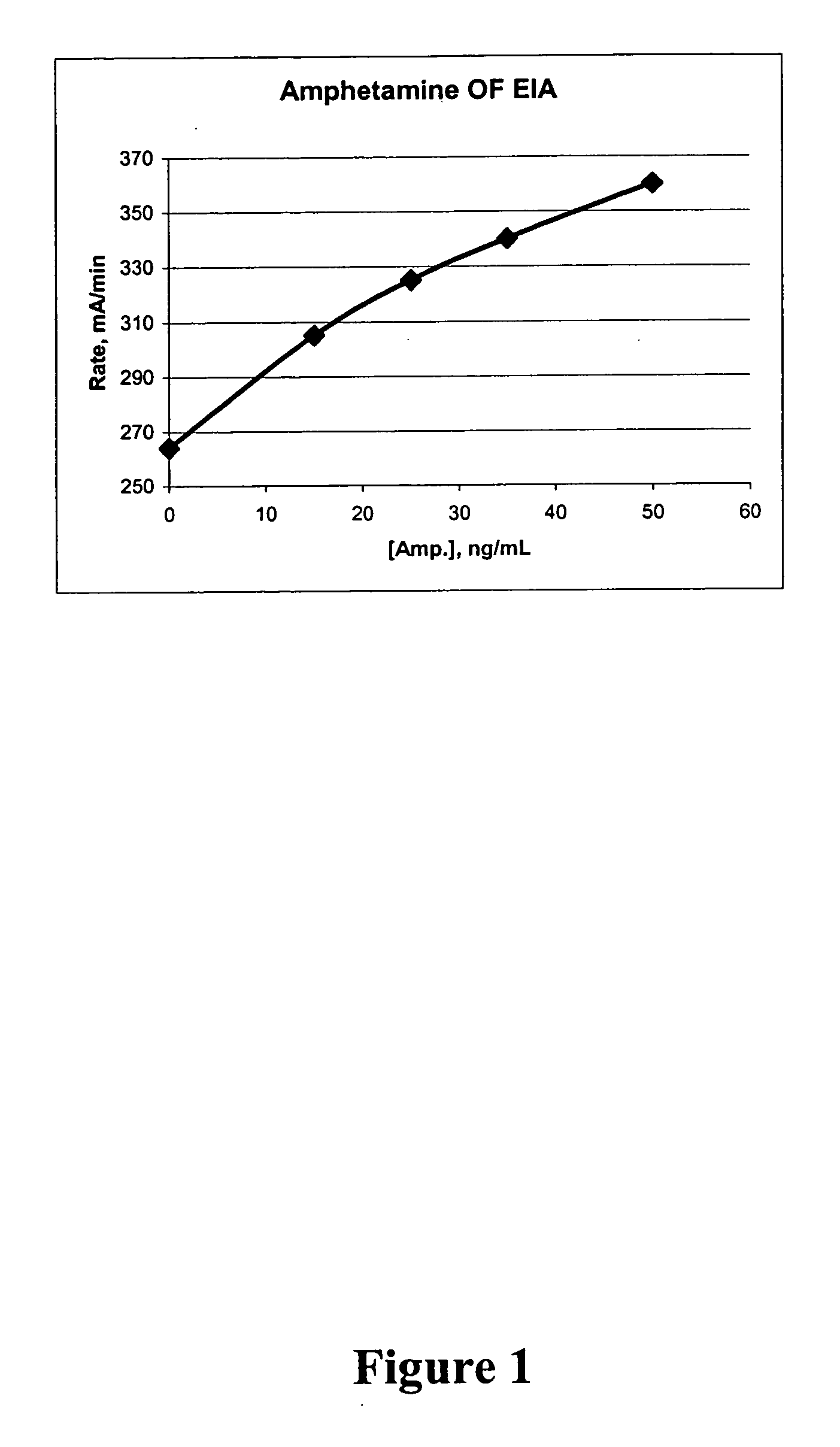
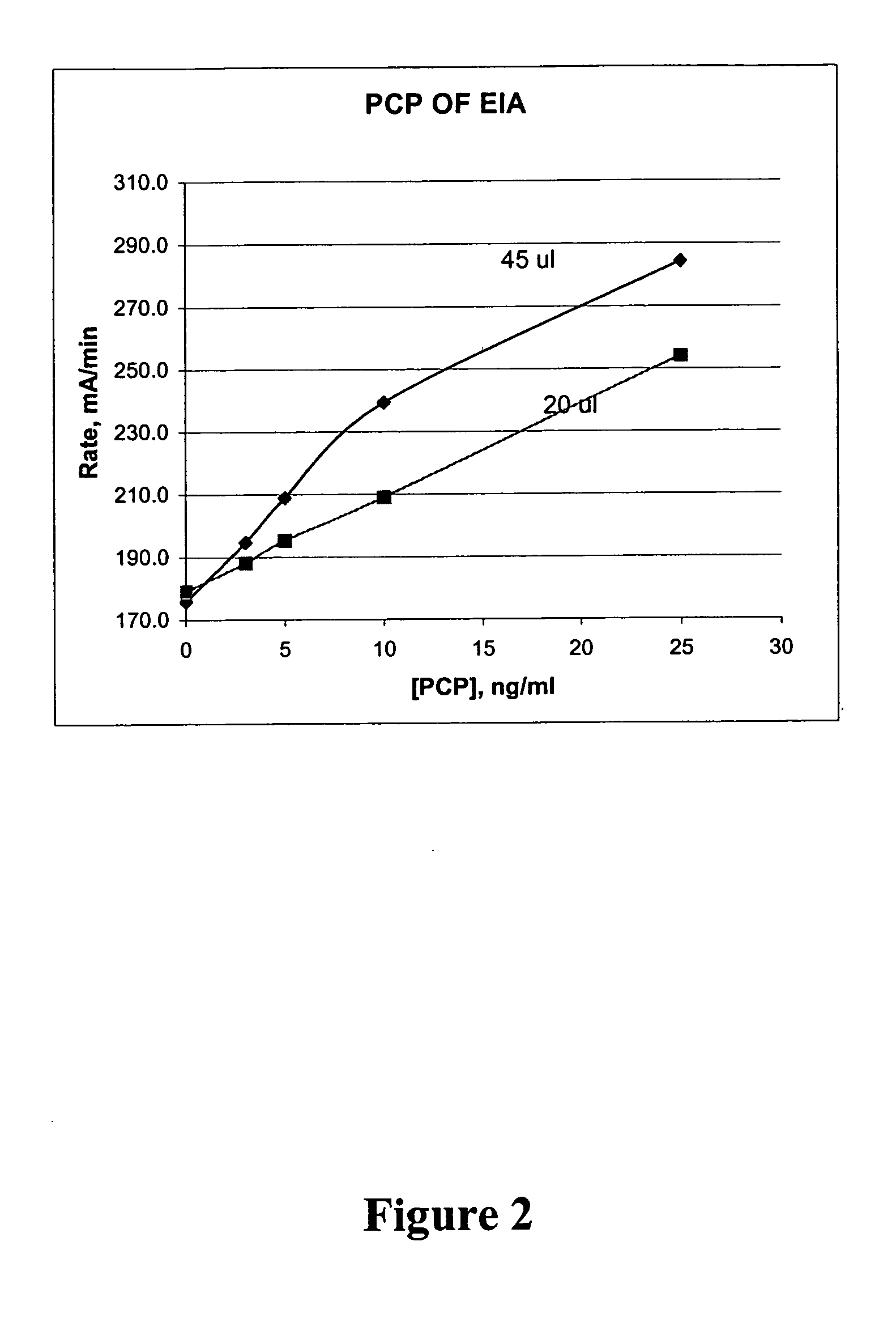
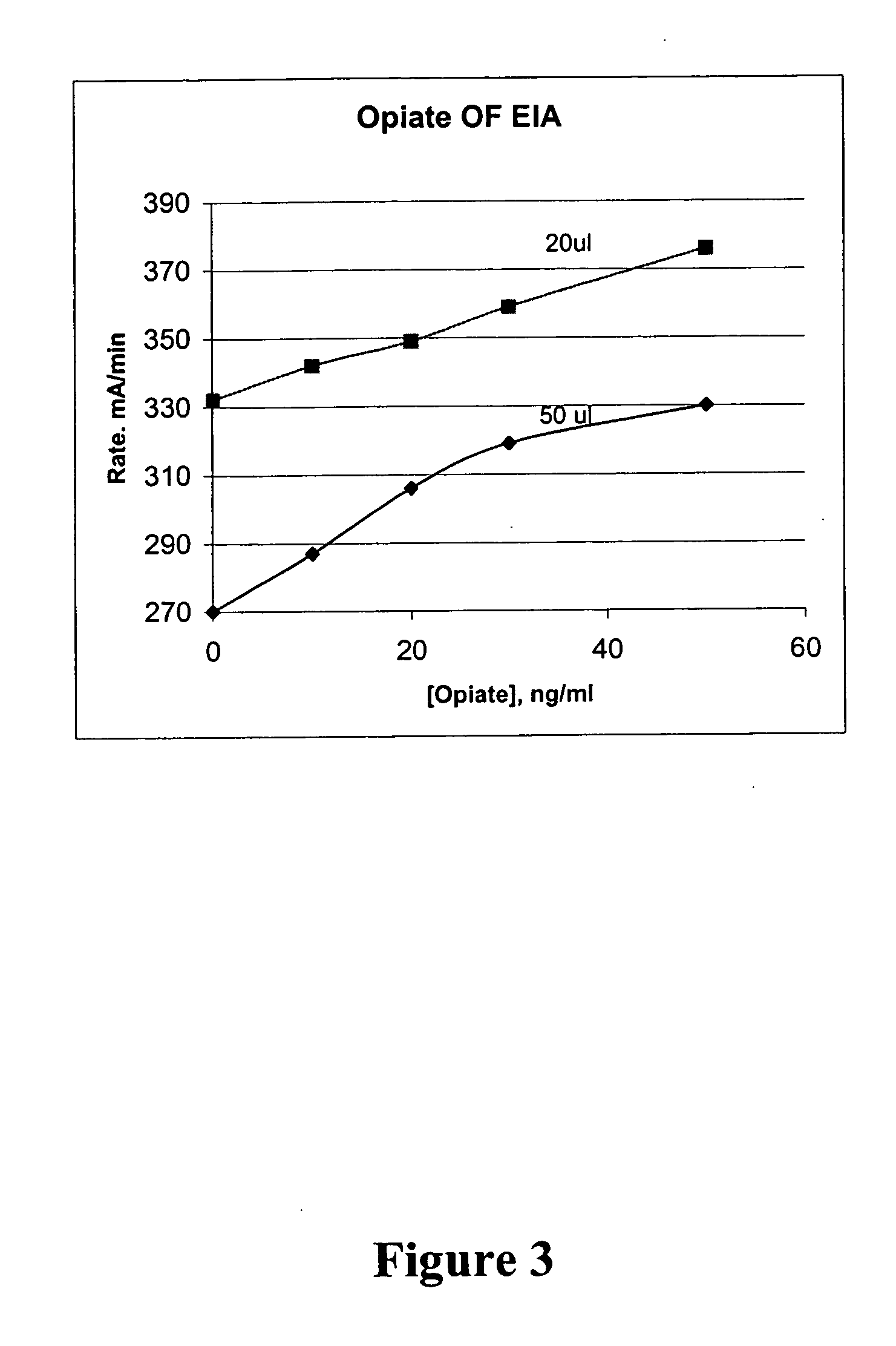
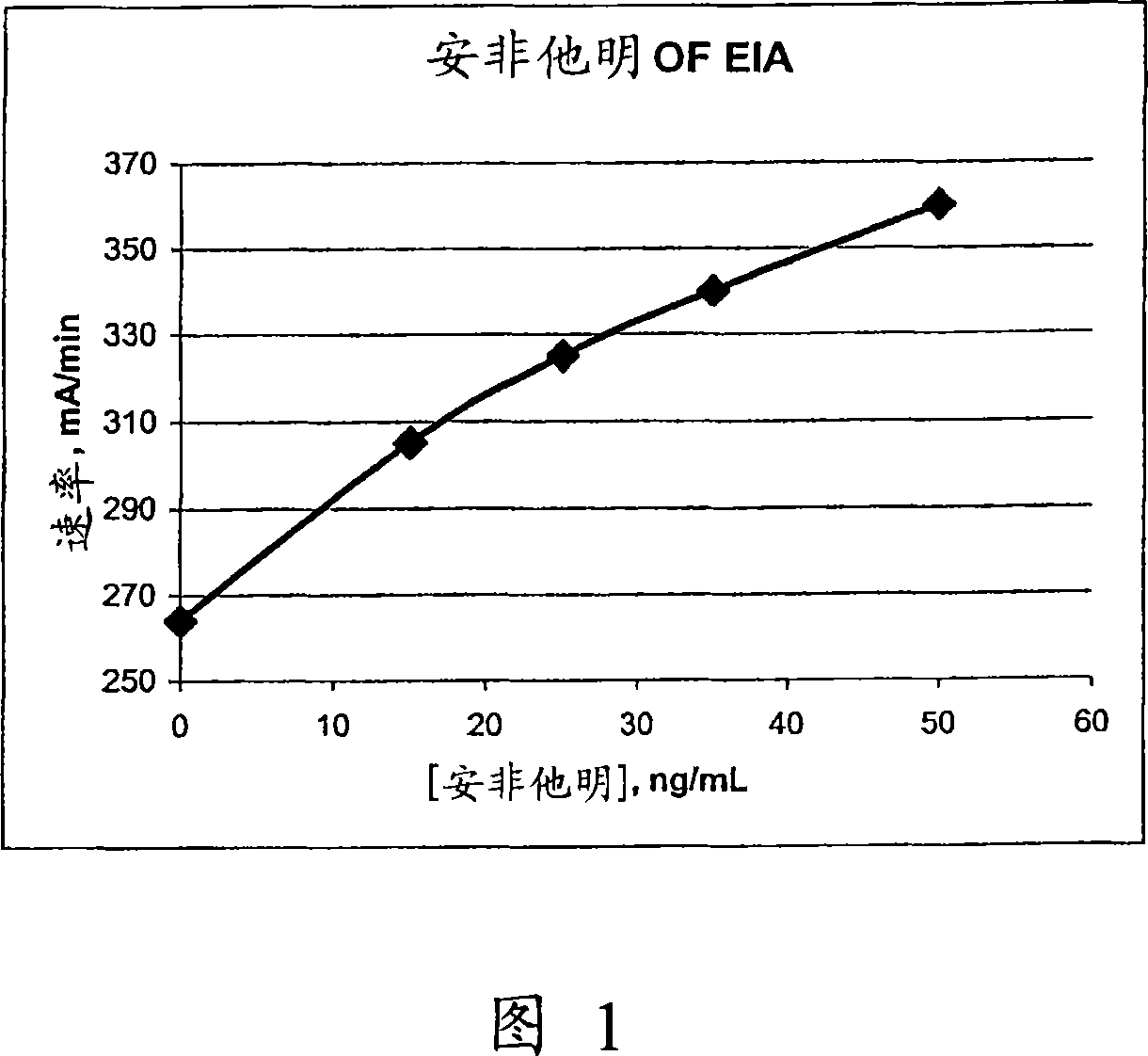
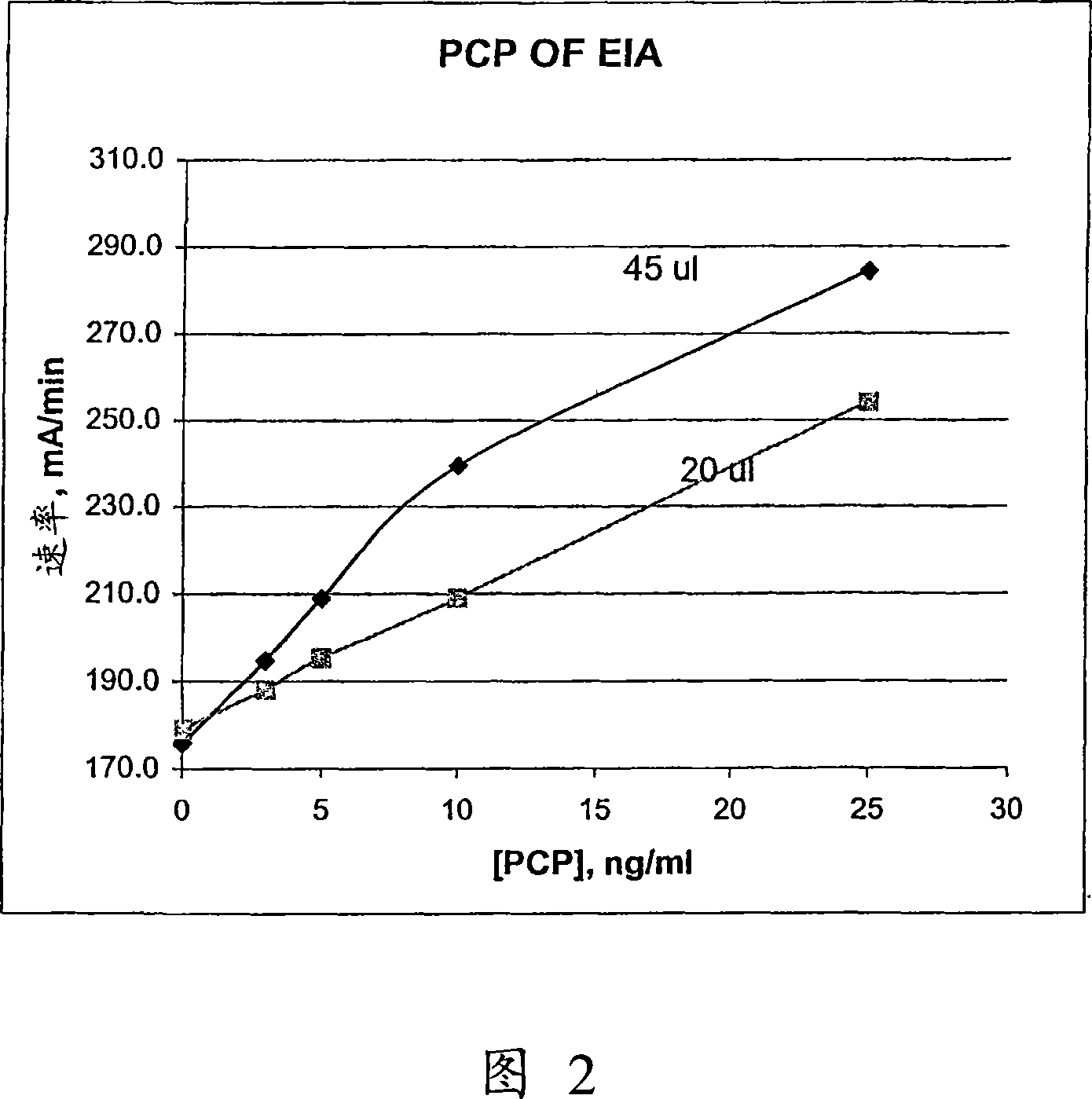
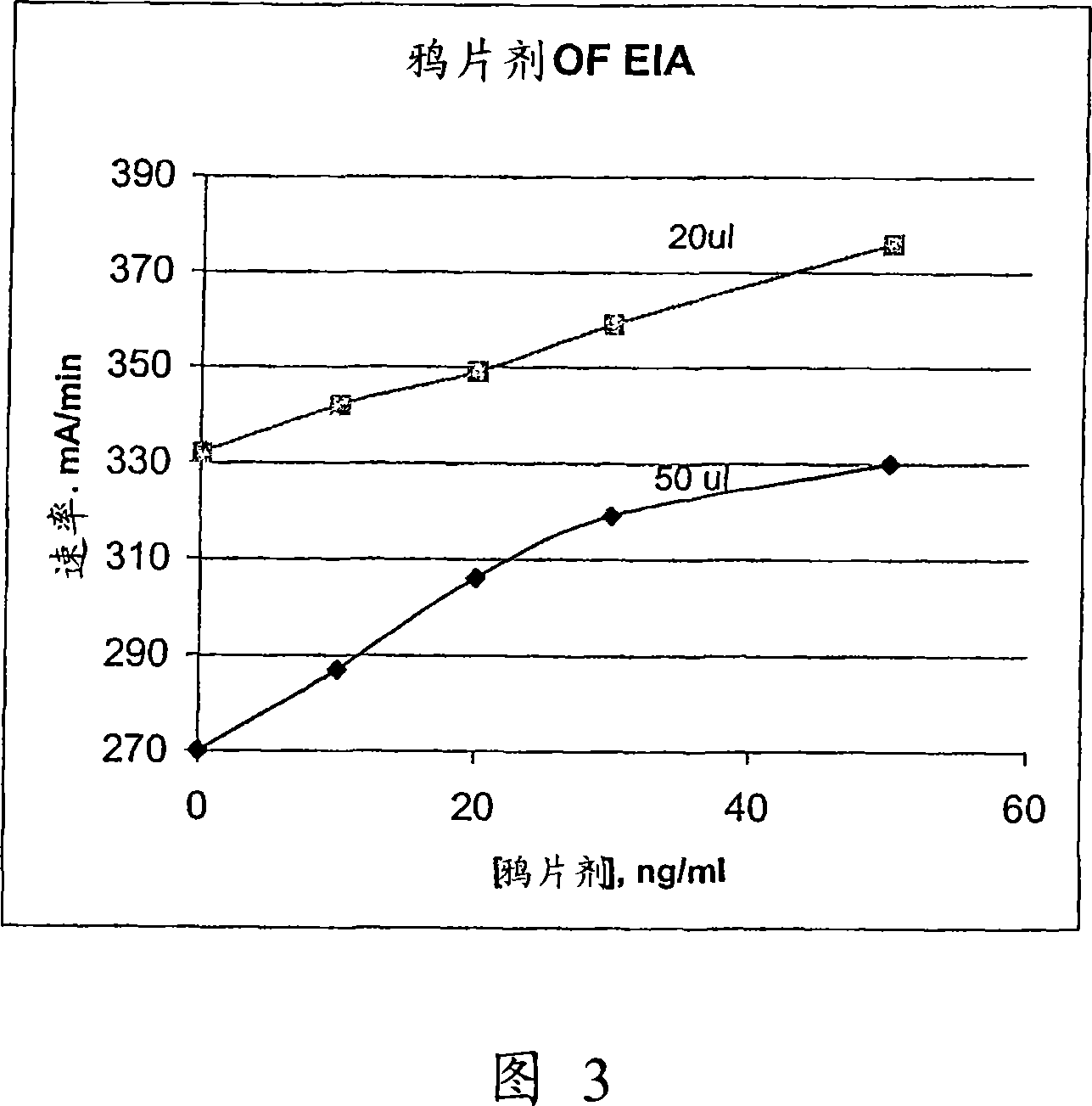
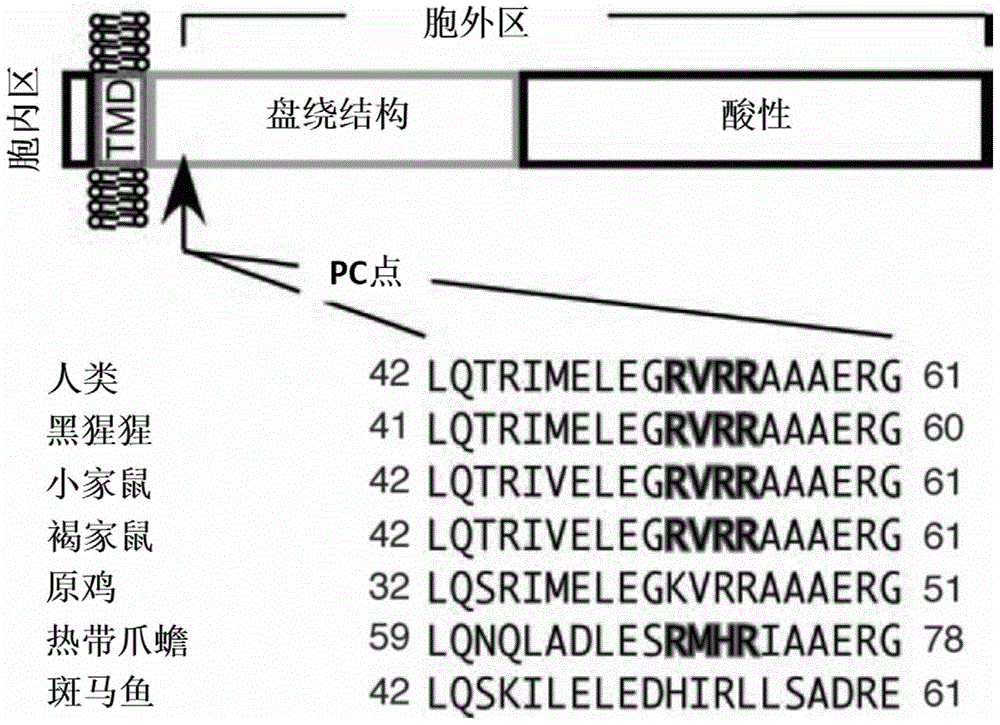
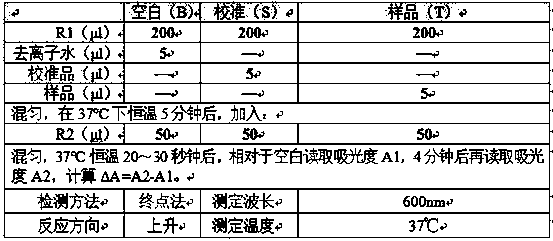
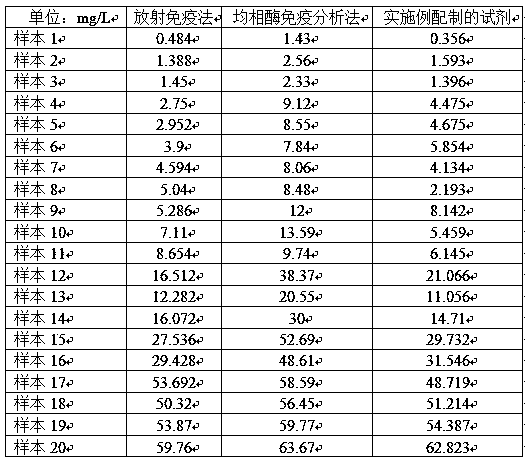
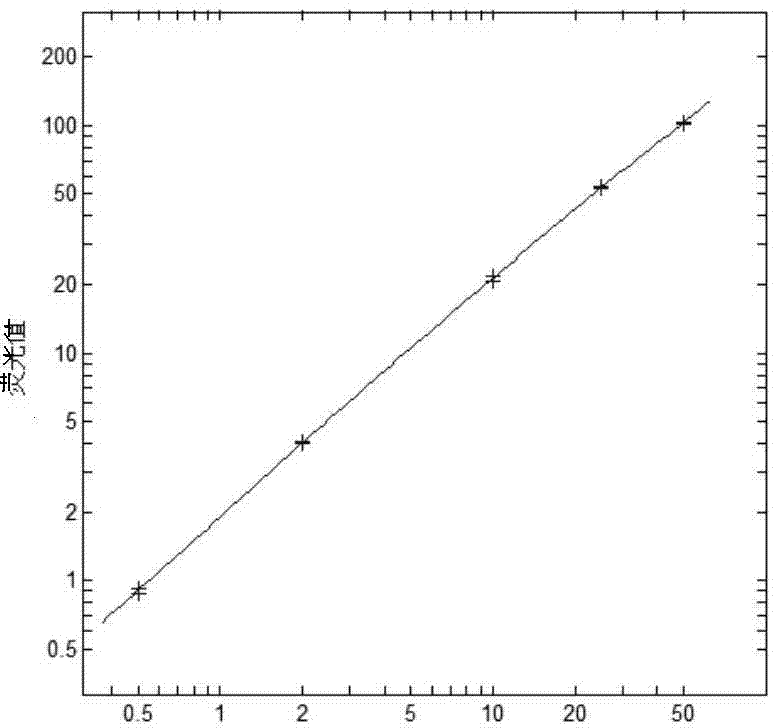
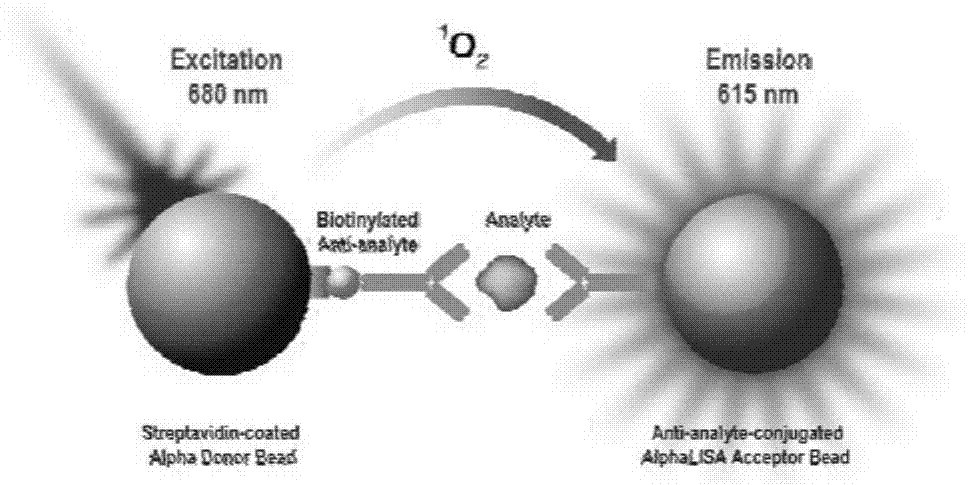
Patents
Literature
256 results about "Enzyme immunoassays" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
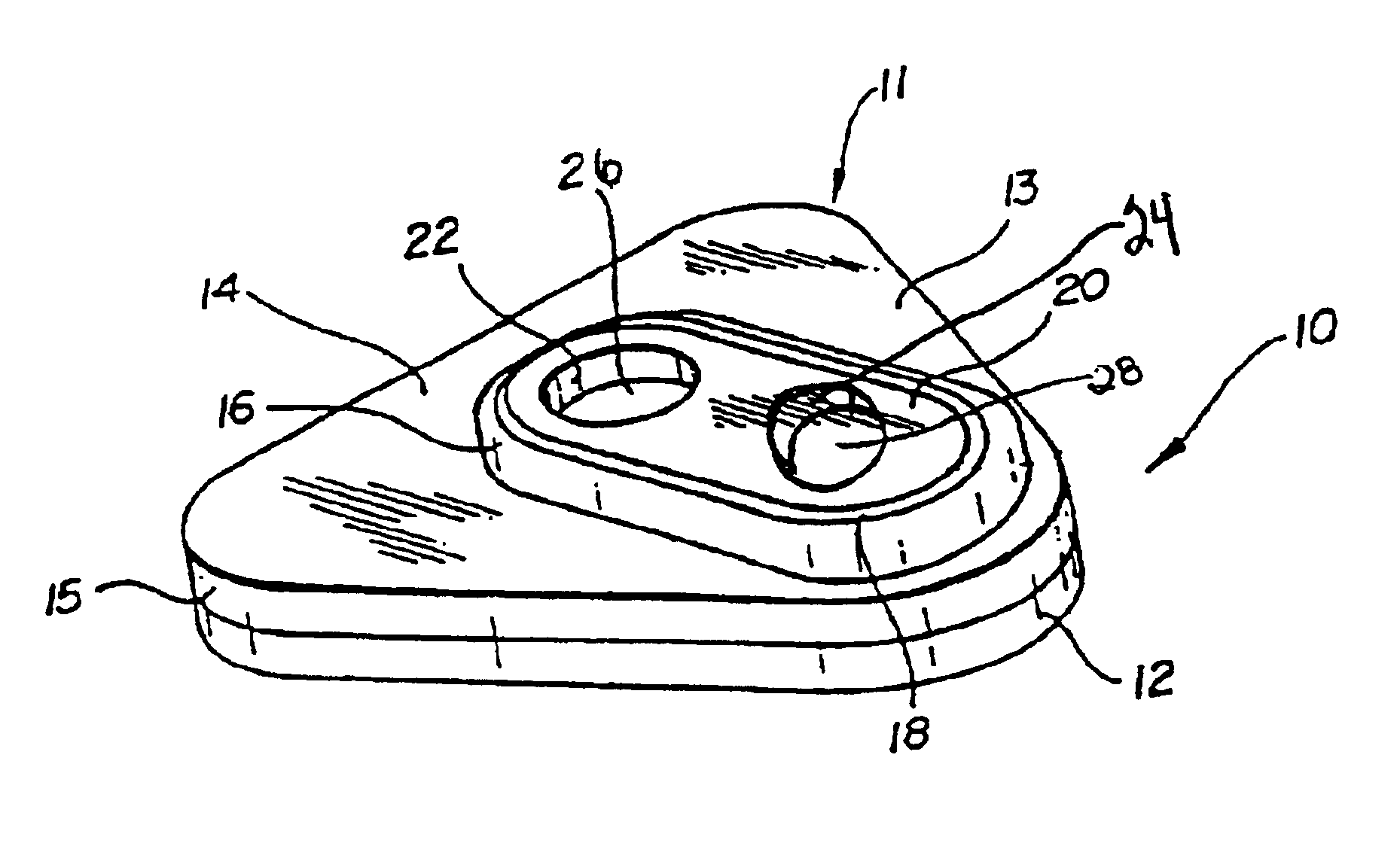
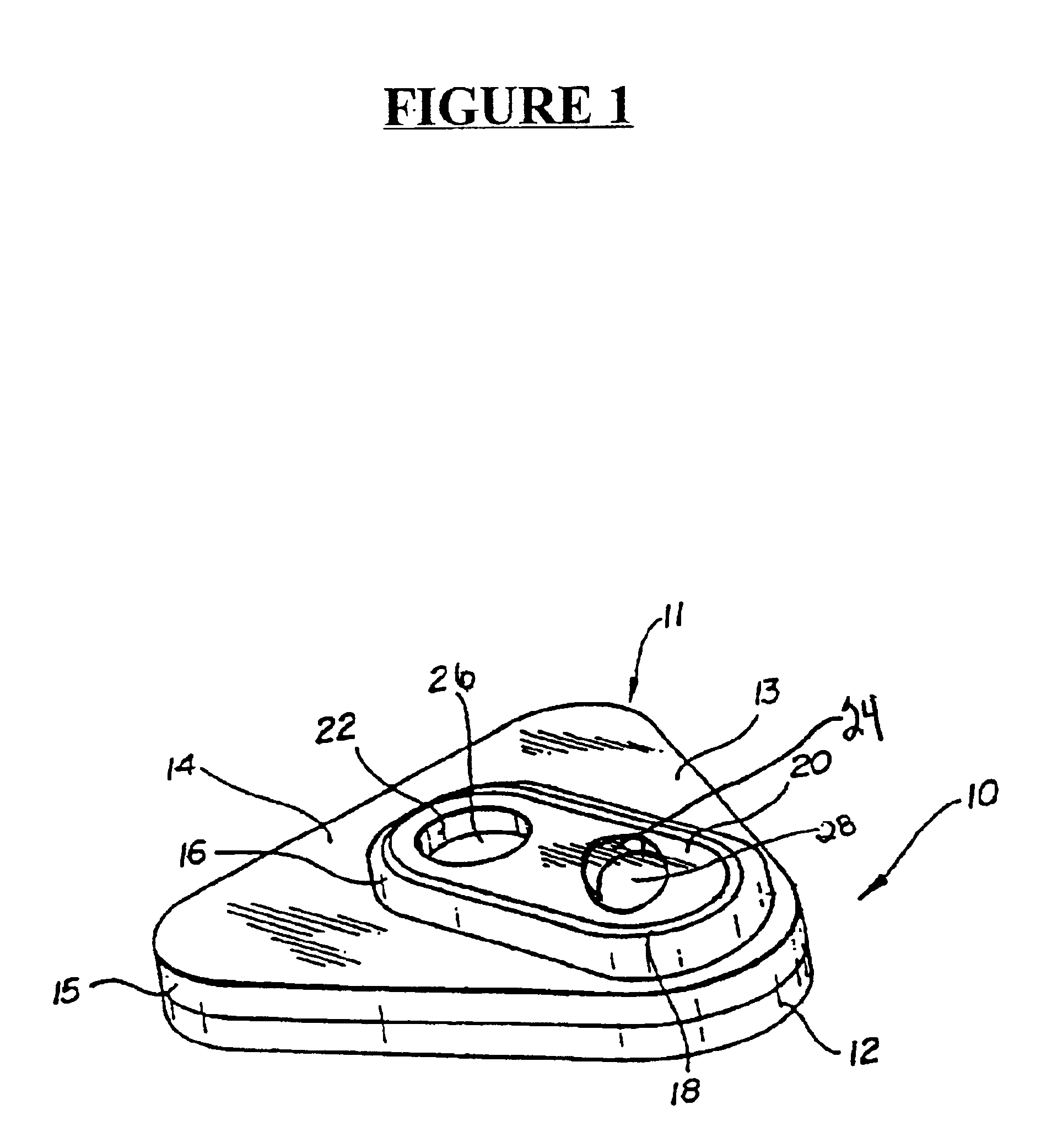
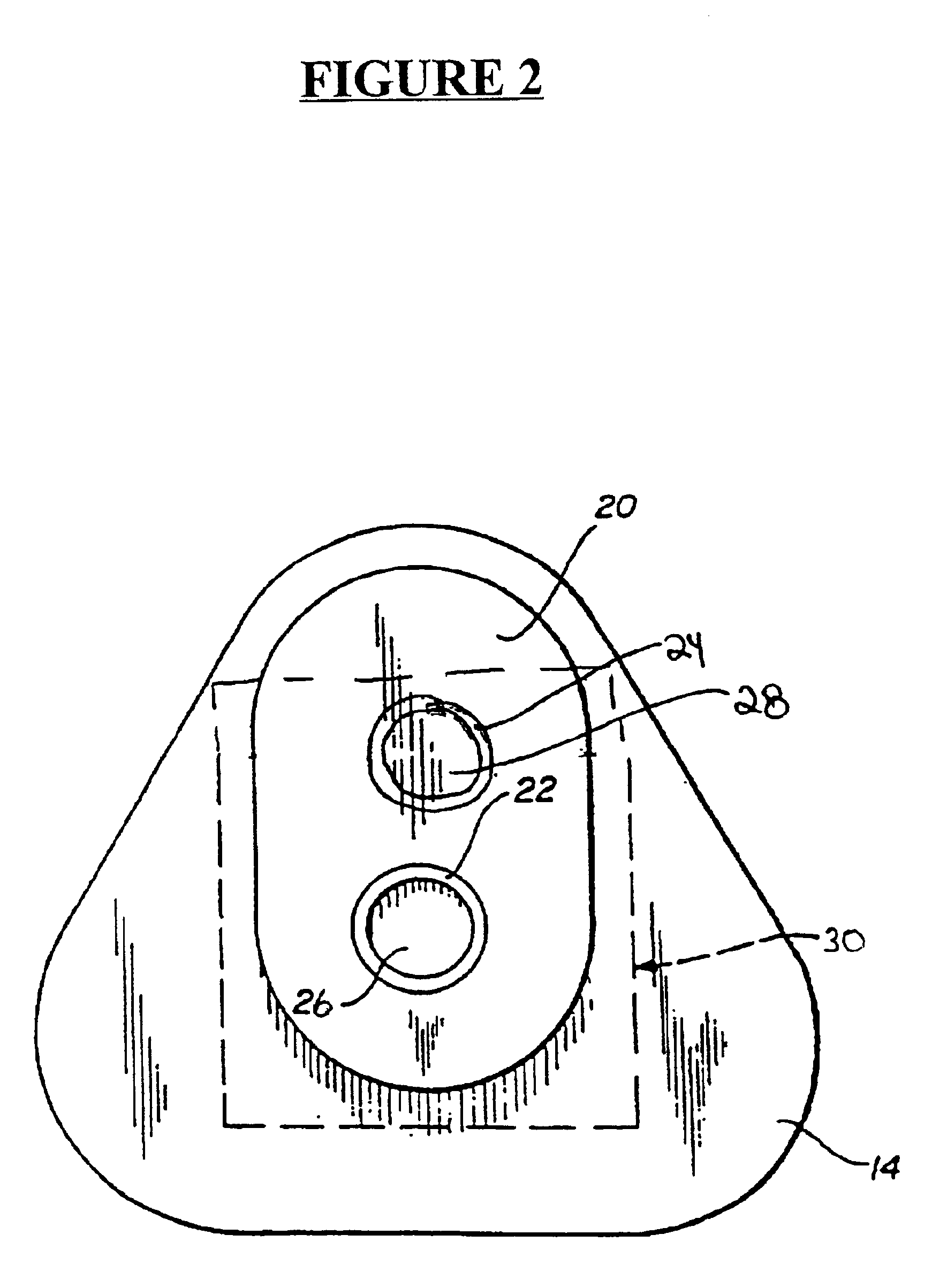
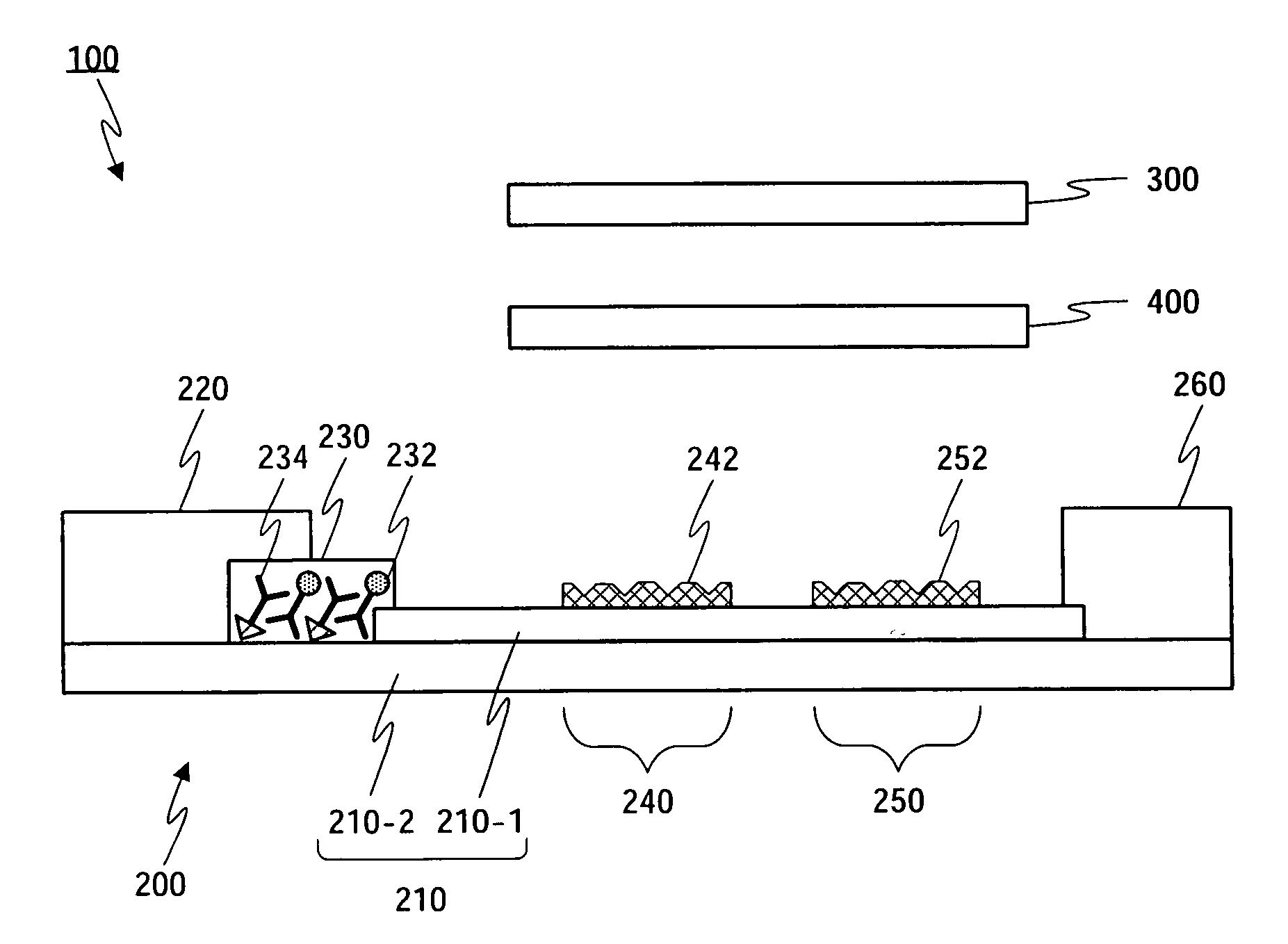
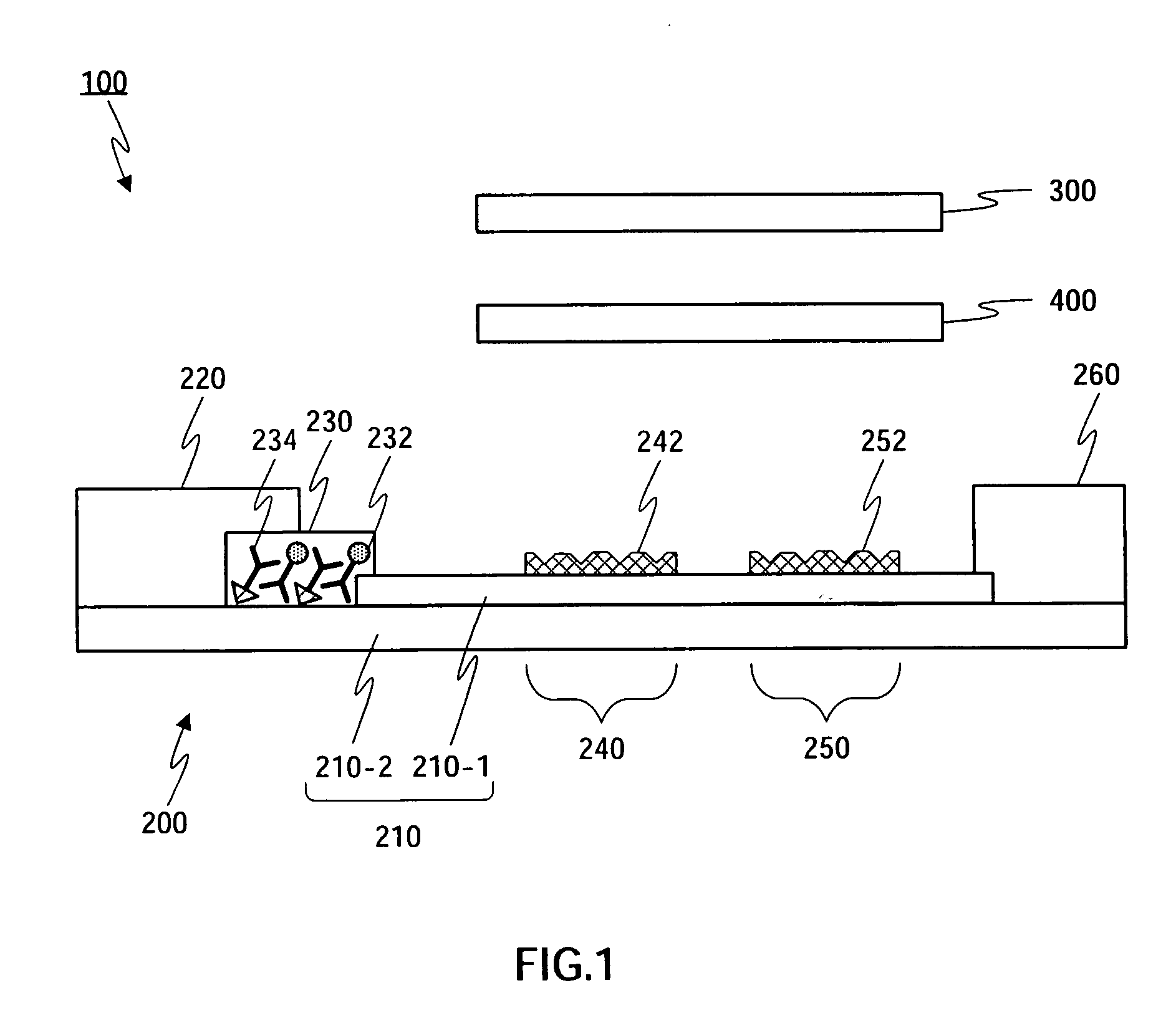
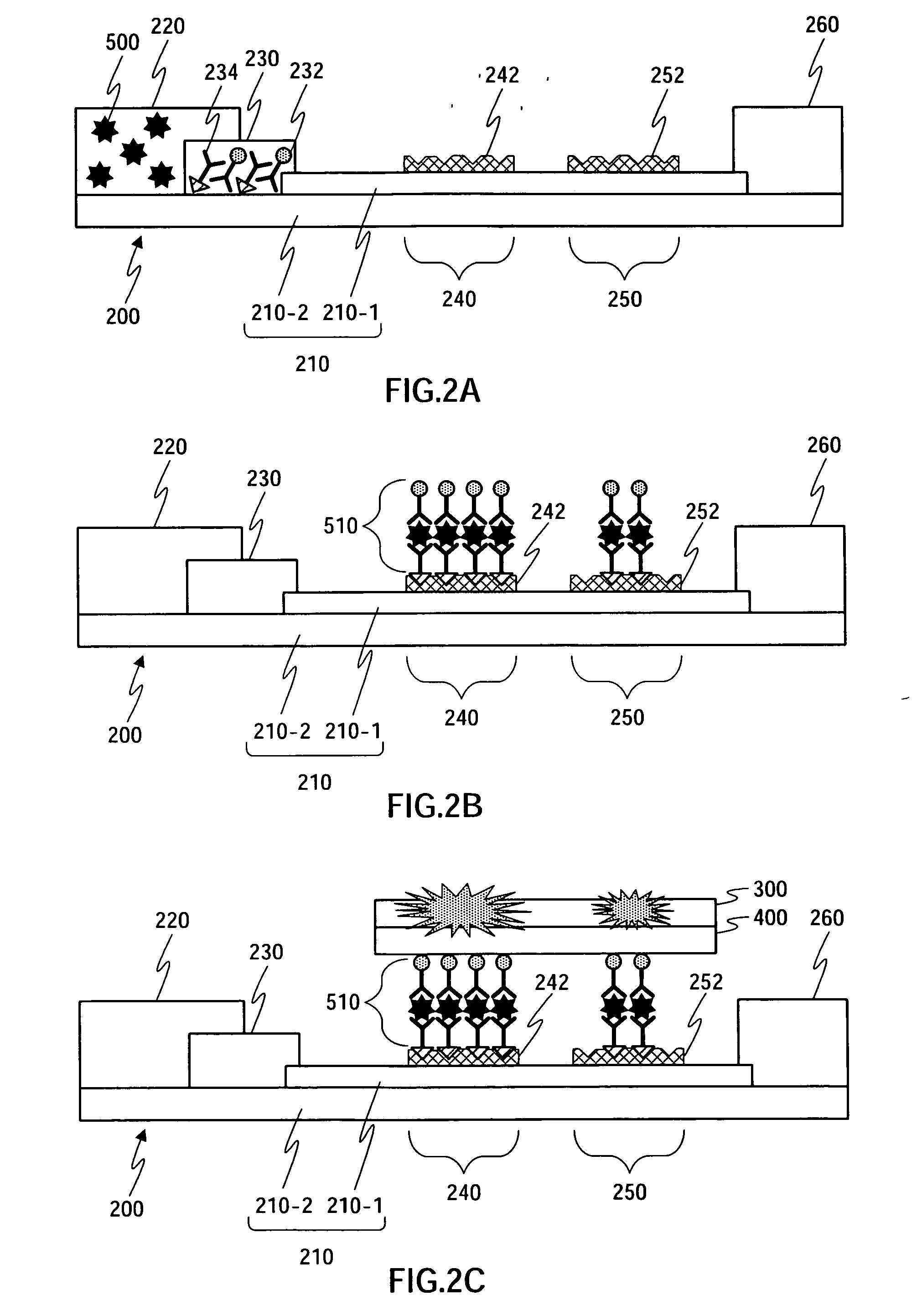
Detection of multiple analytes from a single sample using a multi-well, multi-analyte flow-through diagnostic test device
InactiveUS7052831B2Bioreactor/fermenter combinationsBiological substance pretreatmentsSingle sampleDiagnostic test
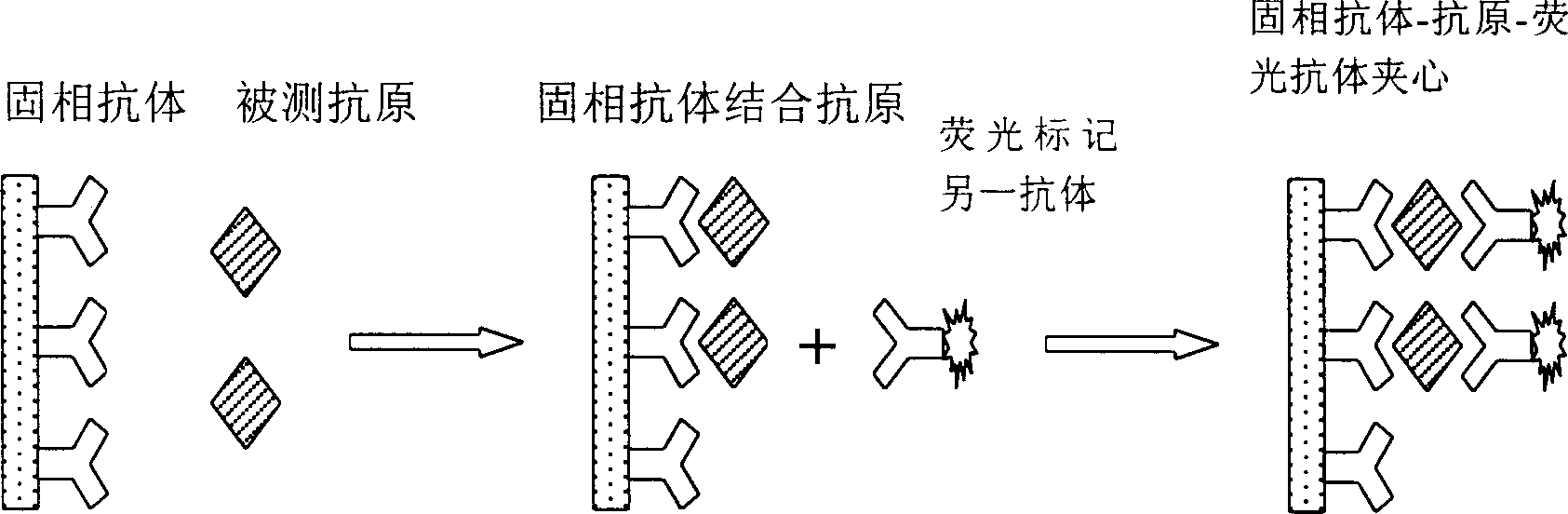
The present invention provides a method and device for conducting a rapid in vitro enzyme immunoassay test for the direct and qualitative detection of two or more viral antigens from specimens of symptomatic patients. The method for immunoassay of viral antigens is performed on a membrane. Non-immunological capture of viral antigens takes place by absorption onto the membrane. Captured antigen binds to a detection reagent that includes a label conjugated to a specific antibody. The test is a differentiated test such that two or more viral antigens may be distinguished from each other in a single test. The invention also includes a kit for performing an assay in accordance with the method of the present invention, wherein the kit comprises the device of the present invention.
Owner:BECTON DICKINSON & CO
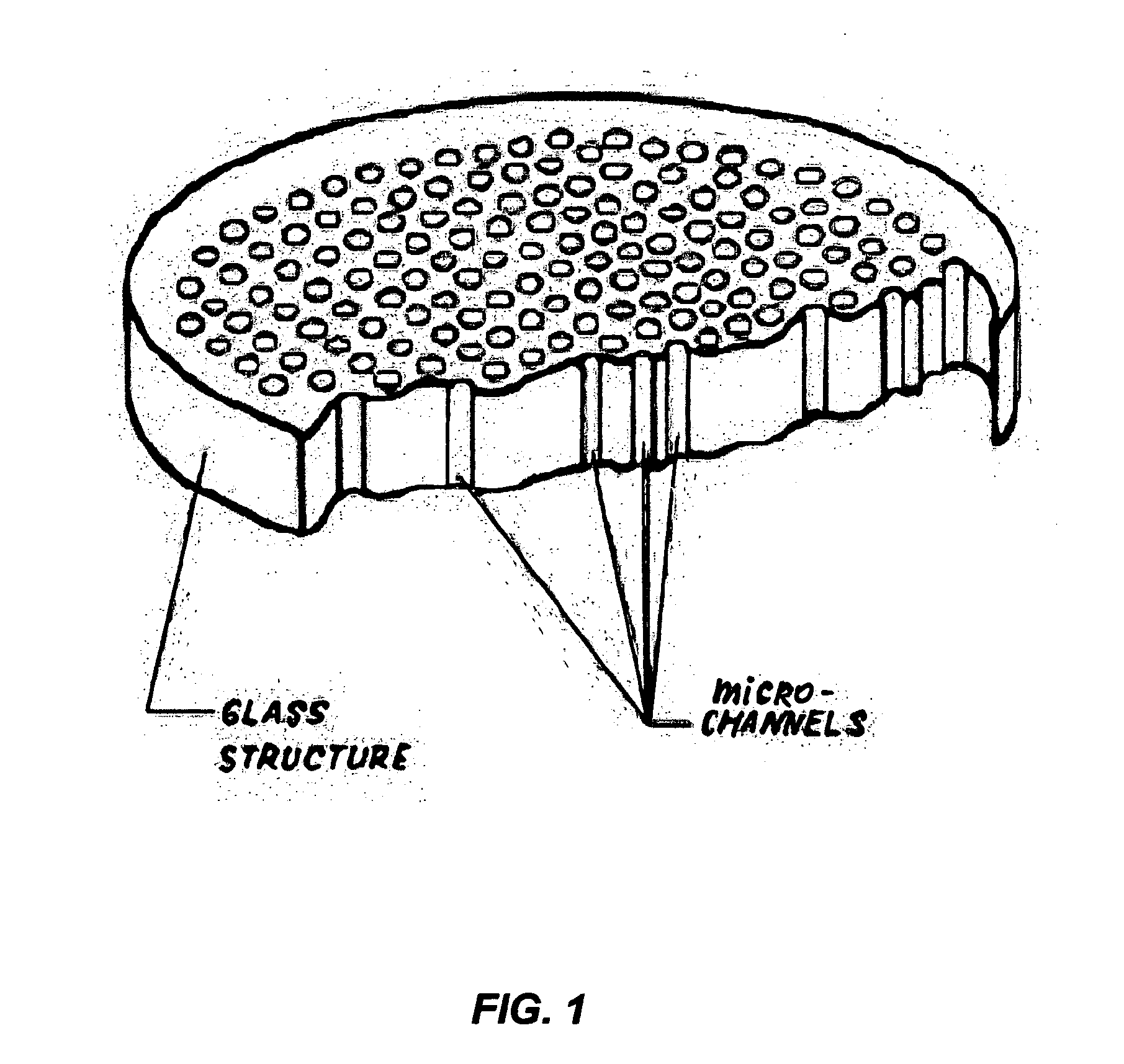
Device for rapid detection and identification of single microorganisms without preliminary growth
InactiveUS20050221403A1Rapid and reliable detectionRapid and reliable and identificationBioreactor/fermenter combinationsBiological substance pretreatmentsMicroorganismFiltration
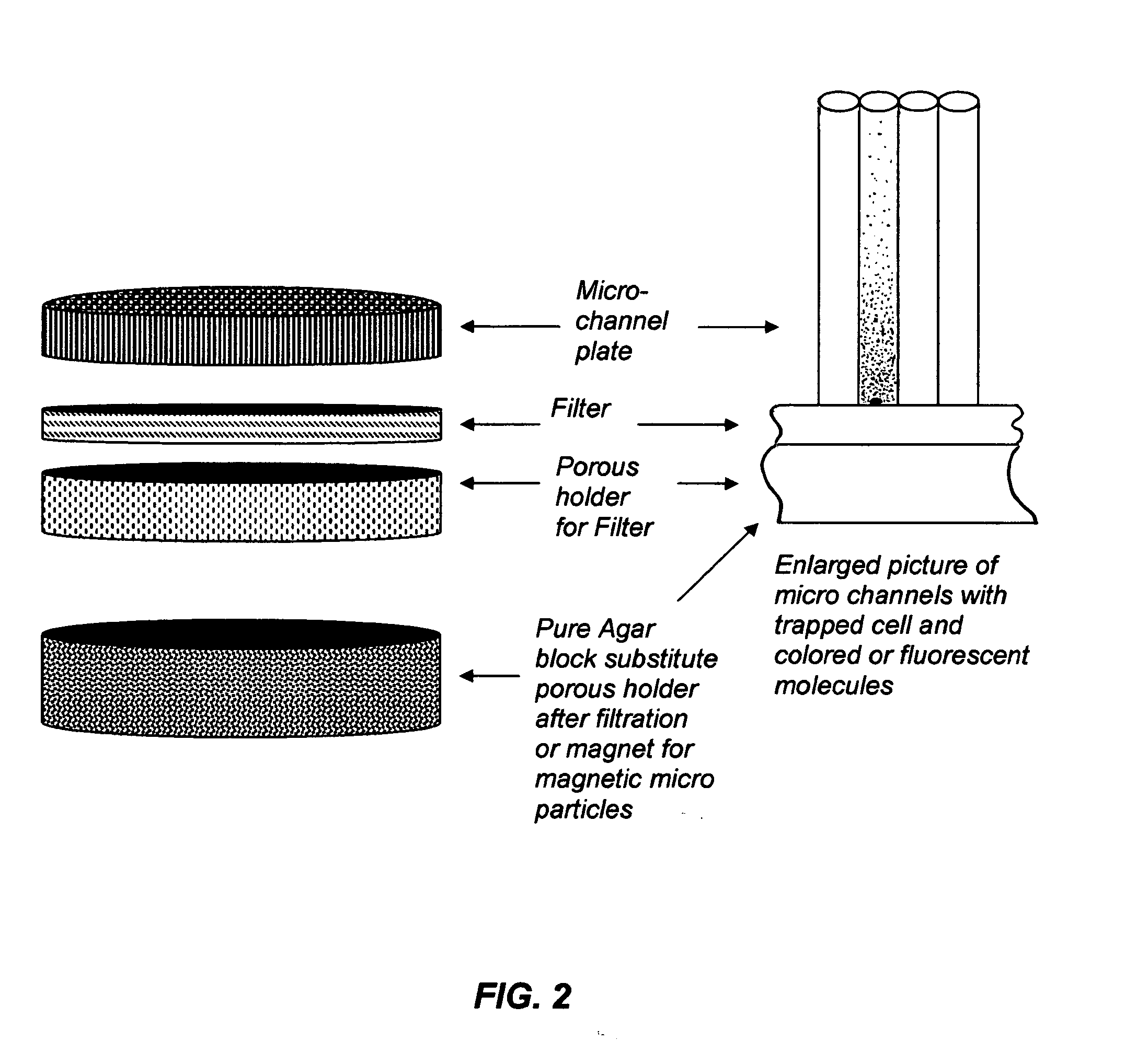
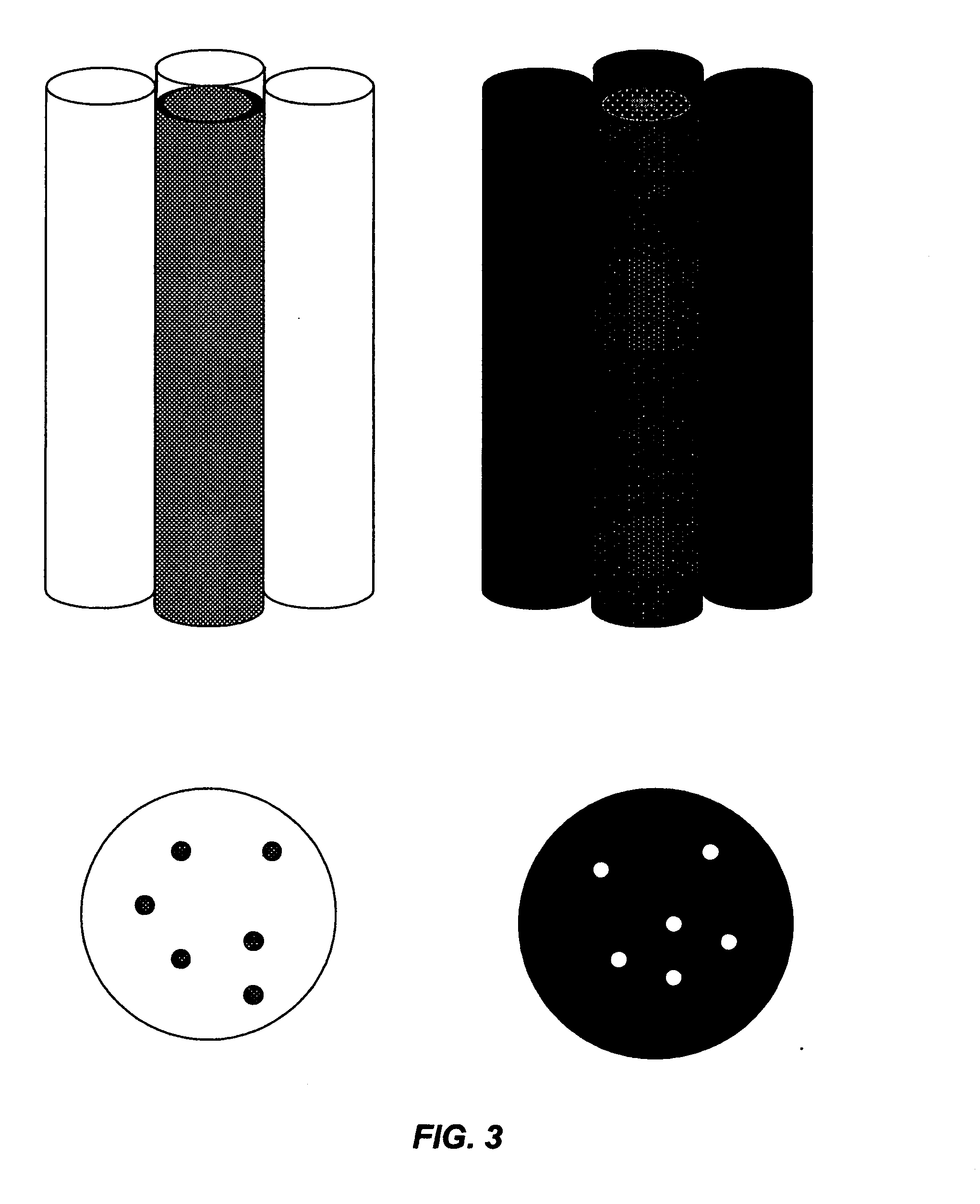
This invention describes a device consisting of a micro channel plate, filter, and porous holder for filter, which is substituted by a pure agar block during method performance, and supportive structural elements. The device is intended for rapid detection and / or identification of microorganisms. Microorganisms are trapped by filtration in long (diameter / length=1 / 10-1 / 100), cylindrical, parallel, micro-channels that are open from both sides and attached to a filter from one side. A micro channel plate houses a multiplicity of micro channels (possible diameter of each channel=1-30 μm, length 100-1000 μm, and number on centimeter2−100,000-1,000,000). The micro channel plate with cells trapped on the surface of the filter is attached to an agar block impregnated by artificial substrate(s) so that the molecules of the artificial substrates will fill all micro channels. Trapped cells produce colored or fluorescent molecules from artificial substrates. These molecules are collected in the very small volume of a micro channel. The extremely small volume of a micro channel (1 / 25 million part of milliliter) allows it to collect a detectable concentration of color or fluorescent substances in a very short time (several minutes). Even one cell from a filtrated sample can be detected by the enzyme—artificial substrate method and / or identified by enzyme immunoassay.
Owner:NANOLOGIX INC
Magnetic microparticle chemiluminescence enzyme immune analytic reagent kit for detecting saccharide antigen and its use method
InactiveCN101324579AQuantitative detection of carbohydrate antigen contentLow pre-processing requirementsMaterial analysisCarbohydrate antigenMicroparticle
The invention relates to a magnetic corpuscule chemiluminescent enzyme immunoassay kit for detecting carbohydrate antigen and the application method thereof. The kit comprises FITC antibody-coated magnetic corpuscules; a marker solution prepared by mixing the FITC-marked carbohydrate antigen monoclonal antibody and the enzyme-marked carbohydrate antigen monoclonal antibody; a carbohydrate antigen standard sample solution; a concentrated washing solution; and a luminescent substrate solution, wherein carbohydrate antigen optionally adopts one of CA72-4, CA50, CA19-9, CA242, CA15-3, CA27-29 and CA125. The enzyme-marked antibody and the FITC-marked antibody are the monoclonal antibodies corresponding to the antigens. The FITC antibody coating the magnetic corpuscules adopts a polyclonal antibody or a monoclonal antibody. The marker solution is prepared by mixing an FITC-marked capture antibody working solution and an enzyme-marked antibody pair working solution by the volume ratio of 1:(1-3). Compared with the known kit for mensurating the carbohydrate antigen, the kit has the advantages of high flux, high sensitivity, wide linear range, rapidness, etc., and has a wide application prospect for the clinical inspection, etc.
Owner:TSINGHUA UNIV
Chemiluminescent reagents and chemiluminescence analysis methods with the use of the same
InactiveUS6395503B1Easy to produceShort timeMicrobiological testing/measurementChemiluminescene/bioluminescencePeroxidaseCharge-transfer complex
The present invention provides a novel chemiluminescent reagent producing chemiluminescence in the presence of hydrogen peroxide, extent of which depends on peroxidase concentration, chemiluminescent analysis method using the same, in particular useful for detection and quantitative analysis of various types of materials by measuring peroxidase enzyme activity or enzyme immunoassay with peroxidase enzyme as the marker.More particularly, the present invention provides a chemiluminescent reagent containing, as the major ingredients, a charge-transferring complex of N,N'-disubstituted-9,9'-bisacridinium salt and N,N-disubstituted carboxylic amide compound; chemiluminescent reagent containing further containing a specific aminoalcohol compound, in addition to the above; and method for measuring peroxidase activity at a high sensitivity in the presence of a peroxide, using the above chemiluminescent reagent.Moreover, the novel chemiluminescent reagent of the present invention can enhance sensitivity of the enzyme immunoassay with peroxidase enzyme as the marker by its chemiluminescent reaction.
Owner:DAINICHISEIKA COLOR & CHEM MFG CO LTD
Kit for detecting non-pathogenic or pathogenic influenza a subtype h5 virus
InactiveUS20040142319A1High sensitivityStrong specificitySugar derivativesMicrobiological testing/measurementReverse transcriptaseHaemagglutination inhibition
Current methods for detecting influenza A subtype H5 virus, for example cell culture, haemagglutination-inhibition, fluorescent antibody and enzyme immunoassay, and reverse transcriptase polymerase chain reaction (RT-PCR) may have the disadvantages of low sensitivity and low specificity. Furthermore, such methods are relatively difficult to use, and may not be suitable for routine detection on a daily basis. The kit for detecting H5 virus of this invention may provide a user-friendly alternative that is relatively more sensitive and specific to H5 virus. The detection kit utilizes two specially designed primers A and B for the replication of H5 virus, and a specific capture probe for immobilizing the amplified viral RNA. An additional primer C is also designed for the detection of pathogenic H5 virus. The detection of H5 virus by the detection kit may be accomplished within one day if desired.
Owner:HAI KANG LIFE
Diagnostic kit for determination of serum total IgE, preparation method and application method
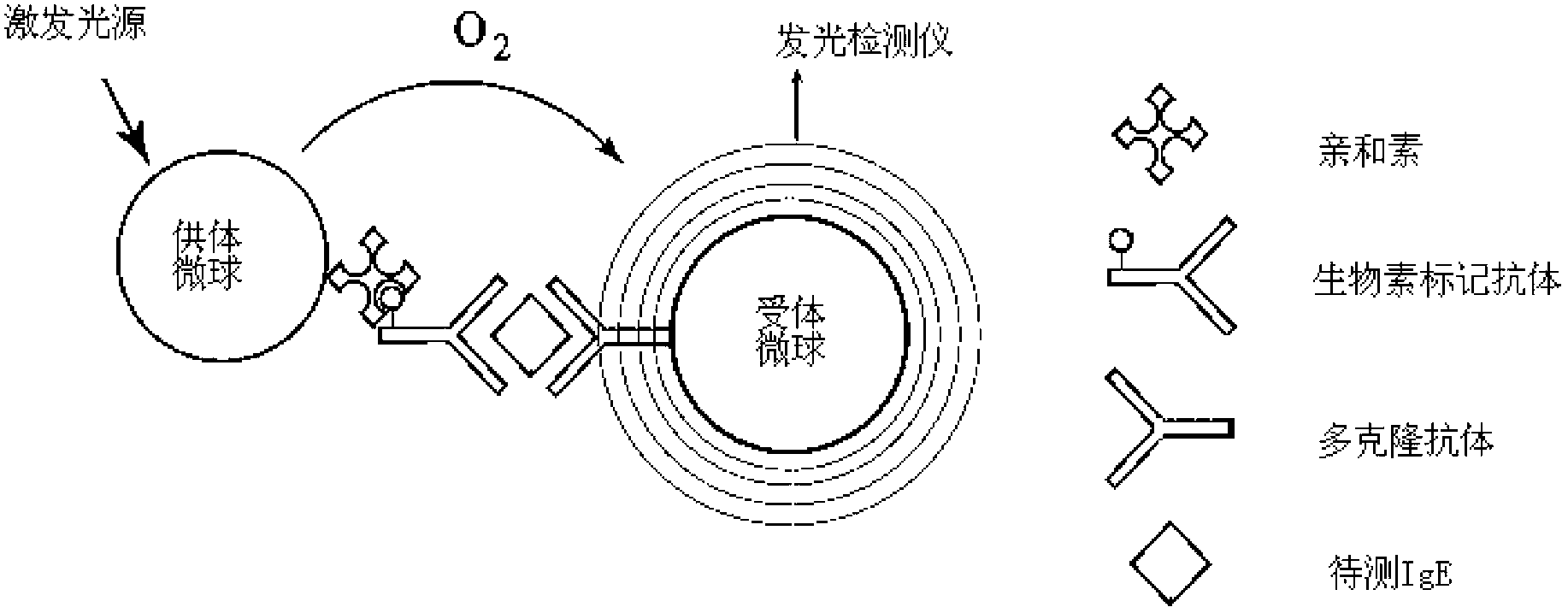
InactiveCN102798725AShorten detection timeReduce biasBiological testingFluorescence/phosphorescenceBiotin-streptavidin complexMicrosphere
The invention provides a diagnostic kit for determination of serum total IgE, a preparation method and an application method. The kit comprises an IgE standard substance, a biotin labeled rate anti-human IgE monoclonal antibody solution, a rabbit anti-human IgE polyclonal antibody coated acceptor microspheres solution and a streptavidin donor microspheres solution. The kit provided by the present invention solves a tedious washing step of current heterogeneous immunoassay kits, overcomes the defects of environmental pollution caused by radioimmunoassay and short shelf life, and overcomes the defects of poor repeatability of enzyme immunoassay analysis and easy hook effect generation, and the detection precision is higher than that of immuno-turbidimetric analysis.
Owner:天津中企华科生物科技发展有限公司
Electrochemiluminescent assays
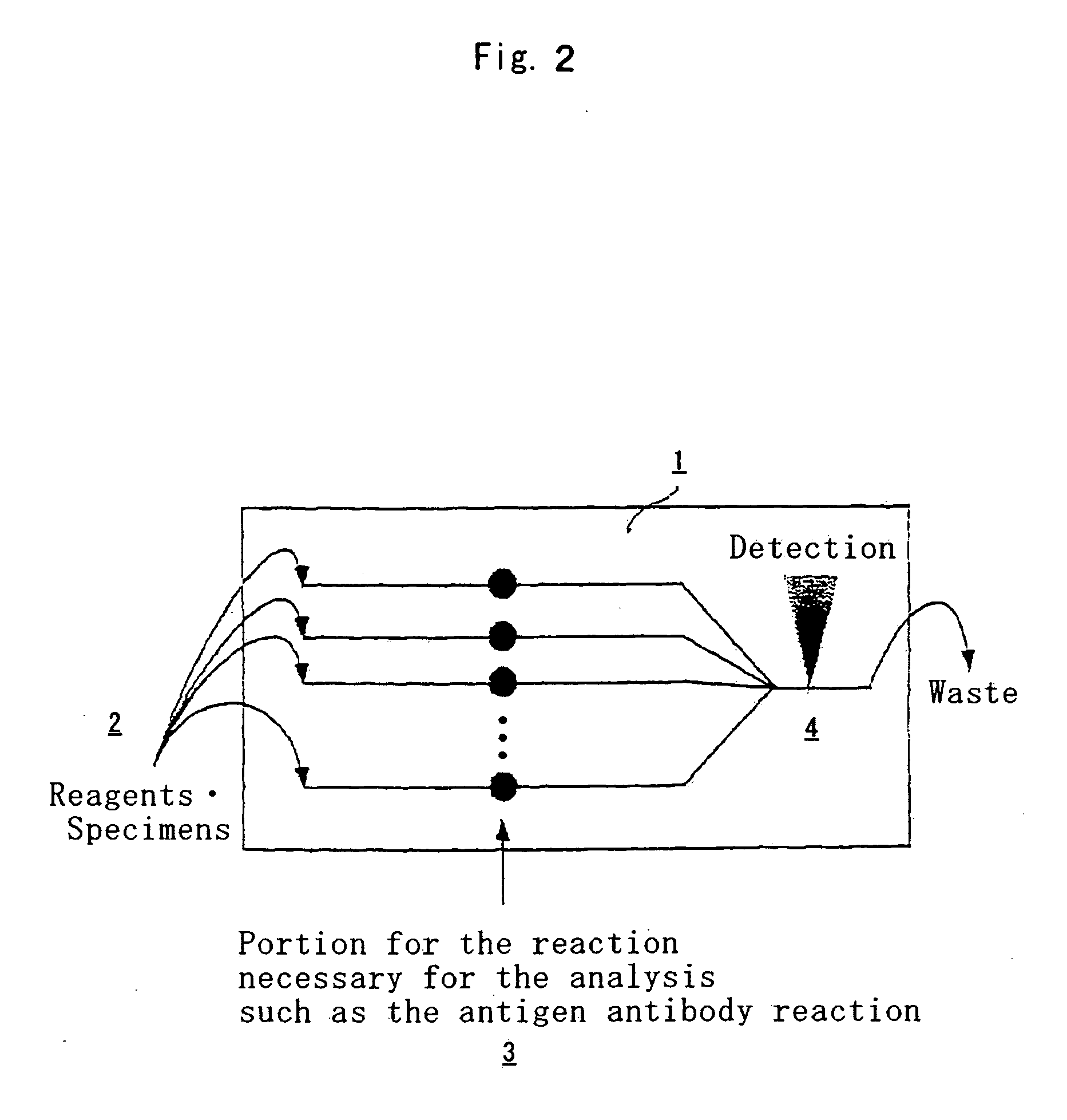
Qualitative and quantitative electrochemiluminescent assays for analytes of interest present in multicomponent liquids are provided. These methods comprise contacting a sample with a reagent labeled with an electrochemiluminescent chemical moiety and capable of combining with the analyte of interest, exposing the resulting sample to electrochemical energy and detecting electromagnetic radiation emitted by the electrochemiluminescent chemical moiety. Further provided are methods for detecting and identifying the presence of a multiplicity of analytes in a liquid food or food homogenate. These methods comprise immersing a diagnostic reagent holder, provided with a multiplicity of reagents, into the food or food homogenate, removing the diagnostic reagent holder from the liquid food or food homogenate, and detecting and identifying the presence of a multiplicity of analytes of interest bound to the diagnostic reagent holder, thereby detecting and identifying the presence of a multiplicity of analytes of interest in the food or food homogenate. The invention further provides an enzyme immunoassay for coliform bacteria. This assay comprises inoculating a sample into a suitable medium for coliform reproduction, immobilizing coliforms present in the medium to a suitable surface, treating the surface with an antibody directed to the immobilized coliforms and detecting the presence of the immobilized coliforms immobilized to a suitable surface.
Owner:BIOVERIS CORP
Homogeneous enzyme immunoassay for oral fluid
The present invention discloses homogeneous enzyme immunoassay systems, methods and kits useful for the qualitatively and quantitatively determination of analytes in oral fluid samples. The system involves a competitive enzyme immunoassay employing a conjugate comprising glucose-6-phosphate dehydrogenase (G6PDH) and an analyte. The methods and kits are particularly useful in the detection of recent drug use and for fast determination of analytes using auto-analyzers.
Owner:LIN ZHI INT
Malachite green vestigial ELISA detection kit and usage method thereof
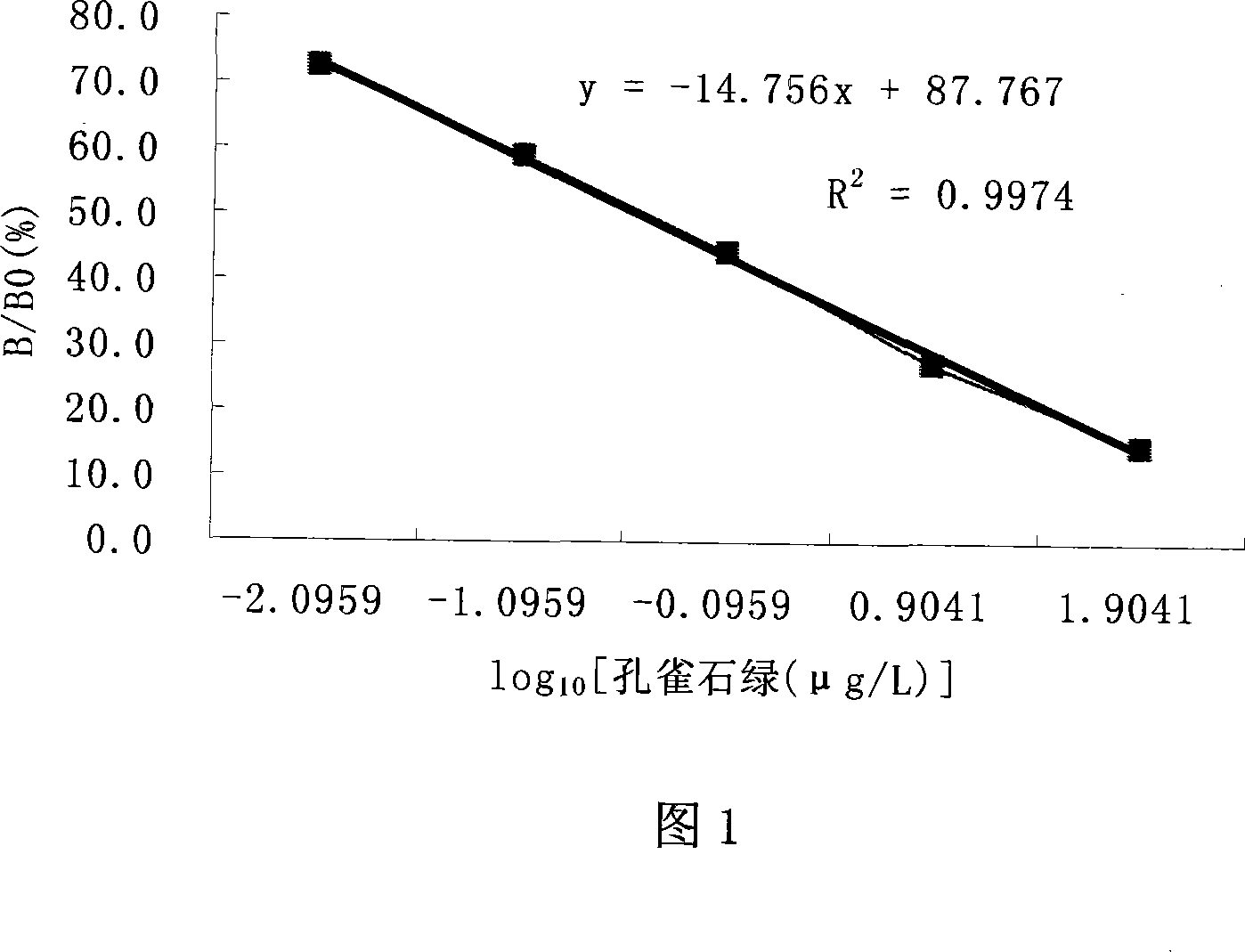
The invention discloses an enzyme immunoassay of testing the bice green residues in animal derived food, which comprises an enzyme label plate covering bice green antigen, enzyme label bice green antibody working solution, bice green standard solution, substrate solution, substrate buffer solution, reaction termination solution, concentration washing liquid and sample dilute solution. The invention further discloses a method for using the immunoassay to test bice green residues, which comprises sample pretreatment, testing via the immunoassay, processing and analyzing result. The inventive immunoassay of bice green test uses direct competition enzyme-linked immunoassay adsorption analysis technique, with high sensitivity, high stability, simplified operation, reduced reaction time, reduced error caused by complex operation, reduced cost, wide application for testing samples and high practicality.
Owner:SOUTH CHINA AGRI UNIV
Homogeneous enzyme immunoassay for oral fluid
The present invention discloses homogeneous enzyme immunoassay systems, methods and kits useful for the qualitatively and quantitatively determination of analytes in oral fluid samples. The system involves a competitive enzyme immunoassay employing a conjugate comprising glucose-6-phosphate dehydrogenase (G6PDH) and an analyte. The methods and kits are particularly useful in the detection of recent drug use and for fast determination of analytes using auto-analyzers.
Owner:LIN ZHI INT
Ultra-sensitive superparamagnetic nano immunization microsphere and GP73 antigen detection method
ActiveCN105699653AThe detection process is fastStrong specificityBiological testingMicrosphereEnzyme immunoassays
The present invention relates to the technical field of diagnostic reagents, and particularly relates to a magnetic immunization in-vitro diagnostic reagent. In order to solve the technical problems of smaller capacity of an immobilized antibody, long reaction time, relatively low sensitivity and low stability of the immobilized antibody of a conventional enzyme immunoassay, the present invention provides a GP73 monoclonal antibody and a superparamagnetic nano immunization microsphere with the GP73 monoclonal antibody coupled with the surface, and a human serum or plasma GP73 antigen detection method using the superparamagnetic nano immunization microsphere by double-antibody sandwich enzyme immunoassay or chemiluminescence method. An antigen for producing the GP73 monoclonal antibody has the following amino acid sequence AAAERGAVELKK. The superparamagnetic nano immunization microsphere has the characteristics of being capable of coupling more antibodies, fast in immunoreaction speed, high in specificity, good in repeatability, low in cost, and simple in experimental condition requirements and the like.
Owner:BEIJING INST OF HEPATOLOGY +1
Detection of multiple analytes from a single sample using a multi-well, multi-analyte flow-through diagnostic test device
InactiveUS20020115062A1Quick distinctionQuick fixBioreactor/fermenter combinationsBiological substance pretreatmentsMulti analyteDiagnostic test
The present invention provides a method and device for conducting a rapid in vitro enzyme immunoassay test for the direct and qualitative detection of two or more viral antigens from specimens of symptomatic patients. The method for immunoassay of viral antigens is performed on a membrane. Non-immunological capture of viral antigens takes place by absorption onto the membrane. Captured antigen binds to a detection reagent that includes a label conjugated to a specific antibody. The test is a differentiated test such that two or more viral antigens may be distinguished from each other in a single test. The invention also includes a kit for performing an assay in accordance with the method of the present invention, wherein the kit comprises the device of the present invention.
Owner:BECTON DICKINSON & CO
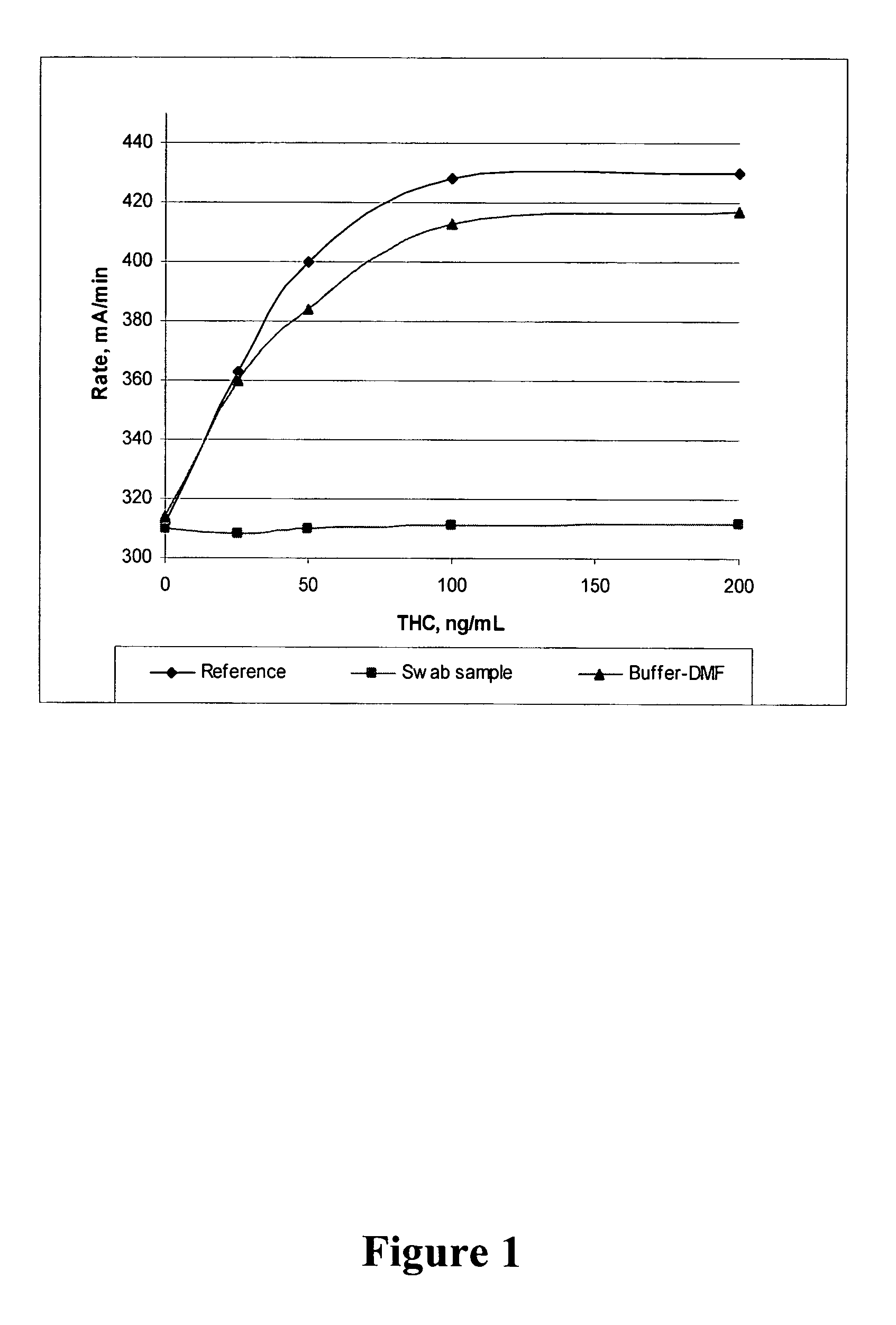
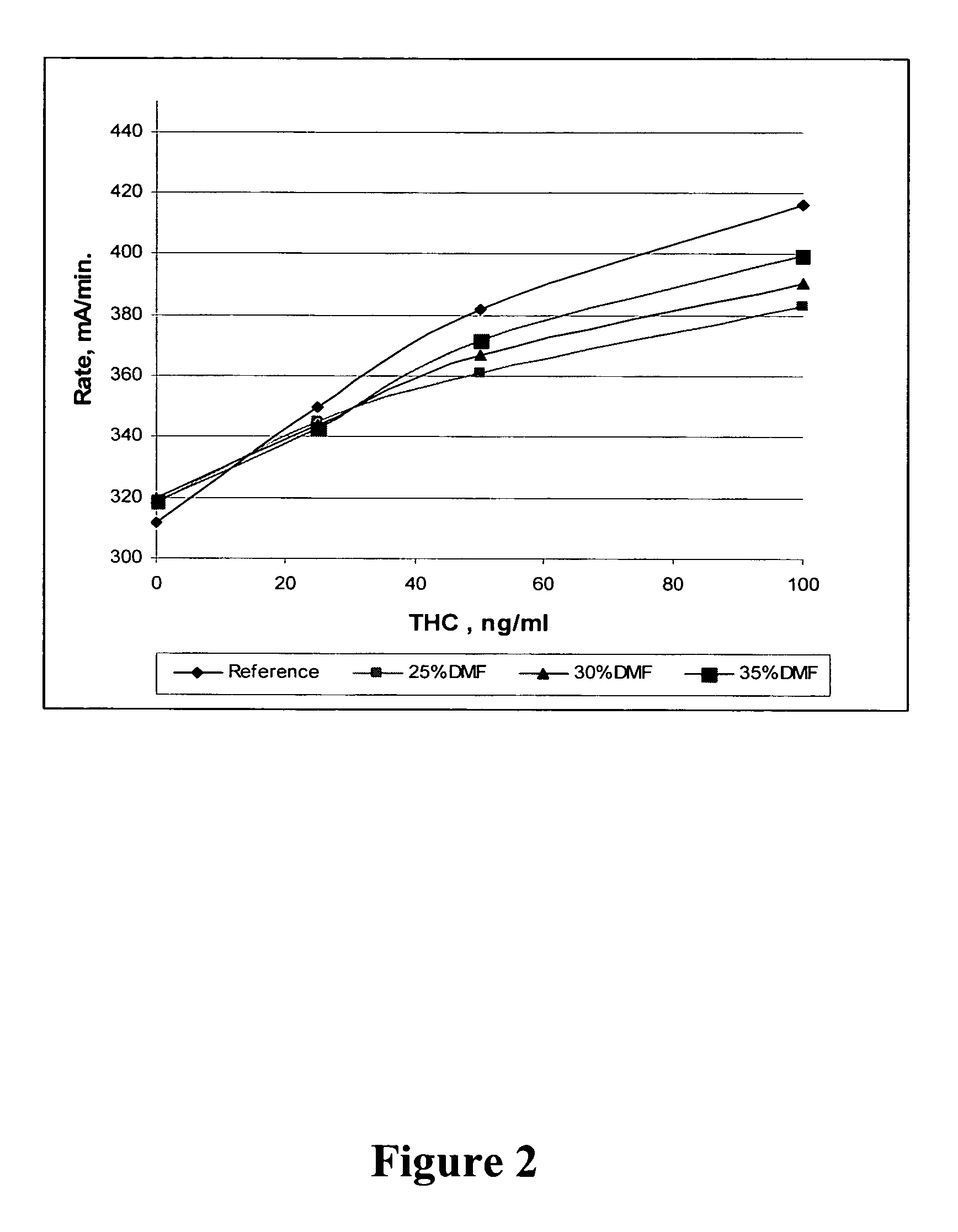
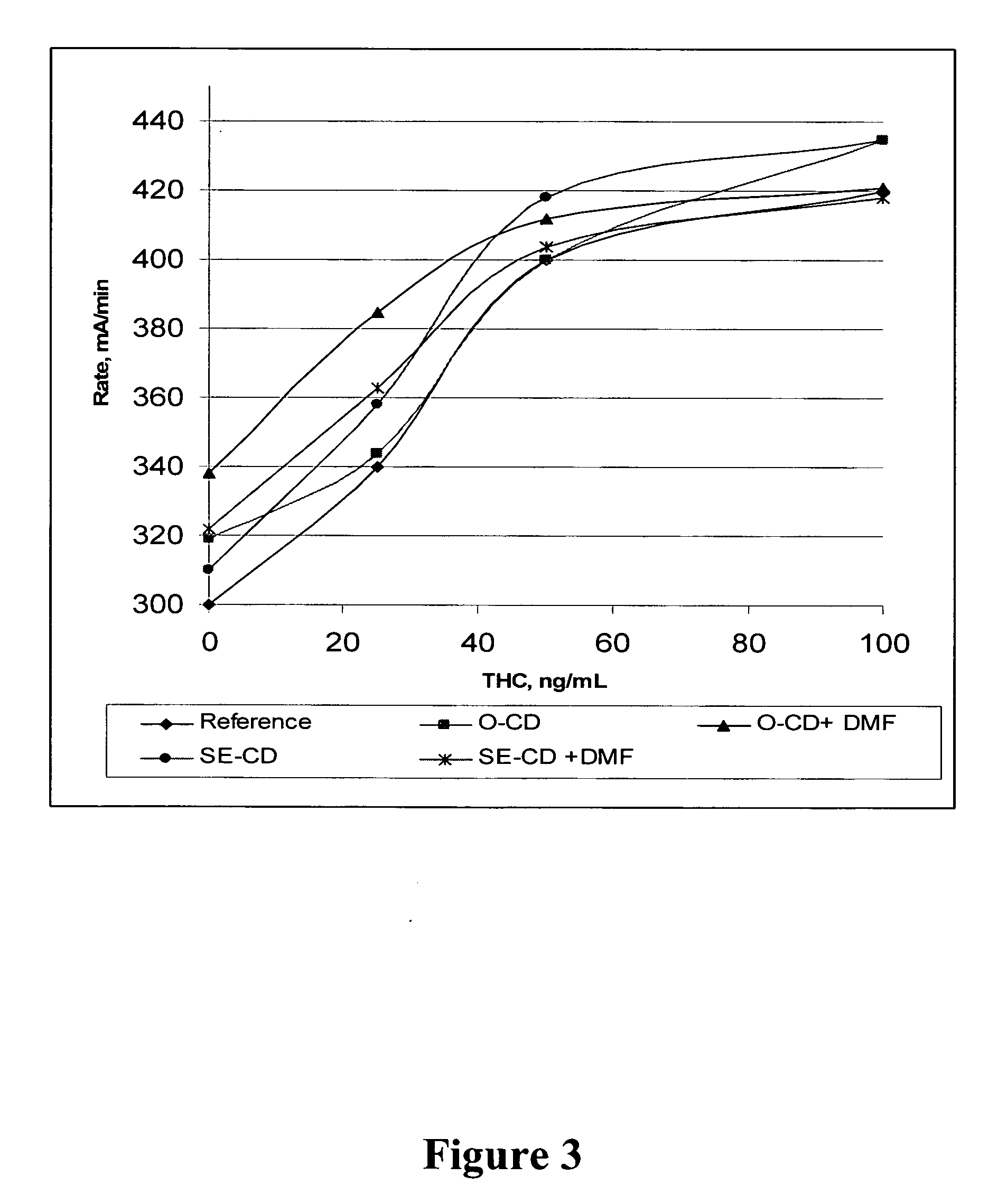
Method for retrieving delta9-THC from oral fluid
The present invention discloses methods and kits for retrieving a cannabinoid from an oral fluid collection device having adsorbed the cannabinoid and to prepare an oral fluid specimen for the determination of Δ9-tetrahydrocaninabinol (Δ9-THC) concentration qualitatively or quantitatively by an enzyme immunoassay. The methods and kits are particularly useful in preserving the Δ9-THC quantitatively from oral fluid samples during the process of collection and preparation.
Owner:LIN ZHI INT
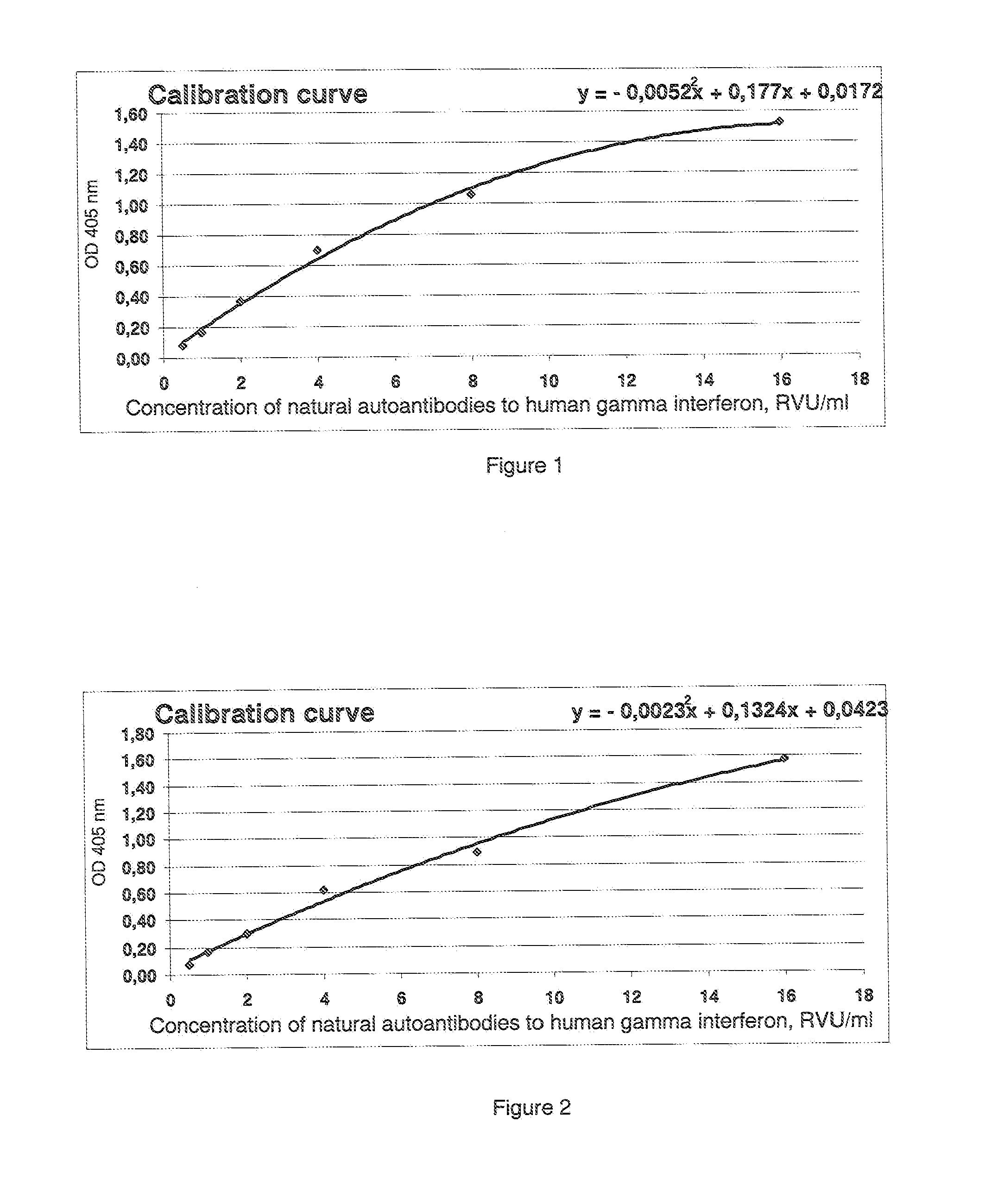
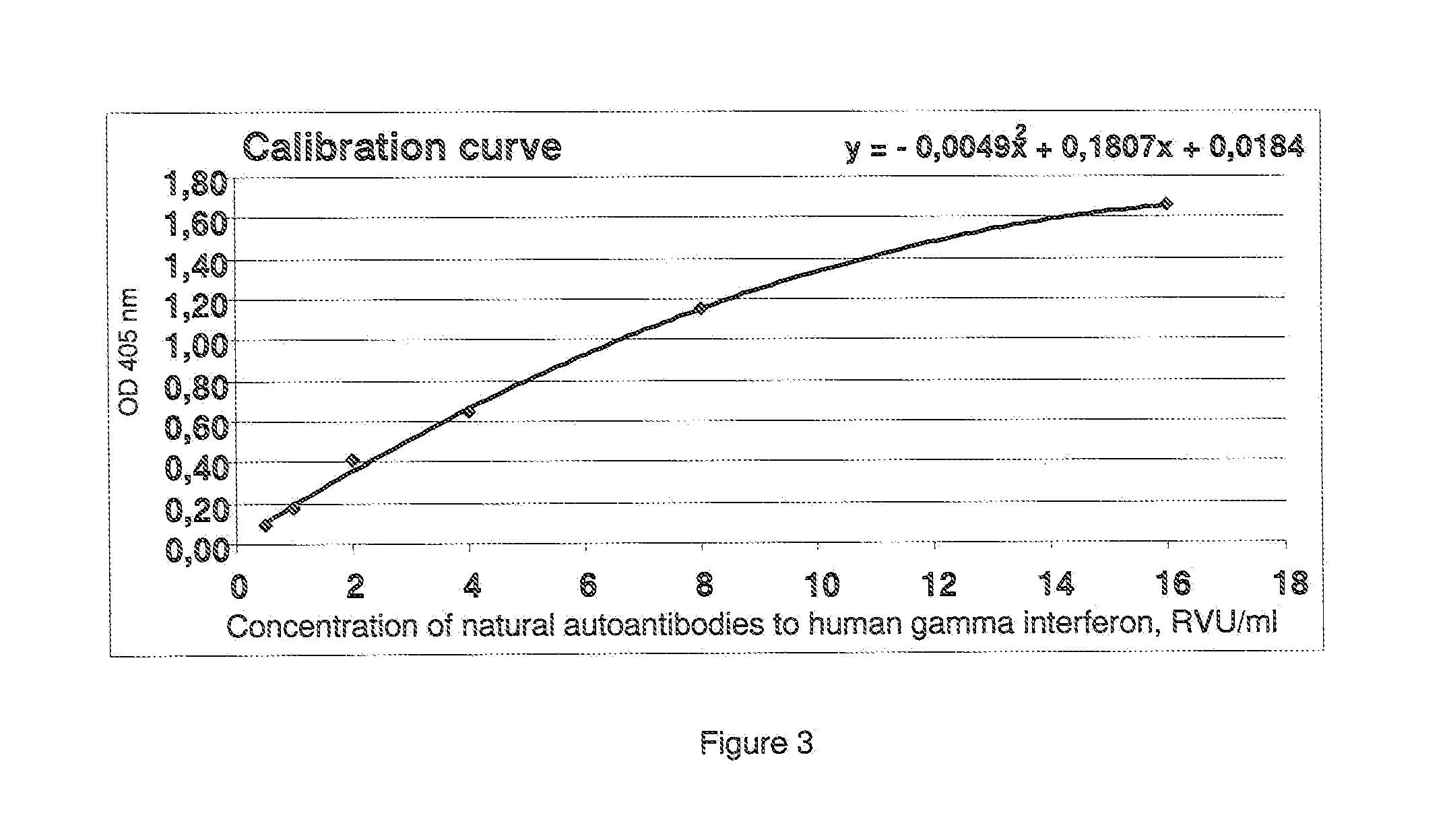
Method of determination of autoantibody level by means of enzyme immunoassay
InactiveUS20130189707A1High sensitivityReduce sensitivityBiological testingBiotin-streptavidin complexSolid phases
The method for quantitative determination of the level of natural autoantibodies in human biological fluids, when as a solid phase of physical sorption is used the solid phase of physical sorption, coated with streptavidin, and the solid phase of physical sorption is treated with preliminary biotinylated antigen and blocking agent for closing the sites of nonspecific binding at the solid phase of physical sorption, for which purpose are used proteins, biotinylated according to standard procedure. As the conjugate-containing solution are used enzyme-labeled monoclonal and polyclonal antibodies, which react with one or all isotypes of human immunoglobulins. In addition, the tested biological fluid is preliminary diluted in a buffer, containing proteins which are used for closing the sites of nonspecific binding at solid phase of physical sorption, and also substances protecting natural autoantibodies from destruction during heat treatment, and subjected to heat treatment. For each tested specimen of biological fluid, a control solid phase of physical sorption is used, and the number of natural autoantibodies is determined with the use of a calibration curve which is plotted using monoclonal or polyclonal antibodies to antigen.
Owner:SERGEEVA SVETLANA ALEXANDROVNA +3
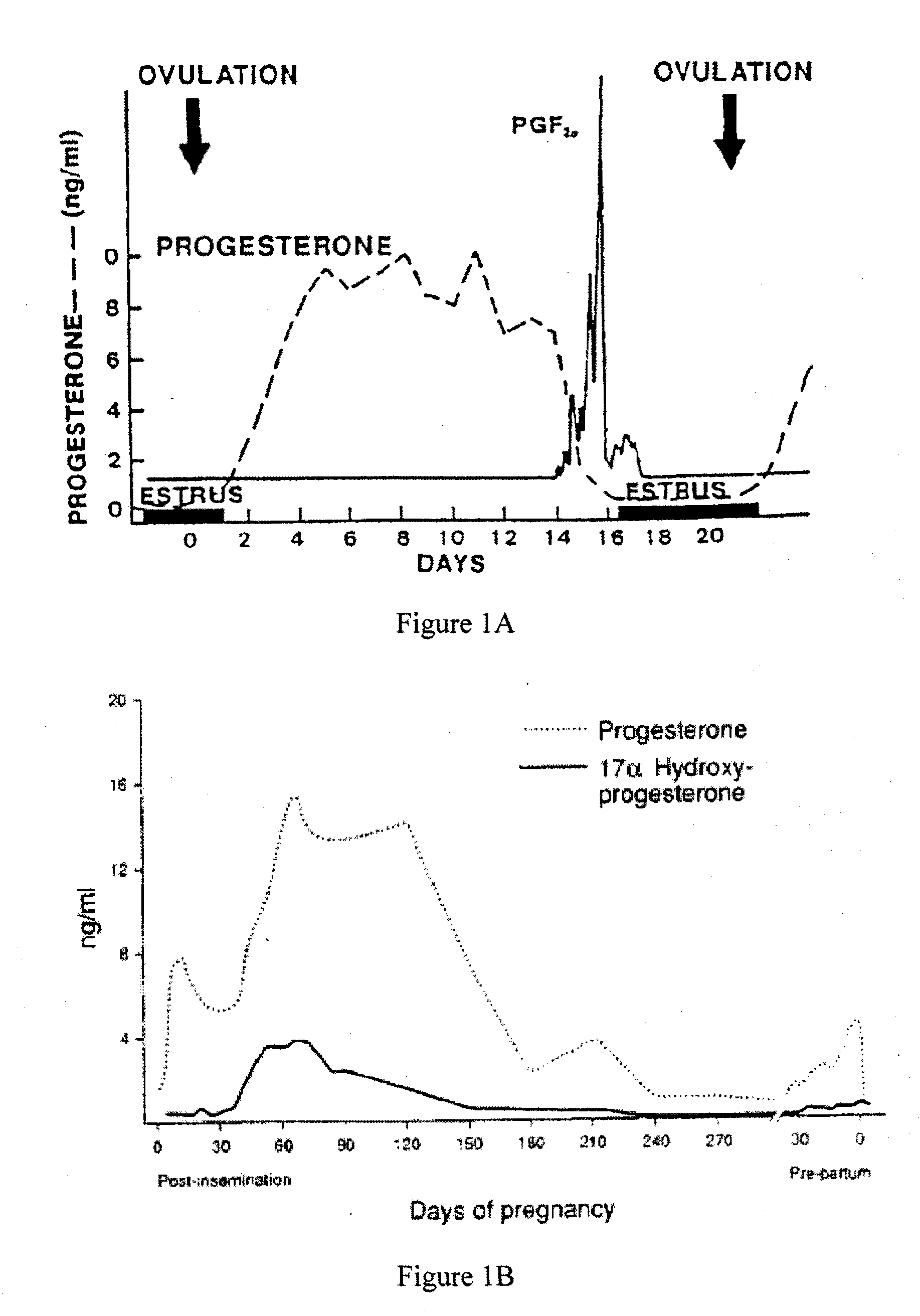
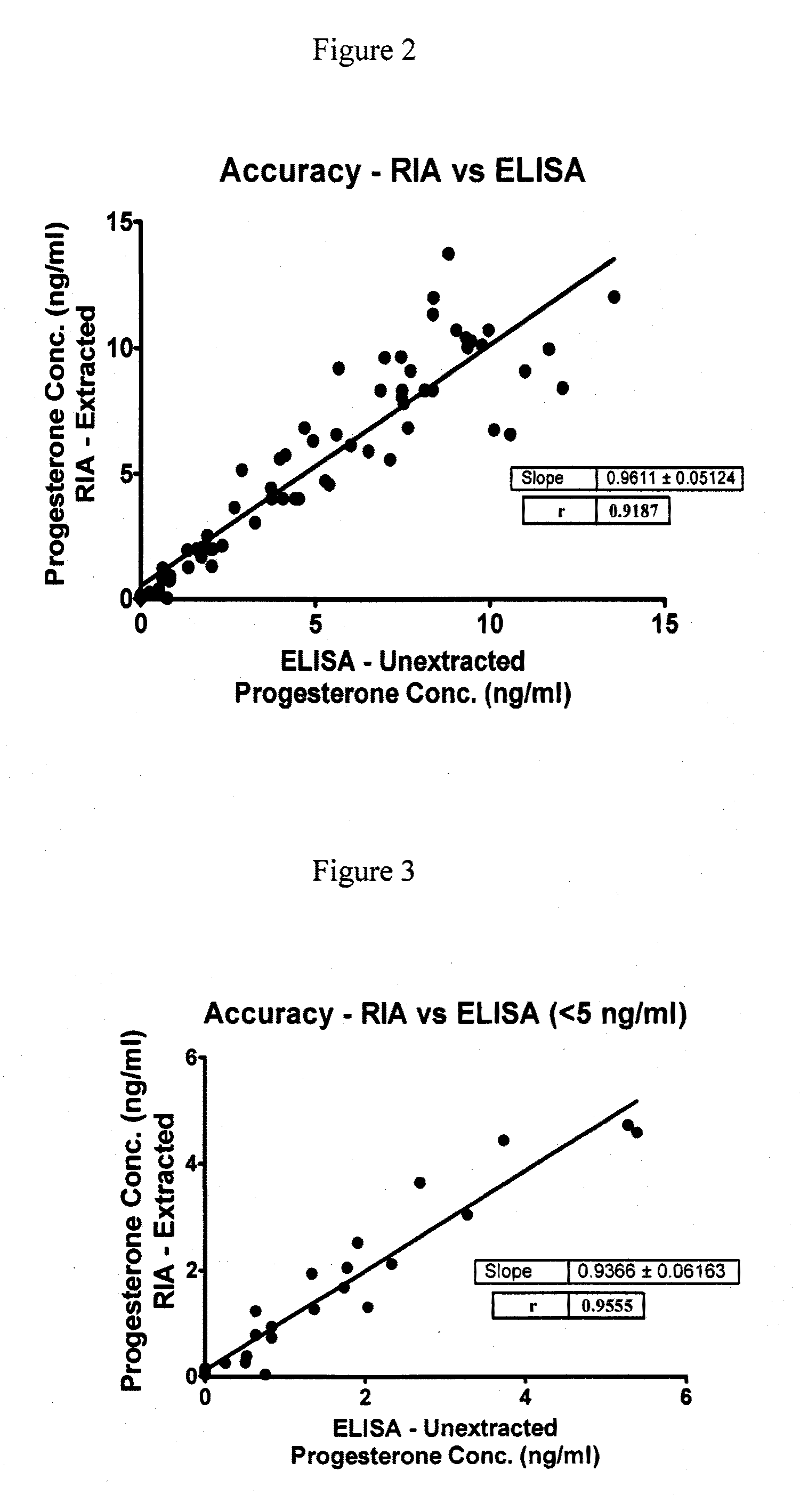
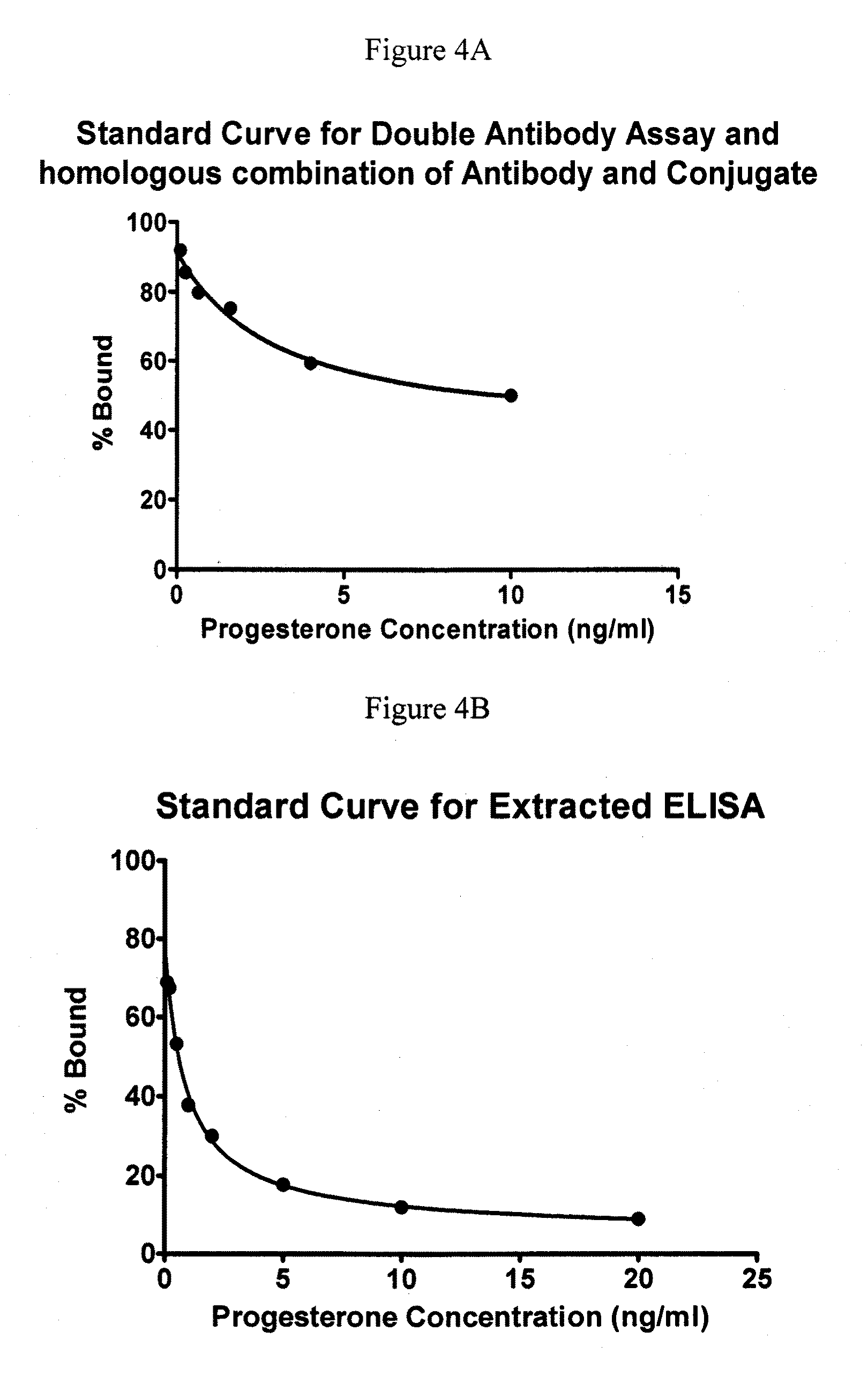
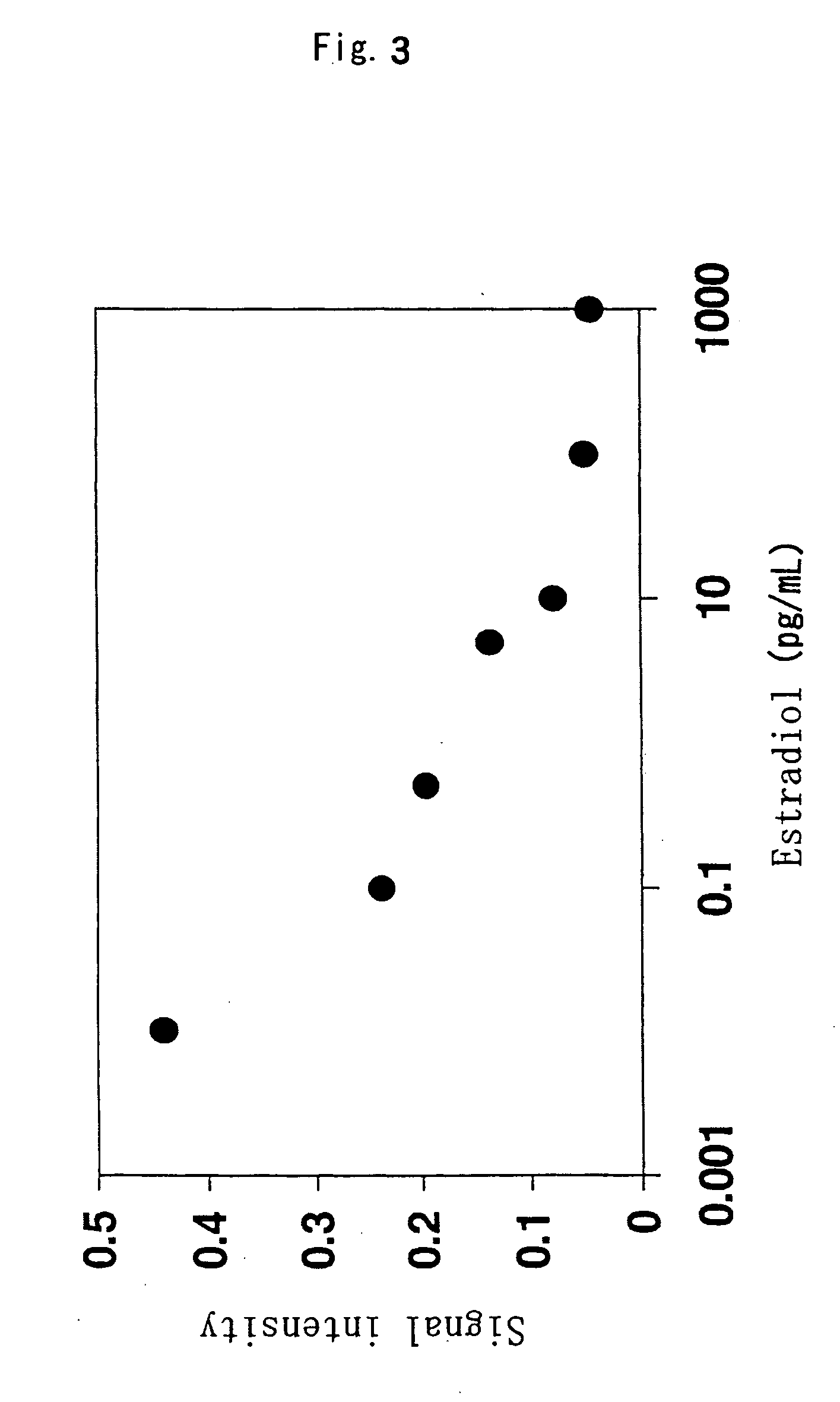
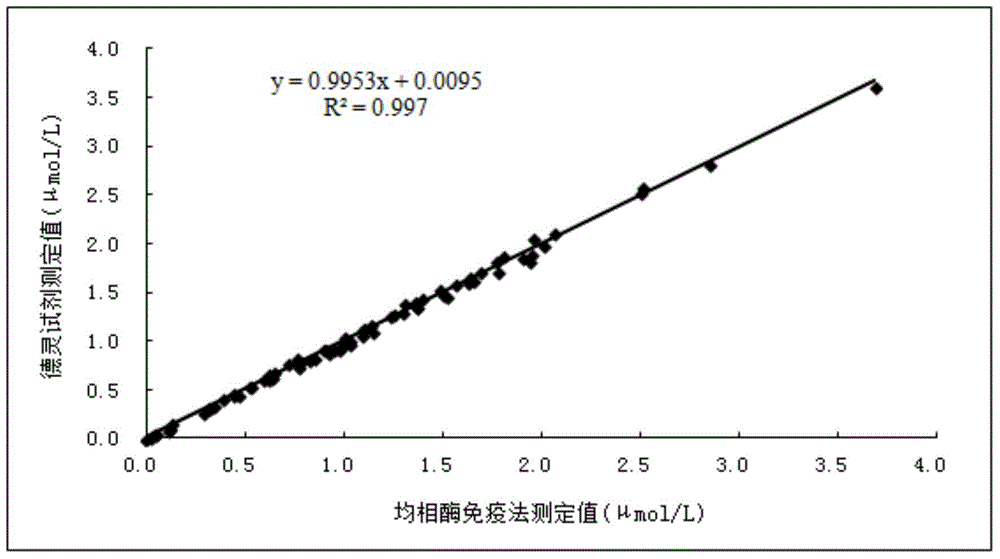
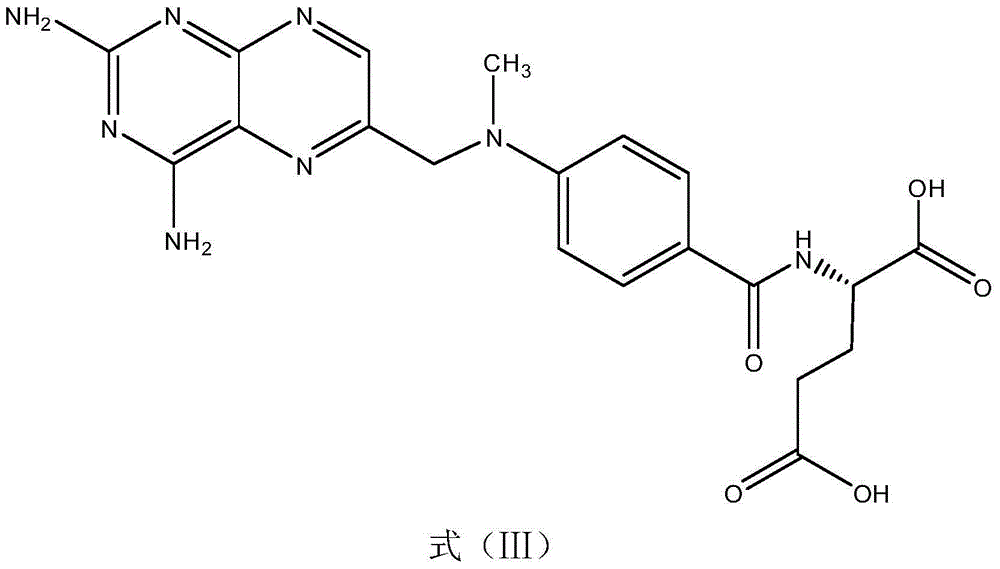
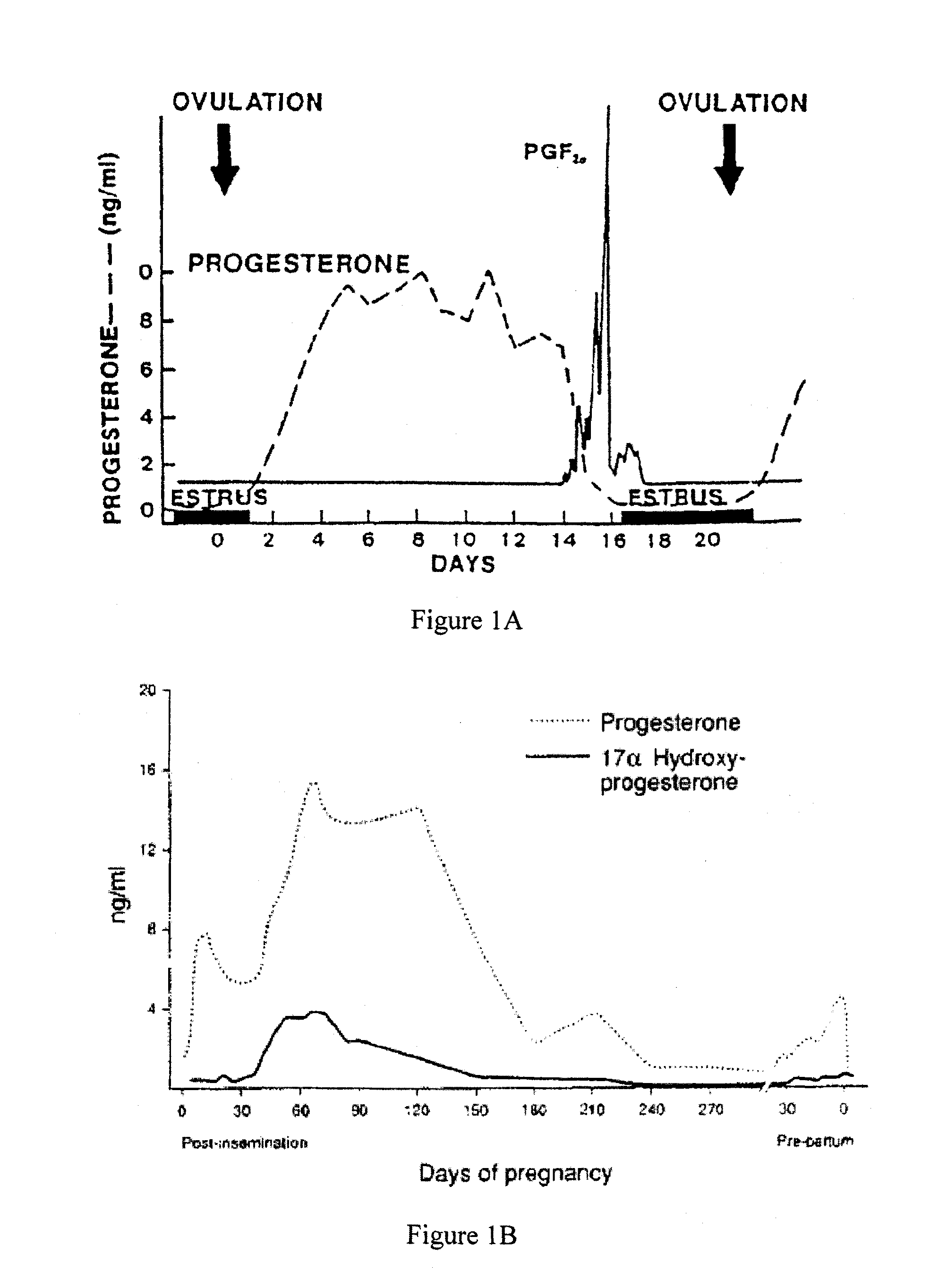
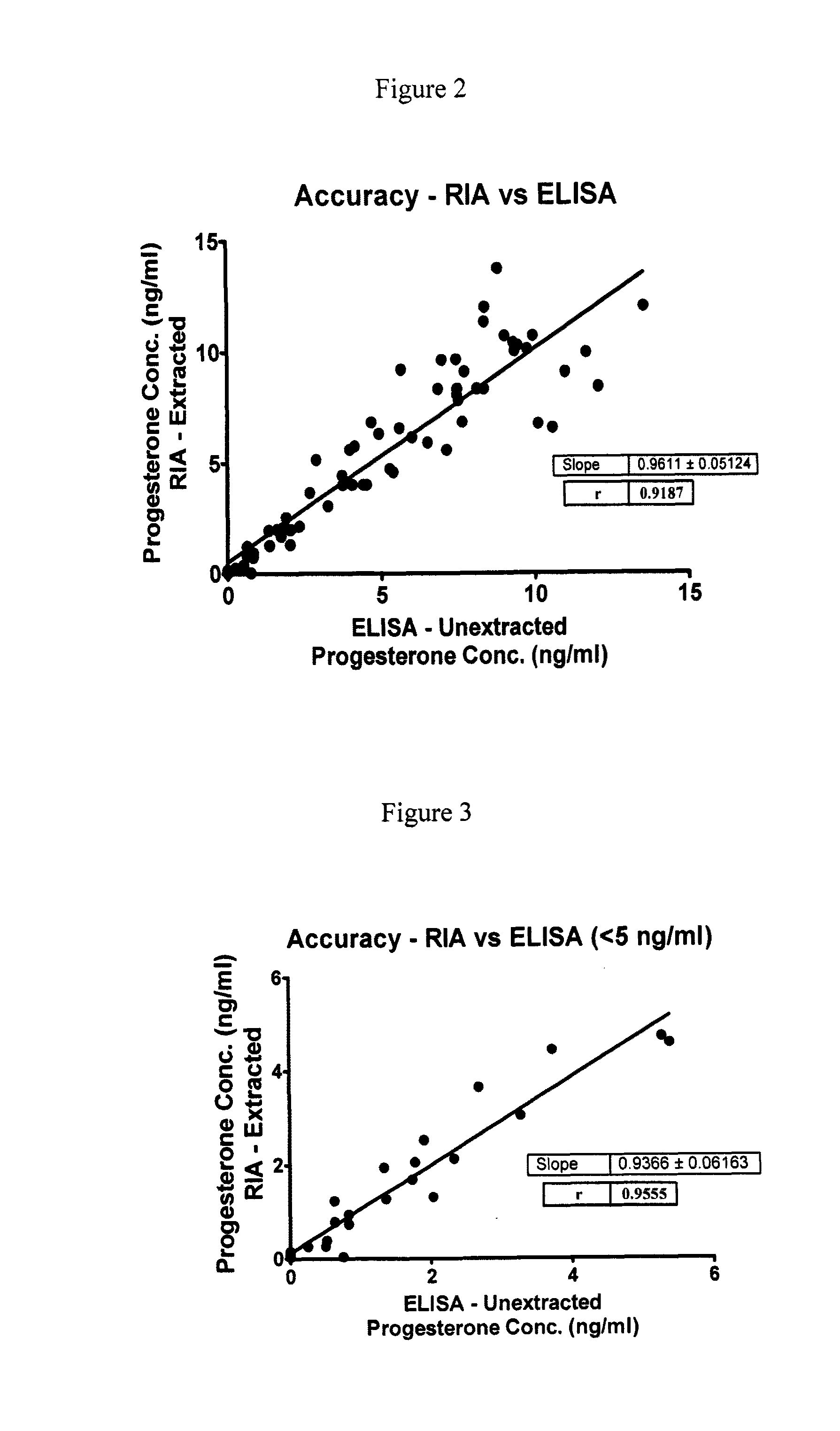
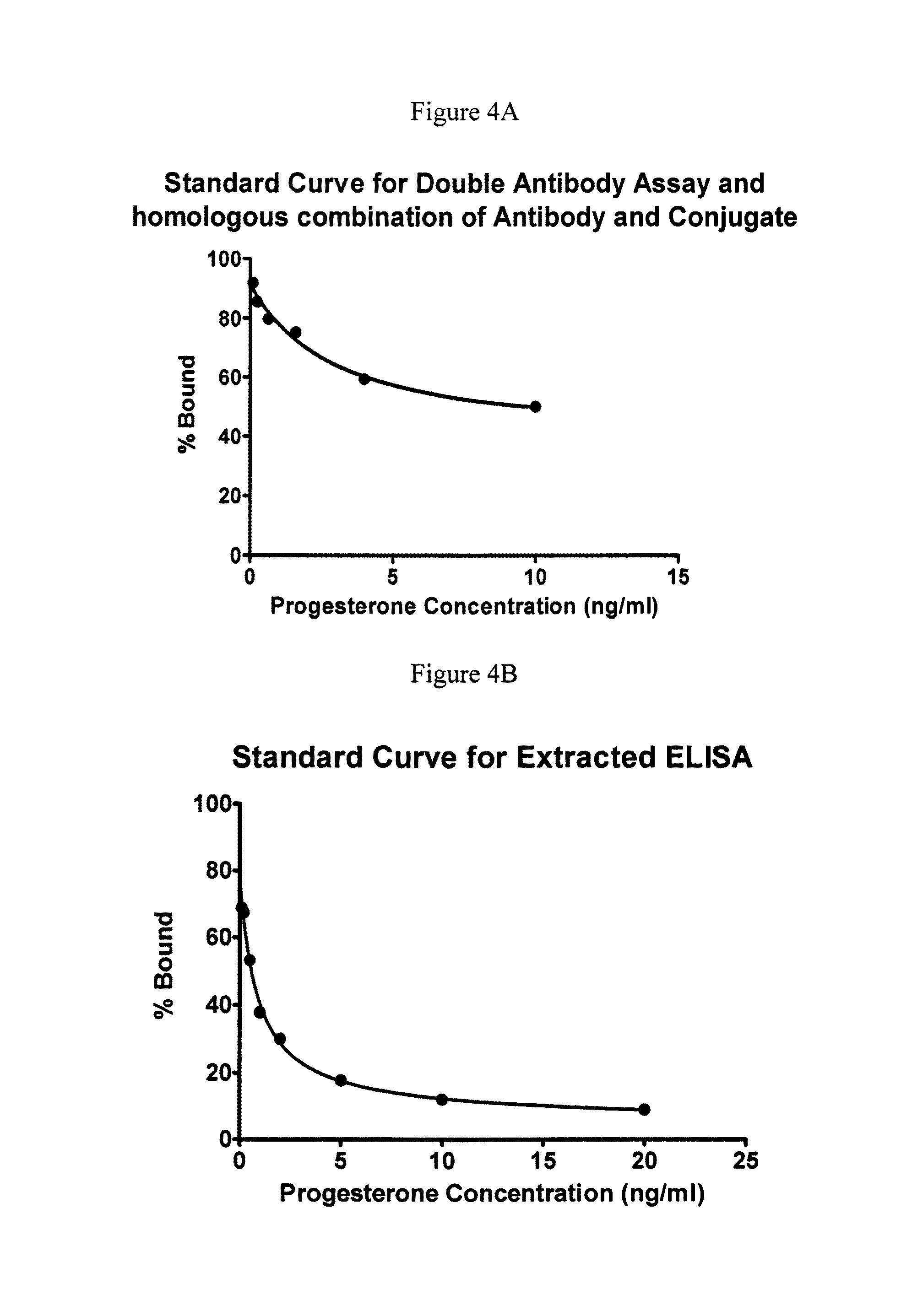
Direct Enzyme Immunoassay and Kit for Measurement of Serum Progesterone Levels
InactiveUS20110020847A1Biological material analysisImmunoglobulins against hormonesSerum progesterone levelEnzyme immunoassays
Owner:COLORADO STATE UNIVERSITY
Clenbterol hydrochloride enzyme immunoassay kit and its immnoassay method
This invention relates to hydrochloric acid CLB enzyme immunity test reagent box and its test method adopting ELISA competition to test CLB and using CLB and OA coupler as CLB antigen immunity rabbits to small mouses to get multiclonal containing CLB antibody or monoclonal CLB antiserum then to be put on enzyme specimen board after separation, purification and dilution to be warm cultivated, washed, added with sample diluent solution and enzyme specimen antigen to be reacted, washed and added with enzyme substrate to display color. It is an easy test method with simple pretreatment especially for testing remaining CLB of meat food.
Owner:JIANGNAN UNIV
Polynary immunoassay board
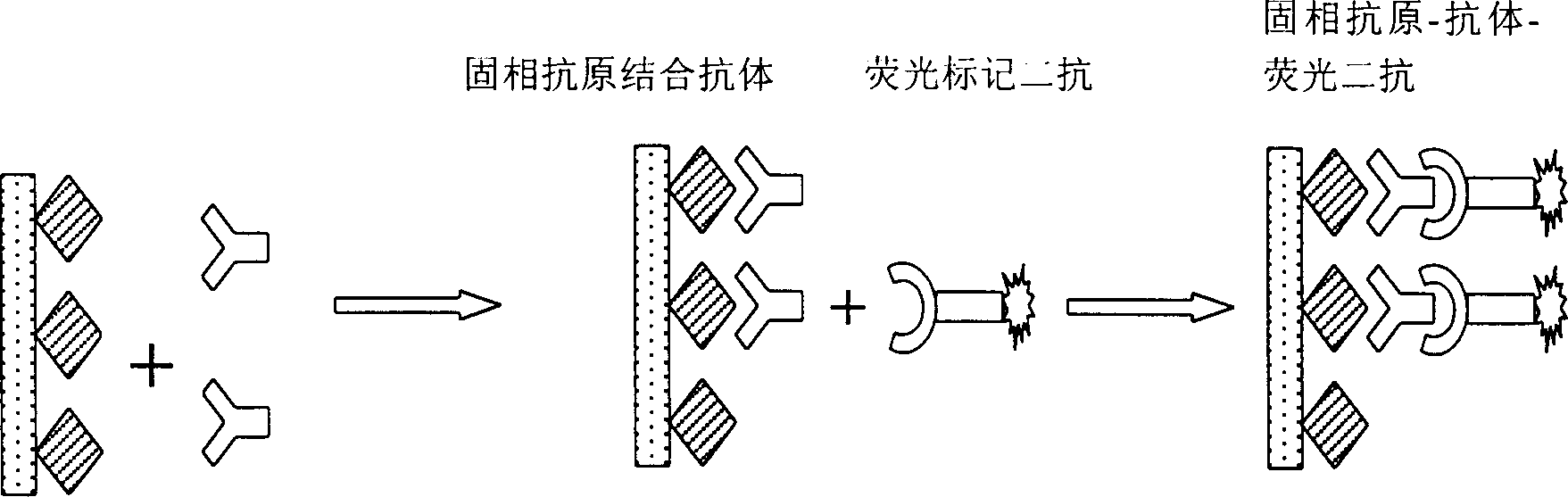
The invented polynary immunoassay plate relates to an enzyme immunoassay chip reaction device which is applied widely to the medical detection, analysis and other field. The polynary immuoassay plate has several holes with antigen fixed by chemical bond to an orifice plate, the surface of the orifice plate as solid carrier is closed and the sample to be tested is added to produce hybridized reaction with the biomolecules fixed to the orifice plate. After unreacted matter is washed off, the labeled antigen is added for hybridization, and the unreacted matter is washed off again before the label signal is detected. Several indexes may be detected simultaneously. The present invention is flexible and saving in tested sample amount, and some commercial autoamtic instruments may be used.
Owner:GENETECH BIOTECH SHANGHAI
Chip and method for analyzing enzyme immunity
InactiveUS20050142624A1Easy to measureBioreactor/fermenter combinationsBiological substance pretreatmentsMicrochip AnalysisEnzyme immunoassays
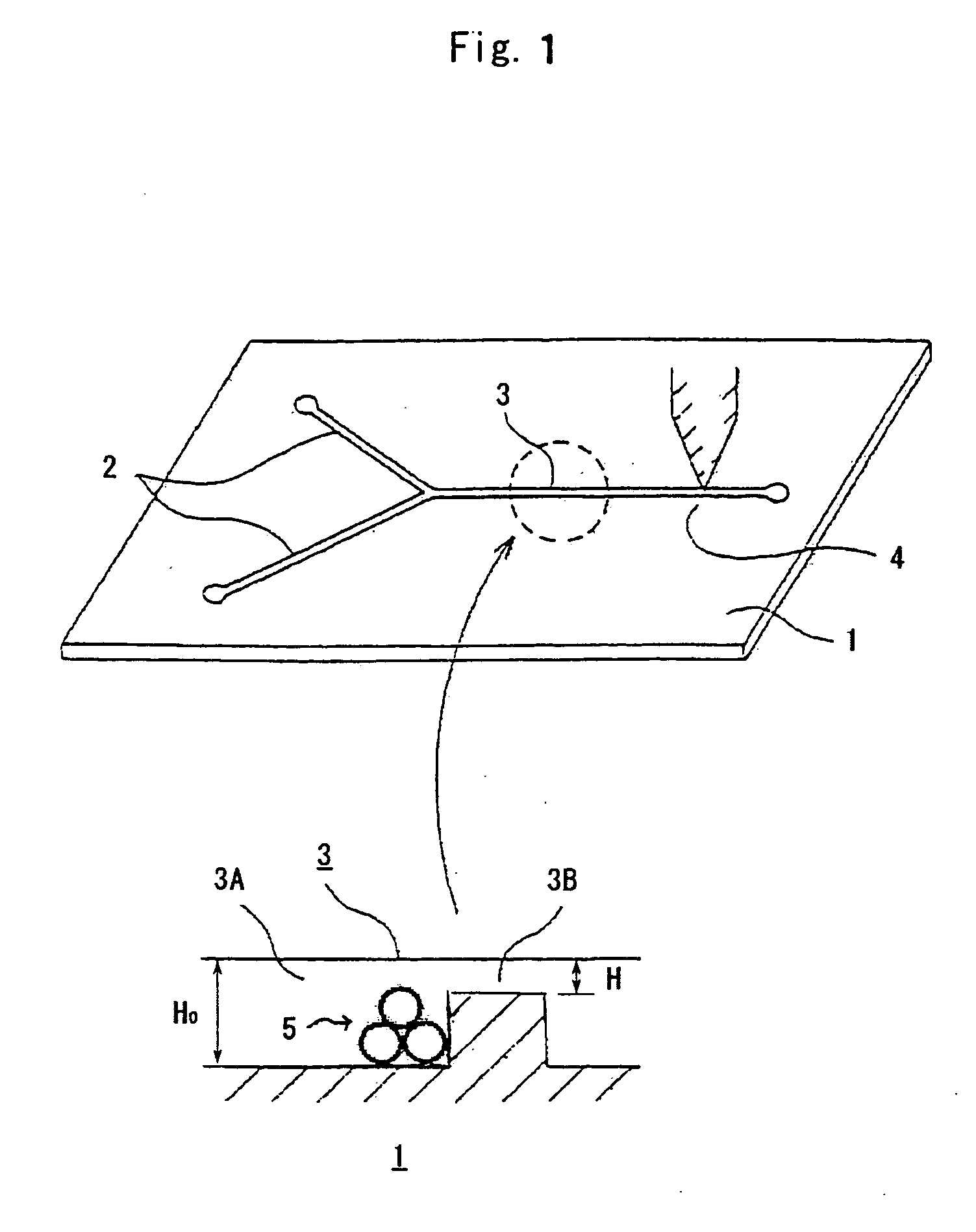
An enzyme immunoassay chip having, as micro channels, a reaction liquid leading-in flow passage part, a reaction flow passage part, and detection flow passage part sequentially disposed on a substrate continuously to each other, comprising an installed part for bead-bodies supporting antibodies and the bead-body flow stopping part formed in the micro channel of the reaction flow passage part, wherein enzyme reactive product flowing beyond the flow stopping part can be analyzed by using the chip.
Owner:KANAGAWA ACADEMY SCI & TECH
Chemiluminiscence enzyme immunoassay fluorescence comprehensive detector
ActiveCN104965075ALow costEasy to carryChemiluminescene/bioluminescenceBiological testingFluorescenceEnzyme immunoassays
The invention relates to a chemiluminiscence enzyme immunoassay fluorescence comprehensive detector which comprises a closed shell, a tray, a chemiluminiscence detection device, an enzyme immunoassay device, a fluorescence detection device and a control device, wherein a moving mechanism is arranged in the closed shell; the tray is suitable for placing an elisa plate and is mounted on the moving mechanism, so that the tray can be driven by the moving mechanism to move along the X-axis and Y-axis directions; the chemiluminiscence detection device is arranged at the first position in the closed shell; the enzyme immunoassay device is arranged at a second position in the closed shell; the fluorescence detection device is arranged at a third position in the closed shell; the control device is in signal connection with the moving mechanism so as to control the moving mechanism to transfer the tray to the first position, the second position or the third position for corresponding detection through the chemiluminiscence detection device, the enzyme immunoassay device or the fluorescence detection device. The chemiluminiscence enzyme immunoassay fluorescence comprehensive detector is provided with the chemiluminiscence detection device, the enzyme immunoassay device or the fluorescence detection device which can be selected as required, so that the chemiluminiscence enzyme immunoassay fluorescence comprehensive detector is conveniently and quickly used and can meet the demand.
Owner:深圳德夏科技发展有限公司
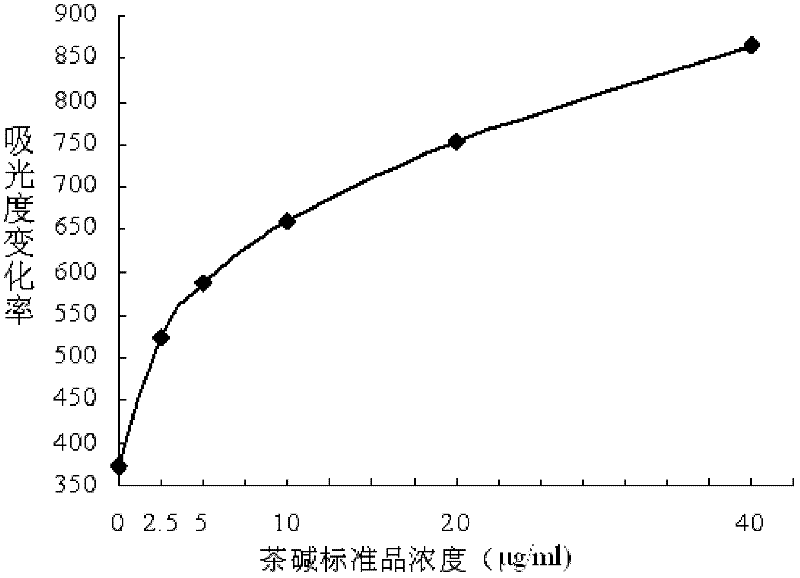
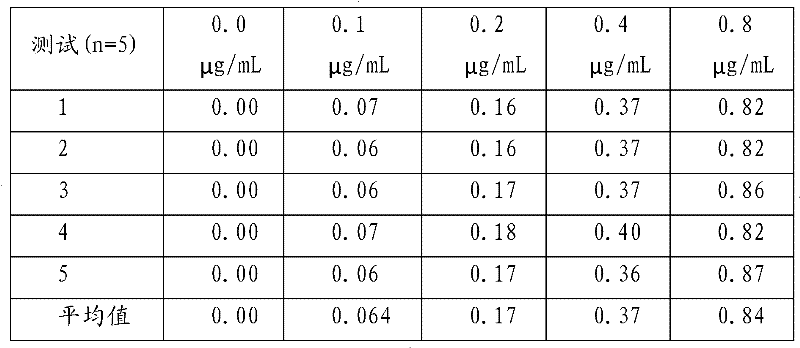
Theophylline homogeneous enzyme immunoassay kit and preparation method thereof
InactiveCN102253215AHigh sensitivityImprove stabilityBiological testingEnzyme immunoassaysImmuno detection
The invention aims to provide simple, rapid, high-sensitivity and full-automation theophylline drug concentration detection equipment, and a preparation method thereof. The invention provides a theophylline homogeneous enzyme immunoassay kit and a preparation method thereof. The preparation method comprises the following steps: synthesizing a theophylline immunogen; preparing a theophylline-resisting specific antibody; preparing an enzyme labeling conjugate; and determining the sample. The equipment provided by the invention has high sensitivity, and good stability and repetitiveness; the sensitivity can reach 0.1 mu g / mL which is far lower than theophylline clinical drug-used range of 10-20 mu g / mL; the average intraassay and interassay precision is less than 2.0%; and the sample recovery rate is 103.4+ / -1.55%. The equipment provided by the invention can be applied on an automatic biochemical analyzer, is simple in operation, can be used for carrying out high-flux rapid sample detection, and is suitable for clinical detection of conventional treatment drug concentration. The theophylline antibody provided by the invention has strong specificity, has no cross reaction on 31 kinds of tested conventional drugs and compounds in the drug cross reaction test, and is suitable for clinically inspecting the blood concentration of the theophylline.
Owner:济南金域医学检验中心有限公司
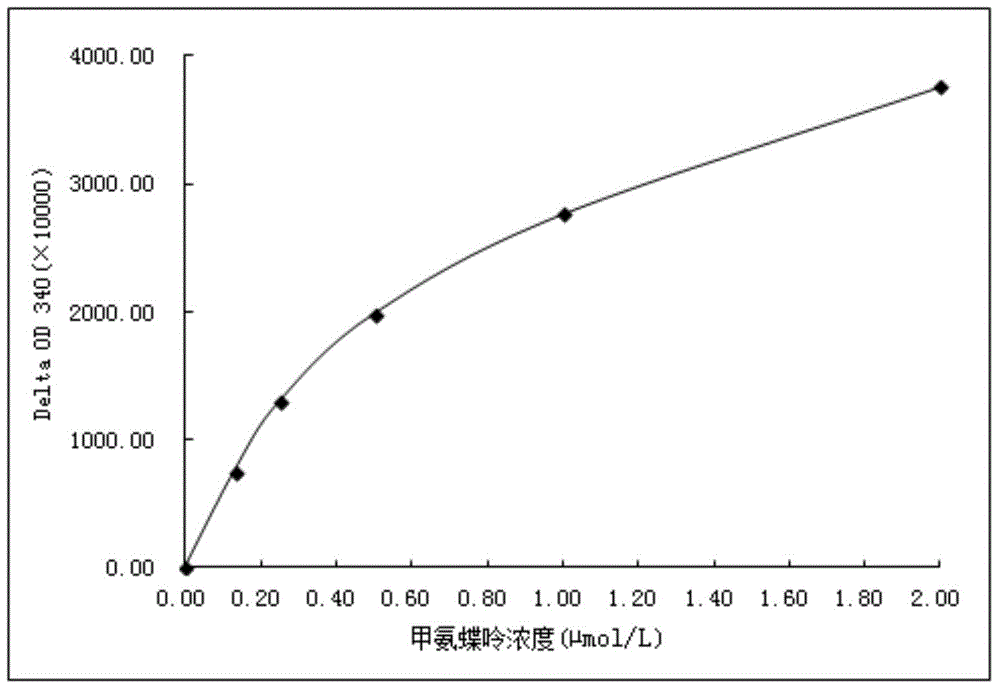
Methotrexate homogenous enzyme immunoassay reagent as well as preparation method and detection method thereof
ActiveCN104569373AStrong immunogen specificityStrong specificityMaterial analysisHigh fluxMethotrexate
The invention relates to a methotrexate detection reagent as well as a preparation method and a detection method thereof, and specifically relates to a methotrexate homogenous enzyme immunoassay reagent as well as a preparation method and a detection method thereof. The methotrexate homogenous enzyme immunoassay reagent comprises an anti-methotrexate specific antibody, and an indication reagent for detecting an anti-methotrexate specific antibody-methotrexate compound, wherein the anti-methotrexate specific antibody is obtained from immune animals with methotrexate immunogen. The methotrexate homogenous enzyme immunoassay reagent disclosed by the invention has the following beneficial effects: the methotrexate immunogen is high in specificity and immunogenicity, and the prepared anti-methotrexate specific antibody is high in specificity and valence, and free from any cross reaction with 62 common medicines; the homogenous enzyme immunoassay reagent containing the anti-methotrexate specific antibody is capable of conveniently, rapidly and accurately determining the content of methotrexate in a sample and measuring a plurality of samples on a fully-automatic biochemical analyser to realize high-flux rapid measurement for methotrexate, is high in accuracy and high in specificity, and is capable of greatly improving the accuracy and the detection efficiency.
Owner:苏州博源医疗科技有限公司
Direct enzyme immunoassay for measurement of serum progesterone levels
InactiveUS9201077B2Biological material analysisImmunoglobulins against hormonesSerum igeSerum progesterone level
Owner:COLORADO STATE UNIVERSITY
Reagent kit and method for detecting thyroglobulin antibody
InactiveCN105334316AOvercoming pollutionOvercome effectivenessDisease diagnosisBiological testingAntigenBiotin
The invention discloses a reagent kit and method for detecting a thyroglobulin antibody. The reagent kit contains a first reagent, a second reagent, a magnetic separation reagent, a chemical light-emitting substrate, a calibration article, a quality control article and cleaning liquid. The first reagent is a biotin reagent marked by thyroglobulin. The second reagent is a thyroglobulin enzyme combination reagent. The magnetic separation reagent is a superparamagnetic nanometer magnetic particle reagent marked by streptavidine, and the reagent kit is adopted for detecting the thyroglobulin antibody. Due to the mode, the defects that radioimmunoassay is high in pollution, the period of validity is short, common microwell plate enzyme immunoassay precision is poor, and sensitivity is poor are overcome, an alkaline phosphatase enzyme catalysis chemiluminescence system, a magnetic particle separation system and an unique antigen and antibody coupling technology are used, and the sensitivity, the precision, the stability, the validity period, the safety and the environment friendliness of the method are greatly improved.
Owner:SUZHOU HAOOUBO BIOPHARML
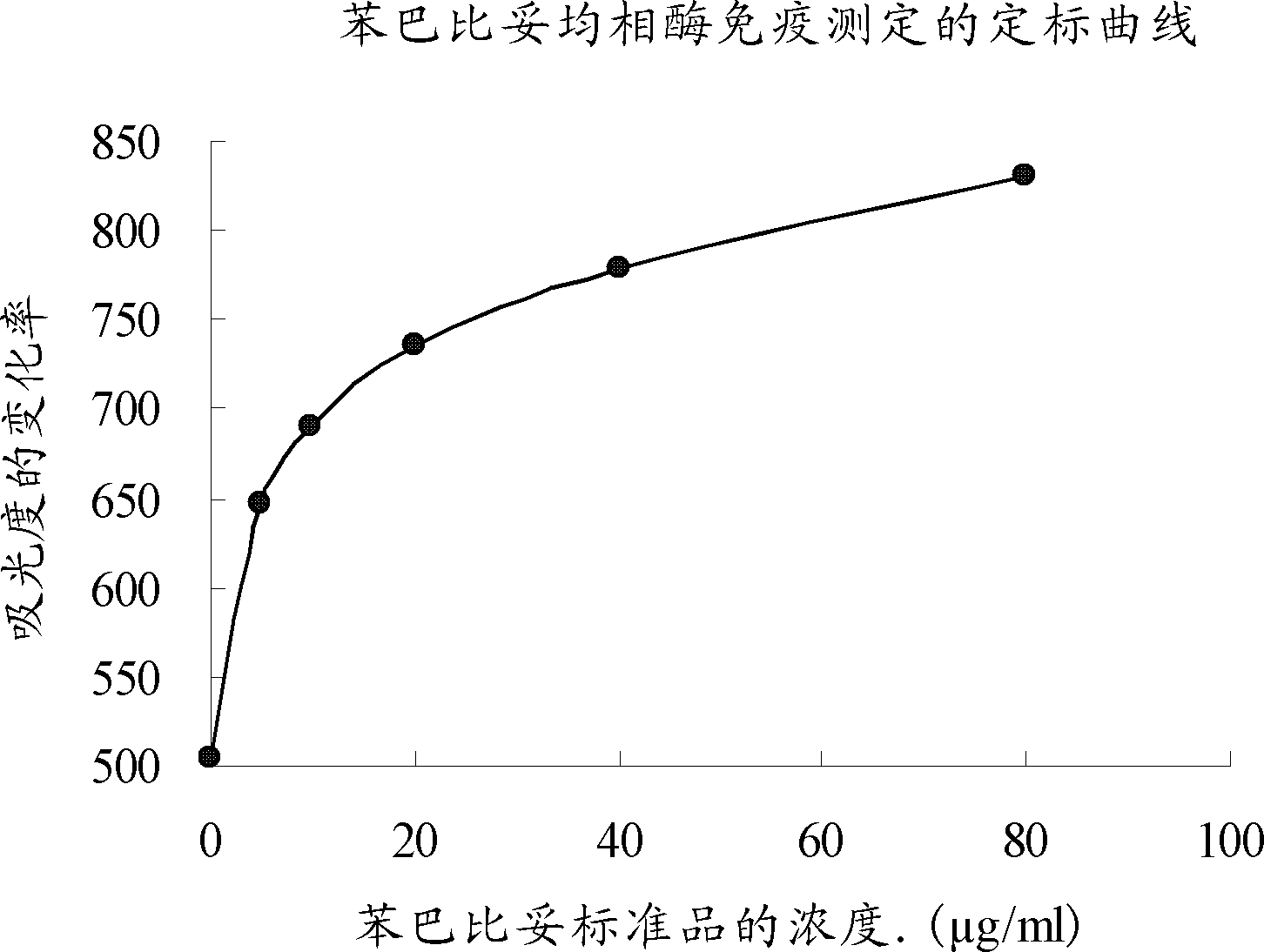
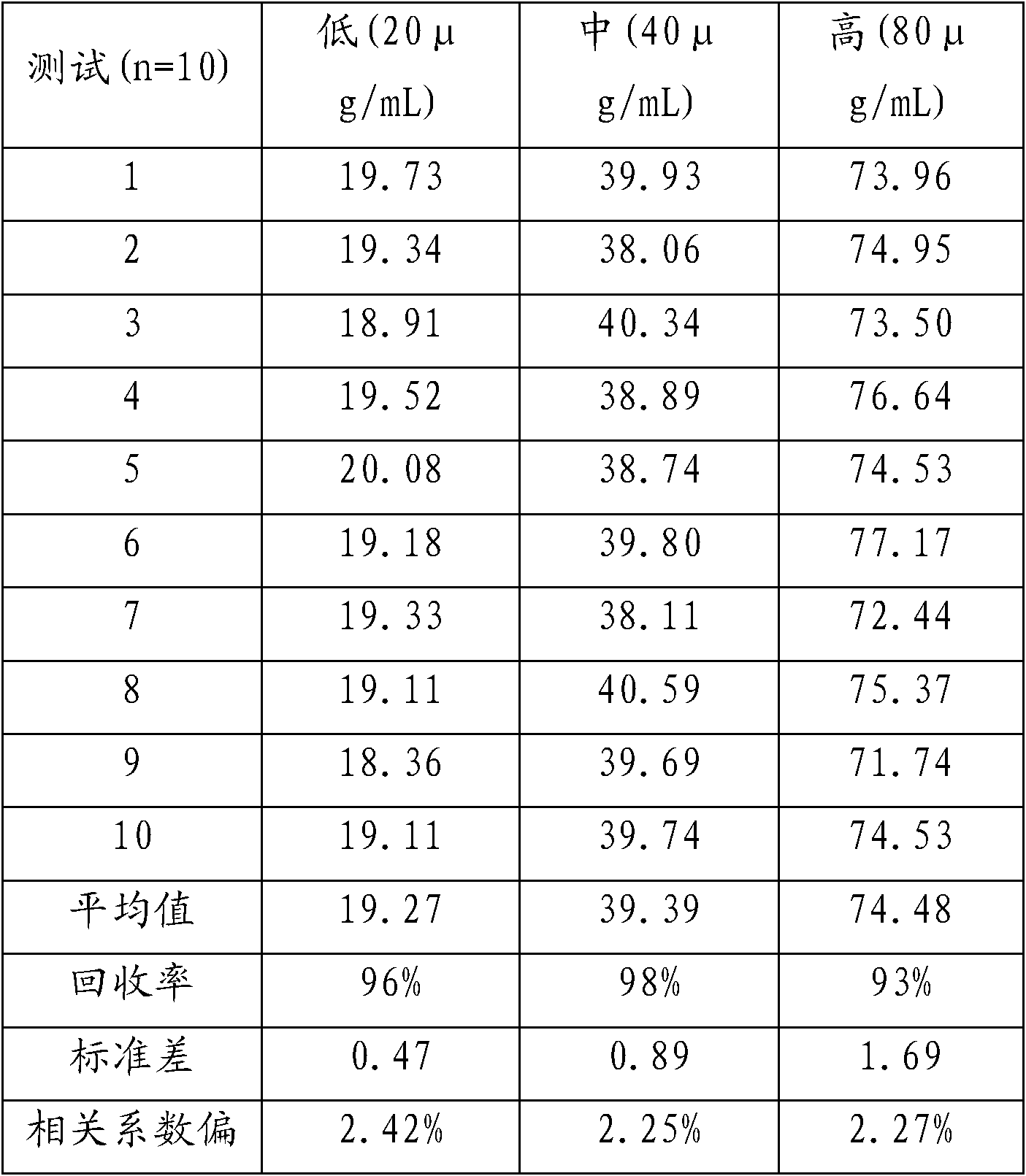
Phenobarbital homogeneous-phase enzyme immunoassay reagent kit and preparation method thereof
ActiveCN102323414AImprove accuracyHigh precisionImmunoglobulinsMaterial analysisDrug interactionEnzyme immunoassays
Owner:西安金域医学检验所有限公司
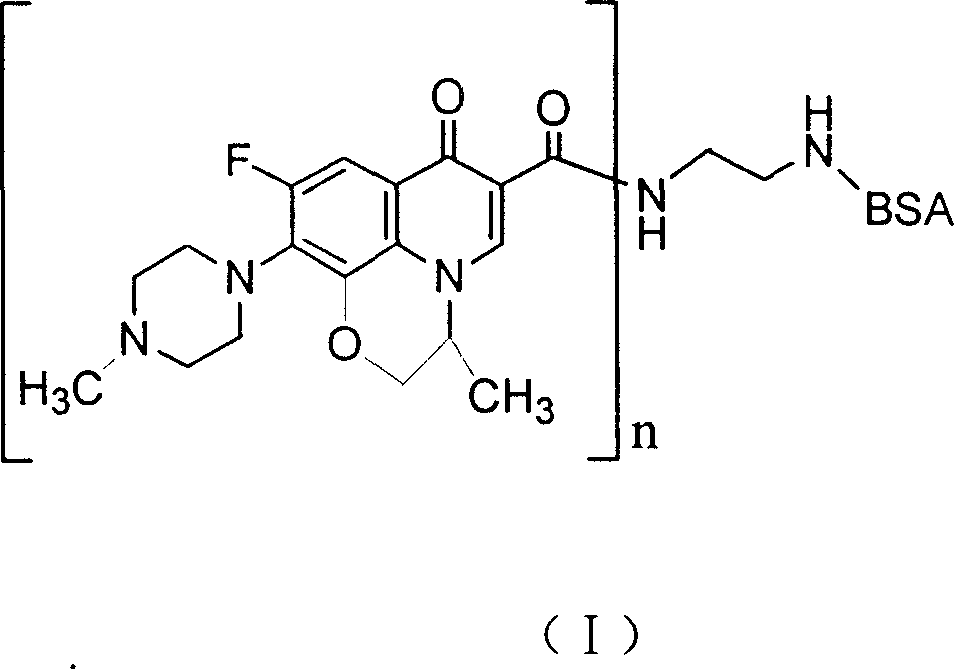
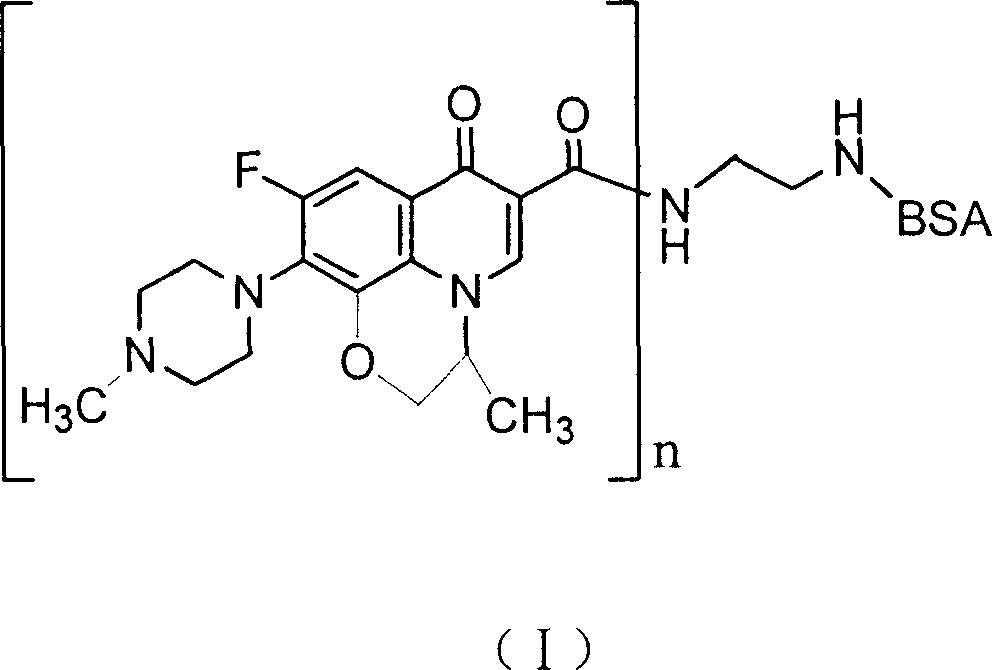
Ofloxacin couple and its preparing method and use
InactiveCN1827642ASimple methodShorten inspection timeImmunoglobulins against animals/humansPeptide preparation methodsBovine serum albuminEnzyme immunoassays
The invention discloses an ofloxacin coupling compound with general formula (I), which comprises coupling compounds of ofloxacin hapten and bovine serum albumin of carrier substance which can produce immunogen or egg albumin. Thereinto, n stands for molecular number of ofloxacin combined with a bovine serum albumin molecule, the said n is an integer between one and twenty, and BSA stands for bovine serum albumin with 6.6KDa-6.9KDa of molecular weight. The invention also discloses a process for preparing the said coupling compound, which consists of joining ofloxacin and carrier substance which can produce immunogen to obtain the said coupling compound which induces immune system of animals to produce antibody. By immuning white rabbits from New Zealand, the ofloxacin coupling compound of this invention has prepared antiserum with 1:512,000 of potency, of which the lowest check limit is 0.1ppb. The invention is characterized in that it is simple, rapid, specific, exact and so on, which provides a foundation for preparing enzyme immunoassay agent box of ofloxacin.
Owner:SHANDONG UNIV
Non-Liquid Phase Type Chemiluminescent Enzyme Immunoassay Method and Assay Kit
InactiveUS20090142781A1Small sizeLow costChemiluminescene/bioluminescenceImmune complex depositionEnzyme immunoassays
A chemiluminescent enzyme immunoassay method whereby a target substance such as a protein is assayed. This chemiluminescent enzyme immunoassay method comprises: the step of capturing an immune complex containing an enzyme-labeled antibody, which is labeled with an enzyme acting a chemiluminescent substrate, and the target substance on a support having no solution layer; the step of overlaying a support membrane containing the chemiluminescent substrate on the immune complex having been captured above; and the step of measuring the luminescence dose caused by the reaction between the enzyme-labeled antibody and the chemiluminescent substrate to thereby quantify the target substance. Since a highly sensitive chemiluminescent enzyme immunoassay is conducted by using a non-liquid phase type reaction system in the chemiluminescent enzyme immunoassay method as described above, multiple items can be assayed by using only a small amount of a specimen and, furthermore, the target substance can be assayed at a high sensitivity thereby without resorting to any troublesome procedures such as pipetting a reagent.
Owner:HOKKAIDO UNIVERSITY +2
Cryptosporidium parvum immune colloidal gold detection test paper strip and production method thereof
InactiveCN102183653AImprove the effect of prevention and controlSuitable for testingImmunoglobulinsFermentationDiseaseProtein.monoclonal
The invention provides a cryptosporidium parvum immune colloidal gold detection test paper strip and a production method thereof. The test paper strip comprises a nitrocellulose membrane, a gold marking pad and an absorption pad, wherein the gold marking pad and the absorption pad are arranged at two ends of the nitrocellulose membrane; the upper end of the gold marking pad is a sample pad, and the gold marking pad is coated with a purified CSpV-S protein monoclonal antibody colloidal gold coupling marker, a detection line is coated with a purified monoclonal antibody, a quality control line is coated with a goat anti mouse IgG antibody, and the absorption pad is attached to the side of the quality control line. The research combines the enzyme-immunoassay principle and colloidal gold chromatography to prepare the colloidal gold test paper strip for detecting C.parvum, which is going to be applied to the clinic to improve the prevention and treatment ability of C.parvum, and the invention has the advantages of simple and rapid operation, clear detection results and easy judgment, high specificity, high sensitivity, no need of instruments and apparatuses or just need of simple instruments and the like, and therefore is especially suitable for clinic sample detection use in sites where diseases occur, outpatient departments, places having no experimental conditions and the like.
Owner:JILIN UNIV
Kit for combined detection of hepatitis C virus antigen and antibody through chemiluminescence
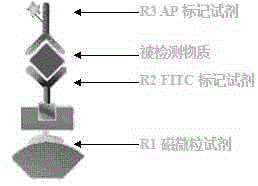
InactiveCN104914244AAvoid missing detectionGuaranteed specificityBiological material analysisEnzyme immunoassaysImmunoresponse
The invention provides a kit for combined detection of hepatitis C virus antigen and antibody through chemiluminescence. According to the invention, the principles of chemiluminescence are utilized; anti-FITC-coated magnetic particles are bonded with an FITC-coated reagent and then undergo immunoreaction with a detected object; after immunoreaction of an AP-labeled substance, an immunoreaction chain (as shown in a figure 1 which is described in the specification) is constructed; luminous sensitivity of a substrate catalyzed by AP is far higher than enzyme immunoassay development, so reaction sensitivity is enhanced; meanwhile, FITC is used to simultaneously marking antigen and antibody, so hepatitis C virus antibody and core antigen are detected at the same time. The kit provided by the invention can more accurately detect hepatitis C, is free of leak detection, achieves the effect of early discovery and has a high application value in prevention of hepatitis C.
Owner:BIOBASE BIODUSTRY (SHANDONG) CO LTD
Reagent for determining content of human cholyglycine by using latex immunoturbidimetry technology
InactiveCN108982860AImprove detection accuracyEliminate distractionsMaterial analysisSerum igePhosphate
The invention discloses a reagent for determining the content of human cholyglycine by using a latex immunoturbidimetry technology. The reagent is prepared from a reagent body 1 and a reagent body 2,wherein the reagent body 1 is prepared by adding 100 mM of an auxiliary agent, a phosphate buffer solution with the pH value of 8.0, a sodium chloride solution with the concentration of 0.9%, BSA withthe concentration of 0.1% and a stabilizer into a latex microsphere-BSA-cholyglycine conjugate with the concentration of 0.04%, and the reagent body 2 is prepared by adding 15 mM of an auxiliary agent, a phosphate buffer solution with the pH value of 7.4, a sodium chloride solution with the concentration of 0.9%, BSA with the concentration of 0.1%, a surfactant with the concentration of 0.1% anda stabilizer into an anti-mouse cholyglycine monoclonal antibody with the concentration of 5%. According to the reagent, latex microspheres are introduced into the reagent, due to the existence of thelatex microspheres, the sensitivity of the detection reagent is greatly improved, and the requirements of clinical use are met. Compared with homogeneous enzyme immunoassay, the reagent has great advantages in stability, under the acceleration condition, and the stability time of the reagent is at least 2 times or above of the stability time of a homogeneous enzyme immunoassay reagent.
Owner:北京安图生物工程有限公司
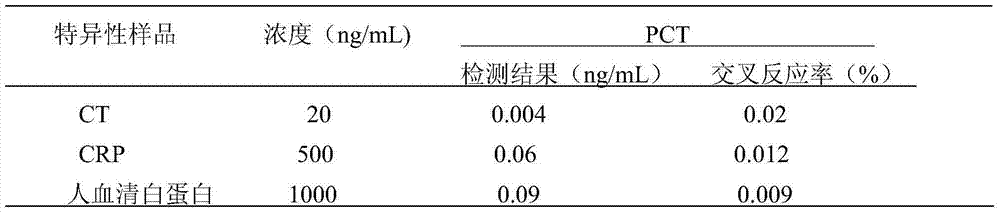
Procalcitonin light-initiated chemiluminescence immunoassay kit and preparation method thereof
The invention discloses a procalcitonin light-initiated chemiluminescence immunoassay kit and a preparation method thereof. The procalcitonin light-initiated chemiluminescence immunoassay kit disclosed by the invention is composed of a white opaque 96-pore plate, a procalcitonin calibrating product, a receptor microsphere coated with an anti-procalcitonin monoclonal antibody, a biotinylated anti-procalcitonin monoclonal antibody, and a streptavidin biotinylated donor microsphere. The procalcitonin light-initiated chemiluminescence immunoassay kit disclosed by the invention has the advantages of rapidness, high sensitivity, wide measuring range, simplicity in operation, and the like, and has higher sensitivity and wider detectability in comparison with an enzyme immunoassay, can be used for diagnosing and identifying individual infectious diseases, and has an application value.
Owner:GUANGZHOU DARUI BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com