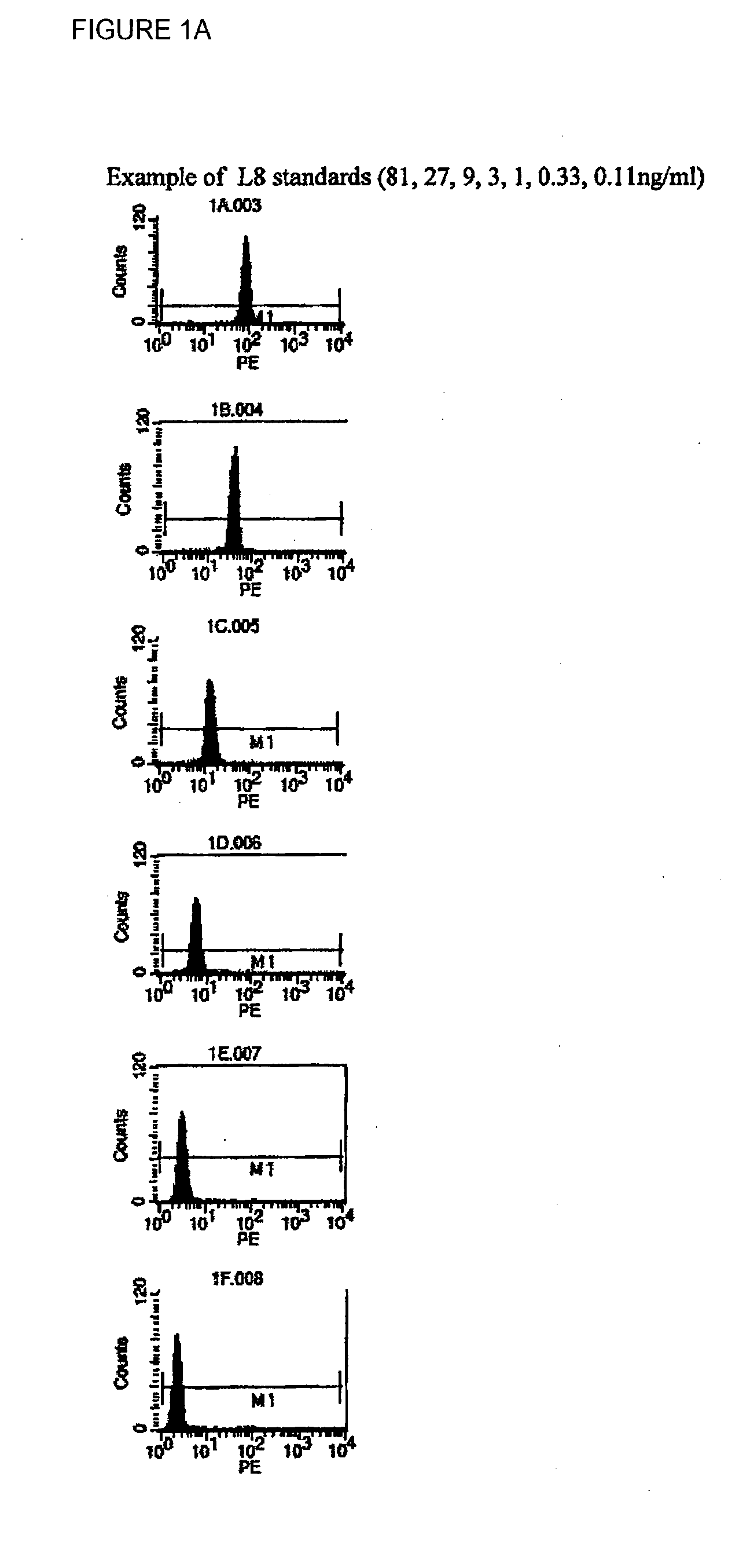
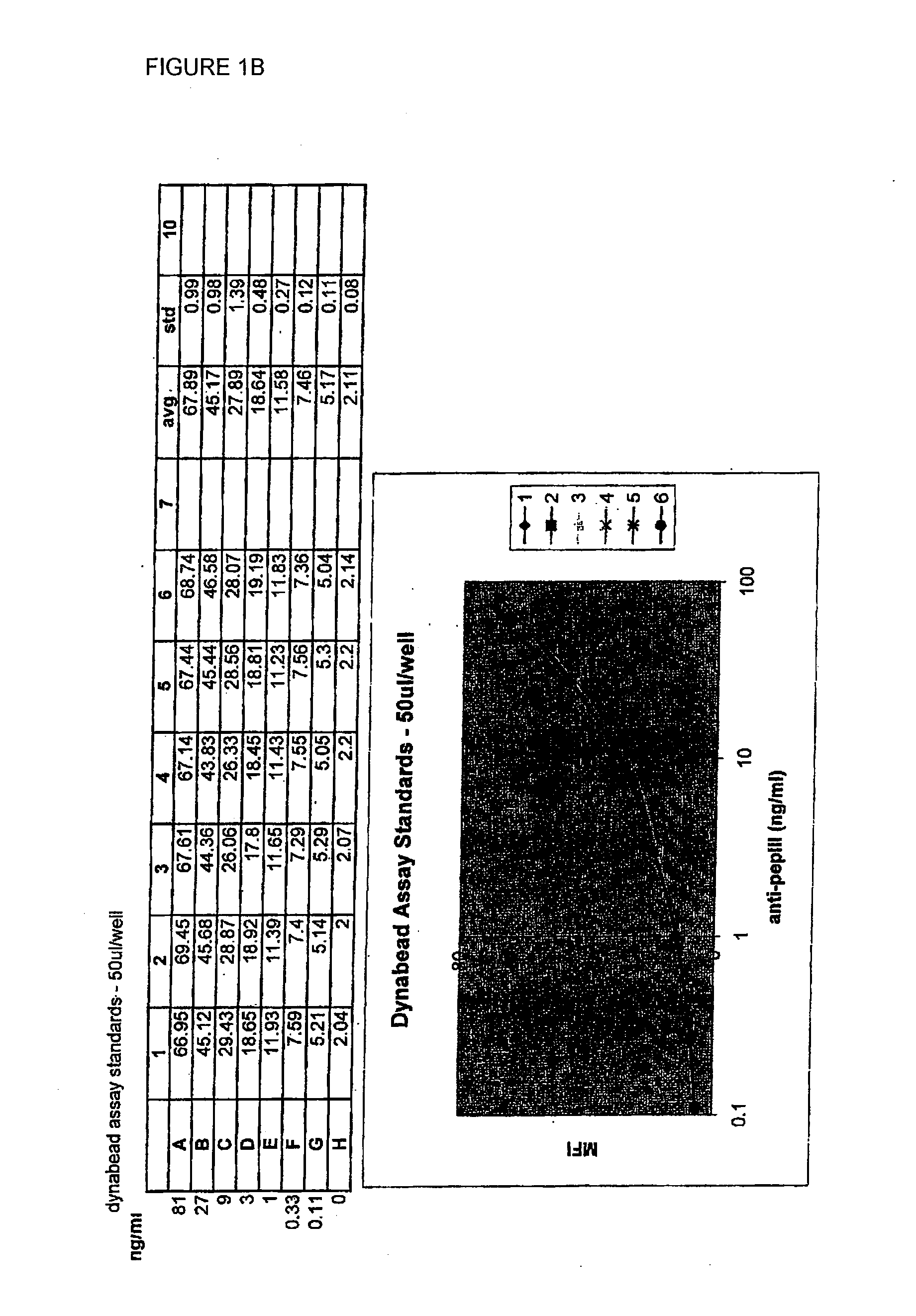
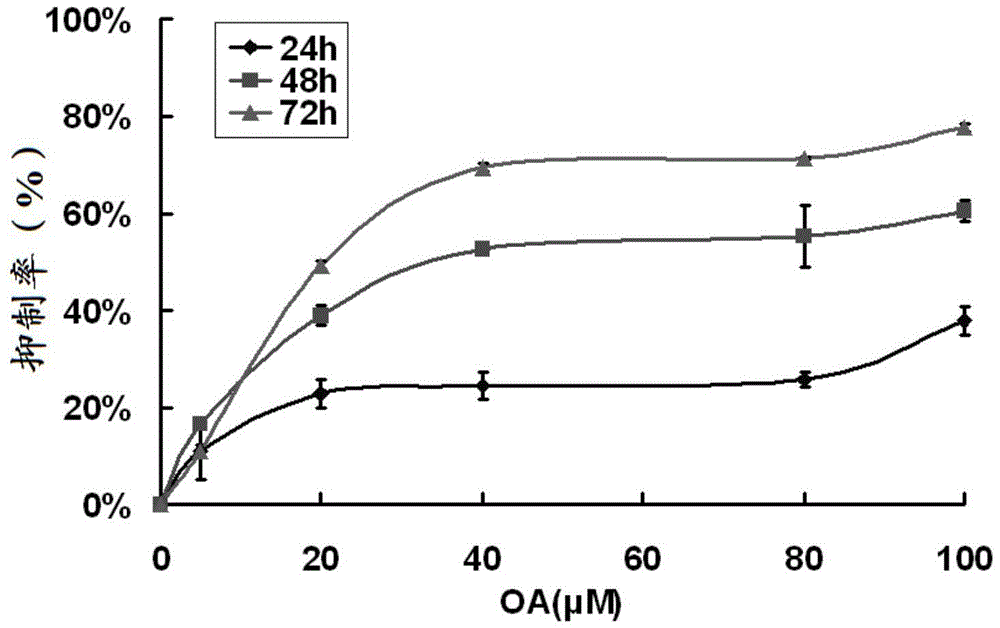
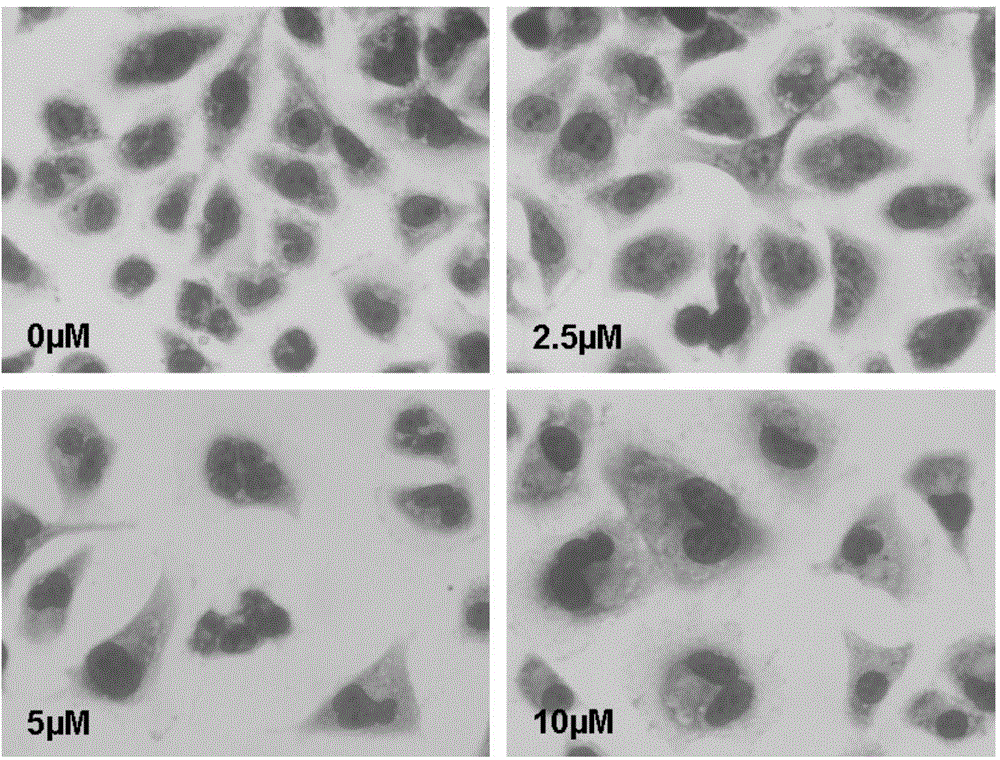
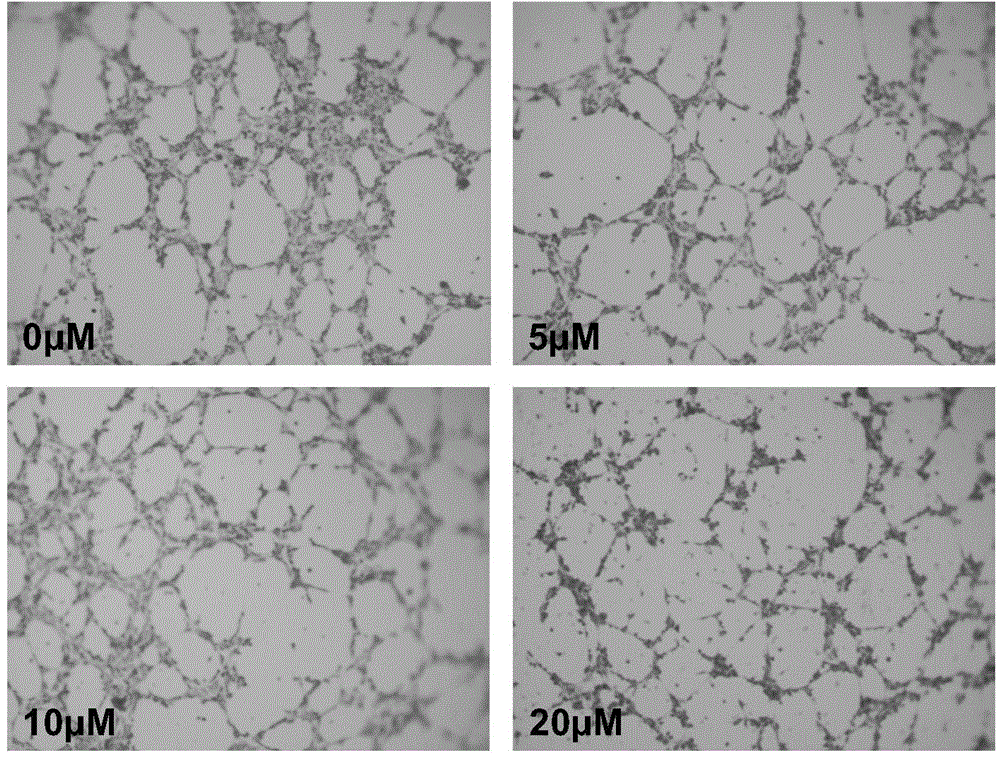
Patents
Literature
78 results about "Tumor burden" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Tumor burden. tu·mor bur·den. the total mass of tumor tissue carried by a patient with a malignancy. tu·mor bur·den. The total mass of tumor tissue carried by a patient with cancer. Synonym(s): tumour burden. tumor burden. The sum of cancer cells present in the body.
Stabilization of cells and biological specimens for analysis
InactiveUS20050181353A1Improve stabilityMaintain qualityOrganic active ingredientsBiocideAbnormal tissue growthIn vivo
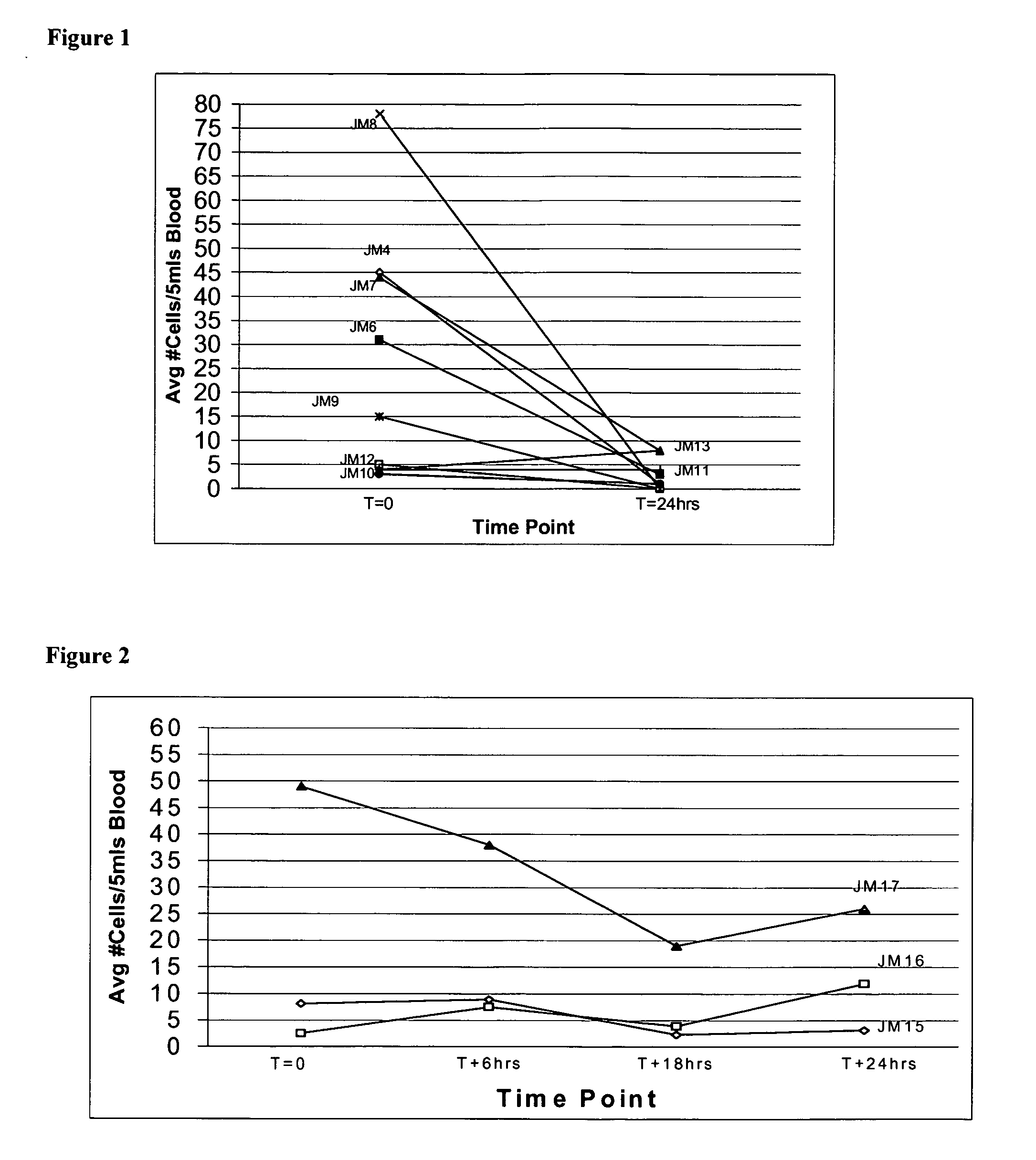
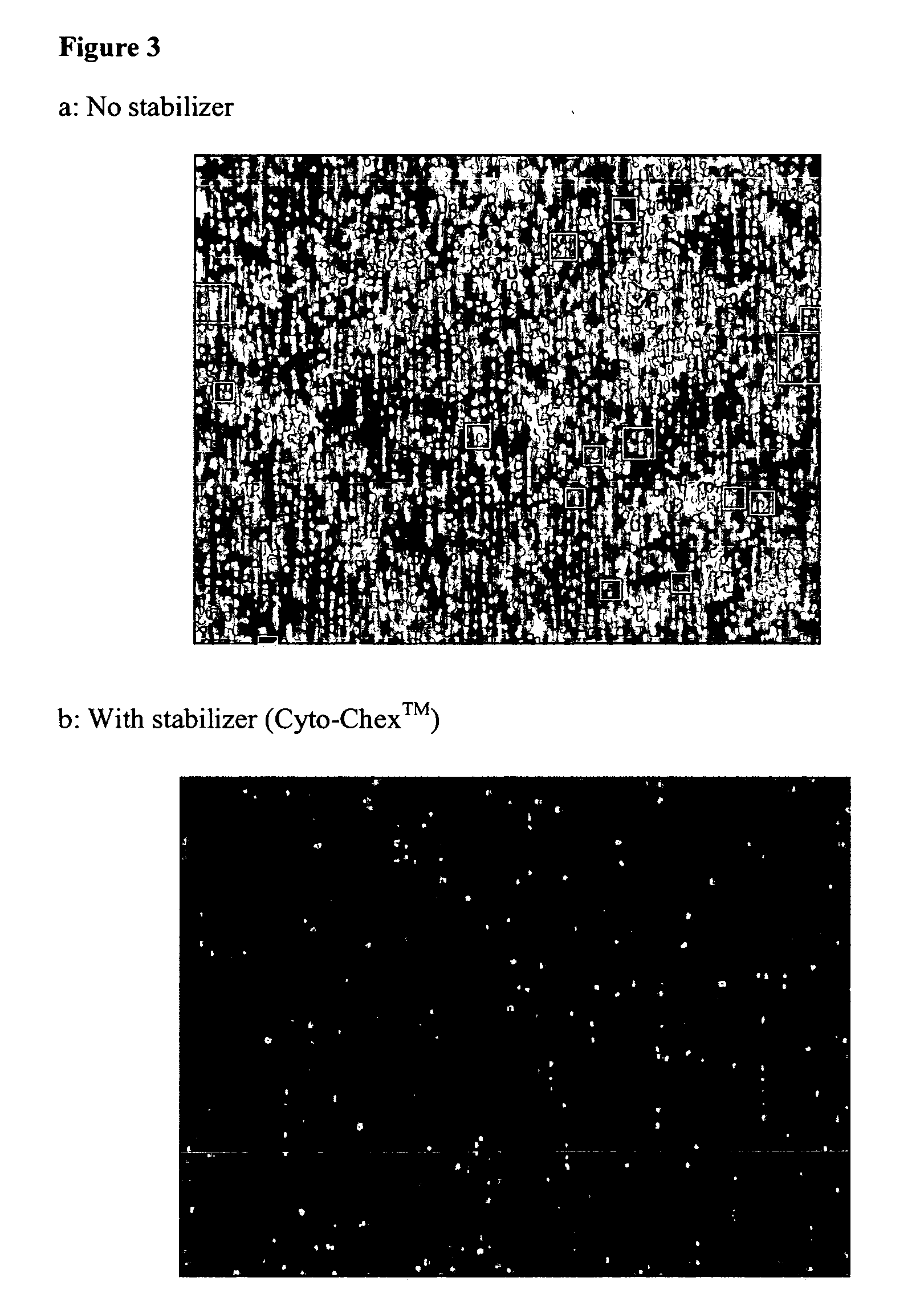
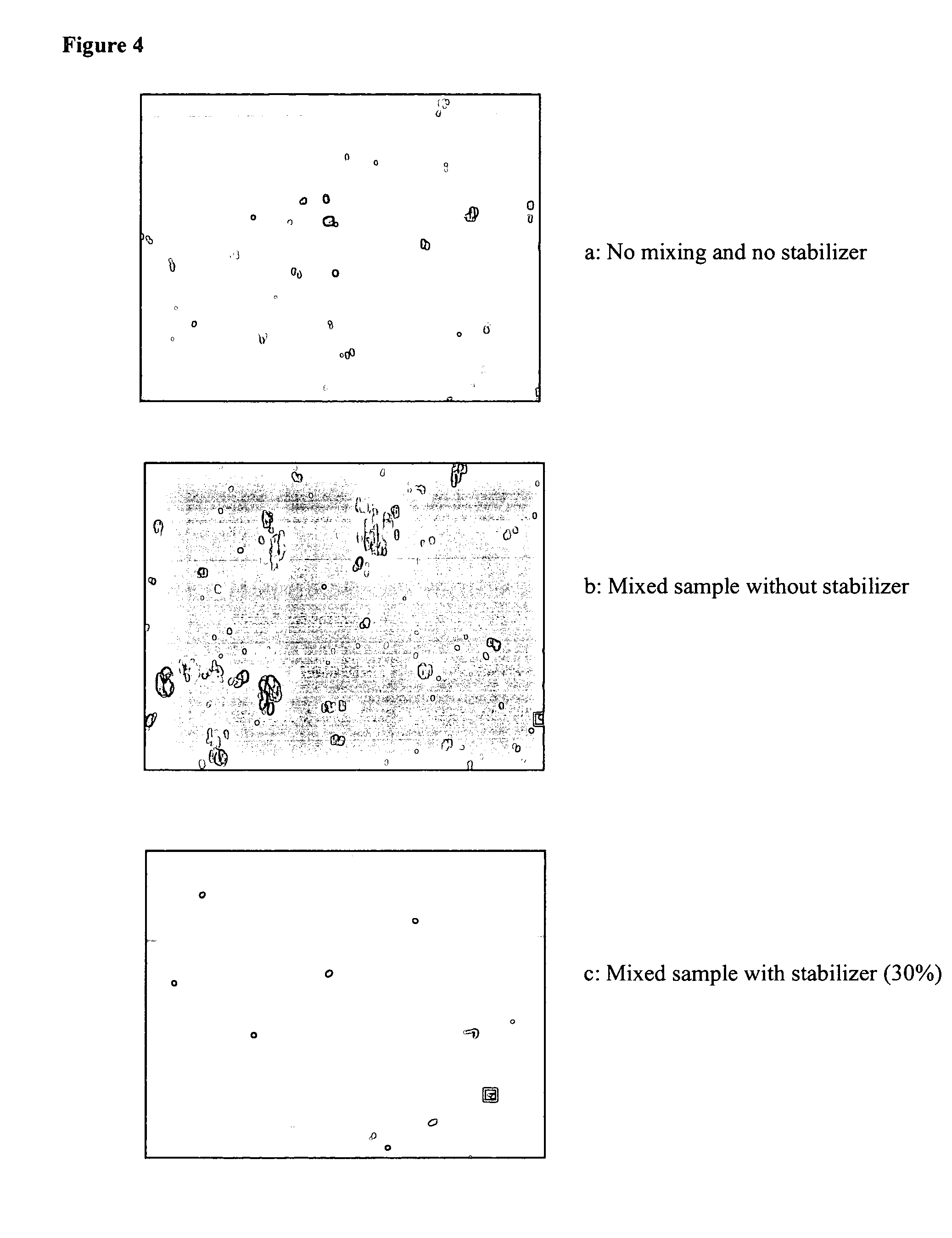
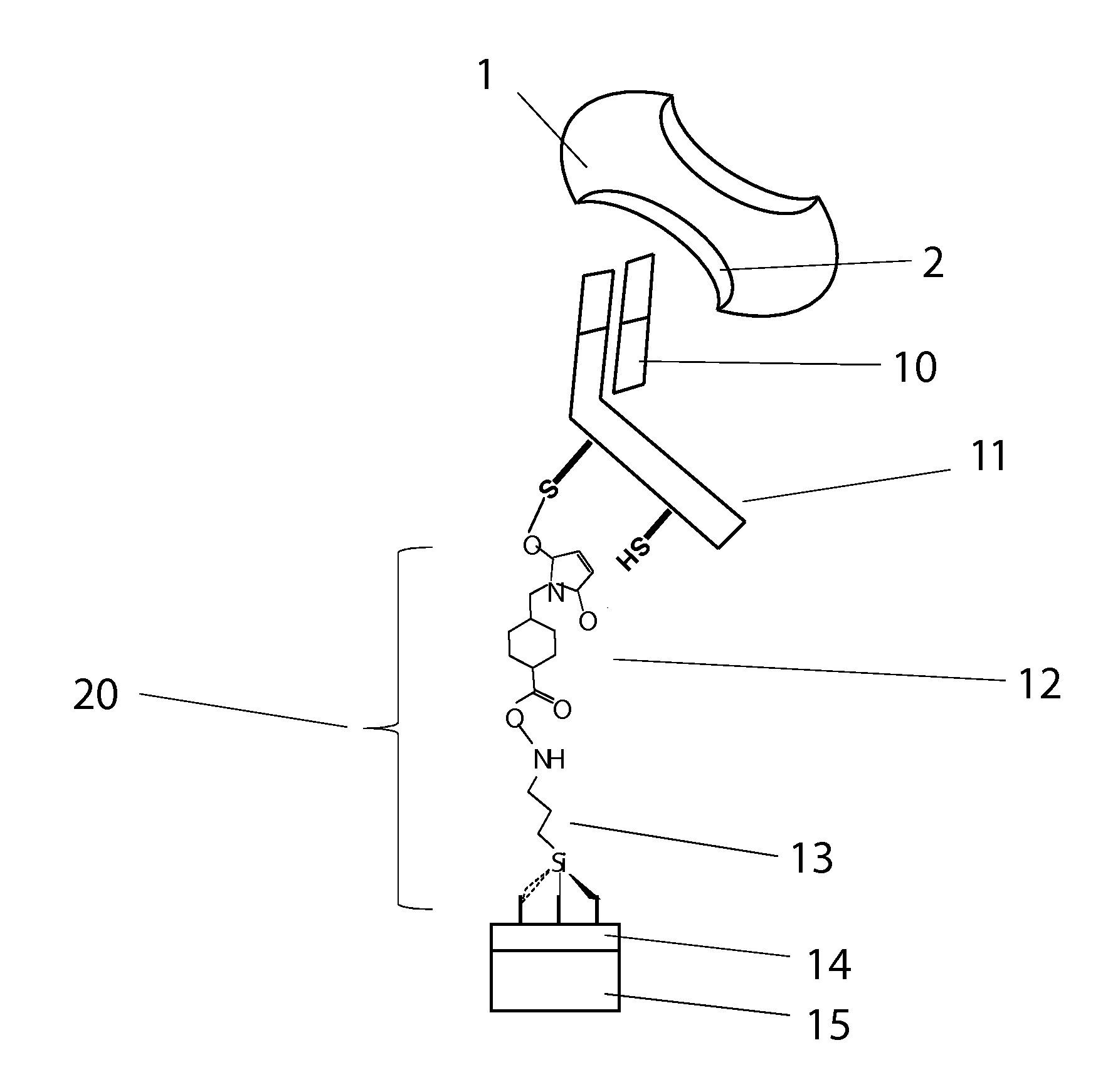
Compositions and methods for stabilizing rare cells in blood specimens, preserving the quality of blood specimens, and also serving as cell fixatives are disclosed which minimize losses of target cells (for example, circulating tumor cells) and formation of debris and aggregates from target cells, non-target cells and plasma components, thereby allowing more accurate analysis and classification of circulating tumor cells (CTC) and, ultimately, of tumor burdens in cancer patients. Stabilization of specimens is particularly desirable in protocols requiring rare cell enrichment from blood specimens drawn from cancer patients. Exposure of such specimens to potentially stressful conditions encountered, for example, in normal processing, mixing, shaking, delays due to transporting the blood, has been observed to not only diminish the number of CTC but also to generate debris and aggregates in the blood specimens that were found to interfere with accurate enumeration of target cells, if present. Stabilizers are necessary to discriminate between in vivo CTC disintegration and in vitro sample degredation.
Owner:VERIDEX LCC
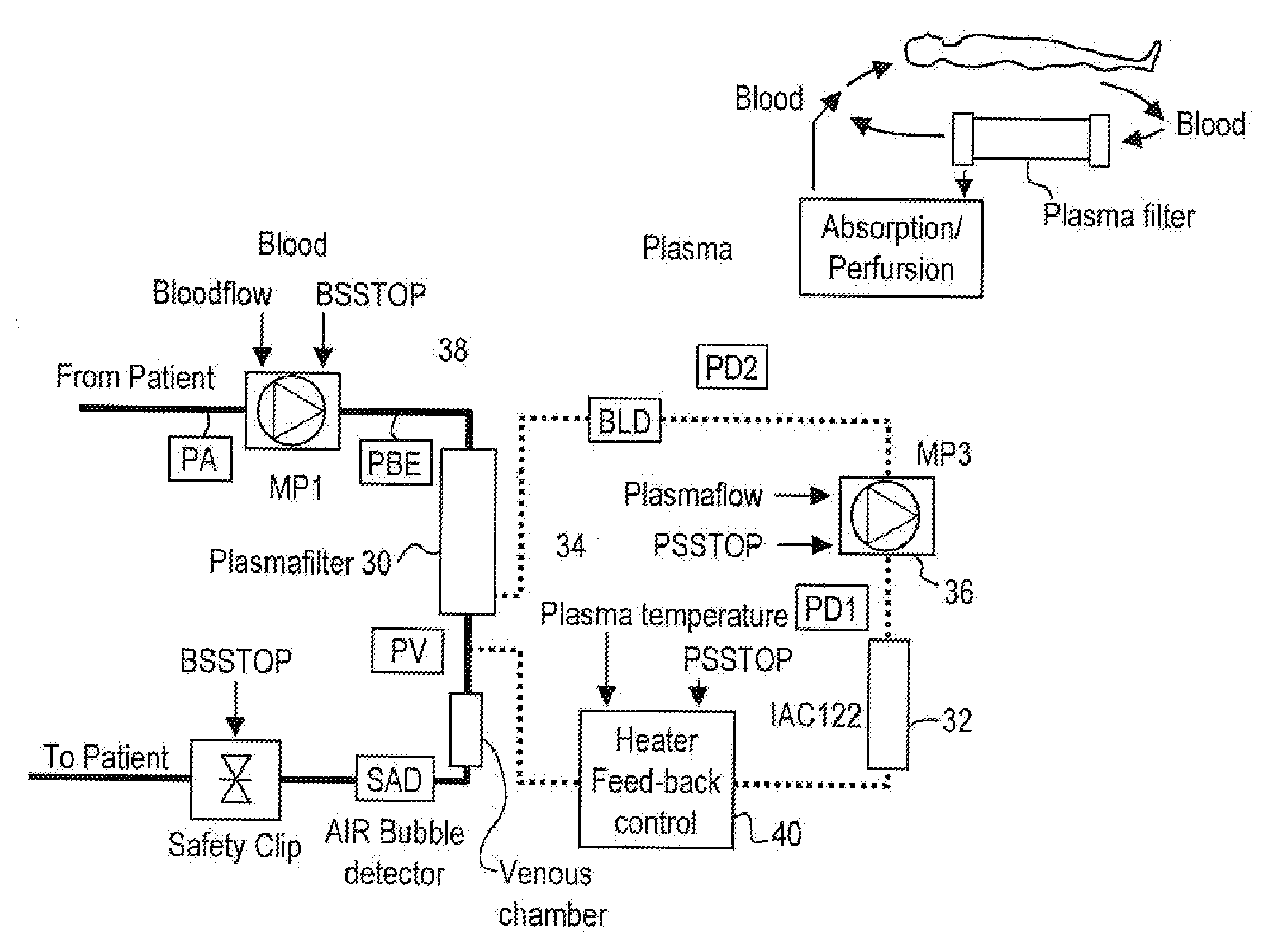
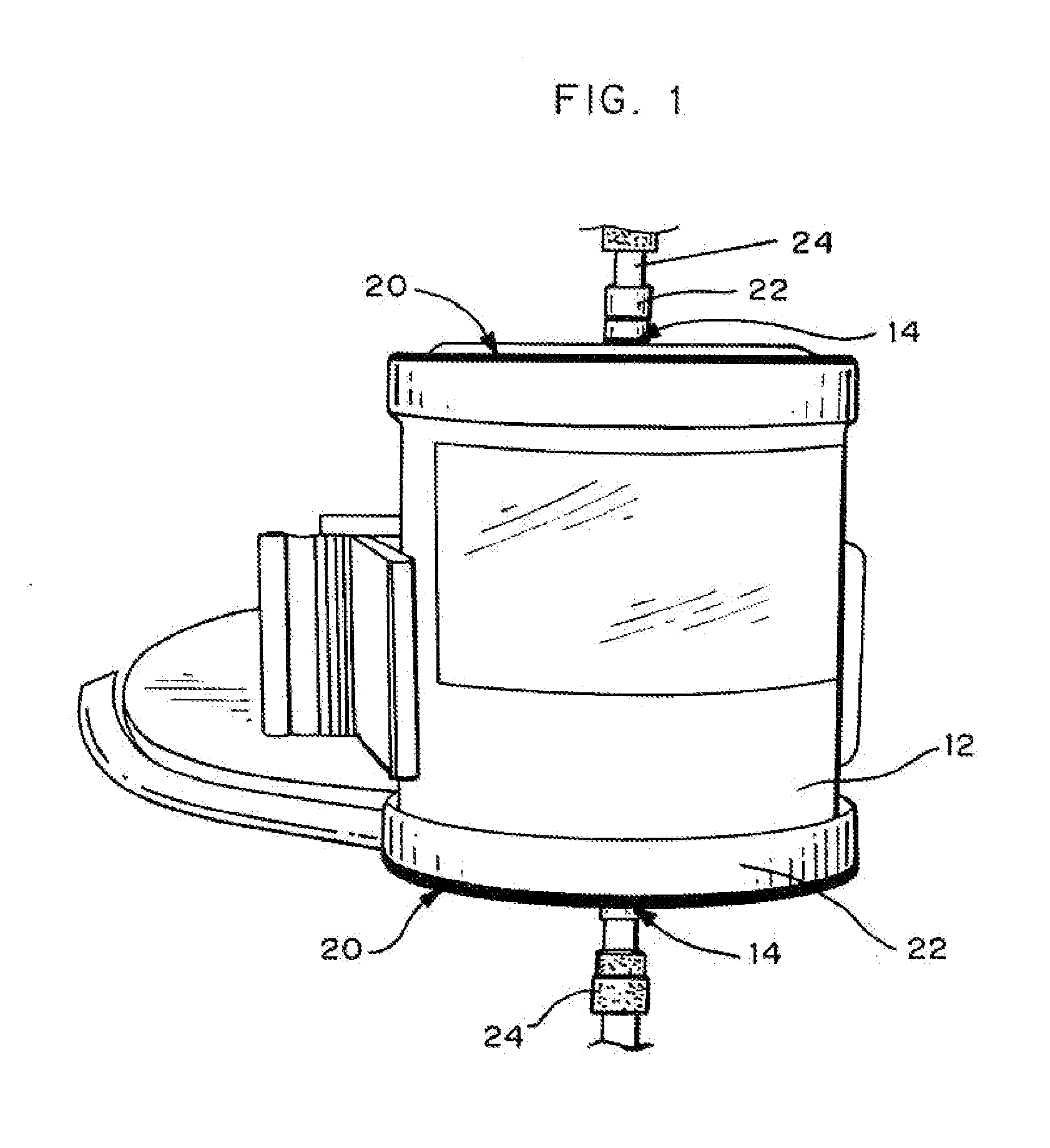
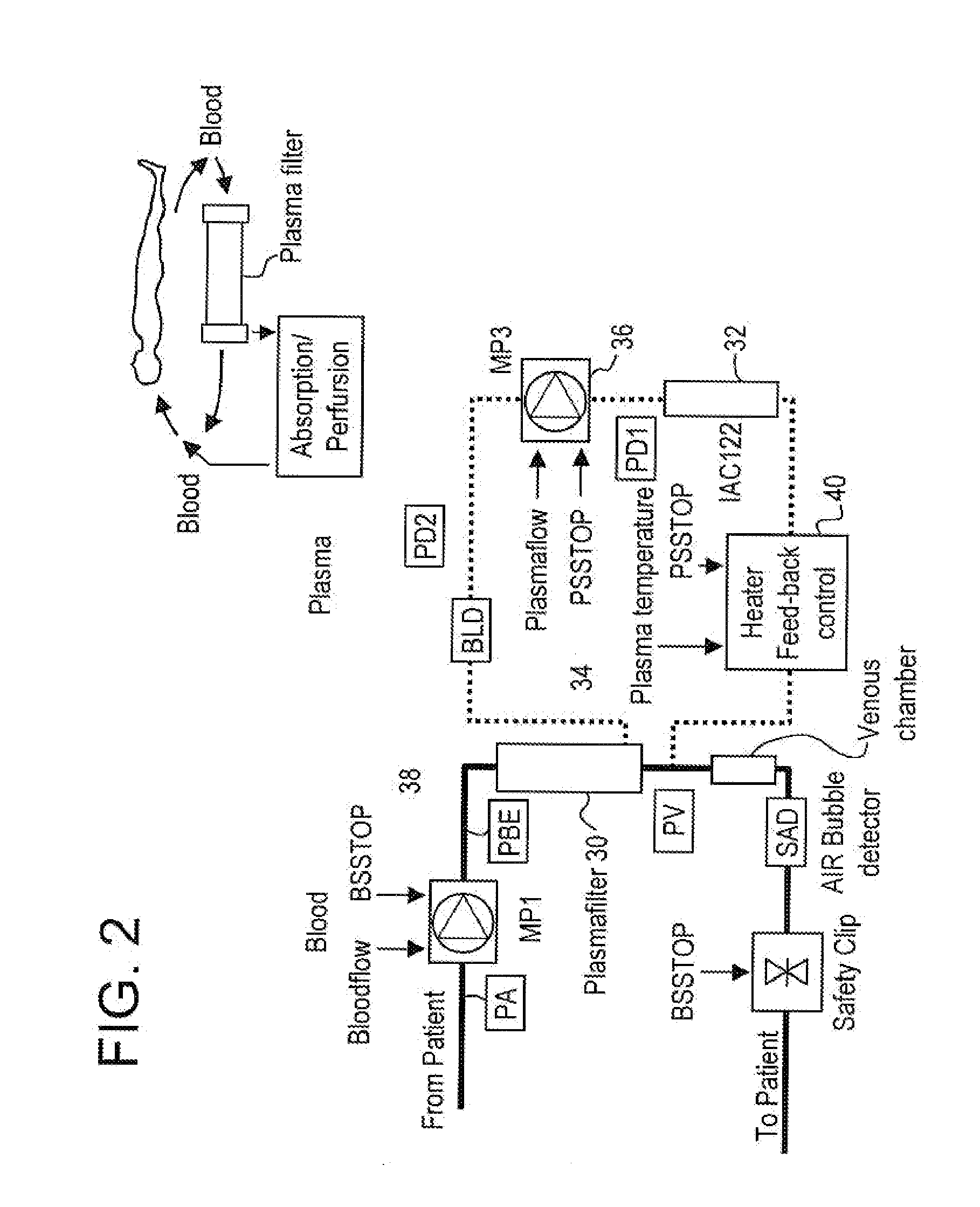
Method and system to remove soluble TNFR1, TNFR2, and IL2 in patients
InactiveUS20050265996A1Induce remissionPeptide/protein ingredientsHaemofiltrationDiseaseAntiendomysial antibodies
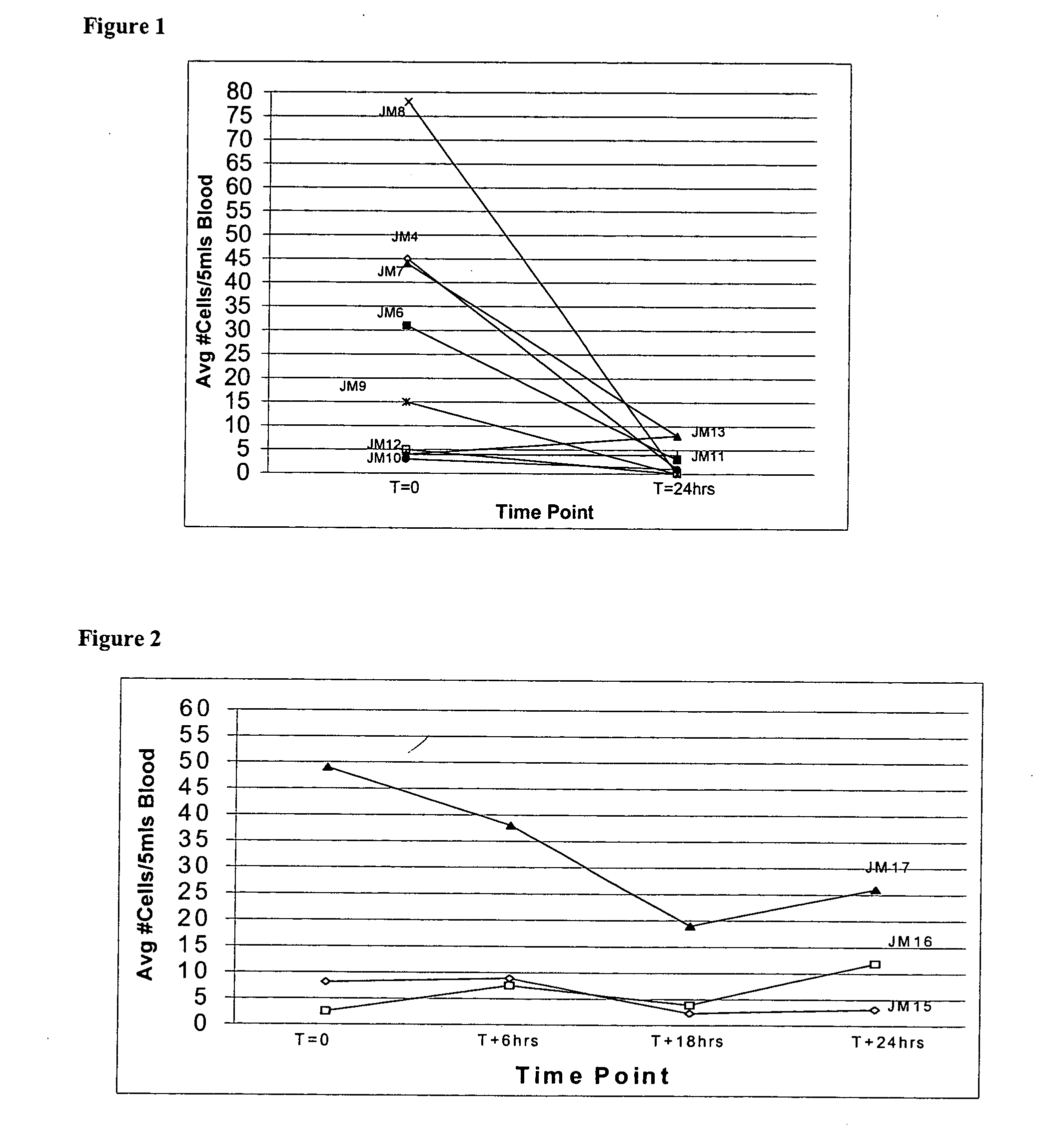
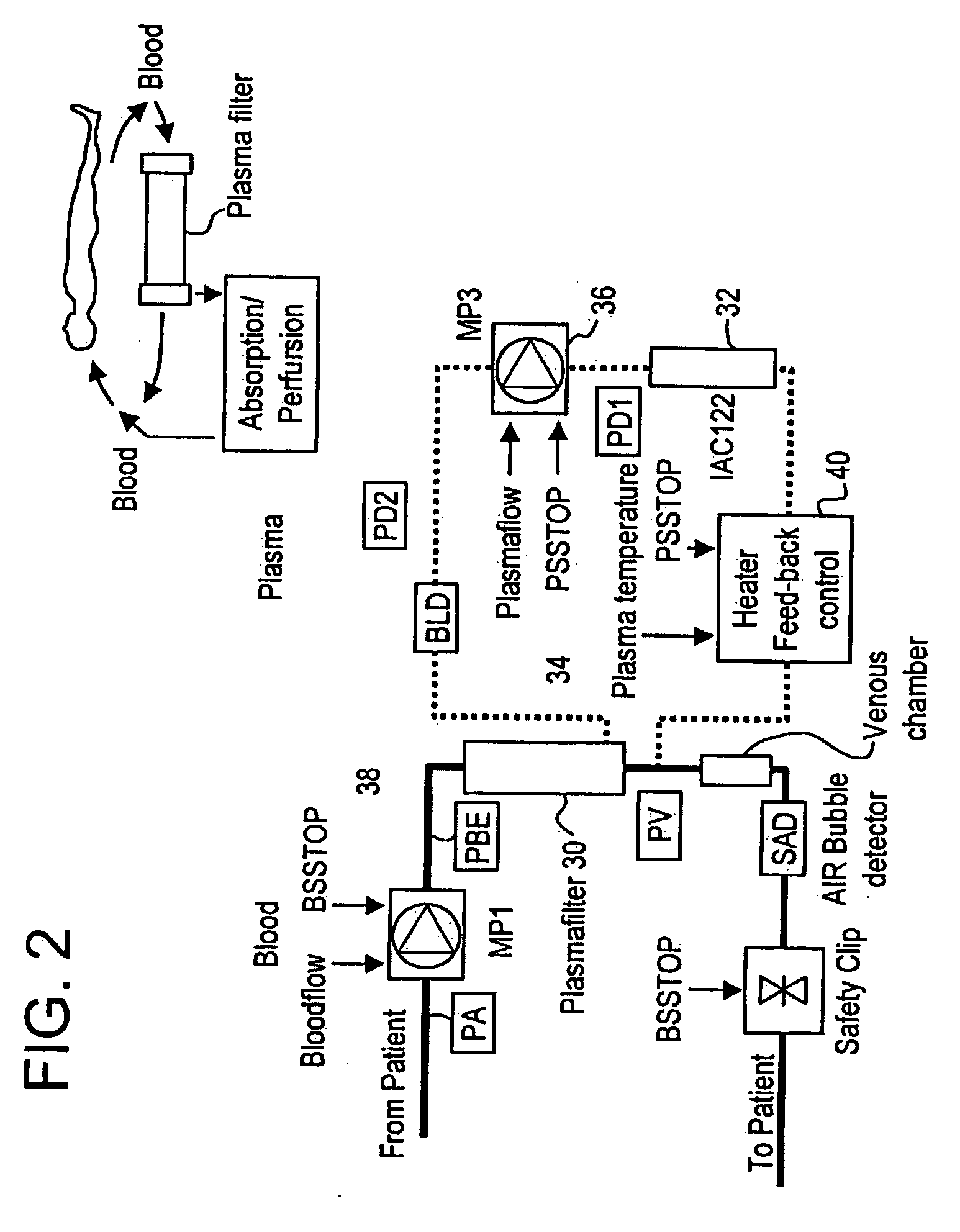
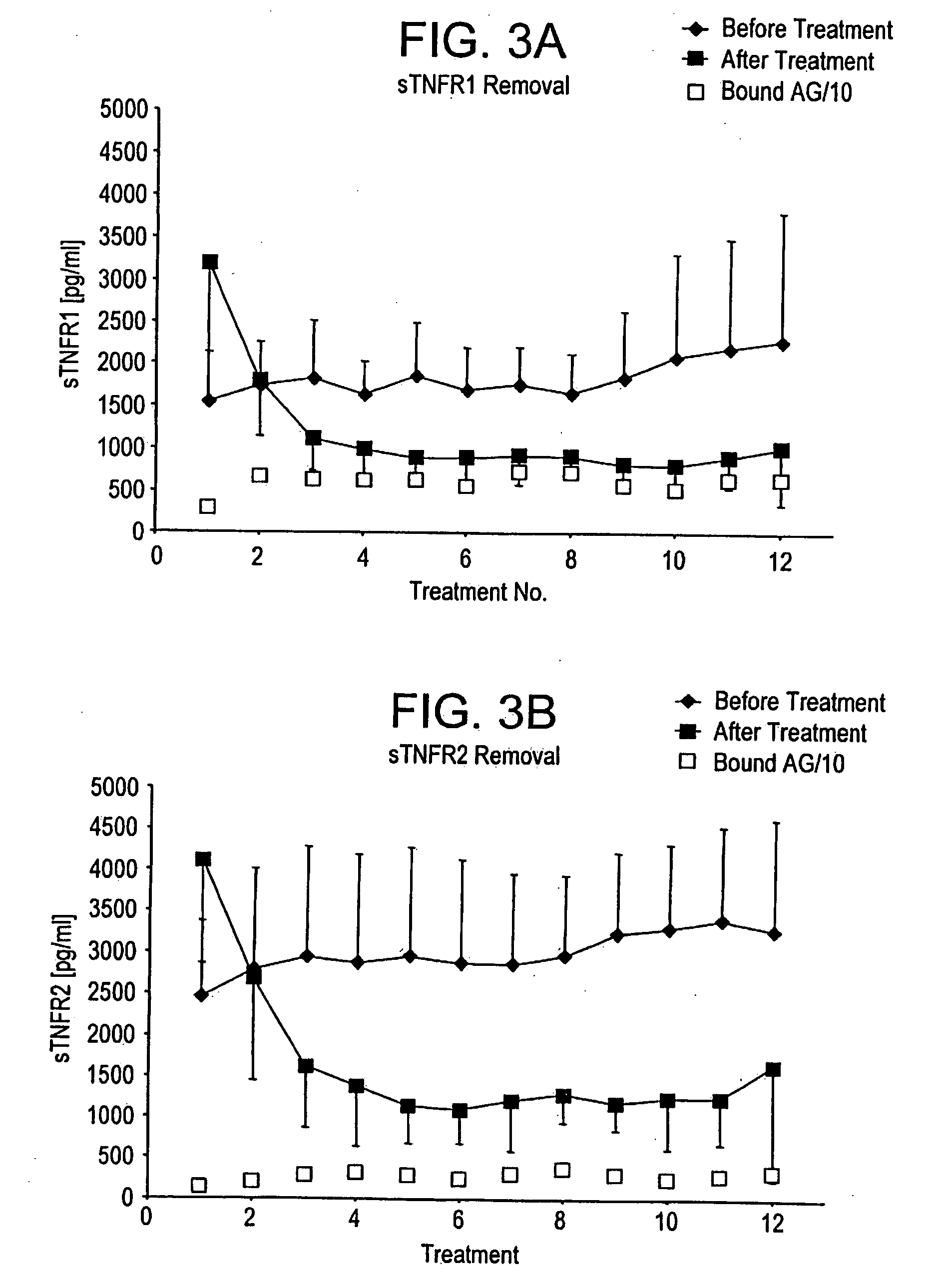
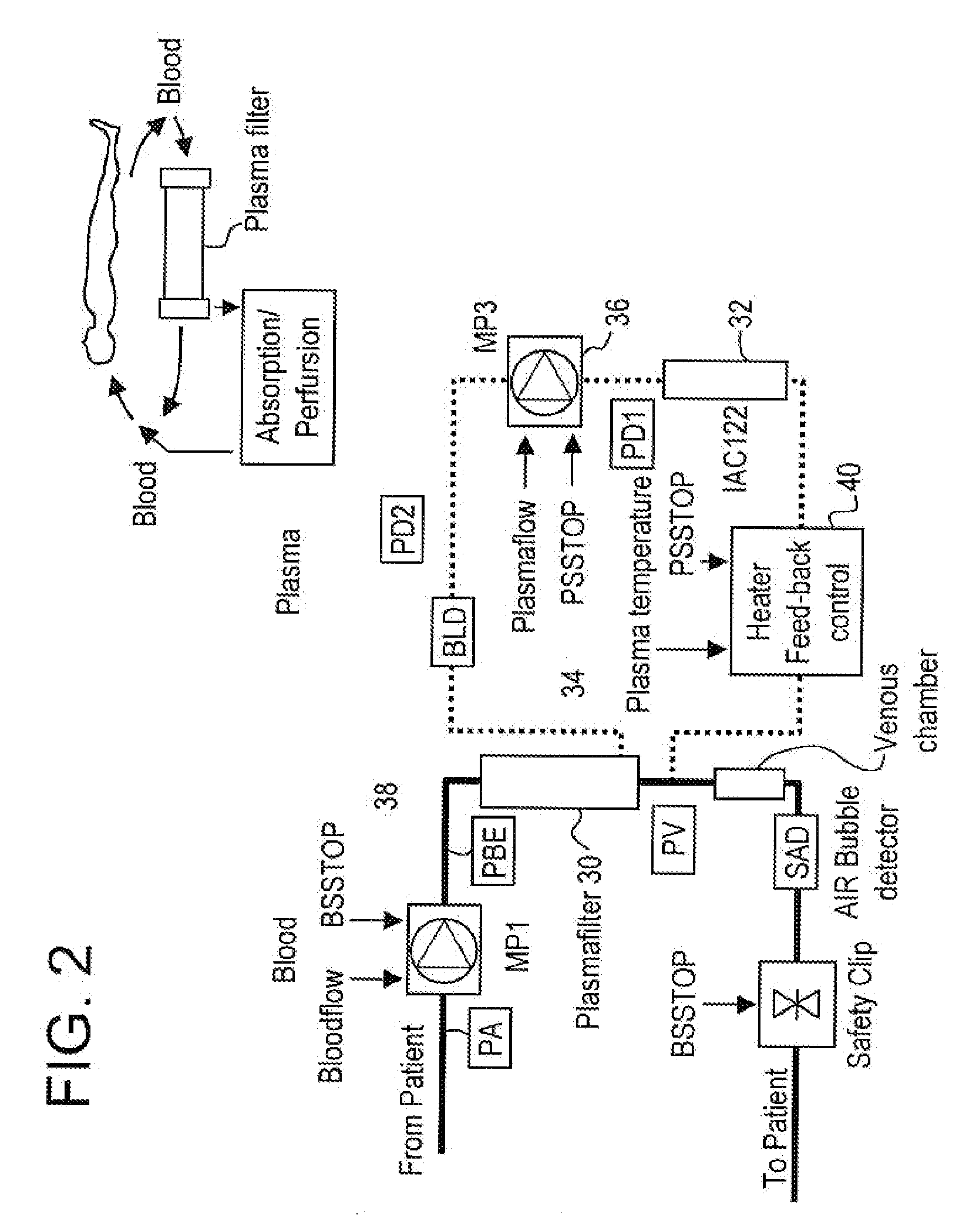
A method, and system, to induce remission in diseases characterized by excess production of sTNR and interleukin 2 has been developed. In the most preferred embodiment, the system consists of antibodies to sTNFR1, sTNFR2 and sIL2R immobilized in a column containing a material such as SEPHAROSE™. The patient is connected to a pheresis machine which separates the blood into the plasma and red cells, and the plasma is circulated through the column until the desired reduction in levels of sTNFR1, sTNFR2, and IL2 is achieved, preferably to less than normal levels. In the preferred method, patients are treated three times a week for four weeks. This process can be repeated after a period of time. Clinical studies showed reduction in tumor burden in patients having failed conventional chemotherapy and radiation treatments.
Owner:INNATUS CORP
Stabilization of cells and biological specimens for analysis
InactiveUS20060194192A1Improve stabilityMaintain qualityOrganic active ingredientsDead animal preservationAbnormal tissue growthIn vivo
Owner:JANSSEN DIAGNOSTICS LLC
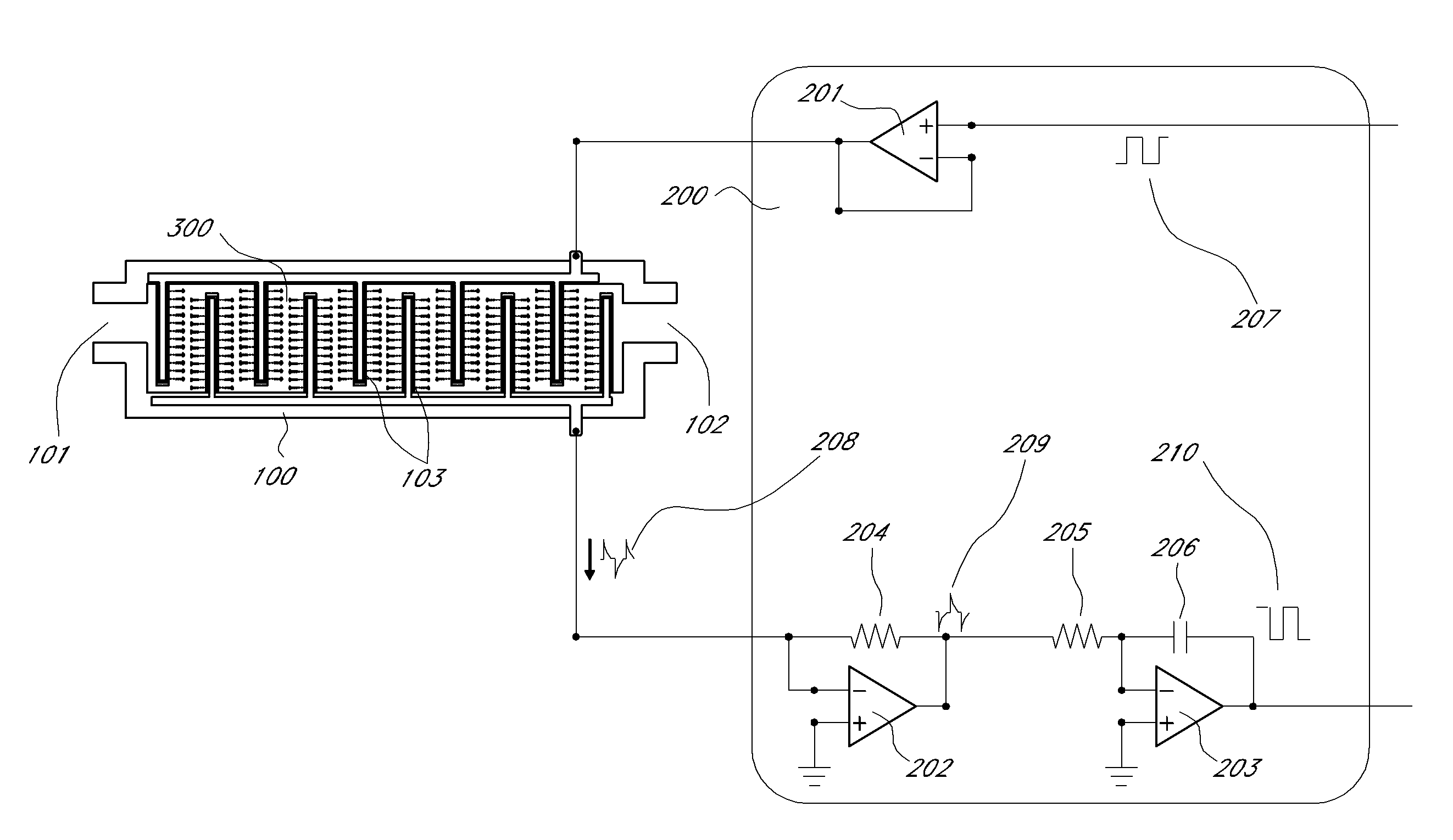
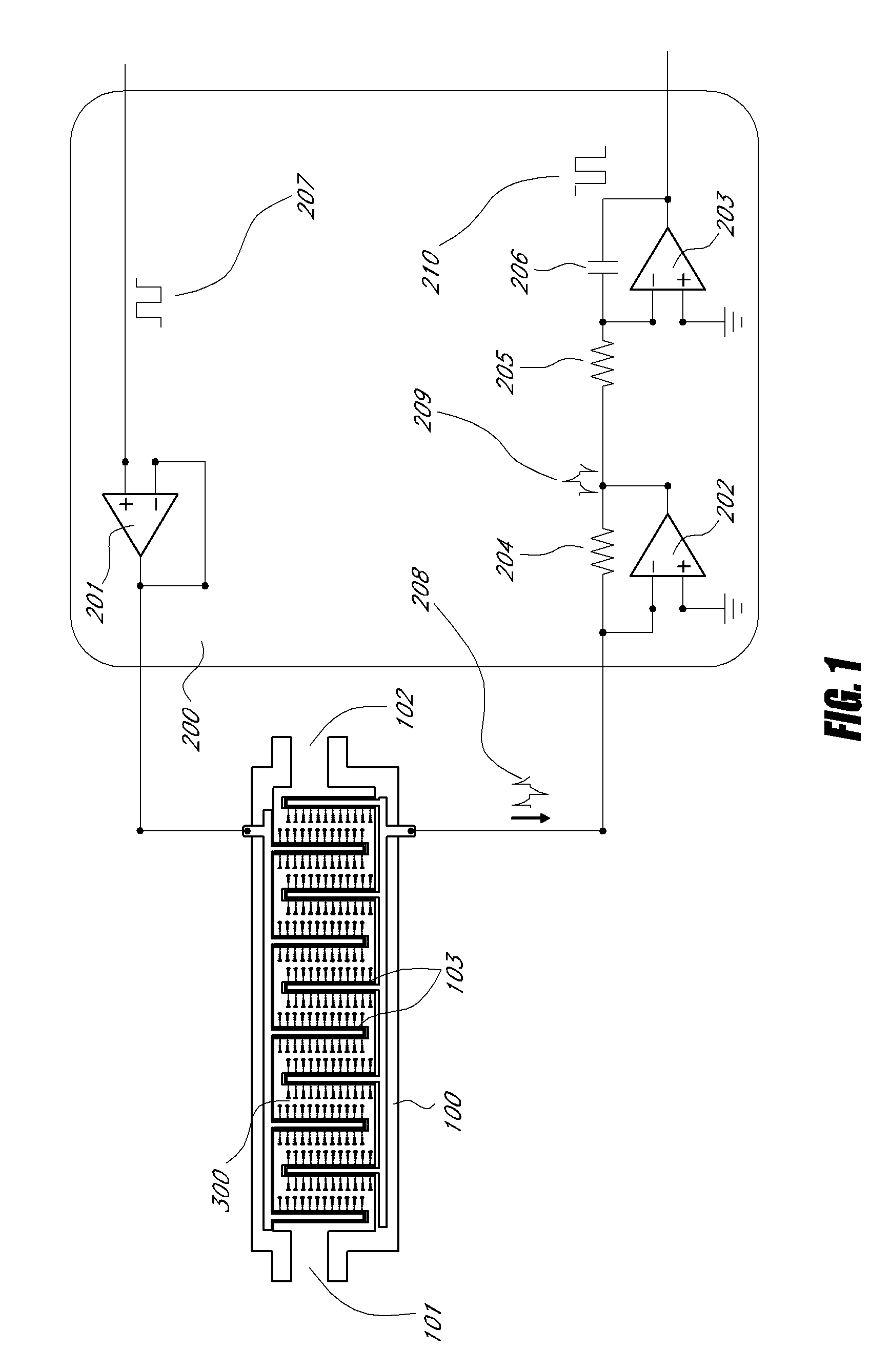
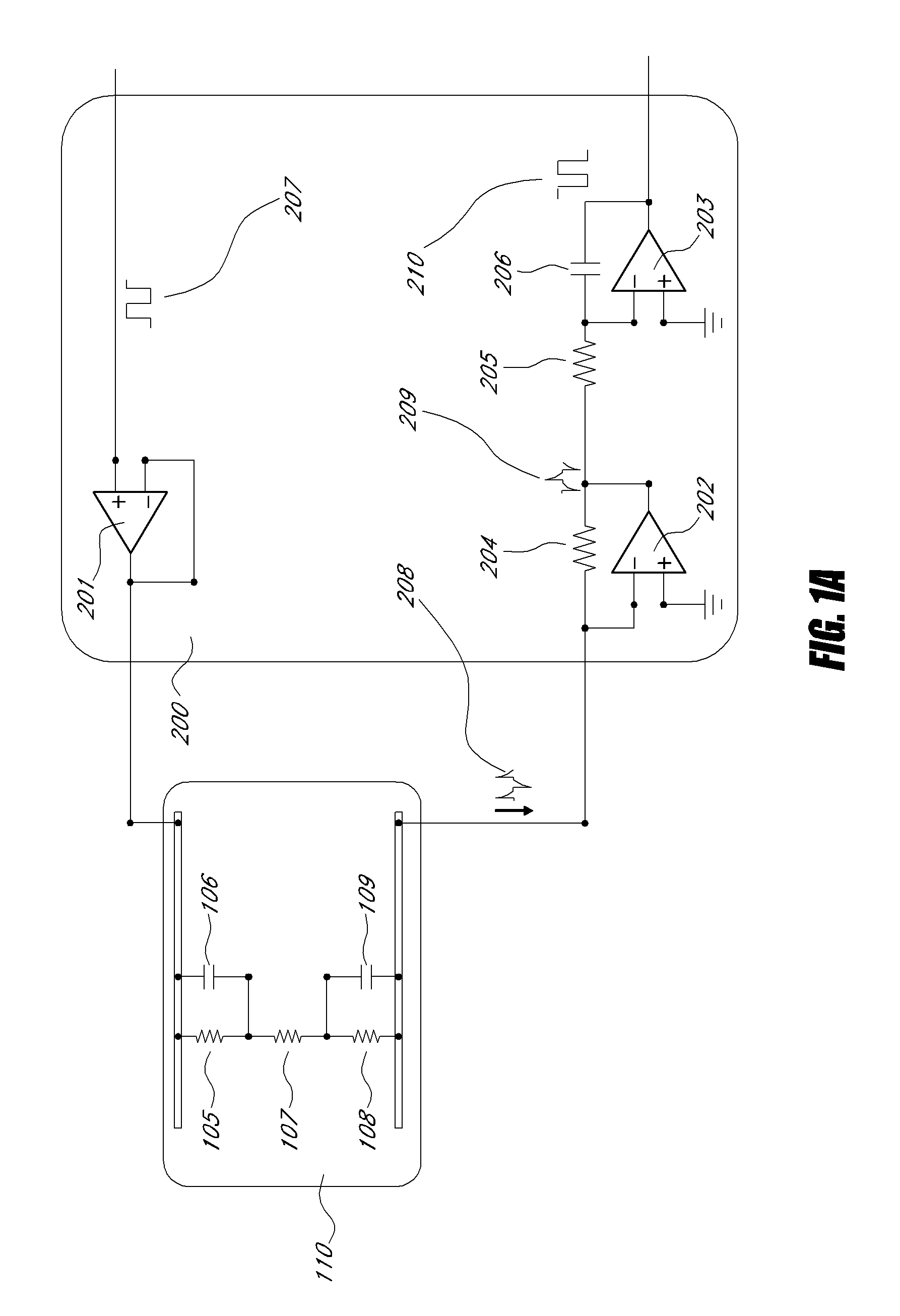
Method and apparatus for detecting and regulating vascular endothelial growth factor (VEGF) by forming a homeostatic loop employing a half-antibody biosensor
ActiveUS20100260679A1Maximize detection surface areaPeptide librariesBiological material analysisAbnormal tissue growthIn vivo
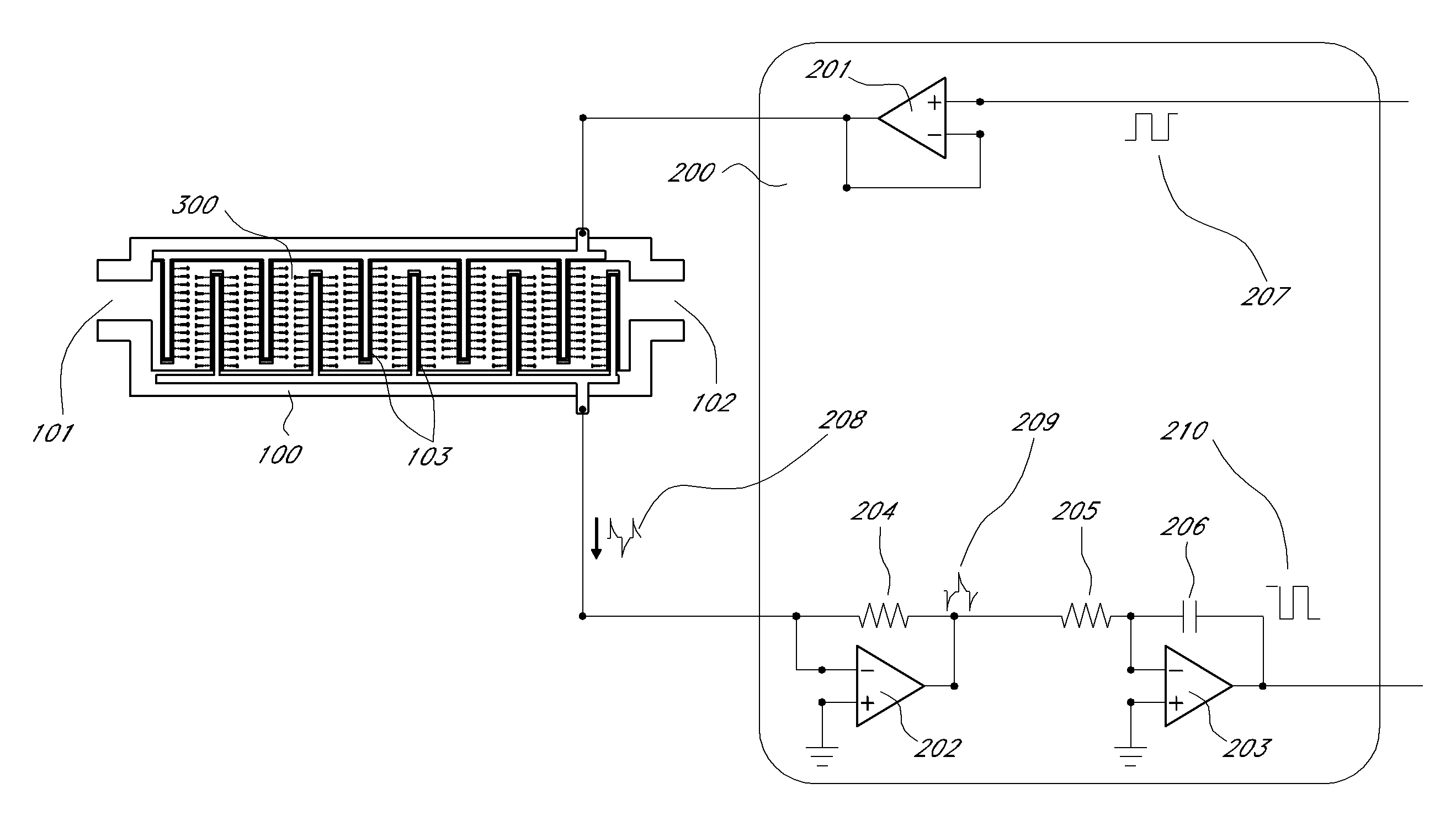
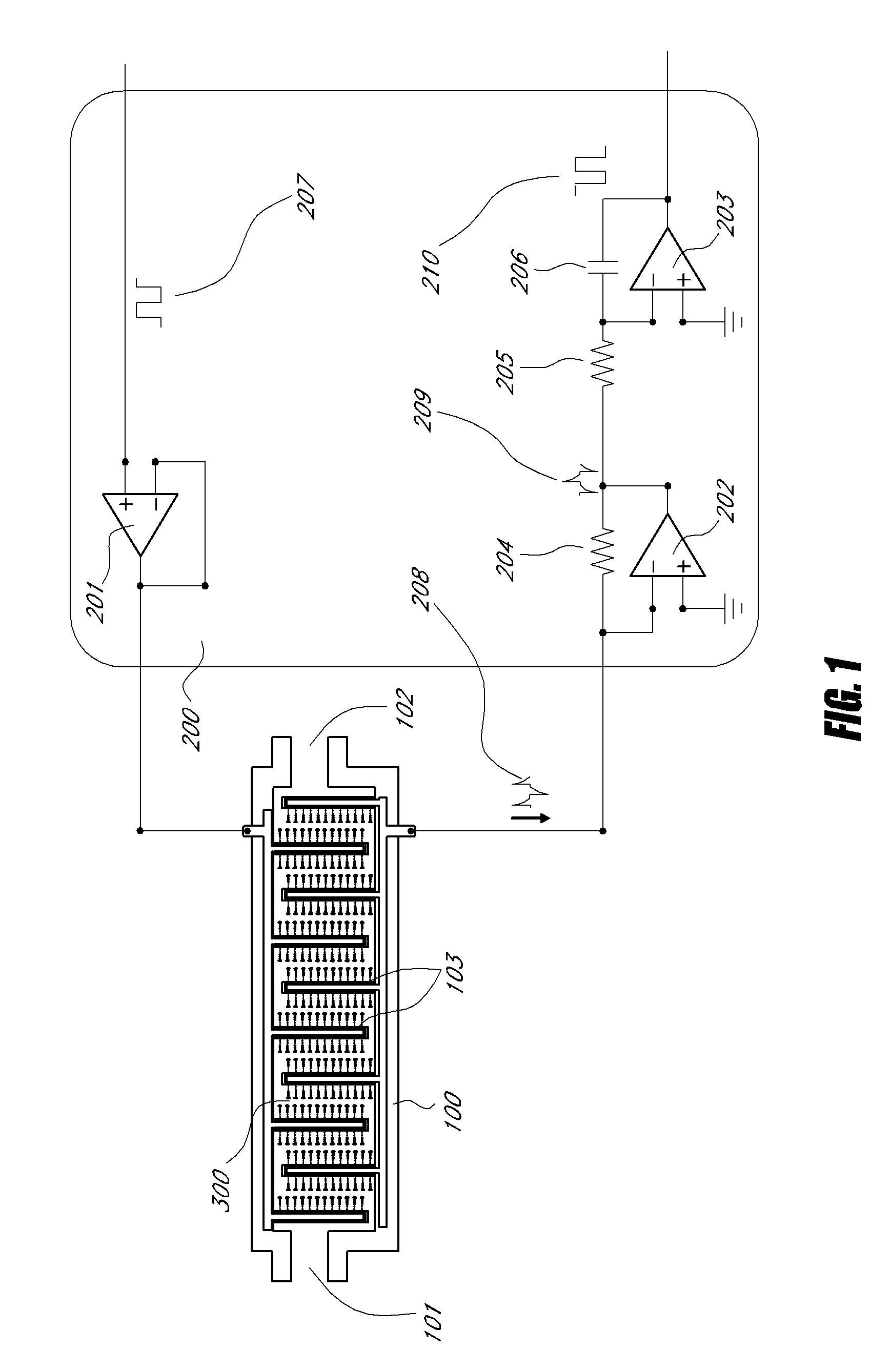
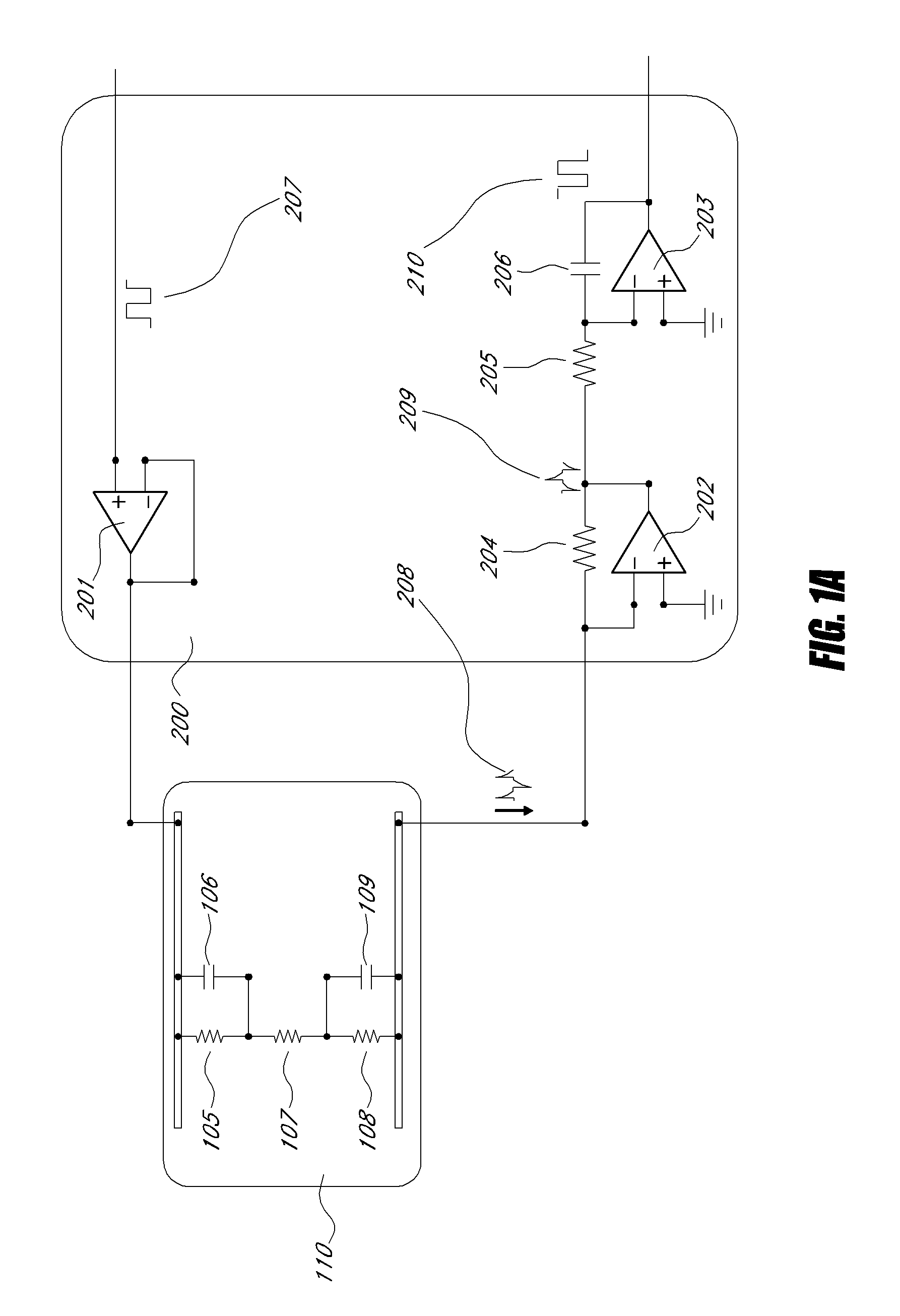
A biosensor for detection of vascular endothelial growth factor (VEGF) hybridization uses an array of parallel capacitors to detect electrochemical binding of circulating VEGF to immobilized anti-VEGF monoclonal half-antibodies (a-VEGF mhAb). Binding of a-VEGF mhAb modulates the threshold voltage of a circuit, changing the impedance of the circuit. An electrode coated with a p-Si substrate enhances the affinity between the VEGF molecules. A fluid cell delivers VEGF samples onto the active surface of the chip. An array of parallel capacitors arranged in an interdigitated pattern detects the VEGF in the fluid. The detector provides an accurately measured and quantifiable rate of change of the VEGF molecules in vivo, providing real time feedback which is used to measure response of the tumor to delivered chemotherapeutic agents and biological response modifiers (BRMs) for the purpose of determining tumor burden and efficacy of the chemotherapy as part of a homeostatic loop for chemotherapy.
Owner:SENSOR KINESIS
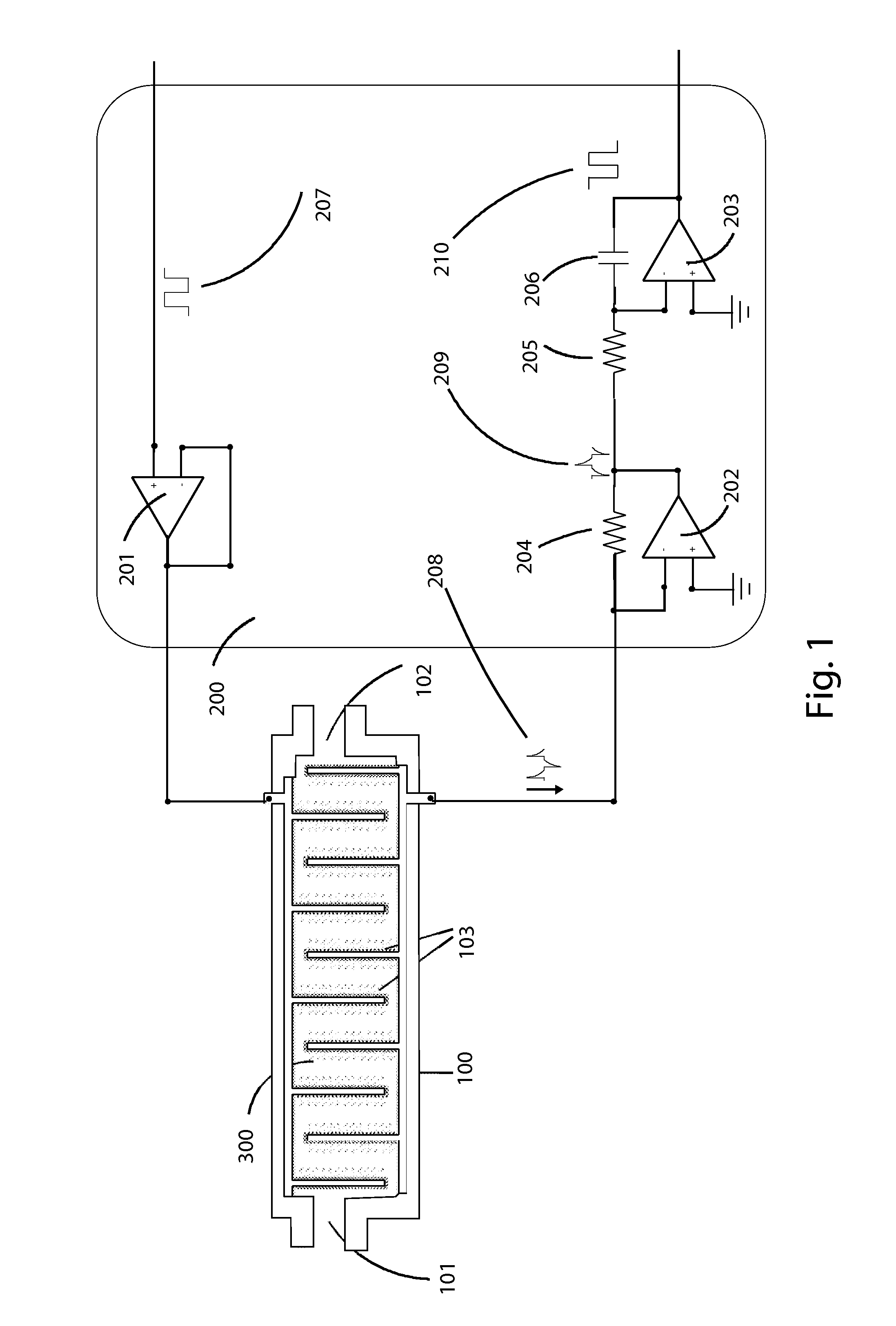
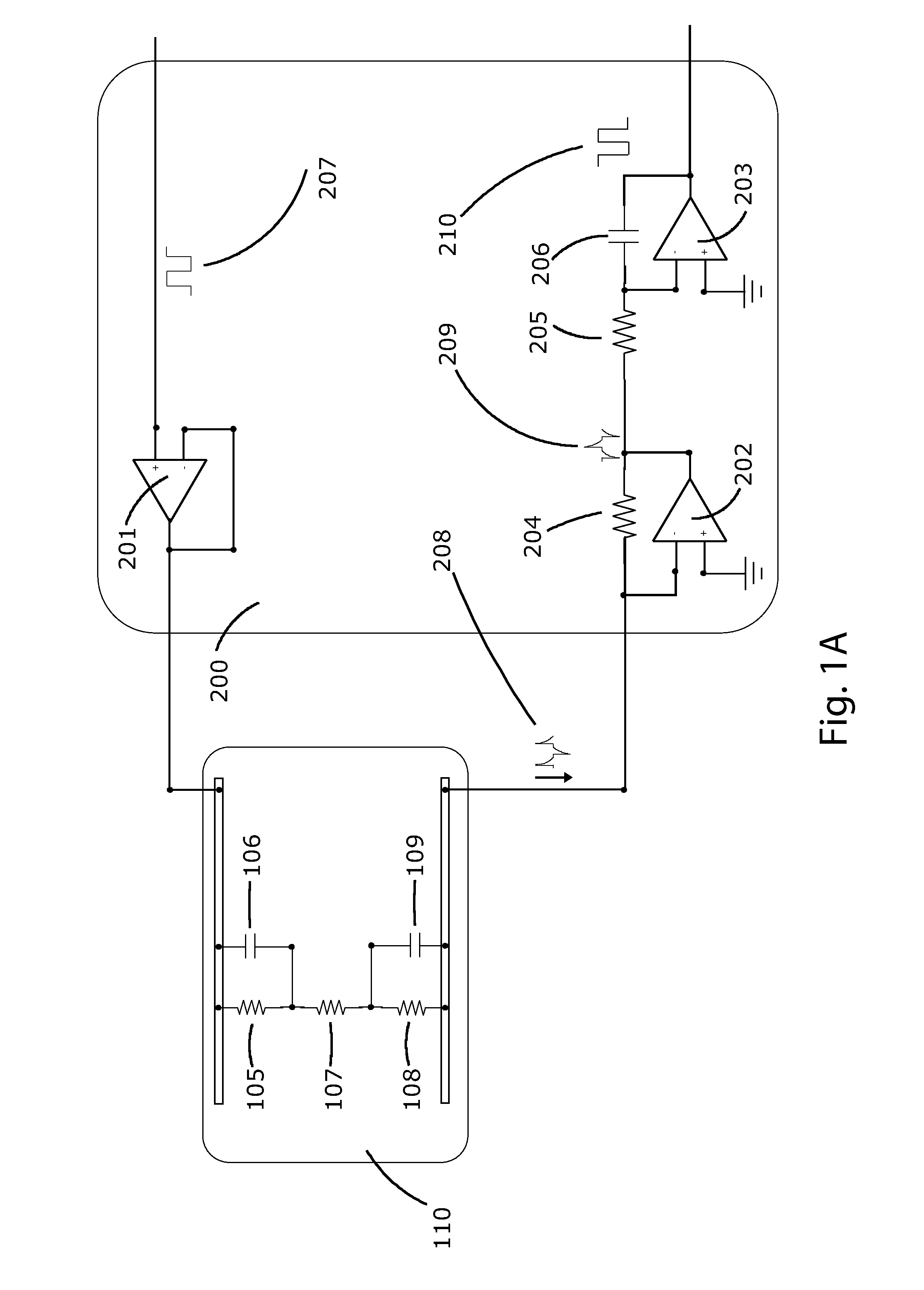
Method for measuring tumor burden in patient derived xenograft (PDX) mice
ActiveUS20180288982A1Easy to observeMicrobiological testing/measurementScreening processCirculating tumor DNAHuman patient
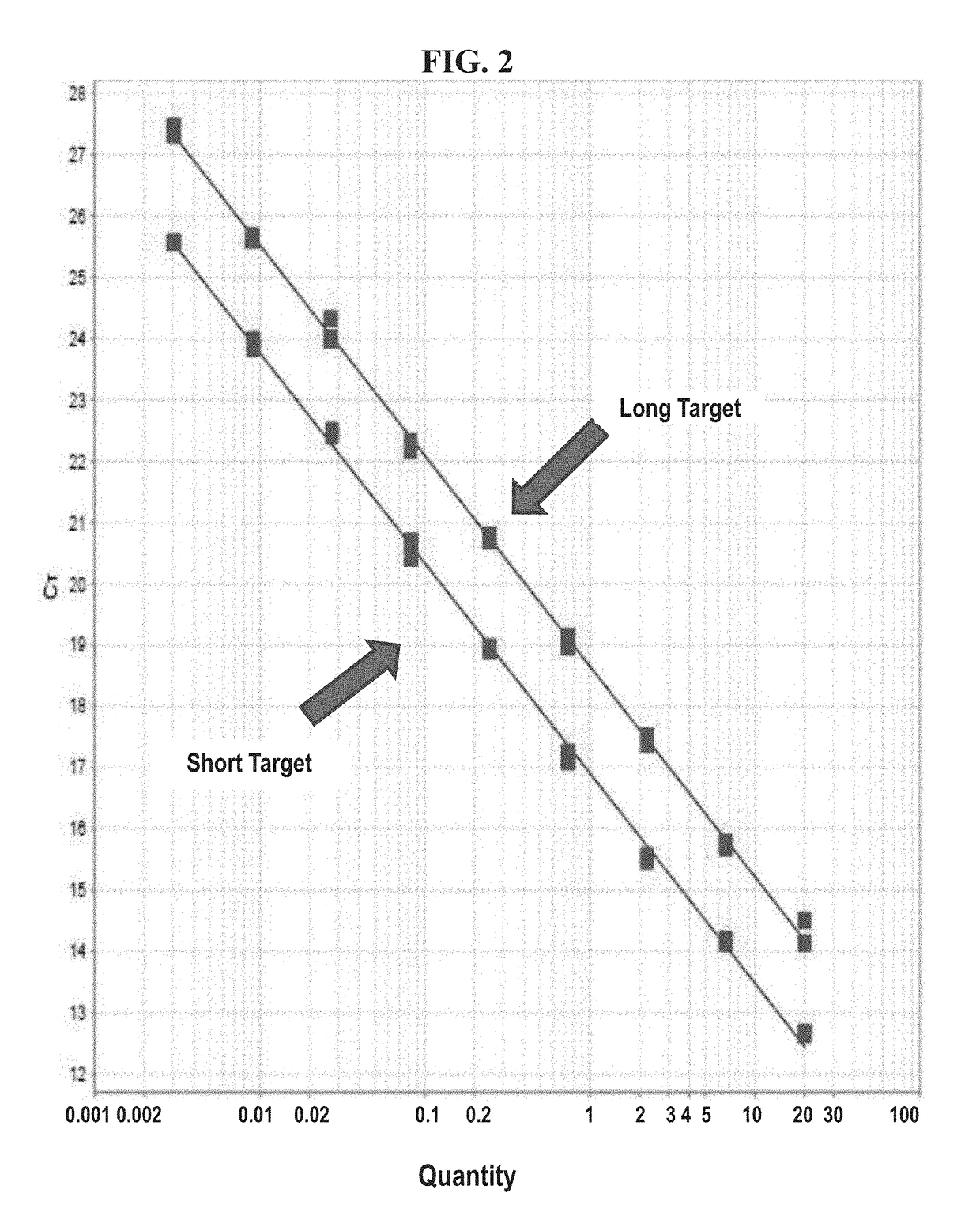
Kits and methods providing measurement of tumor burden in a patient derived xenograft (PDX) mouse are described. Exemplary embodiments contemplate taking a sample, typically a blood sample, from a PDX mouse and using a real-time polymerase chain reaction (PCR) system to quantitate both human patient circulating tumor DNA (ctDNA) and mouse DNA. In preferred embodiments, both PCR amplifications are done simultaneously in a multiplex, and a highly polymorphic human DNA target sequence is amplified for high sensitivity, allowing for small volume samples, typically 50-100 μL, of mouse blood. Serial evaluations are possible because the mouse can survive withdrawal of these small volumes of blood. A related method allows for quantitation of ctDNA in the presence of human immune cells added to a “humanized” mouse. These relatively quick and easy methods of determining tumor burden in PDX mice can have predictive value for the efficacy of cancer treatments in human patients.
Owner:LIFE GENETICS LAB
Elisa assay of serum soluble cd22 to assess tumor burnden/relapse in subjects with leukemia and lymphoma
InactiveUS20050244828A1Microbiological testing/measurementBiological material analysisAbnormal tissue growthTumor Load
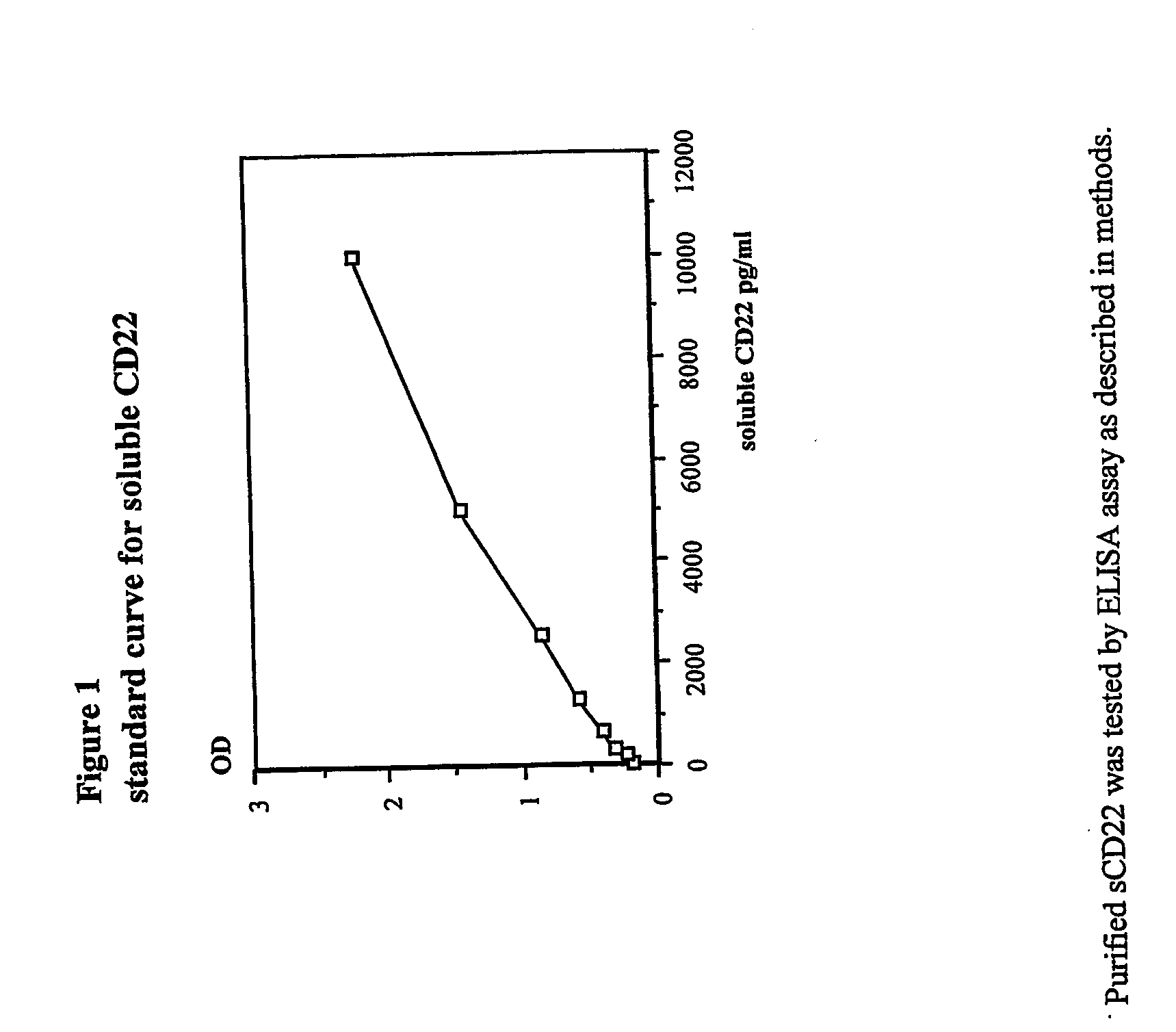
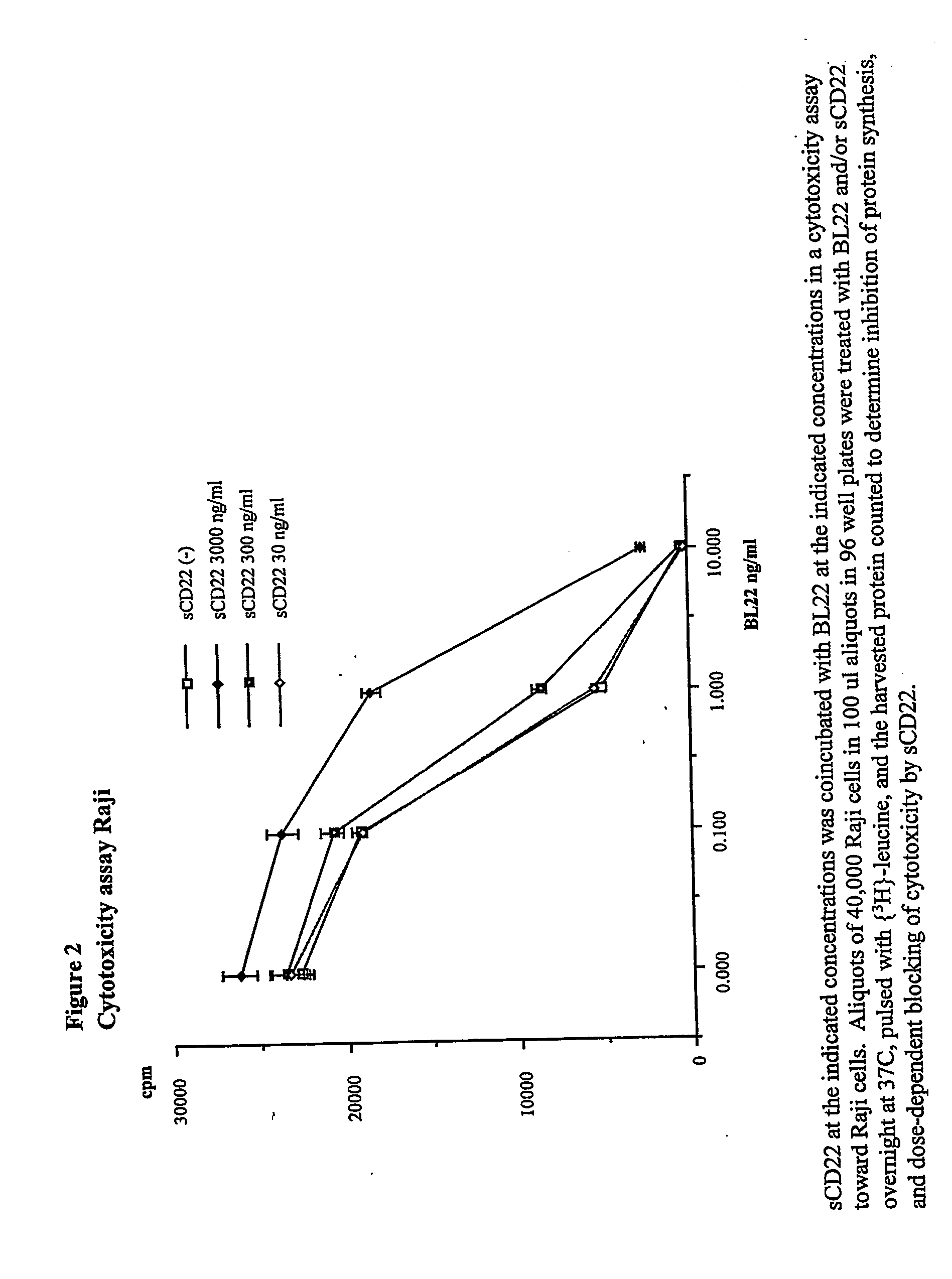
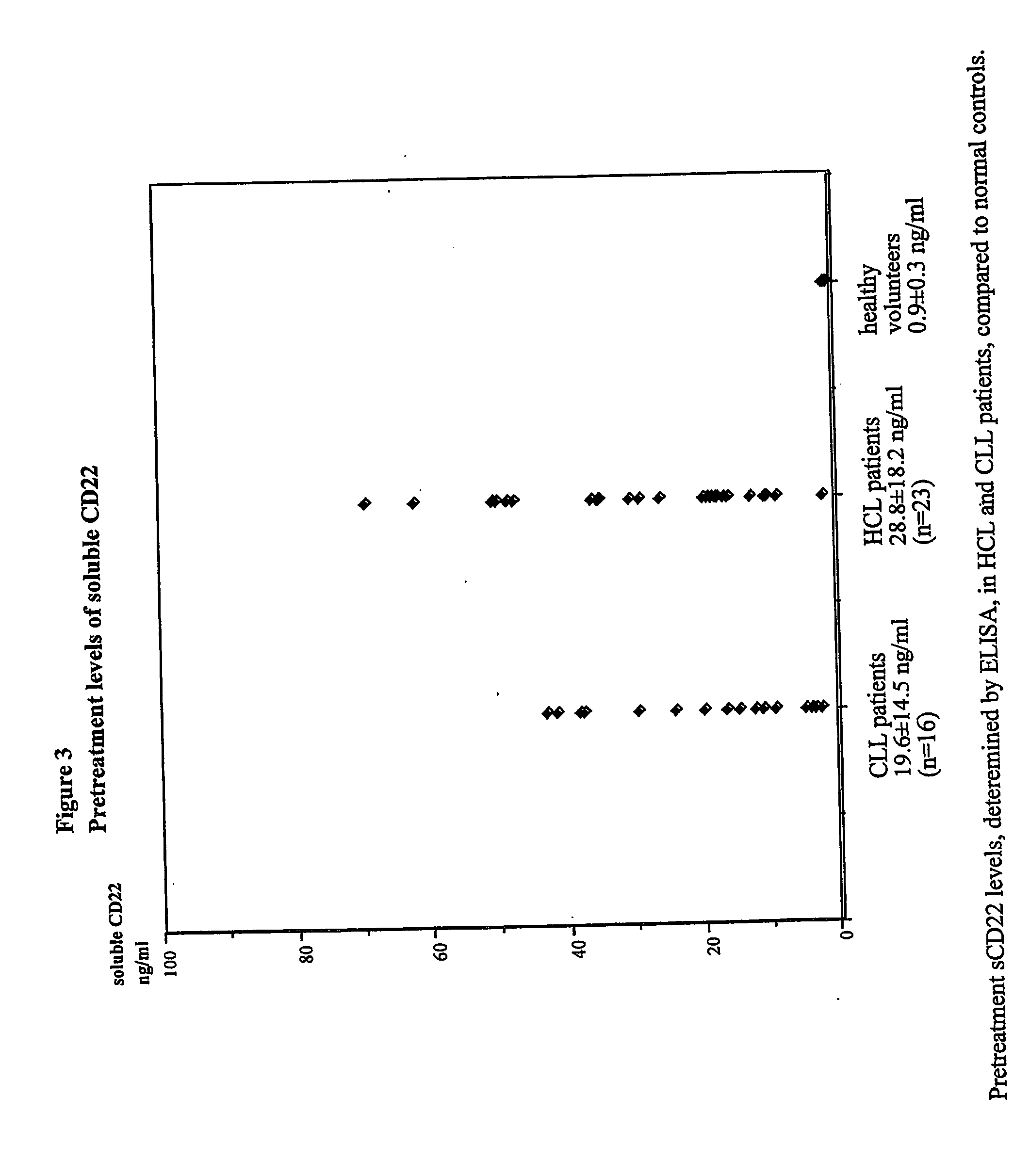
Disclosed herein are methods of using previously unknown soluble forms of CD22 (sCD22) present in the serum of subjects with B-cell leukemias and lymphomas to assess tumor burden in the subjects. Also disclosed are methods of diagnosing or prognosing development or progression of a B-cell lymphoma or leukemia in a subject, including detecting sCD22 in a body fluid sample taken or derived from the subject, for instance serum.
Owner:GOVERNMENT OF THE UNITED STATES OF AMERICAS AS REPRESENTED BY THE SEC OF THE DEPT OF HEALTH & HUMAN SERVICES THE
Regulated genes in cervical cancer
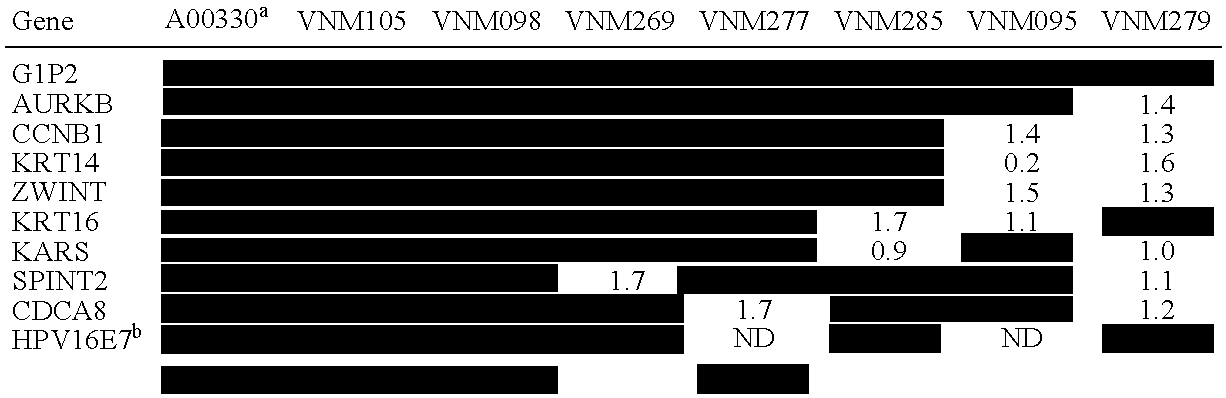
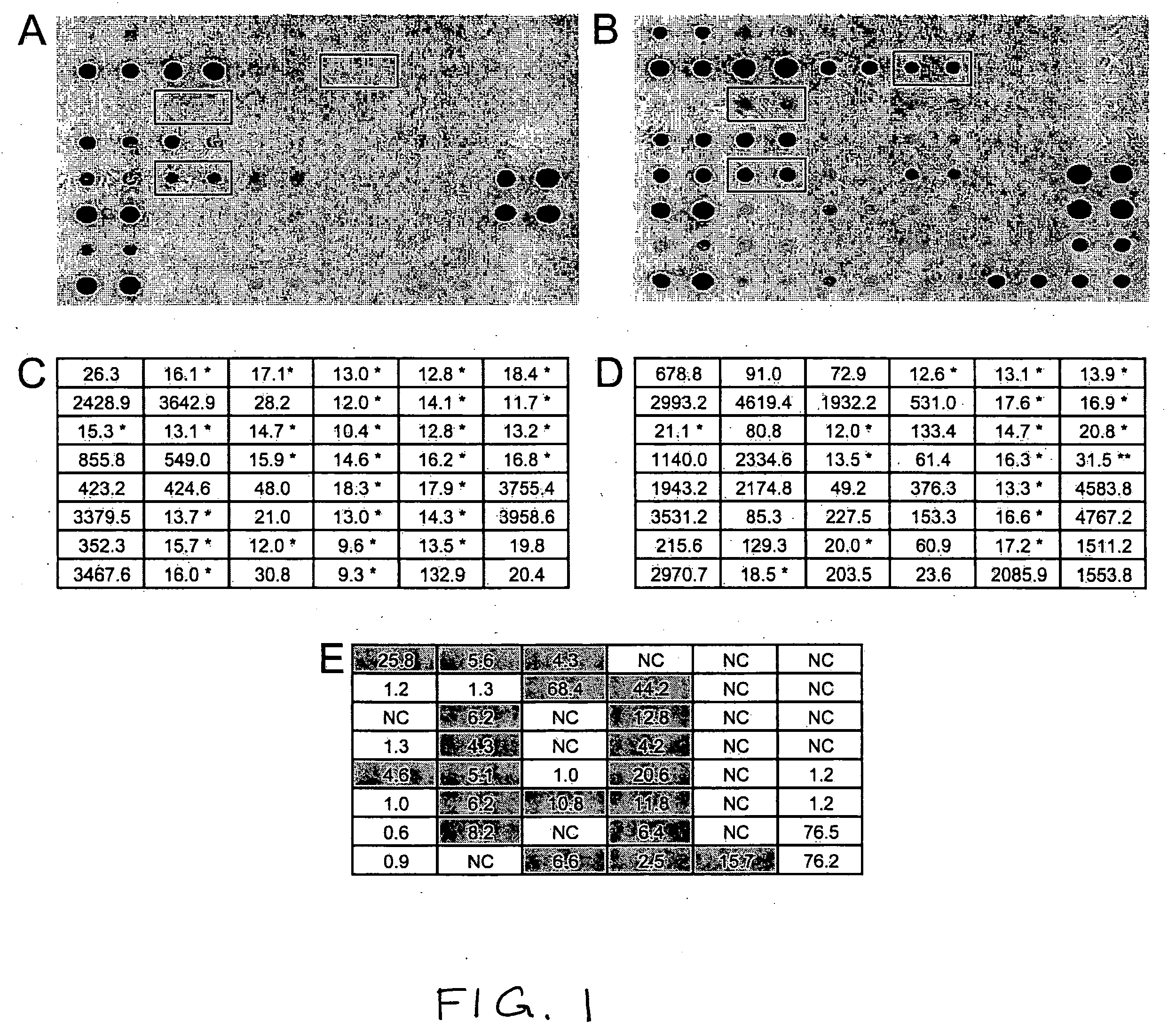
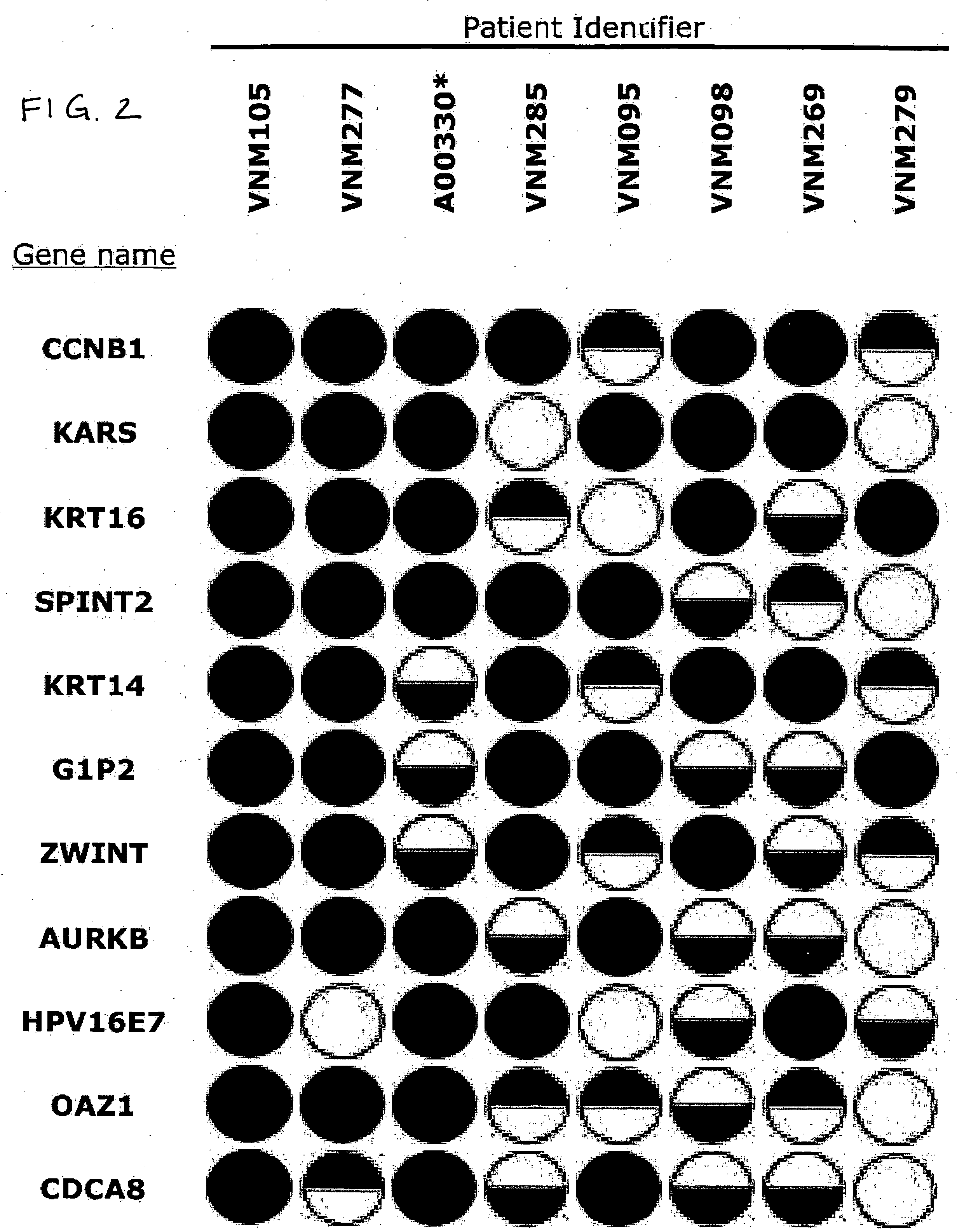
Polynucleotides, as well as polypeptides encoded thereby, that are differentially expressed in SCCC cells are provided. The polynucleotides find use in diagnosis of cancer, and classification of cancer cells according to expression profiles. The methods are useful for detecting cervical cancer cells, facilitating diagnosis of cervical cancer and the severity of the cancer (e.g., tumor grade, tumor burden, and the like) in a subject, facilitating a determination of the prognosis of a subject, and assessing the responsiveness of the subject to therapy.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
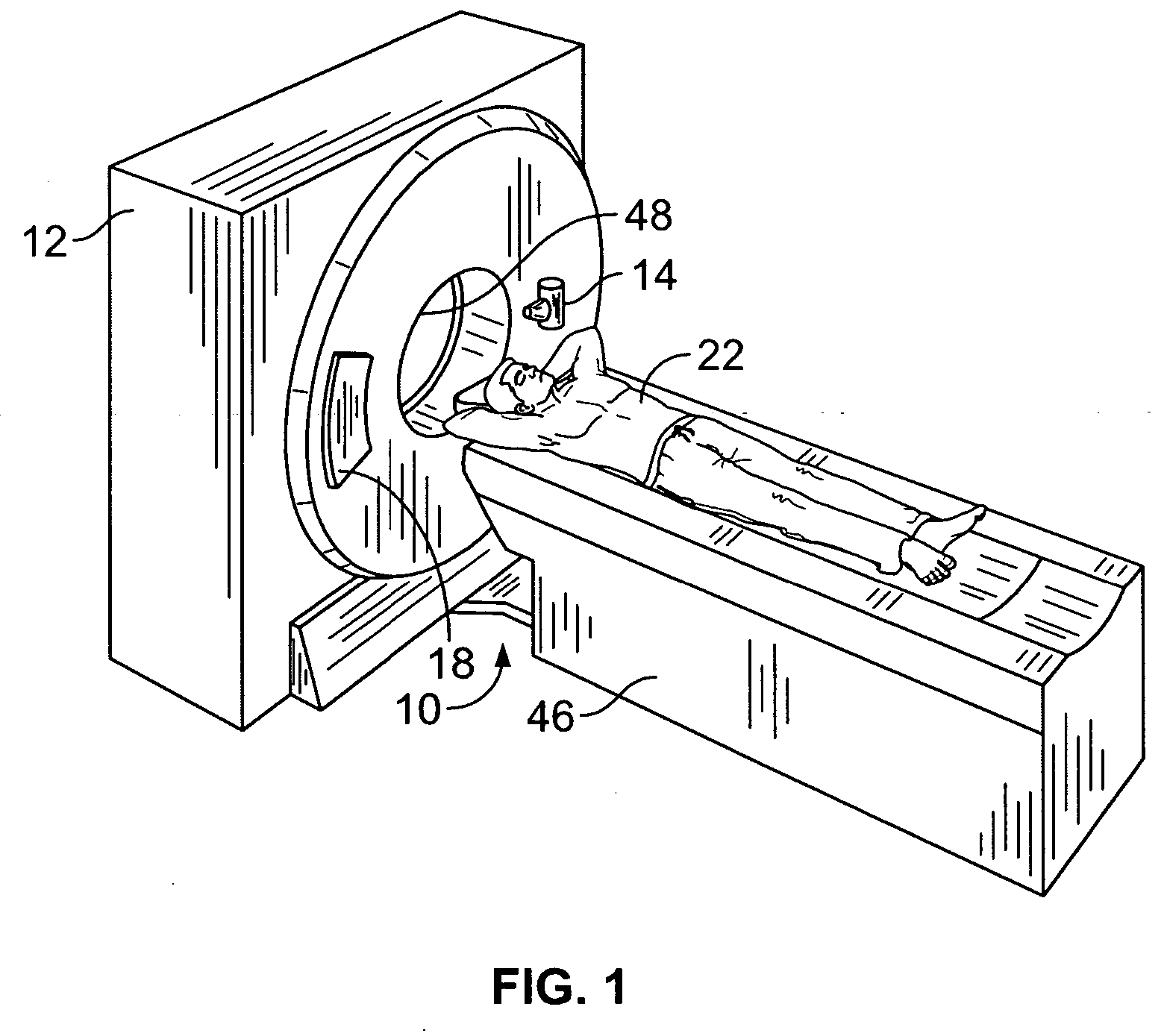
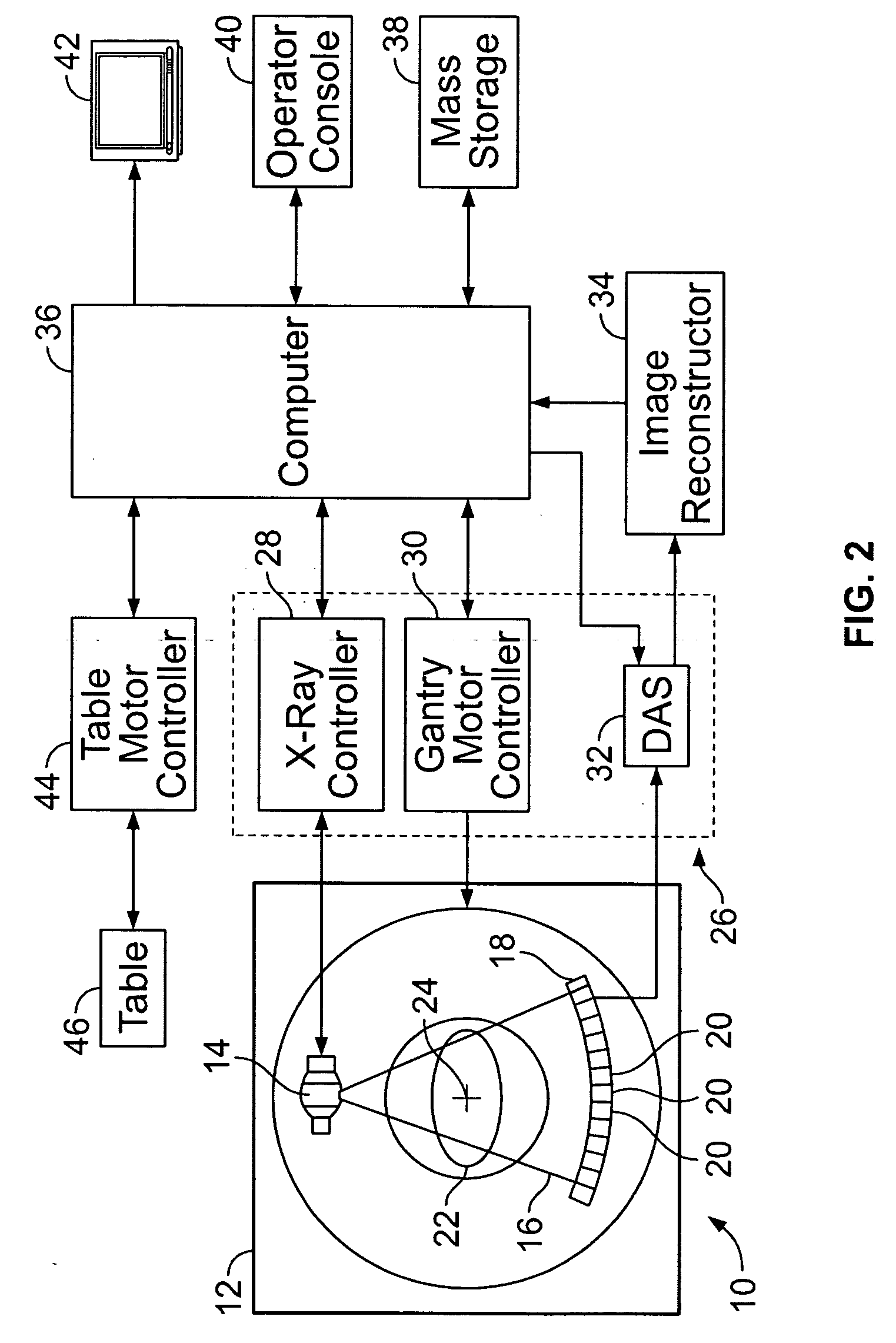
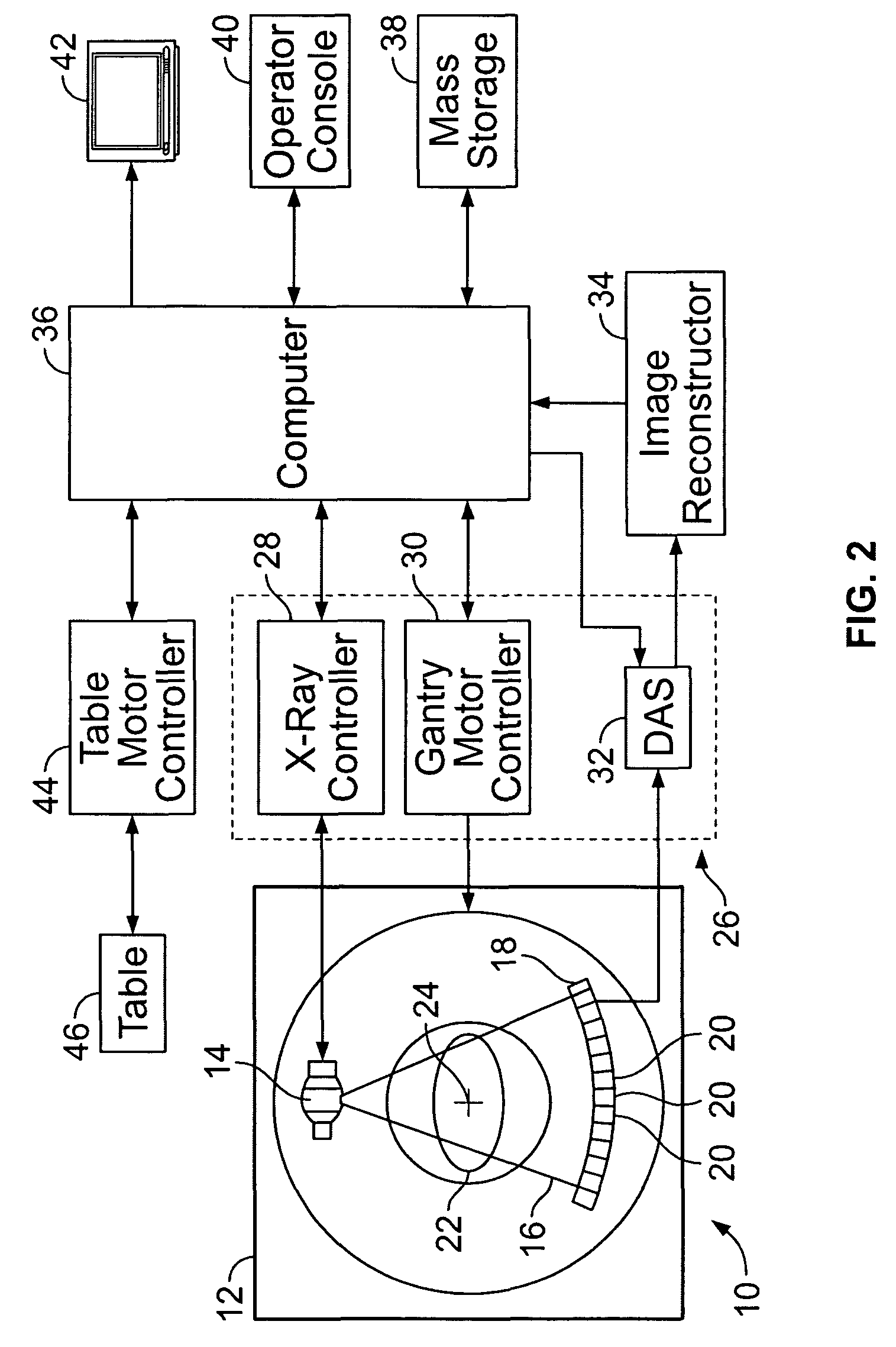
Methods and systems for monitoring tumor burden
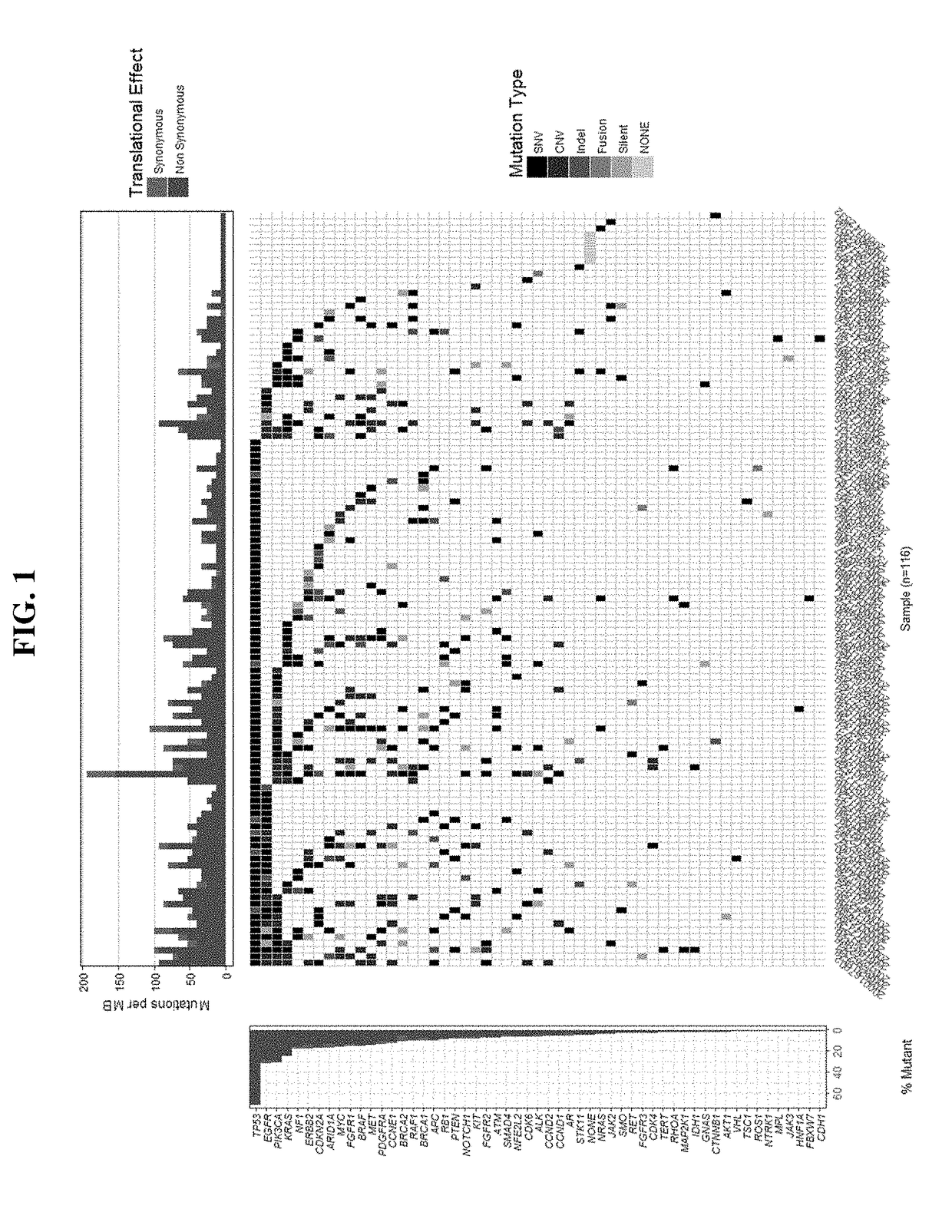
Methods and systems for quantification of a selected attribute of an image volume are provided. The system is configured to receive an image dataset for a volume of interest, process the dataset for a selected attribute based at least on one of shape and texture to obtain a plurality of responses, and compute an index of an aggregate of a plurality of obtained responses.
Owner:GENERAL ELECTRIC CO
Method and system to remove soluble tnfr1, tnfr2, and il2 in patients
A method, and system, to induce remission in diseases characterized by excess production of sTNR and interleukin 2 has been developed. In the most preferred embodiment, the system consists of antibodies to sTNFR1, sTNFR2 and sIL2R immobilized in a column containing a material such as SEPHAROSE™. The patient is connected to a pheresis machine which separates the blood into the plasma and red cells, and the plasma is circulated through the column until the desired reduction in levels of sTNFR1, sTNFR2, and IL2 is achieved, preferably to less than normal levels. In the preferred method, patients are treated three times a week for four weeks. This process can be repeated after a period of time. Clinical studies showed reduction in tumor burden in patients having failed conventional chemotherapy and radiation treatments.
Owner:INNATUS CORP
Method and apparatus for forming a homeostatic loop employing an aptamer biosensor
ActiveUS8145434B2Enhanced signalReliably detect in operationResistance/reactance/impedenceError detection/correctionCapacitanceElectrochemistry
Owner:SENSOR KINESIS
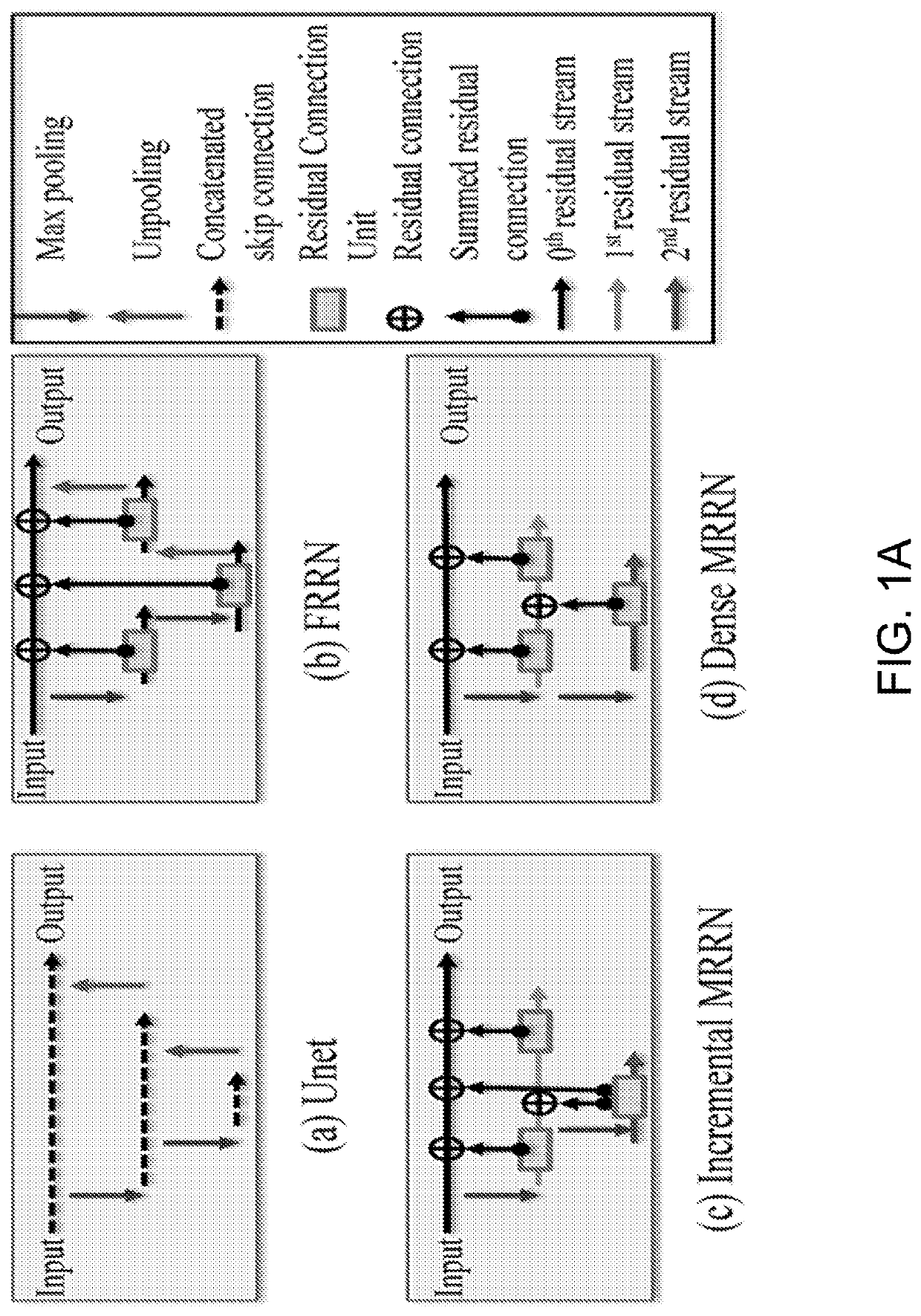
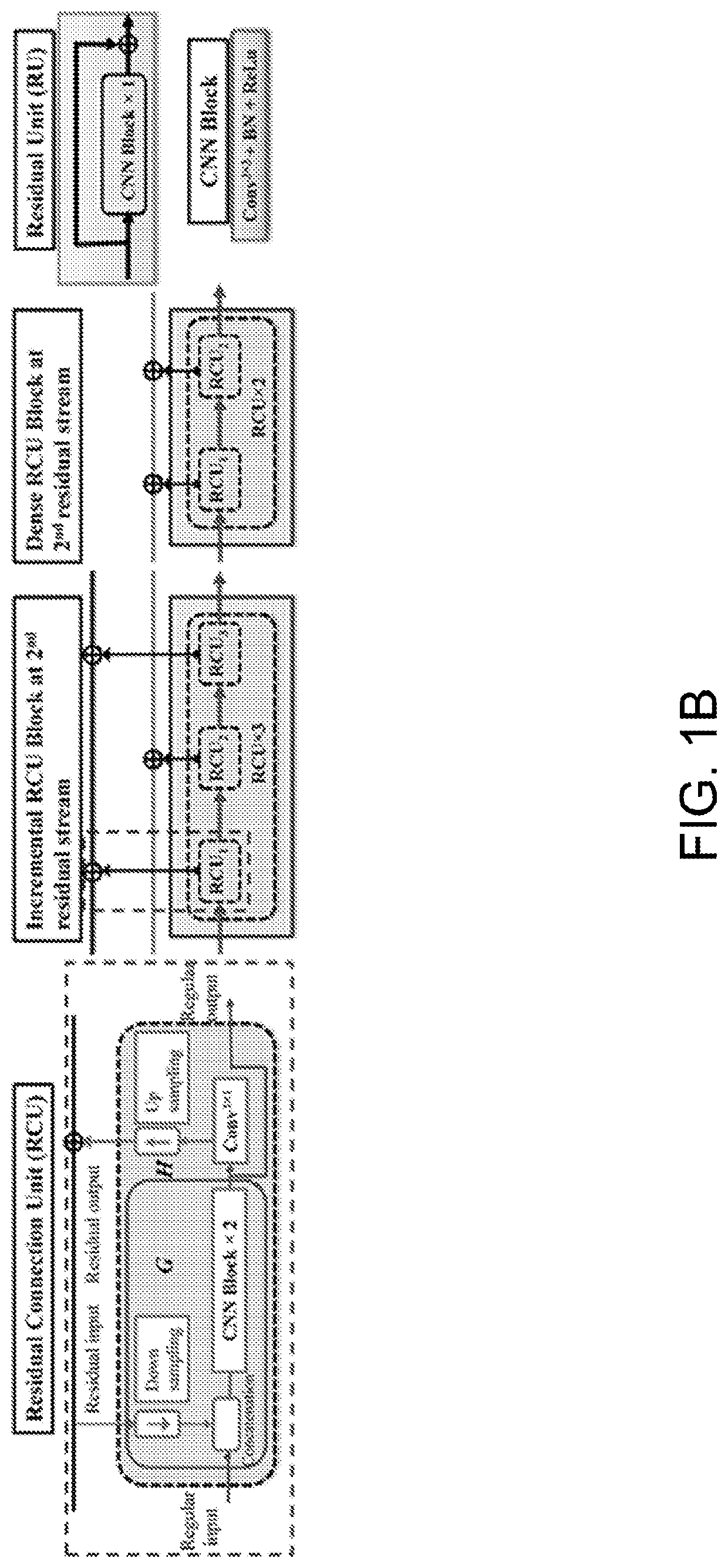
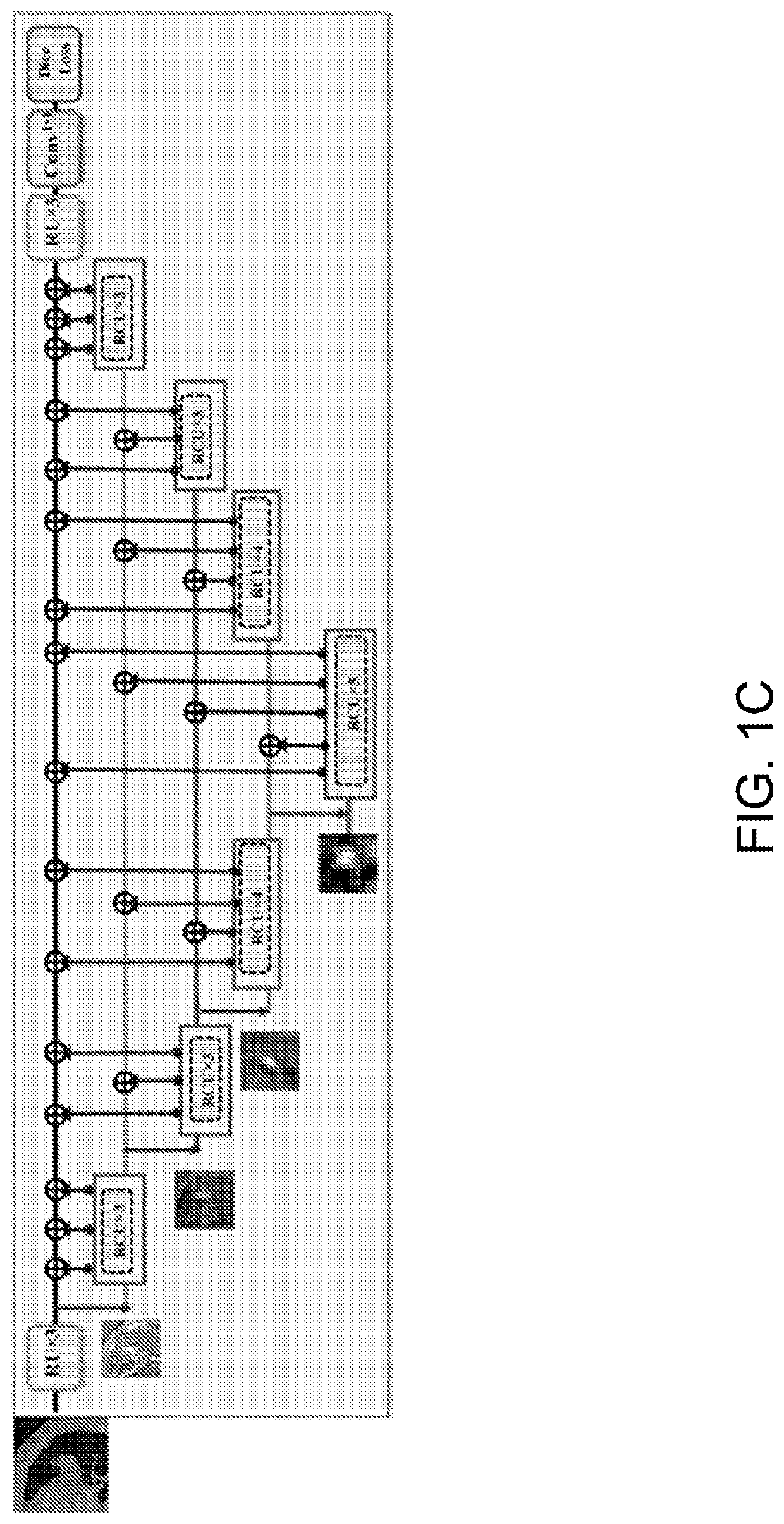
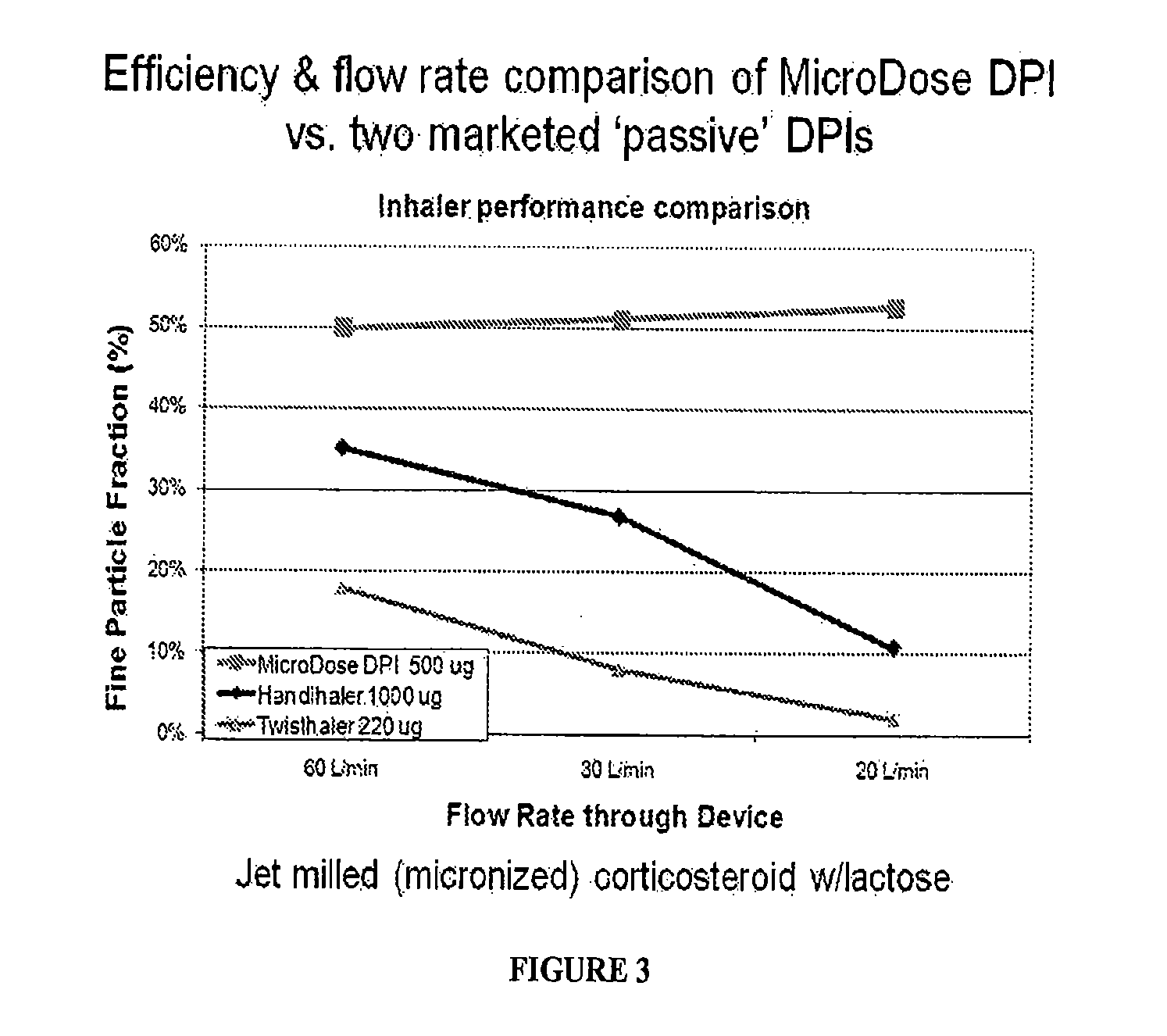
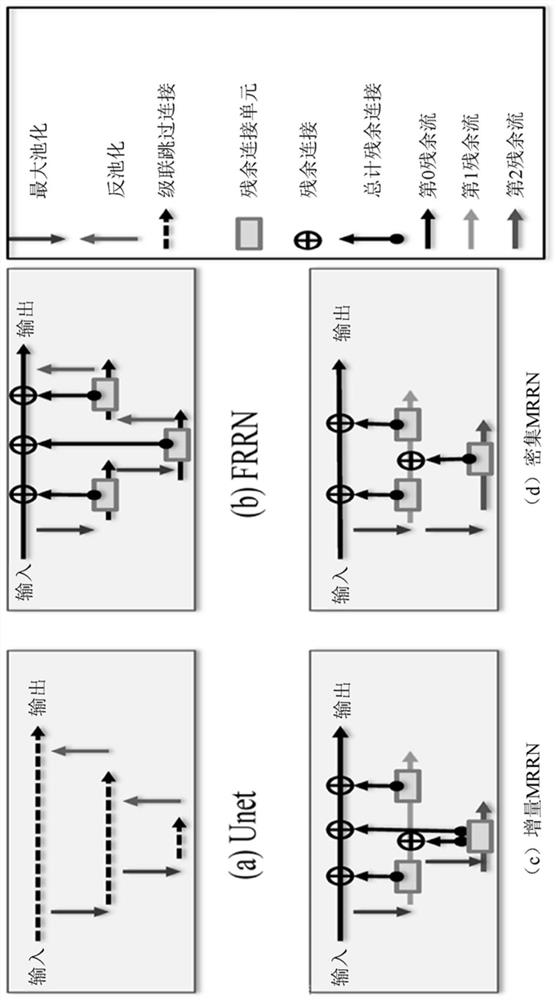
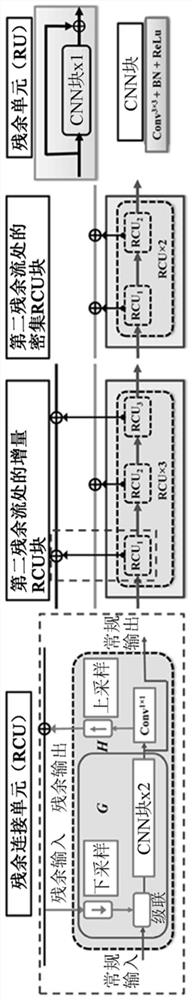
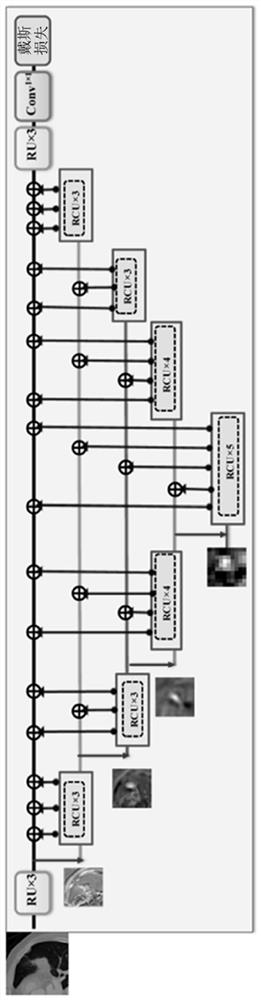
Multi-modal, multi-resolution deep learning neural networks for segmentation, outcomes prediction and longitudinal response monitoring to immunotherapy and radiotherapy
PendingUS20210383538A1Faster training convergencePrevent overfittingImage enhancementImage analysisRadical radiotherapyAdversarial network
Systems and methods for multi-modal, multi-resolution deep learning neural networks for segmentation, outcomes prediction and longitudinal response monitoring to immunotherapy and radiotherapy are detailed herein. A structure-specific Generational Adversarial Network (SSGAN) is used to synthesize realistic and structure-preserving images not produced using state-of-the art GANs and simultaneously incorporate constraints to produce synthetic images. A deeply supervised, Multi-modality, Multi-Resolution Residual Networks (DeepMMRRN) for tumor and organs-at-risk (OAR) segmentation may be used for tumor and OAR segmentation. The DeepMMRRN may combine multiple modalities for tumor and OAR segmentation. Accurate segmentation is may be realized by maximizing network capacity by simultaneously using features at multiple scales and resolutions and feature selection through deep supervision. DeepMMRRN Radiomics may be used for predicting and longitudinal monitoring response to immunotherapy. Auto-segmentations may be combined with radiomics analysis for predicting response prior to treatment initiation. Quantification of entire tumor burden may be used for automatic response assessment.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
Method and system to remove soluble tnfr1, tnfr2, and il2 in patients
InactiveUS20110129441A1Peptide/protein ingredientsIon-exchanger regenerationDiseaseConventional chemotherapy
A method, and system, to induce remission in diseases characterized by excess production of sTNR and interleukin 2 has been developed. In the most preferred embodiment, the system consists of antibodies to sTNFR1, sTNFR2 and sIL2R immobilized in a column containing a material such as SEPHAROSE™. The patient is connected to a pheresis machine which separates the blood into the plasma and red cells, and the plasma is circulated through the column until the desired reduction in levels of sTNFR1, sTNFR2, and IL2 is achieved, preferably to less than normal levels. In the preferred method, patients are treated three times a week for four weeks. This process can be repeated after a period of time. Clinical studies showed reduction in tumor burden in patients having failed conventional chemotherapy and radiation treatments.
Owner:INNATUS CORP
Hyperthermia and immunotherapy for leukemias lymphomas, and solid tumors
An improved method of inducing whole-body hyperthermia and enhanced anti-tumor immune response through inoculation of a fever virus with nil mortality and subsequent injection of irradiated tumor cells derived from the patient. This therapy will safely reduce the tumor burden by 90-99.9% by physical means (fever), before raising interferon levels to over 250 times baseline. The Activated Lymphokine Killer cells produced by these high interferon levels are capable of killing any cell expressing viral or tumor antigens, even those which had previously escaped immune surveillance. As a final step in the process, a specific class of Cytotoxic T Lymphocytes programmed to destroy the patient's own cancer cells will be produced by repeated inoculation of irradiated cancer cells harvested from the patient. Through a combination of three methods of therapy never previously integrated into a single regimen, it is logical to state that this therapy has a high probability of completely eradicating cancer cells from the patient. In addition, this therapy provides for life-long immunity to the reoccurrence of the disease.
Owner:RANDY K BROWN +1
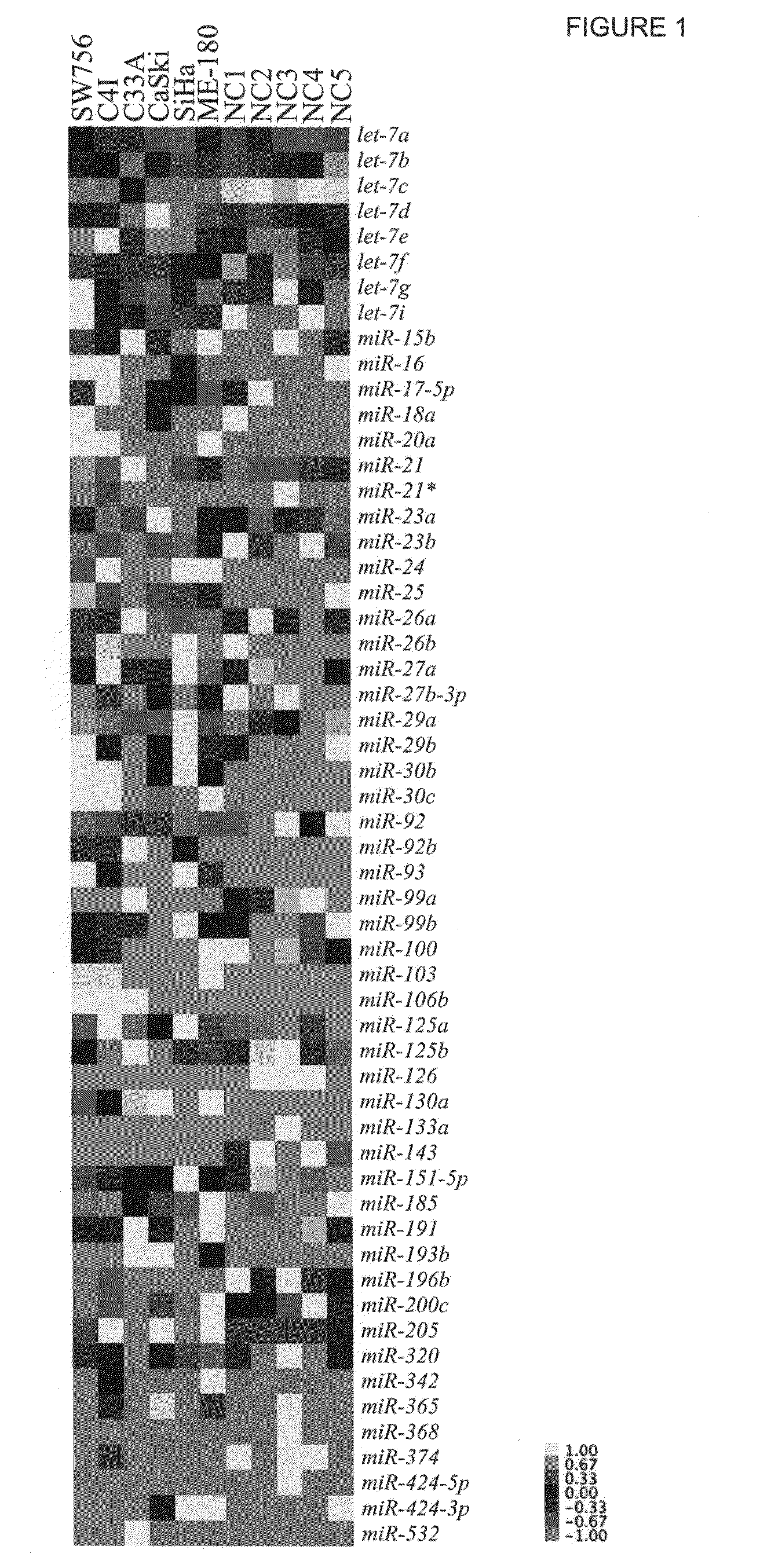
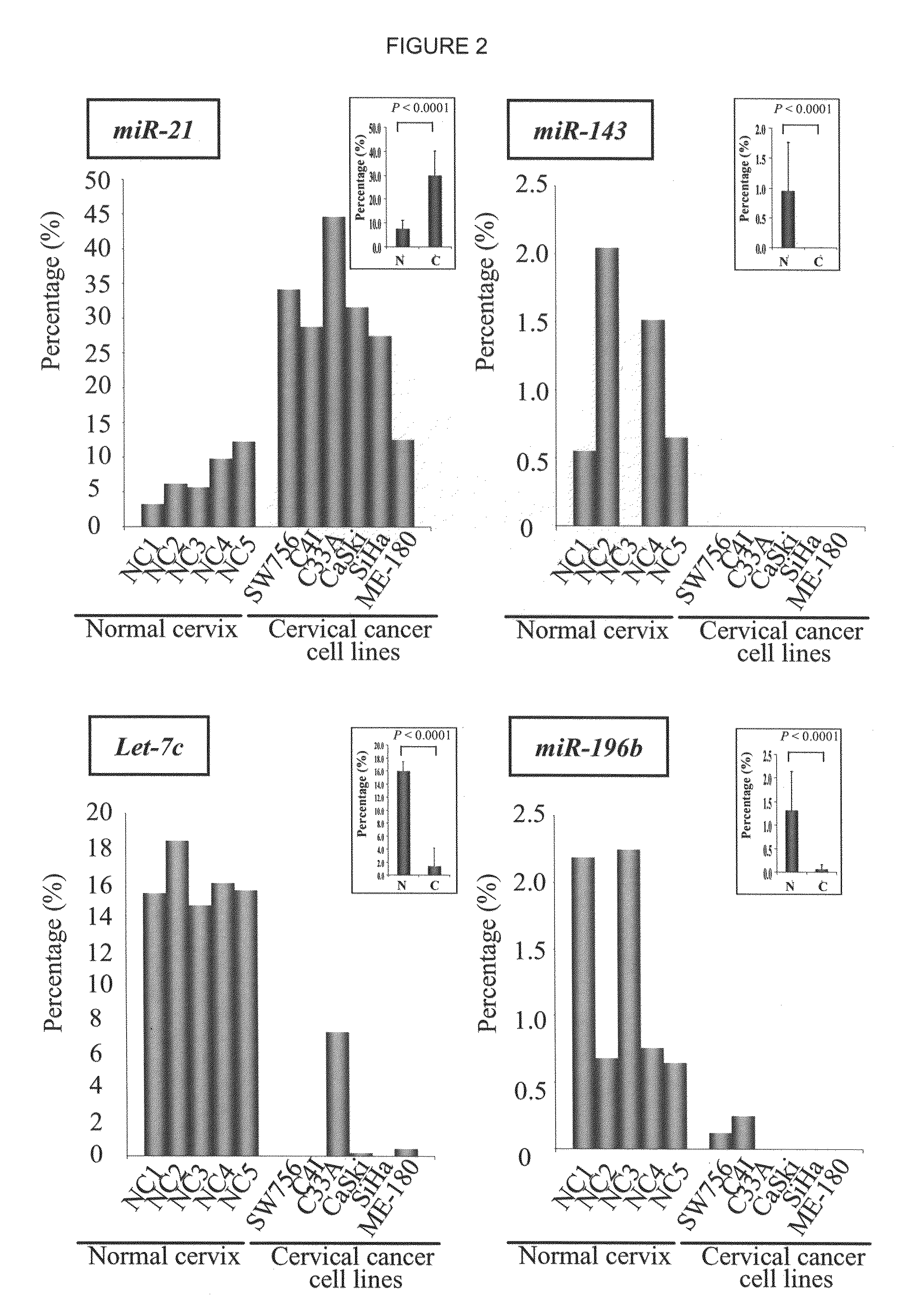
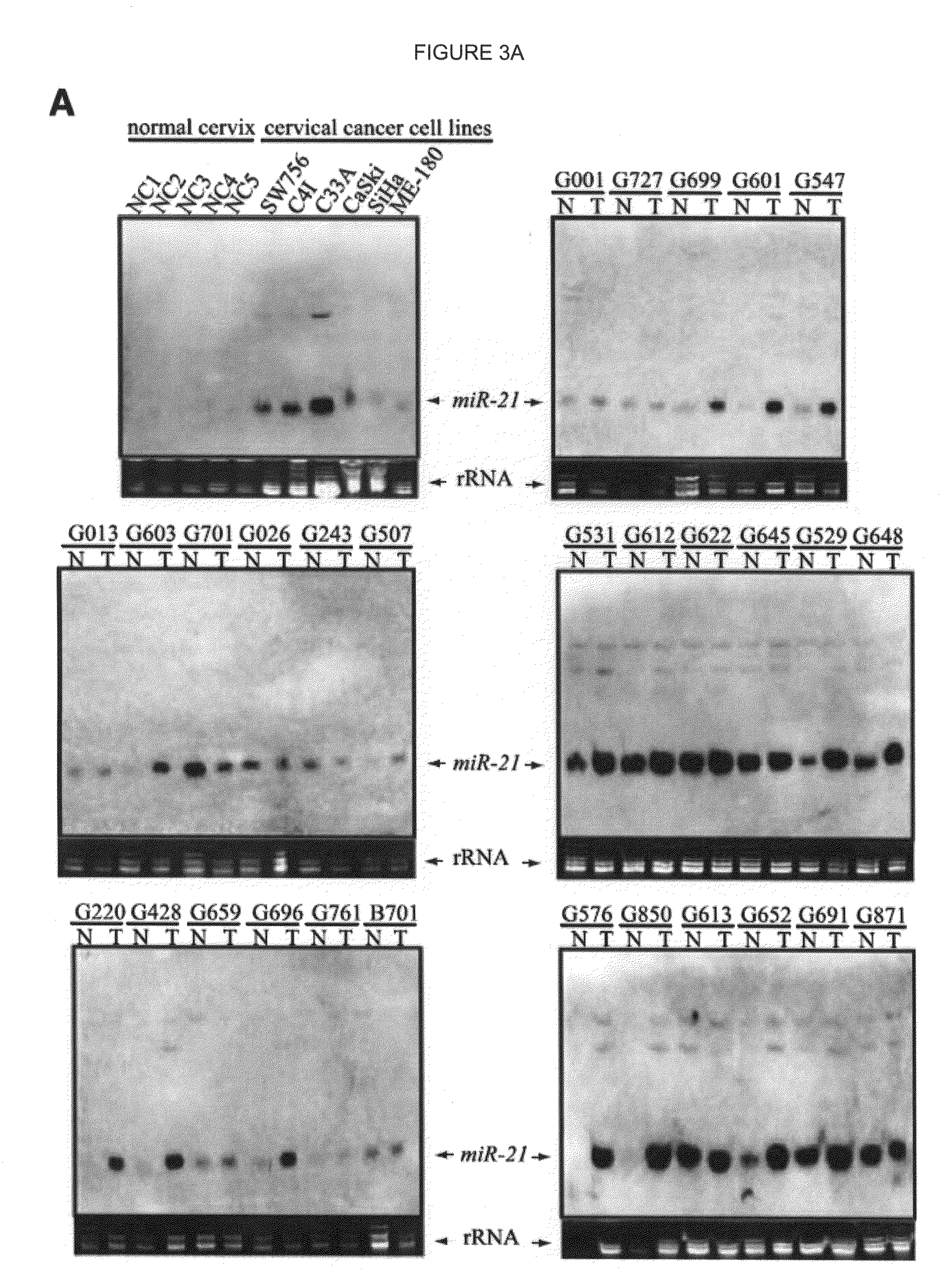
Patterns of known and novel small RNAS in human cervical cancer
InactiveUS20100234445A1Organic active ingredientsMicrobiological testing/measurementTumor LoadCancer cell
Small RNA sequences that are differentially expressed in SCCC cells are provided. The sequences find use in diagnosis of cancer, and classification of cancer cells according to expression profiles. The methods are useful for detecting cervical cancer cells, facilitating diagnosis of cervical cancer and the severity of the cancer (e.g., tumor grade, tumor burden, and the like) in a subject, facilitating a determination of the prognosis of a subject, and assessing the responsiveness of the subject to therapy.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
C-erbB-2 external domain: GP75
InactiveUS7282345B1Reduce chanceShortened time to relapseBiological testingImmunoassaysDiseaseProteinoid
Disclosed are methods and compositions for identifying malignant tumors that overexpress the c-erbB-2 oncogene. Assays useful for diagnosis and prognosis of neoplastic disease are provided which detect the external domain of c-erbB-2, the glycoprotein gp75 and quantitate the level of gp75 in the biological fluids of mammals carrying a tumor burden.Further disclosed are recombinant, synthetically and otherwise biologically produced novel proteins and polypeptides which are encoded by the external domain DNA sequence of the c-erbB-2 oncogene (the gp75 gene) or fragments thereof. Such gp75 proteins and polypeptides are useful as vaccines, therapeutically in the treatment of cancer either alone or in combination with chemotherapeutic agents.Also disclosed are antibodies to such gp75 proteins and polypeptides which are useful diagnostically and therapeutically. Still further disclosed are test kits embodying the assays of this invention.
Owner:SIEMENS HEALTHCARE DIAGNOSTICS GMBH
Method and apparatus for forming a homeostatic loop employing an aptamer biosensor
ActiveUS20100262375A1Accurate measurementEasy diagnosisResistance/reactance/impedenceBiological testingCapacitanceIn vivo
A novel architecture solid-state biosensor for label-free detection of vascular endothelial growth factor (VEGF) hybridization is presented. The new device is realized by forming a matrix array of parallel capacitors, thus allowing the realization of low-cost, portable, fully integrated devices. The detection mechanism is based on an electrochemical binding of circulating VEGF to an immobilized VEGF aptamer; whereby binding of these two compounds modulates the threshold voltage of a novel circuit, changing the impedance (capacitance) of the circuit. This novel circuit is further characterized by an electrode coded with a p-Si substrate, enhancing the affinity between the VEGF molecules and the aptamer. An apparatus forming a fluid cell is configured so as to enable the flow for delivering VEGF samples onto the active surface of the chip. The device has an array of parallel capacitors which act as an integrated, individual counter-electrode, computational apparatus which employs the sensory output over the time domain so as to enable detection, reporting and formation of a homeostatic loop for VEGF measurements. Moreover, this detector is able to provide an accurately measured and quantifiable rate of change of the VEGF molecules in-vivo, providing real time feedback of this important biomarker which may be used to measure response of the tumor to delivered chemotherapeutic agents and biological response modifiers (BRMs) for the purpose of determining tumor burden.
Owner:SENSOR KINESIS
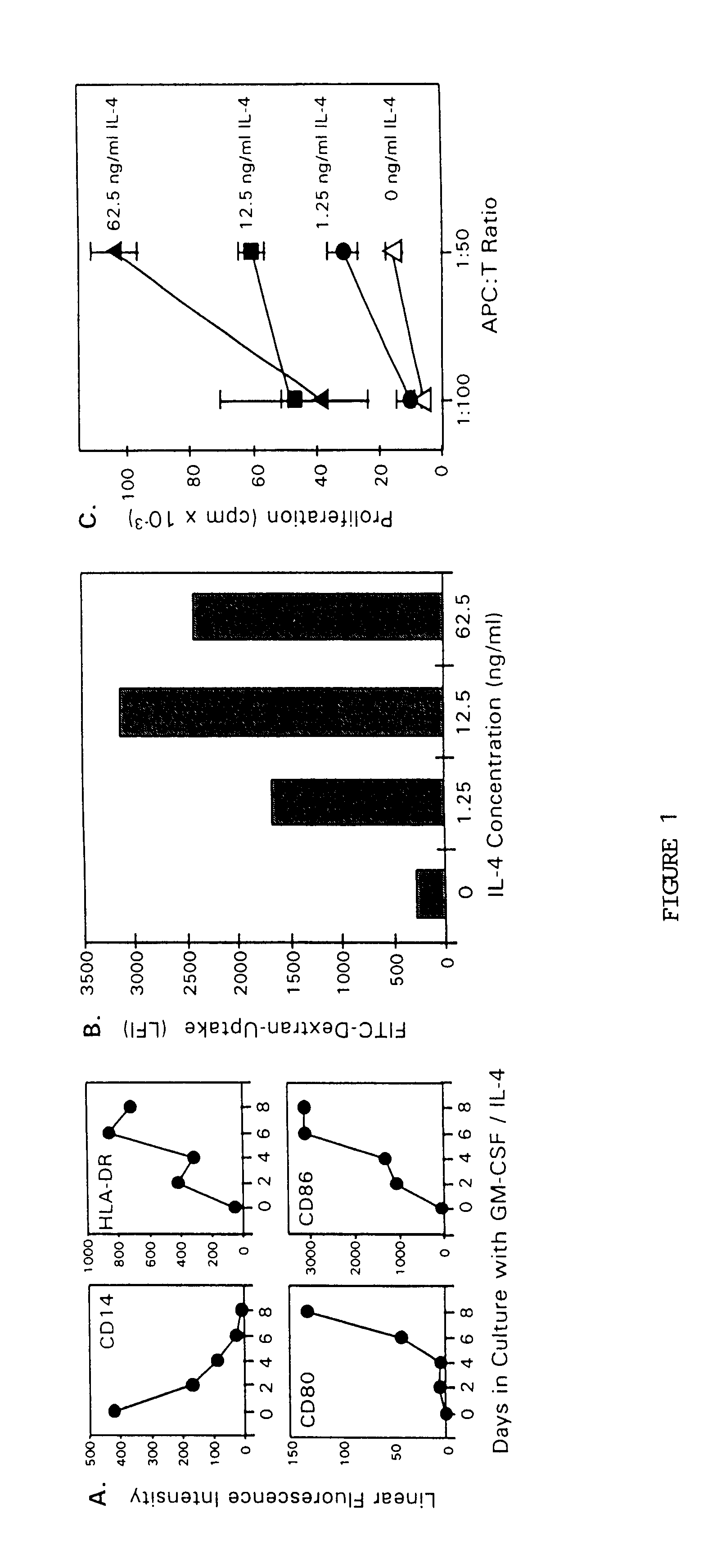
Methods for enhancing antigen-presenting cells and anti-tumor responses in a human patient
InactiveUS6838081B1Improve developmentReduce tumor burdenBiocidePeptide/protein ingredientsTumor responseCo administration
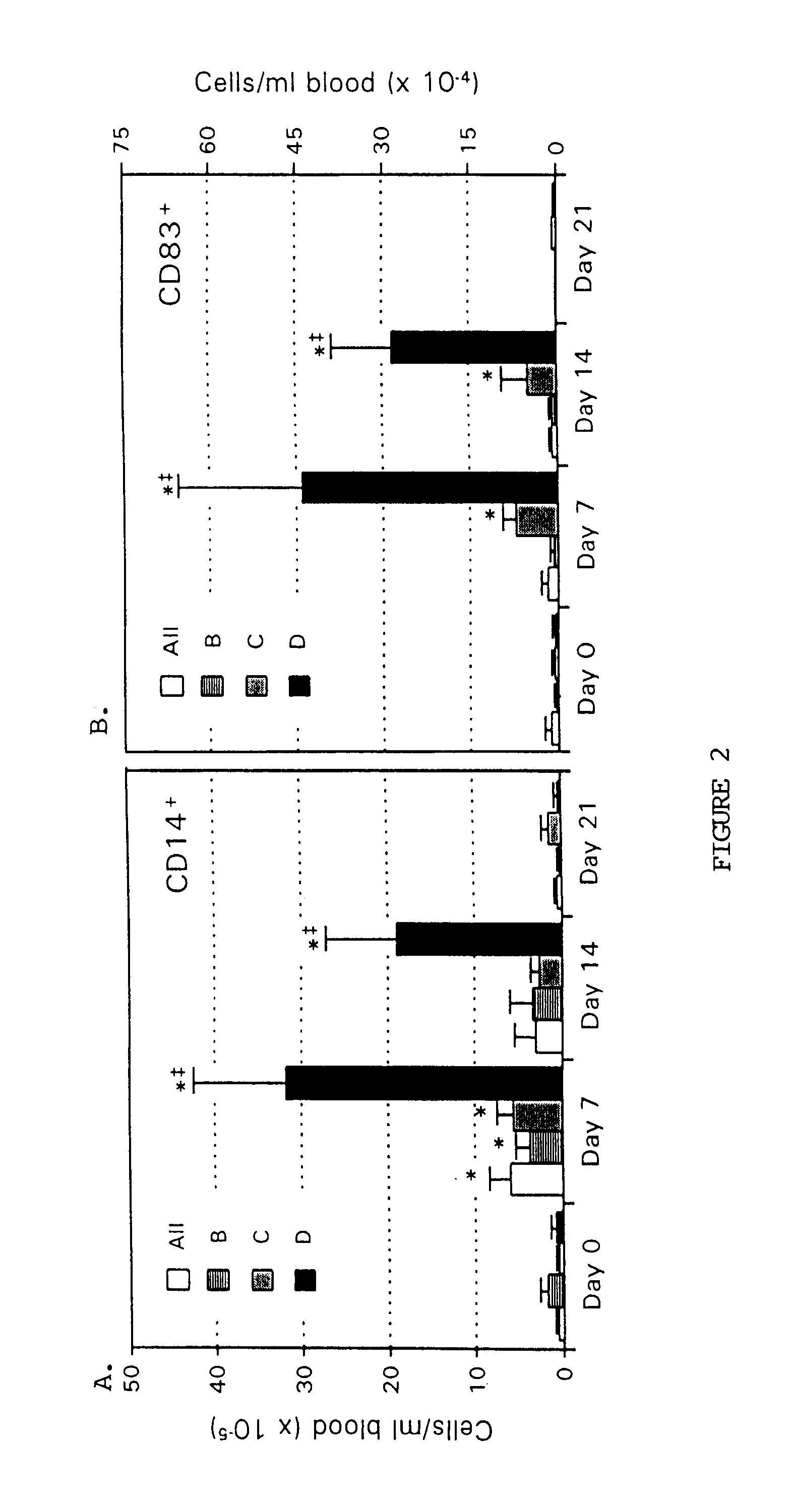
The present invention provides methods for enhancing the development of APC from precursor cells by administering a combination of GM-CSF and IL-4. The precursor cells include: cells contained in peripheral blood, CD14+ cells and precursors in bone marrow. Thus, administration of GM-CSF and IL-4 can be used as a form of cytokine immunotherapy. One embodiment of the present invention involves systemic administration of GM-CSF and IL-4. In this embodiment, APC are required to directly access tumor antigens as they exist in vivo within the patient. A further embodiment of the present invention involves co-administration of a tumor-associated or tumor-specific antigen, with GM-CSF and IL-4, to induce antigen-specific immunity mediated by APC. Yet another embodiment of the present invention describes systemic administration of GM-CSF and IL-4 to achieve reduced tumor burden.
Owner:RGT UNIV OF CALIFORNIA
Use of mks inhibitor peptide-containing compositions for treating non-small cell lung cancer with same
InactiveUS20160263187A1Inhibitory activityInhibition is effectivePowder deliveryHeavy metal active ingredientsPharmaceutical medicineCancer cell proliferation
The described invention provides pharmaceutical compositions, systems and methods for treating a non-small cell lung cancer (NSCLC) solid tumor comprising a population of tumor cells. The method includes administering a pharmaceutical composition comprising a therapeutic amount of a polypeptide having the amino acid sequence YARAAARQARAKALARQLGVAA (SEQ ID NO: 1) or functional equivalent thereof, and a pharmaceutically acceptable carrier, wherein therapeutic amount of the polypeptide is effective to inhibit a kinase activity in the population of tumor cells and to reduce cancer cell proliferation, to reduce tumor size, to reduce tumor burden, to induce tumor cell death, to overcome tumor chemoresistance, to enhance tumor chemosensitivity, or a combination thereof.
Owner:MOERAE MATRIX
Multi-modal, multi-resolution deep learning neural networks for segmentation, outcomes prediction and longitudinal response monitoring to immunotherapy and radiotherapy
PendingCN112771581AImage enhancementImage analysisGenerative adversarial networkRadical radiotherapy
Systems and methods for multi-modal, multi-resolution deep learning neural networks for segmentation, outcomes prediction and longitudinal response monitoring to immunotherapy and radiotherapy are detailed herein. A structure-specific Generational Adversarial Network (SSGAN) is used to synthesize realistic and structure-preserving images not produced using state-of-the art GANs and simultaneously incorporate constraints to produce synthetic images. A deeply supervised, Multi-modality, Multi-Resolution Residual Networks (DeepMMRRN) for tumor and organs-at-risk (OAR) segmentation may be used for tumor and OAR segmentation. The DeepMMRRN may combine multiple modalities for tumor and OAR segmentation. Accurate segmentation is may be realized by maximizing network capacity by simultaneously using features at multiple scales and resolutions and feature selection through deep supervision. DeepMMRRN Radiomics may be used for predicting and longitudinal monitoring response to immunotherapy. Auto-segmentations may be combined with radiomics analysis for predicting response prior to treatment initiation. Quantification of entire tumor burden may be used for automatic response assessment.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
Hyperthermia and immunotherapy for cancer
InactiveUS6048686AReduce tumor burdenWithout riskSsRNA viruses positive-senseViral antigen ingredientsAbnormal tissue growthDisease
An improved method of inducing whole-body hyperthermia and enhanced anti-tumor immune response through inoculation of a fever virus with nil mortality and subsequent injection of irradiated tumor cells derived from the patient. This therapy will safely reduce the tumor burden by 90-99.9% by physical means (fever), before raising interferon levels to over 250 times baseline. The Activated Lymphokine Killer cells produced by these high interferon levels are capable of killing any cell expressing viral or tumor antigens, even those which had previously escaped immune surveillance. As a final step in the process, a specific class of Cytotoxic T Lymphocytes programmed to destroy the patient's own cancer cells will be produced by repeated inoculation of irradiated cancer cells harvested from the patient. Through a combination of three methods of therapy never previously integrated into a single regimen, it is logical to state that this therapy has a high probability of completely eradicating cancer cells from the patient. In addition, this therapy provides for life-long immunity to the reoccurrence of the disease. It is understood that the examples and embodiments described herein are for illustrative purposes only and that various changes or modifications in light thereof will be suggested to persons skilled in the art and are to be included within the spirit and purview of this application and scope of the appended claims.
Owner:AUNER MARIA CARMEN VIVIAN +1
ASSAY FOR ANTI-EGFRvIII ANTIBODIES
Detection of human antibodies directed against the tumor-specific protein Epidermal Growth Factor Receptor variant Class III (EGFRvIII) provide information on tumor burden and vaccine response. The methods of the invention permit the specific identification of antibodies that are able to bind to EGFRvIII. The methods are useful in determining the presence of an EGFRvIII-expressing tumor and in detecting immune responses following immunization with EGFRvIII-derived peptide as part of a cancer immunotherapy regimen.
Owner:DUKE UNIV
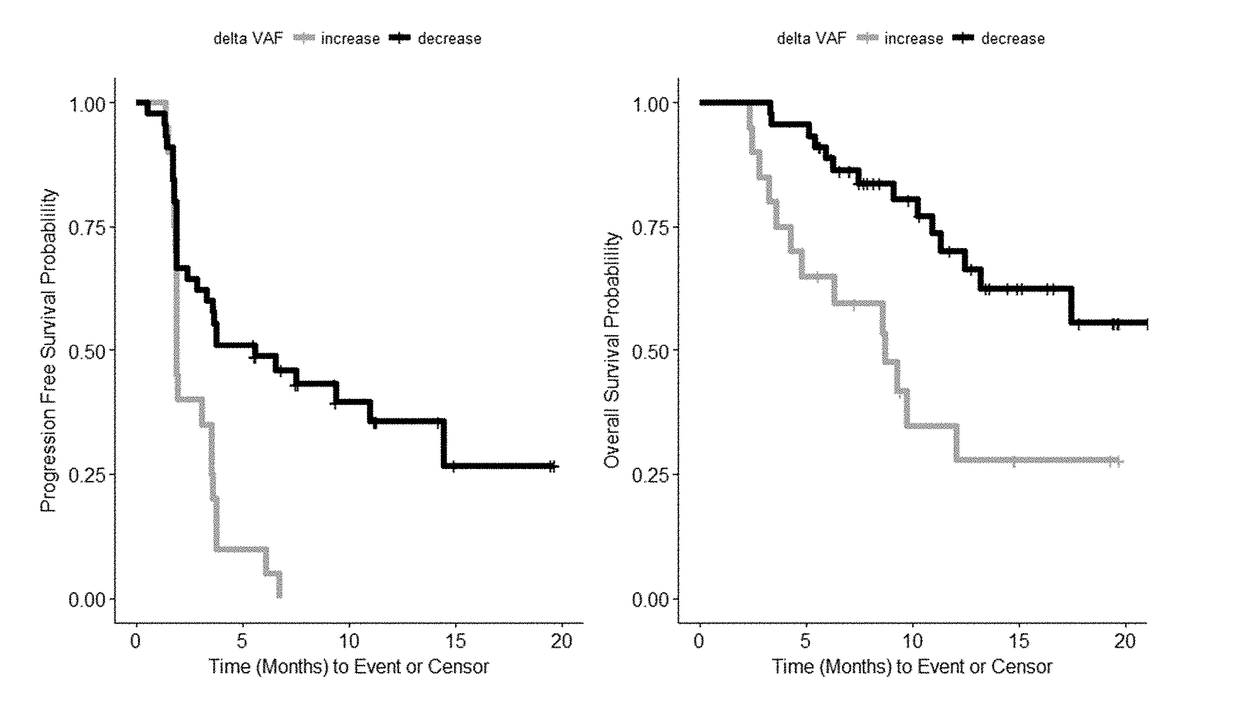
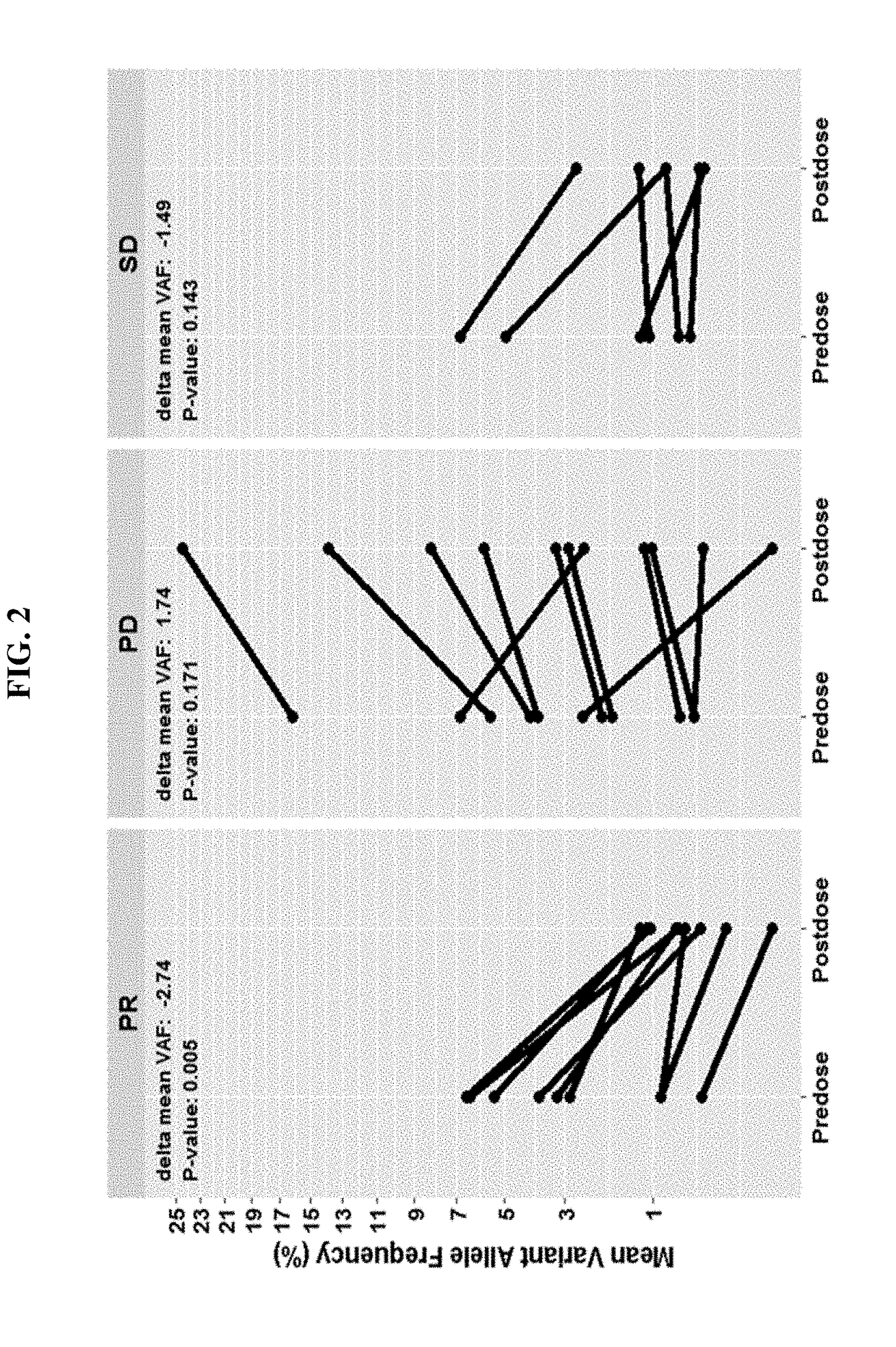
Tumor burden as measured by cell free DNA
InactiveUS20180282417A1Good chanceLong survivalMicrobiological testing/measurementImmunoglobulins against cell receptors/antigens/surface-determinantsCell freeAllele frequency
Disclosed are methods for treating cancer (e.g., solid tumor cancers, lung cancer, bladder head and neck cancer) with an anti-PD-L1 antibody in a patient identified as being responsive to anti-PD-L1 antibody therapy by detecting a mutation in one or more disclosed circulating tumor DNA (ctDNA) markers. Also disclosed are methods for determining the efficacy of anti-PD-L1 therapeutic antibody treatment in a patient having lung cancer or bladder cancer comprising detecting variant allele frequency in ctDNA in plasma samples and determining the difference of the variant allele frequency in ctDNA between the first and at least second plasma samples, wherein a decrease in the variant allele frequency in the at least second plasma sample relative to the first plasma sample identifies the anti-PD-L1 antibody treatment as effective. The disclosure also provides methods of identifying a subject having a cancer responsive to a therapy comprising an anti-PD-L1 antibody by detecting the expression of a mutation in one or more circulating tumor DNA (ctDNA) markers.
Owner:MEDIMMUNE LLC
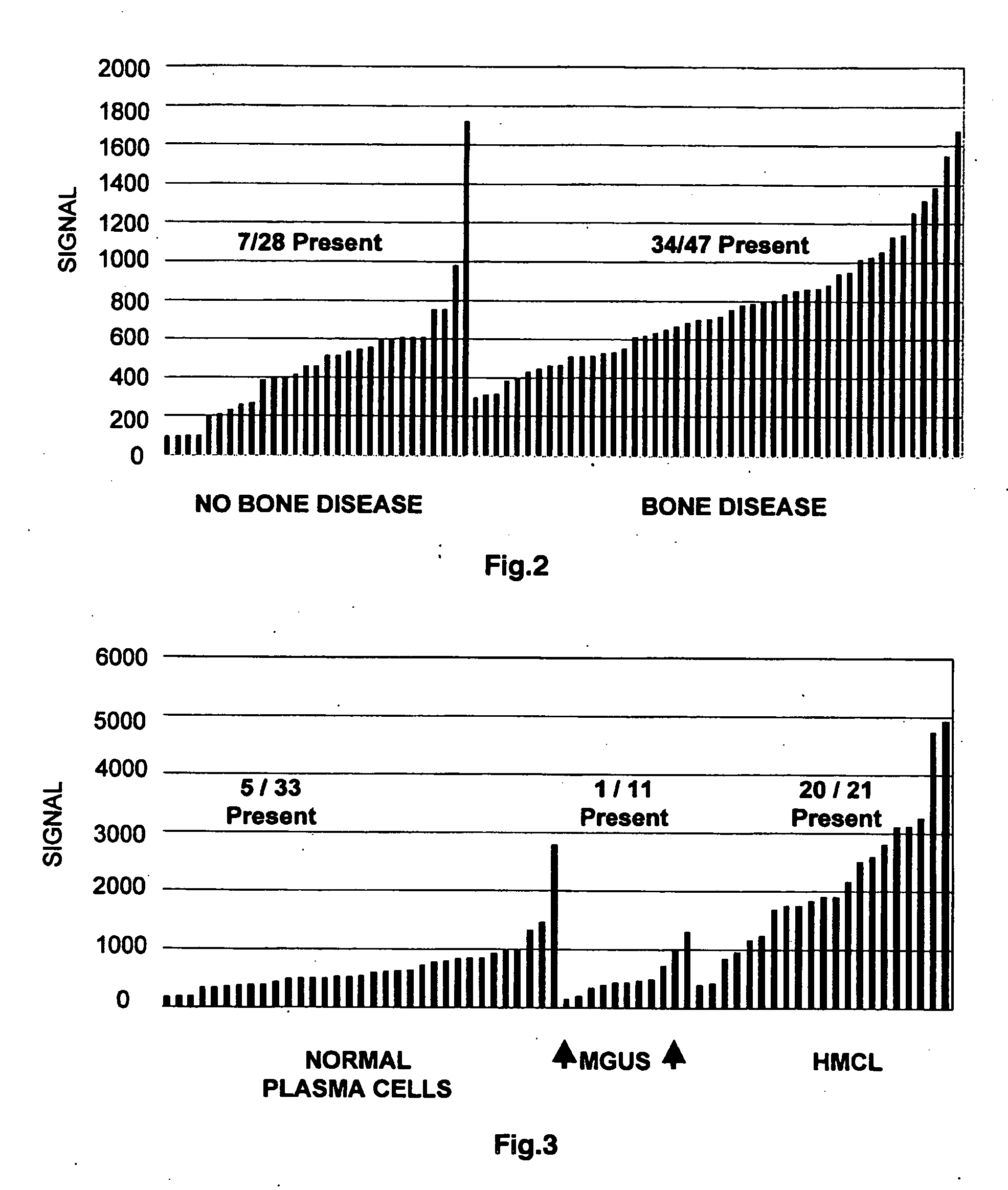
Molecular determinants of myeloma bone disease and use thereof
InactiveUS20070066558A1Increase bone massBone lossGenetic material ingredientsMicrobiological testing/measurementNewly diagnosedNormal bone
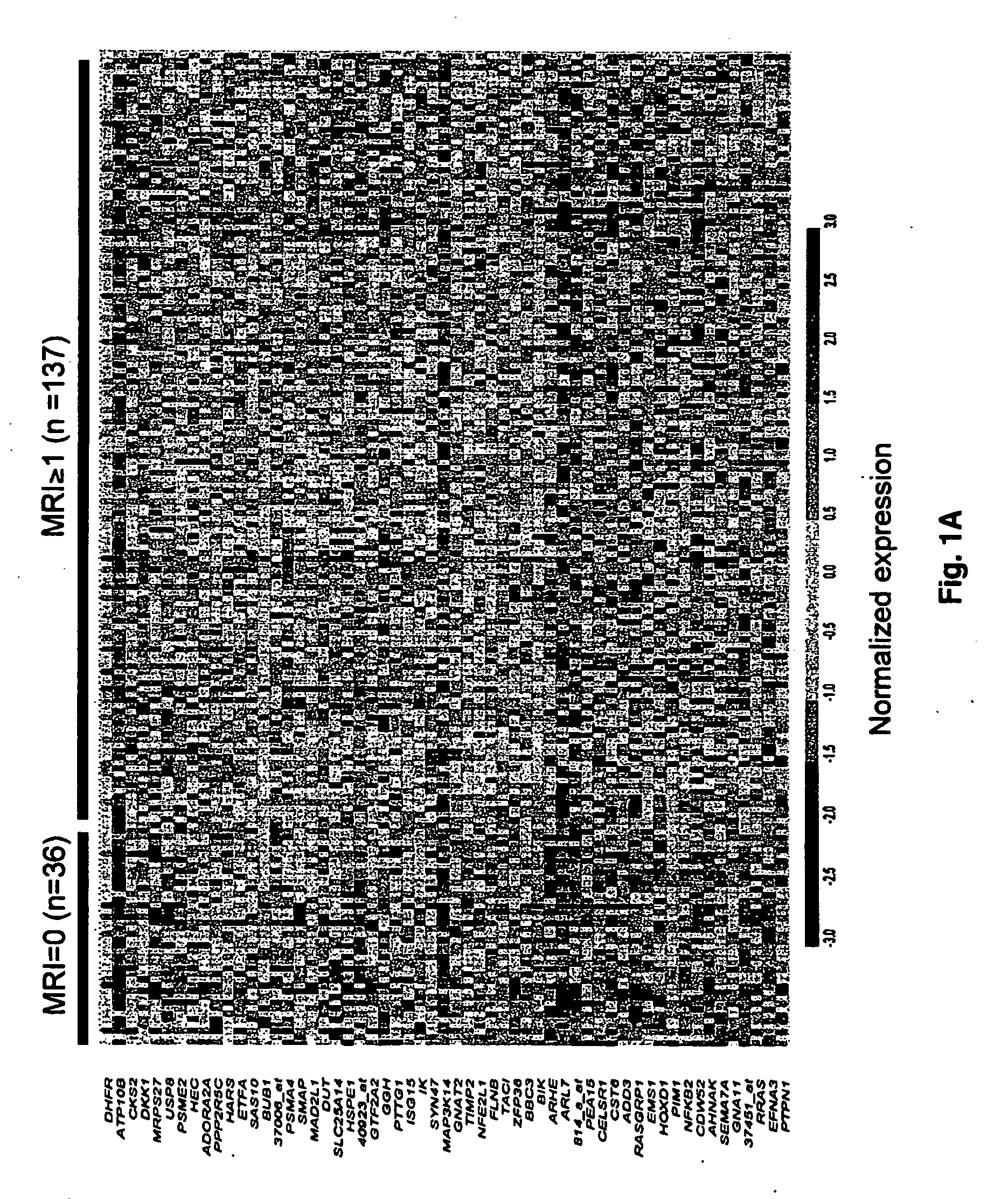
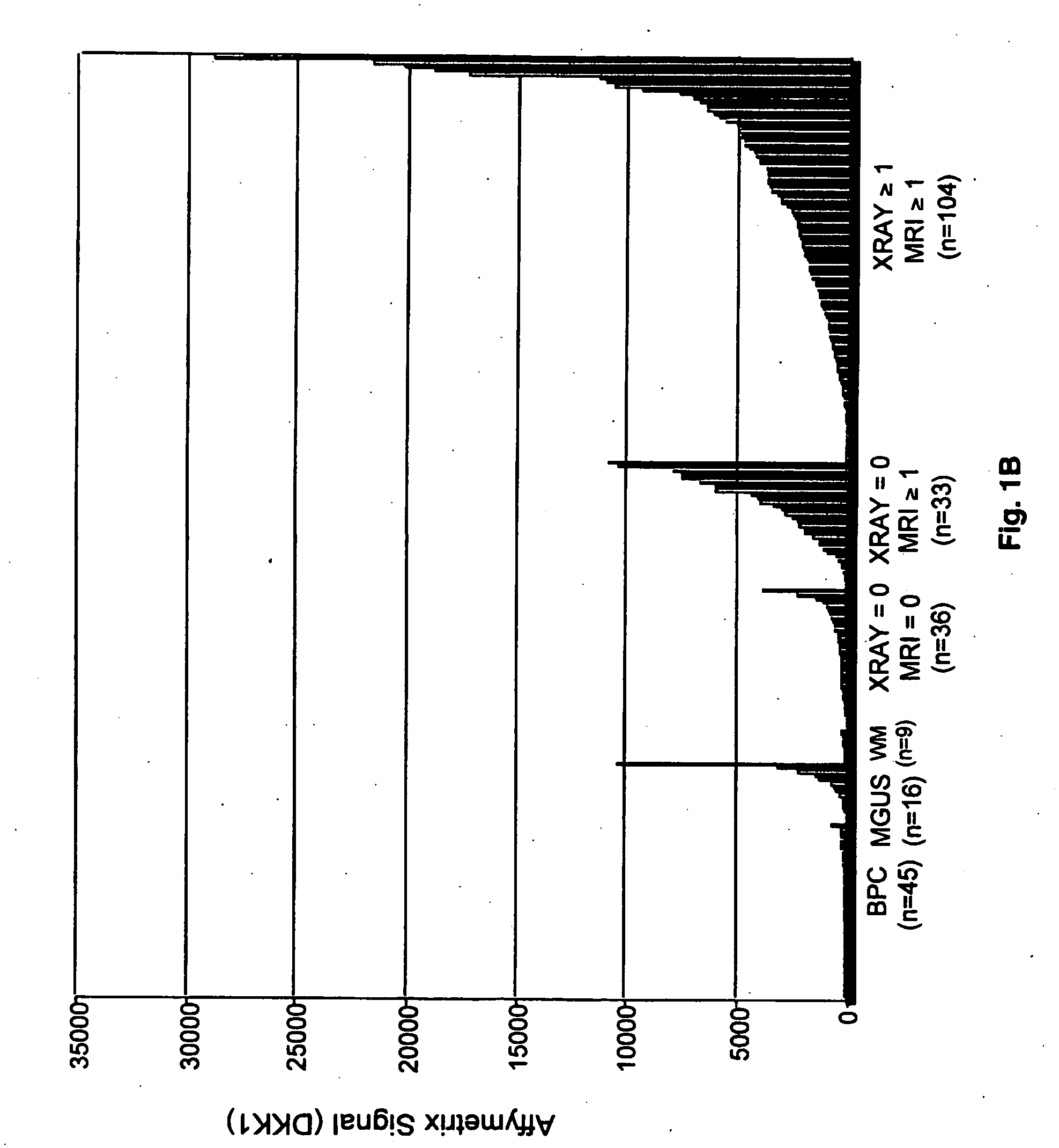
To identify molecular determinants of lytic bone disease in multiple myeloma, the expression profiles of ˜12,000 genes in CD138-enriched plasma cells from newly diagnosed multiple myeloma patients exhibiting no radiological evidence of lytic lesions (n=28) were compared to those with ≧3 lytic lesions (n=47). Two secreted WNT signaling antagonists, soluble frizzled related protein 3 (SFRP-3 / FRZB) and the human homologue of Dickkopf-1 (DKK1), were expressed in 40 of 47 with lytic bone lesions, but only 16 of 28 lacking bone lesions (P<0.05). DKK1 and FRZB were not expressed in plasma cells from 45 normal bone marrow donors or 10 Waldenstrom's macroglobulinemia, a related plasma cells malignancy that lacks bone disease. These data indicate that these factors are important mediators of multiple myeloma bone disease, and inhibitors of these proteins may be used to block bone disease reduce tumor burden in multiple myeloma and to block bone disease.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ARKANSAS
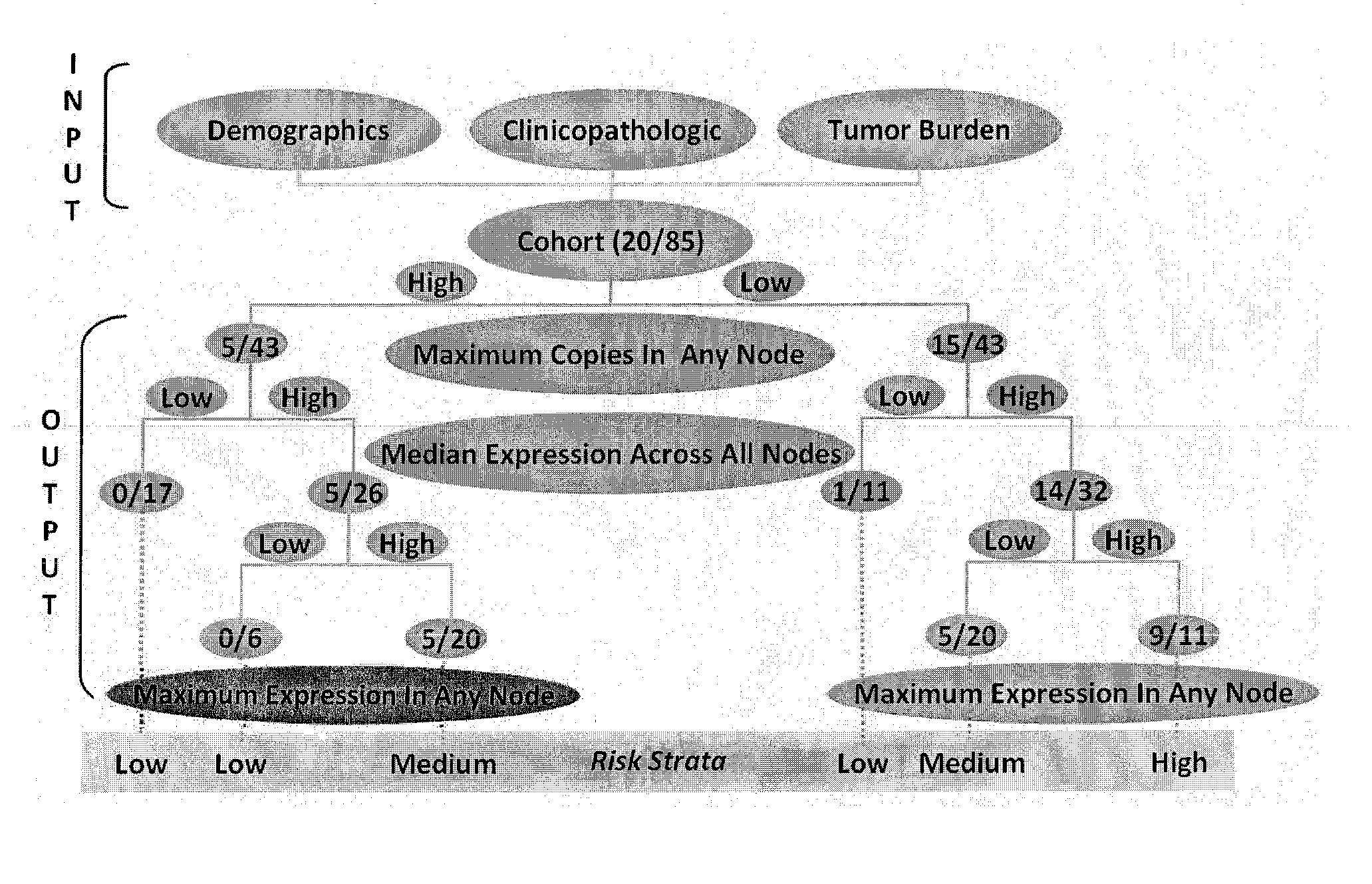
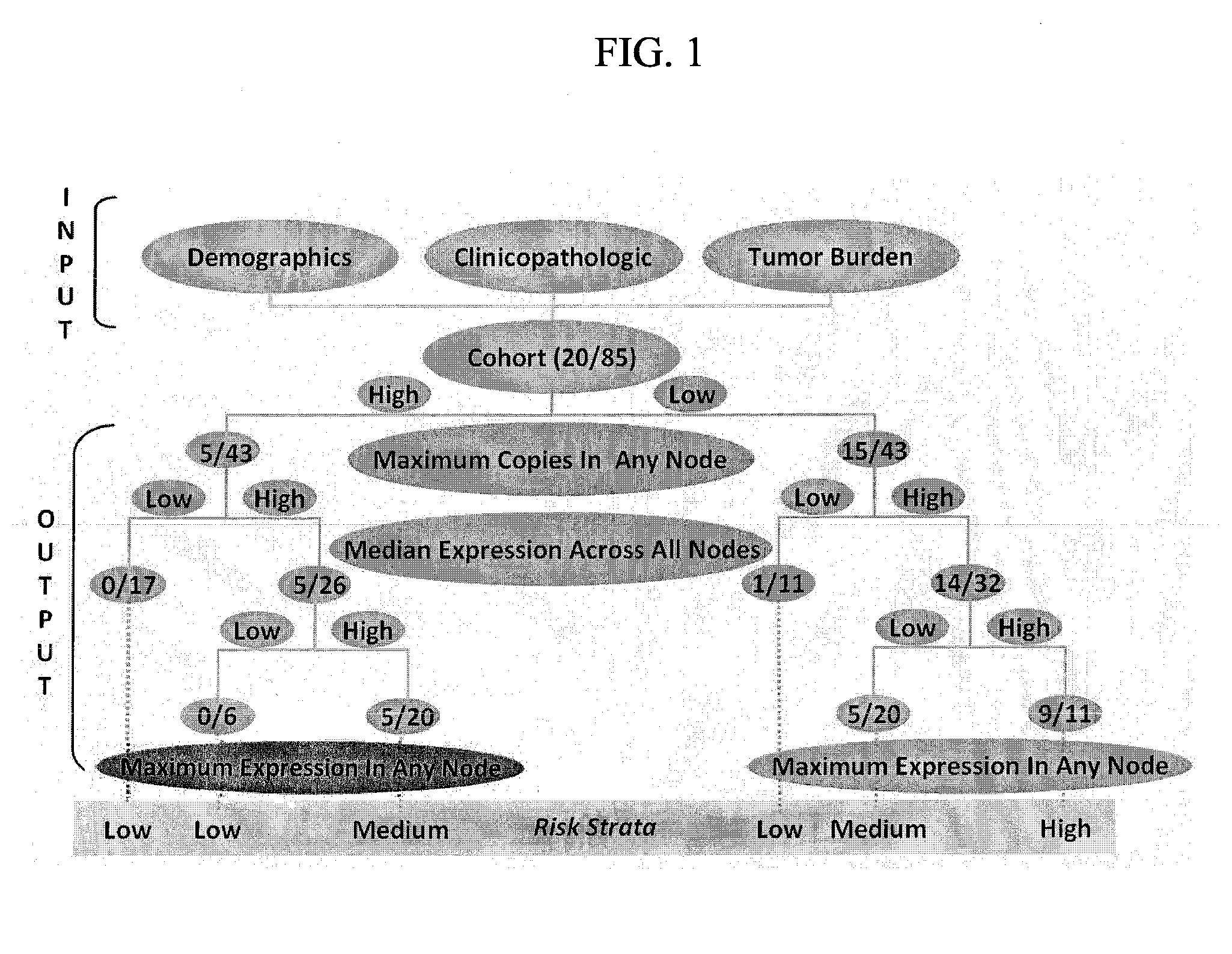
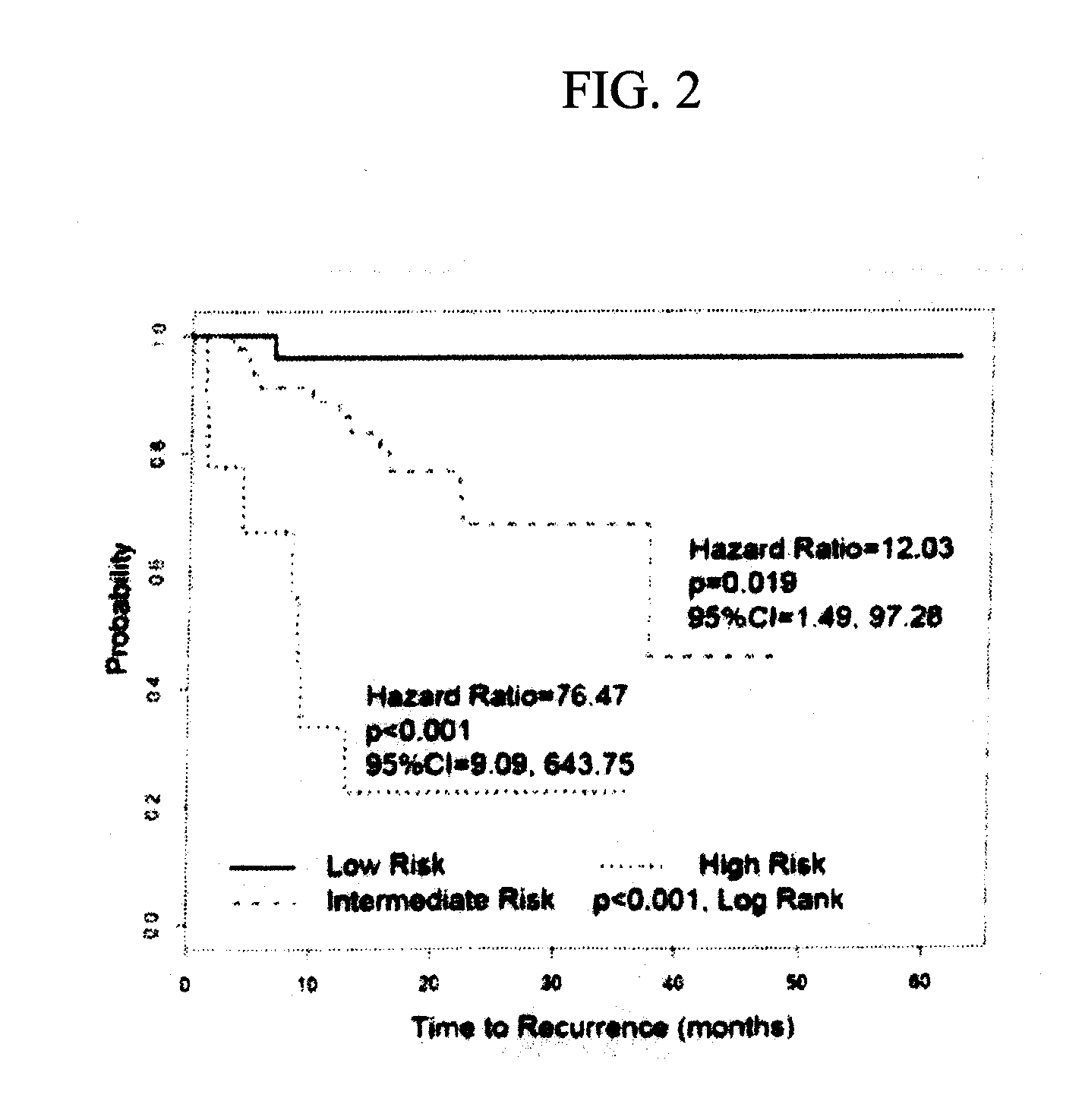
System for and method of determining cancer prognosis and predicting response to therapy
A database for predicting clinical outcomes based upon quantitative tumor burden in lymph node samples from an individual is provided. The database comprises data sets from a plurality of individuals. The data sets include clinical outcome data and data regarding number of lymph nodes evaluated, maximum number of biomarker detected in any single node, median normalized expression levels detected across all evaluated lymph nodes and the maximum normalized expression levels detected in any evaluated lymph nodes and the database also includes stratified risk categories based upon recursive partitioning of data. A system for predicting clinical outcomes based upon quantitative tumor burden in lymph node samples from an individual is provided which includes the database linked to a data processor, an input interface and an output interface. Method of preparing a database and method for predicting clinical outcome for a test patient based upon quantitative tumor burden in lymph node samples from an individual using a system that includes the database linked to a data processor, an input interface and an output interface. The method comprises measuring quantitative tumor burden in a plurality of lymph node samples from an individual, inputting the results into the system and processing with data in the database. The results of the processing of the data is the assignment of data test patient to a stratified risk category. Output is produced that displays test patient's identity and assigned stratified risk category.
Owner:THOMAS JEFFERSON UNIV
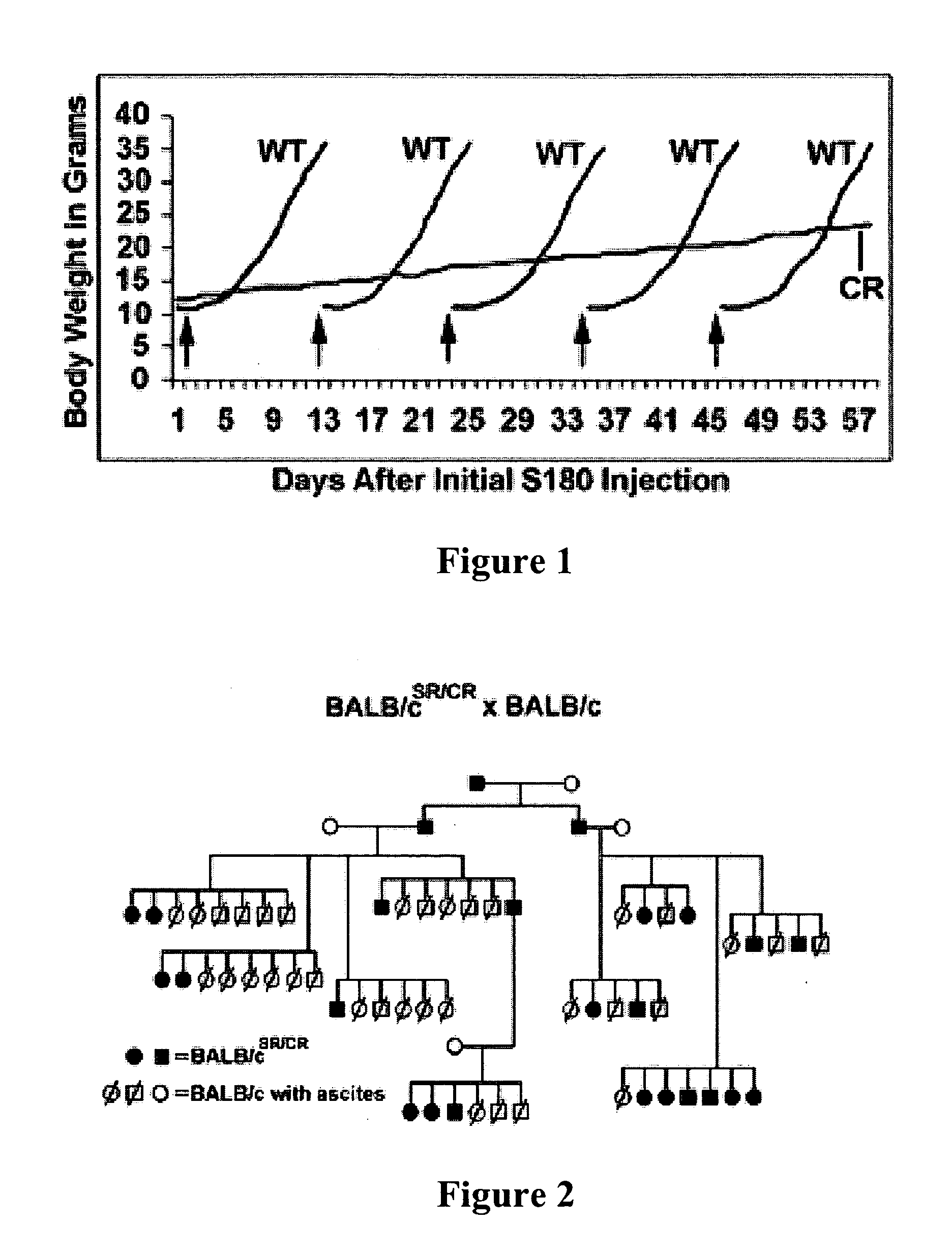
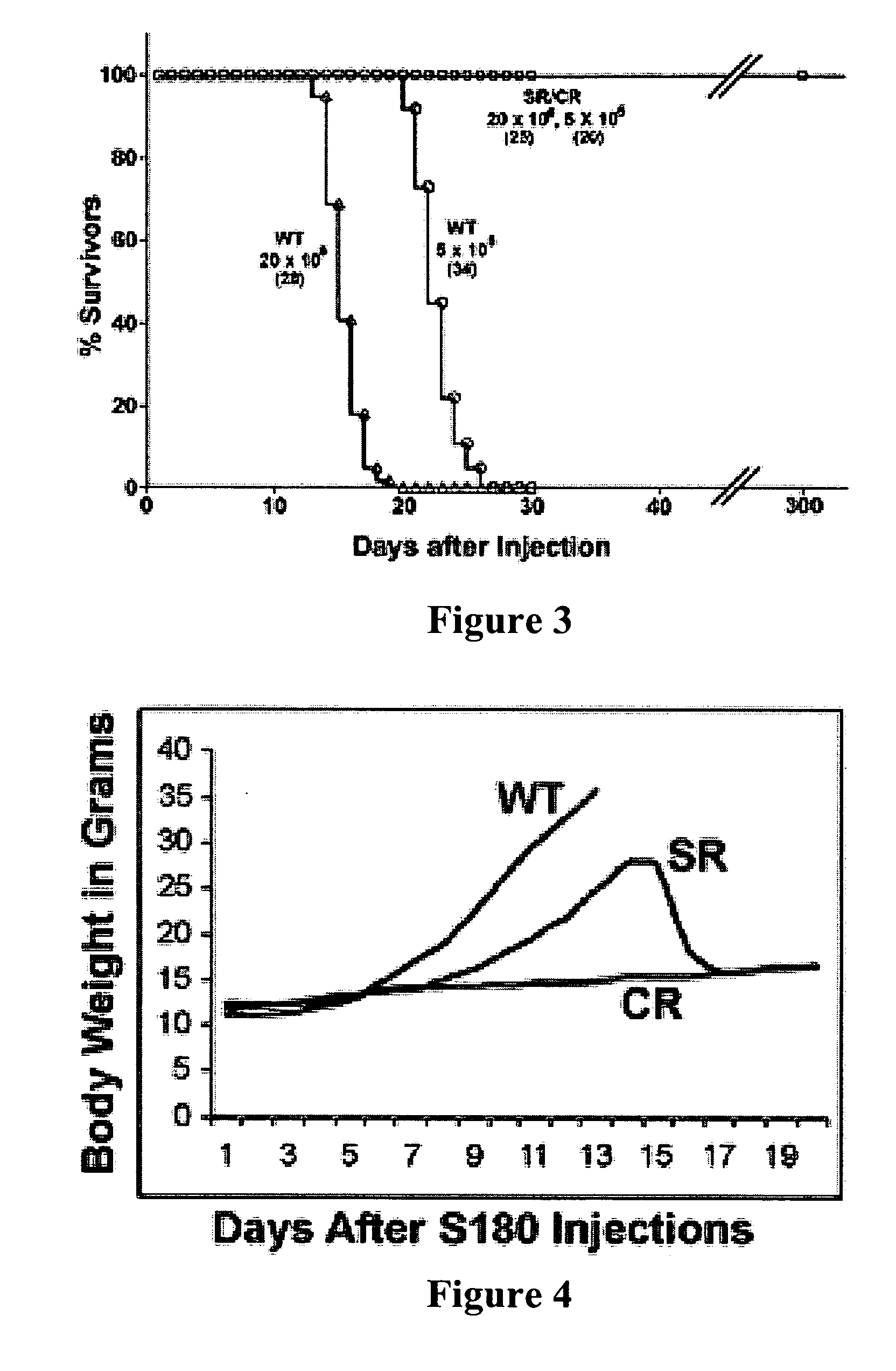
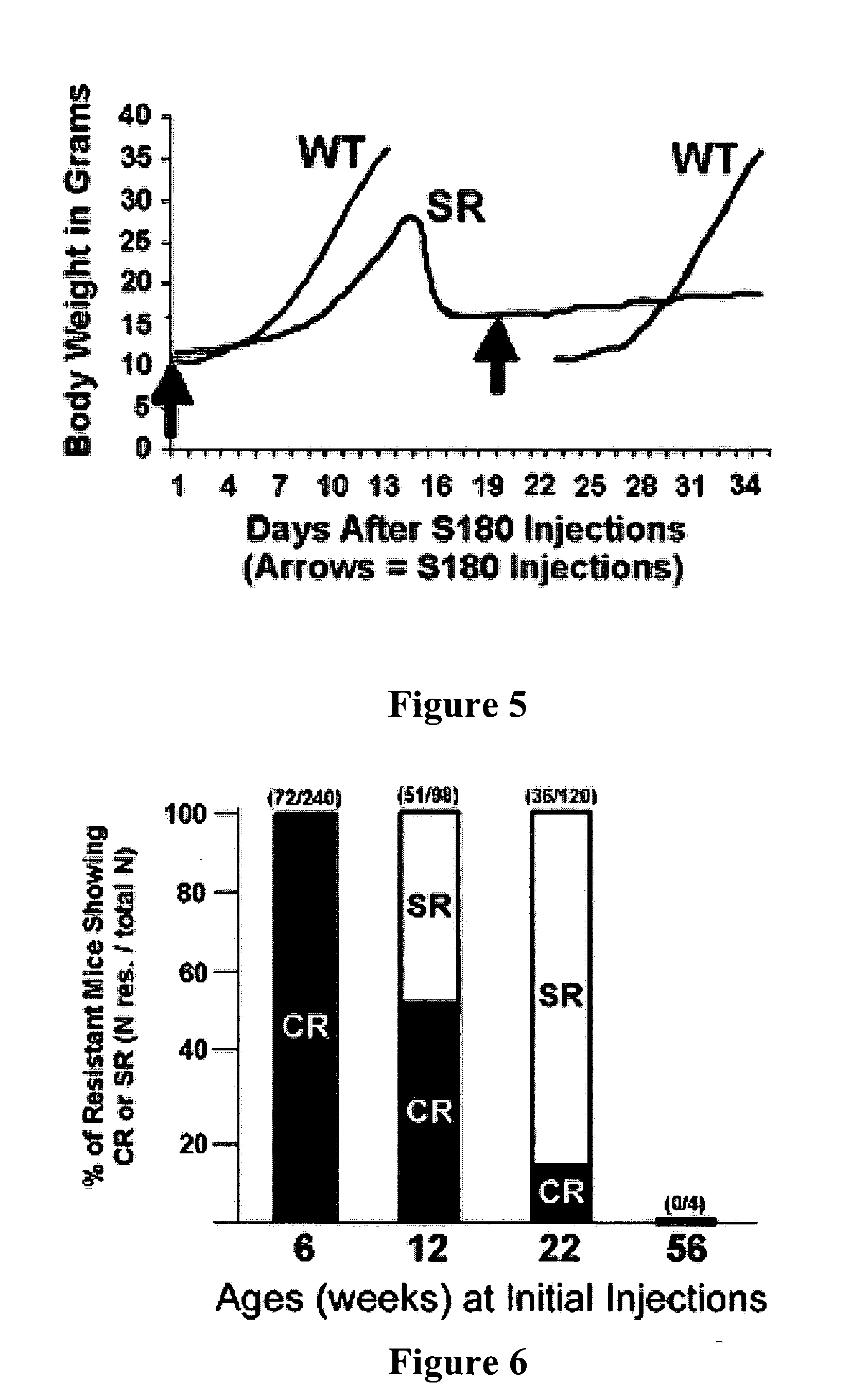
Genetically determined mouse model of resistance to transplantable cancers
We have established and studied a colony of mice with a unique trait of host resistance to both ascites and solid cancers induced by transplantable cells. One dramatic manifestation of this trait is age-dependent spontaneous regression of advanced cancers. This powerful resistance segregates as a single-locus dominant trait, is independent of tumor burden and is effective against cell lines from multiple types of cancer. During spontaneous regression or immediately following exposure, cancer cells provoke a massive infiltration of host leukocytes which form aggregates and rosettes with tumor cells. The cytolytic destruction of cancer cells by innate leukocytes is rapid and specific without apparent damage to normal cells. The mice are healthy, cancer-free and have a normal life span. These observations suggest a previously unrecognized mechanism of immune surveillance that may have potential for therapy or prevention of cancer.
Owner:WAKE FOREST UNIV HEALTH SCI INC
Blood micro-RNA colorectal cancer diagnosis molecule combination
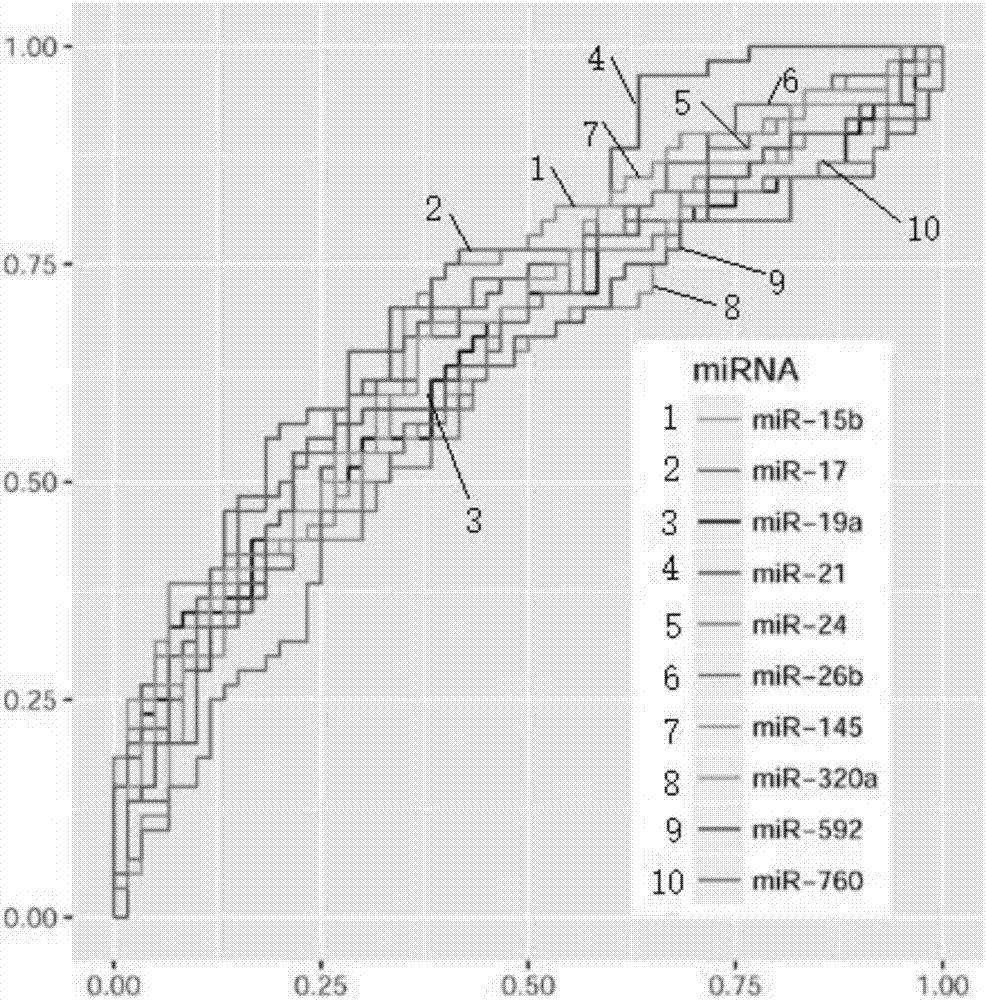
InactiveCN106950370AStrong specificityImprove diagnostic efficiencyMaterial analysisColo-rectal cancerBiomarker (petroleum)
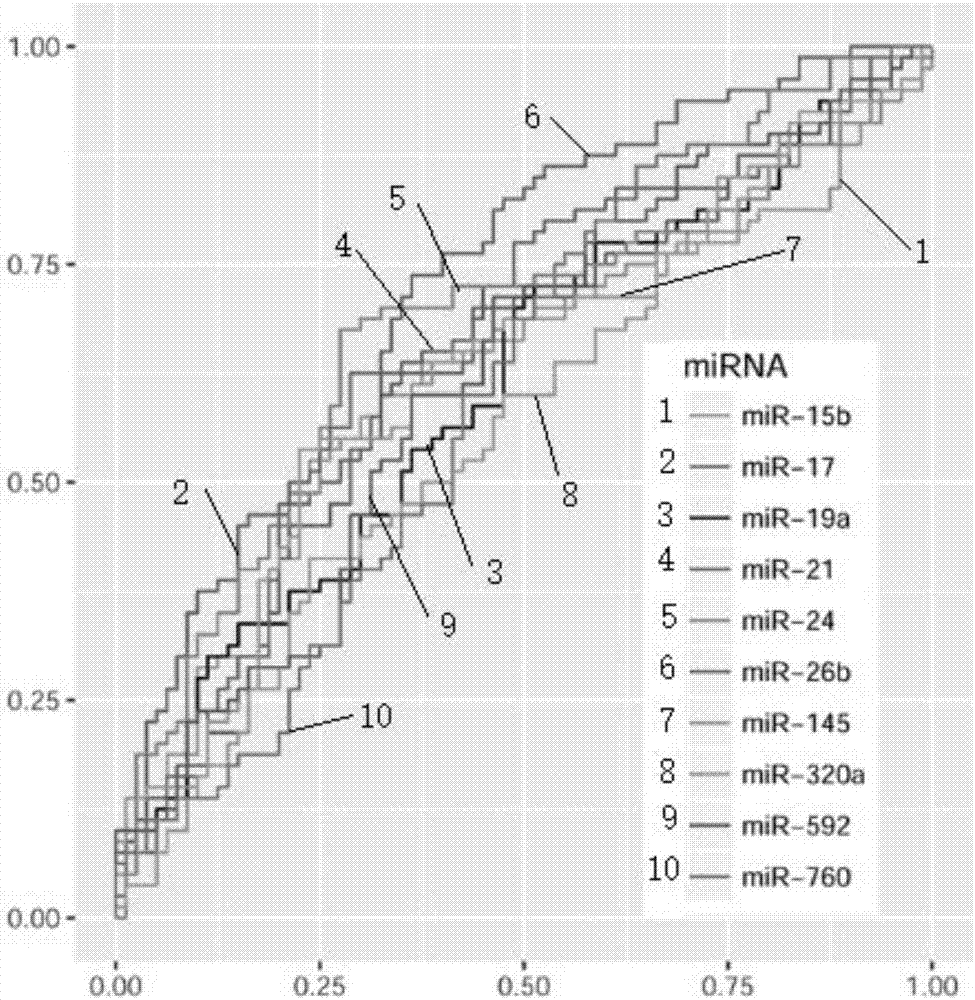
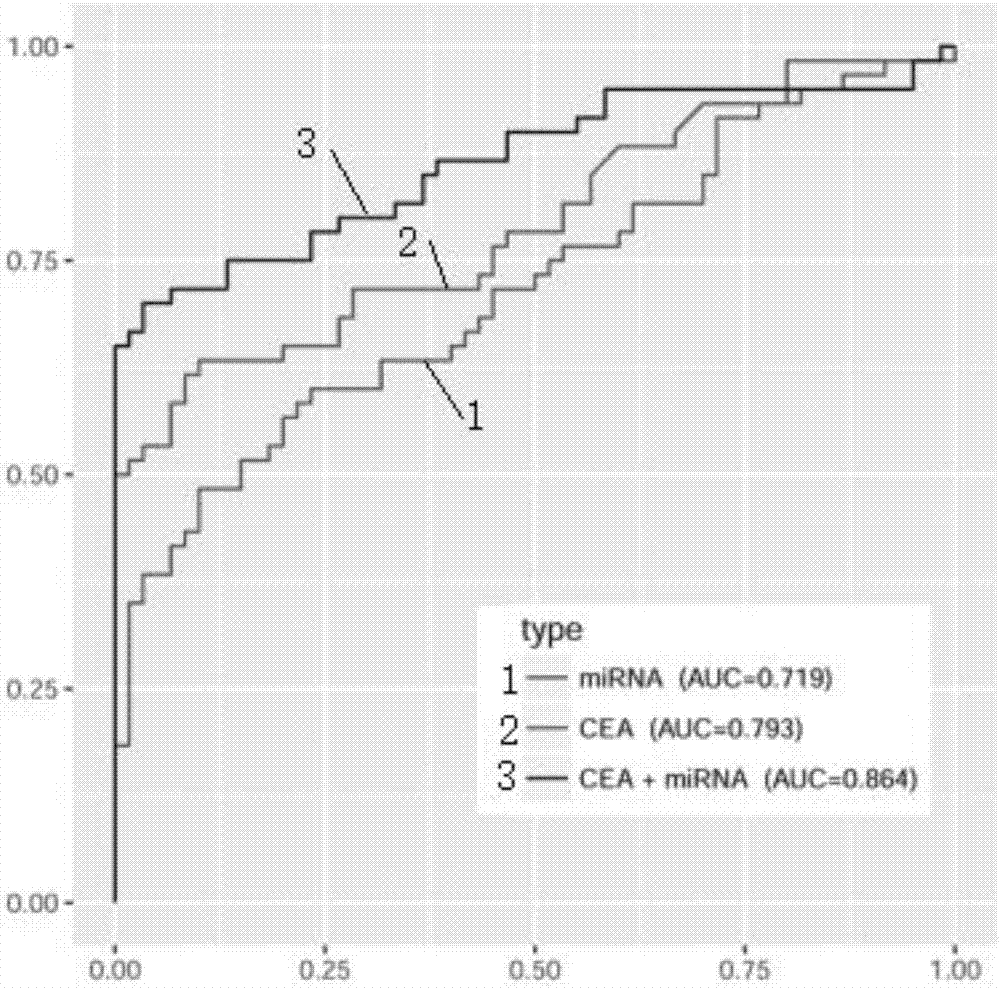
The invention discloses a blood micro-RNA colorectal cancer diagnosis molecule combination including one or more selected from: hsa-miR-15b, hsa-miR-17, hsa-miR-19a, hsa-miR-21, hsa-miR-24, hsa-miR-26b, hsa-miR-145, hsa-miR-592 and hsa-miR-760. An optimum combination of a micro-RNA biomarker is designed by analyzing the markers hereinabove and is used as a biomarker detection model for the colorectal cancer. Especially, the invention relates to two micro-RNA markers, hsa-miR-17 and hsa-miR-26b, which have specificity and fetal origins of the colorectal cancer, wherein the hsa-miR-26b can be used for evaluating differentiated degree, lesion position and tumor burden of the colorectal cancer.
Owner:SHANGHAI TENTH PEOPLES HOSPITAL
Methods and systems for monitoring tumor burden
Owner:GENERAL ELECTRIC CO
Method and apparatus for forming a homeostatic loop employing an aptamer biosensor
ActiveCN102803944AOperable smallSmall fluid volumeBiological testingMaterial electrochemical variablesCapacitanceAptamer
A novel architecture solid-state biosensor for label-free detection of vascular endothelial growth factor (VEGF) hybridization is presented. The new device is realized by forming a matrix array of parallel capacitors, thus allowing the realization of low-cost, portable, fully integrated devices. The detection mechanism is based on an electrochemical binding of circulating VEGF to an immobilized VEGF aptamer; whereby binding of these two compounds modulates the threshold voltage of a novel circuit, changing the impedance (capacitance) of the circuit. This novel circuit is further characterized by an electrode coded with a p-Si substrate, enhancing the affinity between the VEGF molecules and the aptamer. An apparatus forming a fluid cell is configured so as to enable the flow for delivering VEGF samples onto the active surface of the chip. The device has an array of parallel capacitors which act as an integrated, individual counter-electrode, computational apparatus which employs the sensory output over the time domain so as to enable detection, reporting and formation of a homeostatic loop for VEGF measurements. Moreover, this detector is able to provide an accurately measured and quantifiable rate of change of the VEGF molecules in- vivo, providing real time feedback of this important biomarker which may be used to measure response of the tumor to delivered chemotherapeutic agents and biological response modifiers (BRMs) for the purpose of determining tumor burden.
Owner:PHARMACO KINESIS +4
Application of oroxin A in preparation of medicines for treating cancer
ActiveCN104586873AGrowth inhibitionSuppress generationOrganic active ingredientsAntineoplastic agentsCell adhesionTumor cell adhesion
The invention relates to the technical field of traditional Chinese medicines, and in particular relates to an application of oroxin A in preparation of medicines for treating cancer. According to the application disclosed by the invention, research results show that oroxin A has the effect of inhibiting tumor angiogenesis, and can be used for significantly inhibiting the proliferation and growth of tumor cells, inducing the tumor cells to achieve aging heath, cycle arresting and cell apoptosis, and inhibiting the tumor cell adhesion, migration, invasion and angiogenesis; and oroxin A has very good inhibition effects on tumor growth of tumor-burdened mice.
Owner:SUZHOU UNIV
Chinese herbal compound for treating liver cancer
InactiveCN101991807AImprove the quality of lifeFunction increaseAnthropod material medical ingredientsDigestive systemAcute toxicity testingLife quality
The invention relates to a Chinese herbal compound for treating liver cancer, comprising a main prescription and two auxiliary prescriptions, wherein the main prescription comprises 2-18 parts of herba scutellariae barbatae, 1-9 parts of rhizoma zedoariae, 1-4 parts of radix bupleuri, 2-18 parts of radix codonopsitis; the two auxiliary prescriptions comprise 1-9 parts of herba lobeliae chinensis, 1-6 parts of woodlouse, 1-4 parts of raw liquorice, 1-9 parts of atractylodes macrocephaia, 1-9 parts of Tuckahoe and 1-4 parts of honey-fried licorice root. The invention is characterized in that corresponding treatment medicines are applised according to different kinds of pathogenesis emphasis (depression of the liver, insufficiency of the spleen and stagnating stasis in the body) in a developing process of the liver cancer. The traditional research prompts that the two auxiliary prescriptions which use the main prescription as a base can obviously improve the life quality and the liver function of liver cancer patients, can obviously prolong the life time of the tumor-burdened rat, can obviously confront the generation and the development of the dyscrasia of the tumor-burdened rat, which are resulted from the transplanted tumor and can prolong the survive time of the rat with tumors; and an animal acute toxicity test proves that no animal death occurs. The Chinese herbal compound for treating the liver cancer is safe and convenient to use and has determined curative effect.
Owner:陈泽雄 +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com