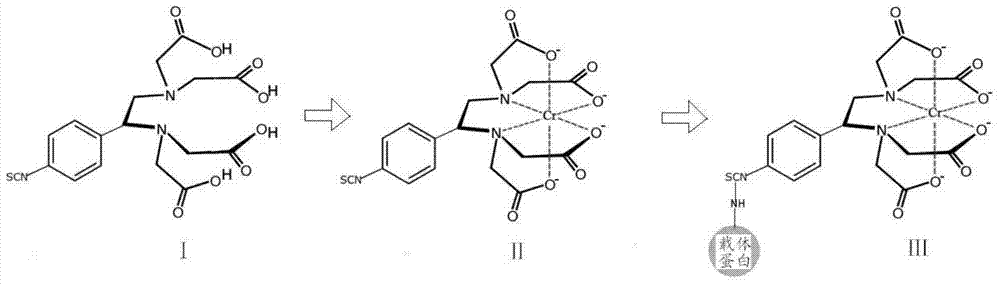
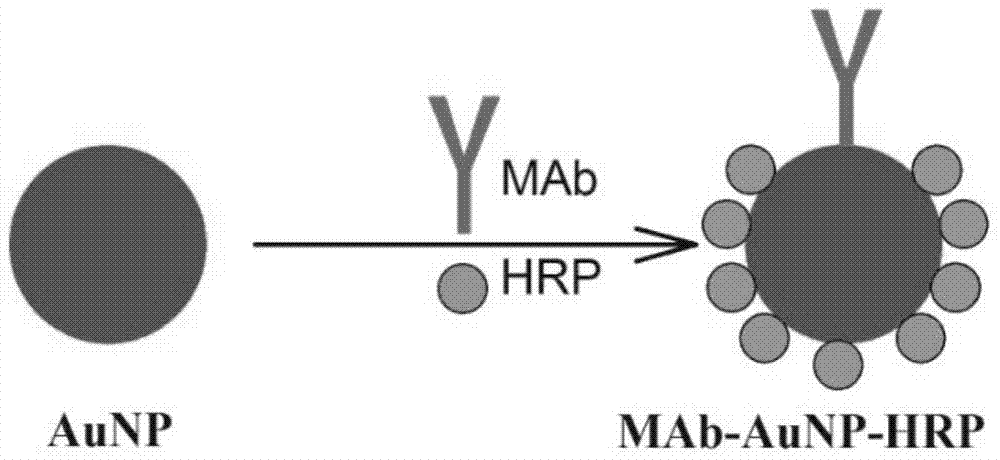
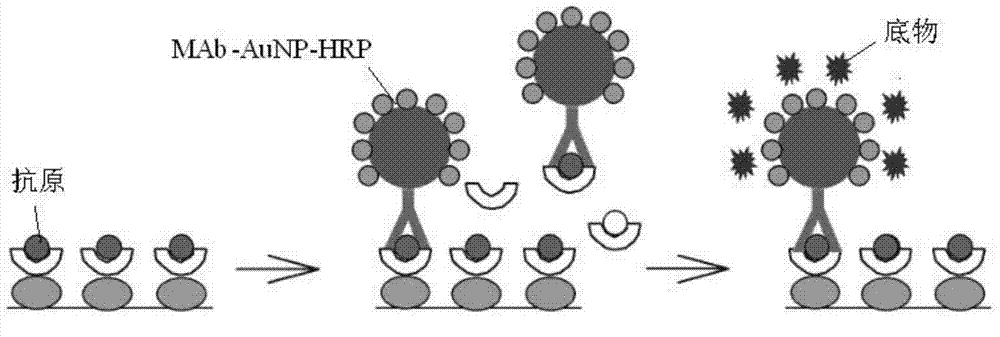
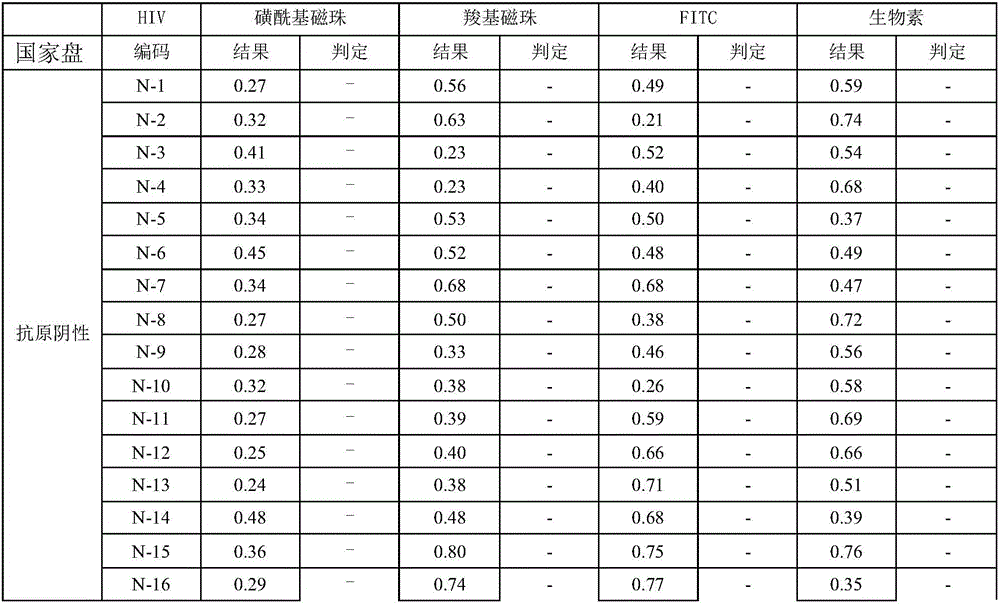
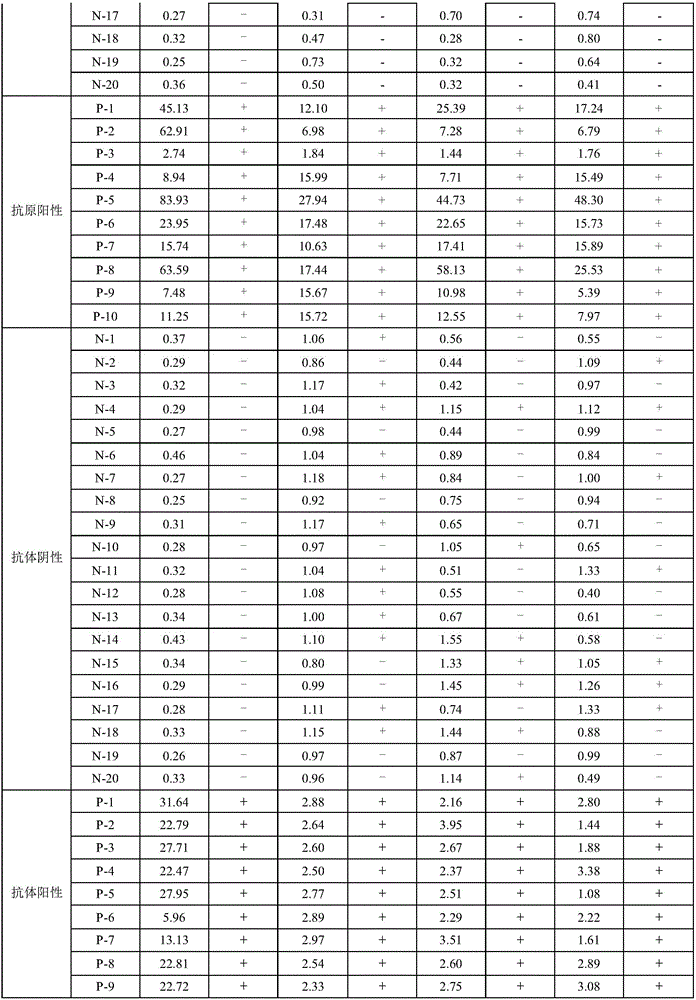
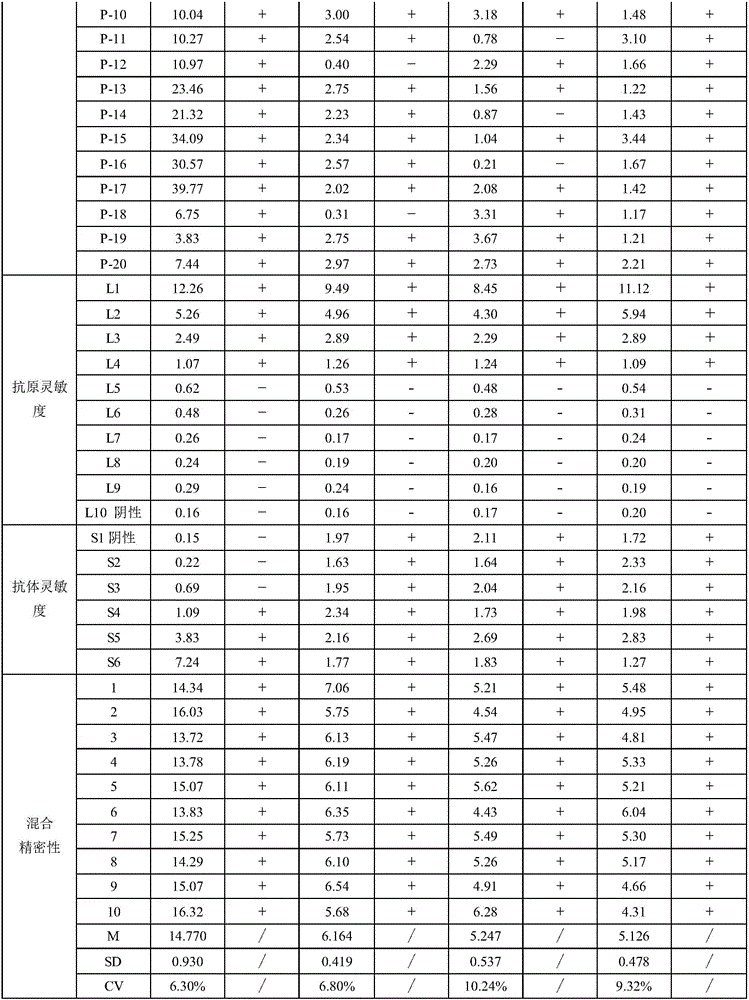
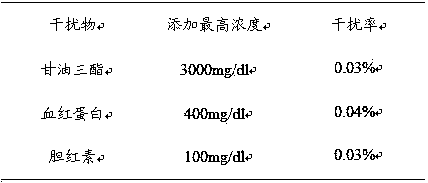
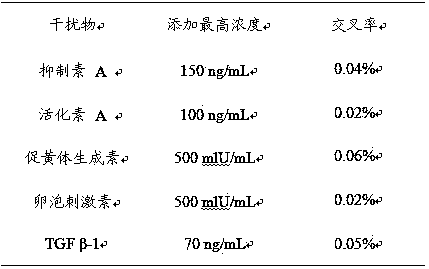
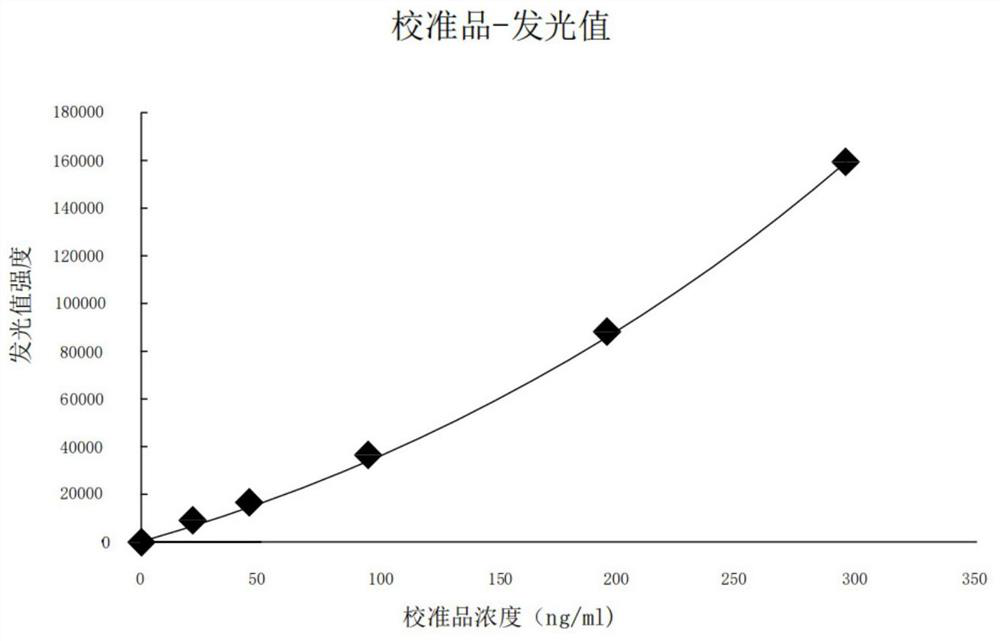
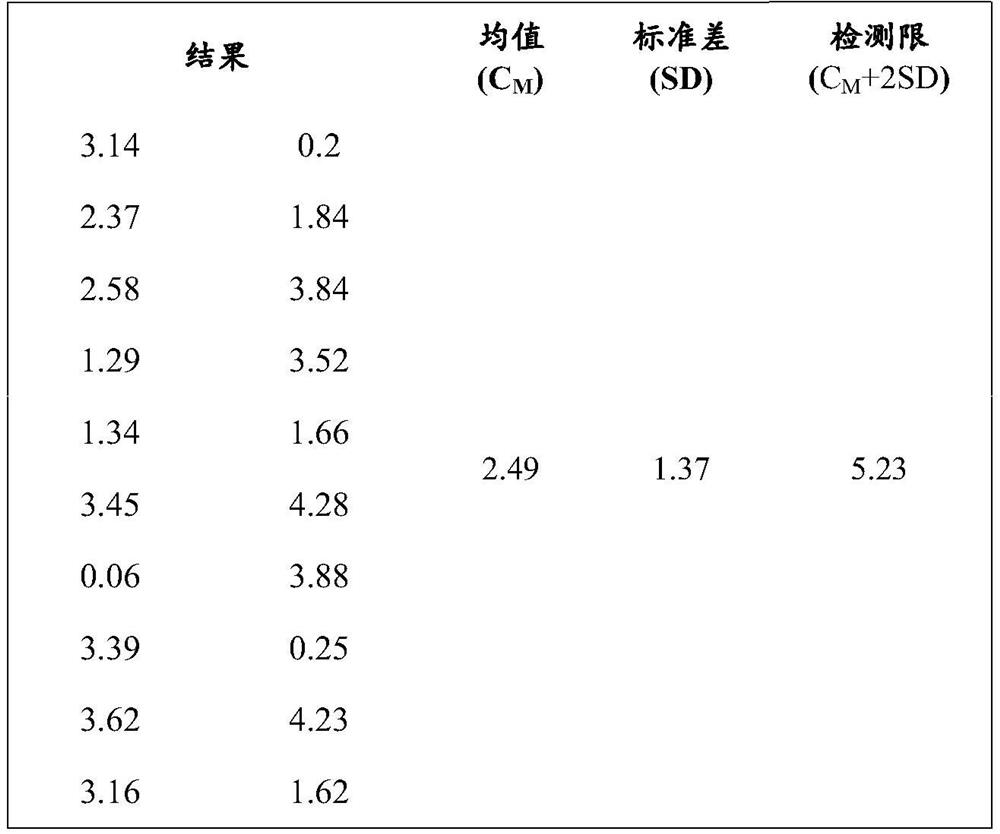
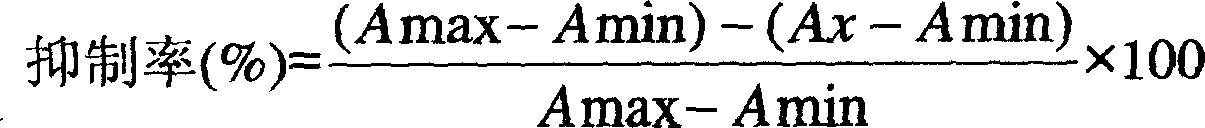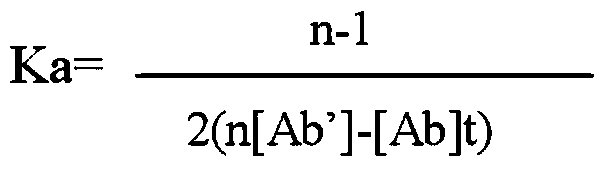Patents
Literature
54 results about "Enzyme immunoassay method" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
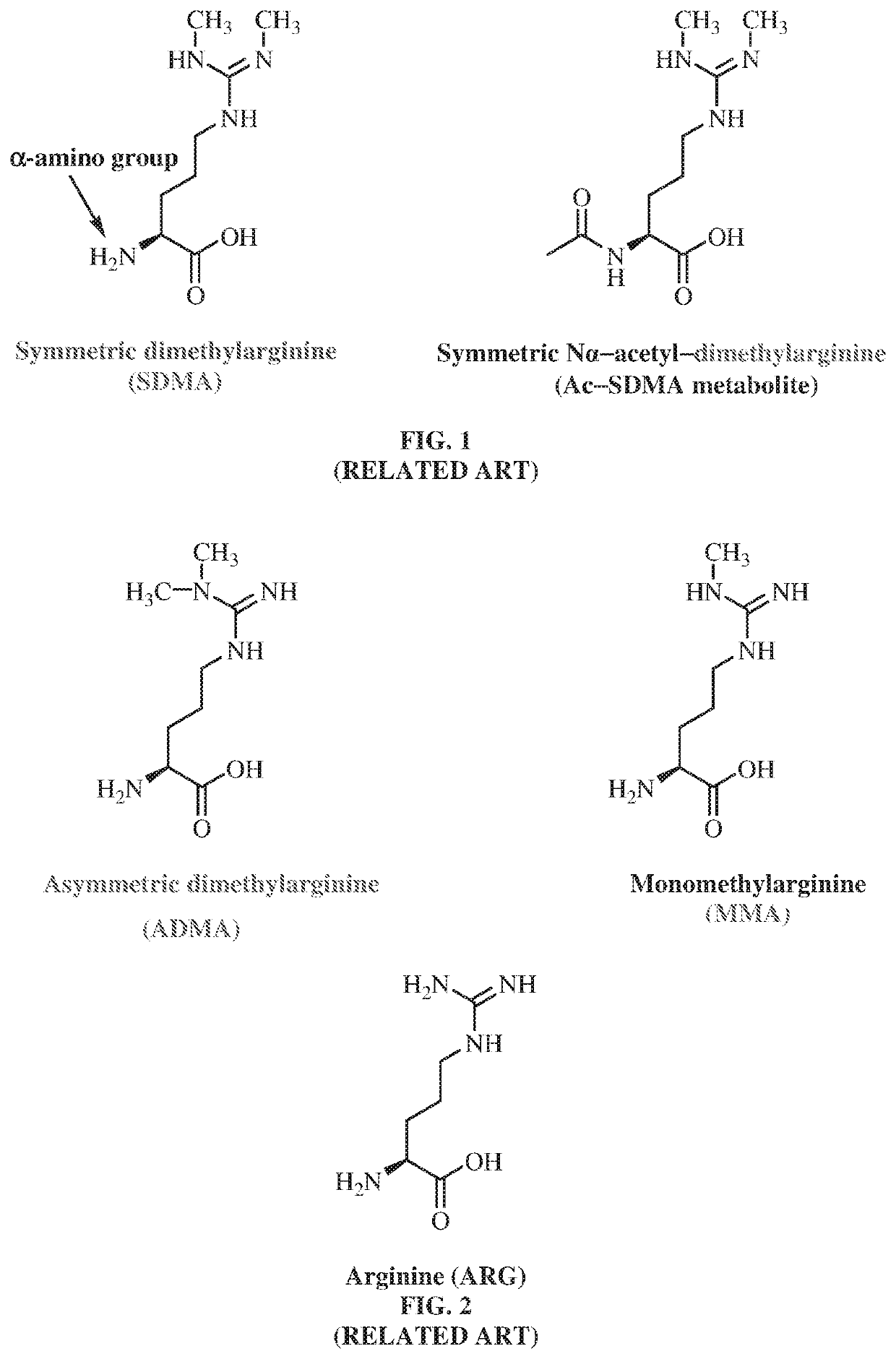
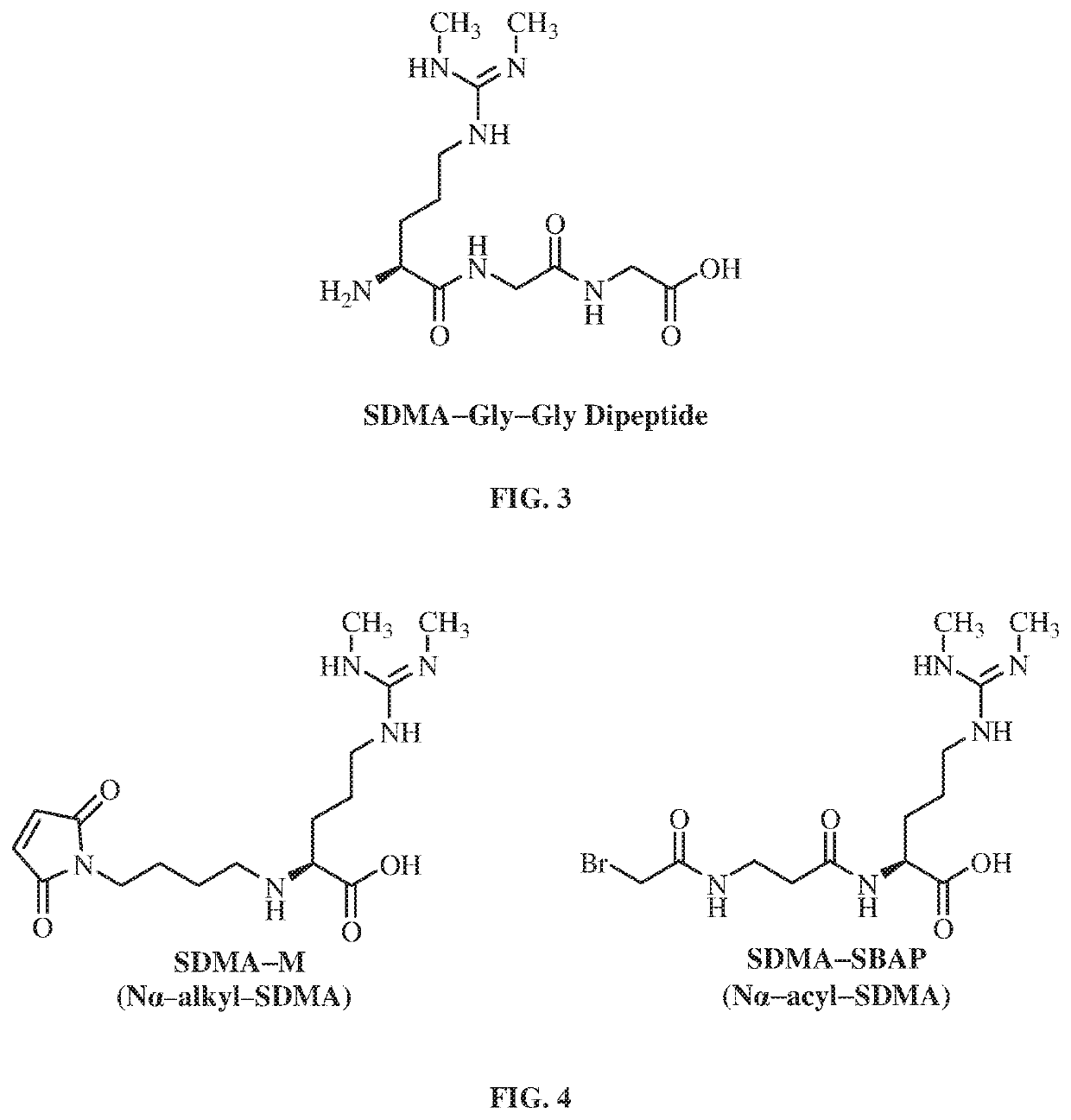
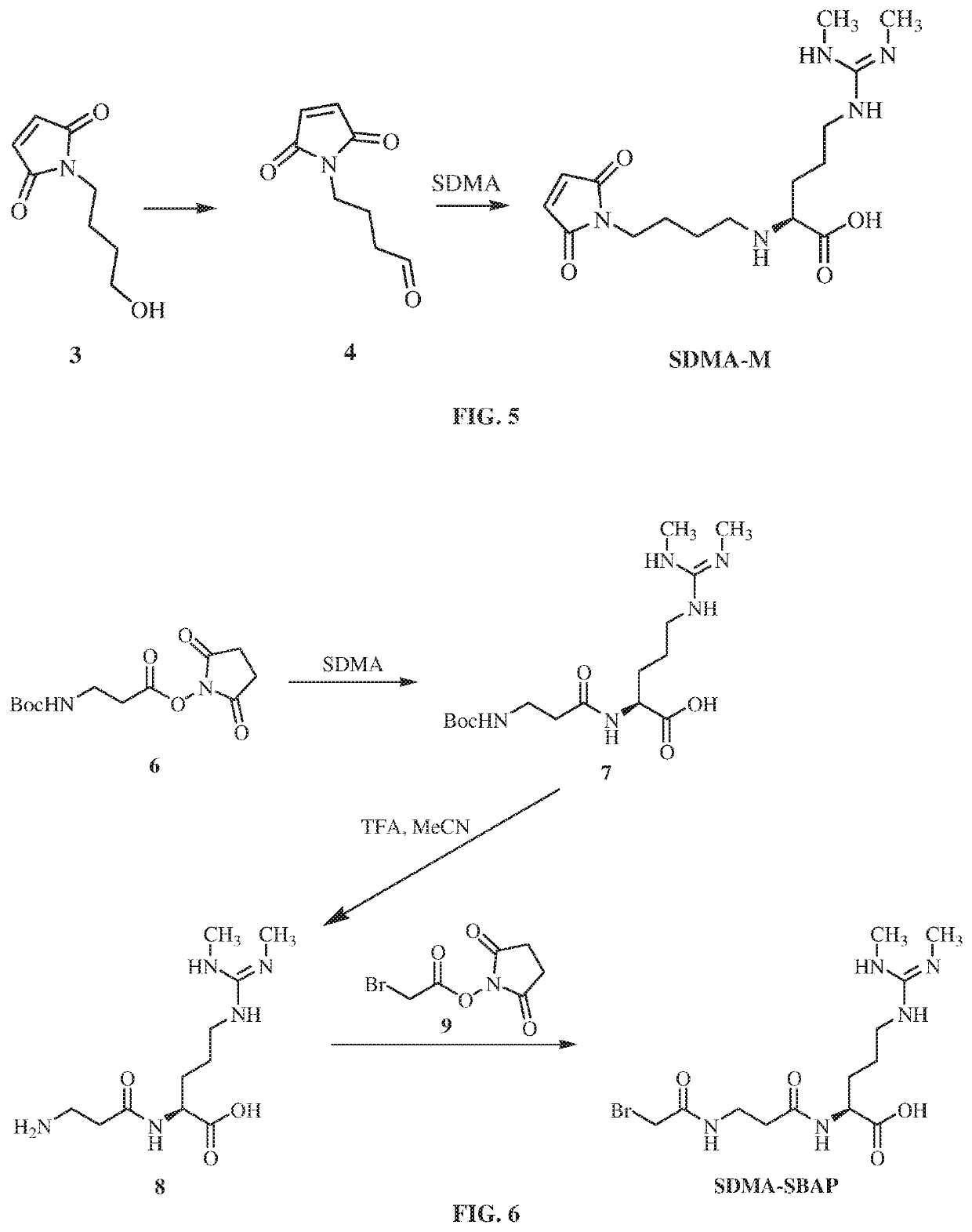
Enzyme immunoassay. A rapid enzyme immunochemical method for determining the presence of an antigen, antibody, or hapten in the blood. In EIA an antigen or antibody is bound to an enzyme, e.g., horseradish peroxidase or alkaline phosphatase.
Quantitative determination RBP4 kit by chemiluminescence magnetic enzymoimmune method
ActiveCN101452001AExtended storage timeStable LuminescenceChemiluminescene/bioluminescenceBiological testingImmunocompetenceMagnetic bead
The invention relates to a medical testing kit for performing quantitative detection on human serum RBP4 using chemiluminescence magnetic-enzyme immunotherapy. The kit is composed of four reagent parts: specificity mouse anti-human RBP4 custodite immunomagnetic beads, enzyme labelling specificity mouse anti-human RBP4 antibody II, chemiluminescence substrate, corresponding titer and quality control liquid. The using method of the kit comprises: using bead particulates as solid phase carrier, combining specificity mouse anti-human RBP4 antibody I on the surface, forming RBP4 specificity immunocompetence beads, capturing antigen RBP4 to be detected in the enzyme labelling specificity mouse anti-human RBP4 antibody II, forming double antibody sandwich composite on the surface of the beads, wherein enzyme marked on the composite reacts with corresponding irradiance substrate in the reaction system to form stable luminous signals, thereby reaching quantitative detection and analysis on RBP4 through strength of the detection light signals. The invention has the advantages of high sensitivity, high specificity, simple and fast operation.
Owner:WUHAN EASYDIAGNOSIS BIOMEDICINE
Hepatits B virus e antibody assay kit and assay method thereof
The invention relates to a diagnostic reagent for hepatits B, and discloses a hepatits B virus e antibody assay kit and an assay method thereof. The invention adopts light initiated chemiluminescence assay technology, and discloses the hepatits B virus e antibody assay kit, which comprises luminescent particles coated by anti-HBe antibody, anti-HBe antibody marked by biotin and neutralized e antigen. The invention also discloses a method for qualitatively or quantitatively assaying hepatits B e antibody by the light initiated chemiluminescence assay technology. The assay kit can be combined with other serum and clinical information for diagnosing infectious conditions of acute or chronic hepatits B of an individual, and also can be used for screening hepatits B of female in perinatal period to judge the hazard that neonates are infected with the hepatits B. Besides, the kit has the characteristics of high sensitivity, wide assay range and the like, and the assay method has higher sensitivity and better assay range than an enzyme-immunoassay method methodologically.
Owner:BEYOND DIAGNOSTICS (SHANGHAI) CO LTD
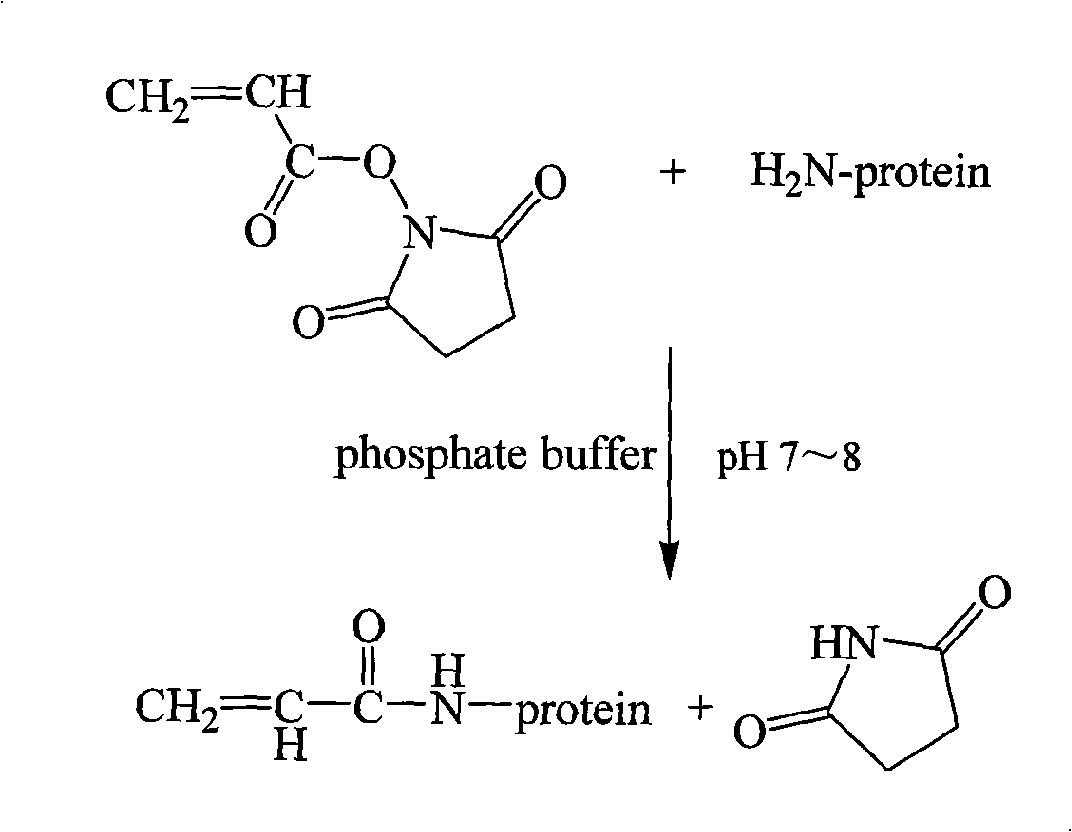
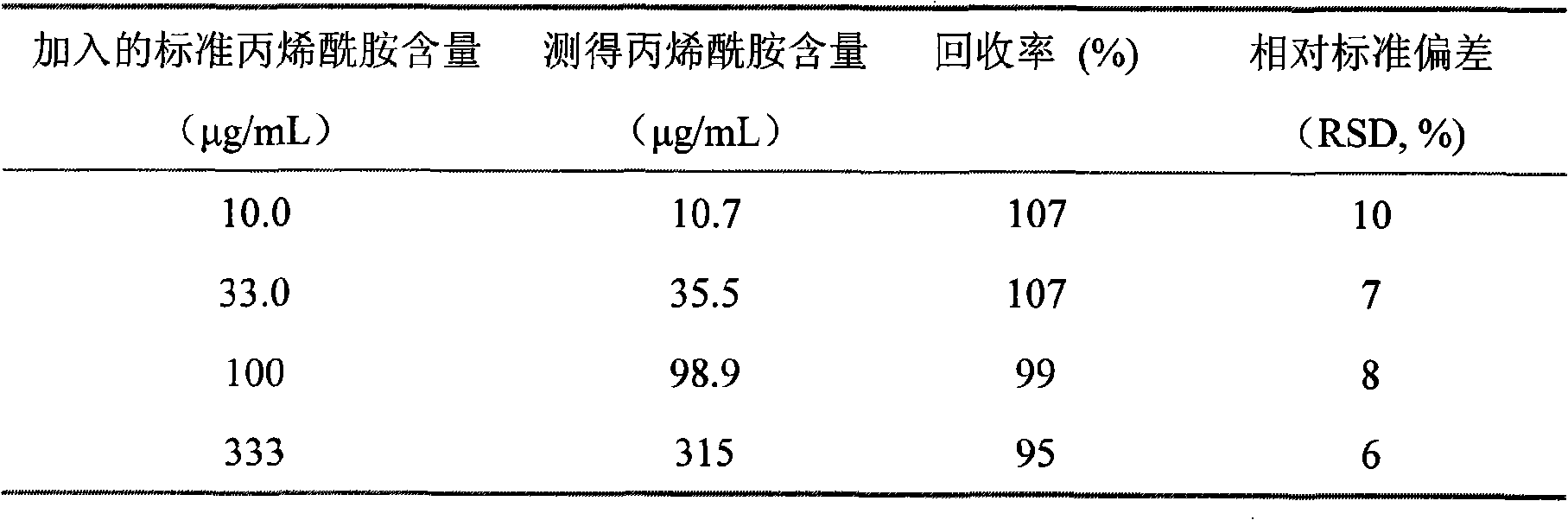
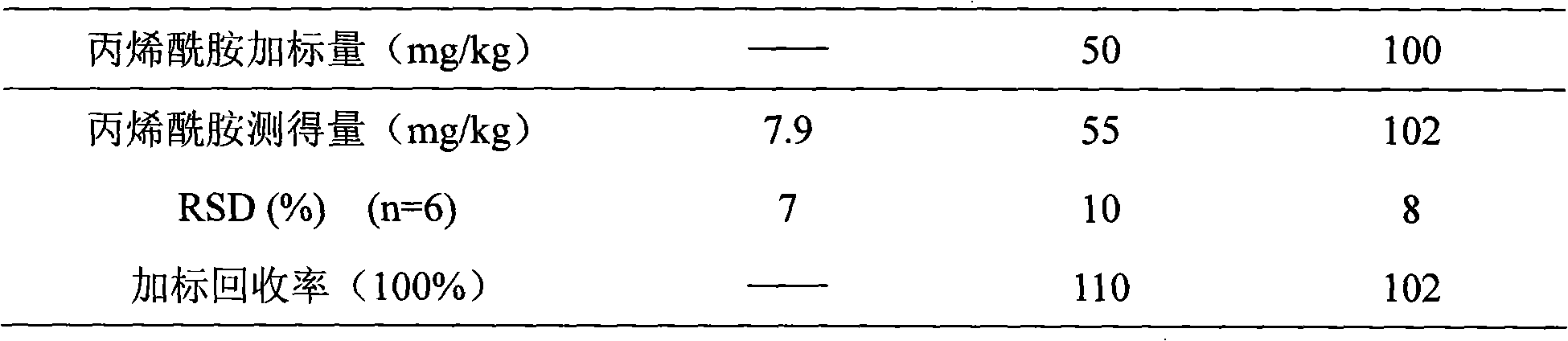
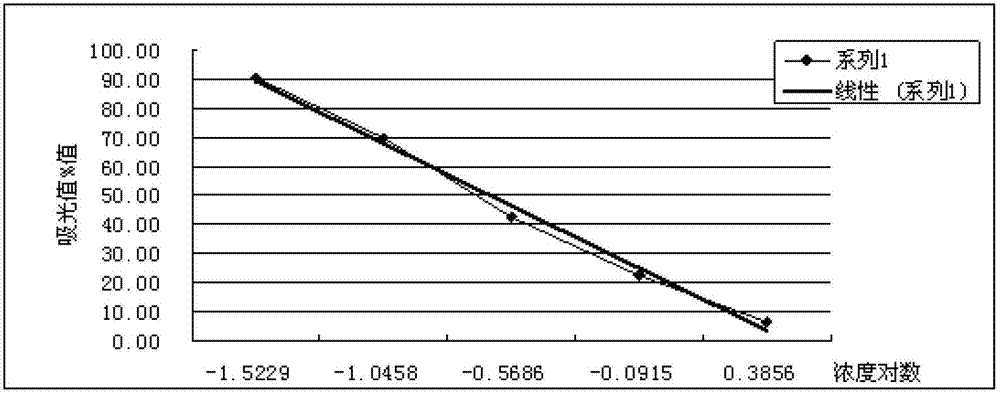
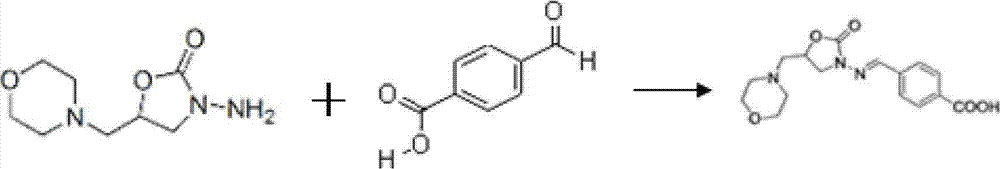
Acrylic amide complete antigen preparation and its ELISA quantitative determination method
The invention provides a method for preparing acrylamide complete antigen. The method is as follows: amine uncoupling reaction is directly carried out between N-acryloyloxy succinimide and the surface amino group of carrier protein molecule in a neutral or weakly alkaline phosphate buffer solution, thereby forming acrylamide complete antigen. Polyclonal antibody capable of realizing specificity identification of acrylamide is obtained through a complete antigen immune animal, so as to establish an enzyme immunoassay method suitable for measuring the content of acrylamide in an aqueous solution or food and to provide a corresponding reagent box. The method has simple operation and does not need other reaction reagent, thereby furthest maintaining the structural integrity of acrylamide; moreover, the method ensures that a coupling position is at a maximum distance from a feature group so as to fully expose the feature structure of acrylamide molecule. The established enzyme immunoassay method of acrylamide and the reagent box have strong specificity, high sensitivity and convenient operation, and are suitable to realize massive sample detection which has low cost and is quick and simple.
Owner:PEKING UNIV
Monoclonal antibody, enzyme-linked immunosorbent assay method and kit for detecting furaltadone residue marker AMOZ
ActiveCN102766213AImprove detection efficiencyHigh precisionMicroorganism based processesTissue cultureAbzymeFuran
The invention discloses a monoclonal antibody capable of identifying a furaltadone residue marker 3-amino-5-morpholinomethyl-2-oxazolidinone (AMOZ). The monoclonal antibody is secreted by a hybridoma cell AMOZ; and the hybridoma is preserved in China Center for Type Culture Collection, and has a preservation number of CCTCC NO:C201188. The invention also discloses an enzyme immunoassay method and a reagent kit for detecting furaltadone marker residue marker AMOZ, and application thereof to detection of the furanketone residue marker AMOZ. Compared with prior art, the monoclonal antibody prepared by the invention, the ELISA Kit and the ELISA method have high detection sensitivity, high precision and good accuracy; a hapten synthesis process is simple, and has high synthesis efficiency; and a derivatization reagent for sample pretreatment is benzene formaldehyde, which has advantage of small toxicity compared with other commonly used derivative reagent like o-nitrobenzaldehyde.
Owner:WUHAN SHANGCHENG BIOTECH
Non-Liquid Phase Type Chemiluminescent Enzyme Immunoassay Method and Assay Kit
InactiveUS20090142781A1Small sizeLow costChemiluminescene/bioluminescenceImmune complex depositionEnzyme immunoassays
A chemiluminescent enzyme immunoassay method whereby a target substance such as a protein is assayed. This chemiluminescent enzyme immunoassay method comprises: the step of capturing an immune complex containing an enzyme-labeled antibody, which is labeled with an enzyme acting a chemiluminescent substrate, and the target substance on a support having no solution layer; the step of overlaying a support membrane containing the chemiluminescent substrate on the immune complex having been captured above; and the step of measuring the luminescence dose caused by the reaction between the enzyme-labeled antibody and the chemiluminescent substrate to thereby quantify the target substance. Since a highly sensitive chemiluminescent enzyme immunoassay is conducted by using a non-liquid phase type reaction system in the chemiluminescent enzyme immunoassay method as described above, multiple items can be assayed by using only a small amount of a specimen and, furthermore, the target substance can be assayed at a high sensitivity thereby without resorting to any troublesome procedures such as pipetting a reagent.
Owner:HOKKAIDO UNIVERSITY +2
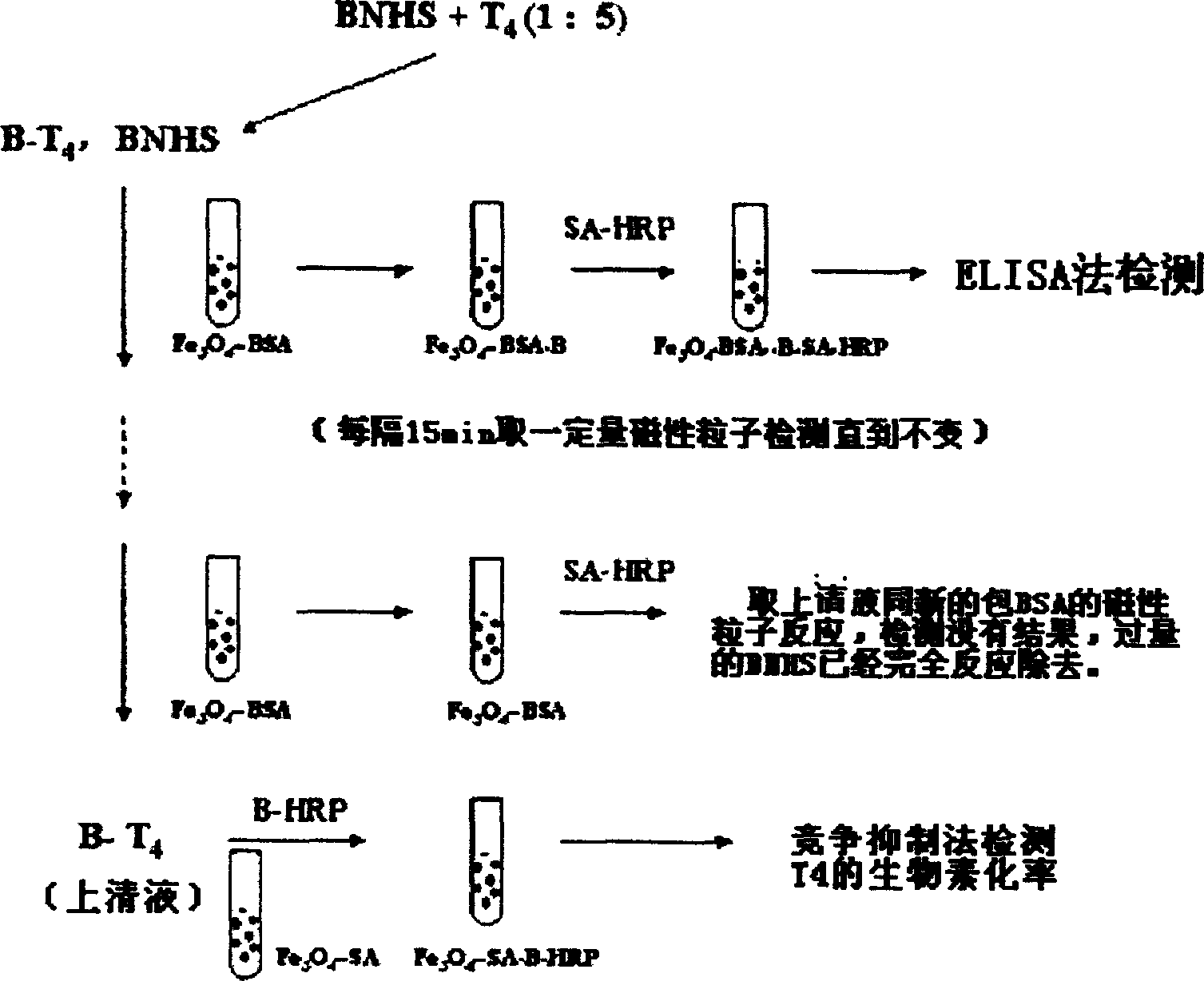
Quick magnetic method for separating and purifying thyroxin marked-by biotin
InactiveCN1540348AHigh purityThe separation effect is the samePeptide preparation methodsMaterial analysisBiotinDegree of reaction
After reaction between bovine serum albumin (bsa) on magnetic particles and biotin label of thyroxin, superfluous biotin reagent in mixed liquor and n-hydroxyl succimide ester generates compound of magnetic particle-bsa-biotin. Under action of external magnetic field, superfluous biotin reagent is removed. Degree of reaction between n-hydroxyl succimide ester and magnetic particles is tested by enzyme immunoassay method. It is determined that final obtained solution is high-purified thyroxin labeled by biotin. Advantages of the invention are simple, quick and high efficiency. The invention is also applicable for separating and purifying biomoleculars of protein, polypeptide, oligonucleotide etc. labeled by biotin.
Owner:SHANGHAI JIAO TONG UNIV
Reagent for determining content of human cholyglycine by using latex immunoturbidimetry technology
InactiveCN108982860AImprove detection accuracyEliminate distractionsMaterial analysisSerum igePhosphate
The invention discloses a reagent for determining the content of human cholyglycine by using a latex immunoturbidimetry technology. The reagent is prepared from a reagent body 1 and a reagent body 2,wherein the reagent body 1 is prepared by adding 100 mM of an auxiliary agent, a phosphate buffer solution with the pH value of 8.0, a sodium chloride solution with the concentration of 0.9%, BSA withthe concentration of 0.1% and a stabilizer into a latex microsphere-BSA-cholyglycine conjugate with the concentration of 0.04%, and the reagent body 2 is prepared by adding 15 mM of an auxiliary agent, a phosphate buffer solution with the pH value of 7.4, a sodium chloride solution with the concentration of 0.9%, BSA with the concentration of 0.1%, a surfactant with the concentration of 0.1% anda stabilizer into an anti-mouse cholyglycine monoclonal antibody with the concentration of 5%. According to the reagent, latex microspheres are introduced into the reagent, due to the existence of thelatex microspheres, the sensitivity of the detection reagent is greatly improved, and the requirements of clinical use are met. Compared with homogeneous enzyme immunoassay, the reagent has great advantages in stability, under the acceleration condition, and the stability time of the reagent is at least 2 times or above of the stability time of a homogeneous enzyme immunoassay reagent.
Owner:北京安图生物工程有限公司
Enzyme-linked immune analytic method for detecting carbofuran
InactiveCN1987469AWeakened color reactionHigh inhibition rateMaterial analysis by observing effect on chemical indicatorBiological testingCarbofuranMonoclonal antibody
Enzyme immunoassay method for detecting Kebaiwei includes (1) synthesizing half antigen and artificial antigen; (2) preparing polyclonal antibody, monoclonal antibody, or gene engineering antibody of possessing specificity to Kebaiwei; (3) preparing enzyme labeled antigen; (4) using second antibody to coat enzyme labeled plate and close the plate to save Kebaiwei; reaction is carried out between anti Kebaiwei antibody and second antibody and fixed on the enzyme labeled plate; (5) washing out free object, adding sample to be tested, and enzyme labeled antigen or Kebaiwei labeled sample, and enzyme labeled antigen; (6) washing out free object, adding substrate of enzyme and developer. Intensity of enzyme promoting color reaction is proportional to quantity of enzyme labeled antigen bound on antibody, and is inversely proportional to content of Kebaiwei in sample. Features are: long term storage, increasing sensitivity, accuracy, simple and quick test operation.
Owner:SOUTH CHINA AGRI UNIV
Immune colloidal gold chromatography method for detecting food allergy to aquatic products
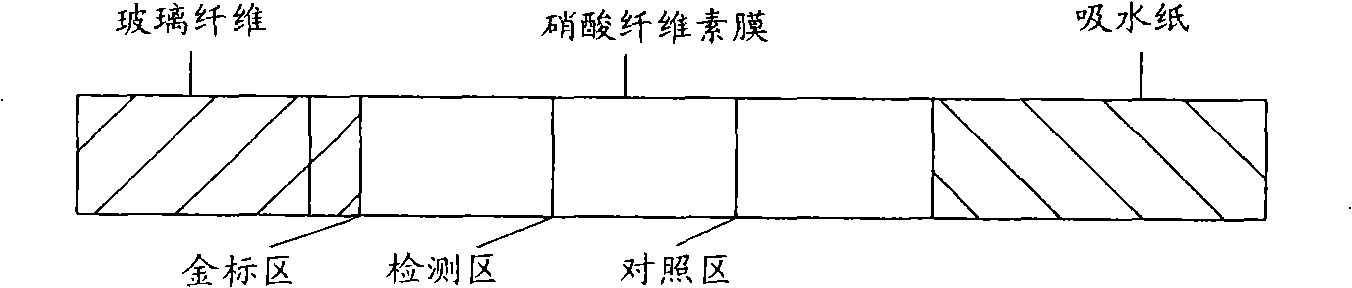
The present invention discloses a gold-immunochromatography assay method which is used for detecting the aquatic product food allergy. The method comprises preparing colloidal gold, labeling protein with the colloidal gold, detecting with the gold-immunochromatography assay, and other steps. The invention adopts the gold-immunochromatography assay technology to detect the aquatic product food allergy; the technology not only has the advantages of high specificity and high sensitivity of the enzyme immunoassay, but also has the advantages of rapid and simple detection of the prick test; and the technology can rapidly detect whether the food has aquatic product allergens or aquatic product allergen antibodies.
Owner:JIMEI UNIV
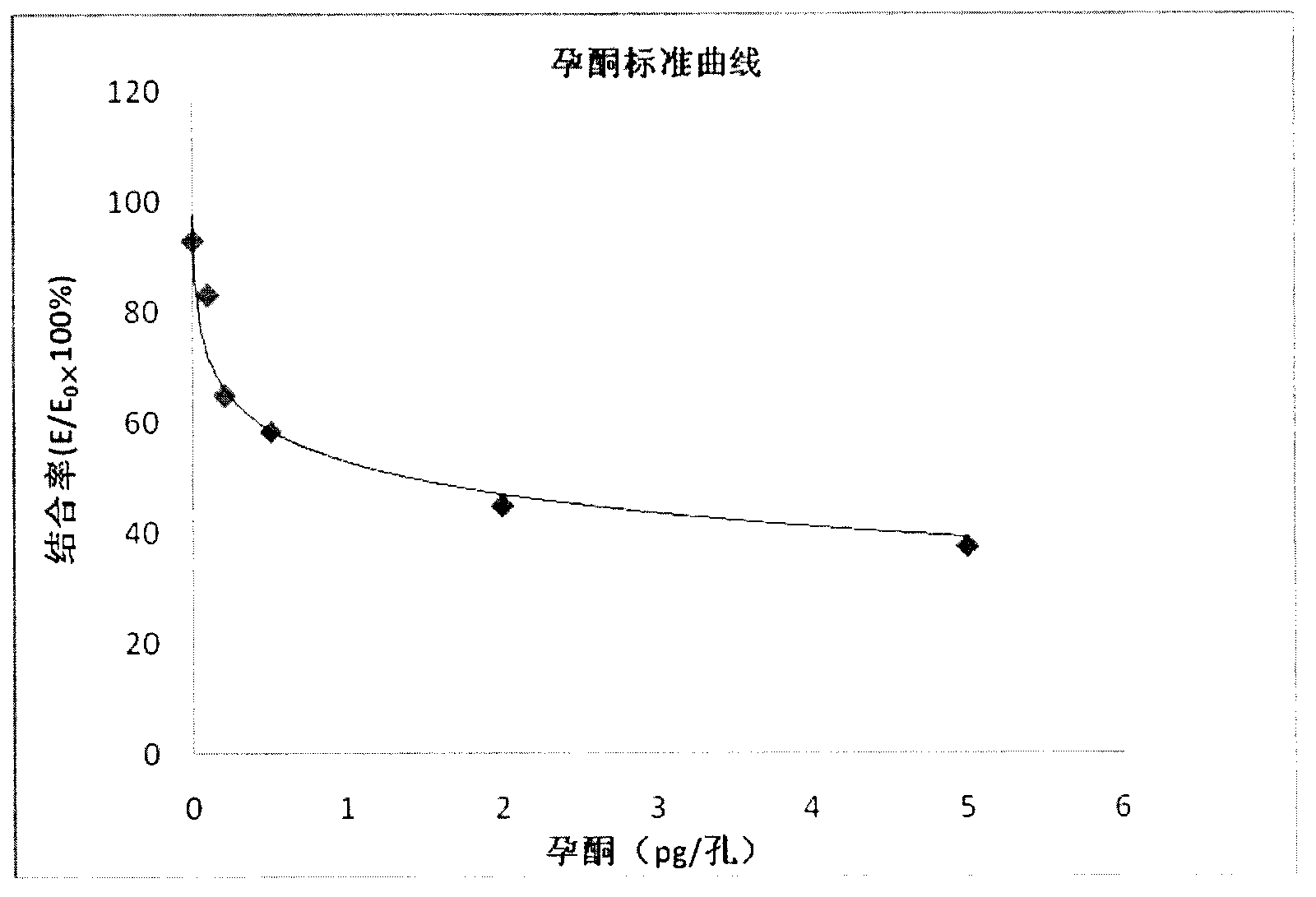
Enzyme immunoassay method for progestational hormone in excrement of sows and method for detecting oestrous cycle of sow
InactiveCN103175960ANo harmSimple equipment requirementsColor/spectral properties measurementsBiological testingEstriolFeces
The invention discloses an enzyme immunoassay method for progestational hormone in excrement of sows. The method comprises the steps of: (1) sample collection: collecting the excrement of the sows, weighing and dissolving 0.5 g of excrement of the sows into 20 ml of measuring buffer liquid, carrying out centrifuging, taking and placing supernatant at 4 DEG C to be detected; and (2) measuring content of progestational hormone in a sample by utilizing competitive ELISA (enzyme-linked immuno sorbent assay): measuring according to an ELISA process. According to the enzyme immunoassay method for the progestational hormone in the excrement of the sows, the detection sensitivity of progesterone reaches 0.2 ng / ml or 8 ng / g; the recovery rate of the progesterone is 95.78%; inter-assay and intra-assay variable coefficients are respectively 6.8% (n is equal to 9) and 11.23% (n is equal to 8); the specificity of the progesterone is that the cross reaction rates of the progesterone to estradiol, estriol and oestrone are all less than 0.01%, and the cross reaction rate of the progesterone to testosterone is 0.35%; the stability is good; the required sample is easy to collect, animals are not injured, a measured result is reliable, and a method for measuring blood of the animals can be replaced to monitor a series of reproductive activities of the animals.
Owner:NANJING AGRICULTURAL UNIVERSITY
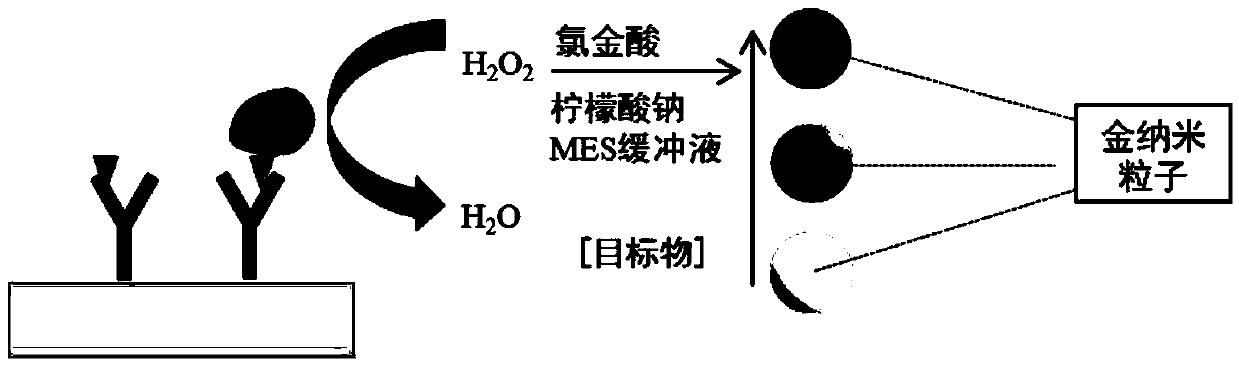
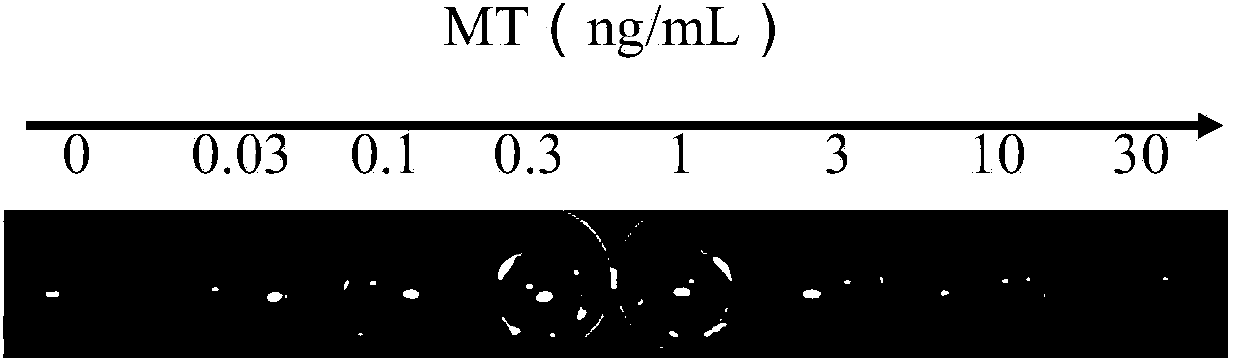
Direct competitive enzyme immunoassay method based on nano-gold plasma excimer
ActiveCN104049083ALow costEasy to operateColor/spectral properties measurementsImmune profilingAntiendomysial antibodies
The invention discloses a direct competitive enzyme immunoassay method based on a nano-gold plasma excimer, and immunoassay visible detection on small-molecule compounds can be realized. The direct competitive enzyme immunoassay method comprises the steps of competitively combining a test sample possibly containing the small-molecule compounds and haptens labeled with catalase with small-molecule compound antibodies covering a solid phase, decomposing hydrogen peroxide through the combined enzyme-labeled antigens, reducing chloroauric acid into nano-gold through the hydrogen peroxide under a certain buffering condition, and detecting the small-molecule compounds in the sample by observing the color or the light absorption intensity of a developing liquid. The method disclosed by the invention has the advantages of high sensitivity, simplicity in operation, low cost, visible result distinguishing and the like, and the shortcomings of the conventional small-molecule compound enzyme-linked immunoassay detection technology are overcome.
Owner:JIANGNAN UNIV
Synthesis methods of sunset yellow hapten and artificial antigen
The invention relates to synthesis methods of a sunset yellow hapten and an artificial antigen, and particularly discloses synthesis methods of the sunset yellow hapten and an antigen thereof, belonging to the technical fields of organic chemistry and immunochemistry. A disodium salt of sunset yellow is converted to sulfonyl chloride, then sulfonyl chloride reacts with different amino-substituted carboxylic ester hydrochlorides, and hydrolysis is carried out to get rid of methyl ester protection so as to obtain the sunset yellow hapten. Then the carbodiimide method is adopted to couple the sunset yellow hapten with carrier protein bovine serum albumin (BSA) to obtain the complete antigen of sunset yellow, which can be used to prepare the corresponding antibody and can provide a technical foundation for the establishment of the relevant enzyme immunoassay method.
Owner:江苏南通维立科化工有限公司 +1
Enzyme immunoassay method for heavy metal chromium detection and enzyme immunoassay detection reagent kit
The invention provides an enzyme immunoassay method for heavy metal chromium detection and an enzyme immunoassay detection reagent kit. Colloidal gold particles are coupled with horse radish peroxidase and anti-chromium monoclonal antibodies for forming enzyme marker compounds, and a detection method is built; through preprocessing chromium in samples and detecting original competitive enzyme-linked labelled antibody compounds, the detection on the chromium content in the samples to be tested is realized through chromogenic reaction amplification signals. The enzyme immunoassay method for chromium detection belongs to a one-step process competitive enzyme-linked immunoassay method, and belongs to a simple convenient fast and high-flux detection method.
Owner:深圳市三方圆生物科技股份有限公司
Human immunodeficiency virus antigen and antibody determination kit and preparation method
InactiveCN106290864AMinimal loss of antigenic activityWide linear rangeChemiluminescene/bioluminescenceAntigenParticulates
The invention discloses a human immunodeficiency virus antigen and antibody determination kit obtained through a magnetism particulate immuno chemistry luminescence method. The kit comprises a magnetic separation reagent, a conjugate reagent, a positive reference substance, a negative reference substance and a calibration solution; the magnetic separation reagent is prepared by combining magnetic particles with an envelope antigen and an envelope antibody, and the conjugate reagent is prepared by coupling a detection antigen and a detection antibody with alkaline phosphatase, wherein both the envelope antigen and the detection antigen are recombinant antigens, and both the envelope antibody and the detection antibody are mouse monoclonal antibodies; the calibration solution contains a to-be-detected antigen and a to-be-detected antibody; the magnetic particles have the hydrophobic surfaces and are modified by sulfonyl groups and combined with the antigen or antibody through an adsorption effect. The kit not only has the advantage that little activity of the antigen activity is lost through an envelope method, but also has the advantages that the magnetic particles achieve chemiluminescence and are easy to wash and wide in linear range; the advantages of little antigen loss and short reaction time and the advantages of being easy to wash and wide in linear range are combined for the first time, an inspection result can be consistent with that obtained through an enzyme-immunoassay method, and the reaction time is obviously shortened.
Owner:JIANGSU ZECEN BIOTECH CO LTD
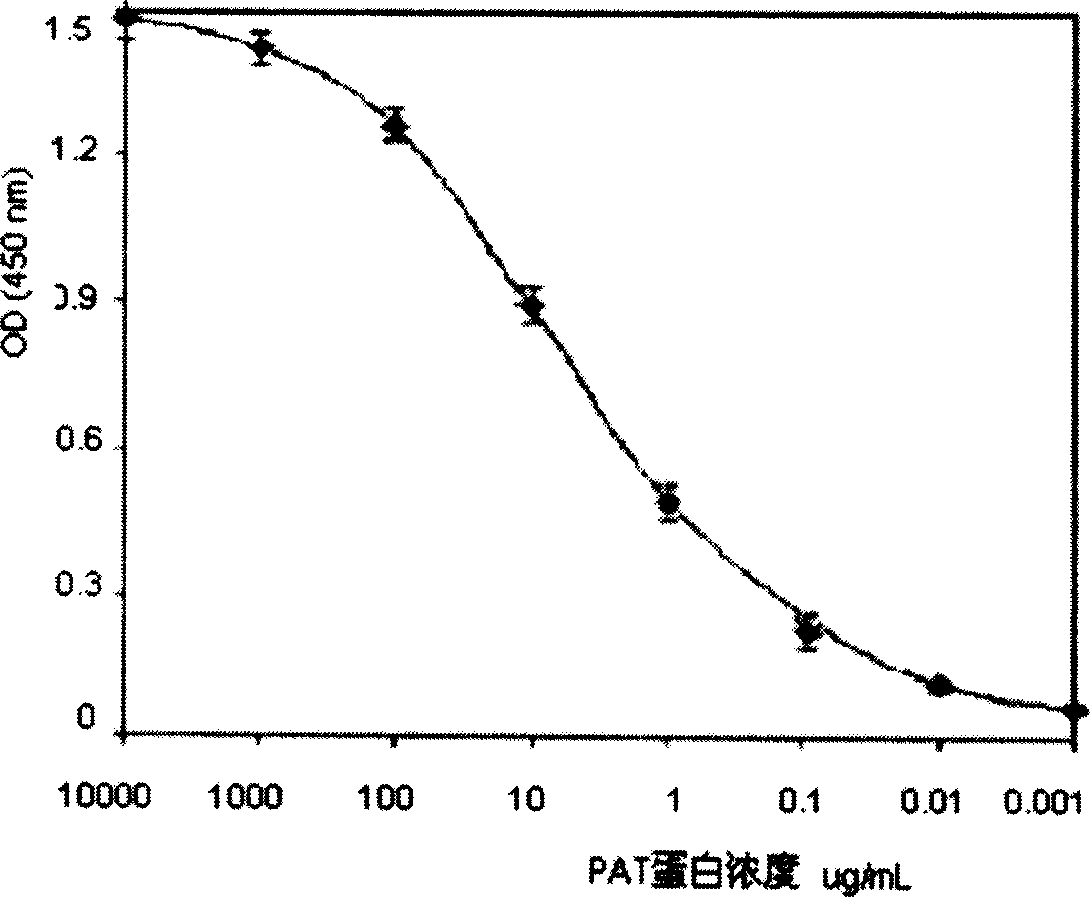
Affinity chromatography-enzyme immunoassay method for PAT protein, and dedicated kit
InactiveCN1696698AAchieving Specific DetectionAddress the need for quantitative testingColor/spectral properties measurementsPolyclonal antibodiesImmuno detection
A detection method includes using PAT protein as antigen to immunize animal for obtaining specific poly antibody, cross - linking the antibody on agarose gel to prepare immuiaffinity chromatographic column, using affinity column to enrich PAT protein after detected sample is processed, collecting affinity column eluent and carrying out enzyme - linked immunosorbant assay for confirming PAT protein content in it. The kit of the method is also disclosed.
Owner:CHINA AGRI UNIV
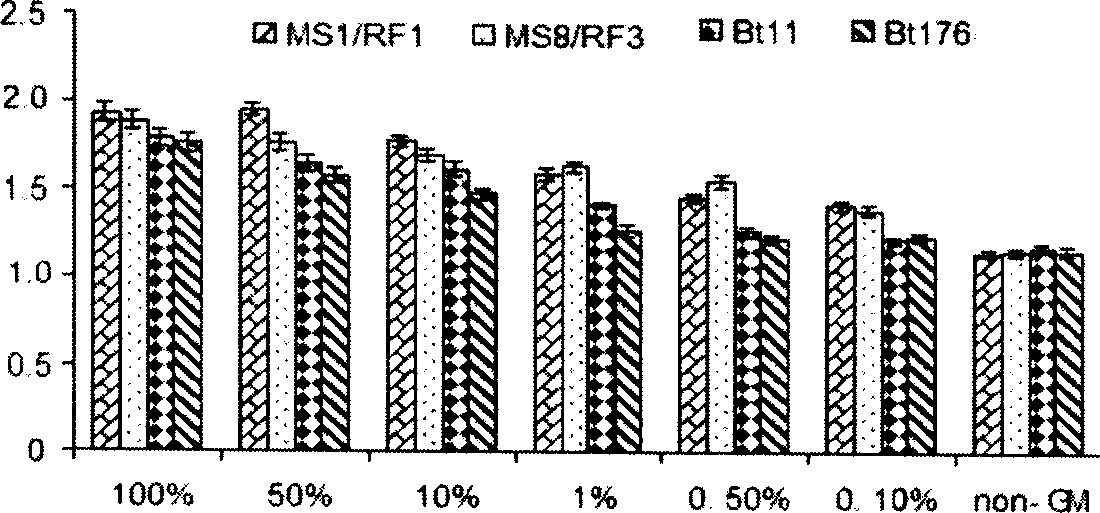
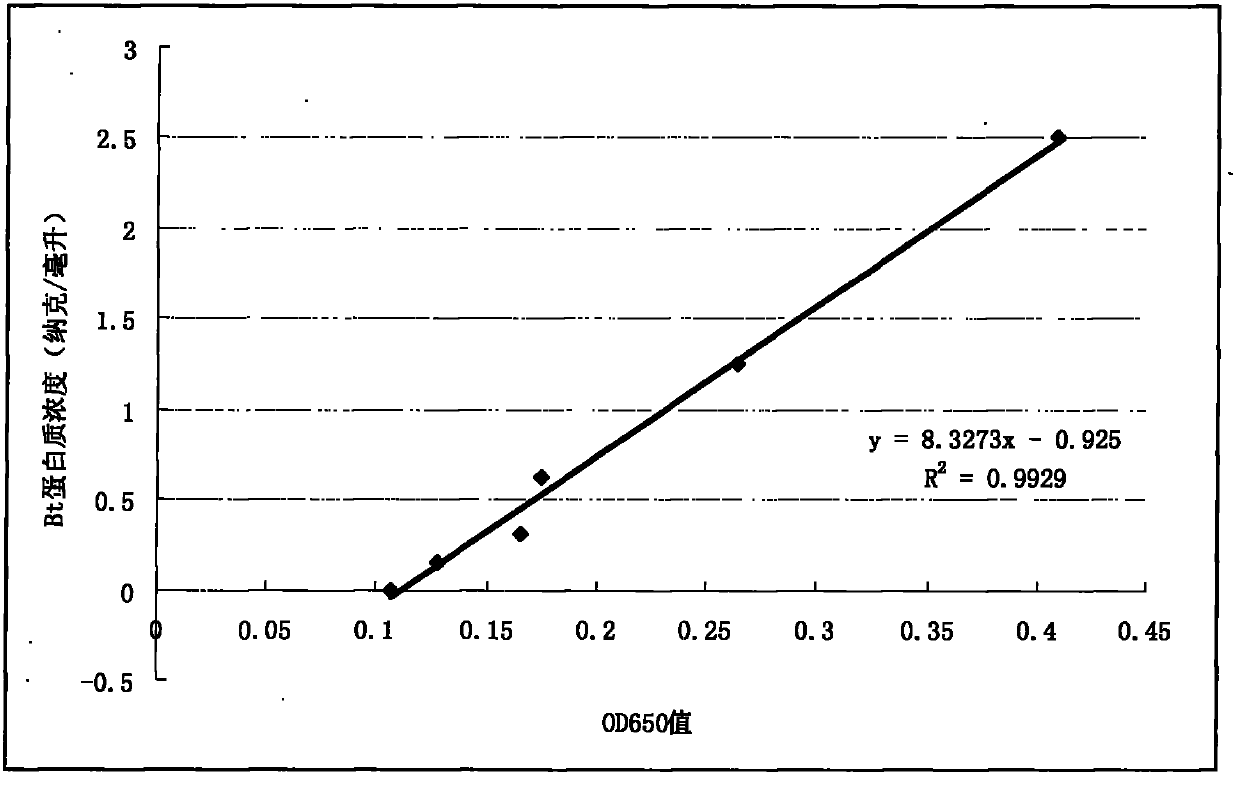
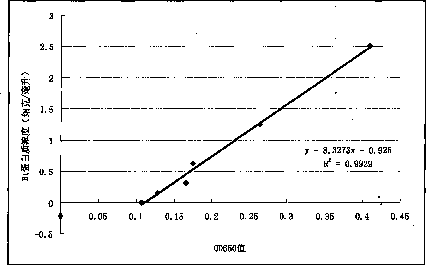
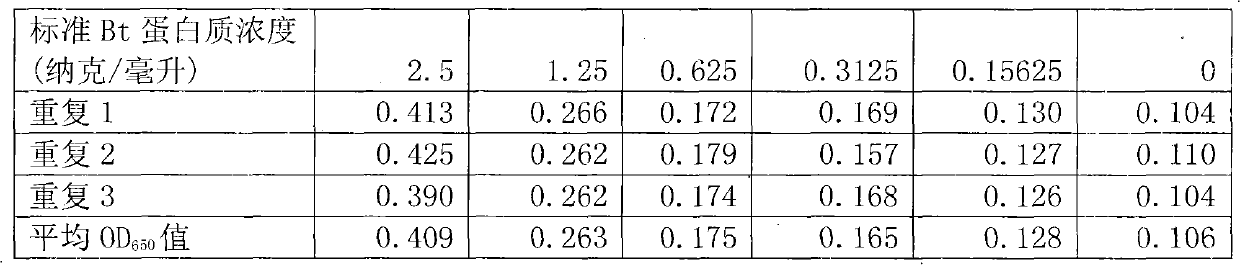
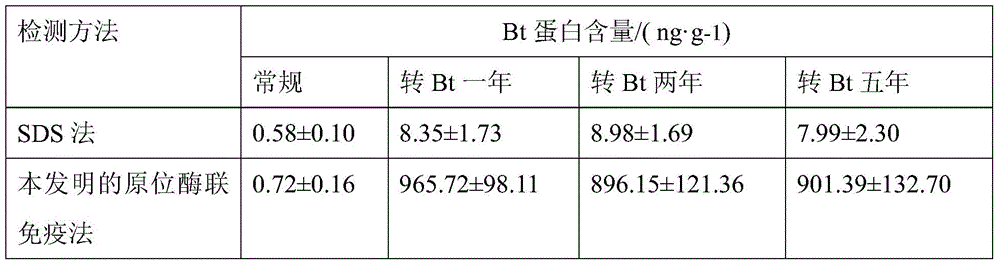
Method for testing trace Bt (bacillus thuringiensis) toxic protein in bodies of insects in transgenic Bt crop habitat
InactiveCN102590523AHigh sensitivityImprove linearityBiological testingBacillus thuringiensisToxic proteins
A method for testing trace Bt toxic protein in the bodies of insects in a transgenic Bt crop habitat includes the following steps: in the first step, collecting insects to be tested growing in a transgenic Bt crop field, and preparing insect liquid to be tested; in the second step, collecting insects growing on non-transgenic Bt plants in a lab, which belong to the same species or variety as the insects to be tested, and preparing contrast insect liquid; in the third step, using the contrast insect liquid to prepare standard Bt toxic proteins with a series of concentrations; in the fourth step, utilizing an enzyme immunoassay method to determine the optical density values of the standard Bt toxic proteins and the insect liquid to be tested at the wavelength of 650 nanometers; in the fifth step, obtaining a standard equation according to the concentrations of the standard Bt toxic proteins and the corresponding optical density values, and calculating the Bt toxic protein concentration in the insect liquid to be tested according to the standard equation. The test method has extremely high sensitivity and good repeatability.
Owner:宋敦伦
One-step chemiluminescence/enzyme immunoassay method and kit for detecting residue of malachite green
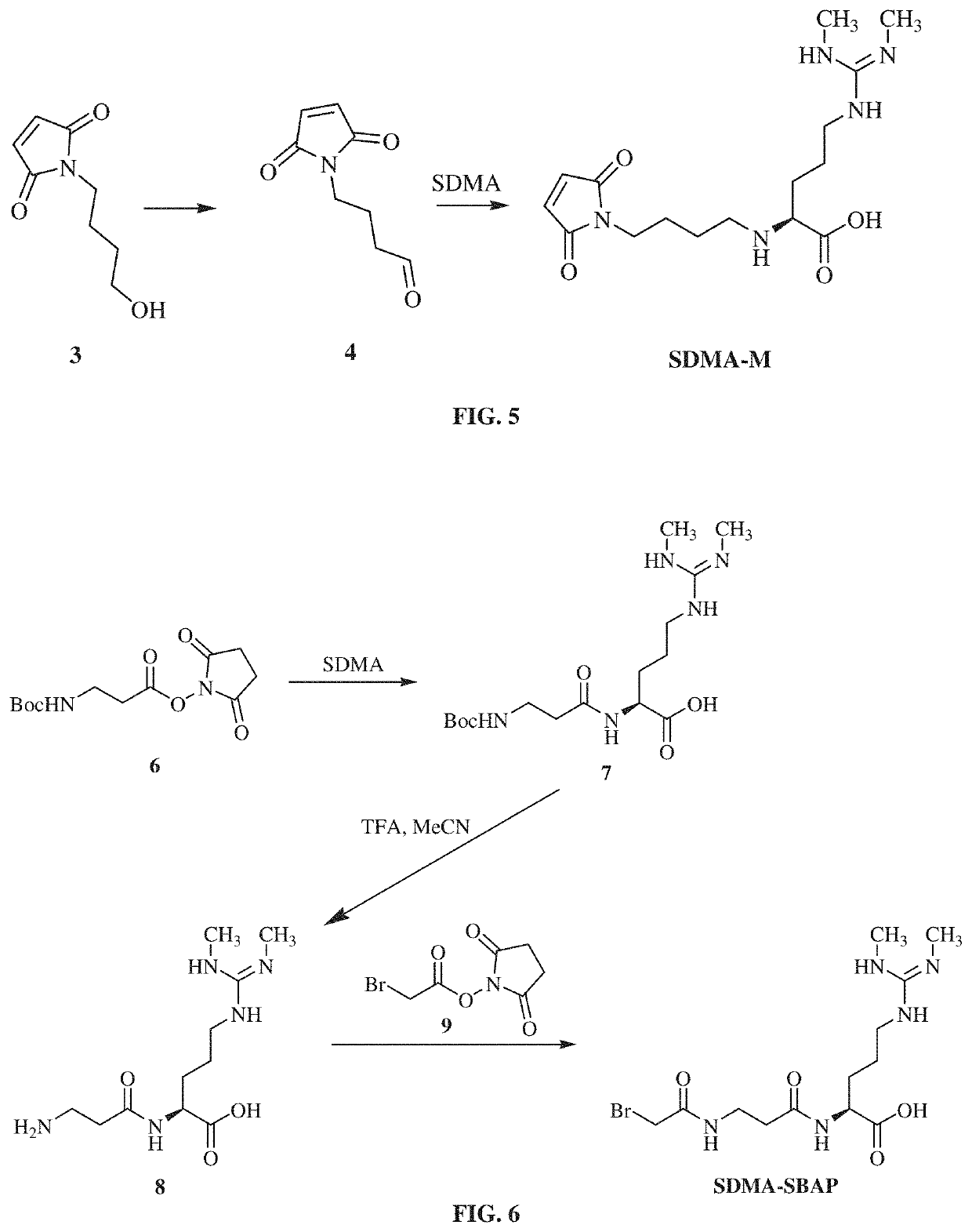
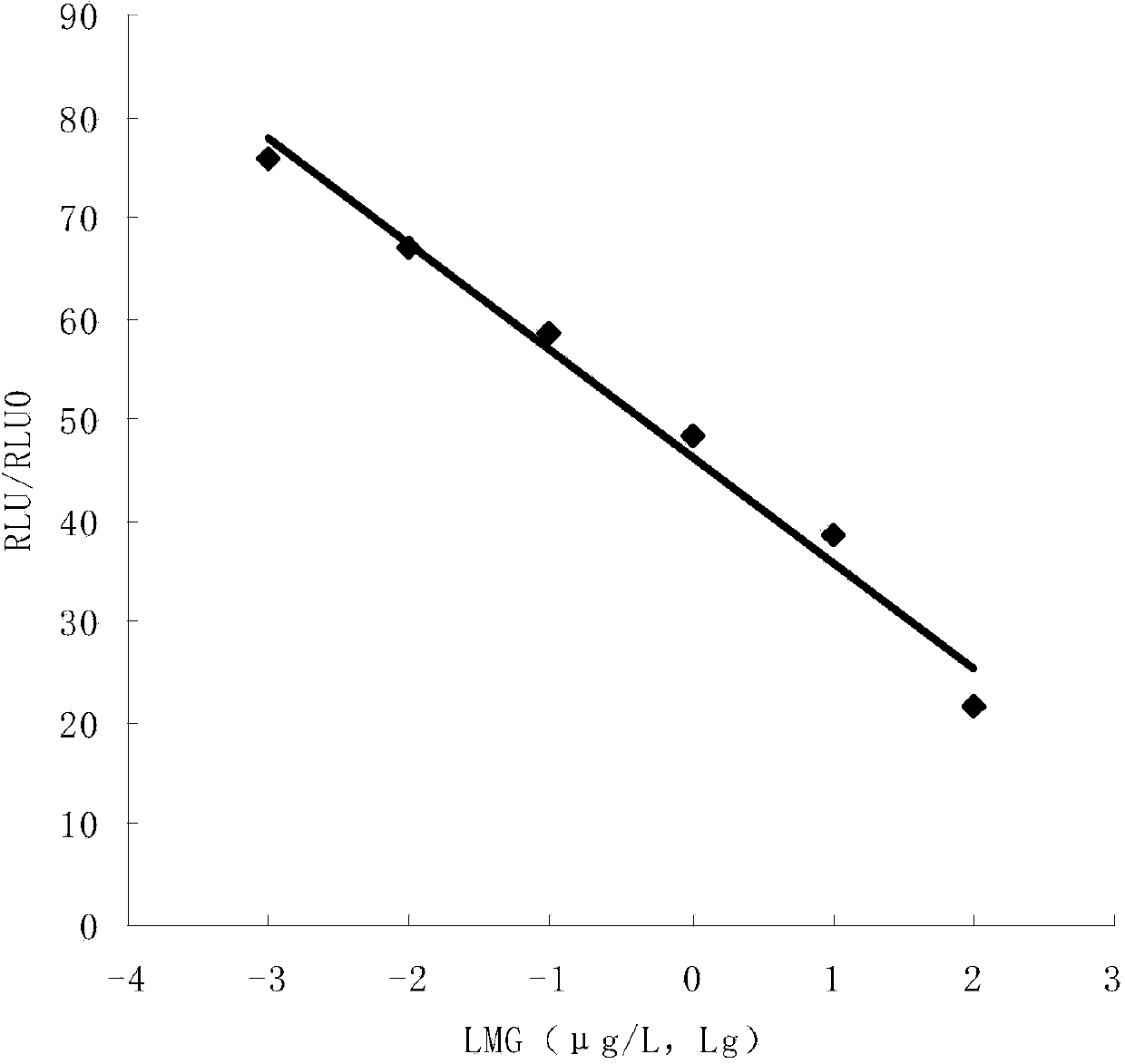
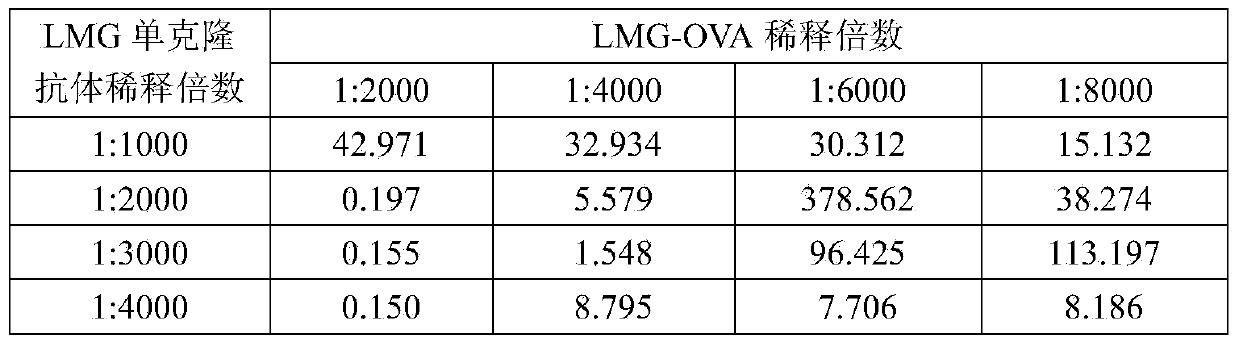
ActiveCN103472228AEasy to operateReduce experimental errorChemiluminescene/bioluminescenceMalachite greenHigh concentration
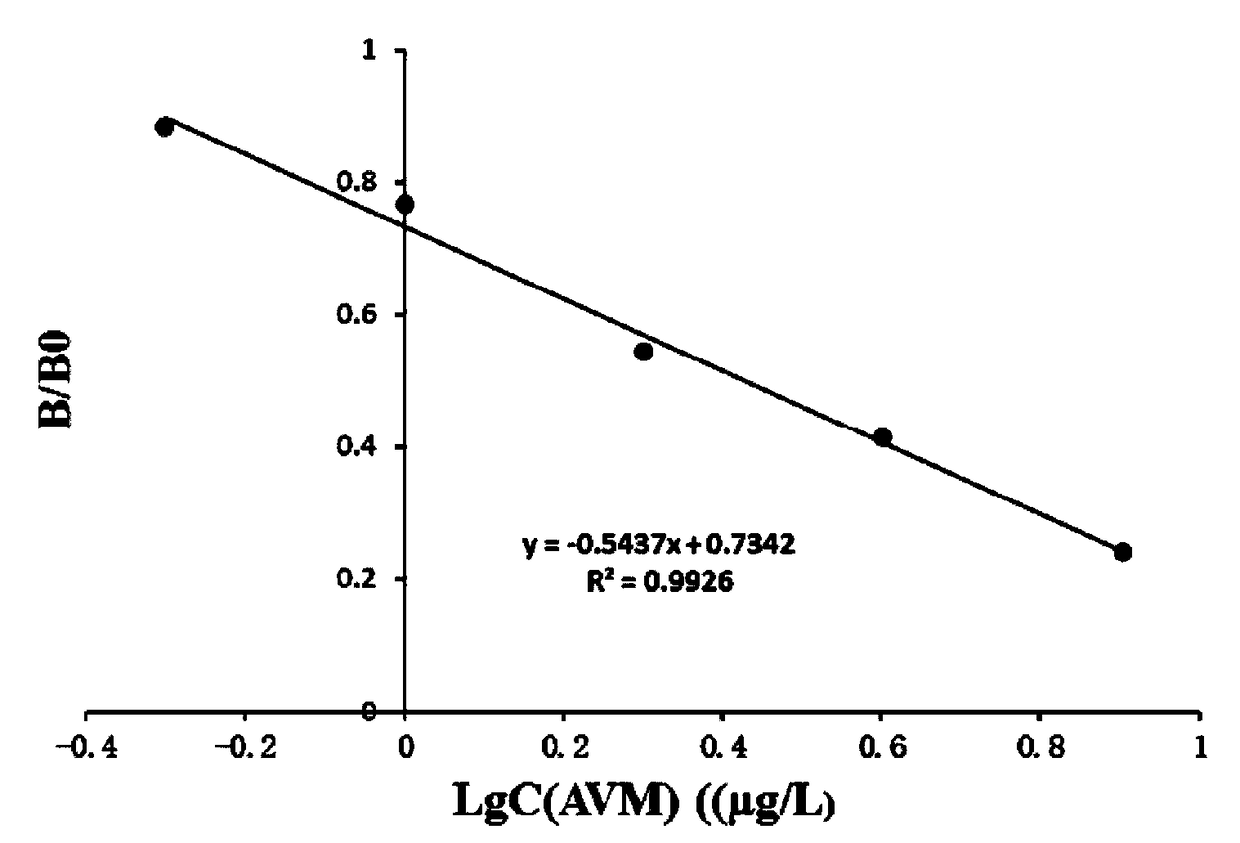
The invention discloses a one-step chemiluminescence / enzyme immunoassay method and kit for detecting residue of malachite green. The method combines indirect enzyme immunoreaction and chemiluminescence technologies, simplifies the traditional two-step chemiluminescence / enzyme immunoassay method into a one-step method without additional preparation of an enzyme-labeled monoclonal antibody, and uses 15-35% acetonitrile as a sample dissolving solution based on the characteristic that the 15-35% acetonitrile has limited dissolving power of high-concentration colorless malachite green. The method is used for detecting residue of malachite green in animal-derived foods, has the characteristics of quickness, convenience, specificity, sensitivity, accuracy, wide detection range and the like, better meets the requirements of quick detection, and has favorable application prospects. The IC50 of the kit is 0.45 mu g / L, the recovery rate is 86.37-116.84%, and the accordance rate with a standard detection method is 100%, thus ensuring that the kit is suitable for trace analysis and batch detection of malachite green.
Owner:GUANGXI VETERINARY RES INST
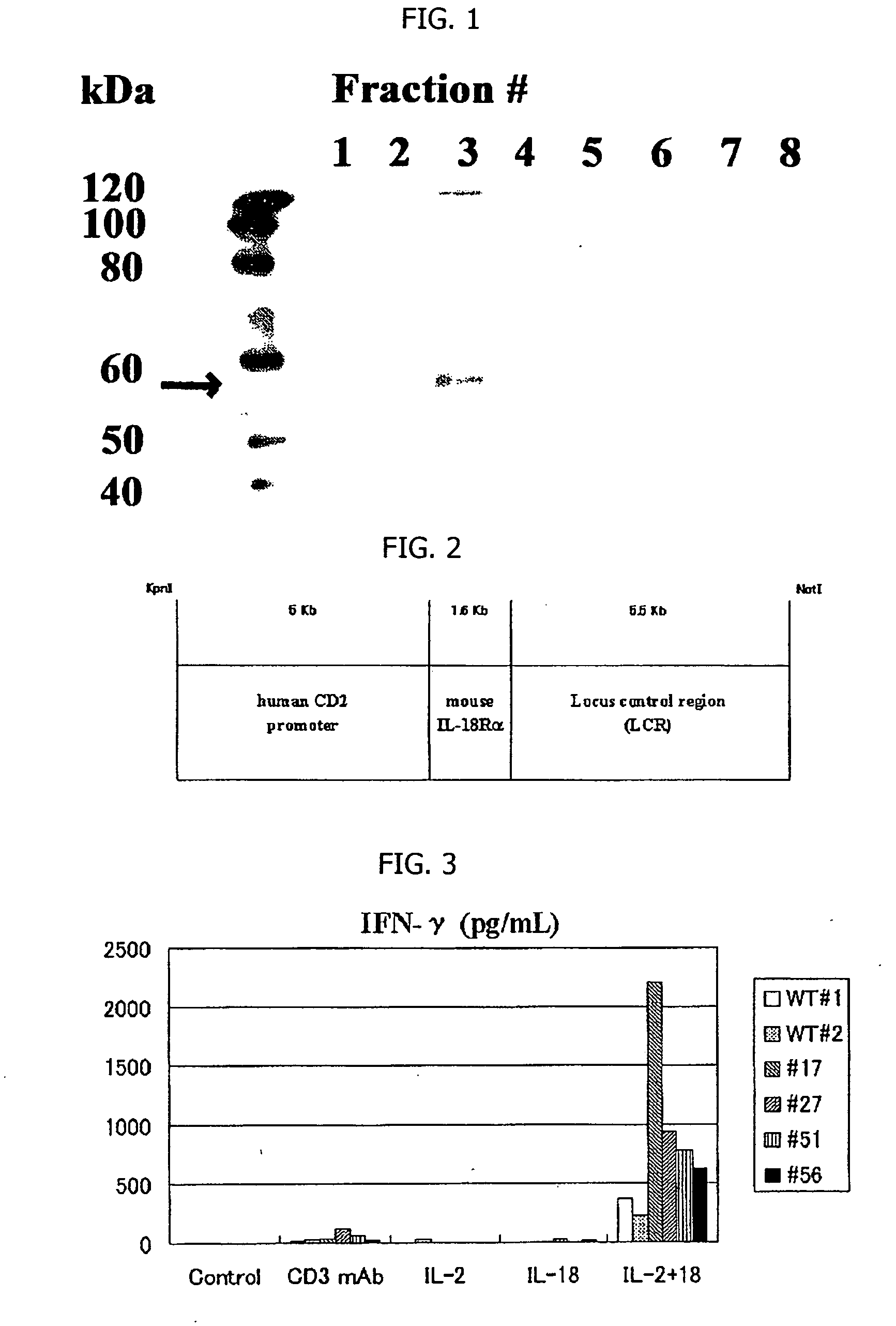
Soluble human interleukin 18 receptor-alpha, method of assaying the same, assay kit and medicinal composition
InactiveUS20060241036A1Simplifying assayEfficient use ofBiocideOrganic active ingredientsAntiendomysial antibodiesReceptor
[PROBLEMS] To confirm the presence of a solubilized human IL-18 receptor-α by a novel ELISA method and provide an assay kit and a medicinal composition containing the solubilized human IL-18 receptor-α as the active ingredient. [MEANS FOR SOLVING PROBLEMS] A solubilized human interleukin-18 receptor-α, a method of assaying the solubilized human interleukin-18 receptor-α by an enzyme immunoassay method characterized by using the following antibody (A), a kit for assaying the solubilized human interleukin-18 receptor-α and a medicinal composition containing the solubilized human IL-18 receptor-α. (A) An anti-human interleukin-18 receptor-α monoclonal antibody capable of recognizing the same epitope as an H44 mouse anti-human interleukin-18 receptor-α monoclonal antibody.
Owner:HOSHINO TOMOAKI
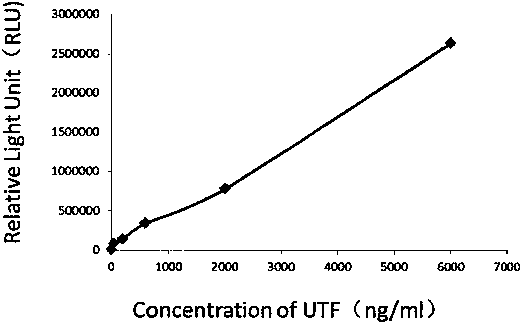
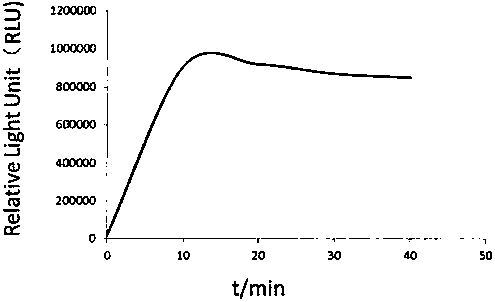
Kit for detecting urine transferrin through chemiluminescence enzyme immunoassay method, and preparation method thereof
InactiveCN107643282AHigh sensitivityStrong specificityChemiluminescene/bioluminescenceBiological testingImmune complex depositionEnzyme immunoassays
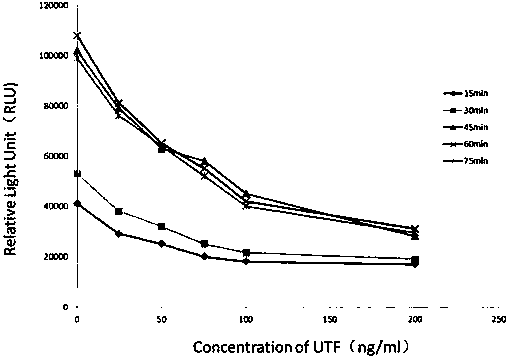
The invention relates to a kit for detecting urine transferrin through a chemiluminescence enzyme immunoassay method, and a preparation method thereof. The kit comprises an antibody-coated plate, a urine transferrin enzyme antibody, a coating buffer solution, a blocking solution, a urine transferrin standard substance, a standard substance dilution solution, a washing solution, a sample dilution solution and a chemiluminescent substrate solution. According to the present invention, the kit can detect the UTF concentration level of human based on chemiluminescence enzyme immunoassay; the chemiluminescent substrate catalytic enzyme HRP and the anti-UTF antibody are conjugated, the obtained conjugate reacts with the sample or the antigen to form the immune complexes, the chemiluminescent substrate solution is added, the relative light unit of the chemiluminescent reaction is measured, the UTF antigen content in the standard substance / the sample is proportional to the relative light unit measured by the optical system, and through standard curve fitting, the UTF content in the urine sample is determined; and the kit of the invention has advantages of high sensitivity, strong specificity, easy operation, low cost and the like.
Owner:长春市诺克因生物科技有限公司
Ivermectin derivative, monoclonal antibody for resisting abamectin type drug and application thereof
ActiveCN109180760ACross-reactivity rate noSensitive detectionSugar derivativesImmunoglobulinsAvermectinMonoclonal antibody
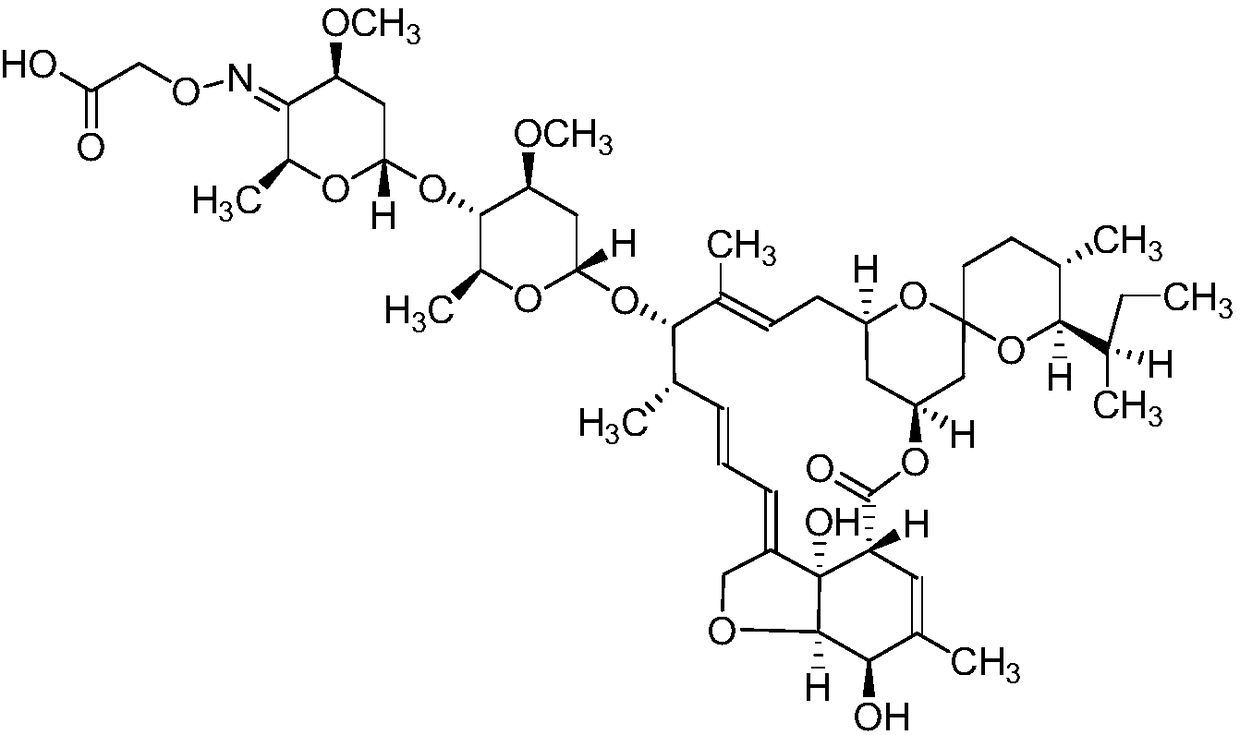
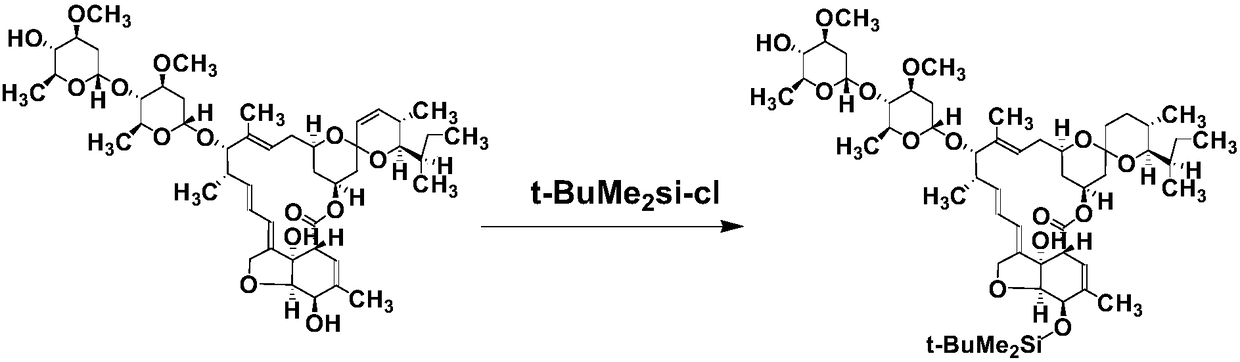
The invention discloses a novel ivermectin hapten. The novel ivermectin hapten is obtained through reacting with carboxymethoxylamine hemihydrochloride at the C4-OH position of ivermectin and substituting hydroxyl on C4 with carboxymethyl hydroxyamino; the derivative is coupled with carrier protein to be used as an immunogen, and can be used for preparing a monoclonal antibody for resisting an abamectin type drug. The invention further discloses one broad-spectrum monoclonal antibody for resisting the abamectin type drug; the monoclonal antibody can be used for simultaneously and specificallyidentifying abamectin, ivermectin, eprinomectin and emamectin; and an enzyme immunoassay method and a kit, which are established by utilizing the monoclonal antibody are suitable for detecting residues of the abamectin type drug in animal tissues and have the advantages of sensitivity in detection, high accuracy, good precision and the like.
Owner:HUAZHONG AGRI UNIV
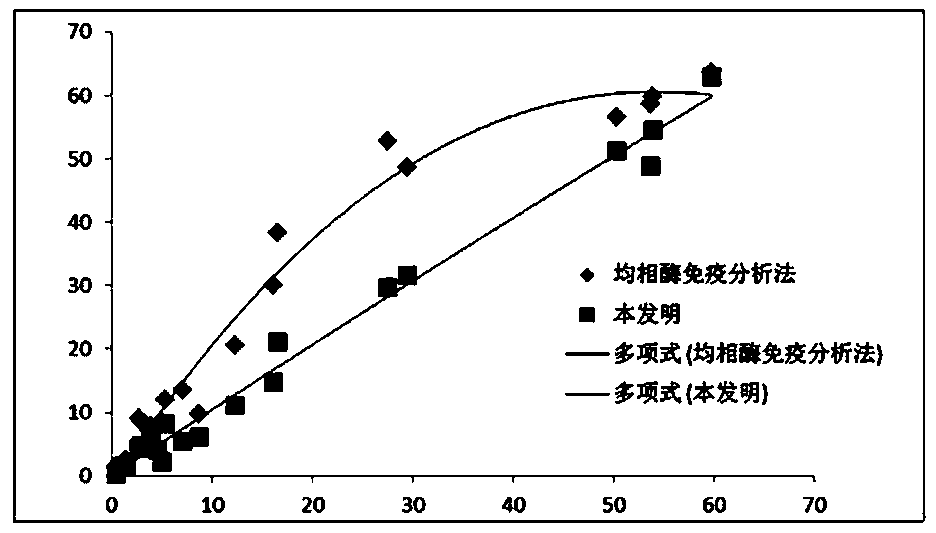
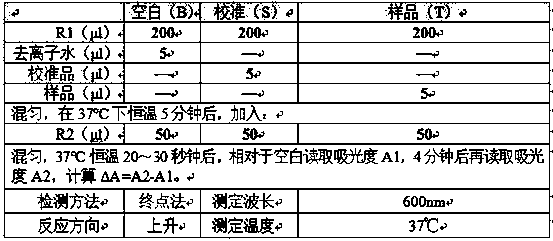
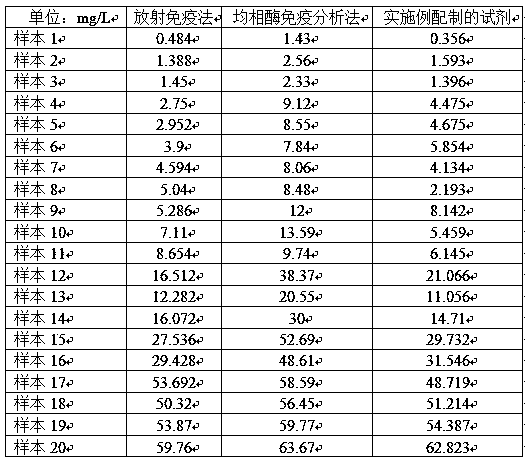
Application of thionicotinamide adenine dinucleotide I to homogeneous enzyme immunodiagnostic reagent
ActiveCN109884317AImprove stabilityGood repeatabilityBiological testingEnzyme immunoassaysGlycocholic acid
The invention relates to the technical field of in vitro diagnosis and detection, in particular to an application of a thionicotinamide adenine dinucleotide I to a homogeneous enzyme immunodiagnosticreagent. The diagnostic reagent is a glycocholic acid detection reagent and a CMPF reagent. The defect of relatively poor accuracy and repeatability of a detection result caused by relatively poor stability of the glycocholic acid reagent and the CMPF reagent in the prior art is overcome; a nicotinamide adenine dinucleotide II in the homogeneous enzyme immunodiagnostic reagent for homogeneous enzyme immunoassay is replaced with the thionicotinamide adenine dinucleotide I, so that the problem of relatively poor stability of the homogeneous enzyme immunodiagnostic reagent is effectively solved,the repeatability of the detection result of the reagent is improved, and the accuracy of the detection result is improved; and meanwhile, the anti-hemoglobin interference resistance of the reagent isimproved, the influence of a hemolysis phenomenon of a clinical sample on the detection result is weakened, and the detectability of the homogeneous enzyme immunodiagnostic reagent is improved, so that the test reagent is suitable for a conventional microplate reader, the detection cost is effectively reduced, and meanwhile, the usage amount of the reagent and raw materials is reduced.
Owner:HANGZHOU BOPU MEDICAL TECH
Kit for quickly detecting human AMH (Anti-Mullerian Hormone) by magnetic particle chemiluminescent immunoassay
InactiveCN109212195AOvercome instabilityOvercome rangeChemiluminescene/bioluminescenceBiological testingFluorescenceSorbent
The invention discloses a kit for quickly detecting human AMH (Anti-Mullerian Hormone) by magnetic particle chemiluminescent immunoassay. The kit comprises a magnetic particle coated with a goat anti-human AMH polyclonal antibody, a modified mouse anti-human AMH monoclonal antibody labeled by enzyme, an AMH calibration product, concentration lotion and a substrate. The kit has the advantage that exiting chemiluminescent immunoassay which is quickly promoted and applied and is advanced is adopted. Compared with the prior art, the chemiluminescent immunoassay disclosed by the invention overcomesthe deficiency of RIA (radioimmunoassay) radioactive contamination and the defects of the instability and the narrow quantification range of an enzyme marker in an enzyme linked immunosorbent assay,meanwhile, the difficulty in an ELISA (Enzyme-Linked Immuno Sorbent Assay) method that a fluorescent marker is likely to be interfered by environment and the defects of the indirect marking and the like of an ECLI (Electro-Chemi-Luminescence Immunoassay) are overcome, the detection sensitivity and accuracy of the kit can be higher than an enzyme immunoassay method, a fluorescence method and the like by several orders of magnitude, detection steps and reaction time are reduced, an application range is wide, and the kit can be applied to an automatic and semi-automatic instruments, and has a good development prospect.
Owner:AUTOBIO DIAGNOSTICS CO LTD
Heparin-binding protein assay kit as well as preparation method and assay method thereof
PendingCN111929443AStrong specificityHigh detection sensitivityChemiluminescene/bioluminescenceDisease diagnosisImmune profilingEnzyme immunoassays
The invention discloses a heparin-binding protein assay kit applying a magnetic particle chemiluminescence enzyme immunoassay method. The heparin-binding protein assay kit comprises avidin-labeled magnetic particles, a biotin-labeled HBP antibody 1, a horseradish peroxidase-labeled HBP antibody 2, an HBP calibrator, a concentrated washing liquid and a substrate. The invention also discloses a preparation method and a determination method of the kit. The novel immunoassay kit disclosed by the invention is used for quantitatively assaying HBP in human body fluid in vitro, overcomes personal errors of a traditional enzyme immunoassay (EIA) method in the aspect of operation, can quantitatively and objectively reflect the existence of HBP, and provides a more powerful experimental diagnosis basis for clinical diagnosis, curative effect observation and prognosis judgment. The heparin binding protein determination method provided by the invention is high in specificity, good in repeatability,high in detection sensitivity, wide in linear range, high in automation degree and simple, convenient and rapid to operate; compared with an EIA method, the method has the advantages of rapidness, simplicity, convenience, accuracy and small interference.
Owner:HANGZHOU JOINSTAR BIOTECH
A kind of preparation method of silk fibroin antibody of silkworm
ActiveCN104447989BStrong specificityStrong immune responseSerum immunoglobulinsImmunoglobulins against animals/humansEnzyme immunoassaysTitin Antibody
The invention discloses a method for preparing silkworm silk fibroin antibody. The calcium chloride ethanol system is used to extract the silk fibroin, and then the silk fibroin is injected into rabbits as a complete antigen for animal immunization to obtain the silk fibroin antibody. The steps are as follows: silk fibroin extraction: extraction with calcium chloride ethanol system; peptide immunization and antiserum preparation: mixing and emulsifying silk fibroin powder with Freund's adjuvant, injecting multiple points subcutaneously in rabbits, and repeatedly boosting immunization until Take antiserum to detect that the antibody titer reaches the standard and stop immunization; antibody purification: after the antibody titer of the rabbit reaches the standard, take blood to make the blood clot fully coagulate, and separate the antiserum; the effectiveness of the silk fibroin antibody is determined by the ELISA step For identification, the obtained silk fibroin antibody is used as the primary antibody, and the standard enzyme-linked immunosorbent method is used to confirm that the silk fibroin antibody can interact with silk fibroin, which can be used in the enzyme-linked immunosorbent assay.
Owner:ZHEJIANG SCI-TECH UNIV
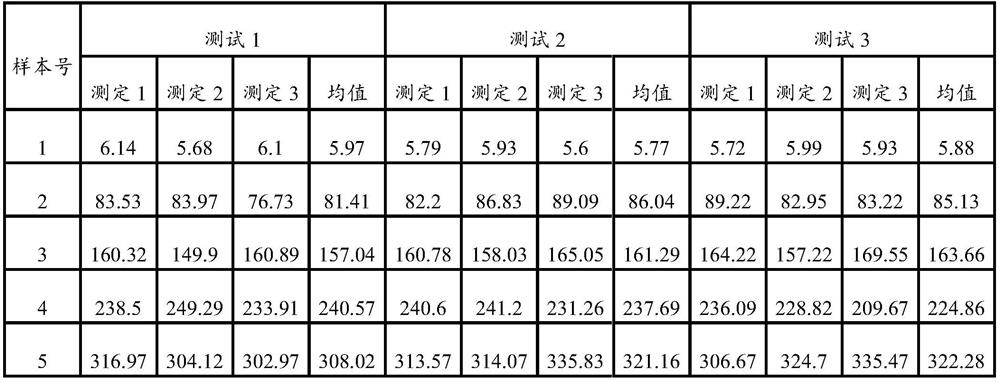
A kind of in situ ELISA quantitative detection method of residual bt protein in soil
The invention discloses an in-situ enzyme linked immunosorbent assay quantitative determination method of a Bt protein residual in soil. The method comprises 1, removing animal and plant residues in a soil sample to be detected, carrying out full grinding, and screening the soil sample by a sieve of 50-60 meshes so that soil particle sizes are 0.2mm or less, 2, carrying out sterilization on the ground soil sample under conditions of high pressure, dampness and heat for 15-20min, 3, weighing 1-2g of the soil sample subjected to sterilization, putting the soil sample into a centrifuge tube with a volume of 10ml, and carrying out washing to remove impurities, 4, enclosing the surface of the soil sample without impurities, and 5, carrying out antibody fixation detection on the enclosed soil sample. The method provides a novel approach for monitoring soil Bt protein residue and Bt protein residue-caused influence on a soil ecosystem, can be used for detecting soil Bt protein and can also be used for detecting various soil residual protein contents after replacement with different antibodies.
Owner:NANJING INST OF ENVIRONMENTAL SCI MINIST OF ECOLOGY & ENVIRONMENT OF THE PEOPLES REPUBLIC OF CHINA
Monoclonal antibody and ELISA method and kit for detecting carbadox and cydox metabolites
InactiveCN104558186BHigh sensitivityStrong specificityMicroorganism based processesTissue cultureMetaboliteTrue positive rate
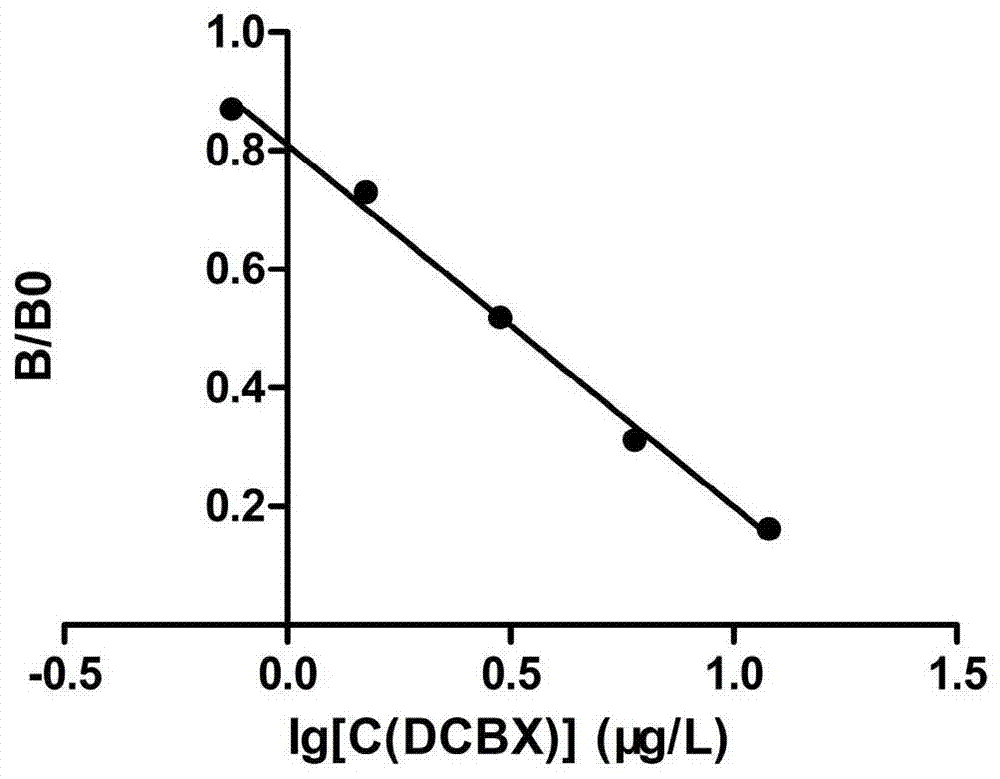
The invention discloses a specificity monoclonal antibody capable of distinguishing various carbadox and cyadox metabolism products and an enzyme linked immunosorbent assay method and kit for detecting the carbadox and cyadox metabolism products. According to the invention, the monoclonal antibody is secreted by a hybridoma cell strain DCBX5B1 of which the preservation number is CCTCC NO: C201495. Compared with the prior art, the monoclonal antibody disclosed by the invention can distinguish DCBX, bisdesoxy cyadox and desoxy cyadox (N4) at the same time, and has higher sensitivity and specificity. The ELISA method and the kit, disclosed by the invention, can detect the residue of the carbadox and cyadox metabolism products in meat food at the same time, and have the advantages of high detection efficiency, high sensitivity, high precision, high accuracy and the like.
Owner:HUAZHONG AGRI UNIV
Monoclonal antibody, ELISA method and kit for detecting benzodiazepines
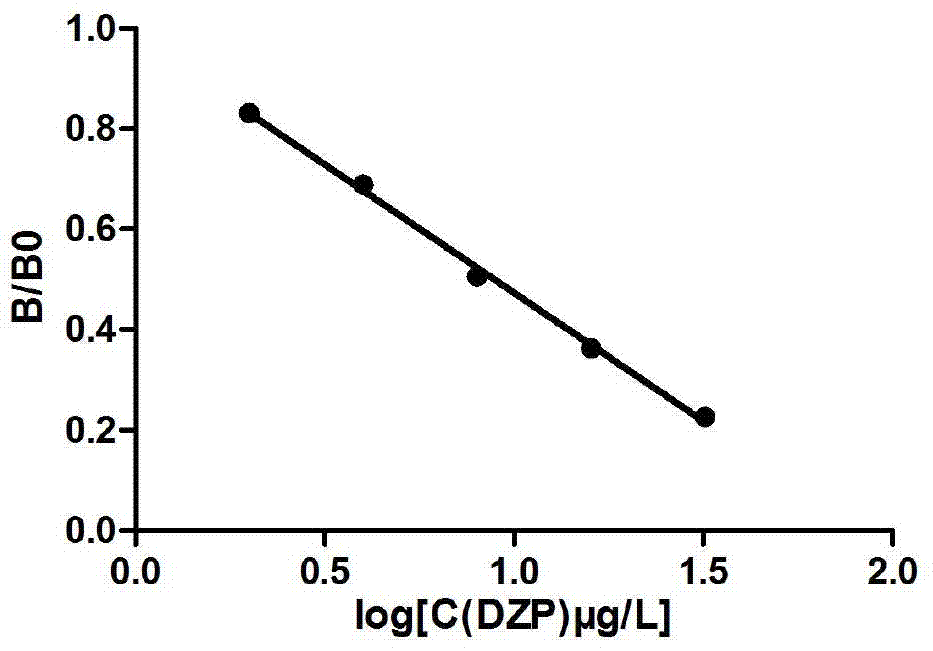
ActiveCN104530240BIncreased cross-reactivityExcellent cross-reactivity rateMicroorganism based processesTissue cultureBenzodiazepineNitrazepam
Owner:HUAZHONG AGRI UNIV
Application of oxidized thio-coenzyme I in homogeneous enzyme immunodiagnostic reagents
The invention relates to the technical field of in vitro diagnosis and detection, in particular to the application of oxidized thio-coenzyme I in homogeneous enzyme immunodiagnostic reagents, and the diagnostic reagents are glycocholic acid detection reagents and CMPF reagents. The present invention overcomes the poor stability of glycocholic acid reagent and CMPF reagent in the prior art, resulting in the defects of poor detection result accuracy and repeatability, by combining the oxidative Type coenzyme II is replaced by oxidized thio-coenzyme I, which effectively solves the problem of poor stability of homogeneous enzyme immunodiagnostic reagents, improves the repeatability of reagent test results, and improves the accuracy of test results; at the same time, it improves the anti-hemoglobin of the reagent. Interference ability, weaken the influence of clinical sample hemolysis on the test results, improve the detectability of homogeneous enzyme immunodiagnostic reagents, make the test reagents suitable for conventional microplate readers, effectively reduce the cost of testing, and save the amount of reagents and raw materials.
Owner:HANGZHOU BOPU MEDICAL TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com