Novel iridoids compound and neuroprotective effect thereof
A technology of compounds and drugs, which is applied in the field of preparing drugs for the treatment and prevention of central nervous system diseases, and can solve problems such as compounds that have not yet been seen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The preparation of structural formula I compound
[0030] The dry rhizome powder (20kg) of spider incense (Valeriana jatamansi Jones) was used as raw material, extracted by cold soaking with 95% industrial ethanol for 4 times, 24h each time, and the extract was concentrated under reduced pressure to obtain 2.8kg brown viscous extract. The extract was dissolved in distilled water, extracted successively with petroleum ether, ethyl acetate, and n-butanol to obtain 450 g of ethyl acetate extract. Take 400g of ethyl acetate extract, go through silica gel (100-200 mesh) column chromatography, elution with chloroform-methanol system gradient (100:1-1:1), and thin-layer chromatography to detect and combine similar parts, collect and combine into Fractions 1, 2, 3, and 4, the second fraction (130g) was chromatographed on a silica gel (100-200 mesh) column again, and eluted with petroleum ether-acetone system gradient (30:1-1:1), Thin-layer chromatographic detection combined si...
Embodiment 2
[0032] Identification of Compounds of Structural Formula I
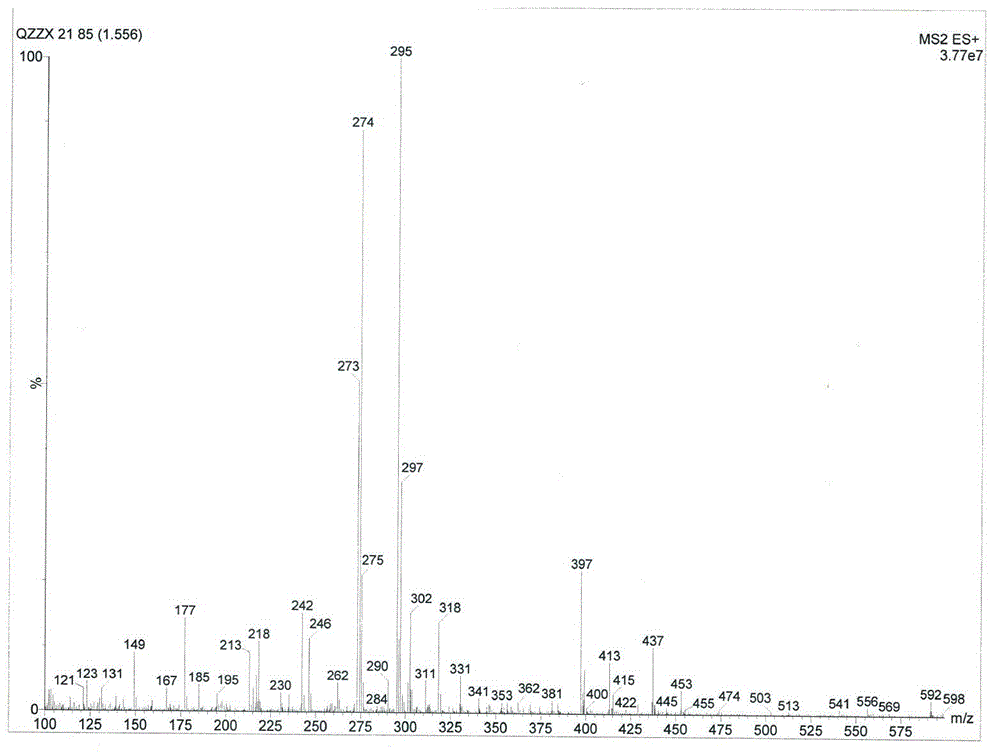
[0033] Compound of structural formula Ⅰ, yellow oil, high resolution mass spectrometry HRESIMS (see figure 1 ) gives an m / z of 390.5, confirming its molecular formula as C 17 h 23 ClO 7 , with an unsaturation of 6.
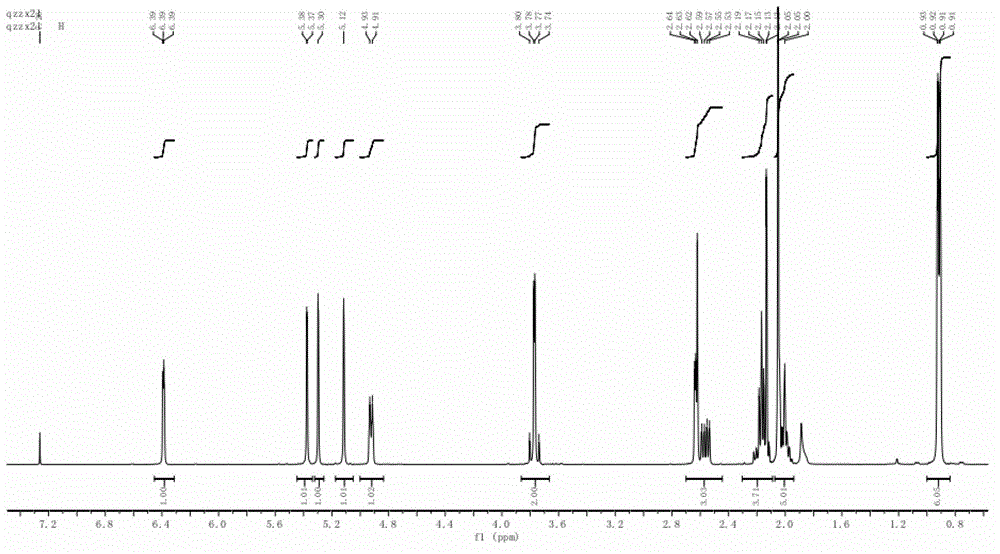
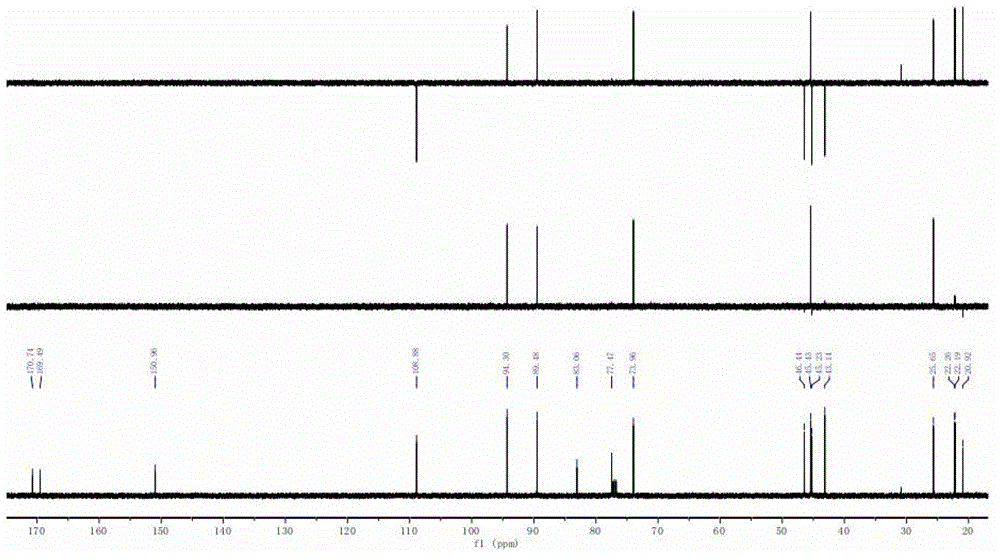
[0034] according to 1 H NMR (see figure 2 )with 13 C NMR (see image 3 ) can be judged as the iridoid compound skeleton, combined with the molecular weight, its molecular formula is determined to be C 17 h 23 ClO 7 . Through two-dimensional NMR HMBC (see Figure 4 ) and COZY (see Figure 5 ) spectrum can see that H-3 is related to C-1 / C-4 / C-11 / C-8 / C-5, and it can be judged that the 3 and 8 positions are connected by epoxy bonds, and H-10 and C- 7 / C-8 / C-9 are correlated, H-1 / H-9 and H-6 / H-7 are COZY correlated. δ H1 6.41(d, 3.4) and δ H9 The coupling constants of 2.65(d, 3.4) are both 3.4. According to the literature, H-1 / H-9 should be trans.
[0035] Based on the analysis of the above d...
Embodiment 3
[0041] Tablet preparation
[0042]
[0043] Take prescription amount of compound of structural formula Ⅰ and mix evenly with prescription amount of lactose, add 3% povidone ethanol solution to make soft material, pass through 18 mesh sieve to make granules, dry at 50°C for 30-45 minutes, granulate, add magnesium stearate, mix Evenly, press into tablets. Each tablet contains 20mg of the compound.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com