Pepsinogen I and pepsinogen II combined detection kit and preparation method and detection method thereof
A pepsinogen and combined detection technology, applied in the field of in vitro diagnostic reagents, can solve the problems of large error in detection results, time-consuming and laborious, complicated operation steps and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] The preparation steps of the enzyme-linked plate coated with anti-PGⅠ and anti-PGⅡ monoclonal antibodies are as follows:
[0070] 1) Spotting: adjust PGⅠ monoclonal antibody and PGⅡ monoclonal antibody to 0.1mg / mL, 0.2mg / mL, 0.3mg / mL, 0.4mg / mL, 0.5mg / mL, 0.6mg / mL respectively with spotting buffer. mL, 0.7mg / mL, 0.8mg / mL, 0.9mg / mL, 1.0mg / mL, preferably 0.4mg / mL; through the GeSim Nano-PlotterTM chip sampling system, absorb PGⅠantibody and PGⅡantibody solutions and add them to the enzyme-linked plate In the same well, PGⅠ and PGⅡ are three replicate points each, and the sample volume of each point is 2nl, 4nl, 6nl, 8nl, 10nl, preferably 8nl, arranged in order according to the spot shape;
[0071] 2) Fixation: Cover the wells of the enzyme-linked plate with a cover film, place at 37°C for 3 hours, overnight at -20°C, and then place at -80°C for 2 hours for antibody fixation;
[0072] 3) Blocking: add blocking solution according to the amount of 200ul per well, block overn...
Embodiment 2
[0074] The preparation steps of the enzyme conjugate labeled with another anti-PGⅠ and another anti-PGⅡ monoclonal antibody are as follows (the PGⅠ antibody and the PGⅡ antibody are labeled separately):
[0075] 1) Weigh 0.4 mg of horseradish peroxidase (HRP) and dissolve in 80 μL of 0.2 M, pH 5.6 acetate buffer to an enzyme concentration of 5 mg / mL;
[0076] 2) Add 22.4 μL of 0.1M sodium periodate, at this time the solution changes from original brown to dark green, and react in the dark at 4°C for 25 minutes;
[0077] 3) Add 16 μL of 2.5% ethylene glycol solution, vortex and mix well, and stop the reaction at 4°C in the dark for 1 hour;
[0078] 4) Take the corresponding antibody, add the antibody solution according to the mass ratio of HRP enzyme and antibody at 1:1, 2:1, 3:1, preferably 2:1, buffer with 0.2M carbonate pH9.5 Adjust the pH value of the solution to 9.0-9.5, and then dialyze through 0.01M carbonate buffer at 4°C in the dark overnight;
[0079] 5) Add 24 μL o...
Embodiment 3
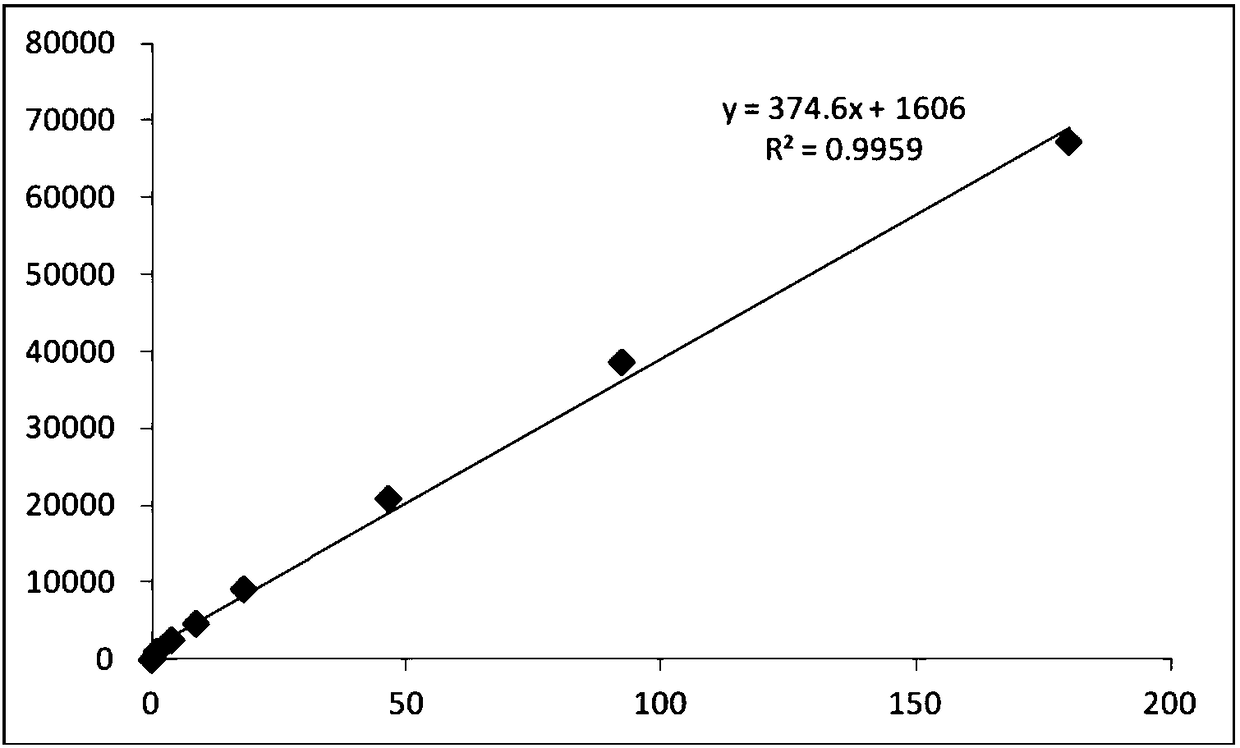
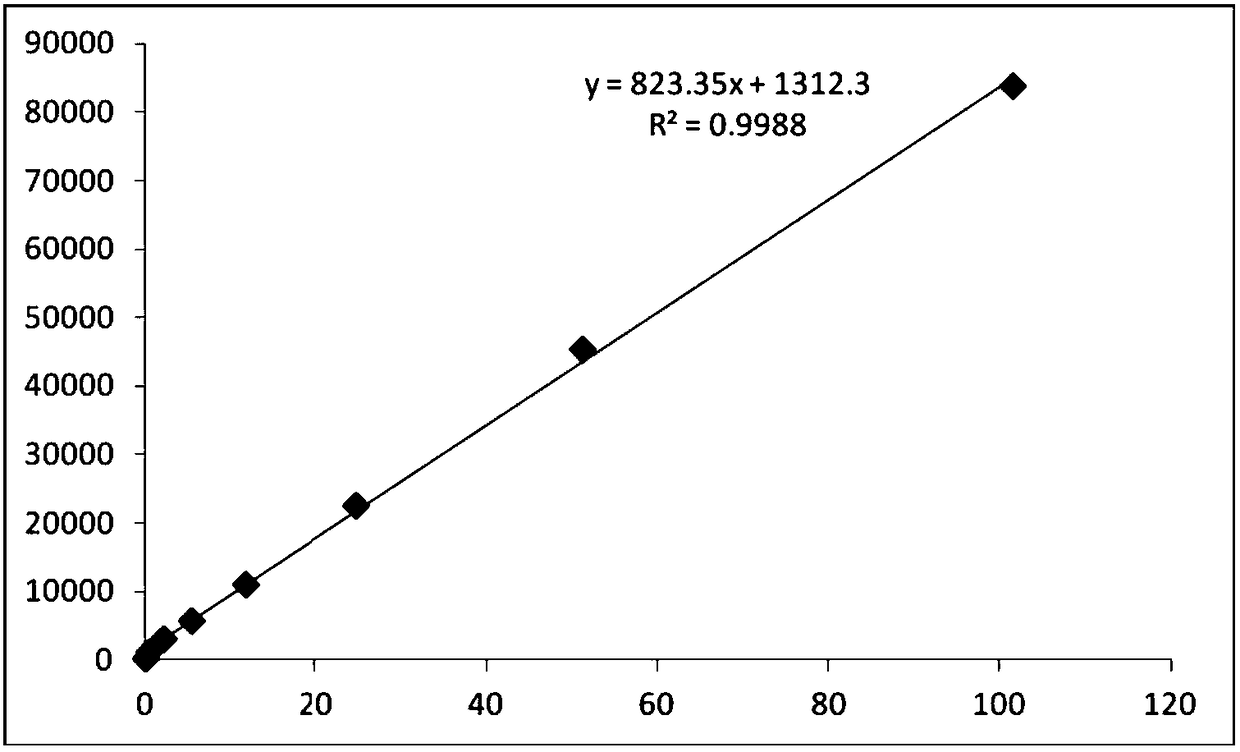
[0086] The preparation steps of PGⅠ and PGⅡ calibrator dilutions are as follows:
[0087] 1) Weigh 8 g of disodium hydrogen phosphate, 0.27 g of sodium dihydrogen phosphate, 8 g of sodium chloride, and 0.2 g of potassium chloride in a 1L container, add an appropriate amount of purified water and stir to dissolve completely;
[0088] 2) Use a pipette to pipette 1mL ProClin 300 into a beaker of 10mL purified water, dissolve it completely and pour it into the above 1L container, add purified water to 900mL and stir thoroughly;
[0089] 3) Adjust the pH meter to measure the pH value of the solution, and adjust the pH value to be controlled at 7.2-7.4;
[0090] 4) Weigh 30g of bovine serum albumin and 1g of protein protectant GH, add them to the above 1L container, stir evenly, and dissolve them fully;
[0091] 5) Dilute to 1L, filter with a 0.2μm filter, label and store under a sterile environment at 2-8°C after filtration.
[0092] Table 2 Calibrator diluent formula
[0093] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com