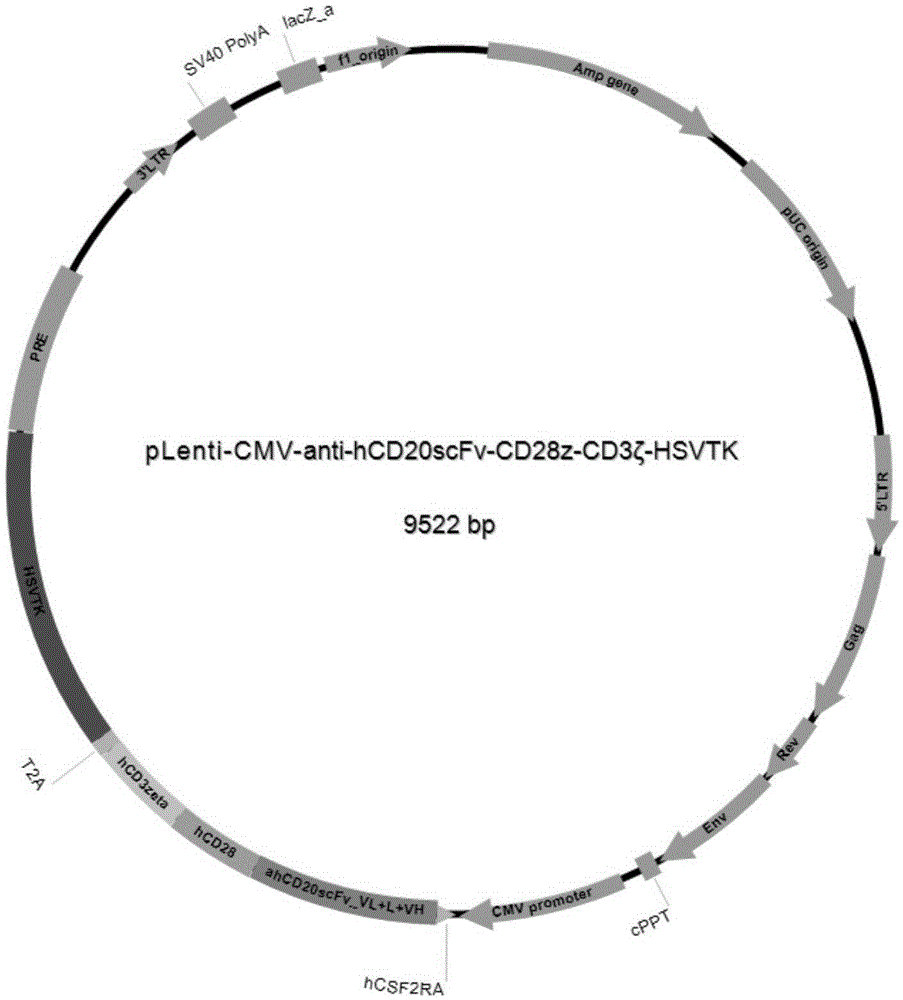
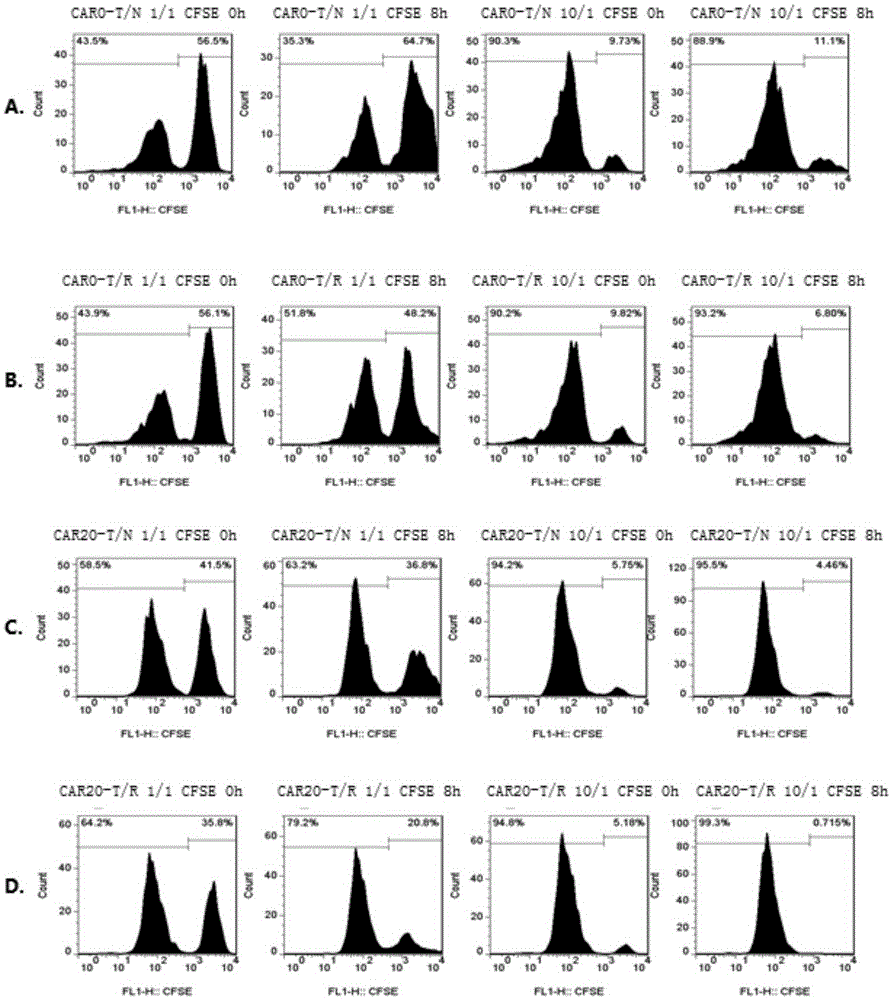
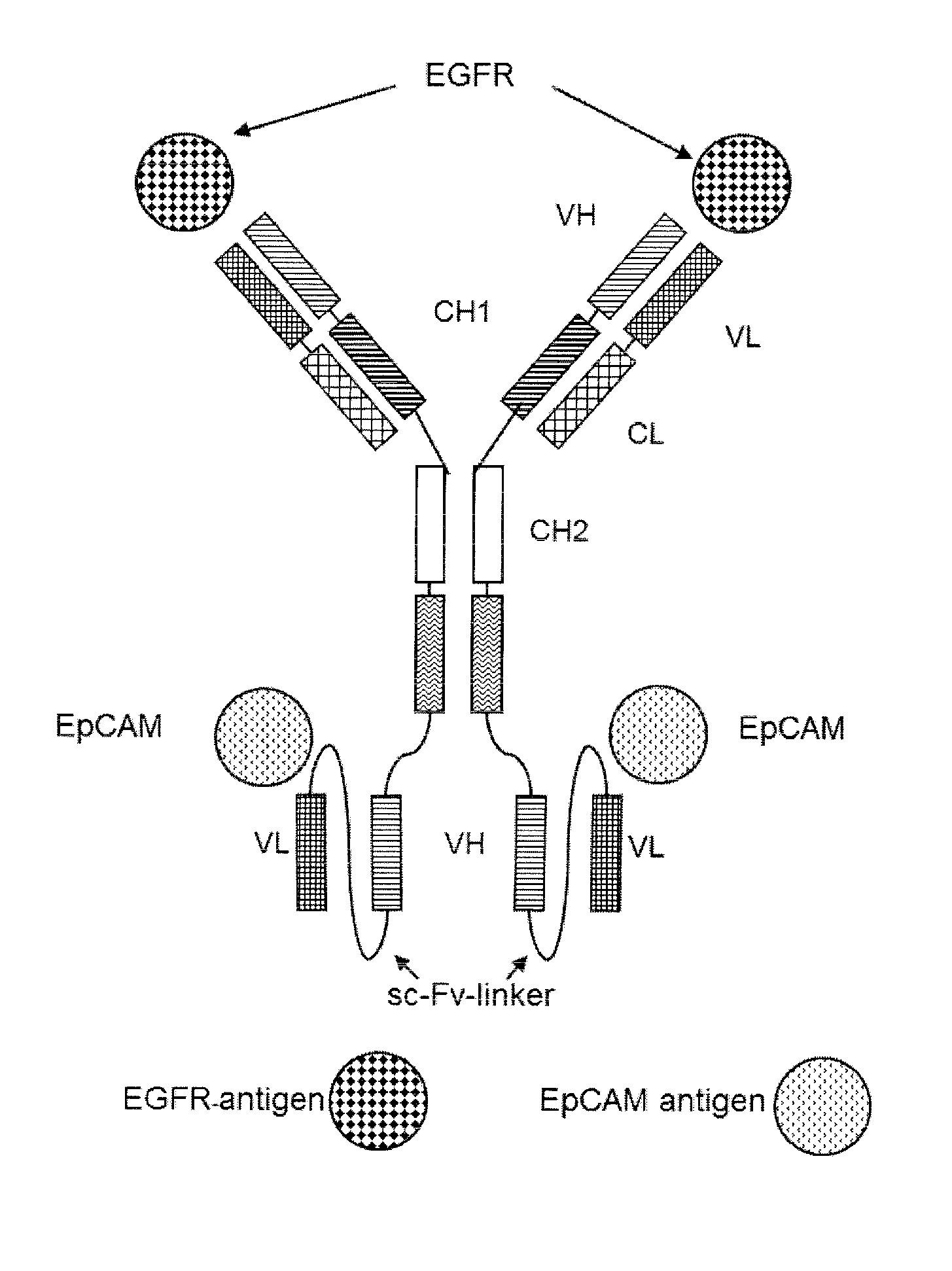
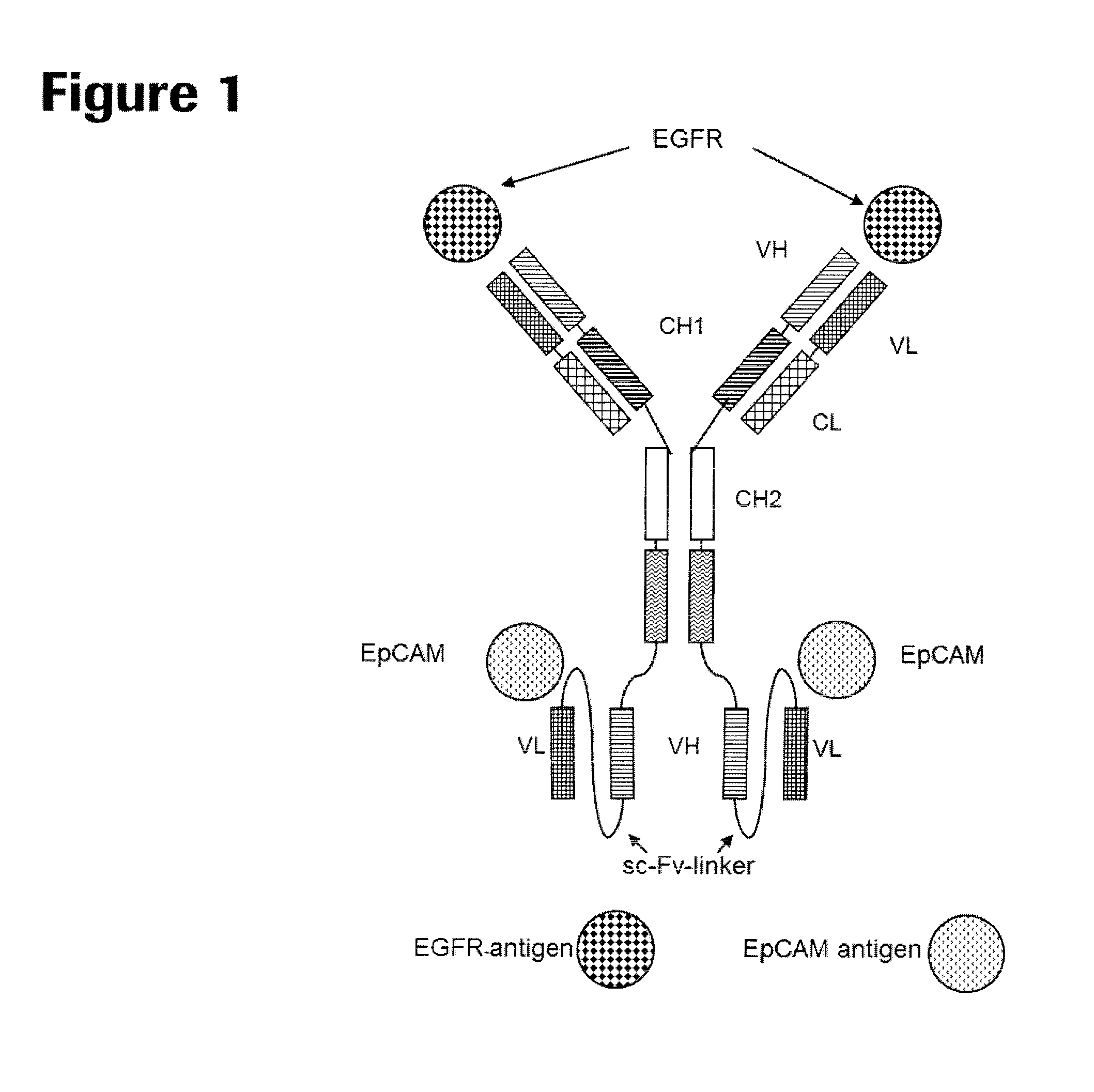
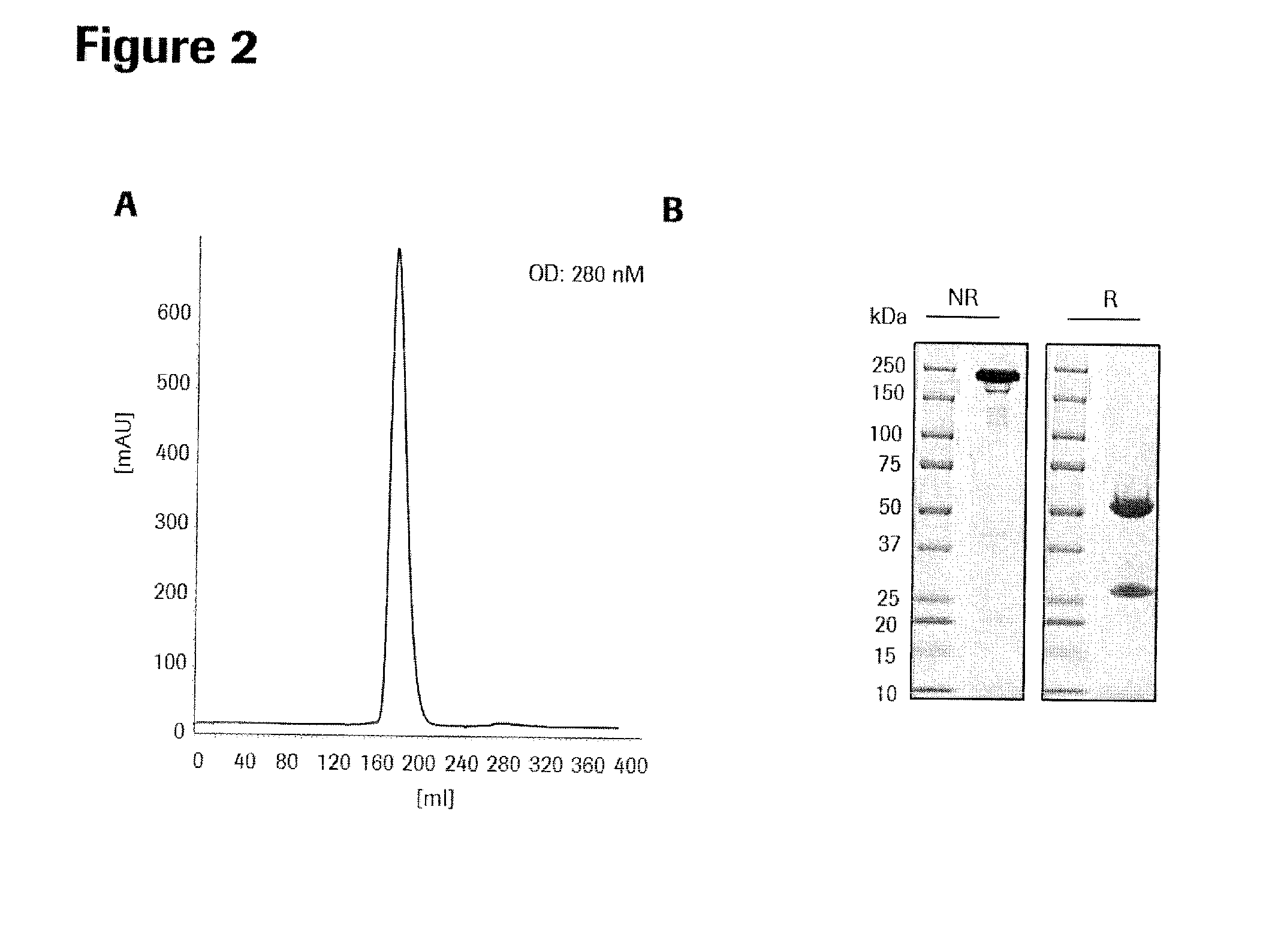
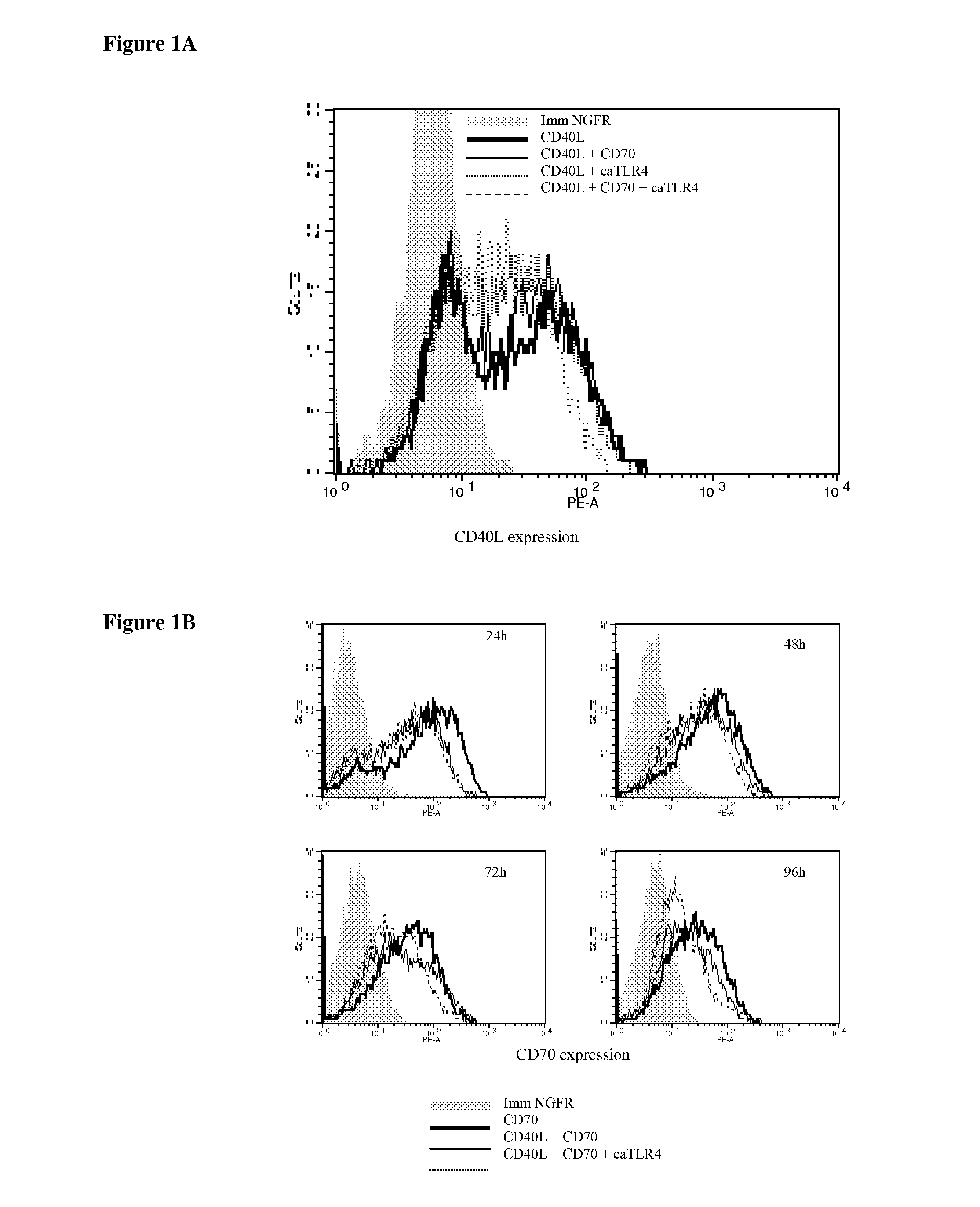
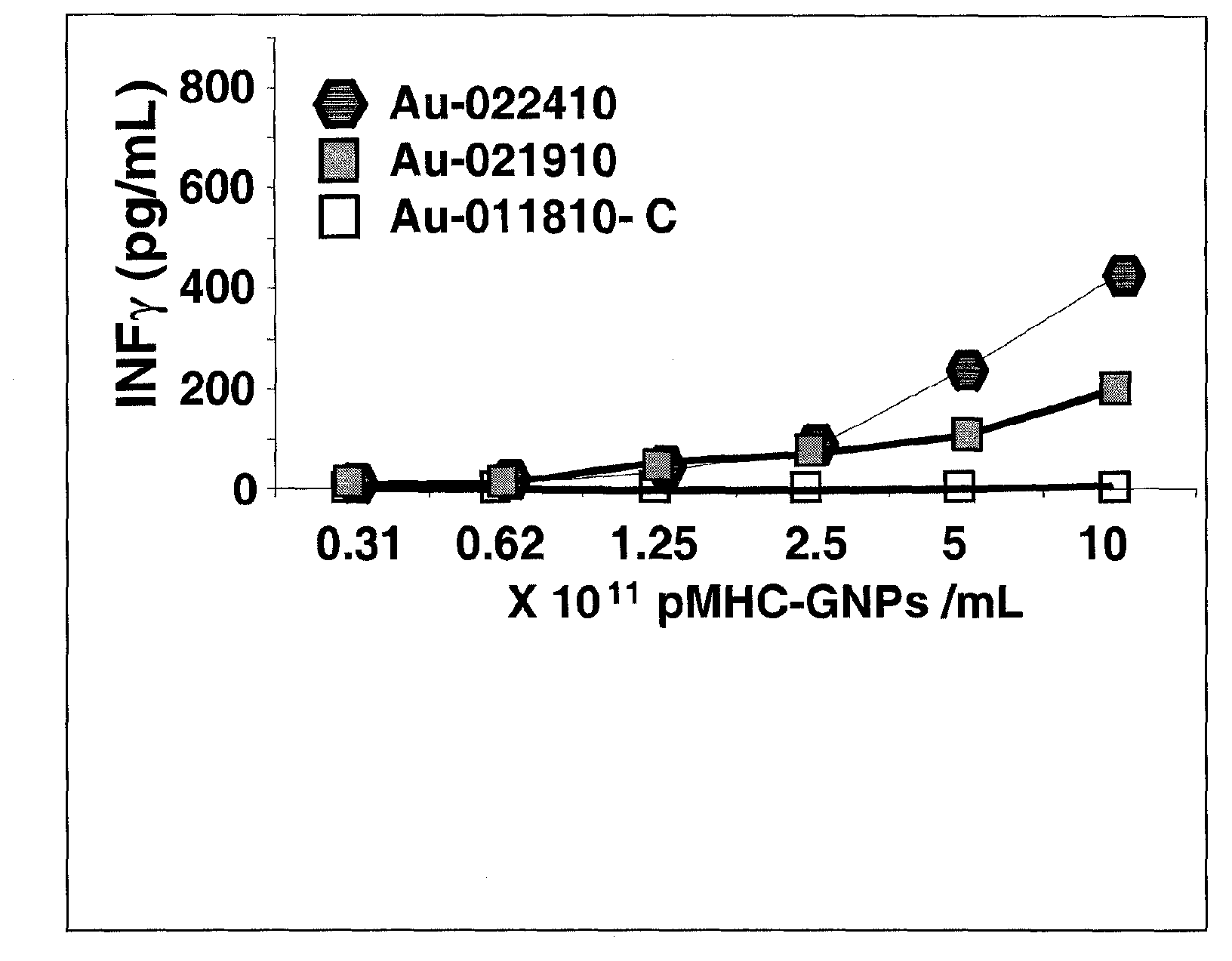
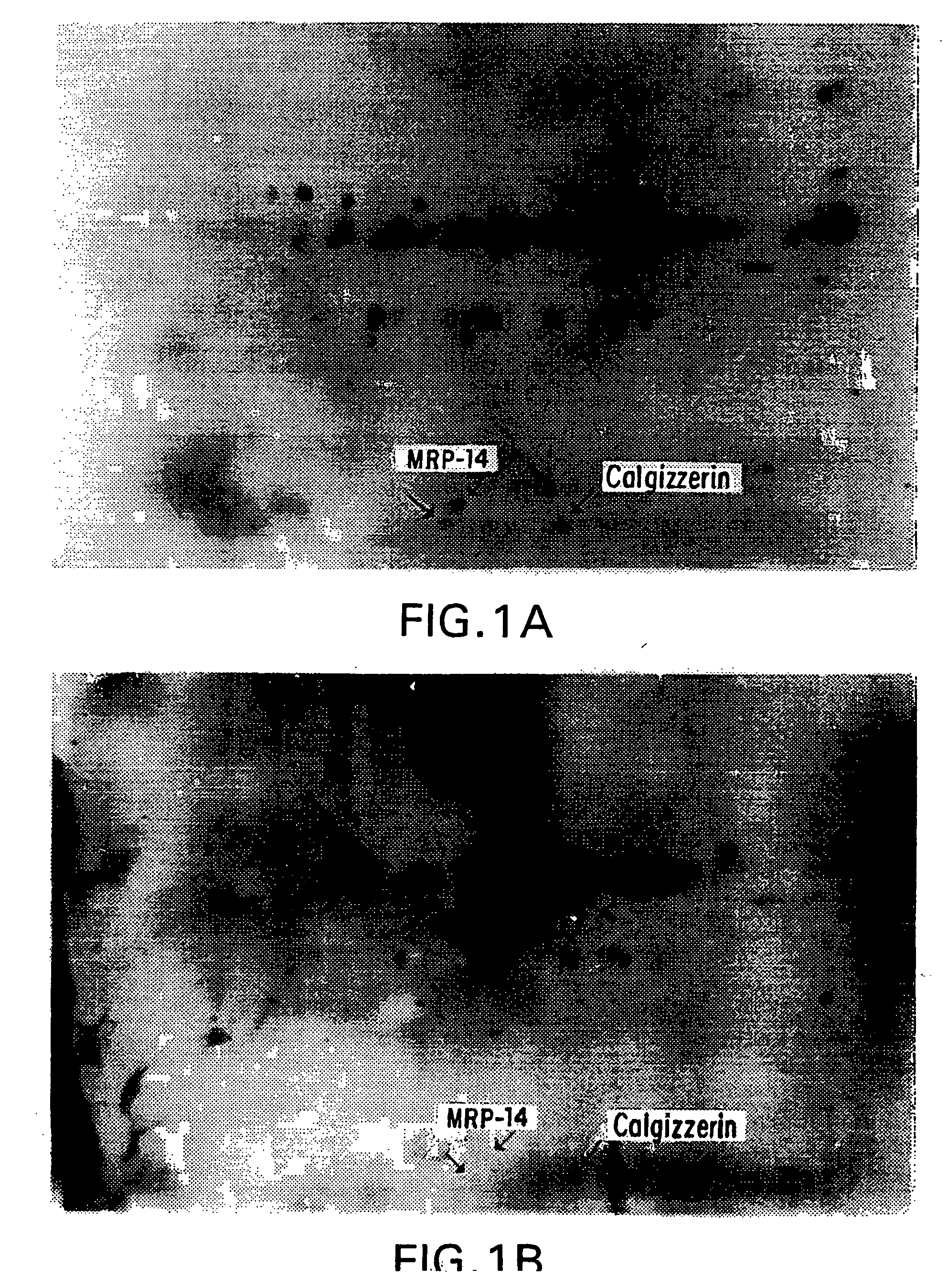
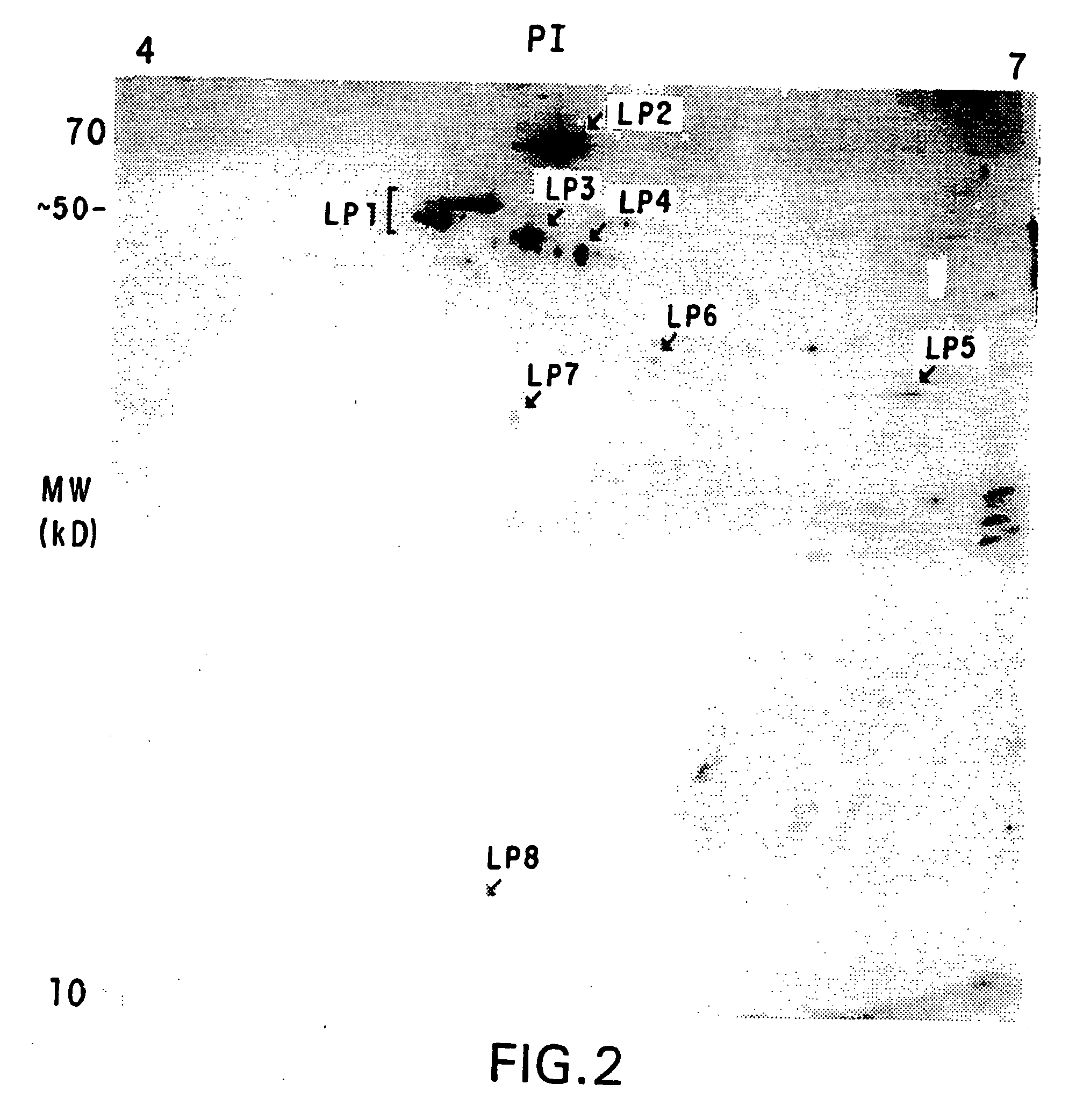
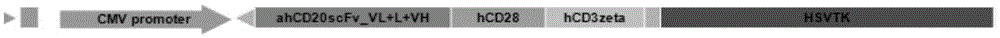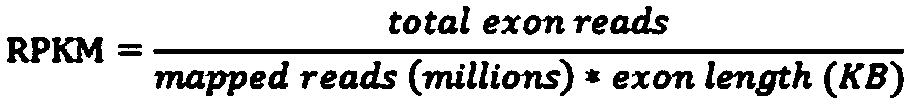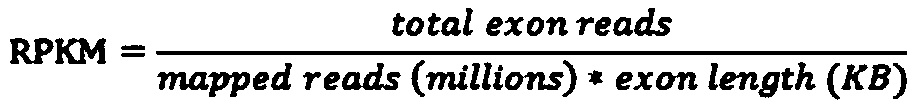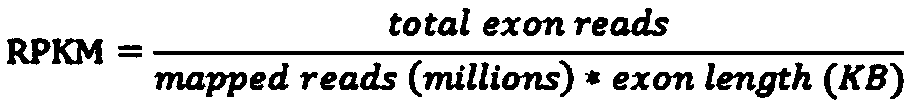Patents
Literature
132 results about "Tumor-specific antigen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
NCI Dictionary of Cancer Terms. ... tumor-specific antigen listen (TOO-mer-speh-SIH-fik AN-tih-jen) A protein or other molecule that is found only on cancer cells and not on normal cells. Tumor-specific antigens can help the body make an immune response against cancer cells.
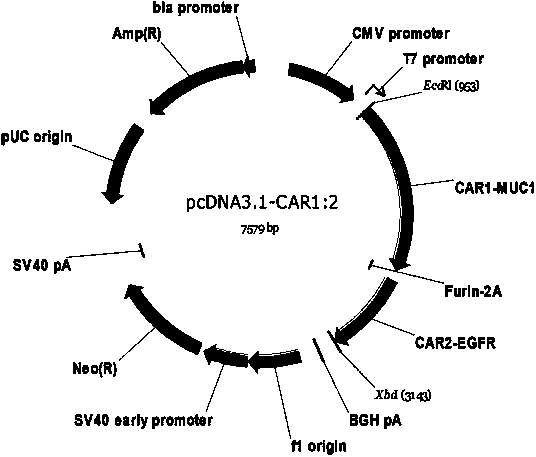
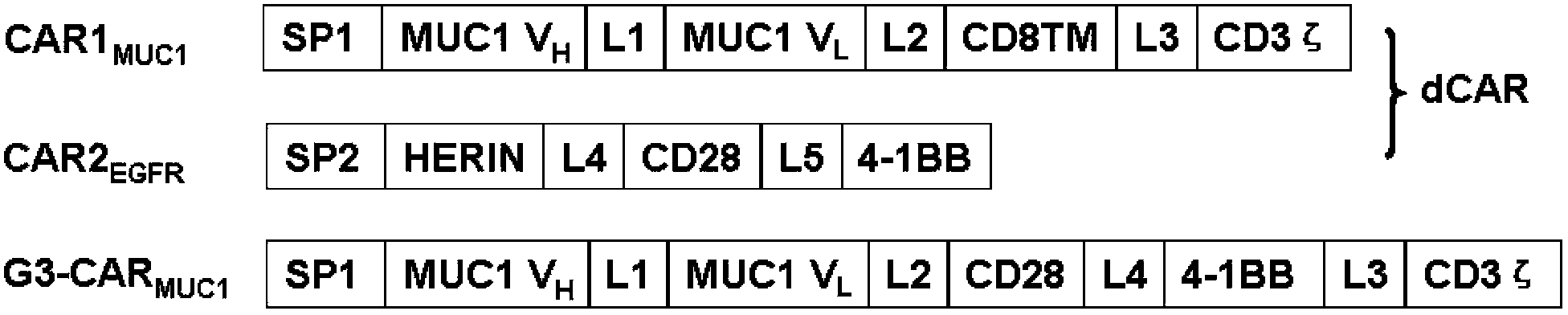
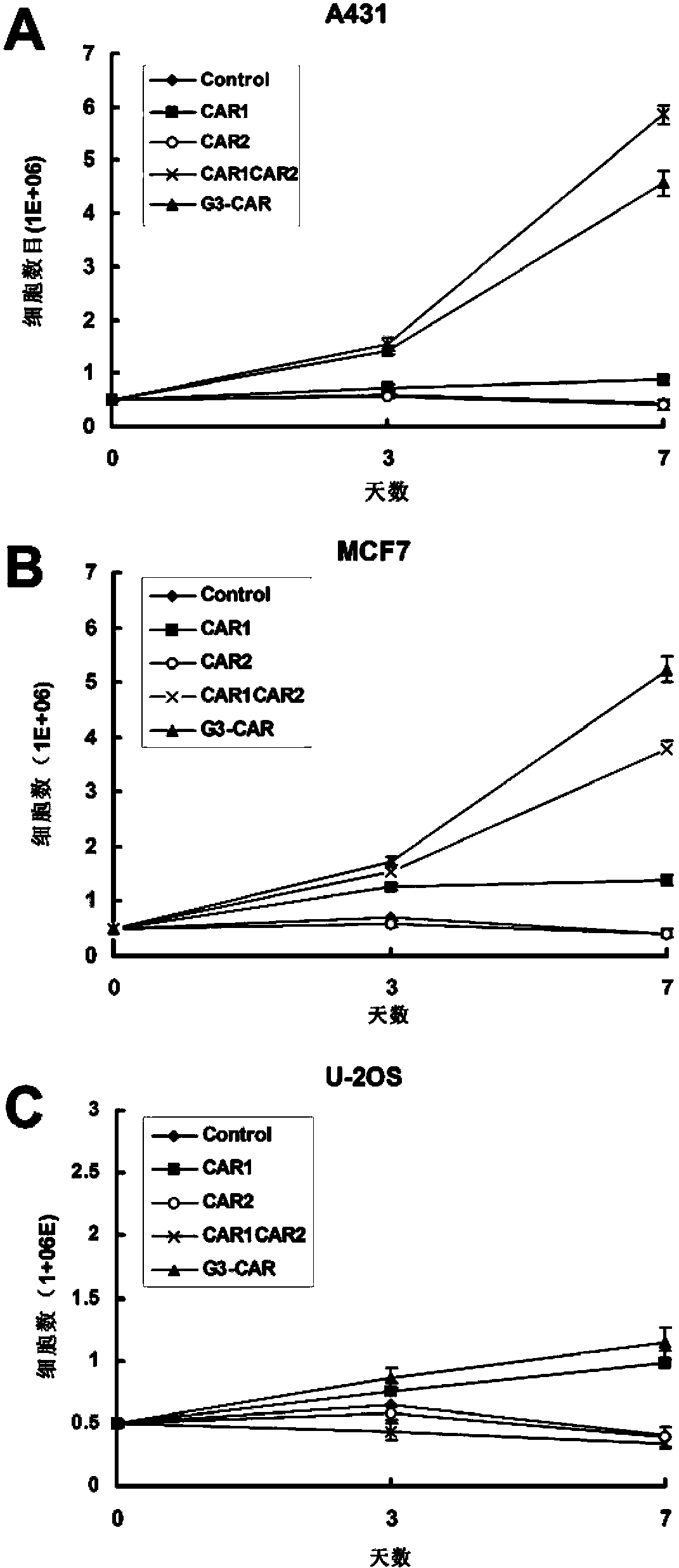
Dual-signal independent chimeric antigen receptors (dsCAR) and uses thereof
ActiveCN103483452APromote proliferationHigh activityPeptide/protein ingredientsAntibody mimetics/scaffoldsAntigen receptorsViral infectious disease
The invention relates to chimeric antigen receptors (CAR), particularly relates to dual-signal independent chimeric antigen receptors (dsCAR), and also relates to immune response cells of the dual-signal independent chimeric antigen receptors (dsCAR) and uses of the immune response cells in preparation of drugs for treatment of malignant tumor and virus infected diseases. In detail, the dual-signal independent chimeric antigen receptors (dsCAR) can respectively identify two different family antigens of tumor cells and can respectively transmit two T-cell-activation related signals. One of the CAR can transmit a first T-cell-activation related signal by combing a ligand of a tumor specific antigen or a tumor-associated antigen to decide T-cell killing specificity, and the other CAR can transmit a second T-cell-activation related signal by combing a ligand of a membrane receptor (such as EGFR (epidermal growth factor receptor) family protein) widely expressed by the tumor cells to promote T cell activation, proliferation and survival. The dual-signal independent chimeric antigen receptors (dsCAR) can avoid the potential safety problems on the basis of maintaining curative effects of second generation and third generation CAR.
Owner:SHANGHAI CELL THERAPY GRP CO LTD
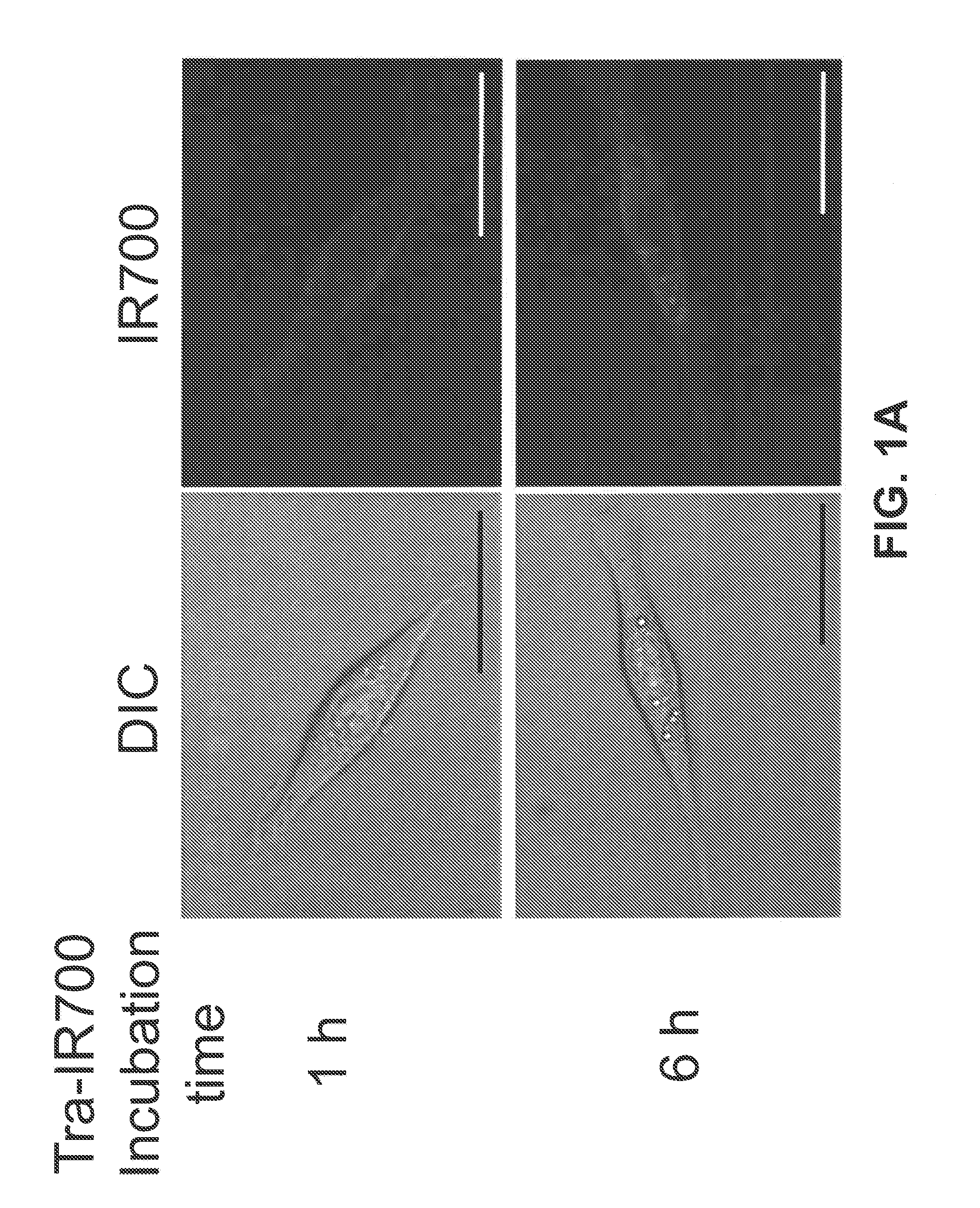
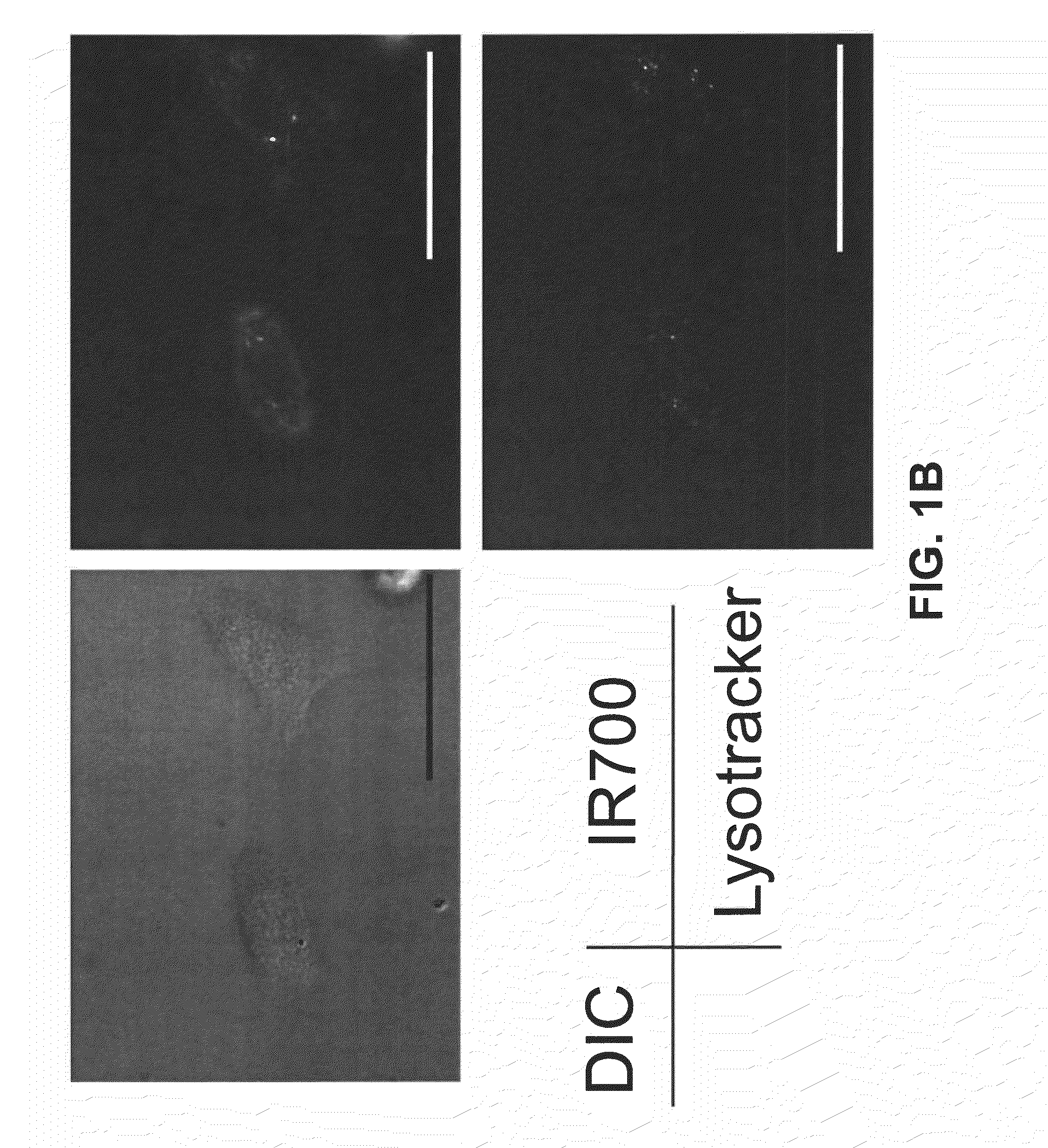
Photosensitizing antibody-fluorophore conjugates
ActiveUS8524239B2Permit treatmentUltrasonic/sonic/infrasonic diagnosticsOrganic active ingredientsCell Surface ProteinsFluorophore
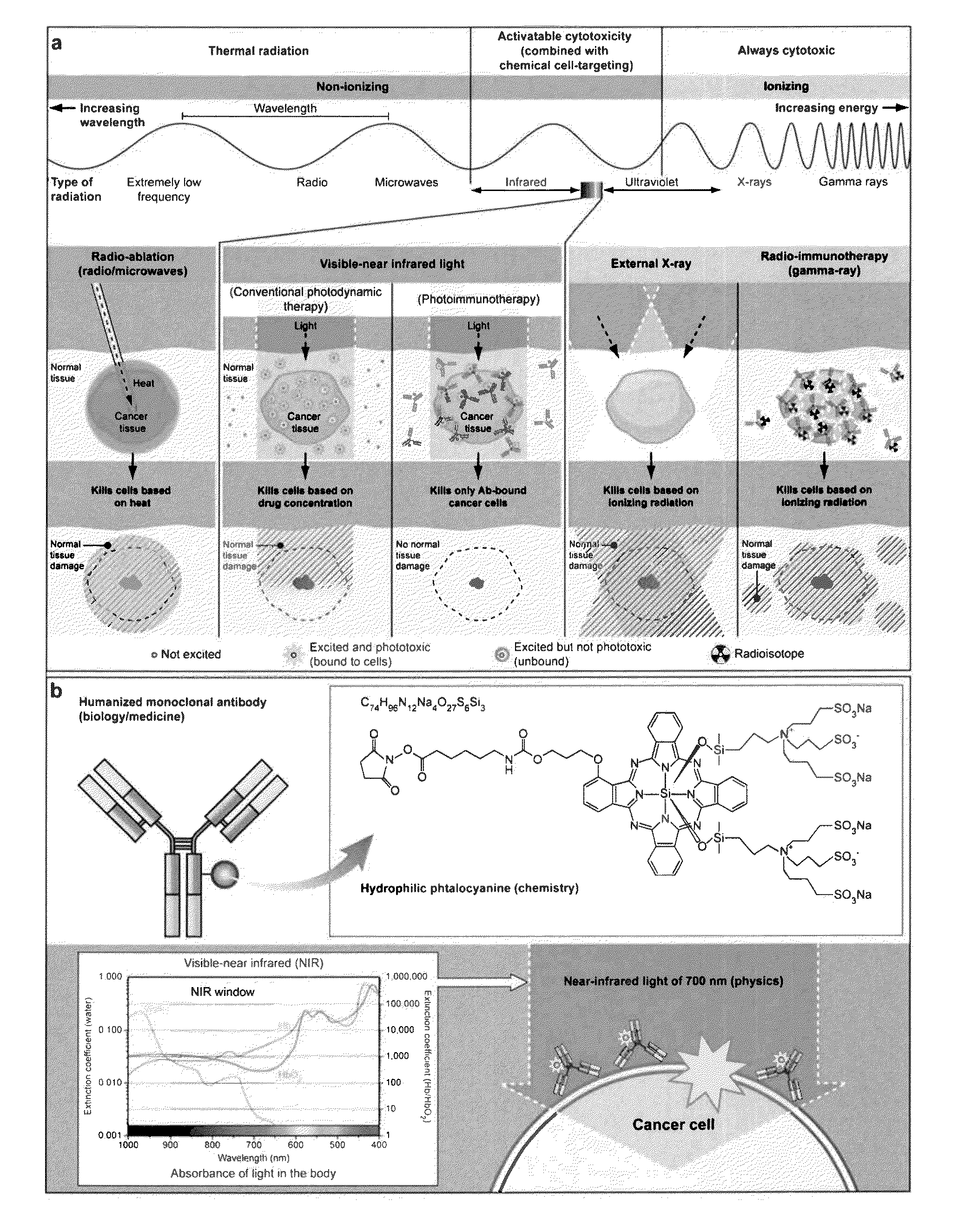
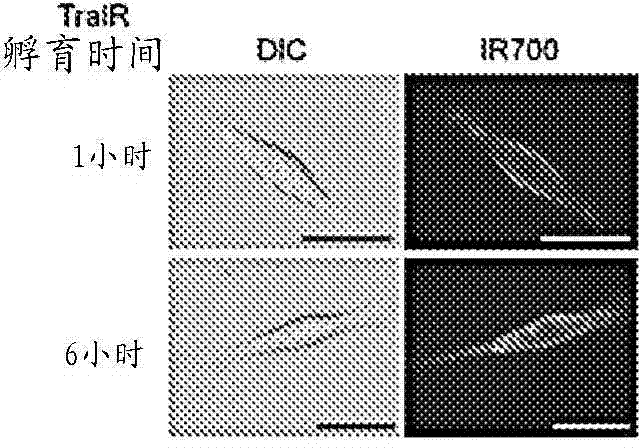
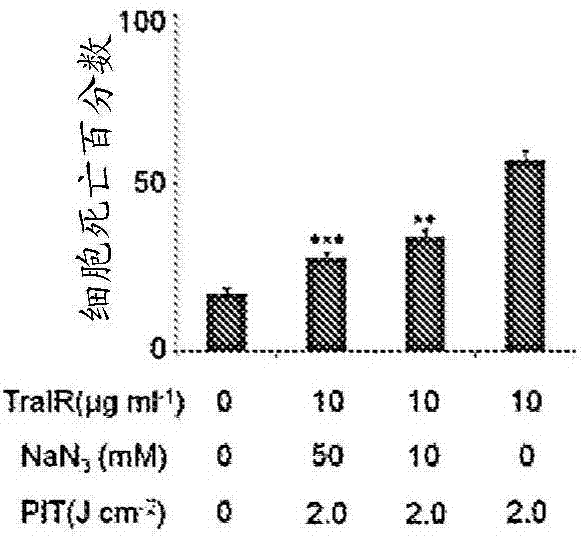
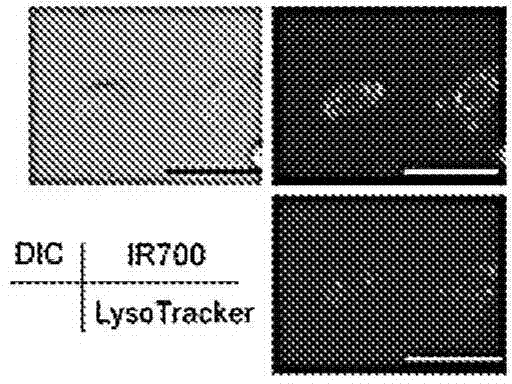
The present disclosure relates to compositions and methods of killing cells in vitro or in vivo. In particular examples, the method includes contacting a cell having a cell surface protein with a therapeutically effective amount of an antibody-IR700 molecule, wherein the antibody specifically binds to the cell surface protein. In particular examples the antibody recognizes a tumor-specific antigen on the surface of a tumor cell. The cell is subsequently irradiated, such as at a wavelength of 660 to 740 nm at a dose of at least 1 J cm−2, thereby killing the cell. Also provided are wearable devices that include an article of clothing, jewelry, or covering; and an NIR LED incorporated into the article, which can be used with the disclosed methods.
Owner:UNITED STATES OF AMERICA
Photosensitizing antibody-fluorophore conjugates
ActiveUS20120010558A1Permit treatmentOrganic active ingredientsGarment special featuresCell Surface ProteinsFluorophore
The present disclosure relates to compositions and methods of killing cells in vitro or in vivo. In particular examples, the method includes contacting a cell having a cell surface protein with a therapeutically effective amount of an antibody-IR700 molecule, wherein the antibody specifically binds to the cell surface protein. In particular examples the antibody recognizes a tumor-specific antigen on the surface of a tumor cell. The cell is subsequently irradiated, such as at a wavelength of 660 to 740 nm at a dose of at least 1 J cm−2, thereby killing the cell. Also provided are wearable devices that include an article of clothing, jewelry, or covering; and an NIR LED incorporated into the article, which can be used with the disclosed methods.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
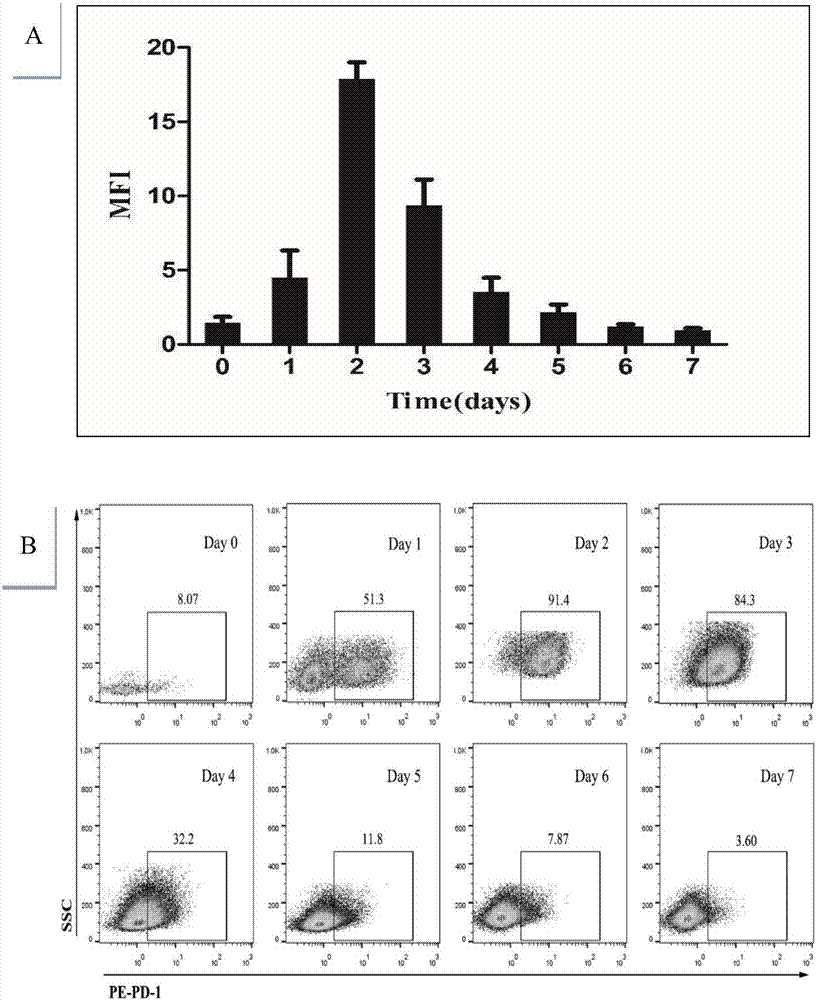
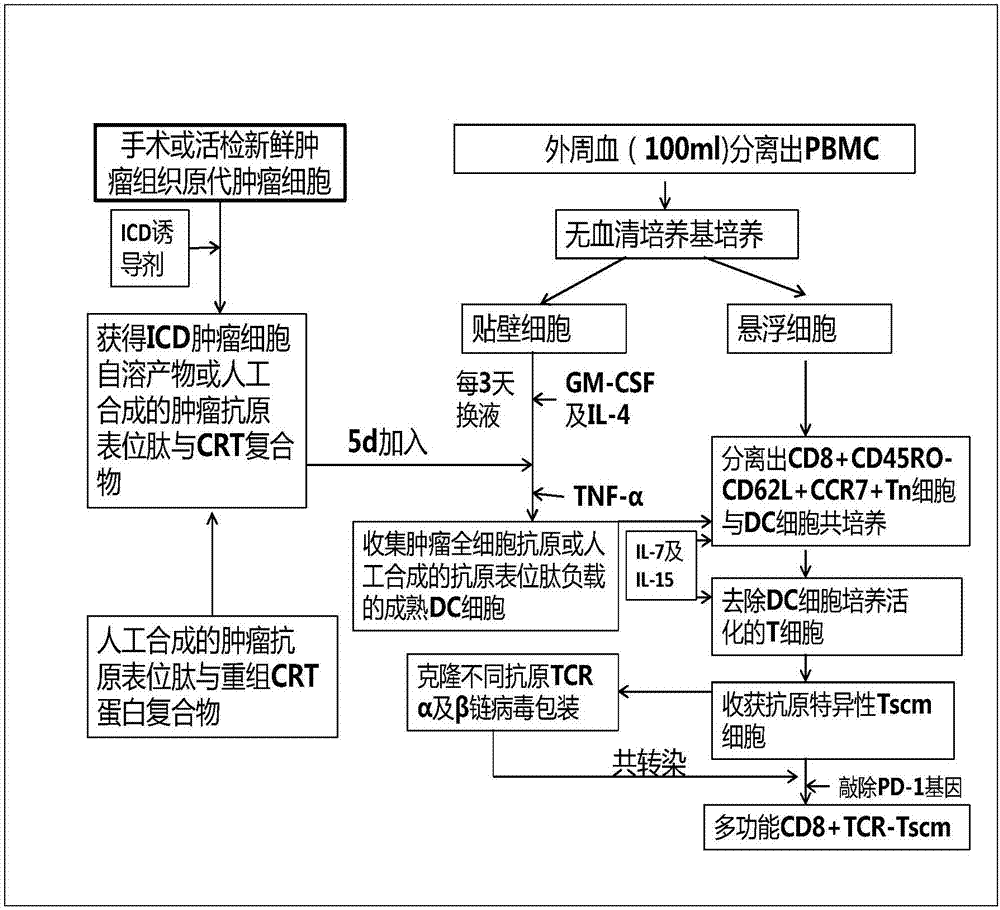
Preparation method and use of TCR gene modified CD8+T memory stem cell
InactiveCN107541498AOvercoming heterogeneityIncrease intakeMammal material medical ingredientsAntineoplastic agentsCell specificDendritic cell
The invention belongs to the technical fields of immunology and tumor therapy, and relates to a preparation method and a use of a TCR gene modified CD8+T memory stem cell. The method comprises the following steps: co-incubating a tumor antigen and an immature dendritic cell to obtain a specific tumor antigen-supported mature dendritic cell, co-culturing the specific tumor antigen-supported maturedendritic cell and a CD8+ naive T cell, and adding a stem cell differentiation inhibitor to promote the generation of a CD8+ stem cell-like memory T lymphocyte; separating the CD8+ stem cell-like memory T lymphocyte; and cloning a T cell receptor gene (TCR) for specifically identifying a specific antigenic epitope, and carrying out lentivirus packaging or retroviral vector cotransfection on an autologous CD8+ Tscm cell to in-vitro prepare a CD8+ TCR-Tscm cell specific for different tumor specific antigens. The CD8+ TCR-Tscm cell prepared by the method has the advantages of overcoming of the tumor heterogeneity, high specificity, few adverse reactions, and highly-efficient and lasting tumor preventing and treating effects.
Owner:FUDAN UNIV SHANGHAI CANCER CENT
Chimeric antigen receptor immune cell provided with safety switch as well as preparation method and application of chimeric antigen receptor immune cell
InactiveCN106755023AImprove effectivenessImprove securityGenetic material ingredientsMammal material medical ingredientsAbnormal tissue growthAntigen receptors
The invention relates to a chimeric antigen receptor (CAR) immune cell provided with a safety switch as well as a preparation method and an application of the CAR immune cell. The CAR immune cell carrying the safety mechanism (the safety switch) comprises a CAR coding nucleotide sequence, wherein the structure of the nucleotide sequence comprises a receptor structural domain for recognizing tumor-specific antigen or tumor-associated antigen, a transmembrane-stimulation structural domain, a CD3[zeta] stimulating signal transduction region and a suicide gene region. The CAR immune cell can be obtained as different immunological effect cells are amplified and a CAR sequence carrying a suicide mechanism is transduced; corresponding antigens of tumor cells are recognized by virtue of CAR; and the CAR immune cell can generate a specific killing effect on the tumor cells. The CAR immune cell, when used, is infused in a gradient mode and dynamic change in related cell factor levels is monitored; in case of need, a suicide gene can be started by virtue of drugs to scavenge the immunological effect cells, so that optimal balance between safety and a curative effect is achieved; therefore, the safety of the technology applied to the treatment of tumors in the clinical field is guaranteed to the greatest extent.
Owner:AFFILIATED HOSPITAL CHINA ACADEMY OF MILITARY MEDICAL SCI +1
Transcriptome-based tumor neoantigen identification method
ActiveCN108491689AImprove accuracyComprehensive identificationSequence analysisSpecial data processing applicationsSample sequenceTumor-specific antigen
The invention discloses a transcriptome-based tumor antigen identification method. The method comprises four steps of: obtaining an RNA sample of a patient tumor tissue, and carrying out library construction and amplification on the RNA sample to obtain an RNA sample sequencing result of the tumor tissue; aligning short read segments of the RNA sample sequencing result to a human reference genometo obtain an RNA alignment result; calculating gene expression quantity according to the RNA alignment result, and carrying out mutation detection and prediction of fusion gene events according to theRNA alignment result; and predicting transcriptome HLA typing according to the alignment result, wherein calculation of the gene expression quantity, mutation detection and prediction of the fusion gene events are carried out according to a specified order or simultaneously carried out; and using the gene expression quantity of a transcriptome sample, depth of transcriptome mutation sites in a whole-exon sequencing sample and binding force of neonatal short peptides and the patient HLA typing as an analysis result to submit the same to a downstream analyst. The invention provides the method capable of identifying a tumor-specific antigen of an individual sample from tumor patient transcriptome NGS data.
Owner:HANGZHOU NEOANTIGEN THERAPEUTICS CO LTD
Photosensitive antibody-phuorophore conjugates
InactiveCN103781495AAllow treatmentGarment special featuresEnergy modified materialsFluorescenceCell Surface Proteins
The present disclosure relates to compositions and methods of killing cells. In particular examples, the method includes contacting a cell having a cell surface protein with a therapeutically effective amount of an antibody-IR700 molecule, wherein the antibody specifically binds to the cell surface protein, such as a tumor-specific antigen on the surface of a tumor cell. The cell is subsequently irradiated, such as at a wavelength of 660 to 740 nm at a dose of at least 1 J cm<-2>. The cell is also contacted with one or more therapeutic agents (such as an anti-cancer agent), for example about 0 to 8 hours after irradiating the cell, thereby killing the cell. Also provided are methods of imaging cell killing in real time, using fluorescence lifetime imaging. Also provided are wearable devices that include an article of clothing, jewelry, or covering; and an NIR LED incorporated into the article, which can be used with the disclosed methods.
Owner:UNITED STATES OF AMERICA
Bispecific antibody molecules with antigen-transfected t-cells and their use in medicine
ActiveUS20150010567A1Simple and rapid screeningAnimal cellsImmunoglobulins against cell receptors/antigens/surface-determinantsDiseaseCD8
The present invention relates to a bispecific (monoclonal) antibody molecule with a first binding domain binding an antigen on CD8+ T-cells that does not naturally occur in and / or on CD8+ T-cells and a second binding domain binding to a tumor specific antigen naturally occurring on the surface of a tumor cell. The bispecific (monoclonal) antibody molecules are particularly useful in combination with transduced CD8+ T-cells comprising an antigen which does not naturally occur in and / or on CD8+ T-cells and / or a T-cell receptor. The invention provides the use of said (bispecific) antibody molecules as a medicament, the (bispecific) antibody molecules for use in a method for the treatment of particular diseases as well as a pharmaceutical composition / medicament comprising said (bispecific) antibody molecules, wherein said (bispecific) antibody molecules are to be administered in combination with transduced CD8+ T-cells comprising an antigen which does not naturally occur in and / or on CD8+ T-cells and / or a T-cell receptor in a specific treatment regimen. Further aspects of the invention are nucleic acid sequences encoding said bispecific (monoclonal) antibody molecules, vectors. host cells, methods for the production of the (bispecific) antibody molecule as well as a kit comprising the (bispecific) antibody molecule of the invention.
Owner:F HOFFMANN LA ROCHE & CO AG +1
Enhancing the t-cells stimulatory capacity of human antigen presenting cells and their use in vaccination
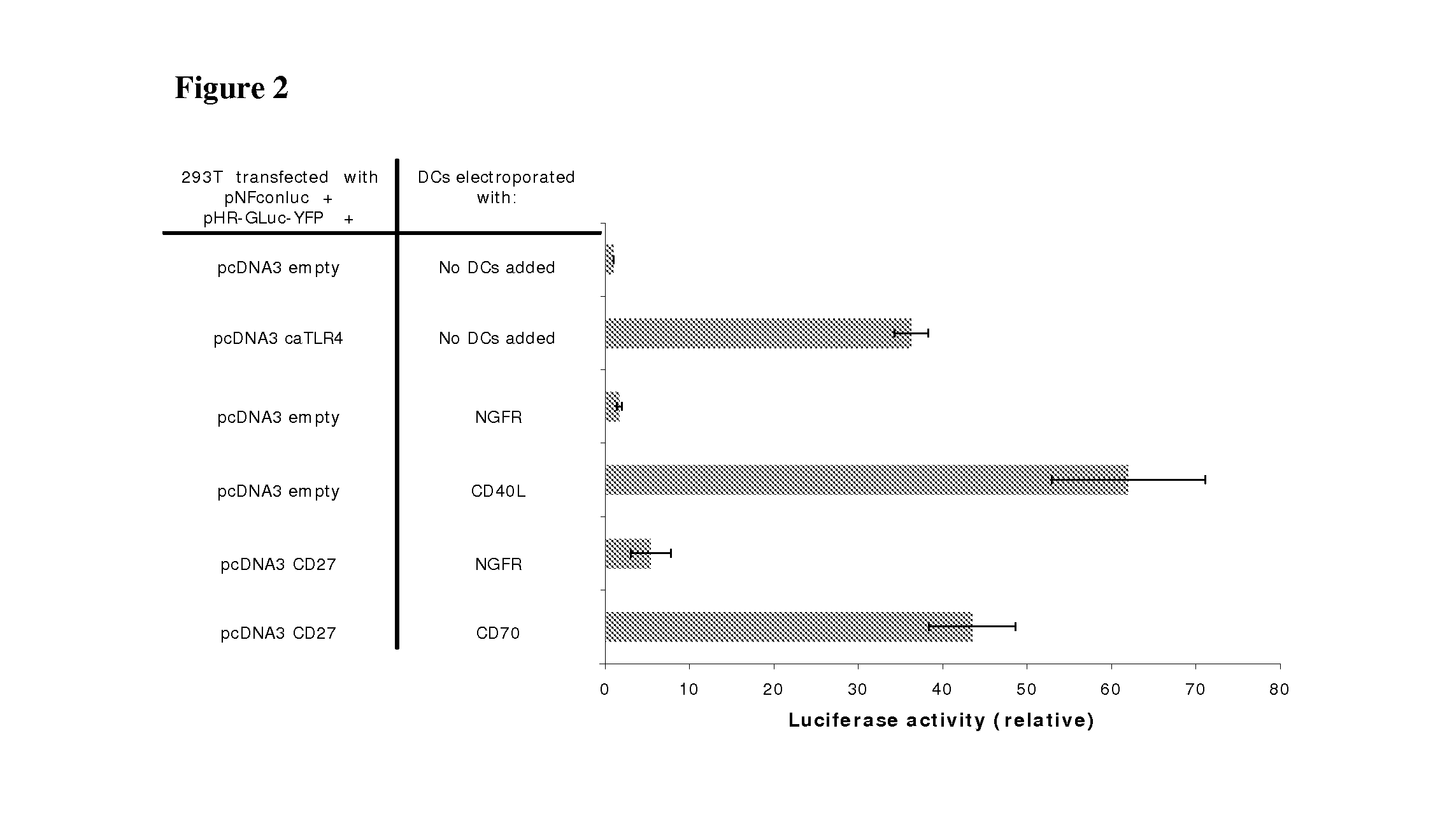
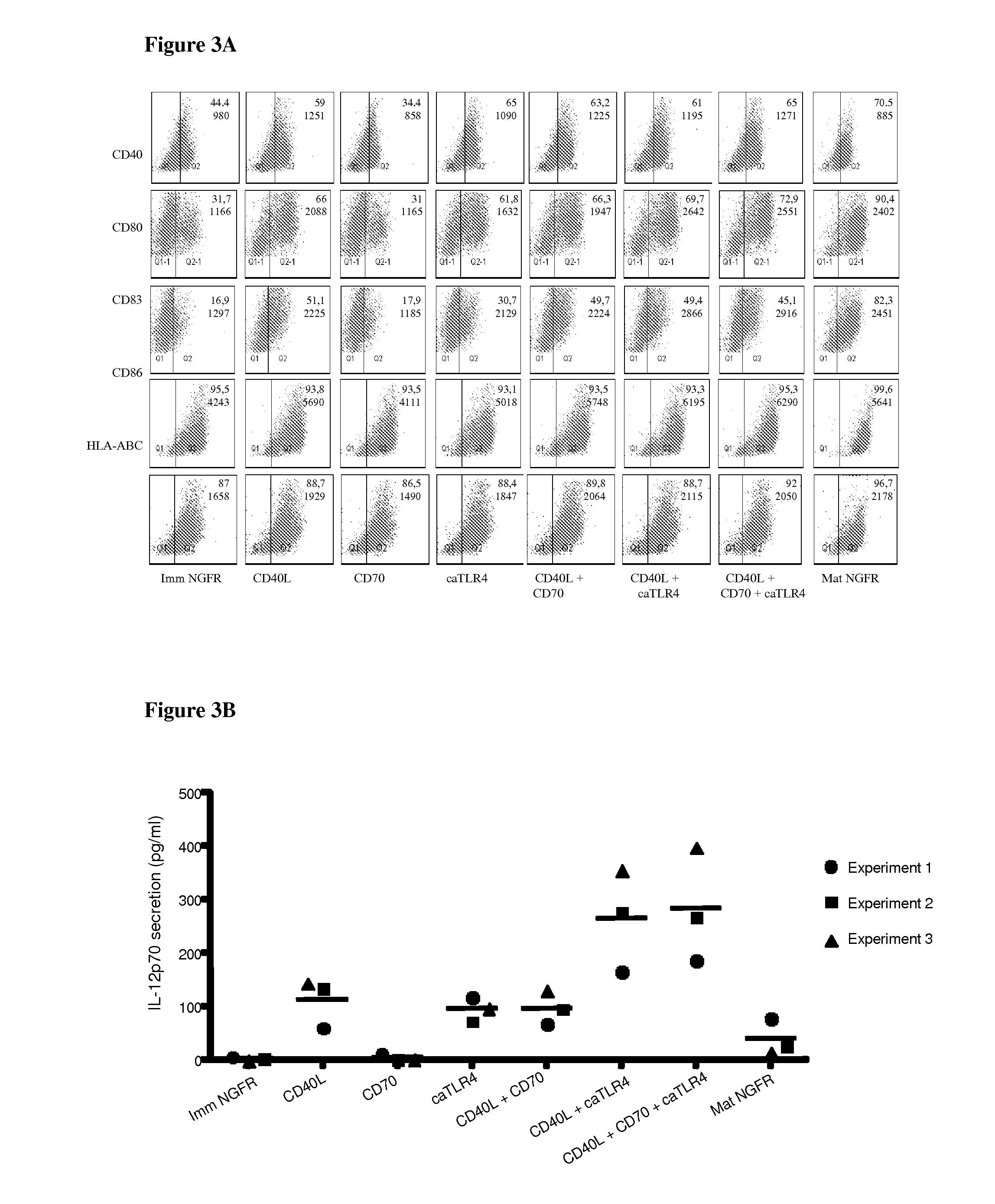
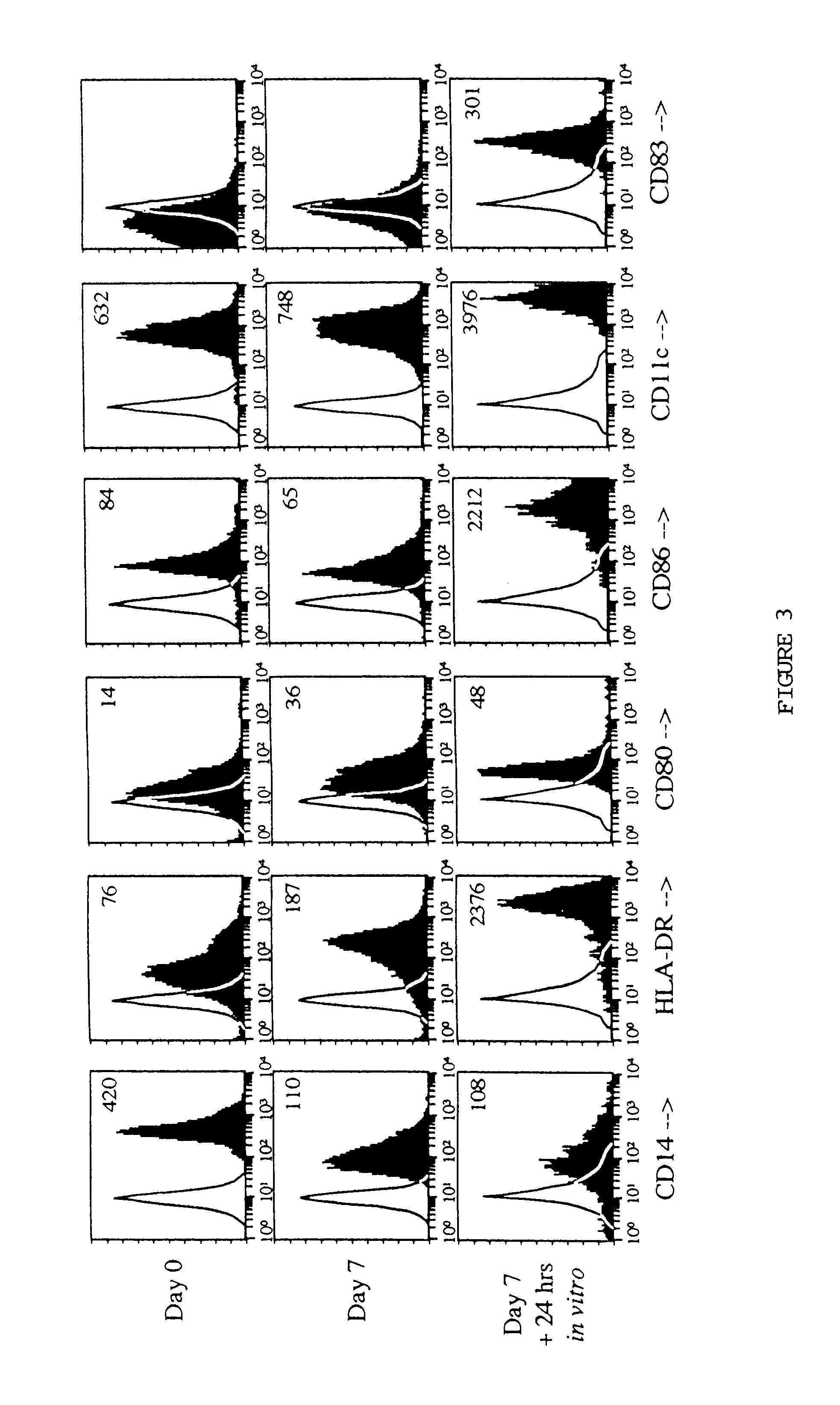
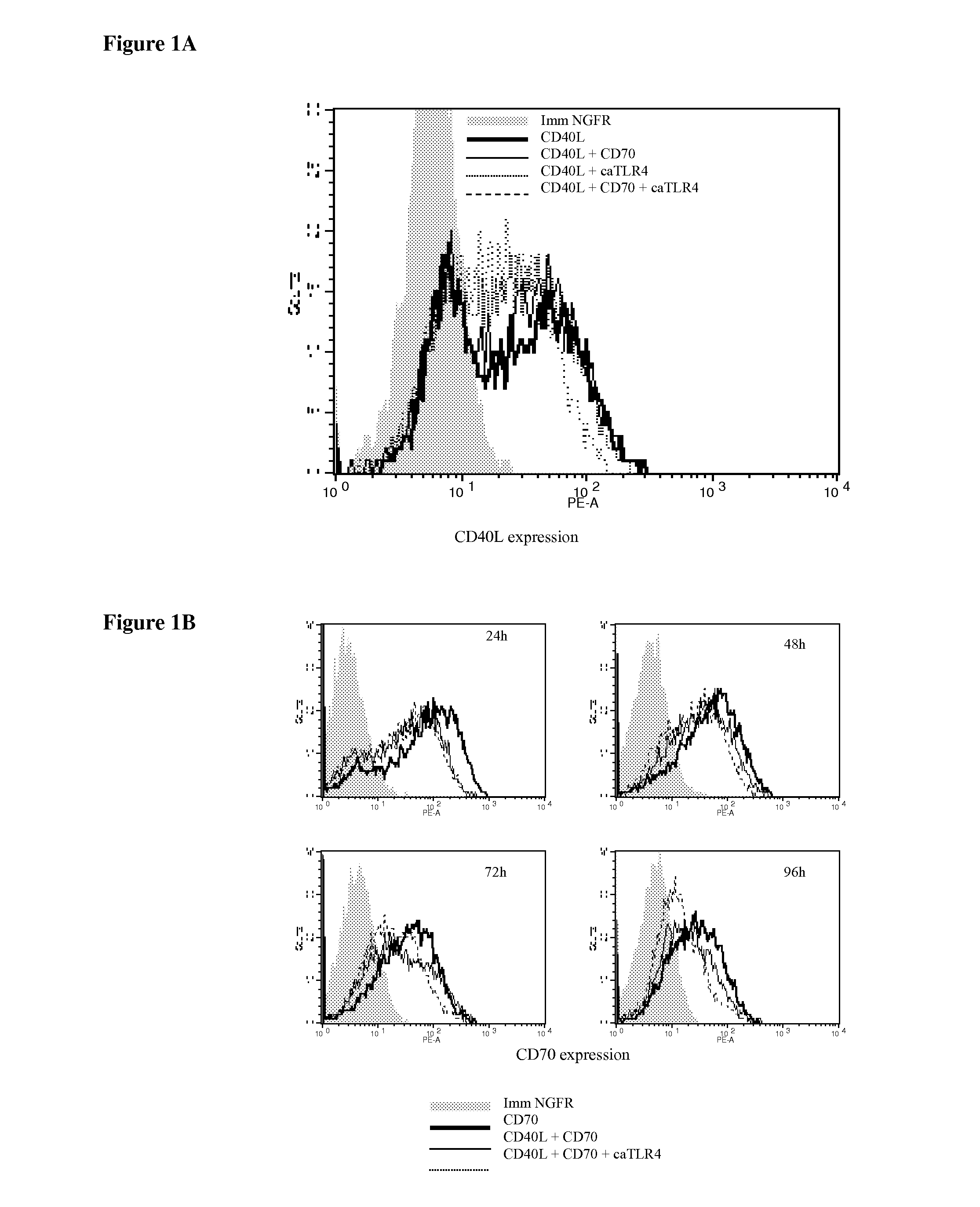
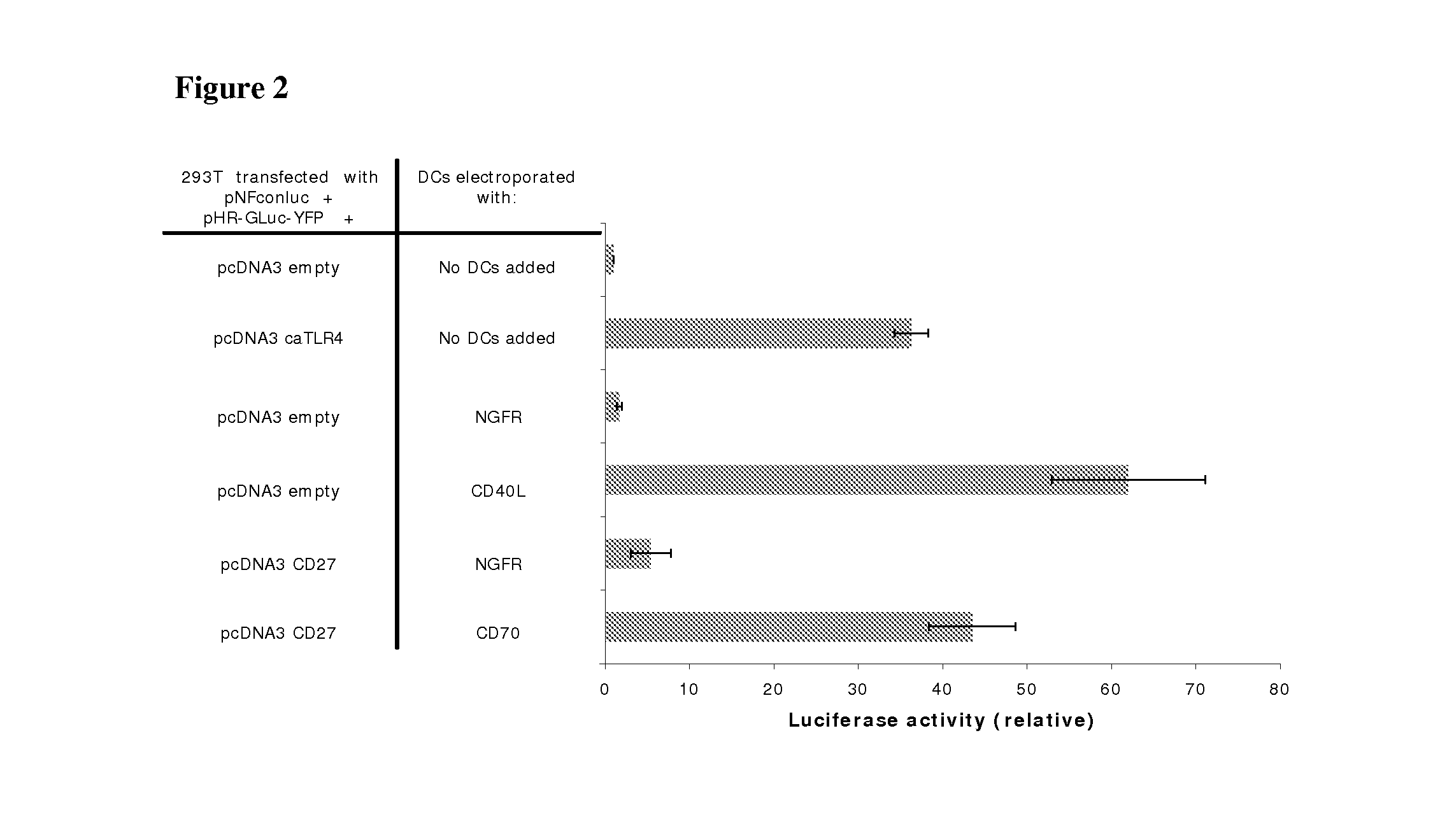
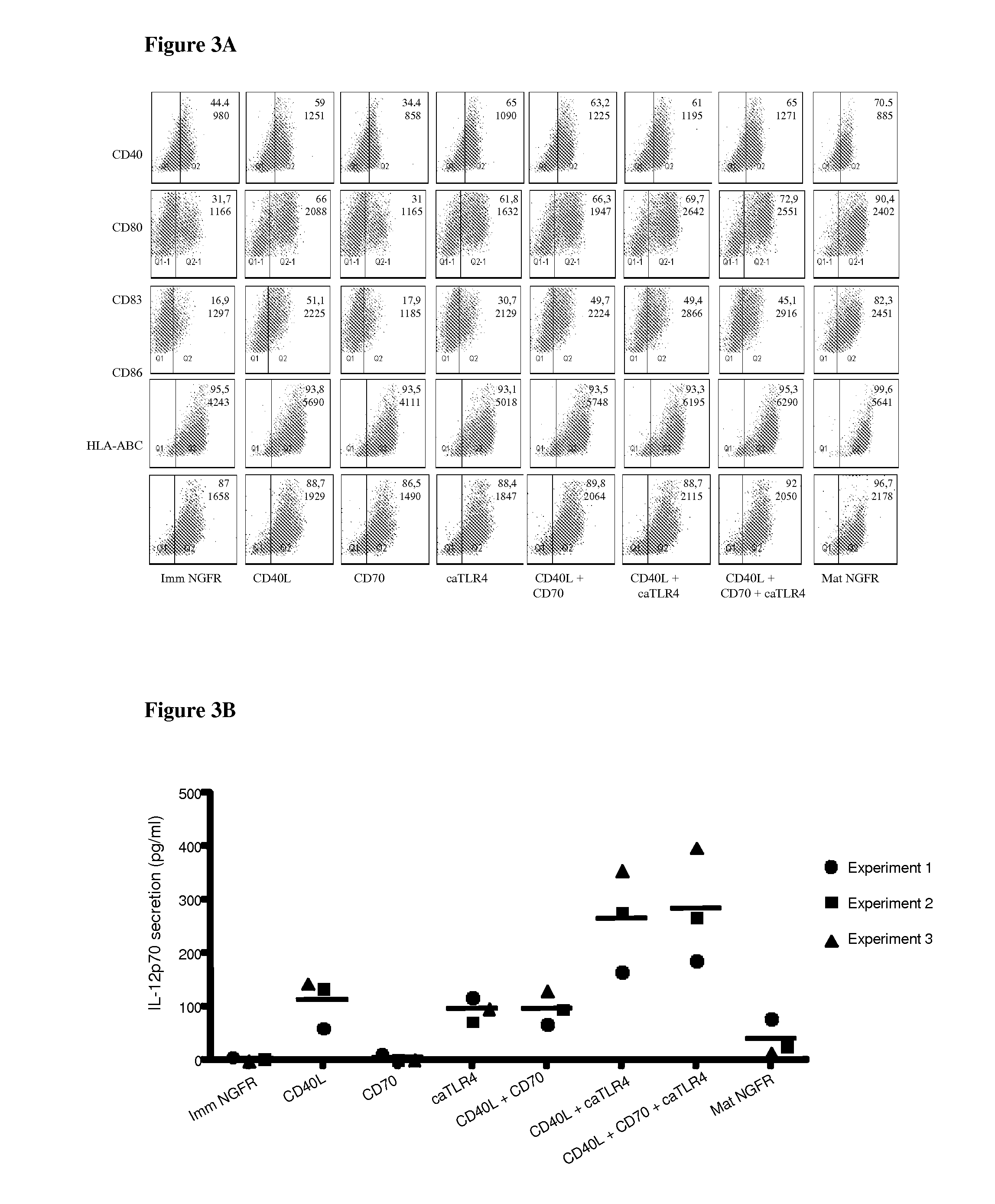
With the current invention, we provide new methods of enhancing the T-cell stimulatory capacity of human dendritic cells (DCs) and their use in cancer vaccination. The method comprises the introduction of different molecular adjuvants to human DCs through transfection with at least two mRNA or DNA molecules encoding markers selected from the group of: CD40L, CD70, constitutively active TLR4 (caTLR4), IL-12p70, EL-selectin, CCR7 and / or 4-1 BBL; or in combination with inhibition of SOCS, A20, PD-L1 and / or STAT3 expression, for example through siRNA transfection. We could show a clear increase in the immunostimulatory capacity of DCs obtained in this way, enabling them to elicit an unexpectedly high T-cell immune response in vitro. Introduction of at least two of the above molecules, in combination with a tumor-specific antigen enables the DCs to elicit a significant host-mediated T-cell immune response in vivo against the tumor antigen and thus makes them very attractive in the manufacturing of anti-cancer vaccines.
Owner:VRIJE UNIV BRUSSEL
Methods for enhancing antigen-presenting cells and anti-tumor responses in a human patient
InactiveUS6838081B1Improve developmentReduce tumor burdenBiocidePeptide/protein ingredientsTumor responseCo administration
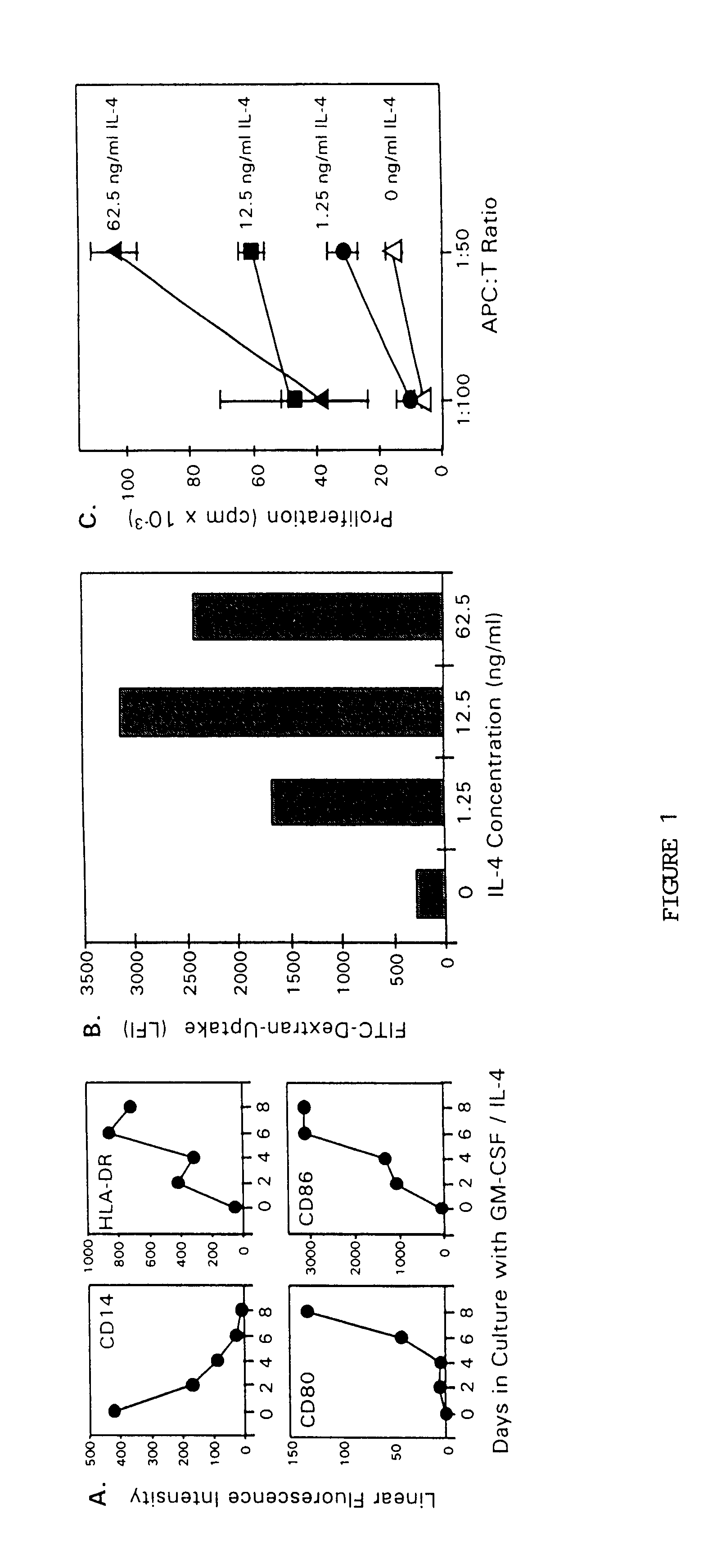
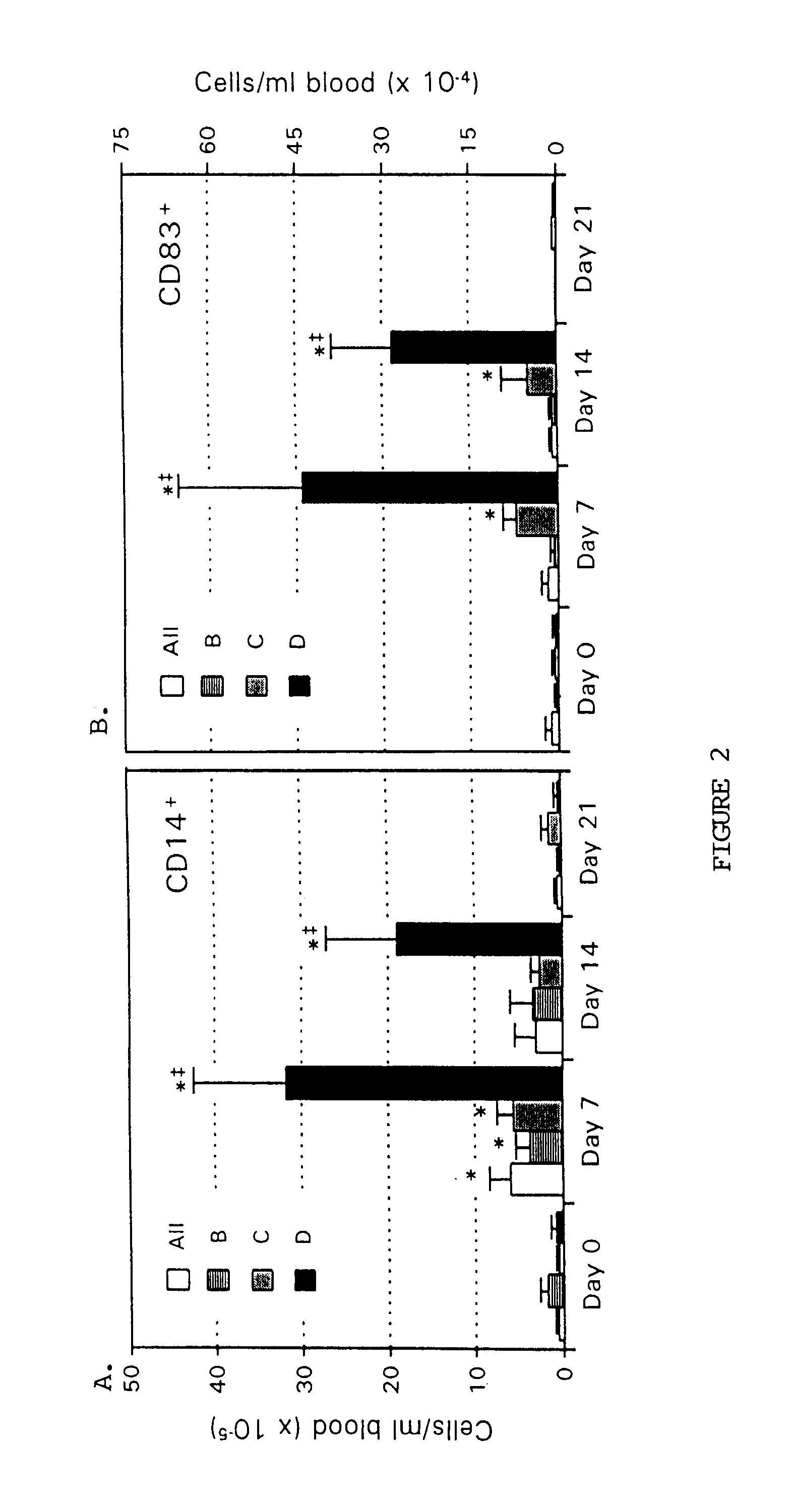
The present invention provides methods for enhancing the development of APC from precursor cells by administering a combination of GM-CSF and IL-4. The precursor cells include: cells contained in peripheral blood, CD14+ cells and precursors in bone marrow. Thus, administration of GM-CSF and IL-4 can be used as a form of cytokine immunotherapy. One embodiment of the present invention involves systemic administration of GM-CSF and IL-4. In this embodiment, APC are required to directly access tumor antigens as they exist in vivo within the patient. A further embodiment of the present invention involves co-administration of a tumor-associated or tumor-specific antigen, with GM-CSF and IL-4, to induce antigen-specific immunity mediated by APC. Yet another embodiment of the present invention describes systemic administration of GM-CSF and IL-4 to achieve reduced tumor burden.
Owner:RGT UNIV OF CALIFORNIA
Enhancing the T-cells stimulatory capacity of human antigen presenting cells and their use in vaccination
With the current invention, we provide new methods of enhancing the T-cell stimulatory capacity of human dendritic cells (DCs) and their use in cancer vaccination. The method comprises the introduction of different molecular adjuvants to human DCs through transfection with at least two mRNA or DNA molecules encoding markers selected from the group of: CD40L, CD70, constitutively active TLR4 (caTLR4), IL-12p70, EL-selectin, CCR7 and / or 4-1 BBL; or in combination with inhibition of SOCS, A20, PD-L1 and / or STAT3 expression, for example through siRNA transfection. We could show a clear increase in the immunostimulatory capacity of DCs obtained in this way, enabling them to elicit an unexpectedly high T-cell immune response in vitro. Introduction of at least two of the above molecules, in combination with a tumor-specific antigen enables the DCs to elicit a significant host-mediated T-cell immune response in vivo against the tumor antigen and thus makes them very attractive in the manufacturing of anti-cancer vaccines.
Owner:VRIJE UNIV BRUSSEL
Preparation method for dendritic cell of umbilical cord blood source and dendritic cell vaccine
ActiveCN102676455ABlood/immune system cellsAntibody medical ingredientsHematopoietic cellCell culture media
The invention discloses a preparation method for the dendritic cell (DC) of an umbilical cord blood source and a dendritic cell (DC) vaccine, which relates to a preparation method for the dendritic cell. According to the method, various cell factors are adopted to induce DC obtained by umbilical cord blood separation, and then the DC is stimulated by a tumor specific antigen so as to improve the specific antigen presentation capability of the DC; and a stem cell growth factor and Flt3-L are added into a cell culture medium so as to effectively accelerate a hematopoietic cell in the umbilical cord blood to induce and proliferate to an immune cell. The DC vaccine prepared with the method has the specific antigen presentation capability, can be combined with a CIK (cytokine induced killer) cell to mutually treat the malignant tumor when being used as a tumor immunotherapy product, and is used as an important adjuvant therapy after operations and chemoradiotherapy. Recurrence and metastasis after the operations can be effectively prevented, and toxic and side effects caused by the chemoradiotherapy on patients are lowered so as to improve the treatment effect.
Owner:北京和泽普瑞生物科技有限公司 +1
Preparation method of large-scale culture dendritic cell vaccine and application thereof
InactiveCN103301449ABlood/immune system cellsAntibody medical ingredientsWhite blood cellTumor necrosis factor alpha
The invention provides a preparation method of a large-scale culture dendritic cell vaccine. The preparation method comprises the following process steps of: performing in-vitro separation and purification on collected white blood cells in human peripheral blood by adopting a blood cell separation machine to obtain a large number of mononuclear cells, wherein the order of magnitude can be above 10<9>; using a human granulocyte-macrophage colony stimulating factor and interleukin 4 to induce the culture to obtain a large number of immature dendritic cells, wherein the order of magnitude can be above 10<8>; and loading a tumor specific antigen in the immature dendritic cells, and then obtaining the mature dendritic cell vaccine with an anti-tumor effect under the induction culture conditions of a tumor necrosis factor alpha and lipopolysaccharides. The invention further provides a multi-time and multi-treatment course clinical application of the dendritic cell vaccine obtained through the method.
Owner:泰州市数康生物科技有限公司
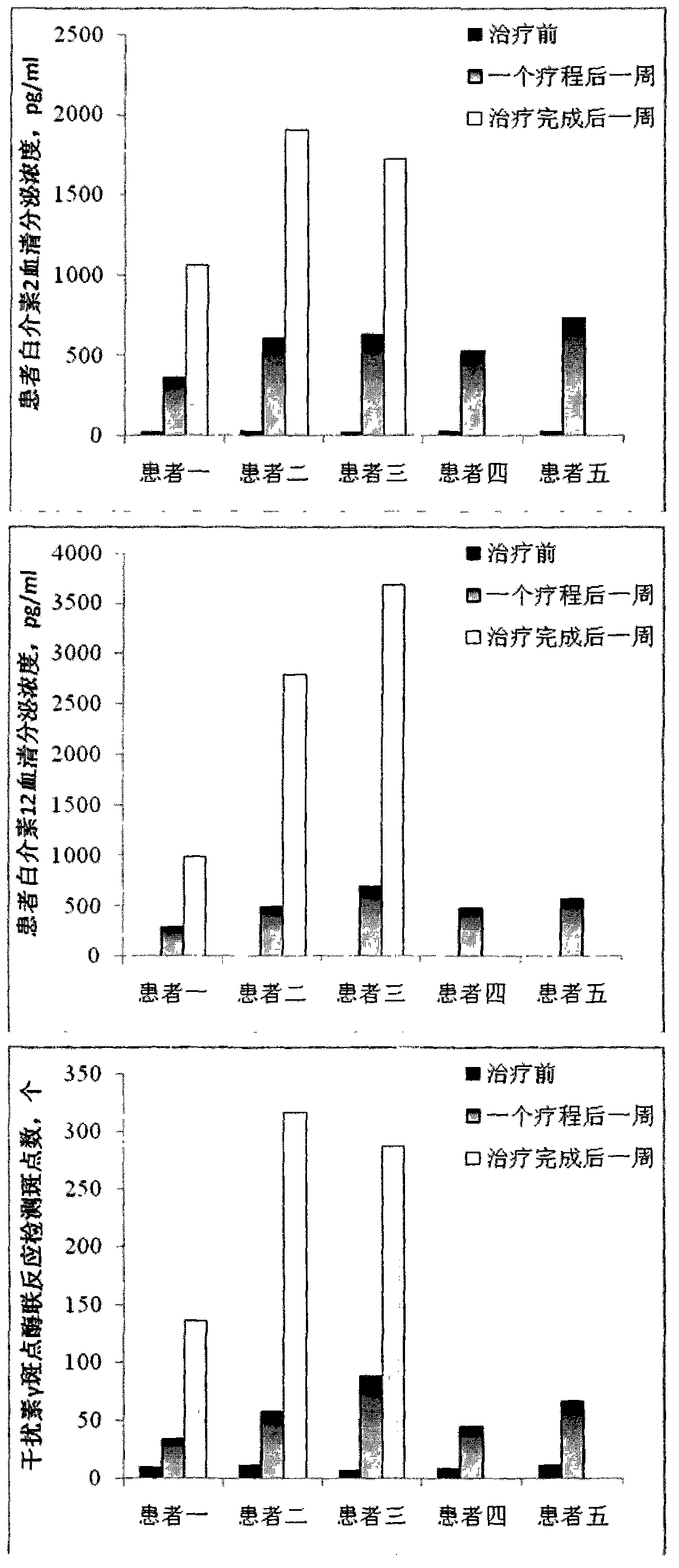
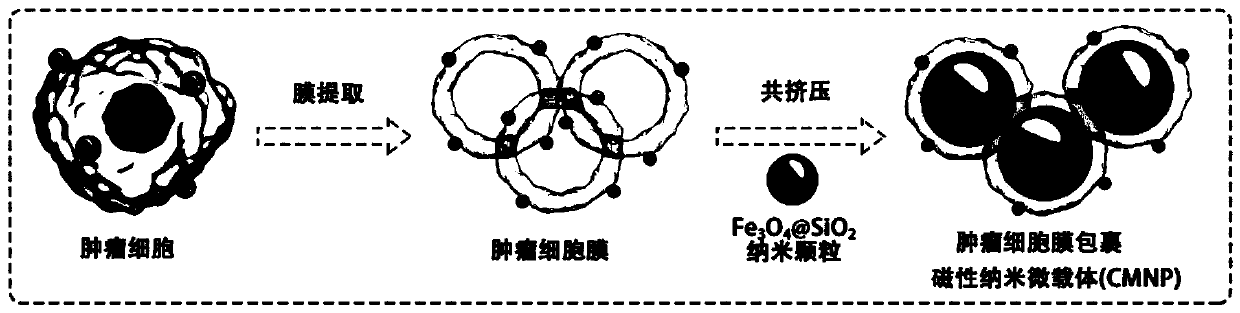
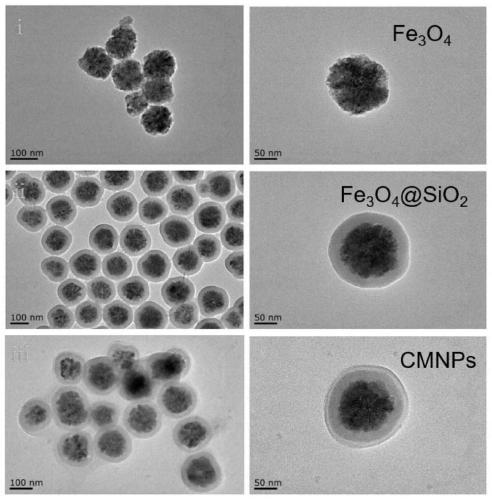
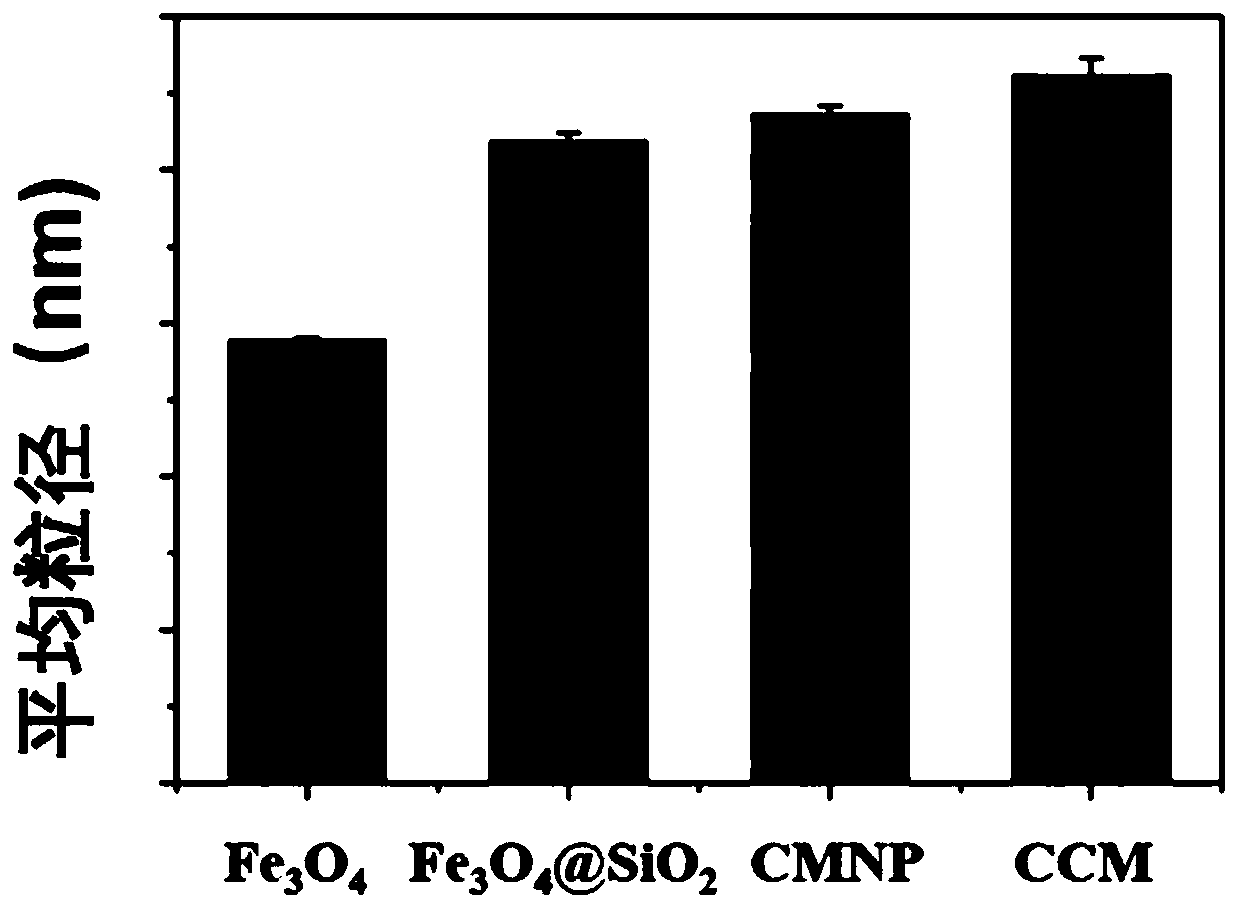
Preparation method and application of magnetic nano microcarrier for wrapping tumor cell membrane
InactiveCN111467483AIntegrity guaranteedGood biocompatibilityNanomagnetismInorganic non-active ingredientsIn vitro stimulationCell membrane
The invention discloses a preparation method and application of a magnetic nano microcarrier for wrapping a tumor cell membrane. The method is based on the tumor cell membrane, and the surface of a ferroferric oxide nano particle modified by silicon dioxide is wrapped with the cell membrane derived from a tumor cell through a membrane-nano coextrusion and centrifugation method, so that the Fe3O4@SiO2 magnetic nano microcarrier (CMNPs) wrapped with the tumor cell membrane is formed. The magnetic nano microcarrier contains a tumor cell membrane surface antigen, has superparamagnetism, and can beenriched and purified by a magnetic field, so that the magnetic nano microcarrier can stimulate activation of immune cells in vitro or is injected into a body to be captured by macrophages to improvea tumor specific antigen, and the anti-tumor effect is enhanced. The preparation method of the microcarrier based on the cell membrane has the advantages of simplicity and easiness in operation, lowcost, wide application and the like.
Owner:NANJING DRUM TOWER HOSPITAL
Recombinant oncolytic virus, and application thereof
PendingCN107164338AStrong targetingImprove securityViral/bacteriophage medical ingredientsImmunoglobulinsSingle-Chain AntibodiesTumor-specific antigen
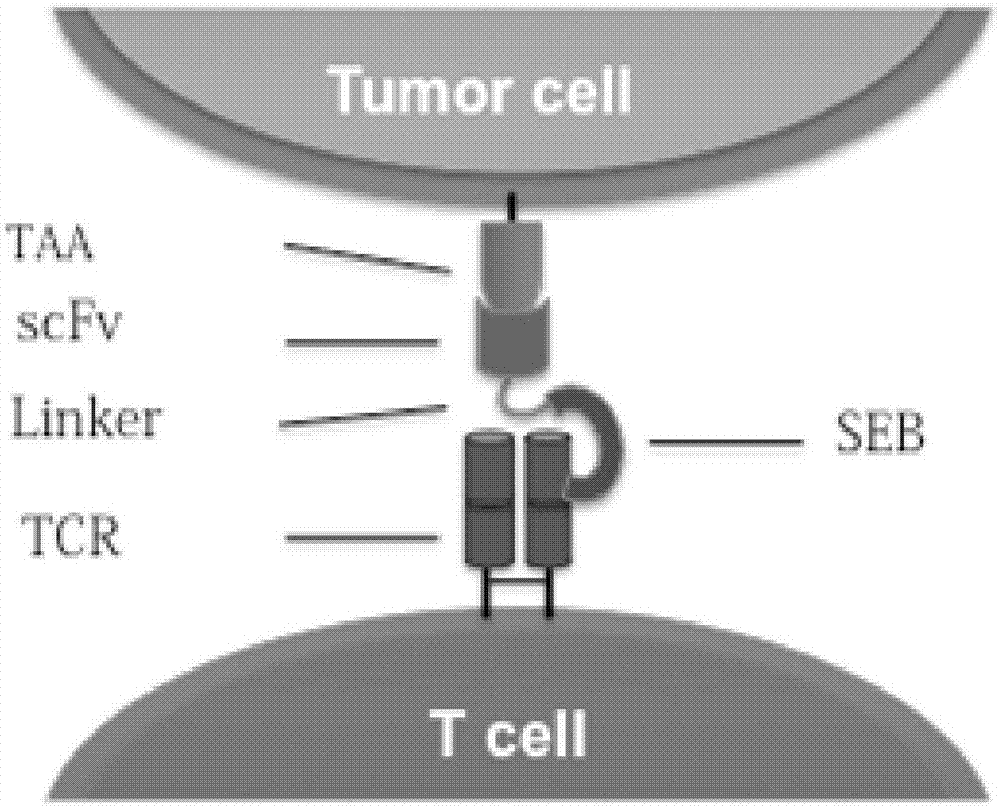
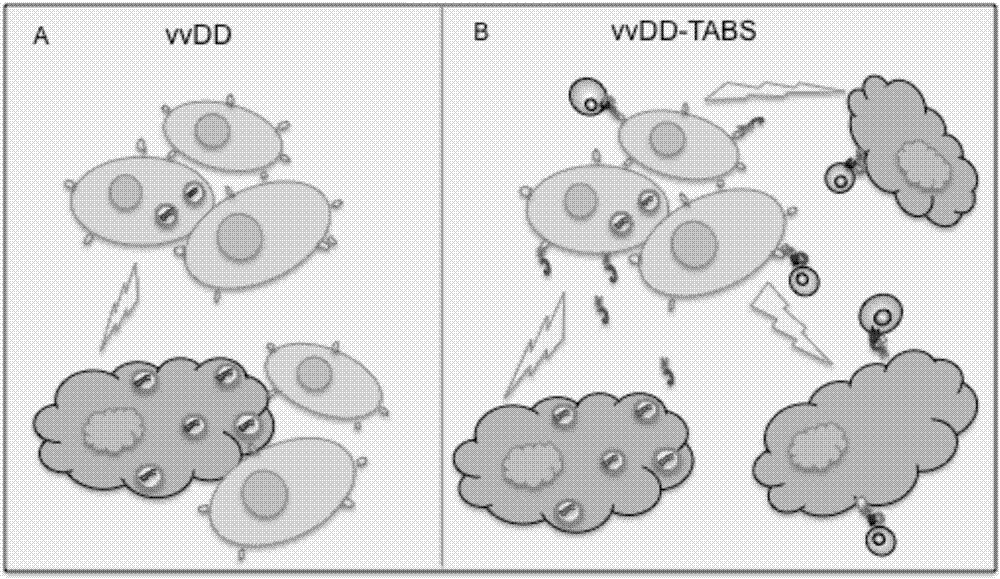
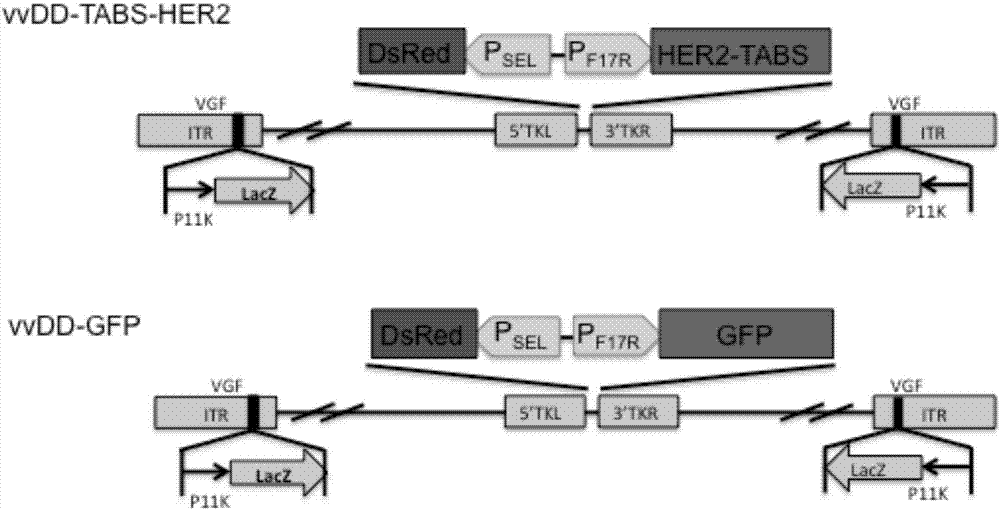
The invention belongs to the fields of biotechnology and gene therapy, and concretely to a recombinant oncolytic virus, and an application thereof. The recombinant oncolytic virus is a oncolytic virus carrying a TABS fusion protein gene, one end of the TABS fusion protein can specifically bind to tumor antigens, and the other end of the TABS fusion protein can specifically bind to the TCR of T cells. The single-chain fusion protein comprises a single chain antibody (scFv) which can specifically recognize a tumor associated antigen (TAA) or a tumor specific antigen (TSA), a linker polypeptide and a superantigen which specifically binds to a TCR complex or component from the amino terminal to the carboxy terminal, and a virus promoter in front of the TABS fusion protein gene is F17R. OVV-TABS prepared in the invention effectively combines the oncolytic effect of the virus and the anticancer effect of T cells, and has a remarkable anticancer effect, so the oncolytic virus has great clinical application prospect.
Owner:镇江市卫克生物科技有限公司
Vaccine
InactiveUS20170042997A1Effective immunityMammal material medical ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsDiseaseVaccination
The present invention relates to a pharmaceutical combination of compositions for use in the treatment or prevention of a disease having cells bearing a target antigen as a vaccine and to a method for vaccination of a mammal, especially of a human for raising a cellular immune response directed against cells of the mammalian recipient, especially human recipient, which cells express a target antigen. The target antigen can e.g. be an autoantigen like a malignant antigen, i.e. a tumour-specific antigen. The pharmaceutical combination of compositions comprises a first composition and a second composition, wherein the second composition is for administration to recipient subsequent to the administration of the first composition, e.g. 2 to 10 days after the first composition. The pharmaceutical combination of compositions has the advantage of raising an effective antigen-specific T-cell response against cells bearing a target antigen that can be a malignant autoantigen, e.g. for raising an antigen-specific T-cell response against cells bearing a tumour-antigen. A further advantage is that the pharmaceutical combination of compositions can raise an antigen-specific T-cell response within a comparatively short time.
Owner:MEDIZINISCHE HOCHSCHULE HANNOVER
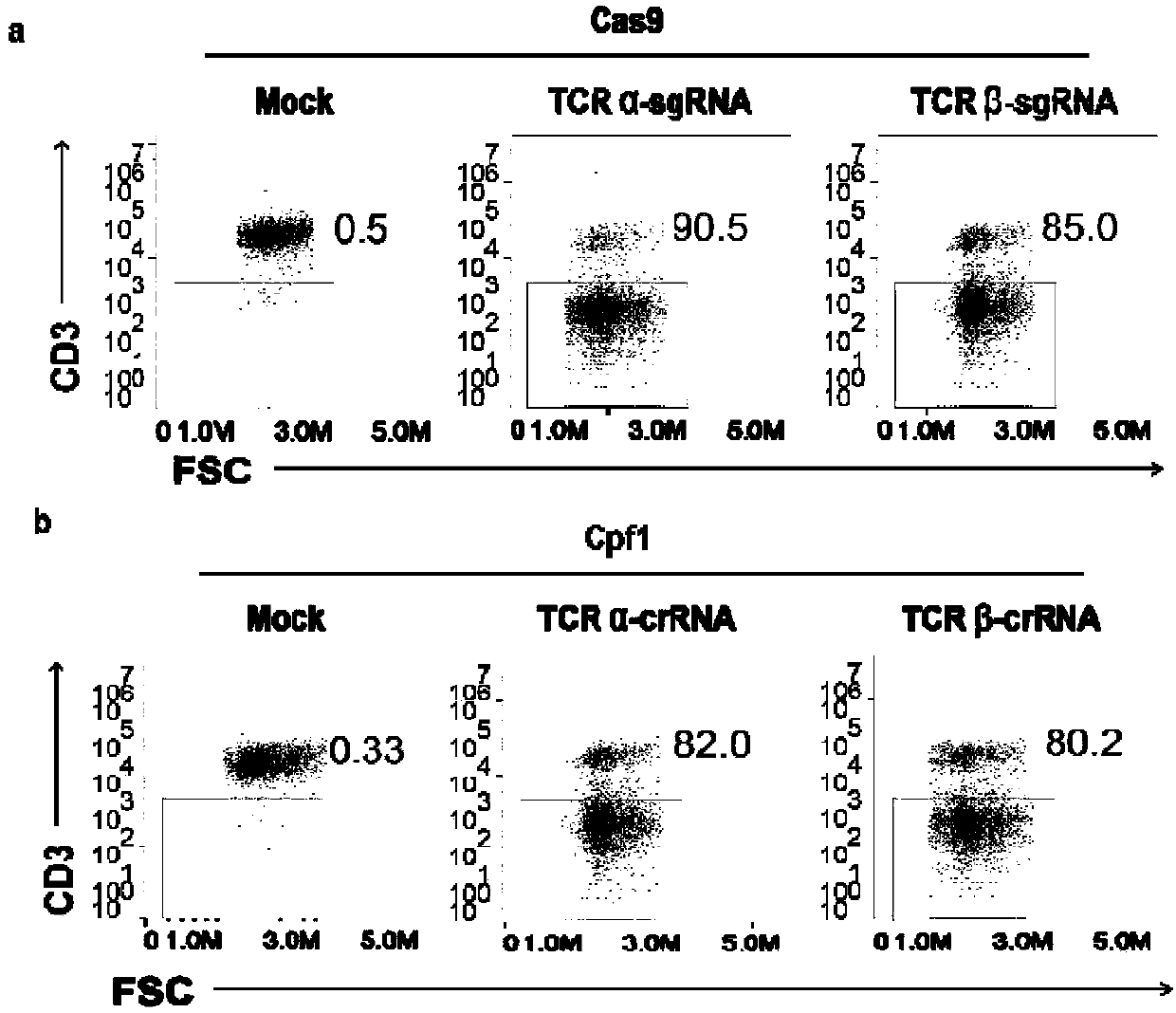
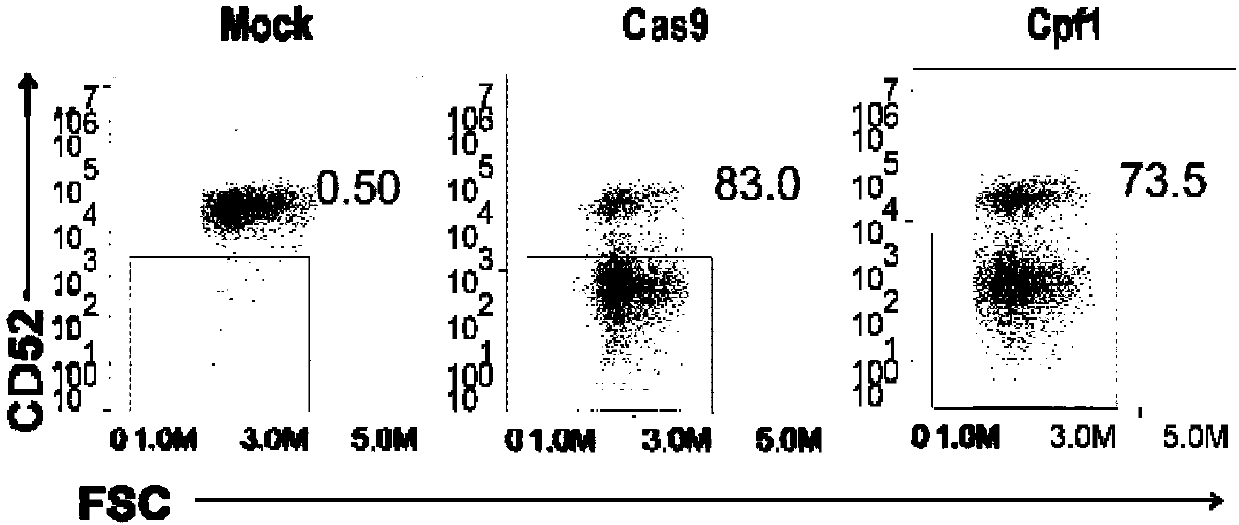
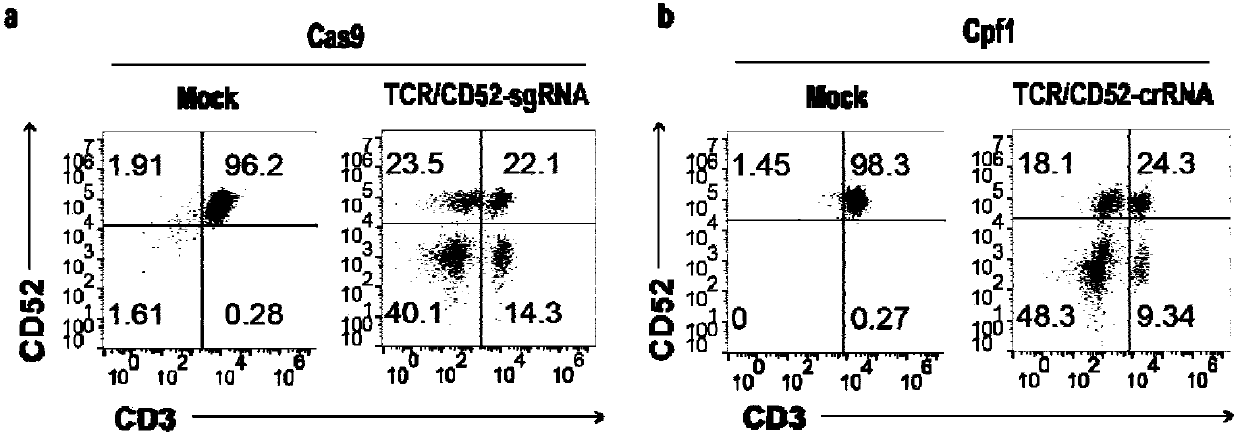
General type CART/TCRT cells with antibody drug resistance as well as construction method of general type CART/TCRT cells
ActiveCN107828730APreserve targetingAvoid exclusionImmunoglobulin superfamilyMammal material medical ingredientsAntigenAntigen receptors
The invention belongs to the fields of genetic engineering and synthetic biology, and in particular relates to general type CART / TCRT cells with antibody drug resistance as well as a construction method of the general type CART / TCRT cells. The general type CART / TCRT cells are allogeneic T cells having chimeric antigen receptors and T cell receptors, and alpha and beta chains and CD52 molecules ofthe T cell receptors are knocked out; a technology for knocking out the alpha and beta chains and the CD52 molecules of the T cell receptors is a CRISPR technology; the general type CART / TCRT cells not only retain the targeting of tumor specific antigen, but also eliminate the problems of GvHD and rejection, and weaken the sensitivity to an antibody drug Alemtuzumab; the general type CART / TCRT cells can be used as a general type simple, low-cost and high-activity CART / TCRT cell preparation.
Owner:NANJING BIOHENG BIOTECH CO LTD
Dual delivery system for heterologous antigens
Provided herein are recombinant Listeria strains expressing a tumor-specific antigenic polypeptide and, optionally, an angiogenic polypeptide wherein a nucleic acid molecule encoding at least one of the polypeptides is operably integrated into the Listeria genome in an open reading frame with a nucleic acid sequence encoding a PEST-containing polypeptide, methods of preparing same, and methods of inducing an immune response, and treating, inhibiting, or suppressing cancer or tumors comprising administering same.
Owner:ADVAXIS
Chimeric antigen receptor, NKG2D CAR-NK cell expressing chimeric antigen receptor, preparation method and application thereof
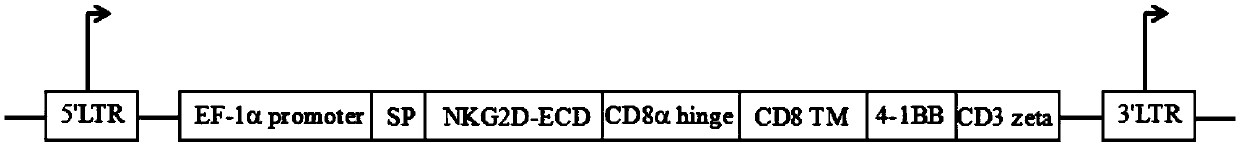
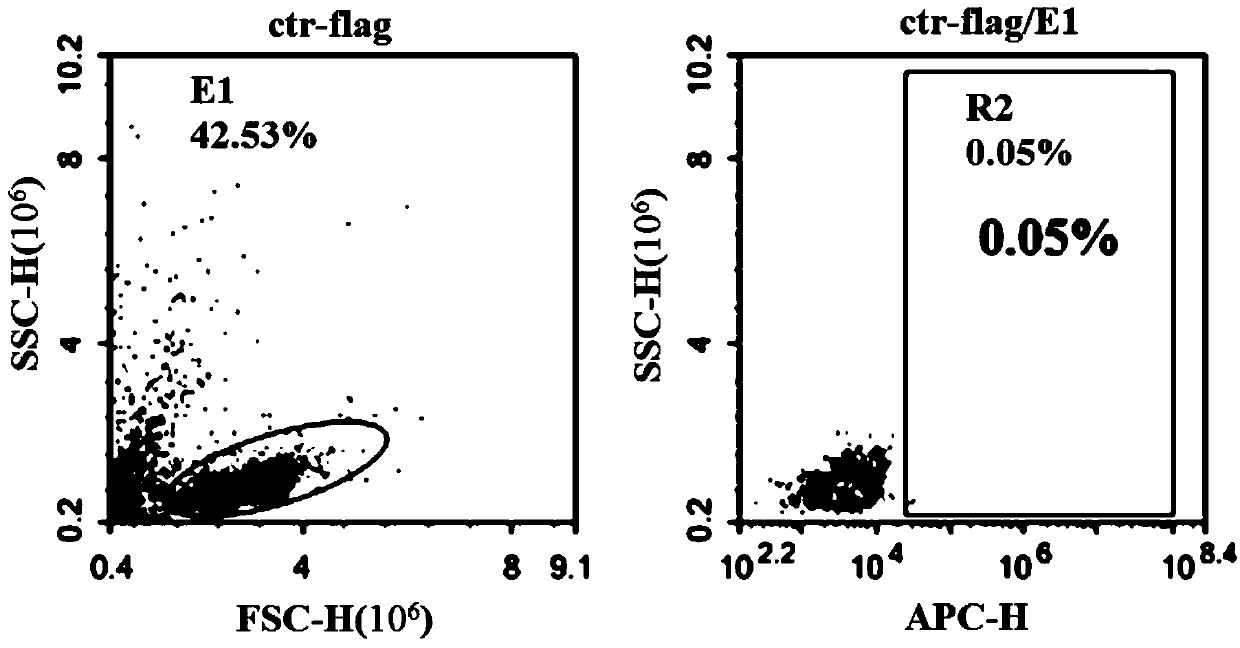
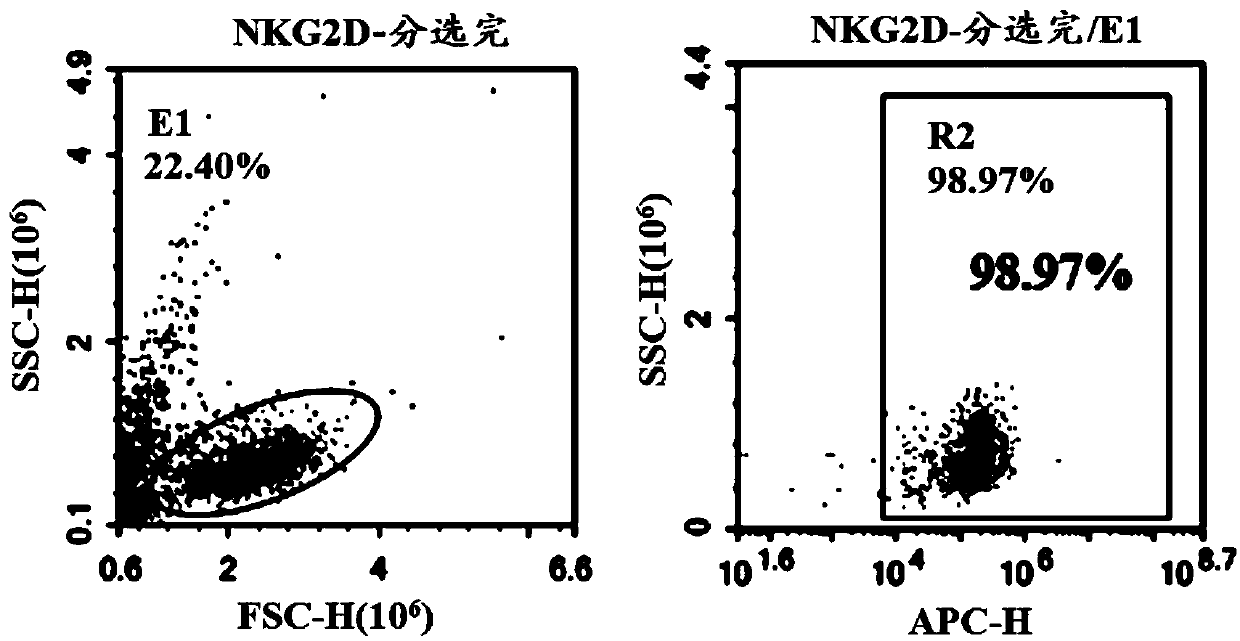
ActiveCN110028589AImprove anti-tumor effectExcellent anti-tumor performanceVirusesAntibody mimetics/scaffoldsDiseaseNkg2d ligands
The invention provides a chimeric antigen receptor, a NKG2D CAR-NK cell expressing the chimeric antigen receptor, a preparation method and an application thereof. The chimeric antigen receptor comprises an antigen binding domain, a transmembrane domain, and a costimulatory signaling domain, and the antigen binding domain is capable of specifically binding to a tumor specific antigen NKG2D ligand and activating NK cells through the transmembrane domain and the costimulatory signaling domain. The CAR-NK cell uses the NKG2D ligand as a target antigen and specifically uses the NKG2D CAR-NK cells to kill tumor cells. The cell can be used as a therapeutic drug for tumor diseases for the treatment of tumors with high expression of the ligand of NKG2D molecules, and provides a novel method for tumor prevention and treatment.
Owner:ASCLEPIUS SUZHOU TECH CO GRP CO LTD
Antitumor vaccination using allogeneic tumor cells expressing alpha (1,3)-galactosyltransferase
The invention relates to methods and compositions for causing the selective targeting and killing of tumor cells. Through ex vivo gene therapy protocols tumor cells are engineered to express an α (1,3) galactosyl epitope. The cells are then irradiated or otherwise killed and administered to a patient. The α galactosyl epitope causes opsonization of the tumor cell enhancing uptake of the opsonized tumor cell by antigen presenting cells which results in enhanced tumor specific antigen presentation. The animal's immune system thus is stimulated to produce tumor specific cytotoxic cells and antibodies which will attack and kill tumor cells present in the animal.
Owner:CENT IOWA HEALTH SYST
Compositions and methods for the prevention and treatment of cancer
Conventional cancer immunotherapy falls short at efficiently expanding T cells that specifically target cancerous cells in numbers sufficient to significantly reduce the tumor size or cancerous cell number in vivo. To overcome this limitation, provided herein are nanoparticles coated with MHC class I and / or class II molecules presenting tumor-specific antigens and co-stimulatory molecules and their use to expand antigen-specific anti-tumorigenic T cellsto levels not achieved in current immunotherapeutic techniques. These antigen-specific anti-tumorigenic T cells include cytotoxic T cells, effector T cells, memory T cells, and helper T cells that are necessary to initiate and maintain a substantial immune response against metastatic or non- metastatic cancerous, pre-cancerous, or neoplastic cells in vivo.; The present invention describes a systemic approach to targeting cancerous or pre-cancerous cells that are circulating cells, as in lymphomas, migratory metastatic cells, and solid tumors.
Owner:UTI LLP
Recombinant plasmid, recombinant malaria parasite and its application
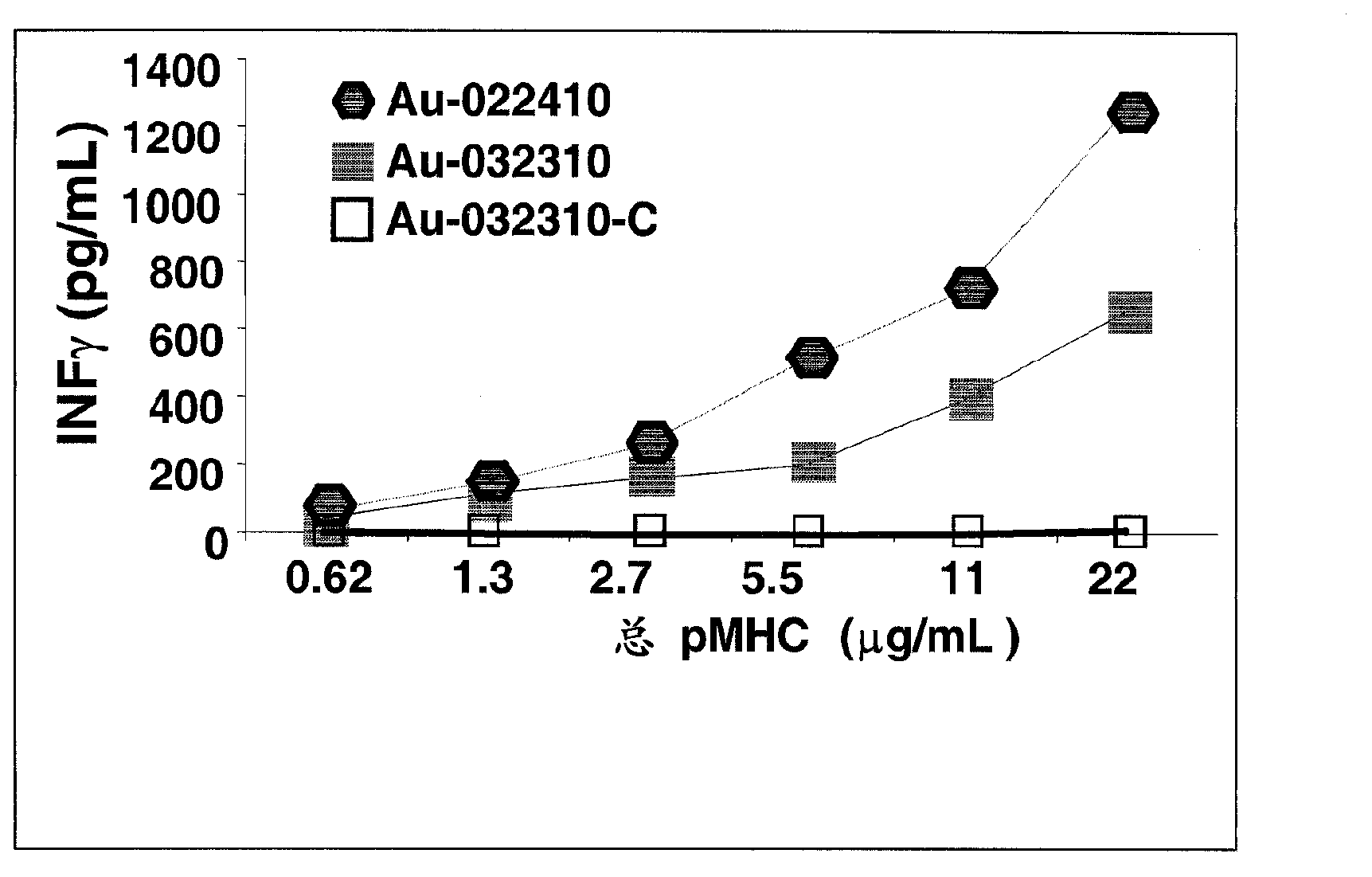
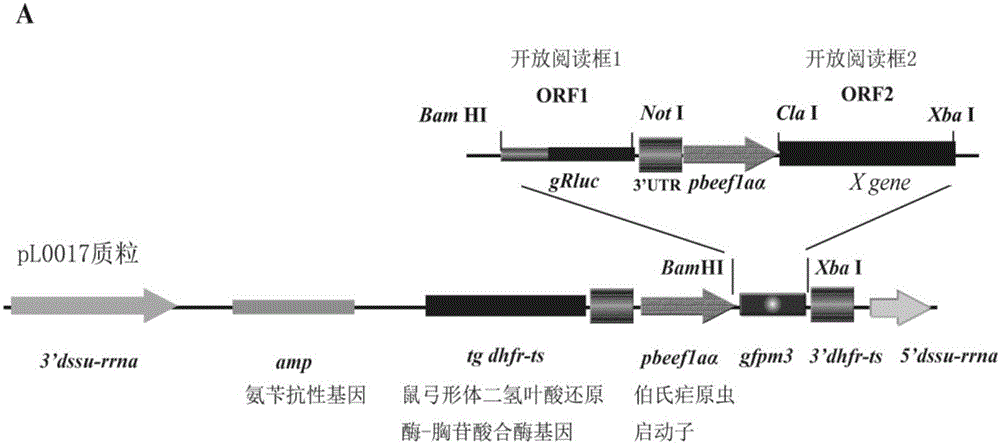
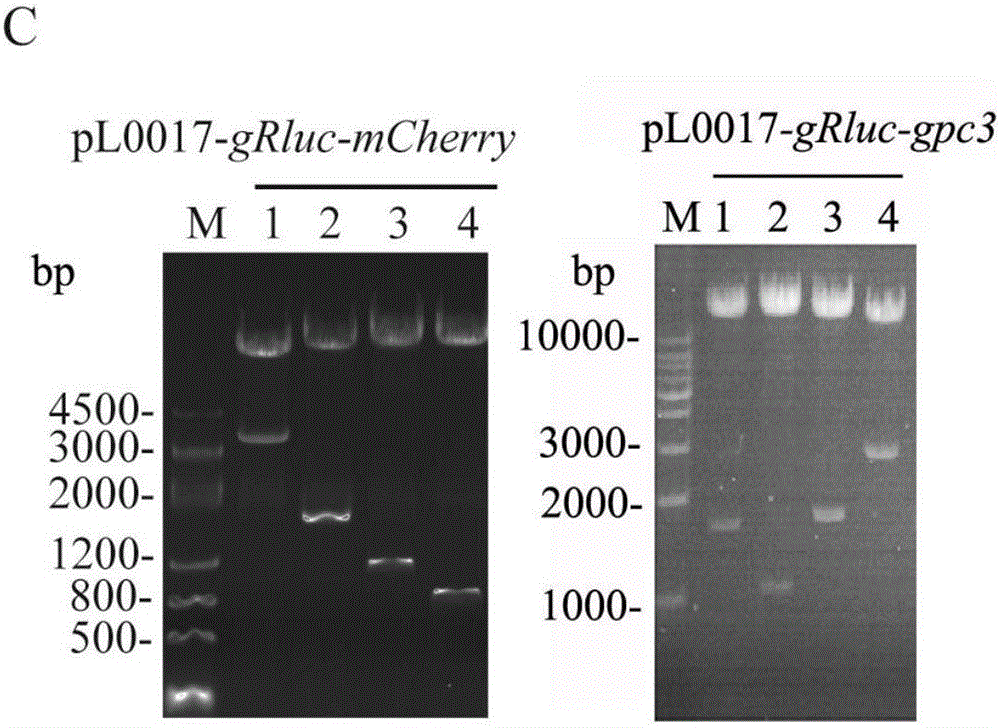
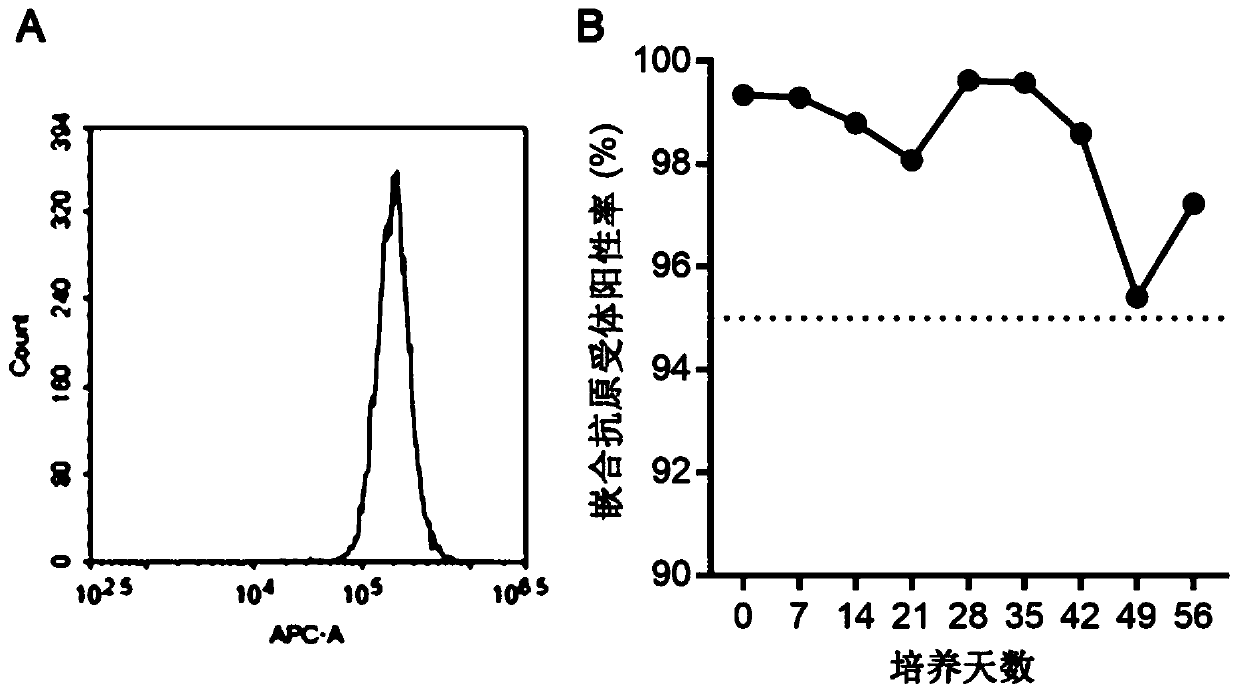
ActiveCN106687592AConducive to long-term existenceFacilitated releaseTumor rejection antigen precursorsProtozoaBacteroidesMalaria
The present invention relates to the field of cellular immunotherapy for tumors and, more particularly, to a recombinant plasmid for the construction of recombinant malaria parasites and their use, wherein the recombinant plasmid is a tumorigenin-specific antigen gene inserted into the pL0017 plasmid, Recombinant malaria parasite, comprising the recombinant plasmid. Compared with plasmid DNA and RNA vector, the recombinant malaria parasite can be multiplied by the proliferation of Plasmodium, which is beneficial to the increase of the antigen in vivo. Compared with the defective virus and bacterial carrier, it survives in the red blood cells of the body longer, not short-term by the body's immune system to clear, long-term effective expression of exogenous tumor antigen, is conducive to long-term antigen and immune stimulation. The recombinant malaria parasite is capable of activating the high expression of Th1-related cytokines in vivo and increasing the proportion of CD8a + DCs in total CD11c + DCs and further activating specific cytotoxic T lymphocyte responses against tumor antigens, which is beneficial to the antitumor effect of the vaccine.
Owner:BLUE ELEGANT BIOTECH CO LTD
Chimeric antigen receptor capable of specifically activating NK cells and application thereof
ActiveCN110627909AIncrease lethalityGood antitumor activityBlood/immune system cellsImmunoglobulinsAntigenNatural Killer Cell Inhibitory Receptors
The invention relates to the field of biological medicines, in particular to a chimeric antigen receptor specifically optimized according to NK cell signal transduction characteristics and an application thereof. The chimeric antigen receptor scFv is composed of a light chain variable region and a heavy chain variable region and connected to an NKp44 transmembrane region through a CD8 hinge structure, and an intracellular segment activation signal transduction structural domain is composed of a CD3 zeta ITAM motif, a 2B4 activation motif, a DNAM1 activation motif and a DAP10 activation motif which are connected in series. The chimeric antigen receptor is used for modifying natural killer (NK) cells, and the modified NK cells (CAR-NK) can be used for treating tumors with positive tumor specific antigens. In a killing test, compared with a classic third-generation T-CAR structure, the structure has the advantages that the killing capability of the NK cells on tumor cells is obviously enhanced, and good anti-tumor activity is shown in an in-vivo model.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Vaccine preparation for cancer treatment
InactiveCN103957930AHigh cancer treatment effectTumor rejection antigen precursorsAntibody mimetics/scaffoldsVaccine antigenCD8
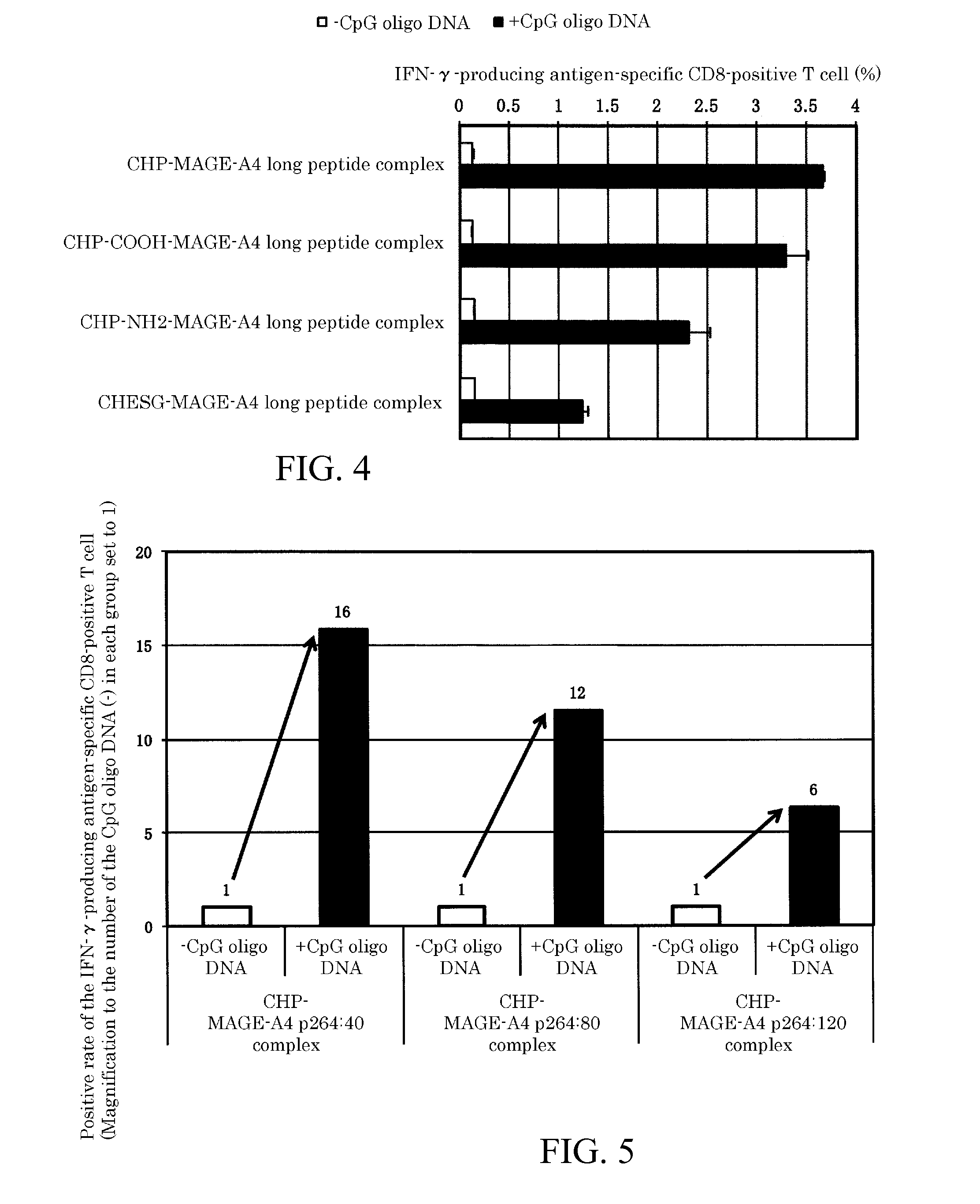
An object of the invention is to provide a method of clearly deriving killer T cells and helper T cells in a cancer treatment vaccine in which the vaccine antibody is a synthetic long peptide which is derived from a tumor-specific antigen protein and / or pathogen-derived protein and which simultaneously contains a CD8-positive, cytotoxic T-cell recognizing epitope and a CD4-positive, helper T-cell recognizing epitope peptide. By simultaneously dosing an immunopotentiating agent with a conjugate of a hydrophobized polysaccharide (the antigen delivery system) to the synthetic long peptide, it is possible to achieve clear derivation of antigen-specific killer T-cells and helper T-cells from the cancer treatment vaccine in which the synthetic long peptide is the antigen.
Owner:MIE UNIVERSITY
Combined factor for inducing tumor specific T cells and method for obtaining tumor specific T cells
InactiveCN105349488ALow CTL contentProliferation effect is goodMammal material medical ingredientsBlood/immune system cellsAbnormal tissue growthLymphocyte culture
The invention discloses a combined factor for inducing tumor specific T cells and a method for obtaining the tumor specific T cells. The combined factor comprises a tumor specific antigen and cell factors IL-2, IL-7 and IL-15. The method comprises the following steps: suspending peripheral blood PBMC with a fresh serum-free lymphocyte culture solution, wherein the cell density is 2*10<6>-5*10<6> / ml; adding a stimulus of the combined factor for inducing the tumor specific T cells, conducting culturing in a 5% CO2 culturing box at 37 DEG C for 2-10 days, and separating the IFN gamma positive antigen specific T cells through an IFN gamma magnetic bead enrichment method. The method can be used for obtaining the high-purity tumor antigen specific T cells, and is simple, and low in cost. The prepared tumor antigen specific T cells can be applied to preparation of medicines or vaccines for clinical cell treatment and tumor immunization treatment.
Owner:SYZ CELL THERAPY +1
Vaccine and preparation method thereof
PendingCN111358942AImprove securityEasy to manufactureCancer antigen ingredientsAntineoplastic agentsAntigenSpecific immunity
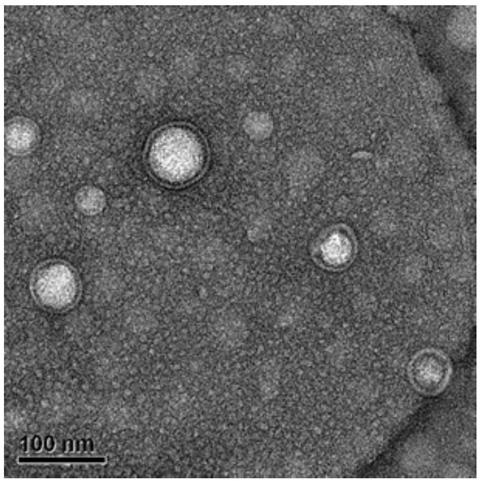
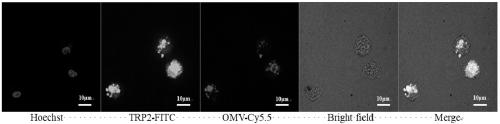
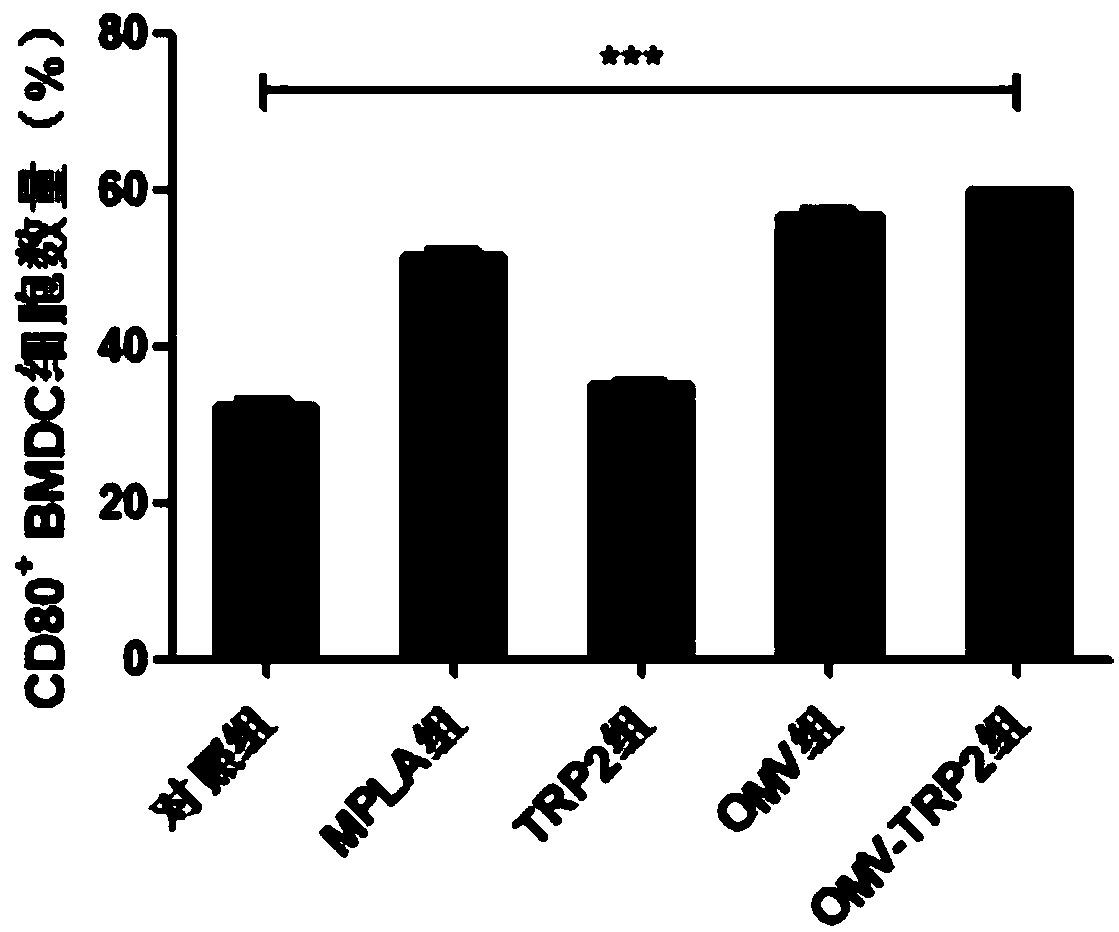
The invention provides a vaccine and a preparation method thereof. The vaccine comprises bacterial outer membrane vesicles and antigen, wherein the antigen is combined with the bacterial outer membrane vesicles to form nanoparticles. The bacterial outer membrane vesicles are combined with a tumor specific antigen to prepare an anti-tumor vaccine, and an innate immune response and a specific immuneresponse are caused at the same time, so that the target of treating tumors by an autoimmune system of a body is achieved; and the vaccine is high in specificity, small in side effect and has a broadapplication prospect and great market value.
Owner:THE NAT CENT FOR NANOSCI & TECH NCNST OF CHINA
Method for screening tumor specific T cell from TIL (tumor infiltrating lymphocyte)
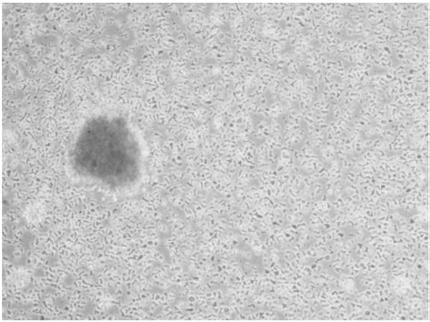
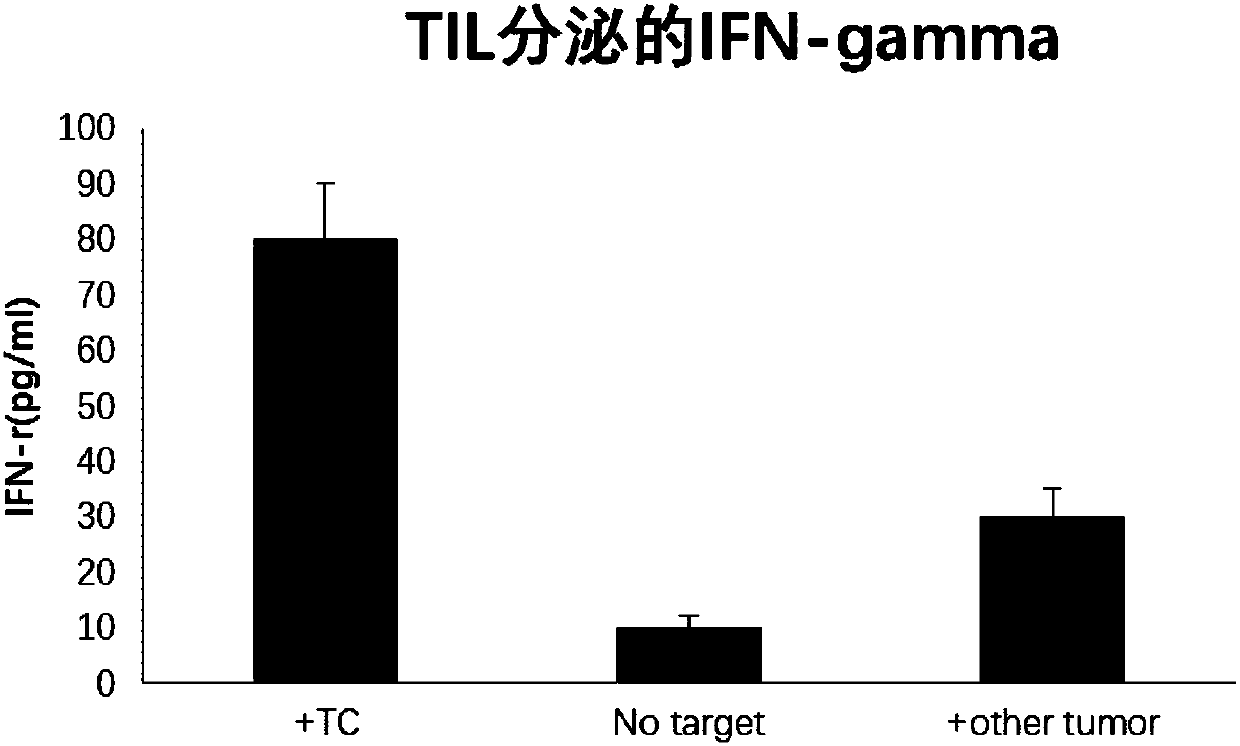
PendingCN109988748AGrowth inhibitionEfficient screeningCell dissociation methodsMammal material medical ingredientsLymphocyteTumor-specific antigen
The invention discloses a method for screening a tumor specific T cell from a TIL (tumor infiltrating lymphocyte). The method includes the steps of screening a tumor specific antigen peptide of a to-be-tested tumor patient; constructing a composite of an MHC (major histocompatibility complex) and the tumor specific antigen peptide, wherein the MHC and antigen peptide composite can be combined witha TCR (T cell receptor) of a T cell of a tumor specific antigen; adding an IFN-r positive TIL of the to-be-tested tumor patient to the MHC and antigen peptide composite, performing incubation, and screening the tumor specific T cell. The invention also provides a tumor specific polypeptide screened by the method, and application of the screened tumor specific T cell and the polypeptide in products related to cancer therapy. By the method, the tumor specific T cell and the tumor specific polypeptide can be efficiently screened out, and accordingly, an effective choice for tumor immunotherapy is provided.
Owner:SHENZHEN HUADA GENE INST
Vaccine preparation for cancer treatment
InactiveUS20140322344A1High encapsulation efficiencyTumor rejection antigen precursorsAntibody mimetics/scaffoldsCD8Tumor-specific antigen
A vaccine preparation for treating cancer includes a complex of a hydrophobized polysaccharide and at least one synthetic long peptide derived from a tumor-specific antigenic protein and / or a pathogen-derived antigenic protein. The at least one synthetic long peptide contains at least one CD8+ cytotoxic T-cell recognition epitope and at least one CD4+ helper T-cell recognition epitope. The complex is simultaneously administered to the patient with at least one immunopotentiating agent.
Owner:MIE UNIVERSITY +1
Compositions and methods useful in pretargeted imaging
InactiveUS20060057062A1Improve target-to-background ratioHybrid immunoglobulinsIn-vivo radioactive preparationsCarrier proteinMethod of images
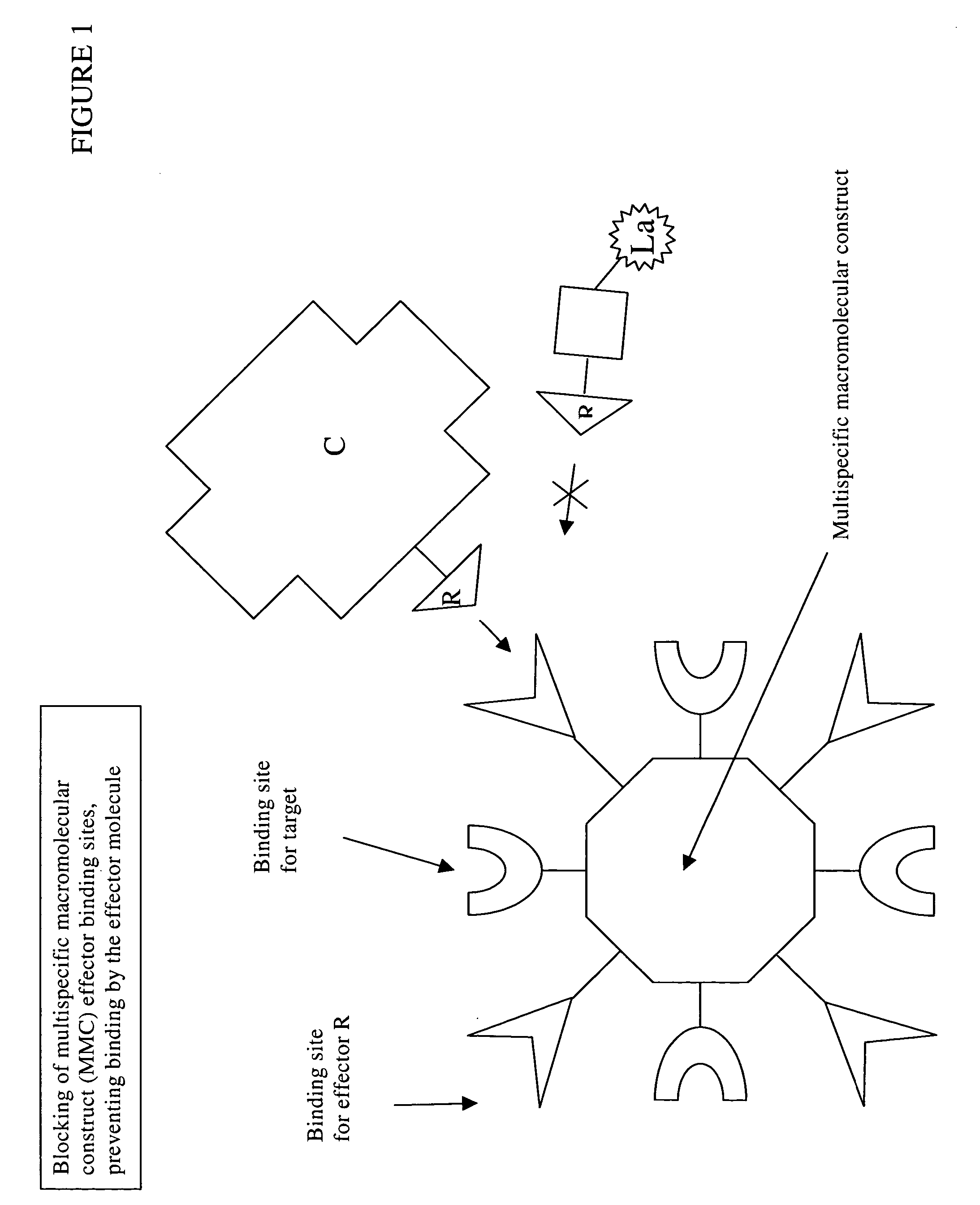
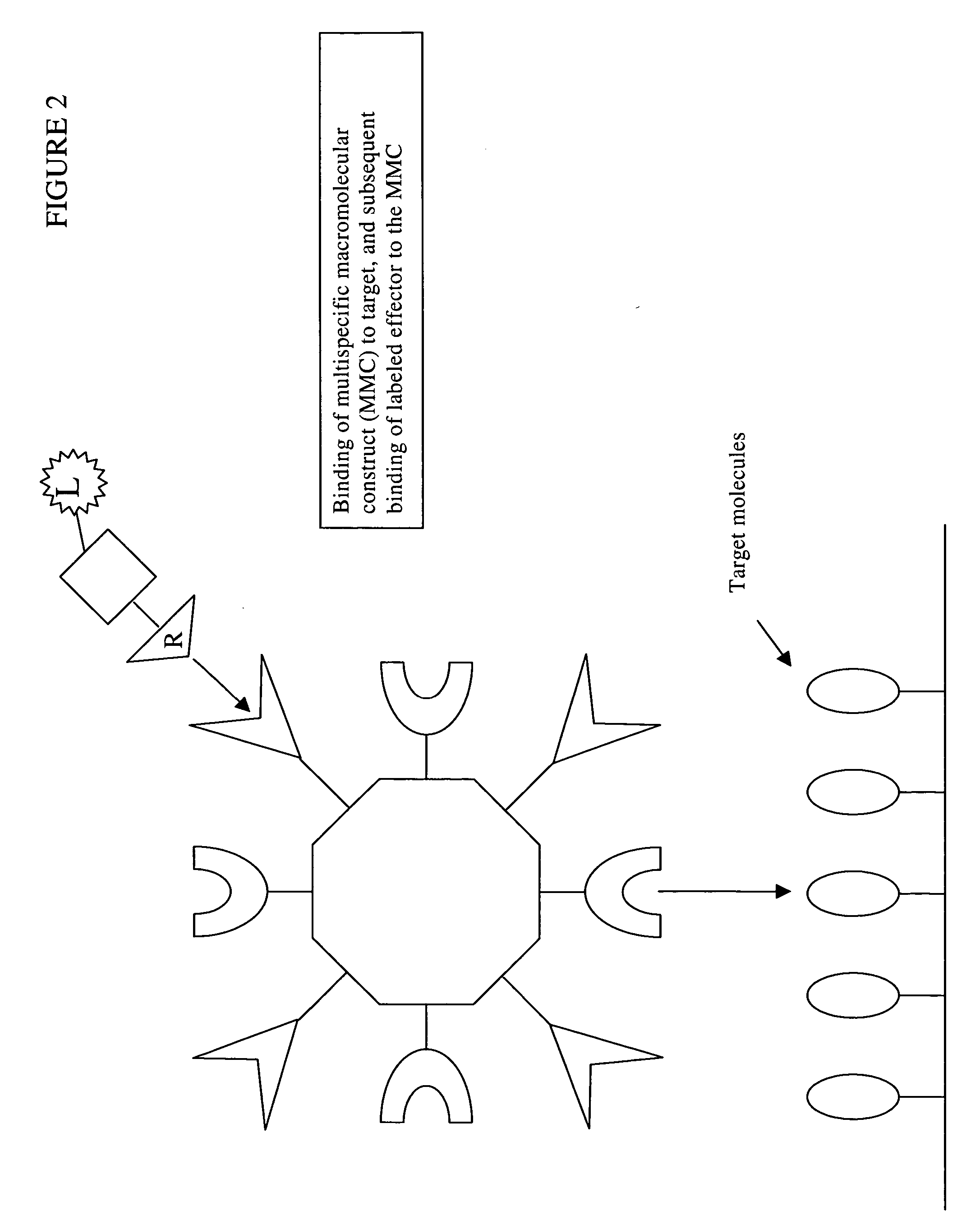
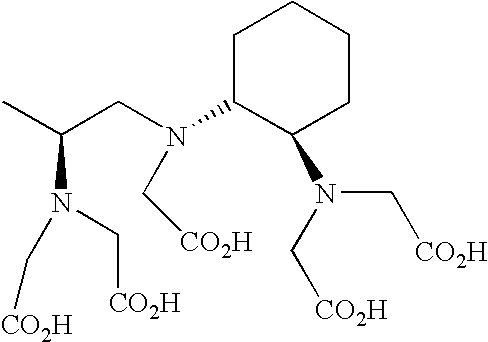
Disclosed are multispecific macromolecular constructs, blocking agents and radiolabeled effector molecules, as well as kits and methods for imaging tissue of interest in a mammalian subject. The multispecific macromolecular construct is capable of binding a radiolabeled effector molecule that can be imaged, as well as a disease marker such as for example a tumor specific antigen expressed on the surface of tumor tissue. The blocking agent comprises, or alternatively consists of, an unlabeled form of the radiolabeled effector conjugated to a carrier protein or polypeptide, said carrier protein or polypeptide preferably being non-immunogenic or having low immunogenicity. The invention further contemplates methods of imaging diseases or disorders in a mammalian subject using said compositions
Owner:GENERAL ELECTRIC CO
Method for identification of cellular protein antigens and presence of antibodies to specific cellular protein antigens in serum
InactiveUS20040191841A1Facilitating tumor cell killingInhibit tumor cell growthMaterial analysisDiseaseADAMTS Proteins
The present invention relates to a method for identification of cellular protein antigens to which patients with cancer, or patients at risk for cancer, may develop autoantibodies. The method of the invention involves the use of patient derived sera for the identification of the cellular protein antigens using two-dimensional gel electrophoresis followed by Western Blot analysis. The identification of such protein antigens provides novel markers that can be utilized for screening, for diagnostics and prognosis of disease. The invention also provides for the use of the identified protein antigens in immunoassays designed to detect the presence of serum antibodies to the specific protein antigens in sera from individuals that may harbor such antibodies. The invention further relates to the use of the identified antigens as immunogens for stimulation of an immune response in patients expressing such protein antigens. The invention is demonstrated by way of example in which elevated levels of circulating autoantibodies reactive against a tumor specific antigen were identified in sera derived from a lung cancer patient. In addition, elevated levels of circulating autoantibodies reactive against several specific beta-tubulin isoforms were detected in the sera of neuroblastoma patients.
Owner:RGT UNIV OF MICHIGAN
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com