Medicinal tropisetron hydrochloride composition dry suspension for treating nausea and emesis
A technology of tropisetron hydrochloride and dry suspension, applied in the field of medicine, can solve the problems of difficult preparation of preparations, unsatisfactory hygroscopicity of impurity content crystal form, influence on stability, etc., and achieves good fluidity and safe and reliable clinical application. , good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1: Preparation of Tropisetron Hydrochloride Crystals
[0027] (1) Take tropisetron hydrochloride crude drug and add it to deionized water, the volume dosage of deionized water is 5 times of the mass of tropisetron hydrochloride;
[0028] (2) Stir until completely dissolved, and adjust the pH to 6-9;
[0029] (3) Add activated carbon for decolorization and filter to obtain a clear solution;
[0030] (4) Move the clarified solution into a pressure vessel, add 2°C isopropanol dropwise under the condition that the pressure in the pressure vessel is controlled at 2.0Mpa and stirred, the stirring speed is controlled at 35rmp, and the volume of isopropanol is de 3 times the volume of ionized water;
[0031] (5) Release the pressure after the dropwise addition, cool the solution to -5°C at a rate of 10°C / min, let it stand for 2 hours, filter, wash with ethanol, and dry under reduced pressure to obtain tropisetron hydrochloride crystals.
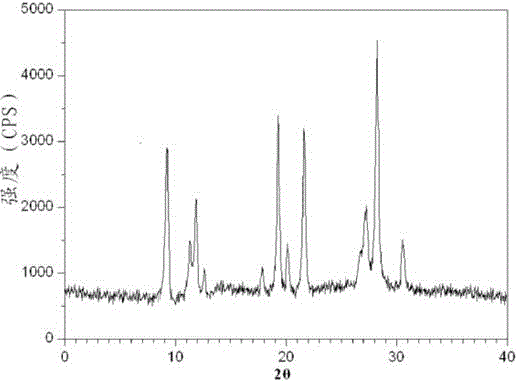
[0032] The prepared tropis...
Embodiment 2
[0033] Example 2: Preparation of Tropisetron Hydrochloride Dry Suspension
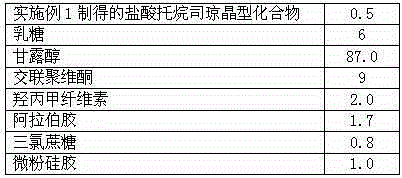
[0034] Prescription: in parts by weight as shown in Table 1
[0035] Table 1 Tropisetron Hydrochloride Composition Prescription
[0036]
[0037] Preparation:
[0038] 1) Weighing: Weigh the raw and auxiliary materials according to the prescription process;
[0039] 2) Pre-mixing and crushing: add the prescribed amount of tropisetron hydrochloride and lactose in equal amounts and mix them evenly, and crush them through a 100-mesh sieve after mixing;
[0040] 3) Total mixing: add the premixed and pulverized tropisetron hydrochloride, lactose and prescribed amount of mannitol, crospovidone, hypromellose, gum arabic, sucralose, and micronized silica gel to the three-dimensional mixer In the middle, the mixing speed is 12r / min, and the mixer is turned on for 60 minutes;
[0041] 4) Subpackage: Add the granules to the granule packaging machine for subpackage, and control the difference in the filli...
Embodiment 3
[0042] Example 3: Preparation of Tropisetron Hydrochloride Dry Suspension
[0043] Prescription: in parts by weight as shown in Table 2
[0044] Table 2 Tropisetron Hydrochloride Composition Prescription
[0045]
[0046] Preparation:
[0047] 1) Weighing: Weigh the raw and auxiliary materials according to the prescription process;
[0048] 2) Pre-mixing and crushing: add the prescribed amount of tropisetron hydrochloride and lactose in equal amounts and mix them evenly, and crush them through a 100-mesh sieve after mixing;
[0049] 3) Total mixing: add the premixed and pulverized tropisetron hydrochloride, lactose and prescribed amount of mannitol, crospovidone, hypromellose, gum arabic, sucralose, and micronized silica gel to the three-dimensional mixer In the middle, the mixing speed is 12r / min, and the mixer is turned on for 60 minutes;
[0050] 4) Subpackage: Add the granules to the granule packaging machine for subpackage, and control the difference in the filli...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com