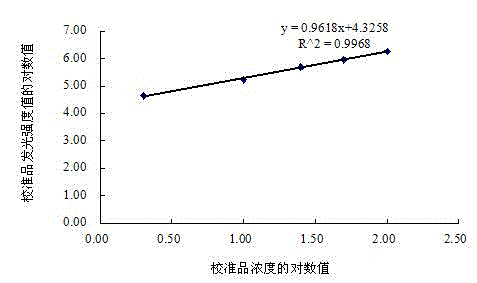
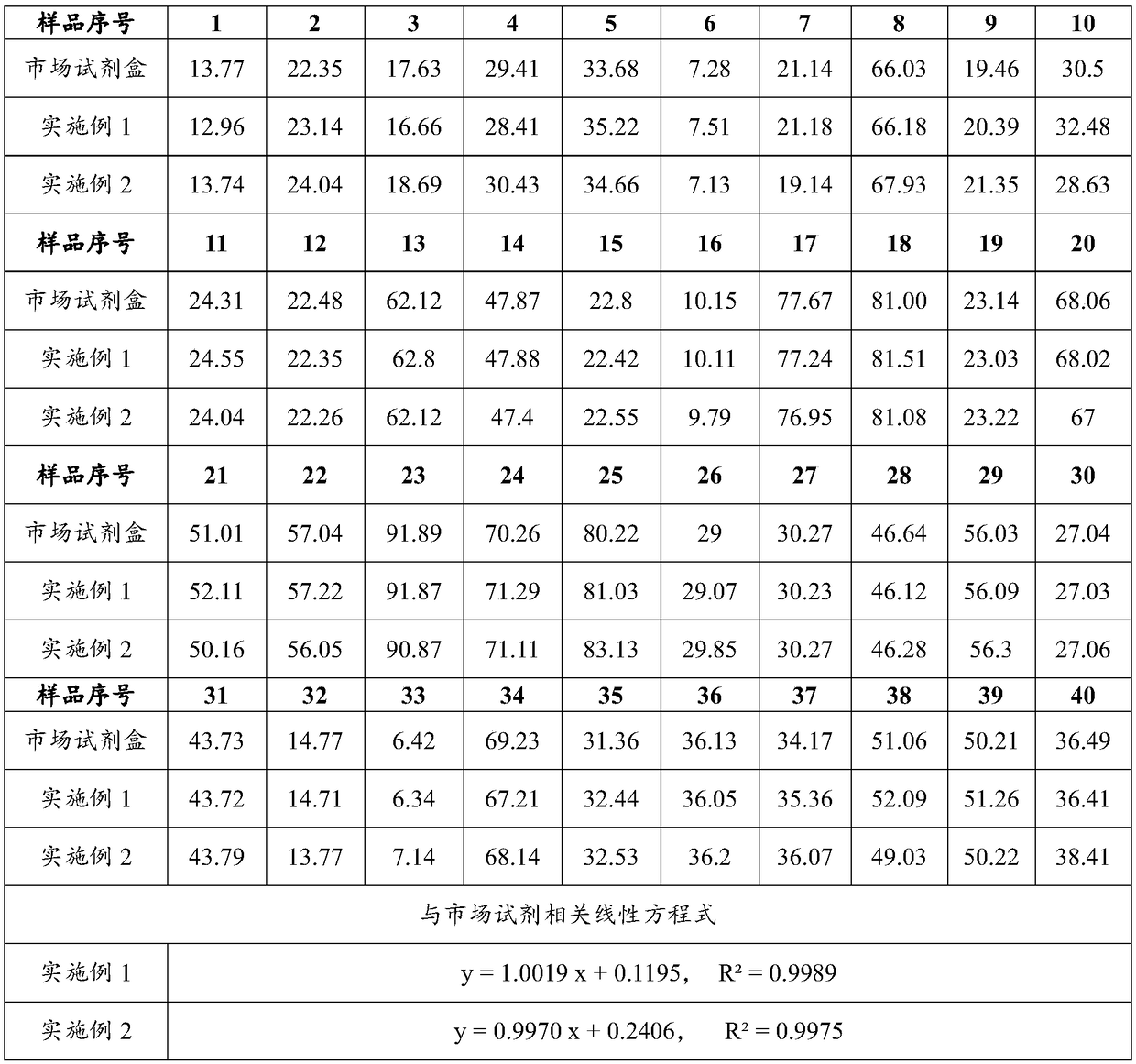
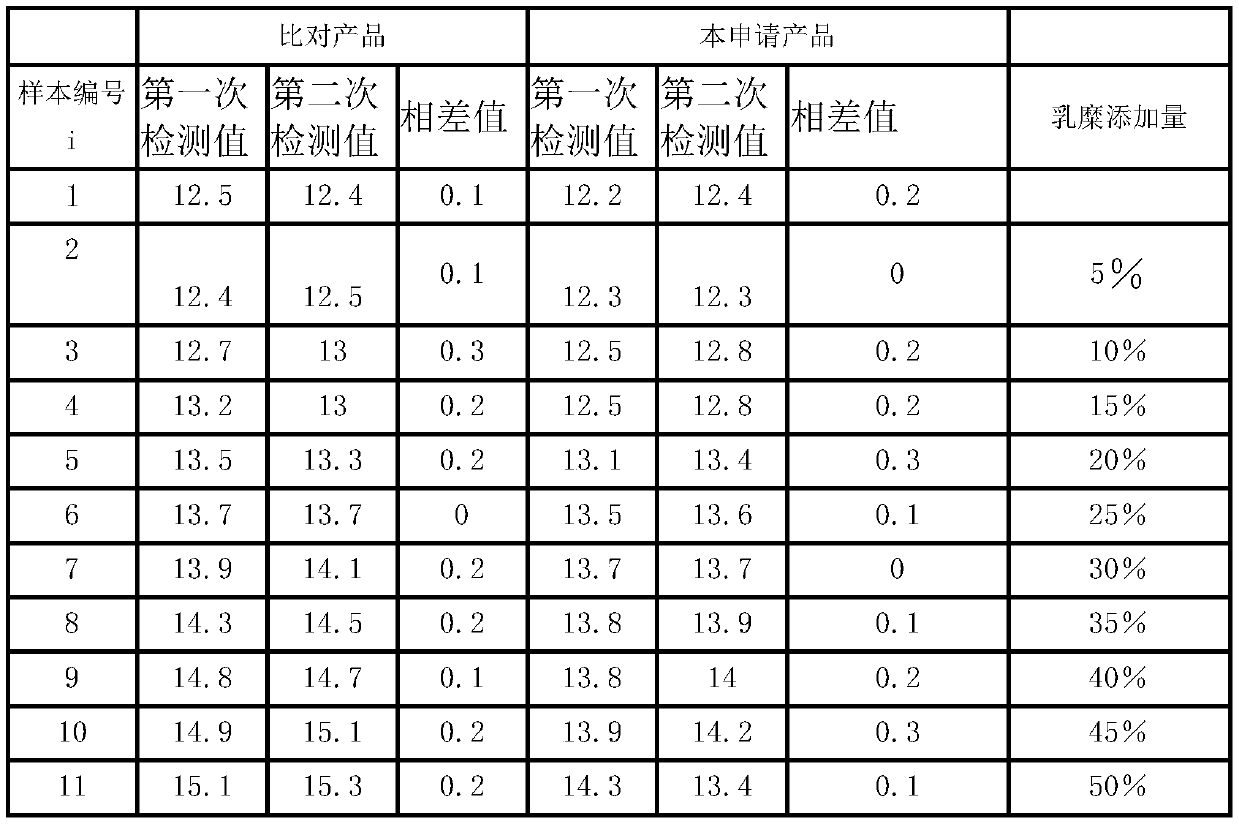
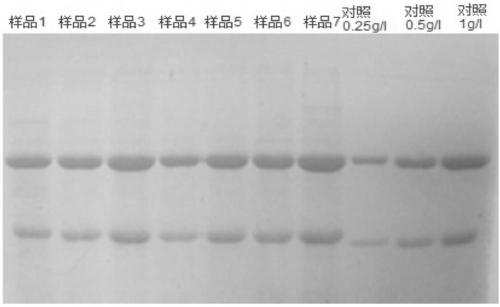
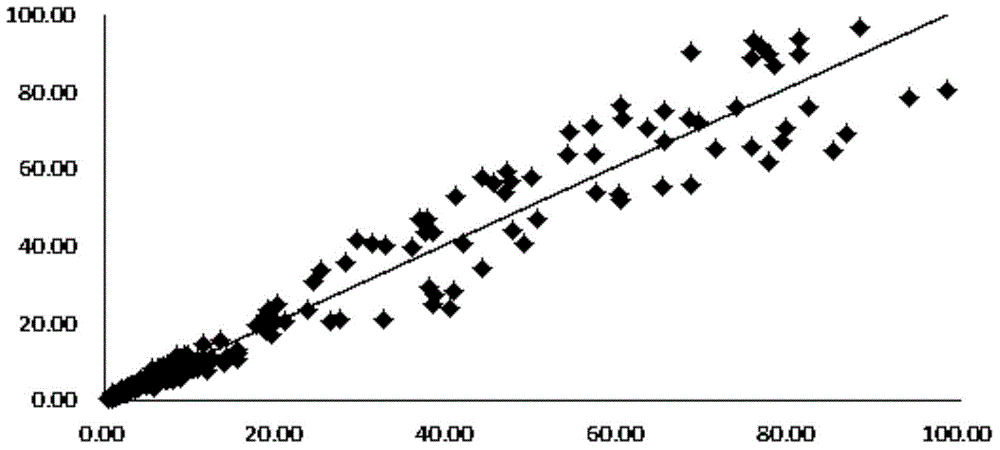
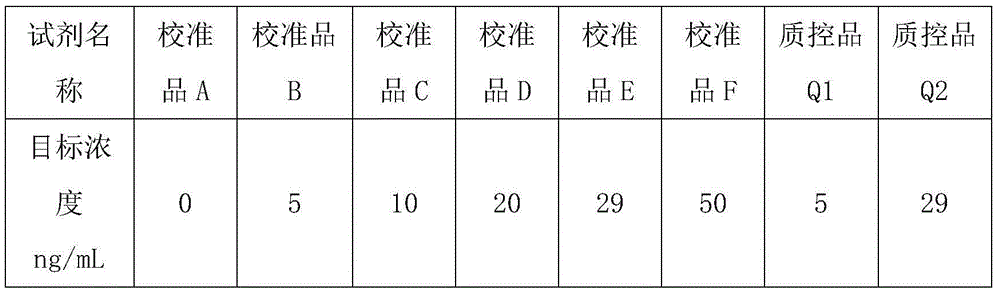
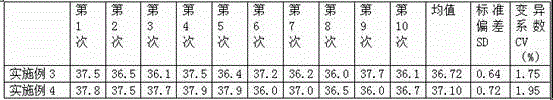
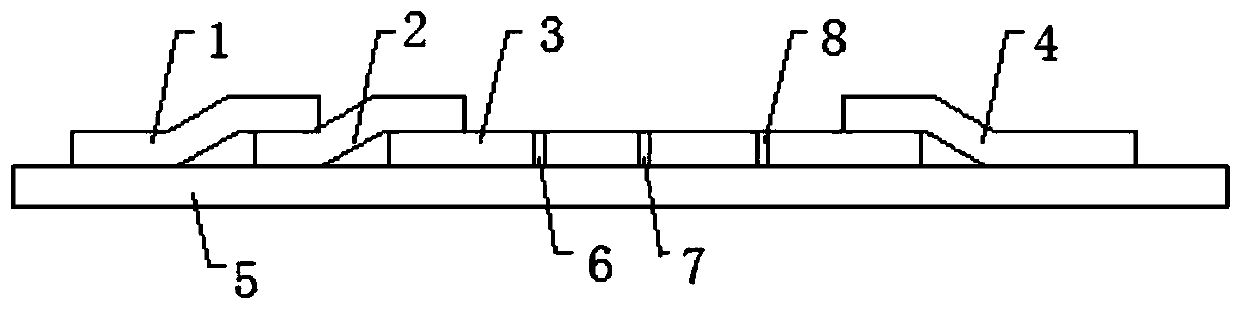
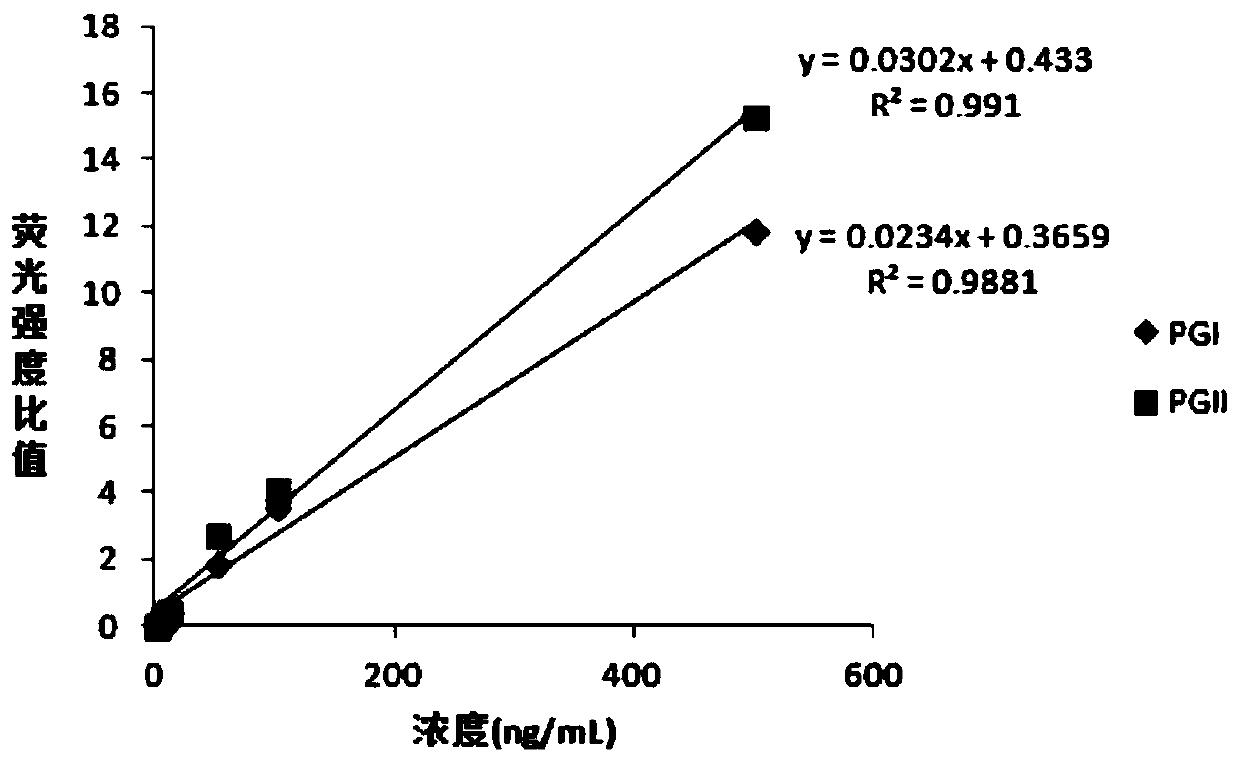
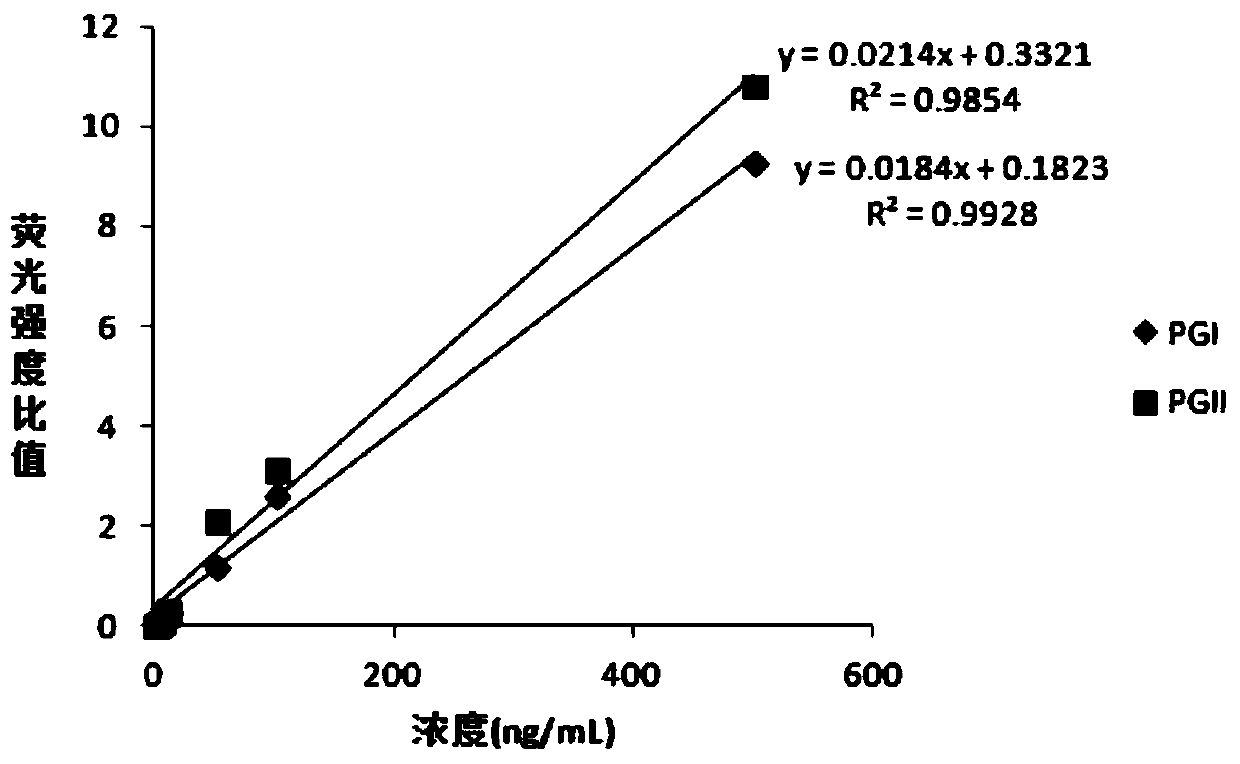
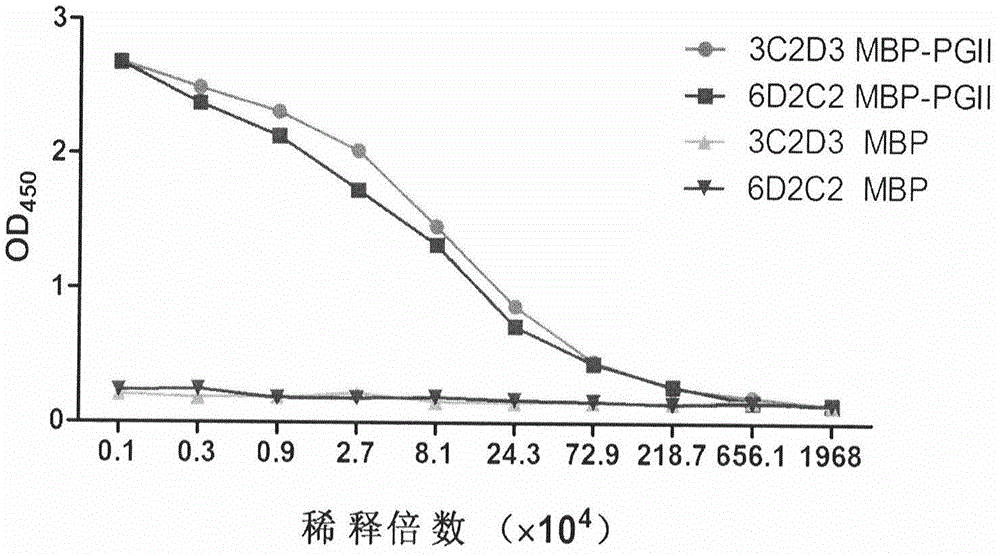
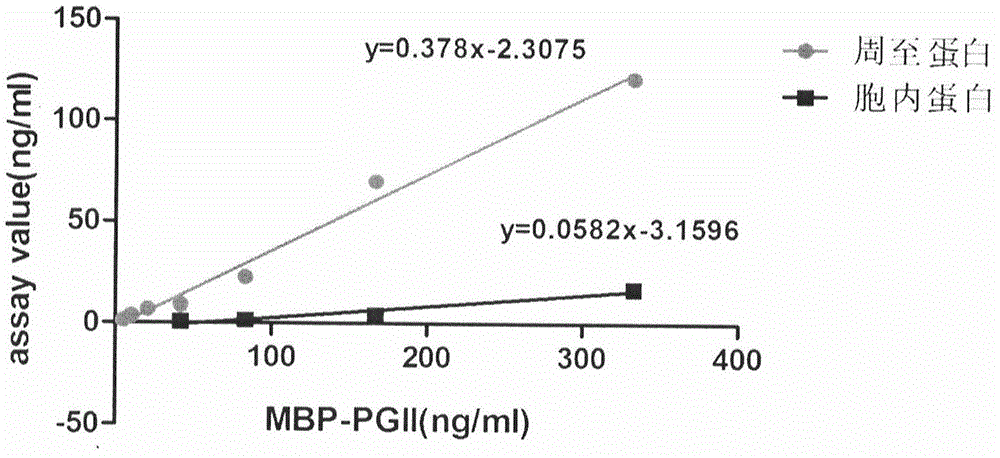
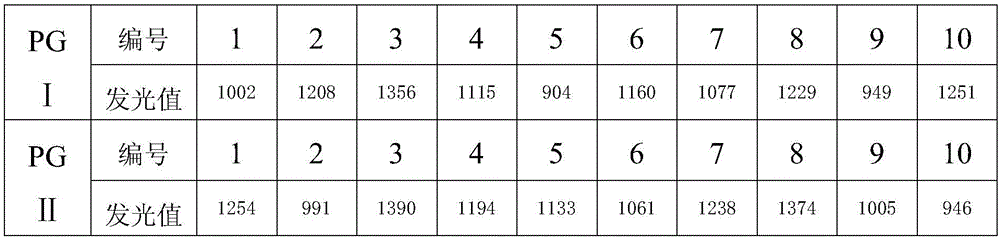
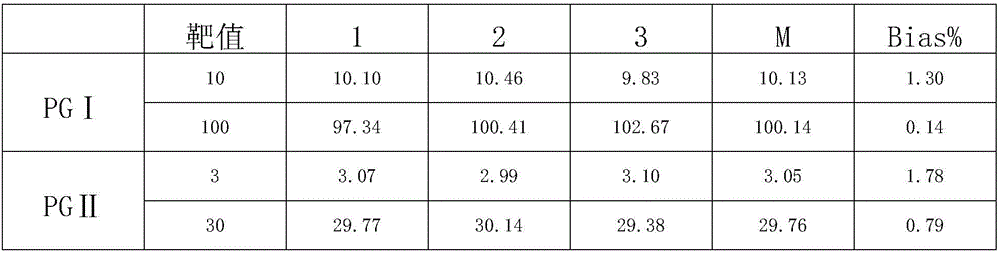
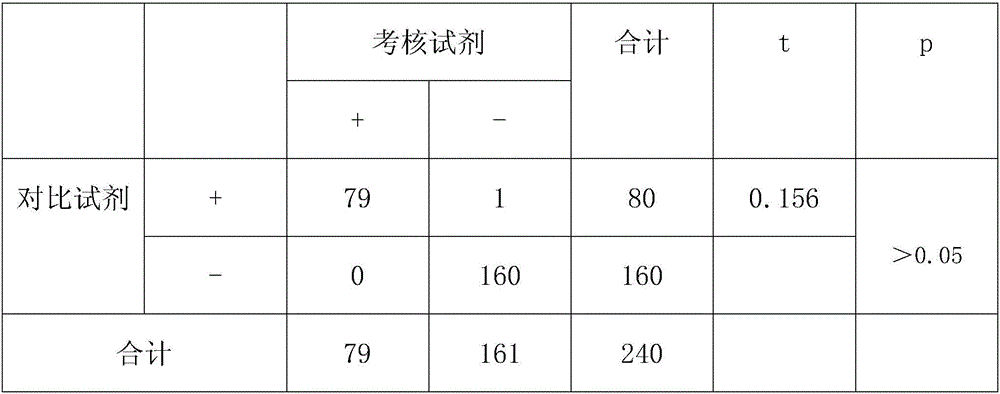
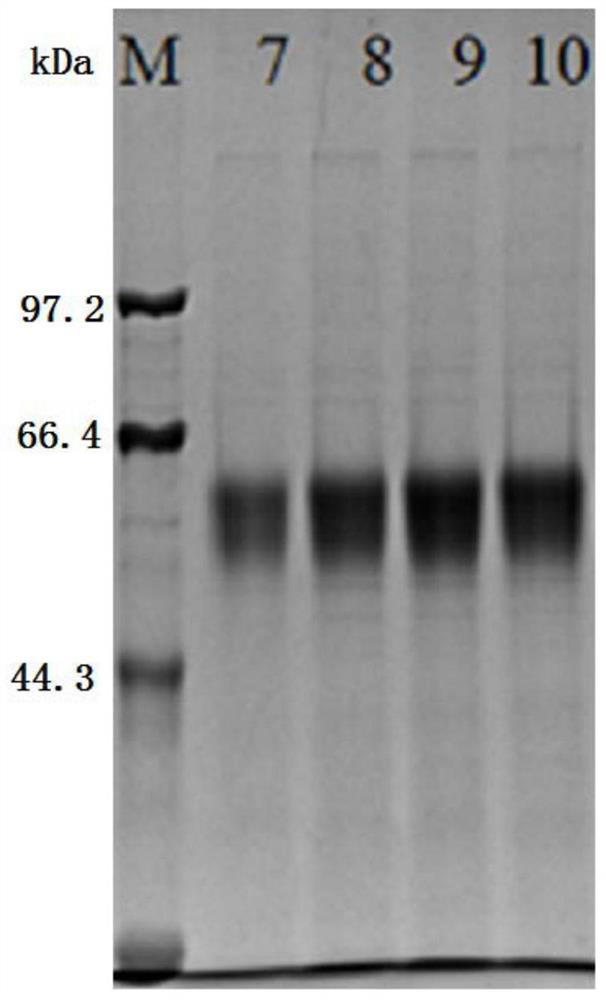
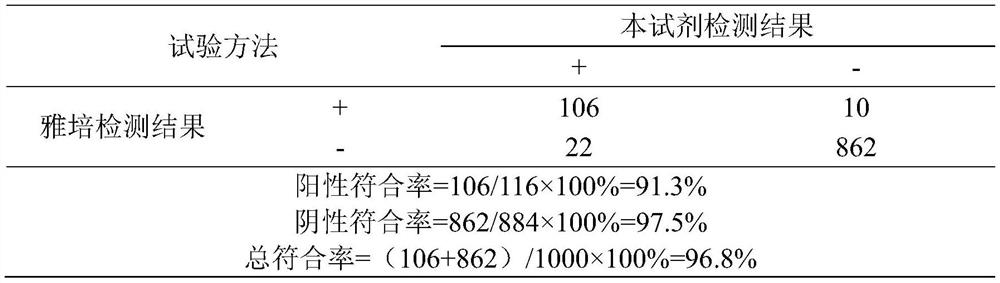
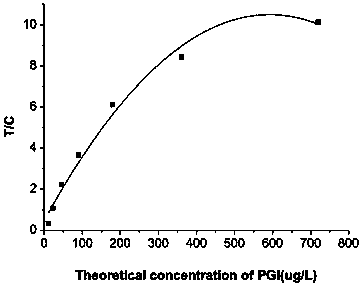
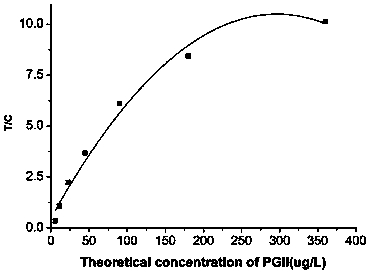
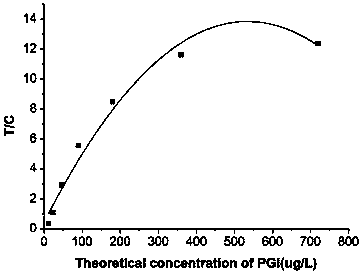
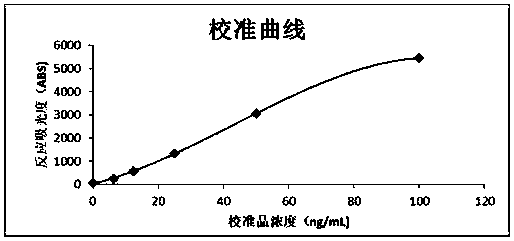
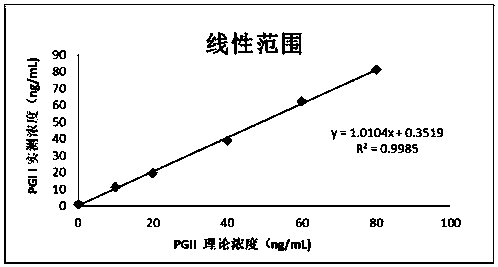
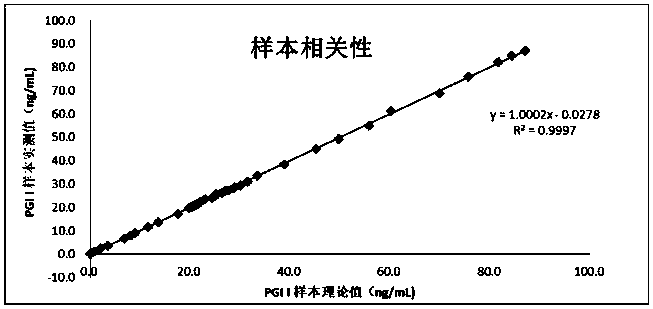
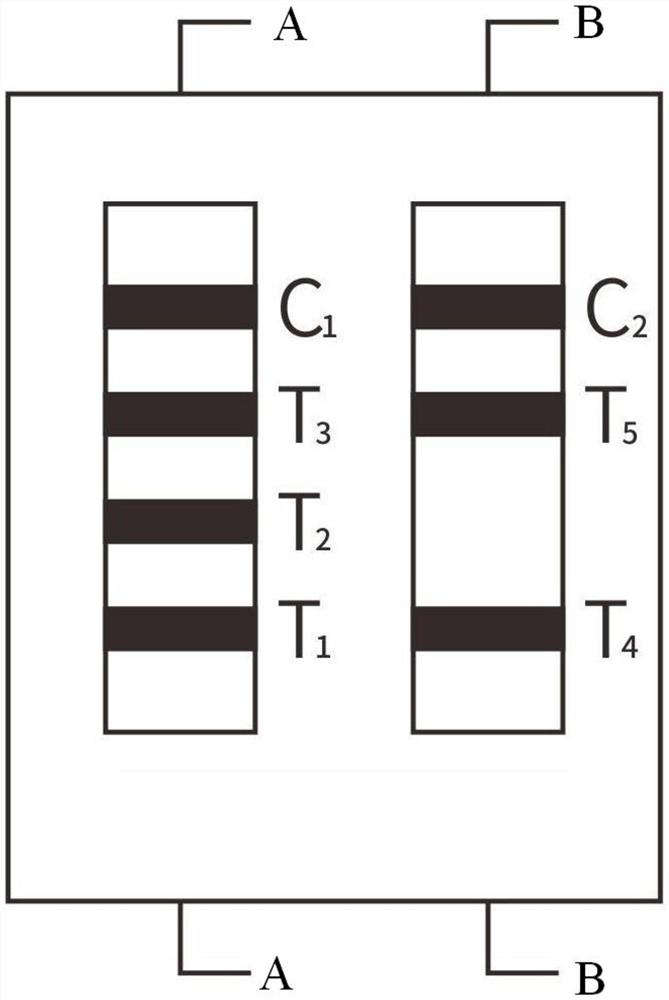
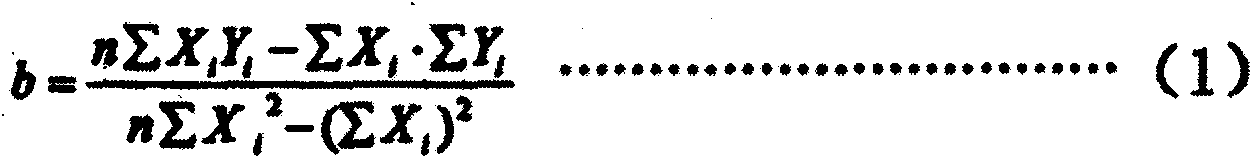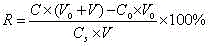Patents
Literature
57 results about "Pepsinogen II" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
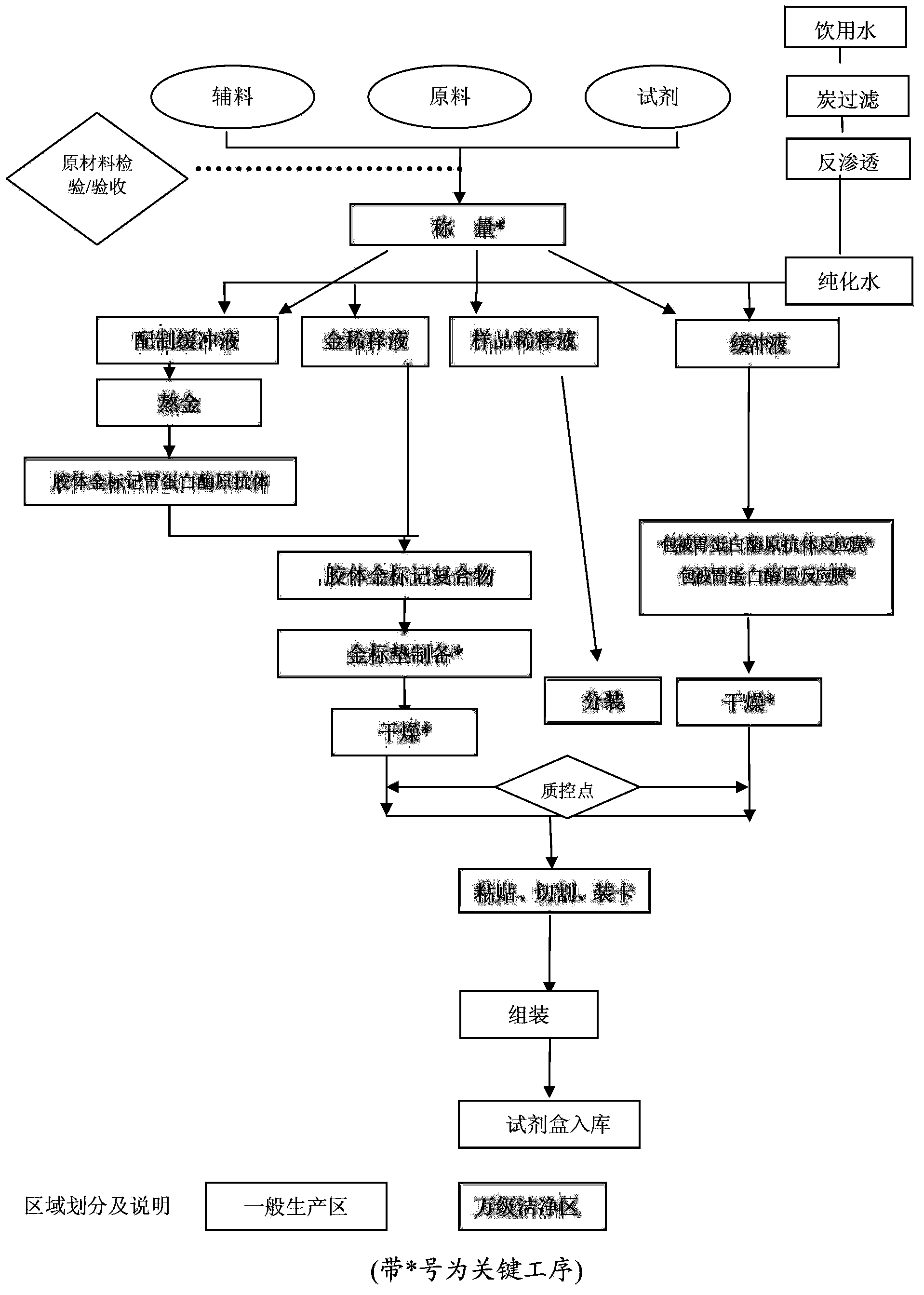
Latex enhanced turbidimetric immunoassay kit for diagnosing gastric diseases or gastric cancer, preparation method thereof and application
The invention discloses a latex enhanced turbidimetric immunoassay kit for diagnosing gastric diseases or gastric cancer, a preparation method thereof and application. Components of the kit include diluent, blank solution and a latex reagent of an antibody of pepsinogen I or an antibody of pepsinogen II and can further include a calibrator and quality control serum. The latex reagent contains nanoparticles coupled with the antibody of the pepsinogen I or the antibody of the pepsinogen II, and the particle sizes of the nanoparticles are different. The examination sensitivity cannot meet requirements when latex with the single particle size lower than 100nm is used for examination, the linear range is small when latex with the single particle size of about 200nm is used for examination, and accordingly the examination sensitivity and the linear range cannot meet requirements when latex with the single particle size is used for examination. After composite latex of the kit is used for marking, the examination sensitivity can be improved, the linear examination range can be broadened, and the latex enhanced turbidimetric immunoassay kit has the advantages of fast examination, high sensitivity and specificity, good accuracy and the like in terms of gastric disease or gastric cancer examination.
Owner:BEIJING MOKOBIO LIFE SCI CO LTD
Enzyme linked immunosorbent assay kit for combined diagnosis of gastrosis or evaluation of gastric cancer risks
InactiveCN102087279AIncreased sensitivityImprove featuresComponent separationTissue cultureAntigenPepsinogen I
The invention discloses an enzyme linked immunosorbent assay kit for combined diagnosis of the gastrosis or evaluation of gastric cancer risks and a preparation method thereof. The kit comprises a micropore plate coated with an antibody against a pepsin antigen I or an antibody against a pepsin antigen II, an enzyme labeled antibody, a color-developing agent, a stop solution and a concentrated cleaning solution, wherein the pepsin antigen I or the pepsin antigen II is a natural protein obtained from extraction of human gastric mucosa tissue. The kit disclosed by the invention adopts a mouse immunized with pepsinogen I and pepsinogen II which are separated from human gastric mucosa to prepare immunogen of a monoclonal antibody, the used standard sample also adopts the pepsin antigen I or the pepsin antigen II separated from the human gastric mucosa, thereby the defects caused by adopting different structures of animal pepsinogen and human pepsinogen are filled. The kit can be used for accurately diagnosing the gastrosis or early gastric cancer and has the advantages of high sensitivity, strong specificity, good accuracy and the like.
Owner:BEIJING MOKOBIO LIFE SCI CO LTD
Latex immunoturbidimetry type pepsinogen II detection kit capable of eliminating chyle interference
ActiveCN103713140AStrong interference abilityHigh sensitivityBiological material analysisBiological testingPepsinogen IILatex particle
The invention relates to the technical field of medical examination and particularly relates to a latex immunoturbidimetry type pepsinogen II detection kit capable of eliminating chyle interference. The kit provided by the invention comprises: (1) a pepsinogen II calibrator; (2) a reagent 1 with a preset chyle remover; and (3) a reagent 2 containing a monoclonal antibody of anti-human pepsinogen II and polyclonal antibody coated latex particles. According to the kit using the method, a conjugation reaction of substance to be detected in a sample and specific antibodies in the reagents is amplified by a latex agglutination effect, turbidity formed by the reaction is associated with the content of the substance to be detected under a given wavelength, so that the content of the substance to be detected can be calculated. The kit provided by the invention is used for detecting the content of pepsinogen II in human serum, and the kit is high in sensitivity, good in specificity, capable of eliminating chyle interference in the sample, quick and convenient to operate, strong in practicability and is wide in application range.
Owner:北京万泰德瑞诊断技术有限公司
Recombinant human pepsinogen II isozyme chimeric protein, and preparation method and applications thereof
ActiveCN103387971AHigh sensitivityIncrease catch rateHydrolasesSerum immunoglobulinsPepsinogen IIIsozyme
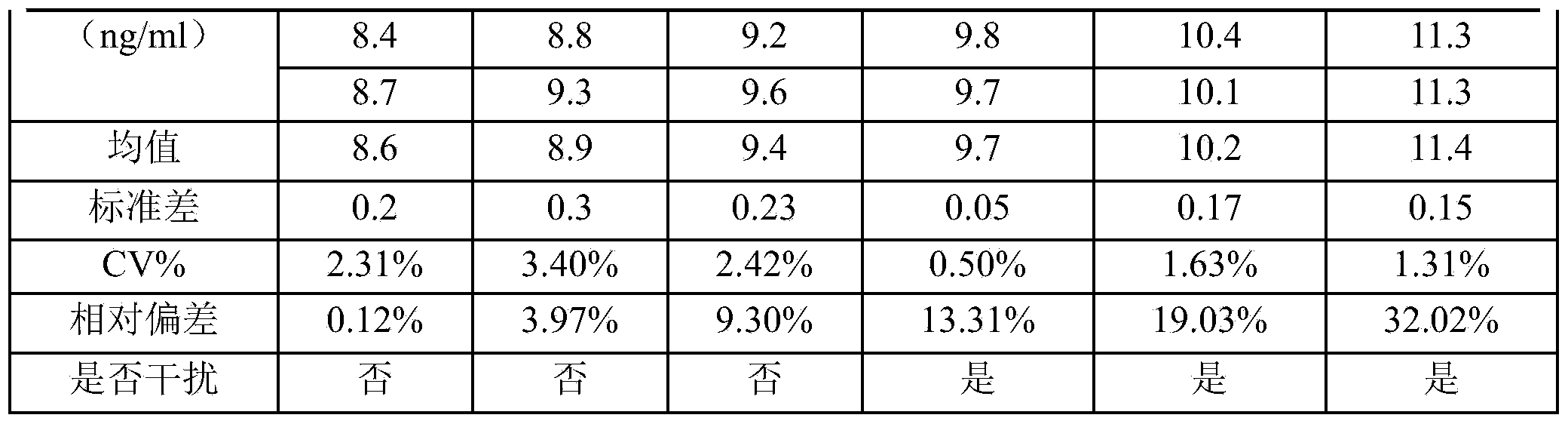
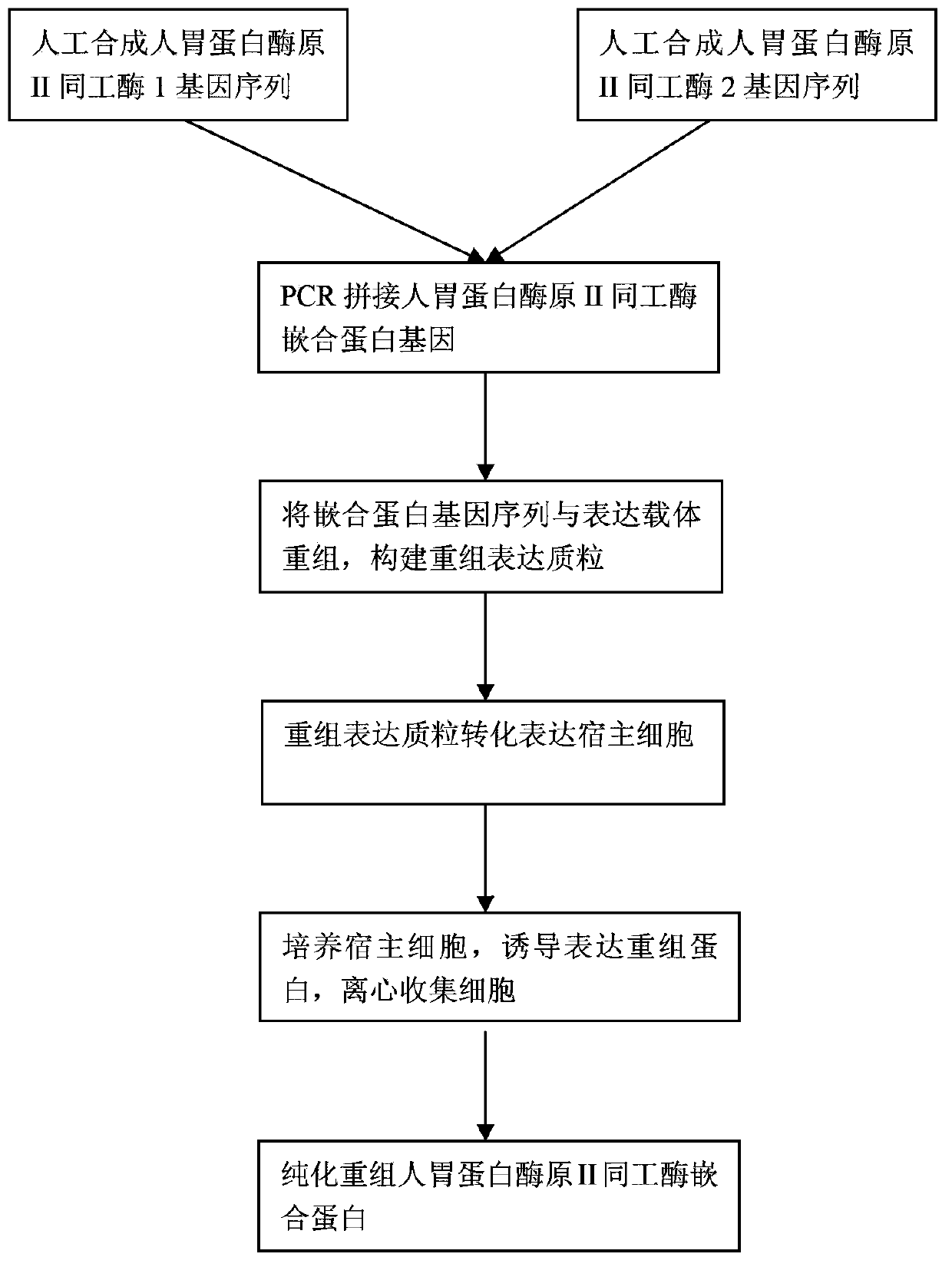
The invention discloses a recombinant human pepsinogen II isozyme chimeric protein, and a preparation method and applications thereof. The preparation method comprises the steps as follows: by taking gene sequences of two isozymes of recombinant human pepsinogen II as a template, carrying out PCR (polymerase chain reaction) splicing to obtain a chimeric protein coding gene sequence, constructing recombinant expression plasmids, transforming the screened positive clone plasmids into expression host cells, screening an efficiently-expressed recombinant chimeric protein strain, carrying out enlargement culture on the cells and inducing expression chimeric protein, and purifying to obtain the recombinant human pepsinogen II isozyme chimeric protein. The invention further discloses the applications of the recombinant human pepsinogen II isozyme chimeric protein in preparing monoclonal antibodies and multiresistant serums or pepsinogen II kit calibration products. A great amount of stably expressed recombinant human pepsinogen II isozyme chimeric protein can be produced by utilizing a genetic engineering technology, and one protein has two chimeric protein sequences of the human pepsinogen II.
Owner:常州爱复康生物科技有限公司
Test paper strip using colloidal gold immunochromatographic technology for quantitative determination of serum pepsinogen and preparation method and application thereof
The invention discloses a test paper strip using colloidal gold immunochromatographic technology for quantitative determination of serum pepsinogen and a preparation method and application thereof. A kit comprises the test paper strip respectively used for detection of pepsinogen I and pepsinogen II, the test paper strip orderly comprises a sample pad, a gold labeled pad, a cellulose nitrate film and a water absorbent pad according to the order of connection, and also includes a plastic bottom lining located below, the role of the plastic bottom lining is to provide a platform for assembling, the cellulose nitrate film is provided with a pepsinogen I or pepsinogen II spraying quality control line, and a pepsinogen I or pepsinogen II second antibody spraying test line, the gold labeled pad is coated with a colloidal gold labeled pepsinogen I or pepsinogen II monoclonal antibody, and the sample pad provides a to-be-tested sample adding position. The kit can be used for direct quantitative detection of the pepsinogen without need of professional training, is convenient and fast in operation, and suitable for promotion and application in primary hospitals and medical centers.
Owner:BEIJING MOKOBIO LIFE SCI CO LTD
Latex enhanced immunoturbidimetry assay kit for pepsinogen I/II
InactiveCN104111335AHigh detection sensitivityEasy to operateDisease diagnosisBiological testingPepsinogen IPepsinogen II
The invention relates to a kit for measurement of the contents of pepsinogen I (PGI) and pepsinogen II (PGII) in serum. To overcome a technical problem, the invention provides the PGI and PGII content measurement kit applicable to a nephelometric instrument or fully automatic biochemical analyzer. The kit comprises the following components: a, R1, which is composed of a buffer solution, an accelerator and a surfactant, with the balance being purified water; b, R2-I, which is composed of a buffer solution and a latex microsphere binding with an antibody against human PGI; c, R2-II, which is composed of a buffer solution and a latex microsphere binding with an antibody against human PGII; d, a calibrator 1, which is composed of a buffer solution, a stabilizing agent, an antiseptic and a certain amount of pure recombinant PGI protein, with the balance being purified water; and e, a calibrator 2, which is composed of a buffer solution, a stabilizing agent, an antiseptic and a certain amount of pure recombinant PGII protein, with the balance being purified water Through combination of the above-mentioned reagents, the kit can rapidly measure the contents of PGI and PGII in serum.
Owner:唐勇
Chemiluminescent quantitative determination kit for pepsinogen II and preparation method of chemiluminescent quantitative determination kit
InactiveCN104569415AStrong specificityHigh sensitivityChemiluminescene/bioluminescencePepsinogen IPepsinogen II
The invention relates to the field of immunodetection, in particular to a chemiluminescent quantitative determination kit for pepsinogen II and a preparation method of the chemiluminescent quantitative determination kit. The chemiluminescent quantitative determination kit for the pepsinogen II comprises (1) a pepsinogen II antibody coated micropore plate, (2) an HRP-labeled pepsinogen II antibody, (3) series pepsinogen II calibration materials diluted by a calibration material diluent, (4) a luminescent substrate A, (5) a luminescent substrate B and (6) a solid washing liquid. The kit disclosed by the invention is capable of detecting the content of the pepsinogen II in serum; and the level of the pepsinogen II in the serum is in positively relevant to atrophic gastritis and peptic ulcer; in treatment of peptic ulcer, the treatment result can be judged by monitoring the change of the degermed pepsinogen; and the chemiluminescent quantitative determination kit is noninvasive, simple and fast in detection method, high in sensitivity, wide in detection range and the like.
Owner:HENAN MAINCARE BIOLOGICAL TECH
Kit for detecting pepsinogen II
InactiveCN108982850AHigh sensitivityImprove stabilityBiological material analysisPepsinogen IISucrose
The invention discloses a kit for detecting pepsinogen II, belonging to the technical field of biological detection. The kit comprises a reagent R1 and a reagent R2, wherein the reagent R1 comprises components of the following concentrations: buffer of 10-200mmoL / L, surfactant of 0.01-5g / L, accelerator of 5-20g / L, and preservative of 0.05-2g / L, wherein a component concentration in the reagent R1 is the final concentration of a component in the reagent R1; reagent R2 comprises the components of the following concentrations: buffer of 80-160mmoL / L, stabilizer of 10-120g / L, blocking agent5-30g / L, anti-human pepsinogen II antibody latex solution of 0.5-3.0g / L, and preservative of 0.05-2g / L, wherein a component concentration in the reagent R2 is the final concentration of a componentin the reagent R2; the stabilizer is at least one of glycerin, sucrose, trehalose and glucose. The kit of the invention has the advantages of high sensitivity, high precision, stable reagents and thelike.
Owner:广州市伊川生物科技有限公司
Pepsinogen II detection kit and preparation method thereof
PendingCN111122866AElimination of chylolysisGuaranteed stabilityDisease diagnosisPepsinogen IPepsinogen II
The invention discloses a pepsinogen II detection kit, which comprises a reagent R1 and a reagent R2, wherein the reagent R1 is prepared from 50-150 mmol / L of sodium phosphate buffer solution, 2-3 g / Lof bovine serum albumin, methylisothiazolinone with the concentration of 2-3%, 1.0-5.0 g / L of fatty alcohol and ethylene oxide condensate, 3-5 mmol / L of magnesium chloride and 20-30 mmol / L of ammonium formate, and the balance of deionized water.; and the reagent R2 is prepared from 1.3-2 mg / ml of pepsinogen II antibody emulsion solution, 2-3 g / L of bovine serum albumin, methylisothiazolinone withthe concentration of 2-3%, and the balance of deionized water. The prepared pepsinogen II detection kit and the preparation method of the pepsinogen II detection kit have the advantages that the chyle problem can be effectively solved, the anti-interference capability is high, and meanwhile, the stability and the sensitivity are high.
Owner:浙江强盛生物科技有限公司
Pepsinogen II detection kit
InactiveCN109307765AEliminates heterophile antibodiesImprove anti-interference abilityChemiluminescene/bioluminescenceBiological testingAntigenPepsinogen II
The invention discloses a pepsinogen II detection kit. The kit comprises a magnetic particle coated with a pepsinogen II monoclonal antibody, an enzyme diluent containing a horseradish peroxidase (HRP) labeled pepsinogen II monoclonal antibody, and a calibrator and a sample diluent containing a pepsinogen II antigen. A blocker component is contained in the sample diluent. The blocker is one or more human anti-mouse antibodies which neutralizes interfere of the HAMA. The method has the advantages that the high-sensitivity chemiluminescence technology and the magnetic particle preparation technology are combined, and an AutoLumo full-automatic detection analyzer is used in cooperation, so that a full-automatic detection is realized; meanwhile, the blocker component is added into the sample diluent, so that the heterophile antibody in the sample can be eliminated, and the anti-interference capability of the kit is greatly improved.
Owner:AUTOBIO DIAGNOSTICS CO LTD
Recombinant antibody against human pepsinogen II
The invention relates to a recombinant antibody against human pepsinogen II (PG II), and studies preparation and application of the recombinant antibody. The binding protein has strong activity, has high affinity with human PG II protein, and can be widely used in the field of detection of the PG II protein.
Owner:DONGGUAN PENGZHI BIOTECH CO LTD
Test strip for detecting pepsinogen I and pepsinogen II as well as detection method and application of test strip
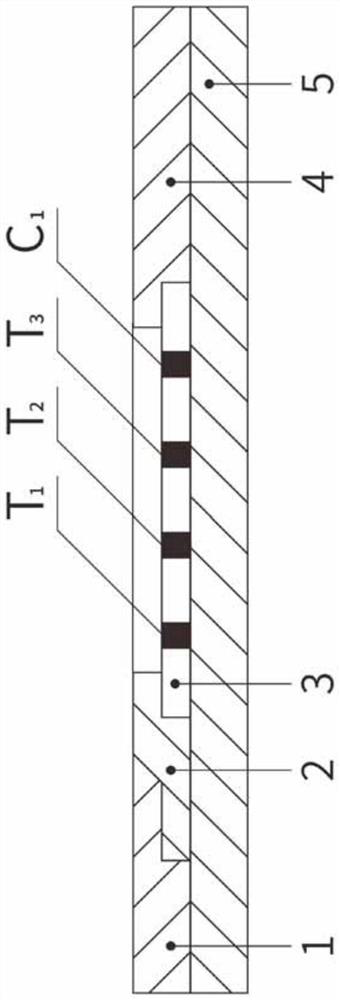
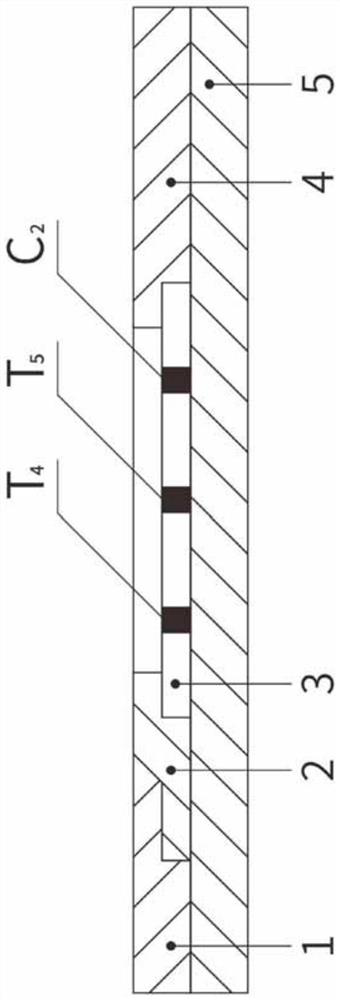
The invention relates to a time resolution fluorescence immunochromatographic test strip and test card for simultaneously and quantitatively detecting contents of pepsinogen I and pepsinogen II as well as an application of the test strip. According to the test strip disclosed by the invention, a nitrocellulose membrane is provided with a first detection zone, a second detection zone and a quality control zone which are sprayed at intervals, the first detection zone is coated with a pepsinogen I monoclonal antibody I, the second detection zone is coated with a pepsinogen II monoclonal antibody II, the quality control zone is coated with a rabbit anti-mouse polyclonal antibody, and a bonding pad is coated with a macromolecular nano microspherical coating with the surface decorated by the pepsinogen I monoclonal antibody I and the pepsinogen I monoclonal antibody and containing rare-earth ions; the test strip can simultaneously detect the contents of the pepsinogen I and the pepsinogen II, the problems that a pepsinogen test box cannot simultaneously detect the pepsinogen I and the pepsinogen II in the prior art can be solved, the high accuracy and the small error in the detection result of the pepsinogen I and the pepsinogen II in same sample liquid can be guaranteed, and the great convenience can be provided for the clinical use.
Owner:无锡市江原实业技贸有限公司
Pepsinogen I and pepsinogen II combined detection kit and application thereof
PendingCN111398591AShorten the timeReduce testing costsDisease diagnosisPepsinogen ITime resolved fluorescence immunoassay
The invention discloses a pepsinogen I and pepsinogen II combined detection kit and application thereof. The invention discloses a pepsinogen I and II combined detection kit for the first time. The pepsinogen I and II combined detection kit comprises a coating antibody and a labeled antibody, wherein the coating antibody is an anti-PGI monoclonal antibody named as a monoclonal antibody IA and an anti-PGII monoclonal antibody named as a monoclonal antibody IIA; wherein the labeled antibody is an anti-PG I monoclonal antibody named as a monoclonal antibody IB and an anti-PG II monoclonal antibody named as a monoclonal antibody IIB. The invention further discloses application of the kit. According to the invention, the pepsinogen I and II combined detection kit is combined with a high-sensitivity inductively coupled plasma mass spectrometry detection technology on the basis of time-resolved fluorescence immunoassay. Detection of two indexes of PGI and PGII can be completed at the same time in one cycle, the time for clinically obtaining a PGI value and a PGI / PGII ratio result is shortened, and the detection cost is greatly reduced.
Owner:北京清分稳同科技有限公司
Pepsinogen II detection method and kit thereof
InactiveCN104502592ARealize in vitro quantitative detectionAccurately determine the qualityDisease diagnosisPepsinogen IIFreeze-drying
The invention relates to a pepsinogen II detection method and a kit, and especially relates to a dual wavelength fluorescence immunity chromatography detection method of pepsinogen II and a detection kit thereof. The method comprises the following steps: 1)preparing an immunity chromatography test strip; 2)preparing a freeze-dried probe; 3)preparing a sample weak solution; and 4)examining the sample. The pepsinogen II detection method and the kit have the advantages of high sensitivity, high accuracy, simple operation and low cost.
Owner:江苏宏泰格尔生物医学工程有限公司
Fluorescent microsphere detection device for gastric function and gastric cancer risk and preparation method thereof
ActiveCN111638369ASimple structureNovel ideaDisease diagnosisBiological testingPepsinogen ICellulose
The invention relates to a fluorescent microsphere detection device for gastric function and gastric cancer risk and a preparation method thereof, and belongs to the field of medical detection equipment. The device is prepared by adhering a nitrocellulose membrane with solid phase containing high-specificity gastrin 17, pepsinogen I, pepsinogen II antibody, anti-human IgG antibody, anti-human IgMantibody and goat-anti-mouse IgG polyclonal antibody, glass fibers adsorbed with fluorescent microsphere labeled gastrin 17, pepsinogen I, pepsinogen II antibodies and helicobacter pylori urease antigens, a sample pad, absorbent paper and other auxiliary materials. The reaction sensitivity is effectively improved; under the same threshold value, the dosage of the immunofluorescence microspheres can be reduced, the cost is saved; five gastric cancer risk markers including gastrin 17, pepsinogen I, pepsinogen II, helicobacter pylori urease IgG antibody and helicobacter pylori urease IgM antibodyin a specimen can be detected at the same time, and the complexity of production operation is not increased. The detection test paper is high in sensitivity, strong in specificity and strong in practicability.
Owner:JILIN PROVINCE GERUISITE BIOTECHNOLOGY CO LTD
Pepsinogen II (PGII) quantitative assaying kit, preparing method thereof and detecting method thereof
InactiveCN105372426AHigh sensitivityEasy to mixChemiluminescene/bioluminescencePepsinogen IIMagnetic bead
The invention discloses a pepsinogen II (PGII) quantitative assaying kit. The kit comprises a calibrated material, a quality control material, a resistance reagent, a magnetic particle reagent and a light emitting substrate. The invention further discloses a preparing method of the kit and a method for detecting PGII through the kit. According to the kit, the preparing method and the method, the resistance reagent is prepared from a PGII coated antibody labeled through fluorescein isothiocyanate and a PGII labeled antibody labeled through alkaline phosphatase, the magnetic particle reagent is prepared in the mode that the fluorescein isothiocyanate resistant antibody is coupled with carboxyl magnetic beads, even mixing and separation of an immunoreaction are easier accordingly, and the reaction speed is greatly increased; the novel chemical light emitting substrate APLS serves as the substrate, and therefore the sensitivity and the specificity of the kit are improved. The detecting kit is reliable in performance, high in sensitivity, wide in linear range and capable of being used in cooperation with semi-automatic and full-automatic instruments.
Owner:JIANGSU ZECEN BIOTECH CO LTD
Kit for determining pepsinogen II and preparation method thereof
InactiveCN105911279AImprove suspension stabilityImprove accuracyBiological material analysisInorganic saltsPepsinogen A
The invention discloses a kit for determining pepsinogen II and a preparation method thereof. The kit comprises double liquid components including a reagent R1 and a reagent R2 which are independent from each other, wherein the reagent R1 is prepared from a buffering solution, inorganic salt, an accelerant and a preservative; and the reagent R2 is prepared from a buffering solution, a stabilizer, a preservative and an emulsion-coated anti-human pepsinogen II antibody. The preparation method comprises the following steps: preparing the reagents according to the content of the components; mixing a sample to be detected with the reagent R1 and the reagent R2 to achieve sufficient reaction; determining a reacted absorbance difference value by utilizing a full-automatic biochemical analyzer; and calculating the concentration of the pepsinogen A in a sample according to an absorbance change value. The kit gas has the advantages of high detection accuracy and the like.
Owner:ANHUI IPROCOM BIOTECH CO LTD
Composite quality control product for gastric function detection and detection kit
InactiveCN111024964AMeet stability requirementsShelf life stableDisease diagnosisBiological testingPepsinogen IPepsinogen II
The invention relates to a pepsinogen I, pepsinogen II and gastrin-17 composite quality control product. The composite quality control material comprises a protein preserving solution, and pepsinogenI, pepsinogen II and gastrin-17 which are dissolved in the protein preserving solution, wherein the total volume of the protein preserving solution is calculated; wherein the protein preserving solution contains 0.8 to 3.5 g / L of AEP-HBC, 0.002 to 0.006 g / L of fibrinogen, 0.05 to 0.15 g / L of gelatin, 2 to 8 g / L of trehalose, 0.05 to 0.25 g / L of tetrapolyphosphate, 5-25 mmol / L of 2-(N-morpholine) ethanesulfonic acid, 0.5-4 g / L of ethylenediaminetetraacetic acid or ethylenediaminetetraacetic acid salt, 1-20 mmol / L of cysteine, methionine or arginine and 0.05-0.10% of a preservative,. The pepsinogen I, pepsinogen II and gastrin-17 composite quality control product is initiated at home, and can be stably stored for 14 months at the temperature of 2-8 DEG C. The invention further discloses a preparation method of the pepsinogen I, pepsinogen II and gastrin-17 composite quality control product. The invention also provides a detection kit containing the composite quality control product, andthe detection kit can be used for clinical detection of gastric functions.
Owner:深圳市蔚景生物科技有限公司
Combined detection method for pepsinogen I and pepsinogen II
The invention discloses a combined detection method for pepsinogen I and pepsinogen II. The method comprises the steps of immunochromatography test strip assembly, antibody coating, sample diluent preparation, sample detection and the like. The combined detection method for pepsinogen I and pepsinogen II utilizes a fluorescence immunochromatographic technique and adopts a double-antibody sandwichmethod, can detect the content of pepsinogen I / II in serum or plasma 10 minutes after sample adding, has the advantages of simplicity, convenience and rapidness, and avoids the damage of X-ray to human body and the inconvenience of gastroscope; and meanwhile, through the detection of samples with different concentrations, the sensitivity is higher, and the correlation is good.
Owner:NINGBO AUCHEER BIOTECHNOLOGY CO LTD
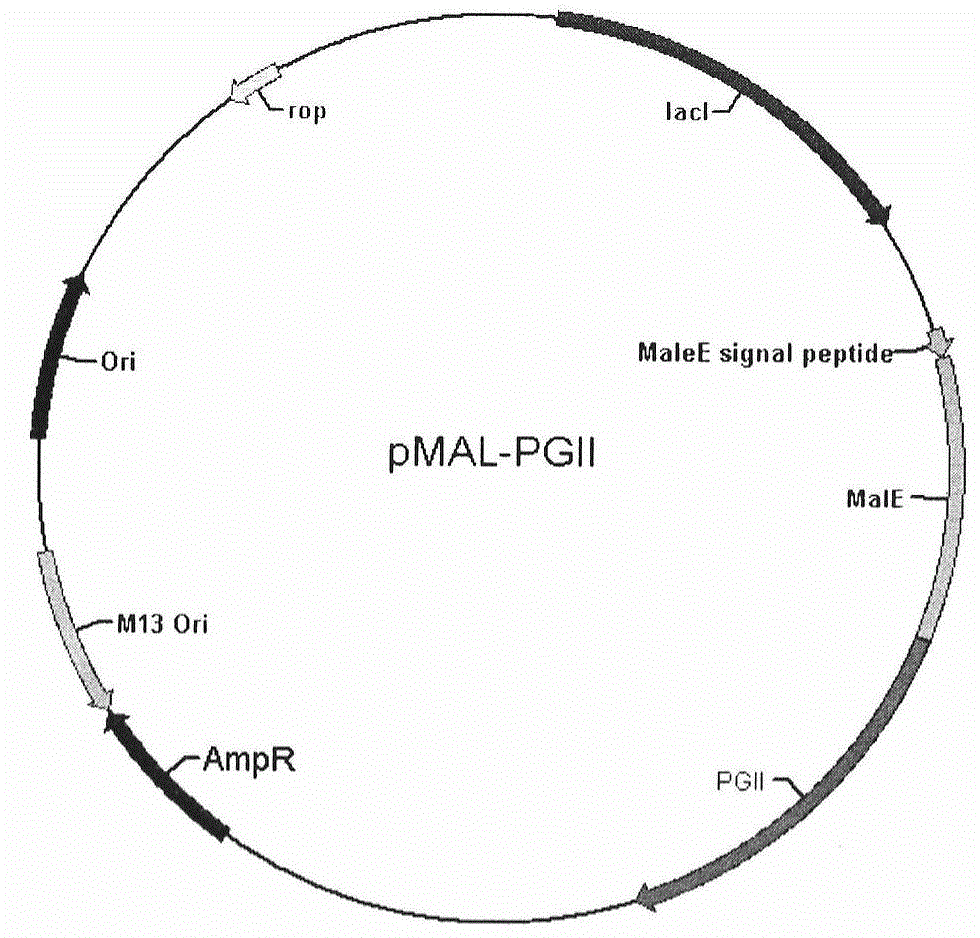
Soluble secretory expression of PG II-MBP fusion protein and application thereof
The invention belongs to the technical field of bioengineering and particularly relates to soluble secretory expression of a fusion protein of a human pepsinogen II (PG II) and a maltose binding protein (MBP) in escherichia coli, provides a preparation method of the fusion protein, and also discloses application of a recombinant BMP-PG II fusion protein to preparation of monoclonal antibodies and calibration materials of pepsinogen II kits. Through the adoption of elements such as a Ptac promoter, an malE signal peptide and the maltose-binding protein, the soluble secretory expression of PG II from periphery to inside of escherichia coli is implemented; the immunogen activity of the recombinant human PG II protein from a prokaryotic expression system is greatly improved; and the preparation cost of the recombinant PG II is effectively reduced.
Owner:BEIJING JIAWAN BIOTECH CO LTD
Pepsinogen I and pepsinogen II combined detection kit and preparation method and detection method thereof
The invention discloses a pepsinogen I and pepsinogen II combined detection kit and a preparation method and detection method thereof. On the basis of enzyme immunoassay, a high sensitivity chemiluminescence detection technology is adopted, PG I and PG II can be simultaneously measured in one circulation test; compared with a method that measures PG I and PG II individually in sequence, the PG I / PG II detection time is shortened in clinic; furthermore, universal reagents are used during whole detection process, the detection cost is largely reduced; a Hamliton automatic detection and analysisinstrument is used, automation (from sample loading to result analysis) is realized; the interference of manual operation is avoided maximally; the time and error of operation such as sample loading,and the like are both reduced; the repeatability is strong, the detection becomes faster, and the results are more stable and reliable.
Owner:上海铭源数康生物芯片有限公司
Pepsinogen I monoclonal antibody and application thereof
ActiveCN113004412AHigh sensitivityHigh precisionBiological material analysisImmunoglobulins against enzymesPepsinogen IPepsinogen II
The invention relates to the technical field of biology, in particular to a pepsinogen I monoclonal antibody and application thereof, and discloses a group of pepsinogen I monoclonal antibodies which can be used in a matched mode. The pepsinogen I monoclonal antibodies have high specificity and stability when being combined with PGI, cannot generate cross reaction with pepsinogen II, and are suitable for various methodologies, and high-sensitivity and high-precision detection of PGI is realized.
Owner:重庆艾生斯生物工程有限公司
Pepsinogen II monoclonal antibody and application thereof
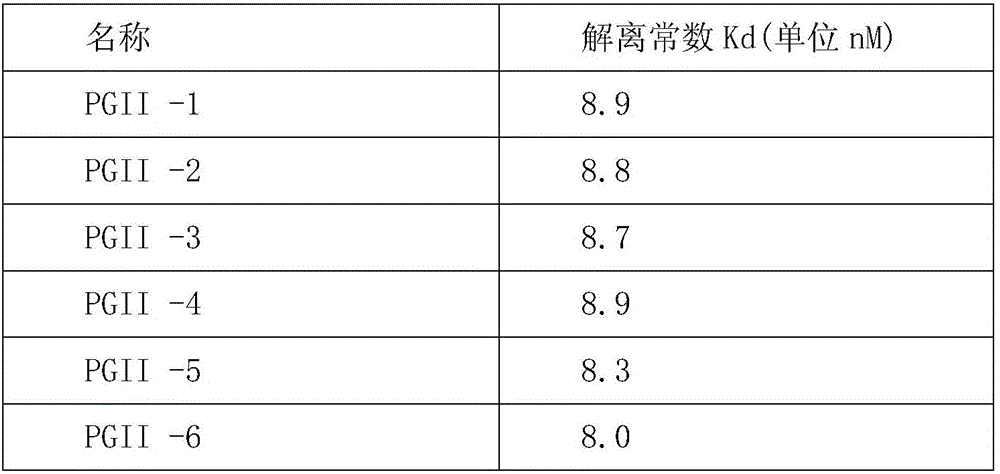
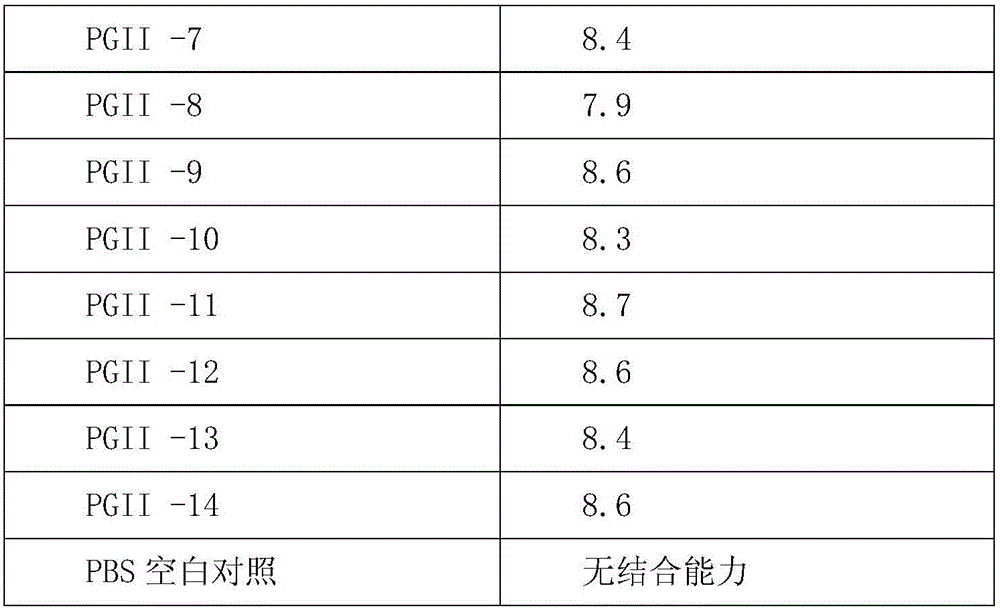
ActiveCN113045666AWith anti-interference abilityAccurate measurementDisease diagnosisImmunoglobulins against enzymesPepsinogen IPepsinogen II
The invention relates to the technical field of biology, in particular relates to a pepsinogen II monoclonal antibody and application thereof, and discloses a group of pepsinogen II monoclonal antibodies which can be used in cooperation, which can be applied to different methodologies, accurate measurement of PGII is achieved, interference of other impurities can be avoided, and high-specificity binding is achieved. The monoclonal antibody provided by the invention can assist in research and development of medical diagnostic reagents, and has great significance.
Owner:重庆艾生斯生物工程有限公司
Stable protein solution, preparation method thereof and detection kit
InactiveCN111024959AImprove stabilityAvoid sex changeBiological material analysisBiological testingProtein solutionPepsinogen I
The invention provides a stable protein solution. The protein solution comprises a buffer solution and a protein containing a heme auxiliary group or a protein conjugate of the protein containing theheme auxiliary group and other proteins. Ferrous ions in the heme auxiliary group are combined with carbon monoxide, so that the protein or the protein conjugate can be prevented from being oxidized and denatured in the storage process, and the stability of the protein solution is improved. The protein solution may also include other stabilizers. The protein solution disclosed by the invention canbe stably stored for at least one year at 2-8 DEG C, has good stability, and can be used for preparing a chemiluminescence detection kit or an enzyme-linked immunosorbent assay kit, such as a detection kit for detecting gastrin 17, pepsinogen I and pepsinogen II.
Owner:深圳市蔚景生物科技有限公司
Chemiluminiscence detection kit for diagnosing gastropathy in combined manner and preparation and application method thereof
The invention discloses a chemiluminiscence detection kit for diagnosing gastropathy in a combined manner. The detection kit is mainly used for performing pepsinogen I detection and pepsinogen II detection. On the basis of the detection kit, the invention further provides a marker combined standard value, which is applicable to the detection kit and can provide reference for gastropathy detection, for detection. The detection kit has the advantages that the detection kit is wide in linearity range, high in specificity, convenient to operate, and the like in terms of gastropathy diagnosis and early gastric cancer screening; in addition, the detection kit is accurate and detailed in gastropathy diagnosis and high in market popularization value, and damage, caused by X-rays, on human bodies and the inconvenience of a gastroscope are avoided.
Owner:山东康华生物医疗科技股份有限公司
Pepsinogen II recombinant protein and its monoclonal antibody, preparation method and application thereof
InactiveCN113046324AIncrease productionImprove controllabilityVector-based foreign material introductionPeptidasesAntigen epitopeAntigen
The invention discloses a CHO-K1 cell strain, which contains a gene capable of efficiently secreting and expressing PG II recombinant protein. The invention also discloses a method for preparing the CHO-K1 cell strain, and the method comprises the following steps: 1) cloning a gene sequence as shown in SEQ ID NO.1 into an eukaryotic expression vector to obtain a recombinant plasmid containing the gene encoding the PG II recombinant protein; (2) transfecting the recombinant plasmid into a CHO-K1 cell, so as to obtain a CHO-K1 cell strain; and 3) culturing, screening and domesticating the CHO-K1 cell strain in the step 2) to obtain the cell strain capable of efficiently secreting and expressing the PG II recombinant protein. The invention also discloses an anti-PG II monoclonal antibody, wherein the antigen epitope combined with a monoclonal antibody 1 is located at aa78-aa90 of the pepsinogen II; and the antigen epitope combined with a monoclonal antibody 2 is located at aa280-aa291 of the pepsinogen II. The protein provided by the invention is high in expression quantity, good in quality and low in cost; the monoclonal antibody can be paired and used for detecting the PG II protein, and is good in specificity and high in sensitivity.
Owner:黎榕萍
Pepsinogen immunochromatographic detection kit based on carbon quantum dots, and preparation method thereof
ActiveCN110208547AHigh fluorescence intensityHigh detection sensitivityBiological testingFluorescence/phosphorescenceEthylenediamineCellulose
The invention discloses a pepsinogen immunochromatographic detection kit based on carbon quantum dots, and a preparation method thereof. The pepsinogen immunochromatographic detection kit comprises aplastic plate and an adsorption layer fixed on the plastic plate, wherein the adsorption layer is composed of a sample pad, a glass cellulose film, a nitrocellulose film and a water absorbent pad which are in lap joint with each other from the test end in sequence; the glass cellulose film is coated with carbon quantum dot-labeled pepsinogen I and pepsinogen II; the nitrocellulose film is providedwith a detection line 1, a detection line 2 and a control line, the detection line 1 is coated with pepsinogen I secondary antibodies, the detection line 2 is coated with pepsinogen II secondary antibodies, and the control line is coated with goat-anti-mouse IgG; and the carbon quantum dots are respectively modified by means of ethylenediamine and N-hydroxysuccinimide. The pepsinogen immunochromatographic detection kit of the invention can be used for combined detection of the pepsinogen I and the pepsinogen II, and has the advantages of detecting pepsinogen in serum efficiently in a low-toxicity manner.
Owner:北京柏海达科技有限公司
Pepsinogen II (PGII) detection kit and production technology
ActiveCN109490533AHigh detection sensitivityImprove measurement repeatabilityMaterial analysisPepsinogen APepsinogen II
The invention provides a pepsinogen II (PGII) detection kit. The pepsinogen I detection kit comprises a reagent R1 and a reagent R2 which are independent to each other, wherein the reagent R1 comprises the following components: 1 to 10g / L of a trihytdroxy methyl aminomethane buffering solution, 1 to 20g / L of sodium chloride, 0.1 to 10mg / L of Tween 20, 10 to 50g / L of polyethylene glycol 20000 and 0.1 to 10g / L of sodium azide; the reagent R2 comprises the following components: 1 to 10g / L of the trihytdroxy methyl aminomethane buffering solution, 10 to 100g / L of trehalose, 2 to 10ml / L of a surfactant, 10 to 1000mg / L of a pepsinogen II (PGII)antibody and 0.1 to 10g / L of the sodium azide. The pepsinogen II (PGII) detection kit is ready to use and does not need to be prepared; and the pepsinogenII (PGII) detection kit has high detection sensitivity and good measurement repeatability and has relatively good stability when being stored for a long period. The invention further provides a production technology of the pepsinogen II (PGII) detection kit.
Owner:NANJING AUBRIME ABM BIOTECH
Stomach function and stomach cancer risk detection device and preparation method thereof
ActiveCN111638370AImprove reasonable comprehensive judgmentSensitive detectionDisease diagnosisBiological testingPepsinogen ICellulose
The invention relates to a stomach function and stomach cancer risk detection device and a preparation method thereof, and belongs to the field of medical detection equipment. The device is prepared by adhering a nitrocellulose membrane with a solid phase containing high-specificity gastrin 17, pepsinogen I, pepsinogen II antibody, anti-human IgG antibody, anti-human IgM antibody and goat-anti-mouse IgG polyclonal antibody, a glass fiber membrane adsorbed with colloidal gold labeled G-17, PGI and PGII antibodies and HP urease antigens, a sample pad, absorbent paper and other auxiliary materials. According to the invention, on the basis of ensuring complete release of immune colloidal gold, the reaction sensitivity is effectively improved; under the same threshold value, the dosage of immune colloidal gold can be reduced, the cost is saved, five gastric function evaluation and gastric cancer risk markers of G-17, PGI, PGII, HP urease IgG antibodies and IgM antibodies in a specimen can be detected at the same time, and the complexity of production operation is not increased. The test paper is high in sensitivity, strong in specificity, simple and convenient to operate, time-saving and strong in practicability.
Owner:JILIN PROVINCE GERUISITE BIOTECHNOLOGY CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com