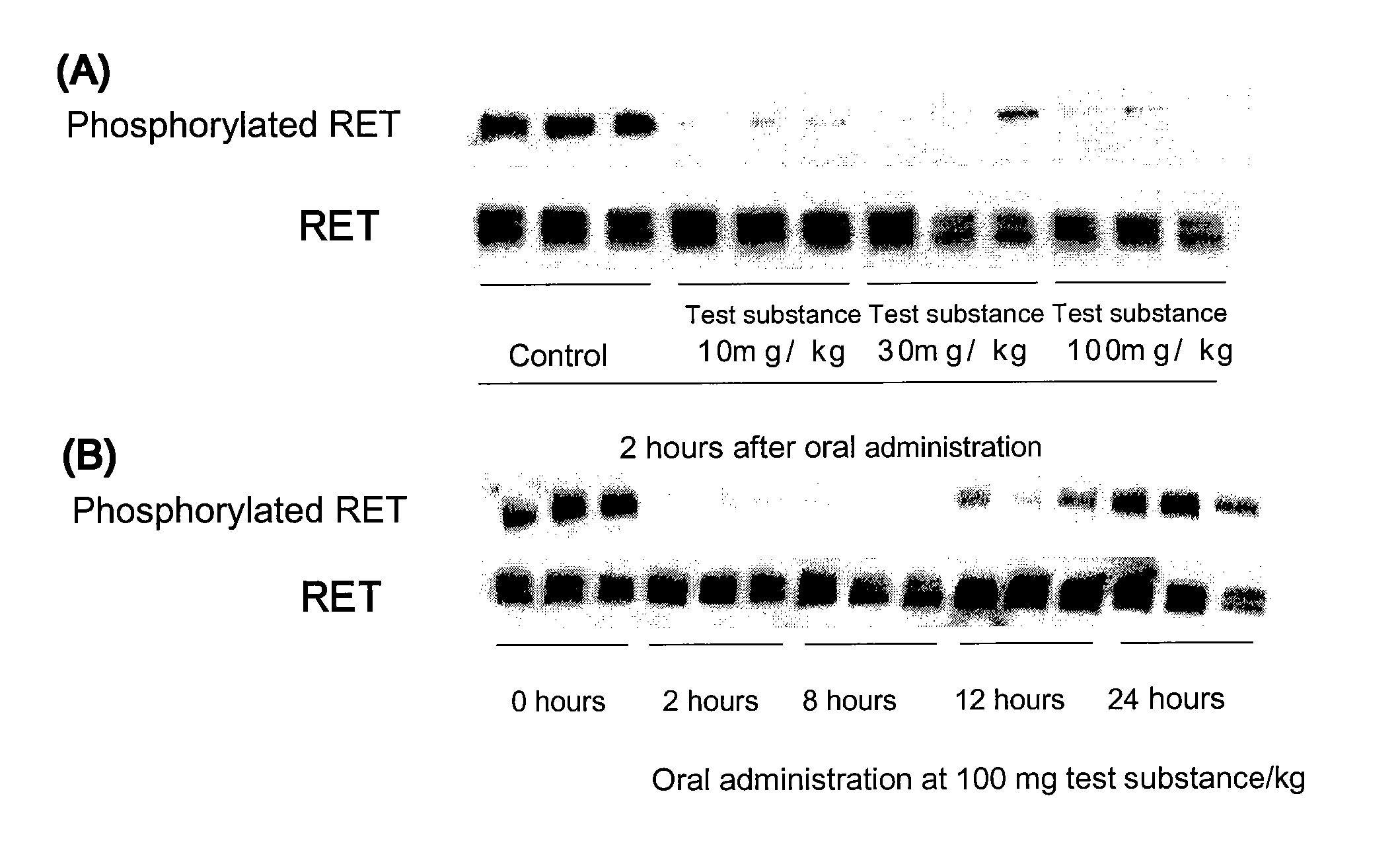
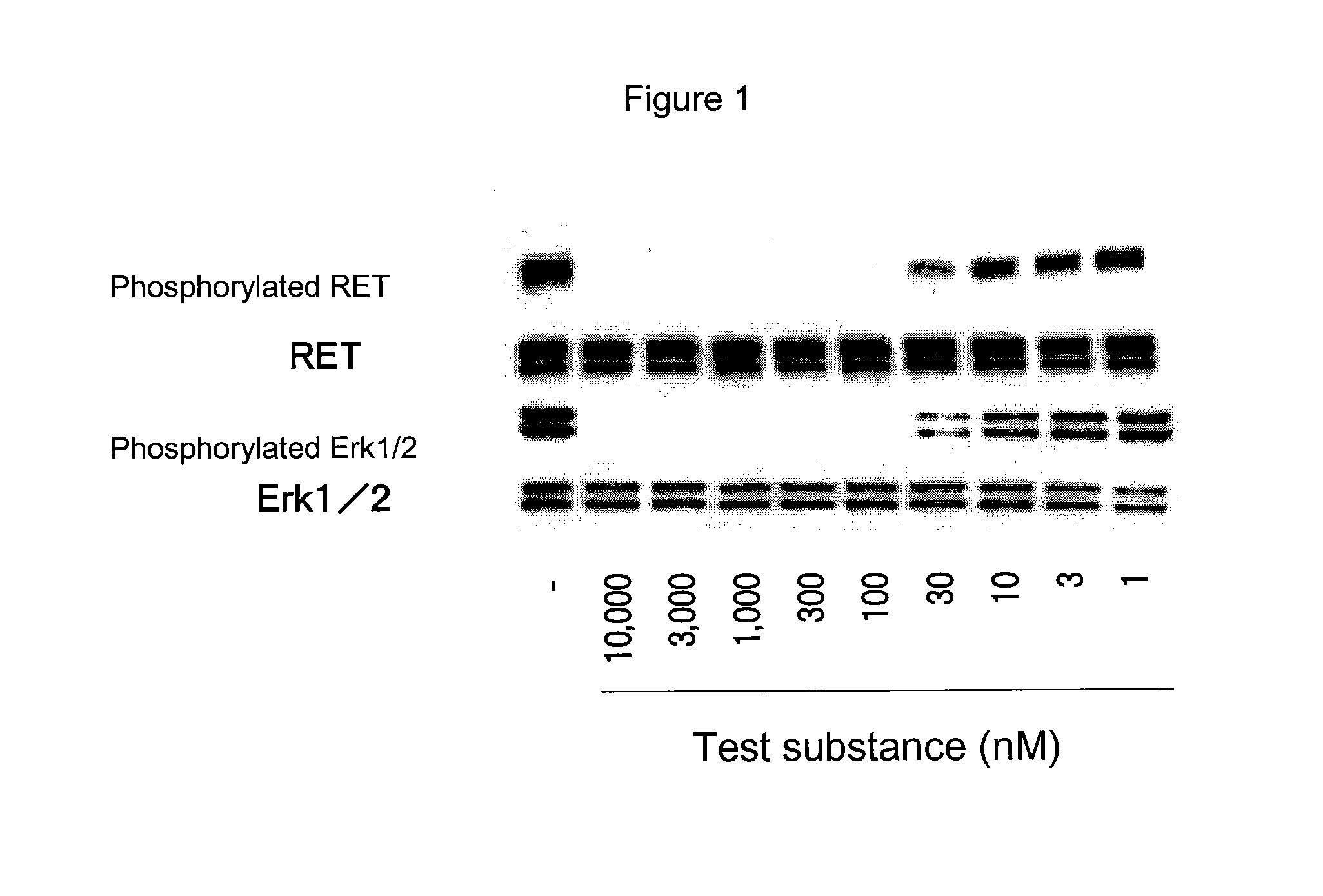
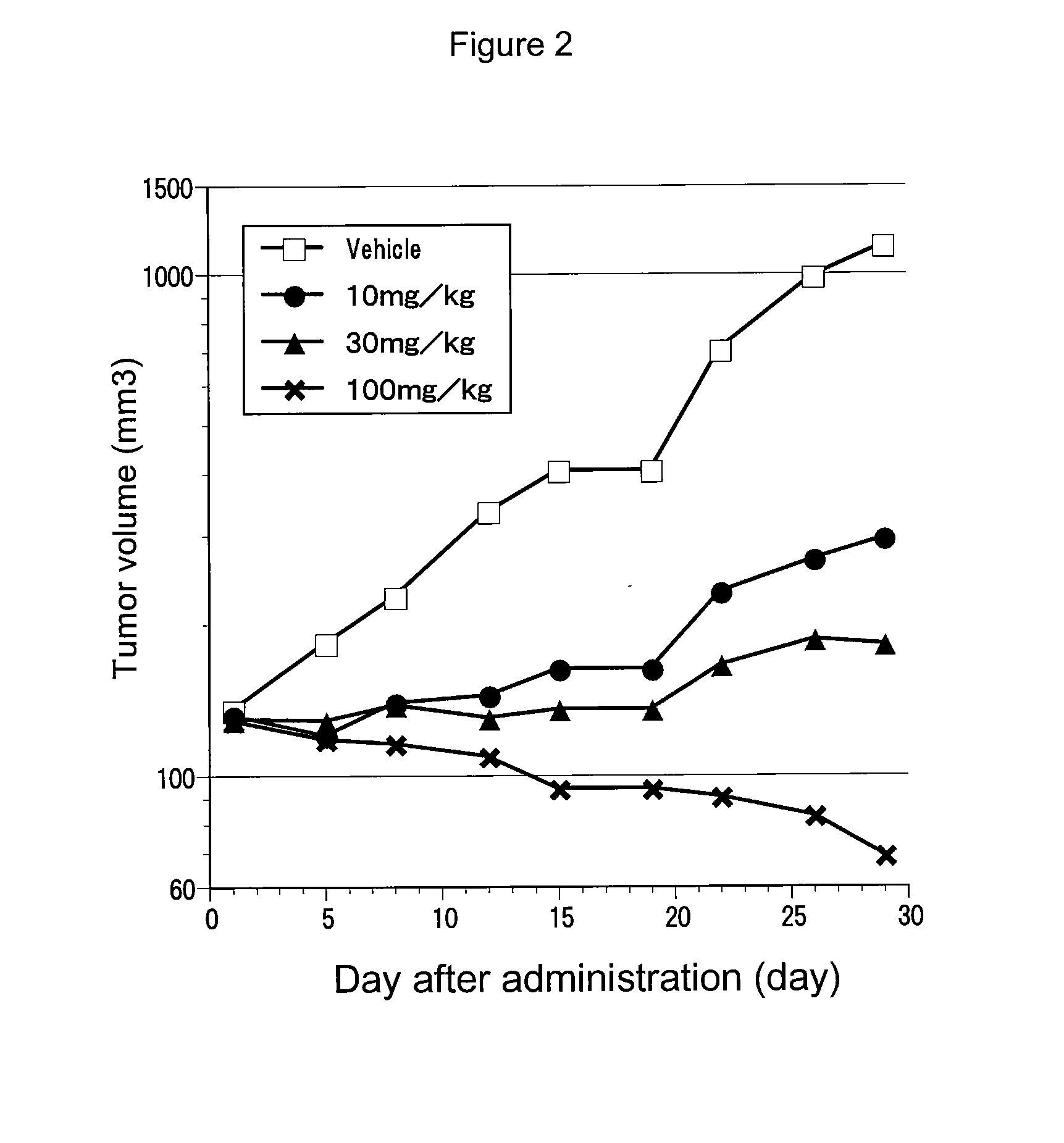
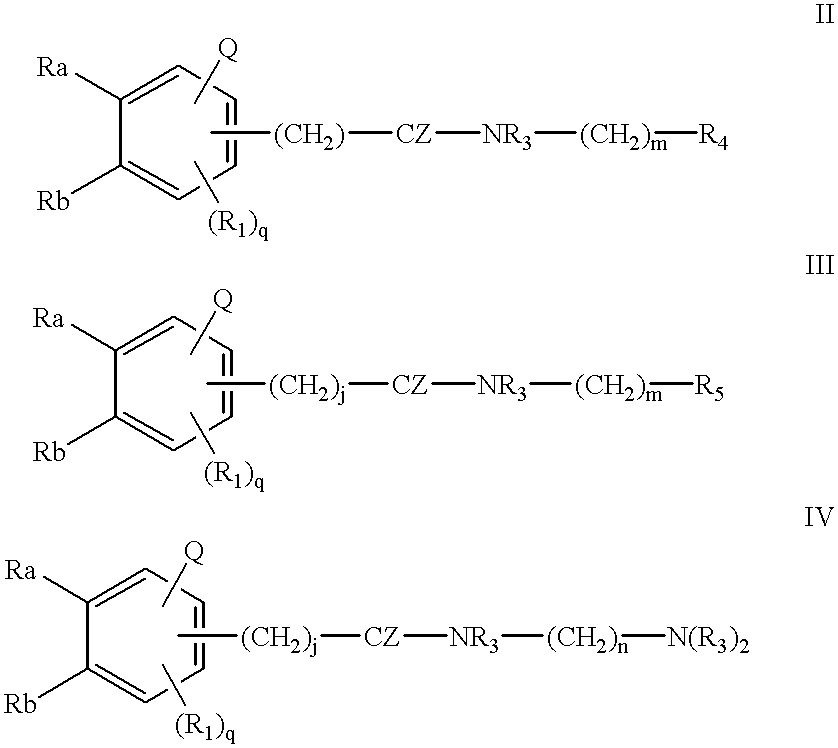
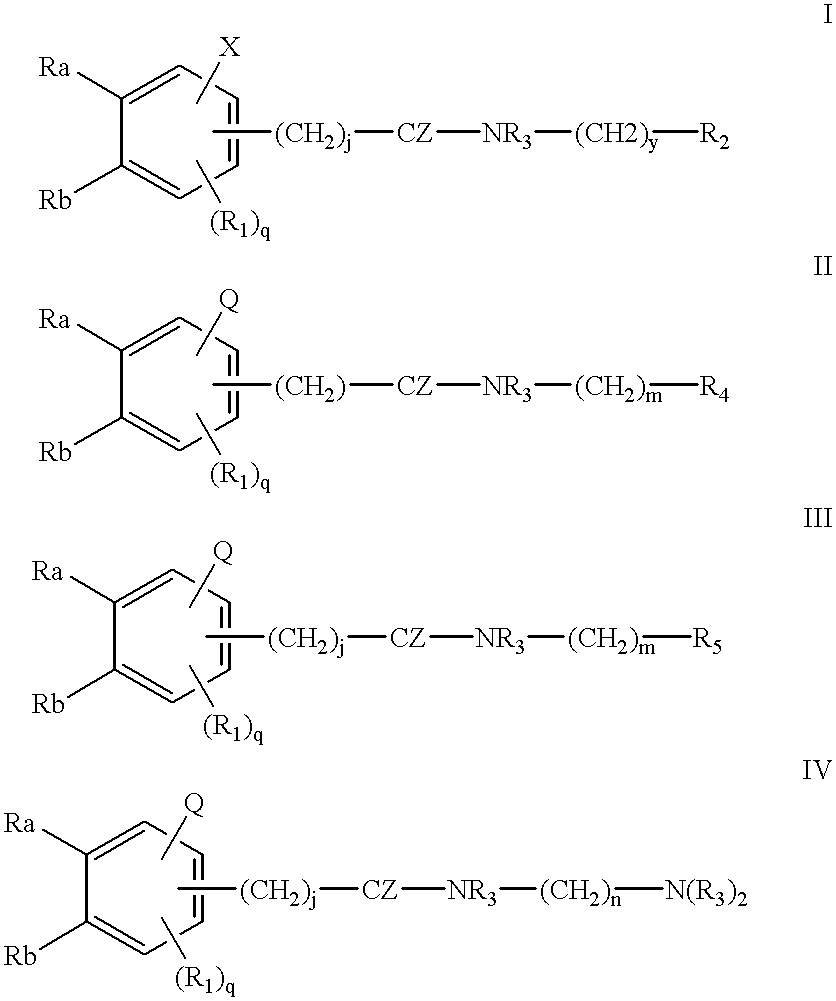
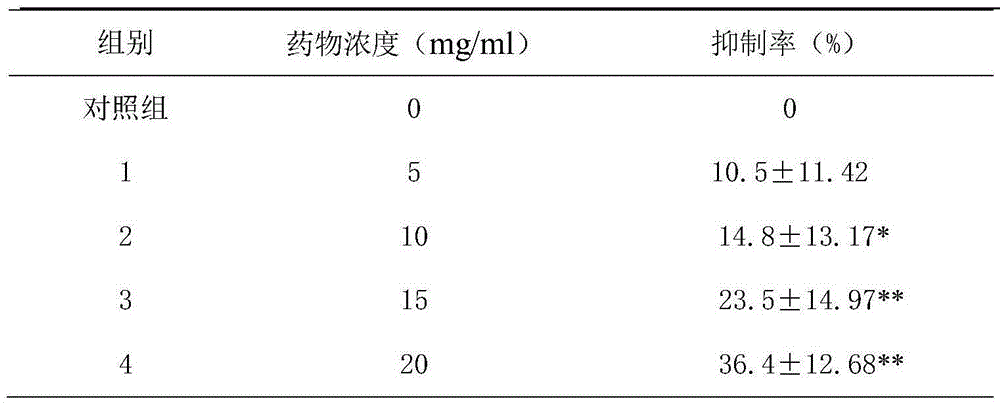
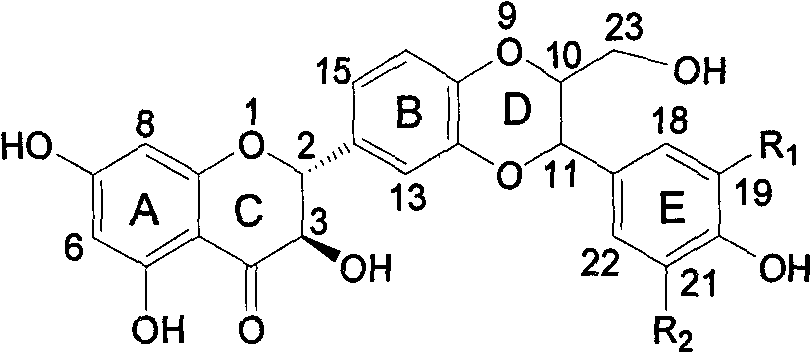
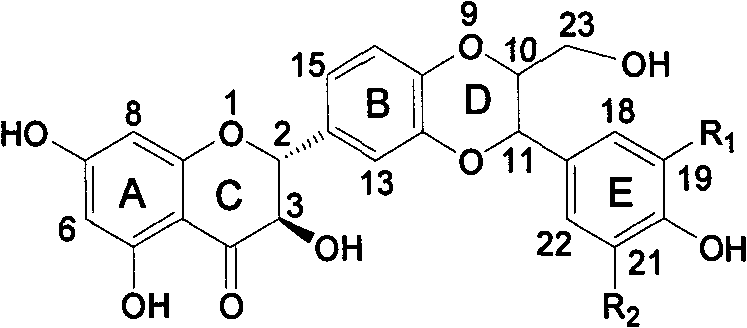
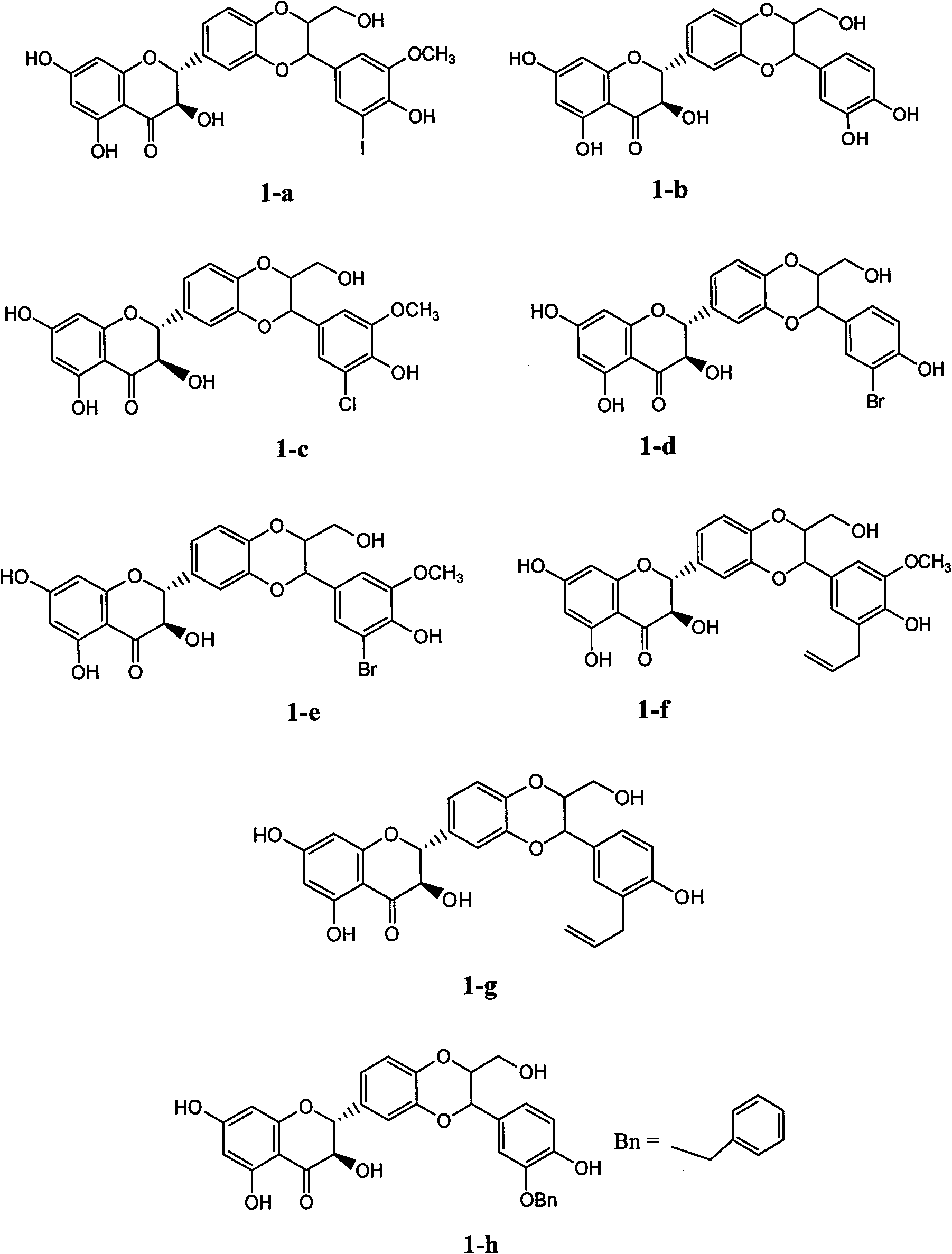
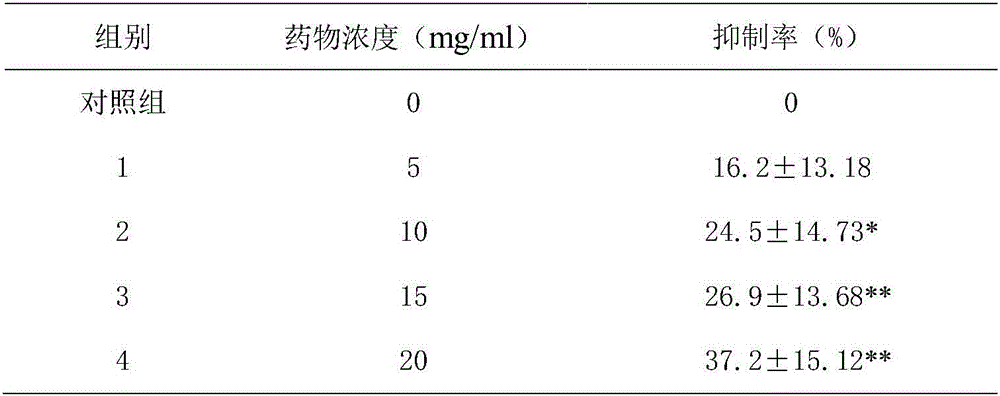
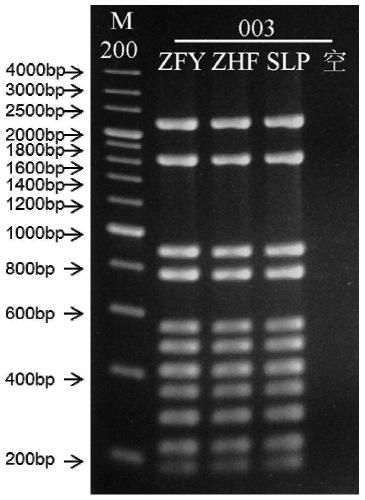
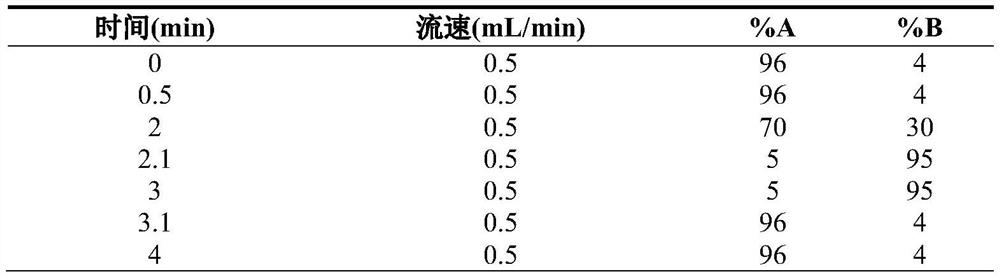
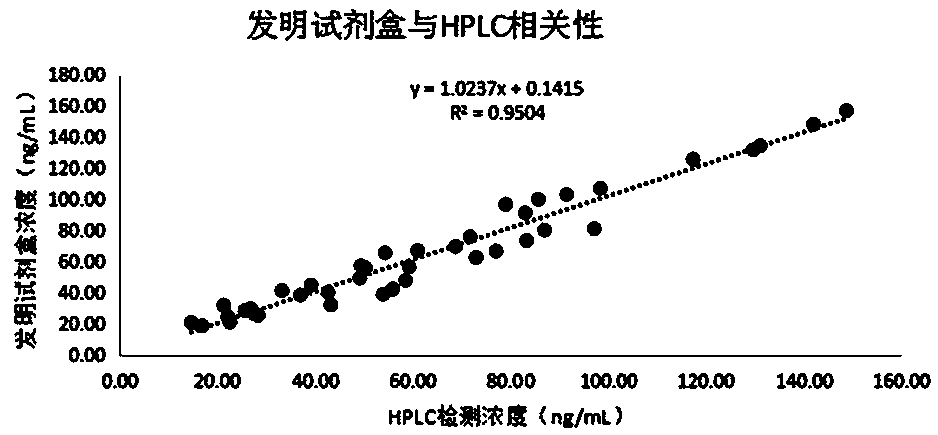
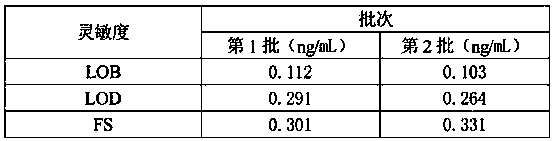
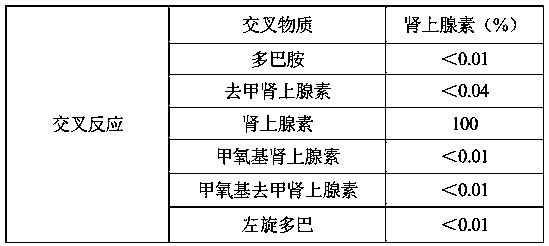
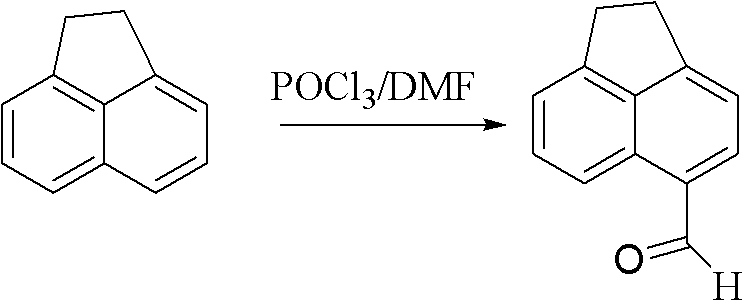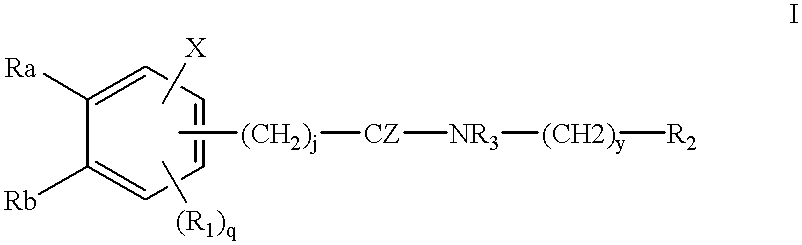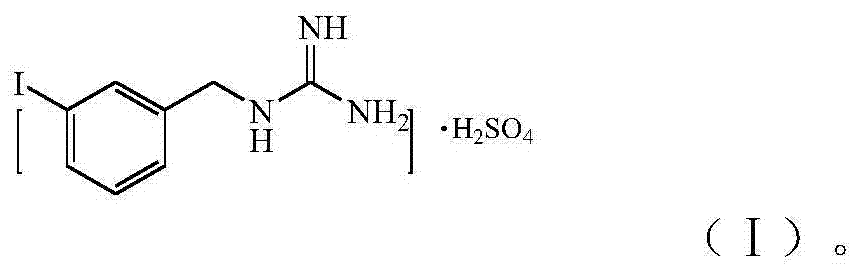Patents
Literature
49 results about "Pheochromocytoma" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A tumor originating in cells of the adrenal gland that causes overproduction of certain hormones.
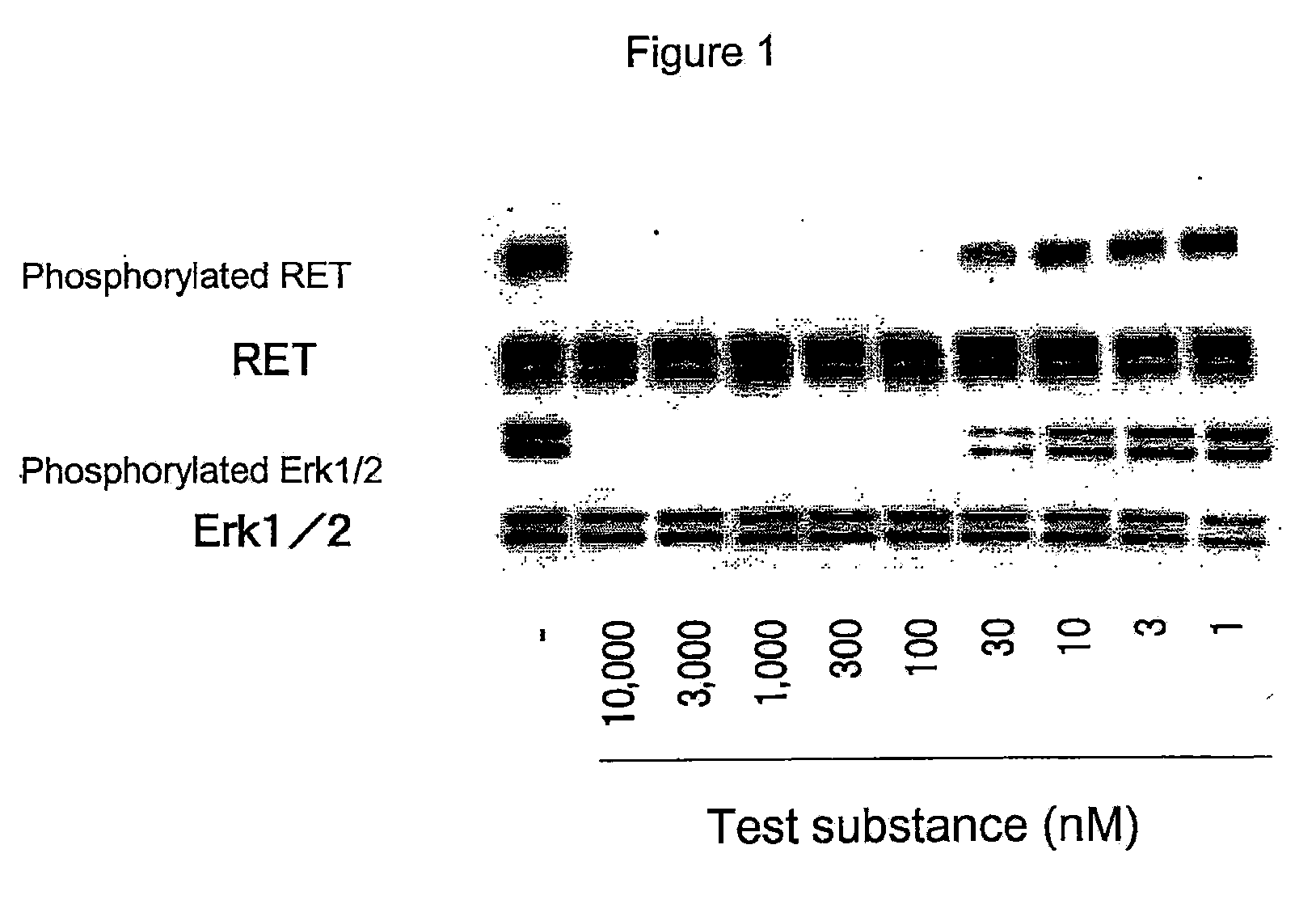
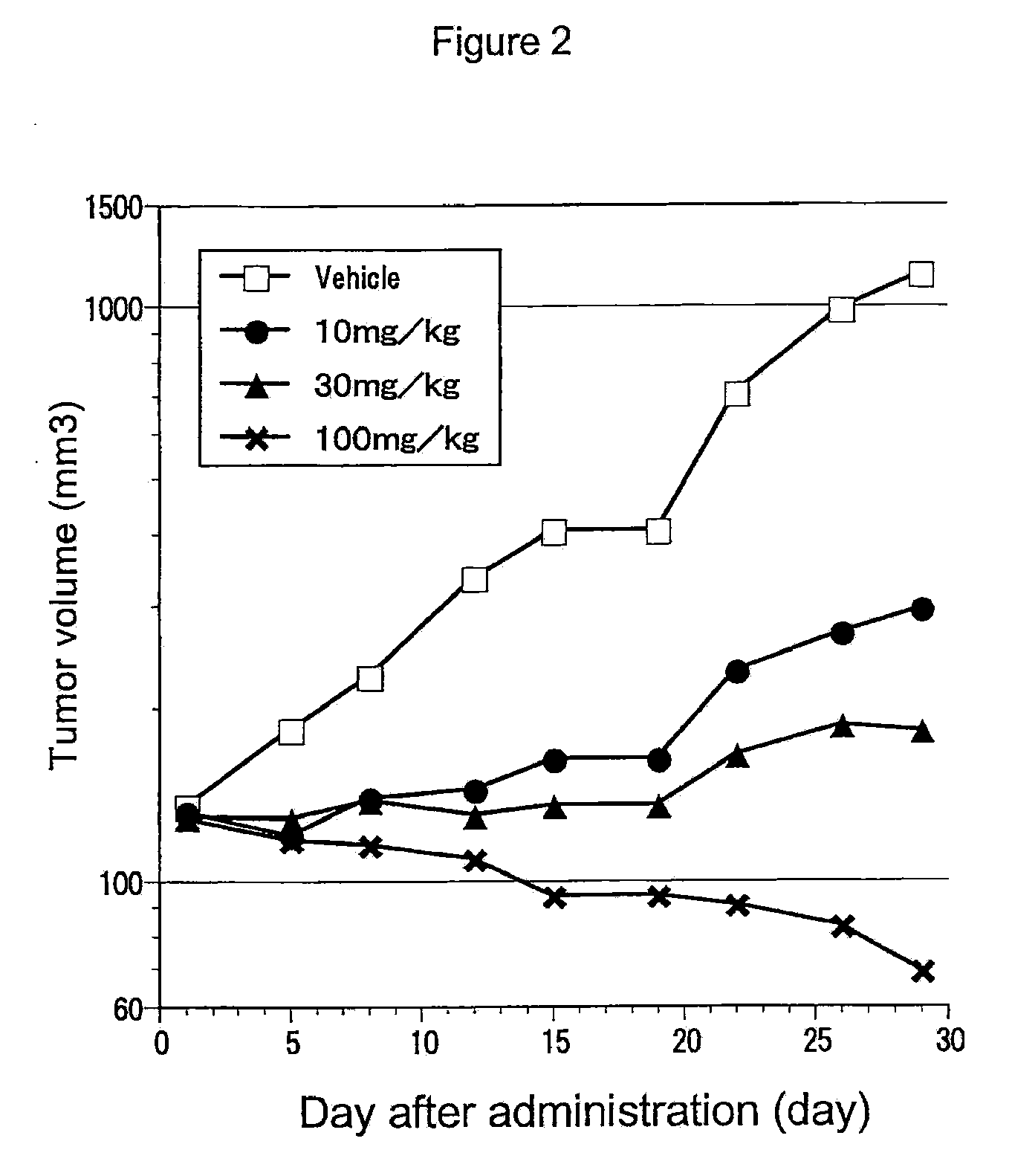
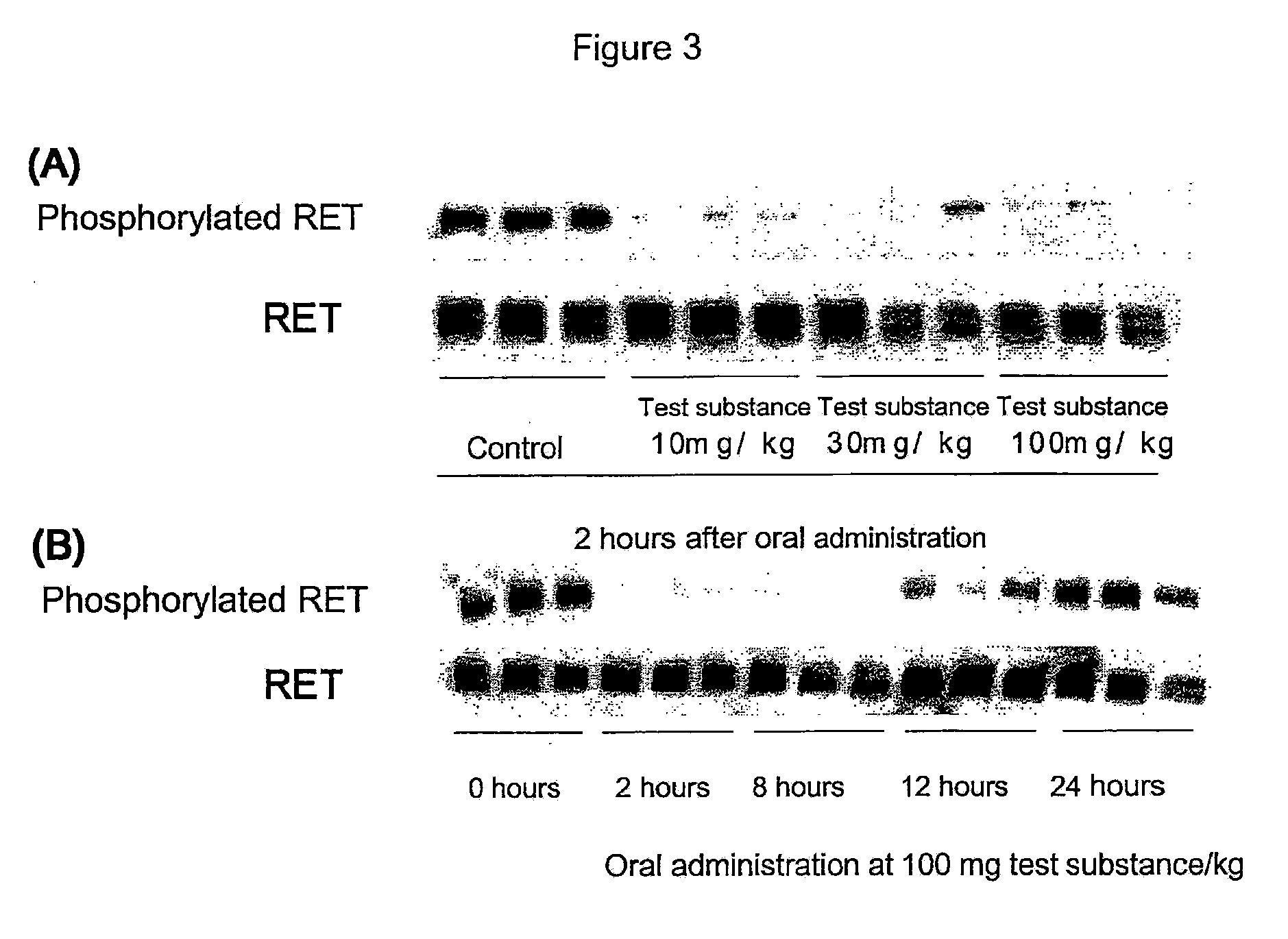
Antitumor agent for thyroid cancer
The objective of the present invention is to provide a pharmaceutical composition and a therapeutic method that are specifically effective against at least one disease selected from multiple endocrine neoplasia, type IIA, multiple endocrine neoplasia, type IIB, familial medullary thyroid carcinoma, thyroid carcinoma, papillary thyroid carcinoma, sporadic medullary thyroid carcinoma, Hirschsprung disease, pheochromocytoma, parathyroid hyperplasia and mucosal neuromas of the gastrointestinal tract.4-(3-chloro-4-(cyclopropylaminocarbonyl)aminophenoxy)-7-methoxy-6-quinolinecarboxamide and analogs thereof are specifically effective against at least one disease selected from multiple endocrine neoplasia, type IIA, multiple endocrine neoplasia, type IIB, familial medullary thyroid carcinoma, thyroid carcinoma, papillary thyroid carcinoma, sporadic medullary thyroid carcinoma, Hirschsprung disease, pheochromocytoma, parathyroid hyperplasia and mucosal neuromas of the gastrointestinal tract.
Owner:EISIA R&D MANAGEMENT CO LTD
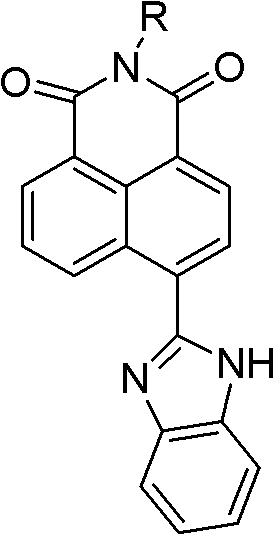
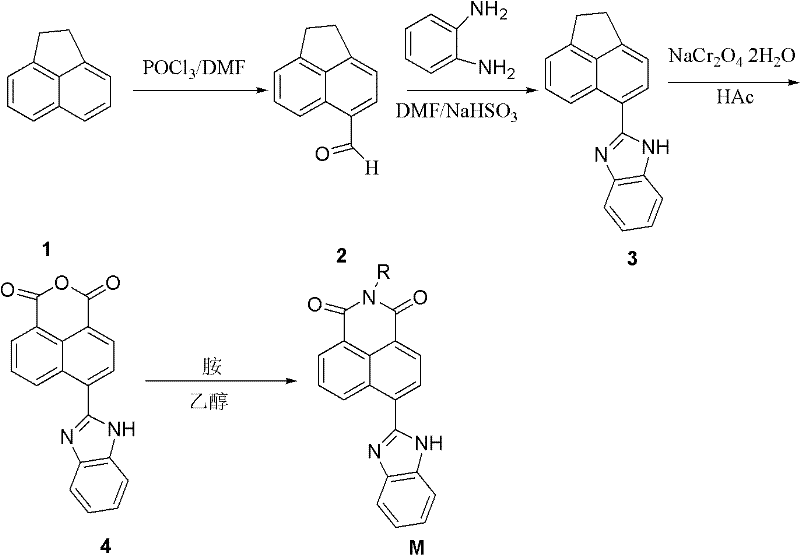
Synthesis of benzimidazole-containing naphthalimide derivatives and applications of benzimidazole-containing naphthalimide derivatives on cancer resistance
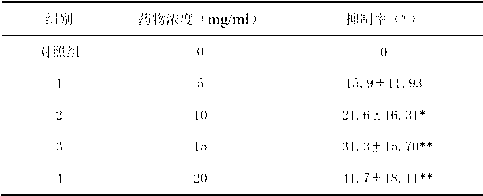
InactiveCN102206203AGood choiceGood tumor suppressor activityOrganic active ingredientsOrganic chemistryPheochromocytomaOrganic synthesis
The invention relates to synthesis of benzimidazole-containing naphthalimide derivatives and applications of the benzimidazole-containing naphthalimide derivatives on cancer resistance, and belongs to the field of organic synthesis and pharmaceutical chemistry technology. The benzimidazole-containing naphthalimide derivatives are obtained through a method that a benzimidazole group is introduced into a fourth position of a naphthalene ring of naphthalimide. Experiments of proliferation inhibiting effects of the benzimidazole-containing naphthalimide derivatives on cancer cells adopt a microculture tetrozolium (MTT) reduction method and aim at MCF-7 human breast cancer cells, Hela human cervical cancer cells and PC12 rat adrenal medullary pheochromocytoma differentiated cells. Results of the experiments show that the benzimidazole-containing naphthalimide derivatives have the advantages of good inhibitory activity against cancer cells and good selectivity on cancer cells.
Owner:DALIAN UNIV OF TECH
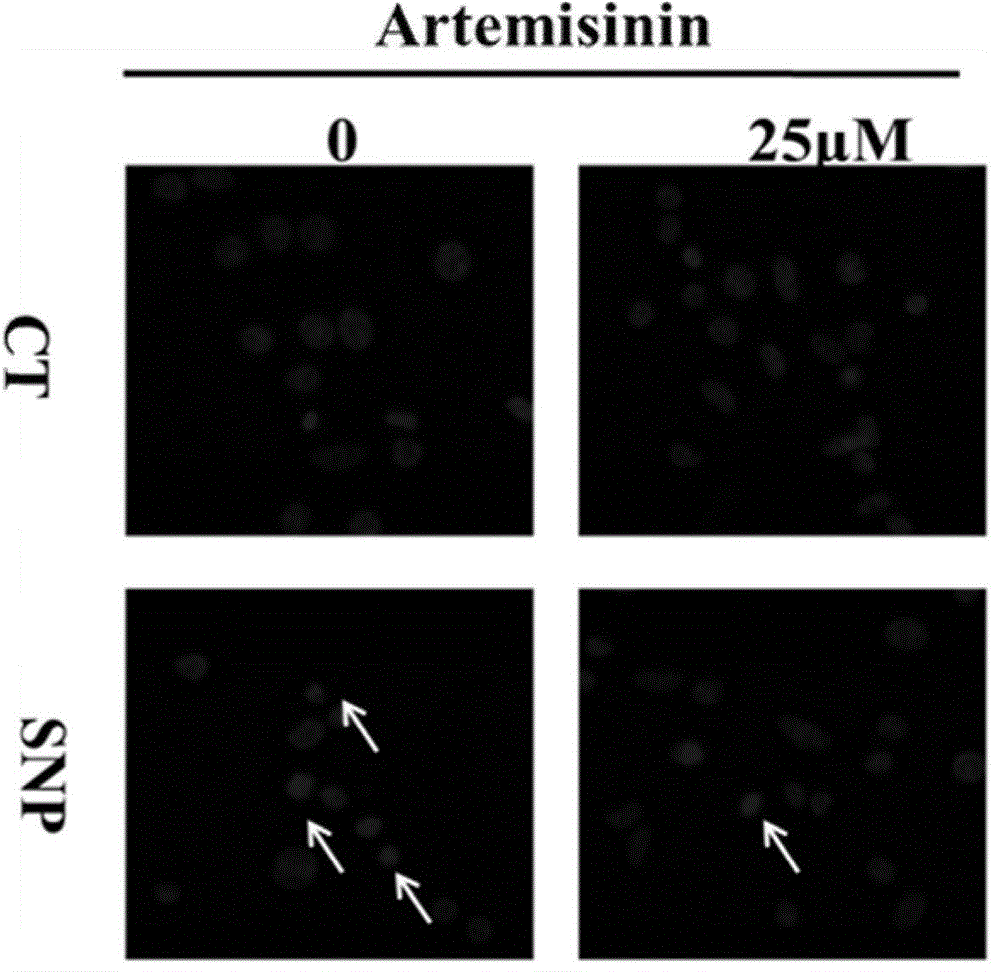
Application of artemisinin in preparing medicament for preventing and treating neurological diseases
ActiveCN103948585AProtectiveAchieve protectionOrganic active ingredientsSenses disorderPheochromocytomaArtemisinins
The invention discloses application of artemisinin and derivatives thereof in preparing medicaments for preventing and treating neurological diseases. In the application, the nerve cell line of rat adrenal pheochromocytoma PC12, retinal nerve cell line RGC-5 and primary cortical neuron are on behalf of experiment objects, sodium nitroprusside and hydrogen peroxide are used to simulate and induce oxidative stress, cell apoptosis and other cell injuries of nerve cells. Researches discover that the artemisinin has a protective effect on sodium nitroprusside and hydrogen peroxide induced cell injury, the protective effect mechanism of the artemisinin and derivatives thereof is primarily discussed, and results indicate that the artemisinin and derivatives thereof can be used as nerve protection medicaments for preventing and treating various neurological diseases, such as various neurodegenerative diseases, acute and chronic neurodegenerative diseases, neurological eye disease and the those neurological diseases mainly related to oxidative stress injuries and cell apoptosis. The application brings a new direction to treatment and prevention of neurological diseases.
Owner:ZHONGSHAN OPHTHALMIC CENT SUN YAT SEN UNIV
Antitumor agent for thyroid cancer
The objective of the present invention is to provide a pharmaceutical composition and a therapeutic method that are specifically effective against at least one disease selected from multiple endocrine neoplasia, type IIA, multiple endocrine neoplasia, type IIB, familial medullary thyroid carcinoma, thyroid carcinoma, papillary thyroid carcinoma, sporadic medullary thyroid carcinoma, Hirschsprung disease, pheochromocytoma, parathyroid hyperplasia and mucosal neuromas of the gastrointestinal tract.4-(3-chloro-4-(cyclopropylaminocarbonyl)aminophenoxy)-7-methoxy-6-quinolinecarboxamide and analogs thereof are specifically effective against at least one disease selected from multiple endocrine neoplasia, type IIA, multiple endocrine neoplasia, type IIB, familial medullary thyroid carcinoma, thyroid carcinoma, papillary thyroid carcinoma, sporadic medullary thyroid carcinoma, Hirschsprung disease, pheochromocytoma, parathyroid hyperplasia and mucosal neuromas of the gastrointestinal tract.
Owner:EISIA R&D MANAGEMENT CO LTD
Preparation method and application of brain-soothing hypertension pill
ActiveCN102988577AReduce dosageHeavy metal active ingredientsNervous disorderPheochromocytomaRat Adrenals
The invention provides a preparation method of a brain-soothing hypertension pill. The brain-soothing hypertension pill is prepared by using 100g of baical skullcap roots, 60g of common selfheal fruit-spike, 60g of sophora flower bud, 60g of forged lodestone, 60g of twotooth achyranthes roots, 100g of Chinese angelica, 40g of rehmannia, 40g of danshen roots, 20g of sanguisuga, 60g of uncaria rhynchophylla, 100g of semen cassia, 20g of lumbricus and 40g of nacre as bulk drugs and adopting supercritical extraction and microwave-assisted extraction modes. By adopting the method, the content of baicalin is greatly improved; and the invention also provides application of the brain-soothing hypertension pill in preparation of drugs for inhibiting cell proliferation of rat adrenal pheochromocytoma PC-12.
Owner:TONGHUA YUSHENG PHARMA
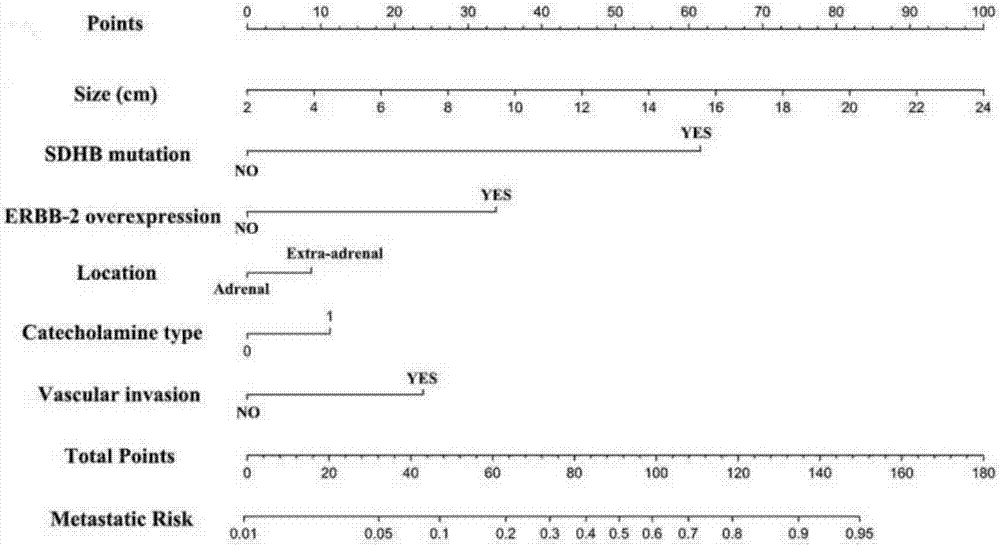
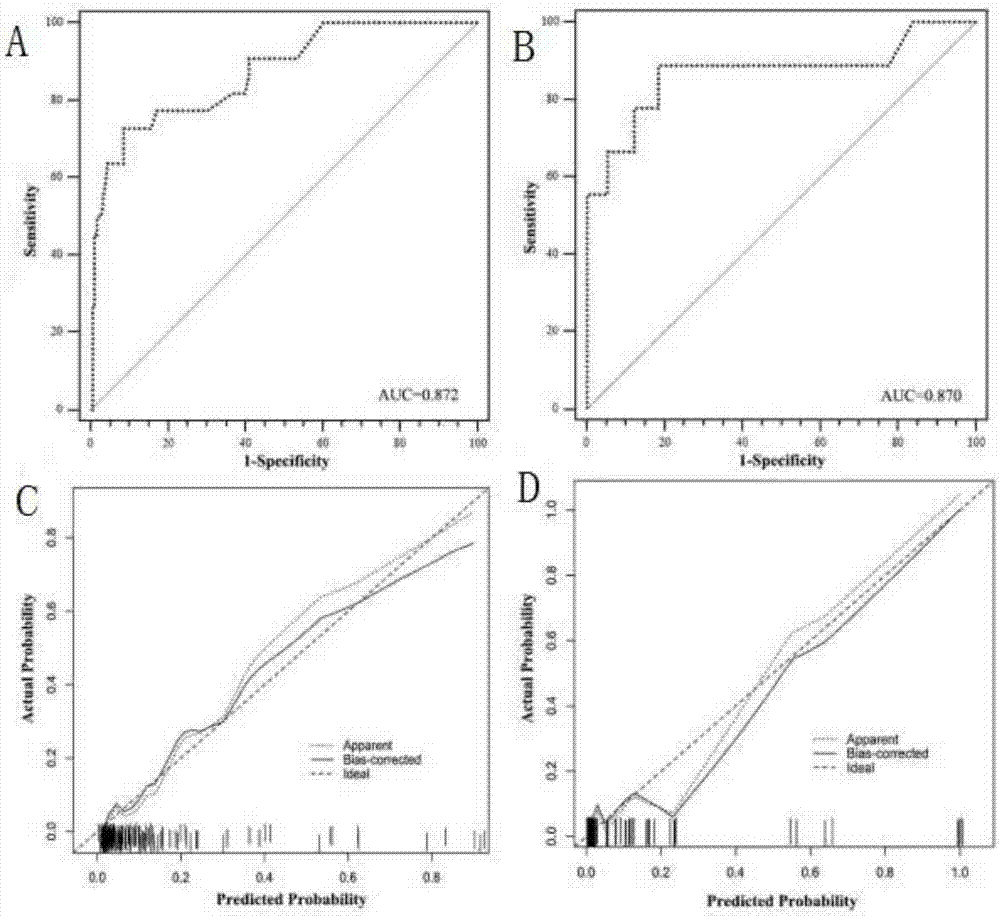
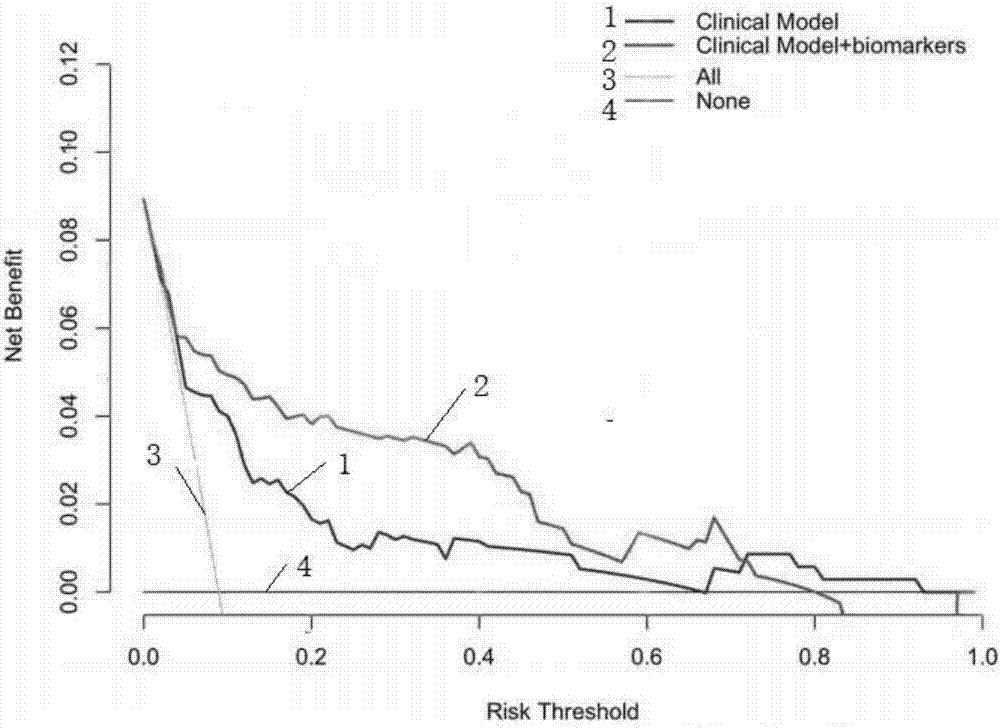
Pheochromocytoma metastasis prediction system based on molecular marker
ActiveCN107545144AEasy to distinguishEasy CalibrationSpecial data processing applicationsPheochromocytomaTotal risk
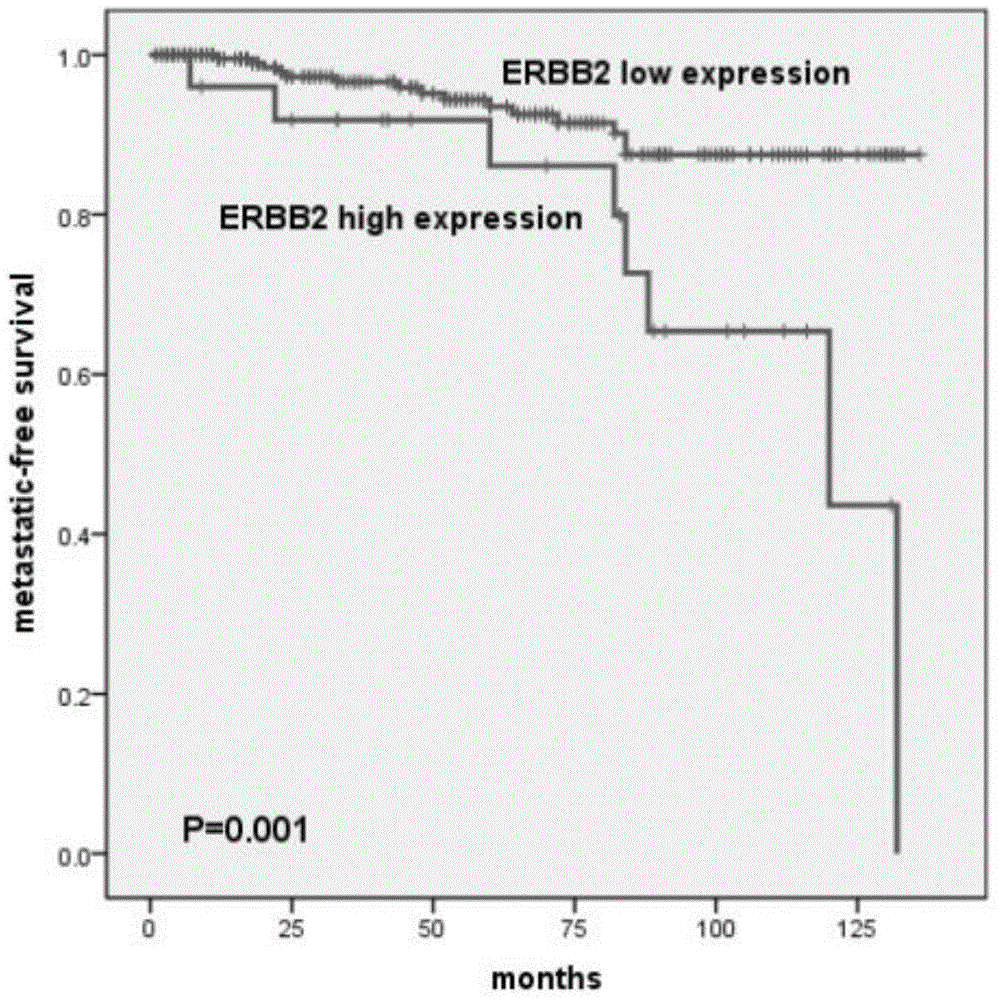
The invention discloses a pheochromocytoma metastasis prediction system based on a molecular marker. The system is characterized in that the system comprises a variable input submodule, an analysis module and an output module; the variable input submodule comprises a tumour primary diameter input submodule, a primary tumour part input submodule, a catecholamine secretion type input submodule, a blood vessel invasion state input submodule, an ERBB-2 overexpression state input submodule and an SDHB mutation state input submodule; the analysis module can build a metastasis probability alignment chart and calculate a total risk value based on variables input by the variable input submodule and can calculate a pheochromocytoma metastasis predicted value of a pheochromocytoma patient according to the total risk value; the output module is used for outputting the pheochromocytoma metastasis predicted value of the pheochromocytoma patient. According to the pheochromocytoma metastasis prediction system based on the molecular marker, SDHB germ-line gene mutation and primary tumour ERBB-2 protein high-expression, the diameter and position of a primary tumour, blood vessel invasion and the catecholamine secretion type are combined, and accordingly the pheochromocytoma metastasis prediction system is built and shows more excellent prediction accuracy compared with separately used clinical risk factors.
Owner:SHANGHAI INST FOR ENDOCRINE & METABOLIC DISEASES +1
Compounds for cancer imaging and therapy
InactiveUS20010006619A1BiocideGroup 3/13 element organic compoundsAbnormal tissue growthPheochromocytoma
The present invention relates to a class of compounds having affinity for certain cancer cells, e.g. lung carcinomas, colon carcinomas, renal carcinomas, prostate carcinomas, breast carcinomas, malignant melanomas, gliomas, neuroblastomas and pheochromocytomas. The compounds of the present invention can also bind with high specificity to cell surface sigma receptors and can therefore be used for diagnostic imaging of any tissue having an abundance of cells with sigma receptors. The present invention provides such compounds as agents for diagnostic imaging and for detecting and treating tumors containing the cancer cells described above.
Owner:RES CORP TECH INC
Novel iridoids compound and neuroprotective effect thereof
The invention discloses a novel iridoids compound with a structure shown in formula I and a neuroprotective effect thereof, a preparation method of the compound I and an application of a pharmaceutically acceptable salt of the compound in the preparing of medicines for treating and preventing central nervous system diseases, and an application of the compound or the pharmaceutically acceptable salt thereof in the preparing of medicines with nerve cell protection effects. The cell survival rate of a 18mg / L group of the compound with the structure shown in formula I is obviously higher than a glutamic acid group (P(0.01)), so that the compound with the structure shown in formula I has an obvious protection effect on PC12 (pheochromocytoma-12) cells damaged by glutamic acid and can be used for preventing and treating central nervous system diseases such as cerebral infarction, Alzheimer disease, Parkinson disease, anxiety disorder and the like.
Owner:SOUTHWEST JIAOTONG UNIV
Phentolamine Mesylate freeze-dried powder injection preparation and preparation method thereof
InactiveCN101099726AAvoid high heat decomposition and deteriorationQuick recovery featureOrganic active ingredientsPowder deliveryPheochromocytomaFreeze-drying
The present invention discloses a phentolamine mesylate freeze-dried powder preparation and its preparation method. Said preparation contains the phentolamine mesylate as active component and pharmaceutically-acceptable carrier. Said preparation can be used for curing pheochromocytoma and preoperative preparation.
Owner:上海复旦复华药业有限公司
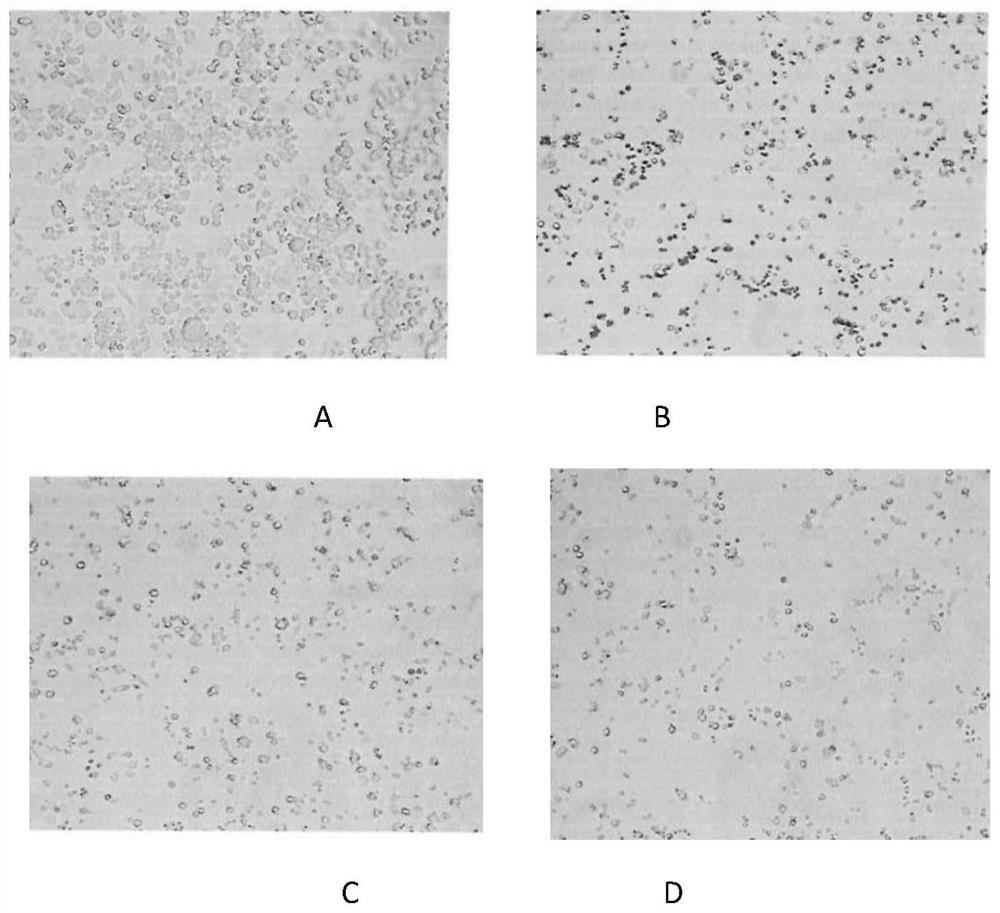
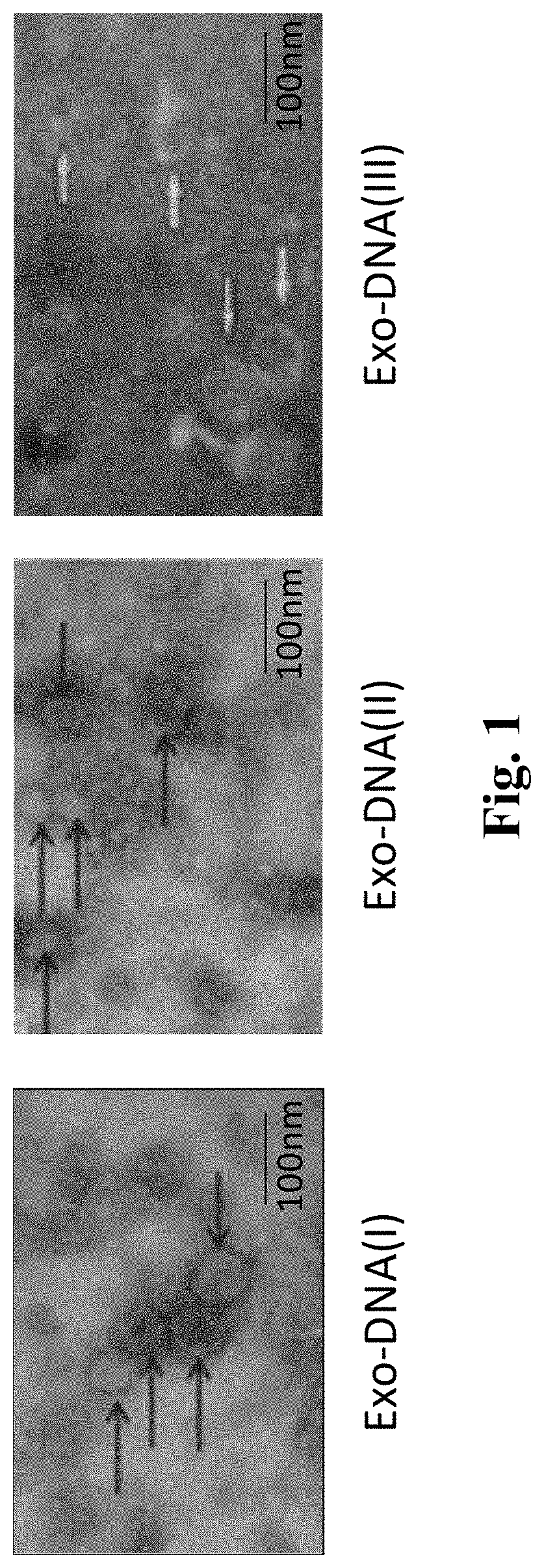
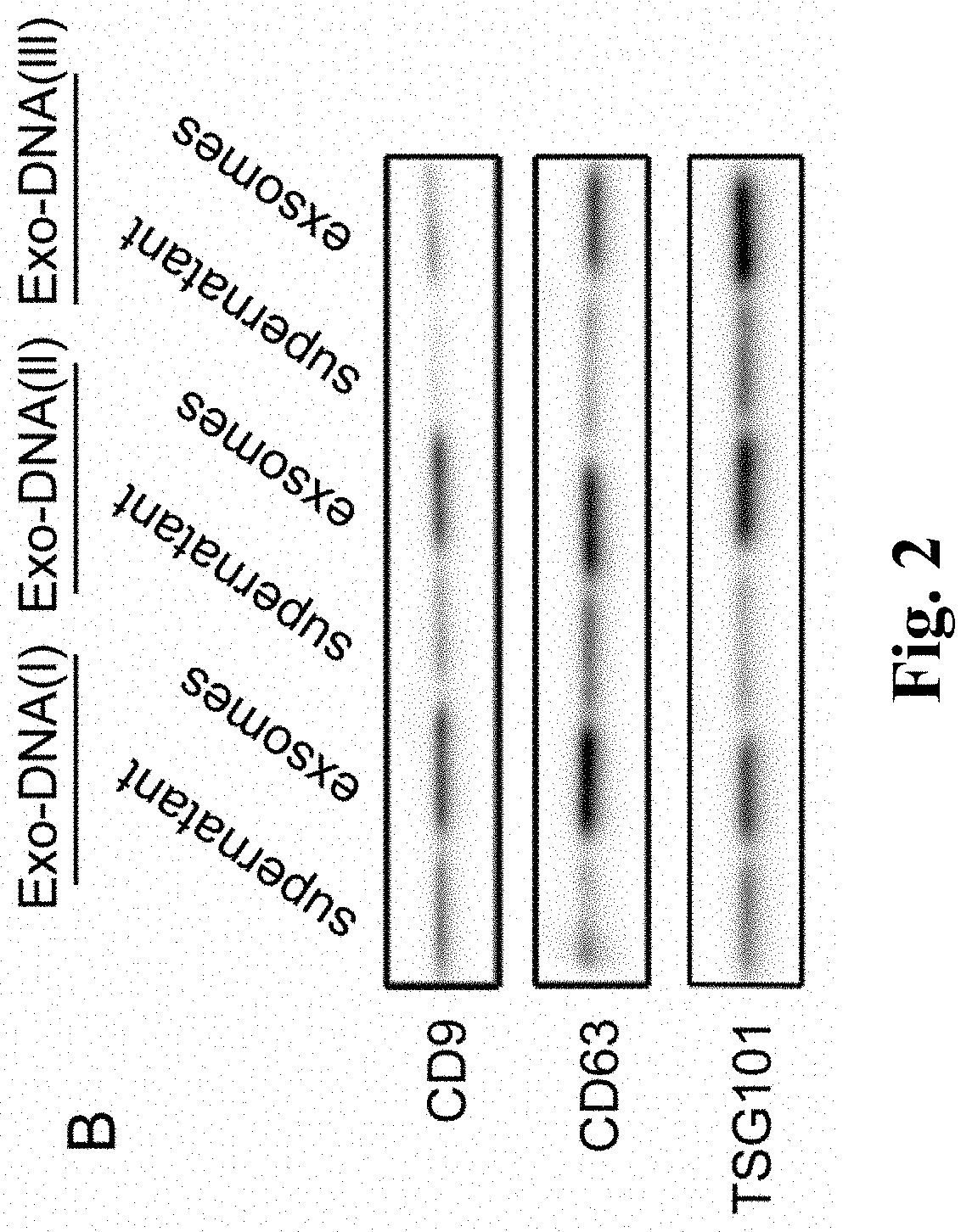
Biomarker applied to early diagnosis and preoperative evaluation of pheochromocytoma/paraganglioma as well as application thereof
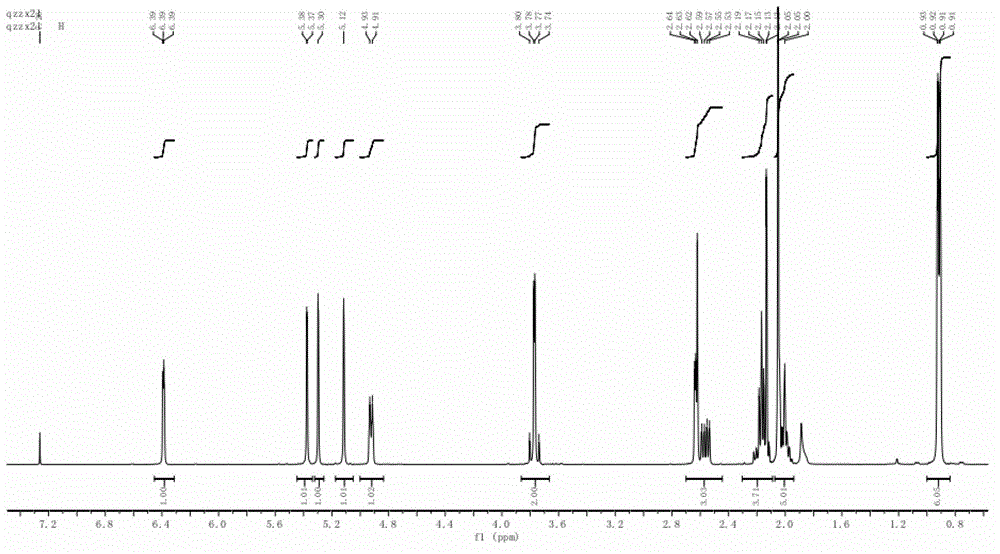
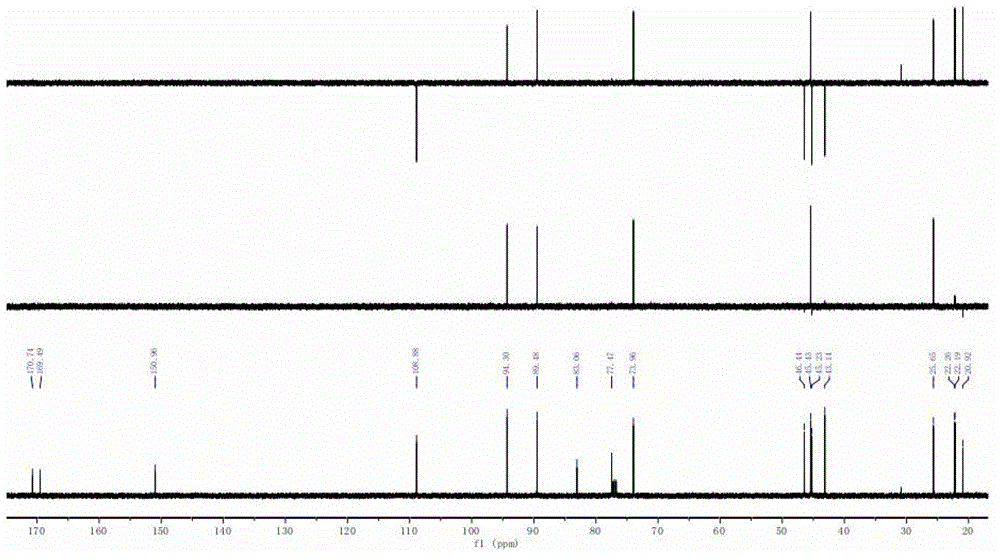
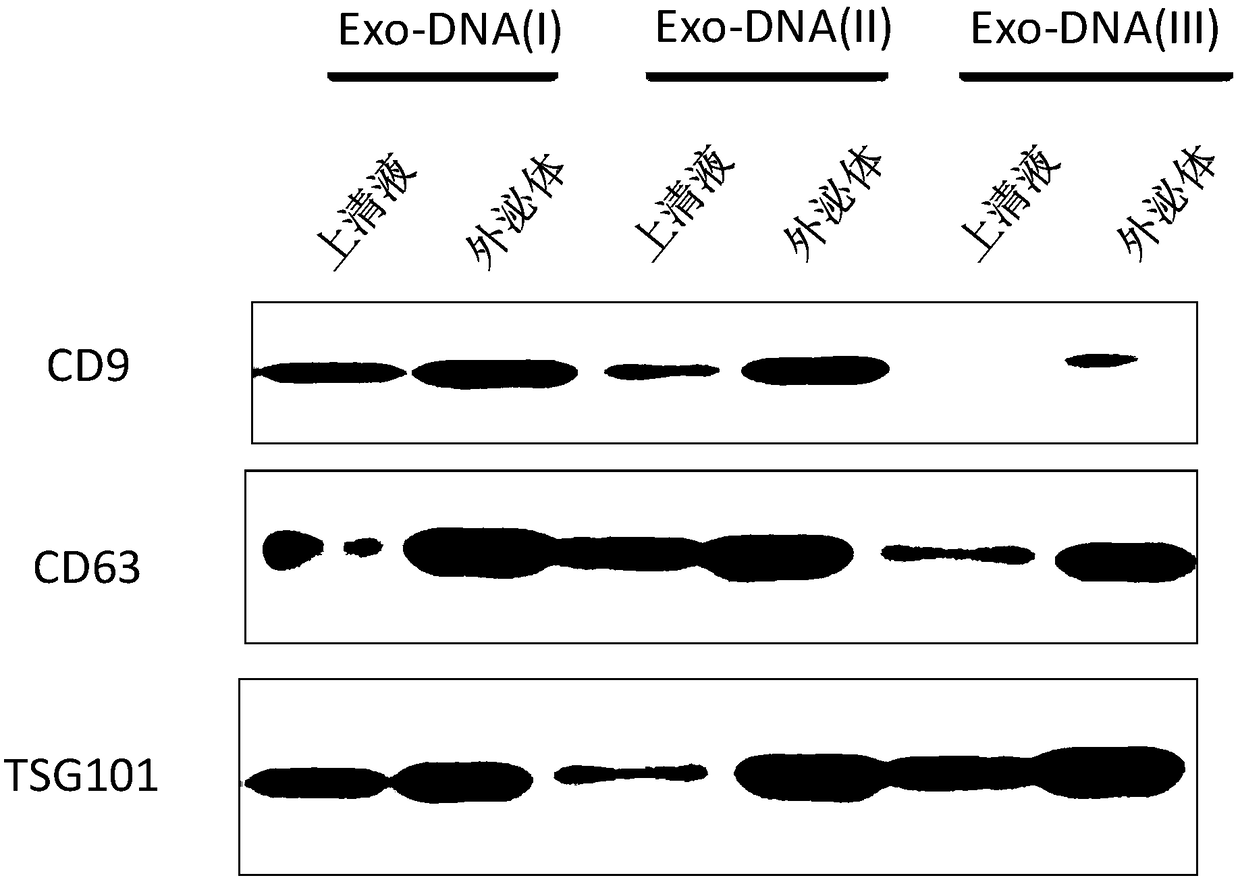
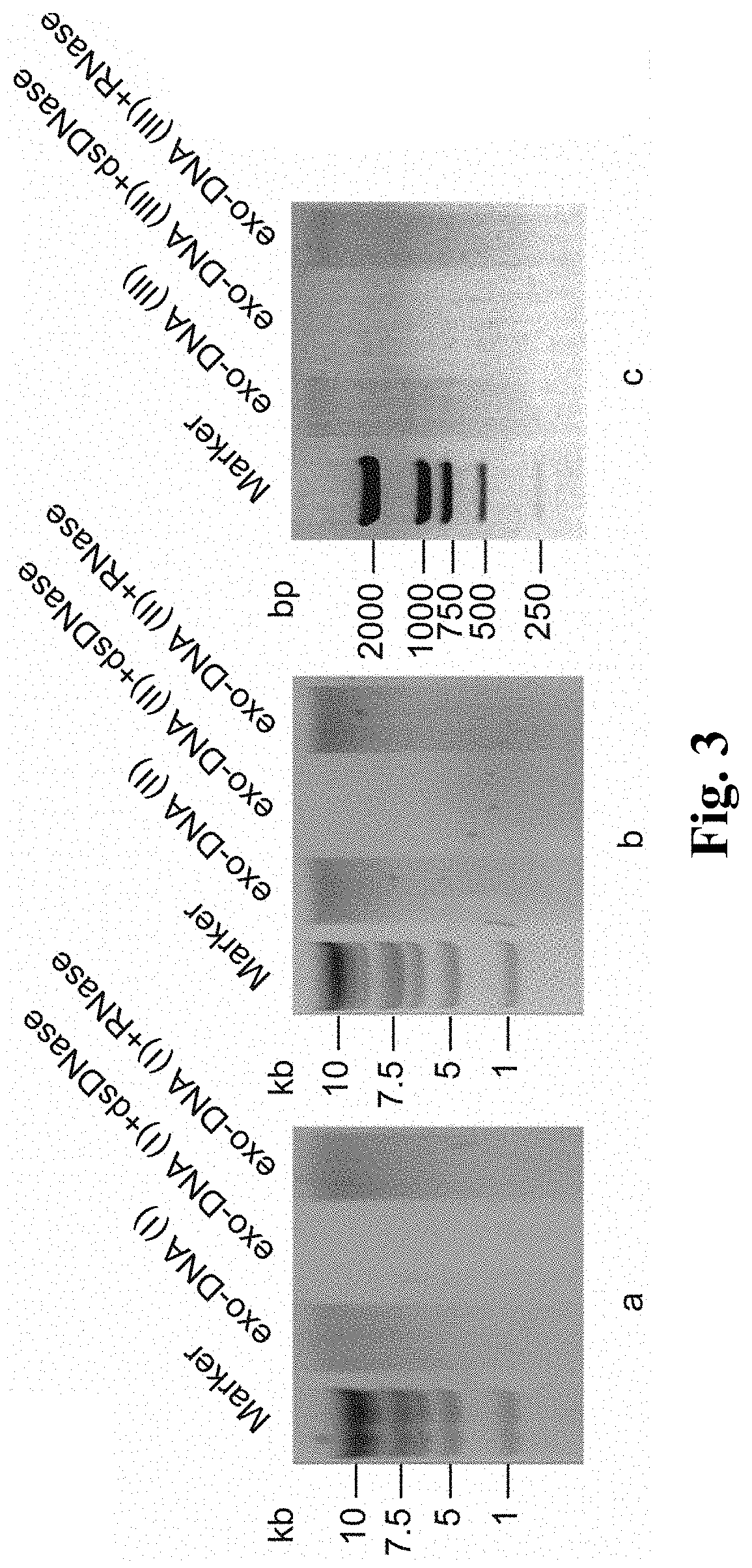
InactiveCN108588224AImprove consistencyMicrobiological testing/measurementDNA/RNA fragmentationPheochromocytomaMutation frequency
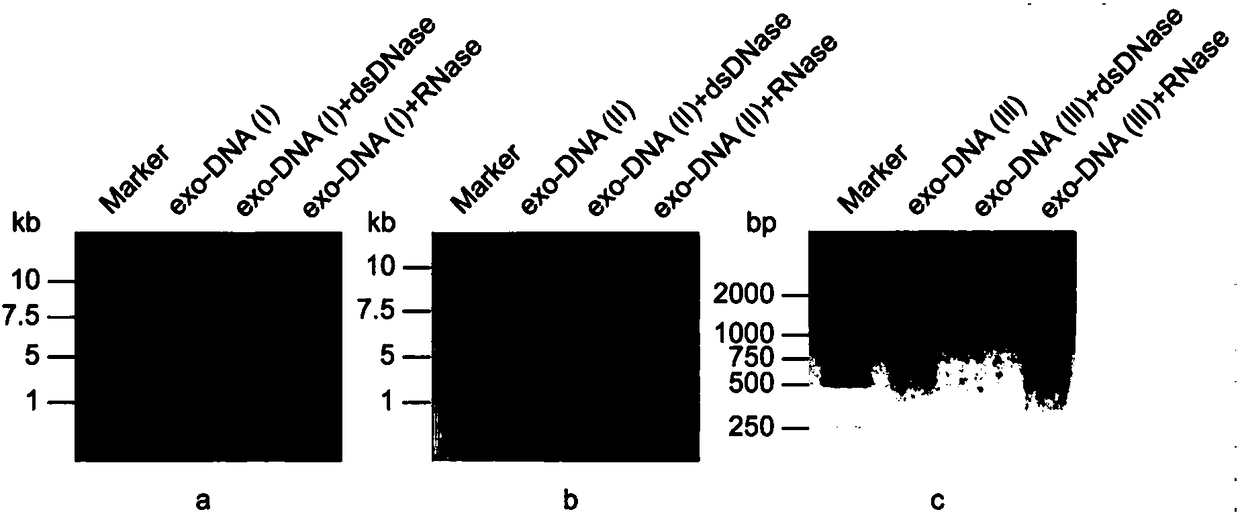
The invention discloses a double-chain DNA biomarker applied to early diagnosis and preoperative evaluation of pheochromocytoma / paraganglioma as well as application thereof. The biomarker is a genomedouble-chain DNA fragment specifically derived from pheochromocytoma / paraganglioma serum exosome. The double-chain DNA can represent the variation condition of RET, VHL and HIF2A with high body cell mutation frequency in a pheochromocytoma / paraganglioma susceptible gene as well as SDHB related to metastatic phenotype. The exosome DNA circulated in peripheral blood of patients comprises 97 percentchromosome locus information with the same source tumor cell. The invention provides the existence basis of the double-chain DNA in the pheochromocytoma / paraganglioma serum exosome; the double-chain DNA can be used for authenticating the mutation existing in the tumor cell; and a noninvasive molecular marker is provided for clinical diagnosis and preoperative evaluation of the pheochromocytoma / paraganglioma.
Owner:FIRST AFFILIATED HOSPITAL OF DALIAN MEDICAL UNIV
Cannabis based compositions and methods of treating hypertension
InactiveUS10105343B2Improve stabilityOrganic active ingredientsMagnoliophyta medical ingredientsCannabisPheochromocytoma
The invention relates to a Cannabis-based pharmaceutical composition for the treatment of hypertensive disorders by submucosal delivery comprising a pharmaceutically acceptable base and an effective amount of at least one cannabinoid or endocannabinoid containing extract of a cloned hybrid of the plant Cannabis sativa, subspecies sativa and Cannabis sativa, subspecies indica of the CTSX-ISS lineage; and methods of treatment of primary and secondary hypertension, the secondary hypertension resulting from pheochromocytoma, primary hyperaldosteronism, adrenal hyperplasia, pulmonary hypertension, portal hypertension, folate deficiency hypertension, arterial hypertension or familial hypertension by administration between one and eight times per day.
Owner:KUBBY PATENT & LICENSES LLC
Novel application of PAK3 as biological marker of neuroendocrine tumor
InactiveCN101520457AMature technology platformProcess specificationMicrobiological testing/measurementColor/spectral properties measurementsPheochromocytomaAbnormal tissue growth
The method relates to novel application of PAK3 as a biological marker of a neuroendocrine tumor. Through protein expression level detection of PAK3 on thymic carcinoid, pheochromocytoma, parathyroid adenoma, pituitary tumor, adrenal adenoma, cell strains of islet cell tumor, cell strains of small cell lung cancer and other neuroendocrine tumor tissues and cells, the PAK3 highly expresses and covers neuroendocrine tumors common in clinics and relates to each tissue and gland of body; the novel application initially finds that the PAK3 is relevant to tumors, in particular to the neuroendocrine tumor; the novel application discloses that the PAK3 can obviously increase the transferring capacity of the cell and shows that the PAK3 plays an important role in the developing process of the neuroendocrine tumor; and the result shows that the PAK3 can be used as the biological marker of the neuroendocrine tumor.
Owner:宁光
Application of ERBB2 protein in preparation of reagent for detecting pheochromocytoma metastasis
InactiveCN104062437ARapid diagnosisAccurate diagnosisBiological testingPheochromocytomaClinical manifestation
The invention belongs to the field of biological medicines, and particularly relates to application of ERBB2 protein in preparation of a reagent for detecting pheochromocytoma metastasis. 262 patients suffering from pheochromocytoma, confirmed through operations and pathology, are selected, and the pheochromocytoma metastasis is not found in the patients when the patients are hospitalized. Basic clinical data of the patients are collected, such as genders, ages, clinical manifestations, biochemical detection results, pathological results, imaging data and pathology results. Experimental results show that the high expression of the ERBB2 protein in cases of pheochromocytoma metastasis is achieved, so that the close relationship exists between the ERBB2 protein and the pheochromocytoma metastasis. The invention verifies that the pheochromocytoma metastasis has a close relationship with the ERBB2 protein expression, and the pheochromocytoma metastasis is confirmed rapidly and accurately by detection on ERBB2. The invention provides experimental data and a theoretical basis for clinic application of the ERBB2 protein in the detection on the pheochromocytoma metastasis.
Owner:RUIJIN HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
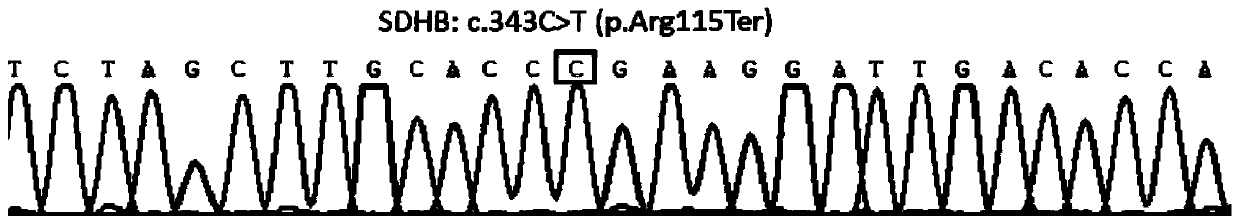
Detection method of gene mutation of mitochondrial succinate dehydrogenase and kit
InactiveCN101684486AStrong specificityHigh amplification efficiencyMicrobiological testing/measurementDNA preparationPheochromocytomaSuccinate dehydrogenase
The invention discloses a reagent for detecting whether gene mutation of mitochondrial succinate dehydrogenase (SDH) exists in samples and a kit containing the reagent. The reagent or the kit can be used for susceptibility analysis of pheochromocytoma. The invention also discloses an optimized method by which SDH gene amplification products are obtained and a method for determining whether gene mutation of SDH exists in the nucleic acid samples to be tested.
Owner:RUIJIN HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
Extracting method and application of plant composite containing gastrodia elata
The invention provides an extracting method of a plant composite containing gastrodia elata. The plant composite is prepared from the raw material herbs of 100 g of gastrodia elata, 60 g of pteris biaurita, 50 g of ageratum conyzoides and 150 g of lysimachia fortunei maxim. The plant composite is prepared by the adoption of the microwave extracting method, in this way, the content is greatly increased, and the usage amount is reduced. The invention further provides an application of the plant composite containing the gastrodia elata in preparation of medicine for restraining rat adrenal medulla pheochromocytoma PC12 cell proliferation.
Owner:南京多宝生物科技有限公司
E-ring substituted silybin derivative and preparation method and medical application thereof
InactiveCN101781292AImproves antioxidant activityReduce oxidative damageOrganic active ingredientsNervous disorderPheochromocytomaDisease
The invention relates to an E-ring substituted silybin derivative and a preparation method and medical application thereof, in particular to synthesis of the E-ring substituted silybin derivative and medical application of the compound or the pharmaceutically acceptable salts of the compound in preparing the medicines for preventing physiological disorders or diseases caused by oxidative stress. The compound has the activity of protecting the hydrogen peroxide induced rat adrenal pheochromocytoma (PC12) cell from injury, namely having the anti-oxidative injury protection effect on the PC12 cell simulating the brain nerve cell, and also has the activity of suppressing xanthine oxidase, thus the compound can be expectedly developed to have the medical applications in preparing the medicines for preventing the neurodegenerative diseases such as oxidation of the brain nerve cell, senile dementia and the like, and the medicines for preventing the diseases caused by xanthine oxidase, such as gout.
Owner:DALI UNIV
Chromogranin A detection reagent, detection reference interval and detection method
InactiveCN109507432AImprove detection accuracyShorten the detection cycleBiological testingChromogranin APheochromocytoma
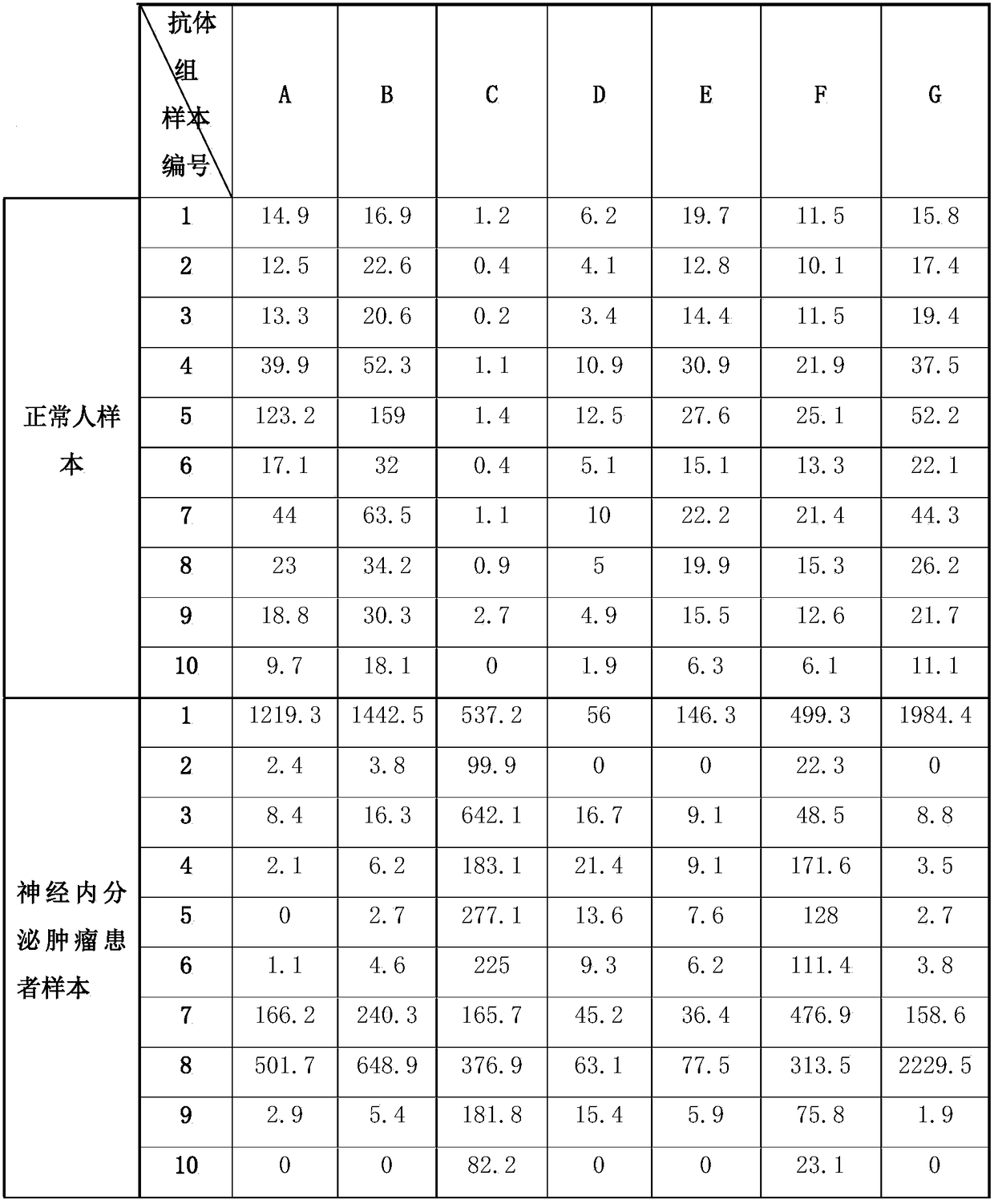
The invention discloses a chromogranin A detection reagent. The chromogranin A detection reagent comprises an immune complex and an antibody detection system, wherein the immune complex is formed by compounding a marked first specific antibody, a marked second specific antibody, a chromogranin A calibration product, a chromogranin A quality control product and a sample to be detected; the first specific antibody and the second specific antibody are formed by carrying out epitope specific binding on antibodies and amino acid sites in a human chromogranin A amino acid sequence; the amino acid sites comprise: 1st to 113rd sites, 131st to 143rd sites, 337th to 364th sites and 373rd to 439th sites; the detection reagent can be used for quantitatively detecting the concentration of chromograninA in human blood plasma and is clinically mainly used for auxiliary diagnosis and differential diagnosis on pheochromocytoma and neuroendocrine neoplasm; the reference interval of the invention has ahigh conformity degree with clinical diagnosis, and the accuracy of the auxiliary diagnosis is improved; a detection method developed by a full-automatic magnetic bead chemiluminescence platform is further utilized by the invention and the detection period is shortened.
Owner:EPITOPE BIOTECH
Preparation method of metaiodobenzylguanidine (MIBG) sulphate
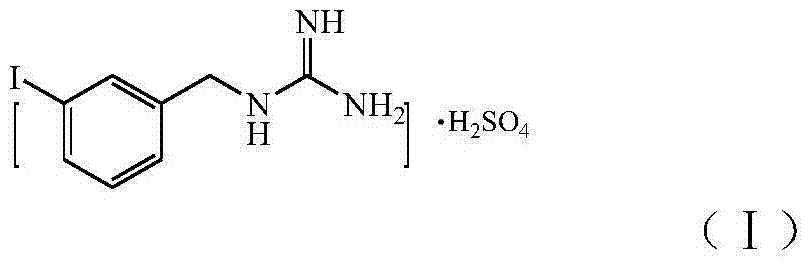
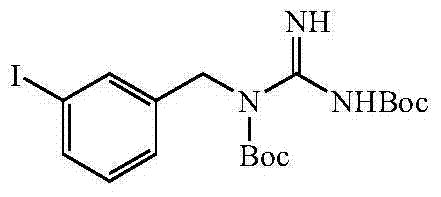
InactiveCN104496856ALow skill level requiredImprove securityOrganic chemistryOrganic compound preparationPheochromocytomaNeuroblastic Tumor
The invention relates to the technical field of radiopharmaceuticals and in particular relates to a preparation method of a radiopharmaceutical *I-metaidobenzylguanidine labelled precursor metaiodobenzylguaidine sulphate applied in the aspects of treatment and diagnosis of neuroendocrine carcinoma including human pheochromocytoma, neuroblastoma and the like. By applying the provided preparation method of MIBG sulphate, MIBG sulphate purity reaches up more than 95%, a technology is simple and optimized compared with the existing MIBG sulphate preparation method, and economic cost is greatly reduced, so that the provided preparation method of MIBG sulphate is applicable to industrial large-scale production application.
Owner:BEIJING ZHIBO BIO MEDICAL TECH +1
Application of needle-leaved polyprenol in preparing medicines for preventing and treating Alzheimer disease
InactiveCN102028674AHas a preventive effectNo side effectsNervous disorderHydroxy compound active ingredientsPheochromocytomaLarch
The invention belongs to the technical field of medicines, and discloses an application of needle-leaved polyprenol in preparing medicines for preventing and treating Alzheimer disease. The needle-leaved polyprenol is obtained from needle leaves of pinaceae plants such as pinus, picea, larch, fir and cedar and the like by extraction, separation and purification. The needle-leaved polyprenol is long-chain polyterpene compound formed by 14-24 isopentene group units. Cell and animal experimental results indicates that the needle-leaved polyprenol has functions of preventing and treating the Alzheimer disease, i.e., the needle-leaved polyprenol has a protection function for damage of pheochromocytoma (PC) 12 nerve cells caused by amyloid-beta protein, and can improve the passive avoidance memory capability and space learning memory capability of demented mice. Therefore, the needle-leaved polyprenol can be used for preparing the medicines for preventing and treating the Alzheimer disease.
Owner:INST OF CHEM IND OF FOREST PROD CHINESE ACAD OF FORESTRY
Extraction method and application of gastrodia elata oligosaccharide GEP-2
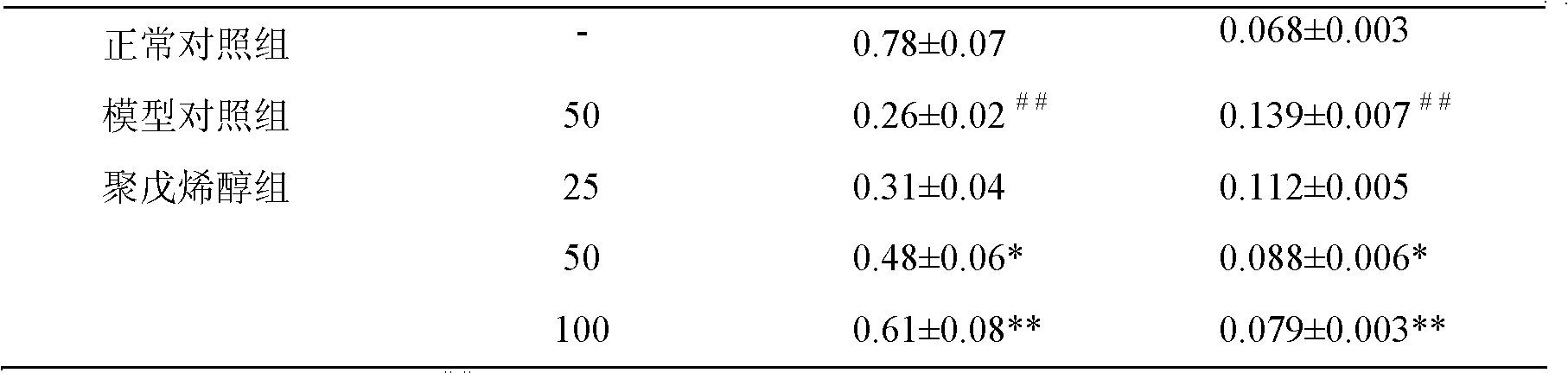
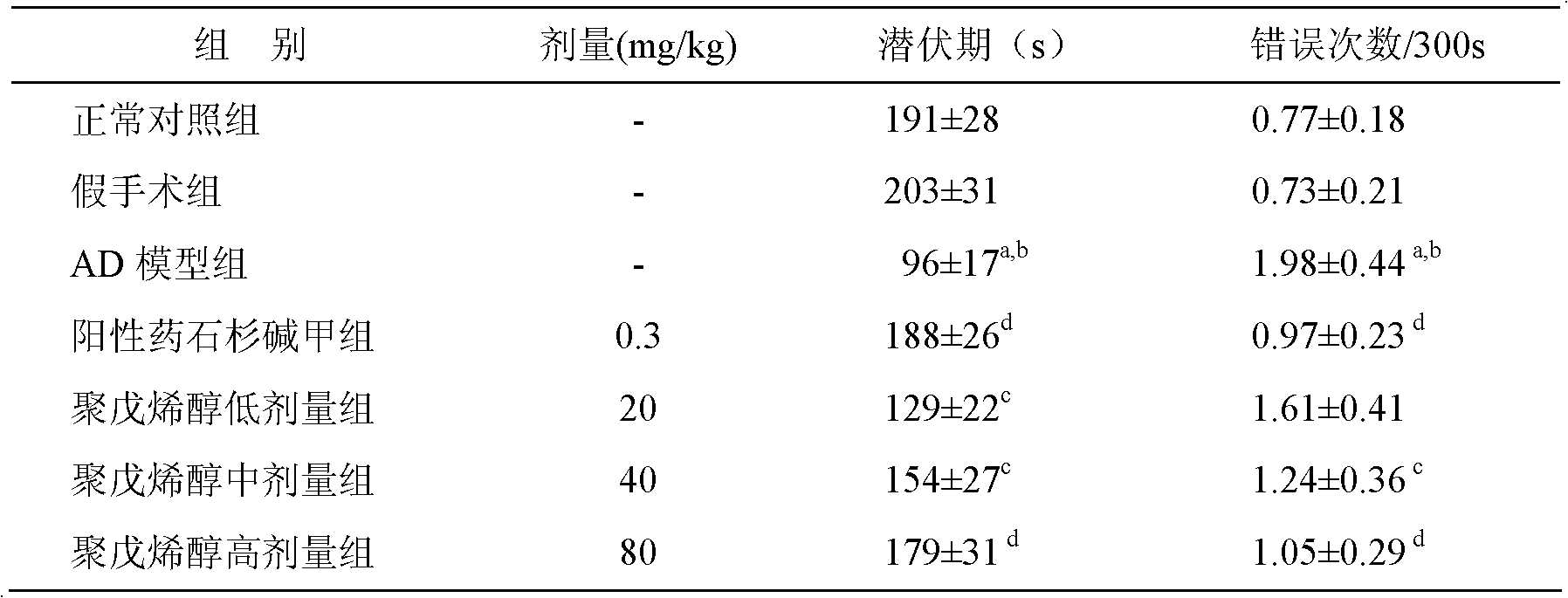
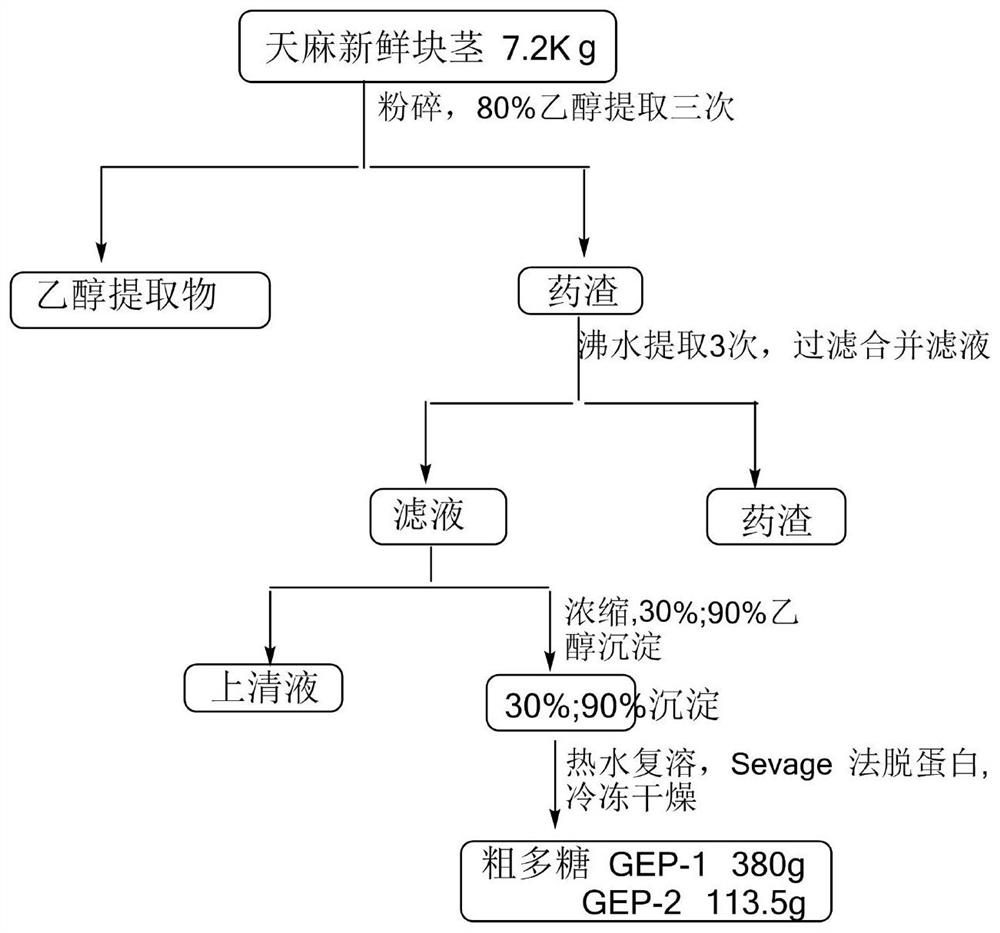
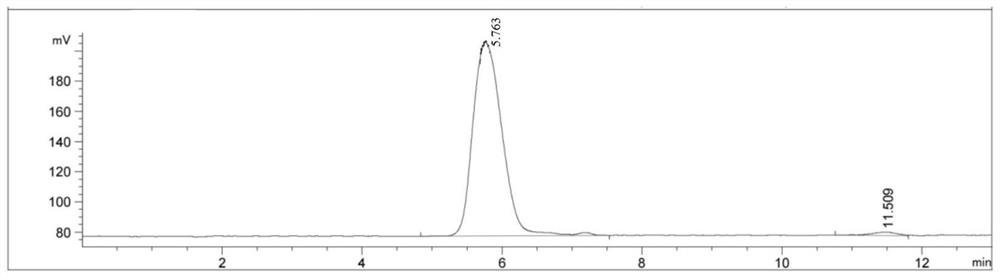
ActiveCN114133461AExcellent NGF activityImprove dissolution stateNervous disorderFood ingredient functionsPheochromocytomaPharmaceutical drug
The invention belongs to the technical field of medicines and biology, and particularly relates to an extraction method and application of gastrodia elata oligosaccharide GEP-2. The invention provides an extraction method of gastrodia elata oligosaccharide GEP-2. The gastrodia elata oligosaccharide GEP-2 provided by the invention has a similar net-shaped structure for preparing a dissolution assisting platform, and the dissolution state of an extract can be remarkably improved. Meanwhile, the gastrodia elata oligosaccharide GEP-2 also has excellent activity similar to nerve growth factors (NGF), and can induce nerve cell differentiation and promote pheochromocytoma (PC12) differentiation. Tests prove that the gastrodia elata oligosaccharide GEP-2 provided by the invention can effectively improve the differentiation rate of nerve cells.
Owner:KUNMING INST OF BOTANY - CHINESE ACAD OF SCI
Cannabis based compositions and methods of treating hypertension
InactiveUS20180104213A1Improve stabilityOrganic active ingredientsMagnoliophyta medical ingredientsPheochromocytomaCannabis
The invention relates to a Cannabis-based pharmaceutical composition for the treatment of hypertensive disorders by submucosal delivery comprising a pharmaceutically acceptable base and an effective amount of at least one cannabinoid or endocannabinoid containing extract of a cloned hybrid of the plant Cannabis sativa, subspecies sativa and Cannabis sativa, subspecies indica of the CTSX-ISS lineage; and methods of treatment of primary and secondary hypertension, the secondary hypertension resulting from pheochromocytoma, primary hyperaldosteronism, adrenal hyperplasia, pulmonary hypertension, portal hypertension, folate deficiency hypertension, arterial hypertension or familial hypertension by administration between one and eight times per day.
Owner:KUBBY PATENT & LICENSES LLC
Catecholamine specific response type iron-doped carbon nanodot as well as preparation method and application thereof
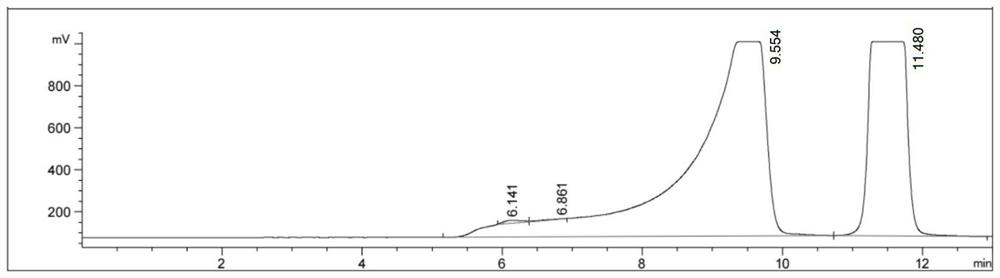
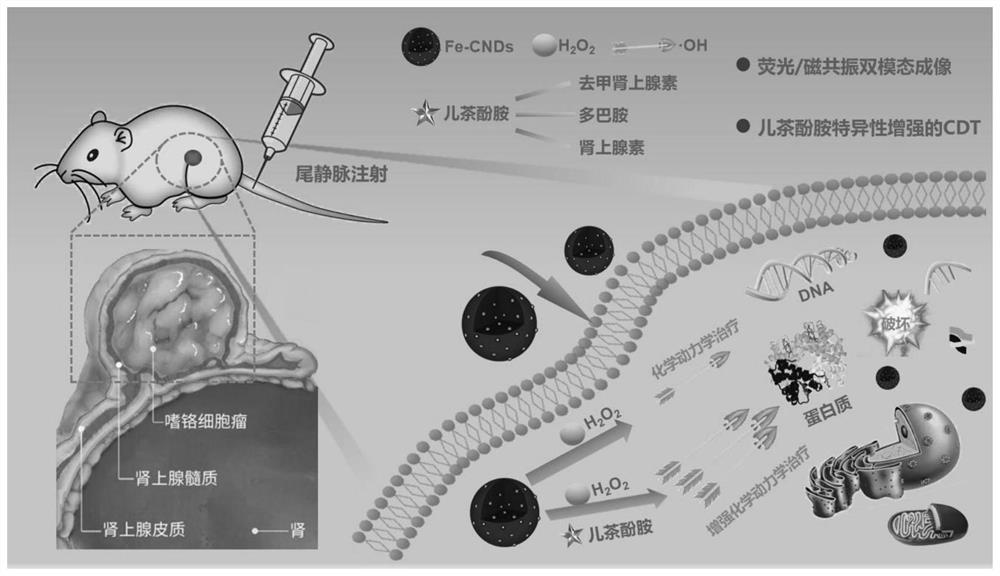
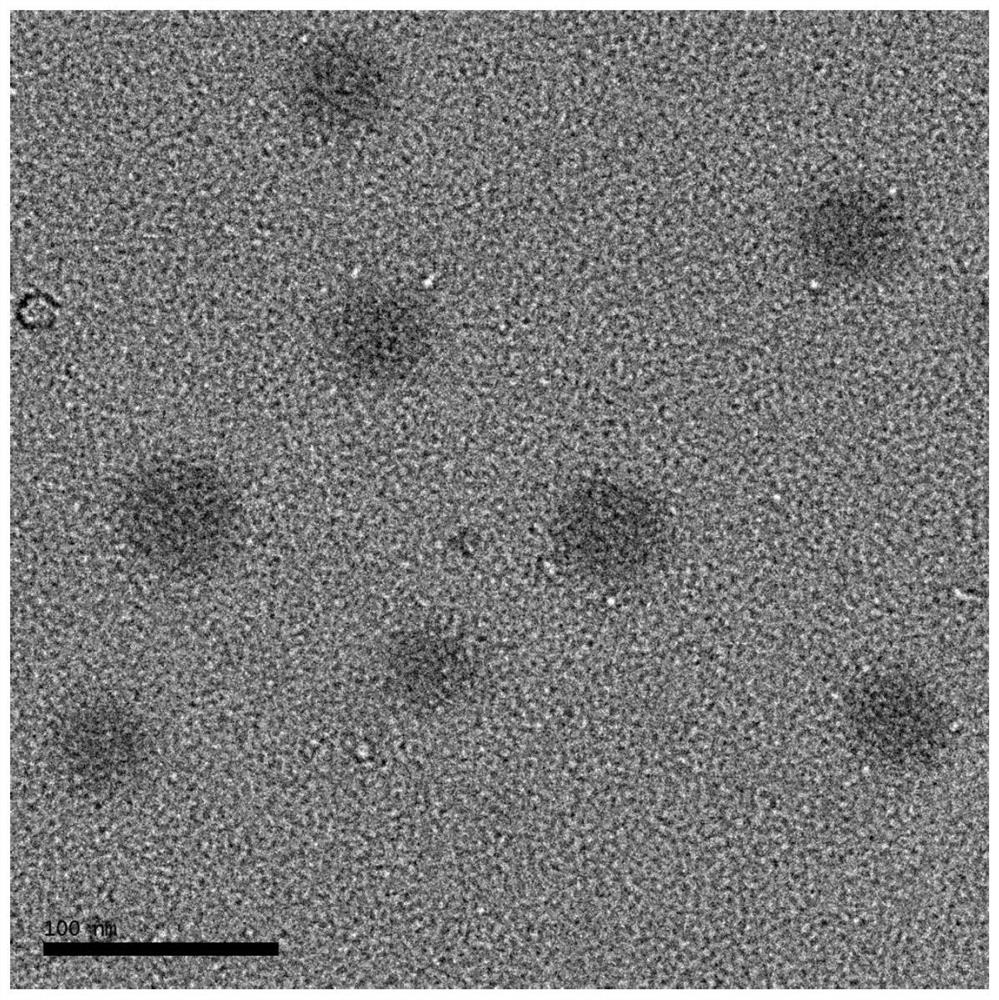
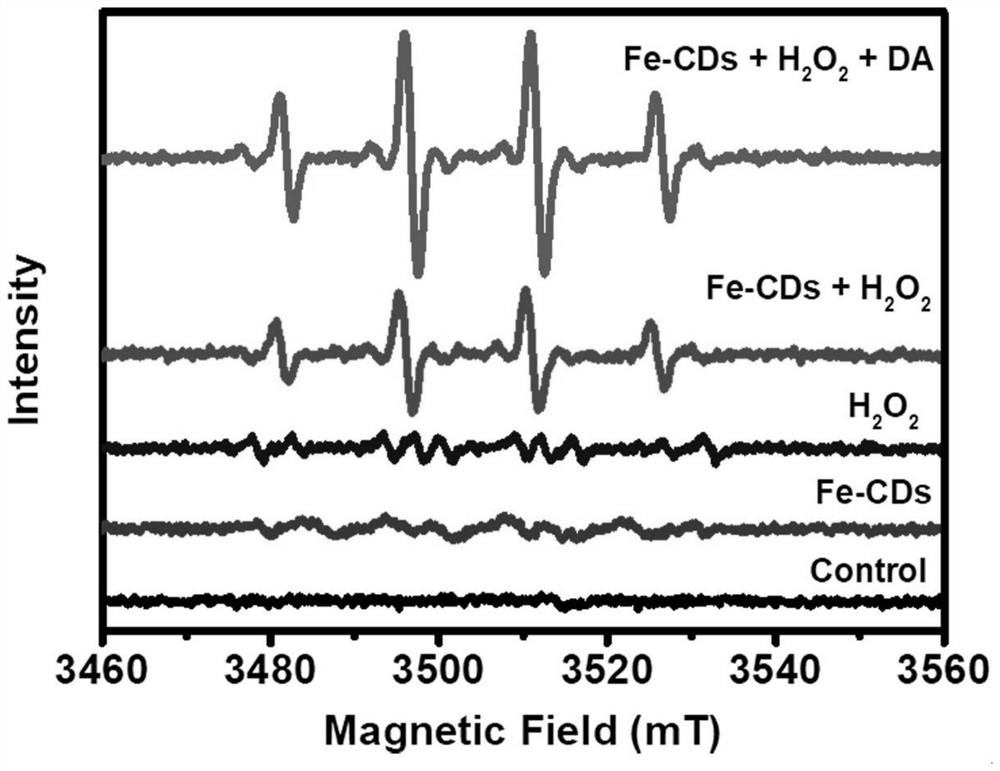
ActiveCN113801657AConvenient diagnosis and treatmentGood biocompatibilityMaterial nanotechnologyGeneral/multifunctional contrast agentsPheochromocytomaSodium acetate
The invention provides a catecholamine specific response type iron-doped carbon nanodot as well as a preparation method and application thereof, and solves the technical problems that the existing carbon nanodot is limited by an external light source when being used for treating tumors, pheochromocytoma and the like are difficult to locate and diagnose, the tumor is difficult to treat timely and effectively due to easy metastasis, and the tumor which is easy to relapse after operation cannot have a good positioning, diagnosis and treatment effect. The carbon nanodot contains ferrous ions and is prepared from the following components in parts by weight: 20-200 parts of a carbon source, 20-200 parts of an iron source, 20-200 parts of a nitrogen source, 20-200 parts of sodium acetate, 20-200 parts of a passivating agent and 40-160 parts of a solvent.
Owner:NORTHWESTERN POLYTECHNICAL UNIV
Noon tea granules and preparation method and application thereof
InactiveCN106110093ASimple structureEasy to operatePharmaceutical product form changeGranular deliveryPheochromocytomaAlcohol
The invention provides noon tea granules. The noon tea granules are prepared through the steps that 50 g of rhizoma atractylodis, 50 g of radix bupleuri, 50 g of notopterygium roots, 50 g of radix saposhnikoviae, 50 g of radix angelicae, 50 g of rhizoma chuanxiong, 50 g of herba pogostemonis, 50 g of radix peucedani, 50 g of fructus forsythiae, 50 g of pericarpium citri reticulatae, 50 g of fructus crataegi, 50 g of fructus aurantii immaturus, 75 g of roasted malt, 50 g of licorice roots, 75 g of radix platycodonis, 50 g of stir-fried medicated leaven, 75 g of folium perillae, 75 g of cortex magnoliae officinalis and 1,600 g of black tea are taken, water extraction and alcohol precipitation are conducted to obtain a concentrated solution, supercritical extraction is conducted to obtain a supercritical extract, and the noon tea granules are prepared through a conventional method. The prepared noon tea granules are high in platycodin D content, and application of the noon tea granules in preparation of drugs for inhibiting rat adrenal pheochromocytoma PC-12 cell proliferation is found.
Owner:NANJING ZHENGKUAN MEDICAL TECH
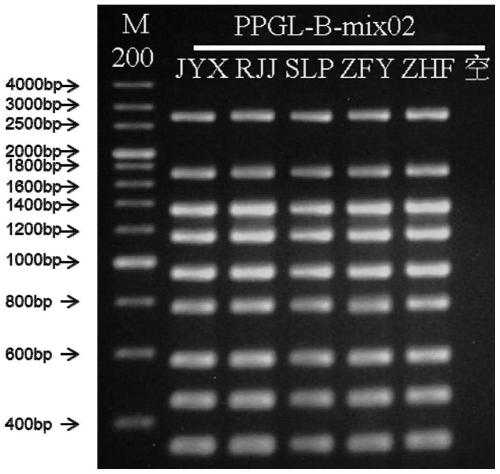
Primer group for detecting pathogenic gene mutation of pheochromocytoma and paraganglioma and application method of primer group
PendingCN111500726AHigh detection throughputImprove detection efficiencyMicrobiological testing/measurementDNA/RNA fragmentationPheochromocytomaGenes mutation
The invention provides a primer group for detecting pathogenic gene mutation of pheochromocytoma and paraganglioma and an application method of the primer group. The primer group comprises at least one primer pair of twenty primer pairs from SEQ ID NO.1 to SEQ ID NO.40 and a corresponding sequencing primer. According to the scheme, the detection flux of pathogenic gene mutation of the pheochromocytoma and paraganglioma can be improved.
Owner:BEIJING HARMONY HEALTH MEDICAL DIAGNOSTICS CO LTD
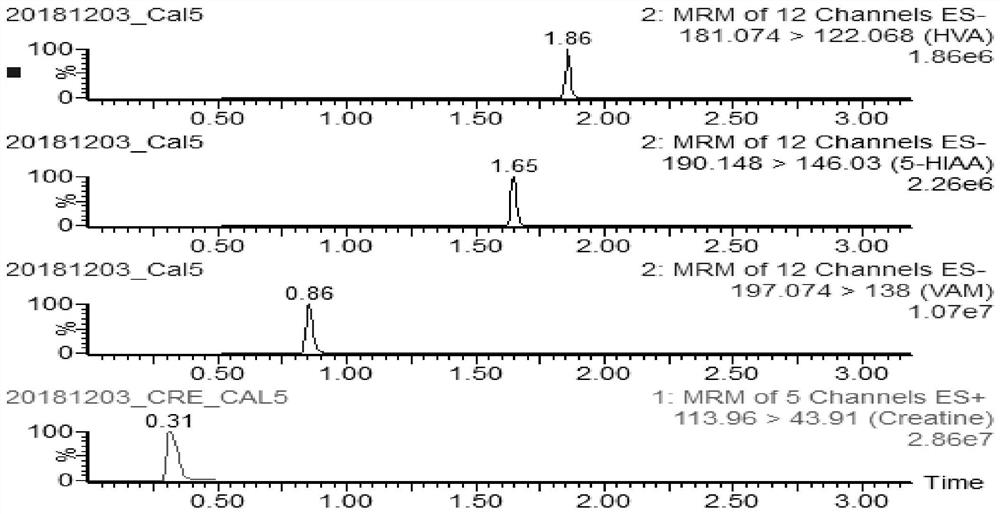
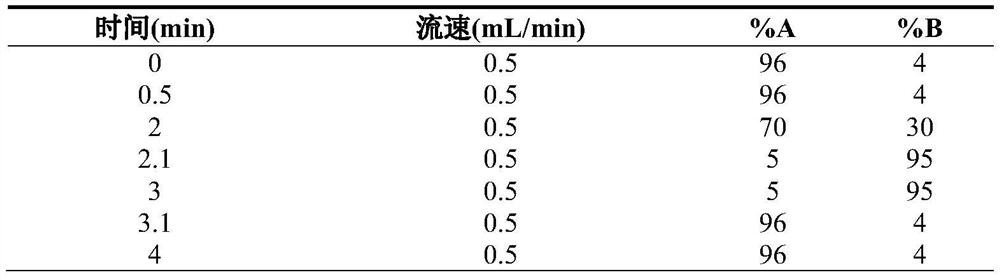
Method and kit for rapidly detecting homovanillic acid, vanillylmandelic acid, 5-hydroxyindoleacetic acid and creatinine in human urine
PendingCN112946150ARapid quantitationImprove throughputComponent separationPheochromocytomaHuman body
The invention discloses a method and a kit for rapidly detecting homovanillic acid, vanillylmandelic acid, 5-hydroxyindoleacetic acid and creatinine in human urine. The method comprises the following steps of 1) preparing a sample dilution working solution, 2) under a dark condition, taking a urine sample, adding EDTA and Na2S2O3 into the urine sample, then adding acetic acid for acidification, and uniformly mixing to obtain a to-be-detected urine sample, and (3) mixing a to-be-detected urine sample, a calibration product, a urine quality control product and the sample diluent working solution, sealing a film, vorticing, centrifuging, taking supernate, and detecting by adopting liquid chromatography-mass spectrometry. When a urine sample prepared from random urine is detected, homovanillic acid, vanillylmandelic acid, 5-hydroxyindoleacetic acid and creatinine can be quantified at a time only by 10 microliters of urine sample, the pretreatment method is simple and rapid, the operation time is saved, neuroblastoma and carcinoid in a human body can be effectively screened and diagnosed according to the indexes, and the pheochromocytoma can be screened in an auxiliary manner.
Owner:BGI CLINICAL LAB (SHENZHEN) CO LTD
Epinephrine luminescence immunodetection kit
ActiveCN109444436AAccurate measurementHigh sensitivityChemiluminescene/bioluminescenceBiological testingPheochromocytomaBlood plasma
The invention discloses an epinephrine luminescence immunodetection kit. The epinephrine luminescence immunodetection kit comprises a detection system and a sample pretreatment system, wherein the detection system comprises a solid phase carrier which is coated with an epinephrine antibody directly or indirectly, an epinephrine antibody solution, an avidin connection tracer solution and a calibration product; the sample pretreatment system comprises an enrichment material, an acylating agent and methylase. By adopting the epinephrine luminescence immunodetection kit, the defects and disadvantages in the existing epinephrine detection method are overcome. Firstly, epinephrine in urea or plasma is subjected to enrichment, acylation and methylation pretreatment, and is adsorbed and separatedwith a cis-diol specific affine medium. The scheme of acylation with the acylating agent having biotin at one end is adopted, so that the content of epinephrine in a sample is accurately determined inconjunction with avidin connected with a tracing marker after the acylated or methylated epinephrine is recognized by an antibody and reacted. The kit has the advantages of high sensitivity and precision, detection automation is realized by aluminescence technology, and the clinical diagnosis of pheochromocytoma is assisted.
Owner:AUTOBIO DIAGNOSTICS CO LTD
Pharmaceutical composition containing monosialotetrahexosyl ganglioside sodium and application thereof
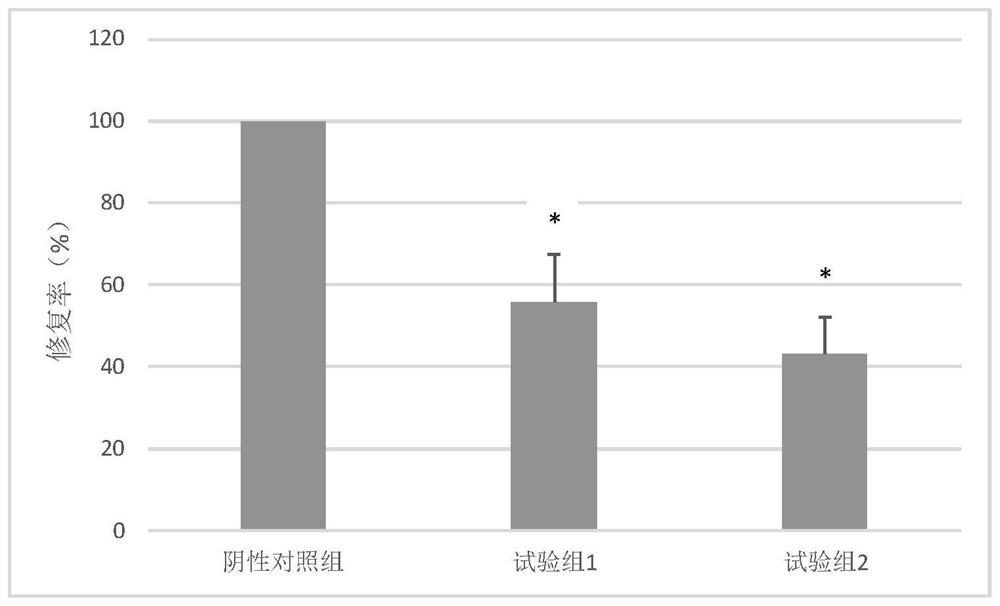
ActiveCN114272268ATo promote metabolismImprove protectionOrganic active ingredientsSenses disorderPheochromocytomaBULK ACTIVE INGREDIENT
The invention relates to a pharmaceutical composition containing monosialotetrahexosyl ganglioside sodium, the active ingredients of the composition comprise 0.1-1% (w / w) of monosialotetrahexosyl ganglioside sodium and a rabbit meat extract containing compound amino acid with the concentration of 1-20 mg / ml, the pH value of the composition is 6-8, and the content of the compound amino acid is 1-20 mg / ml. The mass ratio of the monosialotetrahexosyl ganglioside sodium to the compound amino acid in the composition is 1: (1-10). The pharmaceutical composition disclosed by the invention has the beneficial effects of reducing the volume of encephaledema, improving ischemia and hypoxia conditions of a central nervous system, repairing and protecting nerves, remarkably improving the repair rate of pheochromocytoma damaged cells and the like.
Owner:BEIJING SIHUAN PHARMA +1
Biomarker for Early Diagnosis and Preoperative Assessment of Pheochromocytoma/Paraganglioma, and Application thereof
A double-stranded DNA biomarker derived from exosomes is useful in early diagnosis and preoperative evaluation of pheochromocytoma and paraganglioma, and applications thereof. The biomarker is genome double-stranded DNA fragment specifically derived from exosomes in blood serum of pheochromocytoma and paraganglioma patients. The double-stranded DNA can represent variations of RET, VHL, and HIF2A with high frequency of somatic cell mutation, and metastatic phenotype-related SDHB, which are susceptibility genes of PCCs and PGLs. The circulating exosome DNA in patient's peripheral blood contains 97% of the same chromosomal point mutation information as the tumor cells from which the DNA originated. There is evidence off the existence of double-stranded DNA in the serum exosomes of PCCs and PGLs. The double-stranded DNA can be used to identify mutations in tumor cells and provide a noninvasive molecular marker for the clinical diagnosis and preoperative evaluation of PCCs and PGLs.
Owner:FIRST AFFILIATED HOSPITAL OF DALIAN MEDICAL UNIV
Detection method of gene mutation of mitochondrial succinate dehydrogenase and kit
InactiveCN101684486BStrong specificityHigh amplification efficiencyMicrobiological testing/measurementDNA preparationPheochromocytomaMitophagy
The invention discloses a reagent for detecting whether gene mutation of mitochondrial succinate dehydrogenase (SDH) exists in samples and a kit containing the reagent. The reagent or the kit can be used for susceptibility analysis of pheochromocytoma. The invention also discloses an optimized method by which SDH gene amplification products are obtained and a method for determining whether gene mutation of SDH exists in the nucleic acid samples to be tested.
Owner:RUIJIN HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
Application of total flavonoid extract of bunge pricklyash leaves in anti-hypoxia
InactiveCN110327395AGood treatment effectAntinoxious agentsPlant ingredientsPheochromocytomaCell damage
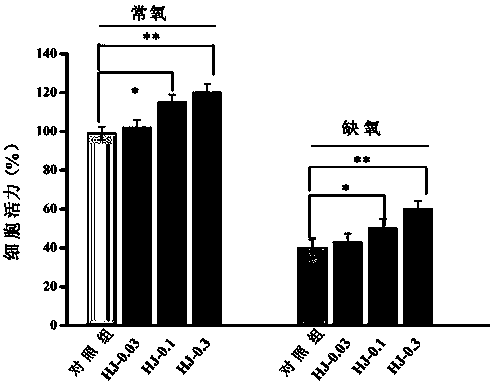
The invention provides a total flavonoid extract of bunge pricklyash leaves with anti-hypoxia activity. A rat adrenal pheochromocytoma PC12 cell experiment shows that the total flavonoid extract of the bunge pricklyash leaves has a protective effect on PC12 cell damages caused by hypoxia, the cell viability under the condition of improving the hypoxia is achieved, brain tissue damages caused by the hypoxia are alleviated, the obvious anti-hypoxia activity is achieved, the decrease of the cell viability under the hypoxic condition can further be significantly reversed, and therefore, the totalflavonoid extract can be used as an active substance for the preparation of anti-hypoxia drugs.
Owner:LANZHOU UNIVERSITY +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com