Pharmaceutical composition containing monosialotetrahexosyl ganglioside sodium and application thereof
A technology of sodium ganglioside and monosialic acid, applied in the field of medicine, can solve the problem that the repair rate of pheochromocytoma cells needs to be improved, and achieve the effects of promoting brain nerve metabolism, good quality of extract and better cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
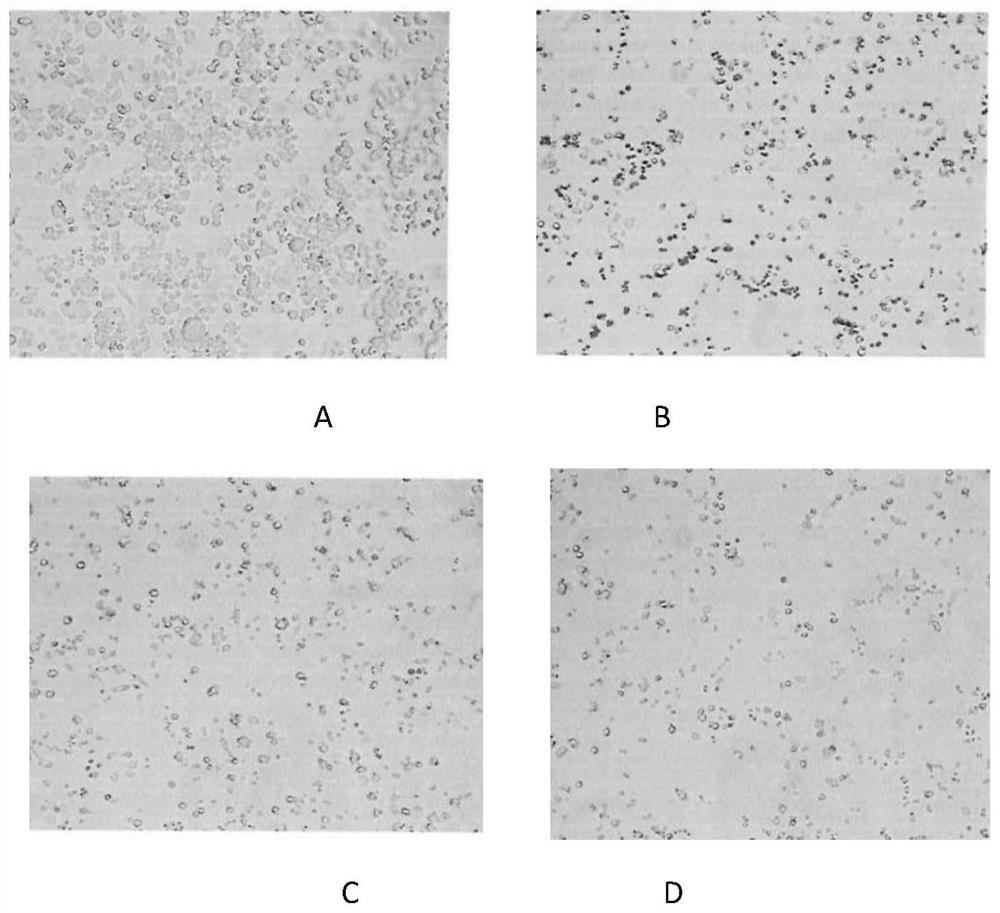
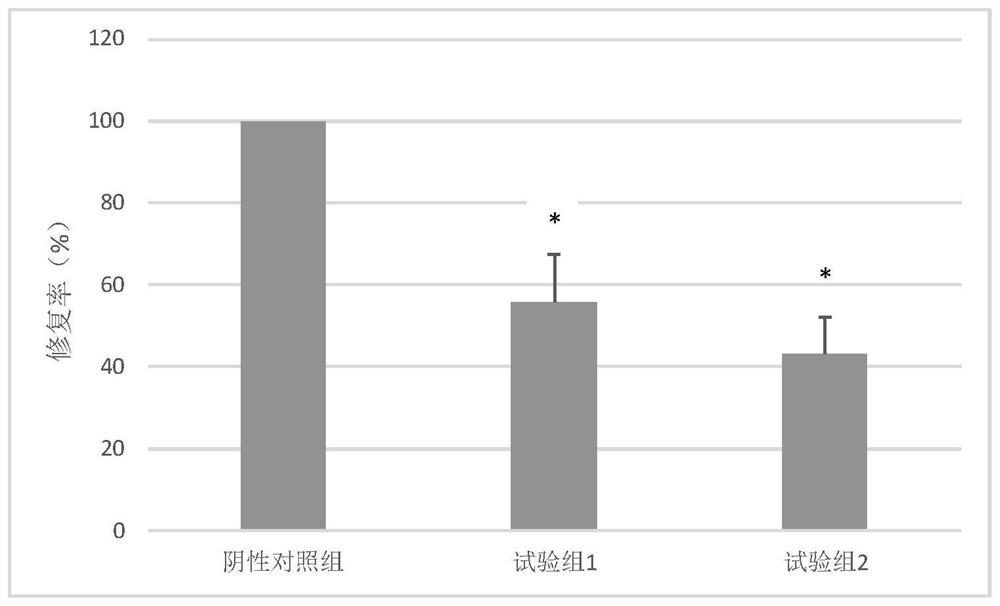
Image
Examples
Embodiment 1
[0069] Example 1 Preparation of the pharmaceutical composition containing monosialotetrahexosyl ganglioside sodium of the present invention
[0070] The preparation of the pharmaceutical composition containing monosialotetrahexosyl ganglioside sodium of the present invention comprises the following steps:
[0071] (1) Mince 10kg of pretreated rabbit muscle until the volume is not higher than 1cm 3 After mixing the obtained minced muscle with water for injection at a ratio of 1:2 (w / w), homogenate, place the obtained muscle homogenate below -10°C and freeze it for 6 days, and then place it in Melt at 9°C for 40 hours, then heat the melted rabbit muscle homogenate to 75°C, keep it warm for 20 minutes, then cool it down to room temperature, and centrifuge to obtain a filtrate;
[0072] (2) Adjust the pH of the filtrate once to 2.5, heat it to 37°C, add 0.1% pepsin, keep it at 37°C for enzymolysis for 2 hours, then adjust the pH of the liquid to 8, heat it at 75°C for 20 minute...
Embodiment 2
[0079] Example 2 Preparation of the pharmaceutical composition containing monosialotetrahexosyl ganglioside sodium of the present invention
[0080] The preparation of the pharmaceutical composition containing monosialotetrahexosyl ganglioside sodium of the present invention comprises the following steps:
[0081] (1) Mince 10kg of pretreated rabbit muscle until the volume is ≤1cm 3 After mixing the obtained minced muscle with water for injection at a ratio of 1:1 (w / w), homogenate, place the obtained muscle homogenate below -10°C and freeze it for 5 days, and then place it in Melt at 8°C for 36 hours, then heat the melted rabbit muscle homogenate to 70°C, keep it warm for 15 minutes, then cool it down to room temperature, and centrifuge to obtain a filtrate;
[0082] (2) Adjust the pH of the filtrate once to 2.0, heat it to 35°C, add 0.05% pepsin, keep it at 35°C for 0.5 hours, then adjust the pH of the liquid to 7.5, heat it at 70°C for 15 minutes, and cool down to 35°C,...
Embodiment 3
[0089] Example 3 Preparation of the pharmaceutical composition containing monosialotetrahexosyl ganglioside sodium of the present invention
[0090] The preparation of the pharmaceutical composition containing monosialotetrahexosyl ganglioside sodium of the present invention comprises the following steps:
[0091] (1) Mince 10kg of pretreated rabbit muscle until the volume is not higher than 1cm 3 After mixing the obtained minced muscle with water for injection at a ratio of 1:3 (w / w), homogenate, place the obtained muscle homogenate below -10°C and freeze it for 7 days, then place it in Melt at 10°C for 48 hours, then heat the melted rabbit muscle homogenate to 80°C, keep it warm for 25 minutes, then cool it down to room temperature, and centrifuge to obtain a filtrate;
[0092] (2) Adjust the pH of the filtrate once to 3.0, heat it to 40°C, add 0.5% pepsin, keep it at 40°C for enzymolysis for 3 hours, then adjust the pH of the liquid to 8.5, heat it at 80°C for 30 minutes...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com