Stable protein solution, preparation method thereof and detection kit
A protein solution and detection kit technology, applied in biological testing, measuring devices, material inspection products, etc., to achieve the effect of improving stability and preventing denaturation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
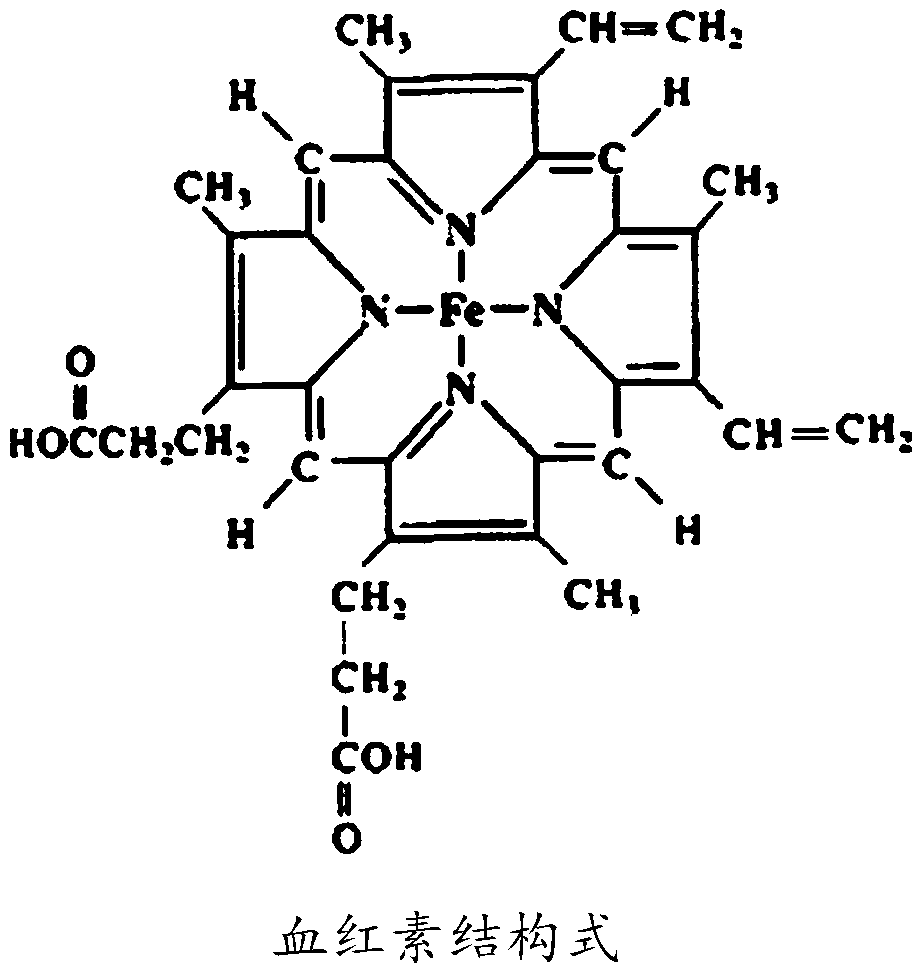
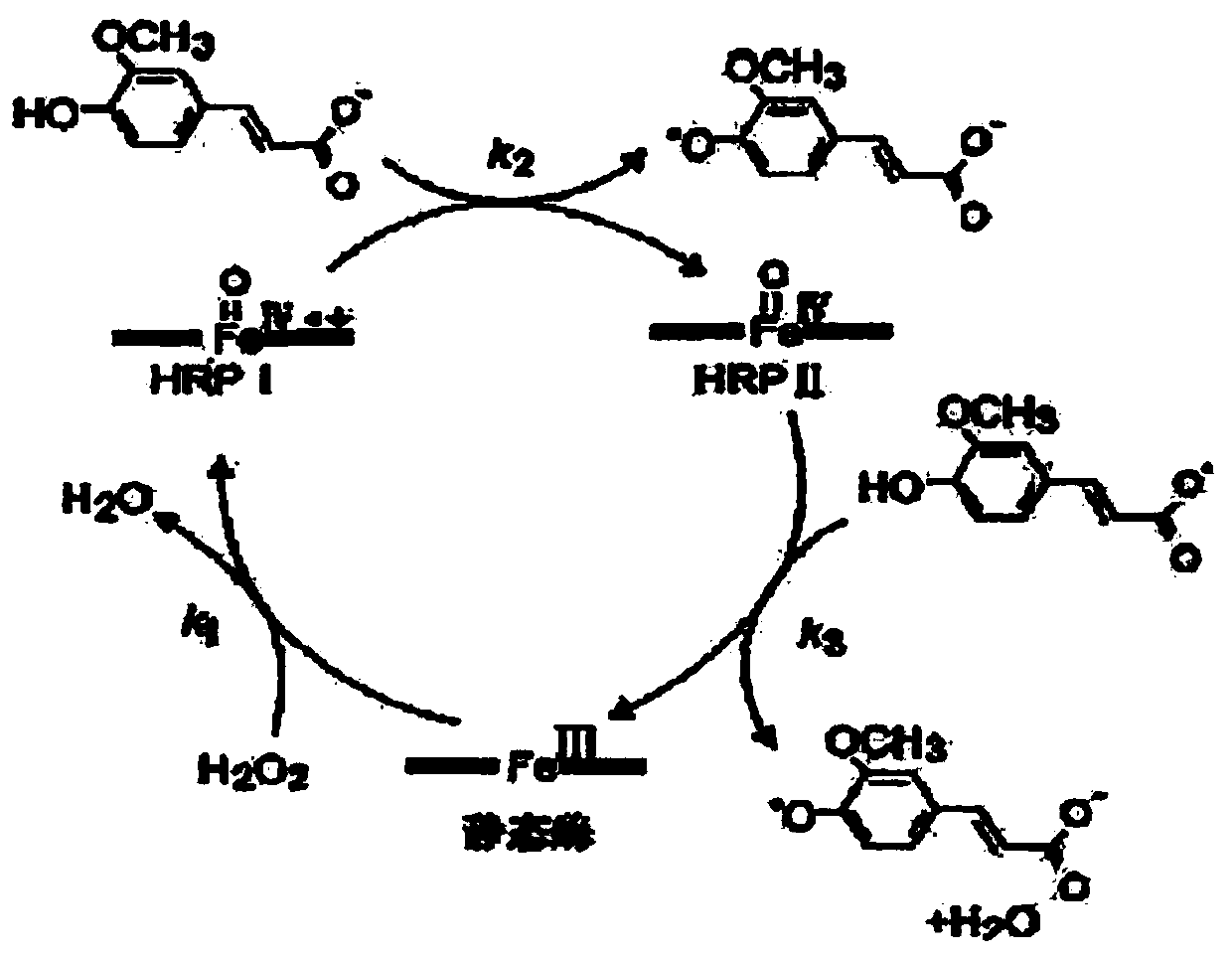
[0050] In this example, HRP (Sinopharm Group Chemical Reagent Co., Ltd.)-labeled anti-gastrin-17 antibody (stock solution of HRP-labeled antibody reagent) was used for the test. Carbon monoxide (CO) was passed through the HRP-labeled antibody reagent stock solution at a flow rate of 2-4 bubbles / second for 1 hour to treat the heme in the HRP. Then, the treated stock solution of the HRP-labeled antibody reagent was added to the protein diluent formula 1 shown in Table 1 at a ratio of 1:2000 (volume ratio) to prepare the HRP-labeled antibody dilution solution.
[0051] Table 1: Protein Diluent Recipe 1
[0052] components Dosage per liter Na 2 HPO 4 12H 2 o
5.2g NaH 2 PO 4 2H 2 o
0.62g Sodium chloride 8.5g casein 1g BSA 20g Proclin-300 0.5mL purified water 1000 pH 7.4±0.2
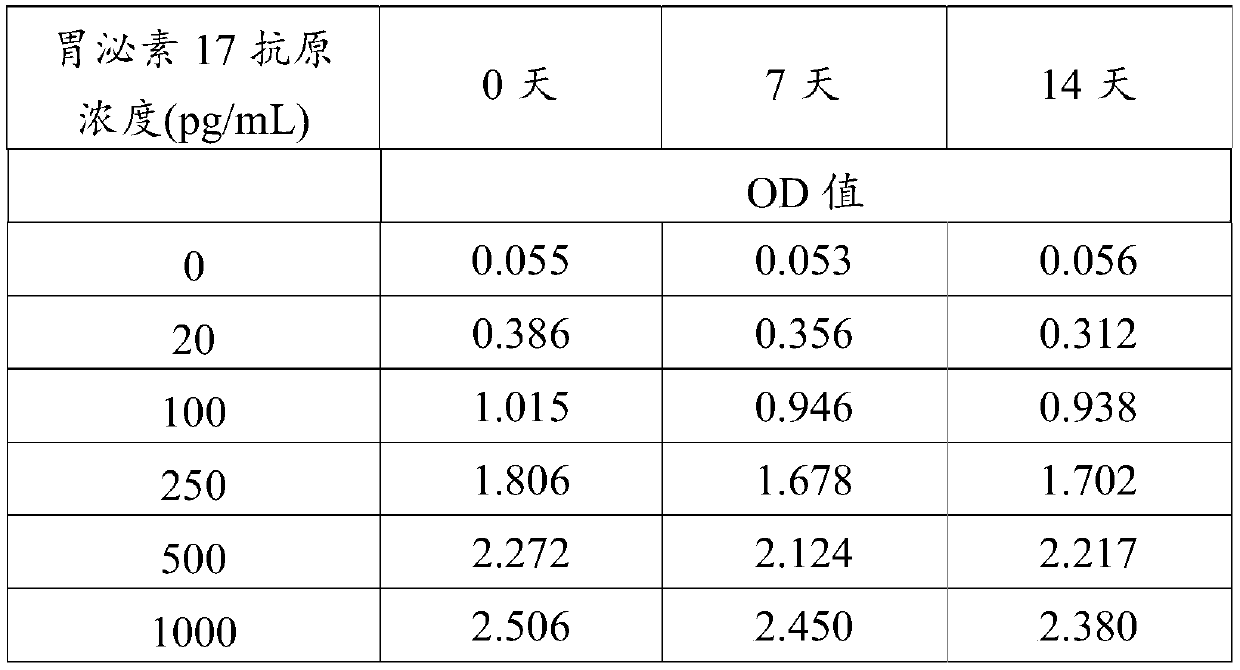
[0053] The prepared HRP-labeled antibody dilution solution was used for accelerated stability test at 37°C, on day 0, day 7 and ...
Embodiment 2
[0073] This example is the same as Example 1, including carbon monoxide (CO) treatment of the stock solution of the HRP-labeled antibody reagent, the difference is that the protein diluent is formulated as the HRP-labeled antibody dilution solution using the protein diluent formula 2 shown in Table 4.
[0074] Table 4: Protein Diluent Recipe 2
[0075]
[0076]
[0077] In the same manner as in Example 1, the prepared HRP-labeled antibody dilution solution was subjected to a 37°C accelerated stability test, and the results are shown in Table 5.
[0078] Table 5: 37°C accelerated stability test results of Example 2
[0079]
[0080]It can be seen from Table 5 that the stock solution of the HRP-labeled antibody reagent in this example was treated with carbon monoxide (CO), and the prepared HRP-labeled antibody dilution solution had no obvious OD value until the 14th day in the accelerated stability test at 37°C. decreased, indicating that carbon monoxide (CO) treatment...
Embodiment 3
[0082] In this example, the stock solution of the HRP-labeled antibody reagent was also treated with carbon monoxide (CO), and the protein diluent formula 2 in Example 2 was used to prepare the HRP-labeled antibody dilution solution, which was a test based on the accelerated stability of Example 2. Accelerated stability is used to quickly screen the best formula, and long-term stability is the final confirmation of the formula, and each performance index can be confirmed more specifically. The long-term stability test is carried out under the environment of 2-8 ℃, and the accuracy (recovery rate), minimum detection limit, precision and linear relationship of the test are investigated.
[0083] 1. Accuracy
[0084] Select 3mL of low-concentration test sample and divide it into 3 parts, each 1mL. Add 0.1mL high-value standard solution (400pg / mL, 800pg / mL) of different concentrations of the analyte to two of the samples, so that the final concentration is between 50-1000pg / mL, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com