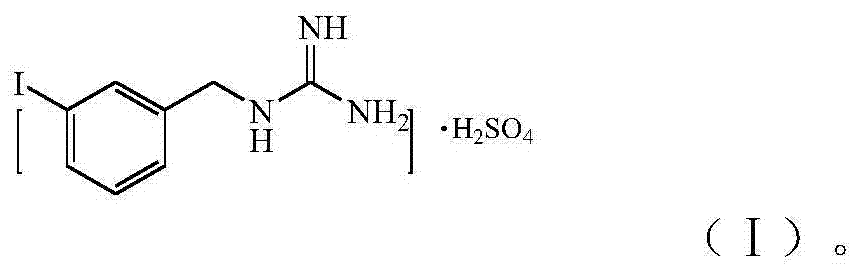Preparation method of metaiodobenzylguanidine (MIBG) sulphate
The technology of m-iodobenzylguanidine sulfate and m-iodobenzyl bromide is applied in the field of preparation of labeling precursor m-iodobenzylguanidine sulfate, and can solve the problem that the health of production personnel is greatly affected, the residual highly toxic substances in the final product are unfavorable, and the Large-scale product preparation and other problems, to achieve the effect of reducing the risk of highly toxic substance residues, simple production operation, and easy large-scale preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0045] The preparation method of m-iodobenzylguanidine sulfate of the present invention prepares the synthetic MIBG sulfate as the labeling precursor of the radiopharmaceutical m-iodobenzylguanidine used in the treatment and diagnosis of neuroendocrine tumors such as human pheochromocytoma and neuroblastoma, Higher security, as can be seen from the above-mentioned preparation method of the present invention, the present invention has abandoned the use of highly toxic reagents such as cyanide, greatly reducing the impact of the production process on personnel and the environment, and at the same time, reducing the highly toxic substances in the final product Residual risk, thus greatly improving the safety of medicines; In addition, the present invention removes the operation of silica gel separation, adopts a large number of recrystallization methods, makes the production operation simple, easy to prepare on a large scale, and has low requirements for the production personnel's ...
Embodiment 1
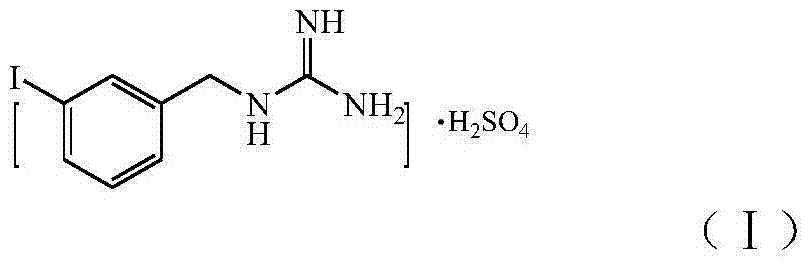
[0051] Synthetic MIBG sulfate as shown in formula (I),
[0052]
[0053] The synthesis reaction is as follows:
[0054]
[0055] The preparation method is as follows:
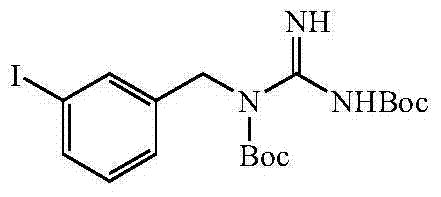
[0056] (1) Synthetic intermediate N, N'-(tert-butyloxycarbonyl)-N-(3-iodobenzyl)guanidine
[0057] (1.1) Synthesis of m-iodobromobenzyl
[0058] Take m-iodotoluene 124mmol, N-bromosuccinimide 204mmol and 2,2'-azobisisobutyronitrile 3.42mmol and dissolve in 100mL CCl 4 In the process, the heating temperature is controlled at 80°C to 100°C, and the reflux is performed for 3 to 5 hours; suction filtration, distillation under reduced pressure to remove the solvent, separation by column chromatography, and a light yellow powder product is obtained, the structure of which is the compound metaiodine shown in the following formula (II): Benzyl bromide, the productive rate is 40%; 1 HNMR (400MHz, CDCl 3 )δ7.74(t, J=1.6Hz, 1H), 7.63(d, J=7.9Hz, 1H), 7.36(d, J=7.7Hz, 1H), 7.08(t, J=7.8Hz, 1H) ,4.40(s,2H);
[...
Embodiment 2
[0073] Synthetic MIBG vitriol salt as shown in formula (I), preparation method is as follows:
[0074] (1) Synthetic intermediate N, N'-(tert-butyloxycarbonyl)-N-(3-iodobenzyl)guanidine
[0075] (1.1) Synthesis
[0076] Take m-iodotoluene 124mmol, N-bromosuccinimide 204mmol and 2,2'-azobisisobutyronitrile 3.42mmol and dissolve in 100mL CCl 4 , the heating temperature was controlled at 85°C to 95°C, and refluxed for 4 hours; suction filtration, decompression distillation to remove the solvent, and column chromatography separation gave a light yellow powder product, the structure of which was the compound shown in the following formula (II), and the yield was 39.8%; 1 H NMR (400MHz, CDCl 3 )δ7.74(t, J=1.6Hz, 1H), 7.63(d, J=7.9Hz, 1H), 7.36(d, J=7.7Hz, 1H), 7.08(t, J=7.8Hz, 1H) ,4.40(s,2H);
[0077]
[0078] (1.2) Synthesis
[0079] (1.2.1) Synthesis
[0080] Dissolve 50mmol of S-methylisothiourea sulfate and 100mmol of di-tert-butyl dicarbonate in 125ml of dichlo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com