Cisatracurium besilate composition for injection and preparation method and application thereof
A technology of cisatracurium besylate and cisatracurium, which is applied in the field of cisatracurium besylate for injection and its preparation, can solve the problems of limited development and achieve convenient operation, simple composition, Low product-related substances and good reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0144]
[0145] 【Preparation】
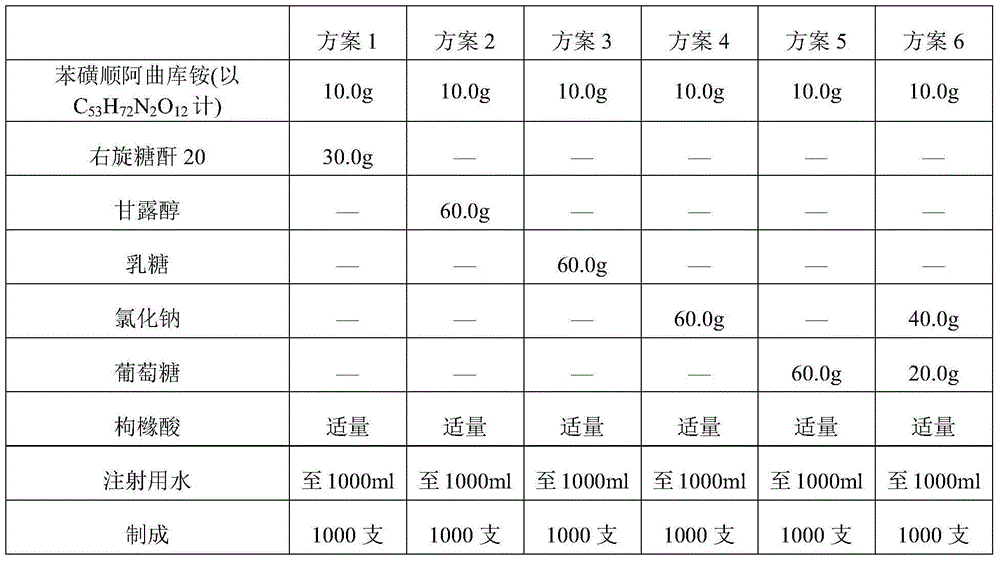
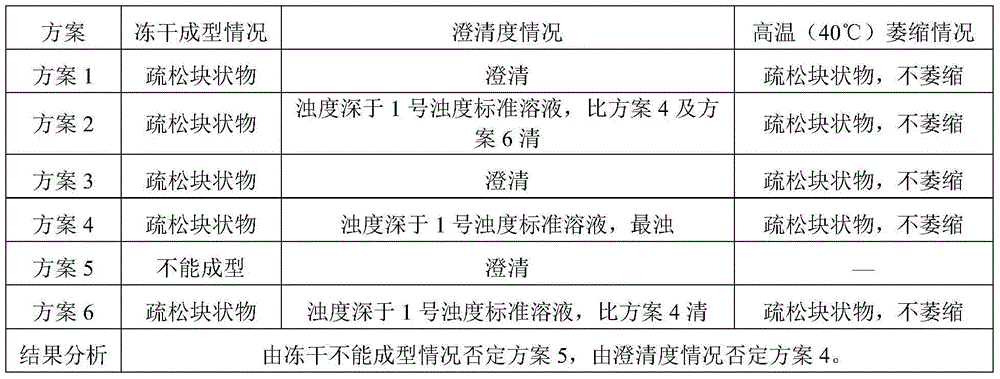
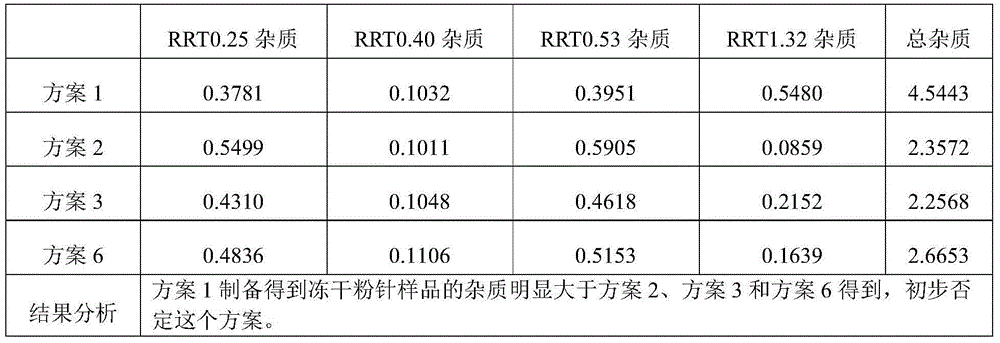
[0146] Take cisatracurium besylate and add 50% of the total amount of water for injection at 30°C, stir to dissolve, add activated carbon for needles with a volume of 0.05% of the liquid medicine, stir for 15 minutes, filter and decarbonize, and obtain the liquid medicine.
[0147] Add sodium chloride and glucose into 25% of the total amount of water for injection at 30° C., stir and dissolve to obtain an excipient solution.
[0148] Mix the medicinal solution and the auxiliary material solution, add 7% hydrochloric acid solution to adjust the pH value of the solution to 2.2, add water for injection to the full amount, and add 7% hydrochloric acid solution to adjust the pH value to 2.3.
[0149] After passing the intermediate inspection, use 0.22μm filter material to sterilize and filter, quantitatively fill in 7ml vials (theoretical loading is 0.5ml), press the rubber stopper halfway, put it in a freeze-drying box, and open the circulation p...
Embodiment 2
[0151]
[0152] 【Preparation】
[0153] Add cisatracurium besylate to 50% of the total amount of water for injection at 40°C, stir to dissolve, add activated carbon for needles with a volume of 0.05% of the liquid medicine, stir for 15 minutes, filter and decarbonize, and obtain the liquid medicine.
[0154] Add 25% of the total amount of sodium chloride and glucose into water for injection at 40° C., stir and dissolve to obtain an excipient solution.
[0155] The medicinal solution is mixed with the auxiliary material solution, and the pH value is adjusted to 2.5 by adding 10% citric acid solution, and the water for injection is added to the full amount, and the pH value is adjusted to 2.5 by adding 10% citric acid solution.
[0156] After passing the intermediate inspection, use 0.22μm filter material to sterilize and filter, quantitatively fill in 7ml vials (theoretical loading is 1ml), press the rubber stopper halfway, put it in a freeze-drying box, and turn on the circu...
Embodiment 3
[0158]
[0159] 【Preparation】
[0160] Add cisatracurium besylate to 50% of the total amount of water for injection at 50°C, stir to dissolve, add activated carbon for needles with a volume of 0.05% of the liquid medicine, stir for 15 minutes, filter and decarbonize, and obtain the liquid medicine.
[0161] Add 25% of the total amount of sodium chloride and glucose into water for injection at 50° C., stir and dissolve to obtain an excipient solution.
[0162] The medicinal solution is mixed with the auxiliary material solution, 7% lactic acid solution is added to adjust the pH value to 2.8, water for injection is added to the full amount, and 7% lactic acid solution is added to adjust the pH value to 3.0.
[0163] After passing the intermediate inspection, use 0.22μm filter material to sterilize and filter, quantitatively fill in 7ml vials (theoretical loading is 2ml), half-press the rubber stopper, put it in a freeze-drying box, and turn on the circulation pump for shelf c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com