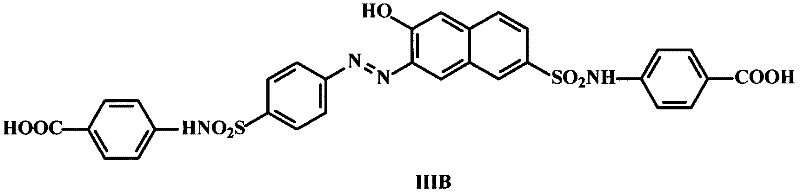
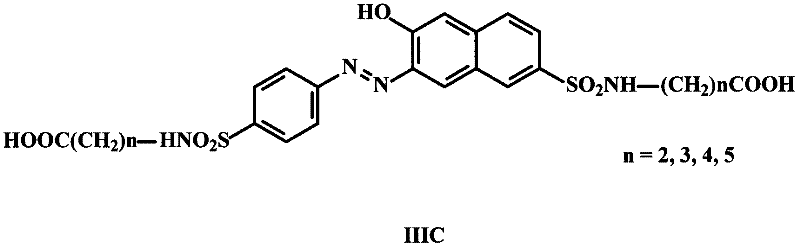
Synthesis methods of sunset yellow hapten and artificial antigen
A synthesis method, technology of sunset yellow, applied in chemical instruments and methods, preparation of azo dyes, animal/human proteins, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
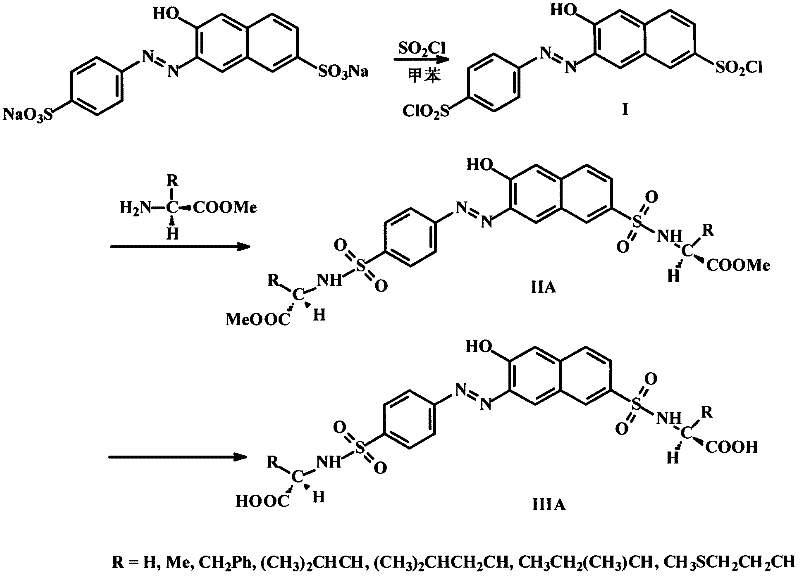
[0023] Add 2.0g (4.4mmol) of sunset yellow and 25ml of anhydrous toluene into a 100ml round bottom flask, slowly add 2.0ml (28.1mmol) of thionyl chloride under electromagnetic stirring, then drop a few drops of anhydrous DMF, and stop after reflux for 2h reaction. Remove the remaining thionyl chloride and toluene by rotary evaporation, add petroleum ether, filter with suction, and dry to obtain compound I as a dark red solid.
Embodiment 2
[0025] Add 0.17g (1.35mmol) glycine methyl ester hydrochloride, 20ml anhydrous acetone, 0.27g triethylamine to a 50ml round bottom flask, stir to dissolve, add 0.2g (0.45mmol) sunset yellow sulfonyl chloride I, and stir at room temperature for 1h. Acetone was distilled off and separated by silica gel column chromatography to obtain product IIA (R=H).
[0026] 1 H NMR (CDCl 3 , 400M) δ8.67 (d, 1H, J=8.8Hz, Ar-H 5 ), 8.24(s, 1H, Ar-H 3 ), 8.14 (d, 1H, J=9.6Hz, Ar-H 2 ), 8.05(d, 2H, J=8.4Hz, Ar-H 6,6’ ), 7.95(s, 1H, Ar-H 4 ), 7.91 (d, 2H, J=8.4Hz, Ar-H 7,7’ ), 6.96 (d, 1H, J=9.6Hz, Ar-H 1 ), 3.77 (d, 4H, J=7.2Hz, CH 2 ×2), 3.54(s, 3H, -OCH 3 ), 3.51(s, 3H, -OCH 3 ),.
[0027] HRMS (ESR): m / z=573.0726 (caled.for573.0720[M+Na] + )
Embodiment 3
[0029] Add 0.29g (1.35mmol) L-phenylalanine methyl ester hydrochloride, 20ml anhydrous acetone, 0.27g triethylamine into a 50ml single-necked round bottom flask, stir until dissolved, add 0.2g (0.45mmol) sunset yellow Acid chloride I, stirred at room temperature for 1h. Acetone was distilled off and separated by column chromatography to obtain the product IIA (R=CH 2 Ph).
[0030] 1 H NMR (CDCl 3 , 400M) δ16.13 (s, 1H, OH), 8.41 (d, 1H, J=8.4Hz, Ar-H 5 ), 7.91(s, 1H, Ar-H 3 ), 7.78 (d, 3H, J=8.4Hz, Ar-H 6,6’,2 ), 7.63 (d, 3H, J=8.4Hz, Ar-H 7,7’,4 ), 7.18(d, 3H, J=6.8Hz, Ar-H), 7.13(d, 3H, J=7.2Hz, Ar-H), 7.05(d, 1H, J=7.2Hz, Ar-H), 6.90(d, 1H, J=8.4Hz, Ar-H 1 ), 5.82-5.88(m, 2H, 2×NH), 4.23-4.26(m, 2H, 2×CH), 3.51(s, 3H, -OCH 3 ), 3.46(s, 3H, -OCH 3 ), 2.96-3.08 (m, 2H, 2×CH 2 ).
[0031] HRMS (ESR): m / z = 753.1654 (caled. for 753.1659 [M+Na] + )
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com