Pharmaceutical composition of humanized antibody for vascular endothelial growth factor
A composition and drug technology, applied in the field of medicine and chemical industry, can solve the problems of affecting product activity, potential safety risks, unsatisfactory stability of Avastin, etc., and achieve the effects of reducing polymers and degradants and improving stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] The preparation of embodiment 1 bevacizumab injection
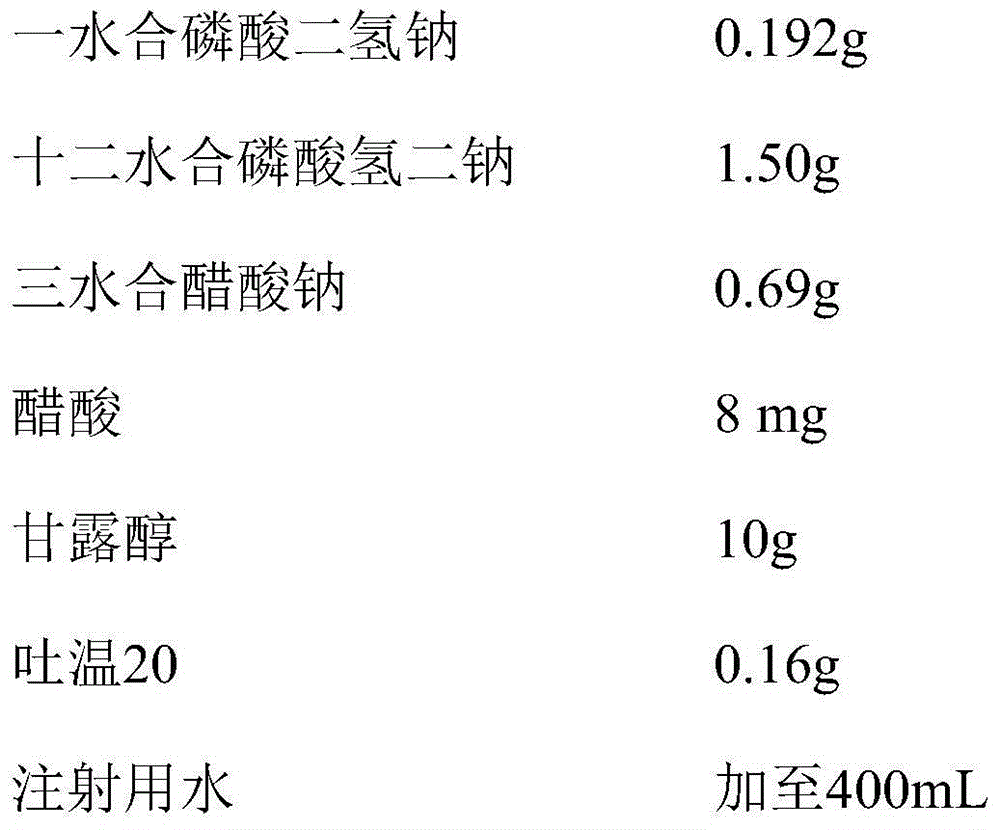
[0094] prescription:
[0095]
[0096]
[0097] Preparation method: According to the above formula, a blank solution without bevacizumab was prepared, and the pH value was 5.2. The purified bevacizumab monoantigen solution was replaced with a blank solution for 6 times to prepare a solution of the above formula, sterilized and filtered, aseptically subpackaged, and passed the visual inspection to obtain the product.
Embodiment 2
[0098] The preparation of embodiment 2 bevacizumab injection
[0099] prescription:
[0100]
[0101] Preparation method: According to the above formula, a blank solution without bevacizumab was prepared, and the pH value was 5.2. The purified bevacizumab monoantigen solution was replaced with a blank solution for 6 times to prepare a solution of the above formula, sterilized and filtered, aseptically subpackaged, and passed the visual inspection to obtain the product.
Embodiment 3
[0102] The preparation of embodiment 3 bevacizumab injection
[0103] prescription:
[0104]
[0105] Preparation method: According to the above formula, a blank solution without bevacizumab was prepared, and the pH value was 5.2. The purified bevacizumab monoantigen solution was replaced with a blank solution for 6 times to prepare a solution of the above formula, sterilized and filtered, aseptically subpackaged, and passed the visual inspection to obtain the product.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com