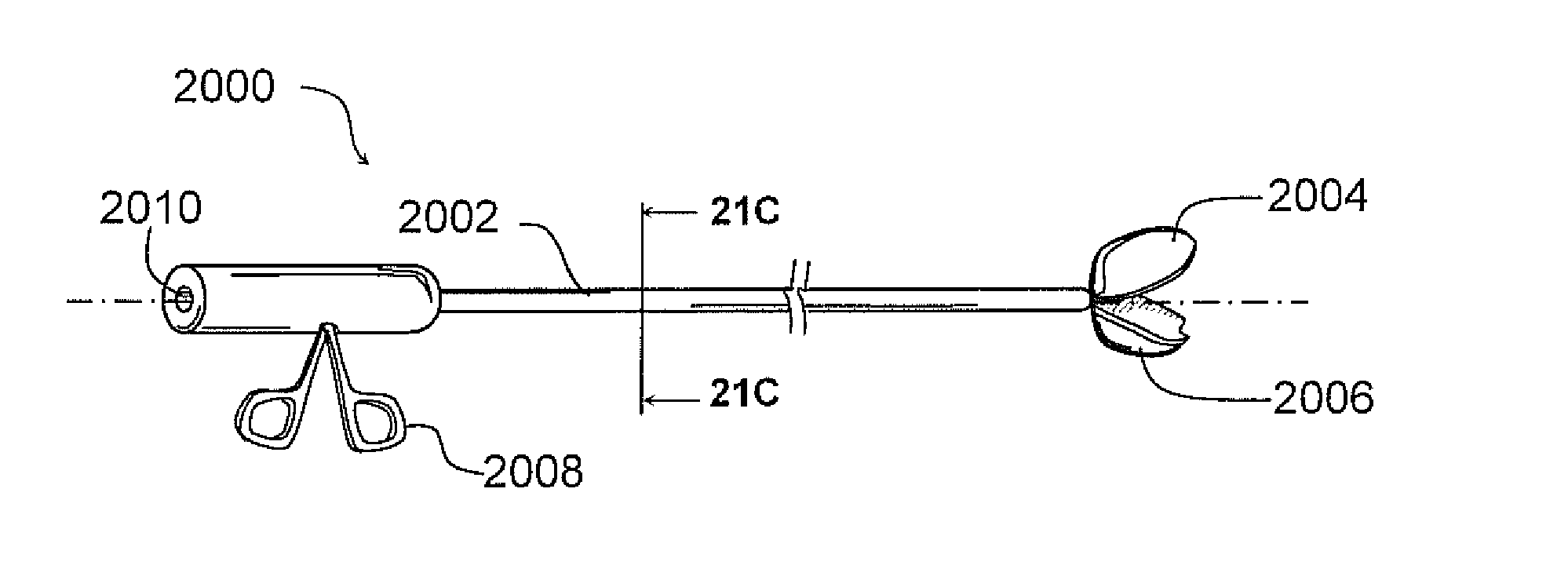
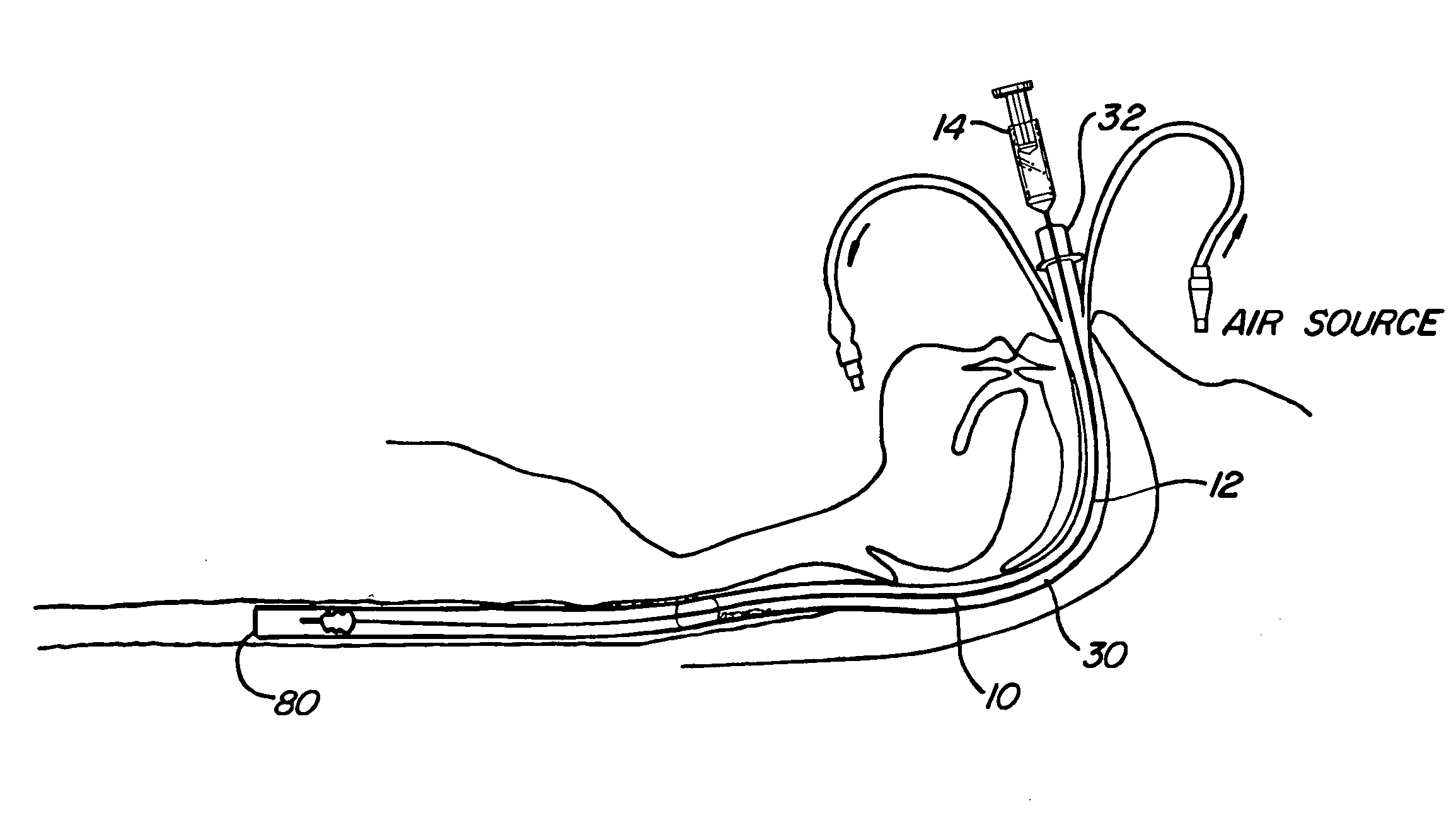
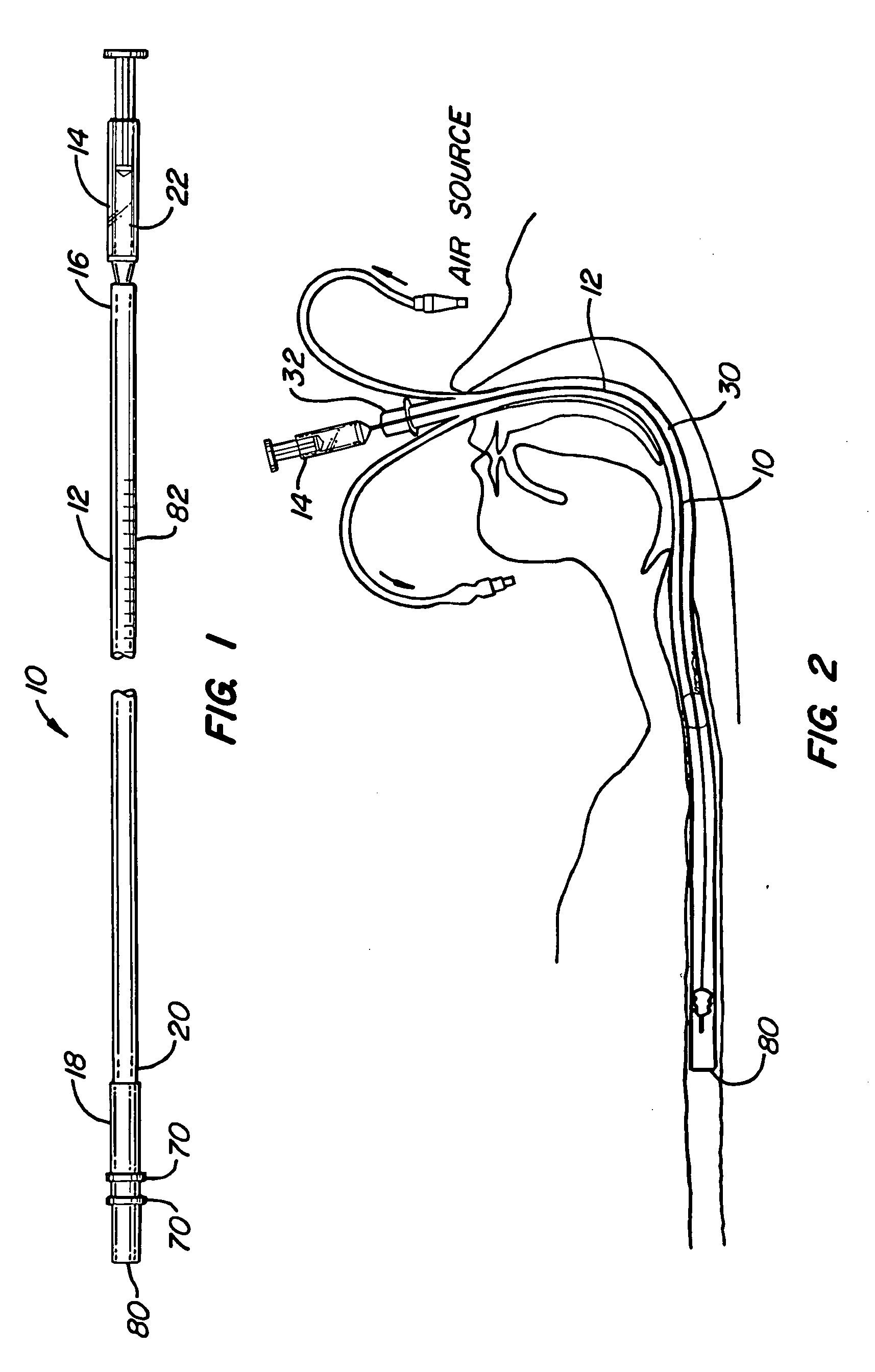
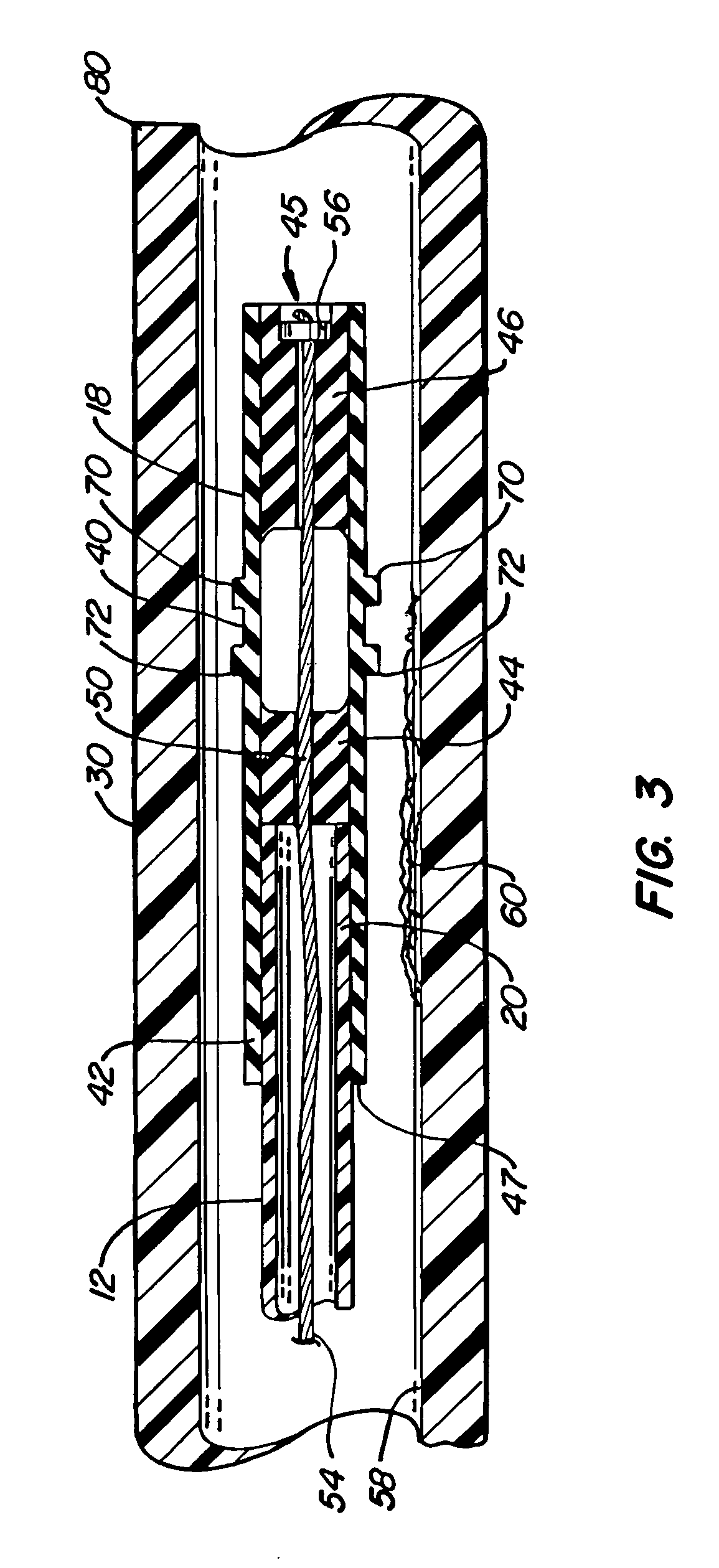
Mucus shaving apparatus for endotracheal tubes
An endotracheal tube cleaning apparatus 10 which can be periodically inserted into the inside of an endotracheal tube 30 to shave away mucus deposits. In a preferred embodiment, this cleaning apparatus 10 comprises a flexible central tube 12 with an inflatable balloon 40 at its distal end. Affixed to the inflatable balloon are one or more shaving rings 70, each having a squared leading edge 72 to shave away mucus accumulations 60. In operation, the uninflated cleaning apparatus 10 of the present invention is inserted into the endotracheal tube until its distal end is properly aligned with or slightly beyond the distal end of the endotracheal tube. After proper alignment, the balloon 40 is inflated by a suitable inflation device, such as a syringe 14, until the balloon's shaving rings are pressed against the inside surface of the endotracheal tube. The cleaning apparatus is then pulled out of the endotracheal tube and, in the process, the balloon's shaving rings shave off the mucus deposits from the inside of the endotracheal tube.
Owner:DEPT OF HEALTH & HUMAN SERVICES GOVERNMENT OF THE UNITED STATES AS REPRESENTED BY THE SEC OF THE +1
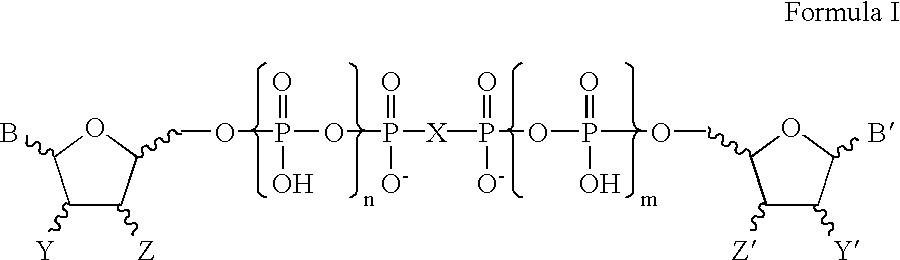
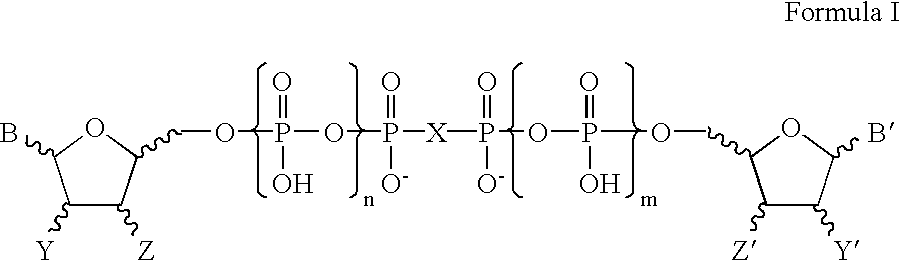
Certain dinucleotides and their use as modulators of mucociliary clearance and ciliary beat frequency
The present invention relates to certain novel dinucleotides and formulations thereof which are highly selective agonists of the P2Y2 and / or P2Y4 purinergic receptor. They are useful in the treatment of chronic obstructive pulmonary diseases such as chronic bronchitis, PCD, cystic fibrosis, as well as prevention of pneumonia due to immobility. Furthermore, because of their general ability to clear retained mucus secretions and stimulate ciliary beat frequency, the compounds of the present invention are also useful in the treatment of sinusitis, otitis media and nasolacrimal duct obstruction. They are also useful for treatment of dry eye disease and retinal detachment as well as facilitating sputum induction and expectoration.
Owner:MERCK SHARP & DOHME CORP
Acoustic respiratory therapy apparatus
Owner:VIBRALUNG
Vaginal health products
InactiveUS7485666B2Minor side effectsPromote cell growthBiocideEther/acetal active ingredientsCell turnoverMammal
Owner:KIMBERLY-CLARK WORLDWIDE INC
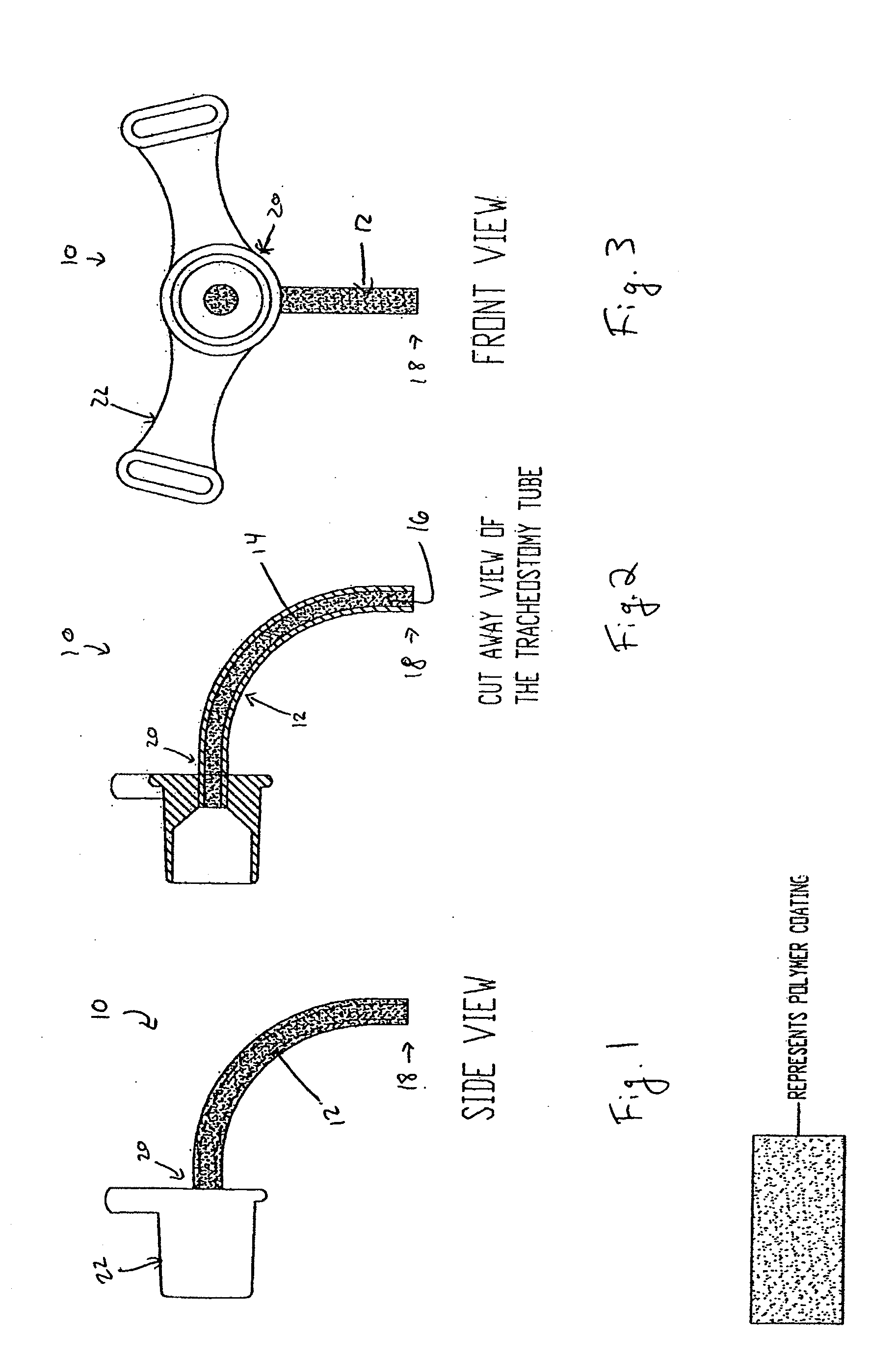
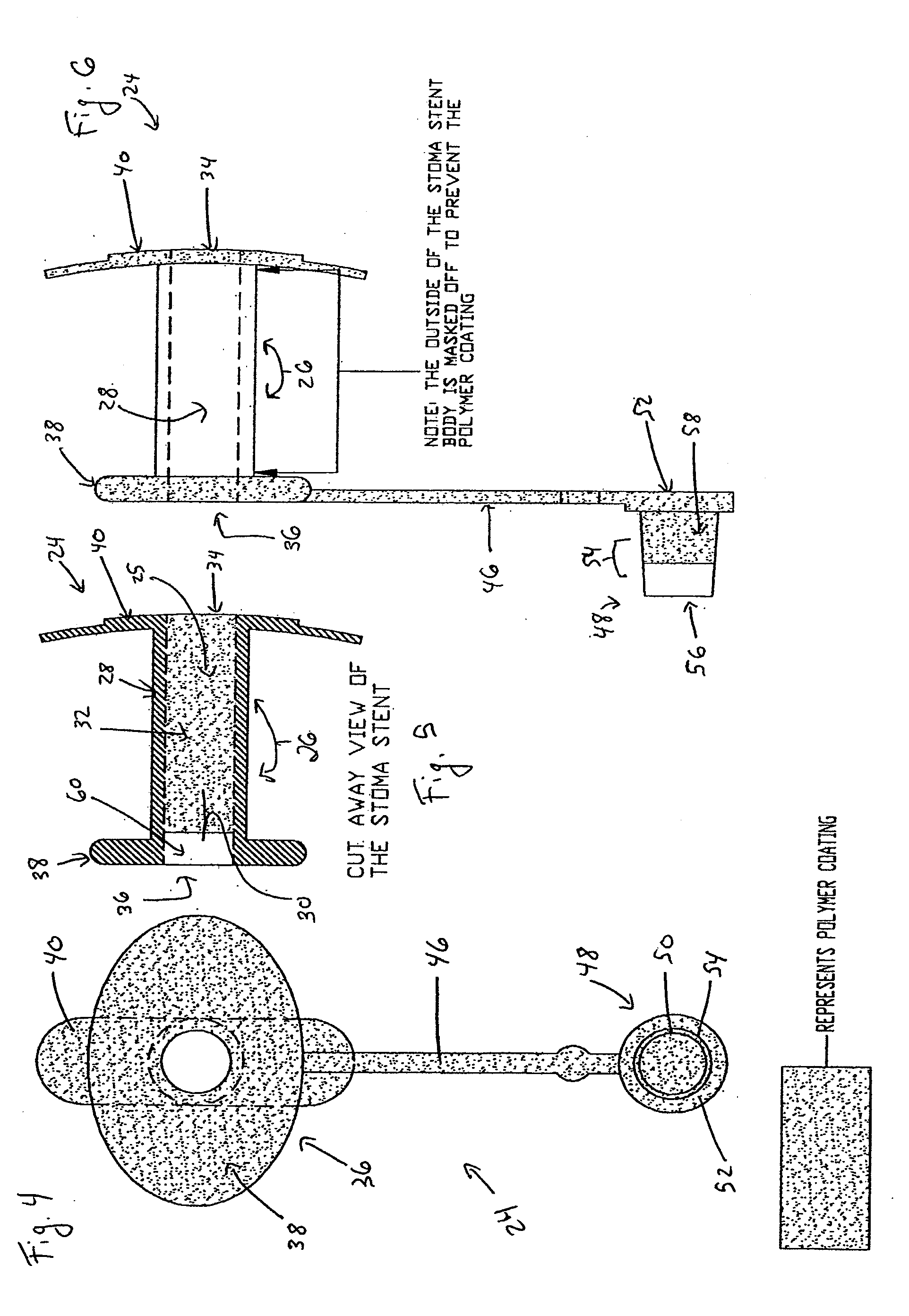
Coated Tracheostomy Tube and Stoma Stent or Cannula
The present invention relates to devices used in the management of bodily airways including tracheostomy tubes, laryngectomy tubes, bronchial stents, bronchial Y-tubes, bronchial TY-tubes, and nasal stents. The devices may comprise a protective coating to prevent the accumulation of mucus, crusting and granulation on or around airway management devices, as well as prevent adhesion to tissues which can cause bleeding upon removal, and prevent build-up of blood, or blood clots, to the stent.
Owner:E BENSON HOOD LAB
Use of inhaled gaseous nitric oxide as a mucolytic agent or expectorant
InactiveUS20070104653A1Increased mucociliary clearanceReduce severityRespiratorsBiocideInhalationCompound (substance)
Methods and devices for treating excess mucus accumulation in mammals by administering gaseous inhaled nitric oxide or nitric oxide releasing compounds as a mucolytic agent or expectorant are provided. Delivery of gaseous nitric oxide can be made nasally or orally and is preferably substantially coincident with inhalation of the mammal or based on a synchronous parameter of the mammal's respiratory cycle. Varying therapeutic profiles may be used for the delivery of gaseous nitric oxide depending on the severity of the excess mucus accumulation. Parameters for the therapeutic profiles may include flow rate of nitric oxide containing gas, duration of administration of nitric oxide containing gas, number of breaths for which nitric oxide containing gas is to be administered, and concentrations of therapeutic NO delivered to the airways.
Owner:BEYOND AIR LTD
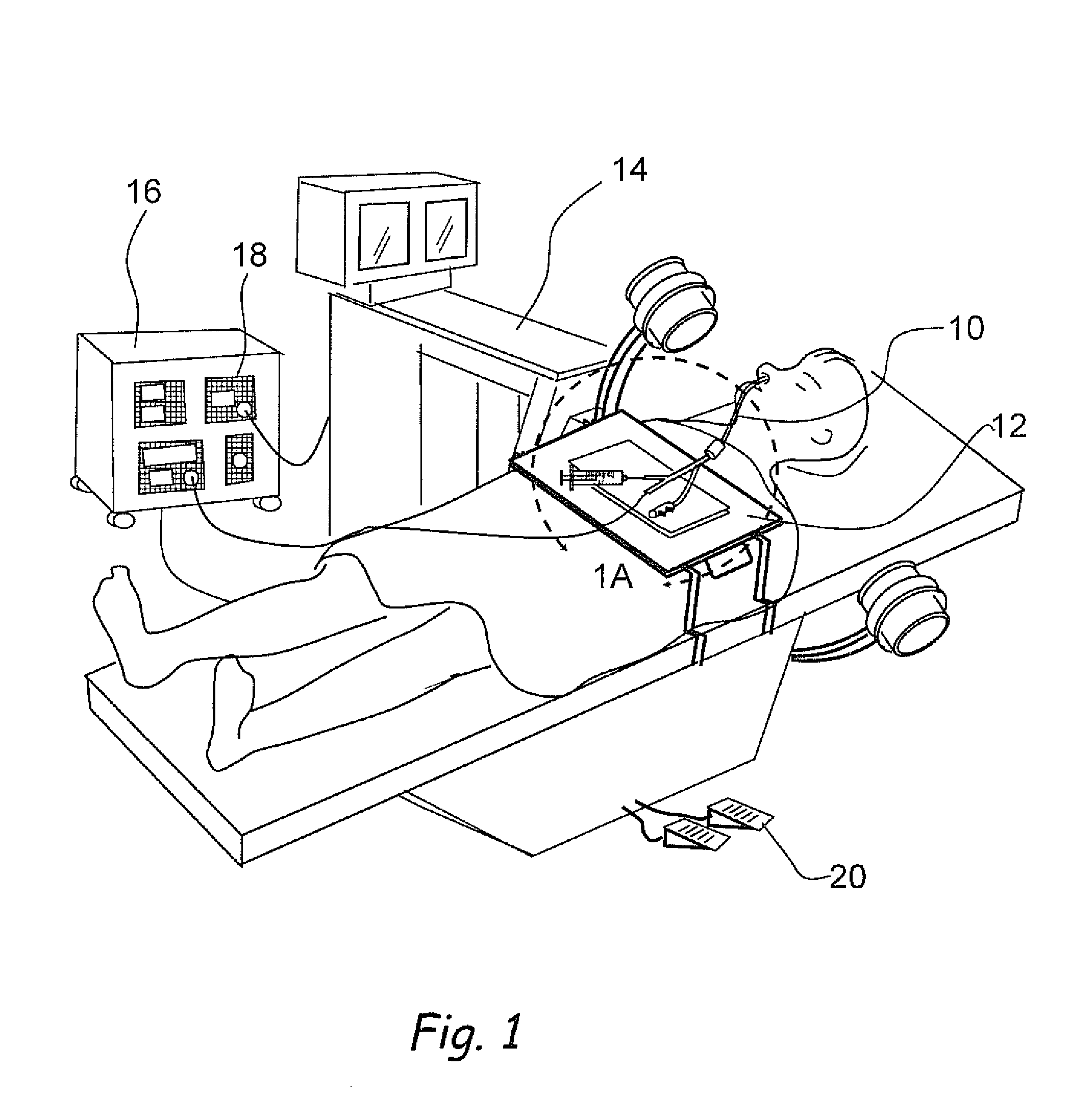
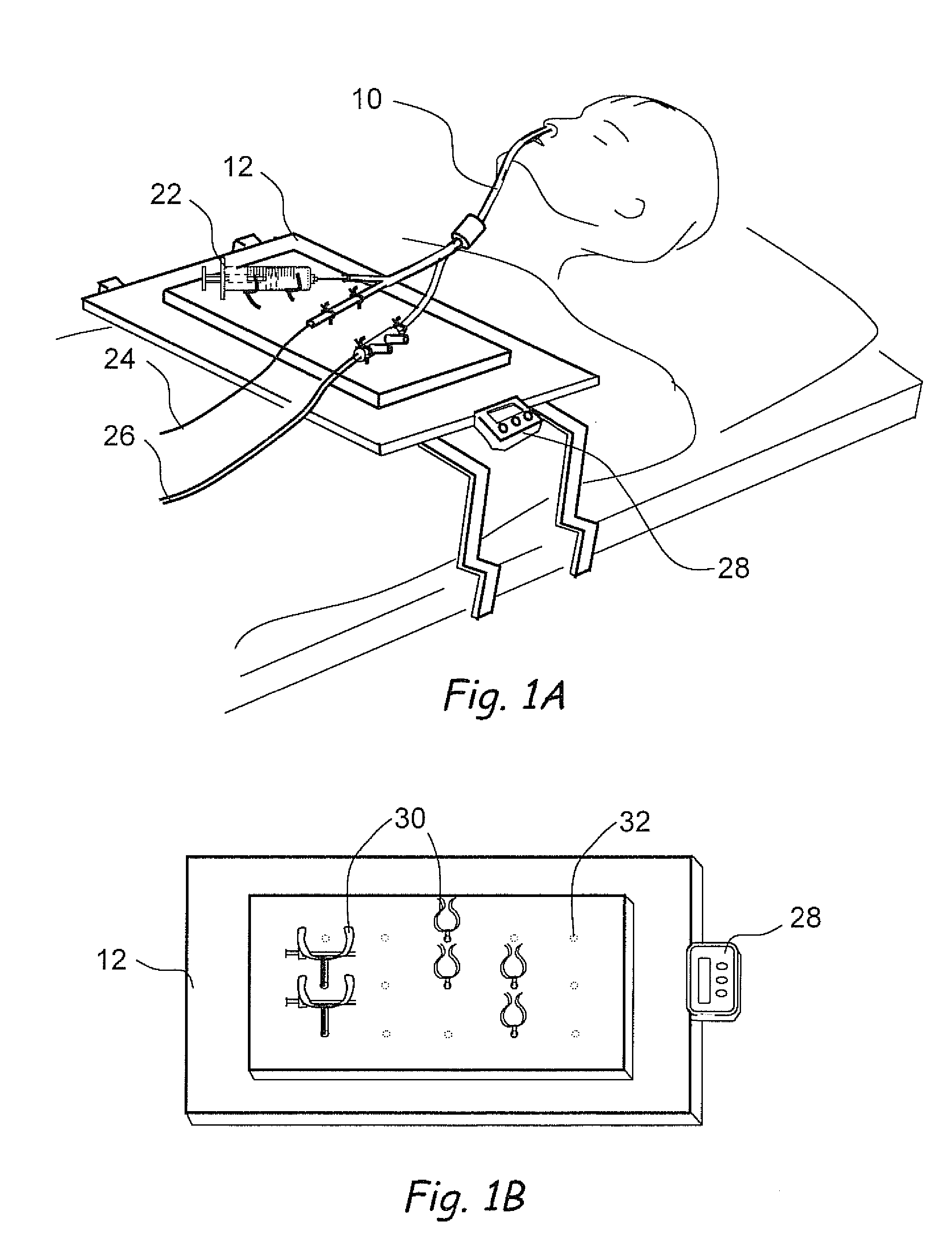
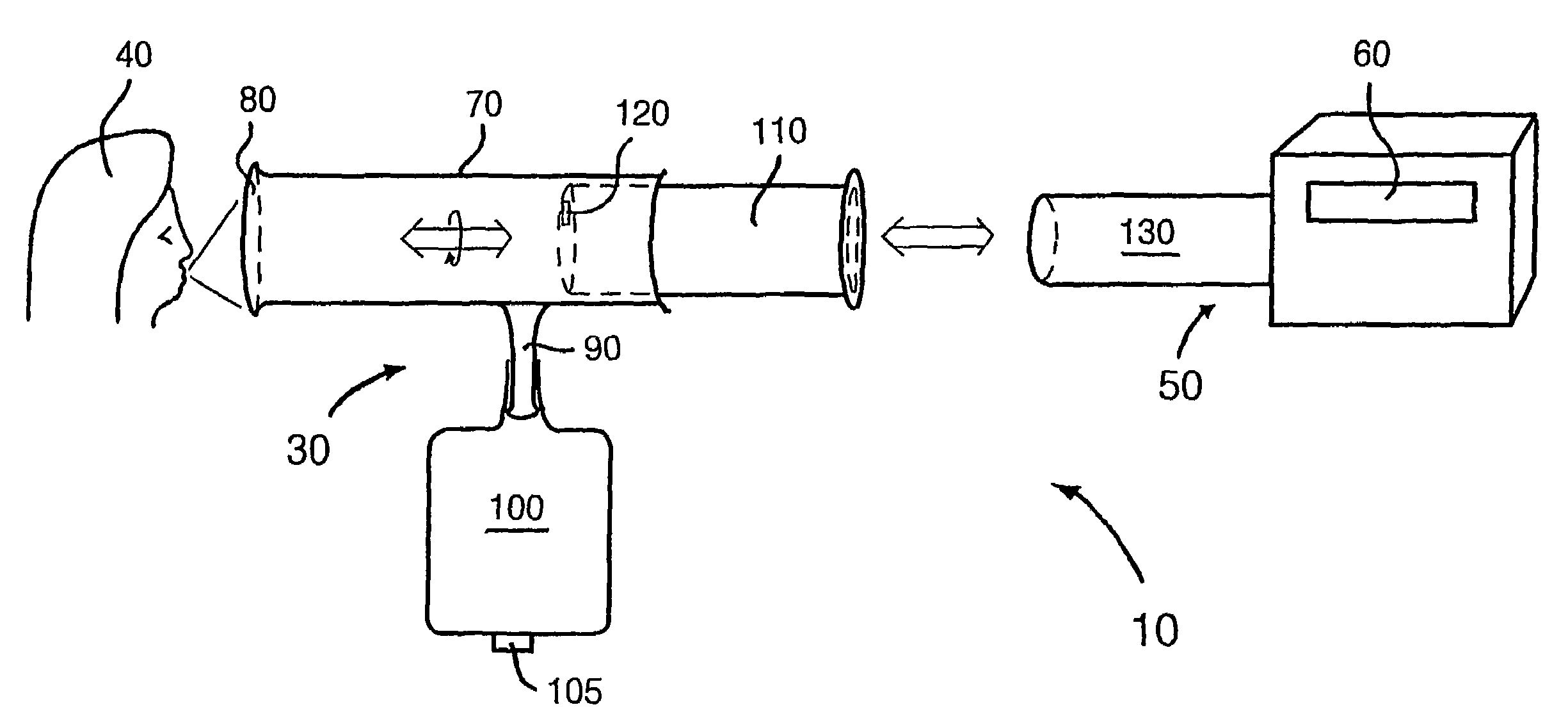
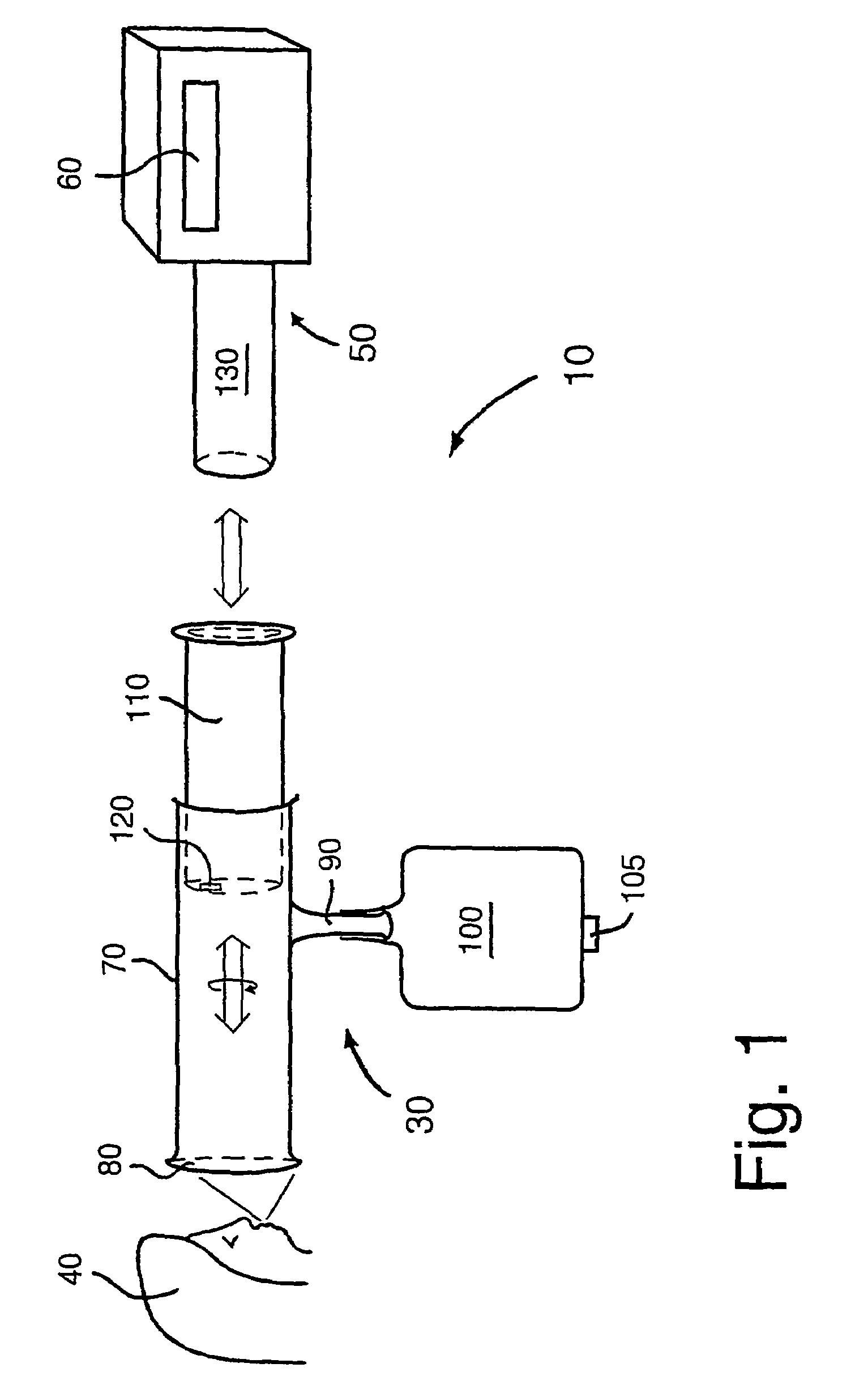
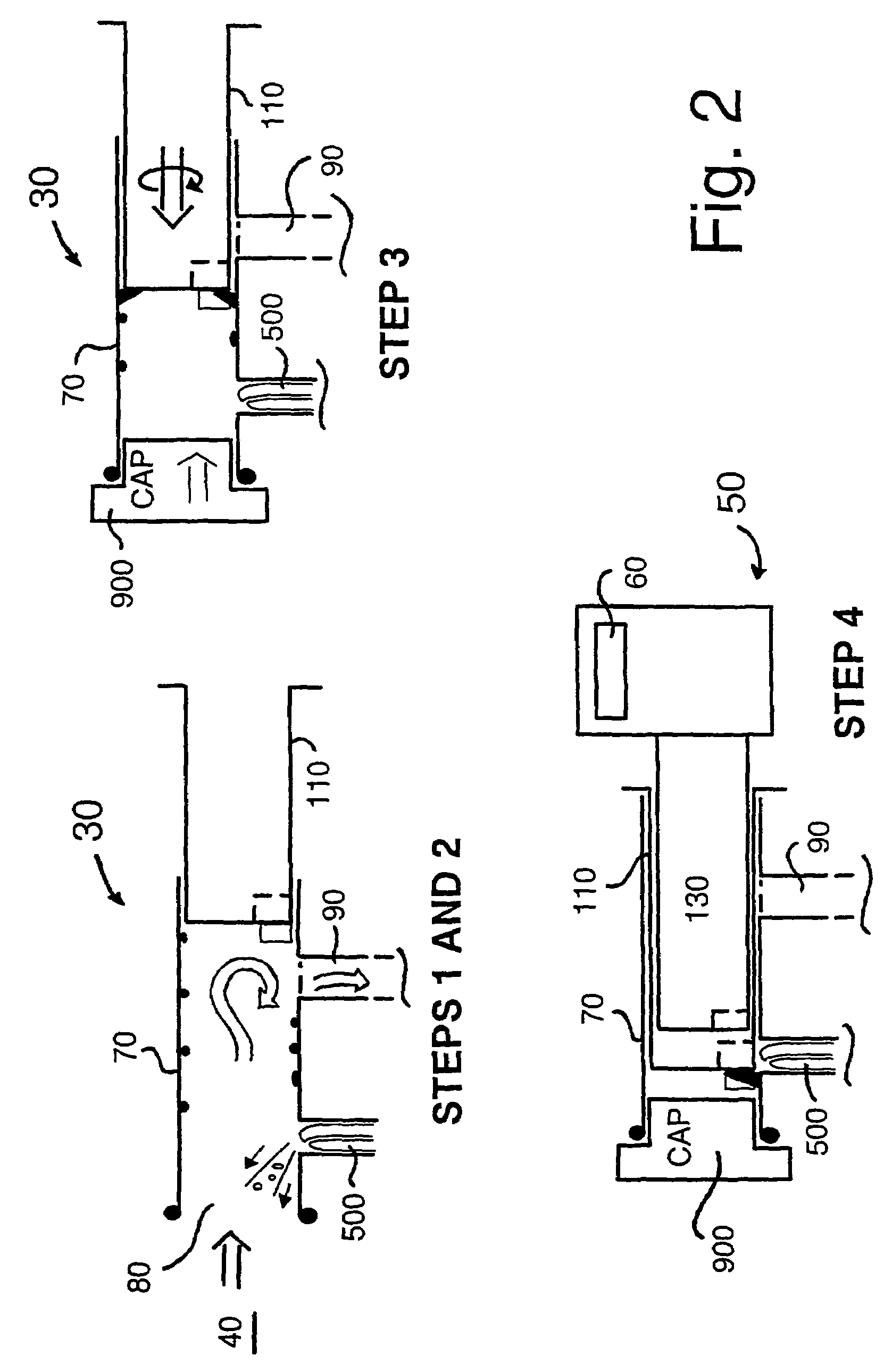
Biological measurement system
InactiveUS7384793B2Maximizes collectableLow costBioreactor/fermenter combinationsBiological substance pretreatmentsMedicineFluorescence
In a biological measurement system (10) for collecting one or more samples of sputum and / or mucus from a patient in the form of an aerosol and for analyzing said one or more samples to detect whether or not pathogens are present therein, the one or more samples are in solution within the system (10) and detection of the pathogens is performed using a fluorescently labeled assay. The system (10) is adapted to detect bacterial pathogens using evanescent-wave spectroscopy preferably by using a single-reflective technique. The one or more samples are advantageously provided to the system (10) in aerosol form. However, the system (10) is capable of being adapted for use in analyzing samples in liquid form. Methods of analyzing said one or more samples in the system (10) are described.
Owner:RAPID BIOSENSOR SYST LTD
Cervical exfoliated cell preservative fluid
InactiveCN101363011AReduce aggregationEfficient killingDead animal preservationTissue cultureRed blood cellCervical cancer screening
The invention relates to a cervical exfoliated cell preservative liquid, the preservative liquid comprises the following components with the contents (by weight): 20 percent to 50 percent of alcohols; 15 percent to 50 percent of anti-aggregation reagent; 5 percent to 10 percent of buffer solution; 1 percent to 20 percent of ion strength maintaining reagent; 0.01 percent to 0.5 percent of anti-microbial reagent; 0.1 to 5 percent of mucus dissolving reagent; and 0 to 0.5 percent of cleaning agent. Compared with the prior art, the preservative liquid can not only lead cells to maintain the shape in an in vitro liquid suspension environment, minimize the protein precipitation, dissolve larger protein substances, such as blood and mucus, and reduce the cell aggregation, but can also selectively eliminate or reduce red cells, effectively kill microbes, prevent the activity of reverse transcriptase and retain the integrity of nucleic acids and proteins for facilitating the later analysis; in addition, the preservative liquid can greatly reduce the costs of consumptive materials for the TCT detection, improve the sensitivity and the specificity of the cervical cancer screening and accelerate the promotion and the popularization of the TCT technology in a medical system.
Owner:SHANGHAI ADICON CLINICAL LAB LNC
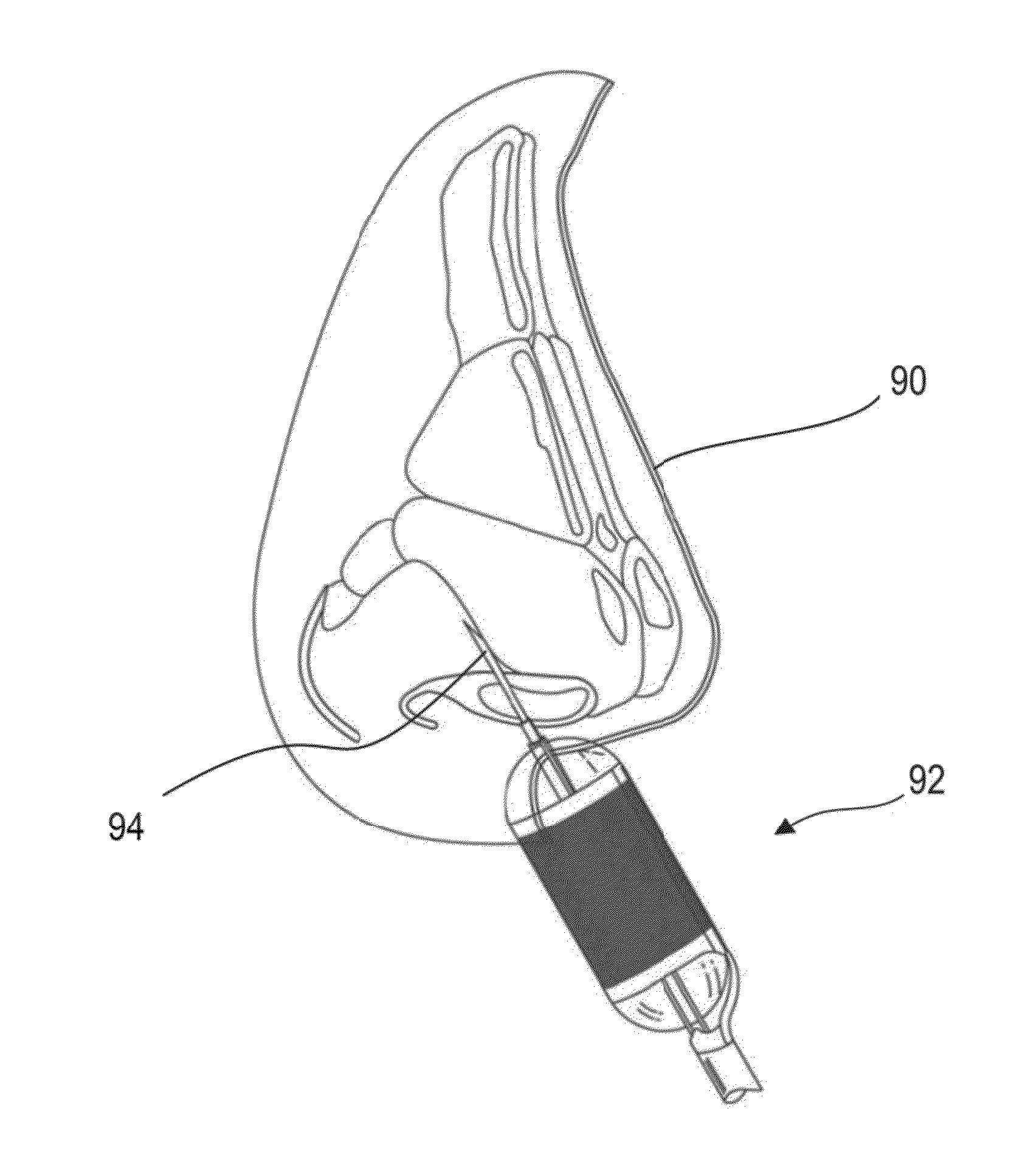
Post nasal drip treatment
ActiveUS20150202003A1Absolute reductionAmeliorate PNDS and/or UACS symptomsUltrasound therapyBalloon catheterNostrilTreatment delivery
Systems and methods for treating a patient's mucus hypersecretion condition are disclosed herein. Certain implementations may involve a method for reducing mucus secretion in an upper airway of a patient to treat at least one of post nasal drip or chronic cough. The method may include advancing a treatment delivery portion of an energy-based treatment device into a nostril of the patient. The treatment delivery portion may contact mucosal tissue of the upper airway without piercing the mucosal tissue. The treatment delivery portion may deliver treatment to at least one tissue selected from the group of the mucosal tissue and another tissue underlying the mucosal tissue to modify a property of the at least one tissue and thus treat at least one of post nasal drip or chronic cough in the patient.
Owner:AERIN MEDICAL
Mucus shaving apparatus for endotracheal tubes
An endotracheal tube cleaning apparatus 10 which can be periodically inserted into the inside of an endotracheal tube 30 to shave away mucus deposits. In a preferred embodiment, this cleaning apparatus 10 comprises a flexible central tube 12 with an inflatable balloon 40 at its distal end. Affixed to the inflatable balloon are one or more shaving rings 70, each having a squared leading edge 72, to shave away mucus accumulations 60. In operation, the uninflated cleaning apparatus 10 is inserted into the endotracheal tube. The balloon 40 is then inflated by a suitable inflation device, such as a syringe 14, until the balloon's shaving rings are pressed against the inside surface of the endotracheal tube. The cleaning apparatus is then pulled out of the endotracheal tube to shave off mucus deposits.
Owner:DEPT OF HEALTH & HUMAN SERVICES GOVERNMENT OF THE UNITED STATES AS REPRESENTED BY THE SEC OF THE +1
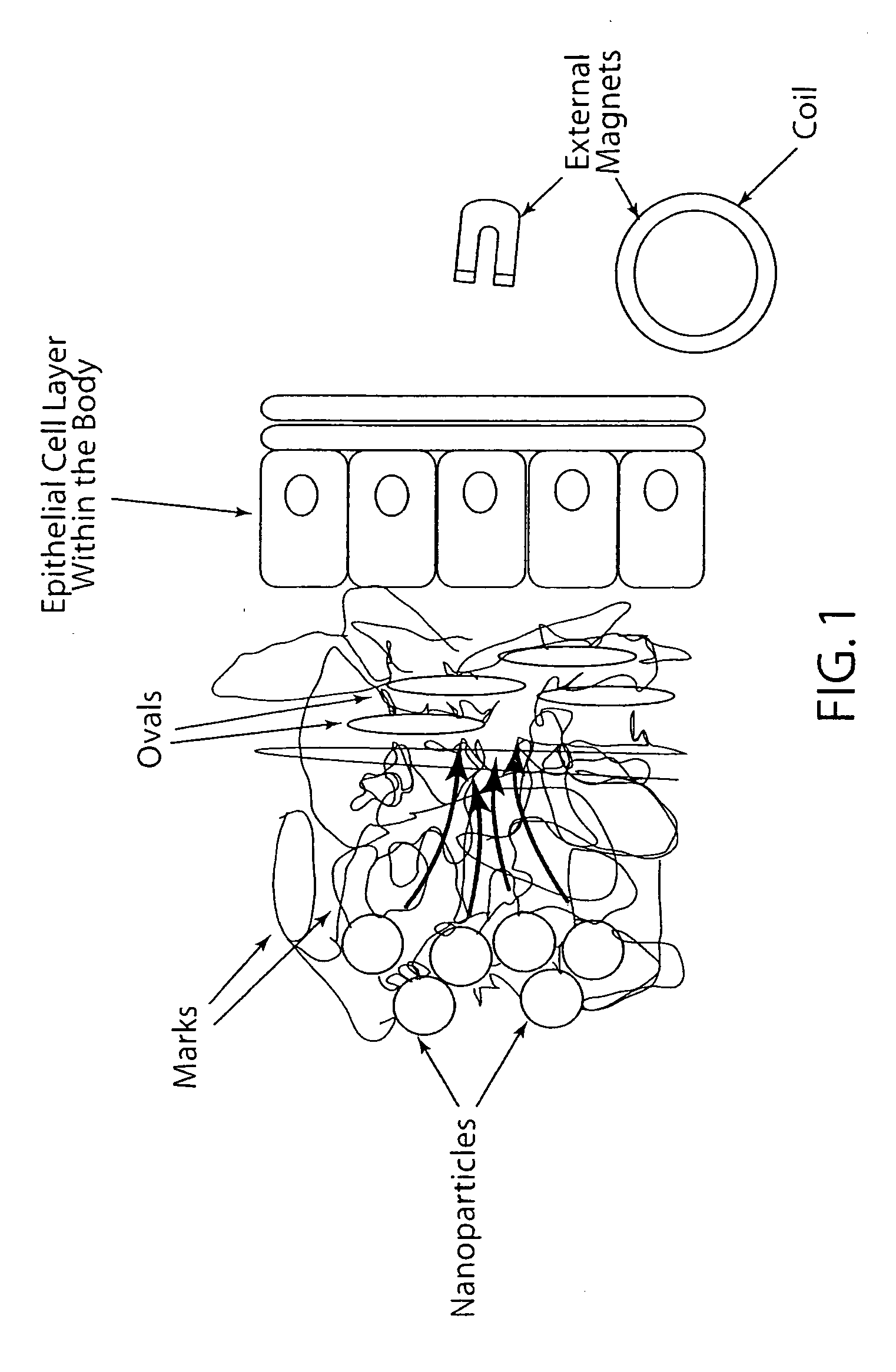
Active nanoparticles and method of using
ActiveUS20090226521A1Improve efficacyImprove distributionPowder deliveryOrganic active ingredientsMultifunctional nanoparticlesGene
Active multifunctional nanoparticles provide significant enhancement of the efficacy of model therapeutic and gene agents due to increased diffusion and penetration through mucus and biological barriers under the influence of a magnetic field.
Owner:STC UNM
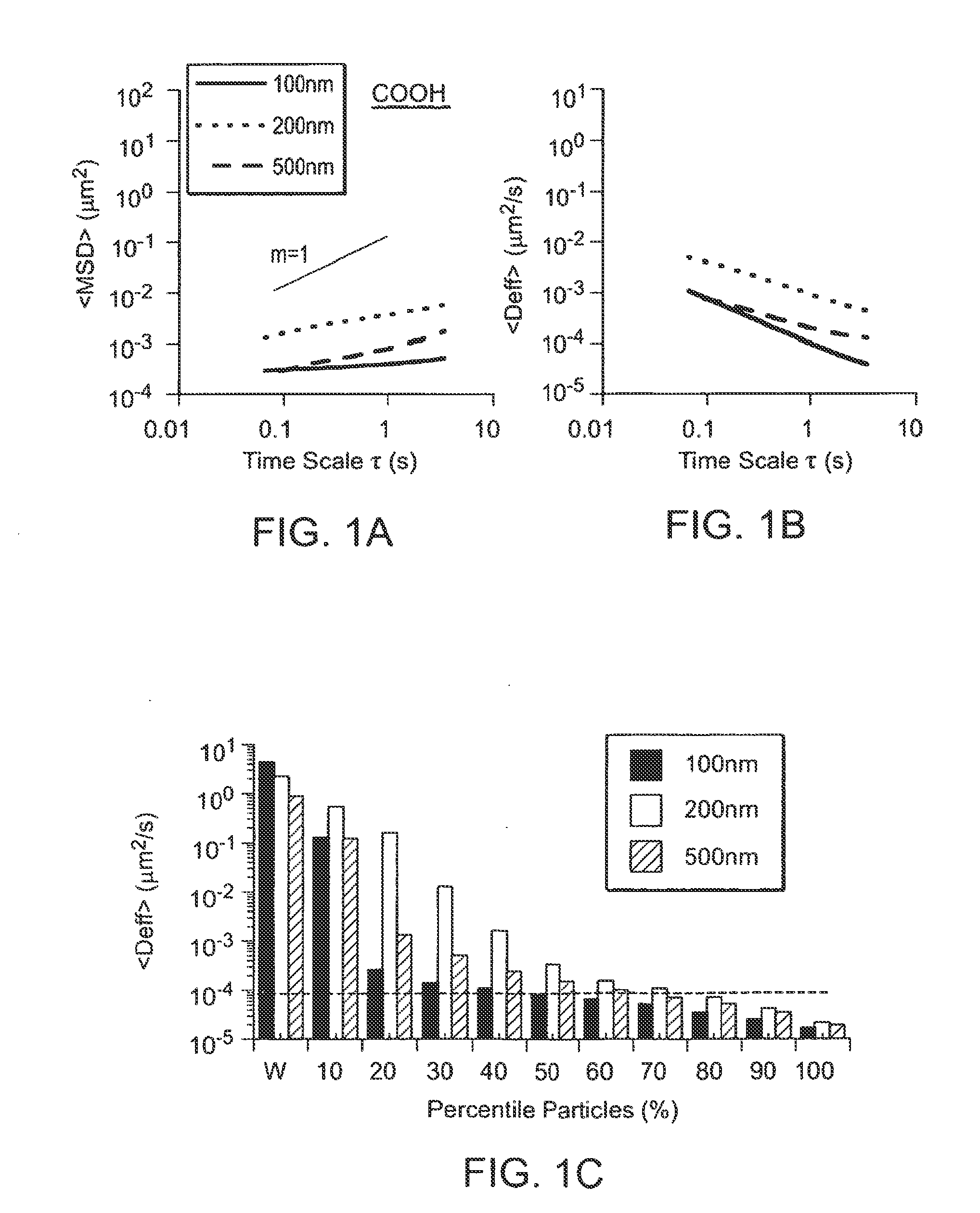
Nanocrystals, compositions, and methods that aid particle transport in mucus
Nanocrystals, compositions, and methods that aid particle transport in mucus are provided. In some embodiments, the compositions and methods involve making mucus-penetrating particles (MPP) without any polymeric carriers, or with minimal use of polymeric carriers. The compositions and methods may include, in some embodiments, modifying the surface coatings of particles formed of pharmaceutical agents that have a low water solubility. Such methods and compositions can be used to achieve efficient transport of particles of pharmaceutical agents though mucus barriers in the body for a wide spectrum of applications, including drug delivery, imaging, and diagnostic applications. In certain embodiments, a pharmaceutical composition including such particles is well-suited for administration routes involving the particles passing through a mucosal barrier.
Owner:ALCON INC +1
Effervescent powders for inhalation
ActiveUS20070031490A1Enhancing loosening, thinning, cleansing, and removing of mucus and extrinsic surface materialsBiocidePowder deliveryInhalable particlesActive agent
Effervescent powders comprising inhalable particles are disclosed, as are methods for preparing these powders. The inhalable carrier particles comprise an inorganic or organic carbonate, and an acid, and exhibit effervescence when exposed to water or humid air. The particles have a mass median aerodynamic diameter suitable for nasal, bronchial, or pulmonary administration. The inhalable particles may be used as carriers for active agents. The inhalable particles may also be used to enhance permeability of mucosal and surface barriers on an inner surface of the nose, mouth, airway, and / or lungs of a patient, as well as to loosen, thin, cleanse, and remove mucus and extrinsic surface materials from an inner surface of the nose, mouth, airway, and / or lungs of a patient in need thereof.
Owner:LOEBENBERG RAIMAR +3
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com