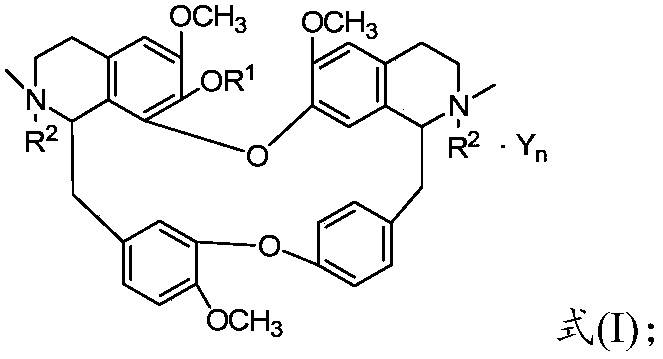
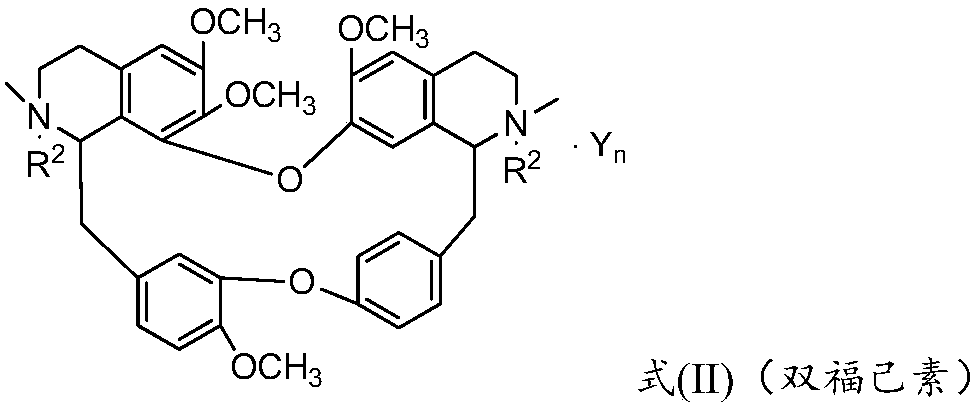
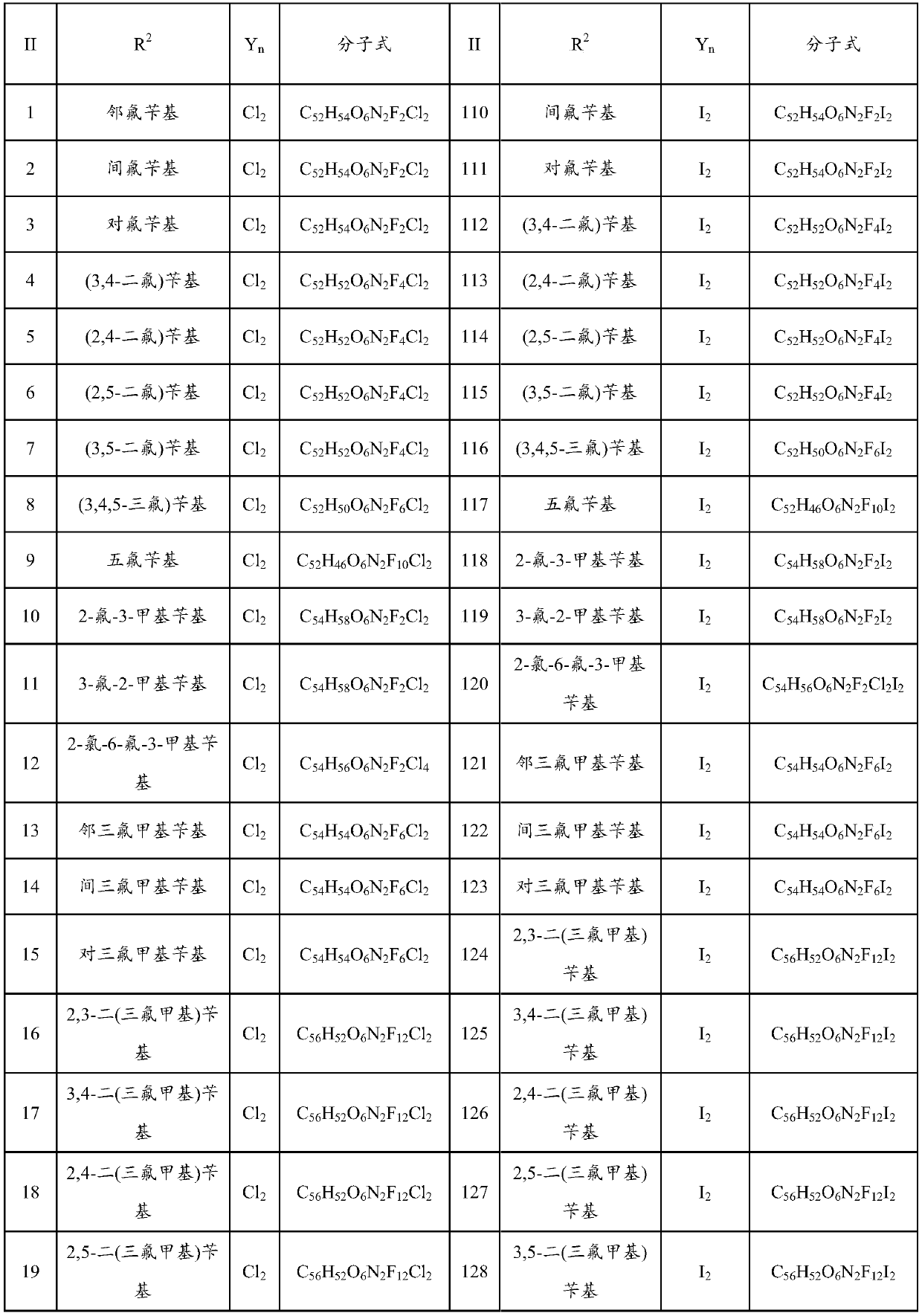
Bisbenzylisoquinoline compound as well as preparation method and application thereof
A technology of bisbenzylisoquinoline and compound, applied in the field of medicine, can solve the problems of poor surgical prognosis, unsatisfactory effect of cholangiocarcinoma and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] Weigh 6.23g of tetrandrine and 0.20g of potassium hydride, dissolve it in 200mL of methanol in a 500mL three-neck flask, add 3.00g of o-fluorobenzyl chloride, stir and heat to 65°C, and keep stirring for 8 hours. TLC detects the complete reaction of tetrandrine , cooled to room temperature, evaporated methanol, added 80 mL of water, extracted 3 times with dichloromethane (100 mL×3), traced the reaction and product separation and purification process by TLC, recovered dichloromethane from the extract, and dried the solid at 60°C for 4 hours , that is, 7.30 g of the product in the form of light yellow powder was obtained, and the yield was 80%. The melting point of the target product: 207.7-209.5°C, 13 C NMR (CDCl 3 ,100MHz)δ160.77,157.87,152.75,150.35,148.34,147.80,147.38,146.09,142.95,137.64,137.51,137.33,133.77,131.71,131.41,129.71,129.65,129.54,128.26,128.06,127.66,127.54,126.90,126.89 ,126.50,123.74,122.12,121.79,120.81,119.34,115.20,113.72,113.51,111.84,110.54,105...
Embodiment 2
[0081] Weigh 18.70g of tetrandrine and 0.20g of sodium ethoxide, dissolve it in 360mL of absolute ethanol in a 500mL three-necked flask, then add 9.00g of p-fluorobenzyl chloride, stir and heat to 78.5°C, and keep stirring for 36h. Alkali reacted completely, distilled out absolute ethanol, cooled to room temperature, added 100mL of water, extracted 3 times with chloroform (150mL×3), traced the separation and purification process of reaction and product by TLC, recovered chloroform from the extract, and the solid was in Dry at 60°C for 4 hours to obtain 16.55 g of a light yellow powder. The melting point of the target product: 208.5-209.9°C, 13 C NMR (CDCl 3 ,100MHz)δ163.55,161.11,153.75,151.39,149.43,148.78,148.52,147.13,143.95,137.41,137.29,136.98,135.12,133.61,133.54,132.65,132.55,132.43,130.09,130.01,128.65,128.43,128.26,127.96 ,127.83,127.26,127.11,122.82,121.91,120.23,116.14,114.89,114.68,112.94,111.59,106.04,73.62,64.07,61.51,56.16,55.93,55.83,55.63,45.65,44.54,44.29,4...
Embodiment 3
[0083] Weigh 6.23g of tetrandrine and 0.50g of potassium carbonate, dissolve in 100mL of propanol in a 500mL three-necked flask, then add 3.60g of 3,5-difluorobenzyl chloride, heat and stir to 98°C, and keep stirring for 24h, TLC Detect the entire reaction of tetrandrine, evaporate the solvent under reduced pressure, cool down to room temperature, add 100 mL of water, extract with acetone for 3 times (150 mL×3), trace the separation and purification process of the reaction and the product by TLC, and use anhydrous Na 2 SO 4 After drying for 8 hours, acetone was recovered, and the solid was dried at 60° C. for 4 hours to obtain 8.22 g of a light yellow powder. The melting point of the target product: 209.2-210.7°C, 13 C NMR (101MHz, CDCl 3 )δ153.87,151.33,151.20,151.04,149.53,148.84,148.74,148.57,147.19,143.91,136.45,135.43,134.98,134.85,134.77,134.56,132.76,130.24,128.86,128.73,128.55,124.02,123.40,122.91,122.00 ,120.28,117.12,116.97,116.74,116.68,116.64,116.62,116.34,113.0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com