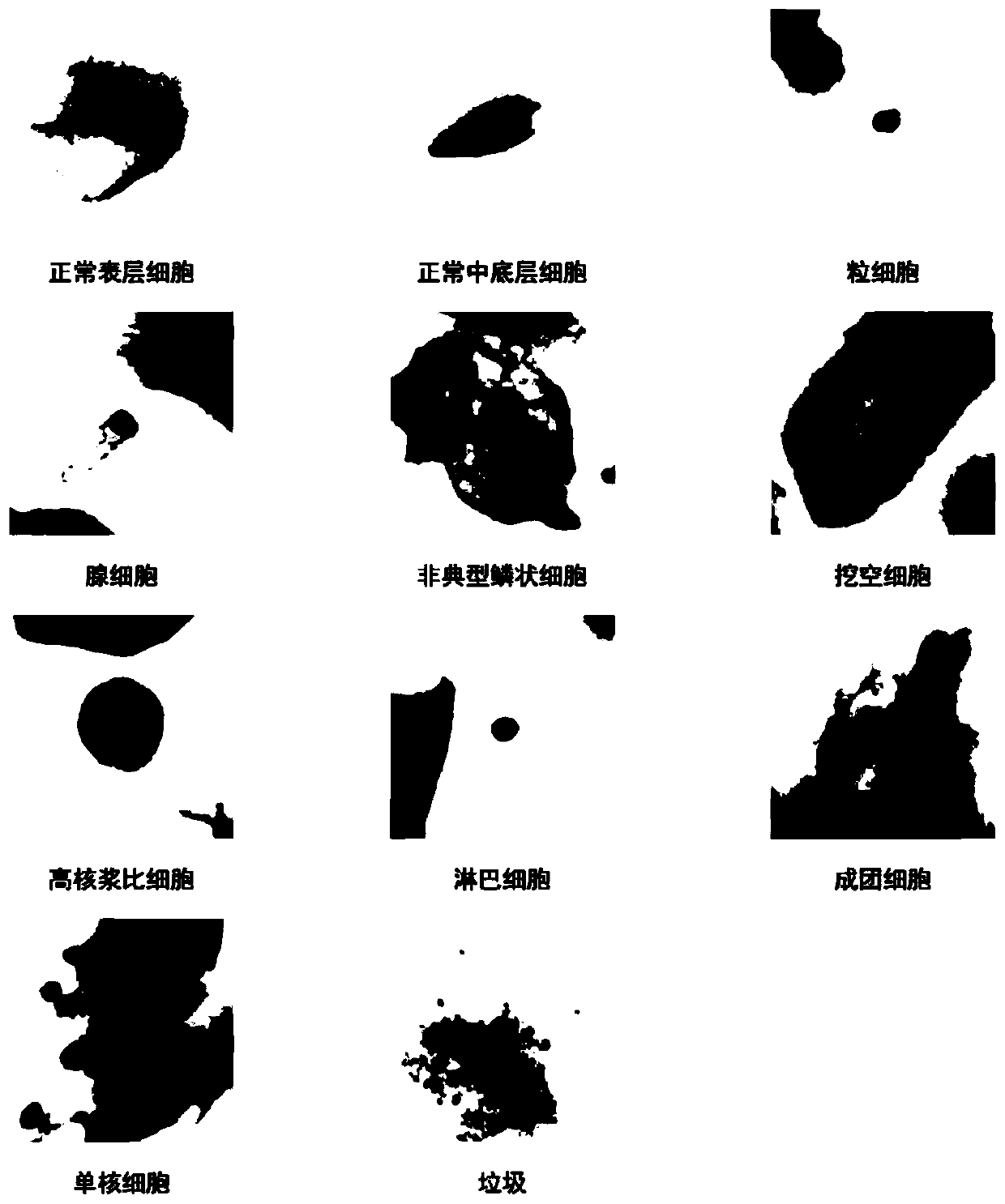
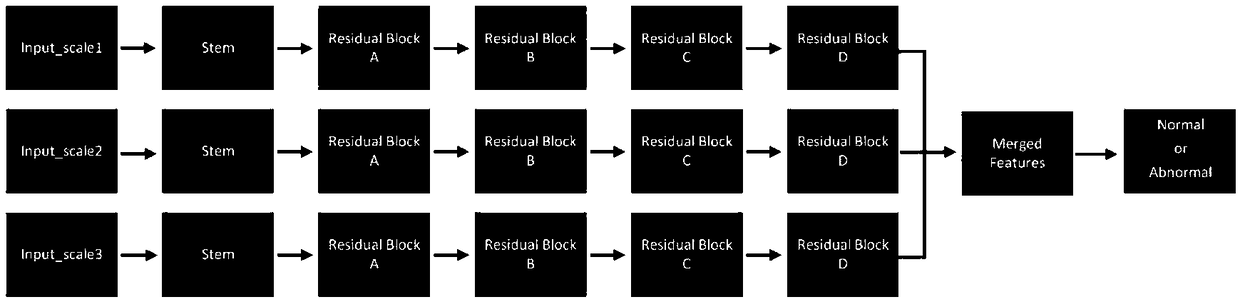
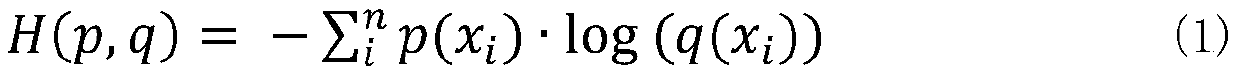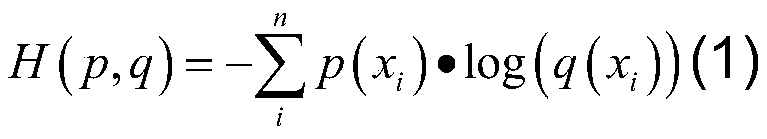Patents
Literature
151 results about "Cervical cell" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cervical dysplasia is a condition in which healthy cells on the cervix undergo some abnormal changes. The cervix is the lower part of the uterus that leads into the vagina. It’s the cervix that dilates during childbirth to allow the fetus to pass through. In cervical dysplasia, the abnormal cells aren’t cancerous,...
Diagnosis and treatment of cervical cancer
InactiveUS20080113340A1Organic active ingredientsPeptide/protein ingredientsCervical lesionCervical cell
In certain aspects, the invention relates to methods of diagnosing cervical cancer by using a combination of certain biomarkers such as hTERT, IGFBP-3, transferrin receptor, beta-catenin, Myc-HPV E6 interaction, HPV E7, and telomere length. In other aspects, the invention relates to methods of detecting immortalization of cervical cells by using a combination of certain biomarkers. In yet other aspects, the invention relates to methods of classifying the grade of a cervical lesion for diagnostic and prognostic purposes in a female. In further aspects, the invention relates to methods of treating cervical cancer by administering a therapeutic agent that targets one or more of these biomarkers.
Owner:GEORGETOWN UNIV
Non-invasive prenatal genetic diagnosis using transcervical cells
InactiveUS20050181429A1Microbiological testing/measurementDisease diagnosisPrenatal diagnosisStaining
A non-invasive, risk-free method of prenatal diagnosis is provided. According to the method of the present invention transcervical specimens are subjected to trophoblast-specific immuno-staining followed by FISH, PRINS, Q-FISH and / or MCB analyses and / or other DNA-based genetic analysis in order to determine fetal gender and / or identify chromosomal and / or DNA abnormalities in a fetus. Also provided is a method of in situ chromosomal, DNA and / or RNA analysis of a prestained specimen by incubating the prestained specimen in ammonium hydroxide. Also provided is a method of identifying embryonic cells according to a nucleus / cytoplasm ratio of at least 1:1 and the presence of at least variably condensed chromatin.
Owner:MONALIZA MEDICAL
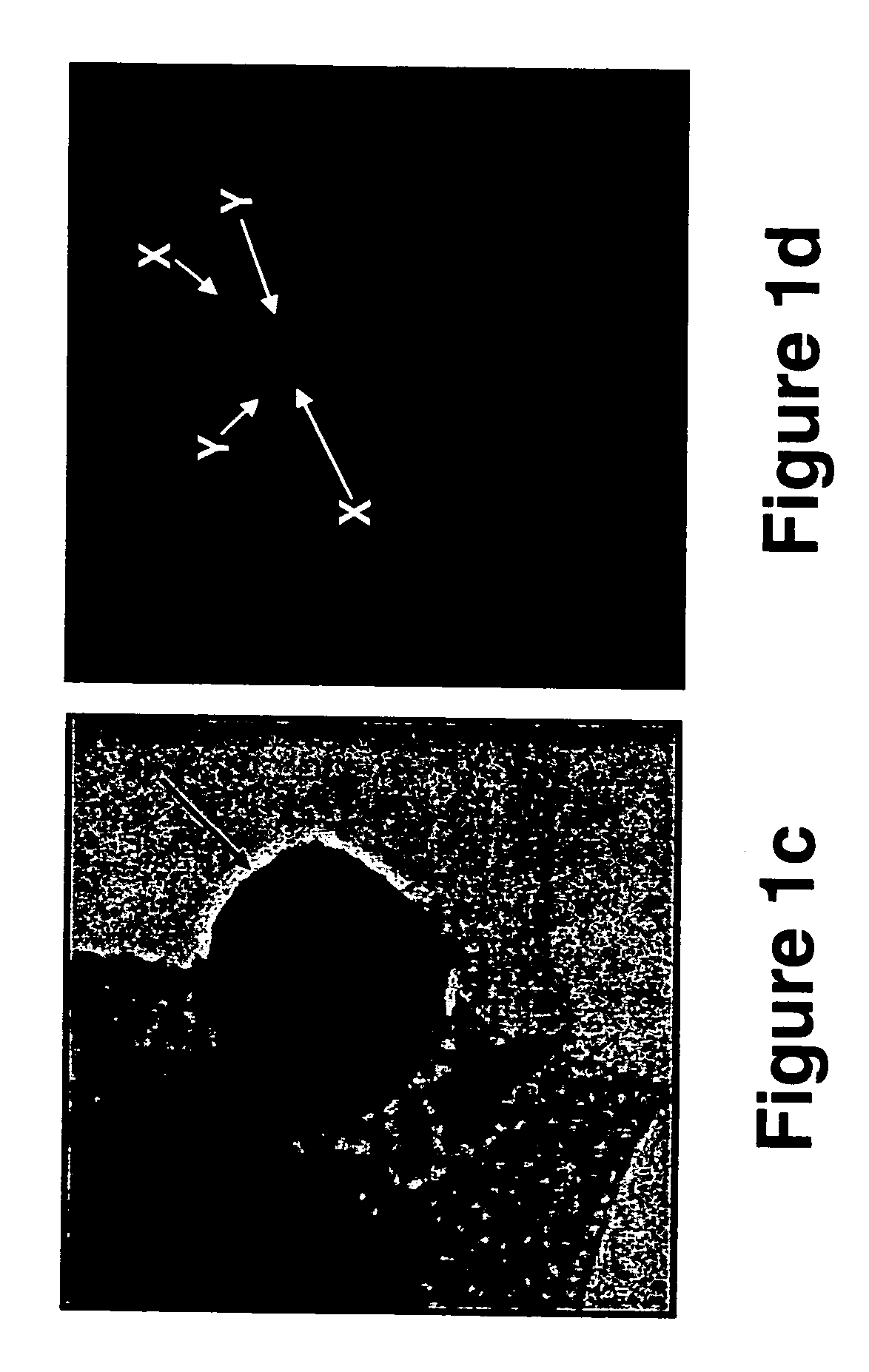
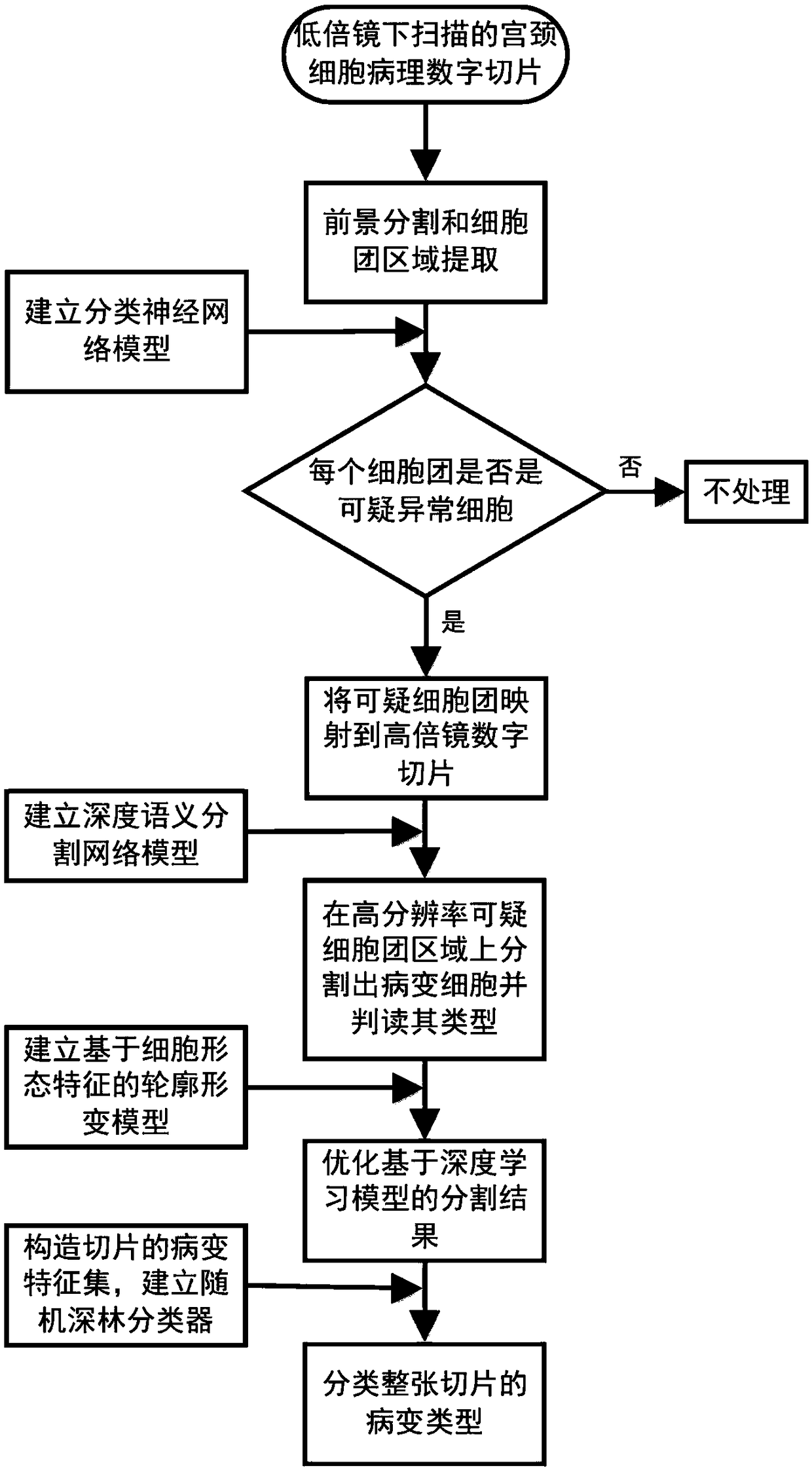
A cervical cell pathological slice classifying method with high and low resolution combination
ActiveCN109034208ASolve the recognition accuracySolve the recognition efficiencyRecognition of medical/anatomical patternsFeature setImage resolution
The invention discloses a cervical cell pathological slice automatic judging method with high and low resolution combination, which is characterized in that the method comprises the following steps: extracting a single cell mass region on a low-resolution cervical pathological slice image; extracting a single cell mass region from the low-resolution cervical pathological slice image; identifying suspicious abnormal cell clusters from low-resolution cell clusters; mapping suspected abnormal cell mass regions into high resolution slice images; semantically segmenting pathological cells from thehigh-resolution region of suspicious cell clusters and interpreting the type; according to the type, quantity and confidence of the segmented lesion cells, establishing the feature set of the slice, and then classifying the lesion type of the whole slice. The invention takes cell cluster as processing and recognition unit, utilizes classification neural network model to quickly identify suspiciousabnormal cell cluster on low-resolution digital slice, then divides out pathological cell on high-resolution suspicious area and judges its type, at the same time, improves recognition accuracy and recognition efficiency.
Owner:怀光智能科技(武汉)有限公司
A Mask-RCNN-based cervical cell smear image segmentation method and system
ActiveCN109886179AGood segmentation resultCell segmentation results are goodCharacter and pattern recognitionICT adaptationPattern recognitionData set
The invention relates to a Mask-RCNN-based cervical cell smear image segmentation method and system, and the method comprises a data set construction step of carrying out the preparation and labelingof a training data set, a verification data set and a test data set, and carrying out the normalization and preprocessing of the data set; b, constructing and training a model, constructing an image segmentation model based on Mask-RCNN, training the model by using the training data set, and verifying an image segmentation result of the model by using the verification data set; and a model verification step of testing the model by using the test data set, and evaluating a segmentation result by using a similarity coefficient. According to the method, the deep neural network model trained by utilizing a large amount of data can be used for modeling and abstracting information contained in the large amount of data, so that the cells and the cell nuclei in the cervical cytology smear image can be positioned, detected and subjected to the instance segmentation through a single model.
Owner:SHENZHEN IMSIGHT MEDICAL TECH CO LTD
Simplified thin-layer liquid-based cytology detection method
InactiveCN1967196ACheap methodGood effectMicrobiological testing/measurementSurgical needlesLiquid base cytologyCervical cells
The invention discloses a simple thin liquid-based cytology examine method applied for the gynecological examination, the cervical cancer general examine and the cervical cancer preceding lesion of selected crowd, the steps including: using the cervical cells brush and the scraper to collect samples, put samples in the liquid-based cell preservation solution, add base liquid cell processing solution, oscillate, centrifugally separate, take and discard the supernatant samples liquid, using adding samples gun to assimilate sediment samples, smear, sheet drying, using 95% ethanol marinate or spray for fixed, washing and dyeing. The invention has simple method, small steps, high positive detection rate, low misdiagnosis rate, low cost, and it can effectively eliminate the red blood cells, mucus and other interference components from the cervical cytology detection specimens, with good sheet-producing result.
Owner:绵竹市人民医院
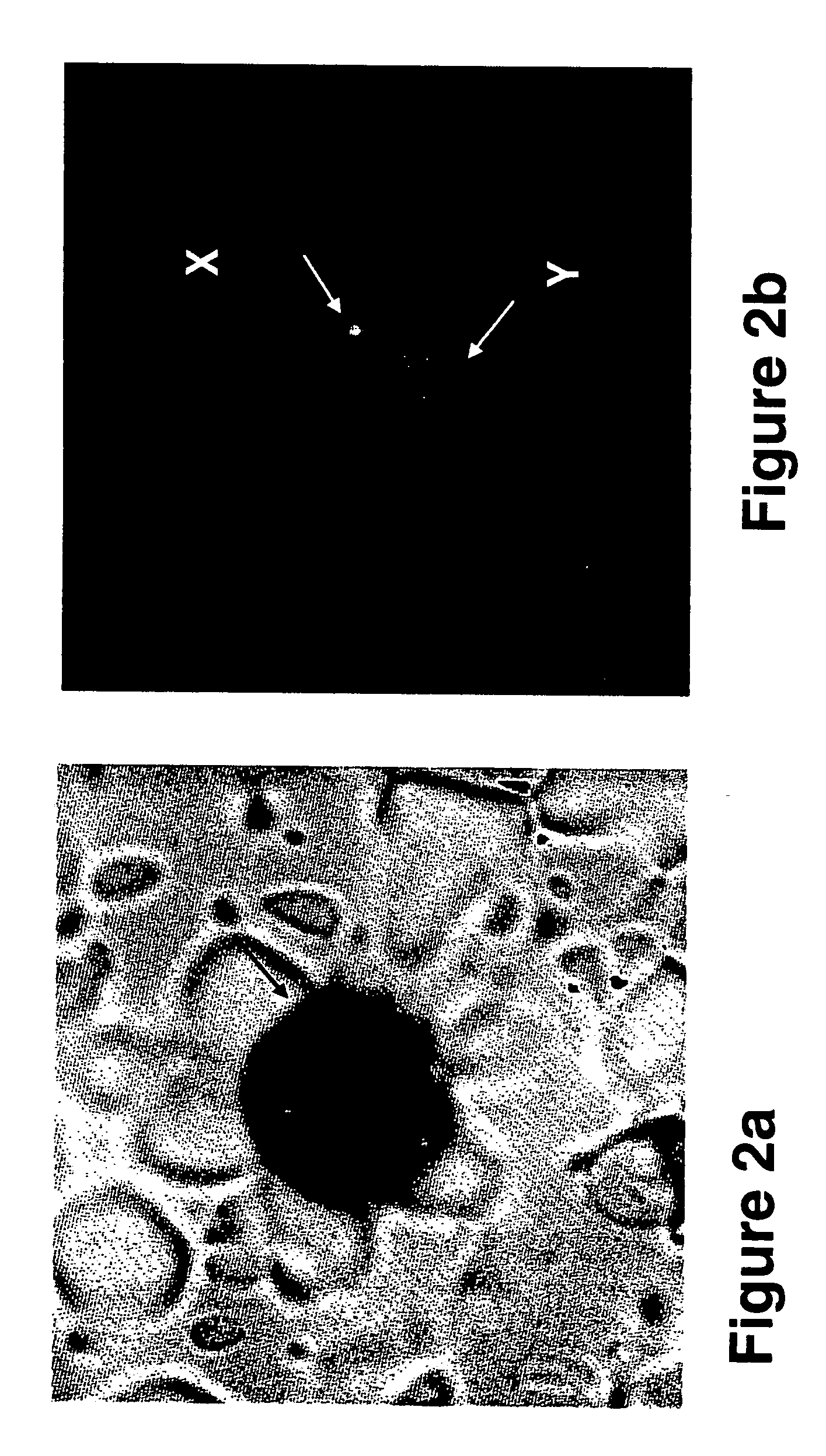
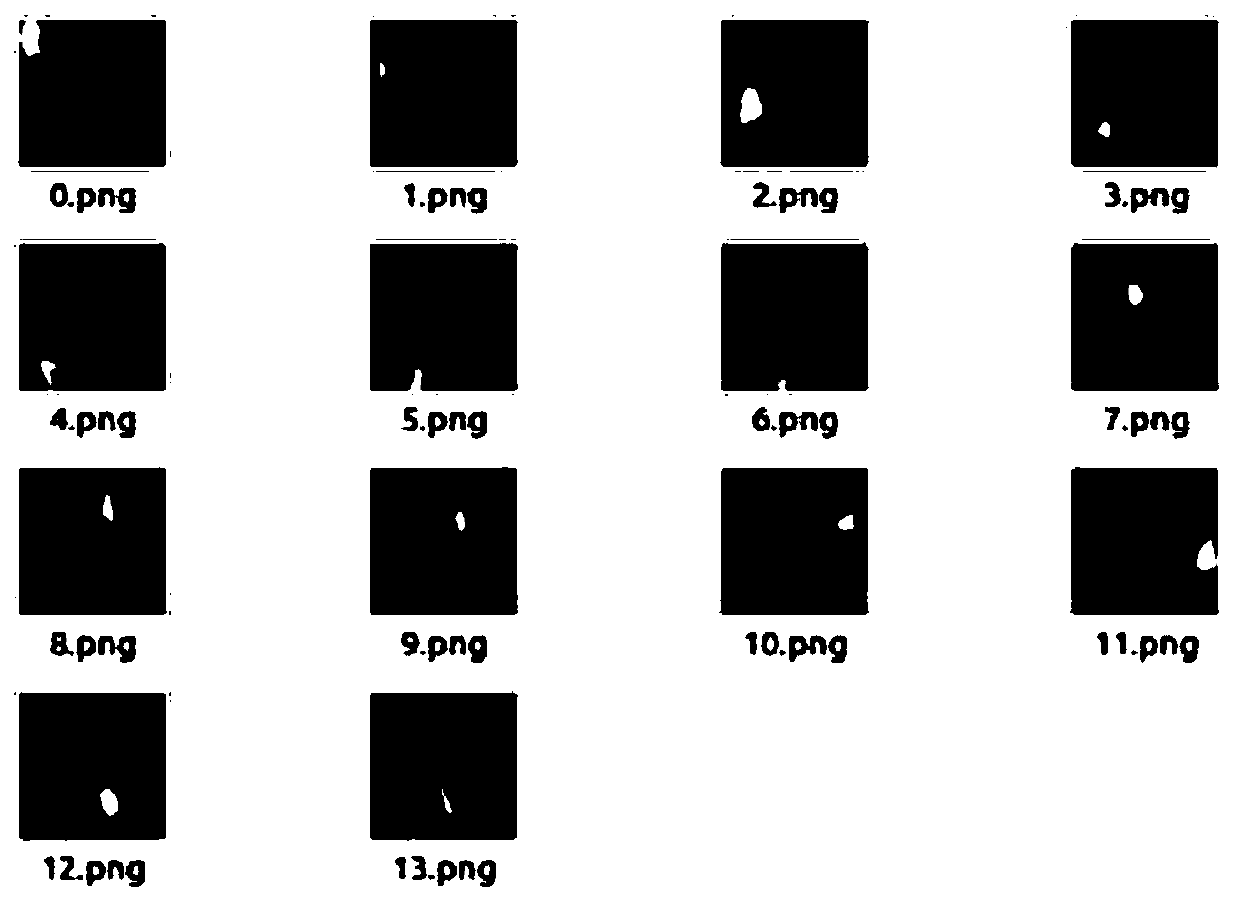
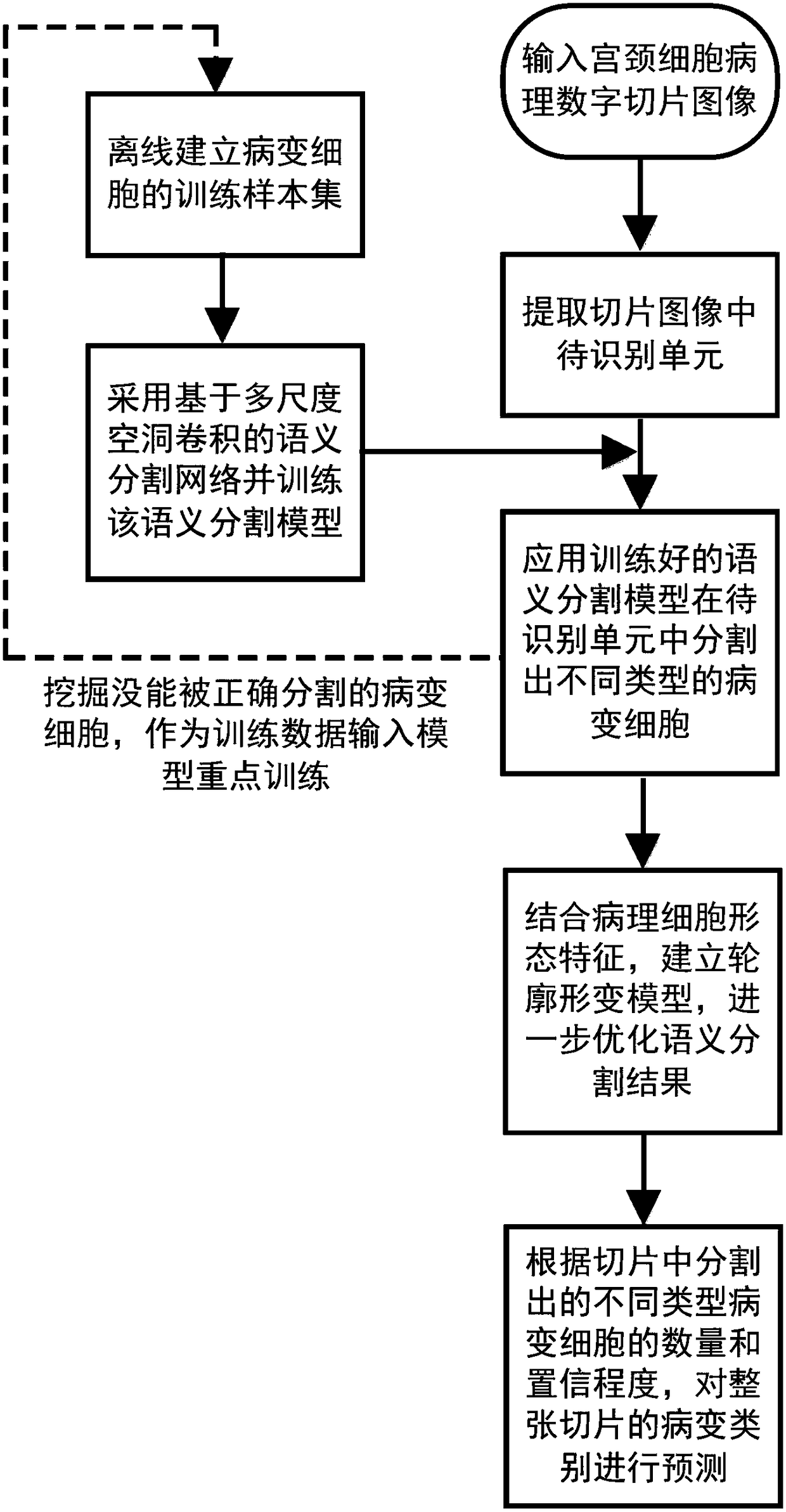
A cervical cell pathological slice pathological cell segmentation method and system
ActiveCN109035269AImprove recognition resultsThe classification method is reasonableImage enhancementImage analysisCervical cellVariational model
The invention discloses a cervical cell pathological slice pathological cell segmentation method and system, in particular, an off-line training sample set of cervical cell pathological slice pathological cell is established, a multi-scale cavity convolution structure semantic segmentation network is introduced on the basis of a depth residual network, and the semantic segmentation model is trained. The unit to be identified is extracted from the cervical pathological slice image, and different types of pathological cells are segmented in the unit to be identified by using the trained semanticsegmentation model. Based on the morphological features of pathological cells, a contour deformation model is established to further optimize the semantic segmentation results. According to the cellnumber and confidence level of different pathological types, the pathological types of the whole slice are predicted. The invention utilizes semantic segmentation network model and deformation variational model to accurately segment different types of pathological cells on the cell pathological slice image, and simultaneously improves the recognition accuracy and the recognition efficiency.
Owner:怀光智能科技(武汉)有限公司
Probe of human papillomavirus and DNA chip comprising the same
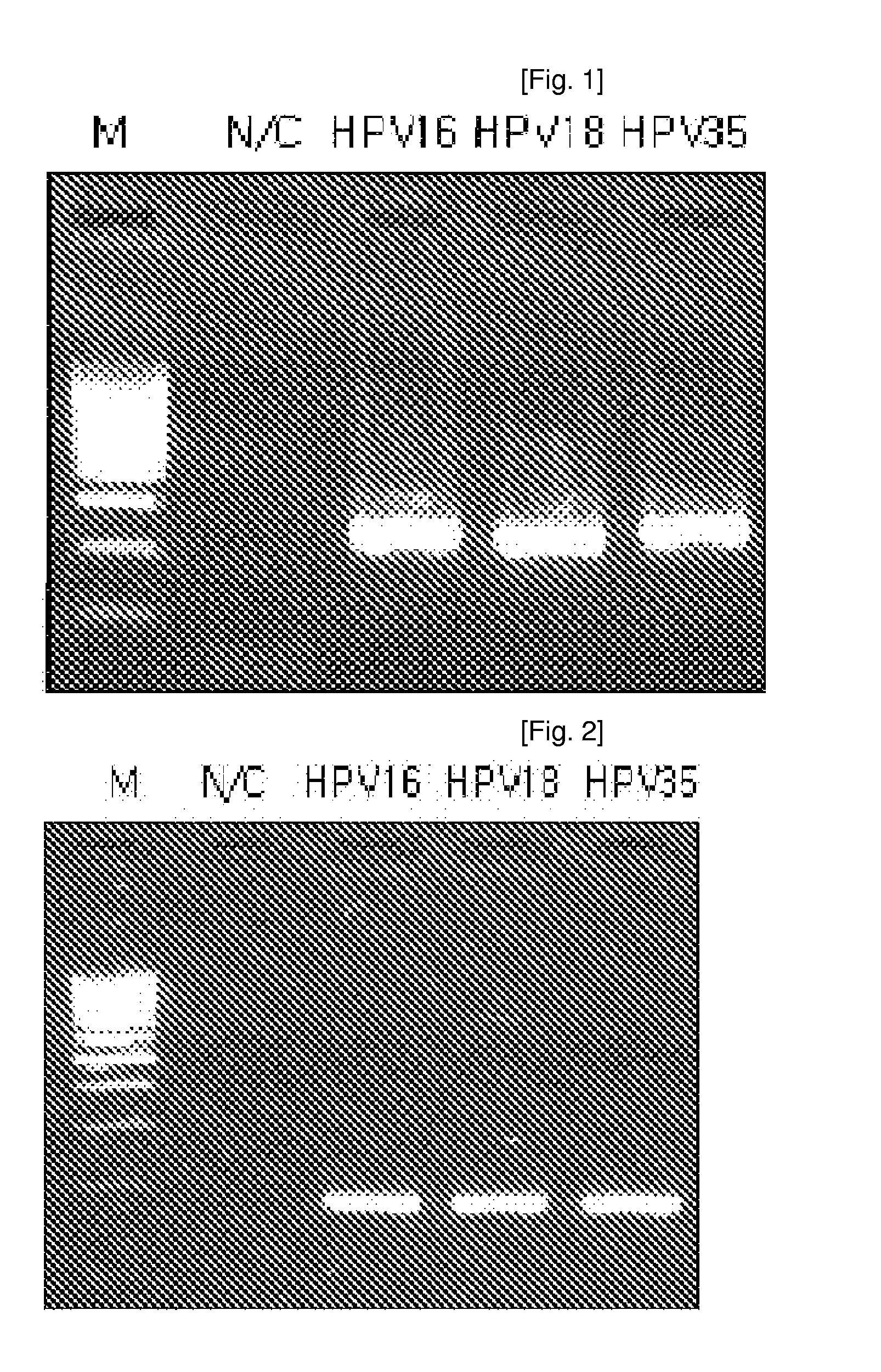
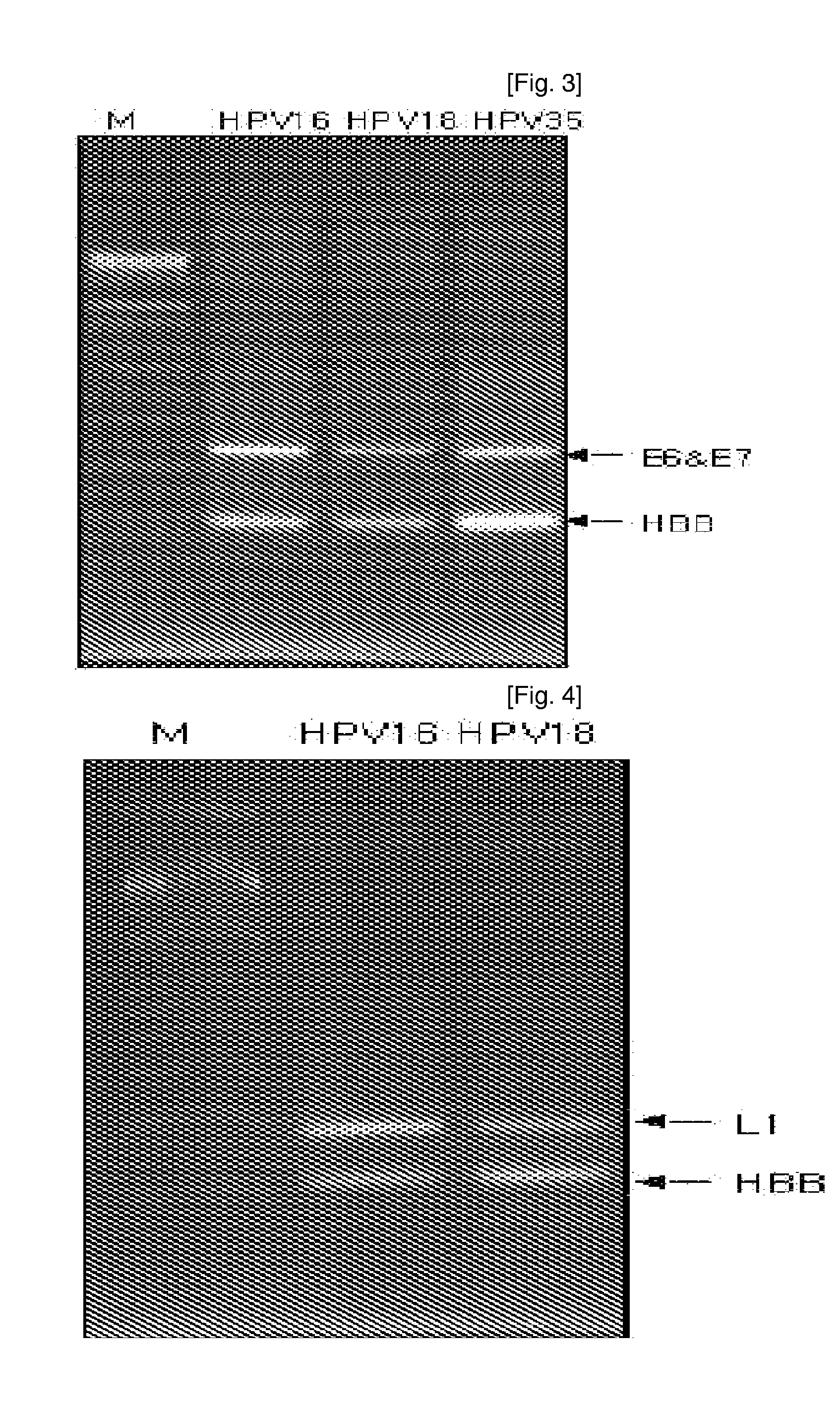
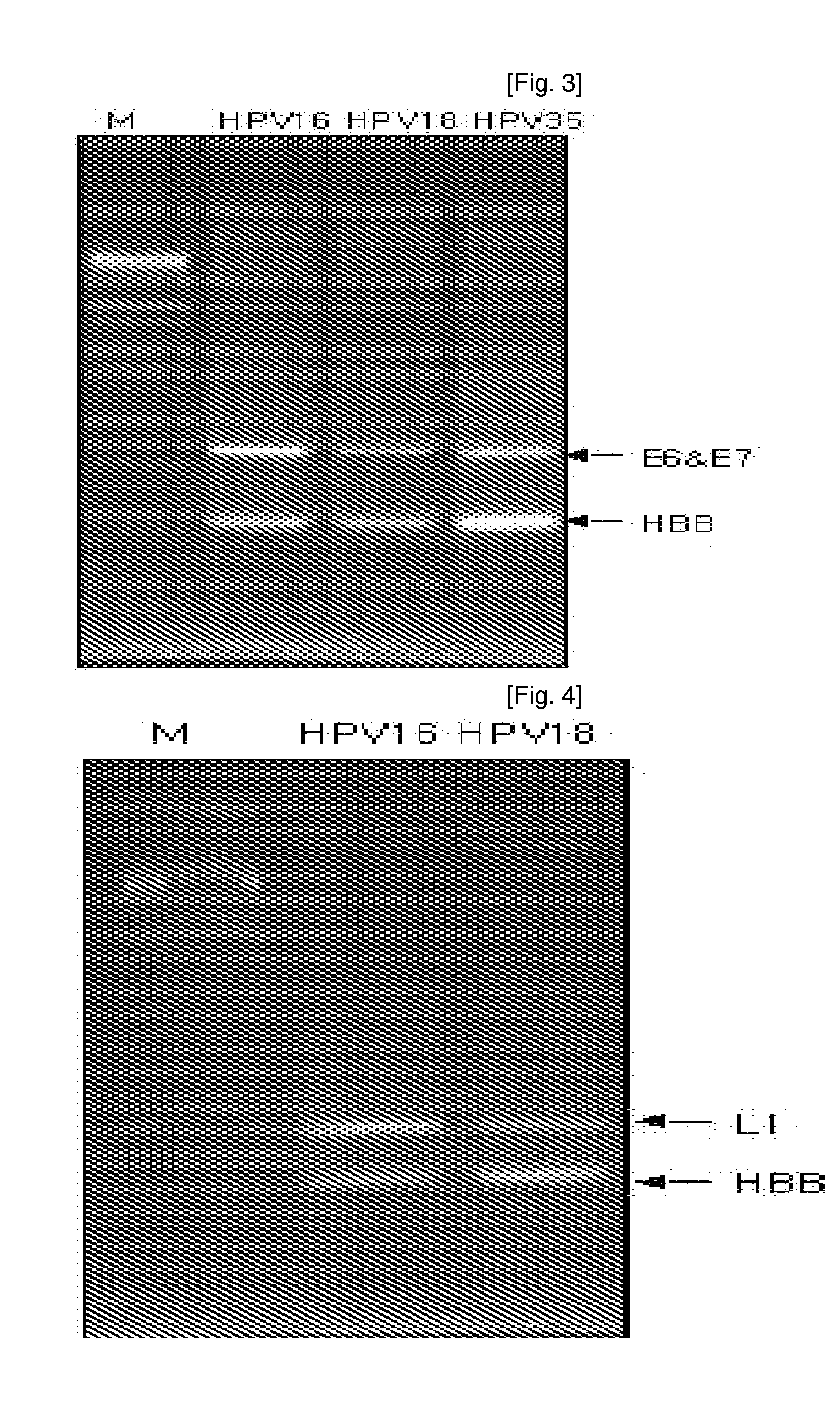
ActiveUS7670774B2High selectivityHigh sensitivityBioreactor/fermenter combinationsBiological substance pretreatmentsHuman papillomavirusBiology
Oligonucleotide probes for analyzing 40 types of HPV were synthesized, and DNA chips were produced by using the oligonucleotide probes. The synthesis of the oligonucleotide probes is based on clones of L1 and E6 / E7 genes of 35 types of HPV obtained from cervical cell specimens from 4,898 Korean adult women and tissue specimens from 68 cervical cancer cases in addition to information based on American and European cases. The DNA chips can analyze the 40 types of HPV found in cervical, diagnose complex infection by at least one type of HPV, and have excellent diagnostic sensitivity and specificity on HPV genetic type up to 100% and reproducibility. Also, the DAN chips are superior to conventional analytic method, and very economical, since they can analyze numerous specimens in shortest time. Accordingly, the DNA chips are useful for predicting cervical cancer and precancerous lesion.
Owner:GOODGENE
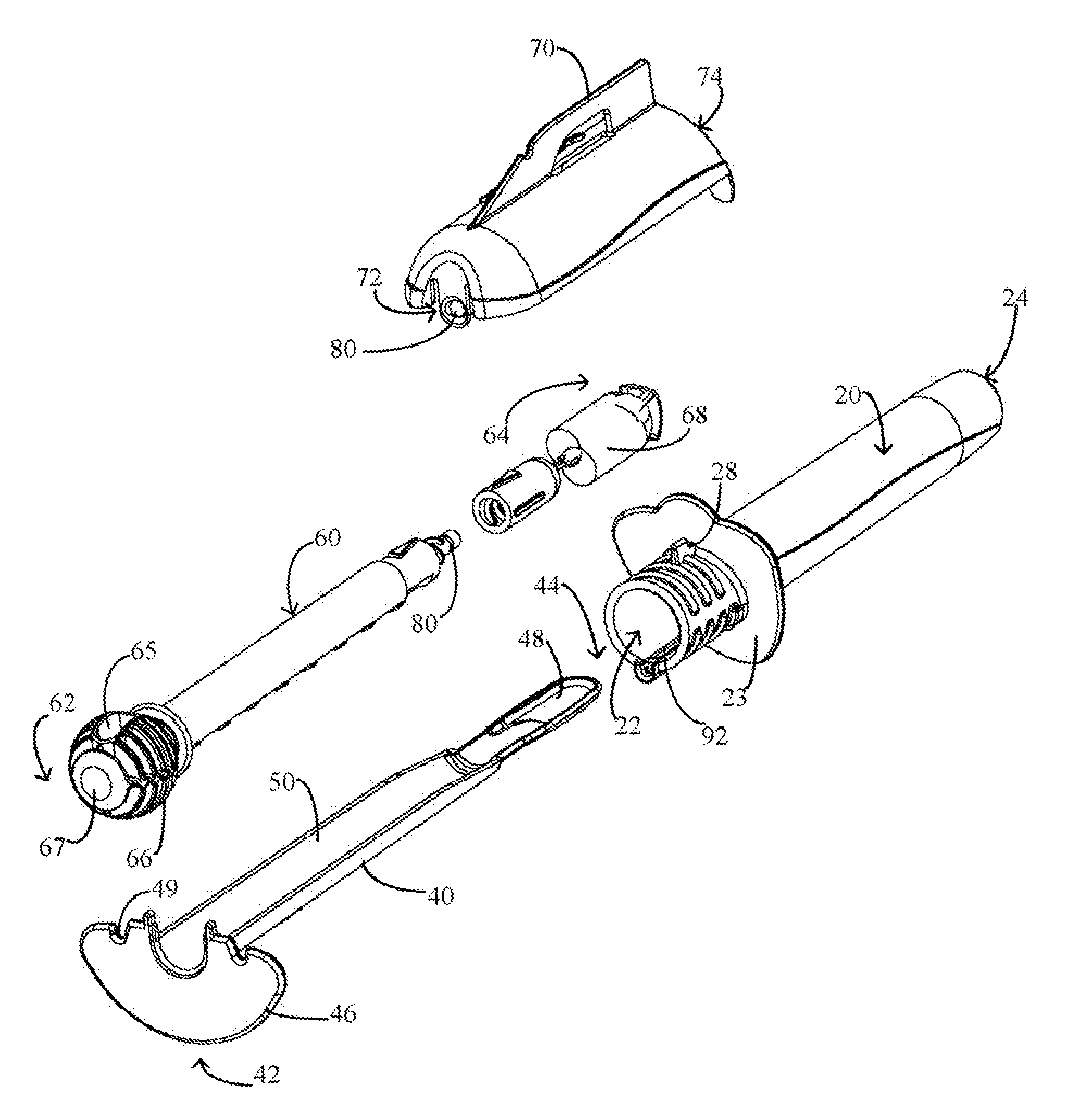
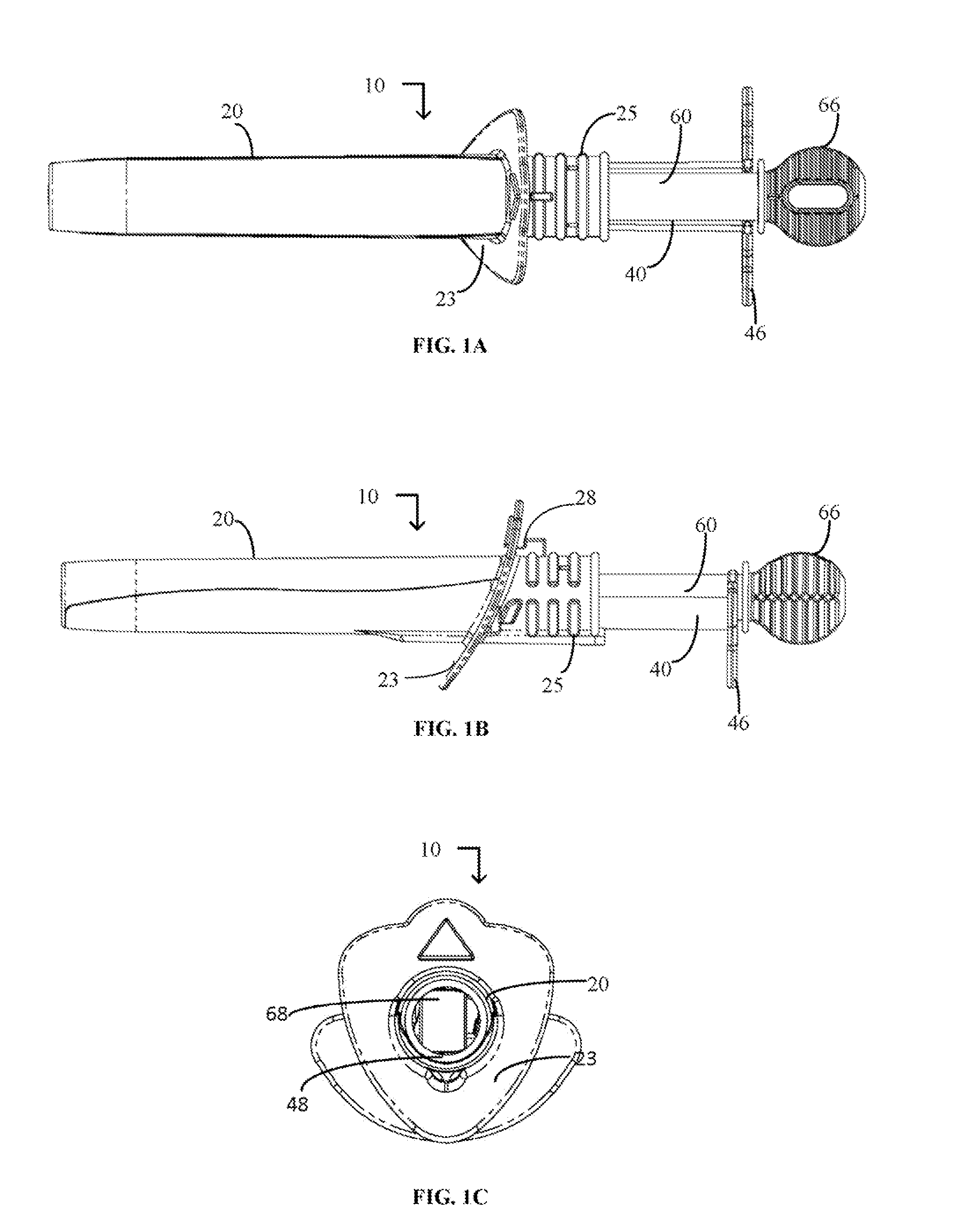
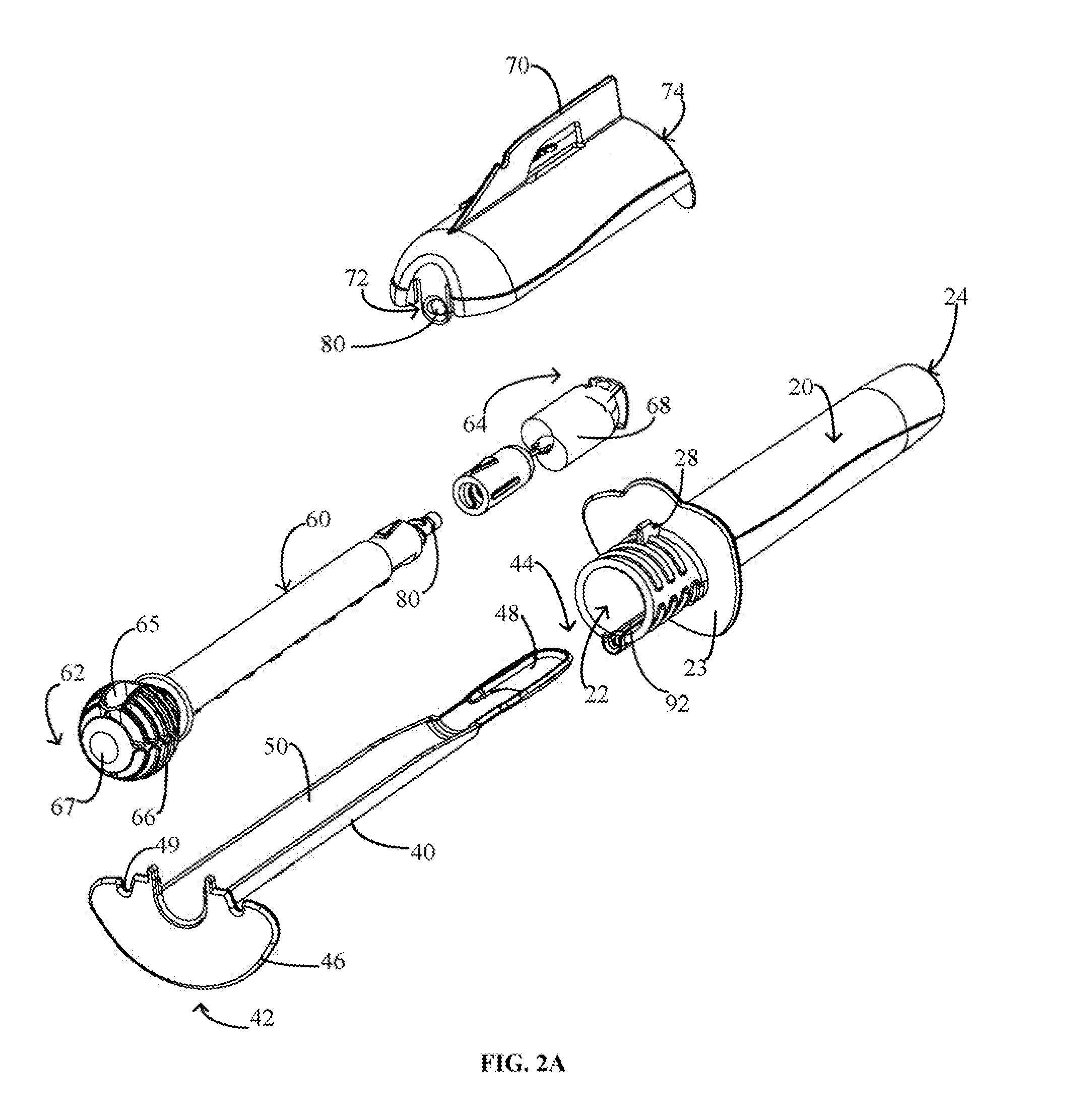
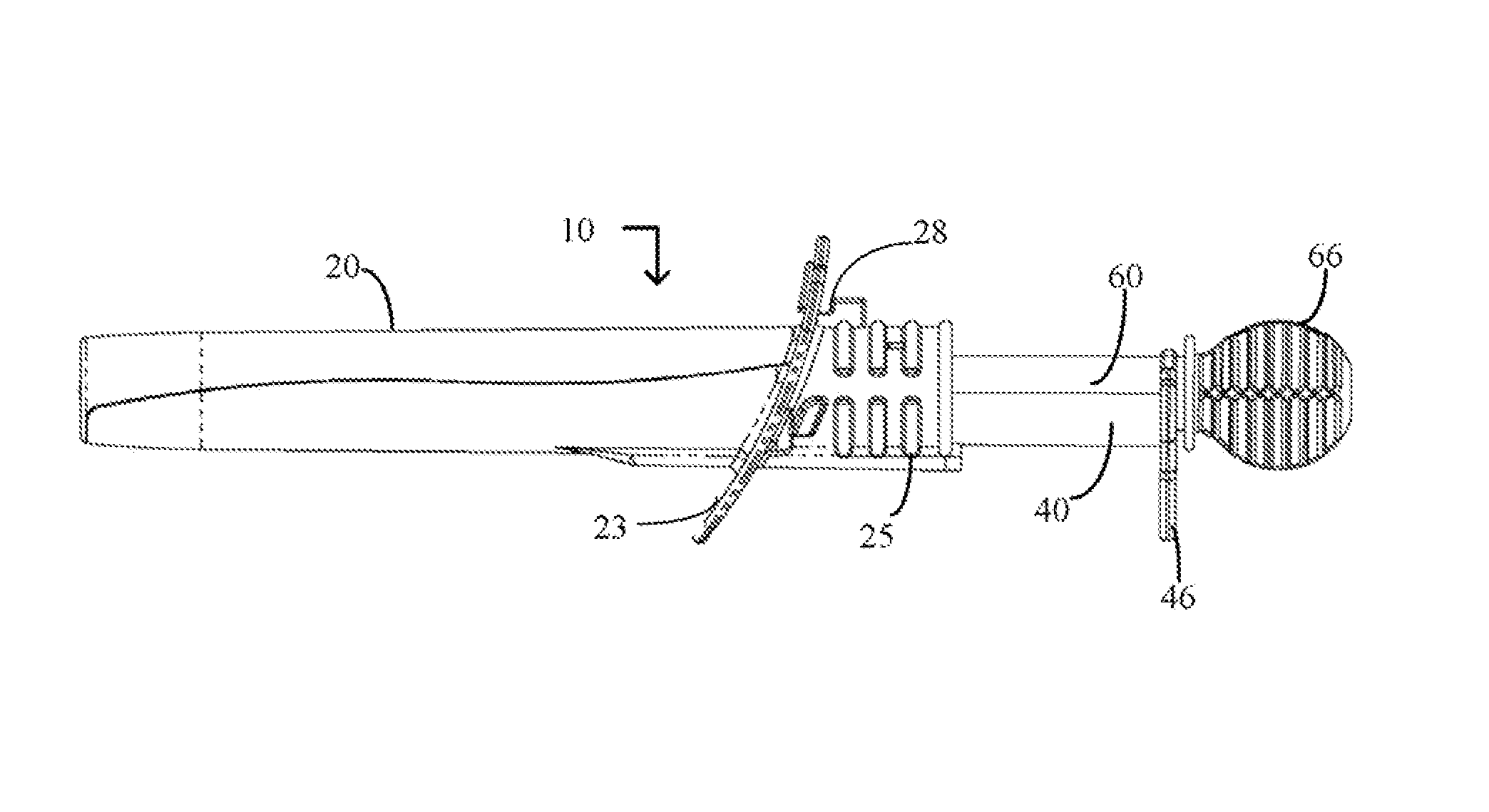
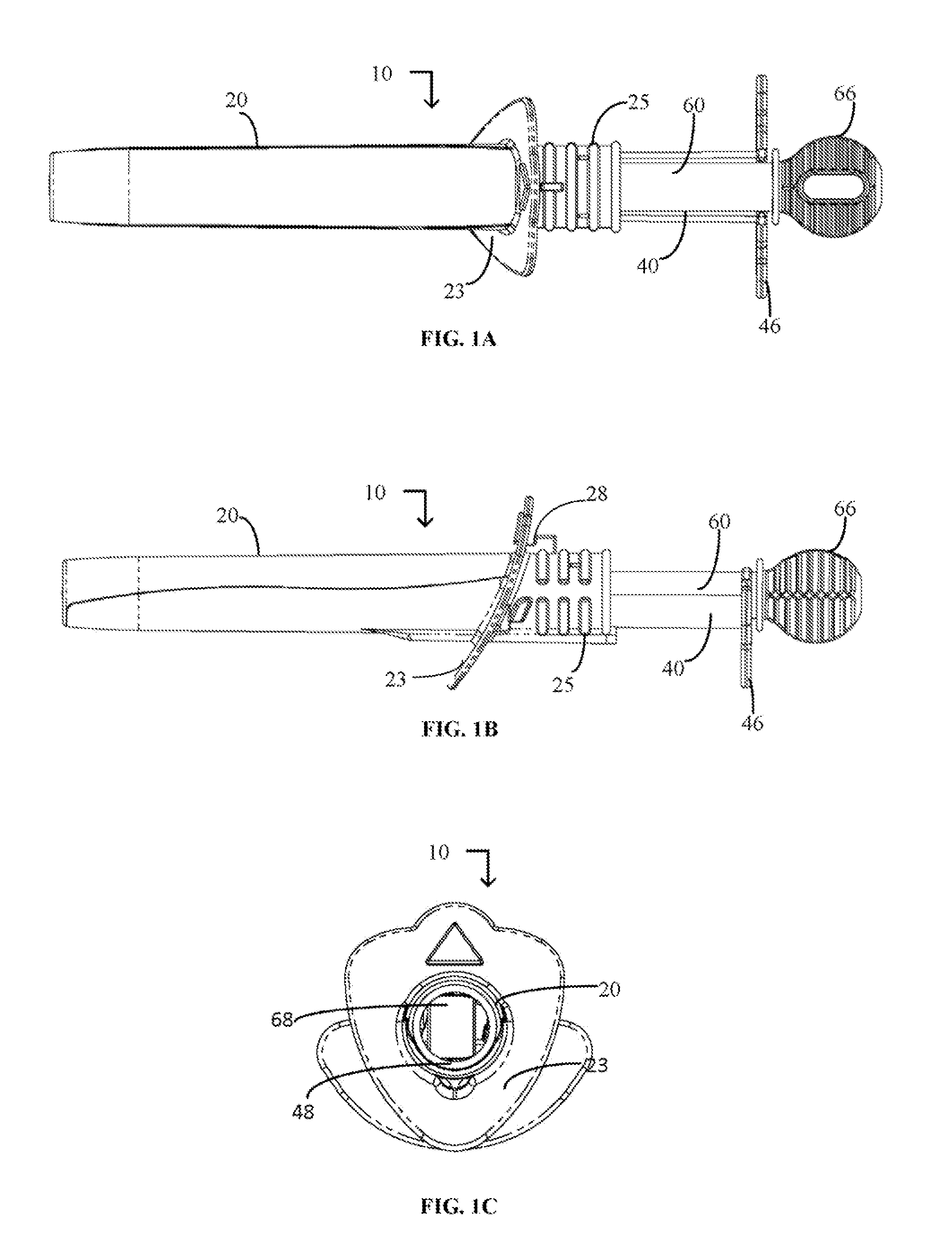
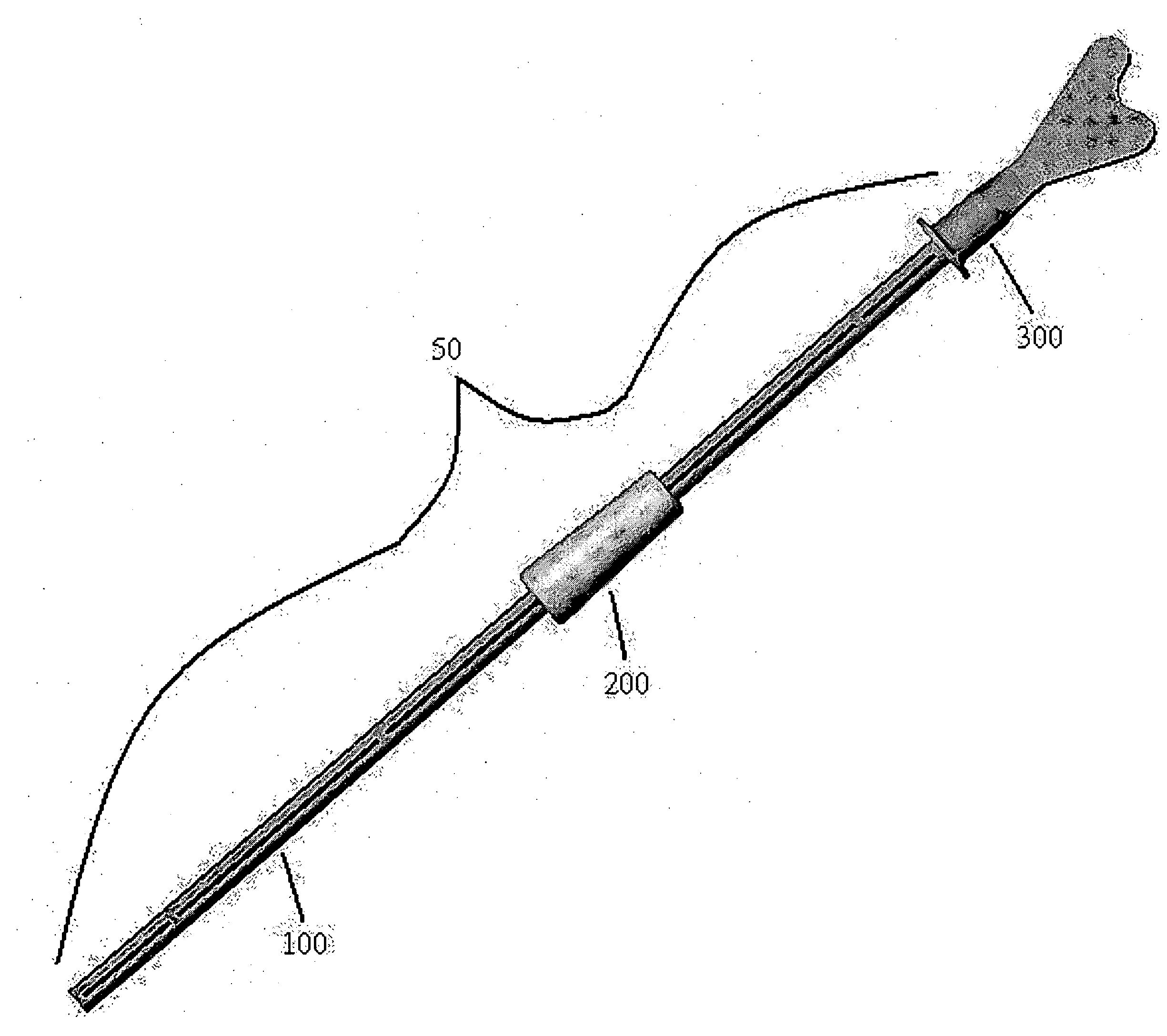
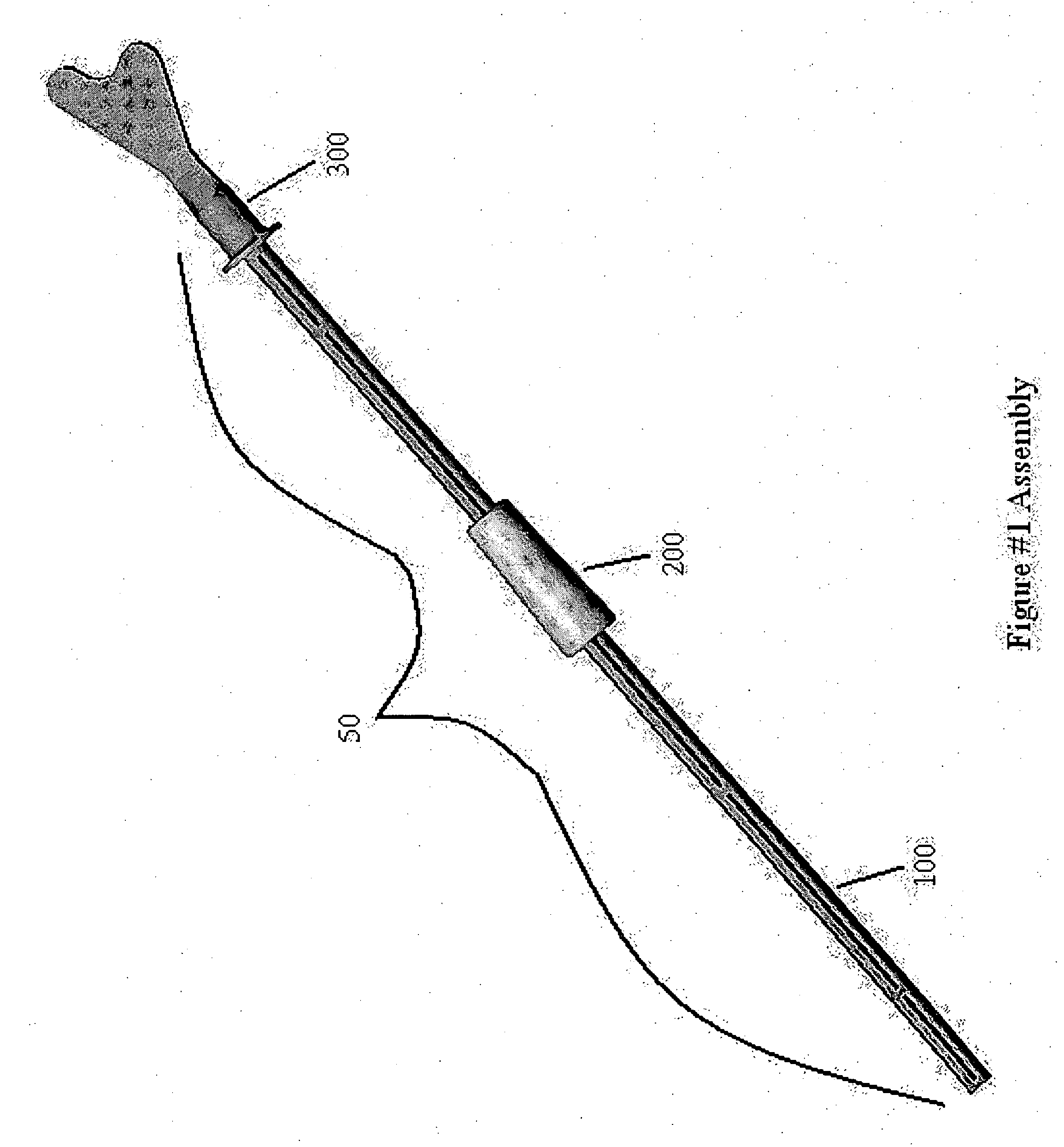
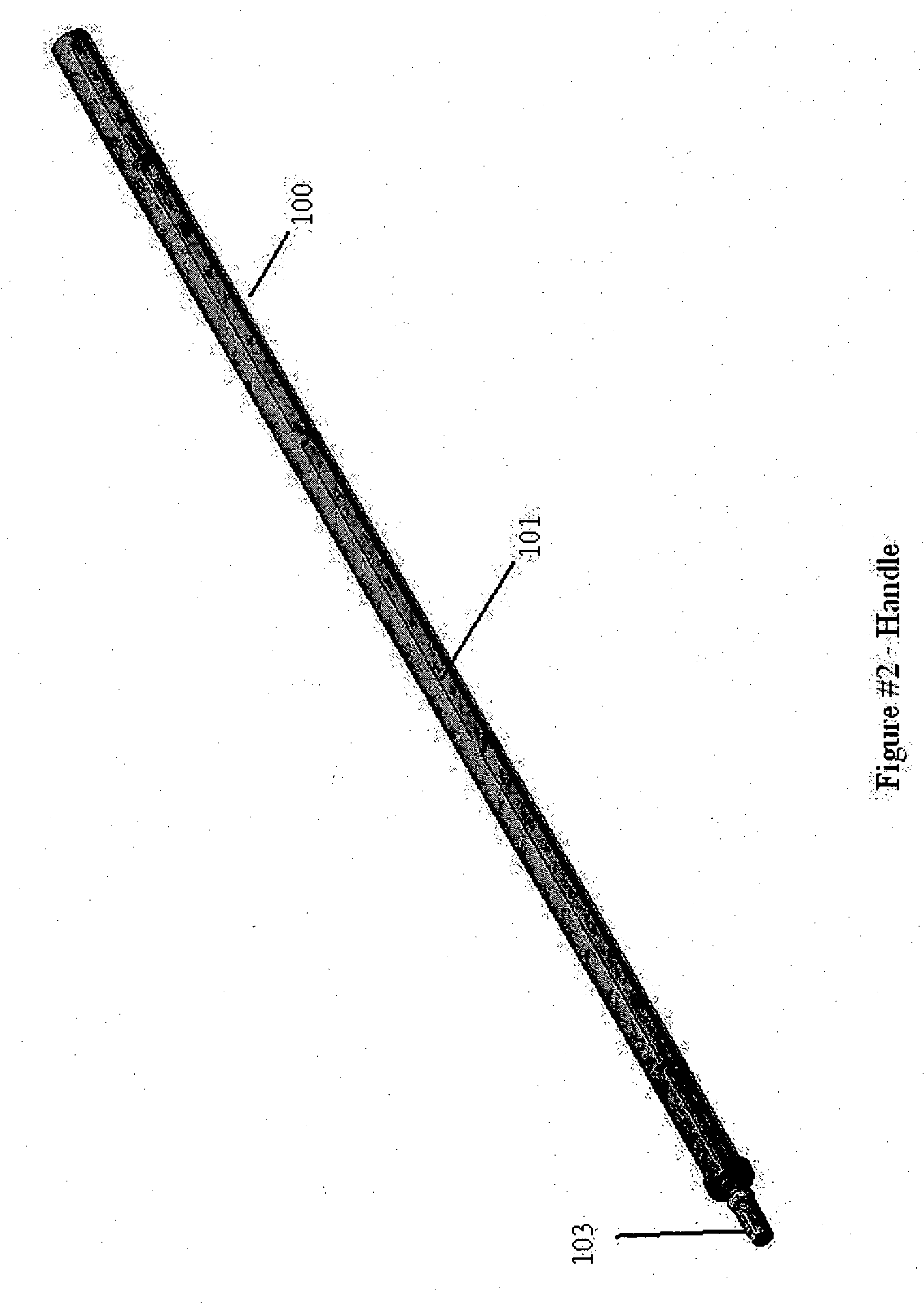
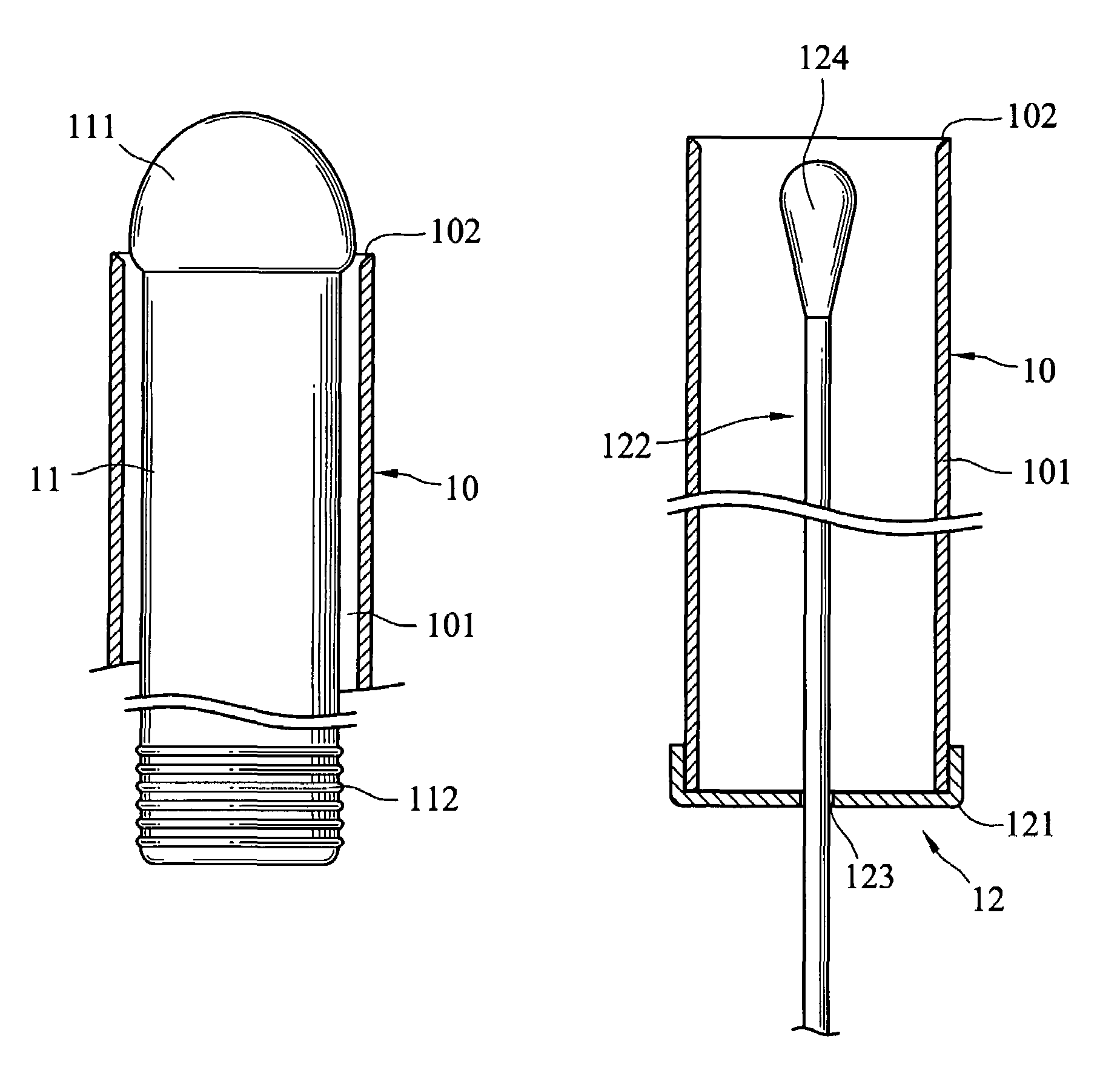
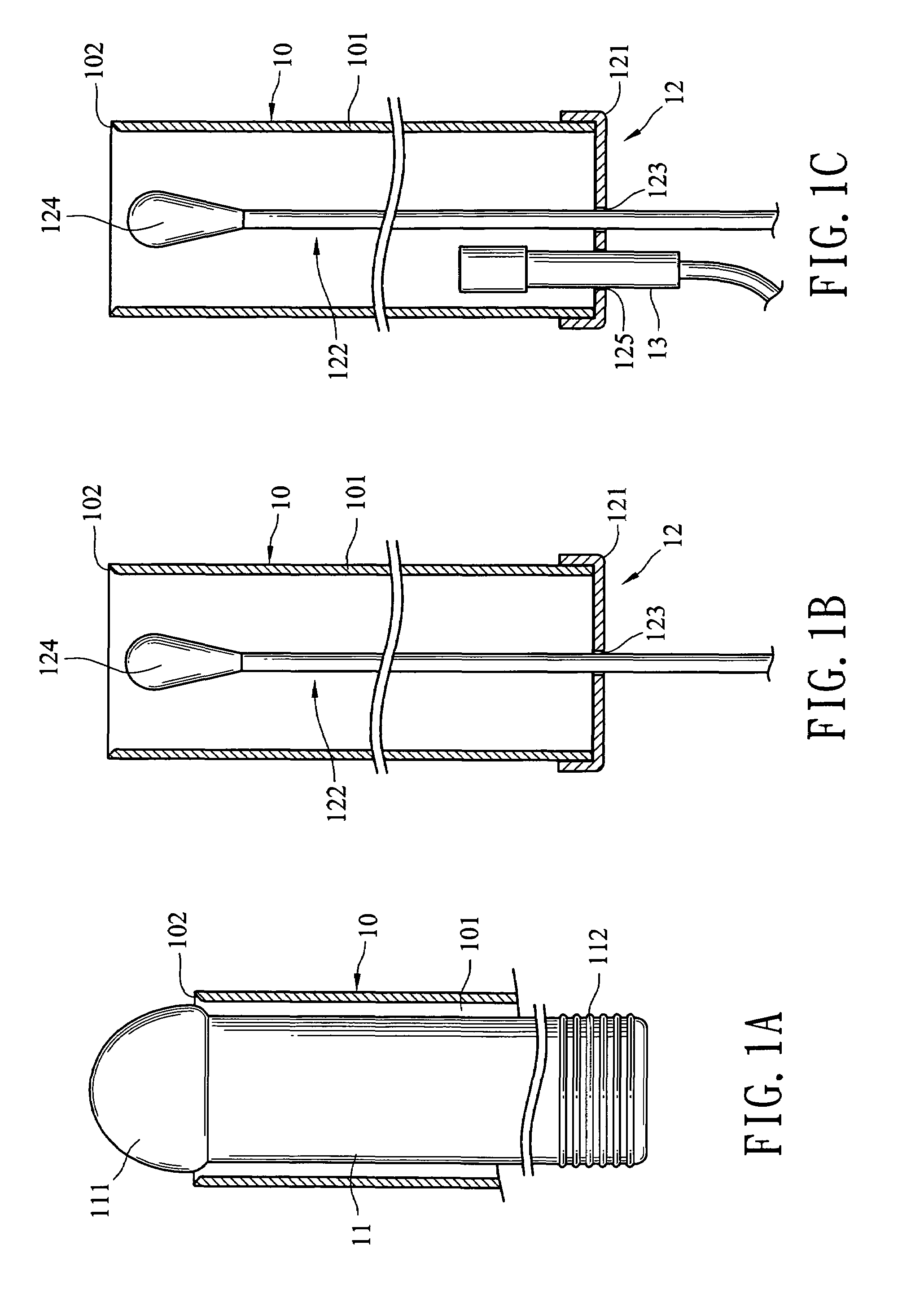
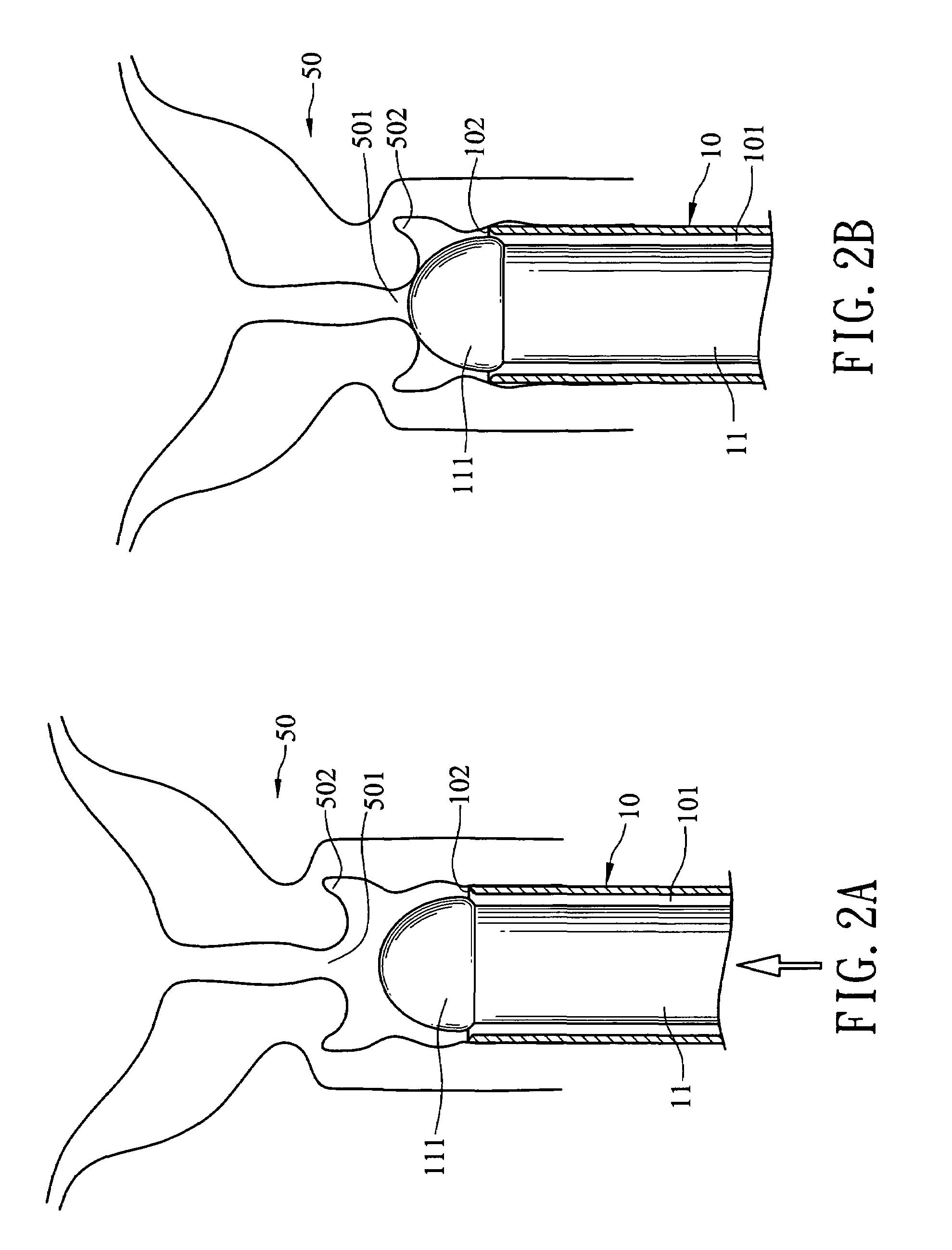
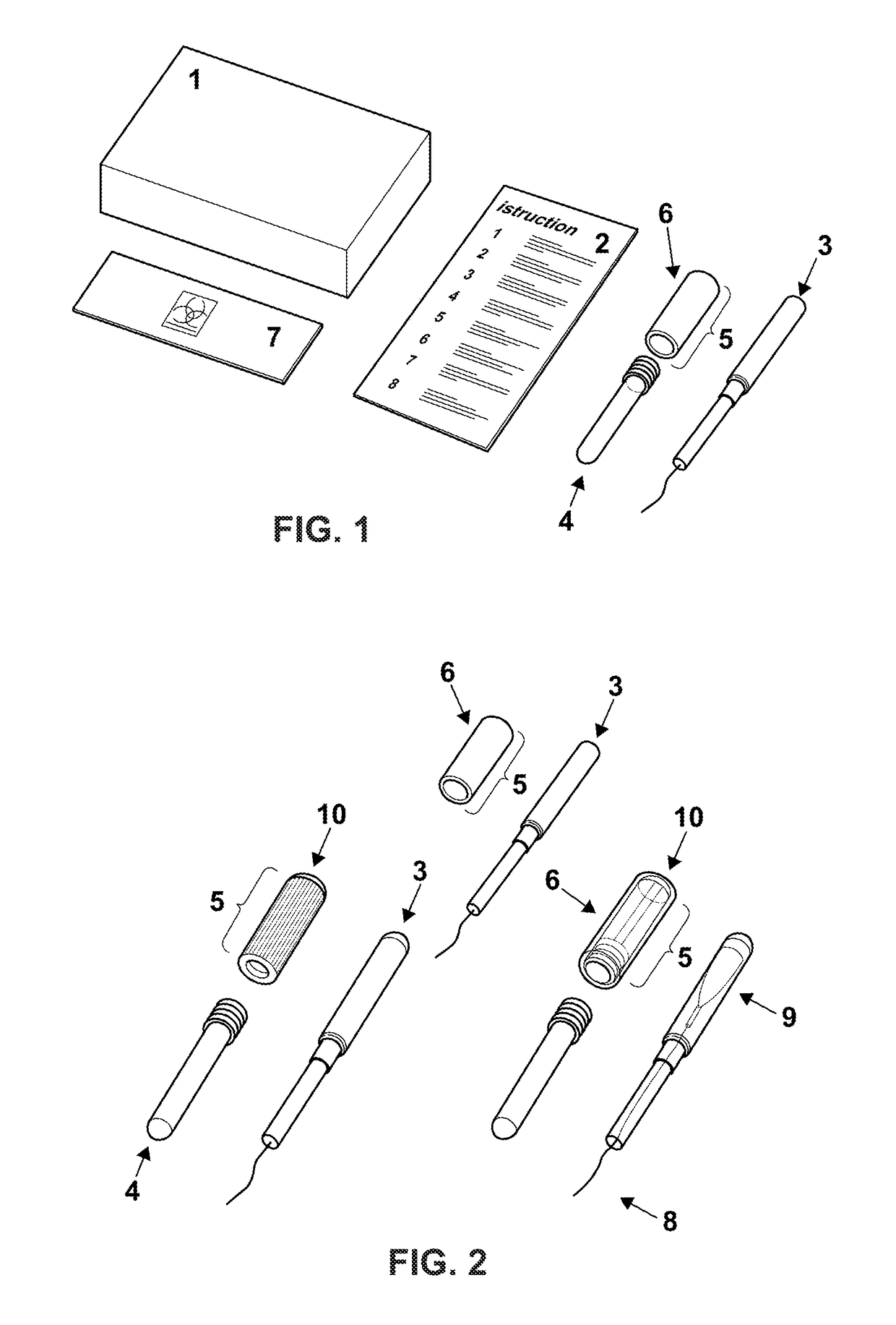
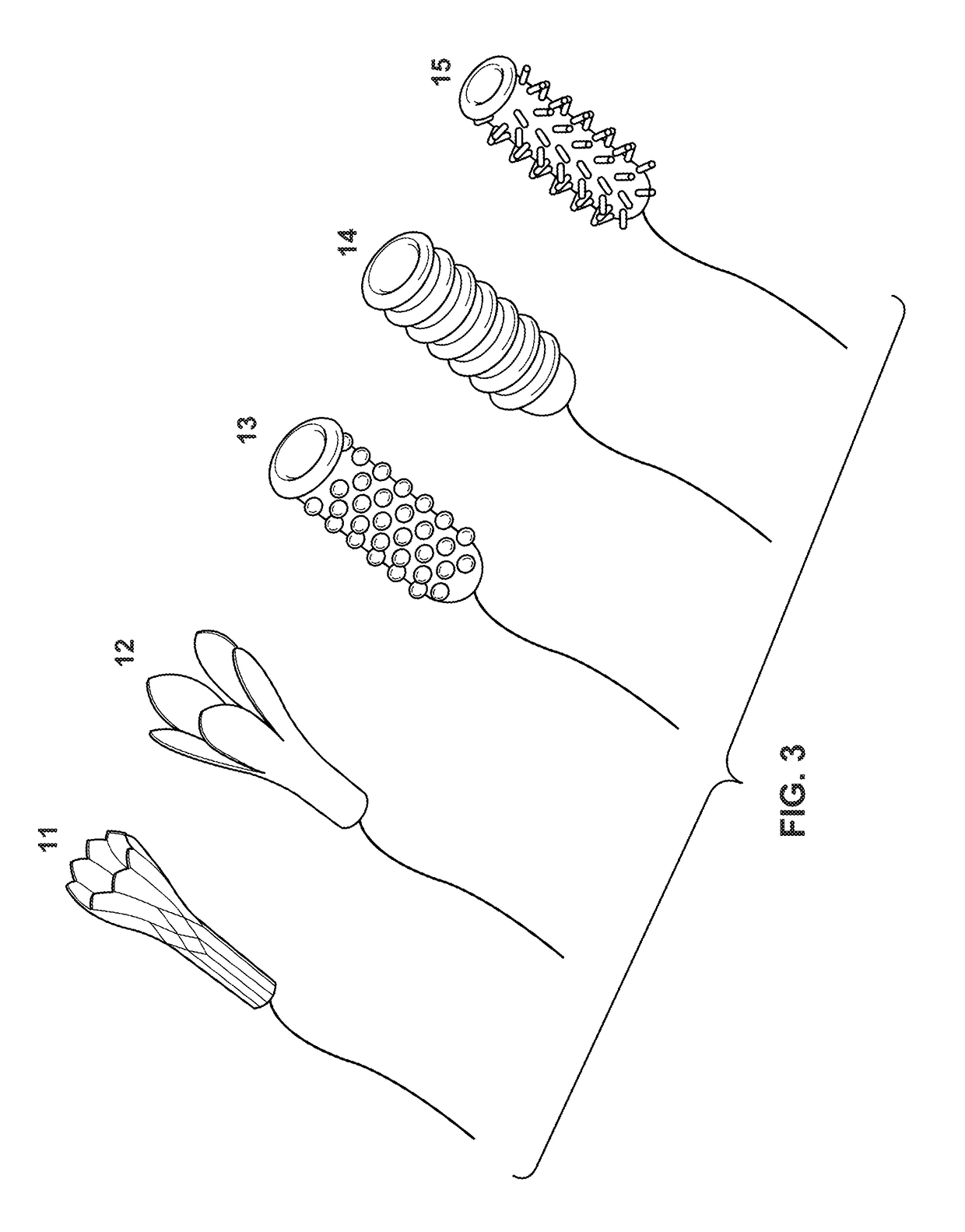
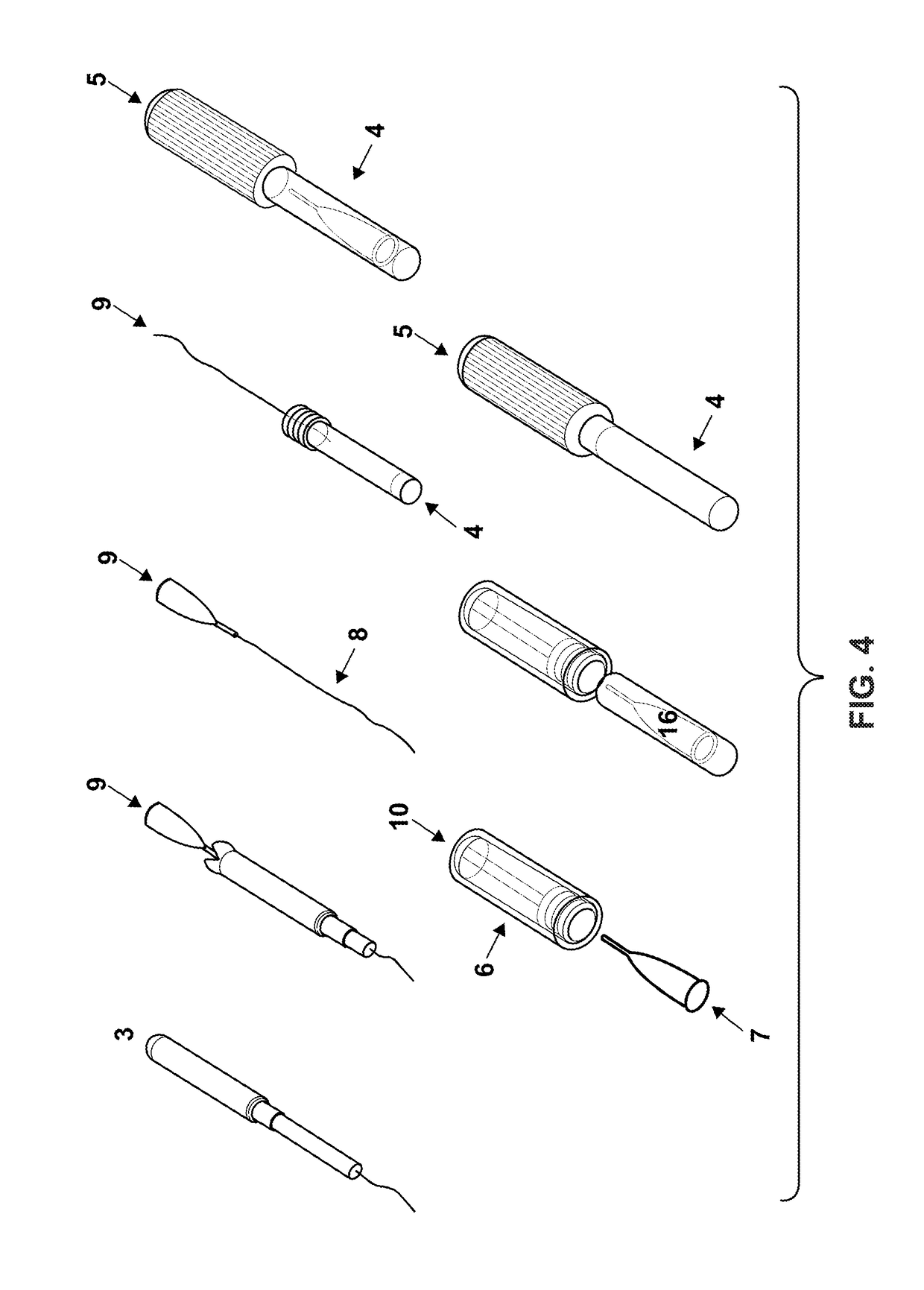
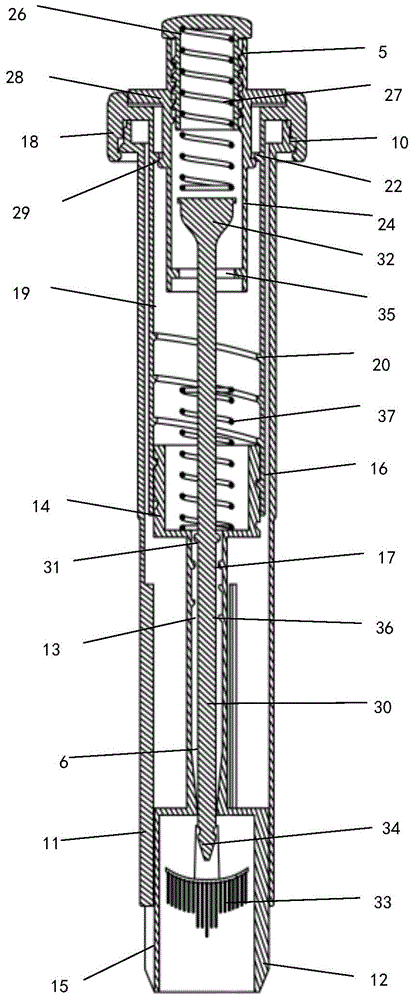
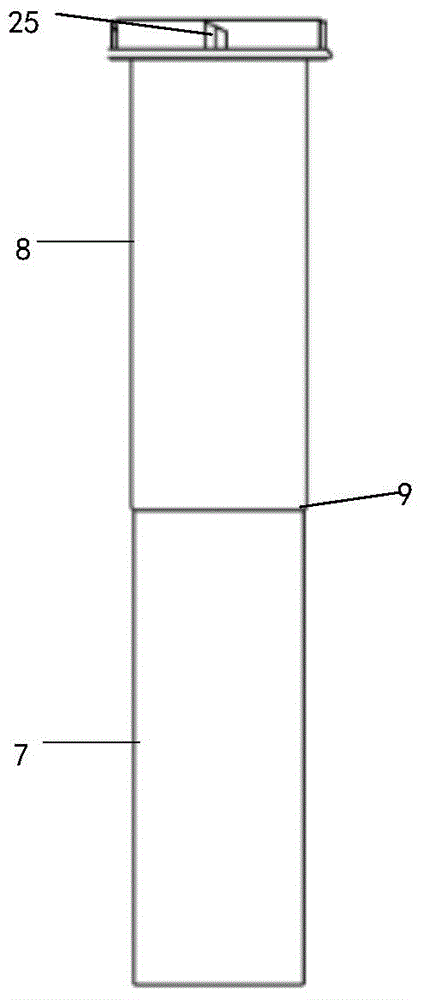
Cervical Cell Tissue Self-Sampling Device
ActiveUS20130066233A1Effective toolHigh densitySurgical needlesVaccination/ovulation diagnosticsCell adhesionSelf sampling
A device, a kit, and a method of use thereof, for self-administration and collection of cervical cell tissue samples such as for Pap smear testing. The device comprises an insertion tube, within which is carried a movable cervical aligning tool with an aligning probe, and a cellular sampling tool with a cellular adhesion surface. The aligning probe and cellular adhesion surface can be selectively movable relative to the insertion tube to improve accuracy of the testing and user safety.
Owner:GYNETECH
Cervical acid phosphatase - papanicolaou (CAP-PAP) test kit, method and accesories, processes for producing and using the same
InactiveUS20040137551A1Improves cell defense mechanismMicrobiological testing/measurementMaterial analysisGynecologyCervical cancer screening
Cervical Acid Phosphatase-Papanicolaou Test Kit (CPK) is an assembly of reagents, controls and instructions for visualization of cervical acid phosphatase on smears or monolayers of cervical specimens, and for performing the CAP-PAP Test (CPT). CPT is a single-slide, double-staining method for demonstration of cervical acid phosphatase activity inside abnormal cervical cells on Papanicolaou stained smears, and a set of criteria for using this test for cervical cancer screening. In previous clinical trials this method was found to enable Pap test screeners to improve test sensitivity (detection of abnormal cells) for more than 10% (from 0.8 to 0.9), and to reduce false negative readings (missing abnormal cells) for more than 50% (from 0.1 to 0.02). Due to better accuracy and the low cost, when approved, CPT may begin to replace current technologies for cervical cancer screening. CPK is designed to meet requirements for testing large series of specimens on regular basis-the usual practice in cytopathology laboratories performing the Pap test. CPK brings consistency for staining and interpretation, makes internal and external controls easier, and improves the test accuracy for lower cost, while increases laboratory productivity for less liability.
Owner:MARKOVIC NENAD S +1
Cervical cell tissue self-sampling device
ActiveUS8460209B2Effective toolHigh densitySurgeryVaccination/ovulation diagnosticsSelf samplingGynecology
A device, a kit, and a method of use thereof, for self-administration and collection of cervical cell tissue samples such as for Pap smear testing. The device comprises an insertion tube, within which is carried a movable cervical aligning tool with an aligning probe, and a cellular sampling tool with a cellular adhesion surface. The aligning probe and cellular adhesion surface can be selectively movable relative to the insertion tube to improve accuracy of the testing and user safety.
Owner:GYNETECH
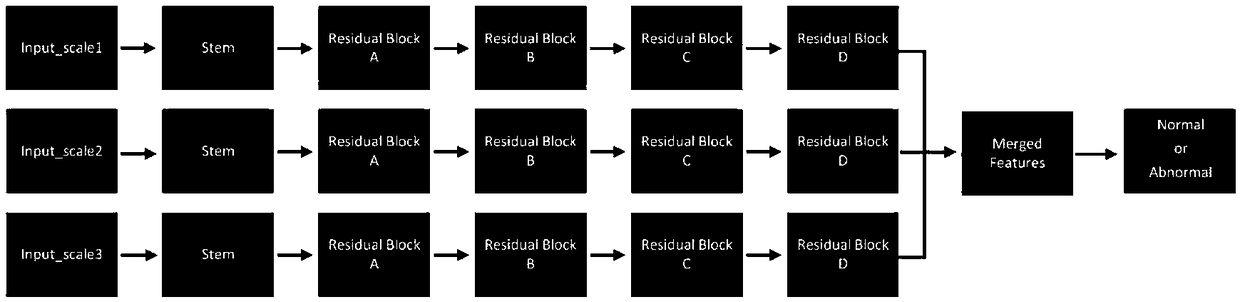
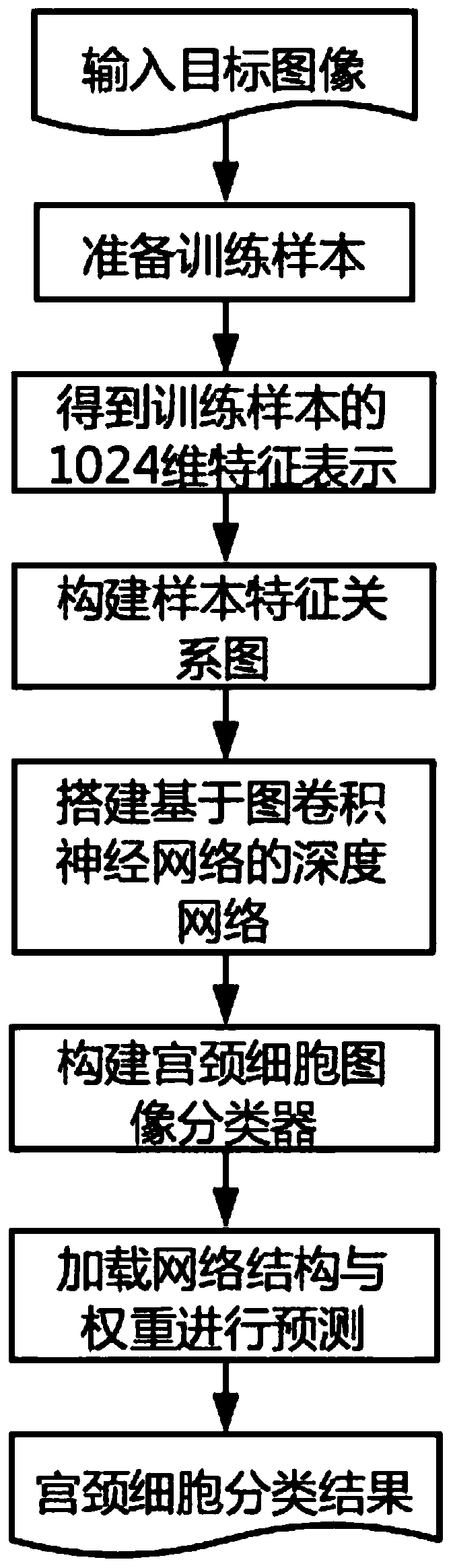
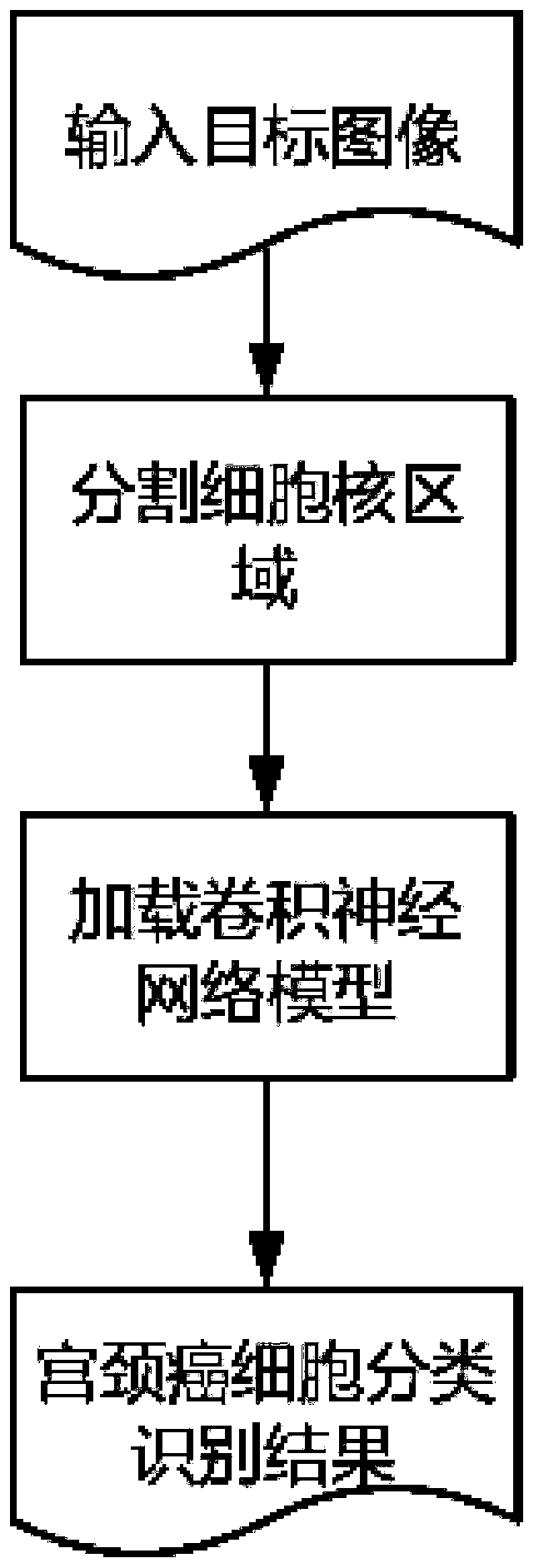
Cervical cell image classification method based on graph convolutional neural network
ActiveCN111274903AImprove diagnostic efficiencyImprove diagnostic accuracyCharacter and pattern recognitionNeural architecturesCervical cellsCervical cell
The invention discloses a cervical cell image classification method based on a graph convolutional neural network. Firstly, a cervix uteri cell image is prepared as a training sample; 1024-dimensionalfeature representation of a sample is acquired, a sample feature relation graph is constructed, a deep network based on a graph convolutional network is constructed, the sample and the sample featurerelation graph are sent to the deep network model for training, training is stopped after iteration is performed for a certain number of times, and network weight parameters are stored. When the method is used, a target image is segmented into a to-be-predicted area with cell nucleuses, then weight parameters and a network structure obtained through training are loaded, the to-be-predicted area is input into the to-be-predicted area, and a classification result can be obtained through calculation. According to the method, the cervical cell diagnosis accuracy and efficiency are improved, the diagnosis process of a pathologist is optimized, and the workload of the pathologist is reduced.
Owner:HEFEI UNIV OF TECH
Pap smear collection device with ejection sleeve
InactiveUS20060200043A1Lower potentialControl releaseSurgeryVaccination/ovulation diagnosticsPregnancy testsPregnancy test
Sample collection for the Pap smear sample is critical for accurate diagnosis. Improper sample collection, poor sampling, and / or cell preservation can render a Pap smear unsatisfactory for evaluation, requiring a repeat smear collection. If the Pap smear does not contain appropriate representative cells from the transformation zone and endocervical canal, the ability of the test to detect disease is very low. Likewise, if the preservation of the sample is compromised, the screener's ability to recognize abnormal cells is greatly diminished. It is generally understood that cervical samples should be harvested by a two-stage technique, which includes sampling of the endocervical canal with a cytobrush and obtaining a sample from the transformation zone with a spatula. The use of either the cytobrush or the spatula alone may be adequate but not as effective as the two-stage technique. Both the Cervex Brush and REG;(Unimar, Inc.) and the Accellon Combi & REG;(Medscand AB) are two collection devices which combine the action of the cytobrush and spatula, thus permitting broader sampling with a one stage technique. The standard method of transferring cervical cells from the collection device or complete transfer of the collection device into the liquid collection vial is often challenging. These challenges include but are not limited to: 1-spilling the sample, 2-dispersing the medium collection such that air born body secretions could contact unprotected health care workers, 3-missing the collection container and contaminating the sample. It is the goal of the current device embodiment to provide a simple and consistent method of transferring the entire collection specimen into the collection container to maximize cell collection while minimizing challenges of head disengagement. Such devices are not limited to the cervical cell cytology collection markets but extend to all cell collection methods were the entire sample is suspended in a liquid or similar medium. Such samples include oral cavity collections (throat swabs, vaginal cavity collections (STDs, pregnancy test), urethral cavity collections (male STDs) fornex collections (Alaph fetal protein), and rectal collections as well as open procedures requiring cell sampling.
Owner:JANNETTY JOSEPH D +1
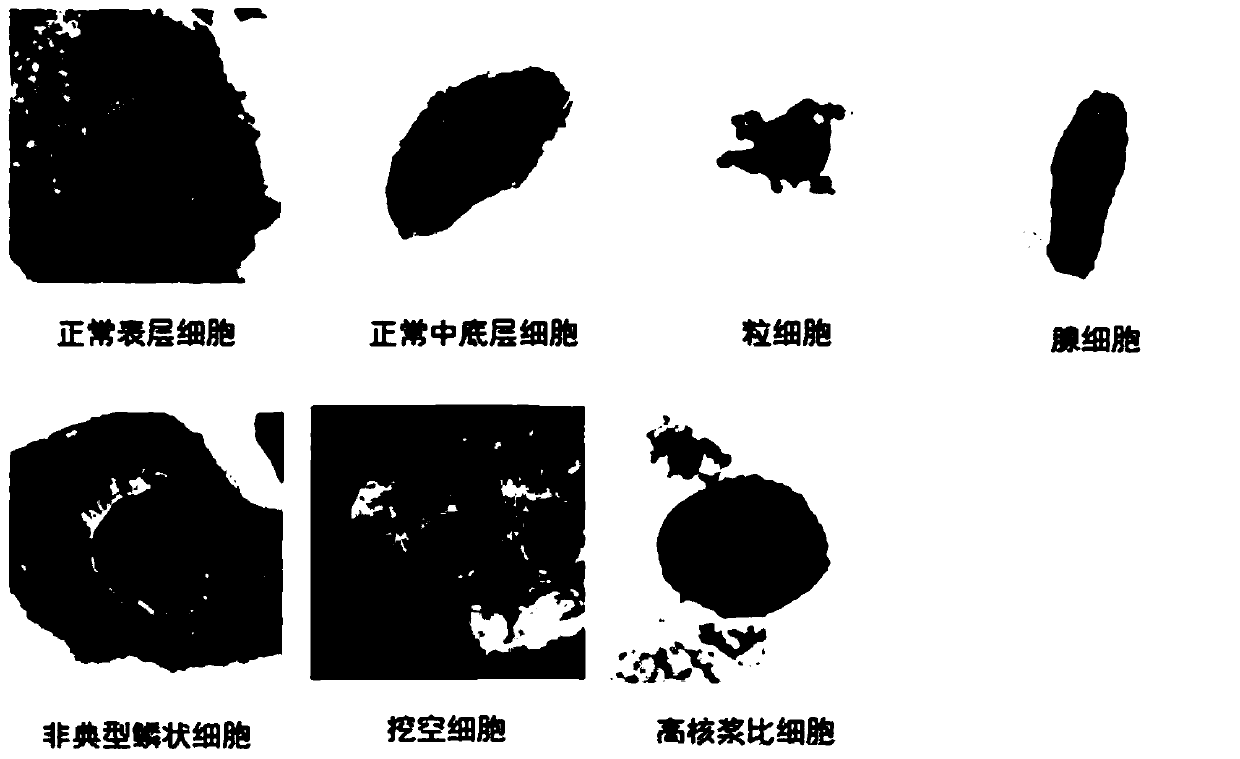
Cervical cell image classification method based on convolutional neural network
InactiveCN110363188AFew parametersImprove diagnostic accuracyAcquiring/recognising microscopic objectsNeural architecturesCervical cellNetwork structure
The invention discloses a cervical cell image classification method based on a convolutional neural network. The method comprises the following steps: preparing a training sample, classifying the training sample to obtain eleven types of samples, constructing a convolutional neural network, inputting the training sample into a convolutional neural network model for training, iterating for a certain number of times, stopping training, and storing network weight parameters. When the method is used, a target image is segmented into a to-be-predicted area with cell nucleuses, and then weight parameters and a network structure obtained through training are loaded. The to-be-predicted area is input into the to-be-predicted area. A classification result can be obtained through calculation. The method improves the accuracy and efficiency of cervical cell diagnosis, and has strong adaptive capacity.
Owner:MOTIC XIAMEN MEDICAL DIAGNOSTICS SYST +1
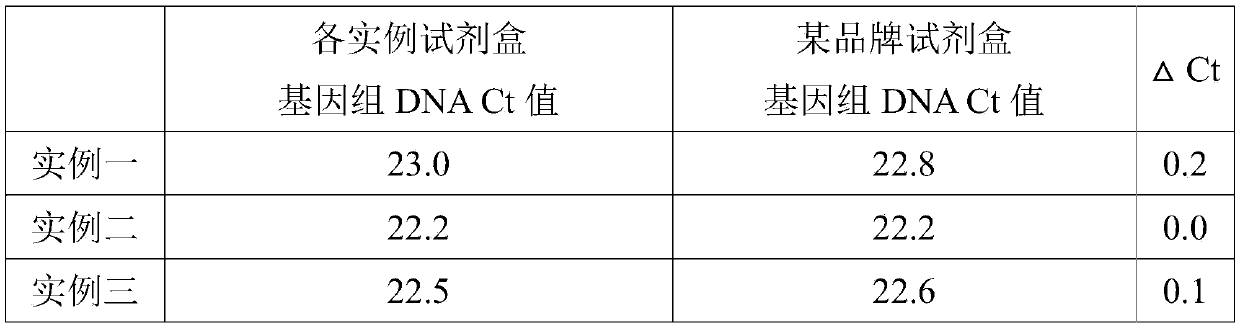
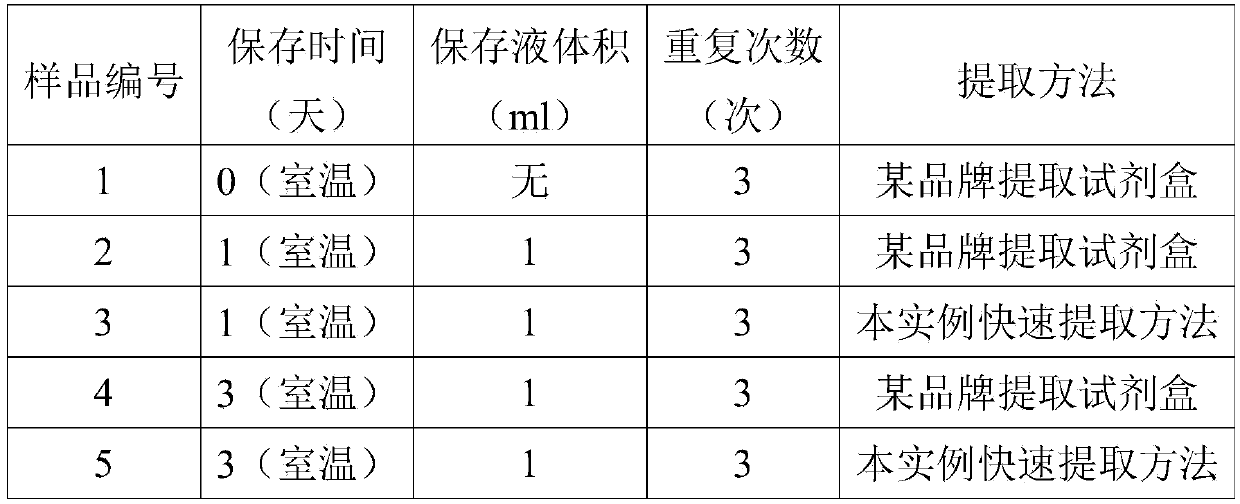
Cervical cell preservation and DNA fast extraction integrated kit and extraction method
ActiveCN105368817AGuaranteed accuracyImprove stabilityDead animal preservationDNA preparationA-DNABiology
The invention provides a cervical cell preservation and DNA fast extraction integrated kit which comprises cell preservation liquid, a first cleaning solution, a second cleaning solution, eluent and a DNA purification column. The cell preservation liquid comprises 2-3mol / L of guanidine hydrochloride or guanidinium thiocyanate, 5-50mmol / L of Tris-HCl, 1-10mmol / L of EDTA and 5-7.5v / v% of Trixon-100. The cervical cell preservation and DNA fast extraction integrated kit has the advantages that cervical sample DNA can be preserved in the cell preservation liquid of the kit for more than a week under room temperature, transportation of collected samples is facilitated, subsequent DNA extract does not need complex splitting anymore, the whole extraction process can be completed in 10 minutes, and DNA extraction efficiency is increased evidently; in addition, the extraction operation only needs a liquid-moving device and a centrifuge, instrument dependence is low, and the extracted DNA is high in purity.
Owner:GWP BIOTECHNOLOGIES INC
Personal cervical cell collector
InactiveUS7144377B2Simple positional functionSurgeryVaccination/ovulation diagnosticsCervical cellPosition effect
Owner:LU LI CHENG
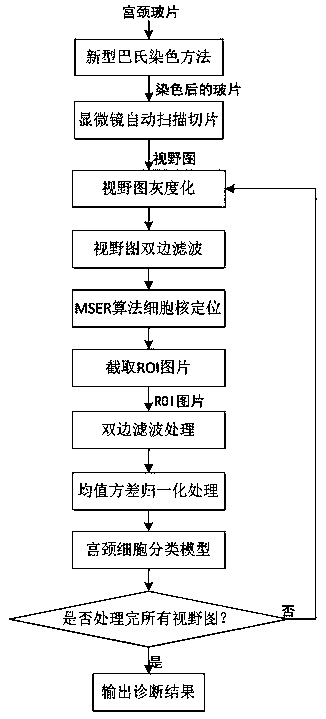
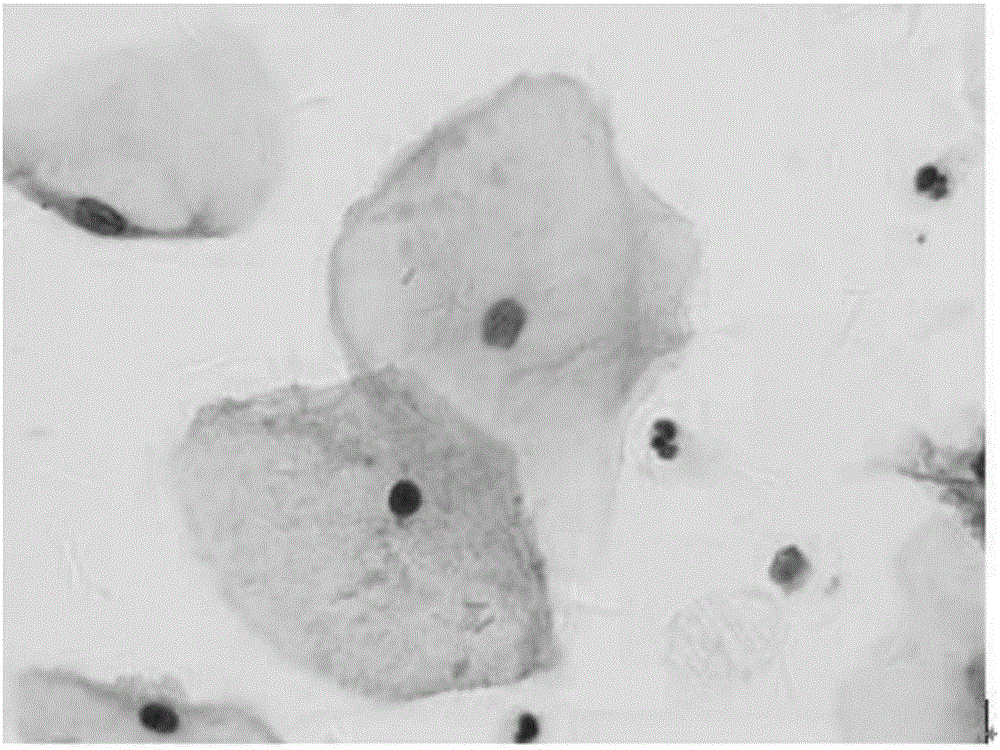
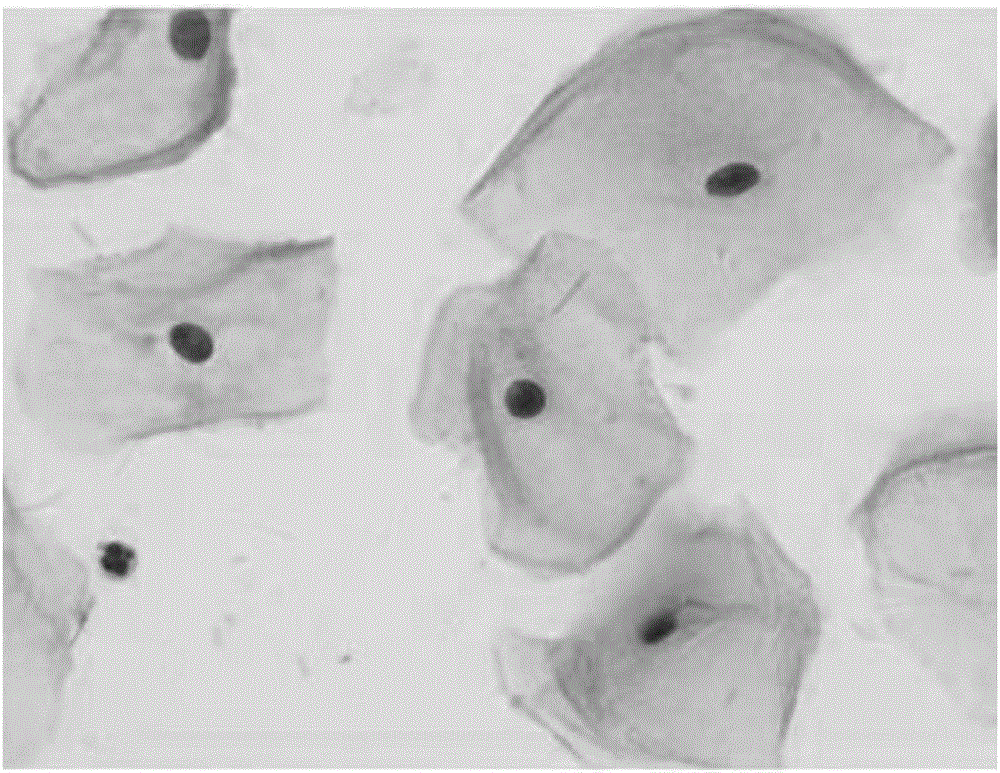
A novel pasteurization method and an abnormal cervical cell automatic identification method
ActiveCN109815888AImprove the degree of stainingIncrease contrastPreparing sample for investigationCharacter and pattern recognitionDiseaseSocial benefits
The invention discloses a novel pasteurization method and an abnormal cervical cell automatic identification method. The novel pasteurization method comprises two modules, the module 1 is used for training a cervical cell classification model based on a mass cervical cell data set, and the module 2 is used for identifying abnormal cervical cells by using the trained classification model. The invention provides the novel pasteurization method, so that the problems existing in the traditional pasteurization method are well solved, and the possibility is provided for realizing automatic and high-precision positioning of cervical cell nuclei by a computer. The invention also provides the abnormal cervical cell automatic identification method, and experiments show that the method realizes the ultrahigh-precision automatic identification of abnormal cervical cells, so that the diagnosis burden of pathologists is greatly reduced, the diagnosis efficiency and the precision of the cervical diseases are improved, and the method has very high practical value and huge social benefits.
Owner:WUHAN LANDING INTELLIGENCE MEDICAL CO LTD
Alkali solution based cell preservation treating fluid and preparation method thereof
ActiveCN106442078AImprove solubilityWell-preservedPreparing sample for investigationCell adhesionRed blood cell
The invention belongs to the field of cell treating fluid, and particularly relates to alkali solution based cell preservation treating fluid and a preparation method thereof. The alkali solution based cell preservation treating fluid is mainly composed of an egg white dissolution agent, a red blood cell cracking agent, an alkaline substance, a corrosion remover and distilled water. The alkali solution based cell preservation treating fluid is mainly used in cooperation with a membrane type liquid based thin-layer cell slide preparation machine and can enhance the cell adhesion capability of a modified glass slide, promote cervical cell enrichment so as to firmly attach cervical cells to the specially-prepared glass slide and effectively reduce the phenomenon of leak detection. Meanwhile, the novel alkali solution based cell preservation treating fluid has the advantages of being free of toxicity, environmentally friendly, capable of rapidly and thoroughly achieving sterilization, low in cost, convenient to configure, safe and efficient.
Owner:SICHUAN KINGMED DIAGNOSTICS CENT
Non-invasive prenatal genetic diagnosis using transcervical cells
InactiveUS20060040305A1Microbiological testing/measurementDisease diagnosisPrenatal diagnosisCervical cell
A non-invasive, risk-free method of prenatal diagnosis is provided. According to the method of the present invention cell specimens are subjected to molecular and morphological methods which allow trophoblast identification. Trophoblasts identified according to the teachings of the present invention can be further examined to thereby prenatally diagnosing a fetus. Also provided is a method of in situ chromosomal, DNA and / or RNA analysis of a prestained specimen by incubating the prestained specimen in ammonium hydroxide. Also provided is a method of identifying embryonic cells according to a nucleus / cytoplasm ratio of at least 0.3 and the presence of at least variably condensed chromatin.
Owner:MONALIZA MEDICAL
System and method for detecting abnormalities in cervical cells
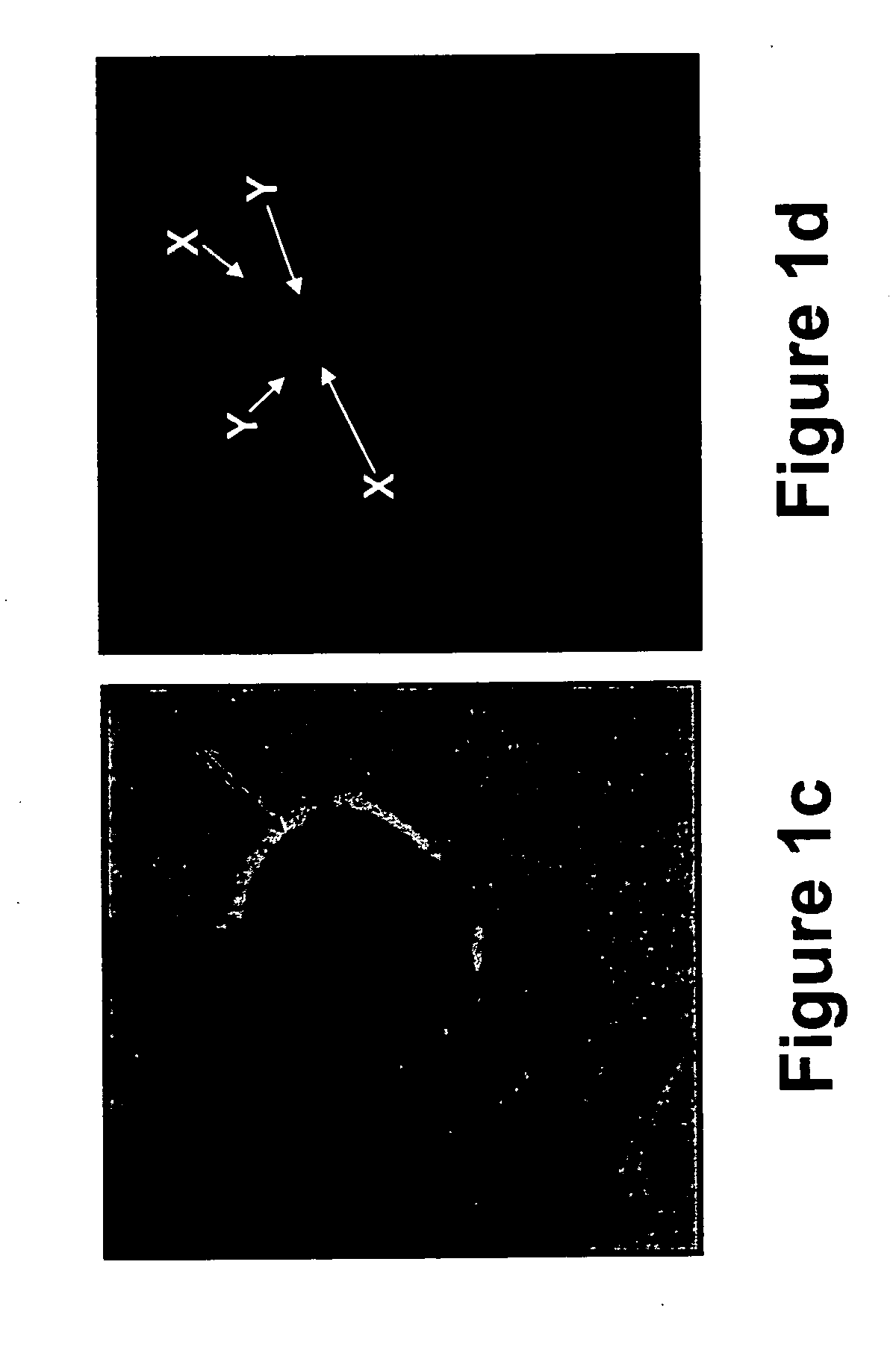
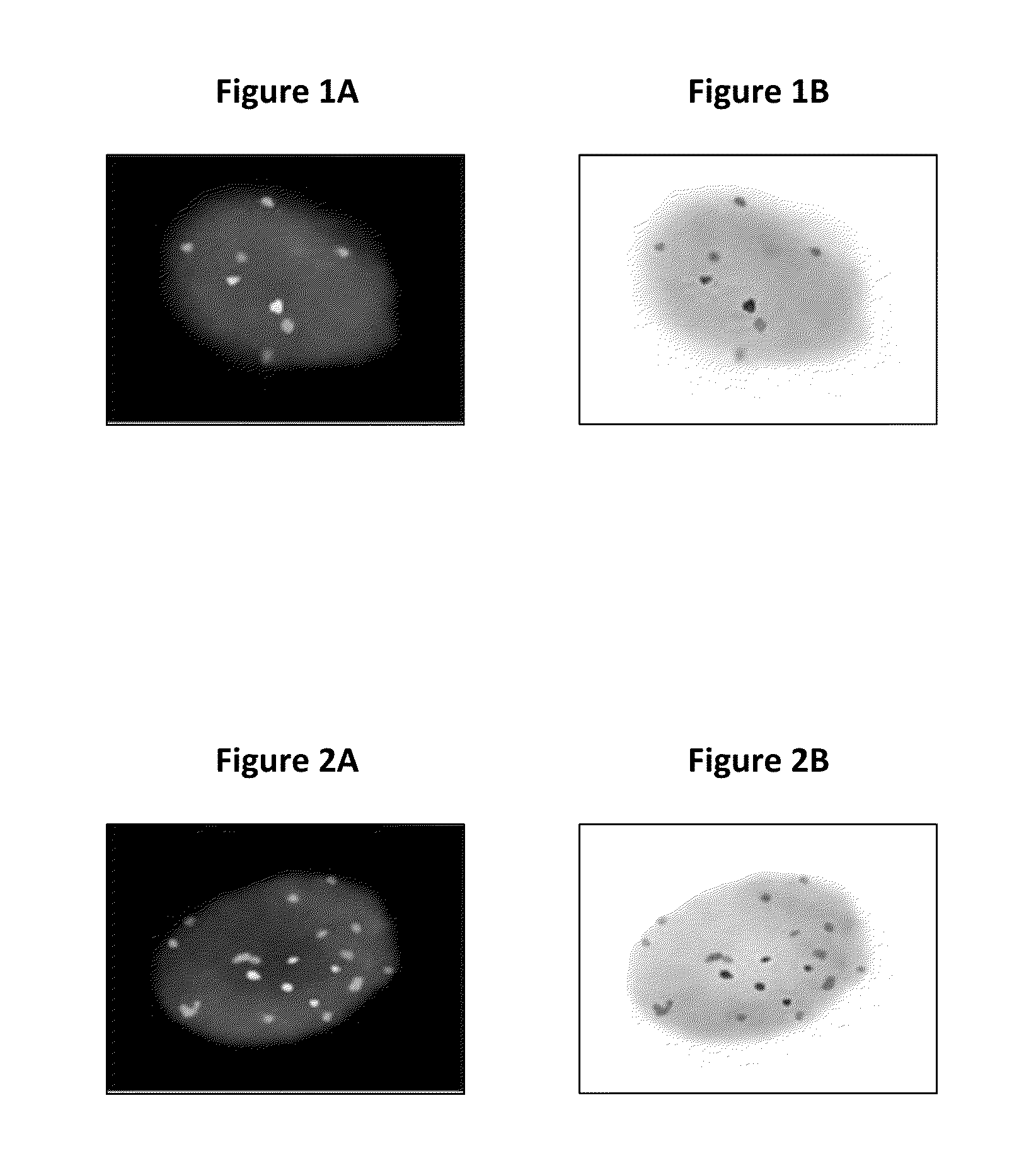
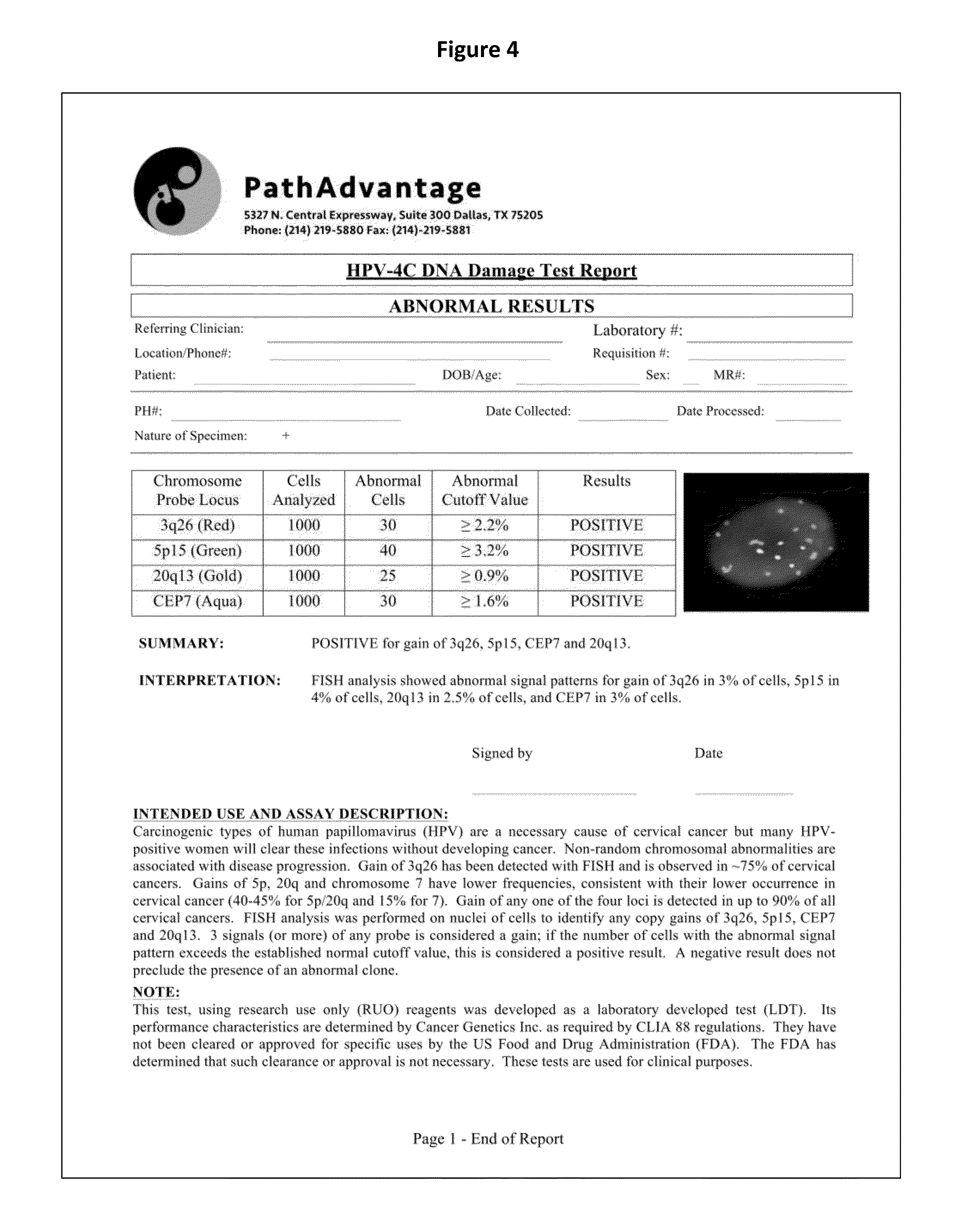
The present disclosure is directed to a method for identifying an abnormal sample of cells by (a) hybridizing a set of chromosomal probes to the sample, wherein the set comprises probes to 3q, 5p, CEP7, and 20; (b) evaluating cells of the sample to detect and quantify the presence of each probe in the set; (c) categorizing the evaluated cells of the sample as normal or abnormal, wherein the normal cells contain exactly two copies of each probe in the set and the abnormal cells do not contain exactly two copies of each probe in the set; (d) calculating the percentage of the abnormal cells in the evaluated cells of the sample; and (e) identifying the sample of cells as abnormal if the percentage of abnormal cells in the evaluated cells is greater than or equal to a predetermined cut-off threshold value.
Owner:PATHADVANTAGE ASSOC
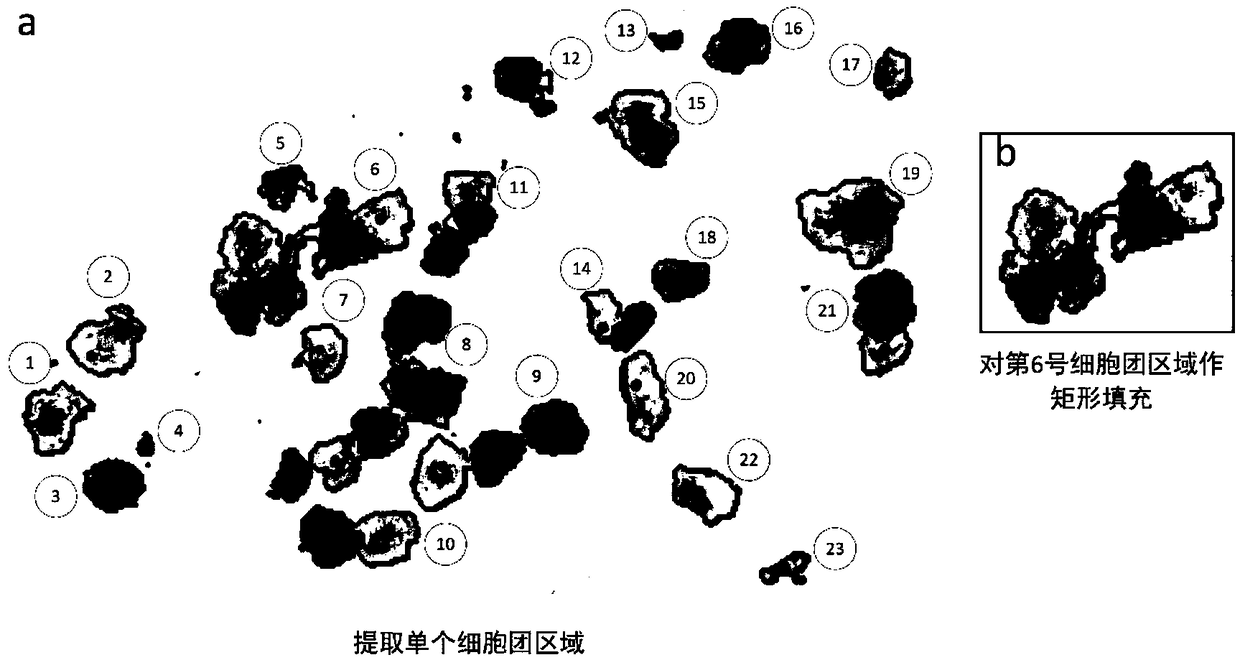
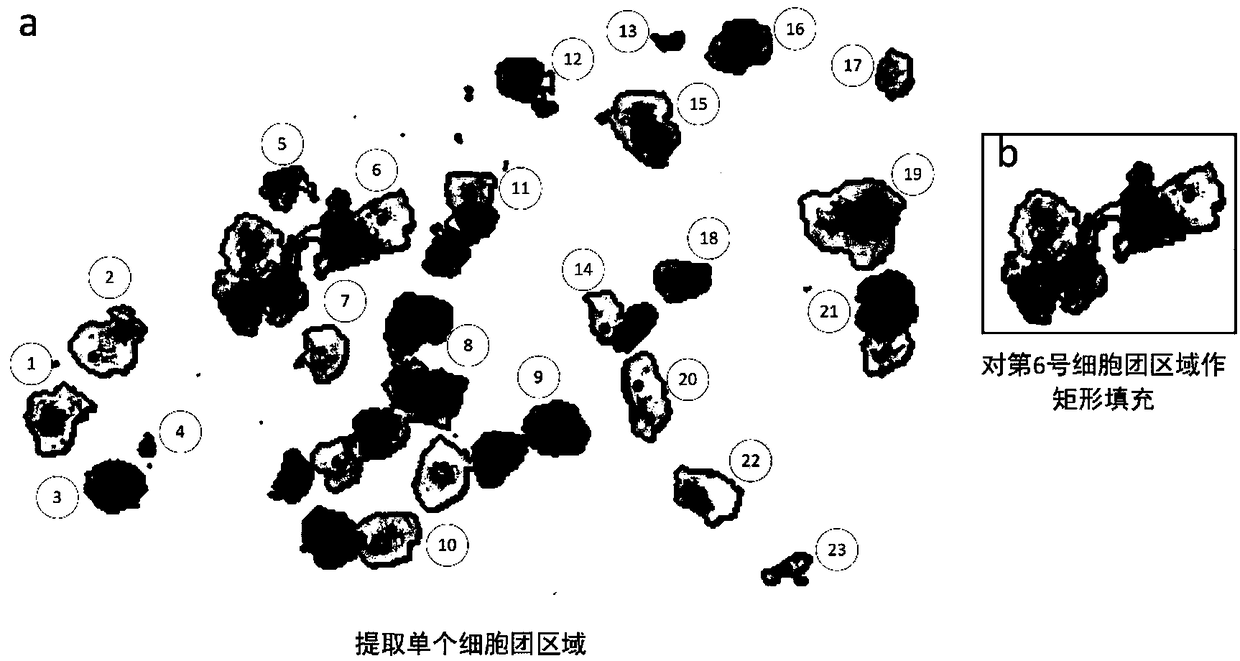
Method and system for recognizing pathological cells of cervical cell pathological section based on cell mass
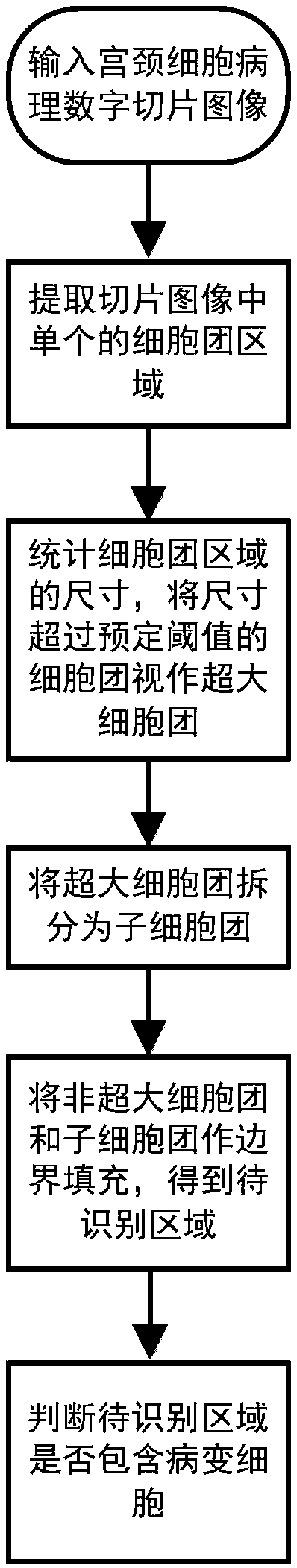
ActiveCN109087283ANo loss of recognition accuracyWithout loss of representationImage enhancementImage analysisCervical cellLarge cell
The invention discloses a cervical cell pathological slice image pathological cell identification method and system based on cell mass, which comprises the following steps: extracting a single cell mass region on the cervical pathological slice image; extracting the single cell mass region on the cervical pathological slice image; counting the size of the cell mass region, and treating the cell mass whose size exceeds a predetermined threshold value as a super-large cell mass; the superlarge cell mass being divided into daughter cell mass; the region to be recognized being obtained by boundaryfilling treatment of the cell cluster region other than the super-large cell cluster and the daughter cell cluster obtained by splitting; a lesion cell being identified in that region to be identified. The invention aims at the whole slice image of the massive pixels, takes the cell cluster as the processing and recognition unit, instead of the conventional image partition and fusion frame, whichis more suitable for the characteristics of the cervical cell pathological slice image, and improves the efficiency and the precision at the same time.
Owner:怀光智能科技(武汉)有限公司
Method and system for image classification of irregular cervical cell mass
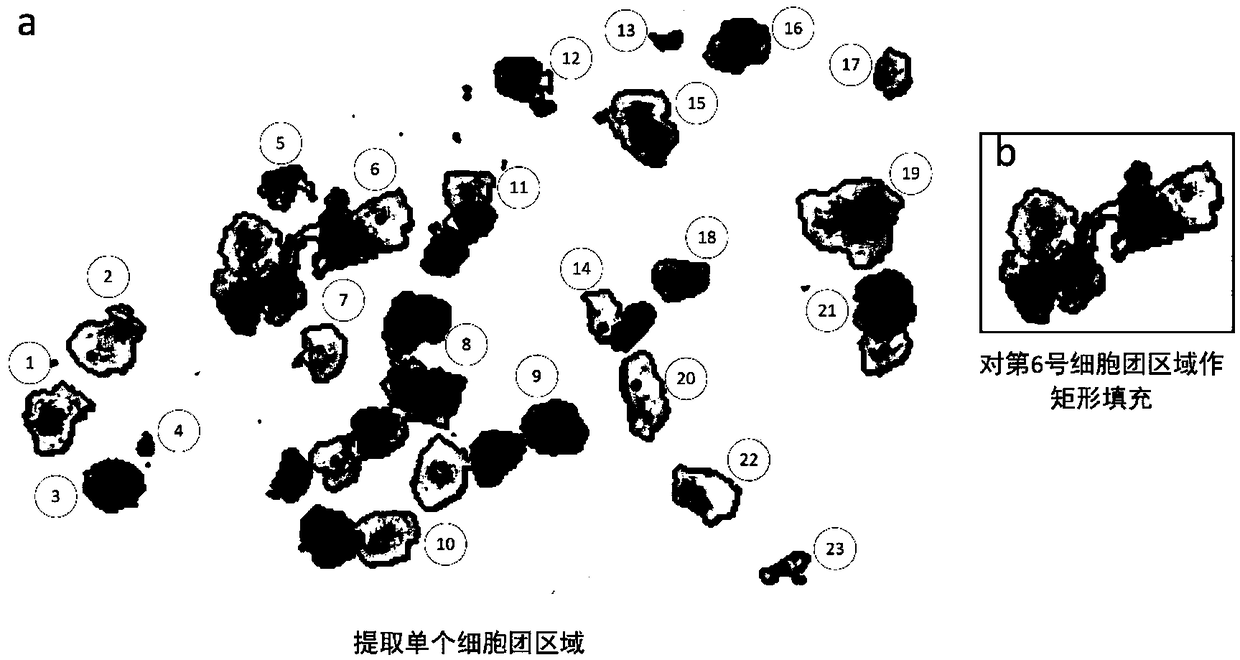
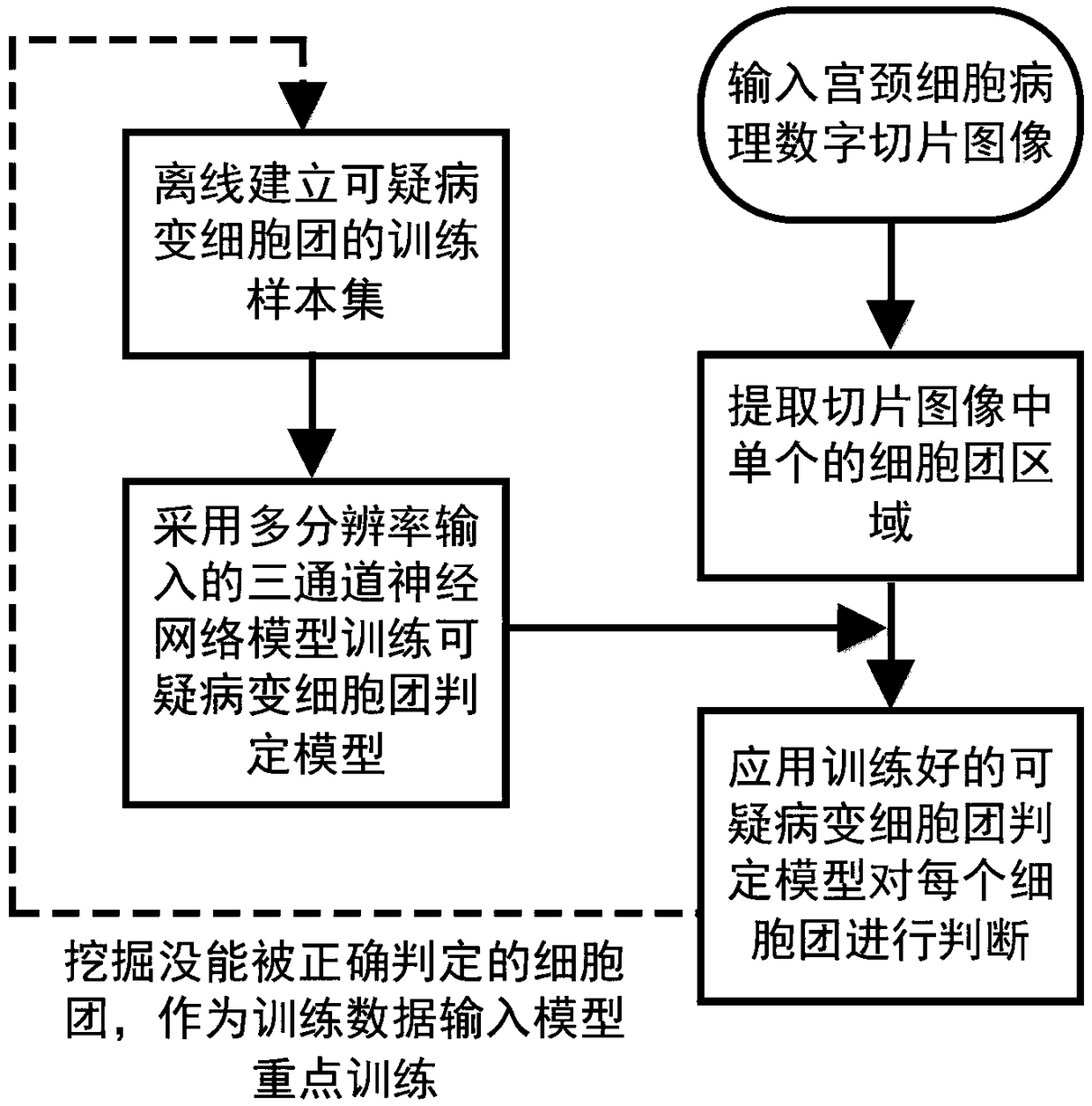
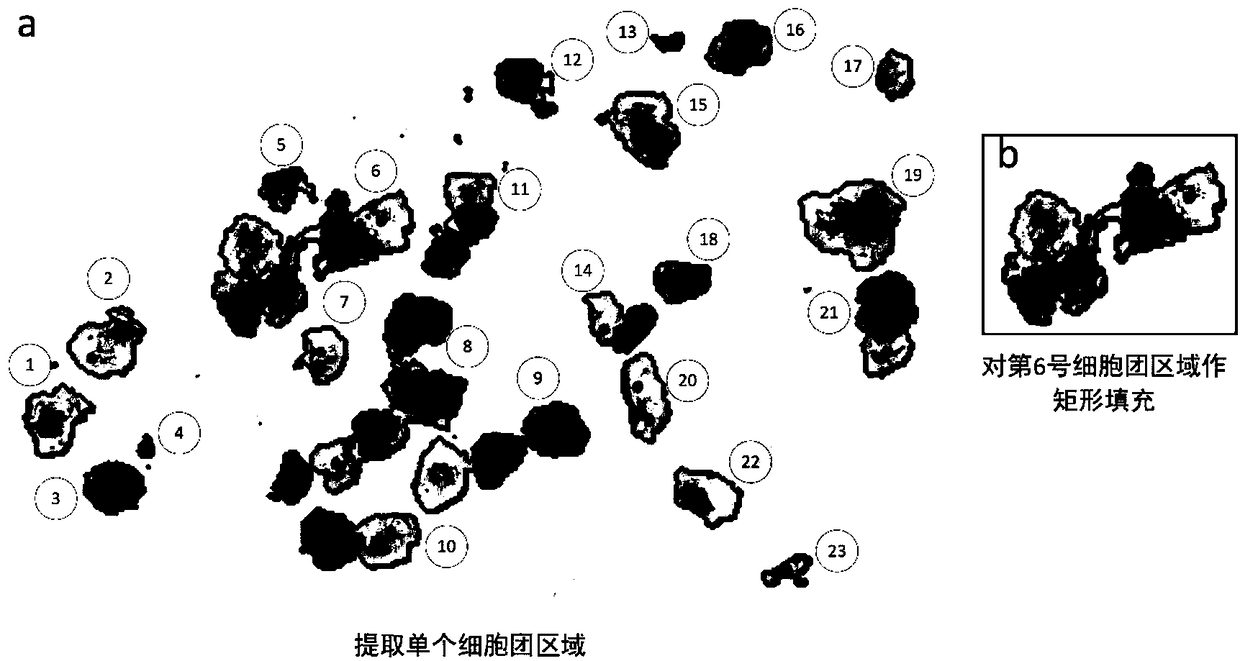
ActiveCN109145941AWithout loss of representationReduce labeling costsCharacter and pattern recognitionNeural learning methodsNerve networkDecision model
The invention discloses an irregular cervical cell cluster image classification method, which is characterized in that the method comprises the following steps: an off-line training sample set of a suspicious cell cluster is established, and a multi-resolution input three-channel neural network model is adopted to train a suspicious pathological cell cluster judgment model; Single cell cluster region was extracted from cervical pathological slice images, and the trained suspicious cell cluster decision model was used to determine the abnormality of each cell cluster. Mining the cell clusters that can not be judged correctly, as the training data input model focus on training. The invention takes the irregular cell cluster as the processing and identification unit, and utilizes the multi-resolution input three-channel neural network model to quickly identify the suspected pathological cell cluster, and simultaneously improves the identification accuracy and the identification efficiency.
Owner:怀光智能科技(武汉)有限公司
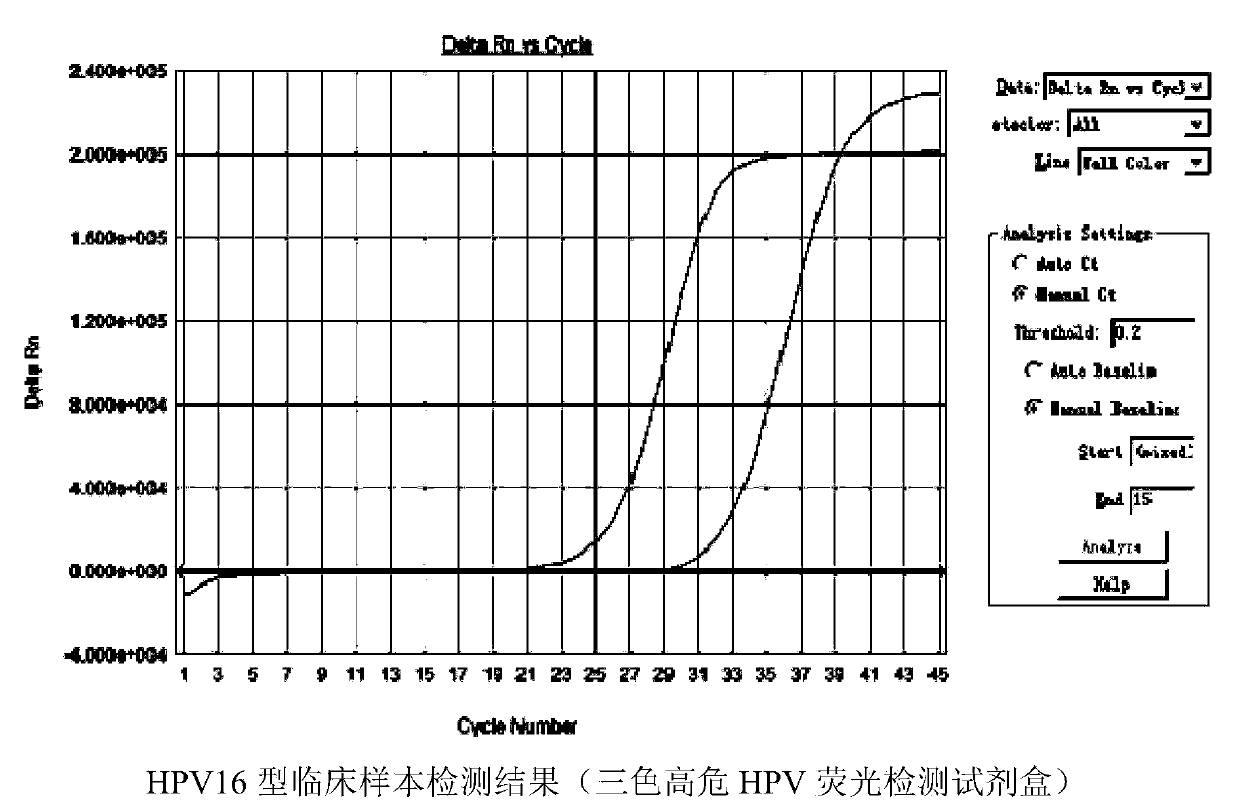
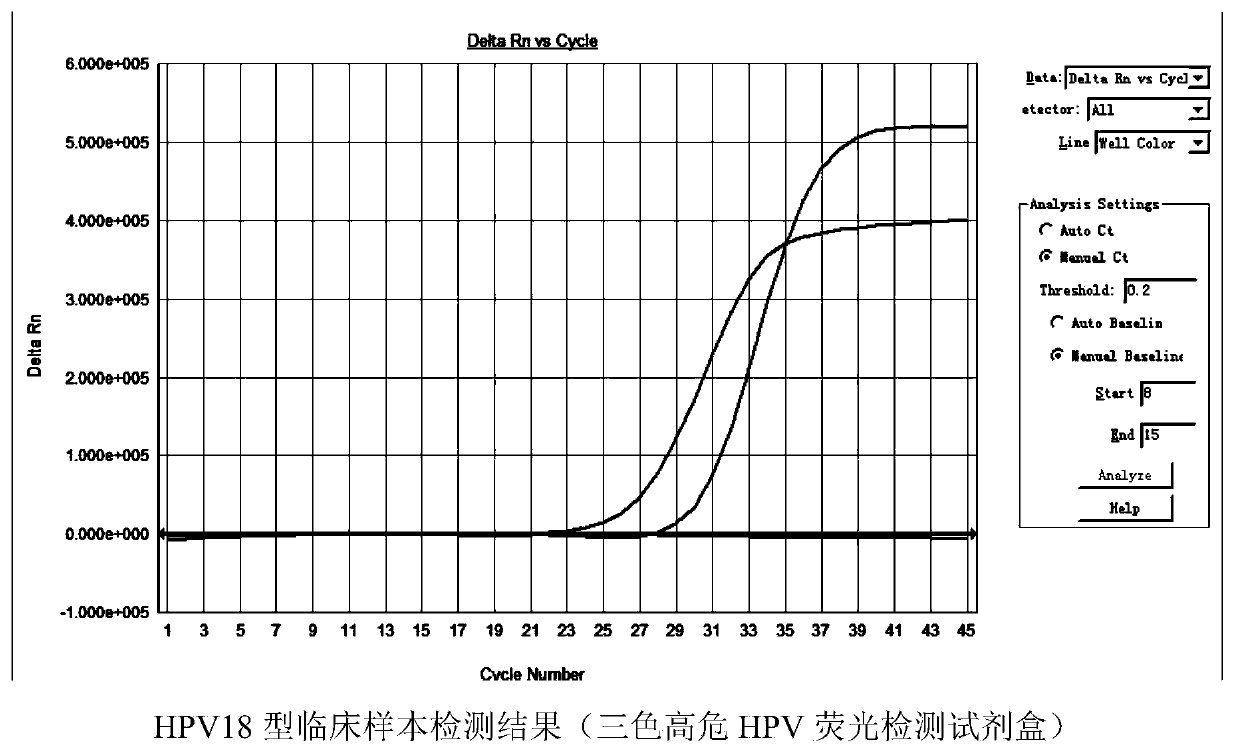
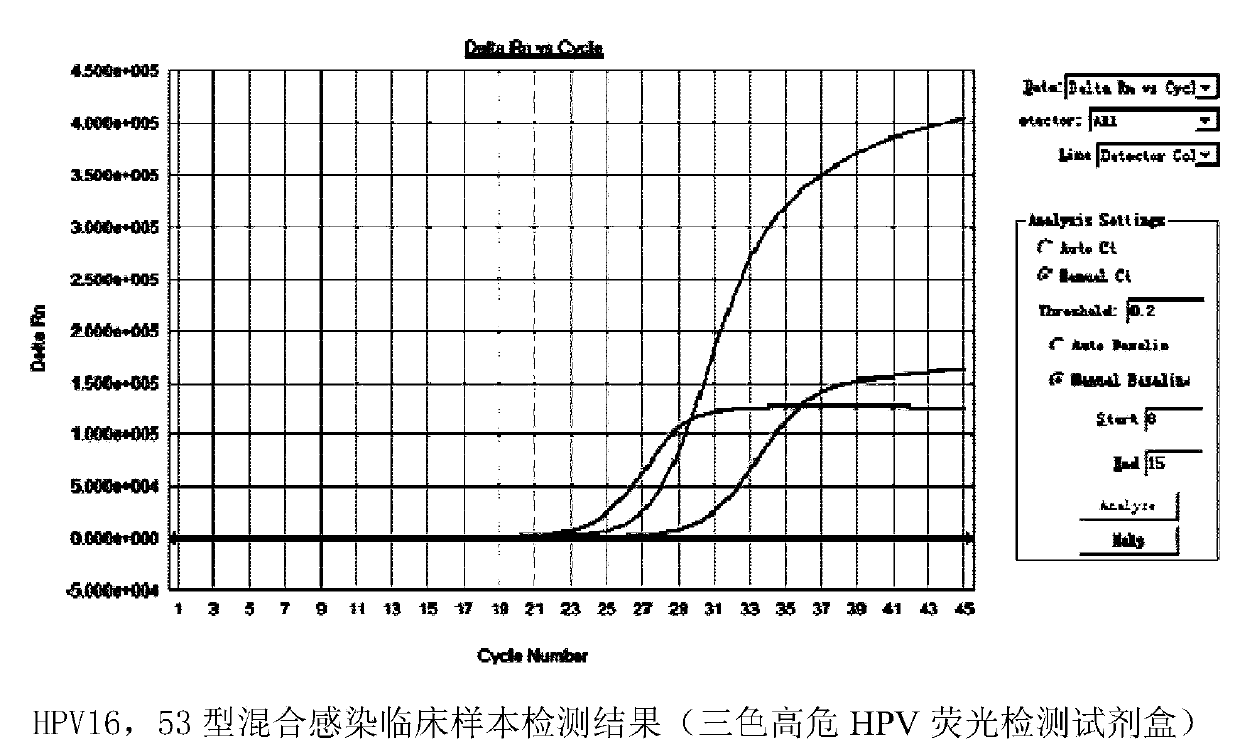
Multicolor high-risk HPV (human papilloma virus) fluorescence detection kit
ActiveCN104195247AHigh detection throughputReduce screening costsMicrobiological testing/measurementMultiplexFluoProbes
The invention discloses a multicolor high-risk HPV (human papilloma virus) fluorescence detection kit which comprises a warm-boot Taq enzyme system, universal primers, fluorescent probes, a negative control and a positive control, wherein the fluorescent probes are HPV-specific MGB probes. The kit is suitable for qualitatively detecting 18 high-risk HPV genotypes capable of causing cervical carcinoma in cervix exfoliated cell and genitourinary tract secretion samples. The kit uses the multiplex fluorescence PCR (polymerase chain reaction) technique to implement detection of 18 HPV genotypes in one tube amplification system. The kit can simultaneously detect 15 common high-risk HPV genotypes (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68 and 82) and can also detect 3 rare high-risk HPV genotypes (26, 53 and 73). The detection kit has the advantage of high detection flux and greatly lowers the detection cost.
Owner:GUANGZHOU HEAS BIOTECH CO LTD
Self-collection device and kit for collecting cervical and vaginal cells
A self-collection device and kit that allow for self-collection of vaginal and cervical cells are provided. The device includes an intra-vaginal insertion member and a shaped collection member for collecting vaginal and cervical cells, resembling a menstrual cup.
Owner:4WOMEN HEALTH INC
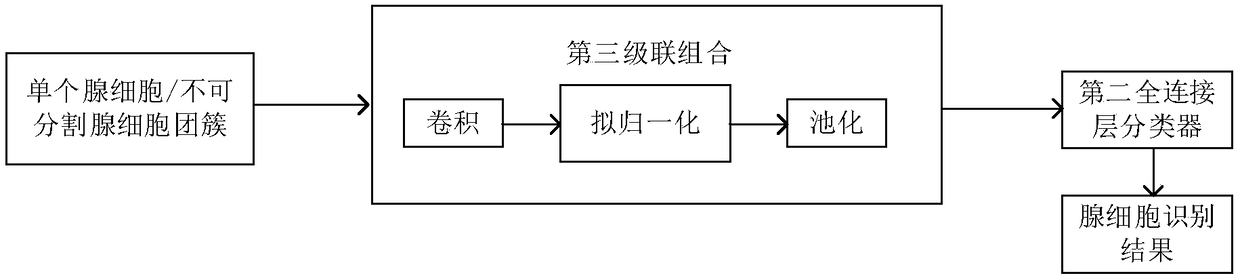
Identification method for atypical abnormal glandular cell in uterine neck cell smear
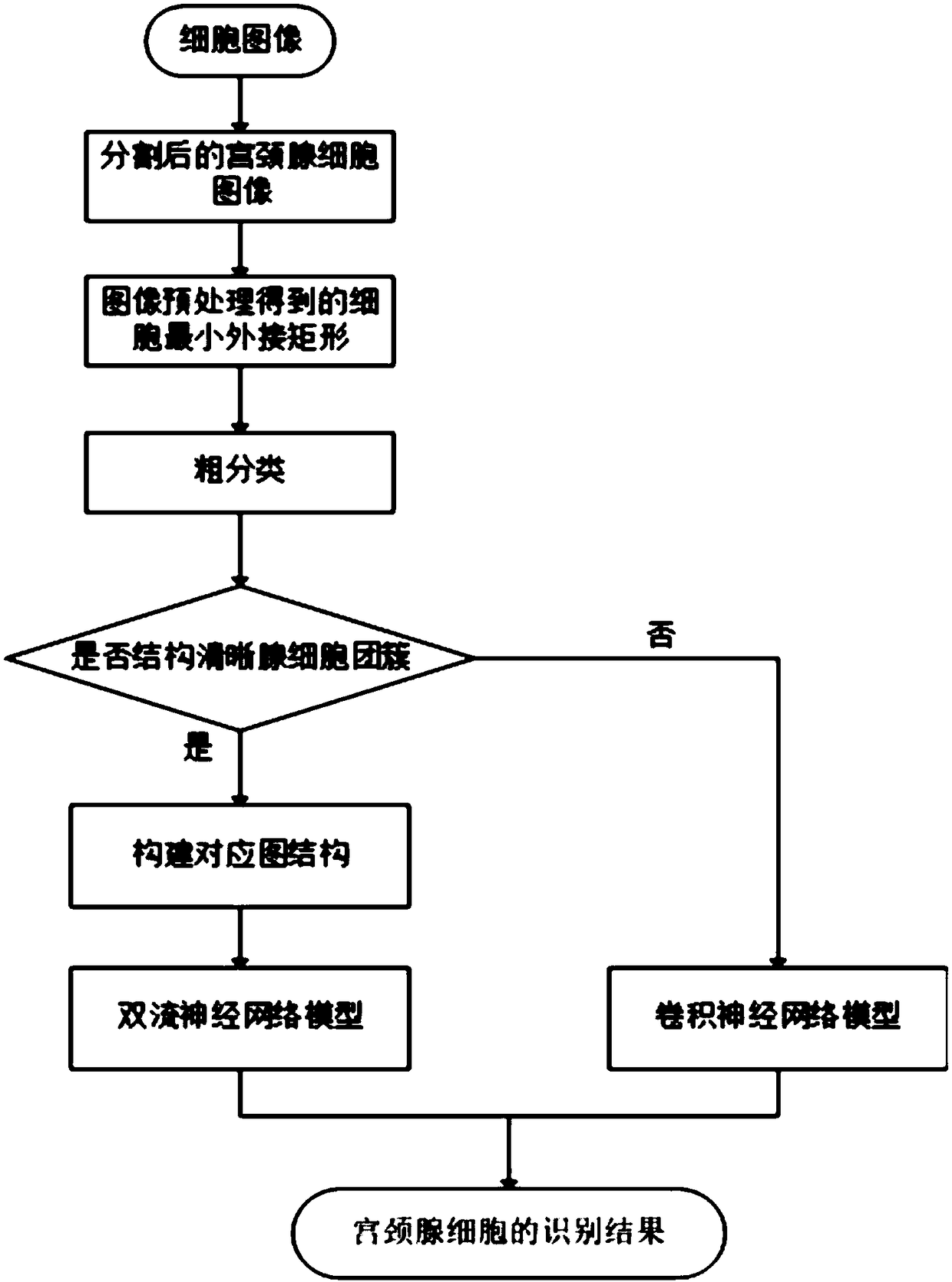
InactiveCN108389198AEasy to identifyImprove completenessImage analysisCharacter and pattern recognitionNeurulationCervical cell
The invention provides an identification method for an atypical abnormal glandular cell in a uterine neck cell smear. According to the method, glandular cells are divided into glandular cell clustersof clear structure, indivisible glandular cell clusters and single glandular cells; for the glandular cell cluster of clear structure, arrangement information and appearance information of the glandular cells serve as two inputs of a double flow neural network model; the single glandular cell and the indivisible glandular cell cluster are input to a convolutional neural network model; and the twomodels output an identification result. The method can be used to improve the identification effect for the glandular cells with obvious arrangement structures, identification is more complete, and misidentification and identification omission are reduced.
Owner:深思考人工智能机器人科技(北京)有限公司
Cervical cancer screening detection kit
InactiveCN108717120AIncrease connection forceAvoid it happening againMaterial analysisWater basedStaining
The invention provides a cervical cancer screening detection kit. The kit comprises a p16 / Ki-67 hybrid primary antibody, a hybrid enzyme-labeled secondary antibody and a staining solution, wherein thestaining solution comprises an AP-Red staining solution and a DAB staining solution, and the preparation method of the AP-Red staining solution comprises chromogen preparation, substrate preparationand staining solution preparation. A novel cervical cancer screening detection kit is provided in the technical scheme. The AP-Red staining solution of a novel formula is used in the kit, the chromogen in the staining solution possibly has multiple reactive groups, and the groups can react with groups on tissues so as to form covalent bonds, so that cervical cell slices using the kit can directlypass through organic reagents after staining, a water-based sealing agent does not need to be used for sealing the slices, bubbles are avoided, the thickness is controlled, the time and labor cost canbe saved, observation of staining results is prevented from being influenced, and the detection efficiency and detection quality are improved.
Owner:FUZHOU MAIXIN BIOTECH CO LTD
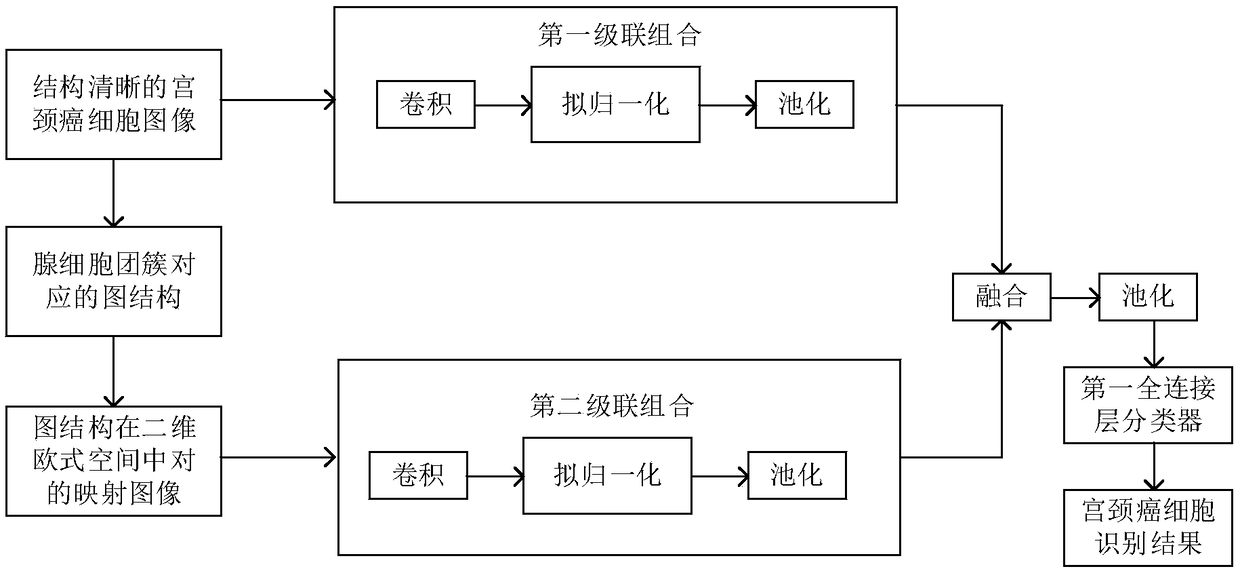
Fine-grained cervical cell image three-stage identification method
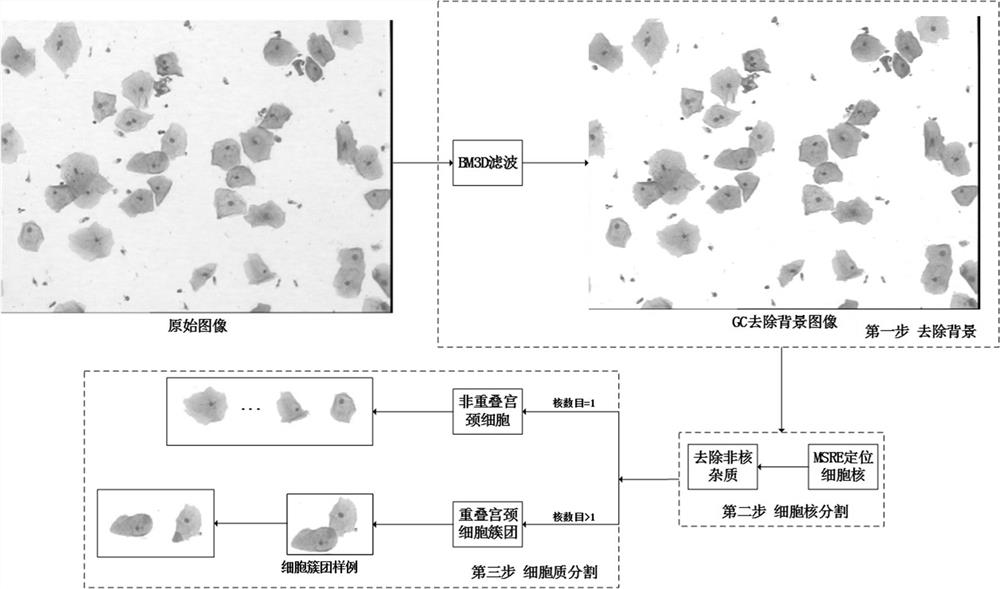
PendingCN111860586AImprove classification recognition accuracyThe identification process system is completeImage enhancementImage analysisCervical cellsImaging quality
The invention provides a fine-grained cervical cell image three-stage identification method, which comprises the following steps: firstly, denoising a whole cervical cell image acquired by electronicimaging equipment to improve the image quality, removing a background to determine a foreground cell region, and segmenting out cell nucleuses and cytoplasm; secondly, extracting shape, color, textureand abstract features from the segmented cell regions, and carrying out selective fusion on the features by adopting a multi-kernel learning method so as to optimize a feature mode; and finally, removing non-cervical cell impurities according to the optimized characteristic mode, and performing multi-classification on the cervical cell images by adopting a cost-sensitive active learning method. The identification process system is complete, can extract medical features of multiple types of cervical cell images, performs selective fusion optimization on feature modes, removes impurities by using a cost-sensitive learning method, solves the problem of unbalanced dichotomy, and can effectively improve the accuracy of cervical cell classification and identification.
Owner:NANTONG UNIVERSITY
Marker, primer probe and kit for early screening and diagnosis of endometrial cancer
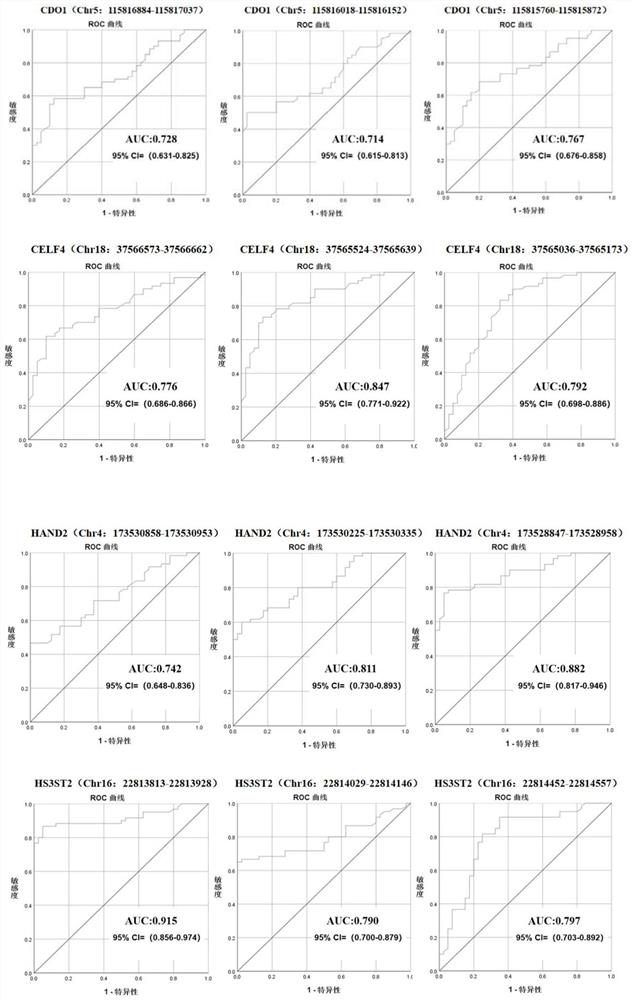
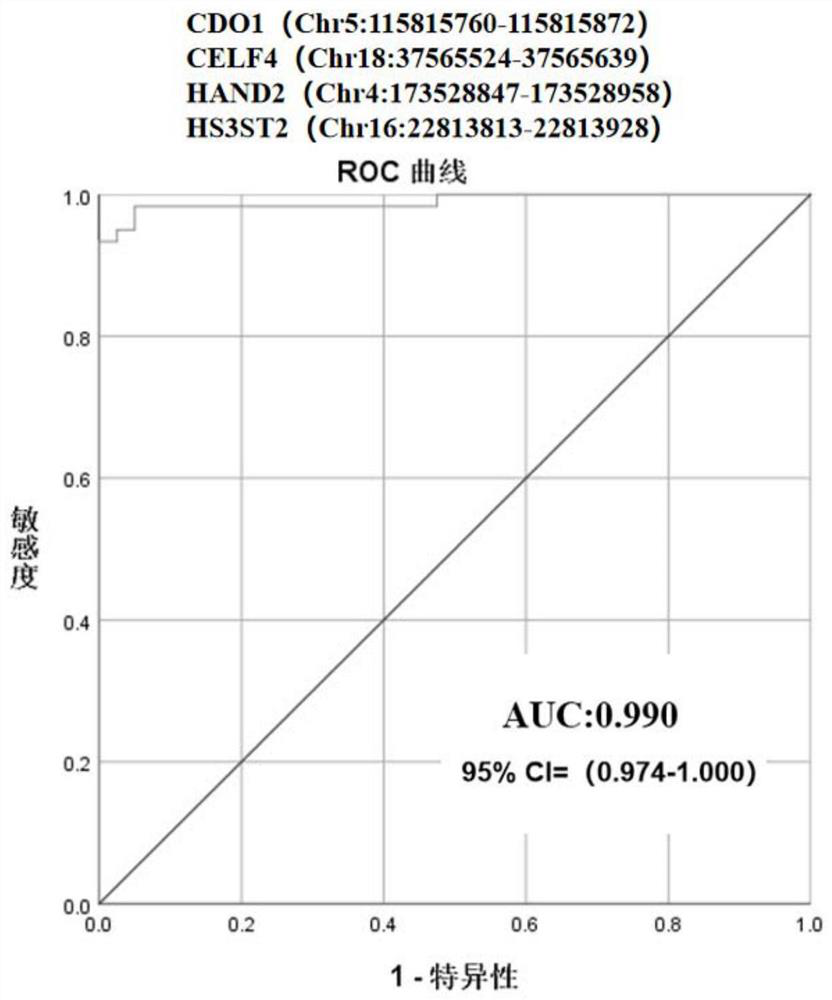
ActiveCN113755603AHigh sensitivityStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationEndometrial CarcinomasCervical cell
The invention discloses a marker, a primer probe and a kit for early screening and diagnosis of endometrial cancer. The marker is partially methylated regions in four genes of CDO1, CELF4, HAND2 and HS3ST2, and detection primers and the probes are designed for the methylated regions and are both of snap ring structures. The kit comprises the primer and the probe. According to the marker, the primer probe and the kit for early screening and diagnosis of the endometrial cancer, the methylated regions are screened and combined to determine the most suitable methylation position for combined diagnosis, so that the sensitivity and specificity of early endometrial cancer detection can be remarkably improved. The kit is particularly suitable for early screening and diagnosis of the endometrial cancer by taking cervical exfoliated cells as a sample, can detect the endometrial cancer even if the concentration of a DNA template is low, has the advantages of noninvasive sampling, high detection speed, higher sensitivity and specificity and the like, can ensure the accuracy of a result, therefore, the purposes of early discovery, early diagnosis and early treatment of the endometrial cancer can be achieved.
Owner:BEIJING ORIGIN-POLY BIO-TEC CO LTD
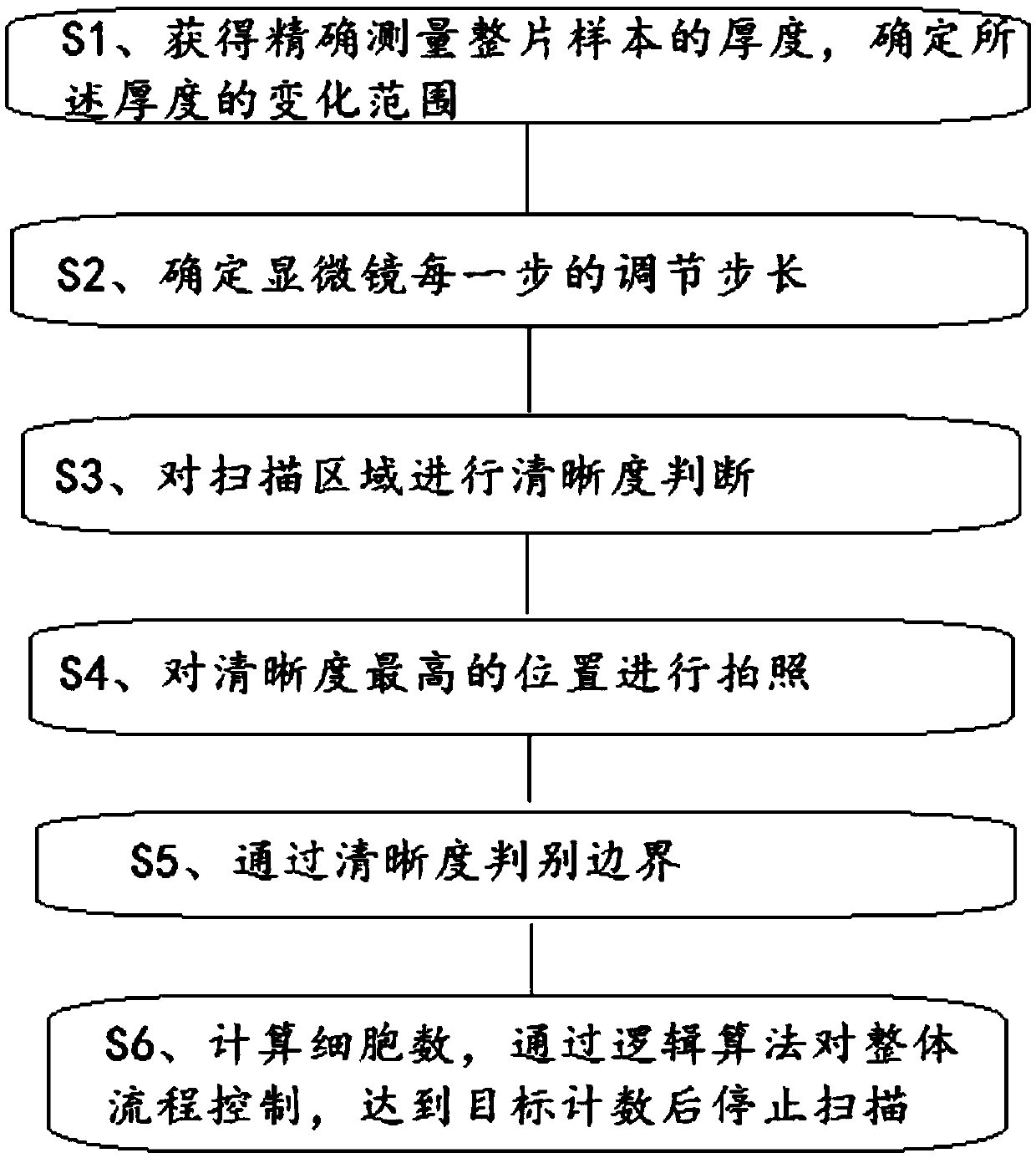
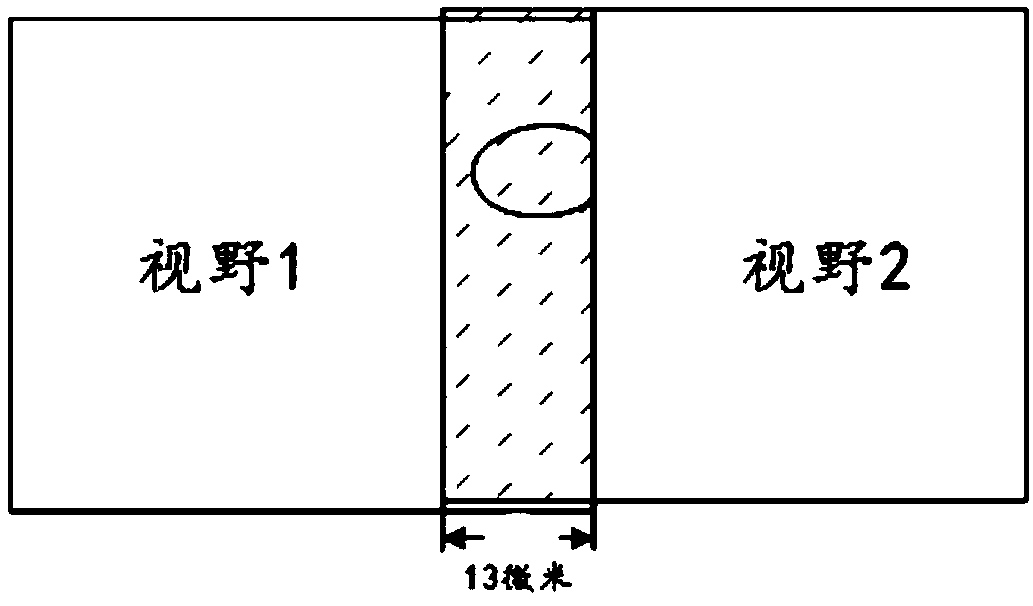
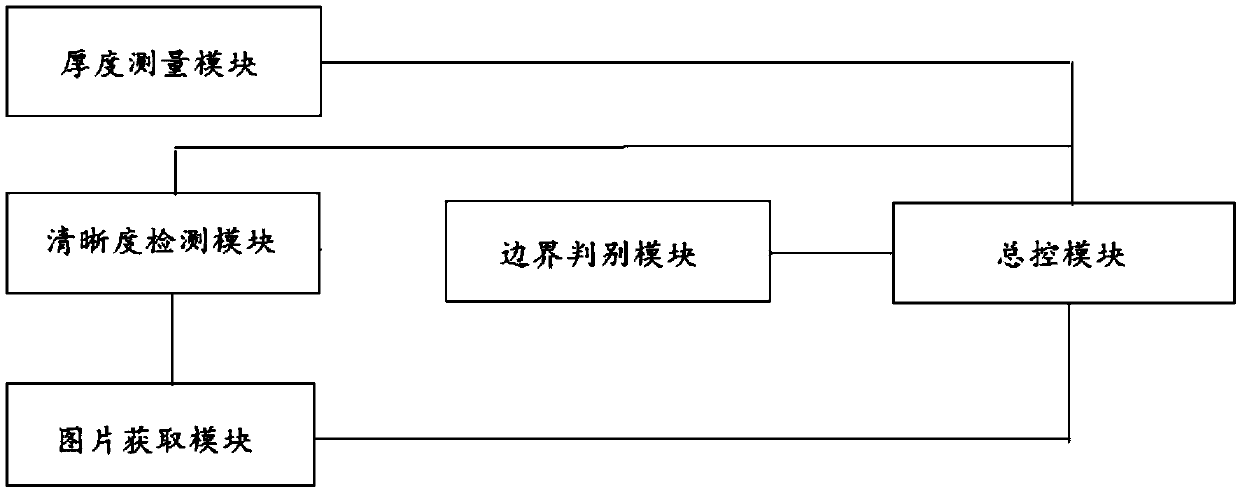
Rapid scanning method and system for cervical exfoliated cell smears
ActiveCN109612992AHigh speedImprove accuracyMaterial analysis by optical meansCell smearGoal attainment
The invention provides a rapid scanning method and system for cervical exfoliated cell smears. An area of a cervical exfoliated cell biological sample is set to be in a circular shape, cell scanning without repeating and missing is achieved through sample boundary judgment, the thickness of the smears is detected to formulate the adjusting step size of a microscope, definition judgment is carriedout on up to seven positions in each scanning area, a camera is called for photographing at the position with the highest definition, the cell number is recorded, and the scanning accuracy is achieved; focusing adjustment is made into a pst file, and correcting and editing are facilitated; and the cell number is recorded synchronously in the scanning process, and scanning is stopped after the target number is reached through logical operation. Thus, the speed and accuracy of the scanning process flow are improved.
Owner:深圳辉煌耀强科技有限公司
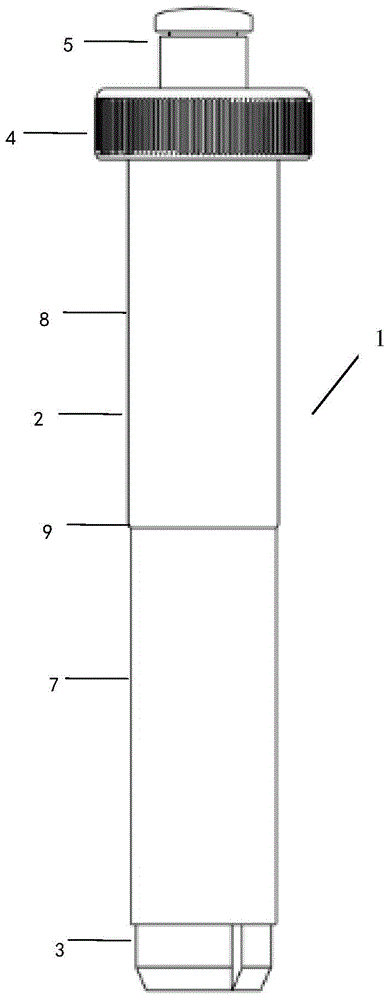
Cervical cell sampling device
ActiveCN104622518AEasy to useAccurate collectionSurgical needlesVaccination/ovulation diagnosticsMedicineCervical cell
The invention discloses a cervical cell sampling device which comprises a sampler master pipe, a power generator, a uterine neck locator and a sampler, wherein the uterine neck locator can be movably arranged in the sampler master pipe under the drive of the power generator; the sampler is movably arranged in the uterine neck locator under the drive of the power generator; the head of the sampler is defined in the uterine neck locator in an initial state; and the head of the sampler extends out of the uterine neck locator under the drive of the power generator in the sampling process.
Owner:HANGZHOU DIAN BIOTECH CO LTD
Probe of Human Papillomavirus and Dna Chip Comprising the Same
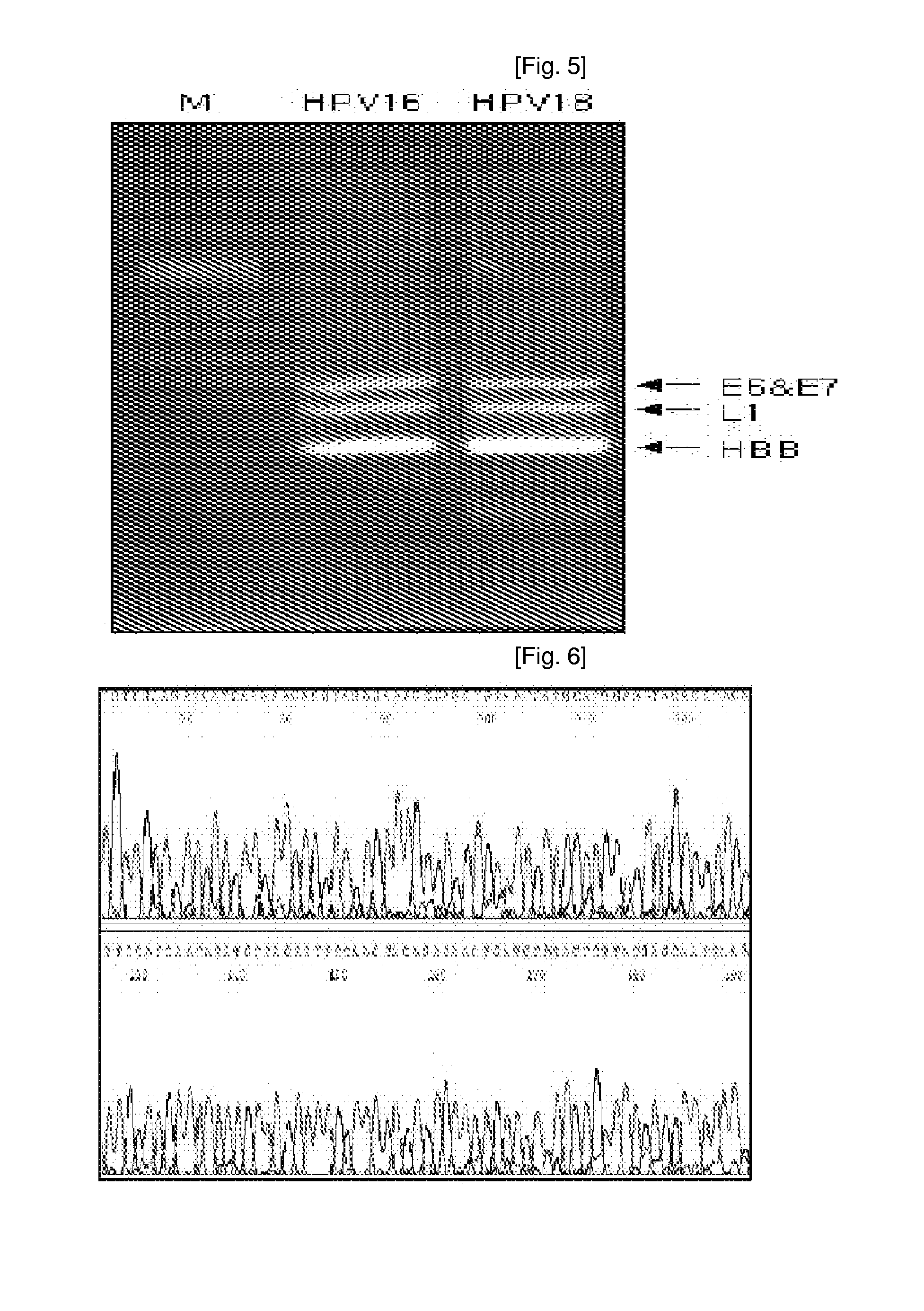
ActiveUS20070248968A1Short timeBioreactor/fermenter combinationsBiological substance pretreatmentsHuman papillomavirusCervical cell
Oligonucleotide probes for analyzing 40 types of HPV were synthesized, and DNA chips were produced by using the oligonucleotide probes. The synthesis of the oligonucleotide probes is based on clones of L1 and E6 / E7 genes of 35 types of HPV obtained from cervical cell specimens from 4,898 Korean adult women and tissue specimens from 68 cervical cancer cases in addition to information based on American and European cases. The DNA chips can analyze the 40 types of HPV found in cervical, diagnose complex infection by at least one type of HPV, and have excellent diagnostic sensitivity and specificity on HPV genetic type up to 100% and reproducibility. Also, the DAN chips are superior to conventional analytic method, and very economical, since they can analyze numerous specimens in shortest time. Accordingly, the DNA chips are useful for predicting cervical cancer and precancerous lesion.
Owner:GOODGENE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com