Microorganisms and Methods in the Fermentation of Benzylisoquinoline Alkaloids
a technology of benzylisoquinoline and microorganisms, which is applied in the field of microorganisms and methods in the fermentation of benzylisoquinoline alkaloids, can solve the problems of low yield and/or cost, no commercial biosynthetically manufactured bias methods, and thebaine and other morphinan alkaloids and their derivatives, etc., to achieve the effect of increasing the efficiency of bia synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Genetically Modified Microorganisms that is Capable of Making High Levels of Salutaridine, Salutaridinol and Thebaine
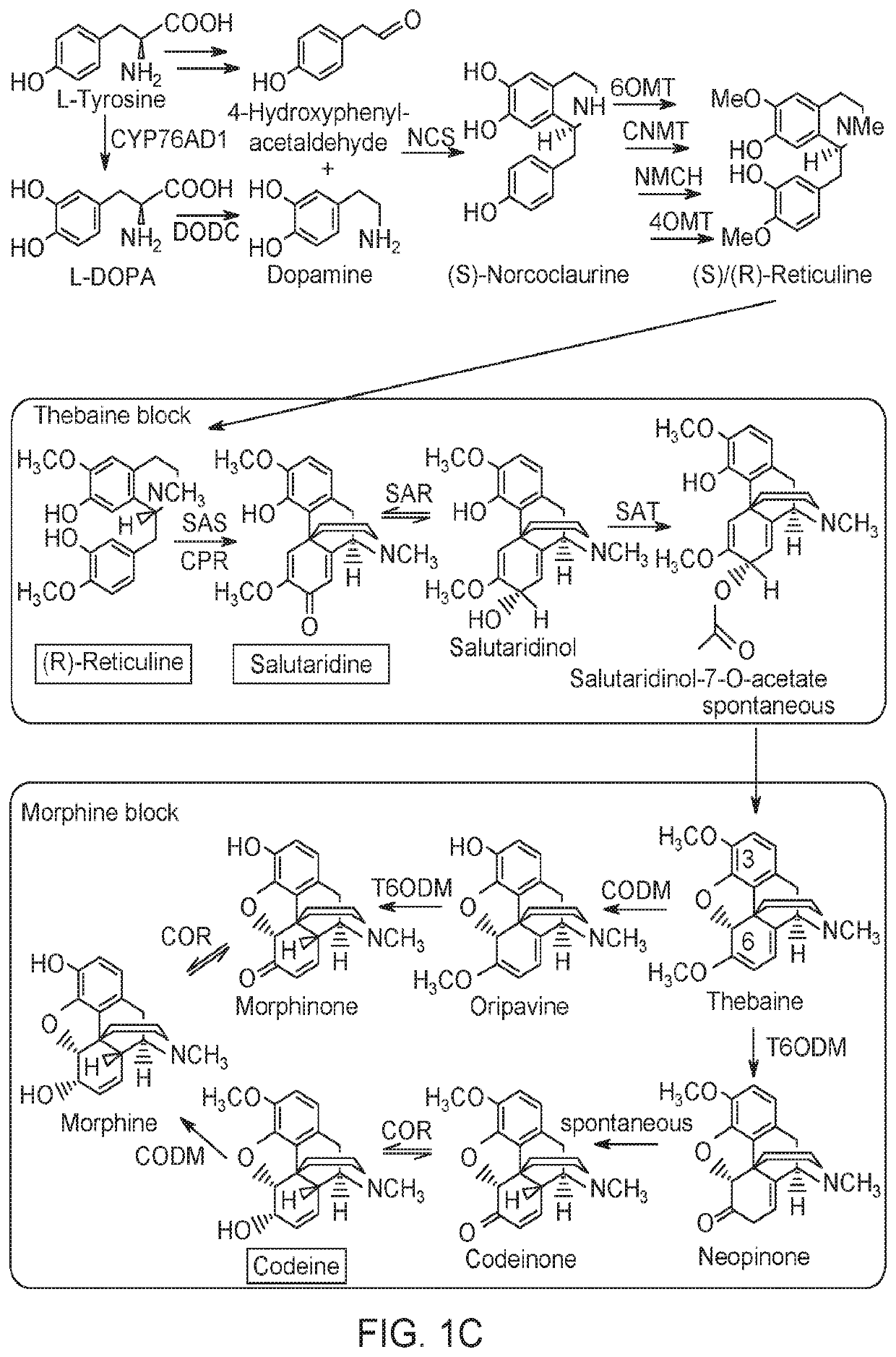
[0224]The S. cerevisiae strain CEN.PK were transformed with nucleic acids encoding for a variety of enzymes that are capable of or take part in the fermentation of sugar to BIAs, such as thebaine. The genotypes of the strains are found in FIG. 4A. The strains contained up to nine chromosomally integrated plant genes encoding BIA biosynthetic enzymes capable of converting (S)-norlaudanosoline to salutaridine, salutaridinol 7-O-acetate, or thebaine. Strain Sc-2 harbored the first seven biosynthetic genes, resulting in the production of salutaridine. Strain Sc-3 contained two additional genes, encoding SalR and SalAT, leading to the formation of salutaridinol 7-O-acetate, whereas strain Sc-4 also included SalR, SalAT and THS2 gene. The sequences of these constructs are presented as SEQ ID NOs. 58 to 63.
example 2
Hydroxylated Byproducts of Salutaridinol 7-O-acetate
[0225]A longstanding dilemma in thebaine biosynthesis involved the formation of the hydrofuran ring in the precursor intermediate salutaridinol. Although salutaridinol undergoes spontaneous allylic elimination at pH <5 in vitro to yield thebaine (FIG. 2A), the possibility that the cyclization of salutaridinol was an enzymatic process remained to be determined. All indications suggested that the cyclization of salutaridinol into thebaine was a spontaneous reaction. First, it was suggested that the C7 hydroxyl of salutaridinol must be functionalized to furnish a better leaving group for intramolecular SN2′ syn displacement. This prediction was realized with the later discovery of salutaridinol 7-O-acetyltransferase (SalAT), which catalyzes the formation of the unstable intermediate salutaridinol 7-O-acetate At pH 8-9 in vitro, but apparently not at lower pH, thebaine was reported to form spontaneously through allylic rearrangement of...
example 3
Thebaine Synthase
[0229]Kinetic analysis of THS2 was performed using a direct assay with (75)-salutaridinol 7-O-acetate as the substrate. (75)-Salutaridinol 7-O-acetate is relatively stable in chloroform but exposure to aqueous conditions caused the rapid (70%) formation of m / z 330-byproduct. To minimize hydroxylated byproduct formation and maintain linear range parameters, the assay was quenched with chloroform after 30 sec (FIG. 3A). The small amount of thebaine formed spontaneously in the absence of THS2 was subtracted from assays containing active enzyme. THS2 displayed positive cooperativity (η=2.3) with a KA of 20 μM, a Vmax of 4.0 nmol min1 μg−1, and a pH optimum of 8.0 (FIG. 3B, FIG. 3C, and FIG. 5). These results suggested that the THS homodimer exhibits allostery with respect to its substrate. The enzymatic reaction mechanism for thebaine formation likely involves deprotonation of the C4 hydroxyl group of (7S)-salutaridinol 7-O-acetate by a catalytic residue acting as a gen...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com