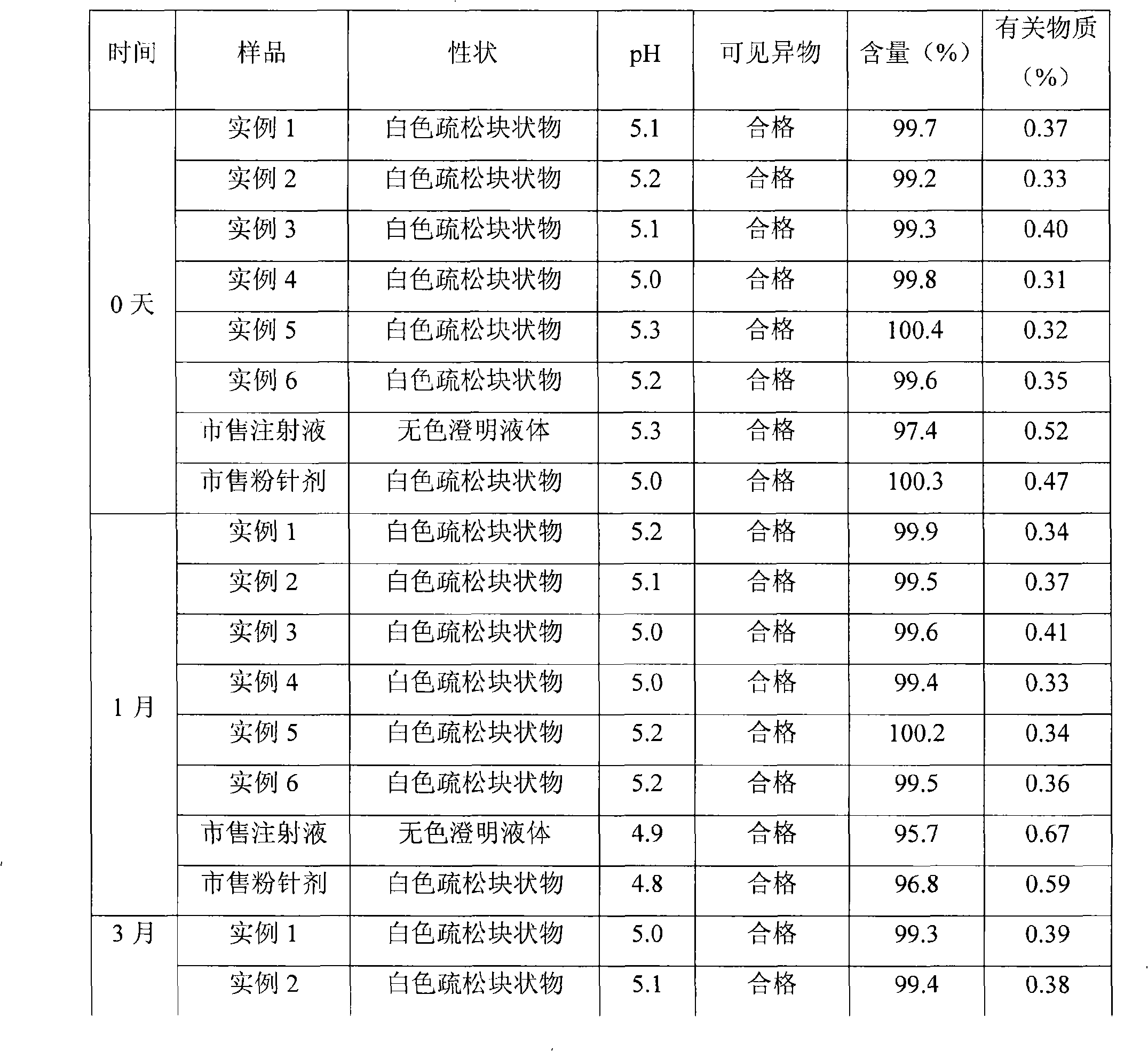
Patents
Literature
92 results about "Bolus injection" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Bolus injection is a delivery of an agent in a single bolus usually over a few seconds as opposed to infusion where the agent is delivered over a longer period and lower flow rate (e.
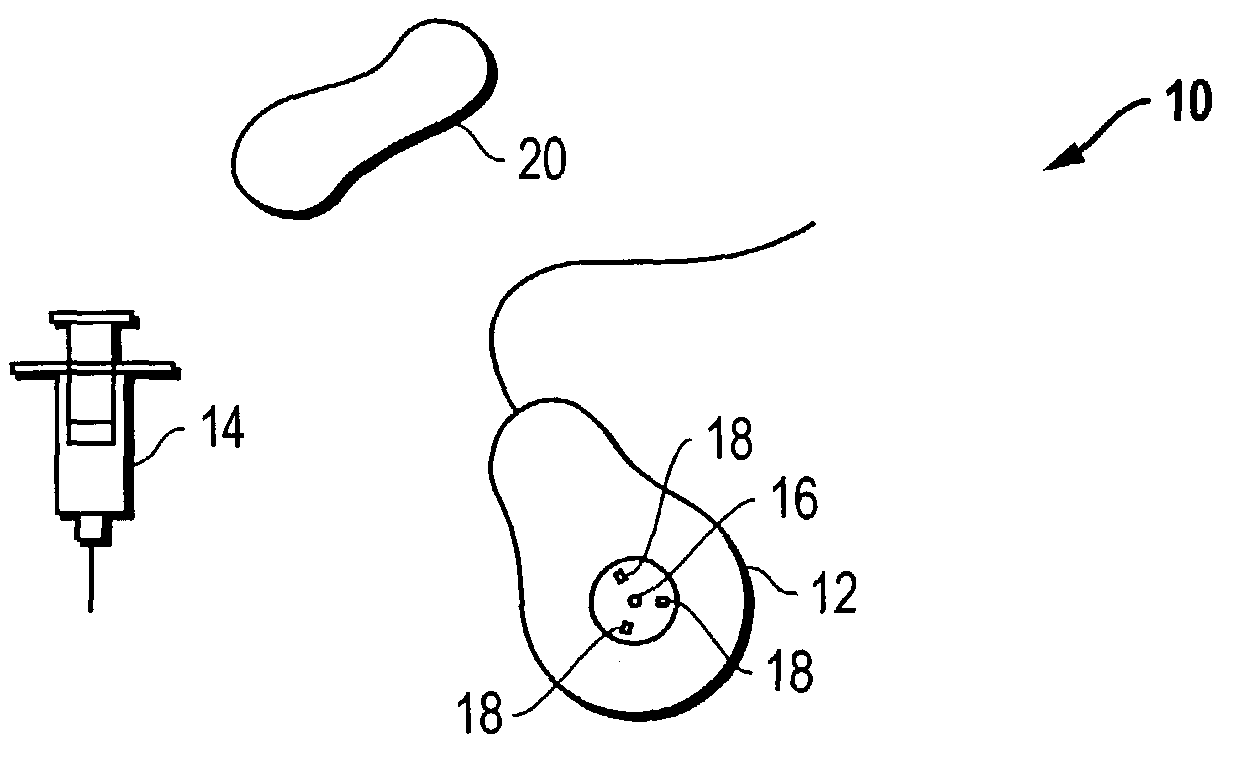
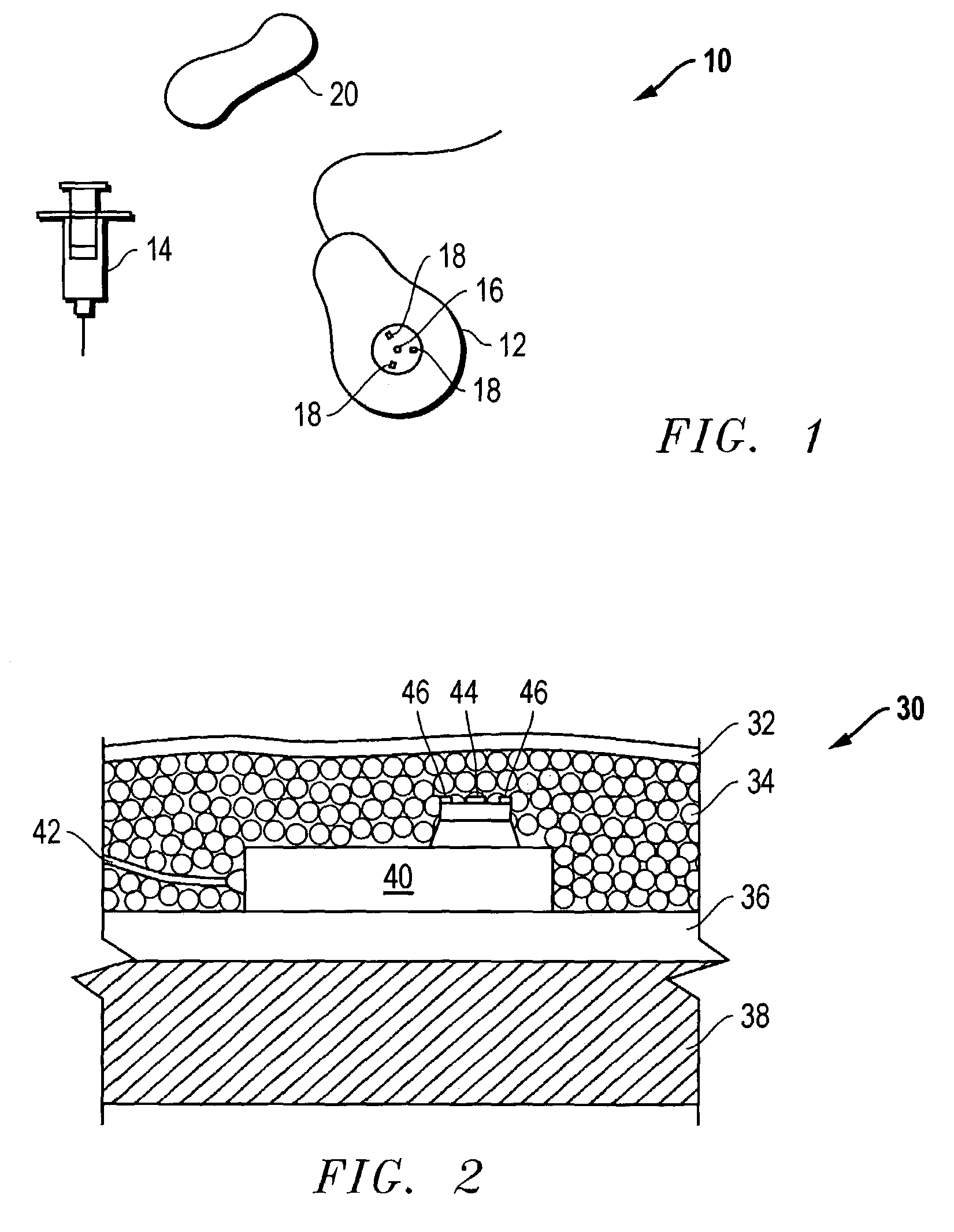
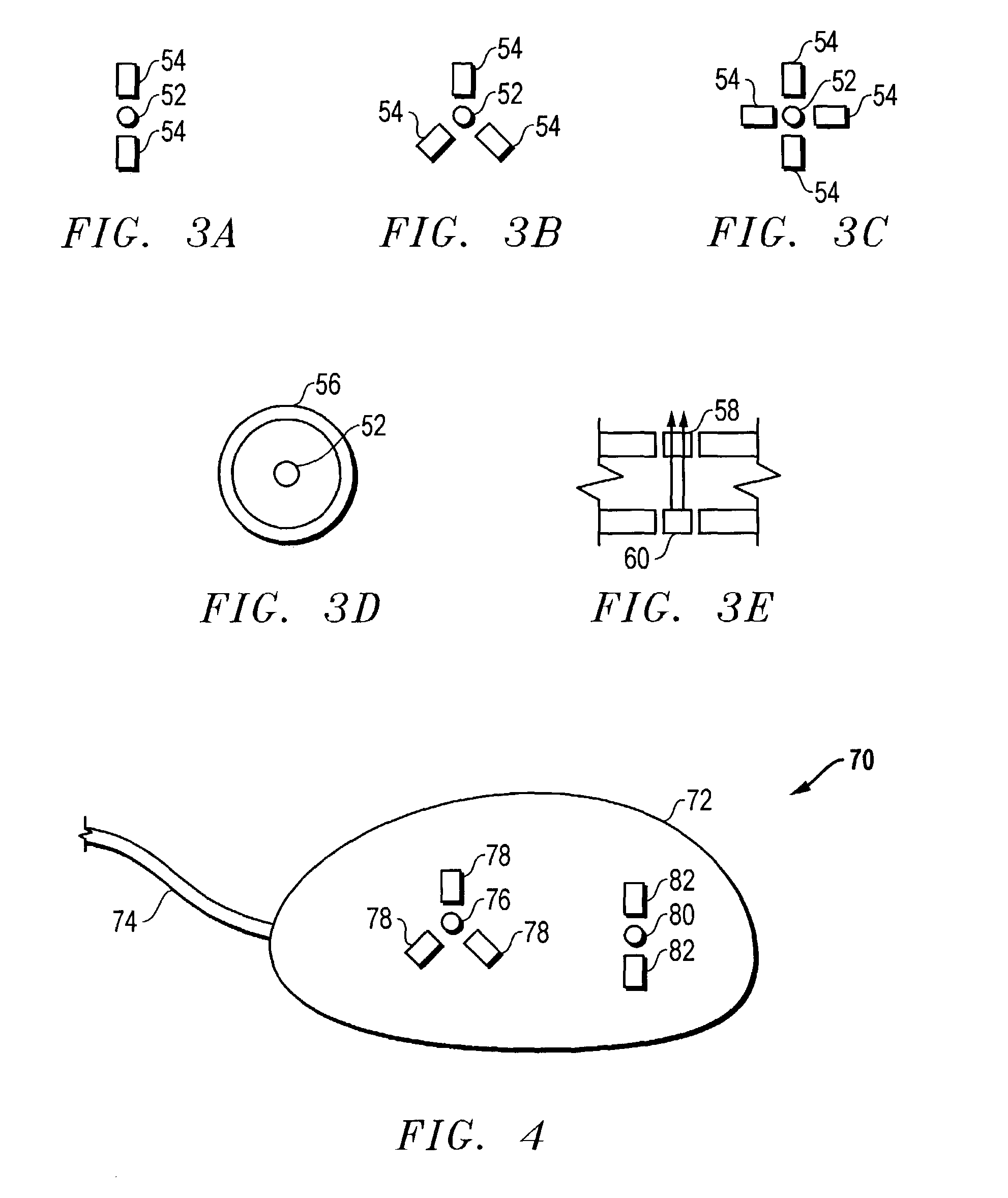
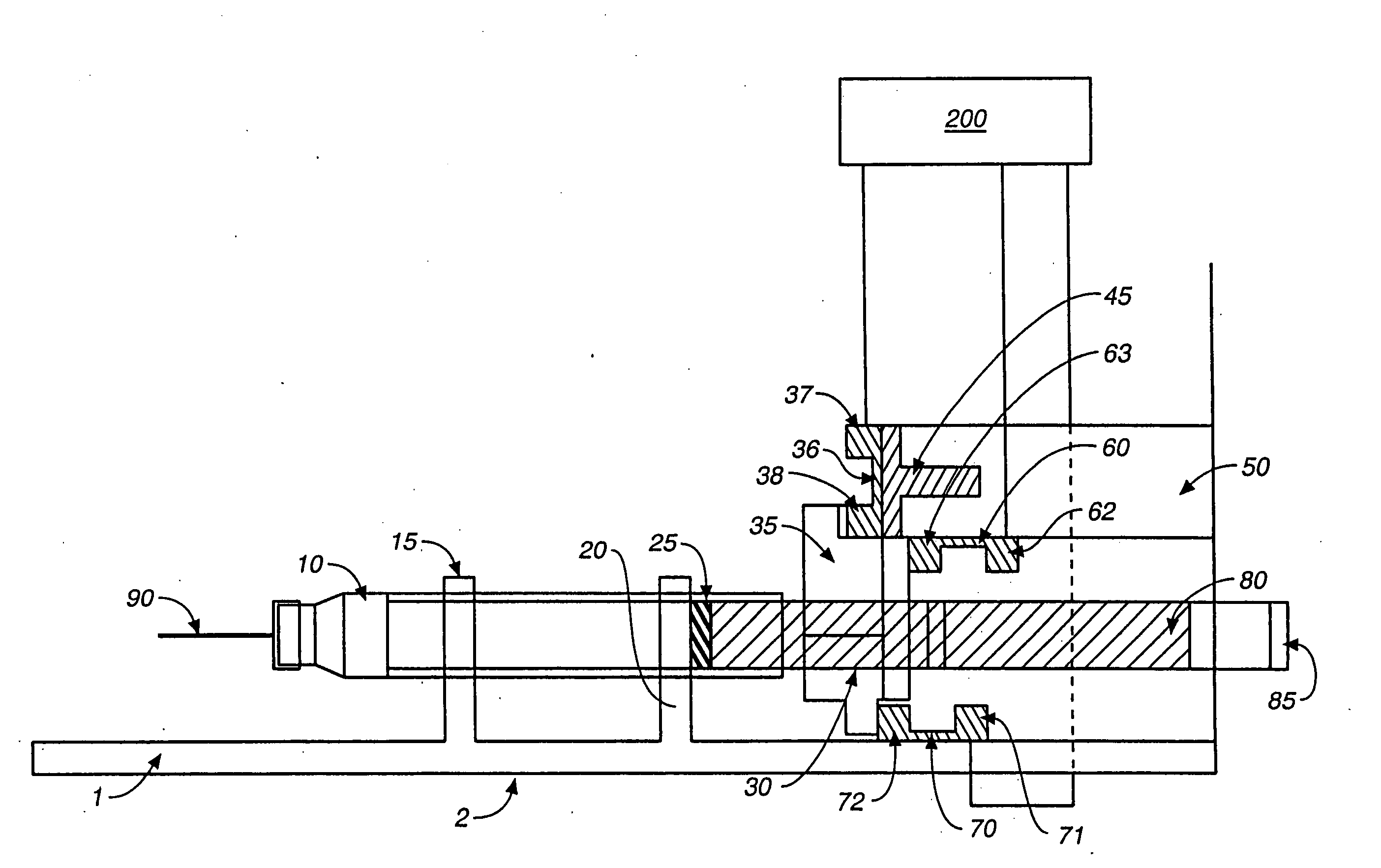
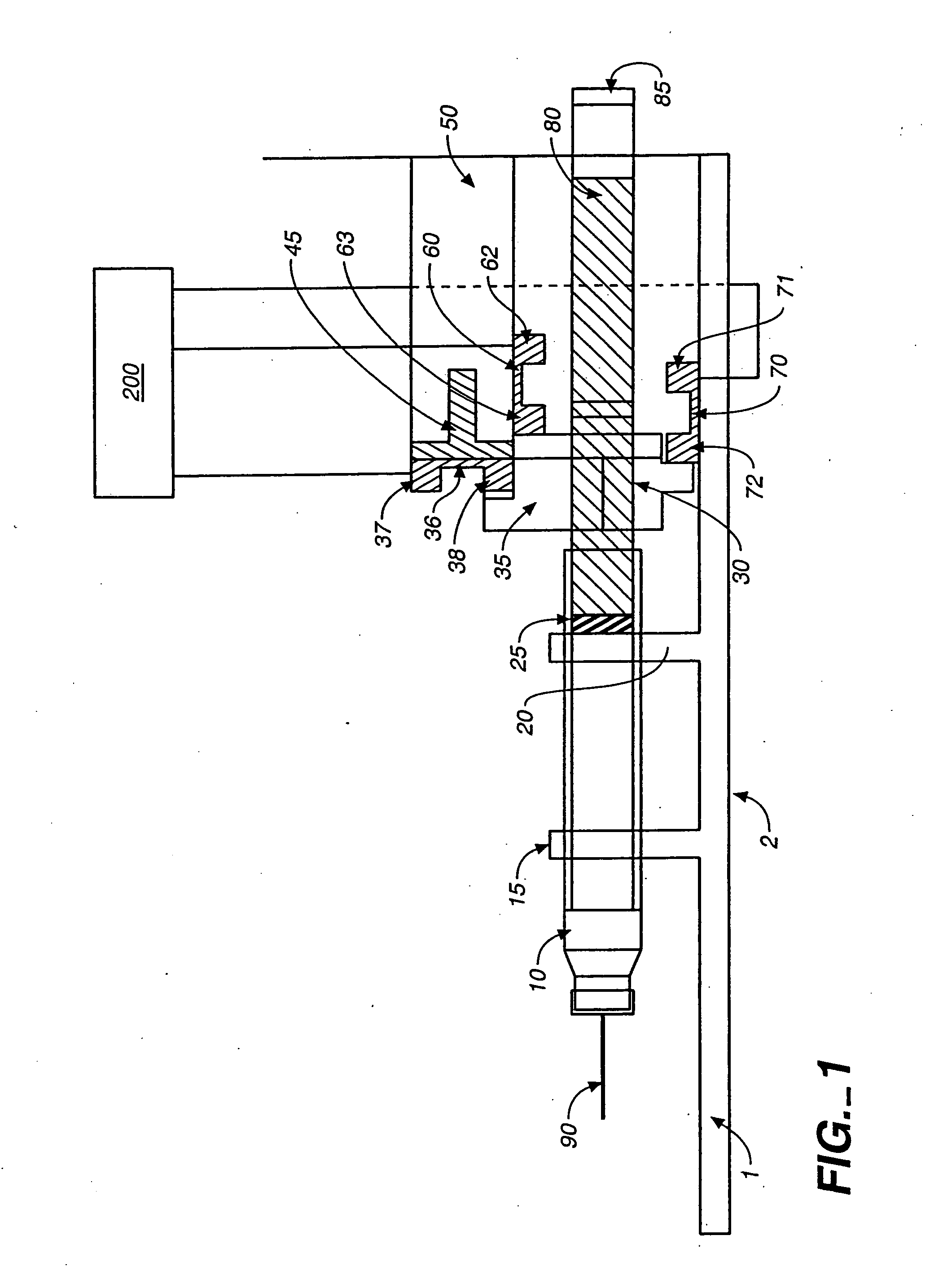
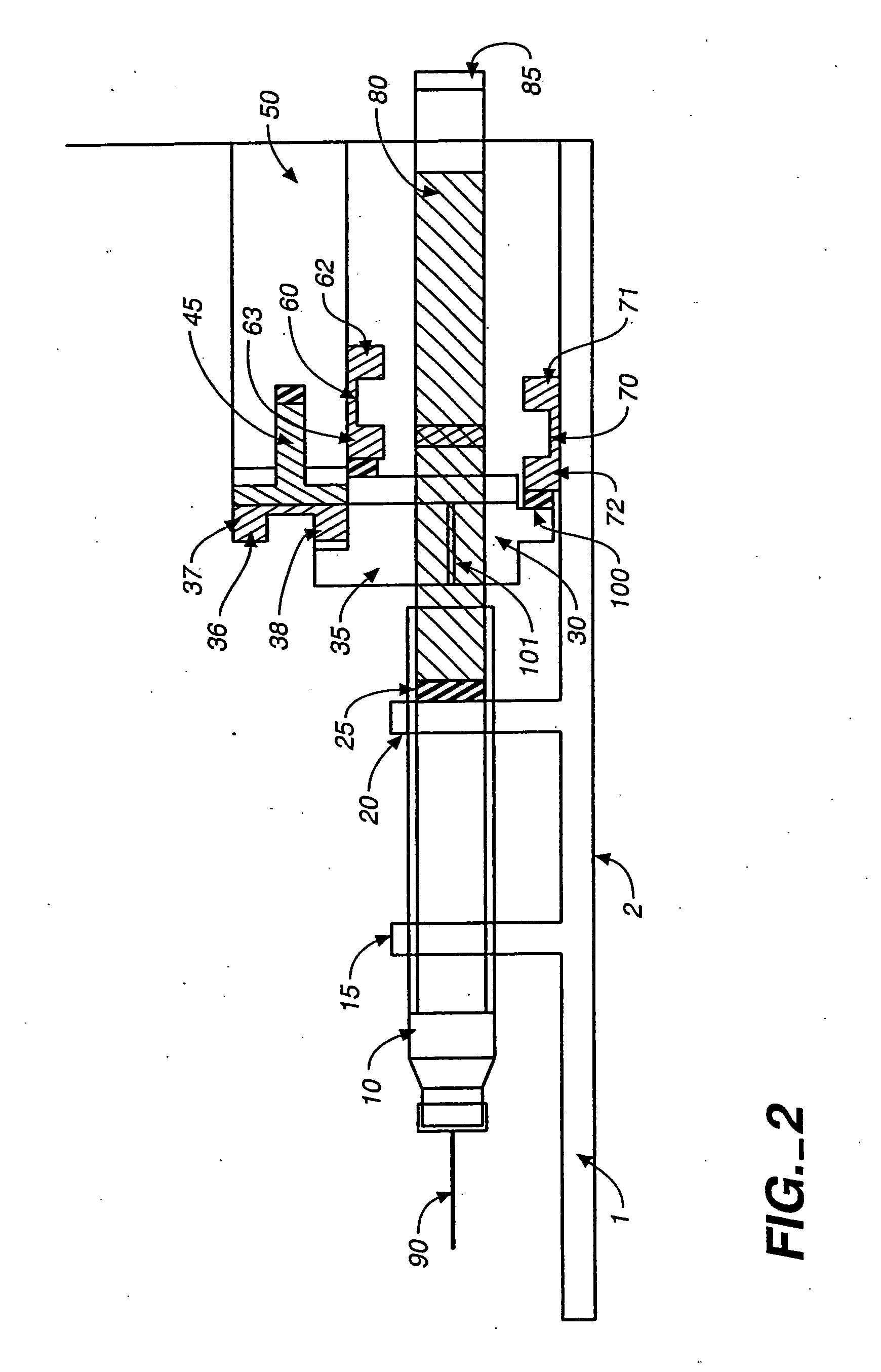
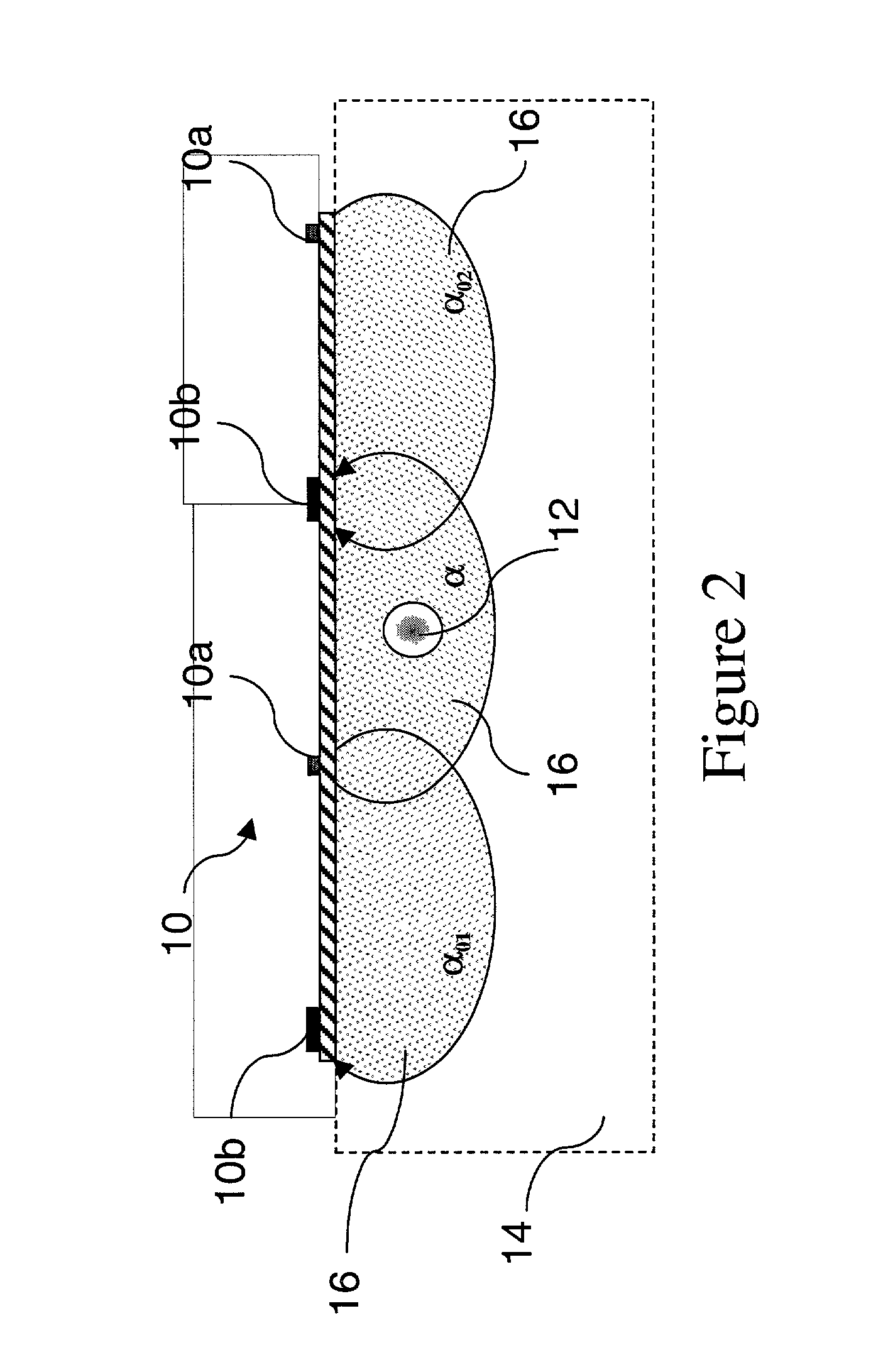
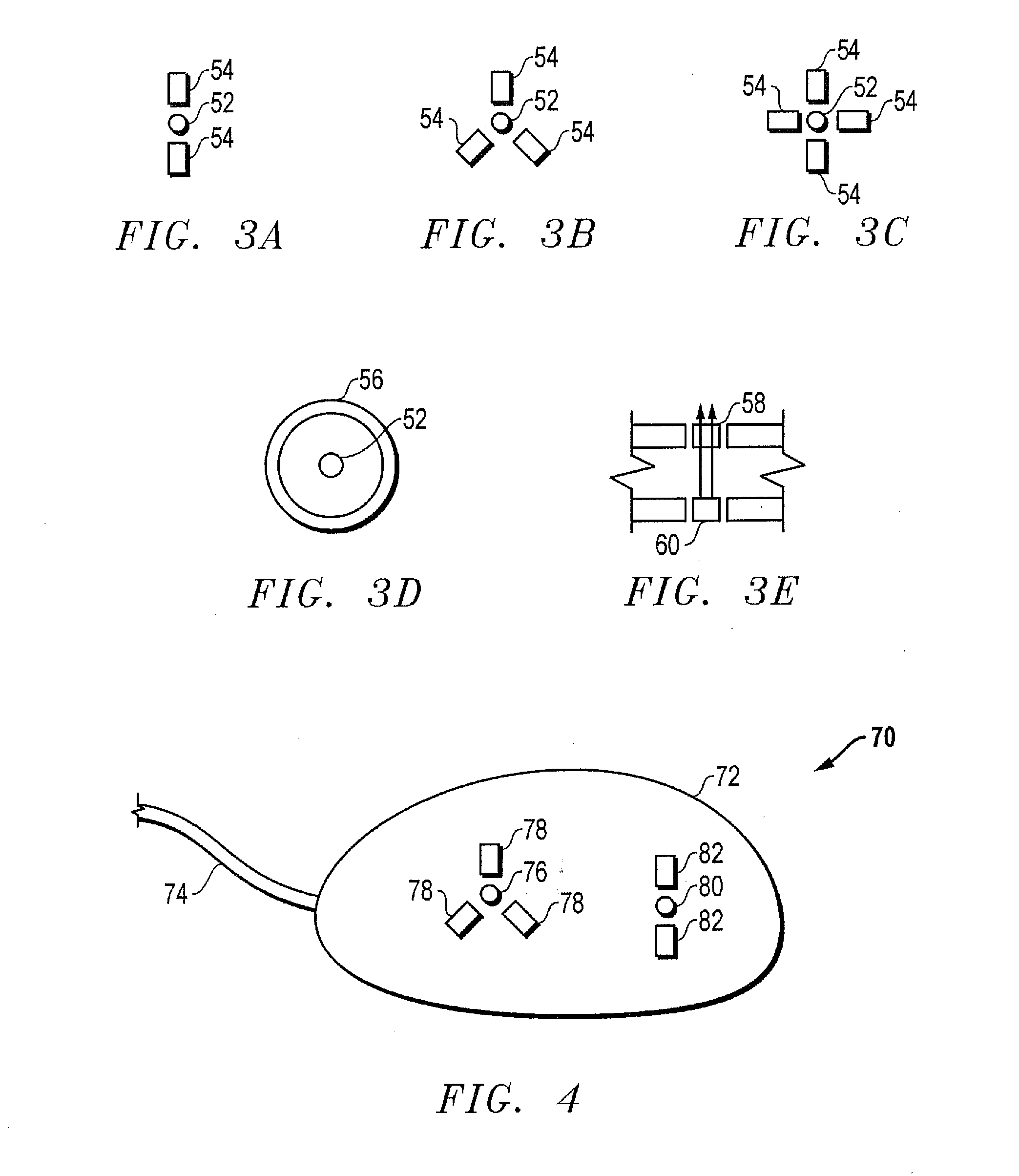
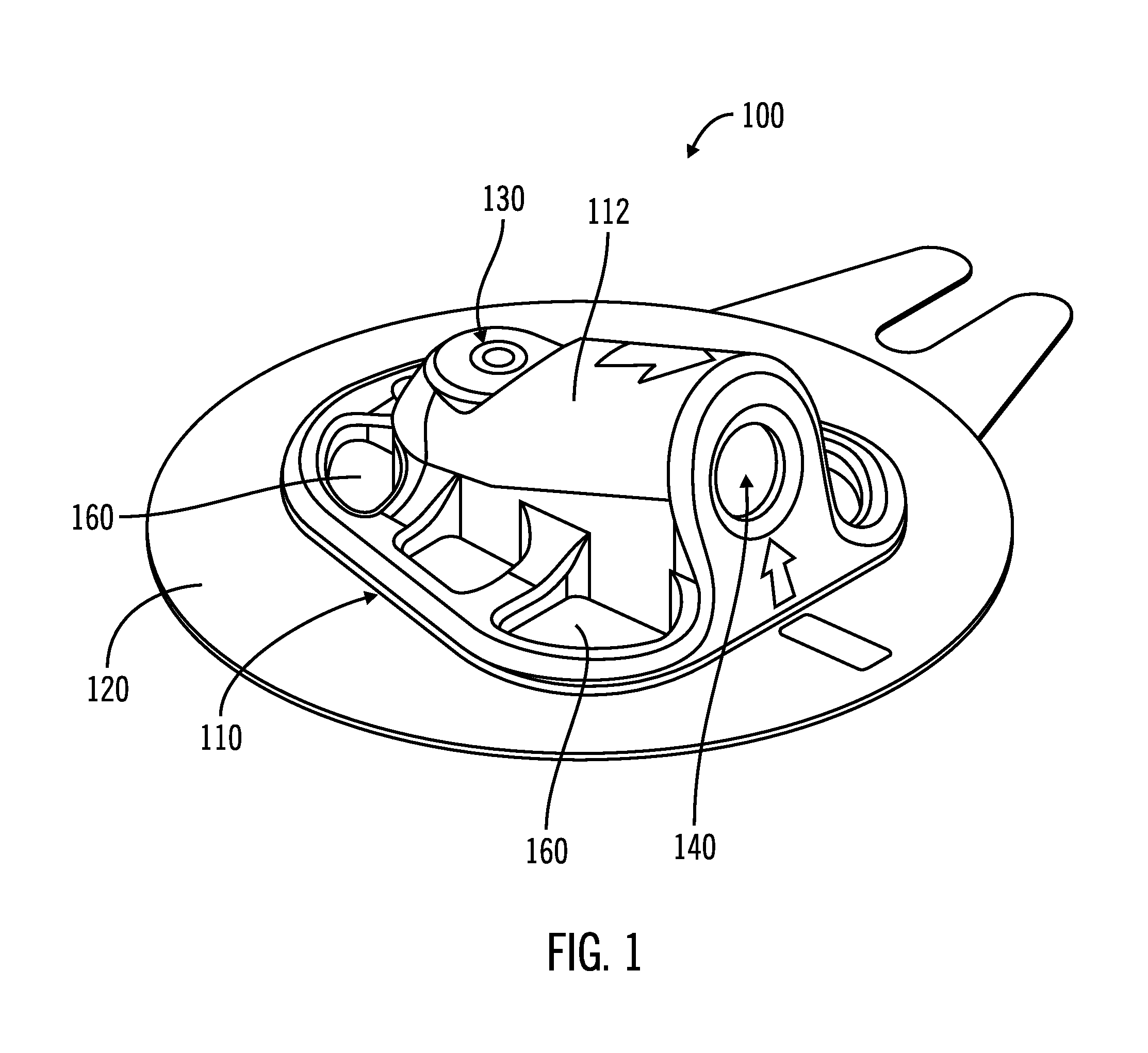
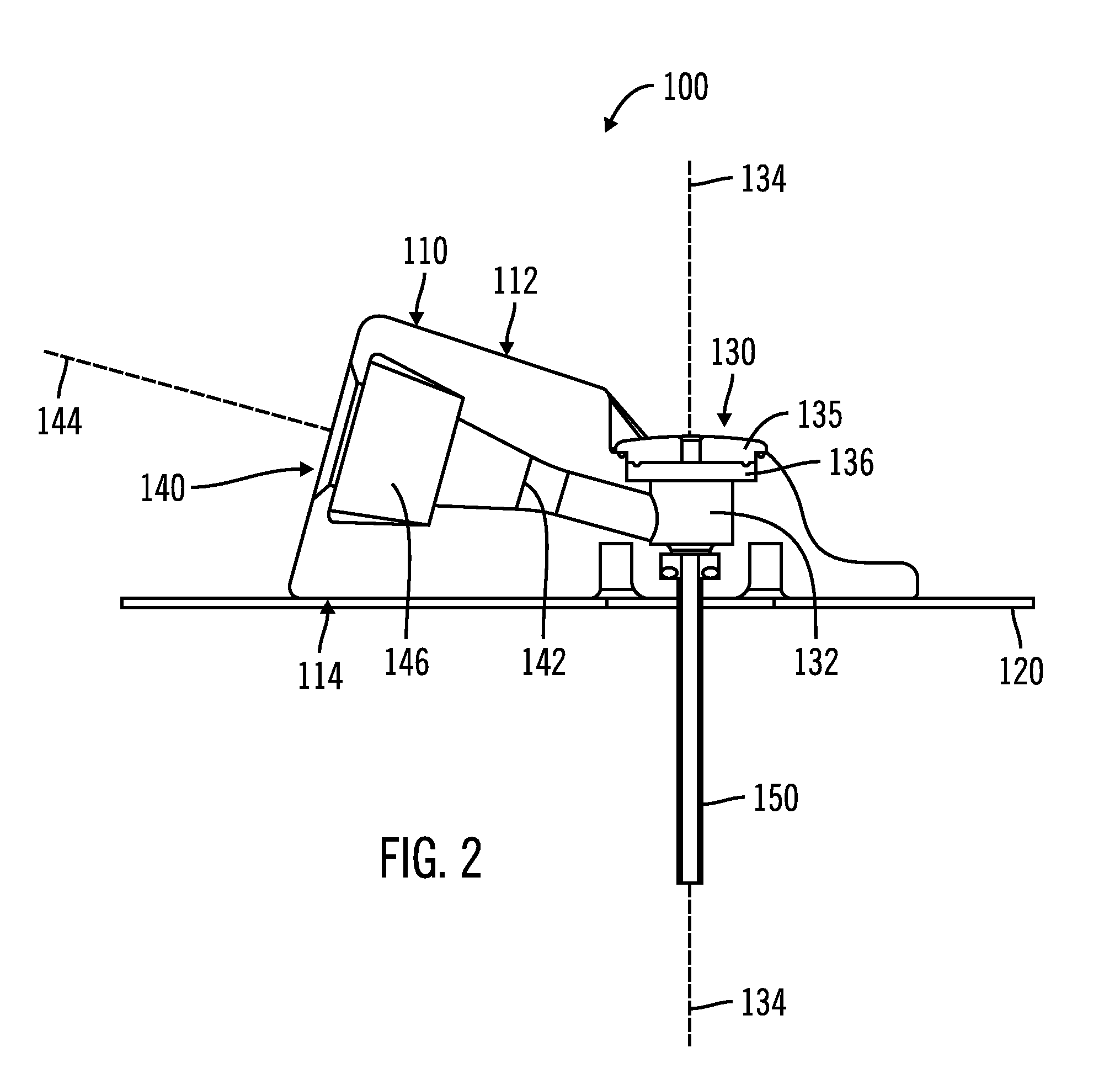
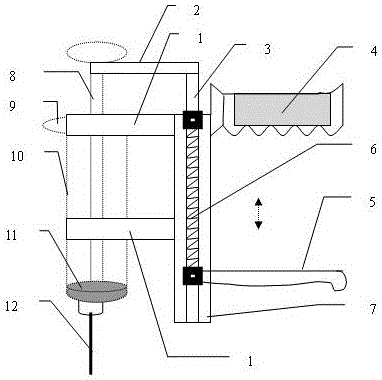
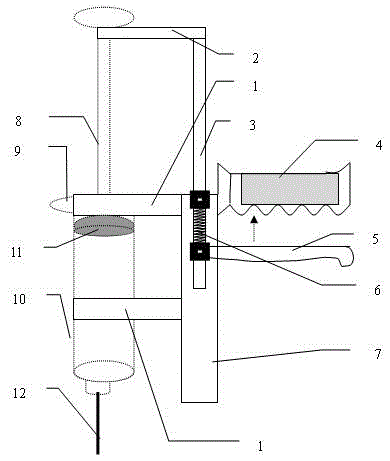
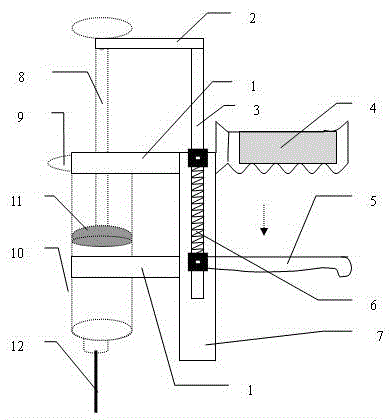
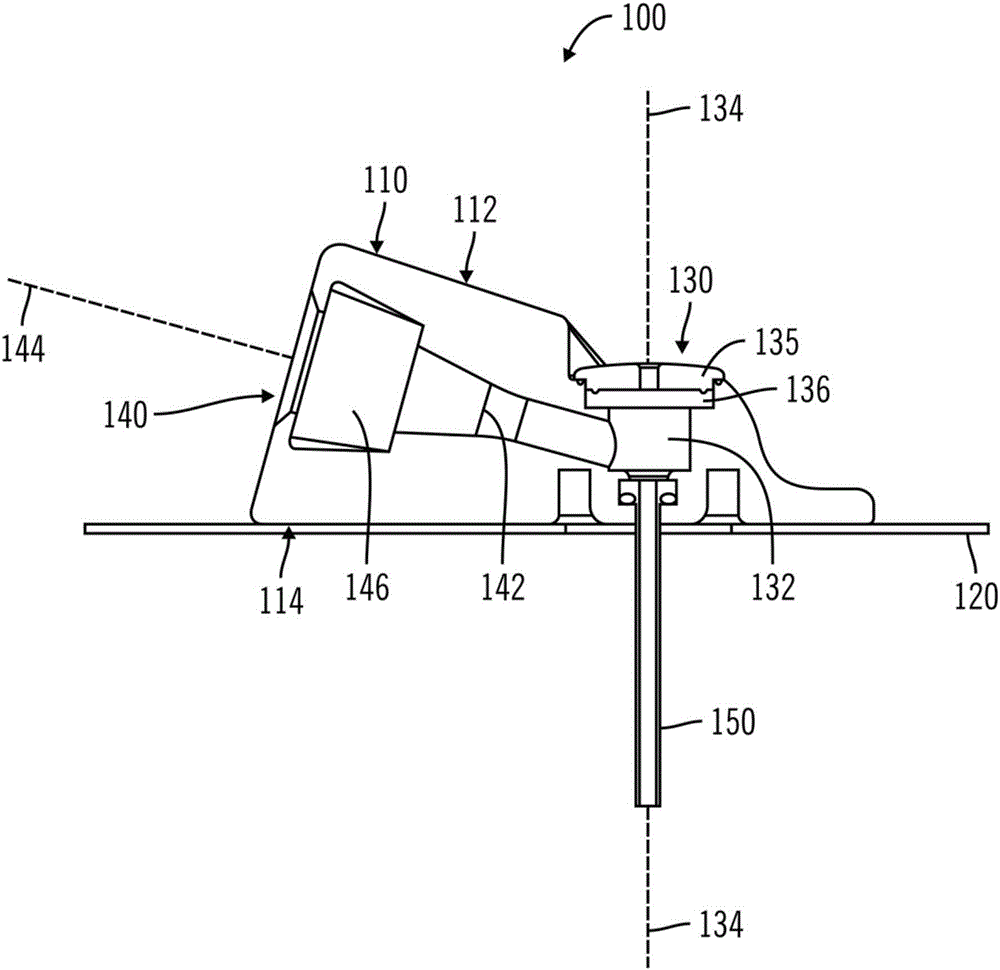
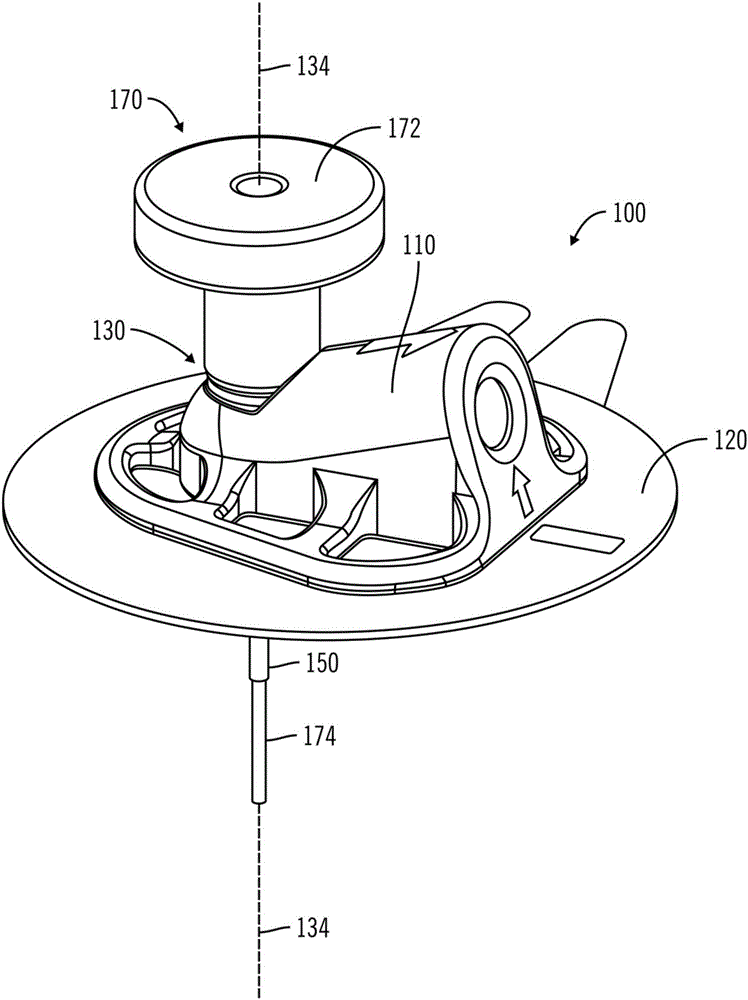
Access port indicator for implantable medical device
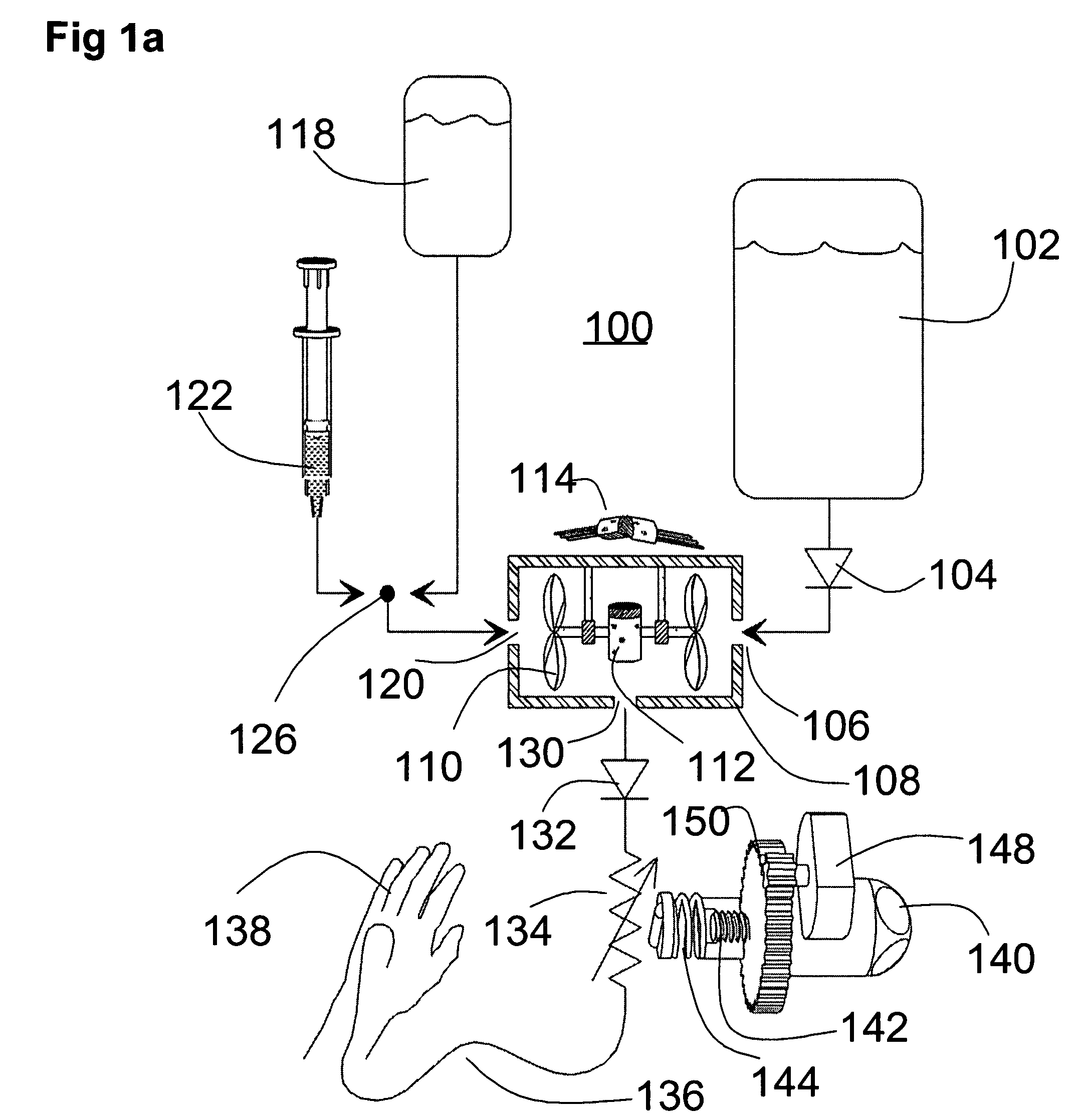
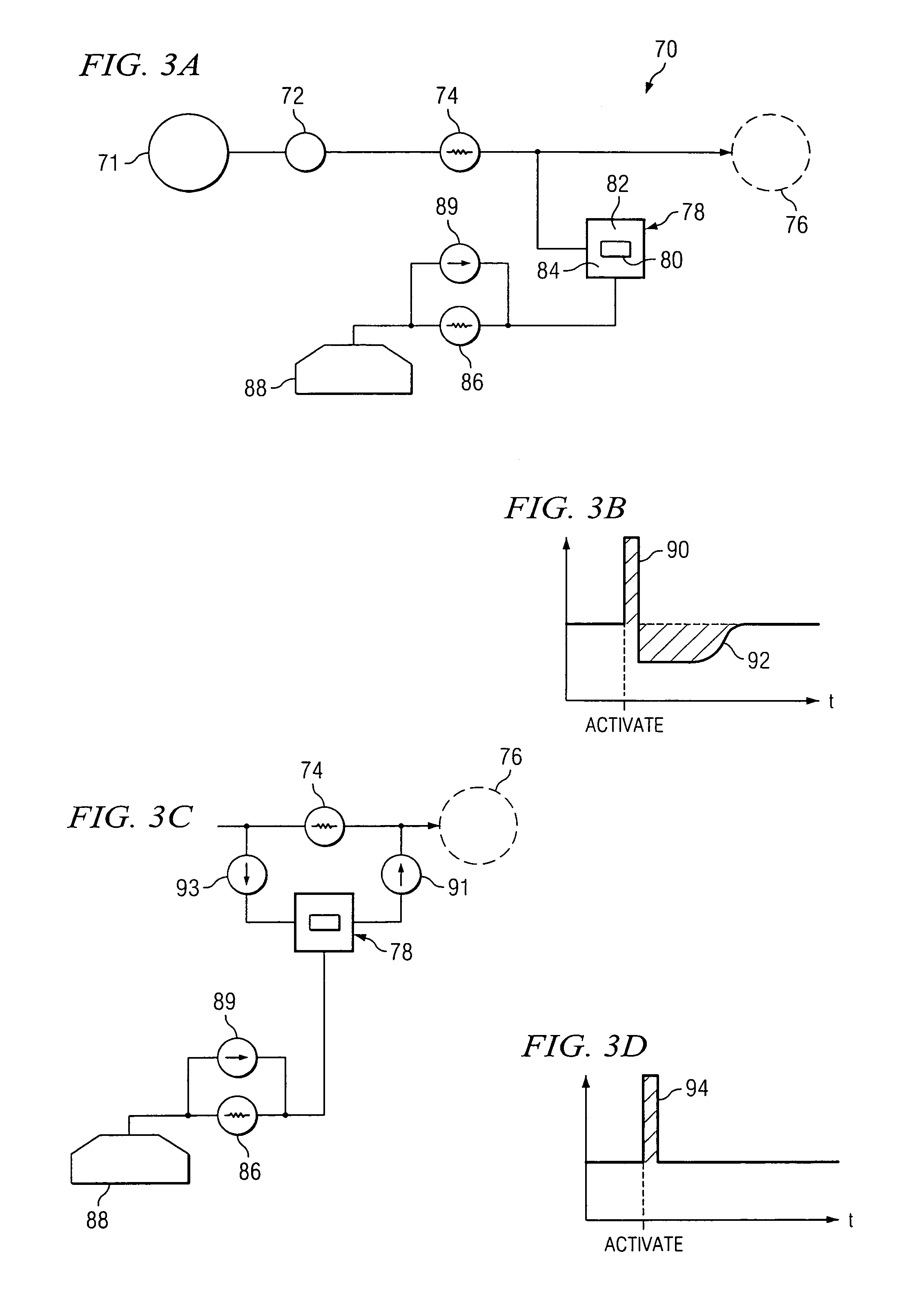
The invention is directed to an implantable pump. The implantable pump has a port with light emitters indicating the location of the port. The port can be useful in providing bolus injections or the refilling of reservoirs. The light emitters can be arranged in various forms about the port including linear, triangular, square, or circular, among others. If more than one port is located on a device, these ports can be differentiated by differing colors and arrangements of emitters. In addition, various circuitries can be used to activate the emitters. These circuitries can include a coil and capacitor arrangement that provide a separate power source from that of the pump.
Owner:ADVANCED NEUROMODULATION SYST INC
Automated fluid flow control system
A device for control of fluid flow with closed loop quasi static adjustment of in-line pressure-based resistance. The disclosed device and methods of using the device provide for the ability to control a flow rate with a highly portable device; the ability for an operator to safely use the device with minimal training; the ability to automatically switch between a primary and secondary fluid; the ability to maintain a flow rate through a tubing set even when the control device is removed; and the ability to measure fluids introduced with a manual bolus injection.
Owner:FRESENIUS KABI USA LLC
Methods for administering aripiprazole
InactiveUS20050032811A1Without complexityWithout expenseOrganic active ingredientsNervous disorderActive agentMicrosphere
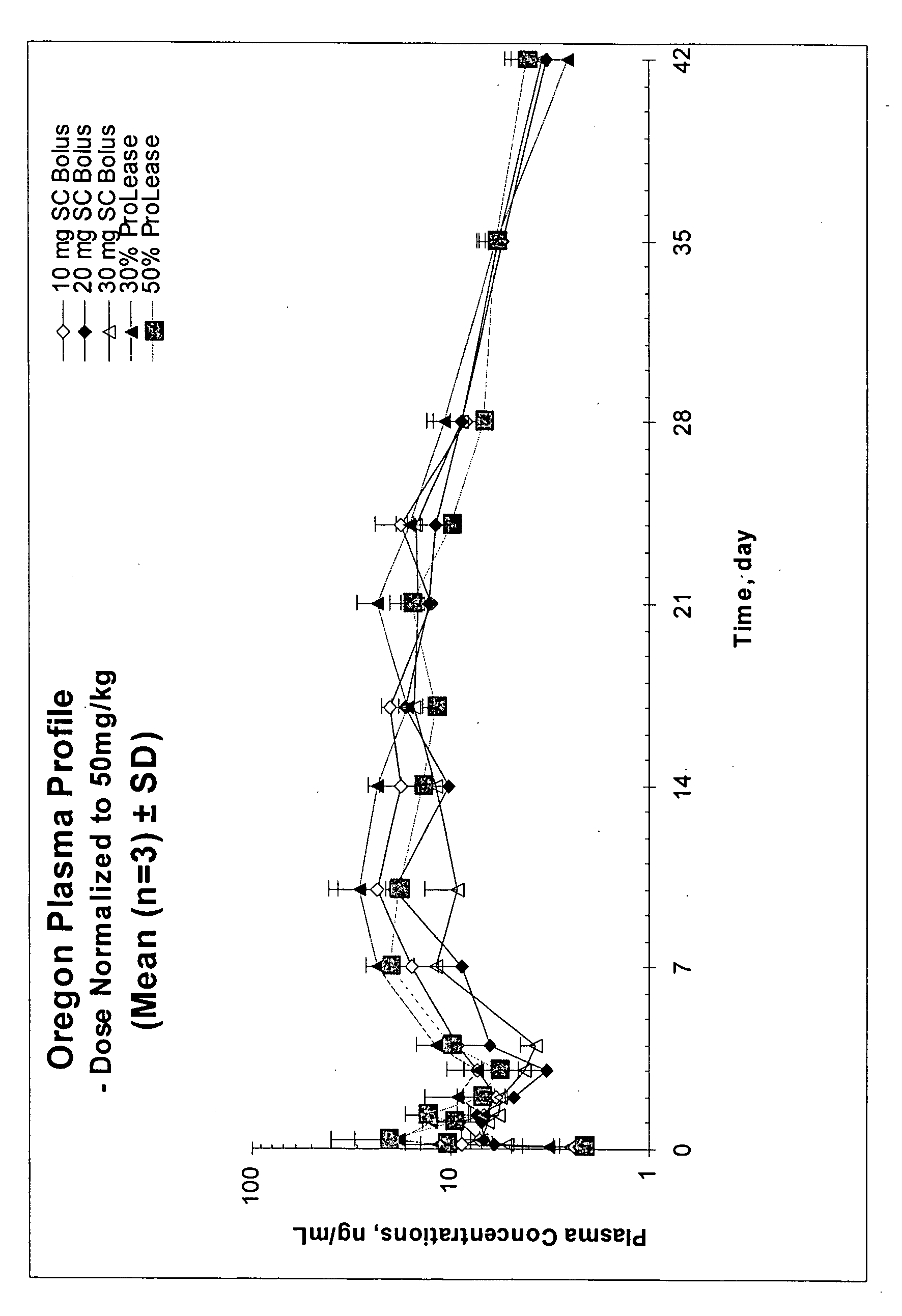
The present invention relates, in part, to the discovery that a pharmaceutical composition comprising aripiprazole and a carrier administered in a bolus injection resulted in an extended release profile similar to that obtained by the injection of a poly lactide-co-glycolide microsphere formulation containing the active agent. This surprising result suggests that pharmacologically beneficial extended release formulations without the complexities and expense associated with the manufacture microspheres.
Owner:ALKERMES INC
Amylin formulations
InactiveUS20080248999A1Reduce in quantityAct quicklyPeptide/protein ingredientsMetabolism disorderGLP-1 MimeticsBefore Breakfast
A combined insulin and amylin and / or GLP-1 mimetic formulation has been developed wherein the pH of the insulin is decreased so that the amylin and / or GLP-1 remains soluble when mixed together with the insulin. In the preferred embodiment, a bolus insulin is administered by injection before breakfast, providing adequate bolus insulin levels to cover the meal, without producing hypoglycemia after the meal. This can be combined with an adequate basal insulin for 24 hours. Lunch and dinner can be covered by two bolus injections of the insulin and amylin and / or GLP-1 mimetic combination. A GLP-1 mimetic may be combined with either rapid acting or basal insulin formulations. As a result, a patient using the combination formulation therapy may only need to inject half as many times per day.
Owner:BIODEL INC
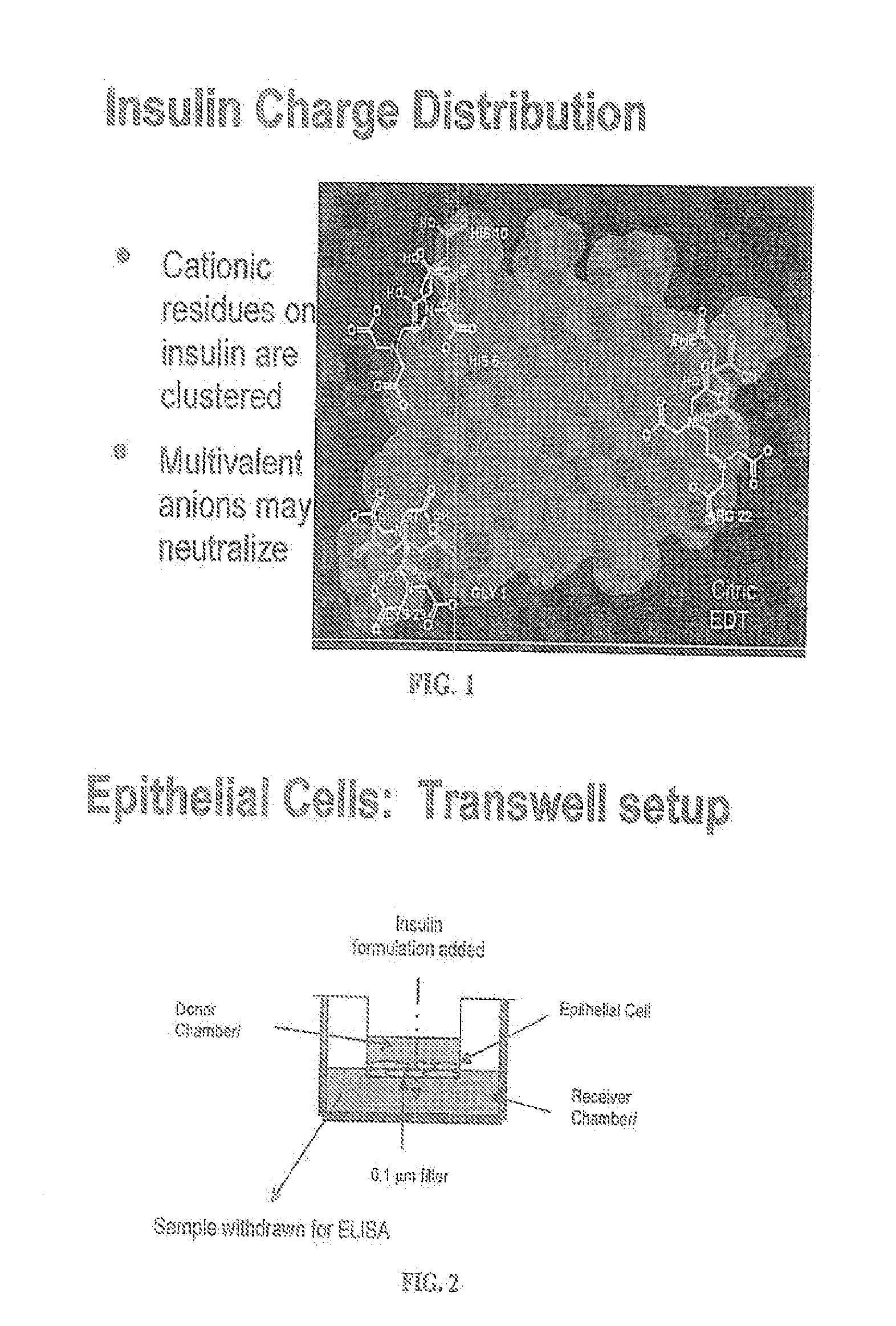
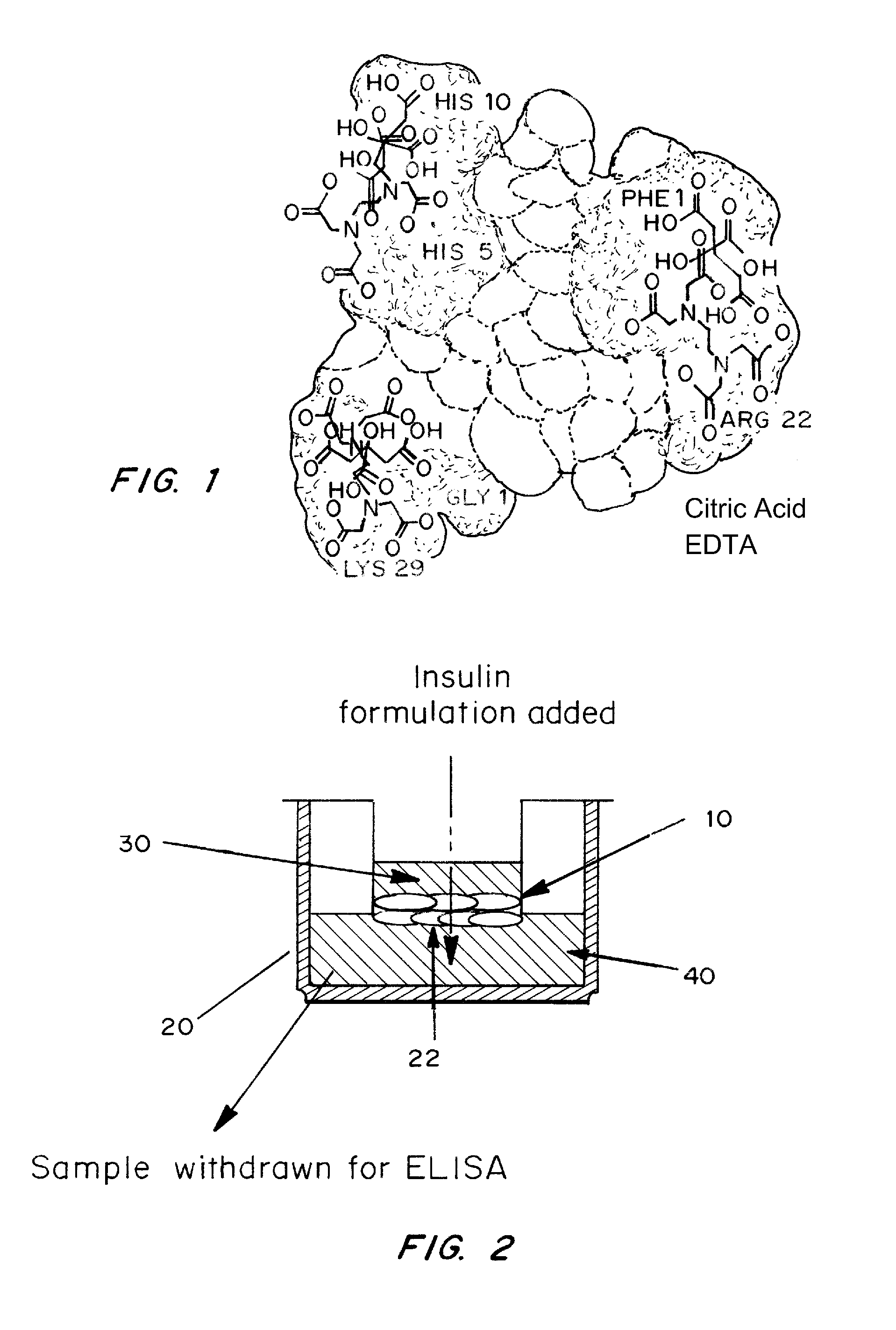
Rapid acting and long acting insulin combination formulations
ActiveUS20080039368A1Increase speedReduce the amount of solutionPeptide/protein ingredientsMetabolism disorderBefore BreakfastIntensive insulinotherapy
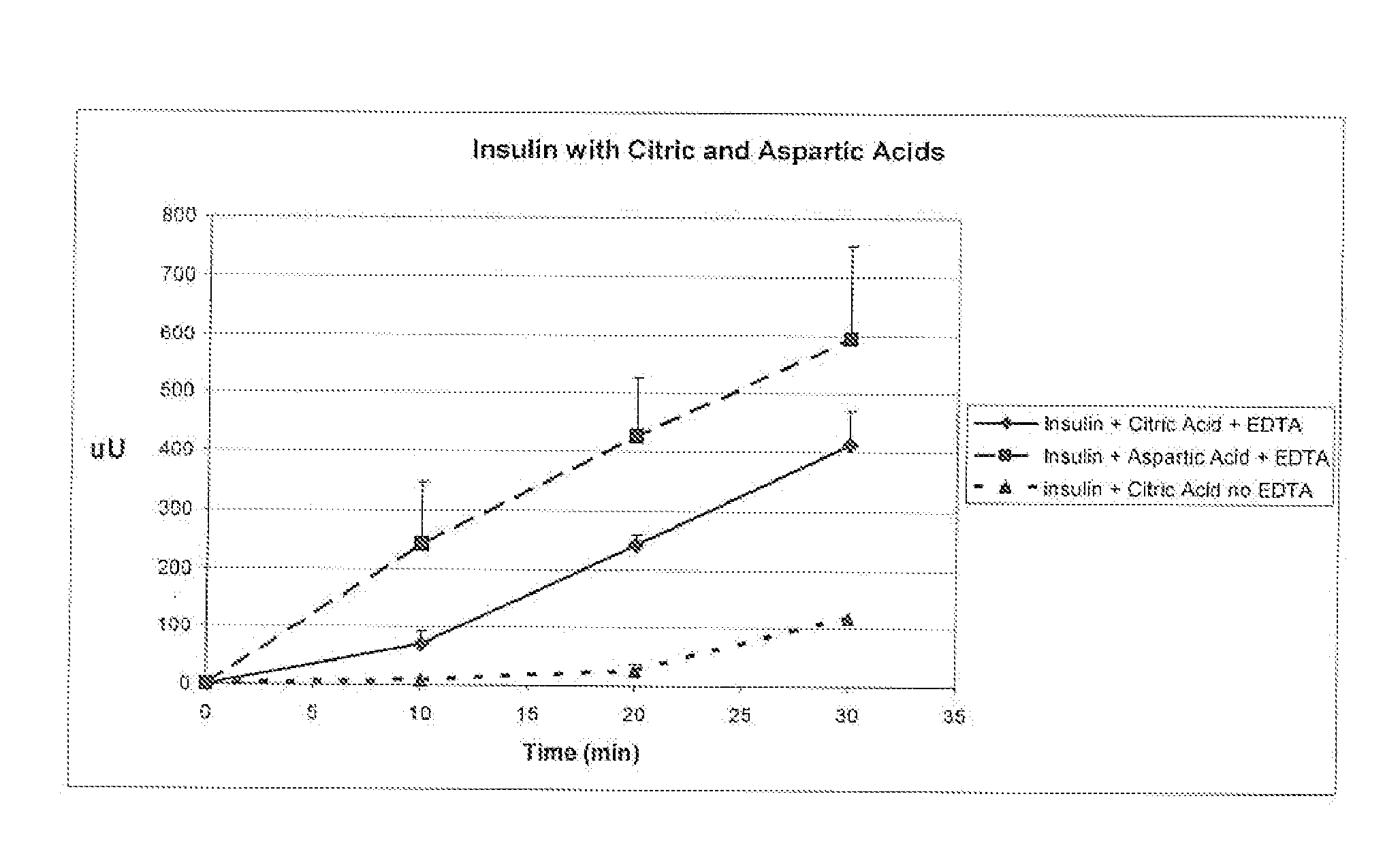
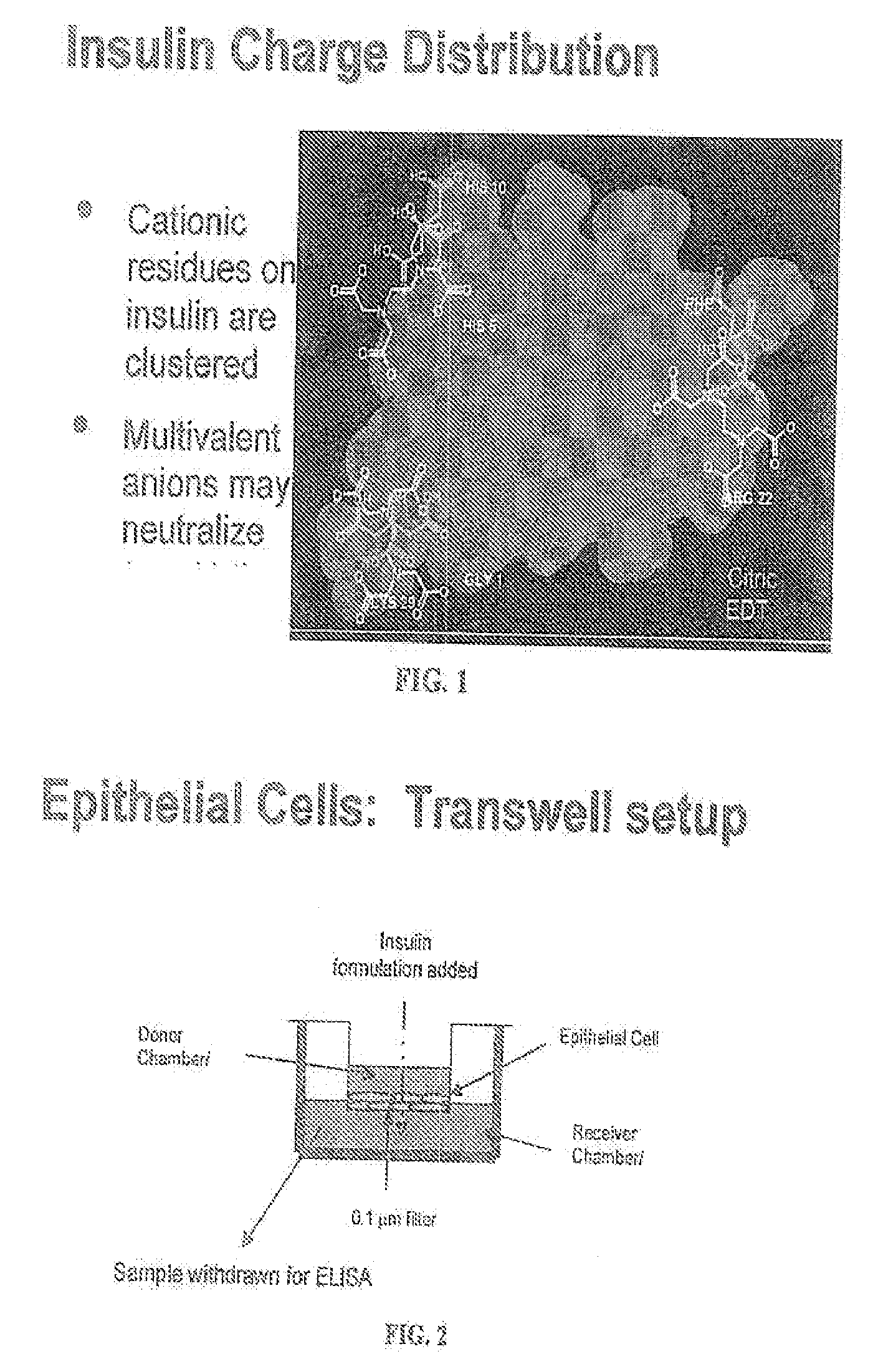
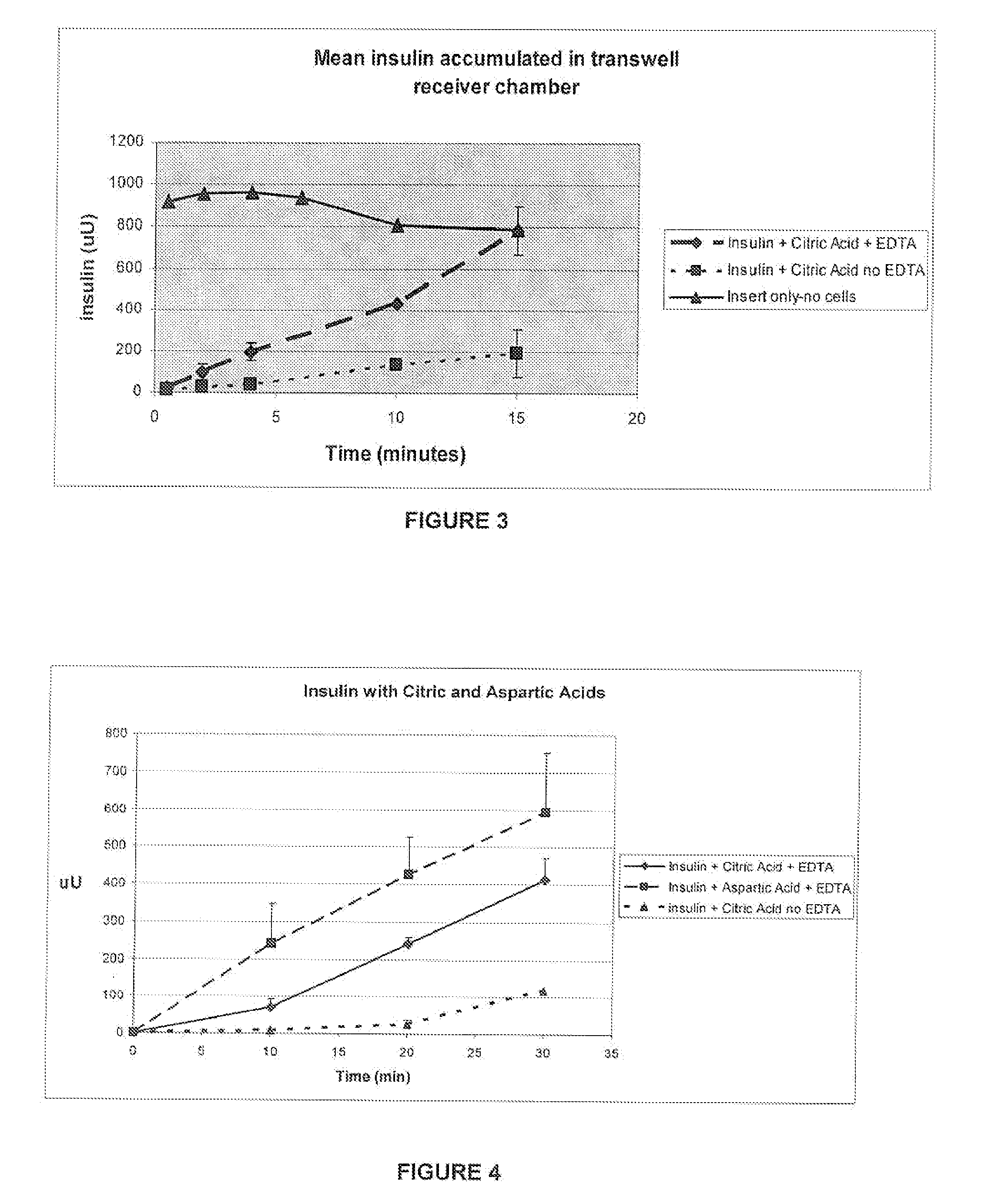
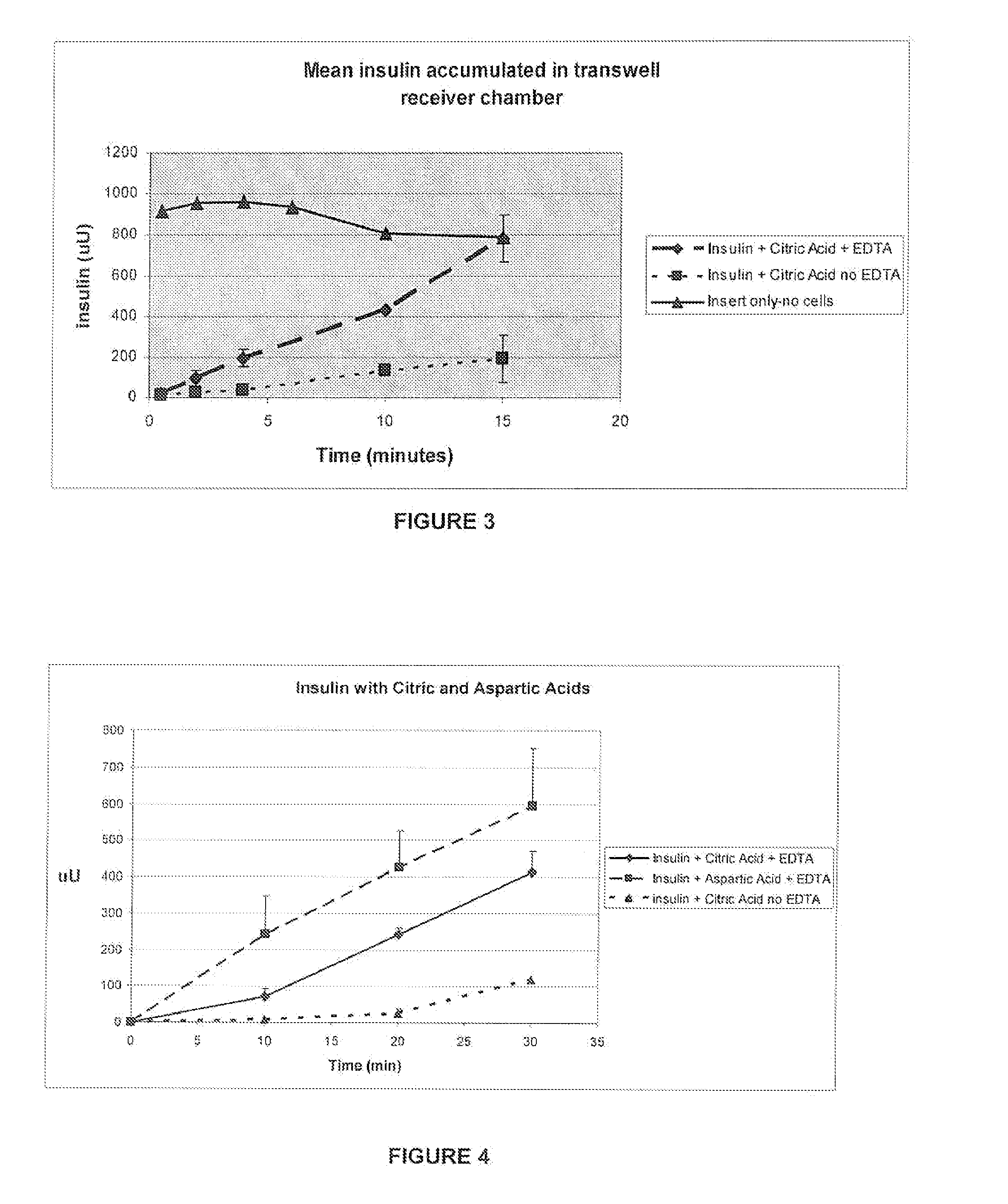
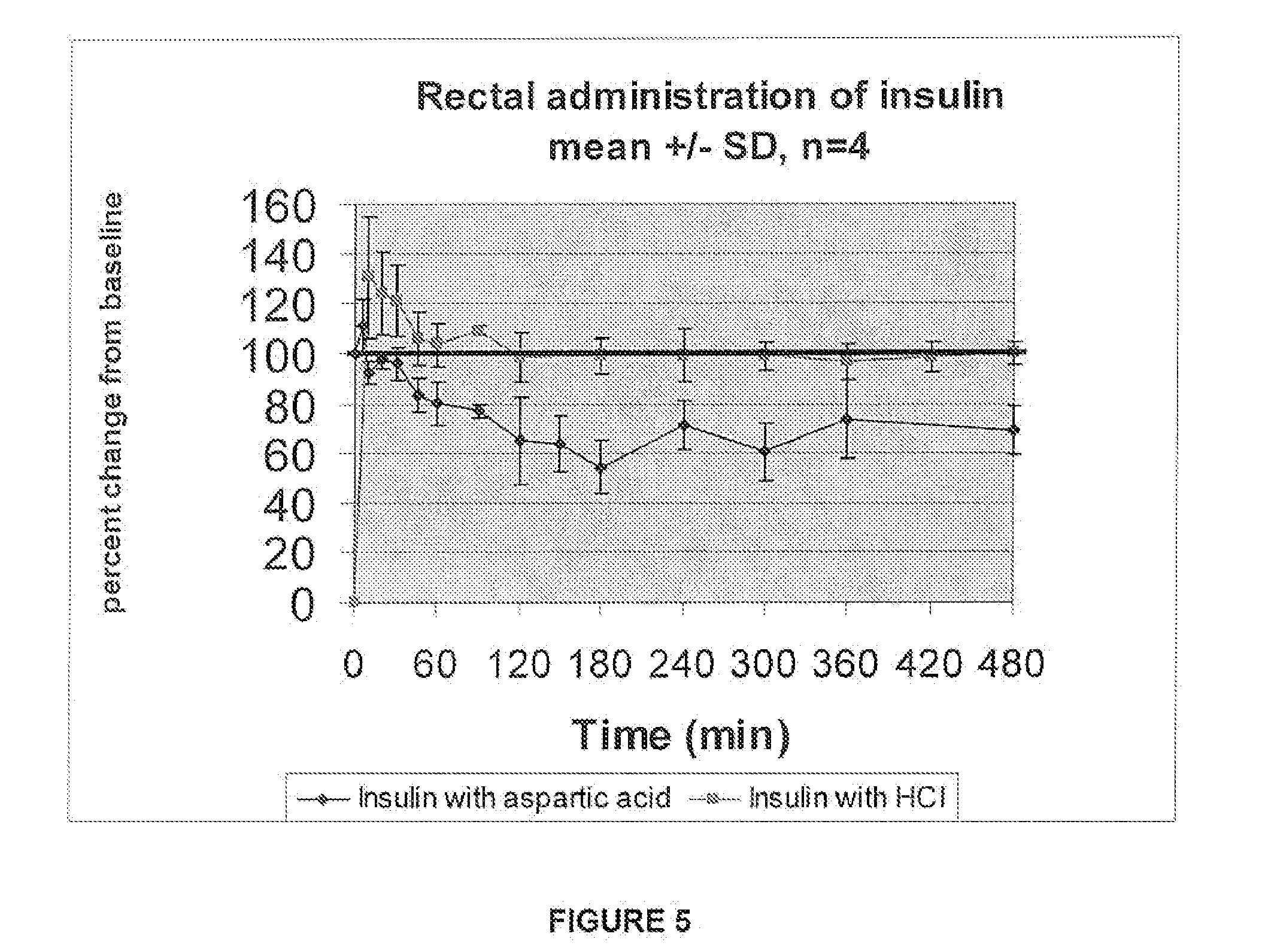
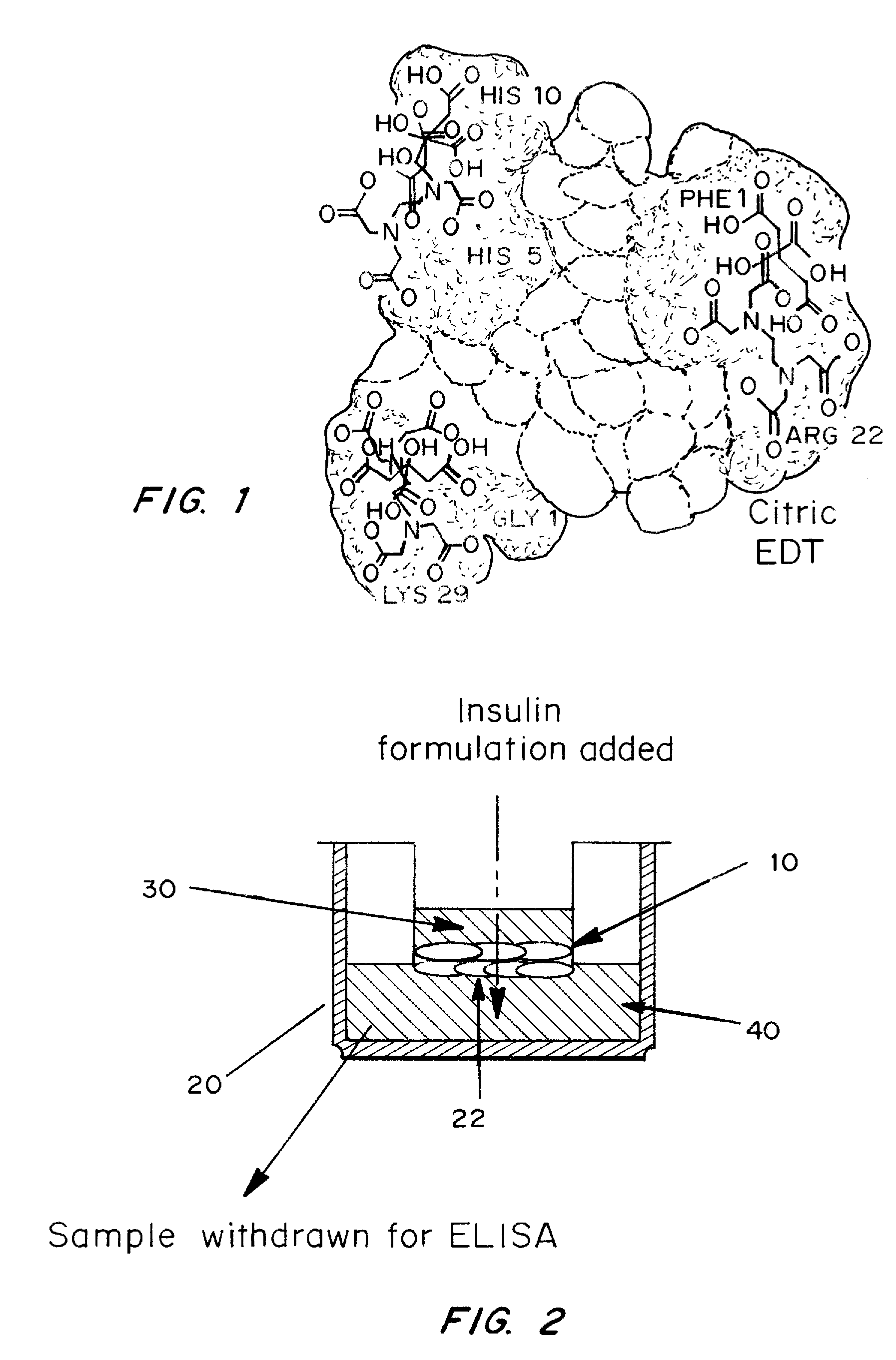
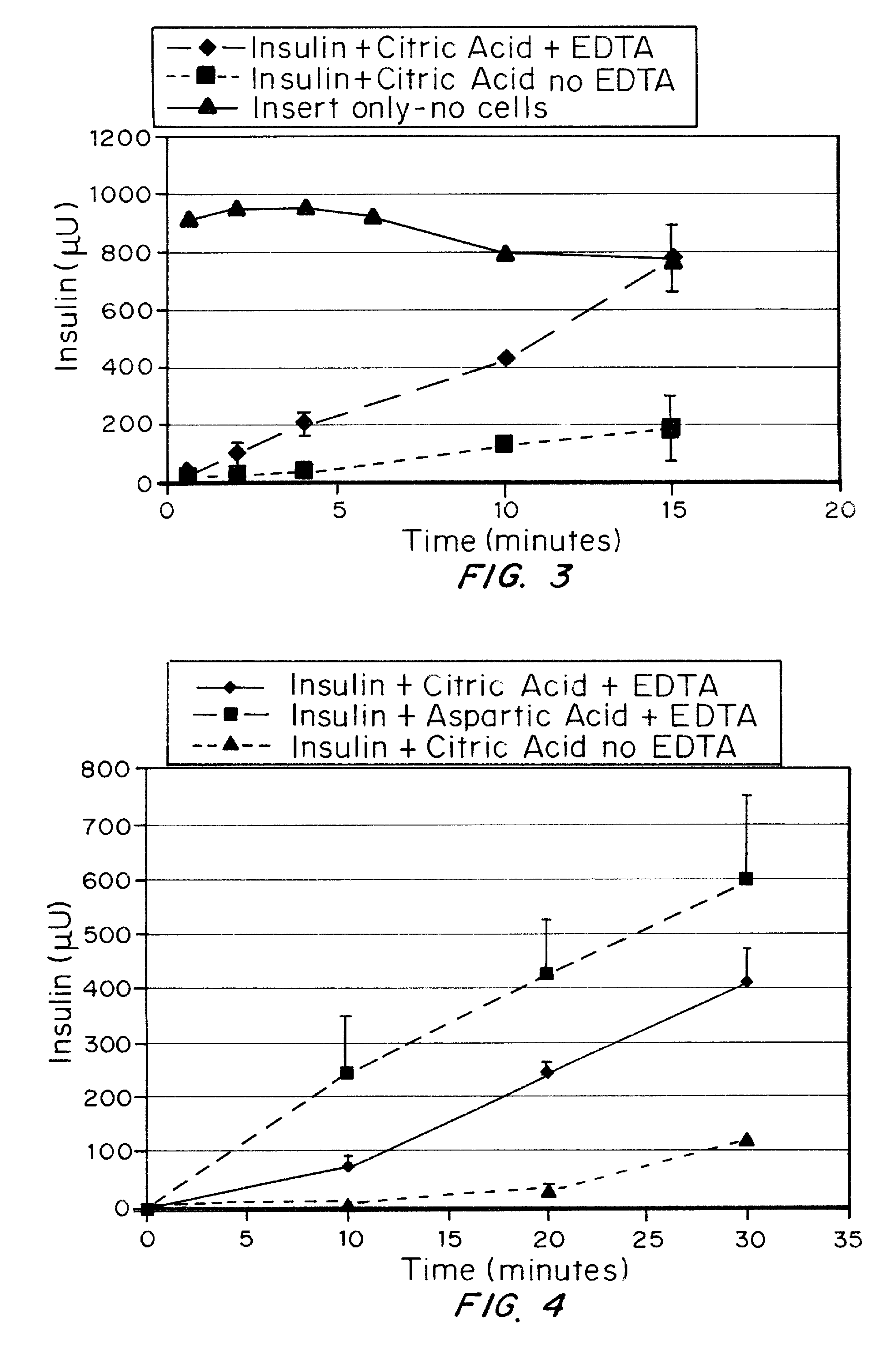
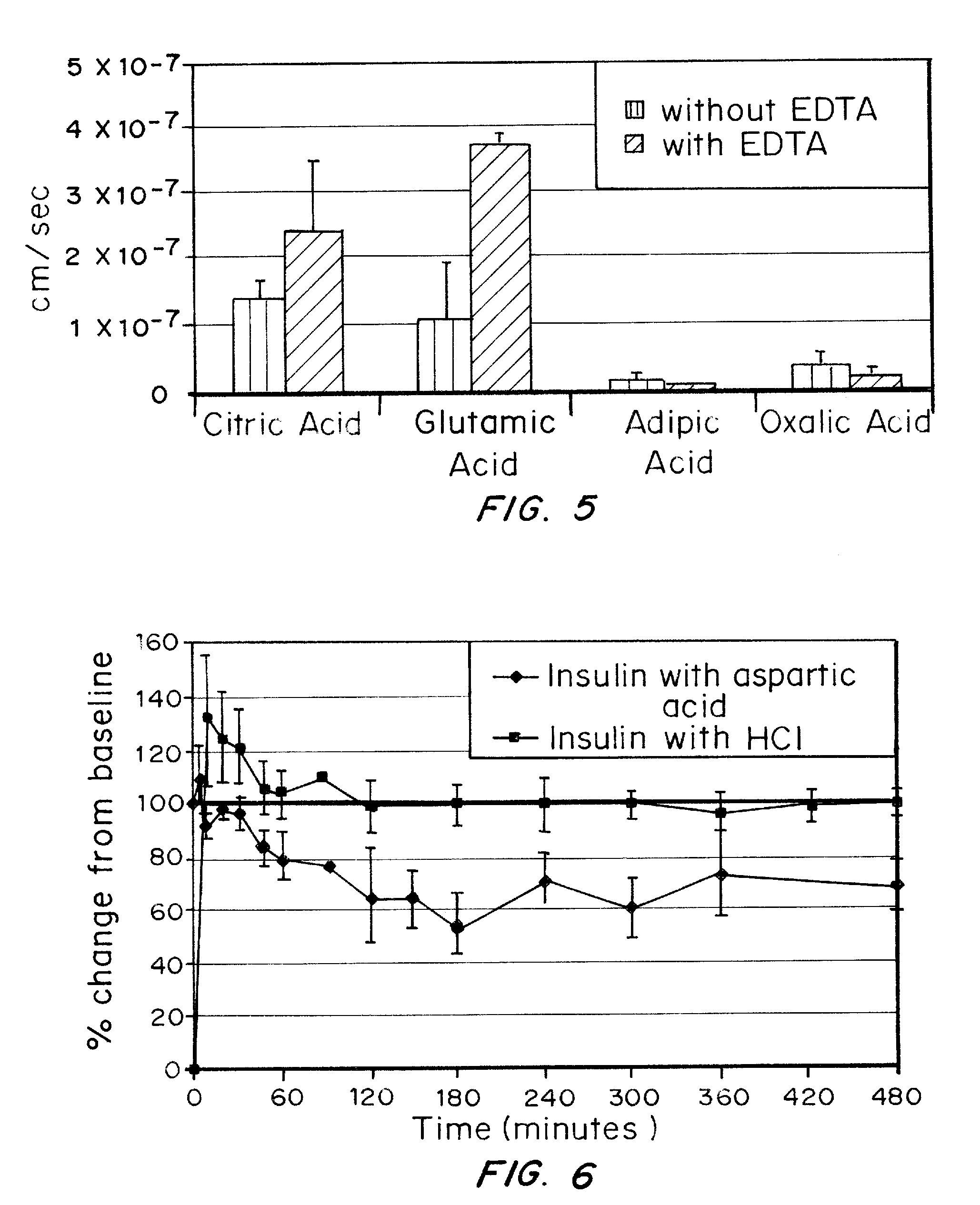
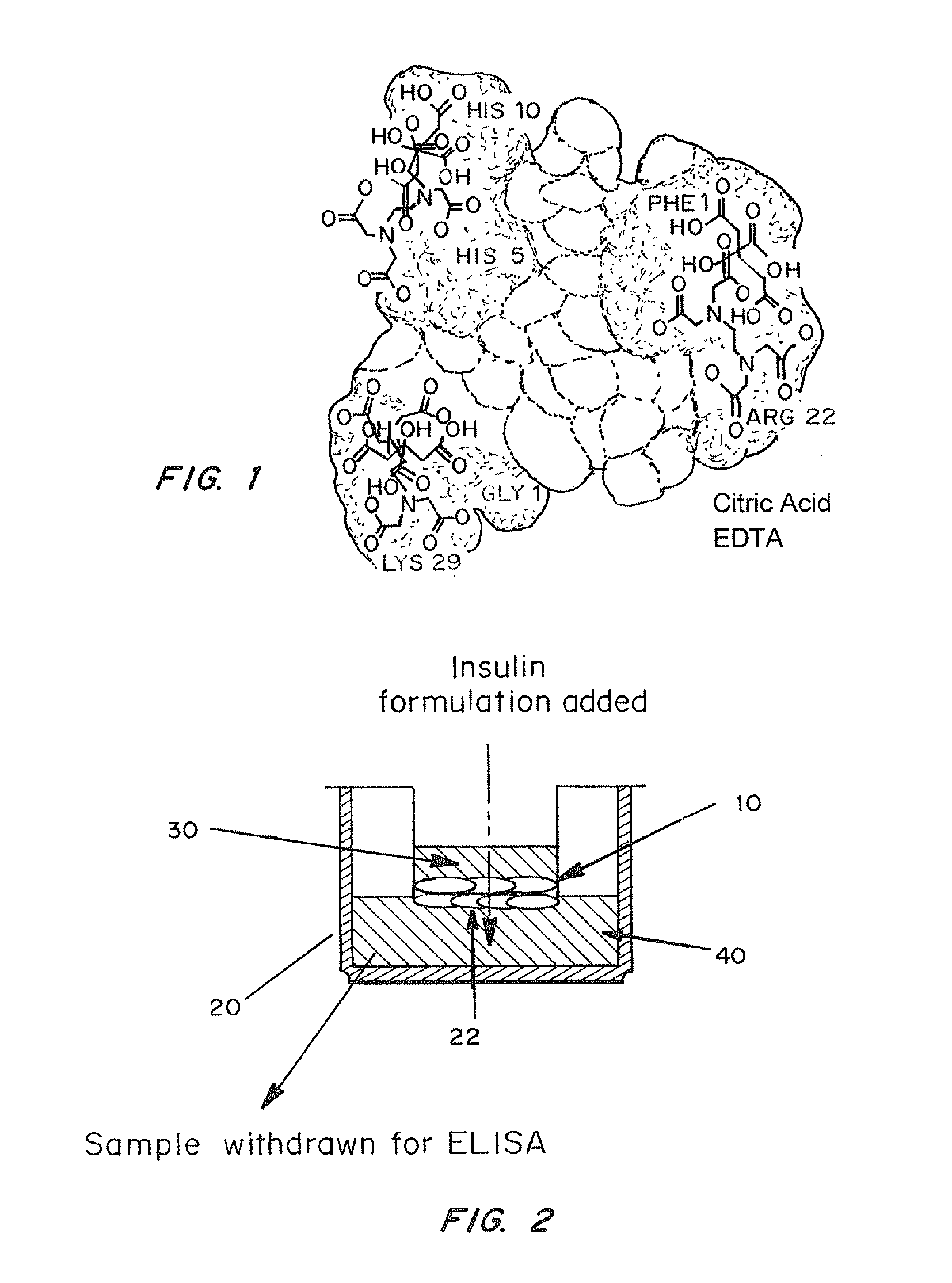
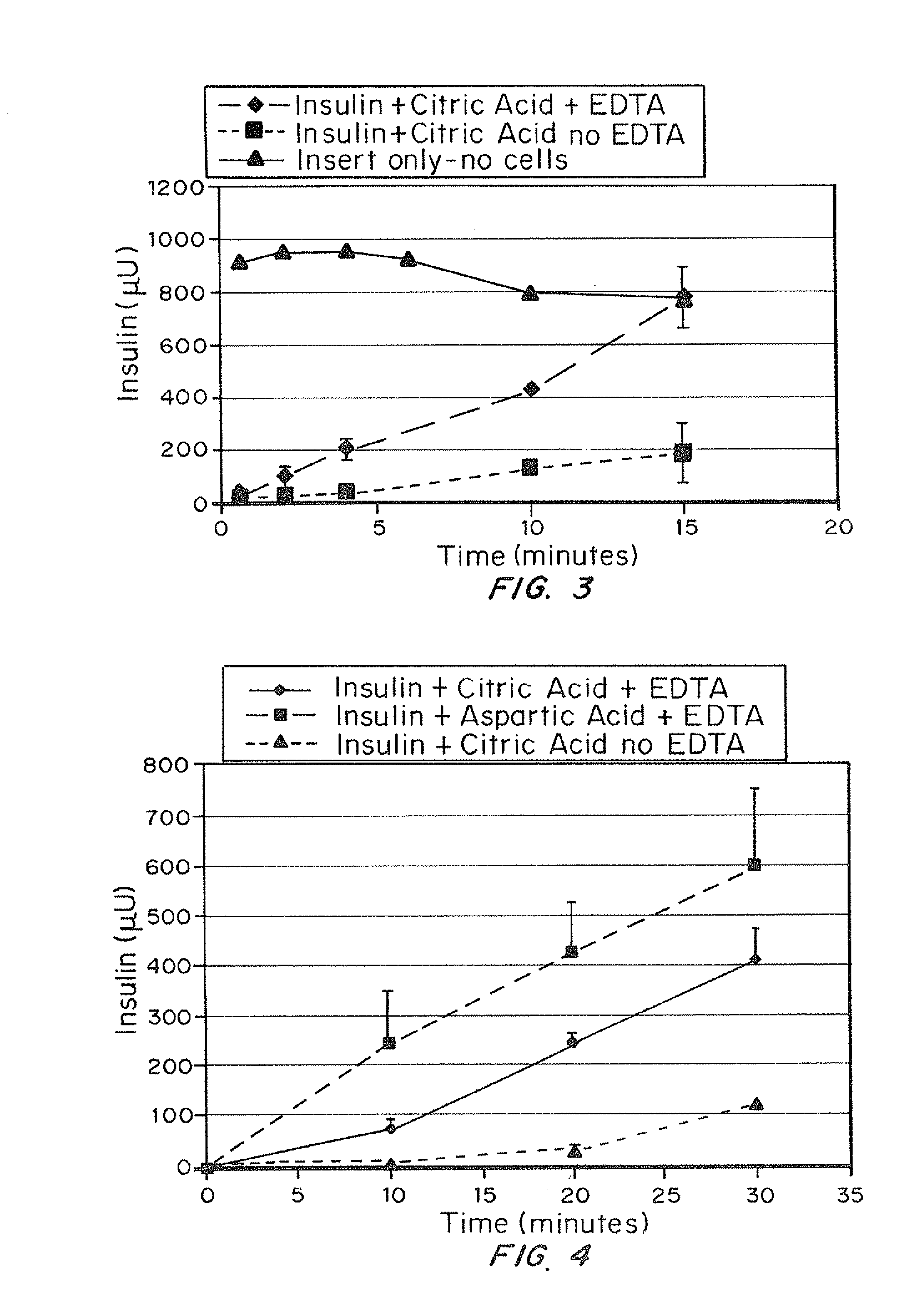
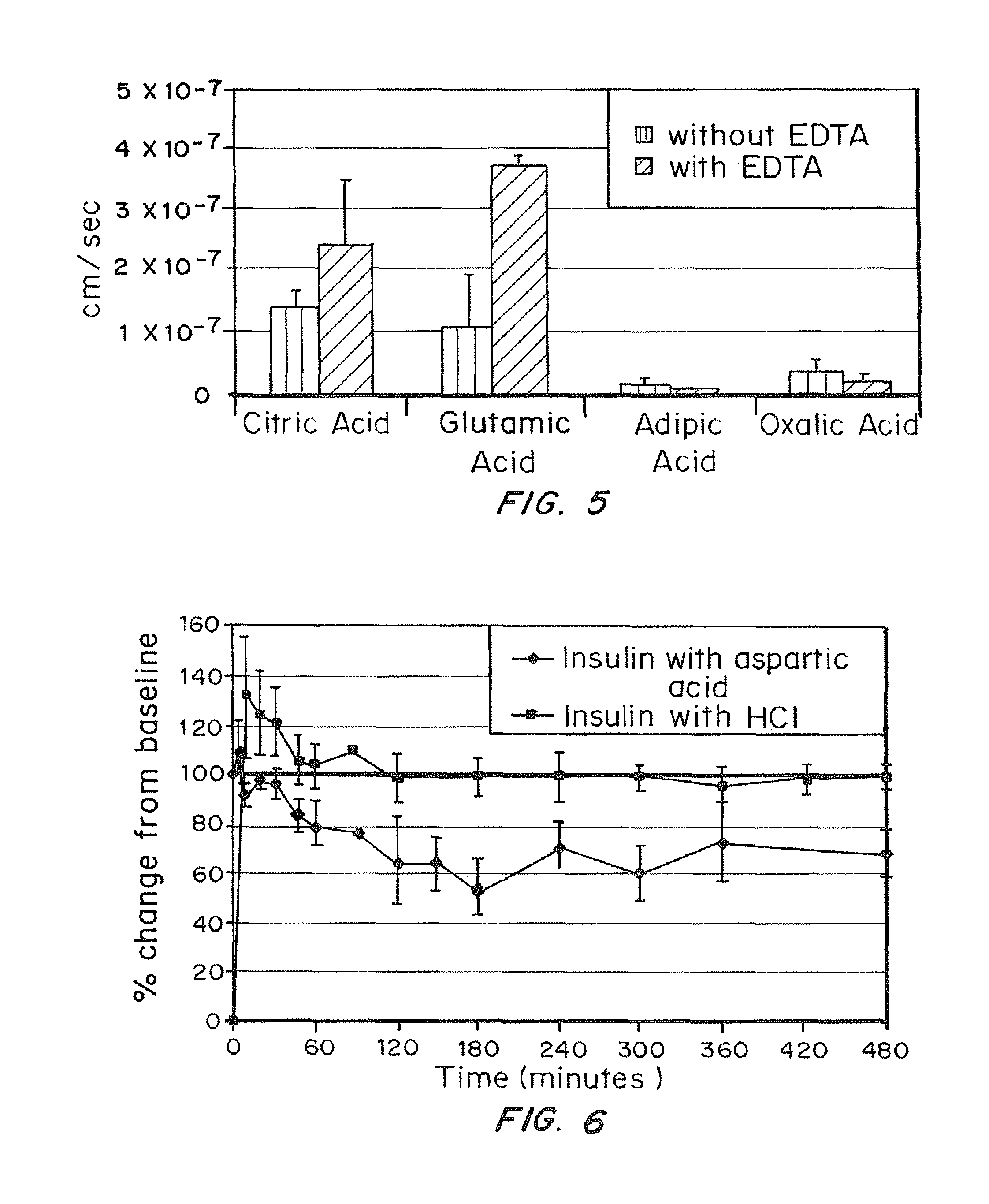
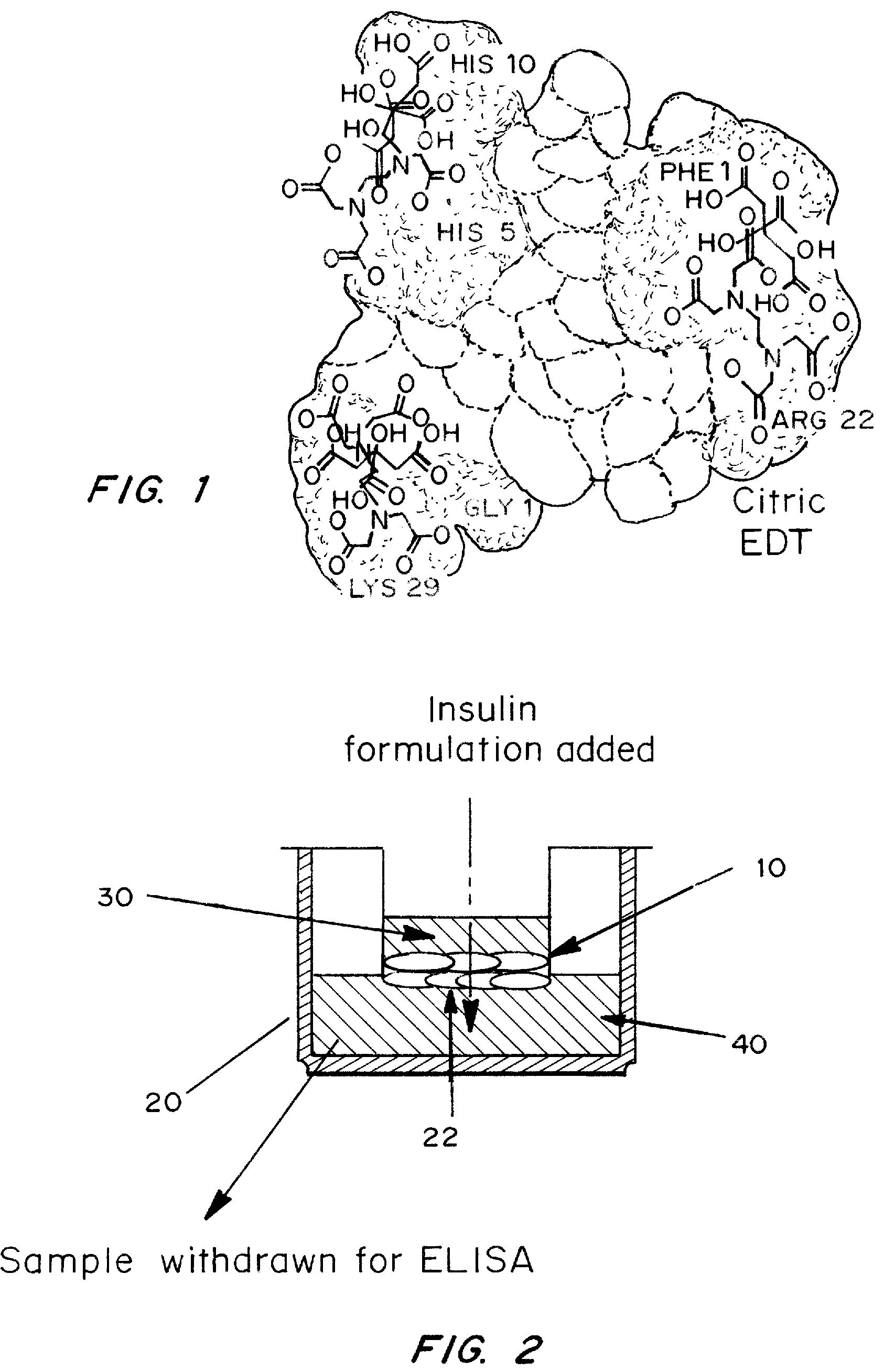
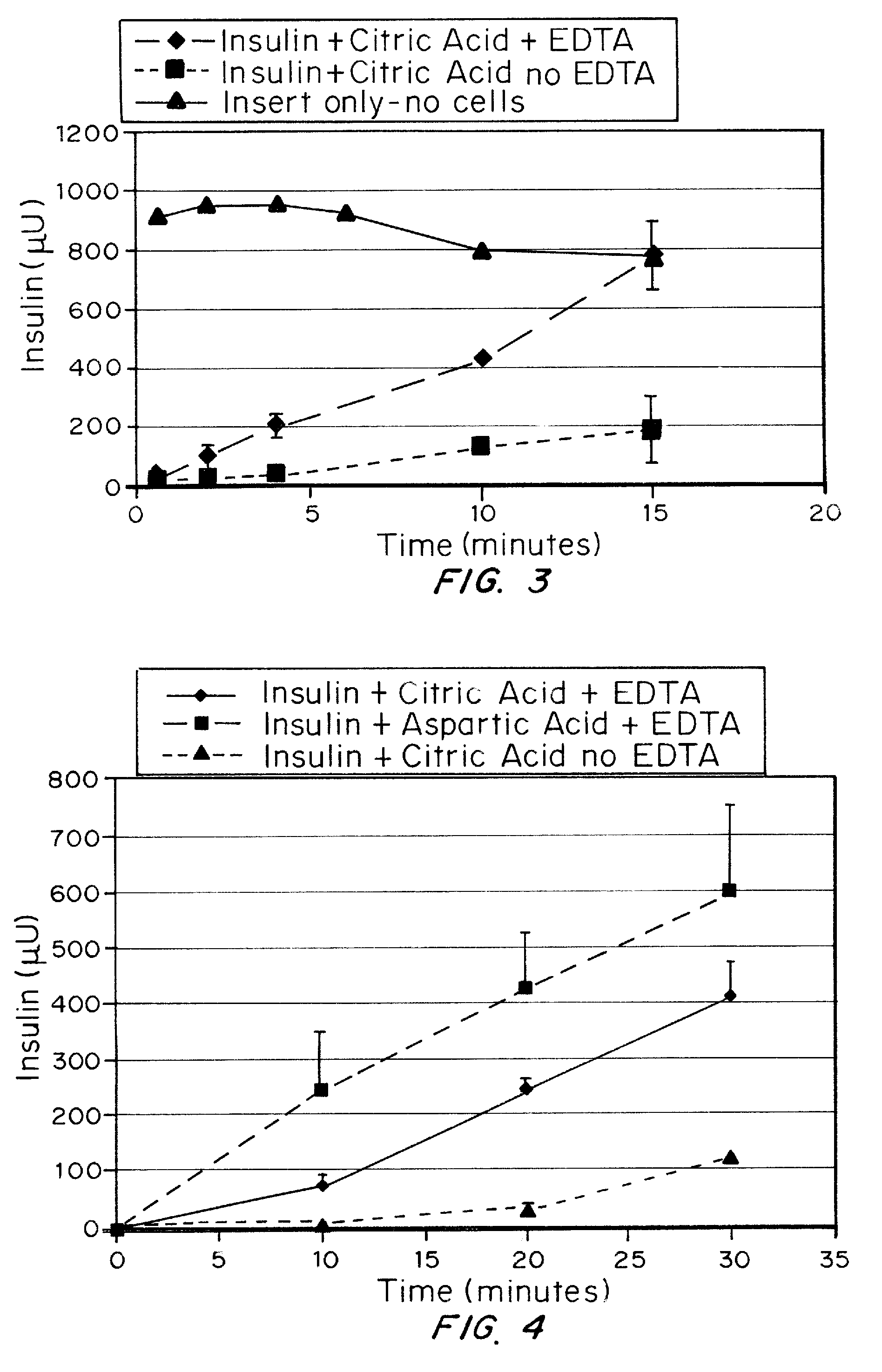
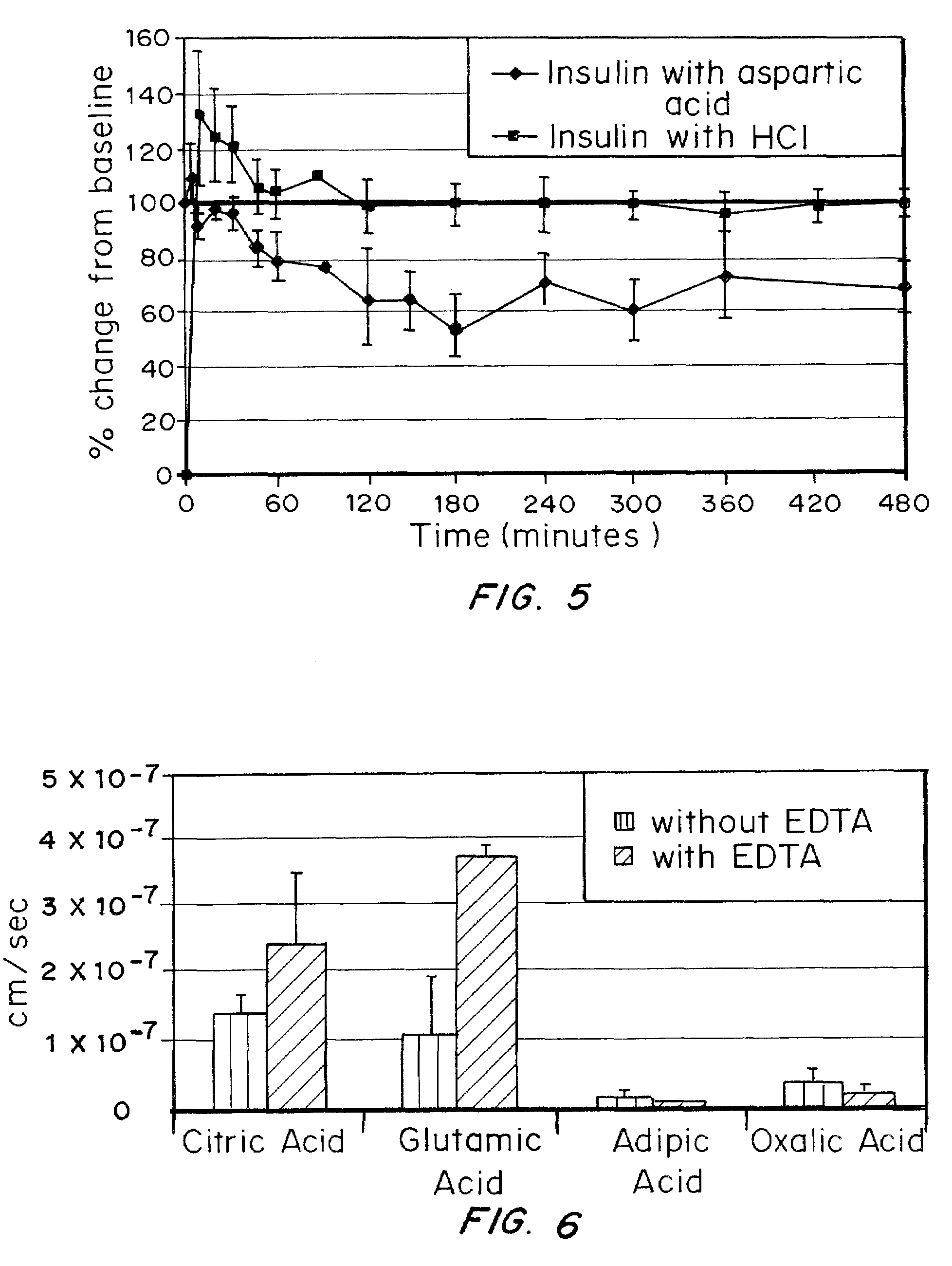
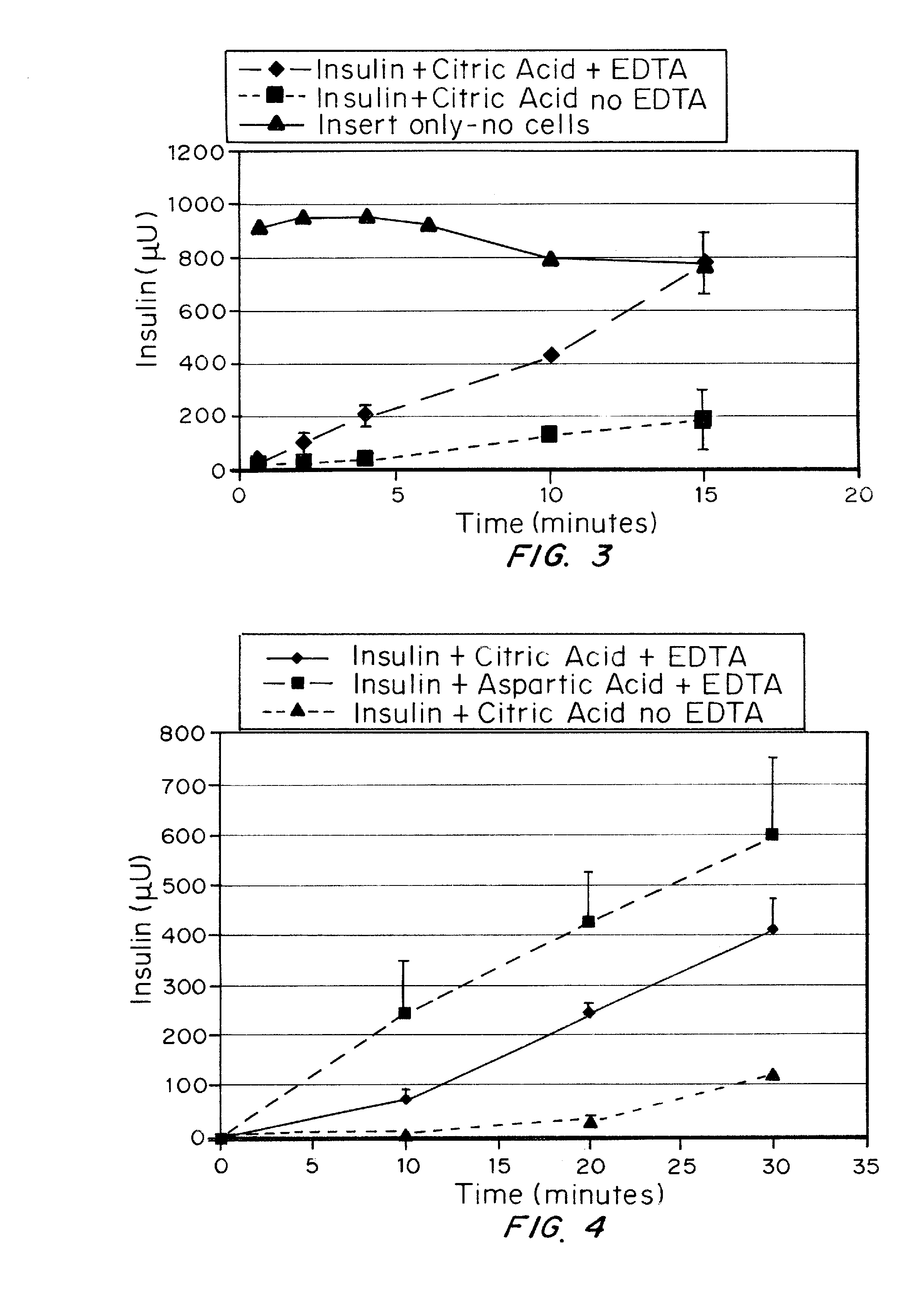
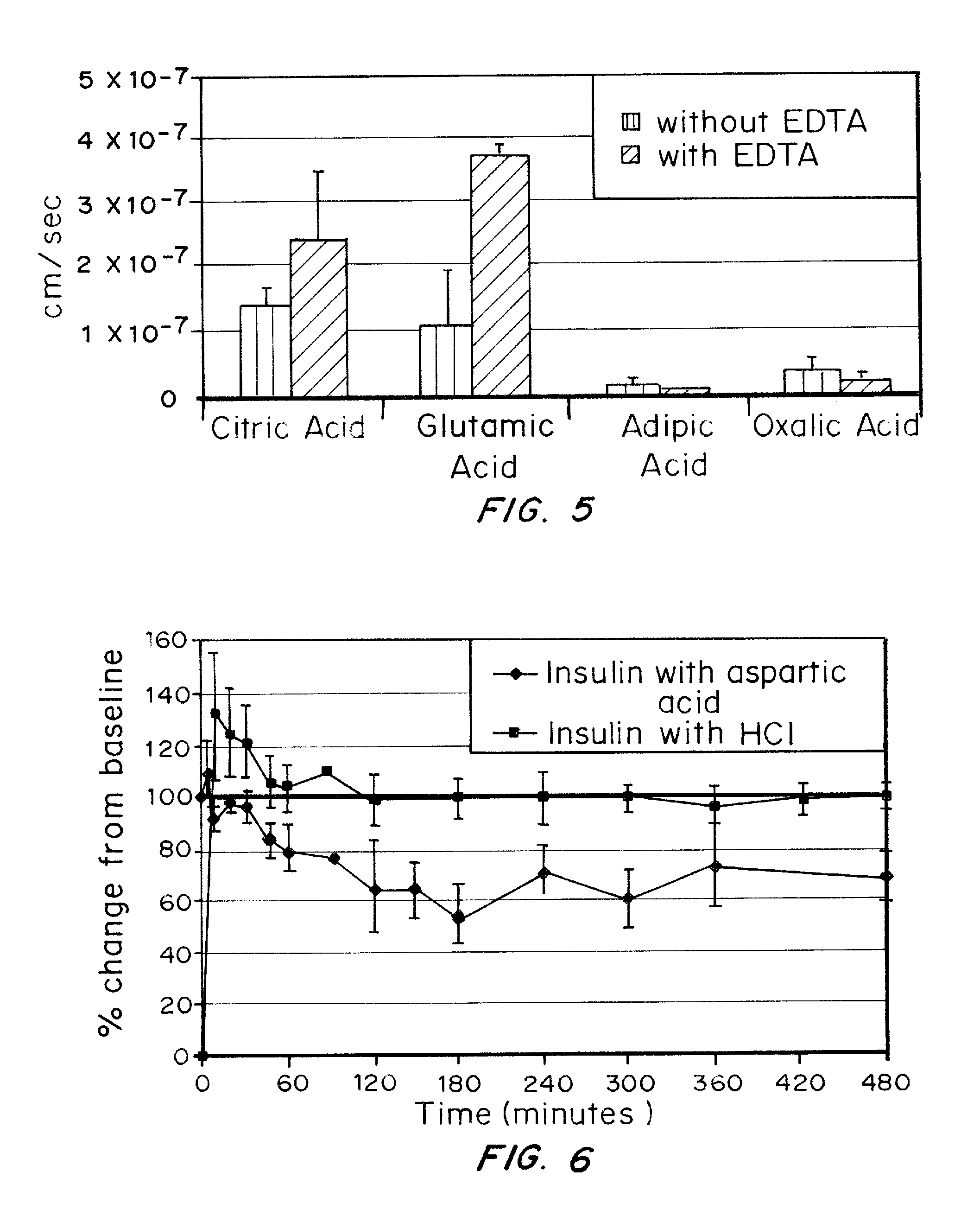
A combined rapid acting-long acting insulin formulation has been developed wherein the pH of the rapid acting insulin is adjusted so that the long acting glargine remains soluble when they are mixed together. In the preferred embodiment, this injectable basal bolus insulin is administered before breakfast, provides adequate bolus insulin levels to cover the meal, does not produce hypoglycemia after the meal and provides adequate basal insulin for 24 hours. Lunch and dinner can be covered by two bolus injections of a fast acting, or a rapid acting or a very rapid acting insulin. As a result, a patient using intensive insulin therapy should only inject three, rather than four, times a day. Experiments have been performed to demonstrate, the importance of the addition of specific acids to hexameric insulin to enhance speed and amount of absorption and preserve bioactivity following dissociation into the monomeric form by addition of a chelator such as EDTA. As shown by the examples, the preferred acids are aspartic, maleic, succinic, glutamic and citric acid. These are added in addition to a chelator, preferably ethylenediaminetetraacetic acid (EDTA). The results show that the citric acid formulation was more effective at dropping the blood glucose rapidly than the identical rapid acting formulation prepared with HCl in swine. Charge masking by the polyacid appears to be responsible for rapid insulin absorption. EDTA was not effective when used with adipic acid, oxalic acid or HCl at hastening the absorption of insulin. These results confirm the results seen in clinical subjects and patients with diabetes treated with the rapid acting insulin in combination with citric acid and EDTA.
Owner:ELI LILLY & CO
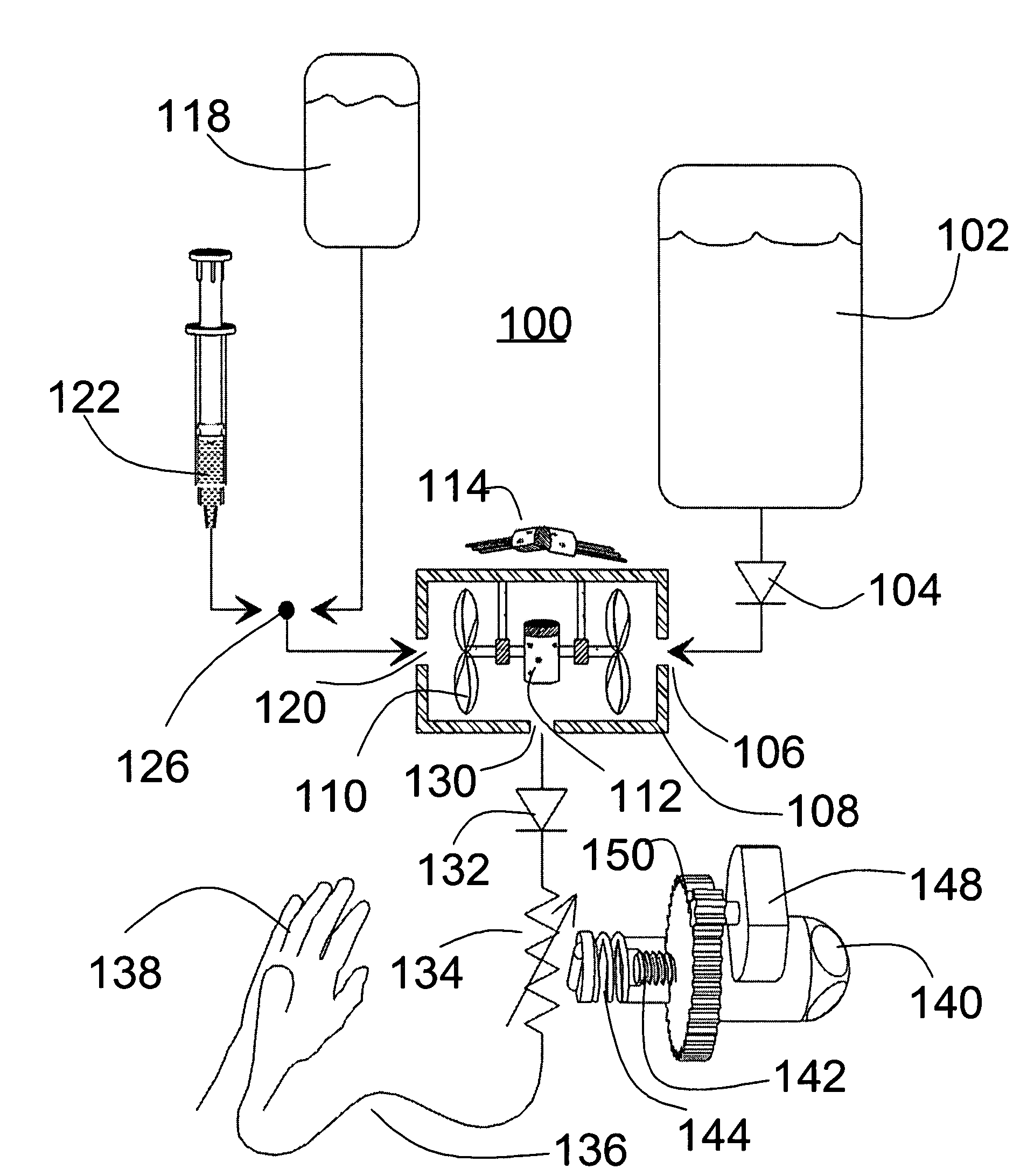
Actuation system and method for an implantable infusion pump
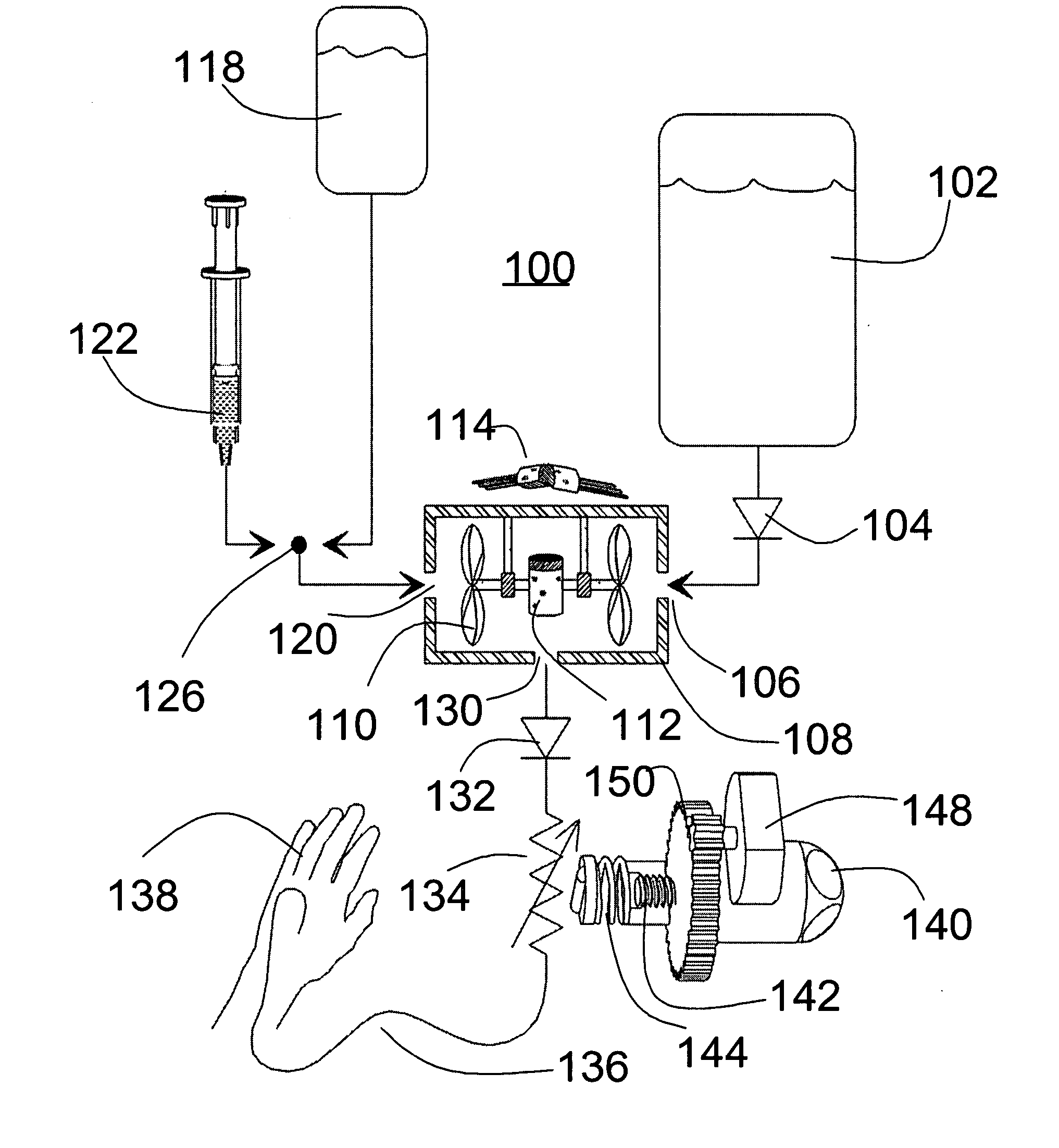
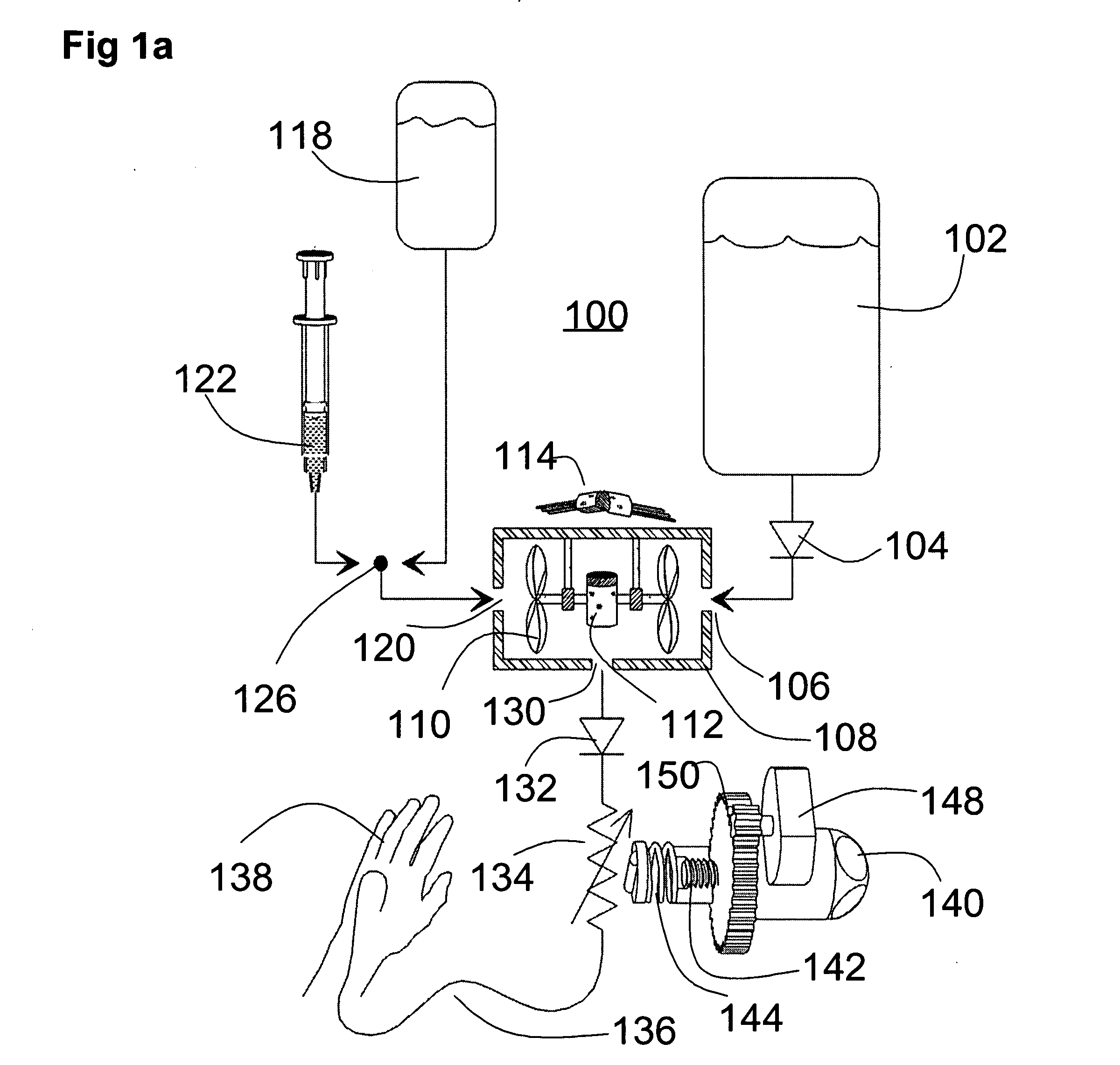
The invention relates to an actuation system and method for an implantable infusion pump. In an example, a working fluid is placed in an actuator. Upon actuation, the working fluid is driven into a piston cylinder. Upon deactuation, the actuator draws the fluid from the cylinder through a restrictor at a rate dictated by the motivating force, fluid viscosity, and restriction. Driving of the piston may produce a bolus dosage or fill a supplemental flow chamber for subsequent delivery. The exemplary system may be configured by selecting a fluid volume and a viscosity. These, in combination, produce a prescribed fluid delivery rate (or recharge rate) and cumulative flow volume provided to a patient over a time period or in a bolus dose. The system may also be configured to limit the total dosage of a bolus injection, or the rate of a supplemental dosage. In this manner, the system is safe, preventing overdose.
Owner:ADVANCED NEUROMODULATION SYST INC
Automated fluid flow control system
ActiveUS20060004330A1Reduce trafficMaximum precisionMedical devicesFlow monitorsControl systemClosed loop
A device for control of fluid flow with closed loop quasi static adjustment of in-line pressure-based resistance. The disclosed device and methods of using the device provide for the ability to control a flow rate with a highly portable device; the ability for an operator to safely use the device with minimal training; the ability to automatically switch between a primary and secondary fluid; the ability to maintain a flow rate through a tubing set even when the control device is removed; and the ability to measure fluids introduced with a manual bolus injection.
Owner:FRESENIUS KABI USA LLC
Micro infusion drug delivery device
InactiveUS20050277887A1Minimize costlyMinimize difficult preparation stepInfusion syringesMedical devicesDrug reservoirMicro actuator
An infusion pump includes a plurality of projections configured so that they enter the subcutaneous region of the patients skin and provides a painless means of creating a breach in the stratum corneum which is sealed against leakage by the skin surrounding each projection and provides a flow path for either a basal and bolus injection of medication. The pump includes a drug reservoir containing a drug. The pump includes a microactuator and includes a housing having a foundation or lid which opens and closes so that the medication container can be inserted and supported in a delivery mode position. A micro actuator is used to advance either a roller or a piston in communication with the medication container. Attached to the micro-actuator is a device that is mounted for movement along the access of the medication container. The device indexes along the medication container that is used to dispense the medication.
Owner:ICU MEDICAL INC
Breviscapine infusion preparation and its preparing method
InactiveCN1425385AInfusion stabilityOvercome the disadvantages of poor clarityOrganic active ingredientsCardiovascular disorderMedicineArginine
The Breviscapine infusion preparation is compounded with Breviscapine as main component, glucose or sodium chloride, propylene glycol, L-arginine, sodium bisulphate, EDTA-2Na and water for injection through certain technological process. The preparation is suitable for great dosage application clinically to avoid cross infection caused by intermediate links. In addition, the present invention is clear and stable.
Owner:上海博泰医药科技有限公司
Rapid Acting and Long Acting Insulin Combination Formulations
InactiveUS20080039365A1Promote absorptionAct quicklyBiocidePeptide/protein ingredientsBefore BreakfastIntensive insulinotherapy
A combined rapid acting-long acting insulin formulation has been developed wherein the pH of the rapid acting insulin is decreased so that the long acting glargine remains soluble when they are mixed together. In the preferred embodiment, this injectable basal bolus insulin is administered before breakfast, provides adequate bolus insulin levels to cover the meal, does not produce hypoglycemia after the meal and provides adequate basal insulin for 24 hours. Lunch and dinner can be covered by two bolus injections of a fast acting, or a rapid acting or a very rapid acting insulin. As a result, a patient using intensive insulin therapy should only inject three, rather than four, times a day. Experiments have been performed to demonstrate the importance of the addition of specific acids to hexameric insulin to enhance speed and amount of absorption and preserve bioactivity following dissociation into the monomeric form by addition of a chelator such as EDTA. As shown by the examples, the preferred acids are aspartic, glutamic and citric acid. These are added in addition to a chelator, preferably ethylenediaminetetraacetic acid (EDTA). The results show that the citric acid formulation was more effective at dropping the blood glucose rapidly than the identical rapid acting formulation prepared with HCl in swine. Charge masking by the polyacid appears to be responsible for rapid insulin absorption. EDTA was not effective when used with adipic acid, oxalic acid or HCl at hastening the absorption of insulin. These results confirm the results seen in clinical subjects and patients with diabetes treated with the rapid acting insulin in combination with citric acid and EDTA.
Owner:ELI LILLY & CO
Myocardial Perfusion Imaging
This invention relates to methods for performing myocardial perfusion imaging for diagnosing and characterizing coronary artery disease using an intravenous (IV) bolus injection of regadenoson while the patient is undergoing sub-maximal exercise.
Owner:TPG AXON LEX SUB TRUST +1
Amylin Formulations
InactiveUS20090192075A1Reduce in quantityAct quicklyPeptide/protein ingredientsMetabolism disorderGLP-1 MimeticsBefore Breakfast
A combined insulin and amylin and / or GLP-1 mimetic formulation has been developed wherein the pH of the insulin is decreased so that the amylin and / or GLP-1 remains soluble when mixed together with the insulin. In the preferred embodiment, a bolus insulin is administered by injection before breakfast, providing adequate bolus insulin levels to cover the meal, without producing hypoglycemia after the meal. This can be combined with an adequate basal insulin for 24 hours. Lunch and dinner can be covered by two bolus injections of the insulin and amylin and / or GLP-1 mimetic combination. A GLP-1 mimetic may be combined with either rapid acting or basal insulin formulations. As a result, a patient using the combination formulation therapy may only need to inject half as many times per day.
Owner:BIODEL INC
Methods for Administering Aripiprazole
ActiveUS20090143403A1Without complexityWithout expenseOrganic active ingredientsNervous disorderActive agentMicrosphere
The present invention relates, in part, to the discovery that a pharmaceutical composition comprising aripiprazole and a carrier administered in a bolus injection resulted in an extended release profile similar to that obtained by the injection of a poly lactide-co-glycolide microsphere formulation containing the active agent. This surprising result suggests that pharmacologically beneficial extended release formulations without the complexities and expense associated with the manufacture microspheres.
Owner:OTSUKA PHARM CO LTD
Rapid acting and long acting insulin combination formulations
ActiveUS7718609B2Increase speedReduce the amount of solutionPeptide/protein ingredientsMetabolism disorderBefore BreakfastIntensive insulinotherapy
A combined rapid acting-long acting insulin formulation has been developed in which the pH of the rapid acting insulin is adjusted so that the long acting glargine remains soluble when they are mixed together. In the preferred embodiment, this injectable basal bolus insulin is administered before breakfast, provides adequate bolus insulin levels to cover the meal, does not produce hypoglycemia after the meal and provides adequate basal insulin for 24 hours. Lunch and dinner can be covered by two bolus injections of a fast acting, or a rapid acting or a very rapid acting insulin. As a result, a patient using intensive insulin therapy should only inject three, rather than four, times a day.
Owner:ELI LILLY & CO
Rapid acting and long acting insulin combination formulations
ActiveUS8084420B2Short durationImprove blood sugar controlPeptide/protein ingredientsMetabolism disorderBefore BreakfastInsulin injection
An injectable formulation containing a rapid acting insulin and a long acting insulin has been developed. The pH of the rapid acting insulin is adjusted so that the long acting insulin, remains soluble when they are mixed together. Preferably, the formulation is administered before breakfast, provides adequate bolus insulin levels to cover the meal and basal insulin for up to 24 hours, and does not produce hypoglycemia after the meal. Lunch and dinner can be covered by two bolus injections of a fast, rapid, or very rapid acting insulin. Alternatively, by adjusting the ratio of rapid to long acting insulin, the long acting insulin may be shortened to a 12 hour formulation, and re-administered to the patient at dinner time, providing a safe and effective basal insulin level until morning. As a result, a patient using intensive insulin therapy should only inject three times a day.
Owner:ELI LILLY & CO
Rapid acting and long acting insulin combination formulations
InactiveUS7713929B2Rapid acting insulin isPromote absorptionBiocidePeptide/protein ingredientsBefore BreakfastIntensive insulinotherapy
A combined rapid acting-long acting insulin formulation has been developed in which the pH of the rapid acting insulin is decreased so that the long acting glargine remains soluble when they are mixed together. In the preferred embodiment, this injectable basal bolus insulin is administered before breakfast, provides adequate bolus insulin levels to cover the meal, does not produce hypoglycemia after the meal and provides adequate basal insulin for 24 hours. Lunch and dinner can be covered by two bolus injections of a fast acting, or a rapid acting or a very rapid acting insulin. As a result, a patient using intensive insulin therapy should only inject three, rather than four, times a day.
Owner:ELI LILLY & CO
Ultrasonic diagnostic imaging system and method for detecting lesions of the liver
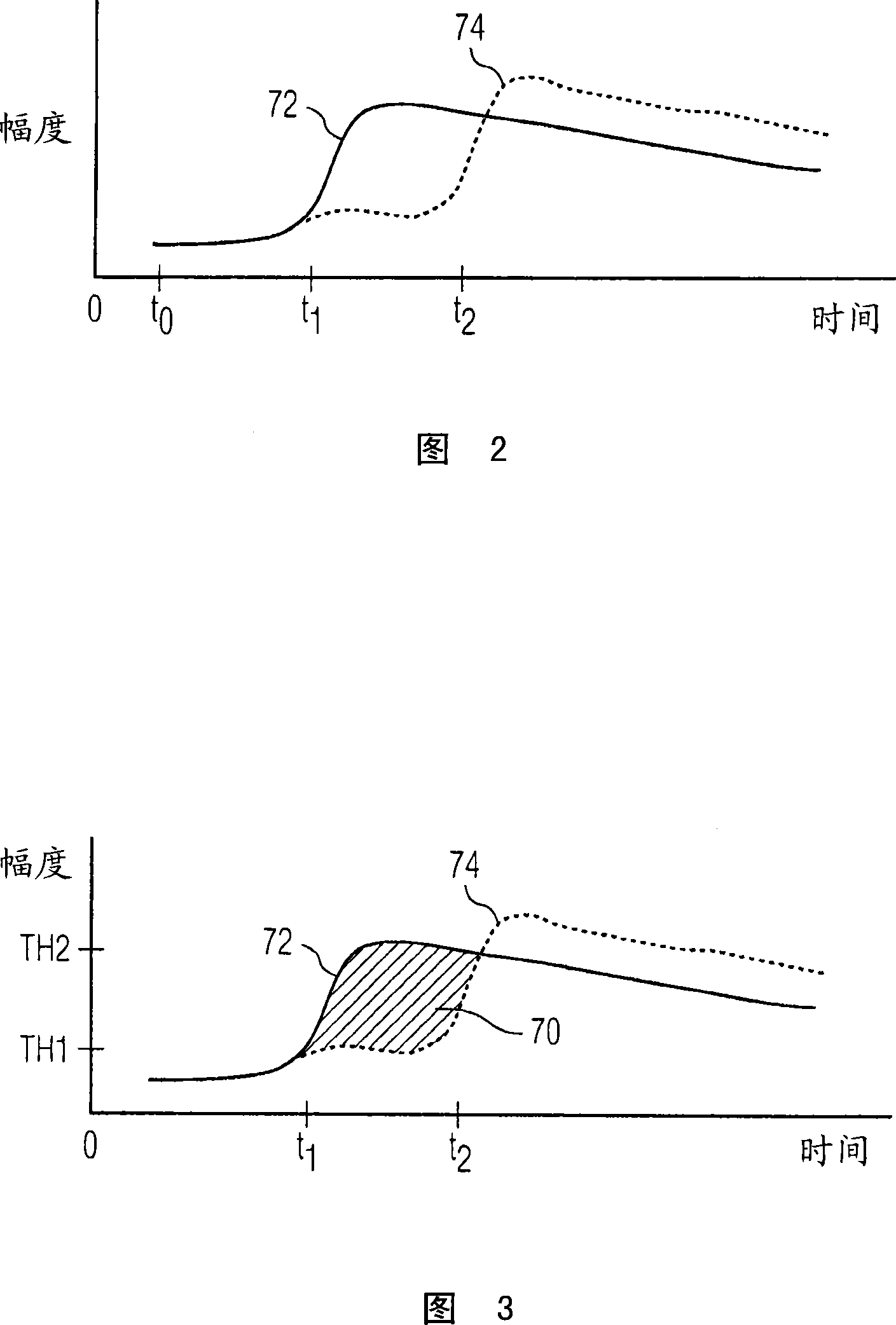
Significant liver growths such as HCC lesions are detected during a contrast agent ultrasound exam by their early reception of contrast and brightening following a bolus injection, as compared with surrounding normal tissue and benign growths. A pixel classifier (30) looks for and identifies pixel or voxel regions where this early wash-in of contrast occurs and denotes these pixel or voxel locations in a parametric image. The pixel classifier analyzes pixel or voxel values from a sequence of images and identifies suspicious regions in an image by uniquely coding the points in a parametric liver image where early wash-in occurs .
Owner:KONINKLIJKE PHILIPS ELECTRONICS NV
Method of measuring transcutaneous access blood flow
InactiveUS20020128545A1Other blood circulation devicesVolume/mass flow measurementIndicator dilutionInjection site
Indicator dilution techniques are used to measure vascular access flow rates during routine hemodialysis. A bolus injection port is used to infuse a specific volume (V.sub.i) of an indicator diluent, such as saline or dye, into the patient cardiovascular circuit by one of the following: 1. Needle injection of a known volume (bolus) of indicator diluent directly into the access site in the presence or absence of the hemodialysis circuit. 2. Infusion of an indicator diluent into the arterial, venous line upstream of the venous needle. 3. Turning the ultrafiltration of the dialysis delivery system from OFF to ON and OFF again over a predetermined time period. 4. In a hemodialysis circuit, turning on the hemodialysis pump and using the priming saline volume as a single saline bolus. A transdermal sensor is used to measure the percent change in a blood parameter. The sensor is positioned directly over the vascular access site a prescribed distance downstream of the injection site and upstream of the access-vein connection. The sensor employs emitter and detector elements at multiple spacings (d.sub.1, d.sub.2) for the purpose of measuring the bulk absorptivity (.alpha.) of the area immediately surrounding and including the access site, and the absorptivity (.alpha..sub.0) of the tissue itself.
Owner:IN LINE DIAGNOSTICS CORP +1
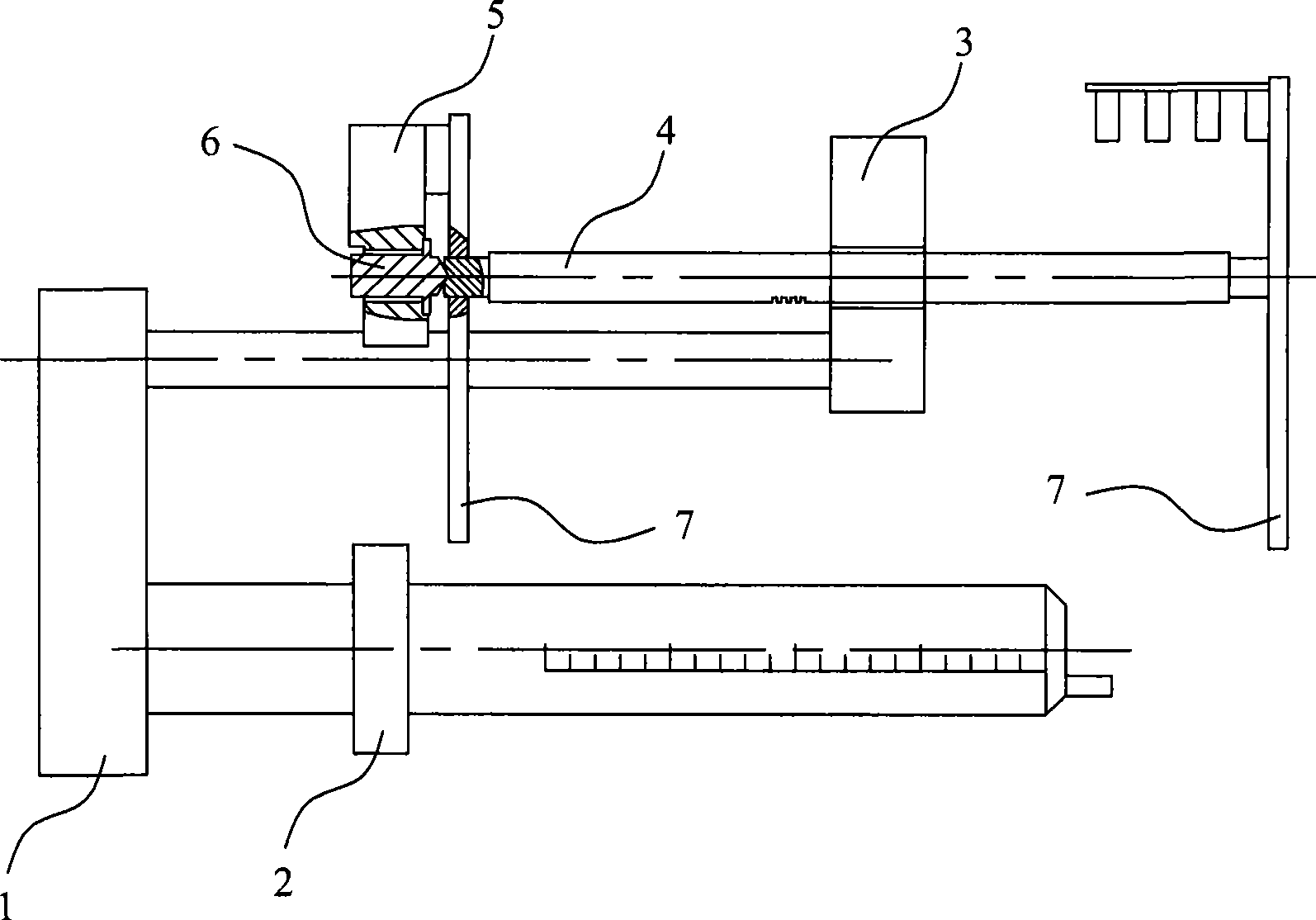
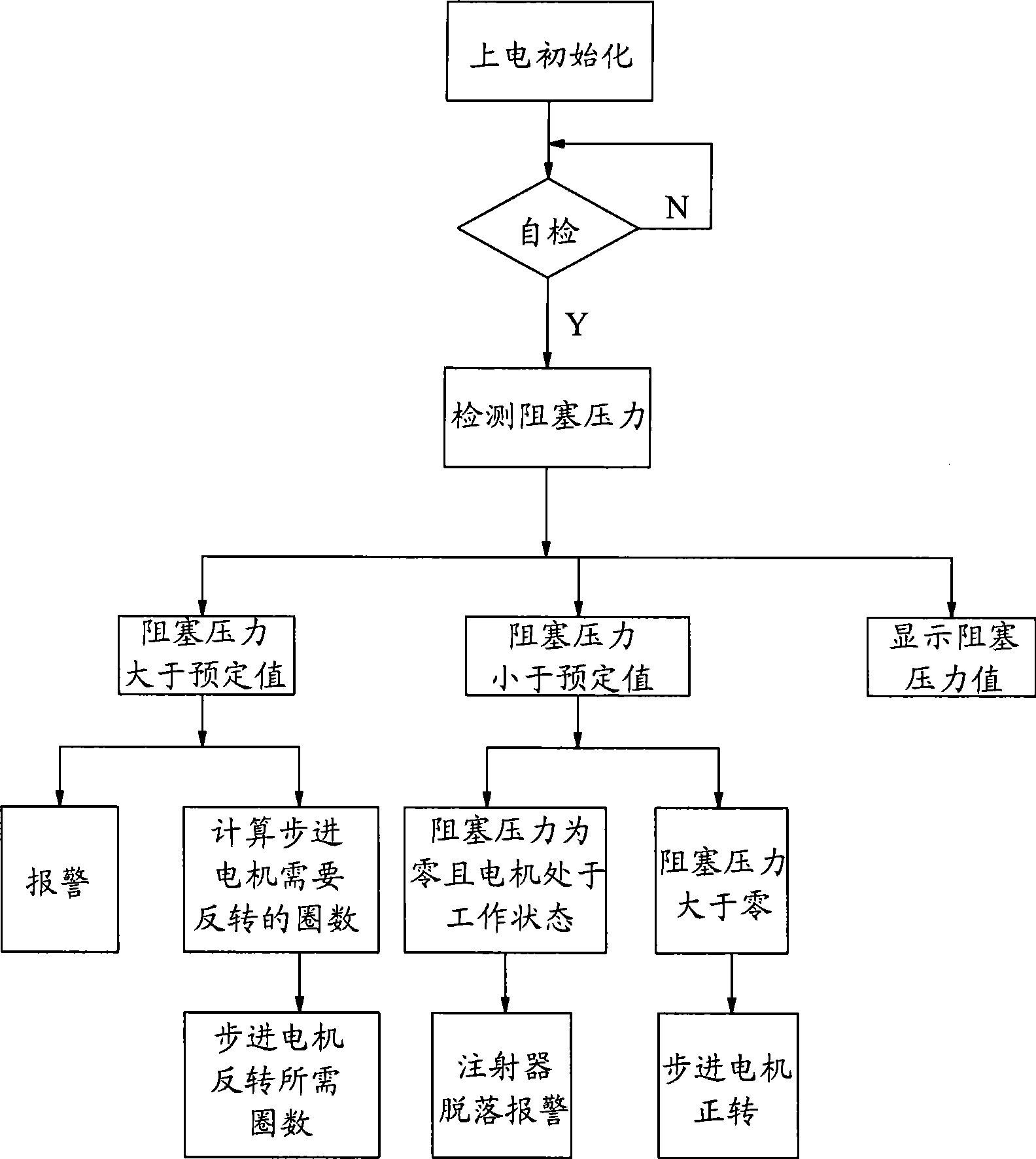
Block pressure detection and processing equipment and method for injection pump
InactiveCN101470036AReal-time detection of occlusion pressureReal-time detection and processing of occlusion pressureInfusion devicesFluid pressure measurementMedicineAxial pressure
The invention discloses a blockage pressure detecting processing device for injection pumps, comprising a frame; a lead screw which is connected with the frame via positive or reversed rotation, is provided with a nut and is connected with the bolus injection head of the injection pump via the nut; a pressure sensor fixed on the frame; and a force transmission component fixed on the pressure sensor, whose first end is axially preloaded on the end of the lead screw for transmitting the axial pressure of the lead screw to the pressure sensor. The invention further discloses a blockage pressure detecting processing method for injection pumps, comprising: detecting the blockage pressure of an injection pump; according to the difference between the blockage pressure and a preset value, controlling a step motor, to preload the force transmission component on the end face of the lead screw in the detection and treatment on the blockage pressure of the injection pump, and keeping the force transmission component against the end face of the lead screw. The invention has high stability, can real-timely detect the blockage pressure of injection pump on the bolus injection head, thereby confirming the performance of injection pumps and alarming for dropped injection pump caused by manual factors.
Owner:BEIJING AEONMED
Constant rate fluid delivery device with selectable flow rate and titratable bolus button
InactiveUS8771227B2Precise and variable flow rate controlLess-expensive to manufactureOther blood circulation devicesPharmaceutical delivery mechanismDrugs infusionElectronic component
A wearable, self-contained drug infusion device is disclosed that is capable of achieving the precise flow rate control needed for dose-critical drugs such as insulin. In preferred embodiments of the device, at least two flow channels are utilized in conjunction with a series of valves for providing a user with selectable, constant flow rate control. The device can be made with small dimensions so that it can be worn by the user with a minimum of discomfort and inconvenience. In addition, the simple mechanical nature of the device provides the user with close control over the flow rate, which is required for safe and effective delivery of insulin and other drugs. Also, the absence of electronic components allows the device to be manufactured inexpensively. The device is provided with a first channel that is long and narrow, functioning as a flow restrictor. The first channel is preferably provided in a serpentine pattern. A second channel is also provided that has a larger cross section so that flow is not restricted. A series of valves are used to force the flow of fluid through a selectable portion of the serpentine portion of the first channel before entering the remainder of the second channel and flowing to the delivery cannula. In one embodiment of the device, a needle port is provided in fluid communication with the delivery cannula for delivering bolus injections. In another embodiment, a bolus button is provided for delivering bolus injections. A flow restrictor is preferably included in the bolus button to limit the rate at which the bolus button refills.
Owner:BECTON DICKINSON & CO
Rapid acting and long acting insulin combination formulations
ActiveUS20090137455A1Short durationImprove blood sugar controlPeptide/protein ingredientsMetabolism disorderBefore BreakfastIntensive insulinotherapy
An injectable formulation containing a combination of a rapid acting insulin and a long acting insulin has been developed wherein the pH of the rapid acting insulin is adjusted so that the long acting insulin, e.g. insulin glargine, remains soluble when they are mixed together. In the preferred embodiment, this injectable basal bolus insulin is administered before breakfast, provides adequate bolus insulin levels to cover the meal, does not produce hypoglycemia after the meal and provides adequate basal insulin for up to 24 hours. Lunch and dinner can be covered by two bolus injections of a fast acting, or a rapid acting or a very rapid acting insulin. Alternatively, through adjustment of the ratio of rapid acting insulin to long acting insulin, the long acting insulin may be shortened to a 12 hour formulation. This rapid and long acting blend is re-administered to the patient at dinner time, providing a safe and effective basal insulin level until morning. As a result, a patient using intensive insulin therapy should only inject three, rather than four, times a day.
Owner:ELI LILLY & CO
Levetiracetam tablet and preparation method
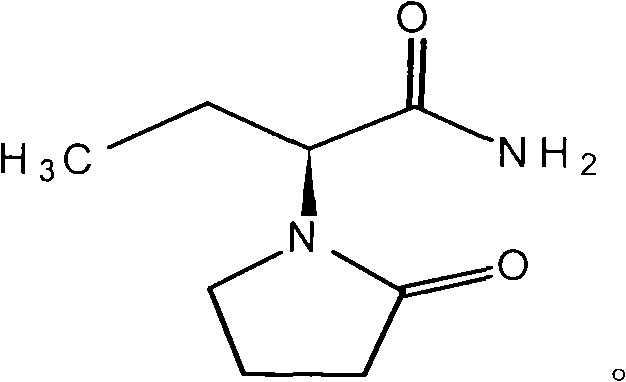
InactiveCN101584673AHigh hardnessPromote dissolutionNervous disorderPharmaceutical non-active ingredientsMedicinePlasticizer
The invention discloses a Levetiracetam tablet and preparation method thereof. Levetiracetam is a bolus injection medicine with bad compressibility and proportion of supplementary materials which can be added is small. Quality of Levetiracetam is unstable and it is hard to produce Levetiracetam industrially. The invention comprises components of weight percentage content: 70% to 80% of Levetiracetam as active component, 1.5% to 8.0% of plasticizer, 9% to 17% of disintegrant, 3% to 14% of filler, 0.1% to 0.5% of bond and 0.1% to 3% of fluidizer. Addition of plasticizer is added by inner way of wet granulation. Levetiracetam as active component has bad mechanic compressibility. The invention adds plasticizer to solve tablet compression difficulty in industrial embodiment thoroughly, which realizes the purpose of being suitable for coating with stable quality and easy industrial application.
Owner:ZHEJIANG JINGXIN PHARMA
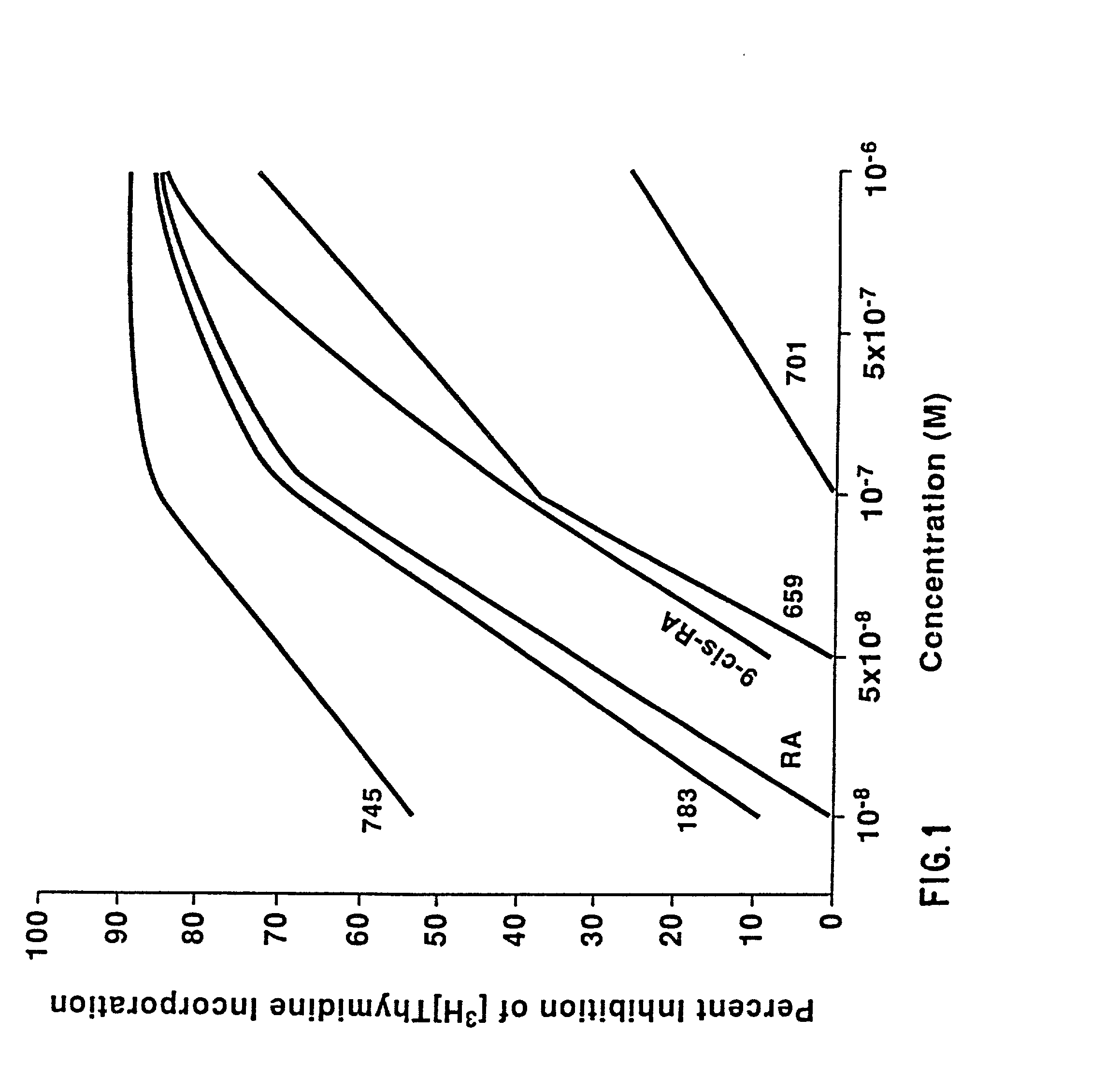
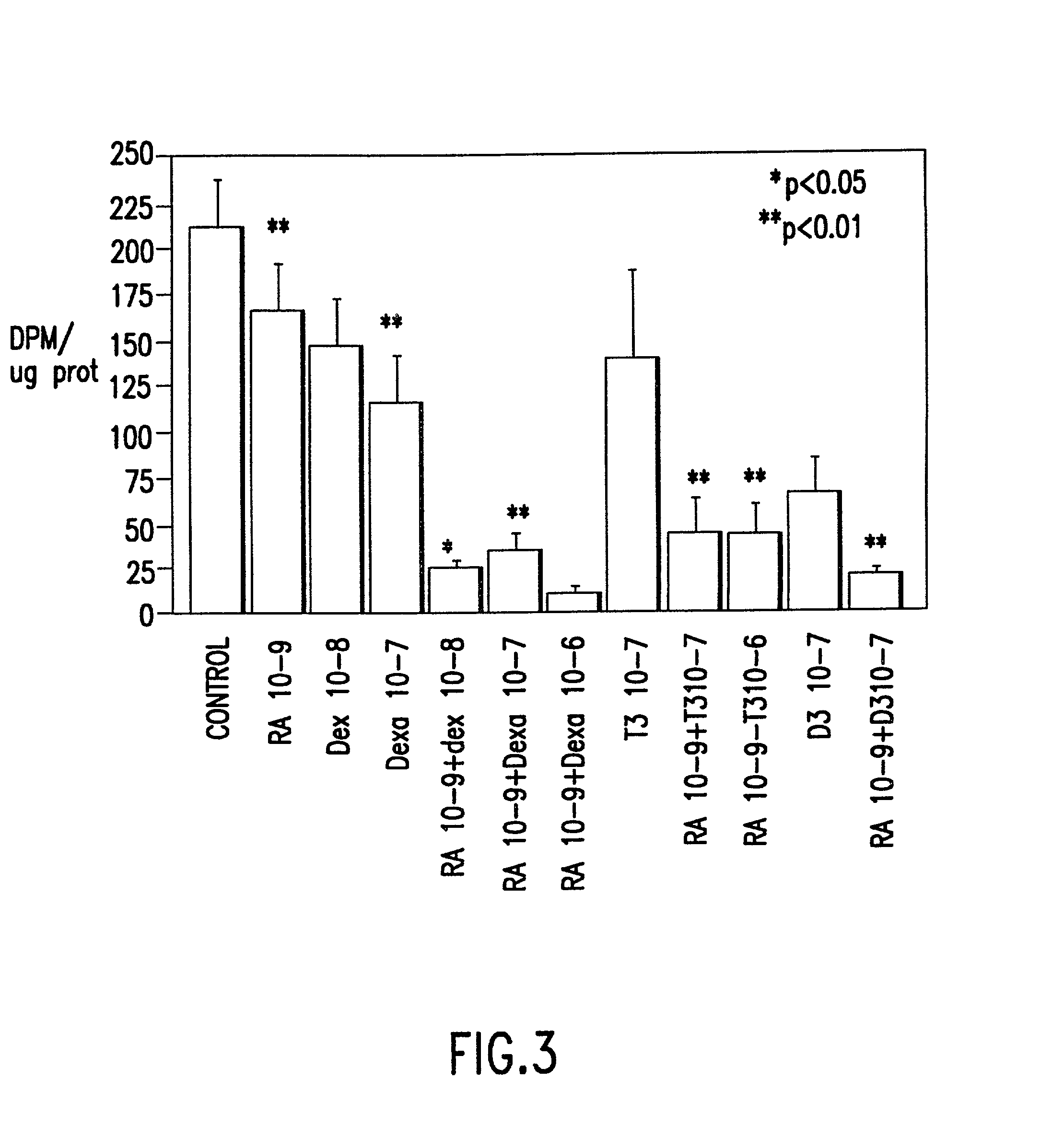
Method of preventing proliferation of retinal pigment epithelium by retinoic acid receptor agonists
InactiveUS20020128291A1Prevent proliferationPotent ability AP1-dependantBiocidePowder deliveryDiseaseLiposome
Proliferation of retinal pigment epithelium following surgery or trauma or resulting in ocular diseases associated with choroidal neovascularization, such as age related macular degeneration and histoplasmosis syndrome, is prevented by contacting retinal pigment epithelium cells with a therapeutic amount of a retinoic acid receptor (RAR agonist, preferably one with specific activity for retinoic acid receptors. Preferably the RAR agonist is also a potent antagonist of AP1-dependent gene expression. Alternatively, the proliferation of retinal pigment epithelium is ameliorated with a therapeutic amount of an AP-1 antagonist, alone or in combination with an RAR agonist. The drug can be administered by bolus injection into the vitreous cavity using a dosage from about 50 to 150 .mu.g. Or by slow release from liposomes or an oil tamponade injected into the vitreous cavity. Formulations for preventing proliferation of retinal pigment epithelium are also provided.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
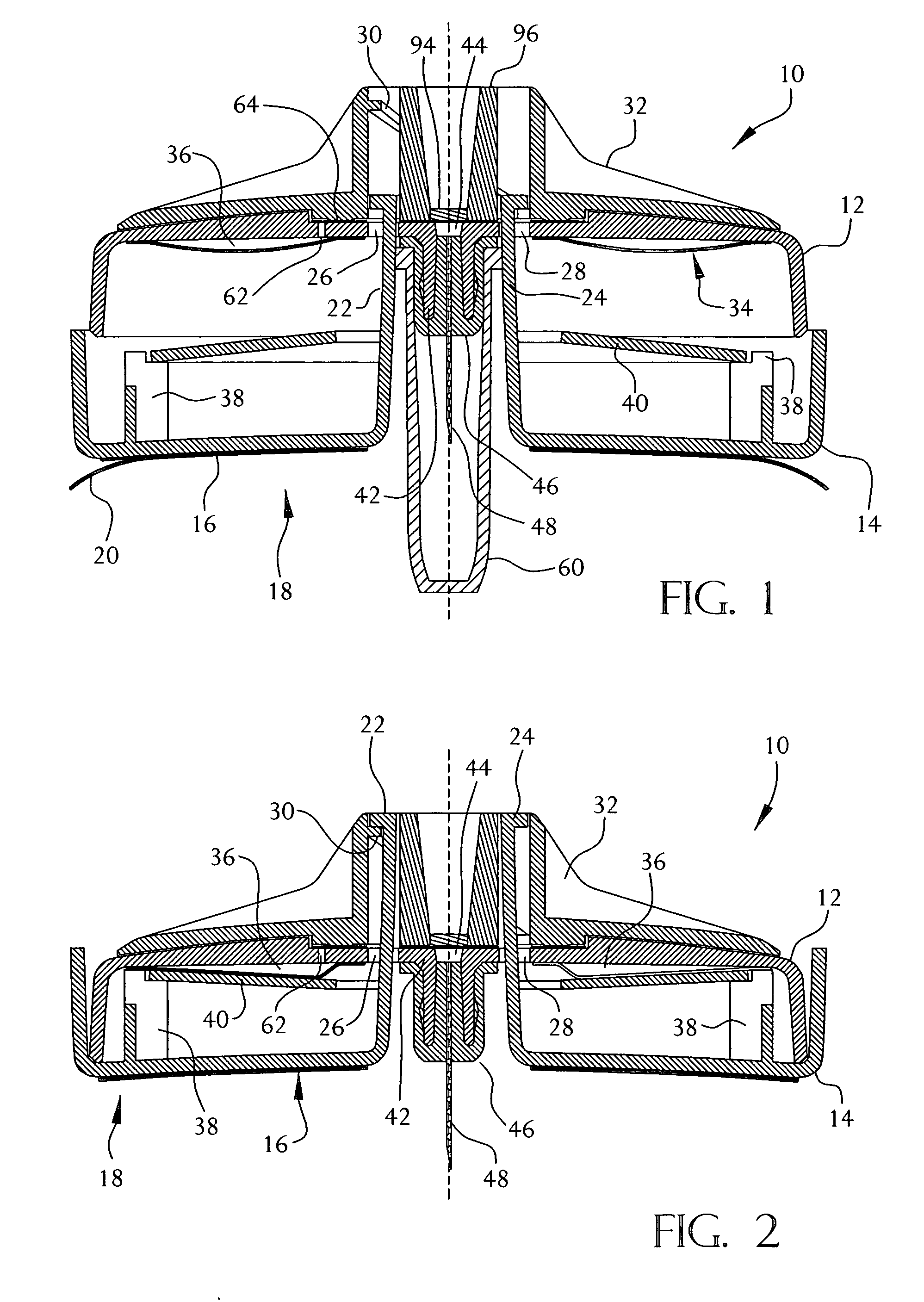
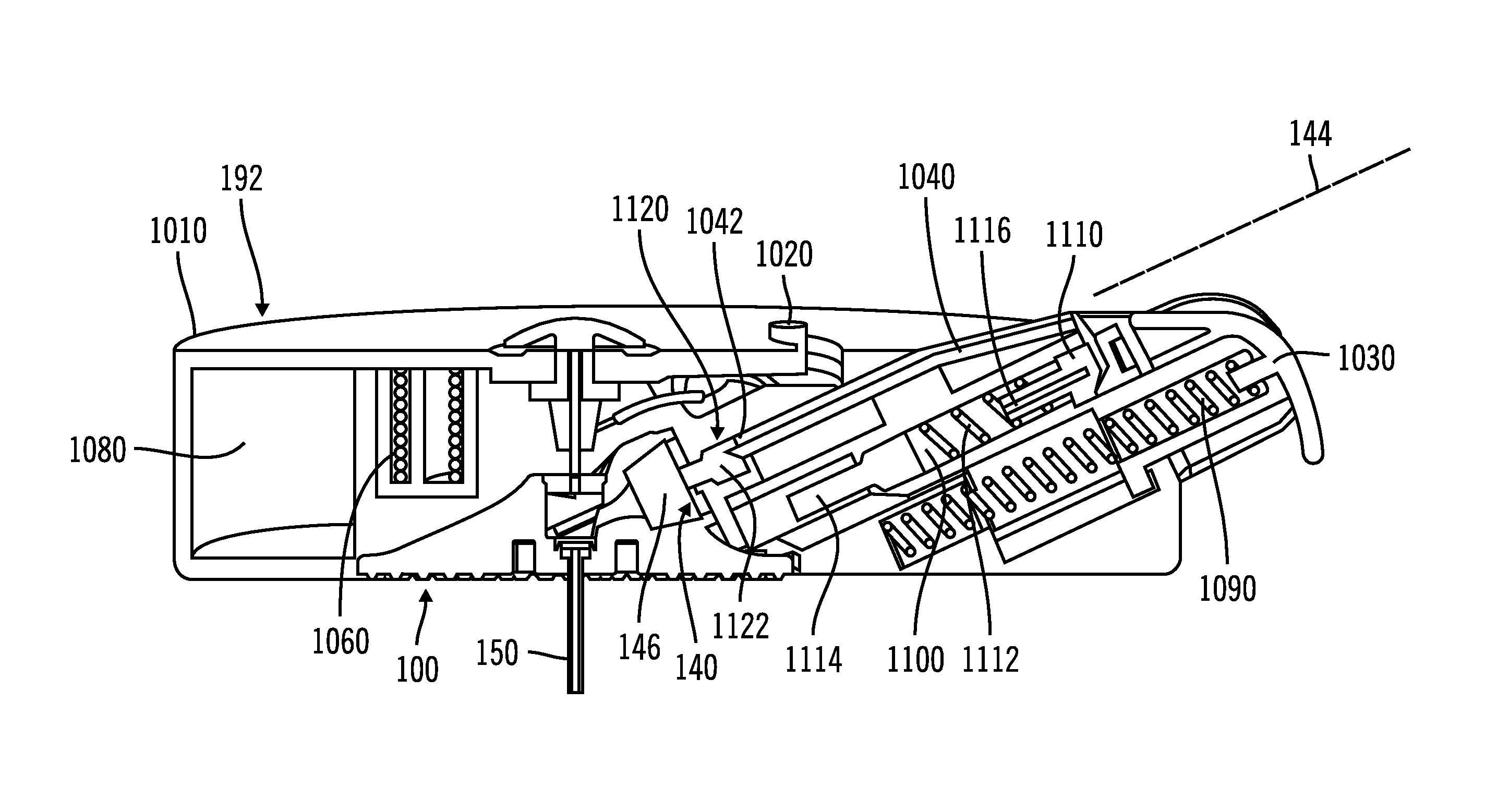
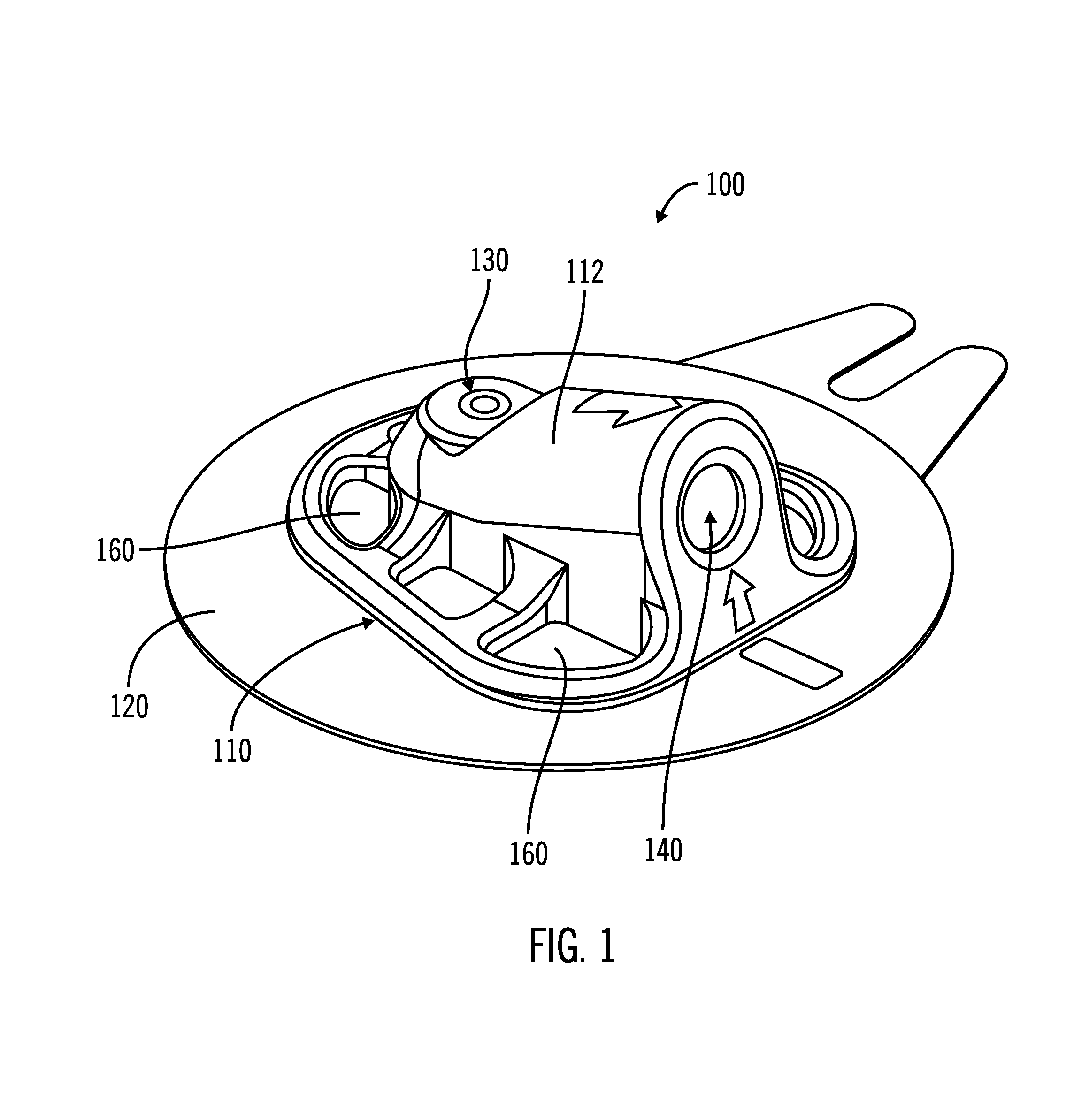
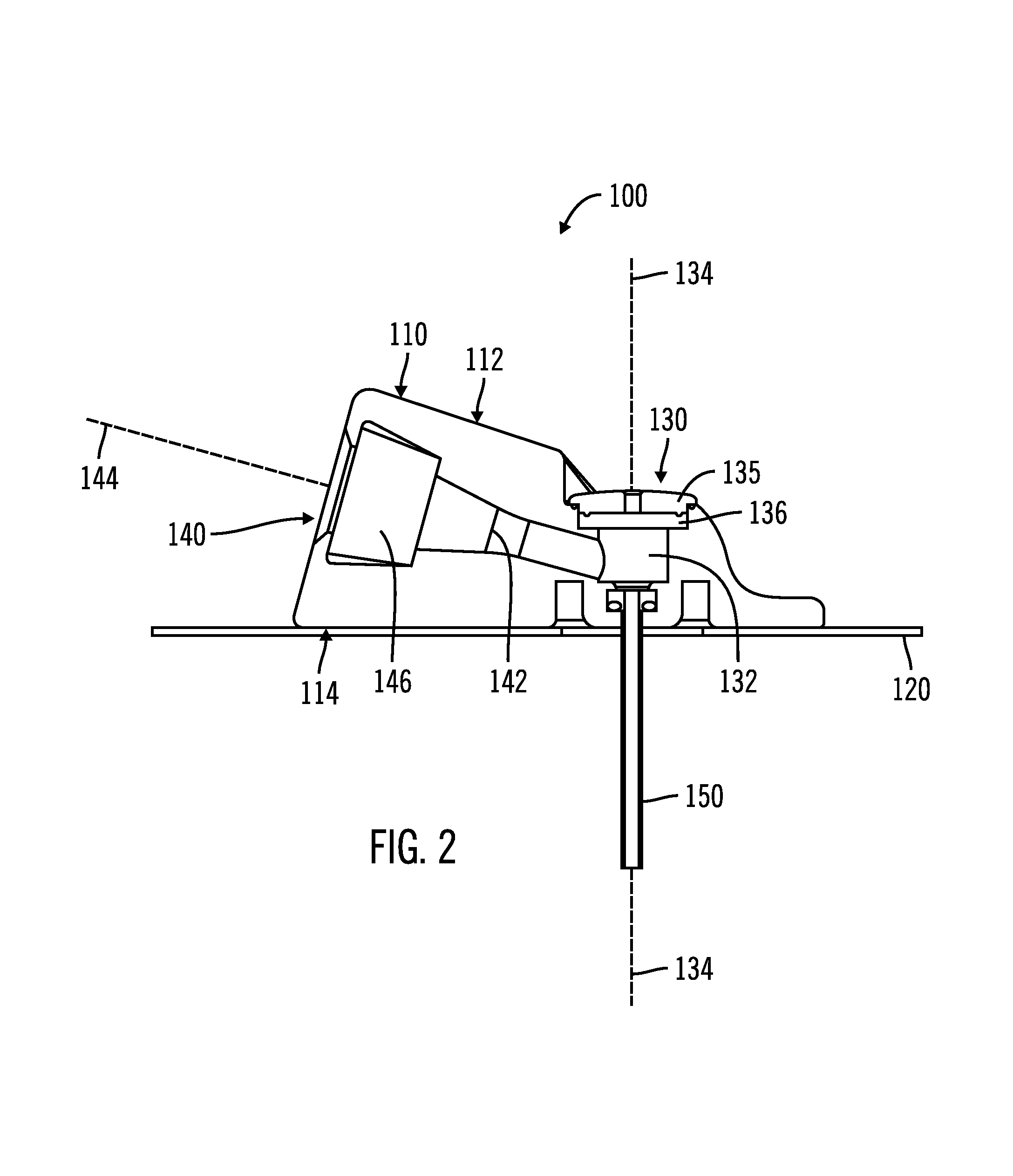
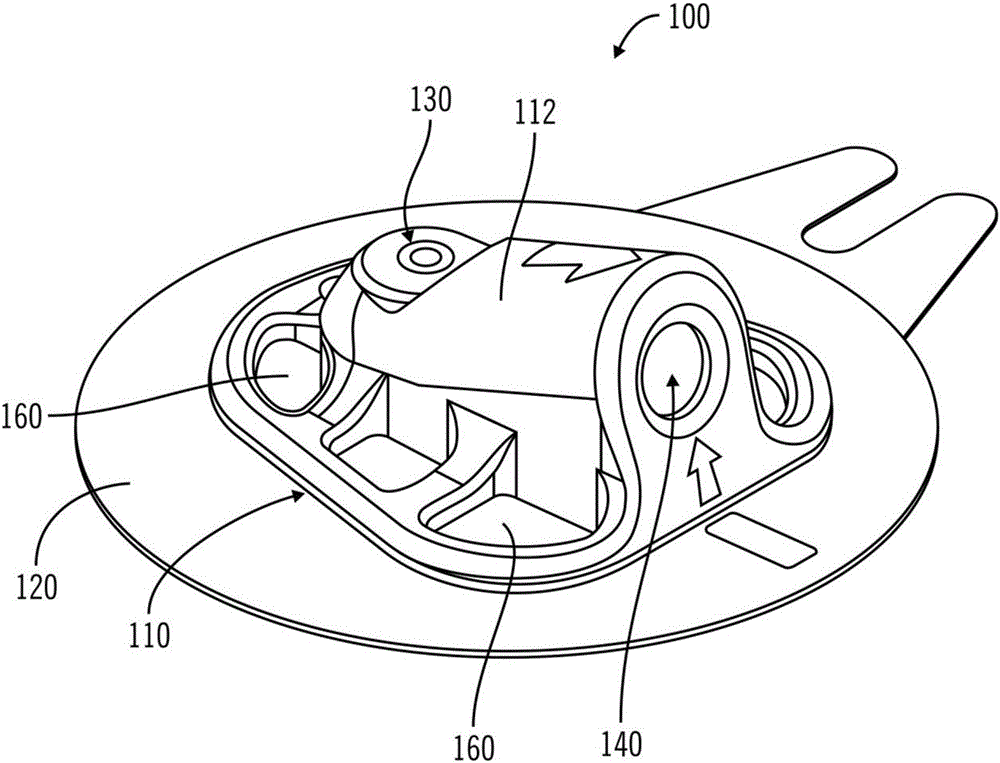
On-body injector and method of use
An on-body injector and method of use including an on-body injector for use with an injection device. The on-body injector includes a bolus reservoir; a bolus injection needle in fluid communication with the bolus reservoir, the bolus injection needle having a bolus injection needle tip aligned with the injection port, the bolus injection needle being slideably biased away from the injection port to define a gap between the bolus injection needle tip and the injection port; and a button operably connected to the bolus injection needle to slide the bolus injection needle along the injection axis. The button is operable to advance the bolus injection needle tip to close the gap and advance the bolus injection needle tip into the injection port. The button is further operable to advance a plunger through the bolus reservoir to deliver a predetermined bolus volume to the patient through the injection flow path.
Owner:MEDTRONIC MIMIMED INC
Access port indicator for implantable medical device
Owner:ADVANCED NEUROMODULATION SYST INC
Electronic injector
An electronic injector including an electronic on-body injector for use with a patient to deliver a fluid through an injection device. The electronic on-body injector includes a fluid reservoir; a MEMS pump; a bolus injection needle, the bolus injection needle having a bolus injection needle tip aligned with the injection port, the bolus injection needle being slideably biased away from the injection port to define a gap; and a bolus needle button operably connected to the bolus injection needle to slide the bolus injection needle along the injection axis. The bolus needle button is operable to advance the bolus injection needle tip to close the gap and advance the bolus injection needle tip into the injection port to form a bolus injection flow path. The bolus needle button is further operable to activate the MEMS pump to deliver a predetermined bolus volume.
Owner:MEDTRONIC MIMIMED INC
Triopisetron Hydrochloride microcapsule and method for preparing injection
InactiveCN101278921AImprove bioavailabilityImprove stabilityPowder deliveryDigestive systemFreeze-dryingHigh pressure
The invention provides a tropisetron hydrochloride freeze-dry preparation for treating emesis, containing tropisetron hydrochloride, gelatin, dextran, oleophilic emulsifier, hydrophilic emulsifier and freeze-dry supporting agent. The invention also relates to a preparation method of the tropisetron hydrochloride freeze-dry preparation, containing the following steps: (1) the tropisetron hydrochloride is added into injection water and completely dissolved; (2) the gelatin and the oleophilic emulsifier are added in solution prepared in step (1) to prepare water-in-oil emulsion; (3) dextran solution and the hydrophilic emulsifier are added in the solution prepared in step (2) to prepare water-in-oil-in-water double emulsion; (4) the prepared double emulsion is dropped by bolus injection into strong base solidified liquor in a high-voltage electrostatic field by an orifice; after solidifying, a microcapsule is filtered and washed by water or PH regulator to lead PH to be 5-7; (5) the freeze-dry supporting agent is dissolved in the injection water and mixed with the microcapsule evenly; after subpackage and freeze drying, the tropisetron hydrochloride freeze-dry preparation is obtained.
Owner:HAINAN LINGKANG PHARMA CO LTD
Ultrasound monitored continuous anesthesia nerve conduction apparatus and method by bolus injection
ActiveUS9668654B2Easy to handlePressure infusionDiagnostic recording/measuringAnesthetic AgentMedicine
The invention generally relates to a continuous anesthesia nerve conduction apparatus and method thereof, and more particularly to a method and system for use in administering a continuous flow or intermittent bolus of anesthetic agent to facilitate a continuous or prolonged nerve block. In one embodiment, the apparatus includes a sheath having a proximal end, a distal end and at least one lumen extending from the proximal end to the distal end. The sheath also includes an embedded conductive element for transmitting an electrical signal from a proximal portion of the sheath to a distal portion of the sheath. A cannula is arranged in the at least one lumen of the sheath and has a distal end protruding from a distal portion of the sheath. The cannula is electrically coupled to at least a portion of the embedded conductive element and is configured to provide nerve stimulation.
Owner:SOLODEX LLC
Syringe gripper capable of preventing contamination of medicine dispensing
InactiveCN106726568APrevent pollution risksEliminate medical safety hazardsPharmaceutical containersMedical packagingIntravenous InfusionsSyringe needle
The invention discloses a syringe gripper capable of preventing contamination of medicine dispensing. The syringe gripper capable of preventing the contamination of medicine dispensing is matched with an existing disposable syringe and consists of needle cylinder fixing nozzles, a pintle fixing nozzle, a lifting rod, a fixed handle, a free handle, a spring and a gripper body. The pintle of the syringe is locked temporarily by the fixed nozzle of the pintle located at the top of the gripper body, then a syringe needle cylinder bulge and a syringe needle cylinder are respectively locked temporarily by taking advantage of the two needle cylinder fixing nozzles located in the middle of the gripper body, when the free handle is pulled, the pintle of the syringe is lifted by the effect of a lifting rod which is formed by the connection of the fixed nozzle of the pintle and the free handle to achieve the suction of liquid; when the free handle is loosened, the pintle of the syringe is lowered under the action of the spring to complete the bolus injection of the liquid. Fingers of an operator will not touch the pintle during the operation, so that the liquid contamination risk can be effectively prevented during the configuration of the intravenous infusion, the triggered pyrogenic transfusion response and the hidden medical security danger which endangers the life safety of patients are resolved.
Owner:李建国
On-body injector and method of use
An on-body injector and method of use including an on-body injector for use with an injection device. The on-body injector includes a bolus reservoir; a bolus injection needle in fluid communication with the bolus reservoir, the bolus injection needle having a bolus injection needle tip aligned with the injection port, the bolus injection needle being slideably biased away from the injection port to define a gap between the bolus injection needle tip and the injection port; and a button operably connected to the bolus injection needle to slide the bolus injection needle along the injection axis. The button is operable to advance the bolus injection needle tip to close the gap and advance the tip into the injection port. The button is further operable to advance a plunger through the bolus reservoir to deliver a predetermined bolus volume to the patient through the injection flow path.
Owner:MEDTRONIC MIMIMED INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com