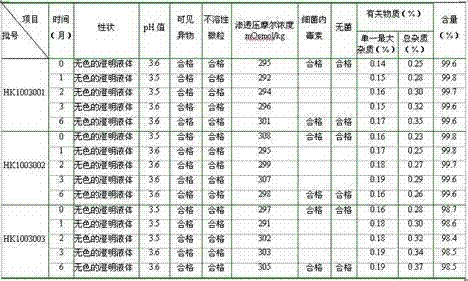
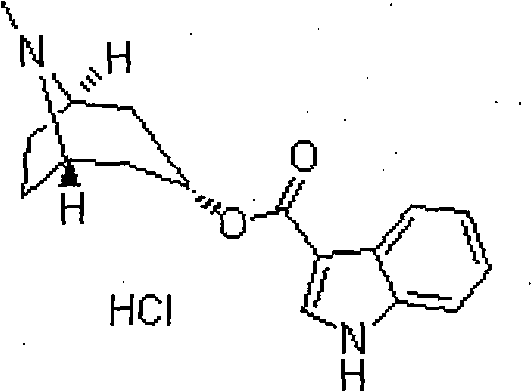
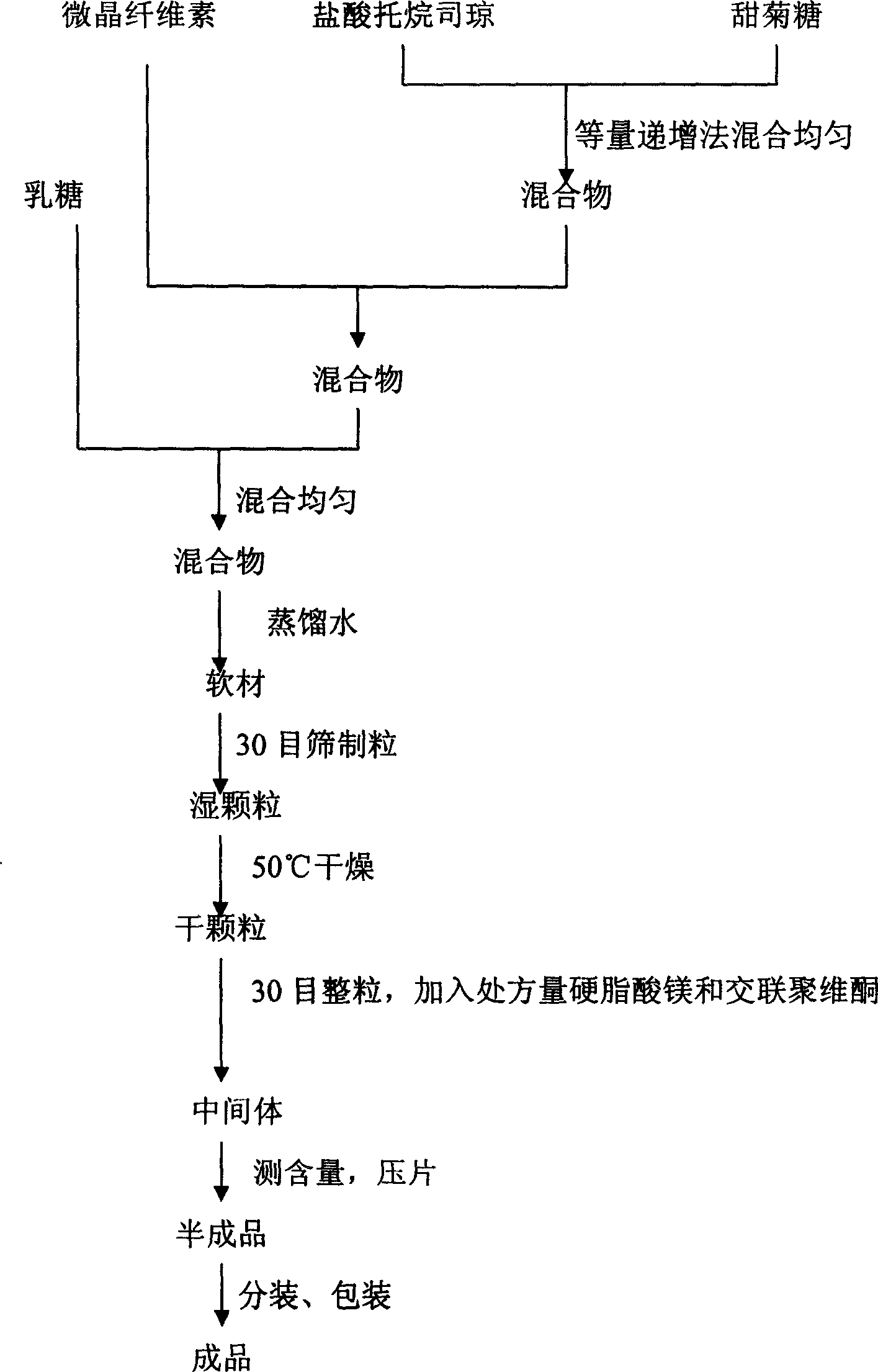
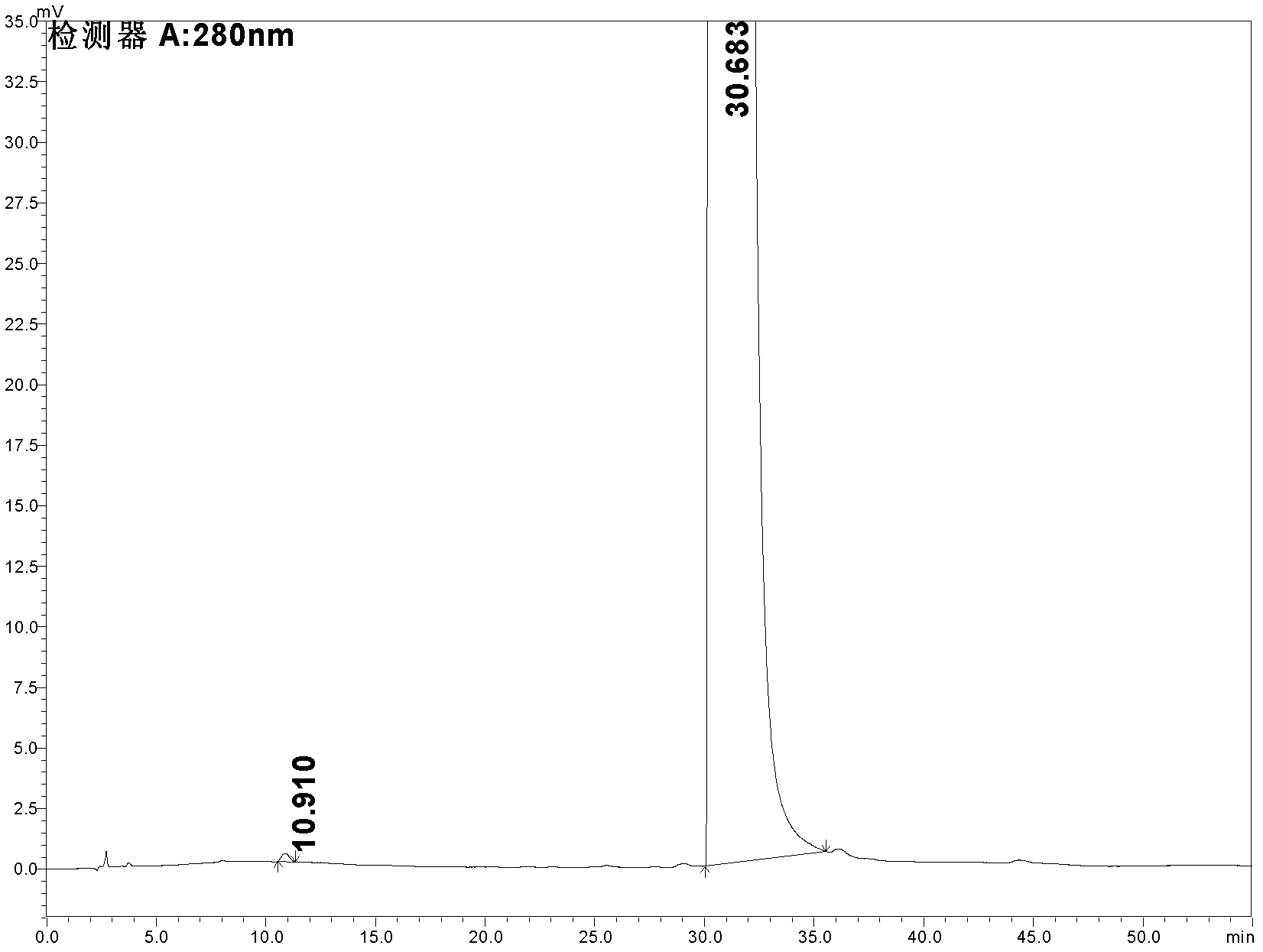
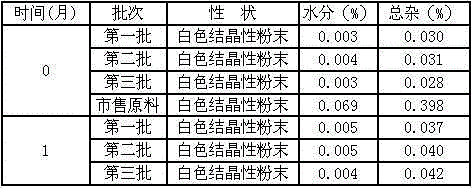
Patents
Literature
49 results about "Tropisetron Hydrochloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
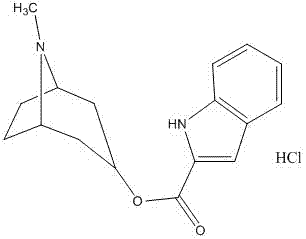
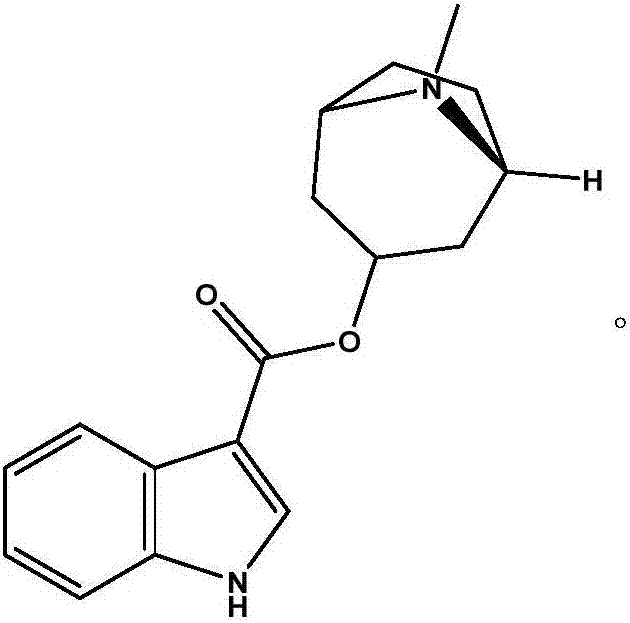
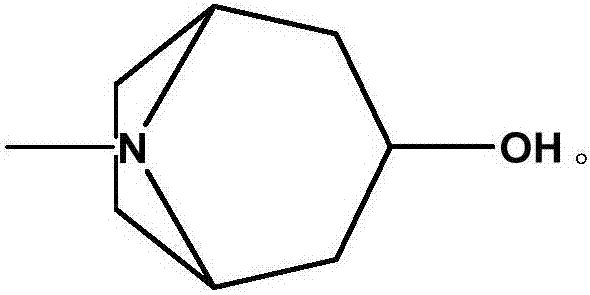
The hydrochloride salt form of tropisetron, a selective, competitive serotonin 5-hydroxytryptamine type 3 (5-HT3) receptor antagonist, with antinauseant and antiemetic activities. Tropisetron competitively binds to and blocks the action of serotonin at 5HT3 receptors peripherally on vagus nerve terminals located in the gastrointestinal (GI) tract as well as centrally in the chemoreceptor trigger zone (CTZ) of the area postrema of the central nervous system (CNS). This results in the suppression of chemotherapy- and radiotherapy-induced nausea and vomiting.
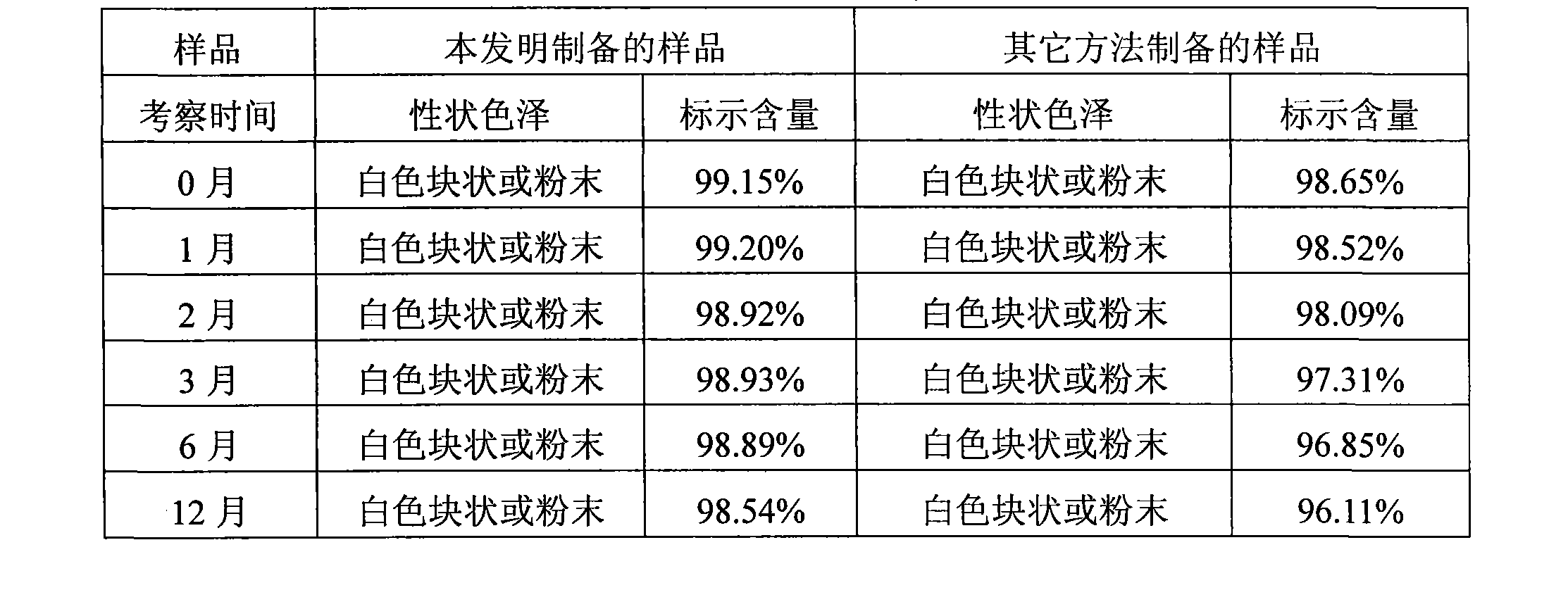
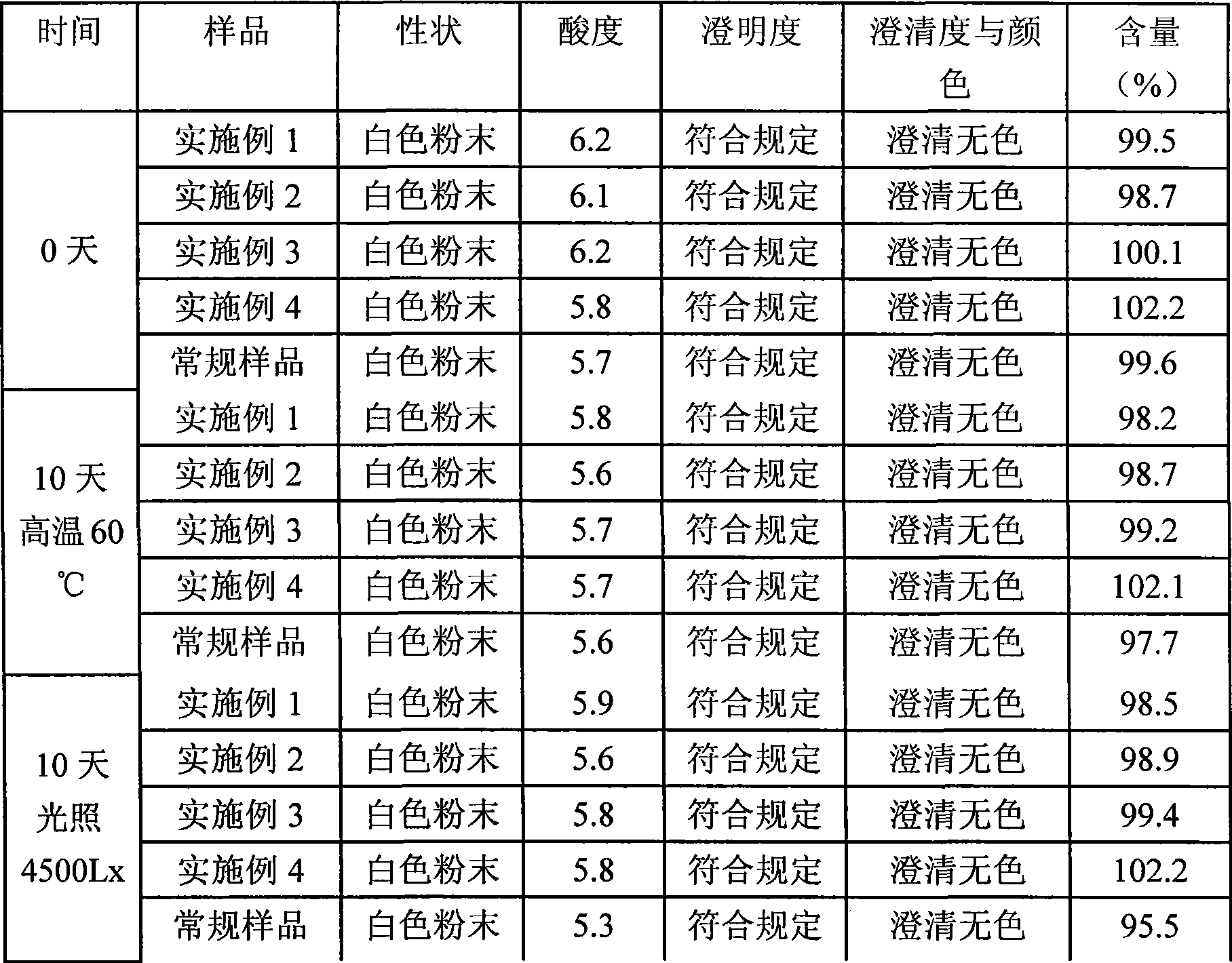
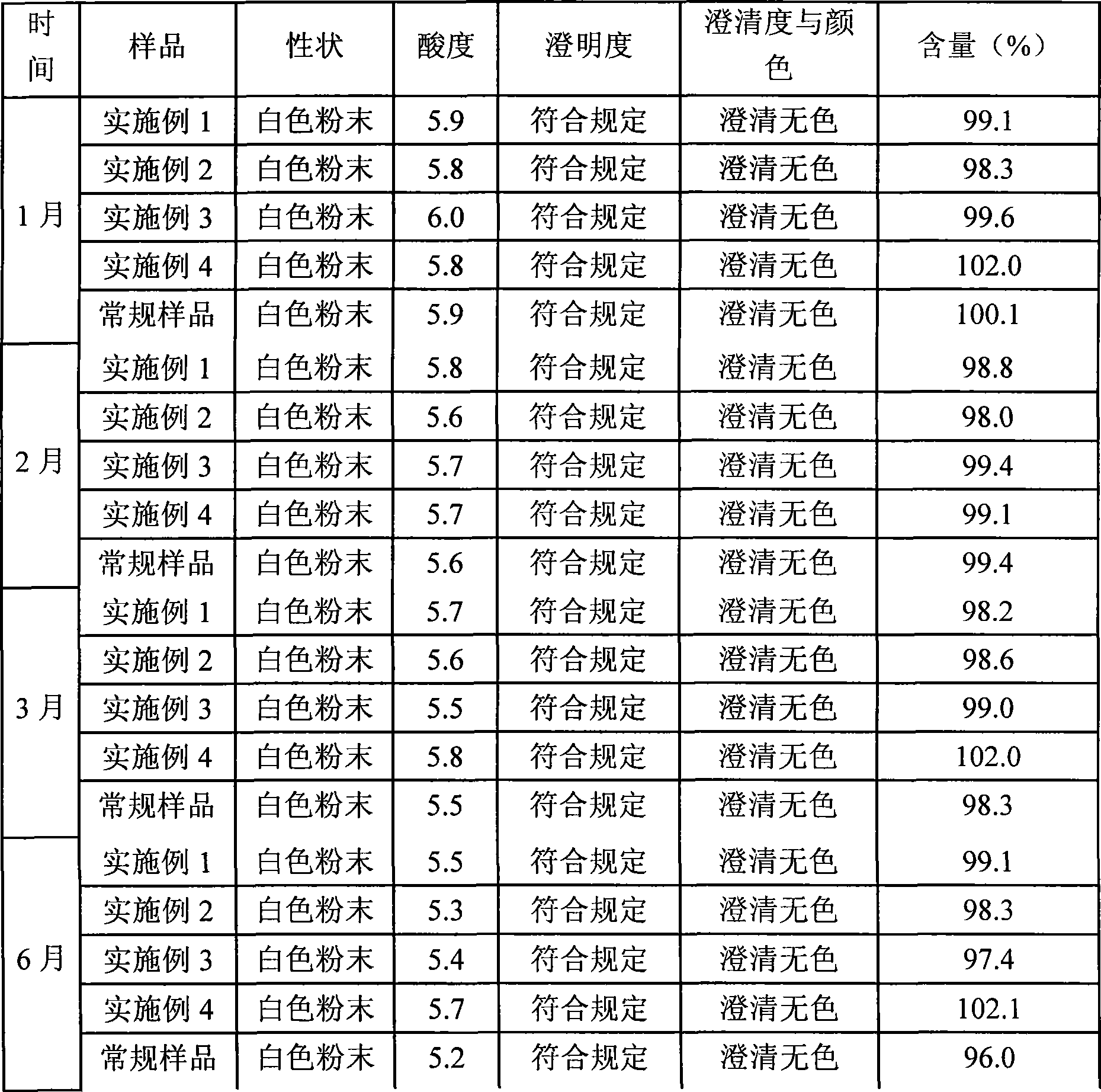
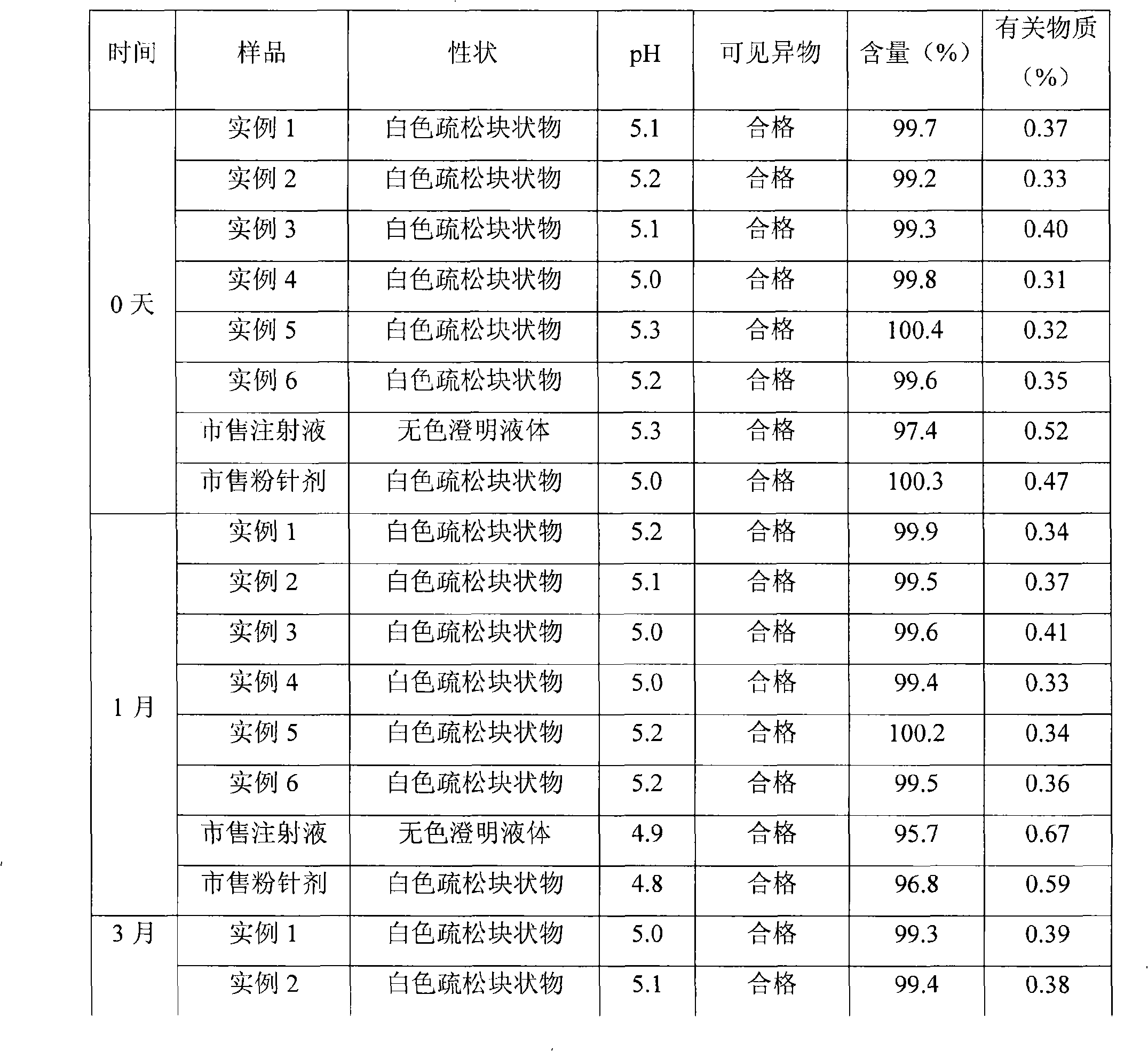
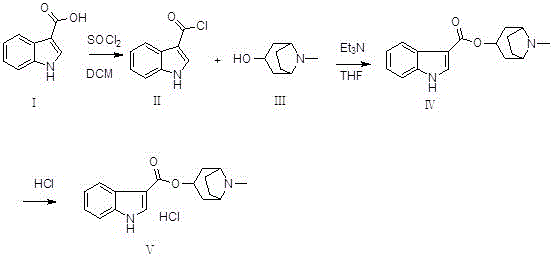
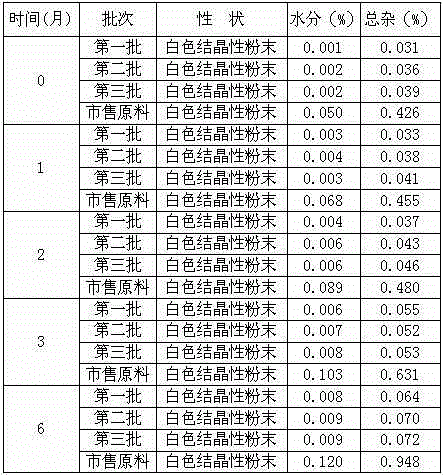
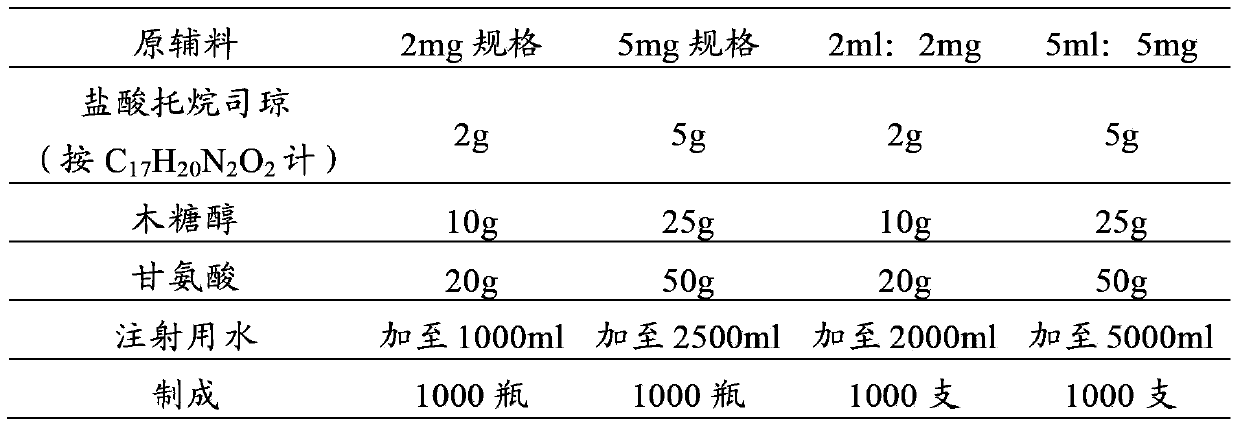
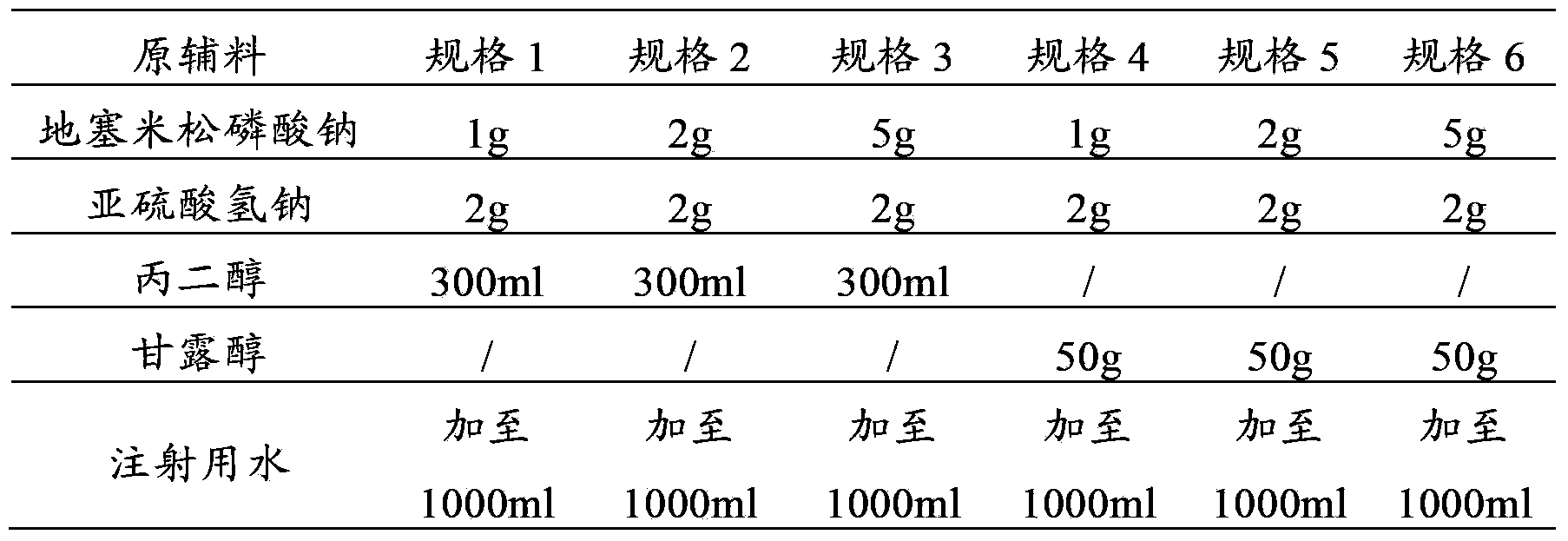
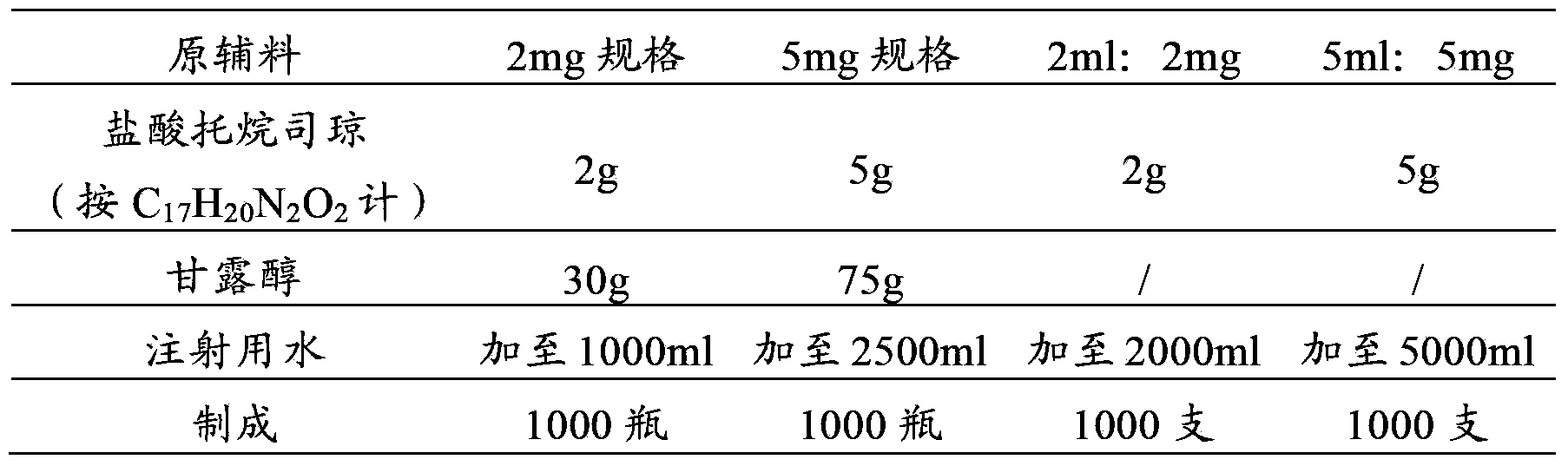
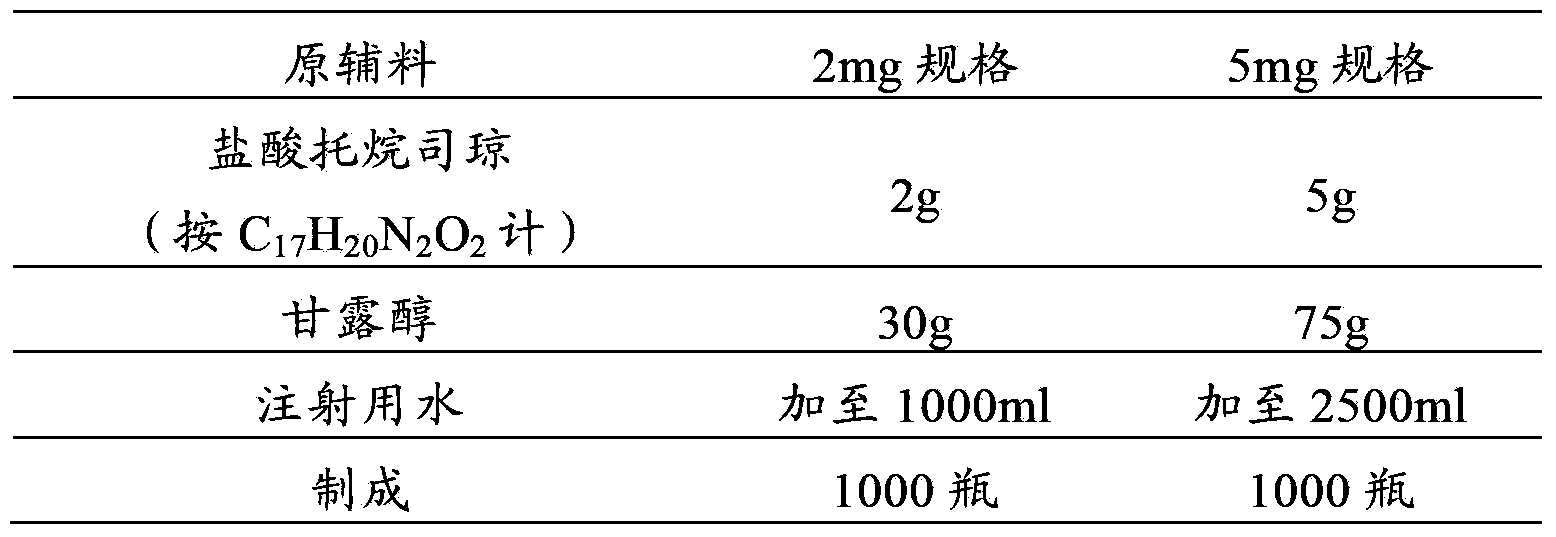
Tropisetron preparation for injection and preparation method thereof
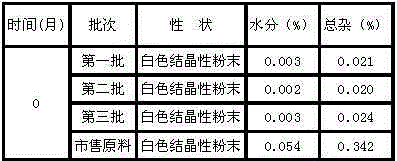
InactiveCN101444508AFlat surfaceNot crackedDigestive systemMacromolecular non-active ingredientsMANNITOL/SORBITOLActive component
The invention provides a tropisetron preparation for injection. The main active components of the tropisetron preparation are tropisetron, beta-cyclodextrin or hydroxypropyl-beta-cyclodextrin and mannitol. The method comprises the following steps: dissolving tropisetron hydrochloride and the beta-cyclodextrin or the hydroxypropyl-beta-cyclodextrin, then adding the mannitol for dissolving, adjusting the pH value with citric acid-disodium hydrogen phosphate buffer solution to obtain a liquid medicine, quickly prefreezing the liquid medicine after filling, and then lyophilizing to obtain the tropisetron preparation. The tropisetron preparation for injection has flat surface, is fine and smooth and uniform, and is free from crack, breakage and sticking to bottles. The tropisetron preparation is a white loose block which is well formed and very easily dissolved, has good redissolution performance, clean and transparent solution, stable product quality, and practicability.
Owner:海南瑞基药物研究有限公司
Tropisetron hydrochloride compound
Belonging to the field of medical technologies, the invention specifically relates to a tropisetron hydrochloride compound. The invention also relates to application of an injection containing the tropisetron hydrochloride compound in the crystal form of the invention in preparing medicaments for treating nausea and vomiting.
Owner:TIANJIN HANKANG PHARMA BIOTECH
High-purified tropisetron hydrochloride compound
InactiveCN101787021AHigh purityImprove product qualityOrganic chemistryActivated carbonAcid–base reaction
The invention relates to a tropisetron hydrochloride compound. The purity of the tropisetron hydrochloride is greatly improved by acid-base reaction, activated carbon adsorption and separation and purification prepared chromatographic columns, thus optimizing the quality of preparation products and the safety of clinical medication. The method has simple process, low cost and high yield, and is applicable to industrial production.
Owner:HAINAN LINGKANG PHARMA CO LTD
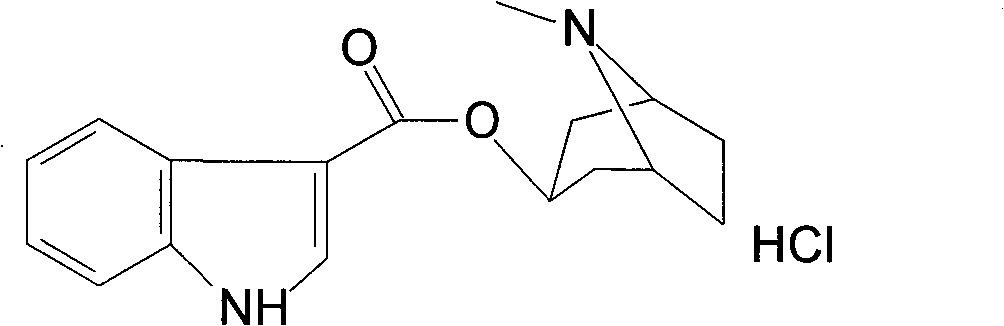
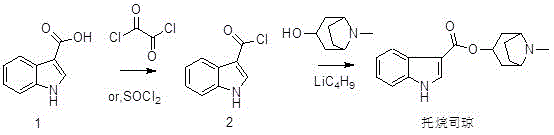
Synthetic method of tropisetron and prepare method of hydrochloric acid tropisetron
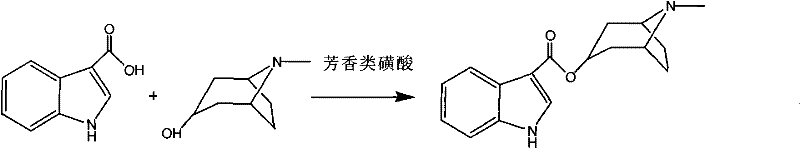
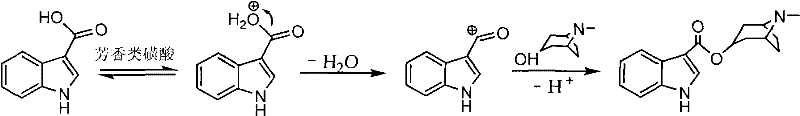
The invention relates to a synthetic method of tropisetron and a preparing method of hydrochloric acid tropisetron. The synthetic method of the tropisetron hydrochloride comprises enabling benzpyrole-3-formic acid and tropine to directly conduct esterification in atent solvent under effects of an aromatic organic sulfoacid compound catalytic agent. The tropisetron synthesized by using the method is then acidized, alkali adjusted, and refined to obtain the target product hydrochloric acid tropisetron. The synthetic method is simple in process, convenient to operate, small in pollution, high in product purity, ideal in yield, and applicable to large-scale industrialization production.
Owner:NEW FOUNDER HLDG DEV LLC +2
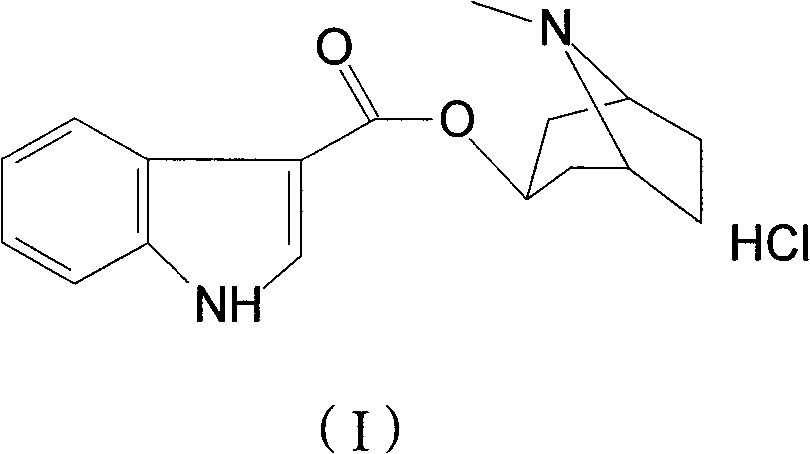
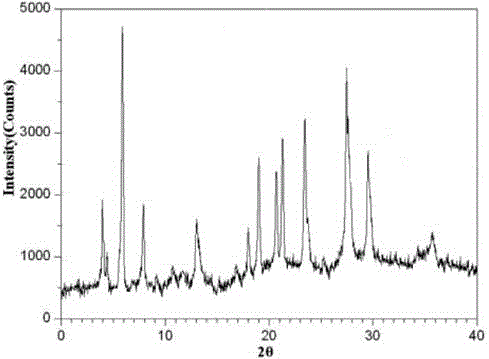
Tropisetron hydrochloride compound, its preparation method, and pharmaceutical composition containing the same
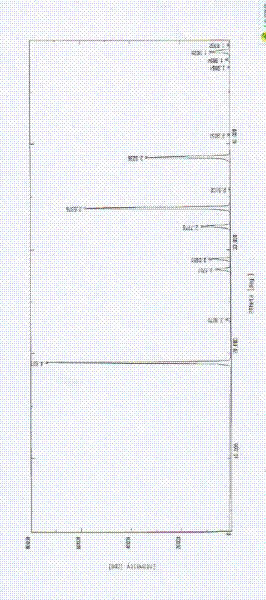
ActiveCN103360386AImprove stabilityQuality improvementOrganic chemistryDigestive systemStructural formulaCharacteristic X-ray
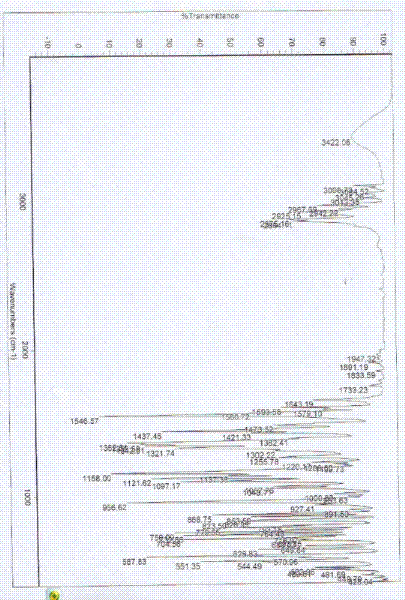
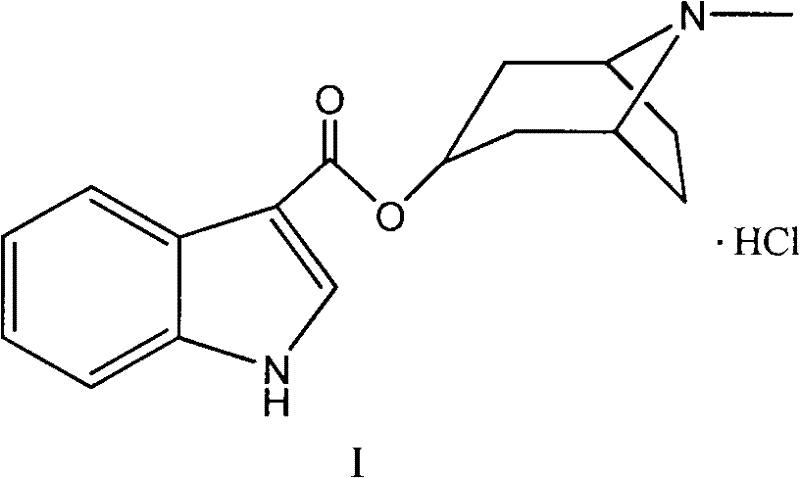
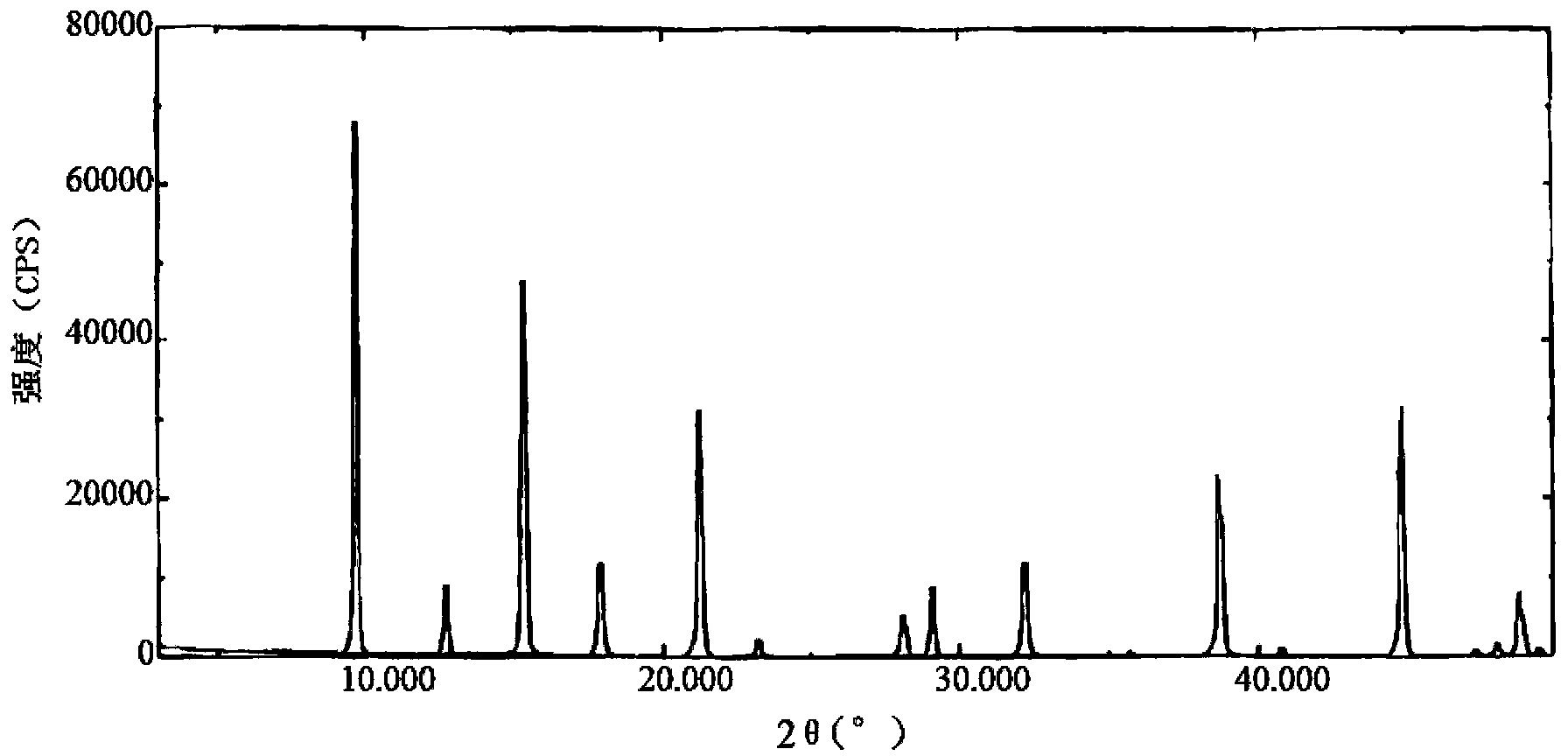
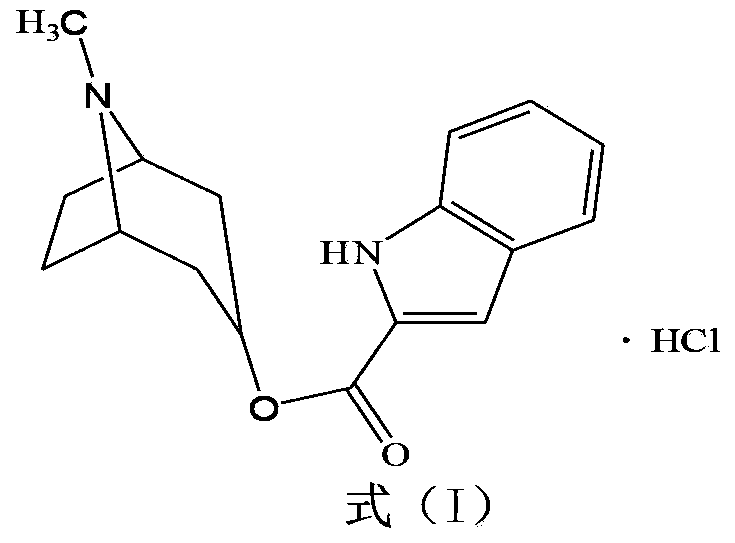
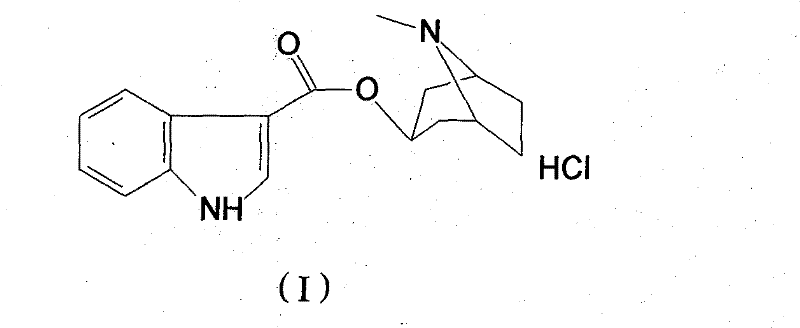
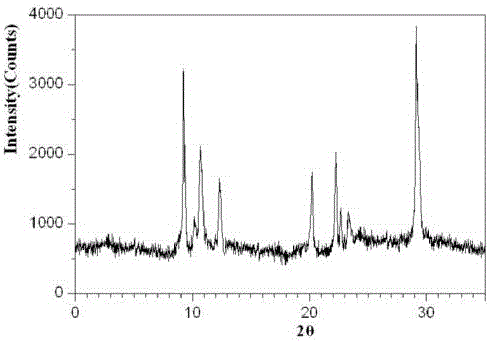
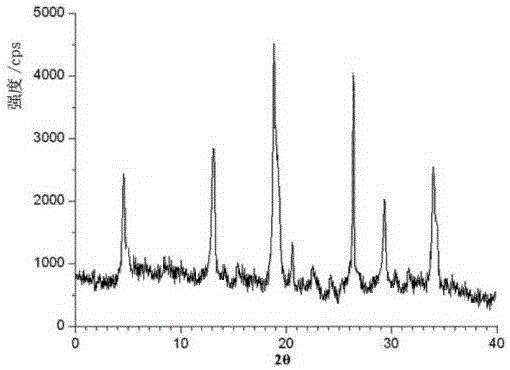
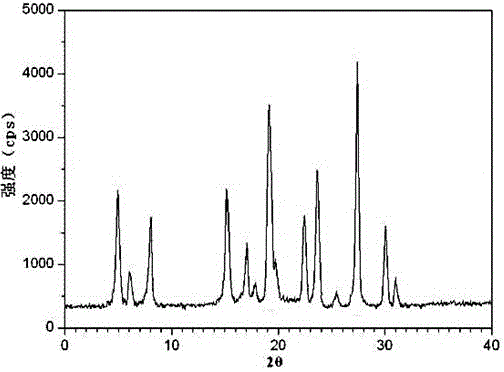
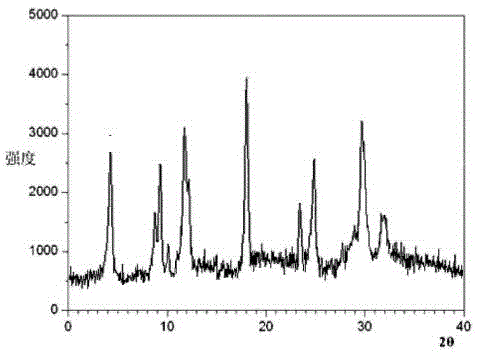
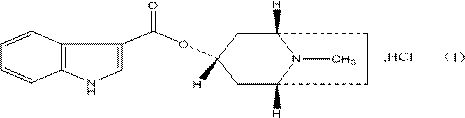
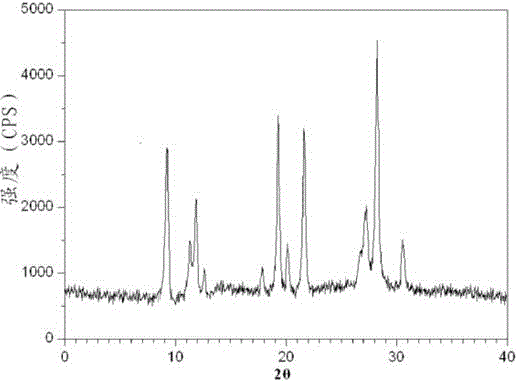
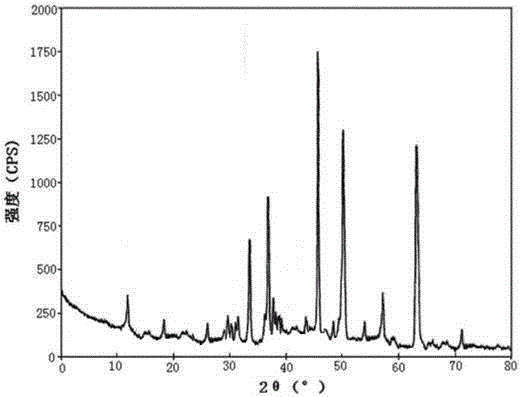
Belonging to the technical field of medicines, the invention particularly relates to a Tropisetron hydrochloride compound, its preparation method, and a pharmaceutical composition containing the same. The Tropisetron hydrochloride compound has a structural formula shown as formula (I), wherein, the Tropisetron hydrochloride compound adopts Cu-K alpha ray for characteristic X ray powder determination, the X ray powder atlas is as shown in figure 1. Compared with the prior art, the Tropisetron hydrochloride compound provided in the invention has a different crystal form, which has good stability and improved liquidity at the same time, thus improving the preparation quality.
Owner:金鸿药业股份有限公司
Triopisetron Hydrochloride microcapsule and method for preparing injection
InactiveCN101278921AImprove bioavailabilityImprove stabilityPowder deliveryDigestive systemFreeze-dryingHigh pressure
The invention provides a tropisetron hydrochloride freeze-dry preparation for treating emesis, containing tropisetron hydrochloride, gelatin, dextran, oleophilic emulsifier, hydrophilic emulsifier and freeze-dry supporting agent. The invention also relates to a preparation method of the tropisetron hydrochloride freeze-dry preparation, containing the following steps: (1) the tropisetron hydrochloride is added into injection water and completely dissolved; (2) the gelatin and the oleophilic emulsifier are added in solution prepared in step (1) to prepare water-in-oil emulsion; (3) dextran solution and the hydrophilic emulsifier are added in the solution prepared in step (2) to prepare water-in-oil-in-water double emulsion; (4) the prepared double emulsion is dropped by bolus injection into strong base solidified liquor in a high-voltage electrostatic field by an orifice; after solidifying, a microcapsule is filtered and washed by water or PH regulator to lead PH to be 5-7; (5) the freeze-dry supporting agent is dissolved in the injection water and mixed with the microcapsule evenly; after subpackage and freeze drying, the tropisetron hydrochloride freeze-dry preparation is obtained.
Owner:HAINAN LINGKANG PHARMA CO LTD
Tropisetron hydrochloride medicament composition for injection
InactiveCN102302495AFix stability issuesHigh yieldInorganic non-active ingredientsPharmaceutical delivery mechanismPharmaceutical drugMedicinal chemistry
The invention discloses a tropisetron hydrochloride medicament composition for injection. The medicament composition consists of tropisetron hydrochloride, sodium chloride and citric acid, and is characterized in that the weight ratio of tropisetron hydrochloride to citric acid is 1:(0.001-5). The preparation method of the medicament composition comprises the following steps: taking 95% of prescription amount of water for injection; introducing dioxide at the temperature of 30-40 DEG C until the pH value is at the rang of 3.0-4.0; adding prescription amount of sodium chloride and citric acid, stirring and dissolving; adding prescription amount of tropisetron hydrochloride, and stirring to dissolve completely; adding medicinal charcoal in the solution, and standing after stirring uniformly; filtering in vacuum, supplementing water for injection to full amount, and mixing uniformly; measuring the initial pH value, and regulating the pH value to the range of 3.0-4.0 with 4% sodium hydroxide solution and 10% citric acid solution according to the initial pH value; carrying out fine filtering; filling; sterilizing; carrying out lamp inspection; and warehousing, thus the finished product is obtained. The tropisetron hydrochloride medicament composition has good stability to light, has good stability, and has the obvious advantages for improving the product yield, lowering cost, implementing industrialization, and realizing clinical application better.
Owner:TIANJIN HANKANG PHARMA BIOTECH
Tropisetron hydrochloride liposome injection
InactiveCN102327222AQuality improvementImprove bioavailabilityDigestive systemLiposomal deliverySide effectCholesterol
The invention provides a tropisetron hydrochloride liposome injection. The tropisetron hydrochloride liposome injection is mainly prepared from tropisetron hydrochloride, glycocholatesodium, cholesterol, gelatin, antioxygen and additive. The liposome injection improves the quality of preparation products, reduces toxic and side effects, improves bioavailability, and is high in preparation stability; and liposome is not broken due to dehydration, fusion, crystallization and the like during freeze-drying, and the liposome still keeps a good entrapment rate after the liposome is subjected to hydration and dissolution.
Owner:HAINAN LINGKANG PHARMA CO LTD
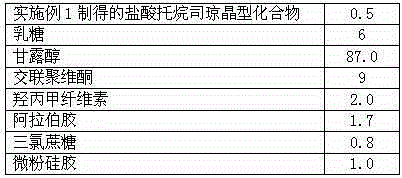
Tropisetron hydrochloride oral disintegration tablet preparation and its preparing method
InactiveCN1682721AEasy to take medicineNo gritOrganic active ingredientsDigestive systemMANNITOL/SORBITOLOrally disintegrating tablet
The present invention relates to medicine preparation, and a kind of orally disintegrated tropisetron hydrochloride tablet and its preparation process. The orally disintegrated tropisetron hydrochloride tablet is prepared with tropisetron hydrochloride as effective component, and lactose, mannitol, crosslinked polyvidon, magnesium stearate, microcrystalline cellulose, distilled water and steviosin as supplementary material, and through mixing in certain proportion and wet tabletting. It has hardness of 1.0-3.5 kg, and may be disintegrated in oral cavity within 1 min. It may be prepared in common tabletting machine.
Owner:王立强
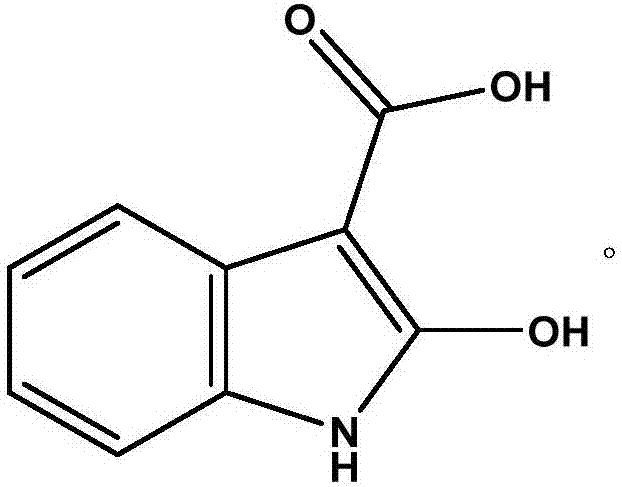
Detecting method for indole-3-carboxylic acid of impurities in tropisetron hydrochloride raw materials and preparations
ActiveCN102507796AEliminate distractionsStrong specificityComponent separationGradient elutionCarboxylic acid
The invention relates to a detecting method for indole-3-carboxylic acid, in particular to a detecting method for indole-3-carboxylic acid of impurities in tropisetron hydrochloride raw materials and preparations. The detecting method includes the steps: (1) taking the tropisetron hydrochloride raw materials or preparations, performing dissolving and diluting to realize constant volume by means of initial flowing phase, preparing liquor containing 0.5mg of tropisetron hydrochloride per milliliter, and using the liquor as sample liquor by tropisetron; (2) taking indole-3-carboxylic acid reference substances, performing dissolving and diluting to realize constant volume by means of initial flowing phase, preparing liquor containing 0.5mug of indole-3-carboxylic acid per milliliter, and using the liquor as reference substance liquor; and (3) respectively filling 50mul of sample liquor prepared in the step (1) and 50mul of reference substance liquor prepared in the step (2) into a high performance liquid chromatograph for gradient elution. The detecting method has the advantages of high specificity, accuracy in quantification, high sensitivity and stability, and the limit of detectingdrug quality is improved to a high standard.
Owner:SHANDONG QIDU PHARMA
Method for detecting alpha-tropine in tropisetron hydrochloride injection
InactiveCN102495170AHigh resolutionHigh sensitivityComponent separationImage resolutionQuality control
The invention relates to a detection method of alpha-tropine, especially to a method for detecting alpha-tropine in a tropisetron hydrochloride injection. The method comprises the steps of: respectively preparing a sample solution and a reference solution, dripping the solutions respectively on one high efficiency silica gel GF254 thin-layer plate, and conducting development, then taking out and air drying the thin-layer plate, which is then dyed in a potassium iodobismuthate solution, and finally viewing the result. If the sample solution shows a speckle at the position of alpha-tropine in the reference solution, the sample solution can be determined to contain alpha-tropine, and the content of alpha-tropine is determined through color strength comparison of the speckle shown by the sample solution and the speckle shown by the reference solution. Characterized by high resolution, high sensitivity, simplicity and speediness, etc., the method of the invention can detect alpha-tropine with content less than 0.30 microgram in a tropisetron hydrochloride injection, thus providing guarantee for quality control during tropisetron hydrochloride injection preparation.
Owner:SHANDONG QIDU PHARMA
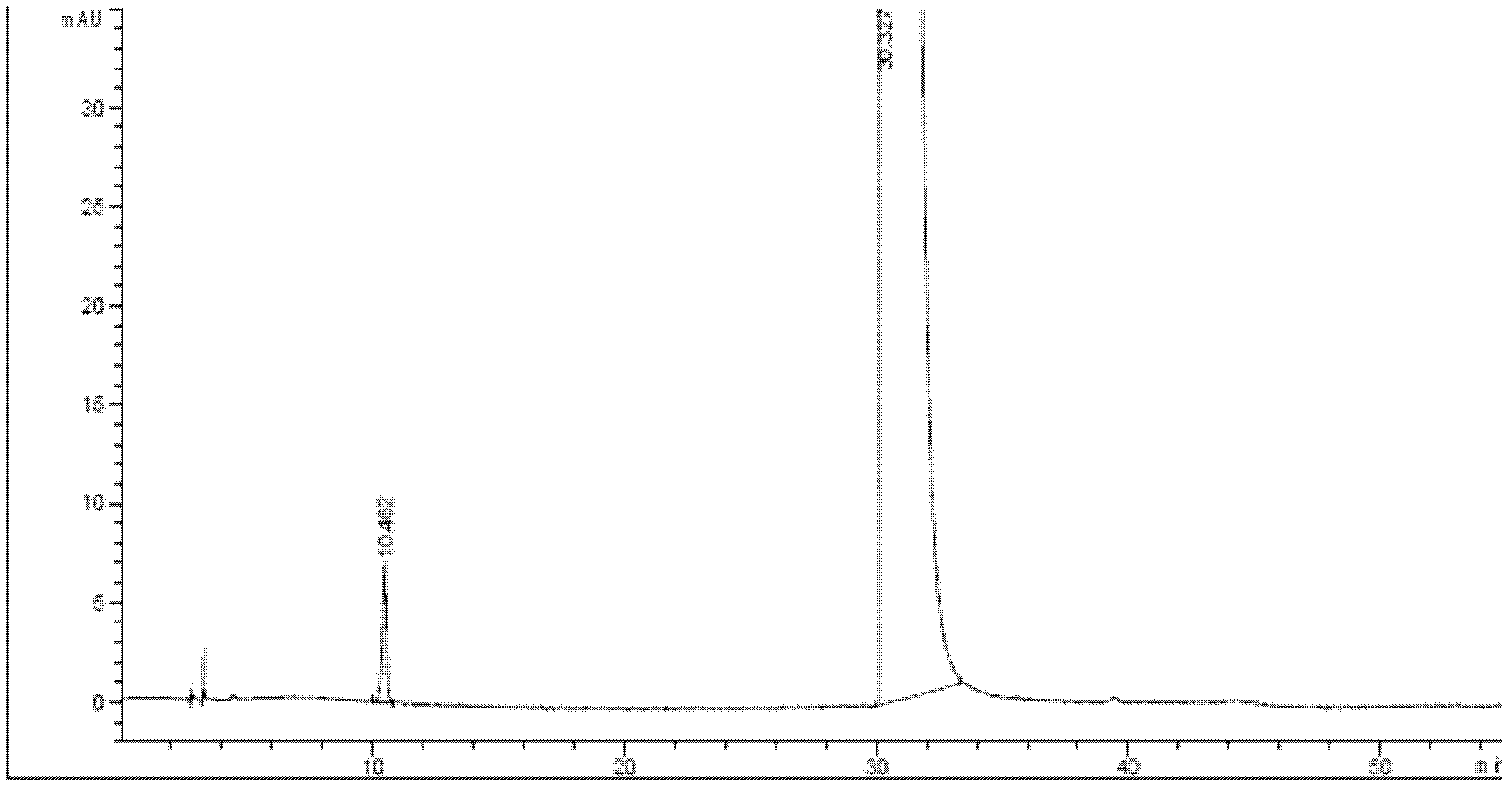
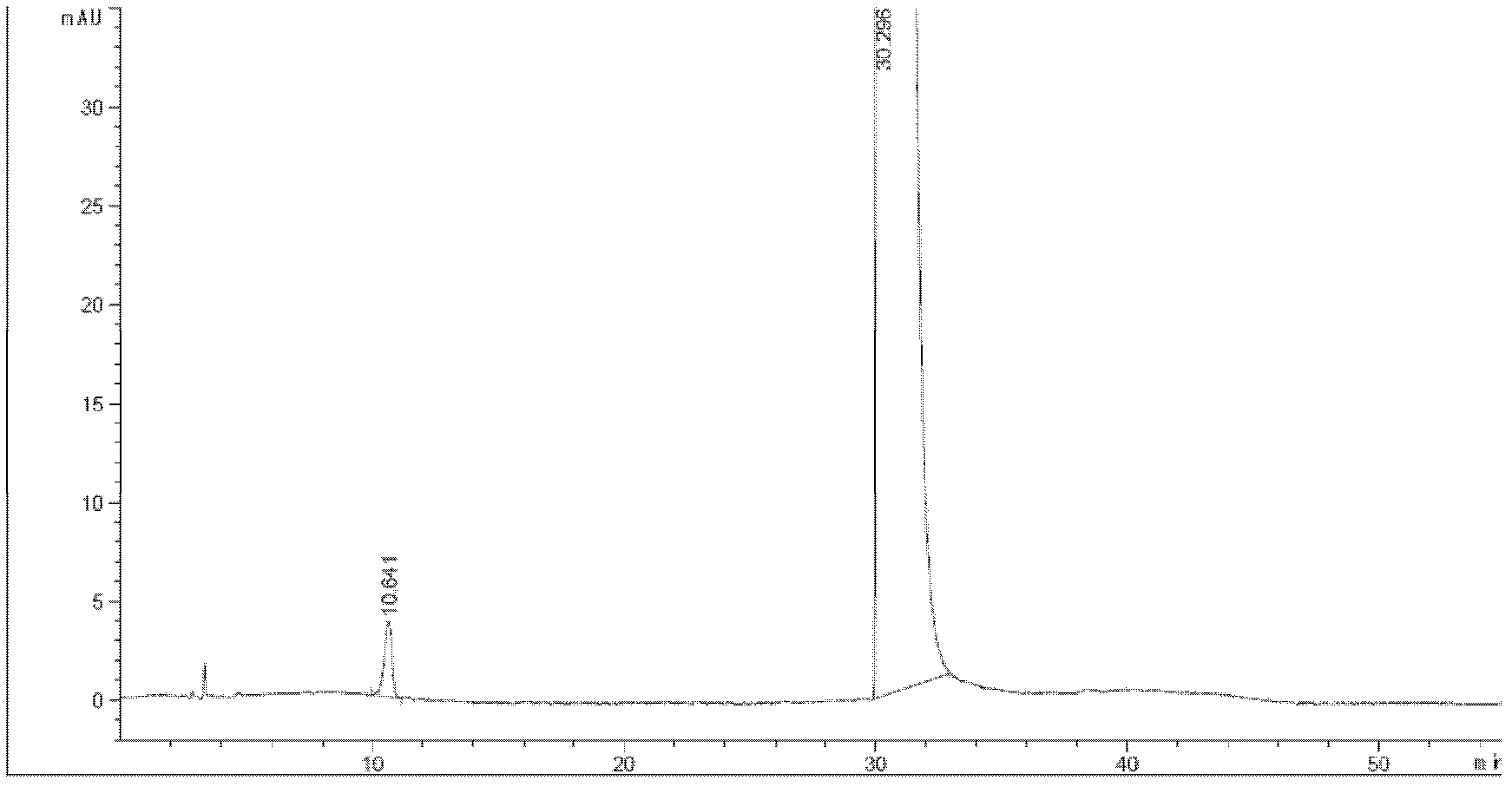
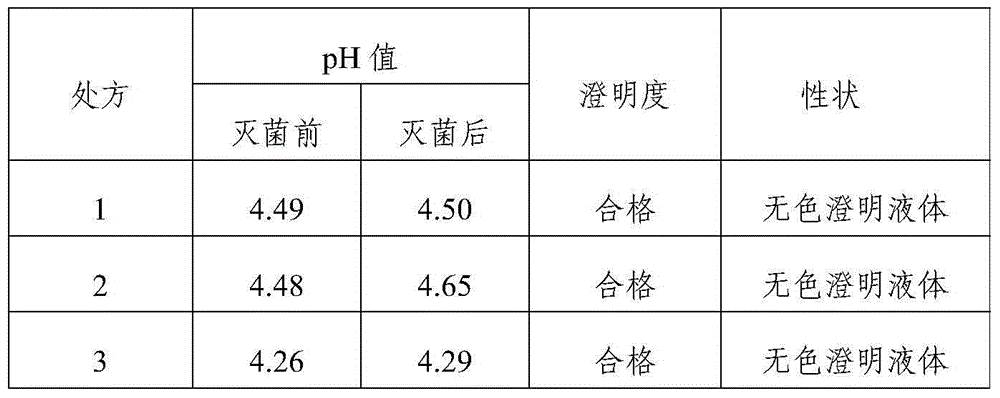
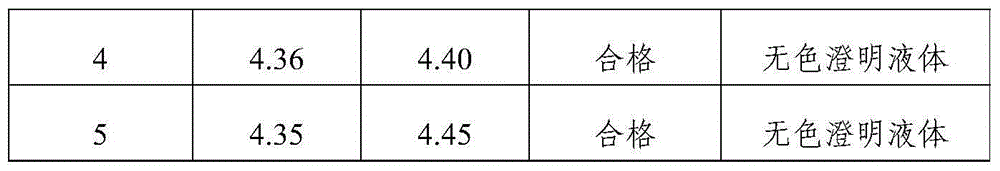
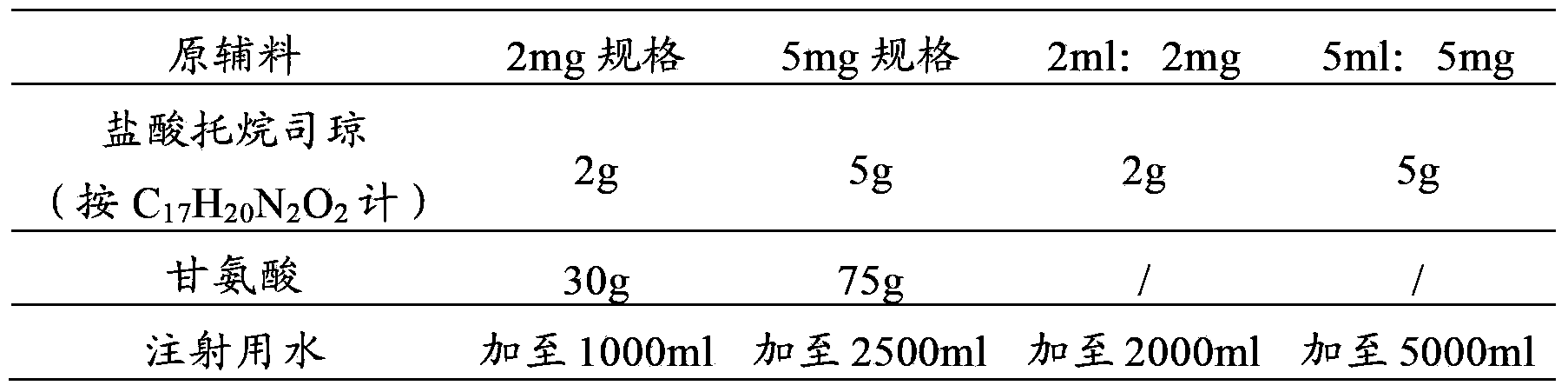
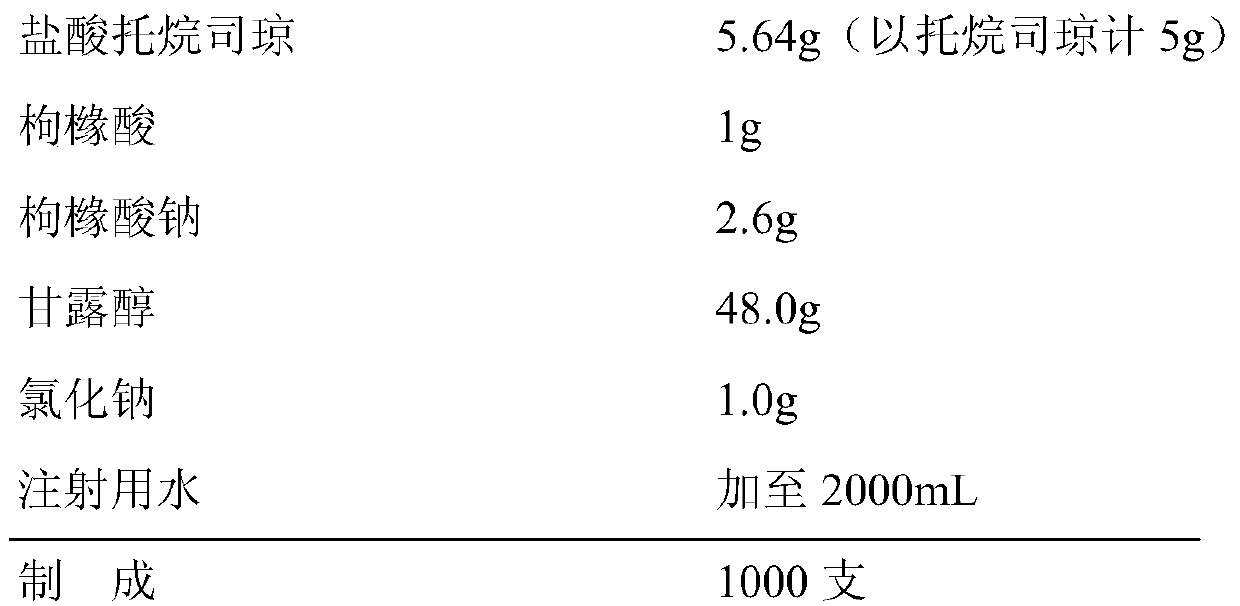
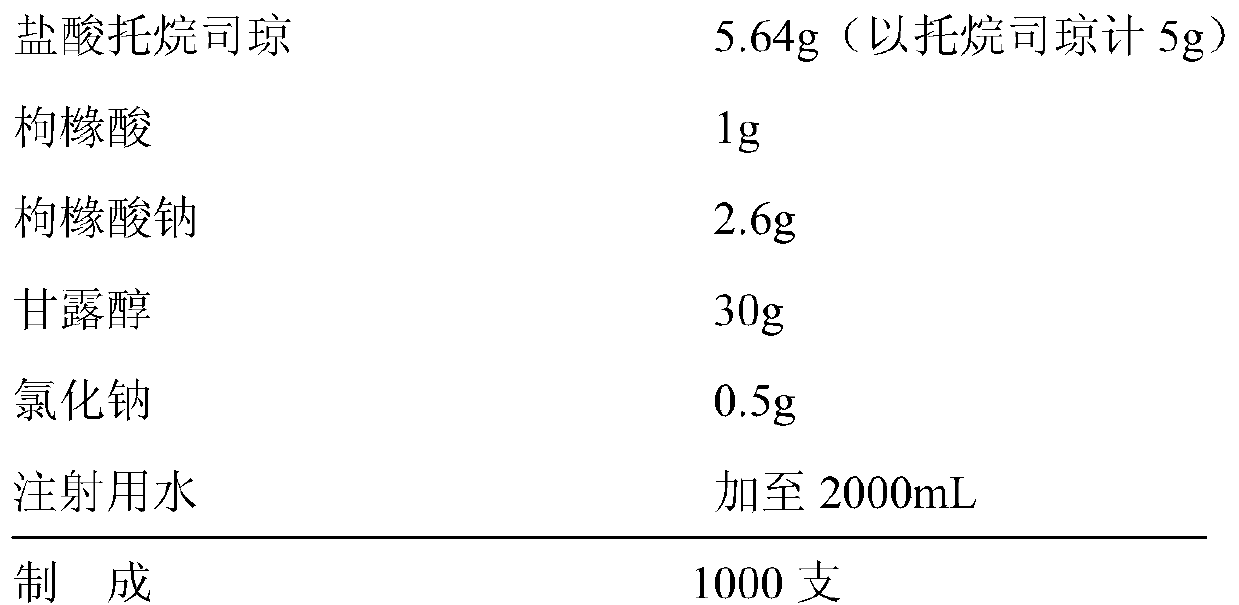
Tropisetron hydrochloride injection and preparation method thereof
ActiveCN104606130AImprove sterilitySimple processDigestive systemPharmaceutical delivery mechanismSolubilitySodium acetate
The invention relates to the field of medicine, in particular to a tropisetron hydrochloride injection and a preparation method thereof. The injection is prepared from the following constituents in parts by weight: 1.13-28.2 parts of tropisetron hydrochloride, 3-75 parts of sodium acetate, 1.7-42.5 parts of glacial acetic acid, 1.2-30 parts of sodium chloride, and 3000-6000 parts of water for injection. The preparation method comprises the following steps: (1) weighing tropisetron hydrochloride, adding the weighed tropisetron hydrochloride into the water for injection, which accounts for 60-95% of the total prescription dosage, adding sodium acetate, glacial acetic acid and sodium chloride to the water, stirring to enable the substances to be dissolved, adding activated carbon for needle, stirring and using a titanium bar to coarsely filtering out and removing carbon; (2) adding the rest water for injection, and sequentially using microfiltration membranes of 0.60-0.45 microns and 0.45-0.10 microns to filter the solution. The tropisetron hydrochloride injection provided by the invention is a colorless clear liquid, high in clarity, good in solubility, low in impurities, free of adverse effects, and convenient and safe to use.
Owner:KAMP PHARMA
High-purified tropisetron hydrochloride compound
InactiveCN101787021BHigh purityImprove product qualityOrganic chemistryActivated carbonAcid–base reaction
The invention relates to a tropisetron hydrochloride compound. The purity of the tropisetron hydrochloride is greatly improved by acid-base reaction, activated carbon adsorption and separation and purification prepared chromatographic columns, thus optimizing the quality of preparation products and the safety of clinical medication. The method has simple process, low cost and high yield, and is applicable to industrial production.
Owner:HAINAN LINGKANG PHARMA CO LTD
Preparation method of tropisetron hydrochloride
InactiveCN106831754ALow investment costEase of industrial productionOrganic chemistry3-indolecarboxylic acidEnergy conservation
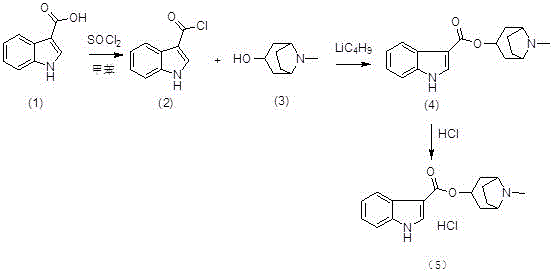
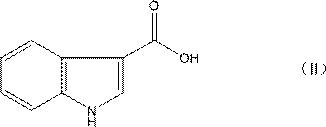
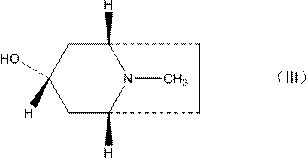
The invention discloses a preparation method of tropisetron hydrochloride. The Preparation method of the tropisetron hydrochloride is characterized by comprising the following preparation steps: (A) performing substation reaction on 3-indolecarboxylic acid (I) serving as a starting material and thionyl chloride by adding a reaction solvent to prepare 3-indoleformyl chloride (II), and directly performing condensation reaction on the 3-indoleformyl chloride (II) and alpha-tropine (III) under the existence of an acid-binding agent triethylamine to produce tropisetron (IV); and (B) performing acid and alkali salt-forming reaction on the tropisetron (IV) and hydrochloric acid in ethanol to produce the tropisetron hydrochloride (V). Raw materials used in the process reactions are the conventional reagents, are easily available on the market and are low in cost; the conditions are simple and not harsh, the conventional equipment can meet production requirements and are easy to control, and industrialized production can be realized; and dichloromethane and tetrahydrofuran have low boiling point and are easily evaporated to dryness, so energy conservation and safety are achieved.
Owner:KAMP PHARMA
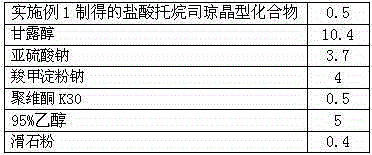
Medicinal tropisetron hydrochloride composition capsule for treating nausea and vomiting
InactiveCN105125518AHigh purityImprove liquidityDigestive systemPharmaceutical non-active ingredientsSulfite saltHave Nausea
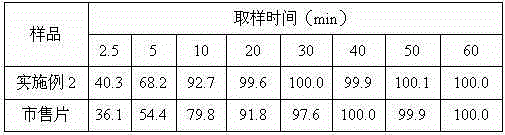
The invention discloses a medicinal tropisetron hydrochloride composition capsule for treating nausea and vomiting, which belongs to the technical field of medicines. The composition is prepared from tropisetron hydrochloride, mannitol, sodium sulfite, carboxymethyl starch sodium, povidone K30, 95 percent ethanol and talc powder, wherein the tropisetron hydrochloride is a new crystalline compound; an X-ray powder diffraction pattern, which is obtained by measurement of Cu-Kalpha rays, of the new crystalline compound, is as shown in figure 1, and the new crystalline compound is tropisetron hydrochloride different from the tropisetron hydrochloride reported in the prior art; tests prove that the new crystalline compound has the advantages of high purity, good flowability, good stability, low content of impurities, low possibility of moisture absorption, and safety and reliability in clinical application; the capsule prepared from the new crystalline compound is high in dissolution rate, good in stability and very suitable for clinical application.
Owner:QINGDAO HUAZHICAO PHARMA CO LTD
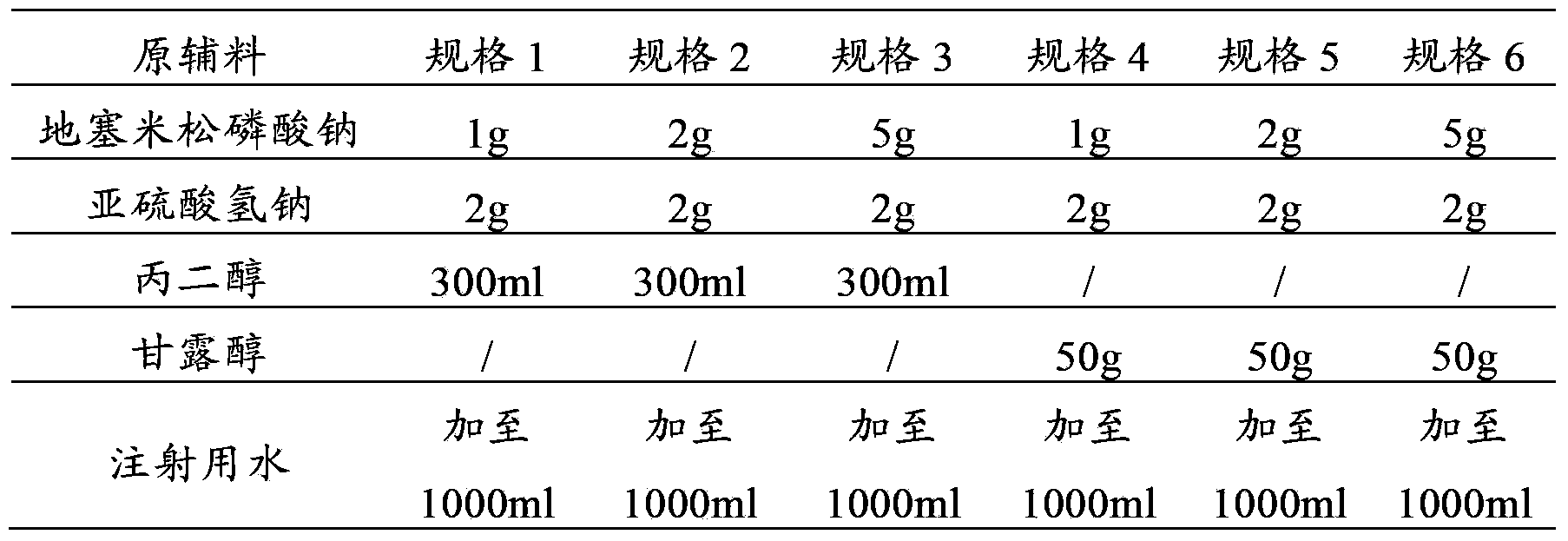
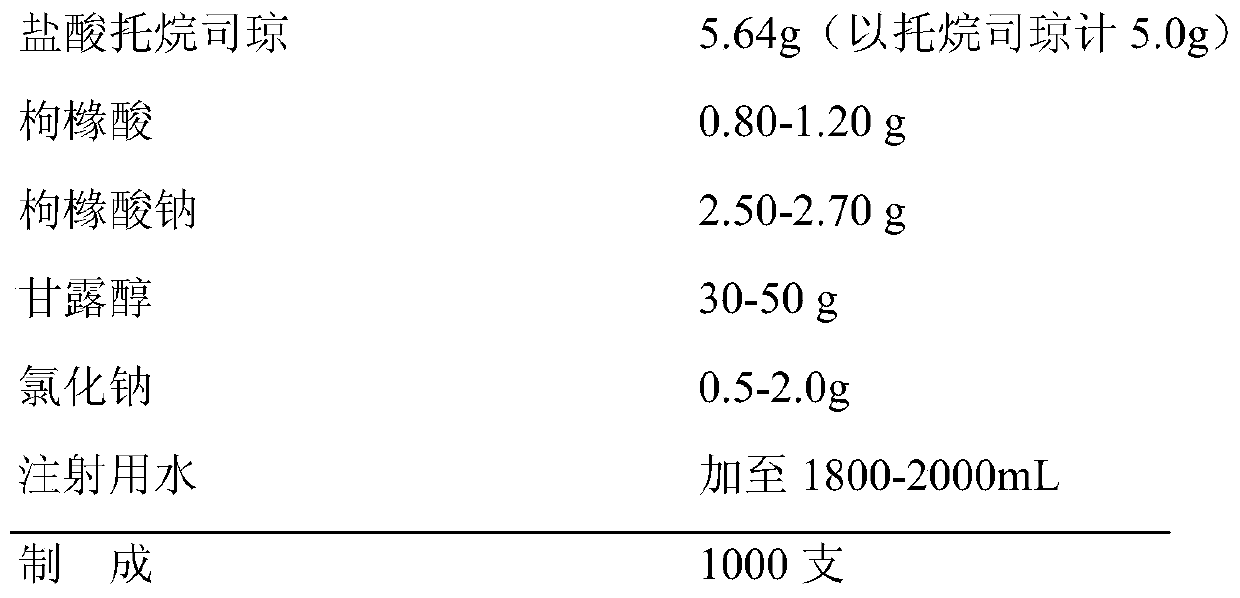
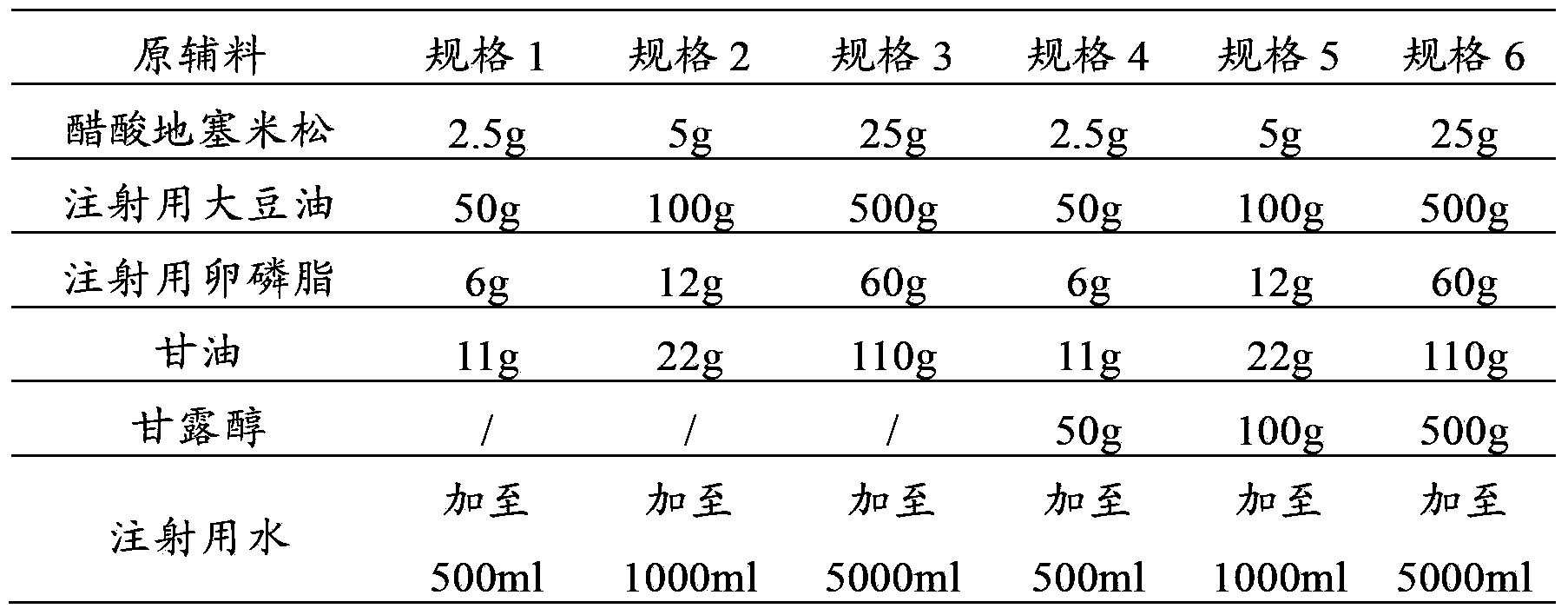
Pharmaceutical composition containing tropisetron hydrochloride and dexamethasone sodium phosphate
InactiveCN103393701AImprove antiemetic efficacyDigestive systemHeterocyclic compound active ingredientsFructoseRinger's solution
The invention relates to a pharmaceutical composition containing containing tropisetron hydrochloride and dexamethasone sodium phosphate, and particularly relates to a combined application package containing a tropisetron hydrochloride injection and a dexamethasone sodium phosphate injection. When the combined application package is in use, the tropisetron hydrochloride injection and the dexamethasone sodium phosphate injection are co-dissolved in normal saline, a Ringer's solution, 5% glucose injection, a fructose solution and the like for intravenous infusion, so that a curative effect of stopping vomit can be increased.
Owner:HAINAN LINGKANG PHARMA CO LTD
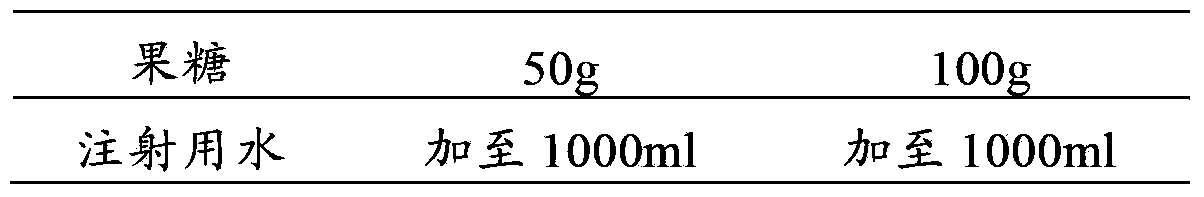
Pharmaceutical composition containing tropisetron hydrochloride and fructose
The invention relates to a pharmaceutical composition containing a tropisetron hydrochloride injection and a fructose injection, and particularly relates to a composite application package comprising the tropisetron hydrochloride injection and the fructose injection. In use, the tropisetron hydrochloride injection is dissolved into the fructose injection, and then the mixture is used for intravenous infusion. The pharmaceutical composition containing the tropisetron hydrochloride injection and the fructose injection has the effect of treating postoperative nausea and emesis, and also is capable of replenishing energy and body fluid of human body.
Owner:HAINAN LINGKANG PHARMA CO LTD
Tropisetron-containing composition for injection
InactiveCN107375208AEase of industrial productionSimple methodDigestive systemPharmaceutical delivery mechanismGlycerolActive ingredient
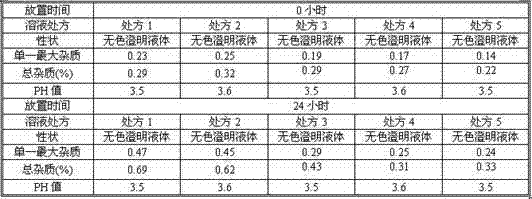
The invention discloses a tropisetron-containing composition for injection, which can enhance the stability of Tropisetron Hydrochloride Injection in the long-term storage process and reduce the degradation of a raw material, namely tropisetron, in the storage process to generate an impurity A and an impurity B. By addition of aspartic acid and sodium salt thereof in the injection, the degradation of tropisetron is effectively inhibited. Meanwhile a stable pH environment is provided, and the storage stability of a final formulation is further enhanced. In the meantime, a problem that salt forms of drug substances are easily dissociated and separated out during long-term storage of the injection to generate insoluble particles, resulting in the unacceptance of visible particles is solved effectively by applying a crystallization inhibitor, namely glycerin, in the injection. At the same time, as a small molecule, the glycerol also plays a role in regulation of osmotic pressure. The obtained tropisetron injection is simple in preparation method, and capable of being produced by using a conventional preparation method, and has obvious advantages of being prone to industrialization, high in production efficiency, good in stability, controllable in quality, and the like.
Owner:天津双硕医药科技有限公司
Tropisetron hydrochloride composition injection capable of treating nausea and vomiting caused by chemotherapy
InactiveCN105078884AHigh purityImprove liquidityOrganic chemistryDigestive systemHave NauseaMoisture absorption
The invention relates to tropisetron hydrochloride composition injection capable of treating nausea and vomiting caused by chemotherapy, belonging to the technical field of medicines. The composition injection is composed of tropisetron hydrochloride and calcium chloride, wherein tropisetron hydrochloride is crystal, and an X-ray powder diffraction diagram obtained by Cu-Kalpha ray measurement is shown as figure I. The novel crystal form of tropisetron hydrochloride provided by the invention is different from the crystal structure in the prior art, through experimental verification, the inventor surprisingly finds that the compound with the novel crystal form is high in purity, good in mobility, high in stability, low in impurity content, and safe and reliable in clinic application, the moisture absorption probability is small, and the injection prepared by adopting the compound with the novel crystal is simple in component and good in stability.
Owner:QINGDAO HUAZHICAO PHARMA CO LTD
Pharmaceutical tropisetron hydrochloride composition dry suspension for treating nausea and vomiting both caused by chemotherapy
InactiveCN105193729AHigh purityImprove liquidityPowder deliveryOrganic chemistryNausea sicknessVomiting medication
The invention belongs to the technical field of medicine, and relates to a pharmaceutical tropisetron hydrochloride composition dry suspension for treating nausea and vomiting both caused by chemotherapy. The composition comprises tropisetron hydrochloride, instant sorbitol, glucose, xanthan gum and aishenglan. The tropisetron hydrochloride is a new crystal-form compound, an X-ray powder diffraction map obtained from Cu-K alpha ray measurement is shown as a map 1, and the tropisetron hydrochloride is different from tropisetron hydrochloride reported in the prior. Experiments show that the new crystal-form compound is high in purity, mobility and stability, low in impurity content, less prone to moisture absorption and safe and reliable in clinical application, and the dry suspension made from the crystal-form compound is high in stability and quite suitable for clinical application.
Owner:杨献美
Detecting method for indole-3-carboxylic acid of impurities in tropisetron hydrochloride raw materials and preparations
ActiveCN102507796BMethod stableStrong specificityComponent separationCarboxylic acidGradient elution
The invention relates to a detecting method for indole-3-carboxylic acid, in particular to a detecting method for indole-3-carboxylic acid of impurities in tropisetron hydrochloride raw materials and preparations. The detecting method includes the steps: (1) taking the tropisetron hydrochloride raw materials or preparations, performing dissolving and diluting to realize constant volume by means of initial flowing phase, preparing liquor containing 0.5mg of tropisetron hydrochloride per milliliter, and using the liquor as sample liquor by tropisetron; (2) taking indole-3-carboxylic acid reference substances, performing dissolving and diluting to realize constant volume by means of initial flowing phase, preparing liquor containing 0.5mug of indole-3-carboxylic acid per milliliter, and using the liquor as reference substance liquor; and (3) respectively filling 50mul of sample liquor prepared in the step (1) and 50mul of reference substance liquor prepared in the step (2) into a high performance liquid chromatograph for gradient elution. The detecting method has the advantages of high specificity, accuracy in quantification, high sensitivity and stability, and the limit of detectingdrug quality is improved to a high standard.
Owner:SHANDONG QIDU PHARMA
Vomit-stopping drug, namely, tropisetron hydrochloride composition tablets
InactiveCN105106212AHigh purityImprove liquidityOrganic chemistryDigestive systemSilicic acidAluminum silicate
The invention discloses a vomit-stopping drug, namely, tropisetron hydrochloride composition tablets, and belongs to the technical field of medicines. The composition is prepared from the following materials: tropisetron hydrochloride, starch, magnesium aluminum silicate, polyvinylpolypyrrolidone, povidone K30, sodium dodecyl benzene sulfonate, purified water and magnesium stearate. Tropisetron hydrochloride is a new crystal compound and different from that reported in the prior art. The X-ray powder diffraction pattern obtained through measurement using Cu-K-alpha rays is shown in the figure 1. Experiments show that the new crystal compound is high in purity, good in fluidity and stability, low in impurity content, not liable to absorb moisture, and safe and reliable in clinical application; the tablets prepared using the new crystal compound is high in dissolution rate, good in stability and quite suitable for clinical applications.
Owner:QINGDAO HUAZHICAO PHARMA CO LTD
Freeze-dried powder injection of tropisetron hydrochloride composition serving as vomit-stopping drug and preparation method of freeze-dried powder injection
InactiveCN104971050AHigh purityImprove liquidityPowder deliveryOrganic chemistryExcipientMedicinal chemistry
The invention relates to a freeze-dried powder injection of a tropisetron hydrochloride composition serving as a vomit-stopping drug and a preparation method of the freeze-dried powder injection, belonging to the technical field of medicines. The composition comprises tropisetron hydrochloride and an excipient, wherein the excipient is trehalose, and the tropisetron hydrochloride is a compound in a novel crystal form; an x-ray powder diffraction diagram obtained through Cu-K alpha ray measurement is as shown in the figure 1; the tropisetron hydrochloride is different from the tropisetron hydrochloride reported by the prior art. Experiments prove that the compound in the novel crystal form is high in purity, good in flowability and stability, low in impurity content, low in moisture absorption possibility as well as safe and reliable in clinical application; and the freeze-dried powder injection prepared from the compound in the novel crystal form is good in stability after being matched with a solvent, extremely low in insoluble particle content and very suitable for clinical application.
Owner:QINGDAO LANSHENGYANG PHARMA & BIOTECH CO LTD
The preparation method of tropisetron hydrochloride
Owner:ZEIN BIOTECHNOLOGY CO LTD +1
Medicinal tropisetron hydrochloride composition dry suspension for treating nausea and emesis
The invention discloses a medicinal tropisetron hydrochloride composition dry suspension for treating nausea and emesis and belongs to the technical field of medicine. The composition is prepared from tropisetron hydrochloride, lactose, mannitol, PVPP, HPMC, Arabic gum, sucralose and aerosol. The tropisetron hydrochloride is a novel crystal-form compound. As shown in Figure 1 of X-ray powder diffraction diagram obtained by means of Cu-K alpha ray measurement, the tropisetron hydrochloride is different from tropisetron hydrochloride reported in the prior art. Tests find that the novel crystal-form compound is high in purity, good in fluidity and stability, low in impurity content, not prone to absorb moisture and safe and reliable in clinical application. The dry suspension prepared through the novel crystal-form compound is good in stability and quite suitable for clinical application.
Owner:QINGDAO HUAZHICAO PHARMA CO LTD
Pharmaceutical composition containing tropisetron hydrochloride and dexamethasone sodium phosphate
InactiveCN103393701BImprove antiemetic efficacyDigestive systemHeterocyclic compound active ingredientsFructoseRinger's solution
Owner:HAINAN LINGKANG PHARMA CO LTD
Tropisetron hydrochloride freeze-dried powder for injection and preparation method thereof
ActiveCN105125505BPrevent oxidationImprove stabilityPowder deliveryDigestive systemFreeze-dryingBULK ACTIVE INGREDIENT
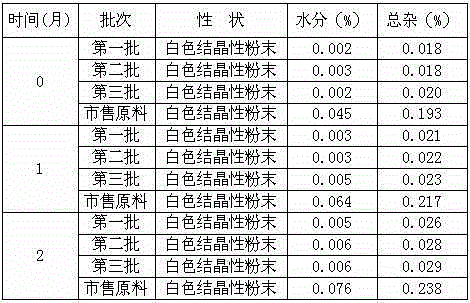
The invention belongs to the technical field of pharmaceutical preparations, and particularly relates to a hydrochloric-acid tropisetron freeze-dried powder injection for injection and a preparation method thereof. The hydrochloric-acid tropisetron freeze-dried powder injection comprises a medicinal active ingredient, excipients and pH (potential of hydrogen) stabilizers, wherein the medicinal active ingredient is hydrochloric-acid tropisetron, the excipients include mannitol and sodium chloride, and the pH stabilizers include citric acid and sodium citrate. According to the hydrochloric-acid tropisetron freeze-dried powder injection, the mannitol and the sodium chloride serve as the excipients, the pH value of a midbody solution is controlled to be stabilized within 4.6-5.2 by citric acid and sodium citrate buffer solutions, oxidation of a main drug is inhibited, and stability of the main drug is improved; in six months of an acceleration test, the injection is stable in content, changes of related substances are small, and the storage period of the injection is prolonged. The preparation method is simple in process and suitable for industrial production.
Owner:REYOUNG PHARMA
Medicine tropisetron hydrochloride composition for treating nausea and emesis
InactiveCN105168212AHigh purityImprove liquidityOrganic chemistryDigestive systemPharmaceutical drugSodium carbonate anhydrous
The invention relates to a medicine tropisetron hydrochloride composition for treating nausea and emesis and belongs to the technical field of medicines. The composition is prepared from tropisetron hydrochloride and anhydrous sodium carbonate, wherein the tropisetron hydrochloride is of a crystal and an X-ray powder diffraction pattern obtained by Cu-K alpha ray measurement is shown as a figure 1. A new crystal form of the tropisetron hydrochloride provided by the invention is different from a crystal form structure in the prior art; experimental verification is carried out to surprisedly find out that the compound with the new crystal form has high purity, good mobility, good stability and low impurity content, does not easily absorb moisture, and is safe and reliable in clinical application; a powder injection prepared from the compound with the new crystal form has good stability; the medicine tropisetron hydrochloride composition has good stability after being matched with a solvent, and the content of insoluble micro-particles is extremely low, so that the composition is very suitable for the clinical application.
Owner:QINGDAO LANSHENGYANG PHARMA & BIOTECH CO LTD
Tropisetron hydrochloride and fructose-sodium chloride containing pharmaceutical composition
The invention provides a pharmaceutical composition of a tropisetron hydrochloride injection and a fructose-sodium chloride injection, and particularly provides a composite application package comprising a tropisetron hydrochloride injection and a fructose-sodium chloride injection. When in use, after the tropisetron hydrochloride injection is dissolved in the fructose-sodium chloride injection, the obtained product is applied to intravenous infusion, so that the pharmaceutical composition not only has an effect of treating postoperative nausea and vomiting, but also can replenish energy and body fluids to a human body.
Owner:HAINAN LINGKANG PHARMA CO LTD
Pharmaceutical composition containing tropisetron hydrochloride and dexamethasone acetate
InactiveCN103393694AImprove antiemetic efficacyPowder deliveryDigestive systemFructoseDexamethasone acetate
The invention relates to a pharmaceutical composition containing tropisetron hydrochloride and dexamethasone acetate, and particularly relates to a combined application package comprising a tropisetron hydrochloride injection and a dexamethasone acetate injection. When the combined application package is in use, the tropisetron hydrochloride injection and a dexamethasone acetate injection are co-dissolved in normal saline, a Ringer's solution, 5% glucose injection, a fructose solution and the like for intravenous infusion, so that a curative effect of stopping vomit can be increased.
Owner:HAINAN LINGKANG PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com