Preparation method of large-scale culture dendritic cell vaccine and application thereof
A dendritic cell, large-scale culture technology, applied in the field of large-scale cultured dendritic cell vaccine preparation, can solve problems such as affecting patient treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
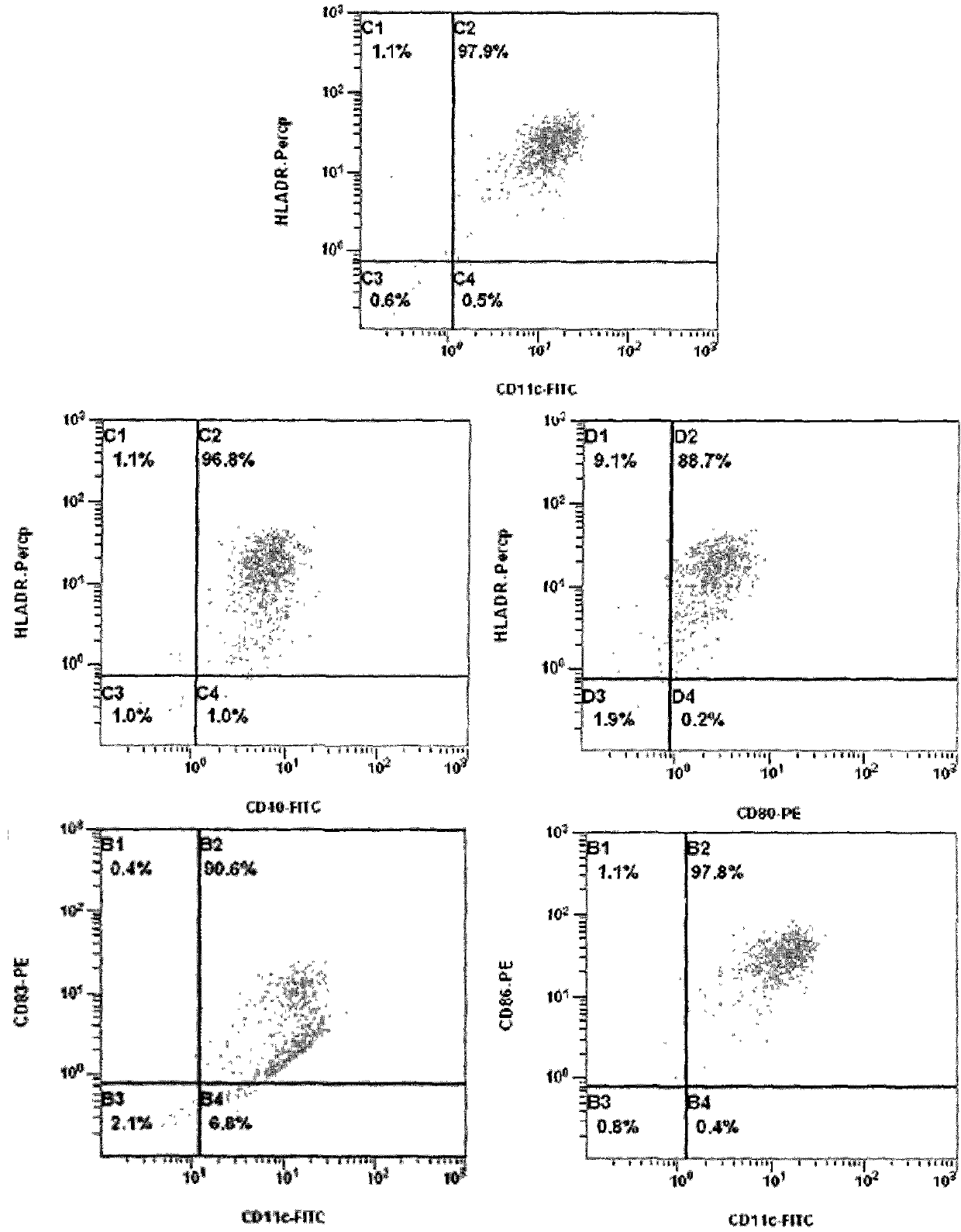
[0030] Large scale culture of dendritic cell vaccine from mononuclear cells separated by blood cell separator
[0031] Gastric cancer patient, male, 35 years old, blood routine test result was 4.2×10 9 white blood cells per liter, 0.5L peripheral blood was collected by a blood cell separator, and the number of collected white blood cells was 2.1×10 9 (Sign an informed consent form with the patient); resuspend the collected white blood cells with 100ml 1×PBS (pH=7.4), add 25ml of 0.6% hydroxyethyl starch saline, mix well and let stand for 30 minutes, absorb as much as possible White cell layer; the white cell layer was subjected to Ficoll-Hypaque density gradient centrifugation to obtain mononuclear cells resuspended in RPMI1640 medium, and the trypan blue staining count was 1.44×10 9 .
[0032] Peripheral blood mononuclear cells were adjusted to a cell concentration of 5×10 with RPMI1640 medium 6 , added to a 2-layer cell culture device (Corning) to adhere to the wall, at 3...
Embodiment 2
[0037] Large-scale culture of dendritic cells obtained from mononuclear cells collected by apheresis
[0038] Patient 1: lung cancer patient, male, 43 years old, blood routine test result was 2.8×10 9 white blood cells per liter, using a blood cell separator to collect 1L of peripheral blood, and the number of collected white blood cells was 2.18×10 9 .
[0039] Patient 2: Breast cancer patient, female, 51 years old, blood routine test result was 3.2×10 9 white blood cells per liter, 0.75L peripheral blood was collected by a blood cell separator, and the number of collected white blood cells was 2.4×10 9 .
[0040] Patient 3: Esophageal cancer patient, male, 31 years old, blood routine test result was 7.0×10 9 white blood cells per liter, 0.5L of peripheral blood was collected using a blood cell separator, and the number of collected white blood cells was 3.5×10 9 .
[0041] The existing technology directly collects the patient's peripheral blood for the cultivation of d...
Embodiment 3
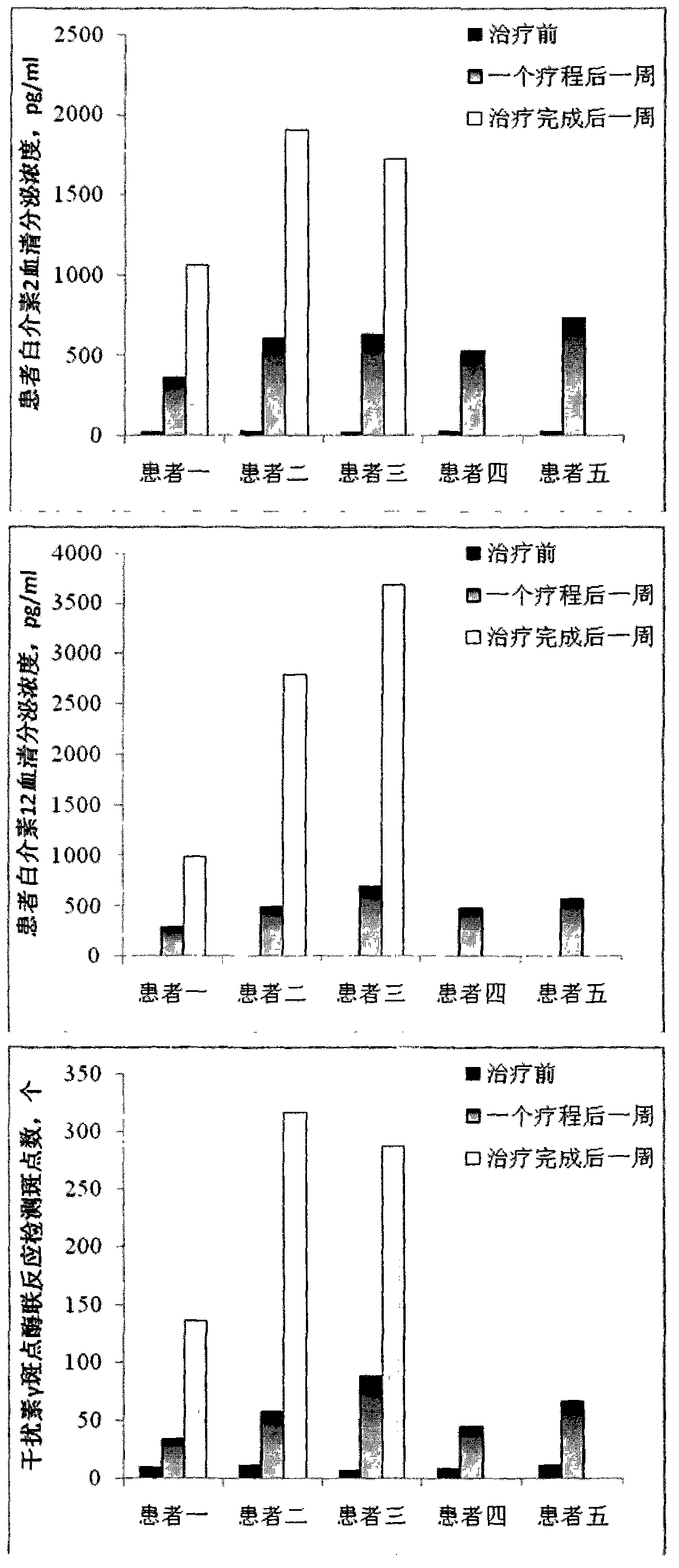
[0051] Clinical experiment of dendritic cell vaccine obtained by large-scale culture of the present invention
[0052] Clinical experiments were performed using the dendritic cell vaccine prepared in Example 2 of the present invention.
[0053] The patient's dendritic cell vaccine obtained by large-scale culture and the patient's dendritic cell vaccine obtained by direct peripheral blood mononuclear cell culture of the present invention are clinically applied to the patient (the informed consent is signed with the patient). The patient received four dendritic cell vaccine treatments in one course of treatment, once every week, and each time the dendritic cells were 5×10 6 , Intradermal injection in the abdominal groove lymph node site. Among them, dendritic cell vaccines obtained through large-scale culture can be used for multiple courses of treatment, and the next course of treatment is performed every two months, a total of 2.5 to 3 courses of treatment; while patients who...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com