Patents
Literature
37 results about "Benzylisoquinoline" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
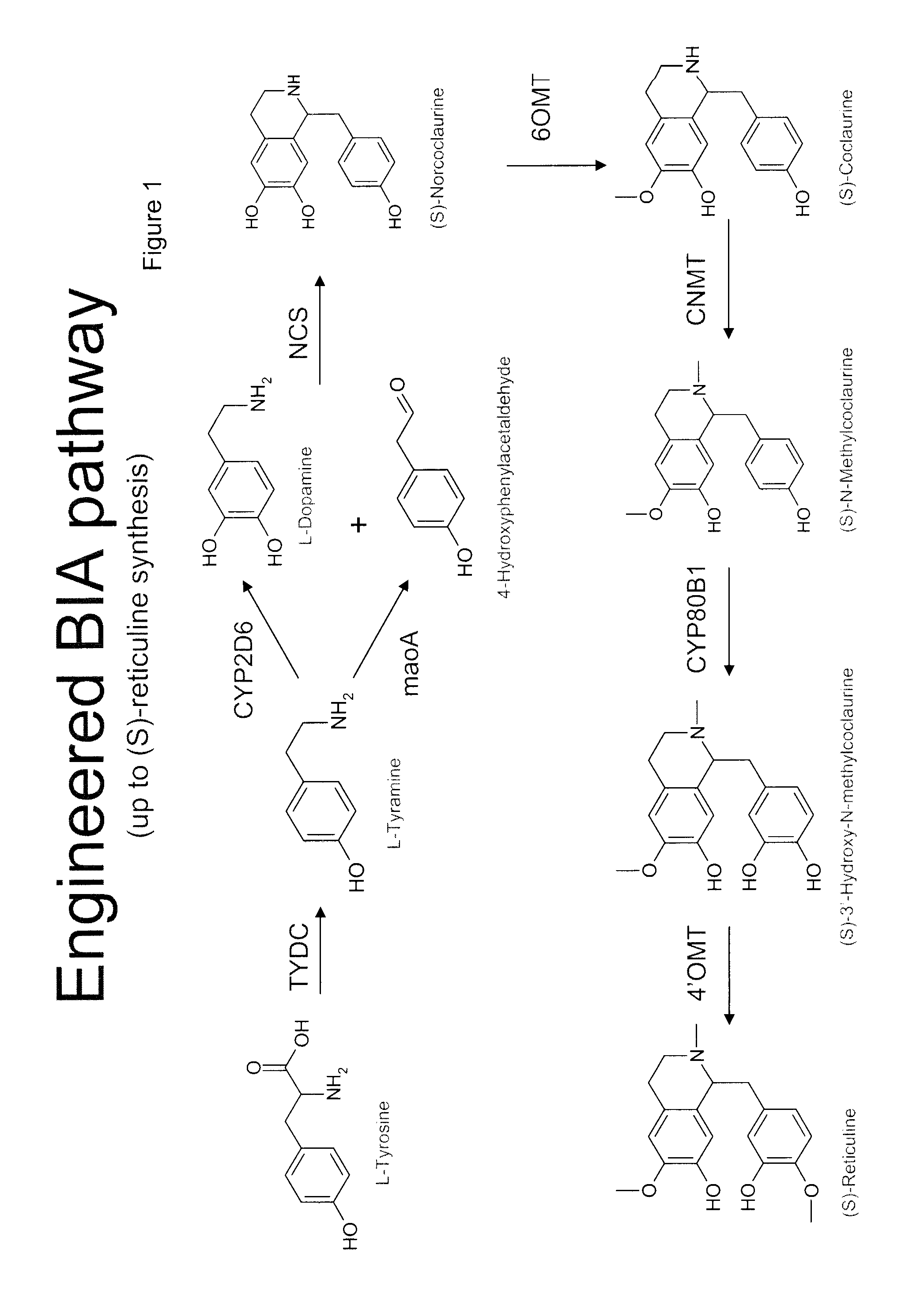
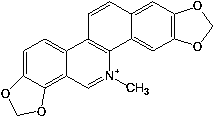
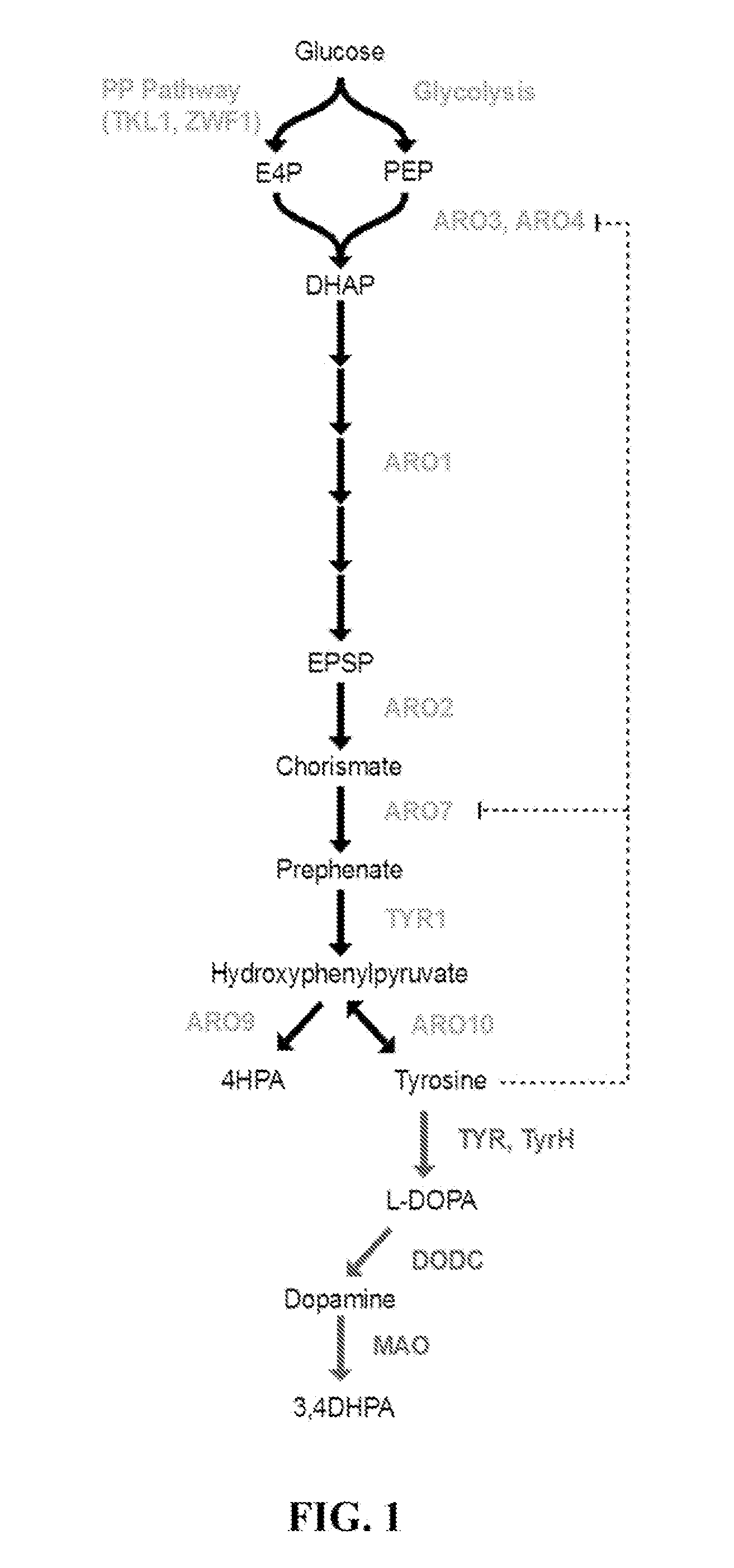
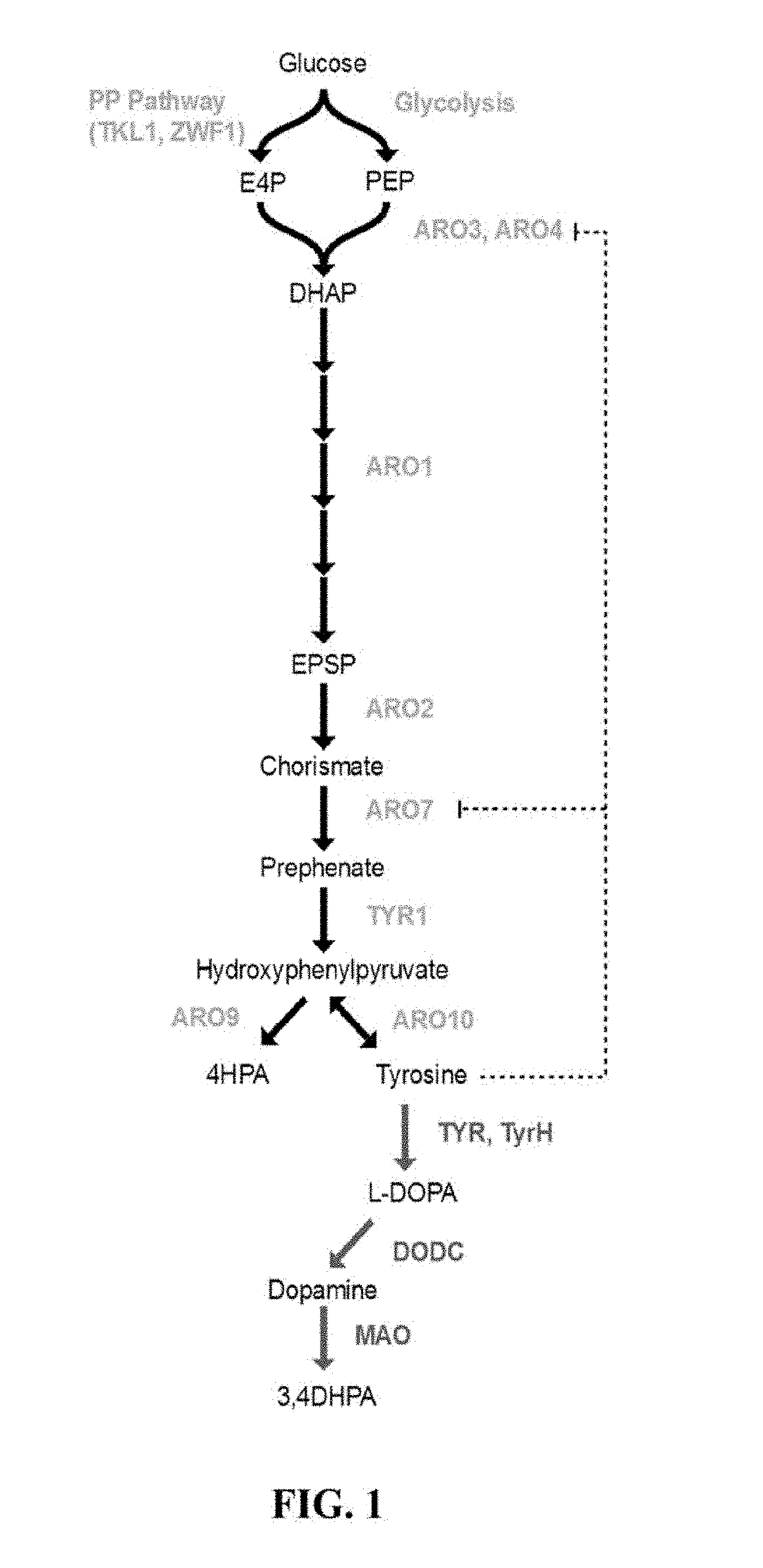
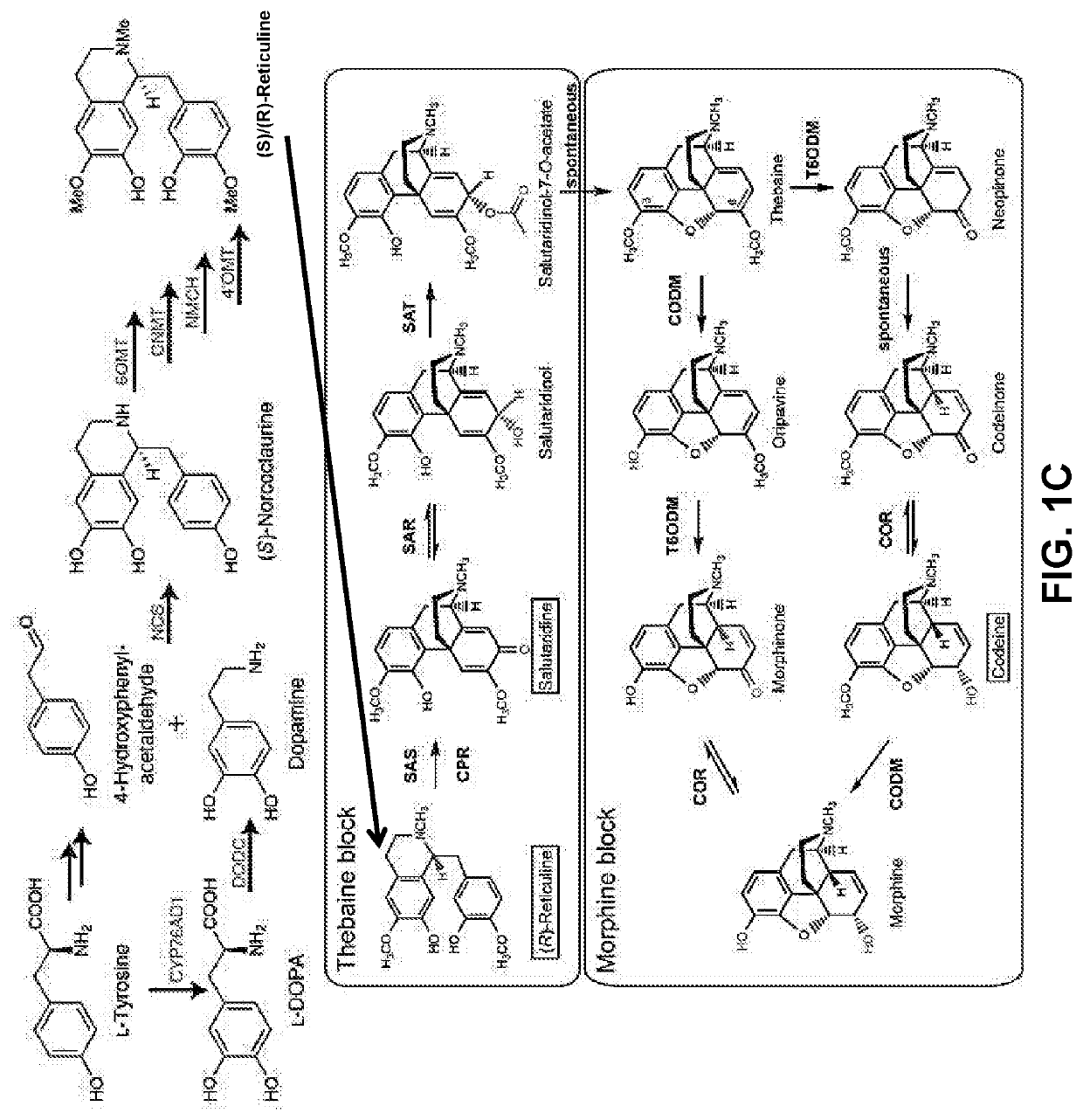
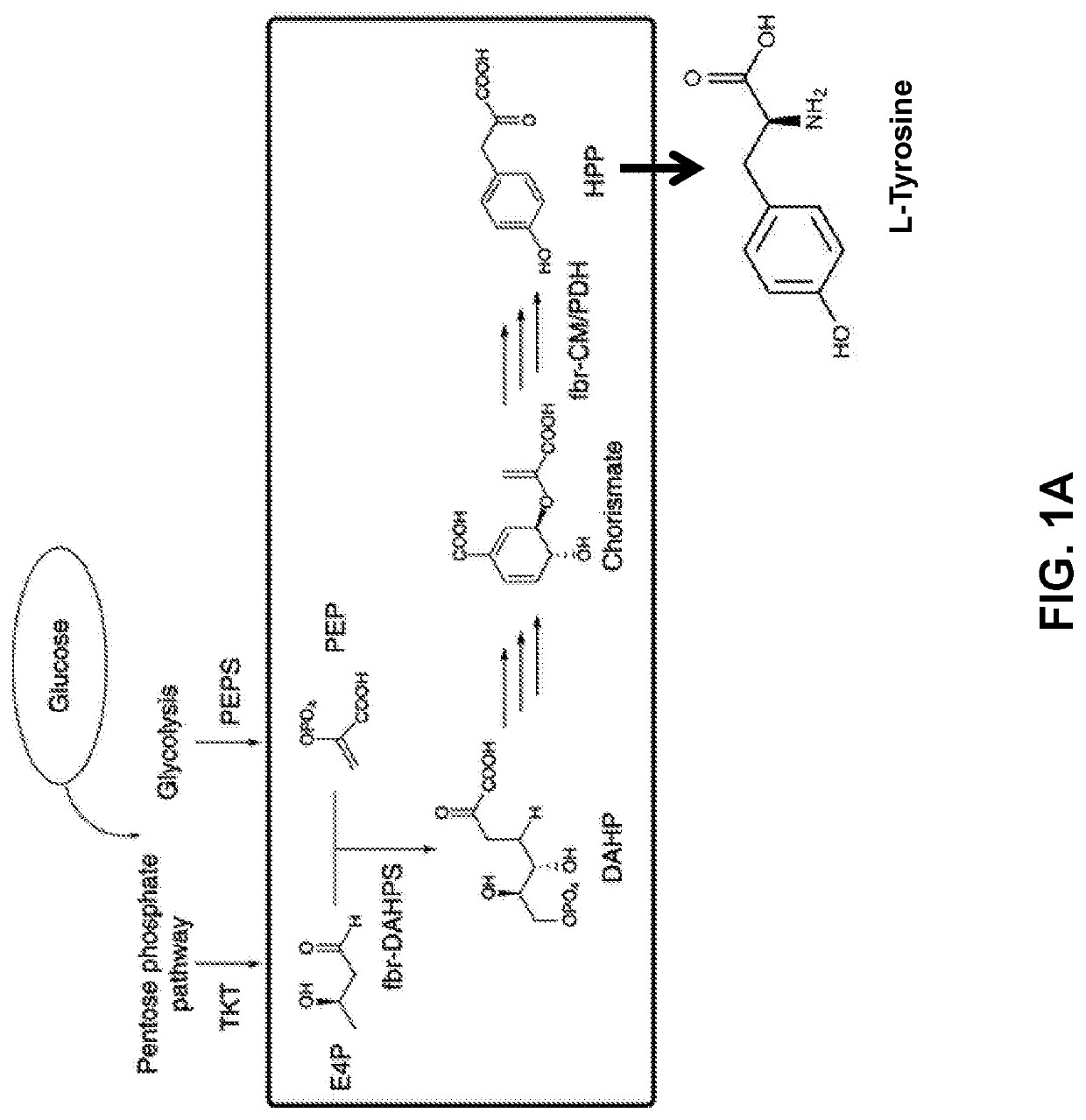
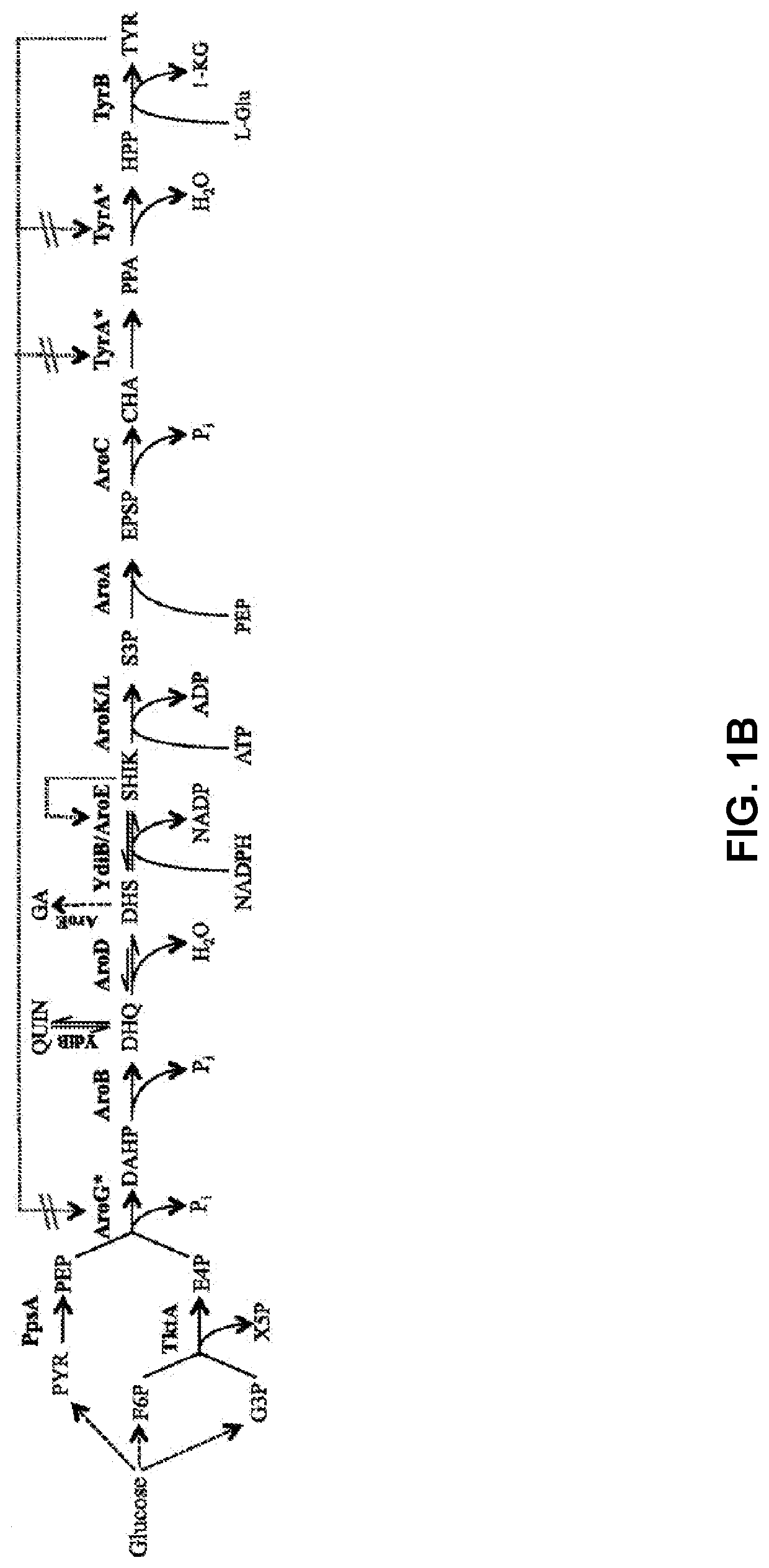
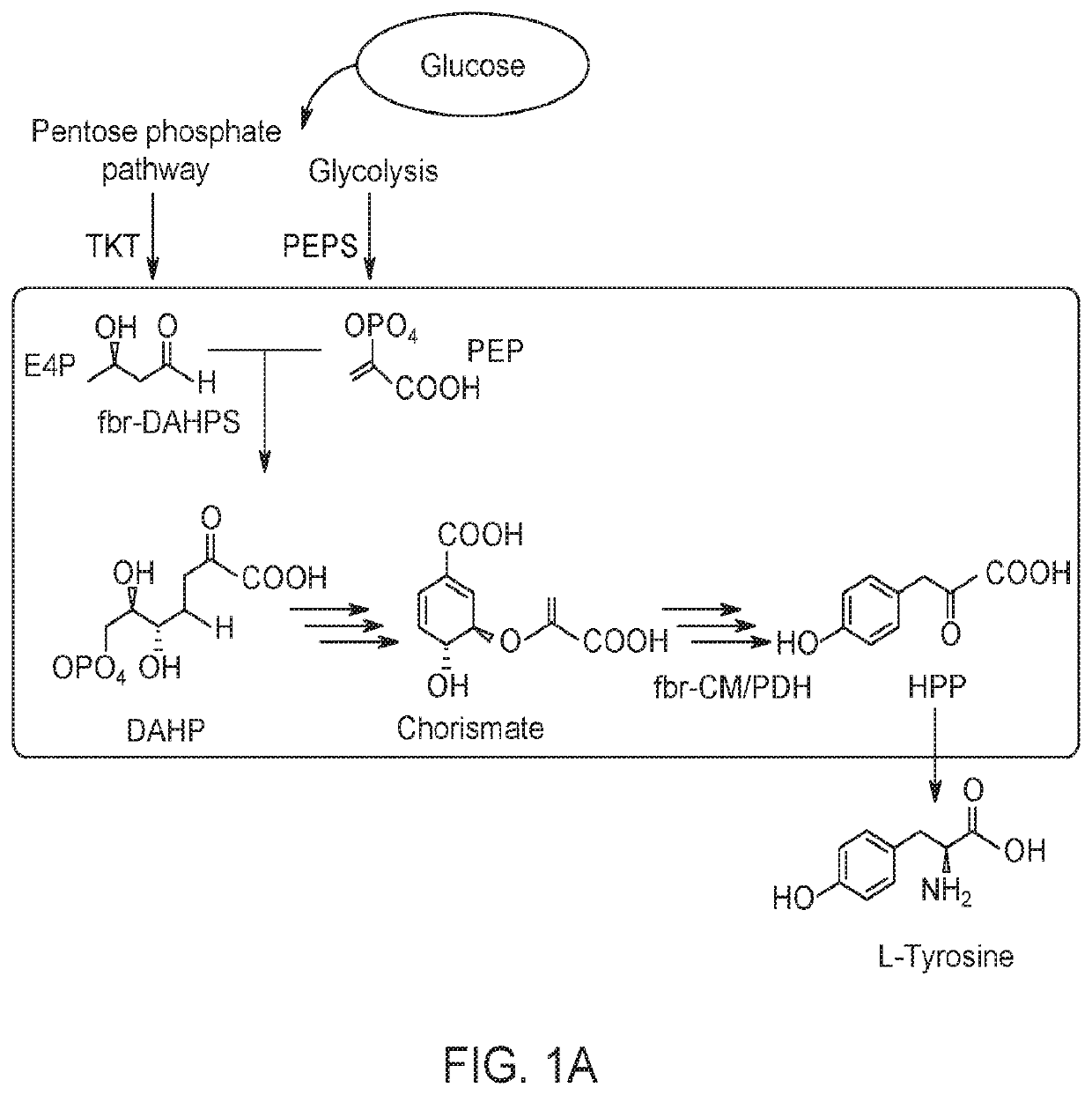
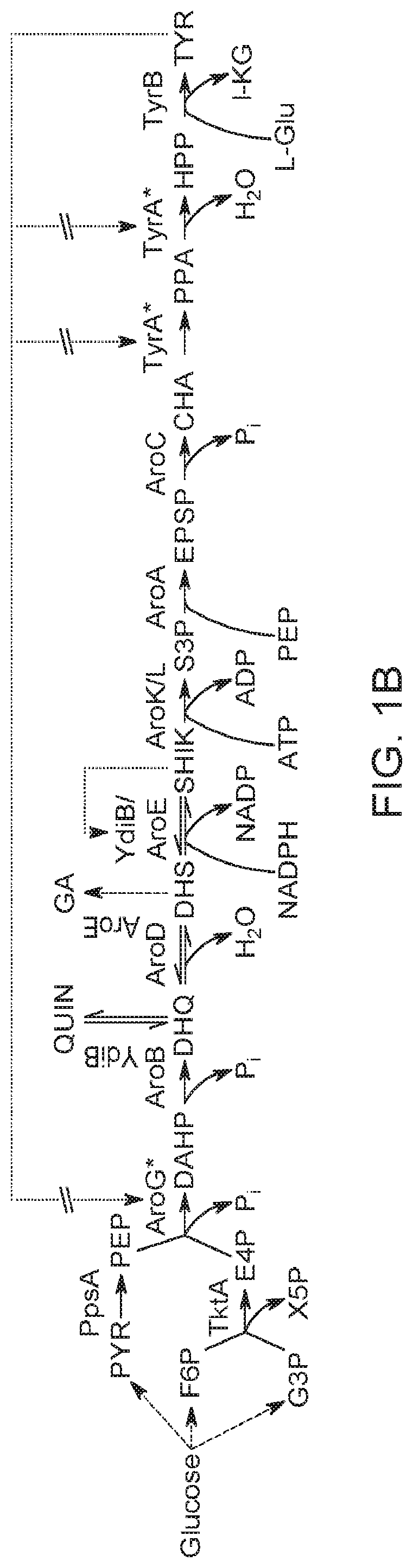
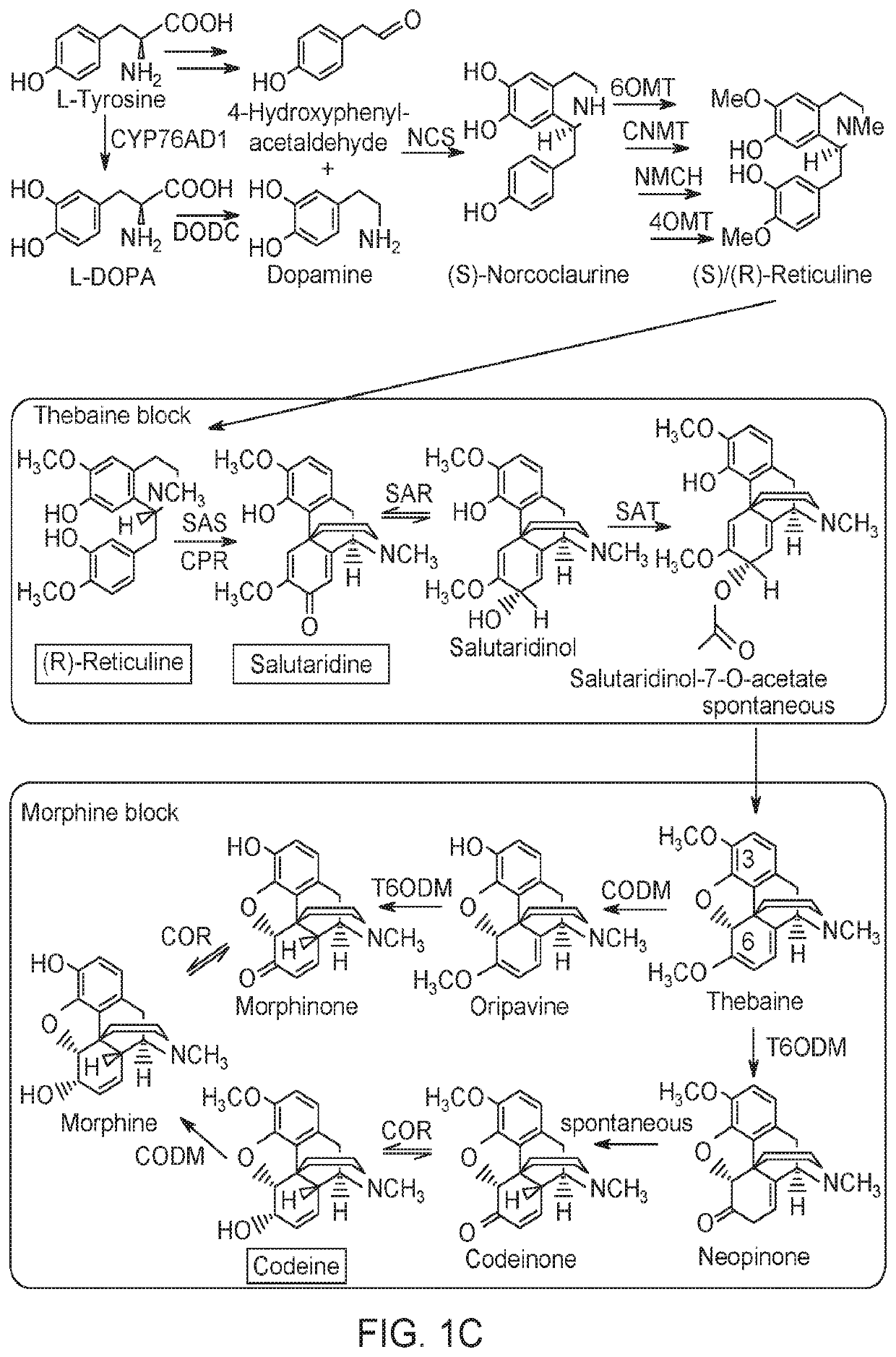
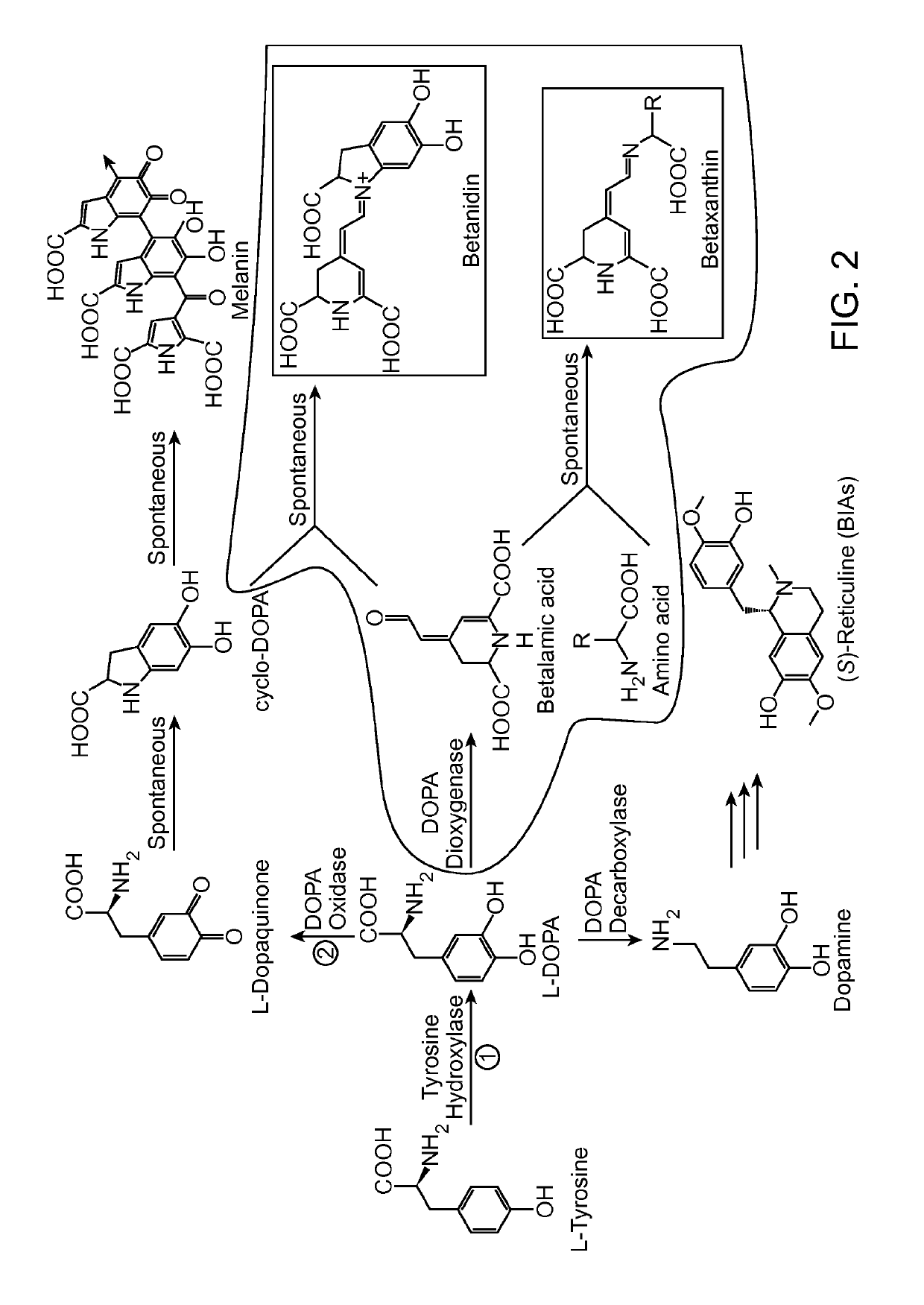
Substitution of the heterocycle isoquinoline at the C1 position by a benzyl group provides 1‑benzylisoquinoline, the most widely examined of the numerous benzylisoquinoline structural isomers. The 1-benzylisoquinoline moiety can be identified within numerous compounds of pharmaceutical interest, such as moxaverine; but most notably it is found within the structures of a wide variety of plant natural products, collectively referred to as benzylisoquinoline alkaloids. This class is exemplified in part by the following compounds: papaverine, noscapine, codeine, morphine, apomorphine, berberine, tubocurarine.
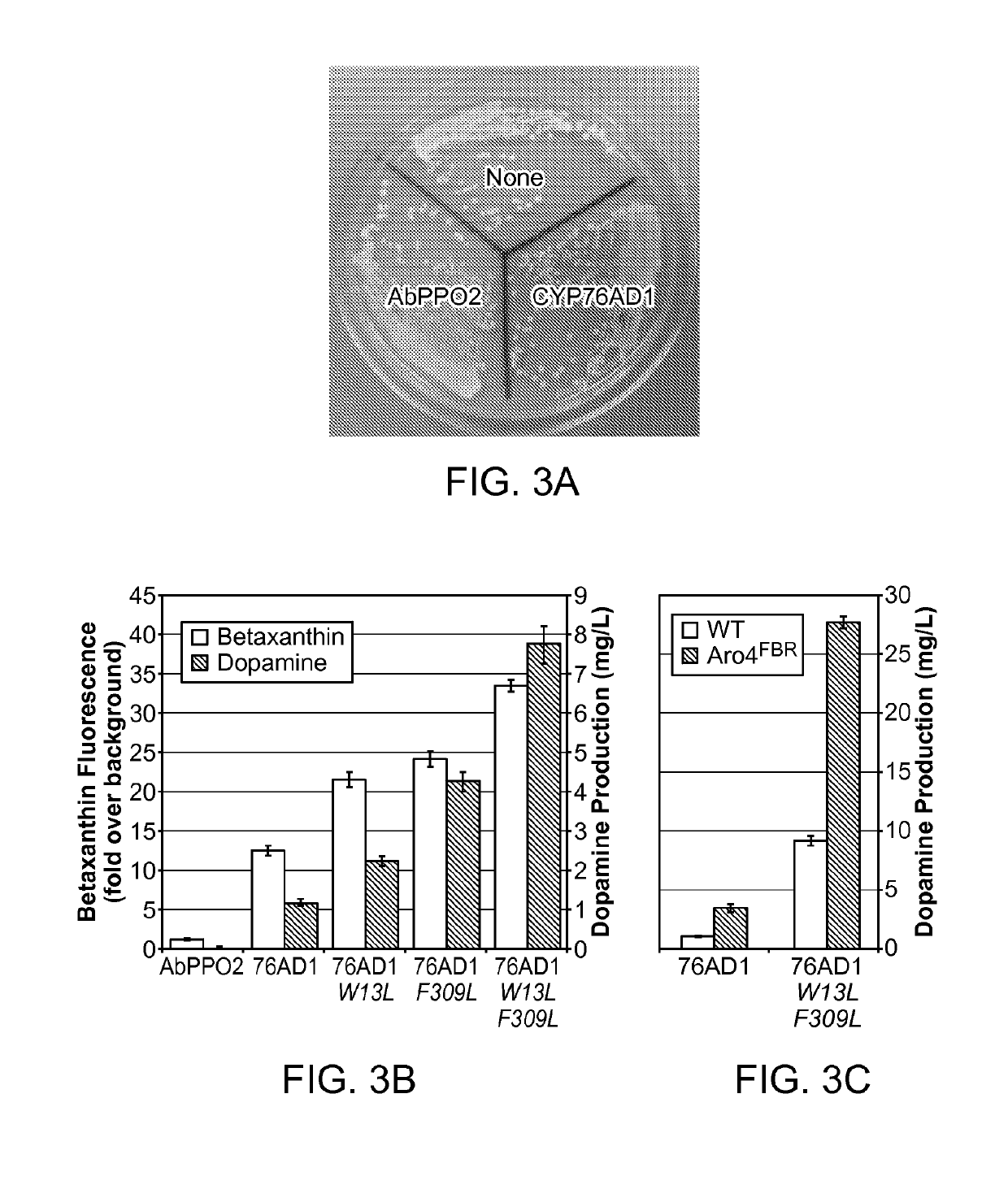
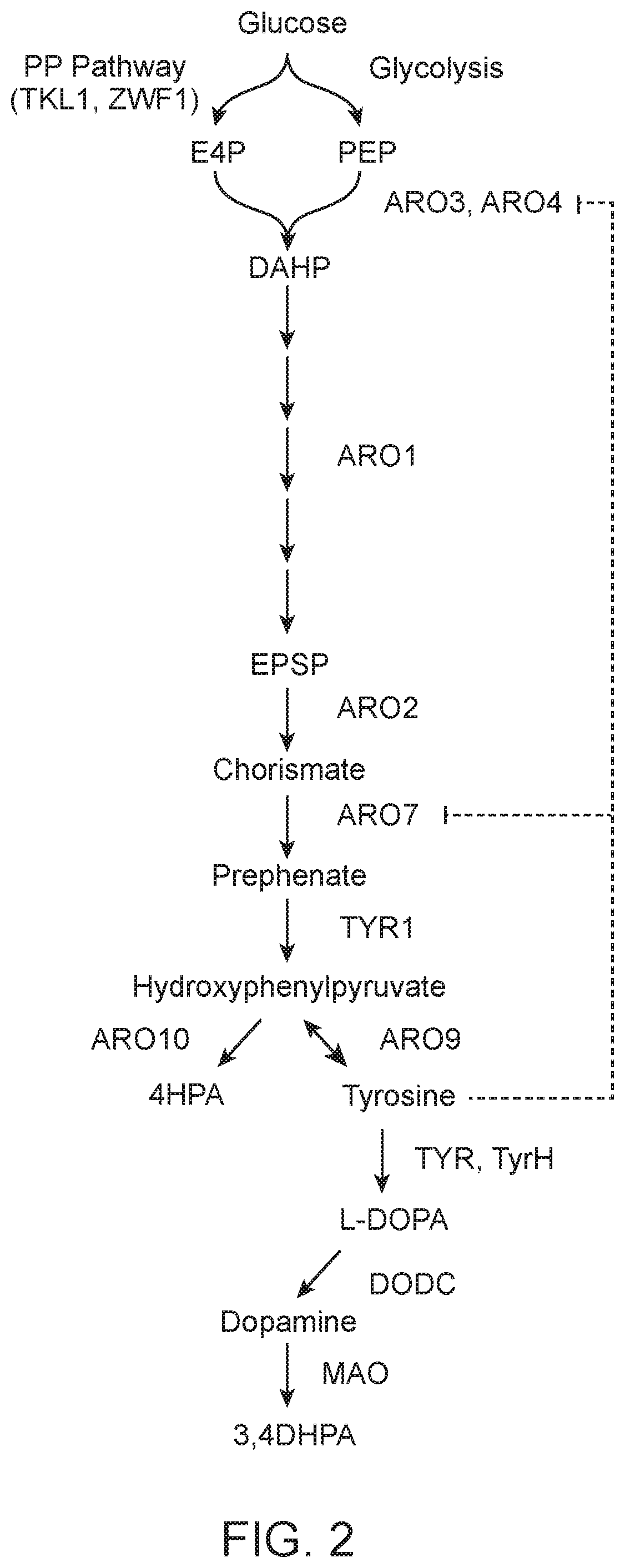
Compositions and methods for producing benzylisoquinoline alkaloids
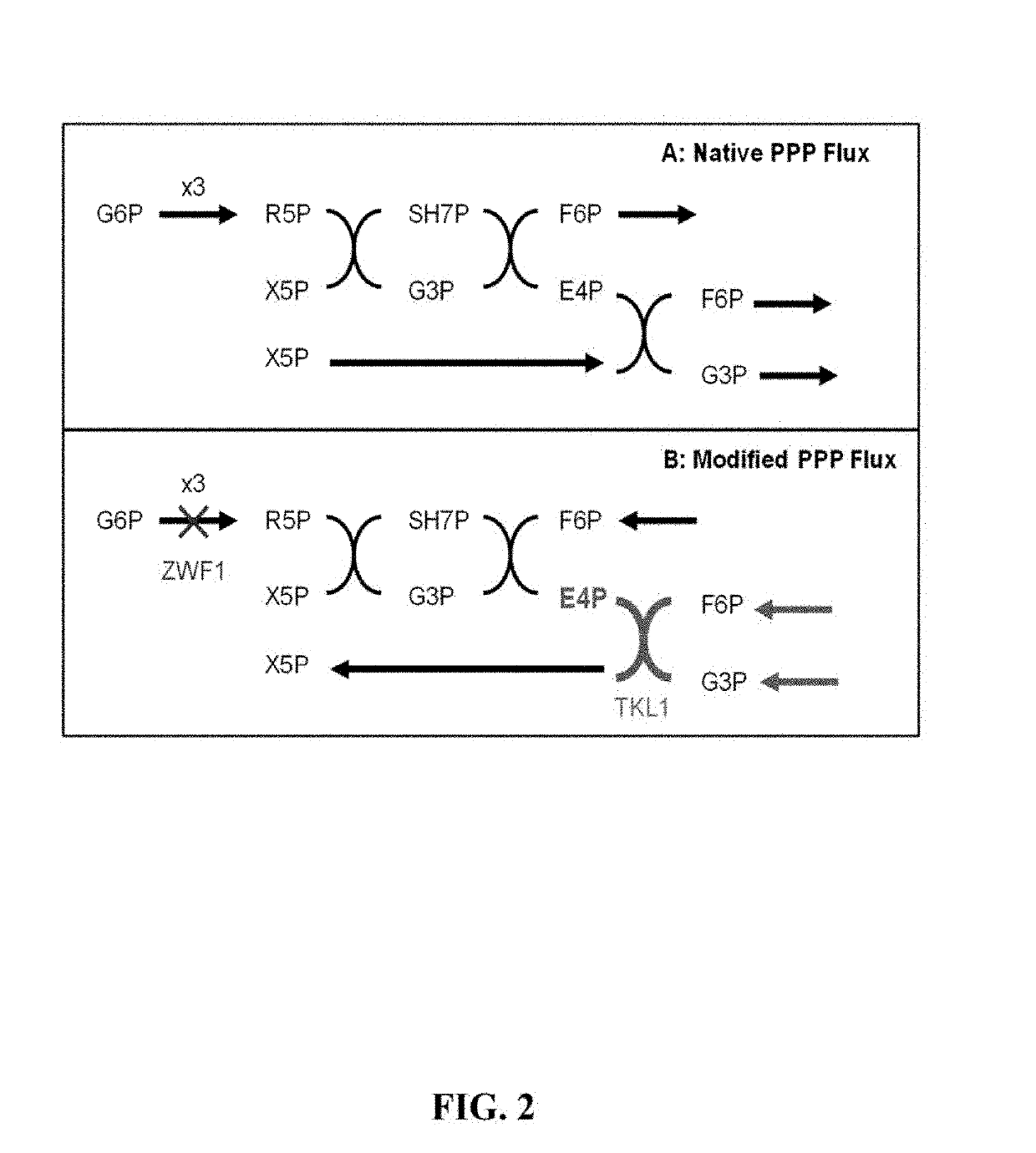
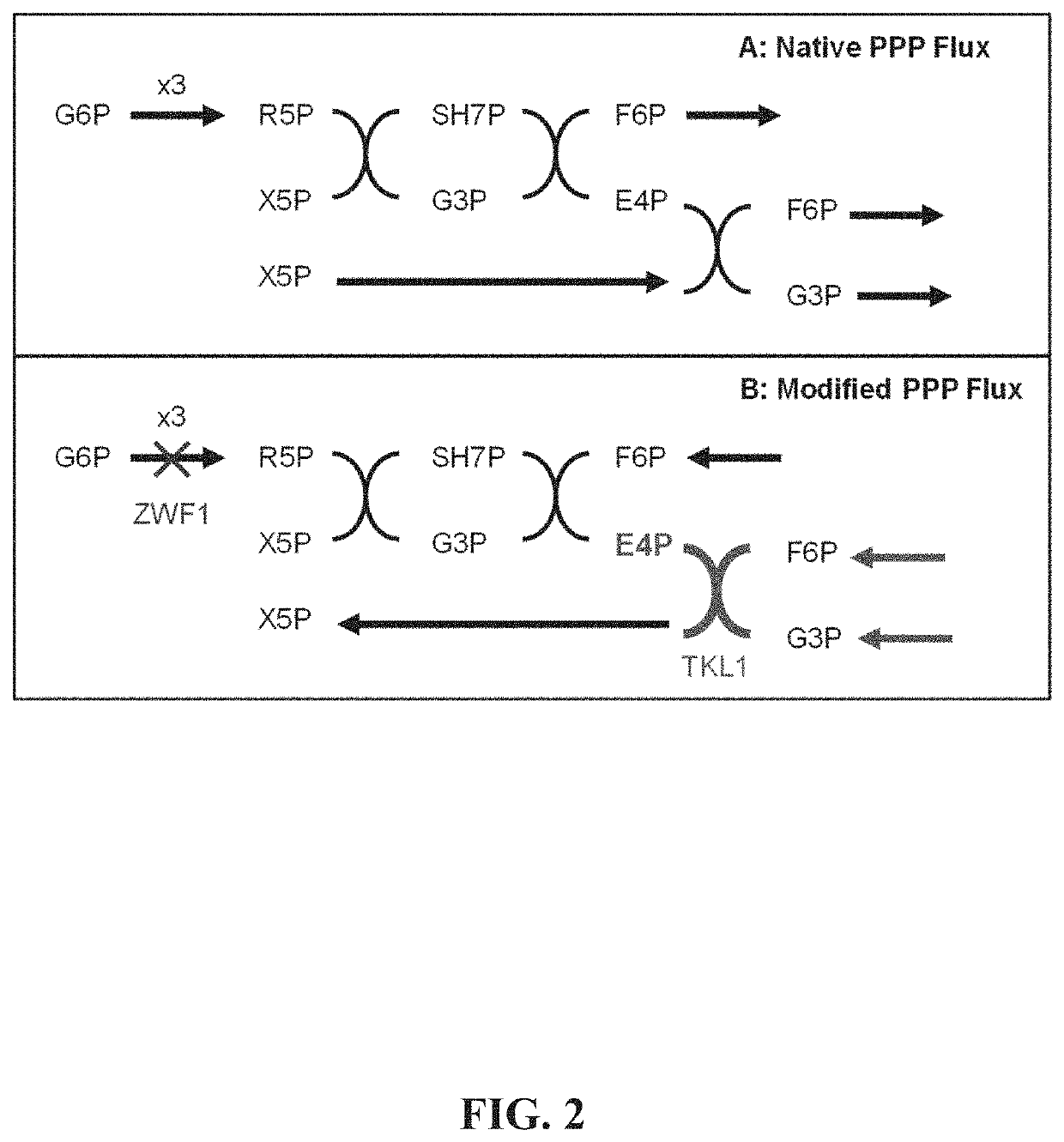
ActiveUS8975063B2Promote expression and activityOptimize growth rateBacteriaSugar derivativesMetabolic pathwayBenzylisoquinoline
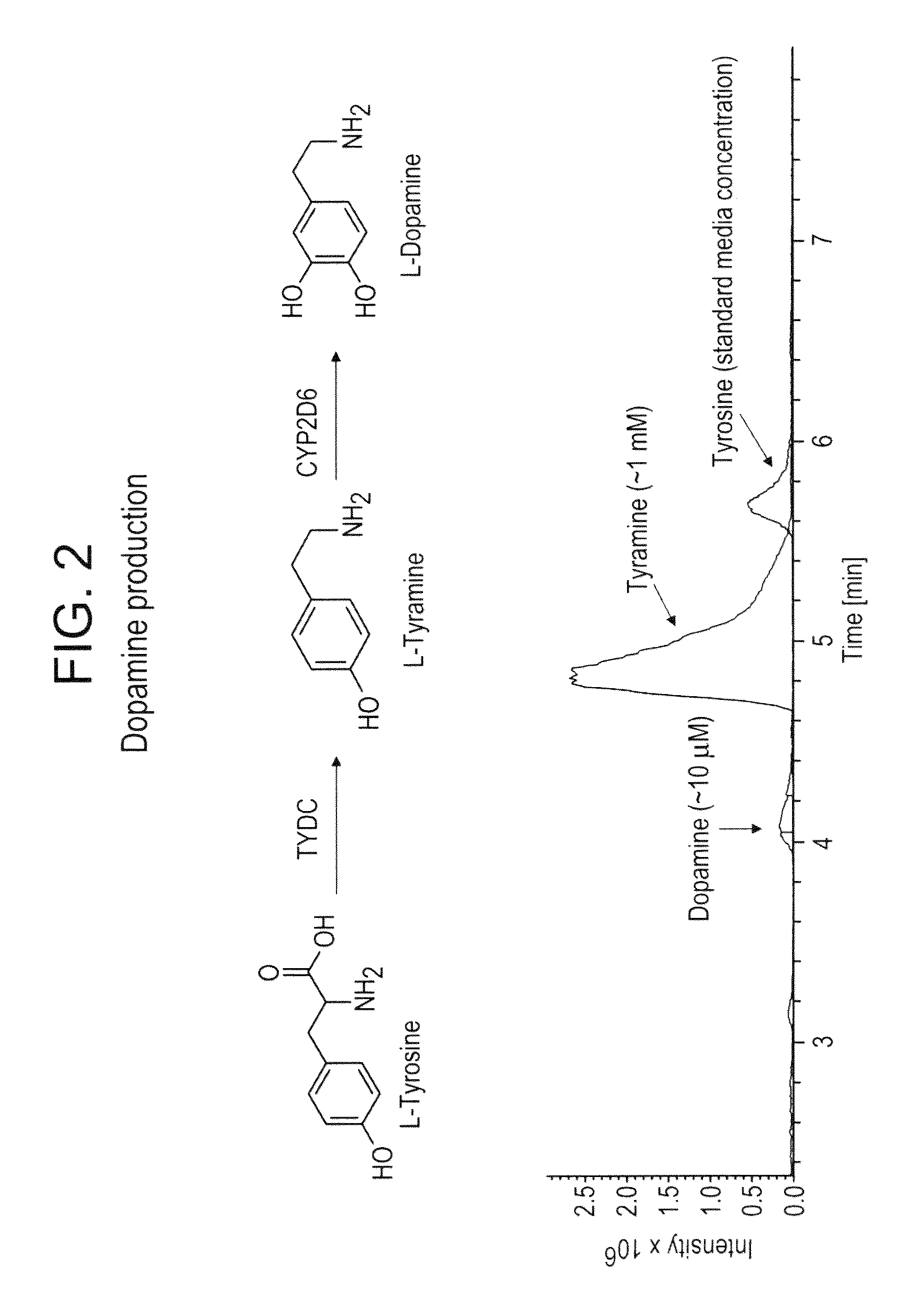
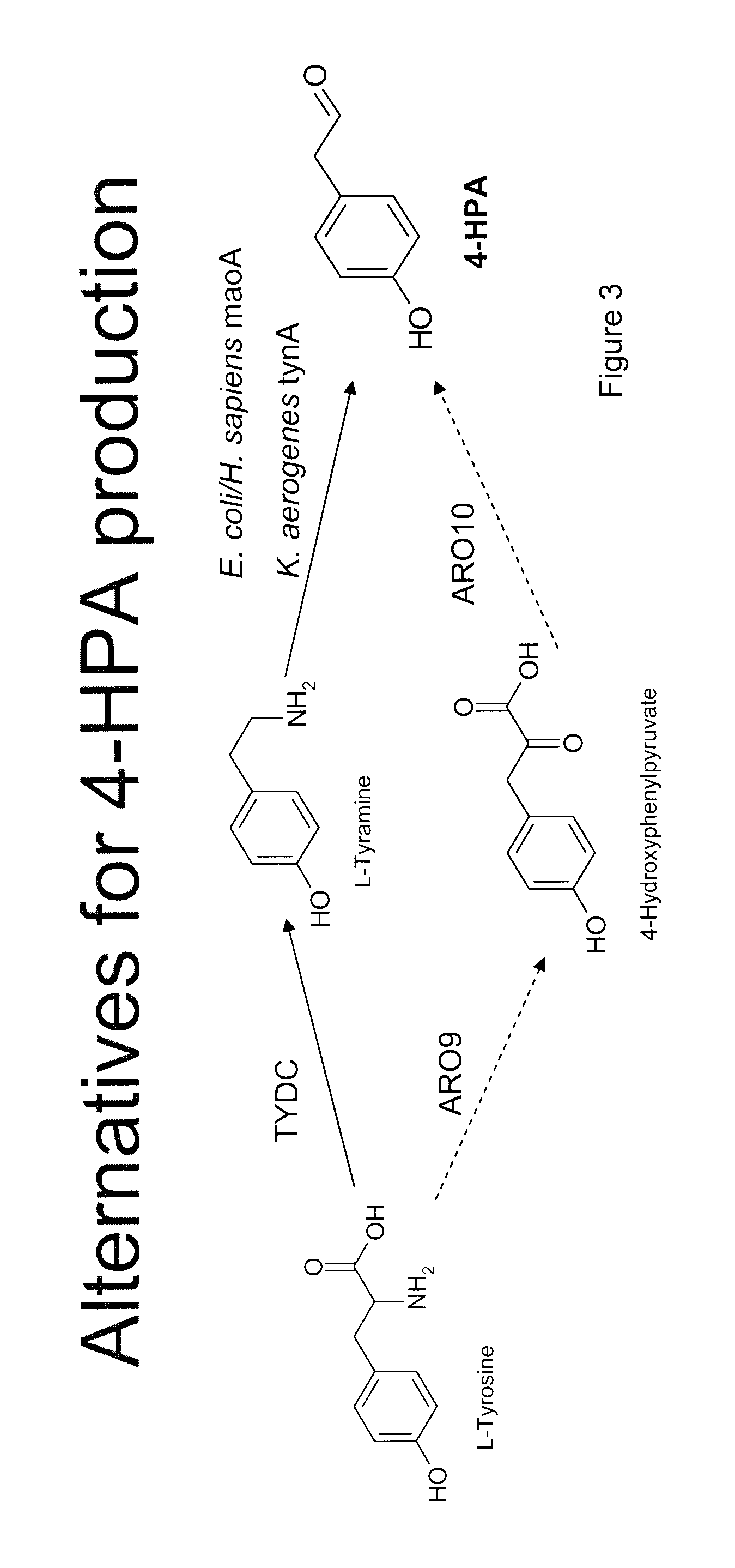
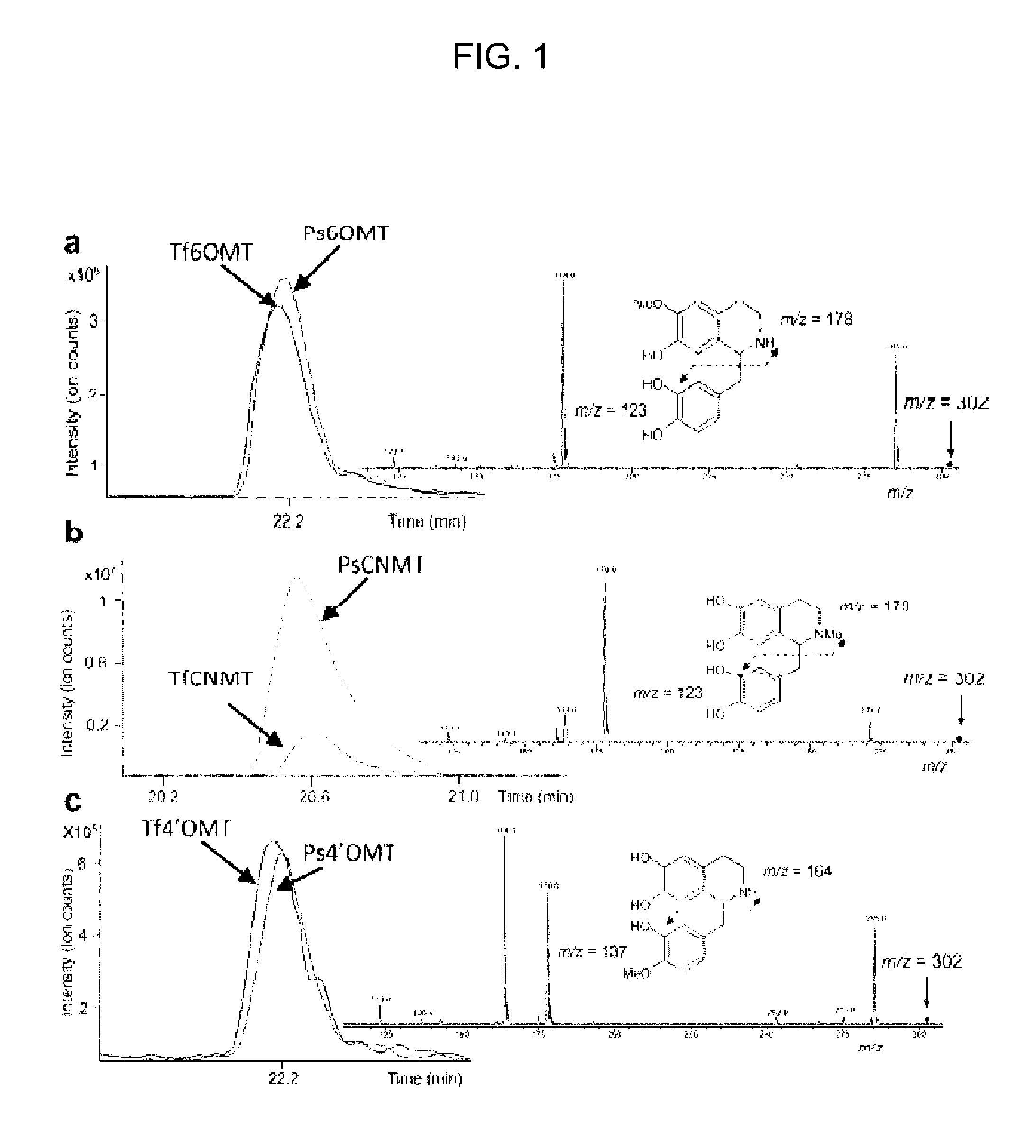
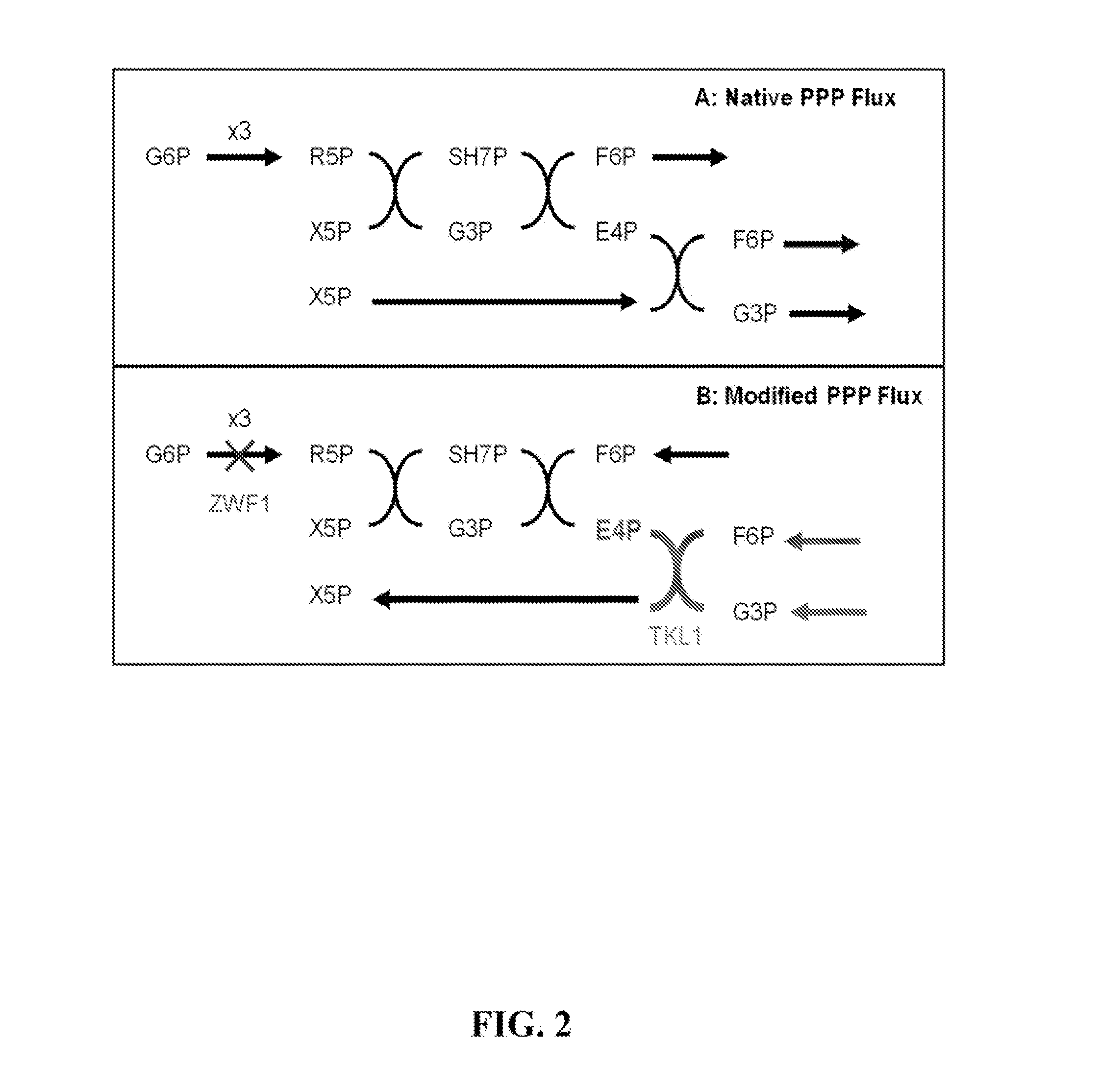
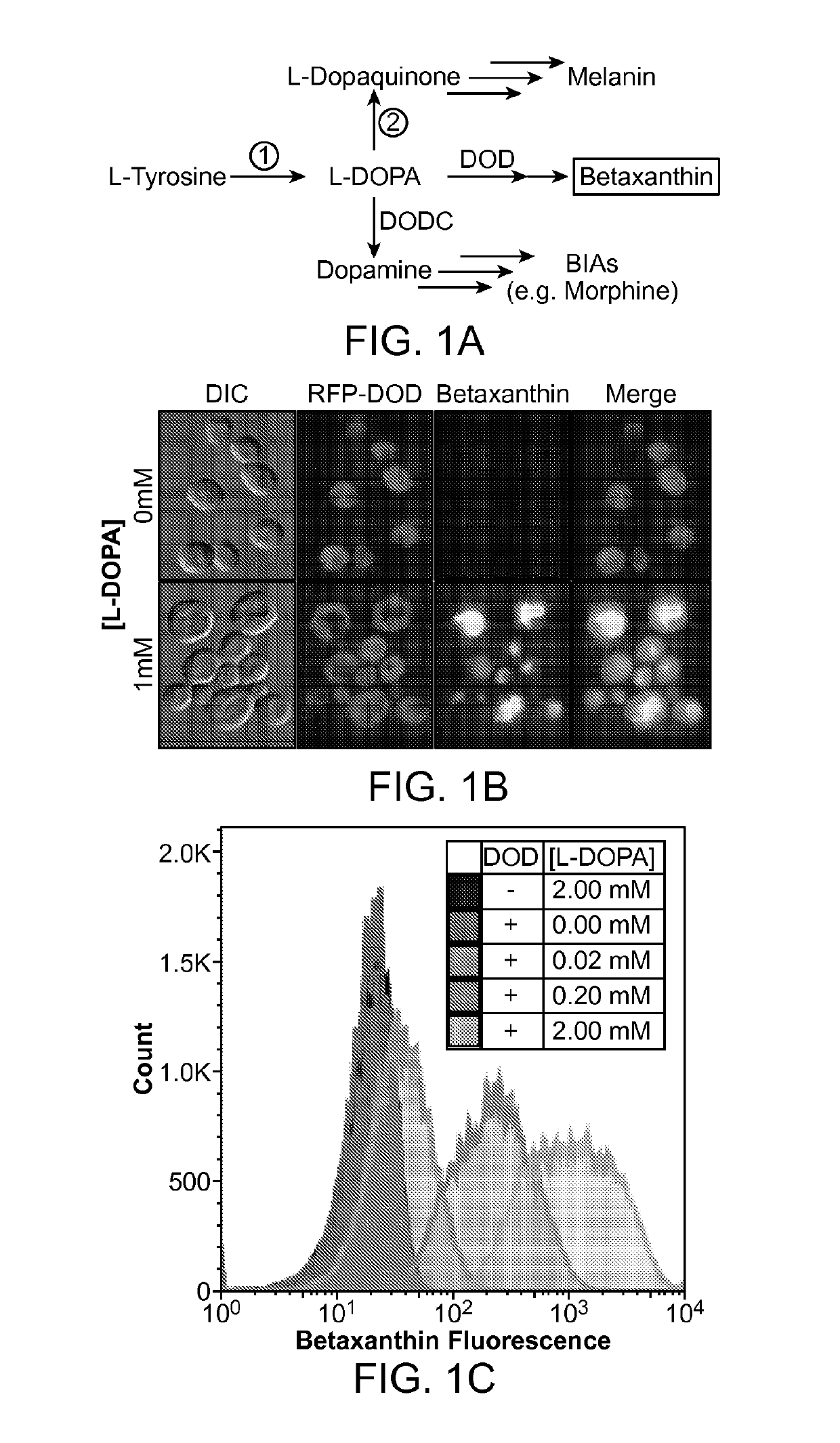
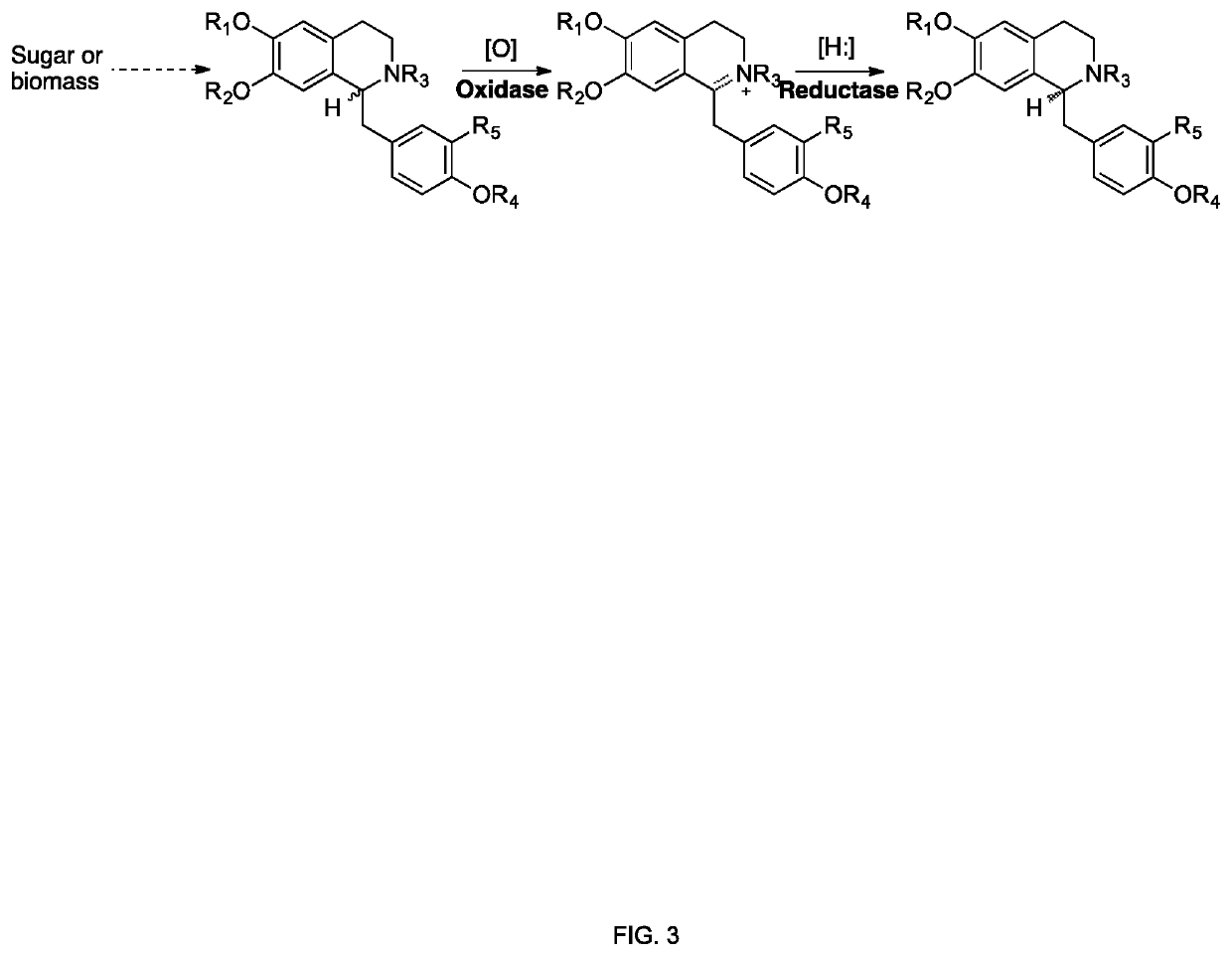
The present invention relates to host cells that produce compounds that are characterized as benzylisoquinolines, as well as select precursors and intermediates thereof. The host cells comprise one, two or more heterologous coding sequences wherein each of the heterologous coding sequences encodes an enzyme involved in the metabolic pathway of a benzylisoquinoline, or its precursors or intermediates from a starting compound. The invention also relates to methods of producing the benzylisoquinoline, as well as select precursors and intermediates thereof by culturing the host cells under culture conditions that promote expression of the enzymes that produce the benzylisoquinoline or precursors or intermediates thereof.
Owner:CALIFORNIA INST OF TECH
Benzylisoquinoline alkaloids (BIA) producing microbes, and methods of making and using the same
Aspects of the invention include host cells that are engineered to produce benzylisoquinoline alkaloids (BIAs). The host cells include heterologous coding sequences for a variety of enzymes involved in synthetic pathways from starting compounds to BIAs of the host cell. Also provided are methods of producing the BIAs of interest by culturing the host cells under culture conditions that promote expression of enzymes encoded by the heterologous coding sequences of the host cells. Aspects of the invention further include compositions, e.g., host cells, starting compounds and kits, etc., that find use in methods of the invention.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Anti-coronavirus macleaya cordata benzylisoquinoline alkaloid and resveratrol composition and application thereof
InactiveCN110960532AEnhanced inhibitory effectHydroxy compound active ingredientsAntiviralsQuinolineChelerythrine
The invention discloses an anti-coronavirus macleaya cordata benzylisoquinoline alkaloid and resveratrol composition and application thereof. The macleaya cordata benzylisoquinoline alkaloid and resveratrol composition is prepared from five compositions including sanguinarine, chelerythrine, protopine, alpha-allocryptoxine and trans-resveratrol. The macleaya cordata benzylisoquinoline alkaloid andresveratrol composition has unique binding activity on a coronavirus related protein target, has a remarkable anti-coronavirus effect, and is expected to become a raw material of a drug for treatingpneumonia caused by human infection of Covid-19 coronavirus especially when concerning to the inhibition effect on Covid-19 coronavirus. The composition can be used for preparing medicines or other products for resisting diseases caused by coronavirus infection.
Owner:金晓飞
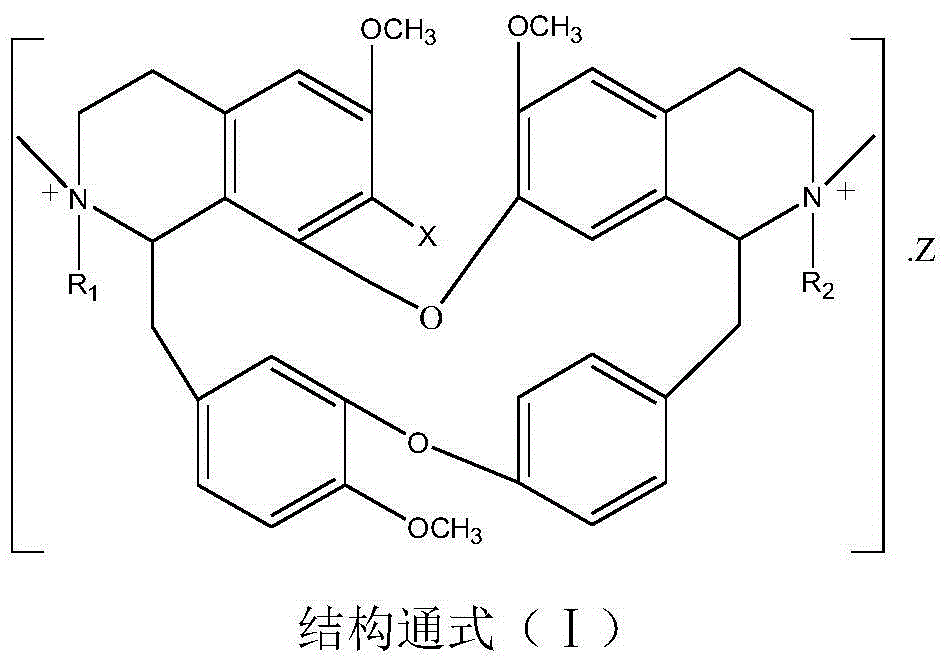
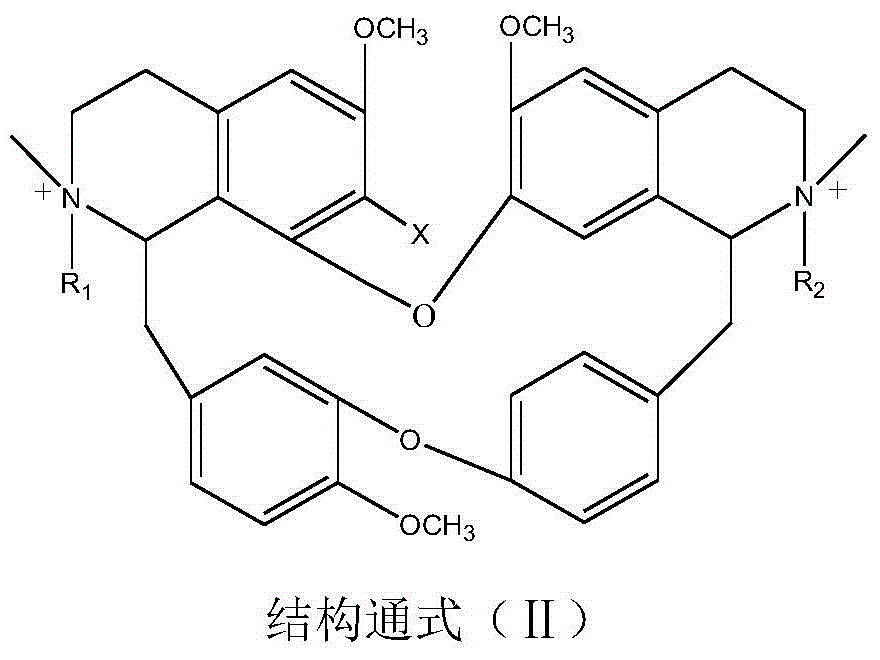
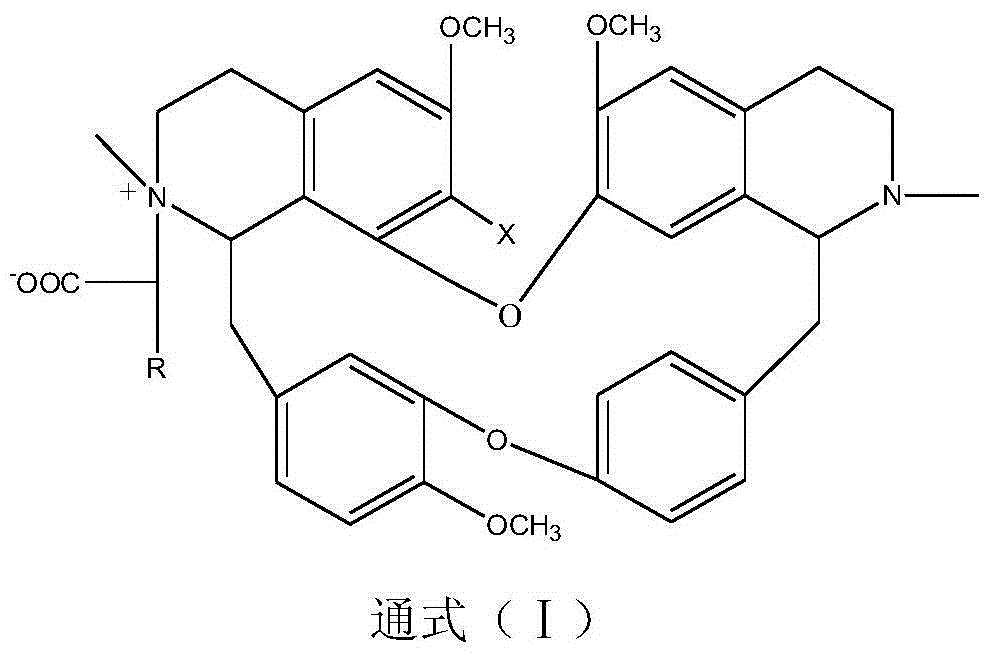
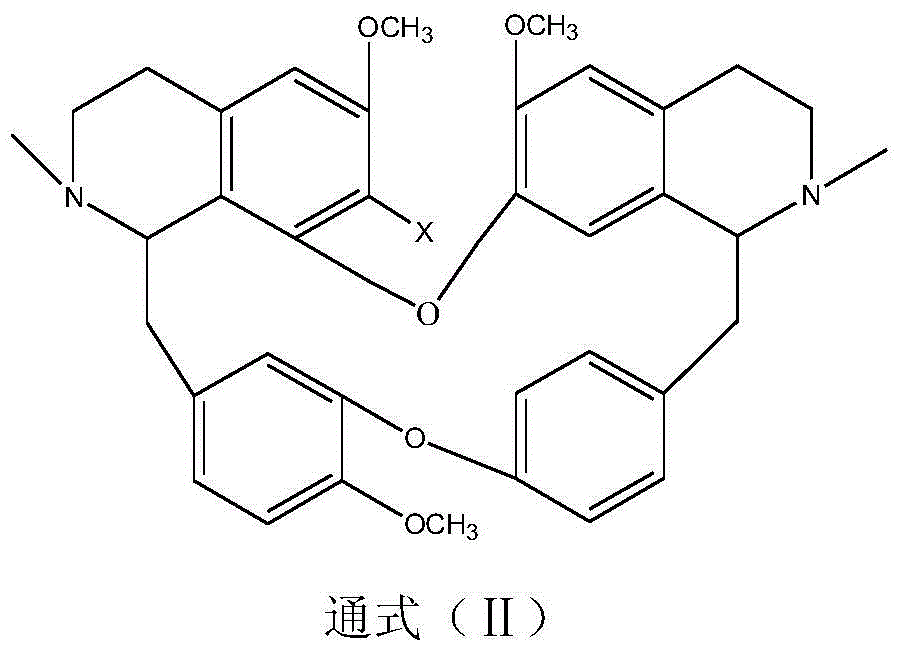
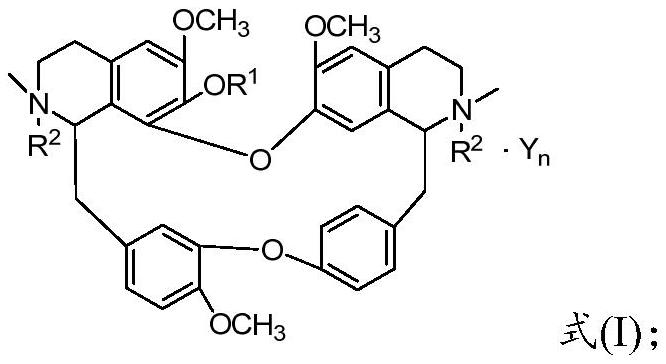
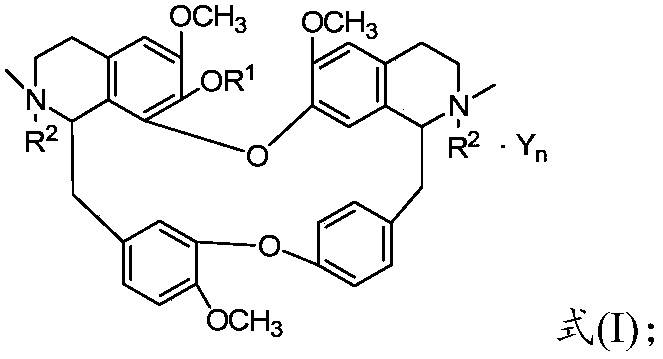
Benzylisoquinoline derivative- or bisbenzylisoquinoline derivative-containing psychotropic agent, analgesic and/or antiphlogistic, and health food
InactiveUS20070027181A1Preventing and alleviating painInflammation can be prevented and alleviatedBiocideNervous disorderPsychotropic medicationAdditive ingredient
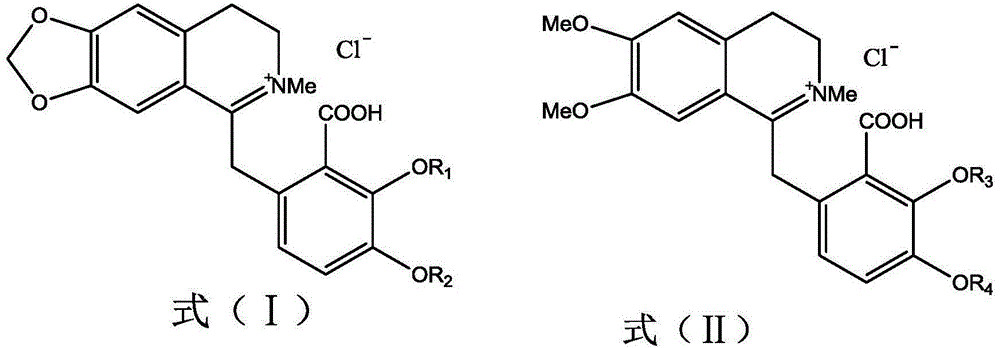
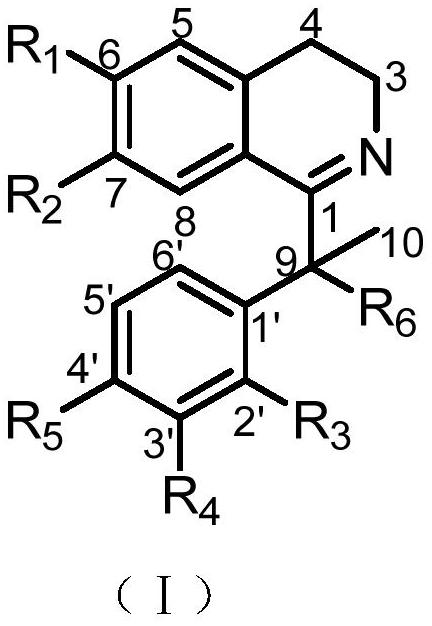
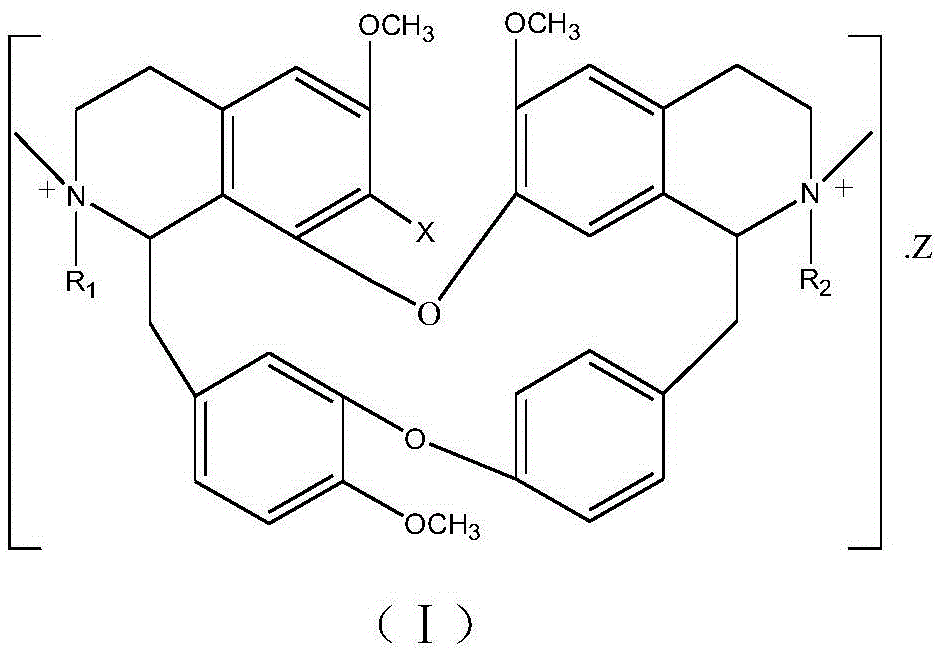
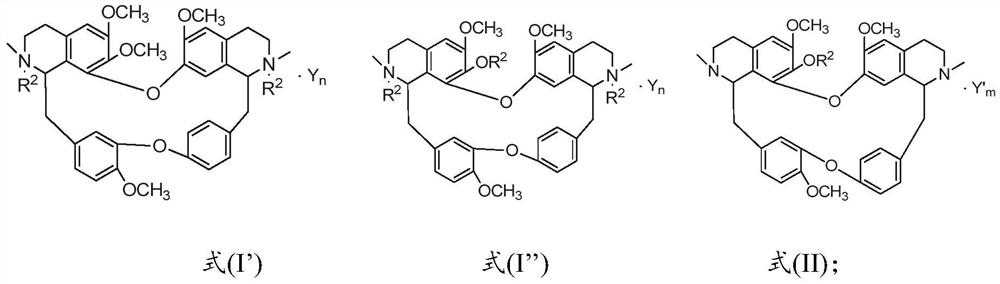
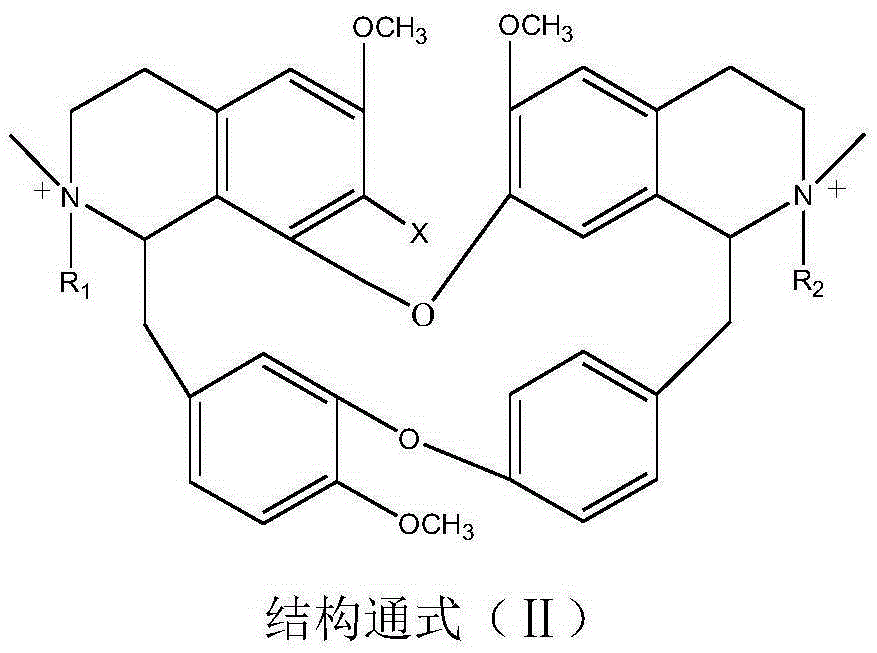
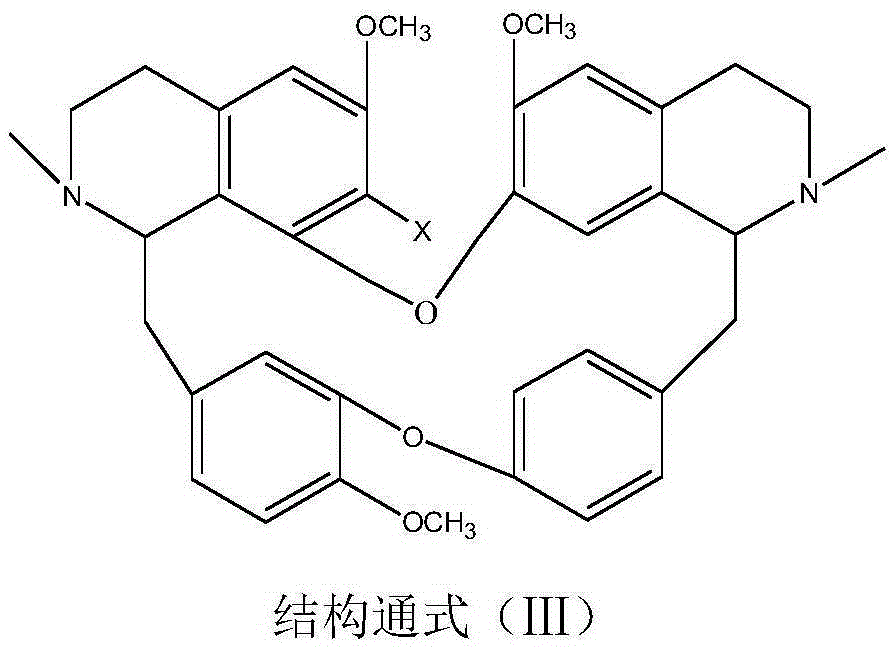
A psychotropic agent, analgesic and / antiinflammatory agent, or health food which is safe even when taken for an extended period of term, comprising as an effective ingredient a benzylisoquinoline derivative represented by general formula (I): or a bisbenzylisoquinoline derivative represented by general formula (II): or a pharmaceutically acceptable salt thereof.
Owner:EDUCATION CENT OF TRADITIONAL CHINESE MEDICINE +1
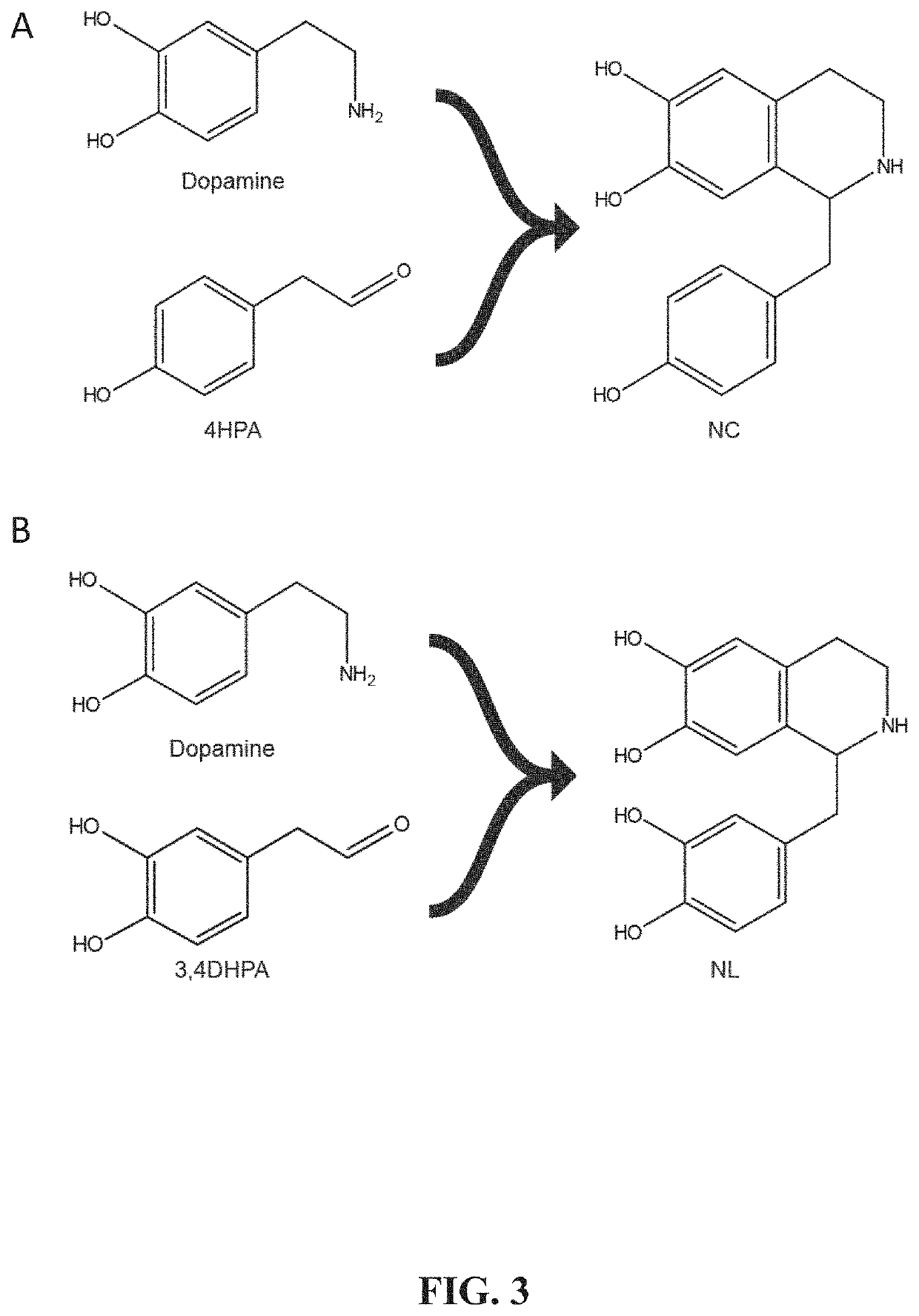
Benzylisoquinoline alkaloid (BIA) precursor producing microbes, and methods of making and using the same
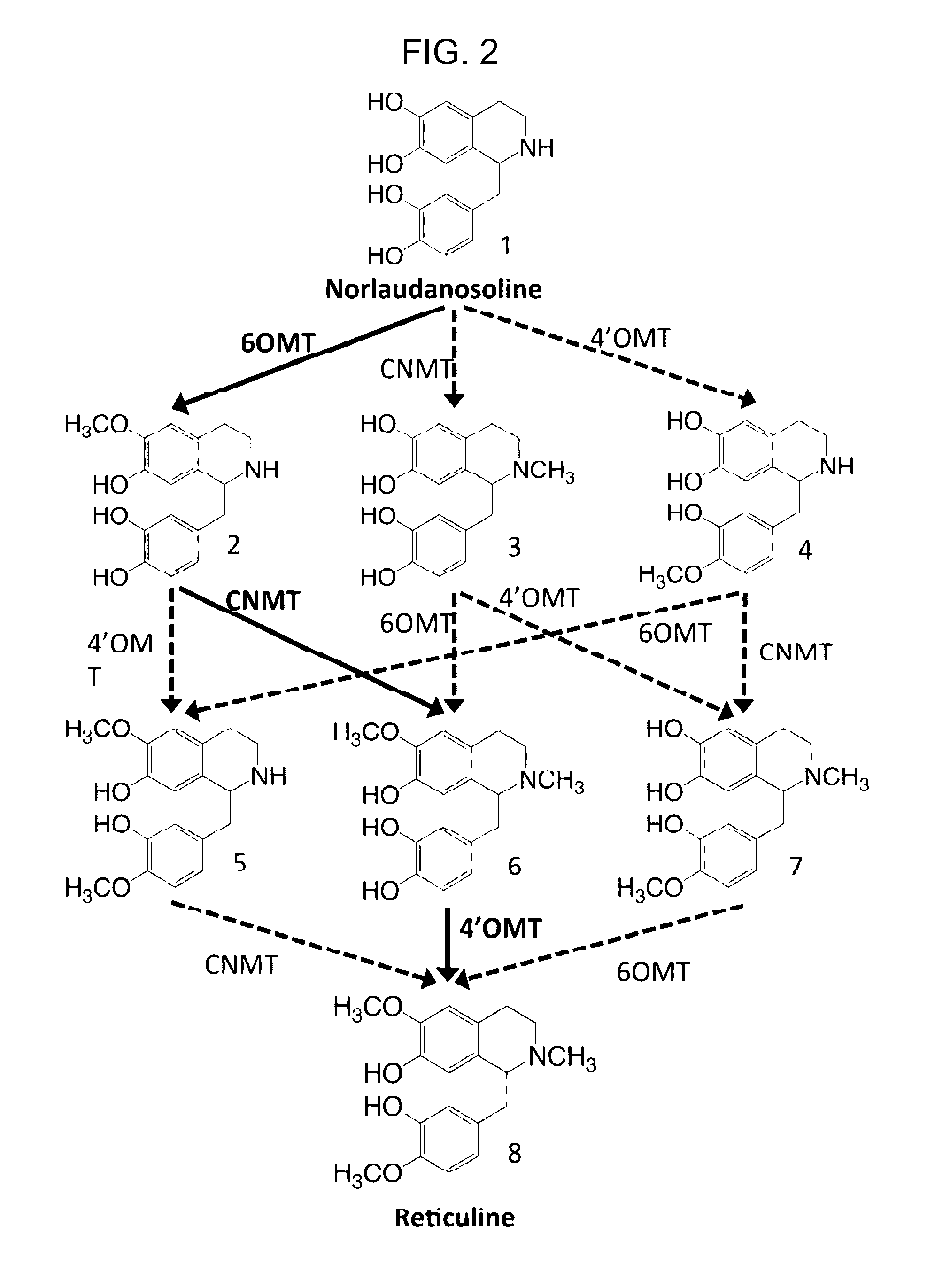
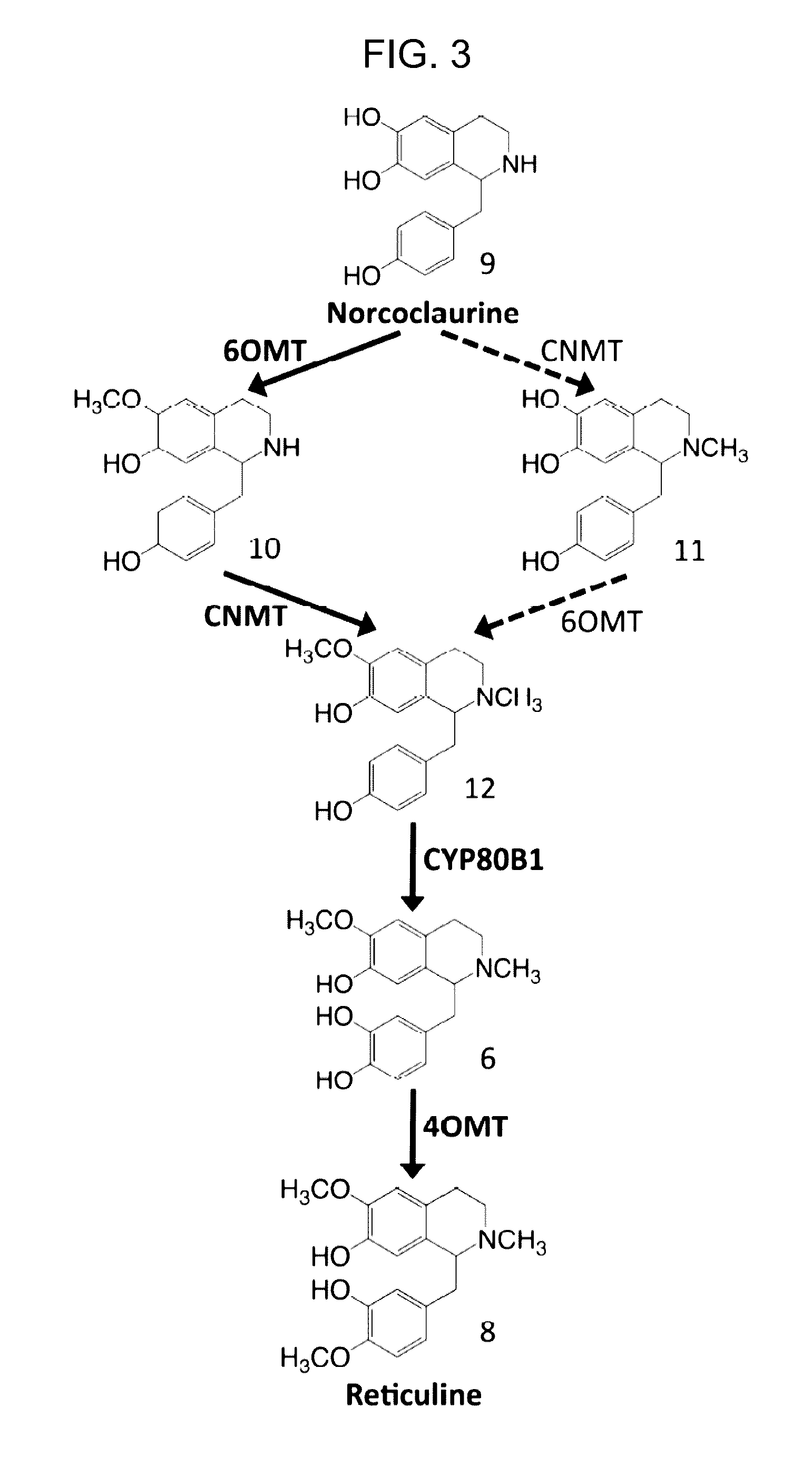
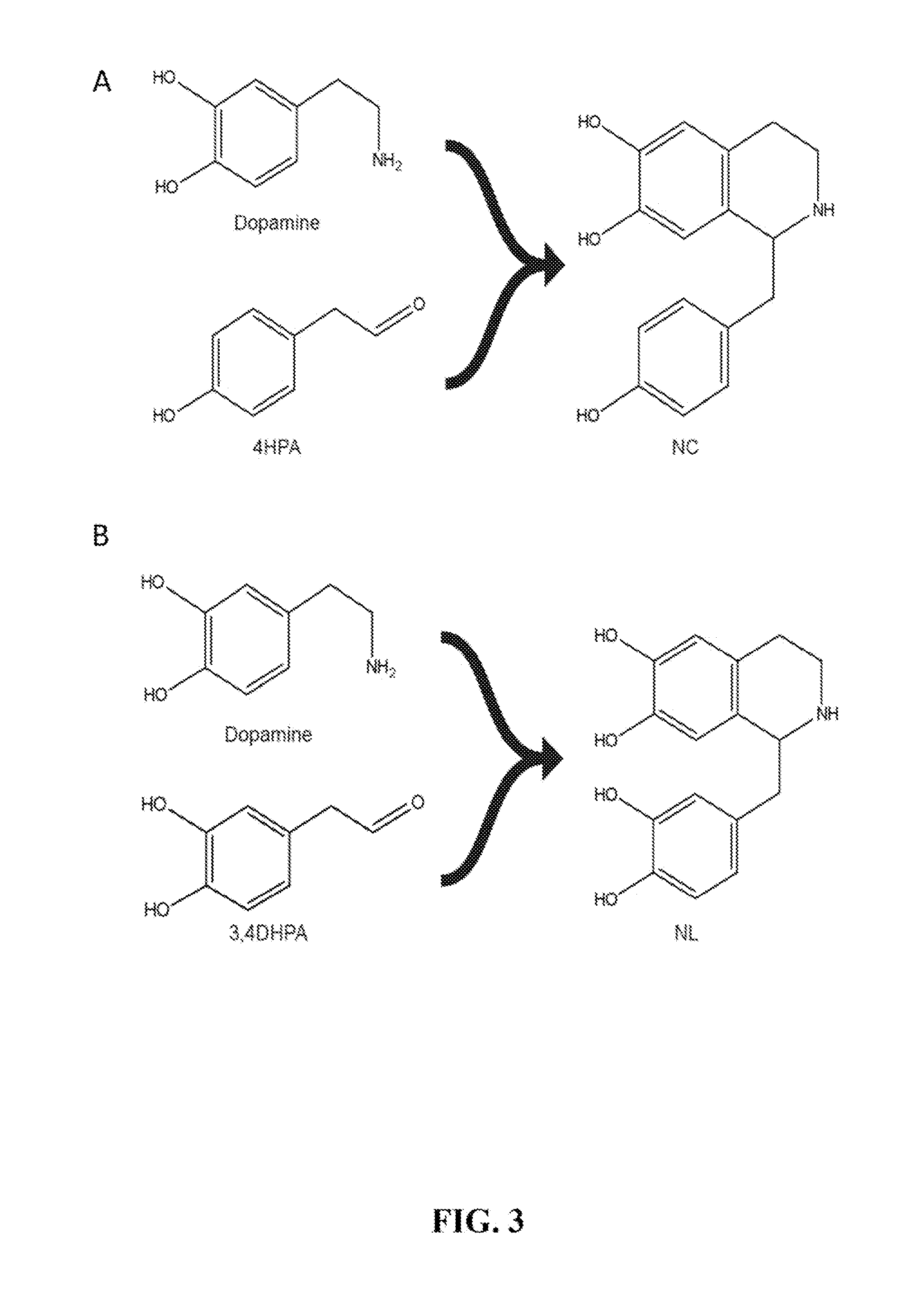
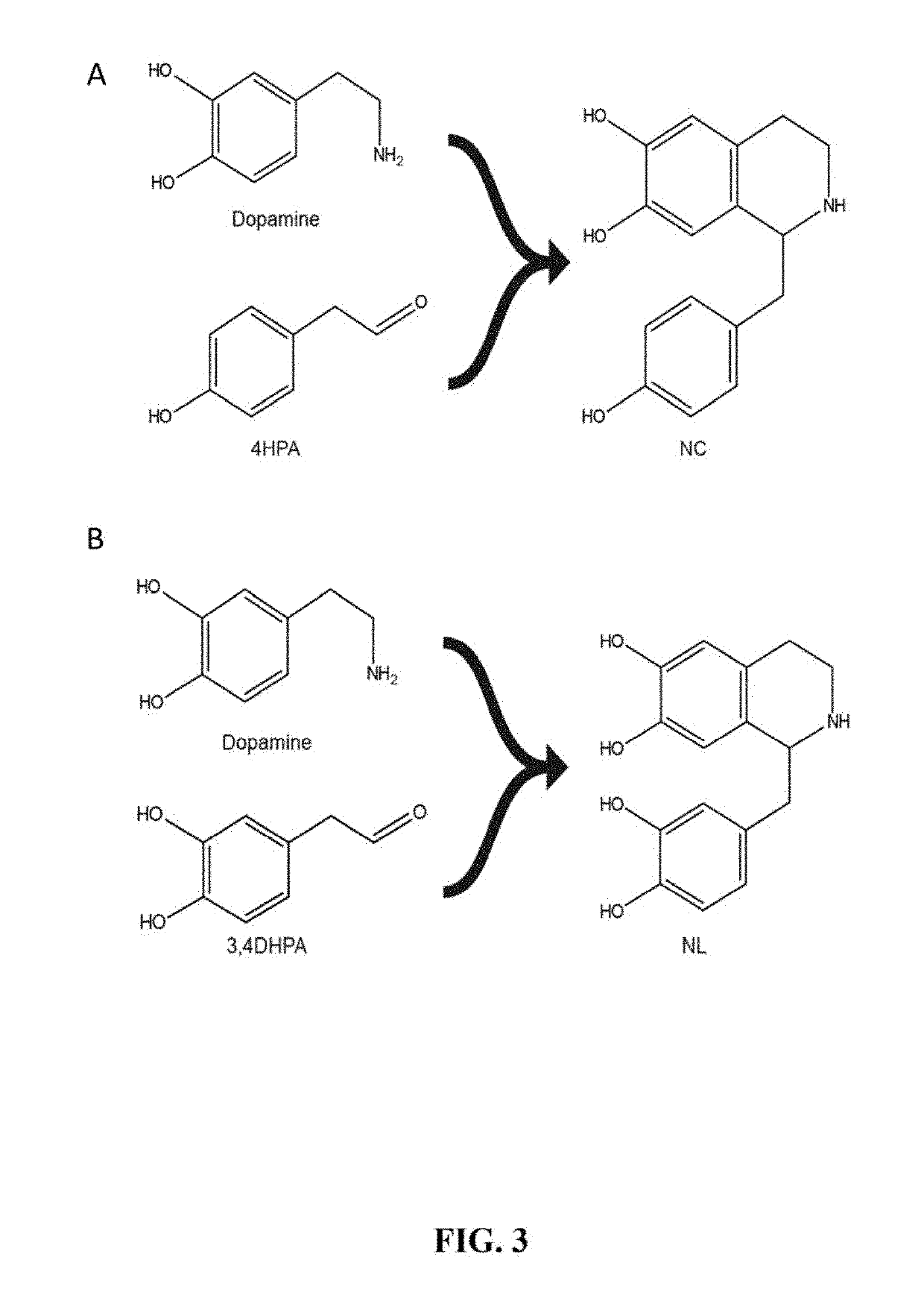
Host cells that are engineered to produce benzylisoquinoline alkaloid (BIAs) precursors, such as norcoclaurine (NC) and norlaudanosoline (NL), are provided. The host cells may have one or more engineered modifications selected from: a feedback inhibition alleviating mutation in a enzyme gene; a transcriptional modulation modification of a biosynthetic enzyme gene; an inactivating mutation in an enzyme; and a heterologous coding sequence. Also provided are methods of producing a BIA of interest or a precursor thereof using the host cells and compositions, e.g., kits, systems etc., that find use in methods of the invention.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Benzylisoquinoline Alkaloid (BIA) Precursor Producing Microbes, and Methods of Making and Using the Same
Host cells that are engineered to produce benzylisoquinoline alkaloid (BIAs) precursors, such as norcodaurine (NC) and norlaudanosoline (NL), are provided. The host cells may have one or more engineered modifications selected from: a feedback inhibition alleviating mutation in a enzyme gene; a transcriptional modulation modification of a biosynthetic enzyme gene; an inactivating mutation in an enzyme; and a heterologous coding sequence. Also provided are methods of producing a BIA of interest or a precursor thereof using the host cells and compositions, e.g., kits, systems etc., that find use in methods of the invention.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Artabotrys pilosus total alkaloid extract and application thereof
InactiveCN104829529AClarify chemical compositionClear chemical compositionOrganic chemistryAntineoplastic agentsDiseaseMiscellaneous antineoplastic
The invention discloses an artabotrys pilosus total alkaloid extract, which is prepared by the following steps: taking dry powder of artabotrys pilosus branches and leaves, carrying out cold soaking extraction with acid water, and mixing acid water extract liquid; filtering, and alkalifying with alkaline water; adding ethyl acetate, extracting, recovering the ethyl acetate in vacuum to obtain an ethyl acetate extract part, namely the total alkaloid part of the artabotrys pilosus branches and leaves, wherein the part mainly comprises 12 alkaloids, including four aporphinoid alkaloids, two benzylisoquinoline alkaloids, two original aporphinoid alkaloids, two morphinane alkaloids and two protoberberine alkaloids. The prepared total alkaloid extract is high in yield, simple in technological process, low in cost and suitable for relatively large-scale preparation, has good antitumor activity, and can be applied to treatment of various tumorous diseases independently or together with other anti-tumor medicines to treat various tumorous diseases.
Owner:HAINAN NORMAL UNIV
Sulfonyl fangchinoline compound as well as preparation method and application thereof
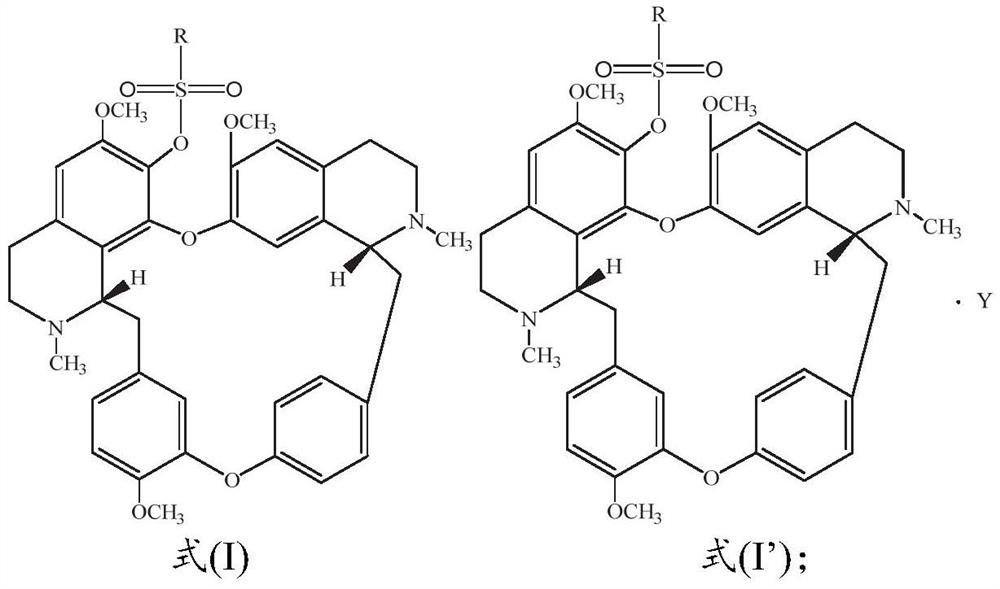
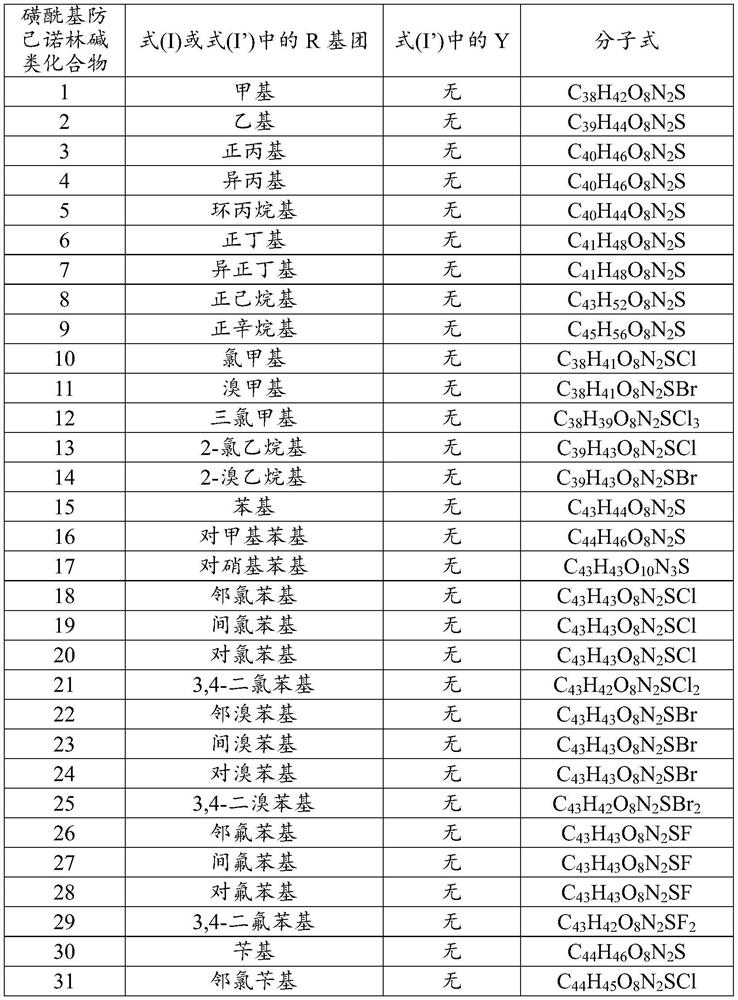
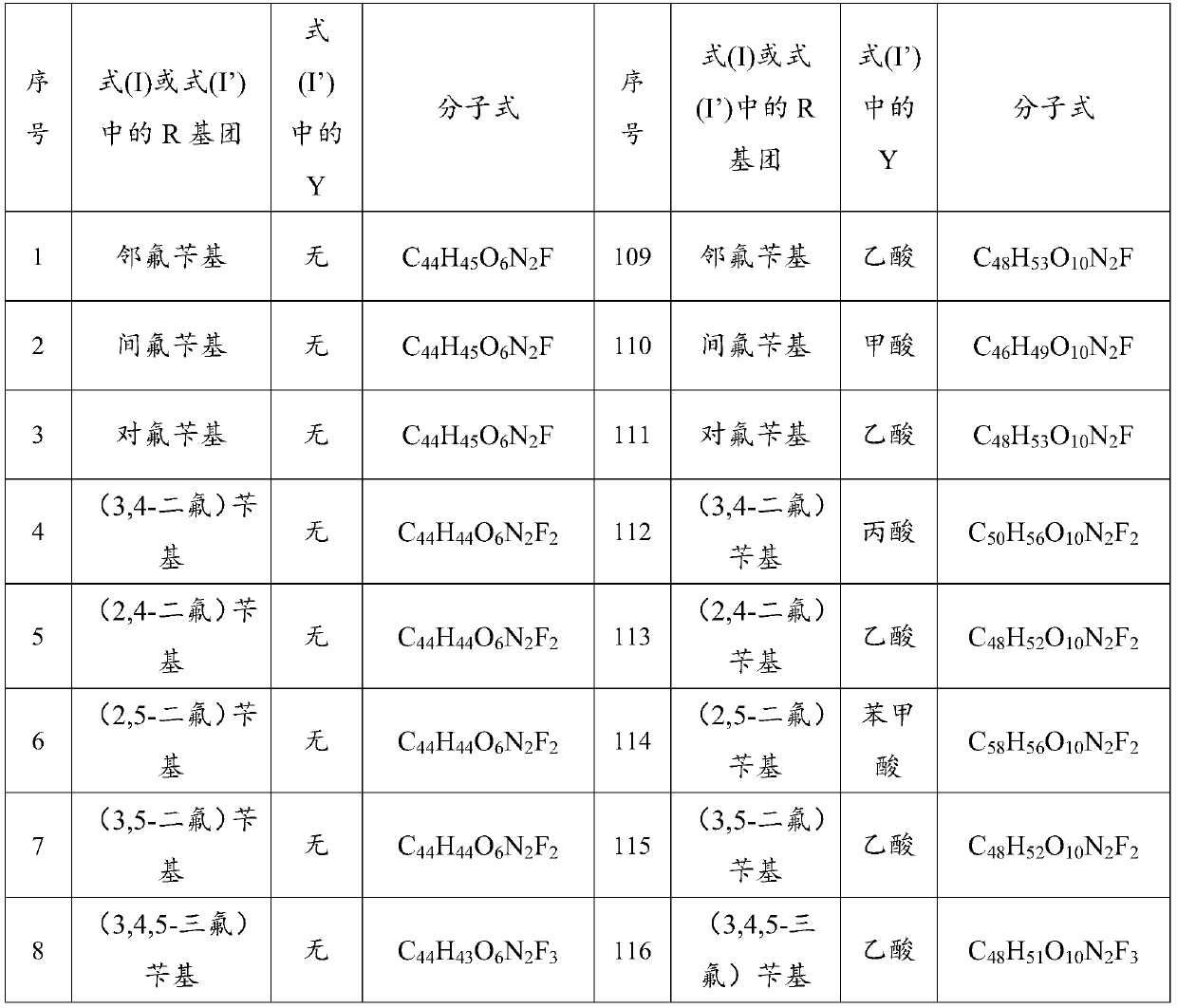
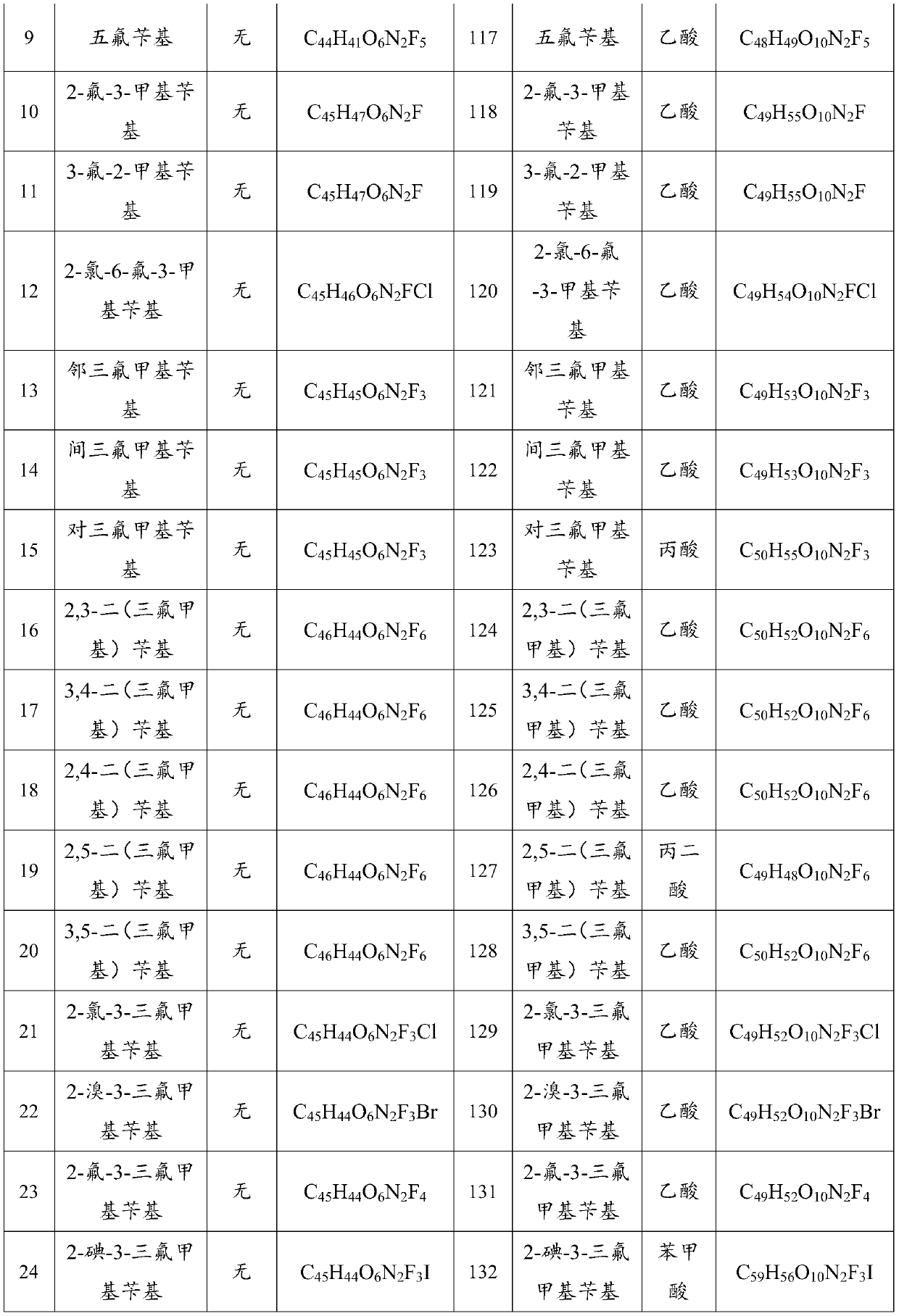
ActiveCN112250690AStrong inhibitory activityHigh inhibition rateOrganic active ingredientsOrganic chemistryOrganic acidFangchinoline
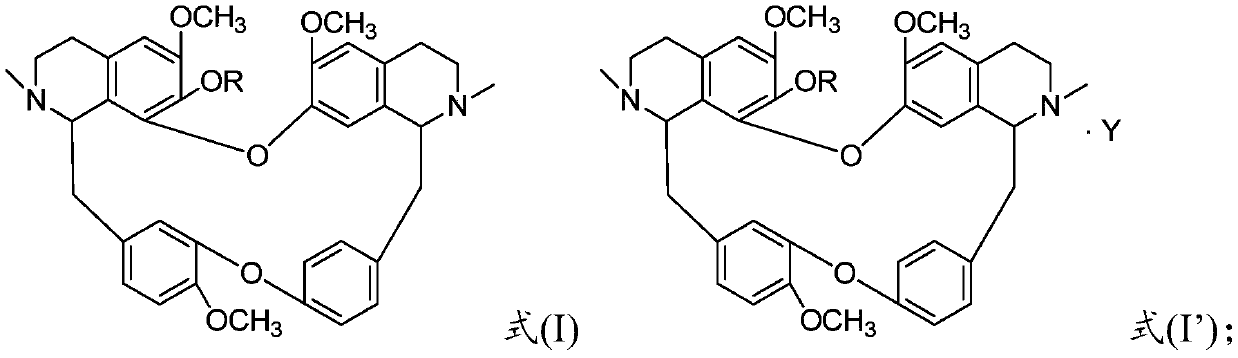
The invention discloses a sulfonyl fangchinoline compound as well as a preparation method and application thereof. The sulfonyl fangchinoline compound has a structure as shown in a formula (I) or a formula (I'). According to the formula (I) or formula (I'), R is selected from alkyl, aryl, benzyl, heterocyclyl, substituted alkyl, substituted aryl, substituted benzyl and substituted heterocyclyl; the substituent group of the substituted alkyl is halogen or nitro; the substituent group of the substituted aryl, the substituent group of the substituted benzyl and the substituent group of the substituted heterocyclyl are one or more of halogen, alkyl and nitro; Y is selected from one-molecule inorganic acid and organic acid or two-molecule inorganic acid and organic acid. The compound has a bisbenzylisoquinoline parent nucleus structure, has sulfonyl characteristics, has a good XOD inhibition effect, and can be used for treating and improving gout, reducing uric acid and the like.
Owner:SHANDONG NORMAL UNIV
Compositions and methods for making benzylisoquinoline alkaloids, morphinan alkaloids, thebaine, and derivatives thereof
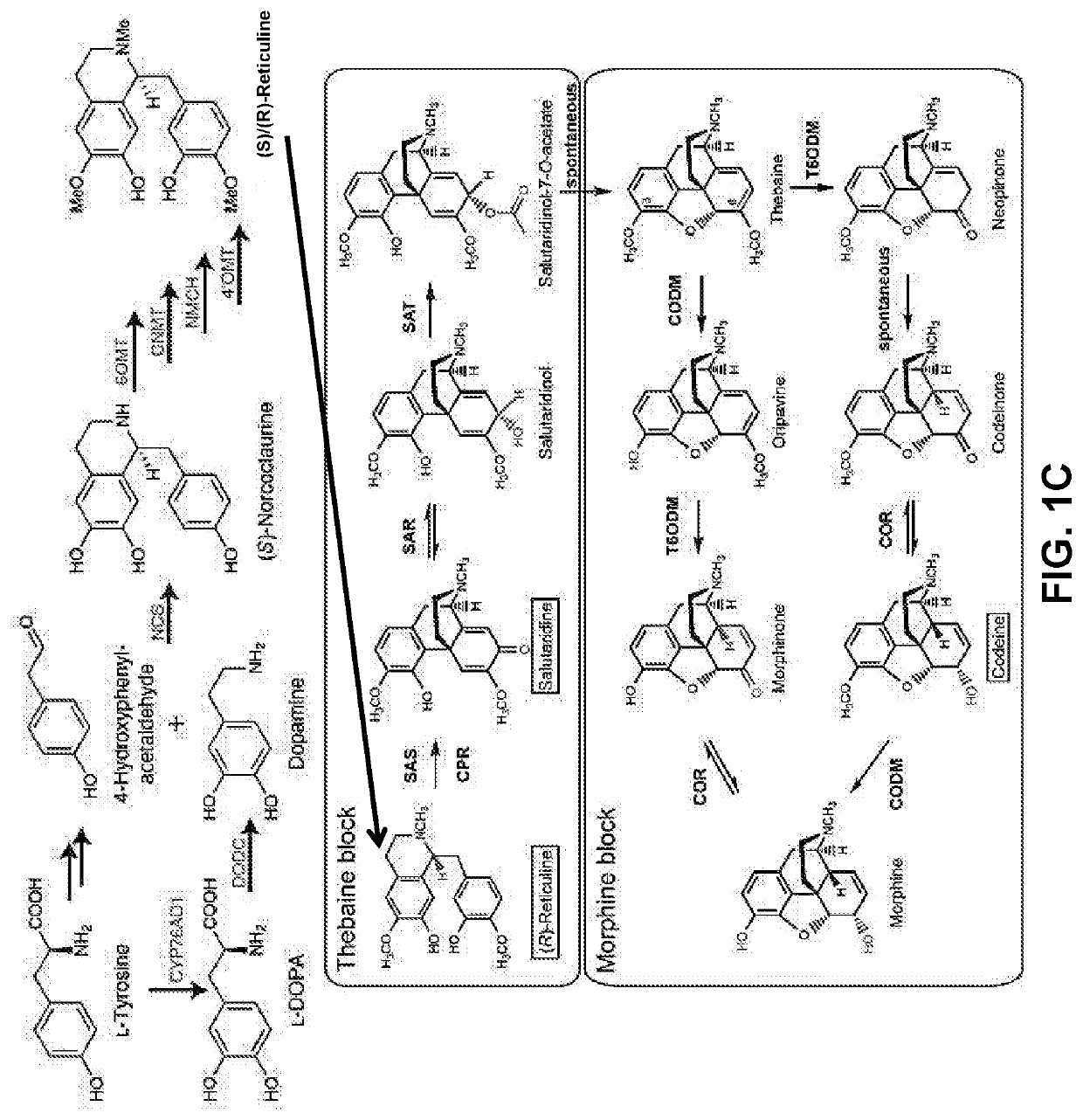
Disclosed herein are methods that may be used for the synthesis of benzylisoquinoline alkaloids (BIAs) such as alkaloid morphinan. The methods disclosed can be used to produce thebaine, oripavine, codeine, morphine, oxycodone, hydrocodone, oxymorphone, hydromorphone, naltrexone, naloxone, hydroxycodeinone, neopinone, and / or buprenorphine. Compositions and organisms useful for the synthesis of BIAs, including thebaine synthesis polypeptides, purine permeases, and polynucleotides encoding the same, are provided.
Owner:ANTHEIA INC
Bisbenzylisoquinoline betaine as well as preparation method thereof and application thereof in preparation of anti-tumor medicament
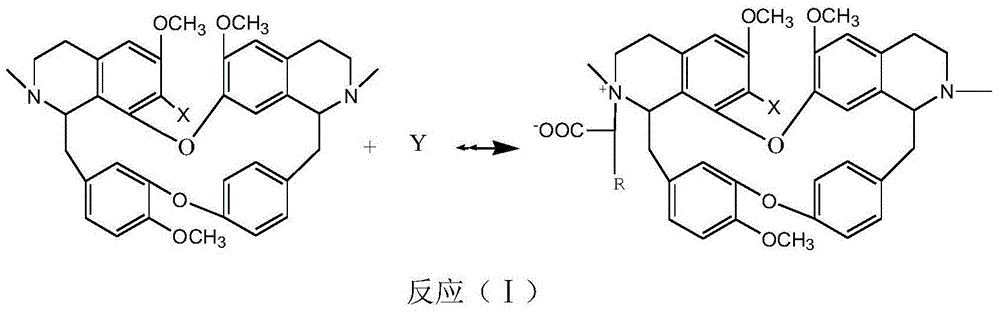
ActiveCN103910741AImprove solubilityNovel chemical structureOrganic active ingredientsOrganic chemistryBetaineQuinoline
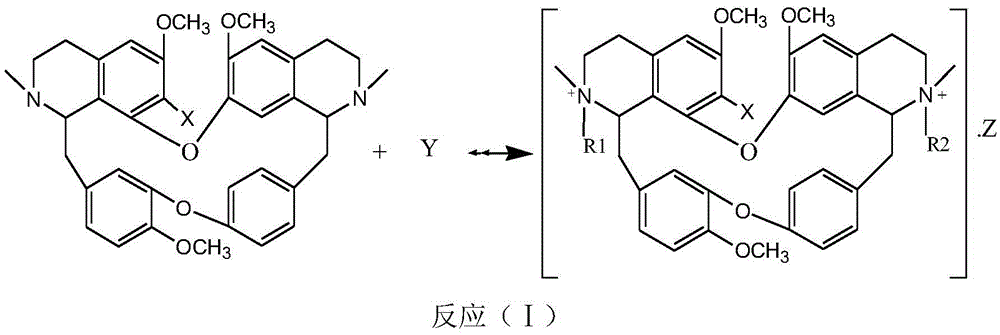
The invention discloses bisbenzylisoquinoline betaine with anti-tumor activity and a preparation method thereof. The compound has the following structural general formula shown in the specification. The preparation method comprises the steps of taking bisbenzylisoquinoline compounds as raw materials, dissolving into a certain solvent, adding a catalyst and a reactant Y, mixing, performing reaction for 0.1-72 hours at -20 DEG C to 300 DEG C, performing hydrolysis and neutralization reaction on the materials after reaction, and then separating and purifying to obtain an objective product. According to the preparation method disclosed by the invention, the prepared bisbenzylisoquinoline betaine has a relatively good inhibiting effect on proliferation of the human liver cancer cell strain HepG2 and leukemia K562 cells, and can be used for preparing anti-tumor medicaments.
Owner:SHANDONG NORMAL UNIV
Novel application of benzylisoquinoline alkaloid
ActiveCN105168214AAbundant sources of raw materialsRich sourcesOrganic active ingredientsMetabolism disorderMedicineFatty acid
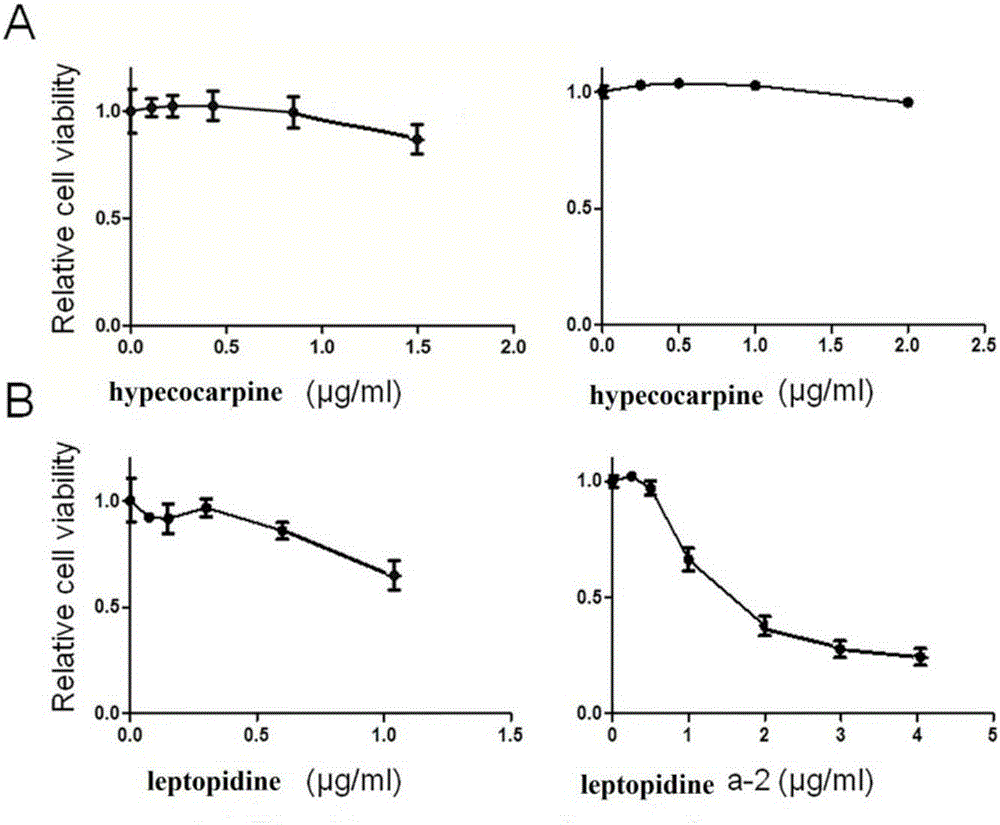
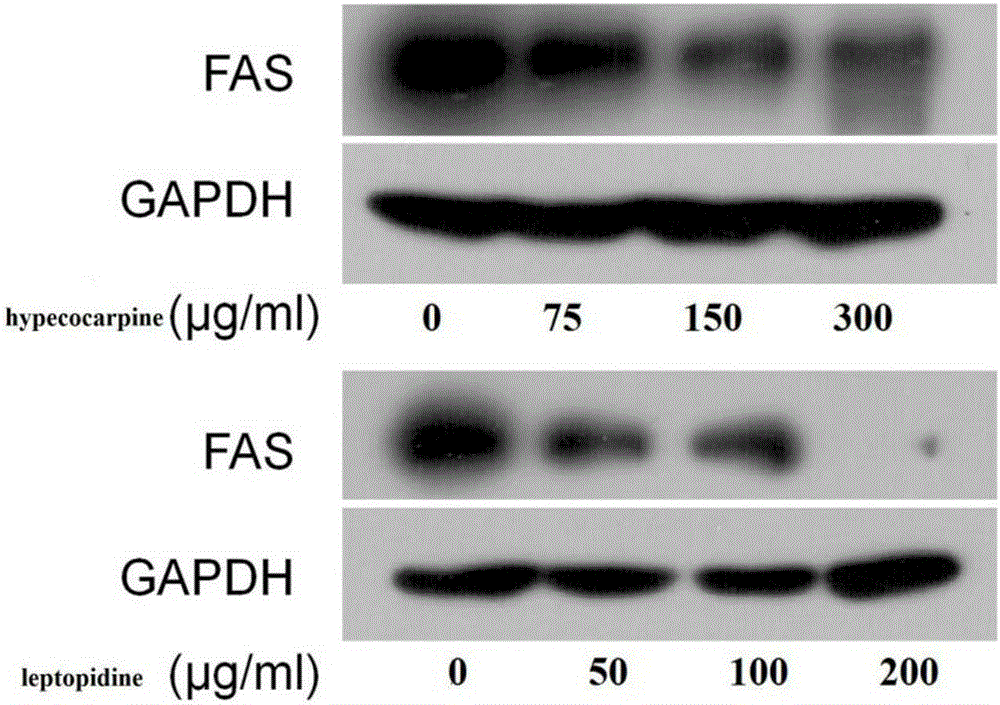
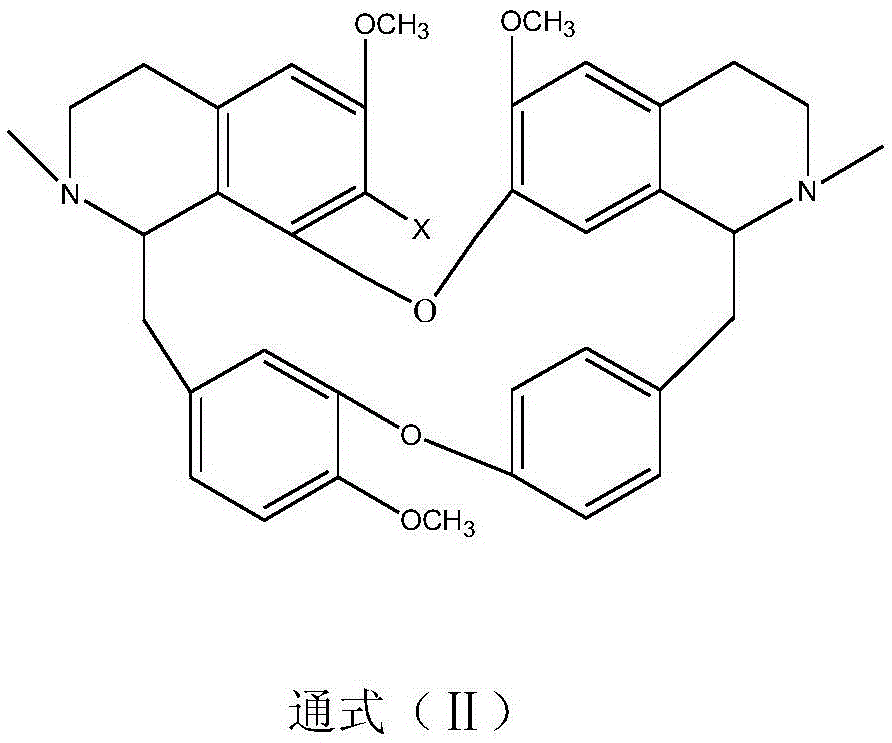
The invention discloses application of benzylisoquinoline alkaloid shown as a formula (I) or a formula (II) or pharmaceutically acceptable salt thereof in preparing fatty acid synthetase (FAS) expression drug. Experiments show that the benzylisoquinoline alkaloid can effectively inhibit FAS expression so as to inhibit proliferation of tumor cells. In addition, the benzylisoquinoline alkaloid is rich in raw material source and has good clinical application prospect.
Owner:CHINA ACAD OF SCI NORTHWEST HIGHLAND BIOLOGY INST
Fujisu and its preparation method and application
ActiveCN109776553BStrong inhibitory activityHigh activityOrganic active ingredientsOrganic chemistryPancreas CancersHuman pancreas
Owner:SHANDONG NORMAL UNIV
Benzylisoquinoline non-depolarization muscle relaxant, and preparation method and use thereof
PendingCN108929269AImprove stabilityHigh purityOrganic active ingredientsOrganic chemistryHigh humidityMoisture
The invention provides a mivacurium chloride hydrate including mivacurium chloride hydrate I and mivacurium chloride hydrate II, and a preparation method and use thereof. The water content of the mivacurium chloride hydrate I is 2.90-3.35 wt%. The mivacurium chloride hydrate disclosed by the invention is good in stability under the conditions of high temperature, high humidity, illumination and acceleration tests; the moisture content is not obviously increased under the condition of a hygroscopicity test, and the product can be stably stored for a long time; and in addition, the preparation process is simple, no special equipment requirement is required, the product is high in yield and purity, the reproducibility is good and the product is suitable for industrial production.
Owner:SICHUAN CREDIT PHARMA
Methods of producing nor-opioid and nal-opioid benzylisoquinoline alkaloids
A method of demethylizing an opioid to a nor-opioid is provided. The method comprises contacting an opioid with at least one enzyme. Contacting the opioid with the at least one enzyme converts the opioid to a nor-opioid. A method of converting a nor-opioid to a nal-opioid is provided. The method comprises contacting a nor-opioid with at least one enzyme. Contacting the nor-opioid with the at least one enzyme converts the nor-opioid to a nal-opioid.
Owner:ANTHEIA INC
Benzylisoquinoline alkaloid with human carboxylesterase 2 inhibition effect and application thereof
ActiveCN112939861AIncrease productionInhibitory activityOrganic active ingredientsMetabolism disorderPharmaceutical drugQuinoline
The invention discloses a benzylisoquinoline alkaloid compound which has a human carboxylesterase 2(hCE2) inhibiting effect and is shown as a formula (I), a plant extract containing the benzylisoquinoline alkaloid compound, a pharmaceutical composition containing the benzylisoquinoline alkaloid compound, and application of the benzylisoquinoline alkaloid compound in preparation of a human carboxylesterase 2(hCE2) inhibitor.
Owner:INST OF MATERIA MEDICA CHINESE ACAD OF MEDICAL SCI
Benzylisoquinoline alkaloid (BIA) precursor producing microbes, and methods of making and using the same
Methods and engineered yeast cells for generating a benzylisoquinoline alkaloid product are provided herein. A method comprises providing engineered yeast cells and a feedstock to a reactor. In the reactor, the engineered yeast cells are subjected to fermentation by incubating the engineered yeast cells for a time period to produce a solution comprising the BIA product and cellular material. The solution comprises not more than one class of molecule selected from the group of protoberberine, morphinan, isopavine, aporphine, and benzylisoquinoline. Additionally, at least one separation unit is used to separate the BIA product from the cellular material to provide the product stream comprising the BIA product.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Bisbenzylisoquinoline glycine betaines as well as preparation method and application thereof in preparing anti-tumor medicines
ActiveCN103910739AEliminate side effectsHigh affinityOrganic active ingredientsOrganic chemistryGlycineSide effect
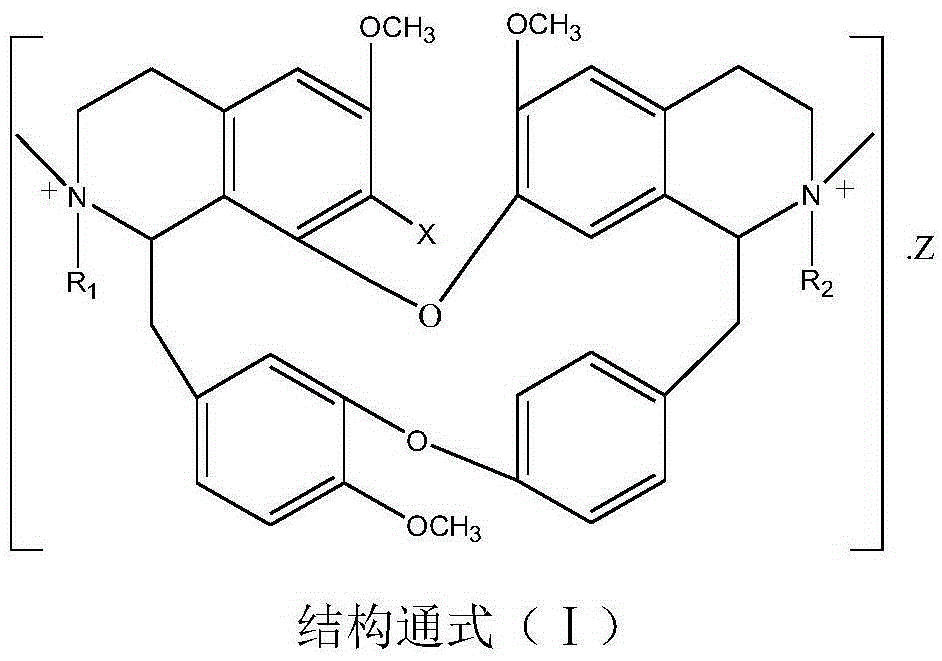
The invention relates to bisbenzylisoquinoline glycine betaines as well as a preparation method thereof and an application in preparing anti-tumor medicines. The structural formula of the bisbenzylisoquinoline glycine betaines is as shown in a general formula (I); substituent groups in the formula are as shown in the specification; the bisbenzylisoquinoline glycine betaines are obtained by reacting bisbenzylisoquinoline or substitutes with a halogenated fatty acid salt in a solvent at a temperature ranging from minus 20 to 300 DEG C. The chemical structural of the bisbenzylisoquinoline glycine betaines enables the affinity to a receptor to be enhanced, and the bisbenzylisoquinoline glycine betaines have relatively high inhibition activity on the proliferation of the human hepatocarcinoma cell line HepG2 and the human colon cancer cells HT29. The anti-liver cancer activity of the bisbenzylisoquinoline glycine betaines is more than 20 times higher than that of the existing tetrandrine compounds, and therefore, the clinical dosage of the medicines can be reduced so that the toxic and side effects of the medicines can be reduced. The general formula is as shown in the specification. The general formula (I) is as shown in the specification.
Owner:SHANDONG NORMAL UNIV
Compositions and methods for making benzylisoquinoline alkaloids, morphinan alkaloids, thebaine, and derivatives thereof
ActiveUS20200140906A1Increased/decreased level of activityTransferasesOxidoreductasesPurineMorphinone
Disclosed herein are methods that may be used for the synthesis of benzylisoquinoline alkaloids (BIAs) such as alkaloid morphinan. The methods disclosed can be used to produce thebaine, oripavine, codeine, morphine, oxycodone, hydrocodone, oxymorphone, hydromorphone, naltrexone, naloxone, hydroxycodeinone, neopinone, and / or buprenorphine. Compositions and organisms useful for the synthesis of BIAs, including thebaine synthesis polypeptides, purine permeases, and polynucleotides encoding the same, are provided.
Owner:ANTHEIA INC
Microorganisms and Methods in the Fermentation of Benzylisoquinoline Alkaloids
Disclosed herein are methods that may be used for the synthesis of benzylisoquinoline alkaloids (“BIAs”) such as thebaine and morphine and their derivatives. The methods disclosed can be used to produce thebaine, oripavine, codeine, morphine, oxycodone, hydrocodone, oxymorphone, hydromorphone, naltrexone, naloxone, hydroxycodeinone, neopinone, and / or buprenorphine. Compositions and organisms useful for the synthesis of BIAs, including thebaine synthases and polynucleotides encoding the same, are provided. Further, methods of adjusting pH to optimize the reaction are disclosed.
Owner:ANTHEIA INC
Tyrosine hydroxylase variants and methods of use thereof
InactiveUS10457918B2Increase tyrosine productionIncreases level and activityMicrobiological testing/measurementOxidoreductasesIncreased tyrosineGene product
Owner:VALORBEC S E C +1
A kind of bisbenzylisoquinoline quaternary ammonium salt and its preparation method and application in the preparation of antitumor drugs
ActiveCN103910738BStrong inhibitory activityHigh activityOrganic active ingredientsOrganic chemistryHepatoma cell linePancreatic cancer cell
The invention relates to bisbenzylisoquinoline quaternary ammonium salt as well as a preparation method and an application thereof in preparing an antitumor drug. A structure of the bisbenzylisoquinoline quaternary ammonium salt is shown in a general formula (I) described in the specification and a substituent group is shown in the specification. The bisbenzylisoquinoline quaternary ammonium salt disclosed by the invention is applied to preparation of an antitumor drug. The compound disclosed by the invention, which has a bisbenzylisoquinoline parent nucleus structure and has the characteristics of ester quaternary ammonium salt, has achieved a pancreatic cancer cell SW1990 proliferation inhibiting rate above 50% and a hepatoma cell line HepG2 inhibiting rate above 50% as well when the compound is 0.5ug / ml, and activity of the compound is much higher than that of an existing tetrandrine compound.
Owner:SHANDONG NORMAL UNIV
Combustion aid
InactiveCN109111962AImprove temperature adaptabilityLow costLiquid carbonaceous fuelsFuel additivesCombustionEngineering
The invention discloses a combustion aid, which comprises the following raw materials by weight: 5-12 parts of acetone, 10-15 parts of benzylisoquinoline, 2-7 parts of isopropanol, 3-5 parts of sodiumoxide, 7-10 parts of glyceryl stearate, 8-10 parts of polyisobutyleneamine, 2-6 parts of metasilicic acid, 2-4 parts of glycerin, and 40-60 parts of nanometer silica. According to the present invention, the combustion aid has advantages of good stability, good temperature adaptability, low-temperature-phase-separation resistance, no high-temperature-air-resistance, low toxicity, low cost, safety,economy and the like.
Owner:南通金庆美术图案设计有限公司
A kind of method for preparing bisbenzylisoquinoline compound
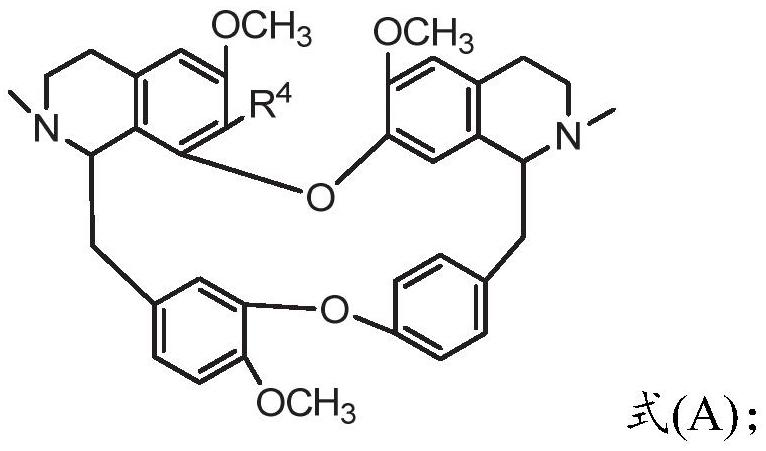
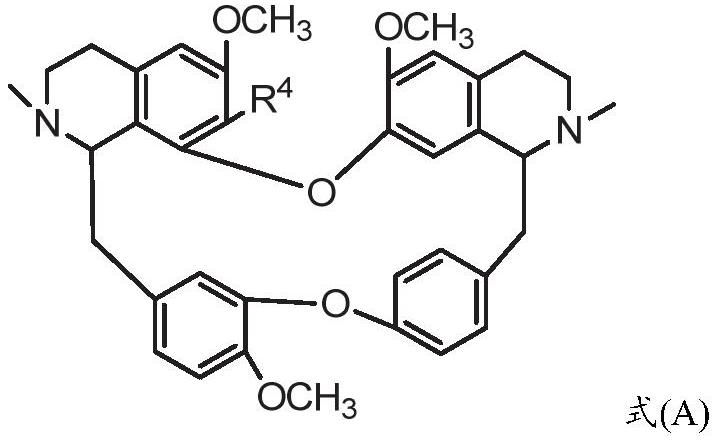
ActiveCN109678871BModerately alkalineImprove catalytic performanceOrganic chemistryIsoquinolineBiochemical engineering
The invention provides a preparation method of bisbenzylisoquinoline compounds. The method comprises the following steps: taking a formula (A) and RX as reactants and carrying out a reaction in a reactor in the presence of ionic liquid or assistants; detecting the reaction progress by chromatography; after the reaction ends, separating and purifying or further adding acid and neutralizing to neutral; then separating and purifying to obtain the bisbenzylisoquinoline compound (The formula is shown in the specification). The method has the advantages of simplicity, environmental protection, highefficiency, as well as high yield which is 90 percent or above, is up to 98 percent or above and is far higher than the yield of a traditional solvent method (20 to 80 percent).
Owner:SHANDONG NORMAL UNIV
Bisbenzylisoquinoline betaine and its preparation method and application in the preparation of antitumor drugs
ActiveCN103910741BHas inhibitory effectLow toxicityOrganic active ingredientsOrganic chemistryBetaineQuinoline
Owner:SHANDONG NORMAL UNIV
Tripolybenzylisoquinoline alkaloid, and preparation method, pharmaceutical composition and application thereof
ActiveCN113214154AGrowth inhibitionGood effectOrganic active ingredientsNervous disorderDiseaseQuinoline
The invention discloses a tripolybenzylisoquinoline alkaloid, and a preparation method, a pharmaceutical composition and application thereof, belongs to the field of medicines, and particularly relates to a group of tripolybenzylisoquinoline alkaloids from Menispermum dauricum DC., which are menidaurine E, menidaurine F, menidaurine G and menidaurine H. The compounds have remarkable anti-tumor and anti-inflammatory activity; and the compound shows a strong inhibition effect on a plurality of tumor cell strains. The invention also relates to the pharmaceutical composition containing an effective dose of the compound, a pharmaceutically acceptable carrier, and an application of the pharmaceutical composition in the field of tumor and immune disease treatment.
Owner:INST OF MATERIA MEDICA CHINESE ACAD OF MEDICAL SCI
Benzyl isoquinoline compounds, preparation method and application thereof
PendingCN111662230AShort duration of muscle relaxationQuick breakdownOrganic active ingredientsSulfonic acids salts preparationMuscle relaxationIsoquinoline
The invention relates to a class of benzyl isoquinoline compounds, a preparation method and application thereof, wherein the structure of the compound is represented by a formula I. The stereoisomer with the structure represented by the formula (I) or the stereoisomer mixture or the pharmaceutically acceptable salt, the solvate of the stereoisomer or the stereoisomer mixture, and the pharmaceutical composition formed by the stereoisomer or the stereoisomer mixture and the pharmaceutically acceptable carrier can generate a neuromuscular junction retardation effect and are applied to the field of preparation of muscle relaxation drugs. Each substituent is consistent with that in the specification.
Owner:SICHUAN DAOZHEN TECH CO LTD
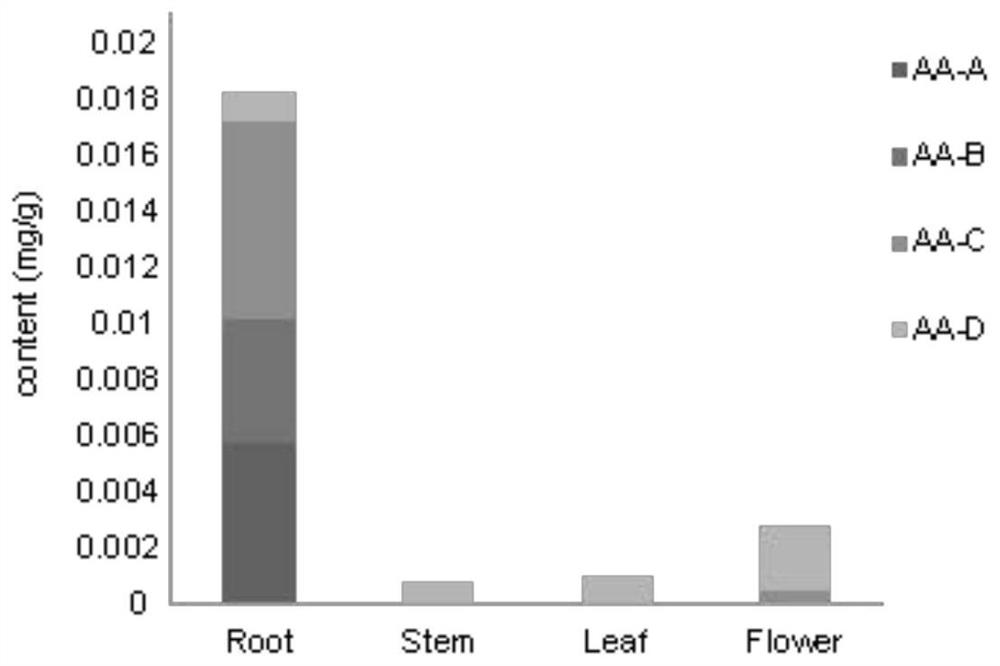
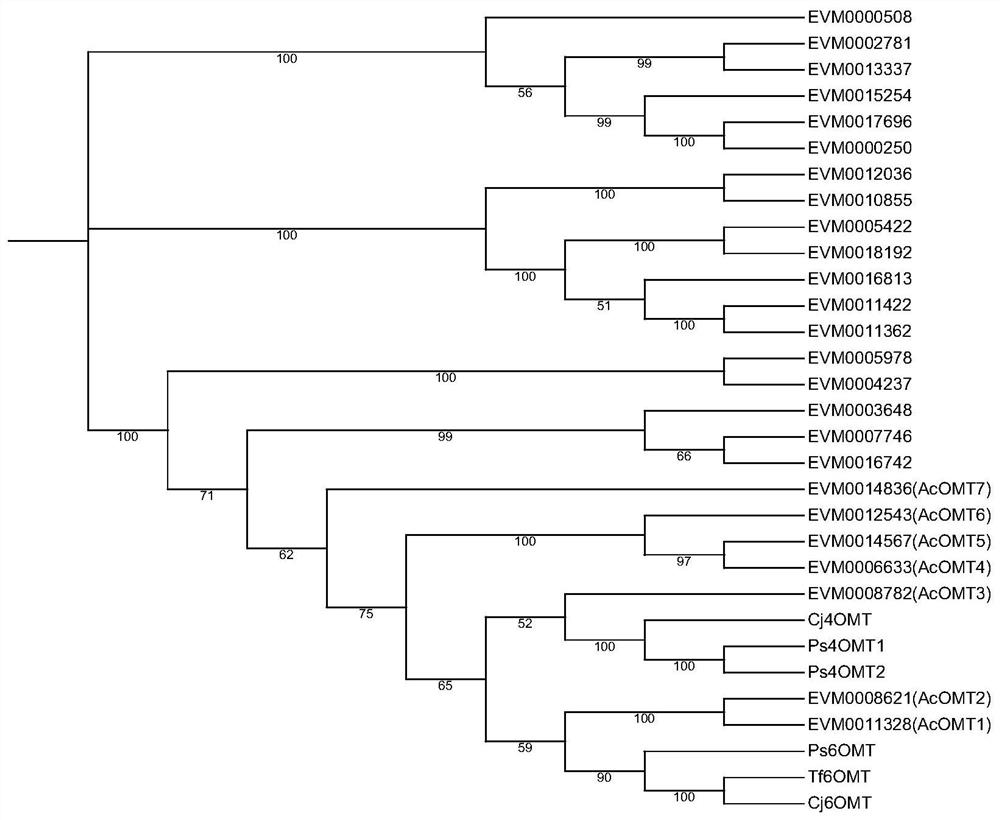
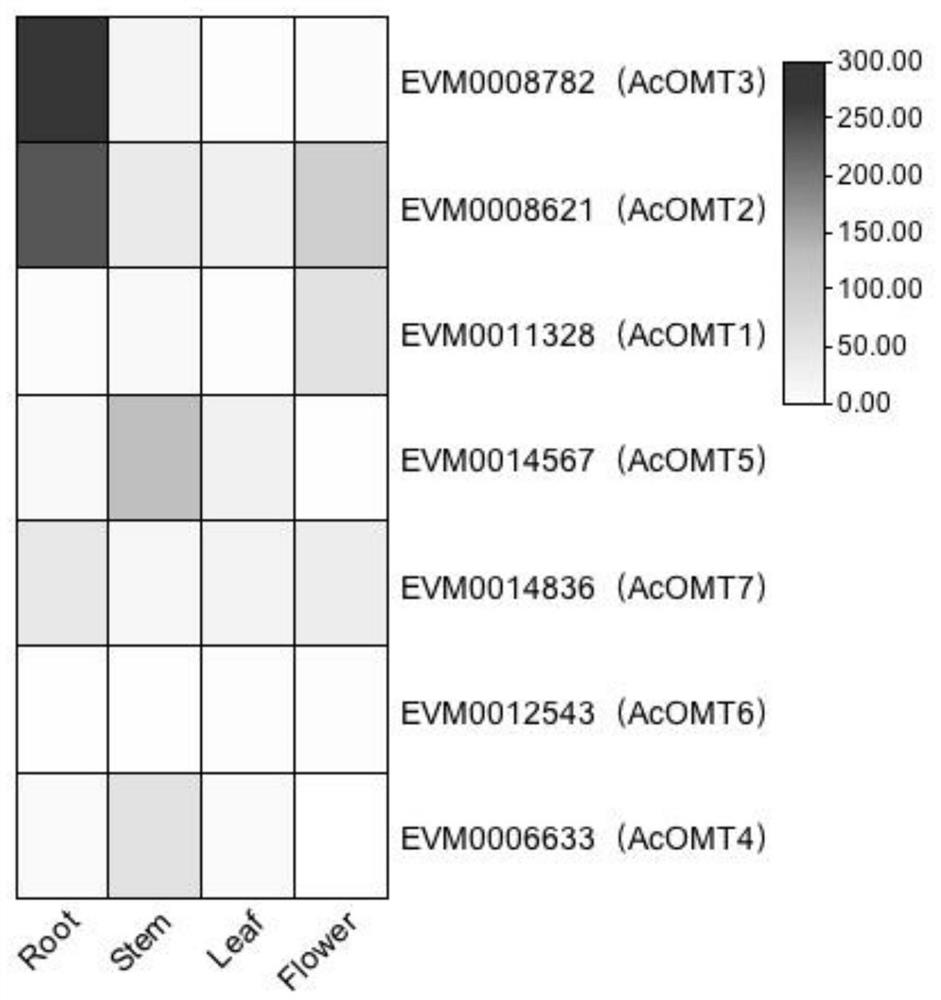
Application of benzylisoquinoline alkaloid and derivative synthetic gene Ac6OMT thereof
The invention discloses an application of an Ac6OMT gene in production of benzylisoquinoline alkaloid and a derivative thereof. The nucleotide sequence of the Ac6OMT gene is as shown in SEQ ID NO. 1; and the synthesized amino acid sequence is as shown in SEQ ID NO. 2. The invention further discloses application of the Ac6OMT gene in breeding transgenic plant varieties which are synthesized or cannot be synthesized with the benzylisoquinoline alkaloid and the derivative thereof, and records a method for regulating organisms to synthesize the benzylisoquinoline alkaloid and the derivative thereof. The Ac6OMT coding gene in a synthetic pathway of the benzylisoquinoline alkaloid and derivatives thereof is subjected to functional identification, and the gene can be applied to biosynthesis and molecular breeding of the aristolochia contorta benzylisoquinoline alkaloid.
Owner:INST OF MEDICINAL PLANT DEV CHINESE ACADEMY OF MEDICAL SCI
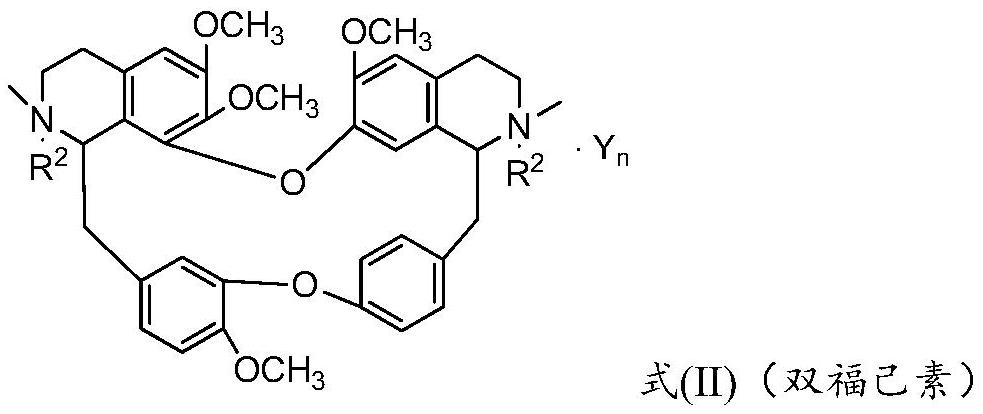
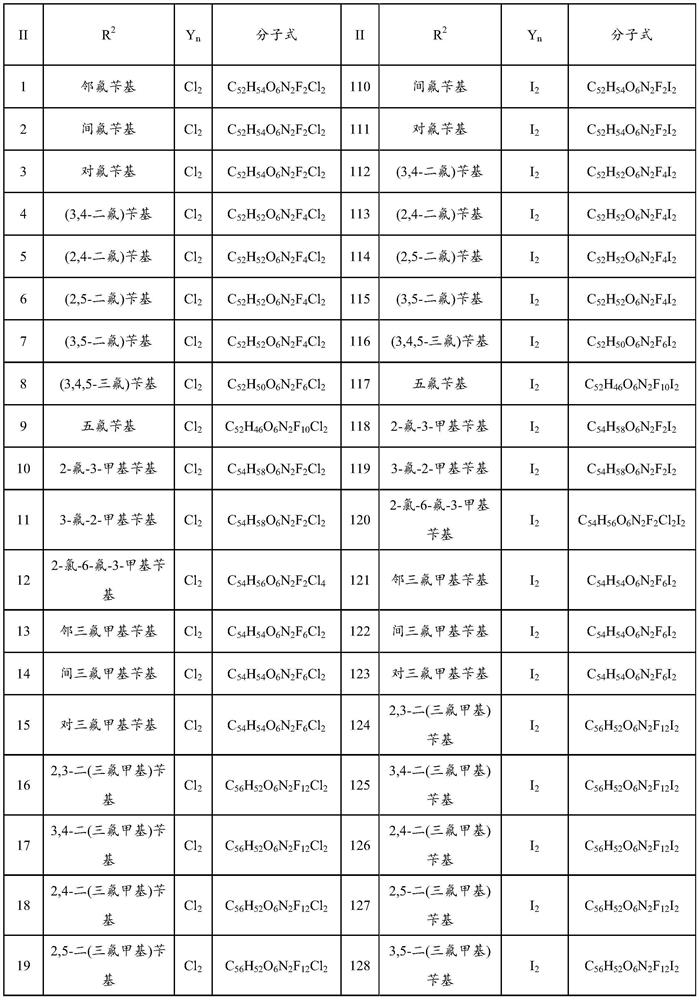
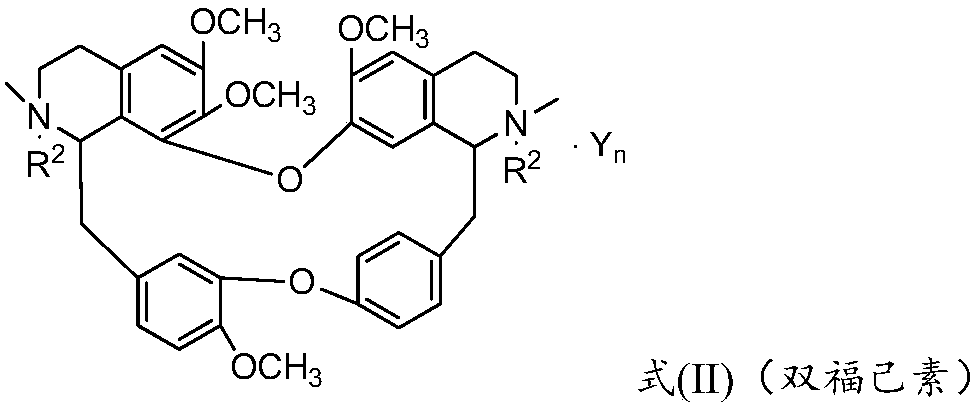
Bisbenzylisoquinoline compound and its preparation method and application
ActiveCN109678872BStrong inhibitory activityHigh activityOrganic active ingredientsOrganic chemistryPancreas CancersCarcinoma bile duct
The invention provides a bisbenzylisoquinoline compound as well as a preparation method and application thereof. The bisbenzylisoquinoline compound has a structure shown in the formula (I), wherein R1and R2 represent same or different groups and are respectively selected from a methyl group; however, R1 and R2 do not represent the methyl group at the same time; R3 represents one or more substituent groups; the R3 group is selected from one or more of halogens, an alkyl group, an alkoxy group and a halogenated alkyl group, wherein R3 represents an F group when R3 is monosubstituted; R3 contains at least one F group or contains the F group when R3 is polysubstituted; Y represents an inorganic acid radical and an organic acid radical, and the value of n is equal to 1 or 2. The inhibition ratio of the type of compounds (concentration: 0.2mug / mL) on proliferation of bile duct carcinoma cells QBC-939 and human pancreatic cancer cells PANC-1 is generally higher than 60%, the inhibition ratioof most of the type of compounds is generally higher than 80%, and the activity of the type of compounds is much higher than that of fluorouracil for current clinical application.
Owner:SHANDONG NORMAL UNIV
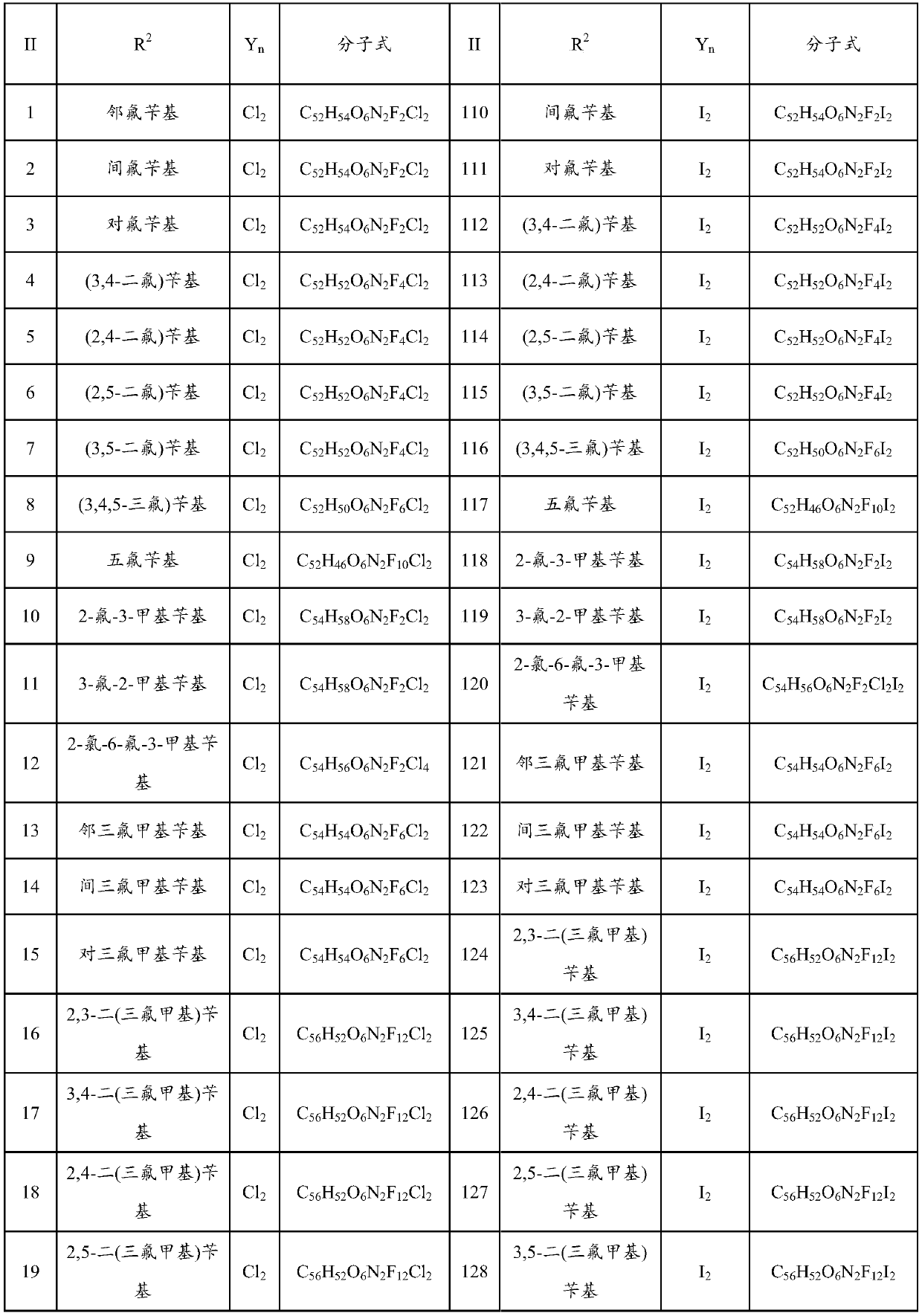
Bisbenzylisoquinoline compound as well as preparation method and application thereof
ActiveCN109678872AStrong inhibitory activityHigh activityOrganic active ingredientsOrganic chemistryHalogenOrganic acid
The invention provides a bisbenzylisoquinoline compound as well as a preparation method and application thereof. The bisbenzylisoquinoline compound has a structure shown in the formula (I), wherein R1and R2 represent same or different groups and are respectively selected from a methyl group; however, R1 and R2 do not represent the methyl group at the same time; R3 represents one or more substituent groups; the R3 group is selected from one or more of halogens, an alkyl group, an alkoxy group and a halogenated alkyl group, wherein R3 represents an F group when R3 is monosubstituted; R3 contains at least one F group or contains the F group when R3 is polysubstituted; Y represents an inorganic acid radical and an organic acid radical, and the value of n is equal to 1 or 2. The inhibition ratio of the type of compounds (concentration: 0.2mug / mL) on proliferation of bile duct carcinoma cells QBC-939 and human pancreatic cancer cells PANC-1 is generally higher than 60%, the inhibition ratioof most of the type of compounds is generally higher than 80%, and the activity of the type of compounds is much higher than that of fluorouracil for current clinical application.
Owner:SHANDONG NORMAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com