Conjugate for the specific targeting of anticancer agents to tumor cells or tumor vasculature and production thereof
a technology of tumor vasculature and anticancer agent, which is applied in the direction of growth factors/regulators, pharmaceutical non-active ingredients, animal/human proteins, etc., can solve the problems of compromising the benefits of treatment, affecting the survival rate of patients, and the failure of existing therapies for such internal cancers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
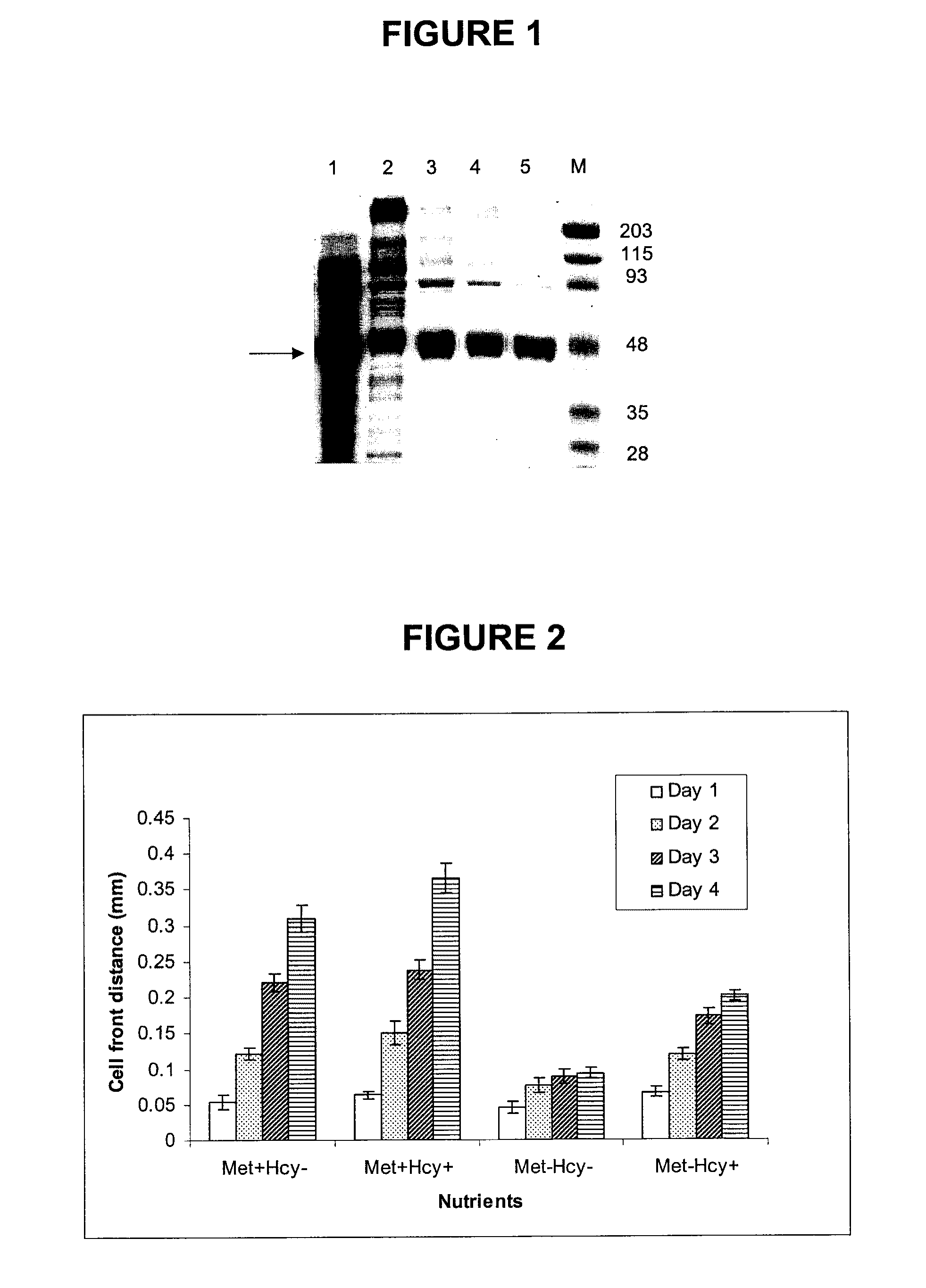
[0104]Expression and Purification of ATF-methioninase. A pKK223-3 plasmid containing the gene for L-methioninase (containing 398 amino acids and with a calculated molecular weight of 42.7 kDa) from Pseudomonas putida was kindly provided by Dr. Dennis Carson of the University of California, San Diego (Hori et al., 1996). Plasmid pULB1221 containing the gene for human urokinase was kindly provided by Dr. Paul Jacobs of the Free University of Brussels, Belgium (Jacobs et al., 1985). Plasmid pKK223-3, with the tac promoter and an ampicillin resistance gene, was obtained from Amersham Biosciences (Piscataway, N.J.). E. coli JM105 was used as the host for both vector construction and protein expression.
[0105]The following fusion protein gene was constructed:
N-(amino acids 1-49 of urokinase A chain)-Gly-Ser-Gly-Ser-Gly-Ser-(L-methioninase)-C
The amino acid sequence of the fusion protein was assigned SEQ ID NO:1, while the nucleic acid sequence of the fusion protein was assigned SEQ ID NO:2....
example 2
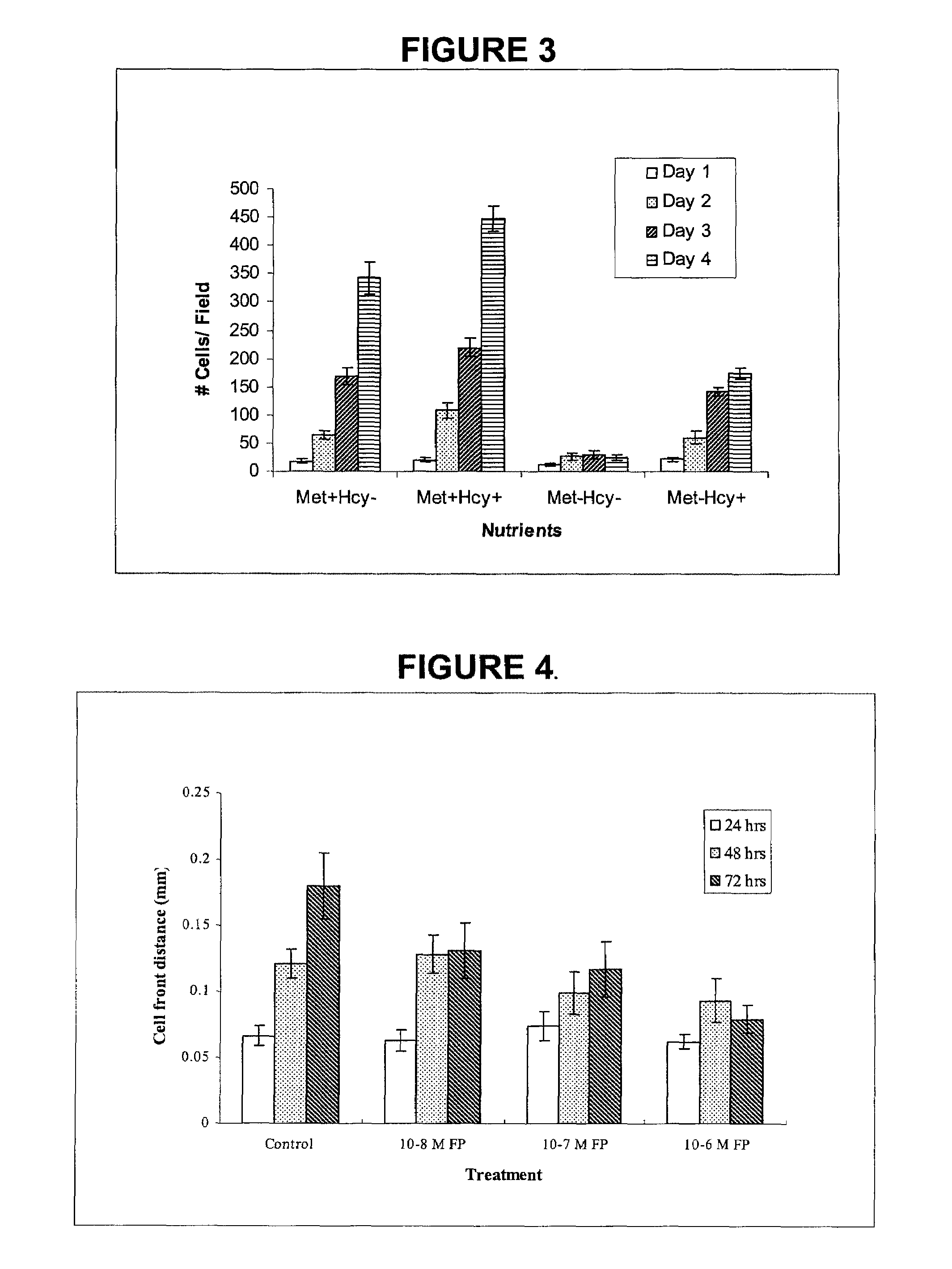
[0130]The work described in Example 1 demonstrates that ATF-methioninase fusion protein specifically binds to the urokinase receptor on MCF-7 breast cancer cells in vitro, based on the measurement of ATF-methioninase displaced by urokinase at various concentrations from the surface of the MCF-7 cells. ATF-methioninase produced a dose-dependent inhibition of both the proliferation and migration of MCF-7 cells in vitro over a period of 1 to 3 days.
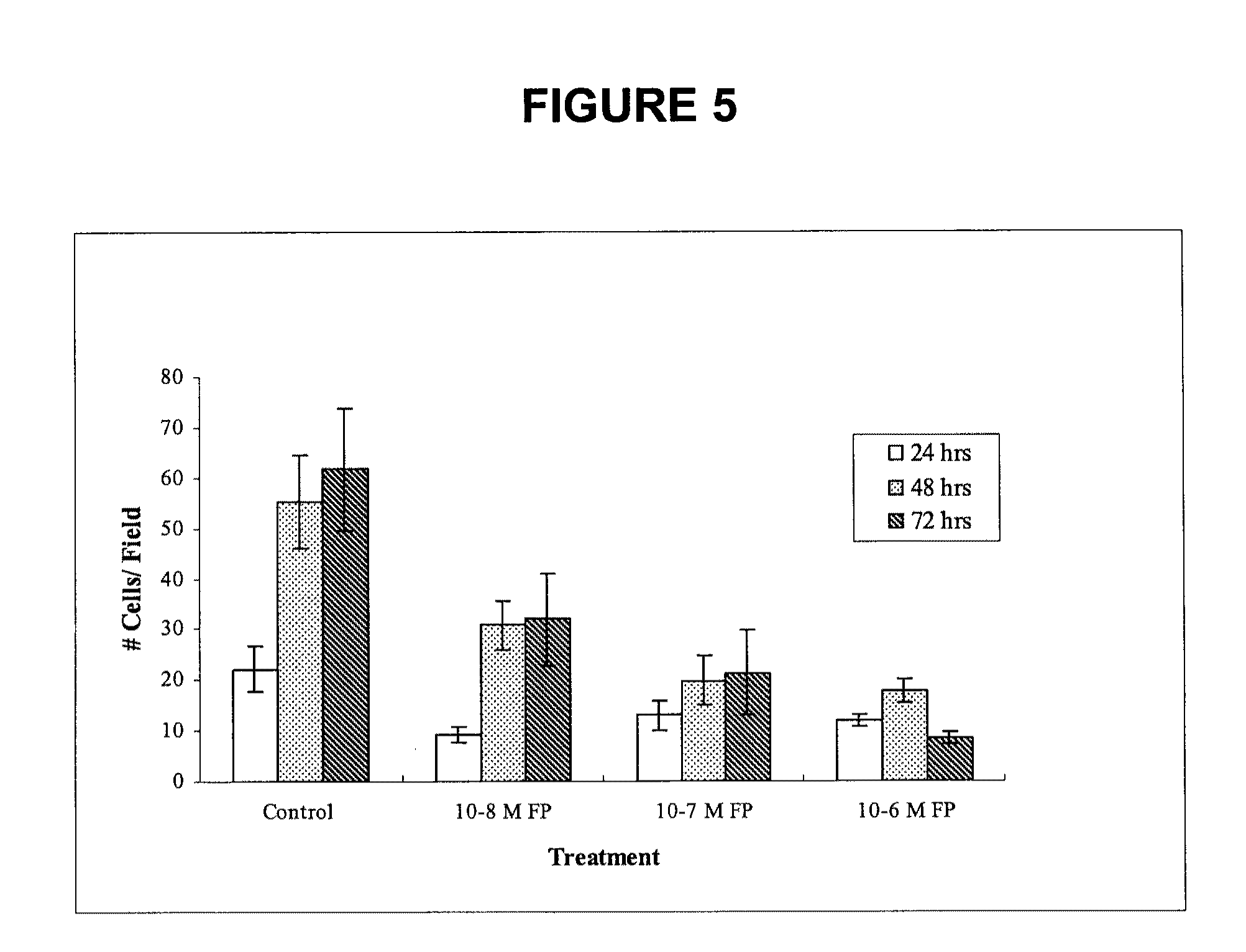
[0131]Because of these results with ATF-methioninase, an expanded study was performed that compares the effect of ATF-methioninase on MCF-7 in vitro cell proliferation and migration to L-methioninase without the receptor targeting peptide added, and to ATF-methioninase with the methioninase mutated so that there is no enzymatic activity. The effect of ATF-methioninase on the cell proliferation and migration of PC-3 prostate cancer and SK-LU-1 lung cancer cells was also studied. To compare the effect of ATF-methioninase with a fusion protein ...
example 3
Binding of Recombinant L-Methioninase-Annexin V to Phosphatidyl Serine Immobilized on Plastic or on the Surface of Cancer Cells
[0166]In this example, the binding of annexin V, part of the fusion protein L-methioninase-annexin V, to phosphatidylserine (PS) by two different methods was demonstrated. In the first method, PS was immobilized on plastic microtiter plates. In the second method, PS was exposed on the surface of human breast cancer cells by the addition of hydrogen peroxide. Binding was measured using an antibody to L-methioninase. The proteins used in this study were produced by recombinant DNA technology in Escherichia Coli bacteria and were purified to homogeneity.
Procedures for Preparation of Proteins
[0167]The protein genes were cloned into E. coli on the vector pET-30 Ek / LIC, which incorporates a His6 tag at the N-terminus and an HRV 3C protease site just before the start of the desired protein. L-methioninase and annexin V are connected by the flexible linker Gly-Ser-G...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com