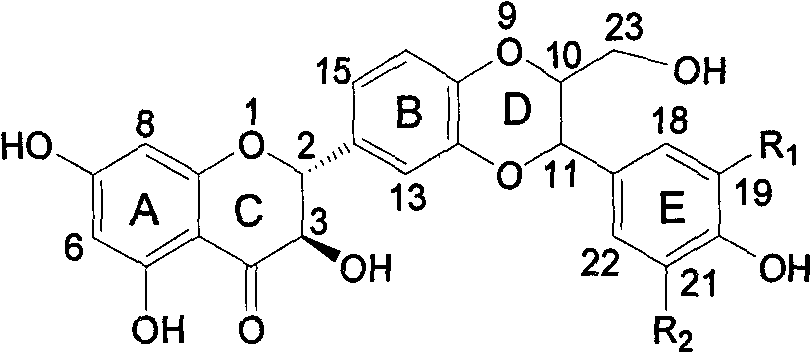
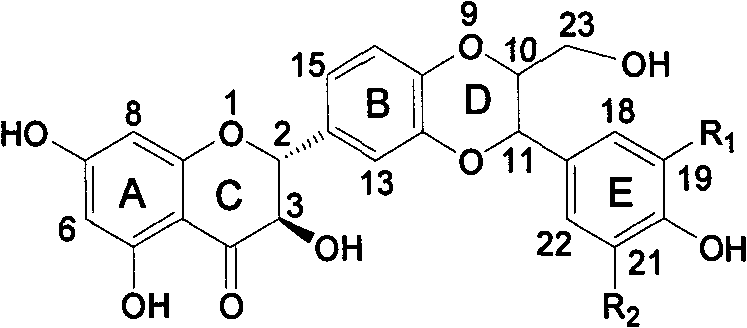
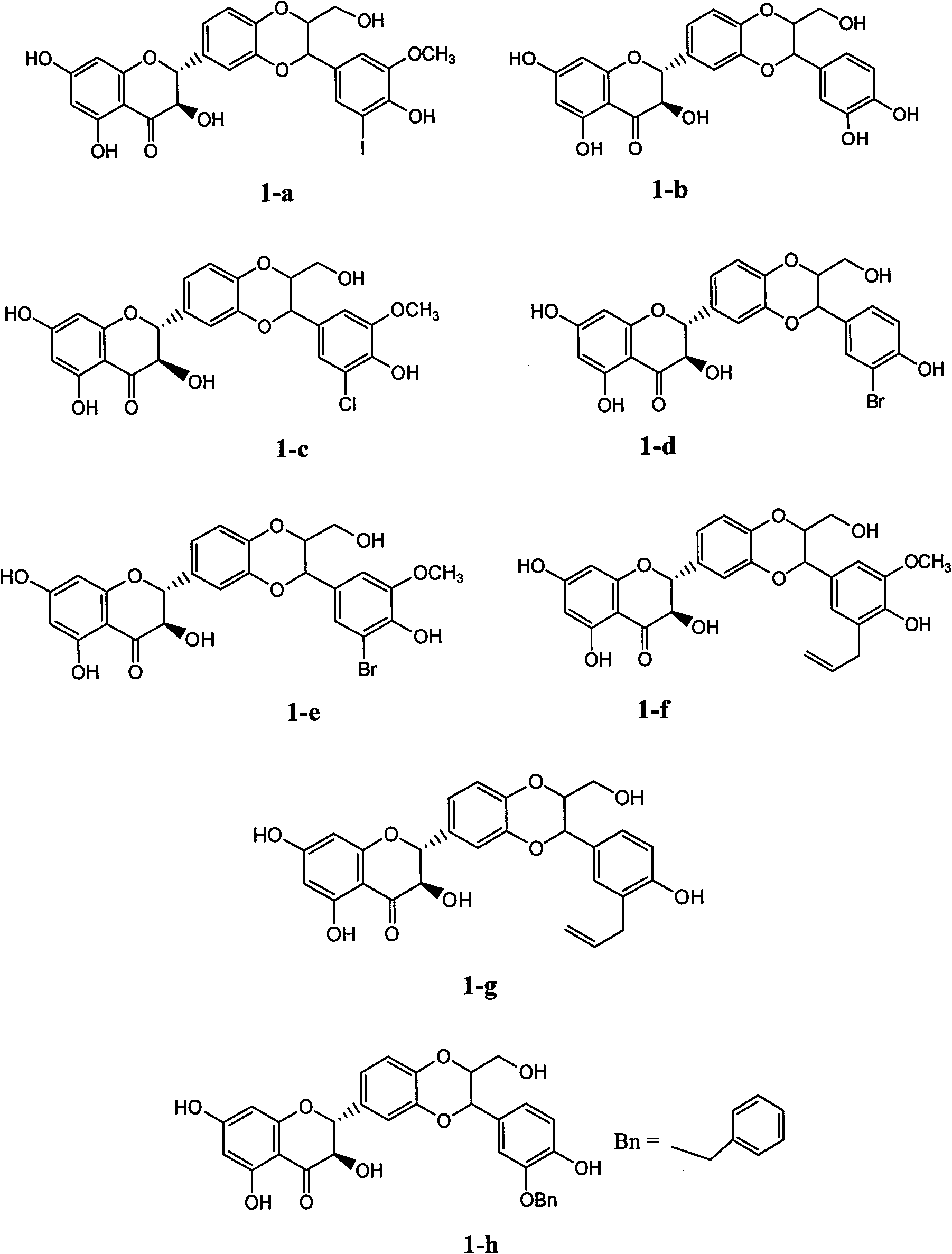
E-ring substituted silybin derivative and preparation method and medical application thereof
A technology of silibinin and derivatives, which is applied in the field of preparation of silybin derivatives, and achieves the effects of simple preparation method, protection of brain nerve cells, and less pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: the preparation of compound I-b-1 (3-allyl-4-hydroxycinnamic acid ethyl ester)
[0035]
[0036] 0.30 g of 3-allyl-4-hydroxybenzaldehyde (2 mmol), 0.70 g of phosphorus ylide (2 mmol) were dissolved in 10 ml of chloroform, heated to reflux for 5 hours, and the solvent was removed by distillation under reduced pressure, and the residue was passed through the column Chromatography gave 0.30 g of a white solid, with a yield of 65%. R f (petroleum ether: ethyl acetate=3:1)=0.29, H NMR spectrum 1 H NMR (400MHz, deuterated chloroform) δ: 1.33(t, J=7.2Hz, 3H, CH 3 ), 3.42 (d, J=6.4Hz, 2H, OCH 2 CH=), 4.25(q, J=7.2Hz, 2H, OCH 2 ), 5.16 (d, J=7.2Hz,=CH 2 a), 5.20(s, 1H, =CH 2 b), 6.00(m, 1H, CH=CH 2 ), 6.29 (d, J=16.0Hz, 1H, H-8), 6.81 (d, J=8.0Hz, 1H, H-6), 7.31 (d, J=8.0Hz, 1H, H-5), 7.31(s, 1H, H-2), 7.61(d, J=16.0Hz, 1H, H-7), electrospray mass spectrometry ESI-MS: 233[M+H] + .
Embodiment 2
[0038] Embodiment 2: the preparation of I-b-2 (3-methoxy-4-hydroxyl-5-allyl cinnamic acid ethyl ester)
[0039]
[0040] Yellow oil; Yield: 74%; R f (petroleum ether: ethyl acetate=3:1)=0.25, H NMR spectrum 1 H NMR (400MHz, deuterated chloroform) δ: 1.33(t, J=7.2Hz, 3H, CH 3 ), 3.40 (d, J=6.8Hz, 2H, OCH 2 CH=), 3.91(s, 3H, OCH 3 ), 4.25 (q, 2H, CH 2 ), 5.09 (m, 2H, CH 2 =), 6.00 (m, 1H, CH 2 =CH), 6.28(d, J=16.0Hz, 1H, H-8), 6.92(d, J=2.0Hz, 1H, H-6), 6.96(d, J=2.0Hz, 1H, H-2 ), 7.59 (d, J=16.0 Hz, 1H, H-7). Embodiment 3: Preparation of I-b-5 (3-chloro-4-hydroxyl-5-methoxycinnamic acid ethyl ester)
Embodiment 3
[0040] Yellow oil; Yield: 74%; R f (petroleum ether: ethyl acetate=3:1)=0.25, H NMR spectrum 1 H NMR (400MHz, deuterated chloroform) δ: 1.33(t, J=7.2Hz, 3H, CH 3 ), 3.40 (d, J=6.8Hz, 2H, OCH 2 CH=), 3.91(s, 3H, OCH 3 ), 4.25 (q, 2H, CH 2 ), 5.09 (m, 2H, CH 2 =), 6.00 (m, 1H, CH 2 =CH), 6.28(d, J=16.0Hz, 1H, H-8), 6.92(d, J=2.0Hz, 1H, H-6), 6.96(d, J=2.0Hz, 1H, H-2 ), 7.59 (d, J=16.0 Hz, 1H, H-7). Embodiment 3: Preparation of I-b-5 (3-chloro-4-hydroxyl-5-methoxycinnamic acid ethyl ester)
[0041]
[0042] Pale yellow solid; Yield: 63%; R f (petroleum ether: ethyl acetate=3:1)=0.40, H NMR spectrum 1 H NMR (400MHz, deuterated chloroform) δ: 1.34(t, J=7.2Hz, 3H, CH 3 ), 3.86(s, 3H, OCH 3 ), 4.27 (q, J=7.2Hz, 2H, CH 2 ), 6.38(d, J=16.0Hz, 1H, H-8), 6.98(d, J=2.0Hz, 1H, H-6), 7.20(d, J=2.0Hz, 1H, H-2), 7.56 (d, J=16.0 Hz, 1H, H-7).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com