Preparation method for linaclotide
A technology of linaclotide and protecting group, which is applied in the field of preparation of linaclotide, can solve the problems of a large amount of time for separation and purification, consumption of large solvent and raw materials, inconvenience of industrial production, etc., and achieves simple operation and high total yield , the effect of improving the yield of cyclization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
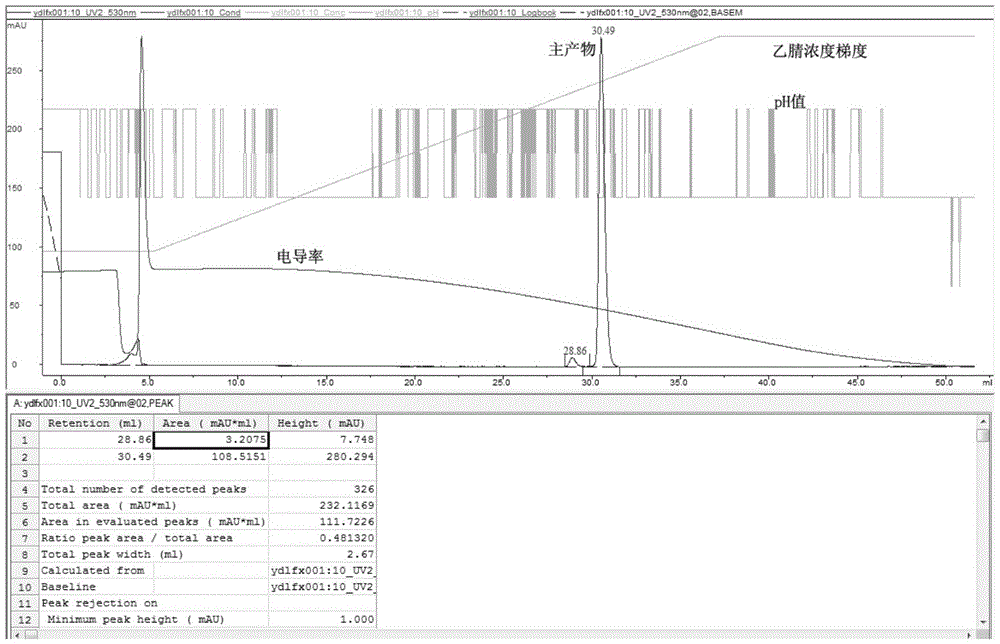
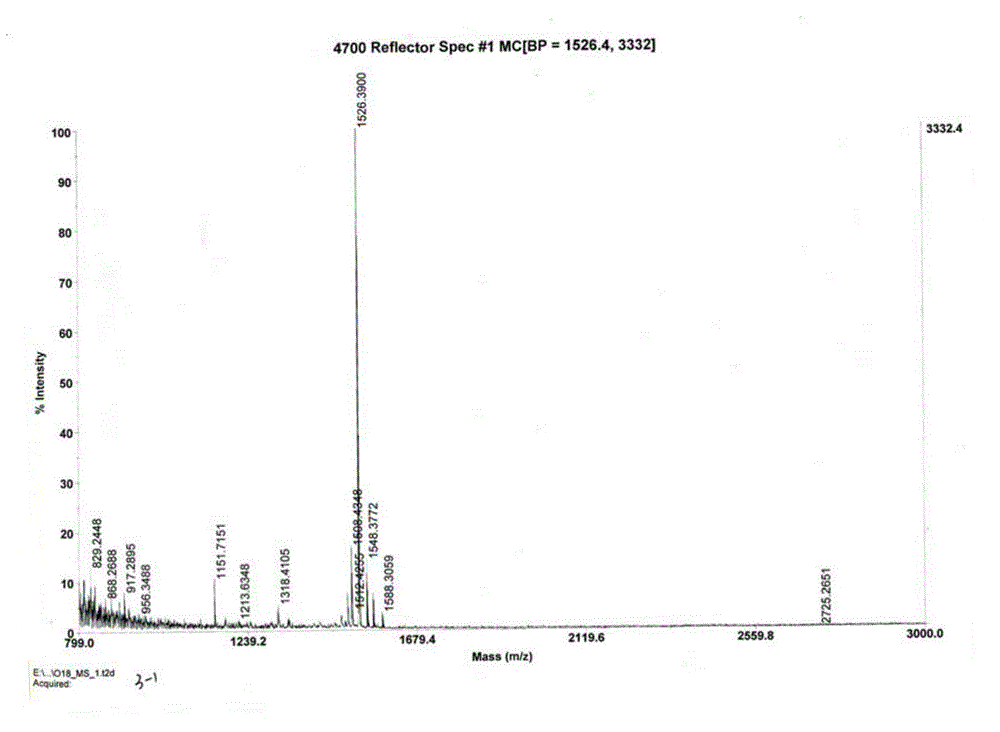
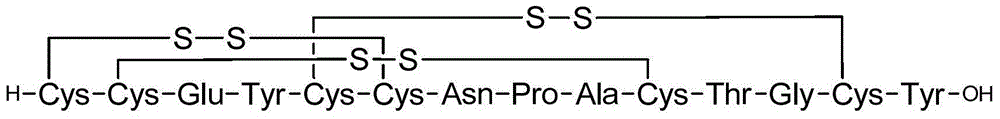
Image
Examples
Embodiment 1
[0050] Step 1 WANG resin (1.28mmol / g, 640mg), tert-butyl (tBu) protected Fmoc tyrosine (Fmoc-Tyr(tBU)-OH) (689.33mg, 1.5mmol) and DIPEA (414μL, 5mmol) , HBTU (113.77mg, 3mmol) was placed in a reaction flask, added DMF (20mL) to dissolve, and stirred for 6-12 hours. After the reaction is complete, let it stand still, drain the DMF, wash the resin with DMF to obtain a yellow resin, then use hexahydropiperidine-DMF (1 / 4; 20mL) to remove the protective group, and react for 20-40 minutes. The synthetic method is carried out with reference to the same steps.
[0051] In step 2, the WANG resin reacted with tyrosine was directly mixed with trityl (Trt)-protected Fmoc cysteine (Fmoc-Cys(Trt)-OH) (878.58 mg, 1.5 mmol) and DIPEA (414 μL, 5mmol), HBTU (113.77mg, 3mmol) was placed in a reaction flask, DMF (20mL) was added to dissolve, and stirred for 6-12 hours. After the reaction is complete, let it stand still, drain the DMF, wash the resin with DMF to obtain a yellow resin, then use...
Embodiment 2
[0067] Step 1 WANG resin (1.28mmol / g, 640mg), tert-butyl (tBu) protected Fmoc tyrosine (Fmoc-Tyr(tBU)-OH) (689.33mg, 1.5mmol) and DIPEA (414μL, 5mmol) , HBTU (113.77mg, 3mmol) was placed in a reaction flask, added DMF (20mL) to dissolve, and stirred for 6-12 hours. After the reaction is complete, let it stand still, drain the DMF, wash the resin with DMF to obtain a yellow resin, then use hexahydropiperidine-DMF (1 / 4; 20mL) to remove the protective group, and react for 20-40 minutes. The synthetic method is carried out with reference to the same steps.
[0068] In step 2, the WANG resin reacted with tyrosine was directly mixed with trityl (Trt)-protected Fmoc cysteine (Fmoc-Cys(Trt)-OH) (878.58 mg, 1.5 mmol) and DIPEA (414 μL, 5mmol), HBTU (113.77mg, 3mmol) was placed in a reaction flask, DMF (20mL) was added to dissolve, and stirred for 6-12 hours. After the reaction is complete, let it stand still, drain the DMF, wash the resin with DMF to obtain a yellow resin, then use...
Embodiment 3
[0084] Step 1 WANG resin (1.28mmol / g, 640mg), tert-butyl (tBu) protected Fmoc tyrosine (Fmoc-Tyr(tBU)-OH) (689.33mg, 1.5mmol) and DIPEA (414μL, 5mmol) , HBTU (113.77mg, 3mmol) was placed in a reaction flask, added DMF (20mL) to dissolve, and stirred for 6-12 hours. After the reaction is complete, let it stand still, drain the DMF, wash the resin with DMF to obtain a yellow resin, then use hexahydropiperidine-DMF (1 / 4; 20mL) to remove the protective group, and react for 20-40 minutes. The synthetic method is carried out with reference to the same steps.
[0085] In step 2, the WANG resin reacted with tyrosine was directly mixed with trityl (Trt)-protected Fmoc cysteine (Fmoc-Cys(Trt)-OH) (878.58 mg, 1.5 mmol) and DIPEA (414 μL, 5mmol), HBTU (113.77mg, 3mmol) was placed in a reaction flask, DMF (20mL) was added to dissolve, and stirred for 6-12 hours. After the reaction is complete, let it stand still, drain the DMF, wash the resin with DMF to obtain a yellow resin, then use...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com