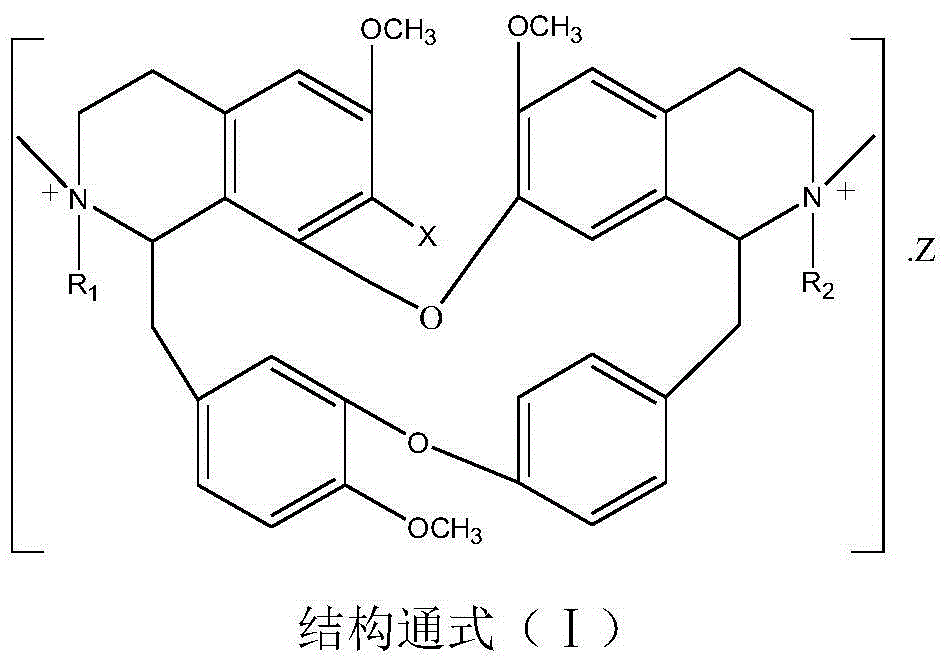
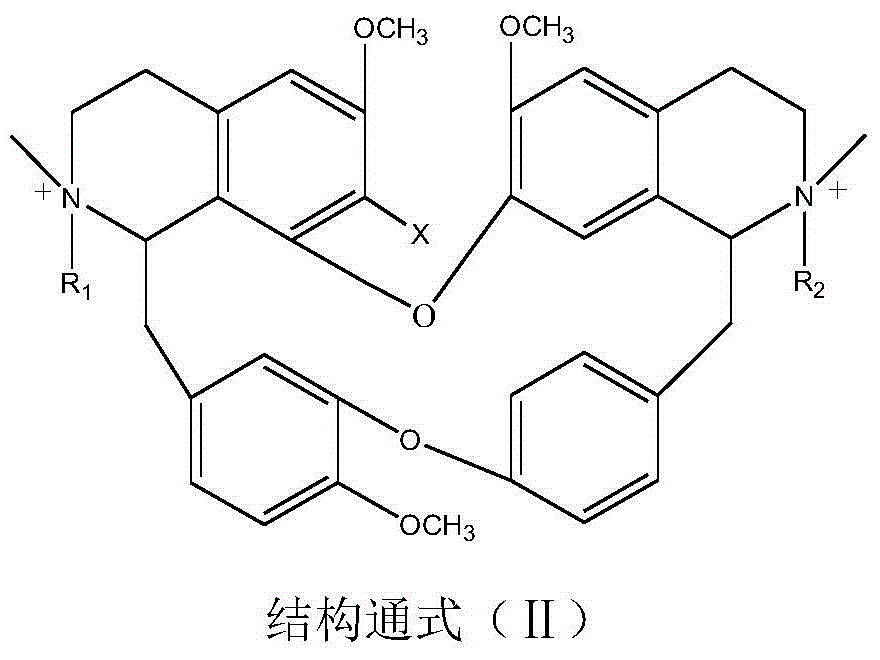
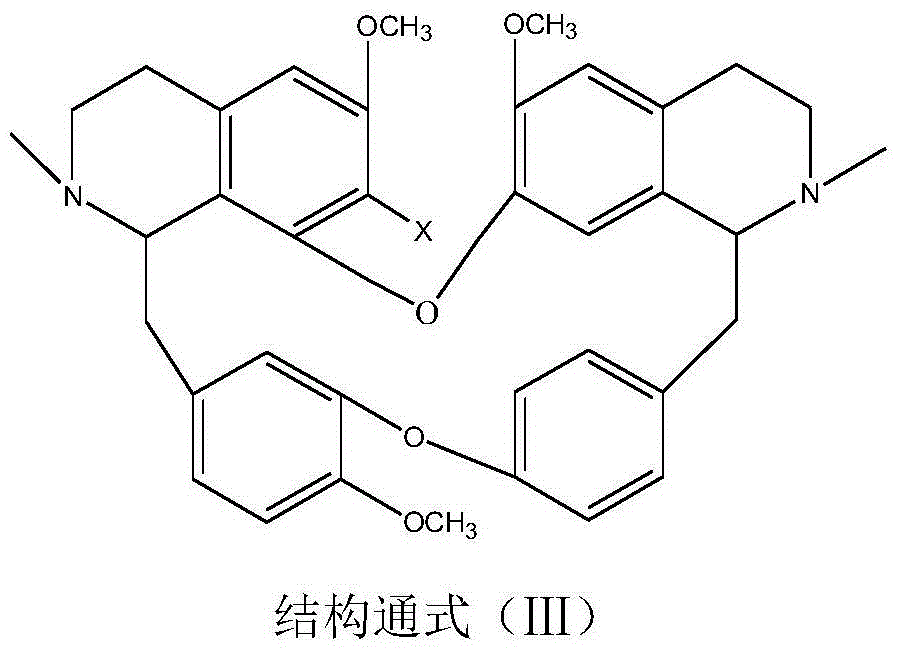
Bisbenzylisoquinoline betaine as well as preparation method thereof and application thereof in preparation of anti-tumor medicament
A technology of bisbenzylisoquinoline and betaine, which is applied in the direction of antineoplastic drugs, drug combinations, and pharmaceutical formulations, can solve problems such as unsatisfactory curative effects, and achieve the effects of strong activity, reduced toxicity, and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Weigh 6.10g of 7-hydroxybisbenzylisoquinoline (in general formula III, X=OH), 3mL of methyl 2-chloroacetate and 1.50g of potassium carbonate, dissolve them in 200mL of ethanol, add them to a 500mL three-necked flask, heat and stir Bring to boil, keep warm and stir for 24 hours, distill ethanol out under reduced pressure, cool down to room temperature, add 50 mL of water, stir at 60 °C for 4 hours, neutralize to neutral with 10% hydrochloric acid, extract 3 times with chloroform (50 mL×3), trace with TLC The separation and purification process of the reaction and the product, the extract is anhydrous Na 2 SO 4 After drying for 8 hours, chloroform was recovered, and the solid was dried at 60°C for 4 hours to obtain 5.87 g of a light yellow powder product with a yield of 83.34% and a purity of 97.23%. The melting point of the target product: 147-148°C, 13 C NMR (75MHz, DMSO-d 6 ): δ20.65(C-4), 23.24(C-4’), 35.67(C-15), 49.70(C-15’), 50.69(NCH 3 ), 53.24 (N'CH 3 ), 53....
Embodiment 2
[0039] Weigh 6.10 g of 7-hydroxybisbenzylisoquinoline (in general formula III, X=OH) and 2.00 g of sodium tert-butoxide, add it to a 500 mL three-necked flask, dissolve it in 100 mL of DMSO, and then add 2-chlorobutyric acid in batches Ethyl ester 50mL, stirred and mixed evenly, stirred and reacted at room temperature for 48h, added water 50mL, stirred at 50°C for 4h, neutralized with dilute hydrochloric acid to neutrality, 200g 200 mesh silica gel column chromatography, washed with dichloromethane-methanol (10:1) Detachment, TLC traced the separation and purification process of the reaction and the product, collected and combined the product fractions, and evaporated the solvent at 60 °C with a rotary evaporator to obtain 4.15 g of a light yellow powder product with a yield of 56.66% and a purity of 95.50 %. The melting point of the target product: 139-141°C, ESI-MS: M / e (348.1706), molecular formula: C 41 h 47 o 8 N 2 Cl, 13 CNMR characterization, namely compound 6 in T...
Embodiment 3
[0041]Weigh 6.10g of 7-hydroxybisbenzylisoquinoline (in general formula III, X=OH), 5mL of methyl 2-chloropropionate and 1.50g of sodium carbonate, dissolve them in 200mL of propanol, add them to a 500mL three-necked flask, Heat and stir until boiling, and keep stirring to react for 12 hours. Evaporate the solvent under reduced pressure, cool down to room temperature, add 50 mL of water, stir at room temperature for 24 hours, neutralize to neutral with 10% hydrochloric acid, and extract 3 times with acetone (60 mL×3), TLC Track the separation and purification process of the reaction and the product, and use anhydrous Na for the extract 2 SO 4 After drying for 8 hours, acetone was recovered, and the solid was dried at 60°C for 4 hours to obtain 5.65 g of a light yellow powder product with a yield of 78.65% and a purity of 95.87%. The melting point of the target product: 145-146°C, 13 CNMR experiment, ESI-MS: M / e (341.1627), molecular formula C 40 h 45 o 8 N 2 Cl, namely c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com