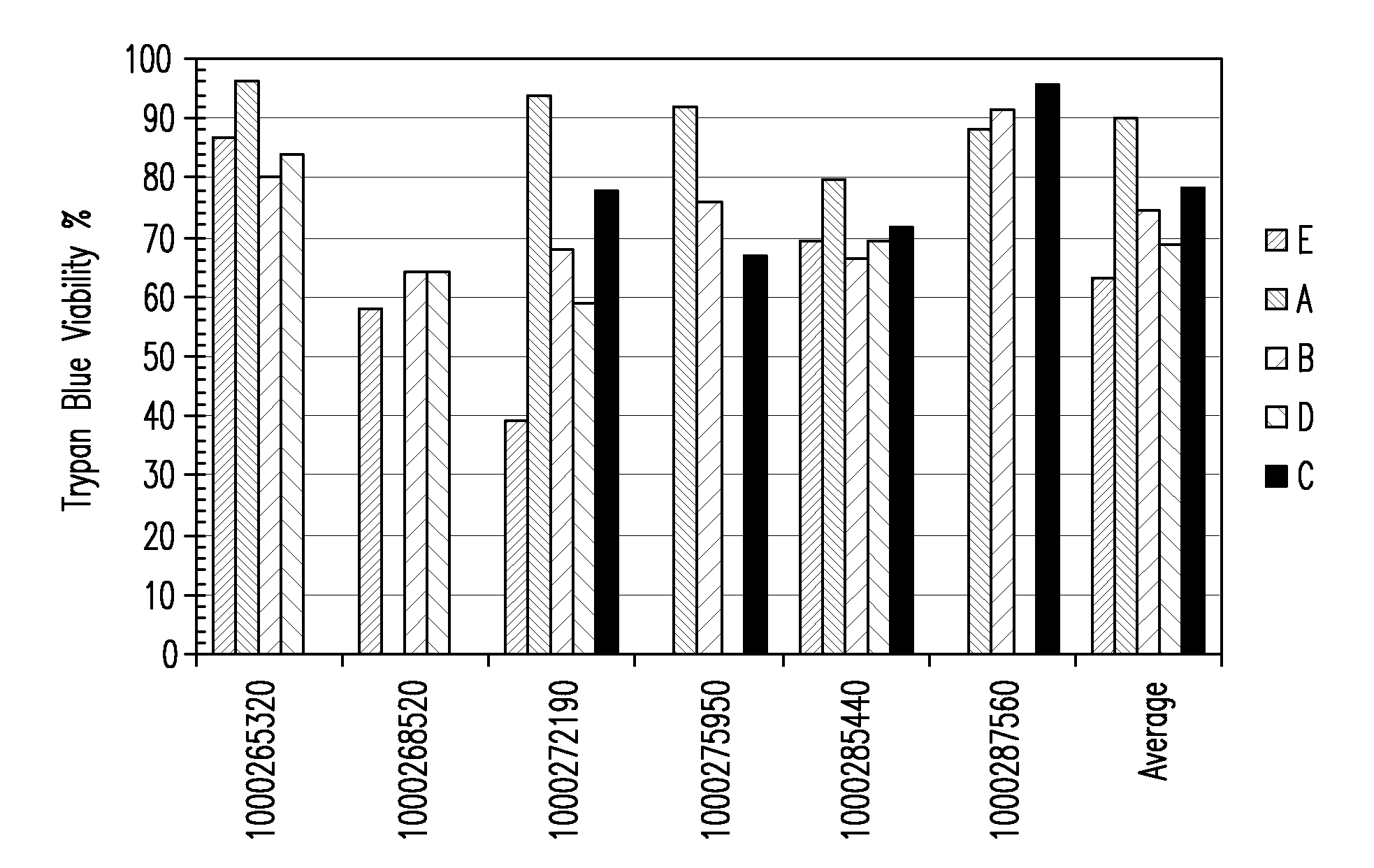
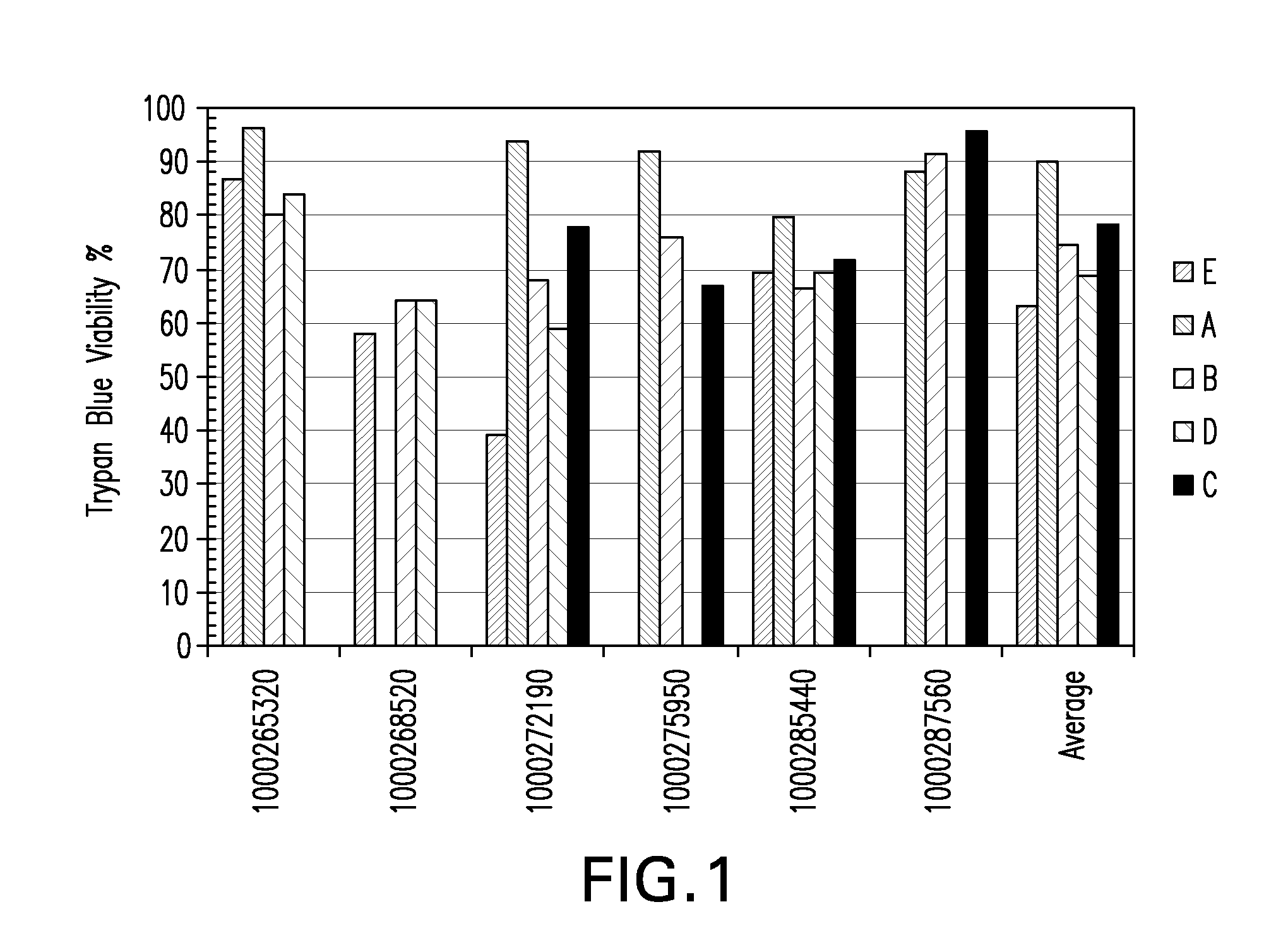
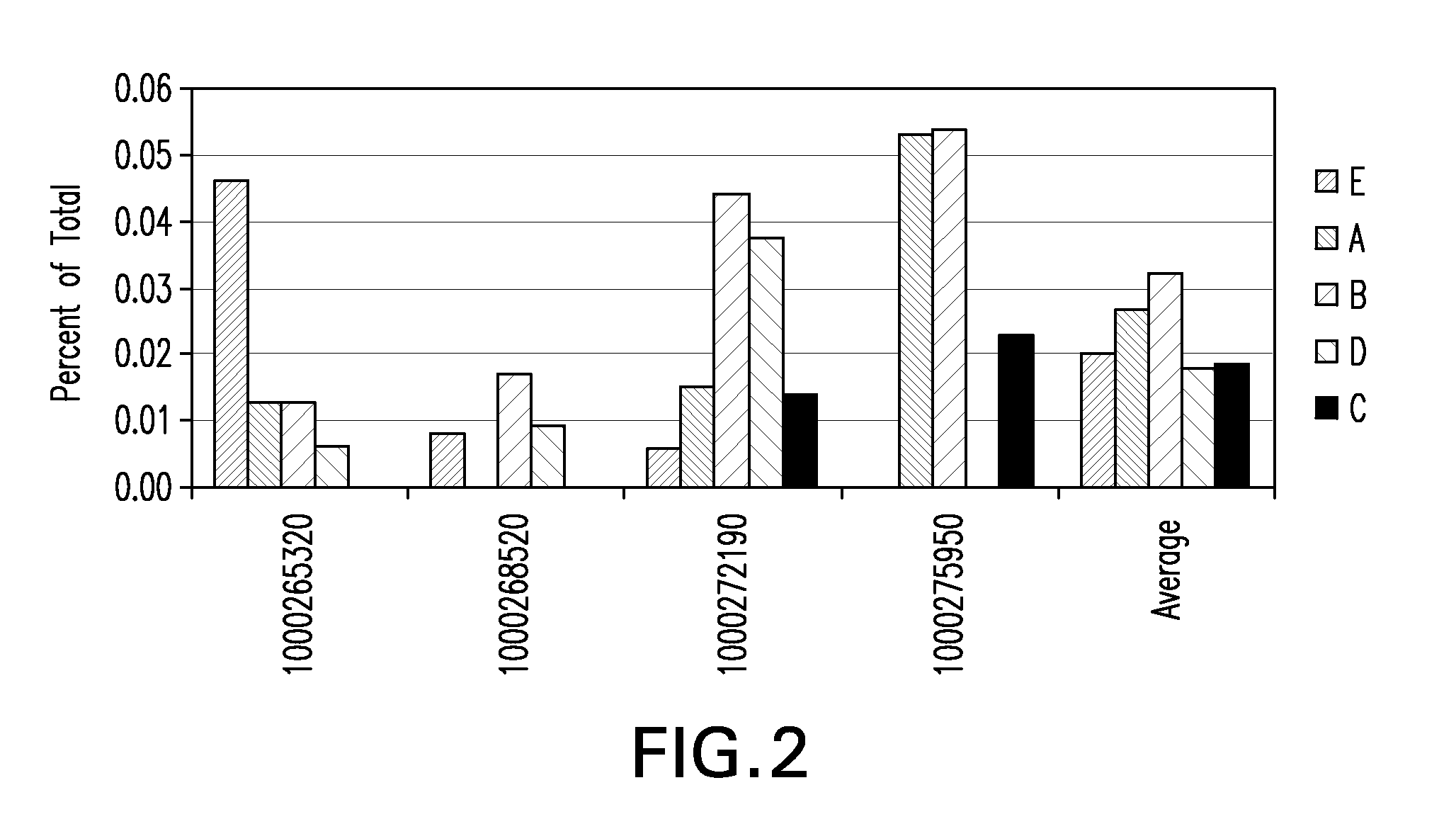
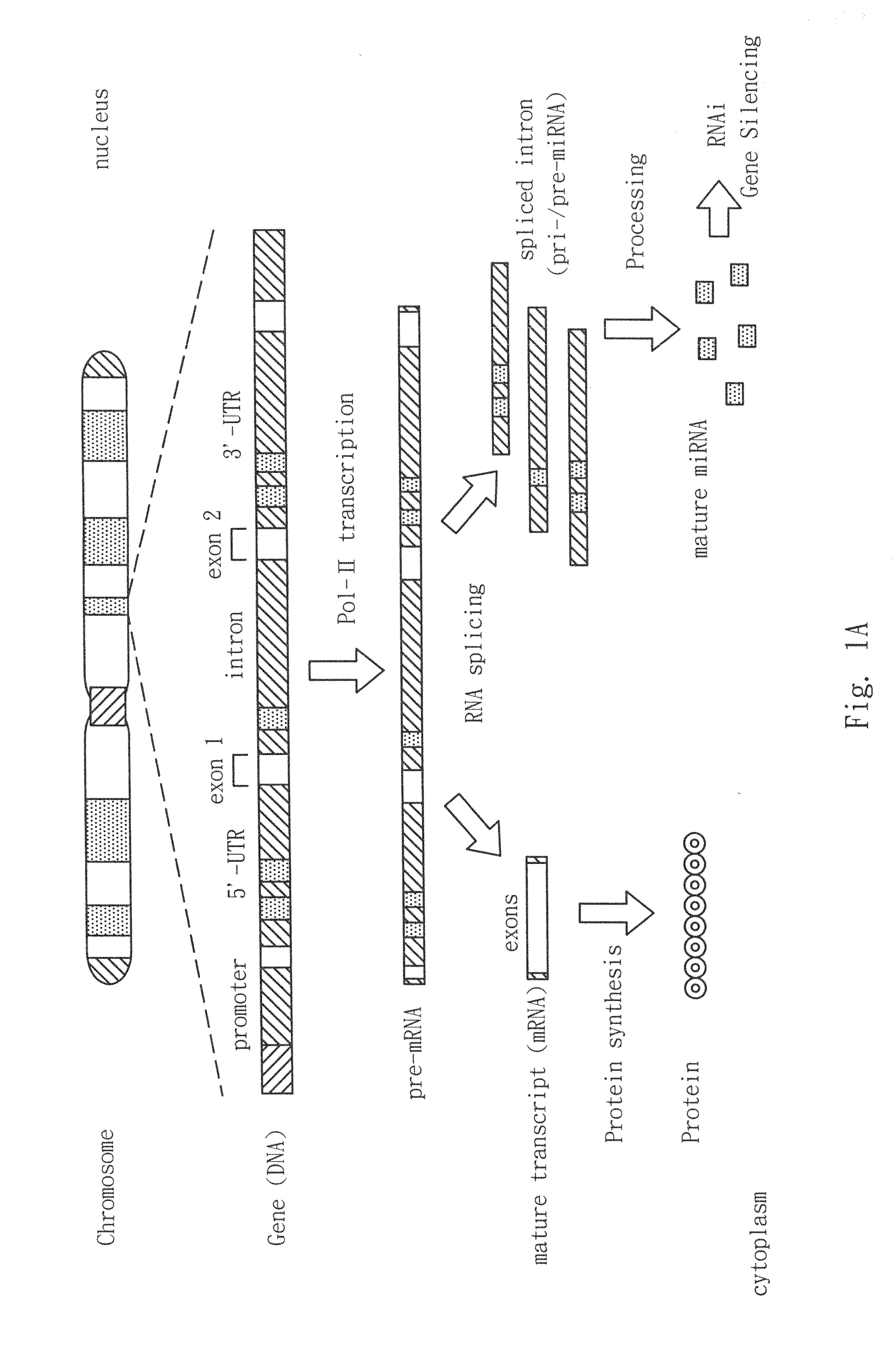
Patents
Literature
585 results about "Wilms tumour" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Wilms' tumor is a malignant tumor containing metanephric blastema, stromal and epithelial derivatives. Characteristic is the presence of abortive tubules and glomeruli surrounded by a spindled cell stroma.
Radioimmunotherapy of lymphoma using anti-CD20 antibodies
Owner:GLAXO SMITHKLINE LLC
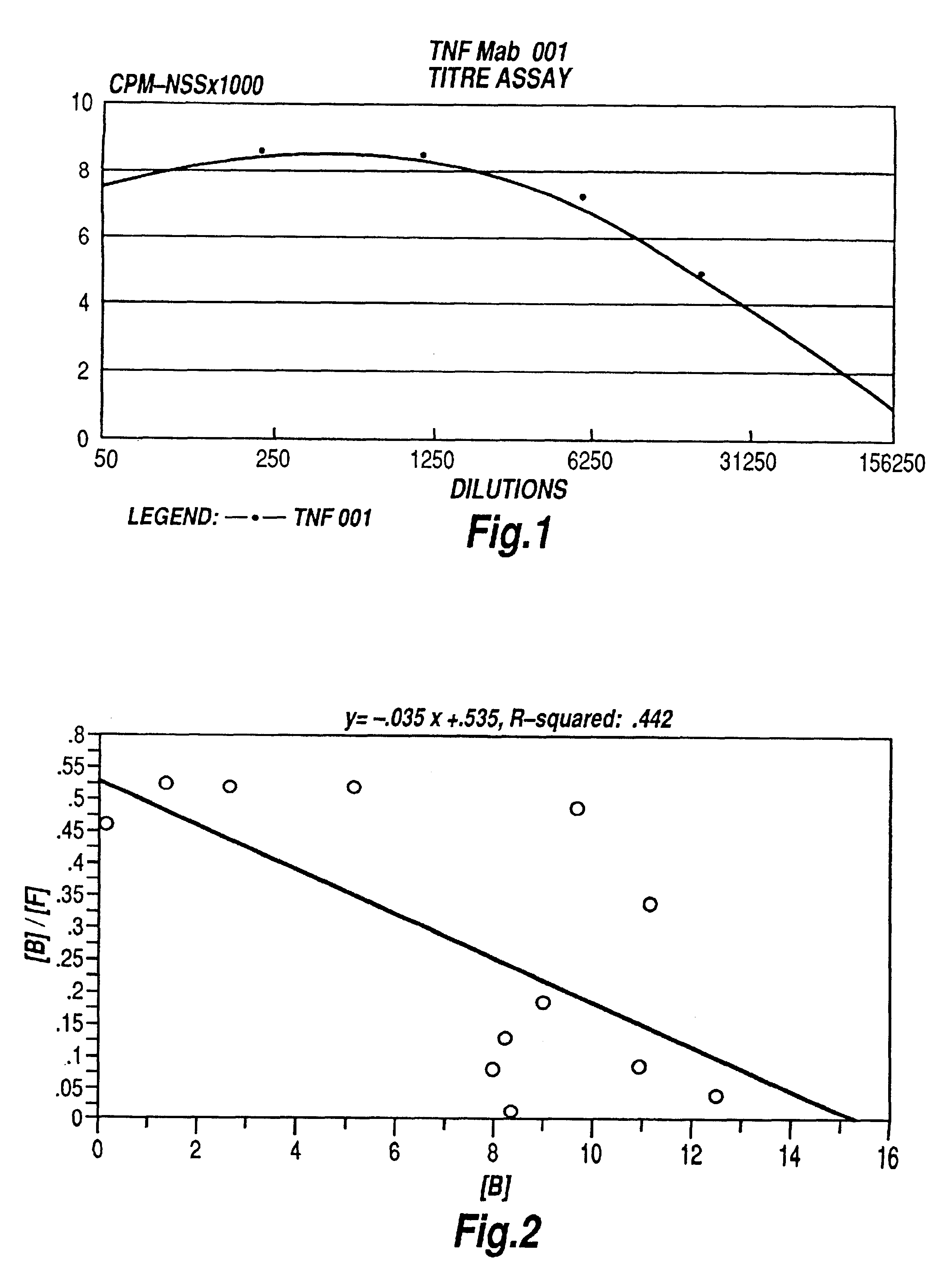
Tumour necrosis factor antibodies
InactiveUS6451983B2Enhance or inhibit TNF alpha activityInduction of endothelial procoagulant activityPeptide/protein ingredientsAntibody mimetics/scaffoldsHuman tumorSingle-Chain Antibodies
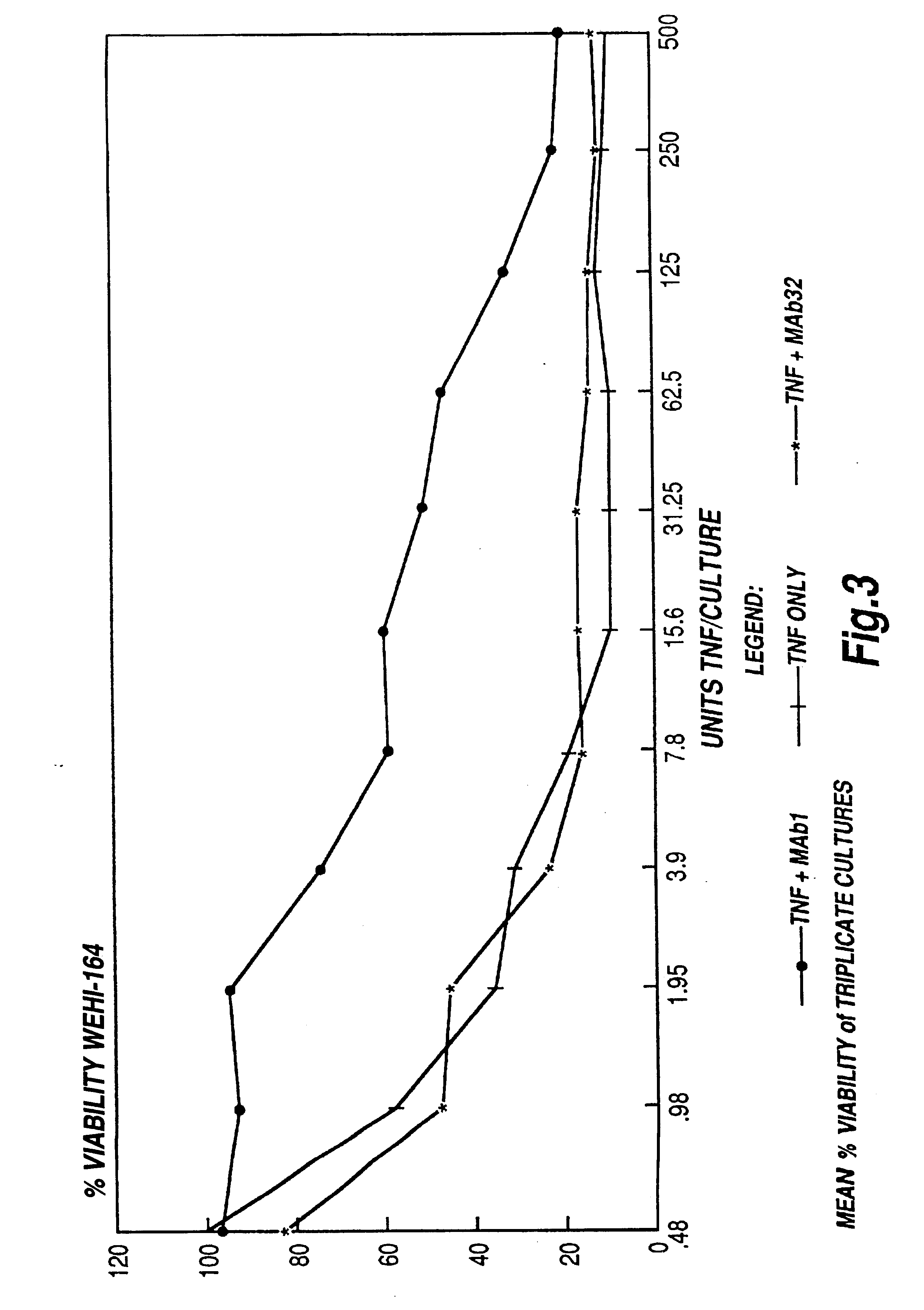
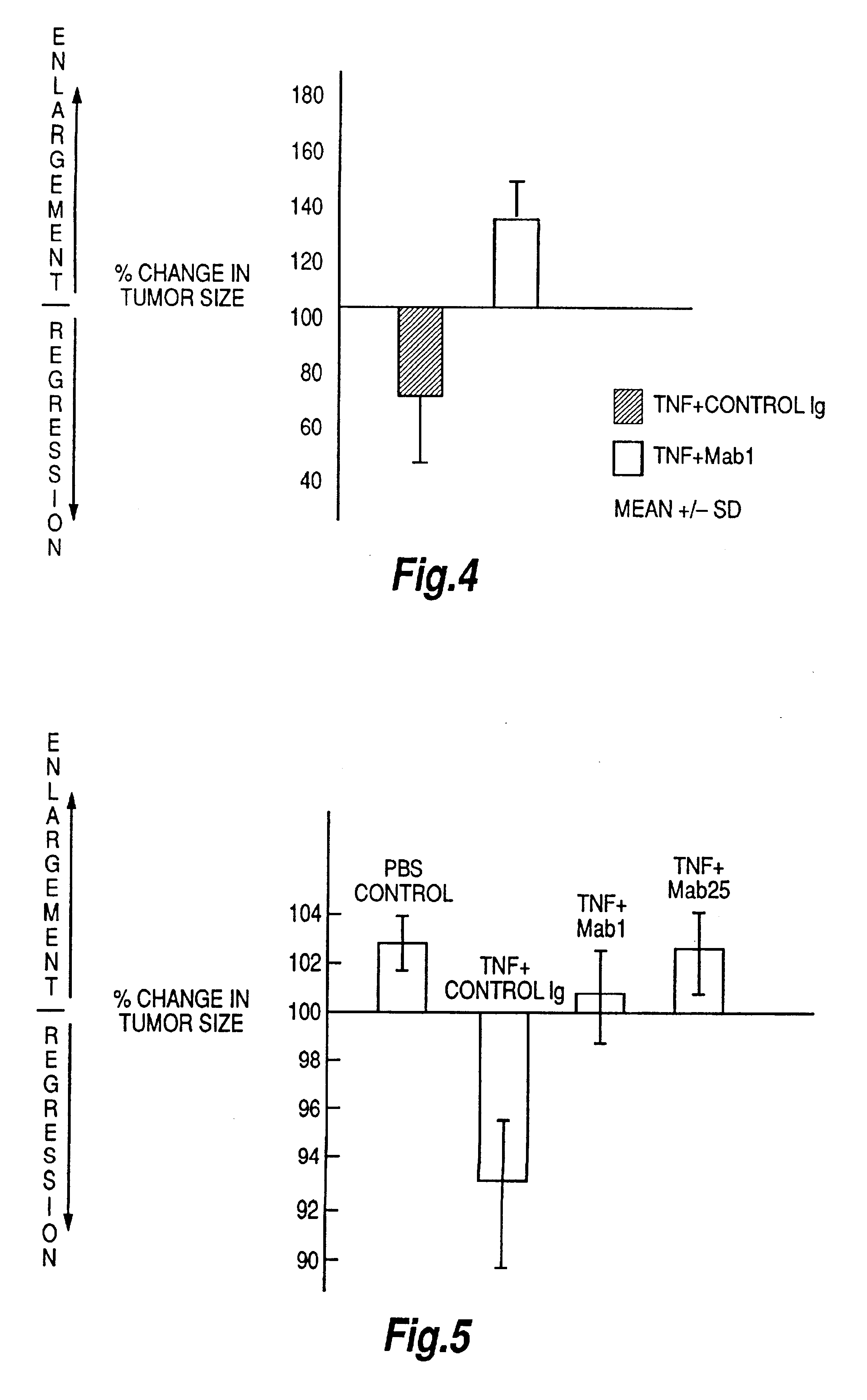
The present invention relates to ligands which bind to human tumor necrosis factor alpha (TNF) in a manner such that upon binding of these ligands to TNF the biological activity of TNF is modified. In preferred forms the ligand binds to TNF in a manner such that the induction of endothelial procoagulant activity of the TNF is inhibited; the binding of TNF to receptors on endothelial cells is inhibited; the induction of fibrin deposition in the tumor and tumor regression activities of the TNF are enhanced; and the cytotoxicity and receptor binding activities of the TNF are unaffected or enhanced on tumor cells. The ligand is preferably an antibody, F(ab) fragment, single domain antibody (dABs) single chain antibody or a serum binding protein. It is preferred, however, that the ligand is a monoclonal antibody or F(ab) fragment thereof.
Owner:CEPHALON AUSTRALIA
Radioimmunotherapy of lymphoma using anti-CD20 antibodies
Methods for the treatment of lymphoma by administration of a B cell-specific antibody are described. The invention encompasses providing to a patient both unlabeled antibodies and antibodies labeled with a radioisotope. A principal advantage of the method is that tumor responses can be obtained in a radiometric dose range that does not require hematopoietic stem cell replacement as an adjunct therapy also described is a composition useful in the treatment of lymphoma.
Owner:RGT UNIV OF MICHIGAN +2
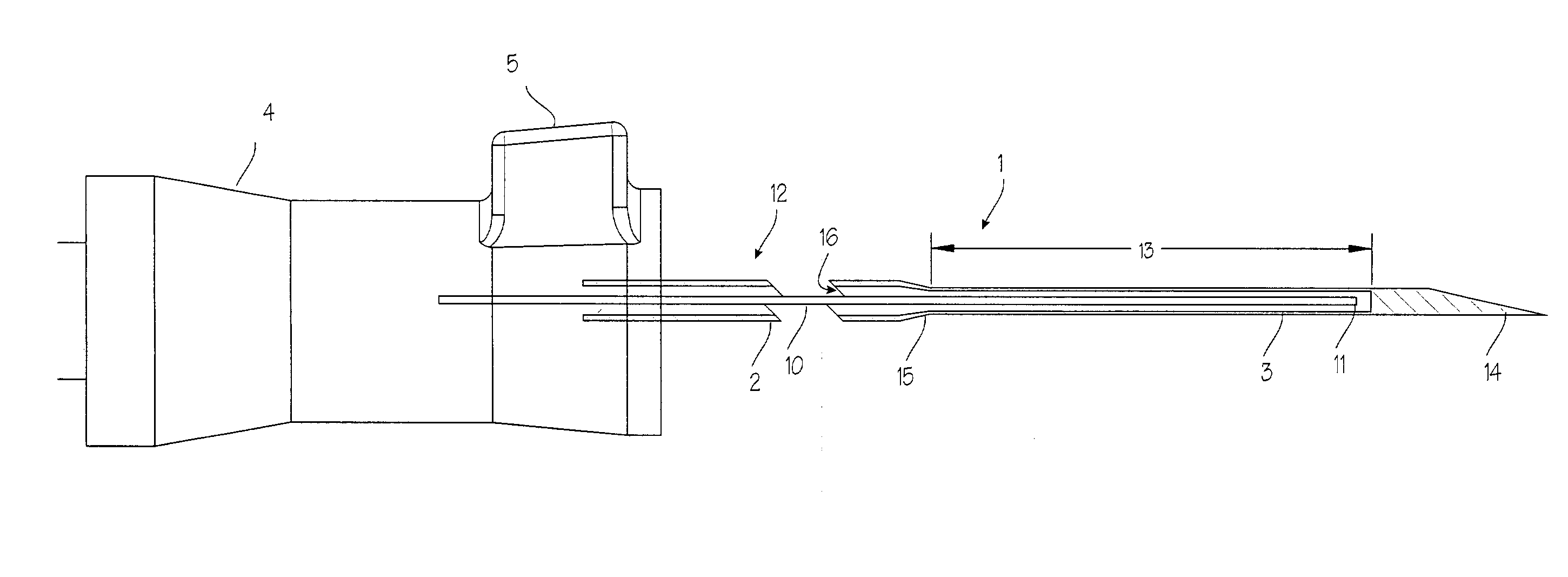
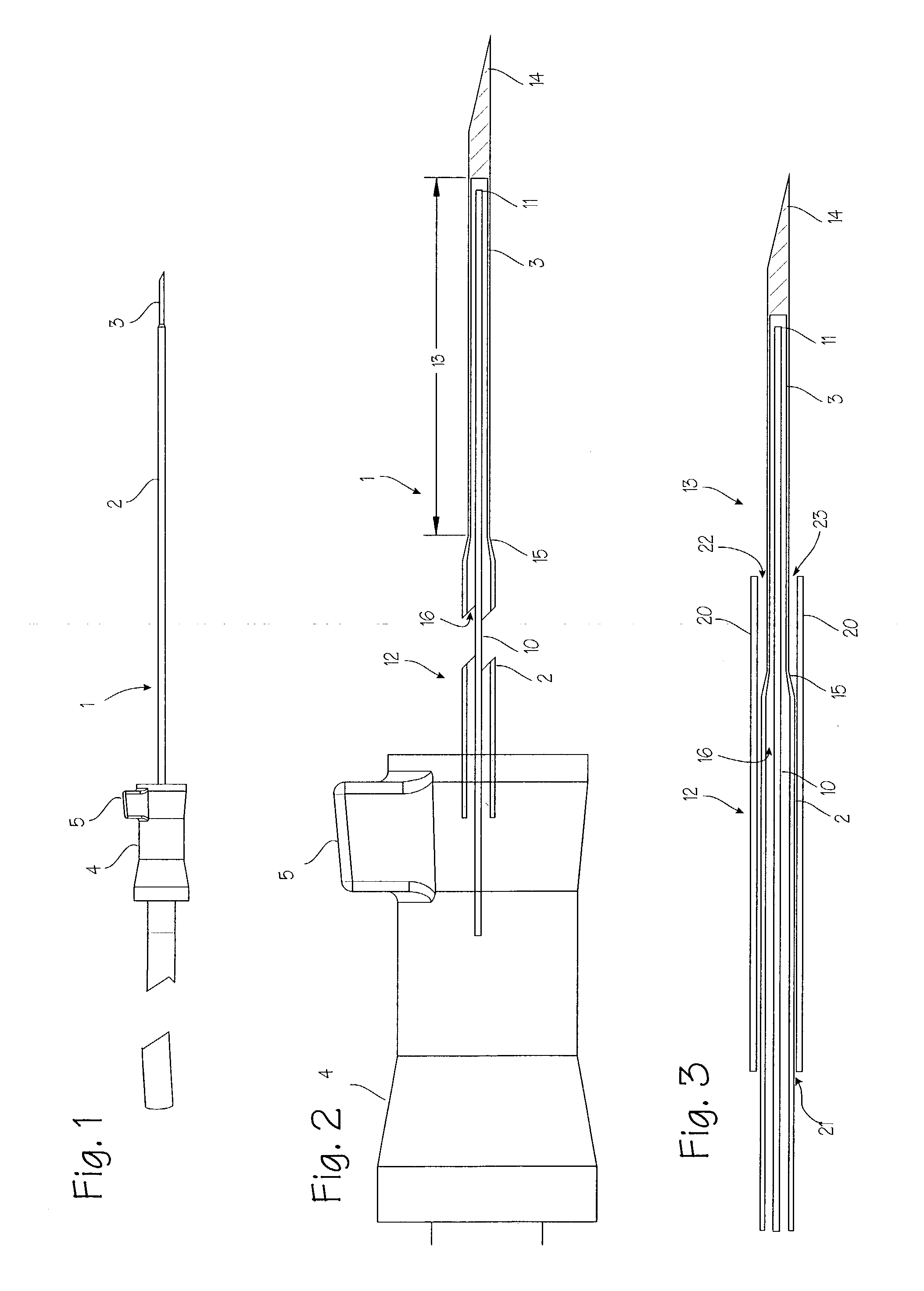
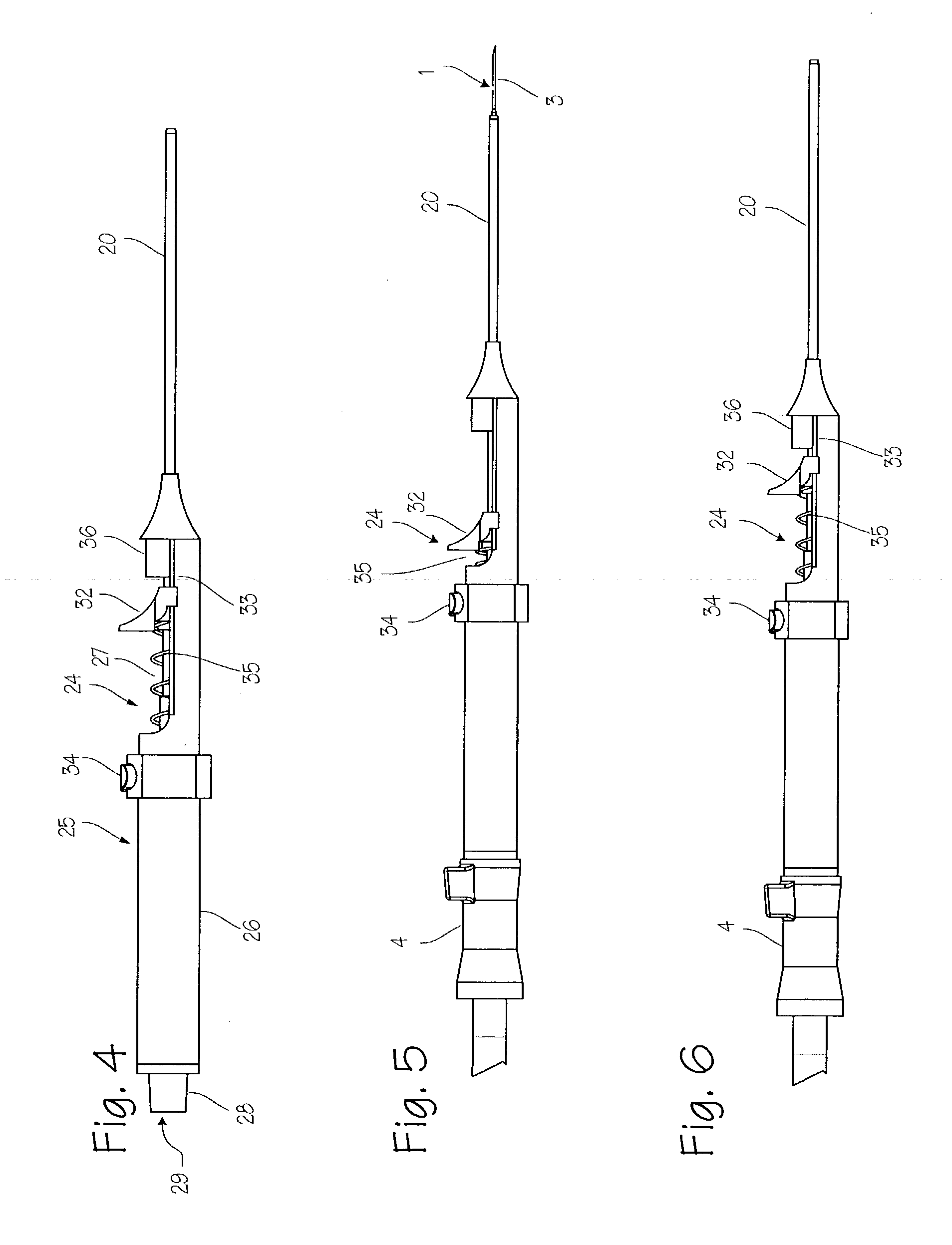
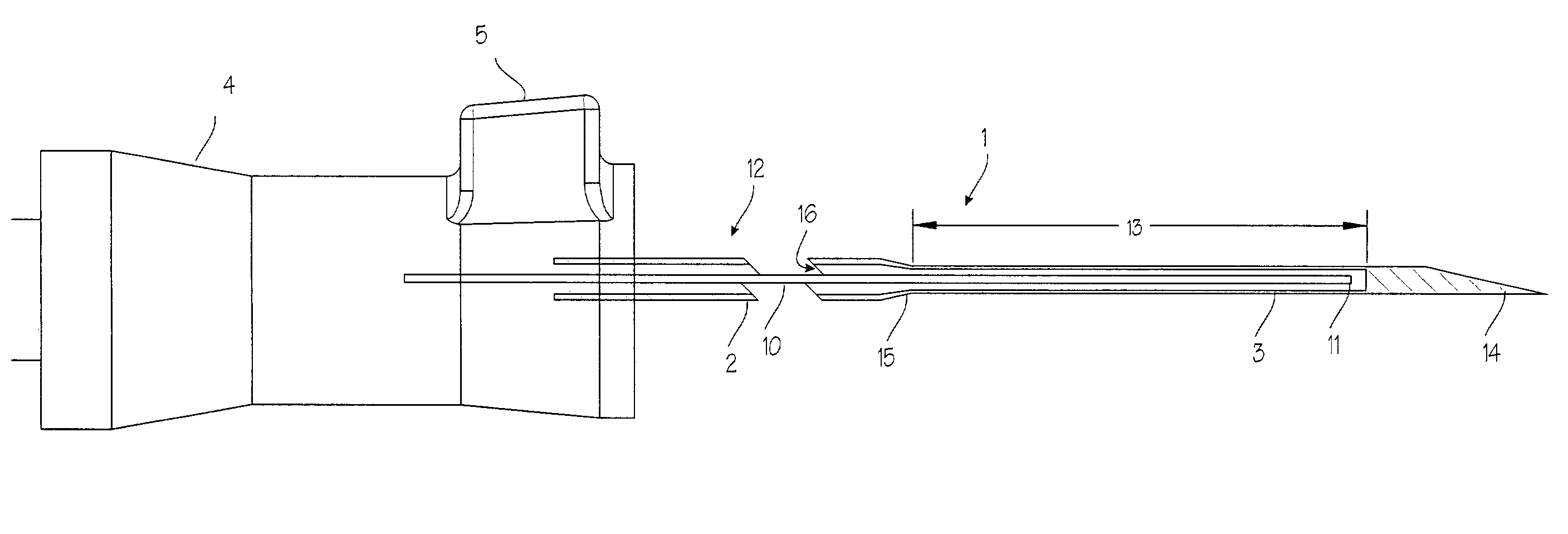
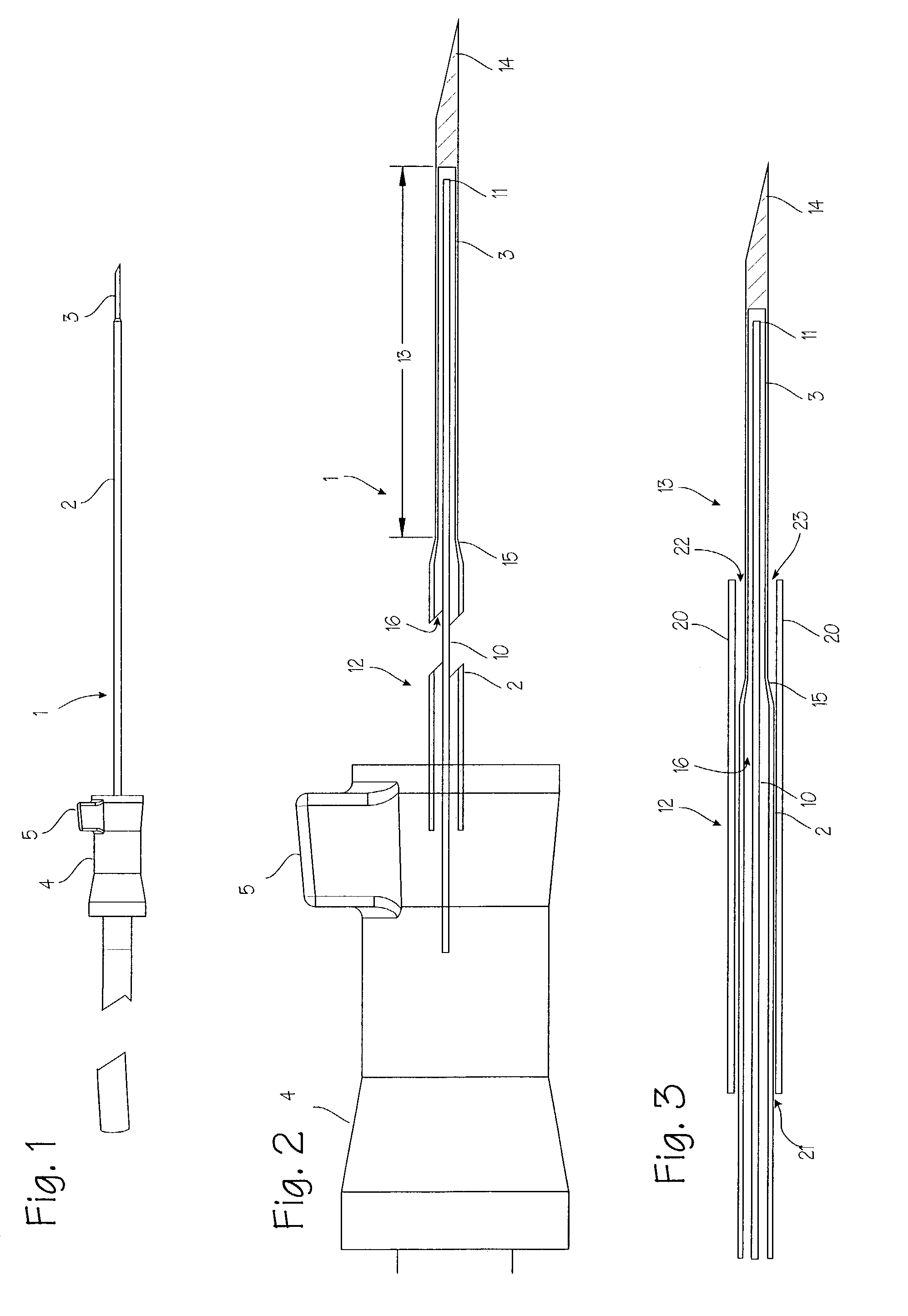
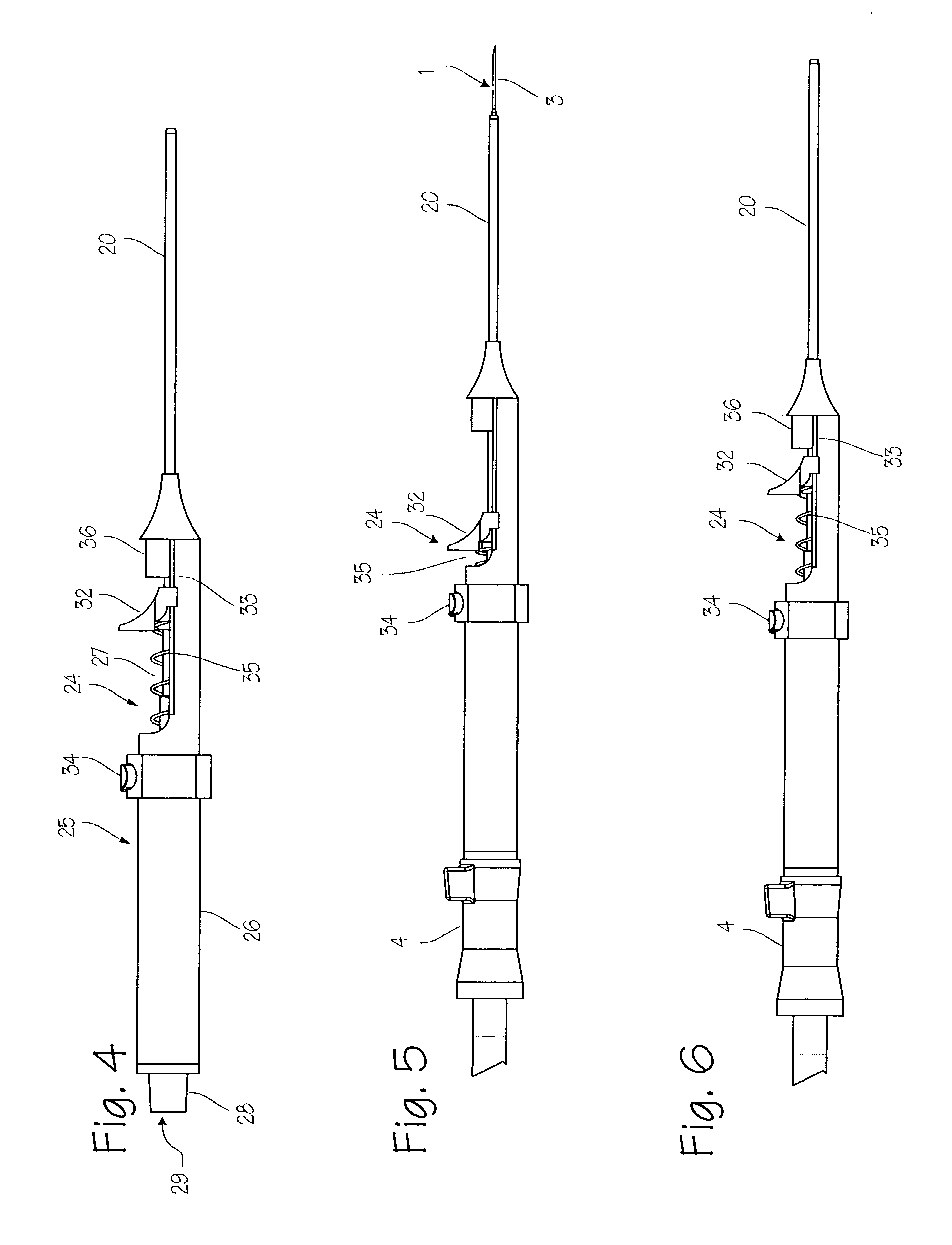
Device for biopsy of tumors
InactiveUS20030195436A1Surgical needlesVaccination/ovulation diagnosticsAbnormal tissue growthCombined use
A device and method of use for securing and coring of tumors within the body during a biopsy of the tumor, specifically breast tumors. An adhesion probe for securing the tumor is described. The probe secures the tumor by piercing the tumor and providing a coolant to the distal tip to cool the tip. The cooled tip adheres to the tumor. A coring instrument adapted for cutting a core sample of the tumor is described. The instrument is provided with a cannula that can cut a core sample of the tumor. The instrument is adapted for use with the probe with the probe fitting within the cannula. The instrument can be used in conjunction with the probe to secure and core a sample of the tumor for biopsy.
Owner:SCION MEDICAL
Tumor suppression using placental stem cells
ActiveUS20080152624A1Promote formationPromote more developedBiocideGenetically modified cellsAbnormal tissue growthPlacental cell
The present invention provides methods of suppression of tumor cell proliferation and tumor growth using placental stem cells and placental stem cell populations. The invention also provides methods of producing and selecting placental cells and cell populations on the basis of tumor suppression, and compositions comprising such cells and cell populations.
Owner:CELULARITY INC
Generation of tumor-free embryonic stem-like pluripotent cells using inducible recombinant RNA agents
InactiveUS20090203141A1Improve target specificityReduce the number of copiesVectorsFermentationCancer cellMammal
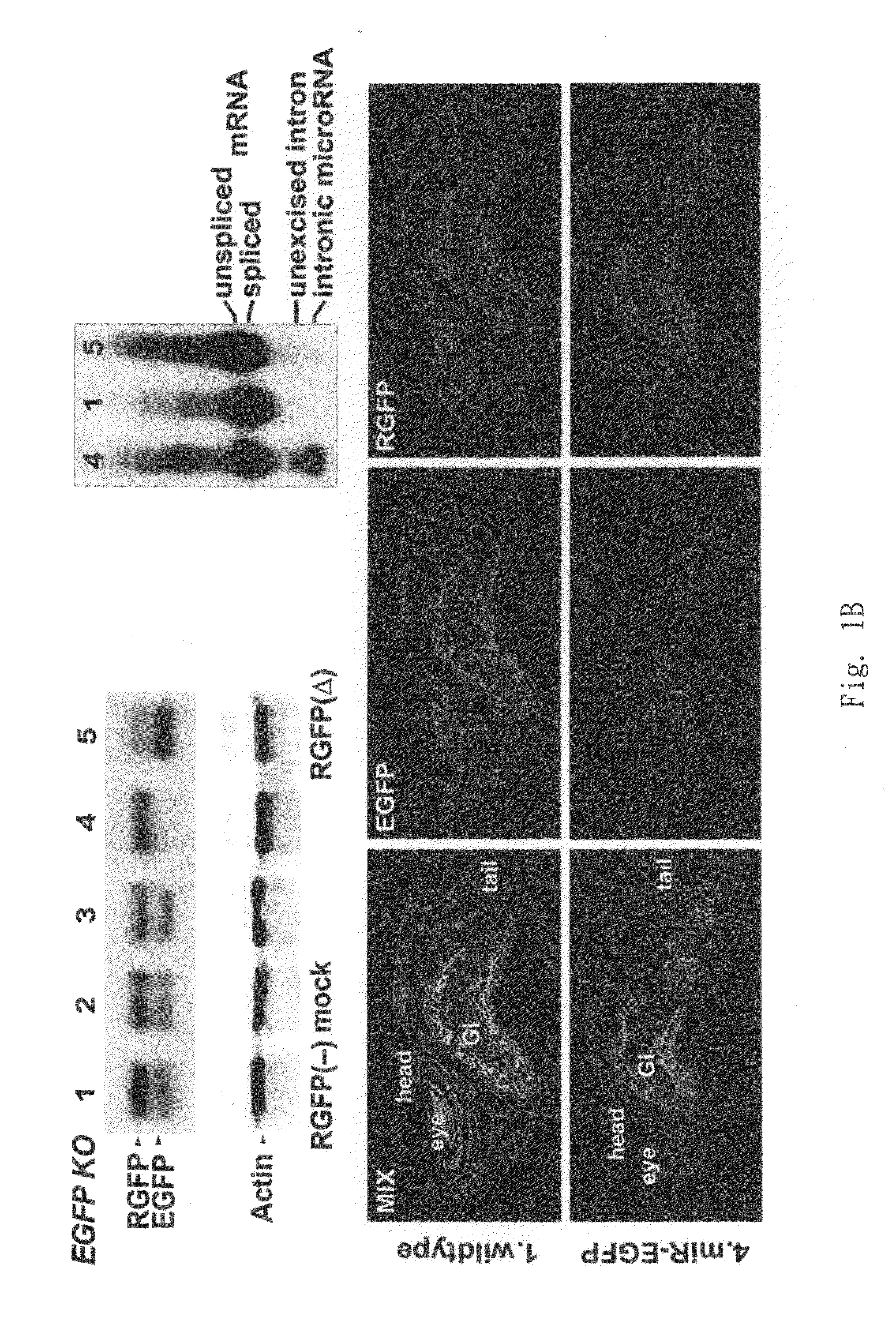
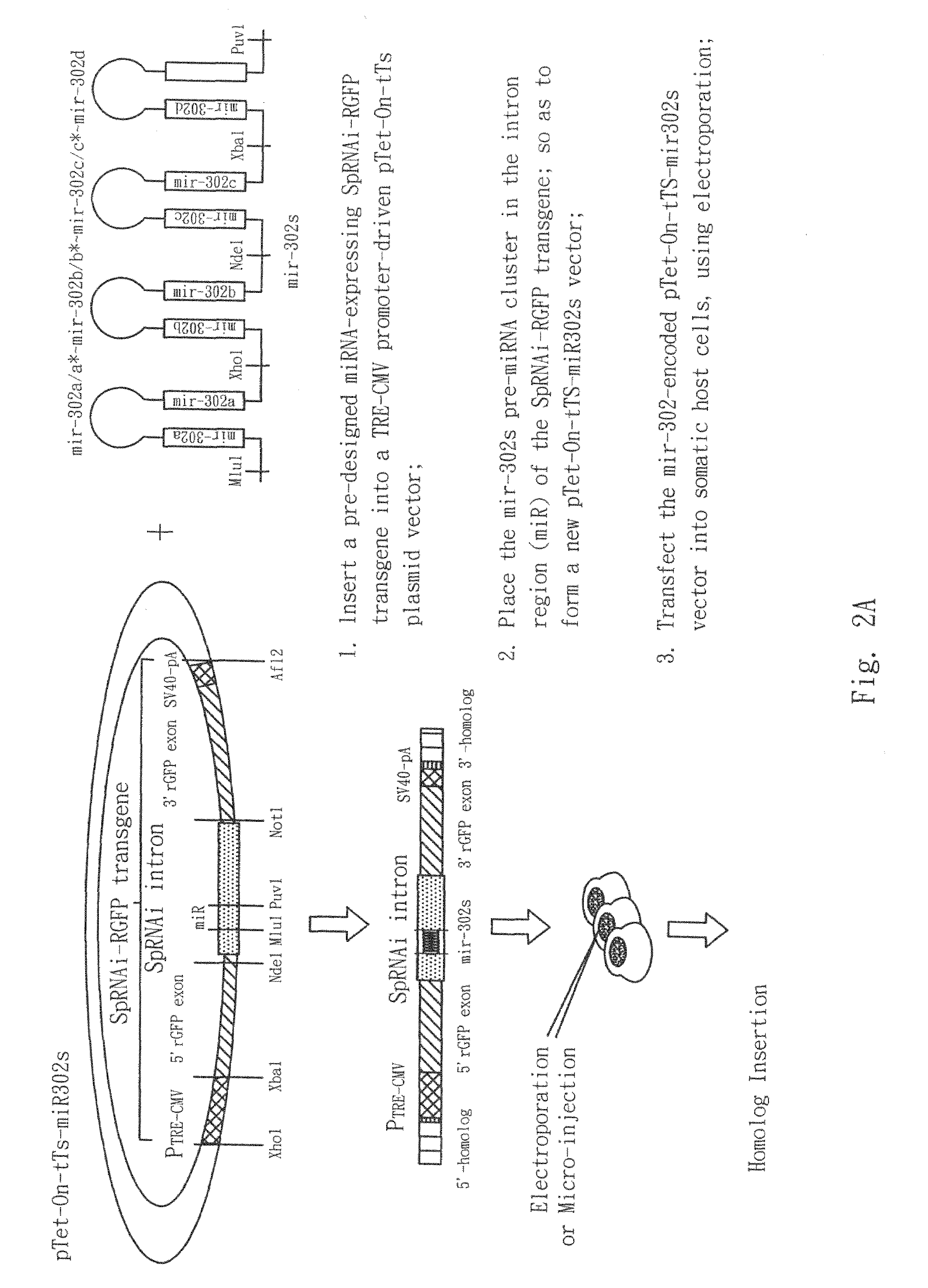
The present invention generally relates to a method for developing, generating and selecting tumor-free embryonic stem (ES)-like pluripotent cells using electroporation delivery of an inducible tumor suppressor mir-302 agent into mammalian cells. More particularly, the present invention relates to a method and composition for generating a Tet-On / Off recombinant transgene capable of expressing a manually re-designed mir-302 microRNA (miRNA) / shRNA agent under the control of doxycyclin (Dox) in human somatic / cancer cells and thus inducing certain specific gene silencing effects on the differentiation-associated genes and oncogenes of the cells, resulting in reprogramming the cells into an ES-like pluripotent state.
Owner:LIN SHI LUNG +1
MN/CA IX/CA9 and Renal Cancer Prognosis
InactiveUS7482129B2Microbiological testing/measurementBiological testingRegimenRenal clear cell carcinoma
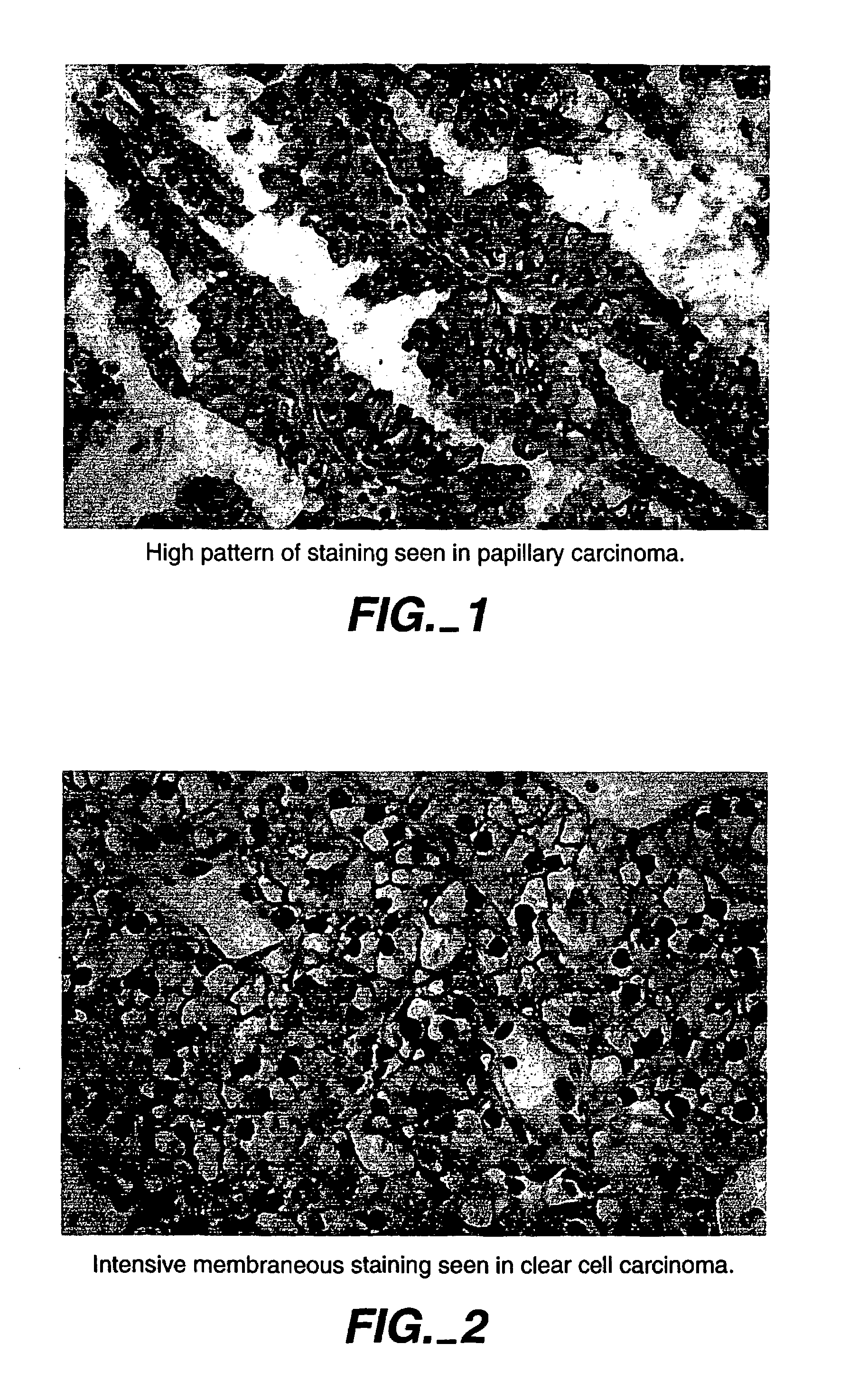
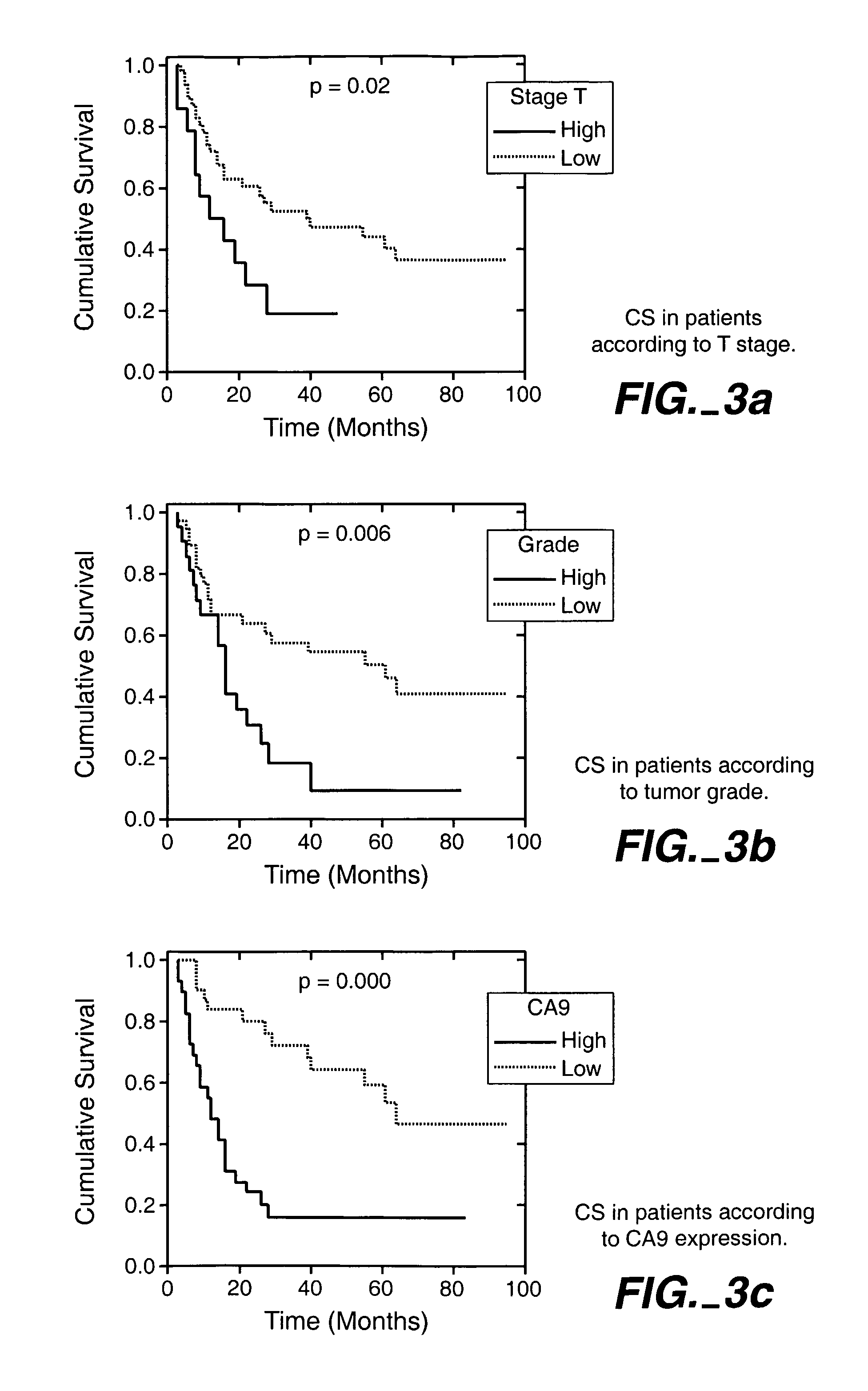
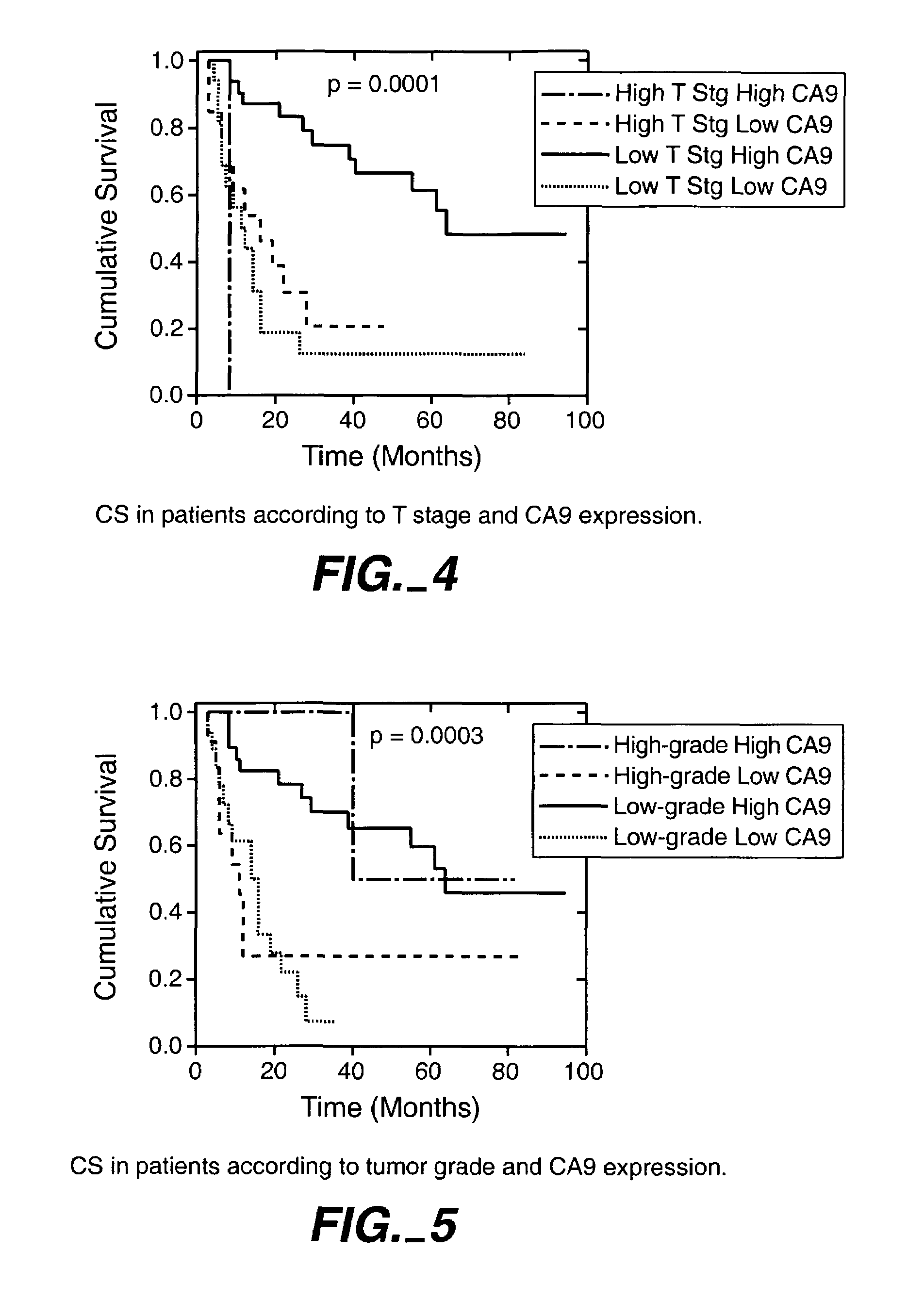
Herein disclosed are methods that are prognostic for renal cell carcinoma, particularly renal clear cell carcinoma, afflicting a vertebrate. An exemplary prognostic method comprises detecting the presence of, and quantitating the level and / or extent of a MN / CA9 gene expression product in a sample from the affected subject, wherein if 50% or fewer cells are found to express the MN / CA9 gene, then the subject is considered to have a poorer prognosis. MN / CA9 gene expression products useful in the prognostic methods include MN protein, MN polypeptide and / or MN nucleic acids. The methods are useful as an aid in the selection of treatment for a patient with renal cell carcinoma, alone or in combination with conventional tumor stage and / or grade information. The methods of the invention can be used, for example, to identify those patients requiring more aggressive therapy regimens, or those patients most likely to respond to adjuvant immunotherapies, particularly MN / CA IX / CA9-targeted therapies.
Owner:BIOMEDICAL RES CENT OF THE SLOVAK ACADEMY OF SCI +1
Adam12 as a biomarker for bladder cancer
InactiveUS20090029372A1Improve the level ofLower Level RequirementsMicrobiological testing/measurementMaterial analysisTissue ArraysAffymetrix genechip
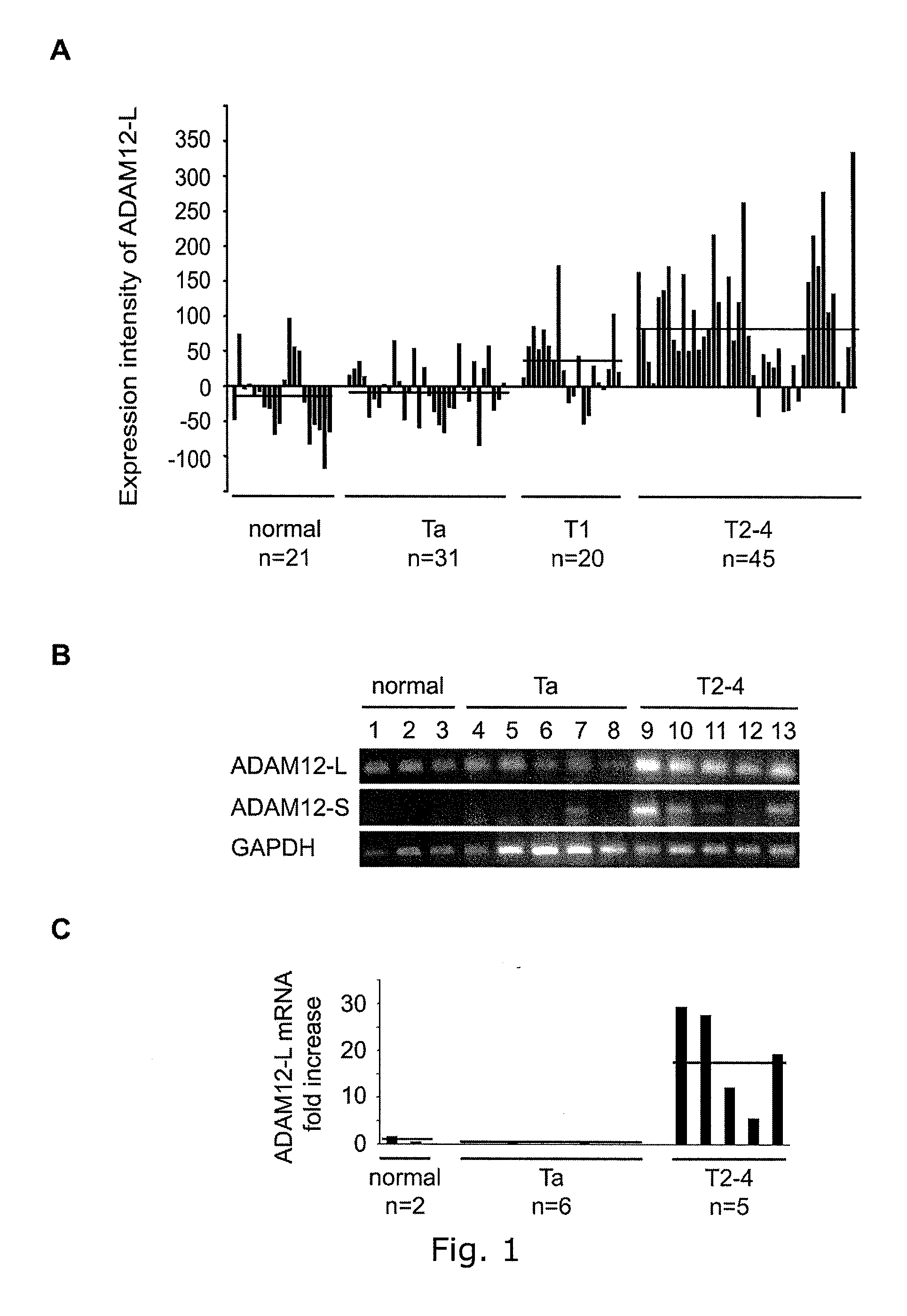
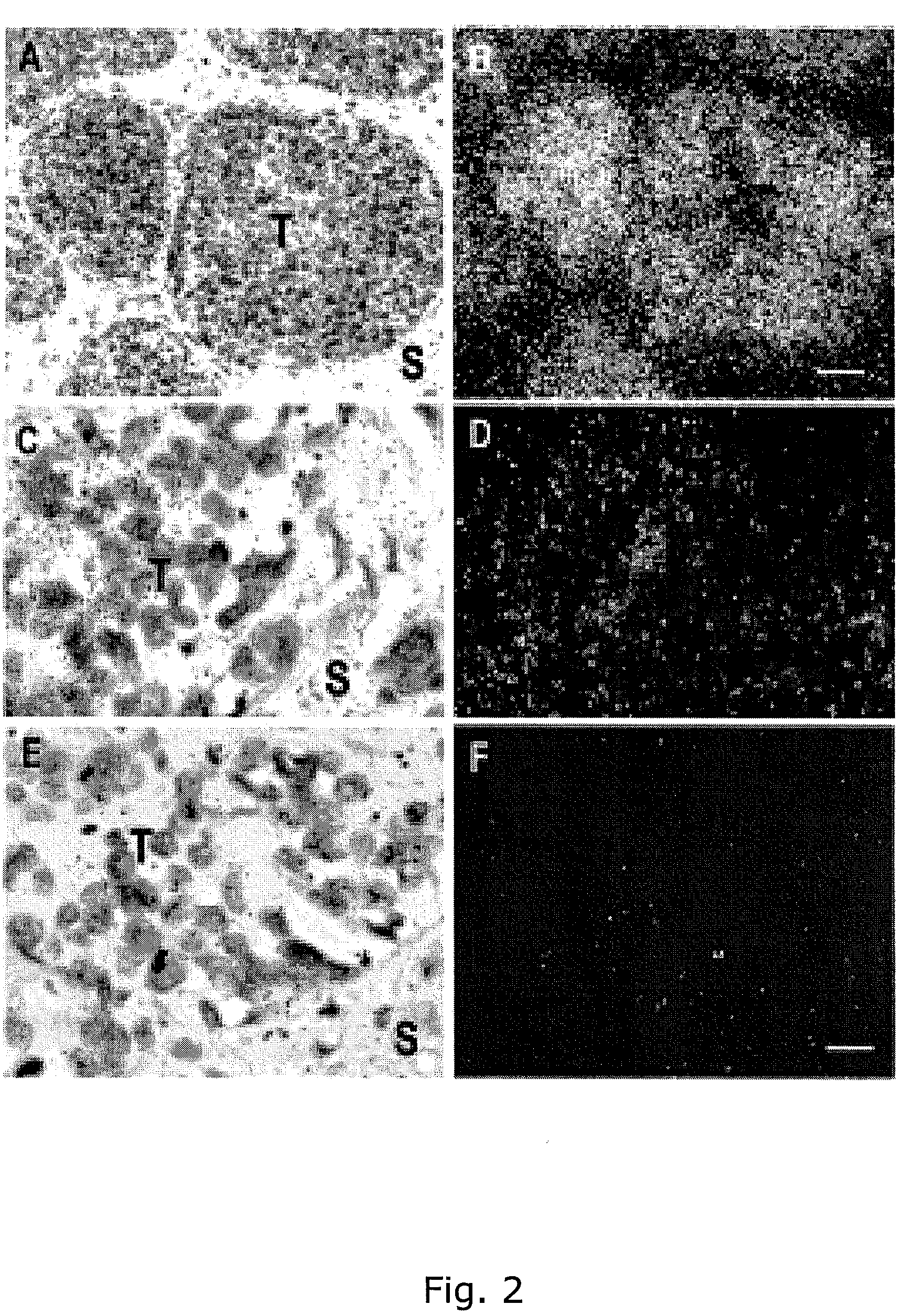
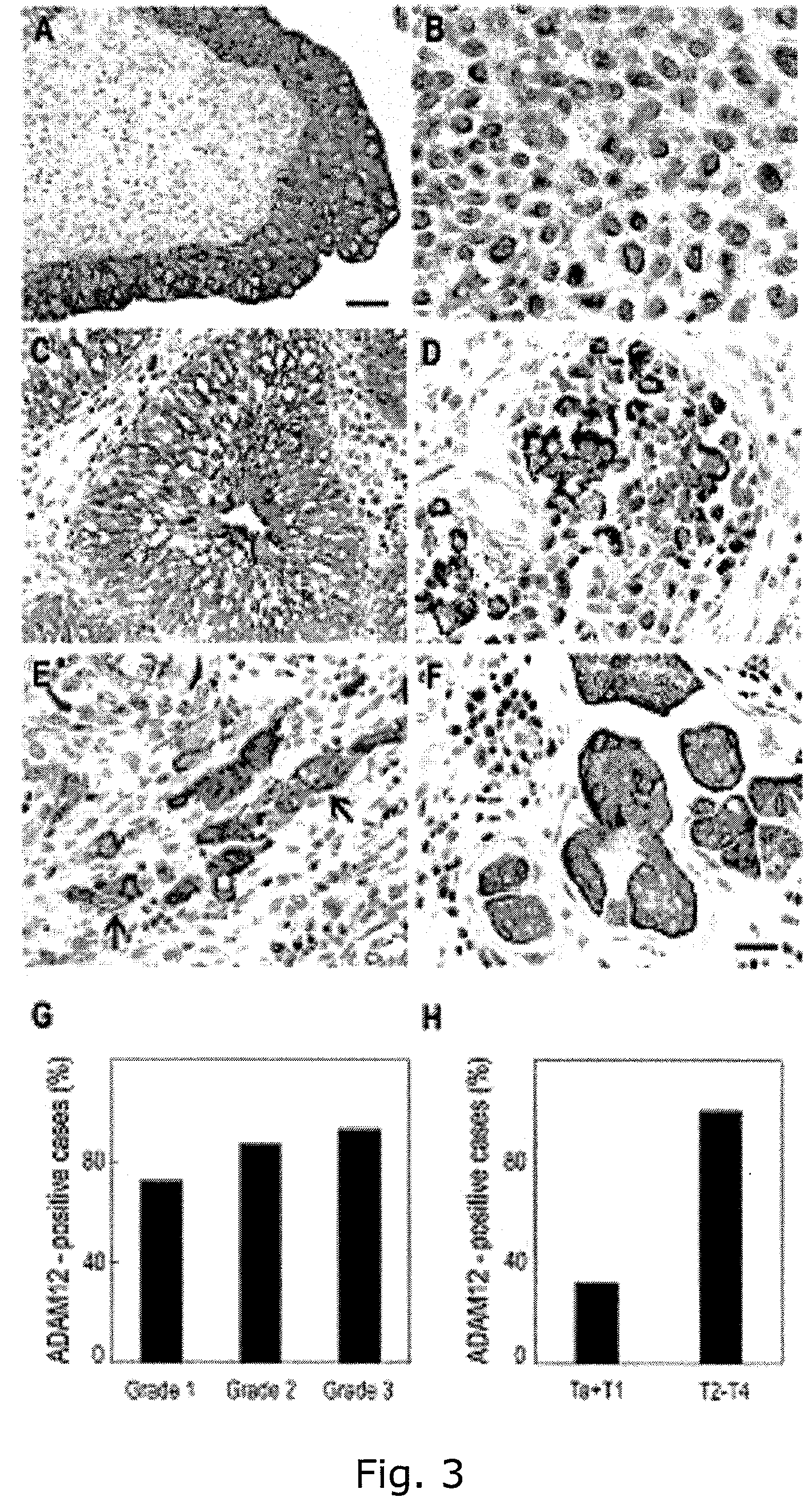
The present inventors have shown that the gene and protein expression profiles of ADAM8, ADAM10 and ADAM12 in different grades and stages of bladder cancer.ADAM12 gene expression was evaluated in tumors from 96 patients with bladder cancer using a customized Affymetrix GeneChip. Gene expression in bladder cancer was validated using reverse transcription-polymerase chain reaction (RT-PCR), quantitative PCR, and in situ hybridization. Protein expression was evaluated by immunohistochemical staining on tissue arrays of bladder cancers.The presence and relative amount of ADAM12 in the urine of cancer patients were determined by Western blotting and densitometric measurements, respectively.Particularly ADAM12 mRNA expression was significantly upregulated in bladder cancer, as determined by microarray analysis, and the level of ADAM12 mRNA correlated with disease stage. ADAM12 protein expression correlated with tumor stage and grade. ADAM12 was present in higher levels in the urine from bladder cancer patients than in urine from healthy individuals. Significantly, following removal of tumor by surgery, in most bladder cancer cases examined the level of ADAM12 in the urine decreased and, upon recurrence of tumor, increased.
Owner:PHYSICIANS CHOICE LAB SERVICES +1
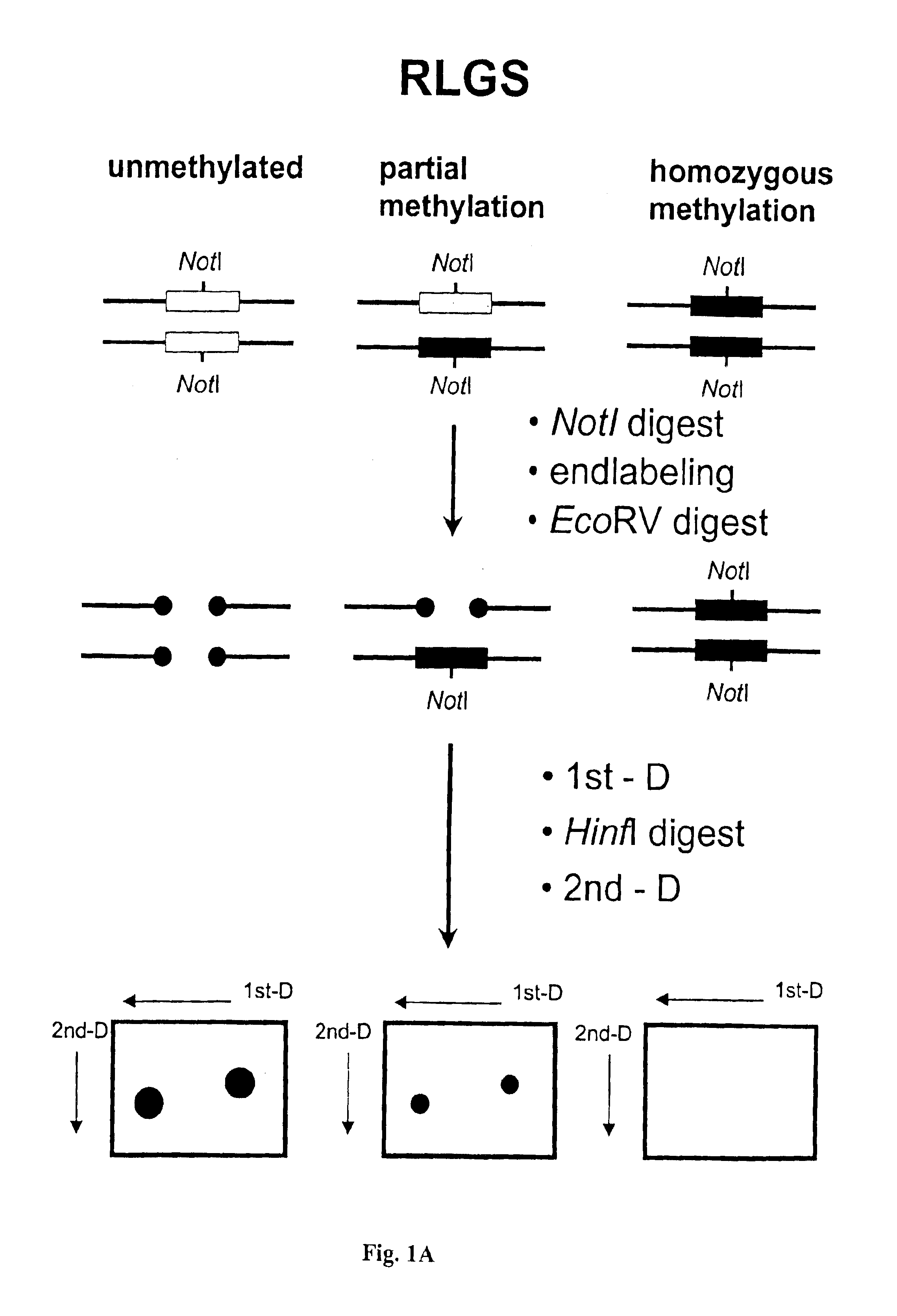
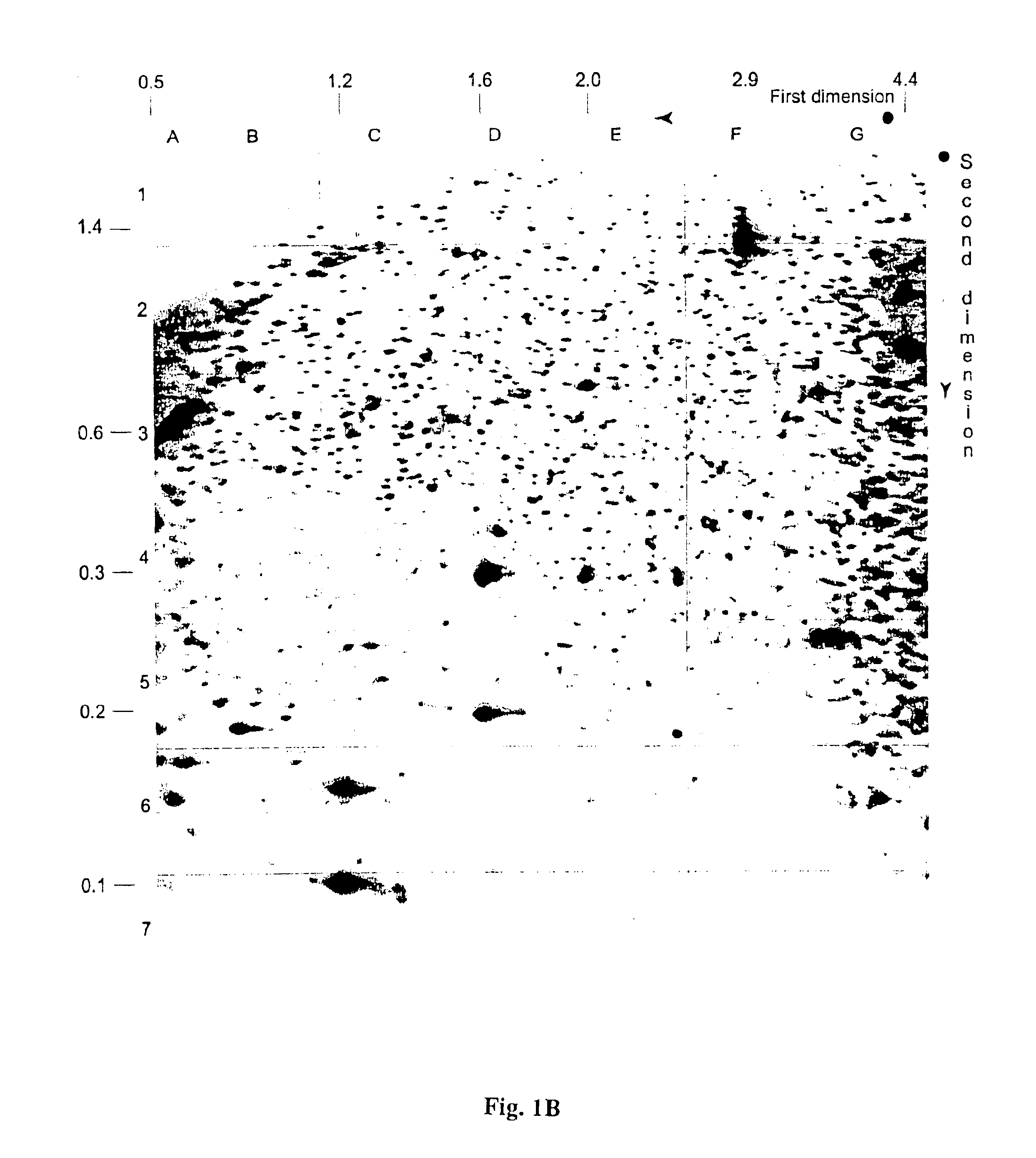
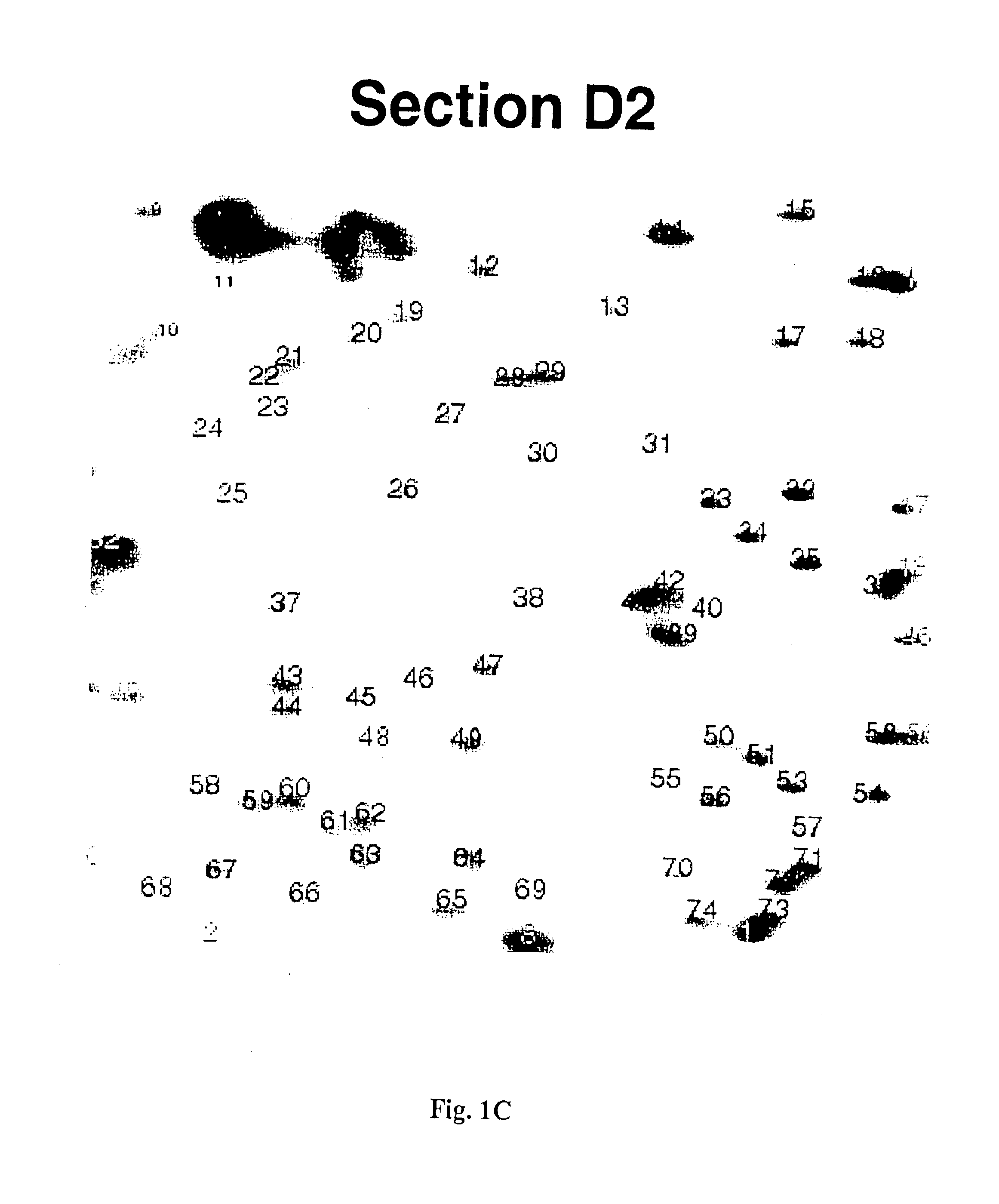
Detection of methylated CpG rich sequences diagnostic for malignant cells
InactiveUS6893820B1Microbiological testing/measurementMaterial analysis by electric/magnetic meansAbnormal tissue growthCancer cell
The present invention provides methods for determining the methylation status of CpG-containing dinucleotides on a genome-wide scale using infrequent cleaving, methylation sensitive restriction endonucleases and two-dimensional gel electrophoretic display of the resulting DNA fragments. Such methods can be used to diagnose cancer, classify tumors and provide prognoses for cancer patients. The present invention also provides isolated polynucleotides and oligonucleotides comprising CpG dinucleotides that are differentially methylated in malignant cells as compared to normal, non-malignant cells. Such polynucleotides and oligonucleotides are useful for diagnosis of cancer. The present invention also provides methods for identifying new DNA clones within a library that contain specific CpG dinucleotides that are differentially methylated in cancer cells as compared to normal cells.
Owner:THE OHIO STATE UNIV RES FOUND
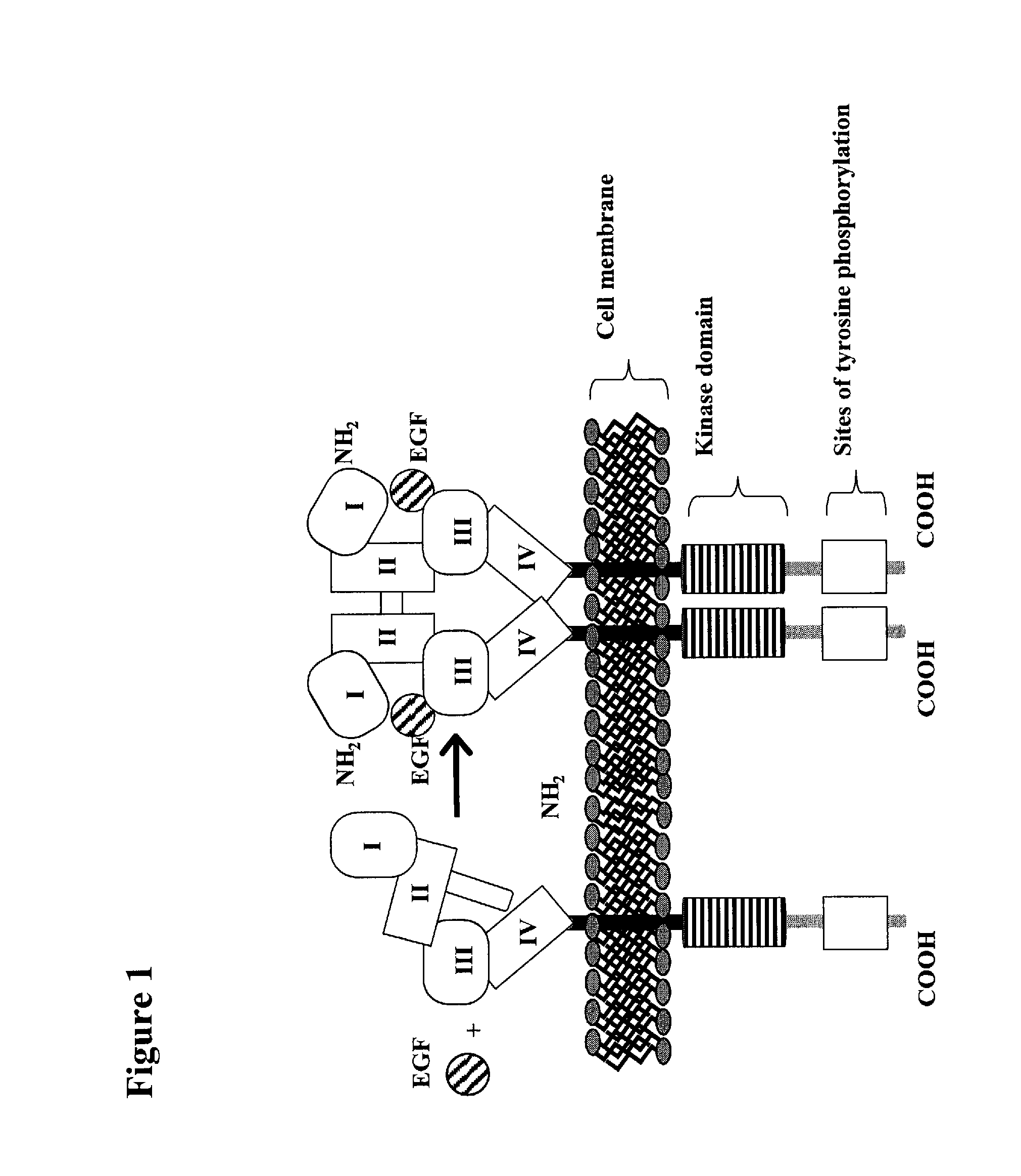
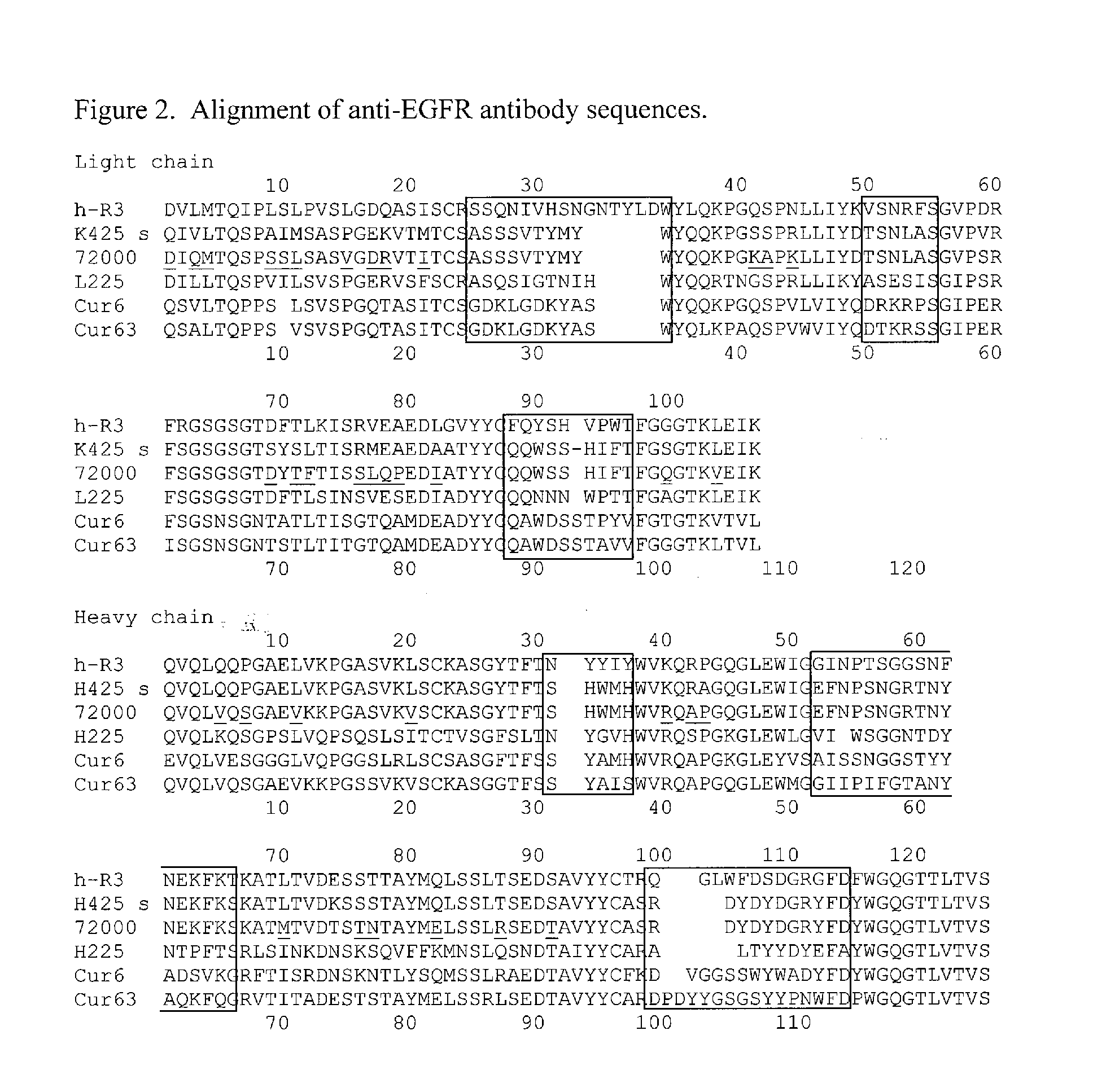
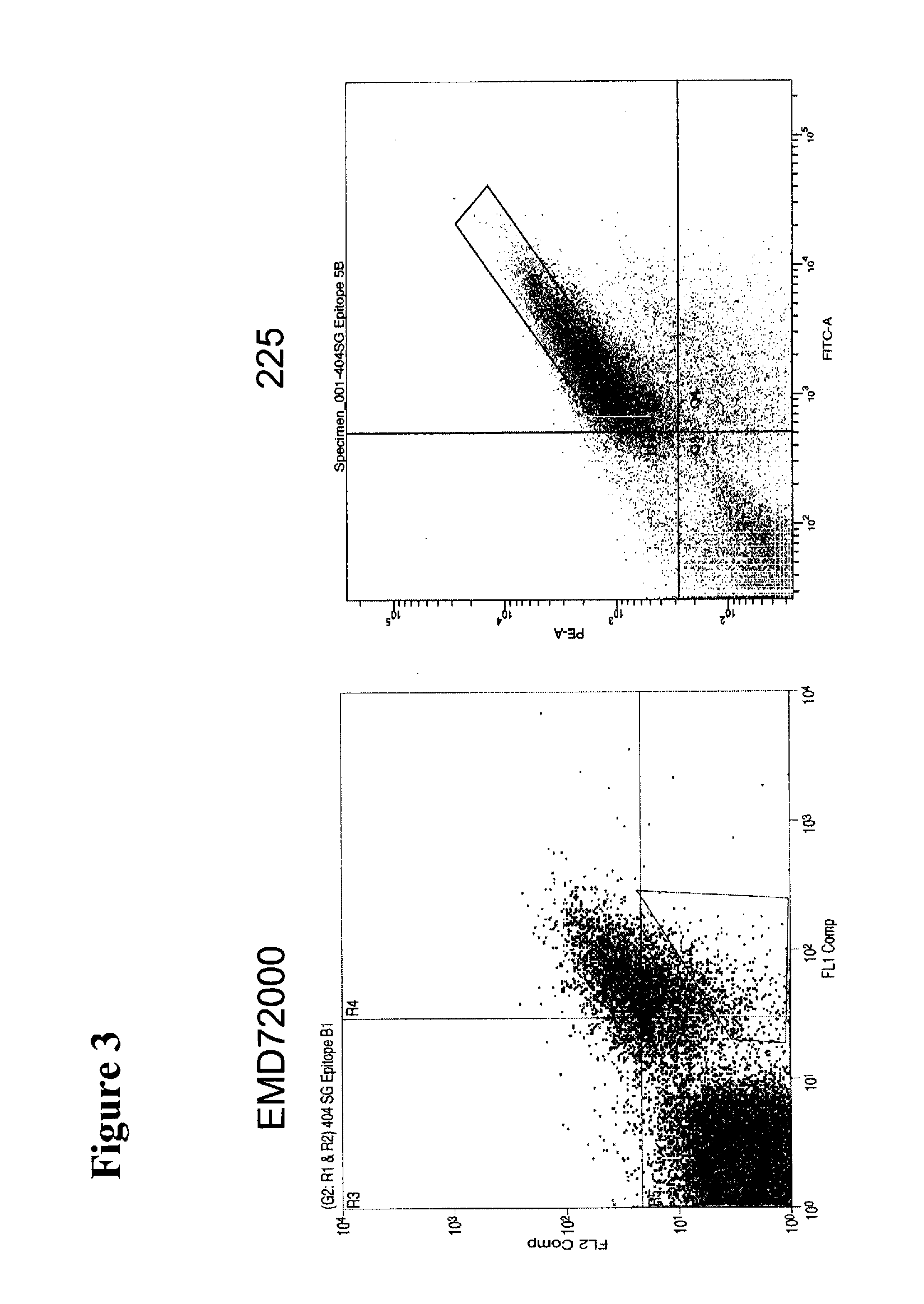
Treatment Of Tumors Expressing Mutant EGF Receptors
InactiveUS20090311803A1Reduces and prevents signalingShrink tumorAntibody ingredientsImmunoglobulinsAntibodyEGF Receptors
The invention discloses methods for identifying antibodies that reduce or prevent signaling by intact epidermal growth factor receptor (EGFR), or mutant EGFRs, such as EGFRvIII.
Owner:WAY JEFFREY C +4
Adjuvant theraphy of G250-expressing tumors
ActiveUS7691375B2Immunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsRenal clear cell carcinomaOncology
The invention relates to a method for the treatment of G250-antigen-expressing tumors, in particular renal clear cell carcinoma comprising the administration of G250-antigen-specific antibodies to high-risk patients diagnosed with non-metastasizing disease.
Owner:WILEX AG
Methods for treatment of cancer or neoplastic disease and for inhibiting growth of cancer cells and neoplastic cells
The present invention provides methods for treating or preventing cancer or neoplastic disease comprising administering to a patient a compound having the features of a pharmacophore for human anti-apotptotic Bcl protein inhibitors or identified by the in vitro methods for identifying anti-apotptotic-Bcl protein inhibitors. Also disclosed are methods for inhibiting the growth of a cancer cell or a neoplastic cell, comprising contacting the cancer cell or neoplastic cell with a compound having the features of a pharmacophore for human anti-apoptotic-Bcl protein inhibitors.
Owner:GEMIN X BIOTECHNOLOGIES INC
Method of assessing metastatic carcinomas from circulating endothelial cells and disseminated tumor cells
InactiveUS20090191535A1Microbiological testing/measurementDead animal preservationCirculating endothelial cellPrimary breast cancer
A method for assessing cancer in test subjects is described based upon enumeration of circulating endothelial cells and / or disseminated tumor cells in a test subject. This method is used to quantify disseminated tumor cells. Correlations with circulating tumor cells provides prognostic information with high accuracy in assessing the risk of recurrence in patients with primary breast cancer.
Owner:JANSSEN DIAGNOSTICS LLC
Device for biopsy of tumors
InactiveUS7311672B2Avoid destructionReduce dispersionSurgical needlesVaccination/ovulation diagnosticsAbnormal tissue growthCombined use
A device and method of use for securing and coring of tumors within the body during a biopsy of the tumor, specifically breast tumors. An adhesion probe for securing the tumor is described. The probe secures the tumor by piercing the tumor and providing a coolant to the distal tip to cool the tip. The cooled tip adheres to the tumor. A coring instrument adapted for cutting a core sample of the tumor is described. The instrument is provided with a cannula that can cut a core sample of the tumor. The instrument is adapted for use with the probe with the probe fitting within the cannula. The instrument can be used in conjunction with the probe to secure and core a sample of the tumor for biopsy.
Owner:SCION MEDICAL
Cancer Metastasis Inhibitor
InactiveUS20120183539A1Promoted angiogenesisPromoted tumor growthImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsHematopoietic cellLymphatic Spread
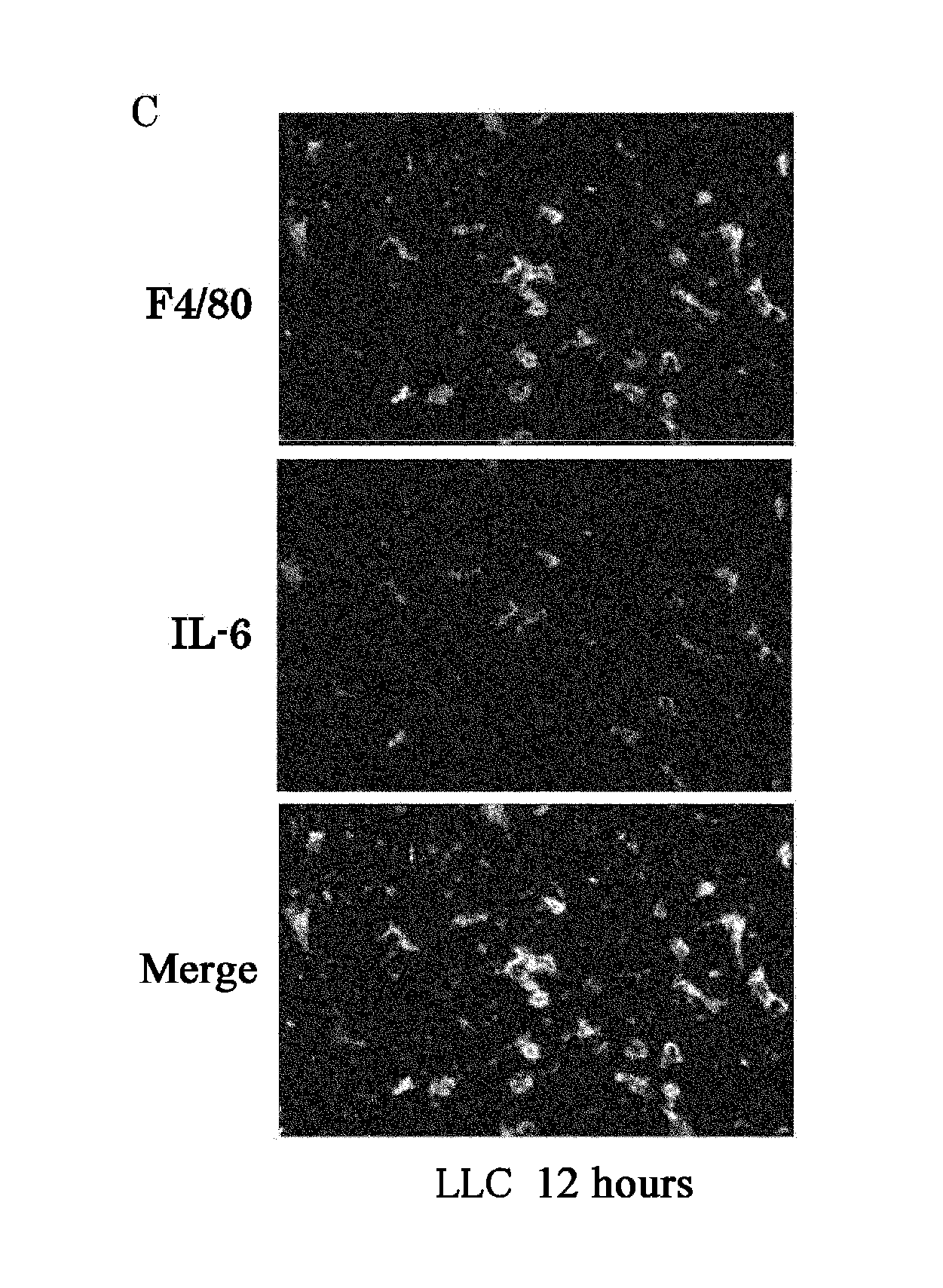
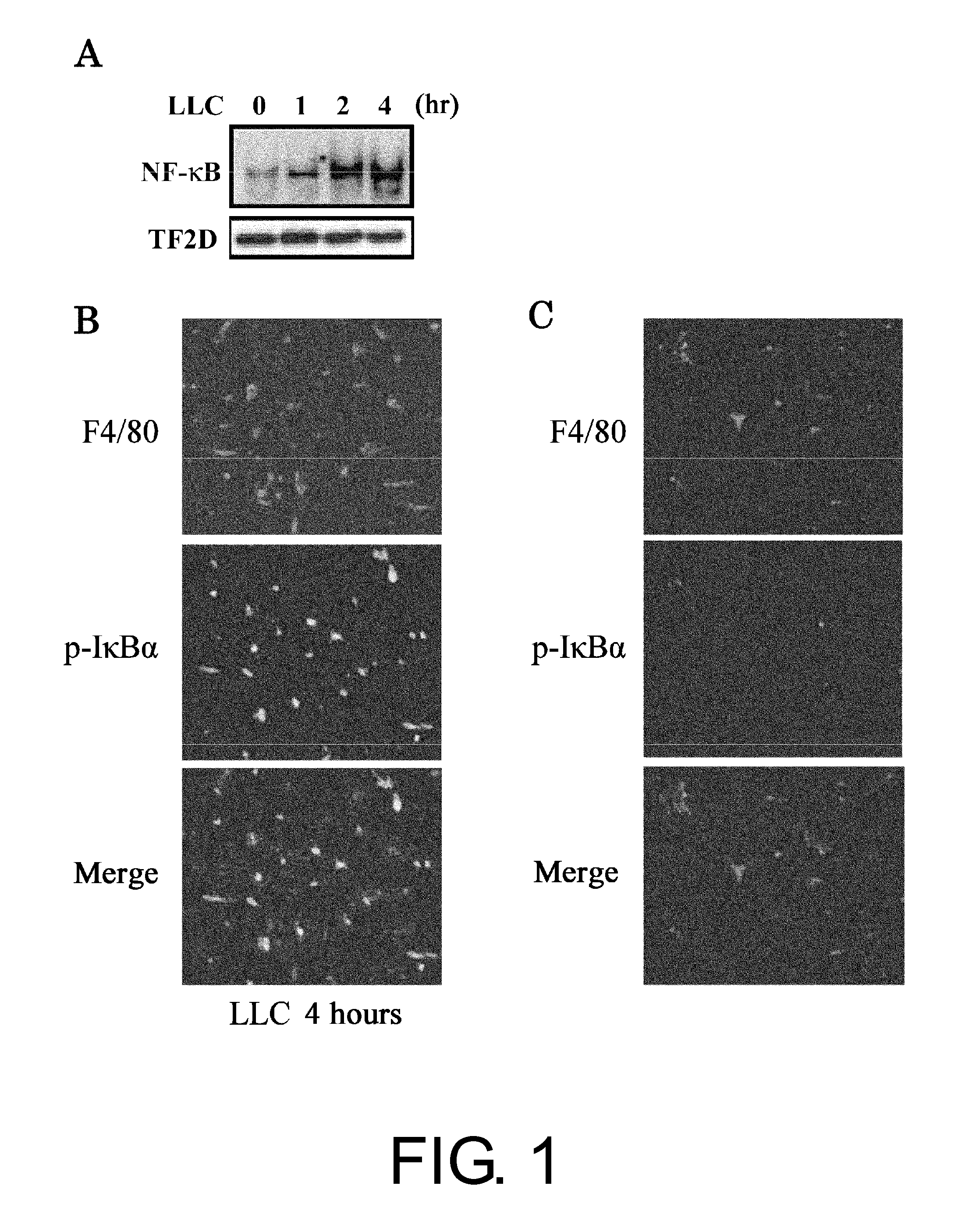
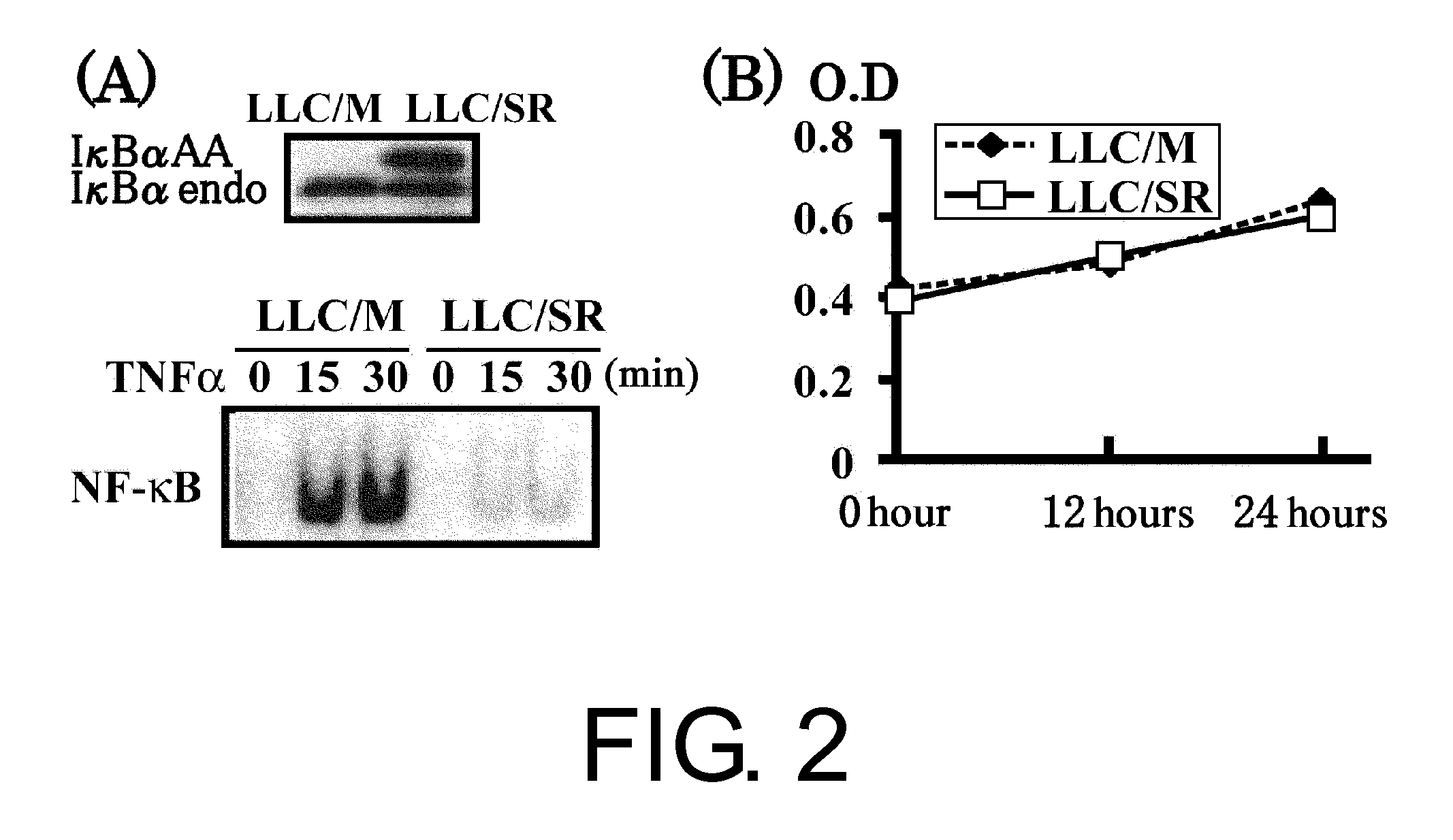
The present inventors used a model of intrasplenically induced liver metastasis to determine whether or not NF-κB activation in the liver is involved in the onset of metastatic tumors. When IKKβ was deleted from both liver cells and hematopoietically-derived cells, the onset of tumors was reduced remarkably. Tumor cells activated neighboring bone marrow cells (Kupffer cells) and produced mitogens such as interleukin (IL)-6, and this promoted angiogenesis and growth of tumors. The mitogen production depended on NF-κB in hematopoietically-derived Kupffer cells. Furthermore, treatment with an anti-IL-6 receptor antibody decreased the degree of metastatic tumor development. That is, the present inventors showed that tumor metastasis depends on inflammation, and proinflammatory intervention that targets Kupffer cells is useful for chemical prevention of metastatic tumors. Furthermore, it was shown that inhibition of the IKKβ / NF-κB signal transduction pathway, in particular IL-6 inhibition, can be utilized for anti-metastasis agents.
Owner:MAEDA CORPORATION
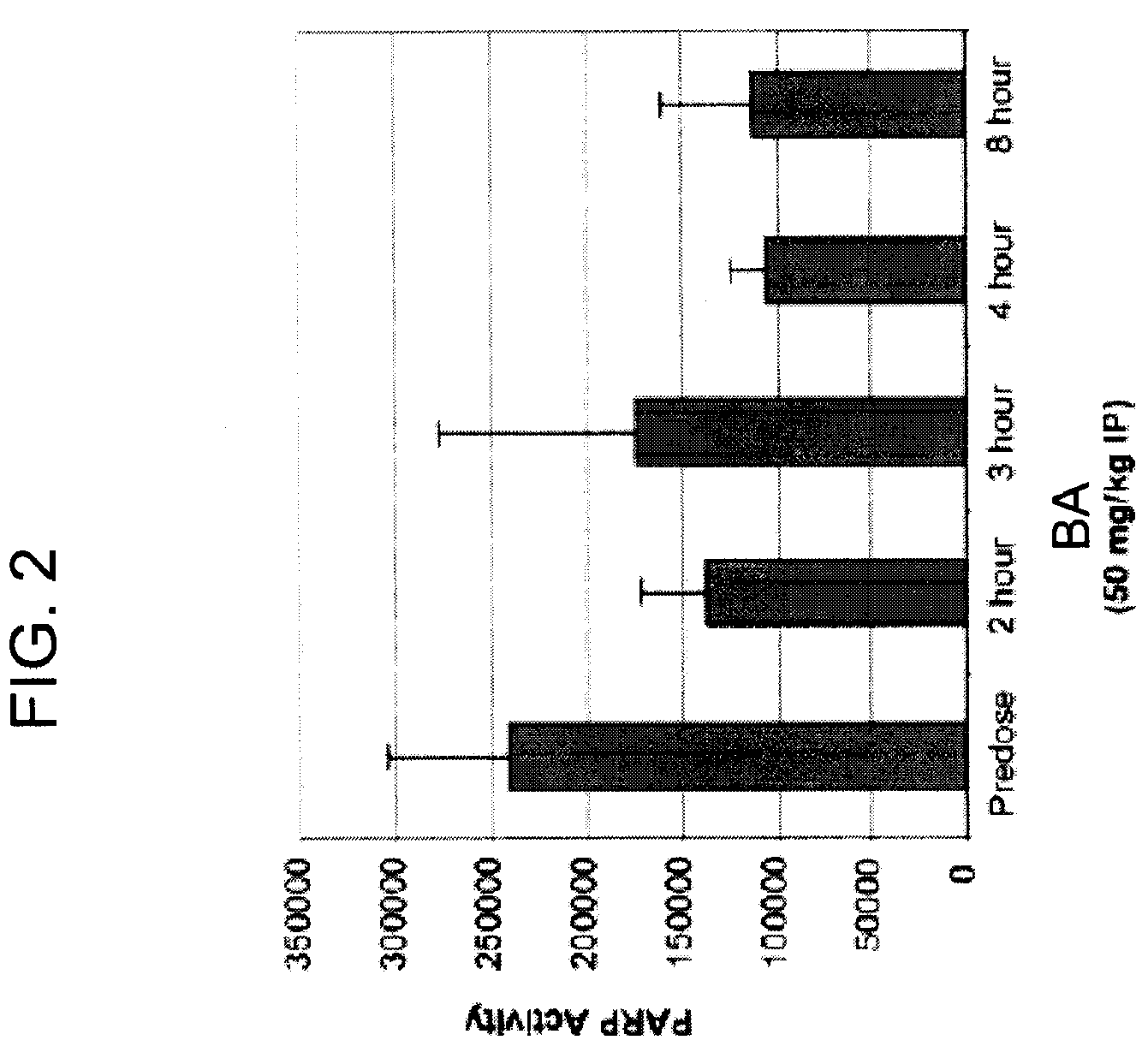
Treatment of uterine cancer and ovarian cancer with a parp inhibitor alone or in combination with Anti-tumor agents
InactiveUS20090123419A1Small sizeImprovement of clinical benefit rateBiocideOrganic active ingredientsPARP inhibitorOvarian cancer
In one aspect, the present invention provides a method of treating uterine cancer, endometrial cancer, or ovarian cancer, comprising administering to a subject at least one PARP inhibitor. In another aspect, the present invention provides a method of treating uterine cancer, endometrial cancer, or ovarian cancer, comprising administering to a subject at least one PARP inhibitor in combination with at least one anti-tumor agent.
Owner:BIPAR SCI INC
Therapeutic and Diagnostic Methods and Compositions Targeting 4Ig-B7-H3 and its Counterpart Nk Cell Receptor
ActiveUS20080081346A1Accurate detectionInhibition effectAntipyreticAntibody mimetics/scaffoldsNatural Killer Cell Inhibitory ReceptorsNK Cell Receptors
The present invention relates to the identification of 4Ig-B7-H3 protein as a tumor associated molecule that imparts protection from NK cell-mediated lysis via a 4Ig-B7-H3 receptor on NK cells. The invention provides compounds that interfere with interactions between the 4Ig-B7-H3 protein and its receptor that can be used to potentiate NK cell cytotoxicity. Also provided are compounds that bind 4Ig-B7-H3-expressing cells so as to inhibit or eliminate them. The compounds are particularly useful in the treatment of tumors, inflammatory conditions, infections and transplantation. Also provided are methods for diagnosing disease by detecting a 4Ig-B7-H3 protein.
Owner:INNATE PHARMA SA +1
Methods of diagnosing or treating prostate cancer using the erg gene, alone or in combination with other over or under expressed genes in prostate cancer
ActiveUS20090170075A1Microbiological testing/measurementImmunoglobulins against cell receptors/antigens/surface-determinantsProstate cancer cellOncology
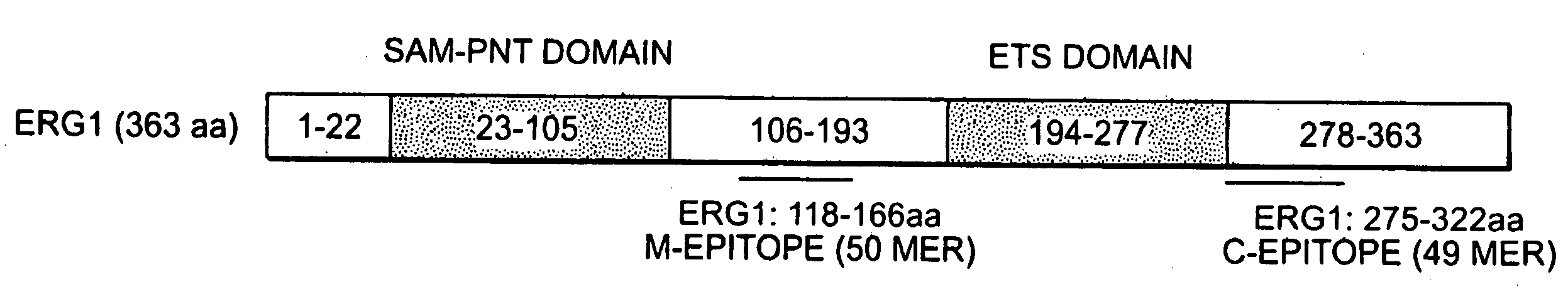
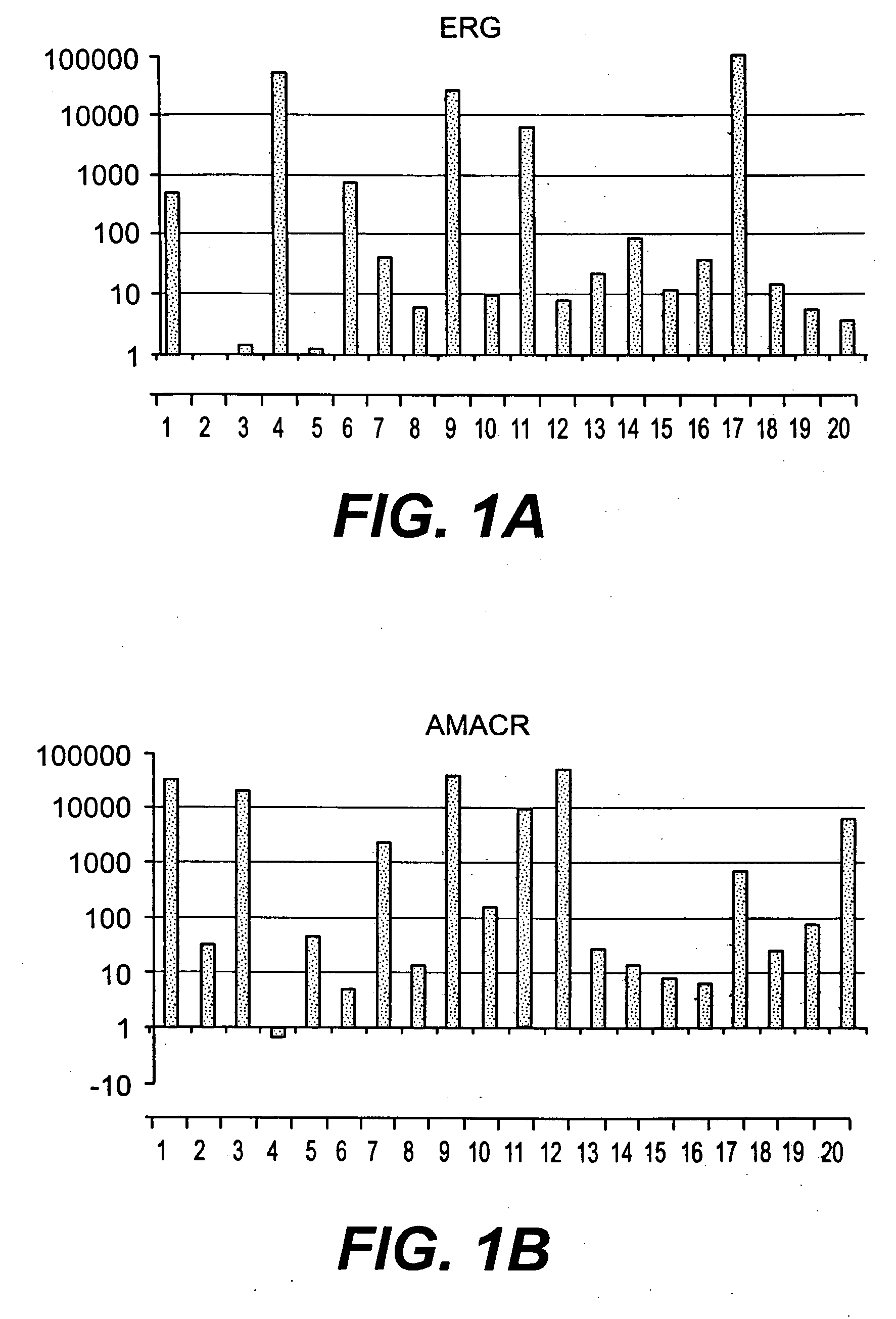
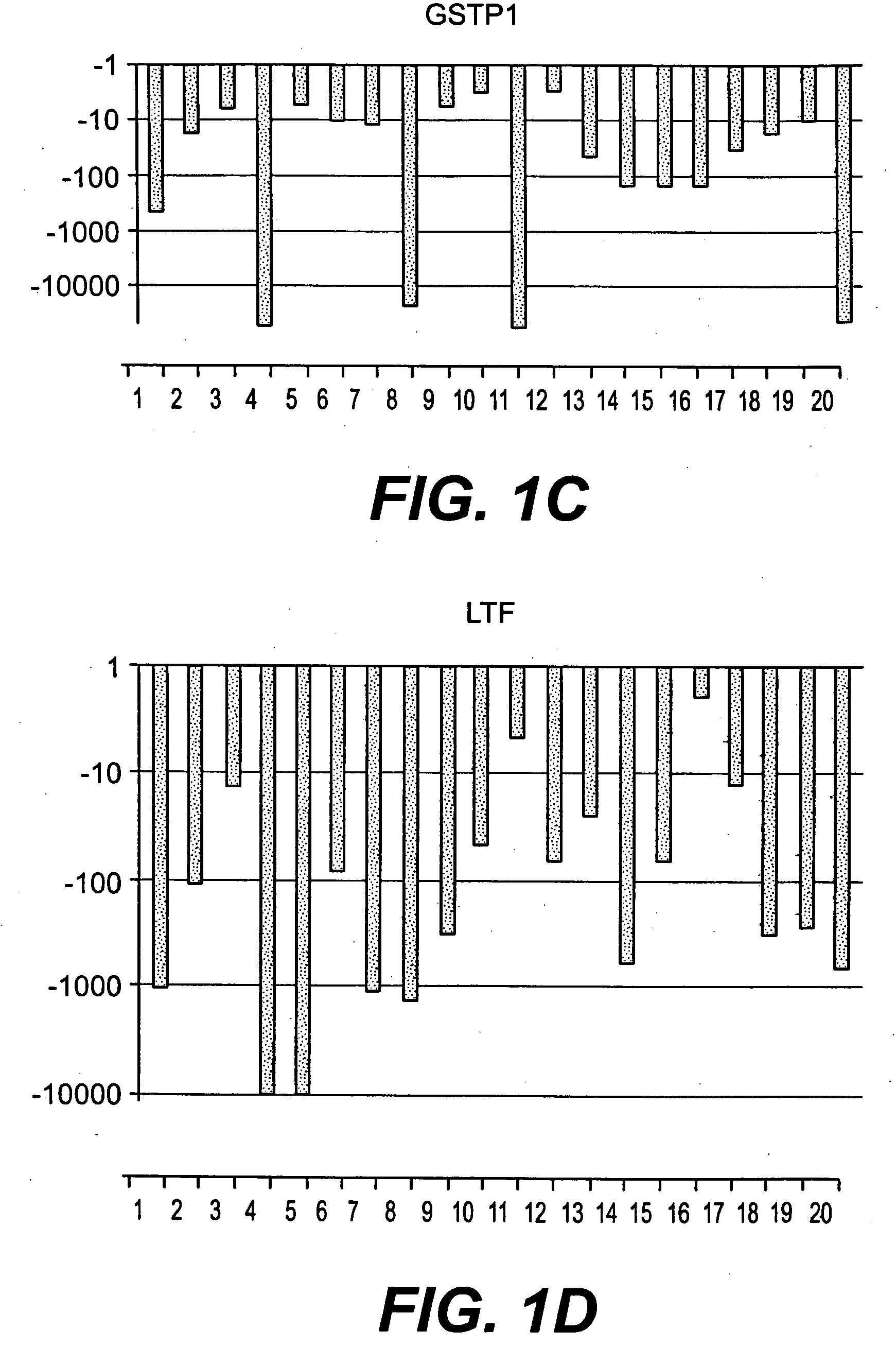
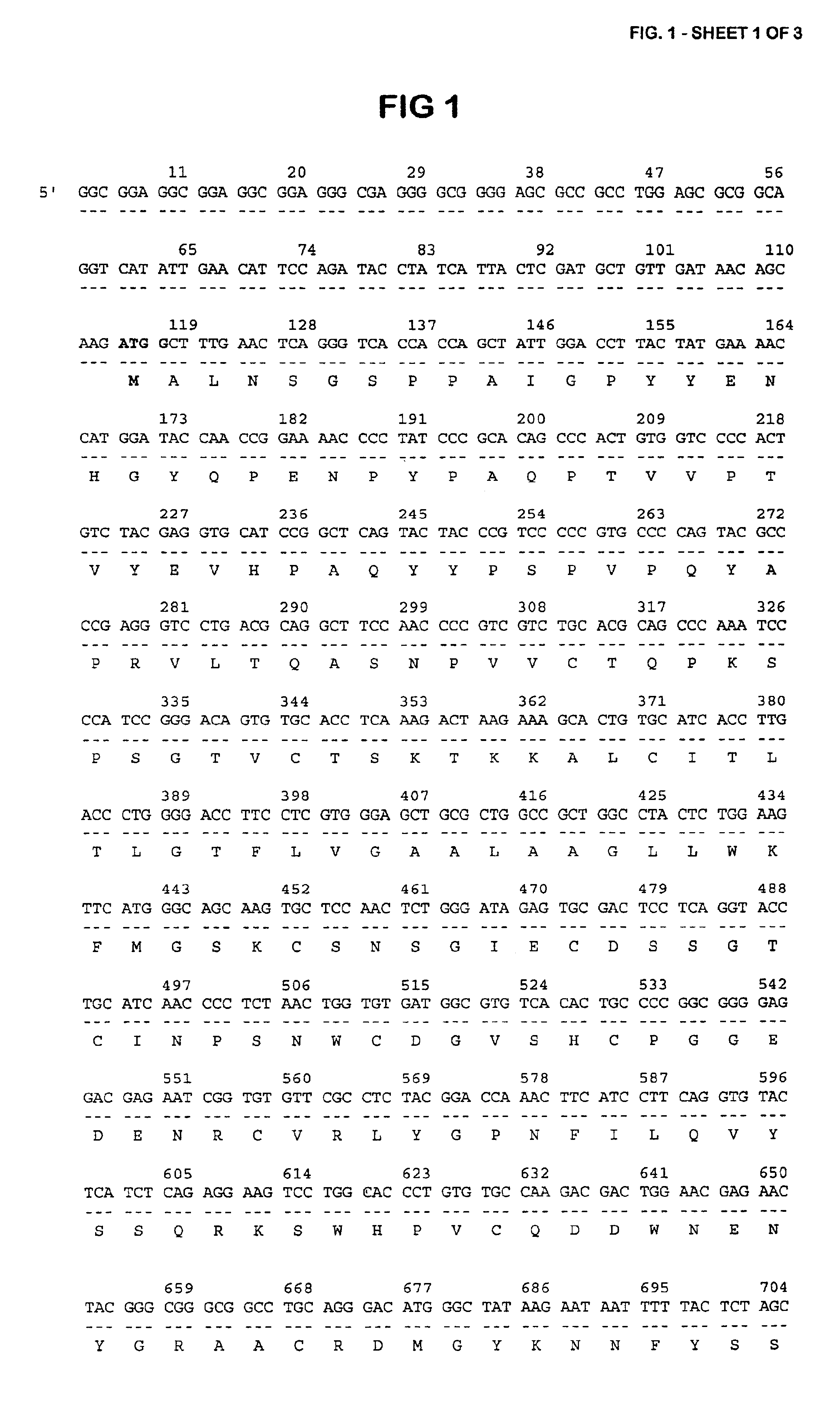
The present invention relates to oncogenes or tumor suppressor genes, as well as other genes, involved in prostate cancer and their expression products, as well as derivatives and analogs thereof. Provided are therapeutic compositions and methods of detecting and treating cancer, including prostate and other related cancers. Also provided are methods of diagnosing and / or prognosing prostate cancer by determining the expression level of at least one prostate cancer-cell-specific gene, including, for example, the ERG gene or the LTF gene alone, or in combination with at least one of the AMACR gene and the DD3 gene.
Owner:THE HENRY M JACKSON FOUND FOR THE ADVANCEMENT OF MILITARY MEDICINE INC
Tumor antigen useful in diagnosis and therapy of prostate and colon cancer
InactiveUS7037667B1Microbiological testing/measurementBiological material analysisCell membraneTumor antigen
Owner:AGENSYS
Antitumor agent for thyroid cancer
The objective of the present invention is to provide a pharmaceutical composition and a therapeutic method that are specifically effective against at least one disease selected from multiple endocrine neoplasia, type IIA, multiple endocrine neoplasia, type IIB, familial medullary thyroid carcinoma, thyroid carcinoma, papillary thyroid carcinoma, sporadic medullary thyroid carcinoma, Hirschsprung disease, pheochromocytoma, parathyroid hyperplasia and mucosal neuromas of the gastrointestinal tract.4-(3-chloro-4-(cyclopropylaminocarbonyl)aminophenoxy)-7-methoxy-6-quinolinecarboxamide and analogs thereof are specifically effective against at least one disease selected from multiple endocrine neoplasia, type IIA, multiple endocrine neoplasia, type IIB, familial medullary thyroid carcinoma, thyroid carcinoma, papillary thyroid carcinoma, sporadic medullary thyroid carcinoma, Hirschsprung disease, pheochromocytoma, parathyroid hyperplasia and mucosal neuromas of the gastrointestinal tract.
Owner:EISIA R&D MANAGEMENT CO LTD
Gene expression profiling in primary ovarian serous papillary tumors and normal ovarian epithelium
InactiveUS20060078941A1Highligthing the divergence of gene expressionMicrobiological testing/measurementBiological testingKallikrein-10Gene family
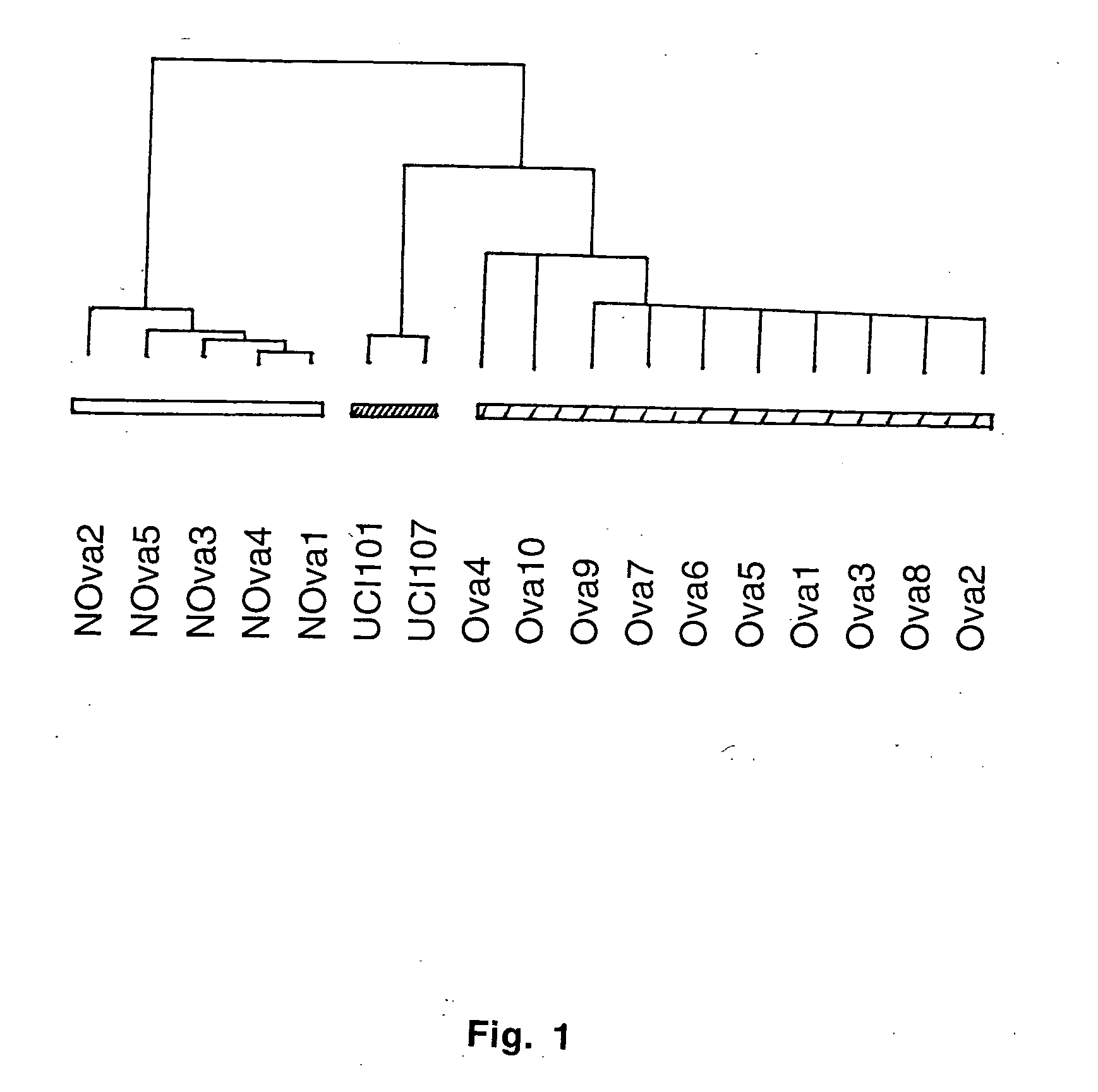
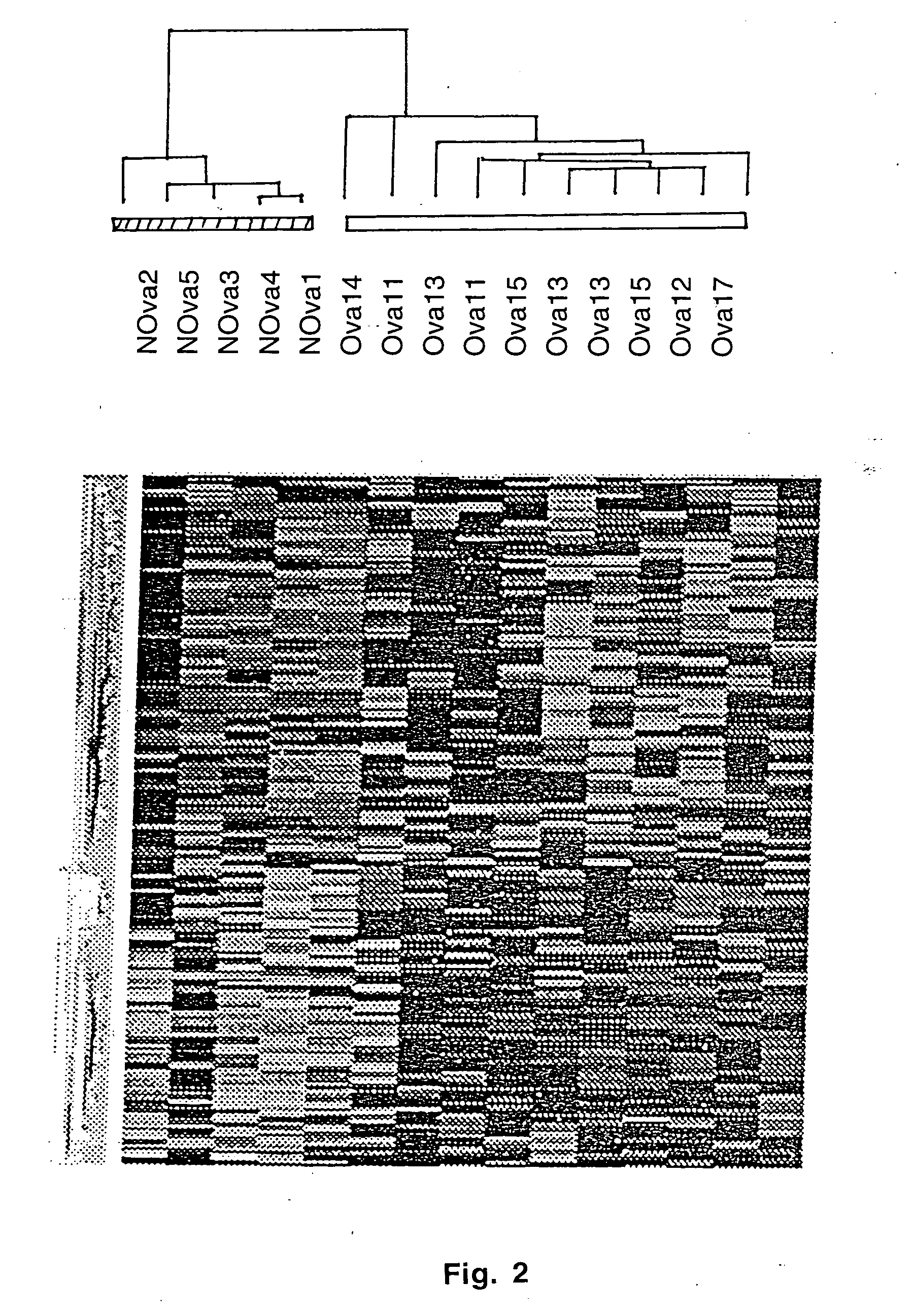
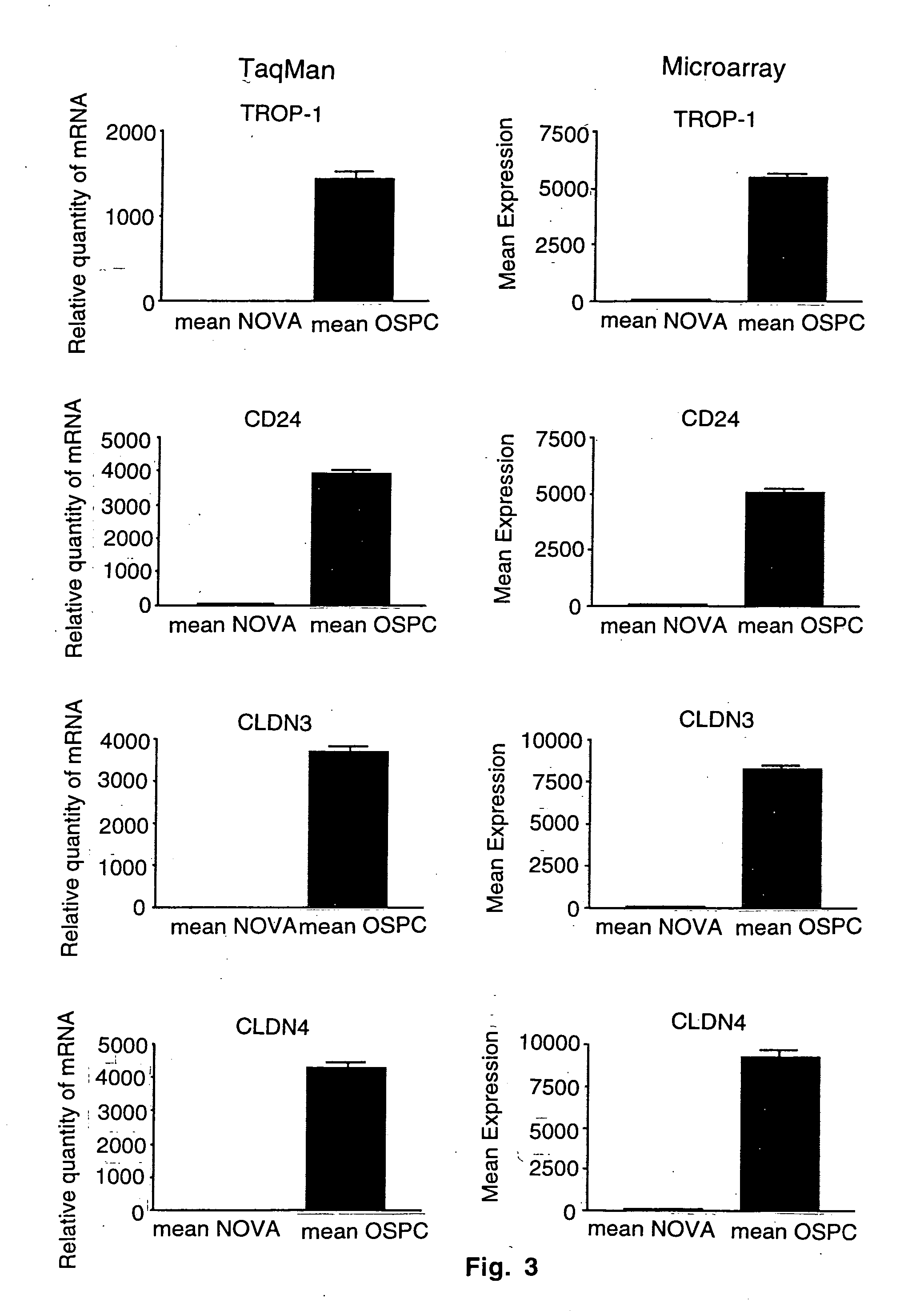
Gene expression profiling and hierarchial clustering analysis readily distinguish normal ovarian epithelial cells from primary ovarian serous papillary carcinomas. Laminin, tumor-associated calcium signal transducer 1 and 2 (TROP-1 / Ep-CAM; TROP-2), claudin 3, claudin 4, ladinin 1, S100A2, SERPIN2 (PAI-2), CD24, lipocalin 2, osteopontin, kallikrein 6 (protease M), kallikrein 10, matriptase and stratifin were found among the most highly overexpressed genes in ovarian serous papillary carcinomas, whereas transforming growth factor beta receptor III, platelet-derived growth factor receptor alpha, SEMACAP3, ras homolog gene family, member I (ARHI), thrombospondin 2 and disabled-2 / differentially expressed in ovarian carcinoma 2 (Dab2 / DOC2) were significantly down-regulated. Therapeutic strategy targeting TROP-1 / Ep-CAM by monoclonal chimeric / humanized antibodies may be beneficial in patients harboring chemotherapy-resistant ovarian serous papillary carcinomas. Claudin-3 and claudin-4 being receptors for Clostridium Perfringens enterotoxin, this toxin may be used as a novel therapeutic agent to treat ovarian serous papillary tumors.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ARKANSAS
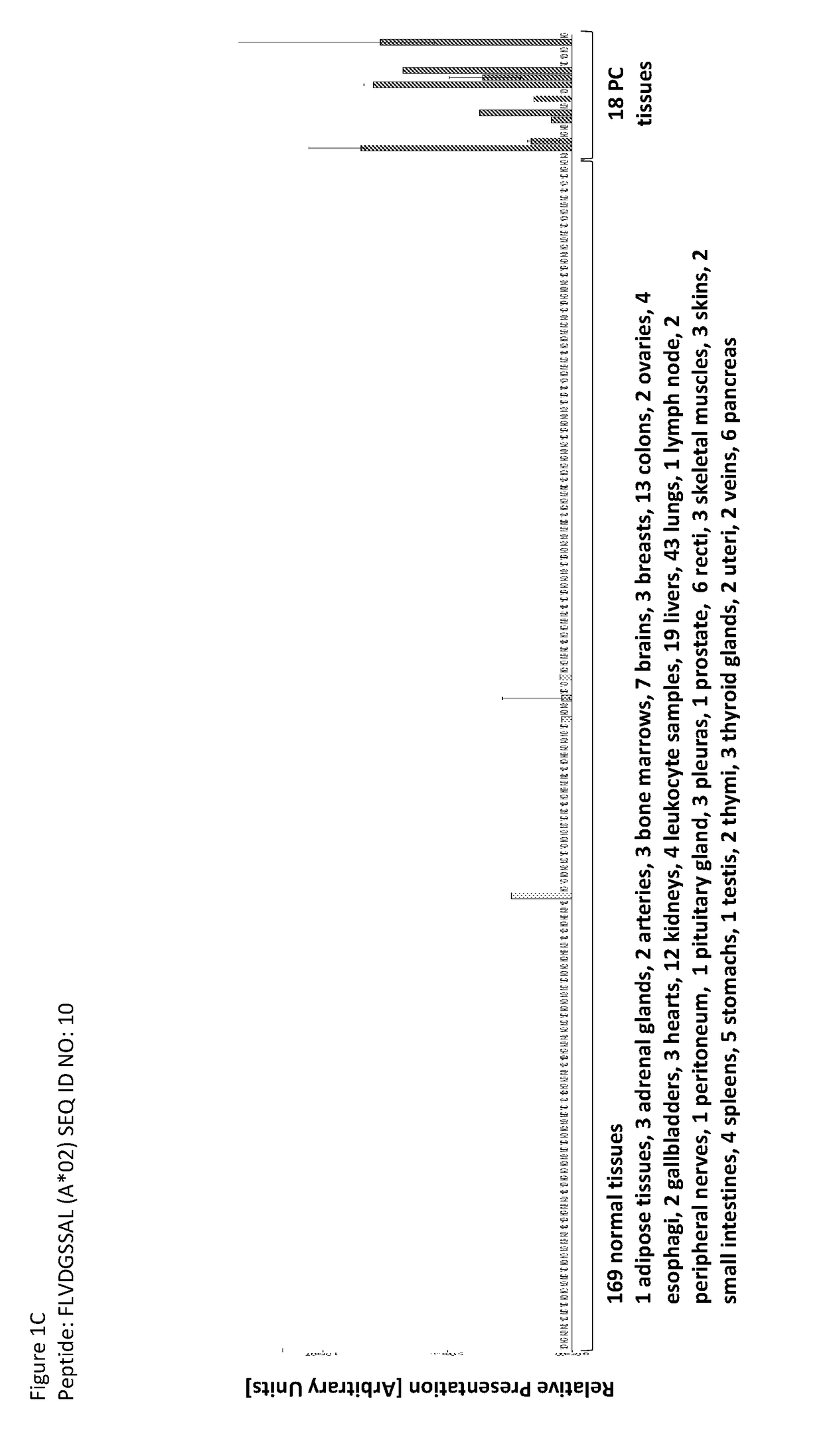
Novel peptides and combination of peptides for use in immunotherapy against pancreatic cancer and other cancers
The present invention relates to peptides, proteins, nucleic acids and cells for use in immunotherapeutic methods. In particular, the present invention relates to the immunotherapy of cancer. The present invention furthermore relates to tumor-associated T-cell peptide epitopes, alone or in combination with other tumor-associated peptides that can for example serve as active pharmaceutical ingredients of vaccine compositions that stimulate anti-tumor immune responses, or to stimulate T cells ex vivo and transfer into patients. Peptides bound to molecules of the major histocompatibility complex (MHC), or peptides as such, can also be targets of antibodies, soluble T-cell receptors, and other binding molecules.
Owner:IMMATICS BIOTECHNOLOGIES GMBH
In vitro culture method of colorectal cancer organoid
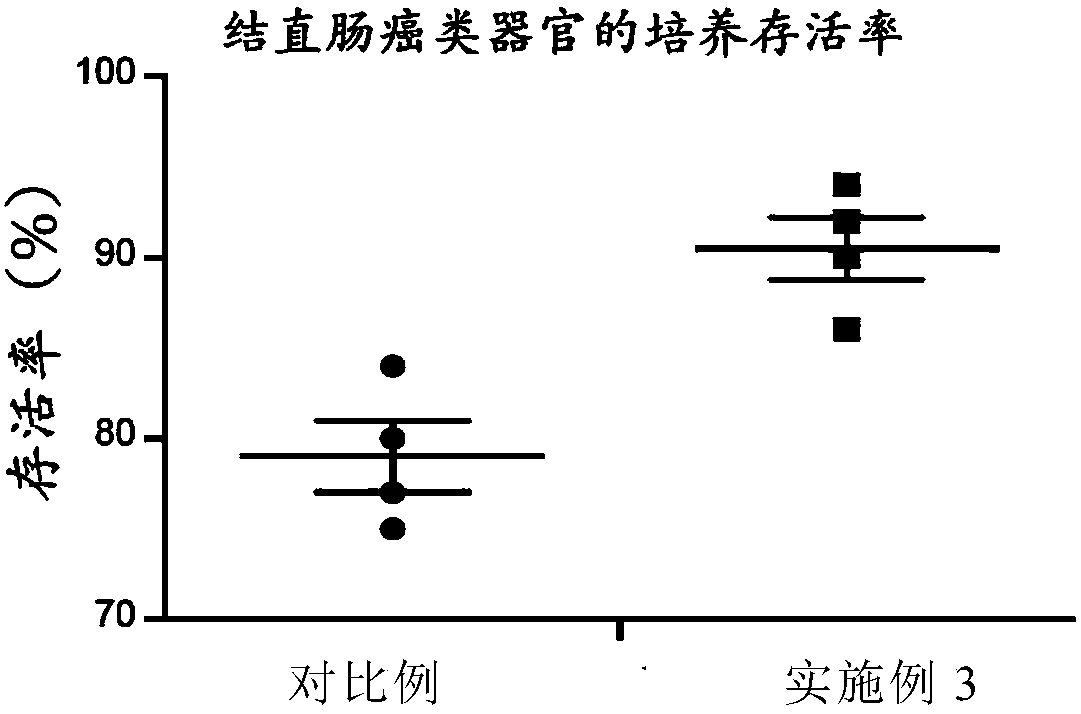
InactiveCN108396010AEffective trainingImprove the cultivation rateCell culture active agentsTumor/cancer cellsBiologyTumor tissue
The invention discloses an in vitro culture method of a colorectal cancer organoid. The in vitro culture method comprises: 1) pretreatment of colorectal cancer biopsy tissue: washing colorectal cancerbiopsy tissue, shearing, and digesting with enzyme to obtain colorectal cancer single cell; and 2) in vitro culture of the colorectal cancer single cell: mixing the colorectal cancer single cell andmatrigel, solidifying, adding a colorectal cancer organoid culture liquid, and culturing to obtain the colorectal cancer organoid. According to the present invention, the method has characteristics ofsimpleness, good stability and high success rate; and the obtained colorectal cancer organoid has a three-dimensional structure, substantially retains the unique morphological characteristics of thepatient tumor tissue, is highly consistent with the pathological features of the biopsy tissue, and provides the material basis for the establishment of the organoid library and the screening of drugs.
Owner:王琼 +1
Method for predicting progression free and overall survival at each follow-up time point during therapy of metastatic breast cancer patients using circulating tumor cells
InactiveUS20090061456A1Less side effectsImprove the quality of lifeDisease diagnosisBiological testingOncologyDisease progression
A cancer test having prognostic utility in predicting time to disease progression, overall survival, and response to therapy in patients with MBC based upon the presence and number of CTC's. The Cell Spotter® System is used to enumerate CTC's in blood. The system immunomagnetically concentrates epithelial cells, fluorescently labels the cells and identifies and quantifies CTC's. The absolute number of CTC's detected in the peripheral blood tumor load is, in part, a factor in prediction of survival, time to progression, and response to therapy. The mean time to survival of patients depended upon a threshold number of 5 CTC's per 7.5 ml of blood. Detection of CTC's in metastatic cancer represents a novel prognostic factor in patients with metastatic cancers, suggests a biological role for the presence of tumor cells in the blood, and indicates that the detection of CTC's could be considered an appropriate surrogate marker for prospective therapeutic clinical trials.
Owner:VERIDEX LCC
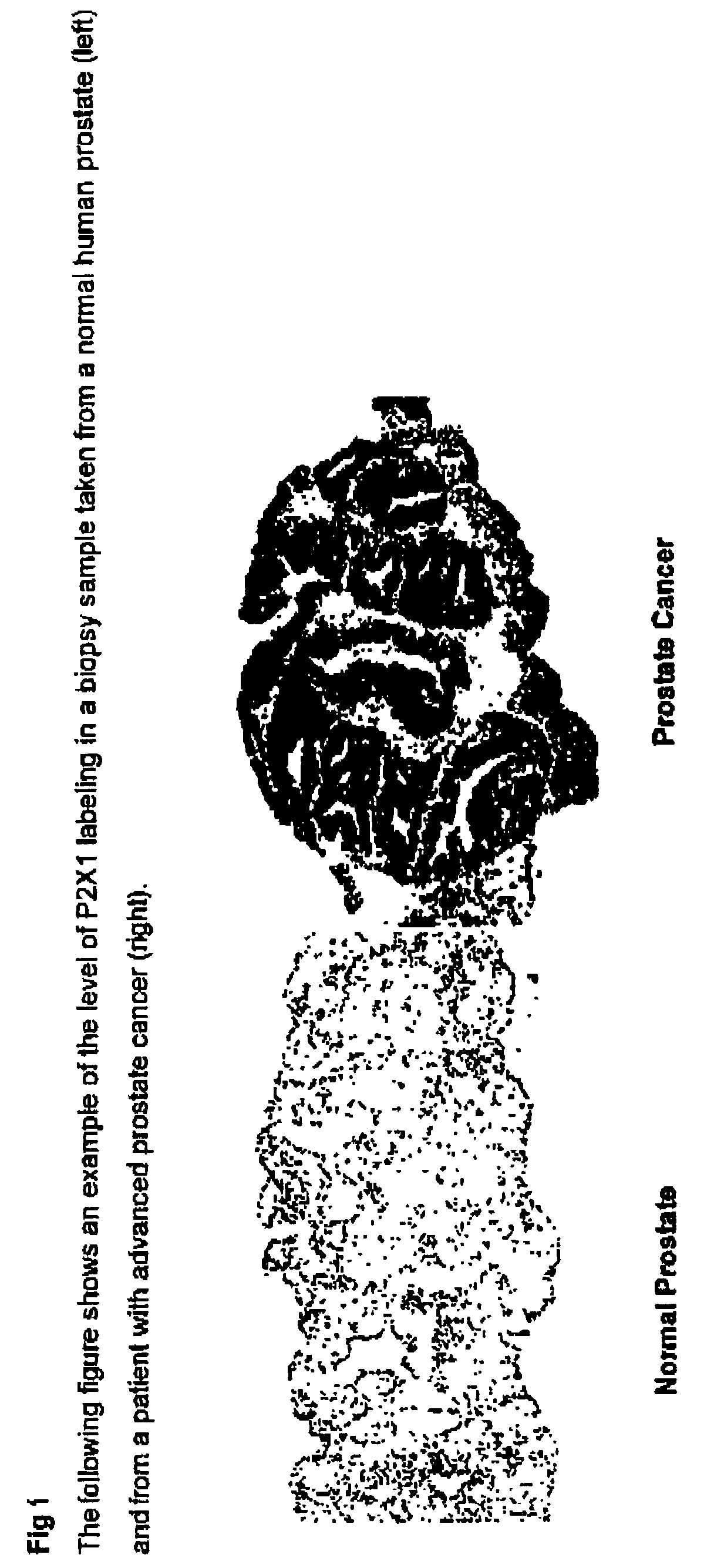
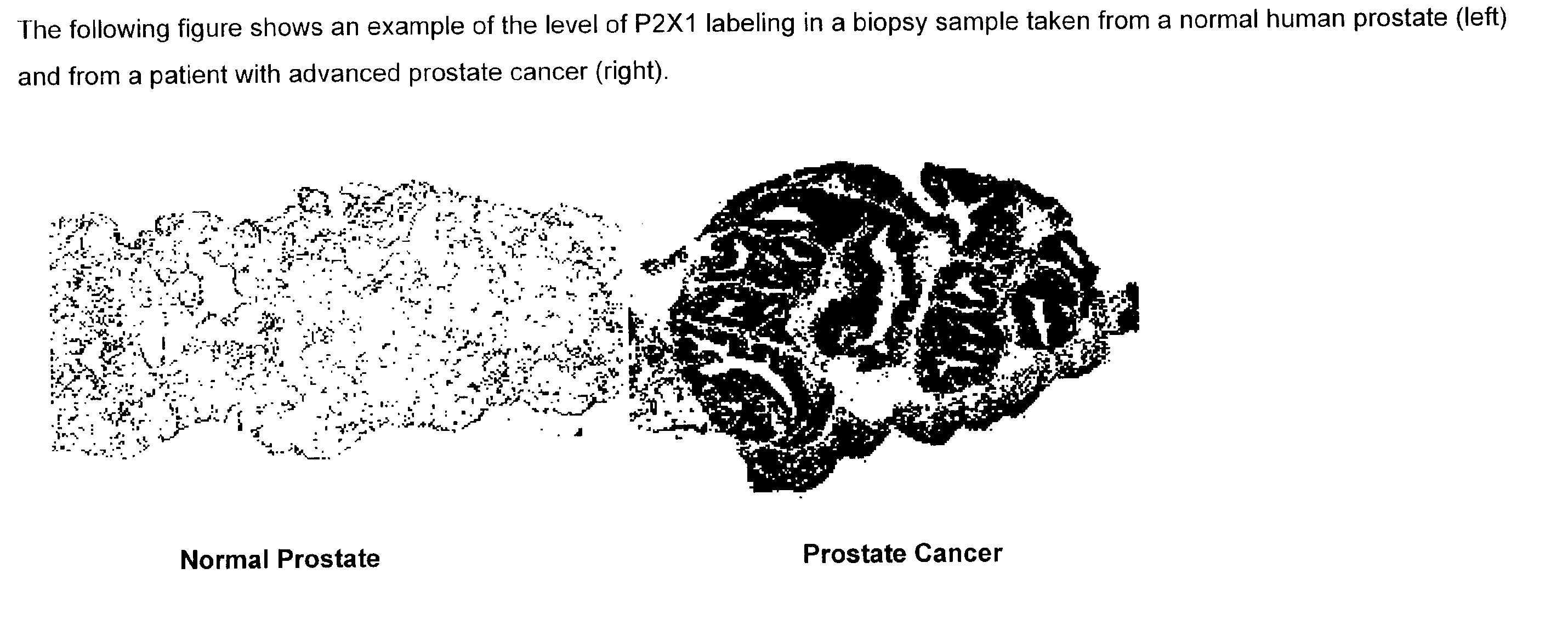
Method for identifying pre-neoplastic and/or neoplastic states in mammals
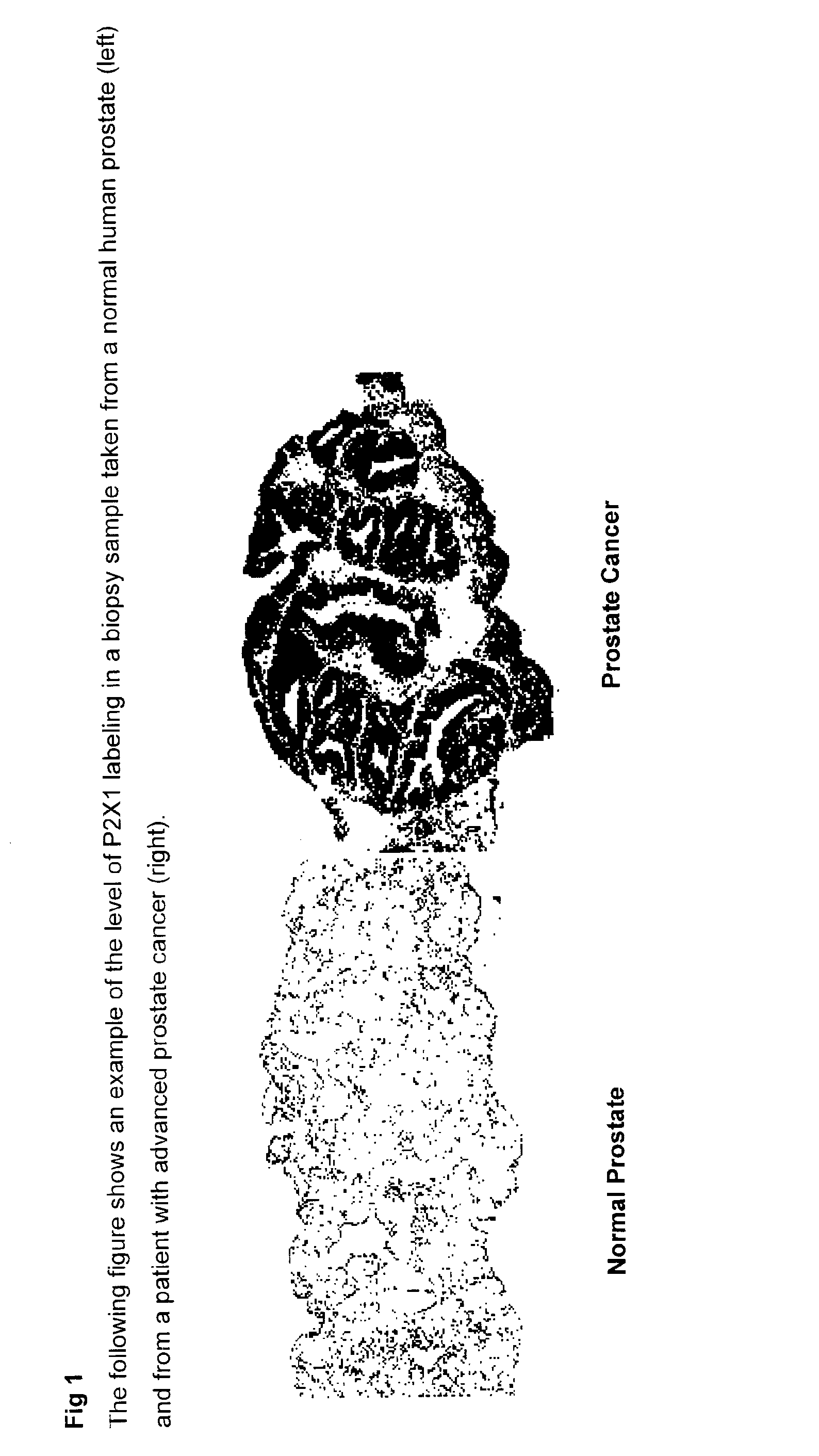
The present invention relates to methods of identifying pre-neoplastic and / or neoplastic states in mammals and in particular to a method for identifying pre-neoplastic and neoplastic cells in tissues and body fluids, based on differential expression of purinergic receptors in these cells.
Owner:BIOSCEPTRE PTY LTD
Imaging and therapy of virus-associated tumors
InactiveUS20110176998A1Easy to detectHigh expressionAntibacterial agentsInorganic boron active ingredientsAbnormal tissue growthViral infection
The present invention features compositions and methods for detecting, selecting a treatment method for, monitoring, and treating a neoplasia associated with a viral infection.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
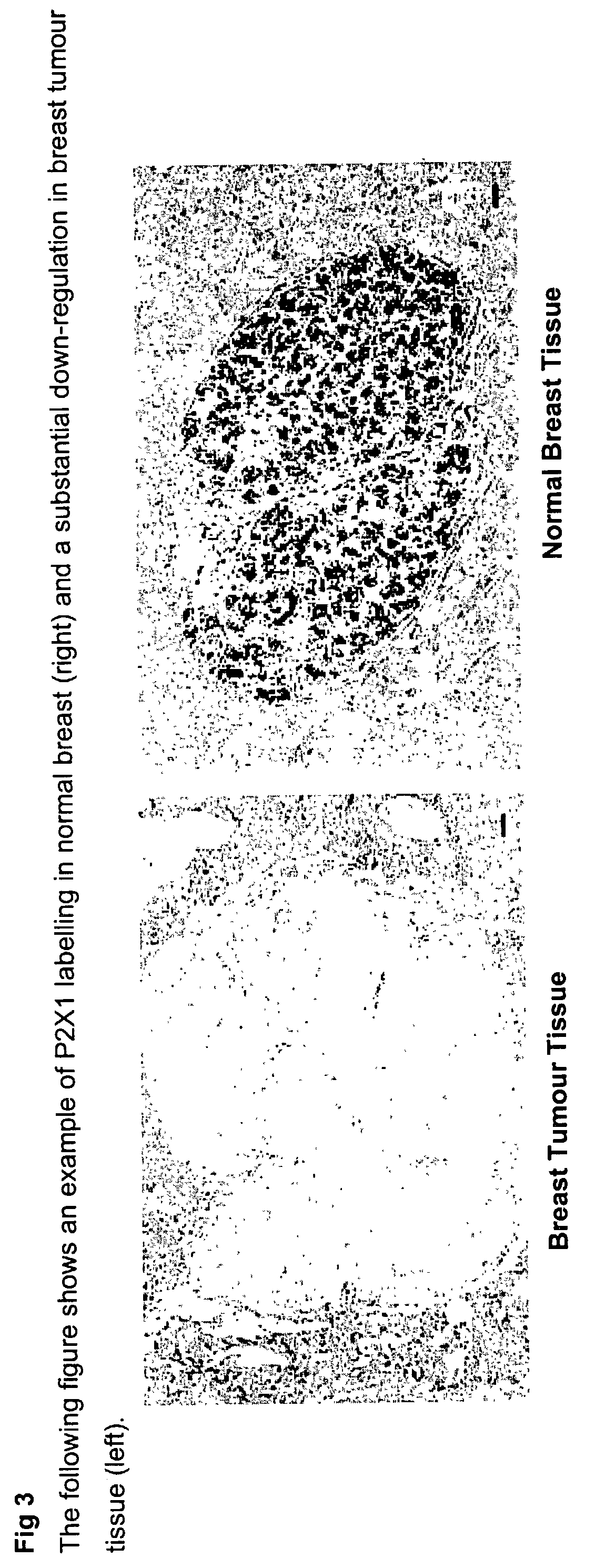
Method for identifying pre-neoplastic and/or neoplastic states in mammals
The present invention relates to methods of identifying pre-neoplastic and / or neoplastic states in mammals and in particular to a method for identifying pre-neoplastic and neoplastic cells in tissues and body fluids, based on differential expression of purinergic receptors in these cells.
Owner:BIOSCEPTRE INT
Tumor marker related to colorectal cancer and application
ActiveCN107043823AMicrobiological testing/measurementDNA/RNA fragmentationLymphatic SpreadNucleotide sequencing
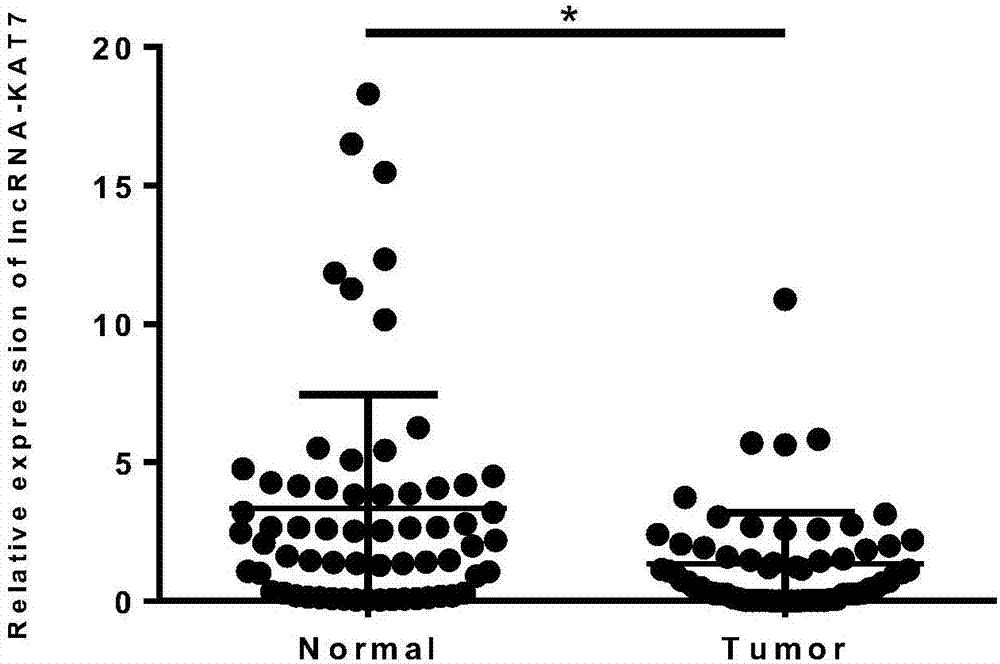
The invention discloses a tumor marker related to colorectal cancer and application. The tumor marker is lncRNA (long noncoding RNA) and is located on the seventeenth chromosome of chr17: 47785696-47797422, a nucleotide sequence of the lncRNA is shown in SEQ ID NO.1 is named as lncRNA-KAT7. According to the lncRNA-KAT7 sequence, specific real-time fluorescent quantitative PCR primer can be designed and synthesized to be used for preparing preparation for colorectal cancer diagnosis or treating effect prediction. By means of the real-time fluorescent quantitative PCR preparation, expression level of lncRNA-KAT7 is detected in colorectal cancer clinical case specimen, expression of the lncRNA-KAT7 in the colorectal cancer is found to be obviously reduced, the lncRNA-KAT7 has obvious statistic significance (p<0.05), and low expression of the lncRNA-KAT7 in the colorectal cancer is closely related to colorectal tumor differentiation, T staging, invasion and metastasis. The result shows that the lncRNA-KAT7 can serve as the tumor marker of the colorectal cancer and can be used for preparing preparation or kits applied to auxiliary diagnosis, treating effect prediction and prognosis of the colorectal cancer.
Owner:CHENZHOU NO 1 PEOPLES HOSPITAL
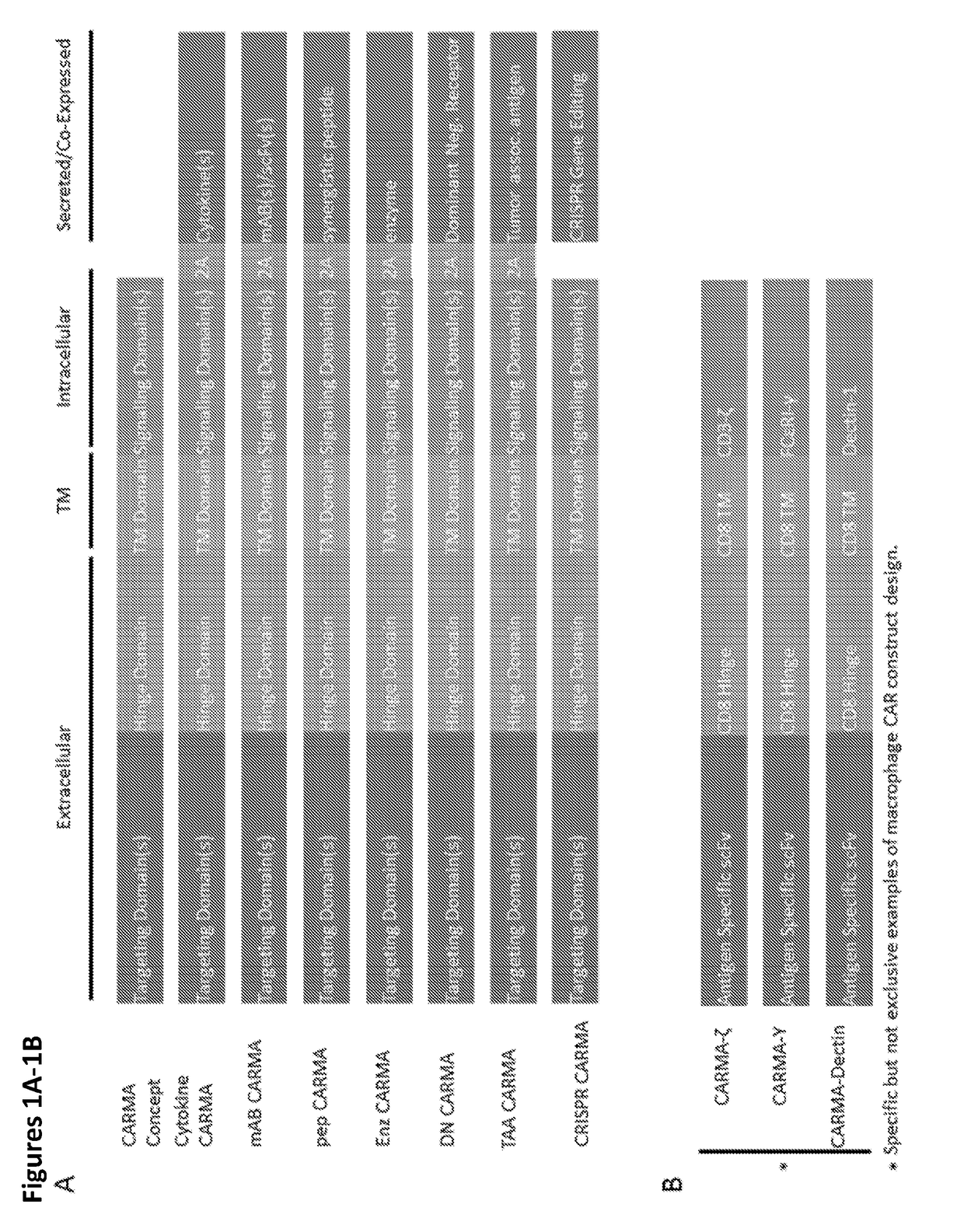
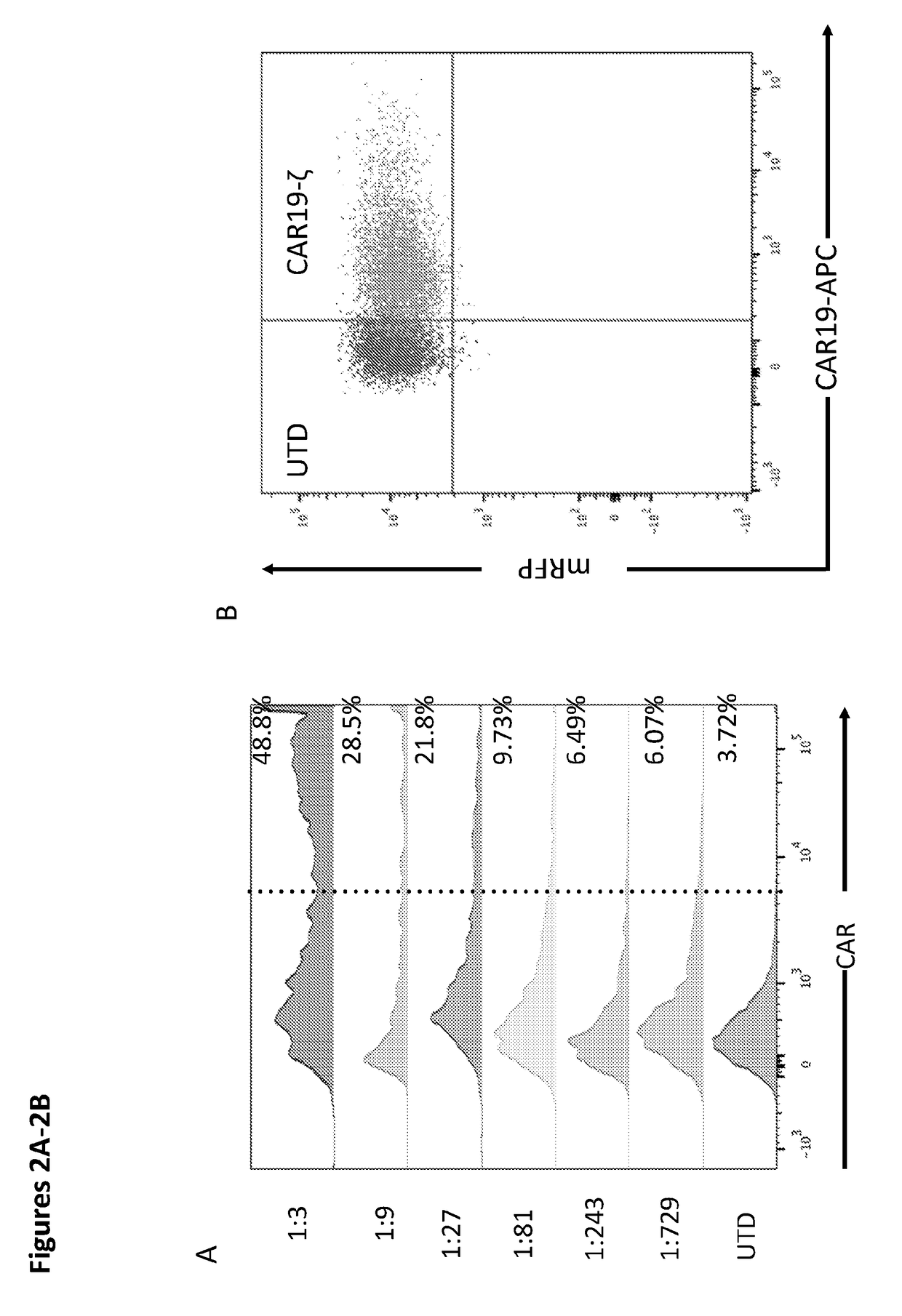
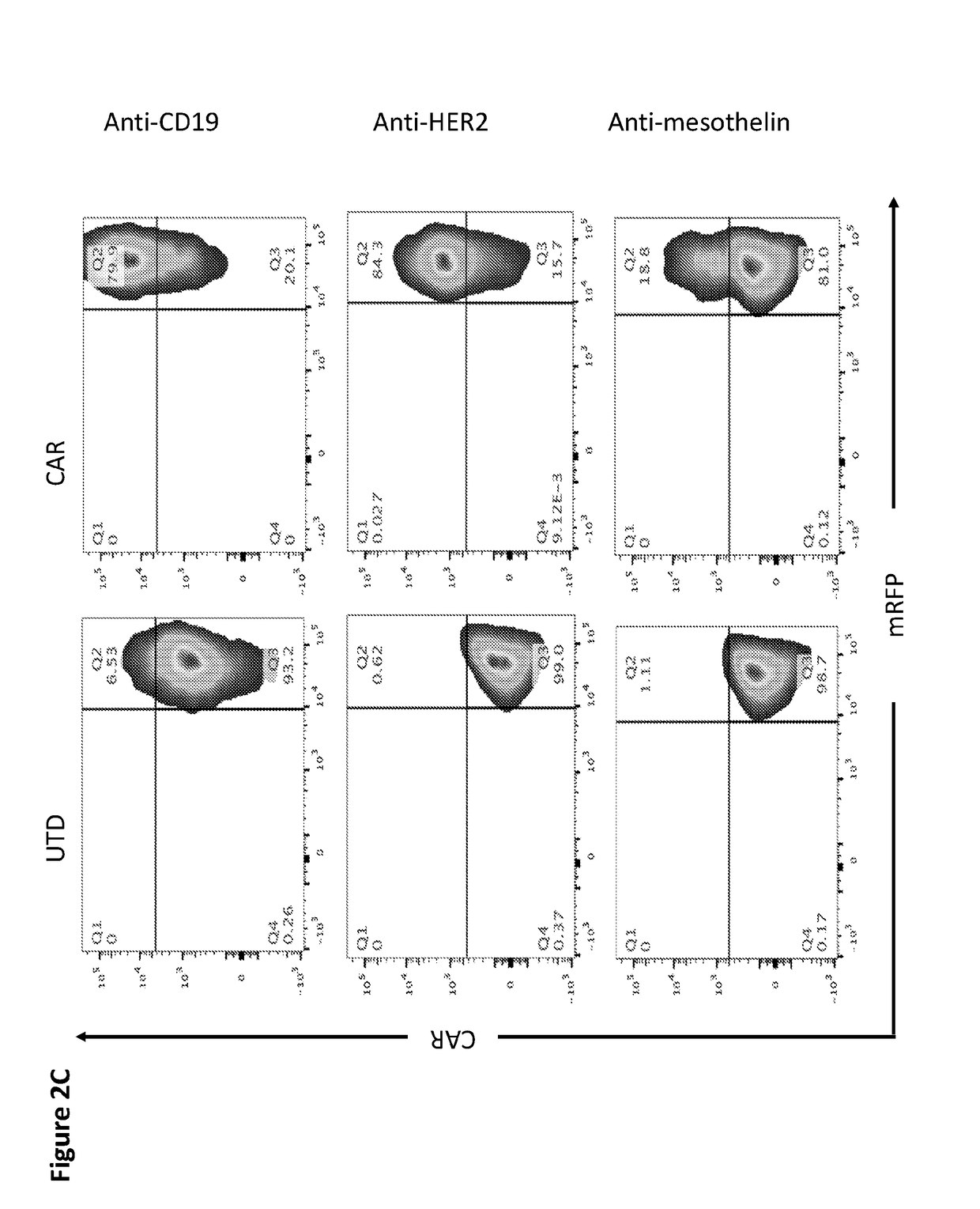
Modified Monocytes/Macrophage Expressing Chimeric Antigen Receptors and Uses Thereof
ActiveUS20180244748A1Stimulate immune responsePeptide/protein ingredientsAntibody mimetics/scaffoldsDendritic cellGackstroemia
The present invention includes methods and compositions for treating cancer, whether a solid tumor or a hematologic malignancy. By expressing a chimeric antigen receptor in a monocyte, macrophage or dendritic cell, the modified cell is recruited to the tumor microenvironment where it acts as a potent immune effector by infiltrating the tumor and killing the target cells. One aspect includes a modified cell and pharmaceutical compositions comprising the modified cell for adoptive cell therapy and treating a disease or condition associated with immunosuppression.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
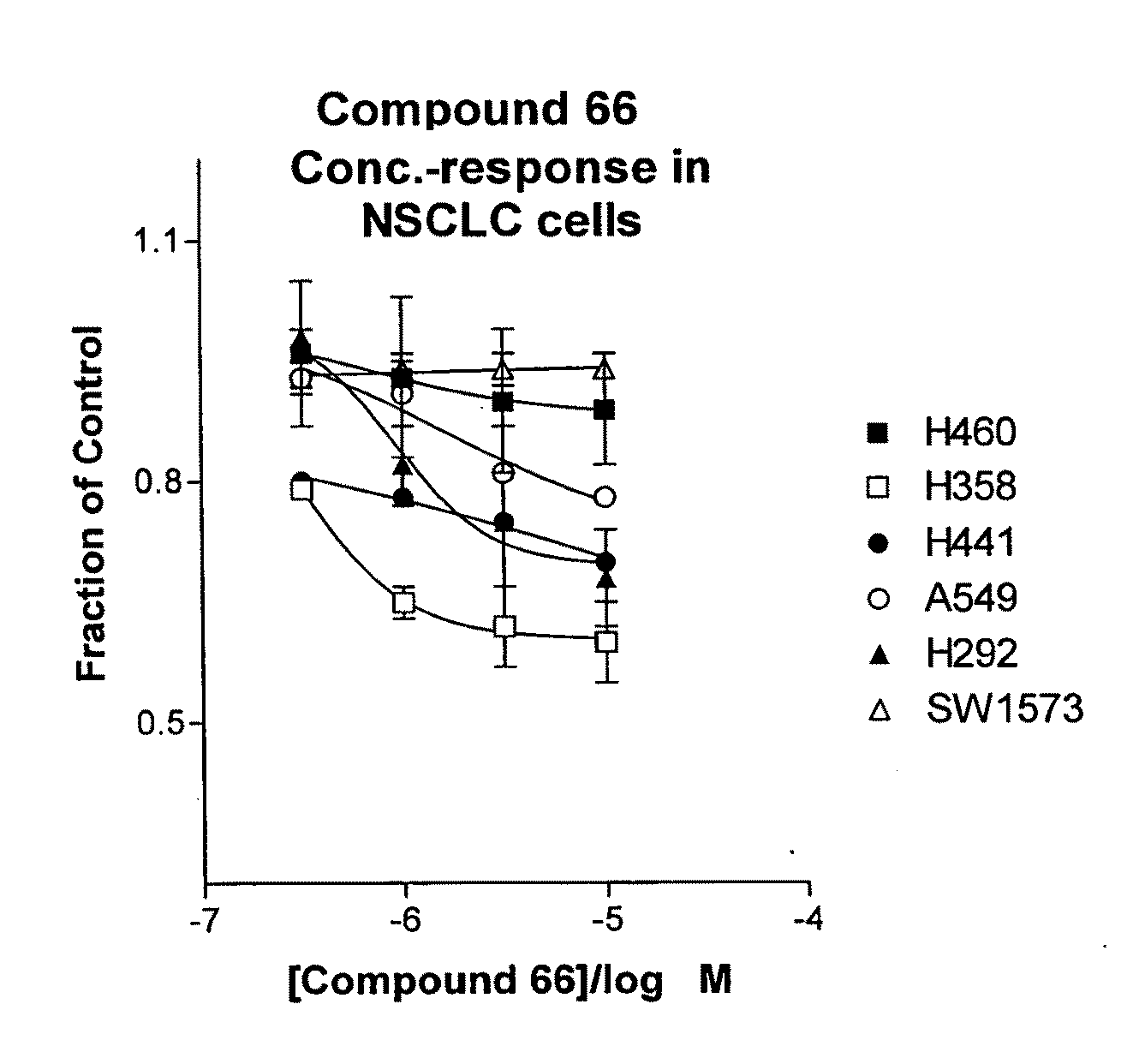
Biological markers predictive of anti-cancer response to insulin-like growth factor-1 receptor kinase inhibitors
ActiveUS20090093488A1High level of biomarkerReduce sensitivityBiocideNervous disorderAbnormal tissue growthCell growth
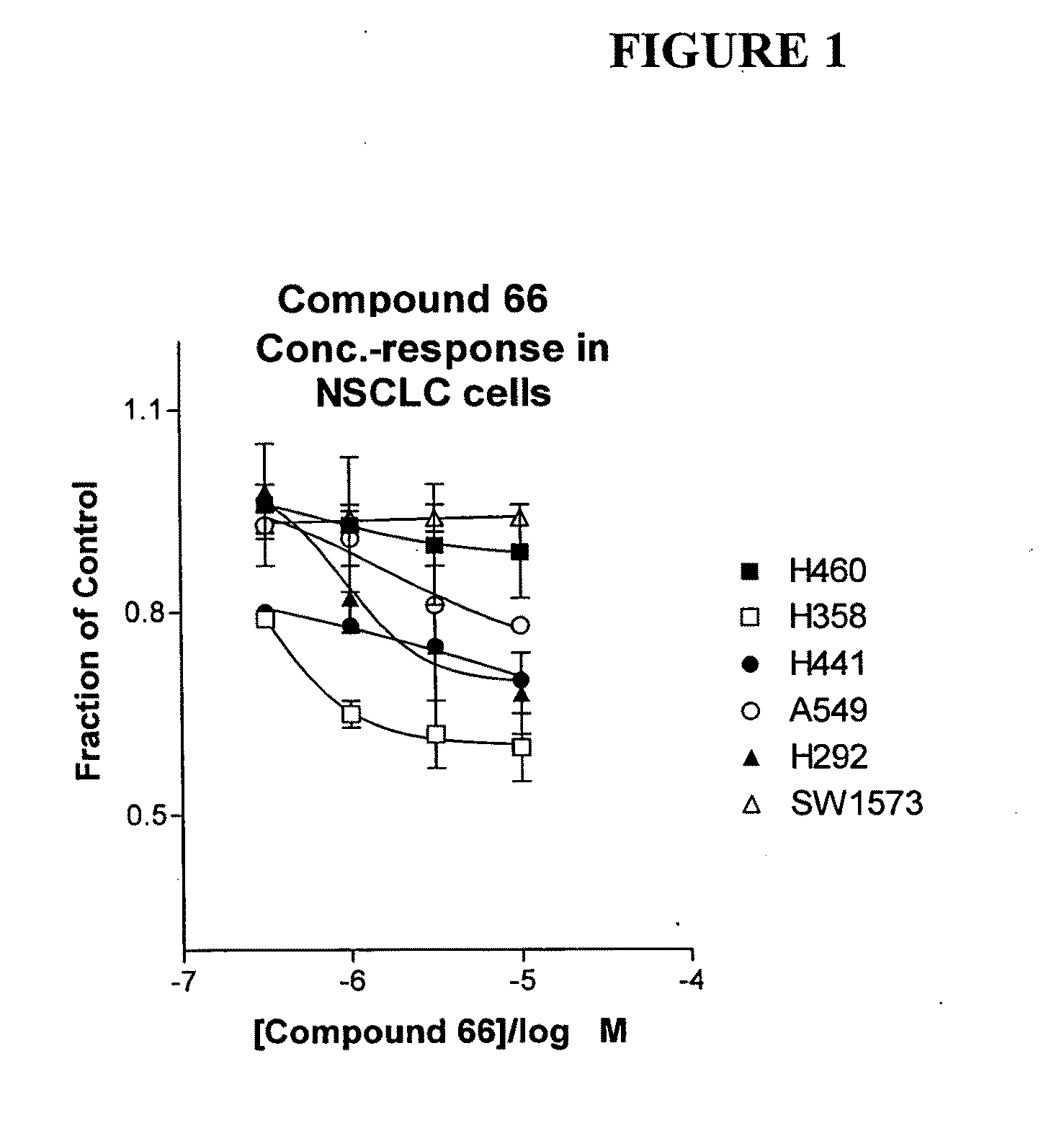
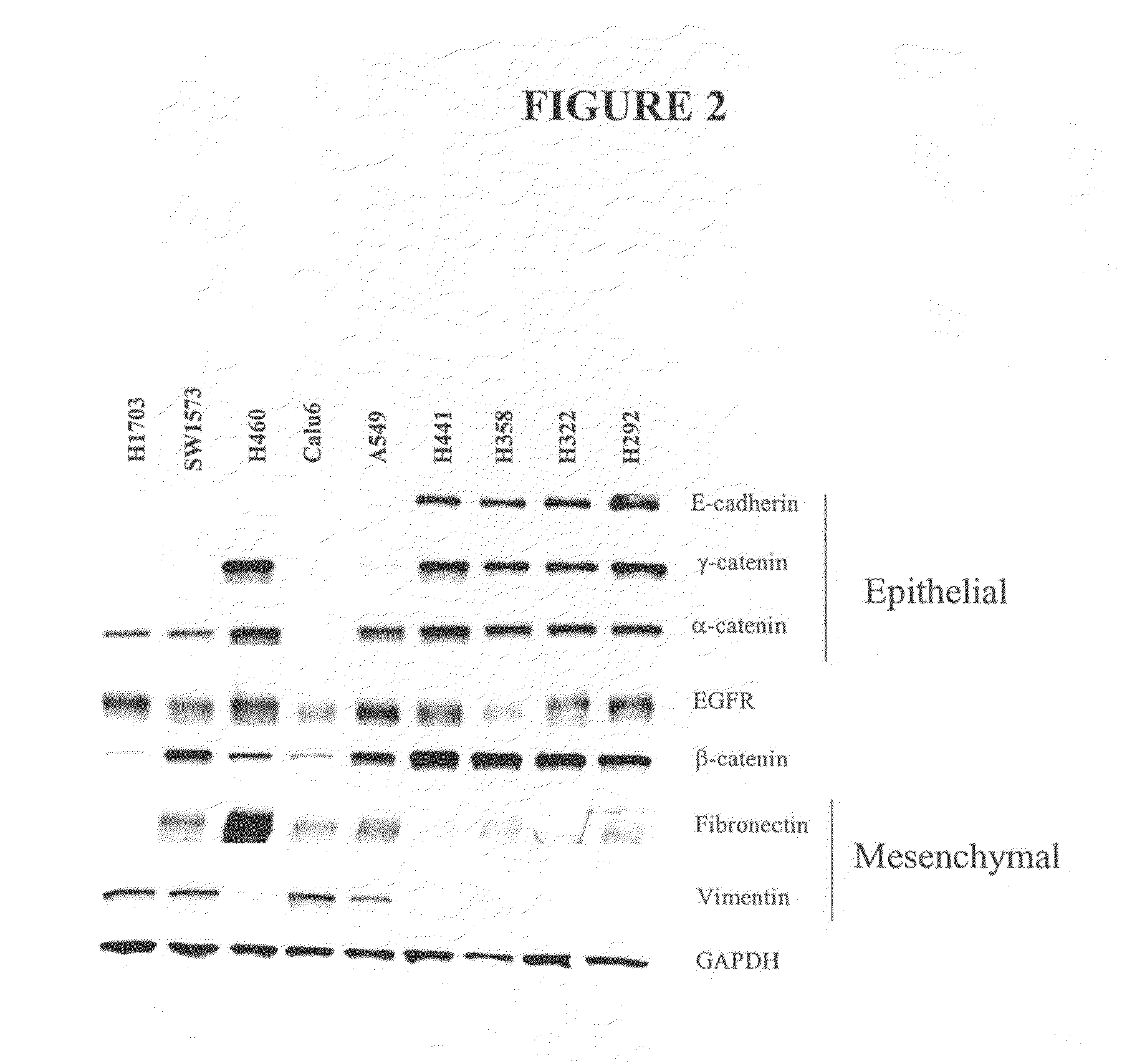
The present invention provides diagnostic and prognostic methods for predicting the effectiveness of treatment of a cancer patient with an IGF-1R kinase inhibitor. Methods are provided for predicting the sensitivity of tumor cell growth to inhibition by an IGF-1R kinase inhibitor, comprising assessing whether the tumor cell has undergone an epithelial to mesenchymal transition (EMT), by determining the expression level of epithelial and / or mesenchymal biomarkers, wherein tumor cells that have undergone an EMT are substantially less sensitive to inhibition by IGF-1R kinase inhibitors. Improved methods for treating cancer patients with IGF-1R kinase inhibitors that incorporate the above methodology are also provided. Additionally, methods are provided for the identification of new biomarkers that are predictive of responsiveness of tumors to IGF-1R kinase inhibitors. Furthermore, methods for the identification of agents that restore the sensitivity of tumor cells that have undergone EMT to inhibition by IGF-1R kinase inhibitors are also provided. pErk, HER3 and pHER are also demonstrated to be effective biomarkers for predicting sensitivity of tumor cells to IGF-1R kinase inhibitors.
Owner:OSI PHARMA INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com