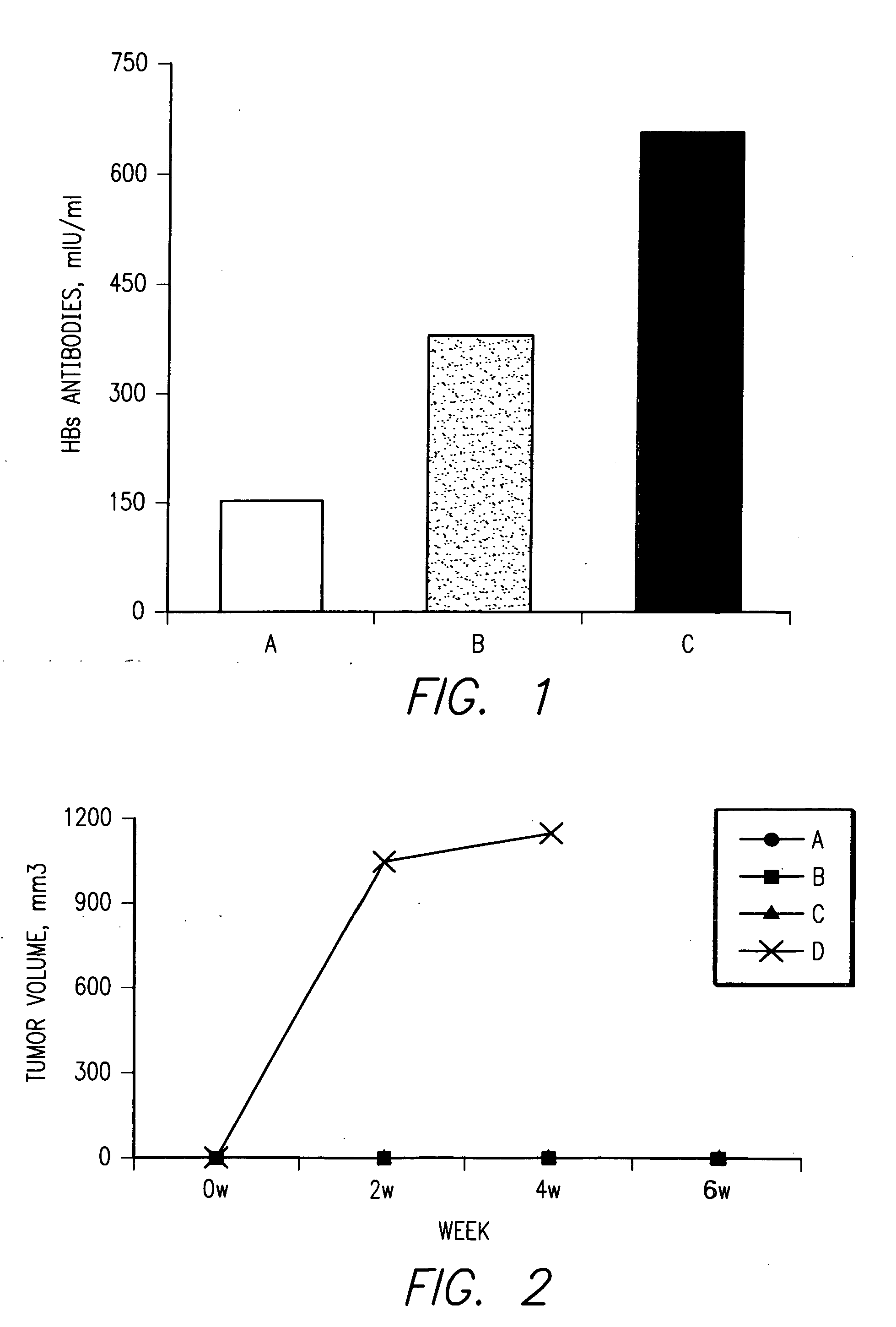
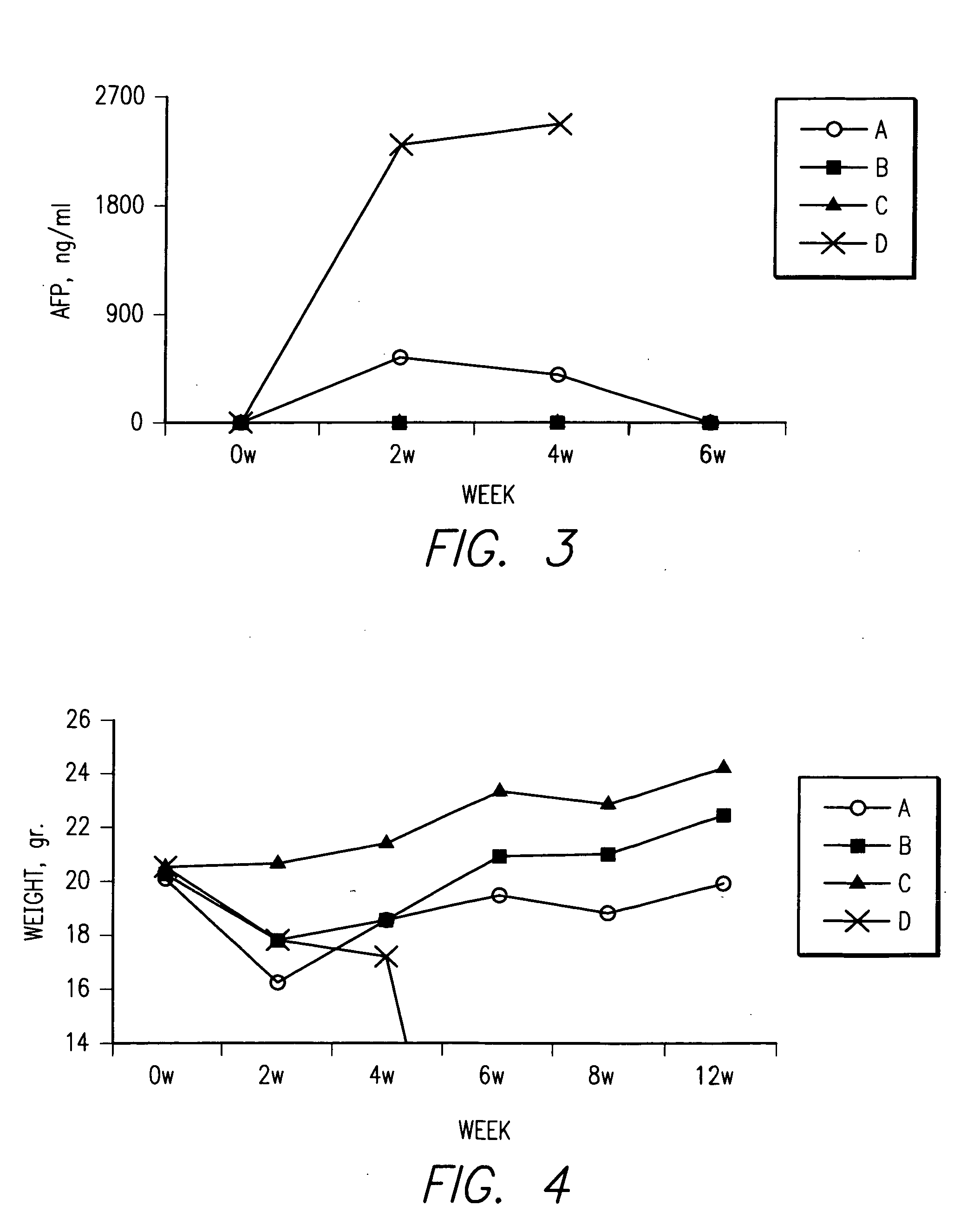
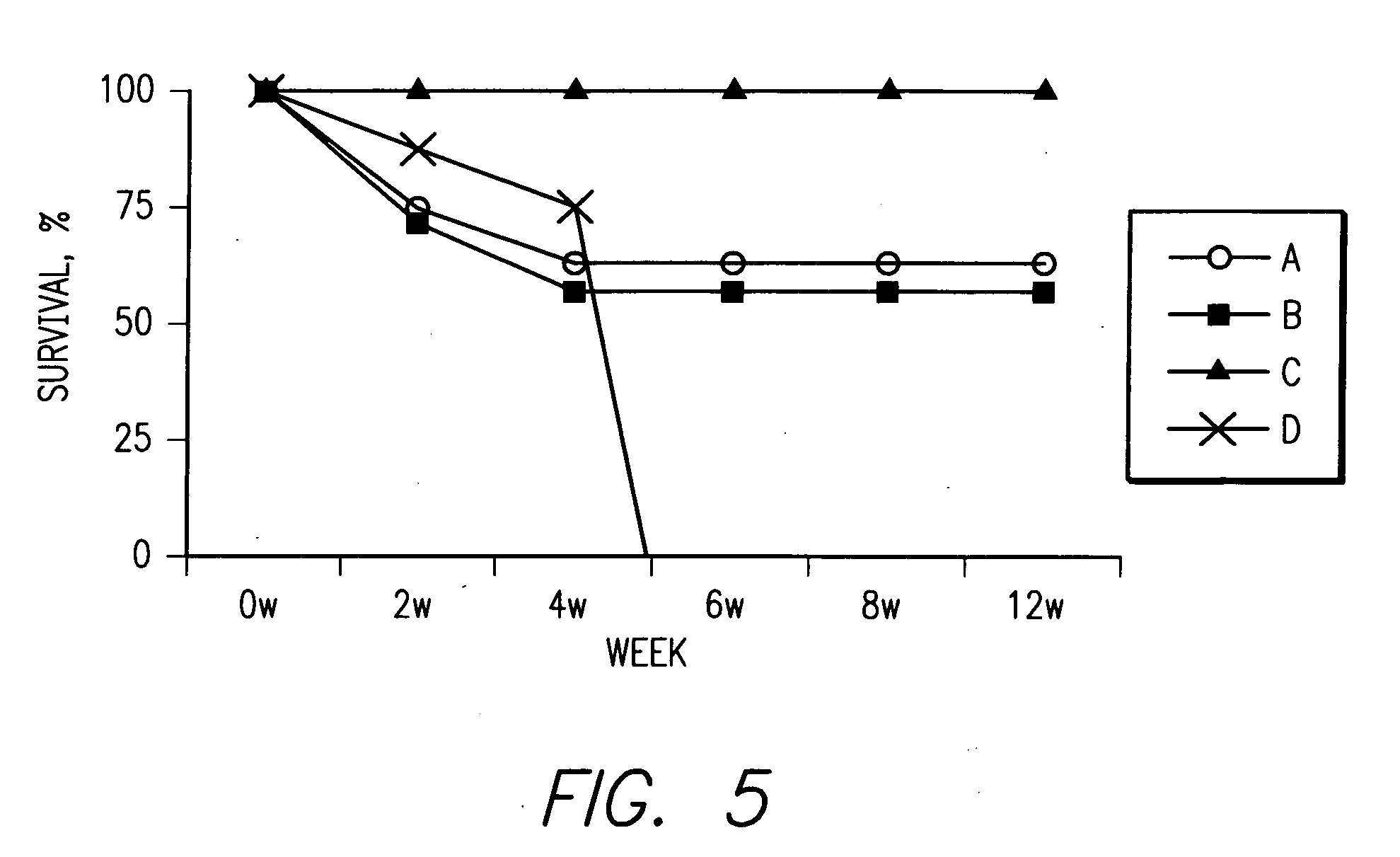
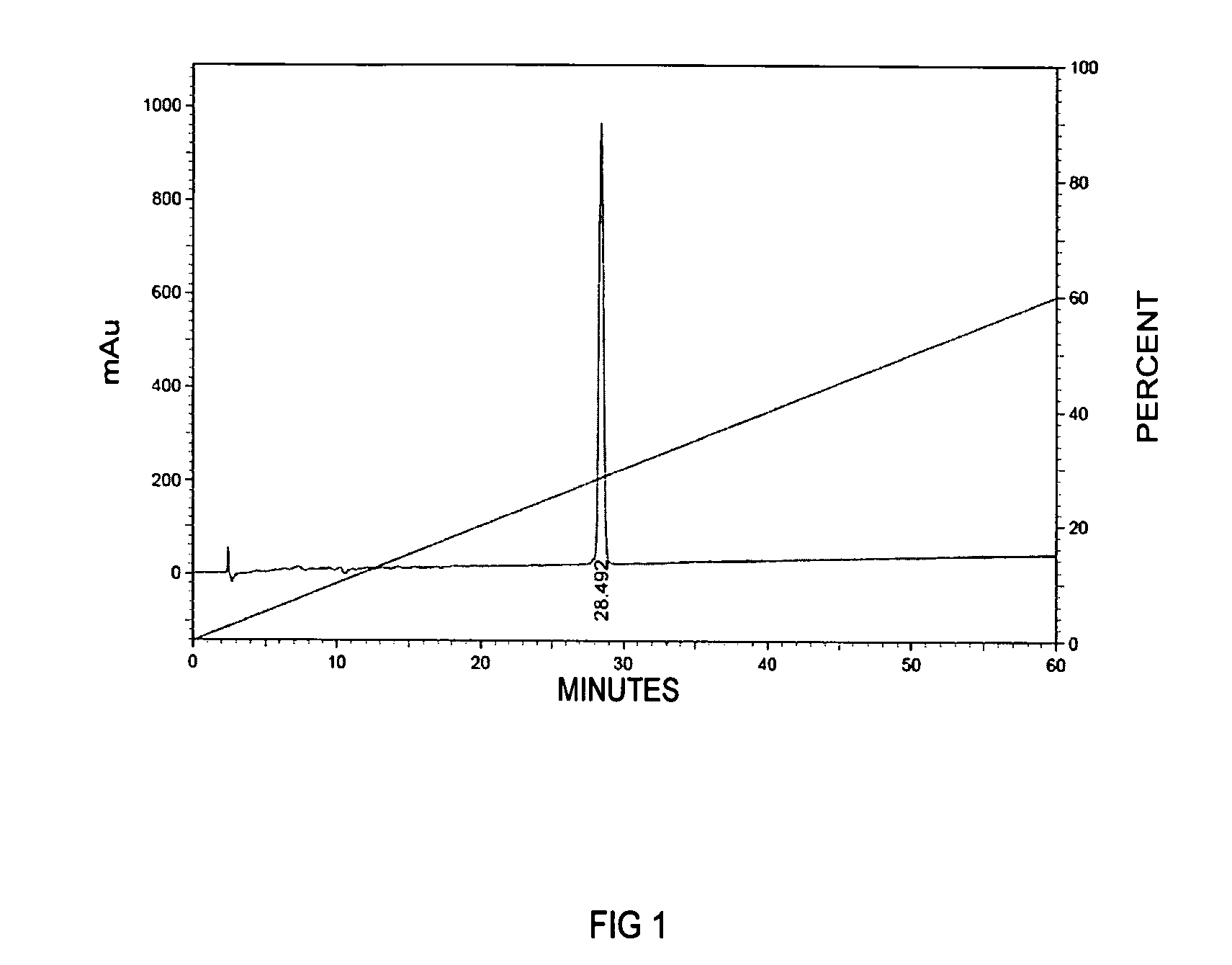
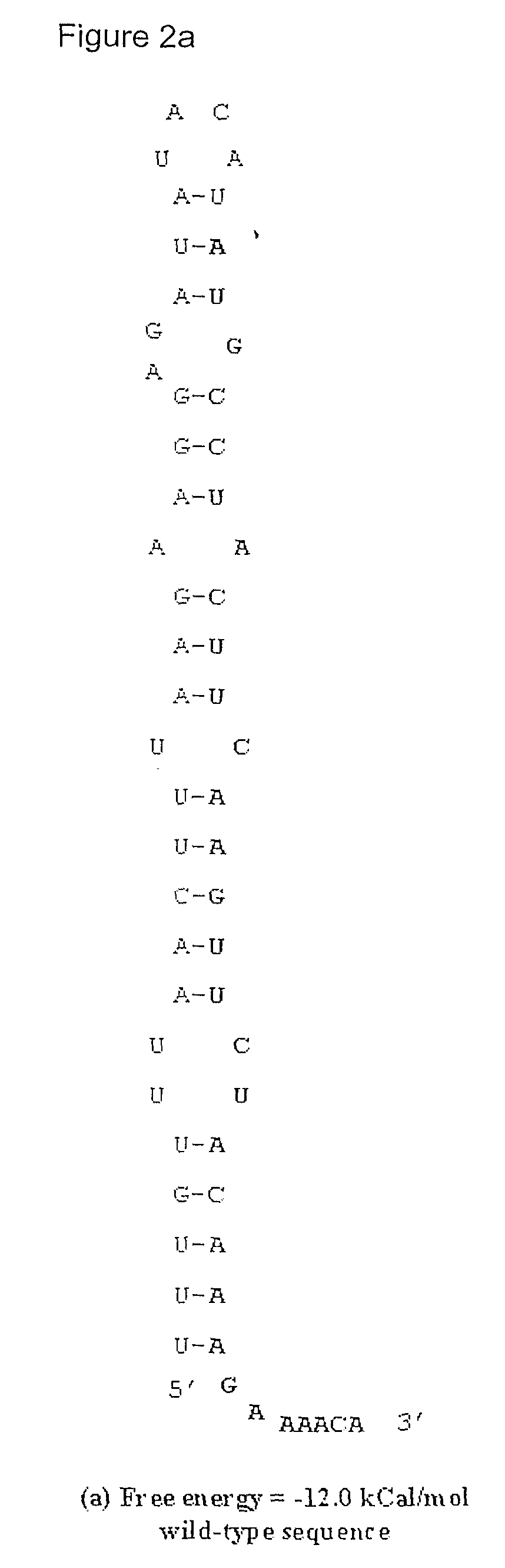Patents
Literature
64 results about "Lymphokine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Lymphokines are a subset of cytokines that are produced by a type of immune cell known as a lymphocyte. They are protein mediators typically produced by T cells to direct the immune system response by signaling between its cells. Lymphokines have many roles, including the attraction of other immune cells, including macrophages and other lymphocytes, to an infected site and their subsequent activation to prepare them to mount an immune response. Circulating lymphocytes can detect a very small concentration of lymphokine and then move up the concentration gradient towards where the immune response is required. Lymphokines aid B cells to produce antibodies.
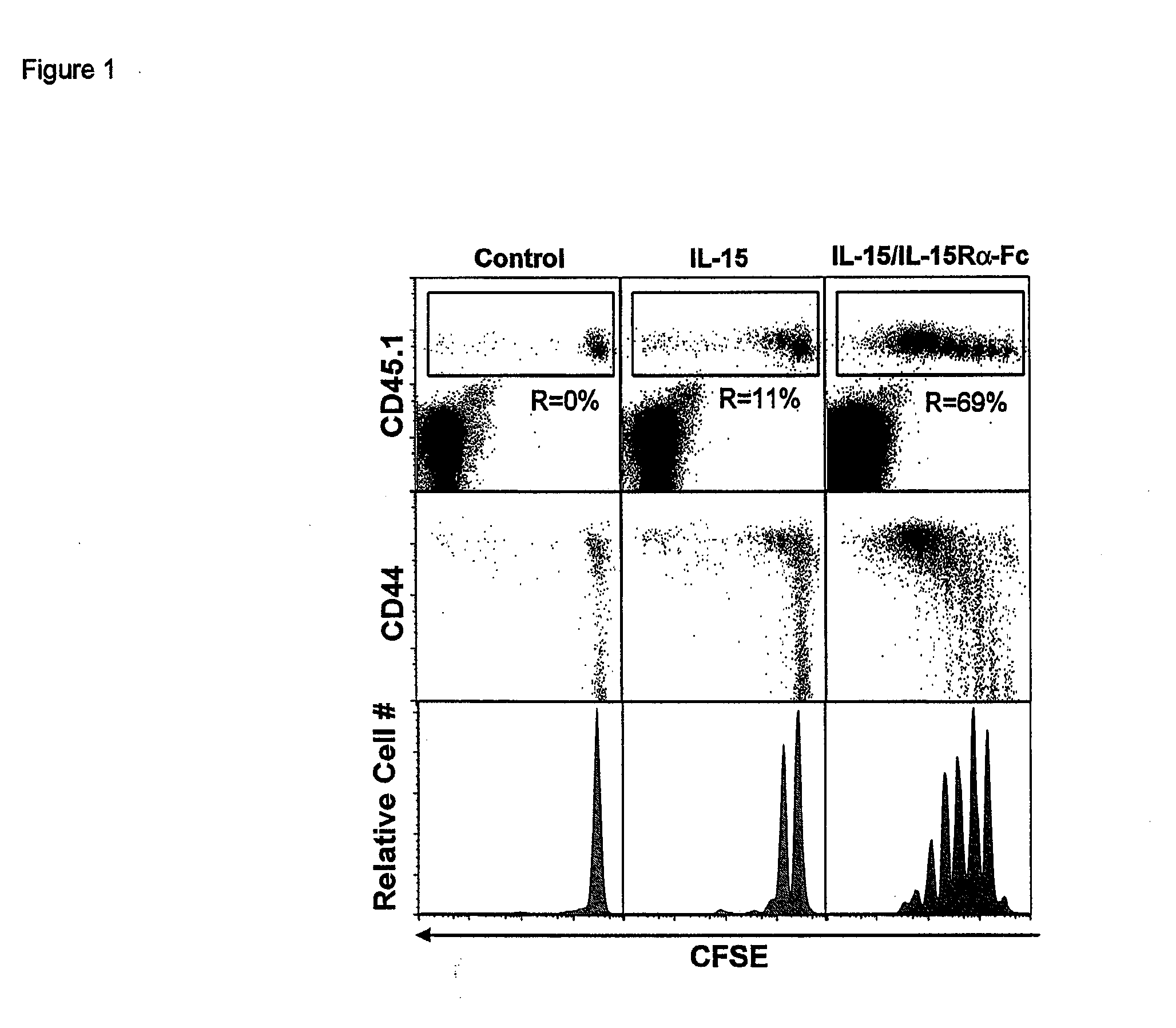
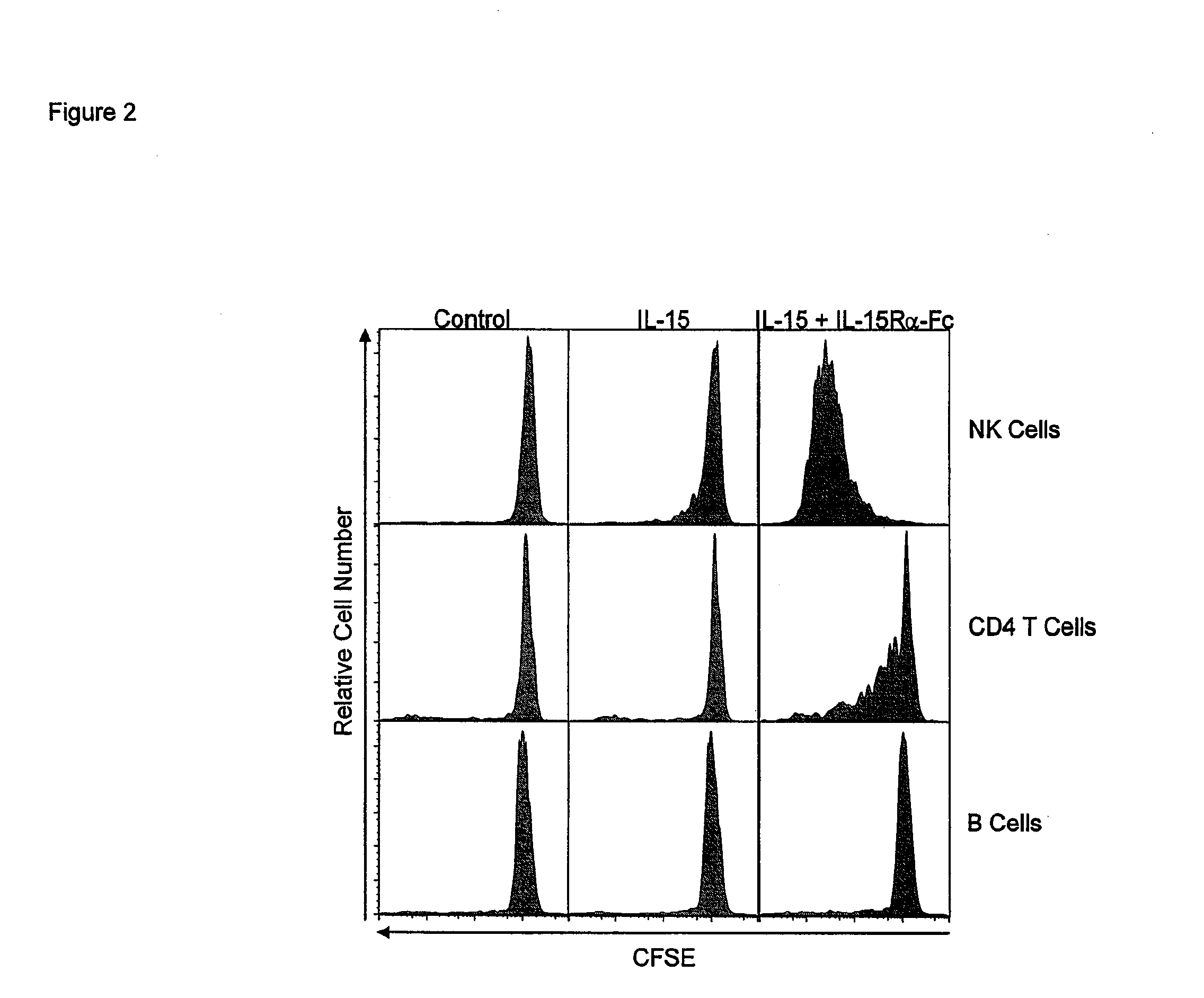
Compositions and methods for immunomodulation in an organism using IL-15 and soluble IL-15Ra
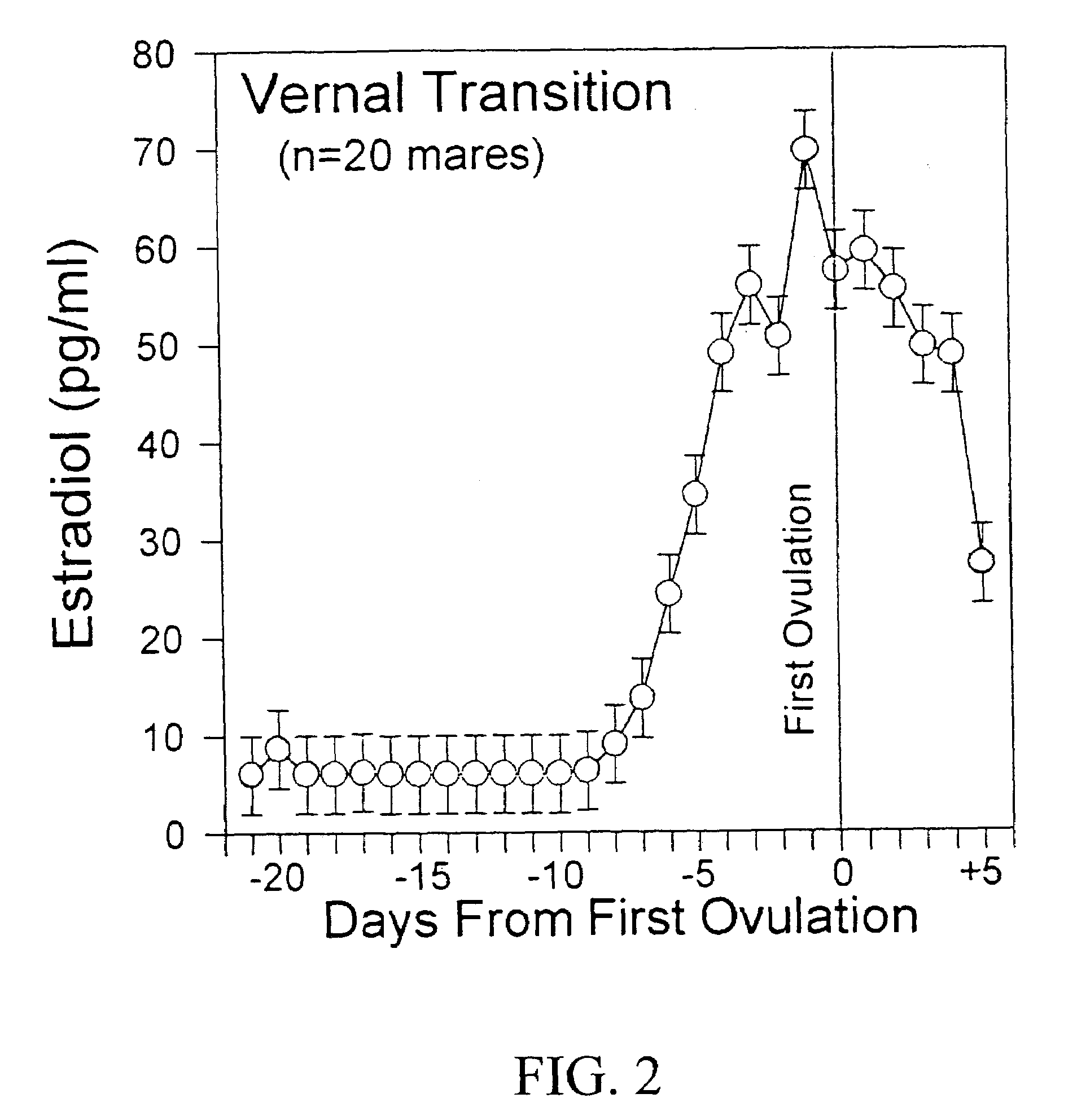
ActiveUS8124084B2Extended half-lifeImprove bioavailabilityPeptide/protein ingredientsAntibody mimetics/scaffoldsBiological bodyVaccination
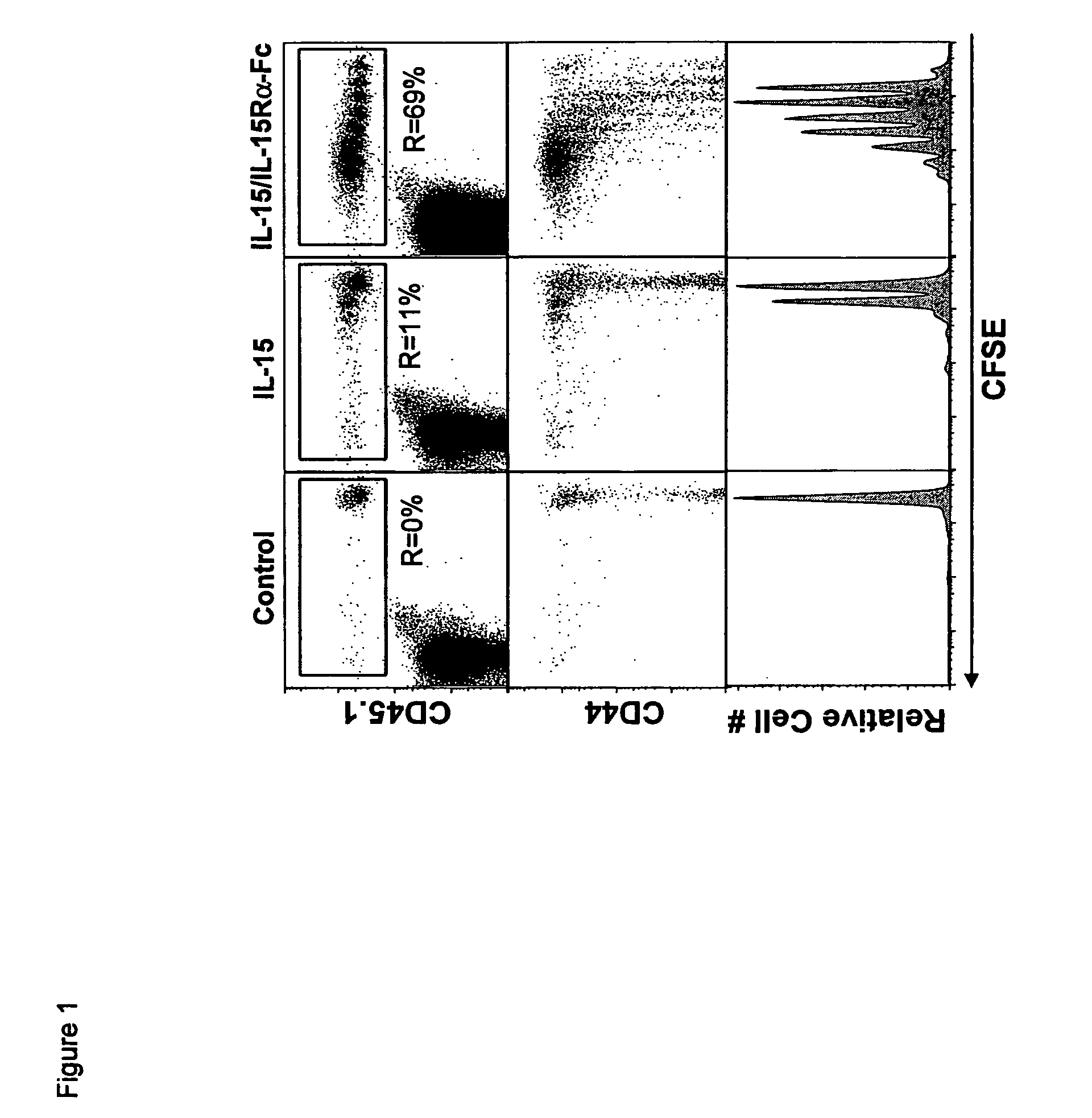
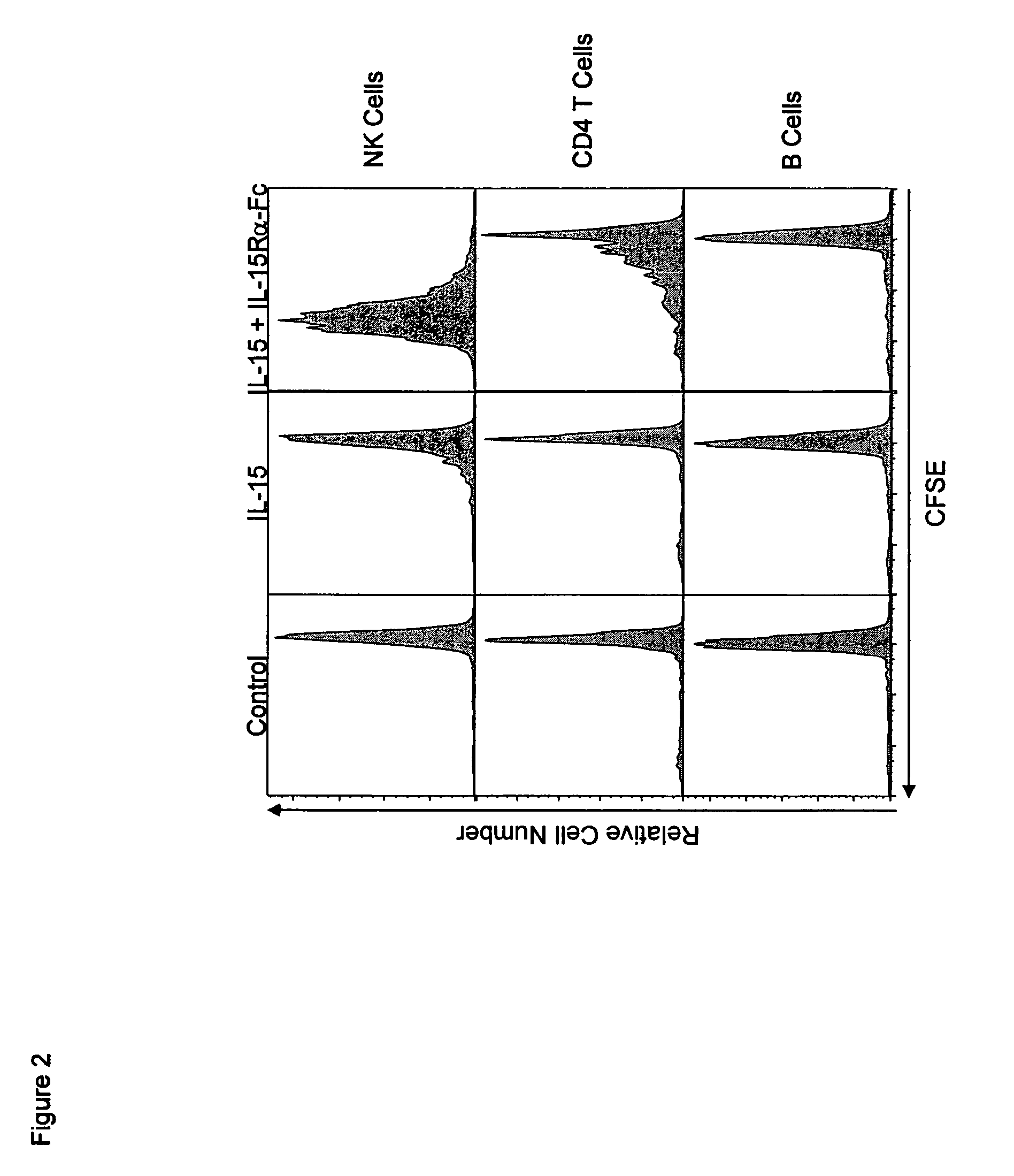
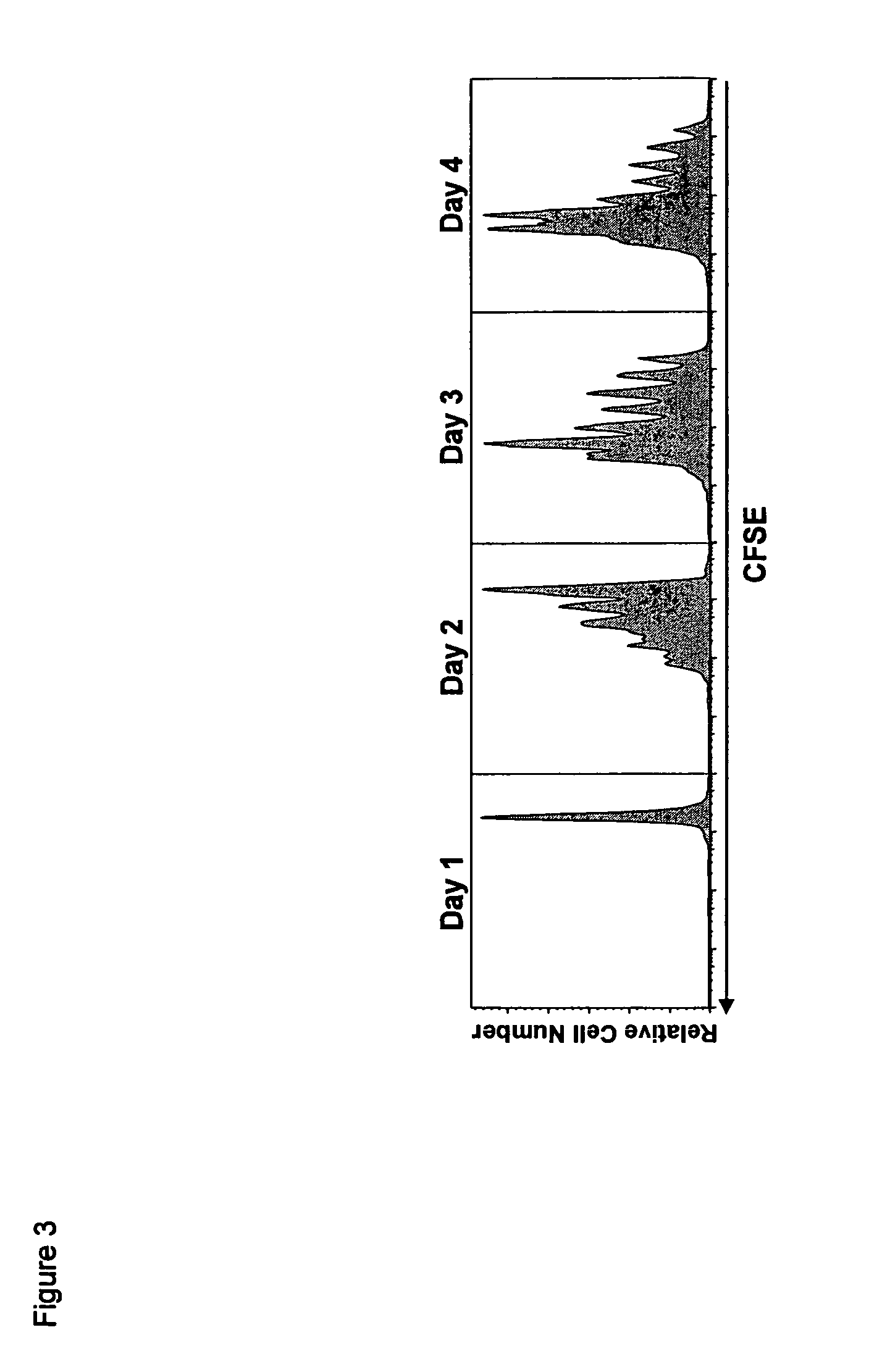
The present invention relates to a therapeutic polypeptide and methods for its creation and use for modulating an immune response in a host organism in need thereof. In particular, the invention relates to the administration to an organism in need thereof, of an effective amount of a pre-coupled polypeptide complex comprising a lymphokine polypeptide portion, for example IL-15 (SEQ ID NO: 5, 6), IL-2 (SEQ ID NO: 10, 12) or combinations of both, and an interleukin receptor polypeptide portion, for example IL-15Ra (SEQ ID NO: 7, 8), IL-2Ra (SEQ ID NO: 9, 11) or combinations of both, for augmenting the immune system in, for example, cancer, SCID, AIDS, or vaccination; or inhibiting the immune system in, for example, rheumatoid arthritis, or Lupus. The therapeutic complex of the invention surprisingly demonstrates increased half-life, and efficacy in vivo.
Owner:UNIV OF CONNECTICUT
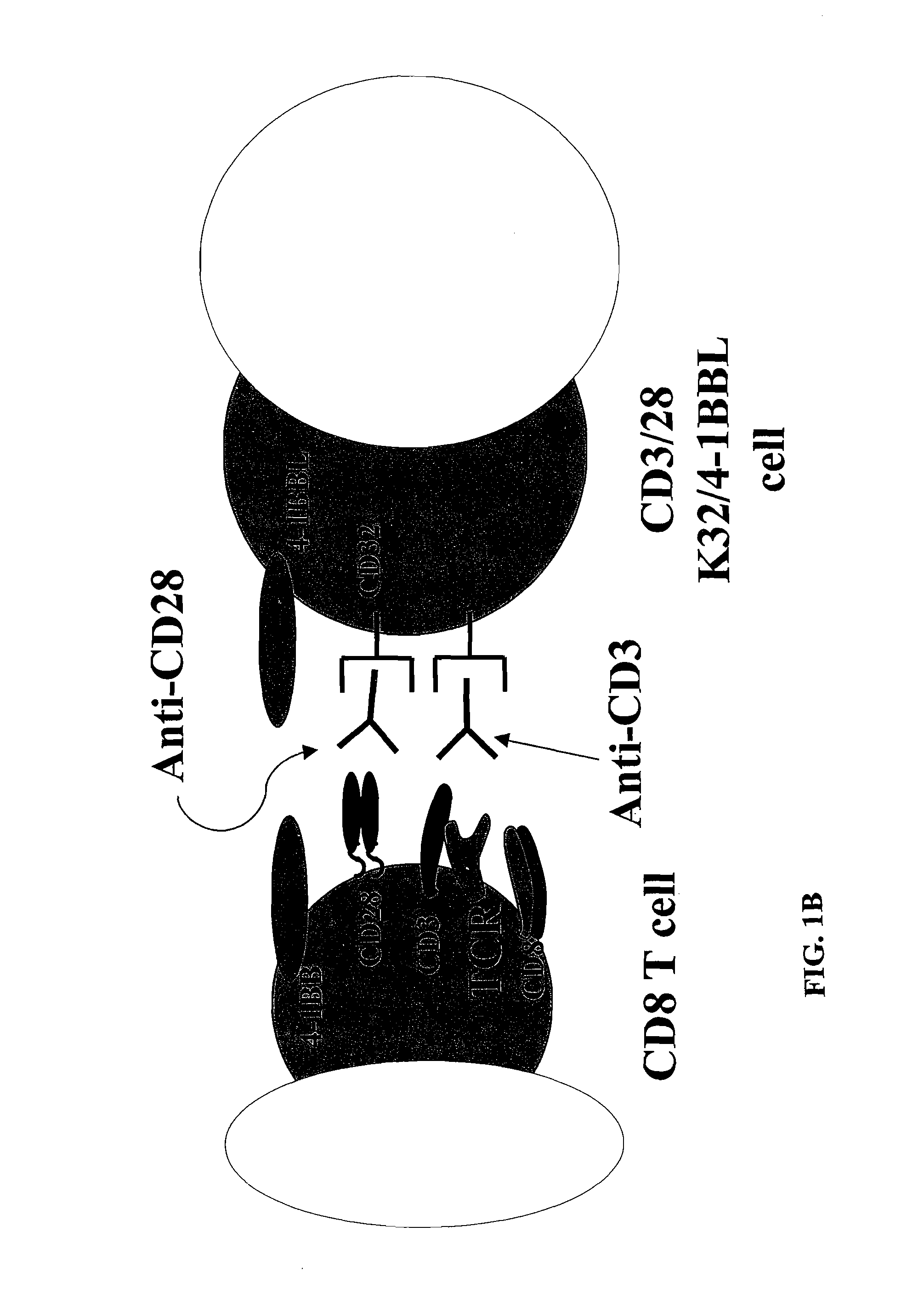
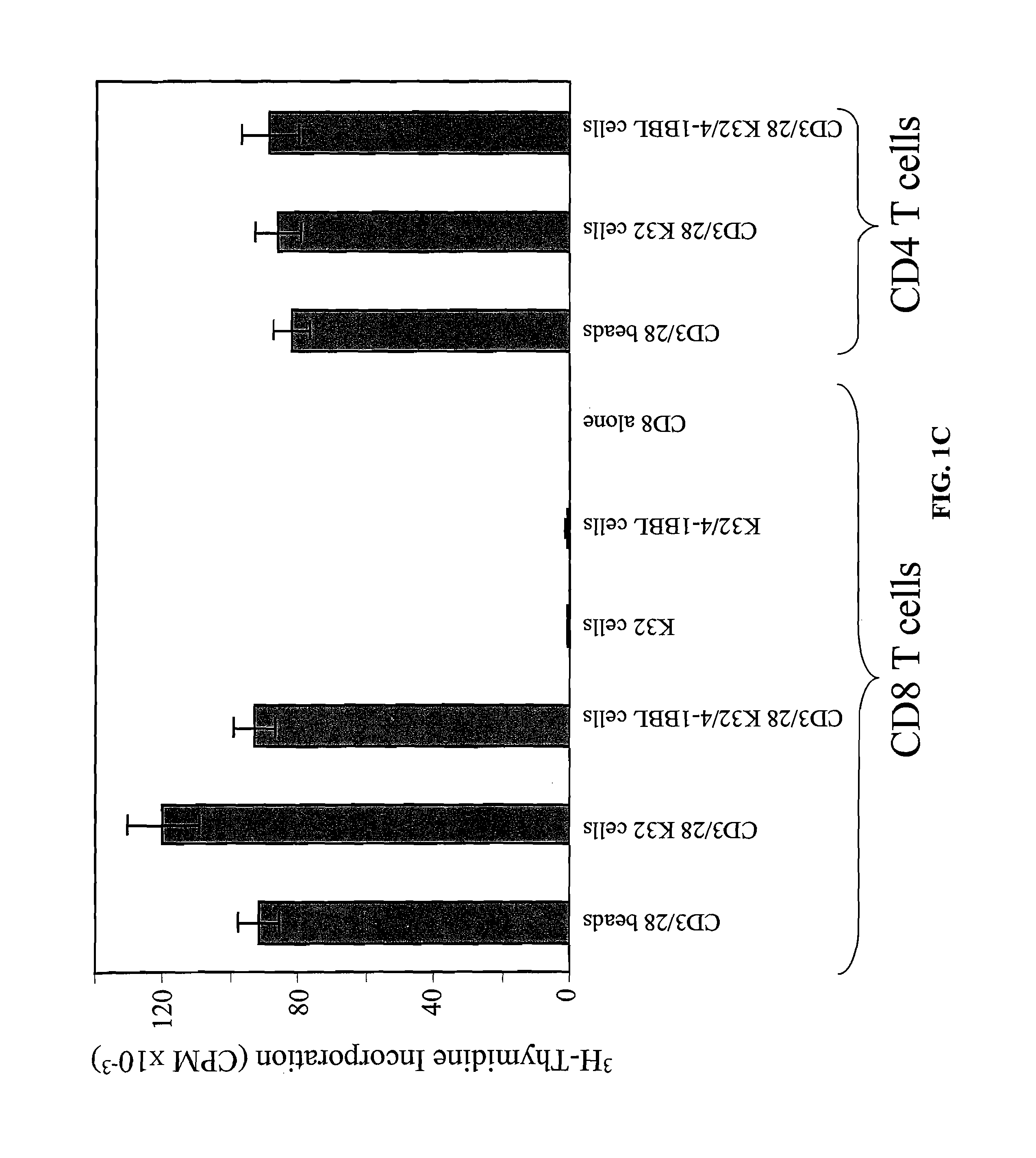
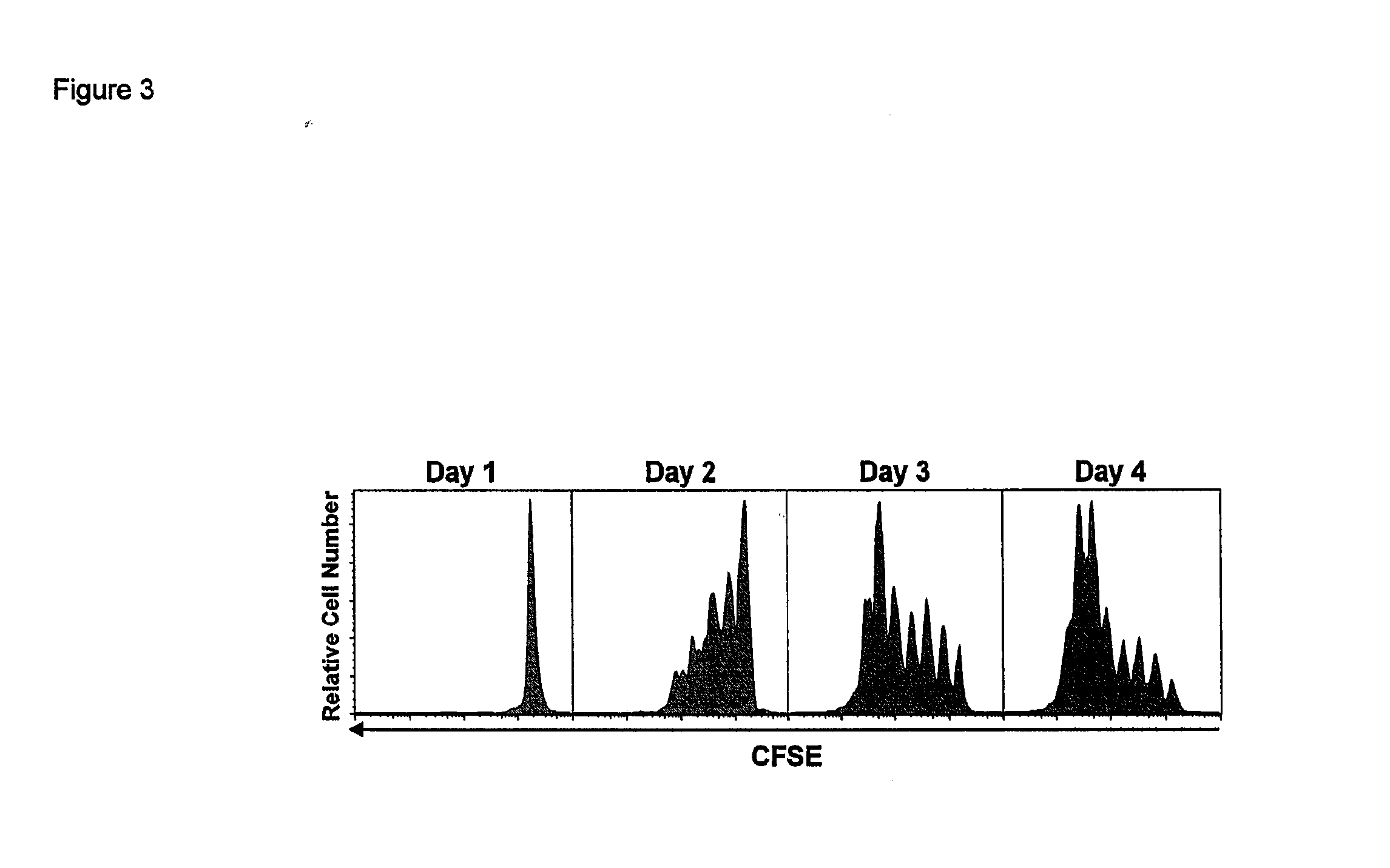
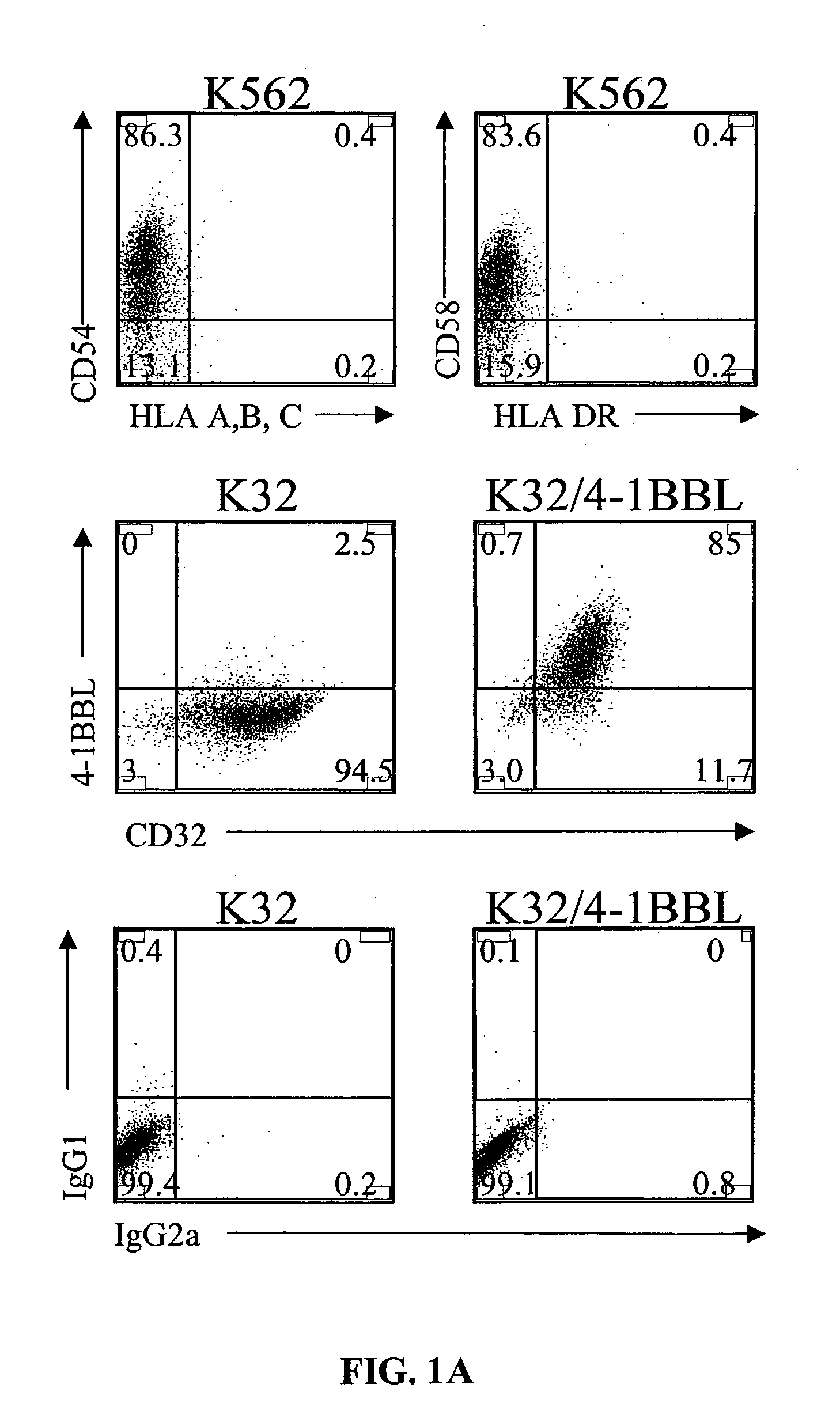
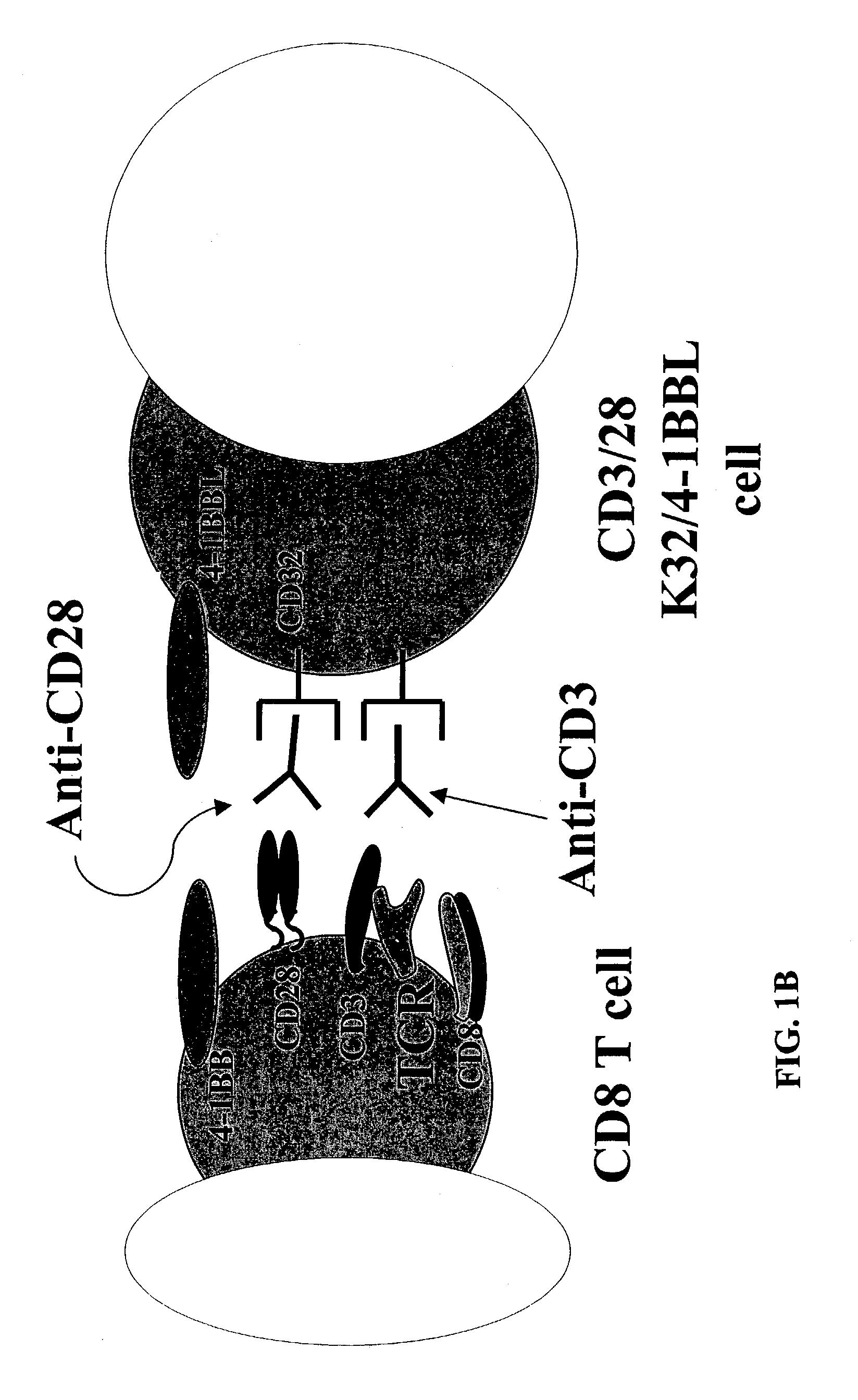
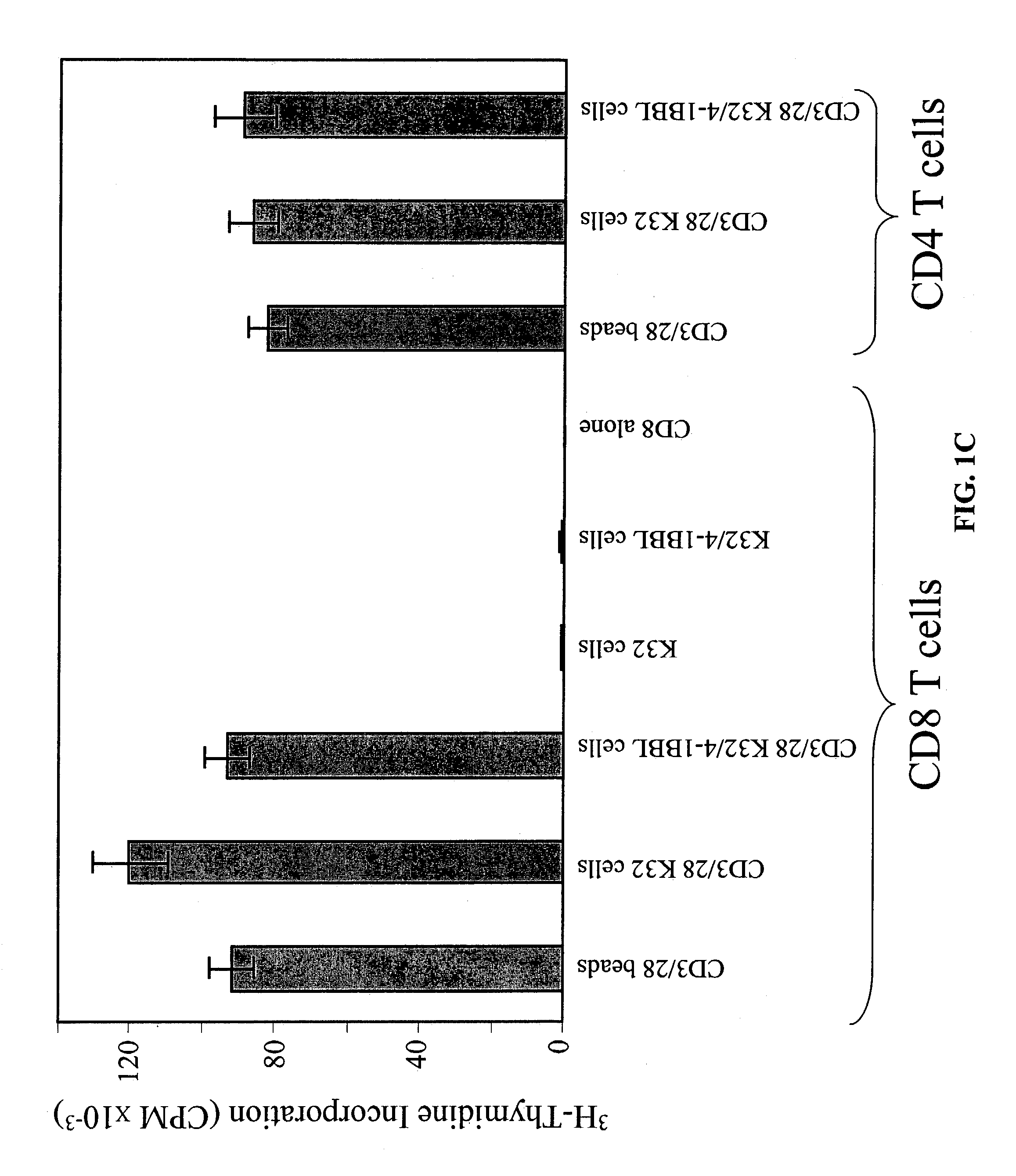
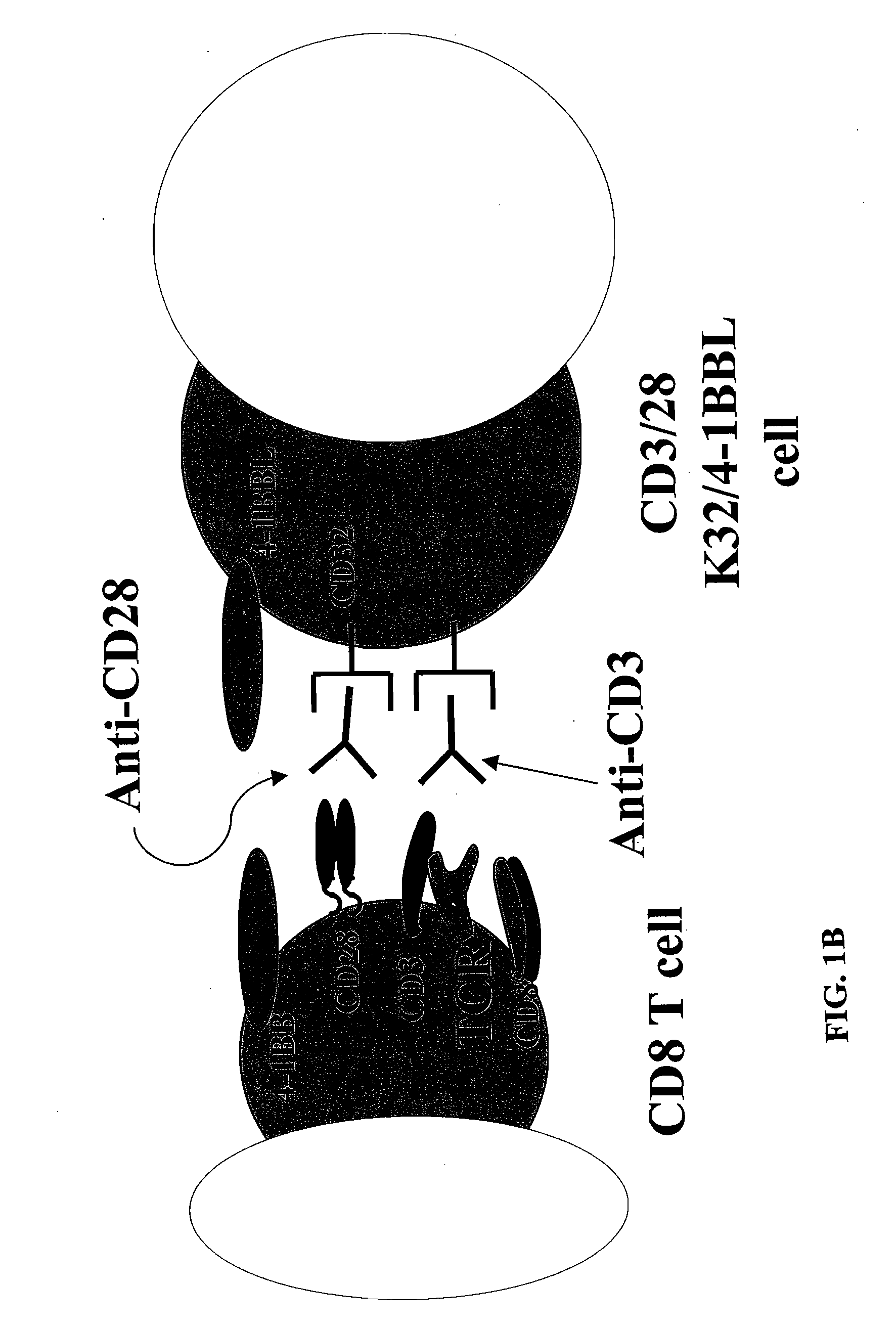
Activation and expansion of T-cells using an engineered multivalent signaling platform as a research tool
InactiveUS8637307B2Maintain their viabilityLower Level RequirementsGenetically modified cellsMammal material medical ingredientsT cellExogenous growth
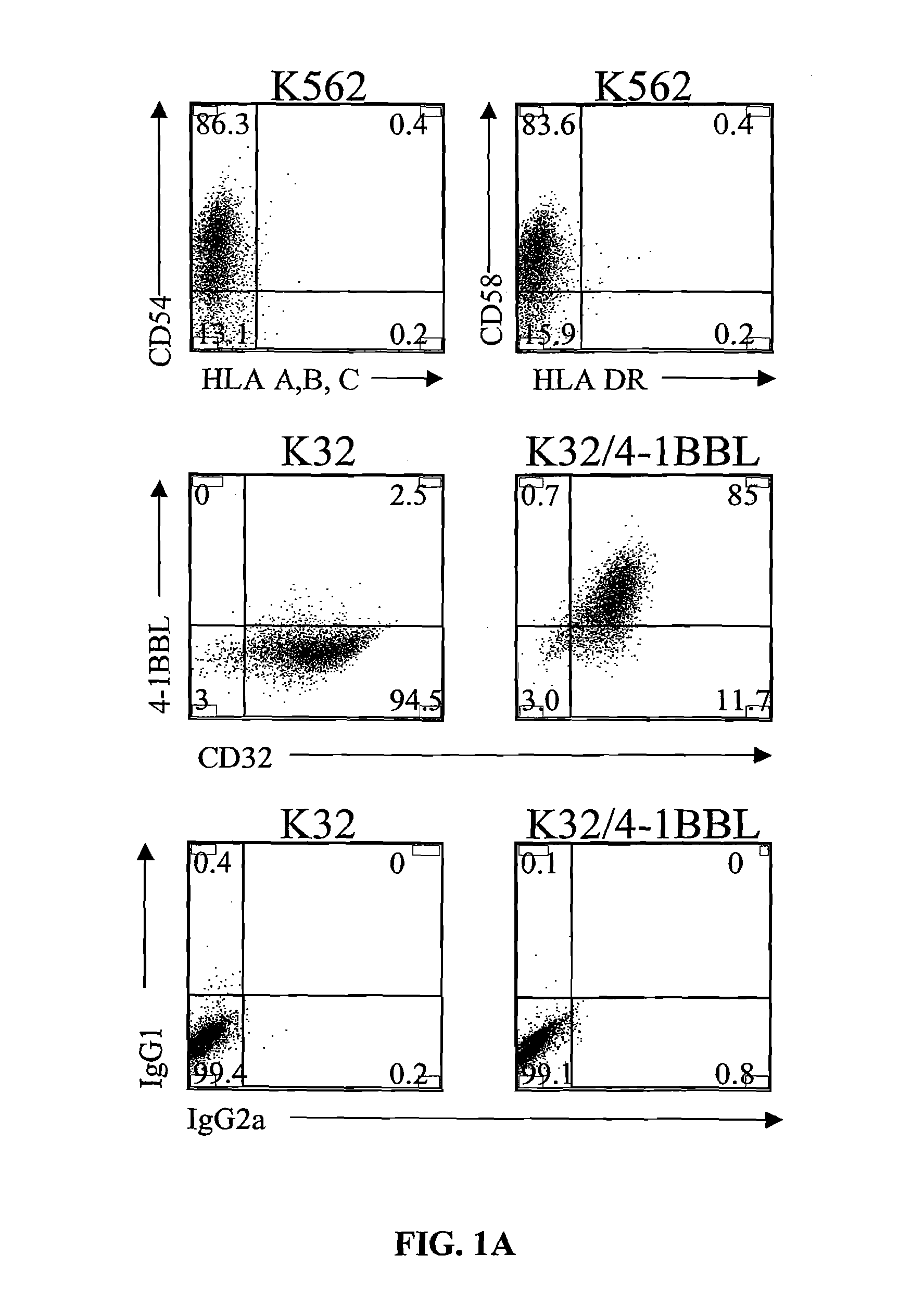
Provided are a system and methods for selectively inducing expansion of a population of T cells in the absence of exogenous growth factors, such as lymphokines, and accessory cells for research purposes. The cell based expansion system and methods permit the long-term growth of CTLs, preferably human CTLs. In addition, T cell proliferation can be induced without the need for antigen, thus providing an expanded T cell population that is polyclonal with respect to antigen reactivity. Further provided are methods for using the system and methods to screen and identify antigens related to specific diseases or conditions, tumors, autoimmune disorders, or an infectious disease or pathogen, and to identify target molecule for research purposes, or for developing a vaccine based thereon.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Compositions and Methods for Immunomodulation in an Organism
ActiveUS20120177598A1Long half-lifeGood treatment effectPolypeptide with localisation/targeting motifPeptide/protein ingredientsVaccinationHalf-life
The present invention relates to a therapeutic polypeptide and methods for its creation and use for modulating an immune response in a host organism in need thereof. In particular, the invention relates to the administration to an organism in need thereof, of an effective amount of a pre-coupled polypeptide complex comprising a lymphokine polypeptide portion, for example IL-15 (SEQ ID NO: 5, 6), IL-2 (SEQ ID NO: 10, 12) or combinations of both, and an interleukin receptor polypeptide portion, for example IL-15Ra (SEQ ID NO: 7, 8), IL-2Ra (SEQ ID NO: 9, 11) or combinations of both, for augmenting the immune system in, for example, cancer, SCID, AIDS, or vaccination; or inhibiting the immune system in, for example, rheumatoid arthritis, or Lupus. The therapeutic complex of the invention surprisingly demonstrates increased half-life, and efficacy in vivo.
Owner:UNIV OF CONNECTICUT
Activation and expansion of T-cells using an engineered multivalent signaling platform as a research tool
ActiveUS7745140B2Decrease in apoptosis levelImprove survivalBiocideGenetic material ingredientsBiological activationExogenous growth
Provided are a system and methods for selectively inducing expansion of a population of T cells in the absence of exogenous growth factors, such as lymphokines, and accessory cells for research purposes. The cell based expansion system and methods permit the long-term growth of CTLs, preferably human CTLs. In addition, T cell proliferation can be induced without the need for antigen, thus providing an expanded T cell population that is polyclonal with respect to antigen reactivity. Further provided are methods for using the system and methods to screen and identify antigens related to specific diseases or conditions, tumors, autoimmune disorders, or an infectious disease or pathogen, and to identify target molecule for research purposes, or for developing a vaccine based thereon.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
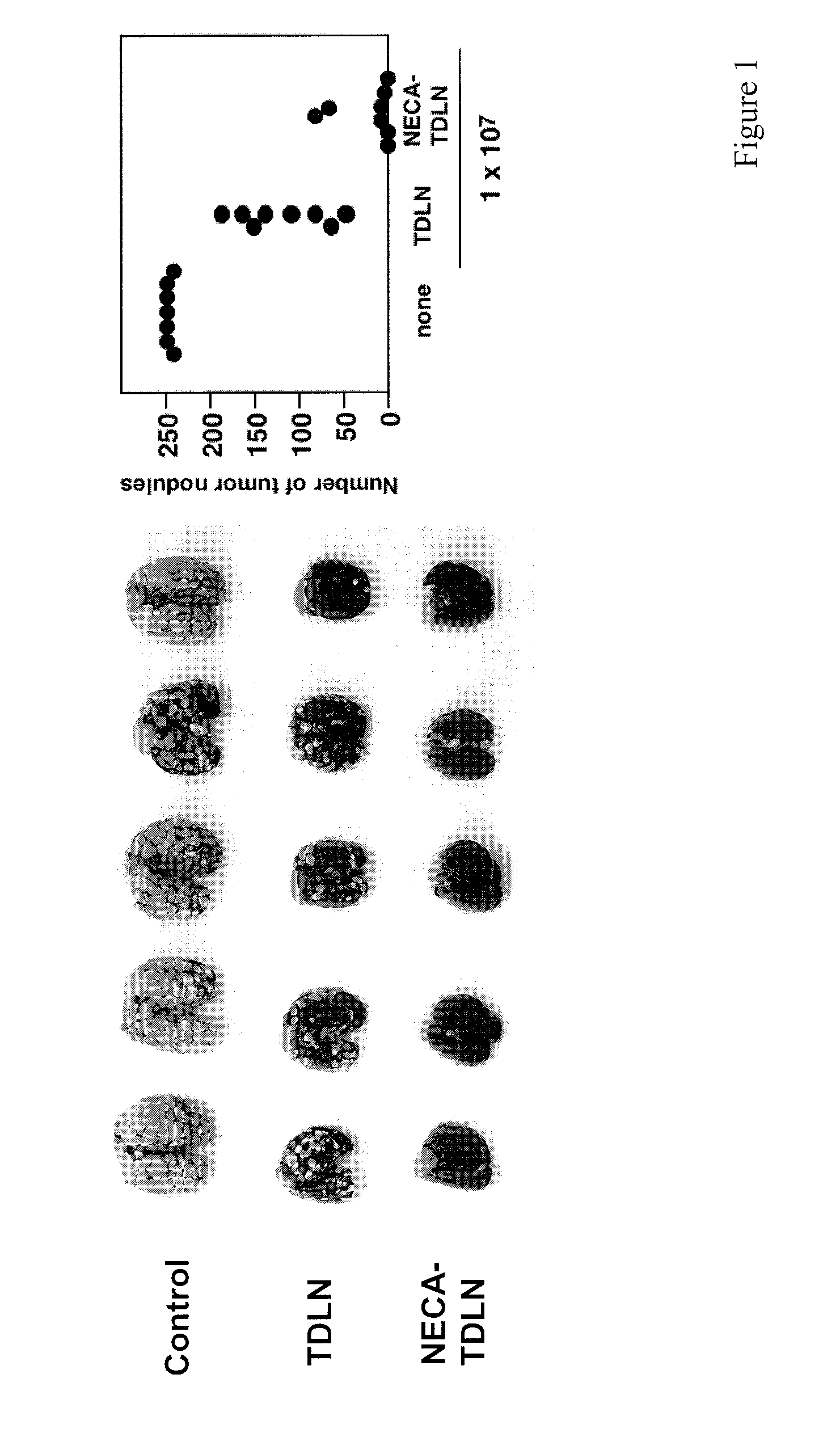
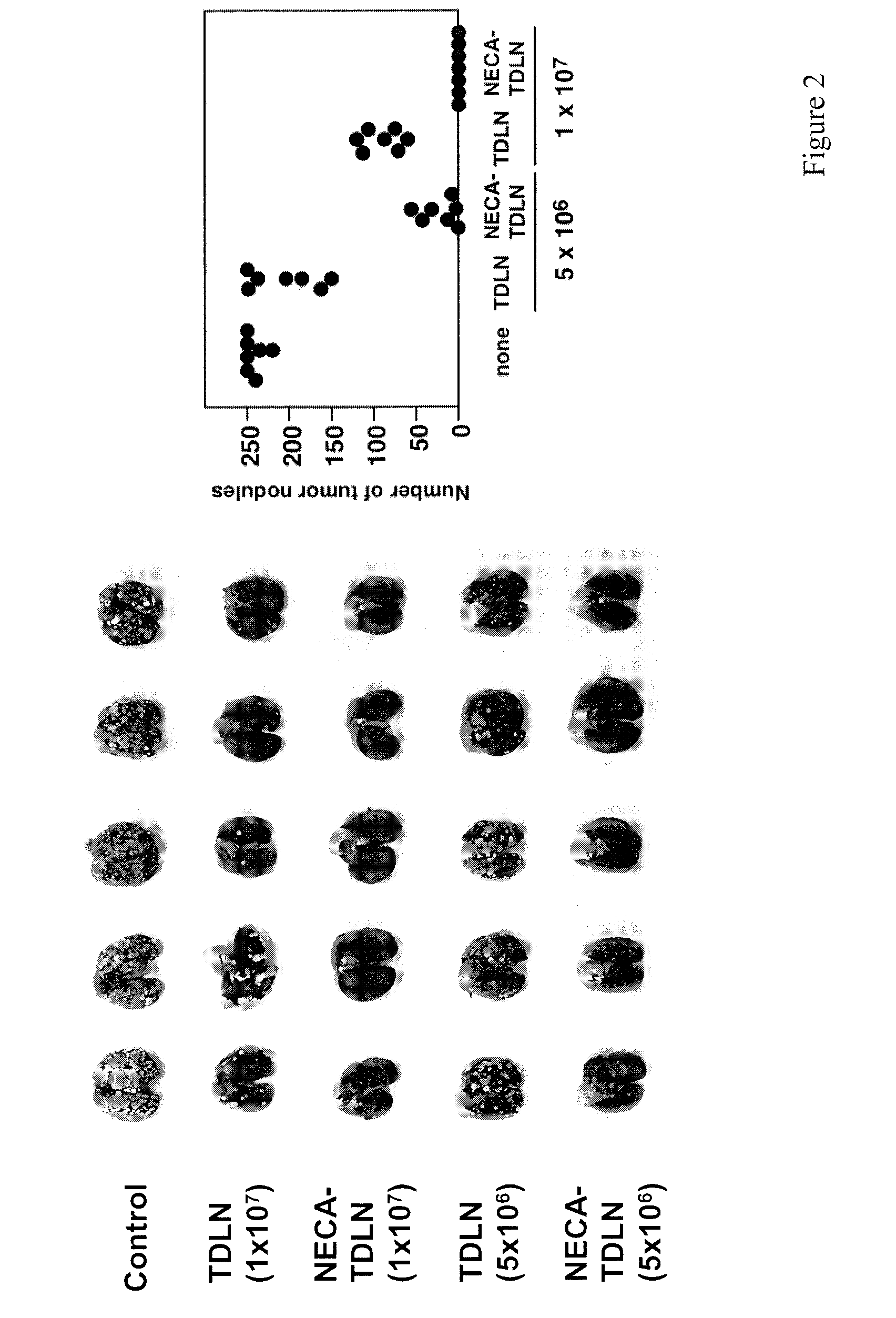
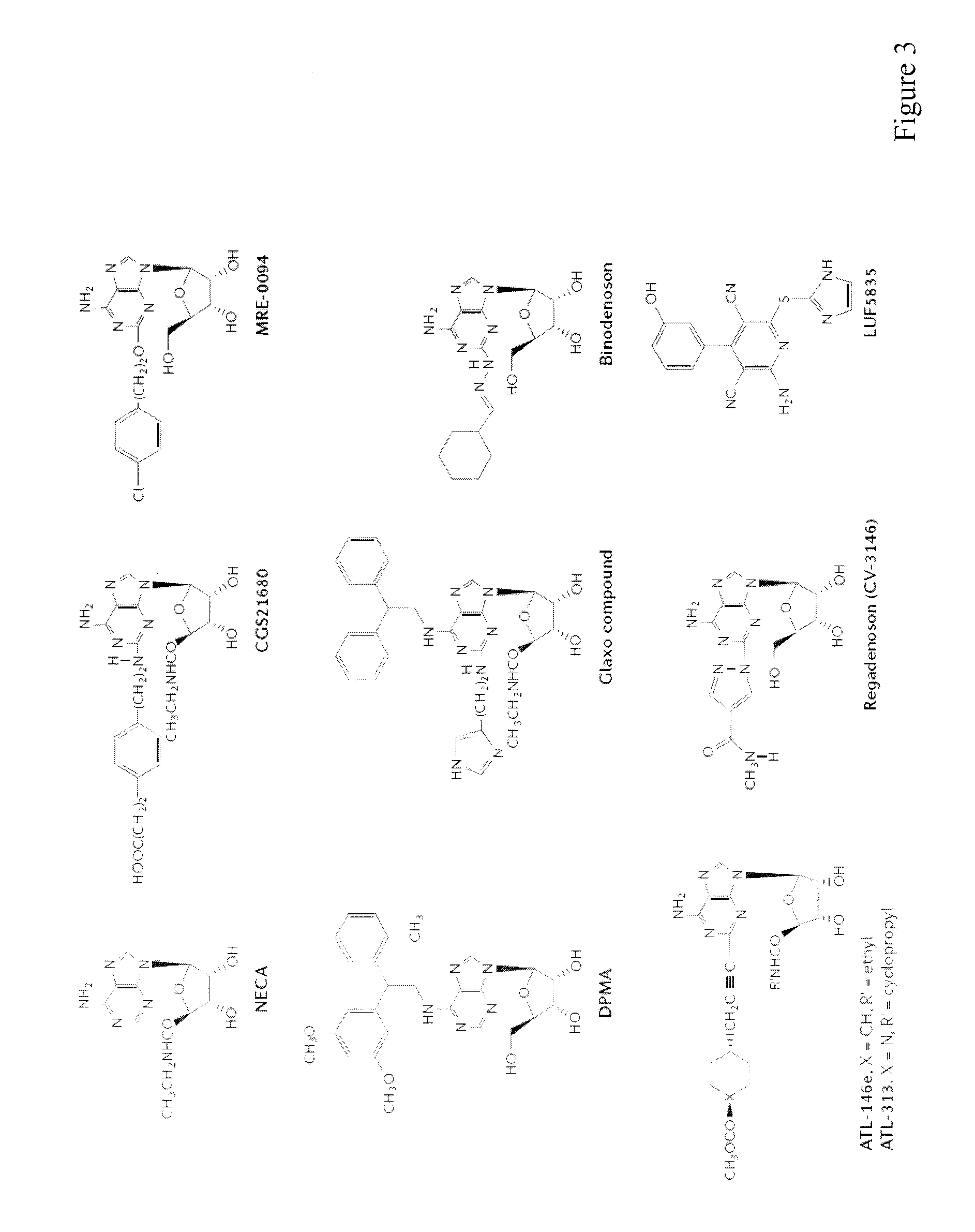
Method of preparing adenosine-resistant anti-tumor T lymphocytes for adoptive immunotherapy
In the past, adoptive immunotherapy often failed because the transferred immune cells were inactive in vivo. This disclosure provides a method of producing immune cells that are highly active in vivo. The immune cells may be expanded in vitro in the presence of an adenosine receptor agonist or an antisense nucleic acid that downregulates expression of an adenosine receptor, for example. The immune cells may be tumor-infiltrating lymphocytes (TIL), cytotoxic T lymphocytes (CTL), natural killer (NK) cells, or lymphokine-activated killer (LAK) cells, for example. The methods described herein may be used to treat a number of diseases including cancer, infectious diseases, and immunodeficiencies.
Owner:NORTHEASTERN UNIV
Immune modulation device for use in animals
InactiveUS6958158B2Minimize irritationEnhance immune responseElectrotherapySnake antigen ingredientsFiberMammal
The present invention is directed to an implantable immune modulation device that is useful for modulating an immune response in mammals, comprising a plurality of fibers, within a porous shell. The fiber filling is loaded with single or multiple antigens, and optionally one or more biologically active compounds such as cytokines (e.g. lymphokines, chemokines etc.), attachment factors, genes, peptides, proteins, nucleotides, carbohydrates or cells depending on the application.
Owner:ORTHO MCNEIL PHARM INC
Activation and Expansion of T-Cells Using An Engineered Multivalent Signaling Platform as a Research Tool
ActiveUS20100261269A1Maintain their viabilityLower Level RequirementsGenetically modified cellsMammal material medical ingredientsT cellExogenous growth
Provided are a system and methods for selectively inducing expansion of a population of T cells in the absence of exogenous growth factors, such as lymphokines, and accessory cells for research purposes. The cell based expansion system and methods permit the long-term growth of CTLs, preferably human CTLs. In addition, T cell proliferation can be induced without the need for antigen, thus providing an expanded T cell population that is polyclonal with respect to antigen reactivity. Further provided are methods for using the system and methods to screen and identify antigens related to specific diseases or conditions, tumors, autoimmune disorders, or an infectious disease or pathogen, and to identify target molecule for research purposes, or for developing a vaccine based thereon.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Hyperthermia and immunotherapy for leukemias lymphomas, and solid tumors
An improved method of inducing whole-body hyperthermia and enhanced anti-tumor immune response through inoculation of a fever virus with nil mortality and subsequent injection of irradiated tumor cells derived from the patient. This therapy will safely reduce the tumor burden by 90-99.9% by physical means (fever), before raising interferon levels to over 250 times baseline. The Activated Lymphokine Killer cells produced by these high interferon levels are capable of killing any cell expressing viral or tumor antigens, even those which had previously escaped immune surveillance. As a final step in the process, a specific class of Cytotoxic T Lymphocytes programmed to destroy the patient's own cancer cells will be produced by repeated inoculation of irradiated cancer cells harvested from the patient. Through a combination of three methods of therapy never previously integrated into a single regimen, it is logical to state that this therapy has a high probability of completely eradicating cancer cells from the patient. In addition, this therapy provides for life-long immunity to the reoccurrence of the disease.
Owner:RANDY K BROWN +1
Adjuvant combination formulations
InactiveUS7611721B1Improve abilitiesSsRNA viruses negative-senseAntibacterial agentsWhite blood cellVertebrate Animals
The use of 3-O-deacylated monophosphoryl lipid A or monophosphoryl lipid A and derivatives and analogs thereof, in combination with a cytokine or lymphokine such as granulocyte macrophage colony stimulating factor or interleukin-12 is useful as an adjuvant combination in an antigenic composition to enhance the immune response in a vertebrate host to a selected antigen.
Owner:ZOETIS SERVICE LLC
Enhanced nasal composition of active peptide
InactiveUS20090035260A1Prevent oxidationImprove stabilityPeptide/protein ingredientsMetabolism disorderNasal cavityGoserelin
A pharmaceutical composition has a therapeutically effective amount of at least one of: a pharmaceutically active nasal peptide, its pharmaceutically acceptable salt and its peptidic fragment. The composition also contains an absorbefacient effective amount of THAM in a pharmaceutically acceptable, aqueous liquid diluent or carrier. The composition is provided in a convenient form for nasal administration. In one embodiment, the peptidic fragment may be selected physiologically active lymphokines and monokines, peptidic enzymes, proteic vaccines, peptidic toxoids and personalized proteins derived from genoma. In another embodiment, the peptidic fragment may be selected from the peptide hormones and hormone antagonists buserelin, desmopressin, vasopressin, angiotensin, felypressin, octreotide, somatropin, thyrotropin (TSH), somatostatin, gosereline, thryptorelin and insulin selected from the group consisting of cow and pig, synthetic and recombinant.
Owner:THERAPICON SRL
Skin and hair care using extract from conditioned medium cultured by mesenchymal stem cells and other regenerative cells
InactiveUS20110177015A1Enable growthEnable maintenanceCosmetic preparationsHair removalHair streamsBiology
A method and composition for treating dermatological conditions and improving skin condition and helping hair to grow or thicken involves putting mesenchymal stem cells or other cells with regenerative properties into culture medium to induce secretion of beneficial factors such as growth factor, regulatory factors, enzymes, hormones, peptides and lymphokines into the culture medium to create conditioned medium and then extracting either a supernatant or a cell free extract of the conditioned medium to use itself or components thereof alone or with other skin or hair care reagents as a topical ointment or formula to apply to the skin or hair topically for therapeutic and cosmetic purposes.
Owner:FRIEDLANDER HYMIE
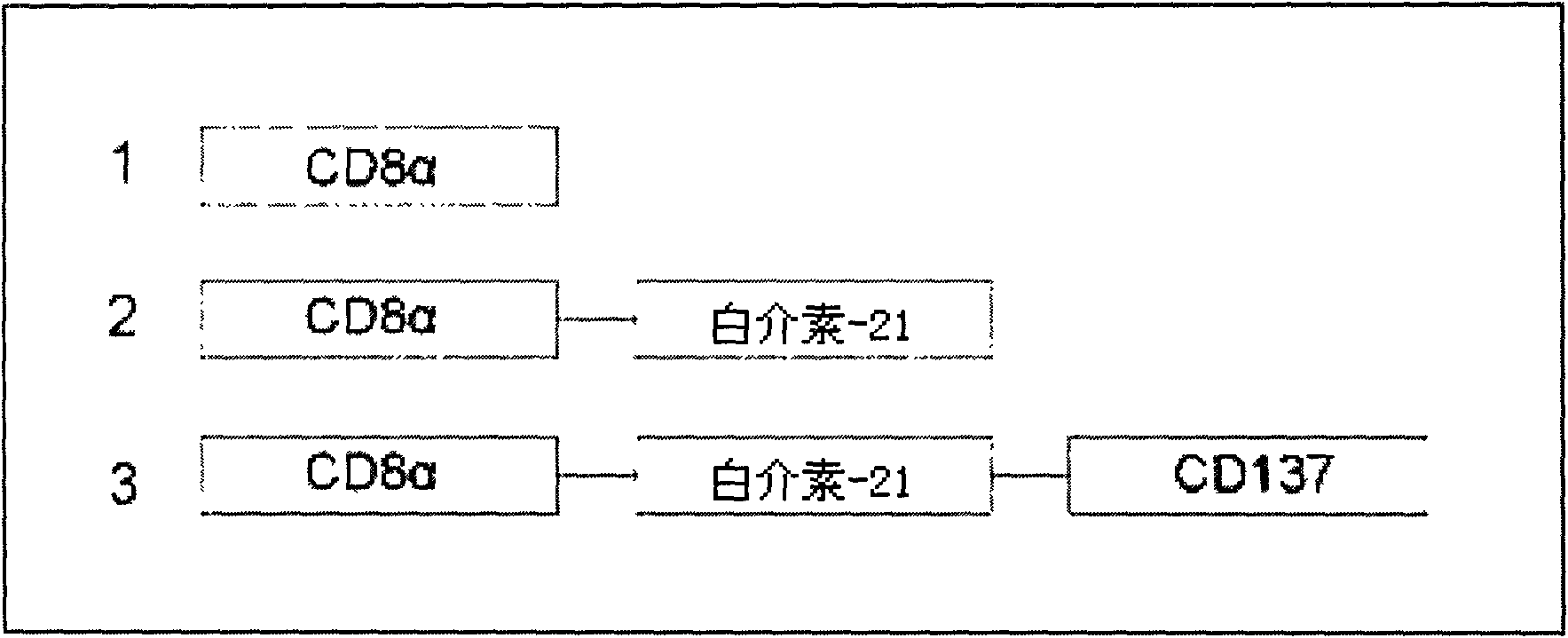
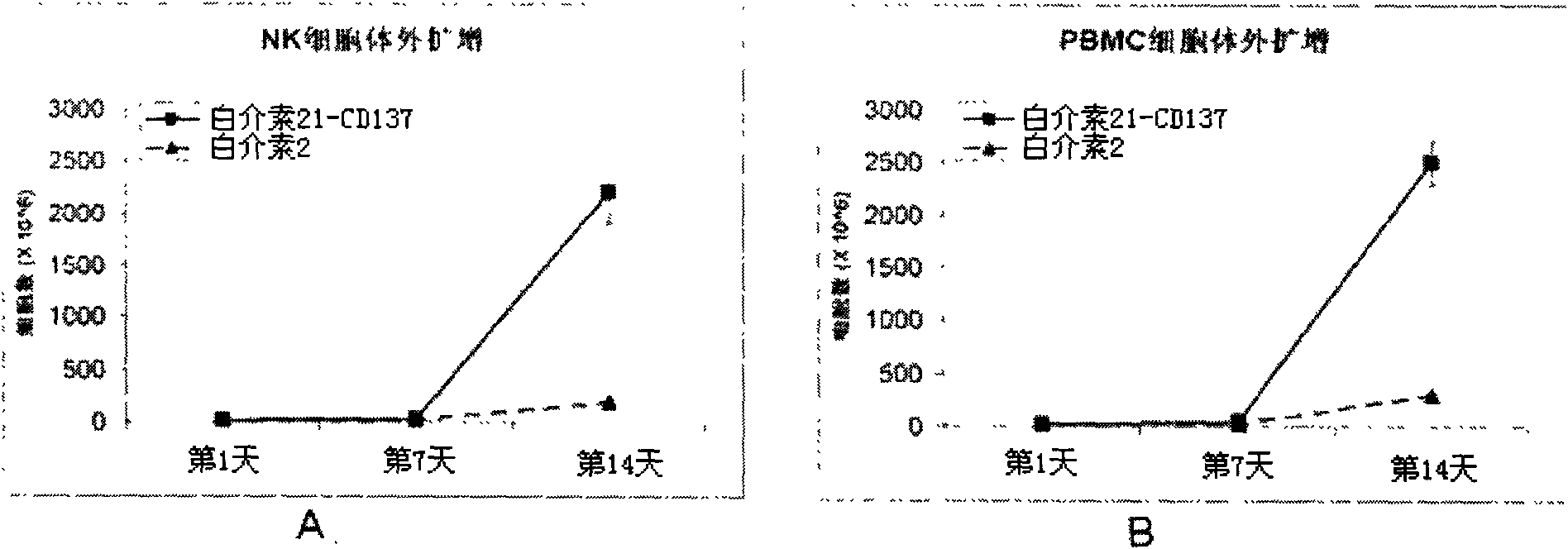
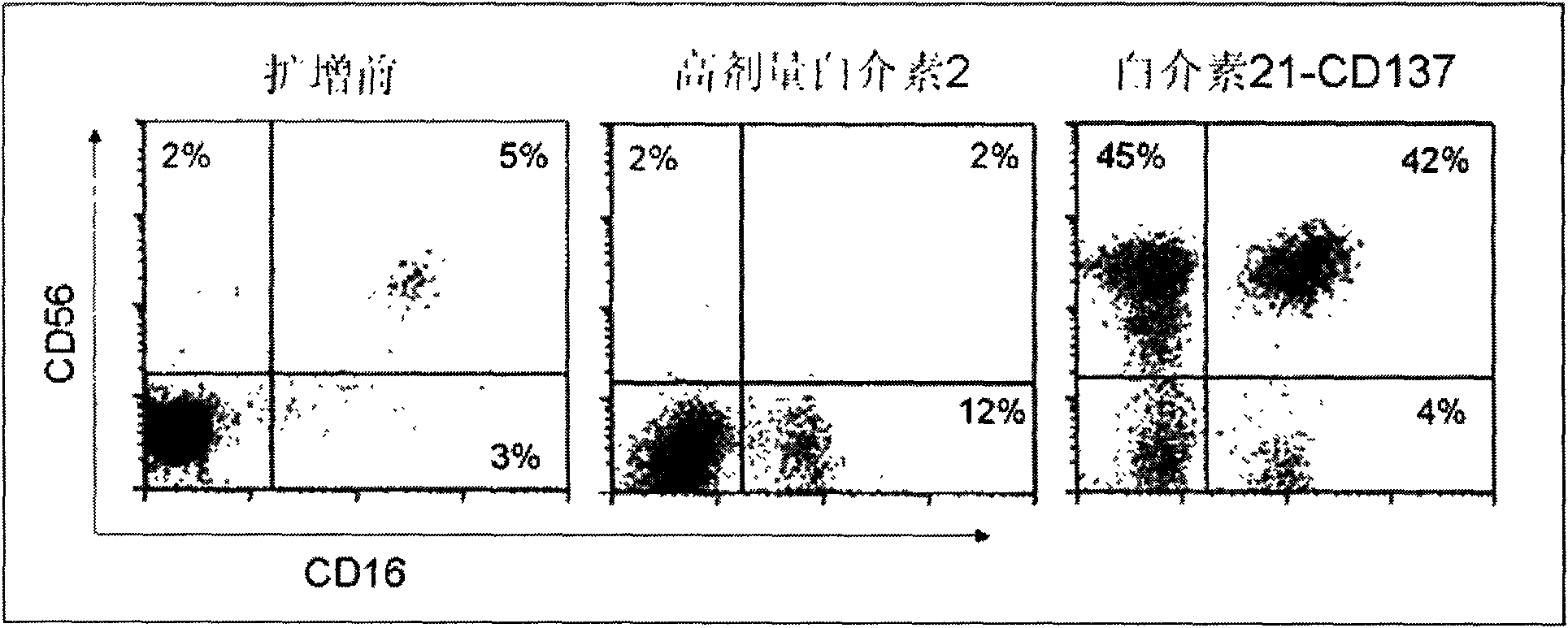
Method for amplifying and activating lymphocyte by using CD8 alpha-interleukin 21-CD137 compound
The invention relates to the field of immunology, in particular to a method for amplifying and activating a natural killer (NK) cell into a lymphokine-activated killer (LAK) cell by using a CD8 alpha-interleukin 21-CD137 compound. The method disclosed by the invention comprises the following steps of: forming the CD8 alpha-interleukin 21-CD137 compound by using CD8 alpha, interleukin 21 and a CD137 functional polypeptide, making an exogenous expression vector enter a host K562 cell, then activating a promoter and culturing a cell to obtain a cell for expressing a transmembrane interleukin 21-CD137 compound; and purifying the compound in the conventional way, and amplifying and activating a lymphocyte by using the purified compound to generate the LAK cell. The method has the advantage that: the LAK cell cultured and amplified by using the transmembrane interleukin 21-CD137 compound and a small dose of interleukin 2 is used for enhancing the immunity of a patient to help the patient resist tumors, viruses and bacteria. The method has a wide clinical using prospect.
Owner:杭州中赢生物医疗科技有限公司
In vitro test system for predicting patient tolerability of therapeutic agents
InactiveUS20060234205A1Simple and efficacious in vitroPredict tolerabilityPeptide/protein ingredientsMicrobiological testing/measurementTolerabilityIn vitro test
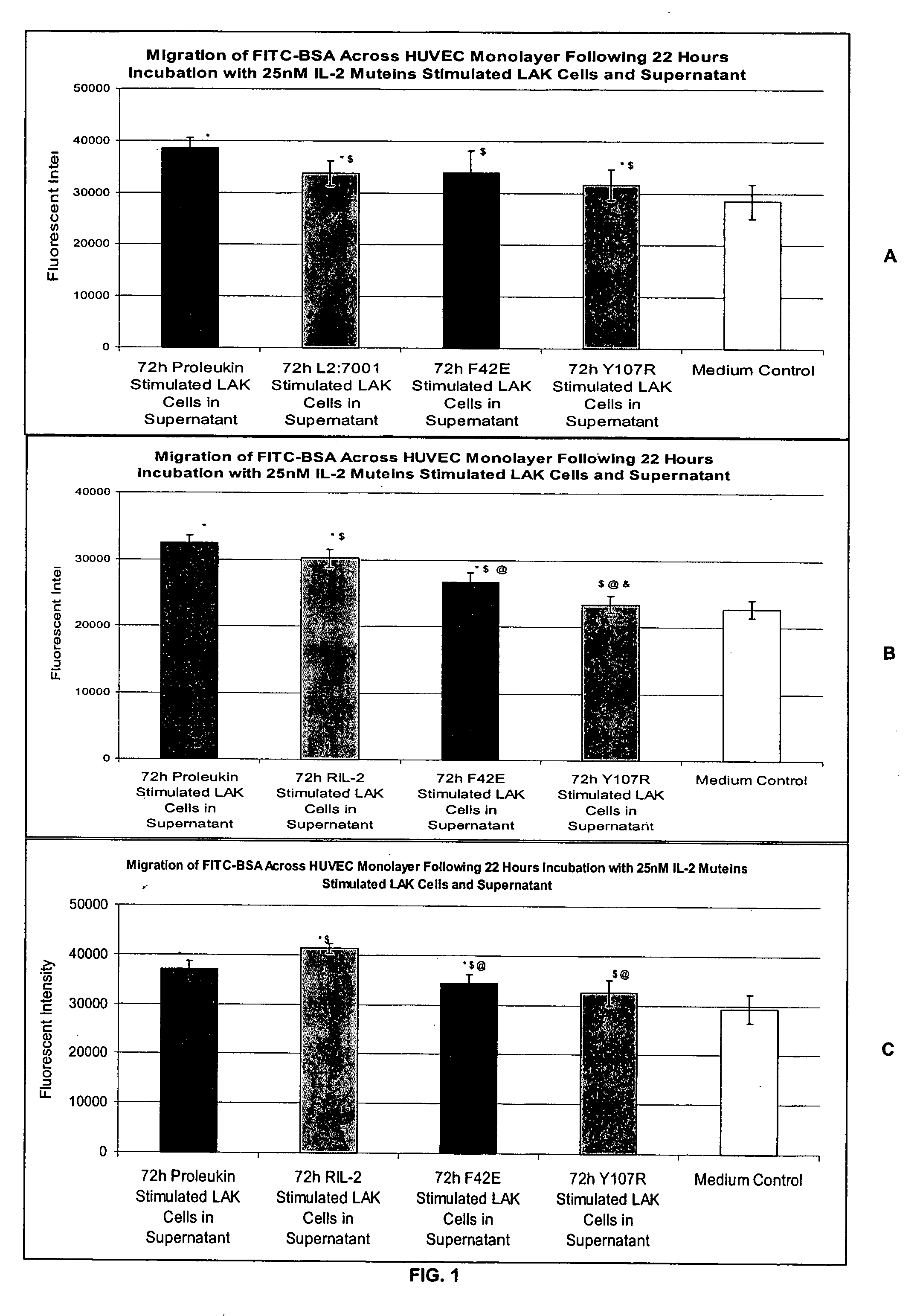
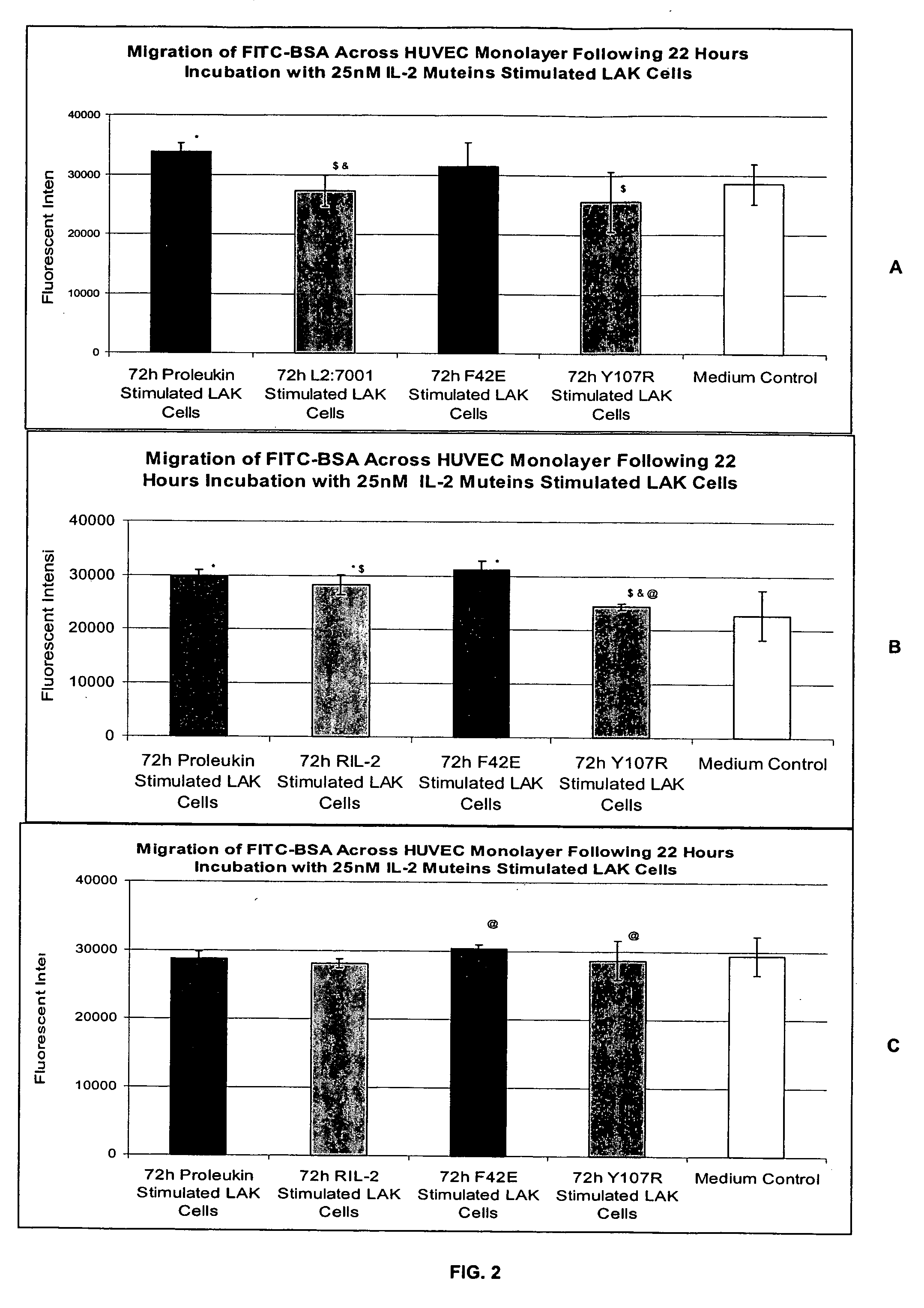
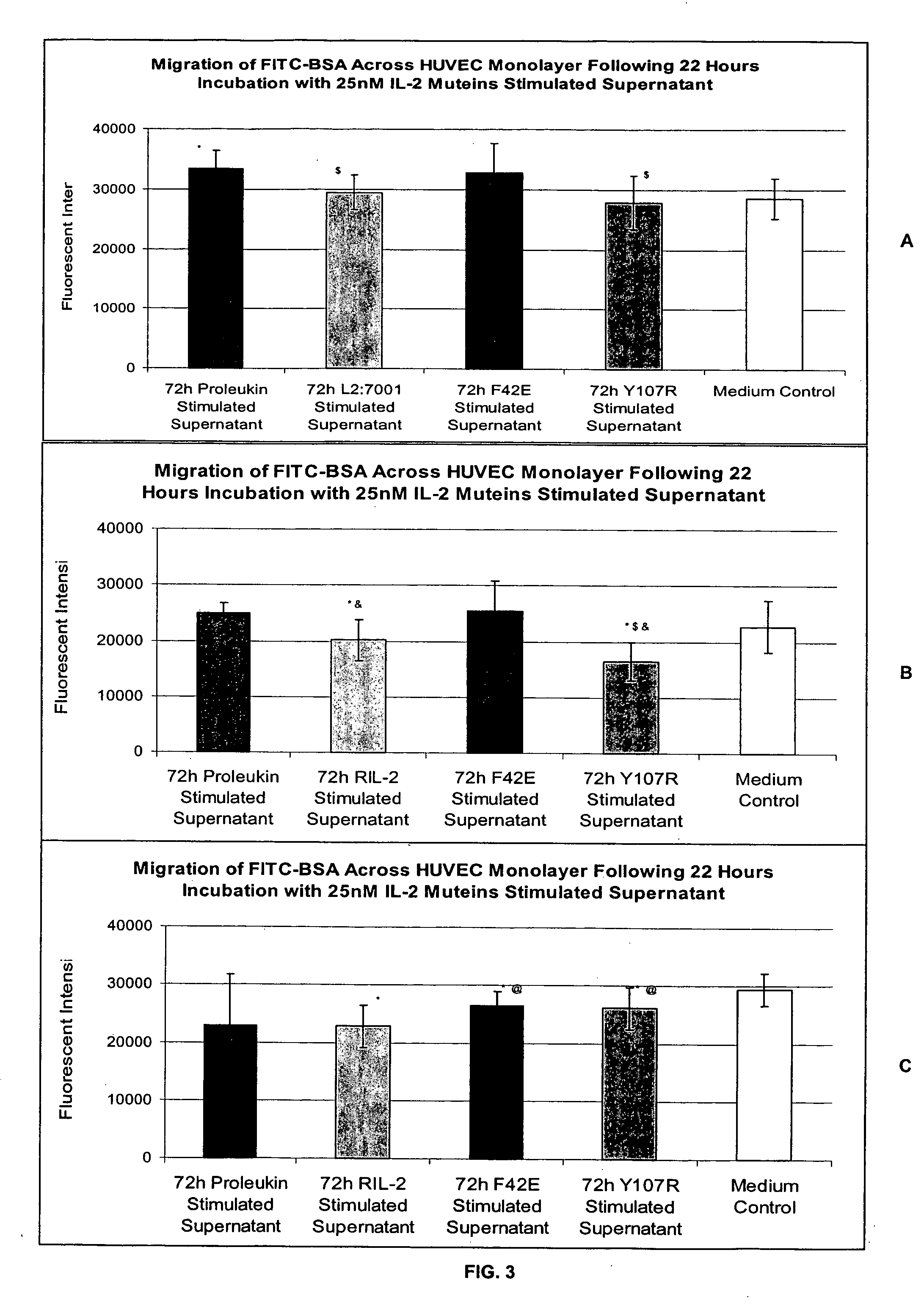
Methods for predicting patient tolerability to therapeutic agents, such as cytokines, lymphokines and immunotoxins, are disclosed. The methods utilize an in vitro model of vascular leak syndrome (VLS) to assess the effect of the agent in question on the permeability of large proteins across confluent monolayers of endothelial cells (EC).
Owner:NOVARTIS VACCINES & DIAGNOSTICS INC
Hyperthermia and immunotherapy for cancer
InactiveUS6048686AReduce tumor burdenWithout riskSsRNA viruses positive-senseViral antigen ingredientsAbnormal tissue growthDisease
An improved method of inducing whole-body hyperthermia and enhanced anti-tumor immune response through inoculation of a fever virus with nil mortality and subsequent injection of irradiated tumor cells derived from the patient. This therapy will safely reduce the tumor burden by 90-99.9% by physical means (fever), before raising interferon levels to over 250 times baseline. The Activated Lymphokine Killer cells produced by these high interferon levels are capable of killing any cell expressing viral or tumor antigens, even those which had previously escaped immune surveillance. As a final step in the process, a specific class of Cytotoxic T Lymphocytes programmed to destroy the patient's own cancer cells will be produced by repeated inoculation of irradiated cancer cells harvested from the patient. Through a combination of three methods of therapy never previously integrated into a single regimen, it is logical to state that this therapy has a high probability of completely eradicating cancer cells from the patient. In addition, this therapy provides for life-long immunity to the reoccurrence of the disease. It is understood that the examples and embodiments described herein are for illustrative purposes only and that various changes or modifications in light thereof will be suggested to persons skilled in the art and are to be included within the spirit and purview of this application and scope of the appended claims.
Owner:AUNER MARIA CARMEN VIVIAN +1
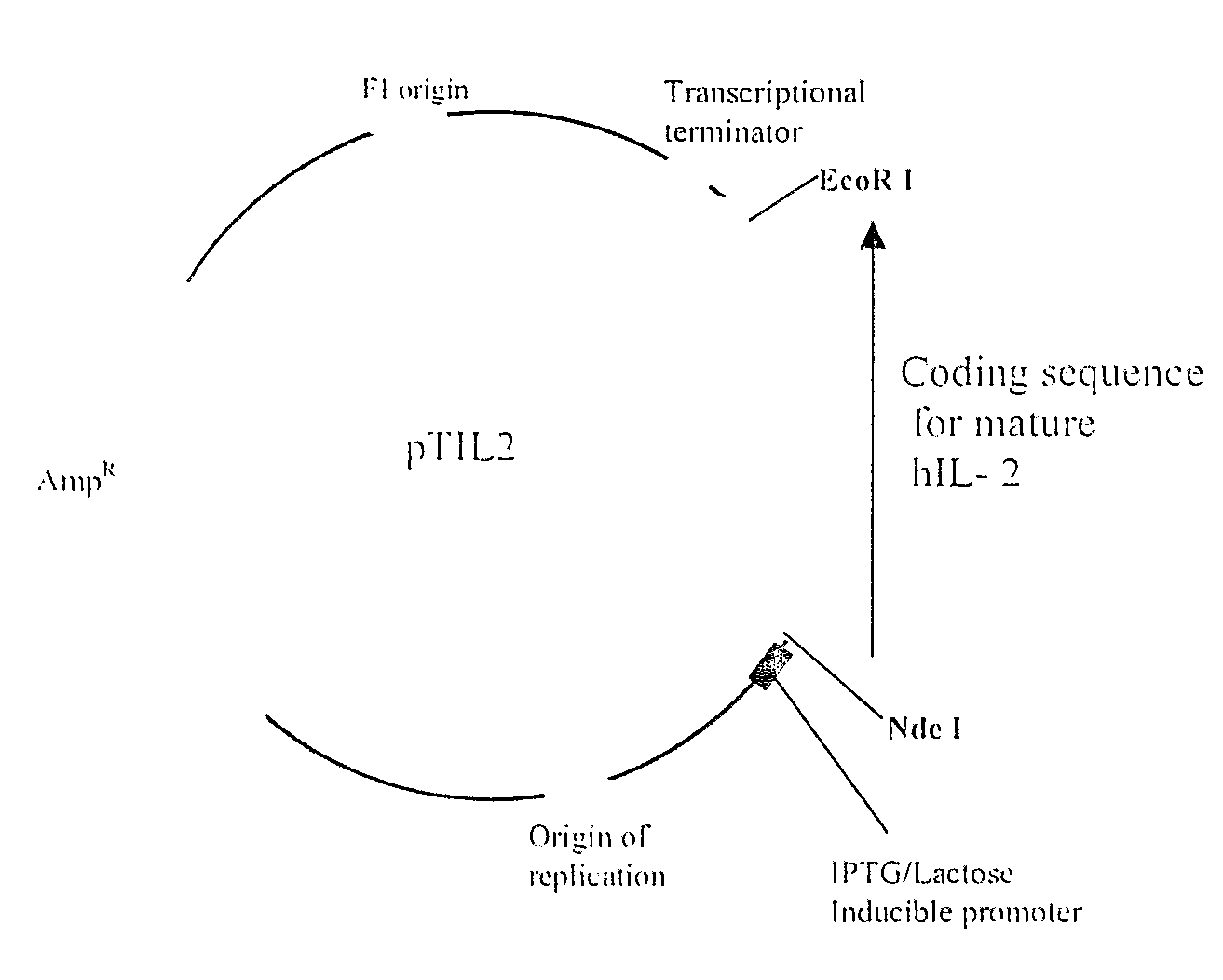
Method for Achieving High-Level Expression of Recombinant Human Interleukin-2 Upon Destabilization of the Rna Secondary Structure
InactiveUS20080268503A1Increase free energyMass productionFermentationVector-based foreign material introductionHigh level expressionFactor ii
The present invention provides a method for achieving high-level expression of the therapeutically important lymphokine (human IL-2). The method comprises of identifying the secondary structure in the 5′ region of human IL-2 mRNA, modifying the 5′ region of the human IL-2 DNA sequence to produce a new DNA sequence wherein the mRNA transcribed from the modified human IL-2 DNA sequence has the predicted 5′ secondary structure destabilized with increased free energy compared to that of the secondary structure of the mRNA transcribed from the native DNA sequence without altering the sequence of the encoded amino acids; and using this modified DNA sequence of human IL-2 for high level recombinant expression in a microbial host for large scale production. This method is also applicable to other expression host like yeasts and mammalian cells.
Owner:ZENOTECH LAB LTD
Novel therapeutic processes and useful compositions therefor
InactiveUS20050147586A1Bacterial antigen ingredientsPeptide/protein ingredientsAbnormal tissue growthCancer cell
This invention provides novel processes for therapeutic applications, including the treatment of subjects carrying infectious agents or having impaired autoimmunity or impaired immune condition. The therapeutic applications disclosed herein are also directed at the treatment of cancerous subjects with malignant tumors containing cancerous cells or malignant or cancerous cells. Vaccination processes for preventing infections in subjects are also provided. The novel processes comprise introducing into or adminstering to a subject one or more antigens, or trained or adopted immune cells. These antigens or immune cells are capable of establishing or increasing at least one first specific immune response and decreasing at least one second specific immune response. Such responses include components, such as cellular immune reaction elements, humoral immune reaction elements and cytokines, the latter also encompassing interferons and lymphokines. Useful compositions are also provided by this invention.
Owner:ENZO THERAPEUTICS ENZO BIOCHEM
Novel therapeutic processes for establishing or enhancing an immunized state, and compositions useful therefor
InactiveUS20050118196A1Bacterial antigen ingredientsPeptide/protein ingredientsAbnormal tissue growthCancer cell
This invention provides novel processes for therapeutic applications, including the treatment of subjects carrying infectious agents or having impaired autoimmunity or impaired immune condition. The therapeutic applications disclosed herein are also directed at the treatment of cancerous subjects with malignant tumors containing cancerous cells or malignant or cancerous cells. Vaccination processes for preventing infections in subjects are also provided. The novel processes comprise introducing into or adminstering to a subject one or more antigens, or trained or adopted immune cells. These antigens or immune cells are capable of establishing or increasing at least one first specific immune response and decreasing at least one second specific immune response. Such responses include components, such as cellular immune reaction elements, humoral immune reaction elements and cytokines, the latter also encompassing interferons and lymphokines. Useful compositions are also provided by this invention.
Owner:ENZO THERAPEUTICS ENZO BIOCHEM
Novel therapeutic vaccination processes, and useful compositions therefor
InactiveUS20050136074A1Peptide/protein ingredientsViral antigen ingredientsAbnormal tissue growthCancer cell
This invention provides novel processes for therapeutic applications, including the treatment of subjects carrying infectious agents or having impaired autoimmunity or impaired immune condition. The therapeutic applications disclosed herein are also directed at the treatment of cancerous subjects with malignant tumors containing cancerous cells or malignant or cancerous cells. Vaccination processes for preventing infections in subjects are also provided. The novel processes comprise introducing into or adminstering to a subject one or more antigens, or trained or adopted immune cells. These antigens or immune cells are capable of establishing or increasing at least one first specific immune response and decreasing at least one second specific immune response. Such responses include components, such as cellular immune reaction elements, humoral immune reaction elements and cytokines, the latter also encompassing interferons and lymphokines. Useful compositions are also provided by this invention.
Owner:ENZO THERAPEUTICS ENZO BIOCHEM
Single Branch Heparin-Binding Growth Factor Analogs
Owner:BIOSURFACE ENG TECH
Method of preparing adenosine-resistant Anti-tumor t lymphocytes for adoptive immunotherapy
InactiveUS20110300183A1Mammal material medical ingredientsBlood/immune system cellsAbnormal tissue growthDisease
In the past, adoptive immunotherapy often failed because the transferred immune cells were inactive in vivo. This disclosure provides a method of producing immune cells that are highly active in vivo. The immune cells may be expanded in vitro in the presence of an adenosine receptor agonist or an antisense nucleic acid that downregulates expression of an adenosine receptor, for example. The immune cells may be tumor-infiltrating lymphocytes (TIL), cytotoxic T lymphocytes (CTL), natural killer (NK) cells, or lymphokine-activated killer (LAK) cells, for example. The methods described herein may be used to treat a number of diseases including cancer, infectious diseases, and immunodeficiencies.
Owner:NORTHEASTERN UNIV
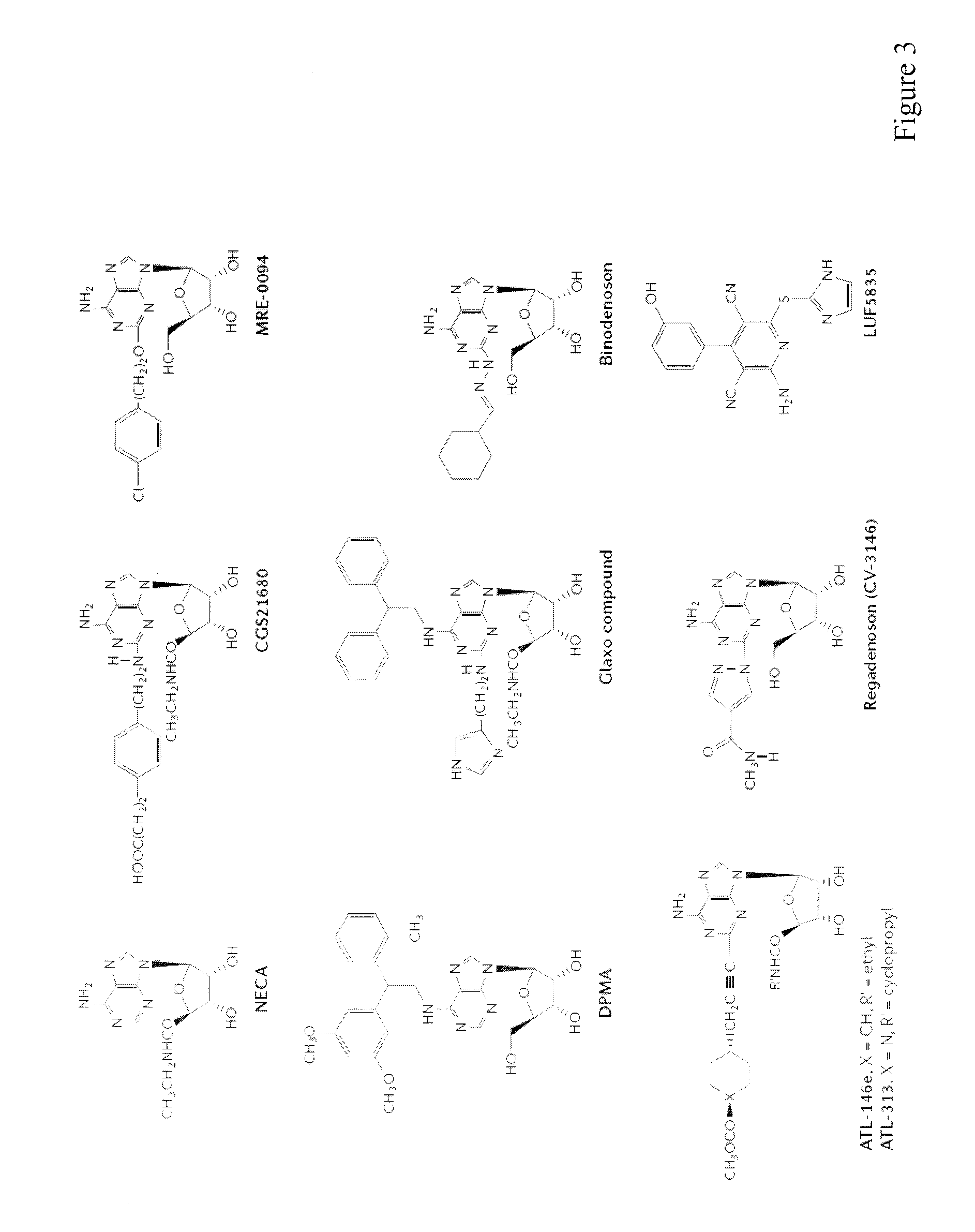
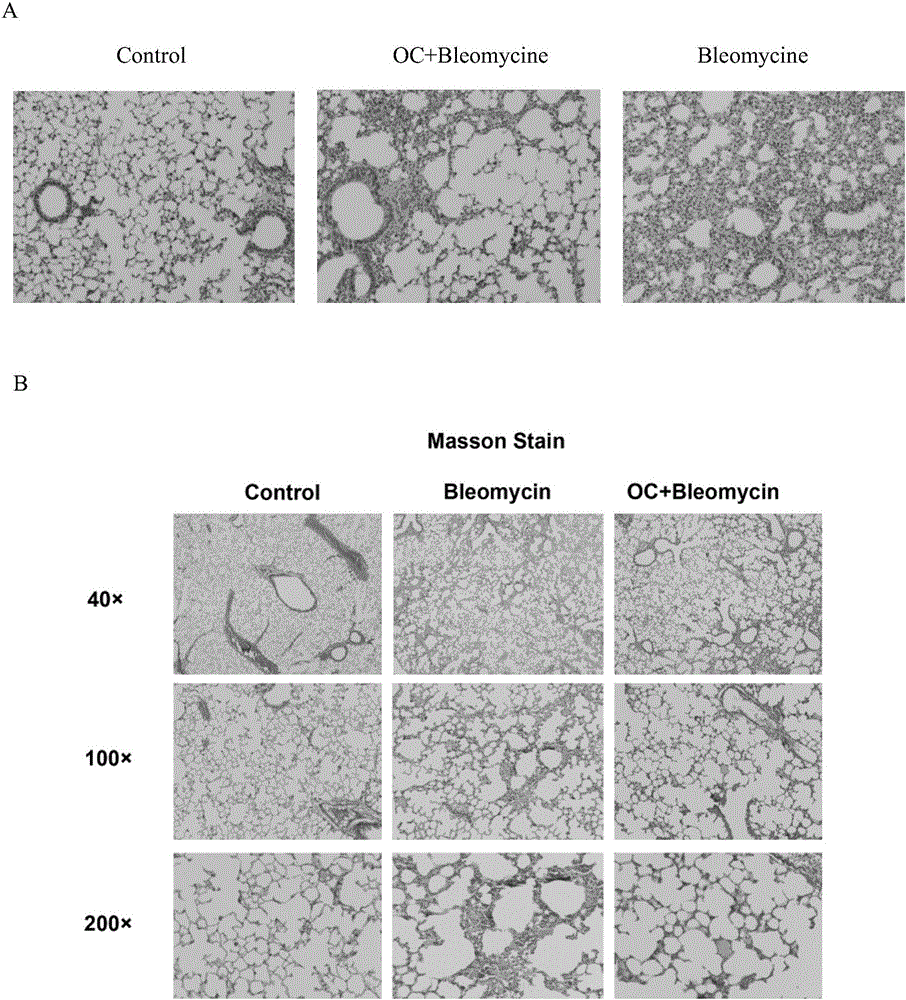
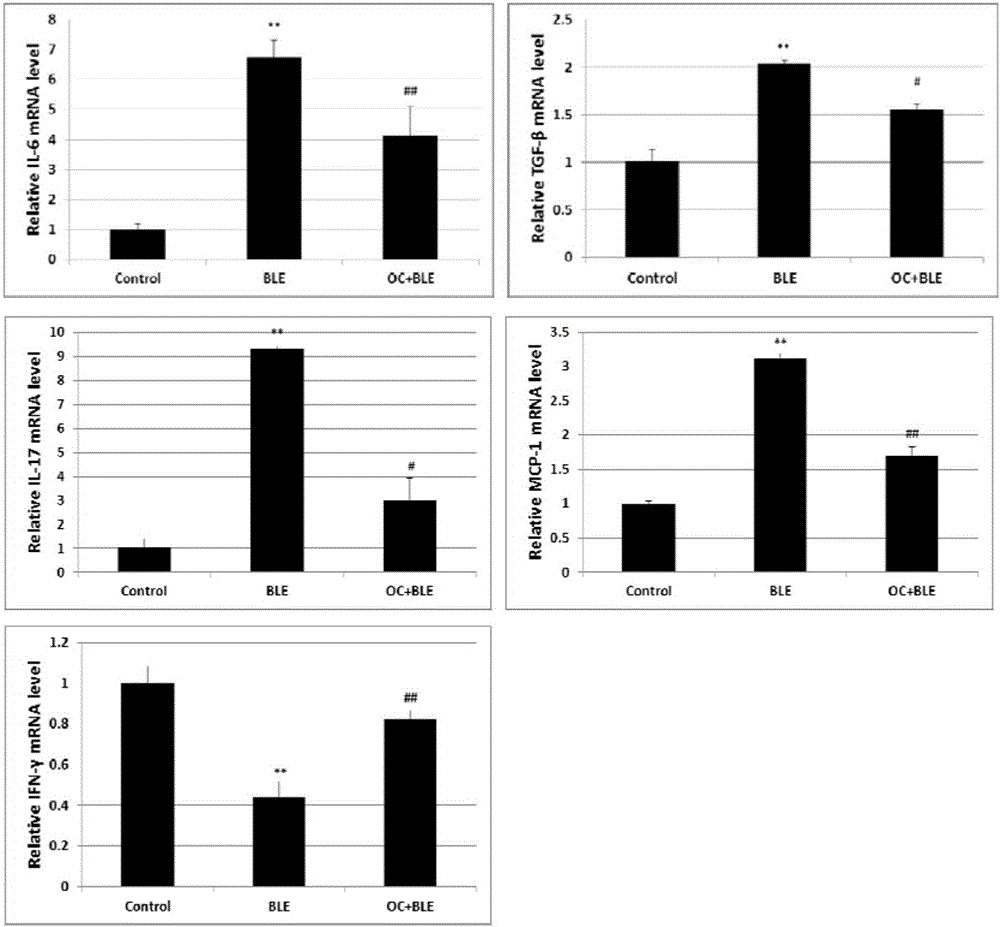
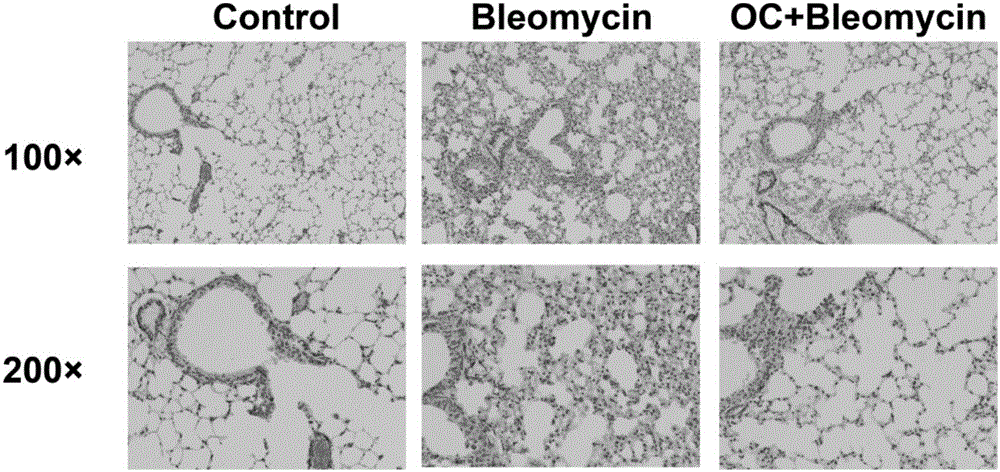
Application of obakunone to preparation of medicine for preventing and treating lung injury and pulmonary fibrosis
ActiveCN105769880ANo damageLow toxicityOrganic active ingredientsPill deliveryKidney injuryPulmonary fibrosis
The invention relates to application of obakunone to preparation of a medicine for preventing and treating lung injury and pulmonary fibrosis. The medicine consists of obakunone and pharmaceutically acceptable auxiliaries and additives, wherein the obakunone accounts for 3-25% by mass of the medicine. The obakunone in the medicine can inhibit mouse lung injury and pulmonary fibrosis induced by Bleomycin and regulate lymphokine expression related to inflammation of mouse lung injury induced by Bleomycin, and not only is remarkable in effects of preventing and treating lung injury and pulmonary fibrosis, but also has the advantages of low toxicity and no liver or kidney injury.
Owner:GUANGDONG HOSPITAL OF TRADITIONAL CHINESE MEDICINE
Anti-tumor chimeric protein, anti-tumor vaccine and anti-tumor vaccine adopting nasal mucosa drug administration mode
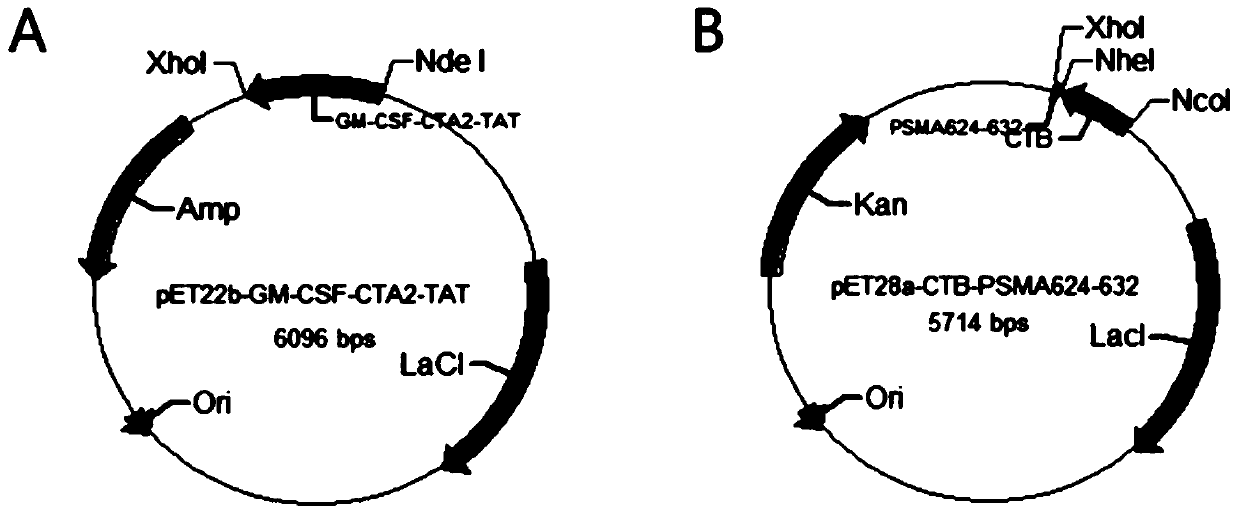
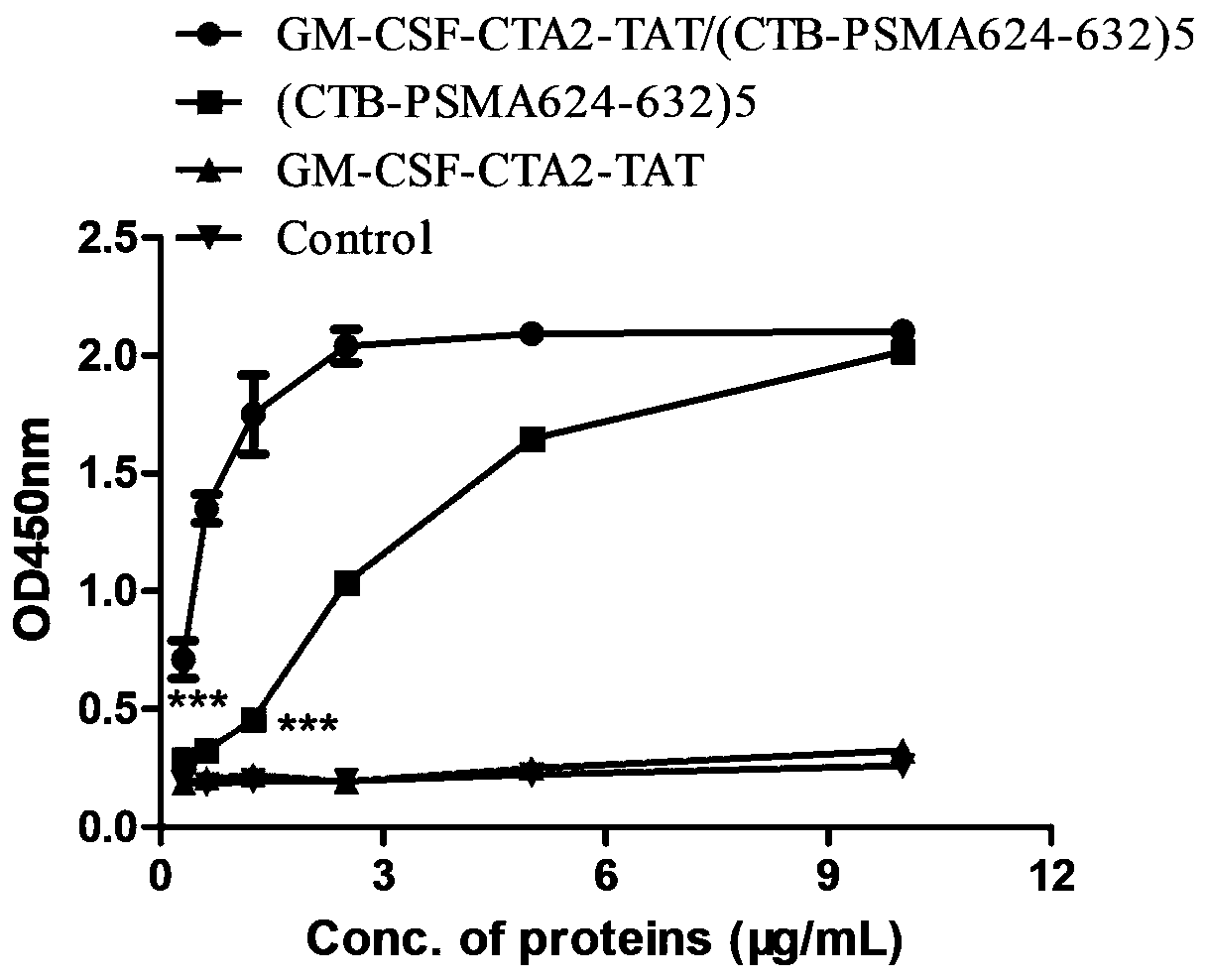
InactiveCN110452302ARapid responseEnhance immune responsePolypeptide with localisation/targeting motifTumor rejection antigen precursorsTumor-specific antigenDrug administration
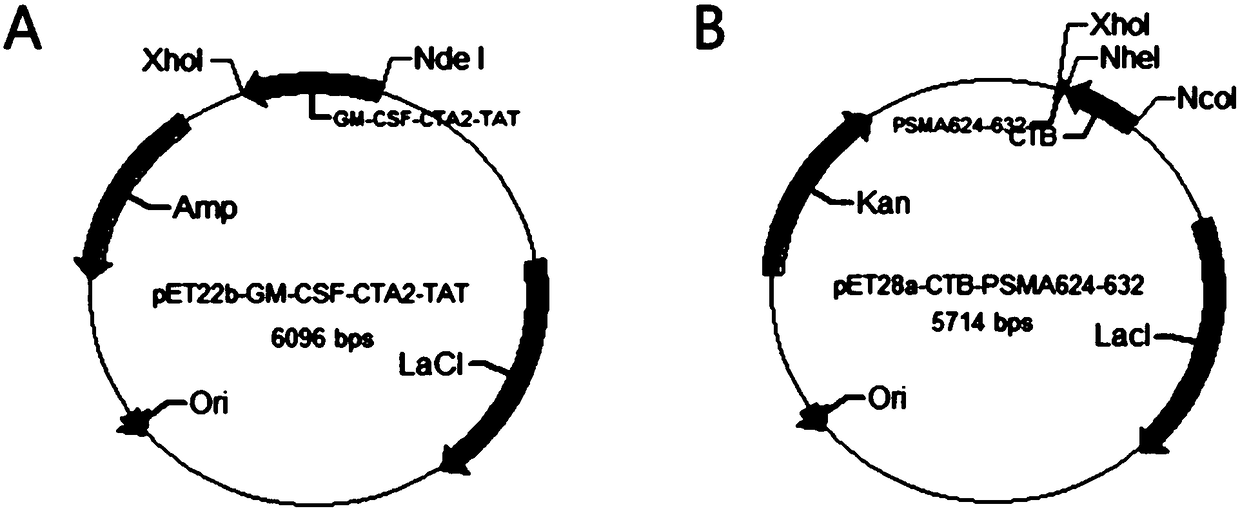
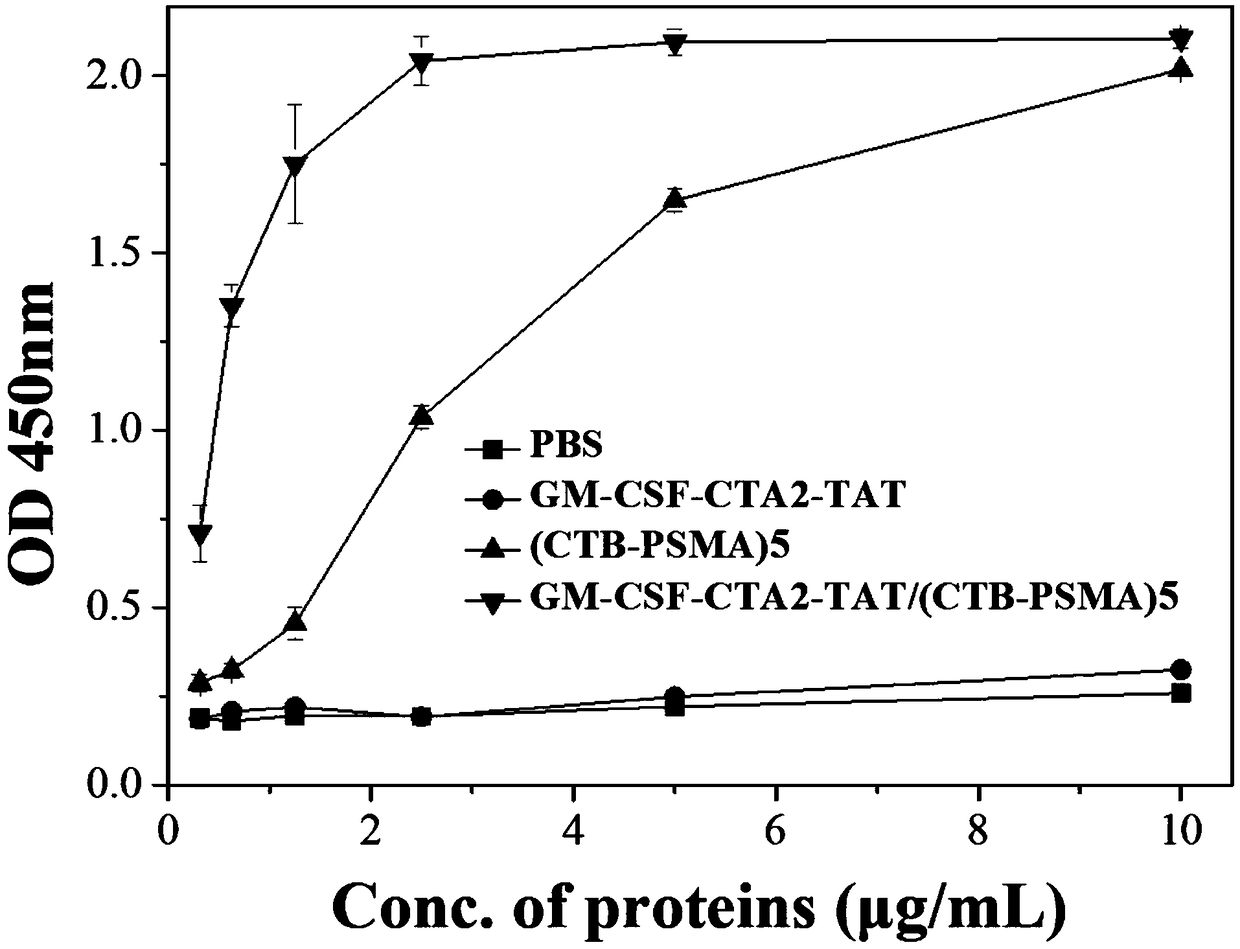
The invention belongs to the technical field of biological medicine, and particularly relates to an anti-tumor chimeric protein, an anti-tumor vaccine and an anti-tumor vaccine adopting a nasal mucosadrug administration mode. The provided anti-tumor chimeric protein comprises first polypeptide comprising a lymphokine sequence used for enhancing an immunoreaction and a CTA2 subunit sequence of a cholera toxin, and second polypeptide comprising a CTB subunit sequence of the cholera toxin and a tumor specific antigen epitope peptide sequence; a CTA2 subunit of the cholera toxin of the first polypeptide and a CTB subunit of the cholera toxin of the second polypeptide are subjected to mutual chimerism to obtain the anti-tumor chimeric protein. The provided anti-tumor chimeric protein, anti-tumor vaccine and anti-tumor vaccine adopting the nasal mucosa drug administration mode are used for overcoming the technical defect that in the prior art, an existing tumor vaccine is poor in immunogenicity.
Owner:GUANGDONG UNIV OF TECH
Materials and methods for detection and quantitation of an analyte
The subject invention pertains to methods and materials for accurately assessing the presence or absence of analytes of interest in samples, particularly in physiological samples. The subject invention involves utilizing a ligand binding domain (LBD) of a receptor to selectively capture the analyte target specific for that LBD. In one embodiment, the receptor is a protein or polypeptide. The ligand binding domain is allowed to react with a sample and the presence or amount of ligand (i.e., target analyte) bound by the LBD is determined. Suitable analytes include soluble analytes such as hormones, enzymes, lipoproteins, bacterial or viral antigens, immunoglobulines, lymphokines, cytokines, drugs, soluble cancer antigens, and the like. The methods of the present invention can be performed in both liquid-phase and solid-phase.
Owner:EQUITECH LAB
Pharmaceutical form for oral administration of a highly controlled and stable dose of nanoparticles or biomacromolecule suspensions
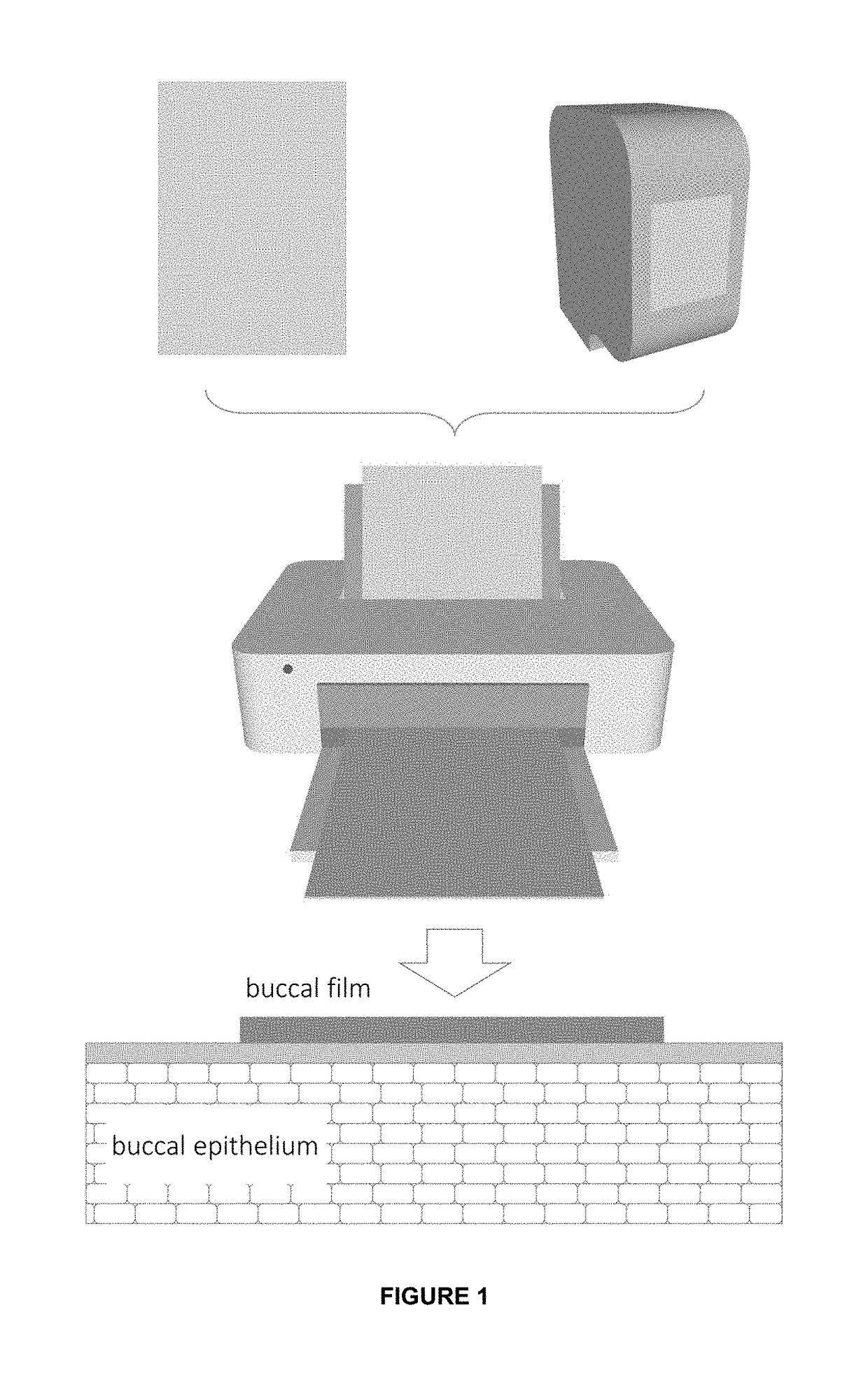
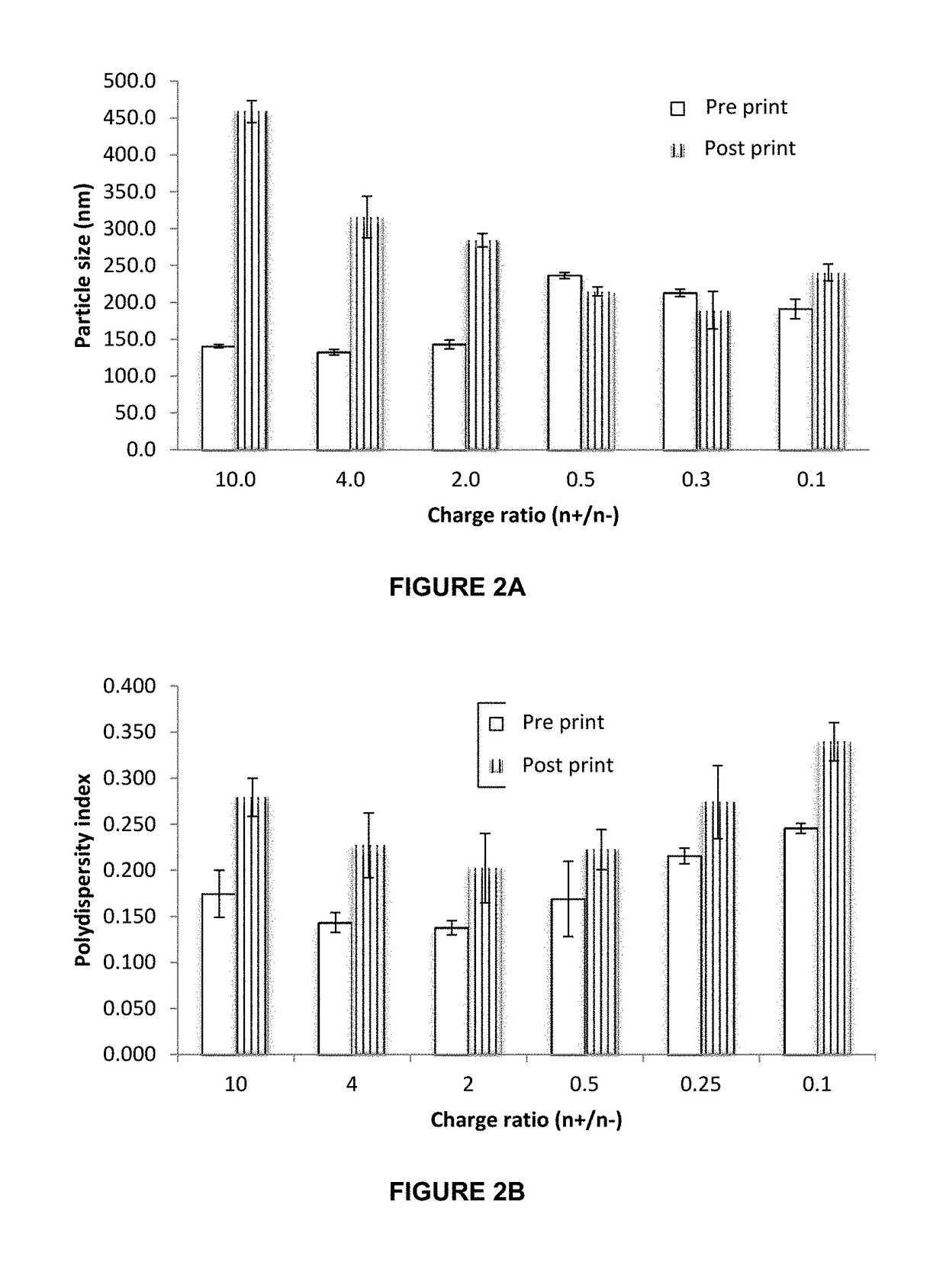
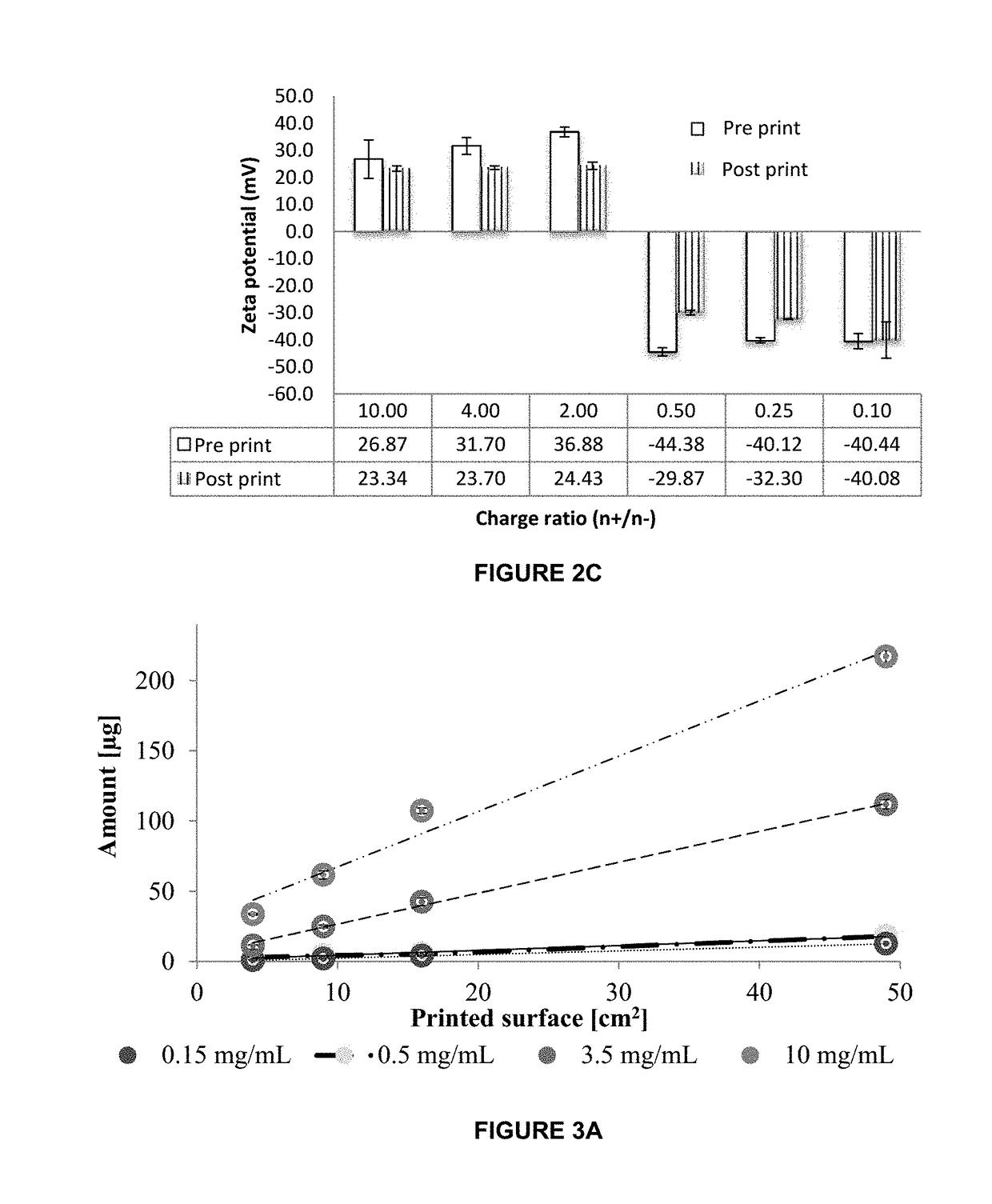
InactiveUS20190015320A1Suitable for printingImprove suppression propertiesAdditive manufacturing apparatusPeptide/protein ingredientsPolymer thin filmsBiological macromolecule
The invention relates to the pharmaceutical industry, particularly to the pharmaceutical industry related to drugs comprising biomacromolecules, or biopharmaceuticals. Even more particularly, the invention relates to a pharmaceutical form comprising biomacromolecules such as lymphokines, hormones, haematopoietic factors, growth factors, antibodies, enzymes, inhibitors, vaccines, and DNA or RNA derivatives. The invention provides a pharmaceutical form comprising biomacromolecules and a method for producing same based on inkjet printing using inks formed by nanosystems comprising the biomacromolecule(s), the drug or biopharmaceutical being administered orally. Even more particularly, the invention relates to a pharmaceutical form for oral administration of a highly controlled, stable controlled release dose of a biomacromolecule comprising: a) a polymer film as a printing substrate, formed by at least one pharmaceutically acceptable excipient; and b) an inkjet ink printed on the polymer film and comprising nanoparticles or nanoparticle suspensions comprising the biomacromolecule and at least one pharmaceutically acceptable excipient.
Owner:UNIVERSITY OF CHILE
Antibacterial adhesive remover for marine organisms
InactiveCN103805375AThe degumming effect is thorough and efficientEfficient sterilizationBiocideDead plant preservationCelluloseBiotin
The invention relates to the technical field of organisms, and particularly relates to an antibacterial adhesive remover for marine organisms applicable to removal of organic adhesive residues such as a sticking adhesive, chewing gum, sealing glue, kinds of oil paints and the like left on the surfaces of kinds of objects in a family or a public place. The formula comprises the following components by weight percent (%): 5.0-6.0% of seaweed lymphokine, 1.0-1.5% of agaroidin gelatum sugar, 7.0-9.5% of dicaproate, 2.0-4.0% of acetone, 2.0-4.0% of mineral oil, 0.5-2.0% of hydroxy propyl cellulose, 2.0-4.0% of sea mud saponin and purified water added to 100%. Pure natural active ingredients of the marine organisms, and nontoxic and nonirritant green marine biotin products which are free of corrosion and fragrant in flavor are adopted, so that the antibacterial adhesive remover has the characteristics of being high in efficiency, low in concentration, good in stability, good in degumming sterilization effect, free of poison and harm, convenient to carry and the like, and has broad applicability.
Owner:QINGDAO ANBEIKANG BIOLOGICAL MEDICINE TECH CO LTD
Purification and characterization of cytotoxic lymphocyte maturation factor and monoclonal antibodies thereto
InactiveUS8110187B2Peptide/protein ingredientsImmunoglobulins against cytokines/lymphokines/interferonsCytotoxic Lymphocyte Maturation FactorT cell
The present invention is a novel cytokine protein called IL-12 or Cytotoxic Lymphocyte Maturation Factor (CLMF) which is produced and synthesized by human NC-37 B lymphoblastoid cells (American Type Culture Collection, Rockville, Md.). CLMF synergistically induces with low concentrations of IL-2 the cytolytic activity of Lymphokine Activated Killer (LAK) cells, and CLMF is capable of stimulating T-cell growth.Also claimed are the cloned gene for CLMF, its recombination in a suitable vector, the transformed cells containing said vector, the recombinant protein produced by the transformed cells and antibodies to CLMF.
Owner:F HOFFMANN LA ROCHE & CO AG
Anti-tumor chimeric protein, anti-tumor vaccine, and anti-tumor vaccine for nasal mucosa administration
InactiveCN109206525APresentation EnhancementImprove immunityPolypeptide with localisation/targeting motifTumor rejection antigen precursorsChrysaora toxinLymphokine
The invention particularly relates to an antitumor chimeric protein, an antitumor vaccine and an antitumor vaccine for nasal mucosa administration, belonging to the technical field of biomedicine. Theanti-tumor chimeric protein provided by the invention comprises: a first polypeptide, which comprises a lymphokine sequence for enhancing an immunoreaction and a CTA2 subunit sequence of cholera toxin; and a second polypeptide, which comprises a CTB subunit sequence of the cholera toxin and a tumor-specific epitope peptide sequence, wherein a CTA2 subunit of the cholera toxin in the first polypeptide and a CTB subunit of the cholera toxin in the second polypeptide are mutually chimeric to form the anti-tumor chimeric protein. The anti-tumor chimeric protein, the anti-tumor vaccine and the anti-tumor vaccine for nasal mucosa administration provided by the invention are used for solving the technical defect of poor immunogenicity of tumor vaccines in the prior art.
Owner:GUANGDONG UNIV OF TECH
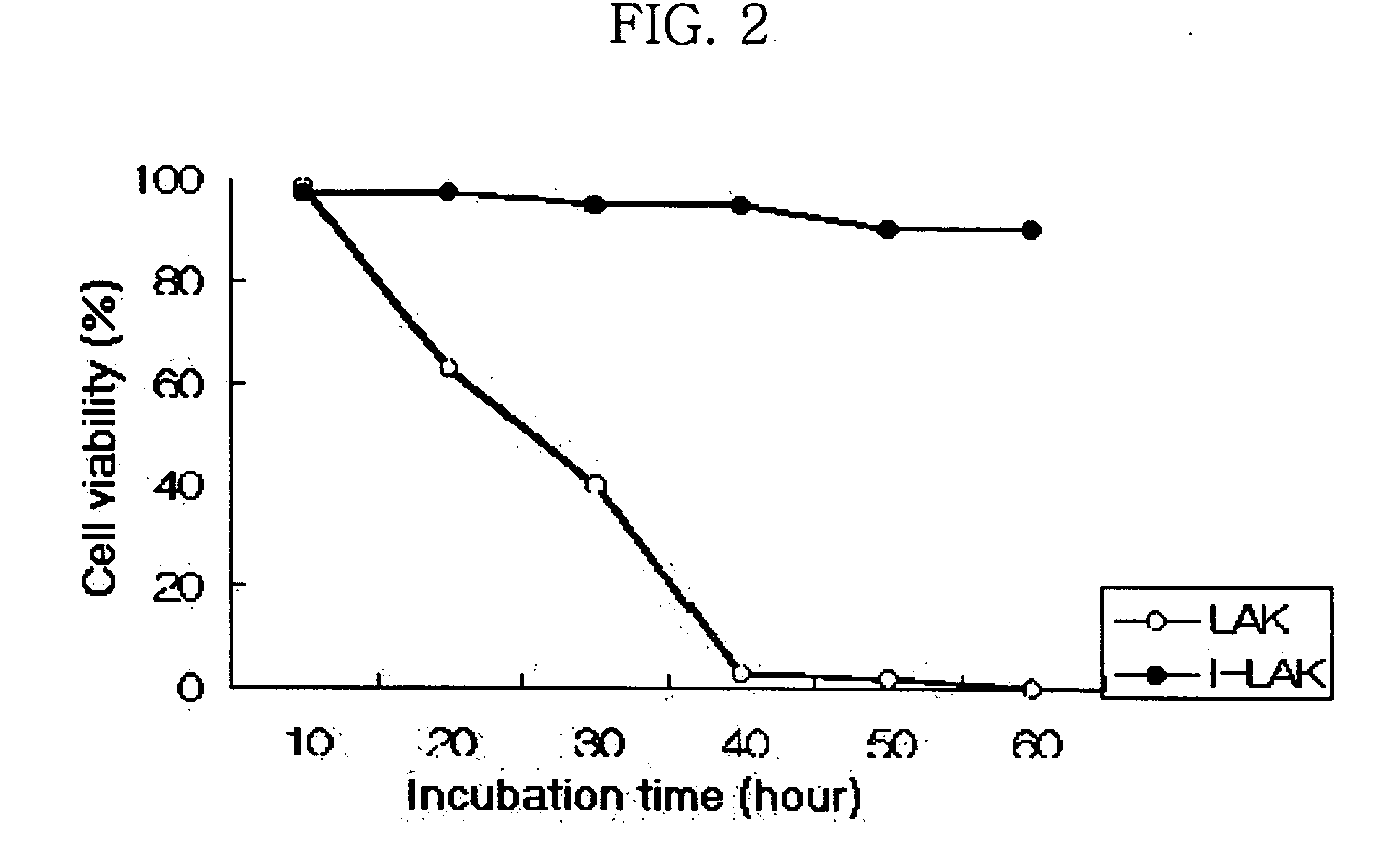
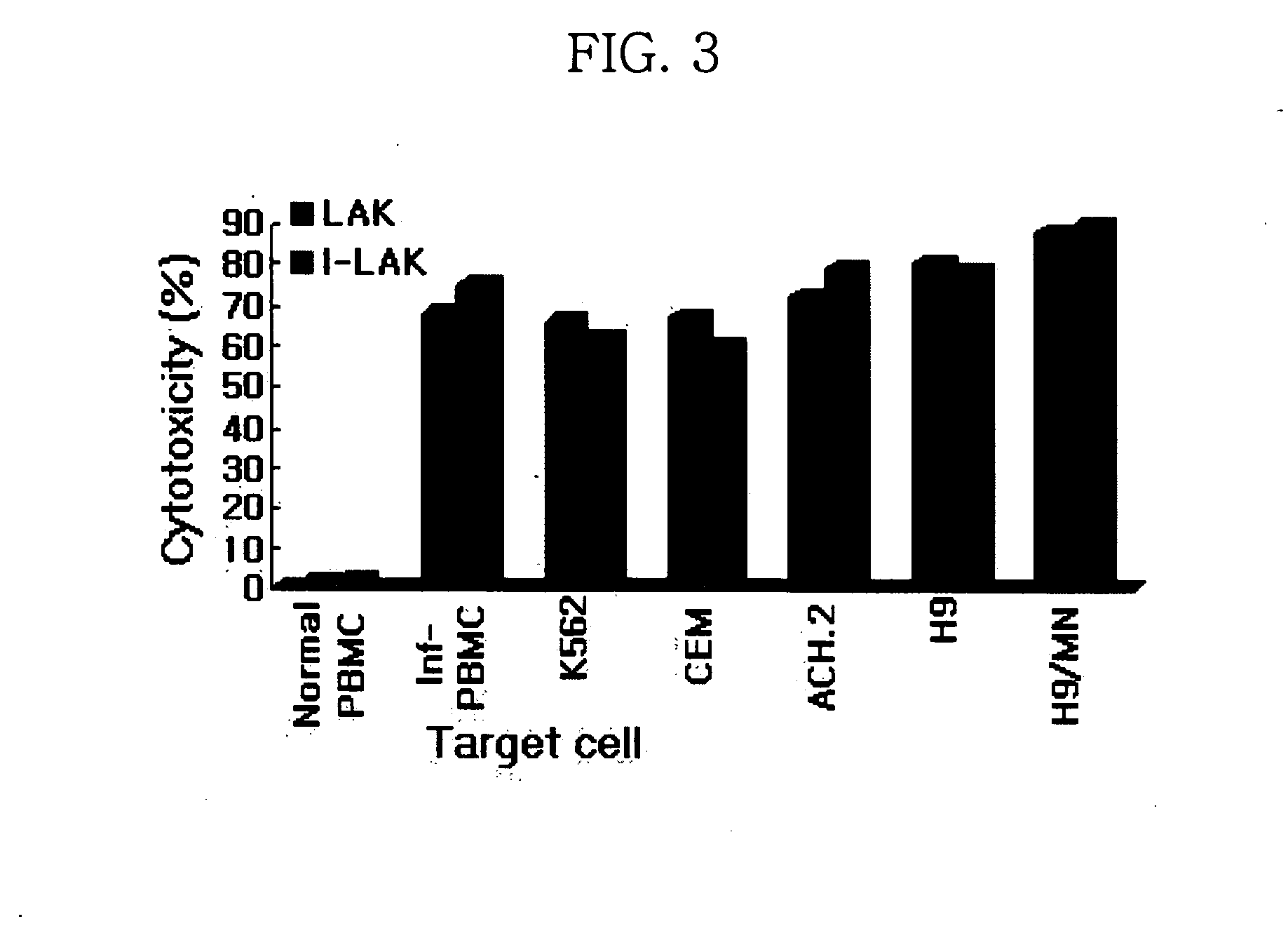
Interleukin-2 gene transferred lymphokine activated killer cells
The present invention relates to a transformed lymphokine activated killer cell (I-LAK cell) useful in the treatment of cancer or virus infection into which a recombinant vector carrying IL-2 gene to which it is operably linked is introduced. Also it relates to a pharmaceutical composition for treating cancer or virus infection disease comprising a sufficient amount of I-LAK cell to elicit an immune response.
Owner:MEDIGENES CO LTD
Cultural method for enhancing killing activity and proliferative activity of LAK (lymphokine-activated killer) cells and application
ActiveCN105002140AHigh activityEnhance killing activityBlood/immune system cellsDendritic cellMicrobiology
The invention relates to a cultural method for enhancing killing activity and proliferative activity of LAK (lymphokine-activated killer) cells and an application. The cultural method for enhancing the killing activity and proliferative activity of the LAK cells is co-culturing the LAK cells and DC (dendritic cells). The application of the DC cells is to enhance the killing activity and proliferative activity of the LAK cells. According to the cultural method, through co-culturing the LAK cells and the DC cells, the proliferation of the LAK cells is promoted, and the killing activity of the LAK cells is enhanced.
Owner:GUANGZHOU SALIAI STEMCELL SCI & TECH CO LTD
HIV vaccine
InactiveUS20090175889A1Well formedImprove stabilityOrganic active ingredientsGenetic material ingredientsInfected cellNucleotide
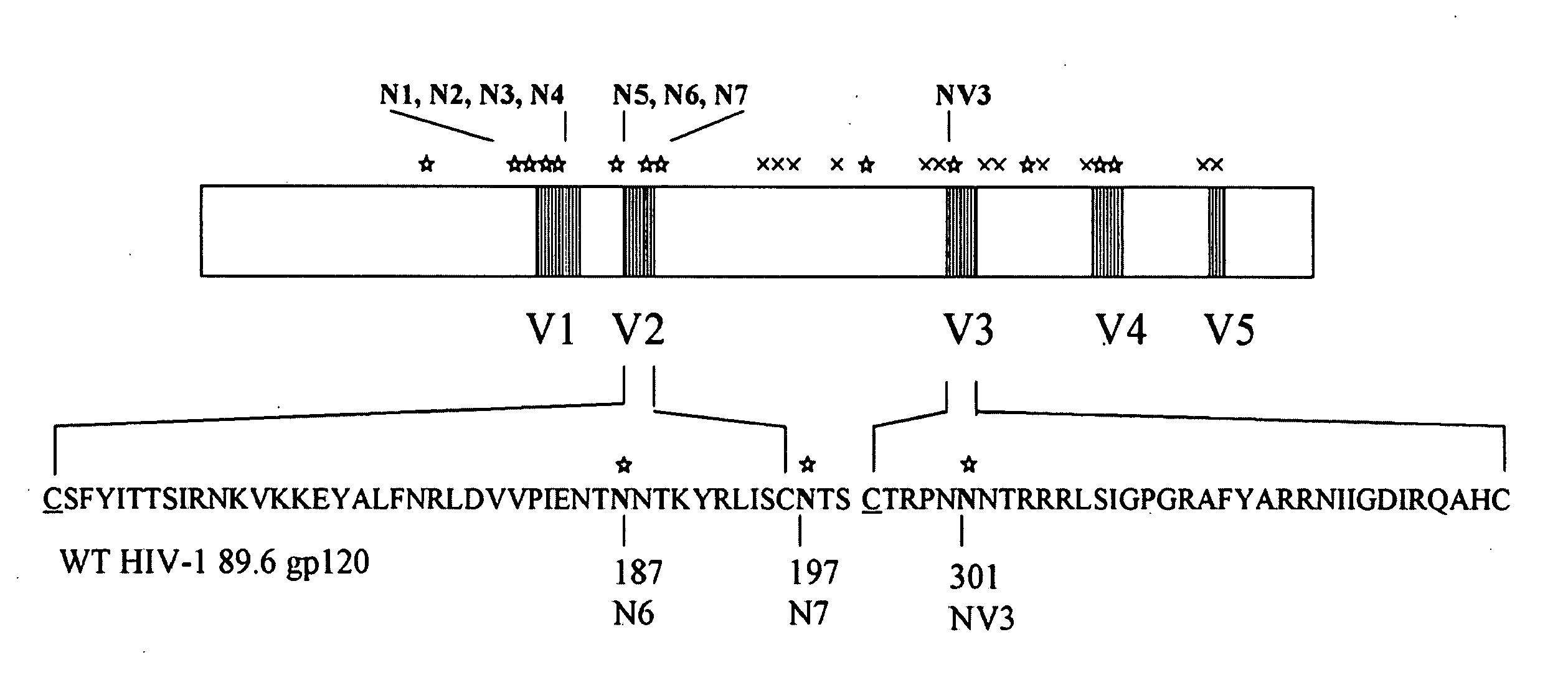
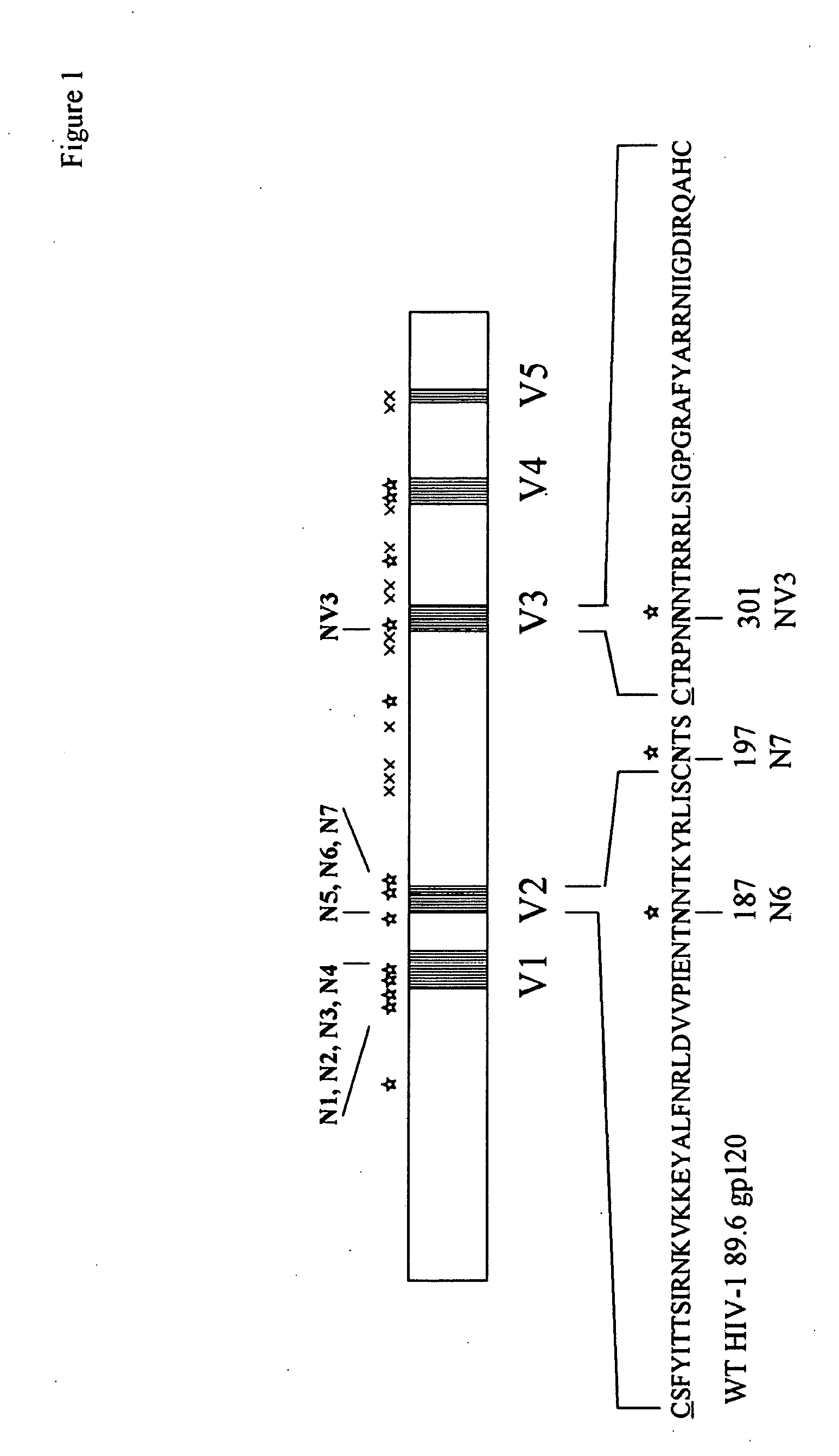
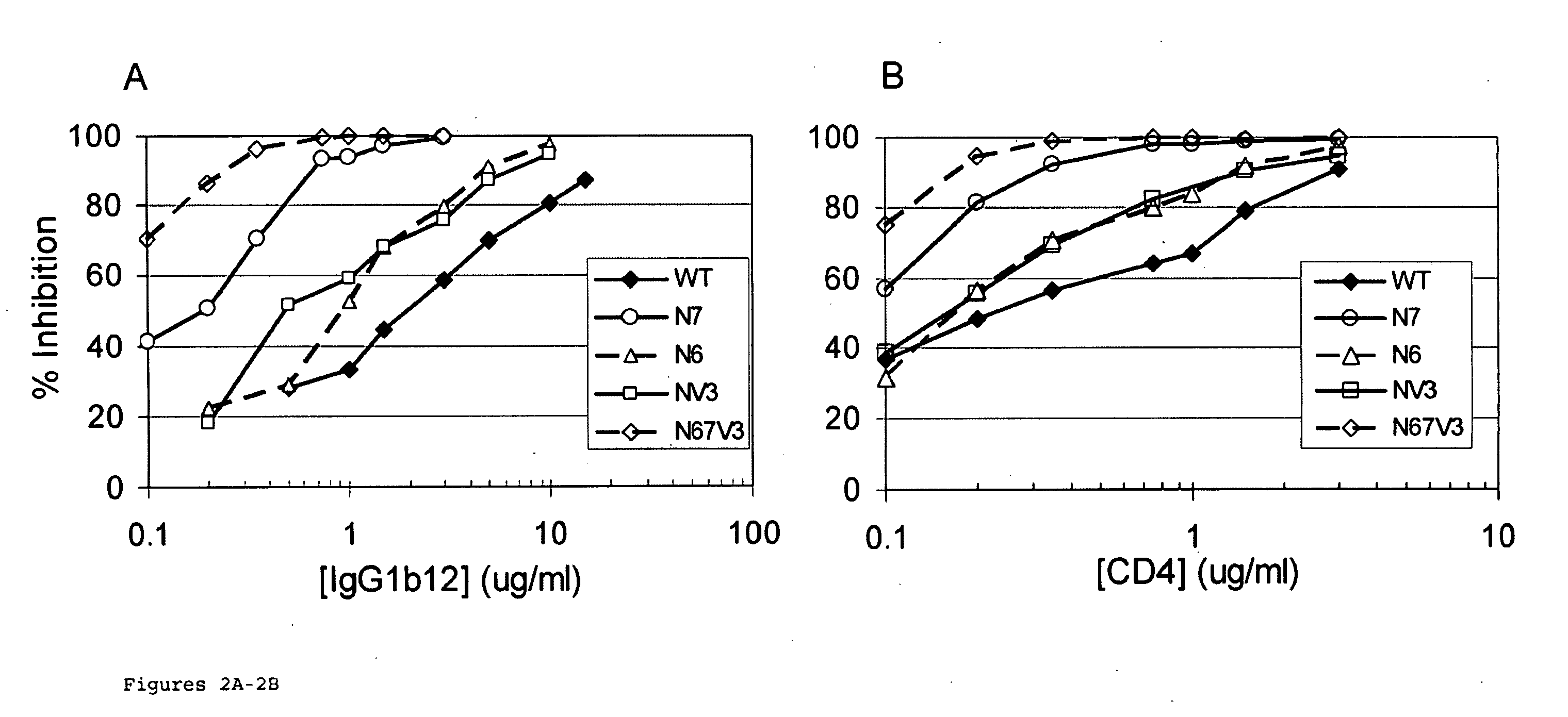
The invention provides a polynucleotide encoding an envelope protein comprising gp120 of human immunodeficiency virus-1 (HIV-1) having a mutation at amino acid residues 197-199 whereby a single N-linked glycan is removed. In a typical embodiment, the mutation replaces the asparagine (N) at residue 197 with another amino acid, such as glutamine (Q), creating an N197Q mutation. Because the N197 glycan is highly conserved among HIV-1 subtypes, this approach is applicable across HIV-1 isolates. The invention provides polypeptides, polynucleotides encoding the polypeptides, vectors, and recombinant viruses containing the polynucleotides, antigen-presenting cells (APCs) presenting the polypeptides, immune cells directed against HIV, and pharmaceutical compositions. The invention additionally provides methods, including methods for preventing and treating infection, for killing infected cells, for inhibiting viral replication, for enhancing secretion of antiviral and / or immunomodulatory lymphokines, and for enhancing production of neutralizing antibody.
Owner:UNIV OF WASHINGTON
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com