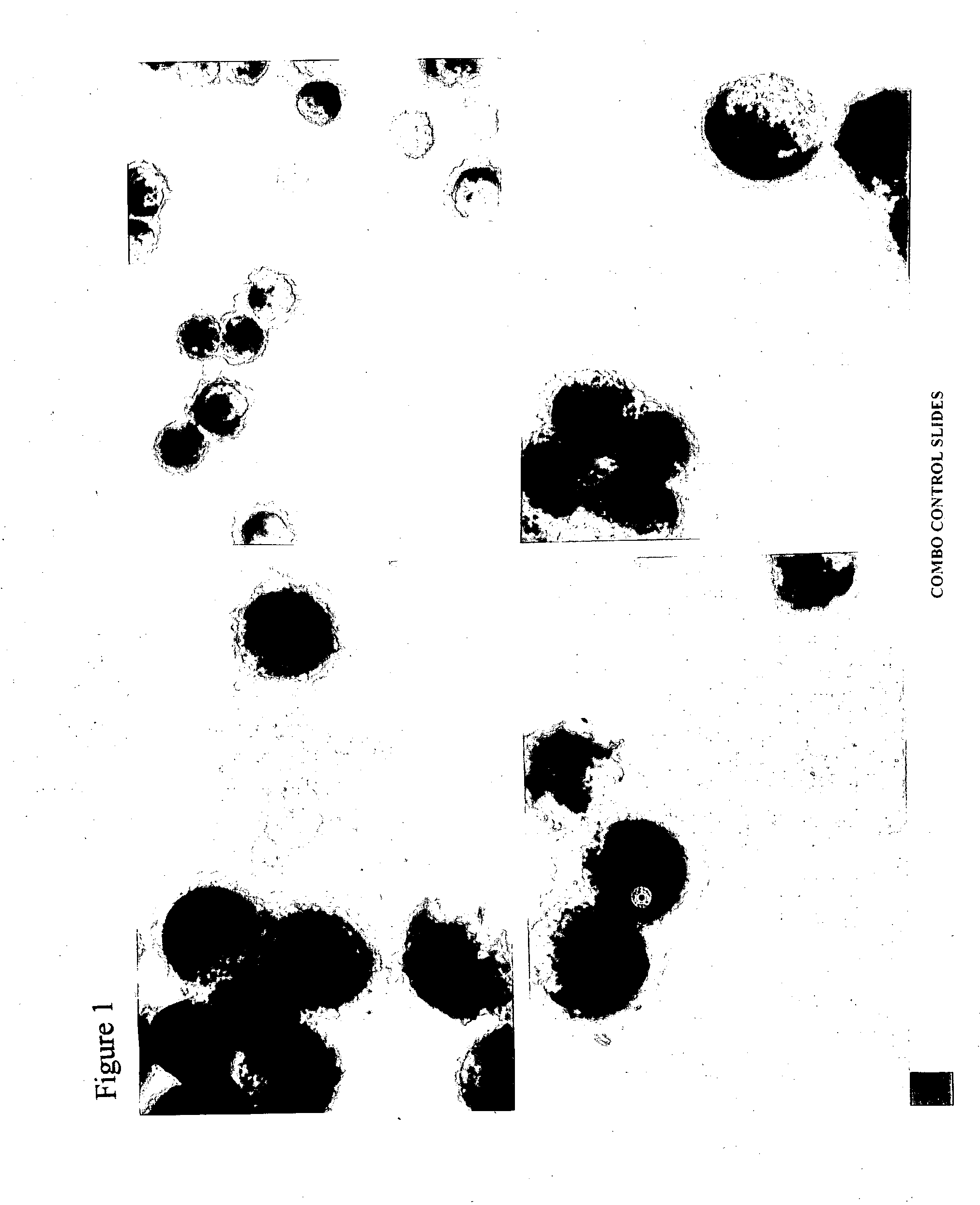
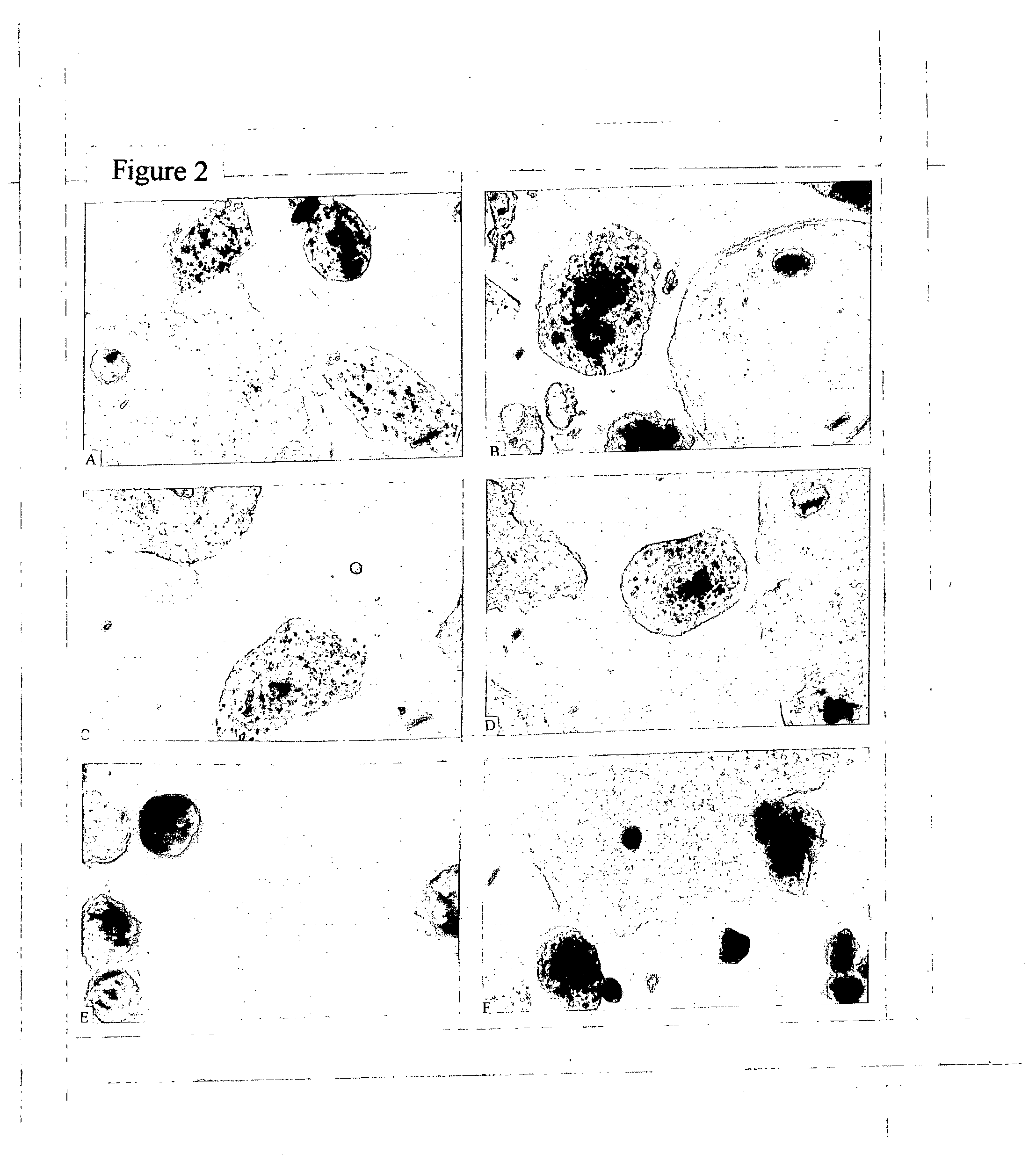
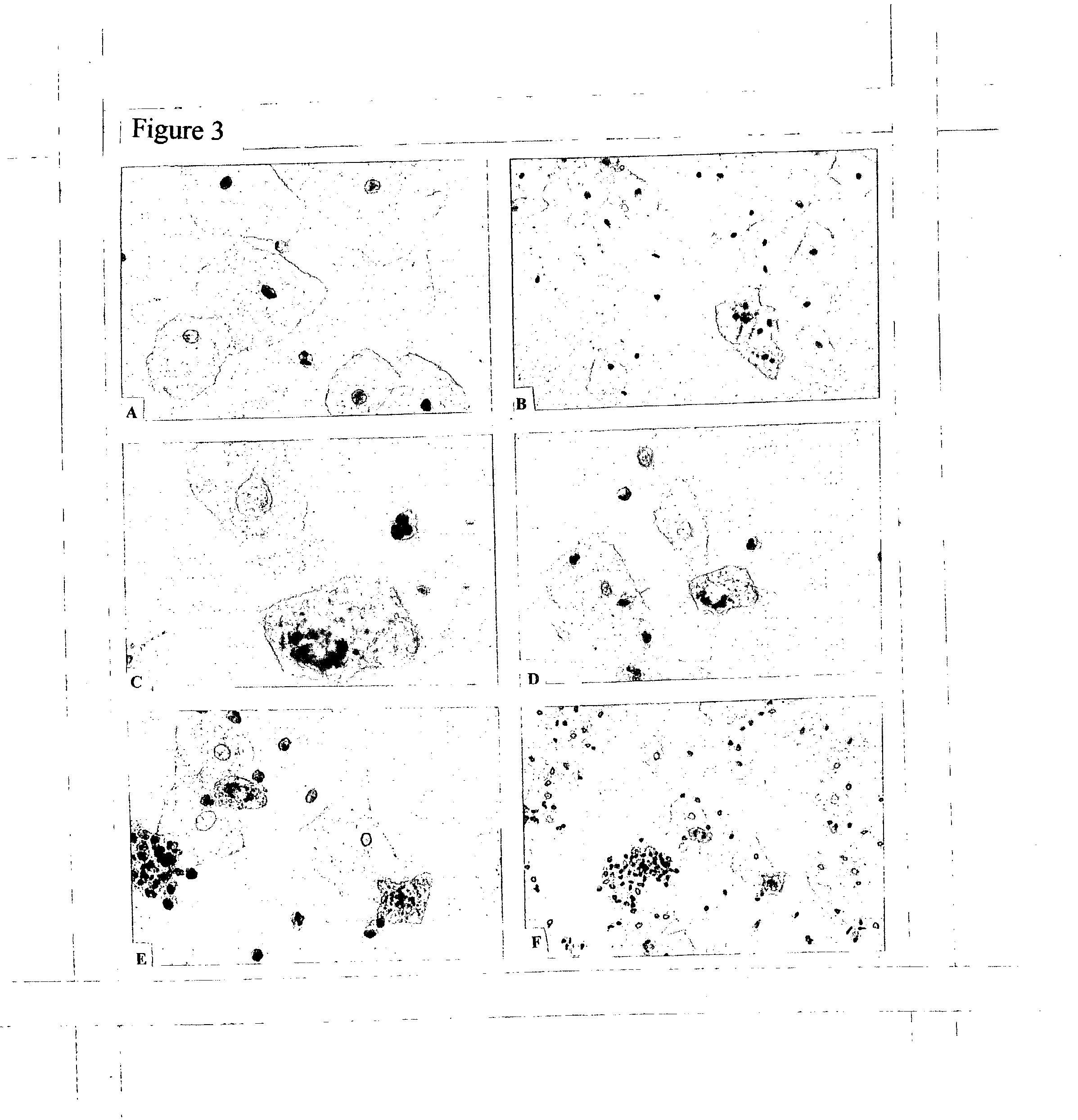
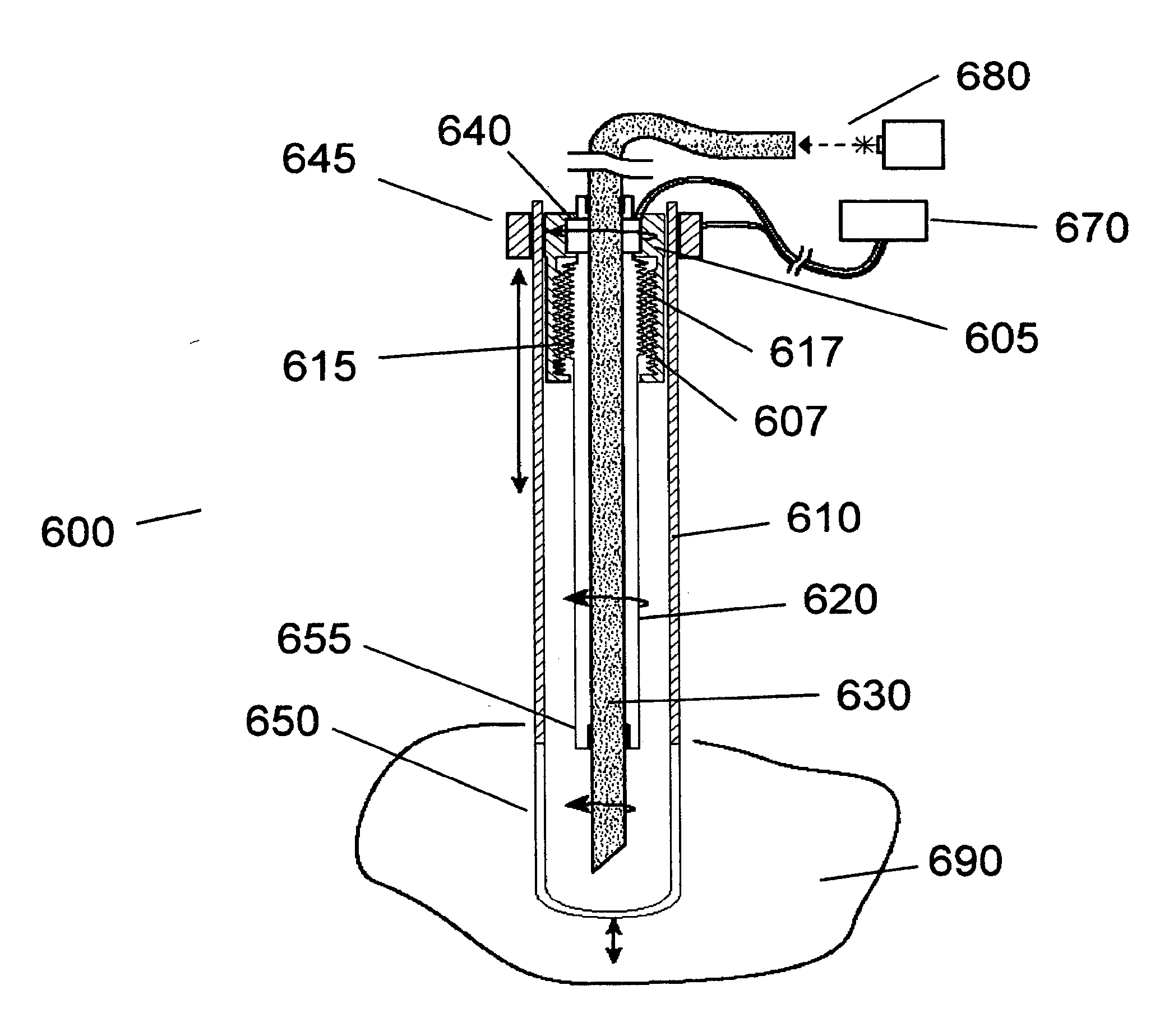
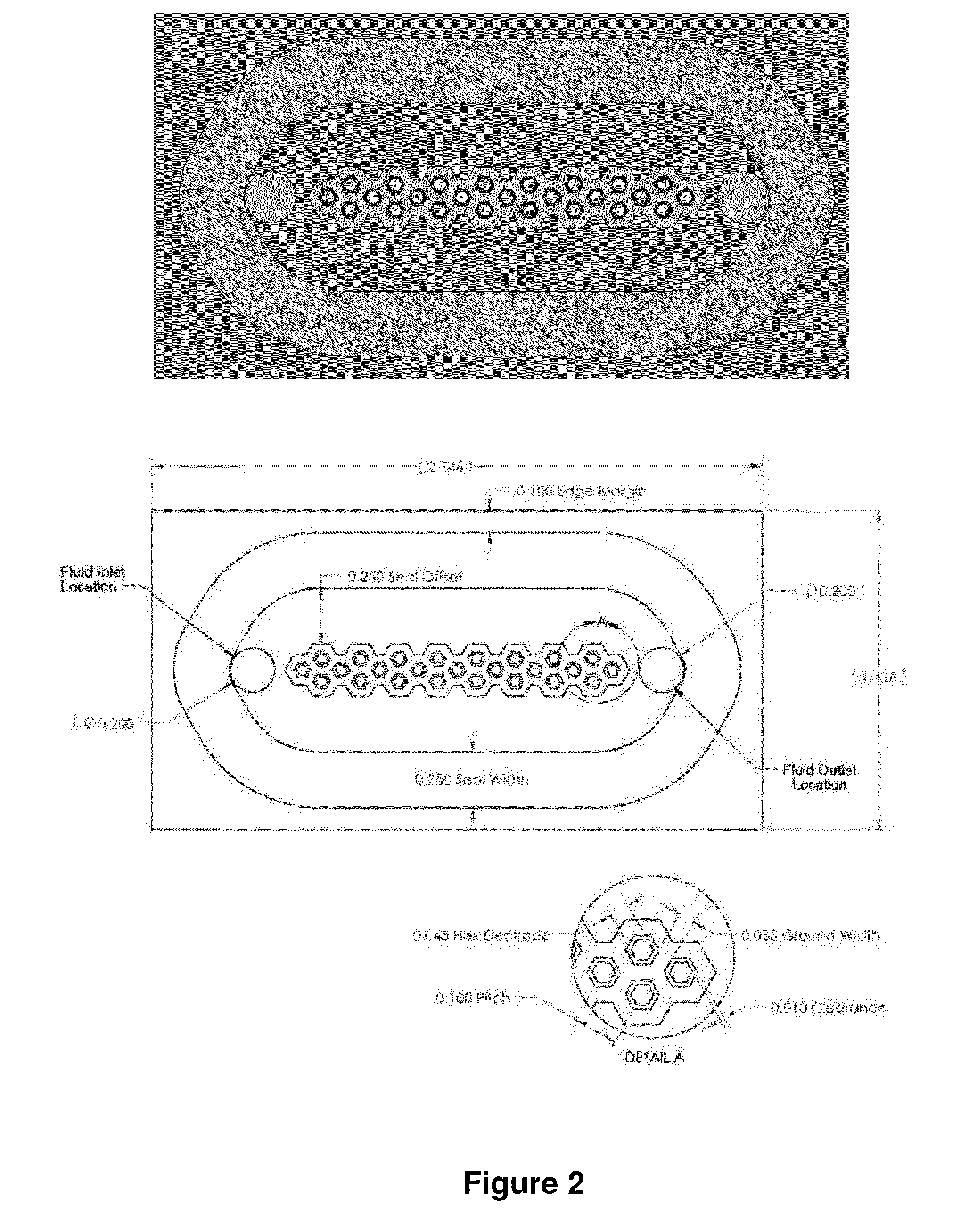
Patents
Literature
81 results about "Cervical cancer screening" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Uterine cervical cancer computer-aided-diagnosis (CAD)
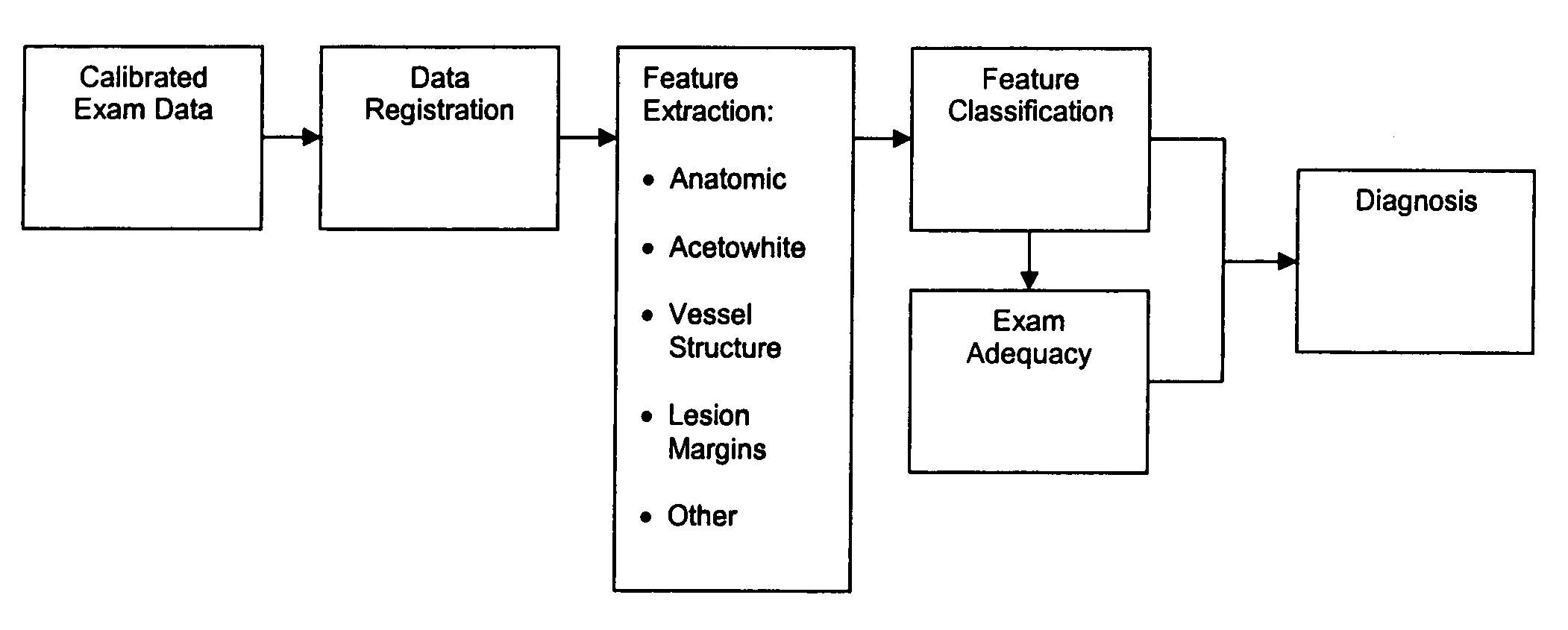
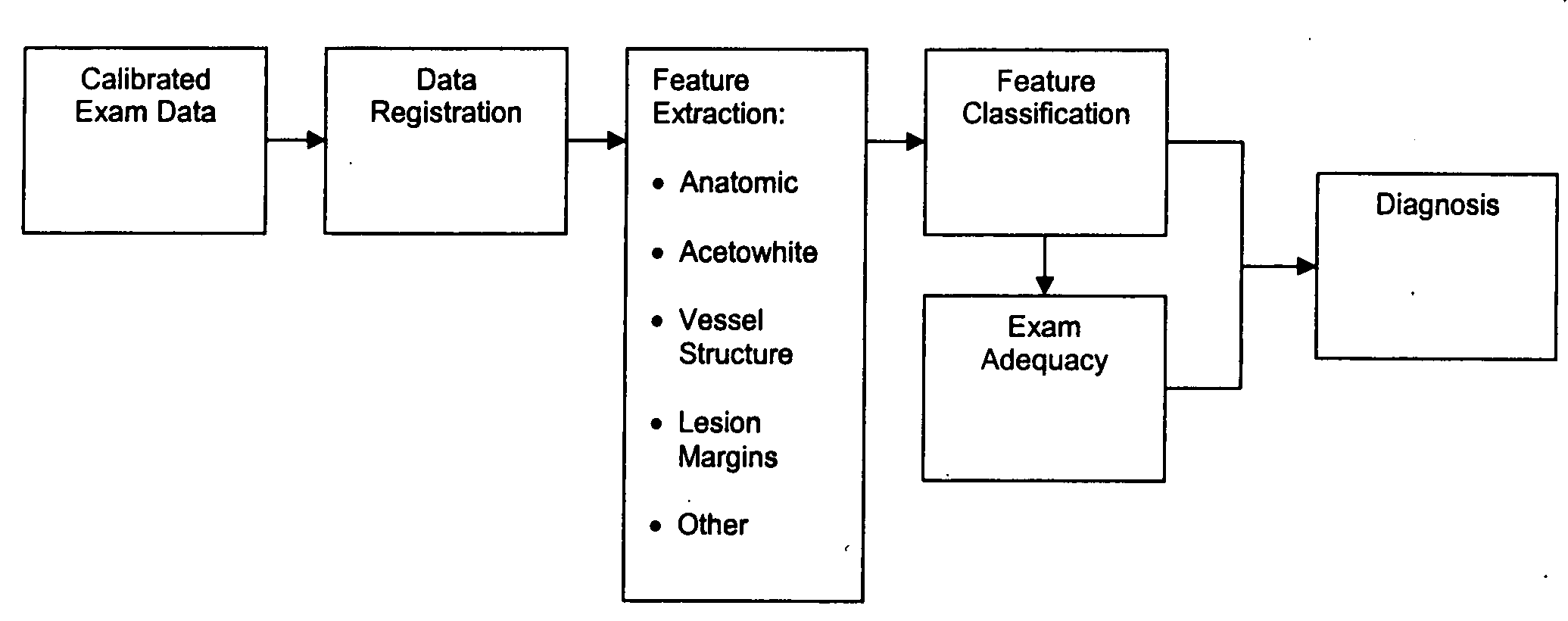
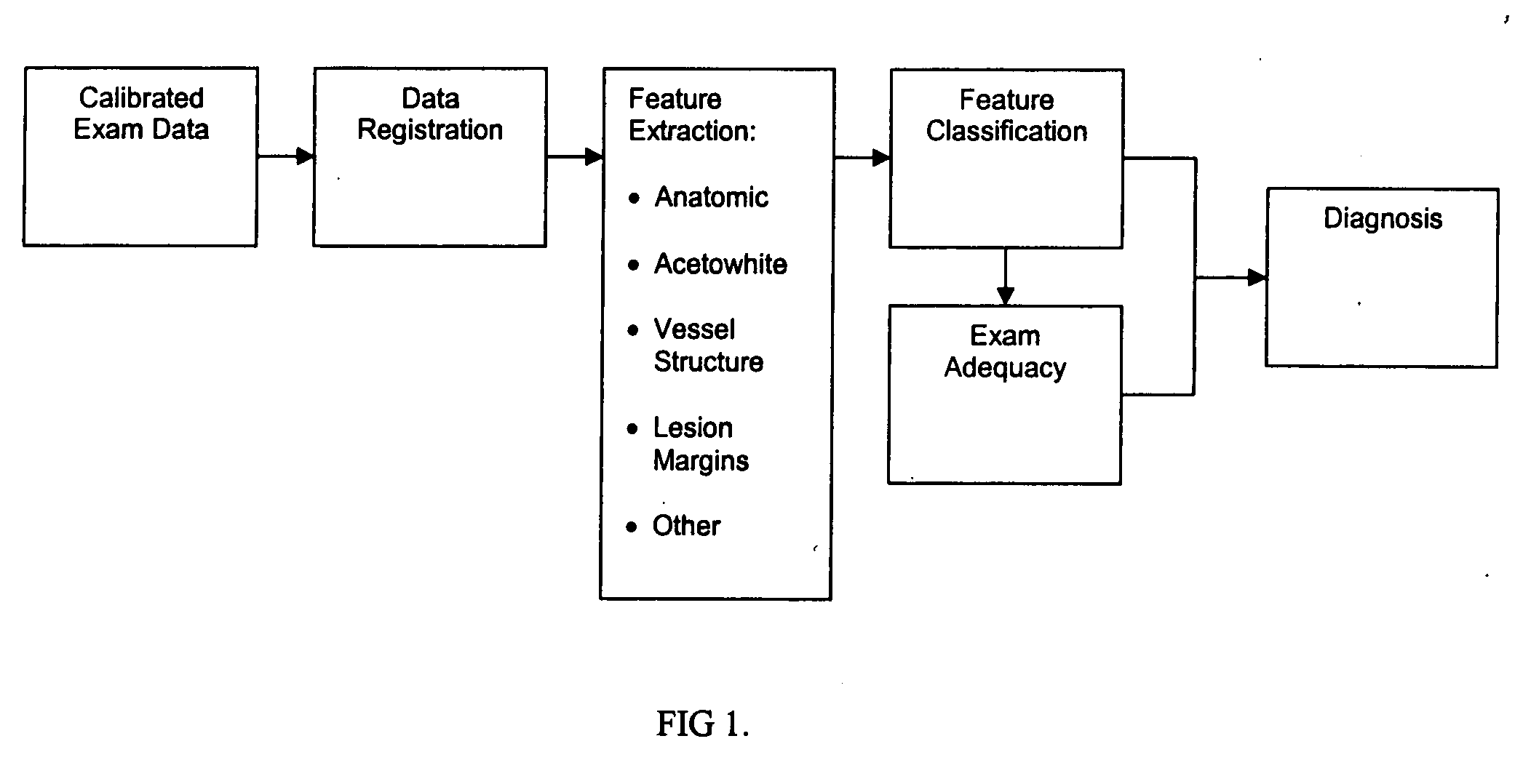
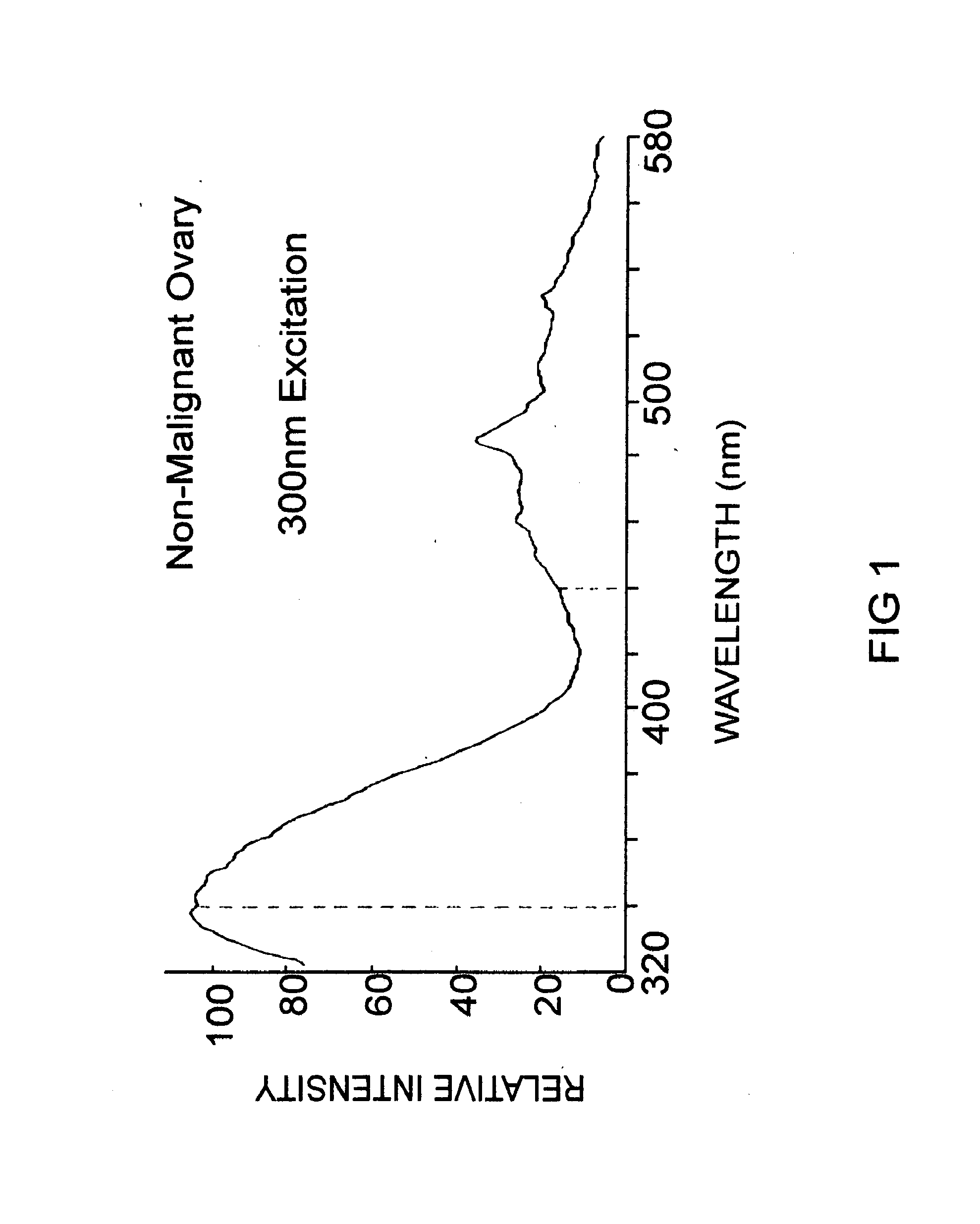
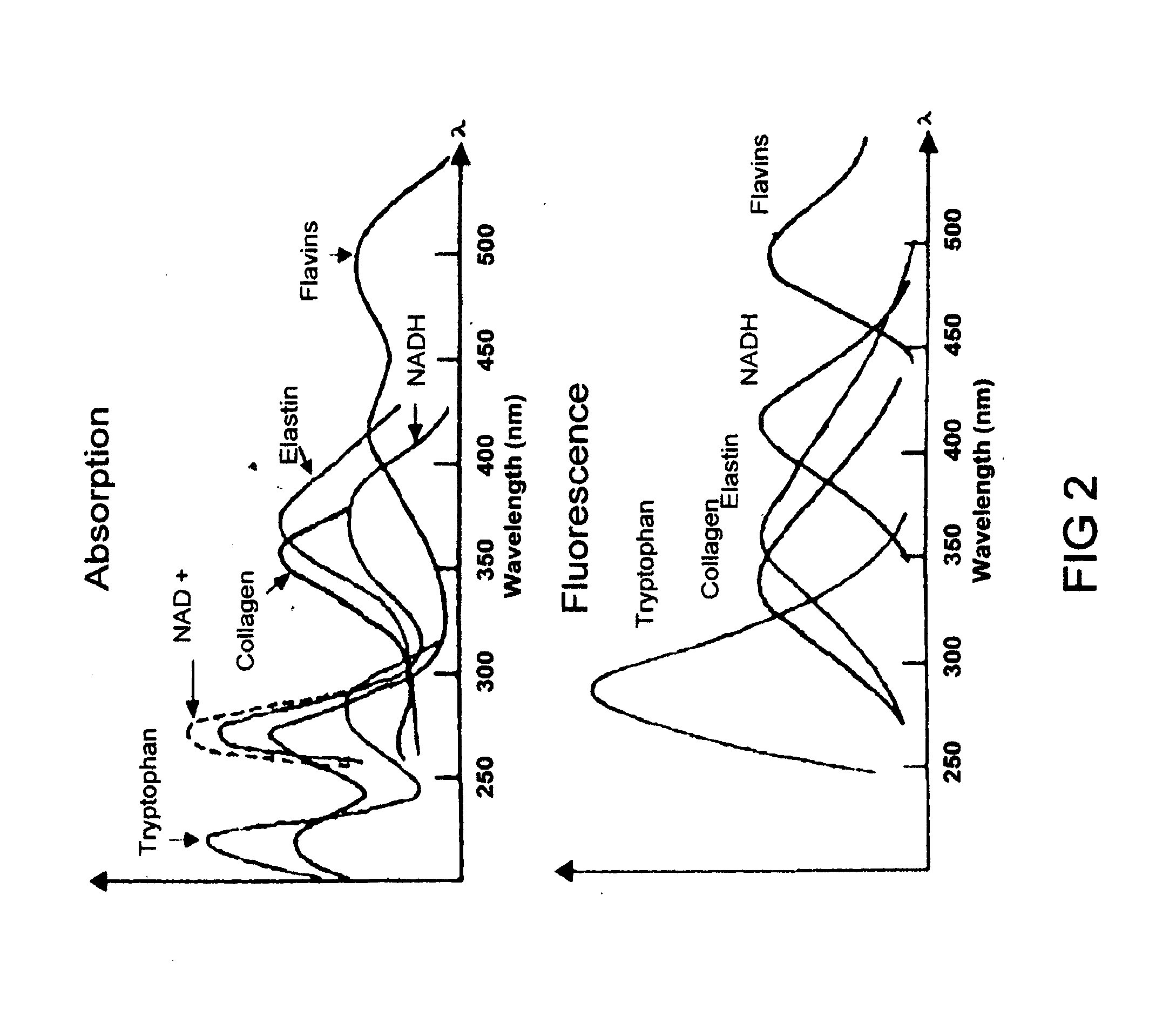
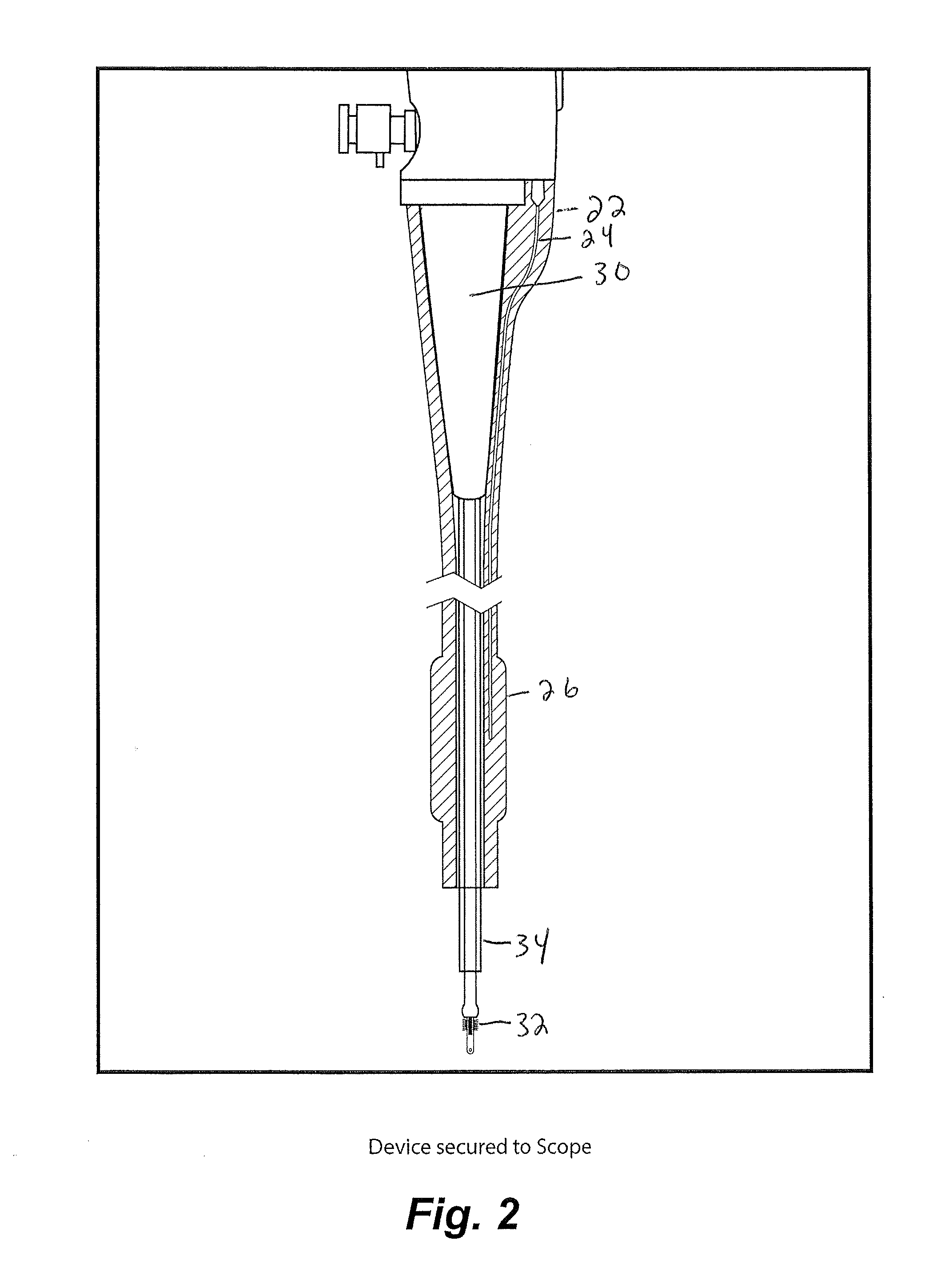
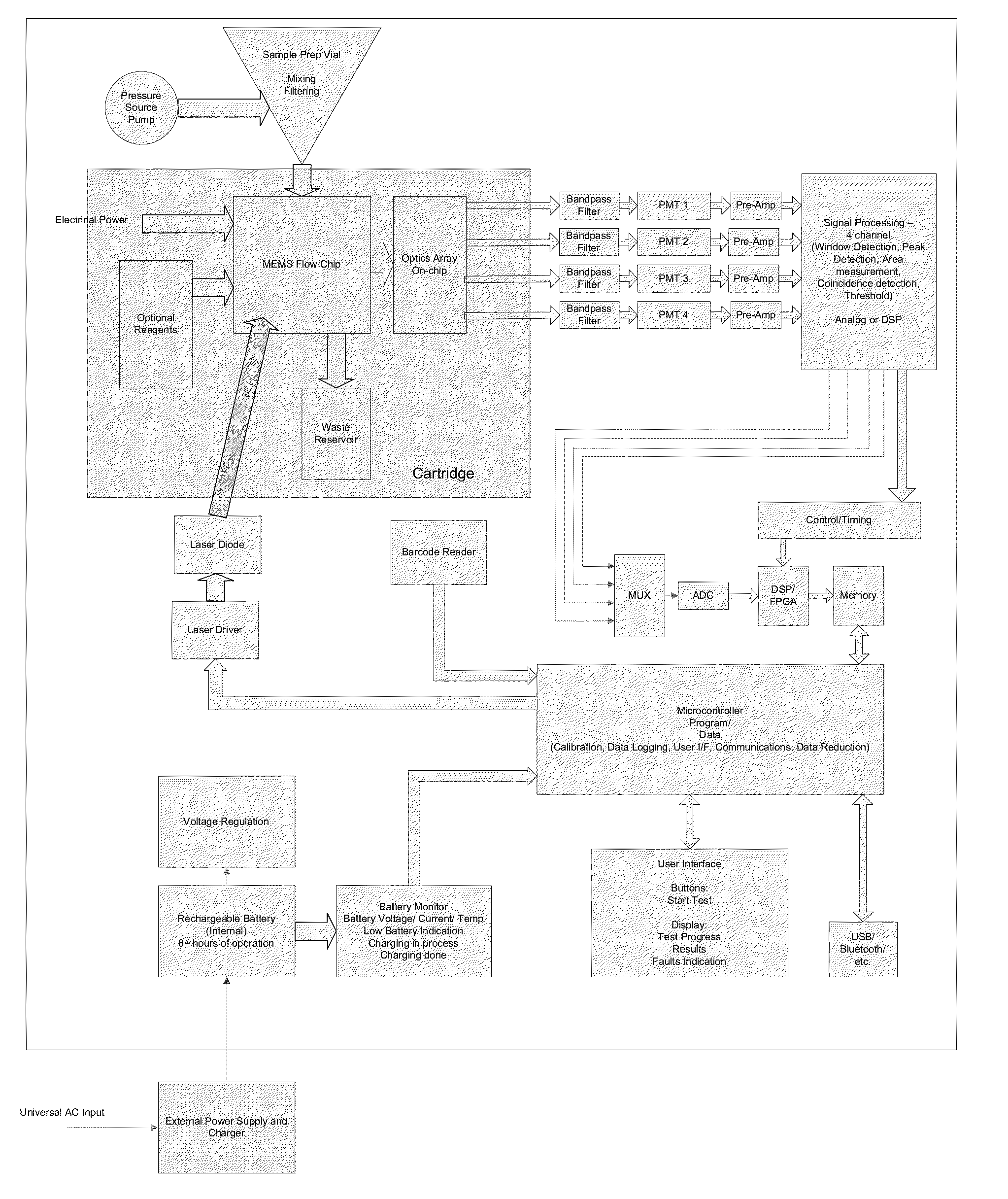
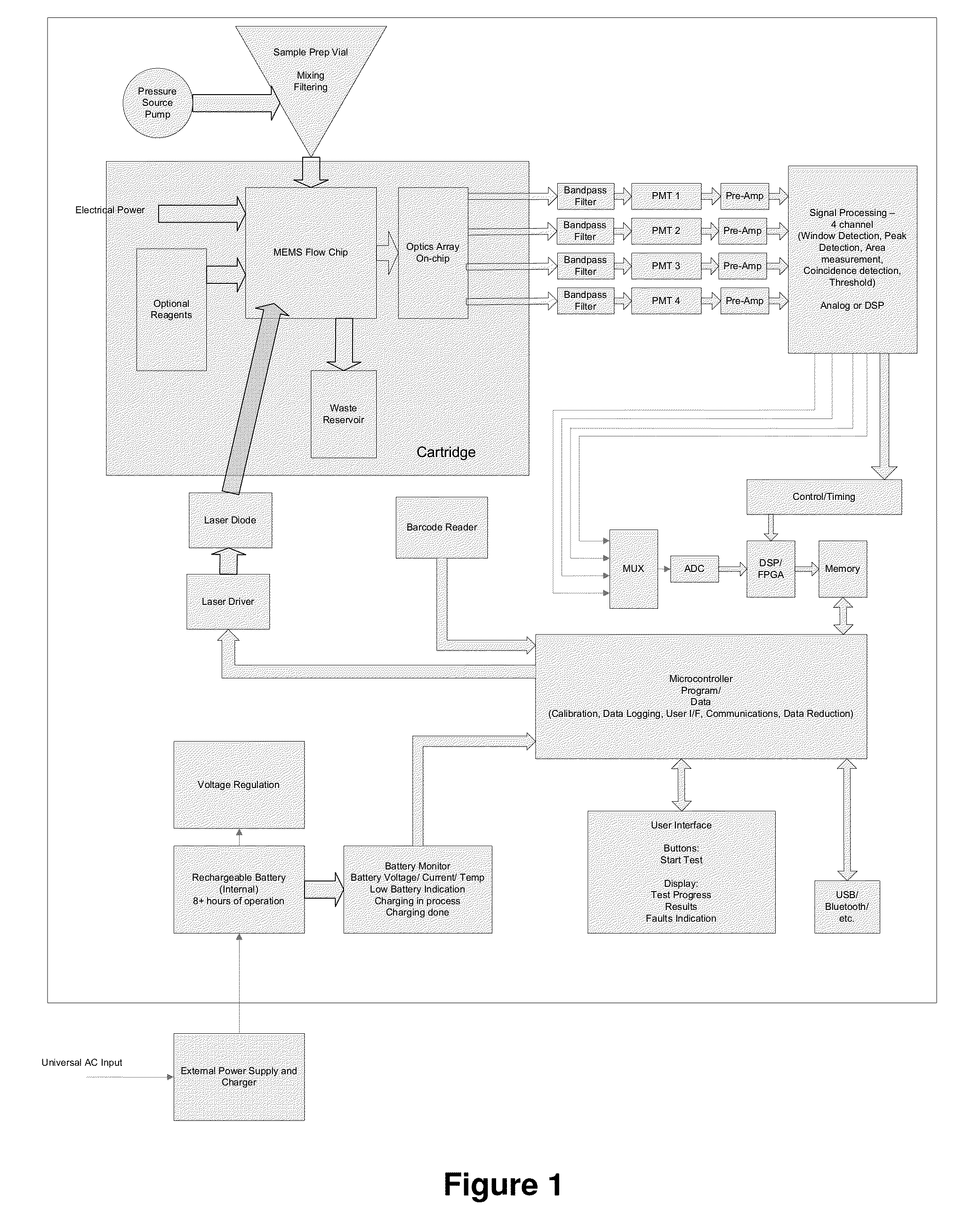
Uterine cervical cancer Computer-Aided-Diagnosis (CAD) according to this invention consists of a core processing system that automatically analyses data acquired from the uterine cervix and provides tissue and patient diagnosis, as well as adequacy of the examination. The data can include, but is not limited to, color still images or video, reflectance and fluorescence multi-spectral or hyper-spectral imagery, coherent optical tomography imagery, and impedance measurements, taken with and without the use of contrast agents like 3-5% acetic acid, Lugol's iodine, or 5-aminolevulinic acid. The core processing system is based on an open, modular, and feature-based architecture, designed for multi-data, multi-sensor, and multi-feature fusion. The core processing system can be embedded in different CAD system realizations. For example: A CAD system for cervical cancer screening could in a very simple version consist of a hand-held device that only acquires one digital RGB image of the uterine cervix after application of 3-5% acetic acid and provides automatically a patient diagnosis. A CAD system used as a colposcopy adjunct could provide all functions that are related to colposcopy and that can be provided by a computer, from automation of the clinical workflow to automated patient diagnosis and treatment recommendation.
Owner:STI MEDICAL SYST
Method and Devices for Screening Cervical Cancer
ActiveUS20080262384A1Bioreactor/fermenter combinationsBiological substance pretreatmentsCancer cellAnalysis method
The present invention provides multi-parameter analysis methods for determining the presence or absence of pre-cancerous or cancerous cells in a cervical sample and for screening cervical abnormality in a cervical sample. The invention also provides an apparatus and automated methods for screening cervical abnormality in a sample. The invention further provides a sampling device and a sample collection assembly for collecting cell samples, including cervical samples.
Owner:SOUTHWEST RES INST
Cervical exfoliated cell preservative fluid
InactiveCN101363011AReduce aggregationEfficient killingDead animal preservationTissue cultureRed blood cellCervical cancer screening
The invention relates to a cervical exfoliated cell preservative liquid, the preservative liquid comprises the following components with the contents (by weight): 20 percent to 50 percent of alcohols; 15 percent to 50 percent of anti-aggregation reagent; 5 percent to 10 percent of buffer solution; 1 percent to 20 percent of ion strength maintaining reagent; 0.01 percent to 0.5 percent of anti-microbial reagent; 0.1 to 5 percent of mucus dissolving reagent; and 0 to 0.5 percent of cleaning agent. Compared with the prior art, the preservative liquid can not only lead cells to maintain the shape in an in vitro liquid suspension environment, minimize the protein precipitation, dissolve larger protein substances, such as blood and mucus, and reduce the cell aggregation, but can also selectively eliminate or reduce red cells, effectively kill microbes, prevent the activity of reverse transcriptase and retain the integrity of nucleic acids and proteins for facilitating the later analysis; in addition, the preservative liquid can greatly reduce the costs of consumptive materials for the TCT detection, improve the sensitivity and the specificity of the cervical cancer screening and accelerate the promotion and the popularization of the TCT technology in a medical system.
Owner:SHANGHAI ADICON CLINICAL LAB LNC
Uterine cervical cancer computer-aided-diagnosis (CAD)
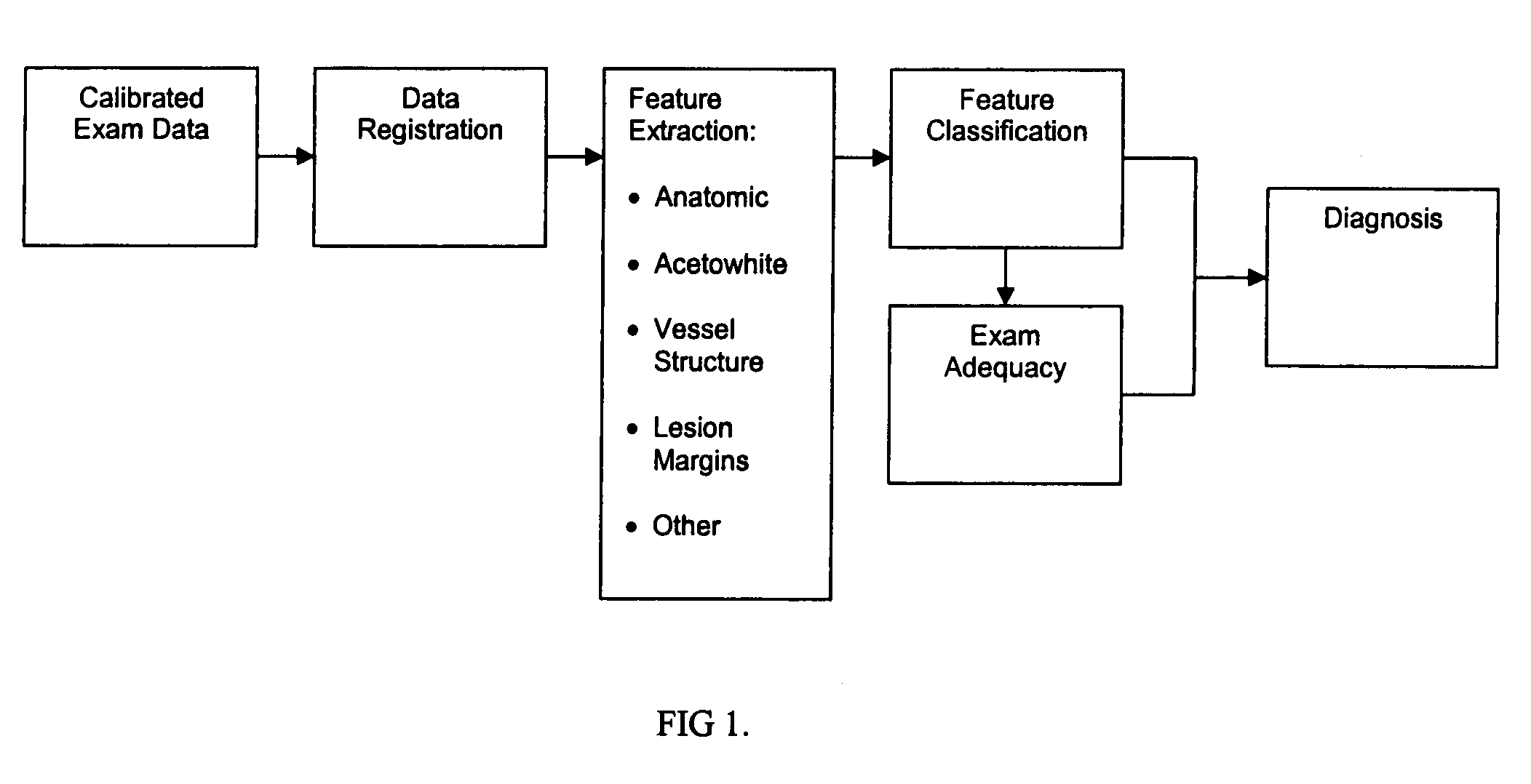
Uterine cervical cancer Computer-Aided-Diagnosis (CAD) according to this invention consists of a core processing system that automatically analyses data acquired from the uterine cervix and provides tissue and patient diagnosis, as well as adequacy of the examination. The data can include, but is not limited to, color still images or video, reflectance and fluorescence multi-spectral or hyper-spectral imagery, coherent optical tomography imagery, and impedance measurements, taken with and without the use of contrast agents like 3-5% acetic acid, Lugol's iodine, or 5-aminolevulinic acid. The core processing system is based on an open, modular, and feature-based architecture, designed for multi-data, multi-sensor, and multi-feature fusion. The core processing system can be embedded in different CAD system realizations. For example: A CAD system for cervical cancer screening could in a very simple version consist of a hand-held device that only acquires one digital RGB image of the uterine cervix after application of 3-5% acetic acid and provides automatically a patient diagnosis. A CAD system used as a colposcopy adjunct could provide all functions that are related to colposcopy and that can be provided by a computer, from automation of the clinical workflow to automated patient diagnosis and treatment recommendation.
Owner:STI MEDICAL SYST
Cervical acid phosphatase - papanicolaou (CAP-PAP) test kit, method and accesories, processes for producing and using the same
InactiveUS20040137551A1Improves cell defense mechanismMicrobiological testing/measurementMaterial analysisGynecologyCervical cancer screening
Cervical Acid Phosphatase-Papanicolaou Test Kit (CPK) is an assembly of reagents, controls and instructions for visualization of cervical acid phosphatase on smears or monolayers of cervical specimens, and for performing the CAP-PAP Test (CPT). CPT is a single-slide, double-staining method for demonstration of cervical acid phosphatase activity inside abnormal cervical cells on Papanicolaou stained smears, and a set of criteria for using this test for cervical cancer screening. In previous clinical trials this method was found to enable Pap test screeners to improve test sensitivity (detection of abnormal cells) for more than 10% (from 0.8 to 0.9), and to reduce false negative readings (missing abnormal cells) for more than 50% (from 0.1 to 0.02). Due to better accuracy and the low cost, when approved, CPT may begin to replace current technologies for cervical cancer screening. CPK is designed to meet requirements for testing large series of specimens on regular basis-the usual practice in cytopathology laboratories performing the Pap test. CPK brings consistency for staining and interpretation, makes internal and external controls easier, and improves the test accuracy for lower cost, while increases laboratory productivity for less liability.
Owner:MARKOVIC NENAD S +1
Method and Apparatus for Cervical Cancer Screening
InactiveUS20120232408A1Reduce fluorescence signalLow costEndoscopesDiagnostics using fluorescence emissionOptical testMedicine
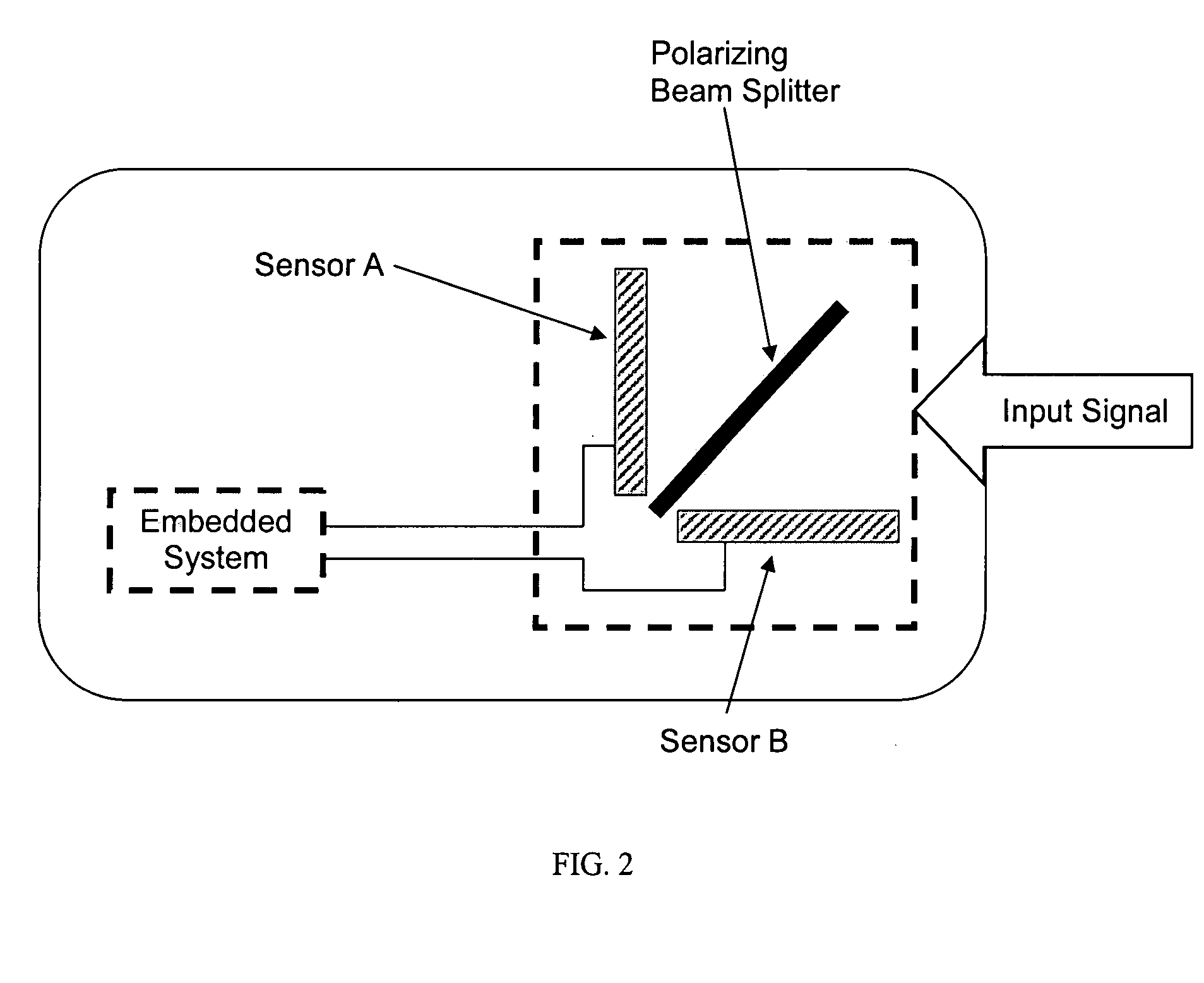
The present invention relates to an apparatus for cervical cancer screening, comprising one or more light sources aligned with a beginning of a first optical test path and a beginning of a second optical test path, one or more optical detectors aligned with an end of the first optical test path and an end of the second optical test path, and a processor coupled to the one or more light sources and the one or more optical detectors and methods for using the same. The present invention further relates to a method for cervical cancer screening.
Owner:WELLER BROPHY LAURA ANN
Composition and reagent kit for early cervical cancer detection
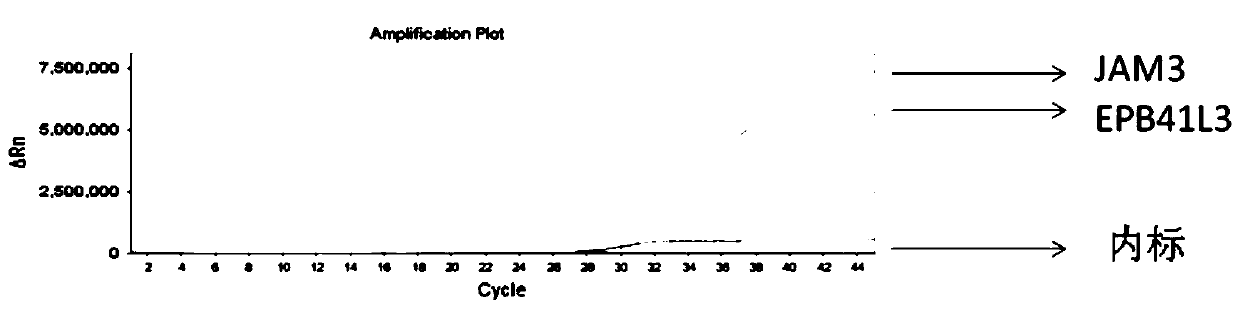
ActiveCN110564857AIncrease coverageHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationCervical cancer screeningNon invasive
The invention discloses a composition and reagent kit for early cervical cancer detection. The composition comprises 3 CpG island regions subjected to high methylation in an EPB41L3 gene and 3 CpG island region amplifying primers subjected to high methylation in a JAM3 gene, 98% or above of different types of cervical cancer can be covered, and detection of polygene polymethylation regions can berealized through a one-pipe reaction and detection. A specific MGB probe and a degenerate closed primer are preferably selected for cooperation, so that sensitivity and accuracy are further increased.The whole detection process is free from wounds, simple and convenient to operate and easy to interpret, universal qPCR equipment can meet detection, a wholly-closed form is adopted in the PCR process, and the possibility of cross-contamination can be avoided; besides, three segments of two markers are detected at the same time, so that the result is more accurate; and all the factors are integrated, so that the composition disclosed by the invention has social promotion properties. Due to high sensitivity of detection, the composition is suitable for non-invasive early cervical cancer screening.
Owner:北京鑫诺美迪基因检测技术有限公司
Apparatus and method of personal screening for cervical cancer conditions in vivo
InactiveUS20040068162A1Early cancer detectionData generationSurgeryVaccination/ovulation diagnosticsGynecologyMenstrual cycle
A method and apparatus for personal screening for early signs of cervical cancer is claimed, whereby the user performs daily or almost daily a diagnostic self-check for some other aspect of reproductive health, and the electronic testing for cervical cancer type of tissue aberration is performed automatically in the background. The screening is invisible to the user, causing no anxiety and no discomfort. The user only becomes alerted to the need to see a physician if a preset condition of reproducibility is reached in the background evaluation of the measurement data if the aberrant pattern has been detected consecutively in a preset number of menstrual cycles, the device prompts the woman to see a physician with a view to undergoing a more demanding definitive diagnostic examination such as colposcopy with biopsy. The invention provides the diagnostic screen in a manner that does not cause the discomfort, anguish and anxiety associated with the Pap smear screen of the prior art.
Owner:KIRSNER VACLAV
Cervical cancer cell detection kit
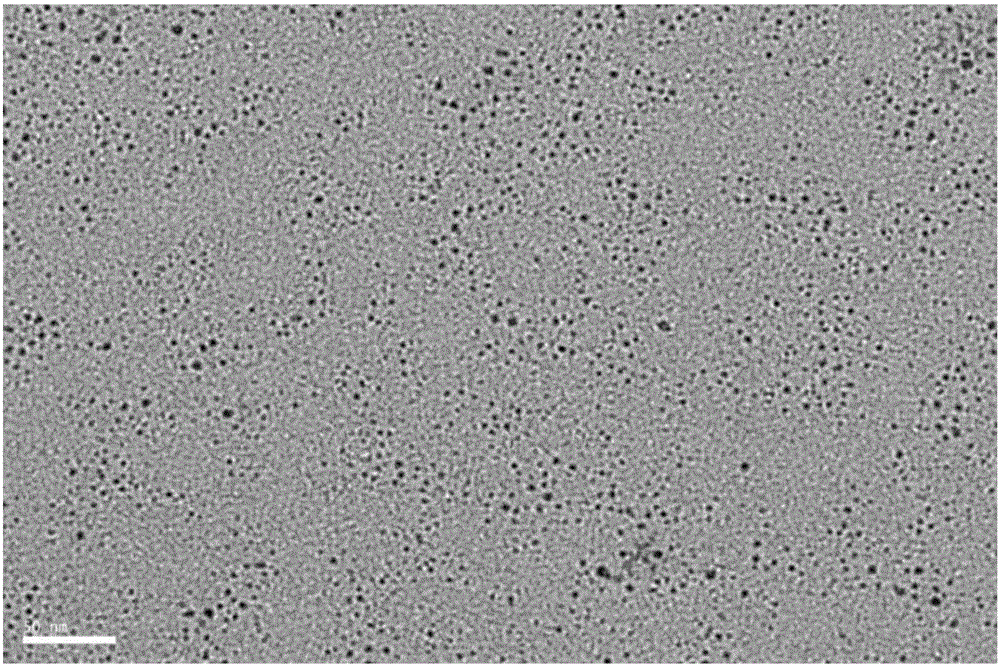
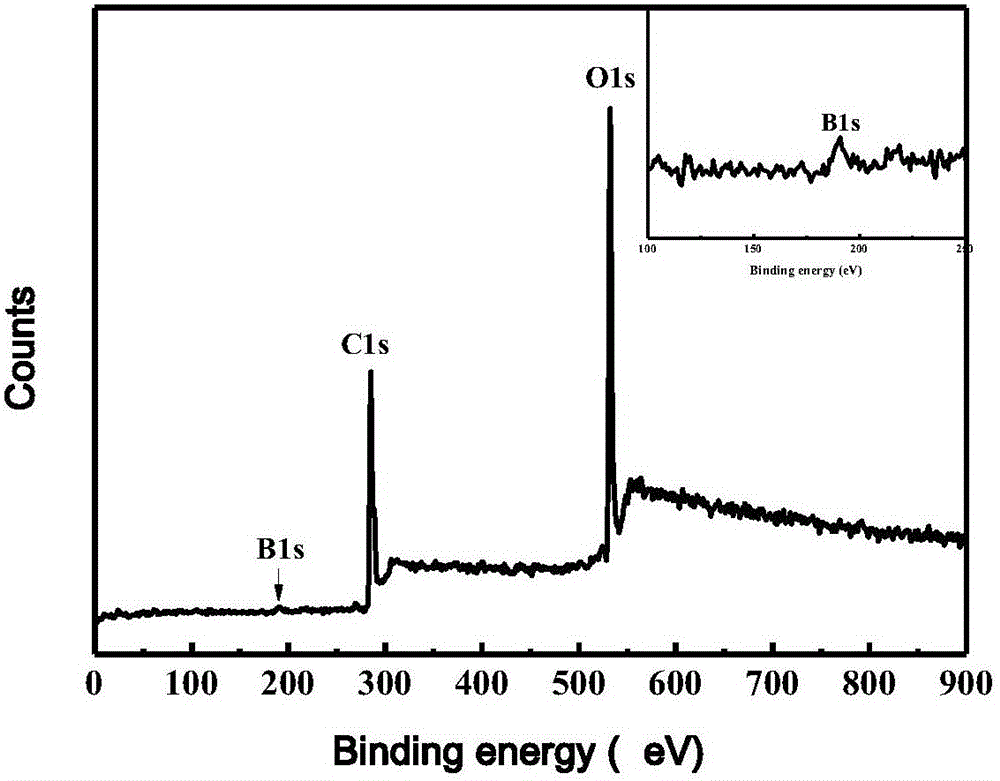
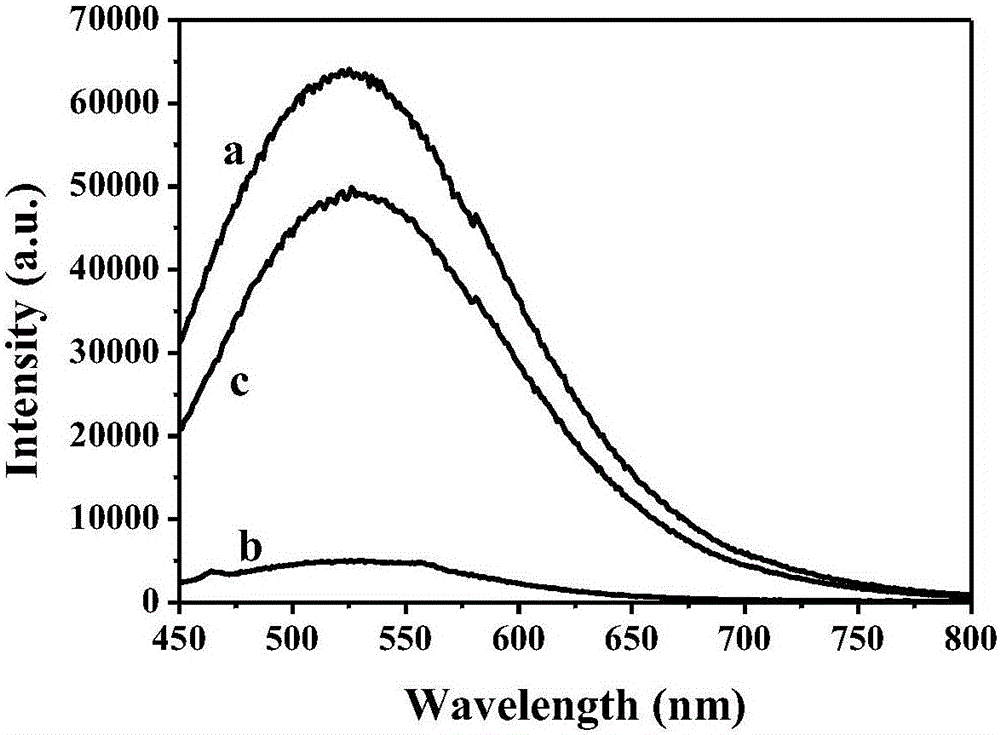
ActiveCN105886596AHigh quantum yieldHigh quantum yield, good for detection sensitivityMicrobiological testing/measurementBiological material analysisCerium nitrateDoped graphene
The invention discloses a cervical cancer cell detection kit based on a boron-doped graphene quantum dot and belongs to the field of biological detection. The cervical cancer cell detection kit comprises reagents A, B and C. The reagent A is a boron-doped graphene quantum dot solution, the reagent B is a cerium nitrate solution, and the reagent C is an adenosine triphosphate solution. Alkaline phosphatase is selected for the kit to serve as a detected targeted goal, the boron-doped graphene quantum dot is adopted as a fluorescent probe, alkaline phosphatase on the surfaces of cervical cancer cells is detected quickly and efficiently through a quantum dot fluorescence analysis method, and the kit can be used in the medical field to detect the cervical cancer cells and concentration thereof. The kit has the advantages of being easy to operate, high in detection result accuracy and sensitivity and low in cost and the like.
Owner:NANJING NORMAL UNIVERSITY
Clinical decision-making system and method for cervical lesions
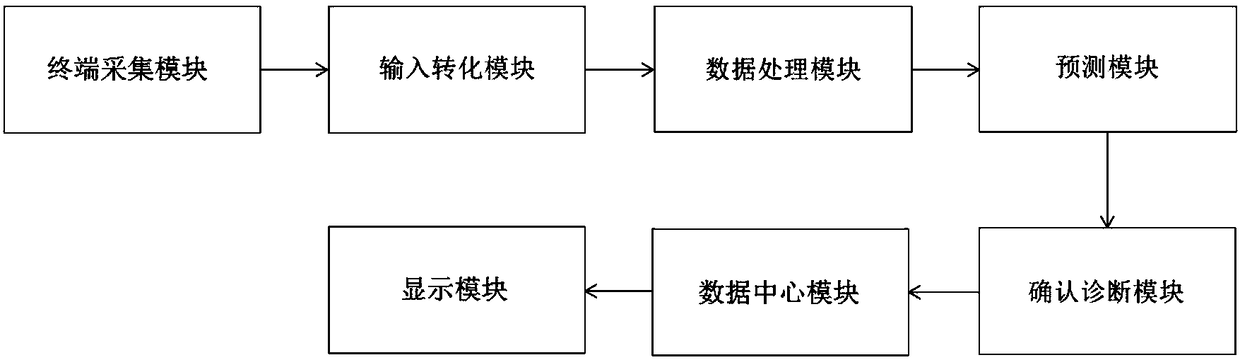
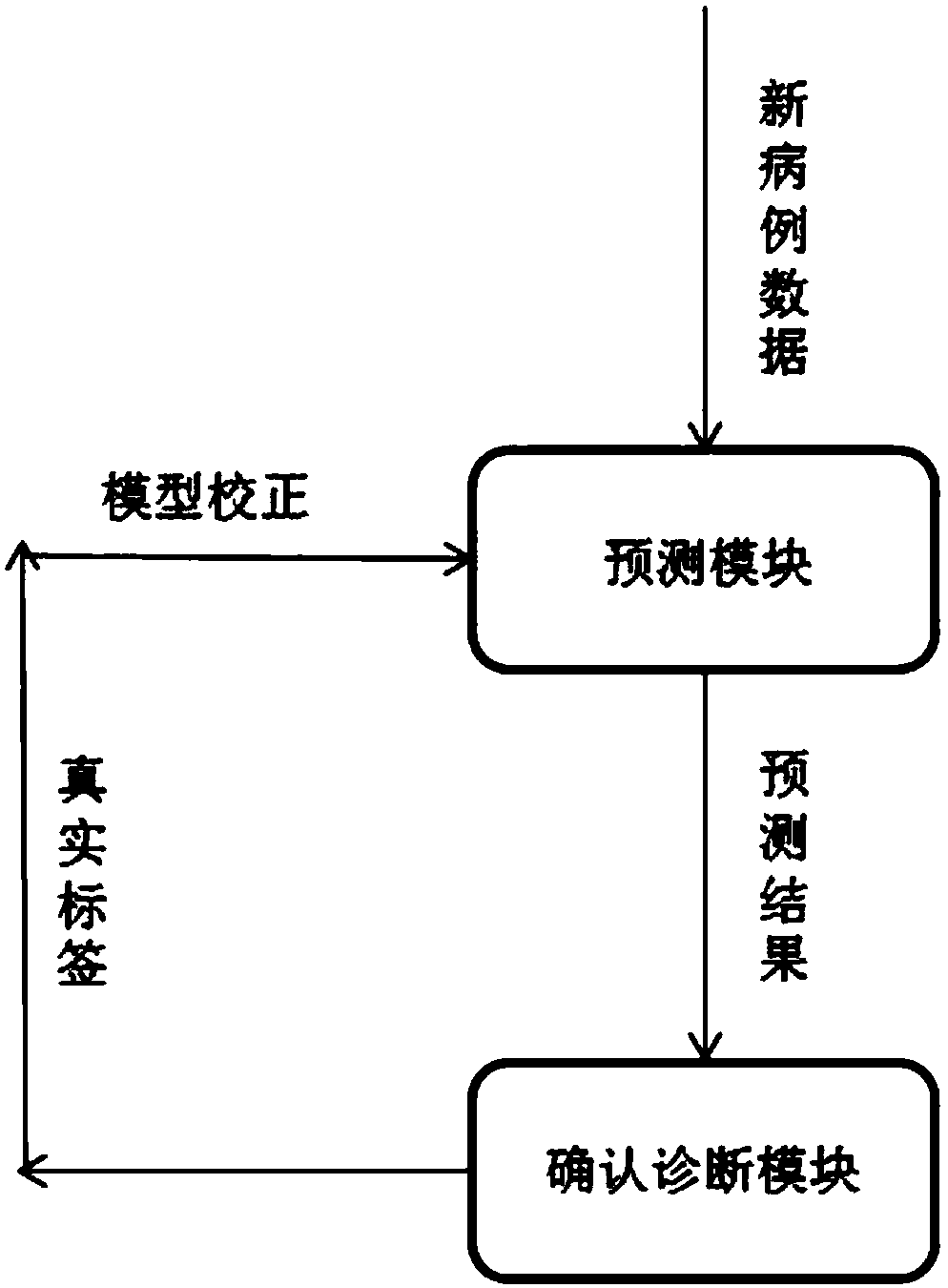
PendingCN108565017AAvoid controversyIncrease initiativeMedical automated diagnosisMedical equipmentCervical lesionData center
The invention discloses a clinical decision-making system and method for cervical lesions. The clinical decision-making system comprises: a terminal acquisition module for acquiring case data of a patient; an input conversion module for extracting the case data of the patient and converting into characteristic variables; a data processing module for respectively performing discretization, sparse coding and binarization processing on the characteristic variables; a prediction module for outputting prediction results after the processed characteristic variables are subjected to weight and offsetdimension assignment processing, rectification function relu transformation and softmax regression operation; a conformation and diagnosis module for confirming final clinical diagnosis results and providing true labels for the prediction results; a data center module for receiving and storing all case data of the patient and periodically iterating the prediction module based on all data; and a display module for displaying the prediction results, the diagnosis results and health guidance advice. The clinical decision-making system and method provided by the invention can improve specificityand accuracy of a current cervical cancer screening strategy, and realize big data sharing and integrated network management of cervical cancer screening.
Owner:明兰世迦(北京)医疗科技有限公司
Apparatus and method for ovarian cancer screening
InactiveUS20150057565A1Low complication rateIncrease volumeBalloon catheterSurgical needlesCervical cancer screeningPeritoneal cavity
An apparatus and method are provided for sampling the distal tube, fimbria and / or ovary, includes advancing a device into the peritoneal cavity and sampling material on or adjacent to the distal tube, fimbria or ovary.
Owner:MKT ENTERPRISES
Detection of Human Papillomavirus
InactiveUS20070292841A1Avoid developmentUnstable cell activity/genomeMicrobiological testing/measurementE6 proteinHigh-Grade Squamous Intraepithelial Lesions
The present invention relates to in vitro methods of screening human subjects for the presence of human papillomavirus (HPV) which exhibits loss of regulation of E6 / E7 mRNA expression and loss of replication and / or expression of a stabilized pre-mRNA encoding full length E6 protein. In particular, the invention provides in vitro methods of screening for persistent cell abnormalities or persistent CIN III lesions, cancer in situ or high-grade squamous intraepithelial lesions (HSIL). The methods are useful in the context of cervical cancer screening.
Owner:NORCHIP AS
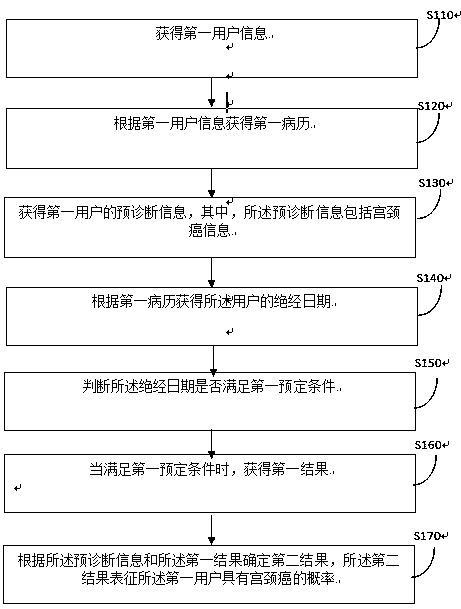
Artificial intelligence cervical cancer screening determination method and device
InactiveCN109887561AIntegrity guaranteedEasy to analyze and judgeMedical communicationHealth-index calculationSystem integrationMedical record
The invention provides an artificial intelligence cervical cancer screening determination method and device. The artificial intelligence cervical cancer screening determination method includes the steps: obtaining first user information; obtaining a first medical record according to the first user information; obtaining pre-diagnosis information of the first user, wherein the pre-diagnosis information includes cervical cancer information; obtaining the menopause date of the user according to the first medical record; determining whether the menopause date satisfies a first predetermined condition; obtaining a first result when the first predetermined condition is satisfied; and determining a second result based on the pre-diagnosis information and the first result, wherein the second result represents the probability of suffering from cervical cancer for the first user. The artificial intelligence cervical cancer screening determination method solves the technical problems that in theprior art, manual detection is required, and many factors that are subject to human influence exist, and the medical data of the patient is fragmented, thus being not conducive to data analysis. The artificial intelligence cervical cancer screening determination method achieves system integration of the patient data, thus being beneficial to management analysis, and the analysis process is completed automatically, without human factors, thus achieving technical effect with higher reliability.
Owner:北京倍肯恒业科技发展股份有限公司
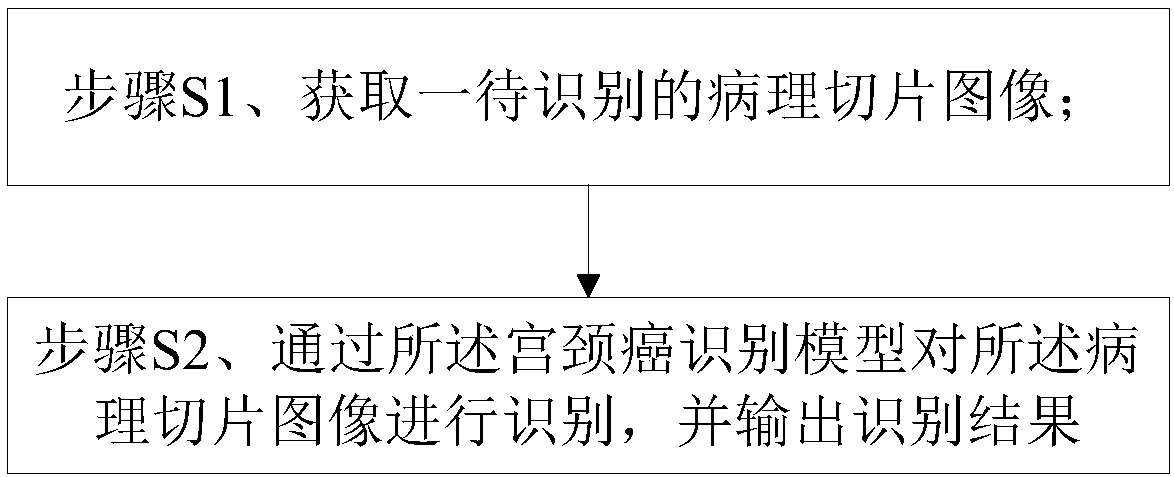
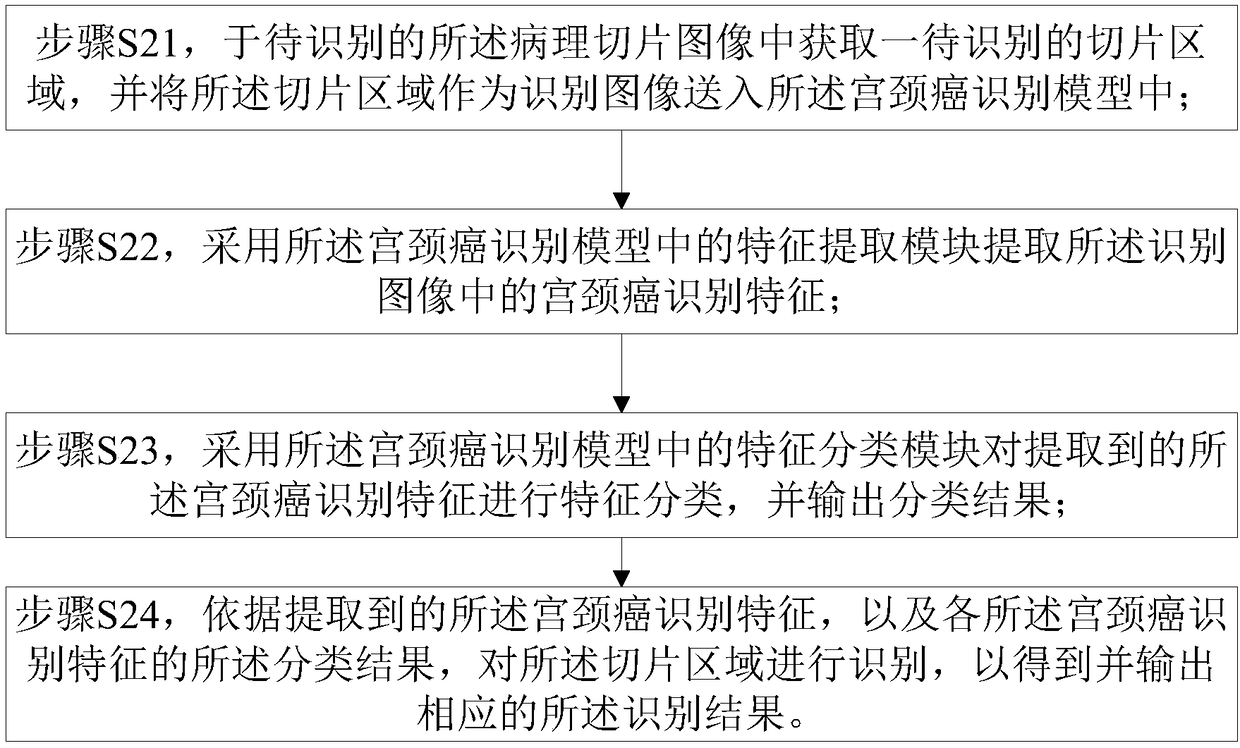
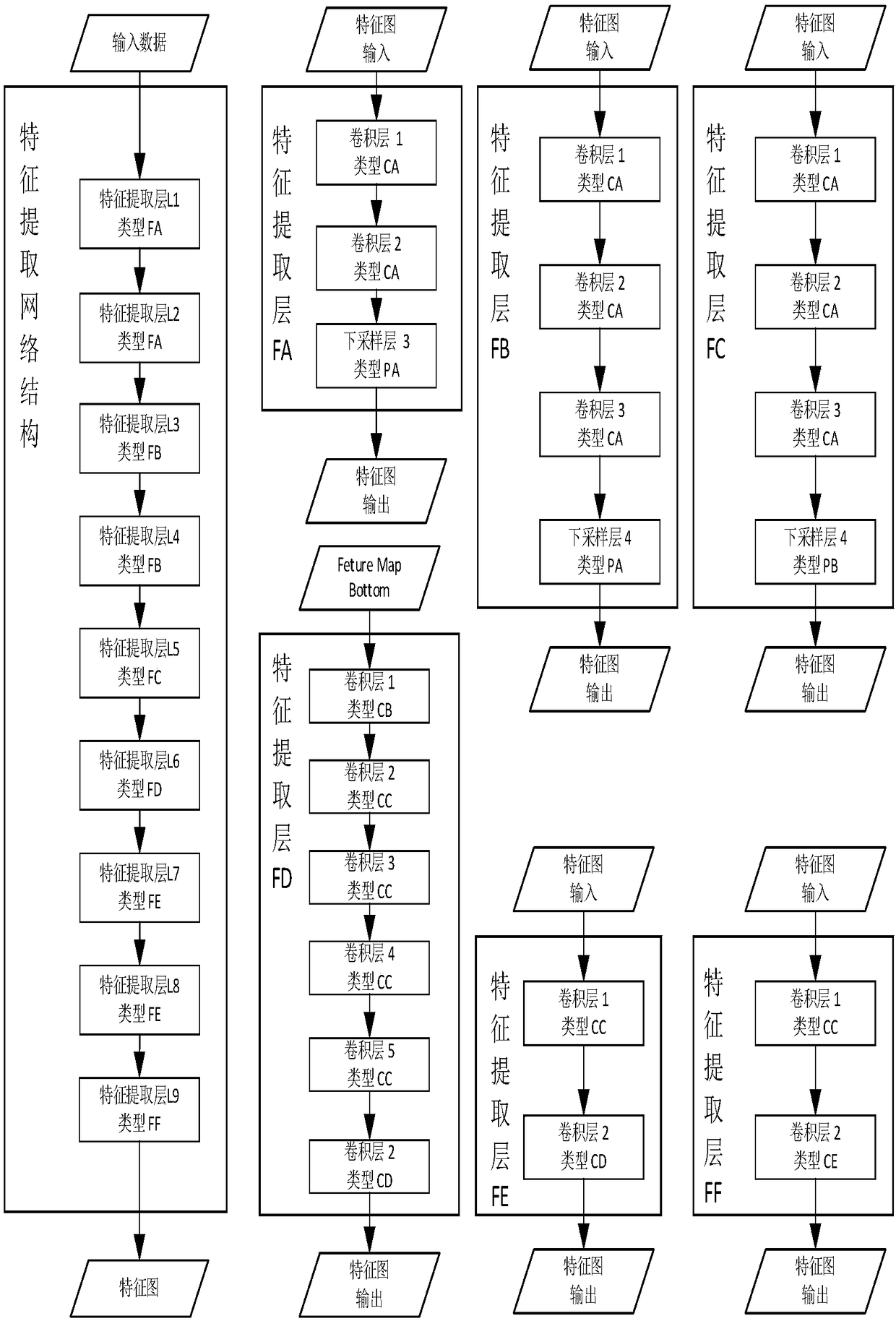
Cervical cancer section recognition method
InactiveCN108345871ARapid positioningReduce redundant informationBiometric pattern recognitionMedicineCervical cancer screening
The invention provides a cervical cancer section recognition method which comprises the following steps of performing the pre-training to form a cervical cancer recognition model according to the pre-prepared training sample associated with a cervical cancer section; and further comprises the following steps of acquiring a pathological section image to be recognized; recognizing the pathological section image through the cervical cancer recognition model, and outputting the recognition result, wherein the recognition result comprises the number of the cervical cancer cells in the pathologicalsection image and the positions of the cervical cancer cells in the pathological section image. The method has the beneficial effects that the suspected cervical cancer cells in the cervical cancer image can be quickly positioned, redundant information is effectively reduced, and the workload in the recognition process is reduced; the digital cervical cancer image can be subjected to statistics instead of a traditional artificial visual mode, the artificial diagnosis process is assisted, and the artificial diagnosis efficiency is greatly improved.
Owner:KONFOONG BIOTECH INT
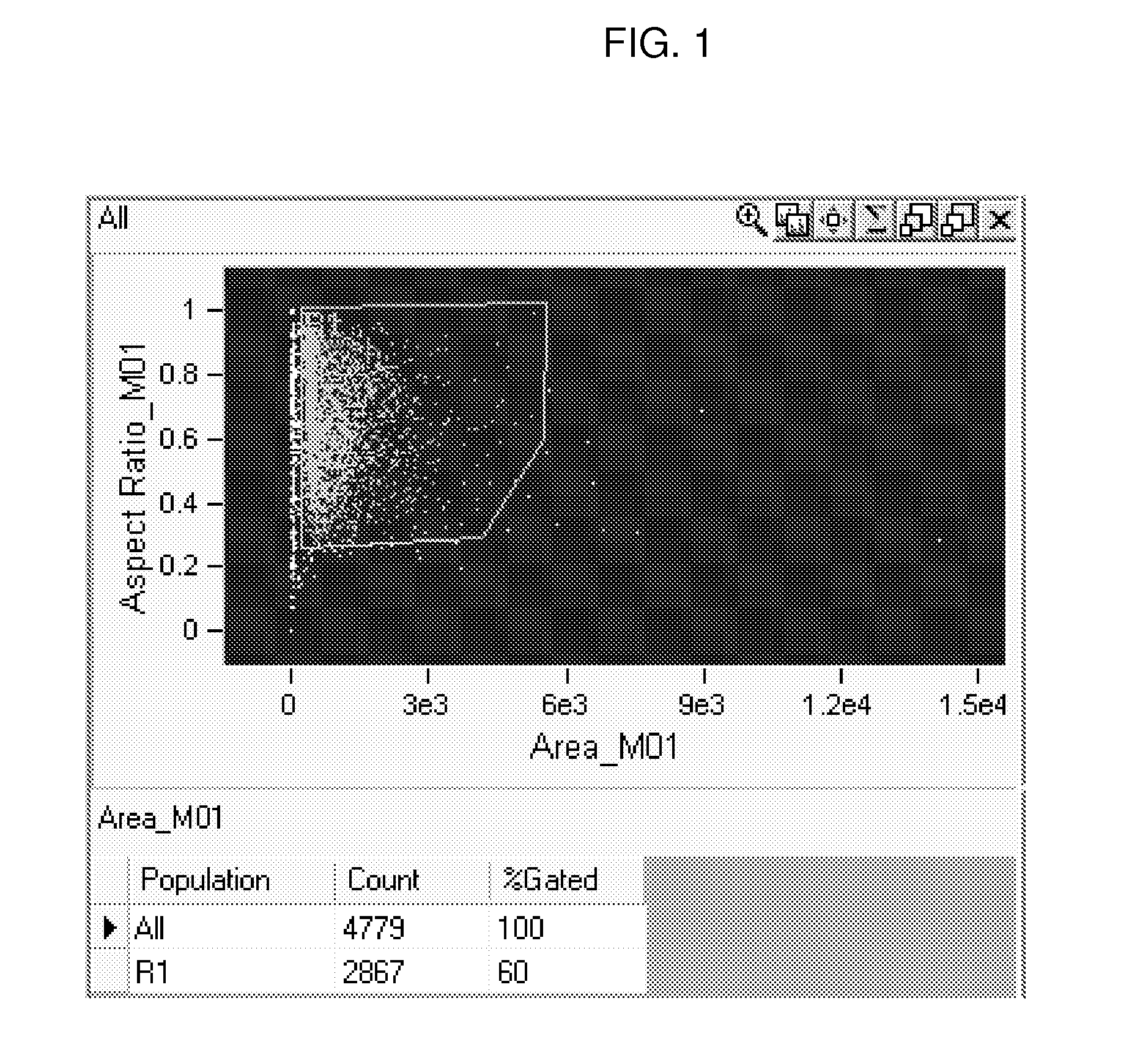
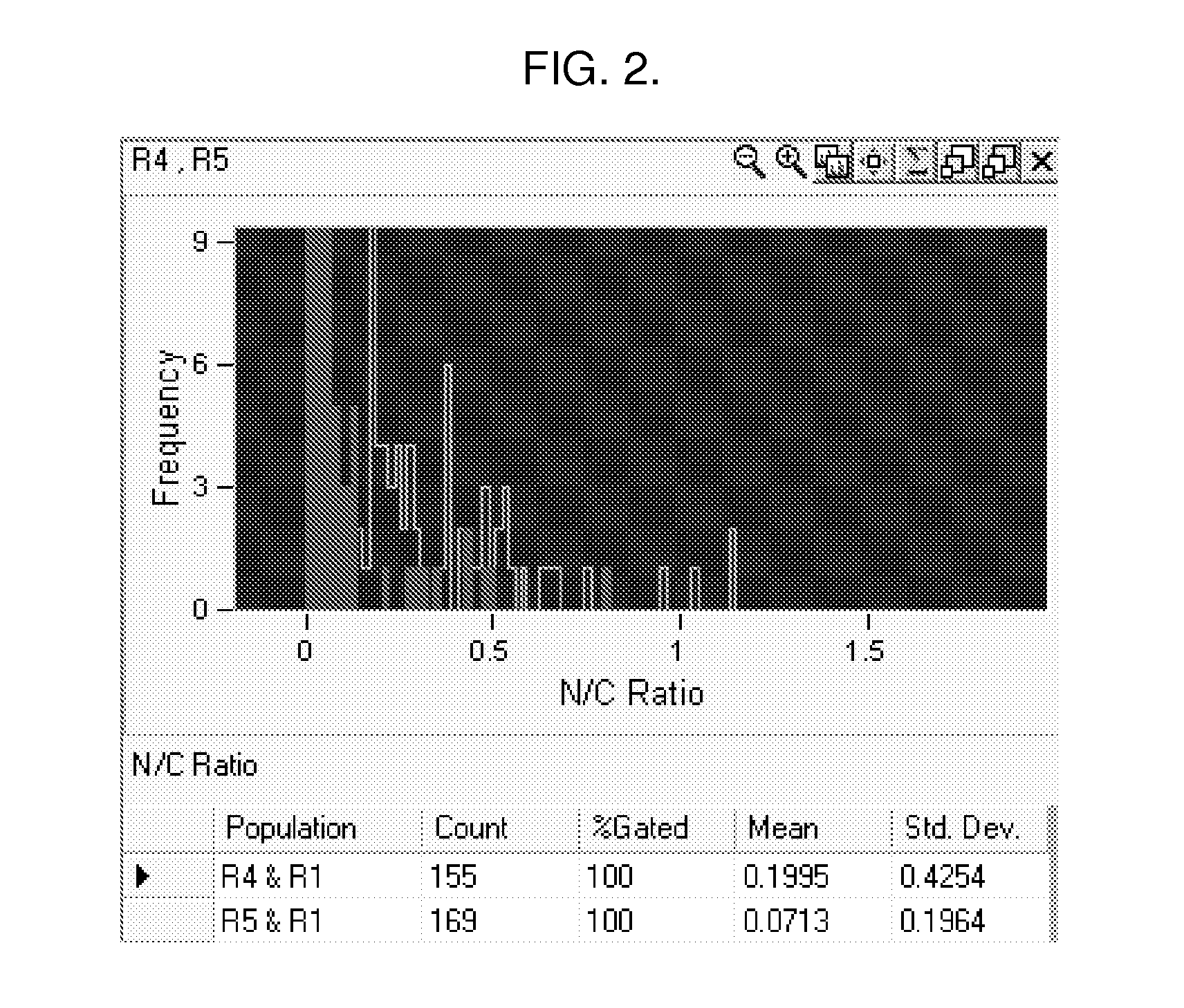
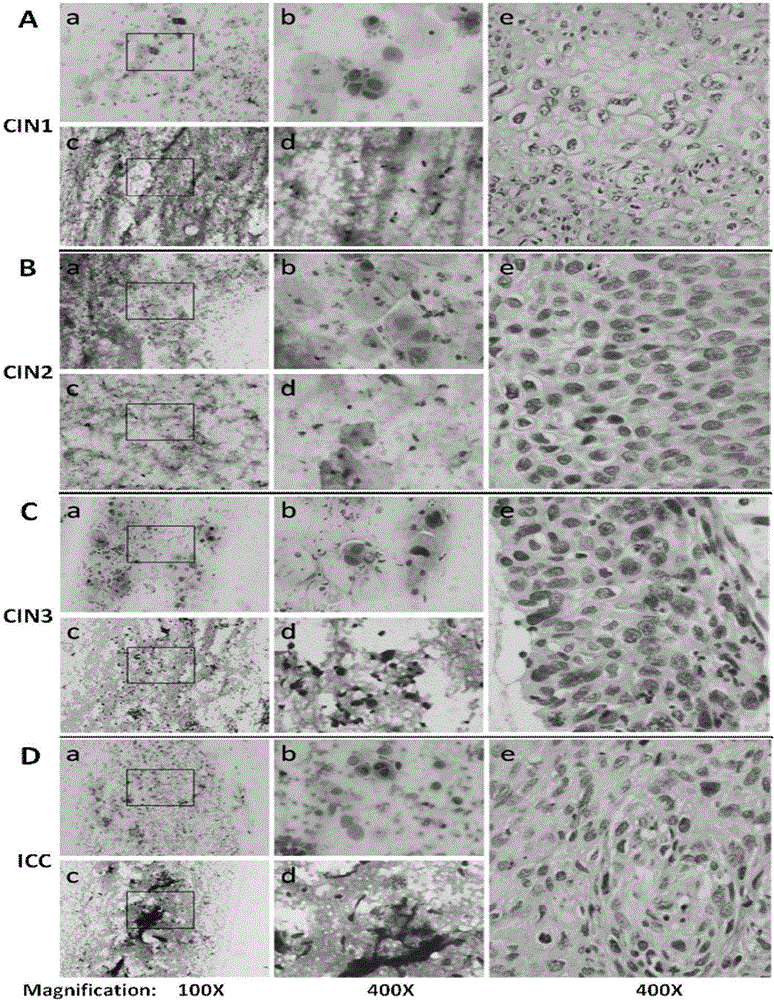
Methods and Systems for Predicting Whether a Subject Has a Cervical Intraepithelial Neoplasia (CIN) Lesion from a Suspension Sample of Cervical Cells
ActiveUS20150259756A1Bioreactor/fermenter combinationsBiological substance pretreatmentsCervical cellCervical cancer screening
Methods of predicting whether a subject has a cervical intraepithelial neoplasia (CIN) lesion are provided. Aspects of the methods include obtaining both morphometric and biomarker data from a liquid cervical cellular sample and then using both types of data to predict whether the subject has a CIN lesion. Also provided are systems that find use in practicing the methods. The methods and systems find use in a variety of applications, including cervical cancer screening applications.
Owner:INCELLDX
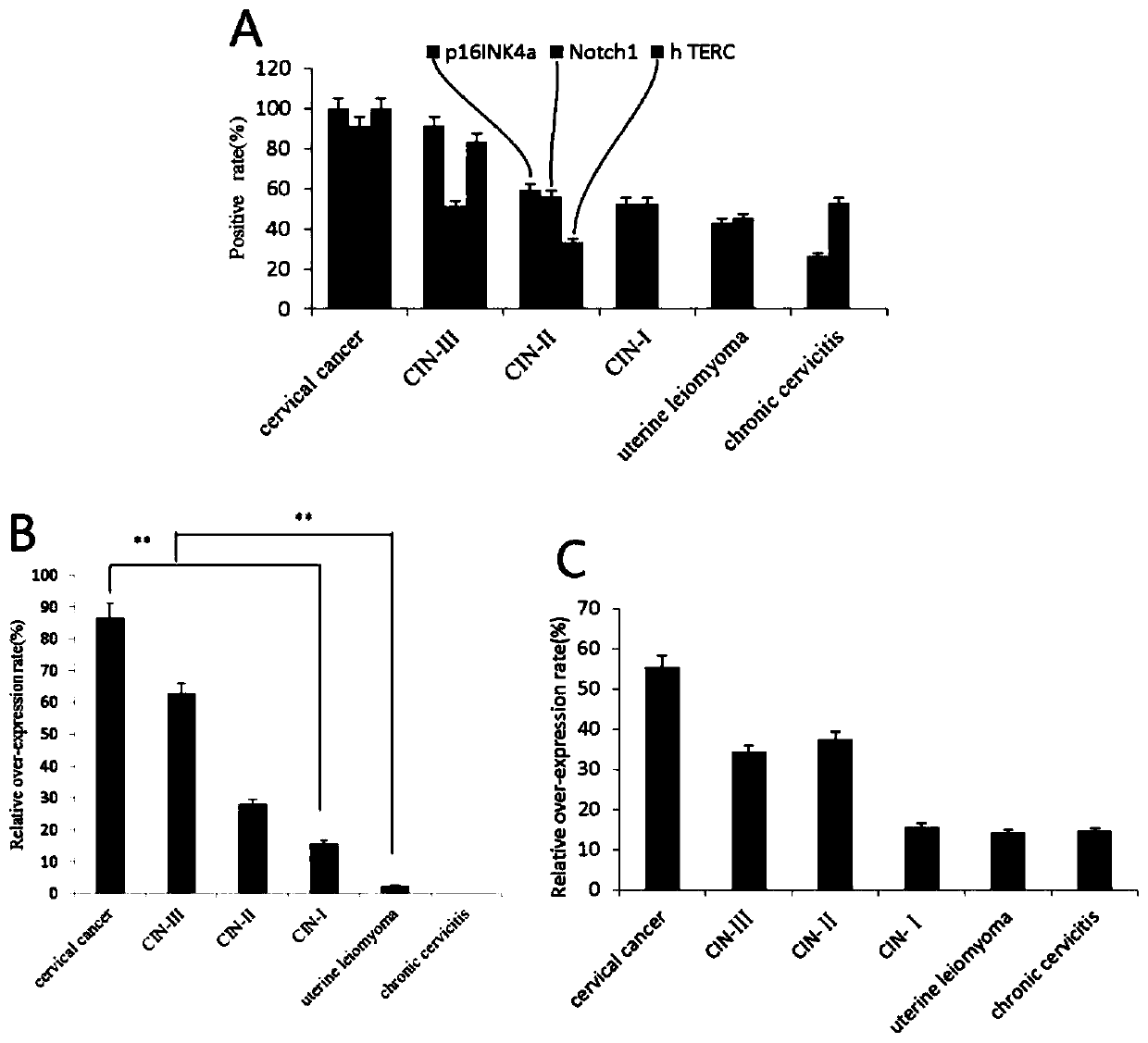
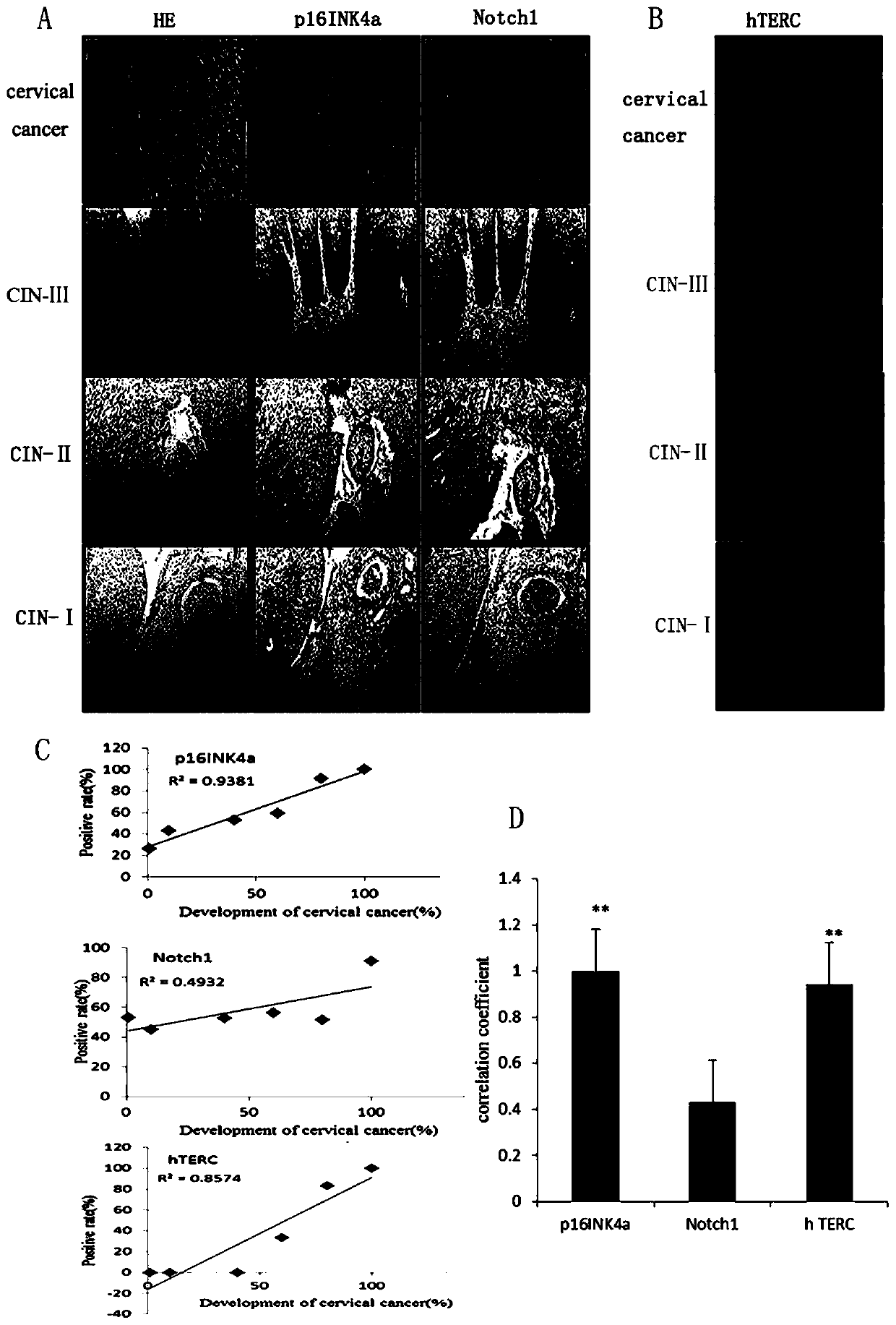
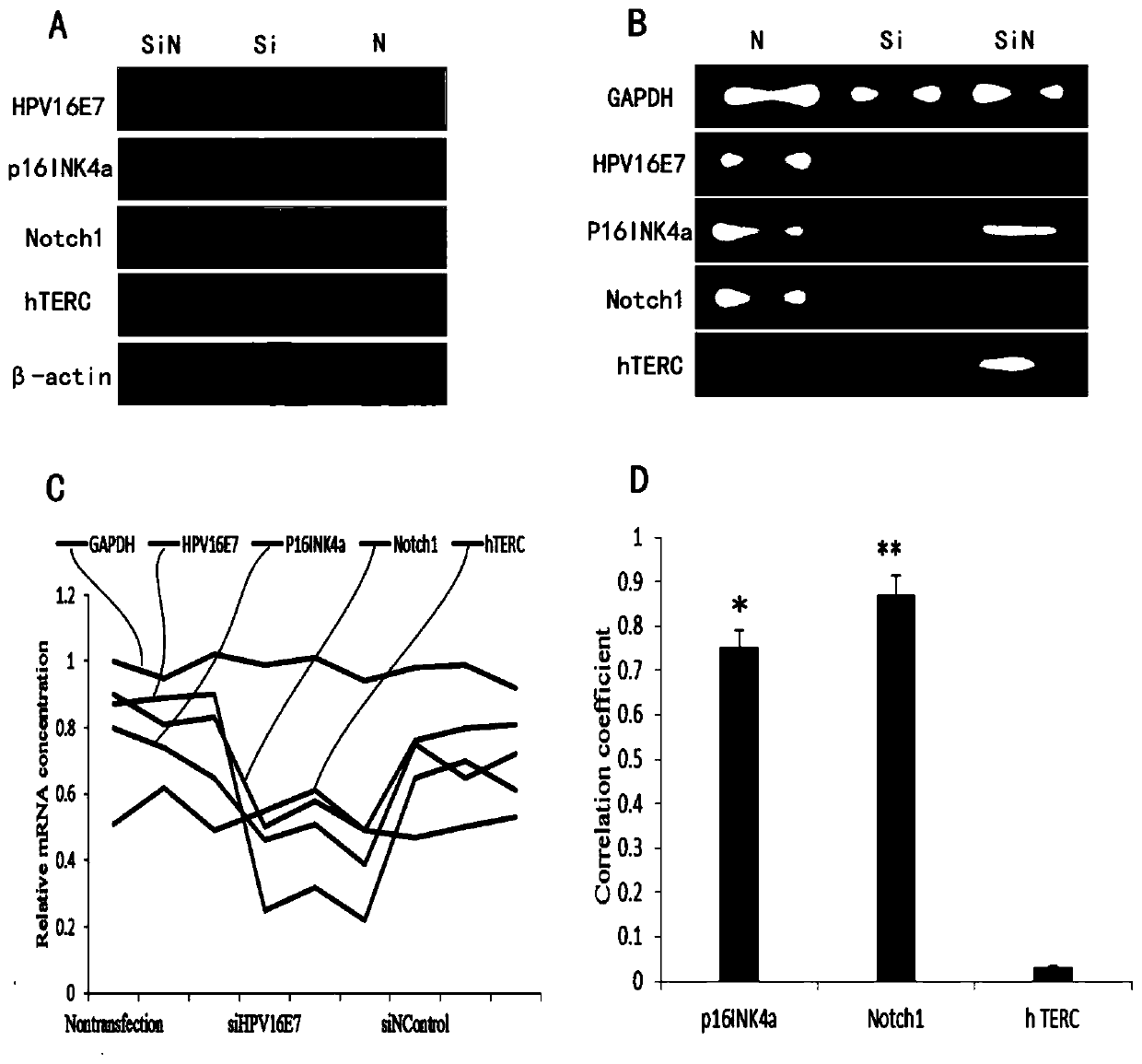
Application of Notch1, p16<INK4a> and hTERC genes as screening markers for cervical cancer
The invention belongs to the technical field of clinical medicine, provides application of Notch1, p16<INK4a> and hTERC genes as screening markers for cervical cancer, and relates to molecular markersfor detecting double or triple genes of cervical precancerous lesion and early diagnosis of the cervical cancer. Patients with cervical disease have no significant symptoms in the early stages of onset, and are easily overlooked and fail to be treated in time, so that the risk of the disease and the difficulty of treatment are increased. It is controversial to use p16<INK4a> protein as a diagnostic screening index for the cervical cancer, and the credibility is not high. The invention provides a set of markers, namely, the application of the Notch1, p16<INK4a> and hTERC genes as the screeningmarkers for the cervical cancer, positive correlation exists between p16<INK4a>, Notch1 and hTERC genes and carcinogenesis of HPV16, and significant positive correlation exists between p16<INK4a> andhTERC genes and the carcinogenesis of the HPV16, and the markers can be used for application of molecular marker reagents for detecting double or triple genes of cervical precancerous lesion and early diagnosis or screening of the cervical cancer.
Owner:INNER MONGOLIA UNIV FOR THE NATITIES
AI (artificial intelligence) cervical cancer screening system
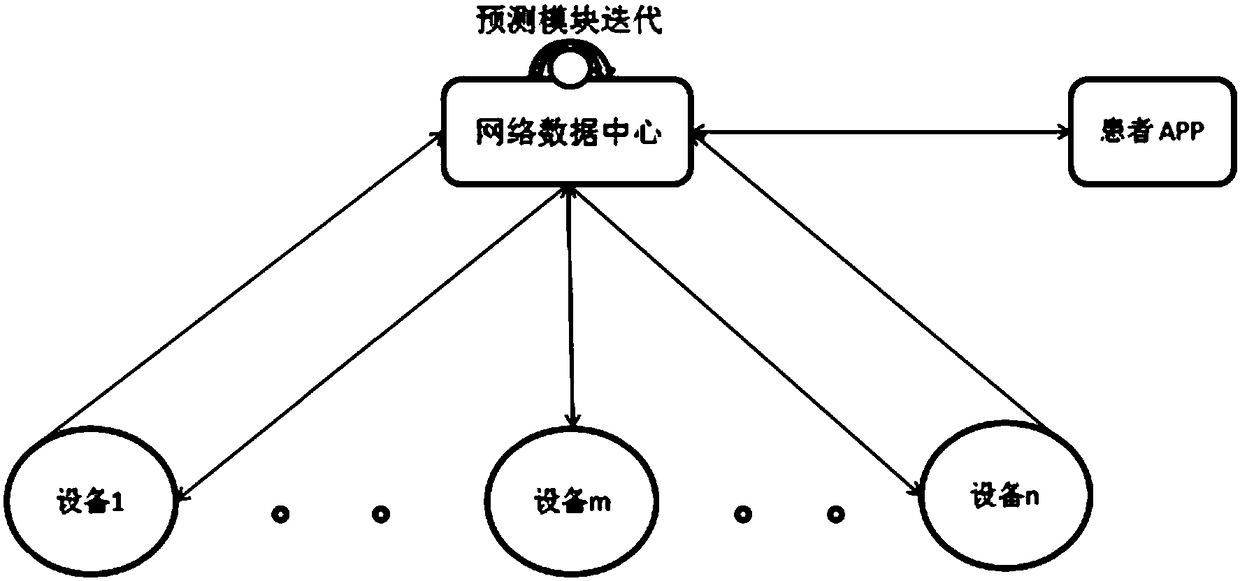
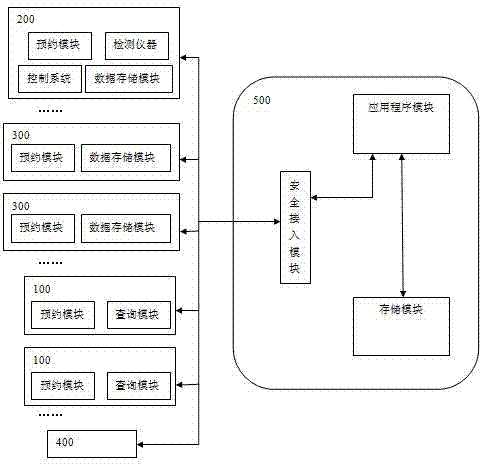
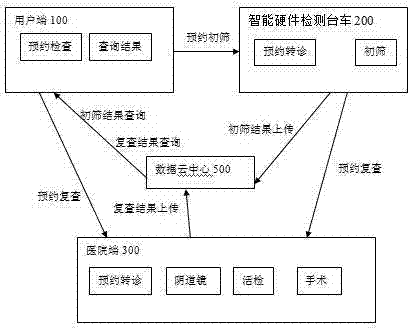
InactiveCN107958295AImprove the efficiency of medical treatmentReduced visit efficiencyReservationsHealthcare resources and facilitiesCervical cancer screeningComputer terminal
An artificial intelligence cervical cancer screening system includes at least one user terminal, at least one intelligent hardware detection trolley, at least one hospital terminal, an intelligent management terminal and a data cloud center, the at least one user terminal, at least one intelligent hardware detection platform The car, at least one hospital terminal and the intelligent management terminal are all networked and connected to the data cloud center through the network. The system has high efficiency in seeing a doctor. Patients can make appointments for various examinations and seeing a doctor through the user terminal, which shortens the efficiency of seeing a doctor and saves waiting time for patients. Moreover, this system realizes the sharing and query of all data, which facilitates the patient's experience in seeing a doctor, and also provides patient data for statistical management by the superior health management department.
Owner:北京倍肯恒业科技发展股份有限公司
Cervical cancer screening kit
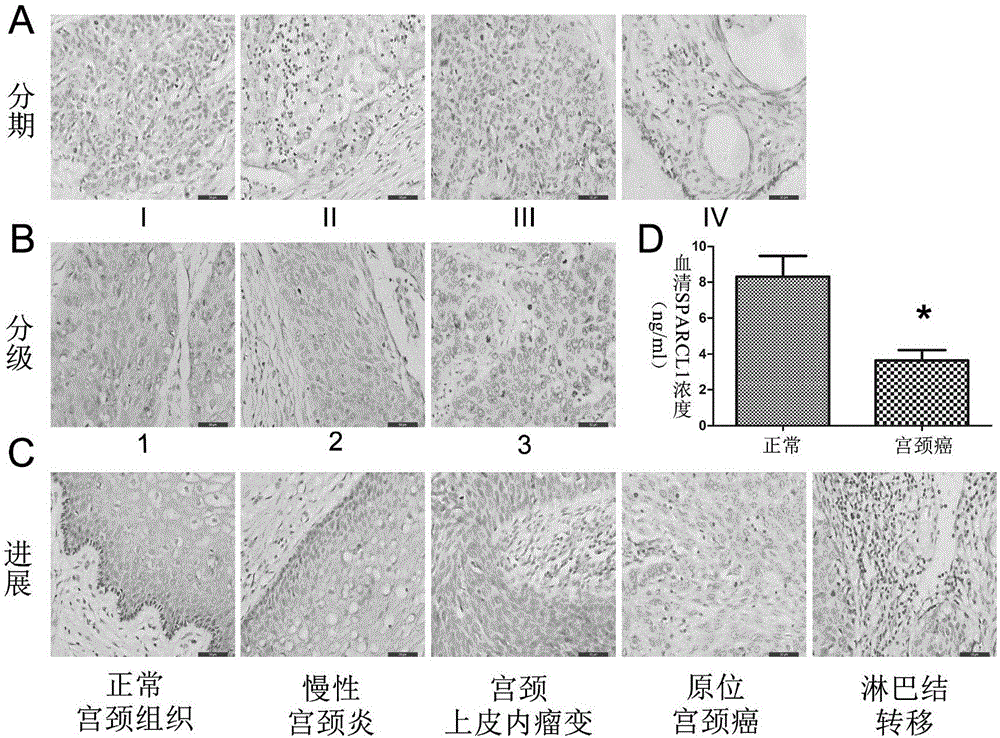
The invention discloses a cervical cancer screening kit, comprising an optional reagent to detect expression level of SPARCL1 in blood. The invention also discloses the application of the reagent to detect the expression level of SPARCL1 in blood, in the preparation of cervical cancer screening reagents. The cervical cancer screening kit can effectively judge the risk of people under test to suffer cercal cancer by detecting the expression level of SPARCL1, is applicable to the clinical assisted diagnosis of cervical cancer, provides effective basis for taking related treatment measures or making decisions for patients, and has a good clinical application prospect.
Owner:成都医学院第一附属医院
Cervical cancer precancerous lesion screening method combining spectrums and images
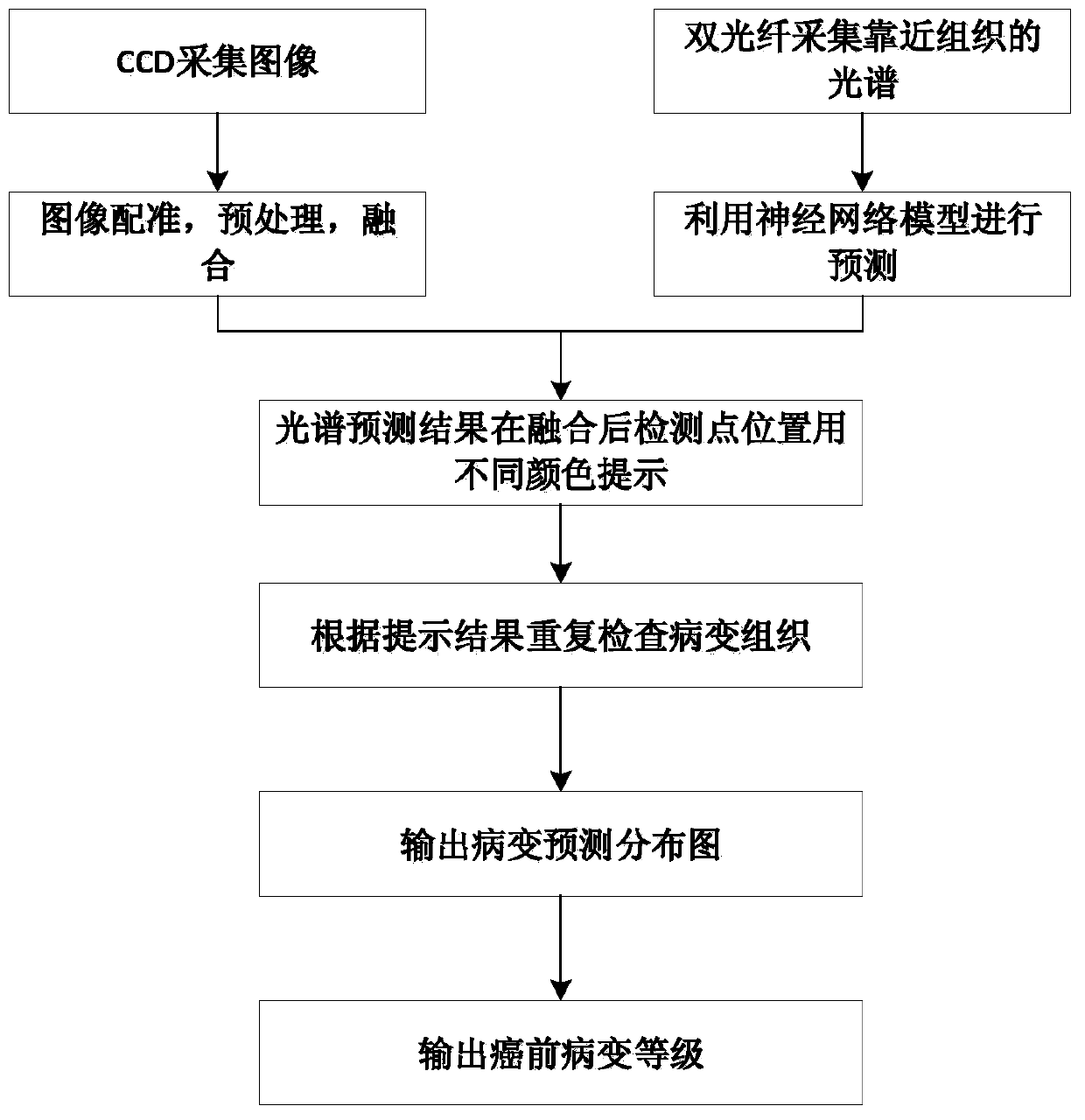
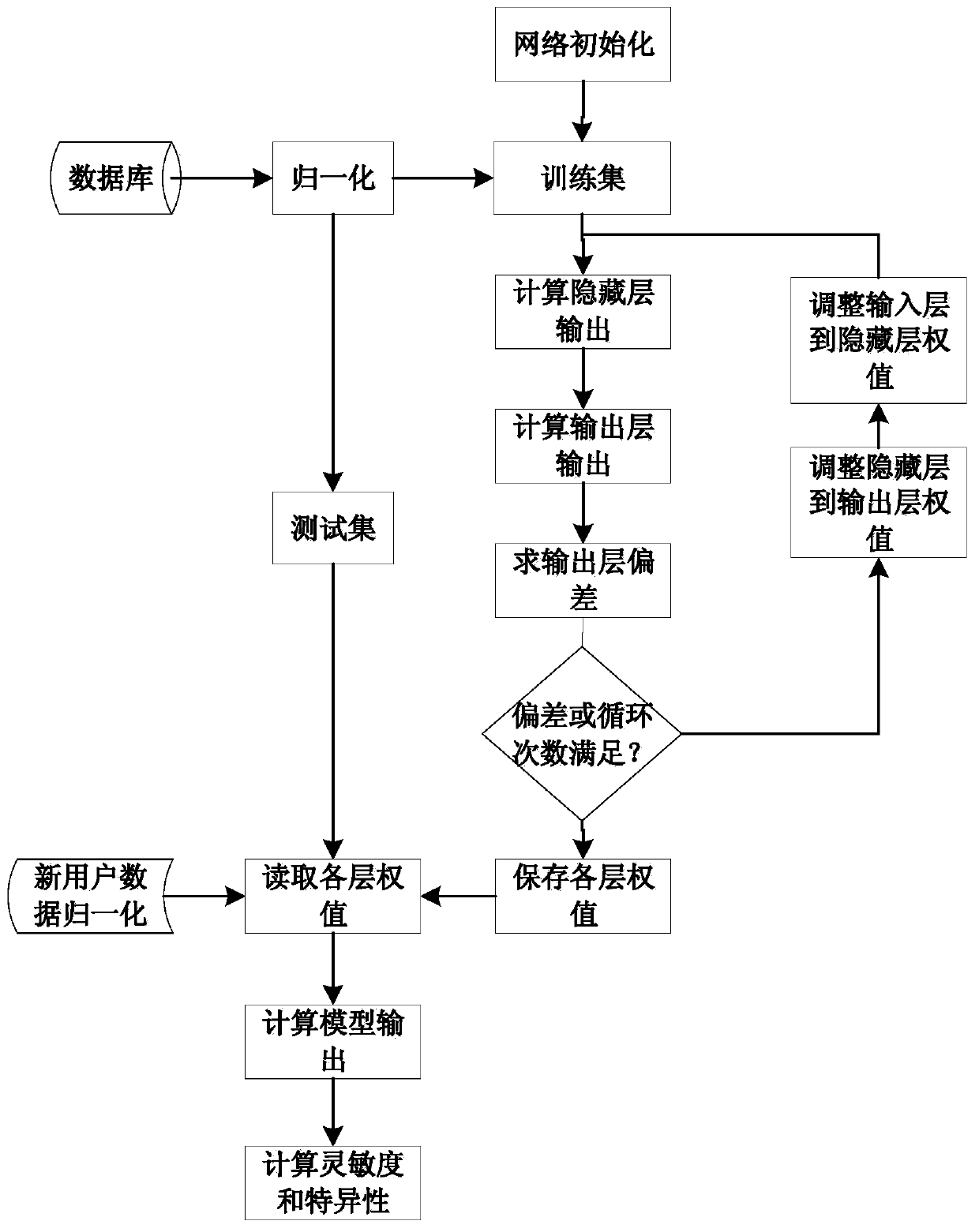
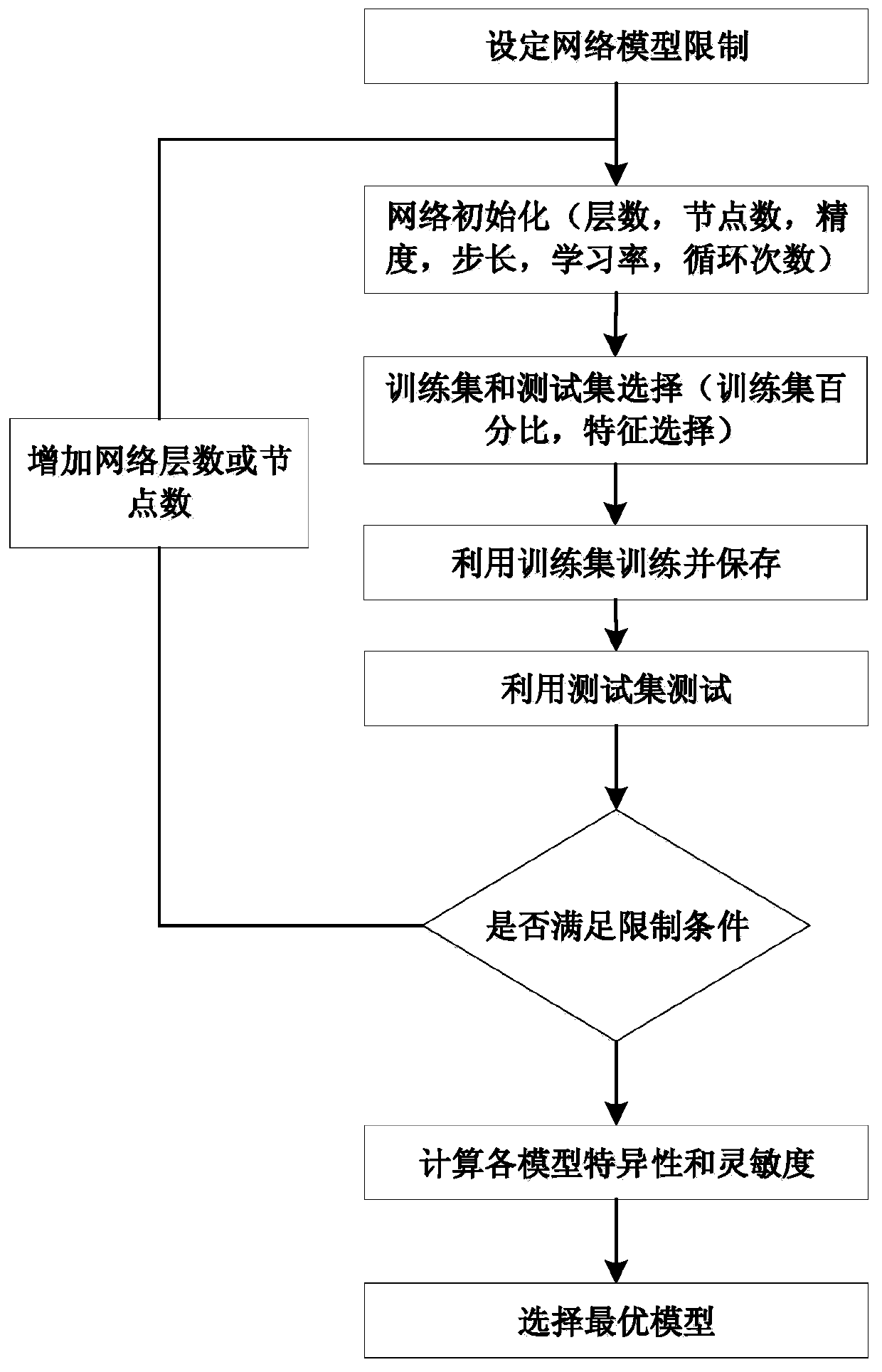
ActiveCN110946552AOverall distribution of lesionsDiagnostic recording/measuringSensorsSpectral databaseCervical cancer screening
The invention relates to a cervical cancer precancerous lesion screening method combining spectrums and images, and belongs to the field of spectral analysis and medical image processing. The method comprises the following steps: constructing a database according to spectrum and image data collected by a spectrum and endoscope combined probe at the same time, constructing a neural network precancerous lesion classification model by using the spectrum database, displaying the spectral diagnosis results of corresponding detection points on the images of the corresponding detection points by using different colors by utilizing image registration and fusion, reconstructing a precancerous lesion distribution diagram, and outputting the diagnosis results according to the distribution diagram. The method can be independently used as a cervical cancer screening algorithm, and can also provide image guidance for further biopsy. The diagnosis result of the method is the cervical cancer precancerous lesion distribution diagram which is objective and direct and not influenced by the technology of operators.
Owner:NANJING UNIV OF AERONAUTICS & ASTRONAUTICS
Cervical carcinoma screening method
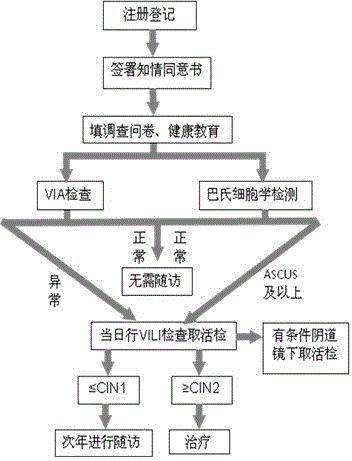
ActiveCN103558150ANo bloodReduce overlapSurgical needlesMaterial analysis by optical meansEarly carcinomaDisease
The invention provides a cervical carcinoma screening method which employs an improved Pap smear for screening. The method comprises the steps: extracting uterine neck secretion, performing conventional staining, film reading and diagnosis; if a patient is diagnosed as ASCUS or a more serious disease by improved Pap smear in cytologic level, performing pathology biopsy under iodine staining inspection. The invention also provides a cervical carcinoma two-method combined screening method. The method comprises: acquiring uterine neck secretion by improved Pap smear, immediately employing an acetic-acid naked-eye inspection method for screening: if a patient is diagnosed as positive by the acetic-acid naked-eye inspection method, performing pathology biopsy at once; and if a patient is diagnosed as negative, waiting for a diagnosis result of the improved Pap smear on site, if the patient is diagnosed as ASCUS or a more serious disease by the improved Pap smear, performing pathology biopsy at once. The methods provided by the invention employ two, but not one, methods for screening on women at the same time, which is not achieved before the methods provided by the invention are disclosed. The methods provided by the invention help to improve detection rate of cervical precancerous lesion and early-stage cancer.
Owner:SHIHEZI UNIVERSITY
Method of detecting human papilloma virus 16/18 type gene in urine by using digital PCR (polymerase chain reaction)
InactiveCN106755583AHigh sensitivityHigh precisionMicrobiological testing/measurementMicroorganism based processesSodium acetateHpv detection
The invention discloses a method of detecting a human papilloma virus 16 / 18 type gene in urine by using digital PCR (polymerase chain reaction). The method comprises the particular steps of extracting DNA (deoxyribonucleic acid) from the urine, preparing a primer reaction solution, preparing a droplet, performing PCR amplification, detecting the droplet, analyzing data and displaying a result, wherein a lysate comprises 10mM of Tris-HCl, 1mM of EDTA (ethylene diamine tetraacetic acid) and 0.5% of SDS (sodium dodecyl sulfonate); a pH (potential of hydrogen) value of a sodium acetate solution is 5.2. The detection mode is free from age limit and available; sampling is noninvasive; the method has high sensitivity and high precision and overcomes the detection difficulty arising from little viral nucleic acid in the urine; HR-HPV detection in the urine can be widely applied to clinical diagnosis and treatment and cervical cancer screening; the method has the advantage that the method has an important significance for prevention and treatment of a cervical cancer.
Owner:安徽安龙基因科技有限公司
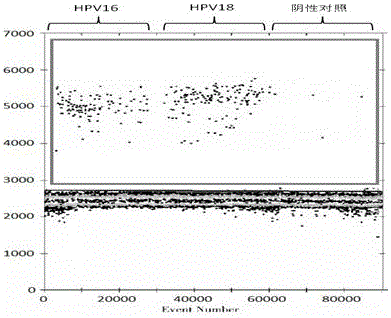
Cervical cancer screening by molecular detection of human papillomavirus-induced neoplasia
InactiveUS20130005026A1Easy to detectImmobilised enzymesBioreactor/fermenter combinationsPoint of careCervical cells
Point-of-care tools for screening biological samples for markers associated with pathogenic microbial infections. In particular, devices and systems for screening cervical cells for the expression of proteins, which occur as a result of human papillomavirus infection and progression to invasive cervical cancer.
Owner:CERMED CORP
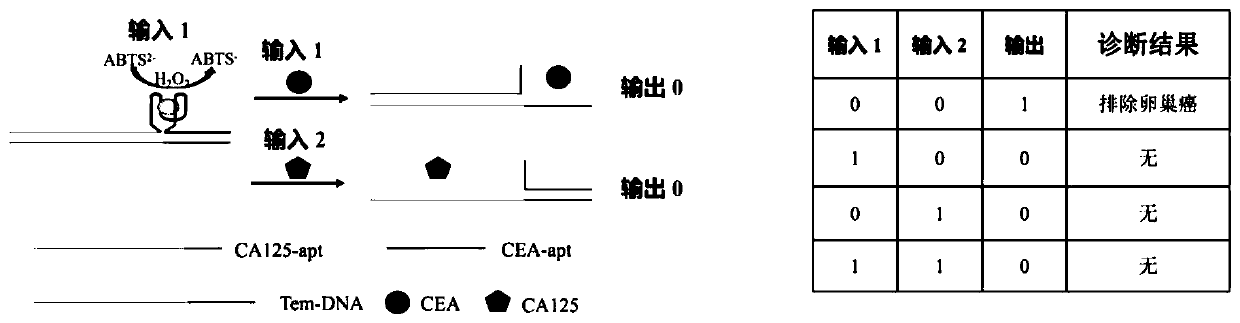
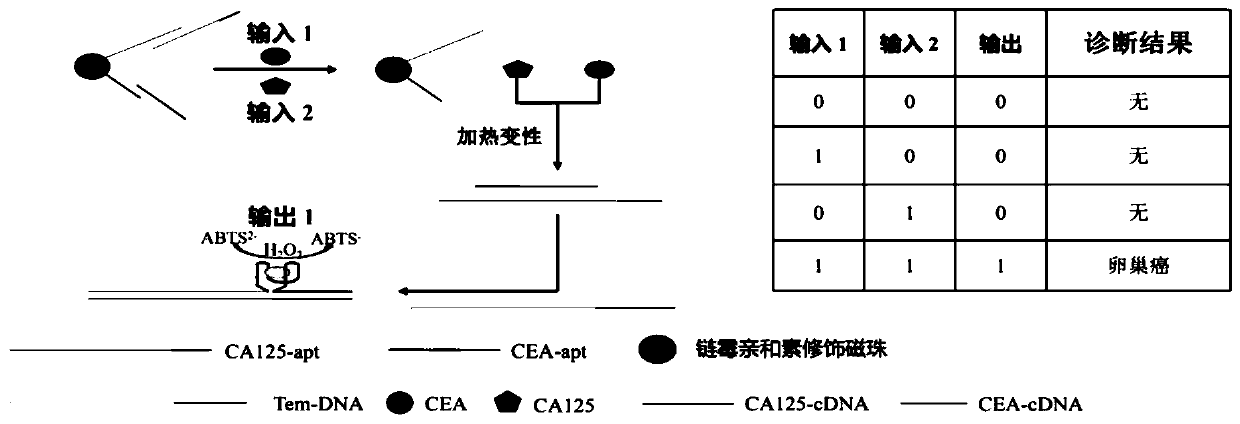
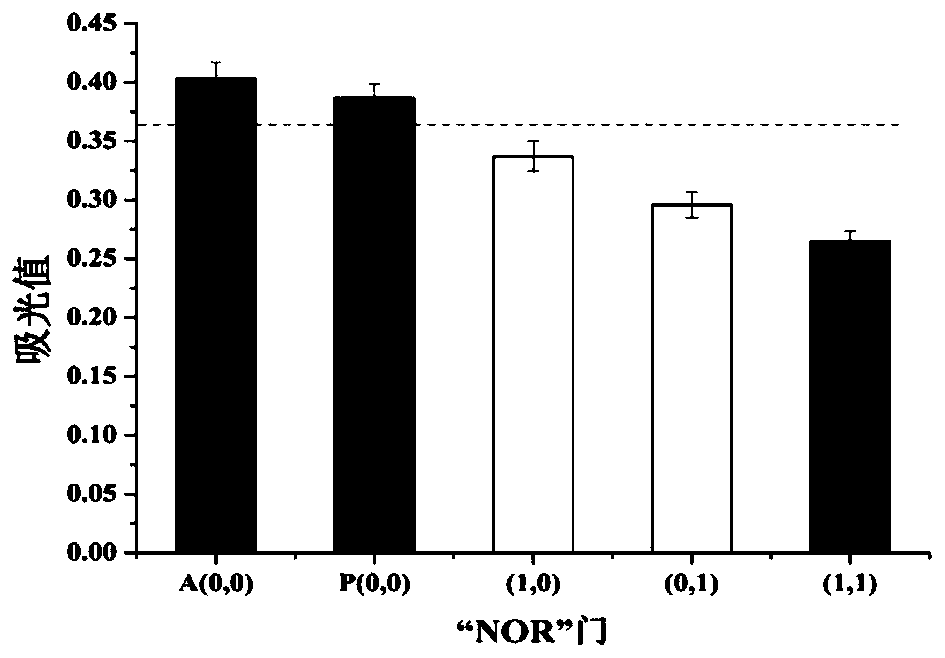
Method for ovarian cancer screening based on ovarian cancer marker and logic gate operation
ActiveCN110426519AHigh sensitivityStrong specificityColor/spectral properties measurementsAptamerObject based
The invention discloses a method for ovarian cancer screening based on an ovarian cancer marker and a logic gate operation, and belongs to the technical field of biomedicine. The protein-nucleic acidrecognition technology, the signal amplification technology and a logic gate are combined, aptamers of CEA and CA125 are used for target recognition, signal transformation and amplification are performed through a G-quadruplex-heme complex, and a method for simultaneous detection of tumor markers is established. According to the method, an optical biosensor with high sensitivity and strong specificity is constructed for achieving the joint detection of the CEA and the CA125, and an ovarian cancer screening result is provided based on the logic gate; through the combination of optical detectionand the signal amplification technology, the detection sensitivity is improved; the universality of the detection principle makes the method also suitable for detection of different objects based ondifferent protein recognition elements.
Owner:JIANGNAN UNIV
Rapid cervical cancer screening system and method
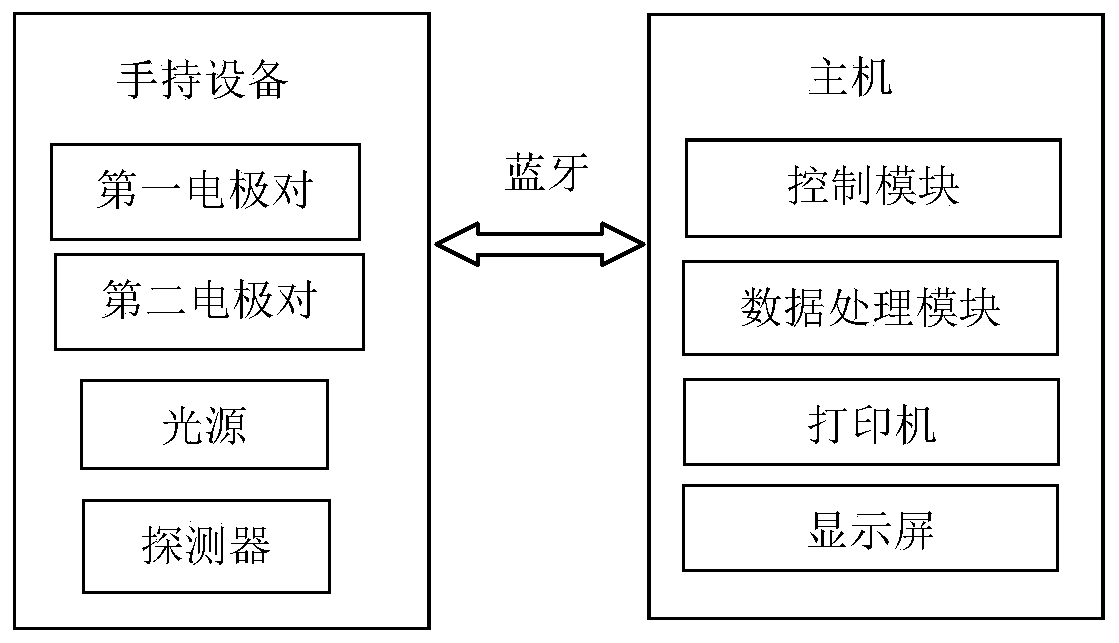
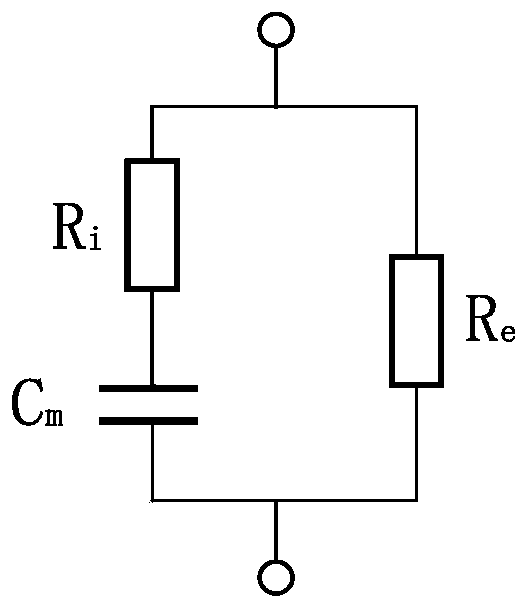
InactiveCN111007111AQuick screeningImprove accuracyScattering properties measurementsMaterial impedanceCervical tissueNormal cervix
The invention discloses a rapid cervical cancer screening system and method. The system comprises a handheld device and a host in communication connection with the handheld device. The handheld deviceis provided with a first electrode pair used as excitation electrodes to generate current signals, a second electrode pair used as measuring electrodes to detect impedance, a light source and a detector. The host comprises a control module and a data processing module, the control module is used for controlling the handheld device, and the data processing module is used for processing detection data of the handheld device to obtain a detection result. According to the method, normal cervical tissues are distinguished from cancerous cervical tissues by detecting the difference of the normal cervical tissue and the cancerous cervical tissue in electrical and optical properties. According to the invention, electrical detection is conducted on the cervical tissue, optical detection is combined to serve as compensation, rapid screening of cervical cancer can be achieved according to the difference of electrical and optical characteristics of normal cervical tissue and abnormal cervical tissue, and the accuracy of diagnosis results can be improved.
Owner:SUZHOU INST OF BIOMEDICAL ENG & TECH CHINESE ACADEMY OF SCI
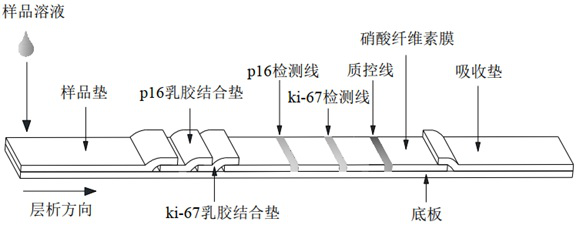
Household cervical cancer screening reagent card
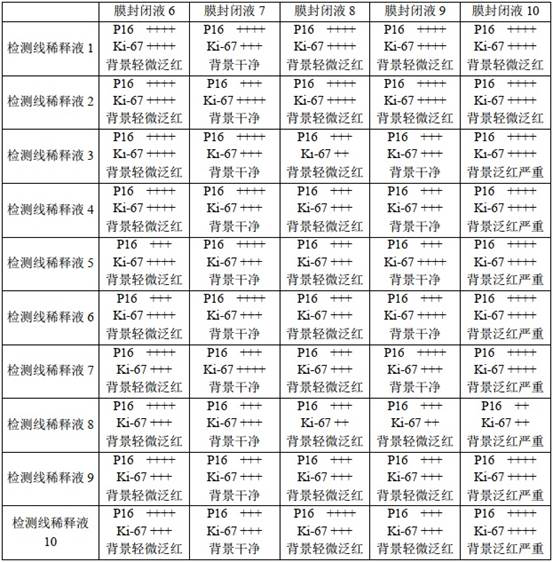
The invention belongs to the technical field of immunology, and particularly relates to a household cervical cancer screening reagent card which comprises a bottom plate, a sample pad, a latex combination pad, a nitrocellulose membrane and an absorption pad, detection lines and a quality control line are arranged on the nitrocellulose membrane, the detection lines comprise a p16 detection line and a ki-67 detection line, and the quality control line comprises a quality control line and a quality control line. The p16 detection line, the ki-67 detection line and the quality control line are parallel to one another, and the p16 detection line and the ki-67 detection line are respectively obtained by spraying a solution of a p16-point membrane antibody and a solution of a ki-67-point membrane antibody on the nitrocellulose membrane. According to the household cervical cancer screening reagent card, the p16 point membrane antibody and the ki-67 point membrane antibody are adopted to obtain the p16 detection line and the ki-67 detection line respectively, detection results of the two detection lines are combined and analyzed, various physical conditions can be evaluated, the detection accuracy is improved, in addition, the reagent card is simple in using method, and the results are simple and easy to understand.
Owner:NANJING LIMING BIO PROD CO LTD
Cervical lesion detector and detection method
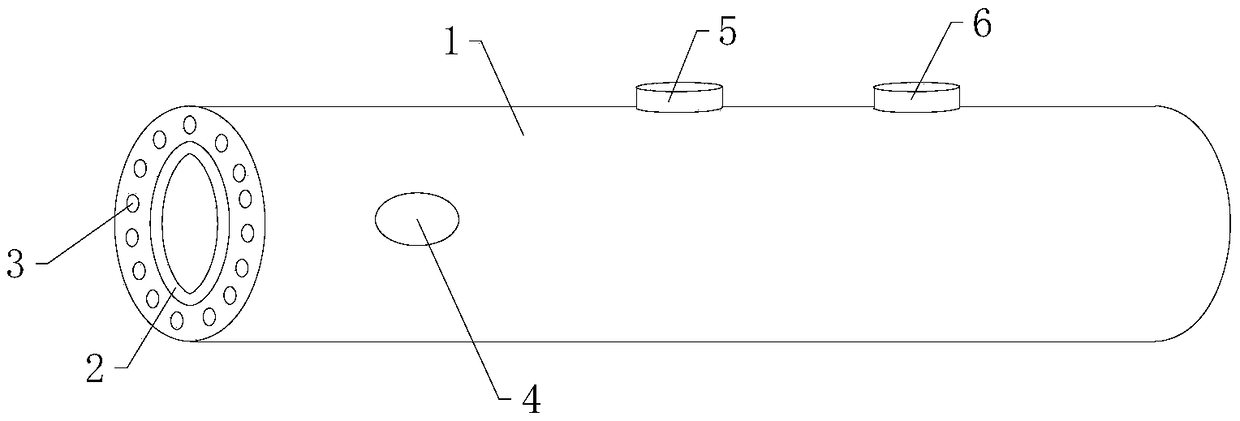
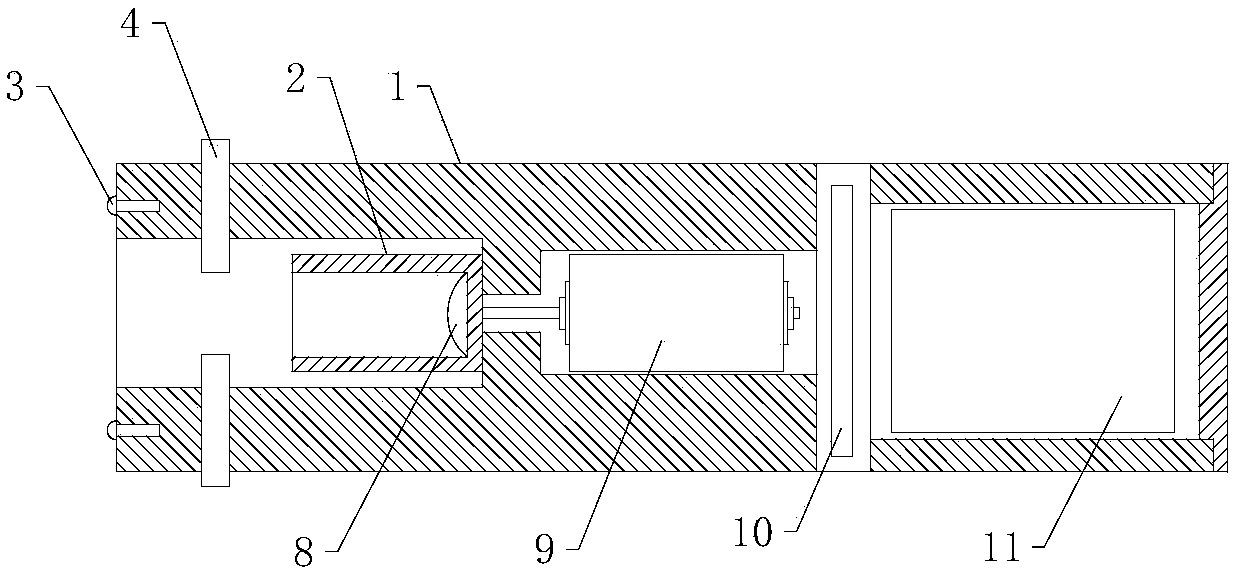
PendingCN108852271AAchieve independent interpretation of resultsEasy to operateEndoscopesDiagnostic recording/measuringCervical lesionCervical cancer screening
The invention belongs to the technical field of medical devices, and discloses a cervical lesion detector which comprises a pressure power device and a detection bar. The pressure power device comprises a holding body, a base hole is formed in the front end of the holding body, a transmission sleeve is arranged in the base hole and rotates relative to the base hole, a wireless pressure sensor is arranged at the bottom of the transmission sleeve, a motor and a main board are arranged in the holding body, the motor is used for driving the transmission sleeve, the main board is used for controlling the motor to run, the detection bar comprises a detection shaft matched with the transmission sleeve, a conical detection head is arranged at the front end of the detection shaft, the rear end of the detection shaft can be inserted into a sleeve, and the pressure is applied to the wireless pressure sensor. According to the detector, a clinician can independently judge and read a result in cervical lesions detection, and the detector is bedside, easy to operate, low in cost, short in time and suitable for popularization and use and solves awkward situations in domestic cervical cancer screening of women.
Owner:康涛
Primer combination, probe combination and human papilloma virus nucleic acid detection kit
PendingCN112575123ALow costStorage temperature requirements are lowMicrobiological testing/measurementDNA/RNA fragmentationColposcopesHuman papilloma virus infection
The invention discloses a primer combination, a probe combination and a human papilloma virus nucleic acid detection kit. The invention relates to the field of biological detection, in particular to aPCR fluorescent probe detection method for diagnosing high-risk human papilloma virus infection, which comprises the following steps: extracting human papilloma virus nucleic acid in cervical exfoliated cells by using a paramagnetic particle method, and carrying out real-time fluorescence polymerase chain reaction to obtain the high-risk human papilloma virus detection kit to detect 14 HPV DNA types in the sample and typing the HPV 16 / 18; and meanwhile, carrying out female cervical cancer screening in combination with cytological examination. The kit can be used for qualitative detection of high-risk human papilloma viruses in cervical cell samples of patients, and typing identification of HPV16 and HPV18 types is realized while HPV31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66 and 68 are detected. The kit is used for qualitatively detecting high-risk HPV DNA in cervical cells of a patient and determining whether the patient needs colposcope examination or not.
Owner:AUTOBIO DIAGNOSTICS CO LTD
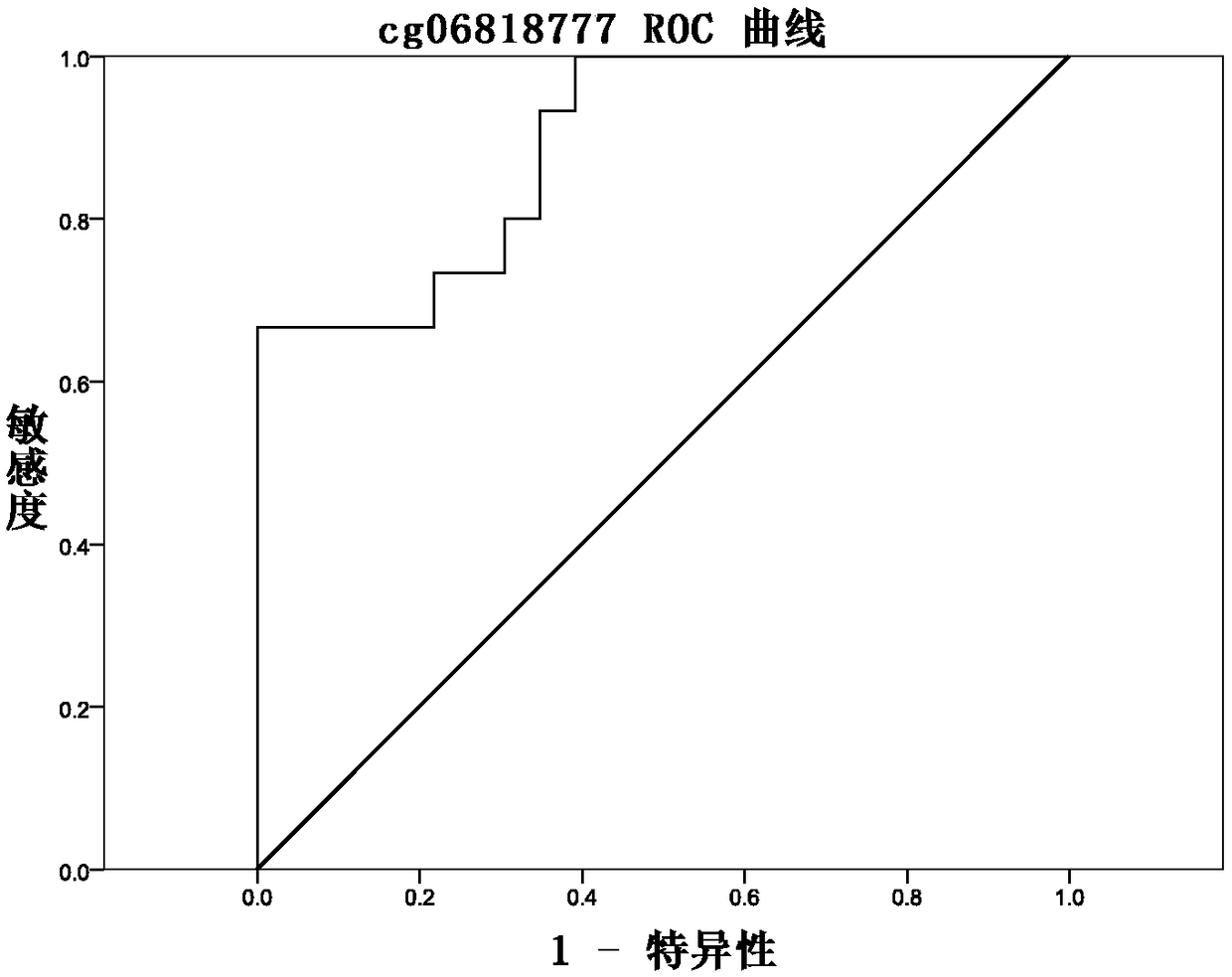
Methylation site for cervical cancer screening and detection primer thereof
ActiveCN109468381AImprove featuresThe result is objectiveMicrobiological testing/measurementDNA/RNA fragmentationCervical cancer screeningMethylation Site
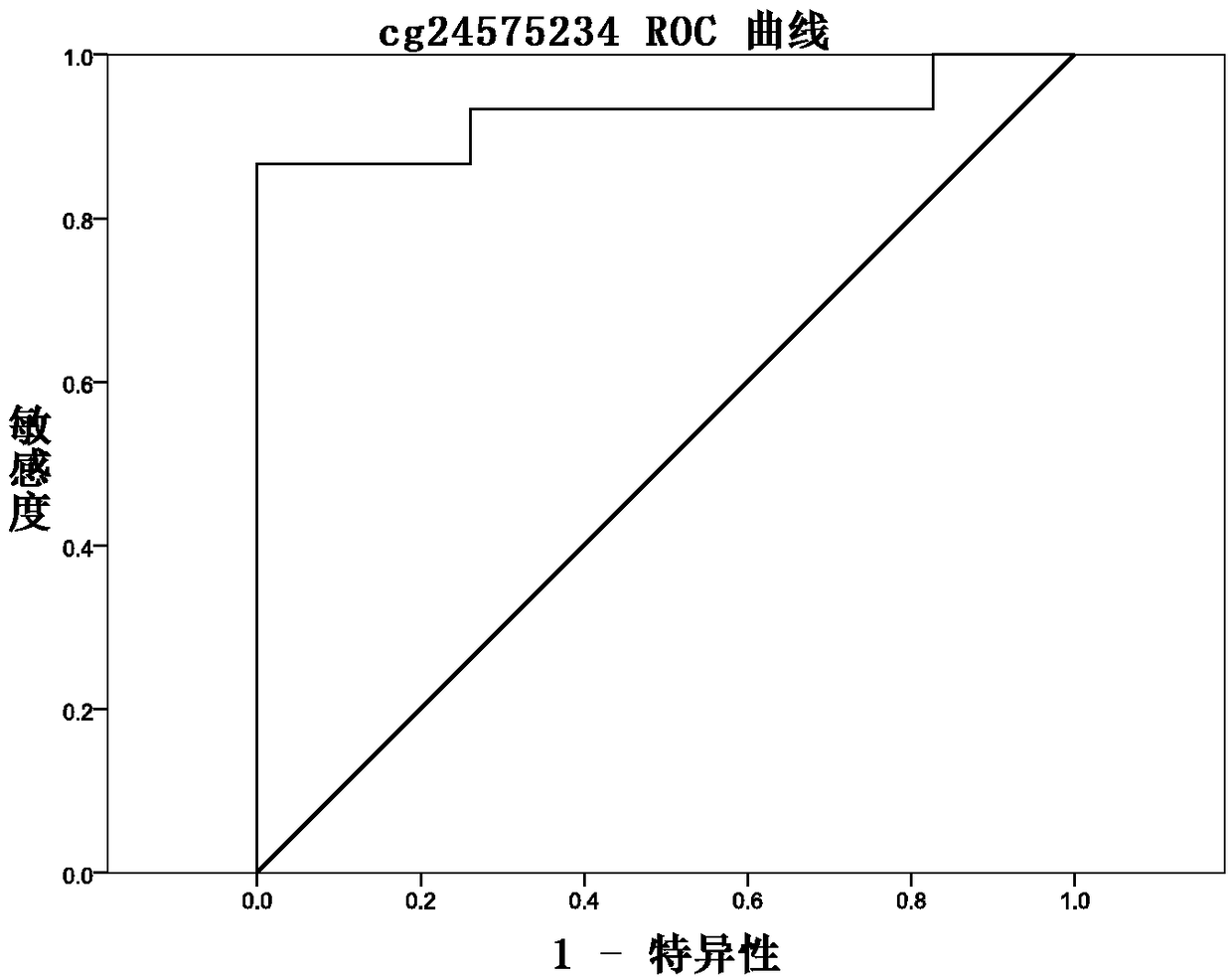
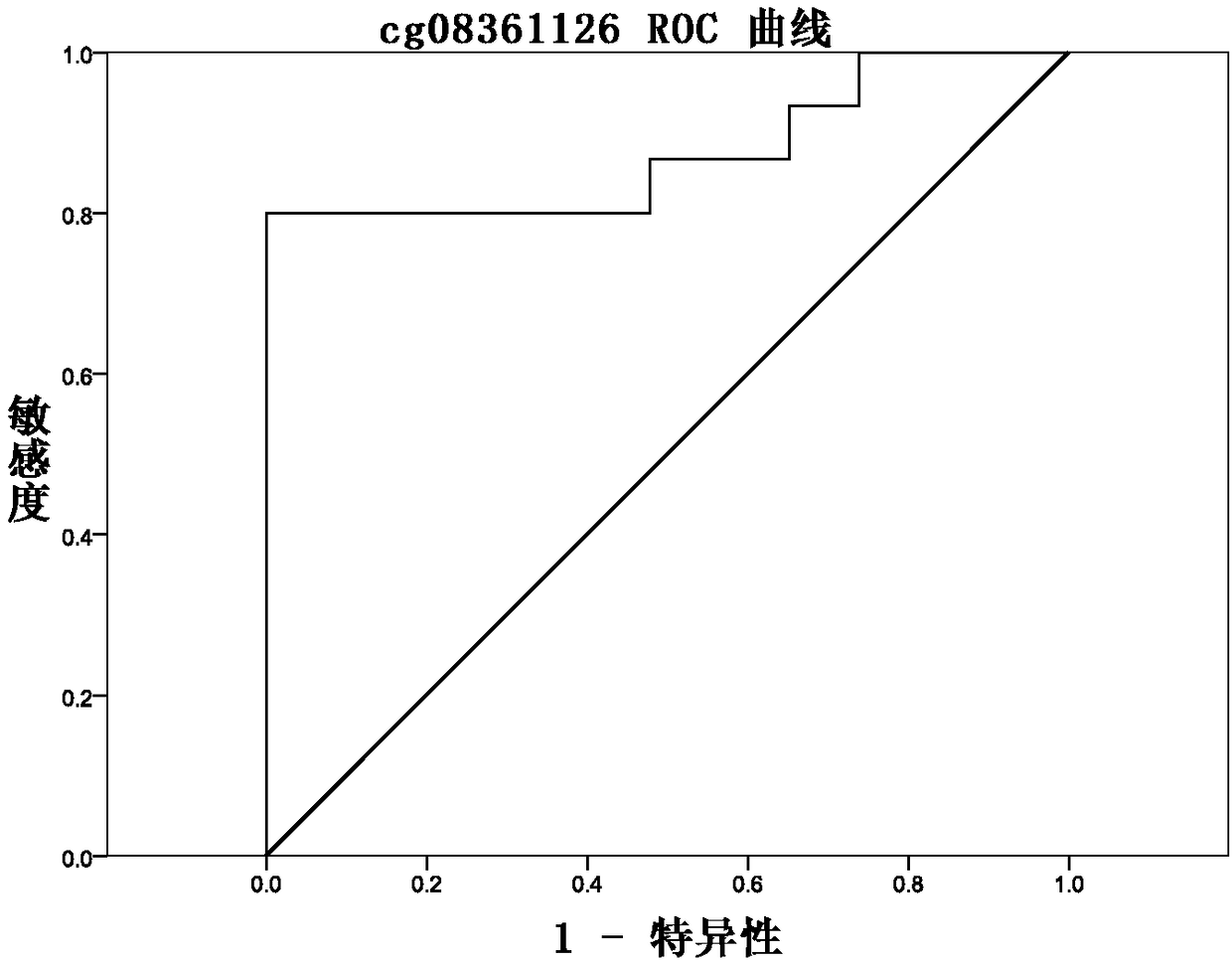
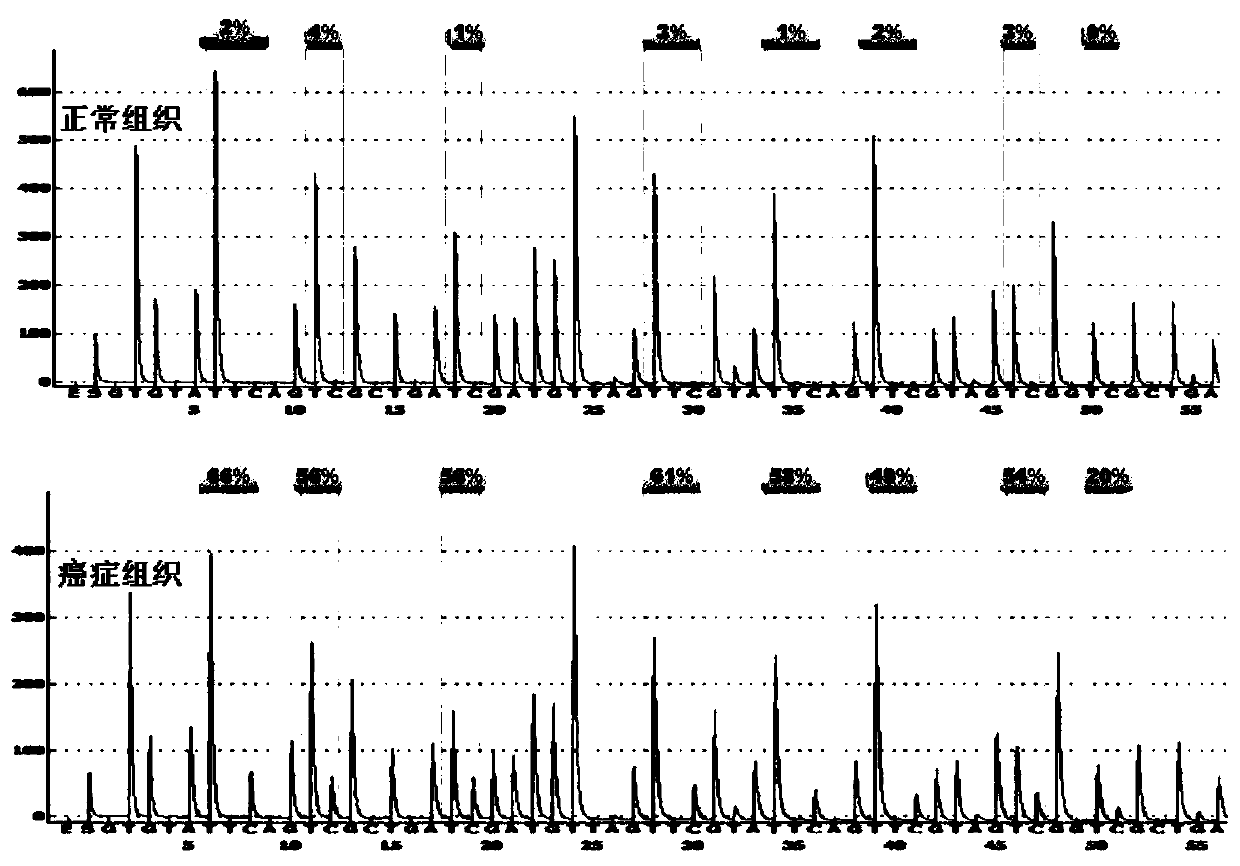
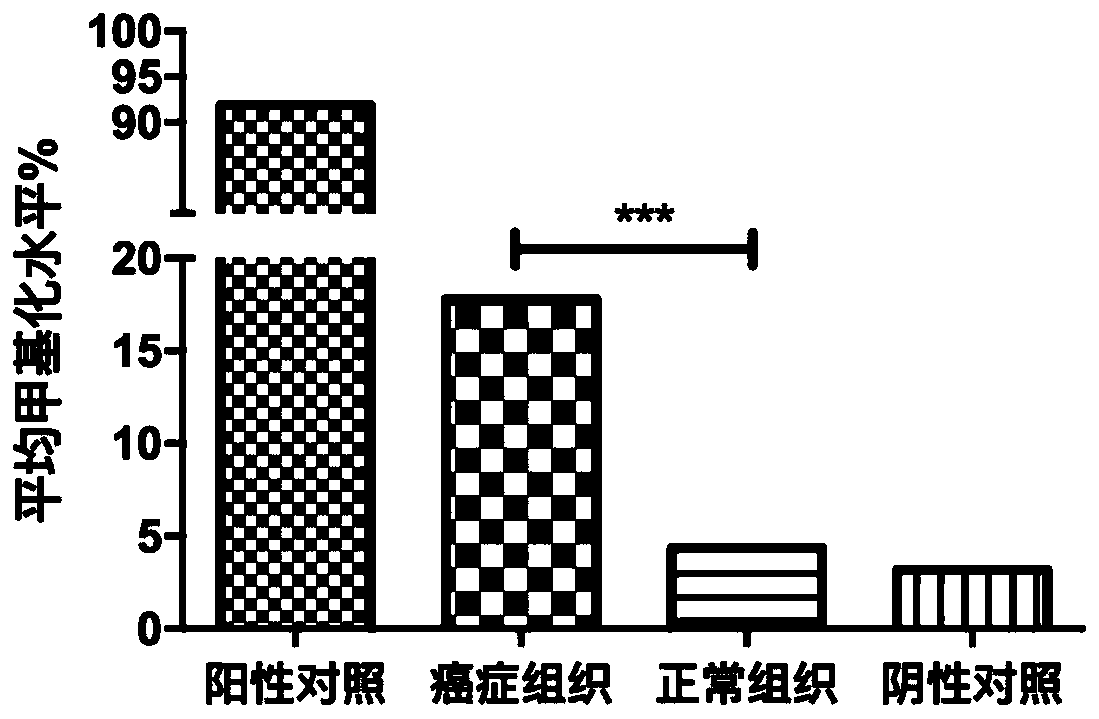
The invention discloses a methylation site for cervical cancer screening. The methylation site is a cg24575234 site of a CHRM2 gene, a cg06818777 site of a CHAD gene, a cg08361126 site of an FGF10 gene, and a cg08976810 site of an ITGA8 gene. The invention also discloses a primer pair included in a kit for detecting a methylation level of a sample gene of cervical cancer. According to the invention, a whole process can be automated, the result is objective and is easy to interpret, and the influence of subjective factors is avoided; and the specificity of the methylation site, provided by theinvention, for detecting and screening cervical cancer is 100%, which is greater than the specificity (80%) of an HPV gene detection method, and the positive prediction value is increased from 0.0474%to 100%, so that the screening result is objective and is easy to interpret. The methylation site provided by the invention can also be used in parallel with a current cervical cancer screening experiment, thereby greatly saving the examination cost and avoiding economic losses and psychological burdens of too many false positive patients due to positive results.
Owner:兰州市第一人民医院
Methylated EphA7 nucleotide fragment and detection method and application thereof
ActiveCN109837344APoor objectivitySolve operational problemsMicrobiological testing/measurementDNA/RNA fragmentationCervical tissueNucleotide
The invention relates to the technical fields of genetic engineering and medical detection, and provides a methylated EphA7 nucleotide fragment as a specific diagnosis marker of cervical cancer, and aprimer set, a probe and a reagent kit relevant with the methylated EphA7 nucleotide fragment, and an application of the methylated EphA7 nucleotide fragment to preparation of a cervical cancer screening and diagnosing preparation. The inventor finds that the methylation degree of a promoter region of EphA7 genes in cervical tissues is in significant correlation with the generation of the cervicalcancer, and the methylated EphA7 nucleotide fragment can be used as a biology marker for assisting in diagnosis of the cervical cancer, so that the human cervical cancer can be timely screened and diagnosed for early stage, and the waste of medical resources is avoided.
Owner:TIANJIN MEDICAL UNIV
Methods and Systems for Predicting Whether a Subject Has a Cervical Intraepithelial Neoplasia (CIN) Lesion from a Suspension Sample of Cervical Cells
PendingUS20170130281A1Microbiological testing/measurementMedical automated diagnosisCervical cellsCervical cancer screening
Methods of predicting whether a subject has a cervical intraepithelial neoplasia (CIN) lesion are provided. Aspects of the methods include obtaining both morphometric and biomarker data from a liquid cervical cellular sample and then using both types of data to predict whether the subject has a CIN lesion. Also provided are systems that find use in practicing the methods. The methods and systems find use in a variety of applications, including cervical cancer screening applications.
Owner:INCELLDX
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com