Patents
Literature
375 results about "Paranasal sinuses" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
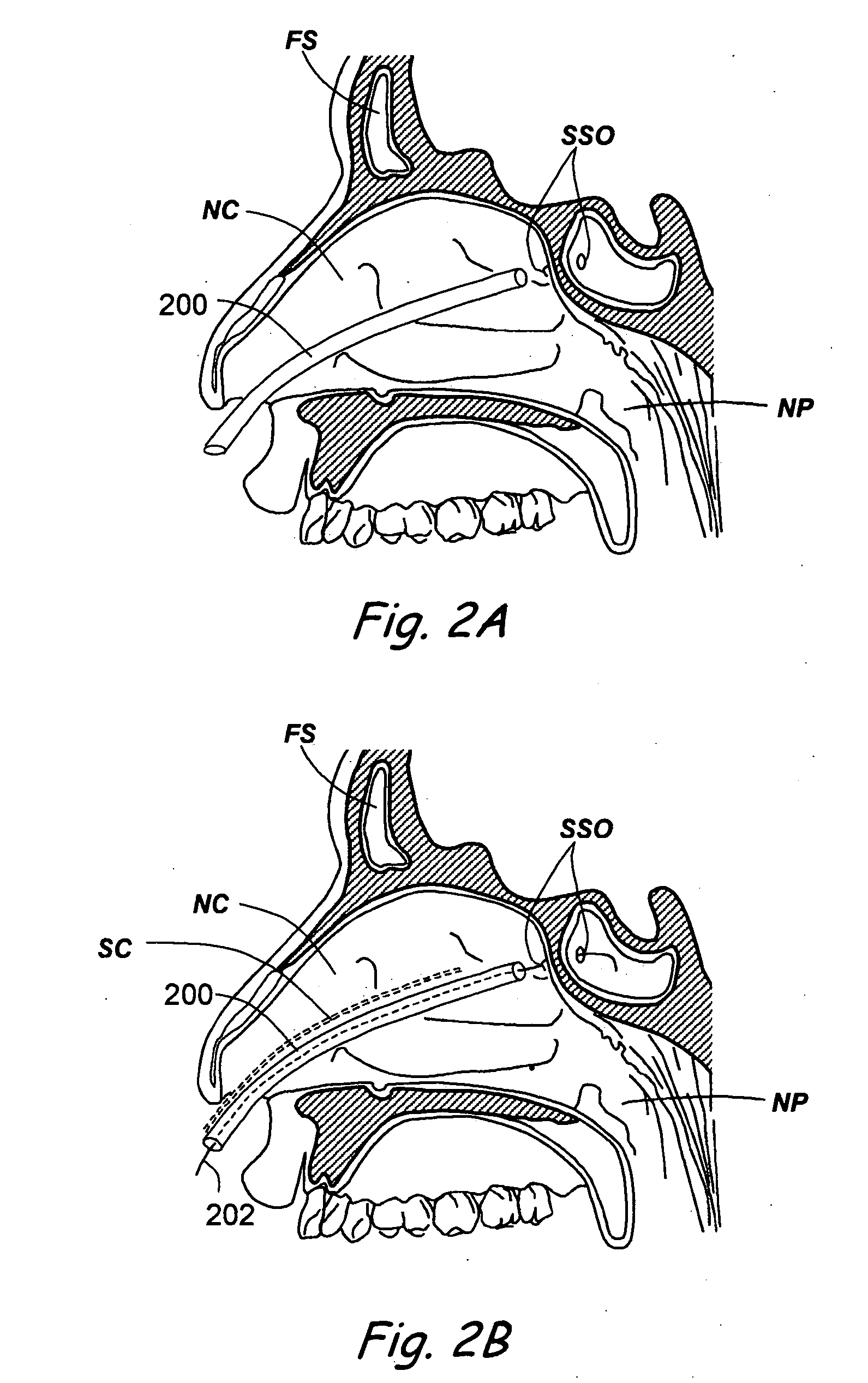
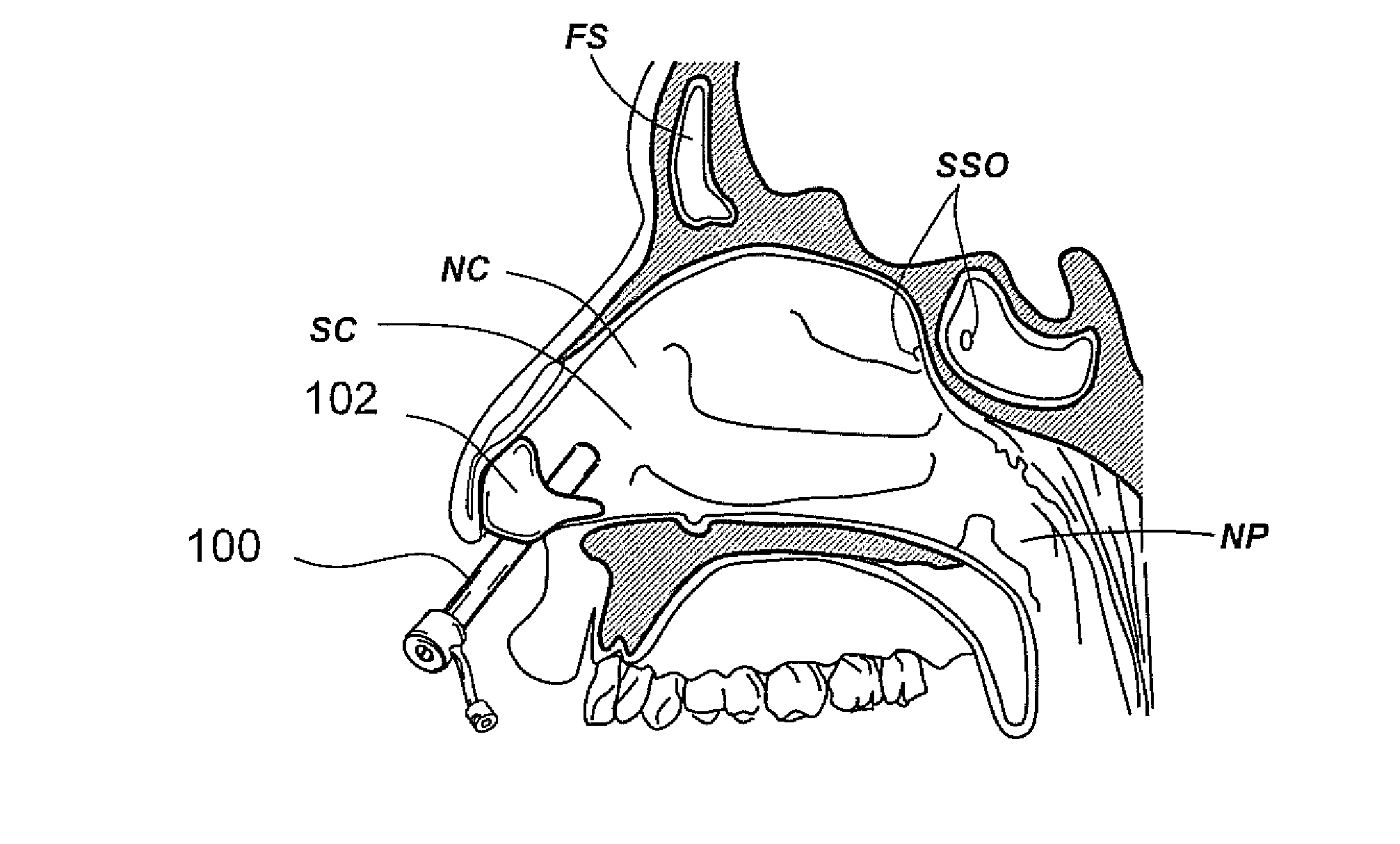
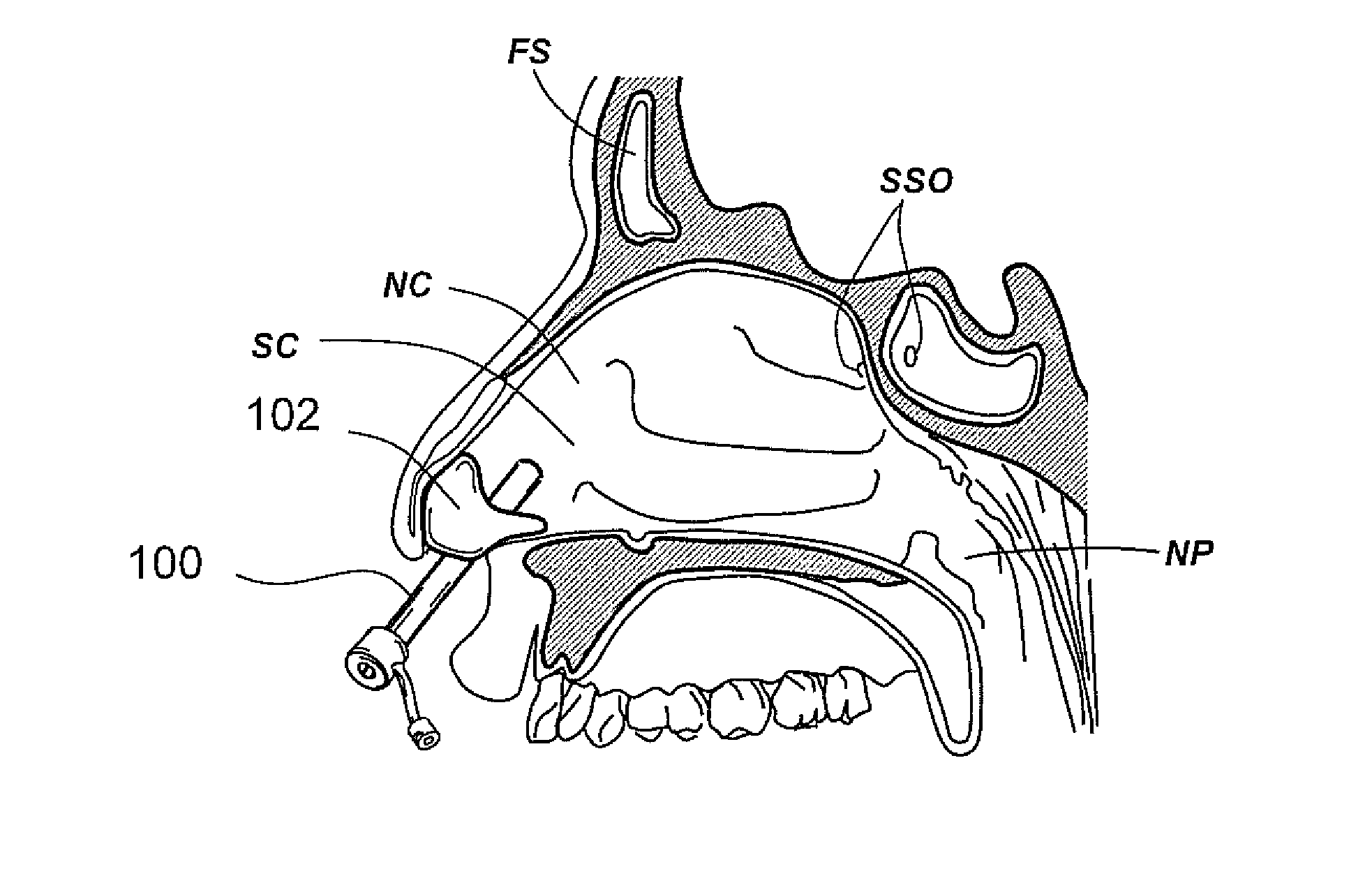
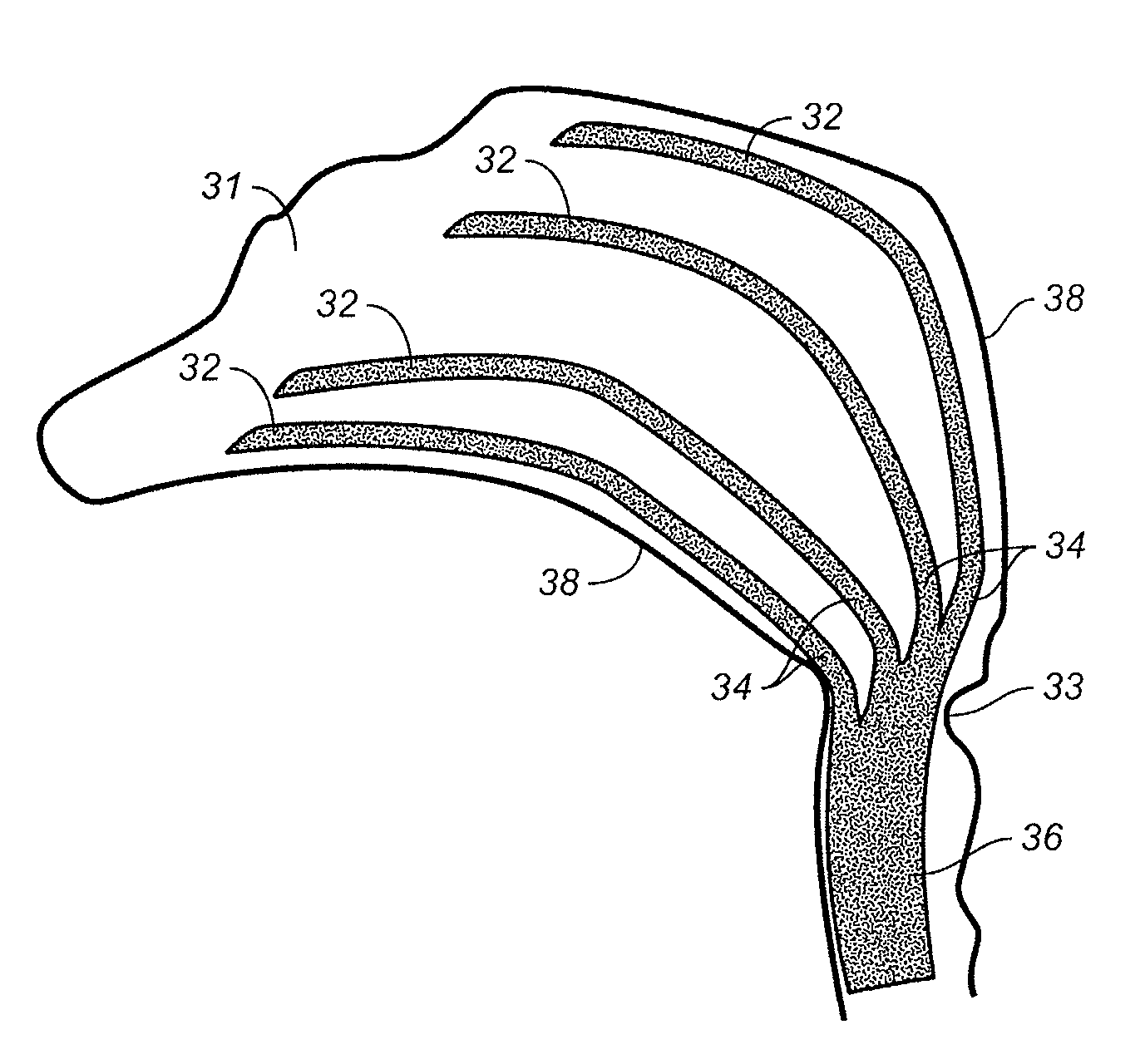
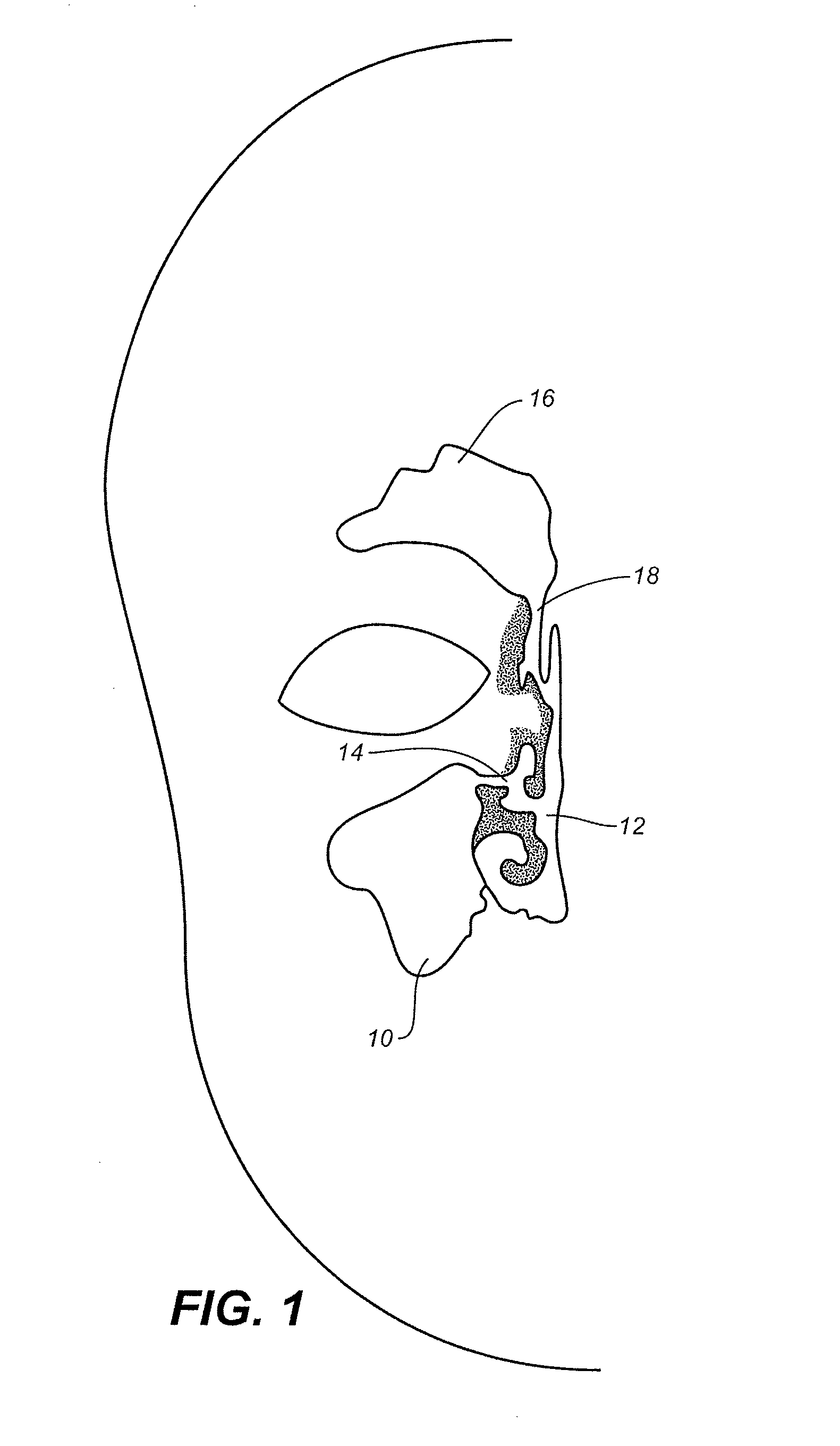
Paranasal sinuses are a group of four paired air-filled spaces that surround the nasal cavity. The maxillary sinuses are located under the eyes; the frontal sinuses are above the eyes; the ethmoidal sinuses are between the eyes and the sphenoidal sinuses are behind the eyes. The sinuses are named for the facial bones in which they are located.
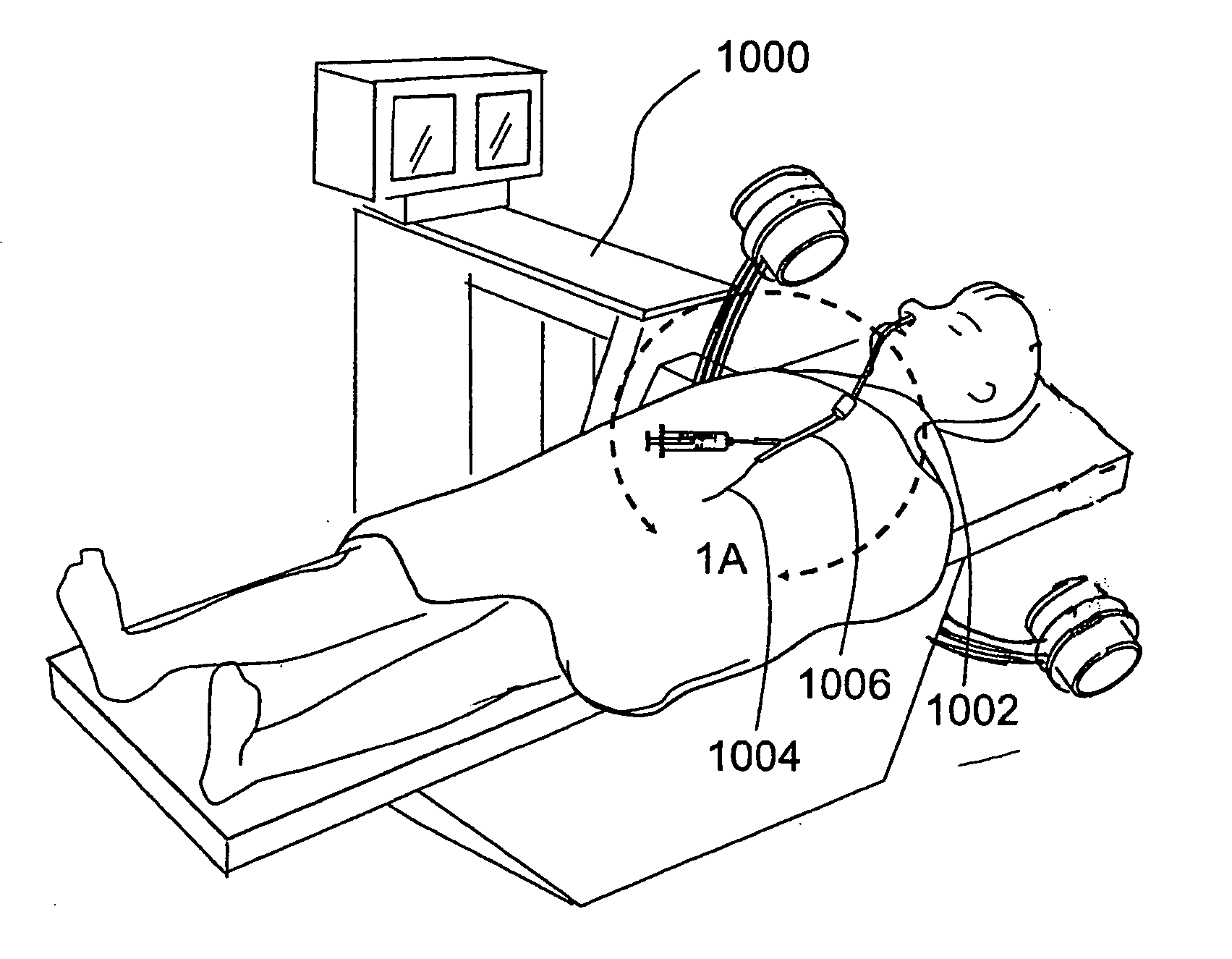
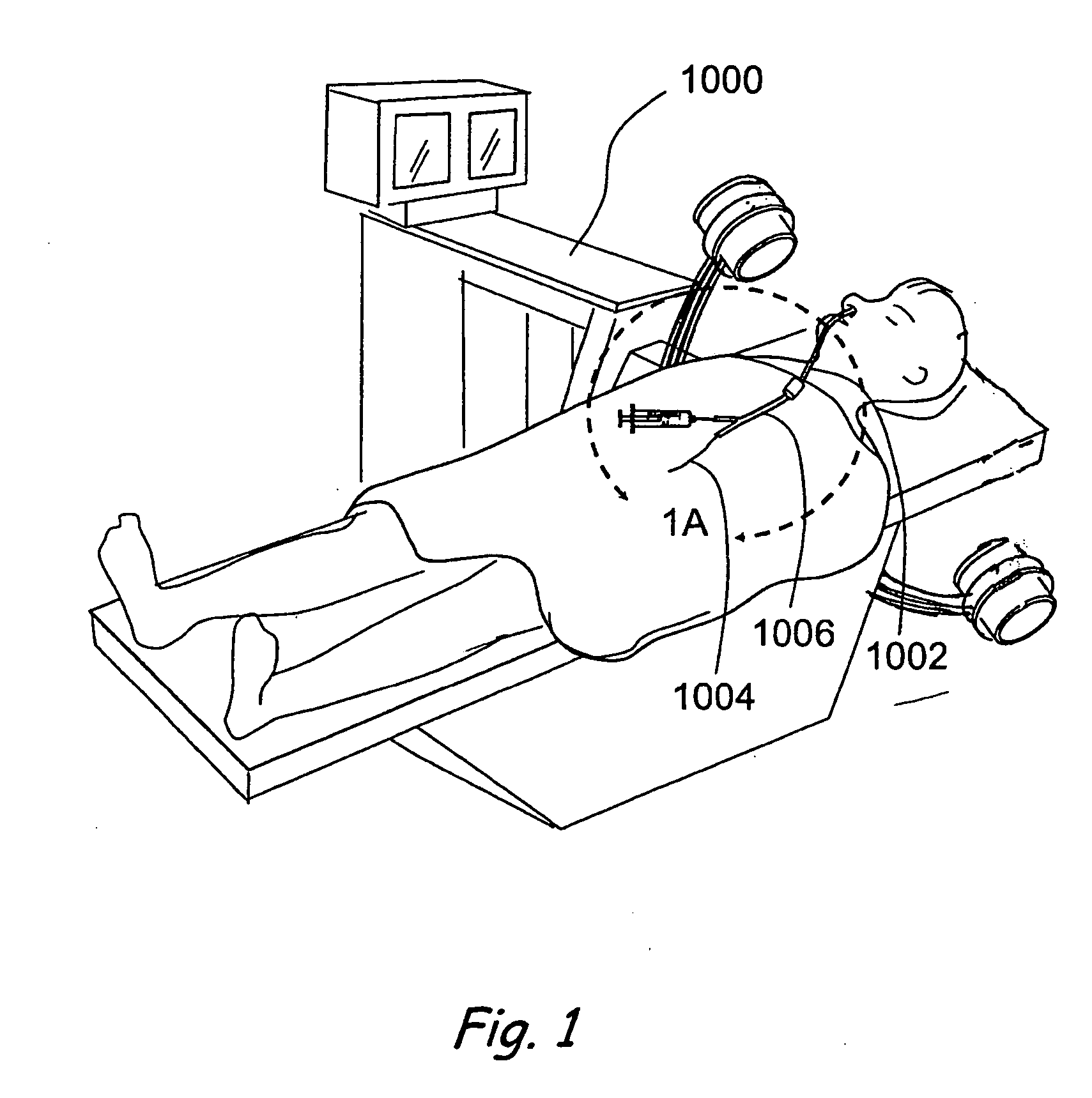
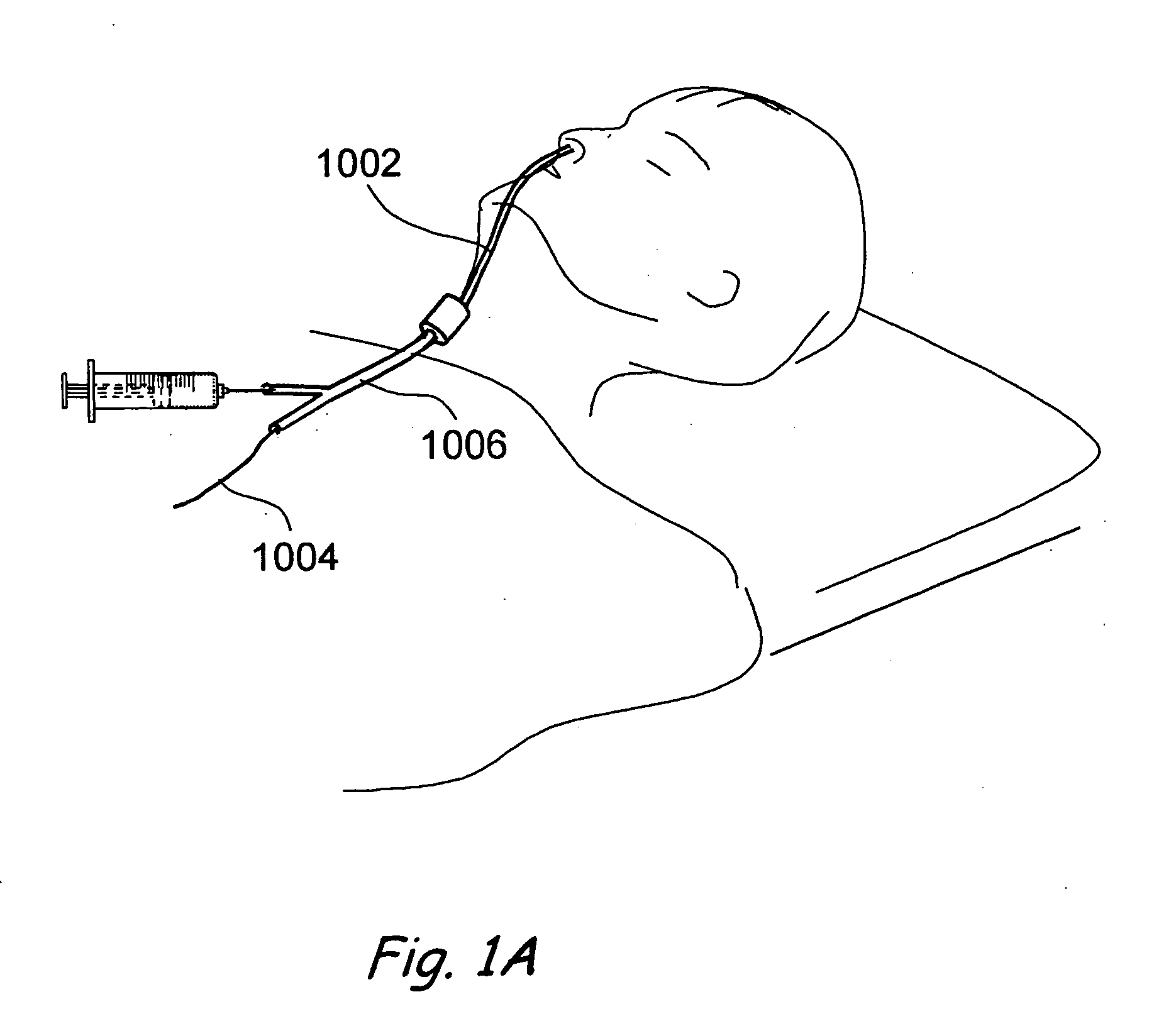
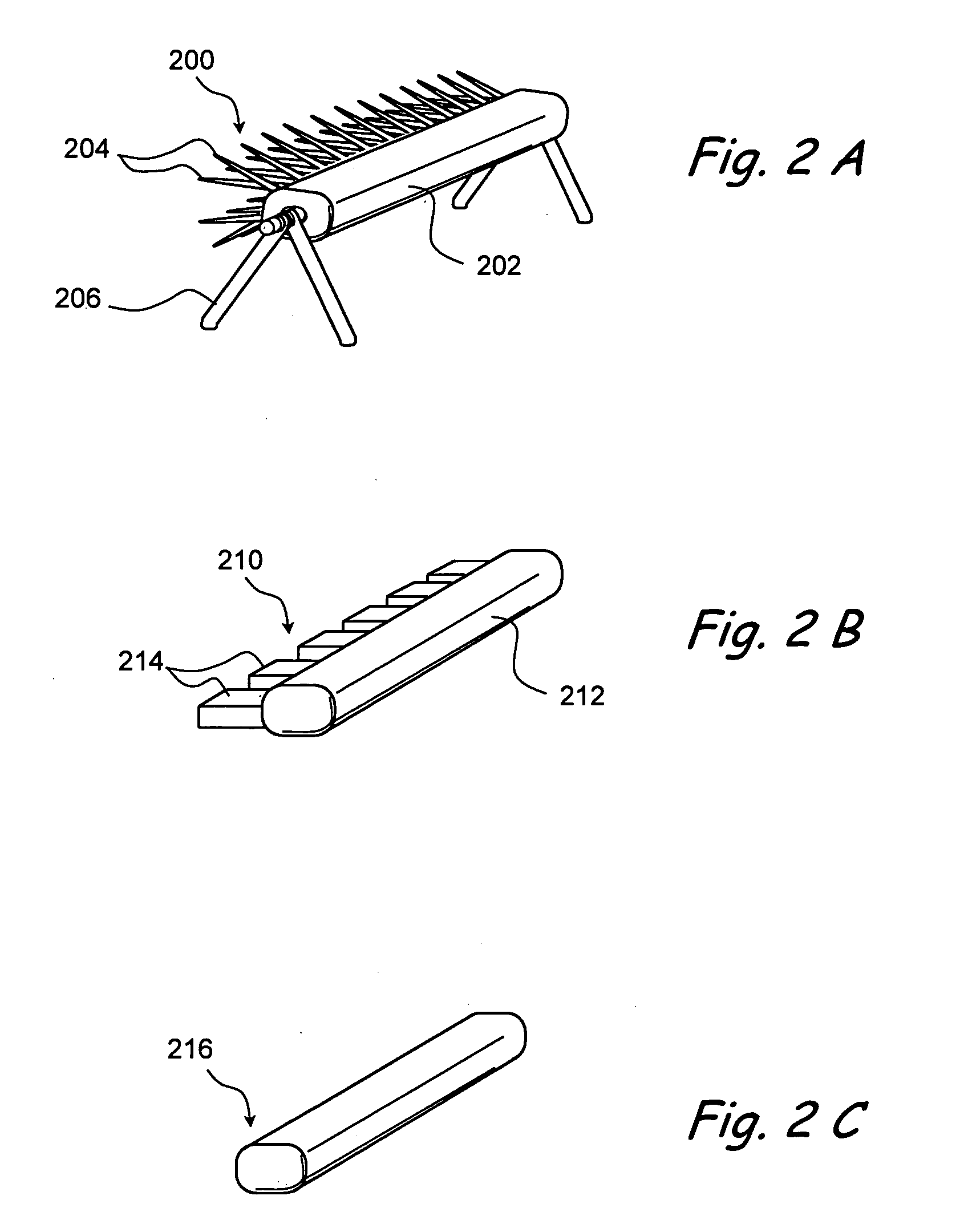
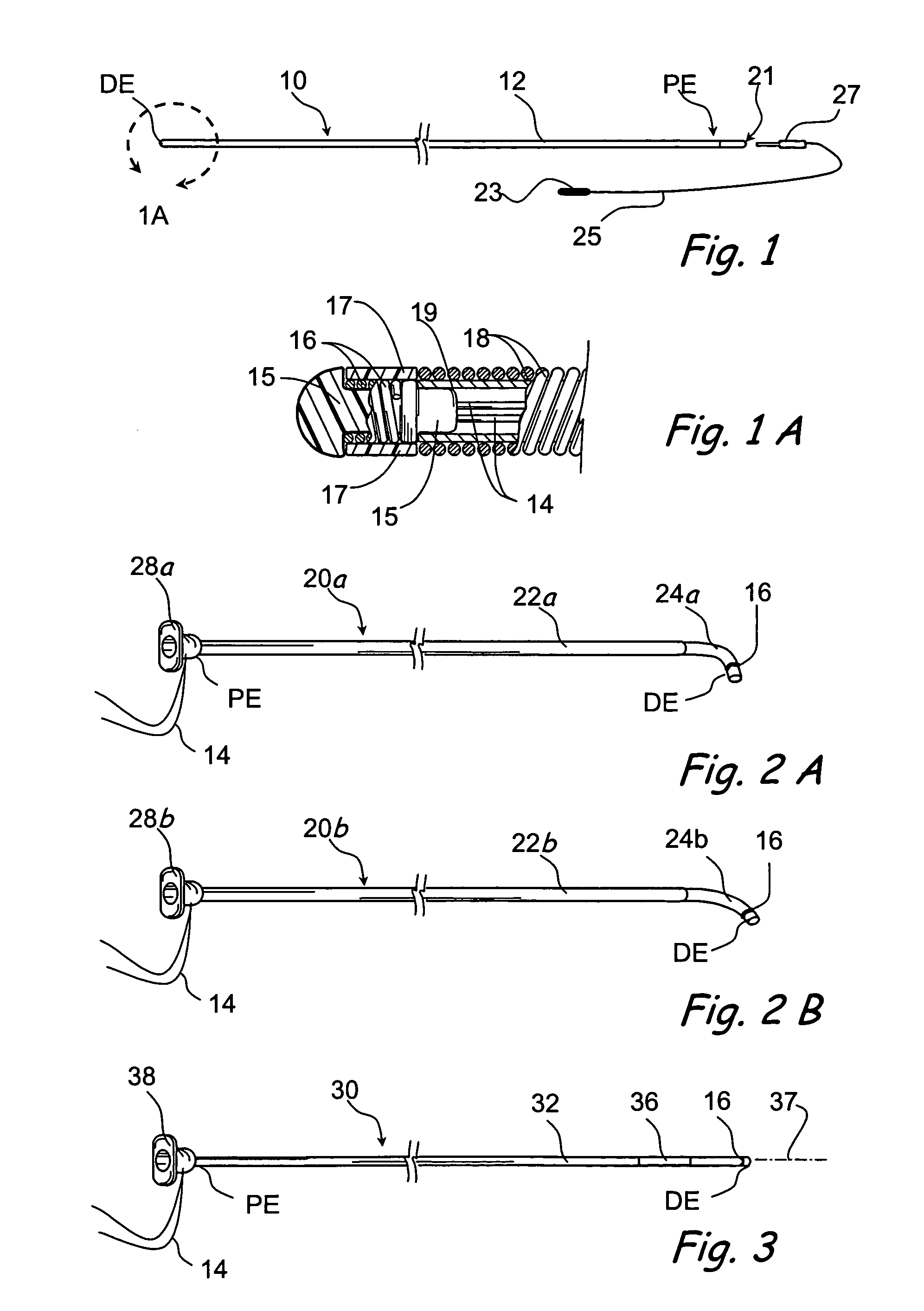
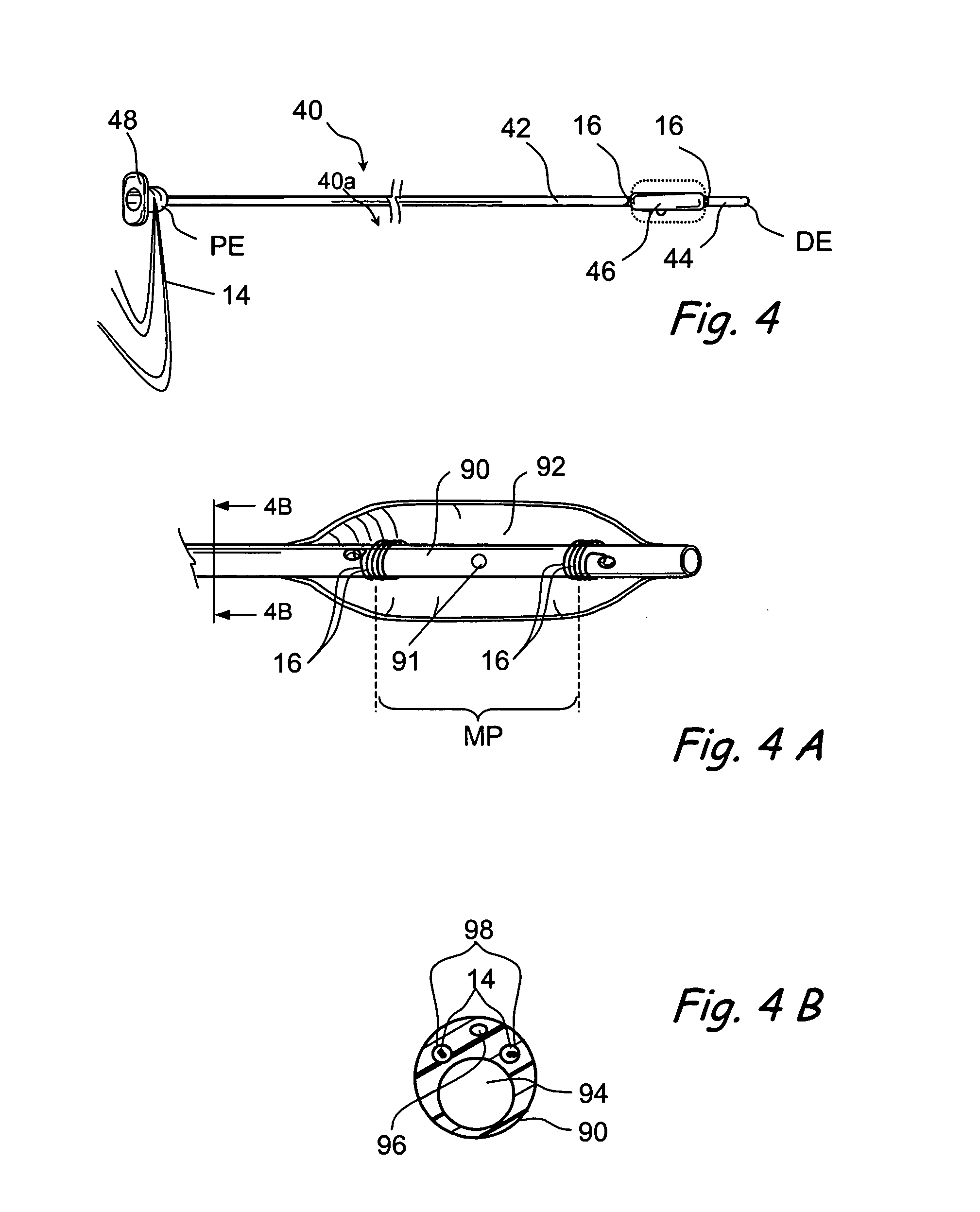
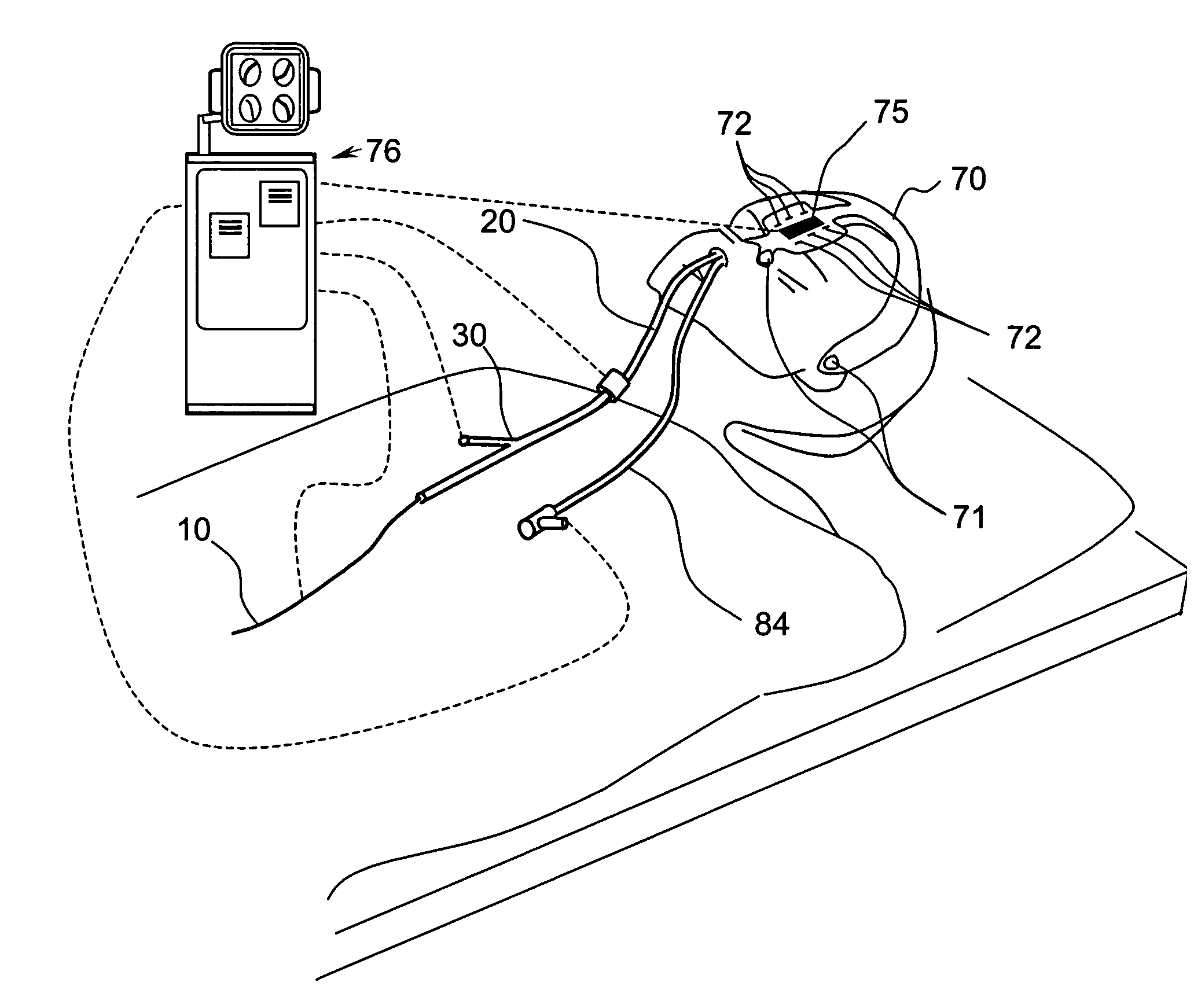
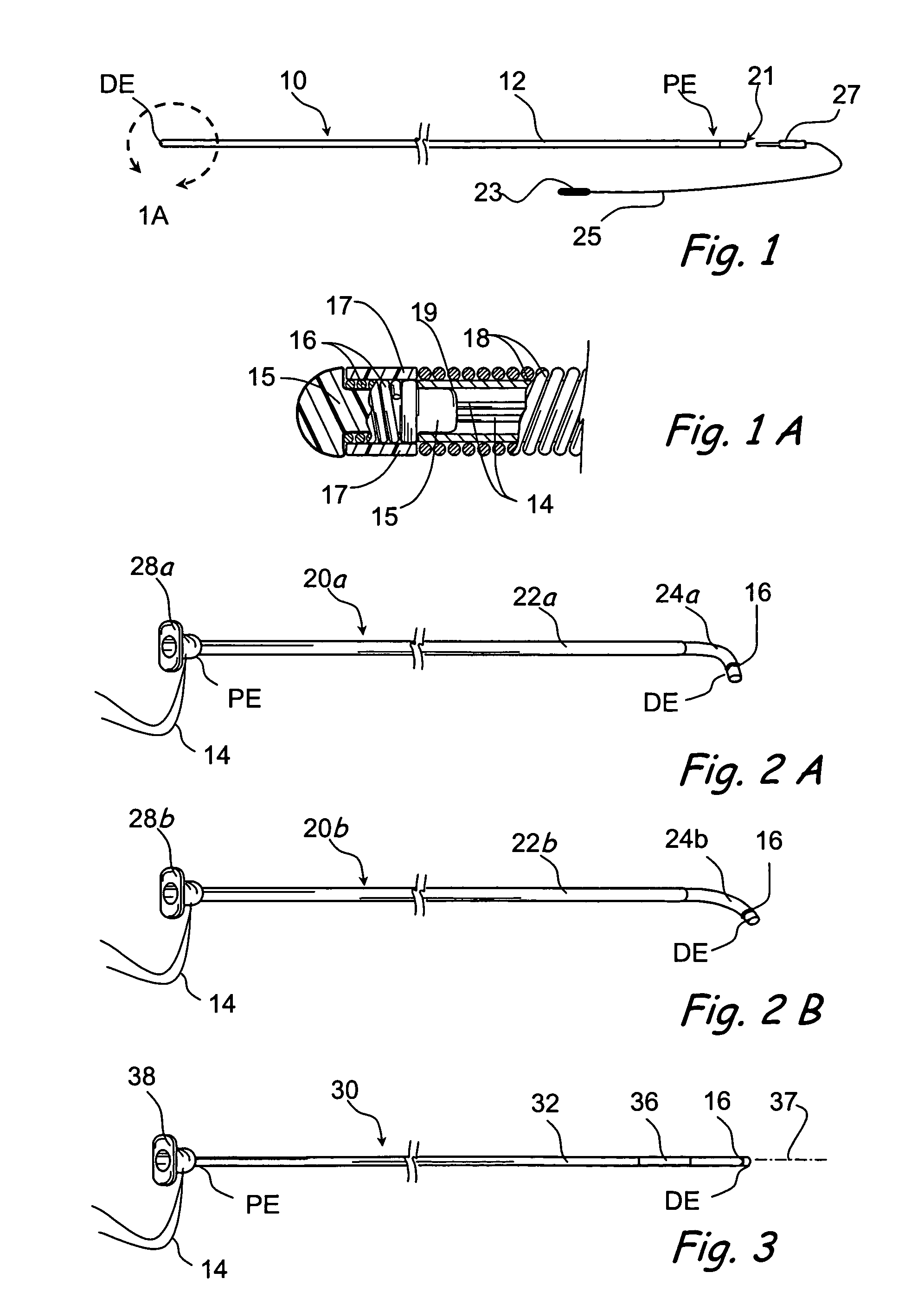
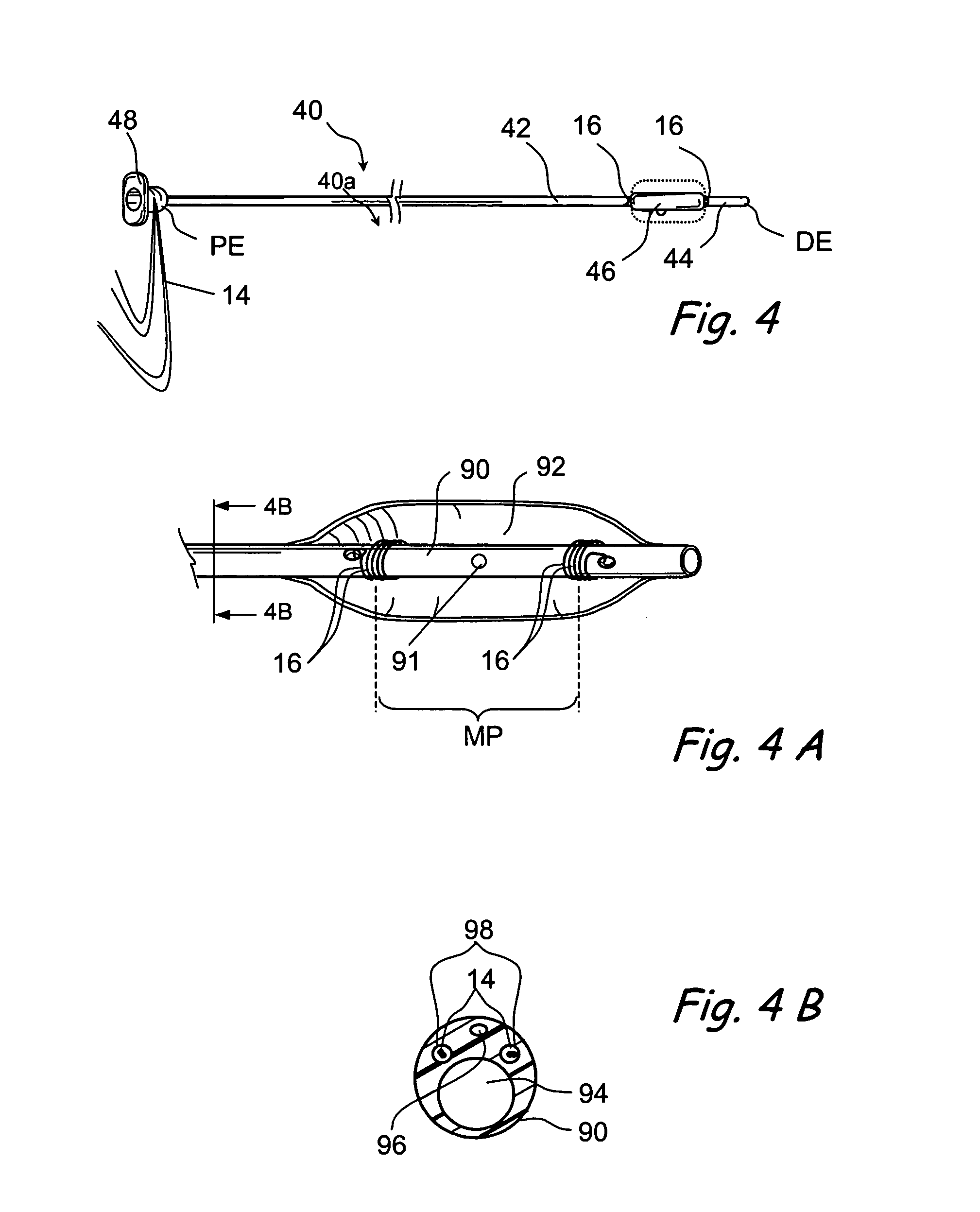
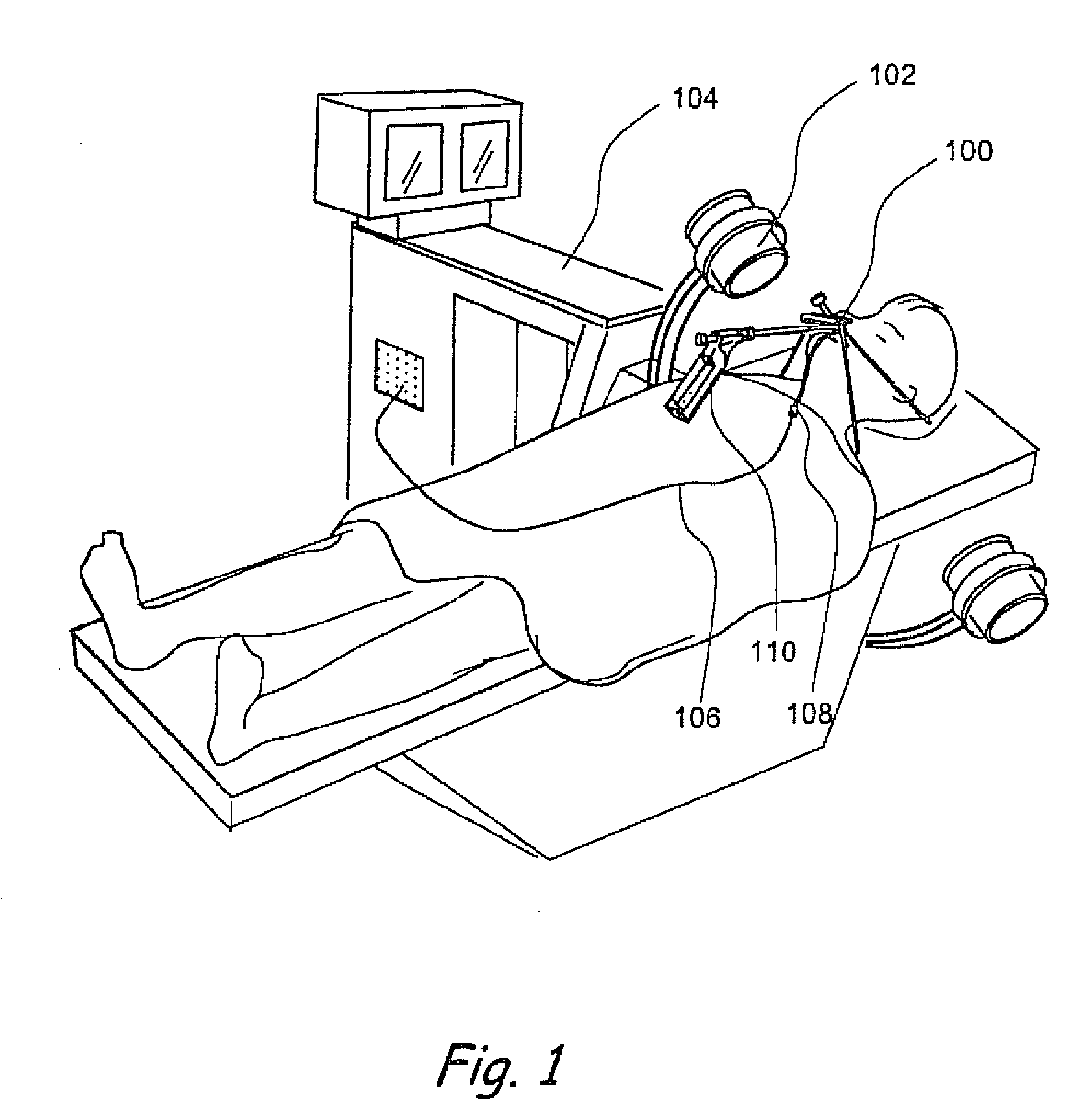
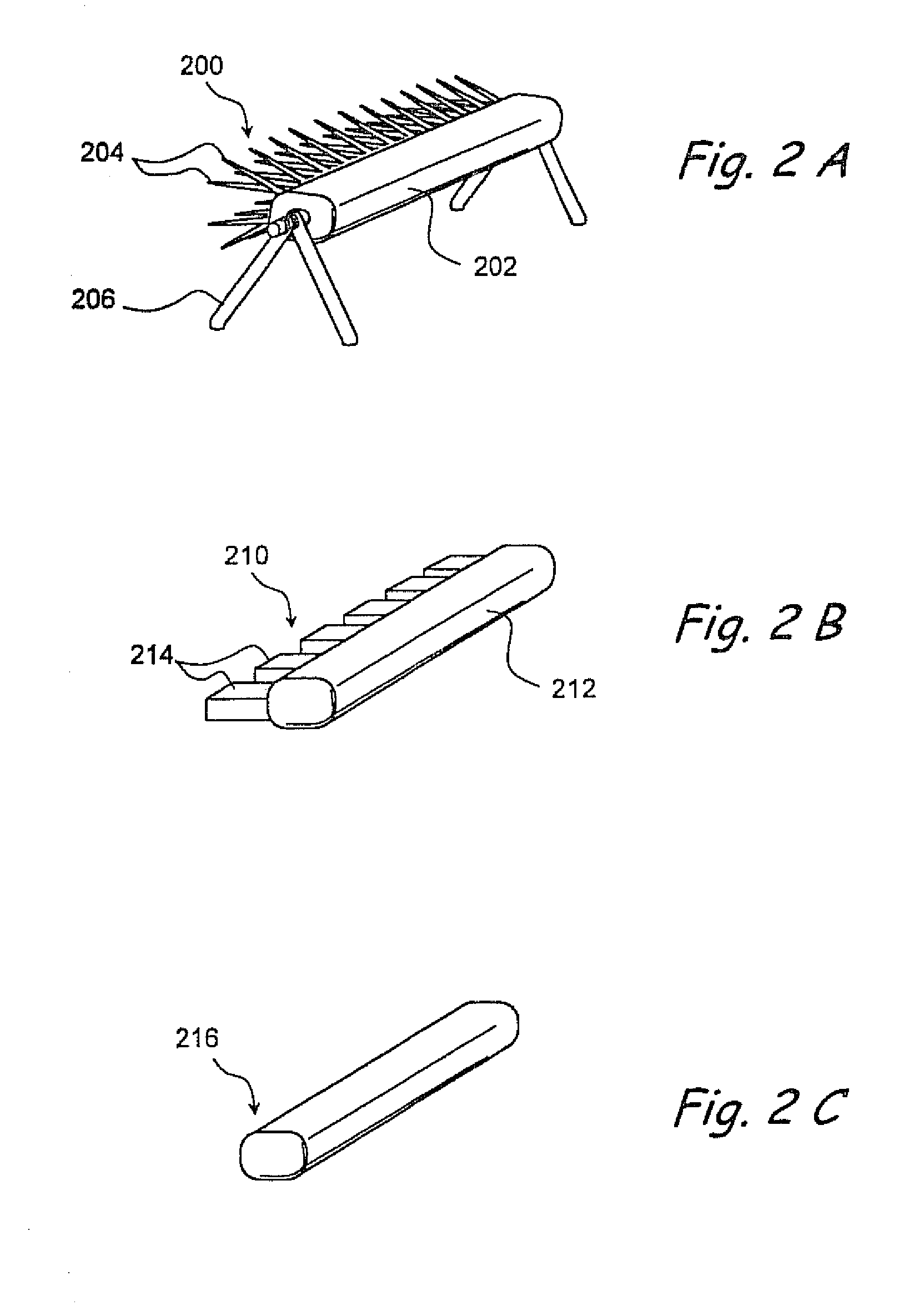
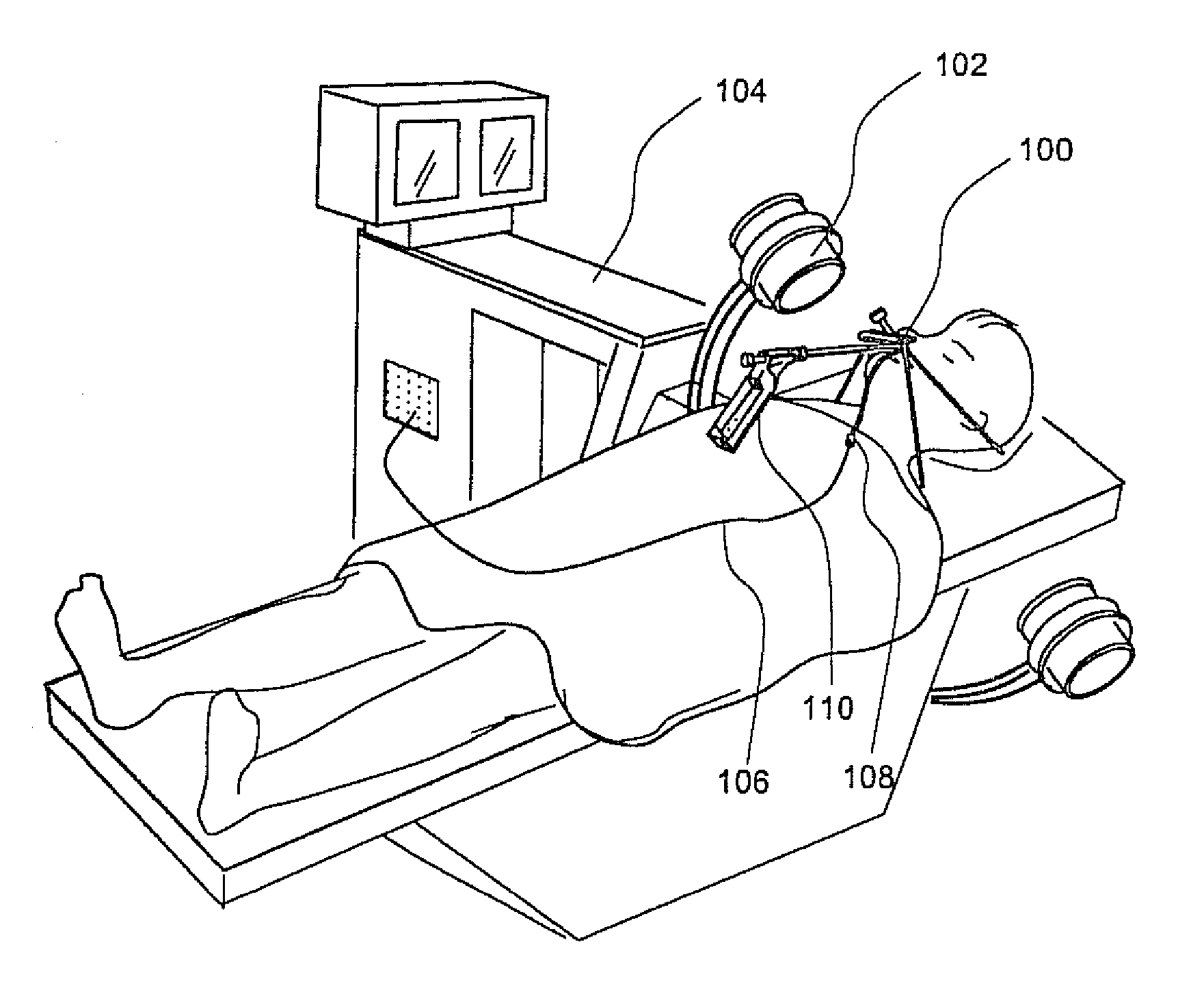
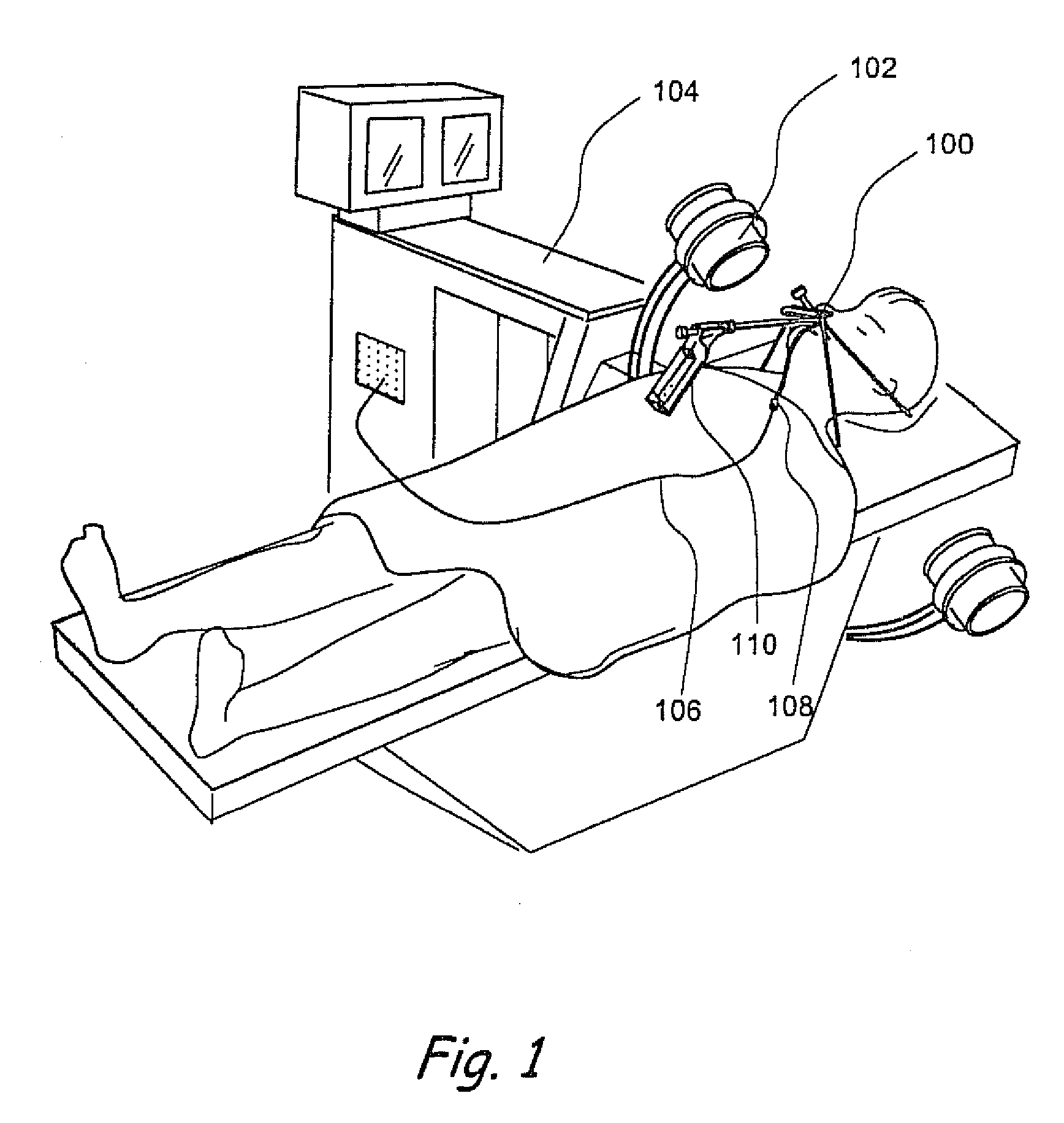
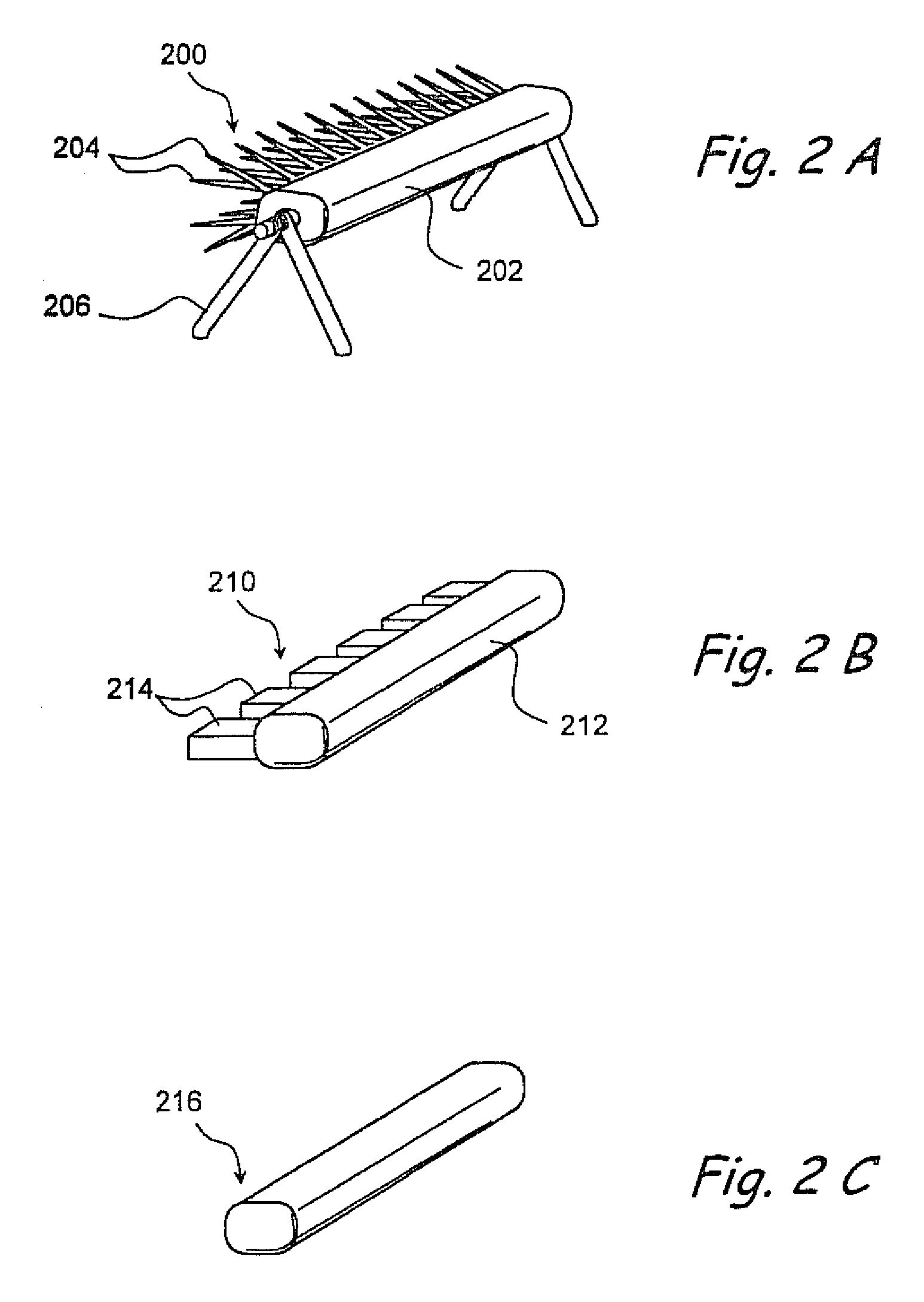
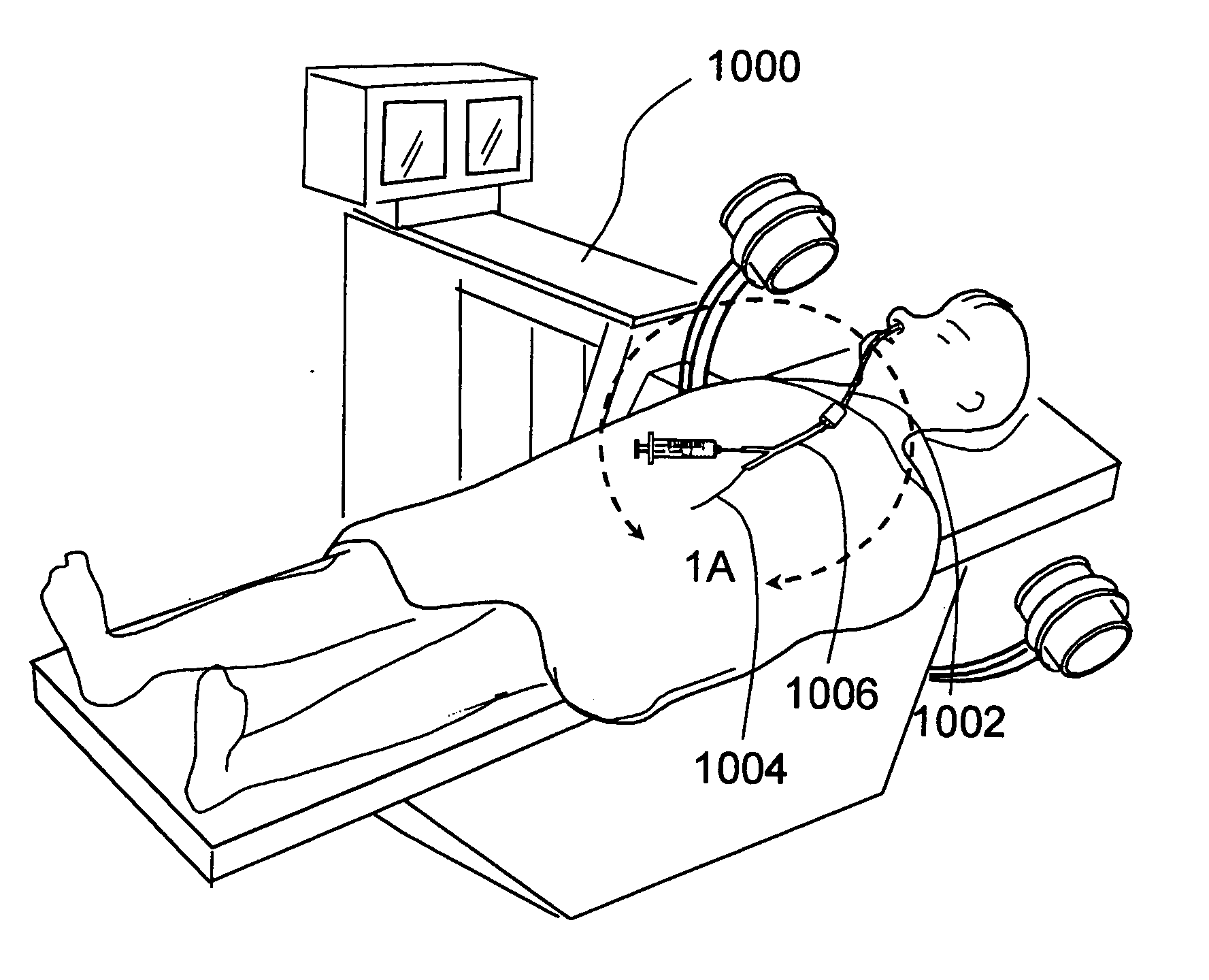
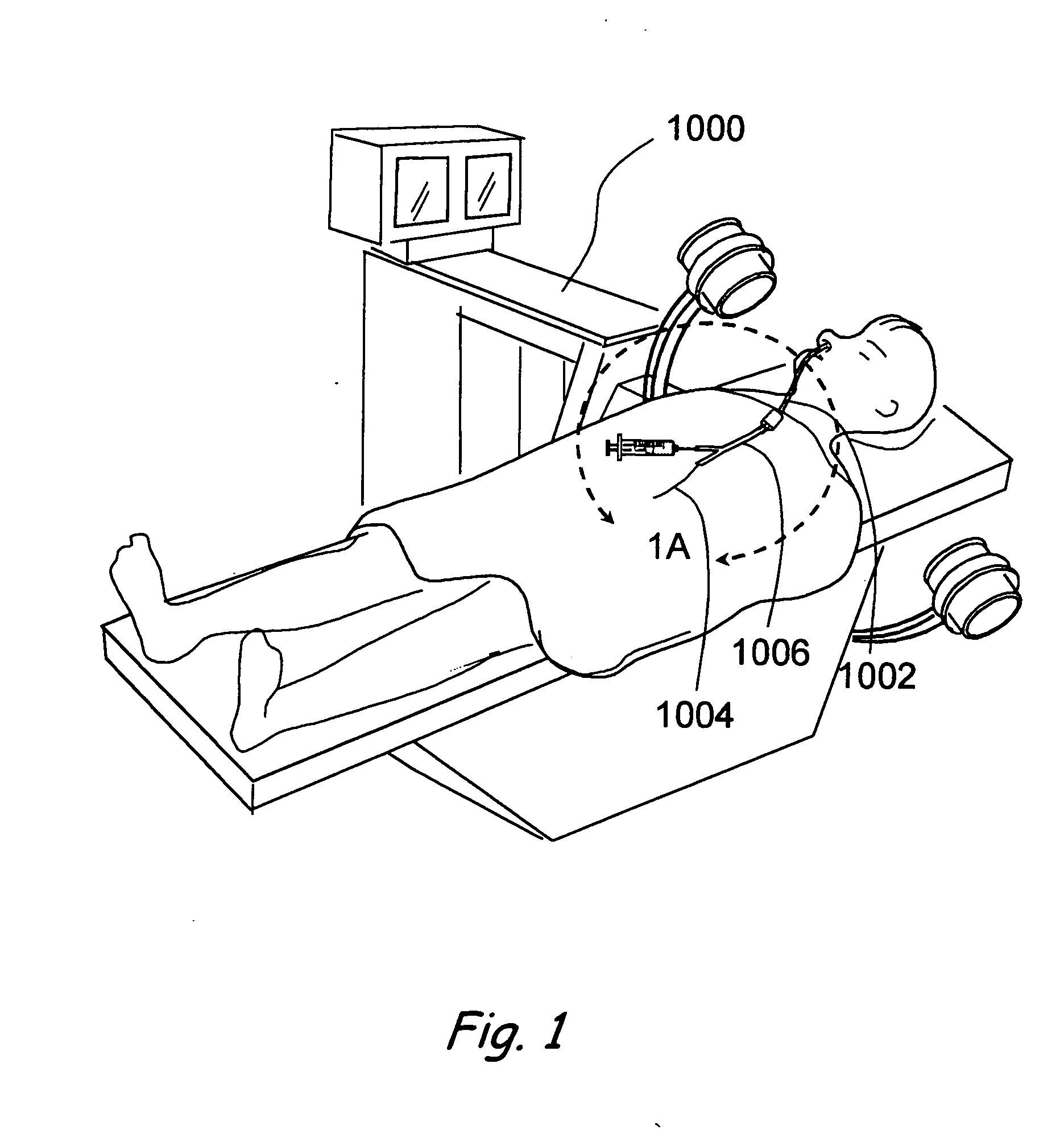
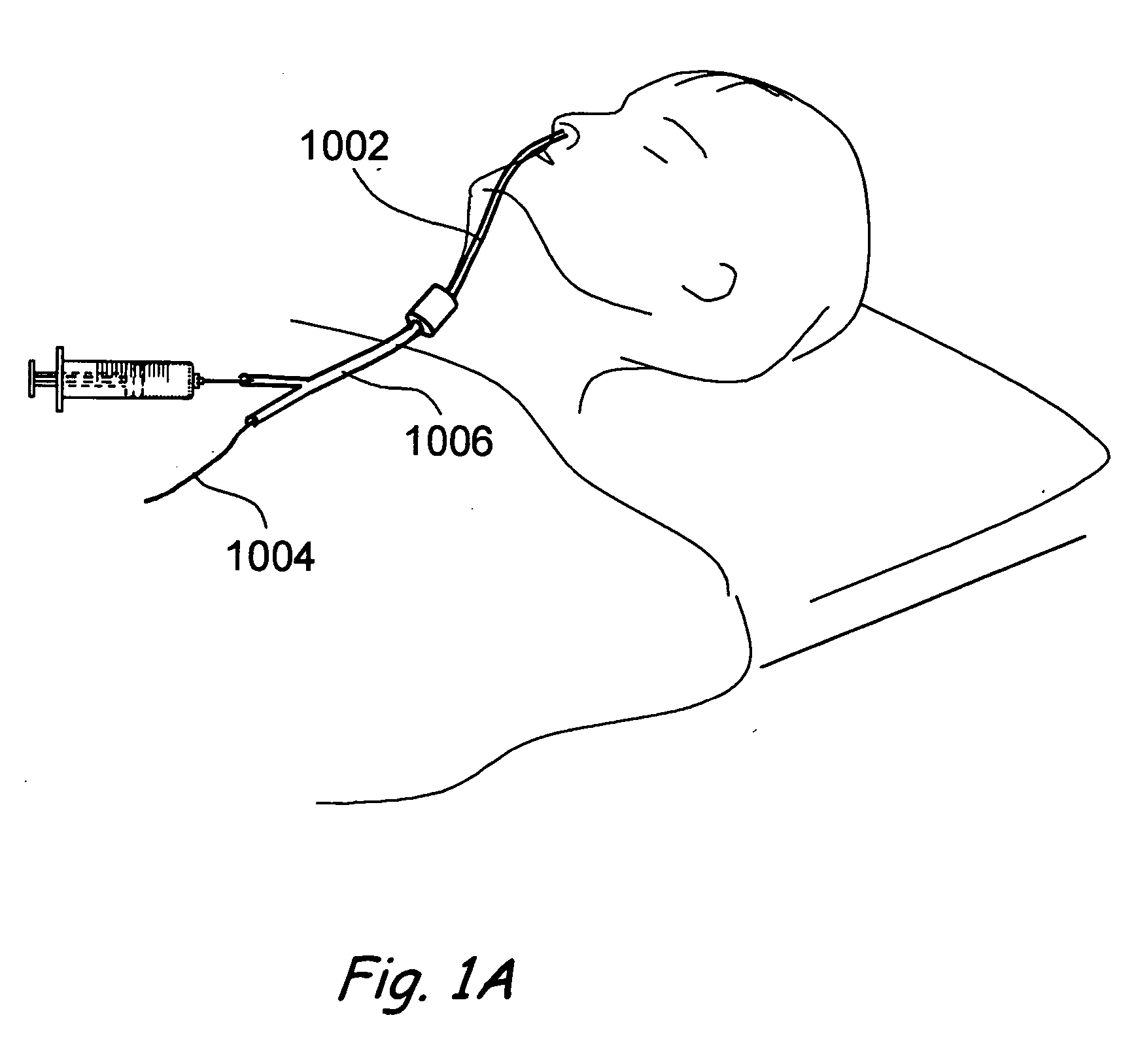
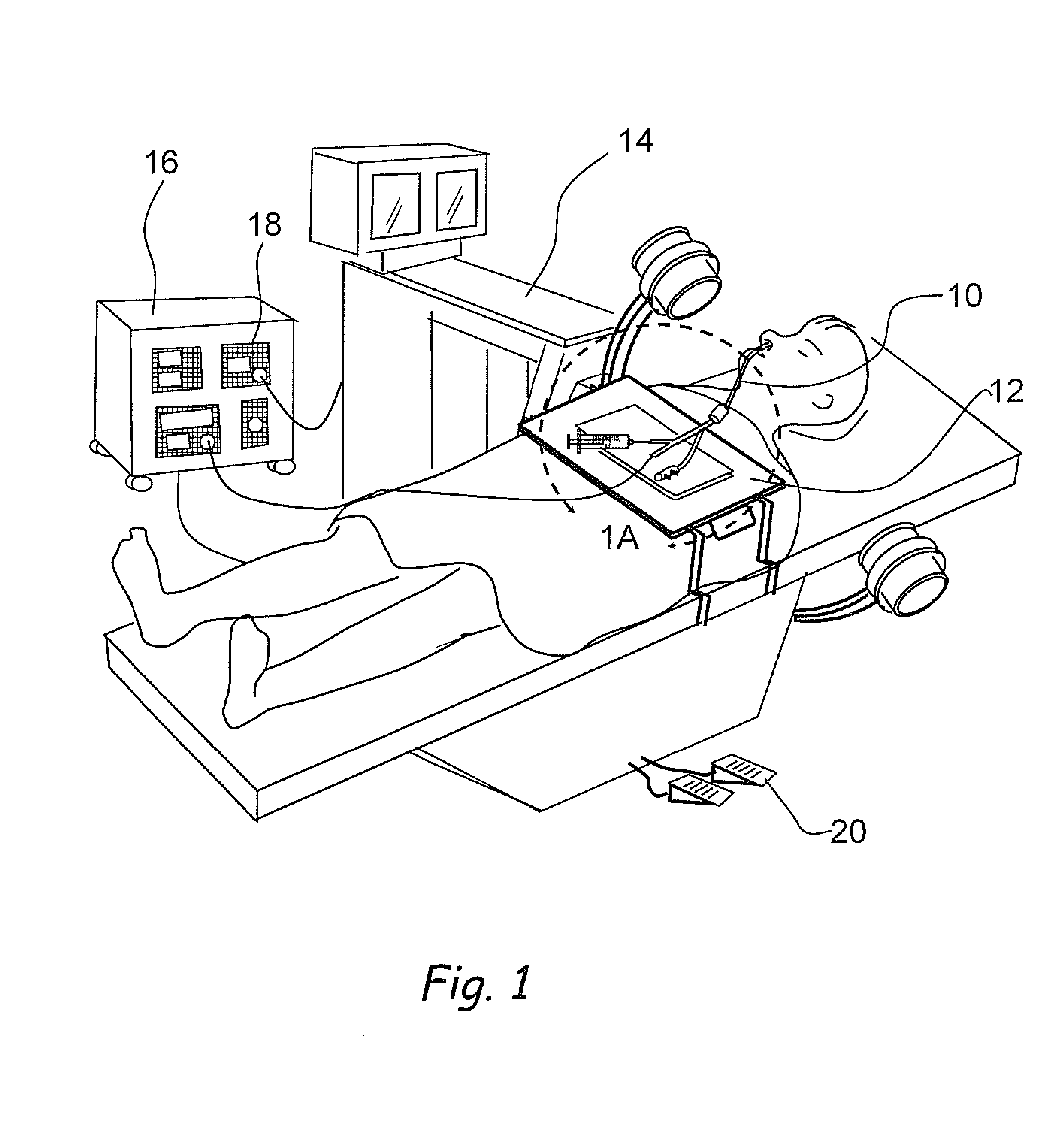
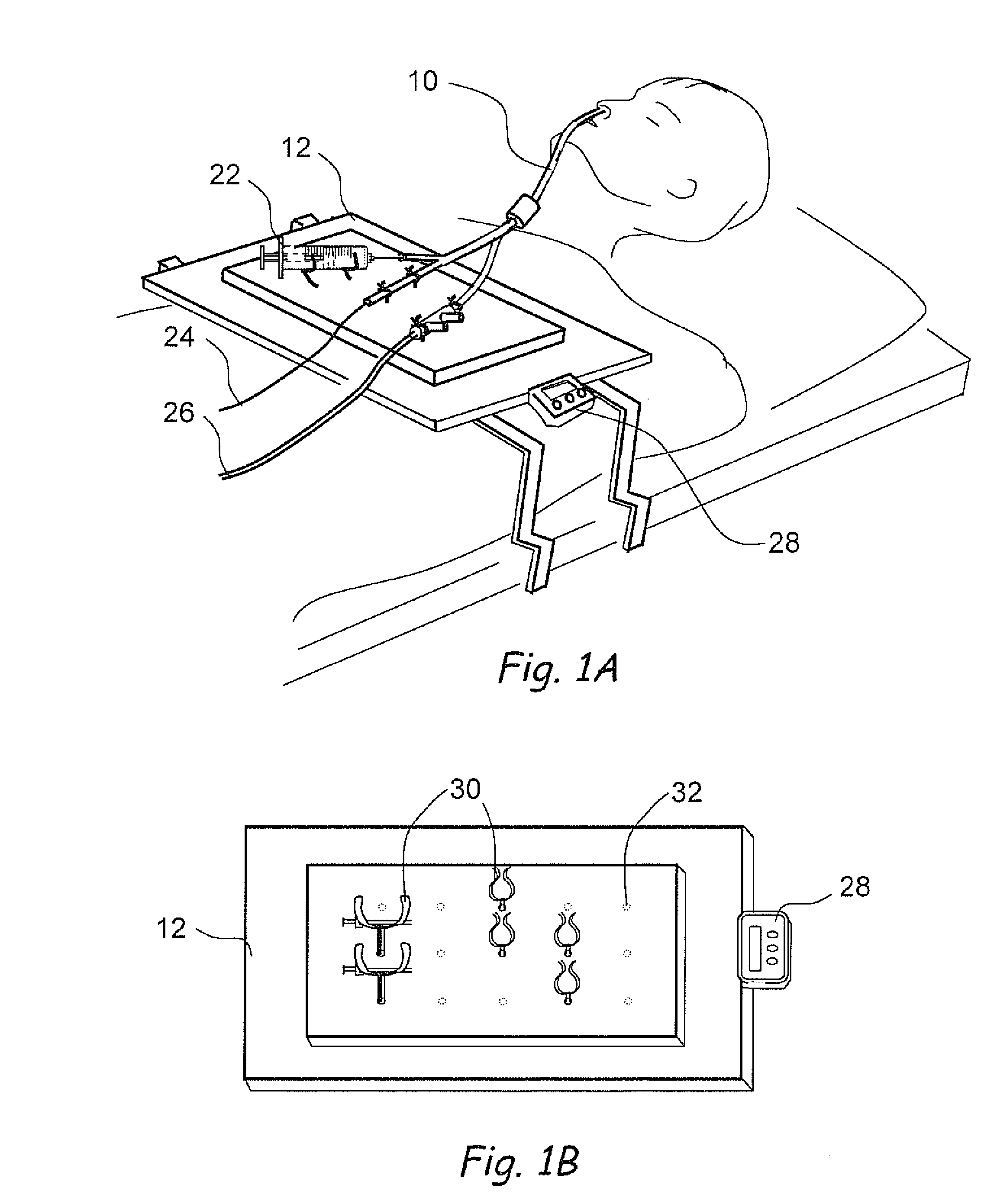
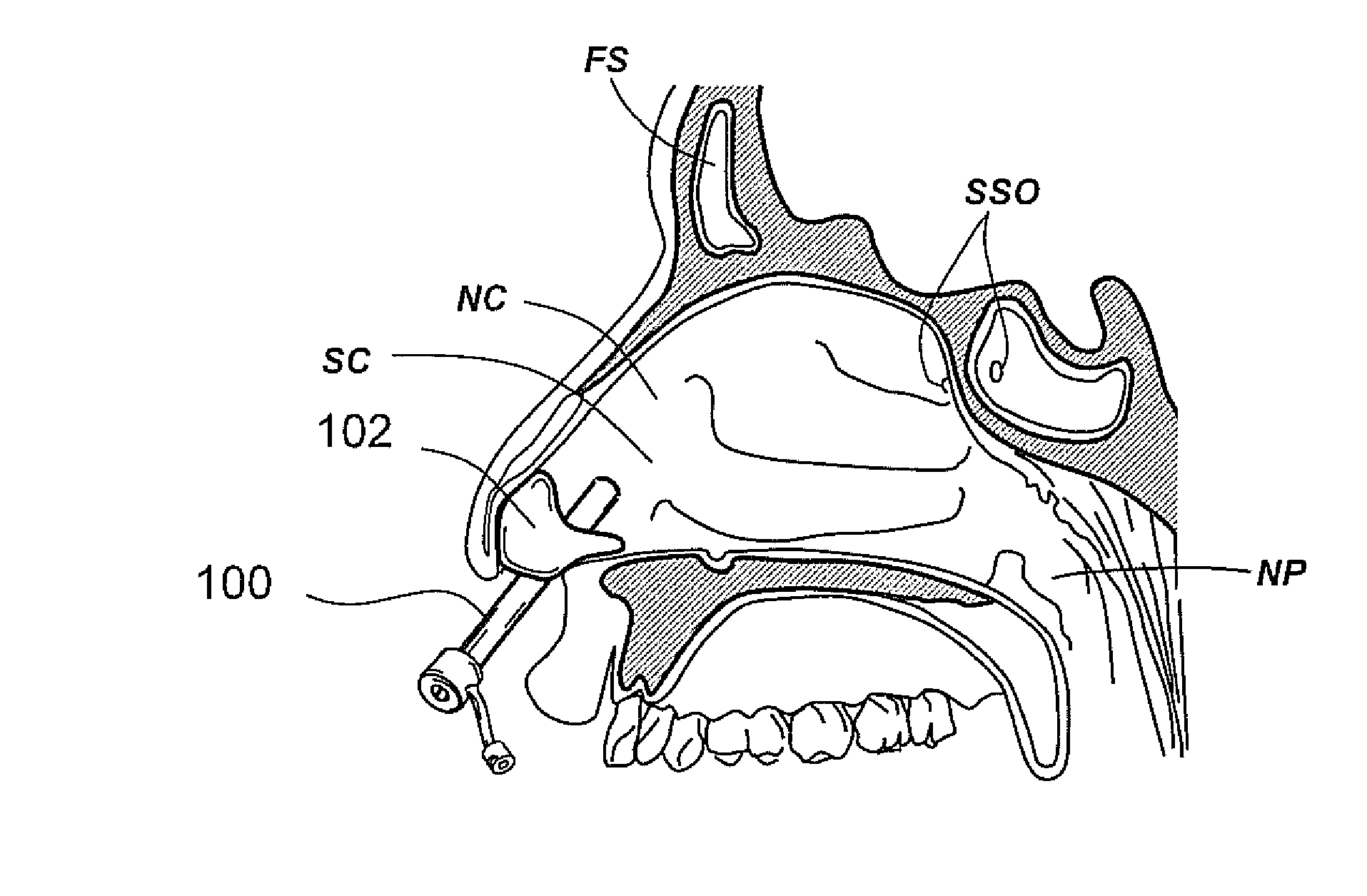
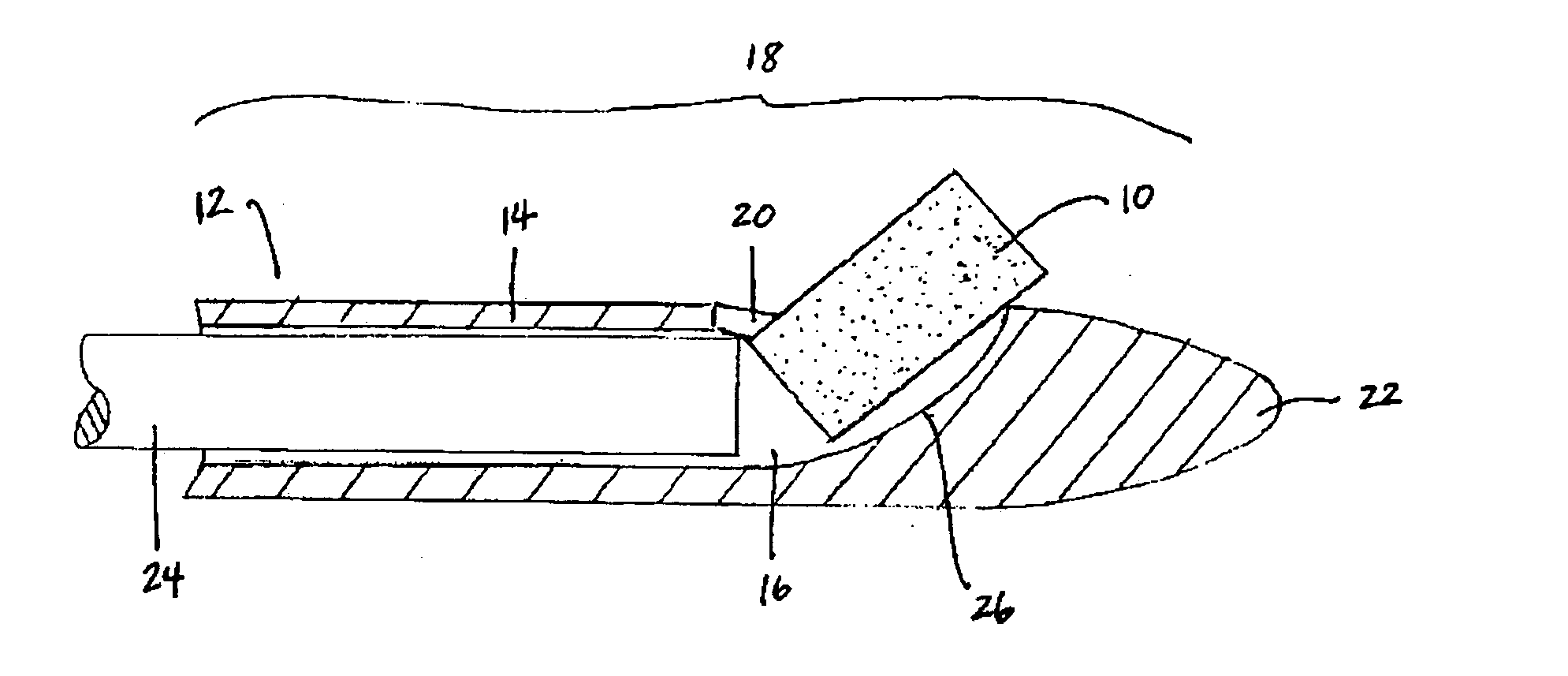
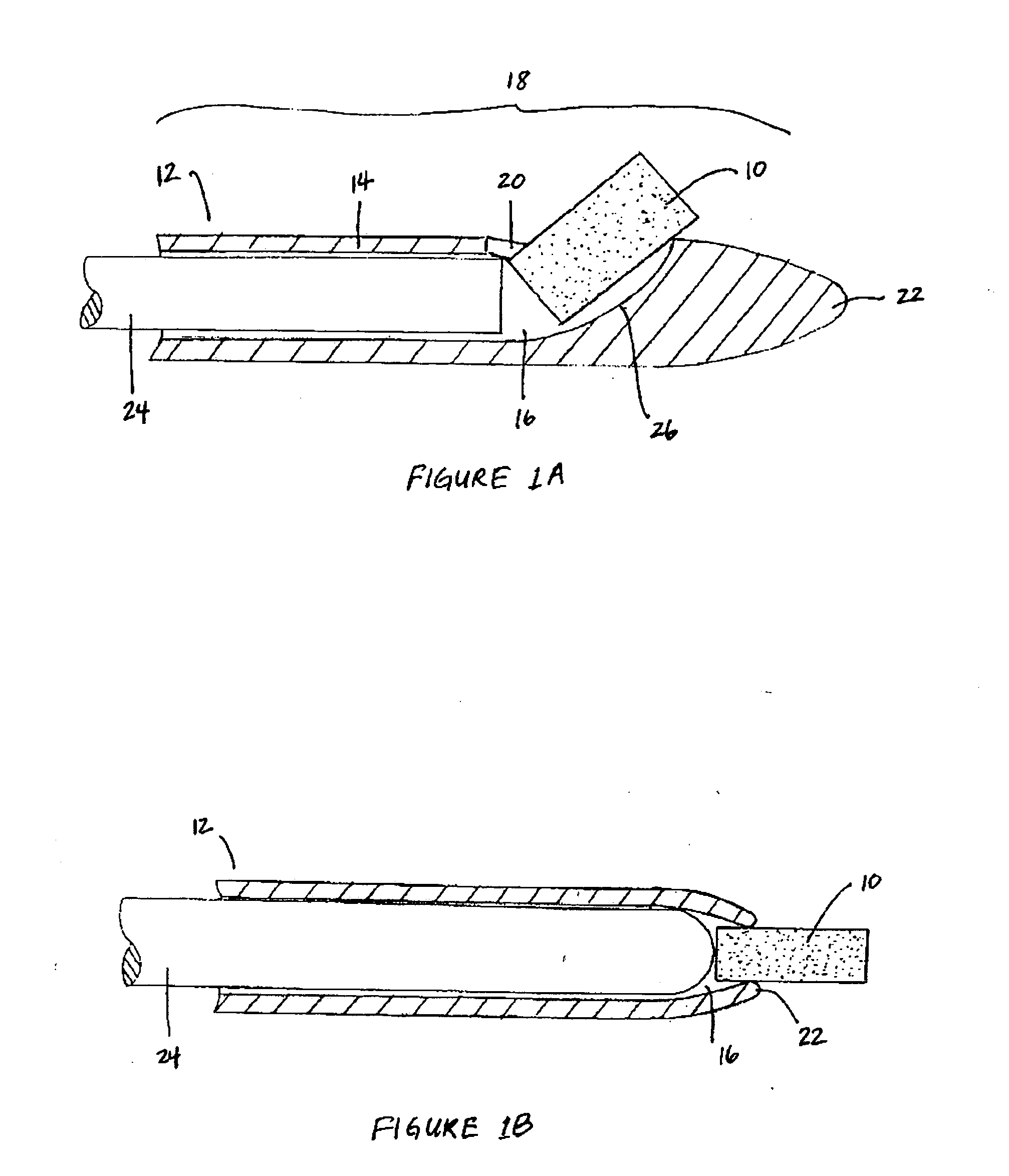
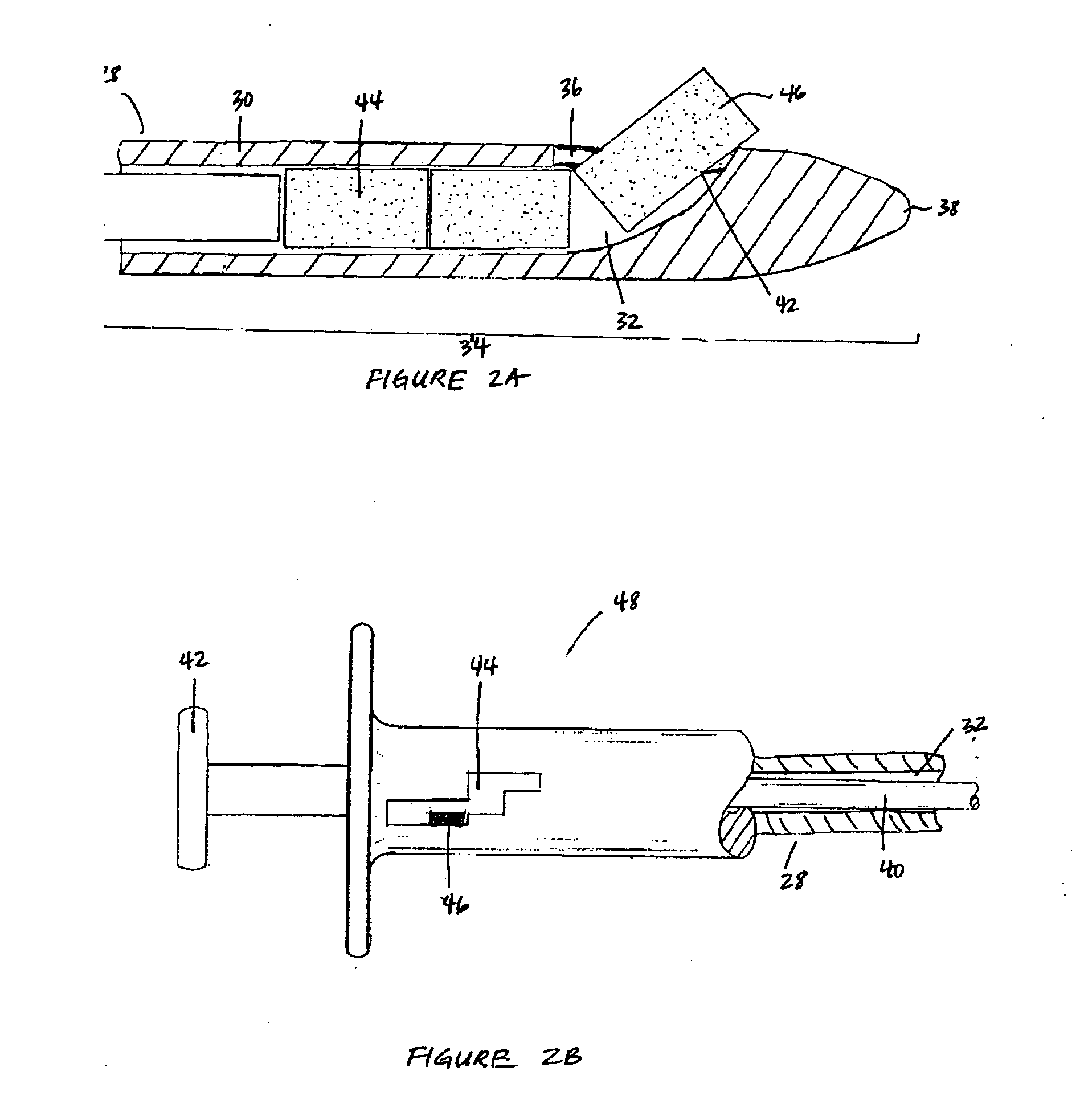
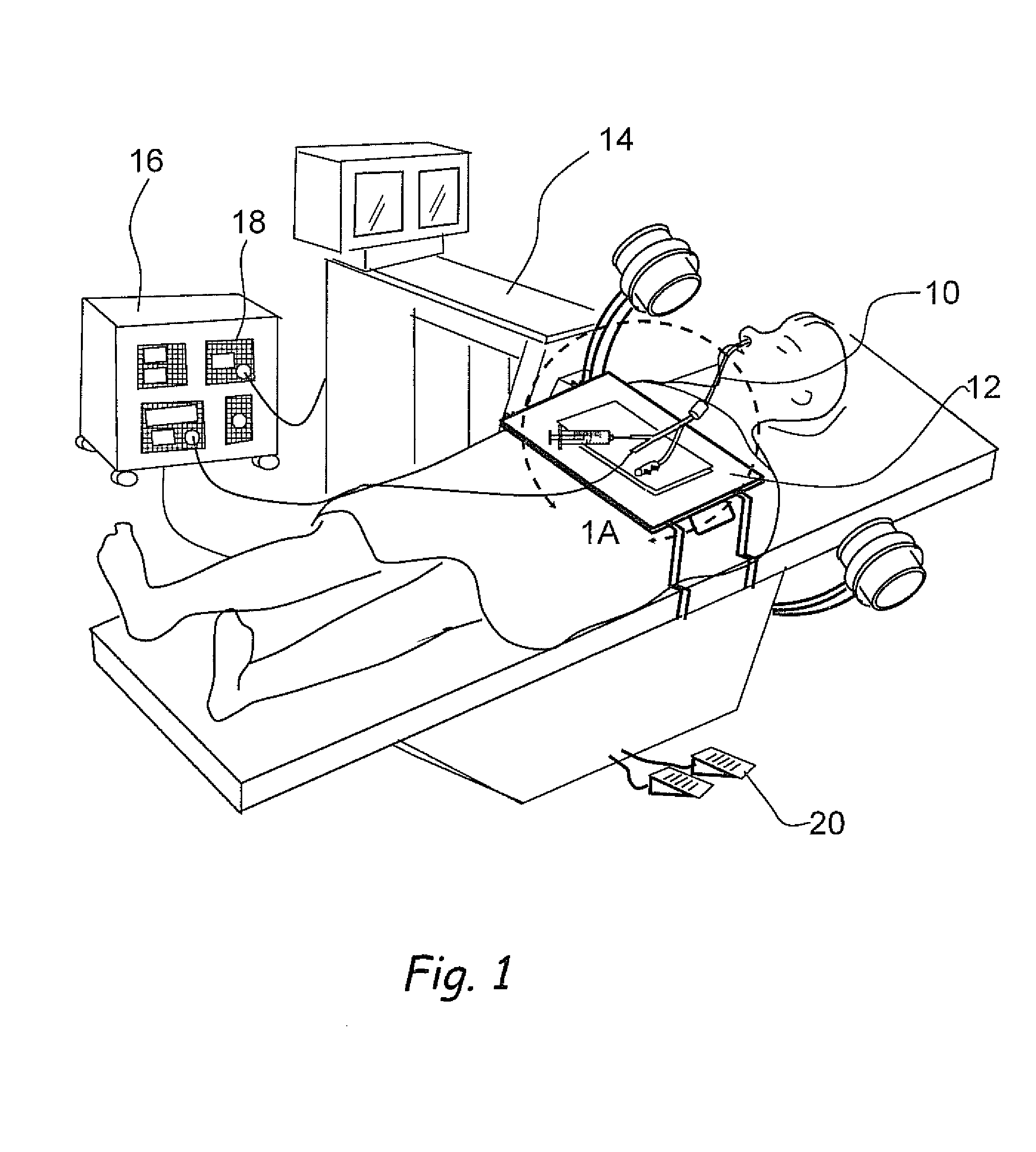
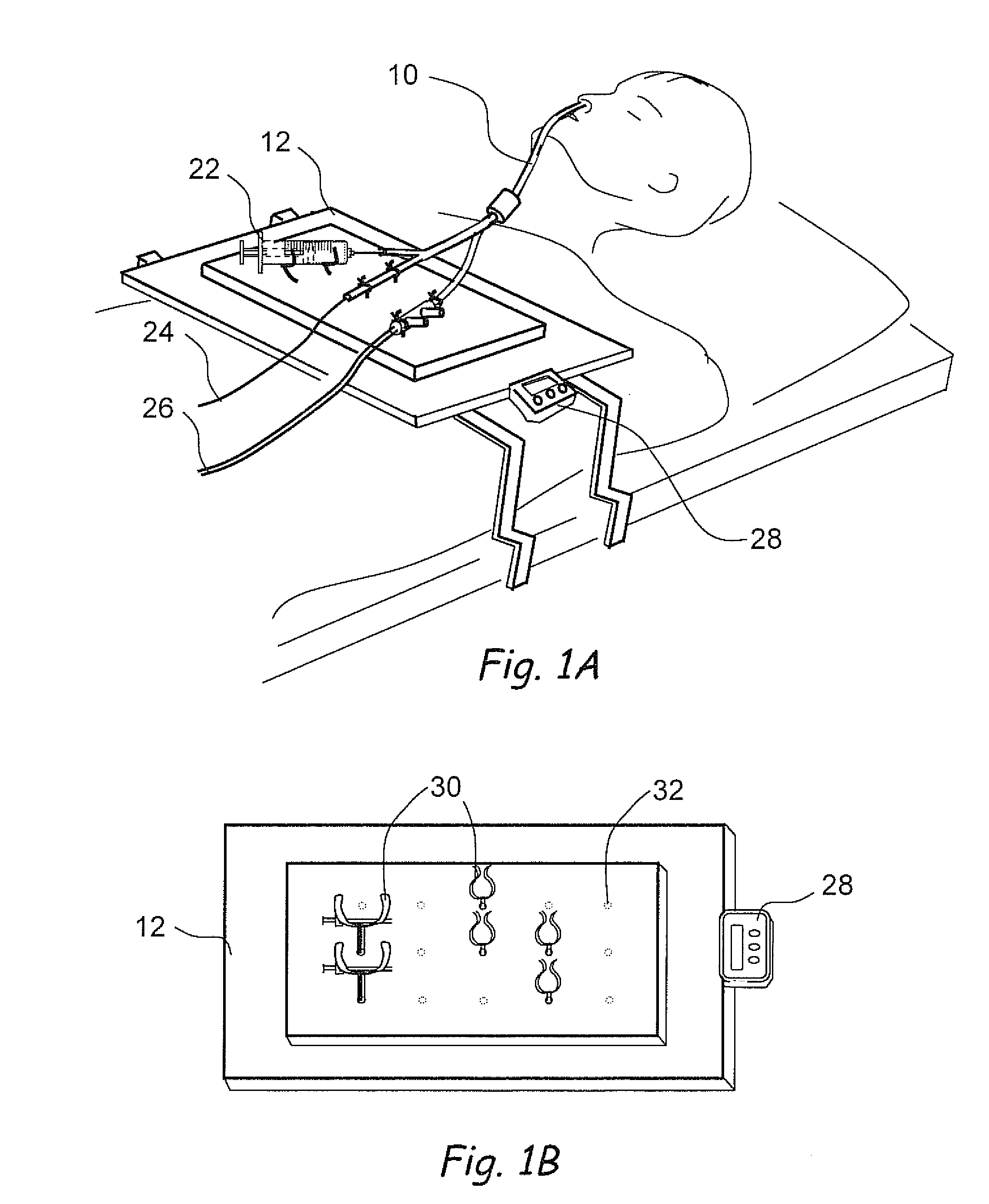
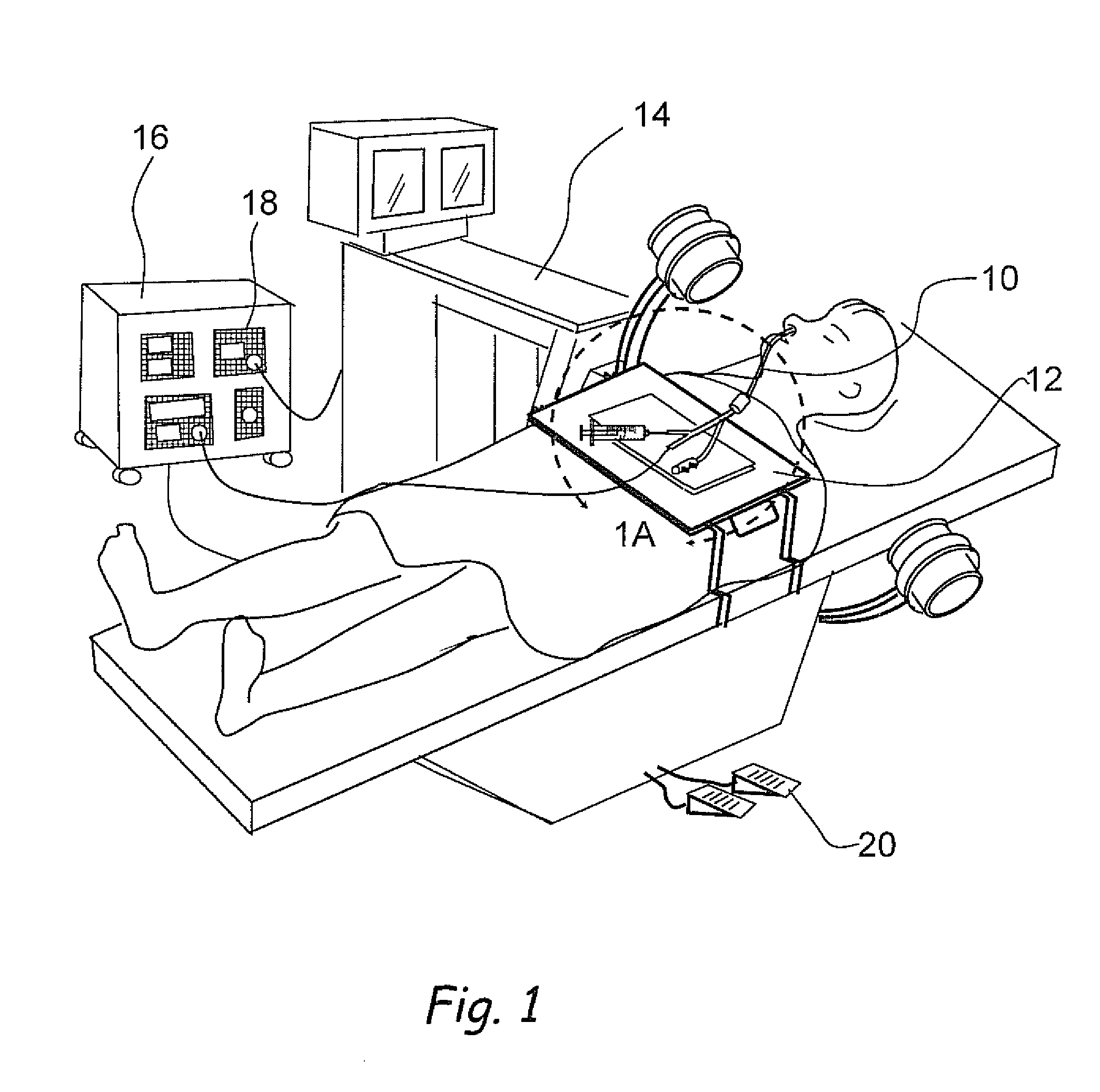
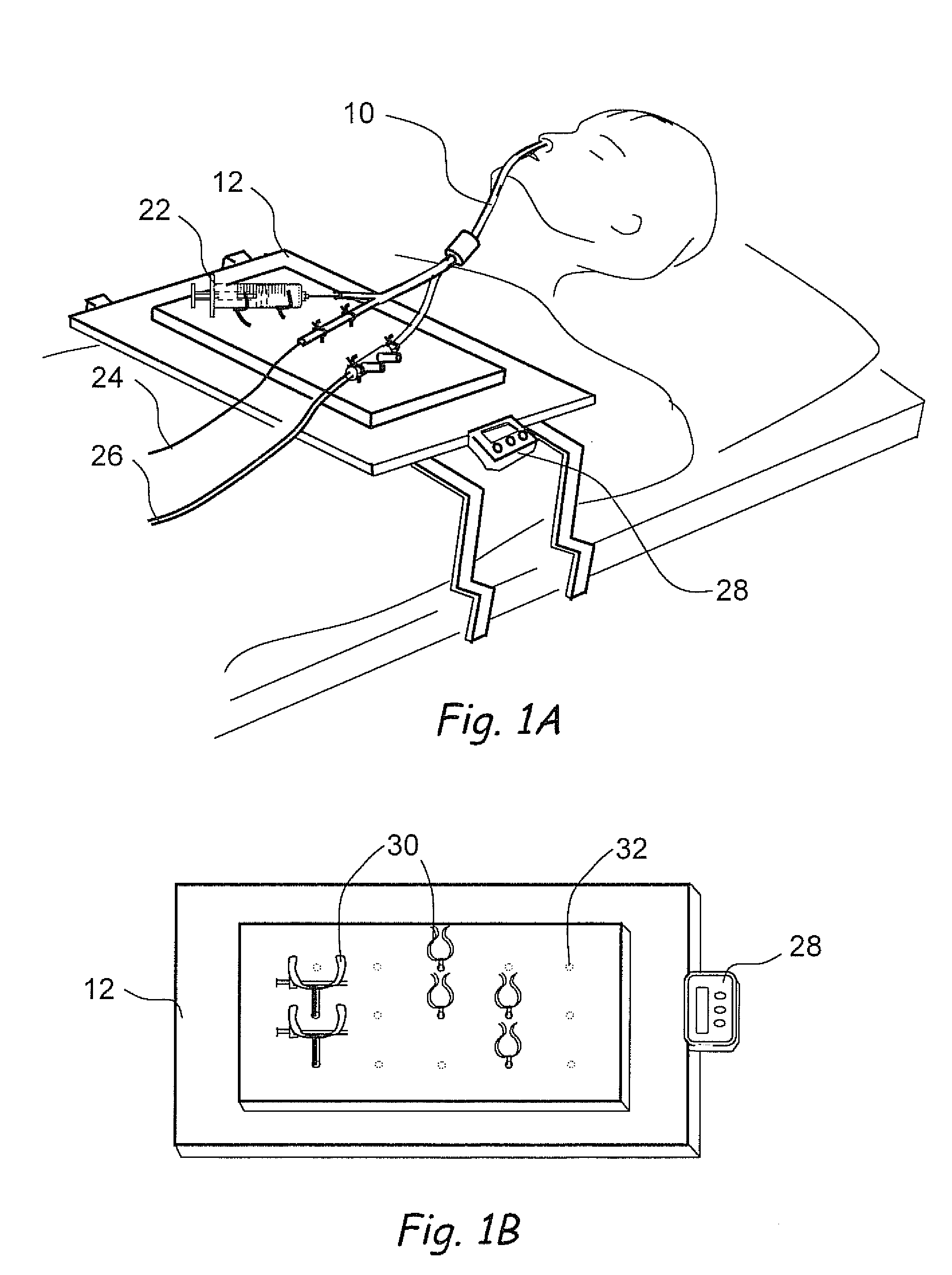
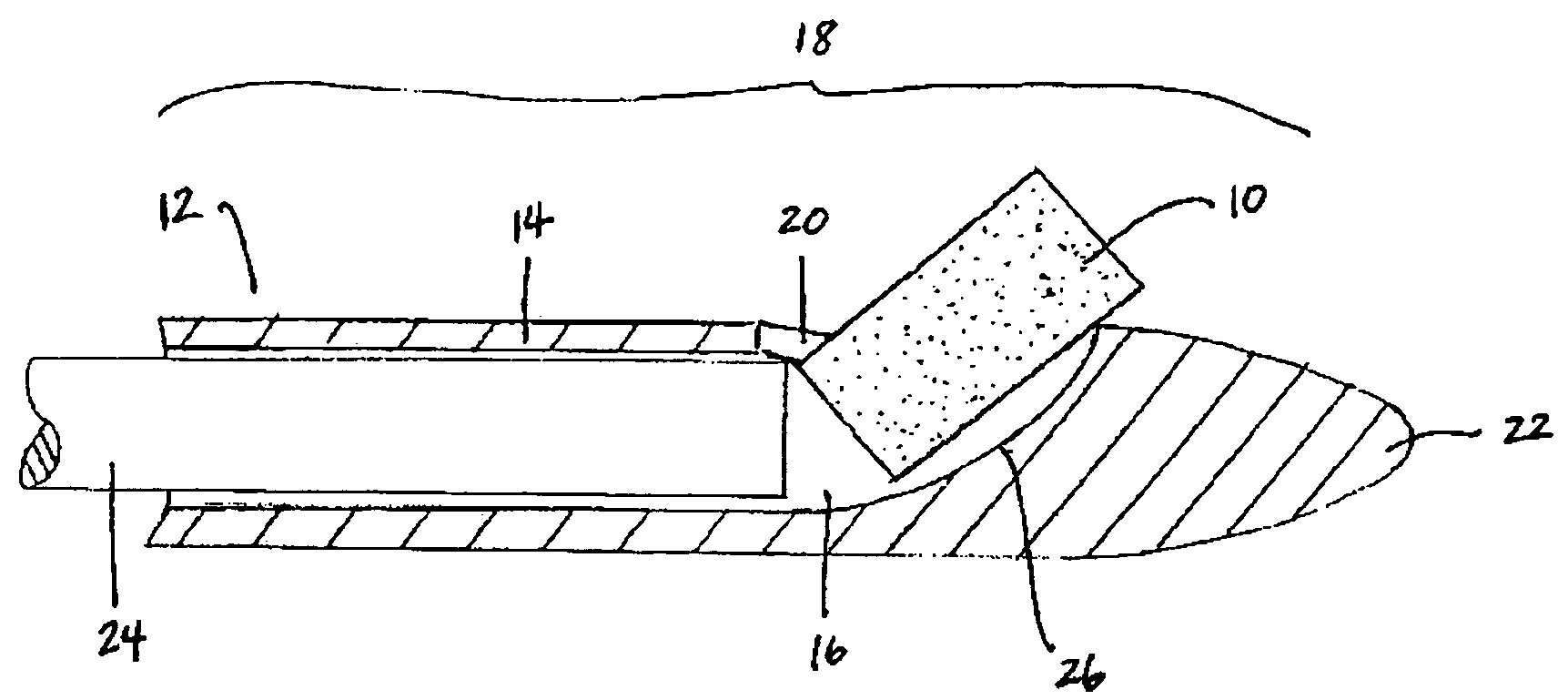
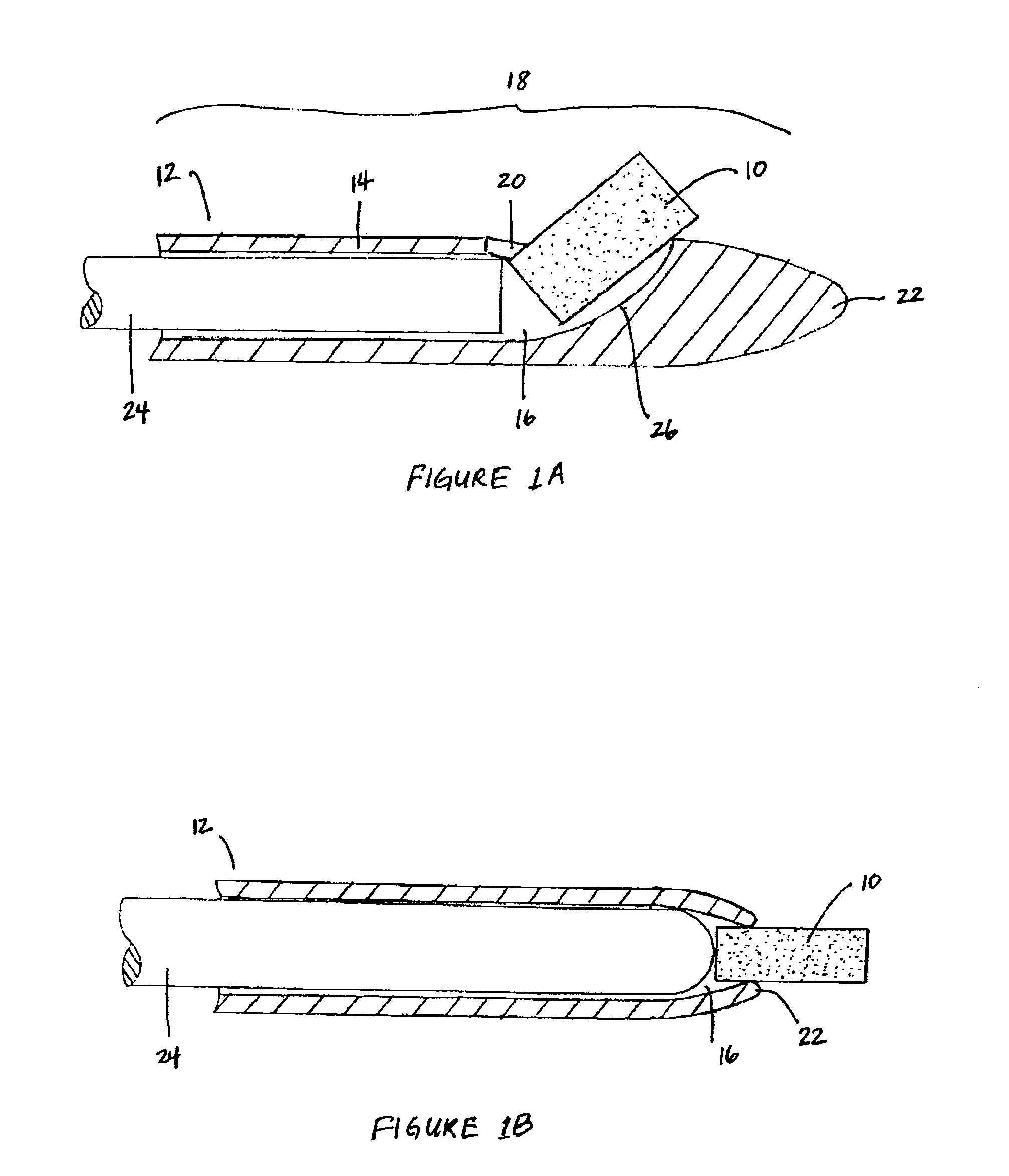
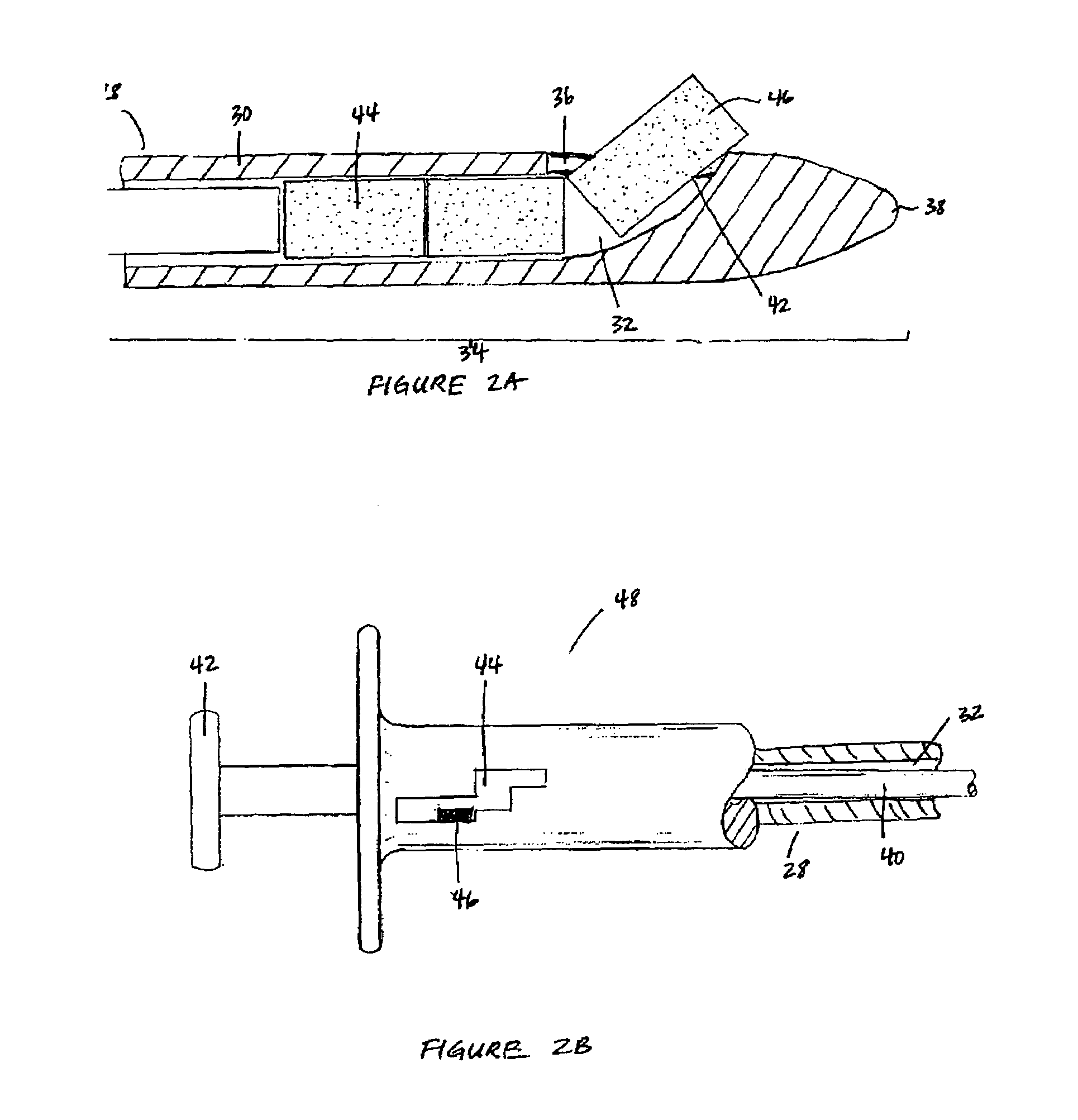
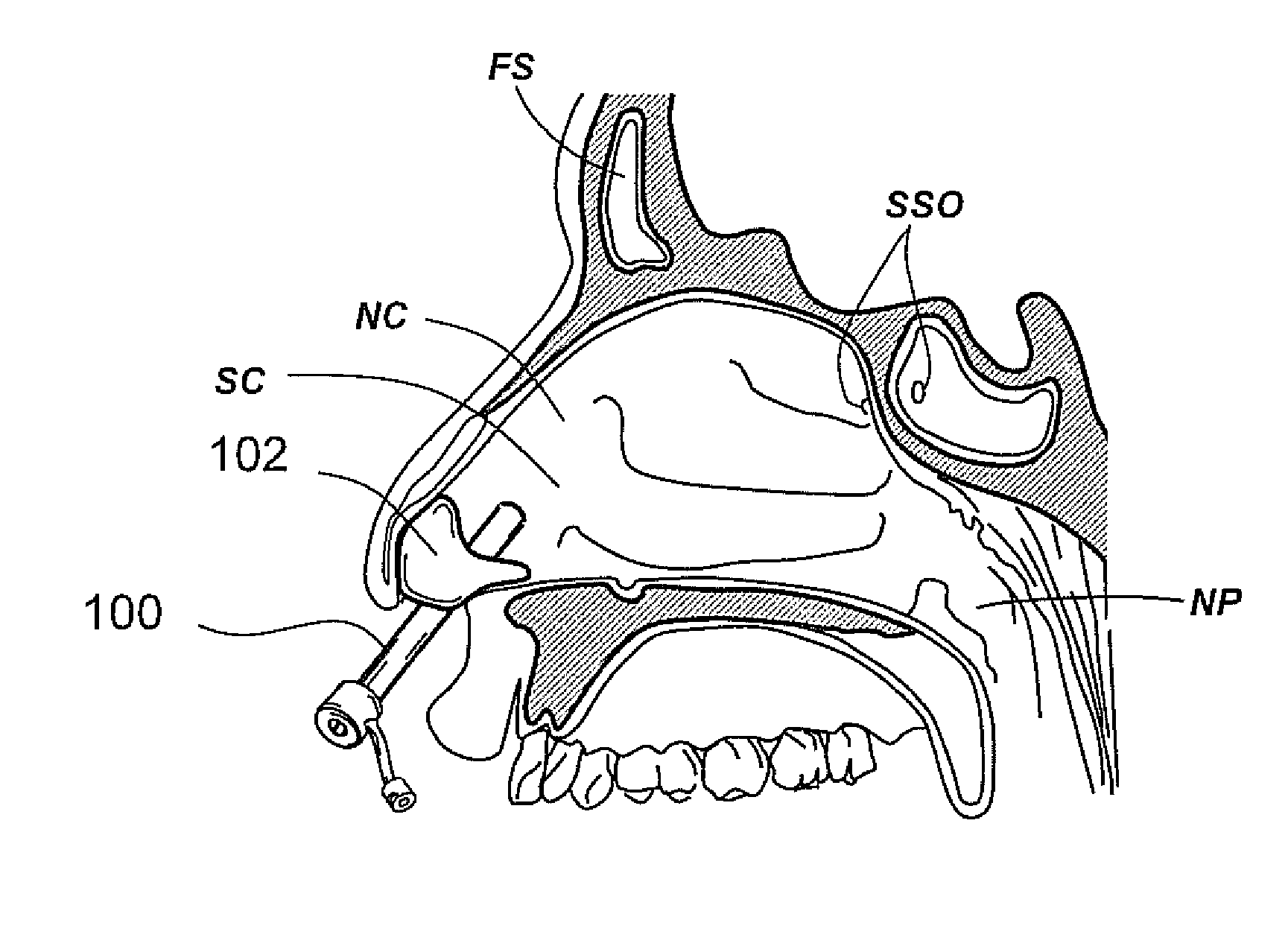
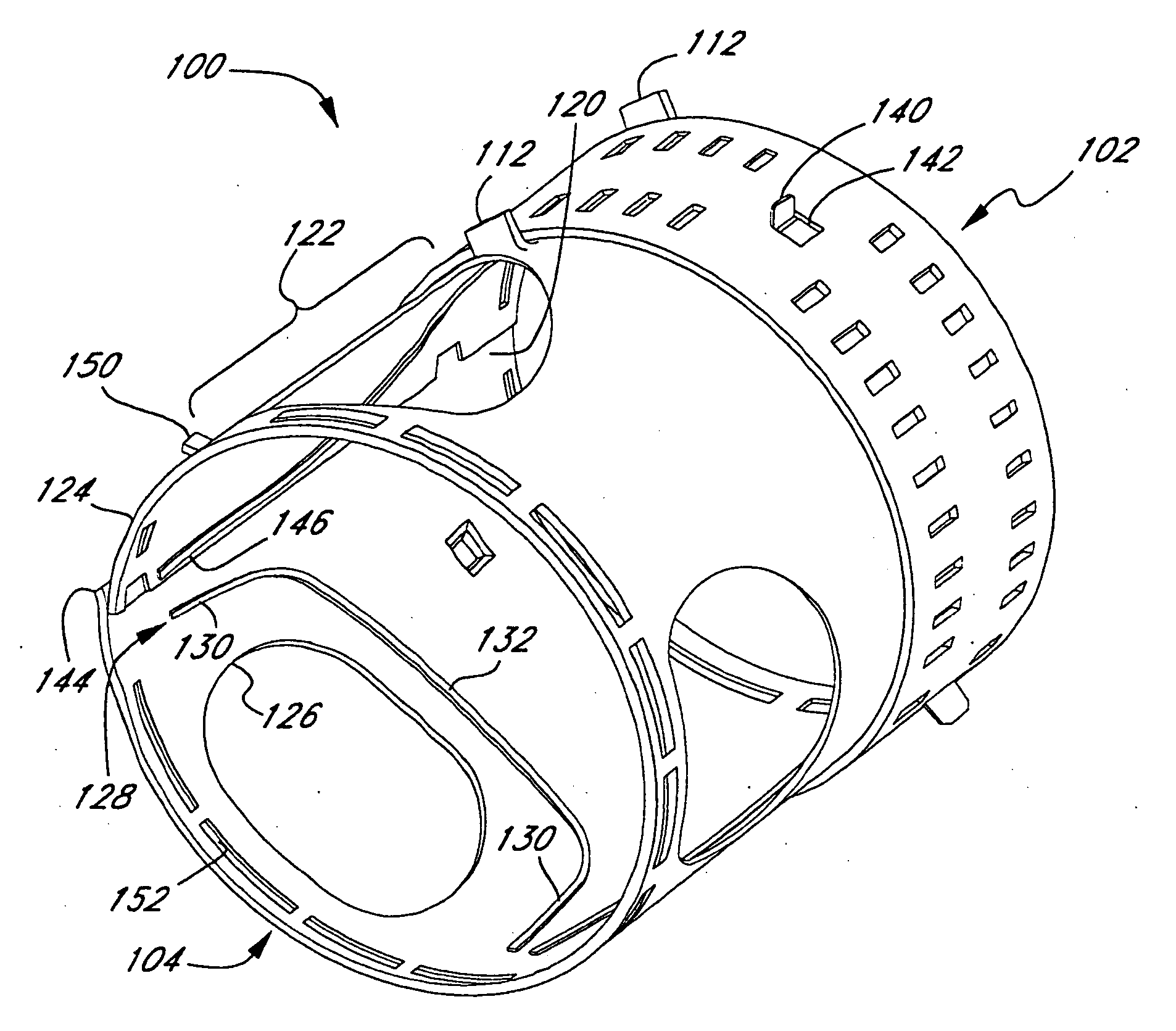
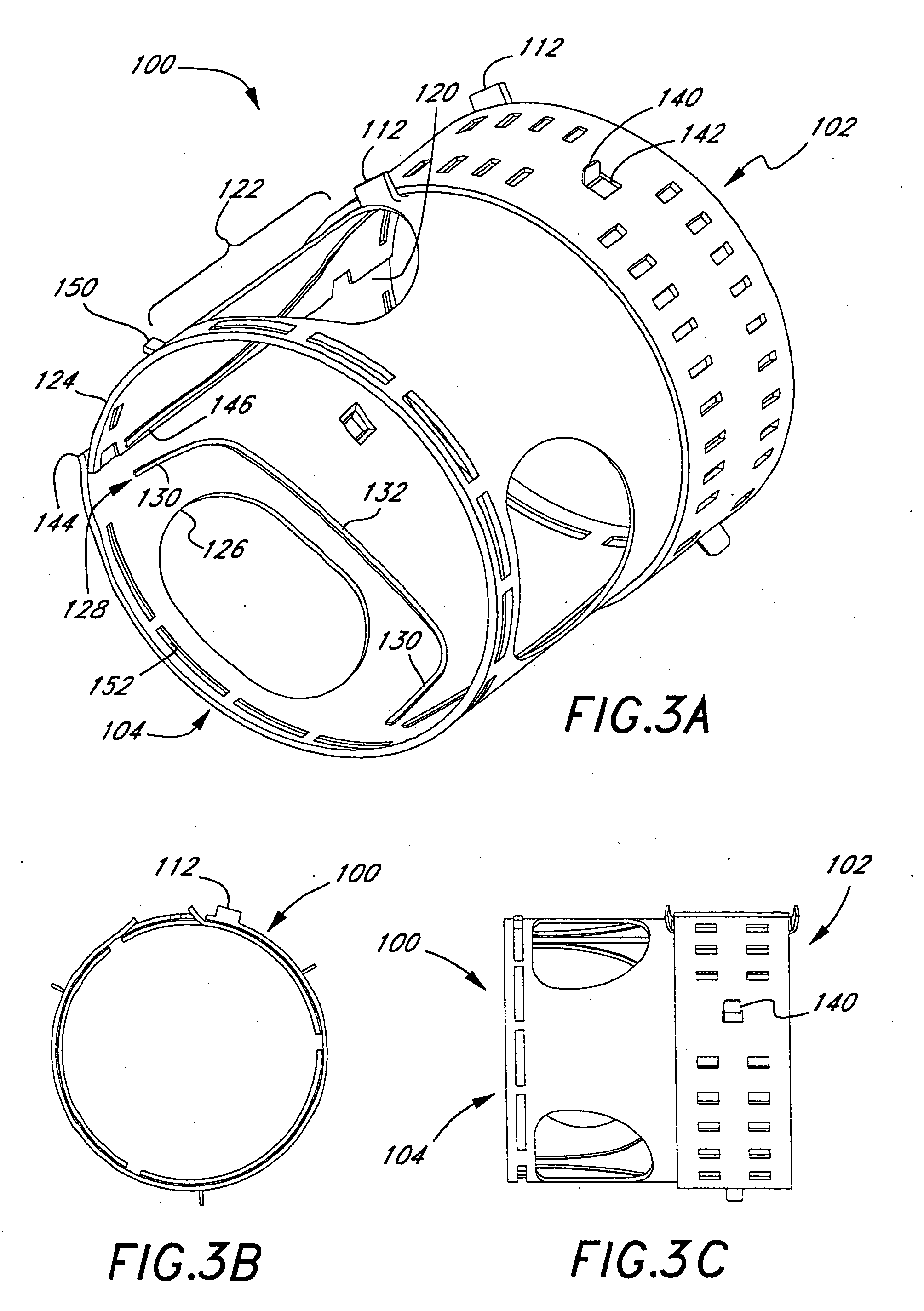
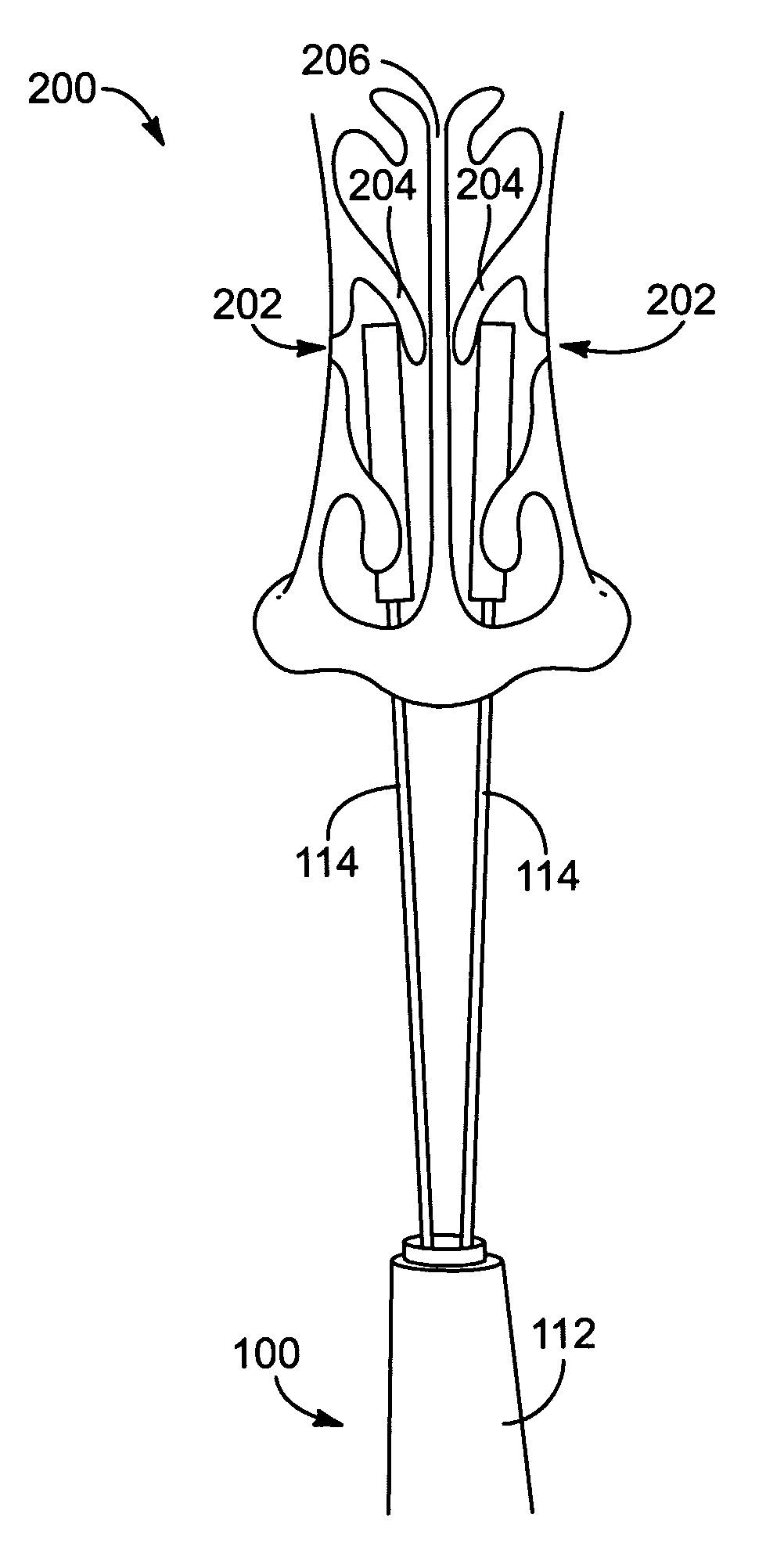
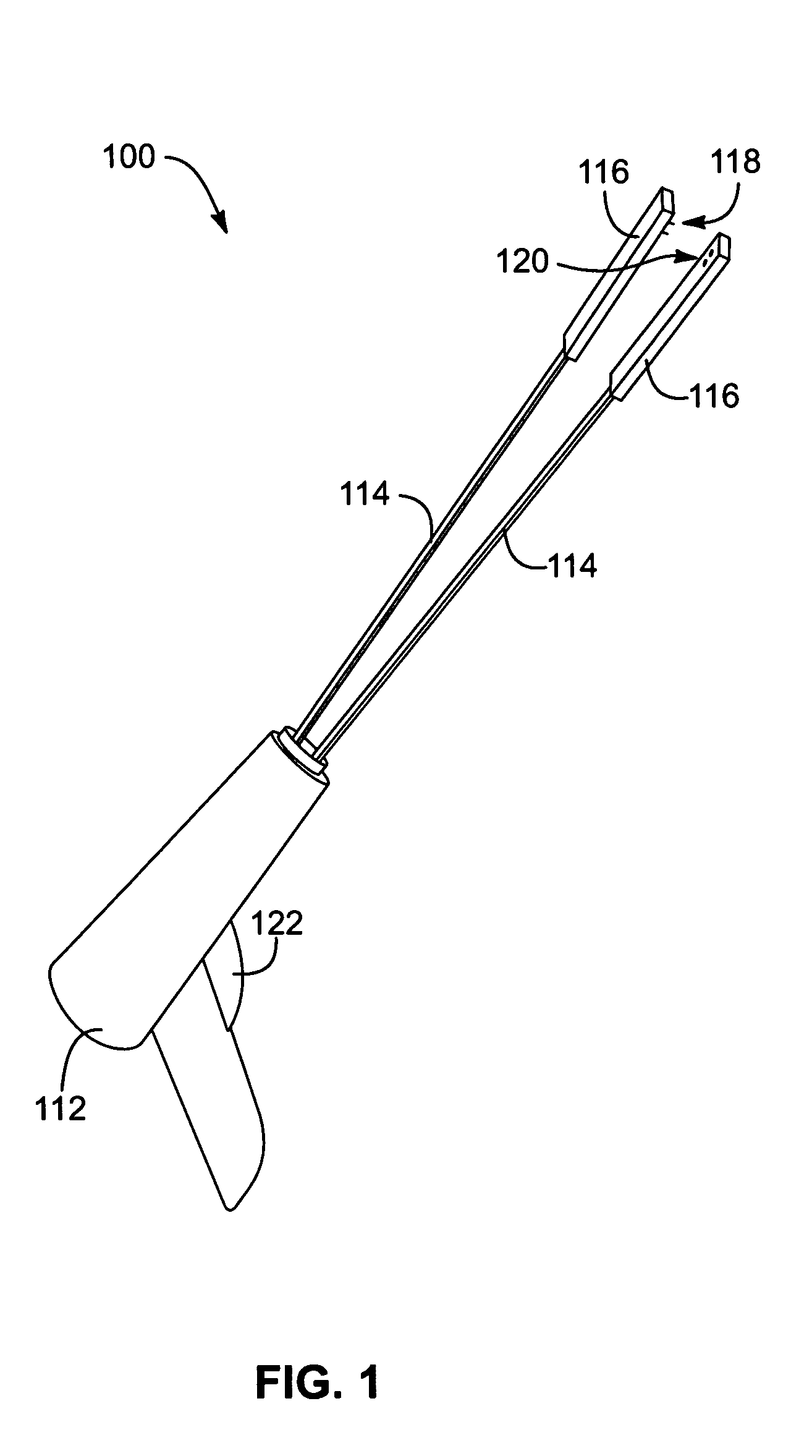
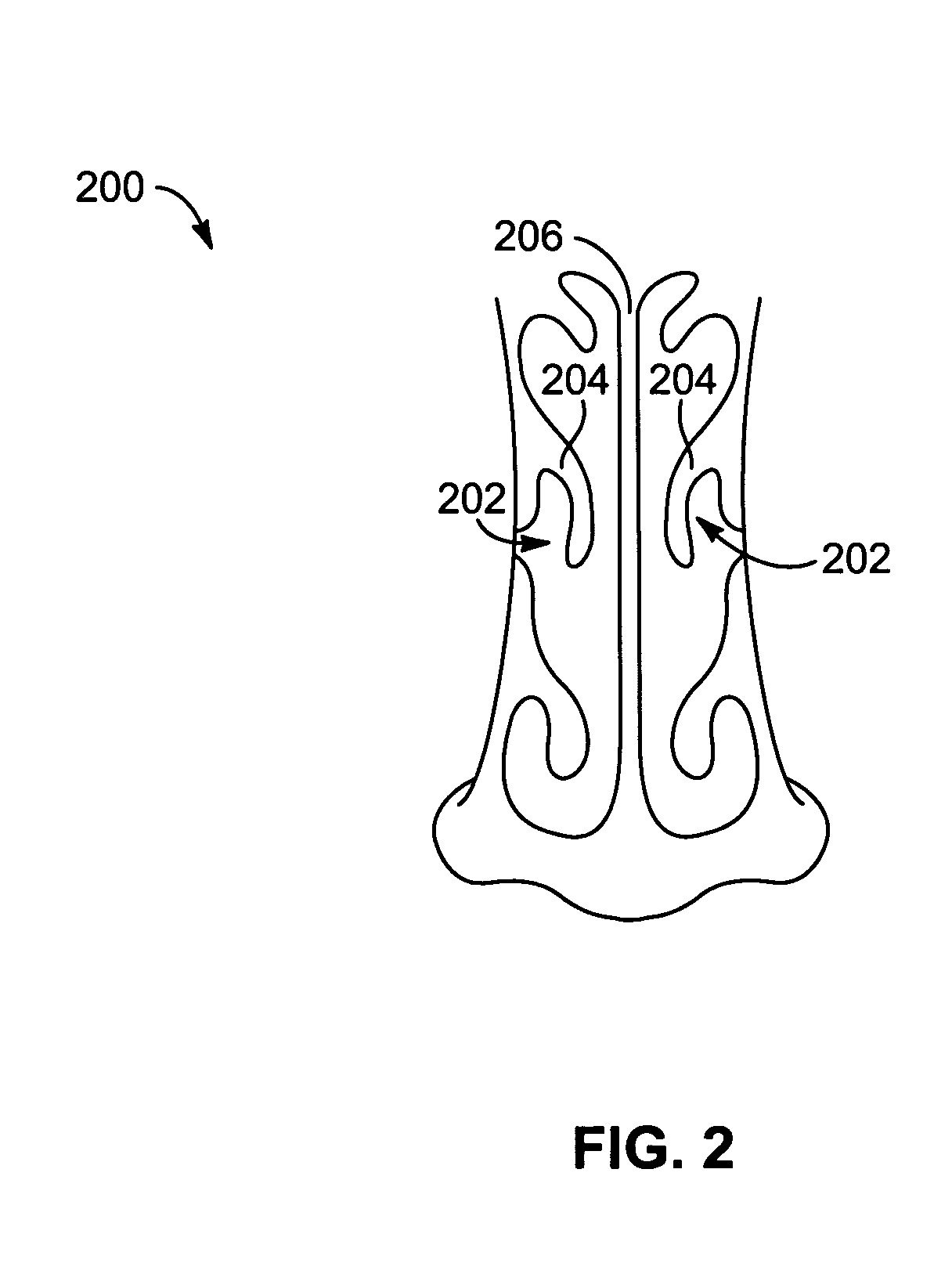
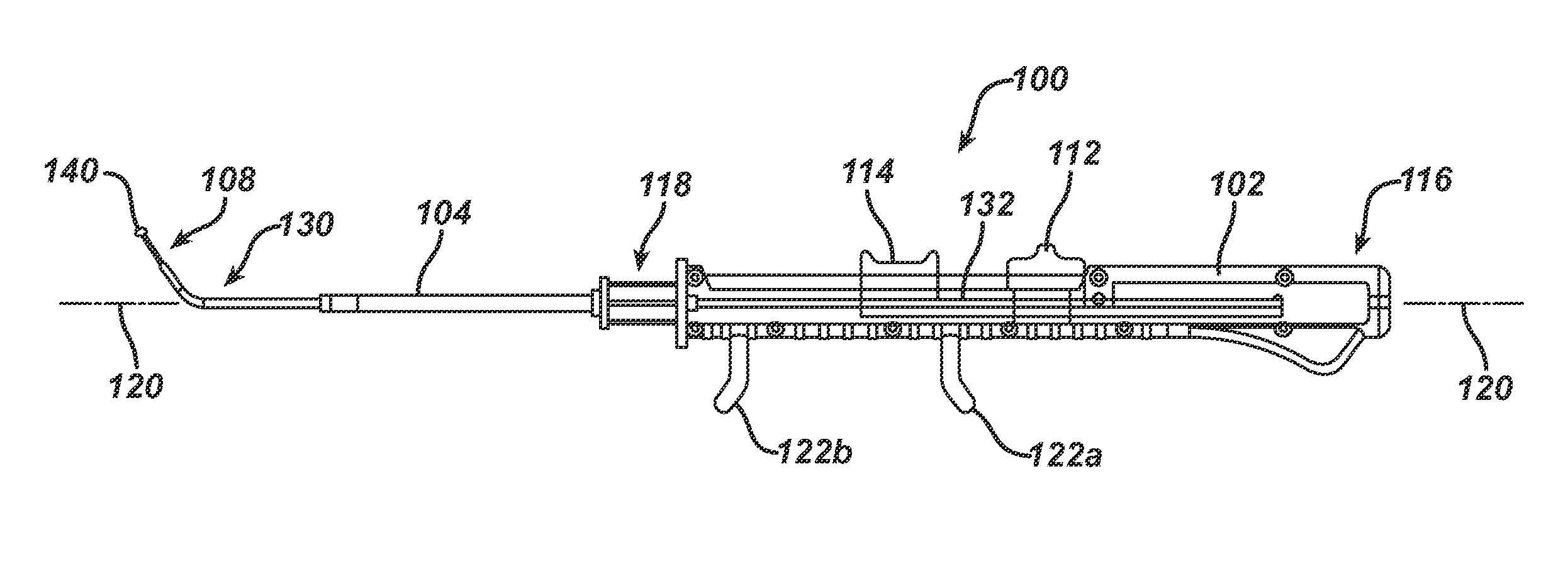
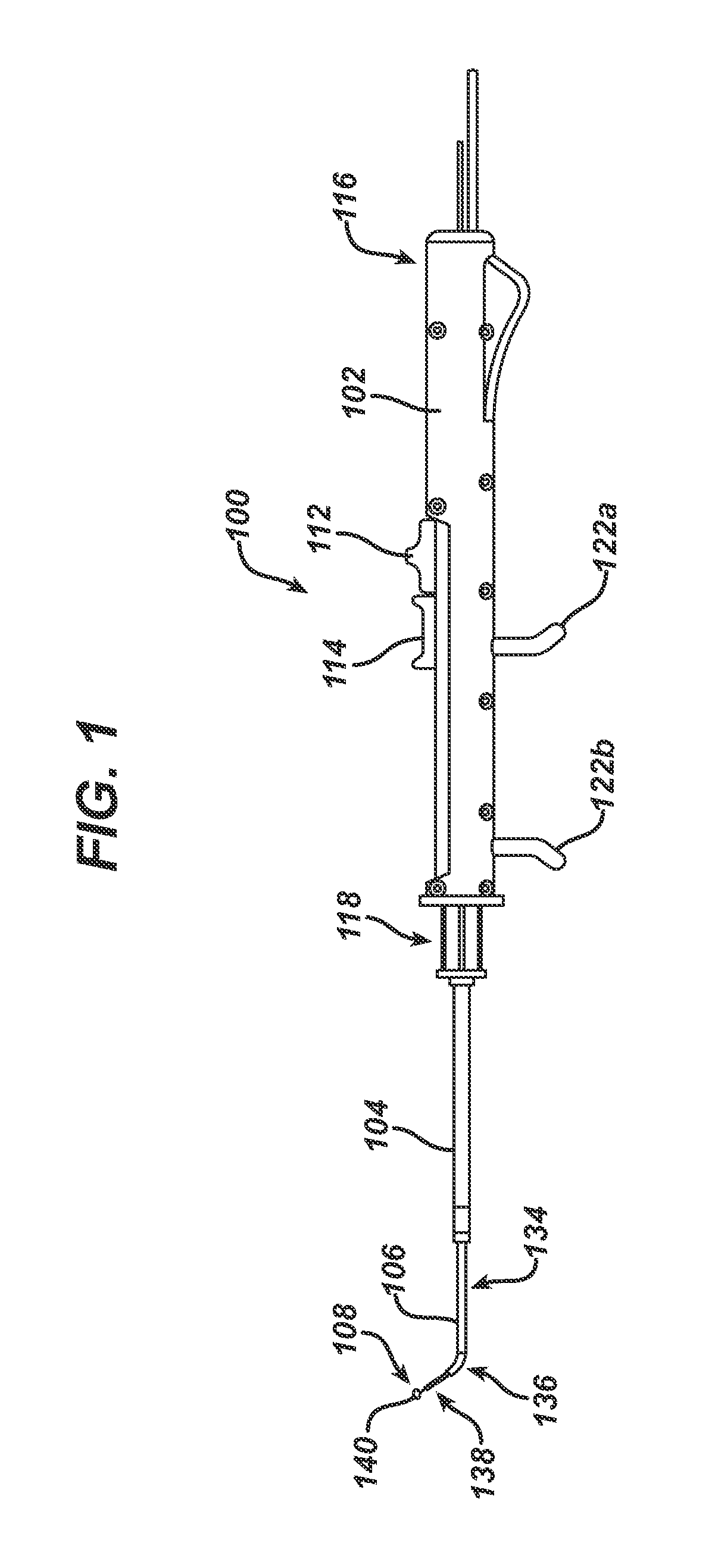
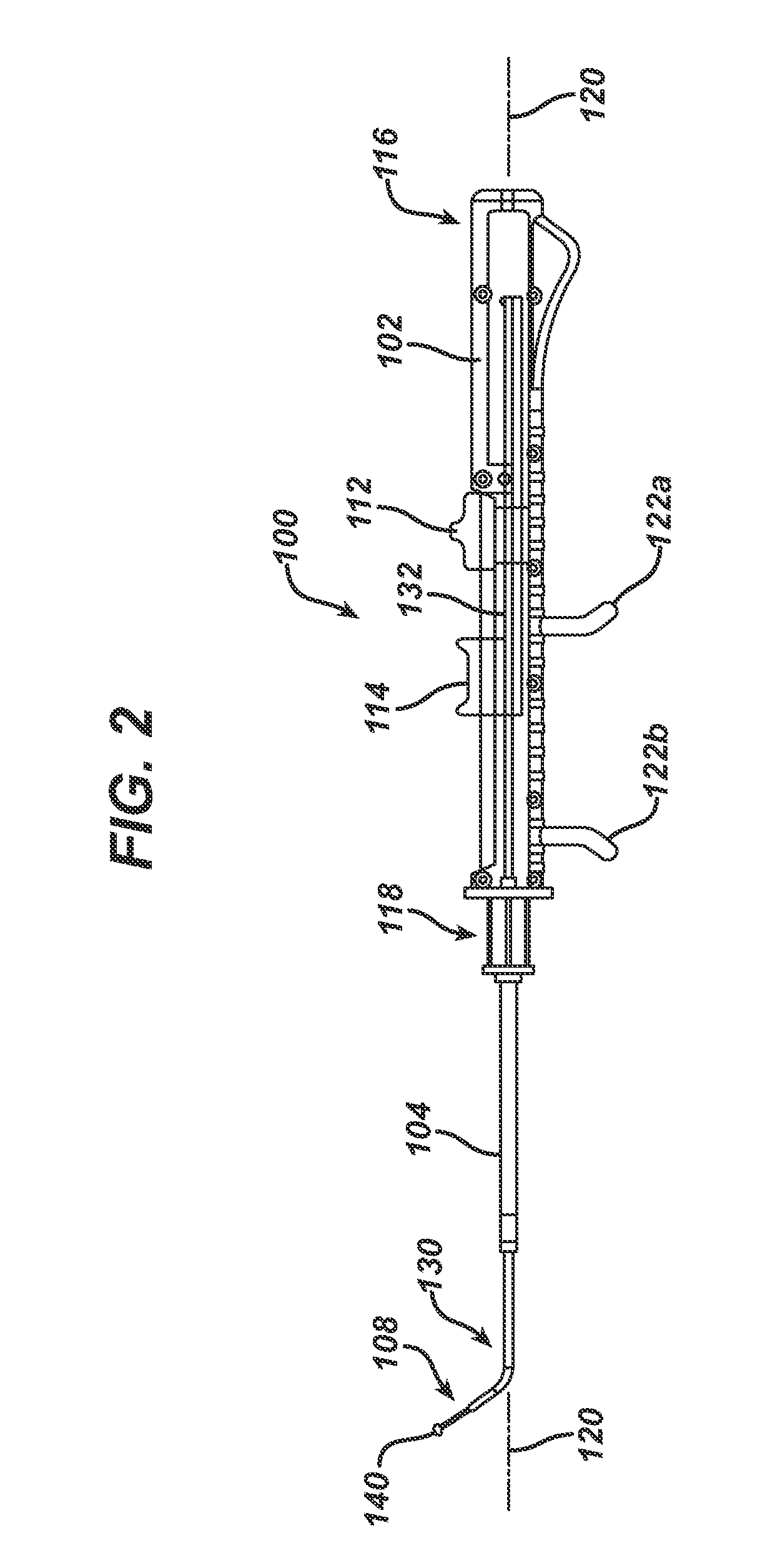
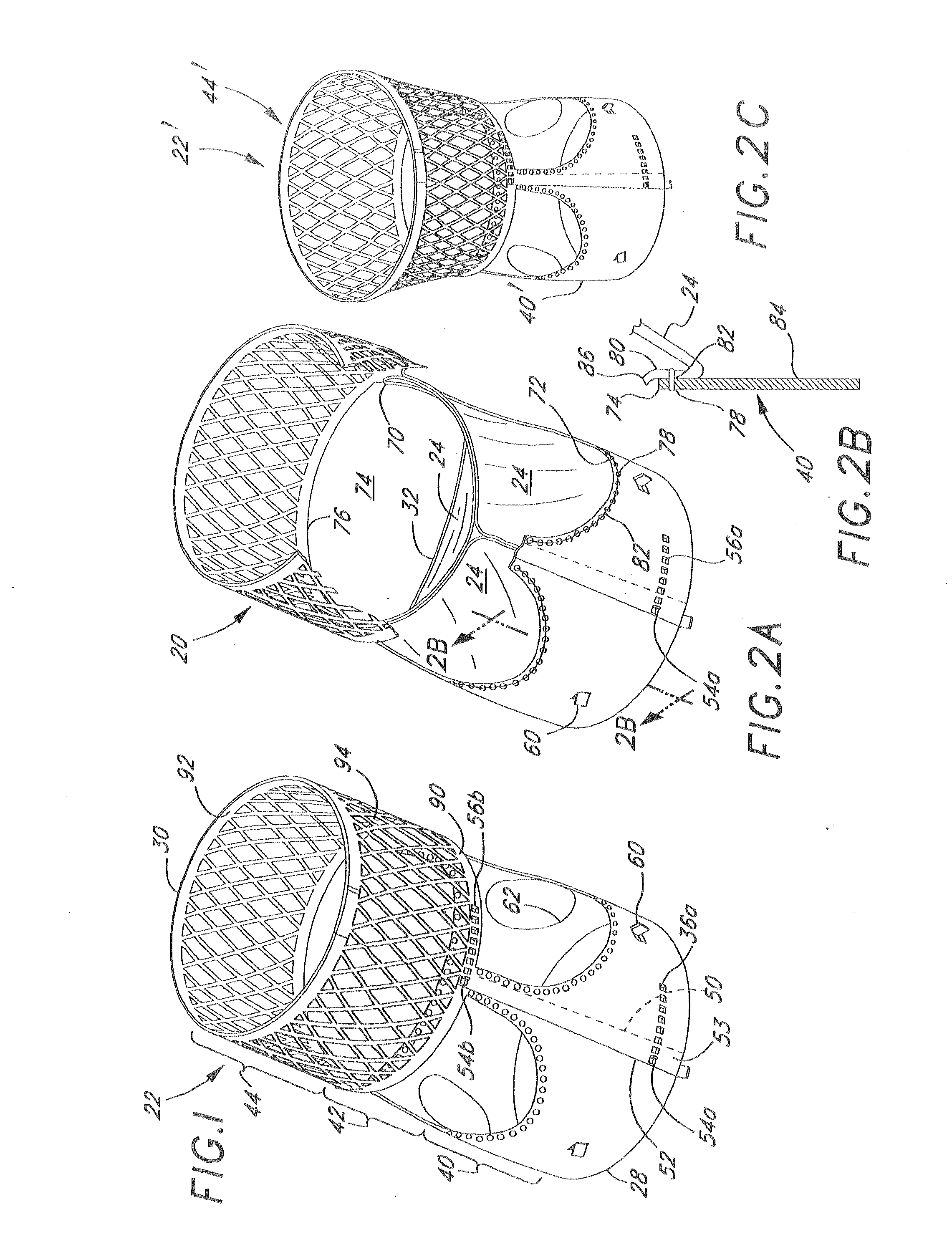
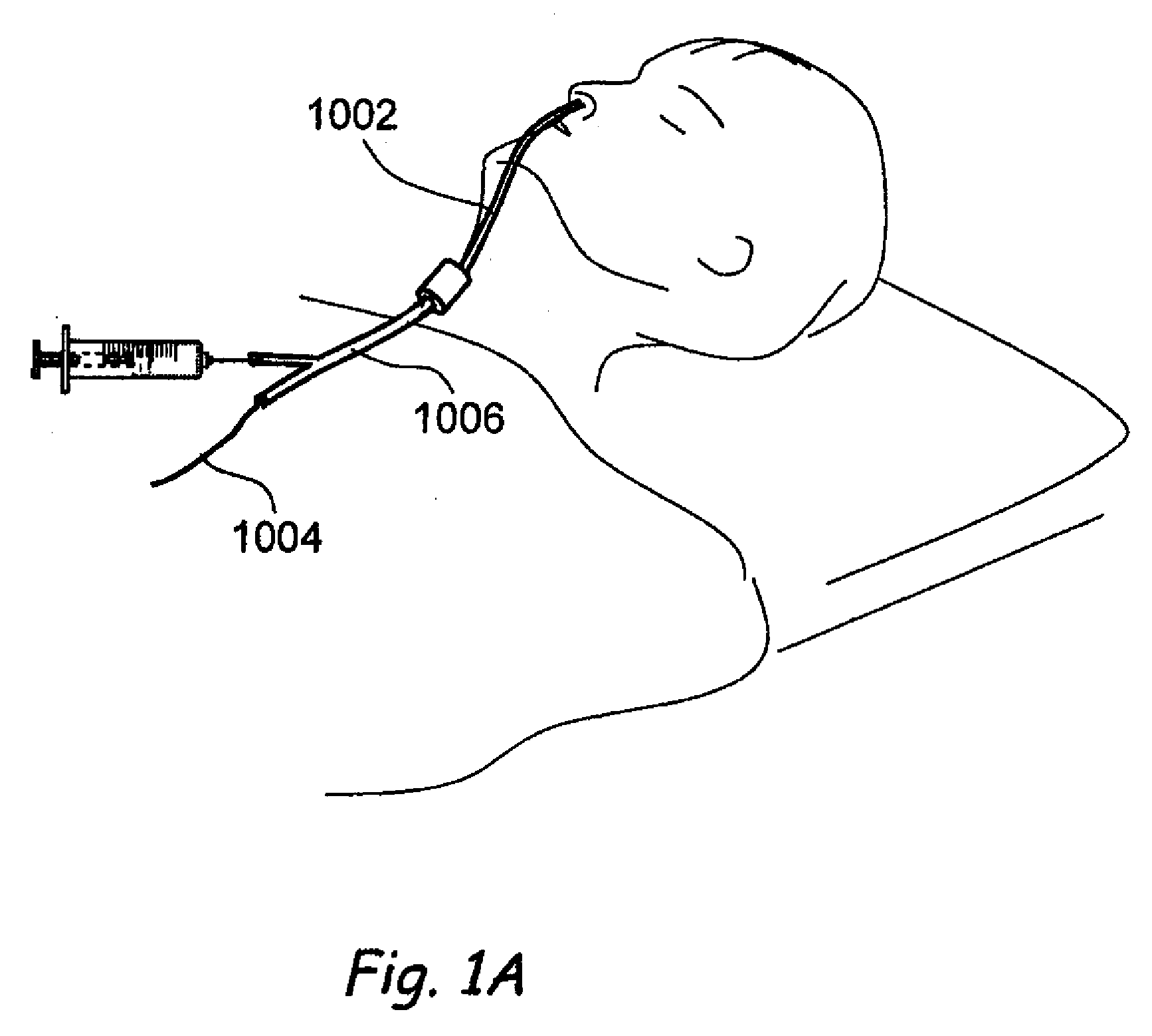
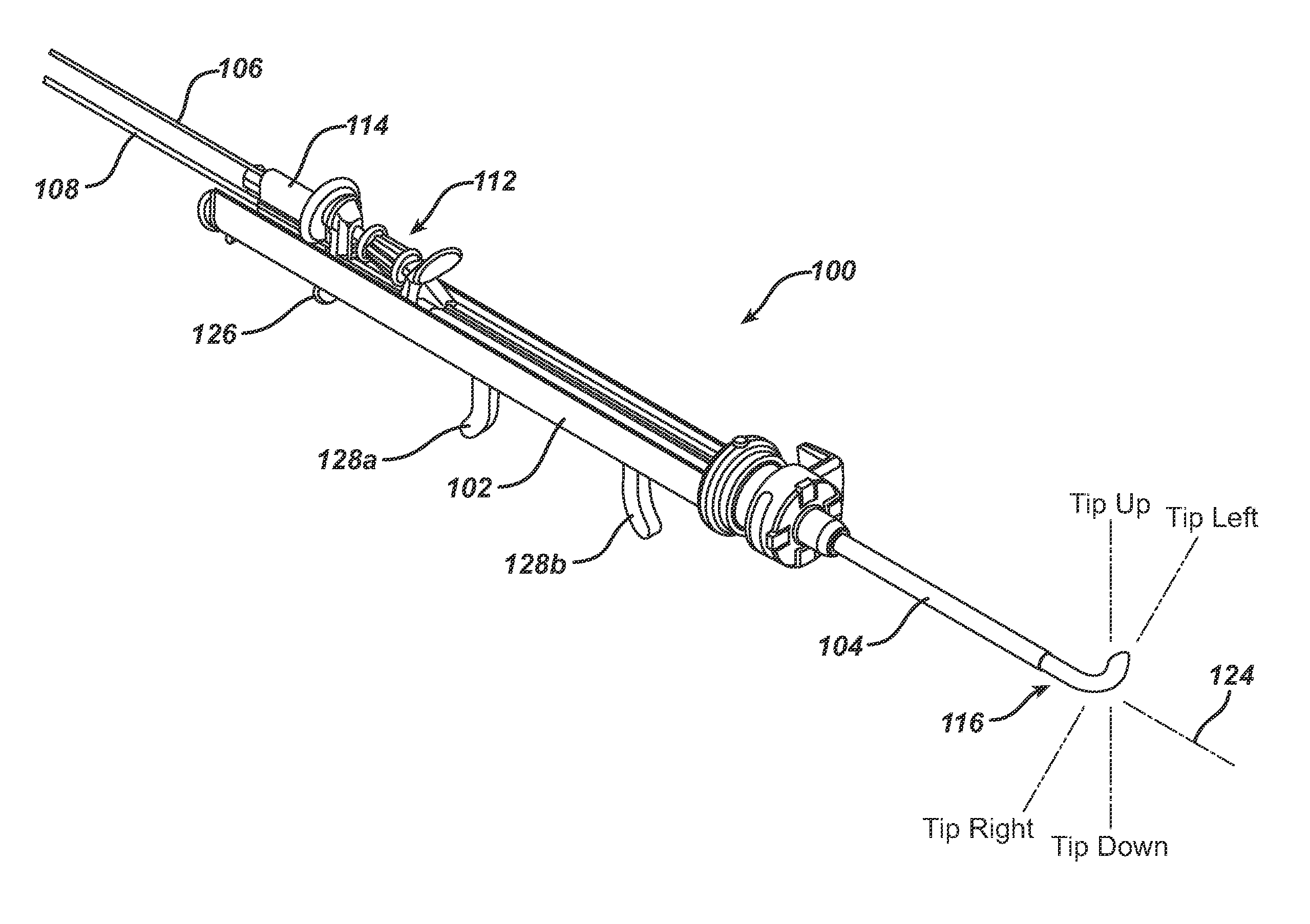
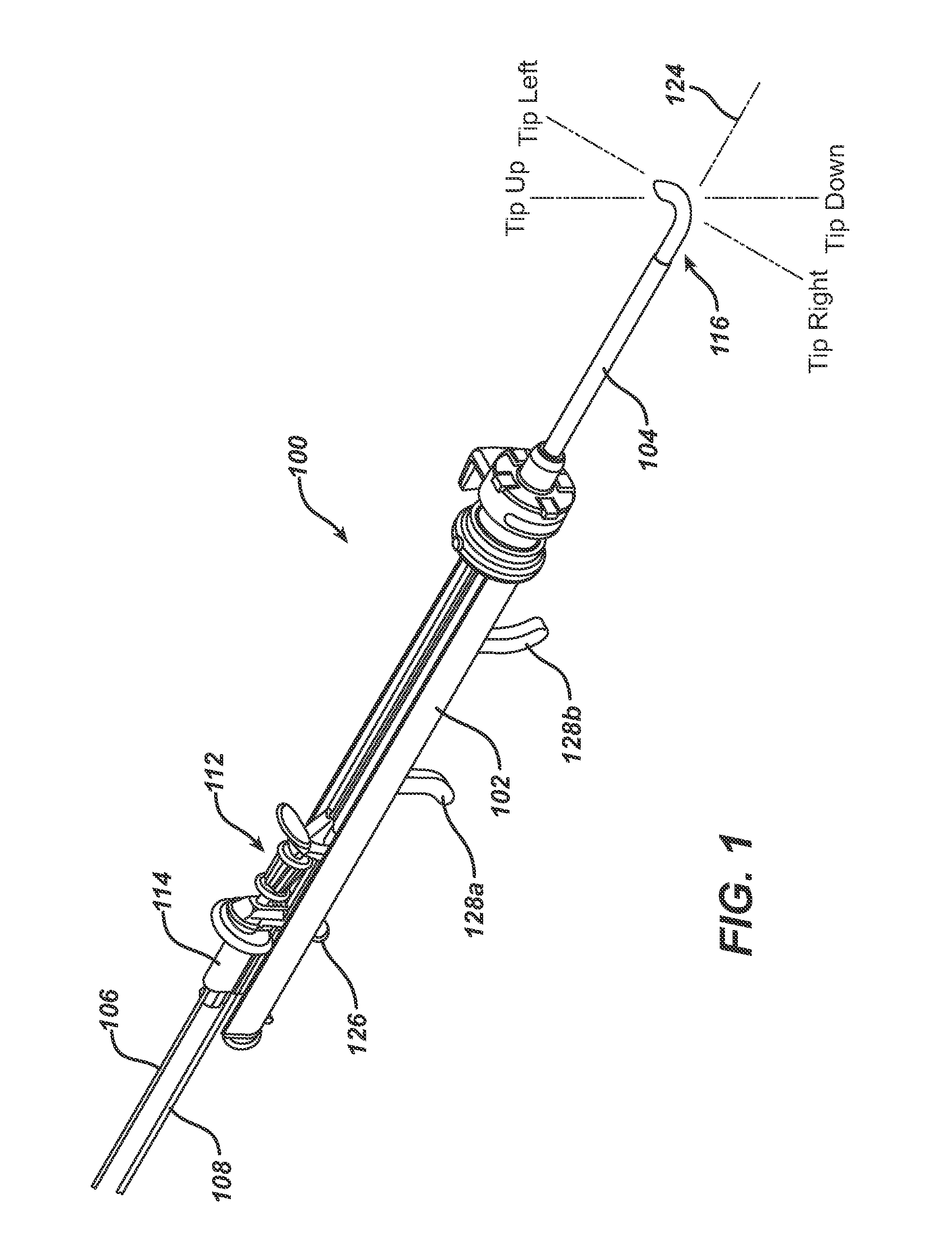
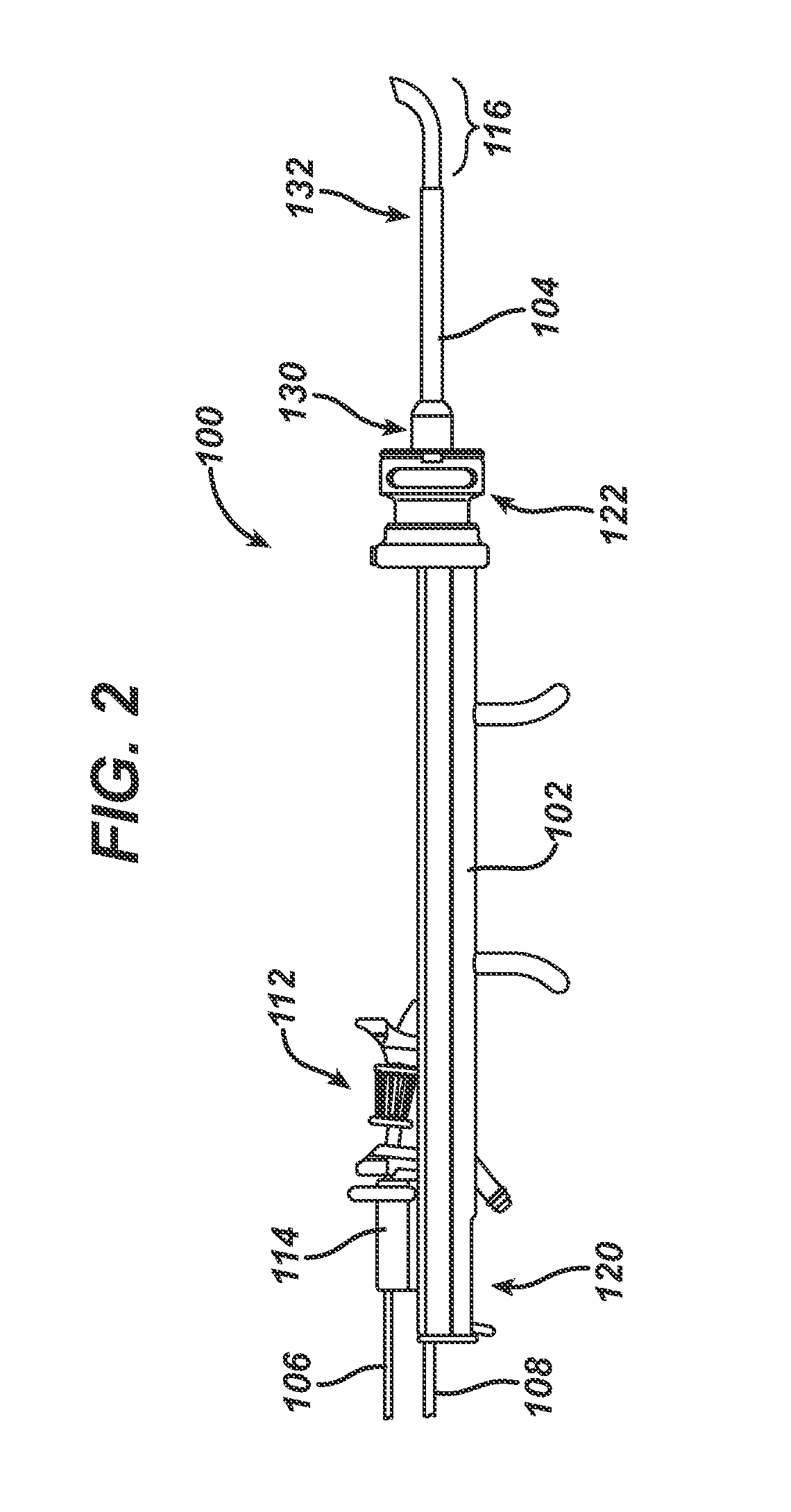
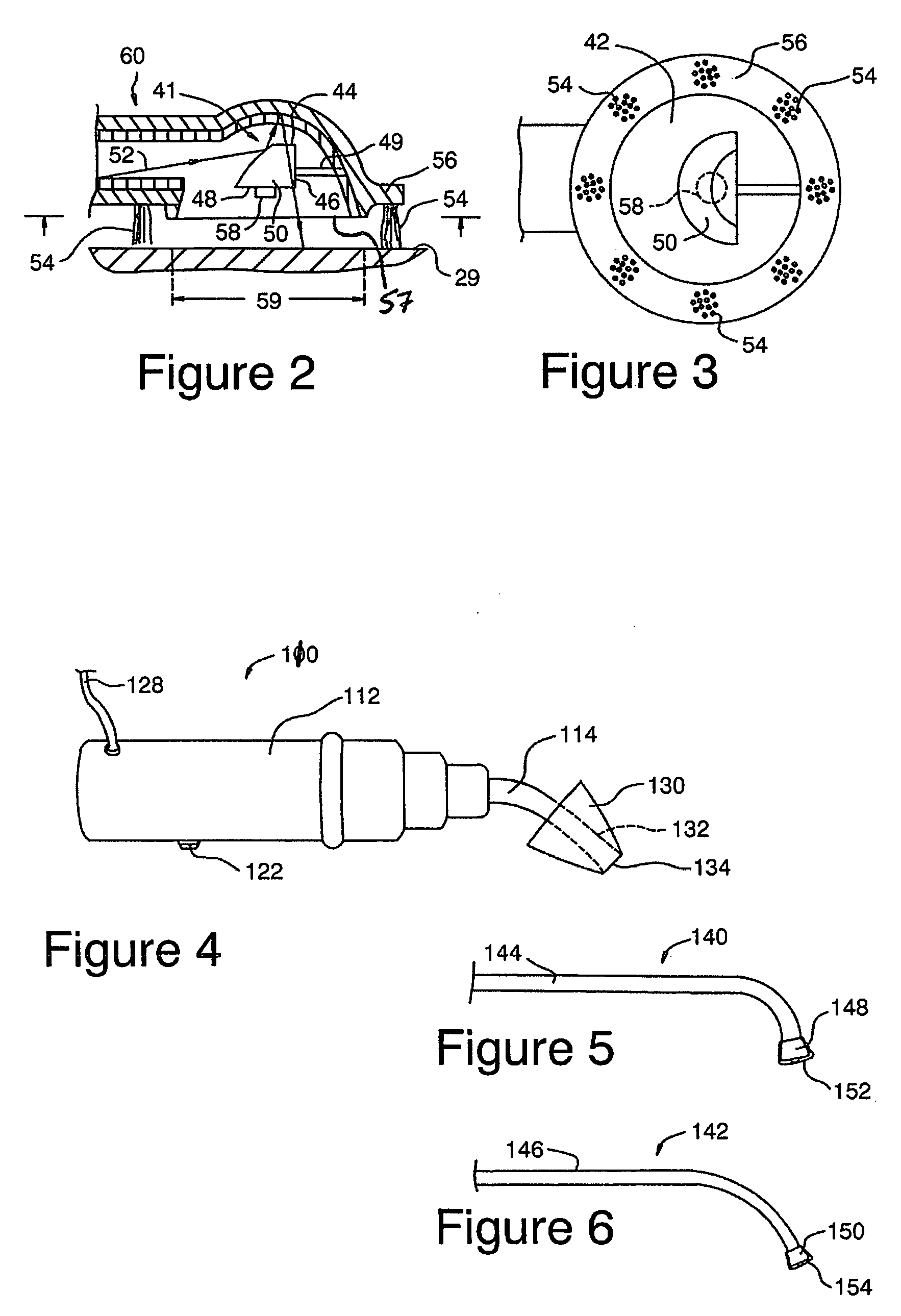
Apparatus and methods for dilating and modifying ostia of paranasal sinuses and other intranasal or paranasal structures
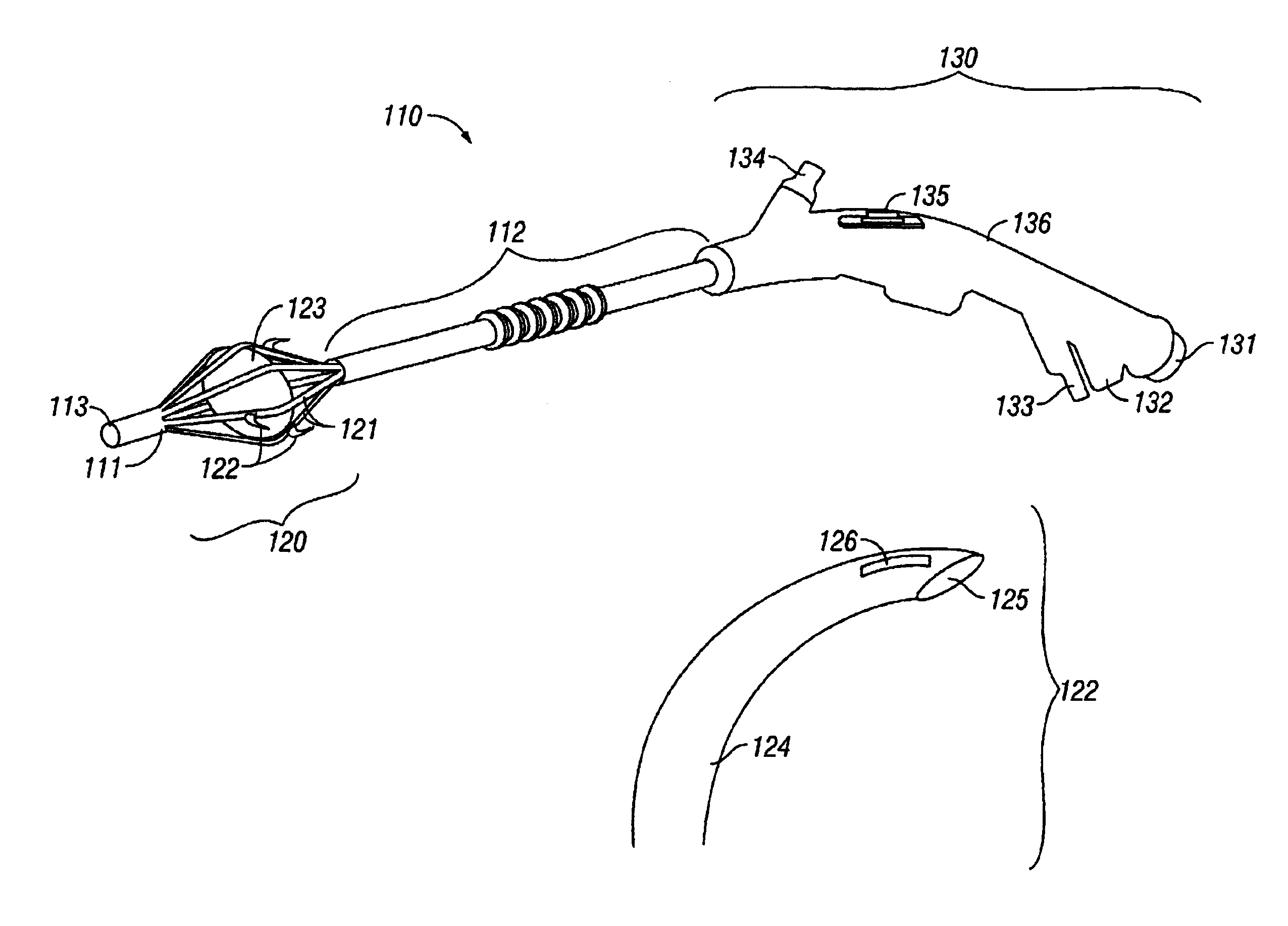
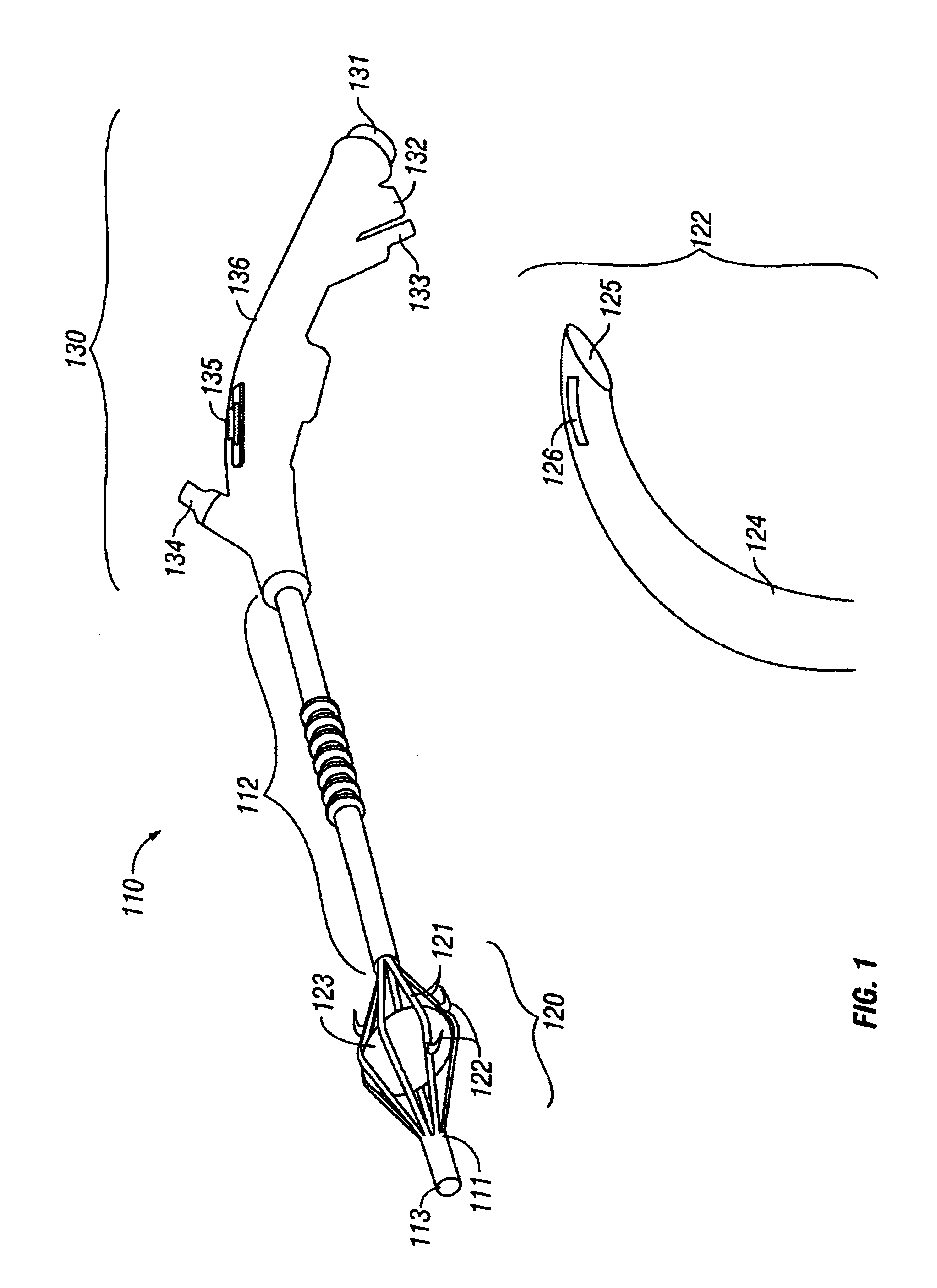
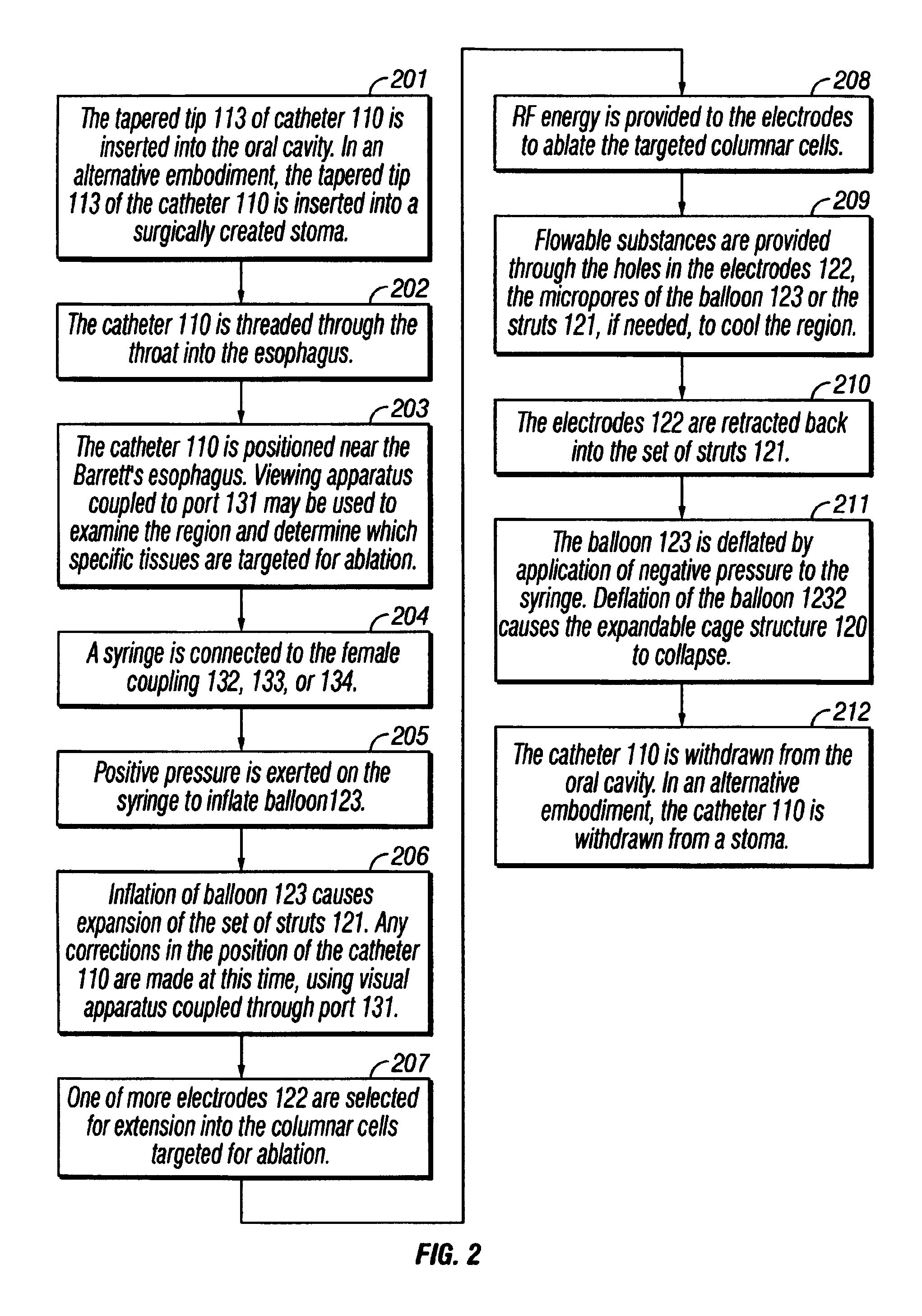
Sinusitis and other disorders of the ear, nose and throat are diagnosed and / or treated using minimally invasive approaches with flexible or rigid instruments. Various methods and devices are used for remodeling or changing the shape, size or configuration of a sinus ostium or duct or other anatomical structure in the ear, nose or throat; implanting a device, cells or tissues; removing matter from the ear, nose or throat; delivering diagnostic or therapeutic substances or performing other diagnostic or therapeutic procedures. Introducing devices (e.g., guide catheters, tubes, guidewires, elongate probes, other elongate members) may be used to facilitate insertion of working devices (e.g. catheters e.g. balloon catheters, guidewires, tissue cutting or remodeling devices, devices for implanting elements like stents, electrosurgical devices, energy emitting devices, devices for delivering diagnostic or therapeutic agents, substance delivery implants, scopes etc.) into the paranasal sinuses or other structures in the ear, nose or throat.
Owner:ACCLARENT INC
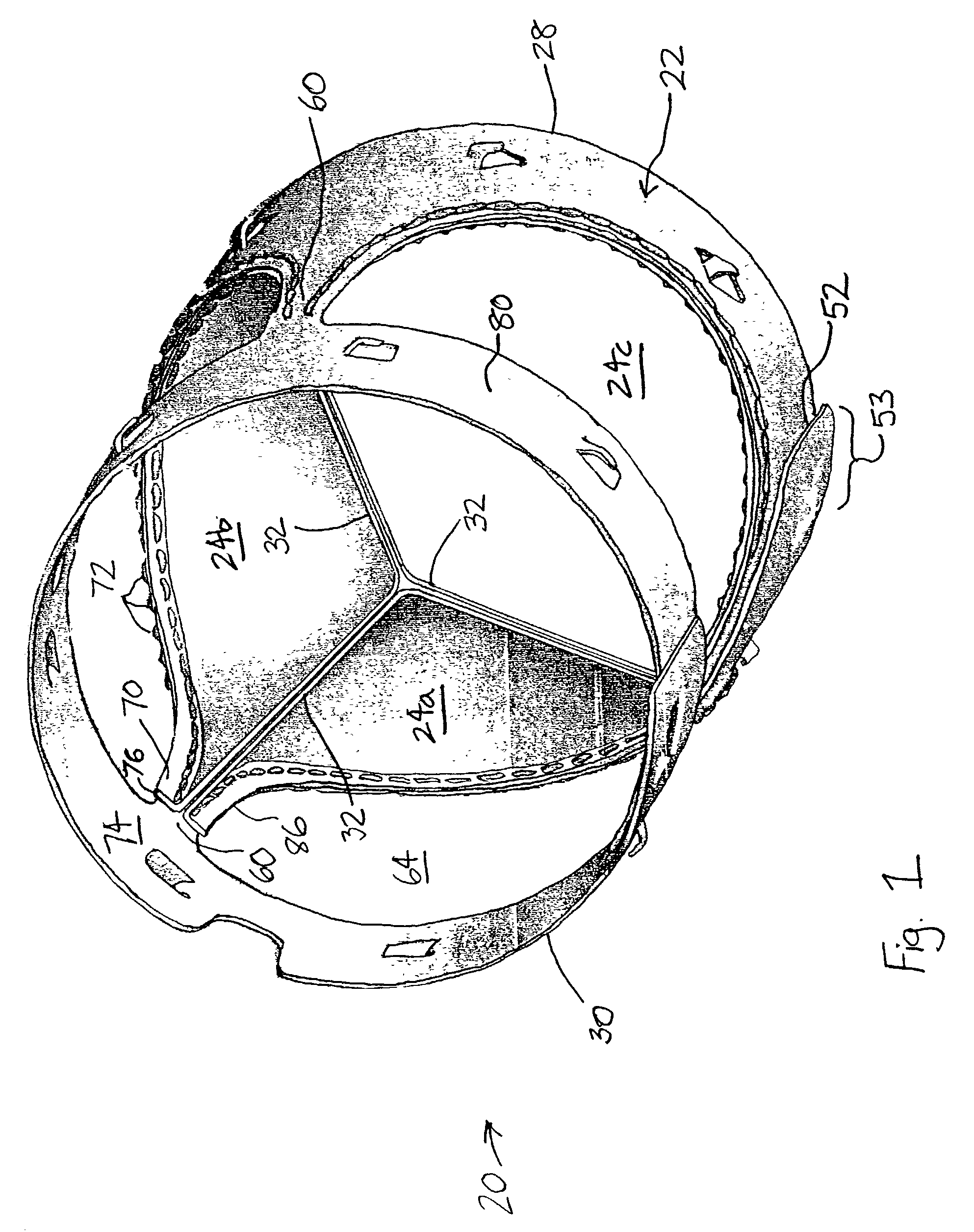
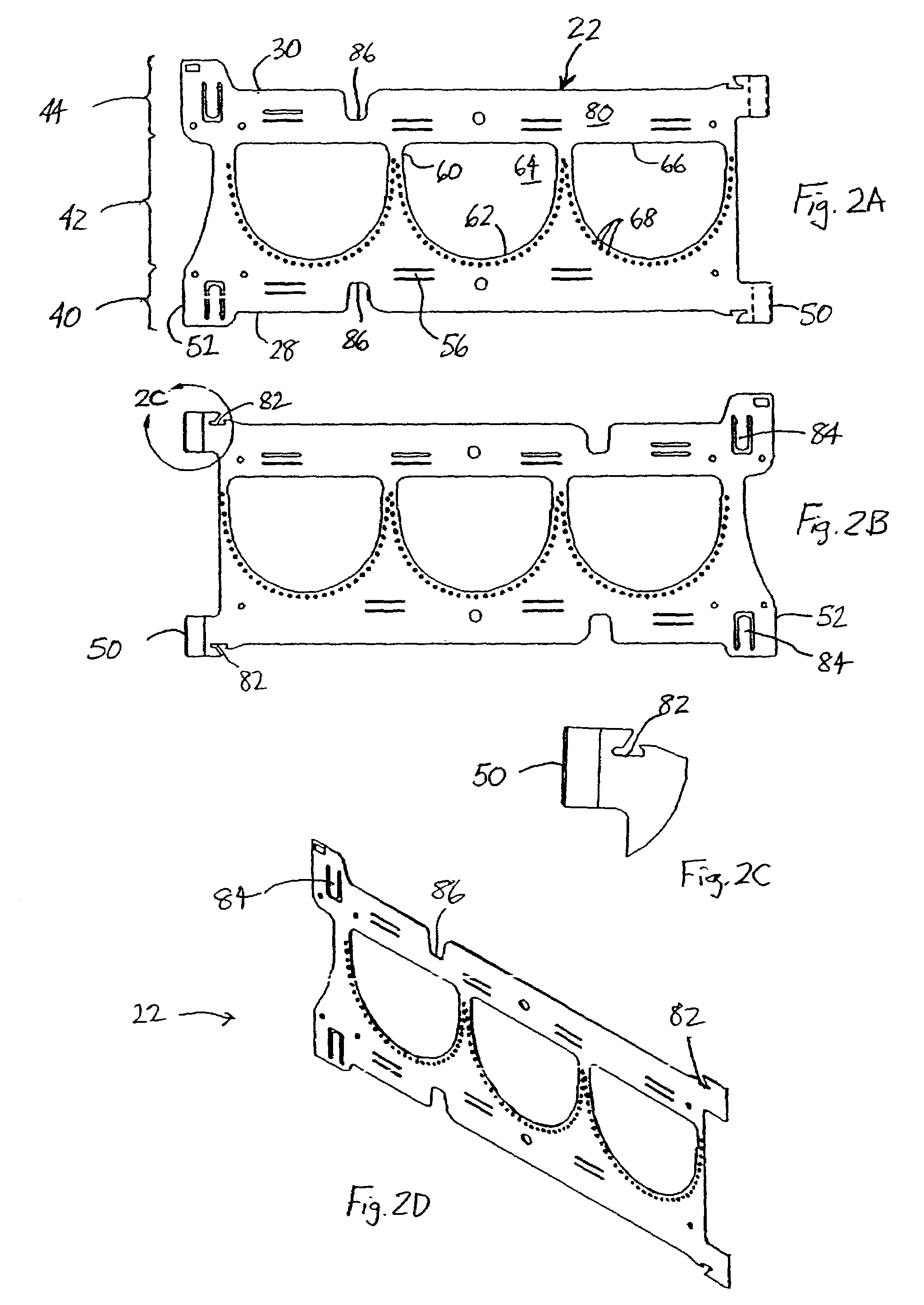
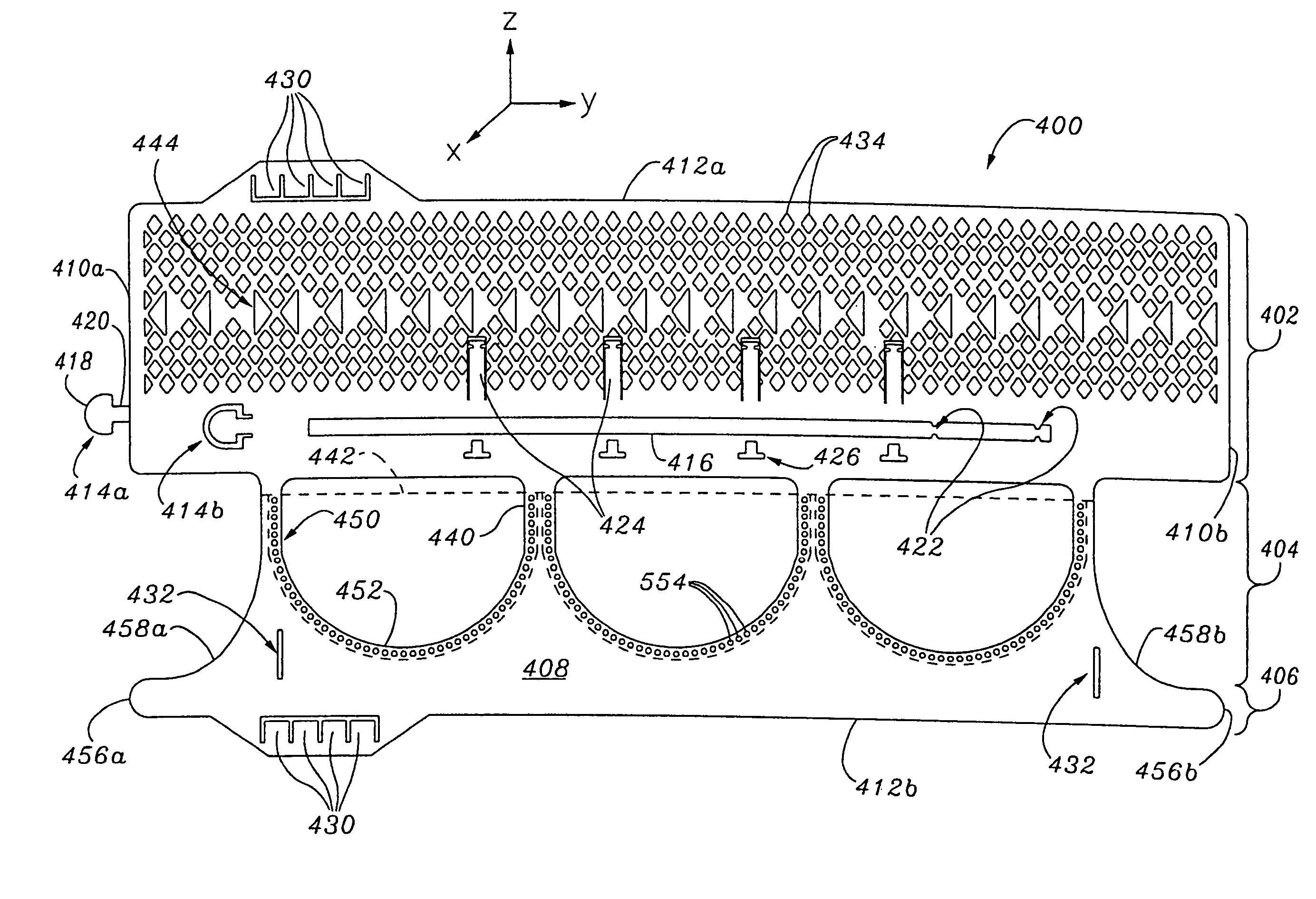
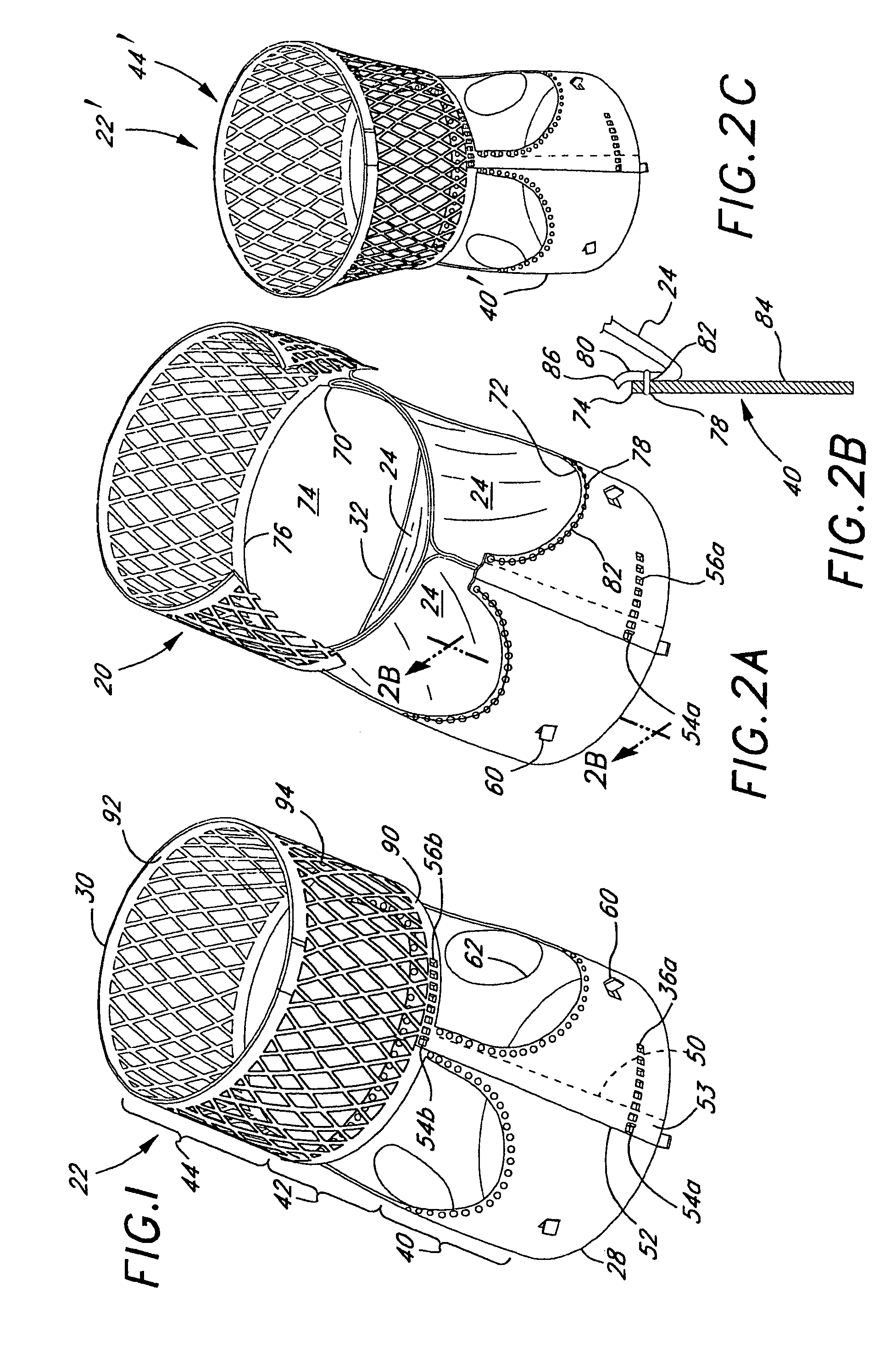
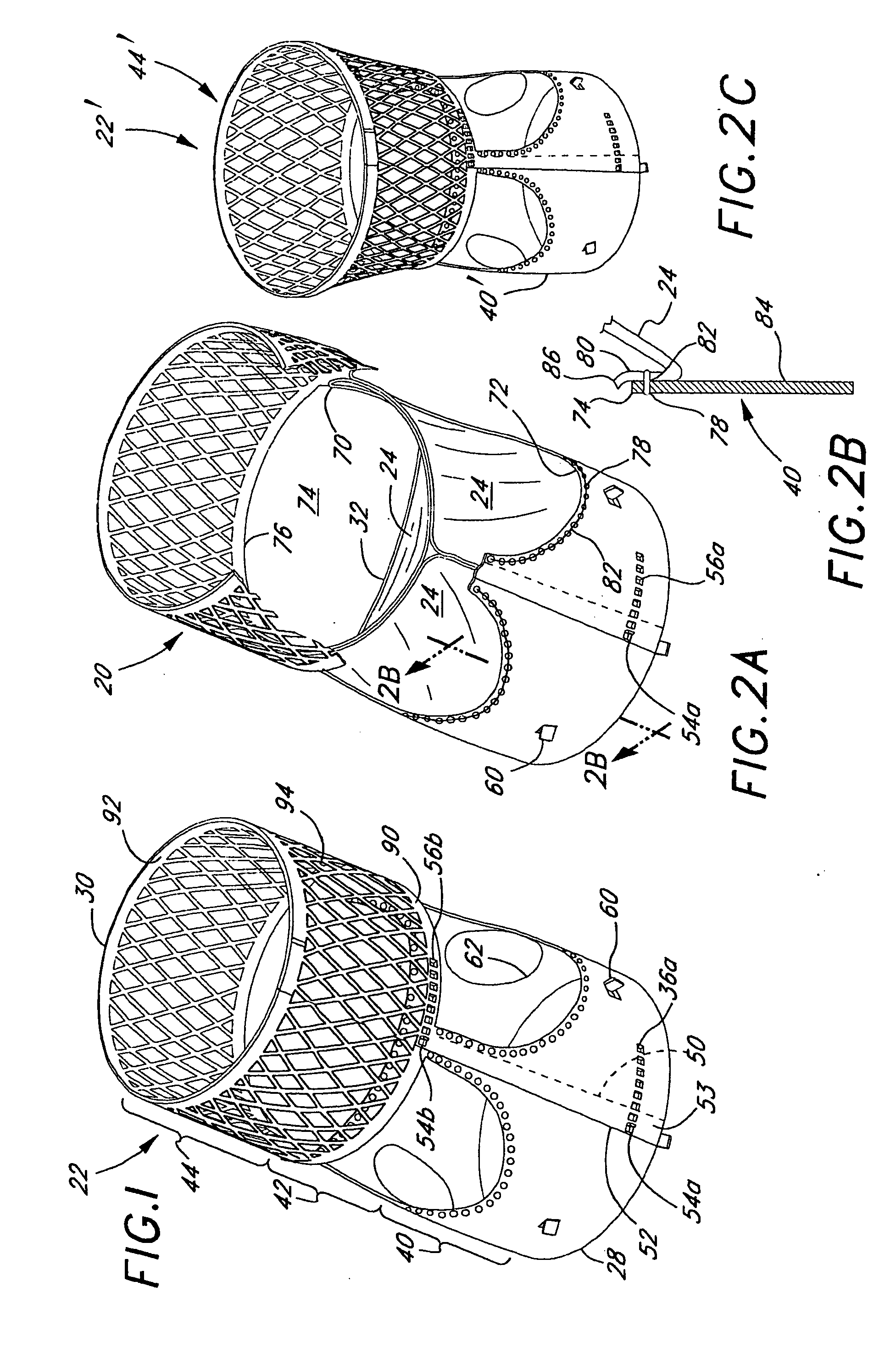
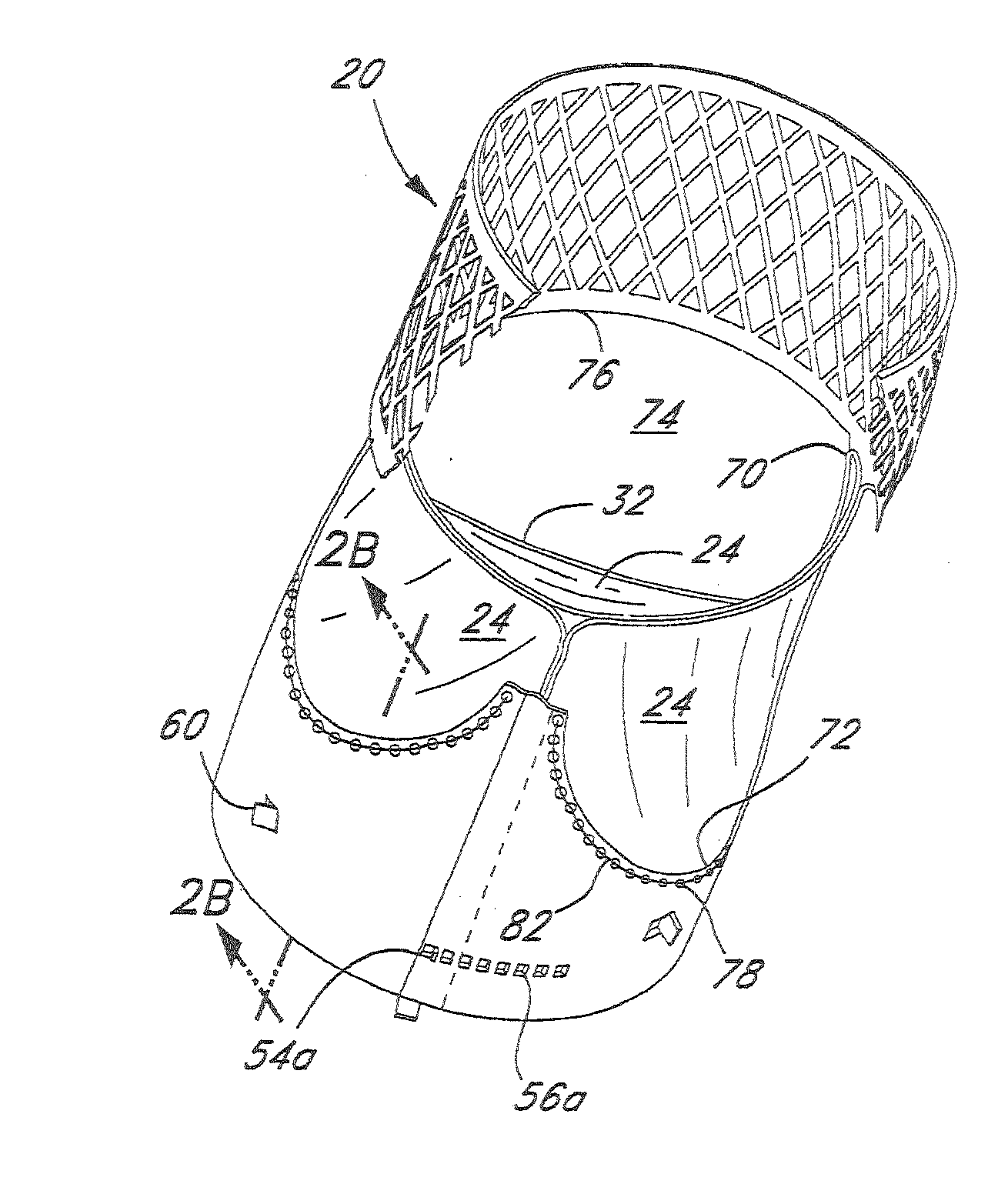
Rolled minimally-invasive heart valves and methods of manufacture
Expandable heart valves for minimally invasive valve replacement surgeries are disclosed. The valves are rolled into a first, contracted configuration for minimally invasive delivery using a catheter, and then unrolled or unfurled at the implantation site. One- and two-piece stents may be used in conjunction with a plurality of flexible leaflet-forming membranes. The stents may include an annulus section, a sinus section with the membranes attached over sinus apertures, and an outflow section. Lockout tabs and making slots secure the stents in their expanded shapes. Alignment structure ensures concentric unfurling of the stent. Anchoring elements at the stent edges or in the stent body secure the valve within the annulus. A method of manufacture includes shape setting the sheet-like stent to ensure an outward bias during deployment. The stent may also include dear tracks for engagement with a gear mechanism for deployment. The stent is desirably made of a superelastic material such as Nitinol and may have areas removed or thinned to reduce the bending stresses when rolled into its small spiral for catheter delivery.
Owner:EDWARDS LIFESCIENCES CORP
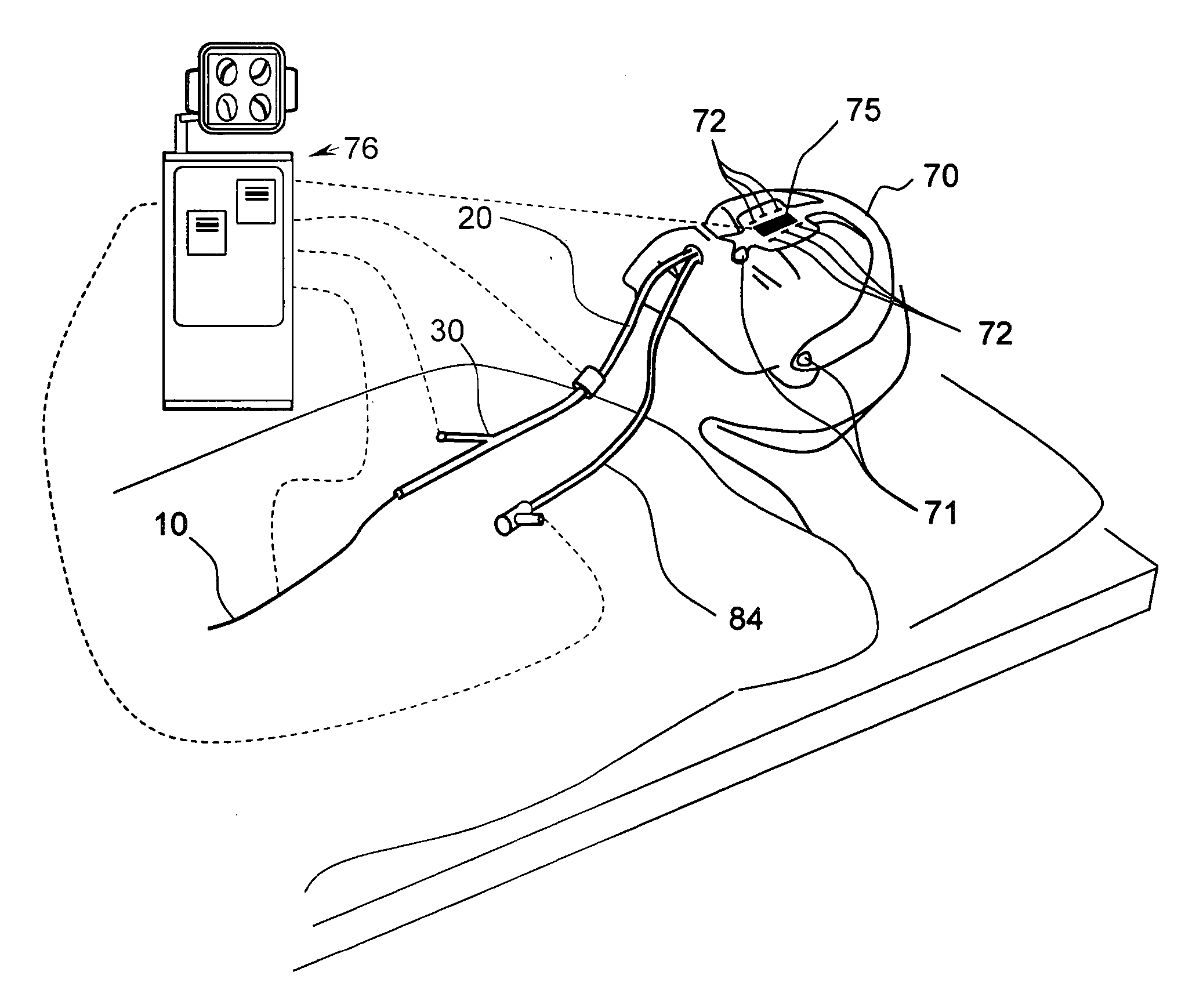
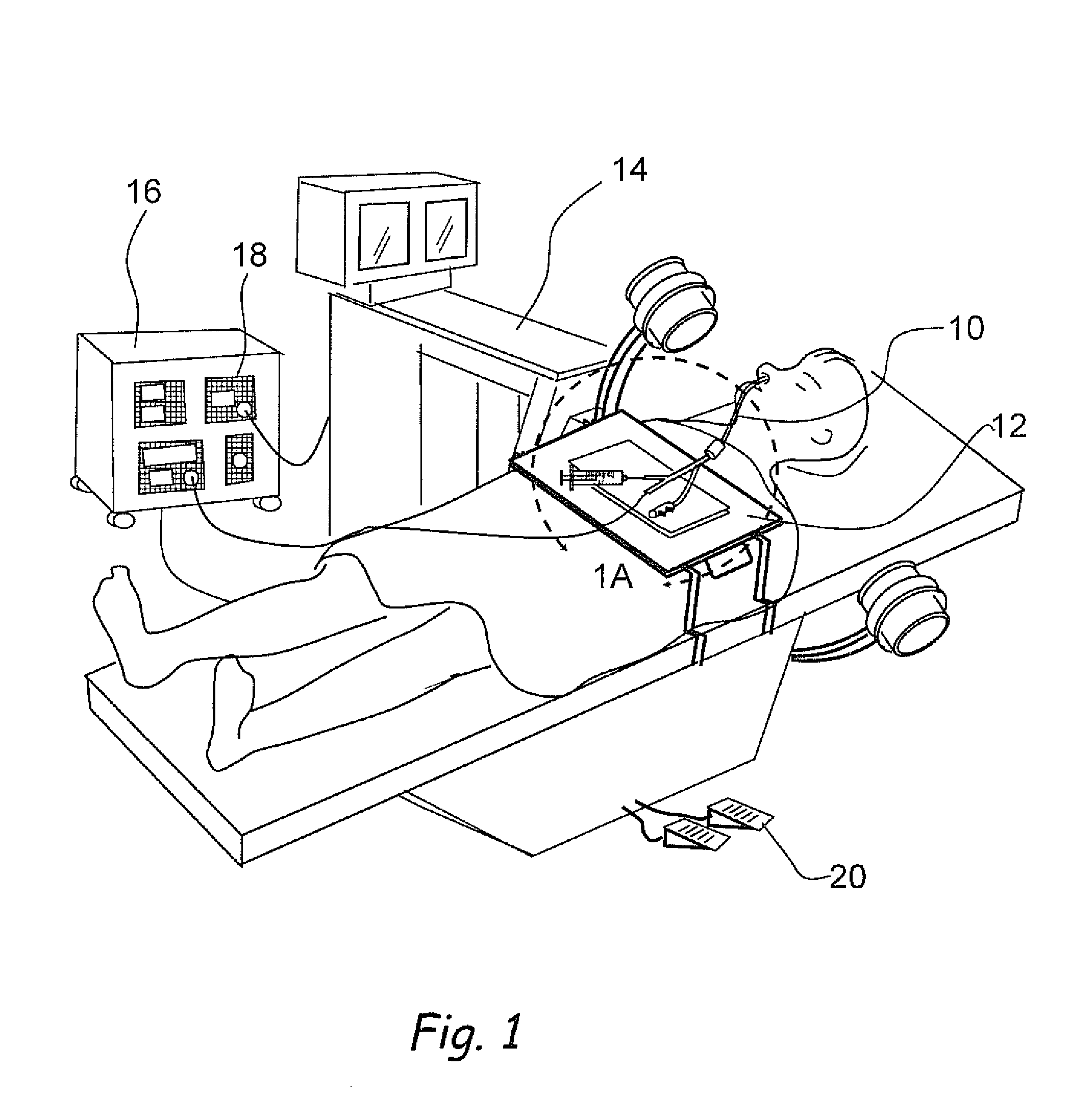
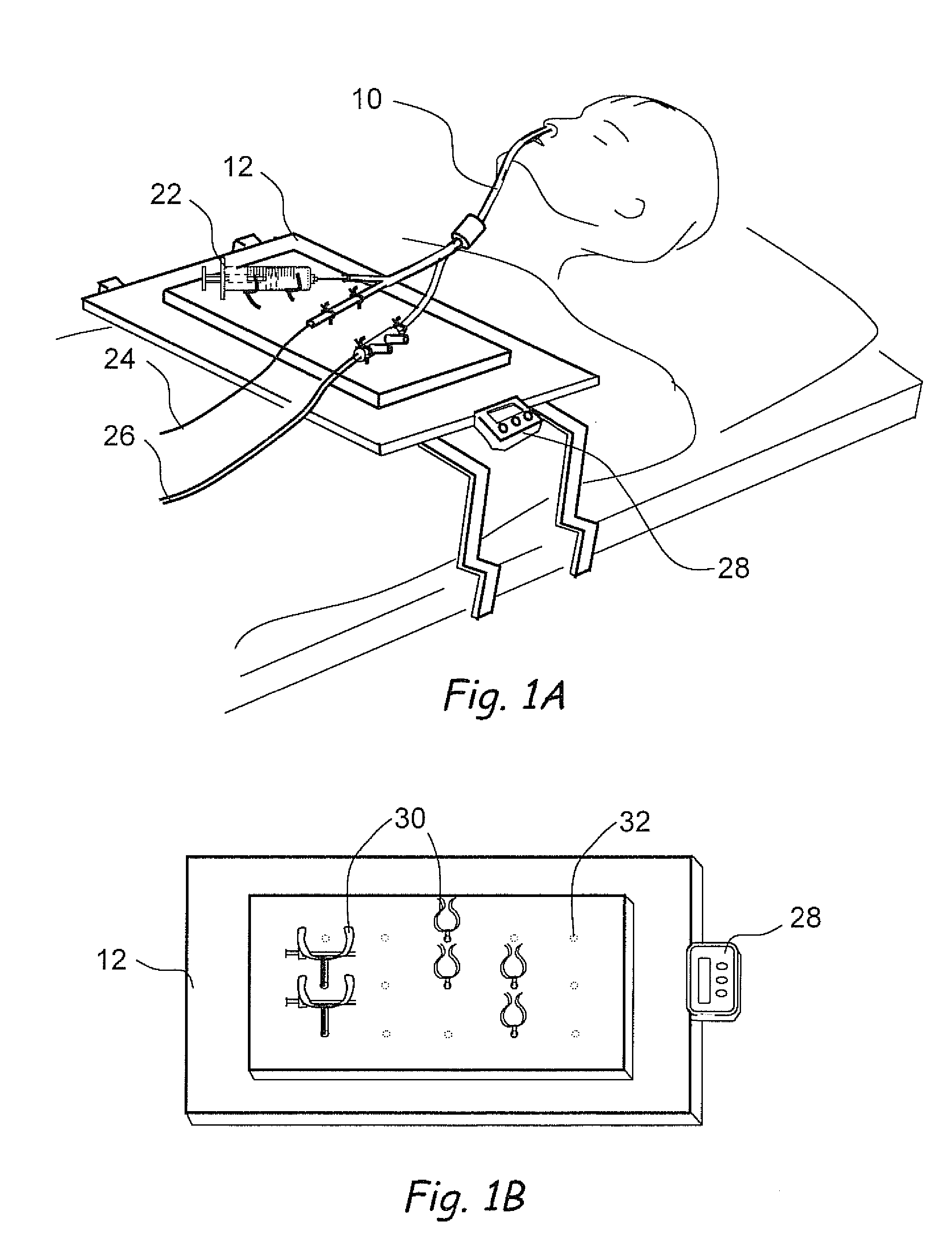
Methods and apparatus for treating disorders of the ear, nose and throat
InactiveUS20060063973A1Improve visualizationEasy accessBronchoscopesLaryngoscopesAnatomical structuresDisease
Methods and apparatus for treating disorders of the ear, nose, throat or paranasal sinuses, including methods and apparatus for dilating ostia, passageways and other anatomical structures, endoscopic methods and apparatus for endoscopic visualization of structures within the ear, nose, throat or paranasal sinuses, navigation devices for use in conjunction with image guidance or navigation system and hand held devices having pistol type grips and other handpieces.
Owner:ACCLARENT INC
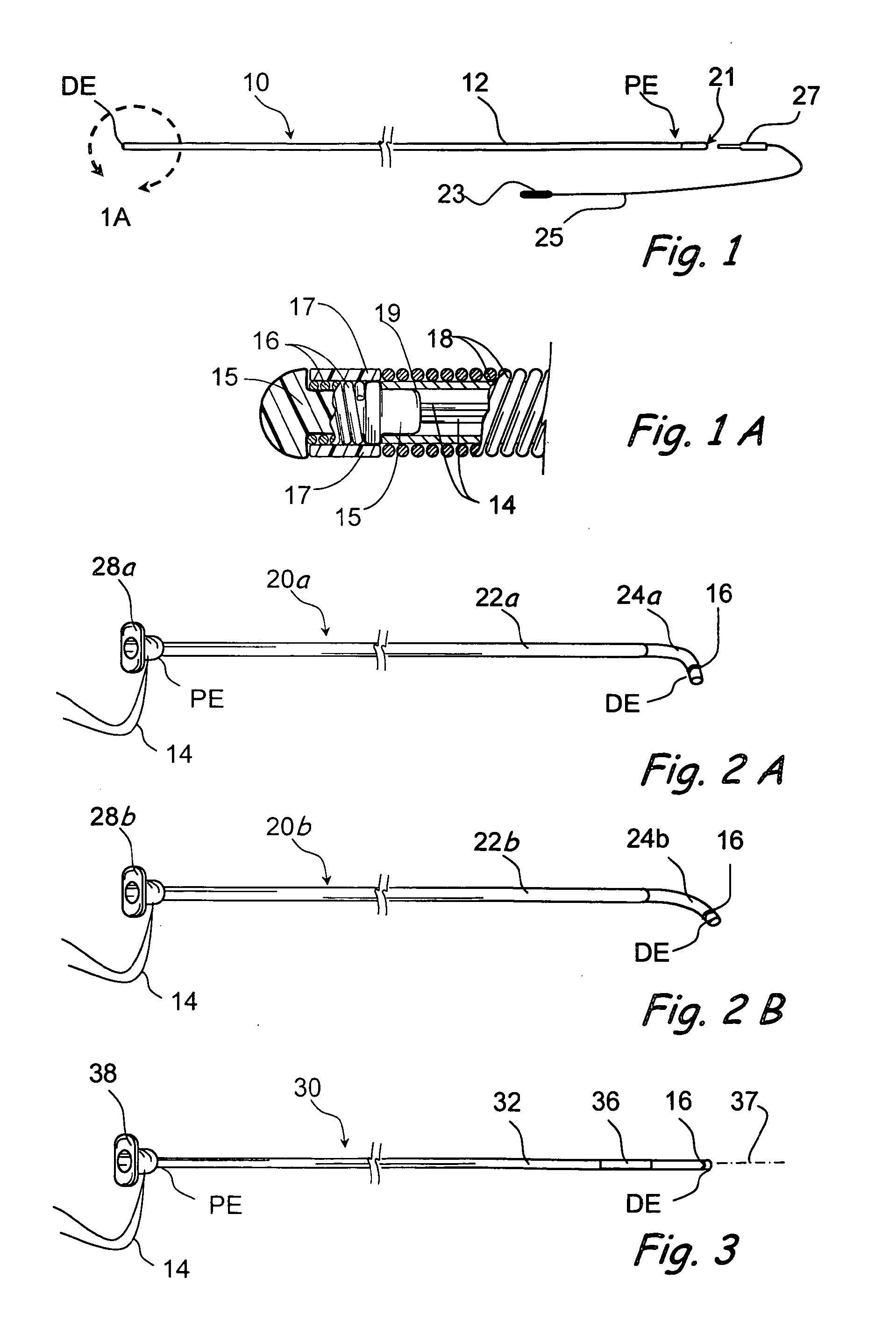
Methods and devices for performing procedures within the ear, nose, throat and paranasal sinuses
Devices, systems and methods for performing image guided interventional and surgical procedures, including various procedures to treat sinusitis and other disorders of the paranasal sinuses, ears, nose or throat.
Owner:ACCLARENT INC
Rolled minimally invasive heart valves
Expandable heart valves for minimally invasive valve replacement surgeries are disclosed. The valves are rolled into a first, contracted configuration for minimally invasive delivery and then unrolled or unfurled at the implantation site. One- and two-piece stents may be used in conjunction with a plurality of flexible leaflet-forming membranes. The one-piece stents may include an annulus anchoring section, a sinus section with the membranes attached over sinus apertures, and a rigid outflow section. The two-piece stent may include a primary stent to provide a tubular base at the annulus, and a secondary stent having the membranes that couples within the primary stent. Lockout tabs to secure the stents in their expanded shapes are provided. Also, alignment structure may be provided to ensure concentric unfurling. Anchoring barbs at the stent edges or in the stent body secure the valve within the annulus. Methods of implantation are also provided.
Owner:EDWARDS LIFESCIENCES CORP
Methods and devices for performing procedures within the ear, nose, throat and paranasal sinuses
Devices, systems and methods for performing image guided interventional and surgical procedures, including various procedures to treat sinusitis and other disorders of the paranasal sinuses, ears, nose or throat.
Owner:ACCLARENT INC
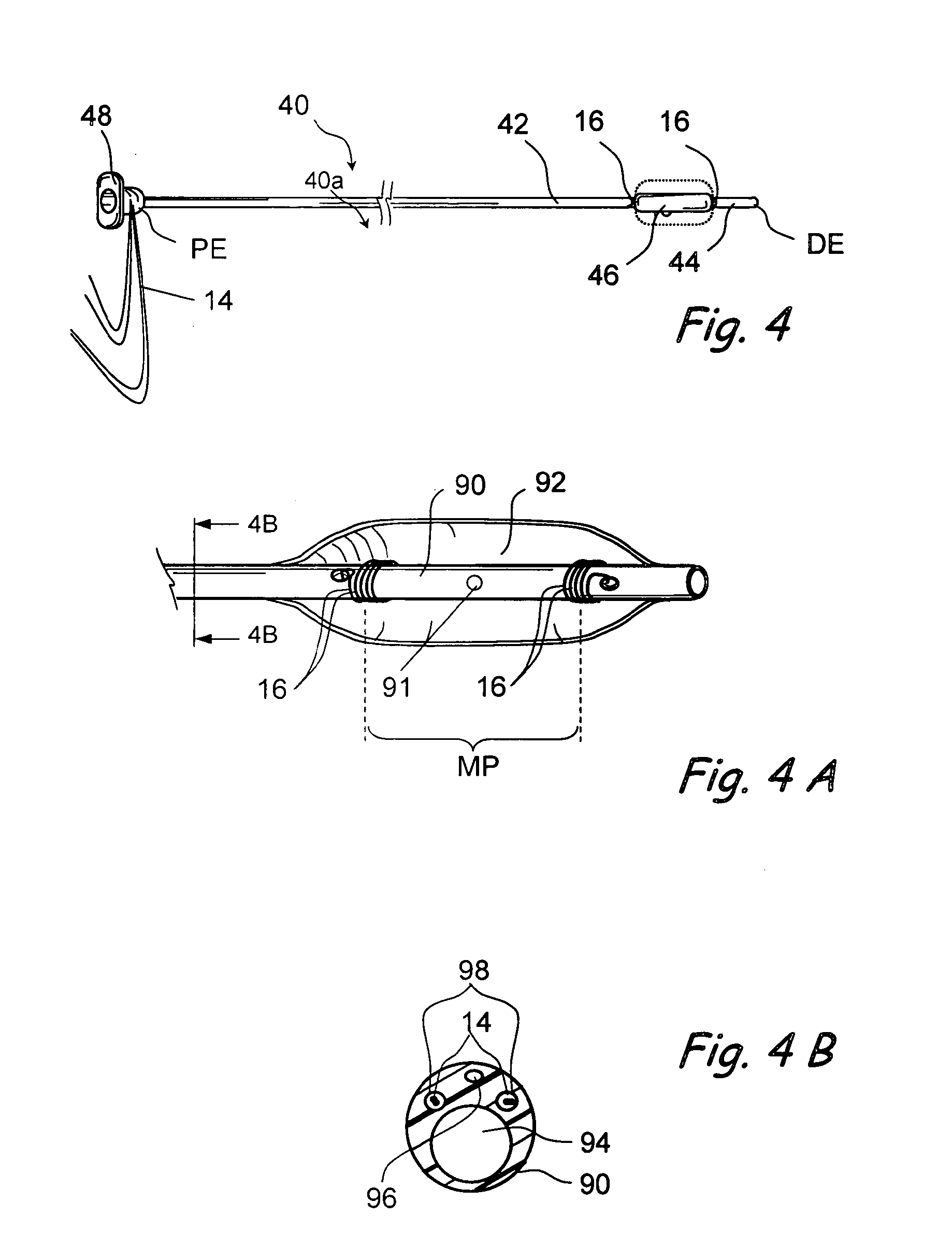
Devices, systems and methods useable for treating sinusitis
Sinusitis and other disorders of the ear, nose and throat are diagnosed and / or treated using minimally invasive approaches with flexible or rigid instruments. Various methods and devices are used for remodeling or changing the shape, size or configuration of a sinus ostium or duct or other anatomical structure in the ear, nose or throat; implanting a device, cells or tissues; removing matter from the ear, nose or throat; delivering diagnostic or therapeutic substances or performing other diagnostic or therapeutic procedures. Introducing devices (e.g., guide catheters, tubes, guidewires, elongate probes, other elongate members) may be used to facilitate insertion of working devices (e.g. catheters e.g. balloon catheters, guidewires, tissue cutting or remodeling devices, devices for implanting elements like stents, electrosurgical devices, energy emitting devices, devices for delivering diagnostic or therapeutic agents, substance delivery implants, scopes etc.) into the paranasal sinuses or other structures in the ear, nose or throat. Specific devices (e.g., tubular guides, guidewires, balloon catheters, tubular sheaths) are provided as are methods for manufacturing and using such devices to treat disorders of the ear, nose or throat.
Owner:ACCLARENT INC
Treatment of tissue in sphincters, sinuses and orifices
Owner:NOVASYS MEDICAL
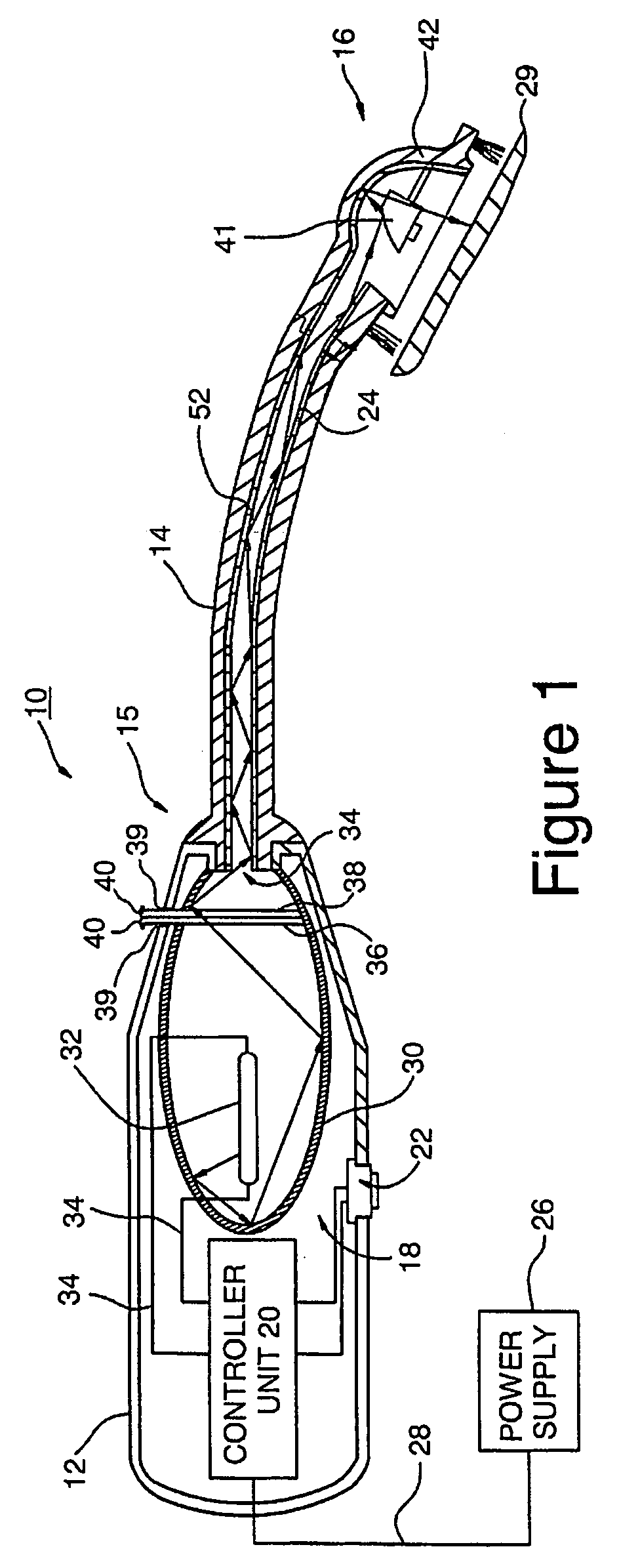
Electrical and magnetic stimulators used to treat migraine/sinus headache and comorbid disorders
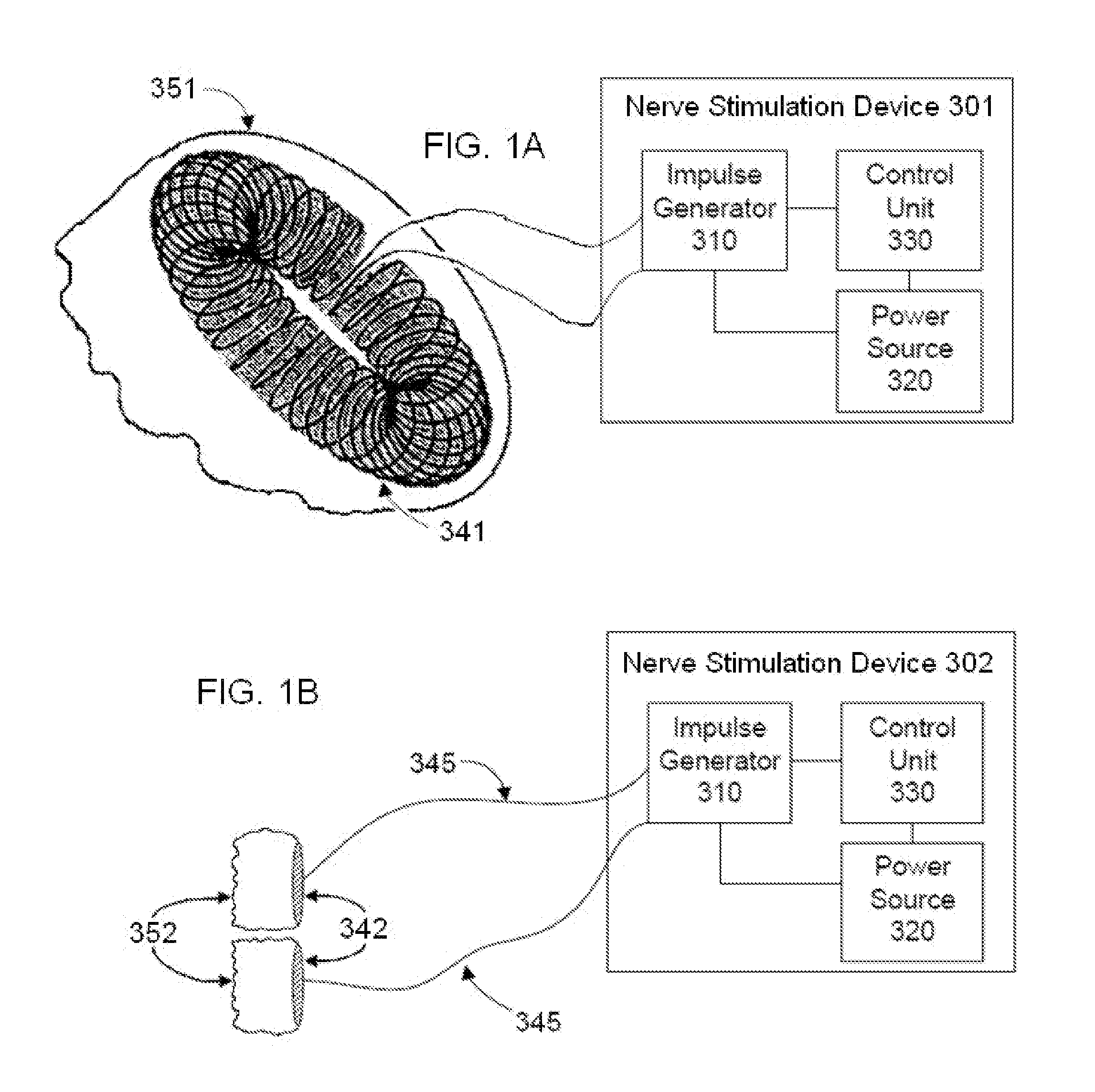
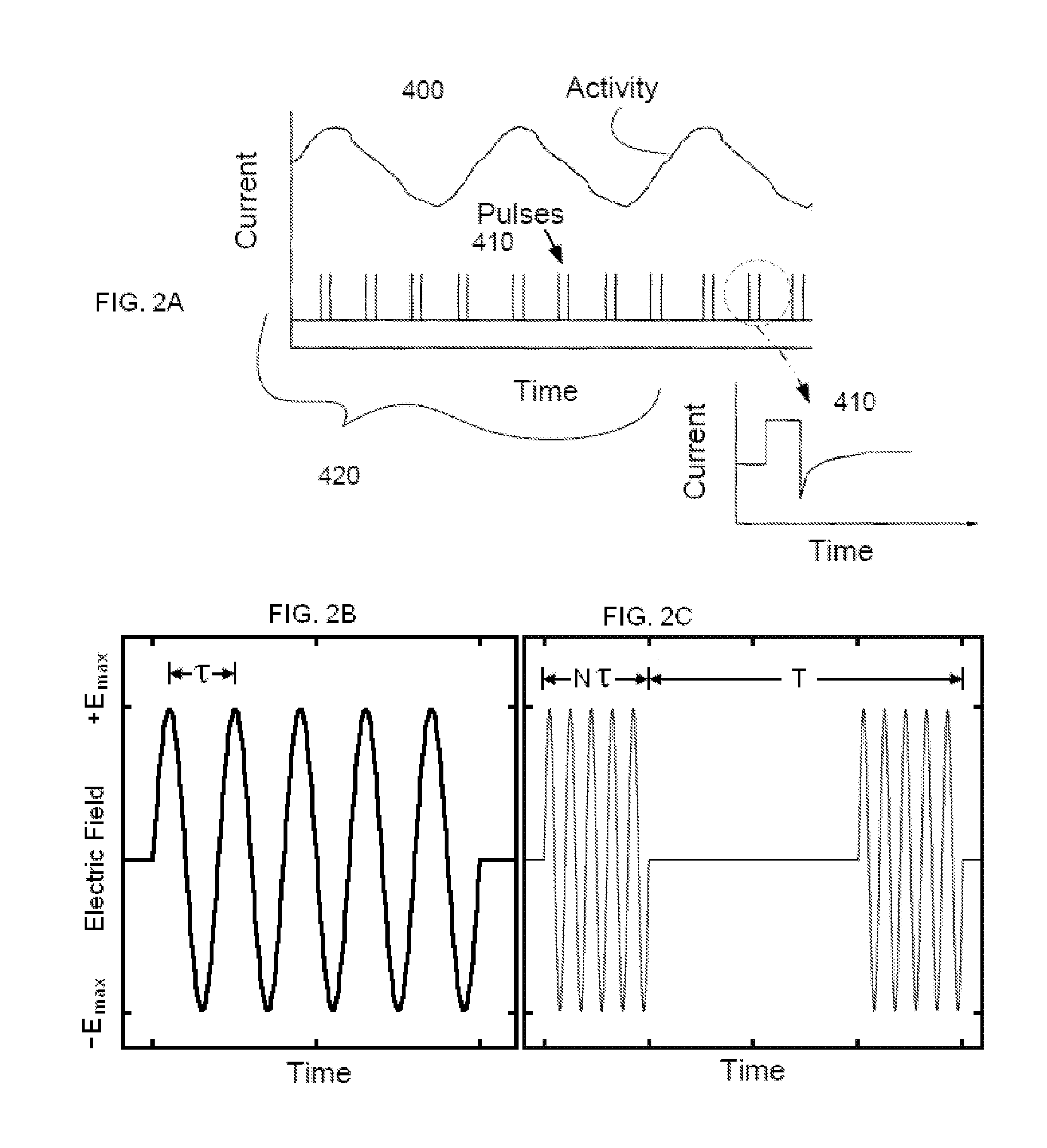
Non-invasive electrical nerve stimulation devices and magnetic stimulation devices are disclosed, along with methods of treating medical disorders using energy that is delivered noninvasively by such devices. The disorders comprise migraine and other primary headaches such as cluster headaches, including sinus symptoms that resemble an immune-mediated response (“sinus” headaches), irrespective of whether those symptoms arise from an allergy that is co-morbid with the headache. The disclosed methods may also be used to treat other disorders that may be co-morbid with migraine headaches, such as anxiety disorders. In preferred embodiments of the disclosed methods, one or both of the patient's vagus nerves are stimulated non-invasively. In other embodiments, parts of the sympathetic nervous system and / or the adrenal glands are stimulated.
Owner:ELECTROCORE
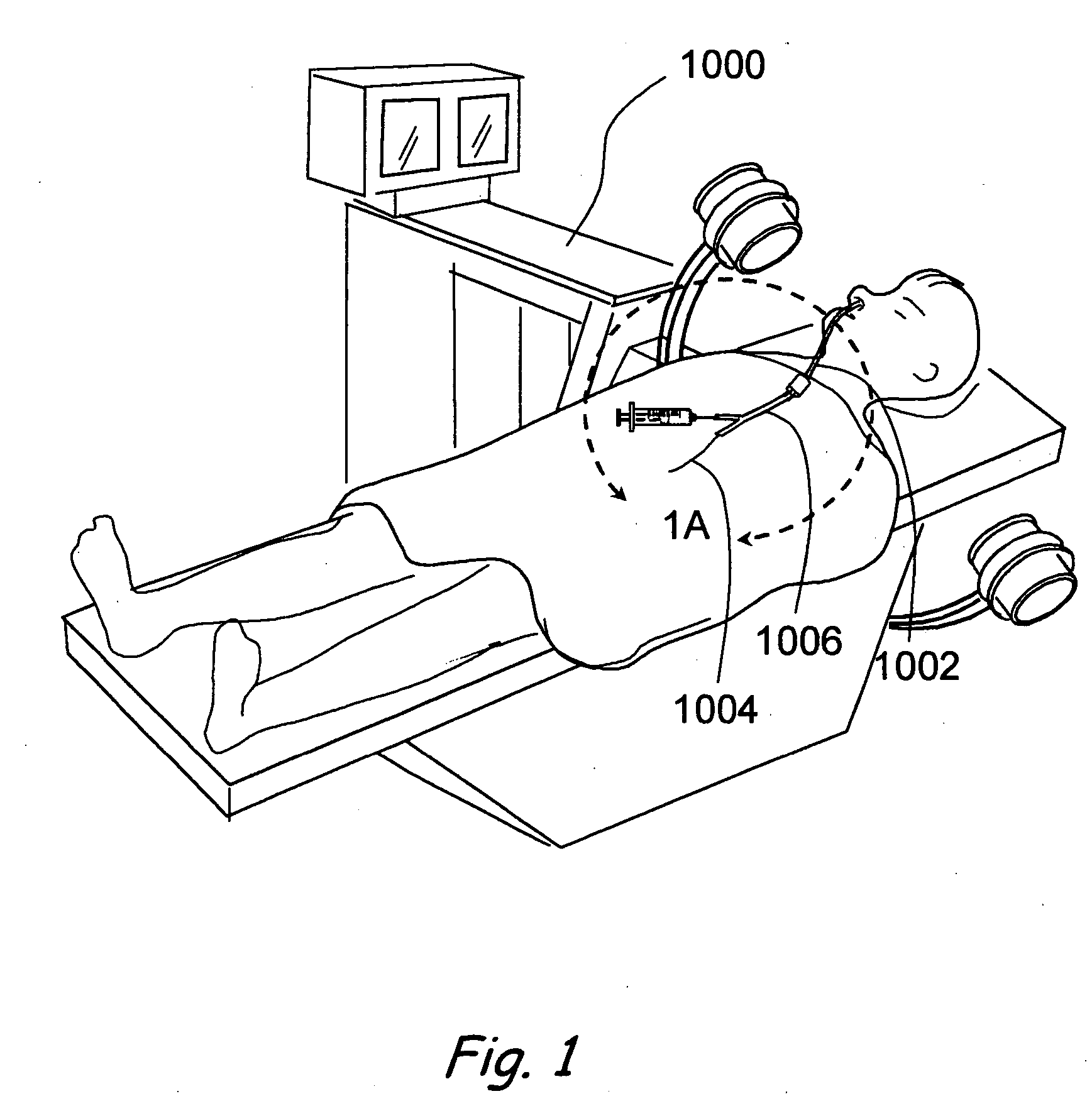
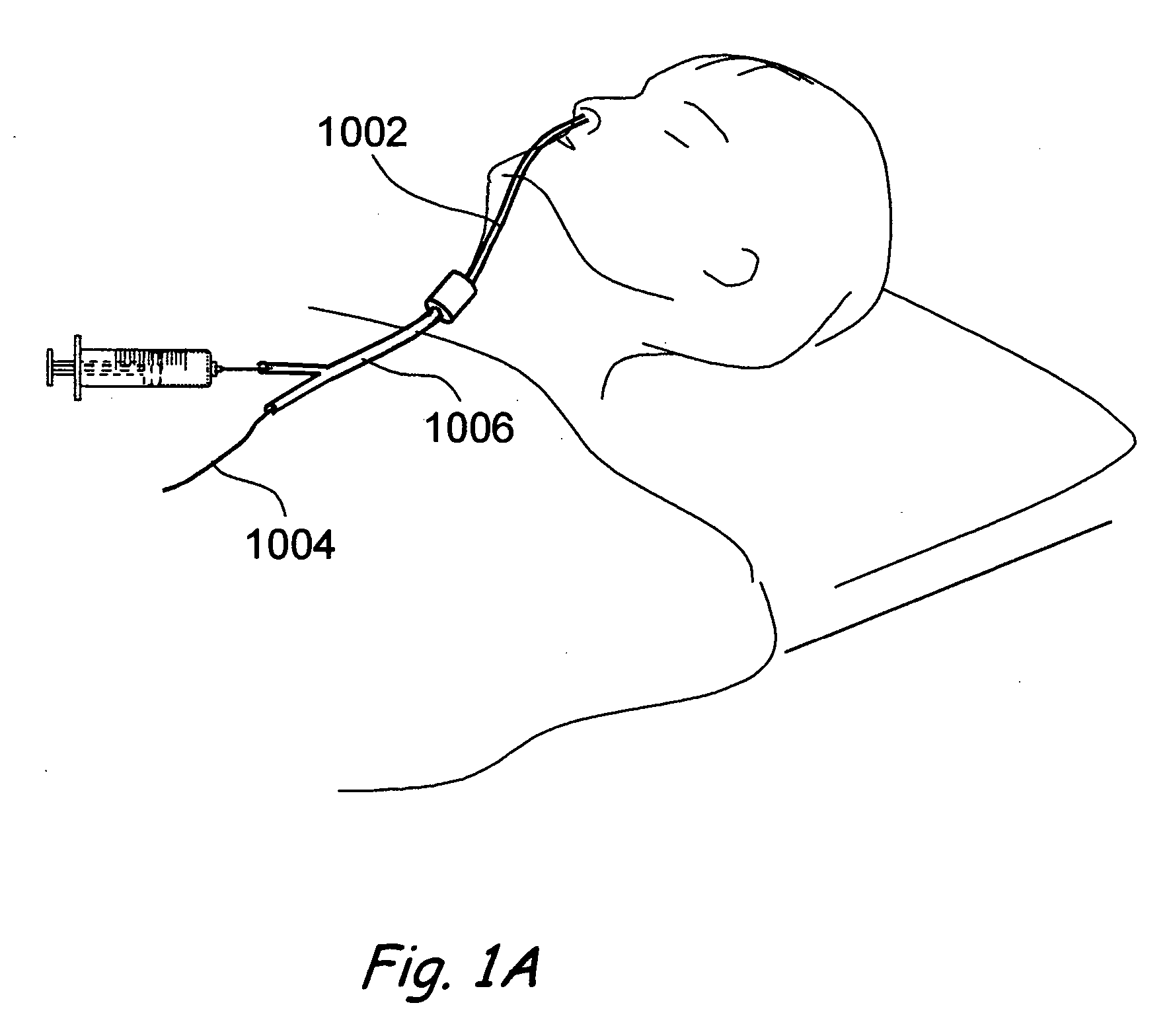
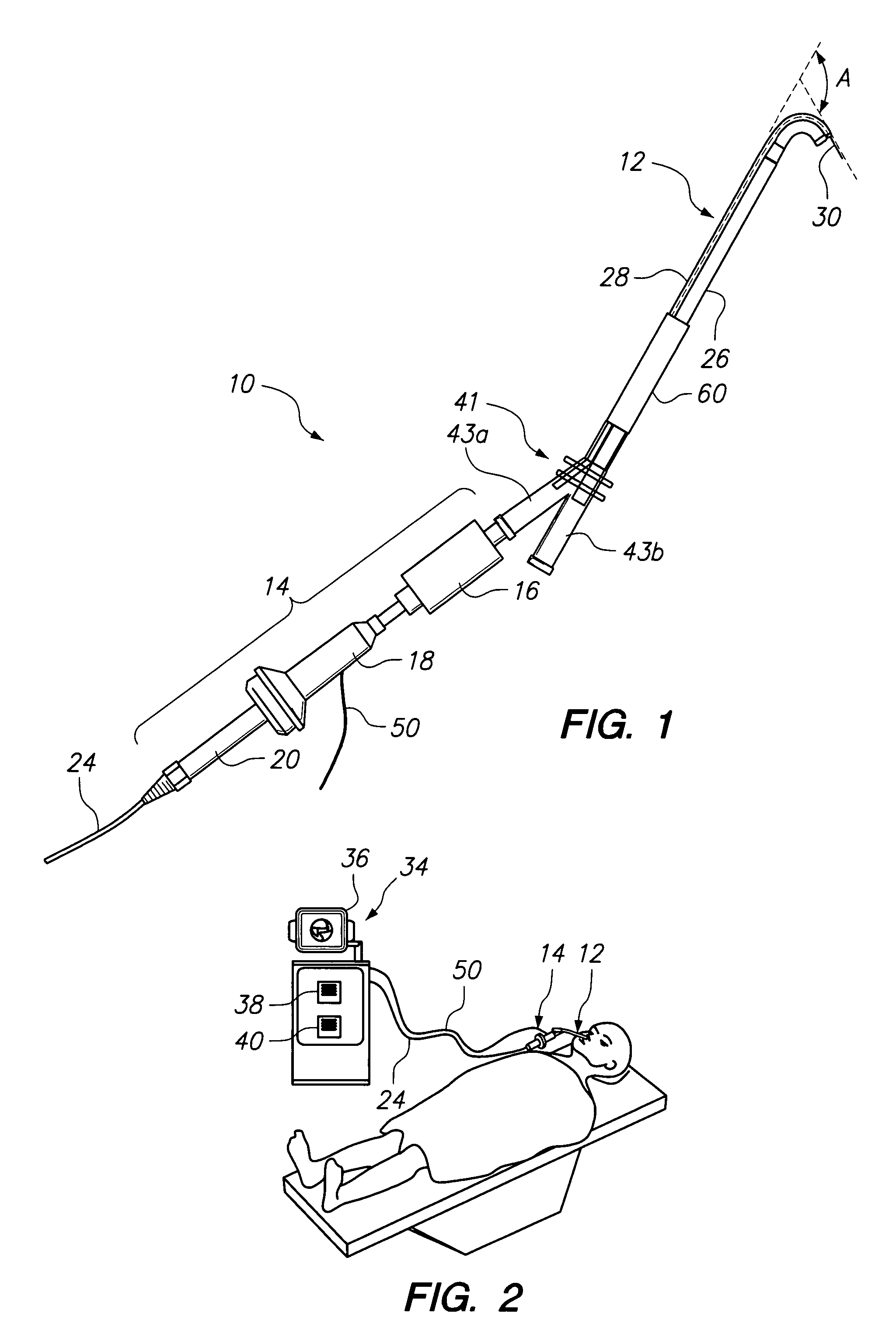
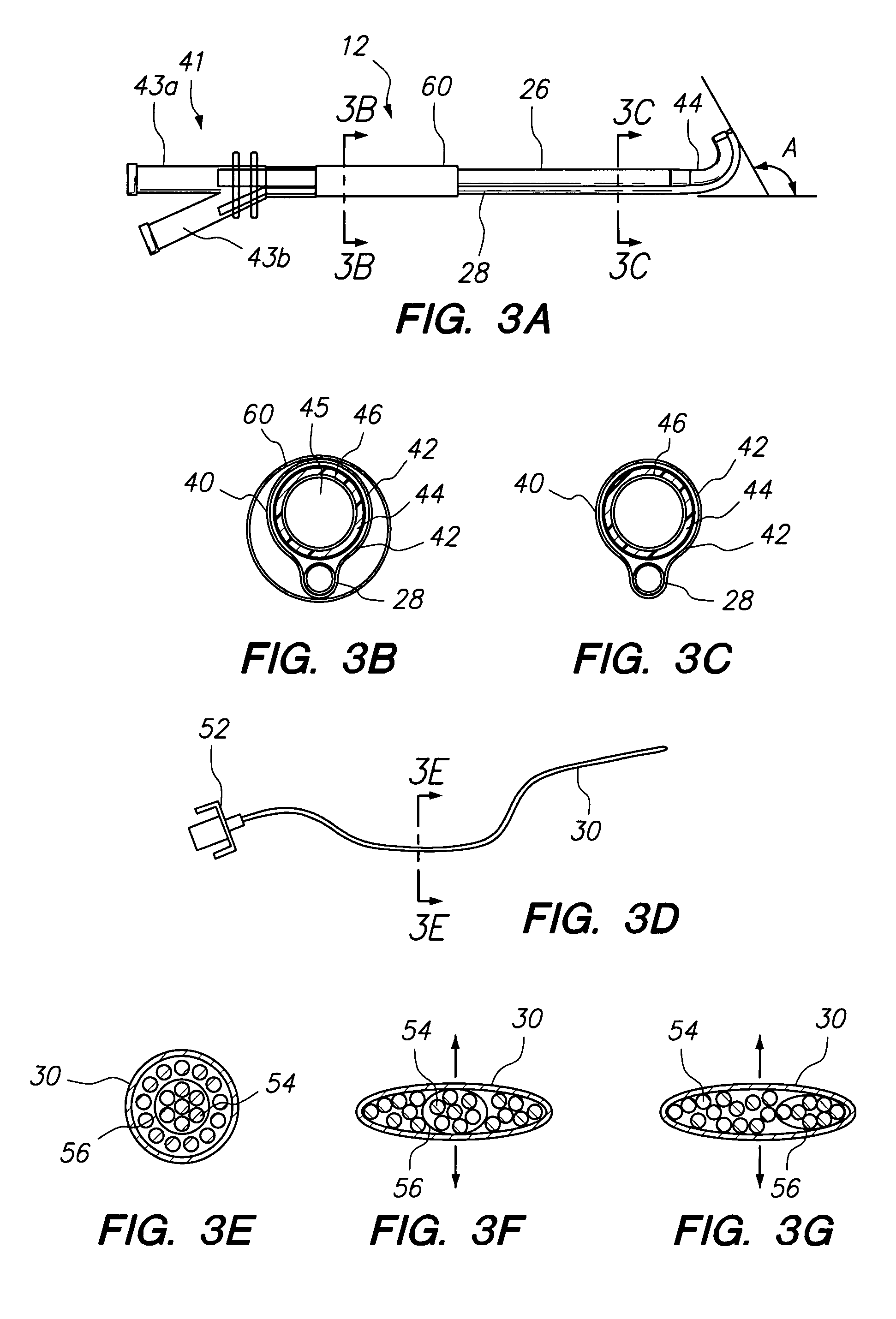
Methods and Apparatus for Treating Disorders of the Ear Nose and Throat
ActiveUS20080097154A1Facilitate precise handling positioningUnwanted movementBronchoscopesLaryngoscopesDiseaseAnatomical structures
Methods and apparatus for treating disorders of the ear, nose, throat or paranasal sinuses, including methods and apparatus for dilating ostia, passageways and other anatomical structures, endoscopic methods and apparatus for endoscopic visualization of structures within the ear, nose, throat or paranasal sinuses, navigation devices for use in conjunction with image guidance or navigation system and hand held devices having pistol type grips and other handpieces.
Owner:ACCLARENT INC
Methods and Apparatus for Treating Disorders of the Ear Nose and Throat
ActiveUS20080103521A1Facilitate precise handling positioningUnwanted movementBronchoscopesLaryngoscopesDiseaseAnatomical structures
Methods and apparatus for treating disorders of the ear, nose, throat or paranasal sinuses, including methods and apparatus for dilating ostia, passageways and other anatomical structures, endoscopic methods and apparatus for endoscopic visualization of structures within the ear, nose, throat or paranasal sinuses, navigation devices for use in conjunction with image guidance or navigation system and hand held devices having pistol type grips and other handpieces.
Owner:ACCLARENT INC
Systems and methods for performing image guided procedures within the ear, nose, throat and paranasal sinuses
Devices, systems and methods for performing image guided interventional and surgical procedures, including various procedures to treat sinusitis and other disorders of the paranasal sinuses, ears, nose or throat.
Owner:ACCLARENT INC
Use of mechanical dilator devices to enlarge ostia of paranasal sinuses and other passages in the ear, nose, throat and paranasal sinuses
Sinusitis and other disorders of the ear, nose and throat are diagnosed and / or treated using minimally invasive approaches with flexible or rigid instruments. Various methods and devices are used for remodeling or changing the shape, size or configuration of a sinus ostium or duct or other anatomical structure in the ear, nose or throat; implanting a device, cells or tissues; removing matter from the ear, nose or throat; delivering diagnostic or therapeutic substances or performing other diagnostic or therapeutic procedures. Introducing devices (e.g., guide catheters, tubes, guidewires, elongate probes, other elongate members) may be used to facilitate insertion of working devices (e.g. catheters e.g. balloon catheters, guidewires, tissue cutting or remodeling devices, devices for implanting elements like stents, electrosurgical devices, energy emitting devices, devices for delivering diagnostic or therapeutic agents, substance delivery implants, scopes etc.) into the paranasal sinuses or other structures in the ear, nose or throat. Specific devices (e.g., tubular guides, guidewires, balloon catheters, tubular sheaths) are provided as are methods for manufacturing and using such devices to treat disorders of the ear, nose or throat.
Owner:ACCLARENT INC
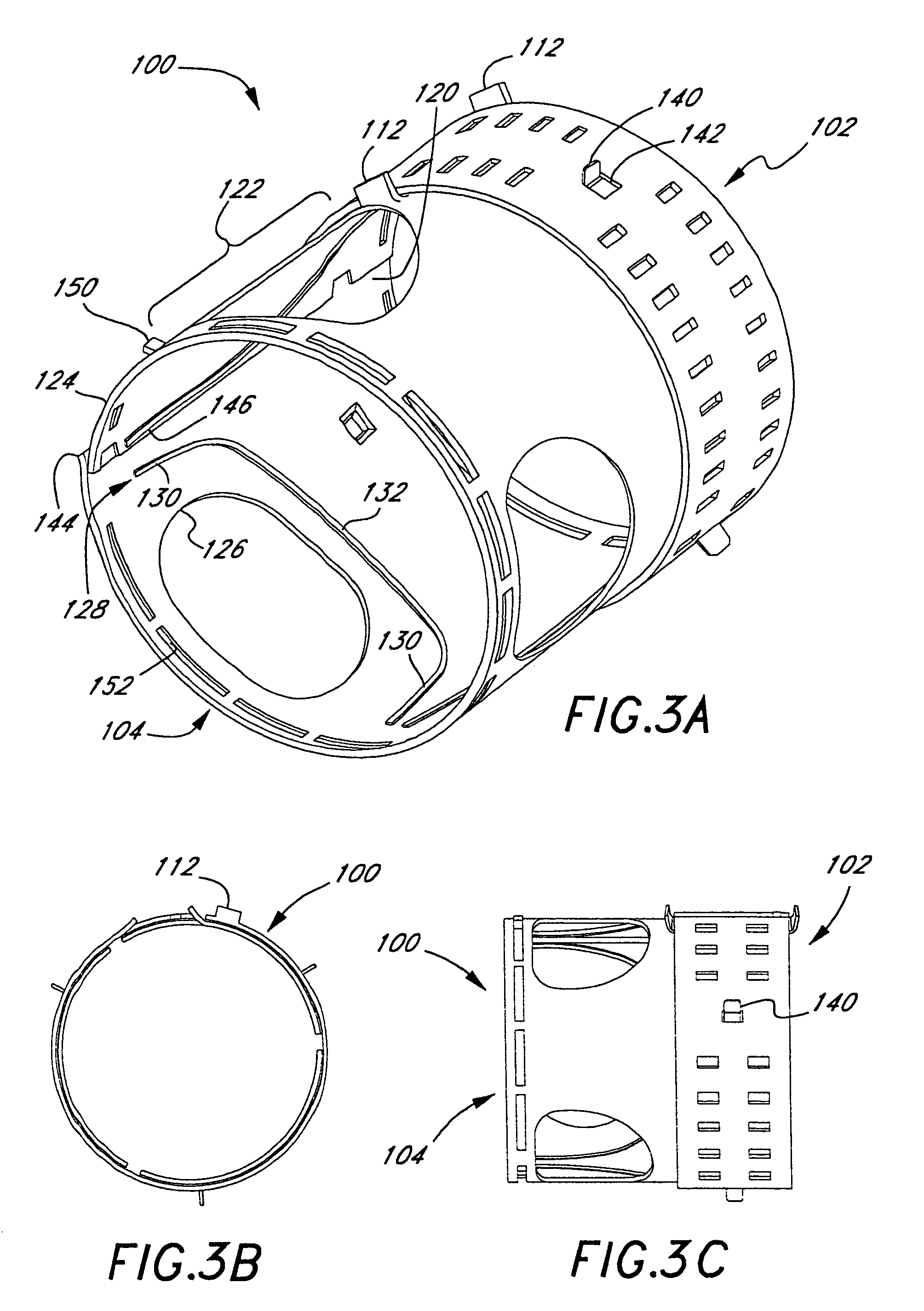
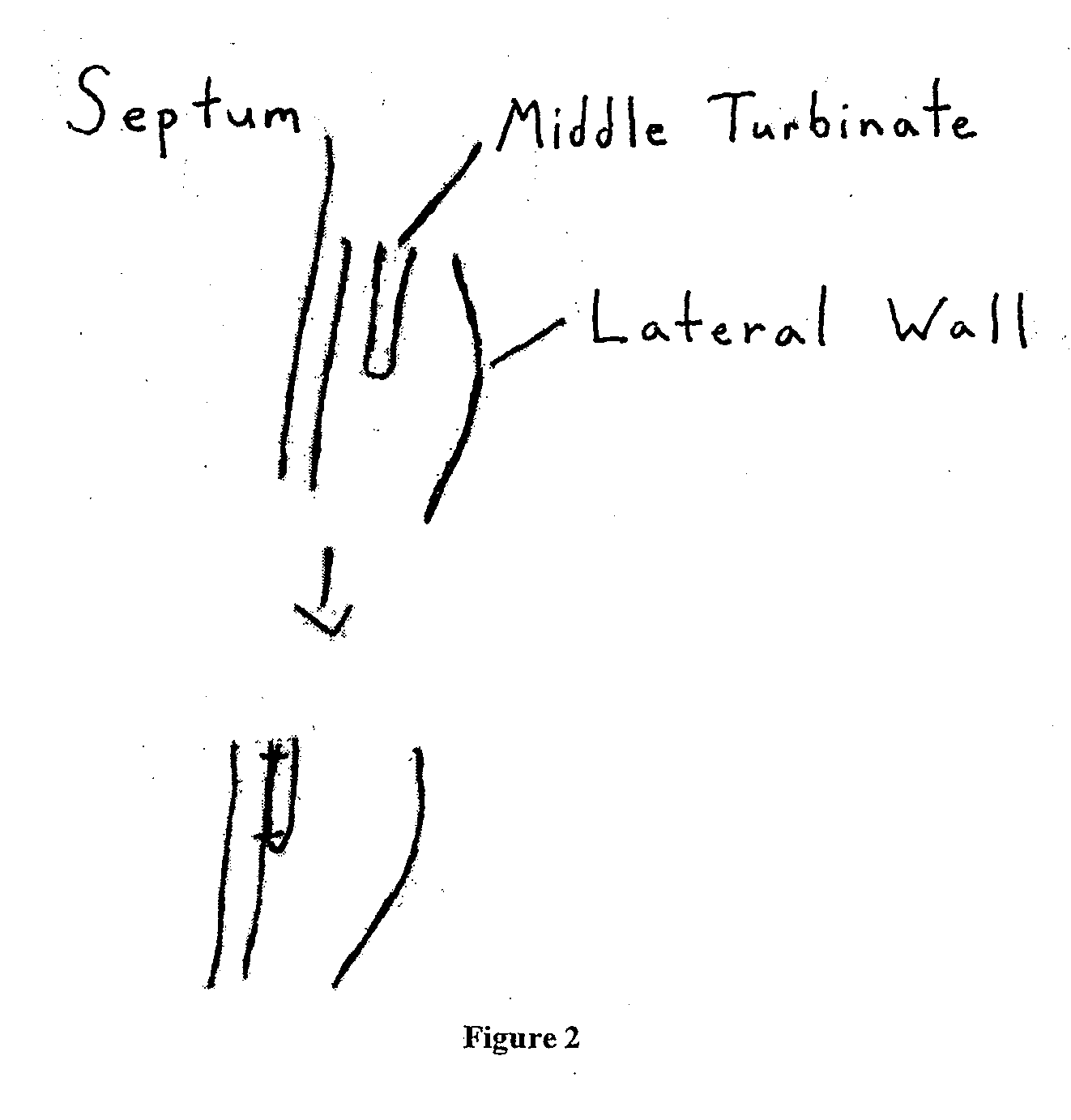
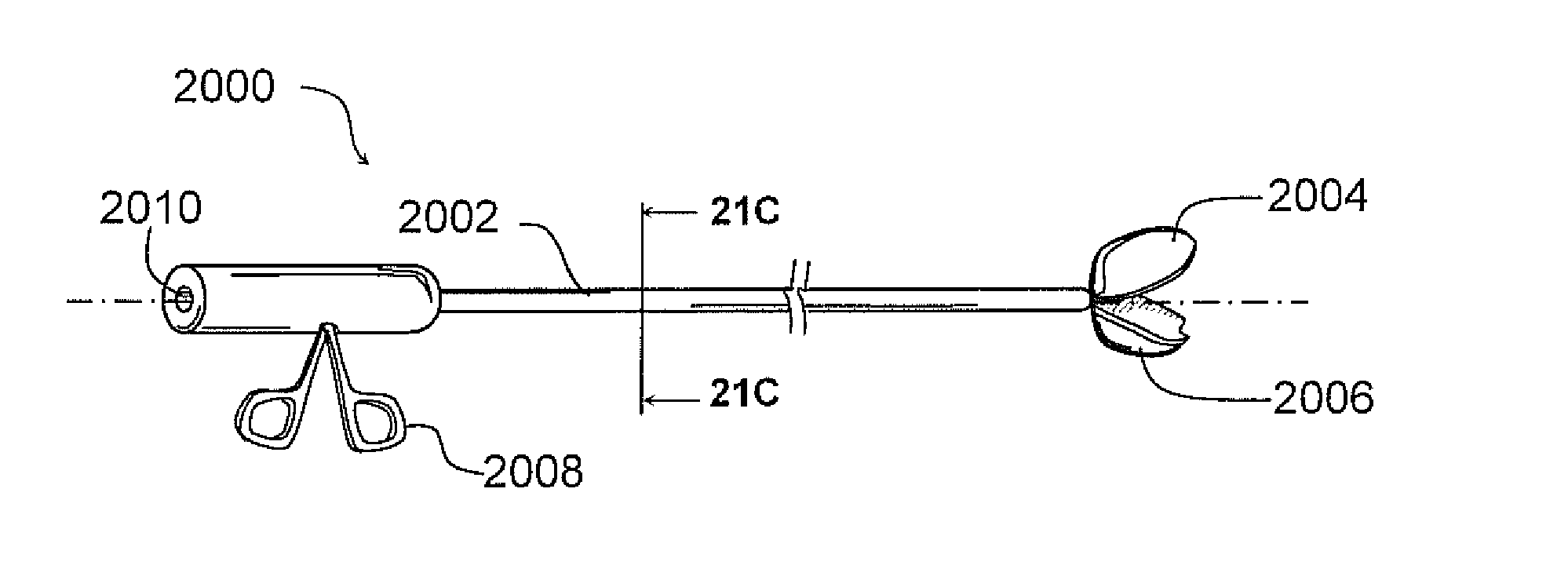
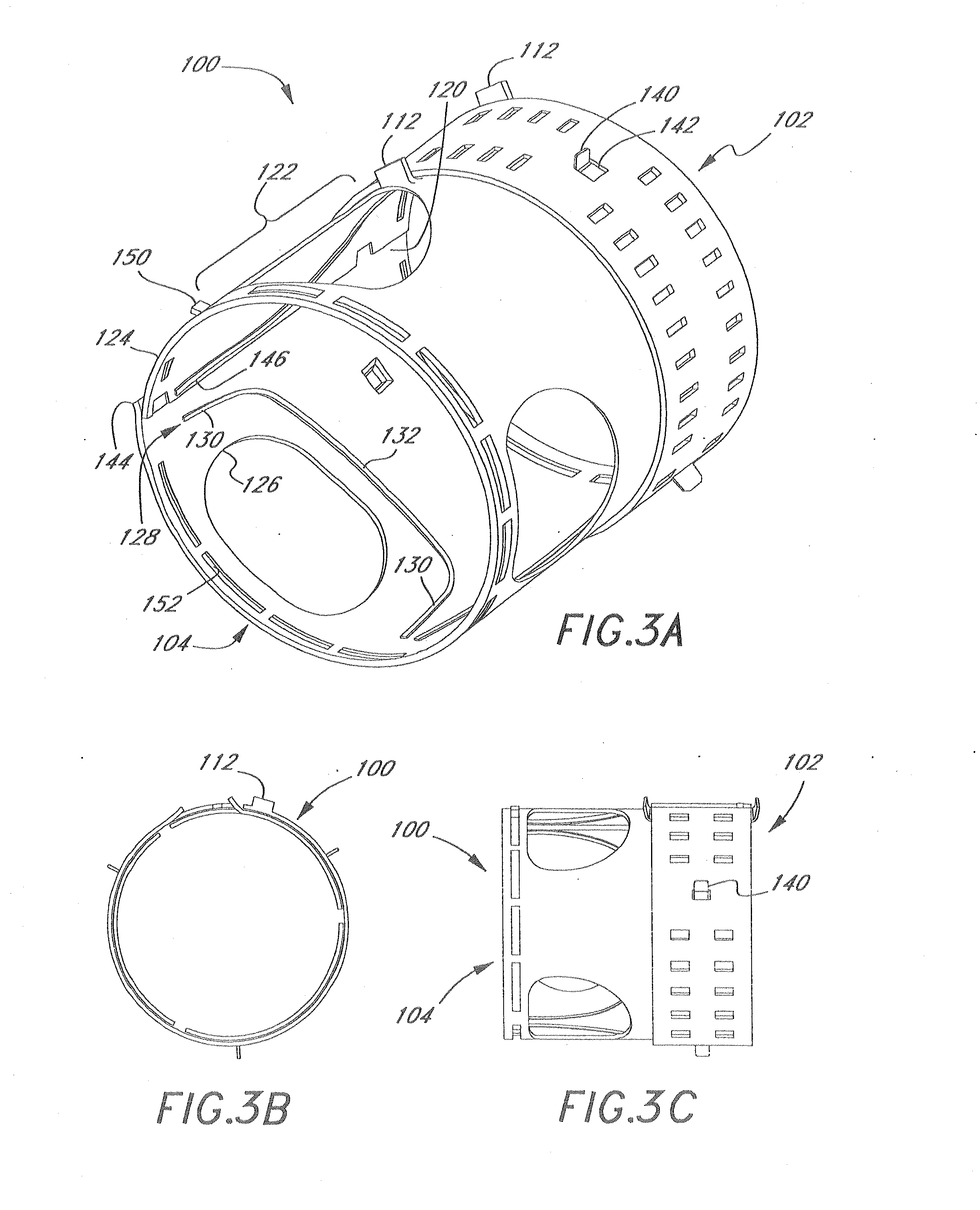
Middle Turbinate Medializer
InactiveUS20070293946A1Avoid stickingRestoring natural anatomySuture equipmentsDiagnosticsPalate muscleMiddle turbinates
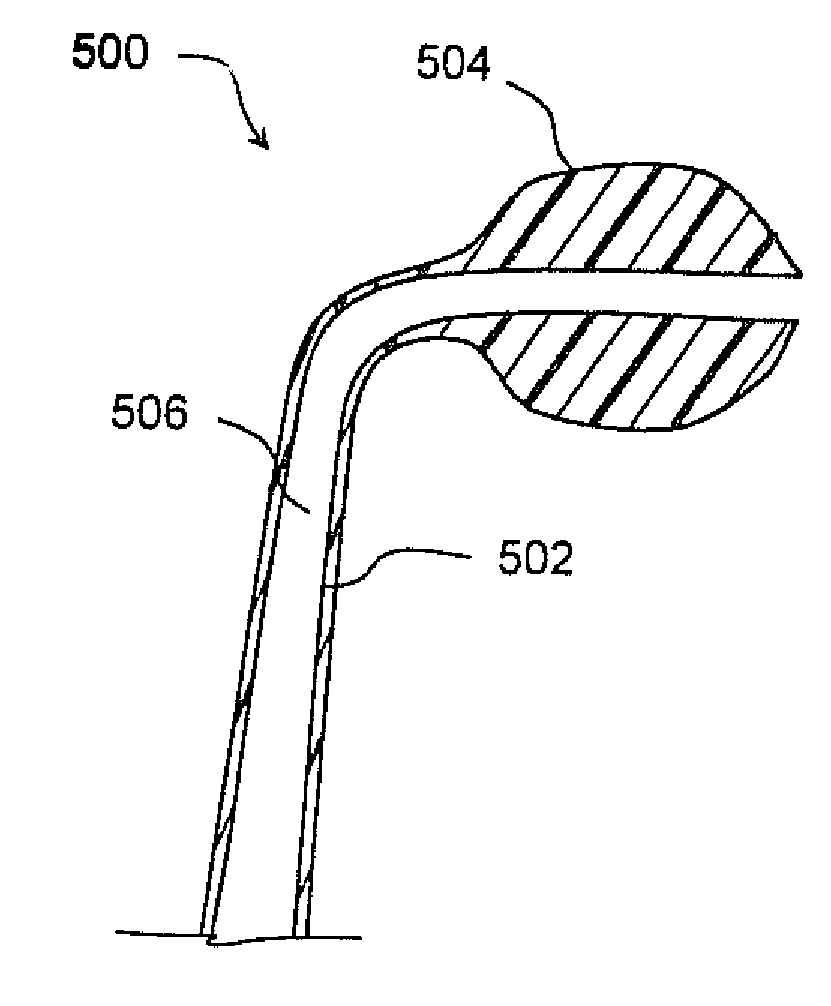
Medializing the middle turbinate in the nose has been realized as a solution to the common complication of adhesions following nasal and sinus surgery. The invention provides a system for medializing the middle turbinate by attaching the middle turbinate temporarily to the nasal septum. The attachment is performed using a wafer with means on both sides for attaching the wafer to a mucosal surface. The attachment may also be performed using a tissue adhesive, pins, or other medical devices described herein. The invention also provides a system for attaching the uvula to the nasopharyngeal side of the soft palate. The invention provides a medical device for use in the inventive procedures as well as methods for the procedures and kits for use by a physician.
Owner:ARTHROCARE
Sinus delivery of sustained release therapeutics
ActiveUS20050043706A1Easy accessReduce inflammationMedical devicesPharmaceutical delivery mechanismSinusitisMicroparticle
The invention provides biodegradable implants for treating sinusitis. The biodegradable implants have a size, shape, density, viscosity, and / or mucoadhesiveness that prevents them from being substantially cleared by the mucociliary lining of the sinuses during the intended treatment period. The biodegradable implants include a sustained release therapeutic, e.g., an antibiotic, a steroidal anti-inflammatory agent, or both. The biodegradable implants may take various forms, such as rods, pellets, beads, strips, or microparticles, and may be delivered into a sinus in various pharmaceutically acceptable carriers.
Owner:INTERSECT ENT INC
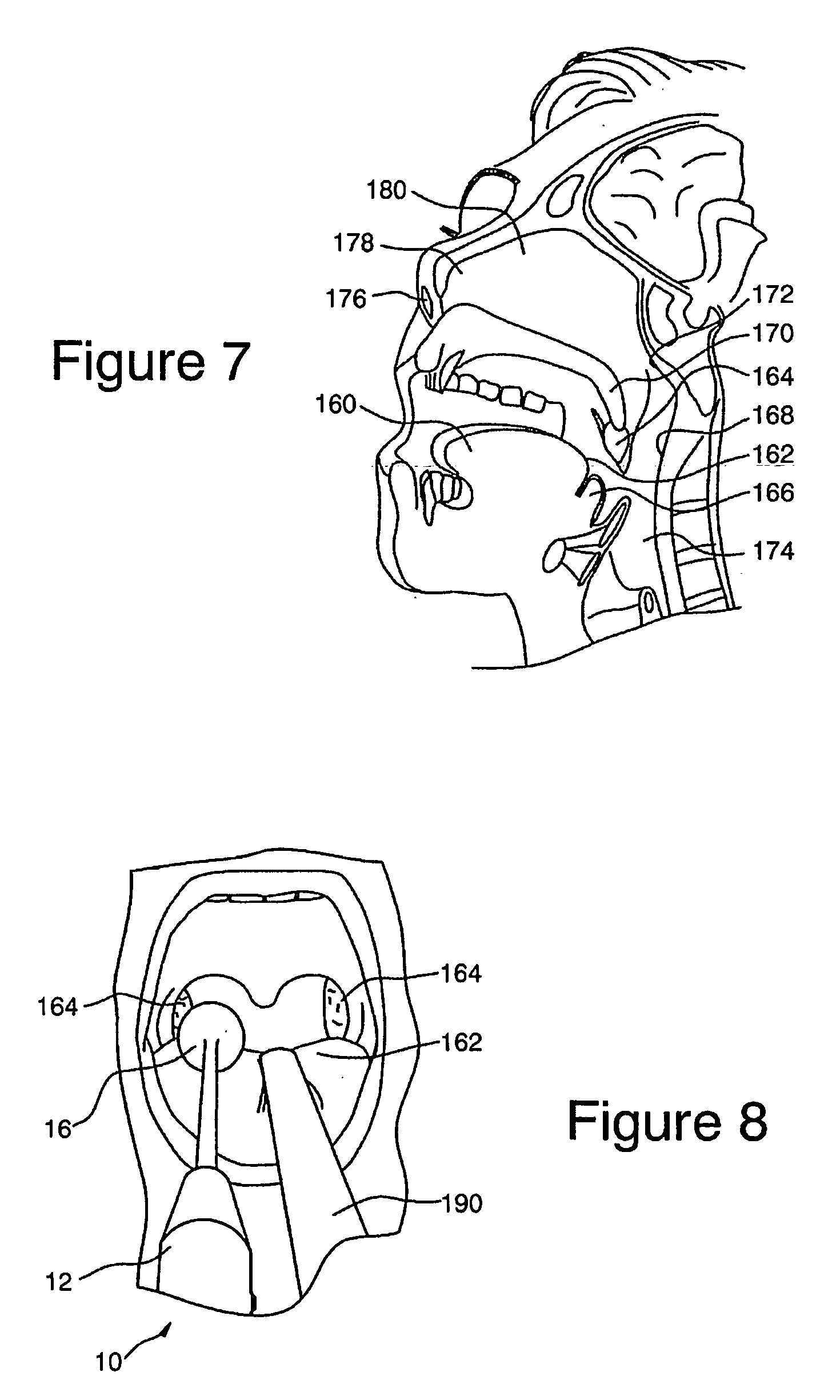
Devices, Systems and Methods for Treating Disorders of the Ear, Nose and Throat
Sinusitis, mucocysts, tumors, infections, hearing disorders, choanal atresia, fractures and other disorders of the paranasal sinuses, Eustachian tubes, Lachrymal ducts and other ear, nose, throat and mouth structures are diagnosed and / or treated using minimally invasive approaches and, in many cases, flexible catheters as opposed to instruments having rigid shafts. Various diagnostic procedures and devices are used to perform imaging studies, mucus flow studies, air / gas flow studies, anatomic dimension studies and endoscopic studies. Access and occluding devices may be used to facilitate insertion of working devices such asendoscopes, wires, probes, needles, catheters, balloon catheters, dilation catheters, dilators, balloons, tissue cutting or remodeling devices, suction or irrigation devices, imaging devices, sizing devices, biopsy devices, image-guided devices containing sensors or transmitters, electrosurgical devices, energy emitting devices, devices for injecting diagnostic or therapeutic agents, devices for implanting devices such as stents, substance eluting or delivering devices and implants, etc.
Owner:ACCLARENT INC
Sinus delivery of sustained release therapeutics
The invention provides biodegradable implants for treating sinusitis. The biodegradable implants have a size, shape, density, viscosity, and / or mucoadhesiveness that prevents them from being substantially cleared by the mucociliary lining of the sinuses during the intended treatment period. The biodegradable implants include a sustained release therapeutic, e.g., an antibiotic, a steroidal anti-inflammatory agent, or both. The biodegradable implants may take various forms, such as rods, pellets, beads, strips, or microparticles, and may be delivered into a sinus in various pharmaceutically acceptable carriers.
Owner:INTERSECT ENT INC
Devices, Systems and Methods for Treating Disorders of the Ear, Nose and Throat
Sinusitis, mucocysts, tumors, infections, hearing disorders, choanal atresia, fractures and other disorders of the paranasal sinuses, Eustachian tubes, Lachrymal ducts and other ear, nose, throat and mouth structures are diagnosed and / or treated using minimally invasive approaches and, in many cases, flexible catheters as opposed to instruments having rigid shafts. Various diagnostic procedures and devices are used to perform imaging studies, mucus flow studies, air / gas flow studies, anatomic dimension studies and endoscopic studies. Access and occluding devices may be used to facilitate insertion of working devices such asendoscopes, wires, probes, needles, catheters, balloon catheters, dilation catheters, dilators, balloons, tissue cutting or remodeling devices, suction or irrigation devices, imaging devices, sizing devices, biopsy devices, image-guided devices containing sensors or transmitters, electrosurgical devices, energy emitting devices, devices for injecting diagnostic or therapeutic agents, devices for implanting devices such as stents, substance eluting or delivering devices and implants, etc.
Owner:ACCLARENT INC
Device and methods for treating paranasal sinus conditions
ActiveUS8025635B2Reduce needRecovery functionAntibacterial agentsOrganic active ingredientsActive agentNose
Described here are paranasal sinus devices for treating paranasal sinus conditions. The devices include a cavity member, ostial member, and nasal portion. One or more of the cavity member, ostial member, and nasal portion may deliver an active agent for sustained release to treat the paranasal sinus condition. Exemplary paranasal sinus conditions are sinus inflammation due to functional endoscopic sinus surgery (FESS) and rhinosinusitis.
Owner:INTERSECT ENT INC
Rolled minimally invasive heart valves
Expandable heart valves for minimally invasive valve replacement surgeries are disclosed. The valves are rolled into a first, contracted configuration for minimally invasive delivery and then unrolled or unfurled at the implantation site. One- and two-piece stents may be used in conjunction with a plurality of flexible leaflet-forming membranes. The one-piece stents may include an annulus anchoring section, a sinus section with the membranes attached over sinus apertures, and a rigid outflow section. The two-piece stent may include a primary stent to provide a tubular base at the annulus, and a secondary stent having the membranes that couples within the primary stent. Lockout tabs to secure the stents in their expanded shapes are provided. Also, alignment structure may be provided to ensure concentric unfurling. Anchoring barbs at the stent edges or in the stent body secure the valve within the annulus. Methods of implantation are also provided.
Owner:EDWARDS LIFESCIENCES CORP
Apparatus, system, and method for middle turbinate medializer
An apparatus, system, and method are disclosed for medializing the middle turbinate following sinus surgery, thus preventing synacheiae, granulation tissue, and other complications of the prior art. The apparatus utilizes a fastening device configured to attach the middle turbinate to the nasal septum, a prong comprising a slender wand portion and a fastening module, and a handle configured to trigger deployment of the fastening means by activating the fastening module. The fastening device may be a staple, rivet, glue, or another similar device.
Owner:TAGGE BRYAN C
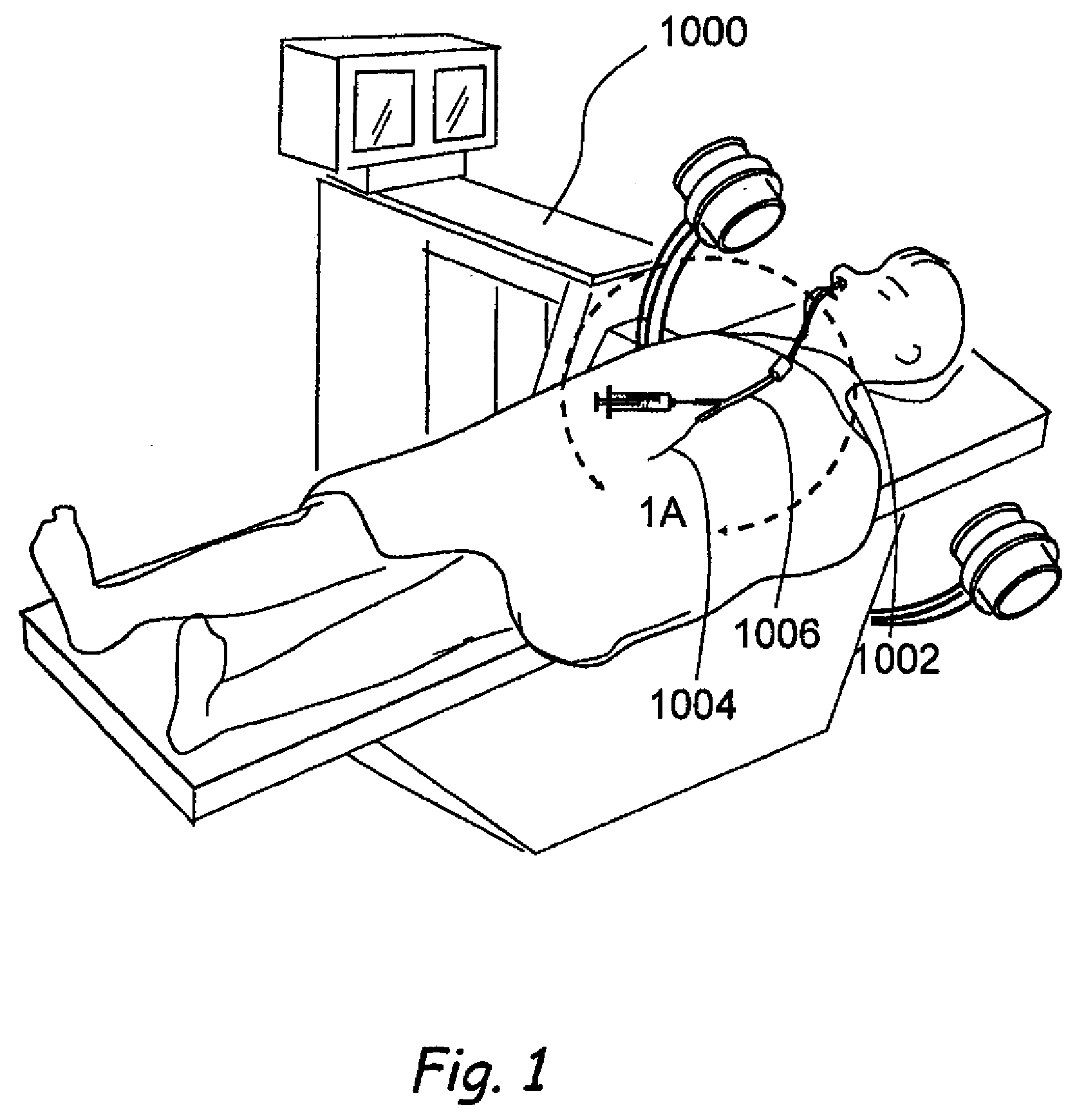
Methods and apparatus for treating disorders of the sinuses
A medical device for the treatment of a sinus opening includes a handle, a grooming sheath, a rail, a guide wire, a balloon catheter and a balloon catheter movement mechanism. A method for treating a sinus opening includes inserting a medical device for the treatment of a sinus opening partially into a patient's anatomy, positioning a guide wire operatively extending from a rail of the medical device into a sinus opening of the patient, advancing a balloon catheter from an annular lumen of the medical device and along both the rail of the medical device and the guide wire with a balloon catheter movement mechanism, and inflating the balloon catheter.
Owner:ACCLARENT INC
Two-part expandable heart valve
Expandable heart valves for minimally invasive valve replacement surgeries are disclosed. The valves are rolled into a first, contracted configuration for minimally invasive delivery and then unrolled or unfurled at the implantation site. One- and two-piece stents may be used in conjunction with a plurality of flexible leaflet-forming membranes. The one-piece stents may include an annulus anchoring section, a sinus section with the membranes attached over sinus apertures, and a rigid outflow section. The two-piece stent may include a primary stent to provide a tubular base at the annulus, and a secondary stent having the membranes that couples within the primary stent. Lockout tabs to secure the stents in their expanded shapes are provided. Also, alignment structure may be provided to ensure concentric unfurling. Anchoring barbs at the stent edges or in the stent body secure the valve within the annulus. Methods of implantation are also provided.
Owner:EDWARDS LIFESCIENCES CORP
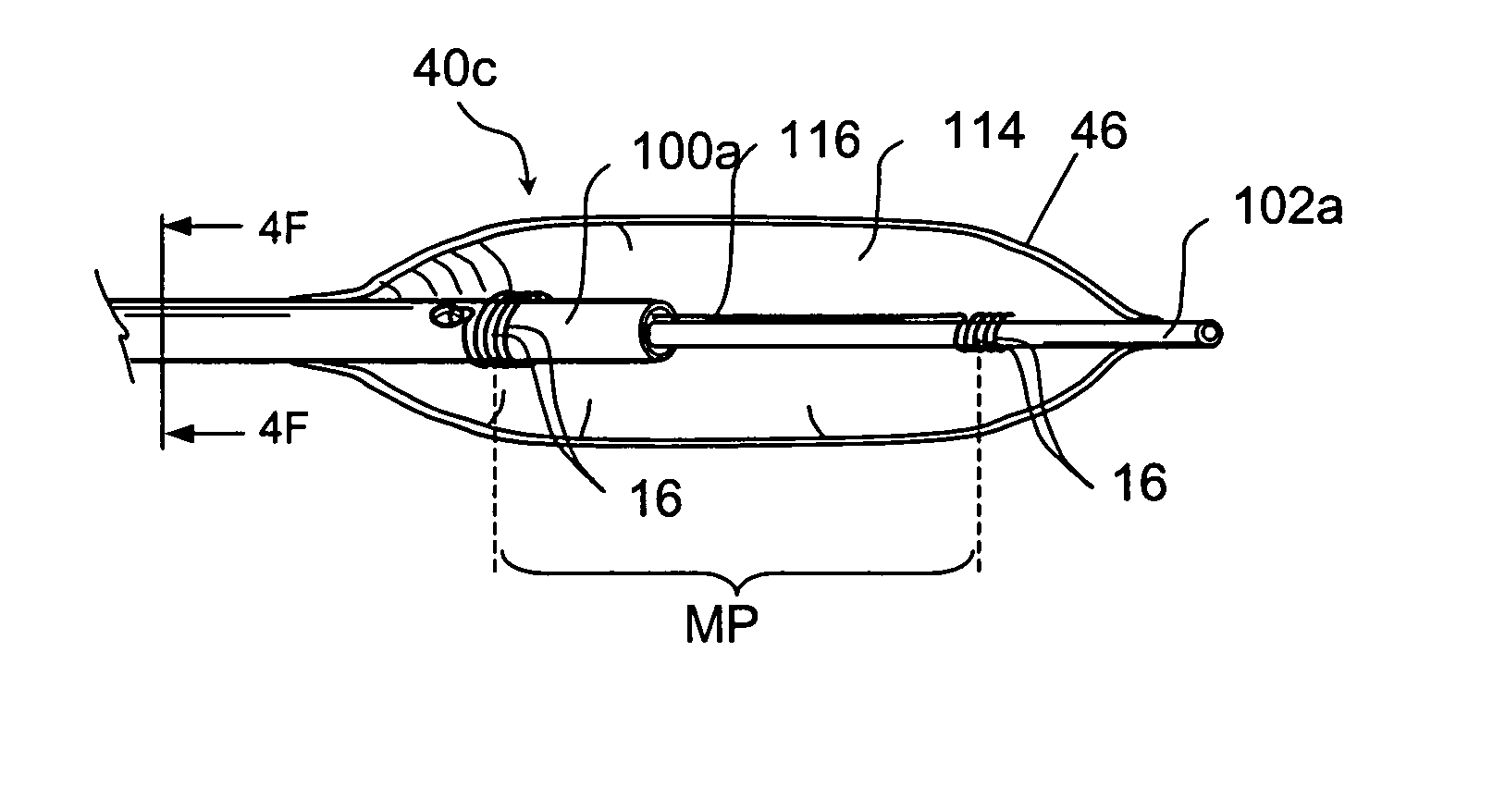
Apparatus and Methods for Dilating and Modifying Ostia of Paranasal Sinuses and Other Intranasal or Paranasal Structures
Sinusitis and other disorders of the ear, nose and throat are diagnosed and / or treated using minimally invasive approaches with flexible or rigid instruments. Various methods and devices are used for remodeling or changing the shape, size or configuration of a sinus ostium or duct or other anatomical structure in the ear, nose or throat; implanting a device, cells or tissues; removing matter from the ear, nose or throat; delivering diagnostic or therapeutic substances or performing other diagnostic or therapeutic procedures. Introducing devices (e.g., guide catheters, tubes, guidewires, elongate probes, other elongate members) may be used to facilitate insertion of working devices (e.g. catheters e.g. balloon catheters, guidewires, tissue cutting or remodeling devices, devices for implanting elements like stents, electrosurgical devices, energy emitting devices, devices for delivering diagnostic or therapeutic agents, substance delivery implants, scopes etc.) into the paranasal sinuses or other structures in the ear, nose or throat.
Owner:ACCLARENT INC
Endoscopic methods and devices for transnasal procedures
Owner:ACCLARENT INC
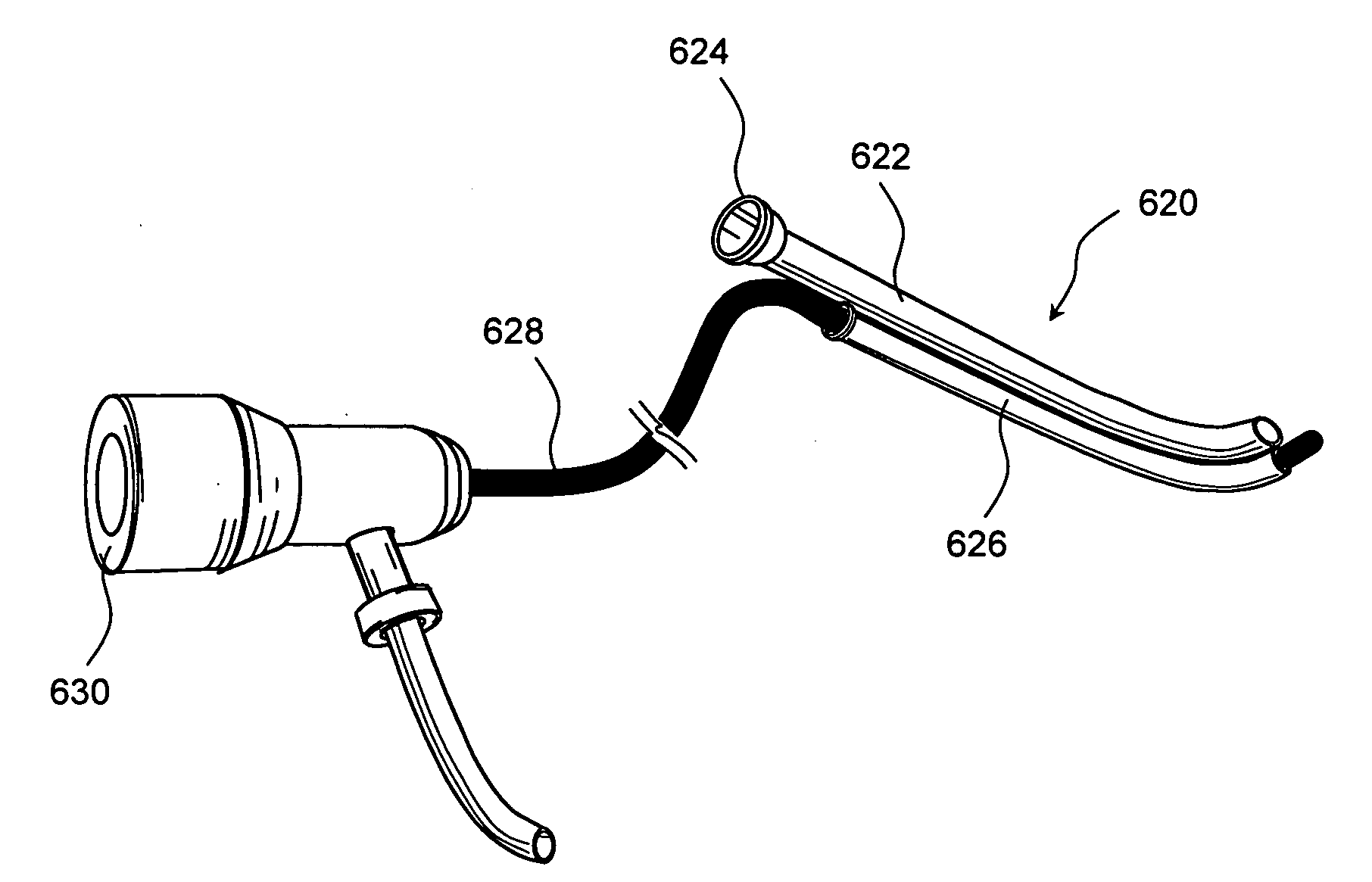
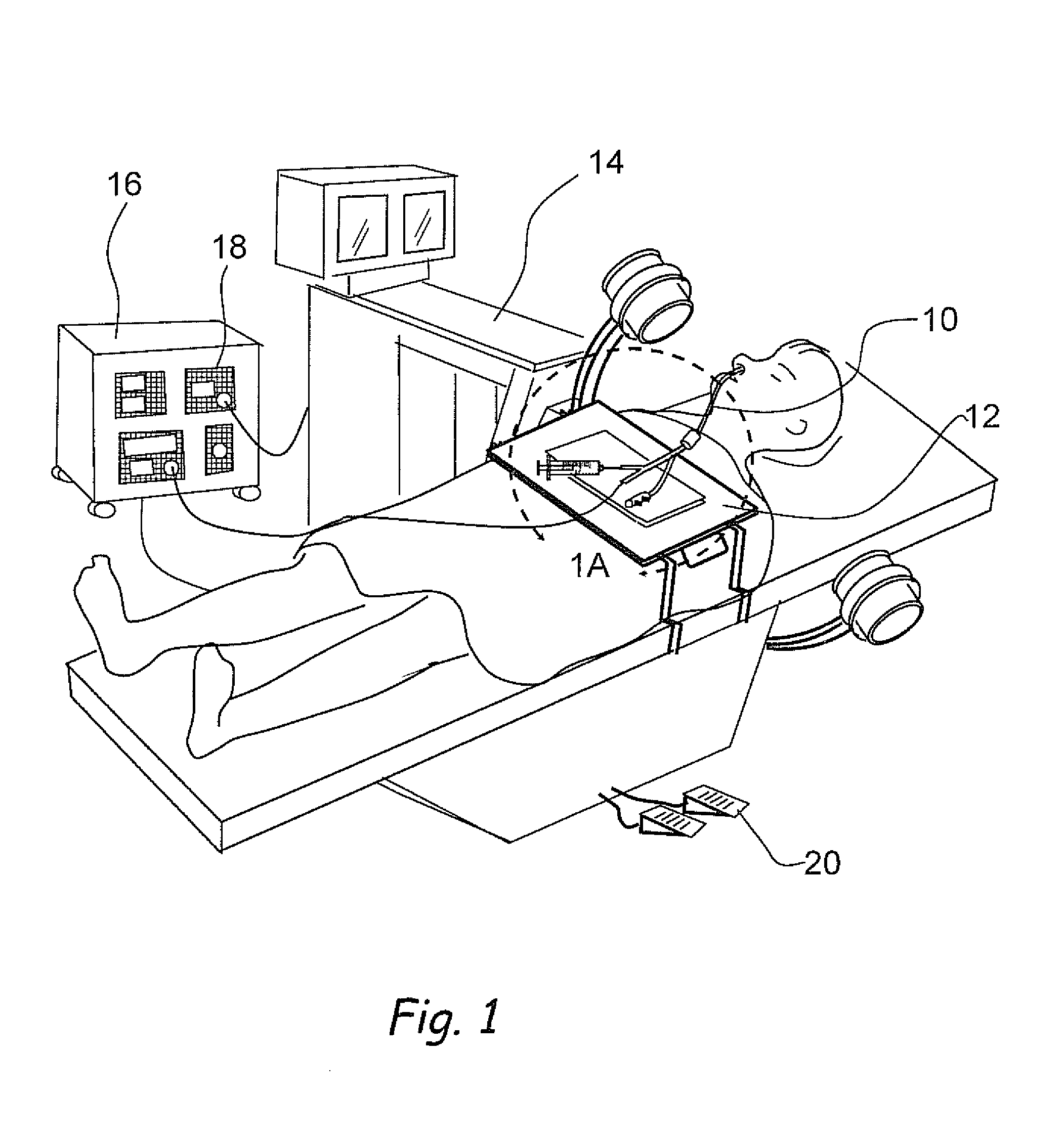
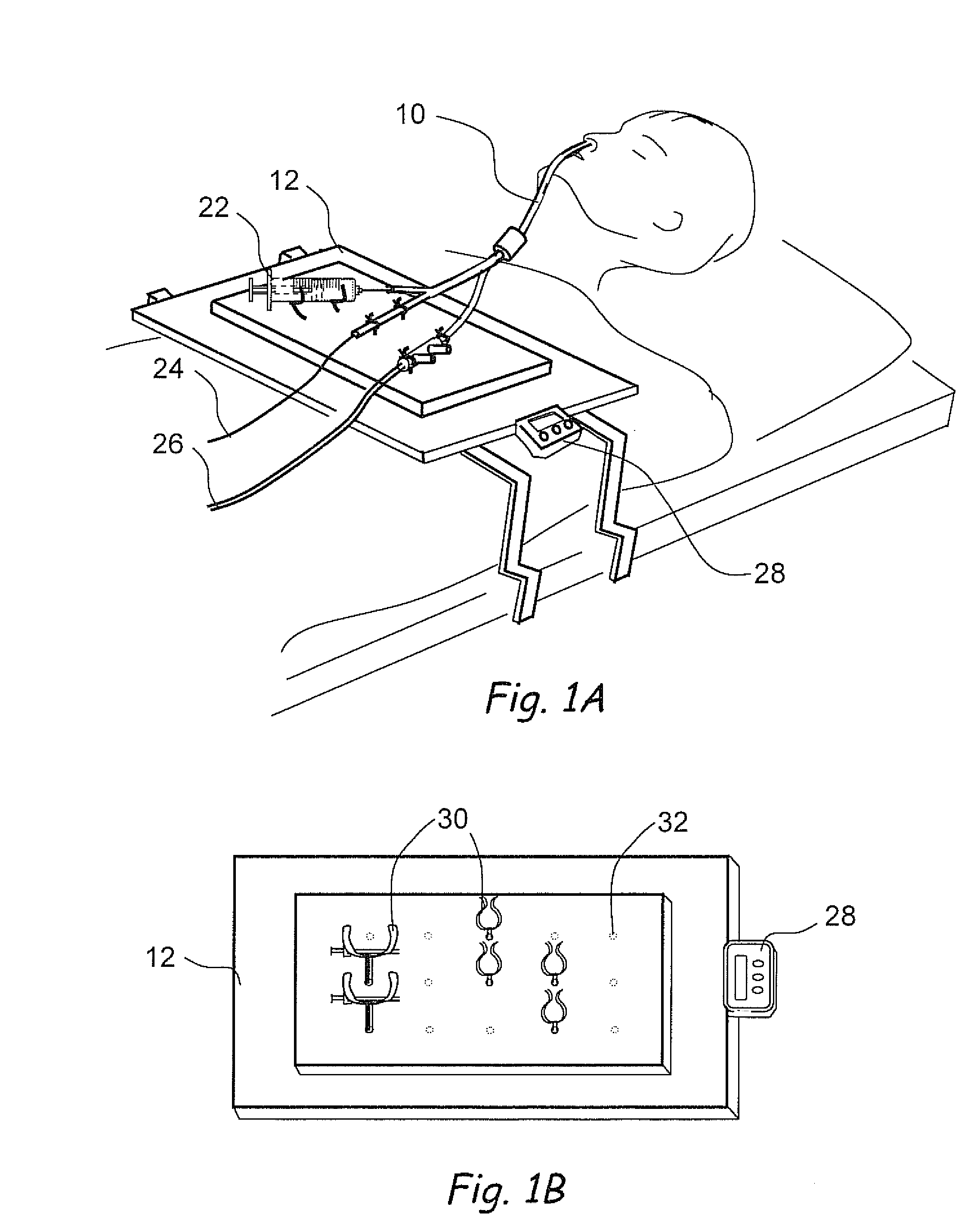
Medical Device and Method for Treatment of a Sinus Opening
A medical device and method for the treatment of a sinus opening includes a handle, a guide catheter, a guide wire, a balloon catheter, a guide wire movement mechanism and a balloon catheter movement mechanism. The handle has proximal and distal ends and a longitudinal axis along the length of the handle. The guide catheter is attached to the distal end of the handle and has a catheter lumen. The guide wire and balloon catheter are both disposed at least partially in the handle and catheter lumen. The guide wire movement mechanism and the balloon catheter movement mechanism are both operatively disposed on the handle. In addition, the guide wire movement mechanism is configured for advancement and retraction of the guide wire through the handle and catheter lumen by user operation of the guide wire movement mechanism. Moreover, the guide wire movement mechanism includes an integrated guide wire locking and rotation mechanism configured for rotation of the guide wire and for securely locking and unlocking the guide wire to the guide wire movement mechanism. Furthermore, the balloon catheter movement mechanism is configured for advancement and retraction of the balloon catheter through the handle and catheter lumen by user operation of the balloon catheter movement mechanism.
Owner:ACCLARENT INC
Control of halitosis-generating and other microorganisms in the non-dental upper respiratory tract
ActiveUS20060047329A1Effective controlReduce the burden onElectrotherapyDiagnosticsNon-ionizing radiationTonsil
Disclosed are safe, simple and effective broad-spectrum treatments for halitosis and other microbial infections of the nondental upper respiratory tract useful to treat bacterial and other microorganism species, including anaerobic bacteria. Electromagnetic radiative energy including visible, and optionally, thermal, RF and / or microwave wavelengths, is topically applied to internal surfaces of the upper respiratory tract to destroy or incapacitate superficial microorganisms without the use of antibiotics. One useful apparatus is a handheld energy applicator having a light output head suitable for treating the back of the tongue and the tonsils and which may be interchangeably provided with extensions to reach the sinuses. The energy applicator can be supported and guided by a mounting device held between the subject's teeth, if desired. Useful embodiments of the invention include preparative treatment of the target surfaces with a photosensitizing agent such as an oxidizing agent or a complementary stain. Optionally a pre-treament procedure may be employed to remove detritus and microfloral overgrowths that may mask more deeply resident target microorganisms. Novel treatments include treatment of halitosis by destruction of bacterial species associated with halitosis, such as Atopobium parvulum, by application of non-ionizing radiative energy to the tonsils and the back of the tongue. Another embodiment comprises a candy bar incorporating a halitosis treatment lamp disposed within the candy.
Owner:VALENT MEDICAL INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com