Interleukin-10 antibodies
a technology of interleukin-10 and specific antibodies, applied in the field of interleukin-10 specific antibodies, can solve the problems of inability to achieve the effect of reducing the immunogenicity of antibodies using antibodies as a therapeutic agent in vivo, affecting the immunogenicity of antibodies, and a minimum loss of therapeutic efficacy, so as to achieve the effect of inhibiting biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example i
General Methods
[0123] Some of the standard methods are described or referenced, e.g., in Maniatis, et al. (1982) MOLECULAR CLONING, A LABORATORY MANUAL, Cold Spring Harbor Laboratory, Cold Spring Harbor Press; Sambrook, et al. (1989) MOLECULAR CLONING: A LABORATORY MANUAL, (2d ed.), vols. 1-3, CSH Press, NY; Ausubel, et al., BIOLOGY, Greene Publishing Associates, Brooklyn, N.Y.; or Ausubel, et al. (1987 and Supplements) CURRENT PROTOCOLS IN MOLECULAR BIOLOGY, Greene / Wiley, New York. Methods for protein purification include such methods as ammonium sulfate precipitation, column chromatography, electrophoresis, centrifugation, crystallization, and others. See, e.g., Ausubel, et al. (1987 and periodic supplements); Deutscher (1990) “Guide to Protein Purification” in METH. ENZYMOL., vol. 182, and other volumes in this series; and manufacturer's literature on use of protein purification products, e.g., Pharmacia, Piscataway, N.J., or Bio-Rad, Richmond, Calif. Combination with recombinan...
example ii
Humanization of Anti-Human IL-10 Antibodies
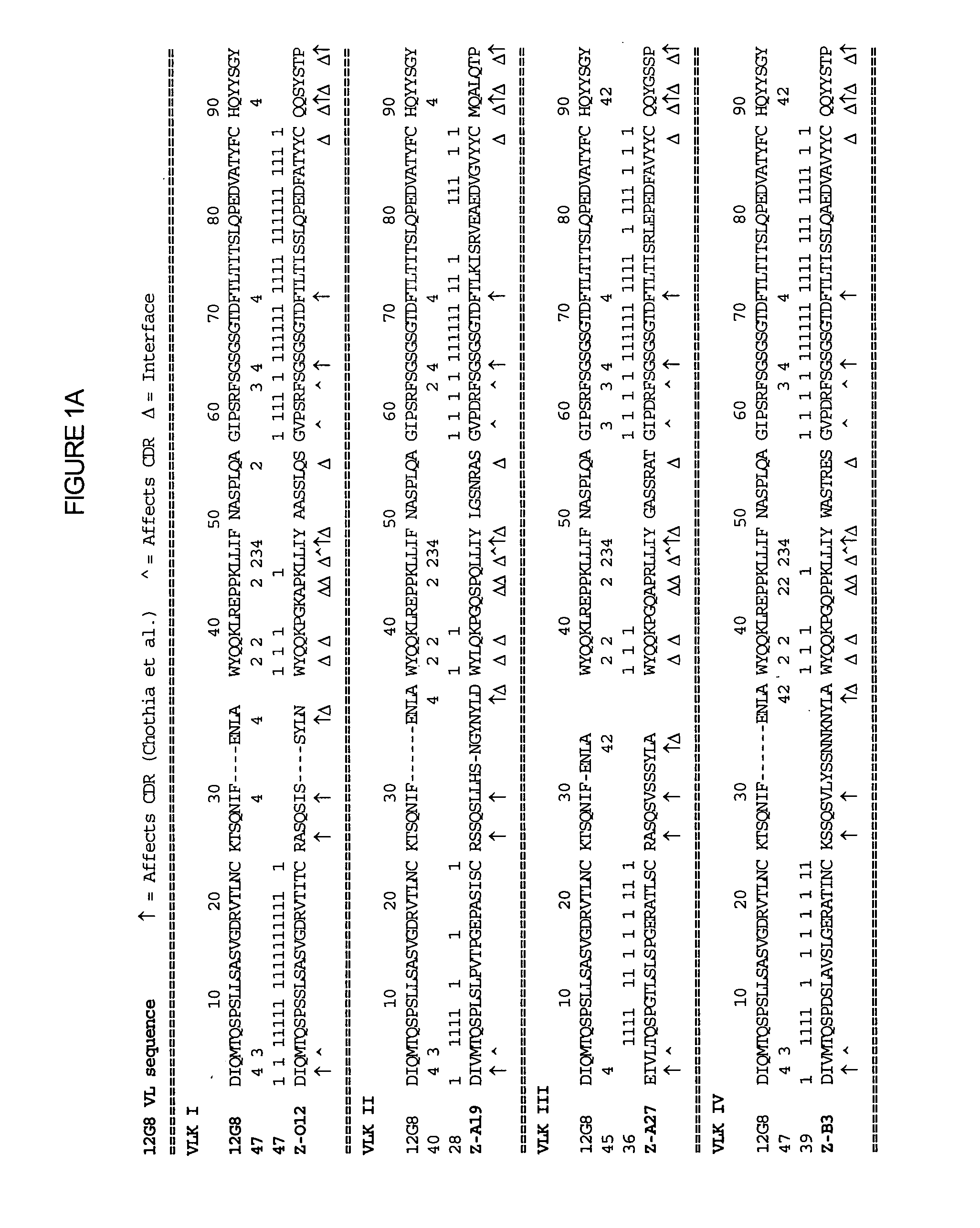
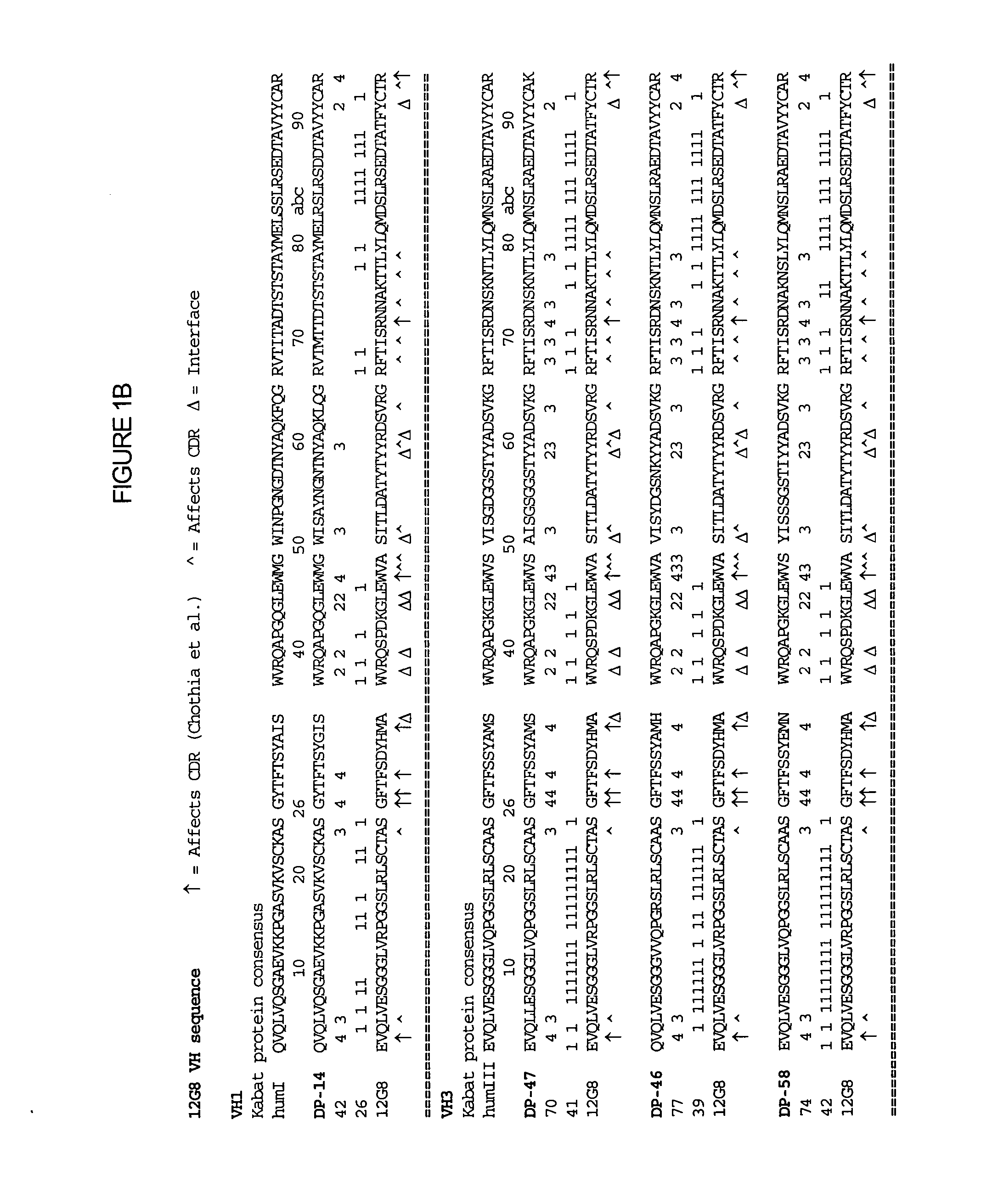
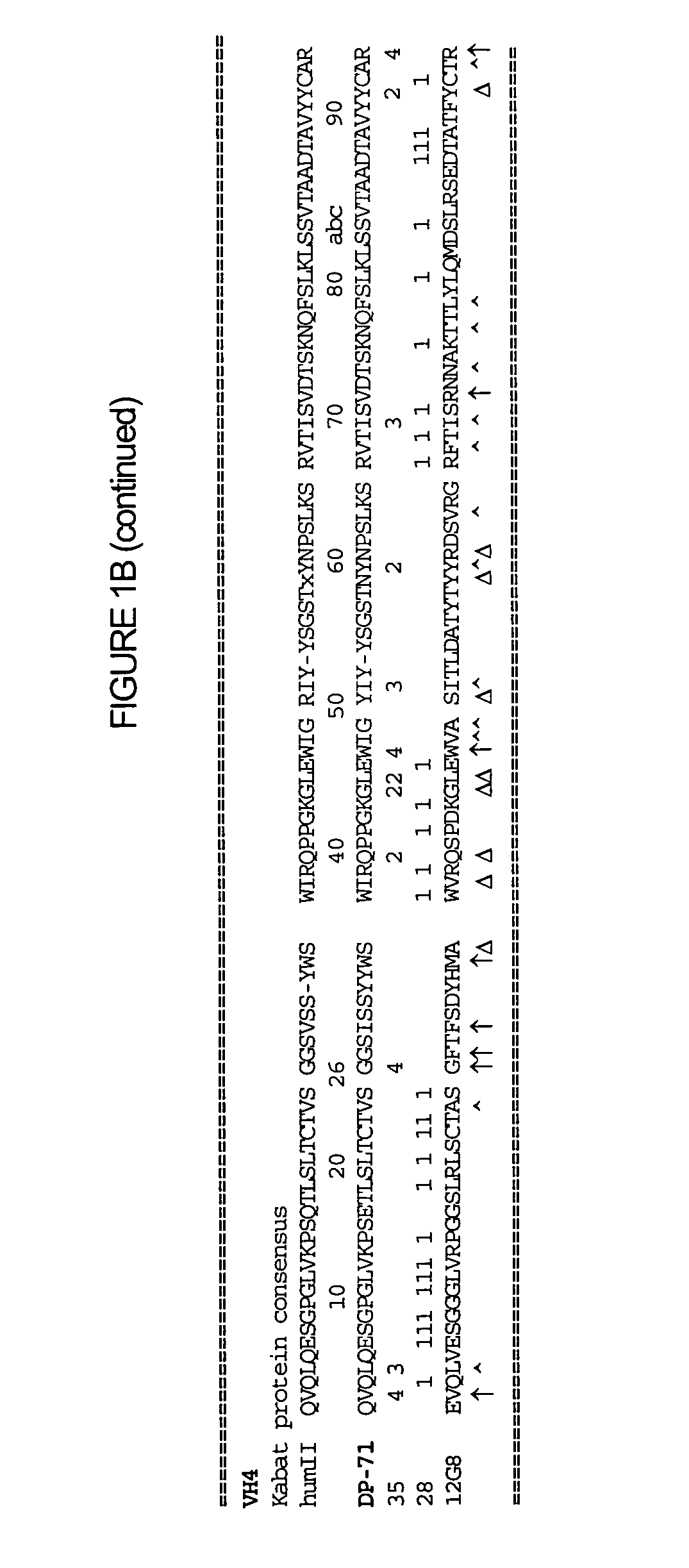
[0125] The humanization of the rat anti-human IL-10 antibody, 12G8, was performed as described in Section C supra. FIG. 1 shows the assignment of the assignment of residue numbers and corresponding numerical scores for residue positions that are identical to the germline sequences being examined. Calculations are shown for the 12G8 variable regions of the light (FIG. 1A) and heavy (FIG. 1B) of the 12G8 anti-human IL-10 antibody and for the variable regions of the light (FIG. 1C) and heavy (FIG. 1D) of the 11D8 anti-human IL-10 antibody.
example iii
Pharmacokinetics of 12G8, an Anti-Human IL-10 Antibody
[0126] Objective: To obtain estimates of in-vivo terminal half-lives and subcutaneous bioavailability for the 12F8 antibody in a murine model.
[0127] Antibody: The antibody is administered in a vehicle of 10 mM Na acetate, 8% sucrose, pH 5.25.
[0128] Mice: Crl:CD-1® (ICR)BR female mice were purchased from Charles River Laboratories.
[0129] Experimental Design: Mice received a single bolus injection of antibody either intravenously (i.v. in lateral tail vein) or subcutaneously (s.c. at nape of neck or mid-scapular or lateral flank). Antibody doses included 0.03, 0.3, 3.0, and 30 mg / kg per mouse. The mice were observed for up to 28 days post-injection. During this time period, mice were weighed and serum samples taken. Serum samples for the 12G8 (SCH 708980) groups (Groups 1-8) were taken at 0.5, 1, 3, 6, 10, 16 hrs, Day 1, 2, 3, 5, 7, 10, 14, 21, and 28 post-injection using 5 mice / time point. In the vehicle group (Group 9), serum...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com