Anti-tumor chimeric protein, anti-tumor vaccine and anti-tumor vaccine adopting nasal mucosa drug administration mode
A chimeric protein, anti-tumor technology, applied in the field of anti-tumor vaccines, can solve the problems of inability to exert immunotherapy, weak immunogenicity, unable to recognize and present to activate toxic T lymphocytes, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
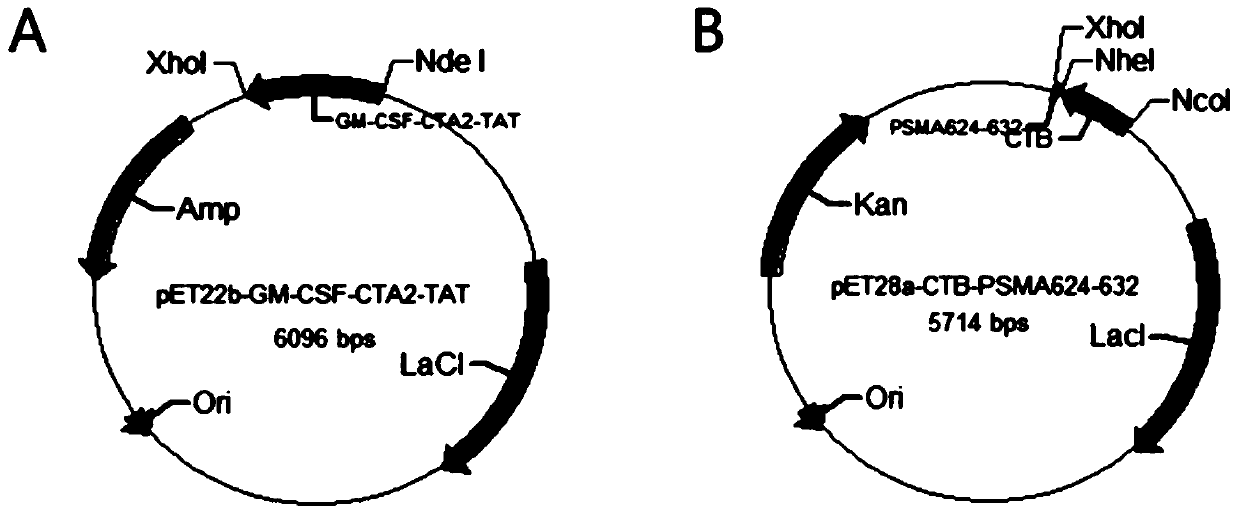
[0055] see figure 1 , this example provides a recombinant engineered bacterium containing the first polypeptide sequence, marked as pET22b-GM-CSF-CTA2-TAT, the construction method is as follows:
[0056] In this example, the commercial carrier pET22b was selected, and the sequence of GM-CSF-CTA2-TAT (GM-CSF, CTA2 subunit sequence of cholera toxin and TAT sequence of cell penetrating peptide sequence) was artificially synthesized and cut with NdeI and XhoI Insert it into the pET22b plasmid to obtain the recombinant plasmid pET22b-GM-CSF-CTA2-TAT for fusion expression, transform Escherichia coli BL21(DE3), and obtain the recombinant engineering bacteria containing the first polypeptide sequence.
[0057] Wherein, the amino acid sequence of GM-CSF-CTA2-TAT is:
[0058] MAPTRSPITVTRPWKHVEAIKEALNLLDDMPVTLNEEVEVVSNEFSFKKLTCVQTRLKIFEQGLRGNFTKLKGALNMTASYYQTYCPPTPETDCETQVTTYADFIDSLKTFLTDIPFECKKPSQKDPTGGASEFELGGGGSMSNTCDEKTQSLGVKFLDEYQSKVKRQIFSHRPHPDLEGTHNRIKDERLVD. The connection bet...
Embodiment 2
[0066] see figure 1 , this embodiment provides a recombinant engineered bacterium containing the second polypeptide sequence, marked as pET28a-CTB-PSMA624-632, the construction method is as follows:
[0067] In this example, the commercial vector pET28a was selected, and the amino acid sequence of CTB was recombined with pET28a through transformation to obtain the recombinant plasmid pET28a-CTB. The PSMA624-632 gene was cut with NheI and XhoI and inserted into the downstream of the CTB gene to form a recombinant plasmid for fusion expression. pET28a-CTB-PSMA624-632 was transformed into Escherichia coli BL21(DE3) to obtain a recombinant engineered bacterium containing the second polypeptide sequence.
[0068] The second polypeptide is a fusion protein, and the amino acid sequence denoted as (CTB-PSMA624-632)5 is:
[0069] MTPQNITDLCAEYHNTQIYTLNDKIFSYTESLAGKREMAIITFKNGAIFQVEVPGSQHIDSQKKAIERMKDTLRIAYLTEAKVEKLCVWNNKTPHAIAAISMANASGGTYSVSFDSLLEHHHHHH. SEQ ID No: 1 is connected to ...
Embodiment 3
[0076] This embodiment provides the expression method of the fusion protein GM-CSF-CTA2-TAT:
[0077] Cultivate the recombinant engineered bacteria containing the first polypeptide sequence of Example 1 at 37°C, with ampicillin and 0.1mmol / L isopropyl-β-D-thiopyran galactobacter at a final concentration of 50 μg / mL Glycoside (IPTG) induces the expression of the fusion protein to obtain the first polypeptide (GM-CSF-CTA2-TAT).
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com