Application of needle-leaved polyprenol in preparing medicines for preventing and treating Alzheimer disease
A technology for Alzheimer's disease and polyprenol, which is applied in the field of medicine to achieve the effects of wide sources, less pollution, and low development and utilization costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Embodiment 1: the extraction of Acerola polyprenol
[0016] The extraction of coniferous polyprenol is the same as the method recorded in Chinese Patent Publication No. CN101654398A. After the fresh conifers are dried and pulverized, they are extracted with petroleum ether, saponified with dilute alkali, extracted with petroleum ether, concentrated under reduced pressure, dissolved in acetone, and removed by cooling. Coniferous polyprenol was prepared after impurity, ethanol extraction, acetone dissolution, cryoprecipitation separation and filter cake vacuum drying.
Embodiment 2
[0017] Embodiment 2: Acerola polyprenol soft capsule
[0018] Coniferous polyprenol is used as the active ingredient of the medicine, and refined vegetable oil, emulsifier, gelatin, glycerin, coloring agent and other auxiliary materials are added, and the soft capsule dosage form is made through batching, gelatinization, pilling, shaping and drying according to the conventional process.
Embodiment 3
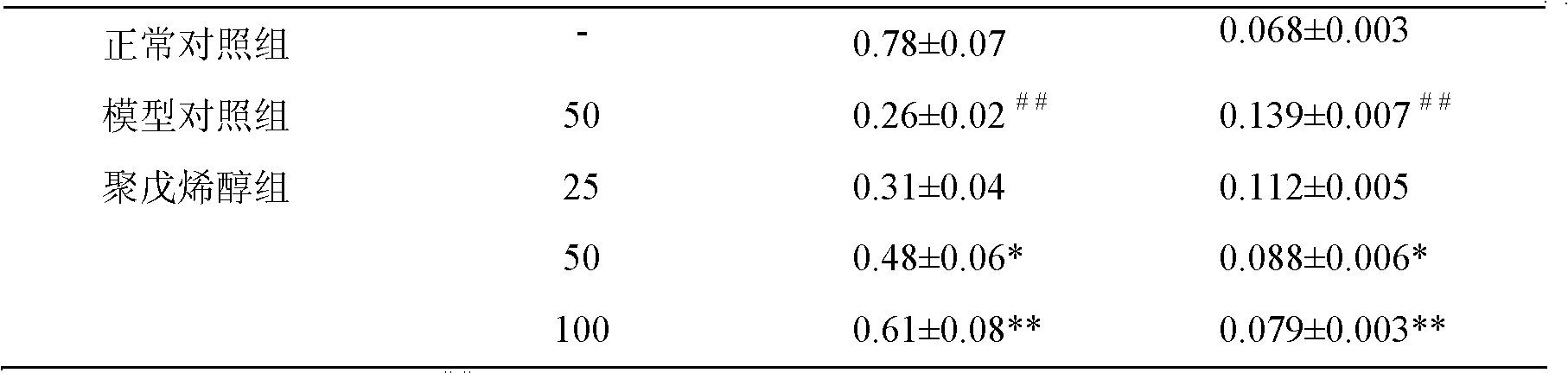
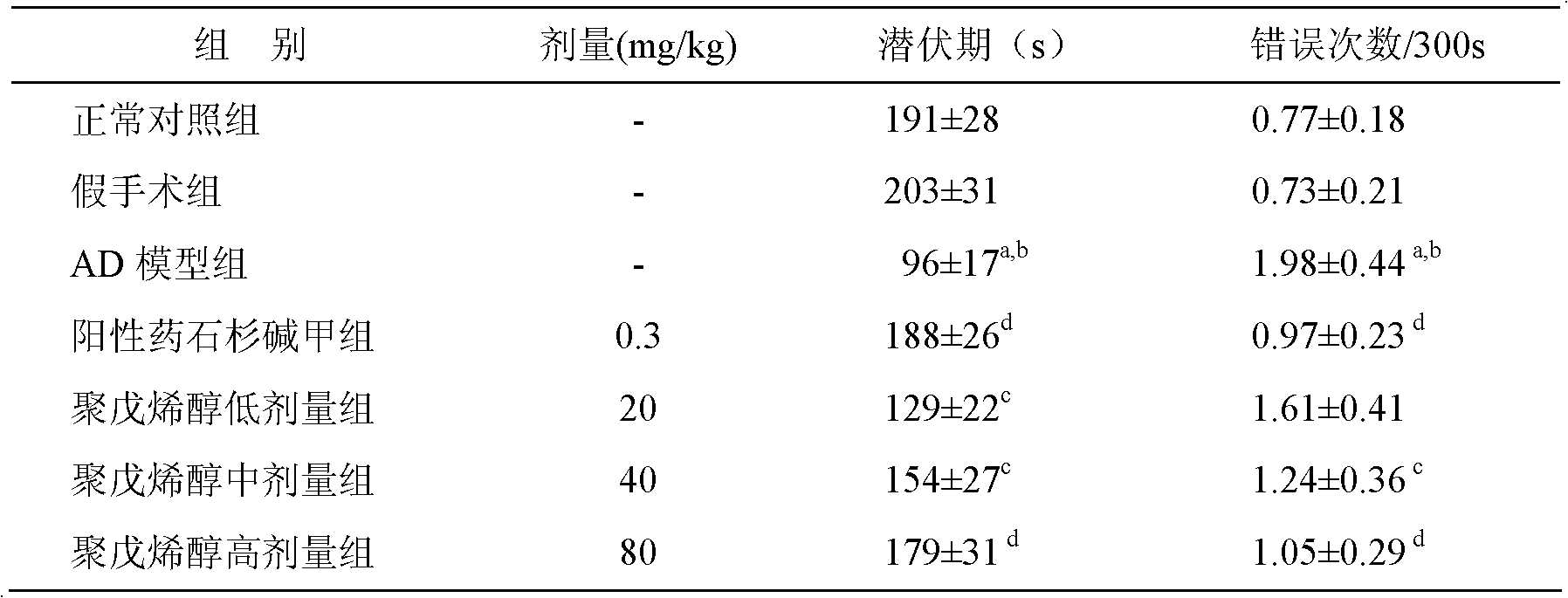
[0019] Example 3: Protective effect of acerola polyprenol on PC12 nerve cell injury induced by β-amyloid protein
[0020] 1 Materials and methods
[0021] 1.1 Experimental materials
[0022] Coniferous polyprenol, 76.2% pure, formulated in DMSO to 10 -2 mol / L solution, stored at 4°C for future use. Aβ 1-42 , purchased from Beijing Boaosen Company, prepared 1g / L mother solution with normal saline, sterilized by filtration with 0.22μm microporous membrane, incubated at 37°C for 7 days, "aged" into a neurotoxic aggregation state, - Store at 20°C for later use.
[0023] 1.2 Drug handling
[0024] After counting PC12 cells in the logarithmic growth phase, 4×10 5 / mL density inoculated in 96-well culture plate, 150 μl per well. The cells were divided into 3 groups: the normal control group, adding an equal amount of DMSO solution; the model control group, adding 7-day-aged Aβ 1-42 Incubate at 50 μmol / L for 48 hours; in the polyprenol group, add coniferous polyprenol at a fin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com