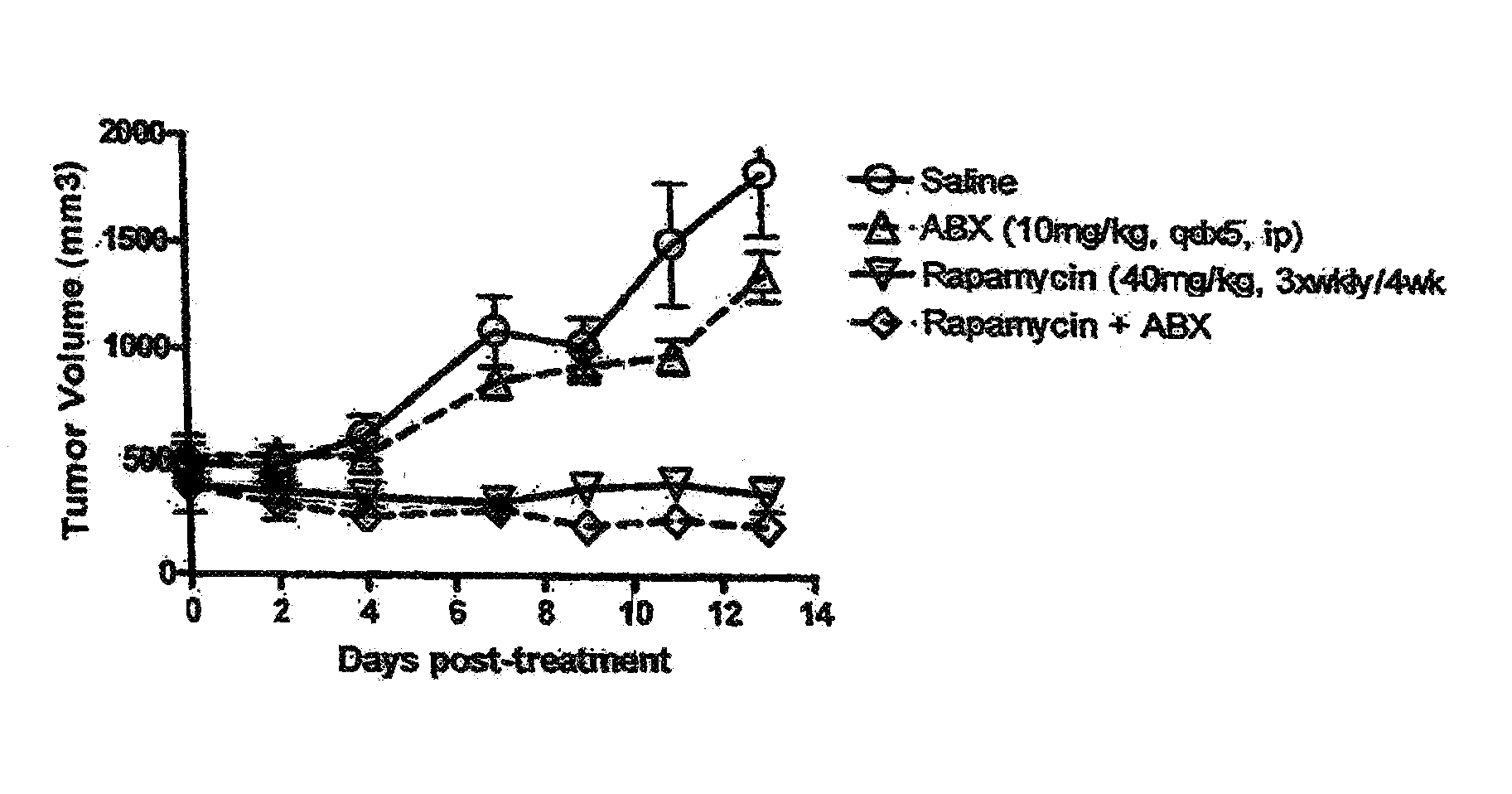
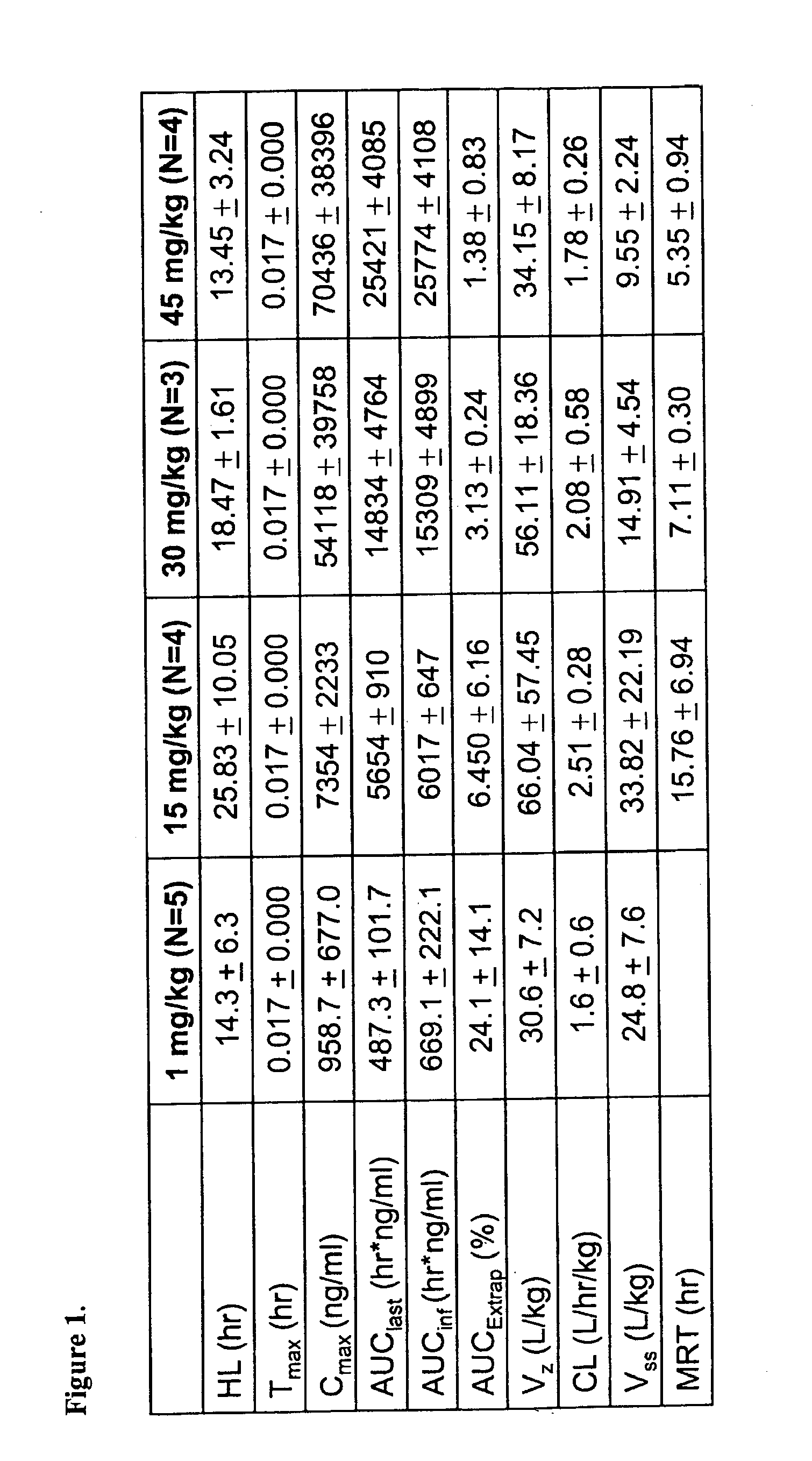
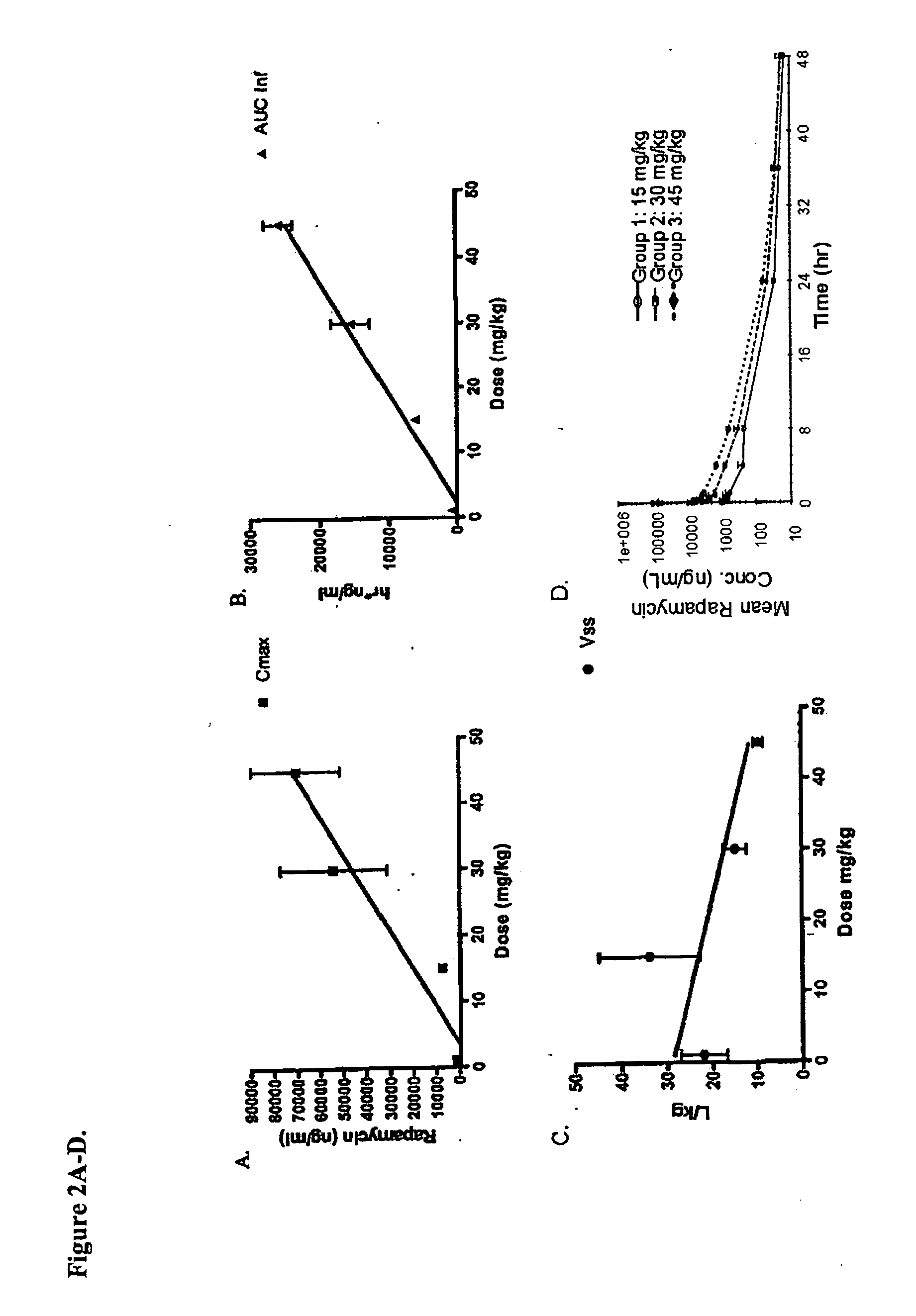
Nanoparticle comprising rapamycin and albumin as anticancer agent
a technology of nanoparticles and tumors, which is applied in the direction of biocide, drug compositions, therapy, etc., can solve the problems of inaccessibility to surgeons, inability to treat tumors located in other areas, and inability to respond to significant numbers of tumors to drugs and/or radiation therapy, etc., to achieve the effect of preventing or retarding the development of tumors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Exemplary Methods for the Formation of Nanoparticle Compositions with Rapamycin and Albumin
example 1-a
[0260]This example demonstrates the preparation of a pharmaceutical composition comprising rapamycin and albumin in which the rapamycin concentration was 8 mg / mL in the emulsion and the formulation was made on a 300 mL scale. Rapamycin (2400 mg) was dissolved in 12 mL of chloroform / t-butanol. The solution was then added into 288 mL of a human serum albumin solution (3% w / v). The mixture was homogenized for 5 minutes at 10,000 rpm (Vitris homogenizer model Tempest I.Q.) in order to form a crude emulsion, and then transferred into a high pressure homogenizer. The emulsification was performed at 20,000 psi while recycling the emulsion. The resulting system was transferred into a Rotavap, and the solvent was rapidly removed at 40° C. at reduced pressure (25 mm of Hg). The resulting dispersion was translucent. At this stage, human serum albumin solution was added to the dispersion to adjust the human serum albumin to rapamycin ratio. The dispersion was serially filtered through multiple ...
example 1-b
[0261]This example demonstrates the preparation of a pharmaceutical composition comprising rapamycin and albumin in which the rapamycin concentration was 8.3 mg / mL in the emulsion and the formulation was made on a 200 mL scale. Rapamycin (1660 mg) was dissolved in 8.5 mL of chloroform / ethanol. The solution was then added into 191.5 mL of a human serum albumin solution (6% w / v). The mixture was homogenized for 5 minutes at 10,000 rpm (Vitris homogenizer model Tempest I.Q.) in order to form a crude emulsion, and then transferred into a high pressure homogenizer. The emulsification was performed at 20,000 psi while recycling the emulsion. The resulting system was transferred into a Rotavap, and the solvent was rapidly removed at 40° C. at reduced pressure (25 mm of Hg). The dispersion was serially filtered. The size of the 0.22 μm filtered formulation was 85 nm (Zav, Malvern Zetasizer). The dispersion was further lyophilized (FTS Systems, Dura-Dry μP, Stone Ridge, New York) for 60 hour...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com