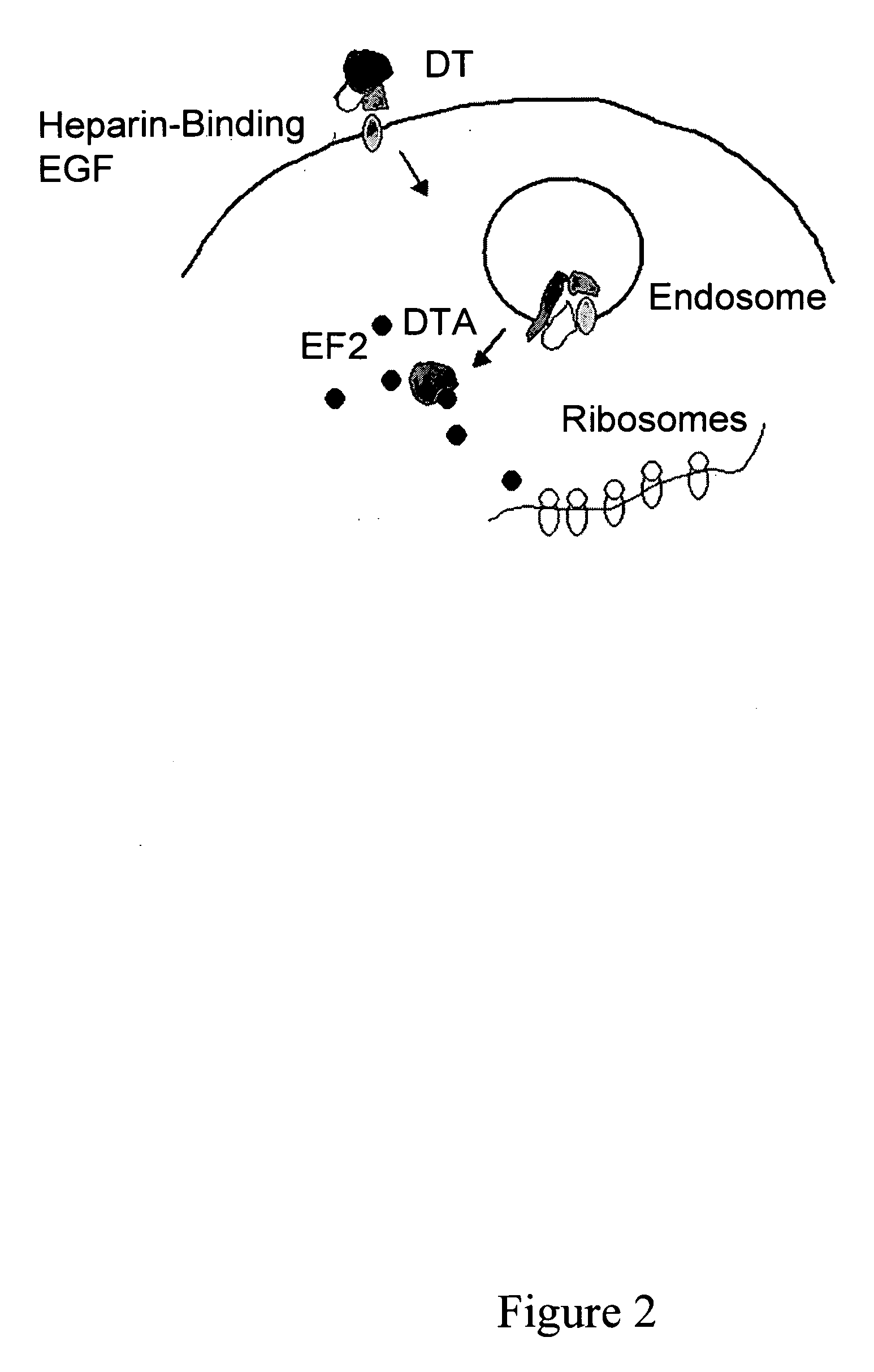
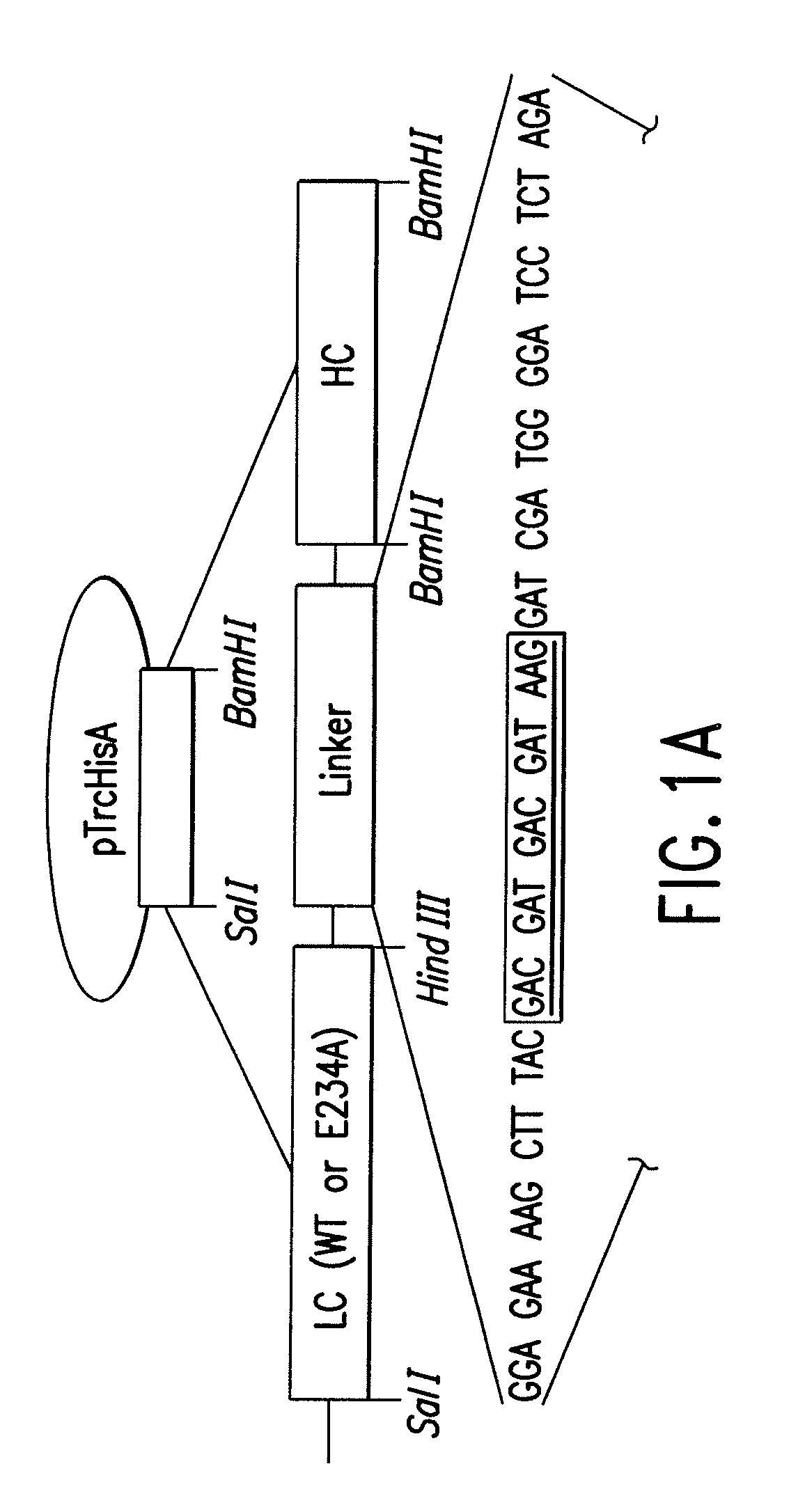
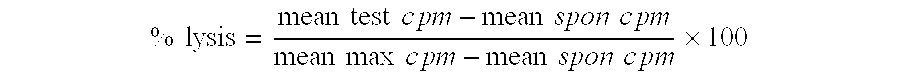Patents
Literature
583results about "Polypeptide with fusion toxin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
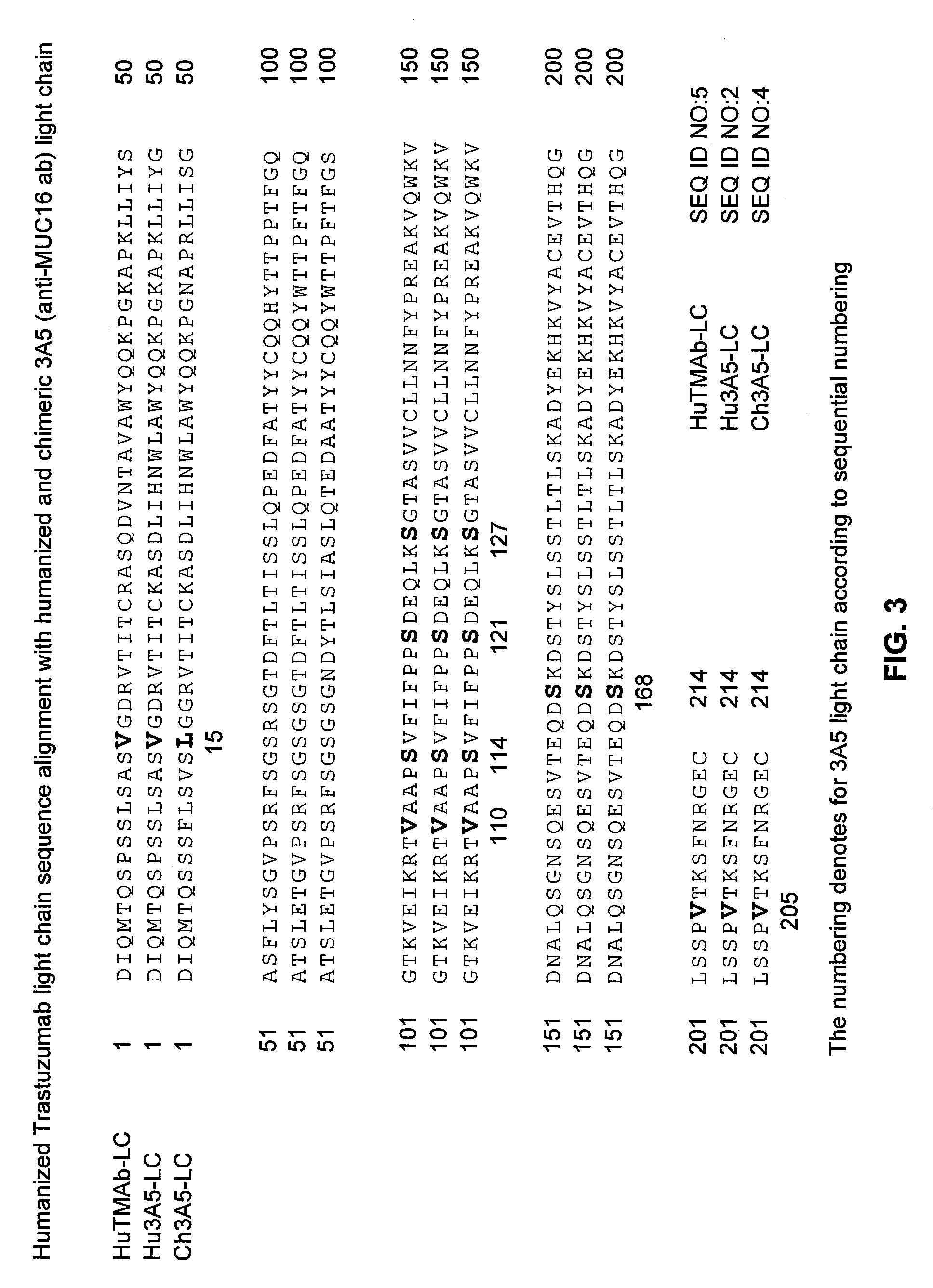
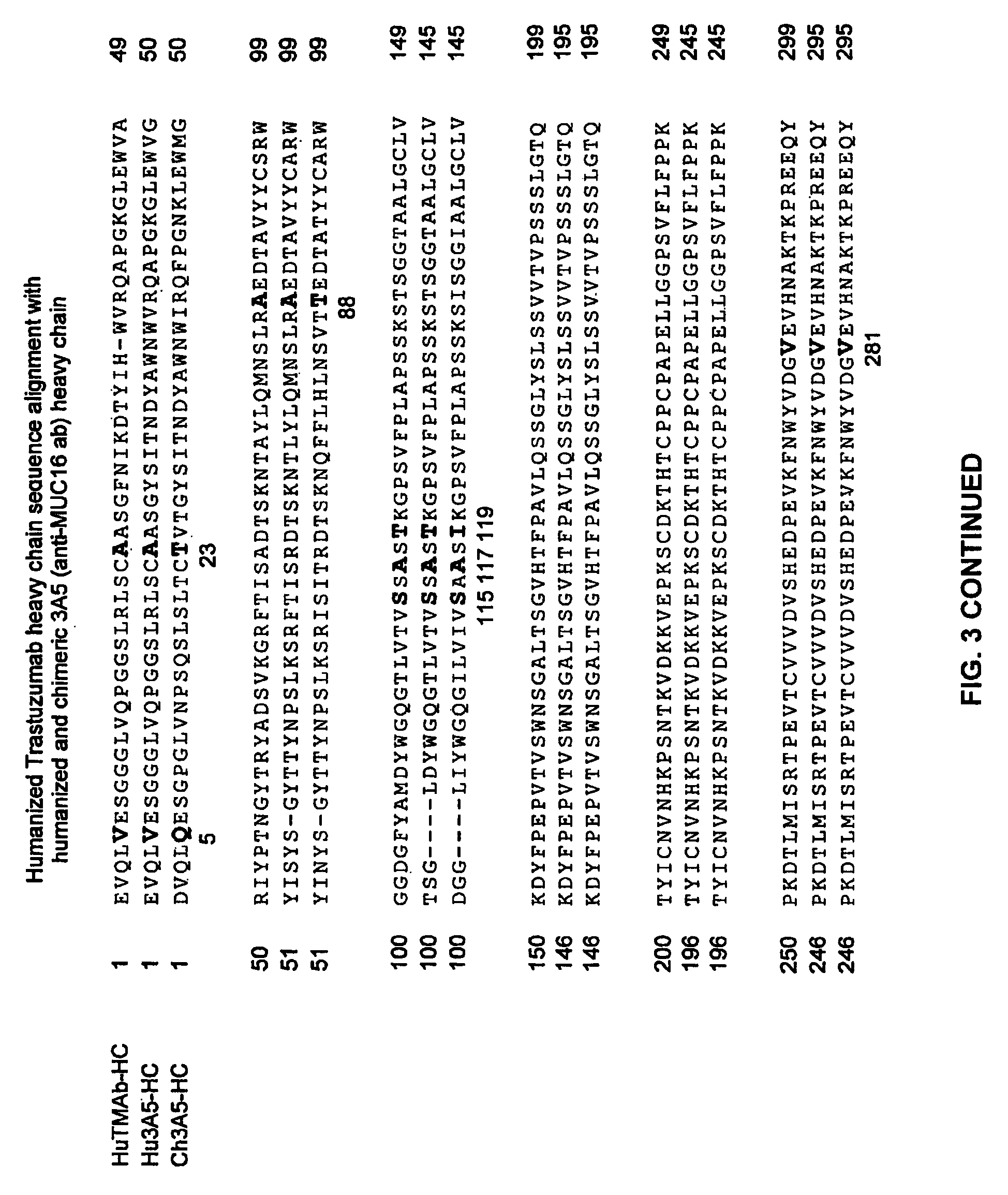
Cysteine engineered anti-MUC16 antibodies and antibody drug conjugates
ActiveUS7723485B2In-vivo radioactive preparationsImmunoglobulins against cell receptors/antigens/surface-determinantsCross-linkDrug compound
Cysteine engineered anti-MUC16 antibodies are engineered by replacing one or more amino acids of a parent anti-MUC16 antibody with non cross-linked, reactive cysteine amino acids. Methods of design, preparation, screening, and selection of the cysteine engineered anti-MUC16 antibodies are provided. Cysteine engineered anti-MUC16 antibodies (Ab) are conjugated with one or more drug moieties (D) through a linker (L) to form cysteine engineered anti-MUC16 antibody-drug conjugates having Formula I:Ab-(L-D)p Iwhere p is 1 to 4. Diagnostic and therapeutic uses for cysteine engineered antibody drug compounds and compositions are disclosed.
Owner:GENENTECH INC
Compositions for targeting the vasculature of solid tumors
InactiveUS6051230AEffectively compete for bindingReduce TEC-4 or TEC-11 bindingHybrid immunoglobulinsPeptide/protein ingredientsAntigenCell Surface Antigens
The present invention relates generally to methods and compositions for targeting the vasculature of solid tumors using immunological- and growth factor-based reagents. In particular aspects, antibodies carrying diagnostic or therapeutic agents are targeted to the vasculature of solid tumor masses through recognition of tumor vasculature-associated antigens, such as, for example, through endoglin binding, or through the specific induction of endothelial cell surface antigens on vascular endothelial cells in solid tumors.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Compositions and methods for treating cancer using immunoconjugates and chemotherapeutic agents
Owner:IMMUNOGEN INC
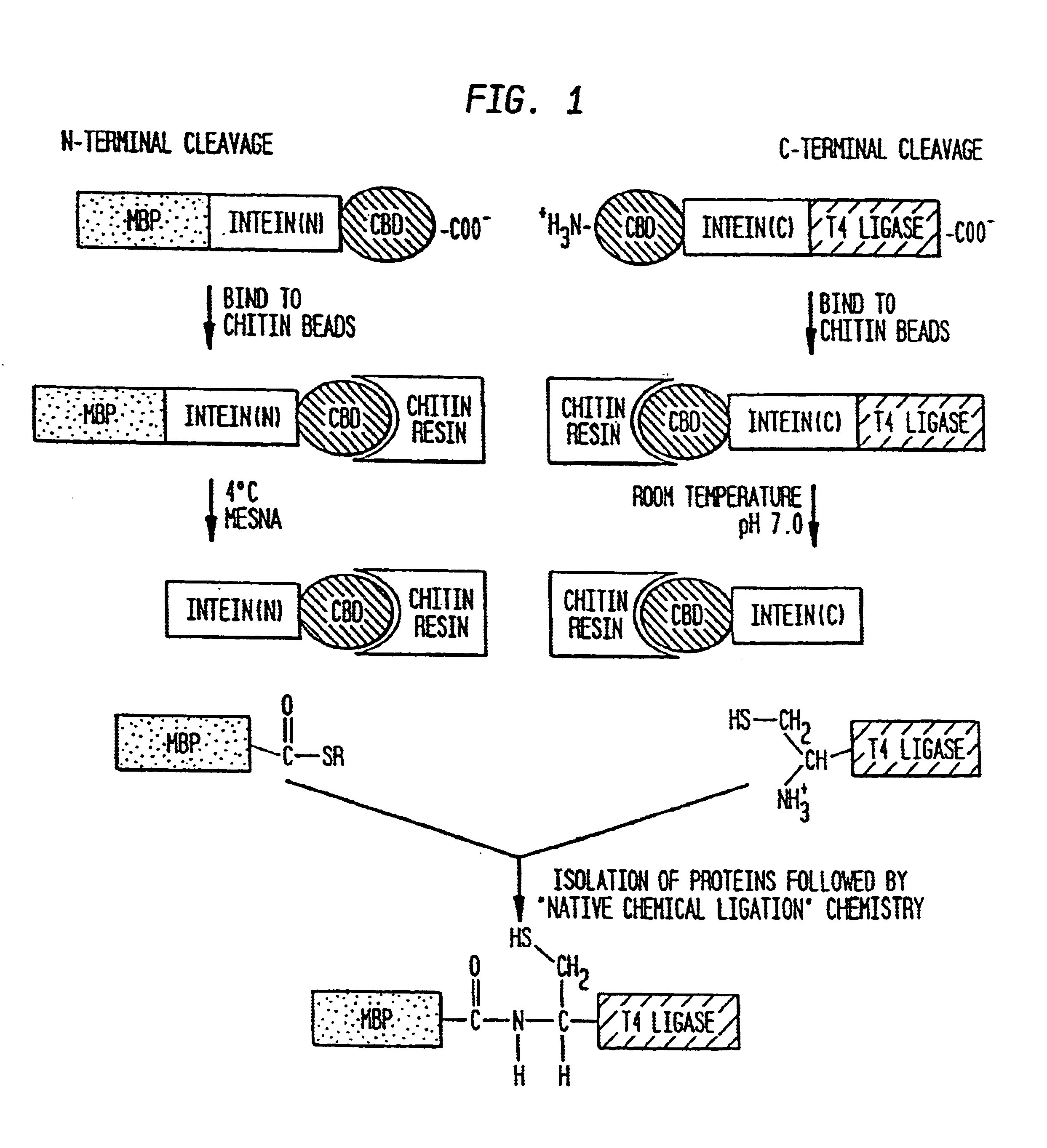
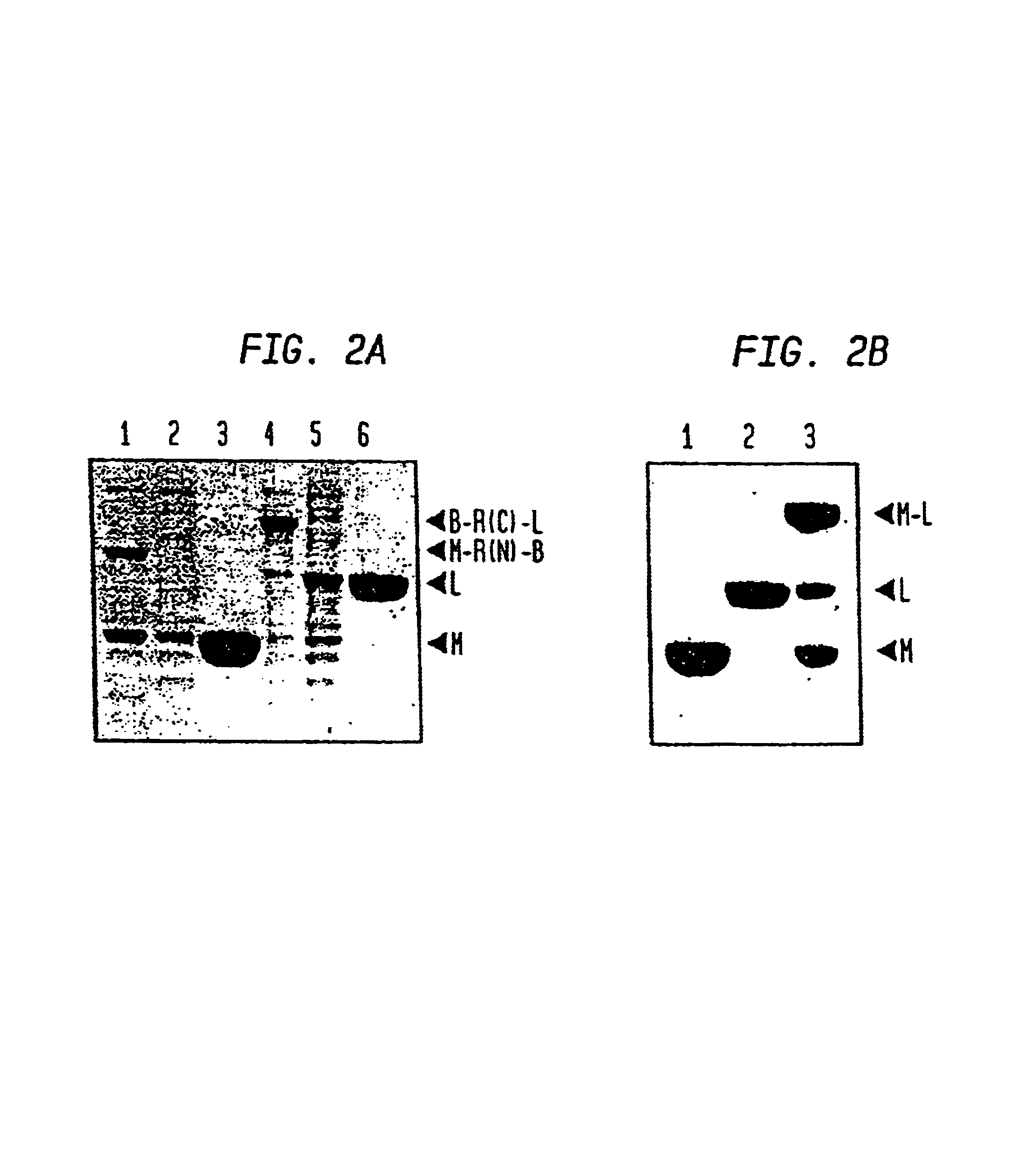
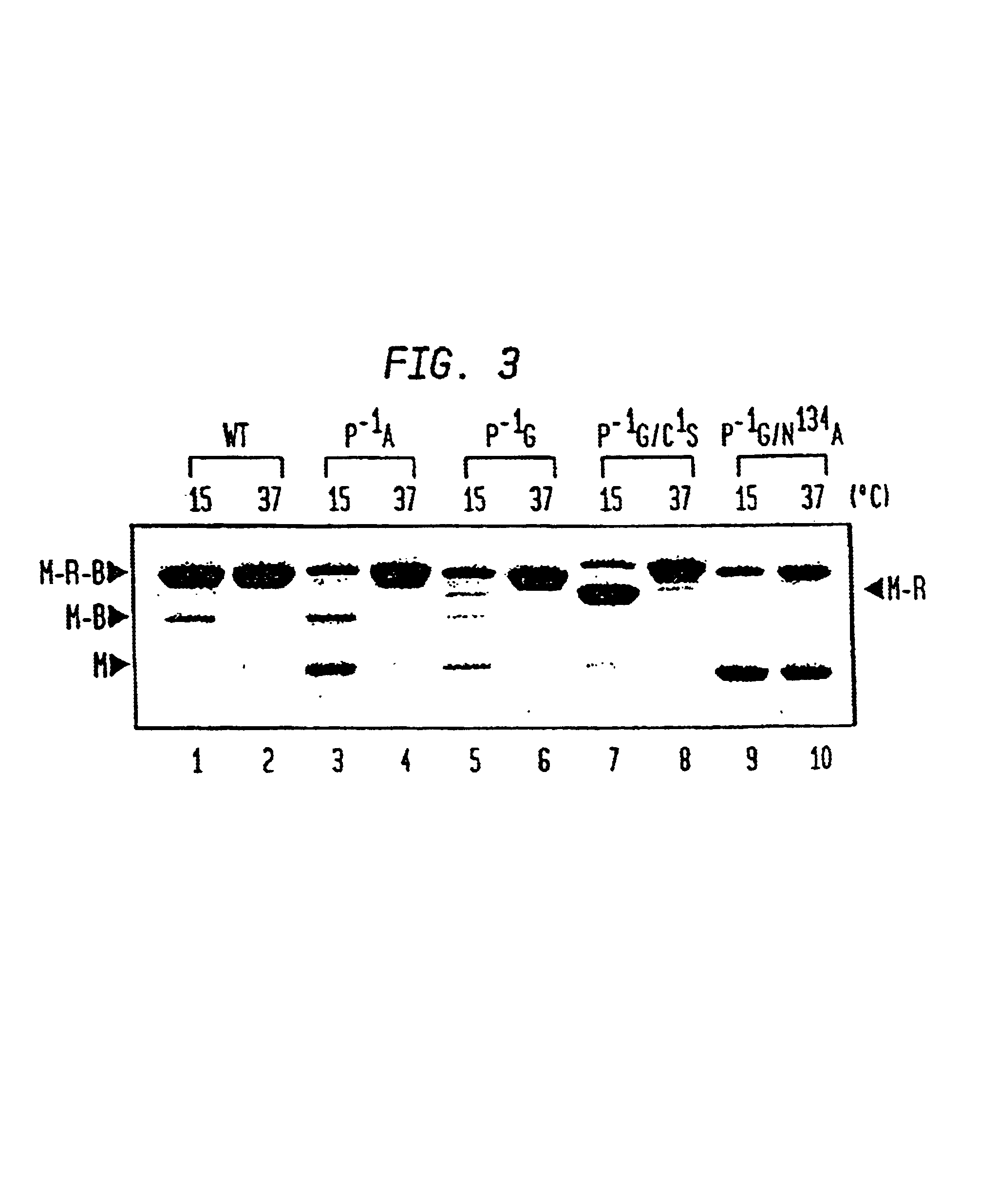
Intein-mediated protein ligation of expressed proteins
InactiveUS6849428B1Eliminate needBacteriaFusion with post-translational modification motifProtein targetIntein
A method for the ligation of expressed proteins which utilizes inteins, for example the RIR1 intein from Methanobacterium thermotrophicum, is provided. Constructs of the Mth RIR1 intein in which either the C-terminal asparagine or N-terminal cysteine of the intein are replaced with alanine enable the facile isolation of a protein with a specified N-terminal, for example, cysteine for use in the fusion of two or more expressed proteins. The method involves the steps of generating a C-terminal thioester-tagged target protein and a second target protein having a specified N-terminal via inteins, such as the modified Mth RIR1 intein, and ligating these proteins. A similar method for producing a cyclic or polymerized protein is provided. Modified inteins engineered to cleave at their C-terminus or N-terminus, respectively, and DNA and plasmids encoding these modified inteins are also provided.
Owner:NEW ENGLAND BIOLABS
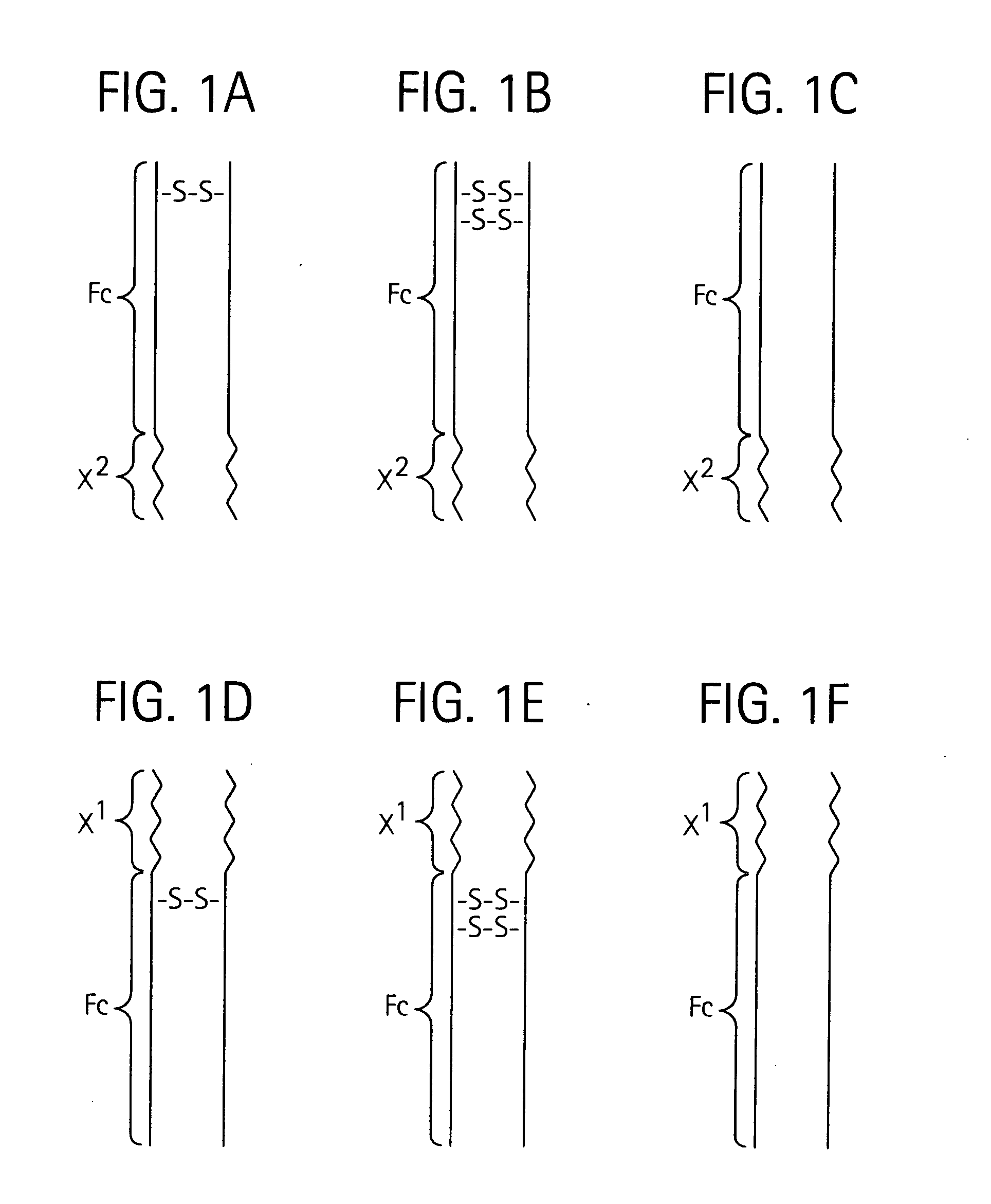
Toxin peptide therapeutic agents
ActiveUS20070071764A1Avoid it happening againRelieve symptomsNervous disorderAntipyreticHalf-lifeSjögren syndrome
Disclosed is a composition of matter of the formula (X1)a—(F1)d—(X2)b—(F2)e—(X3)c (I) and multimers thereof, in which F1 and F2 are half-life extending moieties, and d and e are each independently 0 or 1, provided that at least one of d and e is 1; X1, X2, and X3 are each independently -(L)f-P-(L)g-, and f and g are each independently 0 or 1; P is a toxin peptide of no more than about 80 amino acid residues in length, comprising at least two intrapeptide disulfide bonds; L is an optional linker; and a, b, and c are each independently 0 or 1, provided that at least one of a, b and c is 1. Linkage to the half-life extending moiety or moieties increases the in vivo half-life of the toxin peptide, which otherwise would be quickly degraded. A pharmaceutical composition comprises the composition and a pharmaceutically acceptable carrier. Also disclosed are a DNA encoding the inventive composition of matter, an expression vector comprising the DNA, and a host cell comprising the expression vector. Methods of treating an autoimmune disorder, such as, but not limited to, multiple sclerosis, type 1 diabetes, psoriasis, inflammatory bowel disease, contact-mediated dermatitis, rheumatoid arthritis, psoriatic arthritis, asthma, allergy, restinosis, systemic sclerosis, fibrosis, scleroderma, glomerulonephritis, Sjogren syndrome, inflammatory bone resorption, transplant rejection, graft-versus-host disease, and lupus and of preventing or mitigating a relapse of a symptom of multiple sclerosis are also disclosed.
Owner:AMGEN INC
Targeted antimicrobial moieties
This invention provides novel targeted antimicrobial compositions. In various embodiments chimeric moieties are provided comprising an antimicrobial peptide attached to a peptide targeting moiety that binds a bacterial strain or species.
Owner:C3 JIAN +1
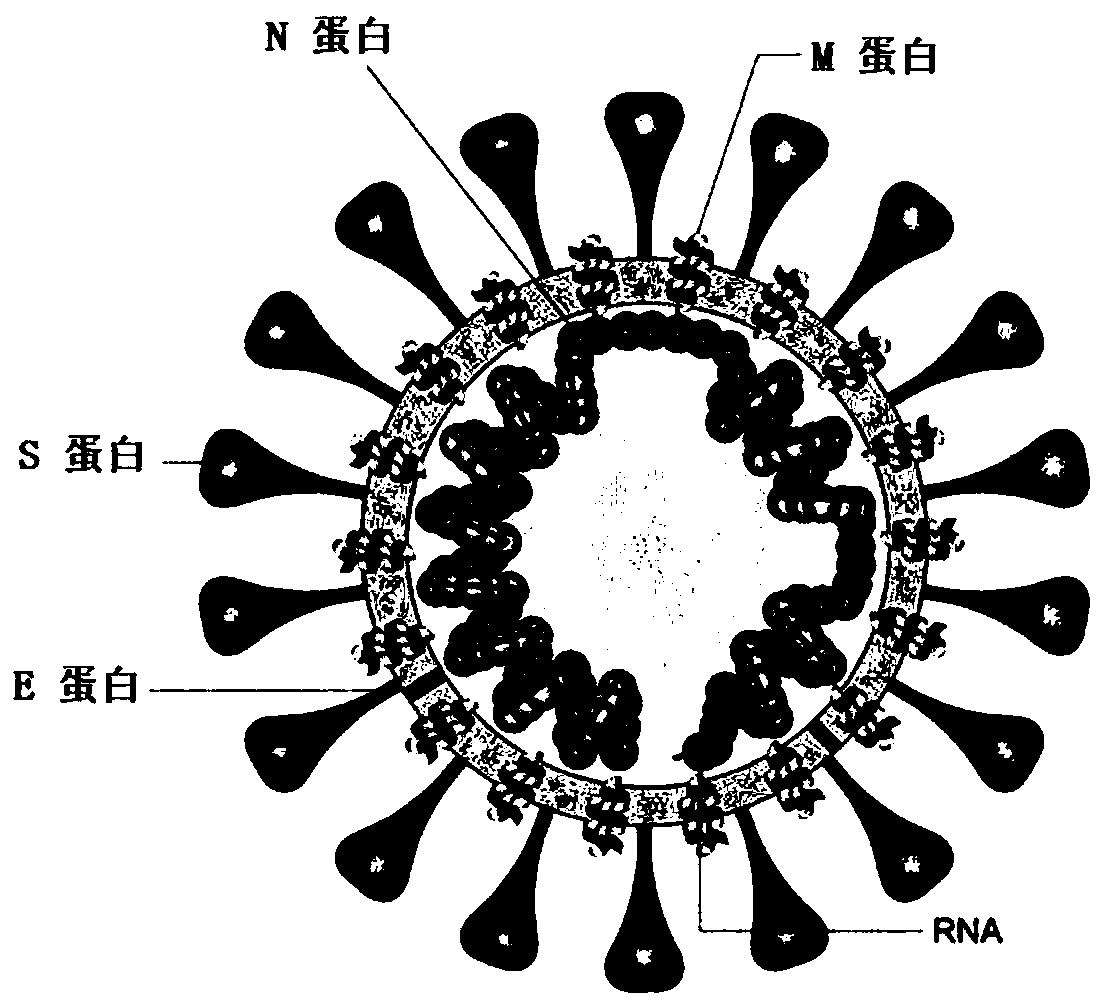
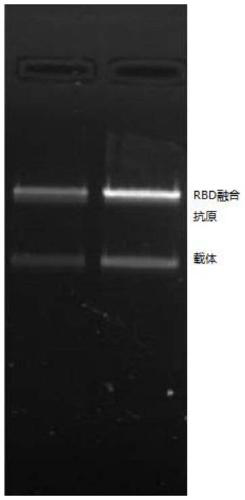
SARS-CoV-2 vaccine and preparation method thereof
ActiveCN111217917AEnter to helpImproving immunogenicityPolypeptide with localisation/targeting motifSsRNA viruses positive-senseAntigenDisease
The invention relates to a preparation method for a vaccine capable of treating and / or preventing SARS-CoV-2 infection or COVID-19 diseases. The core antigen of the vaccine comprises the RBD (receptor binding zone) fusion protein of the SARS-CoV-2, and a vaccine form comprises an RBD fusion protein subunit vaccine, an RBD fusion protein mRNA vaccine or an RBD fusion protein adenovirus vector vaccine. The above vaccine immunizes an organism, and immune reaction for treating and / or preventing the SARS-CoV-2 infection can be generated so as to be used for treating and / or preventing COVID-19. The invention also relates to an RBD fusion gene, the RBD fusion protein, a carrier, a cell, a preparation method, a treatment method or a pharmacy purpose of the SARS-CoV-2.
Owner:CANSINO BIOLOGICS INC
Toxin compounds with enhanced membrane translocation characteristics
InactiveUS20060024331A1Prevent steric hindrancePolypeptide with localisation/targeting motifBacterial antigen ingredientsProtein transduction domainMembrane translocation
The present invention relates to a compound comprising a toxin linked to a translocator. Non-limiting examples of toxins of the present invention are botulimum toxin, butyricum toxin, tetani toxins and the light chains thereof. In some embodiments, the translocator of the present invention comprises a protein transduction domain.
Owner:ALLERGAN INC
Methods and compositions based on diphtheria toxin-interleukin-3 conjugates
ActiveUS20080138313A1Enhance or improve the prophylactic effect(s) of another therapyShorten the durationBiocidePeptide/protein ingredientsCancer cellWhite blood cell
The present invention provides methods for inhibiting interleukin-3 receptor-expressing cells, and, in particular, inhibiting the growth of such cells by using a diphtheria toxin-human interleukin-3 conjugate (DT-IL3) that is toxic to cells expressing the interleukin-3 receptor. In preferred embodiments, the DT-IL3 conjugate is a fusion protein comprising amino acids 1-388 of diphtheria toxin fused via a peptide linker to full-length, human interleukin-3. In certain embodiments, the methods of the present invention relate to the administration of a DT-IL3 conjugate to inhibit the growth of cancer cells and / or cancer stem cells in humans, which cells express one or more subunits of the interleukin-3 receptor. Exemplary cells include myeloid leukemia cancer stem cells. In other embodiments, the methods of the present invention relate to ex vivo purging of bone marrow or peripheral blood to remove cells that express one or more subunits of the interleukin-3 receptor such that the purged bone marrow or peripheral blood is suitable, e.g., for autologous stem cell transplantation to restore hematopoietic function.
Owner:SCOTT & WHITE MEMORIAL HOSPITAL
IL3Ralpha antibody conjugates and uses thereof
ActiveUS20090252742A1Shorten the durationReduce severitySugar derivativesMicrobiological testing/measurementCancer cellAntibody conjugate
The present invention provides antibodies that bind to the IL-3 receptor alpha subunit alpha (Il3Rα) chain, and compositions comprising such antibodies. The present invention provides methods for inhibiting or reducing an IL3Rα-expressing cell population, the methods comprising contacting a population of IL3Rα-expressing cells (e.g., cancer cells and / or cancer stem cells) with an antibody that binds to IL3Rα. The present invention also provides antibody conjugates comprising an antibody that binds to an IL3Rα chain linked to a cytotoxic agent or anticellular agent and compositions comprising such conjugates. The present invention also provides methods for preventing, treating and / or managing a disorder associated with IL3Rα-expressing cells (e.g., a hematological cancer), the methods comprising administering to a subject in need thereof an antibody that binds to IL3Rα.
Owner:STEMLINE THERAPEUTICS
Activatable clostridial toxins
InactiveUS20090018081A1Long duration of therapyOvercome resistanceHydrolasesPeptide/protein ingredientsClostridial toxinNucleic acid
Owner:ALLERGAN INC
Conjugate for the specific targeting of anticancer agents to tumor cells or tumor vasculature and production thereof
InactiveUS20090304666A1Connective tissue peptidesPeptide/protein ingredientsAnticarcinogenCancer cell
A conjugate is disclosed herein, wherein the conjugate includes a ligand having the ability to specifically and stably bind to an external receptor or binding site on a tumor vasculature endothelial cell, wherein the external receptor or binding site is specific for tumor vasculature endothelial cells. The conjugate also includes an anticancer agent that is selectively toxic to cancer cells operatively attached to the ligand. The anticancer agent may be L-methioninase. Pharmaceutical compositions comprising the conjugate are also disclosed, as well as methods of treating a cancer tumor or cancer cells with a therapeutically effective amount of the conjugate.
Owner:THE BOARD OF RGT UNIV OF OKLAHOMA
Circovirus sequences associated with piglet weight loss disease (PWD)
InactiveUS20050084497A1Antibacterial agentsGenetic material ingredientsNucleotide sequencingBody weight
The genome sequences and the nucleotide sequences coding for the PWD circovirus polypeptides, such as the circovirus structural and non-strucutral polypeptides, vectors including the sequences, and cells and animals transformed by the vectors are provided. Methods for detecting the nucleic acids or polypeptides, and kits for diagnosing infection by a PWD circovirus, also are provided. Method for selecting compounds capable of modulating the viral infection are further provided. Pharmaceutical, including vaccines, compositions for preventing and / or treating viral infections caused by PWD circovirus and the use of vectors for preventing and / or treating diseases also are provided.
Owner:ZOETIS SERVICE LLC
Modified pseudomonas exotoxin a
ActiveUS20150099707A1Increased serum half-lifeOptimize treatment planOrganic active ingredientsBacteriaPseudomonas aeruginosa exotoxin APseudomonas
The invention provides a Pseudomonas exotoxin A (PE) comprising an amino acid sequence having a substitution of one or more B-cell and / or T-cell epitopes. The invention further provides related chimeric molecules, as well as related nucleic acids, recombinant expression vectors, host cells, populations of cells, and pharmaceutical compositions. Methods of treating or preventing cancer in a mammal, methods of inhibiting the growth of a target cell, methods of producing the PE, and methods of producing the chimeric molecule are further provided by the invention.
Owner:UNITED STATES OF AMERICA
Targeted biocides
InactiveUS20050014932A1Sure easyPrevent relapseBiocideAntibody mimetics/scaffoldsInnate immune systemReceptor molecule
The present invention relates to retroviral constructs that encode novel monoclonal antibodies, novel fusion proteins, and chimeric monoclonal antibodies and to methods of using and producing the same. In particular, the present invention relates to methods of producing a fusion protein comprising a microorganism targeting molecule (e.g., immunoglobulin or innate immune system receptor molecule) and a biocide (e.g., bactericidal enzymes) in transgenic animals (e.g., bovines) and in cell cultures. The present invention also relates to therapeutic and prophylactic methods of using a fusion protein comprising a microorganism targeting molecule and a biocide in health care (e.g., human and veterinary), agriculture (e.g., animal and plant production), and food processing (e.g., beef carcass processing). The present invention also relates to methods of using a fusion protein comprising a microorganism targeting molecule and a biocide in various diagnostic applications in number of diverse fields such as agriculture, medicine, and national defense.
Owner:IOGENETICS
Molecules that bind to SARS-CoV-2
ActiveUS10822379B1Reduce severityShorten the construction periodSsRNA viruses positive-senseViral antigen ingredientsDiseaseAntigen Binding Fragment
This document provides methods and materials involved in binding a binder (e.g., an antibody, antigen binding fragment, and / or antibody domain) to a SARS-CoV-2 antigen. For example, binders (e.g., antibodies, antigen binding fragments, and antibody domains) that bind to a SARS-CoV-2 polypeptide and methods and materials for using one or more such binding molecules to treat a mammal (e.g., a human) having COVID-19 (or a viral infection caused by SARS-CoV-2) are provided.
Owner:UNIVERSITY OF PITTSBURGH
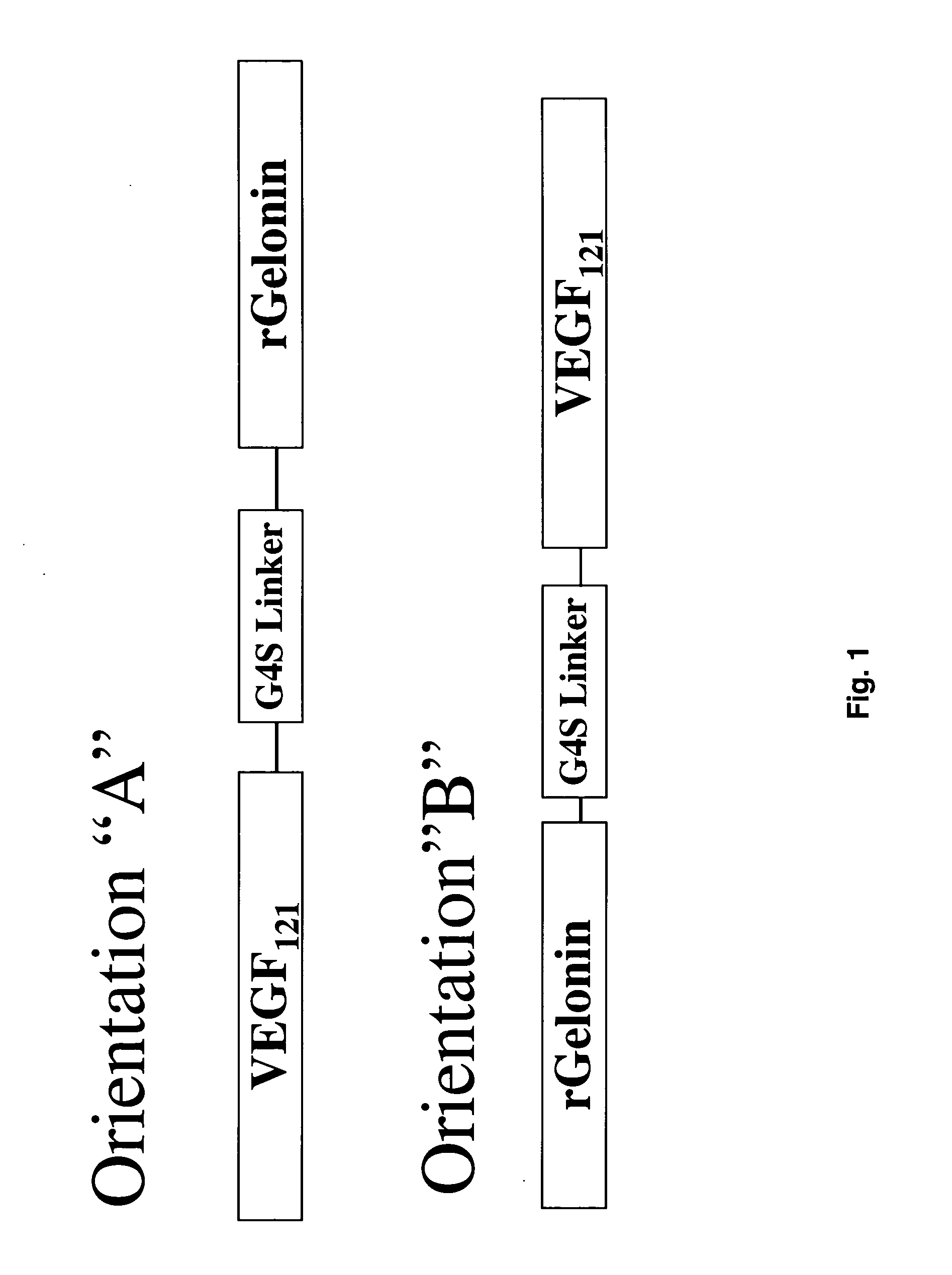
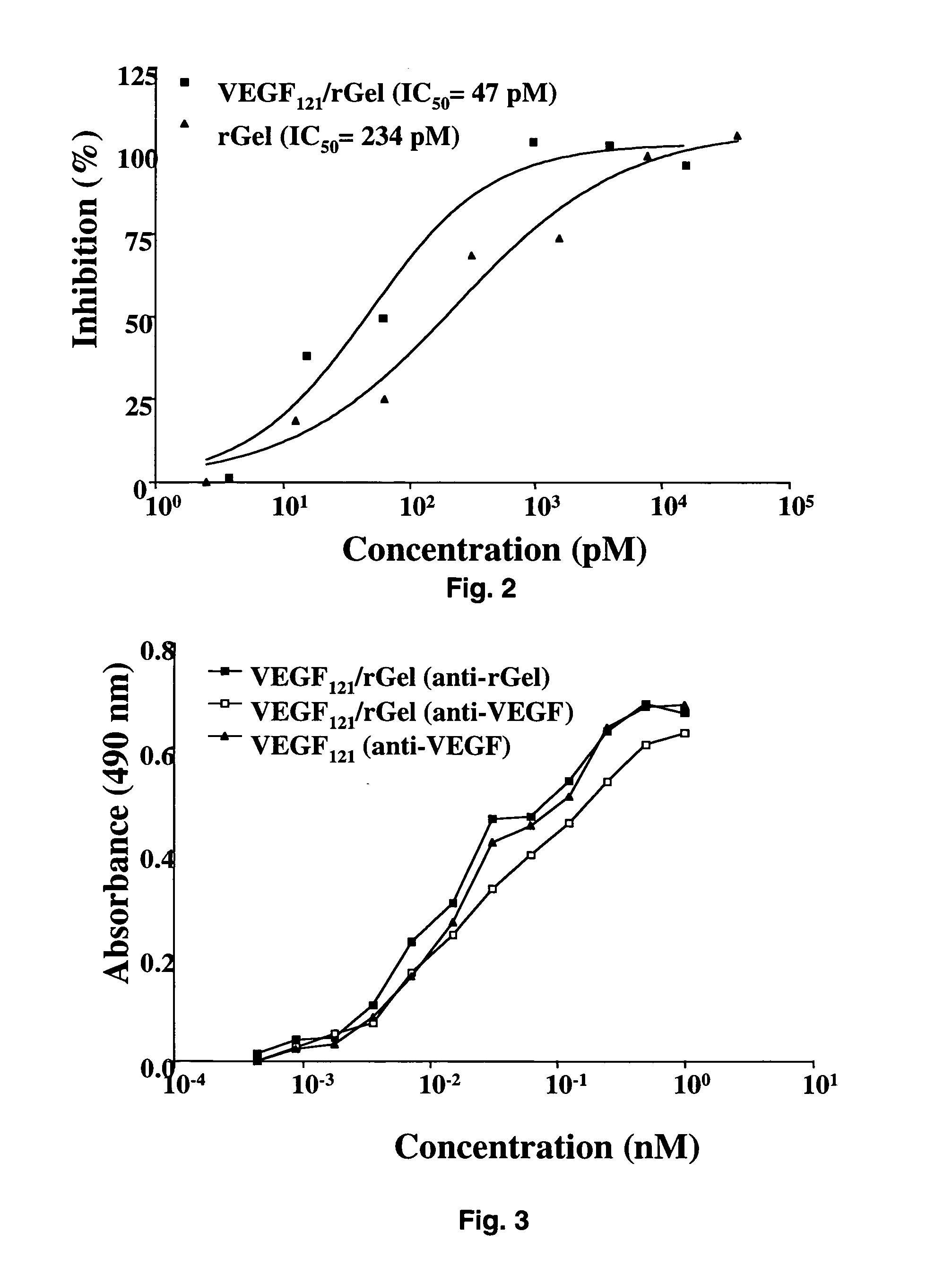
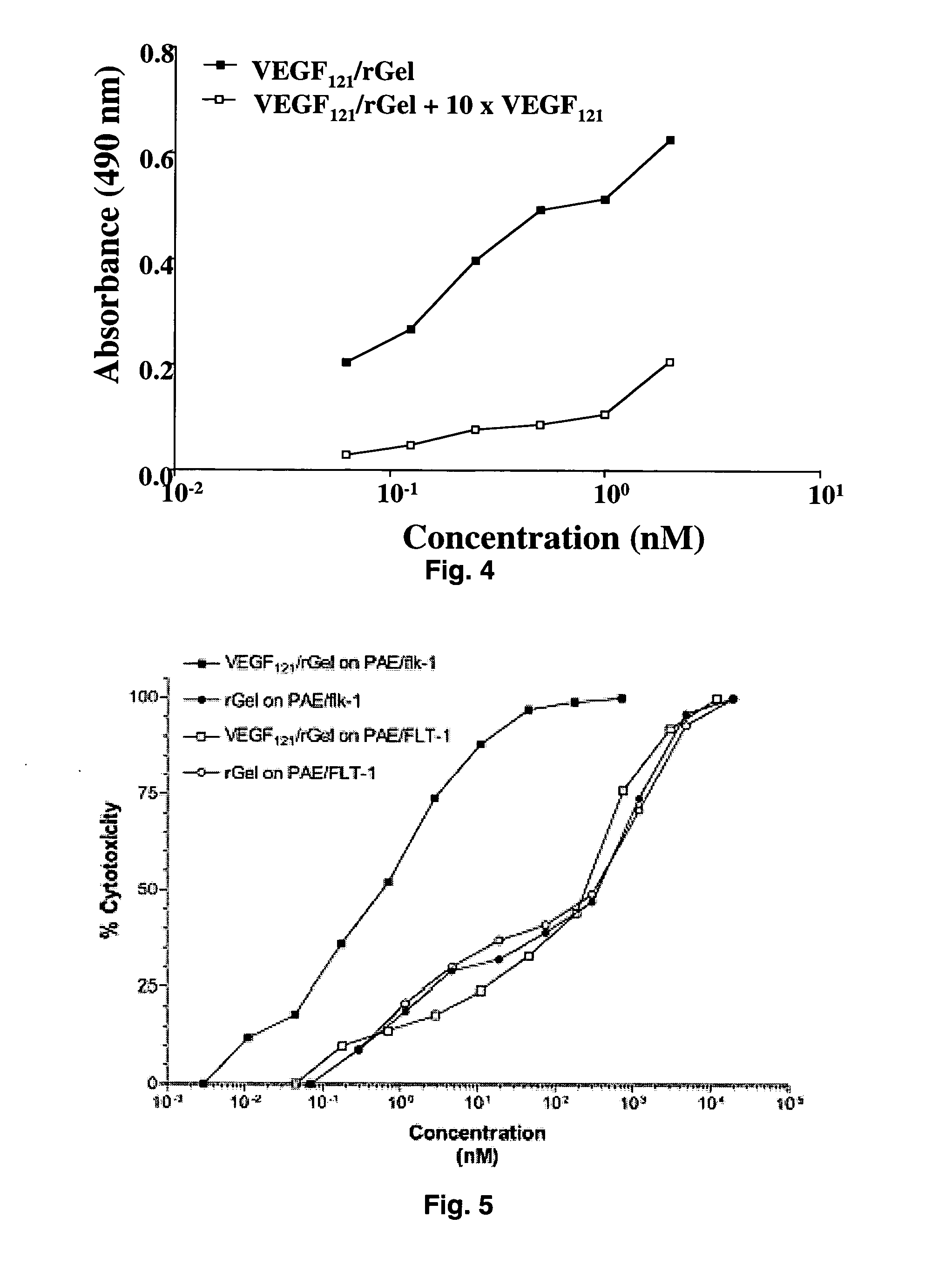
Vascular endothelial growth factor fusion constructs and uses thereof
InactiveUS20050037967A1Inhibiting metastatic spreadInhibiting vascularizationPeptide/protein ingredientsAntibody mimetics/scaffoldsGeloninAbnormal tissue growth
The 121-amino acid isoform of vascular endothelial growth factor (VEGF121) is linked by a flexible G4S tether to a cytotoxic molecule such as toxin gelonin or granzyme B and expressed as a soluble fusion protein. The VEGF12, fusion protein exhibits significant anti-tumor vascular-ablative effects that inhibit the growth of primary tumors and inhibit metastatic spread and vascularization of metastases. The VEGF121 fusion protein also target osteoclast precursor cells in vivo and inhibits osteoclastogenesis.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Toxin peptide therapeutic agents
ActiveUS7833979B2Preventing and mitigating relapseAvoid it happening againNervous disorderAntipyreticHalf-lifeFibrosis
Disclosed is a composition of matter of the formula(X1)a—(F1)d—(X2)b—(F2)e—(X3)c (I)and multimers thereof, in which F1 and F2 are half-life extending moieties, and d and e are each independently 0 or 1, provided that at least one of d and e is 1; X1, X2, and X3 are each independently -(L)f-P-(L)g-, and f and g are each independently 0 or 1; P is a toxin peptide of no more than about 80 amino acid residues in length, comprising at least two intrapeptide disulfide bonds; L is an optional linker; and a, b, and c are each independently 0 or 1, provided that at least one of a, b and c is 1. Linkage to the half-life extending moiety or moieties increases the in vivo half-life of the toxin peptide, which otherwise would be quickly degraded. A pharmaceutical composition comprises the composition and a pharmaceutically acceptable carrier. Also disclosed are a DNA encoding the inventive composition of matter, an expression vector comprising the DNA, and a host cell comprising the expression vector. Methods of treating an autoimmune disorder, such as, but not limited to, multiple sclerosis, type 1 diabetes, psoriasis, inflammatory bowel disease, contact-mediated dermatitis, rheumatoid arthritis, psoriatic arthritis, asthma, allergy, restinosis, systemic sclerosis, fibrosis, scleroderma, glomerulonephritis, Sjogren syndrome, inflammatory bone resorption, transplant rejection, graft-versus-host disease, and lupus and of preventing or mitigating a relapse of a symptom of multiple sclerosis are also disclosed.
Owner:AMGEN INC
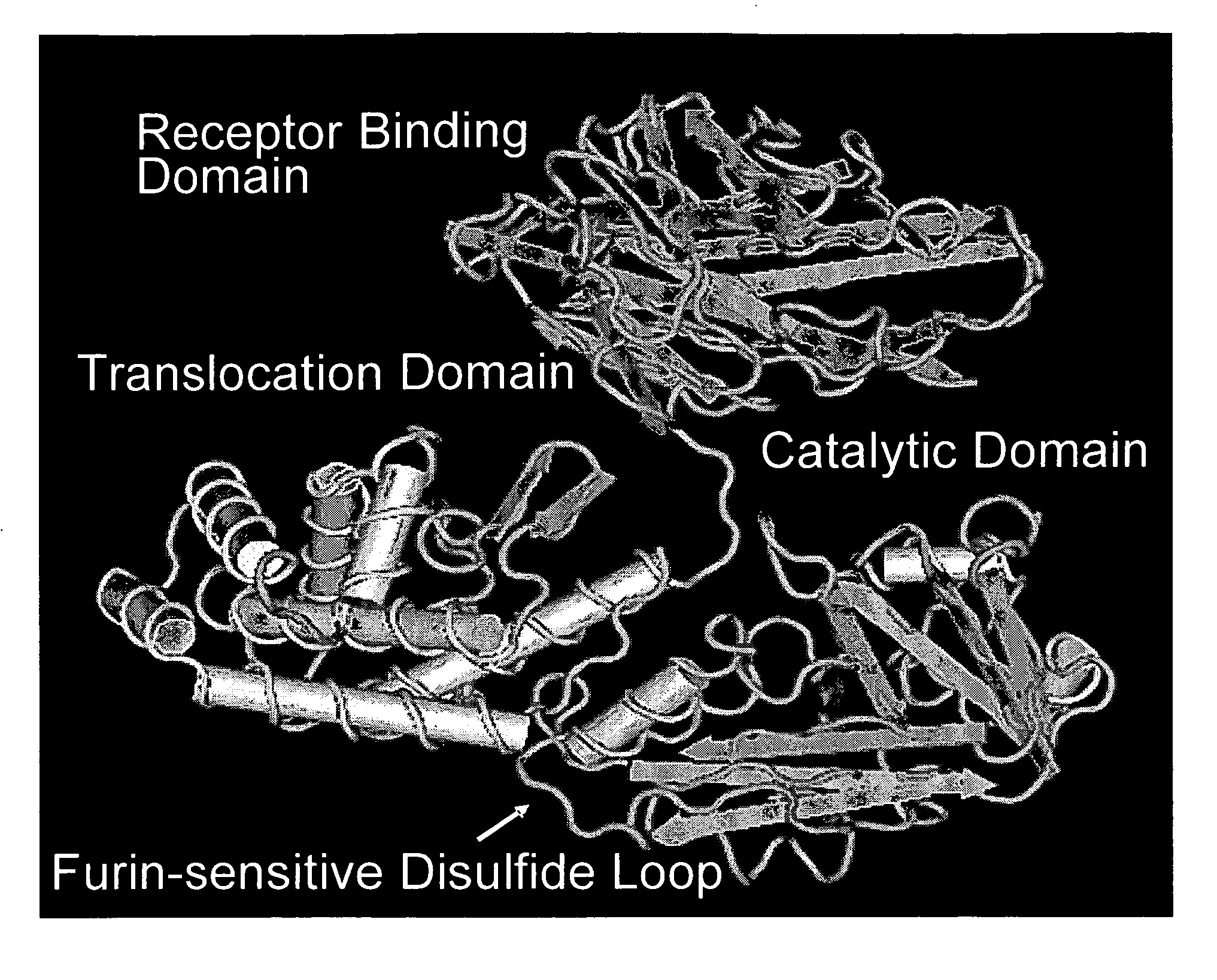
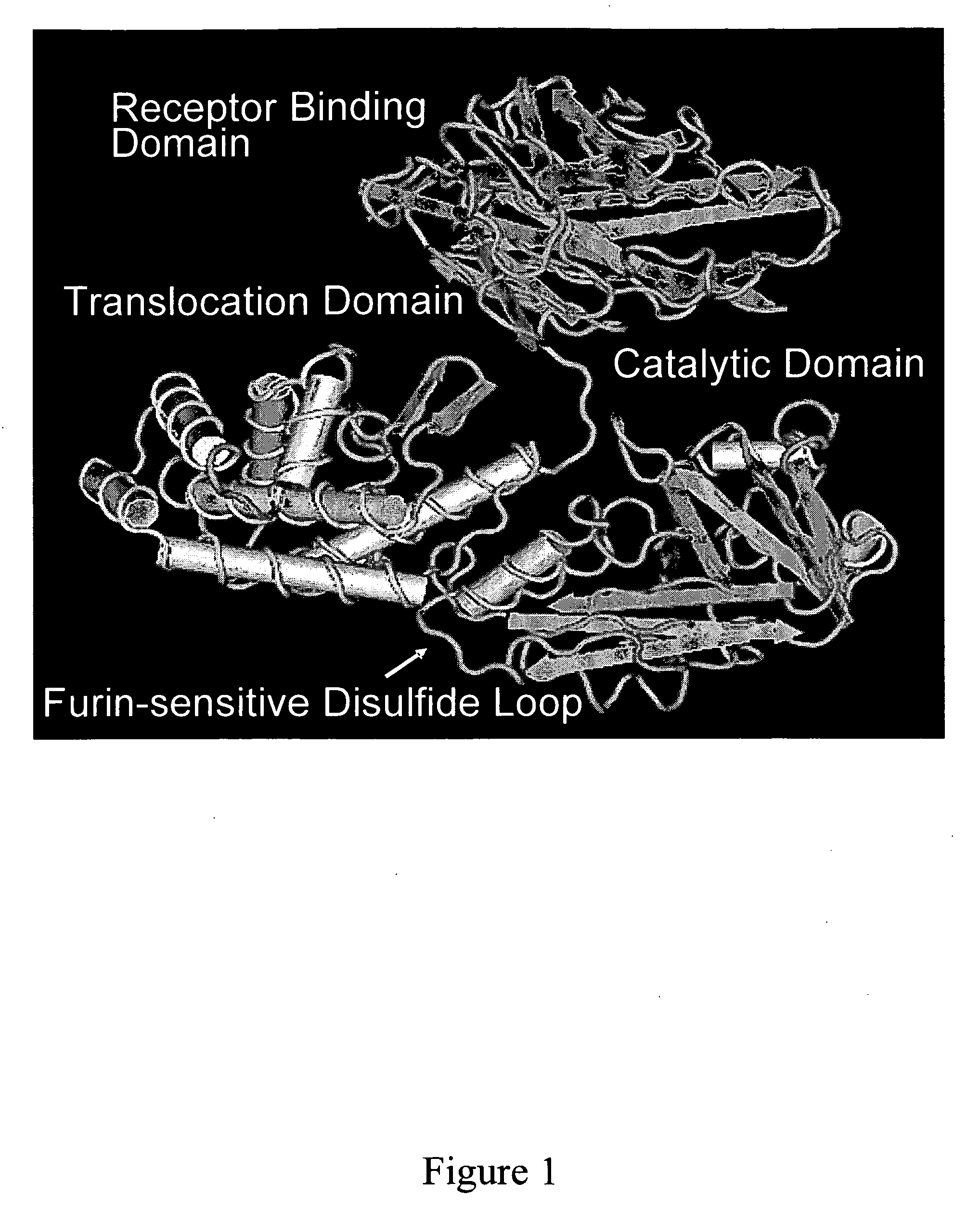
NOVEL PROTEIN DELIVERY SYSTEM TO GENERATE INDUCED PLURIPOTENT STEM (iPS) CELLS OR TISSUE-SPECIFIC CELLS
A novel protein delivery system to generate induced pluripotent stem (iPS) cells is described. The delivery system comprises a construct with a receptor binding domain that recognizes a receptor in a somatic cell, a translocation domain that allows the transfer of an inducer into the cytosolic space, and a cargo bearing domain to which the inducer is attached and facilitates transfer of the inducer into the cell.
Owner:IPSEN PHARMA SAS
Use of human cells of myeloid leukaemia origin for expression of antibodies
ActiveUS20100028947A1High activityIncrease productionPeptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsProtein moleculesHuman cell
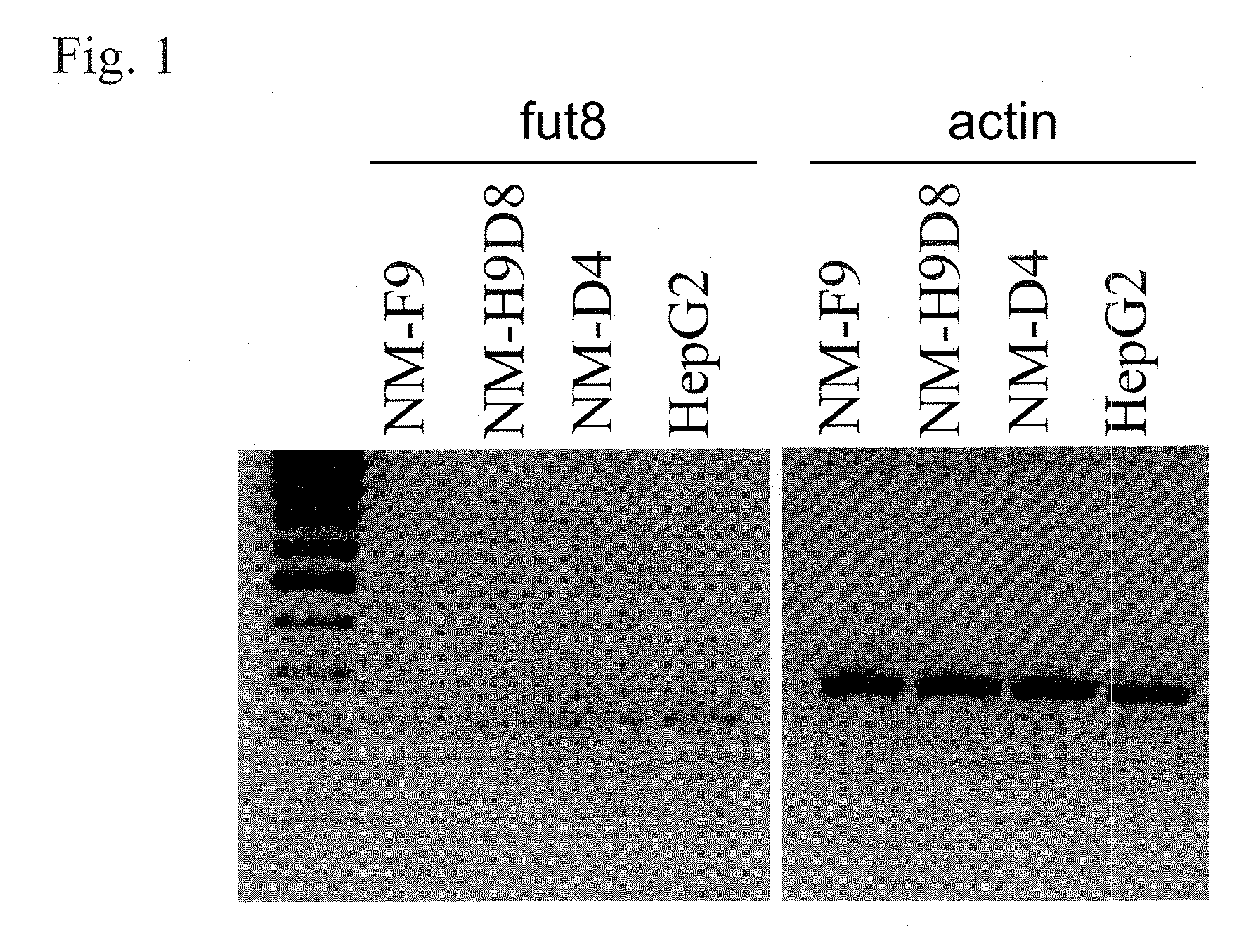
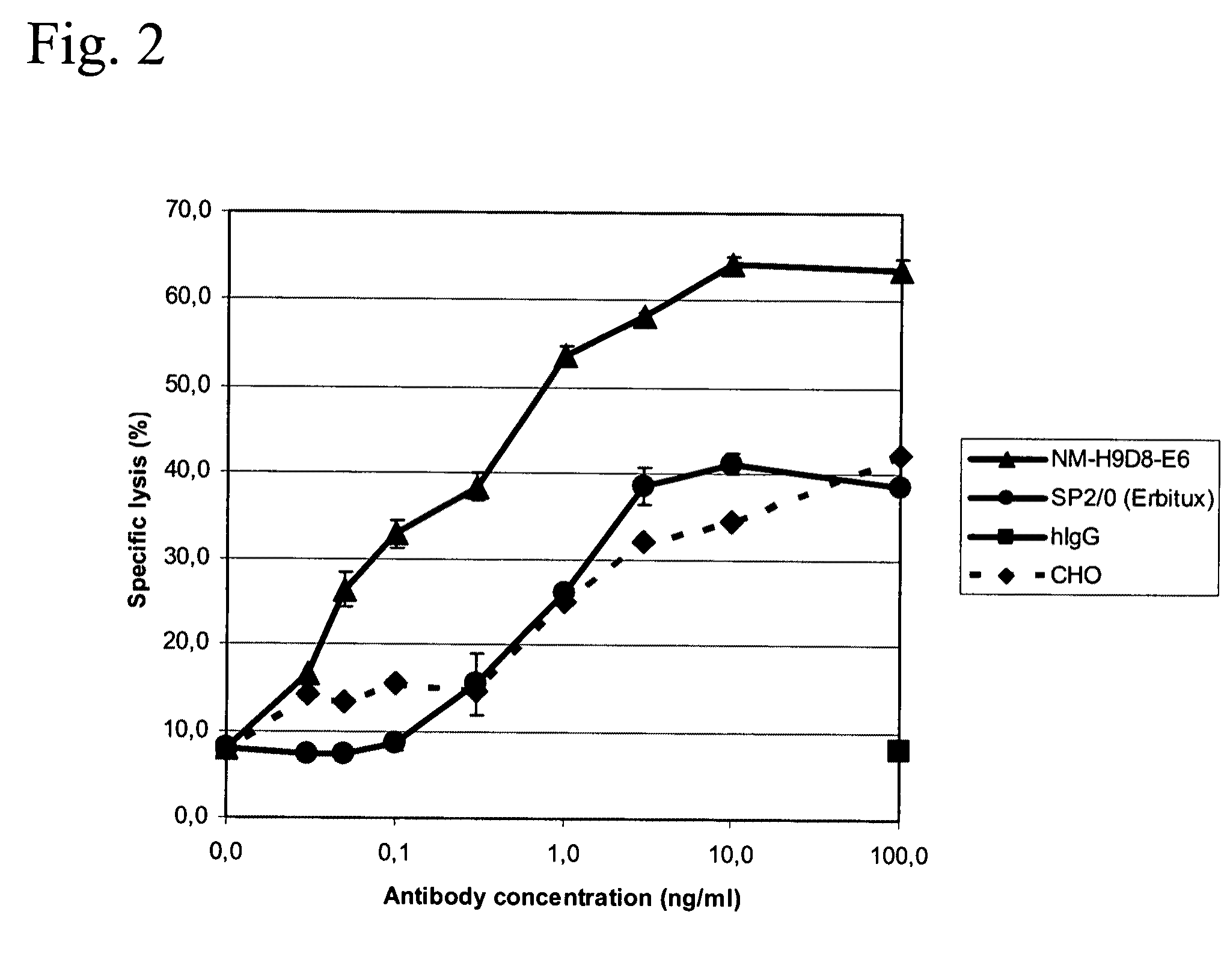
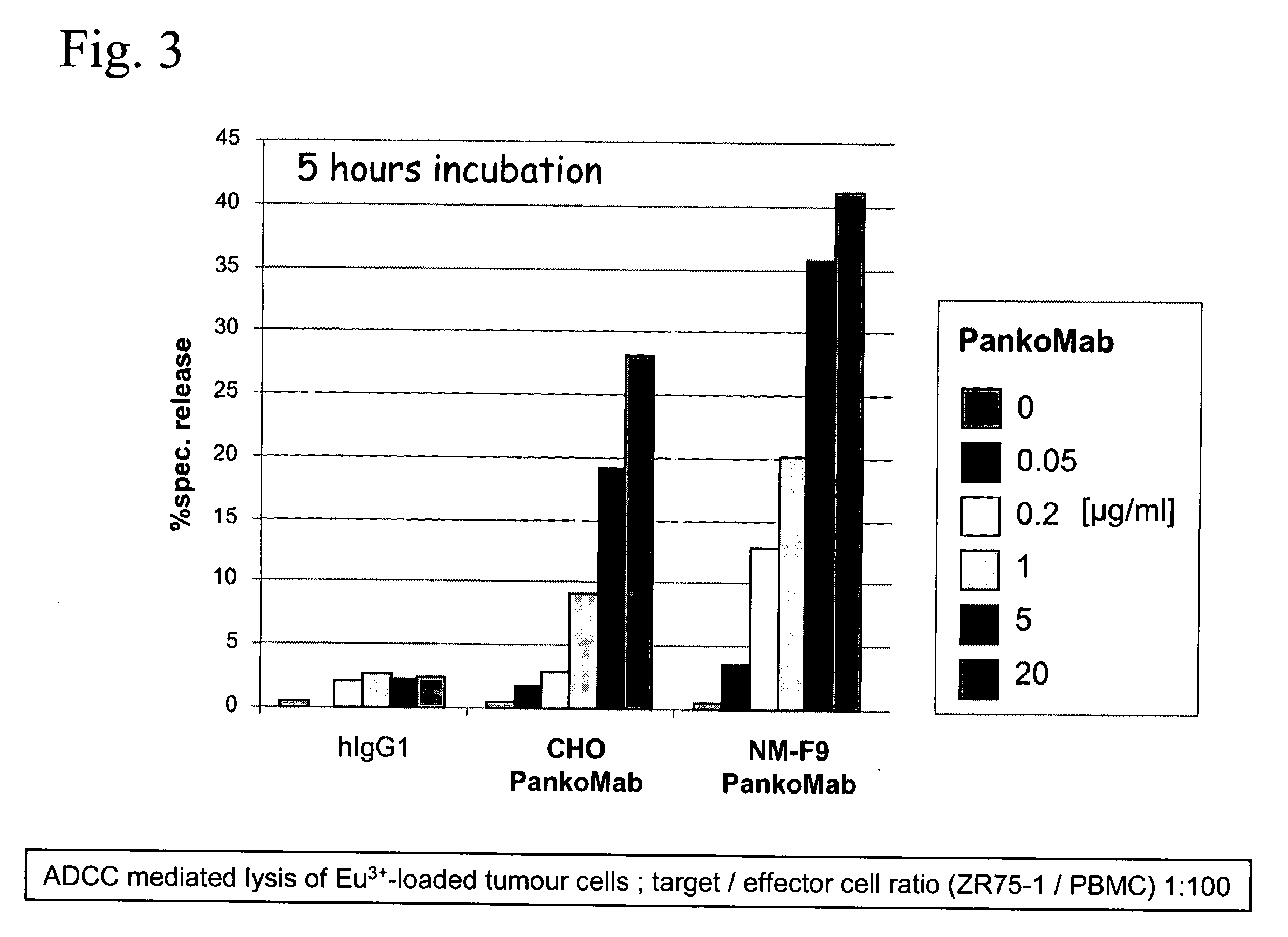
The invention relates to a method for producing a protein molecule composition having a defined glycosylation pattern, comprising (a) introducing in a host cell which is an immortalized human blood cell at least one nucleic acid encoding at least a part of said protein; and (b) culturing said host cell under conditions which permit the production of said protein molecule composition; and (c) isolating said protein molecule composition.
Owner:GLYCOTOPE GMBH
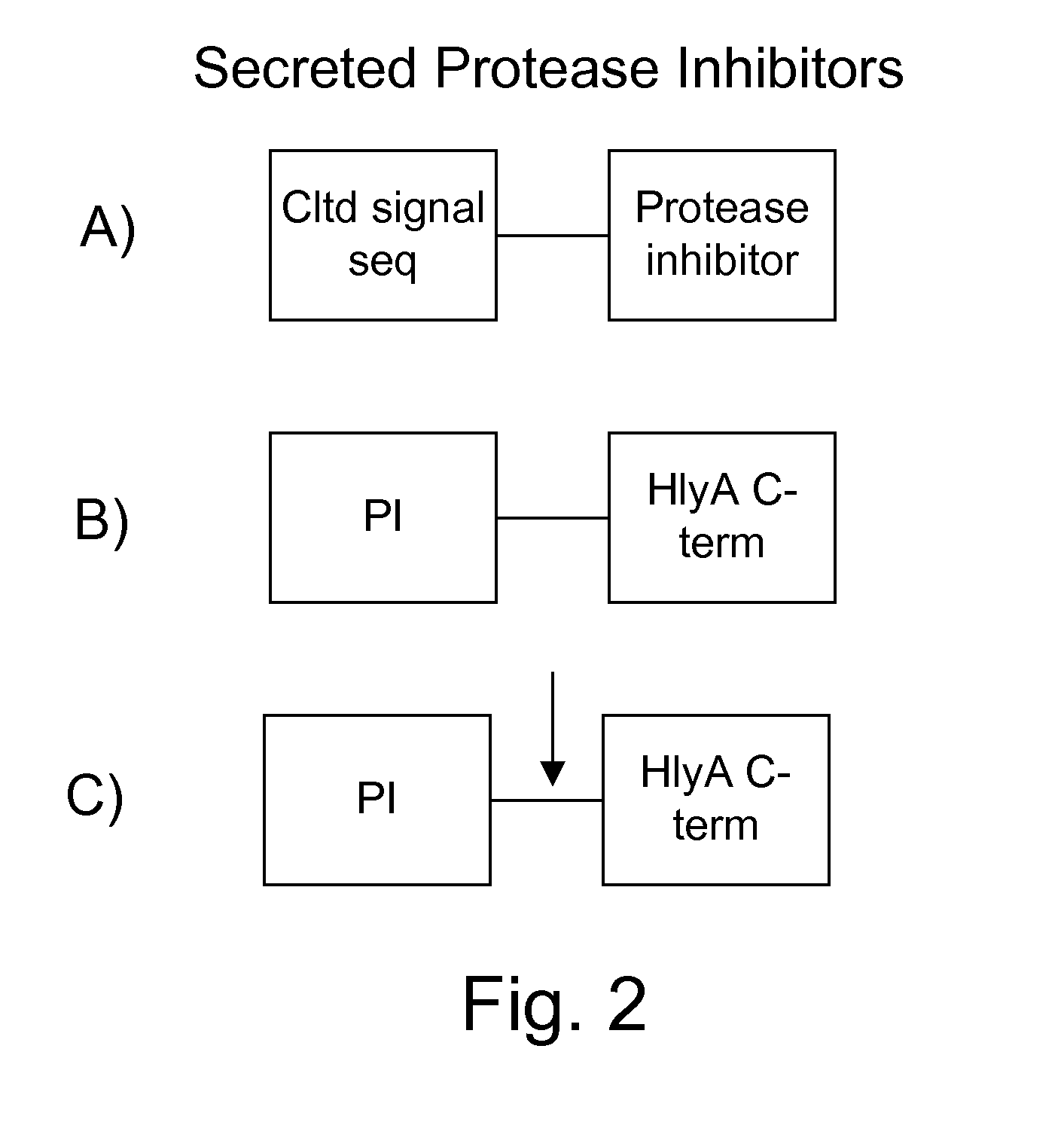
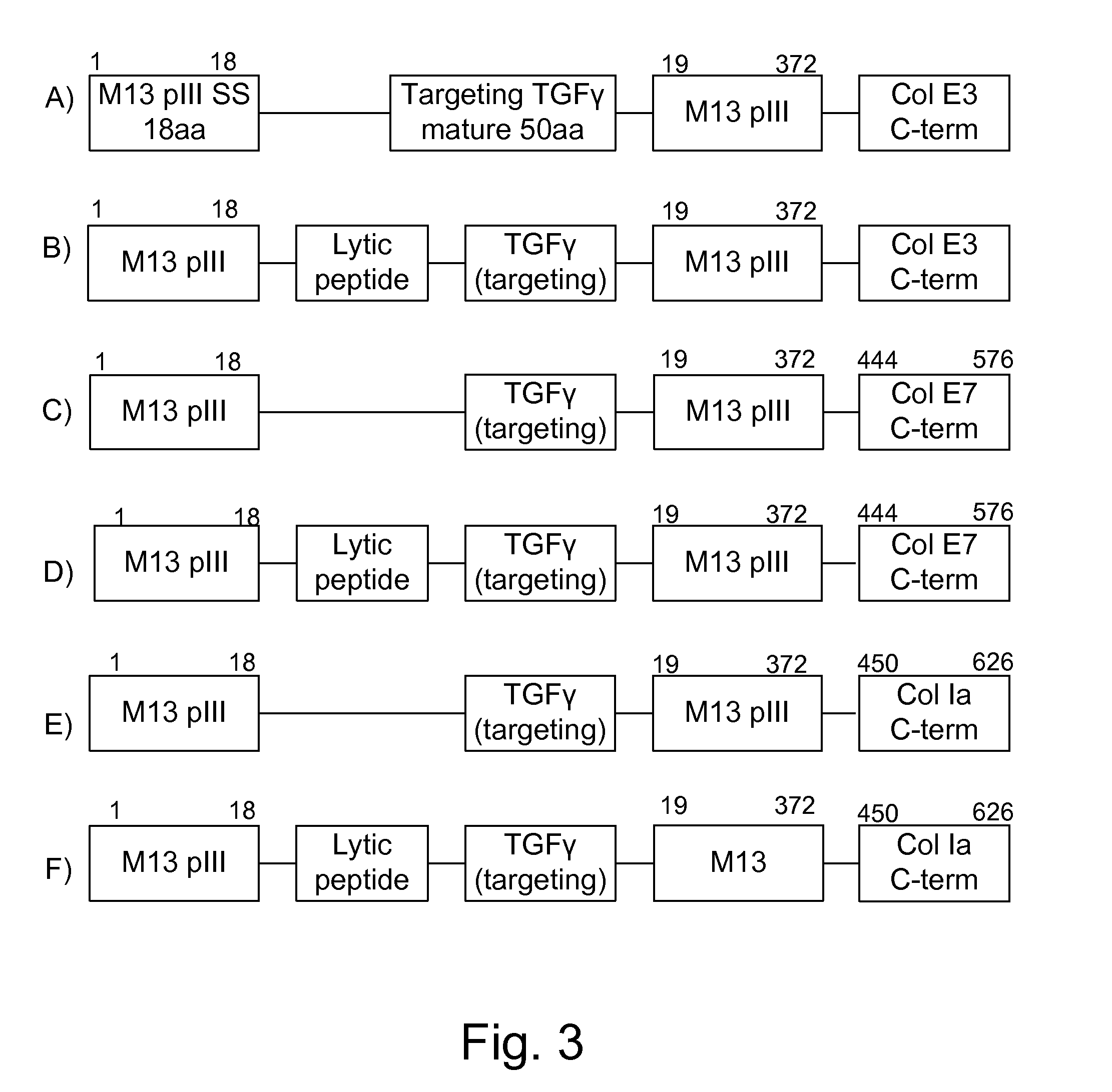
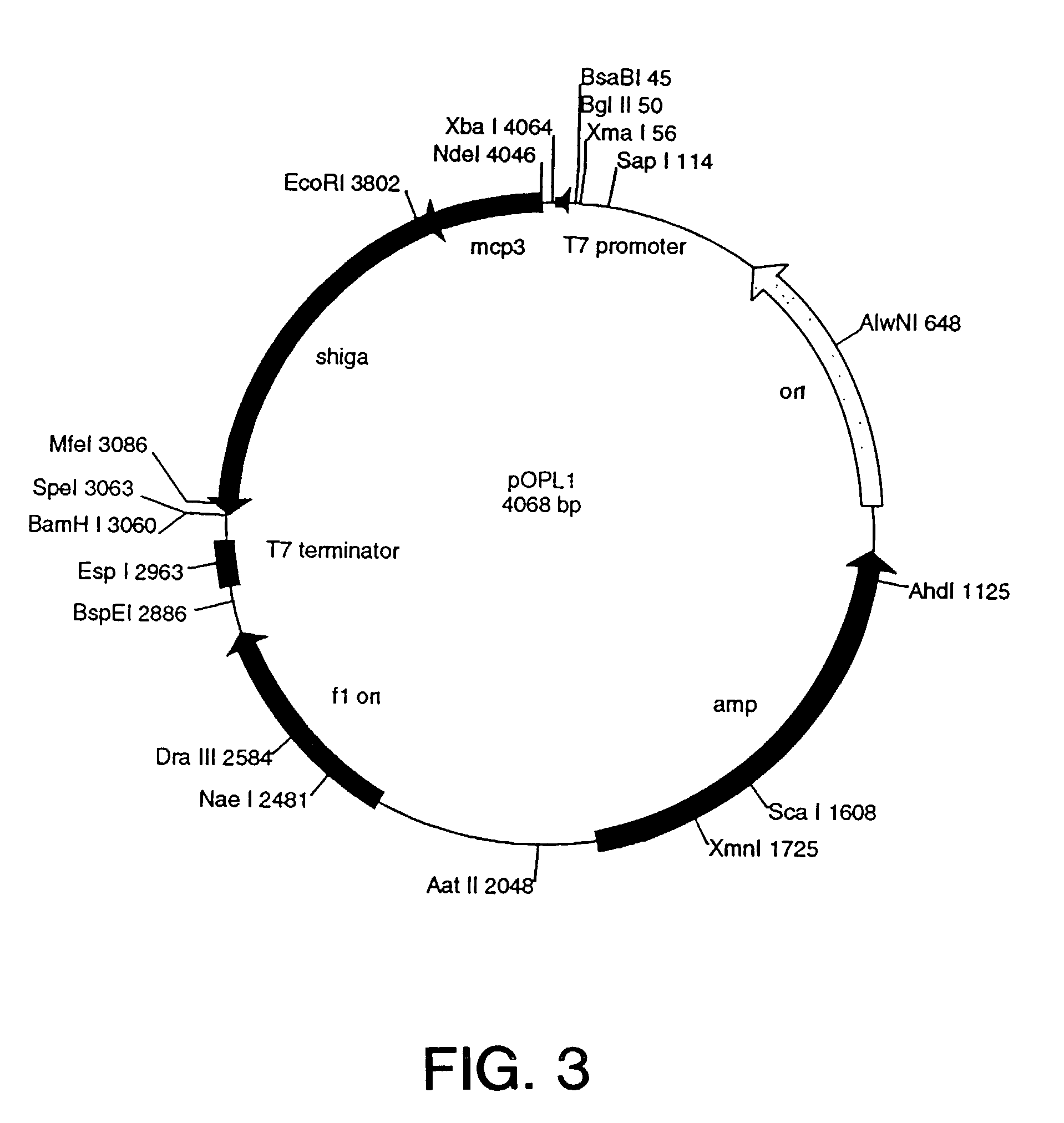
Protease sensitivity expression system
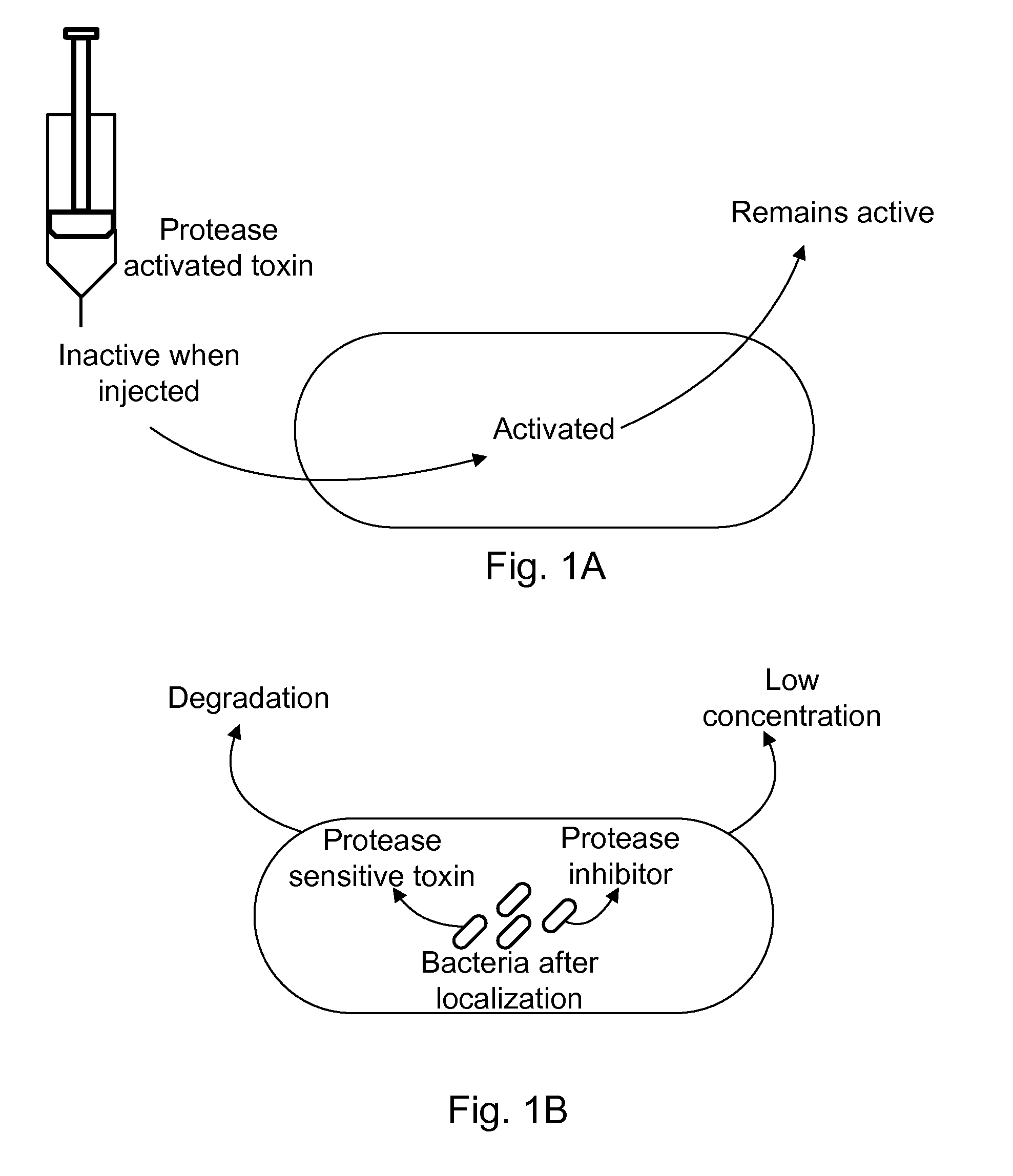
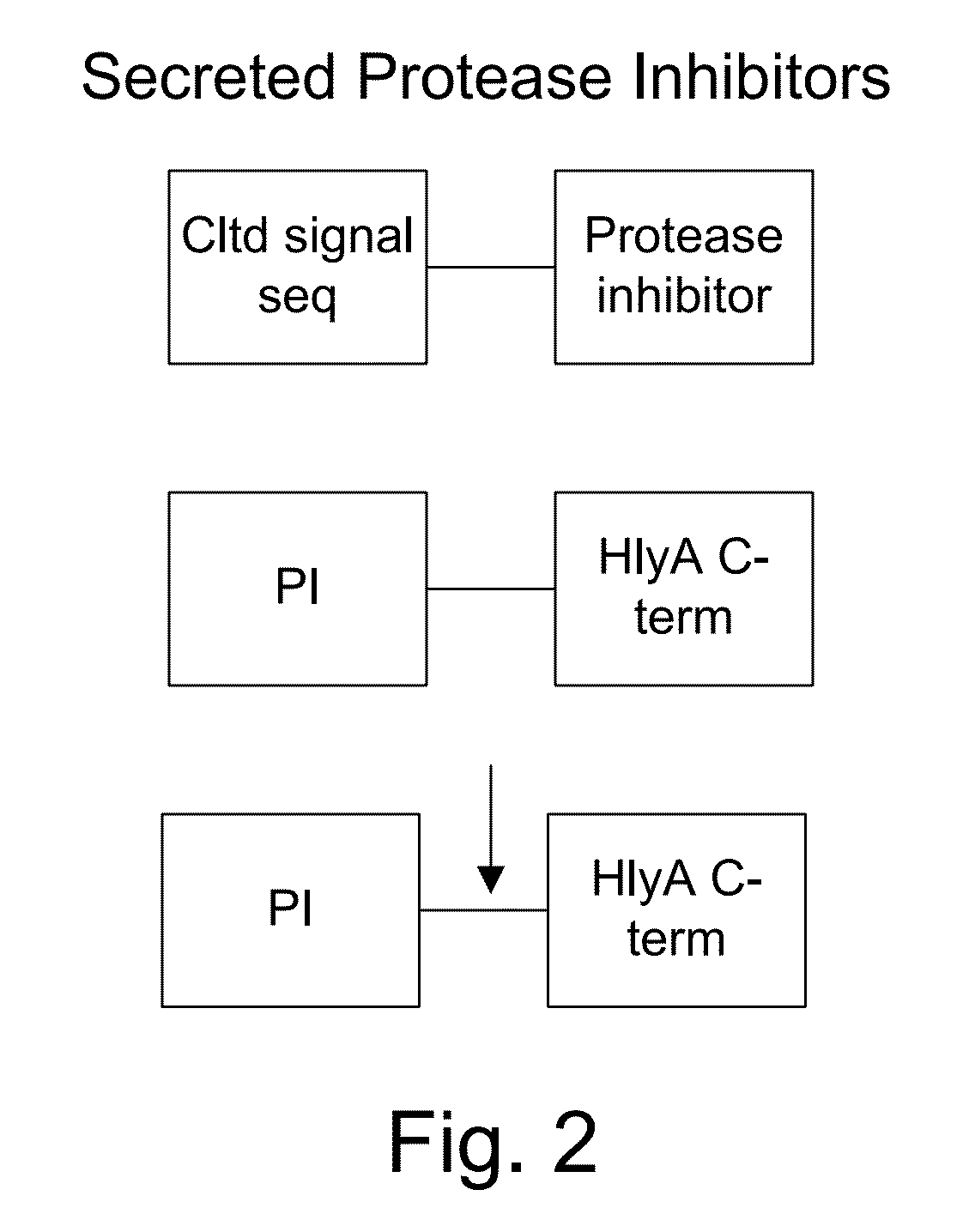
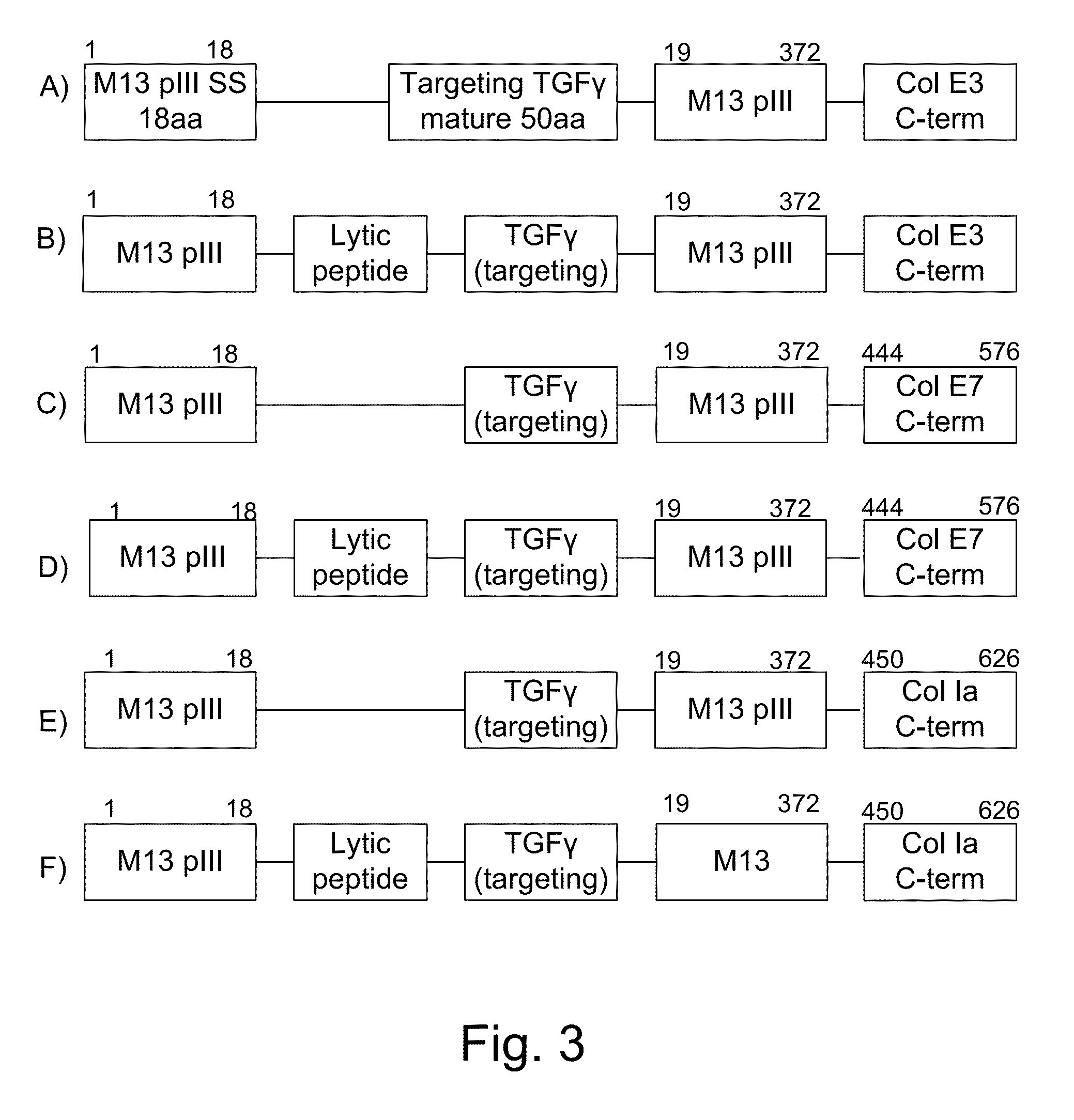
The present invention uses co-expression of protease inhibitors and protease sensitive therapeutic agents that results in their localized production within the target tissue and inactivation outside of the target tissue, thereby increasing therapeutic activity and reducing the systemic toxicity. Inactivation is also accomplished by engineering protease degradation sites within the therapeutic construct for proteases, preferably those that are under-expressed within the target tissue yet present in non-target tissues within the body, resulting in therapeutic activity within the target tissue and inactivation outside of the target tissue. Novel chimeric proteins secreted by bacteria are also described. The chimeric proteins include chimeric toxins targeted to neoplastic cells and cells of the immune system. Novel combination therapies of these protease inhibitor:chimeric toxin-expressing bacteria together with small-molecule and biologic agents are also described. Non-conjugative bacteria capable of delivering phage / phagemids expression cassettes for DNA and RNA-based therapeutics are also described.
Owner:BERMUDES DAVID
Methods and compositions for treating secondary tissue damage and other inflammatory conditions and disorders
InactiveUS7157418B1Enhance and aid in survivalPrevent proliferationAntibacterial agentsBiocidePhagocyteDendritic cell
Conjugates containing as a ligand a chemokine receptor targeting agents, such as chemokines, and a targeted agent, such as a toxin are provided. These conjugates are used to treat inflammatory responses associated with activation, proliferation and migration of immune effector cells, including leukocyte cell types, neutrophiles, macrophages, and eosinophils. The conjugates provided herein are used to lessen or inhibit these processes to prevent or at least lessen the resulting secondary effects. In particular, the conjugates are used to target toxins to receptors on secondary tissue damage-promoting cells. The ligand moiety can be selected to deliver the cell toxin to such secondary tissue damage-promoting cells as mononuclear phagocytes, leukocytes, natural killer cells, dendritic cells, and T and B lymphocytes, thereby suppressing the proliferation, migration, or physiological activity of such cells. Among preferred conjugates are fusion proteins having a chemokine, or a biologically active fragment thereof, as the ligand moiety linked to a cell toxin via a peptide linker of from 2 to about 60 amino acid residues.
Owner:OSPREY PHARMA USA INC
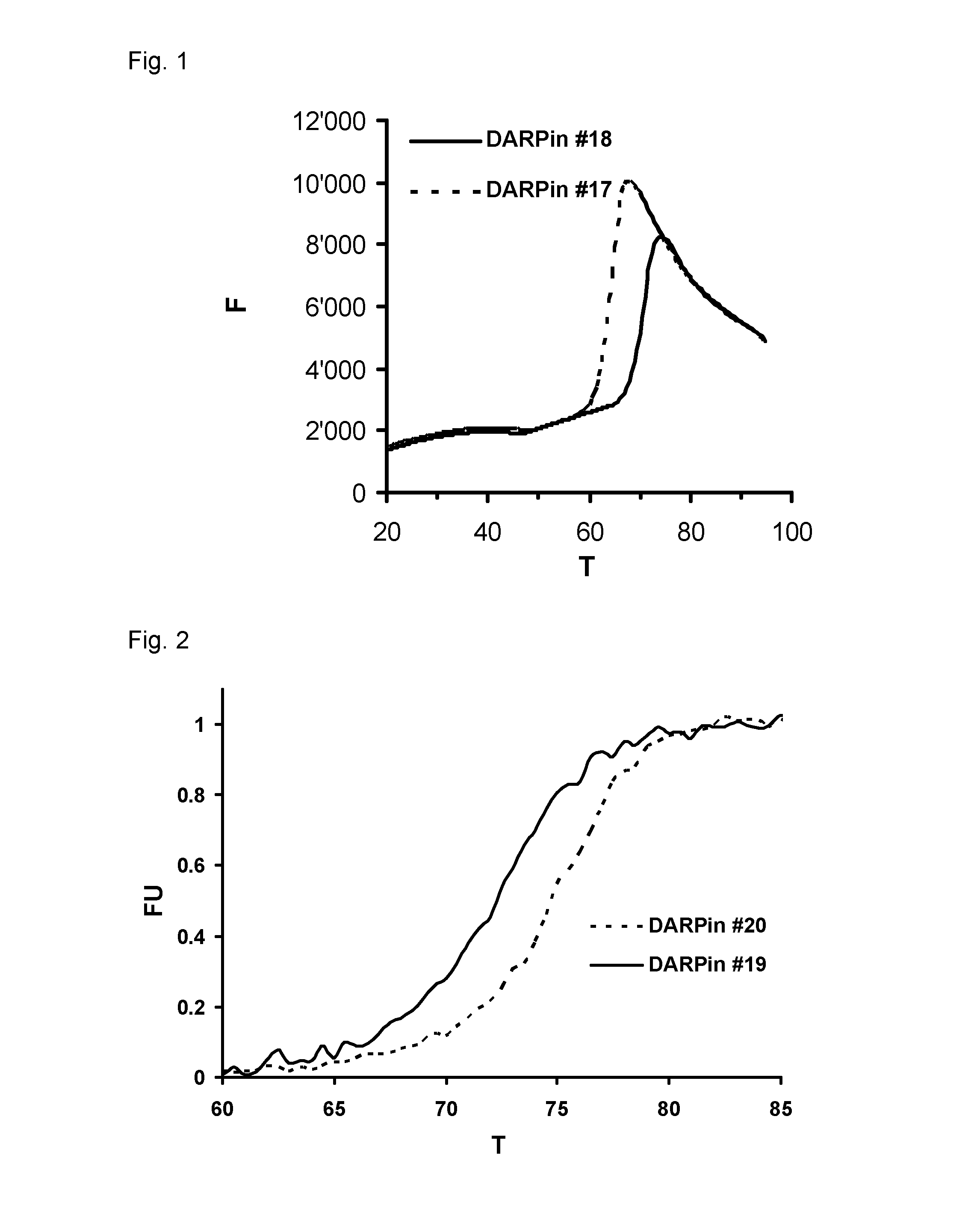
Improved capping modules for designed ankyrin repeat proteins
ActiveUS20130296221A1Improve thermal stabilityPeptide/protein ingredientsAntibody mimetics/scaffoldsBiotechnologyDisease
Improved N-terminal capping modules for designed ankyrin repeat proteins (DARPins) conferring improved thermal stability to the DARPins are described, as well as nucleic acids encoding such proteins, pharmaceutical compositions comprising such proteins and the use of such proteins in the treatment of diseases.
Owner:MOLECULAR PARTNERS AG
Protease inhibitor: protease sensitivity expression system and method improving the therapeutic activity and specificity of proteins and phage and phagemids delivered by bacteria
The present invention uses co-expression of protease inhibitors and protease sensitive therapeutic agents that results in their localized production within the target tissue and inactivation outside of the target tissue, thereby increasing therapeutic activity and reducing the systemic toxicity. Inactivation is also accomplished by engineering protease degradation sites within the therapeutic construct for proteases, preferably those that are under-expressed within the target tissue yet present in non-target tissues within the body, resulting in therapeutic activity within the target tissue and inactivation outside of the target tissue. Novel chimeric proteins secreted by bacteria are also described. The chimeric proteins include chimeric toxins targeted to neoplastic cells and cells of the immune system. Novel combination therapies of these protease inhibitor:chimeric toxin-expressing bacteria together with small-molecule and biologic agents are also described. Non-conjugative bacteria capable of delivering phage / phagemids expression cassettes for DNA and RNA-based therapeutics are also described.
Owner:BERMUDES DAVID GORDON
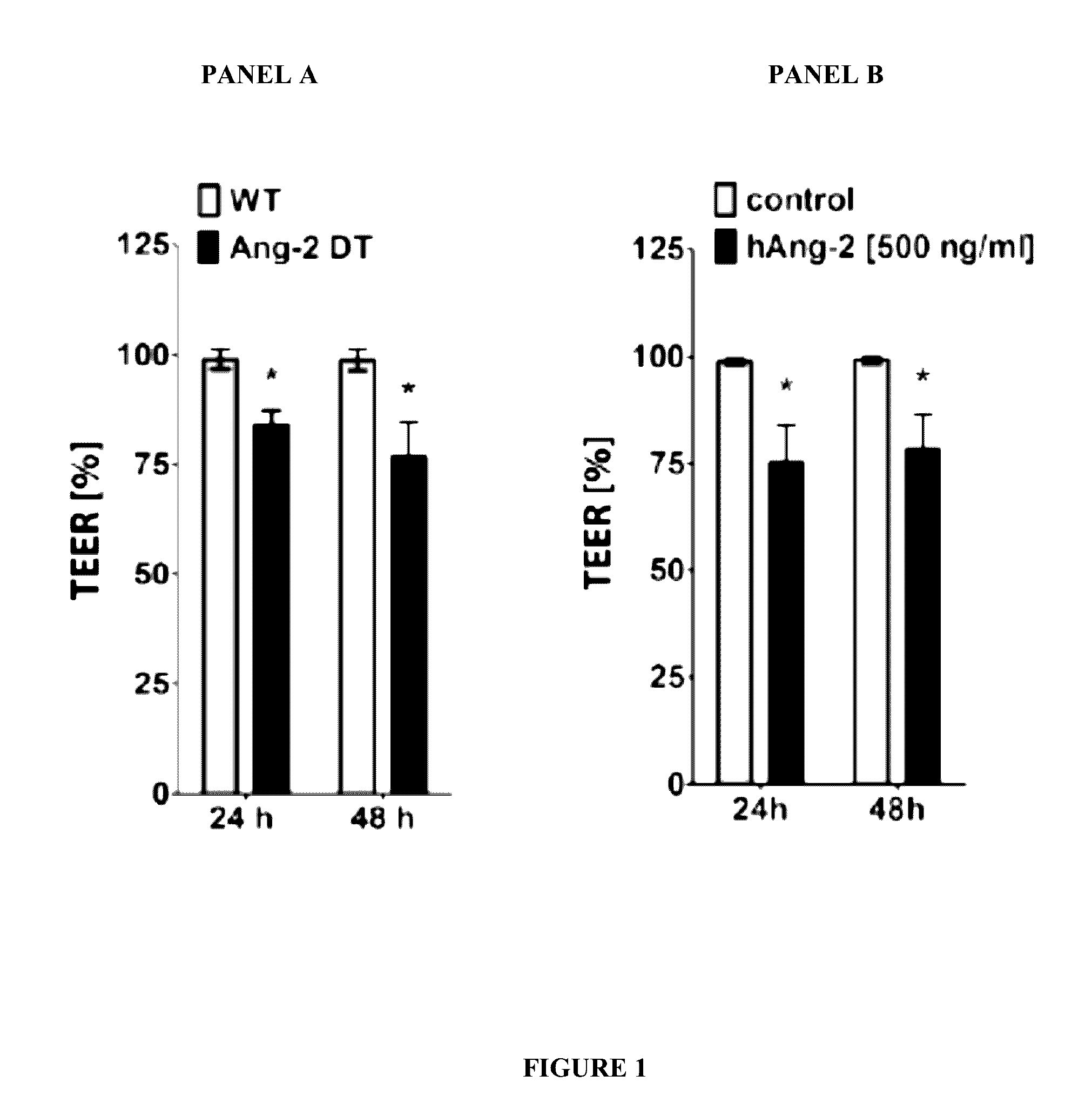
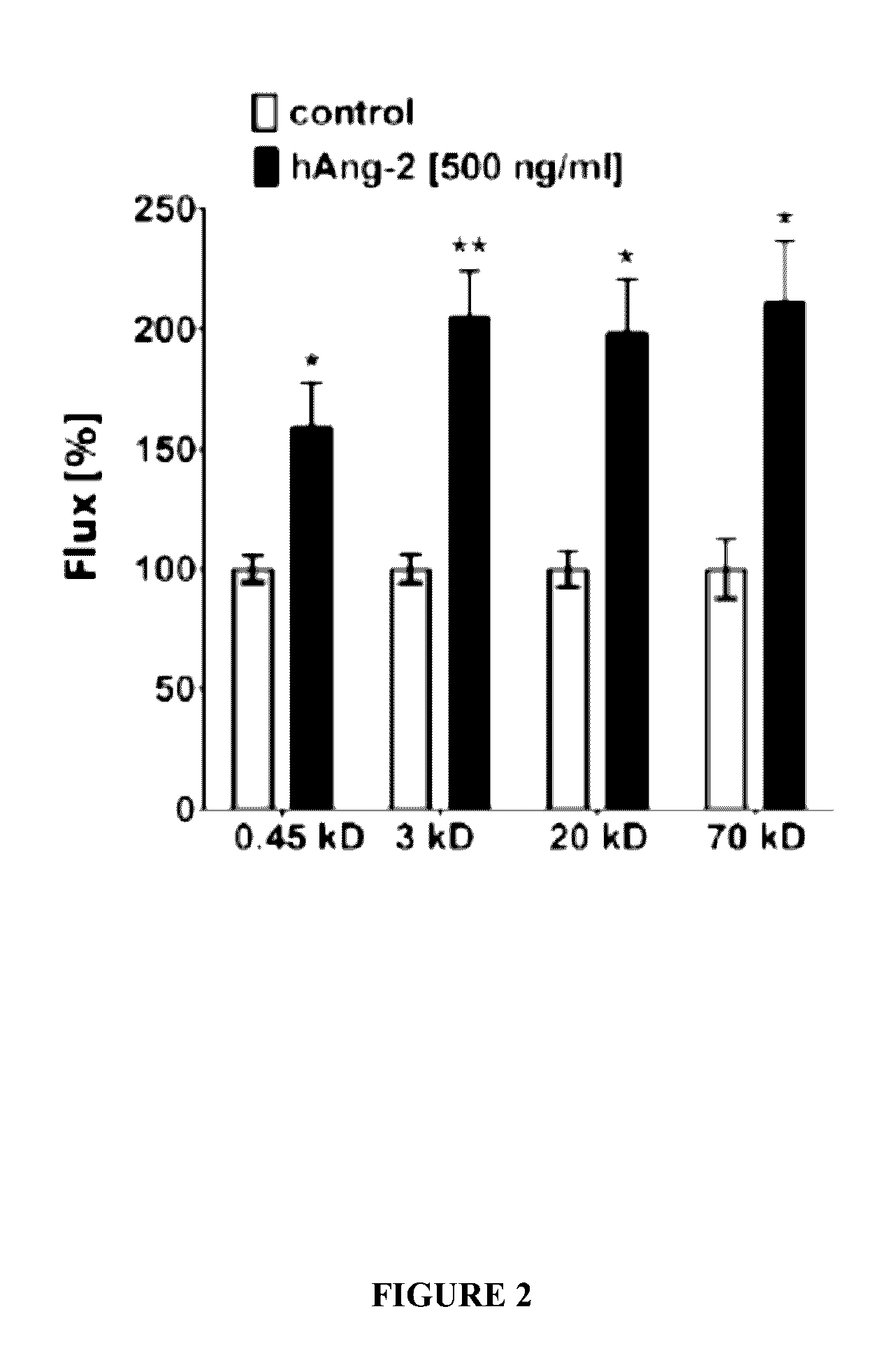
Combination of immunotherapies with activators of Tie-2
The present invention relates to combination therapies comprising at least one activator of Tie-2 and immunotherapies that target immune checkpoints. Combination therapies of the disclosure can provide therapeutic effects not obtained with singly-administered immunotherapies. Combination therapies can be used to increase the therapeutic efficacy of an immunotherapy, to provide lower dosages of an immunotherapy being administered, to lower a toxicity of an immunotherapy, or to manage a side effect of an immunotherapy.
Owner:EYEPOINT PHARMA INC
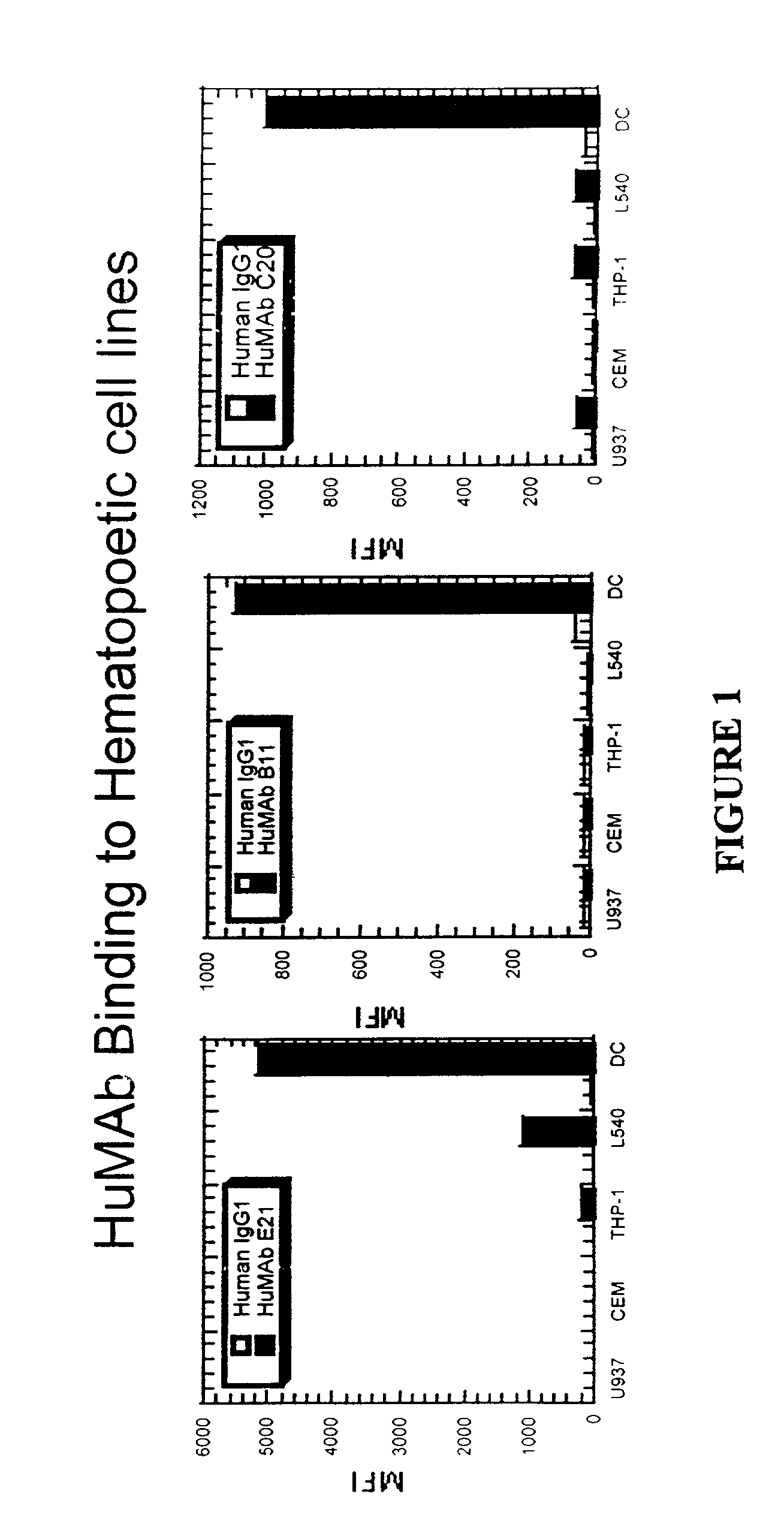
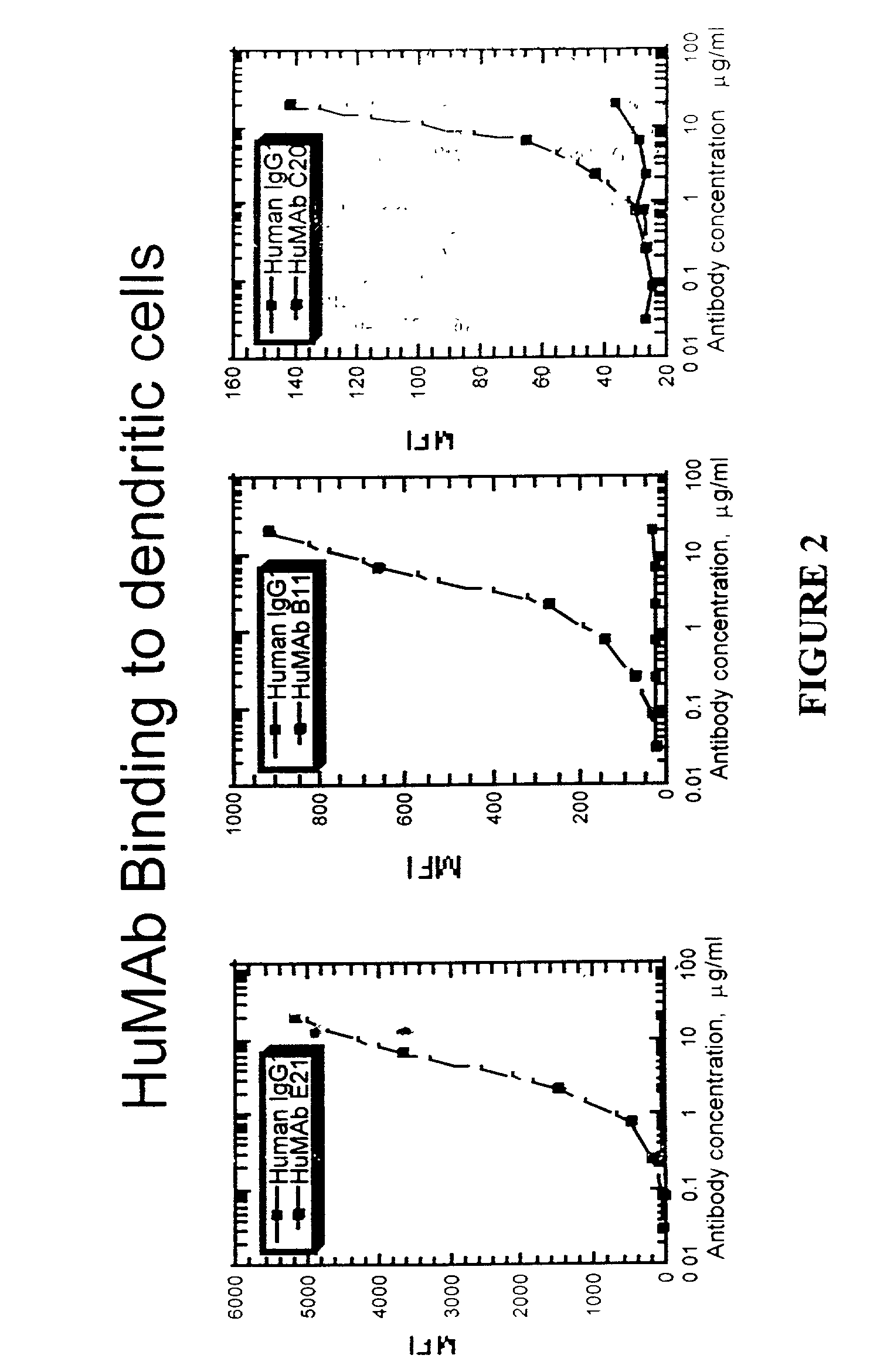
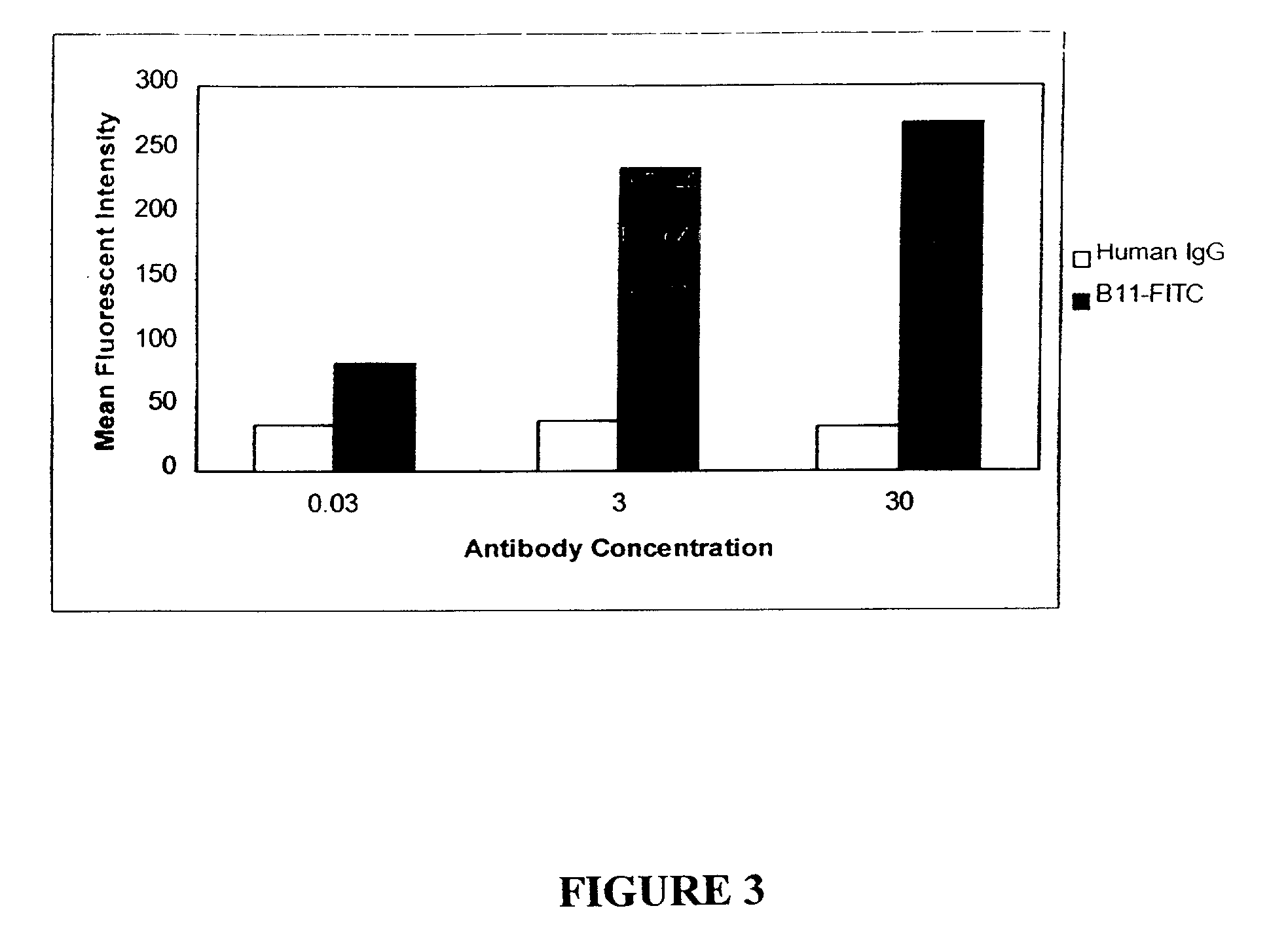
Molecular conjugates comprising human monoclonal antibodies to dendritic cells
InactiveUS7560534B2Enhance antigen presentationStrong immune responseNervous disorderAntipyreticDendritic cellMonoclonal antibody
Molecular conjugates comprising an antigen linked to a human monoclonal antibody that specifically binds to dendritic cells are disclosed. Also disclosed are pharmaceutical compositions comprising the molecular conjugates and therapeutic methods for using the conjugates.
Owner:CELLDEX THERAPEUTICS INC
A fowl adenovirus group I serum type 4 genetic engineering subunit vaccine, and a preparing method and applications thereof
ActiveCN106946995AHigh antigen contentImprove securitySsRNA viruses negative-senseViral antigen ingredientsDiseaseInclusion bodies
A fowl adenovirus group I serum type 4 genetic engineering subunit vaccine, and a preparing method and applications thereof are disclosed. The sequence of an antigen protein in the vaccine is shown as SEQ ID NO:1. The antigen protein has advantages of high safety, high immunity, no pathogenicity for chickens or other animals, and the like. The subunit vaccine can prevent chicken hydropericardium syndrome, inclusion body hepatitis and other diseases which are caused by infection of fowl adenovirus group I serum type 4.
Owner:苏州沃美生物有限公司
Methods and Compositions for Inducing an Immune Response Against Multiple Antigens
InactiveUS20090155297A1Effective and potent immune responseReduce and prevent infectionSugar derivativesAntibody mimetics/scaffoldsHeterologousHeterologous Antigens
Methods and compositions for inducing an immune response against multiple antigens are provided herein. In one aspect, the invention provides a chimeric immunogen, comprising a receptor binding domain, a translocation domain, and more than one non-contiguous heterologous antigen. In other aspects, the invention provides nucleic acids encoding chimeric immunogens of the invention, kits comprising chimeric immunogens of the invention, cells expressing chimeric immunogens of the invention, and methods or using chimeric immunogens of the invention. 1mggkwskssv igwptvrerm rraepaadrvgaasrdlekh gaitssntaa tnaacawlea 61qeeeevgfpv tpqvplrpmt ykaavdlshflkekgglegl ihsqrrqdil dlwiyhtqgy121fpdwqnytpg pgvrypltfg wcyklvpvepdkieeankge ntsllhpvsl hgmddperev181lewrfdsrla fhhvarelhp eyfknc
Owner:TRINITY BIOSYSTEMS INC
Mutant lysenin pores
ActiveUS9777049B2Enhanced ability to interactEasily discriminatedMicrobiological testing/measurementMaterial analysis by electric/magnetic meansChemistry
Owner:OXFORD NANOPORE TECH LTD
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com