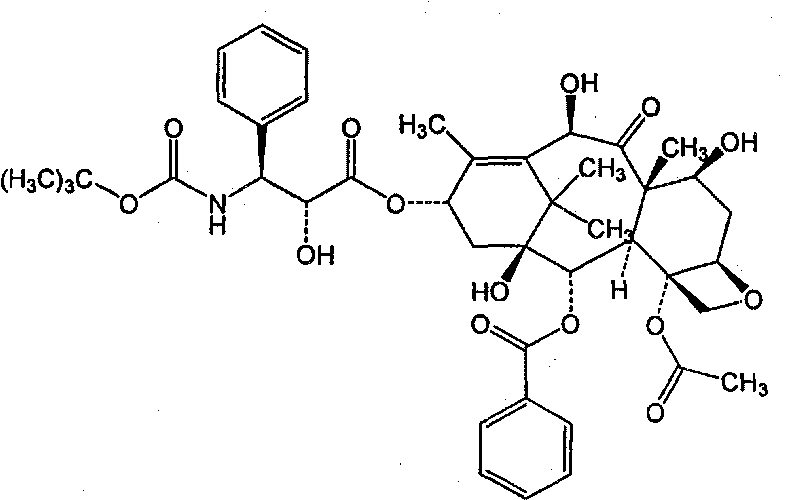
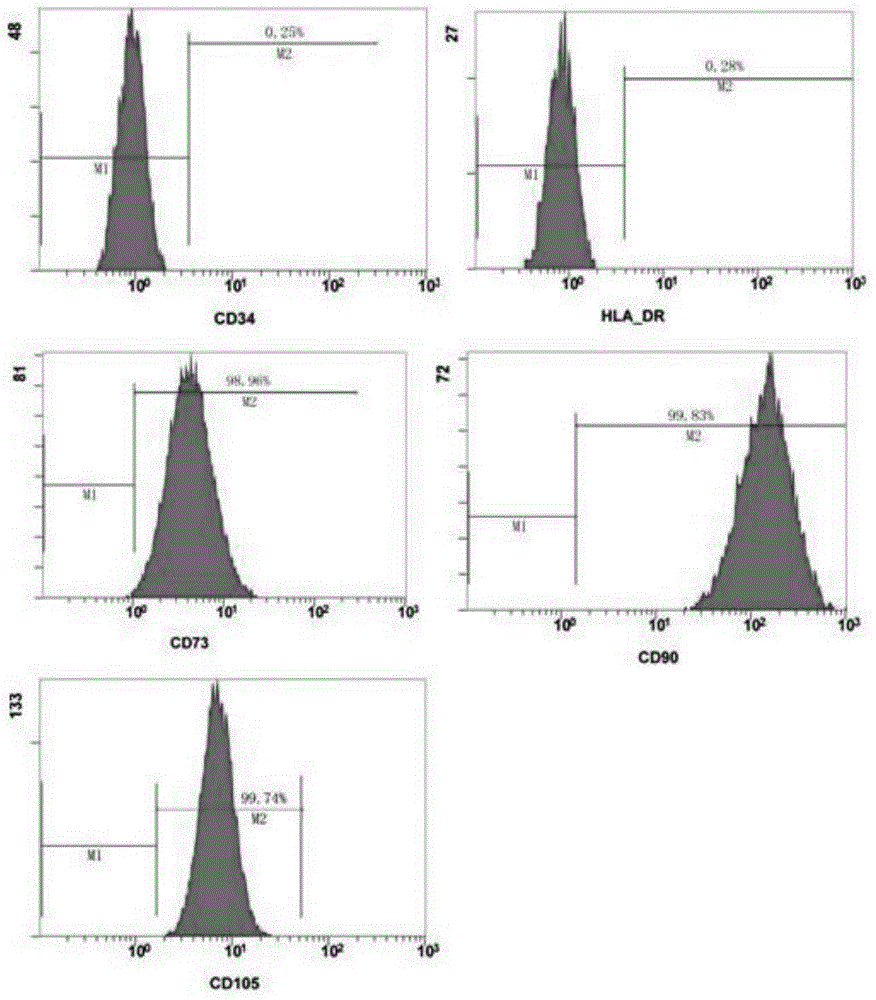
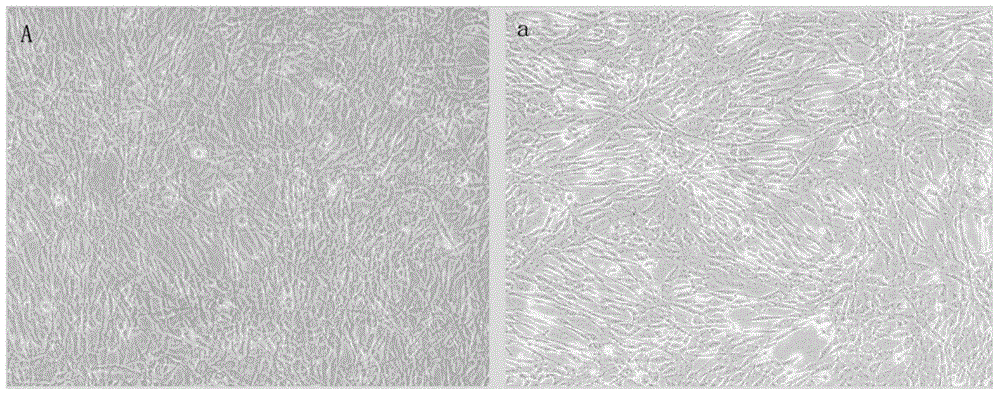
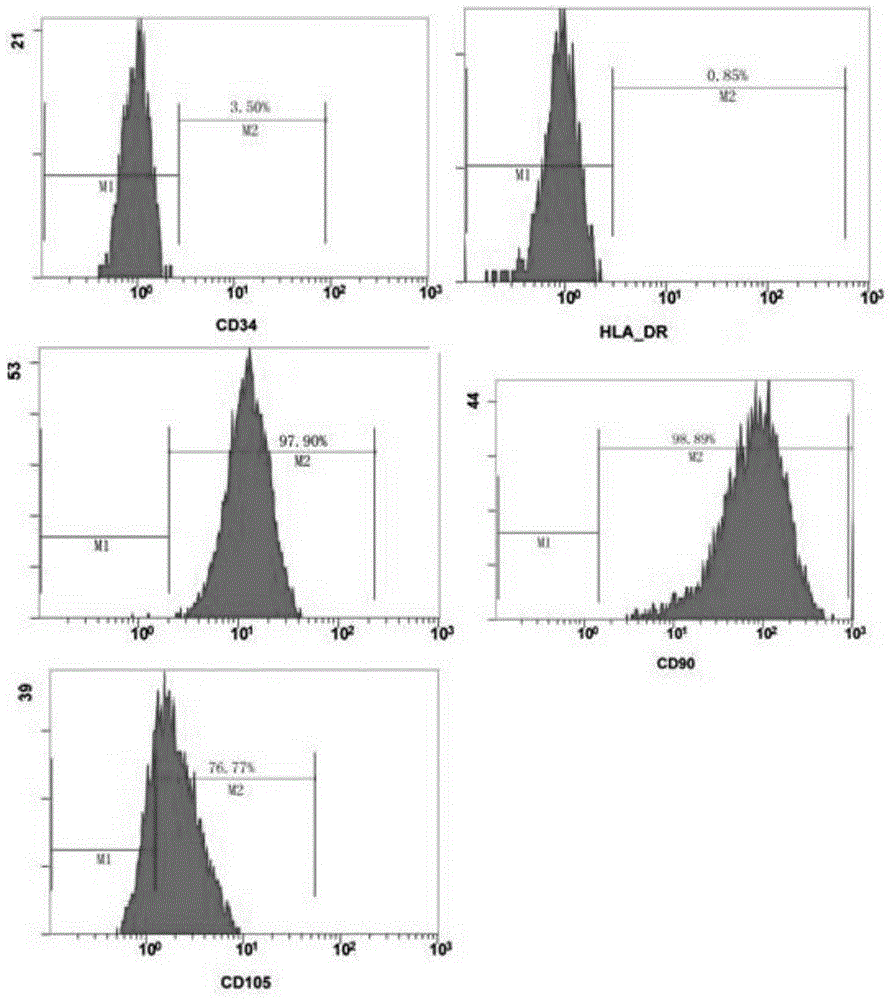
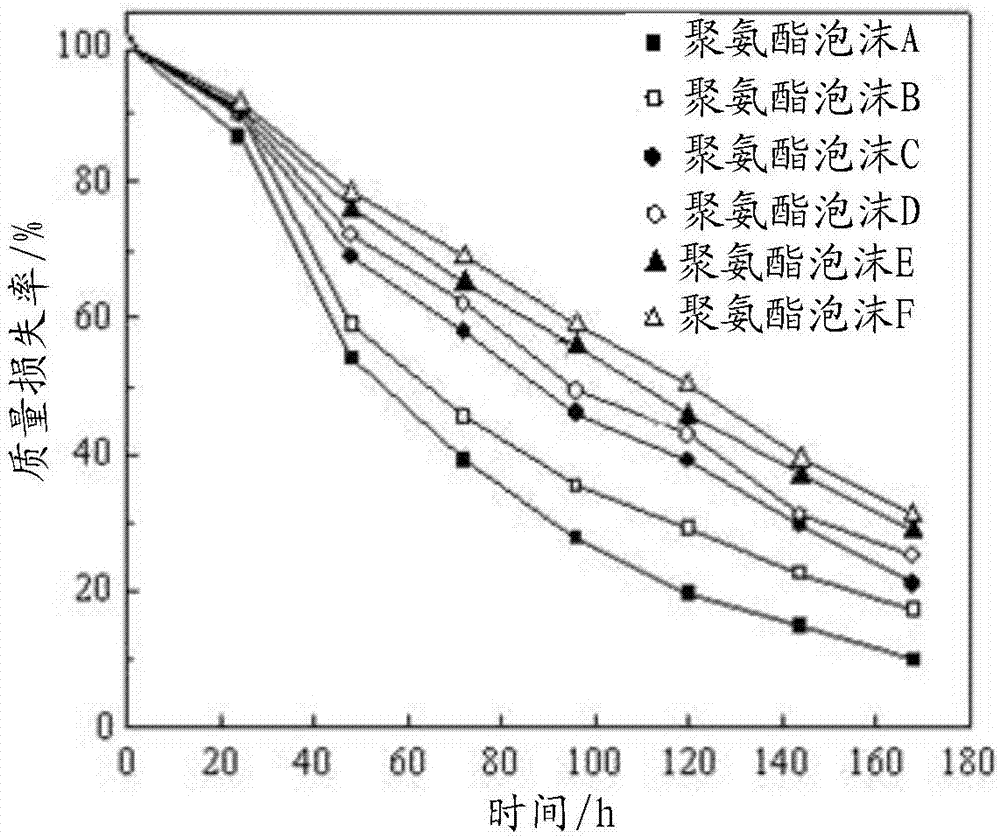
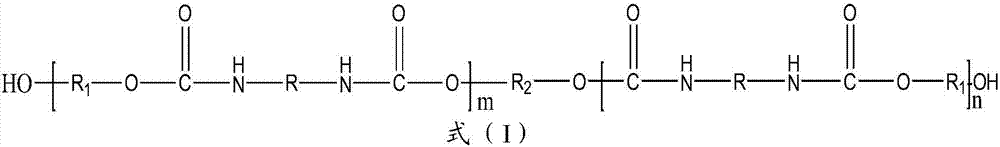
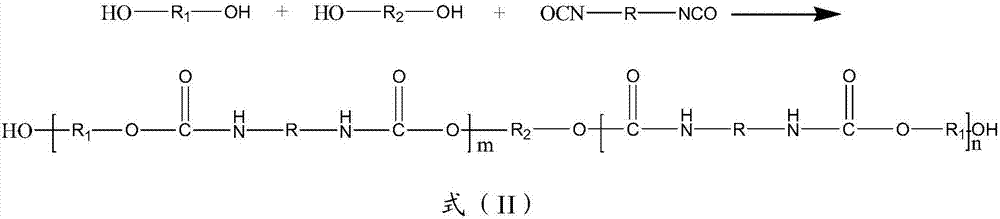
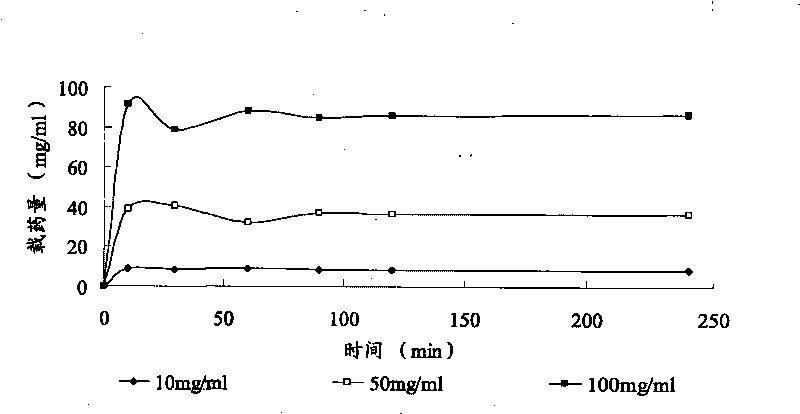
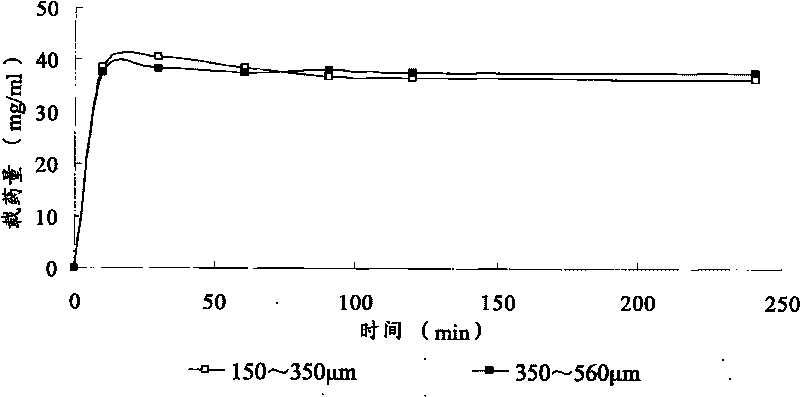
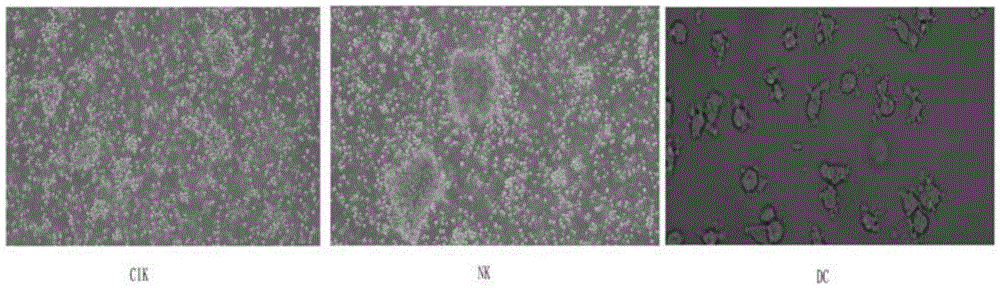
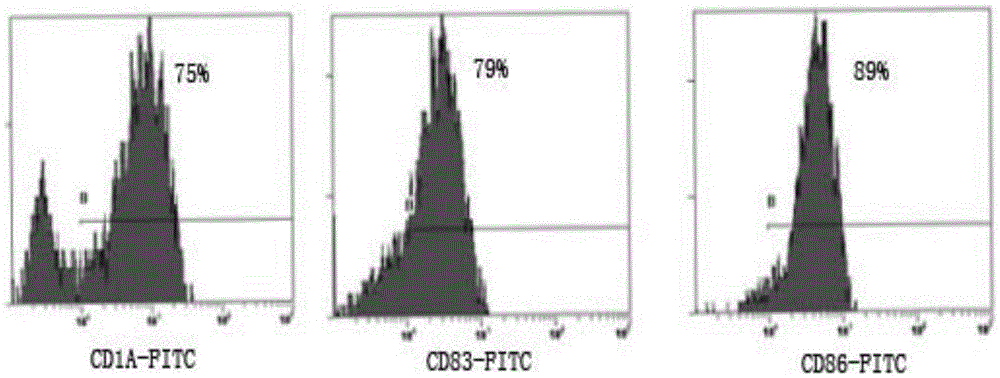
Patents
Literature
475results about How to "Suitable for clinical application" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Particle-enhanced turbidimetric immune assay kit for detecting adiponectin and preparation method thereof
ActiveCN101968492AAccurately reflectHigh sensitivityMaterial analysis by observing effect on chemical indicatorBiological testingPreservativeMonoclonal antibody
The invention relates to a particle-enhanced turbidimetric immune assay kit for detecting adiponectin and a preparation method thereof, in particular to a detection method of a turbidimetric immune assay. The particle-enhanced turbidimetric immune assay kit for detecting the adiponectin comprises a kit body, an application liquid bottle and a latex suspension bottle coating an antiviral adiponectin monoclonal antibody, wherein the application liquid bottle is filled with application liquid; the application liquid comprises a surfactant, a preservative, a macromolecular accelerator, sodium chloride and a buffering agent; the latex suspension bottle coating the antiviral adiponectin monoclonal antibody is filled with a latex suspension coating the antiviral adiponectin monoclonal antibody; and the latex suspension coating the antiviral adiponectin monoclonal antibody comprises latex coating the antiviral adiponectin monoclonal antibody, the surfactant, a stabilizer and the buffering agent. The preparation method comprises the following steps of: preparing a latex antibody; preparing the latex suspension coating the antiviral adiponectin monoclonal antibody; preparing the applicationliquid; and finally preparing adiponectin series standard products.
Owner:ANHUI IPROCOM BIOTECH CO LTD
Flexible massaging robot
ActiveCN104175311AImprove securityEasy to acceptProgramme-controlled manipulatorJointsTorque transmissionDegrees of freedom
The invention provides a flexible massaging robot. The flexible massaging robot comprises a base and a mechanical arm arranged on the base, wherein the mechanical arm comprises a first joint, a first trunk, a second joint, a second trunk, a third joint, a third trunk, a fourth joint and a fourth trunk which are connected in sequence, wherein the first joint is arranged on the base through a joint mounting base; the rotary axes of joint output shafts of the first joint and the third joint are positioned along the length direction of the mechanical arm; the rotary axes of joint output shafts of the second joint and the fourth joint are vertical to the length direction of the mechanical arm; each joint is provided with an elastic torque transmission module for transmitting torque through an elastic part. The flexible massaging robot has four degrees of freedom which are accordant with the degree of freedom distribution of a human arm, and can be used for simulating various massaging ways of a doctor; the structure of a human simulation arm can be accepted more easily by a user; moreover, by using the elastic torque transmission modules, the safety is ensured and the massaging operation is finished at high performance.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
Oxiracetam freeze-drying preparation for injection and preparation method thereof
ActiveCN103446067AStable in natureLong validity periodOrganic active ingredientsPowder deliveryFreeze-dryingImpurity
The invention relates to an oxiracetam freeze-drying preparation for injection and a preparation method thereof, belonging to the technical field of medicines. The freeze-drying preparation is a crystal form compound formed by oxiracetam, D-sorbitol and mannitol in a weight ratio of 1: (0.2-0.25): (0.05-0.1). The oxiracetam freeze-drying preparation has the beneficial effects that the production cycle is shortened by about 20 hours, the rate of finished products is increased by above 99%, the phenomena such as the upward shifting and the eruption of the product are avoided, medicine molecules are constrained in crystal lattices, the water content is extremely low and can be controlled below 0.1%, the preparation is difficult to degrade and has relatively good stability, the period of validity is up to 36 months, the impurity content is greatly reduced, the single maximum impurity is less than 0.1%, and the total impurities are less than 0.5%; no organic solvents are involved in the production process, the energy consumption and the cost are substantially reduced, and the technology has environmentally-friendly effect and is suitable for large-scale industrialization production. Compared with the prior art, the oxiracetam freeze-drying preparation provided by the invention has obviously improved curative effect and is suitable for clinical application.
Owner:CSPC OUYI PHARM CO LTD
Pharmaceutical composition for treating embolism and preparation method thereof
ActiveCN101670095AIncrease concentrationReduce concentrationSurgerySaccharide peptide ingredientsEmbolization AgentDouble bond
The invention provides a pharmaceutical composition for treating embolism and a preparation method thereof, the pharmaceutical composition comprises a hydroxyl-contained biocompatible polymer materialand a monomer containing unsaturated double bonds and anion groups, as well as a polymer generated by polymerization reaction of an optional vinyl monomer, wherein the polymerization is initiated through free radicles, and bleomycin or pingyangmycin is combined on the anion groups of the generated polymer. The preparation method combines the bleomycin or the pingyangmycin on a carrier of the polymer, thereby being capable of fully playing the dual effects of an anti-tumor antibiotic and a hardening agent owned by the bleomycin or the pingyangmycin during the embolism treatment. The anion partof the polymer can be properly combined with the bleomycin or the pingyangmycin which is rich in amino groups, thereby not only realizing the higher drug loading, but also leading the drug in an emboliaztion agent to be exchanged by cation in human body, and further realizing the slow release. In addition, the embolic carrier of the polymer has the advantages of simple prepration technology andlow cost, thereby being applicable to large-scale industrial production.
Owner:HYGEA MEDICAL TECH CO LTD
Nucleic acids of liquid-phase gene chip for synchronously detecting five porcine viruses and detection method thereof
ActiveCN104328218AHigh detection sensitivityStrong specificityMicrobiological testing/measurementMicroorganism based processesClassical swine fever virus CSFVMultiplex
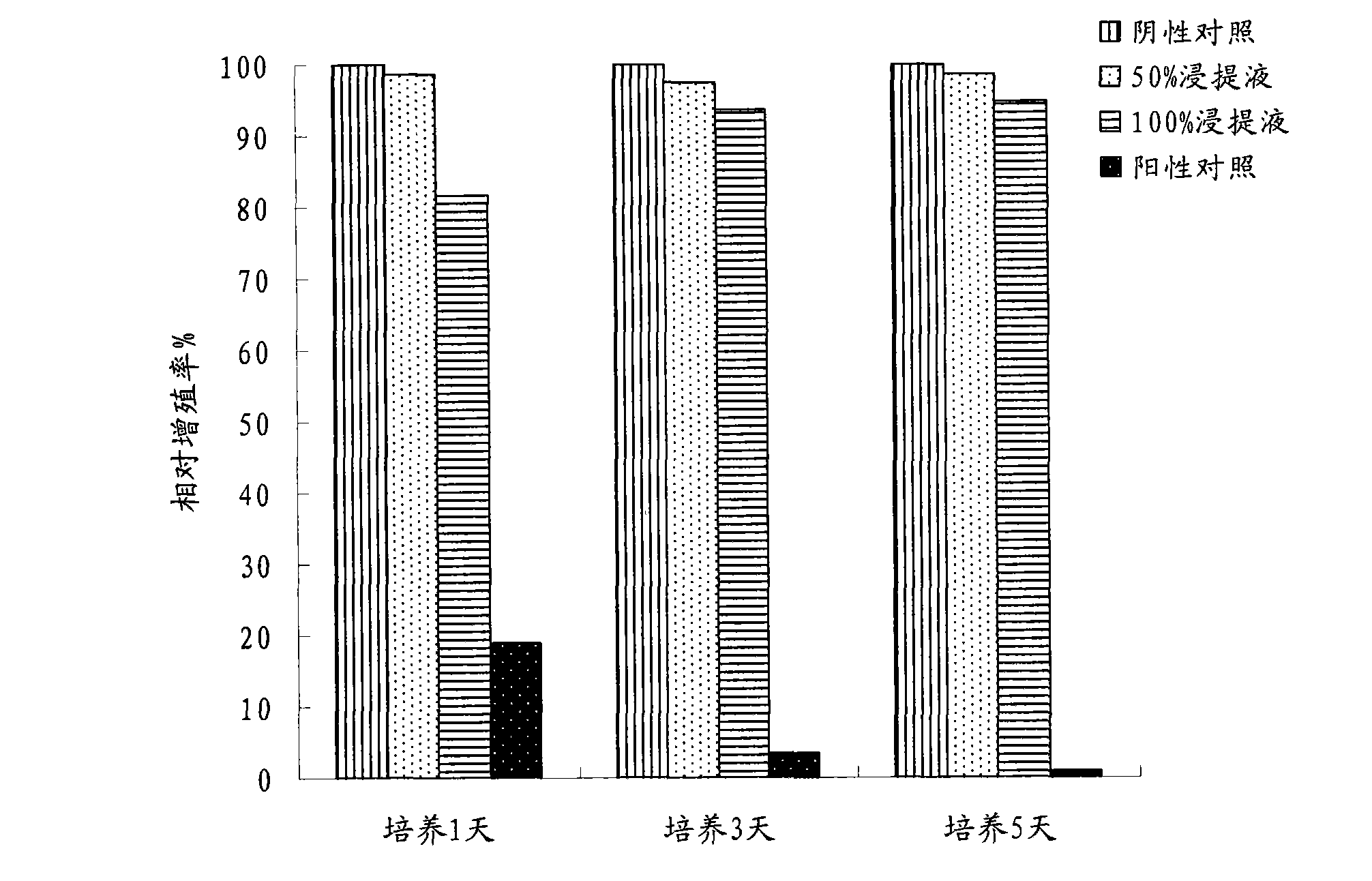
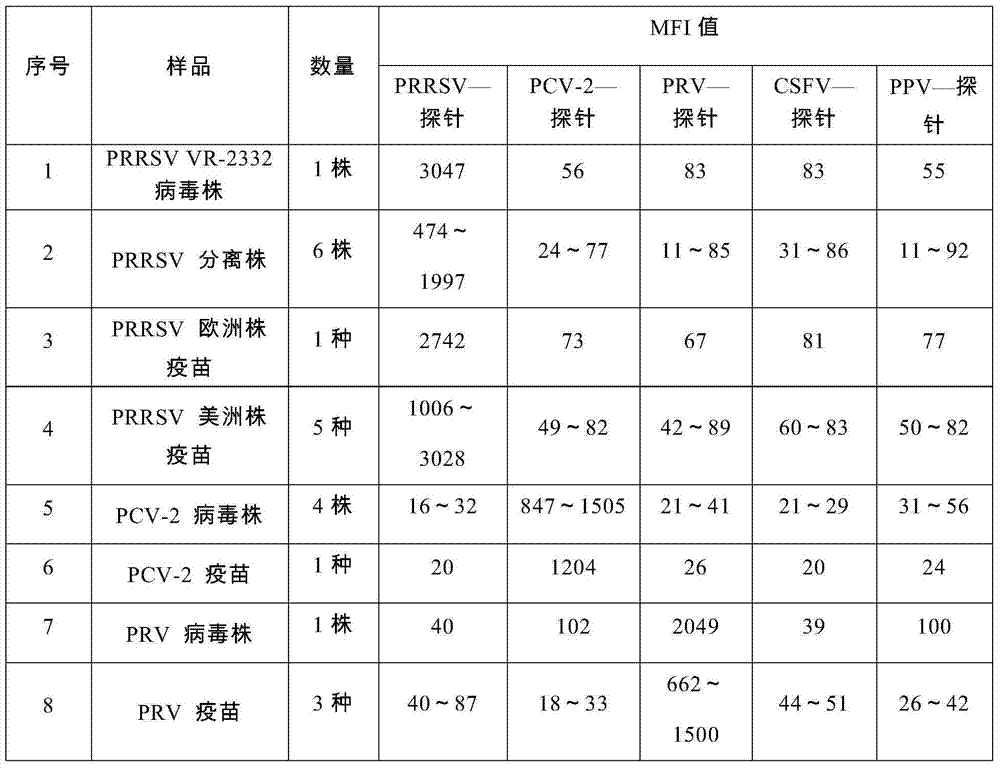
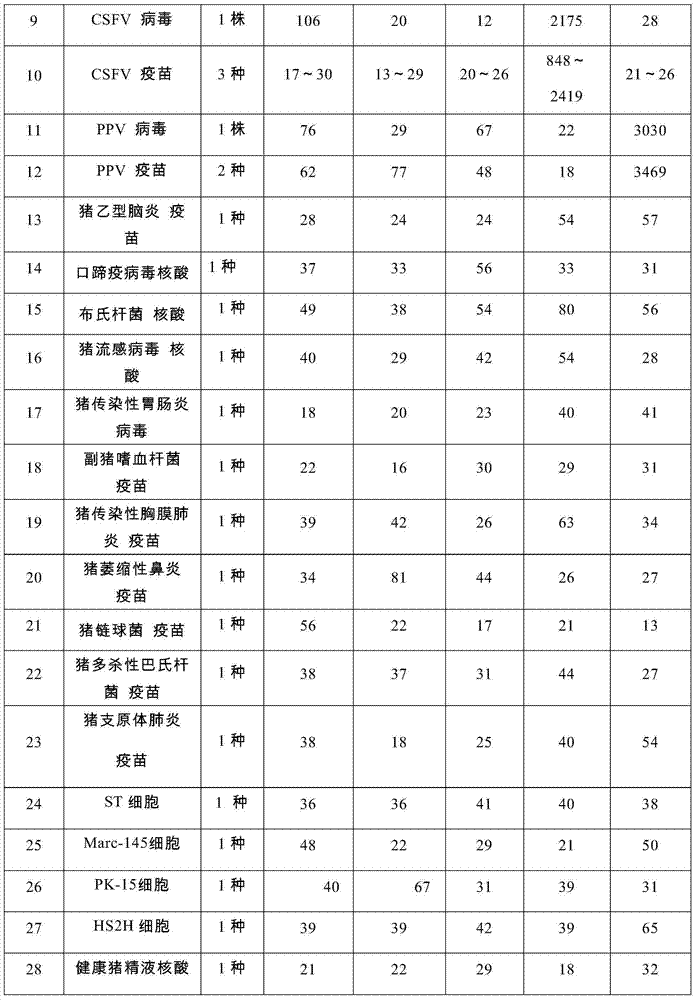
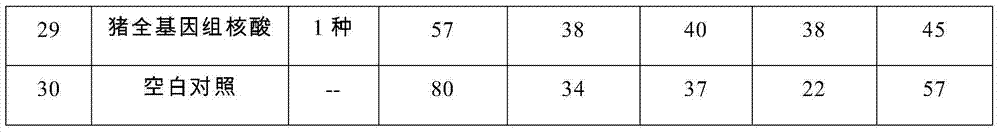
The invention provides a set of nucleic acids of a liquid-phase gene chip for synchronously detecting five porcine viruses, which comprise forward and reverse primers and hybrid probes for porcine reproductive and respiratory syndrome virus (PRRSV), porcine circovirus type 2 (PCV2), porcine pseudorabies virus (PRV), classical swine fever virus (CSFV) and porcine parvovirus (PPV). The invention also provides a multiplex liquid-phase chip high-flux molecular biology detection method of the five porcine viruses. According to the method, porcine virus nucleic acids in the sample to be detected are extracted to perform multiplex unsymmetric nucleic acid amplification / multiplex liquid-phase gene chip (suspension chip) combined detection, thereby synchronously and accurately detecting and identifying the five porcine viruses in the sample to be detected. The method has the advantages of high specificity, high sensitivity, high stability, high flux and high detection speed, and is simple to operate.
Owner:INSPECTION & QUARANTINE TECH CENT OF GUANGDONG ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Method for preparing block polymer micelle freeze-drying preparation carrying docetaxel
InactiveCN101732234AImprove solubilityHigh metabolic stabilityOrganic active ingredientsPharmaceutical delivery mechanismMetabolic stabilityOrganic solvent
The invention discloses a method for preparing a block polymer micelle freeze-drying preparation carrying docetaxel, which uses a PEO-PPO-PEO block polymer as a micelle carrier to wrap the docetaxel inside the micelle and comprises the following steps: (1) dissolving the carrier and the docetaxel into an organic solvent to make the PEO-PPO-PEO block polymer and the docetaxel fully dissolved in the organic solvent, and then preparing aqueous solution of the docetaxel micelle by using the PEO-PPO-PEO block polymer as the carrier; and (2) adding a freeze-drying protective agent into the aqueous solution of the PEO-PPO-PEO block polymer carrying micelle, and filtering, sterilizing and freeze-drying the mixture to obtain freeze-drying preparation of the carrier micelle system. The block polymer micelle freeze-drying preparation carrying the docetaxel can increase the dissolubility, metabolic stability and in vivo circulation time of the docetaxel, reduce the toxicity and improve the bioavailability, and is more suitable for clinical application.
Owner:SHANDONG UNIV
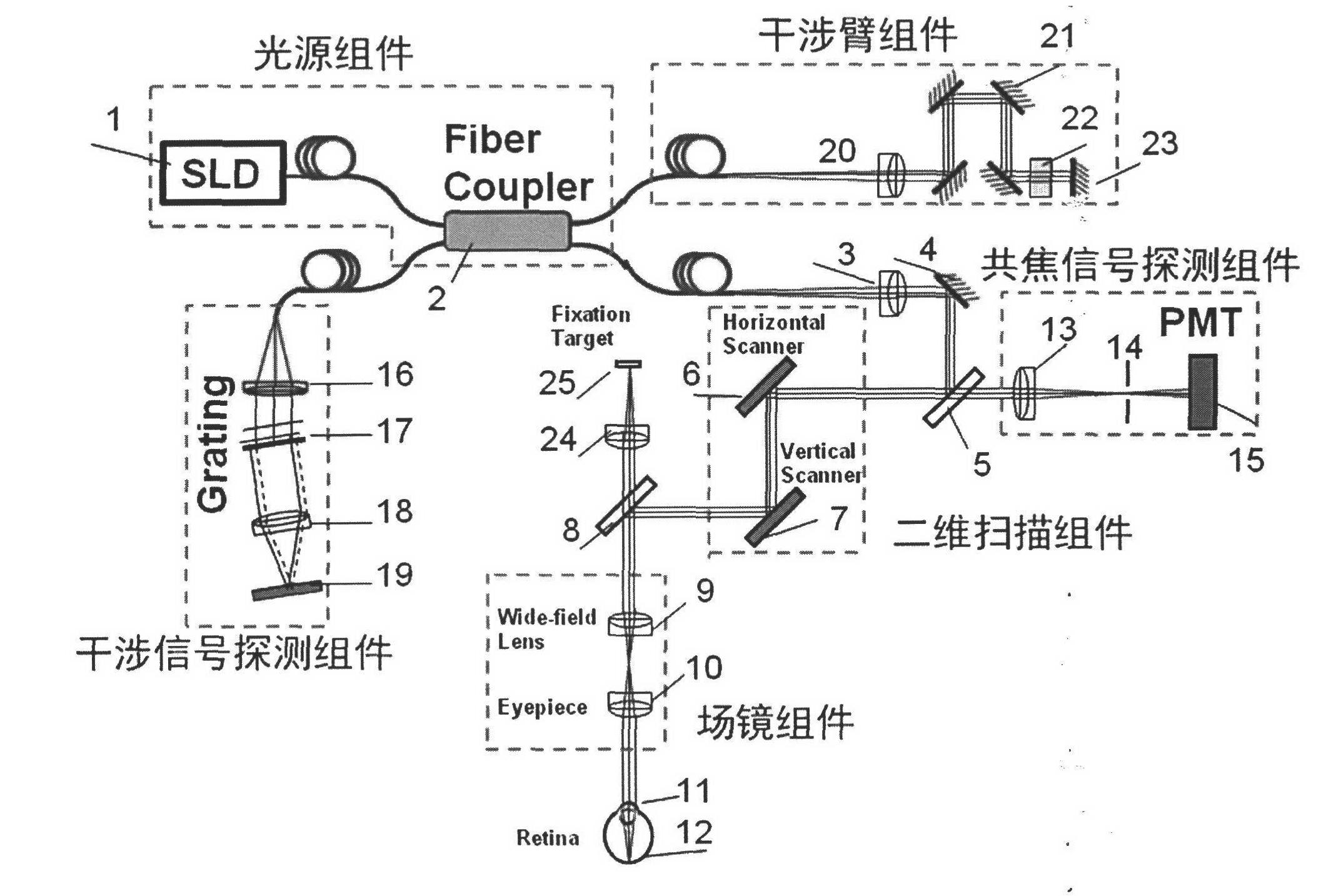
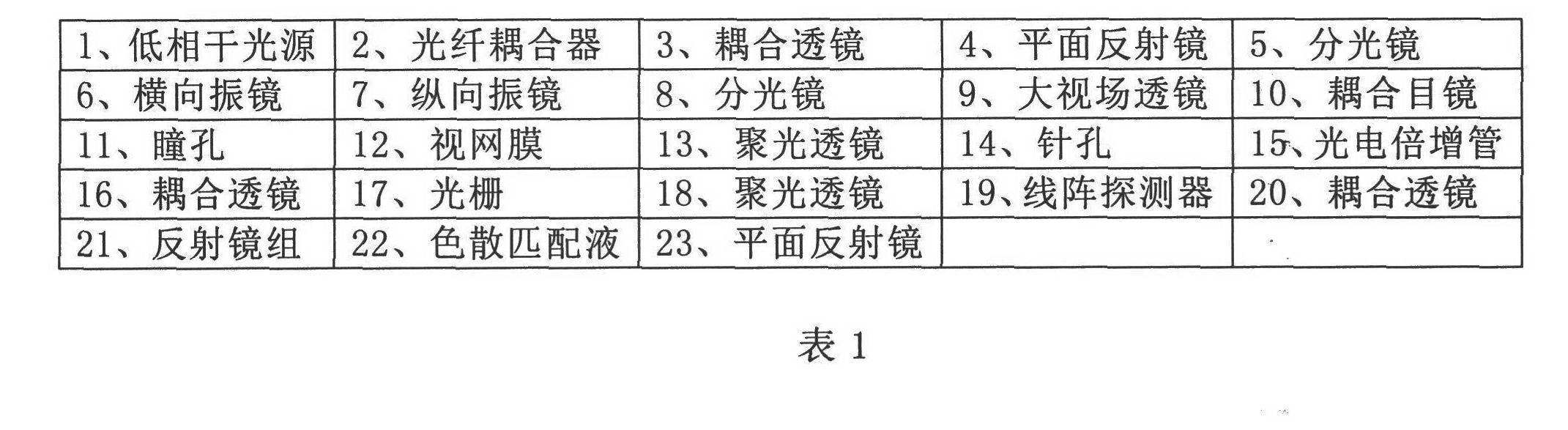
Three-dimensional imaging device for retina
InactiveCN102525406AEliminate aberrationsEliminate non-uniformityOthalmoscopesImage resolutionOptical fiber coupler
The invention discloses a three-dimensional imaging device for a retina. The device consists of a light source component, an interference arm component, a two-dimensional scanning component, a field lens component, a confocal signal detection component and an interference signal detection component. The three-dimensional imaging device for the retina finishes scanning on the retina of a human eye by using the two-dimensional scanning component, extracts a surface image of the retina by using the confocal signal detection component and depth information of the retina by using the interference signal detection component, and jointly finishes the reconstruction of a three-dimension image of the retina through a confocal signal and an interference signal; the system is compact by adopting the design of optical fiber access and an optical fiber coupler; the influence of curvature of field and aberration in the imaging process is reduced through the design of a field lens so as to acquire a large-field-of-view three-dimensional image of the retina of the human eye; and the three-dimensional imaging device for the retina which is compactly designed and is high in imaging resolution and large in imaging field of view is realized, so that the imaging effect of the traditional fundus imaging instrument is greatly improved.
Owner:SUZHOU MICROCLEAR MEDICAL INSTR
Serum-free medium of stem cell
ActiveCN104805054APromote proliferationPromote differentiationSkeletal/connective tissue cellsSerum free mediaVitamin C
The invention discloses a serum-free medium of a stem cell, which comprises a basal medium and an additive, wherein the basal medium is DMEM / F12 (Dulbecco Modified Eagle Medium / F12); the additive comprises 2-15%(v / v) of serum replacement, 20-100ug / ml vitamin C, 0.5-10ng / ml stem cell growth factor, 5-20ng / ml human platelet-derived growth factor and 1-5mmol / ml L-glutamine. The serum-free medium of the stem cell for cell culture has the advantages of short cell cycle, strong multiplication capacity, good cell uniformity and high purity, effectively inhibits differentiation of the stem cell and adherence growth of an endothelial cell, ensures purity and dryness of the stem cell and is free from ingredients of animal origin such as fetal calf serum, and the stem cell obtained by the medium is suitable for clinical application.
Owner:钜威细胞(厦门)医学科技有限公司
Degradable polyurethane foam and application thereof
InactiveCN107286313AImprove mechanical propertiesPromote degradationSurgical adhesivesPharmaceutical delivery mechanismIsocyanateSoft segment
The invention relates to the technical field of biomaterials, in particular to degradable polyurethane foam and an application thereof. A preparation process of the degradable polyurethane foam is simple, shorter in cycle and capable of preventing introduction of impurities; an obtained product has better water absorption property and higher porosity, can be degraded rapidly and is suitable for clinical application. The polyurethane foam is prepared from polyurethane through dissolving in an organic solvent and freeze-drying, wherein polyurethane is prepared from oligomer polyol as a soft segment and polyisocyanates as a hard segment and a chain extender with an ontology one-step process, and the oligomer polyol is a mixture of hydrophilic polyol and degradable polyol. The degradable polyurethane foam in an embodiment of the invention is used for preparing hemostatic materials, wound dressing or ear and nose stuffing.
Owner:SHAANXI RUISHENG BIOTECH
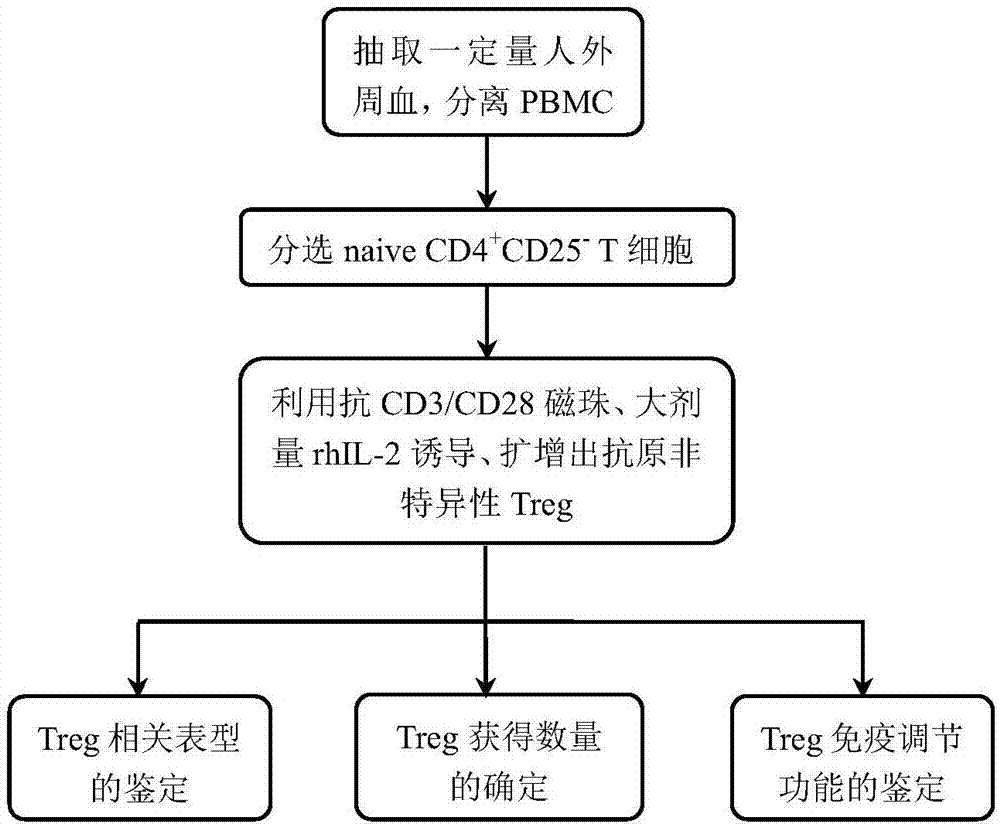
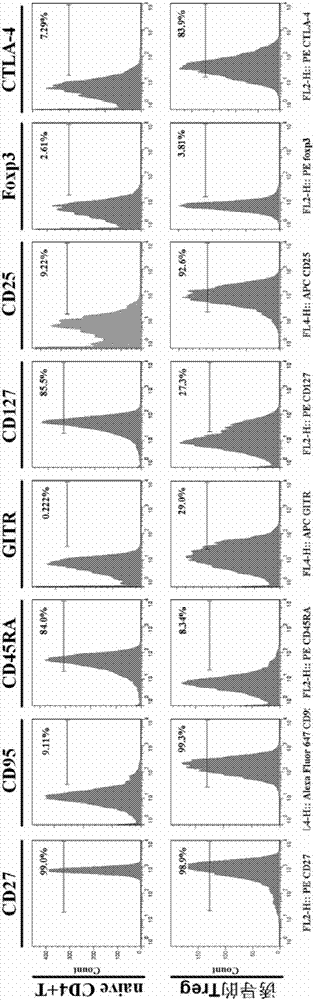
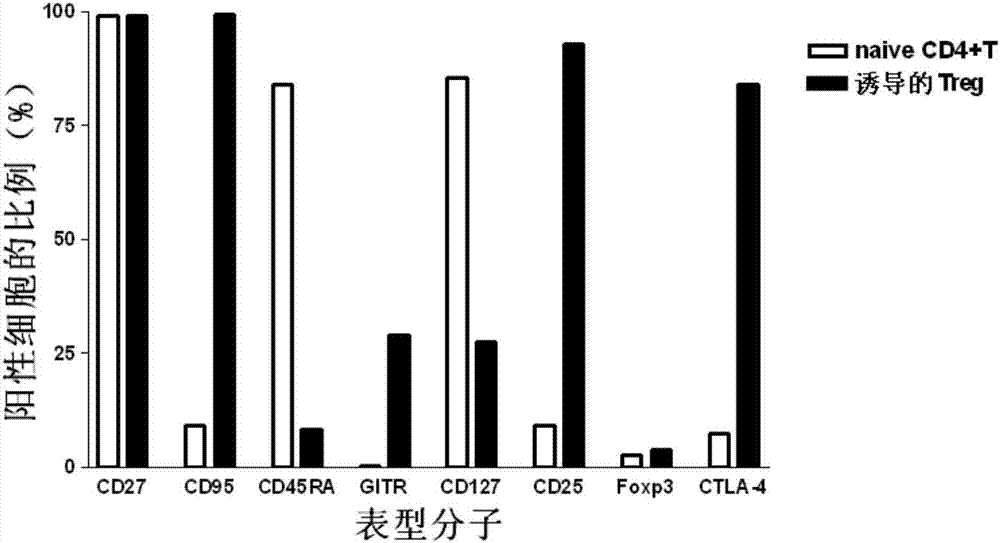
Method for in vitro induction and amplification of human antigen nonspecific regulatory T cell
InactiveCN107083360APhenotype stableStability adjustment functionBlood/immune system cellsCell culture active agentsAntigenMicroorganism
The invention provides a method for in vitro induction and amplification of a human antigen nonspecific regulatory T cell. A human peripheral blood CD4<+> T cell with an abundant source is separated, and a CD4<+>CD25<-> T cell is induced and amplified into a CD4<+>CD25<+>CD127<dim> antigen nonspecific regulatory T cell with high purity and high regulation efficiency within a short period through a simple induction method meeting the Clinical Good Manufacturing Practice (GMP). An enough therapeutic dose of Treg can be obtained through induced cultivation of a week (6-7d), the phenotype and regulation function of the induced Treg are stable, furthermore, the incidence rate of events, such as cell activity reduction and microbial contamination due to long-term culture in vitro is greatly reduced, and the quality control method is specific and fast and is very suitable for clinical application.
Owner:TONGJI HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI TECH +1
Traditional Chinese medicine composition for treating allergic rhinitis
InactiveCN102091128AImprove the immunityPromote blood circulationHydroxy compound active ingredientsRespiratory disorderNostrilSide effect
The invention discloses a traditional Chinese medicine composition for treating allergic rhinitis, comprising the following crude medicines in parts by weight: 28-32 parts of honeysuckle, 28-32 parts of baical skullcap root, 28-32 parts of paniculate swallowwort root, 13-17 parts of xanthium, 13-17 parts of magnolia flower, 7-11 parts of asarum, 7-11 parts of mint, 13-17 parts of angelica, 13-17 parts of centipeda minima, 22-26 parts of chuanxiong rhizome and 16-20 parts of borneol. The preparation method comprises the steps of crushing all components, screening with a sieve of 200 meshes and mixing evenly. When used for treatment, the medicament provided by the invention is put into a medicine bag made of non-woven fabric, then the medicine bag is sown on the inner surface of a disposable mask, and a patient wears the mask and aligns the medicine bag with the nostrils. A course of treatment lasts for 5 days, and generally allergic rhinitis can be cured by two courses of treatment. The medicament provided by the invention is directly inhaled and has the advantages of quick effect taking, convenience in use, safety, reliability, no toxic side effect, low cost and the like.
Owner:QIANFOSHAN HOSPITAL OF SHANDONG
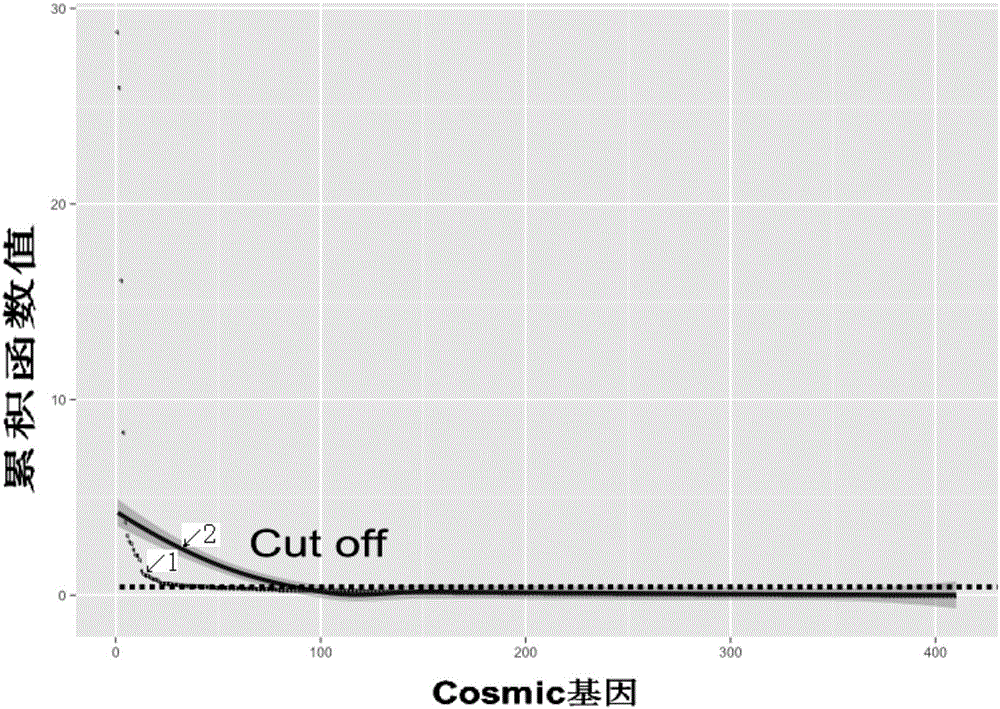
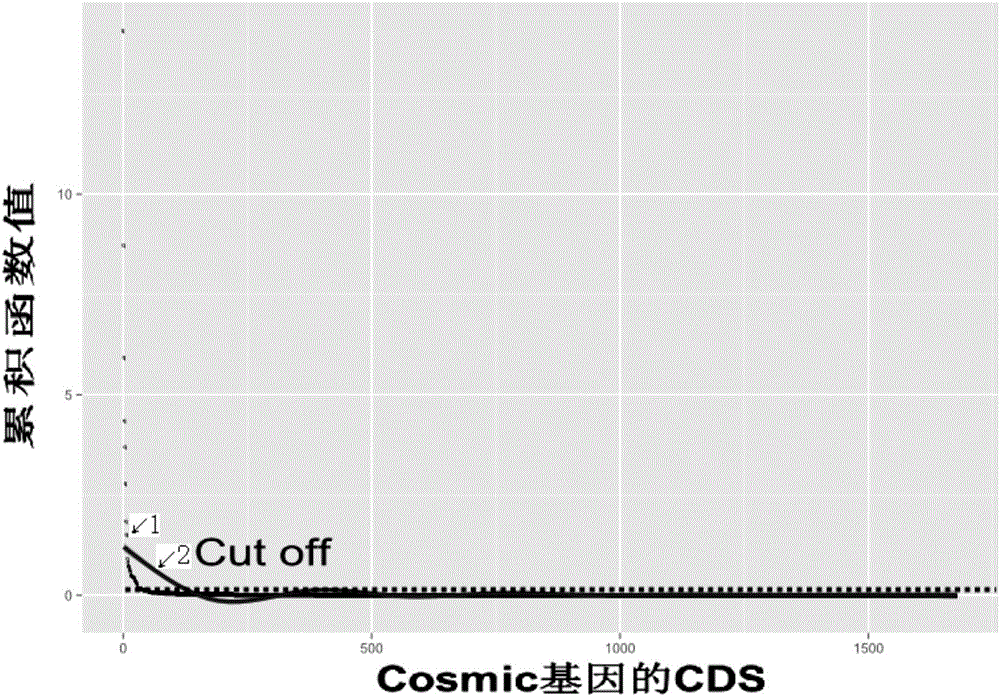
Specificity tumor probe area designing method for acquiring high-throughput sequencing in target area, device and probe
ActiveCN106570349ASequencing range reductionSequencing time and cost reductionMicrobiological testing/measurementHybridisationData validationBiology
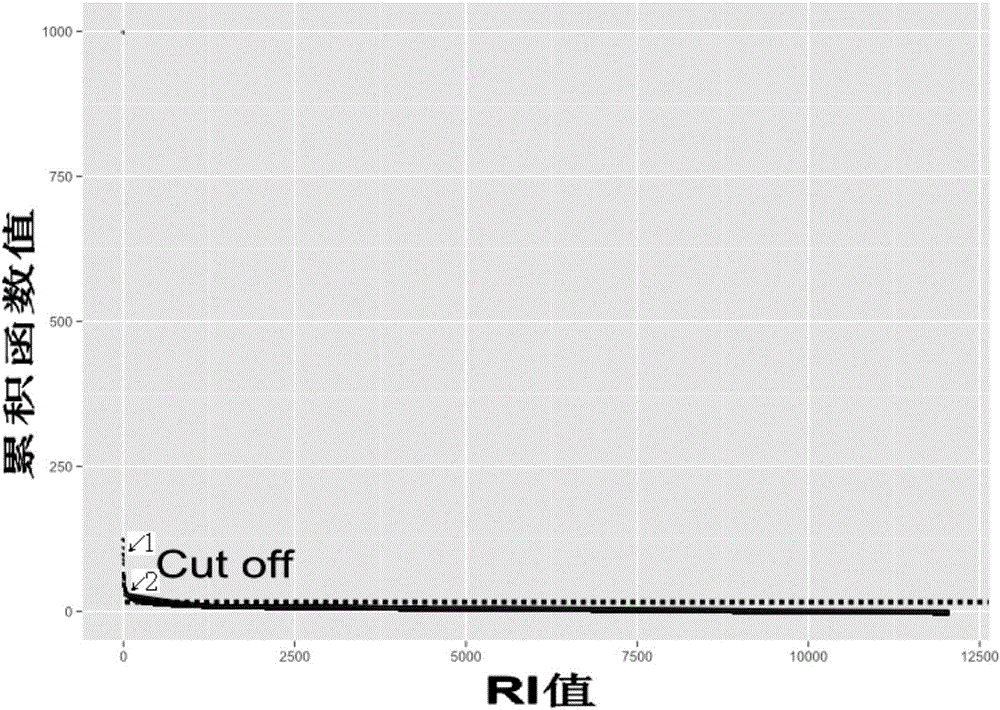
The invention discloses a specificity tumor probe area designing method for acquiring high-throughput sequencing in a target area, a device and a probe. The method comprises steps of calculating a gene and mutation related to a to-be-designed tumor in a first tumor mutation database, looking for a CDS area having the highest mutation frequency and designating the area as a first area, verifying the first area via tumor patient data stored in a second tumor mutation database, maximizing a sample frequency to achieve an area containing the most patients and designing the area as a second area, and calculating sample frequency and a RI value, further optimizing the sample area to achieve a third area. The RI value represents an average mutation number contained in each 1kb. By the use of the method, research is only required for the target area, so sequencing range can be greatly reduced and sequencing cost and time can be reduced; detection period can be obviously reduced; and cost can be lowered, so the specificity tumor probe area designing method for acquiring high-throughput sequencing in the target area is more suitable for clinical application.
Owner:BGI TECH SOLUTIONS
Atracurium freezing-dried composition
ActiveCN101084896AGood water solubilityStable storagePowder deliveryOrganic active ingredientsOrganic acidSolubility
The invention discloses freeze drying composition of atracurium. The composition adopts atracurium or its pharmaceutically acceptable salt or optical isomer as active ingredient, and also comprises sugar and organic acid. The invention also discloses a method for preparing the composition. The inventive composition has good water-solubility and good storing stability, low stimulation to blood vessel, and high safety; is suitable for clinical application.
Owner:SHANGHAI PHARMA DONGYING JIANGSU PHARMA CO LTD
High-sensitivity kit for detecting 5'-nucleotidase
InactiveCN104388534AEasy to operateHigh precisionMicrobiological testing/measurementPurine nucleoside phosphorylaseNucleotidase
The invention relates to the field of an external diagnostic reagent, and particularly relates to a high-sensitivity kit for detecting 5'-nucleotidase. The kit for detecting 5'-nucleotidase comprises a 5'-nucleotidase R1 reagent, a 5'-nucleotidase R2 reagent and a 5'-nucleotidase calibration product, wherein the 5'-nucleotidase R1 reagent comprises a buffer solution, a stabilizing agent, a preservative agent, an enzyme activator, 4-AAP(4-aminoantipyrine), 18-crown ether-6 and UAO (Uricase), PNP (Purine Nucleoside Phosphorylase), XOD (Xanthine Oxidase) and POD (Peroxidase) reaction enzyme systems; the 5'-nucleotidase R2 reagent comprises a buffer solution, a stabilizing agent, a preservative agent, PO3- and a Trinder developing system; and the 5'-nucleotidase calibration product comprises a buffer solution, a stabilizing agent, a preservative agent and 5'-nucleotidase. The detection kit provided by the invention is enhanced in detection sensitivity by 40% compared with a 'PNP-XOD-POD' enzyme system, and has good application prospects.
Owner:CHONGQING ZHONGYUAN BIOLOGICAL TECH
Preparation and purification method of recombinant proserum/growth hormone fusion protein for treating children dwarfism
ActiveCN109851674AHigh expression yieldCorrectly foldedFungiPeptide/protein ingredientsPurification methodsEvery Two Weeks
The invention discloses recombinant proserum / growth hormone fusion protein, a preparation and purification method of the recombinant fusion protein, and the use of the recombinant fusion protein to preparation of medicines for treating children dwarfism. The amino acid sequence of the recombinant proserum / growth hormone fusion protein is SEQID NO.1, and the nucleotide sequence of the recombinant proserum / growth hormone fusion protein is SEQID NO.2. According to a preparation technology of the recombinant proserum / growth hormone fusion protein disclosed by the invention, yeast engineering bacteria are constructed and expressed, so that high-density expression recombinant fusion protein is obtained; and through a purification technology, the recombinant proserum / growth hormone fusion proteinwhich can be used clinically is obtained. The recombinant proserum / growth hormone fusion protein obtained by the preparation and purification method adopts a creative medicine structure for treatingthe children dwarfism, has long residual action that administration can be performed once every two weeks, is more suitable for children medication demands, and has more excellent treatment effects, less administration frequency and lower production cost.
Owner:TIANJIN LINDA SINOBIOTECH CO LTD +1
Large ovarian tissue vitrification freezing carrier and application thereof
PendingCN107535482ALow priceWide selection of materialsDead animal preservationGerm cellsVitrificationEngineering
The invention discloses a large ovarian tissue vitrification freezing carrier. The carrier consists of a carrier inner core and an outer sleeve, wherein the carrier inner core is in threaded connection with the outer sleeve, maintaining high airtightness and preventing cross infection of the ovarian tissue; meanwhile, the user operation is also facilitated; the carrier inner core consists of a handheld part, a cap part, a support part and a bearing part sequentially from top to bottom; the bearing part is clamped and fixed by the support part; when in use, a large ovarian tissue is put on an end part of the bearing part, away from the support part; the end part is a large ovarian tissue loading area; experiments prove that by using the carrier disclosed by the invention, the permeation efficiency of a refrigerant is improved; moreover, the operation is simple, the price is low, batch storage of large ovarian tissues is convenient, and the carrier is suitable for promotion and application.
Owner:SHANDONG UNIV QILU HOSPITAL
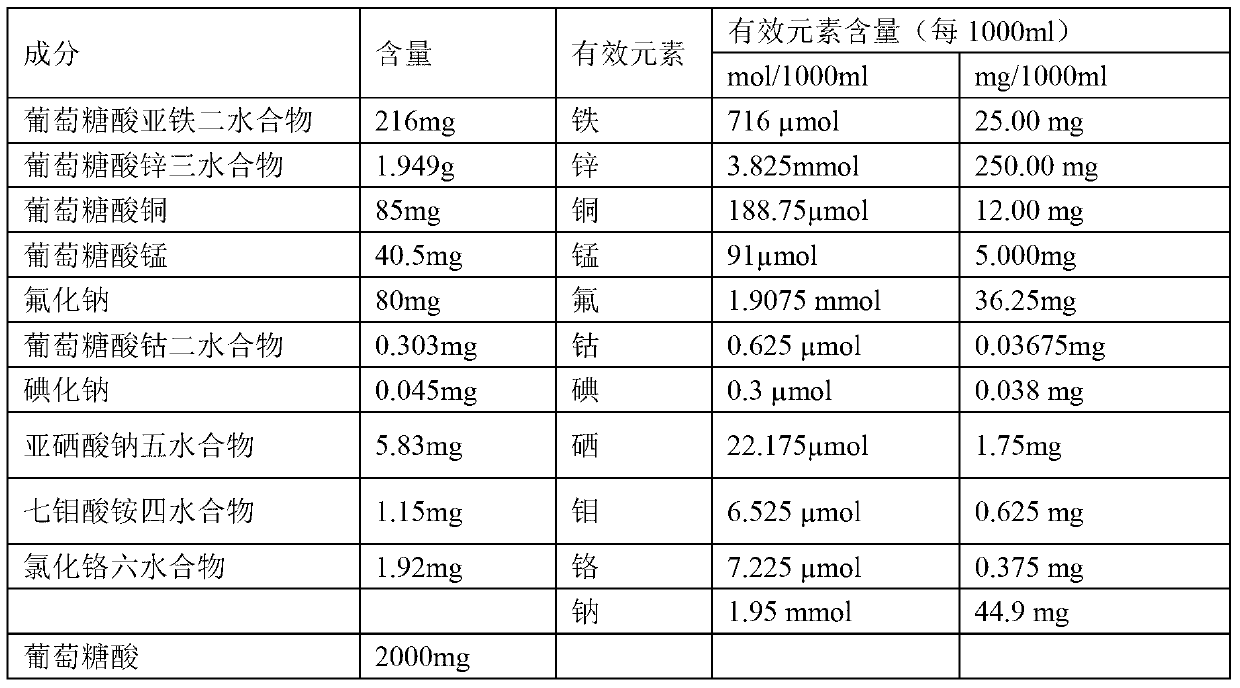
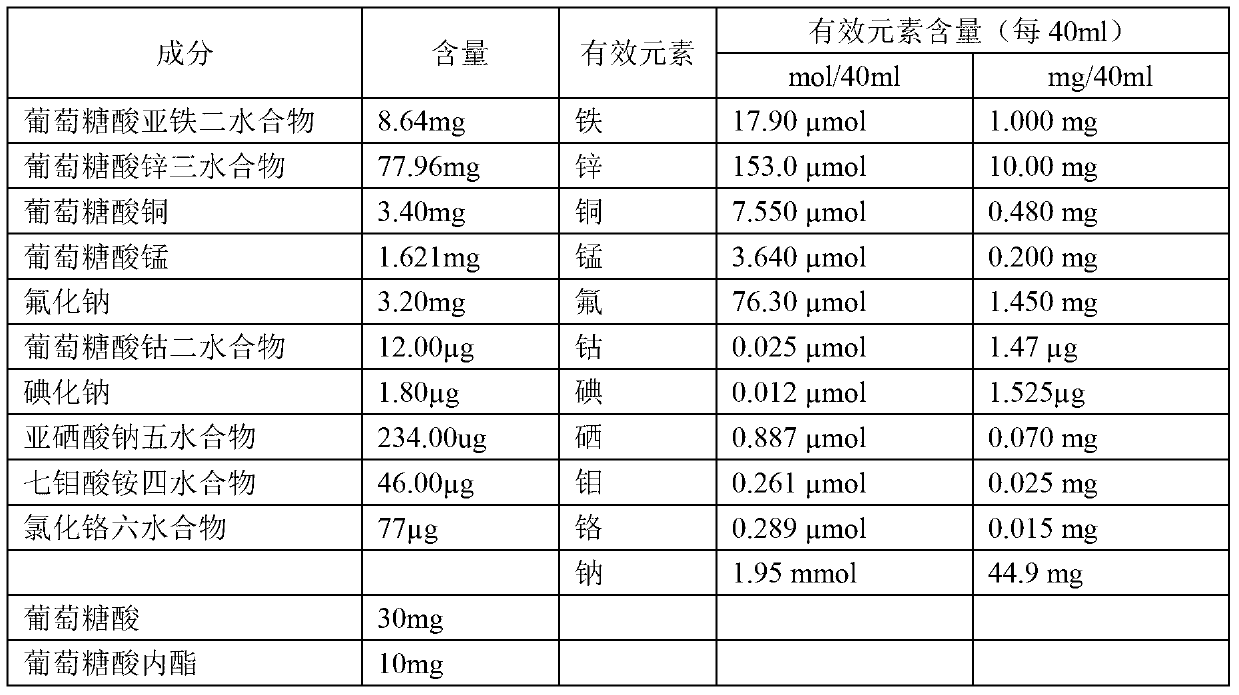
Composition containing various microelements, preparation and preparation method thereof
InactiveCN103340895AImprove stabilitySuitable for clinical applicationHeavy metal active ingredientsMetabolism disorderSodium iodideFreeze-drying
The invention relates to a pharmaceutical composition, and in particular relates to a composition containing various microelements, a preparation and a preparation method thereof. The composition comprises ferrous gluconate dihydrate, a zinc gluconate trihydrate compound, copper gluconate, manganese gluconate, sodium fluoride, cobalt gluconate dihydrate, sodium iodide, sodium iodide pentahydrate, ammonium heptamolybdate tetrahydrate, chromic chloride hexahydrate and a medicine auxiliary material. The form of the pharmaceutical composition can be injection or freeze-dried powder injection. According to the invention, organic iron, organic zinc, organic copper, organic manganese and organic cobalt are adopted to replace chloride, so that not only is the property of the composition more stable, but also the adverse reaction is reduced, and the bioavailability of the medicine is increased. Therefore, the preparation provided by the invention is better in safety and more suitable for clinical application.
Owner:江西博意特科技有限公司
Medicine composite used for embolotherapy and acesodyne and preparation method thereof
ActiveCN101716349ALower drug concentrationSmall side effectsAntipyreticDigestive systemLidocaine HydrochlorideDouble bond
The invention provides a medicine composite used for embolotherapy and acesodyne and a preparation method thereof. The medicine composite comprises a biocompatibility macromolecular compound containing hydroxy, a monomer containing unsaturated double bond and anion group, a polymer, and local anesthetic containing amino group, wherein the polymer is generated through a polymerization reaction of an optional vinyl monomer and the polymerization reaction is initiated by free radicals, and the local anesthetic is combined to an anion group of the generated polymer. In the invention, lidocaine hydrochloride is combined to a polymer carrier; which can give full play to the acesodyne effect of the local anesthetic in the embolotherapy; the anion part of the polymer can properly combine with the local anesthetic containing the amino group, which can both realize higher medicine loading capacity and enable the medicine in an emboliaztion agent to be exchanged by cations in vivo and then slowly released. Moreover, the polymer emboliaztion carrier has simple technology, low cost, and suitability for large scale industrial production.
Owner:HYGEA MEDICAL TECH CO LTD
Titanium casting investment material for oral cavity and application method thereof
InactiveCN101849888AReduced responseReduce the mixing ratioImpression capsDentistry preparationsAluminiumSuperplasticizer
The invention provides a titanium casting investment material for an oral cavity and an application method thereof. A fame-proofing agent thereof adopts ZrO2 powder and Al2O3 powder; a heating expanding agent adopts metal zirconium powder or titanium powder; a bonding agent adopts pure calcium aluminate cement; a curing time modifier adopts LiCO3. The mixing liquid special for water solution with1%-5% of high performance water reducer is added to the above materials by the proportion of 100:15-25. The invention takes heat-resisting zirconia, alumina and the like as the flame-proofing agent, heat-resisting pure calcium aluminate cement as the bonding agent, metal zirconium powder or titanium powder as the heating expanding agent, lithium carbonate as the curing time modifier and water solution with high performance water reducer as the special mixing liquid of the titanium casting investment material for the first time so as to reduce the water mixing ratio and improve the compressionstrength and the heating expansibility, thereby reaching the purpose that the enough expansion volume can be generated to make up the pure titanium casting contraction under the lower temperature.
Owner:BEIJING STOMATOLOGY HOSPITAL CAPITAL MEDICAL UNIV
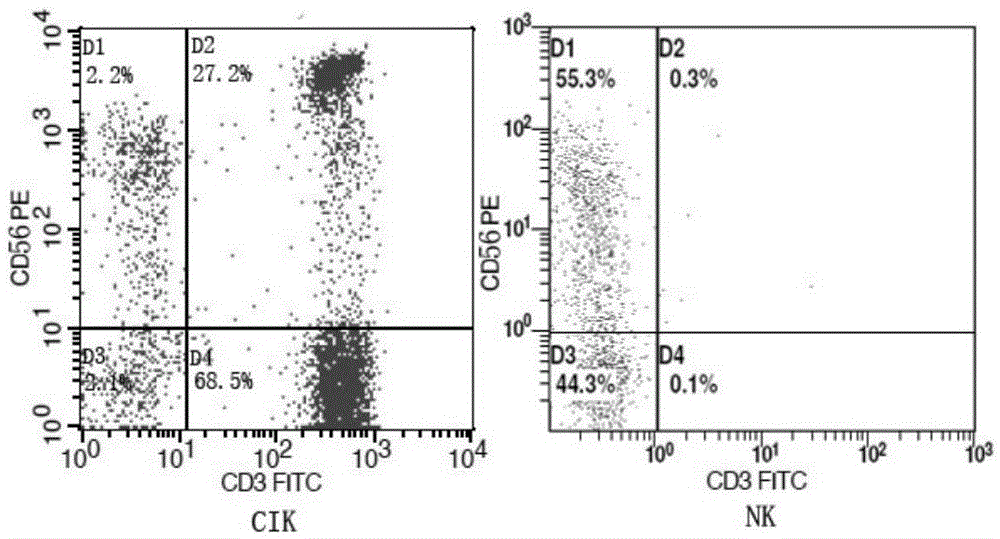
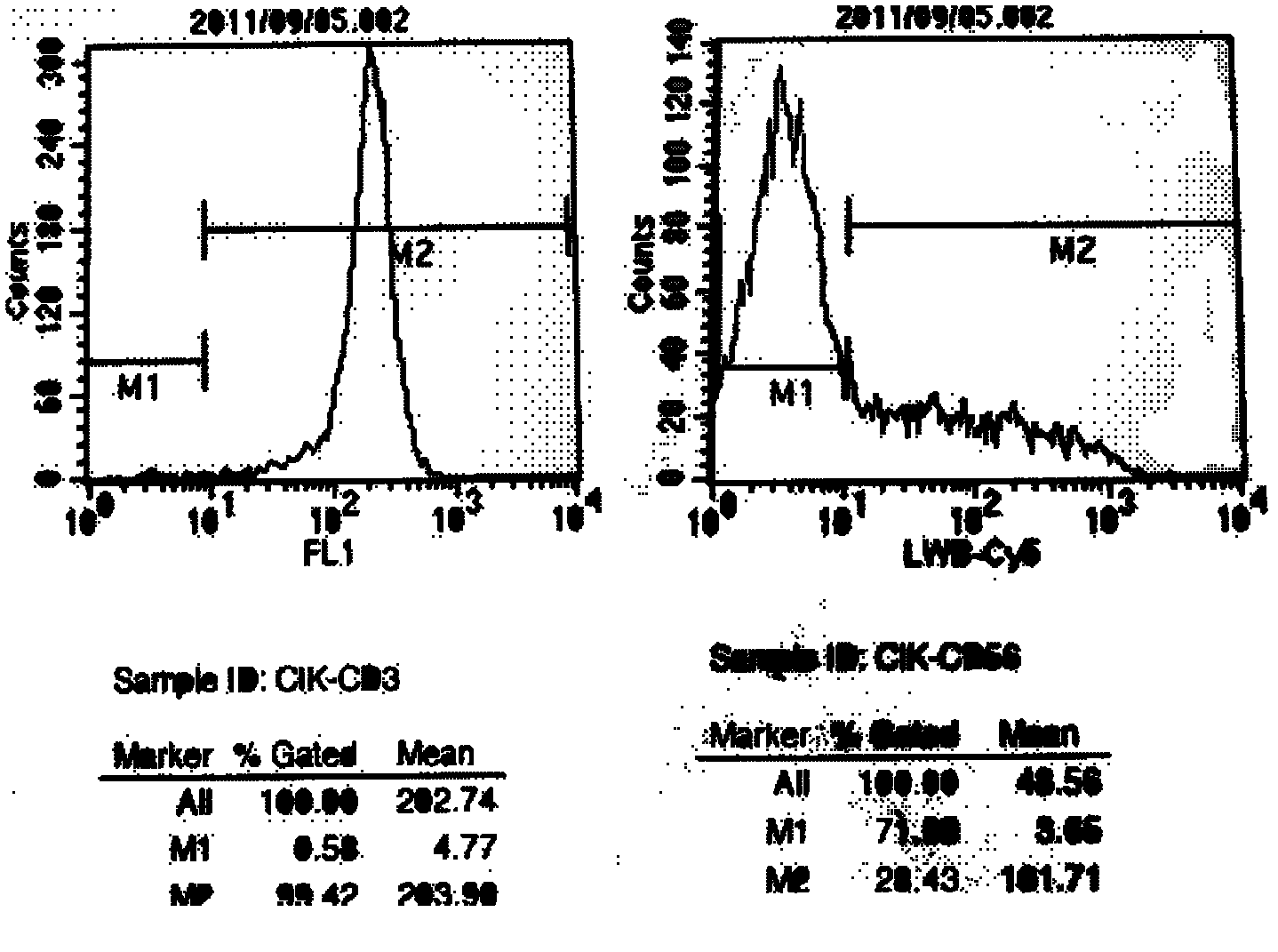
Method for preparing anti-tumor combined immune cells DC (dendritic cell)-CIKs (cytokine induced killers) and NKs (natural killers) simultaneously and prepared combined immune cells
InactiveCN105647865AReduced Chances of ContaminationEnhance tumor killing activityBlood/immune system cellsCell culture active agentsFicollDendritic cell
The invention discloses a method for preparing anti-tumor combined immune cells DC (dendritic cell)-CIKs (cytokine induced killers) and NKs (natural killers) simultaneously and the prepared combined immune cells. Ficoll density gradient centrifugation is used for efficient separation, a mononuclear cell is obtained, sufficient quantities of DCs, CIKs and NKs are obtained through cell culture bags and an immune cell induction culture system, and finally, the induced cells are cultured in a combined manner and applied to clinical treatment, so that a tumor killing effect is realized. The DCs, the CIKs and the NKs are subjected to induction culture respectively with adoption of TexMACS immune cell culture media produced by Miltenyi Biotec, autoserum, various cytokines and a combined culture technology, the cells are mixed for culture and application at certain point in time, application of fetal calf serum is avoided, the pollution rate of an exogenous pyrogen and an exogenous allergen is reduced, and the tumor killing activity of the finally mixed cells is enhanced simultaneously; with adoption of a cell culture bag technology, the cell contamination rate is reduced, and the method is suitable for clinical treatment and application.
Owner:TIANJIN PURUI SAIER BIOLOGICAL TECH CO LTD
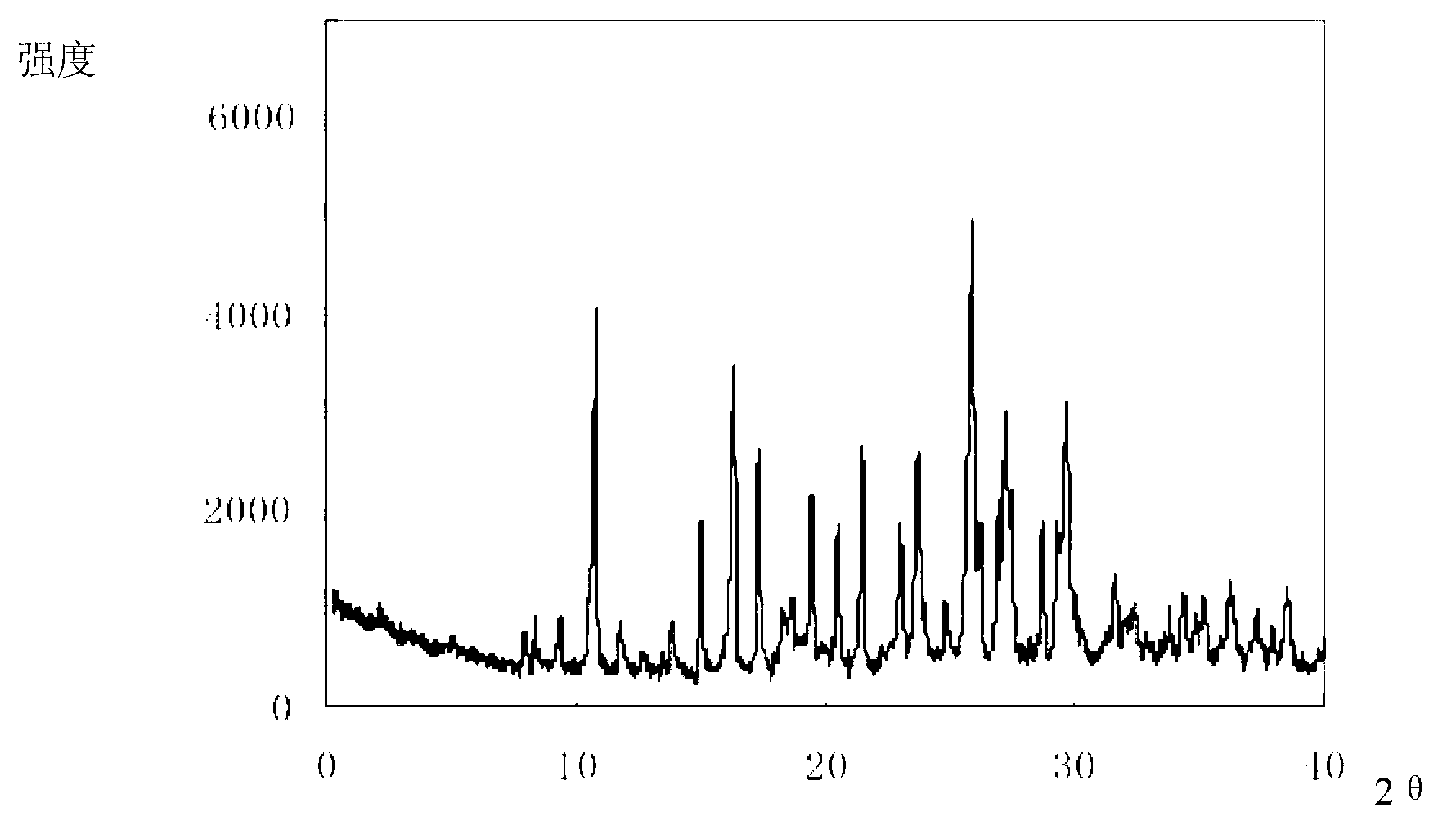
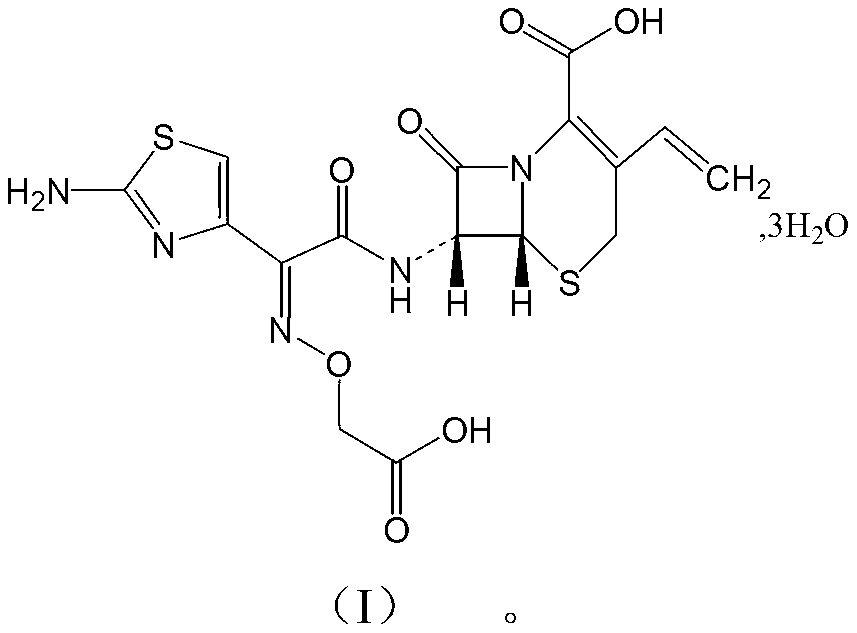
Cefixime compound and pharmaceutical composition thereof
InactiveCN103193798AUniform particle size distributionWell mixedAntibacterial agentsOrganic active ingredientsDrug compoundPharmaceutical Adjuvants
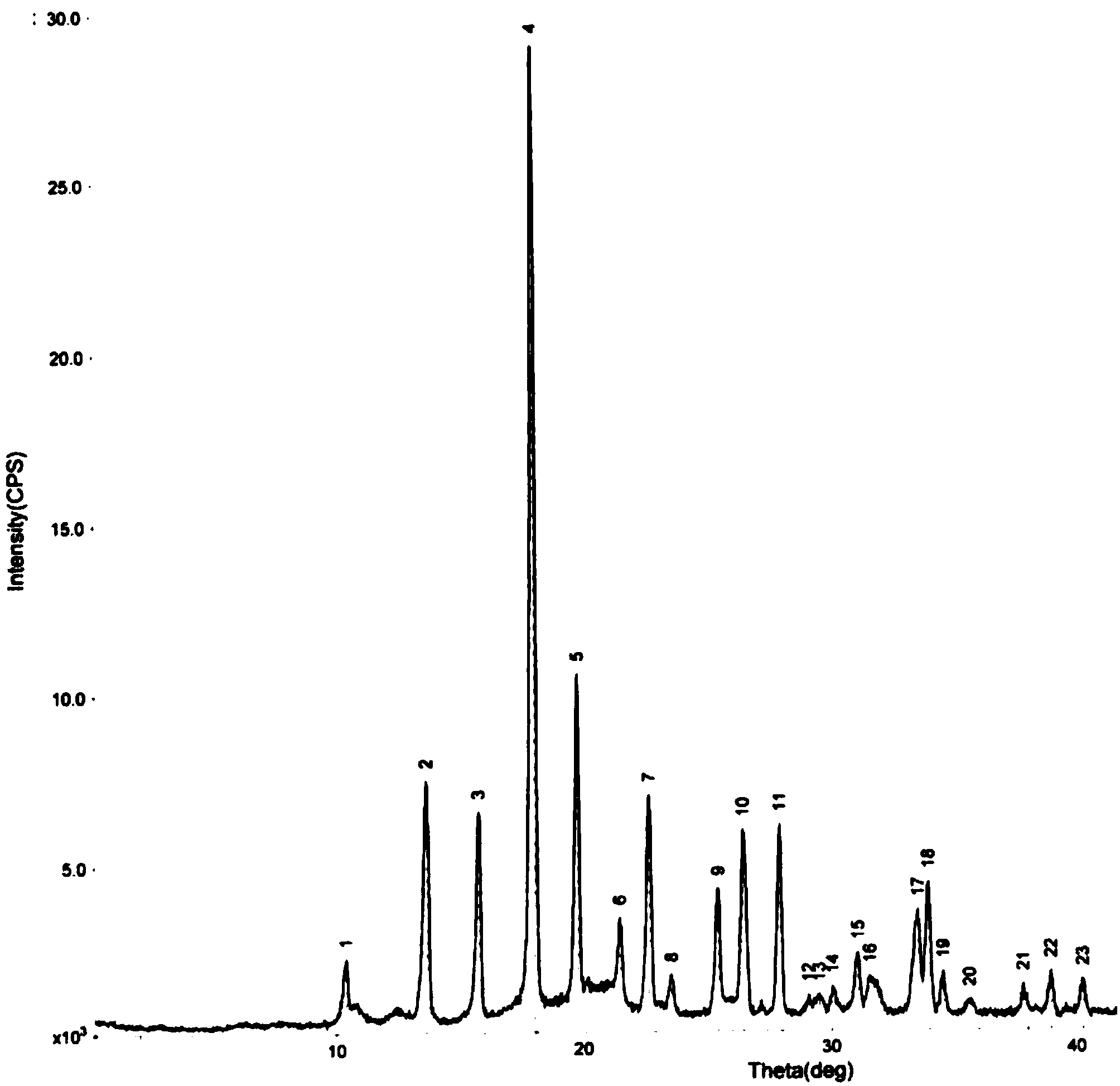
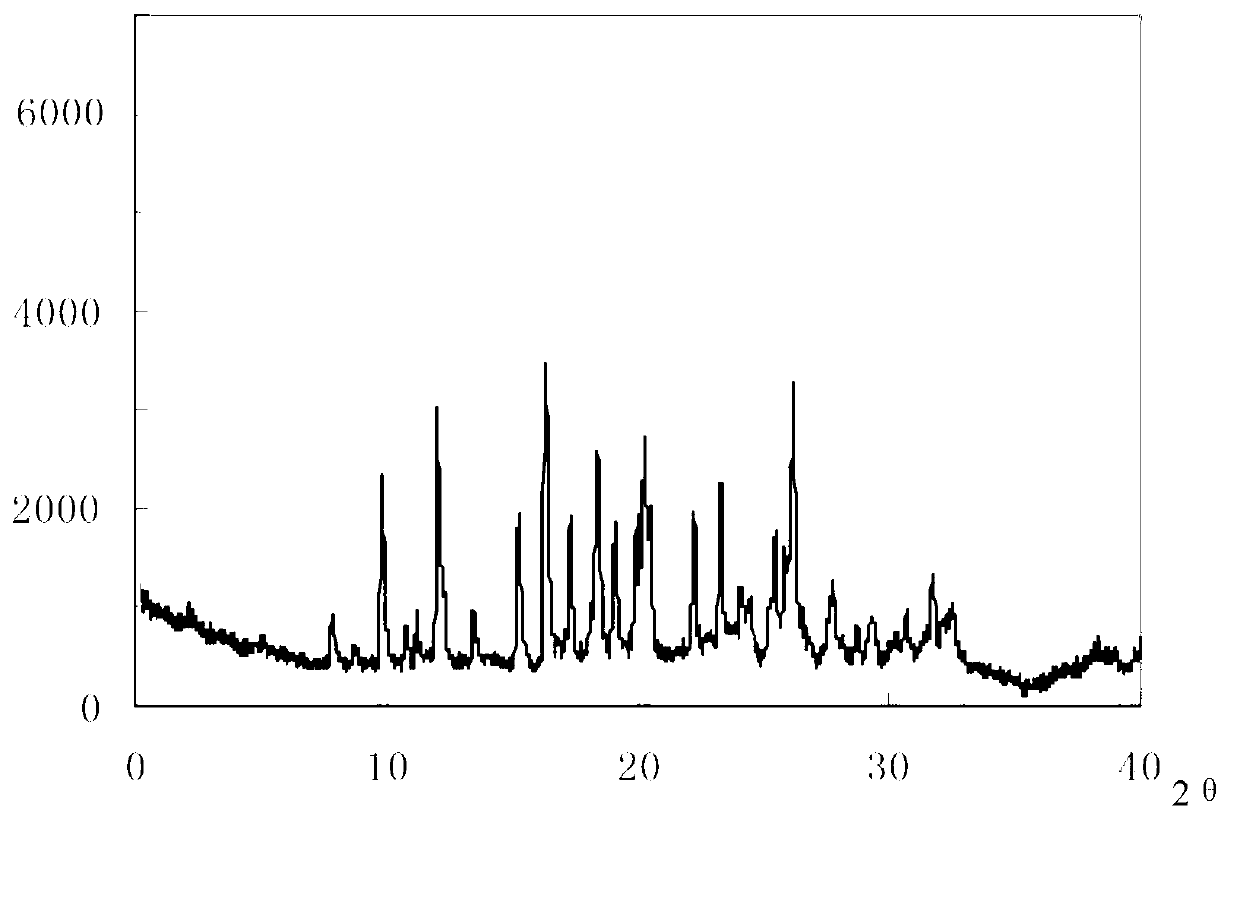
The invention relates to a drug compound, and particularly relates to a cefixime compound. An X-ray powder diffraction pattern of the cefixime compound measured through a Cu-K alpha ray is shown in a figure I. The invention also relates to a pharmaceutical composition containing the cefixime compound. The pharmaceutical composition comprises the cefixime compound and a pharmaceutical adjuvant; the pharmaceutical composition is an oral preparation including an oral normal release preparation and a controlled release preparation; and the oral normal release preparation is selected from a tablet, an enteric-coated tablet, a capsule, a dispersible tablet, dry suspension, a chewable tablet or granules. The cefixime compound disclosed by the invention is high in purity, high in bioavailability and suitable for clinical application.
Owner:四川省惠达药业有限公司
Embolization polymer, novel blood vessel embolization chemotherapy composite as well as preparation and application of novel blood vessel embolization chemotherapy composite
InactiveCN109053953AImprove solubilityThe polymerization process is easy to controlOrganic active ingredientsSurgical adhesivesDiseaseCross-link
The invention provides an embolization polymer. The embolization polymer is prepared by performing polymerization reaction initiated by free radicals on micromolecule monomer containing unsaturated double bonds, monomer containing unsaturated double bonds and an optional cross-linking agent; the cross-linking agent is polyfunctionality water-soluble acrylate or acrylamide. The embolization polymeris an ion exchange microsphere carrier and has higher deformation ability and higher drug trapping efficiency and drug loading capacity; meanwhile, a slow release effect is better. The invention further provides a novel blood vessel embolization chemotherapy composite. The embolization chemotherapy composite disclosed by the invention can jointly deliver an embolization agent and chemotherapy drug to a target blood vessel part through a catheter, so that a curative effect of the chemotherapy drug is fully played, peripheral normal tissues are prevented from being damaged, and disease relapsing is reduced.
Owner:深圳市比德泰克生物医药科技有限公司
Artificial skin and preparation method thereof
InactiveCN105056307ASuitable for clinical applicationVertebrate cellsArtificial cell constructsDiseaseCuticle
The invention belongs to the technical field of tissue engineering, and discloses artificial skin and a preparation method thereof. The artificial skin comprises epithelial cells, dermal cells and a biological bracket material. The preparation method comprises the following steps: compounding the dermal cells into the biological bracket material; inoculating the epithelial cells on the surfaces of the dermal cells; carrying out cultivation. As the artificial skin comprises a corium layer, an epidermal layer and active cells, and the epidermal layer comprises cuticle, the artificial skin is similar to normal skin, human skin with complete functions can be regenerated after the transplanting of the artificial skin, and the regenerated skin comprises skin appurtenant organs such as hair and sebaceous glands. Therefore, the artificial skin can be used for treating skin defect caused by inflammation, ulcer, burning and scalding, and also can be used for preparation of skin models used for disease researches, medicine testing and screening, cosmetic testing, or the like.
Owner:SUZHOU PANSHENG BIOTECH CO LTD
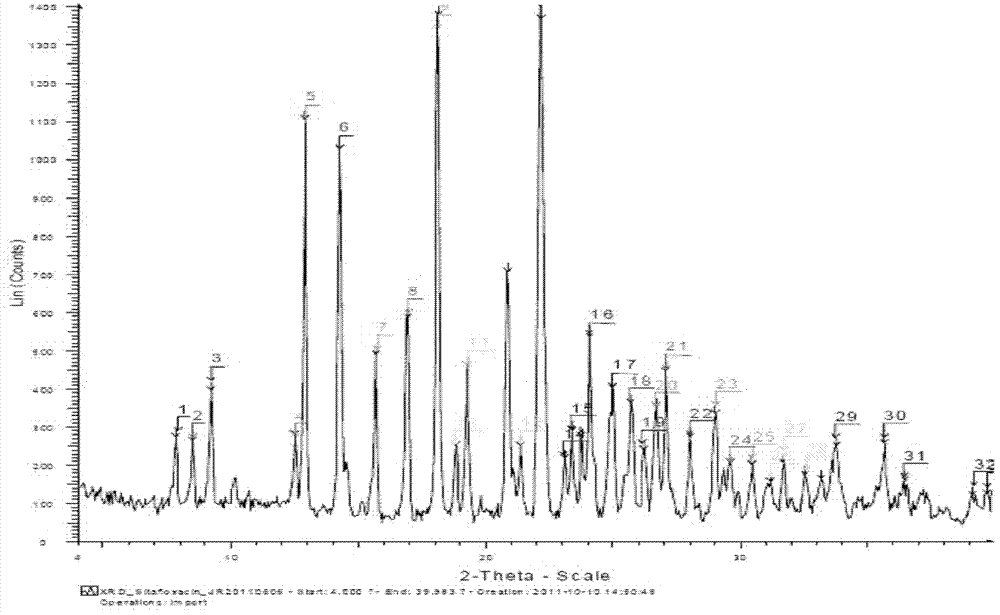
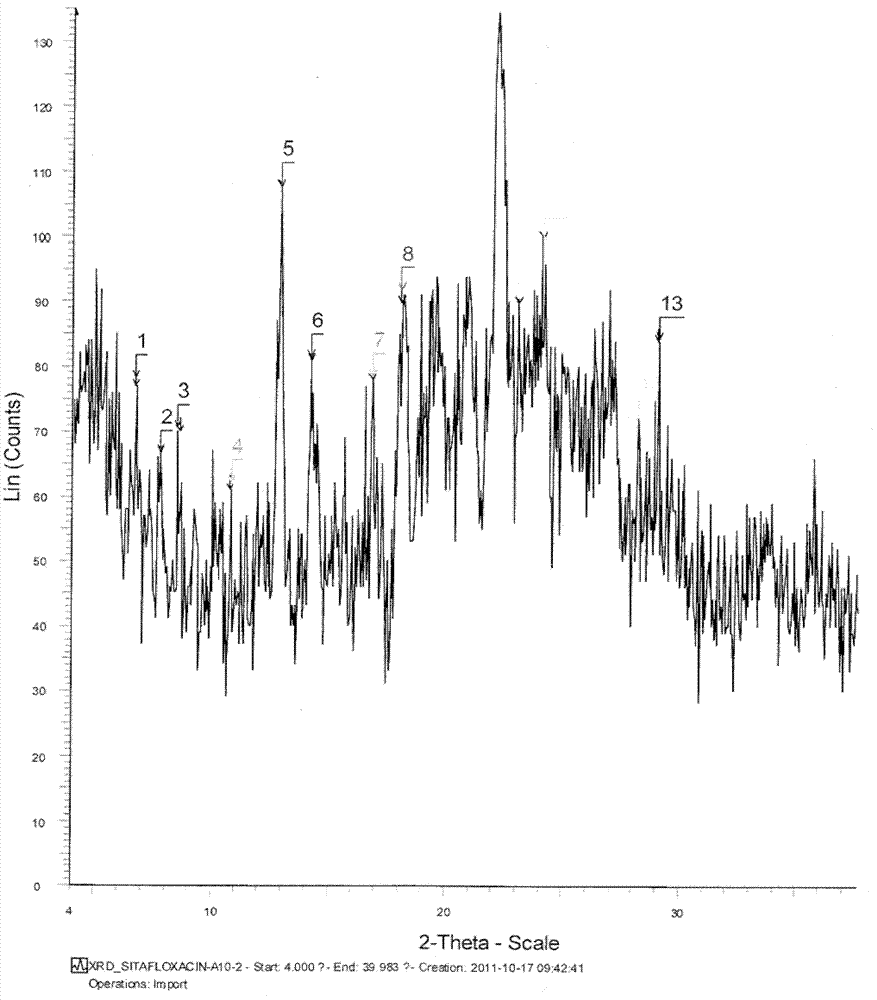
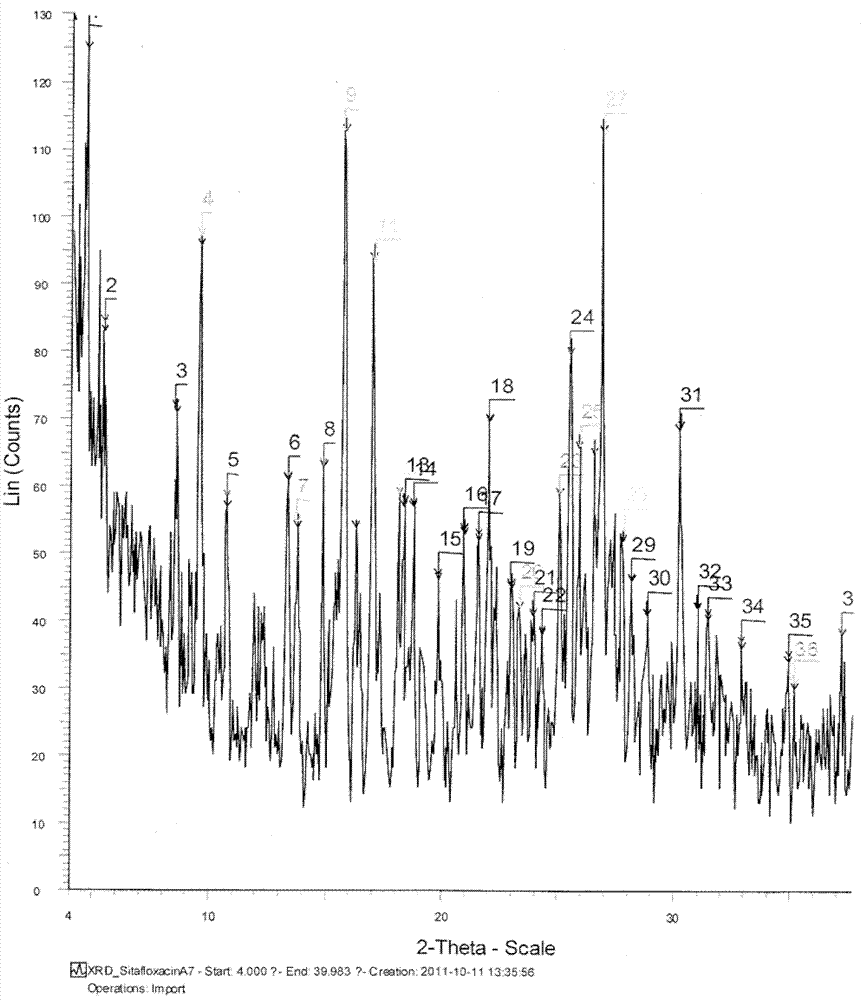
Salts of sitafloxacin and pharmaceutical purposes thereof
ActiveCN103087042AImprove stabilityGood water solubilityAntibacterial agentsOrganic active ingredientsIrritationWater soluble
The invention provides acid addition salts of sitafloxacin represented by the formula, crystal forms and preparation methods of the salts, and pharmaceutical compositions, preparations and pharmaceutical purposes of the salts, wherein A is described in the specification. Both stability and water-solubility of the salts of the sitafloxacin or the crystal forms are better than that of sitafloxacin free alkali; furthermore, the irritation is less than that of the sitafloxacin free alkali; and the compositions or preparations prepared from the salts are applied to clinical applications.
Owner:NANJING YOKO PHARMA GRP CO LTD +2
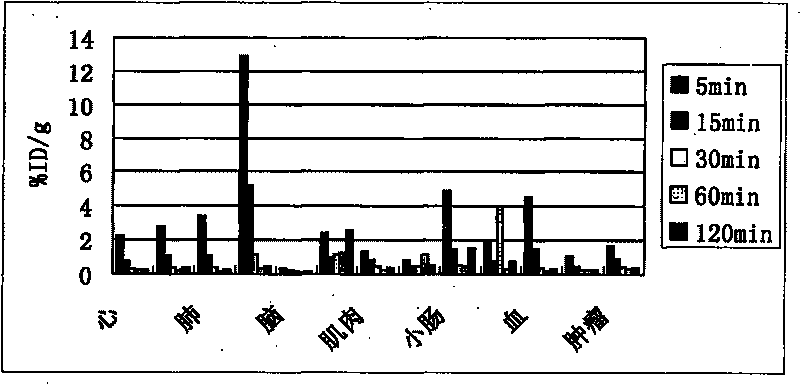
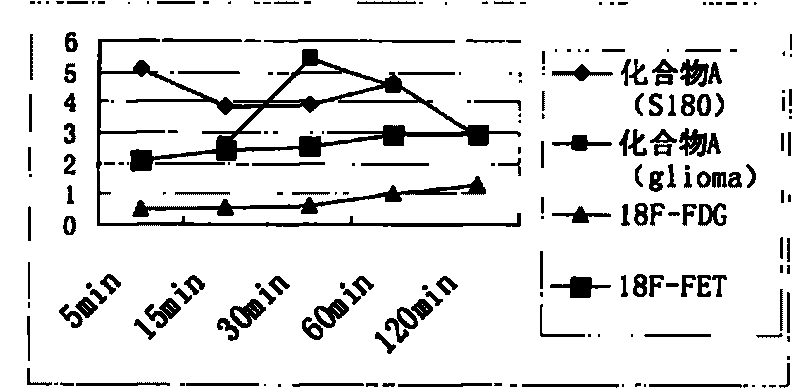
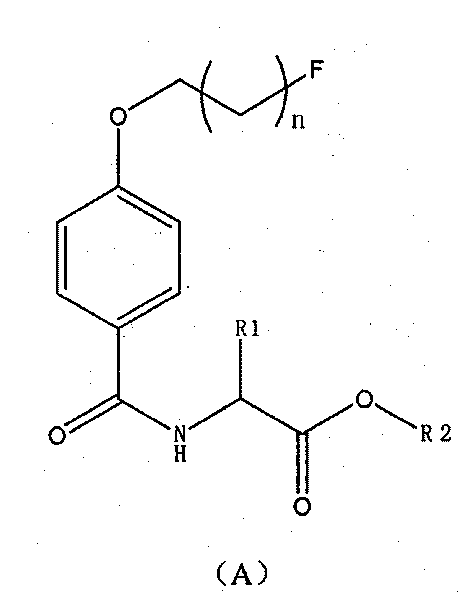
Novel 18F labeled amino acid derivatives, preparation method and application thereof in tumor imaging
InactiveCN101723849AEasy to markEasy to operateOrganic compound preparationRadioactive preparation carriersAbnormal tissue growthImaging agent
The invention discloses novel radioactive 18F labeled amino acid derivatives, which are used in research on tumor positron emission tomography (PET) imaging. The derivatives are characterized in that one end of the derivatives is provided with F substituted alkoxy benzoyl structure, and the other end of the derivatives is provided with alpha-amino acid structure; substituent R1 is positioned on an alpha site of carboxyl group and is hydrogen, methyl group, ethyl group, propyl group, isopropyl group, butyl group, methylthio-ethyl group, methyl acetate group or propionate carbomethoxy group; R2 is methoxy group; and n is a number between 1 and 5. The R1 is hydrogen, methyl group, ethyl group, propyl group, isopropyl group, butyl group, methylthio-ethyl group, methyl acetate group or propionate carbomethoxy group; the R2 is hydrogen; and the n is a number between 1 and 5. Compounds improve fat solubility. Different amino acid structures are introduced into the structure, and F in the structure is 19F and 18F. Compared with the prior art, the 18F labeled amino acid derivatives provided by the invention have better discrimination degree of biological distribution, the potential of being used as a tumor imaging agent (particularly a brain tumor imaging agent), as well as the characteristics of simple preparation and high labeling rate. The R1 is hydrogen, methyl group, ethyl group, propyl group, isopropyl group, butyl group, methylthio-ethyl group, methyl acetate group or propionate carbomethoxy group; the R2 is methoxy group; and the n is a number between 1 and 5. The R1 is hydrogen, methyl group, ethyl group, propyl group, isopropyl group, butyl group, methylthio-ethyl group, methyl acetate group or propionate carbomethoxy group; the R2 is hydrogen; and the n is a number between 1 and 5.
Owner:BEIJING NORMAL UNIVERSITY
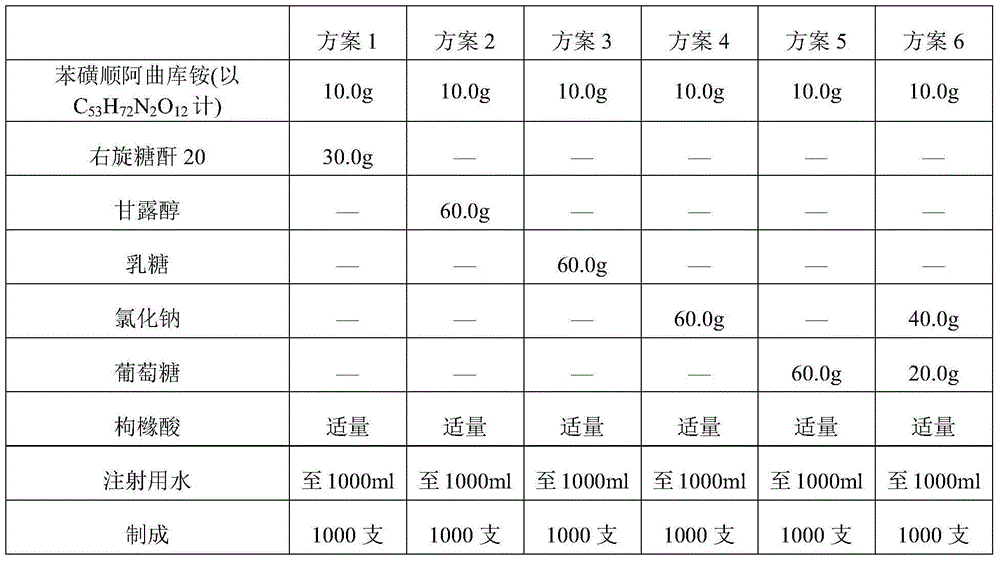
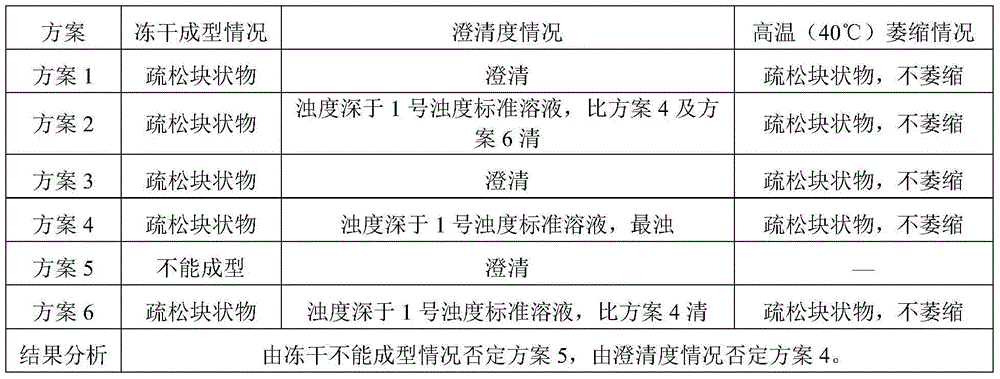
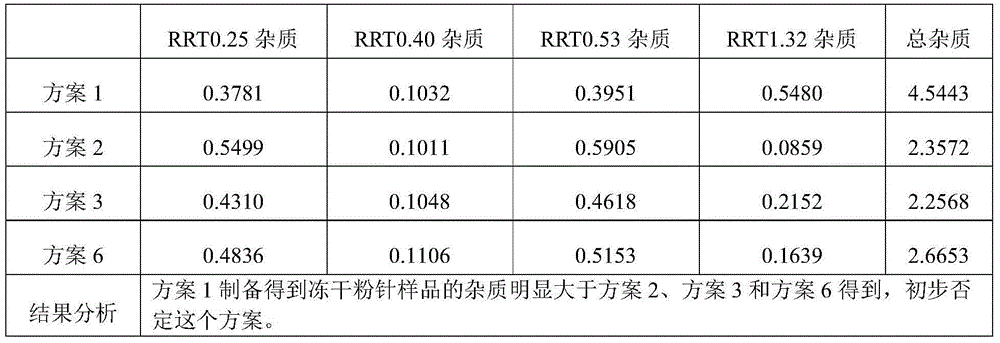
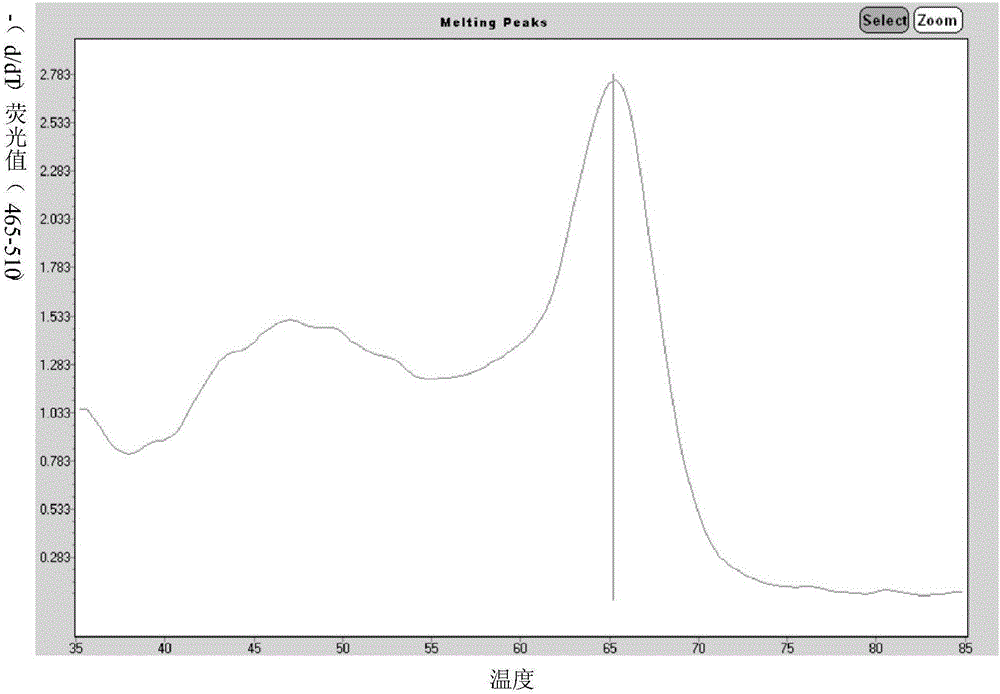
Cisatracurium besilate composition for injection and preparation method and application thereof
ActiveCN104434822AHigh clarityImprove stabilityOrganic active ingredientsPowder deliveryPharmacyMedicine
The invention belongs to the field of chemical pharmacy and in particular relates to a cisatracurium besilate composition for injection and a preparation method and application thereof. The cisatracurium besilate composition for injection comprises cisatracurium besilate, sodium chloride, glucose and a pH regulator, wherein cisatracurium besilate takes cisatracurium as an active ingredient, and cisatracurium, sodium chloride and glucose are combined according to a certain ratio. The cisatracurium besilate composition for injection disclosed by the invention is simple in composition, convenient to operate, simple in freeze-drying process, controllable in quality, low in content of product related substances, high in reproducibility and convenient to popularize and utilize.
Owner:HAINAN XIANTONG PHARMA CO LTD
Preparation method of magnesium-hydroxyapatite/polylactic acid composite molding material
InactiveCN102504508AUniform particle sizeUniformity of dispersionProsthesisOrganic solventBiocompatibility Testing
The invention relates to a preparation method of a magnesium-hydroxyapatite / polylactic acid composite molding material. The preparation method comprises the following steps that: PLA is dissolved in organic solvent, the prepared hydroxyapatite solution is directly fully mixed with the organic solvent dissolved with polylactic acid, then HA / PVA composite is prepared by means of volatilizing and drying the solvent, the composite is dissolved in the organic solvent and is added with M9 powdery particles for intensive mixing, then the magnesium-hydroxyapatite / polylactic acid composite material isobtained by volatilizing and drying the solvent, and finally the magnesium-hydroxyapatite / polylactic acid composite material is molded by injection molding or mold pressing technology. The preparation method provided by the invention has the advantages that the M9-HA / PLA composite material is prepared by the solution blending technology, the dispersible uniformity and the biocompatibility of magnesium and hydroxyapatite in the polylactic acid are improved, the loss of molecular weight of polylactic acid in the preparation process is reduced, the balanced dynamics performance and the degradation rate of the material are guaranteed, and the preparation method is much more applicable to clinical bone repair; in addition, the preparation method is simple in technology and low in cost and is much more suitable for productization production.
Owner:TIANJIN UNIVERSITY OF TECHNOLOGY
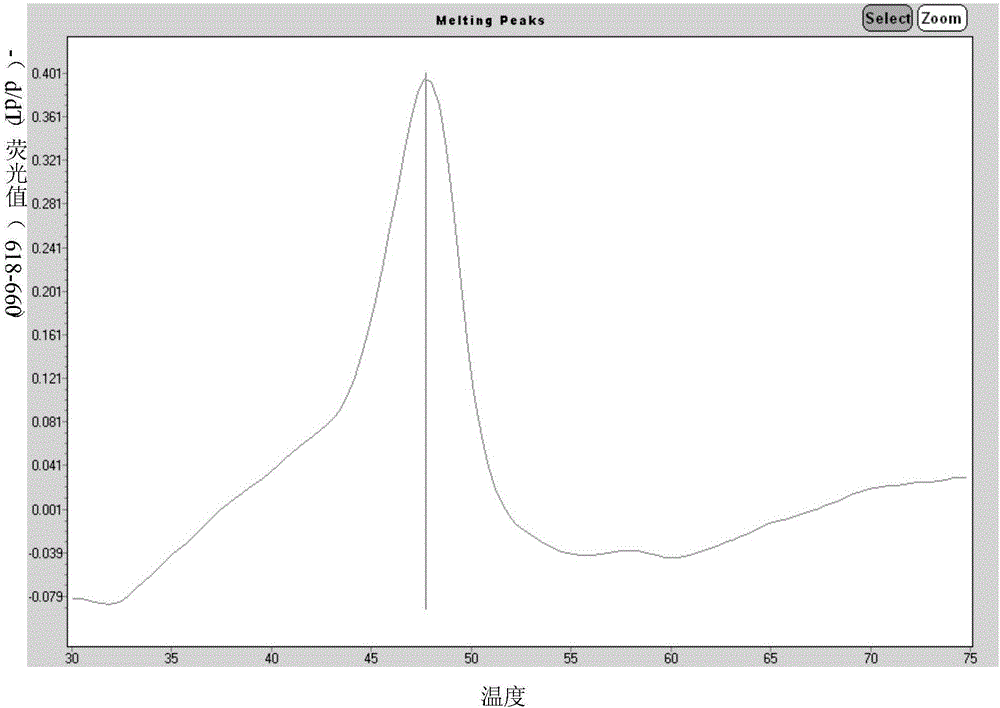
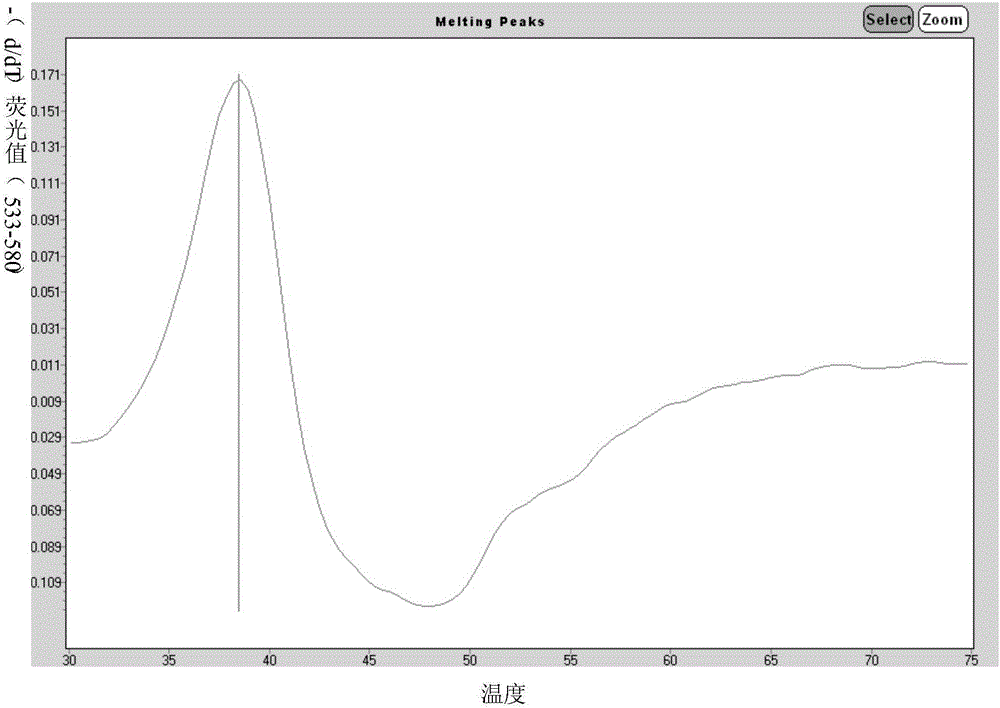
Primer for simultaneously detecting diversified respiratory viruses on basis of melting curve processes and single tube and application of primer
InactiveCN106834546AAvoid pollutionSimple and fast operationMicrobiological testing/measurementDNA/RNA fragmentationFluorescenceRespiratory virus
The invention discloses a primer for simultaneously detecting diversified respiratory viruses on the basis of melting curve processes and a single tube, and belongs to the field of technologies for detecting nucleic acid. The primer comprises reverse transcription primers shown as SEQ ID NO.1-8, amplification primers shown as SEQ ID NO.9-26, amplification starting universal primers shown as SEQ ID NO.27-28 and molecular beacon probes shown as SEQ ID NO.29-35. Fluorescence groups and quenching groups are arranged at two ends of each molecular beacon probe. The primer has the advantages that the primer is based on Tem-PCR (target enriched multiplex-polymerase chain reaction) technologies and melting curve analysis, gene sequences of the diversified viruses are compared in gene sequence libraries of NCBI (national center for biotechnology information), specific primers and the molecular beacon probes are arranged at conservative sites by means of designing, accordingly, 7 types of respiratory viruses can be simultaneously detected by the single tube, and the primer is easy to operate and convenient to apply.
Owner:HANGZHOU D A GENETIC ENG
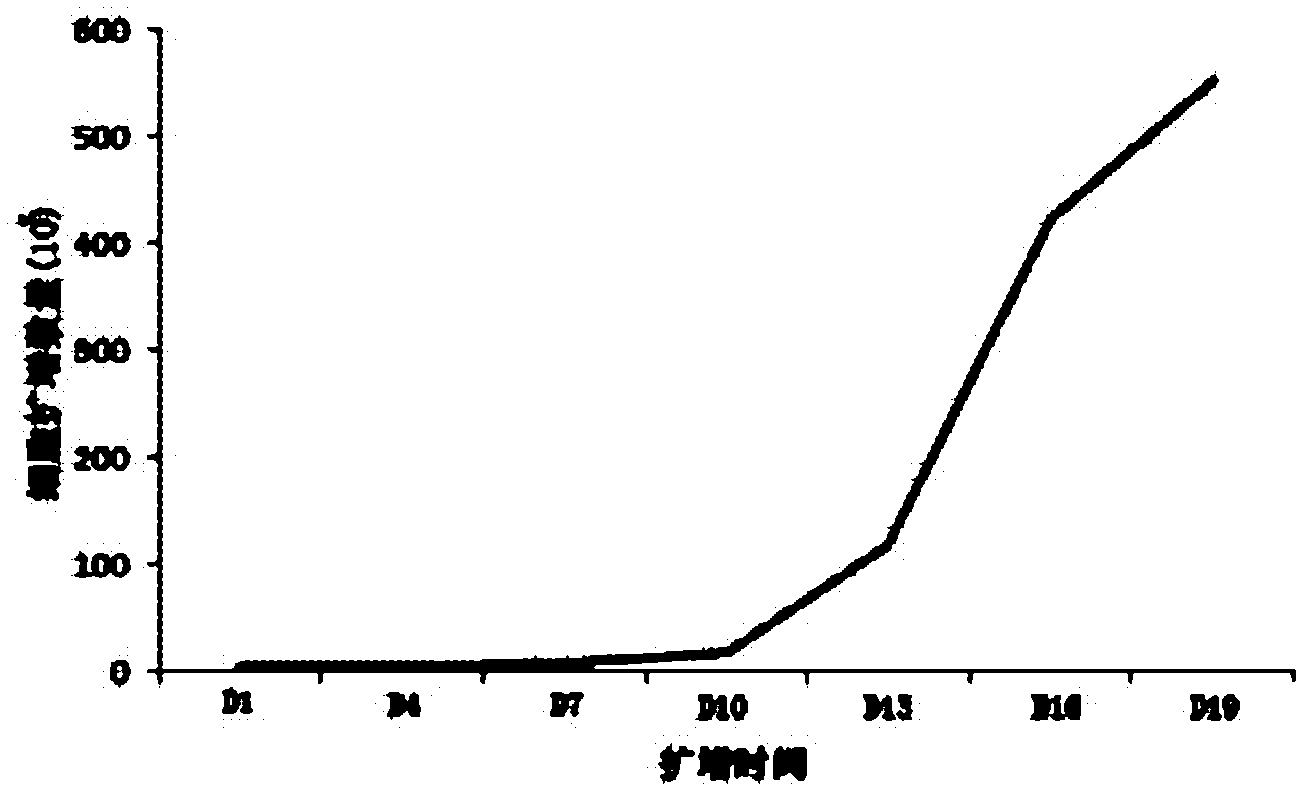
Method for preparing CIK cell with killing effect on tumor cell
InactiveCN103184192ASatisfy the separation effectEasy to separateBlood/immune system cellsFicollLymphocyte culture
The invention discloses a method for preparing CIK cells with a killing effect on tumor cells. The CIK cells are obtained through induced culture of 17-20 days by using a ficoll mononuclear cell separation medium with a density of 1.084 in the PBS system to separate mononuclear cells in peripheral blood or umbilical cord blood. According to the present invention, mononuclear cells are separated and obtained efficiently by using the ficoll density gradient centrifugation method, and a sufficient amount of CIK is obtained by using cell culture bags and a CIK cell culture system to meet the needs of clinical treatment. The Takara lymphocyte culture medium and the autologous serum and cytokine co-culture technique are used in the method, thereby preventing application of fetal bovine serum, reducing contamination risks of exogenous pyrogen and sensitinogen, and while maintaining the advantage of CIK cell efficient proliferation. The cell culture bag technology is used to reduce risk of cell contamination, and is suitable for clinical therapeutic application.
Owner:UNION STEMCELL & GENE ENG
Preparation method of silver ion antibacterial medical dressing
InactiveCN102600497AStrong anti-infection effectPromote wound healingAdhesive dressingsAbsorbent padsIonChemistry
The invention relates to a preparation method of a silver ion antibacterial medical dressing, and the preparation method comprises the following steps: dyeing, attaching a silver ion antibacterial agent, baking, mixing with viscose, weaving, slitting peritoneum, and preparing patch. Compared with similar products, the product has stronger infection resistance, can be used for remarkably accelerating wound healing, shortening the course of treatment and reducing the formation of scars. Clinical application proves that the silver ion antibacterial medical dressing is applicable to treatment of I-type, II-type and III-type cuts after an operation, has the advantages of safety, reliability, efficiency, no allergy occurrence and the like, and is suitable for clinical popularization and application.
Owner:朱建华
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com